User login
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
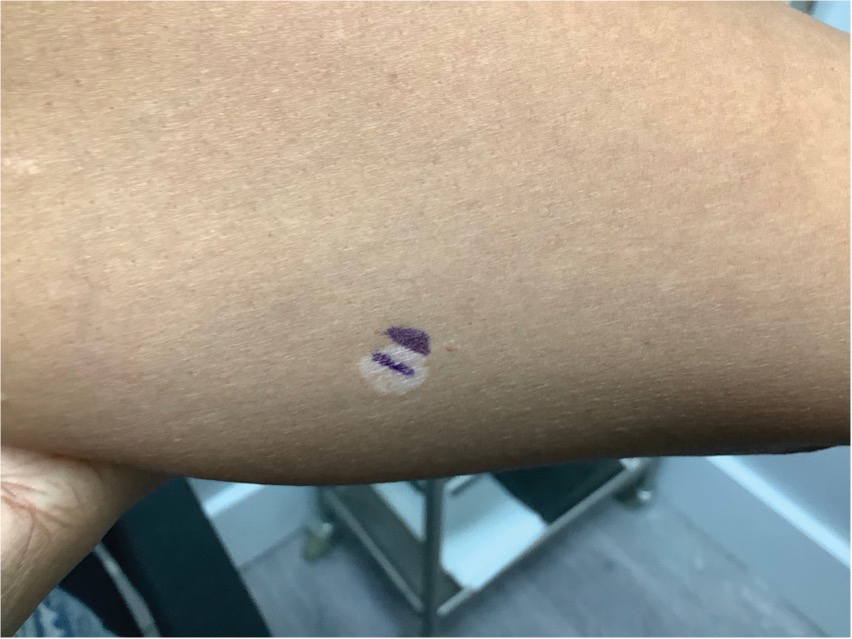
A 52-year-old Black woman presented with self-described whitened spots on the arms and legs of 2 years’ duration. She experienced no improvement with ketoconazole cream and topical calcineurin inhibitors prescribed during a prior dermatology visit at an outside institution. She denied pain or pruritus. A review of systems as well as the patient’s medical history were noncontributory. A prior biopsy at an outside institution revealed an interface dermatitis suggestive of cutaneous lupus erythematosus. The patient noted social drinking and denied tobacco use. She had no known allergies to medications and currently was on tamoxifen for breast cancer following a right mastectomy. Physical examination showed hypopigmented macules and patches on the left upper arm and right proximal leg. The center of the lesions was not erythematous or scaly. Palpation did not reveal enlarged lymph nodes, and laboratory analyses ruled out low levels of red blood cells, white blood cells, or platelets. Punch biopsies from the left arm and right thigh were performed.