User login
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
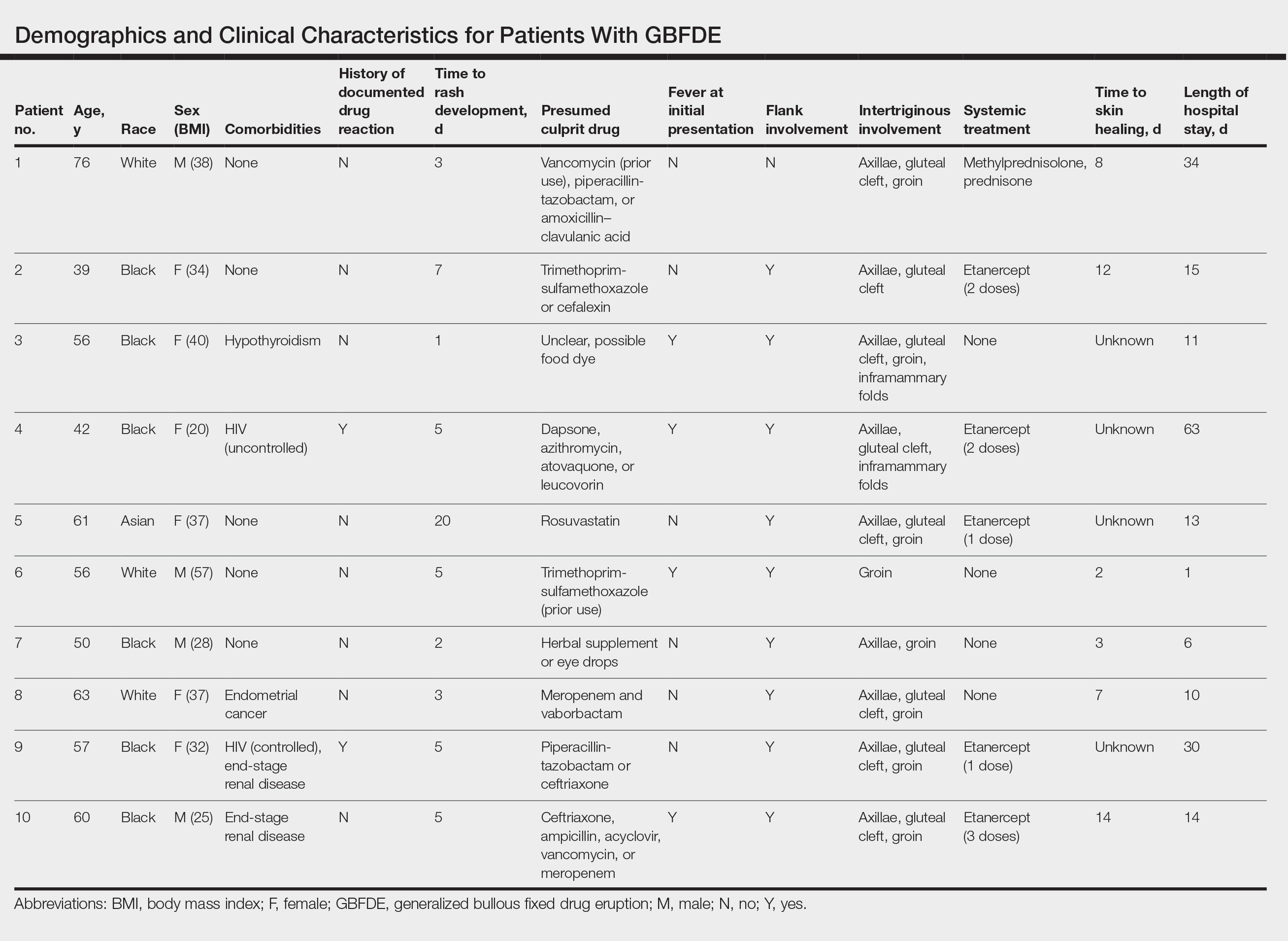
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).
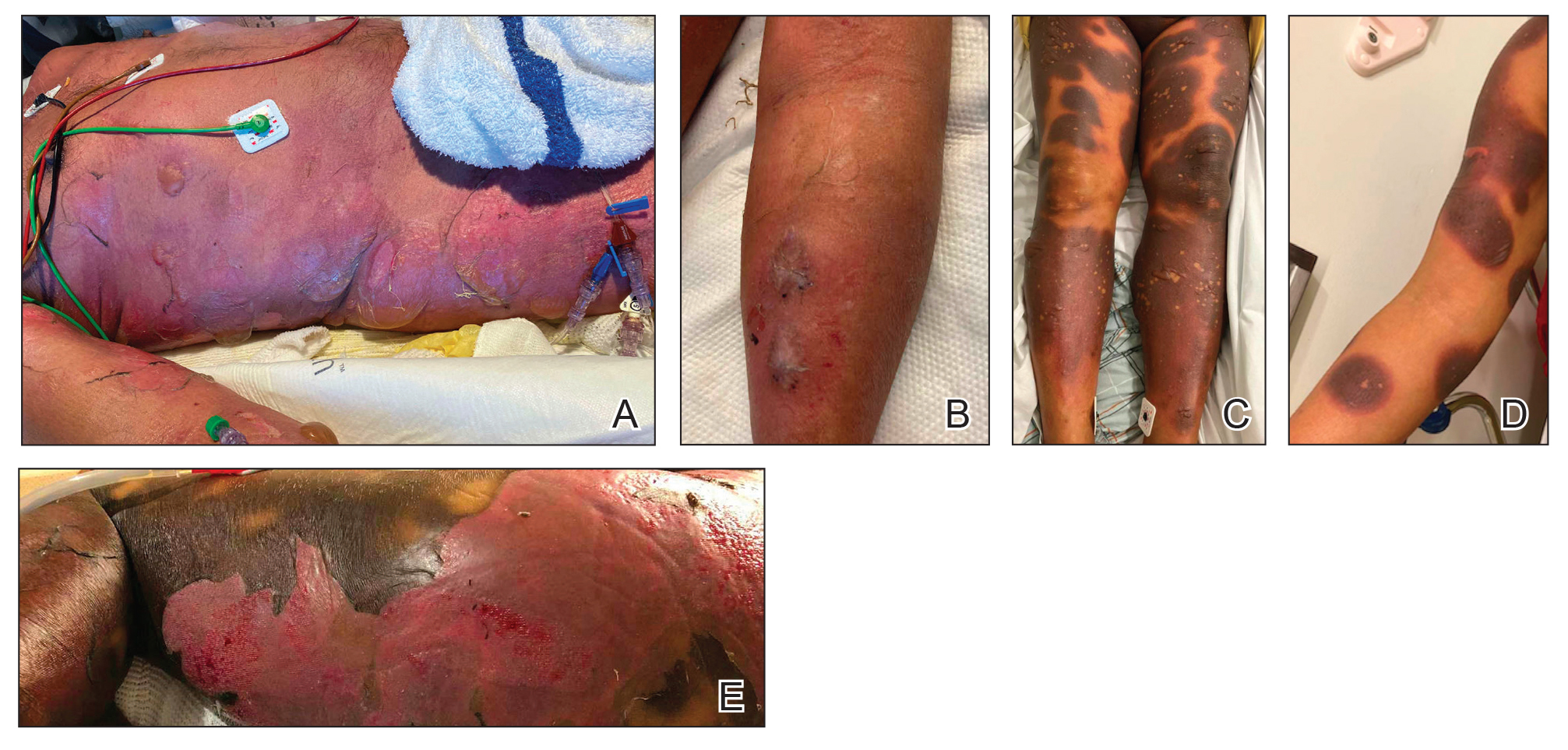
This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).


This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).


This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
PRACTICE POINTS
- Distinguishing features of generalized bullous fixed
drug eruption (GBFDE) may include truncal and proximal predilection with early intertriginous blistering. - Etanercept is a viable treatment option for GBFDE.