User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Take steps to relieve ataxia in patients with alcohol use disorder
Ataxia is a well-known complication of chronic alcohol abuse, which is attributed to degeneration of the cerebellar vermis. However, effective treatment approaches, as well as the timing and level of recovery, remain unclear. One cross-sectional study found that long-term abstainers from alcohol had less severe ataxia than short-term abstainers,1 suggesting that improvement is possible with continued sobriety. However, a recent longitudinal study contradicts this finding, reporting no improvement in ataxia in patients abstinent for 10 weeks to 1 year.2
CASE REPORT
Unable to walk, heavy alcohol use
Mr. G, a 59-year-old white male with a history of daily, heavy alcohol use, presents to the emergency room reporting that he has “not been able to walk right” for 3 weeks. He is in a wheelchair because of ataxia and difficulty balancing. He denies headaches, visual changes, weakness, numbness, and difficulty speaking or swallowing.
Mr. G reports drinking one 40-oz bottle of malt liquor and 2 pints of vodka per day for more than 40 years. His alcohol abuse led to homelessness, unemployment, and divorce. Despite heavy drinking, he denies signs of withdrawal, including shaking, sweating, seizures, and delirium.
Mr. G has no other medical conditions. He denies a family history of neurologic disorders or substance abuse.
His pulse is 100 beats per minute, respirations of 16 breaths per minute, temperature of 37°C, and blood pressure of 143/89 mm Hg. Physical examination reveals a wide-based gait.
Mr. G is admitted to the inpatient psychiatric unit to monitor and treat his alcohol withdrawal and to undergo further workup of the gait disturbance.
A head CT scan shows non-specific changes; an EEG also is within normal limits. Complete blood count, basic metabolic panel, liver function test, HIV test, acute hepatitis panel, thyroid function test, erythrocyte sedimentation rate, and vitamin B12 tests are within normal ranges.
A full neurologic exam reveals a wide-based gait, impaired heel-shin test, and dysmetria on finger-nose-finger test. Mr. G is given a diagnosis of ataxia due to alcoholic cerebellar degeneration. Thiamine repletion is suggested.
Treatment and outcome
Mr. G continues on thiamine, 100 mg, twice daily, and oxazepam, 15 mg, as needed, to manage withdrawal symptoms. He receives gait training 3 times per week.
Approximately 10 days after admission, Mr. G is able to ambulate with a walker. Three weeks after admission, his gait has improved and he walks with a cane. (See the video at CurrentPsychiatry.com for an illustration of this progressive recovery.)
After discharge, Mr. G is referred to an addiction psychiatrist and addiction psychotherapist for ongoing treatment of alcohol use disorder.
Making the diagnosis
In a patient complaining of balance difficulties, consider ataxia secondary to cerebellar degeneration.
- Take a complete history. Ask about the onset and progression of ataxia.
- Obtain a family history. Some types of ataxia are genetic.
- Perform a neurologic examination, which may reveal signs of cerebellar deficits, particularly characteristic wide-based gait. These patients will have difficulty when walking in tandem. Other impairments on the neurologic exam that may raise suspicion for a cerebellar disorder include: impaired heel-shin test, impaired finger-nose-finger test (dysmetria), impaired rapid alternating movements (dysdiadochokinesia), nystagmus, impaired smooth pursuits, intention tremor, or speech abnormalities.
- Perform head imaging, such as a CT scan or MRI. In patients with ataxia secondary to alcohol abuse, imaging might reveal degeneration of the cerebellar vermis.
- Perform laboratory tests, such as inflammatory markers, vitamin levels, and thyroid function testing to detect possible toxic-metabolic or inflammatory causes.
Alcohol-induced ataxia can be diagnosed in patients with a history of heavy drinking if the workup does not reveal another possible cause for the gait disturbance. Other less common deficits associated with alcohol-induced cerebellar injury include:
- dysarthria
- abnormal rate and force of movement
- limb ataxia.3
Recommendations
- Be able to recognize the characteristic gait of patients with alcohol-induced ataxia.
- Provide thiamine supplementation.
- Refer patients to physical therapy.
- Educate your patients that their gait will not improve and may worsen if they continue to drink.
- Refer patients for ongoing treatment for alcohol use disorder, including medication management and psychotherapy.
Our experience suggests that patients with alcohol use disorder with cerebellar ataxia could have a good prognosis for ambulation. Improvement could occur over several weeks; it is unclear whether further gains can be expected with months or years of abstinence.
1. Smith S, Fein G. Persistent but less severe ataxia in long-term versus short-term abstinent alcoholic men and women: a cross-sectional analysis. Alcohol Clin Exp Res. 2011;35(12):2184-2192.
2. Fein G, Greenstein D. Gait and balance deficits in chronic alcoholics: no improvement from 10 weeks through 1 year abstinence. Alcohol Clin Exp Res. 2013;37(1):86-95.
3. Fitzpatrick LE, Jackson M, Crowe SF. Characterization of cerebellar ataxia in chronic alcoholics using the International Cooperative Ataxia Rating Scale (ICARS). Alcohol Clin Exp Res. 2012;36(11):1942-1951.
Ataxia is a well-known complication of chronic alcohol abuse, which is attributed to degeneration of the cerebellar vermis. However, effective treatment approaches, as well as the timing and level of recovery, remain unclear. One cross-sectional study found that long-term abstainers from alcohol had less severe ataxia than short-term abstainers,1 suggesting that improvement is possible with continued sobriety. However, a recent longitudinal study contradicts this finding, reporting no improvement in ataxia in patients abstinent for 10 weeks to 1 year.2
CASE REPORT
Unable to walk, heavy alcohol use
Mr. G, a 59-year-old white male with a history of daily, heavy alcohol use, presents to the emergency room reporting that he has “not been able to walk right” for 3 weeks. He is in a wheelchair because of ataxia and difficulty balancing. He denies headaches, visual changes, weakness, numbness, and difficulty speaking or swallowing.
Mr. G reports drinking one 40-oz bottle of malt liquor and 2 pints of vodka per day for more than 40 years. His alcohol abuse led to homelessness, unemployment, and divorce. Despite heavy drinking, he denies signs of withdrawal, including shaking, sweating, seizures, and delirium.
Mr. G has no other medical conditions. He denies a family history of neurologic disorders or substance abuse.
His pulse is 100 beats per minute, respirations of 16 breaths per minute, temperature of 37°C, and blood pressure of 143/89 mm Hg. Physical examination reveals a wide-based gait.
Mr. G is admitted to the inpatient psychiatric unit to monitor and treat his alcohol withdrawal and to undergo further workup of the gait disturbance.
A head CT scan shows non-specific changes; an EEG also is within normal limits. Complete blood count, basic metabolic panel, liver function test, HIV test, acute hepatitis panel, thyroid function test, erythrocyte sedimentation rate, and vitamin B12 tests are within normal ranges.
A full neurologic exam reveals a wide-based gait, impaired heel-shin test, and dysmetria on finger-nose-finger test. Mr. G is given a diagnosis of ataxia due to alcoholic cerebellar degeneration. Thiamine repletion is suggested.
Treatment and outcome
Mr. G continues on thiamine, 100 mg, twice daily, and oxazepam, 15 mg, as needed, to manage withdrawal symptoms. He receives gait training 3 times per week.
Approximately 10 days after admission, Mr. G is able to ambulate with a walker. Three weeks after admission, his gait has improved and he walks with a cane. (See the video at CurrentPsychiatry.com for an illustration of this progressive recovery.)
After discharge, Mr. G is referred to an addiction psychiatrist and addiction psychotherapist for ongoing treatment of alcohol use disorder.
Making the diagnosis
In a patient complaining of balance difficulties, consider ataxia secondary to cerebellar degeneration.
- Take a complete history. Ask about the onset and progression of ataxia.
- Obtain a family history. Some types of ataxia are genetic.
- Perform a neurologic examination, which may reveal signs of cerebellar deficits, particularly characteristic wide-based gait. These patients will have difficulty when walking in tandem. Other impairments on the neurologic exam that may raise suspicion for a cerebellar disorder include: impaired heel-shin test, impaired finger-nose-finger test (dysmetria), impaired rapid alternating movements (dysdiadochokinesia), nystagmus, impaired smooth pursuits, intention tremor, or speech abnormalities.
- Perform head imaging, such as a CT scan or MRI. In patients with ataxia secondary to alcohol abuse, imaging might reveal degeneration of the cerebellar vermis.
- Perform laboratory tests, such as inflammatory markers, vitamin levels, and thyroid function testing to detect possible toxic-metabolic or inflammatory causes.
Alcohol-induced ataxia can be diagnosed in patients with a history of heavy drinking if the workup does not reveal another possible cause for the gait disturbance. Other less common deficits associated with alcohol-induced cerebellar injury include:
- dysarthria
- abnormal rate and force of movement
- limb ataxia.3
Recommendations
- Be able to recognize the characteristic gait of patients with alcohol-induced ataxia.
- Provide thiamine supplementation.
- Refer patients to physical therapy.
- Educate your patients that their gait will not improve and may worsen if they continue to drink.
- Refer patients for ongoing treatment for alcohol use disorder, including medication management and psychotherapy.
Our experience suggests that patients with alcohol use disorder with cerebellar ataxia could have a good prognosis for ambulation. Improvement could occur over several weeks; it is unclear whether further gains can be expected with months or years of abstinence.
Ataxia is a well-known complication of chronic alcohol abuse, which is attributed to degeneration of the cerebellar vermis. However, effective treatment approaches, as well as the timing and level of recovery, remain unclear. One cross-sectional study found that long-term abstainers from alcohol had less severe ataxia than short-term abstainers,1 suggesting that improvement is possible with continued sobriety. However, a recent longitudinal study contradicts this finding, reporting no improvement in ataxia in patients abstinent for 10 weeks to 1 year.2
CASE REPORT
Unable to walk, heavy alcohol use
Mr. G, a 59-year-old white male with a history of daily, heavy alcohol use, presents to the emergency room reporting that he has “not been able to walk right” for 3 weeks. He is in a wheelchair because of ataxia and difficulty balancing. He denies headaches, visual changes, weakness, numbness, and difficulty speaking or swallowing.
Mr. G reports drinking one 40-oz bottle of malt liquor and 2 pints of vodka per day for more than 40 years. His alcohol abuse led to homelessness, unemployment, and divorce. Despite heavy drinking, he denies signs of withdrawal, including shaking, sweating, seizures, and delirium.
Mr. G has no other medical conditions. He denies a family history of neurologic disorders or substance abuse.
His pulse is 100 beats per minute, respirations of 16 breaths per minute, temperature of 37°C, and blood pressure of 143/89 mm Hg. Physical examination reveals a wide-based gait.
Mr. G is admitted to the inpatient psychiatric unit to monitor and treat his alcohol withdrawal and to undergo further workup of the gait disturbance.
A head CT scan shows non-specific changes; an EEG also is within normal limits. Complete blood count, basic metabolic panel, liver function test, HIV test, acute hepatitis panel, thyroid function test, erythrocyte sedimentation rate, and vitamin B12 tests are within normal ranges.
A full neurologic exam reveals a wide-based gait, impaired heel-shin test, and dysmetria on finger-nose-finger test. Mr. G is given a diagnosis of ataxia due to alcoholic cerebellar degeneration. Thiamine repletion is suggested.
Treatment and outcome
Mr. G continues on thiamine, 100 mg, twice daily, and oxazepam, 15 mg, as needed, to manage withdrawal symptoms. He receives gait training 3 times per week.
Approximately 10 days after admission, Mr. G is able to ambulate with a walker. Three weeks after admission, his gait has improved and he walks with a cane. (See the video at CurrentPsychiatry.com for an illustration of this progressive recovery.)
After discharge, Mr. G is referred to an addiction psychiatrist and addiction psychotherapist for ongoing treatment of alcohol use disorder.
Making the diagnosis
In a patient complaining of balance difficulties, consider ataxia secondary to cerebellar degeneration.
- Take a complete history. Ask about the onset and progression of ataxia.
- Obtain a family history. Some types of ataxia are genetic.
- Perform a neurologic examination, which may reveal signs of cerebellar deficits, particularly characteristic wide-based gait. These patients will have difficulty when walking in tandem. Other impairments on the neurologic exam that may raise suspicion for a cerebellar disorder include: impaired heel-shin test, impaired finger-nose-finger test (dysmetria), impaired rapid alternating movements (dysdiadochokinesia), nystagmus, impaired smooth pursuits, intention tremor, or speech abnormalities.
- Perform head imaging, such as a CT scan or MRI. In patients with ataxia secondary to alcohol abuse, imaging might reveal degeneration of the cerebellar vermis.
- Perform laboratory tests, such as inflammatory markers, vitamin levels, and thyroid function testing to detect possible toxic-metabolic or inflammatory causes.
Alcohol-induced ataxia can be diagnosed in patients with a history of heavy drinking if the workup does not reveal another possible cause for the gait disturbance. Other less common deficits associated with alcohol-induced cerebellar injury include:
- dysarthria
- abnormal rate and force of movement
- limb ataxia.3
Recommendations
- Be able to recognize the characteristic gait of patients with alcohol-induced ataxia.
- Provide thiamine supplementation.
- Refer patients to physical therapy.
- Educate your patients that their gait will not improve and may worsen if they continue to drink.
- Refer patients for ongoing treatment for alcohol use disorder, including medication management and psychotherapy.
Our experience suggests that patients with alcohol use disorder with cerebellar ataxia could have a good prognosis for ambulation. Improvement could occur over several weeks; it is unclear whether further gains can be expected with months or years of abstinence.
1. Smith S, Fein G. Persistent but less severe ataxia in long-term versus short-term abstinent alcoholic men and women: a cross-sectional analysis. Alcohol Clin Exp Res. 2011;35(12):2184-2192.
2. Fein G, Greenstein D. Gait and balance deficits in chronic alcoholics: no improvement from 10 weeks through 1 year abstinence. Alcohol Clin Exp Res. 2013;37(1):86-95.
3. Fitzpatrick LE, Jackson M, Crowe SF. Characterization of cerebellar ataxia in chronic alcoholics using the International Cooperative Ataxia Rating Scale (ICARS). Alcohol Clin Exp Res. 2012;36(11):1942-1951.
1. Smith S, Fein G. Persistent but less severe ataxia in long-term versus short-term abstinent alcoholic men and women: a cross-sectional analysis. Alcohol Clin Exp Res. 2011;35(12):2184-2192.
2. Fein G, Greenstein D. Gait and balance deficits in chronic alcoholics: no improvement from 10 weeks through 1 year abstinence. Alcohol Clin Exp Res. 2013;37(1):86-95.
3. Fitzpatrick LE, Jackson M, Crowe SF. Characterization of cerebellar ataxia in chronic alcoholics using the International Cooperative Ataxia Rating Scale (ICARS). Alcohol Clin Exp Res. 2012;36(11):1942-1951.
No more 'stickies'!: Help your patients bring their ‘to-do’ list into the 21st century
Difficulty with time management and organization is one of the most common complaints of patients with attention-deficit/hyperactivity disorder (ADHD). Being unproductive and inefficient also is anxiety-producing and depressing, leaving patients with additional comorbidity.
Although medication can help improve a person’s focus, if the patient is focusing on a set of poorly designed systems, he (she) will see little improvement. A comprehensive approach to improving day-to-day task management, similar to the one I describe here and use with my patients, is therefore as important as medication.
Needed: An ‘organizing principle’
Imagine that supermarkets displayed food in the order it arrives from the food distributors and producers. You’d walk in to the store and see a display of food that lacks hierarchy—1 random item placed next to another. The experience would be jarring, and shopping would be a much slower chore. Furthermore, what if you had to go to 5 stores to cover all your needs?
Yet, that is how most “to-do” lists are executed: A thought comes in, a thought goes down on paper. Or on a sticky note. Or in an app. Or in a calendar. Or all of the above! Often, there is neither an organizing principle (other than perhaps chronological order) or a central repository. No wonder it’s hard to feel present and clear-minded. Add to this disorganization the volume of information coming in from the environment—e-mails, voice mails, texts, notifications, dings, beeps, buzzes, and maybe even snail mail—and the feeling of being overwhelmed grows.
Unconscious motives for maintaining poor systems also might play a role. People with a “need to please” personality type or who are more passive-aggressive in their communication are more likely to overcommit, and then forget or be late completing their tasks, rather than saying “No” from the outset or delegating the work.
Survival basics for time management
Assuming there is simply a skills deficit, you can teach basic time and project management skills to patients with ADHD (and to any patient with suboptimal executive functioning). Here are basic principles to adopt:
- If you can forget it, you will, so all tasks should go onto the to-do list.
- You should keep only 1 list. Adding on “stickies” is not allowed.
- Your list is like an extra lobe of your brain: It should be present at all times, whether you keep it in “the Cloud,” on your desktop, or on paper.
- Review your list and clean it up at least daily. This takes time, but it also saves time—in spades—when you can call upon the right task, at the right time, with energy and drive.
- The first action you should take in the daily review is to weed out or delegate tasks.
- Next, categorize remaining tasks. (Note: The free smartphone app Evernote allows you to do this with “tags.”) Categorizing allows you to process sets of tasks in buckets that can be tackled as a bundle and, therefore, more efficiently. For example, having all of your errands, items to research, and telephone calls that need to be returned in separate buckets allows for speedier processing—as opposed to veering back and forth between line items.
- Then, move remaining high-priority items to the top of the list. However, remember that, if everything is urgent, nothing is. Items that are low-hanging fruit that you can cross off the list in a matter of minutes can be prioritized even if they are not as urgent. By doing that, your list becomes more manageable and your brain can dive deeper into more complex tasks.
- Block out calendar time for each of your buckets with this formula: (1) Estimate how much time you’ll need to complete the tasks in each bucket, then add 50% for each bucket. (2) Add in commuting, set-up, or wind-down time, if you need it, to the grand total for all buckets, and then add 50% more than you’ve estimated
Set the brain free!
This process will seem like a burden at the beginning, when the synapses underneath it still need to get stronger (much like how the body responds to exercise). However, as long as these principles are put into action daily, they will become a trusted, second-nature system that frees the brain from distraction and anxiety—and, ultimately,
Difficulty with time management and organization is one of the most common complaints of patients with attention-deficit/hyperactivity disorder (ADHD). Being unproductive and inefficient also is anxiety-producing and depressing, leaving patients with additional comorbidity.
Although medication can help improve a person’s focus, if the patient is focusing on a set of poorly designed systems, he (she) will see little improvement. A comprehensive approach to improving day-to-day task management, similar to the one I describe here and use with my patients, is therefore as important as medication.
Needed: An ‘organizing principle’
Imagine that supermarkets displayed food in the order it arrives from the food distributors and producers. You’d walk in to the store and see a display of food that lacks hierarchy—1 random item placed next to another. The experience would be jarring, and shopping would be a much slower chore. Furthermore, what if you had to go to 5 stores to cover all your needs?
Yet, that is how most “to-do” lists are executed: A thought comes in, a thought goes down on paper. Or on a sticky note. Or in an app. Or in a calendar. Or all of the above! Often, there is neither an organizing principle (other than perhaps chronological order) or a central repository. No wonder it’s hard to feel present and clear-minded. Add to this disorganization the volume of information coming in from the environment—e-mails, voice mails, texts, notifications, dings, beeps, buzzes, and maybe even snail mail—and the feeling of being overwhelmed grows.
Unconscious motives for maintaining poor systems also might play a role. People with a “need to please” personality type or who are more passive-aggressive in their communication are more likely to overcommit, and then forget or be late completing their tasks, rather than saying “No” from the outset or delegating the work.
Survival basics for time management
Assuming there is simply a skills deficit, you can teach basic time and project management skills to patients with ADHD (and to any patient with suboptimal executive functioning). Here are basic principles to adopt:
- If you can forget it, you will, so all tasks should go onto the to-do list.
- You should keep only 1 list. Adding on “stickies” is not allowed.
- Your list is like an extra lobe of your brain: It should be present at all times, whether you keep it in “the Cloud,” on your desktop, or on paper.
- Review your list and clean it up at least daily. This takes time, but it also saves time—in spades—when you can call upon the right task, at the right time, with energy and drive.
- The first action you should take in the daily review is to weed out or delegate tasks.
- Next, categorize remaining tasks. (Note: The free smartphone app Evernote allows you to do this with “tags.”) Categorizing allows you to process sets of tasks in buckets that can be tackled as a bundle and, therefore, more efficiently. For example, having all of your errands, items to research, and telephone calls that need to be returned in separate buckets allows for speedier processing—as opposed to veering back and forth between line items.
- Then, move remaining high-priority items to the top of the list. However, remember that, if everything is urgent, nothing is. Items that are low-hanging fruit that you can cross off the list in a matter of minutes can be prioritized even if they are not as urgent. By doing that, your list becomes more manageable and your brain can dive deeper into more complex tasks.
- Block out calendar time for each of your buckets with this formula: (1) Estimate how much time you’ll need to complete the tasks in each bucket, then add 50% for each bucket. (2) Add in commuting, set-up, or wind-down time, if you need it, to the grand total for all buckets, and then add 50% more than you’ve estimated
Set the brain free!
This process will seem like a burden at the beginning, when the synapses underneath it still need to get stronger (much like how the body responds to exercise). However, as long as these principles are put into action daily, they will become a trusted, second-nature system that frees the brain from distraction and anxiety—and, ultimately,
Difficulty with time management and organization is one of the most common complaints of patients with attention-deficit/hyperactivity disorder (ADHD). Being unproductive and inefficient also is anxiety-producing and depressing, leaving patients with additional comorbidity.
Although medication can help improve a person’s focus, if the patient is focusing on a set of poorly designed systems, he (she) will see little improvement. A comprehensive approach to improving day-to-day task management, similar to the one I describe here and use with my patients, is therefore as important as medication.
Needed: An ‘organizing principle’
Imagine that supermarkets displayed food in the order it arrives from the food distributors and producers. You’d walk in to the store and see a display of food that lacks hierarchy—1 random item placed next to another. The experience would be jarring, and shopping would be a much slower chore. Furthermore, what if you had to go to 5 stores to cover all your needs?
Yet, that is how most “to-do” lists are executed: A thought comes in, a thought goes down on paper. Or on a sticky note. Or in an app. Or in a calendar. Or all of the above! Often, there is neither an organizing principle (other than perhaps chronological order) or a central repository. No wonder it’s hard to feel present and clear-minded. Add to this disorganization the volume of information coming in from the environment—e-mails, voice mails, texts, notifications, dings, beeps, buzzes, and maybe even snail mail—and the feeling of being overwhelmed grows.
Unconscious motives for maintaining poor systems also might play a role. People with a “need to please” personality type or who are more passive-aggressive in their communication are more likely to overcommit, and then forget or be late completing their tasks, rather than saying “No” from the outset or delegating the work.
Survival basics for time management
Assuming there is simply a skills deficit, you can teach basic time and project management skills to patients with ADHD (and to any patient with suboptimal executive functioning). Here are basic principles to adopt:
- If you can forget it, you will, so all tasks should go onto the to-do list.
- You should keep only 1 list. Adding on “stickies” is not allowed.
- Your list is like an extra lobe of your brain: It should be present at all times, whether you keep it in “the Cloud,” on your desktop, or on paper.
- Review your list and clean it up at least daily. This takes time, but it also saves time—in spades—when you can call upon the right task, at the right time, with energy and drive.
- The first action you should take in the daily review is to weed out or delegate tasks.
- Next, categorize remaining tasks. (Note: The free smartphone app Evernote allows you to do this with “tags.”) Categorizing allows you to process sets of tasks in buckets that can be tackled as a bundle and, therefore, more efficiently. For example, having all of your errands, items to research, and telephone calls that need to be returned in separate buckets allows for speedier processing—as opposed to veering back and forth between line items.
- Then, move remaining high-priority items to the top of the list. However, remember that, if everything is urgent, nothing is. Items that are low-hanging fruit that you can cross off the list in a matter of minutes can be prioritized even if they are not as urgent. By doing that, your list becomes more manageable and your brain can dive deeper into more complex tasks.
- Block out calendar time for each of your buckets with this formula: (1) Estimate how much time you’ll need to complete the tasks in each bucket, then add 50% for each bucket. (2) Add in commuting, set-up, or wind-down time, if you need it, to the grand total for all buckets, and then add 50% more than you’ve estimated
Set the brain free!
This process will seem like a burden at the beginning, when the synapses underneath it still need to get stronger (much like how the body responds to exercise). However, as long as these principles are put into action daily, they will become a trusted, second-nature system that frees the brain from distraction and anxiety—and, ultimately,
Ataxia due to alcohol abuse
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Forget the myths and help your psychiatric patients quit smoking
The National Ambulatory Medical Care Survey1,2 (NAMCS) indicates that less than 1 out of 4 (23%) psychiatrists provide smoking cessation counseling to their patients, and even fewer prescribe medications.
What gives? How is it that so many psychiatrists endorse having recently helped a patient quit smoking when the data from large-scale surveys1,2 indicate they do not?
From the “glass is half-full” perspective, the discrepancy might indicate that psychiatrists finally have bought into the message put forth 20 years ago when the American Psychiatric Association first published its clinical practice guidelines for treating nicotine dependence.3 Because the figures I cited from NAMCS reflect data from 2006 to 2010, it is possible that in the last 5 years more psychiatrists have started to help their patients quit smoking. Such an hypothesis is further supported by the increasing number of research papers on smoking cessation in individuals with mental illness published over the past 8 years—a period that coincides with the release of the second edition of the Treating tobacco use and dependence clinical practice guideline from the U.S. Agency for Healthcare Research and Quality, which highlighted the need for more research in this population of smokers.4
Regardless of the reason, the fact that my informal surveys indicate a likely uptick in activity among psychiatrists to help their patients quit smoking is welcome news. With nearly 1 out of 2 cigarettes sold in the United States being smoked by individuals with psychiatric and substance use disorders,5 psychiatrists and other mental health professionals play a vital role in addressing this epidemic. That our patients smoke at rates 2- to 4-times that of the general population and die decades earlier than their non-smoking, non-mentally ill counterparts6 are compelling reasons urging us to end our complacency and help our patients quit smoking.
EAGLES trial results help debunk the latest myth about smoking cessation
In an article that I wrote for
In addition to applying the “black-box” warning, the FDA issued a post-marketing requirement to the manufacturers of bupropion and varenicline to conduct a large randomized controlled trial—Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES)—the top-line results of which were published in The Lancet this spring.12
Key results of the EAGLES trial
The researchers found no significant increase in serious neuropsychiatric AEs—a composite measure assessing depression, anxiety, suicidality, and 13 other symptom clusters—attributable to varenicline or bupropion compared with placebo or the nicotine patch in smokers with or without psychiatric disorders. The study did detect a significant difference—approximately 4% (2% in non-psychiatric cohort vs 6% in psychiatric cohort)—in the rate of serious neuropsychiatric AEs regardless of treatment condition. In both cohorts, varenicline was more effective than bupropion, which had similar efficacy to the nicotine patch; all interventions were superior to placebo. Importantly, all 3 medications significantly improved quit rates in smokers with and without psychiatric disorders. Although the efficacy of medications in smokers with or without psychiatric disorders was similar in terms of odds ratios, overall, those with psychiatric disorders had 20% to 30% lower quit rates compared with non-psychiatrically ill smokers.
The EAGLES study results, when viewed in the context of findings from other clinical trials and large-scale observational studies, provide further evidence that smokers with stable mental illness can use bupropion and varenicline safely. It also demonstrates that moderate to severe neuropsychiatric AEs occur during a smoking cessation attempt regardless of the medication used, therefore, monitoring smokers—especially those with psychiatric disorders—is important, a role that psychiatrists are uniquely poised to play.
That all 3 smoking cessation medications are effective in patients with mood, anxiety, and psychotic disorders is good news for our patients. Combined with the EAGLES safety findings, there is no better time to intervene in tobacco dependence
1. Rogers E, Sherman S. Tobacco use screening and treatment by outpatient psychiatrists before and after release of the American Psychiatric Association treatment guidelines for nicotine dependence. Am J Public Health. 2014;104(1):90-95.
2. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228-2230.
3. Practice guideline for the treatment of patients with nicotine dependence. American Psychiatric Association. Am J Psychiatry. 1996;53;153(suppl 10):1-31.
4. U.S. Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Published May 2008. Accessed September 12, 2016.
5. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
6. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
7. Anthenelli RM. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
8. Zyban [package insert]. Research Triangle Park, NC; GlaxoSmithKline; 2016.
9. Chantix [package insert]. New York, NY: Pfizer; 2016.
10. U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: a report of the surgeon general, 2014. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
11. World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. http://www.who.int/tobacco/global_report/2011/en/index.html. Published 2011. Accessed December 1, 2015.
12. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomized, placebo-controlled clinical trial. Lancet. 2016;18;387(10037):2507-2520.
13. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
14. First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
15. First M, Gibbon M, Spitzer RL, et al. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc.; 1997.
16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370.
17. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2001;168(12):1266-1277.
The National Ambulatory Medical Care Survey1,2 (NAMCS) indicates that less than 1 out of 4 (23%) psychiatrists provide smoking cessation counseling to their patients, and even fewer prescribe medications.
What gives? How is it that so many psychiatrists endorse having recently helped a patient quit smoking when the data from large-scale surveys1,2 indicate they do not?
From the “glass is half-full” perspective, the discrepancy might indicate that psychiatrists finally have bought into the message put forth 20 years ago when the American Psychiatric Association first published its clinical practice guidelines for treating nicotine dependence.3 Because the figures I cited from NAMCS reflect data from 2006 to 2010, it is possible that in the last 5 years more psychiatrists have started to help their patients quit smoking. Such an hypothesis is further supported by the increasing number of research papers on smoking cessation in individuals with mental illness published over the past 8 years—a period that coincides with the release of the second edition of the Treating tobacco use and dependence clinical practice guideline from the U.S. Agency for Healthcare Research and Quality, which highlighted the need for more research in this population of smokers.4
Regardless of the reason, the fact that my informal surveys indicate a likely uptick in activity among psychiatrists to help their patients quit smoking is welcome news. With nearly 1 out of 2 cigarettes sold in the United States being smoked by individuals with psychiatric and substance use disorders,5 psychiatrists and other mental health professionals play a vital role in addressing this epidemic. That our patients smoke at rates 2- to 4-times that of the general population and die decades earlier than their non-smoking, non-mentally ill counterparts6 are compelling reasons urging us to end our complacency and help our patients quit smoking.
EAGLES trial results help debunk the latest myth about smoking cessation
In an article that I wrote for
In addition to applying the “black-box” warning, the FDA issued a post-marketing requirement to the manufacturers of bupropion and varenicline to conduct a large randomized controlled trial—Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES)—the top-line results of which were published in The Lancet this spring.12
Key results of the EAGLES trial
The researchers found no significant increase in serious neuropsychiatric AEs—a composite measure assessing depression, anxiety, suicidality, and 13 other symptom clusters—attributable to varenicline or bupropion compared with placebo or the nicotine patch in smokers with or without psychiatric disorders. The study did detect a significant difference—approximately 4% (2% in non-psychiatric cohort vs 6% in psychiatric cohort)—in the rate of serious neuropsychiatric AEs regardless of treatment condition. In both cohorts, varenicline was more effective than bupropion, which had similar efficacy to the nicotine patch; all interventions were superior to placebo. Importantly, all 3 medications significantly improved quit rates in smokers with and without psychiatric disorders. Although the efficacy of medications in smokers with or without psychiatric disorders was similar in terms of odds ratios, overall, those with psychiatric disorders had 20% to 30% lower quit rates compared with non-psychiatrically ill smokers.
The EAGLES study results, when viewed in the context of findings from other clinical trials and large-scale observational studies, provide further evidence that smokers with stable mental illness can use bupropion and varenicline safely. It also demonstrates that moderate to severe neuropsychiatric AEs occur during a smoking cessation attempt regardless of the medication used, therefore, monitoring smokers—especially those with psychiatric disorders—is important, a role that psychiatrists are uniquely poised to play.
That all 3 smoking cessation medications are effective in patients with mood, anxiety, and psychotic disorders is good news for our patients. Combined with the EAGLES safety findings, there is no better time to intervene in tobacco dependence
The National Ambulatory Medical Care Survey1,2 (NAMCS) indicates that less than 1 out of 4 (23%) psychiatrists provide smoking cessation counseling to their patients, and even fewer prescribe medications.
What gives? How is it that so many psychiatrists endorse having recently helped a patient quit smoking when the data from large-scale surveys1,2 indicate they do not?
From the “glass is half-full” perspective, the discrepancy might indicate that psychiatrists finally have bought into the message put forth 20 years ago when the American Psychiatric Association first published its clinical practice guidelines for treating nicotine dependence.3 Because the figures I cited from NAMCS reflect data from 2006 to 2010, it is possible that in the last 5 years more psychiatrists have started to help their patients quit smoking. Such an hypothesis is further supported by the increasing number of research papers on smoking cessation in individuals with mental illness published over the past 8 years—a period that coincides with the release of the second edition of the Treating tobacco use and dependence clinical practice guideline from the U.S. Agency for Healthcare Research and Quality, which highlighted the need for more research in this population of smokers.4
Regardless of the reason, the fact that my informal surveys indicate a likely uptick in activity among psychiatrists to help their patients quit smoking is welcome news. With nearly 1 out of 2 cigarettes sold in the United States being smoked by individuals with psychiatric and substance use disorders,5 psychiatrists and other mental health professionals play a vital role in addressing this epidemic. That our patients smoke at rates 2- to 4-times that of the general population and die decades earlier than their non-smoking, non-mentally ill counterparts6 are compelling reasons urging us to end our complacency and help our patients quit smoking.
EAGLES trial results help debunk the latest myth about smoking cessation
In an article that I wrote for
In addition to applying the “black-box” warning, the FDA issued a post-marketing requirement to the manufacturers of bupropion and varenicline to conduct a large randomized controlled trial—Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES)—the top-line results of which were published in The Lancet this spring.12
Key results of the EAGLES trial
The researchers found no significant increase in serious neuropsychiatric AEs—a composite measure assessing depression, anxiety, suicidality, and 13 other symptom clusters—attributable to varenicline or bupropion compared with placebo or the nicotine patch in smokers with or without psychiatric disorders. The study did detect a significant difference—approximately 4% (2% in non-psychiatric cohort vs 6% in psychiatric cohort)—in the rate of serious neuropsychiatric AEs regardless of treatment condition. In both cohorts, varenicline was more effective than bupropion, which had similar efficacy to the nicotine patch; all interventions were superior to placebo. Importantly, all 3 medications significantly improved quit rates in smokers with and without psychiatric disorders. Although the efficacy of medications in smokers with or without psychiatric disorders was similar in terms of odds ratios, overall, those with psychiatric disorders had 20% to 30% lower quit rates compared with non-psychiatrically ill smokers.
The EAGLES study results, when viewed in the context of findings from other clinical trials and large-scale observational studies, provide further evidence that smokers with stable mental illness can use bupropion and varenicline safely. It also demonstrates that moderate to severe neuropsychiatric AEs occur during a smoking cessation attempt regardless of the medication used, therefore, monitoring smokers—especially those with psychiatric disorders—is important, a role that psychiatrists are uniquely poised to play.
That all 3 smoking cessation medications are effective in patients with mood, anxiety, and psychotic disorders is good news for our patients. Combined with the EAGLES safety findings, there is no better time to intervene in tobacco dependence
1. Rogers E, Sherman S. Tobacco use screening and treatment by outpatient psychiatrists before and after release of the American Psychiatric Association treatment guidelines for nicotine dependence. Am J Public Health. 2014;104(1):90-95.
2. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228-2230.
3. Practice guideline for the treatment of patients with nicotine dependence. American Psychiatric Association. Am J Psychiatry. 1996;53;153(suppl 10):1-31.
4. U.S. Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Published May 2008. Accessed September 12, 2016.
5. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
6. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
7. Anthenelli RM. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
8. Zyban [package insert]. Research Triangle Park, NC; GlaxoSmithKline; 2016.
9. Chantix [package insert]. New York, NY: Pfizer; 2016.
10. U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: a report of the surgeon general, 2014. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
11. World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. http://www.who.int/tobacco/global_report/2011/en/index.html. Published 2011. Accessed December 1, 2015.
12. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomized, placebo-controlled clinical trial. Lancet. 2016;18;387(10037):2507-2520.
13. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
14. First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
15. First M, Gibbon M, Spitzer RL, et al. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc.; 1997.
16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370.
17. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2001;168(12):1266-1277.
1. Rogers E, Sherman S. Tobacco use screening and treatment by outpatient psychiatrists before and after release of the American Psychiatric Association treatment guidelines for nicotine dependence. Am J Public Health. 2014;104(1):90-95.
2. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228-2230.
3. Practice guideline for the treatment of patients with nicotine dependence. American Psychiatric Association. Am J Psychiatry. 1996;53;153(suppl 10):1-31.
4. U.S. Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Published May 2008. Accessed September 12, 2016.
5. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
6. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
7. Anthenelli RM. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
8. Zyban [package insert]. Research Triangle Park, NC; GlaxoSmithKline; 2016.
9. Chantix [package insert]. New York, NY: Pfizer; 2016.
10. U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: a report of the surgeon general, 2014. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
11. World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. http://www.who.int/tobacco/global_report/2011/en/index.html. Published 2011. Accessed December 1, 2015.
12. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomized, placebo-controlled clinical trial. Lancet. 2016;18;387(10037):2507-2520.
13. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
14. First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
15. First M, Gibbon M, Spitzer RL, et al. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc.; 1997.
16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370.
17. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2001;168(12):1266-1277.
Don’t balk at using medical therapy to manage alcohol use disorder
There is ample evidence in the medical literature, as well as clinical experience, that patients seeking help for chemical dependency benefit from pharmacotherapy. It is common, however, for physicians, patients, and family to balk at the idea. Even within the psychiatry community, where there should be better understanding of substance use disorders, many practitioners hesitate to employ medications, especially for alcohol use disorder (AUD).
Efficacy for such FDA-approved medications has been demonstrated in well-designed, randomized controlled trials, but many trainees, and even experienced professionals, have never seen these medications used effectively and appropriately. Medication-assisted treatment (MAT) is not an alternative to biopsychosocial approaches but is an augmentation that can (1) help stabilize the patient until he (she) can be educated in relapse prevention skills and (2) allow the brain to rewire and heal until he regains impulse control.
Diverse presentations
Do you remember that patient who often arrived for appointments intoxicated, promising that he plans to cut down? How about the man you saw in the emergency department with an elevated blood alcohol level, who was constantly endorsing suicidal thoughts that subsided when he reached clinical sobriety? What about the college student who often was treated for alcohol poisoning after binge drinking on weekends, but who never considered this behavior problematic? And, how about the elderly woman who was evaluated for anxiety, but had been drinking 4 beers nightly for the past 30 years?
Despite the diverse presentations, these patients all have a chronic disease and we fail them when we do not apply evidence-based medicine to their treatment.
As psychiatrists, we encounter many patients with AUD as a primary or comorbid diagnosis. This is a global problem associated with significant human and financial cost. With 80% of American adolescents having reported using alcohol in the past year, the problem will continue to grow.1 Furthermore, a greater prevalence of AUD is noted in clinical populations undergoing psychiatric treatment.2 Ongoing alcohol abuse complicates the course of medical and psychiatric conditions and incites significant societal exclusion.
Pharmacotherapy is underutilized
Despite an increase in the use of psychotropic medications for treating psychiatric illness, pharmacotherapy for AUD is underutilized: only 3% of patients have received an FDA-approved treatment.2,3 Nearly one-third of adults are affected by AUD during their lifetime, yet only 20% seek help.3 Management today remains limited to episodic, brief inpatient detoxification and psychosocial therapy.
Recovery rates are highest when addiction treatment that monitors abstinence is continuous; yet, for most part, alcohol addiction is treated in discrete episodes upon relapse. Although MAT is recommended by experts for “moderate” and “severe” substance use disorders, practitioners, in general, have demonstrated considerable resistance to using this modality as part of routine practice.4,5 This is regrettable: Regardless of terminology used to describe their condition, these people suffer a potentially fatal disease characterized by high post-treatment recidivism.
Neuroscience supports the brain disease model of addiction, with neuroplasticity changes being made during phases of drug use. Medications are shown to assist in preventing relapse while the brain is healing and normal emotional and decision-making capacities are being restored.6
Why hesitate to use pharmacotherapeutics?
There are diverse pharmacotherapeutic options that can be pursued for treating AUD with minimal disruption to home and work life. Alarmingly, many trainees have never prescribed or even considered such medications. Despite modest effect sizes in randomized controlled trials, efficacy has been demonstrated in reducing relapse rates and overall severity of drinking days.4,5 So, from where does the ambivalence of patients and providers about using these treatments to achieve lasting recovery stem?
Starting MAT certainly requires both parties to be in agreement. A patient might decline medication because of a fear of dependence or because he overestimates his ability to achieve remission on his own. There also may be financial barriers in a current alcohol treatment system that is traditionally non-medically oriented. Prescribers also fail to offer medications because of:
- lack of familiarity with available agents
- absence of guidelines for use
- disbelief that the condition is treatable.
Given that treatment often is based on a 12-step approach, such as Alcoholics Anonymous (AA), providers might hesitate to prescribe medication for an illness that is thought to be managed through psychosocial interventions, such as group and motivational therapy.
Therapeutic options
Choice of medication depends on the prescriber’s comfort level, reputation of the medication, potential side-effect profile, medical contraindications, and affordability; the most important consideration, however, should be the overall goals and expectations of the patient.
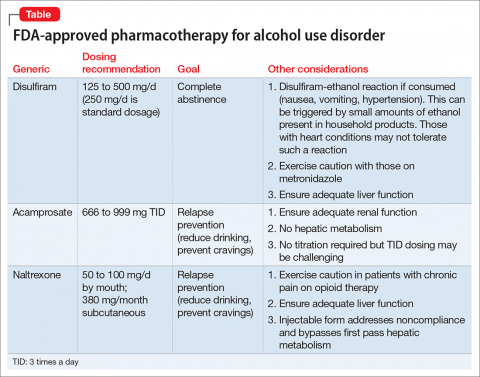
There are 4 FDA-approved medications for AUD (Table); many others are off-label. It is advisable to start with an FDA-approved medication such as disulfiram for the motivated patient who has a collaborator and desires complete abstinence; naltrexone for a patient who wants to cut down on intake (a long-acting formulation can be used for poorly adherent patients); and acamprosate for a patient with at least some established sobriety who needs help with post-withdrawal sleep disturbances.
With regard to off-label medications, topiramate has the highest evidence for efficacy. Gabapentin can augment naltrexone and also helps with sleep, anxiety, withdrawal, and cravings.4,5
Psychosocial interventions
Medications are just 1 tool in recovery; patients should be engaged in a program of counseling. Encourage attendance at AA meetings. An up-and-coming concept is the use of smartphone applications to prevent relapse (or even induce remission); apps that provide an accurate blood alcohol tracking systems and integrated psychosocial therapies are in the pipeline. The novel Reddit online forum r/StopDrinking is a 24-hour peer-support community that relies on
fellowship, accountability, monitoring, and anonymity; the forum can compete with
motivational interviewing for efficacy in increasing abstinence and preventing relapse.
1. Johnson L, O’Malley P, Miech RA, et al. Monitoring the Future national survey results on drug use, 1975-2015: overview, key findings on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Published February 2016. Accessed January 20, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: mental health findings, NSDUH Series H-49, HHS Publication No. (SMA) 14-4887. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Robinson S, Meeks TW, Geniza C. Medication for alcohol use disorder: which agents work best. Current Psychiatry. 2014;13(1):22-29.
5. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
6. Volkow ND, Koob GF, McLellan AT. Neurobiological advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363-371.
Acknowledgment
The authors would like to thank Thomas M. Penders, MS, MD, Medical Director for Consultation-Liaison Psychiatry at Cape Cod Healthcare, Hyannis, Massachusetts, and Affiliate Professor at East Carolina University, Greenville, North Carolina, for all his guidance, support, and mentorship.
There is ample evidence in the medical literature, as well as clinical experience, that patients seeking help for chemical dependency benefit from pharmacotherapy. It is common, however, for physicians, patients, and family to balk at the idea. Even within the psychiatry community, where there should be better understanding of substance use disorders, many practitioners hesitate to employ medications, especially for alcohol use disorder (AUD).
Efficacy for such FDA-approved medications has been demonstrated in well-designed, randomized controlled trials, but many trainees, and even experienced professionals, have never seen these medications used effectively and appropriately. Medication-assisted treatment (MAT) is not an alternative to biopsychosocial approaches but is an augmentation that can (1) help stabilize the patient until he (she) can be educated in relapse prevention skills and (2) allow the brain to rewire and heal until he regains impulse control.
Diverse presentations
Do you remember that patient who often arrived for appointments intoxicated, promising that he plans to cut down? How about the man you saw in the emergency department with an elevated blood alcohol level, who was constantly endorsing suicidal thoughts that subsided when he reached clinical sobriety? What about the college student who often was treated for alcohol poisoning after binge drinking on weekends, but who never considered this behavior problematic? And, how about the elderly woman who was evaluated for anxiety, but had been drinking 4 beers nightly for the past 30 years?
Despite the diverse presentations, these patients all have a chronic disease and we fail them when we do not apply evidence-based medicine to their treatment.
As psychiatrists, we encounter many patients with AUD as a primary or comorbid diagnosis. This is a global problem associated with significant human and financial cost. With 80% of American adolescents having reported using alcohol in the past year, the problem will continue to grow.1 Furthermore, a greater prevalence of AUD is noted in clinical populations undergoing psychiatric treatment.2 Ongoing alcohol abuse complicates the course of medical and psychiatric conditions and incites significant societal exclusion.
Pharmacotherapy is underutilized
Despite an increase in the use of psychotropic medications for treating psychiatric illness, pharmacotherapy for AUD is underutilized: only 3% of patients have received an FDA-approved treatment.2,3 Nearly one-third of adults are affected by AUD during their lifetime, yet only 20% seek help.3 Management today remains limited to episodic, brief inpatient detoxification and psychosocial therapy.
Recovery rates are highest when addiction treatment that monitors abstinence is continuous; yet, for most part, alcohol addiction is treated in discrete episodes upon relapse. Although MAT is recommended by experts for “moderate” and “severe” substance use disorders, practitioners, in general, have demonstrated considerable resistance to using this modality as part of routine practice.4,5 This is regrettable: Regardless of terminology used to describe their condition, these people suffer a potentially fatal disease characterized by high post-treatment recidivism.
Neuroscience supports the brain disease model of addiction, with neuroplasticity changes being made during phases of drug use. Medications are shown to assist in preventing relapse while the brain is healing and normal emotional and decision-making capacities are being restored.6
Why hesitate to use pharmacotherapeutics?
There are diverse pharmacotherapeutic options that can be pursued for treating AUD with minimal disruption to home and work life. Alarmingly, many trainees have never prescribed or even considered such medications. Despite modest effect sizes in randomized controlled trials, efficacy has been demonstrated in reducing relapse rates and overall severity of drinking days.4,5 So, from where does the ambivalence of patients and providers about using these treatments to achieve lasting recovery stem?
Starting MAT certainly requires both parties to be in agreement. A patient might decline medication because of a fear of dependence or because he overestimates his ability to achieve remission on his own. There also may be financial barriers in a current alcohol treatment system that is traditionally non-medically oriented. Prescribers also fail to offer medications because of:
- lack of familiarity with available agents
- absence of guidelines for use
- disbelief that the condition is treatable.
Given that treatment often is based on a 12-step approach, such as Alcoholics Anonymous (AA), providers might hesitate to prescribe medication for an illness that is thought to be managed through psychosocial interventions, such as group and motivational therapy.
Therapeutic options
Choice of medication depends on the prescriber’s comfort level, reputation of the medication, potential side-effect profile, medical contraindications, and affordability; the most important consideration, however, should be the overall goals and expectations of the patient.
There are 4 FDA-approved medications for AUD (Table); many others are off-label. It is advisable to start with an FDA-approved medication such as disulfiram for the motivated patient who has a collaborator and desires complete abstinence; naltrexone for a patient who wants to cut down on intake (a long-acting formulation can be used for poorly adherent patients); and acamprosate for a patient with at least some established sobriety who needs help with post-withdrawal sleep disturbances.
With regard to off-label medications, topiramate has the highest evidence for efficacy. Gabapentin can augment naltrexone and also helps with sleep, anxiety, withdrawal, and cravings.4,5
Psychosocial interventions
Medications are just 1 tool in recovery; patients should be engaged in a program of counseling. Encourage attendance at AA meetings. An up-and-coming concept is the use of smartphone applications to prevent relapse (or even induce remission); apps that provide an accurate blood alcohol tracking systems and integrated psychosocial therapies are in the pipeline. The novel Reddit online forum r/StopDrinking is a 24-hour peer-support community that relies on
fellowship, accountability, monitoring, and anonymity; the forum can compete with
motivational interviewing for efficacy in increasing abstinence and preventing relapse.
There is ample evidence in the medical literature, as well as clinical experience, that patients seeking help for chemical dependency benefit from pharmacotherapy. It is common, however, for physicians, patients, and family to balk at the idea. Even within the psychiatry community, where there should be better understanding of substance use disorders, many practitioners hesitate to employ medications, especially for alcohol use disorder (AUD).
Efficacy for such FDA-approved medications has been demonstrated in well-designed, randomized controlled trials, but many trainees, and even experienced professionals, have never seen these medications used effectively and appropriately. Medication-assisted treatment (MAT) is not an alternative to biopsychosocial approaches but is an augmentation that can (1) help stabilize the patient until he (she) can be educated in relapse prevention skills and (2) allow the brain to rewire and heal until he regains impulse control.
Diverse presentations
Do you remember that patient who often arrived for appointments intoxicated, promising that he plans to cut down? How about the man you saw in the emergency department with an elevated blood alcohol level, who was constantly endorsing suicidal thoughts that subsided when he reached clinical sobriety? What about the college student who often was treated for alcohol poisoning after binge drinking on weekends, but who never considered this behavior problematic? And, how about the elderly woman who was evaluated for anxiety, but had been drinking 4 beers nightly for the past 30 years?
Despite the diverse presentations, these patients all have a chronic disease and we fail them when we do not apply evidence-based medicine to their treatment.
As psychiatrists, we encounter many patients with AUD as a primary or comorbid diagnosis. This is a global problem associated with significant human and financial cost. With 80% of American adolescents having reported using alcohol in the past year, the problem will continue to grow.1 Furthermore, a greater prevalence of AUD is noted in clinical populations undergoing psychiatric treatment.2 Ongoing alcohol abuse complicates the course of medical and psychiatric conditions and incites significant societal exclusion.
Pharmacotherapy is underutilized
Despite an increase in the use of psychotropic medications for treating psychiatric illness, pharmacotherapy for AUD is underutilized: only 3% of patients have received an FDA-approved treatment.2,3 Nearly one-third of adults are affected by AUD during their lifetime, yet only 20% seek help.3 Management today remains limited to episodic, brief inpatient detoxification and psychosocial therapy.
Recovery rates are highest when addiction treatment that monitors abstinence is continuous; yet, for most part, alcohol addiction is treated in discrete episodes upon relapse. Although MAT is recommended by experts for “moderate” and “severe” substance use disorders, practitioners, in general, have demonstrated considerable resistance to using this modality as part of routine practice.4,5 This is regrettable: Regardless of terminology used to describe their condition, these people suffer a potentially fatal disease characterized by high post-treatment recidivism.
Neuroscience supports the brain disease model of addiction, with neuroplasticity changes being made during phases of drug use. Medications are shown to assist in preventing relapse while the brain is healing and normal emotional and decision-making capacities are being restored.6
Why hesitate to use pharmacotherapeutics?
There are diverse pharmacotherapeutic options that can be pursued for treating AUD with minimal disruption to home and work life. Alarmingly, many trainees have never prescribed or even considered such medications. Despite modest effect sizes in randomized controlled trials, efficacy has been demonstrated in reducing relapse rates and overall severity of drinking days.4,5 So, from where does the ambivalence of patients and providers about using these treatments to achieve lasting recovery stem?
Starting MAT certainly requires both parties to be in agreement. A patient might decline medication because of a fear of dependence or because he overestimates his ability to achieve remission on his own. There also may be financial barriers in a current alcohol treatment system that is traditionally non-medically oriented. Prescribers also fail to offer medications because of:
- lack of familiarity with available agents
- absence of guidelines for use
- disbelief that the condition is treatable.
Given that treatment often is based on a 12-step approach, such as Alcoholics Anonymous (AA), providers might hesitate to prescribe medication for an illness that is thought to be managed through psychosocial interventions, such as group and motivational therapy.
Therapeutic options
Choice of medication depends on the prescriber’s comfort level, reputation of the medication, potential side-effect profile, medical contraindications, and affordability; the most important consideration, however, should be the overall goals and expectations of the patient.
There are 4 FDA-approved medications for AUD (Table); many others are off-label. It is advisable to start with an FDA-approved medication such as disulfiram for the motivated patient who has a collaborator and desires complete abstinence; naltrexone for a patient who wants to cut down on intake (a long-acting formulation can be used for poorly adherent patients); and acamprosate for a patient with at least some established sobriety who needs help with post-withdrawal sleep disturbances.
With regard to off-label medications, topiramate has the highest evidence for efficacy. Gabapentin can augment naltrexone and also helps with sleep, anxiety, withdrawal, and cravings.4,5
Psychosocial interventions
Medications are just 1 tool in recovery; patients should be engaged in a program of counseling. Encourage attendance at AA meetings. An up-and-coming concept is the use of smartphone applications to prevent relapse (or even induce remission); apps that provide an accurate blood alcohol tracking systems and integrated psychosocial therapies are in the pipeline. The novel Reddit online forum r/StopDrinking is a 24-hour peer-support community that relies on
fellowship, accountability, monitoring, and anonymity; the forum can compete with
motivational interviewing for efficacy in increasing abstinence and preventing relapse.
1. Johnson L, O’Malley P, Miech RA, et al. Monitoring the Future national survey results on drug use, 1975-2015: overview, key findings on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Published February 2016. Accessed January 20, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: mental health findings, NSDUH Series H-49, HHS Publication No. (SMA) 14-4887. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Robinson S, Meeks TW, Geniza C. Medication for alcohol use disorder: which agents work best. Current Psychiatry. 2014;13(1):22-29.
5. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
6. Volkow ND, Koob GF, McLellan AT. Neurobiological advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363-371.
Acknowledgment
The authors would like to thank Thomas M. Penders, MS, MD, Medical Director for Consultation-Liaison Psychiatry at Cape Cod Healthcare, Hyannis, Massachusetts, and Affiliate Professor at East Carolina University, Greenville, North Carolina, for all his guidance, support, and mentorship.
1. Johnson L, O’Malley P, Miech RA, et al. Monitoring the Future national survey results on drug use, 1975-2015: overview, key findings on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Published February 2016. Accessed January 20, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: mental health findings, NSDUH Series H-49, HHS Publication No. (SMA) 14-4887. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Robinson S, Meeks TW, Geniza C. Medication for alcohol use disorder: which agents work best. Current Psychiatry. 2014;13(1):22-29.
5. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
6. Volkow ND, Koob GF, McLellan AT. Neurobiological advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363-371.
Acknowledgment
The authors would like to thank Thomas M. Penders, MS, MD, Medical Director for Consultation-Liaison Psychiatry at Cape Cod Healthcare, Hyannis, Massachusetts, and Affiliate Professor at East Carolina University, Greenville, North Carolina, for all his guidance, support, and mentorship.
Use these tips to choose the best neuroimaging modality for your pediatric patient
Social media: Potential pitfalls for psychiatrists
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
‘Enough!’ We need to take back our profession; More unresolved questions about psychiatry
‘Enough!’ We need to take back our profession
Every day, I am grateful that I became a physician and a psychiatrist. Every minute that I spend with patients is an honor and a privilege. I have never forgotten that. But it is heartbreaking to see my precious profession being destroyed by bureaucrats.
An example: I am concerned about the effect that passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) will have on physicians. I read articles telling us how we should handle this new plan for reimbursement, but I also read that 86% of physicians are not in favor of MACRA. How did we get stuck with it?
Another example of why it has become virtually impossible to do our job: I spend a fair amount of time obtaining prior authorization for generic medications that are available at big-box stores for $10 or $15; often, these authorizations need approval by the medical director. I have been beaten down enough over the years to learn that I should no longer prescribe brand-name medications—only generic medications (which still require authorization!), even when my patient has been taking the medication for 10 or 15 years. The last time I sought authorization to prescribe a medication, the reviewer asked me why I had not tried 3 different generics over the past year. I had to remind her that I had an active prior authorization in place from the year before, and so why would I do what she was proposing?
Physicians are some of the most highly trained professionals. It takes 7 to 15 years to be able to be somewhat proficient at the job, then another 30 or 40 years of practice to become really good at it. But we’ve become technicians at the mercy of business executives: We go to our office and spend our time checking off boxes, trying to figure out proper coding and the proper diagnosis, so that we can get an appropriate amount of money for the service we’re providing. How has it come to this? Why can’t we take back our profession?
Another problem is that physicians are being paid for their performance and the outcomes they produce. But people are not refrigerators: We can do everything right and the patient still dies. I have a number of patients who have no insight into their psychiatric illness; no matter what I say, or do, or how much time I spend with them, they are nonadherent. How is this my fault?
Physicians are not given the opportunity to think for themselves, or to prescribe treatments that they see fit and document in ways that they were trained. Where is the American Medical Association, the Connecticut State Medical Society, the Hartford County Medical Association, and all the other associations that supposedly represent us? How have they allowed this to happen?
In the future, health care will be provided by physician assistants and nurse practitioners; physicians will provide background supervision, or perform surgery, but the patient will never meet them. I respect NPs and PAs, but they do not have the rigorous training that physicians have. But they’re less expensive—and isn’t that what it’s all about?
If we are not going to speak up, or if we are not going to elect officials to truly represent us and advocate for us, then we have nobody to blame but ourselves.
Carole Black Cohen, MD
Private psychiatric practice
Farmington, Connecticut
More unresolved questions about psychiatry
In Dr. Nasrallah’s August essay (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
Dr. Moffic is spot-on about the escalating rate of burnout among physicians, including psychiatrists. The reason I did not include burnout in the list of questions is because I intended to pose questions related to external forces that interfere with patient care. Burnout is a vicious internal typhoon of emotional turmoil that might be related to multiple idiosyncratic personal variables and only partially to frustrations in clinical practice.
Burnout is, one might say, a subcortical event (generated in the amygdala?)—not a cortical process like the “why” questions that beg for answers. Admittedly, however, the cumulative burden of practice frustrations—especially the inability to erase the personal, social, financial, and vocational stigmata that plague our patients’ lives—can, eventually, take a toll on our morale and quality of life.
Fortunately, we psychiatrists generally are a resilient breed. We can manage personal stress using techniques that we employ in our practices. That might be why burnout is lower in psychiatry than it is in other medical specialties.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
‘Enough!’ We need to take back our profession
Every day, I am grateful that I became a physician and a psychiatrist. Every minute that I spend with patients is an honor and a privilege. I have never forgotten that. But it is heartbreaking to see my precious profession being destroyed by bureaucrats.
An example: I am concerned about the effect that passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) will have on physicians. I read articles telling us how we should handle this new plan for reimbursement, but I also read that 86% of physicians are not in favor of MACRA. How did we get stuck with it?
Another example of why it has become virtually impossible to do our job: I spend a fair amount of time obtaining prior authorization for generic medications that are available at big-box stores for $10 or $15; often, these authorizations need approval by the medical director. I have been beaten down enough over the years to learn that I should no longer prescribe brand-name medications—only generic medications (which still require authorization!), even when my patient has been taking the medication for 10 or 15 years. The last time I sought authorization to prescribe a medication, the reviewer asked me why I had not tried 3 different generics over the past year. I had to remind her that I had an active prior authorization in place from the year before, and so why would I do what she was proposing?
Physicians are some of the most highly trained professionals. It takes 7 to 15 years to be able to be somewhat proficient at the job, then another 30 or 40 years of practice to become really good at it. But we’ve become technicians at the mercy of business executives: We go to our office and spend our time checking off boxes, trying to figure out proper coding and the proper diagnosis, so that we can get an appropriate amount of money for the service we’re providing. How has it come to this? Why can’t we take back our profession?
Another problem is that physicians are being paid for their performance and the outcomes they produce. But people are not refrigerators: We can do everything right and the patient still dies. I have a number of patients who have no insight into their psychiatric illness; no matter what I say, or do, or how much time I spend with them, they are nonadherent. How is this my fault?
Physicians are not given the opportunity to think for themselves, or to prescribe treatments that they see fit and document in ways that they were trained. Where is the American Medical Association, the Connecticut State Medical Society, the Hartford County Medical Association, and all the other associations that supposedly represent us? How have they allowed this to happen?
In the future, health care will be provided by physician assistants and nurse practitioners; physicians will provide background supervision, or perform surgery, but the patient will never meet them. I respect NPs and PAs, but they do not have the rigorous training that physicians have. But they’re less expensive—and isn’t that what it’s all about?
If we are not going to speak up, or if we are not going to elect officials to truly represent us and advocate for us, then we have nobody to blame but ourselves.
Carole Black Cohen, MD
Private psychiatric practice
Farmington, Connecticut
More unresolved questions about psychiatry
In Dr. Nasrallah’s August essay (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
Dr. Moffic is spot-on about the escalating rate of burnout among physicians, including psychiatrists. The reason I did not include burnout in the list of questions is because I intended to pose questions related to external forces that interfere with patient care. Burnout is a vicious internal typhoon of emotional turmoil that might be related to multiple idiosyncratic personal variables and only partially to frustrations in clinical practice.
Burnout is, one might say, a subcortical event (generated in the amygdala?)—not a cortical process like the “why” questions that beg for answers. Admittedly, however, the cumulative burden of practice frustrations—especially the inability to erase the personal, social, financial, and vocational stigmata that plague our patients’ lives—can, eventually, take a toll on our morale and quality of life.
Fortunately, we psychiatrists generally are a resilient breed. We can manage personal stress using techniques that we employ in our practices. That might be why burnout is lower in psychiatry than it is in other medical specialties.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
‘Enough!’ We need to take back our profession
Every day, I am grateful that I became a physician and a psychiatrist. Every minute that I spend with patients is an honor and a privilege. I have never forgotten that. But it is heartbreaking to see my precious profession being destroyed by bureaucrats.
An example: I am concerned about the effect that passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) will have on physicians. I read articles telling us how we should handle this new plan for reimbursement, but I also read that 86% of physicians are not in favor of MACRA. How did we get stuck with it?
Another example of why it has become virtually impossible to do our job: I spend a fair amount of time obtaining prior authorization for generic medications that are available at big-box stores for $10 or $15; often, these authorizations need approval by the medical director. I have been beaten down enough over the years to learn that I should no longer prescribe brand-name medications—only generic medications (which still require authorization!), even when my patient has been taking the medication for 10 or 15 years. The last time I sought authorization to prescribe a medication, the reviewer asked me why I had not tried 3 different generics over the past year. I had to remind her that I had an active prior authorization in place from the year before, and so why would I do what she was proposing?
Physicians are some of the most highly trained professionals. It takes 7 to 15 years to be able to be somewhat proficient at the job, then another 30 or 40 years of practice to become really good at it. But we’ve become technicians at the mercy of business executives: We go to our office and spend our time checking off boxes, trying to figure out proper coding and the proper diagnosis, so that we can get an appropriate amount of money for the service we’re providing. How has it come to this? Why can’t we take back our profession?
Another problem is that physicians are being paid for their performance and the outcomes they produce. But people are not refrigerators: We can do everything right and the patient still dies. I have a number of patients who have no insight into their psychiatric illness; no matter what I say, or do, or how much time I spend with them, they are nonadherent. How is this my fault?
Physicians are not given the opportunity to think for themselves, or to prescribe treatments that they see fit and document in ways that they were trained. Where is the American Medical Association, the Connecticut State Medical Society, the Hartford County Medical Association, and all the other associations that supposedly represent us? How have they allowed this to happen?
In the future, health care will be provided by physician assistants and nurse practitioners; physicians will provide background supervision, or perform surgery, but the patient will never meet them. I respect NPs and PAs, but they do not have the rigorous training that physicians have. But they’re less expensive—and isn’t that what it’s all about?
If we are not going to speak up, or if we are not going to elect officials to truly represent us and advocate for us, then we have nobody to blame but ourselves.
Carole Black Cohen, MD
Private psychiatric practice
Farmington, Connecticut
More unresolved questions about psychiatry
In Dr. Nasrallah’s August essay (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
Dr. Moffic is spot-on about the escalating rate of burnout among physicians, including psychiatrists. The reason I did not include burnout in the list of questions is because I intended to pose questions related to external forces that interfere with patient care. Burnout is a vicious internal typhoon of emotional turmoil that might be related to multiple idiosyncratic personal variables and only partially to frustrations in clinical practice.
Burnout is, one might say, a subcortical event (generated in the amygdala?)—not a cortical process like the “why” questions that beg for answers. Admittedly, however, the cumulative burden of practice frustrations—especially the inability to erase the personal, social, financial, and vocational stigmata that plague our patients’ lives—can, eventually, take a toll on our morale and quality of life.
Fortunately, we psychiatrists generally are a resilient breed. We can manage personal stress using techniques that we employ in our practices. That might be why burnout is lower in psychiatry than it is in other medical specialties.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
The psychiatry workforce pool is shrinking. What are we doing about it?
The dilemma of a diminishing workforce pool might seem more the province of medical school deans, psychiatry department chairs, and psychiatry residency training directors, but our ability to recruit and retain psychiatrists is, in reality, everyone’s concern—including hospitals, clinics, and, especially, patients and their families. Even without knowledge of the specialty or any numerical appraisal, for example, it is common knowledge that we have a dire shortage of child and adolescent and geriatric psychiatrists—a topic of widespread interest and great consequence for access to mental health care.
Tracking a decline
The very title of a recent provocative paper1 in Health Affairs says it all: “Population of US practicing psychiatrists declined 2003-13, which may help explain poor access to mental health care.” In an elegant analysis, the authors expose (1) a 10% decline in the number of psychiatrists for every 100,000 people and (2) wide regional variability in the availability of psychiatrists. In stark contrast, the number of neurologists increased by >15% and the primary care workforce remained stable, with a 1.3% increase in the number of physicians, over the same 10 years.
At the beginning of the psychiatry workforce pipeline, the number of medical students who choose psychiatry remains both small (typically, slightly more than 4% of graduating students) and remarkably stable over time. Wilbanks et al,2 in a thoughtful analysis of the 2011 to 2013 Medical School Graduation Questionnaire of the Association of American Medical Colleges, affirm and, in part, explain this consistent pattern. They note that the 4 most important considerations among students who select psychiatry are:
- personality fit
- specialty content
- work–life balance
- role model influences.
Some of these considerations also overlap with those of students in other specialties; the authors also note that older medical students and women are more likely to choose psychiatry.
Here is what we must do to erase the shortage
It does appear that, despite scientific advances in brain and behavior, expanding therapeutic options, and unique patient interactions that, taken together, should make a career in psychiatry exciting and appealing, there are simply not enough of us to meet the population’s mental health needs. This is a serious problem. It is our professional obligation—all of us—that we take on this shortage and develop solutions to it.
At its zenith, only about 7% of medical students chose psychiatry. We need to proactively prime the pump for our specialty by encouraging more observerships and promoting mental health careers through community outreach to high school students.
We must be diligent and effective mentors to medical students; mentorship is a powerful catalyst for career decision-making.
We need to make psychiatry clerkships exciting, to show off the best of what our specialty has to offer, and to cultivate sustained interest among our students in the brain and its psychiatric disorders.
We need to highlight the momentous advances in knowledge, biology, and treatments that now characterize our psychiatric profession. We need to advocate for more of these accomplishments.
We must be public stigma-busters! (Our patients need us to do this, too.)
And there is more to do:
Collaborate. In delivering psychiatric health care, we need to expand our effectiveness to achieve more collaboration, greater extension of effect, and broader outreach. Collaborative care has come of age as a delivery model; it should be embraced more broadly. We need to continue our efforts to bridge the many sister mental health disciplines—psychology, nursing, social work, counseling—that collectively provide mental health care.
Unite. Given the inadequate workforce numbers and enormous need, we will diminish ourselves by “guild infighting” and, consequently, weaken our legislative advocacy and leverage. We need to embrace and support all medical specialties and have them support us as well. We need to grow closer to primary care and support this specialty as the true front line of mental health. We also need to bridge the gap between addiction medicine and psychiatry, especially given the high level of addiction comorbidity in many psychiatric disorders.
Foster innovation. The deficit of psychiatric workers might be buffered by innovations in how we leverage our expertise. Telepsychiatry, for example, is clearly advancing, and brings psychiatry to remote areas where psychiatrists are scarce. Mobile health also has great potential for mental health. As one of us (H.A.N.) highlighted recently,3 as genetics become more molecular, what has been the potential of clinically applicable pharmacogenomics might become reality. Psychiatry needs to make progress toward personalized medicine because the disorders we treat are extremely heterogeneous in their etiology, phenomenology, treatment response, and outcomes.
The appeal of working with mind and brain
The extent to which we can convey unfettered optimism about the role of psychiatry in medicine and the relentless progress in neurobiological research, together, will go a long way toward attracting the best and brightest newly minted physicians to our specialty. The brain is the last frontier in medicine; psychiatry is intimately tethered to its unfolding complexity. With millennials placing a higher premium on work–life issues, the enviable balance and quality of life of a psychiatric career might now be particularly opportune, enhancing the quantity and quality of professionals that we can attract to psychiatry.
1. Bishop TF, Seirup JK, Pincus HA, et al. Population of US practicing psychiatrist declined, 2003-13, which may help explain poor access to mental health care. Health Aff (Millwood). 2016;35(7):1271-1277.
2. Wilbanks L, Spollen J, Messias E. Factors influencing medical school graduates toward a career in psychiatry: analysis from the 2011-2013 Association of American Medical Colleges Graduation Questionnaire. Acad Psychiatry. 2016;40(2):255-260.
3. Nasrallah HA. ‘Druggable’ genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,41.
The dilemma of a diminishing workforce pool might seem more the province of medical school deans, psychiatry department chairs, and psychiatry residency training directors, but our ability to recruit and retain psychiatrists is, in reality, everyone’s concern—including hospitals, clinics, and, especially, patients and their families. Even without knowledge of the specialty or any numerical appraisal, for example, it is common knowledge that we have a dire shortage of child and adolescent and geriatric psychiatrists—a topic of widespread interest and great consequence for access to mental health care.
Tracking a decline
The very title of a recent provocative paper1 in Health Affairs says it all: “Population of US practicing psychiatrists declined 2003-13, which may help explain poor access to mental health care.” In an elegant analysis, the authors expose (1) a 10% decline in the number of psychiatrists for every 100,000 people and (2) wide regional variability in the availability of psychiatrists. In stark contrast, the number of neurologists increased by >15% and the primary care workforce remained stable, with a 1.3% increase in the number of physicians, over the same 10 years.
At the beginning of the psychiatry workforce pipeline, the number of medical students who choose psychiatry remains both small (typically, slightly more than 4% of graduating students) and remarkably stable over time. Wilbanks et al,2 in a thoughtful analysis of the 2011 to 2013 Medical School Graduation Questionnaire of the Association of American Medical Colleges, affirm and, in part, explain this consistent pattern. They note that the 4 most important considerations among students who select psychiatry are:
- personality fit
- specialty content
- work–life balance
- role model influences.
Some of these considerations also overlap with those of students in other specialties; the authors also note that older medical students and women are more likely to choose psychiatry.
Here is what we must do to erase the shortage
It does appear that, despite scientific advances in brain and behavior, expanding therapeutic options, and unique patient interactions that, taken together, should make a career in psychiatry exciting and appealing, there are simply not enough of us to meet the population’s mental health needs. This is a serious problem. It is our professional obligation—all of us—that we take on this shortage and develop solutions to it.
At its zenith, only about 7% of medical students chose psychiatry. We need to proactively prime the pump for our specialty by encouraging more observerships and promoting mental health careers through community outreach to high school students.
We must be diligent and effective mentors to medical students; mentorship is a powerful catalyst for career decision-making.
We need to make psychiatry clerkships exciting, to show off the best of what our specialty has to offer, and to cultivate sustained interest among our students in the brain and its psychiatric disorders.
We need to highlight the momentous advances in knowledge, biology, and treatments that now characterize our psychiatric profession. We need to advocate for more of these accomplishments.
We must be public stigma-busters! (Our patients need us to do this, too.)
And there is more to do:
Collaborate. In delivering psychiatric health care, we need to expand our effectiveness to achieve more collaboration, greater extension of effect, and broader outreach. Collaborative care has come of age as a delivery model; it should be embraced more broadly. We need to continue our efforts to bridge the many sister mental health disciplines—psychology, nursing, social work, counseling—that collectively provide mental health care.
Unite. Given the inadequate workforce numbers and enormous need, we will diminish ourselves by “guild infighting” and, consequently, weaken our legislative advocacy and leverage. We need to embrace and support all medical specialties and have them support us as well. We need to grow closer to primary care and support this specialty as the true front line of mental health. We also need to bridge the gap between addiction medicine and psychiatry, especially given the high level of addiction comorbidity in many psychiatric disorders.
Foster innovation. The deficit of psychiatric workers might be buffered by innovations in how we leverage our expertise. Telepsychiatry, for example, is clearly advancing, and brings psychiatry to remote areas where psychiatrists are scarce. Mobile health also has great potential for mental health. As one of us (H.A.N.) highlighted recently,3 as genetics become more molecular, what has been the potential of clinically applicable pharmacogenomics might become reality. Psychiatry needs to make progress toward personalized medicine because the disorders we treat are extremely heterogeneous in their etiology, phenomenology, treatment response, and outcomes.
The appeal of working with mind and brain
The extent to which we can convey unfettered optimism about the role of psychiatry in medicine and the relentless progress in neurobiological research, together, will go a long way toward attracting the best and brightest newly minted physicians to our specialty. The brain is the last frontier in medicine; psychiatry is intimately tethered to its unfolding complexity. With millennials placing a higher premium on work–life issues, the enviable balance and quality of life of a psychiatric career might now be particularly opportune, enhancing the quantity and quality of professionals that we can attract to psychiatry.
The dilemma of a diminishing workforce pool might seem more the province of medical school deans, psychiatry department chairs, and psychiatry residency training directors, but our ability to recruit and retain psychiatrists is, in reality, everyone’s concern—including hospitals, clinics, and, especially, patients and their families. Even without knowledge of the specialty or any numerical appraisal, for example, it is common knowledge that we have a dire shortage of child and adolescent and geriatric psychiatrists—a topic of widespread interest and great consequence for access to mental health care.
Tracking a decline
The very title of a recent provocative paper1 in Health Affairs says it all: “Population of US practicing psychiatrists declined 2003-13, which may help explain poor access to mental health care.” In an elegant analysis, the authors expose (1) a 10% decline in the number of psychiatrists for every 100,000 people and (2) wide regional variability in the availability of psychiatrists. In stark contrast, the number of neurologists increased by >15% and the primary care workforce remained stable, with a 1.3% increase in the number of physicians, over the same 10 years.
At the beginning of the psychiatry workforce pipeline, the number of medical students who choose psychiatry remains both small (typically, slightly more than 4% of graduating students) and remarkably stable over time. Wilbanks et al,2 in a thoughtful analysis of the 2011 to 2013 Medical School Graduation Questionnaire of the Association of American Medical Colleges, affirm and, in part, explain this consistent pattern. They note that the 4 most important considerations among students who select psychiatry are:
- personality fit
- specialty content
- work–life balance
- role model influences.
Some of these considerations also overlap with those of students in other specialties; the authors also note that older medical students and women are more likely to choose psychiatry.
Here is what we must do to erase the shortage
It does appear that, despite scientific advances in brain and behavior, expanding therapeutic options, and unique patient interactions that, taken together, should make a career in psychiatry exciting and appealing, there are simply not enough of us to meet the population’s mental health needs. This is a serious problem. It is our professional obligation—all of us—that we take on this shortage and develop solutions to it.
At its zenith, only about 7% of medical students chose psychiatry. We need to proactively prime the pump for our specialty by encouraging more observerships and promoting mental health careers through community outreach to high school students.
We must be diligent and effective mentors to medical students; mentorship is a powerful catalyst for career decision-making.
We need to make psychiatry clerkships exciting, to show off the best of what our specialty has to offer, and to cultivate sustained interest among our students in the brain and its psychiatric disorders.
We need to highlight the momentous advances in knowledge, biology, and treatments that now characterize our psychiatric profession. We need to advocate for more of these accomplishments.
We must be public stigma-busters! (Our patients need us to do this, too.)
And there is more to do:
Collaborate. In delivering psychiatric health care, we need to expand our effectiveness to achieve more collaboration, greater extension of effect, and broader outreach. Collaborative care has come of age as a delivery model; it should be embraced more broadly. We need to continue our efforts to bridge the many sister mental health disciplines—psychology, nursing, social work, counseling—that collectively provide mental health care.
Unite. Given the inadequate workforce numbers and enormous need, we will diminish ourselves by “guild infighting” and, consequently, weaken our legislative advocacy and leverage. We need to embrace and support all medical specialties and have them support us as well. We need to grow closer to primary care and support this specialty as the true front line of mental health. We also need to bridge the gap between addiction medicine and psychiatry, especially given the high level of addiction comorbidity in many psychiatric disorders.
Foster innovation. The deficit of psychiatric workers might be buffered by innovations in how we leverage our expertise. Telepsychiatry, for example, is clearly advancing, and brings psychiatry to remote areas where psychiatrists are scarce. Mobile health also has great potential for mental health. As one of us (H.A.N.) highlighted recently,3 as genetics become more molecular, what has been the potential of clinically applicable pharmacogenomics might become reality. Psychiatry needs to make progress toward personalized medicine because the disorders we treat are extremely heterogeneous in their etiology, phenomenology, treatment response, and outcomes.
The appeal of working with mind and brain
The extent to which we can convey unfettered optimism about the role of psychiatry in medicine and the relentless progress in neurobiological research, together, will go a long way toward attracting the best and brightest newly minted physicians to our specialty. The brain is the last frontier in medicine; psychiatry is intimately tethered to its unfolding complexity. With millennials placing a higher premium on work–life issues, the enviable balance and quality of life of a psychiatric career might now be particularly opportune, enhancing the quantity and quality of professionals that we can attract to psychiatry.
1. Bishop TF, Seirup JK, Pincus HA, et al. Population of US practicing psychiatrist declined, 2003-13, which may help explain poor access to mental health care. Health Aff (Millwood). 2016;35(7):1271-1277.
2. Wilbanks L, Spollen J, Messias E. Factors influencing medical school graduates toward a career in psychiatry: analysis from the 2011-2013 Association of American Medical Colleges Graduation Questionnaire. Acad Psychiatry. 2016;40(2):255-260.
3. Nasrallah HA. ‘Druggable’ genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,41.
1. Bishop TF, Seirup JK, Pincus HA, et al. Population of US practicing psychiatrist declined, 2003-13, which may help explain poor access to mental health care. Health Aff (Millwood). 2016;35(7):1271-1277.
2. Wilbanks L, Spollen J, Messias E. Factors influencing medical school graduates toward a career in psychiatry: analysis from the 2011-2013 Association of American Medical Colleges Graduation Questionnaire. Acad Psychiatry. 2016;40(2):255-260.
3. Nasrallah HA. ‘Druggable’ genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,41.