User login
Indeterminate Cell Histiocytosis and a Review of Current Treatment
To the Editor:
Indeterminate cell histiocytosis (ICH) is a rare neoplastic dendritic cell disorder with a poorly understood histogenesis and pathogenesis.1 The clinical manifestation of ICH is broad and can include isolated or multiple papules or nodules on the face, neck, trunk, arms, or legs. Our case demonstrates a rare occurrence of ICH that initially was misdiagnosed and highlights the use of cobimetinib, a MEK inhibitor, as a potential new therapeutic option for ICH.
A 74-year-old man with a history of type 2 diabetes mellitus presented for evaluation of a progressive pruritic rash of approximately 5 years’ duration. The eruption previously had been diagnosed as Langerhans cell histiocytosis. It started on the chest and spread to the face, neck, trunk, and arms. The patient denied systemic symptoms and had no known history of malignancy.
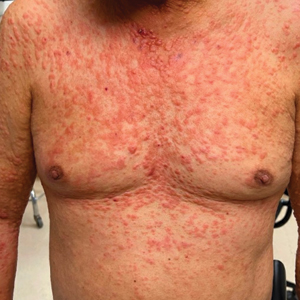
Physical examination revealed pink to orange smooth papules, nodules, and small plaques on the ears, cheeks, trunk, neck, and arms (Figure 1). Baseline laboratory results showed a normal complete blood count and comprehensive metabolic panel, elevated lactate dehydrogenase and erythrocyte sedimentation rate, and hyperlipidemia. Serology for hepatitis B and C was negative. Bone marrow biopsy was normal, and positron emission tomography/ computed tomography demonstrated no evidence of extracutaneous disease. A punch biopsy of a lesion on the left forearm revealed epithelioid histiocytic proliferation in the dermis extending into the subcutis with a background infiltrate of small lymphocytes. Immunohistochemistry was positive for CD1a and CD56 and was variably positive for CD4 but negative for CD163, CD68, S100, Langerin, cyclin D1, myeloperoxidase, CD21, and CD23. No mutation was detected in BRAF codon 600. Given the negative Langerin stain, these findings were compatible with a diagnosis of ICH. After considering the lack of standard treatment options as well as the recent approval of cobimetinib for histiocytic disorders, we initiated treatment with cobimetinib at the standard dose of 60 mg daily for 21 days followed by a 7-day break.

One month into treatment, the patient’s lesions were less erythematous, and he reported improvement in pruritus. Two months into treatment, there was continued improvement in cutaneous symptoms with flattening of the lesions on the chest and back. At this time, the patient developed edema of the face and ears (Figure 2) and reported weakness, blurred vision, and decreased appetite. He was advised to take an additional 7-day treatment break before resuming cobimetinib at a decreased dose of 40 mg daily. The patient returned to the clinic 1 month later with improved systemic symptoms and continued flattening of the lesions. Five months into treatment, the lesions had continued to improve with complete resolution of the facial plaques (Figure 3).


Indeterminate cell histiocytosis is a rarely diagnosed condition characterized by the proliferation of indeterminate histiocytes that morphologically and immunophenotypically resemble Langerhans cells but lack their characteristic Birbeck granules.2 There is no standard treatment for ICH, but previous reports have described improvement with a variety of treatment options including methotrexate,3,4 UVB phototherapy,5 and topical delgocitinib 0.5%.6
Because histiocytic disorders are characterized by mutations in the mitogen-activated protein kinase pathway, it is possible that they would be responsive to MEK inhibition. Cobimetinib, a MEK inhibitor initially approved to treat metastatic melanoma, was approved by the US Food and Drug Administration to treat histiocytic disorders in October 2022.7 The approval followed the release of data from a phase 2 trial of cobimetinib in 18 adults with various histiocytic disorders, which demonstrated an 89% (16/18) overall response rate with 94% (17/18) of patients remaining progression free at 1 year.8 While cobimetinib has not specifically been studied in ICH, given the high response rate in histiocytic disorders and the lack of standard treatment options for ICH, the decision was made to initiate treatment with cobimetinib in our patient. Based on the observed improvement in our patient, we propose cobimetinib as a treatment option for patients with cutaneous ICH and recommend additional studies to confirm its safety and efficacy in patients with this disorder.
- Bakry OA, Samaka RM, Kandil MA, et al. Indeterminate cell histiocytosis with naïve cells. Rare Tumors. 2013;5:e13. doi:10.4081 /rt.2013.e13
- Manente L, Cotellessa C, Schmitt I, et al. Indeterminate cell histiocytosis: a rare histiocytic disorder. Am J Dermatopathol. 1997; 19:276-283. doi:10.1097/00000372-199706000-00014
- Lie E, Jedrych J, Sweren R, et al. Generalized indeterminate cell histiocytosis successfully treated with methotrexate. JAAD Case Rep. 2022;25:93-96. doi:10.1016/j.jdcr.2022.05.027
- Fournier J, Ingraffea A, Pedvis-Leftick A. Successful treatment of indeterminate cell histiocytosis with low-dose methotrexate. J Dermatol. 2011;38:937-939. doi:10.1111/j.1346-8138.2010.01148.x
- Logemann N, Thomas B, Yetto T. Indeterminate cell histiocytosis successfully treated with narrowband UVB. Dermatol Online J. 2013;19:20031. doi:10.5070/D31910020031
- Fujimoto RFT, Miura H, Takata M, et al. Indeterminate cell histiocytosis treated with 0.5% delgocitinib ointment. Br J Dermatol. 2023;188:E39. doi:10.1093/bjd/ljad029
- Diamond EL, Durham B, Dogan A, et al. Phase 2 trial of single-agent cobimetinib for adults with histiocytic neoplasms. Blood. 2023;142:1812. doi:10.1182/blood-2023-187508
- Diamond EL, Durham BH, Ulaner GA, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567:521-524. doi:10.1038/s41586-019-1012-y
To the Editor:
Indeterminate cell histiocytosis (ICH) is a rare neoplastic dendritic cell disorder with a poorly understood histogenesis and pathogenesis.1 The clinical manifestation of ICH is broad and can include isolated or multiple papules or nodules on the face, neck, trunk, arms, or legs. Our case demonstrates a rare occurrence of ICH that initially was misdiagnosed and highlights the use of cobimetinib, a MEK inhibitor, as a potential new therapeutic option for ICH.
A 74-year-old man with a history of type 2 diabetes mellitus presented for evaluation of a progressive pruritic rash of approximately 5 years’ duration. The eruption previously had been diagnosed as Langerhans cell histiocytosis. It started on the chest and spread to the face, neck, trunk, and arms. The patient denied systemic symptoms and had no known history of malignancy.
Physical examination revealed pink to orange smooth papules, nodules, and small plaques on the ears, cheeks, trunk, neck, and arms (Figure 1). Baseline laboratory results showed a normal complete blood count and comprehensive metabolic panel, elevated lactate dehydrogenase and erythrocyte sedimentation rate, and hyperlipidemia. Serology for hepatitis B and C was negative. Bone marrow biopsy was normal, and positron emission tomography/ computed tomography demonstrated no evidence of extracutaneous disease. A punch biopsy of a lesion on the left forearm revealed epithelioid histiocytic proliferation in the dermis extending into the subcutis with a background infiltrate of small lymphocytes. Immunohistochemistry was positive for CD1a and CD56 and was variably positive for CD4 but negative for CD163, CD68, S100, Langerin, cyclin D1, myeloperoxidase, CD21, and CD23. No mutation was detected in BRAF codon 600. Given the negative Langerin stain, these findings were compatible with a diagnosis of ICH. After considering the lack of standard treatment options as well as the recent approval of cobimetinib for histiocytic disorders, we initiated treatment with cobimetinib at the standard dose of 60 mg daily for 21 days followed by a 7-day break.

One month into treatment, the patient’s lesions were less erythematous, and he reported improvement in pruritus. Two months into treatment, there was continued improvement in cutaneous symptoms with flattening of the lesions on the chest and back. At this time, the patient developed edema of the face and ears (Figure 2) and reported weakness, blurred vision, and decreased appetite. He was advised to take an additional 7-day treatment break before resuming cobimetinib at a decreased dose of 40 mg daily. The patient returned to the clinic 1 month later with improved systemic symptoms and continued flattening of the lesions. Five months into treatment, the lesions had continued to improve with complete resolution of the facial plaques (Figure 3).


Indeterminate cell histiocytosis is a rarely diagnosed condition characterized by the proliferation of indeterminate histiocytes that morphologically and immunophenotypically resemble Langerhans cells but lack their characteristic Birbeck granules.2 There is no standard treatment for ICH, but previous reports have described improvement with a variety of treatment options including methotrexate,3,4 UVB phototherapy,5 and topical delgocitinib 0.5%.6
Because histiocytic disorders are characterized by mutations in the mitogen-activated protein kinase pathway, it is possible that they would be responsive to MEK inhibition. Cobimetinib, a MEK inhibitor initially approved to treat metastatic melanoma, was approved by the US Food and Drug Administration to treat histiocytic disorders in October 2022.7 The approval followed the release of data from a phase 2 trial of cobimetinib in 18 adults with various histiocytic disorders, which demonstrated an 89% (16/18) overall response rate with 94% (17/18) of patients remaining progression free at 1 year.8 While cobimetinib has not specifically been studied in ICH, given the high response rate in histiocytic disorders and the lack of standard treatment options for ICH, the decision was made to initiate treatment with cobimetinib in our patient. Based on the observed improvement in our patient, we propose cobimetinib as a treatment option for patients with cutaneous ICH and recommend additional studies to confirm its safety and efficacy in patients with this disorder.
To the Editor:
Indeterminate cell histiocytosis (ICH) is a rare neoplastic dendritic cell disorder with a poorly understood histogenesis and pathogenesis.1 The clinical manifestation of ICH is broad and can include isolated or multiple papules or nodules on the face, neck, trunk, arms, or legs. Our case demonstrates a rare occurrence of ICH that initially was misdiagnosed and highlights the use of cobimetinib, a MEK inhibitor, as a potential new therapeutic option for ICH.
A 74-year-old man with a history of type 2 diabetes mellitus presented for evaluation of a progressive pruritic rash of approximately 5 years’ duration. The eruption previously had been diagnosed as Langerhans cell histiocytosis. It started on the chest and spread to the face, neck, trunk, and arms. The patient denied systemic symptoms and had no known history of malignancy.
Physical examination revealed pink to orange smooth papules, nodules, and small plaques on the ears, cheeks, trunk, neck, and arms (Figure 1). Baseline laboratory results showed a normal complete blood count and comprehensive metabolic panel, elevated lactate dehydrogenase and erythrocyte sedimentation rate, and hyperlipidemia. Serology for hepatitis B and C was negative. Bone marrow biopsy was normal, and positron emission tomography/ computed tomography demonstrated no evidence of extracutaneous disease. A punch biopsy of a lesion on the left forearm revealed epithelioid histiocytic proliferation in the dermis extending into the subcutis with a background infiltrate of small lymphocytes. Immunohistochemistry was positive for CD1a and CD56 and was variably positive for CD4 but negative for CD163, CD68, S100, Langerin, cyclin D1, myeloperoxidase, CD21, and CD23. No mutation was detected in BRAF codon 600. Given the negative Langerin stain, these findings were compatible with a diagnosis of ICH. After considering the lack of standard treatment options as well as the recent approval of cobimetinib for histiocytic disorders, we initiated treatment with cobimetinib at the standard dose of 60 mg daily for 21 days followed by a 7-day break.

One month into treatment, the patient’s lesions were less erythematous, and he reported improvement in pruritus. Two months into treatment, there was continued improvement in cutaneous symptoms with flattening of the lesions on the chest and back. At this time, the patient developed edema of the face and ears (Figure 2) and reported weakness, blurred vision, and decreased appetite. He was advised to take an additional 7-day treatment break before resuming cobimetinib at a decreased dose of 40 mg daily. The patient returned to the clinic 1 month later with improved systemic symptoms and continued flattening of the lesions. Five months into treatment, the lesions had continued to improve with complete resolution of the facial plaques (Figure 3).


Indeterminate cell histiocytosis is a rarely diagnosed condition characterized by the proliferation of indeterminate histiocytes that morphologically and immunophenotypically resemble Langerhans cells but lack their characteristic Birbeck granules.2 There is no standard treatment for ICH, but previous reports have described improvement with a variety of treatment options including methotrexate,3,4 UVB phototherapy,5 and topical delgocitinib 0.5%.6
Because histiocytic disorders are characterized by mutations in the mitogen-activated protein kinase pathway, it is possible that they would be responsive to MEK inhibition. Cobimetinib, a MEK inhibitor initially approved to treat metastatic melanoma, was approved by the US Food and Drug Administration to treat histiocytic disorders in October 2022.7 The approval followed the release of data from a phase 2 trial of cobimetinib in 18 adults with various histiocytic disorders, which demonstrated an 89% (16/18) overall response rate with 94% (17/18) of patients remaining progression free at 1 year.8 While cobimetinib has not specifically been studied in ICH, given the high response rate in histiocytic disorders and the lack of standard treatment options for ICH, the decision was made to initiate treatment with cobimetinib in our patient. Based on the observed improvement in our patient, we propose cobimetinib as a treatment option for patients with cutaneous ICH and recommend additional studies to confirm its safety and efficacy in patients with this disorder.
- Bakry OA, Samaka RM, Kandil MA, et al. Indeterminate cell histiocytosis with naïve cells. Rare Tumors. 2013;5:e13. doi:10.4081 /rt.2013.e13
- Manente L, Cotellessa C, Schmitt I, et al. Indeterminate cell histiocytosis: a rare histiocytic disorder. Am J Dermatopathol. 1997; 19:276-283. doi:10.1097/00000372-199706000-00014
- Lie E, Jedrych J, Sweren R, et al. Generalized indeterminate cell histiocytosis successfully treated with methotrexate. JAAD Case Rep. 2022;25:93-96. doi:10.1016/j.jdcr.2022.05.027
- Fournier J, Ingraffea A, Pedvis-Leftick A. Successful treatment of indeterminate cell histiocytosis with low-dose methotrexate. J Dermatol. 2011;38:937-939. doi:10.1111/j.1346-8138.2010.01148.x
- Logemann N, Thomas B, Yetto T. Indeterminate cell histiocytosis successfully treated with narrowband UVB. Dermatol Online J. 2013;19:20031. doi:10.5070/D31910020031
- Fujimoto RFT, Miura H, Takata M, et al. Indeterminate cell histiocytosis treated with 0.5% delgocitinib ointment. Br J Dermatol. 2023;188:E39. doi:10.1093/bjd/ljad029
- Diamond EL, Durham B, Dogan A, et al. Phase 2 trial of single-agent cobimetinib for adults with histiocytic neoplasms. Blood. 2023;142:1812. doi:10.1182/blood-2023-187508
- Diamond EL, Durham BH, Ulaner GA, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567:521-524. doi:10.1038/s41586-019-1012-y
- Bakry OA, Samaka RM, Kandil MA, et al. Indeterminate cell histiocytosis with naïve cells. Rare Tumors. 2013;5:e13. doi:10.4081 /rt.2013.e13
- Manente L, Cotellessa C, Schmitt I, et al. Indeterminate cell histiocytosis: a rare histiocytic disorder. Am J Dermatopathol. 1997; 19:276-283. doi:10.1097/00000372-199706000-00014
- Lie E, Jedrych J, Sweren R, et al. Generalized indeterminate cell histiocytosis successfully treated with methotrexate. JAAD Case Rep. 2022;25:93-96. doi:10.1016/j.jdcr.2022.05.027
- Fournier J, Ingraffea A, Pedvis-Leftick A. Successful treatment of indeterminate cell histiocytosis with low-dose methotrexate. J Dermatol. 2011;38:937-939. doi:10.1111/j.1346-8138.2010.01148.x
- Logemann N, Thomas B, Yetto T. Indeterminate cell histiocytosis successfully treated with narrowband UVB. Dermatol Online J. 2013;19:20031. doi:10.5070/D31910020031
- Fujimoto RFT, Miura H, Takata M, et al. Indeterminate cell histiocytosis treated with 0.5% delgocitinib ointment. Br J Dermatol. 2023;188:E39. doi:10.1093/bjd/ljad029
- Diamond EL, Durham B, Dogan A, et al. Phase 2 trial of single-agent cobimetinib for adults with histiocytic neoplasms. Blood. 2023;142:1812. doi:10.1182/blood-2023-187508
- Diamond EL, Durham BH, Ulaner GA, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567:521-524. doi:10.1038/s41586-019-1012-y
Indeterminate Cell Histiocytosis and a Review of Current Treatment
Indeterminate Cell Histiocytosis and a Review of Current Treatment
PRACTICE POINTS
- Indeterminate cell histiocytosis (ICH) is a rare neoplastic dendritic cell disorder that can manifest as isolated or multiple papules or nodules on the face, neck, trunk, arms, or legs.
- Although there is no standard treatment for ICH, histiocytic disorders are characterized by mutations in the mitogen-activated protein kinase pathway and may be responsive to MEK inhibition.
- Cobimetinib, a MEK inhibitor initially approved to treat metastatic melanoma, was approved by the US Food and Drug Administration to treat histiocytic disorders in October 2022.