User login
The Food and Drug Administration on Feb. 4 gave clearance to an application for Apple’s iPad and iPhone that will allow physicians to review radiology images in the absence of a standard workstation.
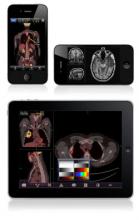
The FDA cleared the app, named Mobile MIM, for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and nuclear medicine technology such as positron emission tomography.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image’s luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine whether he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site that the Mobile MIM should be available in Apple’s App Store the week of Feb. 7.
The Food and Drug Administration on Feb. 4 gave clearance to an application for Apple’s iPad and iPhone that will allow physicians to review radiology images in the absence of a standard workstation.
The FDA cleared the app, named Mobile MIM, for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and nuclear medicine technology such as positron emission tomography.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image’s luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine whether he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site that the Mobile MIM should be available in Apple’s App Store the week of Feb. 7.
The Food and Drug Administration on Feb. 4 gave clearance to an application for Apple’s iPad and iPhone that will allow physicians to review radiology images in the absence of a standard workstation.
The FDA cleared the app, named Mobile MIM, for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and nuclear medicine technology such as positron emission tomography.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image’s luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine whether he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site that the Mobile MIM should be available in Apple’s App Store the week of Feb. 7.
FROM THE FOOD AND DRUG ADMINISTRATION