User login
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
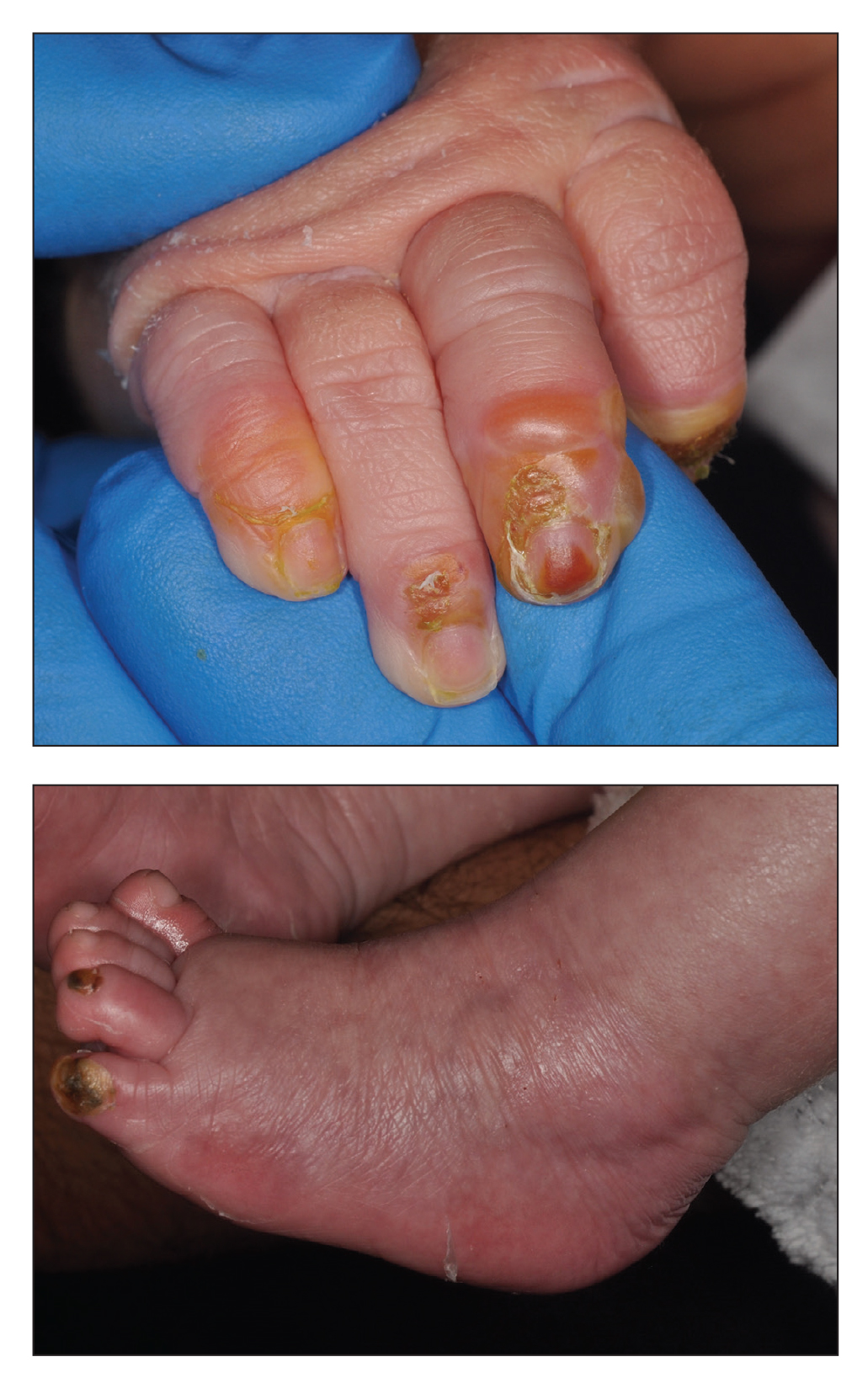
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
A 4-day-old infant boy presented with blisters on the skin. He was born at 36 weeks’ gestation by cesarean delivery to a nulliparous mother who received appropriate prenatal care. On day 2 of life, the patient developed bullae with breakdown of the skin on the bilateral heels and on the skin surrounding intravenous injection sites. Similar blisters subsequently developed on the fingers (top), thighs, groin, and toes (bottom), sparing the oral mucosa and trunk. He remained afebrile and stable and was started on ampicillin, gentamicin, and acyclovir with continued development of blisters. Two weeks later he developed painful ulcers on the tongue that bled upon scraping.