User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Study points to causal role for Lp(a) in atrial fibrillation
Although lipoprotein(a) is causally related to coronary artery disease and aortic valve stenosis – two known risk factors for atrial fibrillation (AFib) – evidence linking Lp(a) to a causal role in the development of AFib has been lukewarm at best.
A recent Mendelian randomization study showed only a nominally significant effect of Lp(a) on AFib, whereas an ARIC substudy showed high levels of Lp(a) to be associated with elevated ischemic stroke risk but not incident AFib.
A new study that adds the heft of Mendelian randomization to large observational and genetic analyses, however, implicates Lp(a) as a potential causal mediator of AFib, independent of its known effects on atherosclerotic cardiovascular disease (ASCVD).
“Why this is exciting is because it shows that Lp(a) has effects beyond the arteries and beyond the aortic valve, and that provides two things,” senior author Guillaume Paré, MD, MSc, Population Health Research Institute, Hamilton, Ontario, told this news organization.
“First, it provides a potential means to decrease the risk, because there are all these Lp(a) inhibitors in development,” he said. “But I think the other thing is that it just points to a new pathway that leads to atrial fibrillation development that could potentially be targeted with other drugs when it’s better understood. We don’t pretend that we understand the biology there, but it opens this possibility.”
The results were published in the Journal of the American College of Cardiology.
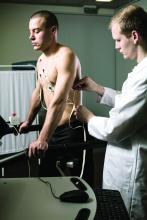
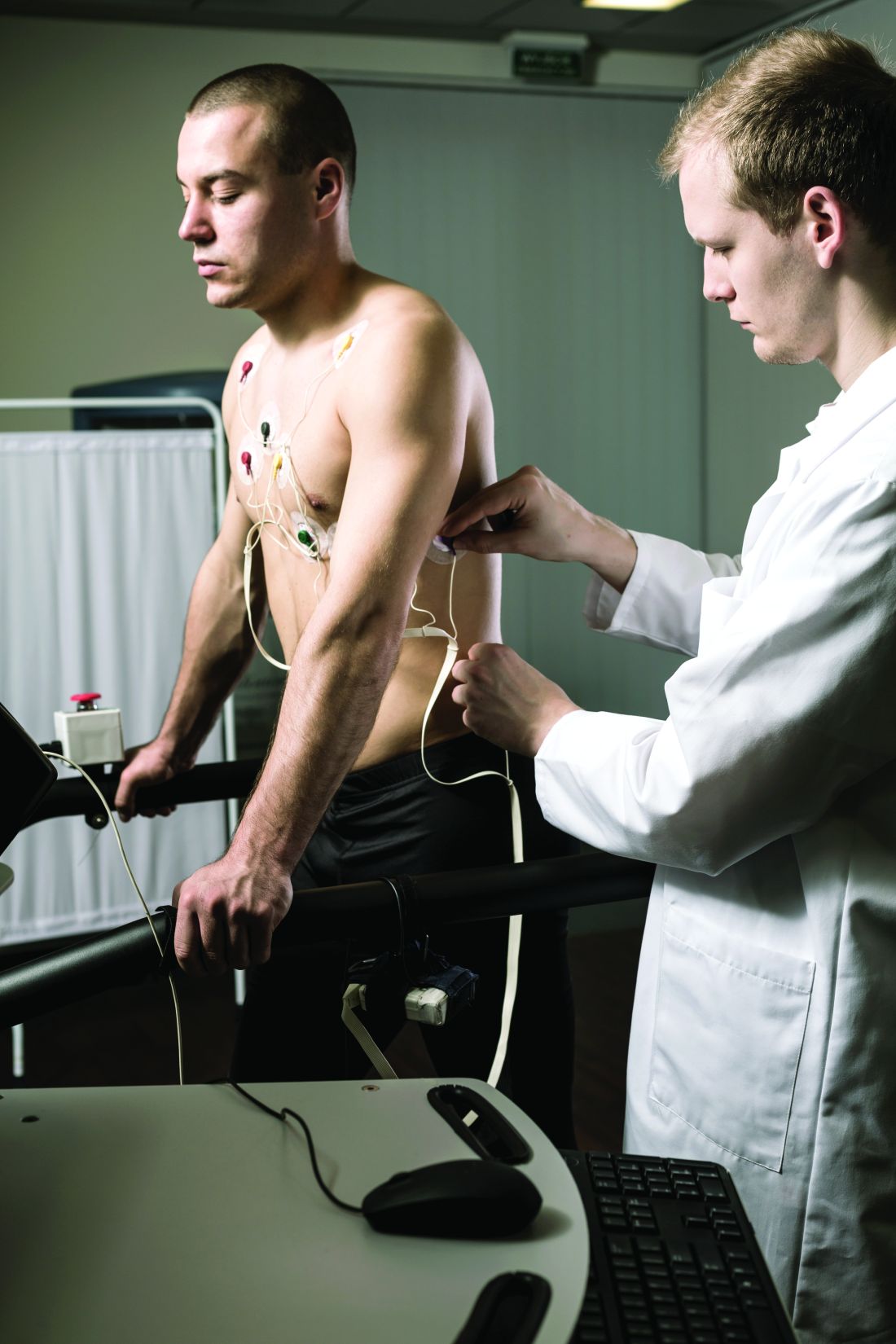
Using data from 435,579 participants in the UK Biobank, the researchers identified 20,432 cases of incident AFib over a median of 11 years of follow-up. They also constructed a genetic risk score for Lp(a) using genetic variants within 500 kb of the LPA gene.
After common AFib risk factors were controlled for, results showed a 3% increased risk for incident AFib per 50 nmol/L increase in Lp(a) at enrollment (hazard ratio, 1.03; 95% confidence interval, 1.02-1.05).
A Mendelian randomization analysis showed a similar association between genetically predicted Lp(a) and AFib (odds ratio, 1.03; 95% CI, 1.02-1.05).
To replicate the results, the investigators performed separate Mendelian randomization analyses using publicly available genome-wide association study (GWAS) statistics from the largest GWAS of AFib involving more than 1 million participants and from the FinnGen cohort involving more than 114,000 Finnish residents.
The analyses showed a 3% increase in risk for AFib in the genome-wide study (OR, 1.03; 95% CI, 1.02-1.05) and an 8% increase in risk in the Finnish study (OR, 1.08; 95% CI, 1.04-1.12) per 50 nmol/L increase in Lp(a).
There was no evidence that the effect of observed or genetically predicted Lp(a) was modified by prevalent ischemic heart disease or aortic stenosis.
Further, MR analyses revealed no risk effect of low-density-lipoprotein cholesterol or triglycerides on AFib.
Notably, only 39% of Lp(a) was mediated through ASCVD, suggesting that Lp(a) partly influences AFib independent of its known effect on ASCVD.
“To me, the eureka moment is when we repeated the same analysis for LDL cholesterol and it had absolutely no association with AFib,” Dr. Paré said. “Because up to that point, there was always this lingering doubt that, well, it’s because of coronary artery disease, and that’s logical. But the signal is completely flat with LDL, and we see this strong signal with Lp(a).”
Another ‘red flag’
Erin D. Michos, MD, MHS, senior author of the ARIC substudy and associate director of preventive cardiology at Johns Hopkins School of Medicine, Baltimore, said the findings are “another red flag that lipoprotein(a) is a marker we need to pay attention to and potentially needs treatment.”
“The fact that it was Mendelian randomization does suggest that there’s a causal role,” she said. “I think the relationship is relatively modest compared to its known risk for stroke, ASCVD, coronary disease, and aortic stenosis, ... which may be why we didn’t see it in the ARIC cohort with 12,000 participants. You needed to have a million participants and 60,000 cases to see an effect here.”
Dr. Michos said she hopes the findings encourage increased testing, particularly with multiple potential treatments currently in the pipeline. She pointed out that the researchers estimated that the experimental antisense agent pelacarsen, which lowers Lp(a) by about 80%, would translate into about an 8% reduction in AFib risk, or “the same effect as 2 kg of weight loss or a 5 mm Hg reduction in blood pressure, which we do think are meaningful.”
Adding to this point in an accompanying editorial, Daniel Seung Kim, MD, PhD, and Abha Khandelwal, MD, MS, Stanford University School of Medicine, California, say that “moreover, reduction of Lp(a) levels would have multifactorial effects on CAD, cerebrovascular/peripheral artery disease, and AS risk.
“Therefore, approaches to reduce Lp(a) should be prioritized to further reduce the morbidity and mortality of a rapidly aging population,” they write.
The editorialists also join the researchers in calling for inclusion of AFib as a secondary outcome in ongoing Lp(a) trials, in addition to cerebrovascular disease and peripheral vascular disease.
Unanswered questions
As to what’s driving the risk effect of Lp(a), first author Pedrum Mohammadi-Shemirani, PhD, also from the Population Health Research Institute, explained that in aortic stenosis, “mechanical stress increases endothelial permeability, allowing Lp(a) to infiltrate valvular tissue and induce gene expression that results in microcalcifications and cell death.”
“So, in theory, a similar sort of mechanism could be at play in atrial tissue that may lead to damage and the electrical remodeling that causes atrial fibrillation,” he told this news organization.
Dr. Mohammadi-Shemirani also noted that Lp(a) has proinflammatory properties, but added that any potential mechanisms are “speculative and require further research to disentangle.”
Dr. Paré and colleagues say follow-up studies are also warranted, noting that generalizability of the results may be limited because AFib cases were found using electronic health records in the population-scale cohorts and because few UK Biobank participants were of non-European ancestry and Lp(a) levels vary among ethnic groups.
Another limitation is that the number of kringle IV type 2 domain repeats within the LPA gene, the largest contributor to genetic variation in Lp(a), could not be directly measured. Still, 71.4% of the variation in Lp(a) was explained using the genetic risk score alone, they say.
Dr. Paré holds the Canada Research Chair in Genetic and Molecular Epidemiology and Cisco Systems Professorship in Integrated Health Biosystems. Dr. Mohammadi-Shemirani is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institute of Health Research. Dr. Michos reports consulting for Novartis and serving on advisory boards for Novartis, AstraZeneca, Bayer, and Boehringer Ingelheim. Dr. Kim reports grant support from the National Institutes of Health and the American Heart Association. Dr. Khandelwal serves on the advisory board of Amgen and has received funding from Novartis CTQJ and Akcea.
A version of this article first appeared on Medscape.com.
Although lipoprotein(a) is causally related to coronary artery disease and aortic valve stenosis – two known risk factors for atrial fibrillation (AFib) – evidence linking Lp(a) to a causal role in the development of AFib has been lukewarm at best.
A recent Mendelian randomization study showed only a nominally significant effect of Lp(a) on AFib, whereas an ARIC substudy showed high levels of Lp(a) to be associated with elevated ischemic stroke risk but not incident AFib.
A new study that adds the heft of Mendelian randomization to large observational and genetic analyses, however, implicates Lp(a) as a potential causal mediator of AFib, independent of its known effects on atherosclerotic cardiovascular disease (ASCVD).
“Why this is exciting is because it shows that Lp(a) has effects beyond the arteries and beyond the aortic valve, and that provides two things,” senior author Guillaume Paré, MD, MSc, Population Health Research Institute, Hamilton, Ontario, told this news organization.
“First, it provides a potential means to decrease the risk, because there are all these Lp(a) inhibitors in development,” he said. “But I think the other thing is that it just points to a new pathway that leads to atrial fibrillation development that could potentially be targeted with other drugs when it’s better understood. We don’t pretend that we understand the biology there, but it opens this possibility.”
The results were published in the Journal of the American College of Cardiology.
Using data from 435,579 participants in the UK Biobank, the researchers identified 20,432 cases of incident AFib over a median of 11 years of follow-up. They also constructed a genetic risk score for Lp(a) using genetic variants within 500 kb of the LPA gene.
After common AFib risk factors were controlled for, results showed a 3% increased risk for incident AFib per 50 nmol/L increase in Lp(a) at enrollment (hazard ratio, 1.03; 95% confidence interval, 1.02-1.05).
A Mendelian randomization analysis showed a similar association between genetically predicted Lp(a) and AFib (odds ratio, 1.03; 95% CI, 1.02-1.05).
To replicate the results, the investigators performed separate Mendelian randomization analyses using publicly available genome-wide association study (GWAS) statistics from the largest GWAS of AFib involving more than 1 million participants and from the FinnGen cohort involving more than 114,000 Finnish residents.
The analyses showed a 3% increase in risk for AFib in the genome-wide study (OR, 1.03; 95% CI, 1.02-1.05) and an 8% increase in risk in the Finnish study (OR, 1.08; 95% CI, 1.04-1.12) per 50 nmol/L increase in Lp(a).
There was no evidence that the effect of observed or genetically predicted Lp(a) was modified by prevalent ischemic heart disease or aortic stenosis.
Further, MR analyses revealed no risk effect of low-density-lipoprotein cholesterol or triglycerides on AFib.
Notably, only 39% of Lp(a) was mediated through ASCVD, suggesting that Lp(a) partly influences AFib independent of its known effect on ASCVD.
“To me, the eureka moment is when we repeated the same analysis for LDL cholesterol and it had absolutely no association with AFib,” Dr. Paré said. “Because up to that point, there was always this lingering doubt that, well, it’s because of coronary artery disease, and that’s logical. But the signal is completely flat with LDL, and we see this strong signal with Lp(a).”
Another ‘red flag’
Erin D. Michos, MD, MHS, senior author of the ARIC substudy and associate director of preventive cardiology at Johns Hopkins School of Medicine, Baltimore, said the findings are “another red flag that lipoprotein(a) is a marker we need to pay attention to and potentially needs treatment.”
“The fact that it was Mendelian randomization does suggest that there’s a causal role,” she said. “I think the relationship is relatively modest compared to its known risk for stroke, ASCVD, coronary disease, and aortic stenosis, ... which may be why we didn’t see it in the ARIC cohort with 12,000 participants. You needed to have a million participants and 60,000 cases to see an effect here.”
Dr. Michos said she hopes the findings encourage increased testing, particularly with multiple potential treatments currently in the pipeline. She pointed out that the researchers estimated that the experimental antisense agent pelacarsen, which lowers Lp(a) by about 80%, would translate into about an 8% reduction in AFib risk, or “the same effect as 2 kg of weight loss or a 5 mm Hg reduction in blood pressure, which we do think are meaningful.”
Adding to this point in an accompanying editorial, Daniel Seung Kim, MD, PhD, and Abha Khandelwal, MD, MS, Stanford University School of Medicine, California, say that “moreover, reduction of Lp(a) levels would have multifactorial effects on CAD, cerebrovascular/peripheral artery disease, and AS risk.
“Therefore, approaches to reduce Lp(a) should be prioritized to further reduce the morbidity and mortality of a rapidly aging population,” they write.
The editorialists also join the researchers in calling for inclusion of AFib as a secondary outcome in ongoing Lp(a) trials, in addition to cerebrovascular disease and peripheral vascular disease.
Unanswered questions
As to what’s driving the risk effect of Lp(a), first author Pedrum Mohammadi-Shemirani, PhD, also from the Population Health Research Institute, explained that in aortic stenosis, “mechanical stress increases endothelial permeability, allowing Lp(a) to infiltrate valvular tissue and induce gene expression that results in microcalcifications and cell death.”
“So, in theory, a similar sort of mechanism could be at play in atrial tissue that may lead to damage and the electrical remodeling that causes atrial fibrillation,” he told this news organization.
Dr. Mohammadi-Shemirani also noted that Lp(a) has proinflammatory properties, but added that any potential mechanisms are “speculative and require further research to disentangle.”
Dr. Paré and colleagues say follow-up studies are also warranted, noting that generalizability of the results may be limited because AFib cases were found using electronic health records in the population-scale cohorts and because few UK Biobank participants were of non-European ancestry and Lp(a) levels vary among ethnic groups.
Another limitation is that the number of kringle IV type 2 domain repeats within the LPA gene, the largest contributor to genetic variation in Lp(a), could not be directly measured. Still, 71.4% of the variation in Lp(a) was explained using the genetic risk score alone, they say.
Dr. Paré holds the Canada Research Chair in Genetic and Molecular Epidemiology and Cisco Systems Professorship in Integrated Health Biosystems. Dr. Mohammadi-Shemirani is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institute of Health Research. Dr. Michos reports consulting for Novartis and serving on advisory boards for Novartis, AstraZeneca, Bayer, and Boehringer Ingelheim. Dr. Kim reports grant support from the National Institutes of Health and the American Heart Association. Dr. Khandelwal serves on the advisory board of Amgen and has received funding from Novartis CTQJ and Akcea.
A version of this article first appeared on Medscape.com.
Although lipoprotein(a) is causally related to coronary artery disease and aortic valve stenosis – two known risk factors for atrial fibrillation (AFib) – evidence linking Lp(a) to a causal role in the development of AFib has been lukewarm at best.
A recent Mendelian randomization study showed only a nominally significant effect of Lp(a) on AFib, whereas an ARIC substudy showed high levels of Lp(a) to be associated with elevated ischemic stroke risk but not incident AFib.
A new study that adds the heft of Mendelian randomization to large observational and genetic analyses, however, implicates Lp(a) as a potential causal mediator of AFib, independent of its known effects on atherosclerotic cardiovascular disease (ASCVD).
“Why this is exciting is because it shows that Lp(a) has effects beyond the arteries and beyond the aortic valve, and that provides two things,” senior author Guillaume Paré, MD, MSc, Population Health Research Institute, Hamilton, Ontario, told this news organization.
“First, it provides a potential means to decrease the risk, because there are all these Lp(a) inhibitors in development,” he said. “But I think the other thing is that it just points to a new pathway that leads to atrial fibrillation development that could potentially be targeted with other drugs when it’s better understood. We don’t pretend that we understand the biology there, but it opens this possibility.”
The results were published in the Journal of the American College of Cardiology.
Using data from 435,579 participants in the UK Biobank, the researchers identified 20,432 cases of incident AFib over a median of 11 years of follow-up. They also constructed a genetic risk score for Lp(a) using genetic variants within 500 kb of the LPA gene.
After common AFib risk factors were controlled for, results showed a 3% increased risk for incident AFib per 50 nmol/L increase in Lp(a) at enrollment (hazard ratio, 1.03; 95% confidence interval, 1.02-1.05).
A Mendelian randomization analysis showed a similar association between genetically predicted Lp(a) and AFib (odds ratio, 1.03; 95% CI, 1.02-1.05).
To replicate the results, the investigators performed separate Mendelian randomization analyses using publicly available genome-wide association study (GWAS) statistics from the largest GWAS of AFib involving more than 1 million participants and from the FinnGen cohort involving more than 114,000 Finnish residents.
The analyses showed a 3% increase in risk for AFib in the genome-wide study (OR, 1.03; 95% CI, 1.02-1.05) and an 8% increase in risk in the Finnish study (OR, 1.08; 95% CI, 1.04-1.12) per 50 nmol/L increase in Lp(a).
There was no evidence that the effect of observed or genetically predicted Lp(a) was modified by prevalent ischemic heart disease or aortic stenosis.
Further, MR analyses revealed no risk effect of low-density-lipoprotein cholesterol or triglycerides on AFib.
Notably, only 39% of Lp(a) was mediated through ASCVD, suggesting that Lp(a) partly influences AFib independent of its known effect on ASCVD.
“To me, the eureka moment is when we repeated the same analysis for LDL cholesterol and it had absolutely no association with AFib,” Dr. Paré said. “Because up to that point, there was always this lingering doubt that, well, it’s because of coronary artery disease, and that’s logical. But the signal is completely flat with LDL, and we see this strong signal with Lp(a).”
Another ‘red flag’
Erin D. Michos, MD, MHS, senior author of the ARIC substudy and associate director of preventive cardiology at Johns Hopkins School of Medicine, Baltimore, said the findings are “another red flag that lipoprotein(a) is a marker we need to pay attention to and potentially needs treatment.”
“The fact that it was Mendelian randomization does suggest that there’s a causal role,” she said. “I think the relationship is relatively modest compared to its known risk for stroke, ASCVD, coronary disease, and aortic stenosis, ... which may be why we didn’t see it in the ARIC cohort with 12,000 participants. You needed to have a million participants and 60,000 cases to see an effect here.”
Dr. Michos said she hopes the findings encourage increased testing, particularly with multiple potential treatments currently in the pipeline. She pointed out that the researchers estimated that the experimental antisense agent pelacarsen, which lowers Lp(a) by about 80%, would translate into about an 8% reduction in AFib risk, or “the same effect as 2 kg of weight loss or a 5 mm Hg reduction in blood pressure, which we do think are meaningful.”
Adding to this point in an accompanying editorial, Daniel Seung Kim, MD, PhD, and Abha Khandelwal, MD, MS, Stanford University School of Medicine, California, say that “moreover, reduction of Lp(a) levels would have multifactorial effects on CAD, cerebrovascular/peripheral artery disease, and AS risk.
“Therefore, approaches to reduce Lp(a) should be prioritized to further reduce the morbidity and mortality of a rapidly aging population,” they write.
The editorialists also join the researchers in calling for inclusion of AFib as a secondary outcome in ongoing Lp(a) trials, in addition to cerebrovascular disease and peripheral vascular disease.
Unanswered questions
As to what’s driving the risk effect of Lp(a), first author Pedrum Mohammadi-Shemirani, PhD, also from the Population Health Research Institute, explained that in aortic stenosis, “mechanical stress increases endothelial permeability, allowing Lp(a) to infiltrate valvular tissue and induce gene expression that results in microcalcifications and cell death.”
“So, in theory, a similar sort of mechanism could be at play in atrial tissue that may lead to damage and the electrical remodeling that causes atrial fibrillation,” he told this news organization.
Dr. Mohammadi-Shemirani also noted that Lp(a) has proinflammatory properties, but added that any potential mechanisms are “speculative and require further research to disentangle.”
Dr. Paré and colleagues say follow-up studies are also warranted, noting that generalizability of the results may be limited because AFib cases were found using electronic health records in the population-scale cohorts and because few UK Biobank participants were of non-European ancestry and Lp(a) levels vary among ethnic groups.
Another limitation is that the number of kringle IV type 2 domain repeats within the LPA gene, the largest contributor to genetic variation in Lp(a), could not be directly measured. Still, 71.4% of the variation in Lp(a) was explained using the genetic risk score alone, they say.
Dr. Paré holds the Canada Research Chair in Genetic and Molecular Epidemiology and Cisco Systems Professorship in Integrated Health Biosystems. Dr. Mohammadi-Shemirani is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institute of Health Research. Dr. Michos reports consulting for Novartis and serving on advisory boards for Novartis, AstraZeneca, Bayer, and Boehringer Ingelheim. Dr. Kim reports grant support from the National Institutes of Health and the American Heart Association. Dr. Khandelwal serves on the advisory board of Amgen and has received funding from Novartis CTQJ and Akcea.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Mediterranean diet linked to lower risk for preeclampsia
Pregnant women who had a higher adherence to a Mediterranean-style diet had a lower risk of preeclampsia, according to the results of a new study.
“As an observational study, it obviously has limitations that need to be considered, but these results build on other evidence that Mediterranean diet reduces cardiovascular risk and extends those findings to pregnancy as preeclampsia is a cardiovascular outcome,” senior author Noel T. Mueller, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview.
The study was published online April 20 in the Journal of the American Heart Association.
The authors noted that preeclampsia, characterized by a range of symptoms including hypertension, proteinuria, and end-organ dysfunction, is a disorder that occurs in up to 5%-10% of all pregnant women worldwide, and is more common in Black women. It is a major cause of maternal and fetal morbidity and raises the risk for long-term cardiovascular disease (CVD), including chronic hypertension, coronary artery disease, ischemic stroke, and heart failure.
Children born to mothers with preeclampsia are at an elevated risk of having higher blood pressure and other abnormal cardiometabolic parameters.
The authors noted that multiple studies have demonstrated the benefit of the Mediterranean diet – characterized primarily by high intake of vegetables, fruits, and unsaturated fats – in reducing cardiovascular risk in the nonpregnant population. The current study was conducted to investigate whether benefits could also be seen in pregnant women in the form of a reduced risk of preeclampsia.
For the study, which used data from the Boston Birth Cohort, maternal sociodemographic and dietary data were obtained from 8,507 women via interview and food frequency questionnaire within 24-72 hours of giving birth. A Mediterranean-style diet score was calculated from the food frequency questionnaire. Additional clinical information, including physician diagnoses of preexisting conditions and preeclampsia, were extracted from medical records.
Of the women in the sample, 848 developed preeclampsia, of whom 47% were Black, and 28% were Hispanic.
After multivariable adjustment, the greatest adherence to a Mediterranean-style diet was associated with lower odds of developing preeclampsia (adjusted odds ratio comparing tertile 3 to tertile 1, 0.78; 95% confidence interval [CI], 0.64-0.96).
A subgroup analysis of Black women demonstrated a similar benefit with an adjusted odds ratio comparing tertile 3 to tertile 1 of 0.74 (95% CI, 0.76-0.96).
“In this racially and ethnically diverse cohort, women who had greater adherence to a Mediterranean-style diet during pregnancy had a greater than 20% lower odds of developing preeclampsia, after [adjustment] for potential confounders. In addition, the evidence for the protective effect of a Mediterranean-style diet against the odds of developing preeclampsia remained present in a subgroup analysis of Black women,” the researchers concluded.
Asked whether this would be enough evidence to recommend a Mediterranean diet to pregnant women, Dr. Mueller said that the organizations that issue dietary guidelines would probably require replication of these results and also possibly a randomized trial in a diverse population group before advocating such a diet.
“That is something we would like to do but this will take time and money,” he added.
Lead study author Anum Minhas, MD, Johns Hopkins University, Baltimore, said that in the meantime she would be recommending a Mediterranean diet to her pregnant patients.
“The Mediterranean diet is a very healthy way of eating. I can’t see any downside of following such a diet in pregnancy, especially for high-risk women – those with obesity, hypertension or gestational diabetes, and there are likely other potential benefits such as reduced weight gain and reduced gestational diabetes,” she said.
Dr. Mueller said he appreciated this pragmatic approach. “Sometimes there can be hesitation on making recommendations from observational studies, but the alternative to recommending this diet is either no recommendations on diet or recommending an alternative diet,” he said. “The Mediterranean diet or the DASH diet, which is quite similar, have shown by far the most evidence of cardioprotection of any diets. They have been shown to reduce blood pressure and lipids and improve cardiovascular risk, and I think we can now assume that that likely extends to pregnancy. I feel comfortable for this diet to be recommended to pregnant women.”
But he added: “Having said that, there is still a need for a randomized trial in pregnancy. We think it works but until we have a randomized trial we won’t know for sure, and we won’t know how much of a benefit we can get.”
Commenting on the study, JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, pointed out that this type of observational study is important for hypothesis generation but cannot prove cause and effect relationships.
“The evidence is promising enough,” said Dr. Manson, who was not involved with this study. But she added that to move forward, a randomized trial in women at elevated risk of preeclampsia would be needed, beginning in early pregnancy, if not earlier.
“In the meantime,” she noted, “several large-scale cohorts could be leveraged to look at diet assessed before or during pregnancy to see if this dietary pattern is prospectively related to lower risk of preeclampsia.
“With additional supportive data, and in view of the diet’s safety and general cardiovascular benefits, it could become a major tool for preventing adverse pregnancy outcomes.”
The Boston Birth Cohort study was supported in part by grants from the March of Dimes, the National Institutes of Health, and the Health Resources and Services Administration of the U.S. Department of Health and Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women who had a higher adherence to a Mediterranean-style diet had a lower risk of preeclampsia, according to the results of a new study.
“As an observational study, it obviously has limitations that need to be considered, but these results build on other evidence that Mediterranean diet reduces cardiovascular risk and extends those findings to pregnancy as preeclampsia is a cardiovascular outcome,” senior author Noel T. Mueller, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview.
The study was published online April 20 in the Journal of the American Heart Association.
The authors noted that preeclampsia, characterized by a range of symptoms including hypertension, proteinuria, and end-organ dysfunction, is a disorder that occurs in up to 5%-10% of all pregnant women worldwide, and is more common in Black women. It is a major cause of maternal and fetal morbidity and raises the risk for long-term cardiovascular disease (CVD), including chronic hypertension, coronary artery disease, ischemic stroke, and heart failure.
Children born to mothers with preeclampsia are at an elevated risk of having higher blood pressure and other abnormal cardiometabolic parameters.
The authors noted that multiple studies have demonstrated the benefit of the Mediterranean diet – characterized primarily by high intake of vegetables, fruits, and unsaturated fats – in reducing cardiovascular risk in the nonpregnant population. The current study was conducted to investigate whether benefits could also be seen in pregnant women in the form of a reduced risk of preeclampsia.
For the study, which used data from the Boston Birth Cohort, maternal sociodemographic and dietary data were obtained from 8,507 women via interview and food frequency questionnaire within 24-72 hours of giving birth. A Mediterranean-style diet score was calculated from the food frequency questionnaire. Additional clinical information, including physician diagnoses of preexisting conditions and preeclampsia, were extracted from medical records.
Of the women in the sample, 848 developed preeclampsia, of whom 47% were Black, and 28% were Hispanic.
After multivariable adjustment, the greatest adherence to a Mediterranean-style diet was associated with lower odds of developing preeclampsia (adjusted odds ratio comparing tertile 3 to tertile 1, 0.78; 95% confidence interval [CI], 0.64-0.96).
A subgroup analysis of Black women demonstrated a similar benefit with an adjusted odds ratio comparing tertile 3 to tertile 1 of 0.74 (95% CI, 0.76-0.96).
“In this racially and ethnically diverse cohort, women who had greater adherence to a Mediterranean-style diet during pregnancy had a greater than 20% lower odds of developing preeclampsia, after [adjustment] for potential confounders. In addition, the evidence for the protective effect of a Mediterranean-style diet against the odds of developing preeclampsia remained present in a subgroup analysis of Black women,” the researchers concluded.
Asked whether this would be enough evidence to recommend a Mediterranean diet to pregnant women, Dr. Mueller said that the organizations that issue dietary guidelines would probably require replication of these results and also possibly a randomized trial in a diverse population group before advocating such a diet.
“That is something we would like to do but this will take time and money,” he added.
Lead study author Anum Minhas, MD, Johns Hopkins University, Baltimore, said that in the meantime she would be recommending a Mediterranean diet to her pregnant patients.
“The Mediterranean diet is a very healthy way of eating. I can’t see any downside of following such a diet in pregnancy, especially for high-risk women – those with obesity, hypertension or gestational diabetes, and there are likely other potential benefits such as reduced weight gain and reduced gestational diabetes,” she said.
Dr. Mueller said he appreciated this pragmatic approach. “Sometimes there can be hesitation on making recommendations from observational studies, but the alternative to recommending this diet is either no recommendations on diet or recommending an alternative diet,” he said. “The Mediterranean diet or the DASH diet, which is quite similar, have shown by far the most evidence of cardioprotection of any diets. They have been shown to reduce blood pressure and lipids and improve cardiovascular risk, and I think we can now assume that that likely extends to pregnancy. I feel comfortable for this diet to be recommended to pregnant women.”
But he added: “Having said that, there is still a need for a randomized trial in pregnancy. We think it works but until we have a randomized trial we won’t know for sure, and we won’t know how much of a benefit we can get.”
Commenting on the study, JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, pointed out that this type of observational study is important for hypothesis generation but cannot prove cause and effect relationships.
“The evidence is promising enough,” said Dr. Manson, who was not involved with this study. But she added that to move forward, a randomized trial in women at elevated risk of preeclampsia would be needed, beginning in early pregnancy, if not earlier.
“In the meantime,” she noted, “several large-scale cohorts could be leveraged to look at diet assessed before or during pregnancy to see if this dietary pattern is prospectively related to lower risk of preeclampsia.
“With additional supportive data, and in view of the diet’s safety and general cardiovascular benefits, it could become a major tool for preventing adverse pregnancy outcomes.”
The Boston Birth Cohort study was supported in part by grants from the March of Dimes, the National Institutes of Health, and the Health Resources and Services Administration of the U.S. Department of Health and Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women who had a higher adherence to a Mediterranean-style diet had a lower risk of preeclampsia, according to the results of a new study.
“As an observational study, it obviously has limitations that need to be considered, but these results build on other evidence that Mediterranean diet reduces cardiovascular risk and extends those findings to pregnancy as preeclampsia is a cardiovascular outcome,” senior author Noel T. Mueller, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview.
The study was published online April 20 in the Journal of the American Heart Association.
The authors noted that preeclampsia, characterized by a range of symptoms including hypertension, proteinuria, and end-organ dysfunction, is a disorder that occurs in up to 5%-10% of all pregnant women worldwide, and is more common in Black women. It is a major cause of maternal and fetal morbidity and raises the risk for long-term cardiovascular disease (CVD), including chronic hypertension, coronary artery disease, ischemic stroke, and heart failure.
Children born to mothers with preeclampsia are at an elevated risk of having higher blood pressure and other abnormal cardiometabolic parameters.
The authors noted that multiple studies have demonstrated the benefit of the Mediterranean diet – characterized primarily by high intake of vegetables, fruits, and unsaturated fats – in reducing cardiovascular risk in the nonpregnant population. The current study was conducted to investigate whether benefits could also be seen in pregnant women in the form of a reduced risk of preeclampsia.
For the study, which used data from the Boston Birth Cohort, maternal sociodemographic and dietary data were obtained from 8,507 women via interview and food frequency questionnaire within 24-72 hours of giving birth. A Mediterranean-style diet score was calculated from the food frequency questionnaire. Additional clinical information, including physician diagnoses of preexisting conditions and preeclampsia, were extracted from medical records.
Of the women in the sample, 848 developed preeclampsia, of whom 47% were Black, and 28% were Hispanic.
After multivariable adjustment, the greatest adherence to a Mediterranean-style diet was associated with lower odds of developing preeclampsia (adjusted odds ratio comparing tertile 3 to tertile 1, 0.78; 95% confidence interval [CI], 0.64-0.96).
A subgroup analysis of Black women demonstrated a similar benefit with an adjusted odds ratio comparing tertile 3 to tertile 1 of 0.74 (95% CI, 0.76-0.96).
“In this racially and ethnically diverse cohort, women who had greater adherence to a Mediterranean-style diet during pregnancy had a greater than 20% lower odds of developing preeclampsia, after [adjustment] for potential confounders. In addition, the evidence for the protective effect of a Mediterranean-style diet against the odds of developing preeclampsia remained present in a subgroup analysis of Black women,” the researchers concluded.
Asked whether this would be enough evidence to recommend a Mediterranean diet to pregnant women, Dr. Mueller said that the organizations that issue dietary guidelines would probably require replication of these results and also possibly a randomized trial in a diverse population group before advocating such a diet.
“That is something we would like to do but this will take time and money,” he added.
Lead study author Anum Minhas, MD, Johns Hopkins University, Baltimore, said that in the meantime she would be recommending a Mediterranean diet to her pregnant patients.
“The Mediterranean diet is a very healthy way of eating. I can’t see any downside of following such a diet in pregnancy, especially for high-risk women – those with obesity, hypertension or gestational diabetes, and there are likely other potential benefits such as reduced weight gain and reduced gestational diabetes,” she said.
Dr. Mueller said he appreciated this pragmatic approach. “Sometimes there can be hesitation on making recommendations from observational studies, but the alternative to recommending this diet is either no recommendations on diet or recommending an alternative diet,” he said. “The Mediterranean diet or the DASH diet, which is quite similar, have shown by far the most evidence of cardioprotection of any diets. They have been shown to reduce blood pressure and lipids and improve cardiovascular risk, and I think we can now assume that that likely extends to pregnancy. I feel comfortable for this diet to be recommended to pregnant women.”
But he added: “Having said that, there is still a need for a randomized trial in pregnancy. We think it works but until we have a randomized trial we won’t know for sure, and we won’t know how much of a benefit we can get.”
Commenting on the study, JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, pointed out that this type of observational study is important for hypothesis generation but cannot prove cause and effect relationships.
“The evidence is promising enough,” said Dr. Manson, who was not involved with this study. But she added that to move forward, a randomized trial in women at elevated risk of preeclampsia would be needed, beginning in early pregnancy, if not earlier.
“In the meantime,” she noted, “several large-scale cohorts could be leveraged to look at diet assessed before or during pregnancy to see if this dietary pattern is prospectively related to lower risk of preeclampsia.
“With additional supportive data, and in view of the diet’s safety and general cardiovascular benefits, it could become a major tool for preventing adverse pregnancy outcomes.”
The Boston Birth Cohort study was supported in part by grants from the March of Dimes, the National Institutes of Health, and the Health Resources and Services Administration of the U.S. Department of Health and Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Myocarditis higher with Moderna COVID vax in young men
One of the largest studies to date on myocarditis after COVID-19 vaccination confirms an increased risk with both the Pfizer and Moderna vaccines in young men and shows that the risk is higher with the Moderna than with the Pfizer vaccine.
The study also suggests for the first time that in young men 16 to 24 years of age, the risk for myocarditis after vaccination with either the Pfizer or Moderna vaccine is higher than the risk for myocarditis after COVID-19 infection.
The population-based study involved data on 23.1 million residents across four Scandinavian countries – Denmark, Finland, Norway, and Sweden – 74% of whom had received two vaccine doses and 7% of whom had received one dose.
By linking data from high-quality nationwide health registers on COVID-19 vaccination, infection rates, and myocarditis diagnoses, the researchers were able to evaluate the risk for myocarditis by vaccine product, vaccination dose number, sex, and age.
The study was published online in JAMA Cardiology.
The results confirm that the risk for myocarditis after COVID-19 mRNA vaccines is highest in young men 16 to 24 years of age after the second dose.
For men in this age group who received two doses of the same vaccine, data were compatible, with between four and seven excess myocarditis events in 28 days per 100,000 individuals after the second dose of the Pfizer vaccine, and between nine and 28 per 100,000 individuals after the second dose of the Moderna vaccine.
“This is one of the largest studies on this topic to date. The first population studies were in Israel, with 5 million individuals, and looked at just the Pfizer vaccine. We have data on 23 million people from Scandinavia that include both the Pfizer and Moderna vaccines,” senior author Rickard Ljung, MD, Swedish Medical Products Agency, told this news organization.
“We show a clearly higher risk of myocarditis after the Moderna vaccine than after the Pfizer vaccine. This has been suggested before, but our data confirm definitively that the Moderna vaccine has a higher risk of myocarditis than the Pfizer vaccine,” he added.
“In the group at highest risk of myocarditis after COVID vaccination – young men aged 16 to 24 – the Pfizer vaccine shows a five times higher risk of myocarditis versus the unvaccinated cohort, while the Moderna vaccine shows a 15 times higher risk,” Dr. Ljung noted.
After seeing these data, the Swedish regulatory authority is no longer recommending use of the Moderna vaccine for people younger than 30 years, Dr. Ljung said. Similar recommendations have been made in Norway and Finland.
The researchers report that their finding of a higher risk for myocarditis after the Moderna vaccine than after the Pfizer vaccine in young men is in line with data from the Canada, France, the United Kingdom, and the United States. But they point out that, compared with previous studies, the current study had the advantage of data analyzed according to a common protocol from four different countries and that showed similar directions of associations, despite considerable differences in previous COVID-19 infection levels and lockdown policies.
Risk higher with vaccination than infection?
For what is believed to be the first time, the Scandinavian data also suggest a higher risk for myocarditis after COVID-19 vaccination with both the Pfizer and Moderna vaccines than after COVID-19 infection in young men 16 to 24 years.
Although previous studies have shown that males in this age group have the highest risk for myocarditis after vaccination, it has always been suggested that the risk after vaccination is lower than the risk after infection. The Scandinavian data suggest otherwise for this age group.
Dr. Ljung explained that the myocarditis risk after COVID infection is very hard to study.
“It is highly dependent on the testing strategy,” he said. “For example, in the first half of 2020, the only people being tested were those admitted to hospital, so studies would have included the sickest patients and would therefore likely have found a higher rate of myocarditis. But this current Scandinavian dataset only included individuals with a positive COVID test after August 2020, reflecting a broader range of people.”
The researchers found an excess rate of myocarditis of 3.26 per 100,000 individuals within 28 days of a positive COVID-19 test among all males, and 1.37 per 100,000 individuals among males 16 to 24 years of age.
“We show that the risk of myocarditis after COVID infection is lower in younger people and higher in older people, but the opposite is true after COVID vaccination, where the risk of myocarditis is higher in younger people and lower in older people,” Dr. Ljung said.
The study was not able to look at severity of myocarditis but did record length of hospital stay, which was similar in patients who developed myocarditis after vaccination and those in the unvaccinated cohort (4 to 5 days). Deaths were rare, with no deaths in people younger than 40 years.
“I think we can say that in people aged over 40, the risk of myocarditis is greater with infection than with vaccination, but in those under 40, it is not so clear. And our data suggest that for young men aged 16 to 24 years, the risk of myocarditis after COVID vaccination with either the Pfizer or Moderna vaccine is higher than after COVID infection,” Dr. Ljung commented.
Although the Swedish regulatory agency has already stopped recommending use of Moderna vaccine in those younger than 30 years on the basis of these data, Dr. Ljung was reluctant to make any recommendations regarding the use of the Pfizer vaccine in young males, saying it was up to individual public-health agencies to makes these decisions.
But he pointed out that the current study only looked at myocarditis, and COVID infection can result in many other complications that can lead to hospitalization and death, which needs to be taken into account when assessing the risk and benefit of vaccination.
Dr. Ljung noted that the current data only applied to the first two doses of the vaccines; data after booster injections have not been included, although the researchers are looking at that now.
What to advise patients?
In an accompanying Editor’s Note, Ann Marie Navar, MD, University of Texas Southwestern Medical Center, Dallas, who is editor of JAMA Cardiology, and Robert Bonow, MD, Northwestern University Feinberg School of Medicine, Chicago, who is deputy editor of JAMA Cardiology, try to explain how these data can inform the way health care professionals communicate with their patients about vaccination.
They point out the “good news,” that older adults who are at highest risk for COVID-19 complications appear to be at extremely low risk for vaccine-associated myocarditis.
They note that for both men and women older than 40 years, the excess number of cases of myocarditis after vaccination was fewer than two in 100,000 vaccinees across all vaccines studied, and the death toll from COVID-19 in the United States as of March was more than 200 per 100,000 population.
“Given the high rates of morbidity and mortality from COVID-19 infection in older adults and the efficacy of the vaccine in preventing severe infection and death, the benefits of immunization in those older than 40 years clearly outweigh the risks,” the editors say.
But given these data in young men, they suggest that health care professionals consider recommending the Pfizer vaccine over the Moderna vaccine for certain populations, including young men and other individuals for whom concerns about myocarditis present a barrier to immunization.
The editors also point out that although the risk for myocarditis after COVID-19 immunization is real, this low risk must be considered in the context of the overall benefit of the vaccine.
“At the individual level, immunization prevents not only COVID-19-related myocarditis but also severe disease, hospitalization, long-term complications after COVID-19 infection, and death. At the population level, immunization helps to decrease community spread, decrease the chances of new variants emerging, protect people who are immunocompromised, and ensure our health care system can continue to provide for our communities,” they conclude.
Dr. Ljung reports grants from Sanofi Aventis paid to his institution outside the submitted work and personal fees from Pfizer outside the submitted work. Dr. Navar reports personal fees from Pfizer and AstraZeneca, outside the scope of this work.
A version of this article first appeared on Medscape.com.
One of the largest studies to date on myocarditis after COVID-19 vaccination confirms an increased risk with both the Pfizer and Moderna vaccines in young men and shows that the risk is higher with the Moderna than with the Pfizer vaccine.
The study also suggests for the first time that in young men 16 to 24 years of age, the risk for myocarditis after vaccination with either the Pfizer or Moderna vaccine is higher than the risk for myocarditis after COVID-19 infection.
The population-based study involved data on 23.1 million residents across four Scandinavian countries – Denmark, Finland, Norway, and Sweden – 74% of whom had received two vaccine doses and 7% of whom had received one dose.
By linking data from high-quality nationwide health registers on COVID-19 vaccination, infection rates, and myocarditis diagnoses, the researchers were able to evaluate the risk for myocarditis by vaccine product, vaccination dose number, sex, and age.
The study was published online in JAMA Cardiology.
The results confirm that the risk for myocarditis after COVID-19 mRNA vaccines is highest in young men 16 to 24 years of age after the second dose.
For men in this age group who received two doses of the same vaccine, data were compatible, with between four and seven excess myocarditis events in 28 days per 100,000 individuals after the second dose of the Pfizer vaccine, and between nine and 28 per 100,000 individuals after the second dose of the Moderna vaccine.
“This is one of the largest studies on this topic to date. The first population studies were in Israel, with 5 million individuals, and looked at just the Pfizer vaccine. We have data on 23 million people from Scandinavia that include both the Pfizer and Moderna vaccines,” senior author Rickard Ljung, MD, Swedish Medical Products Agency, told this news organization.
“We show a clearly higher risk of myocarditis after the Moderna vaccine than after the Pfizer vaccine. This has been suggested before, but our data confirm definitively that the Moderna vaccine has a higher risk of myocarditis than the Pfizer vaccine,” he added.
“In the group at highest risk of myocarditis after COVID vaccination – young men aged 16 to 24 – the Pfizer vaccine shows a five times higher risk of myocarditis versus the unvaccinated cohort, while the Moderna vaccine shows a 15 times higher risk,” Dr. Ljung noted.
After seeing these data, the Swedish regulatory authority is no longer recommending use of the Moderna vaccine for people younger than 30 years, Dr. Ljung said. Similar recommendations have been made in Norway and Finland.
The researchers report that their finding of a higher risk for myocarditis after the Moderna vaccine than after the Pfizer vaccine in young men is in line with data from the Canada, France, the United Kingdom, and the United States. But they point out that, compared with previous studies, the current study had the advantage of data analyzed according to a common protocol from four different countries and that showed similar directions of associations, despite considerable differences in previous COVID-19 infection levels and lockdown policies.
Risk higher with vaccination than infection?
For what is believed to be the first time, the Scandinavian data also suggest a higher risk for myocarditis after COVID-19 vaccination with both the Pfizer and Moderna vaccines than after COVID-19 infection in young men 16 to 24 years.
Although previous studies have shown that males in this age group have the highest risk for myocarditis after vaccination, it has always been suggested that the risk after vaccination is lower than the risk after infection. The Scandinavian data suggest otherwise for this age group.
Dr. Ljung explained that the myocarditis risk after COVID infection is very hard to study.
“It is highly dependent on the testing strategy,” he said. “For example, in the first half of 2020, the only people being tested were those admitted to hospital, so studies would have included the sickest patients and would therefore likely have found a higher rate of myocarditis. But this current Scandinavian dataset only included individuals with a positive COVID test after August 2020, reflecting a broader range of people.”
The researchers found an excess rate of myocarditis of 3.26 per 100,000 individuals within 28 days of a positive COVID-19 test among all males, and 1.37 per 100,000 individuals among males 16 to 24 years of age.
“We show that the risk of myocarditis after COVID infection is lower in younger people and higher in older people, but the opposite is true after COVID vaccination, where the risk of myocarditis is higher in younger people and lower in older people,” Dr. Ljung said.
The study was not able to look at severity of myocarditis but did record length of hospital stay, which was similar in patients who developed myocarditis after vaccination and those in the unvaccinated cohort (4 to 5 days). Deaths were rare, with no deaths in people younger than 40 years.
“I think we can say that in people aged over 40, the risk of myocarditis is greater with infection than with vaccination, but in those under 40, it is not so clear. And our data suggest that for young men aged 16 to 24 years, the risk of myocarditis after COVID vaccination with either the Pfizer or Moderna vaccine is higher than after COVID infection,” Dr. Ljung commented.
Although the Swedish regulatory agency has already stopped recommending use of Moderna vaccine in those younger than 30 years on the basis of these data, Dr. Ljung was reluctant to make any recommendations regarding the use of the Pfizer vaccine in young males, saying it was up to individual public-health agencies to makes these decisions.
But he pointed out that the current study only looked at myocarditis, and COVID infection can result in many other complications that can lead to hospitalization and death, which needs to be taken into account when assessing the risk and benefit of vaccination.
Dr. Ljung noted that the current data only applied to the first two doses of the vaccines; data after booster injections have not been included, although the researchers are looking at that now.
What to advise patients?
In an accompanying Editor’s Note, Ann Marie Navar, MD, University of Texas Southwestern Medical Center, Dallas, who is editor of JAMA Cardiology, and Robert Bonow, MD, Northwestern University Feinberg School of Medicine, Chicago, who is deputy editor of JAMA Cardiology, try to explain how these data can inform the way health care professionals communicate with their patients about vaccination.
They point out the “good news,” that older adults who are at highest risk for COVID-19 complications appear to be at extremely low risk for vaccine-associated myocarditis.
They note that for both men and women older than 40 years, the excess number of cases of myocarditis after vaccination was fewer than two in 100,000 vaccinees across all vaccines studied, and the death toll from COVID-19 in the United States as of March was more than 200 per 100,000 population.
“Given the high rates of morbidity and mortality from COVID-19 infection in older adults and the efficacy of the vaccine in preventing severe infection and death, the benefits of immunization in those older than 40 years clearly outweigh the risks,” the editors say.
But given these data in young men, they suggest that health care professionals consider recommending the Pfizer vaccine over the Moderna vaccine for certain populations, including young men and other individuals for whom concerns about myocarditis present a barrier to immunization.
The editors also point out that although the risk for myocarditis after COVID-19 immunization is real, this low risk must be considered in the context of the overall benefit of the vaccine.
“At the individual level, immunization prevents not only COVID-19-related myocarditis but also severe disease, hospitalization, long-term complications after COVID-19 infection, and death. At the population level, immunization helps to decrease community spread, decrease the chances of new variants emerging, protect people who are immunocompromised, and ensure our health care system can continue to provide for our communities,” they conclude.
Dr. Ljung reports grants from Sanofi Aventis paid to his institution outside the submitted work and personal fees from Pfizer outside the submitted work. Dr. Navar reports personal fees from Pfizer and AstraZeneca, outside the scope of this work.
A version of this article first appeared on Medscape.com.
One of the largest studies to date on myocarditis after COVID-19 vaccination confirms an increased risk with both the Pfizer and Moderna vaccines in young men and shows that the risk is higher with the Moderna than with the Pfizer vaccine.
The study also suggests for the first time that in young men 16 to 24 years of age, the risk for myocarditis after vaccination with either the Pfizer or Moderna vaccine is higher than the risk for myocarditis after COVID-19 infection.
The population-based study involved data on 23.1 million residents across four Scandinavian countries – Denmark, Finland, Norway, and Sweden – 74% of whom had received two vaccine doses and 7% of whom had received one dose.
By linking data from high-quality nationwide health registers on COVID-19 vaccination, infection rates, and myocarditis diagnoses, the researchers were able to evaluate the risk for myocarditis by vaccine product, vaccination dose number, sex, and age.
The study was published online in JAMA Cardiology.
The results confirm that the risk for myocarditis after COVID-19 mRNA vaccines is highest in young men 16 to 24 years of age after the second dose.
For men in this age group who received two doses of the same vaccine, data were compatible, with between four and seven excess myocarditis events in 28 days per 100,000 individuals after the second dose of the Pfizer vaccine, and between nine and 28 per 100,000 individuals after the second dose of the Moderna vaccine.
“This is one of the largest studies on this topic to date. The first population studies were in Israel, with 5 million individuals, and looked at just the Pfizer vaccine. We have data on 23 million people from Scandinavia that include both the Pfizer and Moderna vaccines,” senior author Rickard Ljung, MD, Swedish Medical Products Agency, told this news organization.
“We show a clearly higher risk of myocarditis after the Moderna vaccine than after the Pfizer vaccine. This has been suggested before, but our data confirm definitively that the Moderna vaccine has a higher risk of myocarditis than the Pfizer vaccine,” he added.
“In the group at highest risk of myocarditis after COVID vaccination – young men aged 16 to 24 – the Pfizer vaccine shows a five times higher risk of myocarditis versus the unvaccinated cohort, while the Moderna vaccine shows a 15 times higher risk,” Dr. Ljung noted.
After seeing these data, the Swedish regulatory authority is no longer recommending use of the Moderna vaccine for people younger than 30 years, Dr. Ljung said. Similar recommendations have been made in Norway and Finland.
The researchers report that their finding of a higher risk for myocarditis after the Moderna vaccine than after the Pfizer vaccine in young men is in line with data from the Canada, France, the United Kingdom, and the United States. But they point out that, compared with previous studies, the current study had the advantage of data analyzed according to a common protocol from four different countries and that showed similar directions of associations, despite considerable differences in previous COVID-19 infection levels and lockdown policies.
Risk higher with vaccination than infection?
For what is believed to be the first time, the Scandinavian data also suggest a higher risk for myocarditis after COVID-19 vaccination with both the Pfizer and Moderna vaccines than after COVID-19 infection in young men 16 to 24 years.
Although previous studies have shown that males in this age group have the highest risk for myocarditis after vaccination, it has always been suggested that the risk after vaccination is lower than the risk after infection. The Scandinavian data suggest otherwise for this age group.
Dr. Ljung explained that the myocarditis risk after COVID infection is very hard to study.
“It is highly dependent on the testing strategy,” he said. “For example, in the first half of 2020, the only people being tested were those admitted to hospital, so studies would have included the sickest patients and would therefore likely have found a higher rate of myocarditis. But this current Scandinavian dataset only included individuals with a positive COVID test after August 2020, reflecting a broader range of people.”
The researchers found an excess rate of myocarditis of 3.26 per 100,000 individuals within 28 days of a positive COVID-19 test among all males, and 1.37 per 100,000 individuals among males 16 to 24 years of age.
“We show that the risk of myocarditis after COVID infection is lower in younger people and higher in older people, but the opposite is true after COVID vaccination, where the risk of myocarditis is higher in younger people and lower in older people,” Dr. Ljung said.
The study was not able to look at severity of myocarditis but did record length of hospital stay, which was similar in patients who developed myocarditis after vaccination and those in the unvaccinated cohort (4 to 5 days). Deaths were rare, with no deaths in people younger than 40 years.
“I think we can say that in people aged over 40, the risk of myocarditis is greater with infection than with vaccination, but in those under 40, it is not so clear. And our data suggest that for young men aged 16 to 24 years, the risk of myocarditis after COVID vaccination with either the Pfizer or Moderna vaccine is higher than after COVID infection,” Dr. Ljung commented.
Although the Swedish regulatory agency has already stopped recommending use of Moderna vaccine in those younger than 30 years on the basis of these data, Dr. Ljung was reluctant to make any recommendations regarding the use of the Pfizer vaccine in young males, saying it was up to individual public-health agencies to makes these decisions.
But he pointed out that the current study only looked at myocarditis, and COVID infection can result in many other complications that can lead to hospitalization and death, which needs to be taken into account when assessing the risk and benefit of vaccination.
Dr. Ljung noted that the current data only applied to the first two doses of the vaccines; data after booster injections have not been included, although the researchers are looking at that now.
What to advise patients?
In an accompanying Editor’s Note, Ann Marie Navar, MD, University of Texas Southwestern Medical Center, Dallas, who is editor of JAMA Cardiology, and Robert Bonow, MD, Northwestern University Feinberg School of Medicine, Chicago, who is deputy editor of JAMA Cardiology, try to explain how these data can inform the way health care professionals communicate with their patients about vaccination.
They point out the “good news,” that older adults who are at highest risk for COVID-19 complications appear to be at extremely low risk for vaccine-associated myocarditis.
They note that for both men and women older than 40 years, the excess number of cases of myocarditis after vaccination was fewer than two in 100,000 vaccinees across all vaccines studied, and the death toll from COVID-19 in the United States as of March was more than 200 per 100,000 population.
“Given the high rates of morbidity and mortality from COVID-19 infection in older adults and the efficacy of the vaccine in preventing severe infection and death, the benefits of immunization in those older than 40 years clearly outweigh the risks,” the editors say.
But given these data in young men, they suggest that health care professionals consider recommending the Pfizer vaccine over the Moderna vaccine for certain populations, including young men and other individuals for whom concerns about myocarditis present a barrier to immunization.
The editors also point out that although the risk for myocarditis after COVID-19 immunization is real, this low risk must be considered in the context of the overall benefit of the vaccine.
“At the individual level, immunization prevents not only COVID-19-related myocarditis but also severe disease, hospitalization, long-term complications after COVID-19 infection, and death. At the population level, immunization helps to decrease community spread, decrease the chances of new variants emerging, protect people who are immunocompromised, and ensure our health care system can continue to provide for our communities,” they conclude.
Dr. Ljung reports grants from Sanofi Aventis paid to his institution outside the submitted work and personal fees from Pfizer outside the submitted work. Dr. Navar reports personal fees from Pfizer and AstraZeneca, outside the scope of this work.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Are free lunches back? Docs start seeing drug reps again
In their heyday, drug reps had big expense budgets and would wine and dine physicians, golf with them, and give gifts to their potential physician clients.
But in 2002, pressure from Congress and increased scrutiny from the American Medical Association prompted the Pharmaceutical Research and Manufacturers of America to adopt a set of voluntary ethical codes to regulate the gifts given to physicians. Now, physicians must report even small gifts or meals to the National Practitioner Data Bank.
Before the restrictions, physician/pharmaceutical rep relationships relied on face-to-face meetings. These included lunches with a limited budget or sharing a cup of coffee during a morning visit to a practice. The parties got to know each other, which led to trust and long-term relationships.
During the COVID-19 pandemic, everything changed. “It was culture shock for us,” admitted Craig F, a career pharmaceutical rep. “We didn’t know what we were going to do.”
The pharmaceutical industry pivoted and quickly got up to speed with Zoom, Microsoft Teams, and the like. “We began by reaching out to doctors via email and cell phones to set up virtual meetings,” Craig said. “Most of the doctors were working from home, doing telehealth whenever possible. For new sales reps, this was particularly difficult, because they couldn’t visit offices and get to know doctors.”
Many physicians didn’t want to devote time to Zoom meetings with pharma reps. “We worked around their schedules, and sometimes this even looked like Sunday calls,” he said.
As vaccination levels increased and medical offices began to reopen, so too did some of the old-school, face-to-face pharma rep/doctor meetings. But most proceeded with caution. “Some pharmaceutical companies didn’t put reps back into the field until the fall of 2020,” said Craig. “If we weren’t welcome in an office, we didn’t push it.”
Once much of the population was vaccinated, the thaw began in earnest, although the drug reps continued to tread cautiously, mask up, and respect the wishes of physicians. Today, Craig estimated that about two-thirds of his appointments are in person.
Still, it’s unlikely that the drug rep–supplied “free staff lunch” will ever regain its former popularity. Medical office staff are still keeping distance, owing to COVID; office schedules may be more crowded and may not allow the time; and many physicians are still nervous about having to report “gifts” or “paid lunches” from pharma.
The post-COVID paradigm shift
The pandemic put a dent in the pharma rep/doctor relationship, said Suzy Jackson, managing director of life sciences at Accenture and an author of The “New” Rules of Healthcare Provider Engagement . “COVID started moving power away from reps because they lost the ability to simply wander into a building and have a conversation with a health care provider. We’re seeing the pandemic evolve the meeting model into a hybrid in-person and virtual.”
“Many doctors are operating in a slower fashion because they’re balancing a hybrid model with patients, as well,” said Craig. “Some of my visits now involve talking to nurses or front-office staff, not getting in to see the doctors.”
The push from some doctors to see reps virtually as opposed to in person is a challenge for the pharma companies. “We get more done in person, so virtual is not our favorite way to do business,” said Craig. “But we’re thankful for any time we can get with doctors, so when they ask to do virtual, we agree.”
Still, the Accenture survey offered good news for pharma reps: Only 4% of respondents didn’t want to continue with in-person meetings at all. “I think of this as a positive,” Ms. Jackson said. “It shows that physicians value these relationships, if they’re done in the right way.”
But a survey by Boston Consulting Group confirms that virtual visits are likely to continue. BCG’s Doctors’ Changing Expectations of Pharma Are Here to Stay revealed that three-quarters of respondent physicians prefer to maintain or increase the amount of virtual engagements with pharma reps after becoming accustomed to the practice during the pandemic.
Under these changing scenarios, said Ms. Jackson, pharma reps have to think about more meaningful ways to engage with doctors.
“I feel that doctors are more crunched for time now, managing hybrid environments,” Craig said. “They have less time and want more patient-specific information that leads to fewer calls back to their offices.”
More physicians now value webinars, virtual training, and speaker programs. Virtual channels, the survey found, “give physicians access to the information they need in an easy and convenient manner.”
Still, physicians have noted that the survey indicated that email communications from pharma reps had increased. Often, physicians found the useful information buried in irrelevant “clutter.”
Restrictions on drug reps became tighter
In the 20 years since the guidelines came into existence, PhRMA has continued to strengthen the codes. In 2009, PhRMA issued new recommendations surrounding noneducational gifts and placed a cap of $100 for meals, drug samples, and other items. In 2022, they added layers to the code that focus on speaker programs. For instance, while companies can provide “modest” meals to attendees as an incidental courtesy, pharma reps can no longer pay for or provide alcohol in conjunction with these programs.
The rules vary from state to state. In Minnesota, for instance, gifts from pharma companies cannot exceed $50 per year. Some institutions – such as the Cleveland Clinic – have even stricter rules. “When we have conventions, we put up signage reminding doctors from the strictest states that they can’t even accept a cup of coffee from a rep,” said Craig.
However, COVID hasn’t completely changed doctor/pharma relationships. In Ms. Jackson’s words, “In spite of the shift to a more hybrid model, this is a very human relationship yielding real human results.”
A version of this article first appeared on Medscape.com.
In their heyday, drug reps had big expense budgets and would wine and dine physicians, golf with them, and give gifts to their potential physician clients.
But in 2002, pressure from Congress and increased scrutiny from the American Medical Association prompted the Pharmaceutical Research and Manufacturers of America to adopt a set of voluntary ethical codes to regulate the gifts given to physicians. Now, physicians must report even small gifts or meals to the National Practitioner Data Bank.
Before the restrictions, physician/pharmaceutical rep relationships relied on face-to-face meetings. These included lunches with a limited budget or sharing a cup of coffee during a morning visit to a practice. The parties got to know each other, which led to trust and long-term relationships.
During the COVID-19 pandemic, everything changed. “It was culture shock for us,” admitted Craig F, a career pharmaceutical rep. “We didn’t know what we were going to do.”
The pharmaceutical industry pivoted and quickly got up to speed with Zoom, Microsoft Teams, and the like. “We began by reaching out to doctors via email and cell phones to set up virtual meetings,” Craig said. “Most of the doctors were working from home, doing telehealth whenever possible. For new sales reps, this was particularly difficult, because they couldn’t visit offices and get to know doctors.”
Many physicians didn’t want to devote time to Zoom meetings with pharma reps. “We worked around their schedules, and sometimes this even looked like Sunday calls,” he said.
As vaccination levels increased and medical offices began to reopen, so too did some of the old-school, face-to-face pharma rep/doctor meetings. But most proceeded with caution. “Some pharmaceutical companies didn’t put reps back into the field until the fall of 2020,” said Craig. “If we weren’t welcome in an office, we didn’t push it.”
Once much of the population was vaccinated, the thaw began in earnest, although the drug reps continued to tread cautiously, mask up, and respect the wishes of physicians. Today, Craig estimated that about two-thirds of his appointments are in person.
Still, it’s unlikely that the drug rep–supplied “free staff lunch” will ever regain its former popularity. Medical office staff are still keeping distance, owing to COVID; office schedules may be more crowded and may not allow the time; and many physicians are still nervous about having to report “gifts” or “paid lunches” from pharma.
The post-COVID paradigm shift
The pandemic put a dent in the pharma rep/doctor relationship, said Suzy Jackson, managing director of life sciences at Accenture and an author of The “New” Rules of Healthcare Provider Engagement . “COVID started moving power away from reps because they lost the ability to simply wander into a building and have a conversation with a health care provider. We’re seeing the pandemic evolve the meeting model into a hybrid in-person and virtual.”
“Many doctors are operating in a slower fashion because they’re balancing a hybrid model with patients, as well,” said Craig. “Some of my visits now involve talking to nurses or front-office staff, not getting in to see the doctors.”
The push from some doctors to see reps virtually as opposed to in person is a challenge for the pharma companies. “We get more done in person, so virtual is not our favorite way to do business,” said Craig. “But we’re thankful for any time we can get with doctors, so when they ask to do virtual, we agree.”
Still, the Accenture survey offered good news for pharma reps: Only 4% of respondents didn’t want to continue with in-person meetings at all. “I think of this as a positive,” Ms. Jackson said. “It shows that physicians value these relationships, if they’re done in the right way.”
But a survey by Boston Consulting Group confirms that virtual visits are likely to continue. BCG’s Doctors’ Changing Expectations of Pharma Are Here to Stay revealed that three-quarters of respondent physicians prefer to maintain or increase the amount of virtual engagements with pharma reps after becoming accustomed to the practice during the pandemic.
Under these changing scenarios, said Ms. Jackson, pharma reps have to think about more meaningful ways to engage with doctors.
“I feel that doctors are more crunched for time now, managing hybrid environments,” Craig said. “They have less time and want more patient-specific information that leads to fewer calls back to their offices.”
More physicians now value webinars, virtual training, and speaker programs. Virtual channels, the survey found, “give physicians access to the information they need in an easy and convenient manner.”
Still, physicians have noted that the survey indicated that email communications from pharma reps had increased. Often, physicians found the useful information buried in irrelevant “clutter.”
Restrictions on drug reps became tighter
In the 20 years since the guidelines came into existence, PhRMA has continued to strengthen the codes. In 2009, PhRMA issued new recommendations surrounding noneducational gifts and placed a cap of $100 for meals, drug samples, and other items. In 2022, they added layers to the code that focus on speaker programs. For instance, while companies can provide “modest” meals to attendees as an incidental courtesy, pharma reps can no longer pay for or provide alcohol in conjunction with these programs.
The rules vary from state to state. In Minnesota, for instance, gifts from pharma companies cannot exceed $50 per year. Some institutions – such as the Cleveland Clinic – have even stricter rules. “When we have conventions, we put up signage reminding doctors from the strictest states that they can’t even accept a cup of coffee from a rep,” said Craig.
However, COVID hasn’t completely changed doctor/pharma relationships. In Ms. Jackson’s words, “In spite of the shift to a more hybrid model, this is a very human relationship yielding real human results.”
A version of this article first appeared on Medscape.com.
In their heyday, drug reps had big expense budgets and would wine and dine physicians, golf with them, and give gifts to their potential physician clients.
But in 2002, pressure from Congress and increased scrutiny from the American Medical Association prompted the Pharmaceutical Research and Manufacturers of America to adopt a set of voluntary ethical codes to regulate the gifts given to physicians. Now, physicians must report even small gifts or meals to the National Practitioner Data Bank.
Before the restrictions, physician/pharmaceutical rep relationships relied on face-to-face meetings. These included lunches with a limited budget or sharing a cup of coffee during a morning visit to a practice. The parties got to know each other, which led to trust and long-term relationships.
During the COVID-19 pandemic, everything changed. “It was culture shock for us,” admitted Craig F, a career pharmaceutical rep. “We didn’t know what we were going to do.”
The pharmaceutical industry pivoted and quickly got up to speed with Zoom, Microsoft Teams, and the like. “We began by reaching out to doctors via email and cell phones to set up virtual meetings,” Craig said. “Most of the doctors were working from home, doing telehealth whenever possible. For new sales reps, this was particularly difficult, because they couldn’t visit offices and get to know doctors.”
Many physicians didn’t want to devote time to Zoom meetings with pharma reps. “We worked around their schedules, and sometimes this even looked like Sunday calls,” he said.
As vaccination levels increased and medical offices began to reopen, so too did some of the old-school, face-to-face pharma rep/doctor meetings. But most proceeded with caution. “Some pharmaceutical companies didn’t put reps back into the field until the fall of 2020,” said Craig. “If we weren’t welcome in an office, we didn’t push it.”
Once much of the population was vaccinated, the thaw began in earnest, although the drug reps continued to tread cautiously, mask up, and respect the wishes of physicians. Today, Craig estimated that about two-thirds of his appointments are in person.
Still, it’s unlikely that the drug rep–supplied “free staff lunch” will ever regain its former popularity. Medical office staff are still keeping distance, owing to COVID; office schedules may be more crowded and may not allow the time; and many physicians are still nervous about having to report “gifts” or “paid lunches” from pharma.
The post-COVID paradigm shift
The pandemic put a dent in the pharma rep/doctor relationship, said Suzy Jackson, managing director of life sciences at Accenture and an author of The “New” Rules of Healthcare Provider Engagement . “COVID started moving power away from reps because they lost the ability to simply wander into a building and have a conversation with a health care provider. We’re seeing the pandemic evolve the meeting model into a hybrid in-person and virtual.”
“Many doctors are operating in a slower fashion because they’re balancing a hybrid model with patients, as well,” said Craig. “Some of my visits now involve talking to nurses or front-office staff, not getting in to see the doctors.”
The push from some doctors to see reps virtually as opposed to in person is a challenge for the pharma companies. “We get more done in person, so virtual is not our favorite way to do business,” said Craig. “But we’re thankful for any time we can get with doctors, so when they ask to do virtual, we agree.”
Still, the Accenture survey offered good news for pharma reps: Only 4% of respondents didn’t want to continue with in-person meetings at all. “I think of this as a positive,” Ms. Jackson said. “It shows that physicians value these relationships, if they’re done in the right way.”
But a survey by Boston Consulting Group confirms that virtual visits are likely to continue. BCG’s Doctors’ Changing Expectations of Pharma Are Here to Stay revealed that three-quarters of respondent physicians prefer to maintain or increase the amount of virtual engagements with pharma reps after becoming accustomed to the practice during the pandemic.
Under these changing scenarios, said Ms. Jackson, pharma reps have to think about more meaningful ways to engage with doctors.
“I feel that doctors are more crunched for time now, managing hybrid environments,” Craig said. “They have less time and want more patient-specific information that leads to fewer calls back to their offices.”
More physicians now value webinars, virtual training, and speaker programs. Virtual channels, the survey found, “give physicians access to the information they need in an easy and convenient manner.”
Still, physicians have noted that the survey indicated that email communications from pharma reps had increased. Often, physicians found the useful information buried in irrelevant “clutter.”
Restrictions on drug reps became tighter
In the 20 years since the guidelines came into existence, PhRMA has continued to strengthen the codes. In 2009, PhRMA issued new recommendations surrounding noneducational gifts and placed a cap of $100 for meals, drug samples, and other items. In 2022, they added layers to the code that focus on speaker programs. For instance, while companies can provide “modest” meals to attendees as an incidental courtesy, pharma reps can no longer pay for or provide alcohol in conjunction with these programs.
The rules vary from state to state. In Minnesota, for instance, gifts from pharma companies cannot exceed $50 per year. Some institutions – such as the Cleveland Clinic – have even stricter rules. “When we have conventions, we put up signage reminding doctors from the strictest states that they can’t even accept a cup of coffee from a rep,” said Craig.
However, COVID hasn’t completely changed doctor/pharma relationships. In Ms. Jackson’s words, “In spite of the shift to a more hybrid model, this is a very human relationship yielding real human results.”
A version of this article first appeared on Medscape.com.
CDC panel lists reasons to get second COVID booster
The Centers for Disease Control and Prevention is considering what to tell the public about second booster shots with mRNA vaccinations for COVID-19.
The U.S. Food and Drug Administration in March authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for people aged 50 and older and certain immunocompromised adults, even though many top infectious disease experts questioned the need before the agency’s decision.
In a meeting April 20, the CDC asked its Advisory Committee on Immunization Practices to discuss second booster shots, but did not ask the group of experts to vote on formal recommendations.
Instead,
ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, said she’s concerned about the potential for “booster fatigue.”
“A vaccination program that’s going to require boosting large proportions of the population every 4-6 months is really not sustainable and probably not something that most people want to participate in,” she said.
The benefit of additional COVID-19 shots for now appears to be smaller than what people get from the initial doses, Dr. Bell said.
Earlier in the meeting, CDC staff presented estimates about how well the COVID-19 vaccines work to prevent one case of hospitalization from the disease over 4 months among people aged 50 and older.
The major gain in preventing hospitalizations occurs with the first vaccination series and then wanes, the CDC said.
It appears that one hospitalization is prevented for every 135 people who get the first round of COVID-19 vaccinations. But it takes 674 people getting a first booster dose to prevent one hospitalization. A second booster prevents one hospitalization for every 1,205 people vaccinated.
Dr. Bell said she’s concerned about considering additional doses for “smaller and smaller return and creating an impression that we don’t have a very effective vaccination program,” even though the CDC’s data show a clear benefit.
Reasons to get a second booster
Elisha Hall, PhD, RD, of the CDC presented slides with some factors to help determine the urgency for a person to get a second booster:
- Having certain underlying medical conditions that increase the risk of severe COVID-19 illness.
- Being moderately or severely immunocompromised.
- Living with someone who is immunocompromised, at increased risk for severe disease, or who cannot be vaccinated because of age or contraindication.
- Being at increased risk of exposure to SARS-CoV-2, the virus that causes COVID-19, such as through occupational, institutional, or other activities (e.g., travel or large gatherings).
- Living or working in an area where there is a medium or high level of COVID-19 in the community.
In contrast, people might want to wait if they had been infected with SARS-CoV-2 within the past 3 months, Dr. Hall said in her presentation. Another reason for delay might be a concern that a booster dose may be more important later in the year.
The experts also addressed public confusion over boosters. For the Pfizer and Moderna mRNA vaccines, a second booster is a fourth dose, but for those who received the one-shot J&J vaccine, the second booster is a third dose.
Going forward, it may be easier to refer to subsequent doses as “annual boosters,” the CDC’s Sara Oliver, MD, MSPH, told the panel. It will be important to keep language about subsequent vaccinations clear and easy for the public to follow, she said.
Dr. Oliver also said there’s already been a drop-off in the acceptance of second rounds of COVID-19 vaccinations. CDC data show that 77% of people in the United States have had at least one dose of a COVID-19 vaccine, but only 66% of the population is fully vaccinated, and only 45% have had a first booster dose.
In her presentation, Dr. Oliver said the top priority in COVID-19 vaccination efforts remains initial vaccinations for people who haven’t gotten them.
Kids younger than 5
During the public comment session of the CDC meeting, several people called on the FDA to move quickly to expand authorization of COVID-19 vaccines to children aged 5 years and younger.
“We know that many parents and caregivers and health care providers are anxious to have COVID vaccines available” for young children, said Doran Fink, MD, PhD, a deputy director of the FDA’s vaccines division.
He said the agency is working to be ready to authorize the shots for young children while it awaits research results from the manufacturers.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention is considering what to tell the public about second booster shots with mRNA vaccinations for COVID-19.
The U.S. Food and Drug Administration in March authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for people aged 50 and older and certain immunocompromised adults, even though many top infectious disease experts questioned the need before the agency’s decision.
In a meeting April 20, the CDC asked its Advisory Committee on Immunization Practices to discuss second booster shots, but did not ask the group of experts to vote on formal recommendations.
Instead,
ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, said she’s concerned about the potential for “booster fatigue.”
“A vaccination program that’s going to require boosting large proportions of the population every 4-6 months is really not sustainable and probably not something that most people want to participate in,” she said.
The benefit of additional COVID-19 shots for now appears to be smaller than what people get from the initial doses, Dr. Bell said.
Earlier in the meeting, CDC staff presented estimates about how well the COVID-19 vaccines work to prevent one case of hospitalization from the disease over 4 months among people aged 50 and older.
The major gain in preventing hospitalizations occurs with the first vaccination series and then wanes, the CDC said.
It appears that one hospitalization is prevented for every 135 people who get the first round of COVID-19 vaccinations. But it takes 674 people getting a first booster dose to prevent one hospitalization. A second booster prevents one hospitalization for every 1,205 people vaccinated.
Dr. Bell said she’s concerned about considering additional doses for “smaller and smaller return and creating an impression that we don’t have a very effective vaccination program,” even though the CDC’s data show a clear benefit.
Reasons to get a second booster
Elisha Hall, PhD, RD, of the CDC presented slides with some factors to help determine the urgency for a person to get a second booster:
- Having certain underlying medical conditions that increase the risk of severe COVID-19 illness.
- Being moderately or severely immunocompromised.
- Living with someone who is immunocompromised, at increased risk for severe disease, or who cannot be vaccinated because of age or contraindication.
- Being at increased risk of exposure to SARS-CoV-2, the virus that causes COVID-19, such as through occupational, institutional, or other activities (e.g., travel or large gatherings).
- Living or working in an area where there is a medium or high level of COVID-19 in the community.
In contrast, people might want to wait if they had been infected with SARS-CoV-2 within the past 3 months, Dr. Hall said in her presentation. Another reason for delay might be a concern that a booster dose may be more important later in the year.
The experts also addressed public confusion over boosters. For the Pfizer and Moderna mRNA vaccines, a second booster is a fourth dose, but for those who received the one-shot J&J vaccine, the second booster is a third dose.
Going forward, it may be easier to refer to subsequent doses as “annual boosters,” the CDC’s Sara Oliver, MD, MSPH, told the panel. It will be important to keep language about subsequent vaccinations clear and easy for the public to follow, she said.
Dr. Oliver also said there’s already been a drop-off in the acceptance of second rounds of COVID-19 vaccinations. CDC data show that 77% of people in the United States have had at least one dose of a COVID-19 vaccine, but only 66% of the population is fully vaccinated, and only 45% have had a first booster dose.
In her presentation, Dr. Oliver said the top priority in COVID-19 vaccination efforts remains initial vaccinations for people who haven’t gotten them.
Kids younger than 5
During the public comment session of the CDC meeting, several people called on the FDA to move quickly to expand authorization of COVID-19 vaccines to children aged 5 years and younger.
“We know that many parents and caregivers and health care providers are anxious to have COVID vaccines available” for young children, said Doran Fink, MD, PhD, a deputy director of the FDA’s vaccines division.
He said the agency is working to be ready to authorize the shots for young children while it awaits research results from the manufacturers.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention is considering what to tell the public about second booster shots with mRNA vaccinations for COVID-19.
The U.S. Food and Drug Administration in March authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for people aged 50 and older and certain immunocompromised adults, even though many top infectious disease experts questioned the need before the agency’s decision.
In a meeting April 20, the CDC asked its Advisory Committee on Immunization Practices to discuss second booster shots, but did not ask the group of experts to vote on formal recommendations.
Instead,
ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, said she’s concerned about the potential for “booster fatigue.”
“A vaccination program that’s going to require boosting large proportions of the population every 4-6 months is really not sustainable and probably not something that most people want to participate in,” she said.
The benefit of additional COVID-19 shots for now appears to be smaller than what people get from the initial doses, Dr. Bell said.
Earlier in the meeting, CDC staff presented estimates about how well the COVID-19 vaccines work to prevent one case of hospitalization from the disease over 4 months among people aged 50 and older.
The major gain in preventing hospitalizations occurs with the first vaccination series and then wanes, the CDC said.
It appears that one hospitalization is prevented for every 135 people who get the first round of COVID-19 vaccinations. But it takes 674 people getting a first booster dose to prevent one hospitalization. A second booster prevents one hospitalization for every 1,205 people vaccinated.
Dr. Bell said she’s concerned about considering additional doses for “smaller and smaller return and creating an impression that we don’t have a very effective vaccination program,” even though the CDC’s data show a clear benefit.
Reasons to get a second booster
Elisha Hall, PhD, RD, of the CDC presented slides with some factors to help determine the urgency for a person to get a second booster:
- Having certain underlying medical conditions that increase the risk of severe COVID-19 illness.
- Being moderately or severely immunocompromised.
- Living with someone who is immunocompromised, at increased risk for severe disease, or who cannot be vaccinated because of age or contraindication.
- Being at increased risk of exposure to SARS-CoV-2, the virus that causes COVID-19, such as through occupational, institutional, or other activities (e.g., travel or large gatherings).
- Living or working in an area where there is a medium or high level of COVID-19 in the community.
In contrast, people might want to wait if they had been infected with SARS-CoV-2 within the past 3 months, Dr. Hall said in her presentation. Another reason for delay might be a concern that a booster dose may be more important later in the year.
The experts also addressed public confusion over boosters. For the Pfizer and Moderna mRNA vaccines, a second booster is a fourth dose, but for those who received the one-shot J&J vaccine, the second booster is a third dose.
Going forward, it may be easier to refer to subsequent doses as “annual boosters,” the CDC’s Sara Oliver, MD, MSPH, told the panel. It will be important to keep language about subsequent vaccinations clear and easy for the public to follow, she said.
Dr. Oliver also said there’s already been a drop-off in the acceptance of second rounds of COVID-19 vaccinations. CDC data show that 77% of people in the United States have had at least one dose of a COVID-19 vaccine, but only 66% of the population is fully vaccinated, and only 45% have had a first booster dose.
In her presentation, Dr. Oliver said the top priority in COVID-19 vaccination efforts remains initial vaccinations for people who haven’t gotten them.
Kids younger than 5
During the public comment session of the CDC meeting, several people called on the FDA to move quickly to expand authorization of COVID-19 vaccines to children aged 5 years and younger.
“We know that many parents and caregivers and health care providers are anxious to have COVID vaccines available” for young children, said Doran Fink, MD, PhD, a deputy director of the FDA’s vaccines division.
He said the agency is working to be ready to authorize the shots for young children while it awaits research results from the manufacturers.
A version of this article first appeared on WebMD.com.
RaDonda Vaught: Victim, felon, or both?
For 4 and a half years, I have followed the RaDonda Vaught medication error that led to the unfortunate death of a human being. I am not alone. Nurses across the country have followed the case with anxiety and fear, knowing a guilty verdict might have the potential to challenge basic tenets of care.
According to Kaiser Health News, nurses are “raging and quitting” following the announcement of a guilty verdict for two felonies: criminally negligent homicide and gross neglect of an impaired adult.
Thousands of nurses have claimed they could arrive in Nashville, Tenn., on May 13, the day Ms. Vaught is to be sentenced, to protest the conviction. Others have stated they believe justice is being conducted, as their sympathies lie with the victim, Charlene Murphey, who died 12 hours after being unable to draw breath, paralyzed from the inadvertent dose of vecuronium given intravenously by her nurse.
How should we feel as clinicians? according to sentencing guidelines?
My belief is that it is understandable to feel passionately about this case, including what it could mean to an era of “just culture” that nursing organizations have promoted. The concept of just culture looks at medication/nursing errors as opportunities for growth to avoid future errors, not as scenarios for punitive action. With the guilty verdict in Ms. Vaught’s case, nurses (and facilities) fear that nurses will avoid coming forward after mistakes, leading to cover-ups and a culture perspective.
Will nurses be hesitant to report errors (especially significant errors) that lead to patient harm? Will we fear retribution and reprisal for being truthful?
I believe that Ms. Vaught’s criminal case has changed little in the political landscape of caregiving. Before you let loose with a loud expletive (or two), hear me out.
When a patient dies from unintentional harm, someone must be held accountable. Society needs a scapegoat, and unfortunately, excrement slides downhill to the lowest common denominator, which may be the nurse. Initially, Ms. Vaught was contacted by her state licensing board (Tennessee) and informed there would be no professional repercussions for her mistake. That decision did not hold. She was later indicted criminally for the death of her patient. She also had her nursing license revoked.
Why? The hospital where she worked was threatened with Medicare reprisal if systemic issues were not addressed following the incident; for example, a bar-coding device was not available for Ms. Vaught to use prior to administering the vecuronium, and paralytic agents were stored unsafely in a Pyxis MedStation, readily available for any nurse to obtain via override.
In fact, the number of overrides performed by all nurses caring for Ms. Murphey in the days leading to her death was alarming, leading reviewers to assume that time to acquire medication for inpatients was a problem.
Ms. Vaught herself, stating the obvious on talk shows, said she should not have performed an override, that the situation was “not an emergency” and she should have taken time to check that Versed (midazolam) was available by the generic name and not the “VE” she entered as a search mechanism into the machine. She also stated she was “distracted” by a trainee assigned to her at the time.
We have all been there, feeling rushed to perform a task under stressful situations, skipping safety guidelines to sedate a patient while radiology is waiting. Someone is always on our a**, waiting to get to the next task, the next patient, the next admission, the next pseudo-emergency called nursing workload.
It never ends.
Which is why I wish to emphasize what the Ms. Vaught guilty verdict really means for nurses.
It means we must never forget that our actions have the potential to harm, even kill, our patients.
We must never forget that repercussions and reprisal may occur, whether personal guilt that may prove more damaging than the prison sentence Ms. Vaught might receive, or problems that could result if nurses attempt to hide or subvert medication issues.
In Ms. Vaught’s case, she did not document the medication that had been given to Ms. Murphey, facts the prosecution seized on to proclaim her guilt. Why? We can only guess at this point. But her claims of truthfulness need to be balanced by what occurred, and the facts are that she did not document the error after administering vecuronium that night.
When reflecting on this verdict, we need to remember a patient died, and she did so horribly, being unable to draw breath. This should never happen during our watch, ever, and as clinicians, we need to be vigilant.
In summary, protest if you believe justice has been too harsh or unfair, and that nurses may be fearful as a result. But please spare a moment to realize that someone should protest for Ms. Murphey as well. We cannot bring her back, nor can we right the system issues that may have led to her death.
But we should protest for safer systems, for improved staffing, for a need to catch our collective breaths, and a day to work and nurture patients when someone is not constantly on our a**. Only then will nurses be protected from unjust reprisal, from needing to be the lowest common denominator of guilt.
Ms. Goodman is a researcher and consultant in Libertyville, Ill. She disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
For 4 and a half years, I have followed the RaDonda Vaught medication error that led to the unfortunate death of a human being. I am not alone. Nurses across the country have followed the case with anxiety and fear, knowing a guilty verdict might have the potential to challenge basic tenets of care.
According to Kaiser Health News, nurses are “raging and quitting” following the announcement of a guilty verdict for two felonies: criminally negligent homicide and gross neglect of an impaired adult.
Thousands of nurses have claimed they could arrive in Nashville, Tenn., on May 13, the day Ms. Vaught is to be sentenced, to protest the conviction. Others have stated they believe justice is being conducted, as their sympathies lie with the victim, Charlene Murphey, who died 12 hours after being unable to draw breath, paralyzed from the inadvertent dose of vecuronium given intravenously by her nurse.
How should we feel as clinicians? according to sentencing guidelines?
My belief is that it is understandable to feel passionately about this case, including what it could mean to an era of “just culture” that nursing organizations have promoted. The concept of just culture looks at medication/nursing errors as opportunities for growth to avoid future errors, not as scenarios for punitive action. With the guilty verdict in Ms. Vaught’s case, nurses (and facilities) fear that nurses will avoid coming forward after mistakes, leading to cover-ups and a culture perspective.
Will nurses be hesitant to report errors (especially significant errors) that lead to patient harm? Will we fear retribution and reprisal for being truthful?
I believe that Ms. Vaught’s criminal case has changed little in the political landscape of caregiving. Before you let loose with a loud expletive (or two), hear me out.
When a patient dies from unintentional harm, someone must be held accountable. Society needs a scapegoat, and unfortunately, excrement slides downhill to the lowest common denominator, which may be the nurse. Initially, Ms. Vaught was contacted by her state licensing board (Tennessee) and informed there would be no professional repercussions for her mistake. That decision did not hold. She was later indicted criminally for the death of her patient. She also had her nursing license revoked.
Why? The hospital where she worked was threatened with Medicare reprisal if systemic issues were not addressed following the incident; for example, a bar-coding device was not available for Ms. Vaught to use prior to administering the vecuronium, and paralytic agents were stored unsafely in a Pyxis MedStation, readily available for any nurse to obtain via override.
In fact, the number of overrides performed by all nurses caring for Ms. Murphey in the days leading to her death was alarming, leading reviewers to assume that time to acquire medication for inpatients was a problem.
Ms. Vaught herself, stating the obvious on talk shows, said she should not have performed an override, that the situation was “not an emergency” and she should have taken time to check that Versed (midazolam) was available by the generic name and not the “VE” she entered as a search mechanism into the machine. She also stated she was “distracted” by a trainee assigned to her at the time.
We have all been there, feeling rushed to perform a task under stressful situations, skipping safety guidelines to sedate a patient while radiology is waiting. Someone is always on our a**, waiting to get to the next task, the next patient, the next admission, the next pseudo-emergency called nursing workload.
It never ends.
Which is why I wish to emphasize what the Ms. Vaught guilty verdict really means for nurses.
It means we must never forget that our actions have the potential to harm, even kill, our patients.
We must never forget that repercussions and reprisal may occur, whether personal guilt that may prove more damaging than the prison sentence Ms. Vaught might receive, or problems that could result if nurses attempt to hide or subvert medication issues.
In Ms. Vaught’s case, she did not document the medication that had been given to Ms. Murphey, facts the prosecution seized on to proclaim her guilt. Why? We can only guess at this point. But her claims of truthfulness need to be balanced by what occurred, and the facts are that she did not document the error after administering vecuronium that night.
When reflecting on this verdict, we need to remember a patient died, and she did so horribly, being unable to draw breath. This should never happen during our watch, ever, and as clinicians, we need to be vigilant.
In summary, protest if you believe justice has been too harsh or unfair, and that nurses may be fearful as a result. But please spare a moment to realize that someone should protest for Ms. Murphey as well. We cannot bring her back, nor can we right the system issues that may have led to her death.
But we should protest for safer systems, for improved staffing, for a need to catch our collective breaths, and a day to work and nurture patients when someone is not constantly on our a**. Only then will nurses be protected from unjust reprisal, from needing to be the lowest common denominator of guilt.
Ms. Goodman is a researcher and consultant in Libertyville, Ill. She disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
For 4 and a half years, I have followed the RaDonda Vaught medication error that led to the unfortunate death of a human being. I am not alone. Nurses across the country have followed the case with anxiety and fear, knowing a guilty verdict might have the potential to challenge basic tenets of care.
According to Kaiser Health News, nurses are “raging and quitting” following the announcement of a guilty verdict for two felonies: criminally negligent homicide and gross neglect of an impaired adult.
Thousands of nurses have claimed they could arrive in Nashville, Tenn., on May 13, the day Ms. Vaught is to be sentenced, to protest the conviction. Others have stated they believe justice is being conducted, as their sympathies lie with the victim, Charlene Murphey, who died 12 hours after being unable to draw breath, paralyzed from the inadvertent dose of vecuronium given intravenously by her nurse.
How should we feel as clinicians? according to sentencing guidelines?
My belief is that it is understandable to feel passionately about this case, including what it could mean to an era of “just culture” that nursing organizations have promoted. The concept of just culture looks at medication/nursing errors as opportunities for growth to avoid future errors, not as scenarios for punitive action. With the guilty verdict in Ms. Vaught’s case, nurses (and facilities) fear that nurses will avoid coming forward after mistakes, leading to cover-ups and a culture perspective.
Will nurses be hesitant to report errors (especially significant errors) that lead to patient harm? Will we fear retribution and reprisal for being truthful?
I believe that Ms. Vaught’s criminal case has changed little in the political landscape of caregiving. Before you let loose with a loud expletive (or two), hear me out.
When a patient dies from unintentional harm, someone must be held accountable. Society needs a scapegoat, and unfortunately, excrement slides downhill to the lowest common denominator, which may be the nurse. Initially, Ms. Vaught was contacted by her state licensing board (Tennessee) and informed there would be no professional repercussions for her mistake. That decision did not hold. She was later indicted criminally for the death of her patient. She also had her nursing license revoked.
Why? The hospital where she worked was threatened with Medicare reprisal if systemic issues were not addressed following the incident; for example, a bar-coding device was not available for Ms. Vaught to use prior to administering the vecuronium, and paralytic agents were stored unsafely in a Pyxis MedStation, readily available for any nurse to obtain via override.
In fact, the number of overrides performed by all nurses caring for Ms. Murphey in the days leading to her death was alarming, leading reviewers to assume that time to acquire medication for inpatients was a problem.
Ms. Vaught herself, stating the obvious on talk shows, said she should not have performed an override, that the situation was “not an emergency” and she should have taken time to check that Versed (midazolam) was available by the generic name and not the “VE” she entered as a search mechanism into the machine. She also stated she was “distracted” by a trainee assigned to her at the time.
We have all been there, feeling rushed to perform a task under stressful situations, skipping safety guidelines to sedate a patient while radiology is waiting. Someone is always on our a**, waiting to get to the next task, the next patient, the next admission, the next pseudo-emergency called nursing workload.
It never ends.
Which is why I wish to emphasize what the Ms. Vaught guilty verdict really means for nurses.
It means we must never forget that our actions have the potential to harm, even kill, our patients.
We must never forget that repercussions and reprisal may occur, whether personal guilt that may prove more damaging than the prison sentence Ms. Vaught might receive, or problems that could result if nurses attempt to hide or subvert medication issues.
In Ms. Vaught’s case, she did not document the medication that had been given to Ms. Murphey, facts the prosecution seized on to proclaim her guilt. Why? We can only guess at this point. But her claims of truthfulness need to be balanced by what occurred, and the facts are that she did not document the error after administering vecuronium that night.
When reflecting on this verdict, we need to remember a patient died, and she did so horribly, being unable to draw breath. This should never happen during our watch, ever, and as clinicians, we need to be vigilant.
In summary, protest if you believe justice has been too harsh or unfair, and that nurses may be fearful as a result. But please spare a moment to realize that someone should protest for Ms. Murphey as well. We cannot bring her back, nor can we right the system issues that may have led to her death.
But we should protest for safer systems, for improved staffing, for a need to catch our collective breaths, and a day to work and nurture patients when someone is not constantly on our a**. Only then will nurses be protected from unjust reprisal, from needing to be the lowest common denominator of guilt.
Ms. Goodman is a researcher and consultant in Libertyville, Ill. She disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
Young and older athletes show similar arrhythmia patterns with fQRS
The prevalence of exercise-induced arrhythmias in young athletes with fragmented QRS (fQRS) patterns in lead V1 was 27%, similar to that seen in adult athletes, based on data from nearly 700 individuals.
Recent data suggest that fQRS complex in lead V1 (fQRSV1) in healthy athletes may promote arrhythmias in the context of training-induced right ventricular remodeling, but the prevalence and significance in young athletes has not been well studied, Guilia Quinto, MD, of the University of Padova (Italy) said in a presentation at the annual congress of the European Association of Preventive Cardiology.
Dr. Quinto and colleagues assessed data from of young athletes on ventricular arrhythmias during exercise tests.
The study population included 684 young athletes with a mean age of 15 years; 64% were male. Baseline data collection included medical history, physical exam, resting ECG, standardized maximum exercise tolerance, and echocardiography evaluation.
The overall prevalence of fQRSV1 was 27%. Individuals with fQRSV1 were significantly less likely than those without fQRSV1 to be female (22% vs. 43%), and to present with a lower resting heart rate (66.98 beats per minute vs. 70.08 beats per minute).
Echocardiographic data showed that individuals with fQRSV1 had significantly different morphological and functional right ventricular characteristics.
Notably, right ventricular end-diastolic diameter was 20.42 mm/m2 among individuals with fQRSV1 and 19.81 mm/m2 in those without, a significant difference (P = .019), Dr. Quinto said. Tricuspid annulus plain systolic excursion also differed significantly; 24.33 mm and 23.75 mm for individuals with and without fQRSV1, respectively (P = .013).
However, the individuals with fQRSV1 showed no increased occurrence of any type of exercise-induced arrhythmias regardless of morphology or complexity, said Dr. Quinto.
The prevalence of common and uncommon arrhythmias among individuals with and without fQRSV1 was 31% versus 34% and 13% versus 11%, respectively; these differences were not significant.
The study findings were limited by the relatively small size, but were strengthened by the review of echocardiographic data by two independent physicians, she said.
The results show that the overall prevalence of fQRSV1 in young athletes is comparable with patterns seen in studies of adult athletes, and no differences in exercise-induced arrhythmias occurred despite differences in right ventricular characteristics, she concluded.
Expanded insight into evaluation
The ECG pattern identified in the current study is often encountered in the evaluation of athletes, but its importance was unknown, Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., said in an interview.
“Studies of ECG findings in athletes continues to inform us about which findings are important to evaluate. This study furthers our understanding of how to proceed,” and will serve as a guide for additional testing to reduce athlete risk, he said.
Looking ahead, “this study should guide clinicians about additional testing and evaluation when fQRS is present in adolescent athletes compared to adults,” Dr. Martinez noted. However, additional research is needed to determine which is the next best test, and whether the patient requires ongoing surveillance, or whether a single evaluation is sufficient, he said. “Further study should focus on best practices after fQRS is identified and whether outcomes can be linked to this finding.”
The study received no outside funding. Dr. Quinto and Dr. Martinez had no financial conflicts to disclose.
The prevalence of exercise-induced arrhythmias in young athletes with fragmented QRS (fQRS) patterns in lead V1 was 27%, similar to that seen in adult athletes, based on data from nearly 700 individuals.
Recent data suggest that fQRS complex in lead V1 (fQRSV1) in healthy athletes may promote arrhythmias in the context of training-induced right ventricular remodeling, but the prevalence and significance in young athletes has not been well studied, Guilia Quinto, MD, of the University of Padova (Italy) said in a presentation at the annual congress of the European Association of Preventive Cardiology.
Dr. Quinto and colleagues assessed data from of young athletes on ventricular arrhythmias during exercise tests.
The study population included 684 young athletes with a mean age of 15 years; 64% were male. Baseline data collection included medical history, physical exam, resting ECG, standardized maximum exercise tolerance, and echocardiography evaluation.
The overall prevalence of fQRSV1 was 27%. Individuals with fQRSV1 were significantly less likely than those without fQRSV1 to be female (22% vs. 43%), and to present with a lower resting heart rate (66.98 beats per minute vs. 70.08 beats per minute).
Echocardiographic data showed that individuals with fQRSV1 had significantly different morphological and functional right ventricular characteristics.
Notably, right ventricular end-diastolic diameter was 20.42 mm/m2 among individuals with fQRSV1 and 19.81 mm/m2 in those without, a significant difference (P = .019), Dr. Quinto said. Tricuspid annulus plain systolic excursion also differed significantly; 24.33 mm and 23.75 mm for individuals with and without fQRSV1, respectively (P = .013).
However, the individuals with fQRSV1 showed no increased occurrence of any type of exercise-induced arrhythmias regardless of morphology or complexity, said Dr. Quinto.
The prevalence of common and uncommon arrhythmias among individuals with and without fQRSV1 was 31% versus 34% and 13% versus 11%, respectively; these differences were not significant.
The study findings were limited by the relatively small size, but were strengthened by the review of echocardiographic data by two independent physicians, she said.
The results show that the overall prevalence of fQRSV1 in young athletes is comparable with patterns seen in studies of adult athletes, and no differences in exercise-induced arrhythmias occurred despite differences in right ventricular characteristics, she concluded.
Expanded insight into evaluation
The ECG pattern identified in the current study is often encountered in the evaluation of athletes, but its importance was unknown, Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., said in an interview.
“Studies of ECG findings in athletes continues to inform us about which findings are important to evaluate. This study furthers our understanding of how to proceed,” and will serve as a guide for additional testing to reduce athlete risk, he said.
Looking ahead, “this study should guide clinicians about additional testing and evaluation when fQRS is present in adolescent athletes compared to adults,” Dr. Martinez noted. However, additional research is needed to determine which is the next best test, and whether the patient requires ongoing surveillance, or whether a single evaluation is sufficient, he said. “Further study should focus on best practices after fQRS is identified and whether outcomes can be linked to this finding.”
The study received no outside funding. Dr. Quinto and Dr. Martinez had no financial conflicts to disclose.
The prevalence of exercise-induced arrhythmias in young athletes with fragmented QRS (fQRS) patterns in lead V1 was 27%, similar to that seen in adult athletes, based on data from nearly 700 individuals.
Recent data suggest that fQRS complex in lead V1 (fQRSV1) in healthy athletes may promote arrhythmias in the context of training-induced right ventricular remodeling, but the prevalence and significance in young athletes has not been well studied, Guilia Quinto, MD, of the University of Padova (Italy) said in a presentation at the annual congress of the European Association of Preventive Cardiology.
Dr. Quinto and colleagues assessed data from of young athletes on ventricular arrhythmias during exercise tests.
The study population included 684 young athletes with a mean age of 15 years; 64% were male. Baseline data collection included medical history, physical exam, resting ECG, standardized maximum exercise tolerance, and echocardiography evaluation.
The overall prevalence of fQRSV1 was 27%. Individuals with fQRSV1 were significantly less likely than those without fQRSV1 to be female (22% vs. 43%), and to present with a lower resting heart rate (66.98 beats per minute vs. 70.08 beats per minute).
Echocardiographic data showed that individuals with fQRSV1 had significantly different morphological and functional right ventricular characteristics.
Notably, right ventricular end-diastolic diameter was 20.42 mm/m2 among individuals with fQRSV1 and 19.81 mm/m2 in those without, a significant difference (P = .019), Dr. Quinto said. Tricuspid annulus plain systolic excursion also differed significantly; 24.33 mm and 23.75 mm for individuals with and without fQRSV1, respectively (P = .013).
However, the individuals with fQRSV1 showed no increased occurrence of any type of exercise-induced arrhythmias regardless of morphology or complexity, said Dr. Quinto.
The prevalence of common and uncommon arrhythmias among individuals with and without fQRSV1 was 31% versus 34% and 13% versus 11%, respectively; these differences were not significant.
The study findings were limited by the relatively small size, but were strengthened by the review of echocardiographic data by two independent physicians, she said.
The results show that the overall prevalence of fQRSV1 in young athletes is comparable with patterns seen in studies of adult athletes, and no differences in exercise-induced arrhythmias occurred despite differences in right ventricular characteristics, she concluded.
Expanded insight into evaluation
The ECG pattern identified in the current study is often encountered in the evaluation of athletes, but its importance was unknown, Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., said in an interview.
“Studies of ECG findings in athletes continues to inform us about which findings are important to evaluate. This study furthers our understanding of how to proceed,” and will serve as a guide for additional testing to reduce athlete risk, he said.
Looking ahead, “this study should guide clinicians about additional testing and evaluation when fQRS is present in adolescent athletes compared to adults,” Dr. Martinez noted. However, additional research is needed to determine which is the next best test, and whether the patient requires ongoing surveillance, or whether a single evaluation is sufficient, he said. “Further study should focus on best practices after fQRS is identified and whether outcomes can be linked to this finding.”
The study received no outside funding. Dr. Quinto and Dr. Martinez had no financial conflicts to disclose.
FROM ESC PREVENTIVE CARDIOLOGY 2022
30% of COVID patients in study developed long COVID
University of California, Los Angeles, researchers said in a study published in the Journal of General Internal Medicine.
The UCLA researchers studied 1,038 people enrolled in the UCLA COVID Ambulatory Program between April 2020 and February 2021 and found that 309 developed long COVID.
A long-COVID diagnosis came if a patient answering a questionnaire reported persistent symptoms 60-90 days after they were infected or hospitalized. The most persistent symptoms were fatigue (31%) and shortness of breath (15%) in hospitalized participants. Among outpatients, 16% reported losing sense of smell.
The study’s findings differ from earlier research. The University of California, Davis, for example, estimated that 10% of COVID-19 patients develop long-haul symptoms. A 2021 study from Penn State University found that more than half of worldwide COVID-19 patients would develop long COVID.
Part of the discrepancy can blamed on the fact there is no official, widely accepted definition of long COVID. The Centers for Disease Control and Prevention has said it means patients who experience “new, returning, or ongoing health problems 4 or more weeks after an initial infection” the coronavirus. The UCLA study, meanwhile, included patients still having symptoms 60-90 days after infection.
Still, the UCLA research team looked at demographics and clinical characteristics in an attempt to develop effective treatments.
People with a history of hospitalization, diabetes, and higher body mass index were most likely to develop long COVID, the researchers said. The kind of insurance the patients had also seemed to be a factor, though the researchers didn’t offer a reason why.
“Surprisingly, patients with commercial insurance had double the likelihood of developing [long COVID] compared to patients with Medicaid,” they wrote. “This association will be important to explore further to understand if insurance status in this group is representing unmeasured demographic factors or exposures.”
Older age and socioeconomic status were not associated with long COVID in the study – a surprise because those characteristics are often linked with severe illness and higher risk of death from COVID-19.
Weaknesses in the study included the subjective nature of how patients rated their symptoms and the limited number of symptoms evaluated.
“This study illustrates the need to follow diverse patient populations ... to understand the long COVID disease trajectory and evaluate how individual factors such as preexisting comorbidities, sociodemographic factors, vaccination status and virus variant type affect type and persistence of long COVID symptoms,” said Sun Yoo, MD, health sciences assistant clinical professor at UCLA.
A version of this article first appeared on WebMD.com.
University of California, Los Angeles, researchers said in a study published in the Journal of General Internal Medicine.
The UCLA researchers studied 1,038 people enrolled in the UCLA COVID Ambulatory Program between April 2020 and February 2021 and found that 309 developed long COVID.
A long-COVID diagnosis came if a patient answering a questionnaire reported persistent symptoms 60-90 days after they were infected or hospitalized. The most persistent symptoms were fatigue (31%) and shortness of breath (15%) in hospitalized participants. Among outpatients, 16% reported losing sense of smell.
The study’s findings differ from earlier research. The University of California, Davis, for example, estimated that 10% of COVID-19 patients develop long-haul symptoms. A 2021 study from Penn State University found that more than half of worldwide COVID-19 patients would develop long COVID.
Part of the discrepancy can blamed on the fact there is no official, widely accepted definition of long COVID. The Centers for Disease Control and Prevention has said it means patients who experience “new, returning, or ongoing health problems 4 or more weeks after an initial infection” the coronavirus. The UCLA study, meanwhile, included patients still having symptoms 60-90 days after infection.
Still, the UCLA research team looked at demographics and clinical characteristics in an attempt to develop effective treatments.
People with a history of hospitalization, diabetes, and higher body mass index were most likely to develop long COVID, the researchers said. The kind of insurance the patients had also seemed to be a factor, though the researchers didn’t offer a reason why.
“Surprisingly, patients with commercial insurance had double the likelihood of developing [long COVID] compared to patients with Medicaid,” they wrote. “This association will be important to explore further to understand if insurance status in this group is representing unmeasured demographic factors or exposures.”
Older age and socioeconomic status were not associated with long COVID in the study – a surprise because those characteristics are often linked with severe illness and higher risk of death from COVID-19.
Weaknesses in the study included the subjective nature of how patients rated their symptoms and the limited number of symptoms evaluated.
“This study illustrates the need to follow diverse patient populations ... to understand the long COVID disease trajectory and evaluate how individual factors such as preexisting comorbidities, sociodemographic factors, vaccination status and virus variant type affect type and persistence of long COVID symptoms,” said Sun Yoo, MD, health sciences assistant clinical professor at UCLA.
A version of this article first appeared on WebMD.com.
University of California, Los Angeles, researchers said in a study published in the Journal of General Internal Medicine.
The UCLA researchers studied 1,038 people enrolled in the UCLA COVID Ambulatory Program between April 2020 and February 2021 and found that 309 developed long COVID.
A long-COVID diagnosis came if a patient answering a questionnaire reported persistent symptoms 60-90 days after they were infected or hospitalized. The most persistent symptoms were fatigue (31%) and shortness of breath (15%) in hospitalized participants. Among outpatients, 16% reported losing sense of smell.
The study’s findings differ from earlier research. The University of California, Davis, for example, estimated that 10% of COVID-19 patients develop long-haul symptoms. A 2021 study from Penn State University found that more than half of worldwide COVID-19 patients would develop long COVID.
Part of the discrepancy can blamed on the fact there is no official, widely accepted definition of long COVID. The Centers for Disease Control and Prevention has said it means patients who experience “new, returning, or ongoing health problems 4 or more weeks after an initial infection” the coronavirus. The UCLA study, meanwhile, included patients still having symptoms 60-90 days after infection.
Still, the UCLA research team looked at demographics and clinical characteristics in an attempt to develop effective treatments.
People with a history of hospitalization, diabetes, and higher body mass index were most likely to develop long COVID, the researchers said. The kind of insurance the patients had also seemed to be a factor, though the researchers didn’t offer a reason why.
“Surprisingly, patients with commercial insurance had double the likelihood of developing [long COVID] compared to patients with Medicaid,” they wrote. “This association will be important to explore further to understand if insurance status in this group is representing unmeasured demographic factors or exposures.”
Older age and socioeconomic status were not associated with long COVID in the study – a surprise because those characteristics are often linked with severe illness and higher risk of death from COVID-19.
Weaknesses in the study included the subjective nature of how patients rated their symptoms and the limited number of symptoms evaluated.
“This study illustrates the need to follow diverse patient populations ... to understand the long COVID disease trajectory and evaluate how individual factors such as preexisting comorbidities, sociodemographic factors, vaccination status and virus variant type affect type and persistence of long COVID symptoms,” said Sun Yoo, MD, health sciences assistant clinical professor at UCLA.
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF GENERAL INTERNAL MEDICINE
The Empire strikes out against one physician’s homemade star fighter
The force is with Ukraine, always
Of all the things we could want from Star Wars, a lightsaber is at the top of the list. And someone is working on that. But second is probably the iconic X-wing. It was used to blow up the Death Star after all: Who wouldn’t want one?
A real-life star fighter may be outside our technological capabilities, but Dr. Akaki Lekiachvili of Atlanta has done the next best thing and constructed a two-thirds scale model to encourage kids to enter the sciences and, with the advent of the war in Ukraine, raise money for medical supplies to assist doctors in the embattled country. Perhaps unsurprisingly, Dr. Lekiachvili, originally from Georgia (the country, former Soviet republic, and previous target of Russian aggression in 2008), takes a dim view toward the invasion of Ukraine: “Russia is like the Evil Empire and Ukraine the Rebel Alliance.”
It’s been a long road finishing the X-Wing, as Dr. Lekiachvili started the project in 2016 and spent $60,000 on it, posting numerous updates on social media over that time, even attracting the attention of Luke Skywalker himself, actor Mark Hamill. Now that he’s done, he’s brought his model out to the public multiple times, delighting kids and adults alike. It can’t fly, but it has an engine and wheels so it can move, the wings can lock into attack position, the thrusters light up, and the voices of Obi-Wan Kenobi and R2-D2 guide children along as they sit in the cockpit.
Dr. Lekiachvili hopes to auction off his creation to a collector and donate the proceeds to Ukrainian charities, and we’re sure he’ll receive far more than the $60,000 he spent building his masterpiece. Now, if you’ll excuse us, we’re off to raid our bank accounts. We have a Death Star to destroy.
I’m a doctor, not a hologram
Telemedicine got a big boost during the early phase of the pandemic when hospitals and medical offices were off limits to anyone without COVID-19, but things have cooled off, telemedically speaking, since then. Well, NASA may have heated them up again. Or maybe it was Starfleet. Hmm, wait a second while we check. … No, it was NASA.
The space agency used the Microsoft Hololens Kinect camera and a personal computer with custom software from Aexa Aerospace to “holoport” NASA flight surgeon Josef Schmid up to the International Space Station, where he had a conversation with European Space Agency astronaut Thomas Pesquet, who wore an augmented reality headset that allowed him to see, hear, and interact with a 3D representation of the earthbound medical provider.
“Holoportation has been in use since at least 2016 by Microsoft, but this is the first use in such an extreme and remote environment such as space,” NASA said in a recent written statement, noting that the extreme house call took place on Oct. 8, 2021.
They seem to be forgetting about Star Trek, but we’ll let them slide on that one. Anyway, NASA didn’t share any details of the medical holoconversation – which may have strained the limits of HIPAA’s portability provisions – but Dr. Schmid described it as “a brand-new way of human exploration, where our human entity is able to travel off the planet. Our physical body is not there, but our human entity absolutely is there.”
Boldly doctoring where no doctor has gone before, you might say. You also might notice from the photo that Dr. Schmid went full Trekkie with a genuine Vulcan salute. Live long and prosper, Dr. Schmid. Live long and prosper.
Add electricity for umami
Salt makes everything taste better. Unfortunately, excess salt can cause problems for our bodies down the line, starting with high blood pressure and continuing on to heart disease and strokes. So how do we enjoy our deliciously salty foods without putting ourselves at risk? One answer may be electricity.
Researchers at Meiji University in Tokyo partnered with food and beverage maker Kirin to develop a set of electric chopsticks to boost the taste of salt in foods without the extra sodium. According to codeveloper and Meiji University professor Homei Miyashita, the device, worn like a watch with a wire attached to one of the chopsticks, “uses a weak electrical current to transmit sodium ions from food, through the chopsticks, to the mouth where they create a sense of saltines,” Reuters said.
In a country like Japan, where a lot of food is made with heavily sodium-based ingredients like miso and soy sauce, the average adult consumes 10 g of salt a day. That’s twice the recommended amount proposed by the World Health Organization. To not sacrifice bland food for better health, this device, which enhances the saltiness of the food consumed by 1.5 times, offers a fairly easy solution to a big public health crisis.
The chopsticks were tested by giving participants reduced-sodium miso soup. They told the researchers that the food was improved in “richness, sweetness, and overall tastiness,” the Guardian said.
Worried about having something electric in your mouth? Don’t worry. Kirin said in a statement that the electricity is very weak and not enough to affect the body.
The chopsticks are still in a prototype stage, but you may be able to get your pair as soon as next year. Until then, maybe be a little mindful of the salt.
Pet poop works in mysterious ways
We usually see it as a burden when our pets poop and pee in the house, but those bodily excretions may be able to tell us something about cancer-causing toxins running rampant in our homes.
Those toxins, known as aromatic amines, can be found in tobacco smoke and dyes used in make-up, textiles, and plastics. “Our findings suggest that pets are coming into contact with aromatic amines that leach from products in their household environment,” lead author Sridhar Chinthakindi, PhD, of NYU Langone Health, said in a statement from the university. “As these substances have been tied to bladder, colorectal, and other forms of cancer, our results may help explain why so many dogs and cats develop such diseases.”
Tobacco smoke was not the main source of the aromatic amines found in the poop and urine, but 70% of dogs and 80% of cats had these chemicals in their waste. The researchers looked for 30 types of aromatic amines plus nicotine in the sample and found 8. The chemical concentrations were much higher in cats than in dogs, possibly because of differences in exposure and metabolism between the two species, they suggested.
“If [pets] are getting exposed to toxins in our homes, then we had better take a closer look at our own exposure,” said senior author Kurunthachalam Kannan, PhD, of NYU Langone.
So the next time your pet poops or pees in the house, don’t get mad. Maybe they’re just trying to help you out by supplying some easy-to-collect samples.
The force is with Ukraine, always
Of all the things we could want from Star Wars, a lightsaber is at the top of the list. And someone is working on that. But second is probably the iconic X-wing. It was used to blow up the Death Star after all: Who wouldn’t want one?
A real-life star fighter may be outside our technological capabilities, but Dr. Akaki Lekiachvili of Atlanta has done the next best thing and constructed a two-thirds scale model to encourage kids to enter the sciences and, with the advent of the war in Ukraine, raise money for medical supplies to assist doctors in the embattled country. Perhaps unsurprisingly, Dr. Lekiachvili, originally from Georgia (the country, former Soviet republic, and previous target of Russian aggression in 2008), takes a dim view toward the invasion of Ukraine: “Russia is like the Evil Empire and Ukraine the Rebel Alliance.”
It’s been a long road finishing the X-Wing, as Dr. Lekiachvili started the project in 2016 and spent $60,000 on it, posting numerous updates on social media over that time, even attracting the attention of Luke Skywalker himself, actor Mark Hamill. Now that he’s done, he’s brought his model out to the public multiple times, delighting kids and adults alike. It can’t fly, but it has an engine and wheels so it can move, the wings can lock into attack position, the thrusters light up, and the voices of Obi-Wan Kenobi and R2-D2 guide children along as they sit in the cockpit.
Dr. Lekiachvili hopes to auction off his creation to a collector and donate the proceeds to Ukrainian charities, and we’re sure he’ll receive far more than the $60,000 he spent building his masterpiece. Now, if you’ll excuse us, we’re off to raid our bank accounts. We have a Death Star to destroy.
I’m a doctor, not a hologram
Telemedicine got a big boost during the early phase of the pandemic when hospitals and medical offices were off limits to anyone without COVID-19, but things have cooled off, telemedically speaking, since then. Well, NASA may have heated them up again. Or maybe it was Starfleet. Hmm, wait a second while we check. … No, it was NASA.
The space agency used the Microsoft Hololens Kinect camera and a personal computer with custom software from Aexa Aerospace to “holoport” NASA flight surgeon Josef Schmid up to the International Space Station, where he had a conversation with European Space Agency astronaut Thomas Pesquet, who wore an augmented reality headset that allowed him to see, hear, and interact with a 3D representation of the earthbound medical provider.
“Holoportation has been in use since at least 2016 by Microsoft, but this is the first use in such an extreme and remote environment such as space,” NASA said in a recent written statement, noting that the extreme house call took place on Oct. 8, 2021.
They seem to be forgetting about Star Trek, but we’ll let them slide on that one. Anyway, NASA didn’t share any details of the medical holoconversation – which may have strained the limits of HIPAA’s portability provisions – but Dr. Schmid described it as “a brand-new way of human exploration, where our human entity is able to travel off the planet. Our physical body is not there, but our human entity absolutely is there.”
Boldly doctoring where no doctor has gone before, you might say. You also might notice from the photo that Dr. Schmid went full Trekkie with a genuine Vulcan salute. Live long and prosper, Dr. Schmid. Live long and prosper.
Add electricity for umami
Salt makes everything taste better. Unfortunately, excess salt can cause problems for our bodies down the line, starting with high blood pressure and continuing on to heart disease and strokes. So how do we enjoy our deliciously salty foods without putting ourselves at risk? One answer may be electricity.
Researchers at Meiji University in Tokyo partnered with food and beverage maker Kirin to develop a set of electric chopsticks to boost the taste of salt in foods without the extra sodium. According to codeveloper and Meiji University professor Homei Miyashita, the device, worn like a watch with a wire attached to one of the chopsticks, “uses a weak electrical current to transmit sodium ions from food, through the chopsticks, to the mouth where they create a sense of saltines,” Reuters said.
In a country like Japan, where a lot of food is made with heavily sodium-based ingredients like miso and soy sauce, the average adult consumes 10 g of salt a day. That’s twice the recommended amount proposed by the World Health Organization. To not sacrifice bland food for better health, this device, which enhances the saltiness of the food consumed by 1.5 times, offers a fairly easy solution to a big public health crisis.
The chopsticks were tested by giving participants reduced-sodium miso soup. They told the researchers that the food was improved in “richness, sweetness, and overall tastiness,” the Guardian said.
Worried about having something electric in your mouth? Don’t worry. Kirin said in a statement that the electricity is very weak and not enough to affect the body.
The chopsticks are still in a prototype stage, but you may be able to get your pair as soon as next year. Until then, maybe be a little mindful of the salt.
Pet poop works in mysterious ways
We usually see it as a burden when our pets poop and pee in the house, but those bodily excretions may be able to tell us something about cancer-causing toxins running rampant in our homes.
Those toxins, known as aromatic amines, can be found in tobacco smoke and dyes used in make-up, textiles, and plastics. “Our findings suggest that pets are coming into contact with aromatic amines that leach from products in their household environment,” lead author Sridhar Chinthakindi, PhD, of NYU Langone Health, said in a statement from the university. “As these substances have been tied to bladder, colorectal, and other forms of cancer, our results may help explain why so many dogs and cats develop such diseases.”
Tobacco smoke was not the main source of the aromatic amines found in the poop and urine, but 70% of dogs and 80% of cats had these chemicals in their waste. The researchers looked for 30 types of aromatic amines plus nicotine in the sample and found 8. The chemical concentrations were much higher in cats than in dogs, possibly because of differences in exposure and metabolism between the two species, they suggested.
“If [pets] are getting exposed to toxins in our homes, then we had better take a closer look at our own exposure,” said senior author Kurunthachalam Kannan, PhD, of NYU Langone.
So the next time your pet poops or pees in the house, don’t get mad. Maybe they’re just trying to help you out by supplying some easy-to-collect samples.
The force is with Ukraine, always
Of all the things we could want from Star Wars, a lightsaber is at the top of the list. And someone is working on that. But second is probably the iconic X-wing. It was used to blow up the Death Star after all: Who wouldn’t want one?
A real-life star fighter may be outside our technological capabilities, but Dr. Akaki Lekiachvili of Atlanta has done the next best thing and constructed a two-thirds scale model to encourage kids to enter the sciences and, with the advent of the war in Ukraine, raise money for medical supplies to assist doctors in the embattled country. Perhaps unsurprisingly, Dr. Lekiachvili, originally from Georgia (the country, former Soviet republic, and previous target of Russian aggression in 2008), takes a dim view toward the invasion of Ukraine: “Russia is like the Evil Empire and Ukraine the Rebel Alliance.”
It’s been a long road finishing the X-Wing, as Dr. Lekiachvili started the project in 2016 and spent $60,000 on it, posting numerous updates on social media over that time, even attracting the attention of Luke Skywalker himself, actor Mark Hamill. Now that he’s done, he’s brought his model out to the public multiple times, delighting kids and adults alike. It can’t fly, but it has an engine and wheels so it can move, the wings can lock into attack position, the thrusters light up, and the voices of Obi-Wan Kenobi and R2-D2 guide children along as they sit in the cockpit.
Dr. Lekiachvili hopes to auction off his creation to a collector and donate the proceeds to Ukrainian charities, and we’re sure he’ll receive far more than the $60,000 he spent building his masterpiece. Now, if you’ll excuse us, we’re off to raid our bank accounts. We have a Death Star to destroy.
I’m a doctor, not a hologram
Telemedicine got a big boost during the early phase of the pandemic when hospitals and medical offices were off limits to anyone without COVID-19, but things have cooled off, telemedically speaking, since then. Well, NASA may have heated them up again. Or maybe it was Starfleet. Hmm, wait a second while we check. … No, it was NASA.
The space agency used the Microsoft Hololens Kinect camera and a personal computer with custom software from Aexa Aerospace to “holoport” NASA flight surgeon Josef Schmid up to the International Space Station, where he had a conversation with European Space Agency astronaut Thomas Pesquet, who wore an augmented reality headset that allowed him to see, hear, and interact with a 3D representation of the earthbound medical provider.
“Holoportation has been in use since at least 2016 by Microsoft, but this is the first use in such an extreme and remote environment such as space,” NASA said in a recent written statement, noting that the extreme house call took place on Oct. 8, 2021.
They seem to be forgetting about Star Trek, but we’ll let them slide on that one. Anyway, NASA didn’t share any details of the medical holoconversation – which may have strained the limits of HIPAA’s portability provisions – but Dr. Schmid described it as “a brand-new way of human exploration, where our human entity is able to travel off the planet. Our physical body is not there, but our human entity absolutely is there.”
Boldly doctoring where no doctor has gone before, you might say. You also might notice from the photo that Dr. Schmid went full Trekkie with a genuine Vulcan salute. Live long and prosper, Dr. Schmid. Live long and prosper.
Add electricity for umami
Salt makes everything taste better. Unfortunately, excess salt can cause problems for our bodies down the line, starting with high blood pressure and continuing on to heart disease and strokes. So how do we enjoy our deliciously salty foods without putting ourselves at risk? One answer may be electricity.
Researchers at Meiji University in Tokyo partnered with food and beverage maker Kirin to develop a set of electric chopsticks to boost the taste of salt in foods without the extra sodium. According to codeveloper and Meiji University professor Homei Miyashita, the device, worn like a watch with a wire attached to one of the chopsticks, “uses a weak electrical current to transmit sodium ions from food, through the chopsticks, to the mouth where they create a sense of saltines,” Reuters said.
In a country like Japan, where a lot of food is made with heavily sodium-based ingredients like miso and soy sauce, the average adult consumes 10 g of salt a day. That’s twice the recommended amount proposed by the World Health Organization. To not sacrifice bland food for better health, this device, which enhances the saltiness of the food consumed by 1.5 times, offers a fairly easy solution to a big public health crisis.
The chopsticks were tested by giving participants reduced-sodium miso soup. They told the researchers that the food was improved in “richness, sweetness, and overall tastiness,” the Guardian said.
Worried about having something electric in your mouth? Don’t worry. Kirin said in a statement that the electricity is very weak and not enough to affect the body.
The chopsticks are still in a prototype stage, but you may be able to get your pair as soon as next year. Until then, maybe be a little mindful of the salt.
Pet poop works in mysterious ways
We usually see it as a burden when our pets poop and pee in the house, but those bodily excretions may be able to tell us something about cancer-causing toxins running rampant in our homes.
Those toxins, known as aromatic amines, can be found in tobacco smoke and dyes used in make-up, textiles, and plastics. “Our findings suggest that pets are coming into contact with aromatic amines that leach from products in their household environment,” lead author Sridhar Chinthakindi, PhD, of NYU Langone Health, said in a statement from the university. “As these substances have been tied to bladder, colorectal, and other forms of cancer, our results may help explain why so many dogs and cats develop such diseases.”
Tobacco smoke was not the main source of the aromatic amines found in the poop and urine, but 70% of dogs and 80% of cats had these chemicals in their waste. The researchers looked for 30 types of aromatic amines plus nicotine in the sample and found 8. The chemical concentrations were much higher in cats than in dogs, possibly because of differences in exposure and metabolism between the two species, they suggested.
“If [pets] are getting exposed to toxins in our homes, then we had better take a closer look at our own exposure,” said senior author Kurunthachalam Kannan, PhD, of NYU Langone.
So the next time your pet poops or pees in the house, don’t get mad. Maybe they’re just trying to help you out by supplying some easy-to-collect samples.
Peripheral muscle fatigue limits post-COVID exercise
Peripheral muscle fatigue was the most common cause of exercise limitation in patients recovered from COVID-19 regardless of disease severity, in a study of nearly 300 individuals.
The source and magnitude of exercise intolerance in post–COVID-19 patients has not been well studied, said Mauricio Milani, MD, of Fitcordis Exercise Medicine Clinic, Brasilia, Brazil, in a presentation at the annual congress of the European Association of Preventive Cardiology.
To assess exercise intolerance, the researchers performed cardiopulmonary exercise testing (CPET) on 144 adults who had recovered from COVID-19 and 144 matched controls who had not had COVID-19. The average age of the participants was 43 years, and 57% were male. COVID-19 was defined as mild, moderate, or severe in 60%, 21%, and 19% of the cases, respectively.
Residual symptoms were present in 41% of cases. CPET was performed at roughly 14 weeks after disease onset.
Among the COVID-19 patients, most of the CPET limitations (92%) were caused by muscle fatigue; cardiovascular limitations were noted in 2%, and pulmonary limitations were noted in 6%.
Data from the post-COVID CPET showed differences in peak oxygen consumption, as well as the first and second ventilatory thresholds (VT1 and VT2) between COVID-19 patients and controls, and with lower values related to higher illness severities, Dr. Milani said. Heart rate also varied according to illness severity, with lower values significantly related to higher illness severities and significant differences between COVID patients and controls.
A total of 42 individuals with COVID-19 had previous CPET data for comparison (27 with mild disease and 15 with moderate or severe disease), Dr. Milani said. In the subgroup with mild disease, the only significant difference in CPET results before and after COVID-19 was peak speed. In the moderate/severe group, the researchers observed higher reductions in peak speed and also reductions in oxygen consumption at peak and thresholds.
However, peak oxygen flows were not different before and after COVID-19 in either the mild or moderate/severe subgroups, Dr. Milani said.
The study findings were limited in part by the relatively small study population; however, the results indicate that peripheral muscle fatigue is the primary etiology in exercise limitation in post–COVID-19 patients.
“Our data suggest that treatment should emphasize comprehensive rehabilitation programs, including aerobic and muscle strengthening components,” Dr. Milani concluded.
COVID challenges remain unclear
“After COVID, patients often display a postviral syndrome with a wide range of symptoms,” Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., in an interview said. “These conditions frequently lead to a sense of tiredness and weakness, pain, difficulty concentrating, and headaches that linger after the viral infection has cleared,” and these symptoms may continue for weeks.
However, this scenario is not unique to COVID-19: “This study confirms the importance of muscle fatigue in recovery,” said Dr. Martinez. “Recovery from viral illness requires hydration, sleep and slow progression return to exercise.” Consequently, Dr. Martinez said he was not surprised by the current study findings.
The take-home message for clinicians is to be aware that COVID-19 can have postviral syndrome, as is common after other infections, Dr. Martinez noted. The findings provide a starting point for discussing concerns with patients and explaining that a slow return to normal with usual care is expected. “Time to recovery will vary by individual,” he said. “Additional research is needed to identify which specific therapies are most important to help reduce time to recovery, and what new therapies could be developed to help facilitate muscle fatigue recovery and reduce time needed to recover.”
The study was supported by CAPES and CNPq. Dr. Milani had no financial conflicts to disclose. Dr. Martinez had no financial conflicts to disclose.
Peripheral muscle fatigue was the most common cause of exercise limitation in patients recovered from COVID-19 regardless of disease severity, in a study of nearly 300 individuals.
The source and magnitude of exercise intolerance in post–COVID-19 patients has not been well studied, said Mauricio Milani, MD, of Fitcordis Exercise Medicine Clinic, Brasilia, Brazil, in a presentation at the annual congress of the European Association of Preventive Cardiology.
To assess exercise intolerance, the researchers performed cardiopulmonary exercise testing (CPET) on 144 adults who had recovered from COVID-19 and 144 matched controls who had not had COVID-19. The average age of the participants was 43 years, and 57% were male. COVID-19 was defined as mild, moderate, or severe in 60%, 21%, and 19% of the cases, respectively.
Residual symptoms were present in 41% of cases. CPET was performed at roughly 14 weeks after disease onset.
Among the COVID-19 patients, most of the CPET limitations (92%) were caused by muscle fatigue; cardiovascular limitations were noted in 2%, and pulmonary limitations were noted in 6%.
Data from the post-COVID CPET showed differences in peak oxygen consumption, as well as the first and second ventilatory thresholds (VT1 and VT2) between COVID-19 patients and controls, and with lower values related to higher illness severities, Dr. Milani said. Heart rate also varied according to illness severity, with lower values significantly related to higher illness severities and significant differences between COVID patients and controls.
A total of 42 individuals with COVID-19 had previous CPET data for comparison (27 with mild disease and 15 with moderate or severe disease), Dr. Milani said. In the subgroup with mild disease, the only significant difference in CPET results before and after COVID-19 was peak speed. In the moderate/severe group, the researchers observed higher reductions in peak speed and also reductions in oxygen consumption at peak and thresholds.
However, peak oxygen flows were not different before and after COVID-19 in either the mild or moderate/severe subgroups, Dr. Milani said.
The study findings were limited in part by the relatively small study population; however, the results indicate that peripheral muscle fatigue is the primary etiology in exercise limitation in post–COVID-19 patients.
“Our data suggest that treatment should emphasize comprehensive rehabilitation programs, including aerobic and muscle strengthening components,” Dr. Milani concluded.
COVID challenges remain unclear
“After COVID, patients often display a postviral syndrome with a wide range of symptoms,” Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., in an interview said. “These conditions frequently lead to a sense of tiredness and weakness, pain, difficulty concentrating, and headaches that linger after the viral infection has cleared,” and these symptoms may continue for weeks.
However, this scenario is not unique to COVID-19: “This study confirms the importance of muscle fatigue in recovery,” said Dr. Martinez. “Recovery from viral illness requires hydration, sleep and slow progression return to exercise.” Consequently, Dr. Martinez said he was not surprised by the current study findings.
The take-home message for clinicians is to be aware that COVID-19 can have postviral syndrome, as is common after other infections, Dr. Martinez noted. The findings provide a starting point for discussing concerns with patients and explaining that a slow return to normal with usual care is expected. “Time to recovery will vary by individual,” he said. “Additional research is needed to identify which specific therapies are most important to help reduce time to recovery, and what new therapies could be developed to help facilitate muscle fatigue recovery and reduce time needed to recover.”
The study was supported by CAPES and CNPq. Dr. Milani had no financial conflicts to disclose. Dr. Martinez had no financial conflicts to disclose.
Peripheral muscle fatigue was the most common cause of exercise limitation in patients recovered from COVID-19 regardless of disease severity, in a study of nearly 300 individuals.
The source and magnitude of exercise intolerance in post–COVID-19 patients has not been well studied, said Mauricio Milani, MD, of Fitcordis Exercise Medicine Clinic, Brasilia, Brazil, in a presentation at the annual congress of the European Association of Preventive Cardiology.
To assess exercise intolerance, the researchers performed cardiopulmonary exercise testing (CPET) on 144 adults who had recovered from COVID-19 and 144 matched controls who had not had COVID-19. The average age of the participants was 43 years, and 57% were male. COVID-19 was defined as mild, moderate, or severe in 60%, 21%, and 19% of the cases, respectively.
Residual symptoms were present in 41% of cases. CPET was performed at roughly 14 weeks after disease onset.
Among the COVID-19 patients, most of the CPET limitations (92%) were caused by muscle fatigue; cardiovascular limitations were noted in 2%, and pulmonary limitations were noted in 6%.
Data from the post-COVID CPET showed differences in peak oxygen consumption, as well as the first and second ventilatory thresholds (VT1 and VT2) between COVID-19 patients and controls, and with lower values related to higher illness severities, Dr. Milani said. Heart rate also varied according to illness severity, with lower values significantly related to higher illness severities and significant differences between COVID patients and controls.
A total of 42 individuals with COVID-19 had previous CPET data for comparison (27 with mild disease and 15 with moderate or severe disease), Dr. Milani said. In the subgroup with mild disease, the only significant difference in CPET results before and after COVID-19 was peak speed. In the moderate/severe group, the researchers observed higher reductions in peak speed and also reductions in oxygen consumption at peak and thresholds.
However, peak oxygen flows were not different before and after COVID-19 in either the mild or moderate/severe subgroups, Dr. Milani said.
The study findings were limited in part by the relatively small study population; however, the results indicate that peripheral muscle fatigue is the primary etiology in exercise limitation in post–COVID-19 patients.
“Our data suggest that treatment should emphasize comprehensive rehabilitation programs, including aerobic and muscle strengthening components,” Dr. Milani concluded.
COVID challenges remain unclear
“After COVID, patients often display a postviral syndrome with a wide range of symptoms,” Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., in an interview said. “These conditions frequently lead to a sense of tiredness and weakness, pain, difficulty concentrating, and headaches that linger after the viral infection has cleared,” and these symptoms may continue for weeks.
However, this scenario is not unique to COVID-19: “This study confirms the importance of muscle fatigue in recovery,” said Dr. Martinez. “Recovery from viral illness requires hydration, sleep and slow progression return to exercise.” Consequently, Dr. Martinez said he was not surprised by the current study findings.
The take-home message for clinicians is to be aware that COVID-19 can have postviral syndrome, as is common after other infections, Dr. Martinez noted. The findings provide a starting point for discussing concerns with patients and explaining that a slow return to normal with usual care is expected. “Time to recovery will vary by individual,” he said. “Additional research is needed to identify which specific therapies are most important to help reduce time to recovery, and what new therapies could be developed to help facilitate muscle fatigue recovery and reduce time needed to recover.”
The study was supported by CAPES and CNPq. Dr. Milani had no financial conflicts to disclose. Dr. Martinez had no financial conflicts to disclose.
FROM ESC PREVENTIVE CARDIOLOGY 2022