User login
Nasal Cannula Dislodgement During Sleep in Veterans Receiving Long-term Oxygen Therapy for Hypoxemic Chronic Respiratory Failure
The prevalence of chronic obstructive pulmonary disease (COPD) among male US veterans is higher than in the general population.1 Veterans with COPD have higher rates of comorbidities and increased respiratory-related and all-cause health care use, including the use of long-term oxygen therapy (LTOT).2-5 It has been well established that LTOT reduces all-cause mortality in patients with COPD and
Delivery of domiciliary LTOT entails placing a nasal cannula into both nostrils and loosely securing it around both ears throughout the wake-sleep cycle. Several veterans with hypoxemic CRF due to COPD at the Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, who were receiving LTOT reported nasal cannula dislodgement (NCD) while they slept. However, the clinical significance and impact of these repeated episodes on respiratory-related health care utilization, such as frequent COPD exacerbations with hospitalization, were not recognized.
The purpose of this study was to determine whether veterans with hypoxemic CRF due to COPD and receiving 24-hour LTOT at JBVAMC were experiencing NCD during sleep and, if so, its impact on
METHODS
We reviewed electronic health records (EHRs) of veterans with hypoxemic CRF from COPD who received 24-hour LTOT administered through nasal cannula and were followed
Pertinent patient demographics, clinical and physiologic variables, and hospitalizations with length of JBVAMC stay for each physician-diagnosed COPD exacerbation in the preceding year from the date last seen in the clinic were abstracted from EHRs. Overall hospital cost, defined as a veteran overnight stay in either the medical intensive care unit (MICU) or a general acute medicine bed in a US Department of Veterans Affairs (VA) facility, was calculated for each hospitalization for physician-diagnosed COPD exacerbation using VA Managerial Cost Accounting System National Cost Extracts for inpatient encounters.15 We then contacted each veteran by telephone and asked whether they had experienced NCD and, if so, its weekly frequency ranging from once to nightly.
Data Analysis
Data were reported as mean (SD) where appropriate. The t test and Fisher exact test were used as indicated. P < .05 was considered statistically significant. The study protocol
RESULTS
During the study period,
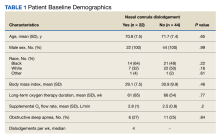
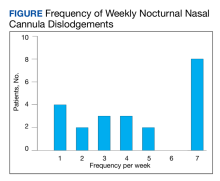
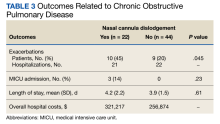
Of the 75 patients, 66 (88%) responded to the telephone survey and 22 patients (33%) reported weekly episodes of NCD while they slept (median, 4 dislodgments per week). (Table 1). Eight patients (36%) reported nightly NCDs (Figure). All 66 respondents were male and 14 of 22 in the NCD group as well as 21 of 44 in the no NCD group were Black veterans. The mean age was similar in both groups: 71 years in the NCD group and 72 years in the no NCD group. There were no statistically significant differences in demographics, including prevalence of obstructive sleep apnea (OSA), supplemental oxygen flow rate, and duration of LTOT, or in pulmonary function test results between patients who did and did not experience NCD while sleeping (Table 2).
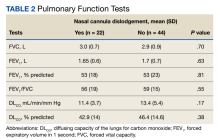
Ten of 22 patients (45%) with NCD and 9 of 44 patients (20%) without NCD were hospitalized at the JBVAMC for ≥ 1 COPD exacerbation in the preceding year that was diagnosed by a physician (P = .045). Three of 22 patients (14%) with NCD and no patients in the no NCD group were admitted to the MICU. No patients required intubation and mechanical ventilation during hospitalization, and no patients died. Overall hospital costs were 25% ($64,342) higher in NCD group compared with the no NCD group and were attributed to the MICU admissions in the NCD group (Table 3). Nine veterans did not respond to repeated telephone calls. One physician-diagnosed COPD exacerbation requiring hospitalization was documented in the nonresponder group; the patient was hospitalized for 2 days. One veteran died before being contacted.
DISCUSSION
There are 3 new findings in this study.
Nocturnal arterial oxygen desaturation in patients with COPD without evidence of OSA may contribute to the frequency of exacerbations.16 Although the mechanism(s) underlying this phenomenon is uncertain, we posit that prolonged nocturnal airway wall hypoxia could amplify underlying chronic inflammation through local generation of reactive oxygen species, thereby predisposing patients to exacerbations. Frequent COPD exacerbations promote disease progression and health status decline and are associated with increased mortality.11,13 Moreover, hospitalization of patients with COPD is the largest contributor to the annual direct cost of COPD per patient.10,12 The higher hospitalization rate observed in the NCD group in our study suggests that interruption of supplemental oxygen delivery while asleep may be a risk factor for COPD exacerbation. Alternatively, an independent factor or factors may have contributed to both NCD during sleep and COPD exacerbation in these patients or an impending exacerbation resulted in sleep disturbances that led to NCD. Additional research is warranted on veterans with hypoxemic CRF from COPD who are receiving LTOT and report frequent NCD during sleep that may support or refute these hypotheses.
To the best of our knowledge, NCD during sleep has not been previously reported in patients
Limitations
This was a small, single-site study, comprised entirely of male patients who are predominantly Black veterans. The telephone interviews with veterans self-reporting NCD during their sleep are prone to recall bias. In addition, the validity and reproducibility of NCD during sleep were not addressed in this study. Missing data from 9 nonresponders may have introduced a nonresponse bias in data analysis and interpretation. The overall hospital cost for a COPD exacerbation at JBVAMC was derived from VA data; US Centers for Medicare & Medicaid Services or commercial carrier data may be different.15,21 Lastly, access to LTOT for veterans with hypoxemic CRF from COPD is regulated and supervised at VA medical facilities.14 This process may be different for patients outside the VA. Taken together, it is difficult to generalize our initial observations to non-VA patients with hypoxemic CRF from COPD who are receiving LTOT. We suggest a large, prospective study of veterans be conducted to determine the prevalence of NCD during sleep and its relationship with COPD exacerbations in veterans receiving LTOT with hypoxemic CRF due to COPD.
CONCLUSIONS
Acknowledgments
We thank Yolanda Davis, RRT, and George Adam for their assistance with this project.
1. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13. doi:10.15620/cdc:101659
2. Sharafkhaneh A, Petersen NJ, Yu H-J, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132. doi:10.2147/copd.s8047
3. LaBedz SL, Krishnan JA, Chung Y-C, et al. Chronic obstructive pulmonary disease outcomes at Veterans Affairs versus non-Veterans Affairs hospitals. Chronic Obstr Pulm Dis. 2021;8(3):306-313. doi:10.15326/jcopdf.2021.0201
4. Darnell K, Dwivedi AK, Weng Z, Panos RJ. Disproportionate utilization of healthcare resources among veterans with COPD: a retrospective analysis of factors associated with COPD healthcare cost. Cost Eff Resour Alloc. 2013;11:13. doi:10.1186/1478-7547-11-13
5. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US Veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
6. Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):CD001744. doi:10.1002/14651858.CD001744.pub2
7. Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(10):1254-1264. doi:10.1164/rccm.201802-0382CI
8. Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(10):e121-e141. doi:10.1164/rccm.202009-3608ST
9. AARC. AARC clinical practice guideline. Oxygen therapy in the home or alternate site health care facility--2007 revision & update. Respir Care. 2007;52(8):1063-1068.
10. Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618
11. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464-471. doi:10.1164/rccm.201710-2029OC
12. Stanford RH, Engel-Nitz NM, Bancroft T, Essoi B. The identification and cost of acute chronic obstructive pulmonary disease exacerbations in a United States population healthcare claims database. COPD. 2020;17(5):499-508. doi:10.1080/15412555.2020.1817357
13. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
14. US Department of Veterans Affairs, Veterans Health Administration. Home oxygen program. VHA Directive 1173.13(1). Published August 5, 2020. Accessed February 28, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8947
15. Phibbs CS, Barnett PG, Fan A, Harden C, King SS, Scott JY. Research guide to decision support system national cost extracts. Health Economics Resource Center of Health Service R&D Services, US Department of Veterans Affairs. September 2010. Accessed February 14, 2024. https://www.herc.research.va.gov/files/book_621.pdf
16. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194. doi:10.1183/09059180.00004311
17. Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373-378. doi:10.1164/rccm.200507-1161WS
18. Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol. 2018;11(3):279-289. doi:10.1080/17512433.2018.1421457
19. Sculley JA, Corbridge SJ, Prieto-Centurion V, et al. Home oxygen therapy for patients with COPD: time for a reboot. Respir Care. 2019;64(12):1574-1585. doi:10.4187/respcare.07135
20. Jacobs SS, Lindell KO, Collins EG, et al. Patient perceptions of the adequacy of supplemental oxygen therapy. Results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15:24-32. doi:10.1513/AnnalsATS.201703-209OC
21. US Centers for Medicare & Medicaid Services. Home use of oxygen. Publication number 100-3. January 3, 2023. Accessed February 14, 2024. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=169
The prevalence of chronic obstructive pulmonary disease (COPD) among male US veterans is higher than in the general population.1 Veterans with COPD have higher rates of comorbidities and increased respiratory-related and all-cause health care use, including the use of long-term oxygen therapy (LTOT).2-5 It has been well established that LTOT reduces all-cause mortality in patients with COPD and
Delivery of domiciliary LTOT entails placing a nasal cannula into both nostrils and loosely securing it around both ears throughout the wake-sleep cycle. Several veterans with hypoxemic CRF due to COPD at the Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, who were receiving LTOT reported nasal cannula dislodgement (NCD) while they slept. However, the clinical significance and impact of these repeated episodes on respiratory-related health care utilization, such as frequent COPD exacerbations with hospitalization, were not recognized.
The purpose of this study was to determine whether veterans with hypoxemic CRF due to COPD and receiving 24-hour LTOT at JBVAMC were experiencing NCD during sleep and, if so, its impact on
METHODS
We reviewed electronic health records (EHRs) of veterans with hypoxemic CRF from COPD who received 24-hour LTOT administered through nasal cannula and were followed
Pertinent patient demographics, clinical and physiologic variables, and hospitalizations with length of JBVAMC stay for each physician-diagnosed COPD exacerbation in the preceding year from the date last seen in the clinic were abstracted from EHRs. Overall hospital cost, defined as a veteran overnight stay in either the medical intensive care unit (MICU) or a general acute medicine bed in a US Department of Veterans Affairs (VA) facility, was calculated for each hospitalization for physician-diagnosed COPD exacerbation using VA Managerial Cost Accounting System National Cost Extracts for inpatient encounters.15 We then contacted each veteran by telephone and asked whether they had experienced NCD and, if so, its weekly frequency ranging from once to nightly.
Data Analysis
Data were reported as mean (SD) where appropriate. The t test and Fisher exact test were used as indicated. P < .05 was considered statistically significant. The study protocol
RESULTS
During the study period,
Of the 75 patients, 66 (88%) responded to the telephone survey and 22 patients (33%) reported weekly episodes of NCD while they slept (median, 4 dislodgments per week). (Table 1). Eight patients (36%) reported nightly NCDs (Figure). All 66 respondents were male and 14 of 22 in the NCD group as well as 21 of 44 in the no NCD group were Black veterans. The mean age was similar in both groups: 71 years in the NCD group and 72 years in the no NCD group. There were no statistically significant differences in demographics, including prevalence of obstructive sleep apnea (OSA), supplemental oxygen flow rate, and duration of LTOT, or in pulmonary function test results between patients who did and did not experience NCD while sleeping (Table 2).
Ten of 22 patients (45%) with NCD and 9 of 44 patients (20%) without NCD were hospitalized at the JBVAMC for ≥ 1 COPD exacerbation in the preceding year that was diagnosed by a physician (P = .045). Three of 22 patients (14%) with NCD and no patients in the no NCD group were admitted to the MICU. No patients required intubation and mechanical ventilation during hospitalization, and no patients died. Overall hospital costs were 25% ($64,342) higher in NCD group compared with the no NCD group and were attributed to the MICU admissions in the NCD group (Table 3). Nine veterans did not respond to repeated telephone calls. One physician-diagnosed COPD exacerbation requiring hospitalization was documented in the nonresponder group; the patient was hospitalized for 2 days. One veteran died before being contacted.
DISCUSSION
There are 3 new findings in this study.
Nocturnal arterial oxygen desaturation in patients with COPD without evidence of OSA may contribute to the frequency of exacerbations.16 Although the mechanism(s) underlying this phenomenon is uncertain, we posit that prolonged nocturnal airway wall hypoxia could amplify underlying chronic inflammation through local generation of reactive oxygen species, thereby predisposing patients to exacerbations. Frequent COPD exacerbations promote disease progression and health status decline and are associated with increased mortality.11,13 Moreover, hospitalization of patients with COPD is the largest contributor to the annual direct cost of COPD per patient.10,12 The higher hospitalization rate observed in the NCD group in our study suggests that interruption of supplemental oxygen delivery while asleep may be a risk factor for COPD exacerbation. Alternatively, an independent factor or factors may have contributed to both NCD during sleep and COPD exacerbation in these patients or an impending exacerbation resulted in sleep disturbances that led to NCD. Additional research is warranted on veterans with hypoxemic CRF from COPD who are receiving LTOT and report frequent NCD during sleep that may support or refute these hypotheses.
To the best of our knowledge, NCD during sleep has not been previously reported in patients
Limitations
This was a small, single-site study, comprised entirely of male patients who are predominantly Black veterans. The telephone interviews with veterans self-reporting NCD during their sleep are prone to recall bias. In addition, the validity and reproducibility of NCD during sleep were not addressed in this study. Missing data from 9 nonresponders may have introduced a nonresponse bias in data analysis and interpretation. The overall hospital cost for a COPD exacerbation at JBVAMC was derived from VA data; US Centers for Medicare & Medicaid Services or commercial carrier data may be different.15,21 Lastly, access to LTOT for veterans with hypoxemic CRF from COPD is regulated and supervised at VA medical facilities.14 This process may be different for patients outside the VA. Taken together, it is difficult to generalize our initial observations to non-VA patients with hypoxemic CRF from COPD who are receiving LTOT. We suggest a large, prospective study of veterans be conducted to determine the prevalence of NCD during sleep and its relationship with COPD exacerbations in veterans receiving LTOT with hypoxemic CRF due to COPD.
CONCLUSIONS
Acknowledgments
We thank Yolanda Davis, RRT, and George Adam for their assistance with this project.
The prevalence of chronic obstructive pulmonary disease (COPD) among male US veterans is higher than in the general population.1 Veterans with COPD have higher rates of comorbidities and increased respiratory-related and all-cause health care use, including the use of long-term oxygen therapy (LTOT).2-5 It has been well established that LTOT reduces all-cause mortality in patients with COPD and
Delivery of domiciliary LTOT entails placing a nasal cannula into both nostrils and loosely securing it around both ears throughout the wake-sleep cycle. Several veterans with hypoxemic CRF due to COPD at the Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, who were receiving LTOT reported nasal cannula dislodgement (NCD) while they slept. However, the clinical significance and impact of these repeated episodes on respiratory-related health care utilization, such as frequent COPD exacerbations with hospitalization, were not recognized.
The purpose of this study was to determine whether veterans with hypoxemic CRF due to COPD and receiving 24-hour LTOT at JBVAMC were experiencing NCD during sleep and, if so, its impact on
METHODS
We reviewed electronic health records (EHRs) of veterans with hypoxemic CRF from COPD who received 24-hour LTOT administered through nasal cannula and were followed
Pertinent patient demographics, clinical and physiologic variables, and hospitalizations with length of JBVAMC stay for each physician-diagnosed COPD exacerbation in the preceding year from the date last seen in the clinic were abstracted from EHRs. Overall hospital cost, defined as a veteran overnight stay in either the medical intensive care unit (MICU) or a general acute medicine bed in a US Department of Veterans Affairs (VA) facility, was calculated for each hospitalization for physician-diagnosed COPD exacerbation using VA Managerial Cost Accounting System National Cost Extracts for inpatient encounters.15 We then contacted each veteran by telephone and asked whether they had experienced NCD and, if so, its weekly frequency ranging from once to nightly.
Data Analysis
Data were reported as mean (SD) where appropriate. The t test and Fisher exact test were used as indicated. P < .05 was considered statistically significant. The study protocol
RESULTS
During the study period,
Of the 75 patients, 66 (88%) responded to the telephone survey and 22 patients (33%) reported weekly episodes of NCD while they slept (median, 4 dislodgments per week). (Table 1). Eight patients (36%) reported nightly NCDs (Figure). All 66 respondents were male and 14 of 22 in the NCD group as well as 21 of 44 in the no NCD group were Black veterans. The mean age was similar in both groups: 71 years in the NCD group and 72 years in the no NCD group. There were no statistically significant differences in demographics, including prevalence of obstructive sleep apnea (OSA), supplemental oxygen flow rate, and duration of LTOT, or in pulmonary function test results between patients who did and did not experience NCD while sleeping (Table 2).
Ten of 22 patients (45%) with NCD and 9 of 44 patients (20%) without NCD were hospitalized at the JBVAMC for ≥ 1 COPD exacerbation in the preceding year that was diagnosed by a physician (P = .045). Three of 22 patients (14%) with NCD and no patients in the no NCD group were admitted to the MICU. No patients required intubation and mechanical ventilation during hospitalization, and no patients died. Overall hospital costs were 25% ($64,342) higher in NCD group compared with the no NCD group and were attributed to the MICU admissions in the NCD group (Table 3). Nine veterans did not respond to repeated telephone calls. One physician-diagnosed COPD exacerbation requiring hospitalization was documented in the nonresponder group; the patient was hospitalized for 2 days. One veteran died before being contacted.
DISCUSSION
There are 3 new findings in this study.
Nocturnal arterial oxygen desaturation in patients with COPD without evidence of OSA may contribute to the frequency of exacerbations.16 Although the mechanism(s) underlying this phenomenon is uncertain, we posit that prolonged nocturnal airway wall hypoxia could amplify underlying chronic inflammation through local generation of reactive oxygen species, thereby predisposing patients to exacerbations. Frequent COPD exacerbations promote disease progression and health status decline and are associated with increased mortality.11,13 Moreover, hospitalization of patients with COPD is the largest contributor to the annual direct cost of COPD per patient.10,12 The higher hospitalization rate observed in the NCD group in our study suggests that interruption of supplemental oxygen delivery while asleep may be a risk factor for COPD exacerbation. Alternatively, an independent factor or factors may have contributed to both NCD during sleep and COPD exacerbation in these patients or an impending exacerbation resulted in sleep disturbances that led to NCD. Additional research is warranted on veterans with hypoxemic CRF from COPD who are receiving LTOT and report frequent NCD during sleep that may support or refute these hypotheses.
To the best of our knowledge, NCD during sleep has not been previously reported in patients
Limitations
This was a small, single-site study, comprised entirely of male patients who are predominantly Black veterans. The telephone interviews with veterans self-reporting NCD during their sleep are prone to recall bias. In addition, the validity and reproducibility of NCD during sleep were not addressed in this study. Missing data from 9 nonresponders may have introduced a nonresponse bias in data analysis and interpretation. The overall hospital cost for a COPD exacerbation at JBVAMC was derived from VA data; US Centers for Medicare & Medicaid Services or commercial carrier data may be different.15,21 Lastly, access to LTOT for veterans with hypoxemic CRF from COPD is regulated and supervised at VA medical facilities.14 This process may be different for patients outside the VA. Taken together, it is difficult to generalize our initial observations to non-VA patients with hypoxemic CRF from COPD who are receiving LTOT. We suggest a large, prospective study of veterans be conducted to determine the prevalence of NCD during sleep and its relationship with COPD exacerbations in veterans receiving LTOT with hypoxemic CRF due to COPD.
CONCLUSIONS
Acknowledgments
We thank Yolanda Davis, RRT, and George Adam for their assistance with this project.
1. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13. doi:10.15620/cdc:101659
2. Sharafkhaneh A, Petersen NJ, Yu H-J, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132. doi:10.2147/copd.s8047
3. LaBedz SL, Krishnan JA, Chung Y-C, et al. Chronic obstructive pulmonary disease outcomes at Veterans Affairs versus non-Veterans Affairs hospitals. Chronic Obstr Pulm Dis. 2021;8(3):306-313. doi:10.15326/jcopdf.2021.0201
4. Darnell K, Dwivedi AK, Weng Z, Panos RJ. Disproportionate utilization of healthcare resources among veterans with COPD: a retrospective analysis of factors associated with COPD healthcare cost. Cost Eff Resour Alloc. 2013;11:13. doi:10.1186/1478-7547-11-13
5. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US Veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
6. Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):CD001744. doi:10.1002/14651858.CD001744.pub2
7. Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(10):1254-1264. doi:10.1164/rccm.201802-0382CI
8. Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(10):e121-e141. doi:10.1164/rccm.202009-3608ST
9. AARC. AARC clinical practice guideline. Oxygen therapy in the home or alternate site health care facility--2007 revision & update. Respir Care. 2007;52(8):1063-1068.
10. Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618
11. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464-471. doi:10.1164/rccm.201710-2029OC
12. Stanford RH, Engel-Nitz NM, Bancroft T, Essoi B. The identification and cost of acute chronic obstructive pulmonary disease exacerbations in a United States population healthcare claims database. COPD. 2020;17(5):499-508. doi:10.1080/15412555.2020.1817357
13. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
14. US Department of Veterans Affairs, Veterans Health Administration. Home oxygen program. VHA Directive 1173.13(1). Published August 5, 2020. Accessed February 28, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8947
15. Phibbs CS, Barnett PG, Fan A, Harden C, King SS, Scott JY. Research guide to decision support system national cost extracts. Health Economics Resource Center of Health Service R&D Services, US Department of Veterans Affairs. September 2010. Accessed February 14, 2024. https://www.herc.research.va.gov/files/book_621.pdf
16. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194. doi:10.1183/09059180.00004311
17. Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373-378. doi:10.1164/rccm.200507-1161WS
18. Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol. 2018;11(3):279-289. doi:10.1080/17512433.2018.1421457
19. Sculley JA, Corbridge SJ, Prieto-Centurion V, et al. Home oxygen therapy for patients with COPD: time for a reboot. Respir Care. 2019;64(12):1574-1585. doi:10.4187/respcare.07135
20. Jacobs SS, Lindell KO, Collins EG, et al. Patient perceptions of the adequacy of supplemental oxygen therapy. Results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15:24-32. doi:10.1513/AnnalsATS.201703-209OC
21. US Centers for Medicare & Medicaid Services. Home use of oxygen. Publication number 100-3. January 3, 2023. Accessed February 14, 2024. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=169
1. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13. doi:10.15620/cdc:101659
2. Sharafkhaneh A, Petersen NJ, Yu H-J, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132. doi:10.2147/copd.s8047
3. LaBedz SL, Krishnan JA, Chung Y-C, et al. Chronic obstructive pulmonary disease outcomes at Veterans Affairs versus non-Veterans Affairs hospitals. Chronic Obstr Pulm Dis. 2021;8(3):306-313. doi:10.15326/jcopdf.2021.0201
4. Darnell K, Dwivedi AK, Weng Z, Panos RJ. Disproportionate utilization of healthcare resources among veterans with COPD: a retrospective analysis of factors associated with COPD healthcare cost. Cost Eff Resour Alloc. 2013;11:13. doi:10.1186/1478-7547-11-13
5. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US Veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
6. Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):CD001744. doi:10.1002/14651858.CD001744.pub2
7. Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(10):1254-1264. doi:10.1164/rccm.201802-0382CI
8. Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(10):e121-e141. doi:10.1164/rccm.202009-3608ST
9. AARC. AARC clinical practice guideline. Oxygen therapy in the home or alternate site health care facility--2007 revision & update. Respir Care. 2007;52(8):1063-1068.
10. Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618
11. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464-471. doi:10.1164/rccm.201710-2029OC
12. Stanford RH, Engel-Nitz NM, Bancroft T, Essoi B. The identification and cost of acute chronic obstructive pulmonary disease exacerbations in a United States population healthcare claims database. COPD. 2020;17(5):499-508. doi:10.1080/15412555.2020.1817357
13. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
14. US Department of Veterans Affairs, Veterans Health Administration. Home oxygen program. VHA Directive 1173.13(1). Published August 5, 2020. Accessed February 28, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8947
15. Phibbs CS, Barnett PG, Fan A, Harden C, King SS, Scott JY. Research guide to decision support system national cost extracts. Health Economics Resource Center of Health Service R&D Services, US Department of Veterans Affairs. September 2010. Accessed February 14, 2024. https://www.herc.research.va.gov/files/book_621.pdf
16. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194. doi:10.1183/09059180.00004311
17. Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373-378. doi:10.1164/rccm.200507-1161WS
18. Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol. 2018;11(3):279-289. doi:10.1080/17512433.2018.1421457
19. Sculley JA, Corbridge SJ, Prieto-Centurion V, et al. Home oxygen therapy for patients with COPD: time for a reboot. Respir Care. 2019;64(12):1574-1585. doi:10.4187/respcare.07135
20. Jacobs SS, Lindell KO, Collins EG, et al. Patient perceptions of the adequacy of supplemental oxygen therapy. Results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15:24-32. doi:10.1513/AnnalsATS.201703-209OC
21. US Centers for Medicare & Medicaid Services. Home use of oxygen. Publication number 100-3. January 3, 2023. Accessed February 14, 2024. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=169
Battlefield Acupuncture vs Ketorolac for Treating Pain in the Emergency Department
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
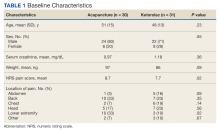
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
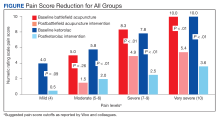
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
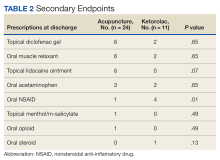
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
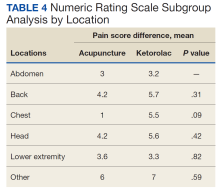
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
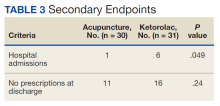
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.