User login
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
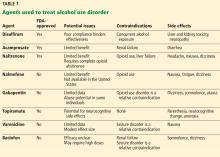
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
KEY POINTS
- Gabapentin has been shown to be safe and effective for mild alcohol withdrawal but is not appropriate as monotherapy for severe withdrawal owing to risk of seizures.
- During early abstinence, gabapentin may improve sleep, cravings, and mood—factors associated with relapse.
- Gabapentin is being used recreationally to achieve or enhance euphoria, but its misuse potential appears to be low when taken at therapeutic doses by patients without a history of drug abuse.