User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
‘Round Face’: A Viral Term’s Real Diagnostic Implications
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.
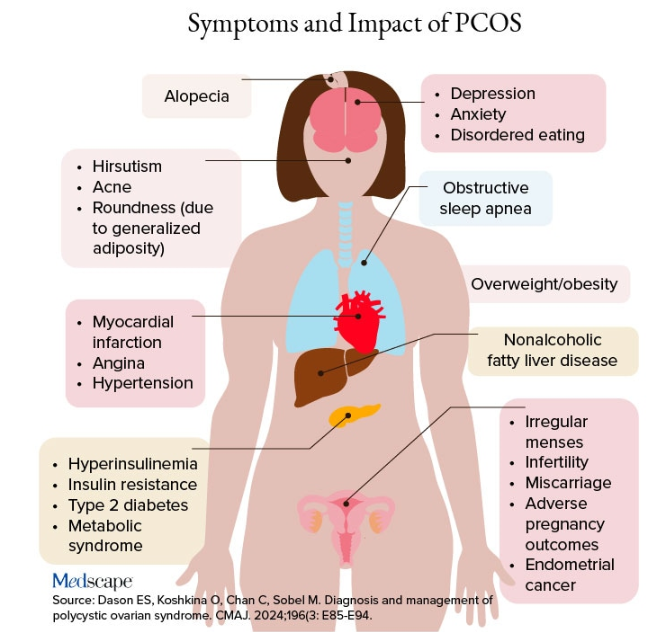
PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
Postpartum Exercise Reduces Depression and Anxiety Symptoms
TOPLINE:
Postpartum exercise reduces the severity of depressive and anxiety symptoms. Initiating exercise within 12 weeks post partum is linked to greater reductions in depressive symptoms.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis including 35 studies with a total of 4072 participants.
- The review included randomized controlled trials and nonrandomized interventions examining the impact of postpartum exercise on depression and anxiety.
- Participants were postpartum individuals within the first year after childbirth, with interventions including various types of exercise.
- Data sources included online databases with data up to January 2024, reference lists, and hand searches.
- The Grading of Recommendations, Assessment, Development, and Evaluation framework was used to assess the certainty of evidence.
TAKEAWAY:
- Postpartum exercise-only interventions resulted in a moderate reduction in the severity of depressive symptoms (standardized mean difference [SMD], –0.52; 95% CI, –0.80 to –0.24).
- Exercise-only interventions were associated with a small reduction in the severity of anxiety symptoms (SMD, –0.25; 95% CI, –0.43 to –0.08).
- Initiating exercise within 12 weeks post partum was associated with a greater reduction in depressive symptoms, compared with starting later.
- Postpartum exercise was associated with a 45% reduction in the odds of developing depression (odds ratio, 0.55; 95% CI, 0.32-0.95).
IN PRACTICE:
“Further investigation should aim to investigate the effects of postpartum exercise in individuals who experienced perinatal complications and in those who had limitations to exercise during pregnancy. Additionally, more investigation is required to address the possible lasting effects of postpartum exercise on maternal mental health as there were very limited studies reporting on this outcome,” the authors of the study wrote.
SOURCE:
This study was led by Margie H. Davenport, University of Alberta in Edmonton, Canada. It was published online in British Journal of Sports Medicine.
LIMITATIONS:
This study’s limitations included high heterogeneity among included studies, small sample sizes in some studies, and the combination of exercise with other interventions in some cases. These factors may have affected the generalizability and precision of the findings.
DISCLOSURES:
This study was funded by the Christenson Professorship in Active Healthy Living. Davenport is funded by a Christenson Professorship in Active Healthy Living. One coauthor is funded by the Université du Québec à Trois-Rivières research chair in physical activity and maternal and neonatal health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Postpartum exercise reduces the severity of depressive and anxiety symptoms. Initiating exercise within 12 weeks post partum is linked to greater reductions in depressive symptoms.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis including 35 studies with a total of 4072 participants.
- The review included randomized controlled trials and nonrandomized interventions examining the impact of postpartum exercise on depression and anxiety.
- Participants were postpartum individuals within the first year after childbirth, with interventions including various types of exercise.
- Data sources included online databases with data up to January 2024, reference lists, and hand searches.
- The Grading of Recommendations, Assessment, Development, and Evaluation framework was used to assess the certainty of evidence.
TAKEAWAY:
- Postpartum exercise-only interventions resulted in a moderate reduction in the severity of depressive symptoms (standardized mean difference [SMD], –0.52; 95% CI, –0.80 to –0.24).
- Exercise-only interventions were associated with a small reduction in the severity of anxiety symptoms (SMD, –0.25; 95% CI, –0.43 to –0.08).
- Initiating exercise within 12 weeks post partum was associated with a greater reduction in depressive symptoms, compared with starting later.
- Postpartum exercise was associated with a 45% reduction in the odds of developing depression (odds ratio, 0.55; 95% CI, 0.32-0.95).
IN PRACTICE:
“Further investigation should aim to investigate the effects of postpartum exercise in individuals who experienced perinatal complications and in those who had limitations to exercise during pregnancy. Additionally, more investigation is required to address the possible lasting effects of postpartum exercise on maternal mental health as there were very limited studies reporting on this outcome,” the authors of the study wrote.
SOURCE:
This study was led by Margie H. Davenport, University of Alberta in Edmonton, Canada. It was published online in British Journal of Sports Medicine.
LIMITATIONS:
This study’s limitations included high heterogeneity among included studies, small sample sizes in some studies, and the combination of exercise with other interventions in some cases. These factors may have affected the generalizability and precision of the findings.
DISCLOSURES:
This study was funded by the Christenson Professorship in Active Healthy Living. Davenport is funded by a Christenson Professorship in Active Healthy Living. One coauthor is funded by the Université du Québec à Trois-Rivières research chair in physical activity and maternal and neonatal health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Postpartum exercise reduces the severity of depressive and anxiety symptoms. Initiating exercise within 12 weeks post partum is linked to greater reductions in depressive symptoms.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis including 35 studies with a total of 4072 participants.
- The review included randomized controlled trials and nonrandomized interventions examining the impact of postpartum exercise on depression and anxiety.
- Participants were postpartum individuals within the first year after childbirth, with interventions including various types of exercise.
- Data sources included online databases with data up to January 2024, reference lists, and hand searches.
- The Grading of Recommendations, Assessment, Development, and Evaluation framework was used to assess the certainty of evidence.
TAKEAWAY:
- Postpartum exercise-only interventions resulted in a moderate reduction in the severity of depressive symptoms (standardized mean difference [SMD], –0.52; 95% CI, –0.80 to –0.24).
- Exercise-only interventions were associated with a small reduction in the severity of anxiety symptoms (SMD, –0.25; 95% CI, –0.43 to –0.08).
- Initiating exercise within 12 weeks post partum was associated with a greater reduction in depressive symptoms, compared with starting later.
- Postpartum exercise was associated with a 45% reduction in the odds of developing depression (odds ratio, 0.55; 95% CI, 0.32-0.95).
IN PRACTICE:
“Further investigation should aim to investigate the effects of postpartum exercise in individuals who experienced perinatal complications and in those who had limitations to exercise during pregnancy. Additionally, more investigation is required to address the possible lasting effects of postpartum exercise on maternal mental health as there were very limited studies reporting on this outcome,” the authors of the study wrote.
SOURCE:
This study was led by Margie H. Davenport, University of Alberta in Edmonton, Canada. It was published online in British Journal of Sports Medicine.
LIMITATIONS:
This study’s limitations included high heterogeneity among included studies, small sample sizes in some studies, and the combination of exercise with other interventions in some cases. These factors may have affected the generalizability and precision of the findings.
DISCLOSURES:
This study was funded by the Christenson Professorship in Active Healthy Living. Davenport is funded by a Christenson Professorship in Active Healthy Living. One coauthor is funded by the Université du Québec à Trois-Rivières research chair in physical activity and maternal and neonatal health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Do Patients on Anti-Obesity Drugs Decrease Alcohol Use?
SAN ANTONIO —
The findings, from surveys of more than 14,000 participants in WeightWatchers’ telehealth weight management program, were presented on November 6 at the Obesity Society’s Obesity Week 2024 meeting by the company’s Chief Nutrition Officer, Michelle I. Cardel, PhD, RD, based in Gainesville, Florida.
Similar reductions in alcohol consumption were seen in people taking different classes of AOMs, suggesting “an additional mechanism by which AOMs reduce energy intake, and also signal a potential role for these medications to reduce alcohol use,” Cardel said, adding “Clinicians treating individuals for obesity may consider anti-obesity medications particularly among those who report higher alcohol intake.”
Asked to comment, session moderator and obesity researcher Joseph A. Skelton, MD, professor of pediatrics at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said, “I think there are some overlapping pathways there, possibly a reward system or something like that in the brain. I don’t think we know exactly what the end result will be as a potential use of the medications. But there’s a signal that needs to be investigated more.”
Cardel noted that there was one previous large cohort study finding that semaglutide was associated with a lower risk for alcohol use disorder, and another study that analyzed social media threads of people saying they’d quit drinking after starting a GLP-1 drug. But this new study is the first to examine the relationship with different classes of AOMs and to quantify the amount of alcohol consumed.
About Half Reported Reduced Alcohol Consumption, Regardless the AOM Class
The study included 14,053 WeightWatchers’ telehealth program participants who initiated an AOM between January 2022 and August 2023 and refilled the same AOM between October and November 2023. Those who had previously used AOMs before coming to the program or who had undergone bariatric surgery were excluded.
Participants had a mean age of 43 years, were 86% women, were 60% White, and had a mean body mass index of 36. They were surveyed about their weekly alcohol use prior to AOM initiation and again at the time of AOM refill.
At baseline, they were divided into categories of 0 (no alcohol use; n = 6562), category 1 (one to three drinks for women and one to six for men; n = 5948), category 2 (4-6 for women and 7-14 for men; n = 1216), and category 3 (≥ 7 for women and ≥ 15 for men; n = 327).
At the second survey, 24% reported decreased drinking after starting an AOM, 71% reported no change, and 4% reported increased drinking (P < .0001). But when just the 7491 individuals who reported any alcohol use at baseline were included, 45% reported decreased drinking after starting an AOM, 52% reported no change, and only 2% reported increased drinking.
The decrease in drinking with AOM use rose with greater alcohol use at baseline, from 37% for category 1, 76% for category 2, and 91% for category 3. The proportions reporting increased drinking were just 3%, 1%, and 0%, respectively. The adjusted odds ratios (ORs) for decreasing drinking were 5.97 for category 2 (P < .0001) and 19.18 for category 3 (P < .0001) vs category 1.
The proportions reporting reduced drinking were similar across AOM classes: 51% for metformin, 46% for bupropion/naltrexone, 46% for first-generation GLP-1s (Saxenda, Trulicity, and Victoza), and 45% for the second-generation GLP-1 drugs (Mounjaro, Ozempic, Rybelsus, Wegovy, and Zepbound). All were statistically significant at P < .0001.
The highest proportion reporting increased drinking was 4% for bupropion/naltrexone. Compared with women, men were significantly more likely to report decreased drinking with AOM use (adjusted OR, 0.74; P < .001), but there were no differences by race/ethnicity or age.
Compared with those who had overweight, those in obesity classes I, II, and III were all more likely to decrease drinking with AOM use, with adjusted ORs of 1.26 (P = .0045), 1.49 (P < .001), and 1.63 (P < .001), respectively.
Mechanisms Appear Both Biological and Behavioral
During the discussion, Cardel said that qualitative assessments with participants suggest that there are at least two mechanisms behind this phenomenon: One biological and the other intentional.
“What we hear from them is twofold, one, particularly amongst those folks on GLP-1 medications, we’re hearing that physiologically, they feel different with the medications, that their cravings for alcohol are decreased, and that when they do choose to drink that there’s often a very much a negative reinforcement ... I’ve had a patient tell me, ‘I used to be able to have two or three margaritas, and maybe I didn’t feel like the best I’d ever felt in the morning, but I was okay. And now if I have two or three drinks, I will be throwing up for 5 hours, and it’s the worst hangover I’ve ever had in my life.’ And so it very much creates that negative reinforcement loop.”
But at the same time, “folks who are coming to us and seeking these medications are very much on a on a health-based journey. That’s what they tell us. The majority of our patients are there to improve their health. We rarely hear about the vanity or aesthetic part of it. So perhaps it’s that, in terms of trying to improve their health, they’re also trying to reduce their alcohol consumption, either just for their overall health or also as a means of trying to decrease their overall calorie consumption.”
In future research, Cardel said, “we want to examine whether the anti-obesity medications are more successful at reducing alcohol use compared to non-pharmacological weight management interventions, as we know that people often reduce their alcohol consumption on a weight management journey as a means of prioritizing their calories for food and decreasing the calories from alcohol.”
Cardel and all the study coauthors were employees and shareholders at WeightWatchers at the time the research was conducted. Skelton is editor in chief of the journal Childhood Obesity.
A version of this article appeared on Medscape.com.
SAN ANTONIO —
The findings, from surveys of more than 14,000 participants in WeightWatchers’ telehealth weight management program, were presented on November 6 at the Obesity Society’s Obesity Week 2024 meeting by the company’s Chief Nutrition Officer, Michelle I. Cardel, PhD, RD, based in Gainesville, Florida.
Similar reductions in alcohol consumption were seen in people taking different classes of AOMs, suggesting “an additional mechanism by which AOMs reduce energy intake, and also signal a potential role for these medications to reduce alcohol use,” Cardel said, adding “Clinicians treating individuals for obesity may consider anti-obesity medications particularly among those who report higher alcohol intake.”
Asked to comment, session moderator and obesity researcher Joseph A. Skelton, MD, professor of pediatrics at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said, “I think there are some overlapping pathways there, possibly a reward system or something like that in the brain. I don’t think we know exactly what the end result will be as a potential use of the medications. But there’s a signal that needs to be investigated more.”
Cardel noted that there was one previous large cohort study finding that semaglutide was associated with a lower risk for alcohol use disorder, and another study that analyzed social media threads of people saying they’d quit drinking after starting a GLP-1 drug. But this new study is the first to examine the relationship with different classes of AOMs and to quantify the amount of alcohol consumed.
About Half Reported Reduced Alcohol Consumption, Regardless the AOM Class
The study included 14,053 WeightWatchers’ telehealth program participants who initiated an AOM between January 2022 and August 2023 and refilled the same AOM between October and November 2023. Those who had previously used AOMs before coming to the program or who had undergone bariatric surgery were excluded.
Participants had a mean age of 43 years, were 86% women, were 60% White, and had a mean body mass index of 36. They were surveyed about their weekly alcohol use prior to AOM initiation and again at the time of AOM refill.
At baseline, they were divided into categories of 0 (no alcohol use; n = 6562), category 1 (one to three drinks for women and one to six for men; n = 5948), category 2 (4-6 for women and 7-14 for men; n = 1216), and category 3 (≥ 7 for women and ≥ 15 for men; n = 327).
At the second survey, 24% reported decreased drinking after starting an AOM, 71% reported no change, and 4% reported increased drinking (P < .0001). But when just the 7491 individuals who reported any alcohol use at baseline were included, 45% reported decreased drinking after starting an AOM, 52% reported no change, and only 2% reported increased drinking.
The decrease in drinking with AOM use rose with greater alcohol use at baseline, from 37% for category 1, 76% for category 2, and 91% for category 3. The proportions reporting increased drinking were just 3%, 1%, and 0%, respectively. The adjusted odds ratios (ORs) for decreasing drinking were 5.97 for category 2 (P < .0001) and 19.18 for category 3 (P < .0001) vs category 1.
The proportions reporting reduced drinking were similar across AOM classes: 51% for metformin, 46% for bupropion/naltrexone, 46% for first-generation GLP-1s (Saxenda, Trulicity, and Victoza), and 45% for the second-generation GLP-1 drugs (Mounjaro, Ozempic, Rybelsus, Wegovy, and Zepbound). All were statistically significant at P < .0001.
The highest proportion reporting increased drinking was 4% for bupropion/naltrexone. Compared with women, men were significantly more likely to report decreased drinking with AOM use (adjusted OR, 0.74; P < .001), but there were no differences by race/ethnicity or age.
Compared with those who had overweight, those in obesity classes I, II, and III were all more likely to decrease drinking with AOM use, with adjusted ORs of 1.26 (P = .0045), 1.49 (P < .001), and 1.63 (P < .001), respectively.
Mechanisms Appear Both Biological and Behavioral
During the discussion, Cardel said that qualitative assessments with participants suggest that there are at least two mechanisms behind this phenomenon: One biological and the other intentional.
“What we hear from them is twofold, one, particularly amongst those folks on GLP-1 medications, we’re hearing that physiologically, they feel different with the medications, that their cravings for alcohol are decreased, and that when they do choose to drink that there’s often a very much a negative reinforcement ... I’ve had a patient tell me, ‘I used to be able to have two or three margaritas, and maybe I didn’t feel like the best I’d ever felt in the morning, but I was okay. And now if I have two or three drinks, I will be throwing up for 5 hours, and it’s the worst hangover I’ve ever had in my life.’ And so it very much creates that negative reinforcement loop.”
But at the same time, “folks who are coming to us and seeking these medications are very much on a on a health-based journey. That’s what they tell us. The majority of our patients are there to improve their health. We rarely hear about the vanity or aesthetic part of it. So perhaps it’s that, in terms of trying to improve their health, they’re also trying to reduce their alcohol consumption, either just for their overall health or also as a means of trying to decrease their overall calorie consumption.”
In future research, Cardel said, “we want to examine whether the anti-obesity medications are more successful at reducing alcohol use compared to non-pharmacological weight management interventions, as we know that people often reduce their alcohol consumption on a weight management journey as a means of prioritizing their calories for food and decreasing the calories from alcohol.”
Cardel and all the study coauthors were employees and shareholders at WeightWatchers at the time the research was conducted. Skelton is editor in chief of the journal Childhood Obesity.
A version of this article appeared on Medscape.com.
SAN ANTONIO —
The findings, from surveys of more than 14,000 participants in WeightWatchers’ telehealth weight management program, were presented on November 6 at the Obesity Society’s Obesity Week 2024 meeting by the company’s Chief Nutrition Officer, Michelle I. Cardel, PhD, RD, based in Gainesville, Florida.
Similar reductions in alcohol consumption were seen in people taking different classes of AOMs, suggesting “an additional mechanism by which AOMs reduce energy intake, and also signal a potential role for these medications to reduce alcohol use,” Cardel said, adding “Clinicians treating individuals for obesity may consider anti-obesity medications particularly among those who report higher alcohol intake.”
Asked to comment, session moderator and obesity researcher Joseph A. Skelton, MD, professor of pediatrics at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said, “I think there are some overlapping pathways there, possibly a reward system or something like that in the brain. I don’t think we know exactly what the end result will be as a potential use of the medications. But there’s a signal that needs to be investigated more.”
Cardel noted that there was one previous large cohort study finding that semaglutide was associated with a lower risk for alcohol use disorder, and another study that analyzed social media threads of people saying they’d quit drinking after starting a GLP-1 drug. But this new study is the first to examine the relationship with different classes of AOMs and to quantify the amount of alcohol consumed.
About Half Reported Reduced Alcohol Consumption, Regardless the AOM Class
The study included 14,053 WeightWatchers’ telehealth program participants who initiated an AOM between January 2022 and August 2023 and refilled the same AOM between October and November 2023. Those who had previously used AOMs before coming to the program or who had undergone bariatric surgery were excluded.
Participants had a mean age of 43 years, were 86% women, were 60% White, and had a mean body mass index of 36. They were surveyed about their weekly alcohol use prior to AOM initiation and again at the time of AOM refill.
At baseline, they were divided into categories of 0 (no alcohol use; n = 6562), category 1 (one to three drinks for women and one to six for men; n = 5948), category 2 (4-6 for women and 7-14 for men; n = 1216), and category 3 (≥ 7 for women and ≥ 15 for men; n = 327).
At the second survey, 24% reported decreased drinking after starting an AOM, 71% reported no change, and 4% reported increased drinking (P < .0001). But when just the 7491 individuals who reported any alcohol use at baseline were included, 45% reported decreased drinking after starting an AOM, 52% reported no change, and only 2% reported increased drinking.
The decrease in drinking with AOM use rose with greater alcohol use at baseline, from 37% for category 1, 76% for category 2, and 91% for category 3. The proportions reporting increased drinking were just 3%, 1%, and 0%, respectively. The adjusted odds ratios (ORs) for decreasing drinking were 5.97 for category 2 (P < .0001) and 19.18 for category 3 (P < .0001) vs category 1.
The proportions reporting reduced drinking were similar across AOM classes: 51% for metformin, 46% for bupropion/naltrexone, 46% for first-generation GLP-1s (Saxenda, Trulicity, and Victoza), and 45% for the second-generation GLP-1 drugs (Mounjaro, Ozempic, Rybelsus, Wegovy, and Zepbound). All were statistically significant at P < .0001.
The highest proportion reporting increased drinking was 4% for bupropion/naltrexone. Compared with women, men were significantly more likely to report decreased drinking with AOM use (adjusted OR, 0.74; P < .001), but there were no differences by race/ethnicity or age.
Compared with those who had overweight, those in obesity classes I, II, and III were all more likely to decrease drinking with AOM use, with adjusted ORs of 1.26 (P = .0045), 1.49 (P < .001), and 1.63 (P < .001), respectively.
Mechanisms Appear Both Biological and Behavioral
During the discussion, Cardel said that qualitative assessments with participants suggest that there are at least two mechanisms behind this phenomenon: One biological and the other intentional.
“What we hear from them is twofold, one, particularly amongst those folks on GLP-1 medications, we’re hearing that physiologically, they feel different with the medications, that their cravings for alcohol are decreased, and that when they do choose to drink that there’s often a very much a negative reinforcement ... I’ve had a patient tell me, ‘I used to be able to have two or three margaritas, and maybe I didn’t feel like the best I’d ever felt in the morning, but I was okay. And now if I have two or three drinks, I will be throwing up for 5 hours, and it’s the worst hangover I’ve ever had in my life.’ And so it very much creates that negative reinforcement loop.”
But at the same time, “folks who are coming to us and seeking these medications are very much on a on a health-based journey. That’s what they tell us. The majority of our patients are there to improve their health. We rarely hear about the vanity or aesthetic part of it. So perhaps it’s that, in terms of trying to improve their health, they’re also trying to reduce their alcohol consumption, either just for their overall health or also as a means of trying to decrease their overall calorie consumption.”
In future research, Cardel said, “we want to examine whether the anti-obesity medications are more successful at reducing alcohol use compared to non-pharmacological weight management interventions, as we know that people often reduce their alcohol consumption on a weight management journey as a means of prioritizing their calories for food and decreasing the calories from alcohol.”
Cardel and all the study coauthors were employees and shareholders at WeightWatchers at the time the research was conducted. Skelton is editor in chief of the journal Childhood Obesity.
A version of this article appeared on Medscape.com.
FROM OBESITY WEEK 2024
Digital Danger: How Cyberattacks Put Patients at Risk
On September 27, 2024, UMC Health System in Lubbock, Texas, experienced an IT outage because of a cybersecurity incident that temporarily diverted patients to other healthcare facilities. So far, in 2024, there have been 386 cyberattacks on healthcare organizations. These high-impact ransomware attacks disrupt and delay patient care.
In recent years, many healthcare systems, including Scripps Health, Universal Health Services, Vastaamo, Sky Lakes, and the University of Vermont, have paid millions — even tens of millions — to recover data after a cyberattack or data breach. When healthcare systems come under cyber fire, the impact extends far past disrupting workflows and compromising data, patient safety can be also be compromised, vital information may be lost, and imaging and lab results can go missing or be held for ransom, making physicians’ job difficult or impossible.
In fact, cyberattacks on hospitals are far more common than you may realize. A new report issued by Ponemon and Proofpoint found that 92% of healthcare organizations have experienced a cyberattack in the past 12 months. Even more sobering is that about half of the organizations affected suffered disruptions in patient care.
Healthcare Systems = ‘Soft Targets’
Healthcare systems are a “soft target” for hackers for several reasons, pointed out Matthew Radolec, vice president, incident response and cloud operations at Varonis, a data security company. “One, they’re usually an amalgamation of many healthcare systems that are interconnected,” said Radolec. “A lot of hospitals are connected to other hospitals or connected to educational institutions, which means their computer vulnerabilities are shared ... and if they have an issue, it could very easily spread to your network.”
Another factor is the cost of securing data. “[With hospitals], they’ll say that a dollar spent on security is a dollar not spent on patient care,” said Radolec. “So the idea of investing in security is really tough from a budget standpoint…they’re choosing between a new MRI machine or better antivirus, backups, or data security.”
Because of the wealth of private data and healthcare information they maintain, hospitals are considered “high impact” for cybercriminals. Attackers know that if they get a foothold in a hospital, it’s more likely to pay — and pay quickly, Radolec told this news organization. Hospitals are also likely to have cyber insurance to help cover the cost of having their data stolen, encrypted, and ransomed.
The 2024 Microsoft Digital Defense Report also found that the bad actors are more sophisticated and better resourced and can challenge even the best cybersecurity. Improved defenses may not be good enough, and the sheer volume of attacks must be met with effective deterrence and government solutions that impose consequences for cybercriminals.
Vulnerable Users
Whether through a phishing email or text, password attack, or web attack, “the moment a ‘threat actor’ gets into your institution and gets credentials ... that’s the Nirvana state of a threat actor,” warned Ryan Witt, chair of the healthcare customer advisory board and vice president of Industry Solutions at Proofpoint, a cybersecurity platform. “They have those credentials and will go into deep reconnaissance mode. It often takes healthcare up to 6 months to even ascertain whether somebody’s actually in the network.” During that time, the hacker is learning how the institution works, what job functions matter, and how best to plan their attack.
“Attackers are getting in because they’re buying databases of usernames and passwords. And they’re trying them by the millions,” added Radolec. “For a sophisticated actor, all it takes is time and motivation. They have the skills. It’s just a matter of how persistent they want to be.”
Certain hospital staff are also more likely to be targeted by cyberhackers than others. “About 10% of a healthcare organization’s user base is much more vulnerable for all sorts of reasons — how they work, the value of their job title and job function, and therefore their access to systems,” said Witt.
High-profile staff are more likely to be targeted than those in lower-level positions; the so-called “CEO attack” is typical. However, staff in other hospital departments are also subject to cybercriminals, including hospice departments/hospice organizations and research arms of hospitals.
The Impact of Cyberattacks on Patients
Physicians and healthcare execs may have considered cybersecurity more of a compliance issue than a true threat to patients in the past. But this attitude is rapidly changing. “We are starting to see a very clear connection between a cyber event and how it can impact patient care and patient safety,” said Witt.
According to the Proofpoint report, cyber breaches can severely affect patient care. In 2024:
- 56% of respondents saw a delay in patient tests/procedures
- 53% experienced increased patient complications from medical procedures
- 52% noted a longer patient length of stay
- 44% saw an increase in patient transfers to other facilities
- 28% had an increase in mortality rate
What Hospitals and Physicians Can Do
Fortunately, hospitals can take measures to better protect their data and their patients. One strategy is segmenting networks to reduce the amount of data or systems one person or system can access. Educating staff about the dangers of phishing and spoofing emails also help protect organizations from ransomware attacks. Having staff avoid reusing passwords and updating logins and passwords frequently helps.
Most hospitals also need more robust security controls. Physicians and healthcare facilities must also embrace the cybersecurity controls found in other industries, said Witt. “Multifactor authentication is one of those things that can cause us frustration,” he said. “The controls can seem onerous, but they’re really valuable overall…and should become standard practice.”
Doctors can also prepare for a ransomware attack and protect patients by practicing some “old-school” medicine, like using paper systems and maintaining good patient notes — often, those notes are synced locally as well as offsite, so you’d be able to access them even during a data breach. “It’s smart to write prescriptions on pads sometimes,” said Radolec. “Don’t forget how to do those things because that will make you more resilient in the event of a ransomware attack.”
A Continuing Threat
Cyberattacks will continue. “When you look at the high likelihood [of success] and the soft target, you end up with ... a perfect storm,” said Radolec. “Hospitals have a lot of vulnerabilities. They have to keep operations going just to receive income, but also to deliver care to people.”
That means that the burden is on healthcare organizations — including physicians, nurses, staff, and C-level execs — to help keep the “security” in cybersecurity. “We are all part of the cybersecurity defense,” said Witt. Helping to maintain that defense has become a critical aspect of caring for patients.
A version of this article first appeared on Medscape.com.
On September 27, 2024, UMC Health System in Lubbock, Texas, experienced an IT outage because of a cybersecurity incident that temporarily diverted patients to other healthcare facilities. So far, in 2024, there have been 386 cyberattacks on healthcare organizations. These high-impact ransomware attacks disrupt and delay patient care.
In recent years, many healthcare systems, including Scripps Health, Universal Health Services, Vastaamo, Sky Lakes, and the University of Vermont, have paid millions — even tens of millions — to recover data after a cyberattack or data breach. When healthcare systems come under cyber fire, the impact extends far past disrupting workflows and compromising data, patient safety can be also be compromised, vital information may be lost, and imaging and lab results can go missing or be held for ransom, making physicians’ job difficult or impossible.
In fact, cyberattacks on hospitals are far more common than you may realize. A new report issued by Ponemon and Proofpoint found that 92% of healthcare organizations have experienced a cyberattack in the past 12 months. Even more sobering is that about half of the organizations affected suffered disruptions in patient care.
Healthcare Systems = ‘Soft Targets’
Healthcare systems are a “soft target” for hackers for several reasons, pointed out Matthew Radolec, vice president, incident response and cloud operations at Varonis, a data security company. “One, they’re usually an amalgamation of many healthcare systems that are interconnected,” said Radolec. “A lot of hospitals are connected to other hospitals or connected to educational institutions, which means their computer vulnerabilities are shared ... and if they have an issue, it could very easily spread to your network.”
Another factor is the cost of securing data. “[With hospitals], they’ll say that a dollar spent on security is a dollar not spent on patient care,” said Radolec. “So the idea of investing in security is really tough from a budget standpoint…they’re choosing between a new MRI machine or better antivirus, backups, or data security.”
Because of the wealth of private data and healthcare information they maintain, hospitals are considered “high impact” for cybercriminals. Attackers know that if they get a foothold in a hospital, it’s more likely to pay — and pay quickly, Radolec told this news organization. Hospitals are also likely to have cyber insurance to help cover the cost of having their data stolen, encrypted, and ransomed.
The 2024 Microsoft Digital Defense Report also found that the bad actors are more sophisticated and better resourced and can challenge even the best cybersecurity. Improved defenses may not be good enough, and the sheer volume of attacks must be met with effective deterrence and government solutions that impose consequences for cybercriminals.
Vulnerable Users
Whether through a phishing email or text, password attack, or web attack, “the moment a ‘threat actor’ gets into your institution and gets credentials ... that’s the Nirvana state of a threat actor,” warned Ryan Witt, chair of the healthcare customer advisory board and vice president of Industry Solutions at Proofpoint, a cybersecurity platform. “They have those credentials and will go into deep reconnaissance mode. It often takes healthcare up to 6 months to even ascertain whether somebody’s actually in the network.” During that time, the hacker is learning how the institution works, what job functions matter, and how best to plan their attack.
“Attackers are getting in because they’re buying databases of usernames and passwords. And they’re trying them by the millions,” added Radolec. “For a sophisticated actor, all it takes is time and motivation. They have the skills. It’s just a matter of how persistent they want to be.”
Certain hospital staff are also more likely to be targeted by cyberhackers than others. “About 10% of a healthcare organization’s user base is much more vulnerable for all sorts of reasons — how they work, the value of their job title and job function, and therefore their access to systems,” said Witt.
High-profile staff are more likely to be targeted than those in lower-level positions; the so-called “CEO attack” is typical. However, staff in other hospital departments are also subject to cybercriminals, including hospice departments/hospice organizations and research arms of hospitals.
The Impact of Cyberattacks on Patients
Physicians and healthcare execs may have considered cybersecurity more of a compliance issue than a true threat to patients in the past. But this attitude is rapidly changing. “We are starting to see a very clear connection between a cyber event and how it can impact patient care and patient safety,” said Witt.
According to the Proofpoint report, cyber breaches can severely affect patient care. In 2024:
- 56% of respondents saw a delay in patient tests/procedures
- 53% experienced increased patient complications from medical procedures
- 52% noted a longer patient length of stay
- 44% saw an increase in patient transfers to other facilities
- 28% had an increase in mortality rate
What Hospitals and Physicians Can Do
Fortunately, hospitals can take measures to better protect their data and their patients. One strategy is segmenting networks to reduce the amount of data or systems one person or system can access. Educating staff about the dangers of phishing and spoofing emails also help protect organizations from ransomware attacks. Having staff avoid reusing passwords and updating logins and passwords frequently helps.
Most hospitals also need more robust security controls. Physicians and healthcare facilities must also embrace the cybersecurity controls found in other industries, said Witt. “Multifactor authentication is one of those things that can cause us frustration,” he said. “The controls can seem onerous, but they’re really valuable overall…and should become standard practice.”
Doctors can also prepare for a ransomware attack and protect patients by practicing some “old-school” medicine, like using paper systems and maintaining good patient notes — often, those notes are synced locally as well as offsite, so you’d be able to access them even during a data breach. “It’s smart to write prescriptions on pads sometimes,” said Radolec. “Don’t forget how to do those things because that will make you more resilient in the event of a ransomware attack.”
A Continuing Threat
Cyberattacks will continue. “When you look at the high likelihood [of success] and the soft target, you end up with ... a perfect storm,” said Radolec. “Hospitals have a lot of vulnerabilities. They have to keep operations going just to receive income, but also to deliver care to people.”
That means that the burden is on healthcare organizations — including physicians, nurses, staff, and C-level execs — to help keep the “security” in cybersecurity. “We are all part of the cybersecurity defense,” said Witt. Helping to maintain that defense has become a critical aspect of caring for patients.
A version of this article first appeared on Medscape.com.
On September 27, 2024, UMC Health System in Lubbock, Texas, experienced an IT outage because of a cybersecurity incident that temporarily diverted patients to other healthcare facilities. So far, in 2024, there have been 386 cyberattacks on healthcare organizations. These high-impact ransomware attacks disrupt and delay patient care.
In recent years, many healthcare systems, including Scripps Health, Universal Health Services, Vastaamo, Sky Lakes, and the University of Vermont, have paid millions — even tens of millions — to recover data after a cyberattack or data breach. When healthcare systems come under cyber fire, the impact extends far past disrupting workflows and compromising data, patient safety can be also be compromised, vital information may be lost, and imaging and lab results can go missing or be held for ransom, making physicians’ job difficult or impossible.
In fact, cyberattacks on hospitals are far more common than you may realize. A new report issued by Ponemon and Proofpoint found that 92% of healthcare organizations have experienced a cyberattack in the past 12 months. Even more sobering is that about half of the organizations affected suffered disruptions in patient care.
Healthcare Systems = ‘Soft Targets’
Healthcare systems are a “soft target” for hackers for several reasons, pointed out Matthew Radolec, vice president, incident response and cloud operations at Varonis, a data security company. “One, they’re usually an amalgamation of many healthcare systems that are interconnected,” said Radolec. “A lot of hospitals are connected to other hospitals or connected to educational institutions, which means their computer vulnerabilities are shared ... and if they have an issue, it could very easily spread to your network.”
Another factor is the cost of securing data. “[With hospitals], they’ll say that a dollar spent on security is a dollar not spent on patient care,” said Radolec. “So the idea of investing in security is really tough from a budget standpoint…they’re choosing between a new MRI machine or better antivirus, backups, or data security.”
Because of the wealth of private data and healthcare information they maintain, hospitals are considered “high impact” for cybercriminals. Attackers know that if they get a foothold in a hospital, it’s more likely to pay — and pay quickly, Radolec told this news organization. Hospitals are also likely to have cyber insurance to help cover the cost of having their data stolen, encrypted, and ransomed.
The 2024 Microsoft Digital Defense Report also found that the bad actors are more sophisticated and better resourced and can challenge even the best cybersecurity. Improved defenses may not be good enough, and the sheer volume of attacks must be met with effective deterrence and government solutions that impose consequences for cybercriminals.
Vulnerable Users
Whether through a phishing email or text, password attack, or web attack, “the moment a ‘threat actor’ gets into your institution and gets credentials ... that’s the Nirvana state of a threat actor,” warned Ryan Witt, chair of the healthcare customer advisory board and vice president of Industry Solutions at Proofpoint, a cybersecurity platform. “They have those credentials and will go into deep reconnaissance mode. It often takes healthcare up to 6 months to even ascertain whether somebody’s actually in the network.” During that time, the hacker is learning how the institution works, what job functions matter, and how best to plan their attack.
“Attackers are getting in because they’re buying databases of usernames and passwords. And they’re trying them by the millions,” added Radolec. “For a sophisticated actor, all it takes is time and motivation. They have the skills. It’s just a matter of how persistent they want to be.”
Certain hospital staff are also more likely to be targeted by cyberhackers than others. “About 10% of a healthcare organization’s user base is much more vulnerable for all sorts of reasons — how they work, the value of their job title and job function, and therefore their access to systems,” said Witt.
High-profile staff are more likely to be targeted than those in lower-level positions; the so-called “CEO attack” is typical. However, staff in other hospital departments are also subject to cybercriminals, including hospice departments/hospice organizations and research arms of hospitals.
The Impact of Cyberattacks on Patients
Physicians and healthcare execs may have considered cybersecurity more of a compliance issue than a true threat to patients in the past. But this attitude is rapidly changing. “We are starting to see a very clear connection between a cyber event and how it can impact patient care and patient safety,” said Witt.
According to the Proofpoint report, cyber breaches can severely affect patient care. In 2024:
- 56% of respondents saw a delay in patient tests/procedures
- 53% experienced increased patient complications from medical procedures
- 52% noted a longer patient length of stay
- 44% saw an increase in patient transfers to other facilities
- 28% had an increase in mortality rate
What Hospitals and Physicians Can Do
Fortunately, hospitals can take measures to better protect their data and their patients. One strategy is segmenting networks to reduce the amount of data or systems one person or system can access. Educating staff about the dangers of phishing and spoofing emails also help protect organizations from ransomware attacks. Having staff avoid reusing passwords and updating logins and passwords frequently helps.
Most hospitals also need more robust security controls. Physicians and healthcare facilities must also embrace the cybersecurity controls found in other industries, said Witt. “Multifactor authentication is one of those things that can cause us frustration,” he said. “The controls can seem onerous, but they’re really valuable overall…and should become standard practice.”
Doctors can also prepare for a ransomware attack and protect patients by practicing some “old-school” medicine, like using paper systems and maintaining good patient notes — often, those notes are synced locally as well as offsite, so you’d be able to access them even during a data breach. “It’s smart to write prescriptions on pads sometimes,” said Radolec. “Don’t forget how to do those things because that will make you more resilient in the event of a ransomware attack.”
A Continuing Threat
Cyberattacks will continue. “When you look at the high likelihood [of success] and the soft target, you end up with ... a perfect storm,” said Radolec. “Hospitals have a lot of vulnerabilities. They have to keep operations going just to receive income, but also to deliver care to people.”
That means that the burden is on healthcare organizations — including physicians, nurses, staff, and C-level execs — to help keep the “security” in cybersecurity. “We are all part of the cybersecurity defense,” said Witt. Helping to maintain that defense has become a critical aspect of caring for patients.
A version of this article first appeared on Medscape.com.
Postpartum Depression Common After Cesarean Delivery
TOPLINE:
About one in six women experience symptoms of postpartum depression (PPD) 2 months after cesarean delivery, with certain obstetric factors such as emergency cesarean delivery before labor, cesarean delivery after labor induction, lack of social support in the operating room, and severe postoperative pain influencing the risk.
METHODOLOGY:
- Researchers conducted a prospective ancillary cohort study of the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery (TRAAP2) trial to examine the prevalence of PPD 2 months after cesarean delivery and associated risk factors.
- A total of 2793 women (median age, 33.5 years) were included who had a cesarean delivery at 34 or more weeks of gestation; they completed the Edinburgh Postnatal Depression Scale (EPDS), a self-administered questionnaire, at 2 months after delivery.
- Information about the cesarean delivery, postpartum blood loss, immediate postpartum period, psychiatric history, and memories of delivery and postoperative pain were prospectively collected.
- Medical records were used to obtain details about characteristics of patients; 5.0% had a psychiatric history (2.4% composed of depression).
- The main endpoint was a positive screening for symptoms consistent with this depression — defined as a PPD diagnosis — 2 months after caesarian delivery, with an EPDS score of 13 or higher.
TAKEAWAY:
- The prevalence of a provisional PPD diagnosis at 2 months after cesarean delivery was 16.4% (95% CI, 14.9-18.0) with an EPDS score of 13 or higher and was 23.1% (95% CI, 21.4-24.9%) with a cutoff value of 11 or higher.
- Women who had an emergency cesarean delivery before labor had a higher risk for PPD than those who had a normal cesarean delivery before labor started (adjusted odds ratio [aOR], 1.70; 95% CI, 1.15-2.50); women who had started labor after induction but then had a cesarean delivery also had a higher risk for PPD than those who had a cesarean delivery before going into labor (aOR, 1.36; 95% CI, 1.03-1.84).
- Severe pain during the postpartum stay (aOR, 1.73; 95% CI, 1.32-2.26) and bad memories of delivery (aOR, 1.67; 95% CI, 1.14-2.45) were also risk factors for PPD.
- However, women who had social support in the operating room showed a 27% lower risk for PPD (P = .02).
IN PRACTICE:
“Identifying subgroups of women at risk for PPD based on aspects of their obstetric experience could help to screen for women who might benefit from early screening and interventions,” the authors wrote.
SOURCE:
This study was led by Alizée Froeliger, MD, MPH, of the Department of Obstetrics and Gynecology at Bordeaux University Hospital in France, and was published online in American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study population was derived from a randomized controlled trial, which may have underestimated the prevalence of PPD. The use of a self-administered questionnaire for PPD screening may not have provided a definitive diagnosis. Moreover, this study did not assess the prevalence of depressive symptoms during pregnancy.
DISCLOSURES:
The TRAAP2 trial was supported by a grant from the French Ministry of Health under its Clinical Research Hospital Program. One author reported carrying out consultancy work and lecturing for Ferring Laboratories, GlaxoSmithKline, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
About one in six women experience symptoms of postpartum depression (PPD) 2 months after cesarean delivery, with certain obstetric factors such as emergency cesarean delivery before labor, cesarean delivery after labor induction, lack of social support in the operating room, and severe postoperative pain influencing the risk.
METHODOLOGY:
- Researchers conducted a prospective ancillary cohort study of the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery (TRAAP2) trial to examine the prevalence of PPD 2 months after cesarean delivery and associated risk factors.
- A total of 2793 women (median age, 33.5 years) were included who had a cesarean delivery at 34 or more weeks of gestation; they completed the Edinburgh Postnatal Depression Scale (EPDS), a self-administered questionnaire, at 2 months after delivery.
- Information about the cesarean delivery, postpartum blood loss, immediate postpartum period, psychiatric history, and memories of delivery and postoperative pain were prospectively collected.
- Medical records were used to obtain details about characteristics of patients; 5.0% had a psychiatric history (2.4% composed of depression).
- The main endpoint was a positive screening for symptoms consistent with this depression — defined as a PPD diagnosis — 2 months after caesarian delivery, with an EPDS score of 13 or higher.
TAKEAWAY:
- The prevalence of a provisional PPD diagnosis at 2 months after cesarean delivery was 16.4% (95% CI, 14.9-18.0) with an EPDS score of 13 or higher and was 23.1% (95% CI, 21.4-24.9%) with a cutoff value of 11 or higher.
- Women who had an emergency cesarean delivery before labor had a higher risk for PPD than those who had a normal cesarean delivery before labor started (adjusted odds ratio [aOR], 1.70; 95% CI, 1.15-2.50); women who had started labor after induction but then had a cesarean delivery also had a higher risk for PPD than those who had a cesarean delivery before going into labor (aOR, 1.36; 95% CI, 1.03-1.84).
- Severe pain during the postpartum stay (aOR, 1.73; 95% CI, 1.32-2.26) and bad memories of delivery (aOR, 1.67; 95% CI, 1.14-2.45) were also risk factors for PPD.
- However, women who had social support in the operating room showed a 27% lower risk for PPD (P = .02).
IN PRACTICE:
“Identifying subgroups of women at risk for PPD based on aspects of their obstetric experience could help to screen for women who might benefit from early screening and interventions,” the authors wrote.
SOURCE:
This study was led by Alizée Froeliger, MD, MPH, of the Department of Obstetrics and Gynecology at Bordeaux University Hospital in France, and was published online in American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study population was derived from a randomized controlled trial, which may have underestimated the prevalence of PPD. The use of a self-administered questionnaire for PPD screening may not have provided a definitive diagnosis. Moreover, this study did not assess the prevalence of depressive symptoms during pregnancy.
DISCLOSURES:
The TRAAP2 trial was supported by a grant from the French Ministry of Health under its Clinical Research Hospital Program. One author reported carrying out consultancy work and lecturing for Ferring Laboratories, GlaxoSmithKline, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
About one in six women experience symptoms of postpartum depression (PPD) 2 months after cesarean delivery, with certain obstetric factors such as emergency cesarean delivery before labor, cesarean delivery after labor induction, lack of social support in the operating room, and severe postoperative pain influencing the risk.
METHODOLOGY:
- Researchers conducted a prospective ancillary cohort study of the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery (TRAAP2) trial to examine the prevalence of PPD 2 months after cesarean delivery and associated risk factors.
- A total of 2793 women (median age, 33.5 years) were included who had a cesarean delivery at 34 or more weeks of gestation; they completed the Edinburgh Postnatal Depression Scale (EPDS), a self-administered questionnaire, at 2 months after delivery.
- Information about the cesarean delivery, postpartum blood loss, immediate postpartum period, psychiatric history, and memories of delivery and postoperative pain were prospectively collected.
- Medical records were used to obtain details about characteristics of patients; 5.0% had a psychiatric history (2.4% composed of depression).
- The main endpoint was a positive screening for symptoms consistent with this depression — defined as a PPD diagnosis — 2 months after caesarian delivery, with an EPDS score of 13 or higher.
TAKEAWAY:
- The prevalence of a provisional PPD diagnosis at 2 months after cesarean delivery was 16.4% (95% CI, 14.9-18.0) with an EPDS score of 13 or higher and was 23.1% (95% CI, 21.4-24.9%) with a cutoff value of 11 or higher.
- Women who had an emergency cesarean delivery before labor had a higher risk for PPD than those who had a normal cesarean delivery before labor started (adjusted odds ratio [aOR], 1.70; 95% CI, 1.15-2.50); women who had started labor after induction but then had a cesarean delivery also had a higher risk for PPD than those who had a cesarean delivery before going into labor (aOR, 1.36; 95% CI, 1.03-1.84).
- Severe pain during the postpartum stay (aOR, 1.73; 95% CI, 1.32-2.26) and bad memories of delivery (aOR, 1.67; 95% CI, 1.14-2.45) were also risk factors for PPD.
- However, women who had social support in the operating room showed a 27% lower risk for PPD (P = .02).
IN PRACTICE:
“Identifying subgroups of women at risk for PPD based on aspects of their obstetric experience could help to screen for women who might benefit from early screening and interventions,” the authors wrote.
SOURCE:
This study was led by Alizée Froeliger, MD, MPH, of the Department of Obstetrics and Gynecology at Bordeaux University Hospital in France, and was published online in American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study population was derived from a randomized controlled trial, which may have underestimated the prevalence of PPD. The use of a self-administered questionnaire for PPD screening may not have provided a definitive diagnosis. Moreover, this study did not assess the prevalence of depressive symptoms during pregnancy.
DISCLOSURES:
The TRAAP2 trial was supported by a grant from the French Ministry of Health under its Clinical Research Hospital Program. One author reported carrying out consultancy work and lecturing for Ferring Laboratories, GlaxoSmithKline, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
When Your Malpractice Insurer Investigates You: What to Know
When psychiatrist Paul Sartain, MD (not his real name), received a letter from his state’s medical board, he was concerned. A patient’s family complained that he made sexual advances to a young woman he treated for psychotic depression.
“There was absolutely no evidence, and the claims were vague,” he said. “I think the family was angry at me and with the system — the woman had not gotten better.” Sartain reviewed his medical records and then called his malpractice insurer.
The insurer asked about his involvement with the patient’s case, if there was anything credible to the patient’s complaint, and if he had thorough documentation. Then, the carrier offered Sartain his choice of several attorneys who could represent him. The medical board ultimately closed the case with no findings against him, and the patient’s family never sued him.
“If I’m wrongly accused, I’m defended (by the carrier). If I had stolen money or had a sexual relationship with the patient, then you’re acting outside the bounds of what is protected (by the carrier),” he said.
How Medical Board and Malpractice Insurer Investigations Differ
Medical board complaints differ from malpractice claims, in which patients seek damages. The investigation process also varies.
When a patient reports a doctor to a state medical board, they may also sue the doctor for monetary damages in civil court. The medical board responds to patient complaints made directly to them, but it also may also initiate its own investigations. Those can be prompted by a malpractice claim resolution, with a court verdict against the doctor, or a settlement recorded in the National Practitioner Data Bank.
Malpractice insurers may offer limited legal representation for medical board investigations, requiring the doctor to report the medical board issue to them before the doctor takes any action. Often, they will cover up to $50,000 in defense costs but not cover any subsequent medical board fines or required classes or medical board fees.
When a doctor contacts the carrier about a medical board investigation, the carrier may ask for the medical board document and the medical records, said Alex Keoskey, a partner in Frier Levitt’s life sciences group.
The carrier may want to ask about the patient, staff members involved, the doctor’s background, if there have been previous medical board investigations or lawsuits against this doctor, and the doctor’s opinion of the allegations. The doctor should be transparent with the carrier, Keoskey said.
Some carriers conduct more in-depth investigations, examining record-keeping, prescription practices, patient consent processes, and continuing medical education status. That’s because the medical board may inquire about these as well should its own investigation expand.
Not all carriers explore cases like these, even if reimbursing for defense costs, said Karen Frisella, director of professional liability claims at BETA Healthcare Group in California. In her experience, a licensing investigation usually follows a claim resolution that was already worked up by the carrier. If a complaint was made directly to the licensing board without an accompanying liability claim, the carrier’s ability to initiate an investigation on the incident depends on the policy terms or coverage available.
“Typically, a professional liability policy requires that the insured report a claim to trigger coverage. The carrier can’t unilaterally decide to open a claim,” she said. A licensing board investigation is not a claim by definition and therefore does not provide a mechanism for the carrier to open a liability claim file, she added.
If the medical board ultimately restricts the doctor’s license or puts the doctor on probation, that becomes public, and the underwriting department may then look into it.
Malpractice insurers routinely monitor licensing board discipline notices. A reprimand or restrictions on a doctor’s license could trigger a review of the physician’s future insurability and lead to higher premiums or even nonrenewal, Frisella said.
If a carrier investigates a reported claim and determines there are issues with the care rendered, whether there is an accompanying medical board action, that also can affect underwriting decisions, Frisella said.
Who Is Your Attorney Really Working for?
The doctor should understand whose interests the attorney represents. In a medical board claim, the attorney — even if defense is paid by the carrier — represents the doctor.
Frisella said her organization provides pass-through coverage, meaning it reimburses the doctor for medical board defense costs. “Because the carrier isn’t directing the medical board defense, it is not generally privy to the work product.”
If a patient files a malpractice claim, however, the attorney ultimately represents the insurance company.
“The panel counsel who works for the insurer does not work for the doctor, and that’s always important to remember,” Keoskey said. While the attorney will do their best to aggressively defend the doctor, “he’s going to protect the insurer’s interest before the doctor’s.”
Physicians who find any conflict of interest with their insurer should seek counsel.
Such conflicts could include:
- Disagreements over the case’s ultimate worth. For example, a physician might want a case to settle for less than their carrier is willing to pay.
- The legal judgment may exceed the carrier’s policy limits, or there are punitive damages or allegations of criminal acts that the insurer does not cover.
In these cases, the insurance company should recommend the doctor get personal counsel. They will send a reservation of rights letter saying they will defend the doctor for now, but if the facts show the doctor committed some type of misconduct, they may decline coverage, said Keoskey. Some states, including California, require that the carrier pay for this independent counsel.
Unless there is a conflict of interest, though, having a personal attorney just makes the situation more complicated, said Frisella.
A version of this article first appeared on Medscape.com.
When psychiatrist Paul Sartain, MD (not his real name), received a letter from his state’s medical board, he was concerned. A patient’s family complained that he made sexual advances to a young woman he treated for psychotic depression.
“There was absolutely no evidence, and the claims were vague,” he said. “I think the family was angry at me and with the system — the woman had not gotten better.” Sartain reviewed his medical records and then called his malpractice insurer.
The insurer asked about his involvement with the patient’s case, if there was anything credible to the patient’s complaint, and if he had thorough documentation. Then, the carrier offered Sartain his choice of several attorneys who could represent him. The medical board ultimately closed the case with no findings against him, and the patient’s family never sued him.
“If I’m wrongly accused, I’m defended (by the carrier). If I had stolen money or had a sexual relationship with the patient, then you’re acting outside the bounds of what is protected (by the carrier),” he said.
How Medical Board and Malpractice Insurer Investigations Differ
Medical board complaints differ from malpractice claims, in which patients seek damages. The investigation process also varies.
When a patient reports a doctor to a state medical board, they may also sue the doctor for monetary damages in civil court. The medical board responds to patient complaints made directly to them, but it also may also initiate its own investigations. Those can be prompted by a malpractice claim resolution, with a court verdict against the doctor, or a settlement recorded in the National Practitioner Data Bank.
Malpractice insurers may offer limited legal representation for medical board investigations, requiring the doctor to report the medical board issue to them before the doctor takes any action. Often, they will cover up to $50,000 in defense costs but not cover any subsequent medical board fines or required classes or medical board fees.
When a doctor contacts the carrier about a medical board investigation, the carrier may ask for the medical board document and the medical records, said Alex Keoskey, a partner in Frier Levitt’s life sciences group.
The carrier may want to ask about the patient, staff members involved, the doctor’s background, if there have been previous medical board investigations or lawsuits against this doctor, and the doctor’s opinion of the allegations. The doctor should be transparent with the carrier, Keoskey said.
Some carriers conduct more in-depth investigations, examining record-keeping, prescription practices, patient consent processes, and continuing medical education status. That’s because the medical board may inquire about these as well should its own investigation expand.
Not all carriers explore cases like these, even if reimbursing for defense costs, said Karen Frisella, director of professional liability claims at BETA Healthcare Group in California. In her experience, a licensing investigation usually follows a claim resolution that was already worked up by the carrier. If a complaint was made directly to the licensing board without an accompanying liability claim, the carrier’s ability to initiate an investigation on the incident depends on the policy terms or coverage available.
“Typically, a professional liability policy requires that the insured report a claim to trigger coverage. The carrier can’t unilaterally decide to open a claim,” she said. A licensing board investigation is not a claim by definition and therefore does not provide a mechanism for the carrier to open a liability claim file, she added.
If the medical board ultimately restricts the doctor’s license or puts the doctor on probation, that becomes public, and the underwriting department may then look into it.
Malpractice insurers routinely monitor licensing board discipline notices. A reprimand or restrictions on a doctor’s license could trigger a review of the physician’s future insurability and lead to higher premiums or even nonrenewal, Frisella said.
If a carrier investigates a reported claim and determines there are issues with the care rendered, whether there is an accompanying medical board action, that also can affect underwriting decisions, Frisella said.
Who Is Your Attorney Really Working for?
The doctor should understand whose interests the attorney represents. In a medical board claim, the attorney — even if defense is paid by the carrier — represents the doctor.
Frisella said her organization provides pass-through coverage, meaning it reimburses the doctor for medical board defense costs. “Because the carrier isn’t directing the medical board defense, it is not generally privy to the work product.”
If a patient files a malpractice claim, however, the attorney ultimately represents the insurance company.
“The panel counsel who works for the insurer does not work for the doctor, and that’s always important to remember,” Keoskey said. While the attorney will do their best to aggressively defend the doctor, “he’s going to protect the insurer’s interest before the doctor’s.”
Physicians who find any conflict of interest with their insurer should seek counsel.
Such conflicts could include:
- Disagreements over the case’s ultimate worth. For example, a physician might want a case to settle for less than their carrier is willing to pay.
- The legal judgment may exceed the carrier’s policy limits, or there are punitive damages or allegations of criminal acts that the insurer does not cover.
In these cases, the insurance company should recommend the doctor get personal counsel. They will send a reservation of rights letter saying they will defend the doctor for now, but if the facts show the doctor committed some type of misconduct, they may decline coverage, said Keoskey. Some states, including California, require that the carrier pay for this independent counsel.
Unless there is a conflict of interest, though, having a personal attorney just makes the situation more complicated, said Frisella.
A version of this article first appeared on Medscape.com.
When psychiatrist Paul Sartain, MD (not his real name), received a letter from his state’s medical board, he was concerned. A patient’s family complained that he made sexual advances to a young woman he treated for psychotic depression.
“There was absolutely no evidence, and the claims were vague,” he said. “I think the family was angry at me and with the system — the woman had not gotten better.” Sartain reviewed his medical records and then called his malpractice insurer.
The insurer asked about his involvement with the patient’s case, if there was anything credible to the patient’s complaint, and if he had thorough documentation. Then, the carrier offered Sartain his choice of several attorneys who could represent him. The medical board ultimately closed the case with no findings against him, and the patient’s family never sued him.
“If I’m wrongly accused, I’m defended (by the carrier). If I had stolen money or had a sexual relationship with the patient, then you’re acting outside the bounds of what is protected (by the carrier),” he said.
How Medical Board and Malpractice Insurer Investigations Differ
Medical board complaints differ from malpractice claims, in which patients seek damages. The investigation process also varies.
When a patient reports a doctor to a state medical board, they may also sue the doctor for monetary damages in civil court. The medical board responds to patient complaints made directly to them, but it also may also initiate its own investigations. Those can be prompted by a malpractice claim resolution, with a court verdict against the doctor, or a settlement recorded in the National Practitioner Data Bank.
Malpractice insurers may offer limited legal representation for medical board investigations, requiring the doctor to report the medical board issue to them before the doctor takes any action. Often, they will cover up to $50,000 in defense costs but not cover any subsequent medical board fines or required classes or medical board fees.
When a doctor contacts the carrier about a medical board investigation, the carrier may ask for the medical board document and the medical records, said Alex Keoskey, a partner in Frier Levitt’s life sciences group.
The carrier may want to ask about the patient, staff members involved, the doctor’s background, if there have been previous medical board investigations or lawsuits against this doctor, and the doctor’s opinion of the allegations. The doctor should be transparent with the carrier, Keoskey said.
Some carriers conduct more in-depth investigations, examining record-keeping, prescription practices, patient consent processes, and continuing medical education status. That’s because the medical board may inquire about these as well should its own investigation expand.
Not all carriers explore cases like these, even if reimbursing for defense costs, said Karen Frisella, director of professional liability claims at BETA Healthcare Group in California. In her experience, a licensing investigation usually follows a claim resolution that was already worked up by the carrier. If a complaint was made directly to the licensing board without an accompanying liability claim, the carrier’s ability to initiate an investigation on the incident depends on the policy terms or coverage available.
“Typically, a professional liability policy requires that the insured report a claim to trigger coverage. The carrier can’t unilaterally decide to open a claim,” she said. A licensing board investigation is not a claim by definition and therefore does not provide a mechanism for the carrier to open a liability claim file, she added.
If the medical board ultimately restricts the doctor’s license or puts the doctor on probation, that becomes public, and the underwriting department may then look into it.
Malpractice insurers routinely monitor licensing board discipline notices. A reprimand or restrictions on a doctor’s license could trigger a review of the physician’s future insurability and lead to higher premiums or even nonrenewal, Frisella said.
If a carrier investigates a reported claim and determines there are issues with the care rendered, whether there is an accompanying medical board action, that also can affect underwriting decisions, Frisella said.
Who Is Your Attorney Really Working for?
The doctor should understand whose interests the attorney represents. In a medical board claim, the attorney — even if defense is paid by the carrier — represents the doctor.
Frisella said her organization provides pass-through coverage, meaning it reimburses the doctor for medical board defense costs. “Because the carrier isn’t directing the medical board defense, it is not generally privy to the work product.”
If a patient files a malpractice claim, however, the attorney ultimately represents the insurance company.
“The panel counsel who works for the insurer does not work for the doctor, and that’s always important to remember,” Keoskey said. While the attorney will do their best to aggressively defend the doctor, “he’s going to protect the insurer’s interest before the doctor’s.”
Physicians who find any conflict of interest with their insurer should seek counsel.
Such conflicts could include:
- Disagreements over the case’s ultimate worth. For example, a physician might want a case to settle for less than their carrier is willing to pay.
- The legal judgment may exceed the carrier’s policy limits, or there are punitive damages or allegations of criminal acts that the insurer does not cover.
In these cases, the insurance company should recommend the doctor get personal counsel. They will send a reservation of rights letter saying they will defend the doctor for now, but if the facts show the doctor committed some type of misconduct, they may decline coverage, said Keoskey. Some states, including California, require that the carrier pay for this independent counsel.
Unless there is a conflict of interest, though, having a personal attorney just makes the situation more complicated, said Frisella.
A version of this article first appeared on Medscape.com.
The Rise of Sham Peer Reviews
While a medical peer review occurs once a patient, fellow doctor, or staff member reports that a physician failed to treat a patient up to standards or acted improperly, a “sham peer review” is undertaken for ulterior motives.
They’re typically undertaken due to professional competition or institutional politics rather than to promote quality care or uphold professional standards.
Physicians should be concerned. In a soon-to-be-published Medscape report on peer reviews, 56% of US physicians surveyed expressed higher levels of concern that a peer review could be misused to punish a physician for reasons unrelated to the matter being reviewed.
This is a troublesome issue, and many doctors may not be aware of it or how often it occurs.
“The biggest misconception about sham peer reviews is a denial of how pervasive they are,” said Andy Schlafly, general counsel for the Association of American Physicians and Surgeons (AAPS), which offers a free legal consultation service for physicians facing a sham peer review. “Many hospital administrations are as dangerous to good physicians as street gangs can be in a crime-ridden neighborhood.”
“Physicians should become aware of whether sham peer reviews are prevalent at their hospital and, if so, those physicians should look to practice somewhere else,” Schlafly said in an interview.
Unfortunately, there are limited data on how often this happens. When it does, it can be a career killer, said Lawrence Huntoon, MD, PhD, who has run the AAPS sham peer review hotline for over 20 years.
The physicians at the most risk for a sham peer review tend to be those who work for large hospital systems — as this is one way for hospitals to get rid of the doctors they don’t want to retain on staff, Huntoon said.
“Hospitals want a model whereby every physician on the medical staff is an employee,” Huntoon added. “This gives them complete power and control over these physicians, including the way they practice and how many patients they see per day, which, for some, is 20-50 a day to generate sufficient revenue.”
Complaints are generally filed via incident reporting software.
“The complaint could be that the physician is ‘disruptive,’ which can include facial expression, tone of voice, and body language — for example, ‘I found his facial expression demeaning’ or ‘I found her tone condescending’ — and this can be used to prosecute a doctor,” Huntoon said.
After the complaint is filed, the leaders of a hospital’s peer review committee meet to discuss the incident, followed by a panel of fellow physicians convened to review the matter. Once the date for a meeting is set, the accused doctor is allowed to testify, offer evidence, and have attorney representation.
The entire experience can take a physician by surprise.
“A sham peer review is difficult to prepare for because no physician thinks this is going to happen to them,” said Laurie L. York, a medical law attorney in Austin, Texas.
York added that there may also be a misperception of what is actually happening.
“When a physician becomes aware of an investigation, it initially may look like a regular peer review, and the physician may feel there has been a ‘misunderstanding’ that they can make right by explaining things,” York said. “The window of opportunity to shut down a sham peer review happens quickly. That’s why the physician needs the help of an experienced attorney as early in the process as possible.”
If You’re a Victim of a Sham Peer Review
Be vigilant. The most important thing you should think about when it comes to sham peer reviews is that this can, indeed, happen to you, Huntoon said. “I’ve written articles to help educate physicians about the tactics that are used,” he said. “You need to be educated and read medical staff bylaws to know your rights before something bad happens.”
Stay in your job. No matter what, if you’re under review, do not resign your position, no matter how difficult this may be. “A resignation during a sham peer review triggers an adverse report to the National Practitioner Data Bank [NPDB],” Schlafly said. The NPDB is a flagging system created by Congress to improve healthcare quality and reduce healthcare fraud and abuse. “A resignation also waives the physician’s right to contest the unfair review. In addition, leverage to negotiate a favorable settlement is lost if the physician simply resigns.”
Get a lawyer on board early. This is the only way to protect your rights. “Don’t wait a year to get an attorney involved,” Huntoon said. But this also can’t be any lawyer. It’s critical to find someone who specializes in sham peer reviews, so be sure to ask about their experience in handling peer review matters in hospitals and how knowledgeable they are about databank reporting requirements. “Sometimes, doctors will hire a malpractice attorney with no knowledge of what happens with sham peer reviews, and they may give bad advice,” he said. “Others may hire an employment attorney and that attorney will be up on employment law but has no experience with peer review matters in hospitals.”
Given the seriousness of a sham peer review, following these guidelines can help.
Contact the AAPA right away. There are things that can be done early on like getting a withdrawal of the request for corrective action as well as obtaining a preliminary injunction. Preparing for the fallout that may occur can be just as challenging.
“After this situation, the doctor is damaged goods,” Huntoon said. “What hospital will want to hire damaged goods to be part of their medical staff? Finding employment is going to be challenging and opening your own practice may also be difficult because the insurers have access to data bank reports.”
Ultimately, the best advice Huntoon can offer is to do your best to stay one step ahead of any work issues that could even lead to a sham peer review.
“Try and shield yourself from a sham peer review and be prepared should it happen,” he said. “I’ve seen careers end in the blink of an eye — wrongfully.”
A version of this article first appeared on Medscape.com.
While a medical peer review occurs once a patient, fellow doctor, or staff member reports that a physician failed to treat a patient up to standards or acted improperly, a “sham peer review” is undertaken for ulterior motives.
They’re typically undertaken due to professional competition or institutional politics rather than to promote quality care or uphold professional standards.
Physicians should be concerned. In a soon-to-be-published Medscape report on peer reviews, 56% of US physicians surveyed expressed higher levels of concern that a peer review could be misused to punish a physician for reasons unrelated to the matter being reviewed.
This is a troublesome issue, and many doctors may not be aware of it or how often it occurs.
“The biggest misconception about sham peer reviews is a denial of how pervasive they are,” said Andy Schlafly, general counsel for the Association of American Physicians and Surgeons (AAPS), which offers a free legal consultation service for physicians facing a sham peer review. “Many hospital administrations are as dangerous to good physicians as street gangs can be in a crime-ridden neighborhood.”
“Physicians should become aware of whether sham peer reviews are prevalent at their hospital and, if so, those physicians should look to practice somewhere else,” Schlafly said in an interview.
Unfortunately, there are limited data on how often this happens. When it does, it can be a career killer, said Lawrence Huntoon, MD, PhD, who has run the AAPS sham peer review hotline for over 20 years.
The physicians at the most risk for a sham peer review tend to be those who work for large hospital systems — as this is one way for hospitals to get rid of the doctors they don’t want to retain on staff, Huntoon said.
“Hospitals want a model whereby every physician on the medical staff is an employee,” Huntoon added. “This gives them complete power and control over these physicians, including the way they practice and how many patients they see per day, which, for some, is 20-50 a day to generate sufficient revenue.”
Complaints are generally filed via incident reporting software.
“The complaint could be that the physician is ‘disruptive,’ which can include facial expression, tone of voice, and body language — for example, ‘I found his facial expression demeaning’ or ‘I found her tone condescending’ — and this can be used to prosecute a doctor,” Huntoon said.
After the complaint is filed, the leaders of a hospital’s peer review committee meet to discuss the incident, followed by a panel of fellow physicians convened to review the matter. Once the date for a meeting is set, the accused doctor is allowed to testify, offer evidence, and have attorney representation.
The entire experience can take a physician by surprise.
“A sham peer review is difficult to prepare for because no physician thinks this is going to happen to them,” said Laurie L. York, a medical law attorney in Austin, Texas.
York added that there may also be a misperception of what is actually happening.
“When a physician becomes aware of an investigation, it initially may look like a regular peer review, and the physician may feel there has been a ‘misunderstanding’ that they can make right by explaining things,” York said. “The window of opportunity to shut down a sham peer review happens quickly. That’s why the physician needs the help of an experienced attorney as early in the process as possible.”
If You’re a Victim of a Sham Peer Review
Be vigilant. The most important thing you should think about when it comes to sham peer reviews is that this can, indeed, happen to you, Huntoon said. “I’ve written articles to help educate physicians about the tactics that are used,” he said. “You need to be educated and read medical staff bylaws to know your rights before something bad happens.”
Stay in your job. No matter what, if you’re under review, do not resign your position, no matter how difficult this may be. “A resignation during a sham peer review triggers an adverse report to the National Practitioner Data Bank [NPDB],” Schlafly said. The NPDB is a flagging system created by Congress to improve healthcare quality and reduce healthcare fraud and abuse. “A resignation also waives the physician’s right to contest the unfair review. In addition, leverage to negotiate a favorable settlement is lost if the physician simply resigns.”
Get a lawyer on board early. This is the only way to protect your rights. “Don’t wait a year to get an attorney involved,” Huntoon said. But this also can’t be any lawyer. It’s critical to find someone who specializes in sham peer reviews, so be sure to ask about their experience in handling peer review matters in hospitals and how knowledgeable they are about databank reporting requirements. “Sometimes, doctors will hire a malpractice attorney with no knowledge of what happens with sham peer reviews, and they may give bad advice,” he said. “Others may hire an employment attorney and that attorney will be up on employment law but has no experience with peer review matters in hospitals.”
Given the seriousness of a sham peer review, following these guidelines can help.
Contact the AAPA right away. There are things that can be done early on like getting a withdrawal of the request for corrective action as well as obtaining a preliminary injunction. Preparing for the fallout that may occur can be just as challenging.
“After this situation, the doctor is damaged goods,” Huntoon said. “What hospital will want to hire damaged goods to be part of their medical staff? Finding employment is going to be challenging and opening your own practice may also be difficult because the insurers have access to data bank reports.”
Ultimately, the best advice Huntoon can offer is to do your best to stay one step ahead of any work issues that could even lead to a sham peer review.
“Try and shield yourself from a sham peer review and be prepared should it happen,” he said. “I’ve seen careers end in the blink of an eye — wrongfully.”
A version of this article first appeared on Medscape.com.
While a medical peer review occurs once a patient, fellow doctor, or staff member reports that a physician failed to treat a patient up to standards or acted improperly, a “sham peer review” is undertaken for ulterior motives.
They’re typically undertaken due to professional competition or institutional politics rather than to promote quality care or uphold professional standards.
Physicians should be concerned. In a soon-to-be-published Medscape report on peer reviews, 56% of US physicians surveyed expressed higher levels of concern that a peer review could be misused to punish a physician for reasons unrelated to the matter being reviewed.
This is a troublesome issue, and many doctors may not be aware of it or how often it occurs.
“The biggest misconception about sham peer reviews is a denial of how pervasive they are,” said Andy Schlafly, general counsel for the Association of American Physicians and Surgeons (AAPS), which offers a free legal consultation service for physicians facing a sham peer review. “Many hospital administrations are as dangerous to good physicians as street gangs can be in a crime-ridden neighborhood.”
“Physicians should become aware of whether sham peer reviews are prevalent at their hospital and, if so, those physicians should look to practice somewhere else,” Schlafly said in an interview.
Unfortunately, there are limited data on how often this happens. When it does, it can be a career killer, said Lawrence Huntoon, MD, PhD, who has run the AAPS sham peer review hotline for over 20 years.
The physicians at the most risk for a sham peer review tend to be those who work for large hospital systems — as this is one way for hospitals to get rid of the doctors they don’t want to retain on staff, Huntoon said.
“Hospitals want a model whereby every physician on the medical staff is an employee,” Huntoon added. “This gives them complete power and control over these physicians, including the way they practice and how many patients they see per day, which, for some, is 20-50 a day to generate sufficient revenue.”
Complaints are generally filed via incident reporting software.
“The complaint could be that the physician is ‘disruptive,’ which can include facial expression, tone of voice, and body language — for example, ‘I found his facial expression demeaning’ or ‘I found her tone condescending’ — and this can be used to prosecute a doctor,” Huntoon said.
After the complaint is filed, the leaders of a hospital’s peer review committee meet to discuss the incident, followed by a panel of fellow physicians convened to review the matter. Once the date for a meeting is set, the accused doctor is allowed to testify, offer evidence, and have attorney representation.
The entire experience can take a physician by surprise.
“A sham peer review is difficult to prepare for because no physician thinks this is going to happen to them,” said Laurie L. York, a medical law attorney in Austin, Texas.
York added that there may also be a misperception of what is actually happening.
“When a physician becomes aware of an investigation, it initially may look like a regular peer review, and the physician may feel there has been a ‘misunderstanding’ that they can make right by explaining things,” York said. “The window of opportunity to shut down a sham peer review happens quickly. That’s why the physician needs the help of an experienced attorney as early in the process as possible.”
If You’re a Victim of a Sham Peer Review
Be vigilant. The most important thing you should think about when it comes to sham peer reviews is that this can, indeed, happen to you, Huntoon said. “I’ve written articles to help educate physicians about the tactics that are used,” he said. “You need to be educated and read medical staff bylaws to know your rights before something bad happens.”
Stay in your job. No matter what, if you’re under review, do not resign your position, no matter how difficult this may be. “A resignation during a sham peer review triggers an adverse report to the National Practitioner Data Bank [NPDB],” Schlafly said. The NPDB is a flagging system created by Congress to improve healthcare quality and reduce healthcare fraud and abuse. “A resignation also waives the physician’s right to contest the unfair review. In addition, leverage to negotiate a favorable settlement is lost if the physician simply resigns.”
Get a lawyer on board early. This is the only way to protect your rights. “Don’t wait a year to get an attorney involved,” Huntoon said. But this also can’t be any lawyer. It’s critical to find someone who specializes in sham peer reviews, so be sure to ask about their experience in handling peer review matters in hospitals and how knowledgeable they are about databank reporting requirements. “Sometimes, doctors will hire a malpractice attorney with no knowledge of what happens with sham peer reviews, and they may give bad advice,” he said. “Others may hire an employment attorney and that attorney will be up on employment law but has no experience with peer review matters in hospitals.”
Given the seriousness of a sham peer review, following these guidelines can help.
Contact the AAPA right away. There are things that can be done early on like getting a withdrawal of the request for corrective action as well as obtaining a preliminary injunction. Preparing for the fallout that may occur can be just as challenging.
“After this situation, the doctor is damaged goods,” Huntoon said. “What hospital will want to hire damaged goods to be part of their medical staff? Finding employment is going to be challenging and opening your own practice may also be difficult because the insurers have access to data bank reports.”
Ultimately, the best advice Huntoon can offer is to do your best to stay one step ahead of any work issues that could even lead to a sham peer review.
“Try and shield yourself from a sham peer review and be prepared should it happen,” he said. “I’ve seen careers end in the blink of an eye — wrongfully.”
A version of this article first appeared on Medscape.com.
On the Road to Care: Travel Nurses Still in Demand
Ashly Doran has worked at seven hospitals in four states since she graduated from nursing school in 2020. No, she isn’t job-hopping. Her travel nursing assignments have ranged from level 1 trauma center emergency rooms in big cities to small medical-surgical units in the suburbs. After each 13-week assignment, Doran packs up her belongings and her cats and moves to a new post.
“Travel nursing is so flexible,” she said. “I decide where I want to go and how much I want to make and start looking for travel contracts in that area.”
Nationwide nursing shortages have forced hospitals to hire travel nurses to fill staffing gaps. During the COVID-19 pandemic, the demand for travel nurses increased by 35%. While there is still a demand for nurses to fill short-term contracts, data show that demand has declined 42% between January and July 2022 and has continued the downward trend.
“What we’re seeing now is a shift…to a pre-pandemic market,” said Rachel Neill, RN, senior clinician advocate at Vivian Health. “Travel [nursing] is not going away — there will always be a need for hospital systems and facilities to fill gaps — but hospitals have shifted more into a traditional ... operational environment.”
Traveling a Different Path
For some registered nurses (RNs), short-term assignments offer opportunities to gain experience in different facilities or explore new locations before settling into permanent positions. Even experienced RNs embrace travel nursing for the flexible schedules and opportunities to take longer breaks between contracts.
Burnout and turnover among nurses are high, and flexible schedules, including controlling when to work, are essential to sustaining a clinical nursing career. In fact, 34% of nurses called travel nursing an “ideal option” for their lifestyle, with 14% viewing it as an option for career progression.
Travel nursing is especially appealing to Millennials and Generation Z, according to Brian Weirich, RN, chief nurse innovation officer at Bon Secours Mercy Health in Cincinnati, Ohio. In fact, the average age of a travel nurse is 35 compared with an average age of 52 for all RNs.
These are generations that are more focused on reducing school loan debt and gaining experience, not 401(k) and health insurance, he said in an interview. Pay is also a factor. The average pay for travel nurses was $2588 per month, compared with $1375 for permanent staff nurses.
During the pandemic, Weirich recalls groups of nurses resigning to take travel assignments together. The RNs picked desirable locations, accepted short-term assignments, and moved together, “making top dollar in locations they wanted to explore with their best friends.”
It’s been more than a decade since Kelly Spurlock traded a permanent nursing role in Lake Placid, Florida, for short-term nursing contracts in intensive care units in 20 states.
Spurlock works with a recruiter at Ingenovis Health to secure new contracts and considers travel assignments “working vacations.” In the process of exploring new places and meeting new people, Spurlock believes that travel nursing allows her to prioritize patient care.
“I can be at the bedside and be an advocate for my patient but also keep out of the spotlight for the political part of what we do,” she explained.
The Road Ahead
The appeal of travel nursing is taking new nursing assignments in different cities and earning higher salaries, but there are downsides, too. Travel nurses often receive fewer benefits than staff nurses and end up with less favorable assignments; their levels of dissatisfaction and burnout are also higher, and their sense of work-life balance is lower than staff nurses.
Most travel contracts last between 4 and 13 weeks. Hospitals often put policies and practices in place that limit the number of back-to-back contracts that traveling nurses can accept, which means that RNs can either convert to core staff or move on to new assignments once their contract term is up.
Weirich noted that some hospitals devote considerable effort to recruiting traveling nurses to full-time roles, adding, “There are active initiatives ... to make it such a good experience that they want to stay.”
On the flip side, contracts can be terminated without notice, leaving traveling nurses scrambling to find a new assignment and a new place to live on short notice.
“You’re there as long as the hospital needs you,” said Neill. “You could sign a 12- or 15-week contract, and their needs change a month in, and ... there are budget cuts, and they can’t pay salaries anymore, so they are laying off their nurses.”
Declining demand for travel nurses has made it harder to line up back-to-back contracts. Despite being available for work, Doran once waited 6 weeks to secure a new assignment and had to live off her savings.
Spurlock believes increased competition and declining wages — pay for travel nurses declined more than 9% from January 2023 to January 2024 — have made travel nursing less attractive.
“There has been such an influx of travel nurses ... because of COVID,” said Spurlock. “The rates have now come down [and] everybody’s fighting for jobs, and ... it’s very difficult to get a job that’s paying decent money.”
Despite the challenges, Spurlock continues learning new things from each assignment and hopes to work as a travel nurse until retirement. Doran has worked at hospitals in Washington, Oregon, California, and Wisconsin and would like to add Montana, Utah, and Nevada to the list. The goal: Continue accepting assignments in different cities and states until she finds the place where she wants to put down roots.
“Nursing is a great job, but it’s a hard job [and] it can take its toll at times,” Neill said. It’s important that nurses know their goals and values to be able to find a good fitting position. “And the beauty of it is that travel can be a great way to explore and add some flexibility.”
A version of this article first appeared on Medscape.com.
Ashly Doran has worked at seven hospitals in four states since she graduated from nursing school in 2020. No, she isn’t job-hopping. Her travel nursing assignments have ranged from level 1 trauma center emergency rooms in big cities to small medical-surgical units in the suburbs. After each 13-week assignment, Doran packs up her belongings and her cats and moves to a new post.
“Travel nursing is so flexible,” she said. “I decide where I want to go and how much I want to make and start looking for travel contracts in that area.”
Nationwide nursing shortages have forced hospitals to hire travel nurses to fill staffing gaps. During the COVID-19 pandemic, the demand for travel nurses increased by 35%. While there is still a demand for nurses to fill short-term contracts, data show that demand has declined 42% between January and July 2022 and has continued the downward trend.
“What we’re seeing now is a shift…to a pre-pandemic market,” said Rachel Neill, RN, senior clinician advocate at Vivian Health. “Travel [nursing] is not going away — there will always be a need for hospital systems and facilities to fill gaps — but hospitals have shifted more into a traditional ... operational environment.”
Traveling a Different Path
For some registered nurses (RNs), short-term assignments offer opportunities to gain experience in different facilities or explore new locations before settling into permanent positions. Even experienced RNs embrace travel nursing for the flexible schedules and opportunities to take longer breaks between contracts.
Burnout and turnover among nurses are high, and flexible schedules, including controlling when to work, are essential to sustaining a clinical nursing career. In fact, 34% of nurses called travel nursing an “ideal option” for their lifestyle, with 14% viewing it as an option for career progression.
Travel nursing is especially appealing to Millennials and Generation Z, according to Brian Weirich, RN, chief nurse innovation officer at Bon Secours Mercy Health in Cincinnati, Ohio. In fact, the average age of a travel nurse is 35 compared with an average age of 52 for all RNs.
These are generations that are more focused on reducing school loan debt and gaining experience, not 401(k) and health insurance, he said in an interview. Pay is also a factor. The average pay for travel nurses was $2588 per month, compared with $1375 for permanent staff nurses.
During the pandemic, Weirich recalls groups of nurses resigning to take travel assignments together. The RNs picked desirable locations, accepted short-term assignments, and moved together, “making top dollar in locations they wanted to explore with their best friends.”
It’s been more than a decade since Kelly Spurlock traded a permanent nursing role in Lake Placid, Florida, for short-term nursing contracts in intensive care units in 20 states.
Spurlock works with a recruiter at Ingenovis Health to secure new contracts and considers travel assignments “working vacations.” In the process of exploring new places and meeting new people, Spurlock believes that travel nursing allows her to prioritize patient care.
“I can be at the bedside and be an advocate for my patient but also keep out of the spotlight for the political part of what we do,” she explained.
The Road Ahead
The appeal of travel nursing is taking new nursing assignments in different cities and earning higher salaries, but there are downsides, too. Travel nurses often receive fewer benefits than staff nurses and end up with less favorable assignments; their levels of dissatisfaction and burnout are also higher, and their sense of work-life balance is lower than staff nurses.
Most travel contracts last between 4 and 13 weeks. Hospitals often put policies and practices in place that limit the number of back-to-back contracts that traveling nurses can accept, which means that RNs can either convert to core staff or move on to new assignments once their contract term is up.
Weirich noted that some hospitals devote considerable effort to recruiting traveling nurses to full-time roles, adding, “There are active initiatives ... to make it such a good experience that they want to stay.”
On the flip side, contracts can be terminated without notice, leaving traveling nurses scrambling to find a new assignment and a new place to live on short notice.
“You’re there as long as the hospital needs you,” said Neill. “You could sign a 12- or 15-week contract, and their needs change a month in, and ... there are budget cuts, and they can’t pay salaries anymore, so they are laying off their nurses.”
Declining demand for travel nurses has made it harder to line up back-to-back contracts. Despite being available for work, Doran once waited 6 weeks to secure a new assignment and had to live off her savings.
Spurlock believes increased competition and declining wages — pay for travel nurses declined more than 9% from January 2023 to January 2024 — have made travel nursing less attractive.
“There has been such an influx of travel nurses ... because of COVID,” said Spurlock. “The rates have now come down [and] everybody’s fighting for jobs, and ... it’s very difficult to get a job that’s paying decent money.”
Despite the challenges, Spurlock continues learning new things from each assignment and hopes to work as a travel nurse until retirement. Doran has worked at hospitals in Washington, Oregon, California, and Wisconsin and would like to add Montana, Utah, and Nevada to the list. The goal: Continue accepting assignments in different cities and states until she finds the place where she wants to put down roots.
“Nursing is a great job, but it’s a hard job [and] it can take its toll at times,” Neill said. It’s important that nurses know their goals and values to be able to find a good fitting position. “And the beauty of it is that travel can be a great way to explore and add some flexibility.”
A version of this article first appeared on Medscape.com.
Ashly Doran has worked at seven hospitals in four states since she graduated from nursing school in 2020. No, she isn’t job-hopping. Her travel nursing assignments have ranged from level 1 trauma center emergency rooms in big cities to small medical-surgical units in the suburbs. After each 13-week assignment, Doran packs up her belongings and her cats and moves to a new post.
“Travel nursing is so flexible,” she said. “I decide where I want to go and how much I want to make and start looking for travel contracts in that area.”
Nationwide nursing shortages have forced hospitals to hire travel nurses to fill staffing gaps. During the COVID-19 pandemic, the demand for travel nurses increased by 35%. While there is still a demand for nurses to fill short-term contracts, data show that demand has declined 42% between January and July 2022 and has continued the downward trend.
“What we’re seeing now is a shift…to a pre-pandemic market,” said Rachel Neill, RN, senior clinician advocate at Vivian Health. “Travel [nursing] is not going away — there will always be a need for hospital systems and facilities to fill gaps — but hospitals have shifted more into a traditional ... operational environment.”
Traveling a Different Path
For some registered nurses (RNs), short-term assignments offer opportunities to gain experience in different facilities or explore new locations before settling into permanent positions. Even experienced RNs embrace travel nursing for the flexible schedules and opportunities to take longer breaks between contracts.
Burnout and turnover among nurses are high, and flexible schedules, including controlling when to work, are essential to sustaining a clinical nursing career. In fact, 34% of nurses called travel nursing an “ideal option” for their lifestyle, with 14% viewing it as an option for career progression.
Travel nursing is especially appealing to Millennials and Generation Z, according to Brian Weirich, RN, chief nurse innovation officer at Bon Secours Mercy Health in Cincinnati, Ohio. In fact, the average age of a travel nurse is 35 compared with an average age of 52 for all RNs.
These are generations that are more focused on reducing school loan debt and gaining experience, not 401(k) and health insurance, he said in an interview. Pay is also a factor. The average pay for travel nurses was $2588 per month, compared with $1375 for permanent staff nurses.
During the pandemic, Weirich recalls groups of nurses resigning to take travel assignments together. The RNs picked desirable locations, accepted short-term assignments, and moved together, “making top dollar in locations they wanted to explore with their best friends.”
It’s been more than a decade since Kelly Spurlock traded a permanent nursing role in Lake Placid, Florida, for short-term nursing contracts in intensive care units in 20 states.
Spurlock works with a recruiter at Ingenovis Health to secure new contracts and considers travel assignments “working vacations.” In the process of exploring new places and meeting new people, Spurlock believes that travel nursing allows her to prioritize patient care.
“I can be at the bedside and be an advocate for my patient but also keep out of the spotlight for the political part of what we do,” she explained.
The Road Ahead
The appeal of travel nursing is taking new nursing assignments in different cities and earning higher salaries, but there are downsides, too. Travel nurses often receive fewer benefits than staff nurses and end up with less favorable assignments; their levels of dissatisfaction and burnout are also higher, and their sense of work-life balance is lower than staff nurses.
Most travel contracts last between 4 and 13 weeks. Hospitals often put policies and practices in place that limit the number of back-to-back contracts that traveling nurses can accept, which means that RNs can either convert to core staff or move on to new assignments once their contract term is up.
Weirich noted that some hospitals devote considerable effort to recruiting traveling nurses to full-time roles, adding, “There are active initiatives ... to make it such a good experience that they want to stay.”
On the flip side, contracts can be terminated without notice, leaving traveling nurses scrambling to find a new assignment and a new place to live on short notice.
“You’re there as long as the hospital needs you,” said Neill. “You could sign a 12- or 15-week contract, and their needs change a month in, and ... there are budget cuts, and they can’t pay salaries anymore, so they are laying off their nurses.”
Declining demand for travel nurses has made it harder to line up back-to-back contracts. Despite being available for work, Doran once waited 6 weeks to secure a new assignment and had to live off her savings.
Spurlock believes increased competition and declining wages — pay for travel nurses declined more than 9% from January 2023 to January 2024 — have made travel nursing less attractive.
“There has been such an influx of travel nurses ... because of COVID,” said Spurlock. “The rates have now come down [and] everybody’s fighting for jobs, and ... it’s very difficult to get a job that’s paying decent money.”
Despite the challenges, Spurlock continues learning new things from each assignment and hopes to work as a travel nurse until retirement. Doran has worked at hospitals in Washington, Oregon, California, and Wisconsin and would like to add Montana, Utah, and Nevada to the list. The goal: Continue accepting assignments in different cities and states until she finds the place where she wants to put down roots.
“Nursing is a great job, but it’s a hard job [and] it can take its toll at times,” Neill said. It’s important that nurses know their goals and values to be able to find a good fitting position. “And the beauty of it is that travel can be a great way to explore and add some flexibility.”
A version of this article first appeared on Medscape.com.
The Bad News Behind the Rise in Locum Tenens
I’ve worked locum tenens off and on since 1982. Flexible schedules allowed me to write several books, pursue a parallel career as a medical journalist, lead medical missions in the Philippines, and develop modest expertise as an underwater photographer.
But the recent rise in locum tenens practitioners signals trouble for medicine.
A Multibillion-Dollar Industry
Roughly 52,000 US doctors work locum tenens full or part time. In annual reports by CHG Healthcare, two thirds of healthcare facilities surveyed report using locums and more than half expect to maintain or increase their use in 2024.
Another measure of the industry’s growth is that membership of The National Association of Locum Tenens Organizations (NALTO), formed in 2001 to lead this fledgling industry, has doubled since 2019. Currently, NALTO has 148 member agencies.
Why Locums?
What used to be the preserve of older physicians transitioning to retirement is now becoming a career choice. According to the 2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals by AMN Healthcare, 81% of respondents said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. What entices doctors to move from place to place, repeatedly adapt to new facilities and electronic medical records, live in cheap hotels, and work without paid vacations, health insurance, or retirement benefits?
Supplemental income is one reason. But the elephant in the room is clearly burnout. Rates of burnout in practicing doctors and physicians-in-training have exceeded 50%. Burnout results in medical errors, malpractice suits, and increased healthcare costs.
A recent Doximity poll of 7590 physicians revealed that 63% would not want their children to pursue a medical career. And in a Medscape survey of 7000 physicians, a third of docs under 40 would not choose medicine again if they had a do-over. If a career in medicine brings high income and privileged status, why do so many physicians regret it and discourage their children from taking the same path?
Where Is Marcus Welby, MD?
Private practice is an endangered species that no one is trying to save. According to a 2022 AMA survey, 44% of physicians owned their practices compared with 76% of physicians in the 1980s. Even fewer younger physicians are choosing private practice. Among physicians under 45 years of age, only 32% owned their practices. Most physicians are now employees, not employers. They have lost control over their duties and work hours.
In 2022, barely 13% of physicians were in solo practice. The iconic Dr Marcus Welby of the 1970s TV series has transmuted from an idealized physician to an implausible figure. (My medical students have never heard of him.)
Hospitals and health systems have purchased many private medical groups. Private-equity companies own close to 1000 physician practices and staff up to 40% of emergency rooms. For these firms, profits are paramount.
Canary in a Coal Mine
Locum tenens offers physicians unprecedented flexibility where they work, when they work, and how much they work. It provides an escape from overwhelming and unsatisfying clinical practice. While some physicians have fled to nonclinical careers, locums physicians can practice medicine without the burdens of administration, hospital politics, and ever-increasing overhead.
The locum tenens paradox is that its successful growth indicates a deteriorating traditional healthcare model. Locum tenens is not the problem, but it’s also not the solution. At best, locums is a pair of crutches that helps the current system limp along.
Healthcare is increasingly controlled by those who prioritize profit, not patients. If physicians become nothing more than complicit cogs in a dysfunctional system, burnout will fester. The profession will fail to attract the best and the brightest, the doctor shortage will increase, and the quality of patient care will decline. Everyone will suffer.
It’s already happening.
Andrew Wilner is an associate professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported conflicts of interest from Accordant Health Services.
A version of this article first appeared on Medscape.com.
I’ve worked locum tenens off and on since 1982. Flexible schedules allowed me to write several books, pursue a parallel career as a medical journalist, lead medical missions in the Philippines, and develop modest expertise as an underwater photographer.
But the recent rise in locum tenens practitioners signals trouble for medicine.
A Multibillion-Dollar Industry
Roughly 52,000 US doctors work locum tenens full or part time. In annual reports by CHG Healthcare, two thirds of healthcare facilities surveyed report using locums and more than half expect to maintain or increase their use in 2024.
Another measure of the industry’s growth is that membership of The National Association of Locum Tenens Organizations (NALTO), formed in 2001 to lead this fledgling industry, has doubled since 2019. Currently, NALTO has 148 member agencies.
Why Locums?
What used to be the preserve of older physicians transitioning to retirement is now becoming a career choice. According to the 2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals by AMN Healthcare, 81% of respondents said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. What entices doctors to move from place to place, repeatedly adapt to new facilities and electronic medical records, live in cheap hotels, and work without paid vacations, health insurance, or retirement benefits?
Supplemental income is one reason. But the elephant in the room is clearly burnout. Rates of burnout in practicing doctors and physicians-in-training have exceeded 50%. Burnout results in medical errors, malpractice suits, and increased healthcare costs.
A recent Doximity poll of 7590 physicians revealed that 63% would not want their children to pursue a medical career. And in a Medscape survey of 7000 physicians, a third of docs under 40 would not choose medicine again if they had a do-over. If a career in medicine brings high income and privileged status, why do so many physicians regret it and discourage their children from taking the same path?
Where Is Marcus Welby, MD?
Private practice is an endangered species that no one is trying to save. According to a 2022 AMA survey, 44% of physicians owned their practices compared with 76% of physicians in the 1980s. Even fewer younger physicians are choosing private practice. Among physicians under 45 years of age, only 32% owned their practices. Most physicians are now employees, not employers. They have lost control over their duties and work hours.
In 2022, barely 13% of physicians were in solo practice. The iconic Dr Marcus Welby of the 1970s TV series has transmuted from an idealized physician to an implausible figure. (My medical students have never heard of him.)
Hospitals and health systems have purchased many private medical groups. Private-equity companies own close to 1000 physician practices and staff up to 40% of emergency rooms. For these firms, profits are paramount.
Canary in a Coal Mine
Locum tenens offers physicians unprecedented flexibility where they work, when they work, and how much they work. It provides an escape from overwhelming and unsatisfying clinical practice. While some physicians have fled to nonclinical careers, locums physicians can practice medicine without the burdens of administration, hospital politics, and ever-increasing overhead.
The locum tenens paradox is that its successful growth indicates a deteriorating traditional healthcare model. Locum tenens is not the problem, but it’s also not the solution. At best, locums is a pair of crutches that helps the current system limp along.
Healthcare is increasingly controlled by those who prioritize profit, not patients. If physicians become nothing more than complicit cogs in a dysfunctional system, burnout will fester. The profession will fail to attract the best and the brightest, the doctor shortage will increase, and the quality of patient care will decline. Everyone will suffer.
It’s already happening.
Andrew Wilner is an associate professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported conflicts of interest from Accordant Health Services.
A version of this article first appeared on Medscape.com.
I’ve worked locum tenens off and on since 1982. Flexible schedules allowed me to write several books, pursue a parallel career as a medical journalist, lead medical missions in the Philippines, and develop modest expertise as an underwater photographer.
But the recent rise in locum tenens practitioners signals trouble for medicine.
A Multibillion-Dollar Industry
Roughly 52,000 US doctors work locum tenens full or part time. In annual reports by CHG Healthcare, two thirds of healthcare facilities surveyed report using locums and more than half expect to maintain or increase their use in 2024.
Another measure of the industry’s growth is that membership of The National Association of Locum Tenens Organizations (NALTO), formed in 2001 to lead this fledgling industry, has doubled since 2019. Currently, NALTO has 148 member agencies.
Why Locums?
What used to be the preserve of older physicians transitioning to retirement is now becoming a career choice. According to the 2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals by AMN Healthcare, 81% of respondents said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. What entices doctors to move from place to place, repeatedly adapt to new facilities and electronic medical records, live in cheap hotels, and work without paid vacations, health insurance, or retirement benefits?
Supplemental income is one reason. But the elephant in the room is clearly burnout. Rates of burnout in practicing doctors and physicians-in-training have exceeded 50%. Burnout results in medical errors, malpractice suits, and increased healthcare costs.
A recent Doximity poll of 7590 physicians revealed that 63% would not want their children to pursue a medical career. And in a Medscape survey of 7000 physicians, a third of docs under 40 would not choose medicine again if they had a do-over. If a career in medicine brings high income and privileged status, why do so many physicians regret it and discourage their children from taking the same path?
Where Is Marcus Welby, MD?
Private practice is an endangered species that no one is trying to save. According to a 2022 AMA survey, 44% of physicians owned their practices compared with 76% of physicians in the 1980s. Even fewer younger physicians are choosing private practice. Among physicians under 45 years of age, only 32% owned their practices. Most physicians are now employees, not employers. They have lost control over their duties and work hours.
In 2022, barely 13% of physicians were in solo practice. The iconic Dr Marcus Welby of the 1970s TV series has transmuted from an idealized physician to an implausible figure. (My medical students have never heard of him.)
Hospitals and health systems have purchased many private medical groups. Private-equity companies own close to 1000 physician practices and staff up to 40% of emergency rooms. For these firms, profits are paramount.
Canary in a Coal Mine
Locum tenens offers physicians unprecedented flexibility where they work, when they work, and how much they work. It provides an escape from overwhelming and unsatisfying clinical practice. While some physicians have fled to nonclinical careers, locums physicians can practice medicine without the burdens of administration, hospital politics, and ever-increasing overhead.
The locum tenens paradox is that its successful growth indicates a deteriorating traditional healthcare model. Locum tenens is not the problem, but it’s also not the solution. At best, locums is a pair of crutches that helps the current system limp along.
Healthcare is increasingly controlled by those who prioritize profit, not patients. If physicians become nothing more than complicit cogs in a dysfunctional system, burnout will fester. The profession will fail to attract the best and the brightest, the doctor shortage will increase, and the quality of patient care will decline. Everyone will suffer.
It’s already happening.
Andrew Wilner is an associate professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported conflicts of interest from Accordant Health Services.
A version of this article first appeared on Medscape.com.
A History of Concussion Linked to Maternal Mental Illness
A history of concussion can have serious long-term mental health implications for women, even years after giving birth, according to a new study.
Researchers looked at all people who delivered babies in Ontario, Canada, and found that those with a predelivery history of concussion were 25% more likely to have a serious mental illness up to 14 years after giving birth than those with no history of concussion.
The findings indicate the need for early identification and screening of women with a history of concussion, as well as ongoing, long-term supports to prevent adverse psychiatric outcomes, wrote the authors.
“I played a lot of sports growing up, and I definitely would not have thought about how a concussion could affect childbearing or parenting,” author Samantha Krueger, RM, MSc, told this news organization. She completed the research as part of her studies at the University of Toronto, Ontario.
The data were published on November 4 in The Journal of Clinical Psychiatry.
Implications for Prevention
“Birthing people, and women in general, are an often-overlooked population in the scientific literature on traumatic brain injury, including concussion. There is a potential interplay between concussion history and the challenges of being a new parent (such as labor and birth, lack of sleep, and increased noise) that make this an important population to study,” said Krueger.
The researchers conducted a population-based cohort study of all women who gave birth in Ontario between 2007 and 2017. Follow-up continued until 2021. The primary outcome was severe maternal mental illness, which was defined as a psychiatric emergency department visit, psychiatric hospital admission, or self-harm or suicide in the 14 years after delivery.
The researchers identified 18,064 women with a predelivery history of concussion and 736,689 women without a history of concussion during the study period. Women with a predelivery history of concussion were more likely than those without such a history to live in a rural area and have a history of assault or mental illness.
Overall, 11.3% (n = 2033) of the women with a predelivery history of concussion developed severe maternal mental illness (14.7 per 1000 person-years), compared with 6.8% (n = 49,928) of the women without a predelivery history of concussion (7.9 per 1000 person-years).
The adjusted hazard ratio (aHR) was 1.25. The association was strongest in women who had a predelivery history of concussion but no history of mental illness (aHR, 1.33).
“We hope to increase awareness of the seriousness of having a concussion, even when it is considered a mild head injury,” Krueger said. “The results have important implications for concussion prevention measures for young people and for the provision of postpartum supports (such as mental health and other social supports like sleep relief) to mitigate the risk of serious mental illness outcomes in birthing people with a history of concussion.”
Healthcare providers, including maternity care providers, should be asking about concussion history and providing mental health screening and supports to clients and their families to detect mental illness before a serious outcome occurs, Krueger added.
“Maternity care providers can help birthing people and their families set up supports for after the baby is born and teach families about mental health symptoms to look out for. It’s also important that providers be certain that their care is trauma informed to avoid triggering a trauma response when providing care,” she said.
Area of Concern
“This research is novel and highlights an area of major concern,” Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization. Sherry did not participate in the study.
“Postpartum depression occurs in approximately 10%-25% of mothers, but it is likely that many more cases go undiagnosed. It is attributed to hormonal changes, genetic predisposition, and environmental factors, and while previous depression or mental illness is frequently considered a risk factor, traumatic brain injuries or concussions usually are not,” Sherry said.
“Mothers are already an at-risk population for mental illness, as illustrated by the high rates of postpartum depression, and so are people with a history of concussion or traumatic brain injury. What sets this study apart is that it shows the heightened risk for women with the combination of those two distinct risk factors. Identifying these risk factors is essential to providing preventive care. If care providers know a patient is at increased risk when starting a pregnancy, then they will likely catch warning signs earlier,” he said.
“Additionally, as the article suggests, maternal mental health often is not studied beyond the first postpartum year,” Sherry said.
“Mental health struggles during the first postpartum year have largely been normalized as part of the transition into parenthood, but mental health issues among parents later in life are less accepted. After birth, so much emphasis is moved from the parent to the child. Parents rightly prioritize their children, but our job as care providers is to ensure we are also prioritizing them. The prolonged period of this study helps illustrate how important the practice of prioritizing mothers’ mental health is,” he added.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The Canadian Institutes of Health Research also supported the study. Krueger is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Masters Award. Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A history of concussion can have serious long-term mental health implications for women, even years after giving birth, according to a new study.
Researchers looked at all people who delivered babies in Ontario, Canada, and found that those with a predelivery history of concussion were 25% more likely to have a serious mental illness up to 14 years after giving birth than those with no history of concussion.
The findings indicate the need for early identification and screening of women with a history of concussion, as well as ongoing, long-term supports to prevent adverse psychiatric outcomes, wrote the authors.
“I played a lot of sports growing up, and I definitely would not have thought about how a concussion could affect childbearing or parenting,” author Samantha Krueger, RM, MSc, told this news organization. She completed the research as part of her studies at the University of Toronto, Ontario.
The data were published on November 4 in The Journal of Clinical Psychiatry.
Implications for Prevention
“Birthing people, and women in general, are an often-overlooked population in the scientific literature on traumatic brain injury, including concussion. There is a potential interplay between concussion history and the challenges of being a new parent (such as labor and birth, lack of sleep, and increased noise) that make this an important population to study,” said Krueger.
The researchers conducted a population-based cohort study of all women who gave birth in Ontario between 2007 and 2017. Follow-up continued until 2021. The primary outcome was severe maternal mental illness, which was defined as a psychiatric emergency department visit, psychiatric hospital admission, or self-harm or suicide in the 14 years after delivery.
The researchers identified 18,064 women with a predelivery history of concussion and 736,689 women without a history of concussion during the study period. Women with a predelivery history of concussion were more likely than those without such a history to live in a rural area and have a history of assault or mental illness.
Overall, 11.3% (n = 2033) of the women with a predelivery history of concussion developed severe maternal mental illness (14.7 per 1000 person-years), compared with 6.8% (n = 49,928) of the women without a predelivery history of concussion (7.9 per 1000 person-years).
The adjusted hazard ratio (aHR) was 1.25. The association was strongest in women who had a predelivery history of concussion but no history of mental illness (aHR, 1.33).
“We hope to increase awareness of the seriousness of having a concussion, even when it is considered a mild head injury,” Krueger said. “The results have important implications for concussion prevention measures for young people and for the provision of postpartum supports (such as mental health and other social supports like sleep relief) to mitigate the risk of serious mental illness outcomes in birthing people with a history of concussion.”
Healthcare providers, including maternity care providers, should be asking about concussion history and providing mental health screening and supports to clients and their families to detect mental illness before a serious outcome occurs, Krueger added.
“Maternity care providers can help birthing people and their families set up supports for after the baby is born and teach families about mental health symptoms to look out for. It’s also important that providers be certain that their care is trauma informed to avoid triggering a trauma response when providing care,” she said.
Area of Concern
“This research is novel and highlights an area of major concern,” Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization. Sherry did not participate in the study.
“Postpartum depression occurs in approximately 10%-25% of mothers, but it is likely that many more cases go undiagnosed. It is attributed to hormonal changes, genetic predisposition, and environmental factors, and while previous depression or mental illness is frequently considered a risk factor, traumatic brain injuries or concussions usually are not,” Sherry said.
“Mothers are already an at-risk population for mental illness, as illustrated by the high rates of postpartum depression, and so are people with a history of concussion or traumatic brain injury. What sets this study apart is that it shows the heightened risk for women with the combination of those two distinct risk factors. Identifying these risk factors is essential to providing preventive care. If care providers know a patient is at increased risk when starting a pregnancy, then they will likely catch warning signs earlier,” he said.
“Additionally, as the article suggests, maternal mental health often is not studied beyond the first postpartum year,” Sherry said.
“Mental health struggles during the first postpartum year have largely been normalized as part of the transition into parenthood, but mental health issues among parents later in life are less accepted. After birth, so much emphasis is moved from the parent to the child. Parents rightly prioritize their children, but our job as care providers is to ensure we are also prioritizing them. The prolonged period of this study helps illustrate how important the practice of prioritizing mothers’ mental health is,” he added.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The Canadian Institutes of Health Research also supported the study. Krueger is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Masters Award. Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A history of concussion can have serious long-term mental health implications for women, even years after giving birth, according to a new study.
Researchers looked at all people who delivered babies in Ontario, Canada, and found that those with a predelivery history of concussion were 25% more likely to have a serious mental illness up to 14 years after giving birth than those with no history of concussion.
The findings indicate the need for early identification and screening of women with a history of concussion, as well as ongoing, long-term supports to prevent adverse psychiatric outcomes, wrote the authors.
“I played a lot of sports growing up, and I definitely would not have thought about how a concussion could affect childbearing or parenting,” author Samantha Krueger, RM, MSc, told this news organization. She completed the research as part of her studies at the University of Toronto, Ontario.
The data were published on November 4 in The Journal of Clinical Psychiatry.
Implications for Prevention
“Birthing people, and women in general, are an often-overlooked population in the scientific literature on traumatic brain injury, including concussion. There is a potential interplay between concussion history and the challenges of being a new parent (such as labor and birth, lack of sleep, and increased noise) that make this an important population to study,” said Krueger.
The researchers conducted a population-based cohort study of all women who gave birth in Ontario between 2007 and 2017. Follow-up continued until 2021. The primary outcome was severe maternal mental illness, which was defined as a psychiatric emergency department visit, psychiatric hospital admission, or self-harm or suicide in the 14 years after delivery.
The researchers identified 18,064 women with a predelivery history of concussion and 736,689 women without a history of concussion during the study period. Women with a predelivery history of concussion were more likely than those without such a history to live in a rural area and have a history of assault or mental illness.
Overall, 11.3% (n = 2033) of the women with a predelivery history of concussion developed severe maternal mental illness (14.7 per 1000 person-years), compared with 6.8% (n = 49,928) of the women without a predelivery history of concussion (7.9 per 1000 person-years).
The adjusted hazard ratio (aHR) was 1.25. The association was strongest in women who had a predelivery history of concussion but no history of mental illness (aHR, 1.33).
“We hope to increase awareness of the seriousness of having a concussion, even when it is considered a mild head injury,” Krueger said. “The results have important implications for concussion prevention measures for young people and for the provision of postpartum supports (such as mental health and other social supports like sleep relief) to mitigate the risk of serious mental illness outcomes in birthing people with a history of concussion.”
Healthcare providers, including maternity care providers, should be asking about concussion history and providing mental health screening and supports to clients and their families to detect mental illness before a serious outcome occurs, Krueger added.
“Maternity care providers can help birthing people and their families set up supports for after the baby is born and teach families about mental health symptoms to look out for. It’s also important that providers be certain that their care is trauma informed to avoid triggering a trauma response when providing care,” she said.
Area of Concern
“This research is novel and highlights an area of major concern,” Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization. Sherry did not participate in the study.
“Postpartum depression occurs in approximately 10%-25% of mothers, but it is likely that many more cases go undiagnosed. It is attributed to hormonal changes, genetic predisposition, and environmental factors, and while previous depression or mental illness is frequently considered a risk factor, traumatic brain injuries or concussions usually are not,” Sherry said.
“Mothers are already an at-risk population for mental illness, as illustrated by the high rates of postpartum depression, and so are people with a history of concussion or traumatic brain injury. What sets this study apart is that it shows the heightened risk for women with the combination of those two distinct risk factors. Identifying these risk factors is essential to providing preventive care. If care providers know a patient is at increased risk when starting a pregnancy, then they will likely catch warning signs earlier,” he said.
“Additionally, as the article suggests, maternal mental health often is not studied beyond the first postpartum year,” Sherry said.
“Mental health struggles during the first postpartum year have largely been normalized as part of the transition into parenthood, but mental health issues among parents later in life are less accepted. After birth, so much emphasis is moved from the parent to the child. Parents rightly prioritize their children, but our job as care providers is to ensure we are also prioritizing them. The prolonged period of this study helps illustrate how important the practice of prioritizing mothers’ mental health is,” he added.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The Canadian Institutes of Health Research also supported the study. Krueger is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Masters Award. Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY