User login
Lung Cancer Exposome in U.S. Military Veterans: Study of Environment and Epigenetic Factors on Risk and Survival
Background
The Exposome—the comprehensive accumulation of environmental exposures from birth to death—provides a framework for linking external risk factors to cancer biology. In U.S. veterans, the exposome includes both military-specific exposures (e.g., asbestos, Agent Orange, burn pits) and postservice socioeconomic and environmental factors. These cumulative exposures may drive tumor development and progression via epigenetic mechanisms, though their impact on lung cancer outcomes remain poorly characterized.
Methods
This is a retrospective cohort study of 71 lung cancer subjects (NSCLC and SCLC) from the Jesse Brown VA Medical Center (IRB# 1586320). We assessed the Area Deprivation Index (ADI), Environmental Burden Index (EBI), and occupational exposure in relation to DNA methylation of CDO1, TAC1, SOX17, and HOXA7. Geospatial data were mapped to US census tracts, and standard statistical analysis were conducted.
Results
NSCLC patients exhibited significantly higher methylation levels across all genes. High EBI exposure was associated with lower SOX17 methylation (p = 0.064) and worse overall survival (p = 0.046). In NSCLC patients, occupational exposure predicted a 7.7-fold increased hazard of death (p = 0.027). SOX17 and TAC1 methylation were independently associated with reduced survival (p = 0.037 and 0.0058, respectively). While ADI did not independently predict survival, it correlated with late-stage presentation and reduced HOXA7 methylation.
Conclusions
Exposome factors such as environmental burden and occupational exposure are biologically embedded in lung cancer cell through gene-specific methylation and significantly impact survival. We posit that integrating exposomic and molecular data could enhance lung precision oncology approaches for high-risk veteran populations.
Background
The Exposome—the comprehensive accumulation of environmental exposures from birth to death—provides a framework for linking external risk factors to cancer biology. In U.S. veterans, the exposome includes both military-specific exposures (e.g., asbestos, Agent Orange, burn pits) and postservice socioeconomic and environmental factors. These cumulative exposures may drive tumor development and progression via epigenetic mechanisms, though their impact on lung cancer outcomes remain poorly characterized.
Methods
This is a retrospective cohort study of 71 lung cancer subjects (NSCLC and SCLC) from the Jesse Brown VA Medical Center (IRB# 1586320). We assessed the Area Deprivation Index (ADI), Environmental Burden Index (EBI), and occupational exposure in relation to DNA methylation of CDO1, TAC1, SOX17, and HOXA7. Geospatial data were mapped to US census tracts, and standard statistical analysis were conducted.
Results
NSCLC patients exhibited significantly higher methylation levels across all genes. High EBI exposure was associated with lower SOX17 methylation (p = 0.064) and worse overall survival (p = 0.046). In NSCLC patients, occupational exposure predicted a 7.7-fold increased hazard of death (p = 0.027). SOX17 and TAC1 methylation were independently associated with reduced survival (p = 0.037 and 0.0058, respectively). While ADI did not independently predict survival, it correlated with late-stage presentation and reduced HOXA7 methylation.
Conclusions
Exposome factors such as environmental burden and occupational exposure are biologically embedded in lung cancer cell through gene-specific methylation and significantly impact survival. We posit that integrating exposomic and molecular data could enhance lung precision oncology approaches for high-risk veteran populations.
Background
The Exposome—the comprehensive accumulation of environmental exposures from birth to death—provides a framework for linking external risk factors to cancer biology. In U.S. veterans, the exposome includes both military-specific exposures (e.g., asbestos, Agent Orange, burn pits) and postservice socioeconomic and environmental factors. These cumulative exposures may drive tumor development and progression via epigenetic mechanisms, though their impact on lung cancer outcomes remain poorly characterized.
Methods
This is a retrospective cohort study of 71 lung cancer subjects (NSCLC and SCLC) from the Jesse Brown VA Medical Center (IRB# 1586320). We assessed the Area Deprivation Index (ADI), Environmental Burden Index (EBI), and occupational exposure in relation to DNA methylation of CDO1, TAC1, SOX17, and HOXA7. Geospatial data were mapped to US census tracts, and standard statistical analysis were conducted.
Results
NSCLC patients exhibited significantly higher methylation levels across all genes. High EBI exposure was associated with lower SOX17 methylation (p = 0.064) and worse overall survival (p = 0.046). In NSCLC patients, occupational exposure predicted a 7.7-fold increased hazard of death (p = 0.027). SOX17 and TAC1 methylation were independently associated with reduced survival (p = 0.037 and 0.0058, respectively). While ADI did not independently predict survival, it correlated with late-stage presentation and reduced HOXA7 methylation.
Conclusions
Exposome factors such as environmental burden and occupational exposure are biologically embedded in lung cancer cell through gene-specific methylation and significantly impact survival. We posit that integrating exposomic and molecular data could enhance lung precision oncology approaches for high-risk veteran populations.
Disparate Prednisone Starting Dosages for Systemic Corticosteroid-Naïve Veterans With Active Sarcoidosis
Disparate Prednisone Starting Dosages for Systemic Corticosteroid-Naïve Veterans With Active Sarcoidosis
Sarcoidosis is a multiorgan granulomatous disorder of unknown etiology that impacts many US veterans.1 At diagnosis, clinical manifestations vary and partially depend on the extent and severity of organ involvement, particularly of the lungs, heart, and eyes.2,3 Sarcoidosis may lead to progressive organ dysfunction, long-term disability, and death.1-3 Clinical practice guidelines recommend prednisone 20 to 40 mg daily or equivalent-prednisone dose followed by a slow tapering, as first-line pharmacotherapy for patients with active sarcoidosis who are naïve to systemic corticosteroids.2-4
Use of prolonged, high-dosage prednisone (> 40 mg daily) is discouraged due to a high risk of corticosteroid-related adverse events and associated health care costs.5,6 Research suggests that initial lower prednisone dosage (< 20 mg daily) may be effective in systemic corticosteroid-naïve patients with active sarcoidosis.3
Adherence to this regimen by specialists (eg, pulmonologists, dermatologists, ophthalmologists, rheumatologists, and cardiologists) has not been established. This study sought to determine the starting dosages for prednisone prescribed at the Jesse Brown Department of Veterans Affairs Medical Center (JBVAMC) to patients with active sarcoidosis who were systemic corticosteroid-naïve.
Methods
Patient data were reviewed from the Computerized Patient Record System (CPRS) for individuals diagnosed with sarcoidosis who were corticosteroid-naïve and prescribed initial prednisone dosages by health care practitioners (HCPs) from several specialties between 2014 and 2023 at JBVAMC. This 200-bed acute care facility serves about 62,000 veterans who live in Illinois or Indiana. JBVAMC is affiliated with the University of Illinois College of Medicine at Chicago, Northwestern University Feinberg School of Medicine, and the University of Chicago Pritzker School of Medicine; many JBVAMC HCPs hold academic appointments with these medical schools.
Patient demographics, prescriber specialty, and daily starting dosage were recorded. The decision to initiate prednisone therapy and its dosage were at the discretion of HCPs who diagnosed active sarcoidosis based on compatible clinical and ancillary test findings as documented in CPRS.2-4,6-10 Statistical analyses were conducted using a t test, and a threshold of P < .05 was considered statistically significant. This study was reviewed and determined to be exempt by the JBVAMC institutional review board.
Results
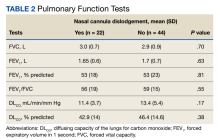
Sixty-eight patients who were systemic corticosteroid- naïve and had sarcoidosis were prescribed prednisone by HCPs at JBVAMC. Fifty-two were Black (76%), 62 were male (91%), and 53 were current or former smokers (78%). The mean (SD) age was 63 (11) years (Table 1). Forty patients (59%) had lung involvement, 6 had eye (9%), 6 had skin (9%), and 5 had musculoskeletal system (7%) involvement.
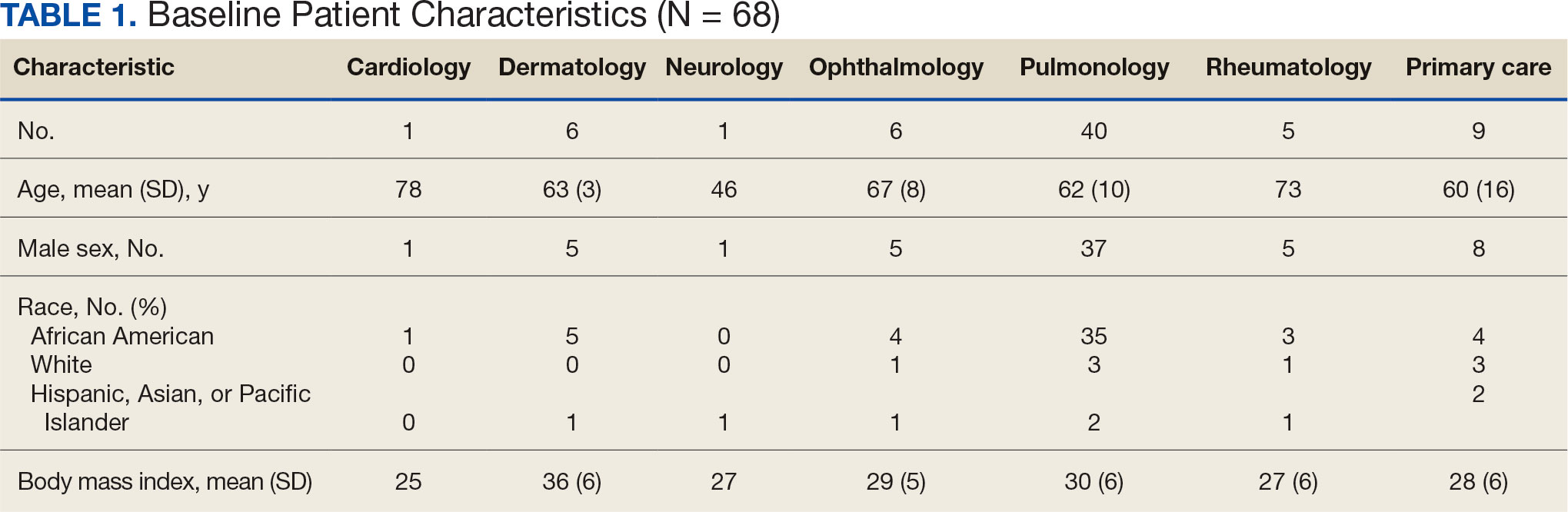
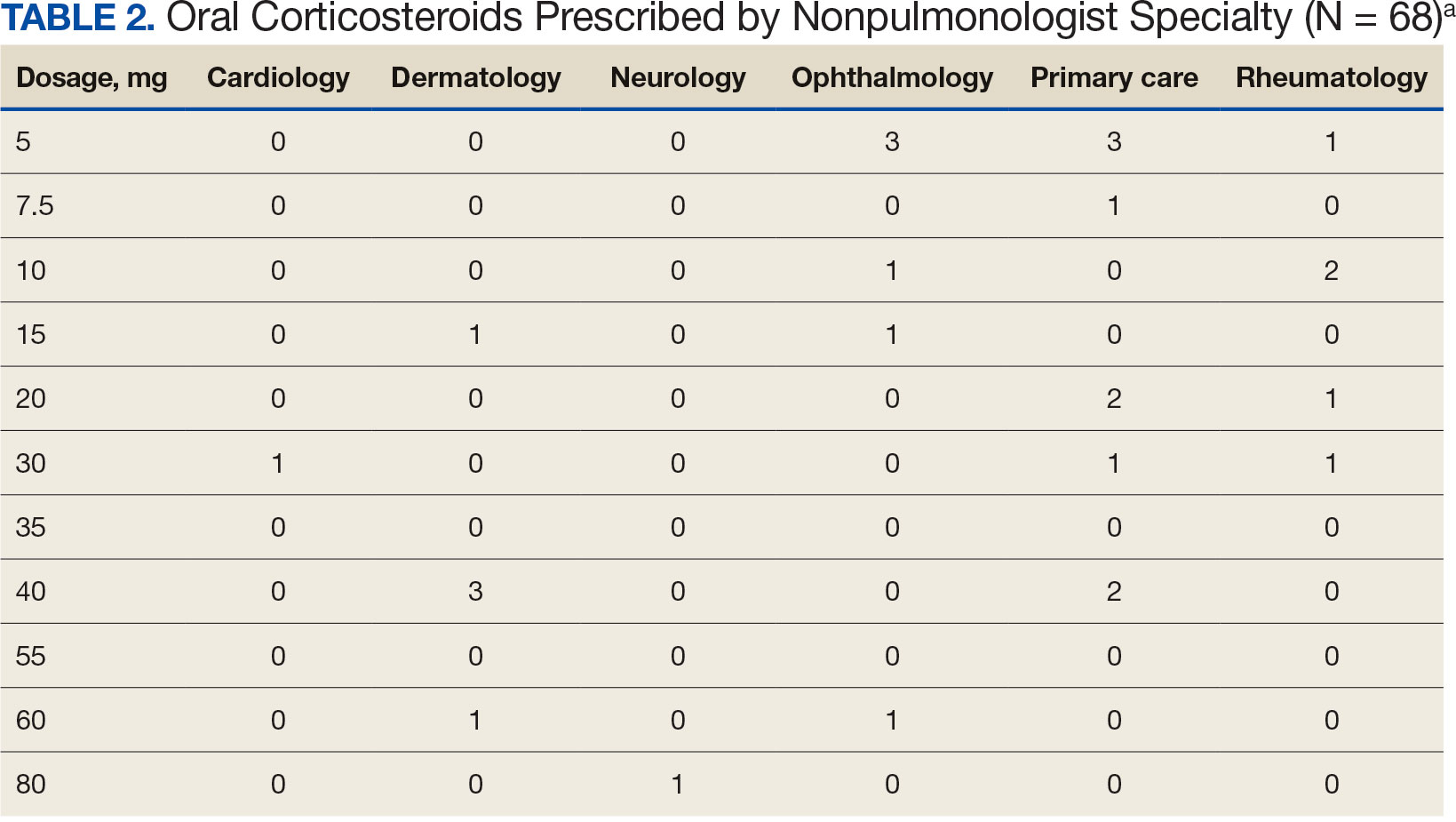
Pulmonologists predominantly prescribed initial dosage of 20 mg to 40 mg (median, 35 mg daily) (Figure). Other HCPs, including primary care, tended to prescribe prednisone < 20 mg (median, 17.5 mg; P < .05) (Table 2). The highest initial prednisone dosage was 80 mg daily, prescribed by a neurologist for a patient with neurosarcoidosis. Voortman et al recommend 20 to 40 mg prednisone daily for neurosarcoidosis.7 Both groups, pulmonologists and nonpulmonologists, had no significant differences in patient characteristics.
Discussion
Disparate prescription patterns of initial prednisone dosages were observed between pulmonologists and nonpulmonologists treating systemic corticosteroid-naïve patients with active sarcoidosis at JBVAMC. This study did not determine the underlying reasons for this phenomenon, nor its impact on patient outcomes.
Clinical practice guidelines have not been independently validated for each organ affected by sarcoidosis.2-4,6-10 Variations in clinical practice for other specialties may account for the variable prednisone starting dosage selection. For example, among 6 patients with active ocular sarcoidosis treated by ophthalmologists, 4 were prescribed an initial prednisone dosage of ≥ 10 mg daily. The American Academy of Ophthalmology recommends an initial short-term course of prednisone at 1 to 1.5 mg/kg daily, tapered down to the lowest effective dosage.10
Limitations
This study used a small, single-center predominantly older Black male patient cohort. The generalizability of these observations is unknown. A larger, multicenter prospective study is warranted to further evaluate these initial observations.
Conclusions
HCPs treating patients who are systemic corticosteroid-naïve with active sarcoidosis for whom prednisone is indicated should adhere to current clinical practice guidelines by prescribing prednisone in the 20 to 40 mg daily range.
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019. Ann Am Thorac Soc. 2023;20(6):797-806. doi:10.1513/AnnalsATS.202206-515OC
- Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58(6):2004079. doi:10.1183/13993003.04079-2020
- Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29(155):190146. doi:10.1183/16000617.0146-2019
- Kwon S, Judson MA. Clinical pharmacology in sarcoidosis: how to use and monitor sarcoidosis medications. J Clin Med. 2024;13(5):1250. doi:10.3390/jcm13051250
- Rice JB, White AG, Johnson M, et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin. 2018;34(8):1519-1527. doi:10.1080/03007995.2018.1474090
- Rice JB, White AG, Johnson M, Wagh A, Qin Y, Bartels-Peculis L, et al. Healthcare resource use and cost associated with varying dosages of extended corticosteroid exposure in a US population. J Med Econ. 2018;21(9):846-852. doi:10.1080/13696998.2018.1474750
- Voortman M, Drent M, Baughman RP. Management of neurosarcoidosis: a clinical challenge. Curr Opin Neurol. 2019;32(3):475-483. doi:10.1097/WCO.0000000000000684
- Cheng RK, Kittleson MM, Beavers CJ, et al. Diagnosis and management of cardiac sarcoidosis: a scientific statement from the American Heart Association. Circulation. 2024;149(21):e1197-e1216. doi:10.1161/CIR.0000000000001240
- Cohen E, Lheure C, Ingen-Housz-Oro S, et al. Which firstline treatment for cutaneous sarcoidosis? A retrospective study of 120 patients. Eur J Dermatol. 2023;33(6):680-685. doi:10.1684/ejd.2023.4584
- American Academy of Ophthalmology. Ocular manifestations of sarcoidosis. EyeWiki. Accessed June 3, 2025. https://eyewiki.org/Ocular_Manifestations_of_Sarcoidosis
Sarcoidosis is a multiorgan granulomatous disorder of unknown etiology that impacts many US veterans.1 At diagnosis, clinical manifestations vary and partially depend on the extent and severity of organ involvement, particularly of the lungs, heart, and eyes.2,3 Sarcoidosis may lead to progressive organ dysfunction, long-term disability, and death.1-3 Clinical practice guidelines recommend prednisone 20 to 40 mg daily or equivalent-prednisone dose followed by a slow tapering, as first-line pharmacotherapy for patients with active sarcoidosis who are naïve to systemic corticosteroids.2-4
Use of prolonged, high-dosage prednisone (> 40 mg daily) is discouraged due to a high risk of corticosteroid-related adverse events and associated health care costs.5,6 Research suggests that initial lower prednisone dosage (< 20 mg daily) may be effective in systemic corticosteroid-naïve patients with active sarcoidosis.3
Adherence to this regimen by specialists (eg, pulmonologists, dermatologists, ophthalmologists, rheumatologists, and cardiologists) has not been established. This study sought to determine the starting dosages for prednisone prescribed at the Jesse Brown Department of Veterans Affairs Medical Center (JBVAMC) to patients with active sarcoidosis who were systemic corticosteroid-naïve.
Methods
Patient data were reviewed from the Computerized Patient Record System (CPRS) for individuals diagnosed with sarcoidosis who were corticosteroid-naïve and prescribed initial prednisone dosages by health care practitioners (HCPs) from several specialties between 2014 and 2023 at JBVAMC. This 200-bed acute care facility serves about 62,000 veterans who live in Illinois or Indiana. JBVAMC is affiliated with the University of Illinois College of Medicine at Chicago, Northwestern University Feinberg School of Medicine, and the University of Chicago Pritzker School of Medicine; many JBVAMC HCPs hold academic appointments with these medical schools.
Patient demographics, prescriber specialty, and daily starting dosage were recorded. The decision to initiate prednisone therapy and its dosage were at the discretion of HCPs who diagnosed active sarcoidosis based on compatible clinical and ancillary test findings as documented in CPRS.2-4,6-10 Statistical analyses were conducted using a t test, and a threshold of P < .05 was considered statistically significant. This study was reviewed and determined to be exempt by the JBVAMC institutional review board.
Results
Sixty-eight patients who were systemic corticosteroid- naïve and had sarcoidosis were prescribed prednisone by HCPs at JBVAMC. Fifty-two were Black (76%), 62 were male (91%), and 53 were current or former smokers (78%). The mean (SD) age was 63 (11) years (Table 1). Forty patients (59%) had lung involvement, 6 had eye (9%), 6 had skin (9%), and 5 had musculoskeletal system (7%) involvement.

Pulmonologists predominantly prescribed initial dosage of 20 mg to 40 mg (median, 35 mg daily) (Figure). Other HCPs, including primary care, tended to prescribe prednisone < 20 mg (median, 17.5 mg; P < .05) (Table 2). The highest initial prednisone dosage was 80 mg daily, prescribed by a neurologist for a patient with neurosarcoidosis. Voortman et al recommend 20 to 40 mg prednisone daily for neurosarcoidosis.7 Both groups, pulmonologists and nonpulmonologists, had no significant differences in patient characteristics.


Discussion
Disparate prescription patterns of initial prednisone dosages were observed between pulmonologists and nonpulmonologists treating systemic corticosteroid-naïve patients with active sarcoidosis at JBVAMC. This study did not determine the underlying reasons for this phenomenon, nor its impact on patient outcomes.
Clinical practice guidelines have not been independently validated for each organ affected by sarcoidosis.2-4,6-10 Variations in clinical practice for other specialties may account for the variable prednisone starting dosage selection. For example, among 6 patients with active ocular sarcoidosis treated by ophthalmologists, 4 were prescribed an initial prednisone dosage of ≥ 10 mg daily. The American Academy of Ophthalmology recommends an initial short-term course of prednisone at 1 to 1.5 mg/kg daily, tapered down to the lowest effective dosage.10
Limitations
This study used a small, single-center predominantly older Black male patient cohort. The generalizability of these observations is unknown. A larger, multicenter prospective study is warranted to further evaluate these initial observations.
Conclusions
HCPs treating patients who are systemic corticosteroid-naïve with active sarcoidosis for whom prednisone is indicated should adhere to current clinical practice guidelines by prescribing prednisone in the 20 to 40 mg daily range.
Sarcoidosis is a multiorgan granulomatous disorder of unknown etiology that impacts many US veterans.1 At diagnosis, clinical manifestations vary and partially depend on the extent and severity of organ involvement, particularly of the lungs, heart, and eyes.2,3 Sarcoidosis may lead to progressive organ dysfunction, long-term disability, and death.1-3 Clinical practice guidelines recommend prednisone 20 to 40 mg daily or equivalent-prednisone dose followed by a slow tapering, as first-line pharmacotherapy for patients with active sarcoidosis who are naïve to systemic corticosteroids.2-4
Use of prolonged, high-dosage prednisone (> 40 mg daily) is discouraged due to a high risk of corticosteroid-related adverse events and associated health care costs.5,6 Research suggests that initial lower prednisone dosage (< 20 mg daily) may be effective in systemic corticosteroid-naïve patients with active sarcoidosis.3
Adherence to this regimen by specialists (eg, pulmonologists, dermatologists, ophthalmologists, rheumatologists, and cardiologists) has not been established. This study sought to determine the starting dosages for prednisone prescribed at the Jesse Brown Department of Veterans Affairs Medical Center (JBVAMC) to patients with active sarcoidosis who were systemic corticosteroid-naïve.
Methods
Patient data were reviewed from the Computerized Patient Record System (CPRS) for individuals diagnosed with sarcoidosis who were corticosteroid-naïve and prescribed initial prednisone dosages by health care practitioners (HCPs) from several specialties between 2014 and 2023 at JBVAMC. This 200-bed acute care facility serves about 62,000 veterans who live in Illinois or Indiana. JBVAMC is affiliated with the University of Illinois College of Medicine at Chicago, Northwestern University Feinberg School of Medicine, and the University of Chicago Pritzker School of Medicine; many JBVAMC HCPs hold academic appointments with these medical schools.
Patient demographics, prescriber specialty, and daily starting dosage were recorded. The decision to initiate prednisone therapy and its dosage were at the discretion of HCPs who diagnosed active sarcoidosis based on compatible clinical and ancillary test findings as documented in CPRS.2-4,6-10 Statistical analyses were conducted using a t test, and a threshold of P < .05 was considered statistically significant. This study was reviewed and determined to be exempt by the JBVAMC institutional review board.
Results
Sixty-eight patients who were systemic corticosteroid- naïve and had sarcoidosis were prescribed prednisone by HCPs at JBVAMC. Fifty-two were Black (76%), 62 were male (91%), and 53 were current or former smokers (78%). The mean (SD) age was 63 (11) years (Table 1). Forty patients (59%) had lung involvement, 6 had eye (9%), 6 had skin (9%), and 5 had musculoskeletal system (7%) involvement.

Pulmonologists predominantly prescribed initial dosage of 20 mg to 40 mg (median, 35 mg daily) (Figure). Other HCPs, including primary care, tended to prescribe prednisone < 20 mg (median, 17.5 mg; P < .05) (Table 2). The highest initial prednisone dosage was 80 mg daily, prescribed by a neurologist for a patient with neurosarcoidosis. Voortman et al recommend 20 to 40 mg prednisone daily for neurosarcoidosis.7 Both groups, pulmonologists and nonpulmonologists, had no significant differences in patient characteristics.


Discussion
Disparate prescription patterns of initial prednisone dosages were observed between pulmonologists and nonpulmonologists treating systemic corticosteroid-naïve patients with active sarcoidosis at JBVAMC. This study did not determine the underlying reasons for this phenomenon, nor its impact on patient outcomes.
Clinical practice guidelines have not been independently validated for each organ affected by sarcoidosis.2-4,6-10 Variations in clinical practice for other specialties may account for the variable prednisone starting dosage selection. For example, among 6 patients with active ocular sarcoidosis treated by ophthalmologists, 4 were prescribed an initial prednisone dosage of ≥ 10 mg daily. The American Academy of Ophthalmology recommends an initial short-term course of prednisone at 1 to 1.5 mg/kg daily, tapered down to the lowest effective dosage.10
Limitations
This study used a small, single-center predominantly older Black male patient cohort. The generalizability of these observations is unknown. A larger, multicenter prospective study is warranted to further evaluate these initial observations.
Conclusions
HCPs treating patients who are systemic corticosteroid-naïve with active sarcoidosis for whom prednisone is indicated should adhere to current clinical practice guidelines by prescribing prednisone in the 20 to 40 mg daily range.
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019. Ann Am Thorac Soc. 2023;20(6):797-806. doi:10.1513/AnnalsATS.202206-515OC
- Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58(6):2004079. doi:10.1183/13993003.04079-2020
- Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29(155):190146. doi:10.1183/16000617.0146-2019
- Kwon S, Judson MA. Clinical pharmacology in sarcoidosis: how to use and monitor sarcoidosis medications. J Clin Med. 2024;13(5):1250. doi:10.3390/jcm13051250
- Rice JB, White AG, Johnson M, et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin. 2018;34(8):1519-1527. doi:10.1080/03007995.2018.1474090
- Rice JB, White AG, Johnson M, Wagh A, Qin Y, Bartels-Peculis L, et al. Healthcare resource use and cost associated with varying dosages of extended corticosteroid exposure in a US population. J Med Econ. 2018;21(9):846-852. doi:10.1080/13696998.2018.1474750
- Voortman M, Drent M, Baughman RP. Management of neurosarcoidosis: a clinical challenge. Curr Opin Neurol. 2019;32(3):475-483. doi:10.1097/WCO.0000000000000684
- Cheng RK, Kittleson MM, Beavers CJ, et al. Diagnosis and management of cardiac sarcoidosis: a scientific statement from the American Heart Association. Circulation. 2024;149(21):e1197-e1216. doi:10.1161/CIR.0000000000001240
- Cohen E, Lheure C, Ingen-Housz-Oro S, et al. Which firstline treatment for cutaneous sarcoidosis? A retrospective study of 120 patients. Eur J Dermatol. 2023;33(6):680-685. doi:10.1684/ejd.2023.4584
- American Academy of Ophthalmology. Ocular manifestations of sarcoidosis. EyeWiki. Accessed June 3, 2025. https://eyewiki.org/Ocular_Manifestations_of_Sarcoidosis
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019. Ann Am Thorac Soc. 2023;20(6):797-806. doi:10.1513/AnnalsATS.202206-515OC
- Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58(6):2004079. doi:10.1183/13993003.04079-2020
- Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29(155):190146. doi:10.1183/16000617.0146-2019
- Kwon S, Judson MA. Clinical pharmacology in sarcoidosis: how to use and monitor sarcoidosis medications. J Clin Med. 2024;13(5):1250. doi:10.3390/jcm13051250
- Rice JB, White AG, Johnson M, et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin. 2018;34(8):1519-1527. doi:10.1080/03007995.2018.1474090
- Rice JB, White AG, Johnson M, Wagh A, Qin Y, Bartels-Peculis L, et al. Healthcare resource use and cost associated with varying dosages of extended corticosteroid exposure in a US population. J Med Econ. 2018;21(9):846-852. doi:10.1080/13696998.2018.1474750
- Voortman M, Drent M, Baughman RP. Management of neurosarcoidosis: a clinical challenge. Curr Opin Neurol. 2019;32(3):475-483. doi:10.1097/WCO.0000000000000684
- Cheng RK, Kittleson MM, Beavers CJ, et al. Diagnosis and management of cardiac sarcoidosis: a scientific statement from the American Heart Association. Circulation. 2024;149(21):e1197-e1216. doi:10.1161/CIR.0000000000001240
- Cohen E, Lheure C, Ingen-Housz-Oro S, et al. Which firstline treatment for cutaneous sarcoidosis? A retrospective study of 120 patients. Eur J Dermatol. 2023;33(6):680-685. doi:10.1684/ejd.2023.4584
- American Academy of Ophthalmology. Ocular manifestations of sarcoidosis. EyeWiki. Accessed June 3, 2025. https://eyewiki.org/Ocular_Manifestations_of_Sarcoidosis
Disparate Prednisone Starting Dosages for Systemic Corticosteroid-Naïve Veterans With Active Sarcoidosis
Disparate Prednisone Starting Dosages for Systemic Corticosteroid-Naïve Veterans With Active Sarcoidosis
Using Telehealth to Increase Lung Cancer Screening Referrals for At-Risk Veterans in Rural Communities
Annual lung cancer screening (LCS) with low-dose computed tomography (LDCT) of the chest has been shown to reduce mortality rates for individuals at risk for lung cancer.1 Despite the benefits, < 5% of those who were eligible for LCS in the United States were screened in 2022.2 Implementation of a LCS program in rural communities is especially challenging because they are sparsely populated, medically underserved, and located far from urban centers.2-7 It is estimated that 1 in 5 people live in rural areas. Rates of tobacco smoking and cancer are higher in rural communities when compared with urban communities.8,9 The scarcity of physicians in rural areas who are familiar with LCS may further impede individuals who are at risk from accessing this life saving service.5,6 As a result, these individuals may not regularly undergo LCS as recommended.9
Telehealth, or the remote delivery of health care services via telecommunications, is an emerging approach for addressing unmet medical needs in rural communities and is being utilized widely by the US Department of Veterans Affairs (VA).4,10-15 The Veterans Integrated Service Network 12 (Great Lakes Network) has established the Clinical Resource Hub (CRH), a telehealth network comprising of licensed independent physicians, nurse practitioners, registered nurses, and ancillary staff. The CRH offers regular, remote health care services to several community-based outpatient clinics (CBOC) primary care clinics located in rural northern Wisconsin and the Upper Peninsula of Michigan.10,14
The utility of telehealth in promoting LCS among at-risk veterans living in rural communities has not been firmly established.4-6 To address this issue, we conducted a proof-of-principle quality improvement project to determine whether a telehealth intervention would increase referrals among at-risk veterans who reside in rural northern Wisconsin and the Upper Peninsula of Michigan who are self-enrolled in a CBOC smoking cessation program in Green Bay, Wisconsin. The CBOC provides primary health care to veterans residing in rural northern Wisconsin and the Upper Peninsula of Michigan as defined by US Department of Agriculture rural-urban commuting area codes.16 The intervention aimed to refer these individuals to the closest available and centralized LCS program, which is located at the Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin.
METHODS
We reviewed electronic health records (EHR) of LCS-eligible veterans treated by 2 authors (SH and TB) who were self-enrolled in the smoking cessation program at the Green Bay CBOC between October 1, 2020, and September 30, 2021. The program provides comprehensive evidence-based tobacco use treatment, online self-help resources, behavioral counseling, and medicines for smoking cessation.17 Veterans aged 50 to 80 years with a smoking history of ≥ 20 pack-years, who currently smoke cigarettes or quit within the past 15 years, were considered at risk for lung cancer and eligible for LCS. After confirming eligibility, pertinent demographic data were abstracted from each EHR.
Telehealth Intervention
The CJZVAMC centralized LCS program manages all delivery processes and has been previously shown to increase uptake of LCS and improve patient outcomes among veterans as compared to a decentralized approach.18,19 In the centralized approach, eligible veterans were referred by a CBOC primary care practitioner (PCP) to a designated centralized LCS program. The centralized LCS program provides further evaluation and disposition, which includes structured and shared decision making, ordering LDCT of the chest, reporting LDCT results to the patient and PCP, devising a goal-directed care plan, and managing follow-up LDCTs as indicated (Figure 1).18,19
This intervention was initiated before other measures aimed to increase the LCS enrollment for at-risk rural veterans at the CBOC, (eg, mailing LCS education fact sheet to veterans).20 After reviewing prospective veterans’ EHRs, 1 author (TB) contacted LCS-eligible veterans by telephone and left a voicemail if contact could not be established. A second telephone call was placed within 2 months of the initial call if no call back was documented in the EHR. When verbal contact was established, the goals of the centralized LCS program were described and the veteran was invited to participate.21
Veterans were seen at CBOCs affiliated with CJZVAMC. The CJZVAMC LCS coordinator was notified whenever a veteran agreed to enroll into LCS and then ordered LDCT, which was performed and read at CJZVAMC. Once LDCT has been ordered, 1 author (TB) reviewed the veteran’s EHR for LDCT completion over the next 4 months.Upon conclusion of the intervention period, the number of veterans referred for LDCT and the number of LDCTs performed were recorded. Each LDCT was reviewed and coded by medical imaging clinicians according to Lung CT Screening Reporting and Data System (Lung-RADS) version 1.1 and coded as 0, 1, 2, 3, or 4 based on the nodule with the highest degree of suspicion.22 The LDCT and reports were also reviewed by pulmonary physicians at the CJZVAMC Lung Nodule Clinic with recommendations issued and reported to the PCP treating the veteran, such as annual follow-up with LDCT or referral to specialty care for further evaluation as indicated.
RESULTS
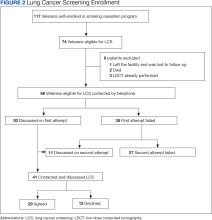
Of 117 veterans enrolled in the smoking cessation program at the CBOC during the intervention period, 74 (63%) were eligible to undergo LCS, and 68 (58%) were contacted by telephone (Figure 2). Eligible patients were primarily White male veterans; their mean (SD) age was 65.0 years (7.6). Participation in LCS was discussed with 41 (60%) veterans either during the initial or second telephone call of which 29 (71%) agreed to enroll and 12 (29%) declined. Veterans did not provide reasons for declining participation at the time of the telephone call.
Among the 74 eligible veterans who attended the smoking cessation program, only 3 had LDCT performed before initiation of this project (4%). At the conclusion of the telehealth intervention period, 19 veterans had LDCT performed (26%). Ten LDCTs were coded Lung-RADS 1, 7 Lung-RADS 2, 1 Lung-RADS 3, and 1 Lung-RADS 4B. In each case, annual follow-up LDCT or referral to a LCS clinician was pursued as indicated.22
DISCUSSION
This proof-of-principle quality improvement project found that a high percentage (66%) of individuals in rural communities who were contacted via telehealth agreed to participate in a regional LCS program. The program reviewed LDCT results, ordered follow-up LDCTs, and recommended further evaluations.18,19 Whether this centralized LCS process could also promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians, if abnormal imaging findings are detected, remains unclear.
It has been well established LDCT LCS reduces lung cancer-specific and overall mortality rates among eligible current and former smokers.1,9,23 The 5-year relative survival rate of veterans diagnosed with localized non-small cell lung cancer is 63%; that number drops to 7% in those with advanced disease attesting to the utility of LCS in detecting early stage lung cancer.2 Despite these favorable observations, however, screening rates with free LDCT remains low in rural communities.3-7
This proof-of-principle quality improvement project found that telehealth intervention may increase referrals of at-risk veterans who reside in rural communities to the closest centralized LCS program located at aregional VAMC. This program is responsible for reviewing the results of the initial LDCT, ordering follow-up LDCT, and recommending further evaluation as indicated.18,19 Whether this centralized LCS process would promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians if abnormal imaging findings are detected is yet to be determined.
We found that among 74 LCS-eligible rural veterans attending a CBOC-based smoking cessation program, only 3 (4%) underwent LDCT screening before this telehealth intervention was launched. This low LCS rate among veterans attempting to quit smoking may have been related, in part, to a lack of awareness of this intervention and/or barriers to LCS access.7,10,21,24 Deploying a telehealth intervention targeting LCS could address this life threatening and unmet medical need in rural communities.25 The results of this proof-of-principle quality improvement project support this contention with the reported increased referrals to and completion of initial LDCT within 4 months of the telehealth encounter.
Limitations
This was a small, single site project composed of predominantly White male rural veterans participating in a smoking cessation program associated with a VA facility.26,27 It is not clear whether similar outcomes would be observed in at-risk veterans who do not participate in a smoking cessation program or in more diverse communities. We were unable to contact 40% of LCS-eligible rural veterans by telephone. Twelve veterans reached by telephone declined to participate in LCS without providing a reason, and only 19 of 68 eligible veterans (28%) underwent LDCT screening during the 4-month telehealth intervention. The reasons underlying this overall low accrual rate and whether rural veterans prefer other means of personal communication regarding LCS were not determined. Lastly, generalizability of our initial observations to other veterans living in rural communities is limited because the project was conducted only in rural northern Wisconsin and the Upper Peninsula of Michigan.
Conclusions
At-risk rural veterans may be willing to participate in a centralized LCS program at a regional VA medical facility when contacted and coordinated using telehealth modalities. These findings offer support for future prospective, multisite, VA telehealth-based studies to be conducted in rural areas. The results of this project also suggest that telehealth intervention could increase referrals of at-risk rural veterans to the closest centralized LCS program located at a regional VA medical facility.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873
2. State of Lung Cancer: 2023 Report. American Lung Association. November 14, 2023. Accessed June 4, 2024. https://www.lung.org/getmedia/186786b6-18c3-46a9-a7e7-810f3ce4deda/SOLC-2023-Print-Report.pdf
3. Okereke IC, Nishi S, Zhou J, Goodwin JS. Trends in lung cancer screening in the United States, 2016-2017. J Thorac Dis. 2019;11(3):873-881. doi:10.21037/jtd.2019.01.105
4. Petraglia AF, Olazagasti JM, Strong A, Dunn B, Anderson RT, Hanley M. Establishing satellite lung cancer screening sites with telehealth to address disparities between high-risk smokers and American College of Radiology-approved Centers of Designation. J Thorac Imaging. 2021;36(1):2-5. doi:10.1097/RTI.0000000000000520
5. Odahowski CL, Zahnd WE, Eberth JM. Challenges and opportunities for lung cancer screening in rural America. J Am Coll Radiol. 2019;16(4 Pt B):590-595. doi:10.1016/j.jacr.2019.01.001
6. Rohatgi KW, Marx CM, Lewis-Thames MW, Liu J, Colditz GA, James AS. Urban-rural disparities in access to low-dose computed tomography lung cancer screening in Missouri and Illinois. Prev Chronic Dis. 2020;17:E140. doi:10.5888/pcd17.200202
7. Boudreau JH, Miller DR, Qian S, Nunez ER, Caverly TJ, Wiener RS. Access to lung cancer screening in the Veterans Health Administration: does geographic distribution match need in the population? Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
8. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2017. National Cancer Institute, US Dept of Health and Human Services; April 15, 2020. Accessed June 4, 2024. https://seer.cancer.gov/archive/csr/1975_2017/index.html
9. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
10. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17(5):582-587. doi:10.1016/j.nurpra.2021.02.020
11. Yacoub JH, Swanson CE, Jay AK, Cooper C, Spies J, Krishnan P. The radiology virtual reading room: during and beyond the COVID-19 pandemic. J Digit Imaging. 2021;34(2):308-319. doi:10.1007/s10278-021-00427-4
12. Beswick DM, Vashi A, Song Y, et al. Consultation via telemedicine and access to operative care for patients with head and neck cancer in a Veterans Health Administration population. Head Neck. 2016;38(6):925-929. doi:10.1002/hed.24386
13. Ruco A, Dossa F, Tinmouth J, et al. Social media and mHealth technology for cancer screening: systematic review and meta-analysis. J Med Internet Res. 2021;23(7):e26759. doi:10.2196/26759
14. Raza T, Joshi M, Schapira RM, Agha Z. Pulmonary telemedicine - a model to access the subspecialist services in underserved rural areas. Int J Med Inform. 2009;78(1):53-59. doi:10.1016/j.ijmedinf.2008.07.010
15. Chen A, Ayub MH, Mishuris RG, et al. Telehealth policy, practice, and education: a position statement of the Society of General Internal Medicine. J Gen Intern Med. 2023;38(11):2613-2620. doi:10.1007/s11606-023-08190-8
16. Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed June 4, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
17. VHA Directive 1056: National Smoking and Tobacco Use Cessation Program. Veterans Health Administration, US Dept of Veterans Affairs; September 5, 2019. Accessed June 4, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8488
18. Smith HB, Ward R, Frazier C, Angotti J, Tanner NT. Guideline-recommended lung cancer screening adherence is superior with a centralized approach. Chest. 2022;161(3):818-825. doi:10.1016/j.chest.2021.09.002
19. Lewis JA, Samuels LR, Denton J, et al. The association of health care system resources with lung cancer screening implementation: a cohort study. Chest. 2022;162(3):701-711. doi:10.1016/j.chest.2022.03.050
20. US Dept of Veterans Affairs. Lung cancer screening: patient education fact sheet. Accessed July 8, 2024. https://www.cancer.va.gov/assets/pdf/survey/LCSflyer.pdf
21. Melzer AC, Golden SE, Ono SS, Datta S, Crothers K, Slatore CG. What exactly is shared decision-making? A qualitative study of shared decision-making in lung cancer screening. J Gen Intern Med. 2020;35(2):546-553. doi:10.1007/s11606-019-05516-3
22. Chelala L, Hossain R, Kazerooni EA, Christensen JD, Dyer DS, White CS. Lung-RADS Version 1.1: challenges and a look ahead, from the AJR special series on radiology reporting and data systems. AJR Am J Roentgenol. 2021;216(6):1411-1422. doi:10.2214/AJR.20.24807
23. Ritzwoller DP, Meza R, Carroll NM, et al. Evaluation of population-level changes associated with the 2021 US Preventive Services Task Force lung cancer screening recommendations in community-based health care systems. JAMA Netw Open. 2021;4(10):e2128176. doi:10.1001/jamanetworkopen.2021.28176
24. Golden SE, Ono SS, Thakurta SG, et al. “I’m putting my trust in their hands”: a qualitative study of patients’ views on clinician initial communication about lung cancer screening. Chest. 2020;158(3):1260-1267. doi:10.1016/j.chest.2020.02.072
25. Park ER, Chiles C, Cinciripini PM, et al. Impact of the COVID-19 pandemic on telehealth research in cancer prevention and care: a call to sustain telehealth advances. Cancer. 2021;127(3):334-338. doi:10.1002/cncr.33227
26. Tremblay A, Taghizadeh N, Huang J, et al. A randomized controlled study of integrated smoking cessation in a lung cancer screening program. J Thorac Oncol. 2019;14(9):1528-1537. doi:10.1016/j.jtho.2019.04.024
27. Neil JM, Marotta C, Gonzalez I, et al. Integrating tobacco treatment into lung cancer screening practices: study protocol for the Screen ASSIST randomized clinical trial. Contemp Clin Trials. 2021;111:106586. doi:10.1016/j.cct.2021.106586
Annual lung cancer screening (LCS) with low-dose computed tomography (LDCT) of the chest has been shown to reduce mortality rates for individuals at risk for lung cancer.1 Despite the benefits, < 5% of those who were eligible for LCS in the United States were screened in 2022.2 Implementation of a LCS program in rural communities is especially challenging because they are sparsely populated, medically underserved, and located far from urban centers.2-7 It is estimated that 1 in 5 people live in rural areas. Rates of tobacco smoking and cancer are higher in rural communities when compared with urban communities.8,9 The scarcity of physicians in rural areas who are familiar with LCS may further impede individuals who are at risk from accessing this life saving service.5,6 As a result, these individuals may not regularly undergo LCS as recommended.9
Telehealth, or the remote delivery of health care services via telecommunications, is an emerging approach for addressing unmet medical needs in rural communities and is being utilized widely by the US Department of Veterans Affairs (VA).4,10-15 The Veterans Integrated Service Network 12 (Great Lakes Network) has established the Clinical Resource Hub (CRH), a telehealth network comprising of licensed independent physicians, nurse practitioners, registered nurses, and ancillary staff. The CRH offers regular, remote health care services to several community-based outpatient clinics (CBOC) primary care clinics located in rural northern Wisconsin and the Upper Peninsula of Michigan.10,14
The utility of telehealth in promoting LCS among at-risk veterans living in rural communities has not been firmly established.4-6 To address this issue, we conducted a proof-of-principle quality improvement project to determine whether a telehealth intervention would increase referrals among at-risk veterans who reside in rural northern Wisconsin and the Upper Peninsula of Michigan who are self-enrolled in a CBOC smoking cessation program in Green Bay, Wisconsin. The CBOC provides primary health care to veterans residing in rural northern Wisconsin and the Upper Peninsula of Michigan as defined by US Department of Agriculture rural-urban commuting area codes.16 The intervention aimed to refer these individuals to the closest available and centralized LCS program, which is located at the Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin.
METHODS
We reviewed electronic health records (EHR) of LCS-eligible veterans treated by 2 authors (SH and TB) who were self-enrolled in the smoking cessation program at the Green Bay CBOC between October 1, 2020, and September 30, 2021. The program provides comprehensive evidence-based tobacco use treatment, online self-help resources, behavioral counseling, and medicines for smoking cessation.17 Veterans aged 50 to 80 years with a smoking history of ≥ 20 pack-years, who currently smoke cigarettes or quit within the past 15 years, were considered at risk for lung cancer and eligible for LCS. After confirming eligibility, pertinent demographic data were abstracted from each EHR.
Telehealth Intervention
The CJZVAMC centralized LCS program manages all delivery processes and has been previously shown to increase uptake of LCS and improve patient outcomes among veterans as compared to a decentralized approach.18,19 In the centralized approach, eligible veterans were referred by a CBOC primary care practitioner (PCP) to a designated centralized LCS program. The centralized LCS program provides further evaluation and disposition, which includes structured and shared decision making, ordering LDCT of the chest, reporting LDCT results to the patient and PCP, devising a goal-directed care plan, and managing follow-up LDCTs as indicated (Figure 1).18,19
This intervention was initiated before other measures aimed to increase the LCS enrollment for at-risk rural veterans at the CBOC, (eg, mailing LCS education fact sheet to veterans).20 After reviewing prospective veterans’ EHRs, 1 author (TB) contacted LCS-eligible veterans by telephone and left a voicemail if contact could not be established. A second telephone call was placed within 2 months of the initial call if no call back was documented in the EHR. When verbal contact was established, the goals of the centralized LCS program were described and the veteran was invited to participate.21
Veterans were seen at CBOCs affiliated with CJZVAMC. The CJZVAMC LCS coordinator was notified whenever a veteran agreed to enroll into LCS and then ordered LDCT, which was performed and read at CJZVAMC. Once LDCT has been ordered, 1 author (TB) reviewed the veteran’s EHR for LDCT completion over the next 4 months.Upon conclusion of the intervention period, the number of veterans referred for LDCT and the number of LDCTs performed were recorded. Each LDCT was reviewed and coded by medical imaging clinicians according to Lung CT Screening Reporting and Data System (Lung-RADS) version 1.1 and coded as 0, 1, 2, 3, or 4 based on the nodule with the highest degree of suspicion.22 The LDCT and reports were also reviewed by pulmonary physicians at the CJZVAMC Lung Nodule Clinic with recommendations issued and reported to the PCP treating the veteran, such as annual follow-up with LDCT or referral to specialty care for further evaluation as indicated.
RESULTS
Of 117 veterans enrolled in the smoking cessation program at the CBOC during the intervention period, 74 (63%) were eligible to undergo LCS, and 68 (58%) were contacted by telephone (Figure 2). Eligible patients were primarily White male veterans; their mean (SD) age was 65.0 years (7.6). Participation in LCS was discussed with 41 (60%) veterans either during the initial or second telephone call of which 29 (71%) agreed to enroll and 12 (29%) declined. Veterans did not provide reasons for declining participation at the time of the telephone call.
Among the 74 eligible veterans who attended the smoking cessation program, only 3 had LDCT performed before initiation of this project (4%). At the conclusion of the telehealth intervention period, 19 veterans had LDCT performed (26%). Ten LDCTs were coded Lung-RADS 1, 7 Lung-RADS 2, 1 Lung-RADS 3, and 1 Lung-RADS 4B. In each case, annual follow-up LDCT or referral to a LCS clinician was pursued as indicated.22
DISCUSSION
This proof-of-principle quality improvement project found that a high percentage (66%) of individuals in rural communities who were contacted via telehealth agreed to participate in a regional LCS program. The program reviewed LDCT results, ordered follow-up LDCTs, and recommended further evaluations.18,19 Whether this centralized LCS process could also promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians, if abnormal imaging findings are detected, remains unclear.
It has been well established LDCT LCS reduces lung cancer-specific and overall mortality rates among eligible current and former smokers.1,9,23 The 5-year relative survival rate of veterans diagnosed with localized non-small cell lung cancer is 63%; that number drops to 7% in those with advanced disease attesting to the utility of LCS in detecting early stage lung cancer.2 Despite these favorable observations, however, screening rates with free LDCT remains low in rural communities.3-7
This proof-of-principle quality improvement project found that telehealth intervention may increase referrals of at-risk veterans who reside in rural communities to the closest centralized LCS program located at aregional VAMC. This program is responsible for reviewing the results of the initial LDCT, ordering follow-up LDCT, and recommending further evaluation as indicated.18,19 Whether this centralized LCS process would promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians if abnormal imaging findings are detected is yet to be determined.
We found that among 74 LCS-eligible rural veterans attending a CBOC-based smoking cessation program, only 3 (4%) underwent LDCT screening before this telehealth intervention was launched. This low LCS rate among veterans attempting to quit smoking may have been related, in part, to a lack of awareness of this intervention and/or barriers to LCS access.7,10,21,24 Deploying a telehealth intervention targeting LCS could address this life threatening and unmet medical need in rural communities.25 The results of this proof-of-principle quality improvement project support this contention with the reported increased referrals to and completion of initial LDCT within 4 months of the telehealth encounter.
Limitations
This was a small, single site project composed of predominantly White male rural veterans participating in a smoking cessation program associated with a VA facility.26,27 It is not clear whether similar outcomes would be observed in at-risk veterans who do not participate in a smoking cessation program or in more diverse communities. We were unable to contact 40% of LCS-eligible rural veterans by telephone. Twelve veterans reached by telephone declined to participate in LCS without providing a reason, and only 19 of 68 eligible veterans (28%) underwent LDCT screening during the 4-month telehealth intervention. The reasons underlying this overall low accrual rate and whether rural veterans prefer other means of personal communication regarding LCS were not determined. Lastly, generalizability of our initial observations to other veterans living in rural communities is limited because the project was conducted only in rural northern Wisconsin and the Upper Peninsula of Michigan.
Conclusions
At-risk rural veterans may be willing to participate in a centralized LCS program at a regional VA medical facility when contacted and coordinated using telehealth modalities. These findings offer support for future prospective, multisite, VA telehealth-based studies to be conducted in rural areas. The results of this project also suggest that telehealth intervention could increase referrals of at-risk rural veterans to the closest centralized LCS program located at a regional VA medical facility.
Annual lung cancer screening (LCS) with low-dose computed tomography (LDCT) of the chest has been shown to reduce mortality rates for individuals at risk for lung cancer.1 Despite the benefits, < 5% of those who were eligible for LCS in the United States were screened in 2022.2 Implementation of a LCS program in rural communities is especially challenging because they are sparsely populated, medically underserved, and located far from urban centers.2-7 It is estimated that 1 in 5 people live in rural areas. Rates of tobacco smoking and cancer are higher in rural communities when compared with urban communities.8,9 The scarcity of physicians in rural areas who are familiar with LCS may further impede individuals who are at risk from accessing this life saving service.5,6 As a result, these individuals may not regularly undergo LCS as recommended.9
Telehealth, or the remote delivery of health care services via telecommunications, is an emerging approach for addressing unmet medical needs in rural communities and is being utilized widely by the US Department of Veterans Affairs (VA).4,10-15 The Veterans Integrated Service Network 12 (Great Lakes Network) has established the Clinical Resource Hub (CRH), a telehealth network comprising of licensed independent physicians, nurse practitioners, registered nurses, and ancillary staff. The CRH offers regular, remote health care services to several community-based outpatient clinics (CBOC) primary care clinics located in rural northern Wisconsin and the Upper Peninsula of Michigan.10,14
The utility of telehealth in promoting LCS among at-risk veterans living in rural communities has not been firmly established.4-6 To address this issue, we conducted a proof-of-principle quality improvement project to determine whether a telehealth intervention would increase referrals among at-risk veterans who reside in rural northern Wisconsin and the Upper Peninsula of Michigan who are self-enrolled in a CBOC smoking cessation program in Green Bay, Wisconsin. The CBOC provides primary health care to veterans residing in rural northern Wisconsin and the Upper Peninsula of Michigan as defined by US Department of Agriculture rural-urban commuting area codes.16 The intervention aimed to refer these individuals to the closest available and centralized LCS program, which is located at the Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin.
METHODS
We reviewed electronic health records (EHR) of LCS-eligible veterans treated by 2 authors (SH and TB) who were self-enrolled in the smoking cessation program at the Green Bay CBOC between October 1, 2020, and September 30, 2021. The program provides comprehensive evidence-based tobacco use treatment, online self-help resources, behavioral counseling, and medicines for smoking cessation.17 Veterans aged 50 to 80 years with a smoking history of ≥ 20 pack-years, who currently smoke cigarettes or quit within the past 15 years, were considered at risk for lung cancer and eligible for LCS. After confirming eligibility, pertinent demographic data were abstracted from each EHR.
Telehealth Intervention
The CJZVAMC centralized LCS program manages all delivery processes and has been previously shown to increase uptake of LCS and improve patient outcomes among veterans as compared to a decentralized approach.18,19 In the centralized approach, eligible veterans were referred by a CBOC primary care practitioner (PCP) to a designated centralized LCS program. The centralized LCS program provides further evaluation and disposition, which includes structured and shared decision making, ordering LDCT of the chest, reporting LDCT results to the patient and PCP, devising a goal-directed care plan, and managing follow-up LDCTs as indicated (Figure 1).18,19
This intervention was initiated before other measures aimed to increase the LCS enrollment for at-risk rural veterans at the CBOC, (eg, mailing LCS education fact sheet to veterans).20 After reviewing prospective veterans’ EHRs, 1 author (TB) contacted LCS-eligible veterans by telephone and left a voicemail if contact could not be established. A second telephone call was placed within 2 months of the initial call if no call back was documented in the EHR. When verbal contact was established, the goals of the centralized LCS program were described and the veteran was invited to participate.21
Veterans were seen at CBOCs affiliated with CJZVAMC. The CJZVAMC LCS coordinator was notified whenever a veteran agreed to enroll into LCS and then ordered LDCT, which was performed and read at CJZVAMC. Once LDCT has been ordered, 1 author (TB) reviewed the veteran’s EHR for LDCT completion over the next 4 months.Upon conclusion of the intervention period, the number of veterans referred for LDCT and the number of LDCTs performed were recorded. Each LDCT was reviewed and coded by medical imaging clinicians according to Lung CT Screening Reporting and Data System (Lung-RADS) version 1.1 and coded as 0, 1, 2, 3, or 4 based on the nodule with the highest degree of suspicion.22 The LDCT and reports were also reviewed by pulmonary physicians at the CJZVAMC Lung Nodule Clinic with recommendations issued and reported to the PCP treating the veteran, such as annual follow-up with LDCT or referral to specialty care for further evaluation as indicated.
RESULTS
Of 117 veterans enrolled in the smoking cessation program at the CBOC during the intervention period, 74 (63%) were eligible to undergo LCS, and 68 (58%) were contacted by telephone (Figure 2). Eligible patients were primarily White male veterans; their mean (SD) age was 65.0 years (7.6). Participation in LCS was discussed with 41 (60%) veterans either during the initial or second telephone call of which 29 (71%) agreed to enroll and 12 (29%) declined. Veterans did not provide reasons for declining participation at the time of the telephone call.
Among the 74 eligible veterans who attended the smoking cessation program, only 3 had LDCT performed before initiation of this project (4%). At the conclusion of the telehealth intervention period, 19 veterans had LDCT performed (26%). Ten LDCTs were coded Lung-RADS 1, 7 Lung-RADS 2, 1 Lung-RADS 3, and 1 Lung-RADS 4B. In each case, annual follow-up LDCT or referral to a LCS clinician was pursued as indicated.22
DISCUSSION
This proof-of-principle quality improvement project found that a high percentage (66%) of individuals in rural communities who were contacted via telehealth agreed to participate in a regional LCS program. The program reviewed LDCT results, ordered follow-up LDCTs, and recommended further evaluations.18,19 Whether this centralized LCS process could also promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians, if abnormal imaging findings are detected, remains unclear.
It has been well established LDCT LCS reduces lung cancer-specific and overall mortality rates among eligible current and former smokers.1,9,23 The 5-year relative survival rate of veterans diagnosed with localized non-small cell lung cancer is 63%; that number drops to 7% in those with advanced disease attesting to the utility of LCS in detecting early stage lung cancer.2 Despite these favorable observations, however, screening rates with free LDCT remains low in rural communities.3-7
This proof-of-principle quality improvement project found that telehealth intervention may increase referrals of at-risk veterans who reside in rural communities to the closest centralized LCS program located at aregional VAMC. This program is responsible for reviewing the results of the initial LDCT, ordering follow-up LDCT, and recommending further evaluation as indicated.18,19 Whether this centralized LCS process would promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians if abnormal imaging findings are detected is yet to be determined.
We found that among 74 LCS-eligible rural veterans attending a CBOC-based smoking cessation program, only 3 (4%) underwent LDCT screening before this telehealth intervention was launched. This low LCS rate among veterans attempting to quit smoking may have been related, in part, to a lack of awareness of this intervention and/or barriers to LCS access.7,10,21,24 Deploying a telehealth intervention targeting LCS could address this life threatening and unmet medical need in rural communities.25 The results of this proof-of-principle quality improvement project support this contention with the reported increased referrals to and completion of initial LDCT within 4 months of the telehealth encounter.
Limitations
This was a small, single site project composed of predominantly White male rural veterans participating in a smoking cessation program associated with a VA facility.26,27 It is not clear whether similar outcomes would be observed in at-risk veterans who do not participate in a smoking cessation program or in more diverse communities. We were unable to contact 40% of LCS-eligible rural veterans by telephone. Twelve veterans reached by telephone declined to participate in LCS without providing a reason, and only 19 of 68 eligible veterans (28%) underwent LDCT screening during the 4-month telehealth intervention. The reasons underlying this overall low accrual rate and whether rural veterans prefer other means of personal communication regarding LCS were not determined. Lastly, generalizability of our initial observations to other veterans living in rural communities is limited because the project was conducted only in rural northern Wisconsin and the Upper Peninsula of Michigan.
Conclusions
At-risk rural veterans may be willing to participate in a centralized LCS program at a regional VA medical facility when contacted and coordinated using telehealth modalities. These findings offer support for future prospective, multisite, VA telehealth-based studies to be conducted in rural areas. The results of this project also suggest that telehealth intervention could increase referrals of at-risk rural veterans to the closest centralized LCS program located at a regional VA medical facility.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873
2. State of Lung Cancer: 2023 Report. American Lung Association. November 14, 2023. Accessed June 4, 2024. https://www.lung.org/getmedia/186786b6-18c3-46a9-a7e7-810f3ce4deda/SOLC-2023-Print-Report.pdf
3. Okereke IC, Nishi S, Zhou J, Goodwin JS. Trends in lung cancer screening in the United States, 2016-2017. J Thorac Dis. 2019;11(3):873-881. doi:10.21037/jtd.2019.01.105
4. Petraglia AF, Olazagasti JM, Strong A, Dunn B, Anderson RT, Hanley M. Establishing satellite lung cancer screening sites with telehealth to address disparities between high-risk smokers and American College of Radiology-approved Centers of Designation. J Thorac Imaging. 2021;36(1):2-5. doi:10.1097/RTI.0000000000000520
5. Odahowski CL, Zahnd WE, Eberth JM. Challenges and opportunities for lung cancer screening in rural America. J Am Coll Radiol. 2019;16(4 Pt B):590-595. doi:10.1016/j.jacr.2019.01.001
6. Rohatgi KW, Marx CM, Lewis-Thames MW, Liu J, Colditz GA, James AS. Urban-rural disparities in access to low-dose computed tomography lung cancer screening in Missouri and Illinois. Prev Chronic Dis. 2020;17:E140. doi:10.5888/pcd17.200202
7. Boudreau JH, Miller DR, Qian S, Nunez ER, Caverly TJ, Wiener RS. Access to lung cancer screening in the Veterans Health Administration: does geographic distribution match need in the population? Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
8. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2017. National Cancer Institute, US Dept of Health and Human Services; April 15, 2020. Accessed June 4, 2024. https://seer.cancer.gov/archive/csr/1975_2017/index.html
9. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
10. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17(5):582-587. doi:10.1016/j.nurpra.2021.02.020
11. Yacoub JH, Swanson CE, Jay AK, Cooper C, Spies J, Krishnan P. The radiology virtual reading room: during and beyond the COVID-19 pandemic. J Digit Imaging. 2021;34(2):308-319. doi:10.1007/s10278-021-00427-4
12. Beswick DM, Vashi A, Song Y, et al. Consultation via telemedicine and access to operative care for patients with head and neck cancer in a Veterans Health Administration population. Head Neck. 2016;38(6):925-929. doi:10.1002/hed.24386
13. Ruco A, Dossa F, Tinmouth J, et al. Social media and mHealth technology for cancer screening: systematic review and meta-analysis. J Med Internet Res. 2021;23(7):e26759. doi:10.2196/26759
14. Raza T, Joshi M, Schapira RM, Agha Z. Pulmonary telemedicine - a model to access the subspecialist services in underserved rural areas. Int J Med Inform. 2009;78(1):53-59. doi:10.1016/j.ijmedinf.2008.07.010
15. Chen A, Ayub MH, Mishuris RG, et al. Telehealth policy, practice, and education: a position statement of the Society of General Internal Medicine. J Gen Intern Med. 2023;38(11):2613-2620. doi:10.1007/s11606-023-08190-8
16. Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed June 4, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
17. VHA Directive 1056: National Smoking and Tobacco Use Cessation Program. Veterans Health Administration, US Dept of Veterans Affairs; September 5, 2019. Accessed June 4, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8488
18. Smith HB, Ward R, Frazier C, Angotti J, Tanner NT. Guideline-recommended lung cancer screening adherence is superior with a centralized approach. Chest. 2022;161(3):818-825. doi:10.1016/j.chest.2021.09.002
19. Lewis JA, Samuels LR, Denton J, et al. The association of health care system resources with lung cancer screening implementation: a cohort study. Chest. 2022;162(3):701-711. doi:10.1016/j.chest.2022.03.050
20. US Dept of Veterans Affairs. Lung cancer screening: patient education fact sheet. Accessed July 8, 2024. https://www.cancer.va.gov/assets/pdf/survey/LCSflyer.pdf
21. Melzer AC, Golden SE, Ono SS, Datta S, Crothers K, Slatore CG. What exactly is shared decision-making? A qualitative study of shared decision-making in lung cancer screening. J Gen Intern Med. 2020;35(2):546-553. doi:10.1007/s11606-019-05516-3
22. Chelala L, Hossain R, Kazerooni EA, Christensen JD, Dyer DS, White CS. Lung-RADS Version 1.1: challenges and a look ahead, from the AJR special series on radiology reporting and data systems. AJR Am J Roentgenol. 2021;216(6):1411-1422. doi:10.2214/AJR.20.24807
23. Ritzwoller DP, Meza R, Carroll NM, et al. Evaluation of population-level changes associated with the 2021 US Preventive Services Task Force lung cancer screening recommendations in community-based health care systems. JAMA Netw Open. 2021;4(10):e2128176. doi:10.1001/jamanetworkopen.2021.28176
24. Golden SE, Ono SS, Thakurta SG, et al. “I’m putting my trust in their hands”: a qualitative study of patients’ views on clinician initial communication about lung cancer screening. Chest. 2020;158(3):1260-1267. doi:10.1016/j.chest.2020.02.072
25. Park ER, Chiles C, Cinciripini PM, et al. Impact of the COVID-19 pandemic on telehealth research in cancer prevention and care: a call to sustain telehealth advances. Cancer. 2021;127(3):334-338. doi:10.1002/cncr.33227
26. Tremblay A, Taghizadeh N, Huang J, et al. A randomized controlled study of integrated smoking cessation in a lung cancer screening program. J Thorac Oncol. 2019;14(9):1528-1537. doi:10.1016/j.jtho.2019.04.024
27. Neil JM, Marotta C, Gonzalez I, et al. Integrating tobacco treatment into lung cancer screening practices: study protocol for the Screen ASSIST randomized clinical trial. Contemp Clin Trials. 2021;111:106586. doi:10.1016/j.cct.2021.106586
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873
2. State of Lung Cancer: 2023 Report. American Lung Association. November 14, 2023. Accessed June 4, 2024. https://www.lung.org/getmedia/186786b6-18c3-46a9-a7e7-810f3ce4deda/SOLC-2023-Print-Report.pdf
3. Okereke IC, Nishi S, Zhou J, Goodwin JS. Trends in lung cancer screening in the United States, 2016-2017. J Thorac Dis. 2019;11(3):873-881. doi:10.21037/jtd.2019.01.105
4. Petraglia AF, Olazagasti JM, Strong A, Dunn B, Anderson RT, Hanley M. Establishing satellite lung cancer screening sites with telehealth to address disparities between high-risk smokers and American College of Radiology-approved Centers of Designation. J Thorac Imaging. 2021;36(1):2-5. doi:10.1097/RTI.0000000000000520
5. Odahowski CL, Zahnd WE, Eberth JM. Challenges and opportunities for lung cancer screening in rural America. J Am Coll Radiol. 2019;16(4 Pt B):590-595. doi:10.1016/j.jacr.2019.01.001
6. Rohatgi KW, Marx CM, Lewis-Thames MW, Liu J, Colditz GA, James AS. Urban-rural disparities in access to low-dose computed tomography lung cancer screening in Missouri and Illinois. Prev Chronic Dis. 2020;17:E140. doi:10.5888/pcd17.200202
7. Boudreau JH, Miller DR, Qian S, Nunez ER, Caverly TJ, Wiener RS. Access to lung cancer screening in the Veterans Health Administration: does geographic distribution match need in the population? Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
8. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2017. National Cancer Institute, US Dept of Health and Human Services; April 15, 2020. Accessed June 4, 2024. https://seer.cancer.gov/archive/csr/1975_2017/index.html
9. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
10. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17(5):582-587. doi:10.1016/j.nurpra.2021.02.020
11. Yacoub JH, Swanson CE, Jay AK, Cooper C, Spies J, Krishnan P. The radiology virtual reading room: during and beyond the COVID-19 pandemic. J Digit Imaging. 2021;34(2):308-319. doi:10.1007/s10278-021-00427-4
12. Beswick DM, Vashi A, Song Y, et al. Consultation via telemedicine and access to operative care for patients with head and neck cancer in a Veterans Health Administration population. Head Neck. 2016;38(6):925-929. doi:10.1002/hed.24386
13. Ruco A, Dossa F, Tinmouth J, et al. Social media and mHealth technology for cancer screening: systematic review and meta-analysis. J Med Internet Res. 2021;23(7):e26759. doi:10.2196/26759
14. Raza T, Joshi M, Schapira RM, Agha Z. Pulmonary telemedicine - a model to access the subspecialist services in underserved rural areas. Int J Med Inform. 2009;78(1):53-59. doi:10.1016/j.ijmedinf.2008.07.010
15. Chen A, Ayub MH, Mishuris RG, et al. Telehealth policy, practice, and education: a position statement of the Society of General Internal Medicine. J Gen Intern Med. 2023;38(11):2613-2620. doi:10.1007/s11606-023-08190-8
16. Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed June 4, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
17. VHA Directive 1056: National Smoking and Tobacco Use Cessation Program. Veterans Health Administration, US Dept of Veterans Affairs; September 5, 2019. Accessed June 4, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8488
18. Smith HB, Ward R, Frazier C, Angotti J, Tanner NT. Guideline-recommended lung cancer screening adherence is superior with a centralized approach. Chest. 2022;161(3):818-825. doi:10.1016/j.chest.2021.09.002
19. Lewis JA, Samuels LR, Denton J, et al. The association of health care system resources with lung cancer screening implementation: a cohort study. Chest. 2022;162(3):701-711. doi:10.1016/j.chest.2022.03.050
20. US Dept of Veterans Affairs. Lung cancer screening: patient education fact sheet. Accessed July 8, 2024. https://www.cancer.va.gov/assets/pdf/survey/LCSflyer.pdf
21. Melzer AC, Golden SE, Ono SS, Datta S, Crothers K, Slatore CG. What exactly is shared decision-making? A qualitative study of shared decision-making in lung cancer screening. J Gen Intern Med. 2020;35(2):546-553. doi:10.1007/s11606-019-05516-3
22. Chelala L, Hossain R, Kazerooni EA, Christensen JD, Dyer DS, White CS. Lung-RADS Version 1.1: challenges and a look ahead, from the AJR special series on radiology reporting and data systems. AJR Am J Roentgenol. 2021;216(6):1411-1422. doi:10.2214/AJR.20.24807
23. Ritzwoller DP, Meza R, Carroll NM, et al. Evaluation of population-level changes associated with the 2021 US Preventive Services Task Force lung cancer screening recommendations in community-based health care systems. JAMA Netw Open. 2021;4(10):e2128176. doi:10.1001/jamanetworkopen.2021.28176
24. Golden SE, Ono SS, Thakurta SG, et al. “I’m putting my trust in their hands”: a qualitative study of patients’ views on clinician initial communication about lung cancer screening. Chest. 2020;158(3):1260-1267. doi:10.1016/j.chest.2020.02.072
25. Park ER, Chiles C, Cinciripini PM, et al. Impact of the COVID-19 pandemic on telehealth research in cancer prevention and care: a call to sustain telehealth advances. Cancer. 2021;127(3):334-338. doi:10.1002/cncr.33227
26. Tremblay A, Taghizadeh N, Huang J, et al. A randomized controlled study of integrated smoking cessation in a lung cancer screening program. J Thorac Oncol. 2019;14(9):1528-1537. doi:10.1016/j.jtho.2019.04.024
27. Neil JM, Marotta C, Gonzalez I, et al. Integrating tobacco treatment into lung cancer screening practices: study protocol for the Screen ASSIST randomized clinical trial. Contemp Clin Trials. 2021;111:106586. doi:10.1016/j.cct.2021.106586
Nasal Cannula Dislodgement During Sleep in Veterans Receiving Long-term Oxygen Therapy for Hypoxemic Chronic Respiratory Failure
The prevalence of chronic obstructive pulmonary disease (COPD) among male US veterans is higher than in the general population.1 Veterans with COPD have higher rates of comorbidities and increased respiratory-related and all-cause health care use, including the use of long-term oxygen therapy (LTOT).2-5 It has been well established that LTOT reduces all-cause mortality in patients with COPD and
Delivery of domiciliary LTOT entails placing a nasal cannula into both nostrils and loosely securing it around both ears throughout the wake-sleep cycle. Several veterans with hypoxemic CRF due to COPD at the Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, who were receiving LTOT reported nasal cannula dislodgement (NCD) while they slept. However, the clinical significance and impact of these repeated episodes on respiratory-related health care utilization, such as frequent COPD exacerbations with hospitalization, were not recognized.
The purpose of this study was to determine whether veterans with hypoxemic CRF due to COPD and receiving 24-hour LTOT at JBVAMC were experiencing NCD during sleep and, if so, its impact on
METHODS
We reviewed electronic health records (EHRs) of veterans with hypoxemic CRF from COPD who received 24-hour LTOT administered through nasal cannula and were followed
Pertinent patient demographics, clinical and physiologic variables, and hospitalizations with length of JBVAMC stay for each physician-diagnosed COPD exacerbation in the preceding year from the date last seen in the clinic were abstracted from EHRs. Overall hospital cost, defined as a veteran overnight stay in either the medical intensive care unit (MICU) or a general acute medicine bed in a US Department of Veterans Affairs (VA) facility, was calculated for each hospitalization for physician-diagnosed COPD exacerbation using VA Managerial Cost Accounting System National Cost Extracts for inpatient encounters.15 We then contacted each veteran by telephone and asked whether they had experienced NCD and, if so, its weekly frequency ranging from once to nightly.
Data Analysis
Data were reported as mean (SD) where appropriate. The t test and Fisher exact test were used as indicated. P < .05 was considered statistically significant. The study protocol
RESULTS
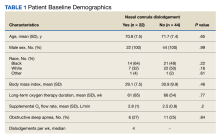
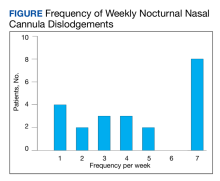
During the study period,
Of the 75 patients, 66 (88%) responded to the telephone survey and 22 patients (33%) reported weekly episodes of NCD while they slept (median, 4 dislodgments per week). (Table 1). Eight patients (36%) reported nightly NCDs (Figure). All 66 respondents were male and 14 of 22 in the NCD group as well as 21 of 44 in the no NCD group were Black veterans. The mean age was similar in both groups: 71 years in the NCD group and 72 years in the no NCD group. There were no statistically significant differences in demographics, including prevalence of obstructive sleep apnea (OSA), supplemental oxygen flow rate, and duration of LTOT, or in pulmonary function test results between patients who did and did not experience NCD while sleeping (Table 2).
Ten of 22 patients (45%) with NCD and 9 of 44 patients (20%) without NCD were hospitalized at the JBVAMC for ≥ 1 COPD exacerbation in the preceding year that was diagnosed by a physician (P = .045). Three of 22 patients (14%) with NCD and no patients in the no NCD group were admitted to the MICU. No patients required intubation and mechanical ventilation during hospitalization, and no patients died. Overall hospital costs were 25% ($64,342) higher in NCD group compared with the no NCD group and were attributed to the MICU admissions in the NCD group (Table 3). Nine veterans did not respond to repeated telephone calls. One physician-diagnosed COPD exacerbation requiring hospitalization was documented in the nonresponder group; the patient was hospitalized for 2 days. One veteran died before being contacted.
DISCUSSION
There are 3 new findings in this study.
Nocturnal arterial oxygen desaturation in patients with COPD without evidence of OSA may contribute to the frequency of exacerbations.16 Although the mechanism(s) underlying this phenomenon is uncertain, we posit that prolonged nocturnal airway wall hypoxia could amplify underlying chronic inflammation through local generation of reactive oxygen species, thereby predisposing patients to exacerbations. Frequent COPD exacerbations promote disease progression and health status decline and are associated with increased mortality.11,13 Moreover, hospitalization of patients with COPD is the largest contributor to the annual direct cost of COPD per patient.10,12 The higher hospitalization rate observed in the NCD group in our study suggests that interruption of supplemental oxygen delivery while asleep may be a risk factor for COPD exacerbation. Alternatively, an independent factor or factors may have contributed to both NCD during sleep and COPD exacerbation in these patients or an impending exacerbation resulted in sleep disturbances that led to NCD. Additional research is warranted on veterans with hypoxemic CRF from COPD who are receiving LTOT and report frequent NCD during sleep that may support or refute these hypotheses.
To the best of our knowledge, NCD during sleep has not been previously reported in patients
Limitations
This was a small, single-site study, comprised entirely of male patients who are predominantly Black veterans. The telephone interviews with veterans self-reporting NCD during their sleep are prone to recall bias. In addition, the validity and reproducibility of NCD during sleep were not addressed in this study. Missing data from 9 nonresponders may have introduced a nonresponse bias in data analysis and interpretation. The overall hospital cost for a COPD exacerbation at JBVAMC was derived from VA data; US Centers for Medicare & Medicaid Services or commercial carrier data may be different.15,21 Lastly, access to LTOT for veterans with hypoxemic CRF from COPD is regulated and supervised at VA medical facilities.14 This process may be different for patients outside the VA. Taken together, it is difficult to generalize our initial observations to non-VA patients with hypoxemic CRF from COPD who are receiving LTOT. We suggest a large, prospective study of veterans be conducted to determine the prevalence of NCD during sleep and its relationship with COPD exacerbations in veterans receiving LTOT with hypoxemic CRF due to COPD.
CONCLUSIONS
Acknowledgments
We thank Yolanda Davis, RRT, and George Adam for their assistance with this project.
1. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13. doi:10.15620/cdc:101659
2. Sharafkhaneh A, Petersen NJ, Yu H-J, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132. doi:10.2147/copd.s8047
3. LaBedz SL, Krishnan JA, Chung Y-C, et al. Chronic obstructive pulmonary disease outcomes at Veterans Affairs versus non-Veterans Affairs hospitals. Chronic Obstr Pulm Dis. 2021;8(3):306-313. doi:10.15326/jcopdf.2021.0201
4. Darnell K, Dwivedi AK, Weng Z, Panos RJ. Disproportionate utilization of healthcare resources among veterans with COPD: a retrospective analysis of factors associated with COPD healthcare cost. Cost Eff Resour Alloc. 2013;11:13. doi:10.1186/1478-7547-11-13
5. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US Veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
6. Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):CD001744. doi:10.1002/14651858.CD001744.pub2
7. Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(10):1254-1264. doi:10.1164/rccm.201802-0382CI
8. Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(10):e121-e141. doi:10.1164/rccm.202009-3608ST
9. AARC. AARC clinical practice guideline. Oxygen therapy in the home or alternate site health care facility--2007 revision & update. Respir Care. 2007;52(8):1063-1068.
10. Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618
11. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464-471. doi:10.1164/rccm.201710-2029OC
12. Stanford RH, Engel-Nitz NM, Bancroft T, Essoi B. The identification and cost of acute chronic obstructive pulmonary disease exacerbations in a United States population healthcare claims database. COPD. 2020;17(5):499-508. doi:10.1080/15412555.2020.1817357
13. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
14. US Department of Veterans Affairs, Veterans Health Administration. Home oxygen program. VHA Directive 1173.13(1). Published August 5, 2020. Accessed February 28, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8947
15. Phibbs CS, Barnett PG, Fan A, Harden C, King SS, Scott JY. Research guide to decision support system national cost extracts. Health Economics Resource Center of Health Service R&D Services, US Department of Veterans Affairs. September 2010. Accessed February 14, 2024. https://www.herc.research.va.gov/files/book_621.pdf
16. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194. doi:10.1183/09059180.00004311
17. Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373-378. doi:10.1164/rccm.200507-1161WS
18. Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol. 2018;11(3):279-289. doi:10.1080/17512433.2018.1421457
19. Sculley JA, Corbridge SJ, Prieto-Centurion V, et al. Home oxygen therapy for patients with COPD: time for a reboot. Respir Care. 2019;64(12):1574-1585. doi:10.4187/respcare.07135
20. Jacobs SS, Lindell KO, Collins EG, et al. Patient perceptions of the adequacy of supplemental oxygen therapy. Results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15:24-32. doi:10.1513/AnnalsATS.201703-209OC
21. US Centers for Medicare & Medicaid Services. Home use of oxygen. Publication number 100-3. January 3, 2023. Accessed February 14, 2024. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=169
The prevalence of chronic obstructive pulmonary disease (COPD) among male US veterans is higher than in the general population.1 Veterans with COPD have higher rates of comorbidities and increased respiratory-related and all-cause health care use, including the use of long-term oxygen therapy (LTOT).2-5 It has been well established that LTOT reduces all-cause mortality in patients with COPD and
Delivery of domiciliary LTOT entails placing a nasal cannula into both nostrils and loosely securing it around both ears throughout the wake-sleep cycle. Several veterans with hypoxemic CRF due to COPD at the Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, who were receiving LTOT reported nasal cannula dislodgement (NCD) while they slept. However, the clinical significance and impact of these repeated episodes on respiratory-related health care utilization, such as frequent COPD exacerbations with hospitalization, were not recognized.
The purpose of this study was to determine whether veterans with hypoxemic CRF due to COPD and receiving 24-hour LTOT at JBVAMC were experiencing NCD during sleep and, if so, its impact on
METHODS
We reviewed electronic health records (EHRs) of veterans with hypoxemic CRF from COPD who received 24-hour LTOT administered through nasal cannula and were followed
Pertinent patient demographics, clinical and physiologic variables, and hospitalizations with length of JBVAMC stay for each physician-diagnosed COPD exacerbation in the preceding year from the date last seen in the clinic were abstracted from EHRs. Overall hospital cost, defined as a veteran overnight stay in either the medical intensive care unit (MICU) or a general acute medicine bed in a US Department of Veterans Affairs (VA) facility, was calculated for each hospitalization for physician-diagnosed COPD exacerbation using VA Managerial Cost Accounting System National Cost Extracts for inpatient encounters.15 We then contacted each veteran by telephone and asked whether they had experienced NCD and, if so, its weekly frequency ranging from once to nightly.
Data Analysis
Data were reported as mean (SD) where appropriate. The t test and Fisher exact test were used as indicated. P < .05 was considered statistically significant. The study protocol
RESULTS
During the study period,
Of the 75 patients, 66 (88%) responded to the telephone survey and 22 patients (33%) reported weekly episodes of NCD while they slept (median, 4 dislodgments per week). (Table 1). Eight patients (36%) reported nightly NCDs (Figure). All 66 respondents were male and 14 of 22 in the NCD group as well as 21 of 44 in the no NCD group were Black veterans. The mean age was similar in both groups: 71 years in the NCD group and 72 years in the no NCD group. There were no statistically significant differences in demographics, including prevalence of obstructive sleep apnea (OSA), supplemental oxygen flow rate, and duration of LTOT, or in pulmonary function test results between patients who did and did not experience NCD while sleeping (Table 2).
Ten of 22 patients (45%) with NCD and 9 of 44 patients (20%) without NCD were hospitalized at the JBVAMC for ≥ 1 COPD exacerbation in the preceding year that was diagnosed by a physician (P = .045). Three of 22 patients (14%) with NCD and no patients in the no NCD group were admitted to the MICU. No patients required intubation and mechanical ventilation during hospitalization, and no patients died. Overall hospital costs were 25% ($64,342) higher in NCD group compared with the no NCD group and were attributed to the MICU admissions in the NCD group (Table 3). Nine veterans did not respond to repeated telephone calls. One physician-diagnosed COPD exacerbation requiring hospitalization was documented in the nonresponder group; the patient was hospitalized for 2 days. One veteran died before being contacted.
DISCUSSION
There are 3 new findings in this study.
Nocturnal arterial oxygen desaturation in patients with COPD without evidence of OSA may contribute to the frequency of exacerbations.16 Although the mechanism(s) underlying this phenomenon is uncertain, we posit that prolonged nocturnal airway wall hypoxia could amplify underlying chronic inflammation through local generation of reactive oxygen species, thereby predisposing patients to exacerbations. Frequent COPD exacerbations promote disease progression and health status decline and are associated with increased mortality.11,13 Moreover, hospitalization of patients with COPD is the largest contributor to the annual direct cost of COPD per patient.10,12 The higher hospitalization rate observed in the NCD group in our study suggests that interruption of supplemental oxygen delivery while asleep may be a risk factor for COPD exacerbation. Alternatively, an independent factor or factors may have contributed to both NCD during sleep and COPD exacerbation in these patients or an impending exacerbation resulted in sleep disturbances that led to NCD. Additional research is warranted on veterans with hypoxemic CRF from COPD who are receiving LTOT and report frequent NCD during sleep that may support or refute these hypotheses.
To the best of our knowledge, NCD during sleep has not been previously reported in patients
Limitations
This was a small, single-site study, comprised entirely of male patients who are predominantly Black veterans. The telephone interviews with veterans self-reporting NCD during their sleep are prone to recall bias. In addition, the validity and reproducibility of NCD during sleep were not addressed in this study. Missing data from 9 nonresponders may have introduced a nonresponse bias in data analysis and interpretation. The overall hospital cost for a COPD exacerbation at JBVAMC was derived from VA data; US Centers for Medicare & Medicaid Services or commercial carrier data may be different.15,21 Lastly, access to LTOT for veterans with hypoxemic CRF from COPD is regulated and supervised at VA medical facilities.14 This process may be different for patients outside the VA. Taken together, it is difficult to generalize our initial observations to non-VA patients with hypoxemic CRF from COPD who are receiving LTOT. We suggest a large, prospective study of veterans be conducted to determine the prevalence of NCD during sleep and its relationship with COPD exacerbations in veterans receiving LTOT with hypoxemic CRF due to COPD.
CONCLUSIONS
Acknowledgments
We thank Yolanda Davis, RRT, and George Adam for their assistance with this project.
The prevalence of chronic obstructive pulmonary disease (COPD) among male US veterans is higher than in the general population.1 Veterans with COPD have higher rates of comorbidities and increased respiratory-related and all-cause health care use, including the use of long-term oxygen therapy (LTOT).2-5 It has been well established that LTOT reduces all-cause mortality in patients with COPD and
Delivery of domiciliary LTOT entails placing a nasal cannula into both nostrils and loosely securing it around both ears throughout the wake-sleep cycle. Several veterans with hypoxemic CRF due to COPD at the Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, who were receiving LTOT reported nasal cannula dislodgement (NCD) while they slept. However, the clinical significance and impact of these repeated episodes on respiratory-related health care utilization, such as frequent COPD exacerbations with hospitalization, were not recognized.
The purpose of this study was to determine whether veterans with hypoxemic CRF due to COPD and receiving 24-hour LTOT at JBVAMC were experiencing NCD during sleep and, if so, its impact on
METHODS
We reviewed electronic health records (EHRs) of veterans with hypoxemic CRF from COPD who received 24-hour LTOT administered through nasal cannula and were followed
Pertinent patient demographics, clinical and physiologic variables, and hospitalizations with length of JBVAMC stay for each physician-diagnosed COPD exacerbation in the preceding year from the date last seen in the clinic were abstracted from EHRs. Overall hospital cost, defined as a veteran overnight stay in either the medical intensive care unit (MICU) or a general acute medicine bed in a US Department of Veterans Affairs (VA) facility, was calculated for each hospitalization for physician-diagnosed COPD exacerbation using VA Managerial Cost Accounting System National Cost Extracts for inpatient encounters.15 We then contacted each veteran by telephone and asked whether they had experienced NCD and, if so, its weekly frequency ranging from once to nightly.
Data Analysis
Data were reported as mean (SD) where appropriate. The t test and Fisher exact test were used as indicated. P < .05 was considered statistically significant. The study protocol
RESULTS
During the study period,
Of the 75 patients, 66 (88%) responded to the telephone survey and 22 patients (33%) reported weekly episodes of NCD while they slept (median, 4 dislodgments per week). (Table 1). Eight patients (36%) reported nightly NCDs (Figure). All 66 respondents were male and 14 of 22 in the NCD group as well as 21 of 44 in the no NCD group were Black veterans. The mean age was similar in both groups: 71 years in the NCD group and 72 years in the no NCD group. There were no statistically significant differences in demographics, including prevalence of obstructive sleep apnea (OSA), supplemental oxygen flow rate, and duration of LTOT, or in pulmonary function test results between patients who did and did not experience NCD while sleeping (Table 2).
Ten of 22 patients (45%) with NCD and 9 of 44 patients (20%) without NCD were hospitalized at the JBVAMC for ≥ 1 COPD exacerbation in the preceding year that was diagnosed by a physician (P = .045). Three of 22 patients (14%) with NCD and no patients in the no NCD group were admitted to the MICU. No patients required intubation and mechanical ventilation during hospitalization, and no patients died. Overall hospital costs were 25% ($64,342) higher in NCD group compared with the no NCD group and were attributed to the MICU admissions in the NCD group (Table 3). Nine veterans did not respond to repeated telephone calls. One physician-diagnosed COPD exacerbation requiring hospitalization was documented in the nonresponder group; the patient was hospitalized for 2 days. One veteran died before being contacted.
DISCUSSION
There are 3 new findings in this study.
Nocturnal arterial oxygen desaturation in patients with COPD without evidence of OSA may contribute to the frequency of exacerbations.16 Although the mechanism(s) underlying this phenomenon is uncertain, we posit that prolonged nocturnal airway wall hypoxia could amplify underlying chronic inflammation through local generation of reactive oxygen species, thereby predisposing patients to exacerbations. Frequent COPD exacerbations promote disease progression and health status decline and are associated with increased mortality.11,13 Moreover, hospitalization of patients with COPD is the largest contributor to the annual direct cost of COPD per patient.10,12 The higher hospitalization rate observed in the NCD group in our study suggests that interruption of supplemental oxygen delivery while asleep may be a risk factor for COPD exacerbation. Alternatively, an independent factor or factors may have contributed to both NCD during sleep and COPD exacerbation in these patients or an impending exacerbation resulted in sleep disturbances that led to NCD. Additional research is warranted on veterans with hypoxemic CRF from COPD who are receiving LTOT and report frequent NCD during sleep that may support or refute these hypotheses.
To the best of our knowledge, NCD during sleep has not been previously reported in patients
Limitations
This was a small, single-site study, comprised entirely of male patients who are predominantly Black veterans. The telephone interviews with veterans self-reporting NCD during their sleep are prone to recall bias. In addition, the validity and reproducibility of NCD during sleep were not addressed in this study. Missing data from 9 nonresponders may have introduced a nonresponse bias in data analysis and interpretation. The overall hospital cost for a COPD exacerbation at JBVAMC was derived from VA data; US Centers for Medicare & Medicaid Services or commercial carrier data may be different.15,21 Lastly, access to LTOT for veterans with hypoxemic CRF from COPD is regulated and supervised at VA medical facilities.14 This process may be different for patients outside the VA. Taken together, it is difficult to generalize our initial observations to non-VA patients with hypoxemic CRF from COPD who are receiving LTOT. We suggest a large, prospective study of veterans be conducted to determine the prevalence of NCD during sleep and its relationship with COPD exacerbations in veterans receiving LTOT with hypoxemic CRF due to COPD.
CONCLUSIONS
Acknowledgments
We thank Yolanda Davis, RRT, and George Adam for their assistance with this project.
1. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13. doi:10.15620/cdc:101659
2. Sharafkhaneh A, Petersen NJ, Yu H-J, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132. doi:10.2147/copd.s8047
3. LaBedz SL, Krishnan JA, Chung Y-C, et al. Chronic obstructive pulmonary disease outcomes at Veterans Affairs versus non-Veterans Affairs hospitals. Chronic Obstr Pulm Dis. 2021;8(3):306-313. doi:10.15326/jcopdf.2021.0201
4. Darnell K, Dwivedi AK, Weng Z, Panos RJ. Disproportionate utilization of healthcare resources among veterans with COPD: a retrospective analysis of factors associated with COPD healthcare cost. Cost Eff Resour Alloc. 2013;11:13. doi:10.1186/1478-7547-11-13
5. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US Veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
6. Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):CD001744. doi:10.1002/14651858.CD001744.pub2
7. Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(10):1254-1264. doi:10.1164/rccm.201802-0382CI
8. Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(10):e121-e141. doi:10.1164/rccm.202009-3608ST
9. AARC. AARC clinical practice guideline. Oxygen therapy in the home or alternate site health care facility--2007 revision & update. Respir Care. 2007;52(8):1063-1068.
10. Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618
11. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464-471. doi:10.1164/rccm.201710-2029OC
12. Stanford RH, Engel-Nitz NM, Bancroft T, Essoi B. The identification and cost of acute chronic obstructive pulmonary disease exacerbations in a United States population healthcare claims database. COPD. 2020;17(5):499-508. doi:10.1080/15412555.2020.1817357
13. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
14. US Department of Veterans Affairs, Veterans Health Administration. Home oxygen program. VHA Directive 1173.13(1). Published August 5, 2020. Accessed February 28, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8947
15. Phibbs CS, Barnett PG, Fan A, Harden C, King SS, Scott JY. Research guide to decision support system national cost extracts. Health Economics Resource Center of Health Service R&D Services, US Department of Veterans Affairs. September 2010. Accessed February 14, 2024. https://www.herc.research.va.gov/files/book_621.pdf
16. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194. doi:10.1183/09059180.00004311
17. Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373-378. doi:10.1164/rccm.200507-1161WS
18. Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol. 2018;11(3):279-289. doi:10.1080/17512433.2018.1421457
19. Sculley JA, Corbridge SJ, Prieto-Centurion V, et al. Home oxygen therapy for patients with COPD: time for a reboot. Respir Care. 2019;64(12):1574-1585. doi:10.4187/respcare.07135
20. Jacobs SS, Lindell KO, Collins EG, et al. Patient perceptions of the adequacy of supplemental oxygen therapy. Results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15:24-32. doi:10.1513/AnnalsATS.201703-209OC
21. US Centers for Medicare & Medicaid Services. Home use of oxygen. Publication number 100-3. January 3, 2023. Accessed February 14, 2024. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=169
1. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13. doi:10.15620/cdc:101659
2. Sharafkhaneh A, Petersen NJ, Yu H-J, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132. doi:10.2147/copd.s8047
3. LaBedz SL, Krishnan JA, Chung Y-C, et al. Chronic obstructive pulmonary disease outcomes at Veterans Affairs versus non-Veterans Affairs hospitals. Chronic Obstr Pulm Dis. 2021;8(3):306-313. doi:10.15326/jcopdf.2021.0201
4. Darnell K, Dwivedi AK, Weng Z, Panos RJ. Disproportionate utilization of healthcare resources among veterans with COPD: a retrospective analysis of factors associated with COPD healthcare cost. Cost Eff Resour Alloc. 2013;11:13. doi:10.1186/1478-7547-11-13
5. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US Veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
6. Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):CD001744. doi:10.1002/14651858.CD001744.pub2
7. Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(10):1254-1264. doi:10.1164/rccm.201802-0382CI
8. Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(10):e121-e141. doi:10.1164/rccm.202009-3608ST
9. AARC. AARC clinical practice guideline. Oxygen therapy in the home or alternate site health care facility--2007 revision & update. Respir Care. 2007;52(8):1063-1068.
10. Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618
11. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464-471. doi:10.1164/rccm.201710-2029OC
12. Stanford RH, Engel-Nitz NM, Bancroft T, Essoi B. The identification and cost of acute chronic obstructive pulmonary disease exacerbations in a United States population healthcare claims database. COPD. 2020;17(5):499-508. doi:10.1080/15412555.2020.1817357
13. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
14. US Department of Veterans Affairs, Veterans Health Administration. Home oxygen program. VHA Directive 1173.13(1). Published August 5, 2020. Accessed February 28, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8947
15. Phibbs CS, Barnett PG, Fan A, Harden C, King SS, Scott JY. Research guide to decision support system national cost extracts. Health Economics Resource Center of Health Service R&D Services, US Department of Veterans Affairs. September 2010. Accessed February 14, 2024. https://www.herc.research.va.gov/files/book_621.pdf
16. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194. doi:10.1183/09059180.00004311
17. Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373-378. doi:10.1164/rccm.200507-1161WS
18. Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol. 2018;11(3):279-289. doi:10.1080/17512433.2018.1421457
19. Sculley JA, Corbridge SJ, Prieto-Centurion V, et al. Home oxygen therapy for patients with COPD: time for a reboot. Respir Care. 2019;64(12):1574-1585. doi:10.4187/respcare.07135
20. Jacobs SS, Lindell KO, Collins EG, et al. Patient perceptions of the adequacy of supplemental oxygen therapy. Results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15:24-32. doi:10.1513/AnnalsATS.201703-209OC
21. US Centers for Medicare & Medicaid Services. Home use of oxygen. Publication number 100-3. January 3, 2023. Accessed February 14, 2024. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=169
Diagnosis of Indolent Clonorchis sinensis and Opisthorchis viverrini Infections as Risk Factors for Cholangiocarcinoma: An Unmet Medical Need
Cholangiocarcinoma is a heterogeneous, highly aggressive cancer of the biliary tract epithelium with an overall 5-year relative survival rate of only 9%.1,2 Although surgical resection of localized, intrahepatic cholangiocarcinoma is associated with improved overall survival, most patients present with advanced disease not amenable to surgery due to a late onset of symptoms.2 Recently, an increased incidence of cholangiocarcinoma has been reported in the United States.3 Although relatively rare in the US, cholangiocarcinoma is prevalent across large parts of Asia, including China, Vietnam, Thailand, South Korea, and Taiwan.2
Risk Factors
To date, risk factors for developing cholangiocarcinoma have not been elucidated. 4,5 However, a growing body of literature suggests that chronic infection of genetically susceptible human subjects with Clonorchis sinensis ( C sinensis ) and Opisthorchis viverrini ( O viverrini ) plays a role. 6,7 The life cycle of these food-borne zoonotic trematodes involves eggs discharged in the stool of infected humans, the definitive host. 6,7 In nature, these eggs are ingested by freshwater snails, the intermediate host, where they undergo several developmental stages to form cercariae. Once released from snails into the water, free-swimming cercariae come in contact and penetrate freshwater fish where they encyst as metacercariae. Infection of humans occurs by ingesting undercooked, salted, pickled, or smoked freshwater fish infested with metacercariae. After ingestion, metacercariae excyst in the duodenum and ascend the biliary tract through the ampulla of Vater. They then mature into adult flukes that reside in small- and medium-sized intrahepatic biliary ducts. 6,7
Although most infected people remain asymptomatic, untreated indolent infections with C sinensis and O viverrini may persist in peripheral intrahepatic bile ducts for as long as 30 years, which is the lifespan of the trematodes.6,7 During this prolonged period, C sinensis and O viverrini feeding activities and their excretory-secretory products may damage bile duct epithelium and promote intense local inflammation.6,7 Conceivably, these pathological processes could then provoke the epithelial desquamation, adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis, and granuloma formation that are conducive to initiation and progression of cholangiocarcinoma in genetically susceptible people.8 Accordingly, the International Agency for Research on Cancer (IARC) has determined that there is sufficient evidence for the carcinogenicity of chronic infections with C sinensis and O viverrini in humans and that chronic infections with these trematodes cause cholangiocarcinoma.9 The IARC concluded that chronic infections with C sinensis and O viverrini are carcinogenic to humans (Group 1).9
Diagnosis
Presently, the diagnosis of C sinensis and O viverrini infection is based on microscopic identification and enumeration of the parasites’ eggs in weighted stool specimens using a formalin-ethyl acetate sedimentation concentration technique. 6,7 This approach requires a labor-intensive test that is conducted by an experienced technician. The test has low specificity and sensitivity because eggs could be confused with those of nonpathogenic intestinal flukes that are morphologically similar and because eggs are not present in feces during all stages of the infection. Although diffuse dilatation of intrahepatic bile ducts by screening sonography is used to diagnose clonorchiasis in endemic areas, it has low sensitivity, particularly in patients with low-level C sinensis and O viverrin i infections. 10
To address the current diagnostic gap, several enzyme-linked immunosorbent assays (ELISA) have been developed for the diagnosis of C sinensis, including monoclonal antibody-based (mAb) ELISA and indirect antibody ELISA.11,12 However, both have important limitations. The mAb ELISA detects only active infections while indirect antibody ELISA cross-reacts with other liver flukes.11,12 Taken together, these data illustrate the difficulties in diagnosing asymptomatic individuals with low-burden C sinensis or O viverrini infections by existing laboratory methods.
Timely serodiagnosis of indolent C sinensis and O viverrini infections is important because these parasites have recently been raised as a risk factor for cholangiocarcinoma in veterans who served in Vietnam.13 The American War Library estimates that as of February 28, 2019, about 610,000 Americans who served on land in Vietnam or in the air over Vietnam between 1954 and 1975 are alive, and about 164,000 Americans who served at sea in Vietnam waters are alive.14 To that end, Psevdos and colleagues screened 97 US veterans who served in Vietnam and identified 50 who reported exposure to raw or undercooked fish while there.13 None had evidence of active C sinensis or O viverrini infection. Blood samples obtained from these veterans were analyzed for circulating C sinensis and O viverrini antibodies using an ELISA developed in South Korea and 12 blood samples tested positive for the trematodes. Imaging of extrahepatic and intrahepatic bile ducts was unyielding in all cases. One veteran diagnosed with cholangiocarcinoma had repeated negative tests. However, the results of this study were challenged by several experts in this field because the authors did not report the sensitivity and specificity of the ELISA assay used.15
Serologic testing of US veterans who served in C sinensis and O viverrini–endemic countries for indolent infections with these parasites is not recommended at present.15 Nevertheless, there is an urgent need to develop sensitive and specific serologic assays, such as ELISA tests with recombinant antigens, to detect both acute and indolent infections caused by each biliary liver fluke in the US, including in patients diagnosed with cholangiocarcinoma. We posit that testing and treatment of high-risk populations could lead to earlier detection and treatment of cholangiocarcinoma, leading to improved overall survival in the population at risk.
1. American Cancer Society. Survival rates for bile duct cancer. Updated March 1, 2023. Accessed March 17, 2023. https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html
2. Vij M, Puri Y, Rammohan A, et al. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14(3):607-627. doi:10.4251/wjgo.v14.i3.607
3. Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117. Published 2016 Sep 21. doi:10.1186/s12876-016-0527-z
4. Rustagi T, Dasanu CA. Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates. J Gastrointest Cancer. 2012;43(2):137-147. doi:10.1007/s12029-011-9284-y
5. Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21(10):754-760. doi:10.1002/jhbp.126
6. Steele JA, Richter CH, Echaubard P, et al. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):44. Published 2018 May 17. doi:10.1186/s40249-018-0434-3.
7. Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 2016;49(11):590-597. doi:10.5483/bmbrep.2016.49.11.109
8. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. Published 2018 Oct 20. doi:10.1186/s12199-018-0740-1
9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt B):1-441.
10. Mairiang E, Laha T, Bethony JM, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61(1):208-211. doi:10.1016/j.parint.2011.07.009
11. Li HM, Qian MB, Yang YC, et al. Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China. Parasit Vectors. 2018;11(1):35. Published 2018 Jan 15. doi:10.1186/s13071-018-2612-3
12. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med. 2017;10:227-237. Published 2017 Aug 10. doi:10.2147/IJGM.S133292
13. Psevdos G, Ford FM, Hong ST. Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war. Infect Dis Clin Pract (Baltim Md). 2018;26(4):208-210. doi:10.1097/IPC.0000000000000611
14. American War Library. In harm’s way... How many real Vietnam vets are alive today? Updated February 28, 2019. Accessed March 17, 2023. https://www.americanwarlibrary.com/personnel/vietvet.htm
15. Nash TE, Sullivan D, Mitre E, et al. Comments on “Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war”. Infect Dis Clin Pract (Baltim Md). 2018;26(4):240-241. doi:10.1097/IPC.0000000000000659
Cholangiocarcinoma is a heterogeneous, highly aggressive cancer of the biliary tract epithelium with an overall 5-year relative survival rate of only 9%.1,2 Although surgical resection of localized, intrahepatic cholangiocarcinoma is associated with improved overall survival, most patients present with advanced disease not amenable to surgery due to a late onset of symptoms.2 Recently, an increased incidence of cholangiocarcinoma has been reported in the United States.3 Although relatively rare in the US, cholangiocarcinoma is prevalent across large parts of Asia, including China, Vietnam, Thailand, South Korea, and Taiwan.2
Risk Factors
To date, risk factors for developing cholangiocarcinoma have not been elucidated. 4,5 However, a growing body of literature suggests that chronic infection of genetically susceptible human subjects with Clonorchis sinensis ( C sinensis ) and Opisthorchis viverrini ( O viverrini ) plays a role. 6,7 The life cycle of these food-borne zoonotic trematodes involves eggs discharged in the stool of infected humans, the definitive host. 6,7 In nature, these eggs are ingested by freshwater snails, the intermediate host, where they undergo several developmental stages to form cercariae. Once released from snails into the water, free-swimming cercariae come in contact and penetrate freshwater fish where they encyst as metacercariae. Infection of humans occurs by ingesting undercooked, salted, pickled, or smoked freshwater fish infested with metacercariae. After ingestion, metacercariae excyst in the duodenum and ascend the biliary tract through the ampulla of Vater. They then mature into adult flukes that reside in small- and medium-sized intrahepatic biliary ducts. 6,7
Although most infected people remain asymptomatic, untreated indolent infections with C sinensis and O viverrini may persist in peripheral intrahepatic bile ducts for as long as 30 years, which is the lifespan of the trematodes.6,7 During this prolonged period, C sinensis and O viverrini feeding activities and their excretory-secretory products may damage bile duct epithelium and promote intense local inflammation.6,7 Conceivably, these pathological processes could then provoke the epithelial desquamation, adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis, and granuloma formation that are conducive to initiation and progression of cholangiocarcinoma in genetically susceptible people.8 Accordingly, the International Agency for Research on Cancer (IARC) has determined that there is sufficient evidence for the carcinogenicity of chronic infections with C sinensis and O viverrini in humans and that chronic infections with these trematodes cause cholangiocarcinoma.9 The IARC concluded that chronic infections with C sinensis and O viverrini are carcinogenic to humans (Group 1).9
Diagnosis
Presently, the diagnosis of C sinensis and O viverrini infection is based on microscopic identification and enumeration of the parasites’ eggs in weighted stool specimens using a formalin-ethyl acetate sedimentation concentration technique. 6,7 This approach requires a labor-intensive test that is conducted by an experienced technician. The test has low specificity and sensitivity because eggs could be confused with those of nonpathogenic intestinal flukes that are morphologically similar and because eggs are not present in feces during all stages of the infection. Although diffuse dilatation of intrahepatic bile ducts by screening sonography is used to diagnose clonorchiasis in endemic areas, it has low sensitivity, particularly in patients with low-level C sinensis and O viverrin i infections. 10
To address the current diagnostic gap, several enzyme-linked immunosorbent assays (ELISA) have been developed for the diagnosis of C sinensis, including monoclonal antibody-based (mAb) ELISA and indirect antibody ELISA.11,12 However, both have important limitations. The mAb ELISA detects only active infections while indirect antibody ELISA cross-reacts with other liver flukes.11,12 Taken together, these data illustrate the difficulties in diagnosing asymptomatic individuals with low-burden C sinensis or O viverrini infections by existing laboratory methods.
Timely serodiagnosis of indolent C sinensis and O viverrini infections is important because these parasites have recently been raised as a risk factor for cholangiocarcinoma in veterans who served in Vietnam.13 The American War Library estimates that as of February 28, 2019, about 610,000 Americans who served on land in Vietnam or in the air over Vietnam between 1954 and 1975 are alive, and about 164,000 Americans who served at sea in Vietnam waters are alive.14 To that end, Psevdos and colleagues screened 97 US veterans who served in Vietnam and identified 50 who reported exposure to raw or undercooked fish while there.13 None had evidence of active C sinensis or O viverrini infection. Blood samples obtained from these veterans were analyzed for circulating C sinensis and O viverrini antibodies using an ELISA developed in South Korea and 12 blood samples tested positive for the trematodes. Imaging of extrahepatic and intrahepatic bile ducts was unyielding in all cases. One veteran diagnosed with cholangiocarcinoma had repeated negative tests. However, the results of this study were challenged by several experts in this field because the authors did not report the sensitivity and specificity of the ELISA assay used.15
Serologic testing of US veterans who served in C sinensis and O viverrini–endemic countries for indolent infections with these parasites is not recommended at present.15 Nevertheless, there is an urgent need to develop sensitive and specific serologic assays, such as ELISA tests with recombinant antigens, to detect both acute and indolent infections caused by each biliary liver fluke in the US, including in patients diagnosed with cholangiocarcinoma. We posit that testing and treatment of high-risk populations could lead to earlier detection and treatment of cholangiocarcinoma, leading to improved overall survival in the population at risk.
Cholangiocarcinoma is a heterogeneous, highly aggressive cancer of the biliary tract epithelium with an overall 5-year relative survival rate of only 9%.1,2 Although surgical resection of localized, intrahepatic cholangiocarcinoma is associated with improved overall survival, most patients present with advanced disease not amenable to surgery due to a late onset of symptoms.2 Recently, an increased incidence of cholangiocarcinoma has been reported in the United States.3 Although relatively rare in the US, cholangiocarcinoma is prevalent across large parts of Asia, including China, Vietnam, Thailand, South Korea, and Taiwan.2
Risk Factors
To date, risk factors for developing cholangiocarcinoma have not been elucidated. 4,5 However, a growing body of literature suggests that chronic infection of genetically susceptible human subjects with Clonorchis sinensis ( C sinensis ) and Opisthorchis viverrini ( O viverrini ) plays a role. 6,7 The life cycle of these food-borne zoonotic trematodes involves eggs discharged in the stool of infected humans, the definitive host. 6,7 In nature, these eggs are ingested by freshwater snails, the intermediate host, where they undergo several developmental stages to form cercariae. Once released from snails into the water, free-swimming cercariae come in contact and penetrate freshwater fish where they encyst as metacercariae. Infection of humans occurs by ingesting undercooked, salted, pickled, or smoked freshwater fish infested with metacercariae. After ingestion, metacercariae excyst in the duodenum and ascend the biliary tract through the ampulla of Vater. They then mature into adult flukes that reside in small- and medium-sized intrahepatic biliary ducts. 6,7
Although most infected people remain asymptomatic, untreated indolent infections with C sinensis and O viverrini may persist in peripheral intrahepatic bile ducts for as long as 30 years, which is the lifespan of the trematodes.6,7 During this prolonged period, C sinensis and O viverrini feeding activities and their excretory-secretory products may damage bile duct epithelium and promote intense local inflammation.6,7 Conceivably, these pathological processes could then provoke the epithelial desquamation, adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis, and granuloma formation that are conducive to initiation and progression of cholangiocarcinoma in genetically susceptible people.8 Accordingly, the International Agency for Research on Cancer (IARC) has determined that there is sufficient evidence for the carcinogenicity of chronic infections with C sinensis and O viverrini in humans and that chronic infections with these trematodes cause cholangiocarcinoma.9 The IARC concluded that chronic infections with C sinensis and O viverrini are carcinogenic to humans (Group 1).9
Diagnosis
Presently, the diagnosis of C sinensis and O viverrini infection is based on microscopic identification and enumeration of the parasites’ eggs in weighted stool specimens using a formalin-ethyl acetate sedimentation concentration technique. 6,7 This approach requires a labor-intensive test that is conducted by an experienced technician. The test has low specificity and sensitivity because eggs could be confused with those of nonpathogenic intestinal flukes that are morphologically similar and because eggs are not present in feces during all stages of the infection. Although diffuse dilatation of intrahepatic bile ducts by screening sonography is used to diagnose clonorchiasis in endemic areas, it has low sensitivity, particularly in patients with low-level C sinensis and O viverrin i infections. 10
To address the current diagnostic gap, several enzyme-linked immunosorbent assays (ELISA) have been developed for the diagnosis of C sinensis, including monoclonal antibody-based (mAb) ELISA and indirect antibody ELISA.11,12 However, both have important limitations. The mAb ELISA detects only active infections while indirect antibody ELISA cross-reacts with other liver flukes.11,12 Taken together, these data illustrate the difficulties in diagnosing asymptomatic individuals with low-burden C sinensis or O viverrini infections by existing laboratory methods.
Timely serodiagnosis of indolent C sinensis and O viverrini infections is important because these parasites have recently been raised as a risk factor for cholangiocarcinoma in veterans who served in Vietnam.13 The American War Library estimates that as of February 28, 2019, about 610,000 Americans who served on land in Vietnam or in the air over Vietnam between 1954 and 1975 are alive, and about 164,000 Americans who served at sea in Vietnam waters are alive.14 To that end, Psevdos and colleagues screened 97 US veterans who served in Vietnam and identified 50 who reported exposure to raw or undercooked fish while there.13 None had evidence of active C sinensis or O viverrini infection. Blood samples obtained from these veterans were analyzed for circulating C sinensis and O viverrini antibodies using an ELISA developed in South Korea and 12 blood samples tested positive for the trematodes. Imaging of extrahepatic and intrahepatic bile ducts was unyielding in all cases. One veteran diagnosed with cholangiocarcinoma had repeated negative tests. However, the results of this study were challenged by several experts in this field because the authors did not report the sensitivity and specificity of the ELISA assay used.15
Serologic testing of US veterans who served in C sinensis and O viverrini–endemic countries for indolent infections with these parasites is not recommended at present.15 Nevertheless, there is an urgent need to develop sensitive and specific serologic assays, such as ELISA tests with recombinant antigens, to detect both acute and indolent infections caused by each biliary liver fluke in the US, including in patients diagnosed with cholangiocarcinoma. We posit that testing and treatment of high-risk populations could lead to earlier detection and treatment of cholangiocarcinoma, leading to improved overall survival in the population at risk.
1. American Cancer Society. Survival rates for bile duct cancer. Updated March 1, 2023. Accessed March 17, 2023. https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html
2. Vij M, Puri Y, Rammohan A, et al. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14(3):607-627. doi:10.4251/wjgo.v14.i3.607
3. Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117. Published 2016 Sep 21. doi:10.1186/s12876-016-0527-z
4. Rustagi T, Dasanu CA. Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates. J Gastrointest Cancer. 2012;43(2):137-147. doi:10.1007/s12029-011-9284-y
5. Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21(10):754-760. doi:10.1002/jhbp.126
6. Steele JA, Richter CH, Echaubard P, et al. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):44. Published 2018 May 17. doi:10.1186/s40249-018-0434-3.
7. Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 2016;49(11):590-597. doi:10.5483/bmbrep.2016.49.11.109
8. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. Published 2018 Oct 20. doi:10.1186/s12199-018-0740-1
9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt B):1-441.
10. Mairiang E, Laha T, Bethony JM, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61(1):208-211. doi:10.1016/j.parint.2011.07.009
11. Li HM, Qian MB, Yang YC, et al. Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China. Parasit Vectors. 2018;11(1):35. Published 2018 Jan 15. doi:10.1186/s13071-018-2612-3
12. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med. 2017;10:227-237. Published 2017 Aug 10. doi:10.2147/IJGM.S133292
13. Psevdos G, Ford FM, Hong ST. Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war. Infect Dis Clin Pract (Baltim Md). 2018;26(4):208-210. doi:10.1097/IPC.0000000000000611
14. American War Library. In harm’s way... How many real Vietnam vets are alive today? Updated February 28, 2019. Accessed March 17, 2023. https://www.americanwarlibrary.com/personnel/vietvet.htm
15. Nash TE, Sullivan D, Mitre E, et al. Comments on “Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war”. Infect Dis Clin Pract (Baltim Md). 2018;26(4):240-241. doi:10.1097/IPC.0000000000000659
1. American Cancer Society. Survival rates for bile duct cancer. Updated March 1, 2023. Accessed March 17, 2023. https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html
2. Vij M, Puri Y, Rammohan A, et al. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14(3):607-627. doi:10.4251/wjgo.v14.i3.607
3. Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117. Published 2016 Sep 21. doi:10.1186/s12876-016-0527-z
4. Rustagi T, Dasanu CA. Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates. J Gastrointest Cancer. 2012;43(2):137-147. doi:10.1007/s12029-011-9284-y
5. Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21(10):754-760. doi:10.1002/jhbp.126
6. Steele JA, Richter CH, Echaubard P, et al. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):44. Published 2018 May 17. doi:10.1186/s40249-018-0434-3.
7. Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 2016;49(11):590-597. doi:10.5483/bmbrep.2016.49.11.109
8. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. Published 2018 Oct 20. doi:10.1186/s12199-018-0740-1
9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt B):1-441.
10. Mairiang E, Laha T, Bethony JM, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61(1):208-211. doi:10.1016/j.parint.2011.07.009
11. Li HM, Qian MB, Yang YC, et al. Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China. Parasit Vectors. 2018;11(1):35. Published 2018 Jan 15. doi:10.1186/s13071-018-2612-3
12. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med. 2017;10:227-237. Published 2017 Aug 10. doi:10.2147/IJGM.S133292
13. Psevdos G, Ford FM, Hong ST. Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war. Infect Dis Clin Pract (Baltim Md). 2018;26(4):208-210. doi:10.1097/IPC.0000000000000611
14. American War Library. In harm’s way... How many real Vietnam vets are alive today? Updated February 28, 2019. Accessed March 17, 2023. https://www.americanwarlibrary.com/personnel/vietvet.htm
15. Nash TE, Sullivan D, Mitre E, et al. Comments on “Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war”. Infect Dis Clin Pract (Baltim Md). 2018;26(4):240-241. doi:10.1097/IPC.0000000000000659
Preliminary Observations of Veterans Without HIV Who Have Mycobacterium avium Complex Pulmonary Disease
Nontuberculous Mycobacterium (NTM) is a ubiquitous organism known to cause a variety of infections in susceptible hosts; however, pulmonary infection is the most common. Mycobacterium avium complex (MAC) is the most prevalent cause of NTM-related pulmonary disease (NTM-PD) and is associated with underlying structural lung disease, such as chronic obstructive pulmonary disease (COPD) and noncystic fibrosis bronchiectasis.1-3
Diagnosis of NTM-PD requires (1) symptoms or radiographic abnormality; and (2) at least 2 sputum cultures positive with the same organism or at least 1 positive culture result on bronchoscopy (wash, lavage, or biopsy).1 Notably, the natural history of untreated NTM-PD varies, though even mild disease may progress substantially.4-6 Progressive disease is more likely to occur in those with a positive smear or more extensive radiographic findings at the initial diagnosis.7 A nationwide Medicare-based study showed that patients with NTM-PD had a higher rate of all-cause mortality than did patients without NTM-PD.8 In a study of 123 patients from Taiwan with MAC-PD, lack of treatment was an independent predictor of mortality.9 Given the risk of progressive morbidity and mortality, recent guidelines recommend initiation of a susceptibility driven, macrolide-based, 3-drug treatment regimen over watchful waiting.10
MAC-PD is increasingly recognized among US veterans.11,12 The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in south/west Chicago serves a large, predominantly Black male population of veterans many of whom are socioeconomically underresourced, and half are aged ≥ 65 years. We observed that initiation of guideline-directed therapy in veterans with MAC-PD at JBVAMC varied among health care professionals (HCPs) in the pulmonary clinic. Therefore, the purpose of this retrospective study was to describe and compare the characteristics of veterans without HIV were diagnosed with MAC-PD and managed at JBVAMC.
Methods
The hospital microbiology department identified veterans diagnosed with NTM at JBVAMC between October 2008 and July 2019. Veterans included in the study were considered to have MAC-PD per American Thoracic Society (ATS)/Infectious Diseases Society of America (ISDA) guidelines and those diagnosed with HIV were excluded from analysis. The electronic health record (EHR) was queried for pertinent demographics, smoking history, comorbidities, and symptoms at the time of a positive mycobacterial culture. Computed tomography (CT) and pulmonary function tests (PFTs) performed within 1 year of diagnosis were included. PFTs were assessed in accordance with Global Initiative for Obstructive Lung Disease (GOLD) criteria, with normal forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) values defined as ≥ 80% and a normal FEV1/FVC ratio defined as ≥ 70. The diffusion capacity of lung for carbon monoxide (DLCO) was assessed per 2017 European Respiratory Society (ERS) technical standards and was considered reduced if below the lower limit of normal.13 Information regarding treatment decisions, initiation, and cessation were collected. All-cause mortality was recorded if available in the EHR at the time of data collection.
Statistical analysis was performed using Mann-Whitney U and Fisher exact tests where appropriate. P < .05 was considered statistically significant. The study was approved by the JBVAMC Institutional Review Board.
Results
We identified 43 veterans who had a positive culture for MAC; however, only 19 veterans met the diagnostic criteria for MAC-PD and were included in the study (Table). The cohort included predominantly Black and male veterans with a median age of 74 years at time of diagnosis (range, 45-92). Sixteen veterans had underlying lung disease (84.2%), and 16 (84.2%) were current or former smokers. Common comorbidities included COPD, obstructive sleep apnea, gastroesophageal reflux disease, and lung cancer. Respiratory symptoms were reported in 17 veterans (89.5%), 15 (78.9%) had a chronic cough, and 10 (52.6%) had dyspnea. Fifteen veterans had a chest CT scan within 1 year of diagnosis: A nodular and tree-in-bud pattern was most commonly found in 13 (86.7%) of veterans. Thirteen veterans had PFTs within 1 year of MAC-PD diagnosis, of whom 6 had a restrictive pattern with percent predicted FVC < 80%, and 9 had evidence of obstruction with FEV1/FVC < 70. DLCO was below the lower limit of normal in 18 veterans. Finally, 6 veterans were deceased at the time of the study.
Of the 19 veterans, guideline-directed, combination antimycobacterial therapy for MAC-PD was initiated in only 10 (52.6%) patients due to presence of symptoms and/or imaging abnormalities. Treatment was deferred due to improved symptoms, concern for adverse events (AEs), or lost to follow-up. Five veterans stopped treatment prematurely due to AEs, lost to follow-up, or all-cause mortality. Assessment of differences between treated and untreated groups revealed no significant difference in race, sex, age, body mass index (BMI), symptom presence, or chest CT abnormalities. There was no statistically significant difference in all-cause mortality (40% and 22.2% in treated and untreated group, respectively).
To further understand the differences of this cohort, the 13 veterans alive at time of the study were compared with the 6 who had since died of all-cause mortality. No statistically significant differences were found.
Discussion
Consistent with previous reports in the literature, veterans in our cohort were predominantly current or former smoking males with underlying COPD and bronchiectasis.1-3,11,12 Chest CT findings varied: Most veterans presented not only with nodules and tree-in-bud opacities, but also a high frequency of fibrosis and emphysema. PFTs revealed a variety of obstruction and restrictive patterns, and most veterans had a reduced DLCO, though it is unclear whether this is reflective of underlying emphysema, fibrosis, or an alternative cardiopulmonary disease.13,14
While underlying structural lung disease may have been a risk factor for MAC-PD in this cohort, the contribution of environmental and domiciliary factors in metropolitan Chicago neighborhoods is unknown. JBVAMC serves an underresourced population who live in the west and south Chicago neighborhoods. Household factors, ambient and indoor air pollution, and potential contamination of the water supply and surface soil may contribute to the prevalence of MAC-PD in this group.15-19 Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.
Recent ATS, European Society of Clinical Microbiology and Infectious Diseases, and IDSA guidelines recommend combination antimycobacterial therapy for patients who meet clinical, radiographic, and microbiologic criteria for the diagnosis of MAC-PD.10 Patients who meet these diagnostic criteria, particularly patients with smear positivity or fibrocavitary disease, should be treated because of risk of unfavorable outcomes.15,20-22 However, we found that the initiation of guideline-recommended antimycobacterial therapy in veterans without HIV with MAC-PD were inconsistent among HCPs. The reasons underlying this phenomenon were not apparent beyond cited reasons for treatment initiation or deference. Despite this inconsistency, there was no clear difference in age, BMI, symptom burden, radiographic abnormality, or all-cause mortality between treatment groups. Existing studies support slow but substantial progression of untreated MAC-PD, and while treatment prevents deterioration of the disease, it does not prevent progression of bronchiectasis.6 The natural history of MAC-PD in this veteran cohort has yet to be fully elucidated. Furthermore, the 50% treatment dropout rate was higher than previously reported rates (11-33%).5 However, the small number of veterans in this study precludes meaningful comparison with similar reports in the literature.
Limitations
The limitations of this small, single-center, retrospective study prevent a robust, generalizable comparison between groups. Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.24-26
Conclusions
These data suggest that clinical, imaging, and treatment attributes of MAC-PD in veterans without HIV who reside in metropolitan Chicago are heterogeneous and are associated with a relatively high mortality rate. Although there was no difference in the attributes or outcomes of veterans who did and did not initiate treatment despite current recommendations, further studies are needed to better explore these relationships.
1. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/ IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction appears in Am J Respir Crit Care Med. 2007 Apr 1;175(7):744-5. Dosage error in article text]. Am J Respir Crit Care Med. 2007;175(4):367-416. doi:10.1164/rccm.200604-571ST
2. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970-976. doi:10.1164/rccm.201002-0310OC
3. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008-2015. Ann Am Thorac Soc. 2020;17(2):178-185. doi:10.1513/AnnalsATS.201804-236OC
4. Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126(2):566-581. doi:10.1378/chest.126.2.566
5. Kimizuka Y, Hoshino Y, Nishimura T, et al. Retrospective evaluation of natural course in mild cases of Mycobacterium avium complex pulmonary disease. PLoS One. 2019;14(4):e0216034. Published 2019 Apr 25. doi:10.1371/journal.pone.0216034
6. Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Clinical findings in relation to mortality in nontuberculous mycobacterial infections: patients with Mycobacterium avium complex have better survival than patients with other mycobacteria. Eur J Clin Microbiol Infect Dis. 2015;34(9):1909-1918. doi:10.1007/s10096-015-2432-8.
7. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017;49(3):1600537. Published 2017 Mar 8. doi:10.1183/13993003.00537-2016
8. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881-886. doi:10.1164/rccm.201111-2016OC
9. Wang PH, Pan SW, Shu CC, et al. Clinical course and risk factors of mortality in Mycobacterium avium complex lung disease without initial treatment. Respir Med. 2020;171:106070. doi:10.1016/j.rmed.2020.106070
10. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ ERS/ESCMID/IDSA Clinical Practice Guideline [published correction appears in Clin Infect Dis. 2020 Dec 31;71(11):3023]. Clin Infect Dis. 2020;71(4):e1-e36. doi:10.1093/cid/ciaa241
11. Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D, Sadikot RT. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17(11):e1000-e1004. doi:10.1016/j.ijid.2013.03.018
12. Oda G, Winters MA, Pacheco SM, et al. Clusters of nontuberculous mycobacteria linked to water sources at three Veterans Affairs medical centers. Infect Control Hosp Epidemiol. 2020;41(3):320-330. doi:10.1017/ice.2019.342
13. Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians [published correction appears in Eur Respir J. 2020 Oct 15;56(4):]. Eur Respir J. 2017;50(3):1700010. Published 2017 Sep 11. doi:10.1183/13993003.00010-2017
14. Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720-735. doi:10.1183/09031936.05.00034905
15. Chalmers JD, Balavoine C, Castellotti PF, et al. European Respiratory Society International Congress, Madrid, 2019: nontuberculous mycobacterial pulmonary disease highlights. ERJ Open Res. 2020;6(4):00317-2020. Published 2020 Oct 19. doi:10.1183/23120541.00317-2020
16. Hamilton LA, Falkinham JO. Aerosolization of Mycobacterium avium and Mycobacterium abscessus from a household ultrasonic humidifier. J Med Microbiol. 2018;67(10):1491-1495. doi:10.1099/jmm.0.000822
17. Hannah CE, Ford BA, Chung J, Ince D, Wanat KA. Characteristics of nontuberculous mycobacterial infections at a midwestern tertiary hospital: a retrospective study of 365 patients. Open Forum Infect Dis. 2020;7(6):ofaa173. Published 2020 May 25. doi:10.1093/ofid/ofaa173
18. Rautiala S, Torvinen E, Torkko P, et al. Potentially pathogenic, slow-growing mycobacteria released into workplace air during the remediation of buildings. J Occup Environ Hyg. 2004;1(1):1-6. doi:10.1080/15459620490250008
19. Tzou CL, Dirac MA, Becker AL, et al. Association between Mycobacterium avium complex pulmonary disease and mycobacteria in home water and soil. Ann Am Thorac Soc. 2020;17(1):57-62. doi:10.1513/AnnalsATS.201812-915OC
20. Daley CL, Winthrop KL. Mycobacterium avium complex: addressing gaps in diagnosis and management. J Infect Dis. 2020;222(suppl 4):S199-S211. doi:10.1093/infdis/jiaa354 21. Kwon BS, Lee JH, Koh Y, et al. The natural history of noncavitary nodular bronchiectatic Mycobacterium avium complex lung disease. Respir Med. 2019;150:45-50. doi:10.1016/j.rmed.2019.02.007
22. Nasiri MJ, Ebrahimi G, Arefzadeh S, Zamani S, Nikpor Z, Mirsaeidi M. Antibiotic therapy success rate in pulmonary Mycobacterium avium complex: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2020;18(3):263- 273. doi:10.1080/14787210.2020.1720650
23. Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18(1):206. Published 2018 May 3. doi:10.1186/s12879-018-3113-x
24. Marras TK, Prevots DR, Jamieson FB, Winthrop KL; Pulmonary MAC Outcomes Group. Opinions differ by expertise in Mycobacterium avium complex disease. Ann Am Thorac Soc. 2014;11(1):17-22. doi:10.1513/AnnalsATS.201305-136OC
25. Plotinsky RN, Talbot EA, von Reyn CF. Proposed definitions for epidemiologic and clinical studies of Mycobacterium avium complex pulmonary disease. PLoS One. 2013;8(11):e77385. Published 2013 Nov 12. doi:10.1371/journal.pone.0077385
26. Swenson C, Zerbe CS, Fennelly K. Host variability in NTM disease: implications for research needs. Front Microbiol. 2018;9:2901. Published 2018 Dec 3. doi:10.3389/fmicb.2018.02901
Nontuberculous Mycobacterium (NTM) is a ubiquitous organism known to cause a variety of infections in susceptible hosts; however, pulmonary infection is the most common. Mycobacterium avium complex (MAC) is the most prevalent cause of NTM-related pulmonary disease (NTM-PD) and is associated with underlying structural lung disease, such as chronic obstructive pulmonary disease (COPD) and noncystic fibrosis bronchiectasis.1-3
Diagnosis of NTM-PD requires (1) symptoms or radiographic abnormality; and (2) at least 2 sputum cultures positive with the same organism or at least 1 positive culture result on bronchoscopy (wash, lavage, or biopsy).1 Notably, the natural history of untreated NTM-PD varies, though even mild disease may progress substantially.4-6 Progressive disease is more likely to occur in those with a positive smear or more extensive radiographic findings at the initial diagnosis.7 A nationwide Medicare-based study showed that patients with NTM-PD had a higher rate of all-cause mortality than did patients without NTM-PD.8 In a study of 123 patients from Taiwan with MAC-PD, lack of treatment was an independent predictor of mortality.9 Given the risk of progressive morbidity and mortality, recent guidelines recommend initiation of a susceptibility driven, macrolide-based, 3-drug treatment regimen over watchful waiting.10
MAC-PD is increasingly recognized among US veterans.11,12 The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in south/west Chicago serves a large, predominantly Black male population of veterans many of whom are socioeconomically underresourced, and half are aged ≥ 65 years. We observed that initiation of guideline-directed therapy in veterans with MAC-PD at JBVAMC varied among health care professionals (HCPs) in the pulmonary clinic. Therefore, the purpose of this retrospective study was to describe and compare the characteristics of veterans without HIV were diagnosed with MAC-PD and managed at JBVAMC.
Methods
The hospital microbiology department identified veterans diagnosed with NTM at JBVAMC between October 2008 and July 2019. Veterans included in the study were considered to have MAC-PD per American Thoracic Society (ATS)/Infectious Diseases Society of America (ISDA) guidelines and those diagnosed with HIV were excluded from analysis. The electronic health record (EHR) was queried for pertinent demographics, smoking history, comorbidities, and symptoms at the time of a positive mycobacterial culture. Computed tomography (CT) and pulmonary function tests (PFTs) performed within 1 year of diagnosis were included. PFTs were assessed in accordance with Global Initiative for Obstructive Lung Disease (GOLD) criteria, with normal forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) values defined as ≥ 80% and a normal FEV1/FVC ratio defined as ≥ 70. The diffusion capacity of lung for carbon monoxide (DLCO) was assessed per 2017 European Respiratory Society (ERS) technical standards and was considered reduced if below the lower limit of normal.13 Information regarding treatment decisions, initiation, and cessation were collected. All-cause mortality was recorded if available in the EHR at the time of data collection.
Statistical analysis was performed using Mann-Whitney U and Fisher exact tests where appropriate. P < .05 was considered statistically significant. The study was approved by the JBVAMC Institutional Review Board.
Results
We identified 43 veterans who had a positive culture for MAC; however, only 19 veterans met the diagnostic criteria for MAC-PD and were included in the study (Table). The cohort included predominantly Black and male veterans with a median age of 74 years at time of diagnosis (range, 45-92). Sixteen veterans had underlying lung disease (84.2%), and 16 (84.2%) were current or former smokers. Common comorbidities included COPD, obstructive sleep apnea, gastroesophageal reflux disease, and lung cancer. Respiratory symptoms were reported in 17 veterans (89.5%), 15 (78.9%) had a chronic cough, and 10 (52.6%) had dyspnea. Fifteen veterans had a chest CT scan within 1 year of diagnosis: A nodular and tree-in-bud pattern was most commonly found in 13 (86.7%) of veterans. Thirteen veterans had PFTs within 1 year of MAC-PD diagnosis, of whom 6 had a restrictive pattern with percent predicted FVC < 80%, and 9 had evidence of obstruction with FEV1/FVC < 70. DLCO was below the lower limit of normal in 18 veterans. Finally, 6 veterans were deceased at the time of the study.
Of the 19 veterans, guideline-directed, combination antimycobacterial therapy for MAC-PD was initiated in only 10 (52.6%) patients due to presence of symptoms and/or imaging abnormalities. Treatment was deferred due to improved symptoms, concern for adverse events (AEs), or lost to follow-up. Five veterans stopped treatment prematurely due to AEs, lost to follow-up, or all-cause mortality. Assessment of differences between treated and untreated groups revealed no significant difference in race, sex, age, body mass index (BMI), symptom presence, or chest CT abnormalities. There was no statistically significant difference in all-cause mortality (40% and 22.2% in treated and untreated group, respectively).
To further understand the differences of this cohort, the 13 veterans alive at time of the study were compared with the 6 who had since died of all-cause mortality. No statistically significant differences were found.
Discussion
Consistent with previous reports in the literature, veterans in our cohort were predominantly current or former smoking males with underlying COPD and bronchiectasis.1-3,11,12 Chest CT findings varied: Most veterans presented not only with nodules and tree-in-bud opacities, but also a high frequency of fibrosis and emphysema. PFTs revealed a variety of obstruction and restrictive patterns, and most veterans had a reduced DLCO, though it is unclear whether this is reflective of underlying emphysema, fibrosis, or an alternative cardiopulmonary disease.13,14
While underlying structural lung disease may have been a risk factor for MAC-PD in this cohort, the contribution of environmental and domiciliary factors in metropolitan Chicago neighborhoods is unknown. JBVAMC serves an underresourced population who live in the west and south Chicago neighborhoods. Household factors, ambient and indoor air pollution, and potential contamination of the water supply and surface soil may contribute to the prevalence of MAC-PD in this group.15-19 Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.
Recent ATS, European Society of Clinical Microbiology and Infectious Diseases, and IDSA guidelines recommend combination antimycobacterial therapy for patients who meet clinical, radiographic, and microbiologic criteria for the diagnosis of MAC-PD.10 Patients who meet these diagnostic criteria, particularly patients with smear positivity or fibrocavitary disease, should be treated because of risk of unfavorable outcomes.15,20-22 However, we found that the initiation of guideline-recommended antimycobacterial therapy in veterans without HIV with MAC-PD were inconsistent among HCPs. The reasons underlying this phenomenon were not apparent beyond cited reasons for treatment initiation or deference. Despite this inconsistency, there was no clear difference in age, BMI, symptom burden, radiographic abnormality, or all-cause mortality between treatment groups. Existing studies support slow but substantial progression of untreated MAC-PD, and while treatment prevents deterioration of the disease, it does not prevent progression of bronchiectasis.6 The natural history of MAC-PD in this veteran cohort has yet to be fully elucidated. Furthermore, the 50% treatment dropout rate was higher than previously reported rates (11-33%).5 However, the small number of veterans in this study precludes meaningful comparison with similar reports in the literature.
Limitations
The limitations of this small, single-center, retrospective study prevent a robust, generalizable comparison between groups. Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.24-26
Conclusions
These data suggest that clinical, imaging, and treatment attributes of MAC-PD in veterans without HIV who reside in metropolitan Chicago are heterogeneous and are associated with a relatively high mortality rate. Although there was no difference in the attributes or outcomes of veterans who did and did not initiate treatment despite current recommendations, further studies are needed to better explore these relationships.
Nontuberculous Mycobacterium (NTM) is a ubiquitous organism known to cause a variety of infections in susceptible hosts; however, pulmonary infection is the most common. Mycobacterium avium complex (MAC) is the most prevalent cause of NTM-related pulmonary disease (NTM-PD) and is associated with underlying structural lung disease, such as chronic obstructive pulmonary disease (COPD) and noncystic fibrosis bronchiectasis.1-3
Diagnosis of NTM-PD requires (1) symptoms or radiographic abnormality; and (2) at least 2 sputum cultures positive with the same organism or at least 1 positive culture result on bronchoscopy (wash, lavage, or biopsy).1 Notably, the natural history of untreated NTM-PD varies, though even mild disease may progress substantially.4-6 Progressive disease is more likely to occur in those with a positive smear or more extensive radiographic findings at the initial diagnosis.7 A nationwide Medicare-based study showed that patients with NTM-PD had a higher rate of all-cause mortality than did patients without NTM-PD.8 In a study of 123 patients from Taiwan with MAC-PD, lack of treatment was an independent predictor of mortality.9 Given the risk of progressive morbidity and mortality, recent guidelines recommend initiation of a susceptibility driven, macrolide-based, 3-drug treatment regimen over watchful waiting.10
MAC-PD is increasingly recognized among US veterans.11,12 The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in south/west Chicago serves a large, predominantly Black male population of veterans many of whom are socioeconomically underresourced, and half are aged ≥ 65 years. We observed that initiation of guideline-directed therapy in veterans with MAC-PD at JBVAMC varied among health care professionals (HCPs) in the pulmonary clinic. Therefore, the purpose of this retrospective study was to describe and compare the characteristics of veterans without HIV were diagnosed with MAC-PD and managed at JBVAMC.
Methods
The hospital microbiology department identified veterans diagnosed with NTM at JBVAMC between October 2008 and July 2019. Veterans included in the study were considered to have MAC-PD per American Thoracic Society (ATS)/Infectious Diseases Society of America (ISDA) guidelines and those diagnosed with HIV were excluded from analysis. The electronic health record (EHR) was queried for pertinent demographics, smoking history, comorbidities, and symptoms at the time of a positive mycobacterial culture. Computed tomography (CT) and pulmonary function tests (PFTs) performed within 1 year of diagnosis were included. PFTs were assessed in accordance with Global Initiative for Obstructive Lung Disease (GOLD) criteria, with normal forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) values defined as ≥ 80% and a normal FEV1/FVC ratio defined as ≥ 70. The diffusion capacity of lung for carbon monoxide (DLCO) was assessed per 2017 European Respiratory Society (ERS) technical standards and was considered reduced if below the lower limit of normal.13 Information regarding treatment decisions, initiation, and cessation were collected. All-cause mortality was recorded if available in the EHR at the time of data collection.
Statistical analysis was performed using Mann-Whitney U and Fisher exact tests where appropriate. P < .05 was considered statistically significant. The study was approved by the JBVAMC Institutional Review Board.
Results
We identified 43 veterans who had a positive culture for MAC; however, only 19 veterans met the diagnostic criteria for MAC-PD and were included in the study (Table). The cohort included predominantly Black and male veterans with a median age of 74 years at time of diagnosis (range, 45-92). Sixteen veterans had underlying lung disease (84.2%), and 16 (84.2%) were current or former smokers. Common comorbidities included COPD, obstructive sleep apnea, gastroesophageal reflux disease, and lung cancer. Respiratory symptoms were reported in 17 veterans (89.5%), 15 (78.9%) had a chronic cough, and 10 (52.6%) had dyspnea. Fifteen veterans had a chest CT scan within 1 year of diagnosis: A nodular and tree-in-bud pattern was most commonly found in 13 (86.7%) of veterans. Thirteen veterans had PFTs within 1 year of MAC-PD diagnosis, of whom 6 had a restrictive pattern with percent predicted FVC < 80%, and 9 had evidence of obstruction with FEV1/FVC < 70. DLCO was below the lower limit of normal in 18 veterans. Finally, 6 veterans were deceased at the time of the study.
Of the 19 veterans, guideline-directed, combination antimycobacterial therapy for MAC-PD was initiated in only 10 (52.6%) patients due to presence of symptoms and/or imaging abnormalities. Treatment was deferred due to improved symptoms, concern for adverse events (AEs), or lost to follow-up. Five veterans stopped treatment prematurely due to AEs, lost to follow-up, or all-cause mortality. Assessment of differences between treated and untreated groups revealed no significant difference in race, sex, age, body mass index (BMI), symptom presence, or chest CT abnormalities. There was no statistically significant difference in all-cause mortality (40% and 22.2% in treated and untreated group, respectively).
To further understand the differences of this cohort, the 13 veterans alive at time of the study were compared with the 6 who had since died of all-cause mortality. No statistically significant differences were found.
Discussion
Consistent with previous reports in the literature, veterans in our cohort were predominantly current or former smoking males with underlying COPD and bronchiectasis.1-3,11,12 Chest CT findings varied: Most veterans presented not only with nodules and tree-in-bud opacities, but also a high frequency of fibrosis and emphysema. PFTs revealed a variety of obstruction and restrictive patterns, and most veterans had a reduced DLCO, though it is unclear whether this is reflective of underlying emphysema, fibrosis, or an alternative cardiopulmonary disease.13,14
While underlying structural lung disease may have been a risk factor for MAC-PD in this cohort, the contribution of environmental and domiciliary factors in metropolitan Chicago neighborhoods is unknown. JBVAMC serves an underresourced population who live in the west and south Chicago neighborhoods. Household factors, ambient and indoor air pollution, and potential contamination of the water supply and surface soil may contribute to the prevalence of MAC-PD in this group.15-19 Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.
Recent ATS, European Society of Clinical Microbiology and Infectious Diseases, and IDSA guidelines recommend combination antimycobacterial therapy for patients who meet clinical, radiographic, and microbiologic criteria for the diagnosis of MAC-PD.10 Patients who meet these diagnostic criteria, particularly patients with smear positivity or fibrocavitary disease, should be treated because of risk of unfavorable outcomes.15,20-22 However, we found that the initiation of guideline-recommended antimycobacterial therapy in veterans without HIV with MAC-PD were inconsistent among HCPs. The reasons underlying this phenomenon were not apparent beyond cited reasons for treatment initiation or deference. Despite this inconsistency, there was no clear difference in age, BMI, symptom burden, radiographic abnormality, or all-cause mortality between treatment groups. Existing studies support slow but substantial progression of untreated MAC-PD, and while treatment prevents deterioration of the disease, it does not prevent progression of bronchiectasis.6 The natural history of MAC-PD in this veteran cohort has yet to be fully elucidated. Furthermore, the 50% treatment dropout rate was higher than previously reported rates (11-33%).5 However, the small number of veterans in this study precludes meaningful comparison with similar reports in the literature.
Limitations
The limitations of this small, single-center, retrospective study prevent a robust, generalizable comparison between groups. Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.24-26
Conclusions
These data suggest that clinical, imaging, and treatment attributes of MAC-PD in veterans without HIV who reside in metropolitan Chicago are heterogeneous and are associated with a relatively high mortality rate. Although there was no difference in the attributes or outcomes of veterans who did and did not initiate treatment despite current recommendations, further studies are needed to better explore these relationships.
1. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/ IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction appears in Am J Respir Crit Care Med. 2007 Apr 1;175(7):744-5. Dosage error in article text]. Am J Respir Crit Care Med. 2007;175(4):367-416. doi:10.1164/rccm.200604-571ST
2. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970-976. doi:10.1164/rccm.201002-0310OC
3. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008-2015. Ann Am Thorac Soc. 2020;17(2):178-185. doi:10.1513/AnnalsATS.201804-236OC
4. Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126(2):566-581. doi:10.1378/chest.126.2.566
5. Kimizuka Y, Hoshino Y, Nishimura T, et al. Retrospective evaluation of natural course in mild cases of Mycobacterium avium complex pulmonary disease. PLoS One. 2019;14(4):e0216034. Published 2019 Apr 25. doi:10.1371/journal.pone.0216034
6. Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Clinical findings in relation to mortality in nontuberculous mycobacterial infections: patients with Mycobacterium avium complex have better survival than patients with other mycobacteria. Eur J Clin Microbiol Infect Dis. 2015;34(9):1909-1918. doi:10.1007/s10096-015-2432-8.
7. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017;49(3):1600537. Published 2017 Mar 8. doi:10.1183/13993003.00537-2016
8. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881-886. doi:10.1164/rccm.201111-2016OC
9. Wang PH, Pan SW, Shu CC, et al. Clinical course and risk factors of mortality in Mycobacterium avium complex lung disease without initial treatment. Respir Med. 2020;171:106070. doi:10.1016/j.rmed.2020.106070
10. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ ERS/ESCMID/IDSA Clinical Practice Guideline [published correction appears in Clin Infect Dis. 2020 Dec 31;71(11):3023]. Clin Infect Dis. 2020;71(4):e1-e36. doi:10.1093/cid/ciaa241
11. Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D, Sadikot RT. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17(11):e1000-e1004. doi:10.1016/j.ijid.2013.03.018
12. Oda G, Winters MA, Pacheco SM, et al. Clusters of nontuberculous mycobacteria linked to water sources at three Veterans Affairs medical centers. Infect Control Hosp Epidemiol. 2020;41(3):320-330. doi:10.1017/ice.2019.342
13. Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians [published correction appears in Eur Respir J. 2020 Oct 15;56(4):]. Eur Respir J. 2017;50(3):1700010. Published 2017 Sep 11. doi:10.1183/13993003.00010-2017
14. Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720-735. doi:10.1183/09031936.05.00034905
15. Chalmers JD, Balavoine C, Castellotti PF, et al. European Respiratory Society International Congress, Madrid, 2019: nontuberculous mycobacterial pulmonary disease highlights. ERJ Open Res. 2020;6(4):00317-2020. Published 2020 Oct 19. doi:10.1183/23120541.00317-2020
16. Hamilton LA, Falkinham JO. Aerosolization of Mycobacterium avium and Mycobacterium abscessus from a household ultrasonic humidifier. J Med Microbiol. 2018;67(10):1491-1495. doi:10.1099/jmm.0.000822
17. Hannah CE, Ford BA, Chung J, Ince D, Wanat KA. Characteristics of nontuberculous mycobacterial infections at a midwestern tertiary hospital: a retrospective study of 365 patients. Open Forum Infect Dis. 2020;7(6):ofaa173. Published 2020 May 25. doi:10.1093/ofid/ofaa173
18. Rautiala S, Torvinen E, Torkko P, et al. Potentially pathogenic, slow-growing mycobacteria released into workplace air during the remediation of buildings. J Occup Environ Hyg. 2004;1(1):1-6. doi:10.1080/15459620490250008
19. Tzou CL, Dirac MA, Becker AL, et al. Association between Mycobacterium avium complex pulmonary disease and mycobacteria in home water and soil. Ann Am Thorac Soc. 2020;17(1):57-62. doi:10.1513/AnnalsATS.201812-915OC
20. Daley CL, Winthrop KL. Mycobacterium avium complex: addressing gaps in diagnosis and management. J Infect Dis. 2020;222(suppl 4):S199-S211. doi:10.1093/infdis/jiaa354 21. Kwon BS, Lee JH, Koh Y, et al. The natural history of noncavitary nodular bronchiectatic Mycobacterium avium complex lung disease. Respir Med. 2019;150:45-50. doi:10.1016/j.rmed.2019.02.007
22. Nasiri MJ, Ebrahimi G, Arefzadeh S, Zamani S, Nikpor Z, Mirsaeidi M. Antibiotic therapy success rate in pulmonary Mycobacterium avium complex: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2020;18(3):263- 273. doi:10.1080/14787210.2020.1720650
23. Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18(1):206. Published 2018 May 3. doi:10.1186/s12879-018-3113-x
24. Marras TK, Prevots DR, Jamieson FB, Winthrop KL; Pulmonary MAC Outcomes Group. Opinions differ by expertise in Mycobacterium avium complex disease. Ann Am Thorac Soc. 2014;11(1):17-22. doi:10.1513/AnnalsATS.201305-136OC
25. Plotinsky RN, Talbot EA, von Reyn CF. Proposed definitions for epidemiologic and clinical studies of Mycobacterium avium complex pulmonary disease. PLoS One. 2013;8(11):e77385. Published 2013 Nov 12. doi:10.1371/journal.pone.0077385
26. Swenson C, Zerbe CS, Fennelly K. Host variability in NTM disease: implications for research needs. Front Microbiol. 2018;9:2901. Published 2018 Dec 3. doi:10.3389/fmicb.2018.02901
1. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/ IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction appears in Am J Respir Crit Care Med. 2007 Apr 1;175(7):744-5. Dosage error in article text]. Am J Respir Crit Care Med. 2007;175(4):367-416. doi:10.1164/rccm.200604-571ST
2. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970-976. doi:10.1164/rccm.201002-0310OC
3. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008-2015. Ann Am Thorac Soc. 2020;17(2):178-185. doi:10.1513/AnnalsATS.201804-236OC
4. Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126(2):566-581. doi:10.1378/chest.126.2.566
5. Kimizuka Y, Hoshino Y, Nishimura T, et al. Retrospective evaluation of natural course in mild cases of Mycobacterium avium complex pulmonary disease. PLoS One. 2019;14(4):e0216034. Published 2019 Apr 25. doi:10.1371/journal.pone.0216034
6. Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Clinical findings in relation to mortality in nontuberculous mycobacterial infections: patients with Mycobacterium avium complex have better survival than patients with other mycobacteria. Eur J Clin Microbiol Infect Dis. 2015;34(9):1909-1918. doi:10.1007/s10096-015-2432-8.
7. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017;49(3):1600537. Published 2017 Mar 8. doi:10.1183/13993003.00537-2016
8. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881-886. doi:10.1164/rccm.201111-2016OC
9. Wang PH, Pan SW, Shu CC, et al. Clinical course and risk factors of mortality in Mycobacterium avium complex lung disease without initial treatment. Respir Med. 2020;171:106070. doi:10.1016/j.rmed.2020.106070
10. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ ERS/ESCMID/IDSA Clinical Practice Guideline [published correction appears in Clin Infect Dis. 2020 Dec 31;71(11):3023]. Clin Infect Dis. 2020;71(4):e1-e36. doi:10.1093/cid/ciaa241
11. Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D, Sadikot RT. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17(11):e1000-e1004. doi:10.1016/j.ijid.2013.03.018
12. Oda G, Winters MA, Pacheco SM, et al. Clusters of nontuberculous mycobacteria linked to water sources at three Veterans Affairs medical centers. Infect Control Hosp Epidemiol. 2020;41(3):320-330. doi:10.1017/ice.2019.342
13. Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians [published correction appears in Eur Respir J. 2020 Oct 15;56(4):]. Eur Respir J. 2017;50(3):1700010. Published 2017 Sep 11. doi:10.1183/13993003.00010-2017
14. Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720-735. doi:10.1183/09031936.05.00034905
15. Chalmers JD, Balavoine C, Castellotti PF, et al. European Respiratory Society International Congress, Madrid, 2019: nontuberculous mycobacterial pulmonary disease highlights. ERJ Open Res. 2020;6(4):00317-2020. Published 2020 Oct 19. doi:10.1183/23120541.00317-2020
16. Hamilton LA, Falkinham JO. Aerosolization of Mycobacterium avium and Mycobacterium abscessus from a household ultrasonic humidifier. J Med Microbiol. 2018;67(10):1491-1495. doi:10.1099/jmm.0.000822
17. Hannah CE, Ford BA, Chung J, Ince D, Wanat KA. Characteristics of nontuberculous mycobacterial infections at a midwestern tertiary hospital: a retrospective study of 365 patients. Open Forum Infect Dis. 2020;7(6):ofaa173. Published 2020 May 25. doi:10.1093/ofid/ofaa173
18. Rautiala S, Torvinen E, Torkko P, et al. Potentially pathogenic, slow-growing mycobacteria released into workplace air during the remediation of buildings. J Occup Environ Hyg. 2004;1(1):1-6. doi:10.1080/15459620490250008
19. Tzou CL, Dirac MA, Becker AL, et al. Association between Mycobacterium avium complex pulmonary disease and mycobacteria in home water and soil. Ann Am Thorac Soc. 2020;17(1):57-62. doi:10.1513/AnnalsATS.201812-915OC
20. Daley CL, Winthrop KL. Mycobacterium avium complex: addressing gaps in diagnosis and management. J Infect Dis. 2020;222(suppl 4):S199-S211. doi:10.1093/infdis/jiaa354 21. Kwon BS, Lee JH, Koh Y, et al. The natural history of noncavitary nodular bronchiectatic Mycobacterium avium complex lung disease. Respir Med. 2019;150:45-50. doi:10.1016/j.rmed.2019.02.007
22. Nasiri MJ, Ebrahimi G, Arefzadeh S, Zamani S, Nikpor Z, Mirsaeidi M. Antibiotic therapy success rate in pulmonary Mycobacterium avium complex: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2020;18(3):263- 273. doi:10.1080/14787210.2020.1720650
23. Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18(1):206. Published 2018 May 3. doi:10.1186/s12879-018-3113-x
24. Marras TK, Prevots DR, Jamieson FB, Winthrop KL; Pulmonary MAC Outcomes Group. Opinions differ by expertise in Mycobacterium avium complex disease. Ann Am Thorac Soc. 2014;11(1):17-22. doi:10.1513/AnnalsATS.201305-136OC
25. Plotinsky RN, Talbot EA, von Reyn CF. Proposed definitions for epidemiologic and clinical studies of Mycobacterium avium complex pulmonary disease. PLoS One. 2013;8(11):e77385. Published 2013 Nov 12. doi:10.1371/journal.pone.0077385
26. Swenson C, Zerbe CS, Fennelly K. Host variability in NTM disease: implications for research needs. Front Microbiol. 2018;9:2901. Published 2018 Dec 3. doi:10.3389/fmicb.2018.02901