User login
The Diagnosis: Cutaneous Blastomycosis
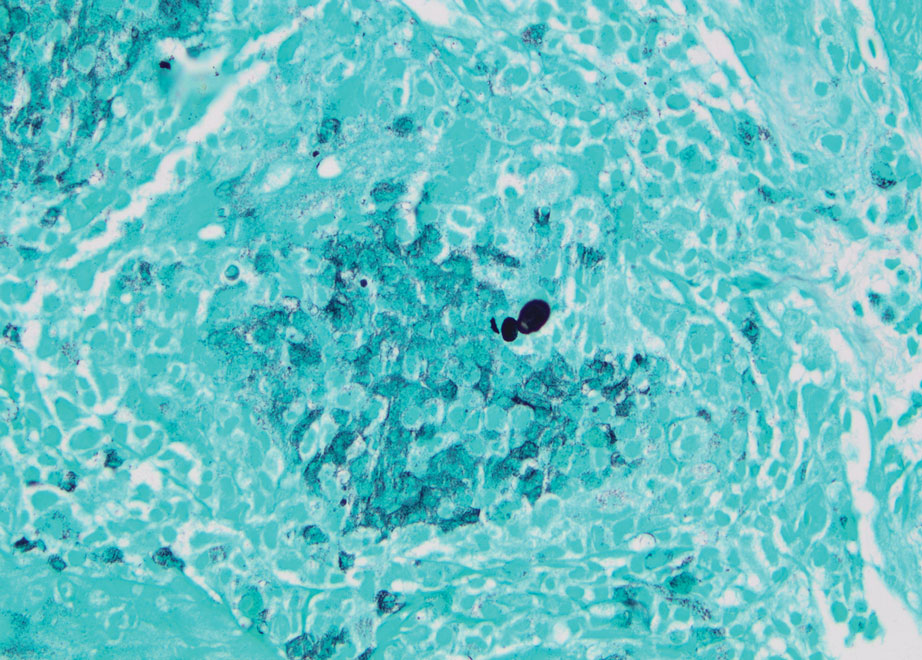
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.
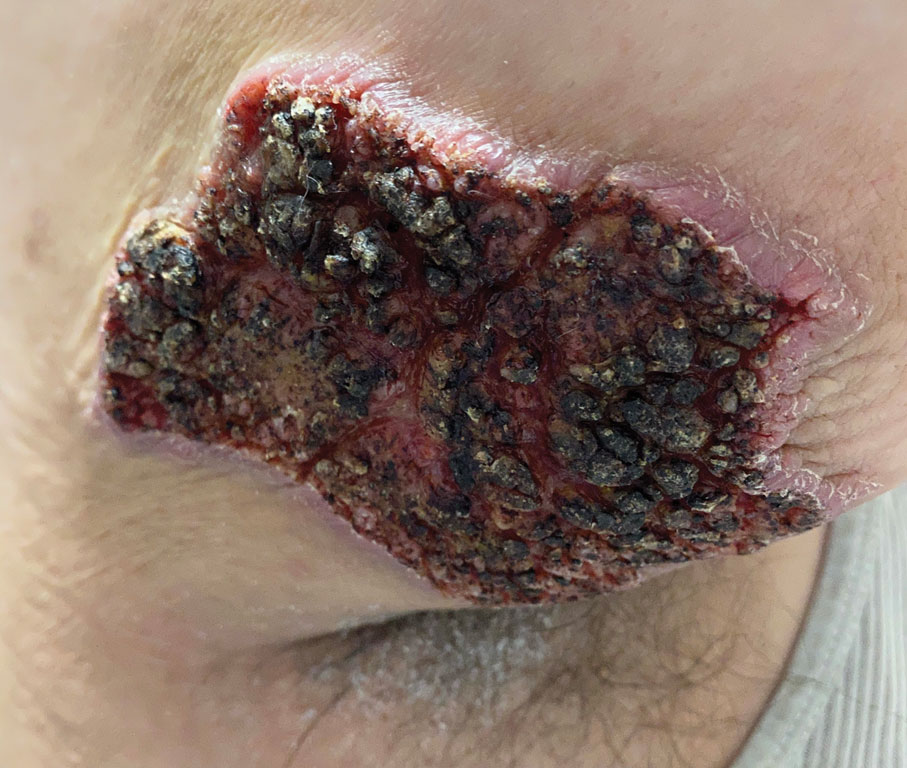
Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1
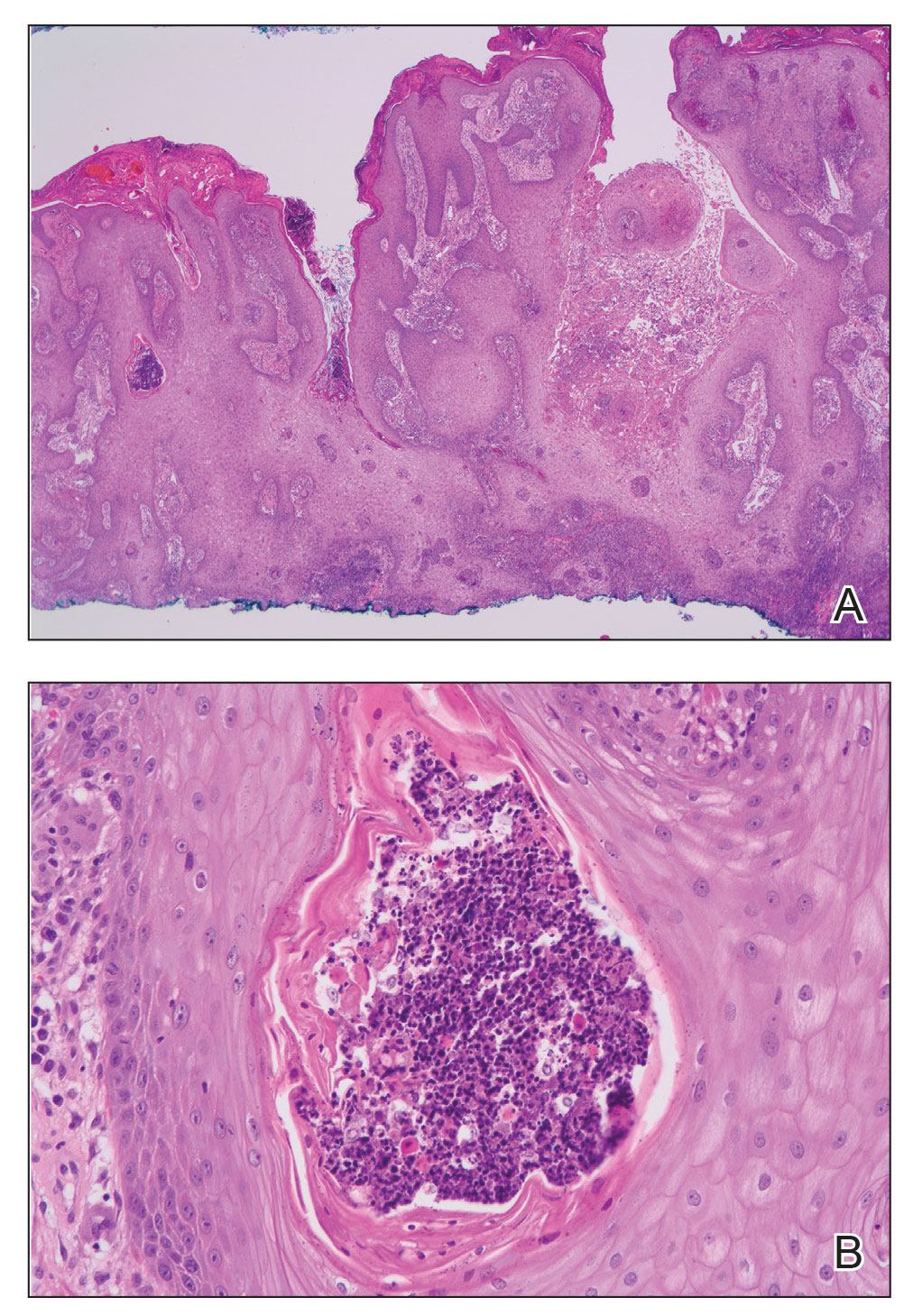
Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
A 39-year-old man from Ohio presented with a tender, 10×6-cm, fungated, eroded plaque on the right medial upper arm that developed over the last 4 years. He initially noticed a firm lump under the right arm 4 years prior that was diagnosed as possible cellulitis at an outside clinic and treated with trimethoprim-sulfamethoxazole. The lesion then began to erode and became a chronic nonhealing wound. Approximately 1 year prior to the current presentation, the patient recalled unloading a truckload of soil around the same time the wound began to enlarge in diameter and depth. He denied any prior or current respiratory or systemic symptoms including fevers, chills, or weight loss.