User login
Ectatic Vessels on the Chest
The Diagnosis: Superior Vena Cava Syndrome
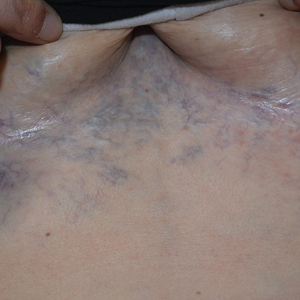
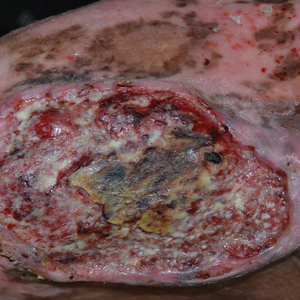
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
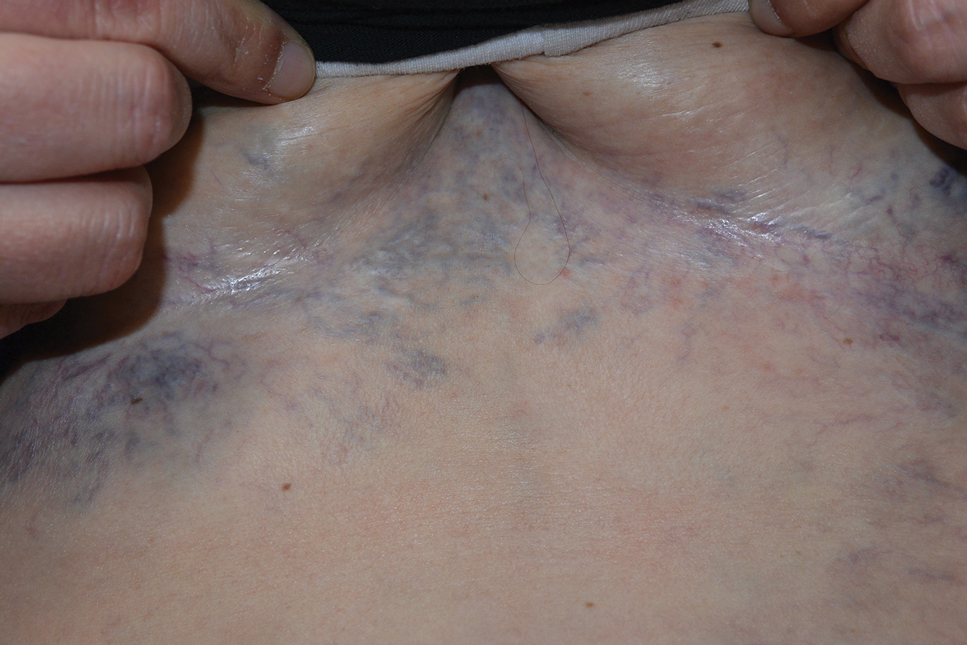
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
A 32-year-old woman presented to vascular surgery for evaluation of spider veins of 2 years’ duration that originated on the breasts but later spread to include the central chest, inframammary folds, and back. She reported associated pain and discomfort as well as intermittent facial swelling and tachycardia but denied pruritus and bleeding. The patient had a history of a kidney transplant 6 months prior, Langerhans cell histiocytosis, and Sjögren syndrome with a left indwelling catheter. Her current medications included systemic immunosuppressive agents. Physical examination revealed blue-purple ectatic vessels on the inframammary folds and central chest extending to the back. Erythema on the face, neck, and arms was not appreciated. No palpable cervical, supraclavicular, or axillary lymph nodes were noted.
Long-term Remission of Pyoderma Gangrenosum, Acne, and Hidradenitis Suppurativa Syndrome
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2 Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.
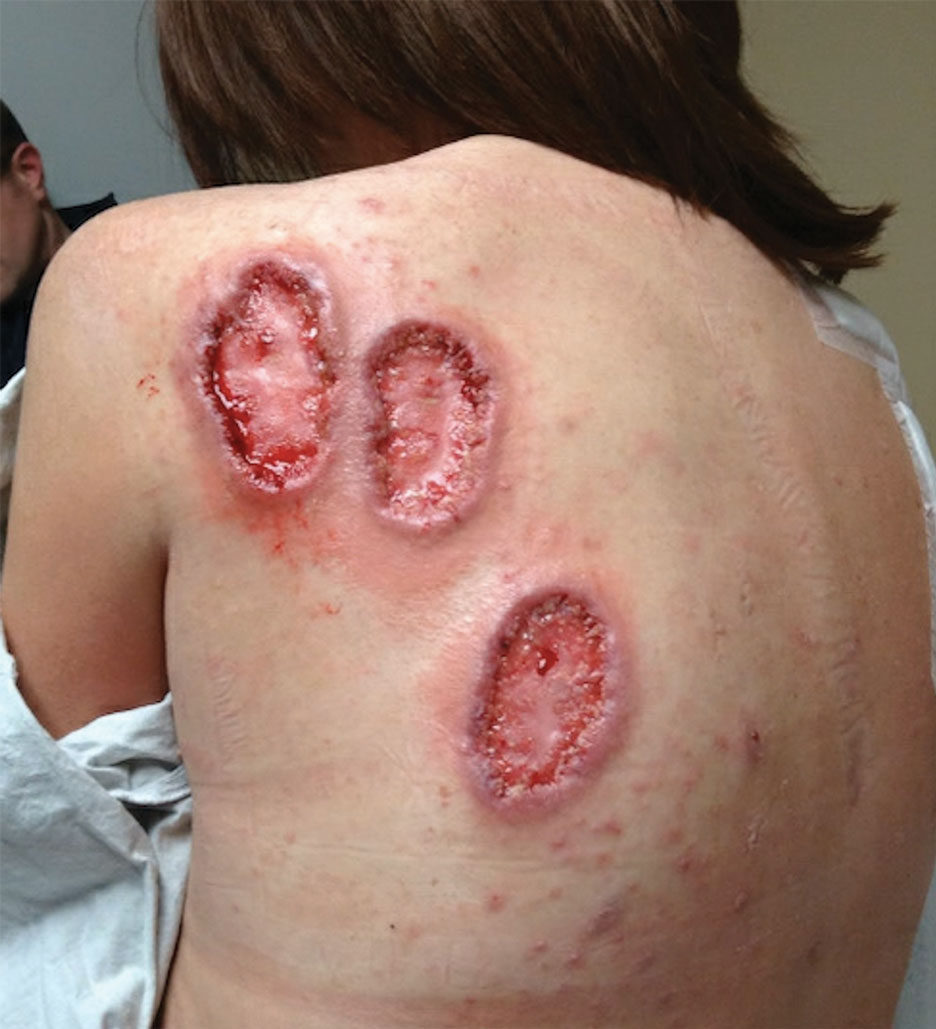
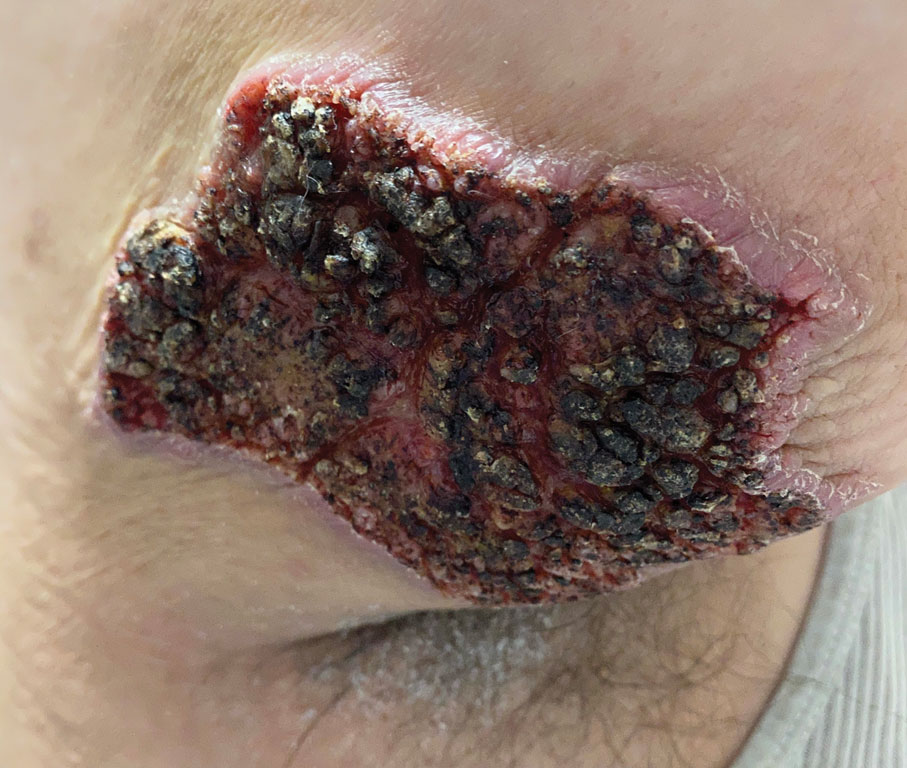
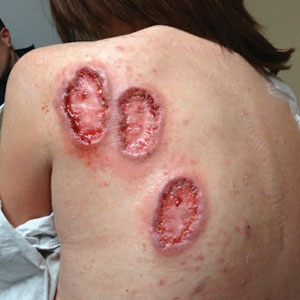
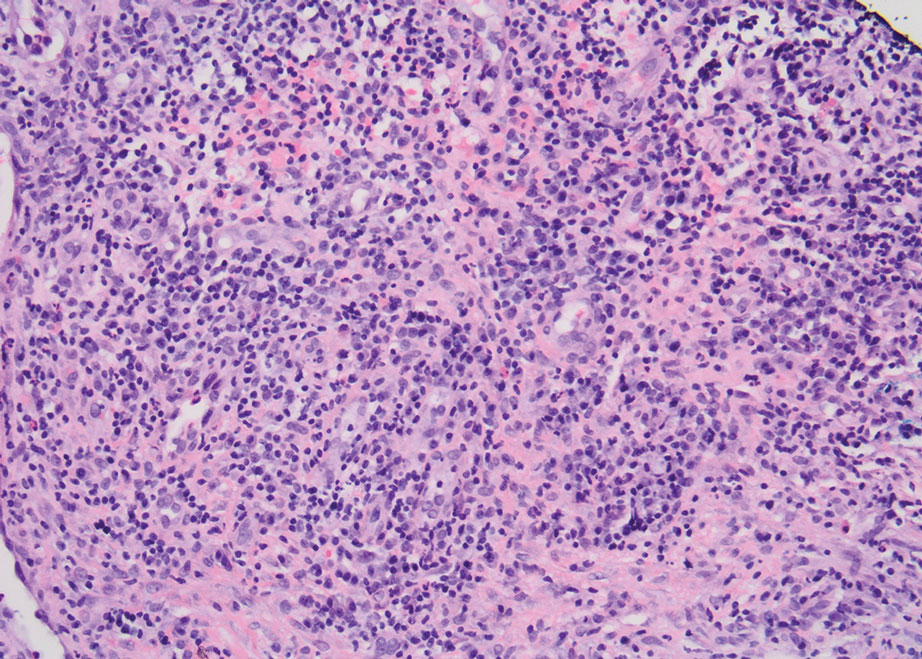
Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.
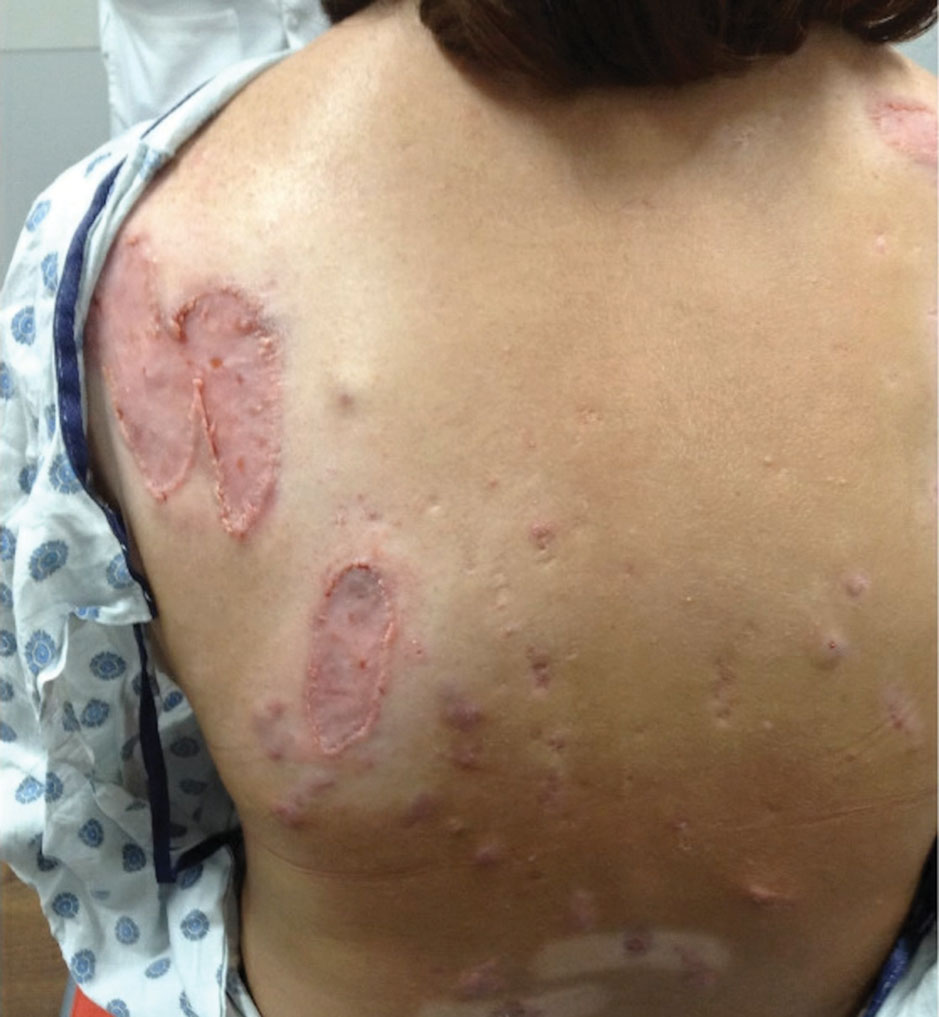
After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.
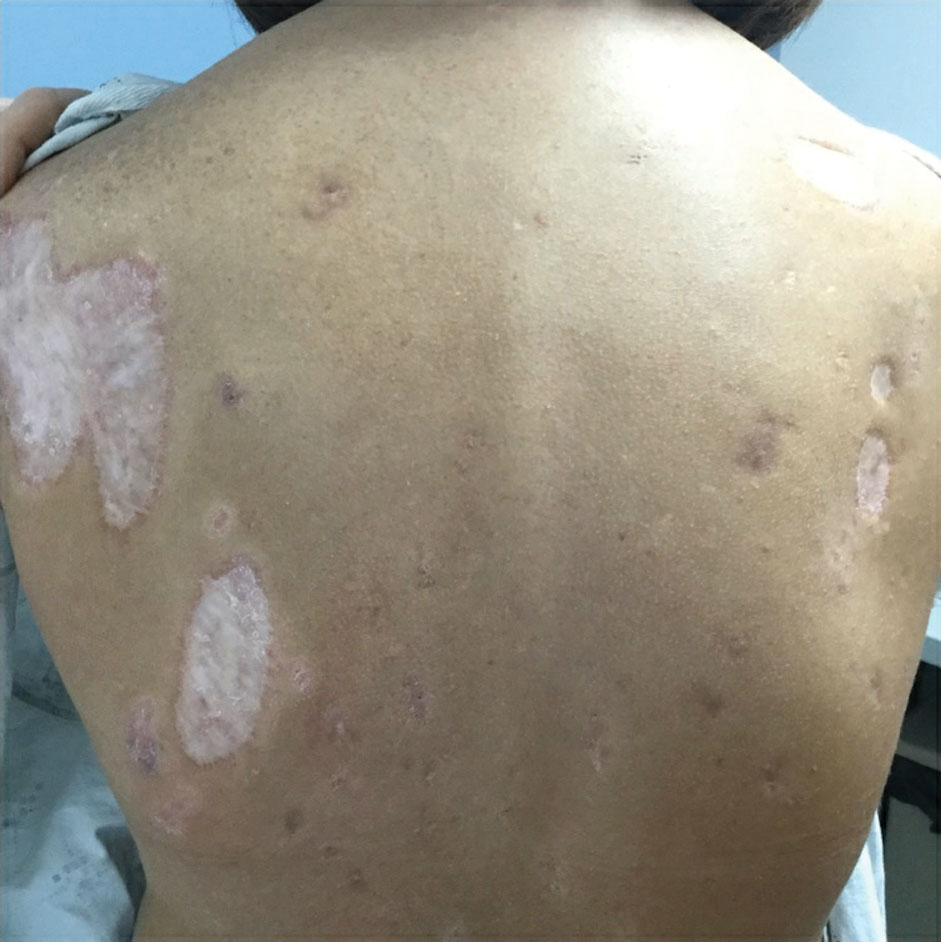
The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.
Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.
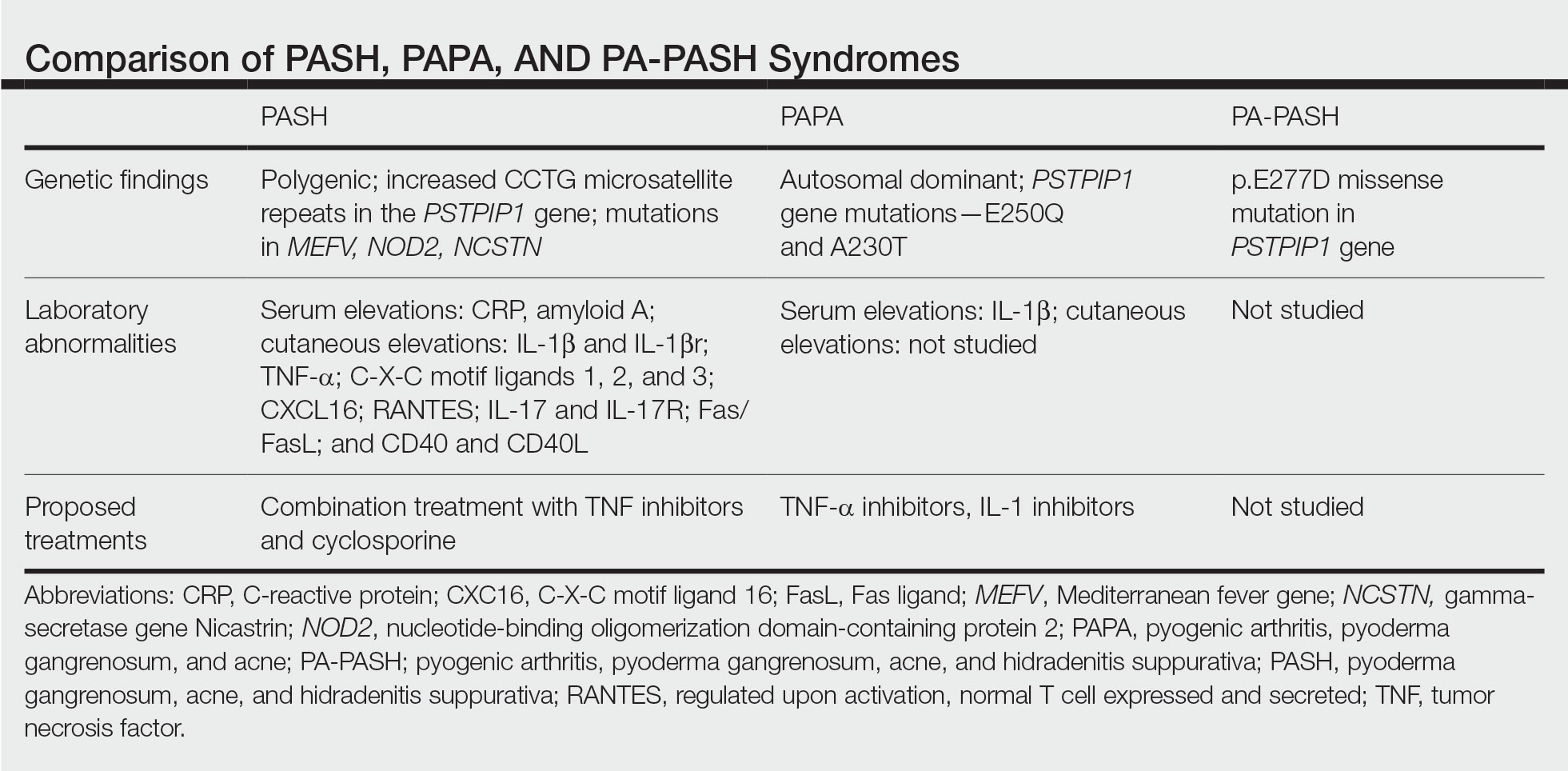
PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2 Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.

Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.

After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.

The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.

Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.

PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2 Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.

Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.

After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.

The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.

Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.

PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
Practice Points
- Despite phenotypic similarities among pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (PASH) syndrome; pyogenic arthritis, PG, and acne syndrome; and pyogenic arthritis–PASH syndrome, there are genotypic differences that contribute to unique inflammatory cytokine patterns and the need for distinct pharmacologic considerations within each entity.
- When formulating therapeutic regimens for patients with PASH syndrome, it is essential for dermatologists to consider the likelihood of hyperactivity in multiple pathways of the innate immune system and utilize a combination of multimodal antiinflammatory therapies.
Multimodal Treatment of Epidermodysplasia Verruciformis in an HIV-Positive Man
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.
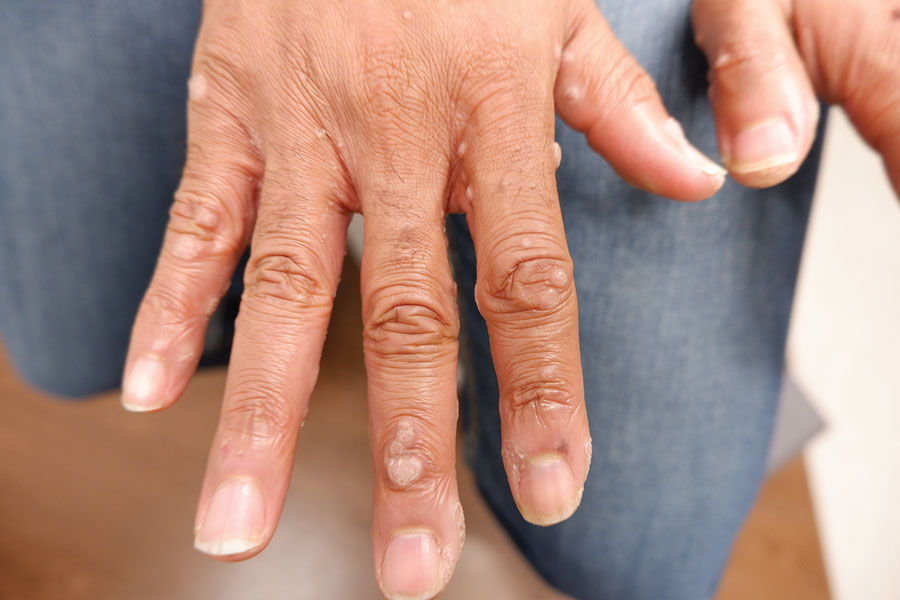
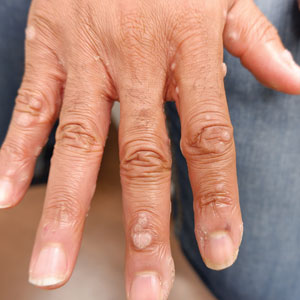
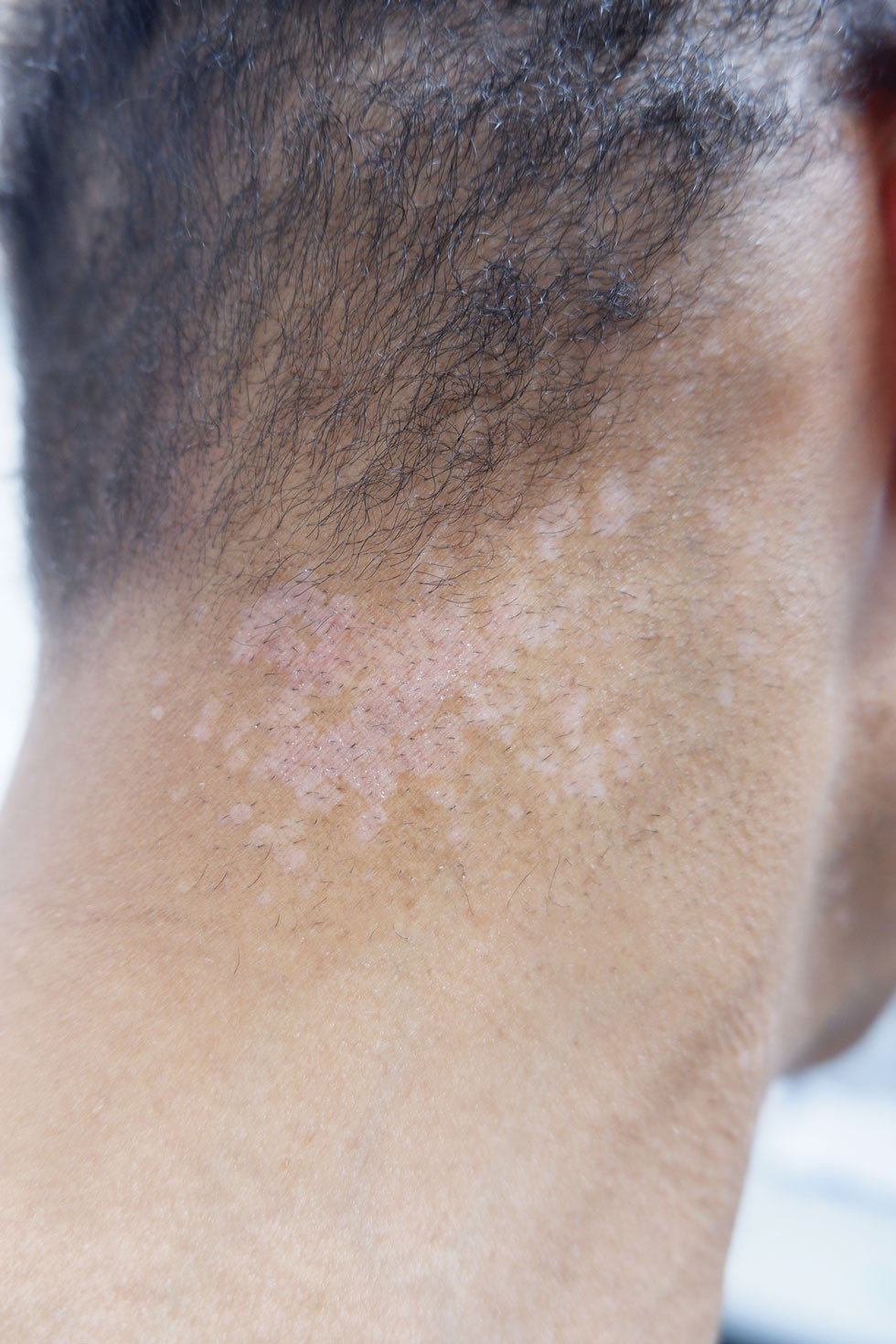
A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.
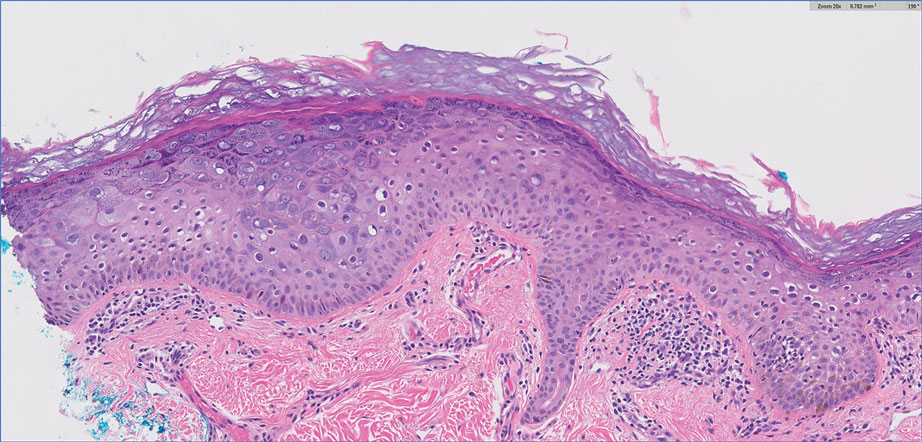
Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.
Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.

A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.

Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.

Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.

A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.

Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.

Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
Practice Points
- Acquired epidermodysplasia verruciformis (EDV) is associated with immunocompromised patients with conditions such as HIV.
- Multimodal treatment of HIV-associated acquired EDV with acitretin, intralesional Candida albicans antigen, and cryotherapy may be efficacious for patients with recalcitrant disease.
Chronic Ulcerative Lesion
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.
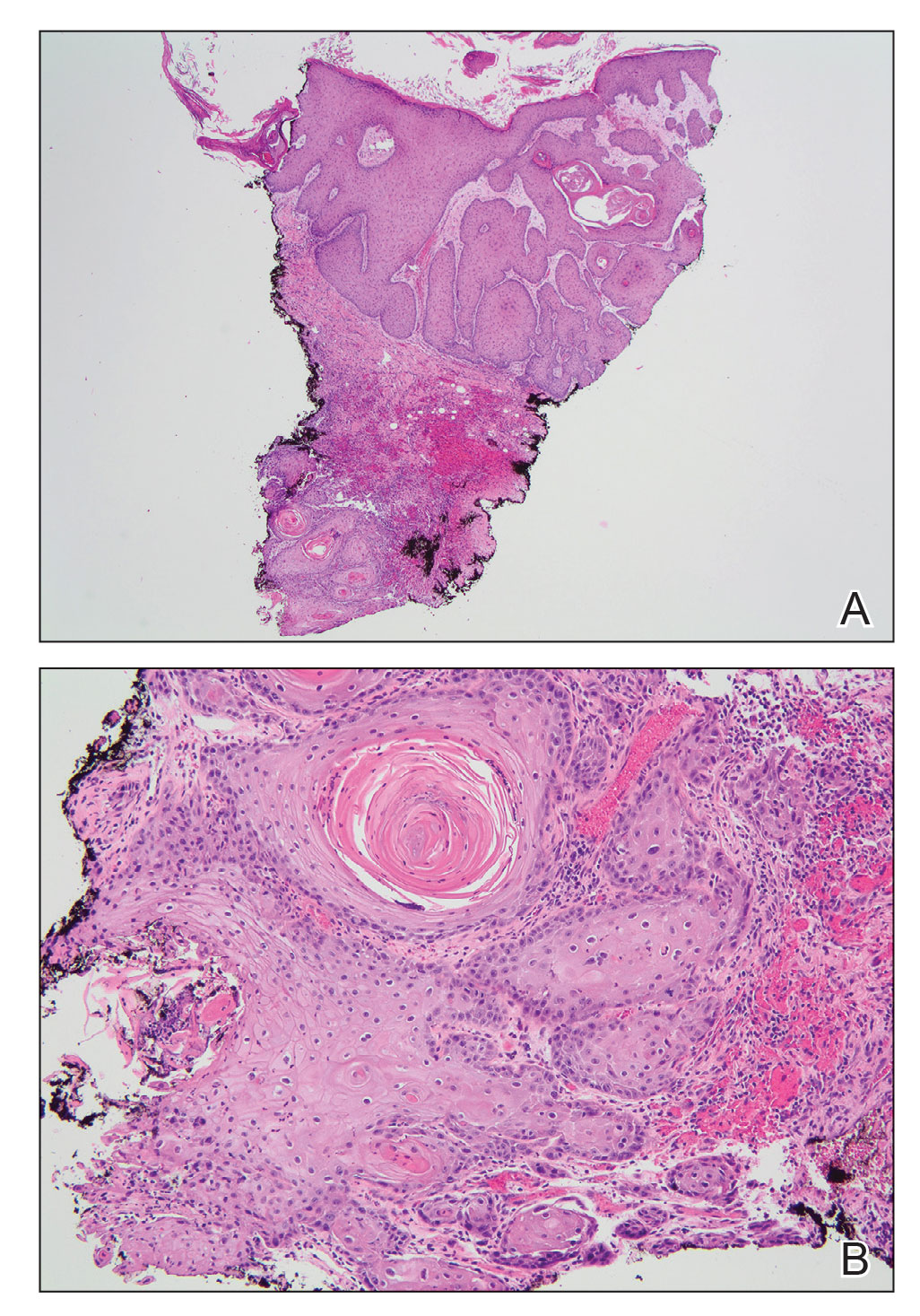
Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.

Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.

Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
A 46-year-old man with a history of a left leg burn during childhood that was unsuccessfully treated with multiple skin grafts presented as a hospital follow-up for outpatient management of an ulcer. The patient had an ulcer that gradually increased in size over 7 years. Over the course of 2 weeks prior to the hospital presentation, he noted increased pain and severe difficulty with ambulation but remained afebrile without other systemic symptoms. Prior to the outpatient follow-up, he had been admitted to the hospital where he underwent imaging, laboratory studies, and skin biopsy, as well as treatment with empiric vancomycin. Physical examination revealed a large undermined ulcer with an elevated peripheral margin and crusting on the left lower leg with surrounding chronic scarring.
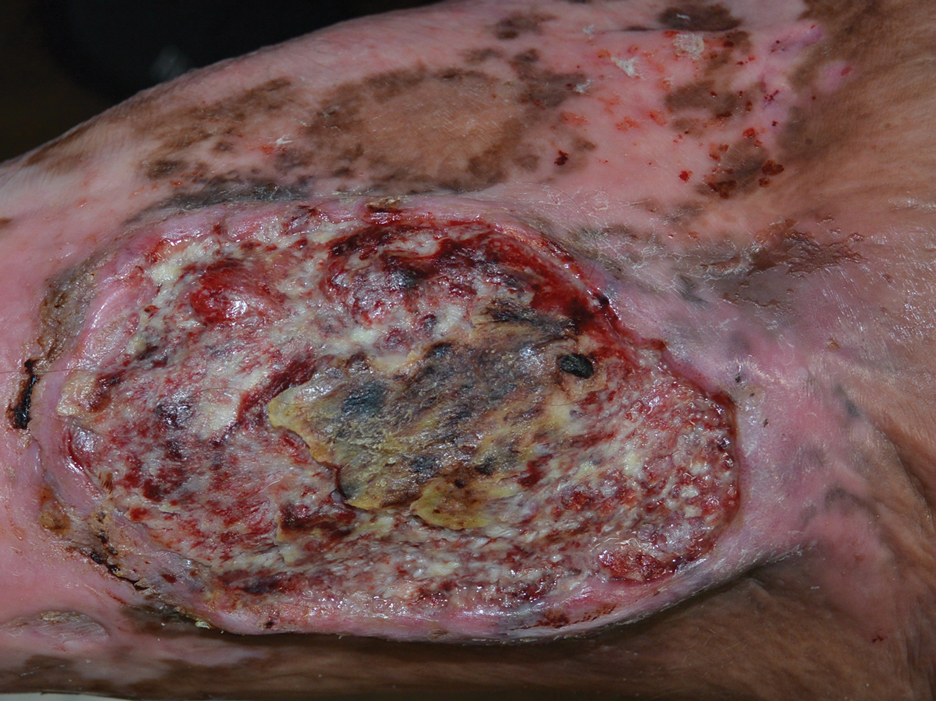
Fungated Eroded Plaque on the Arm
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
A 39-year-old man from Ohio presented with a tender, 10×6-cm, fungated, eroded plaque on the right medial upper arm that developed over the last 4 years. He initially noticed a firm lump under the right arm 4 years prior that was diagnosed as possible cellulitis at an outside clinic and treated with trimethoprim-sulfamethoxazole. The lesion then began to erode and became a chronic nonhealing wound. Approximately 1 year prior to the current presentation, the patient recalled unloading a truckload of soil around the same time the wound began to enlarge in diameter and depth. He denied any prior or current respiratory or systemic symptoms including fevers, chills, or weight loss.