User login
Tips for avoiding nerve injuries in gynecologic surgery
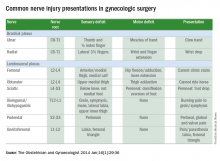
Upper- and lower-extremity injuries can occur during gynecologic surgery. The incidence of lower-extremity injury is 1.1%-1.9% and upper-extremity injuries can occur in 0.16% of cases.1-5 Fortunately, most of the injuries are transient, sensory injuries that resolve spontaneously. However, a small percentage of injuries result in long-term sequelae.
The pathophysiology of the nerve injuries can be mechanistically separated into three categories: neuropraxia, axonotmesis, and neurotmesis. Neuropraxia results from nerve demyelination at the site of injury because of compression and typically resolves within weeks to months as the nerve is remyelinated. Axonotmesis results from severe compression with axon damage. This may take up to a year to resolve as axonal regeneration proceeds at the rate of 1 mm per day. This can be separated into second and third degree and refers to the severity of damage and the resultant persistent deficit. Neurotmesis results from complete transection and is associated with a poor prognosis without reparative surgery.
Brachial plexus
Stretch injury is the most common reason for a brachial plexus injury. This can occur if the arm board is extended to greater than 90 degrees from the patient’s torso or if the patient’s arm falls off of the arm board. Careful positioning and securing the patient’s arm on the arm board before draping can avoid this injury. A brachial plexus injury can also occur if shoulder braces are placed too laterally during minimally invasive surgery. Radial nerve injuries can occur if there is too much pressure on the humerus during positioning. Ulnar injuries arise from pressure placed on the medial aspect of the elbow.
Tip #1: When tucking a patient’s arm for minimally invasive surgery, appropriate padding should be placed around the elbow and wrist, and the arm should be in the “thumbs-up” position.
Tip #2: Shoulder blocks should be placed over the acromioclavicular (AC) joint.
Lumbosacral plexus
The femoral nerve is the nerve most commonly injured during gynecologic surgery and this usually occurs because of compression of the nerve from the lateral blades of self-retaining retractors. One study showed an 8% incidence of injury from self-retaining retractors, compared with less than 1% when the retractors were not used.6 The femoral nerve can also be stretched when patients are placed in the lithotomy position and the hip is hyperflexed.
As with brachial injury prevention, patients should be positioned prior to draping and care must be taken to not hyperflex or externally rotate the hip during minimally invasive surgical procedures. With the introduction of robot-assisted surgery, care must be taken when docking the robot and surgeons must resist excessive movement of the stirrups.
Tip #3: During laparotomy, surgeons should use the shortest blades that allow for adequate visualization and check the blades during the procedure to ensure that excessive pressure is not placed on the psoas muscle. Consider intermittently releasing the pressure on the lateral blades during other portions of the procedure.
Tip #4: Make sure the stirrups are at the same height and that the leg is in line with the patient’s contralateral shoulder.
Obturator nerve injuries can occur during retroperitoneal dissection for pelvic lymphadenectomy (obturator nodes) and can be either a transection or a cautery injury. It can also be injured during urogynecologic procedures including paravaginal defect repairs and during the placement of transobturator tapes.
The sciatic nerve and its branch, the common peroneal, are generally injured because of excessive stretch or pressure. Both nerves can be injured from hyperflexion of the thigh and the common peroneal can suffer a pressure injury as it courses around the lateral head of the fibula. Therefore, care during lithotomy positioning with both candy cane and Allen stirrups is critical during vaginal surgery.
Tip #5: Ensure that the lateral fibula is not touching the stirrup or that padding is placed between the fibular head and the stirrup.
The ilioinguinal and iliohypogastric nerves are typically injured via suture entrapment from low transverse skin incisions, though laparoscopic injury has also been reported. The incidence after a Pfannenstiel incision is about 3.7%.7
Tip #6: Avoid extending the low transverse incision beyond the lateral margin of the rectus muscle, and do not extend the fascial closure suture more than 1.5 cm from the lateral edge of the fascial incision to avoid catching the nerve with the suture.
The pudendal nerve is most commonly injured during vaginal procedures such as sacrospinous fixation. Pain is typically worse when seated.
The genitofemoral nerve is typically injured during retroperitoneal lymph node dissection, particularly the external iliac nodes. The nerve is small and runs lateral to the external iliac artery. It can suffer cautery and transection injuries. Usually, the paresthesias over the mons pubis, labia majora, and medial inner thigh are temporary.
Tip #7: Care should be taken to identify and spare the nerve during retroperitoneal dissection or external iliac node removal.
Nerve injuries during gynecologic surgery are common and are a significant cause of potential morbidity. While occasionally unavoidable and inherent to the surgical procedure, many times the injury could be prevented with proper attention and care to patient positioning and retractor use. Gynecologists should be aware of the risks and have a through understanding of the anatomy. However, should an injury occur, the patient can be reassured that most are self-limited and full recovery is generally expected. In a prospective study, the median time to resolution of symptoms was 31.5 days (range, 1 day to 6 months).5
References
1. The Obstetrician & Gynaecologist 2014;16:29-36.
2. Gynecol Oncol. 1988 Nov;31(3):462-6.
3. Fertil Steril. 1993 Oct;60(4):729-32.
4. J Minim Invasive Gynecol. 2007 Sep-Oct;14(5):664-72.
5. Am J Obstet Gynecol. 2009 Nov;201(5):531.e1-7.
6. Eur J Obstet Gynecol Reprod Biol. 1985 Dec;20(6):385-92.
7. Obstet Gynecol. 2008 Apr;111(4):839-46.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no financial disclosures relevant to this column. Email her at [email protected].
Upper- and lower-extremity injuries can occur during gynecologic surgery. The incidence of lower-extremity injury is 1.1%-1.9% and upper-extremity injuries can occur in 0.16% of cases.1-5 Fortunately, most of the injuries are transient, sensory injuries that resolve spontaneously. However, a small percentage of injuries result in long-term sequelae.
The pathophysiology of the nerve injuries can be mechanistically separated into three categories: neuropraxia, axonotmesis, and neurotmesis. Neuropraxia results from nerve demyelination at the site of injury because of compression and typically resolves within weeks to months as the nerve is remyelinated. Axonotmesis results from severe compression with axon damage. This may take up to a year to resolve as axonal regeneration proceeds at the rate of 1 mm per day. This can be separated into second and third degree and refers to the severity of damage and the resultant persistent deficit. Neurotmesis results from complete transection and is associated with a poor prognosis without reparative surgery.
Brachial plexus
Stretch injury is the most common reason for a brachial plexus injury. This can occur if the arm board is extended to greater than 90 degrees from the patient’s torso or if the patient’s arm falls off of the arm board. Careful positioning and securing the patient’s arm on the arm board before draping can avoid this injury. A brachial plexus injury can also occur if shoulder braces are placed too laterally during minimally invasive surgery. Radial nerve injuries can occur if there is too much pressure on the humerus during positioning. Ulnar injuries arise from pressure placed on the medial aspect of the elbow.
Tip #1: When tucking a patient’s arm for minimally invasive surgery, appropriate padding should be placed around the elbow and wrist, and the arm should be in the “thumbs-up” position.
Tip #2: Shoulder blocks should be placed over the acromioclavicular (AC) joint.
Lumbosacral plexus
The femoral nerve is the nerve most commonly injured during gynecologic surgery and this usually occurs because of compression of the nerve from the lateral blades of self-retaining retractors. One study showed an 8% incidence of injury from self-retaining retractors, compared with less than 1% when the retractors were not used.6 The femoral nerve can also be stretched when patients are placed in the lithotomy position and the hip is hyperflexed.
As with brachial injury prevention, patients should be positioned prior to draping and care must be taken to not hyperflex or externally rotate the hip during minimally invasive surgical procedures. With the introduction of robot-assisted surgery, care must be taken when docking the robot and surgeons must resist excessive movement of the stirrups.
Tip #3: During laparotomy, surgeons should use the shortest blades that allow for adequate visualization and check the blades during the procedure to ensure that excessive pressure is not placed on the psoas muscle. Consider intermittently releasing the pressure on the lateral blades during other portions of the procedure.
Tip #4: Make sure the stirrups are at the same height and that the leg is in line with the patient’s contralateral shoulder.
Obturator nerve injuries can occur during retroperitoneal dissection for pelvic lymphadenectomy (obturator nodes) and can be either a transection or a cautery injury. It can also be injured during urogynecologic procedures including paravaginal defect repairs and during the placement of transobturator tapes.
The sciatic nerve and its branch, the common peroneal, are generally injured because of excessive stretch or pressure. Both nerves can be injured from hyperflexion of the thigh and the common peroneal can suffer a pressure injury as it courses around the lateral head of the fibula. Therefore, care during lithotomy positioning with both candy cane and Allen stirrups is critical during vaginal surgery.
Tip #5: Ensure that the lateral fibula is not touching the stirrup or that padding is placed between the fibular head and the stirrup.
The ilioinguinal and iliohypogastric nerves are typically injured via suture entrapment from low transverse skin incisions, though laparoscopic injury has also been reported. The incidence after a Pfannenstiel incision is about 3.7%.7
Tip #6: Avoid extending the low transverse incision beyond the lateral margin of the rectus muscle, and do not extend the fascial closure suture more than 1.5 cm from the lateral edge of the fascial incision to avoid catching the nerve with the suture.
The pudendal nerve is most commonly injured during vaginal procedures such as sacrospinous fixation. Pain is typically worse when seated.
The genitofemoral nerve is typically injured during retroperitoneal lymph node dissection, particularly the external iliac nodes. The nerve is small and runs lateral to the external iliac artery. It can suffer cautery and transection injuries. Usually, the paresthesias over the mons pubis, labia majora, and medial inner thigh are temporary.
Tip #7: Care should be taken to identify and spare the nerve during retroperitoneal dissection or external iliac node removal.
Nerve injuries during gynecologic surgery are common and are a significant cause of potential morbidity. While occasionally unavoidable and inherent to the surgical procedure, many times the injury could be prevented with proper attention and care to patient positioning and retractor use. Gynecologists should be aware of the risks and have a through understanding of the anatomy. However, should an injury occur, the patient can be reassured that most are self-limited and full recovery is generally expected. In a prospective study, the median time to resolution of symptoms was 31.5 days (range, 1 day to 6 months).5
References
1. The Obstetrician & Gynaecologist 2014;16:29-36.
2. Gynecol Oncol. 1988 Nov;31(3):462-6.
3. Fertil Steril. 1993 Oct;60(4):729-32.
4. J Minim Invasive Gynecol. 2007 Sep-Oct;14(5):664-72.
5. Am J Obstet Gynecol. 2009 Nov;201(5):531.e1-7.
6. Eur J Obstet Gynecol Reprod Biol. 1985 Dec;20(6):385-92.
7. Obstet Gynecol. 2008 Apr;111(4):839-46.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no financial disclosures relevant to this column. Email her at [email protected].
Upper- and lower-extremity injuries can occur during gynecologic surgery. The incidence of lower-extremity injury is 1.1%-1.9% and upper-extremity injuries can occur in 0.16% of cases.1-5 Fortunately, most of the injuries are transient, sensory injuries that resolve spontaneously. However, a small percentage of injuries result in long-term sequelae.
The pathophysiology of the nerve injuries can be mechanistically separated into three categories: neuropraxia, axonotmesis, and neurotmesis. Neuropraxia results from nerve demyelination at the site of injury because of compression and typically resolves within weeks to months as the nerve is remyelinated. Axonotmesis results from severe compression with axon damage. This may take up to a year to resolve as axonal regeneration proceeds at the rate of 1 mm per day. This can be separated into second and third degree and refers to the severity of damage and the resultant persistent deficit. Neurotmesis results from complete transection and is associated with a poor prognosis without reparative surgery.
Brachial plexus
Stretch injury is the most common reason for a brachial plexus injury. This can occur if the arm board is extended to greater than 90 degrees from the patient’s torso or if the patient’s arm falls off of the arm board. Careful positioning and securing the patient’s arm on the arm board before draping can avoid this injury. A brachial plexus injury can also occur if shoulder braces are placed too laterally during minimally invasive surgery. Radial nerve injuries can occur if there is too much pressure on the humerus during positioning. Ulnar injuries arise from pressure placed on the medial aspect of the elbow.
Tip #1: When tucking a patient’s arm for minimally invasive surgery, appropriate padding should be placed around the elbow and wrist, and the arm should be in the “thumbs-up” position.
Tip #2: Shoulder blocks should be placed over the acromioclavicular (AC) joint.
Lumbosacral plexus
The femoral nerve is the nerve most commonly injured during gynecologic surgery and this usually occurs because of compression of the nerve from the lateral blades of self-retaining retractors. One study showed an 8% incidence of injury from self-retaining retractors, compared with less than 1% when the retractors were not used.6 The femoral nerve can also be stretched when patients are placed in the lithotomy position and the hip is hyperflexed.
As with brachial injury prevention, patients should be positioned prior to draping and care must be taken to not hyperflex or externally rotate the hip during minimally invasive surgical procedures. With the introduction of robot-assisted surgery, care must be taken when docking the robot and surgeons must resist excessive movement of the stirrups.
Tip #3: During laparotomy, surgeons should use the shortest blades that allow for adequate visualization and check the blades during the procedure to ensure that excessive pressure is not placed on the psoas muscle. Consider intermittently releasing the pressure on the lateral blades during other portions of the procedure.
Tip #4: Make sure the stirrups are at the same height and that the leg is in line with the patient’s contralateral shoulder.
Obturator nerve injuries can occur during retroperitoneal dissection for pelvic lymphadenectomy (obturator nodes) and can be either a transection or a cautery injury. It can also be injured during urogynecologic procedures including paravaginal defect repairs and during the placement of transobturator tapes.
The sciatic nerve and its branch, the common peroneal, are generally injured because of excessive stretch or pressure. Both nerves can be injured from hyperflexion of the thigh and the common peroneal can suffer a pressure injury as it courses around the lateral head of the fibula. Therefore, care during lithotomy positioning with both candy cane and Allen stirrups is critical during vaginal surgery.
Tip #5: Ensure that the lateral fibula is not touching the stirrup or that padding is placed between the fibular head and the stirrup.
The ilioinguinal and iliohypogastric nerves are typically injured via suture entrapment from low transverse skin incisions, though laparoscopic injury has also been reported. The incidence after a Pfannenstiel incision is about 3.7%.7
Tip #6: Avoid extending the low transverse incision beyond the lateral margin of the rectus muscle, and do not extend the fascial closure suture more than 1.5 cm from the lateral edge of the fascial incision to avoid catching the nerve with the suture.
The pudendal nerve is most commonly injured during vaginal procedures such as sacrospinous fixation. Pain is typically worse when seated.
The genitofemoral nerve is typically injured during retroperitoneal lymph node dissection, particularly the external iliac nodes. The nerve is small and runs lateral to the external iliac artery. It can suffer cautery and transection injuries. Usually, the paresthesias over the mons pubis, labia majora, and medial inner thigh are temporary.
Tip #7: Care should be taken to identify and spare the nerve during retroperitoneal dissection or external iliac node removal.
Nerve injuries during gynecologic surgery are common and are a significant cause of potential morbidity. While occasionally unavoidable and inherent to the surgical procedure, many times the injury could be prevented with proper attention and care to patient positioning and retractor use. Gynecologists should be aware of the risks and have a through understanding of the anatomy. However, should an injury occur, the patient can be reassured that most are self-limited and full recovery is generally expected. In a prospective study, the median time to resolution of symptoms was 31.5 days (range, 1 day to 6 months).5
References
1. The Obstetrician & Gynaecologist 2014;16:29-36.
2. Gynecol Oncol. 1988 Nov;31(3):462-6.
3. Fertil Steril. 1993 Oct;60(4):729-32.
4. J Minim Invasive Gynecol. 2007 Sep-Oct;14(5):664-72.
5. Am J Obstet Gynecol. 2009 Nov;201(5):531.e1-7.
6. Eur J Obstet Gynecol Reprod Biol. 1985 Dec;20(6):385-92.
7. Obstet Gynecol. 2008 Apr;111(4):839-46.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no financial disclosures relevant to this column. Email her at [email protected].
Enhanced recovery pathways in gynecology
Enhanced recovery surgical principles were first described in the 1990s.1 These principles postulate that the body’s stress response to surgical injury and deviation from normal physiology is the source of postoperative morbidity. Thus, enhanced recovery programs are designed around perioperative interventions that mitigate and help the body cope with the surgical stress response.
Many of these interventions run counter to traditional perioperative care paradigms. Enhanced recovery protocols are diverse but have common themes of avoiding preoperative fasting and bowel preparation, early oral intake, limiting use of drains and catheters, multimodal analgesia, early ambulation, and prioritizing euvolemia and normothermia. Individual interventions in these areas are combined to create a master protocol, which is implemented as a bundle to improve surgical outcomes.
Current components
Minimizing preoperative fasting, early postoperative refeeding, and preoperative carbohydrate-loading drinks are all key aspects of enhanced recovery protocols. “NPO after midnight” has been a longstanding rule due to the risk of aspiration with intubation. However, a Cochrane review found no evidence that a shortened period of fasting was associated with an increased risk of aspiration or related morbidity. Currently, the American Society of Anesthesiologists recommends only a 6-hour fast for solid foods and 2 hours for clear liquids.2,3
Preoperative fasting causes depletion of glycogen stores leading to insulin resistance and hyperglycemia, which are both associated with postoperative complications and morbidity.4 Preoperative carbohydrate-loading drinks can reverse some of the effects of limited preoperative fasting including preventing insulin resistance and hyperglycemia.5
Postoperative fasting should also be avoided. Early enteral intake is very important to decrease time spent in a catabolic state and decrease insulin resistance. In gynecology patients, early refeeding is associated with a faster return of bowel function and a decreased length of stay without an increase in postoperative complications.6 Notably, patients undergoing early feeding consistently experience more nausea and vomiting, but this is not associated with complications.7
The fluid management goal in enhanced recovery is to maintain perioperative euvolemia, as both hypovolemia and hypervolemia have negative physiologic consequences. When studied, fluid protocols designed to minimize the use of postoperative fluids have resulted in decreased cardiopulmonary complications, decreased postoperative morbidity, faster return of bowel function, and shorter hospital stays.8 Given the morbidity associated with fluid overload, enhanced recovery protocols recommend that minimal fluids be given in the operating room and intravenous fluids be removed as quickly as possible, often with first oral intake or postoperative day 1 at the latest.
Engagement of the patient in their perioperative recovery with patient education materials and expectations for postoperative tasks, such as early refeeding, spirometry, and ambulation are all important components of enhanced recovery. Patients become partners in achieving postoperative milestones, and this results in improved outcomes such as decreased pain scores and shorter recoveries.
Evidence in gynecology
Enhanced recovery has been studied in many surgical disciplines including urology, colorectal surgery, hepatobiliary surgery, and gynecology. High-quality studies of abdominal and vaginal hysterectomy patients have consistently found a decrease in length of stay with no difference in readmission or postoperative complication rates.9 An interesting study also found that an enhanced recovery program was associated with decreased nursing time required for patient care.10
For ovarian cancer patients, enhanced recovery is associated with decreased length of stay, decreased time to return of bowel function, and improved quality of life. Enhanced recovery is also cost saving, saving $257-$697 per vaginal hysterectomy patient and $5,410-$7,600 per ovarian cancer patient.11
Enhanced recovery protocols are safe, evidenced based, cost saving, and are increasingly being adopted as clinicians and health systems become aware of their benefits.
References
1. Br J Anaesth. 1997 May;78(5):606-17.
2. Cochrane Database Syst Rev. 2003 Oct 20;(4):CD004423.
3. Anesthesiology. 1999 Mar;90(3):896-905.
4. J Am Coll Surg. 2012 Jan;214(1):68-80.
5. Clin Nutr. 1998 Apr;17(2):65-71.
6. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD004508.
7. Obstet Gynecol. 1998 Jul;92(1):94-7.
8. Br J Surg. 2009 Apr;96(4):331-41.
9. Obstet Gynecol. 2013 Aug;122(2 Pt 1):319-28.
10. Qual Saf Health Care. 2009 Jun;18(3):236-40.
11. Gynecol Oncol. 2008 Feb;108(2):282-6.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Barber is a third-year fellow in gynecologic oncology at the university. They reported having no financial disclosures relevant to this column. Email them at [email protected].
Enhanced recovery surgical principles were first described in the 1990s.1 These principles postulate that the body’s stress response to surgical injury and deviation from normal physiology is the source of postoperative morbidity. Thus, enhanced recovery programs are designed around perioperative interventions that mitigate and help the body cope with the surgical stress response.
Many of these interventions run counter to traditional perioperative care paradigms. Enhanced recovery protocols are diverse but have common themes of avoiding preoperative fasting and bowel preparation, early oral intake, limiting use of drains and catheters, multimodal analgesia, early ambulation, and prioritizing euvolemia and normothermia. Individual interventions in these areas are combined to create a master protocol, which is implemented as a bundle to improve surgical outcomes.
Current components
Minimizing preoperative fasting, early postoperative refeeding, and preoperative carbohydrate-loading drinks are all key aspects of enhanced recovery protocols. “NPO after midnight” has been a longstanding rule due to the risk of aspiration with intubation. However, a Cochrane review found no evidence that a shortened period of fasting was associated with an increased risk of aspiration or related morbidity. Currently, the American Society of Anesthesiologists recommends only a 6-hour fast for solid foods and 2 hours for clear liquids.2,3
Preoperative fasting causes depletion of glycogen stores leading to insulin resistance and hyperglycemia, which are both associated with postoperative complications and morbidity.4 Preoperative carbohydrate-loading drinks can reverse some of the effects of limited preoperative fasting including preventing insulin resistance and hyperglycemia.5
Postoperative fasting should also be avoided. Early enteral intake is very important to decrease time spent in a catabolic state and decrease insulin resistance. In gynecology patients, early refeeding is associated with a faster return of bowel function and a decreased length of stay without an increase in postoperative complications.6 Notably, patients undergoing early feeding consistently experience more nausea and vomiting, but this is not associated with complications.7
The fluid management goal in enhanced recovery is to maintain perioperative euvolemia, as both hypovolemia and hypervolemia have negative physiologic consequences. When studied, fluid protocols designed to minimize the use of postoperative fluids have resulted in decreased cardiopulmonary complications, decreased postoperative morbidity, faster return of bowel function, and shorter hospital stays.8 Given the morbidity associated with fluid overload, enhanced recovery protocols recommend that minimal fluids be given in the operating room and intravenous fluids be removed as quickly as possible, often with first oral intake or postoperative day 1 at the latest.
Engagement of the patient in their perioperative recovery with patient education materials and expectations for postoperative tasks, such as early refeeding, spirometry, and ambulation are all important components of enhanced recovery. Patients become partners in achieving postoperative milestones, and this results in improved outcomes such as decreased pain scores and shorter recoveries.
Evidence in gynecology
Enhanced recovery has been studied in many surgical disciplines including urology, colorectal surgery, hepatobiliary surgery, and gynecology. High-quality studies of abdominal and vaginal hysterectomy patients have consistently found a decrease in length of stay with no difference in readmission or postoperative complication rates.9 An interesting study also found that an enhanced recovery program was associated with decreased nursing time required for patient care.10
For ovarian cancer patients, enhanced recovery is associated with decreased length of stay, decreased time to return of bowel function, and improved quality of life. Enhanced recovery is also cost saving, saving $257-$697 per vaginal hysterectomy patient and $5,410-$7,600 per ovarian cancer patient.11
Enhanced recovery protocols are safe, evidenced based, cost saving, and are increasingly being adopted as clinicians and health systems become aware of their benefits.
References
1. Br J Anaesth. 1997 May;78(5):606-17.
2. Cochrane Database Syst Rev. 2003 Oct 20;(4):CD004423.
3. Anesthesiology. 1999 Mar;90(3):896-905.
4. J Am Coll Surg. 2012 Jan;214(1):68-80.
5. Clin Nutr. 1998 Apr;17(2):65-71.
6. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD004508.
7. Obstet Gynecol. 1998 Jul;92(1):94-7.
8. Br J Surg. 2009 Apr;96(4):331-41.
9. Obstet Gynecol. 2013 Aug;122(2 Pt 1):319-28.
10. Qual Saf Health Care. 2009 Jun;18(3):236-40.
11. Gynecol Oncol. 2008 Feb;108(2):282-6.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Barber is a third-year fellow in gynecologic oncology at the university. They reported having no financial disclosures relevant to this column. Email them at [email protected].
Enhanced recovery surgical principles were first described in the 1990s.1 These principles postulate that the body’s stress response to surgical injury and deviation from normal physiology is the source of postoperative morbidity. Thus, enhanced recovery programs are designed around perioperative interventions that mitigate and help the body cope with the surgical stress response.
Many of these interventions run counter to traditional perioperative care paradigms. Enhanced recovery protocols are diverse but have common themes of avoiding preoperative fasting and bowel preparation, early oral intake, limiting use of drains and catheters, multimodal analgesia, early ambulation, and prioritizing euvolemia and normothermia. Individual interventions in these areas are combined to create a master protocol, which is implemented as a bundle to improve surgical outcomes.
Current components
Minimizing preoperative fasting, early postoperative refeeding, and preoperative carbohydrate-loading drinks are all key aspects of enhanced recovery protocols. “NPO after midnight” has been a longstanding rule due to the risk of aspiration with intubation. However, a Cochrane review found no evidence that a shortened period of fasting was associated with an increased risk of aspiration or related morbidity. Currently, the American Society of Anesthesiologists recommends only a 6-hour fast for solid foods and 2 hours for clear liquids.2,3
Preoperative fasting causes depletion of glycogen stores leading to insulin resistance and hyperglycemia, which are both associated with postoperative complications and morbidity.4 Preoperative carbohydrate-loading drinks can reverse some of the effects of limited preoperative fasting including preventing insulin resistance and hyperglycemia.5
Postoperative fasting should also be avoided. Early enteral intake is very important to decrease time spent in a catabolic state and decrease insulin resistance. In gynecology patients, early refeeding is associated with a faster return of bowel function and a decreased length of stay without an increase in postoperative complications.6 Notably, patients undergoing early feeding consistently experience more nausea and vomiting, but this is not associated with complications.7
The fluid management goal in enhanced recovery is to maintain perioperative euvolemia, as both hypovolemia and hypervolemia have negative physiologic consequences. When studied, fluid protocols designed to minimize the use of postoperative fluids have resulted in decreased cardiopulmonary complications, decreased postoperative morbidity, faster return of bowel function, and shorter hospital stays.8 Given the morbidity associated with fluid overload, enhanced recovery protocols recommend that minimal fluids be given in the operating room and intravenous fluids be removed as quickly as possible, often with first oral intake or postoperative day 1 at the latest.
Engagement of the patient in their perioperative recovery with patient education materials and expectations for postoperative tasks, such as early refeeding, spirometry, and ambulation are all important components of enhanced recovery. Patients become partners in achieving postoperative milestones, and this results in improved outcomes such as decreased pain scores and shorter recoveries.
Evidence in gynecology
Enhanced recovery has been studied in many surgical disciplines including urology, colorectal surgery, hepatobiliary surgery, and gynecology. High-quality studies of abdominal and vaginal hysterectomy patients have consistently found a decrease in length of stay with no difference in readmission or postoperative complication rates.9 An interesting study also found that an enhanced recovery program was associated with decreased nursing time required for patient care.10
For ovarian cancer patients, enhanced recovery is associated with decreased length of stay, decreased time to return of bowel function, and improved quality of life. Enhanced recovery is also cost saving, saving $257-$697 per vaginal hysterectomy patient and $5,410-$7,600 per ovarian cancer patient.11
Enhanced recovery protocols are safe, evidenced based, cost saving, and are increasingly being adopted as clinicians and health systems become aware of their benefits.
References
1. Br J Anaesth. 1997 May;78(5):606-17.
2. Cochrane Database Syst Rev. 2003 Oct 20;(4):CD004423.
3. Anesthesiology. 1999 Mar;90(3):896-905.
4. J Am Coll Surg. 2012 Jan;214(1):68-80.
5. Clin Nutr. 1998 Apr;17(2):65-71.
6. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD004508.
7. Obstet Gynecol. 1998 Jul;92(1):94-7.
8. Br J Surg. 2009 Apr;96(4):331-41.
9. Obstet Gynecol. 2013 Aug;122(2 Pt 1):319-28.
10. Qual Saf Health Care. 2009 Jun;18(3):236-40.
11. Gynecol Oncol. 2008 Feb;108(2):282-6.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Barber is a third-year fellow in gynecologic oncology at the university. They reported having no financial disclosures relevant to this column. Email them at [email protected].
The role of lymphadenectomy in endometrial cancer, Part 1
Endometrial cancer is the most common gynecologic malignancy in the United States. Fortunately, most endometrial cancers present at an early stage with excellent overall survival – approximately 85% – in clinical stage I disease. Since 1988, the International Federation of Gynecology and Obstetrics (FIGO) staging of endometrial cancer has required surgical staging reflecting increasing data on the prognostic significance of lymph node metastasis and the treatment implications for node positive cancers.
Indeed, lymph nodes represent the most common location for extrauterine spread in endometrial cancer. The lymphatic drainage from the uterus is to both the pelvic and the para-aortic lymph nodes. Lymphatic channels from the uterus can drain directly from the fundus via the infundibulopelvic ligament to the aortic lymph node chain, thereby bypassing the pelvic lymph nodes. As a result, there is a 2%-3% risk of isolated aortic metastasis with negative pelvic lymph nodes.
The extent of lymph node evaluation required for staging is debatable. The National Comprehensive Cancer Network (NCCN) guidelines recommend complete hysterectomy with bilateral salpingo-oophorectomy and additional procedures based on preoperative and intraoperative findings. During surgery, the surgeon should evaluate all peritoneal surfaces and the retroperitoneal lymphatic chains for abnormalities. All suspicious lymph nodes should be removed, but the extent of lymphadenectomy should be based on the NCCN guidelines.1 The NCCN offers the option for use of sentinel lymph node evaluation with adherence to specific staging algorithms for this technology.
Proponents of lymphadenectomy cite the need for accurate staging to guide adjuvant therapies, to provide prognostic information, and to eradicate metastatic lymph nodes with possible therapeutic benefit. However, criticisms of lymphadenectomy include a lack of randomized studies demonstrating a therapeutic benefit and the morbidity of lymphedema with its corresponding quality of life and cost implications. As a result, practices regarding lymph node evaluation vary widely.
There is conflicting data on whether there is a therapeutic benefit to performing lymphadenectomy. Retrospective studies have shown a benefit, but this was not seen in two prospective trials. There appears to be clear benefit for debulking of clinically enlarged nodal metastasis,2,3 and likely benefit to resection of microscopic metastasis, particularly with combined pelvic and aortic lymphadenectomy in high-risk endometrial cancers.4,5,6,7,8
The ASTEC trial by Kitchener et al and an Italian collaborative trial by Benedetti et al, however, both evaluated the role of lymph node dissection in predominantly low-risk endometrial cancer and found no benefit.9,10 Both studies documented no difference in overall survival, but increased morbidity with lymphadenectomy. No prospective trials have evaluated the role of lymphadenectomy in high-risk endometrial cancers.
Universal use of complete lymphadenectomy in all patients with endometrial cancer would subject a large percent of low risk patients to undo surgical risk. The two most commonly utilized strategies are risk factor based lymphadenectomy and sentinel lymph node evaluation.
Tumors are considered low risk if they are less than 2cm in size, grade 1 or 2, and superficially invasive (less than 50% myometrial invasion).11 The risk of lymph node metastasis in these patients was exceedingly low with no lymph node metastasis detect in more than 400 women who prospectively underwent this evaluation, thus lymphadenectomy can be safely avoided. Utilizing risk factor based lymphadenectomy does require the availability of reliable frozen section pathology evaluation, which may be a limitation for some institutions.
A key argument against routine use of systematic lymphadenectomy is the concern for postoperative complications and lymphedema. The estimated incidence of lymphedema following lymphadenectomy is 20%-30%.12 However, there are challenges in studying lymphedema that likely limit our understanding of the true incidence. The ASTEC trial and Italian cooperative trial have demonstrated that there is an eight-fold increased risk of lymphedema in women who undergo lymphadenectomy, compared with those who do not.13 The development of lymphedema requires ongoing treatment with associated costs of care. Thus, the selective lymphadenectomy or sentinel nodes have the ability to reduce healthcare costs.14 Sentinel lymph nodes will be covered in Part Two of this article.
References
1. J Natl Compr Canc Netw. 2014 Feb;12(2):248-80.
2. Gynecol Oncol. 2005 Dec;99(3):689-95.
3. Int J Gynecol Cancer. 2003 Sep-Oct;13(5):664-72.
4. Gynecol Oncol. 1995 Jan;56(1):29-33.
5. J Clin Oncol. 2005 Jun 1;23(16):3668-75.
6. Lancet. 2010 Apr 3;375(9721):1165-72.
7. Gynecol Oncol. 1998 Dec;71(3):340-3.
8. Cancer. 2006 Oct 15;107(8):1823-30.
9. Lancet. 2009 Jan 10;373(9658):125-36.
10. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
11. Gynecol Oncol. 2008 Apr;109(1):11-8.
12. Obstet Gynecol. 2014 Aug;124(2 Pt 1):307-15.
13. Cochrane Database Syst Rev. 2015 Sep 21;(9):CD007585.
14. Gynecol Oncol. 2014 Dec;135(3):518-24.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Clark is a fellow in the division of gynecologic oncology, department of obstetrics and gynecology at the university. They reported having no financial disclosures relevant to this column. Email them at [email protected].
Endometrial cancer is the most common gynecologic malignancy in the United States. Fortunately, most endometrial cancers present at an early stage with excellent overall survival – approximately 85% – in clinical stage I disease. Since 1988, the International Federation of Gynecology and Obstetrics (FIGO) staging of endometrial cancer has required surgical staging reflecting increasing data on the prognostic significance of lymph node metastasis and the treatment implications for node positive cancers.
Indeed, lymph nodes represent the most common location for extrauterine spread in endometrial cancer. The lymphatic drainage from the uterus is to both the pelvic and the para-aortic lymph nodes. Lymphatic channels from the uterus can drain directly from the fundus via the infundibulopelvic ligament to the aortic lymph node chain, thereby bypassing the pelvic lymph nodes. As a result, there is a 2%-3% risk of isolated aortic metastasis with negative pelvic lymph nodes.
The extent of lymph node evaluation required for staging is debatable. The National Comprehensive Cancer Network (NCCN) guidelines recommend complete hysterectomy with bilateral salpingo-oophorectomy and additional procedures based on preoperative and intraoperative findings. During surgery, the surgeon should evaluate all peritoneal surfaces and the retroperitoneal lymphatic chains for abnormalities. All suspicious lymph nodes should be removed, but the extent of lymphadenectomy should be based on the NCCN guidelines.1 The NCCN offers the option for use of sentinel lymph node evaluation with adherence to specific staging algorithms for this technology.
Proponents of lymphadenectomy cite the need for accurate staging to guide adjuvant therapies, to provide prognostic information, and to eradicate metastatic lymph nodes with possible therapeutic benefit. However, criticisms of lymphadenectomy include a lack of randomized studies demonstrating a therapeutic benefit and the morbidity of lymphedema with its corresponding quality of life and cost implications. As a result, practices regarding lymph node evaluation vary widely.
There is conflicting data on whether there is a therapeutic benefit to performing lymphadenectomy. Retrospective studies have shown a benefit, but this was not seen in two prospective trials. There appears to be clear benefit for debulking of clinically enlarged nodal metastasis,2,3 and likely benefit to resection of microscopic metastasis, particularly with combined pelvic and aortic lymphadenectomy in high-risk endometrial cancers.4,5,6,7,8
The ASTEC trial by Kitchener et al and an Italian collaborative trial by Benedetti et al, however, both evaluated the role of lymph node dissection in predominantly low-risk endometrial cancer and found no benefit.9,10 Both studies documented no difference in overall survival, but increased morbidity with lymphadenectomy. No prospective trials have evaluated the role of lymphadenectomy in high-risk endometrial cancers.
Universal use of complete lymphadenectomy in all patients with endometrial cancer would subject a large percent of low risk patients to undo surgical risk. The two most commonly utilized strategies are risk factor based lymphadenectomy and sentinel lymph node evaluation.
Tumors are considered low risk if they are less than 2cm in size, grade 1 or 2, and superficially invasive (less than 50% myometrial invasion).11 The risk of lymph node metastasis in these patients was exceedingly low with no lymph node metastasis detect in more than 400 women who prospectively underwent this evaluation, thus lymphadenectomy can be safely avoided. Utilizing risk factor based lymphadenectomy does require the availability of reliable frozen section pathology evaluation, which may be a limitation for some institutions.
A key argument against routine use of systematic lymphadenectomy is the concern for postoperative complications and lymphedema. The estimated incidence of lymphedema following lymphadenectomy is 20%-30%.12 However, there are challenges in studying lymphedema that likely limit our understanding of the true incidence. The ASTEC trial and Italian cooperative trial have demonstrated that there is an eight-fold increased risk of lymphedema in women who undergo lymphadenectomy, compared with those who do not.13 The development of lymphedema requires ongoing treatment with associated costs of care. Thus, the selective lymphadenectomy or sentinel nodes have the ability to reduce healthcare costs.14 Sentinel lymph nodes will be covered in Part Two of this article.
References
1. J Natl Compr Canc Netw. 2014 Feb;12(2):248-80.
2. Gynecol Oncol. 2005 Dec;99(3):689-95.
3. Int J Gynecol Cancer. 2003 Sep-Oct;13(5):664-72.
4. Gynecol Oncol. 1995 Jan;56(1):29-33.
5. J Clin Oncol. 2005 Jun 1;23(16):3668-75.
6. Lancet. 2010 Apr 3;375(9721):1165-72.
7. Gynecol Oncol. 1998 Dec;71(3):340-3.
8. Cancer. 2006 Oct 15;107(8):1823-30.
9. Lancet. 2009 Jan 10;373(9658):125-36.
10. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
11. Gynecol Oncol. 2008 Apr;109(1):11-8.
12. Obstet Gynecol. 2014 Aug;124(2 Pt 1):307-15.
13. Cochrane Database Syst Rev. 2015 Sep 21;(9):CD007585.
14. Gynecol Oncol. 2014 Dec;135(3):518-24.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Clark is a fellow in the division of gynecologic oncology, department of obstetrics and gynecology at the university. They reported having no financial disclosures relevant to this column. Email them at [email protected].
Endometrial cancer is the most common gynecologic malignancy in the United States. Fortunately, most endometrial cancers present at an early stage with excellent overall survival – approximately 85% – in clinical stage I disease. Since 1988, the International Federation of Gynecology and Obstetrics (FIGO) staging of endometrial cancer has required surgical staging reflecting increasing data on the prognostic significance of lymph node metastasis and the treatment implications for node positive cancers.
Indeed, lymph nodes represent the most common location for extrauterine spread in endometrial cancer. The lymphatic drainage from the uterus is to both the pelvic and the para-aortic lymph nodes. Lymphatic channels from the uterus can drain directly from the fundus via the infundibulopelvic ligament to the aortic lymph node chain, thereby bypassing the pelvic lymph nodes. As a result, there is a 2%-3% risk of isolated aortic metastasis with negative pelvic lymph nodes.
The extent of lymph node evaluation required for staging is debatable. The National Comprehensive Cancer Network (NCCN) guidelines recommend complete hysterectomy with bilateral salpingo-oophorectomy and additional procedures based on preoperative and intraoperative findings. During surgery, the surgeon should evaluate all peritoneal surfaces and the retroperitoneal lymphatic chains for abnormalities. All suspicious lymph nodes should be removed, but the extent of lymphadenectomy should be based on the NCCN guidelines.1 The NCCN offers the option for use of sentinel lymph node evaluation with adherence to specific staging algorithms for this technology.
Proponents of lymphadenectomy cite the need for accurate staging to guide adjuvant therapies, to provide prognostic information, and to eradicate metastatic lymph nodes with possible therapeutic benefit. However, criticisms of lymphadenectomy include a lack of randomized studies demonstrating a therapeutic benefit and the morbidity of lymphedema with its corresponding quality of life and cost implications. As a result, practices regarding lymph node evaluation vary widely.
There is conflicting data on whether there is a therapeutic benefit to performing lymphadenectomy. Retrospective studies have shown a benefit, but this was not seen in two prospective trials. There appears to be clear benefit for debulking of clinically enlarged nodal metastasis,2,3 and likely benefit to resection of microscopic metastasis, particularly with combined pelvic and aortic lymphadenectomy in high-risk endometrial cancers.4,5,6,7,8
The ASTEC trial by Kitchener et al and an Italian collaborative trial by Benedetti et al, however, both evaluated the role of lymph node dissection in predominantly low-risk endometrial cancer and found no benefit.9,10 Both studies documented no difference in overall survival, but increased morbidity with lymphadenectomy. No prospective trials have evaluated the role of lymphadenectomy in high-risk endometrial cancers.
Universal use of complete lymphadenectomy in all patients with endometrial cancer would subject a large percent of low risk patients to undo surgical risk. The two most commonly utilized strategies are risk factor based lymphadenectomy and sentinel lymph node evaluation.
Tumors are considered low risk if they are less than 2cm in size, grade 1 or 2, and superficially invasive (less than 50% myometrial invasion).11 The risk of lymph node metastasis in these patients was exceedingly low with no lymph node metastasis detect in more than 400 women who prospectively underwent this evaluation, thus lymphadenectomy can be safely avoided. Utilizing risk factor based lymphadenectomy does require the availability of reliable frozen section pathology evaluation, which may be a limitation for some institutions.
A key argument against routine use of systematic lymphadenectomy is the concern for postoperative complications and lymphedema. The estimated incidence of lymphedema following lymphadenectomy is 20%-30%.12 However, there are challenges in studying lymphedema that likely limit our understanding of the true incidence. The ASTEC trial and Italian cooperative trial have demonstrated that there is an eight-fold increased risk of lymphedema in women who undergo lymphadenectomy, compared with those who do not.13 The development of lymphedema requires ongoing treatment with associated costs of care. Thus, the selective lymphadenectomy or sentinel nodes have the ability to reduce healthcare costs.14 Sentinel lymph nodes will be covered in Part Two of this article.
References
1. J Natl Compr Canc Netw. 2014 Feb;12(2):248-80.
2. Gynecol Oncol. 2005 Dec;99(3):689-95.
3. Int J Gynecol Cancer. 2003 Sep-Oct;13(5):664-72.
4. Gynecol Oncol. 1995 Jan;56(1):29-33.
5. J Clin Oncol. 2005 Jun 1;23(16):3668-75.
6. Lancet. 2010 Apr 3;375(9721):1165-72.
7. Gynecol Oncol. 1998 Dec;71(3):340-3.
8. Cancer. 2006 Oct 15;107(8):1823-30.
9. Lancet. 2009 Jan 10;373(9658):125-36.
10. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
11. Gynecol Oncol. 2008 Apr;109(1):11-8.
12. Obstet Gynecol. 2014 Aug;124(2 Pt 1):307-15.
13. Cochrane Database Syst Rev. 2015 Sep 21;(9):CD007585.
14. Gynecol Oncol. 2014 Dec;135(3):518-24.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Clark is a fellow in the division of gynecologic oncology, department of obstetrics and gynecology at the university. They reported having no financial disclosures relevant to this column. Email them at [email protected].
Recognizing granulosa cell ovarian tumors
Granulosa cell tumors arise from ovarian sex cords and make up an estimated 1% of all ovarian cancer cases but comprise more than 70% of all sex cord stromal tumors.
Granulosa cell tumors (GCTs) can be divided into adult and juvenile types. Adult GCTs are much more common, representing 95% of all GCTs. Women diagnosed with adult GCTs are typically younger as compared with those with epithelial ovarian cancer. The average age of diagnosis for adult GCTs is 50 years, and for women with juvenile GCTs, the average age at diagnosis is 20 years.
Granulosa cell tumors have been shown to be more common in nonwhite women, those with a high body mass index, and a family history of breast or ovarian cancer.1
Adult GCTs can be associated with Peutz-Jeghers and Potter syndromes. Juvenile GCTs are exceedingly rare but can also be associated with mesodermal dysplastic syndromes characterized by the presence of enchondromatosis and hemangioma formation, such as Ollier disease or Maffucci syndrome.
Adult granulosa cell tumors are large, hormonally active tumors; typically secreting estrogen and associated with symptoms of hyperestrogenism. In one study, 55% of women with GCTs were reported to have hyperestrogenic findings such as breast tenderness, virulism, abnormal or postmenopausal bleeding, and hyperplasia, and those with juvenile GCTs may present with precocious puberty.2,3
In pregnancy, hormonal symptoms are temporized, thus the most common presentation is acute rupture. Initial evaluation of women with adult GCTs will reveal a palpable unilateral pelvic mass typically larger than 10cm. Juvenile and adult GCTs are unilateral in 95% of cases.4
In women presenting with a large adnexal mass, the appropriate initial clinical evaluation includes radiographic and laboratory studies. Endovaginal ultrasound typically reveals a large adnexal mass with heterogeneous solid and cystic components, areas of hemorrhage or necrosis and increased vascularity on Doppler. Juvenile GCTs have a more distinct appearance of solid growth with focal areas of follicular formation.
Laboratory findings suggestive of GCT include elevated inhibin-A, inhibin-B, anti-Mullerian hormone (AMH), and CA-125. Inhibin-B is the most commonly used tumor marker for the clinical monitoring of adult GCTs, but AMH may be the most specific.5 Lastly, an endometrial biopsy should be considered in all patients with abnormal uterine bleeding and in all postmenopausal women with an adnexal mass and an endometrial stripe greater than 5mm.6
Surgical staging for adult GCTs is the standard of care. For women who do not desire fertility, this includes total hysterectomy, bilateral salpingo-oophorectomy and removal of all gross disease. Comprehensive nodal dissection is not indicated except when necessary for complete cytoreduction. In contrast to epithelial ovarian cancer, approximately 80% of women with adult GCTs are diagnosed with stage I disease. For stage IA disease, treatment with surgery alone is sufficient, yet in women with stage II-IV disease or with tumors that are ruptured intraoperatively, platinum-based chemotherapy is recommended. The most common regimen is bleomycin, etoposide, and cisplatin, though there is increasing experience with an outpatient regimen of paclitaxel and carboplatin.
The gross appearance of both adult and juvenile GCTs are of a large, tan-yellow tumor with cystic, solid, and hemorrhagic components. Microscopically, juvenile GCTs are more distinct than that of adult GCTs. Whereas adult GCTs comprise diffuse cords or trabeculae and small follicles termed Call-Exner bodies of rounded cells with scant cytoplasm and pale “coffee-bean” nuclei, juvenile GCTs have nuclei that are rounded, hyperchromatic with moderate to abundant eosinophilic or vacuolated cytoplasm.
The prognosis of GCTs is largely dependent on the stage at diagnosis and presence of residual disease after debulking. Negative prognostic factors for recurrence include tumor size, rupture, atypia and increased mitotic activity.
There are distinct clinical, radiographic, and laboratory characteristics that may raise the suspicion of the practicing gynecologist for a GCT. In such cases, expedient referral for surgical exploration to a gynecologic oncologist is warranted. If the tumor is encountered inadvertently, intraoperative consultation from a gynecologic oncologist should be requested. If a gynecologic oncologist is not available, it is paramount to optimize surgical exposure to clearly document any abnormal pelvic or intra-abdominal findings, take care to prevent surgical spillage, and preserve fertility if indicated.
If referred appropriately and completely resected, the 5-year overall survival of stage IA disease can be upward of 90%. Recurrences are stage dependent with an average time to recurrence of just under 5 years. When recurrences occur, they tend to happen in the pelvis. All women with a history of GCT will require surveillance and monitoring.
References
1. Gynecol Oncol. 2005 May;97(2):519-23.
2. “Rare and Uncommon Gynecological Cancers: A Clinical Guide” (Heidelberg: Springer, 2011): Reed N.
3. Obstet Gynecol. 1980 Feb;55(2):231-8.
4. “Principles and Practice of Gynecologic Oncology” (Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2013): Barakat R.
5. Indian J Surg Oncol. 2013 Mar;4(1):37-47.
6. “Uncommon Gynecologic Cancers” (Indianapolis: Wiley-Blackwell, 2014): Del Carmen M.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Granulosa cell tumors arise from ovarian sex cords and make up an estimated 1% of all ovarian cancer cases but comprise more than 70% of all sex cord stromal tumors.
Granulosa cell tumors (GCTs) can be divided into adult and juvenile types. Adult GCTs are much more common, representing 95% of all GCTs. Women diagnosed with adult GCTs are typically younger as compared with those with epithelial ovarian cancer. The average age of diagnosis for adult GCTs is 50 years, and for women with juvenile GCTs, the average age at diagnosis is 20 years.
Granulosa cell tumors have been shown to be more common in nonwhite women, those with a high body mass index, and a family history of breast or ovarian cancer.1
Adult GCTs can be associated with Peutz-Jeghers and Potter syndromes. Juvenile GCTs are exceedingly rare but can also be associated with mesodermal dysplastic syndromes characterized by the presence of enchondromatosis and hemangioma formation, such as Ollier disease or Maffucci syndrome.
Adult granulosa cell tumors are large, hormonally active tumors; typically secreting estrogen and associated with symptoms of hyperestrogenism. In one study, 55% of women with GCTs were reported to have hyperestrogenic findings such as breast tenderness, virulism, abnormal or postmenopausal bleeding, and hyperplasia, and those with juvenile GCTs may present with precocious puberty.2,3
In pregnancy, hormonal symptoms are temporized, thus the most common presentation is acute rupture. Initial evaluation of women with adult GCTs will reveal a palpable unilateral pelvic mass typically larger than 10cm. Juvenile and adult GCTs are unilateral in 95% of cases.4
In women presenting with a large adnexal mass, the appropriate initial clinical evaluation includes radiographic and laboratory studies. Endovaginal ultrasound typically reveals a large adnexal mass with heterogeneous solid and cystic components, areas of hemorrhage or necrosis and increased vascularity on Doppler. Juvenile GCTs have a more distinct appearance of solid growth with focal areas of follicular formation.
Laboratory findings suggestive of GCT include elevated inhibin-A, inhibin-B, anti-Mullerian hormone (AMH), and CA-125. Inhibin-B is the most commonly used tumor marker for the clinical monitoring of adult GCTs, but AMH may be the most specific.5 Lastly, an endometrial biopsy should be considered in all patients with abnormal uterine bleeding and in all postmenopausal women with an adnexal mass and an endometrial stripe greater than 5mm.6
Surgical staging for adult GCTs is the standard of care. For women who do not desire fertility, this includes total hysterectomy, bilateral salpingo-oophorectomy and removal of all gross disease. Comprehensive nodal dissection is not indicated except when necessary for complete cytoreduction. In contrast to epithelial ovarian cancer, approximately 80% of women with adult GCTs are diagnosed with stage I disease. For stage IA disease, treatment with surgery alone is sufficient, yet in women with stage II-IV disease or with tumors that are ruptured intraoperatively, platinum-based chemotherapy is recommended. The most common regimen is bleomycin, etoposide, and cisplatin, though there is increasing experience with an outpatient regimen of paclitaxel and carboplatin.
The gross appearance of both adult and juvenile GCTs are of a large, tan-yellow tumor with cystic, solid, and hemorrhagic components. Microscopically, juvenile GCTs are more distinct than that of adult GCTs. Whereas adult GCTs comprise diffuse cords or trabeculae and small follicles termed Call-Exner bodies of rounded cells with scant cytoplasm and pale “coffee-bean” nuclei, juvenile GCTs have nuclei that are rounded, hyperchromatic with moderate to abundant eosinophilic or vacuolated cytoplasm.
The prognosis of GCTs is largely dependent on the stage at diagnosis and presence of residual disease after debulking. Negative prognostic factors for recurrence include tumor size, rupture, atypia and increased mitotic activity.
There are distinct clinical, radiographic, and laboratory characteristics that may raise the suspicion of the practicing gynecologist for a GCT. In such cases, expedient referral for surgical exploration to a gynecologic oncologist is warranted. If the tumor is encountered inadvertently, intraoperative consultation from a gynecologic oncologist should be requested. If a gynecologic oncologist is not available, it is paramount to optimize surgical exposure to clearly document any abnormal pelvic or intra-abdominal findings, take care to prevent surgical spillage, and preserve fertility if indicated.
If referred appropriately and completely resected, the 5-year overall survival of stage IA disease can be upward of 90%. Recurrences are stage dependent with an average time to recurrence of just under 5 years. When recurrences occur, they tend to happen in the pelvis. All women with a history of GCT will require surveillance and monitoring.
References
1. Gynecol Oncol. 2005 May;97(2):519-23.
2. “Rare and Uncommon Gynecological Cancers: A Clinical Guide” (Heidelberg: Springer, 2011): Reed N.
3. Obstet Gynecol. 1980 Feb;55(2):231-8.
4. “Principles and Practice of Gynecologic Oncology” (Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2013): Barakat R.
5. Indian J Surg Oncol. 2013 Mar;4(1):37-47.
6. “Uncommon Gynecologic Cancers” (Indianapolis: Wiley-Blackwell, 2014): Del Carmen M.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Granulosa cell tumors arise from ovarian sex cords and make up an estimated 1% of all ovarian cancer cases but comprise more than 70% of all sex cord stromal tumors.
Granulosa cell tumors (GCTs) can be divided into adult and juvenile types. Adult GCTs are much more common, representing 95% of all GCTs. Women diagnosed with adult GCTs are typically younger as compared with those with epithelial ovarian cancer. The average age of diagnosis for adult GCTs is 50 years, and for women with juvenile GCTs, the average age at diagnosis is 20 years.
Granulosa cell tumors have been shown to be more common in nonwhite women, those with a high body mass index, and a family history of breast or ovarian cancer.1
Adult GCTs can be associated with Peutz-Jeghers and Potter syndromes. Juvenile GCTs are exceedingly rare but can also be associated with mesodermal dysplastic syndromes characterized by the presence of enchondromatosis and hemangioma formation, such as Ollier disease or Maffucci syndrome.
Adult granulosa cell tumors are large, hormonally active tumors; typically secreting estrogen and associated with symptoms of hyperestrogenism. In one study, 55% of women with GCTs were reported to have hyperestrogenic findings such as breast tenderness, virulism, abnormal or postmenopausal bleeding, and hyperplasia, and those with juvenile GCTs may present with precocious puberty.2,3
In pregnancy, hormonal symptoms are temporized, thus the most common presentation is acute rupture. Initial evaluation of women with adult GCTs will reveal a palpable unilateral pelvic mass typically larger than 10cm. Juvenile and adult GCTs are unilateral in 95% of cases.4
In women presenting with a large adnexal mass, the appropriate initial clinical evaluation includes radiographic and laboratory studies. Endovaginal ultrasound typically reveals a large adnexal mass with heterogeneous solid and cystic components, areas of hemorrhage or necrosis and increased vascularity on Doppler. Juvenile GCTs have a more distinct appearance of solid growth with focal areas of follicular formation.
Laboratory findings suggestive of GCT include elevated inhibin-A, inhibin-B, anti-Mullerian hormone (AMH), and CA-125. Inhibin-B is the most commonly used tumor marker for the clinical monitoring of adult GCTs, but AMH may be the most specific.5 Lastly, an endometrial biopsy should be considered in all patients with abnormal uterine bleeding and in all postmenopausal women with an adnexal mass and an endometrial stripe greater than 5mm.6
Surgical staging for adult GCTs is the standard of care. For women who do not desire fertility, this includes total hysterectomy, bilateral salpingo-oophorectomy and removal of all gross disease. Comprehensive nodal dissection is not indicated except when necessary for complete cytoreduction. In contrast to epithelial ovarian cancer, approximately 80% of women with adult GCTs are diagnosed with stage I disease. For stage IA disease, treatment with surgery alone is sufficient, yet in women with stage II-IV disease or with tumors that are ruptured intraoperatively, platinum-based chemotherapy is recommended. The most common regimen is bleomycin, etoposide, and cisplatin, though there is increasing experience with an outpatient regimen of paclitaxel and carboplatin.
The gross appearance of both adult and juvenile GCTs are of a large, tan-yellow tumor with cystic, solid, and hemorrhagic components. Microscopically, juvenile GCTs are more distinct than that of adult GCTs. Whereas adult GCTs comprise diffuse cords or trabeculae and small follicles termed Call-Exner bodies of rounded cells with scant cytoplasm and pale “coffee-bean” nuclei, juvenile GCTs have nuclei that are rounded, hyperchromatic with moderate to abundant eosinophilic or vacuolated cytoplasm.
The prognosis of GCTs is largely dependent on the stage at diagnosis and presence of residual disease after debulking. Negative prognostic factors for recurrence include tumor size, rupture, atypia and increased mitotic activity.
There are distinct clinical, radiographic, and laboratory characteristics that may raise the suspicion of the practicing gynecologist for a GCT. In such cases, expedient referral for surgical exploration to a gynecologic oncologist is warranted. If the tumor is encountered inadvertently, intraoperative consultation from a gynecologic oncologist should be requested. If a gynecologic oncologist is not available, it is paramount to optimize surgical exposure to clearly document any abnormal pelvic or intra-abdominal findings, take care to prevent surgical spillage, and preserve fertility if indicated.
If referred appropriately and completely resected, the 5-year overall survival of stage IA disease can be upward of 90%. Recurrences are stage dependent with an average time to recurrence of just under 5 years. When recurrences occur, they tend to happen in the pelvis. All women with a history of GCT will require surveillance and monitoring.
References
1. Gynecol Oncol. 2005 May;97(2):519-23.
2. “Rare and Uncommon Gynecological Cancers: A Clinical Guide” (Heidelberg: Springer, 2011): Reed N.
3. Obstet Gynecol. 1980 Feb;55(2):231-8.
4. “Principles and Practice of Gynecologic Oncology” (Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2013): Barakat R.
5. Indian J Surg Oncol. 2013 Mar;4(1):37-47.
6. “Uncommon Gynecologic Cancers” (Indianapolis: Wiley-Blackwell, 2014): Del Carmen M.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Management of adnexal masses in pregnancy
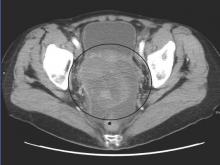
Roughly 1%-2% of pregnancies are complicated by an adnexal mass, and prenatal ultrasound for fetal evaluation has detected more asymptomatic ovarian masses as a result.
The differential diagnosis for adnexal mass is broad and includes follicular or corpus luteum cysts, mature teratoma, theca lutein cyst, hydrosalpinx, endometrioma, cystadenoma, pedunculated leiomyoma, luteoma, as well as malignant neoplasms of epithelial, germ cell, and sex cord–stromal origin (J Ultrasound Med. 2004 Jun;23[6]:805-19). Most masses will be benign neoplasms, with a fraction identified as malignancies.
In 2013, Baser et al. performed a retrospective study of 151 women who underwent surgery of an adnexal mass at time of cesarean delivery. Of the 151 cases reviewed, 148 (98%) of the masses were benign (Int J Gynaecol Obstet. 2013 Nov;123[2]:124-6). Additionally, if the patient presents with pain, diagnoses such as ectopic pregnancy, heterotopic pregnancy, degenerating fibroid, and torsion should also be considered.
Diagnostic evaluation and management
The majority of adnexal masses identified in pregnancy are benign simple cysts measuring less than 5 cm in diameter. Approximately 70% of cystic masses detected in the first trimester will spontaneously resolve by the second trimester (Clin Obstet Gynecol. 2006 Sep;49[3]:492-505). However, for some masses, surgical resection is warranted.
Masses present after the first trimester and that are (1) greater than 10cm in diameter or (2) are solid or contain solid and cystic areas or have septated or papillary areas, are generally managed surgically as these features increase the risk of malignancy or complications such as adnexal torsion, rupture, or labor dystocia (Gynecol Oncol. 2006 May;101(2):315-21).
Adnexal masses without these features often resolve during pregnancy and can be expectantly managed (Obstet Gynecol. 2005 May;105[5 Pt 1]:1098-103). The optimal time for surgical intervention is after the first trimester as organogenesis is largely complete, therefore minimizing the risk of drug-induced teratogenesis, and any necessary cystectomy or oophorectomy will not disrupt the required progesterone production of the corpus luteum as this has been replaced by the placenta.
Preoperative assessment
For most cases, imaging with ultrasound is adequate for preoperative evaluation; however, in some cases, further imaging is needed for appropriate characterization of the mass. In this situation, further imaging with MRI is preferred as this modality has good resolution for visualization of soft tissue pathology and does not expose the patient and fetus to ionizing radiation. Of note, Gadolinium-based contrast should be avoided as effects have not been well established in pregnancy (AJR Am J Roentgenol. 2008 Aug;191[2]:364-70).
If there is concern for malignancy during pregnancy, drawing serum tumor markers preoperatively is typically not suggested. Oncofetal antigens, including alpha fetoprotein (AFP), human chorionic gonadotropin (hCG), carcinoembryonic antigen (CEA), and cancer antigen 125 (CA-125), are elevated during gestation, making them poor markers for malignancy. If malignancy is ultimately diagnosed, then tumor markers can be obtained immediately postoperatively.
Surgical approach and prognosis
If there is low suspicion for malignancy, a laparoscopic approach is preferable and reasonable at all stages of pregnancy, although early second trimester is ideal. Entry at Palmer’s point in the left upper quadrant versus the umbilicus is preferable in order to minimize risk of uterine injury.
If malignancy is suspected, maximum exposure should be obtained with a midline vertical incision. Peritoneal washings should be obtained on immediate entry of the peritoneal cavity, and the contralateral ovary should also be adequately examined along with a general abdominopelvic survey. If the mass demonstrates concerning features, such as solid features or presence of ascites, then the specimen should be sent for intraoperative frozen pathology, and the pathologist should be made aware of the concurrent pregnancy. If malignancy is confirmed on frozen pathology, a full staging procedure should be performed and a gynecologic oncologist consulted.
Roughly three-quarters of invasive ovarian cancers diagnosed in pregnancy are early stage disease, and the 5-year survival of ovarian cancers associated with pregnancy is between 72% and 90% (Int J Gynecol Cancer. 2006 Jan-Feb;16[1]:8-15).
In a retrospective cohort study of 101 pregnant women, 31% of adnexal masses resected in pregnant women greater than 14 weeks gestation were teratomas. In total, 23% of masses were luteal cysts. Less commonly, patients were diagnosed with serous cystadenoma (14%), endometrioma (8%), mucinous cystadenoma (7%), benign cyst (6%), tumor of low malignant potential (5%), and paratubal cyst (3%).
In this study, approximately half of the women underwent minimally invasive surgery and half had surgery via laparotomy. There were more complications in the women undergoing laparotomy (ileus) and there were no differences between the groups with regards to pregnancy and neonatal outcomes (J Minim Invasive Gynecol. 2011 Nov-Dec;18[6]:720-5).
In general, characteristics that are favorable for spontaneous resolution include masses that are simple in nature by ultrasound and less than 5 cm to 6 cm in diameter.
For women with simple-appearing masses on ultrasound, reimaging can occur during the remainder of the pregnancy at the discretion of the physician or during the postpartum period. All women should be provided with torsion and rupture precautions during the pregnancy (Am J Obstet Gynecol. 2011 Aug;205[2]:97-102). For women with more concerning features on ultrasound, referral to a gynecologic oncologist is warranted. If the decision for surgical management is made, minimally invasive surgery should be strongly considered due to minimal maternal and perinatal morbidity.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Roughly 1%-2% of pregnancies are complicated by an adnexal mass, and prenatal ultrasound for fetal evaluation has detected more asymptomatic ovarian masses as a result.
The differential diagnosis for adnexal mass is broad and includes follicular or corpus luteum cysts, mature teratoma, theca lutein cyst, hydrosalpinx, endometrioma, cystadenoma, pedunculated leiomyoma, luteoma, as well as malignant neoplasms of epithelial, germ cell, and sex cord–stromal origin (J Ultrasound Med. 2004 Jun;23[6]:805-19). Most masses will be benign neoplasms, with a fraction identified as malignancies.
In 2013, Baser et al. performed a retrospective study of 151 women who underwent surgery of an adnexal mass at time of cesarean delivery. Of the 151 cases reviewed, 148 (98%) of the masses were benign (Int J Gynaecol Obstet. 2013 Nov;123[2]:124-6). Additionally, if the patient presents with pain, diagnoses such as ectopic pregnancy, heterotopic pregnancy, degenerating fibroid, and torsion should also be considered.
Diagnostic evaluation and management
The majority of adnexal masses identified in pregnancy are benign simple cysts measuring less than 5 cm in diameter. Approximately 70% of cystic masses detected in the first trimester will spontaneously resolve by the second trimester (Clin Obstet Gynecol. 2006 Sep;49[3]:492-505). However, for some masses, surgical resection is warranted.
Masses present after the first trimester and that are (1) greater than 10cm in diameter or (2) are solid or contain solid and cystic areas or have septated or papillary areas, are generally managed surgically as these features increase the risk of malignancy or complications such as adnexal torsion, rupture, or labor dystocia (Gynecol Oncol. 2006 May;101(2):315-21).
Adnexal masses without these features often resolve during pregnancy and can be expectantly managed (Obstet Gynecol. 2005 May;105[5 Pt 1]:1098-103). The optimal time for surgical intervention is after the first trimester as organogenesis is largely complete, therefore minimizing the risk of drug-induced teratogenesis, and any necessary cystectomy or oophorectomy will not disrupt the required progesterone production of the corpus luteum as this has been replaced by the placenta.
Preoperative assessment
For most cases, imaging with ultrasound is adequate for preoperative evaluation; however, in some cases, further imaging is needed for appropriate characterization of the mass. In this situation, further imaging with MRI is preferred as this modality has good resolution for visualization of soft tissue pathology and does not expose the patient and fetus to ionizing radiation. Of note, Gadolinium-based contrast should be avoided as effects have not been well established in pregnancy (AJR Am J Roentgenol. 2008 Aug;191[2]:364-70).
If there is concern for malignancy during pregnancy, drawing serum tumor markers preoperatively is typically not suggested. Oncofetal antigens, including alpha fetoprotein (AFP), human chorionic gonadotropin (hCG), carcinoembryonic antigen (CEA), and cancer antigen 125 (CA-125), are elevated during gestation, making them poor markers for malignancy. If malignancy is ultimately diagnosed, then tumor markers can be obtained immediately postoperatively.
Surgical approach and prognosis
If there is low suspicion for malignancy, a laparoscopic approach is preferable and reasonable at all stages of pregnancy, although early second trimester is ideal. Entry at Palmer’s point in the left upper quadrant versus the umbilicus is preferable in order to minimize risk of uterine injury.
If malignancy is suspected, maximum exposure should be obtained with a midline vertical incision. Peritoneal washings should be obtained on immediate entry of the peritoneal cavity, and the contralateral ovary should also be adequately examined along with a general abdominopelvic survey. If the mass demonstrates concerning features, such as solid features or presence of ascites, then the specimen should be sent for intraoperative frozen pathology, and the pathologist should be made aware of the concurrent pregnancy. If malignancy is confirmed on frozen pathology, a full staging procedure should be performed and a gynecologic oncologist consulted.
Roughly three-quarters of invasive ovarian cancers diagnosed in pregnancy are early stage disease, and the 5-year survival of ovarian cancers associated with pregnancy is between 72% and 90% (Int J Gynecol Cancer. 2006 Jan-Feb;16[1]:8-15).
In a retrospective cohort study of 101 pregnant women, 31% of adnexal masses resected in pregnant women greater than 14 weeks gestation were teratomas. In total, 23% of masses were luteal cysts. Less commonly, patients were diagnosed with serous cystadenoma (14%), endometrioma (8%), mucinous cystadenoma (7%), benign cyst (6%), tumor of low malignant potential (5%), and paratubal cyst (3%).
In this study, approximately half of the women underwent minimally invasive surgery and half had surgery via laparotomy. There were more complications in the women undergoing laparotomy (ileus) and there were no differences between the groups with regards to pregnancy and neonatal outcomes (J Minim Invasive Gynecol. 2011 Nov-Dec;18[6]:720-5).
In general, characteristics that are favorable for spontaneous resolution include masses that are simple in nature by ultrasound and less than 5 cm to 6 cm in diameter.
For women with simple-appearing masses on ultrasound, reimaging can occur during the remainder of the pregnancy at the discretion of the physician or during the postpartum period. All women should be provided with torsion and rupture precautions during the pregnancy (Am J Obstet Gynecol. 2011 Aug;205[2]:97-102). For women with more concerning features on ultrasound, referral to a gynecologic oncologist is warranted. If the decision for surgical management is made, minimally invasive surgery should be strongly considered due to minimal maternal and perinatal morbidity.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Roughly 1%-2% of pregnancies are complicated by an adnexal mass, and prenatal ultrasound for fetal evaluation has detected more asymptomatic ovarian masses as a result.
The differential diagnosis for adnexal mass is broad and includes follicular or corpus luteum cysts, mature teratoma, theca lutein cyst, hydrosalpinx, endometrioma, cystadenoma, pedunculated leiomyoma, luteoma, as well as malignant neoplasms of epithelial, germ cell, and sex cord–stromal origin (J Ultrasound Med. 2004 Jun;23[6]:805-19). Most masses will be benign neoplasms, with a fraction identified as malignancies.
In 2013, Baser et al. performed a retrospective study of 151 women who underwent surgery of an adnexal mass at time of cesarean delivery. Of the 151 cases reviewed, 148 (98%) of the masses were benign (Int J Gynaecol Obstet. 2013 Nov;123[2]:124-6). Additionally, if the patient presents with pain, diagnoses such as ectopic pregnancy, heterotopic pregnancy, degenerating fibroid, and torsion should also be considered.
Diagnostic evaluation and management
The majority of adnexal masses identified in pregnancy are benign simple cysts measuring less than 5 cm in diameter. Approximately 70% of cystic masses detected in the first trimester will spontaneously resolve by the second trimester (Clin Obstet Gynecol. 2006 Sep;49[3]:492-505). However, for some masses, surgical resection is warranted.
Masses present after the first trimester and that are (1) greater than 10cm in diameter or (2) are solid or contain solid and cystic areas or have septated or papillary areas, are generally managed surgically as these features increase the risk of malignancy or complications such as adnexal torsion, rupture, or labor dystocia (Gynecol Oncol. 2006 May;101(2):315-21).
Adnexal masses without these features often resolve during pregnancy and can be expectantly managed (Obstet Gynecol. 2005 May;105[5 Pt 1]:1098-103). The optimal time for surgical intervention is after the first trimester as organogenesis is largely complete, therefore minimizing the risk of drug-induced teratogenesis, and any necessary cystectomy or oophorectomy will not disrupt the required progesterone production of the corpus luteum as this has been replaced by the placenta.
Preoperative assessment
For most cases, imaging with ultrasound is adequate for preoperative evaluation; however, in some cases, further imaging is needed for appropriate characterization of the mass. In this situation, further imaging with MRI is preferred as this modality has good resolution for visualization of soft tissue pathology and does not expose the patient and fetus to ionizing radiation. Of note, Gadolinium-based contrast should be avoided as effects have not been well established in pregnancy (AJR Am J Roentgenol. 2008 Aug;191[2]:364-70).
If there is concern for malignancy during pregnancy, drawing serum tumor markers preoperatively is typically not suggested. Oncofetal antigens, including alpha fetoprotein (AFP), human chorionic gonadotropin (hCG), carcinoembryonic antigen (CEA), and cancer antigen 125 (CA-125), are elevated during gestation, making them poor markers for malignancy. If malignancy is ultimately diagnosed, then tumor markers can be obtained immediately postoperatively.
Surgical approach and prognosis
If there is low suspicion for malignancy, a laparoscopic approach is preferable and reasonable at all stages of pregnancy, although early second trimester is ideal. Entry at Palmer’s point in the left upper quadrant versus the umbilicus is preferable in order to minimize risk of uterine injury.
If malignancy is suspected, maximum exposure should be obtained with a midline vertical incision. Peritoneal washings should be obtained on immediate entry of the peritoneal cavity, and the contralateral ovary should also be adequately examined along with a general abdominopelvic survey. If the mass demonstrates concerning features, such as solid features or presence of ascites, then the specimen should be sent for intraoperative frozen pathology, and the pathologist should be made aware of the concurrent pregnancy. If malignancy is confirmed on frozen pathology, a full staging procedure should be performed and a gynecologic oncologist consulted.
Roughly three-quarters of invasive ovarian cancers diagnosed in pregnancy are early stage disease, and the 5-year survival of ovarian cancers associated with pregnancy is between 72% and 90% (Int J Gynecol Cancer. 2006 Jan-Feb;16[1]:8-15).
In a retrospective cohort study of 101 pregnant women, 31% of adnexal masses resected in pregnant women greater than 14 weeks gestation were teratomas. In total, 23% of masses were luteal cysts. Less commonly, patients were diagnosed with serous cystadenoma (14%), endometrioma (8%), mucinous cystadenoma (7%), benign cyst (6%), tumor of low malignant potential (5%), and paratubal cyst (3%).
In this study, approximately half of the women underwent minimally invasive surgery and half had surgery via laparotomy. There were more complications in the women undergoing laparotomy (ileus) and there were no differences between the groups with regards to pregnancy and neonatal outcomes (J Minim Invasive Gynecol. 2011 Nov-Dec;18[6]:720-5).
In general, characteristics that are favorable for spontaneous resolution include masses that are simple in nature by ultrasound and less than 5 cm to 6 cm in diameter.
For women with simple-appearing masses on ultrasound, reimaging can occur during the remainder of the pregnancy at the discretion of the physician or during the postpartum period. All women should be provided with torsion and rupture precautions during the pregnancy (Am J Obstet Gynecol. 2011 Aug;205[2]:97-102). For women with more concerning features on ultrasound, referral to a gynecologic oncologist is warranted. If the decision for surgical management is made, minimally invasive surgery should be strongly considered due to minimal maternal and perinatal morbidity.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Understanding ovarian germ cell neoplasms
Germ cell neoplasms arise from primordial germ cells of the gonad that differentiate to embryonic and extraembryonic tissues. Approximately 20%-25% of ovarian neoplasms are germ cell in origin but they account for only 3%-5% of ovarian malignancies. Importantly, germ cell neoplasms encompass 70% of the ovarian neoplasms among girls and young women aged 10-30 years, of which approximately one-third are malignant.
Unlike epithelial ovarian cancers, malignant germ cell neoplasms are typically diagnosed at early stages. They can present as a palpable mass or cause acute abdominal pain secondary to their rapid growth, penchant for necrosis, torsion, hemorrhage, infection, or rupture. Since malignant germ cell neoplasms can excrete hormonally-active tumor markers, such as human chorionic gonadotropin (HCG), many women present with menstrual irregularities.
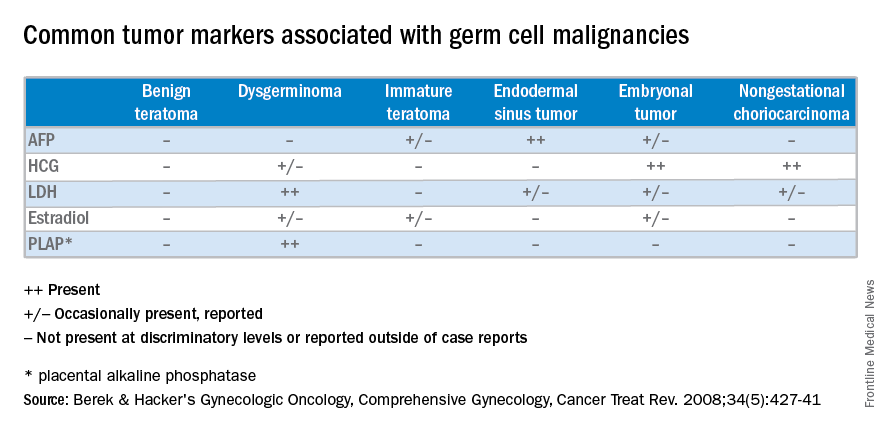
Management of malignant germ cell neoplasms generally includes fertility-sparing surgery – with or without neoadjuvant combination bleomycin, etoposide, and cisplatin (BEP) – and is typically associated with a favorable prognosis (Cancer Treat Rev. 2008 Aug;34[5]:427-41).
Teratomas
Benign teratomas represent about a quarter of all ovarian neoplasms. Benign teratomas can be solid or cystic, and contain components representing all three cell layers: endoderm, mesoderm, and ectoderm. Due to the presence of differentiated adult tissues, benign teratomas have a characteristic radiographic appearance. They can appear as cystic echogenic masses with intense acoustic shadowing, homogenous nodules or bands, and rounded protuberances called “Rokitansky nodules.”
Treatment of benign teratomas in reproductive-age women includes ovarian cystectomy and careful inspection of the contralateral ovary as 10%-15% may be bilateral. In postmenopausal women, there is less than a 2% risk for malignant transformation of the associated cell lines, most commonly a squamous cell carcinoma. In these cases, spread beyond the ovarian capsule is associated with a poor prognosis and chemotherapy and/or radiation are indicated (Int J Gynecol Cancer. 2006 Jan-Feb;16[1]:140-4). Malignant monodermal teratomas are composed of single cell lines with malignant transformations of that tissue type; struma ovarii composed of thyroid tissue, for example, is exceedingly rare.
Dysgerminoma
Dysgerminomas are the most common malignant germ cell neoplasms, accounting for about one-third of cases. They typically present in girls and young women between 10 and 30 years of age, and rarely occur after 50 years of age. As a result, a quarter of cases are identified during pregnancy and another 5% are found in patients presenting with amenorrhea secondary to gonadal dysgenesis, such as is associated with Turner’s syndrome.
At diagnosis, lactate dehydrogenase (LDH) may be elevated and 10% may have an elevation of HCG. Ultrasound findings include a solid, mostly echoic, but heterogeneous mass with apparent lobulations. About two-thirds are stage I at the time of diagnosis, and 10%-15% are bilateral, making dysgerminomas the only malignant germ cell neoplasm with significant risk for bilaterality.
Treatment for early stage dysgerminoma is surgical; young women should have at least unilateral oophorectomy performed; if the contralateral ovary is spared there’s a 10% risk for recurrence over the next 2 years. Comprehensive fertility-sparing surgery is recommended with pelvic and para-aortic lymphadenectomy. Women with gonadal dysgenesis should have a bilateral salpingo-oophorectomy, and those beyond childbearing should undergo a total hysterectomy, with bilateral salpingo-oophorectomy and appropriate staging. BEP should be added for patients with advanced disease.
Other malignant germ cell neoplasms
Uncommon malignant germ cell neoplasms include immature teratomas and endodermal sinus tumors. Though uncommon, the majority of immature teratomas present between the ages of 10 and 20 years and account for nearly 30% of the ovarian cancer deaths in this age group. Immature teratomas usually have negative serum markers, though about one-third will excrete HCG. Immature teratomas are graded by the proportion of neuro-epithelium. Treatment includes unilateral oophorectomy, and surgical staging with adjuvant chemotherapy (BEP) for patients with greater than stage 1A grade 1 disease.
Endodermal sinus tumors are derived from the primitive yolk sac, are unilateral, and most will secrete alpha-fetoprotein (AFP). The median age of diagnosis of an endodermal sinus tumor is 18 years, thus treatment includes unilateral salpingo-oophorectomy and comprehensive fertility-sparing surgical staging followed by BEP.
Embryonal and nongestational choriocarcinomas are rare malignant germ cell neoplasms found in prepubertal girls to young women. Embryonal carcinomas can secrete estrogens, HCG and/or AFP, so patients may present with precocious puberty. Treatment is similar to that of endodermal sinus tumors. Nongestational choriocarcinomas, like the gestational forms, have a poor prognosis and are monitored and treated similarly.
Understanding presentation and treatments for malignant germ cell neoplasms is important in the evaluation of a young patient with a pelvic mass and should prompt the practicing gynecologist to test for AFP and HCG, with or without LDH and CA-125. If encountered inadvertently, every attempt should be made to preserve fertility in these young patients and expedient referral to a gynecologic oncologist, pediatric gynecologist, and/or a reproductive endocrinologist is warranted. Rarely, a second look laparotomy is indicated without obvious intraperitoneal spread and reproductive potential is preserved even in those requiring BEP. If managed appropriately, the overall prognosis remains good for these young women.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Germ cell neoplasms arise from primordial germ cells of the gonad that differentiate to embryonic and extraembryonic tissues. Approximately 20%-25% of ovarian neoplasms are germ cell in origin but they account for only 3%-5% of ovarian malignancies. Importantly, germ cell neoplasms encompass 70% of the ovarian neoplasms among girls and young women aged 10-30 years, of which approximately one-third are malignant.
Unlike epithelial ovarian cancers, malignant germ cell neoplasms are typically diagnosed at early stages. They can present as a palpable mass or cause acute abdominal pain secondary to their rapid growth, penchant for necrosis, torsion, hemorrhage, infection, or rupture. Since malignant germ cell neoplasms can excrete hormonally-active tumor markers, such as human chorionic gonadotropin (HCG), many women present with menstrual irregularities.

Management of malignant germ cell neoplasms generally includes fertility-sparing surgery – with or without neoadjuvant combination bleomycin, etoposide, and cisplatin (BEP) – and is typically associated with a favorable prognosis (Cancer Treat Rev. 2008 Aug;34[5]:427-41).
Teratomas
Benign teratomas represent about a quarter of all ovarian neoplasms. Benign teratomas can be solid or cystic, and contain components representing all three cell layers: endoderm, mesoderm, and ectoderm. Due to the presence of differentiated adult tissues, benign teratomas have a characteristic radiographic appearance. They can appear as cystic echogenic masses with intense acoustic shadowing, homogenous nodules or bands, and rounded protuberances called “Rokitansky nodules.”
Treatment of benign teratomas in reproductive-age women includes ovarian cystectomy and careful inspection of the contralateral ovary as 10%-15% may be bilateral. In postmenopausal women, there is less than a 2% risk for malignant transformation of the associated cell lines, most commonly a squamous cell carcinoma. In these cases, spread beyond the ovarian capsule is associated with a poor prognosis and chemotherapy and/or radiation are indicated (Int J Gynecol Cancer. 2006 Jan-Feb;16[1]:140-4). Malignant monodermal teratomas are composed of single cell lines with malignant transformations of that tissue type; struma ovarii composed of thyroid tissue, for example, is exceedingly rare.
Dysgerminoma
Dysgerminomas are the most common malignant germ cell neoplasms, accounting for about one-third of cases. They typically present in girls and young women between 10 and 30 years of age, and rarely occur after 50 years of age. As a result, a quarter of cases are identified during pregnancy and another 5% are found in patients presenting with amenorrhea secondary to gonadal dysgenesis, such as is associated with Turner’s syndrome.
At diagnosis, lactate dehydrogenase (LDH) may be elevated and 10% may have an elevation of HCG. Ultrasound findings include a solid, mostly echoic, but heterogeneous mass with apparent lobulations. About two-thirds are stage I at the time of diagnosis, and 10%-15% are bilateral, making dysgerminomas the only malignant germ cell neoplasm with significant risk for bilaterality.
Treatment for early stage dysgerminoma is surgical; young women should have at least unilateral oophorectomy performed; if the contralateral ovary is spared there’s a 10% risk for recurrence over the next 2 years. Comprehensive fertility-sparing surgery is recommended with pelvic and para-aortic lymphadenectomy. Women with gonadal dysgenesis should have a bilateral salpingo-oophorectomy, and those beyond childbearing should undergo a total hysterectomy, with bilateral salpingo-oophorectomy and appropriate staging. BEP should be added for patients with advanced disease.
Other malignant germ cell neoplasms
Uncommon malignant germ cell neoplasms include immature teratomas and endodermal sinus tumors. Though uncommon, the majority of immature teratomas present between the ages of 10 and 20 years and account for nearly 30% of the ovarian cancer deaths in this age group. Immature teratomas usually have negative serum markers, though about one-third will excrete HCG. Immature teratomas are graded by the proportion of neuro-epithelium. Treatment includes unilateral oophorectomy, and surgical staging with adjuvant chemotherapy (BEP) for patients with greater than stage 1A grade 1 disease.
Endodermal sinus tumors are derived from the primitive yolk sac, are unilateral, and most will secrete alpha-fetoprotein (AFP). The median age of diagnosis of an endodermal sinus tumor is 18 years, thus treatment includes unilateral salpingo-oophorectomy and comprehensive fertility-sparing surgical staging followed by BEP.
Embryonal and nongestational choriocarcinomas are rare malignant germ cell neoplasms found in prepubertal girls to young women. Embryonal carcinomas can secrete estrogens, HCG and/or AFP, so patients may present with precocious puberty. Treatment is similar to that of endodermal sinus tumors. Nongestational choriocarcinomas, like the gestational forms, have a poor prognosis and are monitored and treated similarly.
Understanding presentation and treatments for malignant germ cell neoplasms is important in the evaluation of a young patient with a pelvic mass and should prompt the practicing gynecologist to test for AFP and HCG, with or without LDH and CA-125. If encountered inadvertently, every attempt should be made to preserve fertility in these young patients and expedient referral to a gynecologic oncologist, pediatric gynecologist, and/or a reproductive endocrinologist is warranted. Rarely, a second look laparotomy is indicated without obvious intraperitoneal spread and reproductive potential is preserved even in those requiring BEP. If managed appropriately, the overall prognosis remains good for these young women.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Germ cell neoplasms arise from primordial germ cells of the gonad that differentiate to embryonic and extraembryonic tissues. Approximately 20%-25% of ovarian neoplasms are germ cell in origin but they account for only 3%-5% of ovarian malignancies. Importantly, germ cell neoplasms encompass 70% of the ovarian neoplasms among girls and young women aged 10-30 years, of which approximately one-third are malignant.
Unlike epithelial ovarian cancers, malignant germ cell neoplasms are typically diagnosed at early stages. They can present as a palpable mass or cause acute abdominal pain secondary to their rapid growth, penchant for necrosis, torsion, hemorrhage, infection, or rupture. Since malignant germ cell neoplasms can excrete hormonally-active tumor markers, such as human chorionic gonadotropin (HCG), many women present with menstrual irregularities.

Management of malignant germ cell neoplasms generally includes fertility-sparing surgery – with or without neoadjuvant combination bleomycin, etoposide, and cisplatin (BEP) – and is typically associated with a favorable prognosis (Cancer Treat Rev. 2008 Aug;34[5]:427-41).
Teratomas
Benign teratomas represent about a quarter of all ovarian neoplasms. Benign teratomas can be solid or cystic, and contain components representing all three cell layers: endoderm, mesoderm, and ectoderm. Due to the presence of differentiated adult tissues, benign teratomas have a characteristic radiographic appearance. They can appear as cystic echogenic masses with intense acoustic shadowing, homogenous nodules or bands, and rounded protuberances called “Rokitansky nodules.”
Treatment of benign teratomas in reproductive-age women includes ovarian cystectomy and careful inspection of the contralateral ovary as 10%-15% may be bilateral. In postmenopausal women, there is less than a 2% risk for malignant transformation of the associated cell lines, most commonly a squamous cell carcinoma. In these cases, spread beyond the ovarian capsule is associated with a poor prognosis and chemotherapy and/or radiation are indicated (Int J Gynecol Cancer. 2006 Jan-Feb;16[1]:140-4). Malignant monodermal teratomas are composed of single cell lines with malignant transformations of that tissue type; struma ovarii composed of thyroid tissue, for example, is exceedingly rare.
Dysgerminoma
Dysgerminomas are the most common malignant germ cell neoplasms, accounting for about one-third of cases. They typically present in girls and young women between 10 and 30 years of age, and rarely occur after 50 years of age. As a result, a quarter of cases are identified during pregnancy and another 5% are found in patients presenting with amenorrhea secondary to gonadal dysgenesis, such as is associated with Turner’s syndrome.
At diagnosis, lactate dehydrogenase (LDH) may be elevated and 10% may have an elevation of HCG. Ultrasound findings include a solid, mostly echoic, but heterogeneous mass with apparent lobulations. About two-thirds are stage I at the time of diagnosis, and 10%-15% are bilateral, making dysgerminomas the only malignant germ cell neoplasm with significant risk for bilaterality.
Treatment for early stage dysgerminoma is surgical; young women should have at least unilateral oophorectomy performed; if the contralateral ovary is spared there’s a 10% risk for recurrence over the next 2 years. Comprehensive fertility-sparing surgery is recommended with pelvic and para-aortic lymphadenectomy. Women with gonadal dysgenesis should have a bilateral salpingo-oophorectomy, and those beyond childbearing should undergo a total hysterectomy, with bilateral salpingo-oophorectomy and appropriate staging. BEP should be added for patients with advanced disease.
Other malignant germ cell neoplasms
Uncommon malignant germ cell neoplasms include immature teratomas and endodermal sinus tumors. Though uncommon, the majority of immature teratomas present between the ages of 10 and 20 years and account for nearly 30% of the ovarian cancer deaths in this age group. Immature teratomas usually have negative serum markers, though about one-third will excrete HCG. Immature teratomas are graded by the proportion of neuro-epithelium. Treatment includes unilateral oophorectomy, and surgical staging with adjuvant chemotherapy (BEP) for patients with greater than stage 1A grade 1 disease.
Endodermal sinus tumors are derived from the primitive yolk sac, are unilateral, and most will secrete alpha-fetoprotein (AFP). The median age of diagnosis of an endodermal sinus tumor is 18 years, thus treatment includes unilateral salpingo-oophorectomy and comprehensive fertility-sparing surgical staging followed by BEP.
Embryonal and nongestational choriocarcinomas are rare malignant germ cell neoplasms found in prepubertal girls to young women. Embryonal carcinomas can secrete estrogens, HCG and/or AFP, so patients may present with precocious puberty. Treatment is similar to that of endodermal sinus tumors. Nongestational choriocarcinomas, like the gestational forms, have a poor prognosis and are monitored and treated similarly.
Understanding presentation and treatments for malignant germ cell neoplasms is important in the evaluation of a young patient with a pelvic mass and should prompt the practicing gynecologist to test for AFP and HCG, with or without LDH and CA-125. If encountered inadvertently, every attempt should be made to preserve fertility in these young patients and expedient referral to a gynecologic oncologist, pediatric gynecologist, and/or a reproductive endocrinologist is warranted. Rarely, a second look laparotomy is indicated without obvious intraperitoneal spread and reproductive potential is preserved even in those requiring BEP. If managed appropriately, the overall prognosis remains good for these young women.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
High-grade cervical dysplasia in pregnancy
Cervical intraepithelial neoplasia (CIN) describes a precancerous lesion of the squamous epithelium of the ectocervix. The cervical cancer screening paradigm in the United States begins with collection of cervical cytology with a Pap smear, frequently in conjunction with human papillomavirus testing. Abnormalities will frequently lead to colposcopy with directed biopsy, which can result in a diagnosis of CIN. There are different grades of severity within CIN, which aids in making treatment recommendations.
Pregnancy is a convenient time to capture women for cervical cancer screening, given the increased contact with health care providers. Routine guidelines should be followed for screening women who are pregnant, as collection of cervical cytology and human papillomavirus (HPV) cotesting is safe.
In women who have been found to have abnormal cytology, CIN or malignancy has been identified in up to 19% of cases (Am J Obstet Gynecol. 2004 Jul;191[1]:105-13). High-grade lesions identified in pregnant women create a unique management dilemma.
Terminology
The Bethesda system describes colposcopic abnormalities as CIN and divides premalignant lesions into grades from 1 to 3 with the highest grade representing more worrisome lesions. CIN2 has been found to have poor reproducibility and likely represents a mix of low- and high-grade lesions. In addition, there is concern that HPV-associated lesions of the lower anogenital tract have incongruent terminology among different specialties that may not accurately represent the current understanding of HPV pathogenesis.
In 2012, the Lower Anogenital Squamous Terminology (LAST) project of the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology (ASCCP) advocated for consistent terminology across all lower anogenital tract lesions with HPV, including CIN (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
With this new terminology, CIN1 is referred to as low-grade squamous intraepithelial lesion (LSIL). CIN2 is characterized by its p16 immunostaining; lesions that are p16 negative are considered LSIL, while those that are positive are considered HSIL (high-grade squamous intraepithelial lesion). While this staining is not universally performed, physicians will start seeing p16 staining results with increasing frequency on their cervical biopsies. CIN3 lesions are referred to as HSIL.
Given the current understanding of HPV-mediated disease, and a commitment to represent the most up-to-date information, the LAST project terminology of HSIL to represent previously identified CIN2 and CIN3 lesions will be used for the remainder of this text.
Diagnosis
There is little data on the natural history of HSIL diagnosed after colposcopy, as most women get some form of therapy. The information that is available suggests that in patients with untreated HSIL, the cumulative incidence of malignancy is as high as 30% at 30 years (Lancet Oncol. 2008 May;9[5]:425-34). Treatment recommendations for excision are aimed at addressing this alarming number; however, care must be individualized, especially in the setting of pregnancy.
If abnormal cervical cytology is obtained on routine screening, appropriate patients should be referred for colposcopic exam. Physicians performing colposcopy should be familiar with the physiologic effects of pregnancy that can obscure the exam, including the increased cervical mucus production, prominence of endocervical glands, and increased vascularity.
Colposcopic-directed ectocervical biopsies have been found to be safe in pregnancy, and these women should be provided the same care as those who are not pregnant (Obstet Gynecol. 1993 Jun;81[6]:915-8). Endocervical sampling and endometrial sampling should not be performed, however, and physicians should remain dedicated to checking pregnancy tests prior to colposcopy.
HSIL cytology should prompt a biopsy in pregnancy; a decision to skip the biopsy and perform an excisional procedure in this setting is not recommended regardless of patient or gestational age. If LSIL (CIN1) is noted on biopsy, reevaluation post partum should be strongly considered, unless a suspicious lesion was felt to be inadequately biopsied.
Management
Managing HSIL in pregnancy focuses on diagnosis and excluding malignancy, while treatment can be reserved for the postpartum period. When choosing a management option, consider individual patient factors such as colposcopic appearance of the lesion, gestational age, and access to health care.
If HSIL is noted on colposcopic-directed biopsy, consider one of several options. The most conservative approach is reevaluation with cytology and colposcopy 6 weeks post partum. This is an option for patients who do not have a colposcopic exam that was concerning for an invasive lesion, were able to be adequately biopsied, and will reliably return for follow-up. Many physicians feel more comfortable with repeat cytology and colposcopy in 3 months from the original biopsy. The most aggressive management would include an excisional procedure during pregnancy.
There are varying rates of regression of biopsy-proven HSIL in pregnancy ranging from 34% to 70% (Obstet Gynecol. 1999 Mar;93[3]:359-62; Acta Obstet Gynecol Scand. 2006;85[9]:1134-7; Reprod Sci. 2009 Nov;16[11]:1034-9). Out of more than 200 patients across these three studies, just two patients were diagnosed with an invasive lesion post partum. Given the low likelihood of progression during pregnancy and the high rate of regression, an excisional procedure should be considered only in cases where there is concern about invasive carcinoma.
In cases where an invasive lesion is suspected, consider an an excisional procedure. While there is some evidence that performing a laser excisional procedure early in pregnancy (18 weeks and earlier) can be safely done, that is not the most common management strategy in the United States (Tumori. 1998 Sep-Oct;84[5]:567-70; Int J Gynecol Cancer. 2007 Jan-Feb;17[1]:127-31). In this circumstance, referral to a gynecologic oncologist is warranted where consideration can be made for performing a cold knife conization. Physicians should be aware of the increased risk of bleeding with this procedure in pregnancy and the potential for preterm birth. There is little literature to guide counseling regarding these risks, and the decision to perform an excisional procedure should be made with a multidisciplinary team (Arch Gynecol Obstet. 2016 Jan 4. doi: 10.1007/s00404-015-3980-y).
The see-and-treat paradigm is not recommended in pregnancy. Those patients with poor follow-up should still undergo colposcopic-directed biopsies prior to any excisional procedure.
Treatment recommendations in pregnancy should be made on the basis of careful consideration of individual patient factors, with strong consideration of repeat testing with cytology and colposcopy prior to an excision procedure.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures. Email them at [email protected].
Cervical intraepithelial neoplasia (CIN) describes a precancerous lesion of the squamous epithelium of the ectocervix. The cervical cancer screening paradigm in the United States begins with collection of cervical cytology with a Pap smear, frequently in conjunction with human papillomavirus testing. Abnormalities will frequently lead to colposcopy with directed biopsy, which can result in a diagnosis of CIN. There are different grades of severity within CIN, which aids in making treatment recommendations.
Pregnancy is a convenient time to capture women for cervical cancer screening, given the increased contact with health care providers. Routine guidelines should be followed for screening women who are pregnant, as collection of cervical cytology and human papillomavirus (HPV) cotesting is safe.
In women who have been found to have abnormal cytology, CIN or malignancy has been identified in up to 19% of cases (Am J Obstet Gynecol. 2004 Jul;191[1]:105-13). High-grade lesions identified in pregnant women create a unique management dilemma.
Terminology
The Bethesda system describes colposcopic abnormalities as CIN and divides premalignant lesions into grades from 1 to 3 with the highest grade representing more worrisome lesions. CIN2 has been found to have poor reproducibility and likely represents a mix of low- and high-grade lesions. In addition, there is concern that HPV-associated lesions of the lower anogenital tract have incongruent terminology among different specialties that may not accurately represent the current understanding of HPV pathogenesis.
In 2012, the Lower Anogenital Squamous Terminology (LAST) project of the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology (ASCCP) advocated for consistent terminology across all lower anogenital tract lesions with HPV, including CIN (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
With this new terminology, CIN1 is referred to as low-grade squamous intraepithelial lesion (LSIL). CIN2 is characterized by its p16 immunostaining; lesions that are p16 negative are considered LSIL, while those that are positive are considered HSIL (high-grade squamous intraepithelial lesion). While this staining is not universally performed, physicians will start seeing p16 staining results with increasing frequency on their cervical biopsies. CIN3 lesions are referred to as HSIL.
Given the current understanding of HPV-mediated disease, and a commitment to represent the most up-to-date information, the LAST project terminology of HSIL to represent previously identified CIN2 and CIN3 lesions will be used for the remainder of this text.
Diagnosis
There is little data on the natural history of HSIL diagnosed after colposcopy, as most women get some form of therapy. The information that is available suggests that in patients with untreated HSIL, the cumulative incidence of malignancy is as high as 30% at 30 years (Lancet Oncol. 2008 May;9[5]:425-34). Treatment recommendations for excision are aimed at addressing this alarming number; however, care must be individualized, especially in the setting of pregnancy.
If abnormal cervical cytology is obtained on routine screening, appropriate patients should be referred for colposcopic exam. Physicians performing colposcopy should be familiar with the physiologic effects of pregnancy that can obscure the exam, including the increased cervical mucus production, prominence of endocervical glands, and increased vascularity.
Colposcopic-directed ectocervical biopsies have been found to be safe in pregnancy, and these women should be provided the same care as those who are not pregnant (Obstet Gynecol. 1993 Jun;81[6]:915-8). Endocervical sampling and endometrial sampling should not be performed, however, and physicians should remain dedicated to checking pregnancy tests prior to colposcopy.
HSIL cytology should prompt a biopsy in pregnancy; a decision to skip the biopsy and perform an excisional procedure in this setting is not recommended regardless of patient or gestational age. If LSIL (CIN1) is noted on biopsy, reevaluation post partum should be strongly considered, unless a suspicious lesion was felt to be inadequately biopsied.
Management
Managing HSIL in pregnancy focuses on diagnosis and excluding malignancy, while treatment can be reserved for the postpartum period. When choosing a management option, consider individual patient factors such as colposcopic appearance of the lesion, gestational age, and access to health care.
If HSIL is noted on colposcopic-directed biopsy, consider one of several options. The most conservative approach is reevaluation with cytology and colposcopy 6 weeks post partum. This is an option for patients who do not have a colposcopic exam that was concerning for an invasive lesion, were able to be adequately biopsied, and will reliably return for follow-up. Many physicians feel more comfortable with repeat cytology and colposcopy in 3 months from the original biopsy. The most aggressive management would include an excisional procedure during pregnancy.
There are varying rates of regression of biopsy-proven HSIL in pregnancy ranging from 34% to 70% (Obstet Gynecol. 1999 Mar;93[3]:359-62; Acta Obstet Gynecol Scand. 2006;85[9]:1134-7; Reprod Sci. 2009 Nov;16[11]:1034-9). Out of more than 200 patients across these three studies, just two patients were diagnosed with an invasive lesion post partum. Given the low likelihood of progression during pregnancy and the high rate of regression, an excisional procedure should be considered only in cases where there is concern about invasive carcinoma.
In cases where an invasive lesion is suspected, consider an an excisional procedure. While there is some evidence that performing a laser excisional procedure early in pregnancy (18 weeks and earlier) can be safely done, that is not the most common management strategy in the United States (Tumori. 1998 Sep-Oct;84[5]:567-70; Int J Gynecol Cancer. 2007 Jan-Feb;17[1]:127-31). In this circumstance, referral to a gynecologic oncologist is warranted where consideration can be made for performing a cold knife conization. Physicians should be aware of the increased risk of bleeding with this procedure in pregnancy and the potential for preterm birth. There is little literature to guide counseling regarding these risks, and the decision to perform an excisional procedure should be made with a multidisciplinary team (Arch Gynecol Obstet. 2016 Jan 4. doi: 10.1007/s00404-015-3980-y).
The see-and-treat paradigm is not recommended in pregnancy. Those patients with poor follow-up should still undergo colposcopic-directed biopsies prior to any excisional procedure.
Treatment recommendations in pregnancy should be made on the basis of careful consideration of individual patient factors, with strong consideration of repeat testing with cytology and colposcopy prior to an excision procedure.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures. Email them at [email protected].
Cervical intraepithelial neoplasia (CIN) describes a precancerous lesion of the squamous epithelium of the ectocervix. The cervical cancer screening paradigm in the United States begins with collection of cervical cytology with a Pap smear, frequently in conjunction with human papillomavirus testing. Abnormalities will frequently lead to colposcopy with directed biopsy, which can result in a diagnosis of CIN. There are different grades of severity within CIN, which aids in making treatment recommendations.
Pregnancy is a convenient time to capture women for cervical cancer screening, given the increased contact with health care providers. Routine guidelines should be followed for screening women who are pregnant, as collection of cervical cytology and human papillomavirus (HPV) cotesting is safe.
In women who have been found to have abnormal cytology, CIN or malignancy has been identified in up to 19% of cases (Am J Obstet Gynecol. 2004 Jul;191[1]:105-13). High-grade lesions identified in pregnant women create a unique management dilemma.
Terminology
The Bethesda system describes colposcopic abnormalities as CIN and divides premalignant lesions into grades from 1 to 3 with the highest grade representing more worrisome lesions. CIN2 has been found to have poor reproducibility and likely represents a mix of low- and high-grade lesions. In addition, there is concern that HPV-associated lesions of the lower anogenital tract have incongruent terminology among different specialties that may not accurately represent the current understanding of HPV pathogenesis.
In 2012, the Lower Anogenital Squamous Terminology (LAST) project of the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology (ASCCP) advocated for consistent terminology across all lower anogenital tract lesions with HPV, including CIN (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
With this new terminology, CIN1 is referred to as low-grade squamous intraepithelial lesion (LSIL). CIN2 is characterized by its p16 immunostaining; lesions that are p16 negative are considered LSIL, while those that are positive are considered HSIL (high-grade squamous intraepithelial lesion). While this staining is not universally performed, physicians will start seeing p16 staining results with increasing frequency on their cervical biopsies. CIN3 lesions are referred to as HSIL.
Given the current understanding of HPV-mediated disease, and a commitment to represent the most up-to-date information, the LAST project terminology of HSIL to represent previously identified CIN2 and CIN3 lesions will be used for the remainder of this text.
Diagnosis
There is little data on the natural history of HSIL diagnosed after colposcopy, as most women get some form of therapy. The information that is available suggests that in patients with untreated HSIL, the cumulative incidence of malignancy is as high as 30% at 30 years (Lancet Oncol. 2008 May;9[5]:425-34). Treatment recommendations for excision are aimed at addressing this alarming number; however, care must be individualized, especially in the setting of pregnancy.
If abnormal cervical cytology is obtained on routine screening, appropriate patients should be referred for colposcopic exam. Physicians performing colposcopy should be familiar with the physiologic effects of pregnancy that can obscure the exam, including the increased cervical mucus production, prominence of endocervical glands, and increased vascularity.
Colposcopic-directed ectocervical biopsies have been found to be safe in pregnancy, and these women should be provided the same care as those who are not pregnant (Obstet Gynecol. 1993 Jun;81[6]:915-8). Endocervical sampling and endometrial sampling should not be performed, however, and physicians should remain dedicated to checking pregnancy tests prior to colposcopy.
HSIL cytology should prompt a biopsy in pregnancy; a decision to skip the biopsy and perform an excisional procedure in this setting is not recommended regardless of patient or gestational age. If LSIL (CIN1) is noted on biopsy, reevaluation post partum should be strongly considered, unless a suspicious lesion was felt to be inadequately biopsied.
Management
Managing HSIL in pregnancy focuses on diagnosis and excluding malignancy, while treatment can be reserved for the postpartum period. When choosing a management option, consider individual patient factors such as colposcopic appearance of the lesion, gestational age, and access to health care.
If HSIL is noted on colposcopic-directed biopsy, consider one of several options. The most conservative approach is reevaluation with cytology and colposcopy 6 weeks post partum. This is an option for patients who do not have a colposcopic exam that was concerning for an invasive lesion, were able to be adequately biopsied, and will reliably return for follow-up. Many physicians feel more comfortable with repeat cytology and colposcopy in 3 months from the original biopsy. The most aggressive management would include an excisional procedure during pregnancy.
There are varying rates of regression of biopsy-proven HSIL in pregnancy ranging from 34% to 70% (Obstet Gynecol. 1999 Mar;93[3]:359-62; Acta Obstet Gynecol Scand. 2006;85[9]:1134-7; Reprod Sci. 2009 Nov;16[11]:1034-9). Out of more than 200 patients across these three studies, just two patients were diagnosed with an invasive lesion post partum. Given the low likelihood of progression during pregnancy and the high rate of regression, an excisional procedure should be considered only in cases where there is concern about invasive carcinoma.
In cases where an invasive lesion is suspected, consider an an excisional procedure. While there is some evidence that performing a laser excisional procedure early in pregnancy (18 weeks and earlier) can be safely done, that is not the most common management strategy in the United States (Tumori. 1998 Sep-Oct;84[5]:567-70; Int J Gynecol Cancer. 2007 Jan-Feb;17[1]:127-31). In this circumstance, referral to a gynecologic oncologist is warranted where consideration can be made for performing a cold knife conization. Physicians should be aware of the increased risk of bleeding with this procedure in pregnancy and the potential for preterm birth. There is little literature to guide counseling regarding these risks, and the decision to perform an excisional procedure should be made with a multidisciplinary team (Arch Gynecol Obstet. 2016 Jan 4. doi: 10.1007/s00404-015-3980-y).
The see-and-treat paradigm is not recommended in pregnancy. Those patients with poor follow-up should still undergo colposcopic-directed biopsies prior to any excisional procedure.
Treatment recommendations in pregnancy should be made on the basis of careful consideration of individual patient factors, with strong consideration of repeat testing with cytology and colposcopy prior to an excision procedure.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures. Email them at [email protected].
A trip through the history of gynecologic oncology
The subspecialty of gynecologic oncology was formalized less than 50 years ago with the creation of the Society of Gynecologic Oncology and subspecialty training and board certification. The formation of the Gynecologic Oncology Group (GOG) – and the many clinical trials spearheaded by that group – has further advanced evidence-based treatments, resulting in improved survival outcomes, quality of life, and preventive strategies.
While it is not possible to provide a comprehensive and exhaustive review of all of the advances, we hope to highlight many of the notable advances in this article.Cervical cancer
Cervical cancer is the fourth most common cancer in women worldwide with 528,000 new cases in 2012. The majority of cervical cancer cases are caused by infection with human papillomavirus (HPV). While the standard therapies for cervical cancer have been long established (radical hysterectomy for stage I and radiation therapy for locally advanced disease), one of the most significant advances in the past 50 years was the addition of radiation-sensitizing chemotherapy (cisplatin) administered concurrently with radiation therapy.
In randomized trials in both early and advanced cervical cancer, the risk of death was reduced by 30%-50%. These studies changed the paradigm for the treatment of cervical cancer (N Engl J Med. 1999 Apr 15;340[15]:1137-43; N Engl J Med. 1999 Apr 15;340[15]:1144-53; J Clin Oncol. 2000 Apr;18[8]:1606-13).
Future studies evaluating biologic adjuncts or additional chemotherapy are currently underway or awaiting data maturation.
The American Society of Clinical Oncology (ASCO) highlighted the “Top 5 advances in 50 years of Modern Oncology” in 2014, and second on the list was the approval of the HPV vaccine to prevent cervical cancer. Vaccines have been developed that can protect against types 2, 4 or 9 of HPV. In a 2014 study, depending on vaccination coverage, the relative number of cervical cancer cases avoided was 34% in Africa, 27% for America, 26% for Asia, 21% for Europe, and worldwide was estimated at 27% (Vaccine. 2014 Feb 3;32[6]:733-9).
While the benefit from HPV vaccination has been proven, in the United States, only about a third of eligible girls and women have been vaccinated. Efforts should focus on expanding vaccination penetration to eligible girls, boys, women, and men.
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy in the United States with an estimated 54,870 cases and 10,170 deaths annually. Notable advances in the management of women with endometrial cancer have arisen because of a better understanding that there are two types of endometrial cancer – type I and type II.
The type I endometrial cancers tend to be associated with lower stage of disease at the time of diagnosis and fewer recurrences, while type II endometrial cancer is associated with worse outcomes.
Tailoring the surgical approaches and adjuvant therapy for women with endometrial cancer has led to improved outcomes. The GOG conducted a large prospective randomized trial of laparotomy versus laparoscopic surgical staging for women with clinical early-stage endometrial cancer (LAP2). Laparoscopy was associated with improved perioperative outcomes and was found to be noninferior to laparotomy with regards to survival outcomes (J Clin Oncol. 2012 Mar 1;30[7]:695-700). Therefore, minimally invasive surgery has become widely accepted for the surgical staging of women with endometrial cancer.
Appropriate surgical staging allows for tailoring of postoperative adjuvant therapy. The current evidence suggests that vaginal brachytherapy should be the adjuvant treatment of choice over whole pelvic radiation in women with early-stage endometrial cancer (Lancet. 2010 Mar 6;375[9717]:816-23). Studies are underway to evaluate the role of both adjuvant radiation and chemotherapy in women with early-stage type II endometrial cancer who are felt to be at high risk for recurrent disease, as well as how to improve on the therapeutic options for women with advanced or recurrent disease.
Ovarian cancer
Epithelial ovarian cancer is the most deadly gynecologic malignancy in the United States with 21,290 cases and 14,180 deaths in 2015. The concept of ovarian tumor debulking was first described by Dr. Joe Meigs in 1934, but did not gain traction until the mid-1970s when Dr. C. Thomas Griffiths published his work (Natl Cancer Inst Monogr. 1975 Oct;42:101-4).
While there are no randomized trials proving that surgical cytoreduction improves overall survival, most retrospective studies support this concept. In 2009, Chi et al. showed improved median survival in women with ovarian cancer based on the increased percentage of women who underwent optimal cytoreduction (Gynecol Oncol. 2009 Jul;114[1]:26-31). This has led to modifications of surgical techniques and surgical goals with an effort to maximally cytoreduce all of the visible disease.
While initial surgical debulking is the goal, there are circumstances when a different approach may be indicated. Vergote et al. conducted a prospective randomized trial of 670 women with advanced ovarian cancer. In this study, neoadjuvant chemotherapy followed by interval debulking was not inferior to primary debulking followed by chemotherapy with regards to progression-free survival and overall survival. However, initial surgery was associated with increased surgical complications and perioperative mortality as compared with interval surgery. Therefore, in women who are not felt to be candidates for optimal cytoreduction, neoadjuvant chemotherapy followed by interval surgery may be an appropriate treatment strategy (N Engl J Med. 2010 Sep 2;363[10]:943-53.).
There have been several notable advances and a series of randomized trials – predominately conducted by the GOG – that have resulted in improved overall survival and progression-free interval in women with ovarian cancer. However, none are as significant as the discovery of paclitaxel and platinum-based chemotherapy (cisplatin and carboplatin).
In 1962, samples of the Pacific Yew’s bark were collected and, 2 years later, the extracts from this bark were found to have cytotoxic activity. There were initial difficulties suspending the drug in solution; however, ultimately a formulation in ethanol, cremophor, and saline was found to be effective. In 1984, the National Cancer Institute began clinical trials of paclitaxel and it was found to be highly effective in ovarian cancer. In 1992, it was approved for the treatment of ovarian cancer.
Cisplatin was approved in 1978. Carboplatin entered clinical trials in 1982 and was approved for women with recurrent ovarian cancer in 1989.
There were a series of trials beginning in the late 1980s that established the role of platinum agents and led us to GOG 111. This trial evaluated cisplatin with either cyclophosphamide or paclitaxel. The paclitaxel combination was superior and in 2003 two trials were published that solidified carboplatin and paclitaxel as the cornerstone in the treatment of women with ovarian cancer (J Clin Oncol. 2003 Sep 1;21[17]:3194-200; J Natl Cancer Inst. 2003 Sep 3;95[17]:1320-9).
What has most recently been debated is the route and schedule for both paclitaxel and the platinum agents. In January 2006, the National Cancer Institute released a Clinical Announcement regarding the role of intraperitoneal (IP) chemotherapy for the treatment of women with optimally debulked ovarian cancer. Of the six trials included in the announcement, four trials showed a benefit for progression-free survival and five studies showed an improvement in overall survival. Armstrong et al (GOG 172) showed a 16-month improvement in overall survival with intravenous (IV) paclitaxel, IP cisplatin, and IP paclitaxel. IP chemotherapy has not been universally embraced by physicians and patients in part because of its toxicity, treatment schedule, and the fact that no IP regimen has been compared with the current standard of IV carboplatin and paclitaxel (N Engl J Med. 2006 Jan 5;354[1]:34-43).
While there have been improvements in 5-year survival over time, most women with advanced ovarian cancer will undergo additional chemotherapy in order to achieve subsequent remissions or maintain stability of disease. Other drugs that have Food and Drug Administration approval in the setting of recurrent ovarian cancer include topotecan, liposomal doxorubicin, gemcitabine, bevacizumab, altretamine, carboplatin, cisplatin, cyclophosphamide, and melphalan. Olaparib was recently approved as monotherapy in women with a germline BRCA-mutation who had received three or more prior lines of chemotherapy.
Minimally invasive surgery
Over the last 30 years, minimally invasive surgery (MIS) in gynecologic oncology, particularly for endometrial cancer, has gone from a niche procedure to the standard of care. The introduction of laparoscopy into gynecologic oncology started in the early 1990s. In a series of 59 women undergoing laparoscopy for endometrial cancer, Childers et al. demonstrated feasibility of the technique and low laparotomy conversion rates (Gynecol Oncol. 1993 Oct;51[1]:33-8.). The GOG trial, LAP2, supported the equivalent oncologic outcomes of MIS versus laparotomy for the treatment of endometrial cancer. While many surgeons and centers offered laparoscopic surgery, there were issues with the learning curve that limited its widespread use.
In 2005, the FDA approval of the robotic platform for gynecologic surgery resulted in at least a doubling of the proportion of endometrial cancer patients treated with MIS (Int J Med Robot. 2009 Dec;5[4]:392-7.). In 2012, the Society of Gynecologic Oncology published a consensus statement regarding robotic-assisted surgery in gynecologic oncology (Gynecol Oncol. 2012 Feb;124[2]:180-4.). This review highlights the advantages of the robotics platform with regards to expanding MIS to women with cervical and ovarian cancer; the improvements in outcomes in the obese woman with endometrial cancer; and that the learning curve for robotic surgery is shorter than for traditional laparoscopy. Issues requiring further research include cost analysis as the cost of the new technology decreases, and opportunities for improvement in patient and physician quality of life.
Sentinel node mapping
The rationale for sentinel node mapping is that if one or more sentinel lymph nodes is/are negative for malignancy, then the other regional lymph nodes will also be negative. This would thereby avoid the need for a complete lymph node dissection and its resultant complications, including chronic lymphedema. Much of the work pioneering this strategy has been in breast cancer and melanoma, but data are rapidly emerging for these techniques in gynecologic malignancies.
Candidates for sentinel lymph node biopsy for vulvar cancer include those with a lesion more than 1mm in depth, a tumor less than 4 cm in size, and no obvious metastatic disease on exam or preoperative imaging. Additionally, recommendations have been made regarding case volume in order to achieve limited numbers of false-negative results and to maintain competency. In the study by Van der Zee et al. of 403 patients (623 groins) who underwent sentinel node procedures, the false-negative rate was 0-2%. The overall survival rate was 97% at 3 years (J Clin Oncol. 2008 Feb 20;26[6]:884-9). However, a more recent data from the Gynecologic Oncology Group (GOG 173) showed a slightly higher false-negative rate of 8% (J Clin Oncol. 2012 Nov 1;30[31]:3786-91). Overall survival data are pending from this study.
While sentinel lymph node mapping for endometrial cancer has been feasible for many years and has been well described, the questioned role of completed lymphadenectomy for early-stage endometrial cancer has led to a resurgence of interest in these techniques. While blue dye and radiolabeled tracer methods have historically been the most popular mapping solutions, the advent of endoscopic near-infrared imaging, with its higher sensitivity and good depth penetration, has added options. Indocyanine green fluorescence can be easily detected during robotic surgery and as experience with these techniques increase, successful mapping and sensitivity will increase.
Genetics
While hereditary cancer syndromes have been recognized for many years, detecting the genetic mutations that may increase an individual’s risk of developing a malignancy were not elucidated until the early 1990s. In gynecologic oncology, the most commonly encountered syndromes involve mutations in BRCA1 and BRCA2 and hereditary non–polyposis colorectal cancer, which causes mutations in DNA mismatch-repair genes and increase the risk of endometrial and ovarian cancer.
The SGO recently published a statement on risk assessment for inherited gynecologic cancer predispositions. In this statement “the evaluation for the presence of a hereditary cancer syndrome enables physicians to provide individualized and quantified assessment of cancer risk, as well as options for tailored screening and preventions strategies that may reduce morbidity associated with the development of malignancy” (Gynecol Oncol. 2015 Jan;136[1]:3-7). Beyond risk-reducing salpingo-oophorectomy, therapeutic strategies targeting patients with germline mutations have been developed (PARP inhibitors in BRCA-mutated women with ovarian cancer).
In August 2015, ASCO released an updated policy statement on genetic and genomic testing for cancer susceptibility and highlighted five key areas: germ-line implications of somatic mutation profiling; multigene panel testing for cancer susceptibility; quality assurance in genetic testing; education for oncology professionals; and access to cancer genetic services.
Antiemetics
Rounding out ASCO’s “Top 5 advances in 50 years of Modern Oncology” was the improvement in patients’ quality of life from supportive therapies, in particular antinausea medications.
Several of the agents commonly used in gynecologic oncology rate high (cisplatin) to moderate (carboplatin, cyclophosphamide, doxorubicin, ifosfamide) with regards to emetogenicity. The advent of 5-HT3 receptor antagonists (for example, ondansetron) has significantly improved the quality of life of patients undergoing cytotoxic chemotherapy. In addition to improving quality of life, the decrease in nausea and vomiting can also decrease life-threatening complications such as dehydration and electrolyte imbalance. Both ASCO and the National Comprehensive Cancer Network both have guidelines for the management of nausea and vomiting in patients undergoing chemotherapy.
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology, and a professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
The subspecialty of gynecologic oncology was formalized less than 50 years ago with the creation of the Society of Gynecologic Oncology and subspecialty training and board certification. The formation of the Gynecologic Oncology Group (GOG) – and the many clinical trials spearheaded by that group – has further advanced evidence-based treatments, resulting in improved survival outcomes, quality of life, and preventive strategies.
While it is not possible to provide a comprehensive and exhaustive review of all of the advances, we hope to highlight many of the notable advances in this article.Cervical cancer
Cervical cancer is the fourth most common cancer in women worldwide with 528,000 new cases in 2012. The majority of cervical cancer cases are caused by infection with human papillomavirus (HPV). While the standard therapies for cervical cancer have been long established (radical hysterectomy for stage I and radiation therapy for locally advanced disease), one of the most significant advances in the past 50 years was the addition of radiation-sensitizing chemotherapy (cisplatin) administered concurrently with radiation therapy.
In randomized trials in both early and advanced cervical cancer, the risk of death was reduced by 30%-50%. These studies changed the paradigm for the treatment of cervical cancer (N Engl J Med. 1999 Apr 15;340[15]:1137-43; N Engl J Med. 1999 Apr 15;340[15]:1144-53; J Clin Oncol. 2000 Apr;18[8]:1606-13).
Future studies evaluating biologic adjuncts or additional chemotherapy are currently underway or awaiting data maturation.
The American Society of Clinical Oncology (ASCO) highlighted the “Top 5 advances in 50 years of Modern Oncology” in 2014, and second on the list was the approval of the HPV vaccine to prevent cervical cancer. Vaccines have been developed that can protect against types 2, 4 or 9 of HPV. In a 2014 study, depending on vaccination coverage, the relative number of cervical cancer cases avoided was 34% in Africa, 27% for America, 26% for Asia, 21% for Europe, and worldwide was estimated at 27% (Vaccine. 2014 Feb 3;32[6]:733-9).
While the benefit from HPV vaccination has been proven, in the United States, only about a third of eligible girls and women have been vaccinated. Efforts should focus on expanding vaccination penetration to eligible girls, boys, women, and men.
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy in the United States with an estimated 54,870 cases and 10,170 deaths annually. Notable advances in the management of women with endometrial cancer have arisen because of a better understanding that there are two types of endometrial cancer – type I and type II.
The type I endometrial cancers tend to be associated with lower stage of disease at the time of diagnosis and fewer recurrences, while type II endometrial cancer is associated with worse outcomes.
Tailoring the surgical approaches and adjuvant therapy for women with endometrial cancer has led to improved outcomes. The GOG conducted a large prospective randomized trial of laparotomy versus laparoscopic surgical staging for women with clinical early-stage endometrial cancer (LAP2). Laparoscopy was associated with improved perioperative outcomes and was found to be noninferior to laparotomy with regards to survival outcomes (J Clin Oncol. 2012 Mar 1;30[7]:695-700). Therefore, minimally invasive surgery has become widely accepted for the surgical staging of women with endometrial cancer.
Appropriate surgical staging allows for tailoring of postoperative adjuvant therapy. The current evidence suggests that vaginal brachytherapy should be the adjuvant treatment of choice over whole pelvic radiation in women with early-stage endometrial cancer (Lancet. 2010 Mar 6;375[9717]:816-23). Studies are underway to evaluate the role of both adjuvant radiation and chemotherapy in women with early-stage type II endometrial cancer who are felt to be at high risk for recurrent disease, as well as how to improve on the therapeutic options for women with advanced or recurrent disease.
Ovarian cancer
Epithelial ovarian cancer is the most deadly gynecologic malignancy in the United States with 21,290 cases and 14,180 deaths in 2015. The concept of ovarian tumor debulking was first described by Dr. Joe Meigs in 1934, but did not gain traction until the mid-1970s when Dr. C. Thomas Griffiths published his work (Natl Cancer Inst Monogr. 1975 Oct;42:101-4).
While there are no randomized trials proving that surgical cytoreduction improves overall survival, most retrospective studies support this concept. In 2009, Chi et al. showed improved median survival in women with ovarian cancer based on the increased percentage of women who underwent optimal cytoreduction (Gynecol Oncol. 2009 Jul;114[1]:26-31). This has led to modifications of surgical techniques and surgical goals with an effort to maximally cytoreduce all of the visible disease.
While initial surgical debulking is the goal, there are circumstances when a different approach may be indicated. Vergote et al. conducted a prospective randomized trial of 670 women with advanced ovarian cancer. In this study, neoadjuvant chemotherapy followed by interval debulking was not inferior to primary debulking followed by chemotherapy with regards to progression-free survival and overall survival. However, initial surgery was associated with increased surgical complications and perioperative mortality as compared with interval surgery. Therefore, in women who are not felt to be candidates for optimal cytoreduction, neoadjuvant chemotherapy followed by interval surgery may be an appropriate treatment strategy (N Engl J Med. 2010 Sep 2;363[10]:943-53.).
There have been several notable advances and a series of randomized trials – predominately conducted by the GOG – that have resulted in improved overall survival and progression-free interval in women with ovarian cancer. However, none are as significant as the discovery of paclitaxel and platinum-based chemotherapy (cisplatin and carboplatin).
In 1962, samples of the Pacific Yew’s bark were collected and, 2 years later, the extracts from this bark were found to have cytotoxic activity. There were initial difficulties suspending the drug in solution; however, ultimately a formulation in ethanol, cremophor, and saline was found to be effective. In 1984, the National Cancer Institute began clinical trials of paclitaxel and it was found to be highly effective in ovarian cancer. In 1992, it was approved for the treatment of ovarian cancer.
Cisplatin was approved in 1978. Carboplatin entered clinical trials in 1982 and was approved for women with recurrent ovarian cancer in 1989.
There were a series of trials beginning in the late 1980s that established the role of platinum agents and led us to GOG 111. This trial evaluated cisplatin with either cyclophosphamide or paclitaxel. The paclitaxel combination was superior and in 2003 two trials were published that solidified carboplatin and paclitaxel as the cornerstone in the treatment of women with ovarian cancer (J Clin Oncol. 2003 Sep 1;21[17]:3194-200; J Natl Cancer Inst. 2003 Sep 3;95[17]:1320-9).
What has most recently been debated is the route and schedule for both paclitaxel and the platinum agents. In January 2006, the National Cancer Institute released a Clinical Announcement regarding the role of intraperitoneal (IP) chemotherapy for the treatment of women with optimally debulked ovarian cancer. Of the six trials included in the announcement, four trials showed a benefit for progression-free survival and five studies showed an improvement in overall survival. Armstrong et al (GOG 172) showed a 16-month improvement in overall survival with intravenous (IV) paclitaxel, IP cisplatin, and IP paclitaxel. IP chemotherapy has not been universally embraced by physicians and patients in part because of its toxicity, treatment schedule, and the fact that no IP regimen has been compared with the current standard of IV carboplatin and paclitaxel (N Engl J Med. 2006 Jan 5;354[1]:34-43).
While there have been improvements in 5-year survival over time, most women with advanced ovarian cancer will undergo additional chemotherapy in order to achieve subsequent remissions or maintain stability of disease. Other drugs that have Food and Drug Administration approval in the setting of recurrent ovarian cancer include topotecan, liposomal doxorubicin, gemcitabine, bevacizumab, altretamine, carboplatin, cisplatin, cyclophosphamide, and melphalan. Olaparib was recently approved as monotherapy in women with a germline BRCA-mutation who had received three or more prior lines of chemotherapy.
Minimally invasive surgery
Over the last 30 years, minimally invasive surgery (MIS) in gynecologic oncology, particularly for endometrial cancer, has gone from a niche procedure to the standard of care. The introduction of laparoscopy into gynecologic oncology started in the early 1990s. In a series of 59 women undergoing laparoscopy for endometrial cancer, Childers et al. demonstrated feasibility of the technique and low laparotomy conversion rates (Gynecol Oncol. 1993 Oct;51[1]:33-8.). The GOG trial, LAP2, supported the equivalent oncologic outcomes of MIS versus laparotomy for the treatment of endometrial cancer. While many surgeons and centers offered laparoscopic surgery, there were issues with the learning curve that limited its widespread use.
In 2005, the FDA approval of the robotic platform for gynecologic surgery resulted in at least a doubling of the proportion of endometrial cancer patients treated with MIS (Int J Med Robot. 2009 Dec;5[4]:392-7.). In 2012, the Society of Gynecologic Oncology published a consensus statement regarding robotic-assisted surgery in gynecologic oncology (Gynecol Oncol. 2012 Feb;124[2]:180-4.). This review highlights the advantages of the robotics platform with regards to expanding MIS to women with cervical and ovarian cancer; the improvements in outcomes in the obese woman with endometrial cancer; and that the learning curve for robotic surgery is shorter than for traditional laparoscopy. Issues requiring further research include cost analysis as the cost of the new technology decreases, and opportunities for improvement in patient and physician quality of life.
Sentinel node mapping
The rationale for sentinel node mapping is that if one or more sentinel lymph nodes is/are negative for malignancy, then the other regional lymph nodes will also be negative. This would thereby avoid the need for a complete lymph node dissection and its resultant complications, including chronic lymphedema. Much of the work pioneering this strategy has been in breast cancer and melanoma, but data are rapidly emerging for these techniques in gynecologic malignancies.
Candidates for sentinel lymph node biopsy for vulvar cancer include those with a lesion more than 1mm in depth, a tumor less than 4 cm in size, and no obvious metastatic disease on exam or preoperative imaging. Additionally, recommendations have been made regarding case volume in order to achieve limited numbers of false-negative results and to maintain competency. In the study by Van der Zee et al. of 403 patients (623 groins) who underwent sentinel node procedures, the false-negative rate was 0-2%. The overall survival rate was 97% at 3 years (J Clin Oncol. 2008 Feb 20;26[6]:884-9). However, a more recent data from the Gynecologic Oncology Group (GOG 173) showed a slightly higher false-negative rate of 8% (J Clin Oncol. 2012 Nov 1;30[31]:3786-91). Overall survival data are pending from this study.
While sentinel lymph node mapping for endometrial cancer has been feasible for many years and has been well described, the questioned role of completed lymphadenectomy for early-stage endometrial cancer has led to a resurgence of interest in these techniques. While blue dye and radiolabeled tracer methods have historically been the most popular mapping solutions, the advent of endoscopic near-infrared imaging, with its higher sensitivity and good depth penetration, has added options. Indocyanine green fluorescence can be easily detected during robotic surgery and as experience with these techniques increase, successful mapping and sensitivity will increase.
Genetics
While hereditary cancer syndromes have been recognized for many years, detecting the genetic mutations that may increase an individual’s risk of developing a malignancy were not elucidated until the early 1990s. In gynecologic oncology, the most commonly encountered syndromes involve mutations in BRCA1 and BRCA2 and hereditary non–polyposis colorectal cancer, which causes mutations in DNA mismatch-repair genes and increase the risk of endometrial and ovarian cancer.
The SGO recently published a statement on risk assessment for inherited gynecologic cancer predispositions. In this statement “the evaluation for the presence of a hereditary cancer syndrome enables physicians to provide individualized and quantified assessment of cancer risk, as well as options for tailored screening and preventions strategies that may reduce morbidity associated with the development of malignancy” (Gynecol Oncol. 2015 Jan;136[1]:3-7). Beyond risk-reducing salpingo-oophorectomy, therapeutic strategies targeting patients with germline mutations have been developed (PARP inhibitors in BRCA-mutated women with ovarian cancer).
In August 2015, ASCO released an updated policy statement on genetic and genomic testing for cancer susceptibility and highlighted five key areas: germ-line implications of somatic mutation profiling; multigene panel testing for cancer susceptibility; quality assurance in genetic testing; education for oncology professionals; and access to cancer genetic services.
Antiemetics
Rounding out ASCO’s “Top 5 advances in 50 years of Modern Oncology” was the improvement in patients’ quality of life from supportive therapies, in particular antinausea medications.
Several of the agents commonly used in gynecologic oncology rate high (cisplatin) to moderate (carboplatin, cyclophosphamide, doxorubicin, ifosfamide) with regards to emetogenicity. The advent of 5-HT3 receptor antagonists (for example, ondansetron) has significantly improved the quality of life of patients undergoing cytotoxic chemotherapy. In addition to improving quality of life, the decrease in nausea and vomiting can also decrease life-threatening complications such as dehydration and electrolyte imbalance. Both ASCO and the National Comprehensive Cancer Network both have guidelines for the management of nausea and vomiting in patients undergoing chemotherapy.
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology, and a professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
The subspecialty of gynecologic oncology was formalized less than 50 years ago with the creation of the Society of Gynecologic Oncology and subspecialty training and board certification. The formation of the Gynecologic Oncology Group (GOG) – and the many clinical trials spearheaded by that group – has further advanced evidence-based treatments, resulting in improved survival outcomes, quality of life, and preventive strategies.
While it is not possible to provide a comprehensive and exhaustive review of all of the advances, we hope to highlight many of the notable advances in this article.Cervical cancer
Cervical cancer is the fourth most common cancer in women worldwide with 528,000 new cases in 2012. The majority of cervical cancer cases are caused by infection with human papillomavirus (HPV). While the standard therapies for cervical cancer have been long established (radical hysterectomy for stage I and radiation therapy for locally advanced disease), one of the most significant advances in the past 50 years was the addition of radiation-sensitizing chemotherapy (cisplatin) administered concurrently with radiation therapy.
In randomized trials in both early and advanced cervical cancer, the risk of death was reduced by 30%-50%. These studies changed the paradigm for the treatment of cervical cancer (N Engl J Med. 1999 Apr 15;340[15]:1137-43; N Engl J Med. 1999 Apr 15;340[15]:1144-53; J Clin Oncol. 2000 Apr;18[8]:1606-13).
Future studies evaluating biologic adjuncts or additional chemotherapy are currently underway or awaiting data maturation.
The American Society of Clinical Oncology (ASCO) highlighted the “Top 5 advances in 50 years of Modern Oncology” in 2014, and second on the list was the approval of the HPV vaccine to prevent cervical cancer. Vaccines have been developed that can protect against types 2, 4 or 9 of HPV. In a 2014 study, depending on vaccination coverage, the relative number of cervical cancer cases avoided was 34% in Africa, 27% for America, 26% for Asia, 21% for Europe, and worldwide was estimated at 27% (Vaccine. 2014 Feb 3;32[6]:733-9).
While the benefit from HPV vaccination has been proven, in the United States, only about a third of eligible girls and women have been vaccinated. Efforts should focus on expanding vaccination penetration to eligible girls, boys, women, and men.
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy in the United States with an estimated 54,870 cases and 10,170 deaths annually. Notable advances in the management of women with endometrial cancer have arisen because of a better understanding that there are two types of endometrial cancer – type I and type II.
The type I endometrial cancers tend to be associated with lower stage of disease at the time of diagnosis and fewer recurrences, while type II endometrial cancer is associated with worse outcomes.
Tailoring the surgical approaches and adjuvant therapy for women with endometrial cancer has led to improved outcomes. The GOG conducted a large prospective randomized trial of laparotomy versus laparoscopic surgical staging for women with clinical early-stage endometrial cancer (LAP2). Laparoscopy was associated with improved perioperative outcomes and was found to be noninferior to laparotomy with regards to survival outcomes (J Clin Oncol. 2012 Mar 1;30[7]:695-700). Therefore, minimally invasive surgery has become widely accepted for the surgical staging of women with endometrial cancer.
Appropriate surgical staging allows for tailoring of postoperative adjuvant therapy. The current evidence suggests that vaginal brachytherapy should be the adjuvant treatment of choice over whole pelvic radiation in women with early-stage endometrial cancer (Lancet. 2010 Mar 6;375[9717]:816-23). Studies are underway to evaluate the role of both adjuvant radiation and chemotherapy in women with early-stage type II endometrial cancer who are felt to be at high risk for recurrent disease, as well as how to improve on the therapeutic options for women with advanced or recurrent disease.
Ovarian cancer
Epithelial ovarian cancer is the most deadly gynecologic malignancy in the United States with 21,290 cases and 14,180 deaths in 2015. The concept of ovarian tumor debulking was first described by Dr. Joe Meigs in 1934, but did not gain traction until the mid-1970s when Dr. C. Thomas Griffiths published his work (Natl Cancer Inst Monogr. 1975 Oct;42:101-4).
While there are no randomized trials proving that surgical cytoreduction improves overall survival, most retrospective studies support this concept. In 2009, Chi et al. showed improved median survival in women with ovarian cancer based on the increased percentage of women who underwent optimal cytoreduction (Gynecol Oncol. 2009 Jul;114[1]:26-31). This has led to modifications of surgical techniques and surgical goals with an effort to maximally cytoreduce all of the visible disease.
While initial surgical debulking is the goal, there are circumstances when a different approach may be indicated. Vergote et al. conducted a prospective randomized trial of 670 women with advanced ovarian cancer. In this study, neoadjuvant chemotherapy followed by interval debulking was not inferior to primary debulking followed by chemotherapy with regards to progression-free survival and overall survival. However, initial surgery was associated with increased surgical complications and perioperative mortality as compared with interval surgery. Therefore, in women who are not felt to be candidates for optimal cytoreduction, neoadjuvant chemotherapy followed by interval surgery may be an appropriate treatment strategy (N Engl J Med. 2010 Sep 2;363[10]:943-53.).
There have been several notable advances and a series of randomized trials – predominately conducted by the GOG – that have resulted in improved overall survival and progression-free interval in women with ovarian cancer. However, none are as significant as the discovery of paclitaxel and platinum-based chemotherapy (cisplatin and carboplatin).
In 1962, samples of the Pacific Yew’s bark were collected and, 2 years later, the extracts from this bark were found to have cytotoxic activity. There were initial difficulties suspending the drug in solution; however, ultimately a formulation in ethanol, cremophor, and saline was found to be effective. In 1984, the National Cancer Institute began clinical trials of paclitaxel and it was found to be highly effective in ovarian cancer. In 1992, it was approved for the treatment of ovarian cancer.
Cisplatin was approved in 1978. Carboplatin entered clinical trials in 1982 and was approved for women with recurrent ovarian cancer in 1989.
There were a series of trials beginning in the late 1980s that established the role of platinum agents and led us to GOG 111. This trial evaluated cisplatin with either cyclophosphamide or paclitaxel. The paclitaxel combination was superior and in 2003 two trials were published that solidified carboplatin and paclitaxel as the cornerstone in the treatment of women with ovarian cancer (J Clin Oncol. 2003 Sep 1;21[17]:3194-200; J Natl Cancer Inst. 2003 Sep 3;95[17]:1320-9).
What has most recently been debated is the route and schedule for both paclitaxel and the platinum agents. In January 2006, the National Cancer Institute released a Clinical Announcement regarding the role of intraperitoneal (IP) chemotherapy for the treatment of women with optimally debulked ovarian cancer. Of the six trials included in the announcement, four trials showed a benefit for progression-free survival and five studies showed an improvement in overall survival. Armstrong et al (GOG 172) showed a 16-month improvement in overall survival with intravenous (IV) paclitaxel, IP cisplatin, and IP paclitaxel. IP chemotherapy has not been universally embraced by physicians and patients in part because of its toxicity, treatment schedule, and the fact that no IP regimen has been compared with the current standard of IV carboplatin and paclitaxel (N Engl J Med. 2006 Jan 5;354[1]:34-43).
While there have been improvements in 5-year survival over time, most women with advanced ovarian cancer will undergo additional chemotherapy in order to achieve subsequent remissions or maintain stability of disease. Other drugs that have Food and Drug Administration approval in the setting of recurrent ovarian cancer include topotecan, liposomal doxorubicin, gemcitabine, bevacizumab, altretamine, carboplatin, cisplatin, cyclophosphamide, and melphalan. Olaparib was recently approved as monotherapy in women with a germline BRCA-mutation who had received three or more prior lines of chemotherapy.
Minimally invasive surgery
Over the last 30 years, minimally invasive surgery (MIS) in gynecologic oncology, particularly for endometrial cancer, has gone from a niche procedure to the standard of care. The introduction of laparoscopy into gynecologic oncology started in the early 1990s. In a series of 59 women undergoing laparoscopy for endometrial cancer, Childers et al. demonstrated feasibility of the technique and low laparotomy conversion rates (Gynecol Oncol. 1993 Oct;51[1]:33-8.). The GOG trial, LAP2, supported the equivalent oncologic outcomes of MIS versus laparotomy for the treatment of endometrial cancer. While many surgeons and centers offered laparoscopic surgery, there were issues with the learning curve that limited its widespread use.
In 2005, the FDA approval of the robotic platform for gynecologic surgery resulted in at least a doubling of the proportion of endometrial cancer patients treated with MIS (Int J Med Robot. 2009 Dec;5[4]:392-7.). In 2012, the Society of Gynecologic Oncology published a consensus statement regarding robotic-assisted surgery in gynecologic oncology (Gynecol Oncol. 2012 Feb;124[2]:180-4.). This review highlights the advantages of the robotics platform with regards to expanding MIS to women with cervical and ovarian cancer; the improvements in outcomes in the obese woman with endometrial cancer; and that the learning curve for robotic surgery is shorter than for traditional laparoscopy. Issues requiring further research include cost analysis as the cost of the new technology decreases, and opportunities for improvement in patient and physician quality of life.
Sentinel node mapping
The rationale for sentinel node mapping is that if one or more sentinel lymph nodes is/are negative for malignancy, then the other regional lymph nodes will also be negative. This would thereby avoid the need for a complete lymph node dissection and its resultant complications, including chronic lymphedema. Much of the work pioneering this strategy has been in breast cancer and melanoma, but data are rapidly emerging for these techniques in gynecologic malignancies.
Candidates for sentinel lymph node biopsy for vulvar cancer include those with a lesion more than 1mm in depth, a tumor less than 4 cm in size, and no obvious metastatic disease on exam or preoperative imaging. Additionally, recommendations have been made regarding case volume in order to achieve limited numbers of false-negative results and to maintain competency. In the study by Van der Zee et al. of 403 patients (623 groins) who underwent sentinel node procedures, the false-negative rate was 0-2%. The overall survival rate was 97% at 3 years (J Clin Oncol. 2008 Feb 20;26[6]:884-9). However, a more recent data from the Gynecologic Oncology Group (GOG 173) showed a slightly higher false-negative rate of 8% (J Clin Oncol. 2012 Nov 1;30[31]:3786-91). Overall survival data are pending from this study.
While sentinel lymph node mapping for endometrial cancer has been feasible for many years and has been well described, the questioned role of completed lymphadenectomy for early-stage endometrial cancer has led to a resurgence of interest in these techniques. While blue dye and radiolabeled tracer methods have historically been the most popular mapping solutions, the advent of endoscopic near-infrared imaging, with its higher sensitivity and good depth penetration, has added options. Indocyanine green fluorescence can be easily detected during robotic surgery and as experience with these techniques increase, successful mapping and sensitivity will increase.
Genetics
While hereditary cancer syndromes have been recognized for many years, detecting the genetic mutations that may increase an individual’s risk of developing a malignancy were not elucidated until the early 1990s. In gynecologic oncology, the most commonly encountered syndromes involve mutations in BRCA1 and BRCA2 and hereditary non–polyposis colorectal cancer, which causes mutations in DNA mismatch-repair genes and increase the risk of endometrial and ovarian cancer.
The SGO recently published a statement on risk assessment for inherited gynecologic cancer predispositions. In this statement “the evaluation for the presence of a hereditary cancer syndrome enables physicians to provide individualized and quantified assessment of cancer risk, as well as options for tailored screening and preventions strategies that may reduce morbidity associated with the development of malignancy” (Gynecol Oncol. 2015 Jan;136[1]:3-7). Beyond risk-reducing salpingo-oophorectomy, therapeutic strategies targeting patients with germline mutations have been developed (PARP inhibitors in BRCA-mutated women with ovarian cancer).
In August 2015, ASCO released an updated policy statement on genetic and genomic testing for cancer susceptibility and highlighted five key areas: germ-line implications of somatic mutation profiling; multigene panel testing for cancer susceptibility; quality assurance in genetic testing; education for oncology professionals; and access to cancer genetic services.
Antiemetics
Rounding out ASCO’s “Top 5 advances in 50 years of Modern Oncology” was the improvement in patients’ quality of life from supportive therapies, in particular antinausea medications.
Several of the agents commonly used in gynecologic oncology rate high (cisplatin) to moderate (carboplatin, cyclophosphamide, doxorubicin, ifosfamide) with regards to emetogenicity. The advent of 5-HT3 receptor antagonists (for example, ondansetron) has significantly improved the quality of life of patients undergoing cytotoxic chemotherapy. In addition to improving quality of life, the decrease in nausea and vomiting can also decrease life-threatening complications such as dehydration and electrolyte imbalance. Both ASCO and the National Comprehensive Cancer Network both have guidelines for the management of nausea and vomiting in patients undergoing chemotherapy.
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology, and a professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Managing menopause symptoms in gynecologic cancer survivors
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies
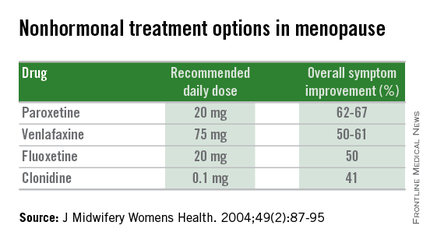
Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies

Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies

Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Managing menopausal symptoms after risk-reducing salpingo-oophorectomy
Compared to the general population, women with mutations in the BRCA1 or BRCA2 genes have a significantly higher lifetime risk of ovarian and breast cancers (Science. 2003 Oct 24;302[5645]:643-6). Since the occurrence of ovarian and breast cancer in BRCA carriers is often prior to menopause, and because we have no screening test to detect early stage ovarian cancer, risk-reducing salpingo-oophorectomy has been recommended around age 40.
It has been shown that risk-reducing salpingo-oophorectomy significantly reduces ovarian cancer risk by 85%-95% in BRCA-affected women. Also, this surgery can reduce breast cancer risk by 53%-68% (N Engl J Med. 2002 May 23;346[21]:1609-15). The 2008 Practice Bulletin from the American College of Obstetricians and Gynecologists recommends that risk-reducing salpingo-oophorectomy should be performed in women with BRCA1 or BRCA2 mutations after the completion of childbearing or age 40 (Obstet Gynecol. 2008 Jan;111[1]:231-41).
Health implications
Nearly 60% of women who have a BRCA1 or BRCA2 mutation will elect to undergo risk-reducing salpingo-oophorectomy between the ages of 35 and 40 years (Open Med. 2007 Aug 13;1[2]:e92-8). As such, surgical menopause can result in hot flashes, vaginal dryness, sexual dysfunction, sleep disturbances, and cognitive changes, which may significantly impact a woman’s quality of life. In addition, increased risk of cardiovascular disease and osteoporosis following bilateral salpingo-oophorectomy may have a significant impact on a woman’s health.
Since these women undergo surgical menopause as opposed to natural menopause, they have an abrupt loss in hormones, and due to their younger age at the time of surgery, they may also have a longer exposure period to the detrimental effects of hypoestrogenism.
Symptom management
Various treatment options exist for relief of menopausal symptoms, including nonhormonal therapies and hormone replacement therapies (HT).
Nonhormonal therapies include serotonin receptor inhibitors (venlafaxine and paroxetine) and alpha-2 adrenergic agonists (clonidine), which are most appropriate for the treatment of vasomotor symptoms. Unfortunately, these options have proved to be as effective as HT. Also, women should be adequately counseled regarding the various side effects of these nonhormonal medications. Alternative approaches such as phytoestrogens are unproven and are still undergoing investigation. As such, HT remains the standard for treatment of menopausal symptoms, and many trials have confirmed that HT can effectively treat menopausal symptoms following risk-reducing salpingo-oophorectomy.
This then raises the question of safety regarding use of HT in this patient population; especially the possibility of increased risk of breast cancer. Interestingly, only 10%-25% of BRCA1 carriers will have estrogen receptor–positive breast cancer, while 65%-79% of BRCA2-associated breast cancers will be positive for the receptor (Clin Cancer Res. 2004 Mar 15;10[6]:2029-34).
Unfortunately, we do not have adequate trials or studies with sufficient long-term follow-up to validate whether HT increases the risk of breast cancer or recurrence. However, the PROSE Study Group did report on a prospective cohort of 462 women with BRCA1 or BRCA2 mutations. In this study, HT did not alter the reduction in breast cancer risk from risk-reducing salpingo-oophorectomy (J Clin Oncol. 2005 Nov 1;23[31]:7804-10). In addition to a paucity of data regarding systemic HT, there is little data in the BRCA-positive population to confirm the safety of local vaginal estrogen for treatment of vaginal atrophy (J Clin Oncol. 2004 Mar 15;22[6]:1045-54).
Understanding the options
Use of HT in women with BRCA1 and BRCA2 mutations requires further investigation. There should be shared decision making between the patient and provider when counseling on the management of menopausal symptoms following risk-reducing salpingo-oophorectomy. Most importantly, women should understand the options of nonhormonal therapies and their specific side effects. They should also understand the lack of significant data regarding use of systemic HT, and that if use is elected, there may be an increased risk of breast cancer.
Women who do elect to use systemic HT following risk-reducing salpingo-oophorectomy have options that can help reduce the risk of HT-associated breast cancer, including a shorter duration of systemic HT or concurrent hysterectomy to allow for estrogen-only HT, which has a decreased risk of breast cancer compared with combined therapies that include progestins. These women may also be considering prophylactic mastectomy, which would change the concerns regarding HT and an increased risk of breast cancer.
Increased awareness of these options among physicians and patients alike can help to decrease unsatisfactory symptoms and improve quality of life in women undergoing risk-reducing salpingo-oophorectomy.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures. Email them at [email protected].
Compared to the general population, women with mutations in the BRCA1 or BRCA2 genes have a significantly higher lifetime risk of ovarian and breast cancers (Science. 2003 Oct 24;302[5645]:643-6). Since the occurrence of ovarian and breast cancer in BRCA carriers is often prior to menopause, and because we have no screening test to detect early stage ovarian cancer, risk-reducing salpingo-oophorectomy has been recommended around age 40.
It has been shown that risk-reducing salpingo-oophorectomy significantly reduces ovarian cancer risk by 85%-95% in BRCA-affected women. Also, this surgery can reduce breast cancer risk by 53%-68% (N Engl J Med. 2002 May 23;346[21]:1609-15). The 2008 Practice Bulletin from the American College of Obstetricians and Gynecologists recommends that risk-reducing salpingo-oophorectomy should be performed in women with BRCA1 or BRCA2 mutations after the completion of childbearing or age 40 (Obstet Gynecol. 2008 Jan;111[1]:231-41).
Health implications
Nearly 60% of women who have a BRCA1 or BRCA2 mutation will elect to undergo risk-reducing salpingo-oophorectomy between the ages of 35 and 40 years (Open Med. 2007 Aug 13;1[2]:e92-8). As such, surgical menopause can result in hot flashes, vaginal dryness, sexual dysfunction, sleep disturbances, and cognitive changes, which may significantly impact a woman’s quality of life. In addition, increased risk of cardiovascular disease and osteoporosis following bilateral salpingo-oophorectomy may have a significant impact on a woman’s health.
Since these women undergo surgical menopause as opposed to natural menopause, they have an abrupt loss in hormones, and due to their younger age at the time of surgery, they may also have a longer exposure period to the detrimental effects of hypoestrogenism.
Symptom management
Various treatment options exist for relief of menopausal symptoms, including nonhormonal therapies and hormone replacement therapies (HT).
Nonhormonal therapies include serotonin receptor inhibitors (venlafaxine and paroxetine) and alpha-2 adrenergic agonists (clonidine), which are most appropriate for the treatment of vasomotor symptoms. Unfortunately, these options have proved to be as effective as HT. Also, women should be adequately counseled regarding the various side effects of these nonhormonal medications. Alternative approaches such as phytoestrogens are unproven and are still undergoing investigation. As such, HT remains the standard for treatment of menopausal symptoms, and many trials have confirmed that HT can effectively treat menopausal symptoms following risk-reducing salpingo-oophorectomy.
This then raises the question of safety regarding use of HT in this patient population; especially the possibility of increased risk of breast cancer. Interestingly, only 10%-25% of BRCA1 carriers will have estrogen receptor–positive breast cancer, while 65%-79% of BRCA2-associated breast cancers will be positive for the receptor (Clin Cancer Res. 2004 Mar 15;10[6]:2029-34).
Unfortunately, we do not have adequate trials or studies with sufficient long-term follow-up to validate whether HT increases the risk of breast cancer or recurrence. However, the PROSE Study Group did report on a prospective cohort of 462 women with BRCA1 or BRCA2 mutations. In this study, HT did not alter the reduction in breast cancer risk from risk-reducing salpingo-oophorectomy (J Clin Oncol. 2005 Nov 1;23[31]:7804-10). In addition to a paucity of data regarding systemic HT, there is little data in the BRCA-positive population to confirm the safety of local vaginal estrogen for treatment of vaginal atrophy (J Clin Oncol. 2004 Mar 15;22[6]:1045-54).
Understanding the options
Use of HT in women with BRCA1 and BRCA2 mutations requires further investigation. There should be shared decision making between the patient and provider when counseling on the management of menopausal symptoms following risk-reducing salpingo-oophorectomy. Most importantly, women should understand the options of nonhormonal therapies and their specific side effects. They should also understand the lack of significant data regarding use of systemic HT, and that if use is elected, there may be an increased risk of breast cancer.
Women who do elect to use systemic HT following risk-reducing salpingo-oophorectomy have options that can help reduce the risk of HT-associated breast cancer, including a shorter duration of systemic HT or concurrent hysterectomy to allow for estrogen-only HT, which has a decreased risk of breast cancer compared with combined therapies that include progestins. These women may also be considering prophylactic mastectomy, which would change the concerns regarding HT and an increased risk of breast cancer.
Increased awareness of these options among physicians and patients alike can help to decrease unsatisfactory symptoms and improve quality of life in women undergoing risk-reducing salpingo-oophorectomy.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures. Email them at [email protected].
Compared to the general population, women with mutations in the BRCA1 or BRCA2 genes have a significantly higher lifetime risk of ovarian and breast cancers (Science. 2003 Oct 24;302[5645]:643-6). Since the occurrence of ovarian and breast cancer in BRCA carriers is often prior to menopause, and because we have no screening test to detect early stage ovarian cancer, risk-reducing salpingo-oophorectomy has been recommended around age 40.
It has been shown that risk-reducing salpingo-oophorectomy significantly reduces ovarian cancer risk by 85%-95% in BRCA-affected women. Also, this surgery can reduce breast cancer risk by 53%-68% (N Engl J Med. 2002 May 23;346[21]:1609-15). The 2008 Practice Bulletin from the American College of Obstetricians and Gynecologists recommends that risk-reducing salpingo-oophorectomy should be performed in women with BRCA1 or BRCA2 mutations after the completion of childbearing or age 40 (Obstet Gynecol. 2008 Jan;111[1]:231-41).
Health implications
Nearly 60% of women who have a BRCA1 or BRCA2 mutation will elect to undergo risk-reducing salpingo-oophorectomy between the ages of 35 and 40 years (Open Med. 2007 Aug 13;1[2]:e92-8). As such, surgical menopause can result in hot flashes, vaginal dryness, sexual dysfunction, sleep disturbances, and cognitive changes, which may significantly impact a woman’s quality of life. In addition, increased risk of cardiovascular disease and osteoporosis following bilateral salpingo-oophorectomy may have a significant impact on a woman’s health.
Since these women undergo surgical menopause as opposed to natural menopause, they have an abrupt loss in hormones, and due to their younger age at the time of surgery, they may also have a longer exposure period to the detrimental effects of hypoestrogenism.
Symptom management
Various treatment options exist for relief of menopausal symptoms, including nonhormonal therapies and hormone replacement therapies (HT).
Nonhormonal therapies include serotonin receptor inhibitors (venlafaxine and paroxetine) and alpha-2 adrenergic agonists (clonidine), which are most appropriate for the treatment of vasomotor symptoms. Unfortunately, these options have proved to be as effective as HT. Also, women should be adequately counseled regarding the various side effects of these nonhormonal medications. Alternative approaches such as phytoestrogens are unproven and are still undergoing investigation. As such, HT remains the standard for treatment of menopausal symptoms, and many trials have confirmed that HT can effectively treat menopausal symptoms following risk-reducing salpingo-oophorectomy.
This then raises the question of safety regarding use of HT in this patient population; especially the possibility of increased risk of breast cancer. Interestingly, only 10%-25% of BRCA1 carriers will have estrogen receptor–positive breast cancer, while 65%-79% of BRCA2-associated breast cancers will be positive for the receptor (Clin Cancer Res. 2004 Mar 15;10[6]:2029-34).
Unfortunately, we do not have adequate trials or studies with sufficient long-term follow-up to validate whether HT increases the risk of breast cancer or recurrence. However, the PROSE Study Group did report on a prospective cohort of 462 women with BRCA1 or BRCA2 mutations. In this study, HT did not alter the reduction in breast cancer risk from risk-reducing salpingo-oophorectomy (J Clin Oncol. 2005 Nov 1;23[31]:7804-10). In addition to a paucity of data regarding systemic HT, there is little data in the BRCA-positive population to confirm the safety of local vaginal estrogen for treatment of vaginal atrophy (J Clin Oncol. 2004 Mar 15;22[6]:1045-54).
Understanding the options
Use of HT in women with BRCA1 and BRCA2 mutations requires further investigation. There should be shared decision making between the patient and provider when counseling on the management of menopausal symptoms following risk-reducing salpingo-oophorectomy. Most importantly, women should understand the options of nonhormonal therapies and their specific side effects. They should also understand the lack of significant data regarding use of systemic HT, and that if use is elected, there may be an increased risk of breast cancer.
Women who do elect to use systemic HT following risk-reducing salpingo-oophorectomy have options that can help reduce the risk of HT-associated breast cancer, including a shorter duration of systemic HT or concurrent hysterectomy to allow for estrogen-only HT, which has a decreased risk of breast cancer compared with combined therapies that include progestins. These women may also be considering prophylactic mastectomy, which would change the concerns regarding HT and an increased risk of breast cancer.
Increased awareness of these options among physicians and patients alike can help to decrease unsatisfactory symptoms and improve quality of life in women undergoing risk-reducing salpingo-oophorectomy.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures. Email them at [email protected].