User login
VALENCIA, SPAIN – Single-pulse transcranial magnetic stimulation shows promise for treatment of migraine with medication overuse headache, according to a pilot study.
Twenty-eight severely affected patients participated in this prospective, open-label study of single-pulse transcranial magnetic stimulation (sTMS). They were instructed to use the device for at least 3 months, with an option to continue for another 3 months.
The proprietary SpringTMS system was approved by the Food and Drug Administration and the U.K.’s National Institute for Clinical Excellence for treatment of acute migraine on the basis of a positive randomized, double-blind, sham-controlled clinical trial (Lancet Neurol. 2010;9:373-80), but it hasn’t previously been evaluated in the common and clinically challenging setting of migraine complicated by medication overuse, Dr. Farooq Maniyar noted at the International Headache Congress.
The reasons participants gave for enrolling in the study varied. Most felt their current medications had intolerable side effects and/or weren’t sufficiently beneficial. Some preferred the possibility of a nondrug method of managing their headaches, explained Dr. Maniyar, a consulting neurologist at Basildon and Thurrock University Hospitals NHS Foundation Trust, Basildon, England, and the Royal London Hospital of Barts Health NHS Trust.
The key results include:
• Twenty-four of 28 patients (86%) reported a reduction in their days of acute medication use per month, while 2 patients reported an increase in acute medications.
• Nineteen patients (68%) experienced fewer migraine days per month, and 7 of the 19 had a 50% or greater reduction.
• The number of patients who rated their migraine pain as excruciating or severe plunged from 19 at baseline to 3 at 3 months, an 84% drop.
• Self-reported headache attack duration, which averaged 1 hour at baseline, decreased in 15 patients, remained unchanged in 9, and increased in 4.
• The Headache Impact Test (HIT-6) score, which reflects patient perception of the impact the migraine headaches have on daily life, was rated severe at baseline – meaning a score of at least 60 – by 26 of 28 participants. At 3 months, this number had decreased from 26 to 18.
“I think it’s fair to say that sTMS may be a useful option in treatment of medication overuse headache,” Dr. Maniyar declared at the meeting sponsored by the International Headache Society and the American Headache Society.
The sTMS regimen was beneficial in migraine patients with or without aura. Although the 12 patients who had migraine with aura showed a consistent trend for better outcomes, Dr. Maniyar said the patient numbers in the study were too small to draw definite conclusions on this score.
After 3 months, five patients discontinued sTMS because of inconvenience or lack of benefit. Four others were lost to follow-up. Of the remaining 19, 16 reported reduced days of acute medication usage at 6 months, compared with baseline. HIT-6 scores in the 19 patients with 6-month treatment data were comparable to their scores at 3 months.
No adverse events occurred in connection with the study.
Dr. Maniyar said the study’s major limitations are its relatively small sample size – the results need a confirmatory study – and the open-label, uncontrolled study design.
“We can’t rule out the possibility of a placebo effect for sTMS,” he observed.
The portable, noninvasive SpringTMS device delivers a brief pulse of magnetic energy to the back of the head in order to disrupt the migraine headache. The daily treatment regimen for the first month involved giving two pulses, followed 15 minutes later by another two, then repeating the sequence roughly 12 hours later. In addition, patients were instructed to use the device at the onset of headaches. Patients were allowed to use a maximum of 16 pulses per day in month 1, 24 per day in month 2, and up to 32 daily during month 3.
The study was sponsored by eNeura, which markets the SpringTMS device. Dr. Maniyar reported serving as a consultant to the company.
VALENCIA, SPAIN – Single-pulse transcranial magnetic stimulation shows promise for treatment of migraine with medication overuse headache, according to a pilot study.
Twenty-eight severely affected patients participated in this prospective, open-label study of single-pulse transcranial magnetic stimulation (sTMS). They were instructed to use the device for at least 3 months, with an option to continue for another 3 months.
The proprietary SpringTMS system was approved by the Food and Drug Administration and the U.K.’s National Institute for Clinical Excellence for treatment of acute migraine on the basis of a positive randomized, double-blind, sham-controlled clinical trial (Lancet Neurol. 2010;9:373-80), but it hasn’t previously been evaluated in the common and clinically challenging setting of migraine complicated by medication overuse, Dr. Farooq Maniyar noted at the International Headache Congress.

The reasons participants gave for enrolling in the study varied. Most felt their current medications had intolerable side effects and/or weren’t sufficiently beneficial. Some preferred the possibility of a nondrug method of managing their headaches, explained Dr. Maniyar, a consulting neurologist at Basildon and Thurrock University Hospitals NHS Foundation Trust, Basildon, England, and the Royal London Hospital of Barts Health NHS Trust.
The key results include:
• Twenty-four of 28 patients (86%) reported a reduction in their days of acute medication use per month, while 2 patients reported an increase in acute medications.
• Nineteen patients (68%) experienced fewer migraine days per month, and 7 of the 19 had a 50% or greater reduction.
• The number of patients who rated their migraine pain as excruciating or severe plunged from 19 at baseline to 3 at 3 months, an 84% drop.
• Self-reported headache attack duration, which averaged 1 hour at baseline, decreased in 15 patients, remained unchanged in 9, and increased in 4.
• The Headache Impact Test (HIT-6) score, which reflects patient perception of the impact the migraine headaches have on daily life, was rated severe at baseline – meaning a score of at least 60 – by 26 of 28 participants. At 3 months, this number had decreased from 26 to 18.
“I think it’s fair to say that sTMS may be a useful option in treatment of medication overuse headache,” Dr. Maniyar declared at the meeting sponsored by the International Headache Society and the American Headache Society.
The sTMS regimen was beneficial in migraine patients with or without aura. Although the 12 patients who had migraine with aura showed a consistent trend for better outcomes, Dr. Maniyar said the patient numbers in the study were too small to draw definite conclusions on this score.
After 3 months, five patients discontinued sTMS because of inconvenience or lack of benefit. Four others were lost to follow-up. Of the remaining 19, 16 reported reduced days of acute medication usage at 6 months, compared with baseline. HIT-6 scores in the 19 patients with 6-month treatment data were comparable to their scores at 3 months.
No adverse events occurred in connection with the study.
Dr. Maniyar said the study’s major limitations are its relatively small sample size – the results need a confirmatory study – and the open-label, uncontrolled study design.
“We can’t rule out the possibility of a placebo effect for sTMS,” he observed.
The portable, noninvasive SpringTMS device delivers a brief pulse of magnetic energy to the back of the head in order to disrupt the migraine headache. The daily treatment regimen for the first month involved giving two pulses, followed 15 minutes later by another two, then repeating the sequence roughly 12 hours later. In addition, patients were instructed to use the device at the onset of headaches. Patients were allowed to use a maximum of 16 pulses per day in month 1, 24 per day in month 2, and up to 32 daily during month 3.
The study was sponsored by eNeura, which markets the SpringTMS device. Dr. Maniyar reported serving as a consultant to the company.
VALENCIA, SPAIN – Single-pulse transcranial magnetic stimulation shows promise for treatment of migraine with medication overuse headache, according to a pilot study.
Twenty-eight severely affected patients participated in this prospective, open-label study of single-pulse transcranial magnetic stimulation (sTMS). They were instructed to use the device for at least 3 months, with an option to continue for another 3 months.
The proprietary SpringTMS system was approved by the Food and Drug Administration and the U.K.’s National Institute for Clinical Excellence for treatment of acute migraine on the basis of a positive randomized, double-blind, sham-controlled clinical trial (Lancet Neurol. 2010;9:373-80), but it hasn’t previously been evaluated in the common and clinically challenging setting of migraine complicated by medication overuse, Dr. Farooq Maniyar noted at the International Headache Congress.

The reasons participants gave for enrolling in the study varied. Most felt their current medications had intolerable side effects and/or weren’t sufficiently beneficial. Some preferred the possibility of a nondrug method of managing their headaches, explained Dr. Maniyar, a consulting neurologist at Basildon and Thurrock University Hospitals NHS Foundation Trust, Basildon, England, and the Royal London Hospital of Barts Health NHS Trust.
The key results include:
• Twenty-four of 28 patients (86%) reported a reduction in their days of acute medication use per month, while 2 patients reported an increase in acute medications.
• Nineteen patients (68%) experienced fewer migraine days per month, and 7 of the 19 had a 50% or greater reduction.
• The number of patients who rated their migraine pain as excruciating or severe plunged from 19 at baseline to 3 at 3 months, an 84% drop.
• Self-reported headache attack duration, which averaged 1 hour at baseline, decreased in 15 patients, remained unchanged in 9, and increased in 4.
• The Headache Impact Test (HIT-6) score, which reflects patient perception of the impact the migraine headaches have on daily life, was rated severe at baseline – meaning a score of at least 60 – by 26 of 28 participants. At 3 months, this number had decreased from 26 to 18.
“I think it’s fair to say that sTMS may be a useful option in treatment of medication overuse headache,” Dr. Maniyar declared at the meeting sponsored by the International Headache Society and the American Headache Society.
The sTMS regimen was beneficial in migraine patients with or without aura. Although the 12 patients who had migraine with aura showed a consistent trend for better outcomes, Dr. Maniyar said the patient numbers in the study were too small to draw definite conclusions on this score.
After 3 months, five patients discontinued sTMS because of inconvenience or lack of benefit. Four others were lost to follow-up. Of the remaining 19, 16 reported reduced days of acute medication usage at 6 months, compared with baseline. HIT-6 scores in the 19 patients with 6-month treatment data were comparable to their scores at 3 months.
No adverse events occurred in connection with the study.
Dr. Maniyar said the study’s major limitations are its relatively small sample size – the results need a confirmatory study – and the open-label, uncontrolled study design.
“We can’t rule out the possibility of a placebo effect for sTMS,” he observed.
The portable, noninvasive SpringTMS device delivers a brief pulse of magnetic energy to the back of the head in order to disrupt the migraine headache. The daily treatment regimen for the first month involved giving two pulses, followed 15 minutes later by another two, then repeating the sequence roughly 12 hours later. In addition, patients were instructed to use the device at the onset of headaches. Patients were allowed to use a maximum of 16 pulses per day in month 1, 24 per day in month 2, and up to 32 daily during month 3.
The study was sponsored by eNeura, which markets the SpringTMS device. Dr. Maniyar reported serving as a consultant to the company.
AT IHC 2015
Key clinical point:The SpringTMS device shows promising results as a safe and effective nondrug therapeutic option in migraine with medication overuse headache.
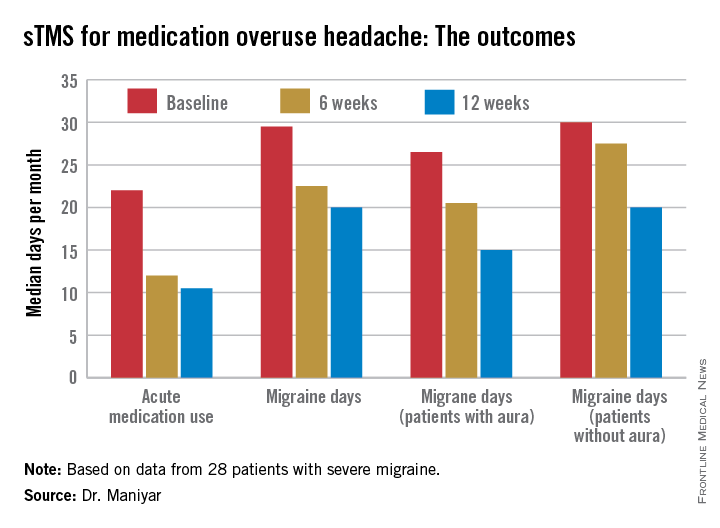
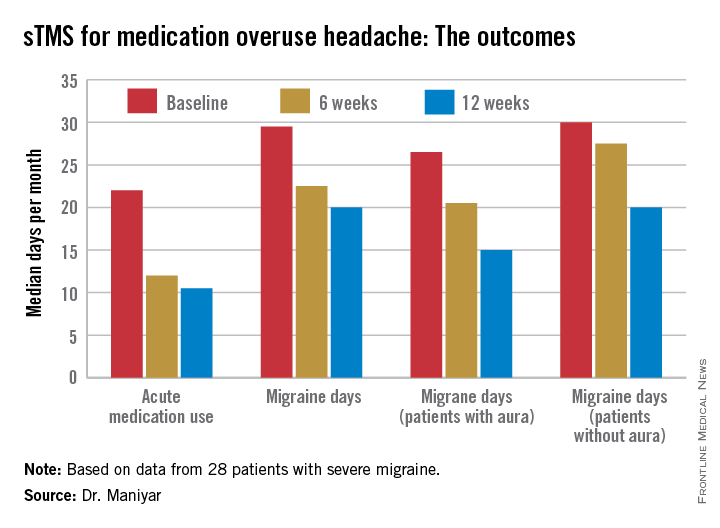
Major finding: The median number of days of acute medication use per month went from 22 at baseline to 12 after 6 weeks of self-treatment with single-pulse transcranial magnetic stimulation to 10.5 days after 3 months.
Data source: This was a prospective, open-label, uncontrolled pilot study involving 28 patients with severe migraine complicated by medication overuse headache.
Disclosures: The study was sponsored by eNeura, which markets the SpringTMS device. The presenter reported serving as a consultant to the company.