User login
To the Editor:
The concept of field cancerization has been well described since its initial proposal by Slaughter et al1 in 1953. It describes a field of genetically altered cells where multiple clonally related neoplasms can develop.2,3 Treatment of patients with multiple neoplasms within an area of field cancerization can be especially challenging. We report a patient with field cancerization who had multiple squamous cell carcinomas (SCCs) and keratoacanthomas (KAs) that arose within the field.
A 78-year-old man initially presented with a papule on the right forearm of 3 months’ duration. He had a medical history of cutaneous SCC, myocardial infarction, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hypercholesterolemia, gout, and diverticulosis. He was not taking any chronic immunosuppressants that may have predisposed him to the development of nonmelanoma skin cancer. The papule was biopsied and diagnosed as a well-differentiated invasive SCC. A month later it was excised with clear margins.
Approximately 5 weeks after the excision, he returned with an enlarging lesion on the right forearm just medial to the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. Two months later the lesion was excised with clear margins. Four weeks later he returned with a new lesion adjacent to the medial aspect of the prior excision. The lesion was biopsied and diagnosed as a well-differentiated SCC. Four weeks later the lesion was excised with clear margins.
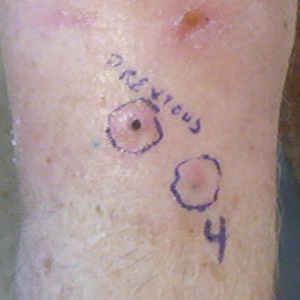
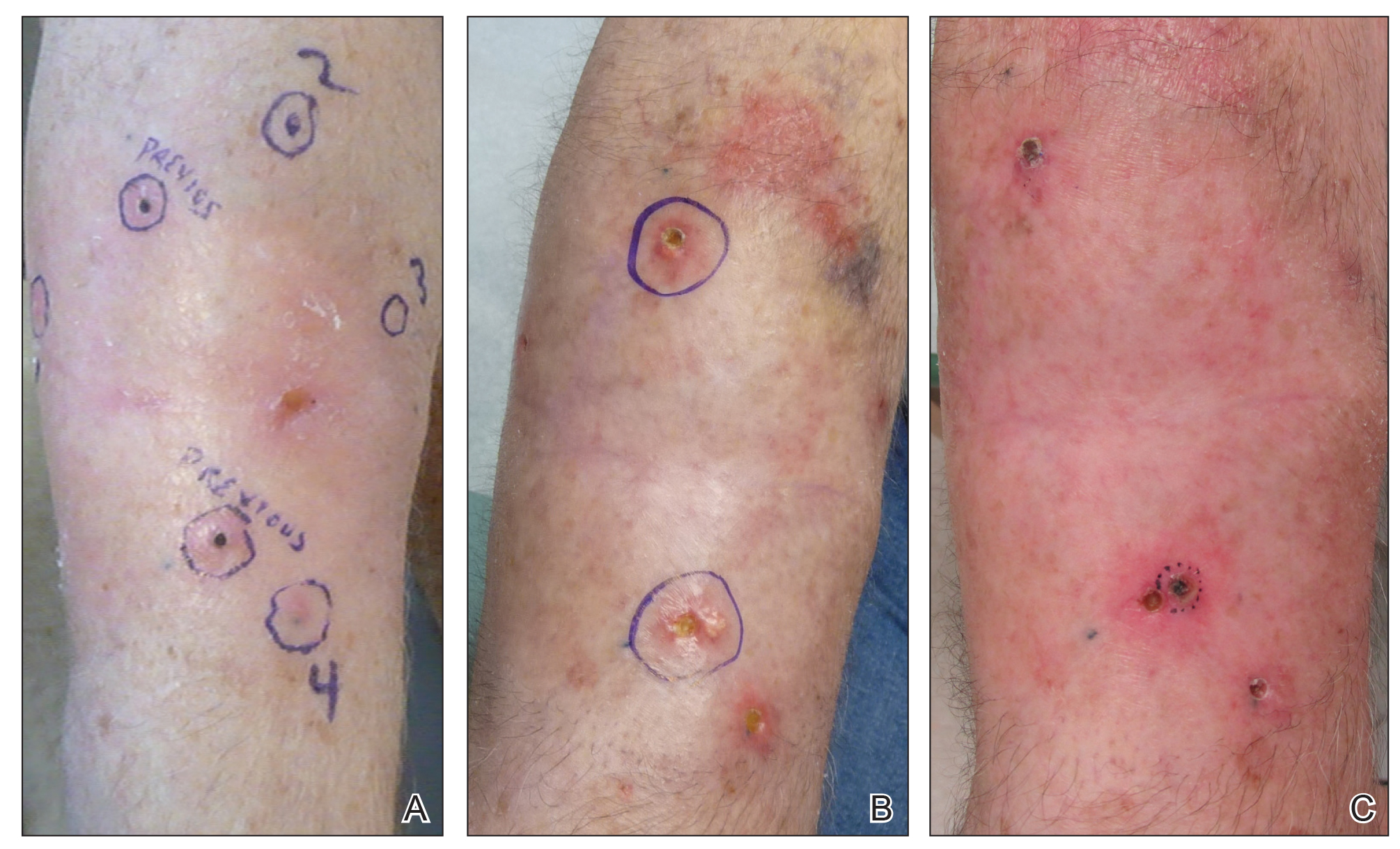
Another 4 weeks later the patient returned with a new lesion on the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. The lesion was treated with radiotherapy, with a 5800-cGy course completed 2 months later. The next month, 2 papules just adjacent to the radiotherapy treatment field were biopsied and diagnosed as well-differentiated SCC, KA type. One week later, 2 additional new papules adjacent to the radiotherapy treatment field were biopsied and diagnosed as moderately differentiated SCC, KA type. At this time, the patient had 4 biopsy-proven KAs on the right forearm in the area of prior radiation (Figure, A). The radiation oncologist felt that further radiation was no longer indicated. A consultation was sought with surgical oncology, and wide excision of the field with sentinel lymph node biopsy and skin grafting was recommended. Computed tomography with contrast of the chest and right arm ordered by surgical oncology did not reveal metastatic disease.
After discussion of the risks, alternatives, and benefits of surgery, the patient elected to try nonsurgical treatment. He was treated with 5-fluorouracil (5-FU) cream 5% twice daily for 4 weeks. It was applied to the right arm from the elbow to the wrist and occluded under an elastic bandage. The patient stated that the biopsy sites became sore and inflamed during the treatment. After 4 weeks of treatment, all 4 KAs had healed without clinical evidence of tumor. During this time, however, the previously treated 2 sites had developed adjacent firm pink papules (Figure, B); these 2 lesions were then treated with intralesional 5-FU 50 mg/mL once weekly to resolution at 4 and 5 weeks, respectively. The proximal lesion was treated with 7.5 mg on week 1 and 5 mg on weeks 2, 3, and 4. The larger distal lesion was treated with 12.5 mg on week 1 and 5 mg on weeks 2, 3, 4, and 5. The volume injected was determined by ability to blanch and indurate the lesion and was decreased due to the shrinking size of the tumor. After 3 injections, both tumors had substantially decreased in size (Figure, C). The patient noted pain during injection but found the procedure tolerable and preferable to surgery. There were no other adverse events. At the end of treatment, both tumors had clinically resolved. No recurrence or development of new tumors was reported over 3 years of follow-up after the last injection.
Field cancerization was the outgrowth of the study of oral SCC in an effort to explain the development of multiple primary tumors and locally recurrent cancer.1,2 Histopathologically, the authors observed that oral cancer developed in multifocal areas of precancerous change, histologically abnormal hyperplastic tissue surrounded the tumors, oral cancer consisted of multiple independent areas that sometimes coalesced, and the persistence of abnormal tissue after surgery might explain local recurrences and the development of new lesions in a previously treated area.1,2 Since then, the concept has been applied to several other organ systems including the lungs, vulva, cervix, breasts, bladder, colon, and skin.2
In the skin, field cancerization involves clusters and contiguous patches of altered cells present in areas of chronic photodamage.2 Genetically altered fields form the foundation in which multiple clonally related neoplastic lesions can develop.2,3 These fields often remain after treatment of the primary tumor and may lead to new cancers that commonly are labeled as a second primary tumor or a local recurrence depending on the exact site and time interval.3 Brennan et al3 found clonal populations of infiltrating tumor cells harboring a p53 gene mutation in more than 50% of histopathologically negative surgical margins of patients with SCC of the head and neck. Furthermore, 40% of the patients with a margin positive for a p53 gene mutation had local recurrence vs none of the patients with negative margins.4 These findings were supported by several other studies where loss of heterozygosity, microsatellite alterations, chromosomal instability, or in situ hybridization was used to demonstrate genetically altered fields.2,4 Histopathologic patterns of epidermolytic hyperkeratosis, focal acantholytic dyskeratosis, and pronounced acantholysis as found in Hailey-Hailey disease may be a consequence of clonal expansion of mutated keratinocytes because of long-term exposure to mutagens such as UV light and human papillomavirus.5
The development of an expanding neoplastic field appears to play an important role in cutaneous carcinogenesis. It is necessary to consider the cutaneous field cancerization as a highly photodamaged area that contains clinical and subclinical lesions.2-4 The treatment of cutaneous neoplasms, SCC in particular, should focus not only on the tumor itself but also on the surrounding tissue. Adjunctive field-directed therapies should be considered after treatment of the primary tumor.4
Our patient continued to develop SCCs on the right forearm after multiple excisions with clear margins and subsequently was treated with radiation therapy. He then developed 4 KAs after radiation therapy to the right forearm. Topical 5-FU is a well-described treatment of field cancerization.2 In our patient, 5-FU cream 5% applied twice daily from the wrist to the elbow under occlusion for 4 weeks led to the involution of all 4 KAs. During this time, our patient developed 2 additional firm pink papules near the previously treated sites, which resolved with intralesional 5-FU weekly for 4 and 5 weeks, respectively.
Intralesional 5-FU has been described for the treatment of multiple and difficult-to-treat KAs. It is an antimetabolite and structural analog of uracil that disrupts DNA and RNA synthesis. It is contraindicated in liver disease, pregnancy or breastfeeding, and allergy to the medication.6 Intralesional 5-FU dosing recommendations for KAs include use of a 50-mg/mL solution and injecting 0.1 to 1 mL until the lesion blanches in color, which may be repeated every 1 to 4 weeks.7,8 The maximum recommended daily dose is 800 mg.6 Pretreatment with intralesional 1% lidocaine has been recommended by some authors due to pain with injection.8 Recommendations for laboratory monitoring include a complete blood cell count with differential at baseline and weekly. Side effects include local pain, erythema, crusting, ulceration, and necrosis. Systemic side effects include cytopenia and gastrointestinal tract upset.6 Intralesional 5-FU has been used successfully in a single dose of 10 mg per lesion in combination with systemic acitretin for the treatment of multiple KAs induced by vemurafenib.9 It also has been effective in the treatment of multiple recurrent reactive KAs developing in surgical margins.7 A review article reported that the use of intralesional 5-FU produced a 98% cure rate in 56 treated KAs.6 Alternative intralesional agents that may be considered for KAs include methotrexate, bleomycin, and interferon alfa-2b.6,7
Field cancerization may cause the development of multiple clonally related neoplasms within a field of genetically altered cells that may continue to develop after excision with clear margins or radiation therapy. Given the success of treatment in our patient, we recommend consideration for topical and intralesional 5-FU in patients who develop SCCs and KAs within an area of field cancerization.
- Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Brennan JA, Mao L, Hruban R, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braakhuis, BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Carlson AJ, Scott D, Wharton J, et al. Incidental histopathologic patterns: possible evidence of “field cancerization” surrounding skin tumors. Am J Dermatopathol. 2001;23:494-496.
- Kirby J, Miller C. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
- Que S, Compton L, Schmults C. Eruptive squamous atypia (also known as eruptive keratoacanthoma): definition of the disease entity and successful management via intralesional 5-fluorouracil. J Am Acad Dermatol. 2019;81:111-122.
- LaPresto L, Cranmer L, Morrison L, et al. A novel therapeutic combination approach for treating multiple vemurafenib-induced keratoacanthomas systemic acitretin and intralesional fluorouracil. JAMA Dermatol. 2013;149:279-281.
To the Editor:
The concept of field cancerization has been well described since its initial proposal by Slaughter et al1 in 1953. It describes a field of genetically altered cells where multiple clonally related neoplasms can develop.2,3 Treatment of patients with multiple neoplasms within an area of field cancerization can be especially challenging. We report a patient with field cancerization who had multiple squamous cell carcinomas (SCCs) and keratoacanthomas (KAs) that arose within the field.
A 78-year-old man initially presented with a papule on the right forearm of 3 months’ duration. He had a medical history of cutaneous SCC, myocardial infarction, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hypercholesterolemia, gout, and diverticulosis. He was not taking any chronic immunosuppressants that may have predisposed him to the development of nonmelanoma skin cancer. The papule was biopsied and diagnosed as a well-differentiated invasive SCC. A month later it was excised with clear margins.
Approximately 5 weeks after the excision, he returned with an enlarging lesion on the right forearm just medial to the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. Two months later the lesion was excised with clear margins. Four weeks later he returned with a new lesion adjacent to the medial aspect of the prior excision. The lesion was biopsied and diagnosed as a well-differentiated SCC. Four weeks later the lesion was excised with clear margins.
Another 4 weeks later the patient returned with a new lesion on the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. The lesion was treated with radiotherapy, with a 5800-cGy course completed 2 months later. The next month, 2 papules just adjacent to the radiotherapy treatment field were biopsied and diagnosed as well-differentiated SCC, KA type. One week later, 2 additional new papules adjacent to the radiotherapy treatment field were biopsied and diagnosed as moderately differentiated SCC, KA type. At this time, the patient had 4 biopsy-proven KAs on the right forearm in the area of prior radiation (Figure, A). The radiation oncologist felt that further radiation was no longer indicated. A consultation was sought with surgical oncology, and wide excision of the field with sentinel lymph node biopsy and skin grafting was recommended. Computed tomography with contrast of the chest and right arm ordered by surgical oncology did not reveal metastatic disease.
After discussion of the risks, alternatives, and benefits of surgery, the patient elected to try nonsurgical treatment. He was treated with 5-fluorouracil (5-FU) cream 5% twice daily for 4 weeks. It was applied to the right arm from the elbow to the wrist and occluded under an elastic bandage. The patient stated that the biopsy sites became sore and inflamed during the treatment. After 4 weeks of treatment, all 4 KAs had healed without clinical evidence of tumor. During this time, however, the previously treated 2 sites had developed adjacent firm pink papules (Figure, B); these 2 lesions were then treated with intralesional 5-FU 50 mg/mL once weekly to resolution at 4 and 5 weeks, respectively. The proximal lesion was treated with 7.5 mg on week 1 and 5 mg on weeks 2, 3, and 4. The larger distal lesion was treated with 12.5 mg on week 1 and 5 mg on weeks 2, 3, 4, and 5. The volume injected was determined by ability to blanch and indurate the lesion and was decreased due to the shrinking size of the tumor. After 3 injections, both tumors had substantially decreased in size (Figure, C). The patient noted pain during injection but found the procedure tolerable and preferable to surgery. There were no other adverse events. At the end of treatment, both tumors had clinically resolved. No recurrence or development of new tumors was reported over 3 years of follow-up after the last injection.

Field cancerization was the outgrowth of the study of oral SCC in an effort to explain the development of multiple primary tumors and locally recurrent cancer.1,2 Histopathologically, the authors observed that oral cancer developed in multifocal areas of precancerous change, histologically abnormal hyperplastic tissue surrounded the tumors, oral cancer consisted of multiple independent areas that sometimes coalesced, and the persistence of abnormal tissue after surgery might explain local recurrences and the development of new lesions in a previously treated area.1,2 Since then, the concept has been applied to several other organ systems including the lungs, vulva, cervix, breasts, bladder, colon, and skin.2
In the skin, field cancerization involves clusters and contiguous patches of altered cells present in areas of chronic photodamage.2 Genetically altered fields form the foundation in which multiple clonally related neoplastic lesions can develop.2,3 These fields often remain after treatment of the primary tumor and may lead to new cancers that commonly are labeled as a second primary tumor or a local recurrence depending on the exact site and time interval.3 Brennan et al3 found clonal populations of infiltrating tumor cells harboring a p53 gene mutation in more than 50% of histopathologically negative surgical margins of patients with SCC of the head and neck. Furthermore, 40% of the patients with a margin positive for a p53 gene mutation had local recurrence vs none of the patients with negative margins.4 These findings were supported by several other studies where loss of heterozygosity, microsatellite alterations, chromosomal instability, or in situ hybridization was used to demonstrate genetically altered fields.2,4 Histopathologic patterns of epidermolytic hyperkeratosis, focal acantholytic dyskeratosis, and pronounced acantholysis as found in Hailey-Hailey disease may be a consequence of clonal expansion of mutated keratinocytes because of long-term exposure to mutagens such as UV light and human papillomavirus.5
The development of an expanding neoplastic field appears to play an important role in cutaneous carcinogenesis. It is necessary to consider the cutaneous field cancerization as a highly photodamaged area that contains clinical and subclinical lesions.2-4 The treatment of cutaneous neoplasms, SCC in particular, should focus not only on the tumor itself but also on the surrounding tissue. Adjunctive field-directed therapies should be considered after treatment of the primary tumor.4
Our patient continued to develop SCCs on the right forearm after multiple excisions with clear margins and subsequently was treated with radiation therapy. He then developed 4 KAs after radiation therapy to the right forearm. Topical 5-FU is a well-described treatment of field cancerization.2 In our patient, 5-FU cream 5% applied twice daily from the wrist to the elbow under occlusion for 4 weeks led to the involution of all 4 KAs. During this time, our patient developed 2 additional firm pink papules near the previously treated sites, which resolved with intralesional 5-FU weekly for 4 and 5 weeks, respectively.
Intralesional 5-FU has been described for the treatment of multiple and difficult-to-treat KAs. It is an antimetabolite and structural analog of uracil that disrupts DNA and RNA synthesis. It is contraindicated in liver disease, pregnancy or breastfeeding, and allergy to the medication.6 Intralesional 5-FU dosing recommendations for KAs include use of a 50-mg/mL solution and injecting 0.1 to 1 mL until the lesion blanches in color, which may be repeated every 1 to 4 weeks.7,8 The maximum recommended daily dose is 800 mg.6 Pretreatment with intralesional 1% lidocaine has been recommended by some authors due to pain with injection.8 Recommendations for laboratory monitoring include a complete blood cell count with differential at baseline and weekly. Side effects include local pain, erythema, crusting, ulceration, and necrosis. Systemic side effects include cytopenia and gastrointestinal tract upset.6 Intralesional 5-FU has been used successfully in a single dose of 10 mg per lesion in combination with systemic acitretin for the treatment of multiple KAs induced by vemurafenib.9 It also has been effective in the treatment of multiple recurrent reactive KAs developing in surgical margins.7 A review article reported that the use of intralesional 5-FU produced a 98% cure rate in 56 treated KAs.6 Alternative intralesional agents that may be considered for KAs include methotrexate, bleomycin, and interferon alfa-2b.6,7
Field cancerization may cause the development of multiple clonally related neoplasms within a field of genetically altered cells that may continue to develop after excision with clear margins or radiation therapy. Given the success of treatment in our patient, we recommend consideration for topical and intralesional 5-FU in patients who develop SCCs and KAs within an area of field cancerization.
To the Editor:
The concept of field cancerization has been well described since its initial proposal by Slaughter et al1 in 1953. It describes a field of genetically altered cells where multiple clonally related neoplasms can develop.2,3 Treatment of patients with multiple neoplasms within an area of field cancerization can be especially challenging. We report a patient with field cancerization who had multiple squamous cell carcinomas (SCCs) and keratoacanthomas (KAs) that arose within the field.
A 78-year-old man initially presented with a papule on the right forearm of 3 months’ duration. He had a medical history of cutaneous SCC, myocardial infarction, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hypercholesterolemia, gout, and diverticulosis. He was not taking any chronic immunosuppressants that may have predisposed him to the development of nonmelanoma skin cancer. The papule was biopsied and diagnosed as a well-differentiated invasive SCC. A month later it was excised with clear margins.
Approximately 5 weeks after the excision, he returned with an enlarging lesion on the right forearm just medial to the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. Two months later the lesion was excised with clear margins. Four weeks later he returned with a new lesion adjacent to the medial aspect of the prior excision. The lesion was biopsied and diagnosed as a well-differentiated SCC. Four weeks later the lesion was excised with clear margins.
Another 4 weeks later the patient returned with a new lesion on the excision site. The lesion was biopsied and diagnosed as a well-differentiated SCC. The lesion was treated with radiotherapy, with a 5800-cGy course completed 2 months later. The next month, 2 papules just adjacent to the radiotherapy treatment field were biopsied and diagnosed as well-differentiated SCC, KA type. One week later, 2 additional new papules adjacent to the radiotherapy treatment field were biopsied and diagnosed as moderately differentiated SCC, KA type. At this time, the patient had 4 biopsy-proven KAs on the right forearm in the area of prior radiation (Figure, A). The radiation oncologist felt that further radiation was no longer indicated. A consultation was sought with surgical oncology, and wide excision of the field with sentinel lymph node biopsy and skin grafting was recommended. Computed tomography with contrast of the chest and right arm ordered by surgical oncology did not reveal metastatic disease.
After discussion of the risks, alternatives, and benefits of surgery, the patient elected to try nonsurgical treatment. He was treated with 5-fluorouracil (5-FU) cream 5% twice daily for 4 weeks. It was applied to the right arm from the elbow to the wrist and occluded under an elastic bandage. The patient stated that the biopsy sites became sore and inflamed during the treatment. After 4 weeks of treatment, all 4 KAs had healed without clinical evidence of tumor. During this time, however, the previously treated 2 sites had developed adjacent firm pink papules (Figure, B); these 2 lesions were then treated with intralesional 5-FU 50 mg/mL once weekly to resolution at 4 and 5 weeks, respectively. The proximal lesion was treated with 7.5 mg on week 1 and 5 mg on weeks 2, 3, and 4. The larger distal lesion was treated with 12.5 mg on week 1 and 5 mg on weeks 2, 3, 4, and 5. The volume injected was determined by ability to blanch and indurate the lesion and was decreased due to the shrinking size of the tumor. After 3 injections, both tumors had substantially decreased in size (Figure, C). The patient noted pain during injection but found the procedure tolerable and preferable to surgery. There were no other adverse events. At the end of treatment, both tumors had clinically resolved. No recurrence or development of new tumors was reported over 3 years of follow-up after the last injection.

Field cancerization was the outgrowth of the study of oral SCC in an effort to explain the development of multiple primary tumors and locally recurrent cancer.1,2 Histopathologically, the authors observed that oral cancer developed in multifocal areas of precancerous change, histologically abnormal hyperplastic tissue surrounded the tumors, oral cancer consisted of multiple independent areas that sometimes coalesced, and the persistence of abnormal tissue after surgery might explain local recurrences and the development of new lesions in a previously treated area.1,2 Since then, the concept has been applied to several other organ systems including the lungs, vulva, cervix, breasts, bladder, colon, and skin.2
In the skin, field cancerization involves clusters and contiguous patches of altered cells present in areas of chronic photodamage.2 Genetically altered fields form the foundation in which multiple clonally related neoplastic lesions can develop.2,3 These fields often remain after treatment of the primary tumor and may lead to new cancers that commonly are labeled as a second primary tumor or a local recurrence depending on the exact site and time interval.3 Brennan et al3 found clonal populations of infiltrating tumor cells harboring a p53 gene mutation in more than 50% of histopathologically negative surgical margins of patients with SCC of the head and neck. Furthermore, 40% of the patients with a margin positive for a p53 gene mutation had local recurrence vs none of the patients with negative margins.4 These findings were supported by several other studies where loss of heterozygosity, microsatellite alterations, chromosomal instability, or in situ hybridization was used to demonstrate genetically altered fields.2,4 Histopathologic patterns of epidermolytic hyperkeratosis, focal acantholytic dyskeratosis, and pronounced acantholysis as found in Hailey-Hailey disease may be a consequence of clonal expansion of mutated keratinocytes because of long-term exposure to mutagens such as UV light and human papillomavirus.5
The development of an expanding neoplastic field appears to play an important role in cutaneous carcinogenesis. It is necessary to consider the cutaneous field cancerization as a highly photodamaged area that contains clinical and subclinical lesions.2-4 The treatment of cutaneous neoplasms, SCC in particular, should focus not only on the tumor itself but also on the surrounding tissue. Adjunctive field-directed therapies should be considered after treatment of the primary tumor.4
Our patient continued to develop SCCs on the right forearm after multiple excisions with clear margins and subsequently was treated with radiation therapy. He then developed 4 KAs after radiation therapy to the right forearm. Topical 5-FU is a well-described treatment of field cancerization.2 In our patient, 5-FU cream 5% applied twice daily from the wrist to the elbow under occlusion for 4 weeks led to the involution of all 4 KAs. During this time, our patient developed 2 additional firm pink papules near the previously treated sites, which resolved with intralesional 5-FU weekly for 4 and 5 weeks, respectively.
Intralesional 5-FU has been described for the treatment of multiple and difficult-to-treat KAs. It is an antimetabolite and structural analog of uracil that disrupts DNA and RNA synthesis. It is contraindicated in liver disease, pregnancy or breastfeeding, and allergy to the medication.6 Intralesional 5-FU dosing recommendations for KAs include use of a 50-mg/mL solution and injecting 0.1 to 1 mL until the lesion blanches in color, which may be repeated every 1 to 4 weeks.7,8 The maximum recommended daily dose is 800 mg.6 Pretreatment with intralesional 1% lidocaine has been recommended by some authors due to pain with injection.8 Recommendations for laboratory monitoring include a complete blood cell count with differential at baseline and weekly. Side effects include local pain, erythema, crusting, ulceration, and necrosis. Systemic side effects include cytopenia and gastrointestinal tract upset.6 Intralesional 5-FU has been used successfully in a single dose of 10 mg per lesion in combination with systemic acitretin for the treatment of multiple KAs induced by vemurafenib.9 It also has been effective in the treatment of multiple recurrent reactive KAs developing in surgical margins.7 A review article reported that the use of intralesional 5-FU produced a 98% cure rate in 56 treated KAs.6 Alternative intralesional agents that may be considered for KAs include methotrexate, bleomycin, and interferon alfa-2b.6,7
Field cancerization may cause the development of multiple clonally related neoplasms within a field of genetically altered cells that may continue to develop after excision with clear margins or radiation therapy. Given the success of treatment in our patient, we recommend consideration for topical and intralesional 5-FU in patients who develop SCCs and KAs within an area of field cancerization.
- Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Brennan JA, Mao L, Hruban R, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braakhuis, BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Carlson AJ, Scott D, Wharton J, et al. Incidental histopathologic patterns: possible evidence of “field cancerization” surrounding skin tumors. Am J Dermatopathol. 2001;23:494-496.
- Kirby J, Miller C. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
- Que S, Compton L, Schmults C. Eruptive squamous atypia (also known as eruptive keratoacanthoma): definition of the disease entity and successful management via intralesional 5-fluorouracil. J Am Acad Dermatol. 2019;81:111-122.
- LaPresto L, Cranmer L, Morrison L, et al. A novel therapeutic combination approach for treating multiple vemurafenib-induced keratoacanthomas systemic acitretin and intralesional fluorouracil. JAMA Dermatol. 2013;149:279-281.
- Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Brennan JA, Mao L, Hruban R, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braakhuis, BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Carlson AJ, Scott D, Wharton J, et al. Incidental histopathologic patterns: possible evidence of “field cancerization” surrounding skin tumors. Am J Dermatopathol. 2001;23:494-496.
- Kirby J, Miller C. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Hadley J, Tristani-Firouzi P, Florell S, et al. Case series of multiple recurrent reactive keratoacanthomas developing at surgical margins. Dermatol Surg. 2009;35:2019-2024.
- Que S, Compton L, Schmults C. Eruptive squamous atypia (also known as eruptive keratoacanthoma): definition of the disease entity and successful management via intralesional 5-fluorouracil. J Am Acad Dermatol. 2019;81:111-122.
- LaPresto L, Cranmer L, Morrison L, et al. A novel therapeutic combination approach for treating multiple vemurafenib-induced keratoacanthomas systemic acitretin and intralesional fluorouracil. JAMA Dermatol. 2013;149:279-281.