User login
Gabapentin for alcohol use disorder: A good option, or cause for concern?
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
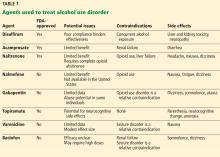
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
KEY POINTS
- Gabapentin has been shown to be safe and effective for mild alcohol withdrawal but is not appropriate as monotherapy for severe withdrawal owing to risk of seizures.
- During early abstinence, gabapentin may improve sleep, cravings, and mood—factors associated with relapse.
- Gabapentin is being used recreationally to achieve or enhance euphoria, but its misuse potential appears to be low when taken at therapeutic doses by patients without a history of drug abuse.
Cannabis for peripheral neuropathy: The good, the bad, and the unknown
Marijuana, which is still illegal under federal law but legal in 30 states for medical purposes as of this writing, has shown promising results for treating peripheral neuropathy. Studies suggest that cannabis may be an option for patients whose pain responds poorly to standard treatments; however, its use may be restricted by cognitive and psychiatric adverse effects, particularly at high doses.1
In this article, we discuss the basic pharmacology of cannabis and how it may affect neuropathic pain. We review clinical trials on its use for peripheral neuropathy and provide guidance for its use.
PERIPHERAL NEUROPATHY IS COMMON AND COMPLEX
An estimated 20 million people in the United States suffer from neuropathic pain. The prevalence is higher in certain populations, with 26% of people over age 65 and 30% of patients with diabetes mellitus affected.2–4
Peripheral neuropathy is a complex, chronic state that occurs when nerve fibers are damaged, dysfunctional, or injured, sending incorrect signals to pain centers in the central nervous system.5 It is characterized by weakness, pain, and paresthesias that typically begin in the hands or feet and progress proximally.4 Symptoms depend on the number and types of nerves affected.
In many cases, peripheral neuropathy is idiopathic, but common causes include diabetes, alcoholism, human immunodeficiency virus (HIV) infection, and autoimmune disease. Others include toxicity from chemotherapy and heavy metals.
Peripheral neuropathy significantly worsens quality of life and function. Many patients experience emotional, cognitive, and functional problems, resulting in high rates of medical and psychiatric comorbidities and occupational impairment.4,6,7 Yet despite its clinical and epidemiologic significance, it is often undertreated.8
STANDARD TREATMENTS INADEQUATE
Peripheral neuropathy occurs in patients with a wide range of comorbidities and is especially difficult to treat. Mainstays of therapy include anticonvulsants, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.9 A more invasive option is spinal cord stimulation.
These treatments can have considerable adverse effects, and response rates remain suboptimal, with pain relief insufficient to improve quality of life for many patients.9,10 Better treatments are needed to improve clinical outcomes and patient experience.11
CANNABIS: A MIX OF COMPOUNDS
Cannabis sativa has been used as an analgesic for centuries. The plant contains more than 400 chemical compounds and is often used for its euphoric properties. Long-term use may lead to addiction and cognitive impairment.12,13
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main components and the 2 best-studied cannabinoids with analgesic effects.
THC is the primary psychoactive component of cannabis. Its effects include relaxation, altered perception, heightened sensations, increased libido, and perceptual distortions of time and space. Temporary effects may include decreased short-term memory, dry mouth, impaired motor function, conjunctival injection, paranoia, and anxiety.
CBD is nonpsychoactive and has anti-inflammatory and antioxidant properties. It has been shown to reduce pain and inflammation without the effects of THC.14
Other compounds in the cannabis plant include phytocannabinoids, flavonoids, and tapenoids, which may produce individual, interactive, or synergistic effects.15 Different strains of cannabis have varying amounts of the individual components, making comparisons among clinical studies difficult.
THE ENDOCANNABINOID SYSTEM
The endogenous mammalian cannabinoid system plays a regulatory role in the development, homeostasis, and neuroplasticity of the central nervous system. It is also involved in modulating pain transmission in the nociceptive pathway.
Two of the most abundant cannabinoid endogenous ligands are anandamide and 2-arachidonylglycerol.9 These endocannabinoids are produced on demand in the central nervous system to reduce pain by acting as a circuit breaker.16–18 They target the G protein-coupled cannabinoid receptors CB1 and CB2, located throughout the central and peripheral nervous system and in organs and tissues.12
CB1 receptors are found primarily in the central nervous system, specifically in areas involved in movement, such as the basal ganglia and cerebellum, as well as in areas involved in memory, such as the hippocampus.12 They are also abundant in brain regions implicated in conducting and modulating pain signals, including the periaqueductal gray and the dorsal horn of the spinal cord.16–20
CB2 receptors are mostly found in peripheral tissues and organs, mainly those involved in the immune system, including splenic, tonsillar, and hematopoietic cells.12 They help regulate inflammation, allodynia, and hyperalgesia.17
Modifying response to injury
Following a nerve injury, neurons along the nociceptive pathway may become more reactive and responsive in a process known as sensitization.21 The process involves a cascade of cellular events that result in sprouting of pain-sensitive nerve endings.21,22
Cannabinoids are thought to reduce pain by modifying these cellular events. They also inhibit nociceptive conduction in the dorsal horn of the spinal cord and in the ascending spinothalamic tract.20 CB1 receptors found in nociceptive terminals along the peripheral nervous system impede pain conduction, while activation of CB2 receptors in immune cells decreases the release of nociceptive agents.
STUDIES OF CANNABIS FOR NEUROPATHIC PAIN
A number of studies have evaluated cannabis for treating neuropathic pain. Overall, available data support the efficacy of smoked or inhaled cannabis in its flower form when used as monotherapy or adjunctive therapy for relief of neuropathic pain of various etiologies. Many studies also report secondary benefits, including better sleep and functional improvement.23,24
However, adverse effects are common, especially at high doses, and include difficulty concentrating, lightheadedness, fatigue, and tachycardia. More serious reported adverse effects include anxiety, paranoia, and psychosis.
Wilsey et al, 2008: Neuropathic pain reduced
Wilsey et al25 conducted a double-blind, placebo-controlled crossover study that assessed the effects of smoking cannabis in 38 patients with central or peripheral neuropathic pain. Participants were assigned to smoke either high- or low-dose cannabis (7% or 3.5% delta-9-THC) or placebo cigarettes. Cigarettes were smoked during treatment sessions using the following regimen: 2 puffs at 60 minutes from baseline, 3 puffs at 120 minutes, and 4 puffs at 180 minutes. Patients were assessed after each set of puffs and for 2 hours afterwards. The primary outcome was spontaneous relief of pain as measured by a visual analog scale.
Pain intensity was comparable and significantly reduced in both treatment groups compared with placebo. At the high dose, some participants experienced neurocognitive impairment in attention, learning, memory, and psychomotor speed; only learning and memory declined at the low dose.
Ellis et al, 2009: Pain reduction in HIV neuropathy
Ellis et al23 conducted a double-blind, placebo-controlled crossover trial in patients with HIV neuropathy that was unresponsive to at least 2 analgesics with different modes of action. During each treatment week, participants were randomly assigned to smoke either active cannabis or placebo, while continuing their standard therapy. Titration started at 4% THC and was adjusted based on tolerability and efficacy. Twenty-eight of the 34 enrolled patients completed both cannabis and placebo treatments. The principal outcome was change in pain intensity from baseline at the end of each week, using the Descriptor Differential Scale of Pain Intensity.
Of the 28 patients, 46% achieved an average pain reduction of 3.3 points (30%). One patient experienced cannabis-induced psychosis, and another developed an intractable cough, which resolved with smoking cessation.
Ware et al, 2010: Reduced posttraumatic or postsurgical neuropathic pain
Ware et al24 performed a randomized crossover trial in 21 patients with posttraumatic or postsurgical neuropathic pain. Participants inhaled 4 different formulations of cannabis (containing 0%, 2.5%, 6.0%, and 9.4% THC) during 4 14-day periods. They inhaled a 25-mg dose through a pipe 3 times a day for the first 5 days of each cycle, followed by a 9-day washout period. Daily average pain intensity was measured using a numeric rating scale. The investigators also assessed mood, sleep, quality of life, and adverse effects.
Patients in the 9.4% THC group reported significantly less pain and better sleep, with average pain scores decreasing from 6.1 to 5.4 on an 11-point scale. Although the benefit was modest, the authors noted that the pain had been refractory to standard treatments.
The number of reported adverse events increased with greater potency and were most commonly throat irritation, burning sensation, headache, dizziness, and fatigue. This study suggests that THC potency affects tolerability, with higher doses eliciting clinically important adverse effects, some of which may reduce the ability to perform activities of daily living, such as driving.
Wilsey et al, 2013: Use in resistant neuropathic pain
Wilsey et al26 conducted another double-blind, placebo-controlled crossover study assessing the effect of vaporized cannabis on central and peripheral neuropathic pain resistant to first-line pharmacotherapies. Dose-effect relationships were explored using medium-dose (3.5%), low-dose (1.3%), and placebo cannabis. The primary outcome measure was a 30% reduction in pain intensity based on a visual analog scale.
In the placebo group, 26% of patients achieved this vs 57% of the low-dose cannabis group and 61% of those receiving the medium dose. No significant difference was found between the 2 active doses in reducing neuropathic pain, and both were more effective than placebo. The number needed to treat to achieve a 30% reduction in pain was about 3 for both cannabis groups compared with placebo. Psychoactive effects were minimal, of short duration, and reversible.
Wallace et al, 2015: Use in diabetic peripheral neuropathy
Wallace et al27 conducted a randomized, double-blind, placebo-controlled crossover study evaluating cannabis for diabetic peripheral neuropathy in 16 patients. Each had experienced at least 6 months of neuropathic pain in their feet. The participants inhaled a single dose of 1%, 4%, or 7% THC cannabis or placebo. Spontaneous pain was reported with a visual analog scale and also tested with a foam brush and von Frey filament at intervals until 4 hours after treatment.
Pain scores were lower with treatment compared with placebo, with high-dose cannabis having the greatest analgesic effect. Pain reduction lasted for the full duration of the test. Cannabis recipients had declines in attention and working memory, with the high-dose group experiencing the greatest impact 15 minutes after treatment. High-dose recipients also had poorer scores on testing of quick task-switching, with the greatest effect at 2 hours.27
Research and market cannabis are not equal
Results of US studies must be qualified. Most have used cannabis provided by the National Institute of Drug Abuse (NIDA),23–26 which differs in potency from commercially available preparations. This limits the clinical usefulness of the analysis of benefits and risks.
Vergara et al28 found that NIDA varieties contained much lower THC levels and as much as 23 times the cannabinol content as cannabis in state-legalized markets.
Studies based on NIDA varieties likely underestimate the risks of consumer-purchased cannabis, as THC is believed to be most responsible for the risk of psychosis and impaired driving and cognition.24,28
CBD MAY PROTECT AGAINST ADVERSE EFFECTS
Studies of CBD alone are limited to preclinical data.29 Evidence suggests that CBD alone or combined with THC can suppress chronic neuropathic pain, and that CBD may have a protective effect after nerve injury.30
Nabiximols, an oromucosal spray preparation with equal amounts of THC and CBD, has been approved in Canada as well as in European countries including the United Kingdom. Although its use has not been associated with many of the adverse effects of inhaled cannabis,30–32 evidence of efficacy from clinical trials has been mixed.
Lynch et al,31 in a 2014 randomized, double-blind, placebo-controlled crossover pilot study31 evaluated nabiximols in 16 patients with neuropathic pain related to chemotherapy. No statistically significant difference was found between treatment and placebo. However, the trial was underpowered.
Serpell et al,32 in a 2014 European randomized, placebo-controlled parallel-group study, evaluated 246 patients with peripheral neuropathy with allodynia, with 128 receiving active treatment (THC-CBD oromucosal spray) and 118 receiving placebo. Over the 15-week study, participants continued their current analgesic treatments.
Pain was reduced in the treatment group, but the difference from placebo was not statistically significant. However, the treatment group reported significantly better sleep quality and Patient Global Impression of Change measures (reflecting a patient’s belief of treatment efficacy).
META-ANALYSES CONFIRM EFFECT
Three meta-analyses of available studies of the effects of cannabis on neuropathic pain have been completed.
Andreae et al, 2015: 5 trials, 178 patients
Andreae et al1 evaluated 5 randomized controlled trials in 178 patients in North America. All had had neuropathy for at least 3 months, with a pain level of at least about 3 on a scale of 10. Two studies had patients with HIV-related neuropathy; the other 3 involved patients with neuropathy related to trauma, diabetes, complex regional pain syndrome, or spinal cord injury. All trials used whole cannabis plant provided by NIDA, and the main outcomes were patient-reported pain scales. No study evaluated pain beyond 2 weeks after trial termination.
They found that 1 of every 5 to 6 patients treated with cannabis had at least a 30% pain reduction.
Nugent et al, 2017: 13 trials, 246 patients
Nugent et al33 reviewed 13 trials in 246 patients that evaluated the effects of different cannabis-based preparations on either central or peripheral neuropathic pain from various conditions. Actively treated patients were more likely to report a 30% improvement in neuropathic pain. Again, studies tended to be small and brief.
Cochrane review, 2018: 16 trials, 1,750 patients
A Cochrane review34 analyzed 16 trials (in 1,750 patients) lasting 2 to 26 weeks. Treatments included an oromucosal spray with a plant-derived combination of THC and CBD, nabilone, inhaled herbal cannabis, and plant-derived THC.
With cannabis-based treatments, significantly more people achieved 50% or greater pain relief than with placebo (21% vs 17%, number needed to treat 20); 30% pain reduction was achieved in 39% of treated patients vs 33% of patients taking placebo (number needed to treat 11).
On the other hand, significantly more participants withdrew from studies because of adverse events with cannabis-based treatments than placebo (10% vs 5%), with psychiatric disorders occurring in 17% of patients receiving active treatment vs 5% of those receiving placebo (number needed to harm 10).
The primary studies suffered from methodologic limitations including small size, short duration, and inconsistency of formulations and study designs. Further evaluation of long-term efficacy, tolerability, and addiction potential is critical to determine the risk-benefit ratio.
RISKS OF CANNABIS USE
Like any drug therapy, cannabis has effects that may limit its use. Cannabis can affect a person’s psyche, physiology, and lifestyle.
Impaired attention, task speed
Neurocognitive changes associated with cannabis use—especially dizziness, fatigue, and slowed task-switching—could affect driving and other complex tasks. Evidence indicates that such activities should be avoided in the hours after treatment.26,27,32,33
Concern over brain development
Most worrisome is the effect of long-term cannabis use on brain development in young adults. Regular use of cannabis at an early age is associated with lower IQ, decline in school performance, and lower rates of high school graduation.35
Avoid in psychiatric patients
It is unlikely that cannabis can be safely used in patients with psychiatric illnesses. Anxiety, depression, and psychotic disorders can be exacerbated by the regular use of cannabis, and the risk of developing these conditions is increased while using cannabis.36,37
High concentrations of THC (the highest concentration used in the above studies was 9.5%) can cause anxiety, paranoia, and psychosis.
Respiratory effects
Long-term cannabis smoking may cause wheezing, cough, dyspnea, and exacerbations of chronic bronchitis. There is some evidence that symptoms improve after stopping smoking.33,38
SHOULD WE RECOMMEND CANNABIS?
Where cannabis can be legally used, doctors should be familiar with the literature and its limitations so that they can counsel patients on the best use and potential risks and benefits of cannabis treatment.
A recent conceptualization of pain suggests that a pain score reflects a composite of sensory factors (eg, tissue damage), cognitive factors (eg, beliefs about pain), and affective factors (eg, anxiety, depression).39 Physicians should keep this in mind when evaluating patients to better assess the risks and benefits of cannabis. While pharmacotherapy may address sensory factors, cognitive behavioral therapy may help alter beliefs about the pain as well as anxiety and depressive symptoms that might influence subjective reports.
Ideally, patients being considered for cannabis treatment would have a type of neuropathic pain proven to respond to cannabis in randomized, controlled studies, as well as evidence of failed first-line treatments.
Relative contraindications include depression, anxiety, substance use, psychotic disorders, and respiratory conditions, and these should also be considered.
Although current research shows an analgesic benefit of cannabis on neuropathic pain comparable to that of gabapentin,40 further investigation is needed to better evaluate long-term safety, efficacy, and interactions with standard therapies. Until we have a more complete picture, we should use the current literature, along with a thorough knowledge of each patient, to determine if the benefits of cannabis therapy outweigh the risks.
Acknowledgments: We thank Camillo Ferrari, BS, and Christina McMahon, BA, for their helpful comments.
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015; 16(12):1221–1232. doi:10.1016/j.jpain.2015.07.009
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet. www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Accessed November 14, 2018.
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older adults. J Am Board Fam Med 2004; 17(5):308–318. doi:10.3122/jabfm.17.5.309
- Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of developing peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig 2014; 5(6):714–721. doi:10.1111/jdi.12223
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
- Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain 2016; 157(suppl 1):S23–S32. doi:10.1097/j.pain.0000000000000428
- Zeng L, Alongkronrusmee D, van Rijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 2017; 10:219–228. doi:10.2147/JPR.S125987
- Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA 2015; 314(20):2172–2181. doi:10.1001/jama.2015.13611
- Adams AS, Callaghan B, Grant RW. Overcoming barriers to diabetic polyneuropathy management in primary care. Healthc (Amst) 2017; 5(4):171–173. doi:10.1016/j.hjdsi.2016.10.003
- Gwak YS, Kim HY, Lee BH, Yang CH. Combined approaches for the relief of spinal cord injury-induced neuropathic pain. Complement Ther Med 2016; 25:27–33. doi:10.1016/j.ctim.2015.12.021
- Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology 2016; 30(11):1020–1029. pmid:27854104
- Grotenhermen F. Cannabinoids and the endocannabinoid system. Cannabinoids 2006; 1(1):10–14.
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA 2015; 313(24):2474–2483. doi:10.1001/jama.2015.6199
- Campos AC, Fogaça MV, Scarante FF, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 2017; 8:269. doi:10.3389/fphar.2017.00269
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163(7):1344–1364. doi:10.1111/j.1476-5381.2011.01238.x
- Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci 2017; Jul 7: 1–20. doi:10.1080/1028415X.2017.1347373
- Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018; 43(1):155–172. doi:10.1038/npp.2017.130
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991; 11(2):563–583. pmid:1992016
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience1998; 83(2):393–411. pmid:9460749
- Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: Deer TR, Leong MS, ed. Comprehensve Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches. New York, NY: Springer; 2013:181–193.
- Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012; 12(4):304–314. pmid:23033930
- Yamanaka H, Noguchi K. Pathophysiology of neuropathic pain: molecular mechanisms underlying central sensitization in the dorsal horn in neuropathic pain. Brain Nerve 2012; 64(11):1255–1265. Japanese. pmid:23131736
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010; 182(14):E694–E701. doi:10.1503/cmaj.091414
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008; 9(6):506–521. doi:10.1016/j.jpain.2007.12.010
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013; 14(2):136–148. doi:10.1016/j.jpain.2012.10.009
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Scientific Reports. 2017; 7(1):1-8. doi:10.1038/srep46528
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterized by allodynia: a randomized, double-blind, placebo-controlled clinical trial. Pain 2007; 133(1–3):210–220. doi:10.1016/j.pain.2007.08.028
- Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017; 158(12):2442–2451. doi:10.1097/j.pain.0000000000001052
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014; 47(1):166–173. doi:10.1016/j.jpainsymman.2013.02.018
- Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
- Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167(5):319–331. doi:10.7326/M17-0155
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Castellanos-Ryan N, Pingault JB, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol 2017; 29(4):1253–1266. doi:10.1017/S0954579416001280
- Karila L, Roux P, Benyamina A, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014; 20(25):4112–4118. pmid:24001294
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001; 178:116–122. pmid:11157424
- National Academies of Science, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academy Press; 2017. doi:10.17226/24625
- Modesto-Lowe V, Griard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J Opioid Manag 2012; 8(3):167–175. doi:10.5055/jom.2012.0113
- Grant I. Medicinal cannabis and painful sensory neuropathy. Virtual Mentor 2013; 15(5):466–469. doi:10.1001/virtualmentor.2013.15.5.oped1-1305
Marijuana, which is still illegal under federal law but legal in 30 states for medical purposes as of this writing, has shown promising results for treating peripheral neuropathy. Studies suggest that cannabis may be an option for patients whose pain responds poorly to standard treatments; however, its use may be restricted by cognitive and psychiatric adverse effects, particularly at high doses.1
In this article, we discuss the basic pharmacology of cannabis and how it may affect neuropathic pain. We review clinical trials on its use for peripheral neuropathy and provide guidance for its use.
PERIPHERAL NEUROPATHY IS COMMON AND COMPLEX
An estimated 20 million people in the United States suffer from neuropathic pain. The prevalence is higher in certain populations, with 26% of people over age 65 and 30% of patients with diabetes mellitus affected.2–4
Peripheral neuropathy is a complex, chronic state that occurs when nerve fibers are damaged, dysfunctional, or injured, sending incorrect signals to pain centers in the central nervous system.5 It is characterized by weakness, pain, and paresthesias that typically begin in the hands or feet and progress proximally.4 Symptoms depend on the number and types of nerves affected.
In many cases, peripheral neuropathy is idiopathic, but common causes include diabetes, alcoholism, human immunodeficiency virus (HIV) infection, and autoimmune disease. Others include toxicity from chemotherapy and heavy metals.
Peripheral neuropathy significantly worsens quality of life and function. Many patients experience emotional, cognitive, and functional problems, resulting in high rates of medical and psychiatric comorbidities and occupational impairment.4,6,7 Yet despite its clinical and epidemiologic significance, it is often undertreated.8
STANDARD TREATMENTS INADEQUATE
Peripheral neuropathy occurs in patients with a wide range of comorbidities and is especially difficult to treat. Mainstays of therapy include anticonvulsants, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.9 A more invasive option is spinal cord stimulation.
These treatments can have considerable adverse effects, and response rates remain suboptimal, with pain relief insufficient to improve quality of life for many patients.9,10 Better treatments are needed to improve clinical outcomes and patient experience.11
CANNABIS: A MIX OF COMPOUNDS
Cannabis sativa has been used as an analgesic for centuries. The plant contains more than 400 chemical compounds and is often used for its euphoric properties. Long-term use may lead to addiction and cognitive impairment.12,13
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main components and the 2 best-studied cannabinoids with analgesic effects.
THC is the primary psychoactive component of cannabis. Its effects include relaxation, altered perception, heightened sensations, increased libido, and perceptual distortions of time and space. Temporary effects may include decreased short-term memory, dry mouth, impaired motor function, conjunctival injection, paranoia, and anxiety.
CBD is nonpsychoactive and has anti-inflammatory and antioxidant properties. It has been shown to reduce pain and inflammation without the effects of THC.14
Other compounds in the cannabis plant include phytocannabinoids, flavonoids, and tapenoids, which may produce individual, interactive, or synergistic effects.15 Different strains of cannabis have varying amounts of the individual components, making comparisons among clinical studies difficult.
THE ENDOCANNABINOID SYSTEM
The endogenous mammalian cannabinoid system plays a regulatory role in the development, homeostasis, and neuroplasticity of the central nervous system. It is also involved in modulating pain transmission in the nociceptive pathway.
Two of the most abundant cannabinoid endogenous ligands are anandamide and 2-arachidonylglycerol.9 These endocannabinoids are produced on demand in the central nervous system to reduce pain by acting as a circuit breaker.16–18 They target the G protein-coupled cannabinoid receptors CB1 and CB2, located throughout the central and peripheral nervous system and in organs and tissues.12
CB1 receptors are found primarily in the central nervous system, specifically in areas involved in movement, such as the basal ganglia and cerebellum, as well as in areas involved in memory, such as the hippocampus.12 They are also abundant in brain regions implicated in conducting and modulating pain signals, including the periaqueductal gray and the dorsal horn of the spinal cord.16–20
CB2 receptors are mostly found in peripheral tissues and organs, mainly those involved in the immune system, including splenic, tonsillar, and hematopoietic cells.12 They help regulate inflammation, allodynia, and hyperalgesia.17
Modifying response to injury
Following a nerve injury, neurons along the nociceptive pathway may become more reactive and responsive in a process known as sensitization.21 The process involves a cascade of cellular events that result in sprouting of pain-sensitive nerve endings.21,22
Cannabinoids are thought to reduce pain by modifying these cellular events. They also inhibit nociceptive conduction in the dorsal horn of the spinal cord and in the ascending spinothalamic tract.20 CB1 receptors found in nociceptive terminals along the peripheral nervous system impede pain conduction, while activation of CB2 receptors in immune cells decreases the release of nociceptive agents.
STUDIES OF CANNABIS FOR NEUROPATHIC PAIN
A number of studies have evaluated cannabis for treating neuropathic pain. Overall, available data support the efficacy of smoked or inhaled cannabis in its flower form when used as monotherapy or adjunctive therapy for relief of neuropathic pain of various etiologies. Many studies also report secondary benefits, including better sleep and functional improvement.23,24
However, adverse effects are common, especially at high doses, and include difficulty concentrating, lightheadedness, fatigue, and tachycardia. More serious reported adverse effects include anxiety, paranoia, and psychosis.
Wilsey et al, 2008: Neuropathic pain reduced
Wilsey et al25 conducted a double-blind, placebo-controlled crossover study that assessed the effects of smoking cannabis in 38 patients with central or peripheral neuropathic pain. Participants were assigned to smoke either high- or low-dose cannabis (7% or 3.5% delta-9-THC) or placebo cigarettes. Cigarettes were smoked during treatment sessions using the following regimen: 2 puffs at 60 minutes from baseline, 3 puffs at 120 minutes, and 4 puffs at 180 minutes. Patients were assessed after each set of puffs and for 2 hours afterwards. The primary outcome was spontaneous relief of pain as measured by a visual analog scale.
Pain intensity was comparable and significantly reduced in both treatment groups compared with placebo. At the high dose, some participants experienced neurocognitive impairment in attention, learning, memory, and psychomotor speed; only learning and memory declined at the low dose.
Ellis et al, 2009: Pain reduction in HIV neuropathy
Ellis et al23 conducted a double-blind, placebo-controlled crossover trial in patients with HIV neuropathy that was unresponsive to at least 2 analgesics with different modes of action. During each treatment week, participants were randomly assigned to smoke either active cannabis or placebo, while continuing their standard therapy. Titration started at 4% THC and was adjusted based on tolerability and efficacy. Twenty-eight of the 34 enrolled patients completed both cannabis and placebo treatments. The principal outcome was change in pain intensity from baseline at the end of each week, using the Descriptor Differential Scale of Pain Intensity.
Of the 28 patients, 46% achieved an average pain reduction of 3.3 points (30%). One patient experienced cannabis-induced psychosis, and another developed an intractable cough, which resolved with smoking cessation.
Ware et al, 2010: Reduced posttraumatic or postsurgical neuropathic pain
Ware et al24 performed a randomized crossover trial in 21 patients with posttraumatic or postsurgical neuropathic pain. Participants inhaled 4 different formulations of cannabis (containing 0%, 2.5%, 6.0%, and 9.4% THC) during 4 14-day periods. They inhaled a 25-mg dose through a pipe 3 times a day for the first 5 days of each cycle, followed by a 9-day washout period. Daily average pain intensity was measured using a numeric rating scale. The investigators also assessed mood, sleep, quality of life, and adverse effects.
Patients in the 9.4% THC group reported significantly less pain and better sleep, with average pain scores decreasing from 6.1 to 5.4 on an 11-point scale. Although the benefit was modest, the authors noted that the pain had been refractory to standard treatments.
The number of reported adverse events increased with greater potency and were most commonly throat irritation, burning sensation, headache, dizziness, and fatigue. This study suggests that THC potency affects tolerability, with higher doses eliciting clinically important adverse effects, some of which may reduce the ability to perform activities of daily living, such as driving.
Wilsey et al, 2013: Use in resistant neuropathic pain
Wilsey et al26 conducted another double-blind, placebo-controlled crossover study assessing the effect of vaporized cannabis on central and peripheral neuropathic pain resistant to first-line pharmacotherapies. Dose-effect relationships were explored using medium-dose (3.5%), low-dose (1.3%), and placebo cannabis. The primary outcome measure was a 30% reduction in pain intensity based on a visual analog scale.
In the placebo group, 26% of patients achieved this vs 57% of the low-dose cannabis group and 61% of those receiving the medium dose. No significant difference was found between the 2 active doses in reducing neuropathic pain, and both were more effective than placebo. The number needed to treat to achieve a 30% reduction in pain was about 3 for both cannabis groups compared with placebo. Psychoactive effects were minimal, of short duration, and reversible.
Wallace et al, 2015: Use in diabetic peripheral neuropathy
Wallace et al27 conducted a randomized, double-blind, placebo-controlled crossover study evaluating cannabis for diabetic peripheral neuropathy in 16 patients. Each had experienced at least 6 months of neuropathic pain in their feet. The participants inhaled a single dose of 1%, 4%, or 7% THC cannabis or placebo. Spontaneous pain was reported with a visual analog scale and also tested with a foam brush and von Frey filament at intervals until 4 hours after treatment.
Pain scores were lower with treatment compared with placebo, with high-dose cannabis having the greatest analgesic effect. Pain reduction lasted for the full duration of the test. Cannabis recipients had declines in attention and working memory, with the high-dose group experiencing the greatest impact 15 minutes after treatment. High-dose recipients also had poorer scores on testing of quick task-switching, with the greatest effect at 2 hours.27
Research and market cannabis are not equal
Results of US studies must be qualified. Most have used cannabis provided by the National Institute of Drug Abuse (NIDA),23–26 which differs in potency from commercially available preparations. This limits the clinical usefulness of the analysis of benefits and risks.
Vergara et al28 found that NIDA varieties contained much lower THC levels and as much as 23 times the cannabinol content as cannabis in state-legalized markets.
Studies based on NIDA varieties likely underestimate the risks of consumer-purchased cannabis, as THC is believed to be most responsible for the risk of psychosis and impaired driving and cognition.24,28
CBD MAY PROTECT AGAINST ADVERSE EFFECTS
Studies of CBD alone are limited to preclinical data.29 Evidence suggests that CBD alone or combined with THC can suppress chronic neuropathic pain, and that CBD may have a protective effect after nerve injury.30
Nabiximols, an oromucosal spray preparation with equal amounts of THC and CBD, has been approved in Canada as well as in European countries including the United Kingdom. Although its use has not been associated with many of the adverse effects of inhaled cannabis,30–32 evidence of efficacy from clinical trials has been mixed.
Lynch et al,31 in a 2014 randomized, double-blind, placebo-controlled crossover pilot study31 evaluated nabiximols in 16 patients with neuropathic pain related to chemotherapy. No statistically significant difference was found between treatment and placebo. However, the trial was underpowered.
Serpell et al,32 in a 2014 European randomized, placebo-controlled parallel-group study, evaluated 246 patients with peripheral neuropathy with allodynia, with 128 receiving active treatment (THC-CBD oromucosal spray) and 118 receiving placebo. Over the 15-week study, participants continued their current analgesic treatments.
Pain was reduced in the treatment group, but the difference from placebo was not statistically significant. However, the treatment group reported significantly better sleep quality and Patient Global Impression of Change measures (reflecting a patient’s belief of treatment efficacy).
META-ANALYSES CONFIRM EFFECT
Three meta-analyses of available studies of the effects of cannabis on neuropathic pain have been completed.
Andreae et al, 2015: 5 trials, 178 patients
Andreae et al1 evaluated 5 randomized controlled trials in 178 patients in North America. All had had neuropathy for at least 3 months, with a pain level of at least about 3 on a scale of 10. Two studies had patients with HIV-related neuropathy; the other 3 involved patients with neuropathy related to trauma, diabetes, complex regional pain syndrome, or spinal cord injury. All trials used whole cannabis plant provided by NIDA, and the main outcomes were patient-reported pain scales. No study evaluated pain beyond 2 weeks after trial termination.
They found that 1 of every 5 to 6 patients treated with cannabis had at least a 30% pain reduction.
Nugent et al, 2017: 13 trials, 246 patients
Nugent et al33 reviewed 13 trials in 246 patients that evaluated the effects of different cannabis-based preparations on either central or peripheral neuropathic pain from various conditions. Actively treated patients were more likely to report a 30% improvement in neuropathic pain. Again, studies tended to be small and brief.
Cochrane review, 2018: 16 trials, 1,750 patients
A Cochrane review34 analyzed 16 trials (in 1,750 patients) lasting 2 to 26 weeks. Treatments included an oromucosal spray with a plant-derived combination of THC and CBD, nabilone, inhaled herbal cannabis, and plant-derived THC.
With cannabis-based treatments, significantly more people achieved 50% or greater pain relief than with placebo (21% vs 17%, number needed to treat 20); 30% pain reduction was achieved in 39% of treated patients vs 33% of patients taking placebo (number needed to treat 11).
On the other hand, significantly more participants withdrew from studies because of adverse events with cannabis-based treatments than placebo (10% vs 5%), with psychiatric disorders occurring in 17% of patients receiving active treatment vs 5% of those receiving placebo (number needed to harm 10).
The primary studies suffered from methodologic limitations including small size, short duration, and inconsistency of formulations and study designs. Further evaluation of long-term efficacy, tolerability, and addiction potential is critical to determine the risk-benefit ratio.
RISKS OF CANNABIS USE
Like any drug therapy, cannabis has effects that may limit its use. Cannabis can affect a person’s psyche, physiology, and lifestyle.
Impaired attention, task speed
Neurocognitive changes associated with cannabis use—especially dizziness, fatigue, and slowed task-switching—could affect driving and other complex tasks. Evidence indicates that such activities should be avoided in the hours after treatment.26,27,32,33
Concern over brain development
Most worrisome is the effect of long-term cannabis use on brain development in young adults. Regular use of cannabis at an early age is associated with lower IQ, decline in school performance, and lower rates of high school graduation.35
Avoid in psychiatric patients
It is unlikely that cannabis can be safely used in patients with psychiatric illnesses. Anxiety, depression, and psychotic disorders can be exacerbated by the regular use of cannabis, and the risk of developing these conditions is increased while using cannabis.36,37
High concentrations of THC (the highest concentration used in the above studies was 9.5%) can cause anxiety, paranoia, and psychosis.
Respiratory effects
Long-term cannabis smoking may cause wheezing, cough, dyspnea, and exacerbations of chronic bronchitis. There is some evidence that symptoms improve after stopping smoking.33,38
SHOULD WE RECOMMEND CANNABIS?
Where cannabis can be legally used, doctors should be familiar with the literature and its limitations so that they can counsel patients on the best use and potential risks and benefits of cannabis treatment.
A recent conceptualization of pain suggests that a pain score reflects a composite of sensory factors (eg, tissue damage), cognitive factors (eg, beliefs about pain), and affective factors (eg, anxiety, depression).39 Physicians should keep this in mind when evaluating patients to better assess the risks and benefits of cannabis. While pharmacotherapy may address sensory factors, cognitive behavioral therapy may help alter beliefs about the pain as well as anxiety and depressive symptoms that might influence subjective reports.
Ideally, patients being considered for cannabis treatment would have a type of neuropathic pain proven to respond to cannabis in randomized, controlled studies, as well as evidence of failed first-line treatments.
Relative contraindications include depression, anxiety, substance use, psychotic disorders, and respiratory conditions, and these should also be considered.
Although current research shows an analgesic benefit of cannabis on neuropathic pain comparable to that of gabapentin,40 further investigation is needed to better evaluate long-term safety, efficacy, and interactions with standard therapies. Until we have a more complete picture, we should use the current literature, along with a thorough knowledge of each patient, to determine if the benefits of cannabis therapy outweigh the risks.
Acknowledgments: We thank Camillo Ferrari, BS, and Christina McMahon, BA, for their helpful comments.
Marijuana, which is still illegal under federal law but legal in 30 states for medical purposes as of this writing, has shown promising results for treating peripheral neuropathy. Studies suggest that cannabis may be an option for patients whose pain responds poorly to standard treatments; however, its use may be restricted by cognitive and psychiatric adverse effects, particularly at high doses.1
In this article, we discuss the basic pharmacology of cannabis and how it may affect neuropathic pain. We review clinical trials on its use for peripheral neuropathy and provide guidance for its use.
PERIPHERAL NEUROPATHY IS COMMON AND COMPLEX
An estimated 20 million people in the United States suffer from neuropathic pain. The prevalence is higher in certain populations, with 26% of people over age 65 and 30% of patients with diabetes mellitus affected.2–4
Peripheral neuropathy is a complex, chronic state that occurs when nerve fibers are damaged, dysfunctional, or injured, sending incorrect signals to pain centers in the central nervous system.5 It is characterized by weakness, pain, and paresthesias that typically begin in the hands or feet and progress proximally.4 Symptoms depend on the number and types of nerves affected.
In many cases, peripheral neuropathy is idiopathic, but common causes include diabetes, alcoholism, human immunodeficiency virus (HIV) infection, and autoimmune disease. Others include toxicity from chemotherapy and heavy metals.
Peripheral neuropathy significantly worsens quality of life and function. Many patients experience emotional, cognitive, and functional problems, resulting in high rates of medical and psychiatric comorbidities and occupational impairment.4,6,7 Yet despite its clinical and epidemiologic significance, it is often undertreated.8
STANDARD TREATMENTS INADEQUATE
Peripheral neuropathy occurs in patients with a wide range of comorbidities and is especially difficult to treat. Mainstays of therapy include anticonvulsants, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.9 A more invasive option is spinal cord stimulation.
These treatments can have considerable adverse effects, and response rates remain suboptimal, with pain relief insufficient to improve quality of life for many patients.9,10 Better treatments are needed to improve clinical outcomes and patient experience.11
CANNABIS: A MIX OF COMPOUNDS
Cannabis sativa has been used as an analgesic for centuries. The plant contains more than 400 chemical compounds and is often used for its euphoric properties. Long-term use may lead to addiction and cognitive impairment.12,13
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main components and the 2 best-studied cannabinoids with analgesic effects.
THC is the primary psychoactive component of cannabis. Its effects include relaxation, altered perception, heightened sensations, increased libido, and perceptual distortions of time and space. Temporary effects may include decreased short-term memory, dry mouth, impaired motor function, conjunctival injection, paranoia, and anxiety.
CBD is nonpsychoactive and has anti-inflammatory and antioxidant properties. It has been shown to reduce pain and inflammation without the effects of THC.14
Other compounds in the cannabis plant include phytocannabinoids, flavonoids, and tapenoids, which may produce individual, interactive, or synergistic effects.15 Different strains of cannabis have varying amounts of the individual components, making comparisons among clinical studies difficult.
THE ENDOCANNABINOID SYSTEM
The endogenous mammalian cannabinoid system plays a regulatory role in the development, homeostasis, and neuroplasticity of the central nervous system. It is also involved in modulating pain transmission in the nociceptive pathway.
Two of the most abundant cannabinoid endogenous ligands are anandamide and 2-arachidonylglycerol.9 These endocannabinoids are produced on demand in the central nervous system to reduce pain by acting as a circuit breaker.16–18 They target the G protein-coupled cannabinoid receptors CB1 and CB2, located throughout the central and peripheral nervous system and in organs and tissues.12
CB1 receptors are found primarily in the central nervous system, specifically in areas involved in movement, such as the basal ganglia and cerebellum, as well as in areas involved in memory, such as the hippocampus.12 They are also abundant in brain regions implicated in conducting and modulating pain signals, including the periaqueductal gray and the dorsal horn of the spinal cord.16–20
CB2 receptors are mostly found in peripheral tissues and organs, mainly those involved in the immune system, including splenic, tonsillar, and hematopoietic cells.12 They help regulate inflammation, allodynia, and hyperalgesia.17
Modifying response to injury
Following a nerve injury, neurons along the nociceptive pathway may become more reactive and responsive in a process known as sensitization.21 The process involves a cascade of cellular events that result in sprouting of pain-sensitive nerve endings.21,22
Cannabinoids are thought to reduce pain by modifying these cellular events. They also inhibit nociceptive conduction in the dorsal horn of the spinal cord and in the ascending spinothalamic tract.20 CB1 receptors found in nociceptive terminals along the peripheral nervous system impede pain conduction, while activation of CB2 receptors in immune cells decreases the release of nociceptive agents.
STUDIES OF CANNABIS FOR NEUROPATHIC PAIN
A number of studies have evaluated cannabis for treating neuropathic pain. Overall, available data support the efficacy of smoked or inhaled cannabis in its flower form when used as monotherapy or adjunctive therapy for relief of neuropathic pain of various etiologies. Many studies also report secondary benefits, including better sleep and functional improvement.23,24
However, adverse effects are common, especially at high doses, and include difficulty concentrating, lightheadedness, fatigue, and tachycardia. More serious reported adverse effects include anxiety, paranoia, and psychosis.
Wilsey et al, 2008: Neuropathic pain reduced
Wilsey et al25 conducted a double-blind, placebo-controlled crossover study that assessed the effects of smoking cannabis in 38 patients with central or peripheral neuropathic pain. Participants were assigned to smoke either high- or low-dose cannabis (7% or 3.5% delta-9-THC) or placebo cigarettes. Cigarettes were smoked during treatment sessions using the following regimen: 2 puffs at 60 minutes from baseline, 3 puffs at 120 minutes, and 4 puffs at 180 minutes. Patients were assessed after each set of puffs and for 2 hours afterwards. The primary outcome was spontaneous relief of pain as measured by a visual analog scale.
Pain intensity was comparable and significantly reduced in both treatment groups compared with placebo. At the high dose, some participants experienced neurocognitive impairment in attention, learning, memory, and psychomotor speed; only learning and memory declined at the low dose.
Ellis et al, 2009: Pain reduction in HIV neuropathy
Ellis et al23 conducted a double-blind, placebo-controlled crossover trial in patients with HIV neuropathy that was unresponsive to at least 2 analgesics with different modes of action. During each treatment week, participants were randomly assigned to smoke either active cannabis or placebo, while continuing their standard therapy. Titration started at 4% THC and was adjusted based on tolerability and efficacy. Twenty-eight of the 34 enrolled patients completed both cannabis and placebo treatments. The principal outcome was change in pain intensity from baseline at the end of each week, using the Descriptor Differential Scale of Pain Intensity.
Of the 28 patients, 46% achieved an average pain reduction of 3.3 points (30%). One patient experienced cannabis-induced psychosis, and another developed an intractable cough, which resolved with smoking cessation.
Ware et al, 2010: Reduced posttraumatic or postsurgical neuropathic pain
Ware et al24 performed a randomized crossover trial in 21 patients with posttraumatic or postsurgical neuropathic pain. Participants inhaled 4 different formulations of cannabis (containing 0%, 2.5%, 6.0%, and 9.4% THC) during 4 14-day periods. They inhaled a 25-mg dose through a pipe 3 times a day for the first 5 days of each cycle, followed by a 9-day washout period. Daily average pain intensity was measured using a numeric rating scale. The investigators also assessed mood, sleep, quality of life, and adverse effects.
Patients in the 9.4% THC group reported significantly less pain and better sleep, with average pain scores decreasing from 6.1 to 5.4 on an 11-point scale. Although the benefit was modest, the authors noted that the pain had been refractory to standard treatments.
The number of reported adverse events increased with greater potency and were most commonly throat irritation, burning sensation, headache, dizziness, and fatigue. This study suggests that THC potency affects tolerability, with higher doses eliciting clinically important adverse effects, some of which may reduce the ability to perform activities of daily living, such as driving.
Wilsey et al, 2013: Use in resistant neuropathic pain
Wilsey et al26 conducted another double-blind, placebo-controlled crossover study assessing the effect of vaporized cannabis on central and peripheral neuropathic pain resistant to first-line pharmacotherapies. Dose-effect relationships were explored using medium-dose (3.5%), low-dose (1.3%), and placebo cannabis. The primary outcome measure was a 30% reduction in pain intensity based on a visual analog scale.
In the placebo group, 26% of patients achieved this vs 57% of the low-dose cannabis group and 61% of those receiving the medium dose. No significant difference was found between the 2 active doses in reducing neuropathic pain, and both were more effective than placebo. The number needed to treat to achieve a 30% reduction in pain was about 3 for both cannabis groups compared with placebo. Psychoactive effects were minimal, of short duration, and reversible.
Wallace et al, 2015: Use in diabetic peripheral neuropathy
Wallace et al27 conducted a randomized, double-blind, placebo-controlled crossover study evaluating cannabis for diabetic peripheral neuropathy in 16 patients. Each had experienced at least 6 months of neuropathic pain in their feet. The participants inhaled a single dose of 1%, 4%, or 7% THC cannabis or placebo. Spontaneous pain was reported with a visual analog scale and also tested with a foam brush and von Frey filament at intervals until 4 hours after treatment.
Pain scores were lower with treatment compared with placebo, with high-dose cannabis having the greatest analgesic effect. Pain reduction lasted for the full duration of the test. Cannabis recipients had declines in attention and working memory, with the high-dose group experiencing the greatest impact 15 minutes after treatment. High-dose recipients also had poorer scores on testing of quick task-switching, with the greatest effect at 2 hours.27
Research and market cannabis are not equal
Results of US studies must be qualified. Most have used cannabis provided by the National Institute of Drug Abuse (NIDA),23–26 which differs in potency from commercially available preparations. This limits the clinical usefulness of the analysis of benefits and risks.
Vergara et al28 found that NIDA varieties contained much lower THC levels and as much as 23 times the cannabinol content as cannabis in state-legalized markets.
Studies based on NIDA varieties likely underestimate the risks of consumer-purchased cannabis, as THC is believed to be most responsible for the risk of psychosis and impaired driving and cognition.24,28
CBD MAY PROTECT AGAINST ADVERSE EFFECTS
Studies of CBD alone are limited to preclinical data.29 Evidence suggests that CBD alone or combined with THC can suppress chronic neuropathic pain, and that CBD may have a protective effect after nerve injury.30
Nabiximols, an oromucosal spray preparation with equal amounts of THC and CBD, has been approved in Canada as well as in European countries including the United Kingdom. Although its use has not been associated with many of the adverse effects of inhaled cannabis,30–32 evidence of efficacy from clinical trials has been mixed.
Lynch et al,31 in a 2014 randomized, double-blind, placebo-controlled crossover pilot study31 evaluated nabiximols in 16 patients with neuropathic pain related to chemotherapy. No statistically significant difference was found between treatment and placebo. However, the trial was underpowered.
Serpell et al,32 in a 2014 European randomized, placebo-controlled parallel-group study, evaluated 246 patients with peripheral neuropathy with allodynia, with 128 receiving active treatment (THC-CBD oromucosal spray) and 118 receiving placebo. Over the 15-week study, participants continued their current analgesic treatments.
Pain was reduced in the treatment group, but the difference from placebo was not statistically significant. However, the treatment group reported significantly better sleep quality and Patient Global Impression of Change measures (reflecting a patient’s belief of treatment efficacy).
META-ANALYSES CONFIRM EFFECT
Three meta-analyses of available studies of the effects of cannabis on neuropathic pain have been completed.
Andreae et al, 2015: 5 trials, 178 patients
Andreae et al1 evaluated 5 randomized controlled trials in 178 patients in North America. All had had neuropathy for at least 3 months, with a pain level of at least about 3 on a scale of 10. Two studies had patients with HIV-related neuropathy; the other 3 involved patients with neuropathy related to trauma, diabetes, complex regional pain syndrome, or spinal cord injury. All trials used whole cannabis plant provided by NIDA, and the main outcomes were patient-reported pain scales. No study evaluated pain beyond 2 weeks after trial termination.
They found that 1 of every 5 to 6 patients treated with cannabis had at least a 30% pain reduction.
Nugent et al, 2017: 13 trials, 246 patients
Nugent et al33 reviewed 13 trials in 246 patients that evaluated the effects of different cannabis-based preparations on either central or peripheral neuropathic pain from various conditions. Actively treated patients were more likely to report a 30% improvement in neuropathic pain. Again, studies tended to be small and brief.
Cochrane review, 2018: 16 trials, 1,750 patients
A Cochrane review34 analyzed 16 trials (in 1,750 patients) lasting 2 to 26 weeks. Treatments included an oromucosal spray with a plant-derived combination of THC and CBD, nabilone, inhaled herbal cannabis, and plant-derived THC.
With cannabis-based treatments, significantly more people achieved 50% or greater pain relief than with placebo (21% vs 17%, number needed to treat 20); 30% pain reduction was achieved in 39% of treated patients vs 33% of patients taking placebo (number needed to treat 11).
On the other hand, significantly more participants withdrew from studies because of adverse events with cannabis-based treatments than placebo (10% vs 5%), with psychiatric disorders occurring in 17% of patients receiving active treatment vs 5% of those receiving placebo (number needed to harm 10).
The primary studies suffered from methodologic limitations including small size, short duration, and inconsistency of formulations and study designs. Further evaluation of long-term efficacy, tolerability, and addiction potential is critical to determine the risk-benefit ratio.
RISKS OF CANNABIS USE
Like any drug therapy, cannabis has effects that may limit its use. Cannabis can affect a person’s psyche, physiology, and lifestyle.
Impaired attention, task speed
Neurocognitive changes associated with cannabis use—especially dizziness, fatigue, and slowed task-switching—could affect driving and other complex tasks. Evidence indicates that such activities should be avoided in the hours after treatment.26,27,32,33
Concern over brain development
Most worrisome is the effect of long-term cannabis use on brain development in young adults. Regular use of cannabis at an early age is associated with lower IQ, decline in school performance, and lower rates of high school graduation.35
Avoid in psychiatric patients
It is unlikely that cannabis can be safely used in patients with psychiatric illnesses. Anxiety, depression, and psychotic disorders can be exacerbated by the regular use of cannabis, and the risk of developing these conditions is increased while using cannabis.36,37
High concentrations of THC (the highest concentration used in the above studies was 9.5%) can cause anxiety, paranoia, and psychosis.
Respiratory effects
Long-term cannabis smoking may cause wheezing, cough, dyspnea, and exacerbations of chronic bronchitis. There is some evidence that symptoms improve after stopping smoking.33,38
SHOULD WE RECOMMEND CANNABIS?
Where cannabis can be legally used, doctors should be familiar with the literature and its limitations so that they can counsel patients on the best use and potential risks and benefits of cannabis treatment.
A recent conceptualization of pain suggests that a pain score reflects a composite of sensory factors (eg, tissue damage), cognitive factors (eg, beliefs about pain), and affective factors (eg, anxiety, depression).39 Physicians should keep this in mind when evaluating patients to better assess the risks and benefits of cannabis. While pharmacotherapy may address sensory factors, cognitive behavioral therapy may help alter beliefs about the pain as well as anxiety and depressive symptoms that might influence subjective reports.
Ideally, patients being considered for cannabis treatment would have a type of neuropathic pain proven to respond to cannabis in randomized, controlled studies, as well as evidence of failed first-line treatments.
Relative contraindications include depression, anxiety, substance use, psychotic disorders, and respiratory conditions, and these should also be considered.
Although current research shows an analgesic benefit of cannabis on neuropathic pain comparable to that of gabapentin,40 further investigation is needed to better evaluate long-term safety, efficacy, and interactions with standard therapies. Until we have a more complete picture, we should use the current literature, along with a thorough knowledge of each patient, to determine if the benefits of cannabis therapy outweigh the risks.
Acknowledgments: We thank Camillo Ferrari, BS, and Christina McMahon, BA, for their helpful comments.
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015; 16(12):1221–1232. doi:10.1016/j.jpain.2015.07.009
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet. www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Accessed November 14, 2018.
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older adults. J Am Board Fam Med 2004; 17(5):308–318. doi:10.3122/jabfm.17.5.309
- Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of developing peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig 2014; 5(6):714–721. doi:10.1111/jdi.12223
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
- Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain 2016; 157(suppl 1):S23–S32. doi:10.1097/j.pain.0000000000000428
- Zeng L, Alongkronrusmee D, van Rijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 2017; 10:219–228. doi:10.2147/JPR.S125987
- Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA 2015; 314(20):2172–2181. doi:10.1001/jama.2015.13611
- Adams AS, Callaghan B, Grant RW. Overcoming barriers to diabetic polyneuropathy management in primary care. Healthc (Amst) 2017; 5(4):171–173. doi:10.1016/j.hjdsi.2016.10.003
- Gwak YS, Kim HY, Lee BH, Yang CH. Combined approaches for the relief of spinal cord injury-induced neuropathic pain. Complement Ther Med 2016; 25:27–33. doi:10.1016/j.ctim.2015.12.021
- Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology 2016; 30(11):1020–1029. pmid:27854104
- Grotenhermen F. Cannabinoids and the endocannabinoid system. Cannabinoids 2006; 1(1):10–14.
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA 2015; 313(24):2474–2483. doi:10.1001/jama.2015.6199
- Campos AC, Fogaça MV, Scarante FF, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 2017; 8:269. doi:10.3389/fphar.2017.00269
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163(7):1344–1364. doi:10.1111/j.1476-5381.2011.01238.x
- Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci 2017; Jul 7: 1–20. doi:10.1080/1028415X.2017.1347373
- Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018; 43(1):155–172. doi:10.1038/npp.2017.130
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991; 11(2):563–583. pmid:1992016
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience1998; 83(2):393–411. pmid:9460749
- Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: Deer TR, Leong MS, ed. Comprehensve Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches. New York, NY: Springer; 2013:181–193.
- Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012; 12(4):304–314. pmid:23033930
- Yamanaka H, Noguchi K. Pathophysiology of neuropathic pain: molecular mechanisms underlying central sensitization in the dorsal horn in neuropathic pain. Brain Nerve 2012; 64(11):1255–1265. Japanese. pmid:23131736
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010; 182(14):E694–E701. doi:10.1503/cmaj.091414
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008; 9(6):506–521. doi:10.1016/j.jpain.2007.12.010
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013; 14(2):136–148. doi:10.1016/j.jpain.2012.10.009
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Scientific Reports. 2017; 7(1):1-8. doi:10.1038/srep46528
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterized by allodynia: a randomized, double-blind, placebo-controlled clinical trial. Pain 2007; 133(1–3):210–220. doi:10.1016/j.pain.2007.08.028
- Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017; 158(12):2442–2451. doi:10.1097/j.pain.0000000000001052
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014; 47(1):166–173. doi:10.1016/j.jpainsymman.2013.02.018
- Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
- Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167(5):319–331. doi:10.7326/M17-0155
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Castellanos-Ryan N, Pingault JB, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol 2017; 29(4):1253–1266. doi:10.1017/S0954579416001280
- Karila L, Roux P, Benyamina A, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014; 20(25):4112–4118. pmid:24001294
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001; 178:116–122. pmid:11157424
- National Academies of Science, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academy Press; 2017. doi:10.17226/24625
- Modesto-Lowe V, Griard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J Opioid Manag 2012; 8(3):167–175. doi:10.5055/jom.2012.0113
- Grant I. Medicinal cannabis and painful sensory neuropathy. Virtual Mentor 2013; 15(5):466–469. doi:10.1001/virtualmentor.2013.15.5.oped1-1305
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015; 16(12):1221–1232. doi:10.1016/j.jpain.2015.07.009
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet. www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Accessed November 14, 2018.
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older adults. J Am Board Fam Med 2004; 17(5):308–318. doi:10.3122/jabfm.17.5.309
- Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of developing peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig 2014; 5(6):714–721. doi:10.1111/jdi.12223
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
- Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain 2016; 157(suppl 1):S23–S32. doi:10.1097/j.pain.0000000000000428
- Zeng L, Alongkronrusmee D, van Rijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 2017; 10:219–228. doi:10.2147/JPR.S125987
- Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA 2015; 314(20):2172–2181. doi:10.1001/jama.2015.13611
- Adams AS, Callaghan B, Grant RW. Overcoming barriers to diabetic polyneuropathy management in primary care. Healthc (Amst) 2017; 5(4):171–173. doi:10.1016/j.hjdsi.2016.10.003
- Gwak YS, Kim HY, Lee BH, Yang CH. Combined approaches for the relief of spinal cord injury-induced neuropathic pain. Complement Ther Med 2016; 25:27–33. doi:10.1016/j.ctim.2015.12.021
- Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology 2016; 30(11):1020–1029. pmid:27854104
- Grotenhermen F. Cannabinoids and the endocannabinoid system. Cannabinoids 2006; 1(1):10–14.
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA 2015; 313(24):2474–2483. doi:10.1001/jama.2015.6199
- Campos AC, Fogaça MV, Scarante FF, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 2017; 8:269. doi:10.3389/fphar.2017.00269
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163(7):1344–1364. doi:10.1111/j.1476-5381.2011.01238.x
- Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci 2017; Jul 7: 1–20. doi:10.1080/1028415X.2017.1347373
- Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018; 43(1):155–172. doi:10.1038/npp.2017.130
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991; 11(2):563–583. pmid:1992016
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience1998; 83(2):393–411. pmid:9460749
- Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: Deer TR, Leong MS, ed. Comprehensve Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches. New York, NY: Springer; 2013:181–193.
- Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012; 12(4):304–314. pmid:23033930
- Yamanaka H, Noguchi K. Pathophysiology of neuropathic pain: molecular mechanisms underlying central sensitization in the dorsal horn in neuropathic pain. Brain Nerve 2012; 64(11):1255–1265. Japanese. pmid:23131736
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010; 182(14):E694–E701. doi:10.1503/cmaj.091414
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008; 9(6):506–521. doi:10.1016/j.jpain.2007.12.010
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013; 14(2):136–148. doi:10.1016/j.jpain.2012.10.009
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Scientific Reports. 2017; 7(1):1-8. doi:10.1038/srep46528
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterized by allodynia: a randomized, double-blind, placebo-controlled clinical trial. Pain 2007; 133(1–3):210–220. doi:10.1016/j.pain.2007.08.028
- Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017; 158(12):2442–2451. doi:10.1097/j.pain.0000000000001052
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014; 47(1):166–173. doi:10.1016/j.jpainsymman.2013.02.018
- Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
- Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167(5):319–331. doi:10.7326/M17-0155
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Castellanos-Ryan N, Pingault JB, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol 2017; 29(4):1253–1266. doi:10.1017/S0954579416001280
- Karila L, Roux P, Benyamina A, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014; 20(25):4112–4118. pmid:24001294
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001; 178:116–122. pmid:11157424
- National Academies of Science, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academy Press; 2017. doi:10.17226/24625
- Modesto-Lowe V, Griard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J Opioid Manag 2012; 8(3):167–175. doi:10.5055/jom.2012.0113
- Grant I. Medicinal cannabis and painful sensory neuropathy. Virtual Mentor 2013; 15(5):466–469. doi:10.1001/virtualmentor.2013.15.5.oped1-1305
KEY POINTS
- Small clinical studies have found that cannabis provides benefits for peripheral neuropathy, including pain reduction, better sleep, and improved function, even in patients with symptoms refractory to standard therapies.
- Adverse effects such as throat irritation, headache, and dizziness are common, and serious neuropsychiatric effects can occur at high doses.
- Safety may not be adequately assessed in US trials because cannabis supplied by the National Institute of Drug Abuse is less potent than commercially available products.
Use and misuse of opioid agonists in opioid addiction
For a patient struggling with opioid addiction, opioid agonist therapy with methadone or buprenorphine can reduce craving and opioid use and may even save his or her life. But many clinicians are unfamiliar with this evidence-based treatment,1,2 which is best started early in the course of addiction.3
This article outlines the pharmacology of these drugs, their clinical uses, and the challenges of using them to treat opioid addiction.
DIAGNOSTIC CRITERIA
Opioid addiction, formally known as opioid use disorder, is a pattern of opioid misuse leading to clinically significant impairment in multiple areas of life. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, lists 11 diagnostic criteria, but only 2 need to be present within the past year to make the diagnosis4:
- Taking opioids longer or in higher doses than was intended
- A persistent desire or unsuccessful efforts to cut down or control opioid use
- Spending a great deal of time obtaining, using, or recovering from using opioids
- Craving opioids
- Repeatedly failing to fulfill obligations at work, school, or home due to opioid use
- Continuing to use opioids even though it causes or exacerbates social or interpersonal problems
- Giving up or curtailing important social, occupational, or recreational activities because of opioid use
- Repeatedly using opioids in situations in which it is physically hazardous
- Continuing to use opioids despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance
- Tolerance
- Withdrawal.
Recent estimates indicate that 2.23 million people in the United States have opioid use disorder (426,000 with heroin and 1.8 million with prescription opioids).5
Progression from prescription opioids to heroin
We have observed that many patients with opioid use disorder start by misusing prescription opioids. Over time, tolerance can develop, which drives patients to use higher and higher doses.6
As the addiction progresses, a subset of prescription opioid users advances to using heroin, which is typically less expensive and easier to obtain.7 Most patients start with the intranasal route but eventually inject it intravenously.6,7
For many addicts, heroin use has medical consequences such as hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infection, psychiatric problems such as depression and anxiety, and illegal activities such as theft and sex work.8 People who use heroin appear to have more severe addiction and a lower socioeconomic status than prescription opioid users.9–11 But recently, a growing number of middle class individuals are becoming addicted to heroin.12
METHADONE
Methadone is a long-acting synthetic opioid that functions as a full agonist on the mu-opioid receptor. The drug binds, occupies, and stimulates the receptor, preventing withdrawal symptoms and reducing opioid cravings for at least 24 hours.13
Adverse effects of methadone
The most common adverse effects include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating.14 Other adverse effects:
Unintentional overdose. The risk is serious, as a single 30-mg dose can be fatal in people who are opioid-naïve.13
QTc prolongation, which can lead to torsade de pointes. This risk, which is dose-related, must be taken into consideration in patients who have any cardiac symptoms (eg, syncope, arrhythmia), pathology (familial QT prolongation), or other risk factors for QTc prolongation (eg, hypokalemia, QTc-prolonging medications).15
Respiratory depression, which can be fatal. This dose-related risk is heightened during the first 4 weeks of treatment if titration is too rapid or if methadone is used in combination with other drugs that cause central nervous system or respiratory depression.13,14
Starting methadone
To prevent respiratory depression and death related to rapid induction, the general rule is to start methadone at a low daily dose (20–30 mg) depending on the patient’s withdrawal symptoms.14 During this period, patients need to be closely monitored and educated on the perils of concomitant use of central nervous system depressants.14
In most patients, the dose is titrated up until their withdrawal symptoms and cravings are eliminated, which generally requires 60 to 120 mg daily.14 Hepatic and renal impairment, pregnancy, and advanced age can alter methadone pharmacokinetics and may therefore necessitate dose adjustment.
BUPRENORPHINE
Buprenorphine is an alkaloid thebaine opioid derivative that acts as a partial mu-opioid agonist and a kappa antagonist.16 Like methadone, buprenorphine is used to manage cravings and withdrawal symptoms.16 Dosages of 4 to 16 mg (up to 32 mg) per day of buprenorphine are usually required to adequately control opioid cravings.16
Sublingual and subdermal products
Buprenorphine is currently available in the United States in sublingual and subdermal formulations.16,17
Sublingual buprenorphine is usually combined with naloxone in a 4:1 ratio to deter intravenous use. Intravenous injection of the combination product can precipitate withdrawal due to the antagonist action of naloxone. (Taken orally or sublingually, naloxone is poorly absorbed and has little or no clinical effect.) Buprenorphine-naloxone is available in tablets, a sublingual film strip, and a buccal film strip. Buprenorphine is also available by itself in a sublingual formulation.
The US Food and Drug Administration has approved a buprenorphine subdermal implant, Probuphine. Four rods, about 1 inch long, are placed under the skin in the inner aspect of the upper arm and provide the equivalent of 8 mg of buprenorphine daily for 6 months.17 However, this method is formulated only for maintenance treatment and cannot be used for induction. Additionally, it is recommended that the implants be surgically removed at the end of 6 months, after which another set of implants can be inserted in the other arm or the patient can switch to sublingual therapy, depending on the clinical situation and patient preference.17
Generally safer than methadone
Buprenorphine works on the same receptor as methadone and therefore has a similar side effect profile. However, buprenorphine has a ceiling effect, which greatly reduces the risk of fatal respiratory depression.18 It also does not cause clinically significant QTc prolongation and is preferable in patients who have cardiac risk factors.18
Another advantage is that buprenorphine has fewer identified medication interactions than methadone.18 Further, induction of buprenorphine in patients with opioid use disorder has been shown to be safer than methadone.19
Although buprenorphine has been found to be 6 times safer than methadone with regard to overdose among the general population,20 it can still cause fatal intoxication if used in combination with central nervous system depressants.21
Buprenorphine has been also associated with hepatotoxicity, though the risk of new-onset liver disease appears to be low.22
NALTREXONE IS LESS EFFECTIVE THAN METHADONE, BUPRENORPHINE
Besides methadone and buprenorphine, the only other approved option for treating opioid use disorder is the opioid antagonist naltrexone.
Naltrexone has significantly less abuse potential, as it provides no euphoria, but patients do not like it. Even with the long-acting formulation (Vivitrol), naltrexone treatment is significantly less effective than methadone or buprenorphine.23–25 Further, although naltrexone is not a controlled substance and so does not face the same scrutiny as the agonist therapies, there are other significant barriers. Additional information on naltrexone is presented in reviews by Modesto-Lowe and Van Kirk24 and Woody.25
OBSTACLES TO TREATMENT
People hold conflicting views about opioid agonist therapy. Some believe that “trading one drug for another” is not a legitimate therapeutic strategy, and they may feel ashamed of being on maintenance therapy.26 Similarly, some argue that the answer to establishing stable abstinence does not lie simply in prescribing medications.
The contrary argument is that these medications, if used appropriately, confer many benefits such as reducing the medical and psychosocial sequelae of opioid addiction.18 In fact, properly treated patients no longer meet the diagnostic criteria of opioid use disorder, and both methadone and buprenorphine are on the World Health Organization’s (WHO) list of essential medicines.27
Despite endorsement by the WHO, the stigma attached to the opioid agonists has been difficult to overcome. Patients with opioid use disorder may be viewed with distrust by healthcare providers and often do not feel welcome in healthcare settings or in self-help recovery groups.28
Barriers to methadone therapy
Federal regulations on methadone prescribing and use were established to promote patient safety and decrease diversion, but they may also complicate access to care.29 They stipulate that to qualify for methadone maintenance, patients need to demonstrate opioid addiction for 1 year, except for pregnant women and those who have been incarcerated in the past 6 months. Patients under the age of 18 must have 2 documented failed treatment episodes as well as approval by a guardian to receive treatment.
Inconvenience. Methadone can be prescribed for opioid dependence only by an accredited treatment program. Patients must therefore travel to the clinic and wait to be evaluated on a daily basis for a minimum of 90 days. Only after they demonstrate consistent responsible behavior and negative results on urine testing do they become eligible to take methadone home.29 If a patient is to travel out of the area during the initial 90 days of treatment, he or she must make arrangements in advance to find a clinic that will provide a “guest dose.”
The inconvenience arising from the regulations may deter some patients from seeking methadone therapy. In spite of this, once patients are started on methadone, more of them continue treatment than with buprenorphine.18 A proposed reason is that methadone is a potent full opioid agonist and therefore relieves withdrawal symptoms and craving more effectively than buprenorphine, which is a partial agonist.30 Another possible reason is the higher level of supervision afforded by methadone clinics, which require daily contact for at least 90 days.
Safety concerns arise from methadone diversion, as illicit use may have lethal consequences. In the past decade, deaths from methadone overdose have risen significantly, most of them due to respiratory depression or torsade de pointes.13 However, most cases of diversion and overdose involve methadone that is prescribed for pain by individual practitioners and not from maintenance programs.13
Advantages of buprenorphine
Together, methadone’s lethality, stigma, and inconvenience may contribute to patients preferring buprenorphine.31
The regulations governing buprenorphine’s use are less restrictive than those with methadone. For example, patients must have a diagnosis of opioid addiction to be prescribed buprenorphine, but they are not required to carry the diagnosis for a year before treatment.31 Additionally, they do not need to travel to a federally approved opioid treatment center daily and can receive buprenorphine directly from a physician in an outpatient setting.
Under the Drug Abuse Treatment Act (DATA) of 2000, any physician can apply for a waiver to prescribe and dispense buprenorphine in his or her office. To qualify for an initial waiver, physicians must either obtain certification in the fields of addiction medicine or addiction psychiatry or complete an approved 8-hour training session.32 Each physician starts with a maximum of 30 patients, but can apply to treat up to 100 patients after 1 year and eventually up to 275 patients. Physicians must document every buprenorphine prescription they write and be able to refer patients for counseling.31
As of February 2017, nurse practitioners and physician assistants can also apply for a DATA 2000 waiver. All waivered providers are subject to unannounced visits from the Drug Enforcement Administration once every 5 years.32
While there are no federal restrictions on the amount of buprenorphine that can be dispensed, some states and some insurance companies have placed restrictions on dose or length of treatment.33 Buprenorphine patients can fill their prescriptions at any pharmacy and are permitted to bring their medication home, which improves access to care. However, office-based outpatient treatment is not without risk, and preventing buprenorphine diversion remains a challenge.34
‘Lending’ buprenorphine is a felony
Addicts have illegally used buprenorphine to self-treat opioid withdrawal, craving, and dependence.35 Its misuse has also been coupled with self-treatment of conditions that include depression and pain.36
A survey found that 83.7% of patients deem buprenorphine diversion to be appropriate; further, most patients said they consider it unethical to withhold prescribed buprenorphine from individuals showing symptoms of withdrawal.34 Physicians who prescribe buprenorphine must inform their patients that even “lending” or giving away their medication is a felony.
Prescribing physicians must also be diligent about monitoring for signs of diversion such as inconsistent urine toxicology screens, “lost” medication, and requests for early refills or escalating doses.37
EVALUATING PATIENTS FOR OPIOID REPLACEMENT THERAPY
In addition to federal regulations, we propose a 4-step approach to evaluate eligibility for opioid replacement therapy based on existing guidelines.37–39
Step 1: History and physical examination
The history should give particular attention to the patient’s cardiac, pulmonary, and hepatic status, with consideration of the risks of any medical comorbidities (eg, bacterial endocarditis, HIV and HCV infection) that might influence treatment.37
It is also essential to evaluate for any contraindications or drug interactions before prescribing methadone or buprenorphine.38
Contraindications to methadone maintenance include40:
- Cor pulmonale
- Methadone hypersensitivity
- Pseudomembranous colitis
- Selegiline use (due to risk of serotonin syndrome)
- Ileum paralyticus.
Contraindications to buprenorphine use include:
- Hypersensitivity to naloxone or buprenorphine
- Impaired liver function (due to the risk of inadvertent overdose associated with slowed metabolism).
Concurrent use of alcohol or illicit benzodiazepines is a relative contraindication to both methadone and buprenorphine due to the risk of respiratory depression and overdose.37 Likewise, avoid coprescribing opioid agonists and benzodiazepines whenever possible. Obtain a complete list of current medications and query a prescription-monitoring database to determine whether any controlled substances are currently prescribed.37
During the physical examination, look for stigmata of intravenous drug use such as track marks or abscesses37 and document any physical findings consistent with intoxication or withdrawal. Patients must be completely detoxed or in withdrawal before beginning buprenorphine induction; premature induction can precipitate withdrawal.38
A discussion of pregnant patients with opioid use disorder is beyond the scope of this paper. However, it is incumbent on the prescriber to inquire whether the client is pregnant or intends to become pregnant and what birth control methods are in place.
Step 2: Assess psychiatric status
Assessment of the patient’s psychiatric status, including an assessment of alcohol and other drug use, will help determine his or her eligibility for opioid agonists.37 To prepare for the need to manage patients with psychiatrically complex issues, it is helpful to develop relationships with addiction specialists and psychiatrists who are familiar with opioid replacement therapy in your area. This will make it easier to collaborate on patients’ care.
Ask all patients directly about suicidal or homicidal ideation. Any patient with active suicidal or homicidal ideation should be assessed for need of immediate hospitalization by a psychiatrist or another qualified mental health professional. Patients with a history of suicidal ideation should be monitored closely by a mental health professional throughout treatment.37
Many if not most patients with opioid use disorder have concurrent psychiatric disorders, and the interplay between these disorders is complex.40,41 Depression, for example, can precede and even precipitate drug use (an observation supporting the “self-medication theory”).42 If the underlying depressive disorder is not addressed, relapse is nearly inevitable.
It has also been shown that both chronic opioid use and withdrawal can exacerbate aversive emotional states. This escalation of symptoms may result from the pharmacologic effects of opioids or from psychosocial sequelae that can arise from chronic opioid use.41 In this situation, maintaining abstinence can lead to resolution of depressive symptoms. As depression and opioid use can occur together, successful treatment requires equal attention to both illnesses.
Other common comorbidities in patients with opioid use disorder include posttraumatic stress disorder, attention deficit hyperactivity disorder, antisocial personality disorder, and concurrent substance abuse disorders.43 The confluence of antisocial personality disorder is particularly important, as patients with antisocial personality disorder display disruptive and maladaptive behaviors.
Identify any psychotropic medication that is prescribed and check carefully for drug interactions. This applies especially to methadone, as many psychiatric medications also prolong the QT interval. Moreover, patients may not be forthcoming about the use of psychiatric medication.
Find out whether the patient is using any other addictive substances, particularly those that affect the central nervous system, as those who use fentanyl, benzodiazepines, or alcohol are at the highest risk of overdose.31 Often the best option for those with concurrent substance use disorders is inpatient detoxification followed by residential rehabilitation care. Either buprenorphine or methadone can then be initiated upon return to an outpatient setting.
Step 3: Assess psychosocial status
To what extent do the patient’s home environment and support systems promote a drug-free lifestyle? Unfortunately, the psychosocial status of many of these patients is fragile, and they may live in areas where illicit drugs are readily available (which can be urban, suburban, or rural), making it difficult to stay substance-free.38
Generally, lifestyle modifications are needed to transform maladaptive behaviors and promote an environment conducive to long-term recovery. Referrals to social services to address housing, vocational needs, and entitlements may be helpful.39
Step 4: Assess readiness to change
According to one model, people go through 5 stages when changing a behavior: precontemplation, contemplation, preparation for action, action, and maintenance.43 In general, the further along the stages a patient is, the more appropriate he or she is for office-based treatment with buprenorphine.39
The level of change can be assessed with tools such as Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES). Use of stage-specific strategies may enhance a patient’s readiness to cease opioid use.43
Precontemplation. Those in the precontemplation stage are not ready to think about changing their behavior.43 They may be unaware of or unwilling to consider the risks associated with their opioid use and resistant to the idea of quitting. Engagement with opioid agonists for individuals in this stage is low and dropout rates are likely high.
Thus, the proper approach for “precontemplators” is to help them develop some ambivalence about their opioid use. One tactic is to involve the patient in a discussion of the personal benefits and risks of opioid use.
Contemplation. Individuals in the contemplation stage have begun to weigh the costs and benefits of opioid use and express ambivalence about it.44 Because the patient is willing to explore the risks of ongoing use and consider the benefits of treatment, the goal in this stage is to elicit a commitment from the individual to seek treatment.
Preparation. The person in this stage moves from thinking about treatment to planning what action to take.45 As the individual prepares to enter treatment, indecision tends to resurface, as well as self-doubt about his or her ability to change. During this stage, it is important for the provider to spell out goals (abstinence) and strategies (eg, counseling, medication) and enhance a sense of self-efficacy.
Action and maintenance. Patients in these stages engage in treatment and employ new strategies to abstain from opioid use. Maintaining these behaviors can be a daily struggle. Expressing confidence in the patient’s ability to abstain from use will support his or her progress. Behavioral interventions such as strategic avoidance of triggers and engagement in alternative activities (eg, support groups, exercise, faith-based practices) will help to maintain abstinence.
A CHRONIC CONDITION
Opioid use disorder, like many chronic illnesses, requires long-term attention to attain successful patient outcomes. The opioid agonists methadone and buprenorphine are the mainstay of treatment for it, conferring benefits such as reducing opioid use and preventing relapse.
Candidates for opioid agonist therapy should undergo a multidisciplinary assessment, including an evaluation on the patient’s readiness to change his or her opioid use.
Patient education should include a discussion of the risks of methadone (eg, respiratory depression, fatal overdose, and QTc prolongation) and buprenorphine (eg hepatotoxicity) and their benefits (eg, controlling craving, decreasing the risk of relapse). Patients should also be educated about overdose and diversion.
Despite the difficulties inherent in treating patients with opioid use disorder, when used appropriately, opioid agonist therapy can be lifesaving for patients struggling with long-term opioid addiction.
Acknowledgment: We thank Katelyn Colosi, BS, and Drs. Susan Wolfe, Dennis Bouffard, and Sinha Shirshendu for their helpful comments.
- Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: results from a survey of general internists. Subst Abus 2016; 37:635–641.
- Samuels EA, Dwyer K, Mello MJ, Baird J, Kellogg AR, Bernstein E. Emergency department-based opioid harm reduction: moving physicians from willing to doing. Acad Emerg Med 2016; 23:455–465.
- Mohlman MK, Tanzman B, Finison K, Pinette M, Jones C. Impact of medication-assisted treatment for opioid addiction on Medicaid expenditures and health services utilization rates in Vermont. J Subst Abuse Treat 2016; 67:9–14.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA, American Psychiatric Association, 2013.
- Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health. www.samhsa.gov/data. Accessed April 6, 2017.
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016; 374:154–163.
- Ruan X, Wyche MQ, Kaye AD. Analyzing the relationship between nonmedical prescription-opioid use and heroin use. J Opioid Manage 2016; 12:11–14.
- Hser YI, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harv Rev Psychiatry 2015; 23:76–89.
- Nielsen S, Hillhouse M, Mooney L, Ang A, Ling W. Buprenorphine pharmacotherapy and behavioral treatment: comparison of outcomes among prescription opioid users, heroin users and combination users. J Subst Abuse Treat 2015; 48:70–76.
- Moore BA, Fiellin DA, Barry DT, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med 2007; 22:527–530.
- Fischer B, Patra J, Cruz MF, Gittins J, Rehm J. Comparing heroin users and prescription opioid users in a Canadian multi-site population of illicit opioid users. Drug Alcohol Rev 2008; 27:625–632.
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016; 374:154–163.
- Jones CM, Baldwin GT, Manocchio T, White JO, Mack KA. Trends in methadone distribution for pain treatment, methadone diversion, and overdose deaths—United States, 2002–2014. MMWR Morb Mortal Wkly Rep 2016; 65:667–671.
- Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med 2013; 7:377–386.
- Alinejad S, Kazemi T, Zamani N, Hoffman RS, Mehrpour O. A systematic review of the cardiotoxicity of methadone. EXCLI J 2015; 14:577–600.
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend 2003; 70(suppl 2):S59–S77.
- Ling W. Buprenorphine implant for opioid addiction. Pain Manage 2012; 2:345–350.
- Saxon AJ, Hser YI, Woody G, Ling W. Medication-assisted treatment for opioid addiction: methadone and buprenorphine. J Food Drug Anal 2013; 21:S69–S72.
- Kimber J, Larney S, Hickman M, Randall D, Degenhardt L. Mortality risk of opioid substitution therapy with methadone versus buprenorphine: a retrospective cohort study. Lancet Psychiatry 2015; 2:901–908.
- Marteau D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open 2015; 5: e007629.
- Kintz P. Deaths involving buprenorphine: a compendium of French cases. Forensic Sci Int 2001; 121:65–69.
- Zuin M, Giorgini A, Selmi C, et al. Acute liver and renal failure during treatment with buprenorphine at therapeutic dose. Dig Liver Dis 2009; 41:e8–e10.
- Klein JW. Pharmacotherapy for substance use disorders. Med Clin North Am 2016; 100:891–910.
- Modesto-Lowe V, Van Kirk J. Clinical uses of naltrexone: a review of the evidence. Exp Clin Psychopharmocol 2002; 10:213–227.
- Woody GE. Agonist models for treating persons with substance use disorders. Curr Psychiatry Rep 2014; 16:489.
- Sanders JJ, Roose RJ, Lubrano MC, Lucan SC. Meaning and methadone: patient perceptions of methadone dose and a model to promote adherence to maintenance treatment. J Addict Med 2013; 7:307–313.
- Herget G. Methadone and buprenorphine added to the WHO list of essential medicines. HIV/AIDS Policy Law Rev 2005; 10:23–24.
- Suzuki J, Dodds T. Clinical recommendation of 12-step meeting attendance and discussion regarding disclosure of buprenorphine use among patients in office-based opioid treatment. Subst Abus 2016; 37:31–34.
- Rettig RA, Yarmolinsky A. Federal Regulation of Methadone Treatment. Washington, DC: National Academies Press; 1995.
- Srivastava A, Kahan M, Nader M. Primary care management of opioid use disorders: abstinence, methadone, or buprenorphine-naloxone? Can Fam Physician 2017; 63:200–205.
- Substance Abuse and Mental Health Services Administration. Federal Guidelines for Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
- Substance Abuse and Mental Health Services Administration SAMSHA. Buprenorphine waiver management. www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management. Accessed April 6, 2017.
- Mark TL, Lubran R, McCance-Kats EF, Chalk M, Richardson J. Medicaid coverage of medications to treat alcohol and opioid dependence. J Subst Abuse Treat 2015; 55:1–5.
- Johnson B, Richert T. Diversion of methadone and buprenorphine from opioid substitution treatment: the importance of patients’ attitudes and norms. J Subst Abuse Treat 2015; 54:50–55.
- Yokell MA, Zaller ND, Green TC, Rich JD. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review. Curr Drug Abuse Rev 2011; 4:28–41.
- Schuman-Olivier Z, Albanese M, Nelson SE, et al. Self-treatment: illicit buprenorphine use by opioid-dependent treatment seekers. J Subst Abuse Treat 2010; 39:41–50.
- American Society of Addiction Medicine. National practice guidelines for the use of medications in the treatment of addiction involving opioid use. www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf. Accessed April 6, 2017.
- McNicholas L. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Service Administration; 2004.
- Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2004. (Treatment Improvement Protocol (TIP) Series, No. 40.) www.ncbi.nlm.nih.gov/books/NBK64245. Accessed April 6, 2017.
- Zippel-Schultz B, Specka M, Cimander K, et al. Outcomes of patients in long-term opioid maintenance treatment. Subst Use Misuse 2016; 51:1493–1503.
- Martins SS, Keyes KM, Storr CL, Zhu H, Chilcoat HD. Pathways between nonmedical opioid use/dependence and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 2009; 103:16–24.
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 1985; 142:1259–1264.
- Belding MA, Iguchi MY, Lamb RJ, Lakin M, Terry R. Stages and processes of change among polydrug users in methadone maintenance treatment. Drug Alcohol Depend 1995; 39:45–53.
- Peteet JR, Brenner S, Curtiss D, Ferrigno M, Kauffman J. A stage of change approach to addiction in the medical setting. Gen Hosp Psychiatry 1998; 20:267–273.
- Vijay A, Bazazi AR, Yee I, Kamarulzaman A, Altice FL. Treatment readiness, attitudes toward, and experiences with methadone and buprenorphine maintenance therapy among people who inject drugs in Malaysia. J Subst Abuse Treat 2015; 54:29–36.
For a patient struggling with opioid addiction, opioid agonist therapy with methadone or buprenorphine can reduce craving and opioid use and may even save his or her life. But many clinicians are unfamiliar with this evidence-based treatment,1,2 which is best started early in the course of addiction.3
This article outlines the pharmacology of these drugs, their clinical uses, and the challenges of using them to treat opioid addiction.
DIAGNOSTIC CRITERIA
Opioid addiction, formally known as opioid use disorder, is a pattern of opioid misuse leading to clinically significant impairment in multiple areas of life. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, lists 11 diagnostic criteria, but only 2 need to be present within the past year to make the diagnosis4:
- Taking opioids longer or in higher doses than was intended
- A persistent desire or unsuccessful efforts to cut down or control opioid use
- Spending a great deal of time obtaining, using, or recovering from using opioids
- Craving opioids
- Repeatedly failing to fulfill obligations at work, school, or home due to opioid use
- Continuing to use opioids even though it causes or exacerbates social or interpersonal problems
- Giving up or curtailing important social, occupational, or recreational activities because of opioid use
- Repeatedly using opioids in situations in which it is physically hazardous
- Continuing to use opioids despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance
- Tolerance
- Withdrawal.
Recent estimates indicate that 2.23 million people in the United States have opioid use disorder (426,000 with heroin and 1.8 million with prescription opioids).5
Progression from prescription opioids to heroin
We have observed that many patients with opioid use disorder start by misusing prescription opioids. Over time, tolerance can develop, which drives patients to use higher and higher doses.6
As the addiction progresses, a subset of prescription opioid users advances to using heroin, which is typically less expensive and easier to obtain.7 Most patients start with the intranasal route but eventually inject it intravenously.6,7
For many addicts, heroin use has medical consequences such as hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infection, psychiatric problems such as depression and anxiety, and illegal activities such as theft and sex work.8 People who use heroin appear to have more severe addiction and a lower socioeconomic status than prescription opioid users.9–11 But recently, a growing number of middle class individuals are becoming addicted to heroin.12
METHADONE
Methadone is a long-acting synthetic opioid that functions as a full agonist on the mu-opioid receptor. The drug binds, occupies, and stimulates the receptor, preventing withdrawal symptoms and reducing opioid cravings for at least 24 hours.13
Adverse effects of methadone
The most common adverse effects include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating.14 Other adverse effects:
Unintentional overdose. The risk is serious, as a single 30-mg dose can be fatal in people who are opioid-naïve.13
QTc prolongation, which can lead to torsade de pointes. This risk, which is dose-related, must be taken into consideration in patients who have any cardiac symptoms (eg, syncope, arrhythmia), pathology (familial QT prolongation), or other risk factors for QTc prolongation (eg, hypokalemia, QTc-prolonging medications).15
Respiratory depression, which can be fatal. This dose-related risk is heightened during the first 4 weeks of treatment if titration is too rapid or if methadone is used in combination with other drugs that cause central nervous system or respiratory depression.13,14
Starting methadone
To prevent respiratory depression and death related to rapid induction, the general rule is to start methadone at a low daily dose (20–30 mg) depending on the patient’s withdrawal symptoms.14 During this period, patients need to be closely monitored and educated on the perils of concomitant use of central nervous system depressants.14
In most patients, the dose is titrated up until their withdrawal symptoms and cravings are eliminated, which generally requires 60 to 120 mg daily.14 Hepatic and renal impairment, pregnancy, and advanced age can alter methadone pharmacokinetics and may therefore necessitate dose adjustment.
BUPRENORPHINE
Buprenorphine is an alkaloid thebaine opioid derivative that acts as a partial mu-opioid agonist and a kappa antagonist.16 Like methadone, buprenorphine is used to manage cravings and withdrawal symptoms.16 Dosages of 4 to 16 mg (up to 32 mg) per day of buprenorphine are usually required to adequately control opioid cravings.16
Sublingual and subdermal products
Buprenorphine is currently available in the United States in sublingual and subdermal formulations.16,17
Sublingual buprenorphine is usually combined with naloxone in a 4:1 ratio to deter intravenous use. Intravenous injection of the combination product can precipitate withdrawal due to the antagonist action of naloxone. (Taken orally or sublingually, naloxone is poorly absorbed and has little or no clinical effect.) Buprenorphine-naloxone is available in tablets, a sublingual film strip, and a buccal film strip. Buprenorphine is also available by itself in a sublingual formulation.
The US Food and Drug Administration has approved a buprenorphine subdermal implant, Probuphine. Four rods, about 1 inch long, are placed under the skin in the inner aspect of the upper arm and provide the equivalent of 8 mg of buprenorphine daily for 6 months.17 However, this method is formulated only for maintenance treatment and cannot be used for induction. Additionally, it is recommended that the implants be surgically removed at the end of 6 months, after which another set of implants can be inserted in the other arm or the patient can switch to sublingual therapy, depending on the clinical situation and patient preference.17
Generally safer than methadone
Buprenorphine works on the same receptor as methadone and therefore has a similar side effect profile. However, buprenorphine has a ceiling effect, which greatly reduces the risk of fatal respiratory depression.18 It also does not cause clinically significant QTc prolongation and is preferable in patients who have cardiac risk factors.18
Another advantage is that buprenorphine has fewer identified medication interactions than methadone.18 Further, induction of buprenorphine in patients with opioid use disorder has been shown to be safer than methadone.19
Although buprenorphine has been found to be 6 times safer than methadone with regard to overdose among the general population,20 it can still cause fatal intoxication if used in combination with central nervous system depressants.21
Buprenorphine has been also associated with hepatotoxicity, though the risk of new-onset liver disease appears to be low.22
NALTREXONE IS LESS EFFECTIVE THAN METHADONE, BUPRENORPHINE
Besides methadone and buprenorphine, the only other approved option for treating opioid use disorder is the opioid antagonist naltrexone.
Naltrexone has significantly less abuse potential, as it provides no euphoria, but patients do not like it. Even with the long-acting formulation (Vivitrol), naltrexone treatment is significantly less effective than methadone or buprenorphine.23–25 Further, although naltrexone is not a controlled substance and so does not face the same scrutiny as the agonist therapies, there are other significant barriers. Additional information on naltrexone is presented in reviews by Modesto-Lowe and Van Kirk24 and Woody.25
OBSTACLES TO TREATMENT
People hold conflicting views about opioid agonist therapy. Some believe that “trading one drug for another” is not a legitimate therapeutic strategy, and they may feel ashamed of being on maintenance therapy.26 Similarly, some argue that the answer to establishing stable abstinence does not lie simply in prescribing medications.
The contrary argument is that these medications, if used appropriately, confer many benefits such as reducing the medical and psychosocial sequelae of opioid addiction.18 In fact, properly treated patients no longer meet the diagnostic criteria of opioid use disorder, and both methadone and buprenorphine are on the World Health Organization’s (WHO) list of essential medicines.27
Despite endorsement by the WHO, the stigma attached to the opioid agonists has been difficult to overcome. Patients with opioid use disorder may be viewed with distrust by healthcare providers and often do not feel welcome in healthcare settings or in self-help recovery groups.28
Barriers to methadone therapy
Federal regulations on methadone prescribing and use were established to promote patient safety and decrease diversion, but they may also complicate access to care.29 They stipulate that to qualify for methadone maintenance, patients need to demonstrate opioid addiction for 1 year, except for pregnant women and those who have been incarcerated in the past 6 months. Patients under the age of 18 must have 2 documented failed treatment episodes as well as approval by a guardian to receive treatment.
Inconvenience. Methadone can be prescribed for opioid dependence only by an accredited treatment program. Patients must therefore travel to the clinic and wait to be evaluated on a daily basis for a minimum of 90 days. Only after they demonstrate consistent responsible behavior and negative results on urine testing do they become eligible to take methadone home.29 If a patient is to travel out of the area during the initial 90 days of treatment, he or she must make arrangements in advance to find a clinic that will provide a “guest dose.”
The inconvenience arising from the regulations may deter some patients from seeking methadone therapy. In spite of this, once patients are started on methadone, more of them continue treatment than with buprenorphine.18 A proposed reason is that methadone is a potent full opioid agonist and therefore relieves withdrawal symptoms and craving more effectively than buprenorphine, which is a partial agonist.30 Another possible reason is the higher level of supervision afforded by methadone clinics, which require daily contact for at least 90 days.
Safety concerns arise from methadone diversion, as illicit use may have lethal consequences. In the past decade, deaths from methadone overdose have risen significantly, most of them due to respiratory depression or torsade de pointes.13 However, most cases of diversion and overdose involve methadone that is prescribed for pain by individual practitioners and not from maintenance programs.13
Advantages of buprenorphine
Together, methadone’s lethality, stigma, and inconvenience may contribute to patients preferring buprenorphine.31
The regulations governing buprenorphine’s use are less restrictive than those with methadone. For example, patients must have a diagnosis of opioid addiction to be prescribed buprenorphine, but they are not required to carry the diagnosis for a year before treatment.31 Additionally, they do not need to travel to a federally approved opioid treatment center daily and can receive buprenorphine directly from a physician in an outpatient setting.
Under the Drug Abuse Treatment Act (DATA) of 2000, any physician can apply for a waiver to prescribe and dispense buprenorphine in his or her office. To qualify for an initial waiver, physicians must either obtain certification in the fields of addiction medicine or addiction psychiatry or complete an approved 8-hour training session.32 Each physician starts with a maximum of 30 patients, but can apply to treat up to 100 patients after 1 year and eventually up to 275 patients. Physicians must document every buprenorphine prescription they write and be able to refer patients for counseling.31
As of February 2017, nurse practitioners and physician assistants can also apply for a DATA 2000 waiver. All waivered providers are subject to unannounced visits from the Drug Enforcement Administration once every 5 years.32
While there are no federal restrictions on the amount of buprenorphine that can be dispensed, some states and some insurance companies have placed restrictions on dose or length of treatment.33 Buprenorphine patients can fill their prescriptions at any pharmacy and are permitted to bring their medication home, which improves access to care. However, office-based outpatient treatment is not without risk, and preventing buprenorphine diversion remains a challenge.34
‘Lending’ buprenorphine is a felony
Addicts have illegally used buprenorphine to self-treat opioid withdrawal, craving, and dependence.35 Its misuse has also been coupled with self-treatment of conditions that include depression and pain.36
A survey found that 83.7% of patients deem buprenorphine diversion to be appropriate; further, most patients said they consider it unethical to withhold prescribed buprenorphine from individuals showing symptoms of withdrawal.34 Physicians who prescribe buprenorphine must inform their patients that even “lending” or giving away their medication is a felony.
Prescribing physicians must also be diligent about monitoring for signs of diversion such as inconsistent urine toxicology screens, “lost” medication, and requests for early refills or escalating doses.37
EVALUATING PATIENTS FOR OPIOID REPLACEMENT THERAPY
In addition to federal regulations, we propose a 4-step approach to evaluate eligibility for opioid replacement therapy based on existing guidelines.37–39
Step 1: History and physical examination
The history should give particular attention to the patient’s cardiac, pulmonary, and hepatic status, with consideration of the risks of any medical comorbidities (eg, bacterial endocarditis, HIV and HCV infection) that might influence treatment.37
It is also essential to evaluate for any contraindications or drug interactions before prescribing methadone or buprenorphine.38
Contraindications to methadone maintenance include40:
- Cor pulmonale
- Methadone hypersensitivity
- Pseudomembranous colitis
- Selegiline use (due to risk of serotonin syndrome)
- Ileum paralyticus.
Contraindications to buprenorphine use include:
- Hypersensitivity to naloxone or buprenorphine
- Impaired liver function (due to the risk of inadvertent overdose associated with slowed metabolism).
Concurrent use of alcohol or illicit benzodiazepines is a relative contraindication to both methadone and buprenorphine due to the risk of respiratory depression and overdose.37 Likewise, avoid coprescribing opioid agonists and benzodiazepines whenever possible. Obtain a complete list of current medications and query a prescription-monitoring database to determine whether any controlled substances are currently prescribed.37
During the physical examination, look for stigmata of intravenous drug use such as track marks or abscesses37 and document any physical findings consistent with intoxication or withdrawal. Patients must be completely detoxed or in withdrawal before beginning buprenorphine induction; premature induction can precipitate withdrawal.38
A discussion of pregnant patients with opioid use disorder is beyond the scope of this paper. However, it is incumbent on the prescriber to inquire whether the client is pregnant or intends to become pregnant and what birth control methods are in place.
Step 2: Assess psychiatric status
Assessment of the patient’s psychiatric status, including an assessment of alcohol and other drug use, will help determine his or her eligibility for opioid agonists.37 To prepare for the need to manage patients with psychiatrically complex issues, it is helpful to develop relationships with addiction specialists and psychiatrists who are familiar with opioid replacement therapy in your area. This will make it easier to collaborate on patients’ care.
Ask all patients directly about suicidal or homicidal ideation. Any patient with active suicidal or homicidal ideation should be assessed for need of immediate hospitalization by a psychiatrist or another qualified mental health professional. Patients with a history of suicidal ideation should be monitored closely by a mental health professional throughout treatment.37
Many if not most patients with opioid use disorder have concurrent psychiatric disorders, and the interplay between these disorders is complex.40,41 Depression, for example, can precede and even precipitate drug use (an observation supporting the “self-medication theory”).42 If the underlying depressive disorder is not addressed, relapse is nearly inevitable.
It has also been shown that both chronic opioid use and withdrawal can exacerbate aversive emotional states. This escalation of symptoms may result from the pharmacologic effects of opioids or from psychosocial sequelae that can arise from chronic opioid use.41 In this situation, maintaining abstinence can lead to resolution of depressive symptoms. As depression and opioid use can occur together, successful treatment requires equal attention to both illnesses.
Other common comorbidities in patients with opioid use disorder include posttraumatic stress disorder, attention deficit hyperactivity disorder, antisocial personality disorder, and concurrent substance abuse disorders.43 The confluence of antisocial personality disorder is particularly important, as patients with antisocial personality disorder display disruptive and maladaptive behaviors.
Identify any psychotropic medication that is prescribed and check carefully for drug interactions. This applies especially to methadone, as many psychiatric medications also prolong the QT interval. Moreover, patients may not be forthcoming about the use of psychiatric medication.
Find out whether the patient is using any other addictive substances, particularly those that affect the central nervous system, as those who use fentanyl, benzodiazepines, or alcohol are at the highest risk of overdose.31 Often the best option for those with concurrent substance use disorders is inpatient detoxification followed by residential rehabilitation care. Either buprenorphine or methadone can then be initiated upon return to an outpatient setting.
Step 3: Assess psychosocial status
To what extent do the patient’s home environment and support systems promote a drug-free lifestyle? Unfortunately, the psychosocial status of many of these patients is fragile, and they may live in areas where illicit drugs are readily available (which can be urban, suburban, or rural), making it difficult to stay substance-free.38
Generally, lifestyle modifications are needed to transform maladaptive behaviors and promote an environment conducive to long-term recovery. Referrals to social services to address housing, vocational needs, and entitlements may be helpful.39
Step 4: Assess readiness to change
According to one model, people go through 5 stages when changing a behavior: precontemplation, contemplation, preparation for action, action, and maintenance.43 In general, the further along the stages a patient is, the more appropriate he or she is for office-based treatment with buprenorphine.39
The level of change can be assessed with tools such as Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES). Use of stage-specific strategies may enhance a patient’s readiness to cease opioid use.43
Precontemplation. Those in the precontemplation stage are not ready to think about changing their behavior.43 They may be unaware of or unwilling to consider the risks associated with their opioid use and resistant to the idea of quitting. Engagement with opioid agonists for individuals in this stage is low and dropout rates are likely high.
Thus, the proper approach for “precontemplators” is to help them develop some ambivalence about their opioid use. One tactic is to involve the patient in a discussion of the personal benefits and risks of opioid use.
Contemplation. Individuals in the contemplation stage have begun to weigh the costs and benefits of opioid use and express ambivalence about it.44 Because the patient is willing to explore the risks of ongoing use and consider the benefits of treatment, the goal in this stage is to elicit a commitment from the individual to seek treatment.
Preparation. The person in this stage moves from thinking about treatment to planning what action to take.45 As the individual prepares to enter treatment, indecision tends to resurface, as well as self-doubt about his or her ability to change. During this stage, it is important for the provider to spell out goals (abstinence) and strategies (eg, counseling, medication) and enhance a sense of self-efficacy.
Action and maintenance. Patients in these stages engage in treatment and employ new strategies to abstain from opioid use. Maintaining these behaviors can be a daily struggle. Expressing confidence in the patient’s ability to abstain from use will support his or her progress. Behavioral interventions such as strategic avoidance of triggers and engagement in alternative activities (eg, support groups, exercise, faith-based practices) will help to maintain abstinence.
A CHRONIC CONDITION
Opioid use disorder, like many chronic illnesses, requires long-term attention to attain successful patient outcomes. The opioid agonists methadone and buprenorphine are the mainstay of treatment for it, conferring benefits such as reducing opioid use and preventing relapse.
Candidates for opioid agonist therapy should undergo a multidisciplinary assessment, including an evaluation on the patient’s readiness to change his or her opioid use.
Patient education should include a discussion of the risks of methadone (eg, respiratory depression, fatal overdose, and QTc prolongation) and buprenorphine (eg hepatotoxicity) and their benefits (eg, controlling craving, decreasing the risk of relapse). Patients should also be educated about overdose and diversion.
Despite the difficulties inherent in treating patients with opioid use disorder, when used appropriately, opioid agonist therapy can be lifesaving for patients struggling with long-term opioid addiction.
Acknowledgment: We thank Katelyn Colosi, BS, and Drs. Susan Wolfe, Dennis Bouffard, and Sinha Shirshendu for their helpful comments.
For a patient struggling with opioid addiction, opioid agonist therapy with methadone or buprenorphine can reduce craving and opioid use and may even save his or her life. But many clinicians are unfamiliar with this evidence-based treatment,1,2 which is best started early in the course of addiction.3
This article outlines the pharmacology of these drugs, their clinical uses, and the challenges of using them to treat opioid addiction.
DIAGNOSTIC CRITERIA
Opioid addiction, formally known as opioid use disorder, is a pattern of opioid misuse leading to clinically significant impairment in multiple areas of life. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, lists 11 diagnostic criteria, but only 2 need to be present within the past year to make the diagnosis4:
- Taking opioids longer or in higher doses than was intended
- A persistent desire or unsuccessful efforts to cut down or control opioid use
- Spending a great deal of time obtaining, using, or recovering from using opioids
- Craving opioids
- Repeatedly failing to fulfill obligations at work, school, or home due to opioid use
- Continuing to use opioids even though it causes or exacerbates social or interpersonal problems
- Giving up or curtailing important social, occupational, or recreational activities because of opioid use
- Repeatedly using opioids in situations in which it is physically hazardous
- Continuing to use opioids despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance
- Tolerance
- Withdrawal.
Recent estimates indicate that 2.23 million people in the United States have opioid use disorder (426,000 with heroin and 1.8 million with prescription opioids).5
Progression from prescription opioids to heroin
We have observed that many patients with opioid use disorder start by misusing prescription opioids. Over time, tolerance can develop, which drives patients to use higher and higher doses.6
As the addiction progresses, a subset of prescription opioid users advances to using heroin, which is typically less expensive and easier to obtain.7 Most patients start with the intranasal route but eventually inject it intravenously.6,7
For many addicts, heroin use has medical consequences such as hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infection, psychiatric problems such as depression and anxiety, and illegal activities such as theft and sex work.8 People who use heroin appear to have more severe addiction and a lower socioeconomic status than prescription opioid users.9–11 But recently, a growing number of middle class individuals are becoming addicted to heroin.12
METHADONE
Methadone is a long-acting synthetic opioid that functions as a full agonist on the mu-opioid receptor. The drug binds, occupies, and stimulates the receptor, preventing withdrawal symptoms and reducing opioid cravings for at least 24 hours.13
Adverse effects of methadone
The most common adverse effects include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating.14 Other adverse effects:
Unintentional overdose. The risk is serious, as a single 30-mg dose can be fatal in people who are opioid-naïve.13
QTc prolongation, which can lead to torsade de pointes. This risk, which is dose-related, must be taken into consideration in patients who have any cardiac symptoms (eg, syncope, arrhythmia), pathology (familial QT prolongation), or other risk factors for QTc prolongation (eg, hypokalemia, QTc-prolonging medications).15
Respiratory depression, which can be fatal. This dose-related risk is heightened during the first 4 weeks of treatment if titration is too rapid or if methadone is used in combination with other drugs that cause central nervous system or respiratory depression.13,14
Starting methadone
To prevent respiratory depression and death related to rapid induction, the general rule is to start methadone at a low daily dose (20–30 mg) depending on the patient’s withdrawal symptoms.14 During this period, patients need to be closely monitored and educated on the perils of concomitant use of central nervous system depressants.14
In most patients, the dose is titrated up until their withdrawal symptoms and cravings are eliminated, which generally requires 60 to 120 mg daily.14 Hepatic and renal impairment, pregnancy, and advanced age can alter methadone pharmacokinetics and may therefore necessitate dose adjustment.
BUPRENORPHINE
Buprenorphine is an alkaloid thebaine opioid derivative that acts as a partial mu-opioid agonist and a kappa antagonist.16 Like methadone, buprenorphine is used to manage cravings and withdrawal symptoms.16 Dosages of 4 to 16 mg (up to 32 mg) per day of buprenorphine are usually required to adequately control opioid cravings.16
Sublingual and subdermal products
Buprenorphine is currently available in the United States in sublingual and subdermal formulations.16,17
Sublingual buprenorphine is usually combined with naloxone in a 4:1 ratio to deter intravenous use. Intravenous injection of the combination product can precipitate withdrawal due to the antagonist action of naloxone. (Taken orally or sublingually, naloxone is poorly absorbed and has little or no clinical effect.) Buprenorphine-naloxone is available in tablets, a sublingual film strip, and a buccal film strip. Buprenorphine is also available by itself in a sublingual formulation.
The US Food and Drug Administration has approved a buprenorphine subdermal implant, Probuphine. Four rods, about 1 inch long, are placed under the skin in the inner aspect of the upper arm and provide the equivalent of 8 mg of buprenorphine daily for 6 months.17 However, this method is formulated only for maintenance treatment and cannot be used for induction. Additionally, it is recommended that the implants be surgically removed at the end of 6 months, after which another set of implants can be inserted in the other arm or the patient can switch to sublingual therapy, depending on the clinical situation and patient preference.17
Generally safer than methadone
Buprenorphine works on the same receptor as methadone and therefore has a similar side effect profile. However, buprenorphine has a ceiling effect, which greatly reduces the risk of fatal respiratory depression.18 It also does not cause clinically significant QTc prolongation and is preferable in patients who have cardiac risk factors.18
Another advantage is that buprenorphine has fewer identified medication interactions than methadone.18 Further, induction of buprenorphine in patients with opioid use disorder has been shown to be safer than methadone.19
Although buprenorphine has been found to be 6 times safer than methadone with regard to overdose among the general population,20 it can still cause fatal intoxication if used in combination with central nervous system depressants.21
Buprenorphine has been also associated with hepatotoxicity, though the risk of new-onset liver disease appears to be low.22
NALTREXONE IS LESS EFFECTIVE THAN METHADONE, BUPRENORPHINE
Besides methadone and buprenorphine, the only other approved option for treating opioid use disorder is the opioid antagonist naltrexone.
Naltrexone has significantly less abuse potential, as it provides no euphoria, but patients do not like it. Even with the long-acting formulation (Vivitrol), naltrexone treatment is significantly less effective than methadone or buprenorphine.23–25 Further, although naltrexone is not a controlled substance and so does not face the same scrutiny as the agonist therapies, there are other significant barriers. Additional information on naltrexone is presented in reviews by Modesto-Lowe and Van Kirk24 and Woody.25
OBSTACLES TO TREATMENT
People hold conflicting views about opioid agonist therapy. Some believe that “trading one drug for another” is not a legitimate therapeutic strategy, and they may feel ashamed of being on maintenance therapy.26 Similarly, some argue that the answer to establishing stable abstinence does not lie simply in prescribing medications.
The contrary argument is that these medications, if used appropriately, confer many benefits such as reducing the medical and psychosocial sequelae of opioid addiction.18 In fact, properly treated patients no longer meet the diagnostic criteria of opioid use disorder, and both methadone and buprenorphine are on the World Health Organization’s (WHO) list of essential medicines.27
Despite endorsement by the WHO, the stigma attached to the opioid agonists has been difficult to overcome. Patients with opioid use disorder may be viewed with distrust by healthcare providers and often do not feel welcome in healthcare settings or in self-help recovery groups.28
Barriers to methadone therapy
Federal regulations on methadone prescribing and use were established to promote patient safety and decrease diversion, but they may also complicate access to care.29 They stipulate that to qualify for methadone maintenance, patients need to demonstrate opioid addiction for 1 year, except for pregnant women and those who have been incarcerated in the past 6 months. Patients under the age of 18 must have 2 documented failed treatment episodes as well as approval by a guardian to receive treatment.
Inconvenience. Methadone can be prescribed for opioid dependence only by an accredited treatment program. Patients must therefore travel to the clinic and wait to be evaluated on a daily basis for a minimum of 90 days. Only after they demonstrate consistent responsible behavior and negative results on urine testing do they become eligible to take methadone home.29 If a patient is to travel out of the area during the initial 90 days of treatment, he or she must make arrangements in advance to find a clinic that will provide a “guest dose.”
The inconvenience arising from the regulations may deter some patients from seeking methadone therapy. In spite of this, once patients are started on methadone, more of them continue treatment than with buprenorphine.18 A proposed reason is that methadone is a potent full opioid agonist and therefore relieves withdrawal symptoms and craving more effectively than buprenorphine, which is a partial agonist.30 Another possible reason is the higher level of supervision afforded by methadone clinics, which require daily contact for at least 90 days.
Safety concerns arise from methadone diversion, as illicit use may have lethal consequences. In the past decade, deaths from methadone overdose have risen significantly, most of them due to respiratory depression or torsade de pointes.13 However, most cases of diversion and overdose involve methadone that is prescribed for pain by individual practitioners and not from maintenance programs.13
Advantages of buprenorphine
Together, methadone’s lethality, stigma, and inconvenience may contribute to patients preferring buprenorphine.31
The regulations governing buprenorphine’s use are less restrictive than those with methadone. For example, patients must have a diagnosis of opioid addiction to be prescribed buprenorphine, but they are not required to carry the diagnosis for a year before treatment.31 Additionally, they do not need to travel to a federally approved opioid treatment center daily and can receive buprenorphine directly from a physician in an outpatient setting.
Under the Drug Abuse Treatment Act (DATA) of 2000, any physician can apply for a waiver to prescribe and dispense buprenorphine in his or her office. To qualify for an initial waiver, physicians must either obtain certification in the fields of addiction medicine or addiction psychiatry or complete an approved 8-hour training session.32 Each physician starts with a maximum of 30 patients, but can apply to treat up to 100 patients after 1 year and eventually up to 275 patients. Physicians must document every buprenorphine prescription they write and be able to refer patients for counseling.31
As of February 2017, nurse practitioners and physician assistants can also apply for a DATA 2000 waiver. All waivered providers are subject to unannounced visits from the Drug Enforcement Administration once every 5 years.32
While there are no federal restrictions on the amount of buprenorphine that can be dispensed, some states and some insurance companies have placed restrictions on dose or length of treatment.33 Buprenorphine patients can fill their prescriptions at any pharmacy and are permitted to bring their medication home, which improves access to care. However, office-based outpatient treatment is not without risk, and preventing buprenorphine diversion remains a challenge.34
‘Lending’ buprenorphine is a felony
Addicts have illegally used buprenorphine to self-treat opioid withdrawal, craving, and dependence.35 Its misuse has also been coupled with self-treatment of conditions that include depression and pain.36
A survey found that 83.7% of patients deem buprenorphine diversion to be appropriate; further, most patients said they consider it unethical to withhold prescribed buprenorphine from individuals showing symptoms of withdrawal.34 Physicians who prescribe buprenorphine must inform their patients that even “lending” or giving away their medication is a felony.
Prescribing physicians must also be diligent about monitoring for signs of diversion such as inconsistent urine toxicology screens, “lost” medication, and requests for early refills or escalating doses.37
EVALUATING PATIENTS FOR OPIOID REPLACEMENT THERAPY
In addition to federal regulations, we propose a 4-step approach to evaluate eligibility for opioid replacement therapy based on existing guidelines.37–39
Step 1: History and physical examination
The history should give particular attention to the patient’s cardiac, pulmonary, and hepatic status, with consideration of the risks of any medical comorbidities (eg, bacterial endocarditis, HIV and HCV infection) that might influence treatment.37
It is also essential to evaluate for any contraindications or drug interactions before prescribing methadone or buprenorphine.38
Contraindications to methadone maintenance include40:
- Cor pulmonale
- Methadone hypersensitivity
- Pseudomembranous colitis
- Selegiline use (due to risk of serotonin syndrome)
- Ileum paralyticus.
Contraindications to buprenorphine use include:
- Hypersensitivity to naloxone or buprenorphine
- Impaired liver function (due to the risk of inadvertent overdose associated with slowed metabolism).
Concurrent use of alcohol or illicit benzodiazepines is a relative contraindication to both methadone and buprenorphine due to the risk of respiratory depression and overdose.37 Likewise, avoid coprescribing opioid agonists and benzodiazepines whenever possible. Obtain a complete list of current medications and query a prescription-monitoring database to determine whether any controlled substances are currently prescribed.37
During the physical examination, look for stigmata of intravenous drug use such as track marks or abscesses37 and document any physical findings consistent with intoxication or withdrawal. Patients must be completely detoxed or in withdrawal before beginning buprenorphine induction; premature induction can precipitate withdrawal.38
A discussion of pregnant patients with opioid use disorder is beyond the scope of this paper. However, it is incumbent on the prescriber to inquire whether the client is pregnant or intends to become pregnant and what birth control methods are in place.
Step 2: Assess psychiatric status
Assessment of the patient’s psychiatric status, including an assessment of alcohol and other drug use, will help determine his or her eligibility for opioid agonists.37 To prepare for the need to manage patients with psychiatrically complex issues, it is helpful to develop relationships with addiction specialists and psychiatrists who are familiar with opioid replacement therapy in your area. This will make it easier to collaborate on patients’ care.
Ask all patients directly about suicidal or homicidal ideation. Any patient with active suicidal or homicidal ideation should be assessed for need of immediate hospitalization by a psychiatrist or another qualified mental health professional. Patients with a history of suicidal ideation should be monitored closely by a mental health professional throughout treatment.37
Many if not most patients with opioid use disorder have concurrent psychiatric disorders, and the interplay between these disorders is complex.40,41 Depression, for example, can precede and even precipitate drug use (an observation supporting the “self-medication theory”).42 If the underlying depressive disorder is not addressed, relapse is nearly inevitable.
It has also been shown that both chronic opioid use and withdrawal can exacerbate aversive emotional states. This escalation of symptoms may result from the pharmacologic effects of opioids or from psychosocial sequelae that can arise from chronic opioid use.41 In this situation, maintaining abstinence can lead to resolution of depressive symptoms. As depression and opioid use can occur together, successful treatment requires equal attention to both illnesses.
Other common comorbidities in patients with opioid use disorder include posttraumatic stress disorder, attention deficit hyperactivity disorder, antisocial personality disorder, and concurrent substance abuse disorders.43 The confluence of antisocial personality disorder is particularly important, as patients with antisocial personality disorder display disruptive and maladaptive behaviors.
Identify any psychotropic medication that is prescribed and check carefully for drug interactions. This applies especially to methadone, as many psychiatric medications also prolong the QT interval. Moreover, patients may not be forthcoming about the use of psychiatric medication.
Find out whether the patient is using any other addictive substances, particularly those that affect the central nervous system, as those who use fentanyl, benzodiazepines, or alcohol are at the highest risk of overdose.31 Often the best option for those with concurrent substance use disorders is inpatient detoxification followed by residential rehabilitation care. Either buprenorphine or methadone can then be initiated upon return to an outpatient setting.
Step 3: Assess psychosocial status
To what extent do the patient’s home environment and support systems promote a drug-free lifestyle? Unfortunately, the psychosocial status of many of these patients is fragile, and they may live in areas where illicit drugs are readily available (which can be urban, suburban, or rural), making it difficult to stay substance-free.38
Generally, lifestyle modifications are needed to transform maladaptive behaviors and promote an environment conducive to long-term recovery. Referrals to social services to address housing, vocational needs, and entitlements may be helpful.39
Step 4: Assess readiness to change
According to one model, people go through 5 stages when changing a behavior: precontemplation, contemplation, preparation for action, action, and maintenance.43 In general, the further along the stages a patient is, the more appropriate he or she is for office-based treatment with buprenorphine.39
The level of change can be assessed with tools such as Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES). Use of stage-specific strategies may enhance a patient’s readiness to cease opioid use.43
Precontemplation. Those in the precontemplation stage are not ready to think about changing their behavior.43 They may be unaware of or unwilling to consider the risks associated with their opioid use and resistant to the idea of quitting. Engagement with opioid agonists for individuals in this stage is low and dropout rates are likely high.
Thus, the proper approach for “precontemplators” is to help them develop some ambivalence about their opioid use. One tactic is to involve the patient in a discussion of the personal benefits and risks of opioid use.
Contemplation. Individuals in the contemplation stage have begun to weigh the costs and benefits of opioid use and express ambivalence about it.44 Because the patient is willing to explore the risks of ongoing use and consider the benefits of treatment, the goal in this stage is to elicit a commitment from the individual to seek treatment.
Preparation. The person in this stage moves from thinking about treatment to planning what action to take.45 As the individual prepares to enter treatment, indecision tends to resurface, as well as self-doubt about his or her ability to change. During this stage, it is important for the provider to spell out goals (abstinence) and strategies (eg, counseling, medication) and enhance a sense of self-efficacy.
Action and maintenance. Patients in these stages engage in treatment and employ new strategies to abstain from opioid use. Maintaining these behaviors can be a daily struggle. Expressing confidence in the patient’s ability to abstain from use will support his or her progress. Behavioral interventions such as strategic avoidance of triggers and engagement in alternative activities (eg, support groups, exercise, faith-based practices) will help to maintain abstinence.
A CHRONIC CONDITION
Opioid use disorder, like many chronic illnesses, requires long-term attention to attain successful patient outcomes. The opioid agonists methadone and buprenorphine are the mainstay of treatment for it, conferring benefits such as reducing opioid use and preventing relapse.
Candidates for opioid agonist therapy should undergo a multidisciplinary assessment, including an evaluation on the patient’s readiness to change his or her opioid use.
Patient education should include a discussion of the risks of methadone (eg, respiratory depression, fatal overdose, and QTc prolongation) and buprenorphine (eg hepatotoxicity) and their benefits (eg, controlling craving, decreasing the risk of relapse). Patients should also be educated about overdose and diversion.
Despite the difficulties inherent in treating patients with opioid use disorder, when used appropriately, opioid agonist therapy can be lifesaving for patients struggling with long-term opioid addiction.
Acknowledgment: We thank Katelyn Colosi, BS, and Drs. Susan Wolfe, Dennis Bouffard, and Sinha Shirshendu for their helpful comments.
- Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: results from a survey of general internists. Subst Abus 2016; 37:635–641.
- Samuels EA, Dwyer K, Mello MJ, Baird J, Kellogg AR, Bernstein E. Emergency department-based opioid harm reduction: moving physicians from willing to doing. Acad Emerg Med 2016; 23:455–465.
- Mohlman MK, Tanzman B, Finison K, Pinette M, Jones C. Impact of medication-assisted treatment for opioid addiction on Medicaid expenditures and health services utilization rates in Vermont. J Subst Abuse Treat 2016; 67:9–14.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA, American Psychiatric Association, 2013.
- Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health. www.samhsa.gov/data. Accessed April 6, 2017.
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016; 374:154–163.
- Ruan X, Wyche MQ, Kaye AD. Analyzing the relationship between nonmedical prescription-opioid use and heroin use. J Opioid Manage 2016; 12:11–14.
- Hser YI, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harv Rev Psychiatry 2015; 23:76–89.
- Nielsen S, Hillhouse M, Mooney L, Ang A, Ling W. Buprenorphine pharmacotherapy and behavioral treatment: comparison of outcomes among prescription opioid users, heroin users and combination users. J Subst Abuse Treat 2015; 48:70–76.
- Moore BA, Fiellin DA, Barry DT, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med 2007; 22:527–530.
- Fischer B, Patra J, Cruz MF, Gittins J, Rehm J. Comparing heroin users and prescription opioid users in a Canadian multi-site population of illicit opioid users. Drug Alcohol Rev 2008; 27:625–632.
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016; 374:154–163.
- Jones CM, Baldwin GT, Manocchio T, White JO, Mack KA. Trends in methadone distribution for pain treatment, methadone diversion, and overdose deaths—United States, 2002–2014. MMWR Morb Mortal Wkly Rep 2016; 65:667–671.
- Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med 2013; 7:377–386.
- Alinejad S, Kazemi T, Zamani N, Hoffman RS, Mehrpour O. A systematic review of the cardiotoxicity of methadone. EXCLI J 2015; 14:577–600.
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend 2003; 70(suppl 2):S59–S77.
- Ling W. Buprenorphine implant for opioid addiction. Pain Manage 2012; 2:345–350.
- Saxon AJ, Hser YI, Woody G, Ling W. Medication-assisted treatment for opioid addiction: methadone and buprenorphine. J Food Drug Anal 2013; 21:S69–S72.
- Kimber J, Larney S, Hickman M, Randall D, Degenhardt L. Mortality risk of opioid substitution therapy with methadone versus buprenorphine: a retrospective cohort study. Lancet Psychiatry 2015; 2:901–908.
- Marteau D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open 2015; 5: e007629.
- Kintz P. Deaths involving buprenorphine: a compendium of French cases. Forensic Sci Int 2001; 121:65–69.
- Zuin M, Giorgini A, Selmi C, et al. Acute liver and renal failure during treatment with buprenorphine at therapeutic dose. Dig Liver Dis 2009; 41:e8–e10.
- Klein JW. Pharmacotherapy for substance use disorders. Med Clin North Am 2016; 100:891–910.
- Modesto-Lowe V, Van Kirk J. Clinical uses of naltrexone: a review of the evidence. Exp Clin Psychopharmocol 2002; 10:213–227.
- Woody GE. Agonist models for treating persons with substance use disorders. Curr Psychiatry Rep 2014; 16:489.
- Sanders JJ, Roose RJ, Lubrano MC, Lucan SC. Meaning and methadone: patient perceptions of methadone dose and a model to promote adherence to maintenance treatment. J Addict Med 2013; 7:307–313.
- Herget G. Methadone and buprenorphine added to the WHO list of essential medicines. HIV/AIDS Policy Law Rev 2005; 10:23–24.
- Suzuki J, Dodds T. Clinical recommendation of 12-step meeting attendance and discussion regarding disclosure of buprenorphine use among patients in office-based opioid treatment. Subst Abus 2016; 37:31–34.
- Rettig RA, Yarmolinsky A. Federal Regulation of Methadone Treatment. Washington, DC: National Academies Press; 1995.
- Srivastava A, Kahan M, Nader M. Primary care management of opioid use disorders: abstinence, methadone, or buprenorphine-naloxone? Can Fam Physician 2017; 63:200–205.
- Substance Abuse and Mental Health Services Administration. Federal Guidelines for Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
- Substance Abuse and Mental Health Services Administration SAMSHA. Buprenorphine waiver management. www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management. Accessed April 6, 2017.
- Mark TL, Lubran R, McCance-Kats EF, Chalk M, Richardson J. Medicaid coverage of medications to treat alcohol and opioid dependence. J Subst Abuse Treat 2015; 55:1–5.
- Johnson B, Richert T. Diversion of methadone and buprenorphine from opioid substitution treatment: the importance of patients’ attitudes and norms. J Subst Abuse Treat 2015; 54:50–55.
- Yokell MA, Zaller ND, Green TC, Rich JD. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review. Curr Drug Abuse Rev 2011; 4:28–41.
- Schuman-Olivier Z, Albanese M, Nelson SE, et al. Self-treatment: illicit buprenorphine use by opioid-dependent treatment seekers. J Subst Abuse Treat 2010; 39:41–50.
- American Society of Addiction Medicine. National practice guidelines for the use of medications in the treatment of addiction involving opioid use. www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf. Accessed April 6, 2017.
- McNicholas L. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Service Administration; 2004.
- Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2004. (Treatment Improvement Protocol (TIP) Series, No. 40.) www.ncbi.nlm.nih.gov/books/NBK64245. Accessed April 6, 2017.
- Zippel-Schultz B, Specka M, Cimander K, et al. Outcomes of patients in long-term opioid maintenance treatment. Subst Use Misuse 2016; 51:1493–1503.
- Martins SS, Keyes KM, Storr CL, Zhu H, Chilcoat HD. Pathways between nonmedical opioid use/dependence and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 2009; 103:16–24.
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 1985; 142:1259–1264.
- Belding MA, Iguchi MY, Lamb RJ, Lakin M, Terry R. Stages and processes of change among polydrug users in methadone maintenance treatment. Drug Alcohol Depend 1995; 39:45–53.
- Peteet JR, Brenner S, Curtiss D, Ferrigno M, Kauffman J. A stage of change approach to addiction in the medical setting. Gen Hosp Psychiatry 1998; 20:267–273.
- Vijay A, Bazazi AR, Yee I, Kamarulzaman A, Altice FL. Treatment readiness, attitudes toward, and experiences with methadone and buprenorphine maintenance therapy among people who inject drugs in Malaysia. J Subst Abuse Treat 2015; 54:29–36.
- Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: results from a survey of general internists. Subst Abus 2016; 37:635–641.
- Samuels EA, Dwyer K, Mello MJ, Baird J, Kellogg AR, Bernstein E. Emergency department-based opioid harm reduction: moving physicians from willing to doing. Acad Emerg Med 2016; 23:455–465.
- Mohlman MK, Tanzman B, Finison K, Pinette M, Jones C. Impact of medication-assisted treatment for opioid addiction on Medicaid expenditures and health services utilization rates in Vermont. J Subst Abuse Treat 2016; 67:9–14.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA, American Psychiatric Association, 2013.
- Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health. www.samhsa.gov/data. Accessed April 6, 2017.
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016; 374:154–163.
- Ruan X, Wyche MQ, Kaye AD. Analyzing the relationship between nonmedical prescription-opioid use and heroin use. J Opioid Manage 2016; 12:11–14.
- Hser YI, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harv Rev Psychiatry 2015; 23:76–89.
- Nielsen S, Hillhouse M, Mooney L, Ang A, Ling W. Buprenorphine pharmacotherapy and behavioral treatment: comparison of outcomes among prescription opioid users, heroin users and combination users. J Subst Abuse Treat 2015; 48:70–76.
- Moore BA, Fiellin DA, Barry DT, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med 2007; 22:527–530.
- Fischer B, Patra J, Cruz MF, Gittins J, Rehm J. Comparing heroin users and prescription opioid users in a Canadian multi-site population of illicit opioid users. Drug Alcohol Rev 2008; 27:625–632.
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016; 374:154–163.
- Jones CM, Baldwin GT, Manocchio T, White JO, Mack KA. Trends in methadone distribution for pain treatment, methadone diversion, and overdose deaths—United States, 2002–2014. MMWR Morb Mortal Wkly Rep 2016; 65:667–671.
- Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med 2013; 7:377–386.
- Alinejad S, Kazemi T, Zamani N, Hoffman RS, Mehrpour O. A systematic review of the cardiotoxicity of methadone. EXCLI J 2015; 14:577–600.
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend 2003; 70(suppl 2):S59–S77.
- Ling W. Buprenorphine implant for opioid addiction. Pain Manage 2012; 2:345–350.
- Saxon AJ, Hser YI, Woody G, Ling W. Medication-assisted treatment for opioid addiction: methadone and buprenorphine. J Food Drug Anal 2013; 21:S69–S72.
- Kimber J, Larney S, Hickman M, Randall D, Degenhardt L. Mortality risk of opioid substitution therapy with methadone versus buprenorphine: a retrospective cohort study. Lancet Psychiatry 2015; 2:901–908.
- Marteau D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open 2015; 5: e007629.
- Kintz P. Deaths involving buprenorphine: a compendium of French cases. Forensic Sci Int 2001; 121:65–69.
- Zuin M, Giorgini A, Selmi C, et al. Acute liver and renal failure during treatment with buprenorphine at therapeutic dose. Dig Liver Dis 2009; 41:e8–e10.
- Klein JW. Pharmacotherapy for substance use disorders. Med Clin North Am 2016; 100:891–910.
- Modesto-Lowe V, Van Kirk J. Clinical uses of naltrexone: a review of the evidence. Exp Clin Psychopharmocol 2002; 10:213–227.
- Woody GE. Agonist models for treating persons with substance use disorders. Curr Psychiatry Rep 2014; 16:489.
- Sanders JJ, Roose RJ, Lubrano MC, Lucan SC. Meaning and methadone: patient perceptions of methadone dose and a model to promote adherence to maintenance treatment. J Addict Med 2013; 7:307–313.
- Herget G. Methadone and buprenorphine added to the WHO list of essential medicines. HIV/AIDS Policy Law Rev 2005; 10:23–24.
- Suzuki J, Dodds T. Clinical recommendation of 12-step meeting attendance and discussion regarding disclosure of buprenorphine use among patients in office-based opioid treatment. Subst Abus 2016; 37:31–34.
- Rettig RA, Yarmolinsky A. Federal Regulation of Methadone Treatment. Washington, DC: National Academies Press; 1995.
- Srivastava A, Kahan M, Nader M. Primary care management of opioid use disorders: abstinence, methadone, or buprenorphine-naloxone? Can Fam Physician 2017; 63:200–205.
- Substance Abuse and Mental Health Services Administration. Federal Guidelines for Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
- Substance Abuse and Mental Health Services Administration SAMSHA. Buprenorphine waiver management. www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management. Accessed April 6, 2017.
- Mark TL, Lubran R, McCance-Kats EF, Chalk M, Richardson J. Medicaid coverage of medications to treat alcohol and opioid dependence. J Subst Abuse Treat 2015; 55:1–5.
- Johnson B, Richert T. Diversion of methadone and buprenorphine from opioid substitution treatment: the importance of patients’ attitudes and norms. J Subst Abuse Treat 2015; 54:50–55.
- Yokell MA, Zaller ND, Green TC, Rich JD. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review. Curr Drug Abuse Rev 2011; 4:28–41.
- Schuman-Olivier Z, Albanese M, Nelson SE, et al. Self-treatment: illicit buprenorphine use by opioid-dependent treatment seekers. J Subst Abuse Treat 2010; 39:41–50.
- American Society of Addiction Medicine. National practice guidelines for the use of medications in the treatment of addiction involving opioid use. www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf. Accessed April 6, 2017.
- McNicholas L. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Service Administration; 2004.
- Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2004. (Treatment Improvement Protocol (TIP) Series, No. 40.) www.ncbi.nlm.nih.gov/books/NBK64245. Accessed April 6, 2017.
- Zippel-Schultz B, Specka M, Cimander K, et al. Outcomes of patients in long-term opioid maintenance treatment. Subst Use Misuse 2016; 51:1493–1503.
- Martins SS, Keyes KM, Storr CL, Zhu H, Chilcoat HD. Pathways between nonmedical opioid use/dependence and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 2009; 103:16–24.
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 1985; 142:1259–1264.
- Belding MA, Iguchi MY, Lamb RJ, Lakin M, Terry R. Stages and processes of change among polydrug users in methadone maintenance treatment. Drug Alcohol Depend 1995; 39:45–53.
- Peteet JR, Brenner S, Curtiss D, Ferrigno M, Kauffman J. A stage of change approach to addiction in the medical setting. Gen Hosp Psychiatry 1998; 20:267–273.
- Vijay A, Bazazi AR, Yee I, Kamarulzaman A, Altice FL. Treatment readiness, attitudes toward, and experiences with methadone and buprenorphine maintenance therapy among people who inject drugs in Malaysia. J Subst Abuse Treat 2015; 54:29–36.
KEY POINTS
- Opioid use disorder is potentially lethal and has become more prevalent in the United States over the past few decades.
- The opioid agonist methadone and the partial agonist buprenorphine are the currently recommended treatments for patients who need opioid maintenance therapy. However, they carry the risk of adverse effects (eg, respiratory depression, QTc interval prolongation, hepatotoxicity), diversion, and overdose.
- Patients being considered for opioid agonist therapy need a comprehensive assessment including a thorough medical history and physical examination, psychiatric evaluation, psychosocial appraisal, and determination of readiness to change.
- When methadone and buprenorphine are properly prescribed they confer significant benefits, including reduction or elimination of opioid use, reductions in overdose risk, and positive changes in behavior and lifestyle.
Universal precautions to reduce stimulant misuse in treating adult ADHD
Children are not the only people affected by attention-deficit/hyperactivity disorder (ADHD). Characterized by high levels of inattention, overactivity, and impulsivity, ADHD affects 5% of school-aged children, but also 4% of adults.1–3 Adults with untreated ADHD are likely to develop serious psychosocial problems that manifest as unemployment, arrests, divorce, underachievement, and psychiatric comorbidities.4,5
However, many clinicians are reluctant to manage adults with ADHD, partly because of concerns about misuse of the stimulant drugs they must prescribe to treat it.
Here, we outline an approach whereby clinicians can diagnose and treat adult ADHD while taking “universal precautions” to discourage misuse of the medications involved.
RECOGNIZING ADHD IN ADULTS
ADHD is characterized by developmentally inappropriate levels of inattention, impulsiveness, and hyperactivity that arise in childhood and result in impairments that often persist.
The presentation of ADHD in adults may be influenced by the longevity of their ADHD, associated sequelae (eg, low self-esteem and interpersonal, educational, and occupational difficulties), and comorbid disorders.6 There are neither reliable biomarkers nor neuropsychological tests for diagnosis, and persons with ADHD typically have a complex presentation with at least one comorbidity.6,7
In patients diagnosed in childhood, difficulties with organization as well as initiating, maintaining, and completing tasks become more prominent in adulthood and hyperactivity tends to subside. Adult impulsivity may present as edginess, shopping sprees, quitting jobs, and risky behaviors.6
Overall, the clinical manifestations of ADHD in adolescents and adults include inattention, difficulties with task completion, disorganization, and executive dysfunction—all skills critical to managing the various roles of adult life.
OBSTACLES TO EFFECTIVE TREATMENT
In the past, ADHD treatment was routinely discontinued during adolescence, as it was unclear whether adults still had significant symptoms or benefited from treatment.8,9 Now, available ADHD guidelines suggest that children and adults who respond to pharmacotherapy should continue it for as long as it remains effective. In this context, there is increasing recognition of adult ADHD as a valid and treatable disorder.10
One of the challenges clinicians face is the reliability of adult recall of childhood ADHD. A controlled, prospective 16-year follow-up study found that of all adults retrospectively given a diagnosis of childhood ADHD, only 27% actually had the disorder.11 This study suggests that retrospective diagnoses of childhood ADHD made solely on the basis of self-reports are unlikely to be valid.
Another obstacle is that traditional medical education has seldom included training in adult ADHD.8,12 In a UK study, clinicians felt that they lacked training and knowledge to assess and manage adult ADHD patients.9
Even if adult ADHD is recognized, diagnosis is just the first step of care.13 These patients require ongoing management and follow-up assessments.
Although practice patterns vary, efforts to encourage doctors to provide adult ADHD care may be hindered by the fact that the gold standard of treatment is stimulant medication.4,10 Medications approved by the US Food and Drug Administration for adult ADHD include the stimulants lisdexamfetamine, osmotic-release methylphenidate, mixed amphetamine salts extended release, dexmethylphenidate extended release, and the nonstimulant atomoxetine.6 While stimulants are generally more efficacious for ADHD symptoms than nonstimulants, they are associated with misuse and diversion.14
UNIVERSAL PRECAUTIONS: A SIMPLIFIED APPROACH
The universal-precautions approach to prescribing stimulants aims to allay physician concerns and promote appropriate medication use to allow for proper management of this disorder.15 These precautions, to be applied to all adult ADHD patients for whom stimulants are being considered, include careful diagnosis and consideration of comorbidities, baseline risk stratification, informed consent processes, treatment agreements, periodic reassessments of treatment response, and meticulous documentation.
DIAGNOSIS
A frequently used screening assessment for adult ADHD is the ADHD Rating Scale (ADHD RS), which consists of two subscales for assessing hyperactivity/impulsivity and inattentiveness.16 ADHD can be classified into one of three subtypes based on symptoms: inattentive, hyperactive, or combined type. Symptoms must persist for at least 6 months for a diagnosis to be made. Other ADHD scales include the Conners Adult ADHD Rating Scales and the Brown Attention-Deficit Disorder Scales.4
High scores on screening scales must be interpreted within the clinical context. Clinicians need to ask about ADHD symptoms, establish their presence in various settings, and determine if these symptoms interfere with functioning. A diagnosis of adult ADHD also requires evidence of symptoms beginning in childhood.17 According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, inattentive or hyperactive-impulsive symptoms must be present before age 12 in two or more settings and interfere with function and development.
Although self-reporting screening tools are helpful, these tests are not reliable for diagnostic purposes, and collateral information is also required.
Neuropsychological testing may detect impairments in persons with ADHD. The most consistently employed neuropsychological tests to evaluate ADHD include the Conners Continuous Performance Test, Stroop Color and Word Test, Trail-making Test, verbal fluency tests, Controlled Oral Word Association Test, and the Weschler Adult Intelligence Scale.6
COMORBIDITY
Epidemiologic studies suggest that adults with ADHD develop many psychiatric problems including anxiety, depression, and substance use disorders.7,16 Table 1 illustrates common comorbidities and their associated prevalence in the ADHD patient.7
Comorbid psychiatric disorders may affect the presentation of adult ADHD. For instance, adults with comorbid depression and ADHD are more likely to present with heightened irritability and difficulties concentrating on tasks than those with either condition alone.18 Similarly, antisocial personality disorder is more common in adults with ADHD.19 Such patients exhibit stable antisocial behavior (lying, stealing, and aggression) as well as medication misuse.5,14,19
While these comorbid disorders may obscure the ADHD diagnosis, their recognition is essential to effectively manage adult ADHD. In sum, a careful evaluation of the adult, including elucidating both ADHD and comorbid symptoms, functionality in several domains, and the degree of impairment, should precede initiating pharmacotherapy for adult ADHD.
BASELINE RISK STRATIFICATION: RISK FACTORS FOR STIMULANT MISUSE
After diagnosing ADHD, the prescriber must assess the risk for misuse of stimulant medications.20
One study revealed that nonmedical use of stimulant medications occurred in only 2% of the 4,300 people surveyed.21 Among the misusers, 66% had obtained medication from family or friends. Another 34% had stolen medication, and 20% had obtained prescriptions from a physician by falsely reporting symptoms. The study also assessed motivation for misuse. In this sample, 40% of misuse was to enhance performance, 34% was for recreation, and 23% was to stay awake.21
Other studies show that misuse of stimulant medications is common among youth in the United States, reporting that 18% of college students use some formulation of prescription stimulants.22
Still more research suggests that childhood conduct disorder or illicit drug use results in a higher risk of stimulant medication misuse.20 Additional risk factors for misuse include male sex, white ethnicity, upper-class background, Jewish or no religious affiliation, affiliation with a sorority or fraternity, off-campus housing, and a low grade-point average.23
Table 2 illustrates clinical interventions providers can use, once they have risk-stratified their patients, to monitor for stimulant misuse.
HOW SHOULD THESE RISK FACTORS AFFECT TREATMENT?
Although no formal scoring system exists to help clinicians risk-stratify these patients, the presence of multiple risk factors suggests the need for vigilance.14 Physicians should prescribe agents with less potential for abuse and monitor these patients more intensely.
Short-acting stimulant medications are the most likely to be abused, as phasic dopamine increase is more reinforcing than therapeutic dopamine release.24 Longer-acting stimulant medications are less likely to be abused, and they provide better symptom relief, as tonic dopamine release maintains a steady state and increases the therapeutic efficacy of these medications.25 For example, methylphenidate extended-release tablets have an osmotic oral controlled-release system and are less likely to be crushed for recreational inhalation.6,14
Lisdexamfetamine is a prodrug therapeutically inert until converted to d-amphetamine when lysine is cleaved from the molecule. This medication may be a good option for patients at high risk of misuse because it is tamper-resistant. However, it still may be subject to misuse for performance enhancement.26,27
The nonstimulant atomoxetine is also approved for ADHD, has no abuse potential, and may be particularly useful when anxiety, mood, and substance use disorders co-occur with ADHD.6 Rarely, atomoxetine can damage the liver, and liver function tests should be monitored if right upper quadrant pain develops.4,10
Other nonapproved agents such as bupropion and desipramine also have been used empirically and off-label for ADHD.4,10
Overall, treatment should be selected according to the risk of misuse of stimulant medication and the patient’s comorbidities.
INFORMED CONSENT
Informed consent may help patients appreciate the risks and benefits of the treatment options and develop realistic expectations about treatment.26 Patients are instructed to take their stimulant medications as prescribed and are informed of the risks of combining stimulants with other substances, particularly those that may interact with stimulants (eg, cocaine) and raise the risk of seizures and cardiovascular complications.
Stimulant medications lead to elevations in blood pressure and heart rate, although large-scale studies have shown no increase in the rate of serious cardiovascular events when these drugs are used appropriately.6 Less serious side effects associated with stimulant medications include insomnia, weight loss, decreased appetite, dry mouth, headache, and rarely, depression and anxiety.6
Patients need to be warned about diversion and abuse liability of stimulant medications, as well as alternative treatments.
The nonstimulant atomoxetine has no reinforcing properties but also raises the blood pressure and heart rate.6 As with stimulants, these elevations are generally minimal, time-limited, and of minor clinical significance.4,10 Frequent reasons to prescribe atomoxetine include poor tolerability of stimulants and a history of substance abuse. In addition, women with ADHD and high levels of emotional dysregulation or social anxiety appear to be particularly responsive to atomoxetine.6
Another consideration is cognitive behavioral therapy, which can augment the effects of pharmacotherapy.4 Cognitive behavioral therapy focuses on time management, prioritization, organization, problem-solving, motivation, and emotional regulation.4
Finally, patients also need to understand the possible consequences of nontreatment.5 Adults with untreated ADHD have high rates of academic and occupational difficulties, anti-social behaviors, and other forms of psychosocial adversity.4,5
Overall, this process should involve discussing risks and benefits of treatment options with the patient and promoting joint decision-making.
TREATMENT AGREEMENTS
Stimulant medications are classified by the US Drug Enforcement Administration as schedule II substances due to their abuse potential.20
It is important to inform patients of the addictive nature of the medication and to instruct them on how to store stimulants safely.27 Patients need to know that giving away or selling these medications is illegal.27
Treatment agreements establish rules for prescribing and are signed by the patient before initiating therapy.28,29 Patients are expected to attend all of their appointments, receive their prescriptions from one doctor, and obtain their medication from one pharmacy. These agreements may also require patients to submit to monitoring with random urine drug screens.29 Overall, they underscore the need for patients to follow a treatment plan in order to continue therapy with controlled substances.29
Manning27 recommends using agreements for high-risk college students prescribed stimulant medications. Red flags for misuse include signs of active substance use (eg, intoxication, a pattern of “lost” prescriptions, and doctor-shopping).27
The effectiveness in reducing risk of misuse in the adult ADHD population has not yet been investigated. Nonetheless, a method of communicating the seriousness of stimulant misuse to adult patients is essential to ADHD care.
STAYING ON TRACK
In the clinical setting, treatment response is measured not just by symptom reduction, but also by functional improvement. Thus, clinicians and patients must set functional goals whenever possible.27 Successful progress toward these goals justifies continuation of therapy, whereas lack of improvement signals the need to reconsider stimulant therapy.27
MONITORING AND DOCUMENTATION
Adults with ADHD present with varying levels of functional impairment and comorbidities, which may require different levels of monitoring.30 Not all patients with ADHD respond optimally to stimulant medications or tolerate them well.31,32 Hence, monitoring parameters for therapeutic change and adverse outcomes are important in that they guide the alteration or even discontinuation of pharmacotherapy.4,6,14
Documenting the decision-making process to continue stimulant medications under certain circumstances is also essential. Documentation should include discussion of goals and expectations, risks and benefits, and alternatives to stimulant use.
In adults, risk of stimulant medication misuse adds a new layer of complexity to monitoring.13,14 Adults may get multiple prescriptions from multiple providers, seek early refills, fill prescriptions at different pharmacies, or alter formulations. Many states track stimulant prescription use, and prescribers can use this information to determine if patients are refilling their prescriptions appropriately or obtaining stimulants from more than one provider.
Although these monitoring strategies are useful,6 it is prudent to structure the level of monitoring according to the patient’s risk of adverse events or medication misuse.14,27 Gourlay and Heit15 proposed the following “four-A” mnemonic for four domains to be explored at each visit in patients receiving pain medicine. This mnemonic can be applied to adult ADHD patients to more accurately monitor the patient throughout treatment.
THE ‘FOUR-A’ MNEMONIC
ADHD symptoms
Several ADHD scales can be used to track symptom changes over time.33 However, these self-report scales may be subject to positive illusory bias, a phenomenon observed in individuals with ADHD in which they tend to overrate their functioning,34 which may limit the accuracy of self-report scales.35
Activities of daily living
Since patients with ADHD tend to overrate their functioning in various aspects of living, collateral information should be gathered to corroborate patient self-reports whenever possible.
Adverse events
Blood pressure, heart rate, and weight should be assessed at baseline and monitored during stimulant treatment. Other symptoms to monitor include gastrointestinal distress, headache, aggression, depression, appetite, and sleeping habits.4,6 More intensive monitoring (eg, electrocardiography) may be indicated for those with hypertension and cardiovascular risk factors.
Aberrant behavior
Monitoring for misuse and diversion of stimulant medications is essential, as ADHD itself is a risk factor for addiction.20,21 Pill counts, prescription monitoring programs, urine drug screens, and collateral informants have all been proposed but not studied in monitoring for the misuse of stimulant medications.27 Before prescribing, it is prudent to check the prescription monitoring program, get a urine drug screen, and discuss any positive findings with the patient.36,37
Treatment agreements ensure that patients are aware of the consequences of misuse and allow the clinician to reference prior discussion when terminating treatment with stimulants.
LIVES CAN BE ENHANCED
ADHD is a common disorder that arises in childhood and can persist throughout life. Adults with untreated ADHD are at risk of severe impairments in various domains of functioning. Stimulant medications are an effective treatment but may be diverted into the street market. Using the universal-precautions model may reduce the risks of both nontreatment of ADHD and misuse of stimulants.
Accordingly, clinicians need to confirm the ADHD diagnosis, assess comorbidities, estimate risk of misuse, and provide informed consent prior to prescribing. Subsequent monitoring should involve the use of treatment agreements and evaluating treatment response, paying particular attention to ADHD symptom control but also to level of function, adverse effects, and aberrant behavior.
With these principles in mind, clinicians can address the risks of misuse and potentially enhance the lives of people who may have been suffering substantially due to lack of appropriate care.
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007; 164:942–948.
- Polanczyk GV, Wilcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 2014; 43:434–442.
- Wilens TE. ADHD: Prevalence, diagnosis, and issues of comorbidity. CNS Spectr 2007; 12(suppl 6):1–5.
- Kooij SJ, Bejerot S, Blackwell A, et al. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry 2010; 10:67.
- Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med 2012;10:99.
- Modesto-Lowe V, Meyer A, Soovajian V. A clinician’s guide to adult attention-deficit hyperactivity disorder. Conn Med 2012; 76:517–523.
- Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006; 163:716–723.
- Goodman DW, Surman CB, Scherer PB, Salinas GD, Brown JJ. Assessment of physician practices in adult attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord 2012; 14(4).
- Hall CL, Newell K, Taylor J, Sayal K, Swift KD, Hollis C. ‘Mind the gap’—mapping services for young people with ADHD transitioning from child to adult mental health services. BMC Psychiatry 2013; 13:186.
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management of ADHD in children, young people and adults. The British Psychological Society and The Royal College of Psychiatrists: United Kingdom; 2009.
- Mannuzza S, Klein RG, Klein DF, Bessler A, Shrout P. Accuracy of adult recall of childhood attention deficit hyperactivity disorder. Am J Psychiatry 2002; 159:1882–1888.
- Wetzel MW. Medical student participation in an adult ADHD outpatient clinic: an ideal setting for education in outpatient psychiatry. Acad Psychiatry 2009; 33:80–81.
- Culpepper L, Mattingly G. Challenges in identifying and managing attention-deficit/hyperactivity disorder in adults in the primary care setting: a review of the literature. Prim Care Companion J Clin Psychiatry 2010; 12(6).
- Rabiner DL. Stimulant prescription cautions: addressing misuse, diversion and malingering. Curr Psychiatry Rep 2013; 15:375.
- Gourlay D, Heit H. Universal precautions: a matter of mutual trust and responsibility. Pain Med 2006; 7:210–211.
- Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005; 35:245–256.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- CADDRA Guidelines Steering Committee. Canadian ADHD practice guidelines: CADDRA 2008. http://www.naceonline.com/AdultADHDtoolkit/professionalresources/caddraguidelines.pdf. Accessed July 10, 2015.
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry 1998; 155:493–498.
- Kaye S, Darke S. The diversion and misuse of pharmaceutical stimulants: what do we know and why should we care? Addiction 2012; 107:467–477.
- Novak SP, Kroutil LA, Williams RL, Van Brunt DL. The nonmedical use of prescription ADHD medications: results from a national Internet panel. Subst Abuse Treat Prev Policy 2007; 2:32.
- Bavarian N, Flay BR, Ketcham P, et al. Using structural equation modeling to understand prescription stimulant misuse: a test of the Theory of Triadic Influence. Drug Alcohol Depend 2014; 138:193–201.
- McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs 2006; 38:43–56.
- Volkow ND. Stimulant medications: how to minimize their reinforcing effects? Am J Psychiatry 2006; 163:359–361.
- Kolar D, Keller A, Golfinopoulos M, Cumyn L, Syer C, Hechtman L. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat 2008; 4:107–121.
- Schachter D, Tharmalingam S, Kleinman I. Informed consent and stimulant medication: adolescents’ and parents’ ability to understand information about benefits and risks of stimulant medication for the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2011; 21:139–148.
- Manning JS. Strategies for managing the risks associated with ADHD medications. J Clin Psychiatry 2013; 74:e19.
- Deep K. Use of narcotics contracts. Virtual Mentor 2013; 15:416–420.
- Cheatle MD, Savage SR. Informed consent in opioid therapy: a potential obligation and opportunity. J Pain Symptom Manage 2012; 44:105–116.
- Dias TG, Kieling C, Graeff-Martins AS, Moriyama TS, Rohde LA, Polanczyk GV. Developments and challenges in the diagnosis and treatment of ADHD. Rev Bras Psiquiatr 2013; 35(suppl 1):S40–S50.
- Mattingly GW, Weisler RH, Young J, et al. Clinical response and symptomatic remission in short- and long-term trials of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2013; 13:39.
- Contini V, Victor MM, Bertuzzi GP, et al. No significant association between genetic variants in 7 candidate genes and response to methylphenidate treatment in adult patients with ADHD. J Clin Psychopharmacol 2012; 32:820–823.
- Rösler M, Retz W, Thome J, Schneider M, Stieglitz RD, Falkai P. Psychopathological rating scales for diagnostic use in adults with attention-deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci 2006; 256(suppl 1):i3–i11.
- Prevatt F, Proctor B, Best L, Baker L, Van Walker J, Taylor NW. The positive illusory bias: does it explain self-evaluations in college students with ADHD? J Atten Disord 2012; 16:235–243.
- Jiang Y, Johnston C. The relationship between ADHD symptoms and competence as reported by both self and others. J Atten Disord 2012; 16:418–426.
- Darredeau C, Barrett SP, Jardin B, Pihl RO. Patterns and predictors of medication compliance, diversion, and misuse in adult prescribed methylphenidate users. Hum Psychopharmacol 2007; 22:529–536.
- Worley J. Prescription drug monitoring programs, a response to doctor shopping: purpose, effectiveness, and directions for future research. Issues Ment Health Nurs 2012; 33:319–328.
Children are not the only people affected by attention-deficit/hyperactivity disorder (ADHD). Characterized by high levels of inattention, overactivity, and impulsivity, ADHD affects 5% of school-aged children, but also 4% of adults.1–3 Adults with untreated ADHD are likely to develop serious psychosocial problems that manifest as unemployment, arrests, divorce, underachievement, and psychiatric comorbidities.4,5
However, many clinicians are reluctant to manage adults with ADHD, partly because of concerns about misuse of the stimulant drugs they must prescribe to treat it.
Here, we outline an approach whereby clinicians can diagnose and treat adult ADHD while taking “universal precautions” to discourage misuse of the medications involved.
RECOGNIZING ADHD IN ADULTS
ADHD is characterized by developmentally inappropriate levels of inattention, impulsiveness, and hyperactivity that arise in childhood and result in impairments that often persist.
The presentation of ADHD in adults may be influenced by the longevity of their ADHD, associated sequelae (eg, low self-esteem and interpersonal, educational, and occupational difficulties), and comorbid disorders.6 There are neither reliable biomarkers nor neuropsychological tests for diagnosis, and persons with ADHD typically have a complex presentation with at least one comorbidity.6,7
In patients diagnosed in childhood, difficulties with organization as well as initiating, maintaining, and completing tasks become more prominent in adulthood and hyperactivity tends to subside. Adult impulsivity may present as edginess, shopping sprees, quitting jobs, and risky behaviors.6
Overall, the clinical manifestations of ADHD in adolescents and adults include inattention, difficulties with task completion, disorganization, and executive dysfunction—all skills critical to managing the various roles of adult life.
OBSTACLES TO EFFECTIVE TREATMENT
In the past, ADHD treatment was routinely discontinued during adolescence, as it was unclear whether adults still had significant symptoms or benefited from treatment.8,9 Now, available ADHD guidelines suggest that children and adults who respond to pharmacotherapy should continue it for as long as it remains effective. In this context, there is increasing recognition of adult ADHD as a valid and treatable disorder.10
One of the challenges clinicians face is the reliability of adult recall of childhood ADHD. A controlled, prospective 16-year follow-up study found that of all adults retrospectively given a diagnosis of childhood ADHD, only 27% actually had the disorder.11 This study suggests that retrospective diagnoses of childhood ADHD made solely on the basis of self-reports are unlikely to be valid.
Another obstacle is that traditional medical education has seldom included training in adult ADHD.8,12 In a UK study, clinicians felt that they lacked training and knowledge to assess and manage adult ADHD patients.9
Even if adult ADHD is recognized, diagnosis is just the first step of care.13 These patients require ongoing management and follow-up assessments.
Although practice patterns vary, efforts to encourage doctors to provide adult ADHD care may be hindered by the fact that the gold standard of treatment is stimulant medication.4,10 Medications approved by the US Food and Drug Administration for adult ADHD include the stimulants lisdexamfetamine, osmotic-release methylphenidate, mixed amphetamine salts extended release, dexmethylphenidate extended release, and the nonstimulant atomoxetine.6 While stimulants are generally more efficacious for ADHD symptoms than nonstimulants, they are associated with misuse and diversion.14
UNIVERSAL PRECAUTIONS: A SIMPLIFIED APPROACH
The universal-precautions approach to prescribing stimulants aims to allay physician concerns and promote appropriate medication use to allow for proper management of this disorder.15 These precautions, to be applied to all adult ADHD patients for whom stimulants are being considered, include careful diagnosis and consideration of comorbidities, baseline risk stratification, informed consent processes, treatment agreements, periodic reassessments of treatment response, and meticulous documentation.
DIAGNOSIS
A frequently used screening assessment for adult ADHD is the ADHD Rating Scale (ADHD RS), which consists of two subscales for assessing hyperactivity/impulsivity and inattentiveness.16 ADHD can be classified into one of three subtypes based on symptoms: inattentive, hyperactive, or combined type. Symptoms must persist for at least 6 months for a diagnosis to be made. Other ADHD scales include the Conners Adult ADHD Rating Scales and the Brown Attention-Deficit Disorder Scales.4
High scores on screening scales must be interpreted within the clinical context. Clinicians need to ask about ADHD symptoms, establish their presence in various settings, and determine if these symptoms interfere with functioning. A diagnosis of adult ADHD also requires evidence of symptoms beginning in childhood.17 According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, inattentive or hyperactive-impulsive symptoms must be present before age 12 in two or more settings and interfere with function and development.
Although self-reporting screening tools are helpful, these tests are not reliable for diagnostic purposes, and collateral information is also required.
Neuropsychological testing may detect impairments in persons with ADHD. The most consistently employed neuropsychological tests to evaluate ADHD include the Conners Continuous Performance Test, Stroop Color and Word Test, Trail-making Test, verbal fluency tests, Controlled Oral Word Association Test, and the Weschler Adult Intelligence Scale.6
COMORBIDITY
Epidemiologic studies suggest that adults with ADHD develop many psychiatric problems including anxiety, depression, and substance use disorders.7,16 Table 1 illustrates common comorbidities and their associated prevalence in the ADHD patient.7
Comorbid psychiatric disorders may affect the presentation of adult ADHD. For instance, adults with comorbid depression and ADHD are more likely to present with heightened irritability and difficulties concentrating on tasks than those with either condition alone.18 Similarly, antisocial personality disorder is more common in adults with ADHD.19 Such patients exhibit stable antisocial behavior (lying, stealing, and aggression) as well as medication misuse.5,14,19
While these comorbid disorders may obscure the ADHD diagnosis, their recognition is essential to effectively manage adult ADHD. In sum, a careful evaluation of the adult, including elucidating both ADHD and comorbid symptoms, functionality in several domains, and the degree of impairment, should precede initiating pharmacotherapy for adult ADHD.
BASELINE RISK STRATIFICATION: RISK FACTORS FOR STIMULANT MISUSE
After diagnosing ADHD, the prescriber must assess the risk for misuse of stimulant medications.20
One study revealed that nonmedical use of stimulant medications occurred in only 2% of the 4,300 people surveyed.21 Among the misusers, 66% had obtained medication from family or friends. Another 34% had stolen medication, and 20% had obtained prescriptions from a physician by falsely reporting symptoms. The study also assessed motivation for misuse. In this sample, 40% of misuse was to enhance performance, 34% was for recreation, and 23% was to stay awake.21
Other studies show that misuse of stimulant medications is common among youth in the United States, reporting that 18% of college students use some formulation of prescription stimulants.22
Still more research suggests that childhood conduct disorder or illicit drug use results in a higher risk of stimulant medication misuse.20 Additional risk factors for misuse include male sex, white ethnicity, upper-class background, Jewish or no religious affiliation, affiliation with a sorority or fraternity, off-campus housing, and a low grade-point average.23
Table 2 illustrates clinical interventions providers can use, once they have risk-stratified their patients, to monitor for stimulant misuse.
HOW SHOULD THESE RISK FACTORS AFFECT TREATMENT?
Although no formal scoring system exists to help clinicians risk-stratify these patients, the presence of multiple risk factors suggests the need for vigilance.14 Physicians should prescribe agents with less potential for abuse and monitor these patients more intensely.
Short-acting stimulant medications are the most likely to be abused, as phasic dopamine increase is more reinforcing than therapeutic dopamine release.24 Longer-acting stimulant medications are less likely to be abused, and they provide better symptom relief, as tonic dopamine release maintains a steady state and increases the therapeutic efficacy of these medications.25 For example, methylphenidate extended-release tablets have an osmotic oral controlled-release system and are less likely to be crushed for recreational inhalation.6,14
Lisdexamfetamine is a prodrug therapeutically inert until converted to d-amphetamine when lysine is cleaved from the molecule. This medication may be a good option for patients at high risk of misuse because it is tamper-resistant. However, it still may be subject to misuse for performance enhancement.26,27
The nonstimulant atomoxetine is also approved for ADHD, has no abuse potential, and may be particularly useful when anxiety, mood, and substance use disorders co-occur with ADHD.6 Rarely, atomoxetine can damage the liver, and liver function tests should be monitored if right upper quadrant pain develops.4,10
Other nonapproved agents such as bupropion and desipramine also have been used empirically and off-label for ADHD.4,10
Overall, treatment should be selected according to the risk of misuse of stimulant medication and the patient’s comorbidities.
INFORMED CONSENT
Informed consent may help patients appreciate the risks and benefits of the treatment options and develop realistic expectations about treatment.26 Patients are instructed to take their stimulant medications as prescribed and are informed of the risks of combining stimulants with other substances, particularly those that may interact with stimulants (eg, cocaine) and raise the risk of seizures and cardiovascular complications.
Stimulant medications lead to elevations in blood pressure and heart rate, although large-scale studies have shown no increase in the rate of serious cardiovascular events when these drugs are used appropriately.6 Less serious side effects associated with stimulant medications include insomnia, weight loss, decreased appetite, dry mouth, headache, and rarely, depression and anxiety.6
Patients need to be warned about diversion and abuse liability of stimulant medications, as well as alternative treatments.
The nonstimulant atomoxetine has no reinforcing properties but also raises the blood pressure and heart rate.6 As with stimulants, these elevations are generally minimal, time-limited, and of minor clinical significance.4,10 Frequent reasons to prescribe atomoxetine include poor tolerability of stimulants and a history of substance abuse. In addition, women with ADHD and high levels of emotional dysregulation or social anxiety appear to be particularly responsive to atomoxetine.6
Another consideration is cognitive behavioral therapy, which can augment the effects of pharmacotherapy.4 Cognitive behavioral therapy focuses on time management, prioritization, organization, problem-solving, motivation, and emotional regulation.4
Finally, patients also need to understand the possible consequences of nontreatment.5 Adults with untreated ADHD have high rates of academic and occupational difficulties, anti-social behaviors, and other forms of psychosocial adversity.4,5
Overall, this process should involve discussing risks and benefits of treatment options with the patient and promoting joint decision-making.
TREATMENT AGREEMENTS
Stimulant medications are classified by the US Drug Enforcement Administration as schedule II substances due to their abuse potential.20
It is important to inform patients of the addictive nature of the medication and to instruct them on how to store stimulants safely.27 Patients need to know that giving away or selling these medications is illegal.27
Treatment agreements establish rules for prescribing and are signed by the patient before initiating therapy.28,29 Patients are expected to attend all of their appointments, receive their prescriptions from one doctor, and obtain their medication from one pharmacy. These agreements may also require patients to submit to monitoring with random urine drug screens.29 Overall, they underscore the need for patients to follow a treatment plan in order to continue therapy with controlled substances.29
Manning27 recommends using agreements for high-risk college students prescribed stimulant medications. Red flags for misuse include signs of active substance use (eg, intoxication, a pattern of “lost” prescriptions, and doctor-shopping).27
The effectiveness in reducing risk of misuse in the adult ADHD population has not yet been investigated. Nonetheless, a method of communicating the seriousness of stimulant misuse to adult patients is essential to ADHD care.
STAYING ON TRACK
In the clinical setting, treatment response is measured not just by symptom reduction, but also by functional improvement. Thus, clinicians and patients must set functional goals whenever possible.27 Successful progress toward these goals justifies continuation of therapy, whereas lack of improvement signals the need to reconsider stimulant therapy.27
MONITORING AND DOCUMENTATION
Adults with ADHD present with varying levels of functional impairment and comorbidities, which may require different levels of monitoring.30 Not all patients with ADHD respond optimally to stimulant medications or tolerate them well.31,32 Hence, monitoring parameters for therapeutic change and adverse outcomes are important in that they guide the alteration or even discontinuation of pharmacotherapy.4,6,14
Documenting the decision-making process to continue stimulant medications under certain circumstances is also essential. Documentation should include discussion of goals and expectations, risks and benefits, and alternatives to stimulant use.
In adults, risk of stimulant medication misuse adds a new layer of complexity to monitoring.13,14 Adults may get multiple prescriptions from multiple providers, seek early refills, fill prescriptions at different pharmacies, or alter formulations. Many states track stimulant prescription use, and prescribers can use this information to determine if patients are refilling their prescriptions appropriately or obtaining stimulants from more than one provider.
Although these monitoring strategies are useful,6 it is prudent to structure the level of monitoring according to the patient’s risk of adverse events or medication misuse.14,27 Gourlay and Heit15 proposed the following “four-A” mnemonic for four domains to be explored at each visit in patients receiving pain medicine. This mnemonic can be applied to adult ADHD patients to more accurately monitor the patient throughout treatment.
THE ‘FOUR-A’ MNEMONIC
ADHD symptoms
Several ADHD scales can be used to track symptom changes over time.33 However, these self-report scales may be subject to positive illusory bias, a phenomenon observed in individuals with ADHD in which they tend to overrate their functioning,34 which may limit the accuracy of self-report scales.35
Activities of daily living
Since patients with ADHD tend to overrate their functioning in various aspects of living, collateral information should be gathered to corroborate patient self-reports whenever possible.
Adverse events
Blood pressure, heart rate, and weight should be assessed at baseline and monitored during stimulant treatment. Other symptoms to monitor include gastrointestinal distress, headache, aggression, depression, appetite, and sleeping habits.4,6 More intensive monitoring (eg, electrocardiography) may be indicated for those with hypertension and cardiovascular risk factors.
Aberrant behavior
Monitoring for misuse and diversion of stimulant medications is essential, as ADHD itself is a risk factor for addiction.20,21 Pill counts, prescription monitoring programs, urine drug screens, and collateral informants have all been proposed but not studied in monitoring for the misuse of stimulant medications.27 Before prescribing, it is prudent to check the prescription monitoring program, get a urine drug screen, and discuss any positive findings with the patient.36,37
Treatment agreements ensure that patients are aware of the consequences of misuse and allow the clinician to reference prior discussion when terminating treatment with stimulants.
LIVES CAN BE ENHANCED
ADHD is a common disorder that arises in childhood and can persist throughout life. Adults with untreated ADHD are at risk of severe impairments in various domains of functioning. Stimulant medications are an effective treatment but may be diverted into the street market. Using the universal-precautions model may reduce the risks of both nontreatment of ADHD and misuse of stimulants.
Accordingly, clinicians need to confirm the ADHD diagnosis, assess comorbidities, estimate risk of misuse, and provide informed consent prior to prescribing. Subsequent monitoring should involve the use of treatment agreements and evaluating treatment response, paying particular attention to ADHD symptom control but also to level of function, adverse effects, and aberrant behavior.
With these principles in mind, clinicians can address the risks of misuse and potentially enhance the lives of people who may have been suffering substantially due to lack of appropriate care.
Children are not the only people affected by attention-deficit/hyperactivity disorder (ADHD). Characterized by high levels of inattention, overactivity, and impulsivity, ADHD affects 5% of school-aged children, but also 4% of adults.1–3 Adults with untreated ADHD are likely to develop serious psychosocial problems that manifest as unemployment, arrests, divorce, underachievement, and psychiatric comorbidities.4,5
However, many clinicians are reluctant to manage adults with ADHD, partly because of concerns about misuse of the stimulant drugs they must prescribe to treat it.
Here, we outline an approach whereby clinicians can diagnose and treat adult ADHD while taking “universal precautions” to discourage misuse of the medications involved.
RECOGNIZING ADHD IN ADULTS
ADHD is characterized by developmentally inappropriate levels of inattention, impulsiveness, and hyperactivity that arise in childhood and result in impairments that often persist.
The presentation of ADHD in adults may be influenced by the longevity of their ADHD, associated sequelae (eg, low self-esteem and interpersonal, educational, and occupational difficulties), and comorbid disorders.6 There are neither reliable biomarkers nor neuropsychological tests for diagnosis, and persons with ADHD typically have a complex presentation with at least one comorbidity.6,7
In patients diagnosed in childhood, difficulties with organization as well as initiating, maintaining, and completing tasks become more prominent in adulthood and hyperactivity tends to subside. Adult impulsivity may present as edginess, shopping sprees, quitting jobs, and risky behaviors.6
Overall, the clinical manifestations of ADHD in adolescents and adults include inattention, difficulties with task completion, disorganization, and executive dysfunction—all skills critical to managing the various roles of adult life.
OBSTACLES TO EFFECTIVE TREATMENT
In the past, ADHD treatment was routinely discontinued during adolescence, as it was unclear whether adults still had significant symptoms or benefited from treatment.8,9 Now, available ADHD guidelines suggest that children and adults who respond to pharmacotherapy should continue it for as long as it remains effective. In this context, there is increasing recognition of adult ADHD as a valid and treatable disorder.10
One of the challenges clinicians face is the reliability of adult recall of childhood ADHD. A controlled, prospective 16-year follow-up study found that of all adults retrospectively given a diagnosis of childhood ADHD, only 27% actually had the disorder.11 This study suggests that retrospective diagnoses of childhood ADHD made solely on the basis of self-reports are unlikely to be valid.
Another obstacle is that traditional medical education has seldom included training in adult ADHD.8,12 In a UK study, clinicians felt that they lacked training and knowledge to assess and manage adult ADHD patients.9
Even if adult ADHD is recognized, diagnosis is just the first step of care.13 These patients require ongoing management and follow-up assessments.
Although practice patterns vary, efforts to encourage doctors to provide adult ADHD care may be hindered by the fact that the gold standard of treatment is stimulant medication.4,10 Medications approved by the US Food and Drug Administration for adult ADHD include the stimulants lisdexamfetamine, osmotic-release methylphenidate, mixed amphetamine salts extended release, dexmethylphenidate extended release, and the nonstimulant atomoxetine.6 While stimulants are generally more efficacious for ADHD symptoms than nonstimulants, they are associated with misuse and diversion.14
UNIVERSAL PRECAUTIONS: A SIMPLIFIED APPROACH
The universal-precautions approach to prescribing stimulants aims to allay physician concerns and promote appropriate medication use to allow for proper management of this disorder.15 These precautions, to be applied to all adult ADHD patients for whom stimulants are being considered, include careful diagnosis and consideration of comorbidities, baseline risk stratification, informed consent processes, treatment agreements, periodic reassessments of treatment response, and meticulous documentation.
DIAGNOSIS
A frequently used screening assessment for adult ADHD is the ADHD Rating Scale (ADHD RS), which consists of two subscales for assessing hyperactivity/impulsivity and inattentiveness.16 ADHD can be classified into one of three subtypes based on symptoms: inattentive, hyperactive, or combined type. Symptoms must persist for at least 6 months for a diagnosis to be made. Other ADHD scales include the Conners Adult ADHD Rating Scales and the Brown Attention-Deficit Disorder Scales.4
High scores on screening scales must be interpreted within the clinical context. Clinicians need to ask about ADHD symptoms, establish their presence in various settings, and determine if these symptoms interfere with functioning. A diagnosis of adult ADHD also requires evidence of symptoms beginning in childhood.17 According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, inattentive or hyperactive-impulsive symptoms must be present before age 12 in two or more settings and interfere with function and development.
Although self-reporting screening tools are helpful, these tests are not reliable for diagnostic purposes, and collateral information is also required.
Neuropsychological testing may detect impairments in persons with ADHD. The most consistently employed neuropsychological tests to evaluate ADHD include the Conners Continuous Performance Test, Stroop Color and Word Test, Trail-making Test, verbal fluency tests, Controlled Oral Word Association Test, and the Weschler Adult Intelligence Scale.6
COMORBIDITY
Epidemiologic studies suggest that adults with ADHD develop many psychiatric problems including anxiety, depression, and substance use disorders.7,16 Table 1 illustrates common comorbidities and their associated prevalence in the ADHD patient.7
Comorbid psychiatric disorders may affect the presentation of adult ADHD. For instance, adults with comorbid depression and ADHD are more likely to present with heightened irritability and difficulties concentrating on tasks than those with either condition alone.18 Similarly, antisocial personality disorder is more common in adults with ADHD.19 Such patients exhibit stable antisocial behavior (lying, stealing, and aggression) as well as medication misuse.5,14,19
While these comorbid disorders may obscure the ADHD diagnosis, their recognition is essential to effectively manage adult ADHD. In sum, a careful evaluation of the adult, including elucidating both ADHD and comorbid symptoms, functionality in several domains, and the degree of impairment, should precede initiating pharmacotherapy for adult ADHD.
BASELINE RISK STRATIFICATION: RISK FACTORS FOR STIMULANT MISUSE
After diagnosing ADHD, the prescriber must assess the risk for misuse of stimulant medications.20
One study revealed that nonmedical use of stimulant medications occurred in only 2% of the 4,300 people surveyed.21 Among the misusers, 66% had obtained medication from family or friends. Another 34% had stolen medication, and 20% had obtained prescriptions from a physician by falsely reporting symptoms. The study also assessed motivation for misuse. In this sample, 40% of misuse was to enhance performance, 34% was for recreation, and 23% was to stay awake.21
Other studies show that misuse of stimulant medications is common among youth in the United States, reporting that 18% of college students use some formulation of prescription stimulants.22
Still more research suggests that childhood conduct disorder or illicit drug use results in a higher risk of stimulant medication misuse.20 Additional risk factors for misuse include male sex, white ethnicity, upper-class background, Jewish or no religious affiliation, affiliation with a sorority or fraternity, off-campus housing, and a low grade-point average.23
Table 2 illustrates clinical interventions providers can use, once they have risk-stratified their patients, to monitor for stimulant misuse.
HOW SHOULD THESE RISK FACTORS AFFECT TREATMENT?
Although no formal scoring system exists to help clinicians risk-stratify these patients, the presence of multiple risk factors suggests the need for vigilance.14 Physicians should prescribe agents with less potential for abuse and monitor these patients more intensely.
Short-acting stimulant medications are the most likely to be abused, as phasic dopamine increase is more reinforcing than therapeutic dopamine release.24 Longer-acting stimulant medications are less likely to be abused, and they provide better symptom relief, as tonic dopamine release maintains a steady state and increases the therapeutic efficacy of these medications.25 For example, methylphenidate extended-release tablets have an osmotic oral controlled-release system and are less likely to be crushed for recreational inhalation.6,14
Lisdexamfetamine is a prodrug therapeutically inert until converted to d-amphetamine when lysine is cleaved from the molecule. This medication may be a good option for patients at high risk of misuse because it is tamper-resistant. However, it still may be subject to misuse for performance enhancement.26,27
The nonstimulant atomoxetine is also approved for ADHD, has no abuse potential, and may be particularly useful when anxiety, mood, and substance use disorders co-occur with ADHD.6 Rarely, atomoxetine can damage the liver, and liver function tests should be monitored if right upper quadrant pain develops.4,10
Other nonapproved agents such as bupropion and desipramine also have been used empirically and off-label for ADHD.4,10
Overall, treatment should be selected according to the risk of misuse of stimulant medication and the patient’s comorbidities.
INFORMED CONSENT
Informed consent may help patients appreciate the risks and benefits of the treatment options and develop realistic expectations about treatment.26 Patients are instructed to take their stimulant medications as prescribed and are informed of the risks of combining stimulants with other substances, particularly those that may interact with stimulants (eg, cocaine) and raise the risk of seizures and cardiovascular complications.
Stimulant medications lead to elevations in blood pressure and heart rate, although large-scale studies have shown no increase in the rate of serious cardiovascular events when these drugs are used appropriately.6 Less serious side effects associated with stimulant medications include insomnia, weight loss, decreased appetite, dry mouth, headache, and rarely, depression and anxiety.6
Patients need to be warned about diversion and abuse liability of stimulant medications, as well as alternative treatments.
The nonstimulant atomoxetine has no reinforcing properties but also raises the blood pressure and heart rate.6 As with stimulants, these elevations are generally minimal, time-limited, and of minor clinical significance.4,10 Frequent reasons to prescribe atomoxetine include poor tolerability of stimulants and a history of substance abuse. In addition, women with ADHD and high levels of emotional dysregulation or social anxiety appear to be particularly responsive to atomoxetine.6
Another consideration is cognitive behavioral therapy, which can augment the effects of pharmacotherapy.4 Cognitive behavioral therapy focuses on time management, prioritization, organization, problem-solving, motivation, and emotional regulation.4
Finally, patients also need to understand the possible consequences of nontreatment.5 Adults with untreated ADHD have high rates of academic and occupational difficulties, anti-social behaviors, and other forms of psychosocial adversity.4,5
Overall, this process should involve discussing risks and benefits of treatment options with the patient and promoting joint decision-making.
TREATMENT AGREEMENTS
Stimulant medications are classified by the US Drug Enforcement Administration as schedule II substances due to their abuse potential.20
It is important to inform patients of the addictive nature of the medication and to instruct them on how to store stimulants safely.27 Patients need to know that giving away or selling these medications is illegal.27
Treatment agreements establish rules for prescribing and are signed by the patient before initiating therapy.28,29 Patients are expected to attend all of their appointments, receive their prescriptions from one doctor, and obtain their medication from one pharmacy. These agreements may also require patients to submit to monitoring with random urine drug screens.29 Overall, they underscore the need for patients to follow a treatment plan in order to continue therapy with controlled substances.29
Manning27 recommends using agreements for high-risk college students prescribed stimulant medications. Red flags for misuse include signs of active substance use (eg, intoxication, a pattern of “lost” prescriptions, and doctor-shopping).27
The effectiveness in reducing risk of misuse in the adult ADHD population has not yet been investigated. Nonetheless, a method of communicating the seriousness of stimulant misuse to adult patients is essential to ADHD care.
STAYING ON TRACK
In the clinical setting, treatment response is measured not just by symptom reduction, but also by functional improvement. Thus, clinicians and patients must set functional goals whenever possible.27 Successful progress toward these goals justifies continuation of therapy, whereas lack of improvement signals the need to reconsider stimulant therapy.27
MONITORING AND DOCUMENTATION
Adults with ADHD present with varying levels of functional impairment and comorbidities, which may require different levels of monitoring.30 Not all patients with ADHD respond optimally to stimulant medications or tolerate them well.31,32 Hence, monitoring parameters for therapeutic change and adverse outcomes are important in that they guide the alteration or even discontinuation of pharmacotherapy.4,6,14
Documenting the decision-making process to continue stimulant medications under certain circumstances is also essential. Documentation should include discussion of goals and expectations, risks and benefits, and alternatives to stimulant use.
In adults, risk of stimulant medication misuse adds a new layer of complexity to monitoring.13,14 Adults may get multiple prescriptions from multiple providers, seek early refills, fill prescriptions at different pharmacies, or alter formulations. Many states track stimulant prescription use, and prescribers can use this information to determine if patients are refilling their prescriptions appropriately or obtaining stimulants from more than one provider.
Although these monitoring strategies are useful,6 it is prudent to structure the level of monitoring according to the patient’s risk of adverse events or medication misuse.14,27 Gourlay and Heit15 proposed the following “four-A” mnemonic for four domains to be explored at each visit in patients receiving pain medicine. This mnemonic can be applied to adult ADHD patients to more accurately monitor the patient throughout treatment.
THE ‘FOUR-A’ MNEMONIC
ADHD symptoms
Several ADHD scales can be used to track symptom changes over time.33 However, these self-report scales may be subject to positive illusory bias, a phenomenon observed in individuals with ADHD in which they tend to overrate their functioning,34 which may limit the accuracy of self-report scales.35
Activities of daily living
Since patients with ADHD tend to overrate their functioning in various aspects of living, collateral information should be gathered to corroborate patient self-reports whenever possible.
Adverse events
Blood pressure, heart rate, and weight should be assessed at baseline and monitored during stimulant treatment. Other symptoms to monitor include gastrointestinal distress, headache, aggression, depression, appetite, and sleeping habits.4,6 More intensive monitoring (eg, electrocardiography) may be indicated for those with hypertension and cardiovascular risk factors.
Aberrant behavior
Monitoring for misuse and diversion of stimulant medications is essential, as ADHD itself is a risk factor for addiction.20,21 Pill counts, prescription monitoring programs, urine drug screens, and collateral informants have all been proposed but not studied in monitoring for the misuse of stimulant medications.27 Before prescribing, it is prudent to check the prescription monitoring program, get a urine drug screen, and discuss any positive findings with the patient.36,37
Treatment agreements ensure that patients are aware of the consequences of misuse and allow the clinician to reference prior discussion when terminating treatment with stimulants.
LIVES CAN BE ENHANCED
ADHD is a common disorder that arises in childhood and can persist throughout life. Adults with untreated ADHD are at risk of severe impairments in various domains of functioning. Stimulant medications are an effective treatment but may be diverted into the street market. Using the universal-precautions model may reduce the risks of both nontreatment of ADHD and misuse of stimulants.
Accordingly, clinicians need to confirm the ADHD diagnosis, assess comorbidities, estimate risk of misuse, and provide informed consent prior to prescribing. Subsequent monitoring should involve the use of treatment agreements and evaluating treatment response, paying particular attention to ADHD symptom control but also to level of function, adverse effects, and aberrant behavior.
With these principles in mind, clinicians can address the risks of misuse and potentially enhance the lives of people who may have been suffering substantially due to lack of appropriate care.
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007; 164:942–948.
- Polanczyk GV, Wilcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 2014; 43:434–442.
- Wilens TE. ADHD: Prevalence, diagnosis, and issues of comorbidity. CNS Spectr 2007; 12(suppl 6):1–5.
- Kooij SJ, Bejerot S, Blackwell A, et al. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry 2010; 10:67.
- Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med 2012;10:99.
- Modesto-Lowe V, Meyer A, Soovajian V. A clinician’s guide to adult attention-deficit hyperactivity disorder. Conn Med 2012; 76:517–523.
- Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006; 163:716–723.
- Goodman DW, Surman CB, Scherer PB, Salinas GD, Brown JJ. Assessment of physician practices in adult attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord 2012; 14(4).
- Hall CL, Newell K, Taylor J, Sayal K, Swift KD, Hollis C. ‘Mind the gap’—mapping services for young people with ADHD transitioning from child to adult mental health services. BMC Psychiatry 2013; 13:186.
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management of ADHD in children, young people and adults. The British Psychological Society and The Royal College of Psychiatrists: United Kingdom; 2009.
- Mannuzza S, Klein RG, Klein DF, Bessler A, Shrout P. Accuracy of adult recall of childhood attention deficit hyperactivity disorder. Am J Psychiatry 2002; 159:1882–1888.
- Wetzel MW. Medical student participation in an adult ADHD outpatient clinic: an ideal setting for education in outpatient psychiatry. Acad Psychiatry 2009; 33:80–81.
- Culpepper L, Mattingly G. Challenges in identifying and managing attention-deficit/hyperactivity disorder in adults in the primary care setting: a review of the literature. Prim Care Companion J Clin Psychiatry 2010; 12(6).
- Rabiner DL. Stimulant prescription cautions: addressing misuse, diversion and malingering. Curr Psychiatry Rep 2013; 15:375.
- Gourlay D, Heit H. Universal precautions: a matter of mutual trust and responsibility. Pain Med 2006; 7:210–211.
- Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005; 35:245–256.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- CADDRA Guidelines Steering Committee. Canadian ADHD practice guidelines: CADDRA 2008. http://www.naceonline.com/AdultADHDtoolkit/professionalresources/caddraguidelines.pdf. Accessed July 10, 2015.
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry 1998; 155:493–498.
- Kaye S, Darke S. The diversion and misuse of pharmaceutical stimulants: what do we know and why should we care? Addiction 2012; 107:467–477.
- Novak SP, Kroutil LA, Williams RL, Van Brunt DL. The nonmedical use of prescription ADHD medications: results from a national Internet panel. Subst Abuse Treat Prev Policy 2007; 2:32.
- Bavarian N, Flay BR, Ketcham P, et al. Using structural equation modeling to understand prescription stimulant misuse: a test of the Theory of Triadic Influence. Drug Alcohol Depend 2014; 138:193–201.
- McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs 2006; 38:43–56.
- Volkow ND. Stimulant medications: how to minimize their reinforcing effects? Am J Psychiatry 2006; 163:359–361.
- Kolar D, Keller A, Golfinopoulos M, Cumyn L, Syer C, Hechtman L. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat 2008; 4:107–121.
- Schachter D, Tharmalingam S, Kleinman I. Informed consent and stimulant medication: adolescents’ and parents’ ability to understand information about benefits and risks of stimulant medication for the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2011; 21:139–148.
- Manning JS. Strategies for managing the risks associated with ADHD medications. J Clin Psychiatry 2013; 74:e19.
- Deep K. Use of narcotics contracts. Virtual Mentor 2013; 15:416–420.
- Cheatle MD, Savage SR. Informed consent in opioid therapy: a potential obligation and opportunity. J Pain Symptom Manage 2012; 44:105–116.
- Dias TG, Kieling C, Graeff-Martins AS, Moriyama TS, Rohde LA, Polanczyk GV. Developments and challenges in the diagnosis and treatment of ADHD. Rev Bras Psiquiatr 2013; 35(suppl 1):S40–S50.
- Mattingly GW, Weisler RH, Young J, et al. Clinical response and symptomatic remission in short- and long-term trials of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2013; 13:39.
- Contini V, Victor MM, Bertuzzi GP, et al. No significant association between genetic variants in 7 candidate genes and response to methylphenidate treatment in adult patients with ADHD. J Clin Psychopharmacol 2012; 32:820–823.
- Rösler M, Retz W, Thome J, Schneider M, Stieglitz RD, Falkai P. Psychopathological rating scales for diagnostic use in adults with attention-deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci 2006; 256(suppl 1):i3–i11.
- Prevatt F, Proctor B, Best L, Baker L, Van Walker J, Taylor NW. The positive illusory bias: does it explain self-evaluations in college students with ADHD? J Atten Disord 2012; 16:235–243.
- Jiang Y, Johnston C. The relationship between ADHD symptoms and competence as reported by both self and others. J Atten Disord 2012; 16:418–426.
- Darredeau C, Barrett SP, Jardin B, Pihl RO. Patterns and predictors of medication compliance, diversion, and misuse in adult prescribed methylphenidate users. Hum Psychopharmacol 2007; 22:529–536.
- Worley J. Prescription drug monitoring programs, a response to doctor shopping: purpose, effectiveness, and directions for future research. Issues Ment Health Nurs 2012; 33:319–328.
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007; 164:942–948.
- Polanczyk GV, Wilcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 2014; 43:434–442.
- Wilens TE. ADHD: Prevalence, diagnosis, and issues of comorbidity. CNS Spectr 2007; 12(suppl 6):1–5.
- Kooij SJ, Bejerot S, Blackwell A, et al. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry 2010; 10:67.
- Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med 2012;10:99.
- Modesto-Lowe V, Meyer A, Soovajian V. A clinician’s guide to adult attention-deficit hyperactivity disorder. Conn Med 2012; 76:517–523.
- Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006; 163:716–723.
- Goodman DW, Surman CB, Scherer PB, Salinas GD, Brown JJ. Assessment of physician practices in adult attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord 2012; 14(4).
- Hall CL, Newell K, Taylor J, Sayal K, Swift KD, Hollis C. ‘Mind the gap’—mapping services for young people with ADHD transitioning from child to adult mental health services. BMC Psychiatry 2013; 13:186.
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management of ADHD in children, young people and adults. The British Psychological Society and The Royal College of Psychiatrists: United Kingdom; 2009.
- Mannuzza S, Klein RG, Klein DF, Bessler A, Shrout P. Accuracy of adult recall of childhood attention deficit hyperactivity disorder. Am J Psychiatry 2002; 159:1882–1888.
- Wetzel MW. Medical student participation in an adult ADHD outpatient clinic: an ideal setting for education in outpatient psychiatry. Acad Psychiatry 2009; 33:80–81.
- Culpepper L, Mattingly G. Challenges in identifying and managing attention-deficit/hyperactivity disorder in adults in the primary care setting: a review of the literature. Prim Care Companion J Clin Psychiatry 2010; 12(6).
- Rabiner DL. Stimulant prescription cautions: addressing misuse, diversion and malingering. Curr Psychiatry Rep 2013; 15:375.
- Gourlay D, Heit H. Universal precautions: a matter of mutual trust and responsibility. Pain Med 2006; 7:210–211.
- Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005; 35:245–256.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- CADDRA Guidelines Steering Committee. Canadian ADHD practice guidelines: CADDRA 2008. http://www.naceonline.com/AdultADHDtoolkit/professionalresources/caddraguidelines.pdf. Accessed July 10, 2015.
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry 1998; 155:493–498.
- Kaye S, Darke S. The diversion and misuse of pharmaceutical stimulants: what do we know and why should we care? Addiction 2012; 107:467–477.
- Novak SP, Kroutil LA, Williams RL, Van Brunt DL. The nonmedical use of prescription ADHD medications: results from a national Internet panel. Subst Abuse Treat Prev Policy 2007; 2:32.
- Bavarian N, Flay BR, Ketcham P, et al. Using structural equation modeling to understand prescription stimulant misuse: a test of the Theory of Triadic Influence. Drug Alcohol Depend 2014; 138:193–201.
- McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs 2006; 38:43–56.
- Volkow ND. Stimulant medications: how to minimize their reinforcing effects? Am J Psychiatry 2006; 163:359–361.
- Kolar D, Keller A, Golfinopoulos M, Cumyn L, Syer C, Hechtman L. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat 2008; 4:107–121.
- Schachter D, Tharmalingam S, Kleinman I. Informed consent and stimulant medication: adolescents’ and parents’ ability to understand information about benefits and risks of stimulant medication for the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2011; 21:139–148.
- Manning JS. Strategies for managing the risks associated with ADHD medications. J Clin Psychiatry 2013; 74:e19.
- Deep K. Use of narcotics contracts. Virtual Mentor 2013; 15:416–420.
- Cheatle MD, Savage SR. Informed consent in opioid therapy: a potential obligation and opportunity. J Pain Symptom Manage 2012; 44:105–116.
- Dias TG, Kieling C, Graeff-Martins AS, Moriyama TS, Rohde LA, Polanczyk GV. Developments and challenges in the diagnosis and treatment of ADHD. Rev Bras Psiquiatr 2013; 35(suppl 1):S40–S50.
- Mattingly GW, Weisler RH, Young J, et al. Clinical response and symptomatic remission in short- and long-term trials of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2013; 13:39.
- Contini V, Victor MM, Bertuzzi GP, et al. No significant association between genetic variants in 7 candidate genes and response to methylphenidate treatment in adult patients with ADHD. J Clin Psychopharmacol 2012; 32:820–823.
- Rösler M, Retz W, Thome J, Schneider M, Stieglitz RD, Falkai P. Psychopathological rating scales for diagnostic use in adults with attention-deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci 2006; 256(suppl 1):i3–i11.
- Prevatt F, Proctor B, Best L, Baker L, Van Walker J, Taylor NW. The positive illusory bias: does it explain self-evaluations in college students with ADHD? J Atten Disord 2012; 16:235–243.
- Jiang Y, Johnston C. The relationship between ADHD symptoms and competence as reported by both self and others. J Atten Disord 2012; 16:418–426.
- Darredeau C, Barrett SP, Jardin B, Pihl RO. Patterns and predictors of medication compliance, diversion, and misuse in adult prescribed methylphenidate users. Hum Psychopharmacol 2007; 22:529–536.
- Worley J. Prescription drug monitoring programs, a response to doctor shopping: purpose, effectiveness, and directions for future research. Issues Ment Health Nurs 2012; 33:319–328.
KEY POINTS
- Untreated adult ADHD is associated with negative outcomes that include unemployment, arrests, divorce, and psychiatric comorbidities.
- Available ADHD guidelines suggest that children and adults who respond to pharmacotherapy should continue it for as long as it remains effective. In this context, there is increasing recognition of adult ADHD as a valid and treatable disorder.
- Following the guidelines of universal precautions in the diagnosis and treatment of adult ADHD can alleviate clinicians’ concerns when diagnosing and treating this disorder.