User login
A 28-year-old man developed fever, night sweats, nausea, headache, reduced appetite, skin rash, and hemoptysis 2 weeks after returning to the United States from Mexico.
The patient had fistulizing Crohn disease and had been taking the tumor necrosis factor alpha (TNF-alpha) blocker adalimumab for the past 3 months. He had no risk factors for human immunodeficiency virus infection, and he had stopped smoking 1 year previously. Chest radiography and a tuberculin skin test before he started adalimumab therapy were negative. While in Mexico, he did not drink more than 1 alcoholic beverage a day.
He had presented recently to his local hospital with the same symptoms and had been prescribed ciprofloxacin, metronidazole, ceftriaxone, vancomycin, and ampicillin, which he was still taking but with no improvement of symptoms. Blood cultures drawn before the start of antibiotic therapy had been negative. Urinalysis, a screen for infectious mononucleosis, and lumbar puncture were also negative. Results of renal function testing were normal except for the anion gap, which was 20.8 mmol/L (reference range 10–20).
INITIAL EVALUATION
On presentation to this hospital, the patient was afebrile but continued to have temperature spikes up to 39.0°C (102.2°F). His heart rate was 90 per minute, blood pressure 104/61 mm Hg, respiratory rate 18 per minute, and oxygen saturation 95% on 2 L of oxygen via nasal cannula.
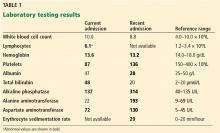
- White blood cell count 10.0 × 109/L (reference range 4.0–10.0 × 109/L)
- Lymphocyte count 6.1 × 109/L (1.2–3.4)
- Hemoglobin level 13.6 g/dL (14.0–18.0)
- Platelet count 87 × 109/L (150–400), reaching a nadir of 62 on hospital day 23
- Albumin 47 g/L (35–50)
- Total bilirubin 48 µmol/L (2–20)
- Alkaline phosphatase 137 U/L (40–135)
- Alanine aminotransferase 22 U/L (9–69)
- Aspartate aminotransferase 72 U/L (5–45).
He continued to have temperature spikes. His alkaline phosphatase level plateaued at 1,015 U/L on day 30, while his alanine aminotransferase and aspartate aminotransferase levels remained stable.
The patient’s ceftriaxone was continued, and the other antibiotics were replaced with doxycycline. Fluconazole was added when sputum culture grew Candida albicans. However, these drugs were later discontinued in view of worsening results on liver enzyme testing.
The evaluation continues
Sputum cultures were negative for acid-fast bacilli on 3 occasions.
Serologic testing was negative for:
- Hepatitis B surface antigen (but hepatitis B surface antibody was positive at > 1,000 IU/L)
- Hepatitis C virus antibody
- Cytomegalovirus immunoglobulin (Ig) G
- Toxoplasma gondii IgG
- Epstein-Barr virus viral capsid antigen IgM
- Rickettsia antibodies
- Antinuclear antibody
- Antineutrophil cytoplasmic antibody
- Antiglomerular basement membrane antibody.
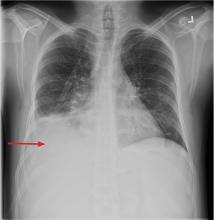
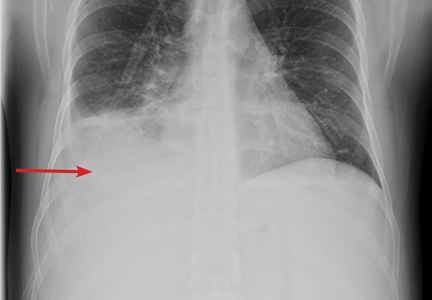
Chest radiography showed blunting of both costophrenic angles and mild prominence of right perihilar interstitial markings and the right hilum.
Computed tomography of the chest, abdomen, and pelvis showed a subpleural density in the lower lobe of the right lung, small bilateral pleural effusions, right hilar lymphadenopathy, and splenomegaly with no specific hepatobiliary abnormality.
A white blood cell nuclear scan found no occult infection.
Abdominal ultrasonography showed a prominent liver and spleen. The liver parenchyma showed diffuse decreased echogenicity, suggestive of hepatitis.
Transesophageal echocardiography showed no vegetations or valvular abnormalities.
Bronchoscopy showed normal airways without evidence of pulmonary hemorrhage. No foci of infection were obtained. A focus of granuloma consisting of epithelioid histiocytes in tight clusters was seen on washings from the right lower lobe, but no malignant cells were seen.
Sections of pathologically enlarged right hilar and subcarinal lymph nodes obtained with transbronchial needle aspiration were sent for cytologic analysis and flow cytometry.
Cultures for tuberculous and fungal organisms were negative.
A clue. On further inquiry, the patient said he had gone swimming in the natural pool, or cenote, under a rock formation at Cenote Maya Park in Mexico.
DIFFERENTIAL DIAGNOSIS
1. Which of the following is not in the differential diagnosis?
- Disseminated tuberculosis
- Coccidioidomycosis
- Subacute infective endocarditis
- Disseminated histoplasmosis
- Blastomycosis
Although the patient has a systemic disease, subacute infective endocarditis is not likely because of a lack of predisposing factors such as a history of endocarditis, abnormal or artificial heart valve, or intravenous drug abuse. Moreover, negative blood cultures and the absence of vegetations on echocardiography make endocarditis very unlikely.
Given that the patient is immunosuppressed, opportunistic infection must be at the top of the differential diagnosis. Histoplasmosis, coccidioidomycosis, and blastomycosis are endemic in Mexico. Disseminated histoplasmosis is the most likely diagnosis; coccidioidomycosis and blastomycosis are less likely, based on the history, signs, and symptoms. Disseminated tuberculosis must be excluded before other diagnostic possibilities are considered.
TUBERCULOSIS IN PATIENTS ON TNF-ALPHA ANTAGONISTS
Tuberculosis has been reported in patients taking TNF-alpha antagonists.1 The frequency of tuberculosis is much higher than that of other opportunistic infections, and over 50% of reported cases involve extrapulmonary tissues in patients treated with TNF-alpha antagonists.2
British Thoracic Society guidelines recommend screening for latent tuberculosis before starting treatment with a TNF-alpha antagonist; the screening should include a history of tuberculosis treatment, a clinical examination, chest radiography, and a tuberculin skin test.3 Patients found to have active tuberculosis should receive a minimum of 2 months of standard treatment before starting a TNF-alpha antagonist. Patients with evidence of past tuberculosis or a history of tuberculosis who received adequate treatment should be monitored regularly. Patients with prior tuberculosis not adequately treated should receive chemoprophylaxis before starting a TNF-alpha antagonist.
Fever, night sweats, and intrathoracic and intra-abdominal lymphadenopathy are common features of disseminated tuberculosis. Upper-lobe cavitary disease or miliary lesions may be seen on chest radiography, but atypical presentations with lower-lobe infiltrate are not uncommon in immunosuppressed patients.4
A negative tuberculin skin test and a normal chest radiograph 3 months ago, along with negative sputum and bronchial lavage fluid cultures and no history of tuberculosis contact, make tuberculosis unlikely in our patient.
COCCIDIOIDOMYCOSIS
Coccidioidomycosis (valley fever) is caused by the fungus Coccidioides immitis, which lives in the soil and is acquired by inhalation of airborne microscopic spores.
Fatigue, cough, fever, shortness of breath, headache, night sweats, muscle or joint pain, and a rash on the upper body or legs are common symptoms. It may cause a self-limiting flulike illness. From 5% to 10% of patients may develop serious long-term lung problems. In a small number of patients, the disease may progress beyond the lungs to involve the central nervous system, spinal cord, skin, bones, and joints.5
Serologic testing is highly useful for the diagnosis. Antigen testing has a sensitivity of 71% and a specificity of 98% for the diagnosis, but cross-reactivity occurs in 10% of patients with other types of mycosis. Respiratory secretions and tissue samples should undergo microscopic study and culture.
BLASTOMYCOSIS
Blastomycosis is caused by the fungus Blastomyces dermatitidis, which lives in soil and in association with decomposing organic matter such as wood and leaves. Inhalation of spores may cause a flulike illness or pneumonia. In serious cases, the disease can spread to skin and bone.
The diagnosis is established with fungal cultures of tissue samples or body fluids (bone marrow, liver tissue, skin, sputum, blood). Rapid diagnosis may be obtained by examination of the secretions under a microscope, where typical broad-based budding yeast can be seen in almost 90% of cases.6 Antigen may also be detected in urine and serum7; the sensitivity of antigen testing is 93% and the specificity is 98%. Serologic testing is not recommended for diagnosis of blastomycosis because of poor sensitivity and specificity.8
NARROWING THE DIFFERENTIAL
Both coccidioidomycosis and blastomycosis should be included in the differential diagnosis of a systemic disease with subacute onset and prominent lung involvement in a patient returning from travel to Mexico. The lack of involvement of the central nervous system, spinal cord, bones, or joints makes these infections less likely in our patient.
However, swimming in a cenote under a rock formation is an important clue to the diagnosis in our patient, as it puts him at risk of inhaling microconidia or hyphal elements of histoplasmosis. This, along with his immunocompromised status, fever, hemoptysis, night sweats, skin and lung features, and the generally subacute course of his illness, make disseminated histoplasmosis the most likely diagnosis.
Radiologic findings of pulmonary infiltrate with effusion and elevated lactate dehydrogenase, aminotransferases, and alkaline phosphatase increase the likelihood of disseminated histoplasmosis.
HISTOPLASMOSIS
Histoplasma capsulatum is a dimorphic fungus that thrives in the soil and caves of regions with moderate climate, especially in soil containing large amounts of bird excreta or bat guano.9 Bats are natural hosts of this organism, and it is endemic in North and Central America, including parts of Mexico. Air currents can carry the microconidia for miles, thus exposing people without direct contact with contaminated sites.
The infection is usually acquired by inhalation of microconidia or small hyphal elements or by reactivation of previously quiescent foci of infection in an immunosuppressed patient. Most patients exposed to H capsulatum remain asymptomatic or develop mild symptoms, which are self-limiting. A small number develop acute pulmonary histoplasmosis or chronic cavitary histoplasmosis. Disseminated disease usually occurs only in an immunosuppressed host.
Acute pulmonary histoplasmosis presents with fever, malaise, headache, weakness, substernal chest pain, and dry cough and may be associated with erythema nodosum, erythema multiforme, and arthralgias. It may be mistaken for sarcoidosis since enlarged hilar and mediastinal lymph nodes are often seen on chest radiography.10
Progressive disseminated histoplasmosis is defined as a clinical illness that does not improve after at least 3 weeks of observation and is associated with physical or radiographic findings with or without laboratory evidence of extrapulmonary involvement.11
Fever, malaise, anorexia, weight loss, night sweats, hepatosplenomegaly, and lymphadenopathy are features of progressive disseminated histoplasmosis.
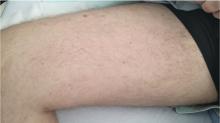
Cutaneous manifestations of disseminated histoplasmosis occur in 10% to 25% of patients with acquired immunodeficiency syndrome and include papules, plaques with or without crust, pustules, nodules, lesions resembling molluscum contagiosum virus infection, acneiform eruptions, erythematous macules, and keratotic plaques.12
TESTING FOR HISTOPLASMOSIS
2. What investigation is least likely to help confirm the diagnosis of disseminated histoplasmosis?
- Polymerase chain reaction (PCR) testing of serum, cerebrospinal fluid, and bronchoalveolar lavage specimens
- Urinary Histoplasma antigen testing
- Serologic testing
- Blood and bronchoalveolar lavage cultures
Urinary Histoplasma antigen has a sensitivity of 90% for the diagnosis of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome.18 It is less useful for pulmonary forms of histoplasmosis: the sensitivity is 75% and may even be less in milder or chronic forms of pneumonia.19 False-positive reactions may occur in patients with other fungal infections such as coccidioidomycosis, blastomycosis, paracoccidioidomycosis and penicilliosis.20 Urine antigen levels can also be used to monitor therapy, since levels decrease during therapy and increase in 90% of those who have a relapse.21
Our patient’s urinary Histoplasma antigen level was greater than 23.0 ng/mL (positive is > 0.50).
Serologic testing. Immunodiffusion immunoglobulin G (IgG) testing for Histoplasma and Blastomyces was negative, as was an enzyme immunoassay for Coccidioides IgG and IgM. However, antibody tests are less useful in immunosuppressed patients,22 and thus a negative result does not rule out histoplasmosis. A fourfold rise in complement fixation antibody titer is diagnostic of acute histoplasmosis. A single complement fixation titer of 1:32 is suggestive but not diagnostic of histoplasmosis. Cross-reactions may occur with other fungal infections like blastomycosis. The immunodiffusion assay has a greater specificity but slightly less sensitivity than the complement fixation assay.19
Culture of H capsulatum is the definitive test to establish a diagnosis of histoplasmosis. Culture can be performed on samples taken from blood, bone marrow, sputum, and bronchoalveolar lavage fluid, or from lung, liver, or lymph node tissue. Cultures are positive in 74% to 82% of cases of progressive disseminated histoplasmosis.13 However, treatment should not await culture results since the fungus may take several weeks to grow.
Back to our patient
Although Histoplasma serologic studies and cultures were negative, the diagnosis of disseminated histoplasmosis was made on the basis of the patient’s immunosuppressed status, travel history, clinical features, and positivity for urine Histoplasma antigen. Though urine histoplama antigen may be falsely positive in other fungal infections such as coccidioidomycosis, paracoccidioidomycosis, and blastomycosis, clinical features and the absence of central nervous system, joint, and bone involvement suggested disseminated histoplasmosis.
TREATMENT
3. What is the appropriate treatment for this patient?
- Amphotericin B followed by oral itraconozole
- Oral fluconazole
- Oral itraconazole
Liposomal amphotericin B or amphotericin B deoxycholate is recommended as initial therapy for moderately severe to severe and progressive disseminated histoplasmosis. It should be continued for 1 to 2 weeks, followed by oral itraconazole (200 mg 3 times daily for 3 days, then 200 mg 2 times daily for at least 12 months).
Monitoring itraconazole therapy through random serum levels is strongly recommended, and a random concentration of at least 1.0 mg/mL is recommended.23
Urine antigen levels should be measured before treatment is started, at 2 weeks, at 1 month, then every 3 months during therapy, continuing for 12 months after treatment is stopped.11
Lifelong suppressive therapy with itraconazole 200 mg daily may be required in immunosuppressed patients and patients who have a relapse despite appropriate therapy.11
While oral itraconazole is used as a sole agent for the treatment of mild to moderate acute pulmonary histoplasmosis and chronic cavitary pulmonary histoplasmosis, oral treatment alone with either fluconazole or itraconazole is not recommended for the treatment of progressive disseminated histoplasmosis.11
COMPLICATIONS OF HISTOPLASMOSIS
4. Which of the following is not a possible complication of histoplasmosis?
- Chronic cavitary pulmonary histoplasmosis
- Fibrosing mediastinitis
- Hypoadrenalism
- Hypothyroidism
Chronic cavitary pulmonary histoplasmosis usually develops in patients with underlying emphysema. Fatigue, night sweats, fever, anorexia, and weight loss are features of chronic cavitary pulmonary histoplasmosis. Progression of necrosis may lead to “marching cavity,” in which necrosis increases the size of the cavity and may consume an entire lobe.10
Fibrosing mediastinitis is an uncommon but often lethal complication of disseminated histoplasmosis. Increasing dyspnea, cough, hemoptysis, and signs of superior vena cava syndrome and right heart failure may develop. However, fibrosing mediastinitis is thought to be due to an exuberant immune response to past Histoplasma infection and would not be expected in an immunocompromised patient.17
Hypoadrenalism. Extensive destruction of the adrenal glands may lead to hypoadrenalism, manifesting as orthostatic hypotension, hyperkalemia, hyponatremia, and evidence of markedly enlarged adrenal glands with central necrosis on computed tomography.24
Hypothyroidism. Acute or disseminated histoplasmosis has not been reported to cause thyroid dysfunction.
CASE CONCLUSION
Our patient was treated with itraconazole 200 mg twice daily for 24 months. Although the literature supports lifelong itraconazole therapy in immunosuppressed patients, our patient was reluctant to do so. He agreed to close monitoring. If symptoms recur, itraconazole will be reinstituted and continued lifelong.
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61:409–417.
- Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis 2003; 3:148–155.
- British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005; 60:800–805.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1998. A 19-year-old man with the acquired immunodeficiency syndrome and persistent fever. N Engl J Med 1998; 339:1835–1843.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Lemos LB, Guo M, Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol 2000; 4:391–406.
- Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Micribiol 2004; 42:4873–4875.
- Wheat LJ. Approach to the diagnosis of the endemic mycoses. Clin Chest Med 2009; 30:379–389.
- Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49:785–798.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Chang P, Rodas C. Skin lesions in histoplasmosis. Clinics Dermatol 2012; 30:592–598.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol 2012; 19:53–56.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am 2016; 30:247–264.
- Stockamp NW, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2016; 30:229–246.
- Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am 2016; 30:207–227.
- Wheat LJ, Garringer T, Drizendine E, Connolly P. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis 2002; 14:1389–1391.
- Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 2008; 21:421–425.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob Agents Chemother 2002; 46:248–250.
- Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol 2003; 11:488–494.
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 1998; 35:461–473.
- Sarosi GA, Voth DW, Dahl BA, Doto IL, Tosh FE. Disseminated histoplasmosis: results of long-term follow-up. Ann Intern Med 1971; 75:511–516.
A 28-year-old man developed fever, night sweats, nausea, headache, reduced appetite, skin rash, and hemoptysis 2 weeks after returning to the United States from Mexico.
The patient had fistulizing Crohn disease and had been taking the tumor necrosis factor alpha (TNF-alpha) blocker adalimumab for the past 3 months. He had no risk factors for human immunodeficiency virus infection, and he had stopped smoking 1 year previously. Chest radiography and a tuberculin skin test before he started adalimumab therapy were negative. While in Mexico, he did not drink more than 1 alcoholic beverage a day.
He had presented recently to his local hospital with the same symptoms and had been prescribed ciprofloxacin, metronidazole, ceftriaxone, vancomycin, and ampicillin, which he was still taking but with no improvement of symptoms. Blood cultures drawn before the start of antibiotic therapy had been negative. Urinalysis, a screen for infectious mononucleosis, and lumbar puncture were also negative. Results of renal function testing were normal except for the anion gap, which was 20.8 mmol/L (reference range 10–20).
INITIAL EVALUATION
On presentation to this hospital, the patient was afebrile but continued to have temperature spikes up to 39.0°C (102.2°F). His heart rate was 90 per minute, blood pressure 104/61 mm Hg, respiratory rate 18 per minute, and oxygen saturation 95% on 2 L of oxygen via nasal cannula.
- White blood cell count 10.0 × 109/L (reference range 4.0–10.0 × 109/L)
- Lymphocyte count 6.1 × 109/L (1.2–3.4)
- Hemoglobin level 13.6 g/dL (14.0–18.0)
- Platelet count 87 × 109/L (150–400), reaching a nadir of 62 on hospital day 23
- Albumin 47 g/L (35–50)
- Total bilirubin 48 µmol/L (2–20)
- Alkaline phosphatase 137 U/L (40–135)
- Alanine aminotransferase 22 U/L (9–69)
- Aspartate aminotransferase 72 U/L (5–45).
He continued to have temperature spikes. His alkaline phosphatase level plateaued at 1,015 U/L on day 30, while his alanine aminotransferase and aspartate aminotransferase levels remained stable.
The patient’s ceftriaxone was continued, and the other antibiotics were replaced with doxycycline. Fluconazole was added when sputum culture grew Candida albicans. However, these drugs were later discontinued in view of worsening results on liver enzyme testing.
The evaluation continues
Sputum cultures were negative for acid-fast bacilli on 3 occasions.
Serologic testing was negative for:
- Hepatitis B surface antigen (but hepatitis B surface antibody was positive at > 1,000 IU/L)
- Hepatitis C virus antibody
- Cytomegalovirus immunoglobulin (Ig) G
- Toxoplasma gondii IgG
- Epstein-Barr virus viral capsid antigen IgM
- Rickettsia antibodies
- Antinuclear antibody
- Antineutrophil cytoplasmic antibody
- Antiglomerular basement membrane antibody.
Chest radiography showed blunting of both costophrenic angles and mild prominence of right perihilar interstitial markings and the right hilum.
Computed tomography of the chest, abdomen, and pelvis showed a subpleural density in the lower lobe of the right lung, small bilateral pleural effusions, right hilar lymphadenopathy, and splenomegaly with no specific hepatobiliary abnormality.
A white blood cell nuclear scan found no occult infection.
Abdominal ultrasonography showed a prominent liver and spleen. The liver parenchyma showed diffuse decreased echogenicity, suggestive of hepatitis.
Transesophageal echocardiography showed no vegetations or valvular abnormalities.
Bronchoscopy showed normal airways without evidence of pulmonary hemorrhage. No foci of infection were obtained. A focus of granuloma consisting of epithelioid histiocytes in tight clusters was seen on washings from the right lower lobe, but no malignant cells were seen.
Sections of pathologically enlarged right hilar and subcarinal lymph nodes obtained with transbronchial needle aspiration were sent for cytologic analysis and flow cytometry.
Cultures for tuberculous and fungal organisms were negative.
A clue. On further inquiry, the patient said he had gone swimming in the natural pool, or cenote, under a rock formation at Cenote Maya Park in Mexico.
DIFFERENTIAL DIAGNOSIS
1. Which of the following is not in the differential diagnosis?
- Disseminated tuberculosis
- Coccidioidomycosis
- Subacute infective endocarditis
- Disseminated histoplasmosis
- Blastomycosis
Although the patient has a systemic disease, subacute infective endocarditis is not likely because of a lack of predisposing factors such as a history of endocarditis, abnormal or artificial heart valve, or intravenous drug abuse. Moreover, negative blood cultures and the absence of vegetations on echocardiography make endocarditis very unlikely.
Given that the patient is immunosuppressed, opportunistic infection must be at the top of the differential diagnosis. Histoplasmosis, coccidioidomycosis, and blastomycosis are endemic in Mexico. Disseminated histoplasmosis is the most likely diagnosis; coccidioidomycosis and blastomycosis are less likely, based on the history, signs, and symptoms. Disseminated tuberculosis must be excluded before other diagnostic possibilities are considered.
TUBERCULOSIS IN PATIENTS ON TNF-ALPHA ANTAGONISTS
Tuberculosis has been reported in patients taking TNF-alpha antagonists.1 The frequency of tuberculosis is much higher than that of other opportunistic infections, and over 50% of reported cases involve extrapulmonary tissues in patients treated with TNF-alpha antagonists.2
British Thoracic Society guidelines recommend screening for latent tuberculosis before starting treatment with a TNF-alpha antagonist; the screening should include a history of tuberculosis treatment, a clinical examination, chest radiography, and a tuberculin skin test.3 Patients found to have active tuberculosis should receive a minimum of 2 months of standard treatment before starting a TNF-alpha antagonist. Patients with evidence of past tuberculosis or a history of tuberculosis who received adequate treatment should be monitored regularly. Patients with prior tuberculosis not adequately treated should receive chemoprophylaxis before starting a TNF-alpha antagonist.
Fever, night sweats, and intrathoracic and intra-abdominal lymphadenopathy are common features of disseminated tuberculosis. Upper-lobe cavitary disease or miliary lesions may be seen on chest radiography, but atypical presentations with lower-lobe infiltrate are not uncommon in immunosuppressed patients.4
A negative tuberculin skin test and a normal chest radiograph 3 months ago, along with negative sputum and bronchial lavage fluid cultures and no history of tuberculosis contact, make tuberculosis unlikely in our patient.
COCCIDIOIDOMYCOSIS
Coccidioidomycosis (valley fever) is caused by the fungus Coccidioides immitis, which lives in the soil and is acquired by inhalation of airborne microscopic spores.
Fatigue, cough, fever, shortness of breath, headache, night sweats, muscle or joint pain, and a rash on the upper body or legs are common symptoms. It may cause a self-limiting flulike illness. From 5% to 10% of patients may develop serious long-term lung problems. In a small number of patients, the disease may progress beyond the lungs to involve the central nervous system, spinal cord, skin, bones, and joints.5
Serologic testing is highly useful for the diagnosis. Antigen testing has a sensitivity of 71% and a specificity of 98% for the diagnosis, but cross-reactivity occurs in 10% of patients with other types of mycosis. Respiratory secretions and tissue samples should undergo microscopic study and culture.
BLASTOMYCOSIS
Blastomycosis is caused by the fungus Blastomyces dermatitidis, which lives in soil and in association with decomposing organic matter such as wood and leaves. Inhalation of spores may cause a flulike illness or pneumonia. In serious cases, the disease can spread to skin and bone.
The diagnosis is established with fungal cultures of tissue samples or body fluids (bone marrow, liver tissue, skin, sputum, blood). Rapid diagnosis may be obtained by examination of the secretions under a microscope, where typical broad-based budding yeast can be seen in almost 90% of cases.6 Antigen may also be detected in urine and serum7; the sensitivity of antigen testing is 93% and the specificity is 98%. Serologic testing is not recommended for diagnosis of blastomycosis because of poor sensitivity and specificity.8
NARROWING THE DIFFERENTIAL
Both coccidioidomycosis and blastomycosis should be included in the differential diagnosis of a systemic disease with subacute onset and prominent lung involvement in a patient returning from travel to Mexico. The lack of involvement of the central nervous system, spinal cord, bones, or joints makes these infections less likely in our patient.
However, swimming in a cenote under a rock formation is an important clue to the diagnosis in our patient, as it puts him at risk of inhaling microconidia or hyphal elements of histoplasmosis. This, along with his immunocompromised status, fever, hemoptysis, night sweats, skin and lung features, and the generally subacute course of his illness, make disseminated histoplasmosis the most likely diagnosis.
Radiologic findings of pulmonary infiltrate with effusion and elevated lactate dehydrogenase, aminotransferases, and alkaline phosphatase increase the likelihood of disseminated histoplasmosis.
HISTOPLASMOSIS
Histoplasma capsulatum is a dimorphic fungus that thrives in the soil and caves of regions with moderate climate, especially in soil containing large amounts of bird excreta or bat guano.9 Bats are natural hosts of this organism, and it is endemic in North and Central America, including parts of Mexico. Air currents can carry the microconidia for miles, thus exposing people without direct contact with contaminated sites.
The infection is usually acquired by inhalation of microconidia or small hyphal elements or by reactivation of previously quiescent foci of infection in an immunosuppressed patient. Most patients exposed to H capsulatum remain asymptomatic or develop mild symptoms, which are self-limiting. A small number develop acute pulmonary histoplasmosis or chronic cavitary histoplasmosis. Disseminated disease usually occurs only in an immunosuppressed host.
Acute pulmonary histoplasmosis presents with fever, malaise, headache, weakness, substernal chest pain, and dry cough and may be associated with erythema nodosum, erythema multiforme, and arthralgias. It may be mistaken for sarcoidosis since enlarged hilar and mediastinal lymph nodes are often seen on chest radiography.10
Progressive disseminated histoplasmosis is defined as a clinical illness that does not improve after at least 3 weeks of observation and is associated with physical or radiographic findings with or without laboratory evidence of extrapulmonary involvement.11
Fever, malaise, anorexia, weight loss, night sweats, hepatosplenomegaly, and lymphadenopathy are features of progressive disseminated histoplasmosis.
Cutaneous manifestations of disseminated histoplasmosis occur in 10% to 25% of patients with acquired immunodeficiency syndrome and include papules, plaques with or without crust, pustules, nodules, lesions resembling molluscum contagiosum virus infection, acneiform eruptions, erythematous macules, and keratotic plaques.12
TESTING FOR HISTOPLASMOSIS
2. What investigation is least likely to help confirm the diagnosis of disseminated histoplasmosis?
- Polymerase chain reaction (PCR) testing of serum, cerebrospinal fluid, and bronchoalveolar lavage specimens
- Urinary Histoplasma antigen testing
- Serologic testing
- Blood and bronchoalveolar lavage cultures
Urinary Histoplasma antigen has a sensitivity of 90% for the diagnosis of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome.18 It is less useful for pulmonary forms of histoplasmosis: the sensitivity is 75% and may even be less in milder or chronic forms of pneumonia.19 False-positive reactions may occur in patients with other fungal infections such as coccidioidomycosis, blastomycosis, paracoccidioidomycosis and penicilliosis.20 Urine antigen levels can also be used to monitor therapy, since levels decrease during therapy and increase in 90% of those who have a relapse.21
Our patient’s urinary Histoplasma antigen level was greater than 23.0 ng/mL (positive is > 0.50).
Serologic testing. Immunodiffusion immunoglobulin G (IgG) testing for Histoplasma and Blastomyces was negative, as was an enzyme immunoassay for Coccidioides IgG and IgM. However, antibody tests are less useful in immunosuppressed patients,22 and thus a negative result does not rule out histoplasmosis. A fourfold rise in complement fixation antibody titer is diagnostic of acute histoplasmosis. A single complement fixation titer of 1:32 is suggestive but not diagnostic of histoplasmosis. Cross-reactions may occur with other fungal infections like blastomycosis. The immunodiffusion assay has a greater specificity but slightly less sensitivity than the complement fixation assay.19
Culture of H capsulatum is the definitive test to establish a diagnosis of histoplasmosis. Culture can be performed on samples taken from blood, bone marrow, sputum, and bronchoalveolar lavage fluid, or from lung, liver, or lymph node tissue. Cultures are positive in 74% to 82% of cases of progressive disseminated histoplasmosis.13 However, treatment should not await culture results since the fungus may take several weeks to grow.
Back to our patient
Although Histoplasma serologic studies and cultures were negative, the diagnosis of disseminated histoplasmosis was made on the basis of the patient’s immunosuppressed status, travel history, clinical features, and positivity for urine Histoplasma antigen. Though urine histoplama antigen may be falsely positive in other fungal infections such as coccidioidomycosis, paracoccidioidomycosis, and blastomycosis, clinical features and the absence of central nervous system, joint, and bone involvement suggested disseminated histoplasmosis.
TREATMENT
3. What is the appropriate treatment for this patient?
- Amphotericin B followed by oral itraconozole
- Oral fluconazole
- Oral itraconazole
Liposomal amphotericin B or amphotericin B deoxycholate is recommended as initial therapy for moderately severe to severe and progressive disseminated histoplasmosis. It should be continued for 1 to 2 weeks, followed by oral itraconazole (200 mg 3 times daily for 3 days, then 200 mg 2 times daily for at least 12 months).
Monitoring itraconazole therapy through random serum levels is strongly recommended, and a random concentration of at least 1.0 mg/mL is recommended.23
Urine antigen levels should be measured before treatment is started, at 2 weeks, at 1 month, then every 3 months during therapy, continuing for 12 months after treatment is stopped.11
Lifelong suppressive therapy with itraconazole 200 mg daily may be required in immunosuppressed patients and patients who have a relapse despite appropriate therapy.11
While oral itraconazole is used as a sole agent for the treatment of mild to moderate acute pulmonary histoplasmosis and chronic cavitary pulmonary histoplasmosis, oral treatment alone with either fluconazole or itraconazole is not recommended for the treatment of progressive disseminated histoplasmosis.11
COMPLICATIONS OF HISTOPLASMOSIS
4. Which of the following is not a possible complication of histoplasmosis?
- Chronic cavitary pulmonary histoplasmosis
- Fibrosing mediastinitis
- Hypoadrenalism
- Hypothyroidism
Chronic cavitary pulmonary histoplasmosis usually develops in patients with underlying emphysema. Fatigue, night sweats, fever, anorexia, and weight loss are features of chronic cavitary pulmonary histoplasmosis. Progression of necrosis may lead to “marching cavity,” in which necrosis increases the size of the cavity and may consume an entire lobe.10
Fibrosing mediastinitis is an uncommon but often lethal complication of disseminated histoplasmosis. Increasing dyspnea, cough, hemoptysis, and signs of superior vena cava syndrome and right heart failure may develop. However, fibrosing mediastinitis is thought to be due to an exuberant immune response to past Histoplasma infection and would not be expected in an immunocompromised patient.17
Hypoadrenalism. Extensive destruction of the adrenal glands may lead to hypoadrenalism, manifesting as orthostatic hypotension, hyperkalemia, hyponatremia, and evidence of markedly enlarged adrenal glands with central necrosis on computed tomography.24
Hypothyroidism. Acute or disseminated histoplasmosis has not been reported to cause thyroid dysfunction.
CASE CONCLUSION
Our patient was treated with itraconazole 200 mg twice daily for 24 months. Although the literature supports lifelong itraconazole therapy in immunosuppressed patients, our patient was reluctant to do so. He agreed to close monitoring. If symptoms recur, itraconazole will be reinstituted and continued lifelong.
A 28-year-old man developed fever, night sweats, nausea, headache, reduced appetite, skin rash, and hemoptysis 2 weeks after returning to the United States from Mexico.
The patient had fistulizing Crohn disease and had been taking the tumor necrosis factor alpha (TNF-alpha) blocker adalimumab for the past 3 months. He had no risk factors for human immunodeficiency virus infection, and he had stopped smoking 1 year previously. Chest radiography and a tuberculin skin test before he started adalimumab therapy were negative. While in Mexico, he did not drink more than 1 alcoholic beverage a day.
He had presented recently to his local hospital with the same symptoms and had been prescribed ciprofloxacin, metronidazole, ceftriaxone, vancomycin, and ampicillin, which he was still taking but with no improvement of symptoms. Blood cultures drawn before the start of antibiotic therapy had been negative. Urinalysis, a screen for infectious mononucleosis, and lumbar puncture were also negative. Results of renal function testing were normal except for the anion gap, which was 20.8 mmol/L (reference range 10–20).
INITIAL EVALUATION
On presentation to this hospital, the patient was afebrile but continued to have temperature spikes up to 39.0°C (102.2°F). His heart rate was 90 per minute, blood pressure 104/61 mm Hg, respiratory rate 18 per minute, and oxygen saturation 95% on 2 L of oxygen via nasal cannula.
- White blood cell count 10.0 × 109/L (reference range 4.0–10.0 × 109/L)
- Lymphocyte count 6.1 × 109/L (1.2–3.4)
- Hemoglobin level 13.6 g/dL (14.0–18.0)
- Platelet count 87 × 109/L (150–400), reaching a nadir of 62 on hospital day 23
- Albumin 47 g/L (35–50)
- Total bilirubin 48 µmol/L (2–20)
- Alkaline phosphatase 137 U/L (40–135)
- Alanine aminotransferase 22 U/L (9–69)
- Aspartate aminotransferase 72 U/L (5–45).
He continued to have temperature spikes. His alkaline phosphatase level plateaued at 1,015 U/L on day 30, while his alanine aminotransferase and aspartate aminotransferase levels remained stable.
The patient’s ceftriaxone was continued, and the other antibiotics were replaced with doxycycline. Fluconazole was added when sputum culture grew Candida albicans. However, these drugs were later discontinued in view of worsening results on liver enzyme testing.
The evaluation continues
Sputum cultures were negative for acid-fast bacilli on 3 occasions.
Serologic testing was negative for:
- Hepatitis B surface antigen (but hepatitis B surface antibody was positive at > 1,000 IU/L)
- Hepatitis C virus antibody
- Cytomegalovirus immunoglobulin (Ig) G
- Toxoplasma gondii IgG
- Epstein-Barr virus viral capsid antigen IgM
- Rickettsia antibodies
- Antinuclear antibody
- Antineutrophil cytoplasmic antibody
- Antiglomerular basement membrane antibody.
Chest radiography showed blunting of both costophrenic angles and mild prominence of right perihilar interstitial markings and the right hilum.
Computed tomography of the chest, abdomen, and pelvis showed a subpleural density in the lower lobe of the right lung, small bilateral pleural effusions, right hilar lymphadenopathy, and splenomegaly with no specific hepatobiliary abnormality.
A white blood cell nuclear scan found no occult infection.
Abdominal ultrasonography showed a prominent liver and spleen. The liver parenchyma showed diffuse decreased echogenicity, suggestive of hepatitis.
Transesophageal echocardiography showed no vegetations or valvular abnormalities.
Bronchoscopy showed normal airways without evidence of pulmonary hemorrhage. No foci of infection were obtained. A focus of granuloma consisting of epithelioid histiocytes in tight clusters was seen on washings from the right lower lobe, but no malignant cells were seen.
Sections of pathologically enlarged right hilar and subcarinal lymph nodes obtained with transbronchial needle aspiration were sent for cytologic analysis and flow cytometry.
Cultures for tuberculous and fungal organisms were negative.
A clue. On further inquiry, the patient said he had gone swimming in the natural pool, or cenote, under a rock formation at Cenote Maya Park in Mexico.
DIFFERENTIAL DIAGNOSIS
1. Which of the following is not in the differential diagnosis?
- Disseminated tuberculosis
- Coccidioidomycosis
- Subacute infective endocarditis
- Disseminated histoplasmosis
- Blastomycosis
Although the patient has a systemic disease, subacute infective endocarditis is not likely because of a lack of predisposing factors such as a history of endocarditis, abnormal or artificial heart valve, or intravenous drug abuse. Moreover, negative blood cultures and the absence of vegetations on echocardiography make endocarditis very unlikely.
Given that the patient is immunosuppressed, opportunistic infection must be at the top of the differential diagnosis. Histoplasmosis, coccidioidomycosis, and blastomycosis are endemic in Mexico. Disseminated histoplasmosis is the most likely diagnosis; coccidioidomycosis and blastomycosis are less likely, based on the history, signs, and symptoms. Disseminated tuberculosis must be excluded before other diagnostic possibilities are considered.
TUBERCULOSIS IN PATIENTS ON TNF-ALPHA ANTAGONISTS
Tuberculosis has been reported in patients taking TNF-alpha antagonists.1 The frequency of tuberculosis is much higher than that of other opportunistic infections, and over 50% of reported cases involve extrapulmonary tissues in patients treated with TNF-alpha antagonists.2
British Thoracic Society guidelines recommend screening for latent tuberculosis before starting treatment with a TNF-alpha antagonist; the screening should include a history of tuberculosis treatment, a clinical examination, chest radiography, and a tuberculin skin test.3 Patients found to have active tuberculosis should receive a minimum of 2 months of standard treatment before starting a TNF-alpha antagonist. Patients with evidence of past tuberculosis or a history of tuberculosis who received adequate treatment should be monitored regularly. Patients with prior tuberculosis not adequately treated should receive chemoprophylaxis before starting a TNF-alpha antagonist.
Fever, night sweats, and intrathoracic and intra-abdominal lymphadenopathy are common features of disseminated tuberculosis. Upper-lobe cavitary disease or miliary lesions may be seen on chest radiography, but atypical presentations with lower-lobe infiltrate are not uncommon in immunosuppressed patients.4
A negative tuberculin skin test and a normal chest radiograph 3 months ago, along with negative sputum and bronchial lavage fluid cultures and no history of tuberculosis contact, make tuberculosis unlikely in our patient.
COCCIDIOIDOMYCOSIS
Coccidioidomycosis (valley fever) is caused by the fungus Coccidioides immitis, which lives in the soil and is acquired by inhalation of airborne microscopic spores.
Fatigue, cough, fever, shortness of breath, headache, night sweats, muscle or joint pain, and a rash on the upper body or legs are common symptoms. It may cause a self-limiting flulike illness. From 5% to 10% of patients may develop serious long-term lung problems. In a small number of patients, the disease may progress beyond the lungs to involve the central nervous system, spinal cord, skin, bones, and joints.5
Serologic testing is highly useful for the diagnosis. Antigen testing has a sensitivity of 71% and a specificity of 98% for the diagnosis, but cross-reactivity occurs in 10% of patients with other types of mycosis. Respiratory secretions and tissue samples should undergo microscopic study and culture.
BLASTOMYCOSIS
Blastomycosis is caused by the fungus Blastomyces dermatitidis, which lives in soil and in association with decomposing organic matter such as wood and leaves. Inhalation of spores may cause a flulike illness or pneumonia. In serious cases, the disease can spread to skin and bone.
The diagnosis is established with fungal cultures of tissue samples or body fluids (bone marrow, liver tissue, skin, sputum, blood). Rapid diagnosis may be obtained by examination of the secretions under a microscope, where typical broad-based budding yeast can be seen in almost 90% of cases.6 Antigen may also be detected in urine and serum7; the sensitivity of antigen testing is 93% and the specificity is 98%. Serologic testing is not recommended for diagnosis of blastomycosis because of poor sensitivity and specificity.8
NARROWING THE DIFFERENTIAL
Both coccidioidomycosis and blastomycosis should be included in the differential diagnosis of a systemic disease with subacute onset and prominent lung involvement in a patient returning from travel to Mexico. The lack of involvement of the central nervous system, spinal cord, bones, or joints makes these infections less likely in our patient.
However, swimming in a cenote under a rock formation is an important clue to the diagnosis in our patient, as it puts him at risk of inhaling microconidia or hyphal elements of histoplasmosis. This, along with his immunocompromised status, fever, hemoptysis, night sweats, skin and lung features, and the generally subacute course of his illness, make disseminated histoplasmosis the most likely diagnosis.
Radiologic findings of pulmonary infiltrate with effusion and elevated lactate dehydrogenase, aminotransferases, and alkaline phosphatase increase the likelihood of disseminated histoplasmosis.
HISTOPLASMOSIS
Histoplasma capsulatum is a dimorphic fungus that thrives in the soil and caves of regions with moderate climate, especially in soil containing large amounts of bird excreta or bat guano.9 Bats are natural hosts of this organism, and it is endemic in North and Central America, including parts of Mexico. Air currents can carry the microconidia for miles, thus exposing people without direct contact with contaminated sites.
The infection is usually acquired by inhalation of microconidia or small hyphal elements or by reactivation of previously quiescent foci of infection in an immunosuppressed patient. Most patients exposed to H capsulatum remain asymptomatic or develop mild symptoms, which are self-limiting. A small number develop acute pulmonary histoplasmosis or chronic cavitary histoplasmosis. Disseminated disease usually occurs only in an immunosuppressed host.
Acute pulmonary histoplasmosis presents with fever, malaise, headache, weakness, substernal chest pain, and dry cough and may be associated with erythema nodosum, erythema multiforme, and arthralgias. It may be mistaken for sarcoidosis since enlarged hilar and mediastinal lymph nodes are often seen on chest radiography.10
Progressive disseminated histoplasmosis is defined as a clinical illness that does not improve after at least 3 weeks of observation and is associated with physical or radiographic findings with or without laboratory evidence of extrapulmonary involvement.11
Fever, malaise, anorexia, weight loss, night sweats, hepatosplenomegaly, and lymphadenopathy are features of progressive disseminated histoplasmosis.
Cutaneous manifestations of disseminated histoplasmosis occur in 10% to 25% of patients with acquired immunodeficiency syndrome and include papules, plaques with or without crust, pustules, nodules, lesions resembling molluscum contagiosum virus infection, acneiform eruptions, erythematous macules, and keratotic plaques.12
TESTING FOR HISTOPLASMOSIS
2. What investigation is least likely to help confirm the diagnosis of disseminated histoplasmosis?
- Polymerase chain reaction (PCR) testing of serum, cerebrospinal fluid, and bronchoalveolar lavage specimens
- Urinary Histoplasma antigen testing
- Serologic testing
- Blood and bronchoalveolar lavage cultures
Urinary Histoplasma antigen has a sensitivity of 90% for the diagnosis of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome.18 It is less useful for pulmonary forms of histoplasmosis: the sensitivity is 75% and may even be less in milder or chronic forms of pneumonia.19 False-positive reactions may occur in patients with other fungal infections such as coccidioidomycosis, blastomycosis, paracoccidioidomycosis and penicilliosis.20 Urine antigen levels can also be used to monitor therapy, since levels decrease during therapy and increase in 90% of those who have a relapse.21
Our patient’s urinary Histoplasma antigen level was greater than 23.0 ng/mL (positive is > 0.50).
Serologic testing. Immunodiffusion immunoglobulin G (IgG) testing for Histoplasma and Blastomyces was negative, as was an enzyme immunoassay for Coccidioides IgG and IgM. However, antibody tests are less useful in immunosuppressed patients,22 and thus a negative result does not rule out histoplasmosis. A fourfold rise in complement fixation antibody titer is diagnostic of acute histoplasmosis. A single complement fixation titer of 1:32 is suggestive but not diagnostic of histoplasmosis. Cross-reactions may occur with other fungal infections like blastomycosis. The immunodiffusion assay has a greater specificity but slightly less sensitivity than the complement fixation assay.19
Culture of H capsulatum is the definitive test to establish a diagnosis of histoplasmosis. Culture can be performed on samples taken from blood, bone marrow, sputum, and bronchoalveolar lavage fluid, or from lung, liver, or lymph node tissue. Cultures are positive in 74% to 82% of cases of progressive disseminated histoplasmosis.13 However, treatment should not await culture results since the fungus may take several weeks to grow.
Back to our patient
Although Histoplasma serologic studies and cultures were negative, the diagnosis of disseminated histoplasmosis was made on the basis of the patient’s immunosuppressed status, travel history, clinical features, and positivity for urine Histoplasma antigen. Though urine histoplama antigen may be falsely positive in other fungal infections such as coccidioidomycosis, paracoccidioidomycosis, and blastomycosis, clinical features and the absence of central nervous system, joint, and bone involvement suggested disseminated histoplasmosis.
TREATMENT
3. What is the appropriate treatment for this patient?
- Amphotericin B followed by oral itraconozole
- Oral fluconazole
- Oral itraconazole
Liposomal amphotericin B or amphotericin B deoxycholate is recommended as initial therapy for moderately severe to severe and progressive disseminated histoplasmosis. It should be continued for 1 to 2 weeks, followed by oral itraconazole (200 mg 3 times daily for 3 days, then 200 mg 2 times daily for at least 12 months).
Monitoring itraconazole therapy through random serum levels is strongly recommended, and a random concentration of at least 1.0 mg/mL is recommended.23
Urine antigen levels should be measured before treatment is started, at 2 weeks, at 1 month, then every 3 months during therapy, continuing for 12 months after treatment is stopped.11
Lifelong suppressive therapy with itraconazole 200 mg daily may be required in immunosuppressed patients and patients who have a relapse despite appropriate therapy.11
While oral itraconazole is used as a sole agent for the treatment of mild to moderate acute pulmonary histoplasmosis and chronic cavitary pulmonary histoplasmosis, oral treatment alone with either fluconazole or itraconazole is not recommended for the treatment of progressive disseminated histoplasmosis.11
COMPLICATIONS OF HISTOPLASMOSIS
4. Which of the following is not a possible complication of histoplasmosis?
- Chronic cavitary pulmonary histoplasmosis
- Fibrosing mediastinitis
- Hypoadrenalism
- Hypothyroidism
Chronic cavitary pulmonary histoplasmosis usually develops in patients with underlying emphysema. Fatigue, night sweats, fever, anorexia, and weight loss are features of chronic cavitary pulmonary histoplasmosis. Progression of necrosis may lead to “marching cavity,” in which necrosis increases the size of the cavity and may consume an entire lobe.10
Fibrosing mediastinitis is an uncommon but often lethal complication of disseminated histoplasmosis. Increasing dyspnea, cough, hemoptysis, and signs of superior vena cava syndrome and right heart failure may develop. However, fibrosing mediastinitis is thought to be due to an exuberant immune response to past Histoplasma infection and would not be expected in an immunocompromised patient.17
Hypoadrenalism. Extensive destruction of the adrenal glands may lead to hypoadrenalism, manifesting as orthostatic hypotension, hyperkalemia, hyponatremia, and evidence of markedly enlarged adrenal glands with central necrosis on computed tomography.24
Hypothyroidism. Acute or disseminated histoplasmosis has not been reported to cause thyroid dysfunction.
CASE CONCLUSION
Our patient was treated with itraconazole 200 mg twice daily for 24 months. Although the literature supports lifelong itraconazole therapy in immunosuppressed patients, our patient was reluctant to do so. He agreed to close monitoring. If symptoms recur, itraconazole will be reinstituted and continued lifelong.
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61:409–417.
- Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis 2003; 3:148–155.
- British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005; 60:800–805.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1998. A 19-year-old man with the acquired immunodeficiency syndrome and persistent fever. N Engl J Med 1998; 339:1835–1843.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Lemos LB, Guo M, Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol 2000; 4:391–406.
- Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Micribiol 2004; 42:4873–4875.
- Wheat LJ. Approach to the diagnosis of the endemic mycoses. Clin Chest Med 2009; 30:379–389.
- Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49:785–798.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Chang P, Rodas C. Skin lesions in histoplasmosis. Clinics Dermatol 2012; 30:592–598.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol 2012; 19:53–56.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am 2016; 30:247–264.
- Stockamp NW, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2016; 30:229–246.
- Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am 2016; 30:207–227.
- Wheat LJ, Garringer T, Drizendine E, Connolly P. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis 2002; 14:1389–1391.
- Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 2008; 21:421–425.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob Agents Chemother 2002; 46:248–250.
- Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol 2003; 11:488–494.
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 1998; 35:461–473.
- Sarosi GA, Voth DW, Dahl BA, Doto IL, Tosh FE. Disseminated histoplasmosis: results of long-term follow-up. Ann Intern Med 1971; 75:511–516.
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61:409–417.
- Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis 2003; 3:148–155.
- British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005; 60:800–805.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1998. A 19-year-old man with the acquired immunodeficiency syndrome and persistent fever. N Engl J Med 1998; 339:1835–1843.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Lemos LB, Guo M, Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol 2000; 4:391–406.
- Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Micribiol 2004; 42:4873–4875.
- Wheat LJ. Approach to the diagnosis of the endemic mycoses. Clin Chest Med 2009; 30:379–389.
- Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49:785–798.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Chang P, Rodas C. Skin lesions in histoplasmosis. Clinics Dermatol 2012; 30:592–598.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol 2012; 19:53–56.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am 2016; 30:247–264.
- Stockamp NW, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2016; 30:229–246.
- Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am 2016; 30:207–227.
- Wheat LJ, Garringer T, Drizendine E, Connolly P. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis 2002; 14:1389–1391.
- Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 2008; 21:421–425.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob Agents Chemother 2002; 46:248–250.
- Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol 2003; 11:488–494.
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 1998; 35:461–473.
- Sarosi GA, Voth DW, Dahl BA, Doto IL, Tosh FE. Disseminated histoplasmosis: results of long-term follow-up. Ann Intern Med 1971; 75:511–516.