User login
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies
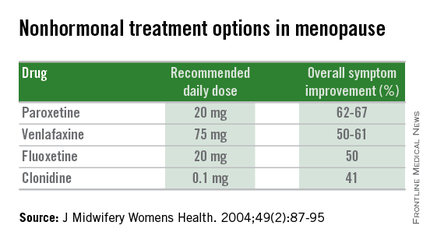
Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies

Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies

Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.


