User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Pregnant and catatonic
CASE: Mute and unresponsive
Mrs. K, a 42-year-old Haitian who is 31 weeks pregnant, presents with a 4-week history of progressive mutism and psychomotor retardation. At inpatient admission, she is awake and alert but does not speak and resists the treatment team’s attempts to engage her. Mrs. K’s eyes are open, but she has a vacant stare and avoids eye contact. Her affect is flat and nonreactive and she appears internally preoccupied. Mrs. K exhibits motoric immobility, displays a rigid posture, and resists attempts to get her to move. Features of catatonic excitement, echo phenomena, posturing, stereotypies, and mannerisms are absent during the initial evaluation.
Mrs. K’s husband reports that they had been on vacation for 6 days before he brought her for psychiatric evaluation. He denies any recent evidence of psychosis or mood disturbance, stating that his wife was excited when she learned of the pregnancy, and attended all prenatal appointments. He reports that when this episode began, Mrs. K stopped talking to her 3-year-old daughter, did not respond to her name, and did not pay attention to those around her.
According to her husband, a similar episode occurred 2 years earlier, during which Mrs. K was selectively mute for approximately 1 month. She did not eat for 5 days and neglected the care of her daughter. There were 2 additional brief periods of prominent psychomotor retardation for which she was hospitalized in Haiti. According to the patient’s aunt, Mrs. K complained that her husband had cast a “voodoo spell” on her because he wanted sole custody of their daughter. Her husband recounted an episode, when they lived in Haiti, during which his wife became paranoid, left the house, and wandered the streets for 2 days.
The medical history is significant for a cervical polyp that was removed 2 years ago. Mrs. K has no history of substance abuse. She was born and raised in Haiti where she studied medicine. Her family reports that Mrs. K’s husband is “controlling,” which causes her distress.
a) major depressive disorder, severe with psychosis, with catatonia
b) schizophrenia, with catatonia
c) conversion disorder, with catatonia
d) bipolar I disorder, with psychosis and catatonia
The authors' observations
Catatonia is a neuropsychiatric syndrome that can occur in schizophrenia, mood disorders, mental retardation, neurologic disease, metabolic conditions, and drug intoxication.1 Catatonia can present in several ways, from catatonic stupor to extreme purposeless agitation; more than 60 catatonic signs and symptoms have been described.1 According to DSM-52 catatonia is characterized by 3 or more of the following symptoms:
• stupor
• catalepsy
• waxy flexibility
• mutism
• negativism
• posturing
• mannerism
• stereotypy
• agitation, not influenced by external stimuli
• grimacing
• echolalia
• echopraxia.
Mrs. K exhibited stupor, mutism, posturing, and grimacing (Table 1).2 We thought that her catatonic features were secondary to schizophrenia because she had paranoid delusions and displayed disorganized behavior while in Haiti. There was no evidence of past or current mood disorder, metabolic condition, neurologic illness, or substance abuse.
Catatonia and pregnancy
There is little available information to guide clinicians who are treating a pregnant woman who has a catatonic syndrome. Espinola-Nadurille and co-workers described a 22-year-old pregnant (21 weeks) woman from rural Mexico who was hospitalized with agitation, disorganized speech, restlessness, and hallucinations after several weeks of alternating agitation and withdrawal with mutism and refusal to eat or drink.3 This patient developed malignant catatonia with creatine phosphokinase elevation and leukocytosis and eventually responded to treatment with lorazepam and electroconvulsive therapy (ECT). She was given a diagnosis of schizophreniform disorder. Treating her catatonic symptoms did not result in any adverse effects on the pregnancy or the fetus.
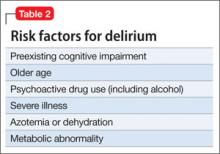
Exacerbation of schizophrenia during pregnancy can lead to neglect of pregnancy and prenatal care,4 imminent harm to the fetus because of malnutrition and dehydration, and risk of preterm delivery and low weight at birth. Prolonged catatonia can cause medical complications such as decubitus ulcers, incontinence, recurrent urinary tract infections, aspiration pneumonia, increased risk of deep venous thrombosis, malnutrition, and ocular complications because of prolonged staring and reduced blinking (Table 25-10). For these reasons, it is important to treat this condition early and aggressively to improve pregnancy outcome and infant well-being.
of Mrs. K’s symptoms?
a) Positive and Negative Symptom Scale
b) The Northoff Catatonia Rating Scale
c) The Bush-Francis Catatonia Rating Scale (BFCRS)
d) The Rogers Catatonia Scale
EVALUATION: Flat affect
The mental status examination on admission describes a tall, black, Haitian woman with unkempt hair and fair hygiene. Mrs. K has a prominent abdomen, consistent with a 31-week pregnancy. She exhibits a blank stare without direct eye contact; she is mute, and exhibits flat affect. We cannot evaluate her thought processes and content because Mrs. K is mute, although she does appear internally preoccupied.
Physical examination on admission is unremarkable; vital signs are stable and within normal limits. Laboratory work-up reveals a urinary tract infection, which is treated with ceftriaxone. Mrs. K also has macrocytic anemia (hemoglobin, 11.7 g/dL; hematocrit, 34.7%; mean corpuscular volume, 99.2 μm3). Albumin is low at 2.6 g/dL. Urine drug toxicology screen is negative. Fingerstick glucose reading is 139 mg/dL. Mrs. K is given a presumptive diagnosis of schizophrenia with catatonia.
Mrs. K’s BFCRS11 score is 22 at admission. She is mute, holds postures for longer than a minute, and is resistant to repositioning. She also has extreme hypoactivity and does not interact with others. She has a fixed, blank stare, and exhibits mild grimacing.
The authors' observations
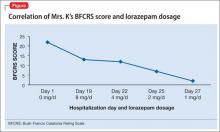
BFCRS defines each catatonic sign, rates its severity, and provides a standardized schema for clinical examination.11 The BFCRS is preferred for routine use because of its validity, reliability, and ease of administration.12 The treatment team rated Mrs. K at admission, during the course of treatment, and at discharge, showing a substantial improvement at the end of the hospitalization (Figure).
a) ECT
b) lorazepam
c) haloperidol
d) olanzapine
The authors' observations
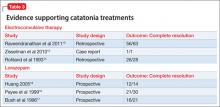
Benzodiazepines (particularly lorazepam) and ECT are considered the treatment of choice for catatonic symptoms.11 More than 72% of patients with catatonic symptoms remit after a trial of a benzodiazepine.1 ECT is considered when patients do not respond to a benzodiazepine after 48 to 72 hours.3 Several cases of complete resolution of catatonic symptoms have been linked to ECT (Table 3).13-18
A recent retrospective review revealed that patients who do not respond to lorazepam are more likely to come from a rural setting, have a longer duration of illness, exhibit mutism, and exhibit third-person auditory hallucinations and made phenomena (the feeling that some aspect of the individual is under the external control of others).19 Case reports of treatment of catatonic patients with ECT vs lorazepam are listed in Table 3.13-18
In pregnancy, ECT can be considered early in the course of illness. A review of the literature on ECT during pregnancy reported at least partial remission in 78% of studies reporting efficacy data.20 Among these 339 patients, there were 25 fetal or neonatal complications—only 11 of these were related to ECT—and 20 maternal complications, of which 18 were related to ECT. The authors of this review concluded that 1) ECT is an effective treatment for severe mental illness during pregnancy and 2) the risks to fetus and mother are low.
A 2007 study identified 1,979 infants whose mothers reported use of benzodiazepines or hypnotic benzodiazepine-receptor agonists during early pregnancy.21 An additional 401 infants born to mothers who were prescribed these medications during late pregnancy also were included in this study. Women who took these medications were at an increased risk for preterm birth and low birth weight. The rate of congenital malformations in this study was moderately increased among infants exposed in early pregnancy (adjusted odds ratio = 1.24 [95% confidence interval, 1.00 to 1.55]).
Because catatonic symptoms can appear during the course of schizophrenia, several antipsychotics have been used to treat this condition. The efficacy and safety of antipsychotics for treating catatonia remains largely unknown, however.1
OUTCOME: Recovery, baby girl
We begin oral haloperidol, 10 mg/d, for Mrs. K, which we then increase to 20 mg/d. Because she shows little response to haloperidol, we suggest a trial of ECT, but her husband refuses to consent. She is started on IM lorazepam, 6 mg/d.
Mrs. K gradually improves and increases her intake of food and liquids. After 10 days of lorazepam treatment, her BFCRS score decreases to 13. Mrs. K begins to speak and her gaze is less fixed. Negativistic behaviors are nearly absent.
Because we are concerned about Mrs. K’s pregnancy, lab tests are repeated. A complete metabolic panel shows an elevated glucose level (122 mg/dL); urinalysis reveals glycosuria (glucose, 1,000 mg/dL), proteinuria (protein, 10 mg/dL), and ketonuria, (ketones, 20 mg/dL). She is transferred to the obstetrics service for evaluation of gestational diabetes.
Psychotropics are continued while Mrs. K is on the obstetrics service; she returns to the inpatient psychiatric unit on an insulin regimen. IM lorazepam is increased to 8 mg/d, and haloperidol is decreased from 20 mg/d, to 10 mg/d, to prevent worsening of catatonia, which can occur when catatonic patients receive a psychotropic.11 Three days later, Mrs. K’s BFCRS score is 12 and she shows only mild rigidity. Mrs. K briefly interacts with staff, particularly when she wants something.
Lorazepam is decreased to 1 mg/d in anticipation of cesarean delivery. Mrs. K becomes more adherent with her medications; often, she takes the oral dose of haloperidol, rather than the IM formulation. On mental status examination she exhibits poor eye contact, rather than a fixed gaze, and her BFCRS score decreases to 7 by day 25.
By the end of lorazepam treatment, Mrs. K has fully recovered from her catatonic state. She interacts with staff, engages with the treatment team, and is excited to go home. At discharge, she is given a diagnosis of schizophrenia with catatonia, and is taking haloperidol, 5 mg, twice a day. She gives birth to a healthy girl.
The authors' observations
Mrs. K was treated initially with haloperidol for several reasons. Haloperidol is relatively safe during pregnancy (FDA pregnancy category C) as shown by a recent multicenter, prospective, cohort study in which babies exposed in utero to haloperidol showed a congenital malformation (limb defect) rate within the expected baseline risk for the general population.22 Lorazepam is FDA category D for use in pregnancy and can cause preterm delivery,23 floppy infant syndrome, and withdrawal syndromes.24 We did not use a second-generation antipsychotic (SGA) because it could have made Mrs. K’s hyperglycemia worse. SGAs can induce gestational diabetes and increase the incidence of large-for-gestational-age newborns, compared with first-generation antipsychotics.24 Last, Mrs. K’s family rejected ECT.
Because of Mrs. K’s poor response to haloperidol, the treatment team decided to start IM lorazepam, which eventually was increased to 8 mg/d. The haloperidol dose was reduced by half to avoid worsening of catatonia and reduce the risk of neuroleptic malignant syndrome.1,25 When clinical response was achieved, lorazepam was tapered and Mrs. K was discharged with only haloperidol.
In the absence of well-designed prospective follow-up studies, information on the potential impact of prenatal exposure to antipsychotics and benzodiazepines on a child’s cognitive development is limited.26 This case adds to the scant literature on the treatment of catatonia during pregnancy and illustrates how the BFCRS can be utilized during serial patient evaluations to monitor clinical improvement.
Bottom Line
Psychosis and catatonia during pregnancy are associated with complications to mother and child. The Bush-Francis Catatonia Rating Scale can be used to identify and track catatonic symptoms. Lorazepam and electroconvulsive therapy have been used safely and with good outcomes in mentally ill pregnant women when used appropriately.
Related Resources
- Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry. 2009;54(7):437-445.
- Seethalakshmi R, Dhavale S, Suggu K, et al. Catatonic syndrome: importance of detection and treatment with lorazepam. Ann Clin Psychiatry. 2008;20(1):5-8.
- Salam S, Kilzieh N. Lorazepam treatment of psychogenic catatonia: an update. J Clin Psychiatry, 1988;49(suppl):16-21.
Drug Brand Names
Ceftriaxone • Rocephin Olanzapine • Zyprexa
Haloperidol • Haldol Short-acting Insulin • Novolin, Humulin
Lorazepam • Ativan
Disclosures
Dr. Runyan receives grant support from Lippincott, Williams, & Wilkins. Drs. Durant, Prudent, and Sotelo report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weder N, Muralee S, Penland H, et al. Catatonia: a review. Ann Clin Psychiatry. 2008;20(2):97-107.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
3. Espinola-Nadurille M, Ramirez-Bermudez J, Fricchione GL. Pregnancy and malignant catatonia. Gen Hosp Psychiatry. 2007;29(1):69-71.
4. Solari H, Dickson KE, Miller L. Understanding and treating women with schizophrenia during pregnancy and postpartum. Can J Clin Pharmacol. 2009;16(1):e23-e32.
5. Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry. 2008;10(12):
153-155.
6. Larsen HH, Ritchie JC, McNutt MD, et al. Pulmonary embolism in a patient with catatonia: an old disease, changing times. Psychosomatics. 2011;52(4):387-391.
7. Lachner C, Sandson NB. Medical complications of catatonia: a case of catatonia-induced deep venous thrombosis. Psychosomatics. 2003;44(6):512-4.
8. Morioka H, Nagatomo I, Yamada K, et al. Deep venous thrombosis of the leg due to psychiatric stupor. Psychiatry Clin Neurosci, 1997;51(5):323-326.
9. Nomoto H, Hatta K, Usui C, et al. Vitamin K deficiency due to prolongation of antibiotic treatment and decrease in food intake in a catatonia patient. Psychosomatics. 2011;52(5):
486-487.
10. Srivastava A, Gupta A, Murthy P, et al. Catatonia and multiple pressure ulcers: a rare complication in psychiatric setting. Indian J Psychiatry. 2009;51(3):206-208.
11. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185.
12. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1):1-9.
13. Zisselman MH, Jaffe RL. ECT in the treatment of a patient with catatonia: consent and complications. Am J Psychiatry. 2010;167(2):127-132.
14. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
15. Raveendranathan D, Narayanaswamy JC, Reddi SV. Response rate of catatonia to electroconvulsive therapy and its clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):425-430.
16. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to lorazepam. Indian J Psychiatry. 1999; 41(1):49-53.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Huang TL. Lorazepam and diazepam rapidly relieve catatonic signs in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(1):52-55.
19. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34:312-316.
20. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71:
235-242.
21. Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:
1203-1210.
22. Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:
518-544.
23. Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201:579e1-8.
24. Howland RH. Prescribing psychotropic medications during pregnancy and lactation: principles and guidelines. J Psychosoc Nurs Ment Health Serv. 2009;47(5):19-23.
25. White DA, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry. 1991; 158:419-421.
26. Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010; 27(7):675-686.
CASE: Mute and unresponsive
Mrs. K, a 42-year-old Haitian who is 31 weeks pregnant, presents with a 4-week history of progressive mutism and psychomotor retardation. At inpatient admission, she is awake and alert but does not speak and resists the treatment team’s attempts to engage her. Mrs. K’s eyes are open, but she has a vacant stare and avoids eye contact. Her affect is flat and nonreactive and she appears internally preoccupied. Mrs. K exhibits motoric immobility, displays a rigid posture, and resists attempts to get her to move. Features of catatonic excitement, echo phenomena, posturing, stereotypies, and mannerisms are absent during the initial evaluation.
Mrs. K’s husband reports that they had been on vacation for 6 days before he brought her for psychiatric evaluation. He denies any recent evidence of psychosis or mood disturbance, stating that his wife was excited when she learned of the pregnancy, and attended all prenatal appointments. He reports that when this episode began, Mrs. K stopped talking to her 3-year-old daughter, did not respond to her name, and did not pay attention to those around her.
According to her husband, a similar episode occurred 2 years earlier, during which Mrs. K was selectively mute for approximately 1 month. She did not eat for 5 days and neglected the care of her daughter. There were 2 additional brief periods of prominent psychomotor retardation for which she was hospitalized in Haiti. According to the patient’s aunt, Mrs. K complained that her husband had cast a “voodoo spell” on her because he wanted sole custody of their daughter. Her husband recounted an episode, when they lived in Haiti, during which his wife became paranoid, left the house, and wandered the streets for 2 days.
The medical history is significant for a cervical polyp that was removed 2 years ago. Mrs. K has no history of substance abuse. She was born and raised in Haiti where she studied medicine. Her family reports that Mrs. K’s husband is “controlling,” which causes her distress.
a) major depressive disorder, severe with psychosis, with catatonia
b) schizophrenia, with catatonia
c) conversion disorder, with catatonia
d) bipolar I disorder, with psychosis and catatonia
The authors' observations
Catatonia is a neuropsychiatric syndrome that can occur in schizophrenia, mood disorders, mental retardation, neurologic disease, metabolic conditions, and drug intoxication.1 Catatonia can present in several ways, from catatonic stupor to extreme purposeless agitation; more than 60 catatonic signs and symptoms have been described.1 According to DSM-52 catatonia is characterized by 3 or more of the following symptoms:
• stupor
• catalepsy
• waxy flexibility
• mutism
• negativism
• posturing
• mannerism
• stereotypy
• agitation, not influenced by external stimuli
• grimacing
• echolalia
• echopraxia.
Mrs. K exhibited stupor, mutism, posturing, and grimacing (Table 1).2 We thought that her catatonic features were secondary to schizophrenia because she had paranoid delusions and displayed disorganized behavior while in Haiti. There was no evidence of past or current mood disorder, metabolic condition, neurologic illness, or substance abuse.
Catatonia and pregnancy
There is little available information to guide clinicians who are treating a pregnant woman who has a catatonic syndrome. Espinola-Nadurille and co-workers described a 22-year-old pregnant (21 weeks) woman from rural Mexico who was hospitalized with agitation, disorganized speech, restlessness, and hallucinations after several weeks of alternating agitation and withdrawal with mutism and refusal to eat or drink.3 This patient developed malignant catatonia with creatine phosphokinase elevation and leukocytosis and eventually responded to treatment with lorazepam and electroconvulsive therapy (ECT). She was given a diagnosis of schizophreniform disorder. Treating her catatonic symptoms did not result in any adverse effects on the pregnancy or the fetus.
Exacerbation of schizophrenia during pregnancy can lead to neglect of pregnancy and prenatal care,4 imminent harm to the fetus because of malnutrition and dehydration, and risk of preterm delivery and low weight at birth. Prolonged catatonia can cause medical complications such as decubitus ulcers, incontinence, recurrent urinary tract infections, aspiration pneumonia, increased risk of deep venous thrombosis, malnutrition, and ocular complications because of prolonged staring and reduced blinking (Table 25-10). For these reasons, it is important to treat this condition early and aggressively to improve pregnancy outcome and infant well-being.
of Mrs. K’s symptoms?
a) Positive and Negative Symptom Scale
b) The Northoff Catatonia Rating Scale
c) The Bush-Francis Catatonia Rating Scale (BFCRS)
d) The Rogers Catatonia Scale
EVALUATION: Flat affect
The mental status examination on admission describes a tall, black, Haitian woman with unkempt hair and fair hygiene. Mrs. K has a prominent abdomen, consistent with a 31-week pregnancy. She exhibits a blank stare without direct eye contact; she is mute, and exhibits flat affect. We cannot evaluate her thought processes and content because Mrs. K is mute, although she does appear internally preoccupied.
Physical examination on admission is unremarkable; vital signs are stable and within normal limits. Laboratory work-up reveals a urinary tract infection, which is treated with ceftriaxone. Mrs. K also has macrocytic anemia (hemoglobin, 11.7 g/dL; hematocrit, 34.7%; mean corpuscular volume, 99.2 μm3). Albumin is low at 2.6 g/dL. Urine drug toxicology screen is negative. Fingerstick glucose reading is 139 mg/dL. Mrs. K is given a presumptive diagnosis of schizophrenia with catatonia.
Mrs. K’s BFCRS11 score is 22 at admission. She is mute, holds postures for longer than a minute, and is resistant to repositioning. She also has extreme hypoactivity and does not interact with others. She has a fixed, blank stare, and exhibits mild grimacing.
The authors' observations
BFCRS defines each catatonic sign, rates its severity, and provides a standardized schema for clinical examination.11 The BFCRS is preferred for routine use because of its validity, reliability, and ease of administration.12 The treatment team rated Mrs. K at admission, during the course of treatment, and at discharge, showing a substantial improvement at the end of the hospitalization (Figure).
a) ECT
b) lorazepam
c) haloperidol
d) olanzapine
The authors' observations
Benzodiazepines (particularly lorazepam) and ECT are considered the treatment of choice for catatonic symptoms.11 More than 72% of patients with catatonic symptoms remit after a trial of a benzodiazepine.1 ECT is considered when patients do not respond to a benzodiazepine after 48 to 72 hours.3 Several cases of complete resolution of catatonic symptoms have been linked to ECT (Table 3).13-18
A recent retrospective review revealed that patients who do not respond to lorazepam are more likely to come from a rural setting, have a longer duration of illness, exhibit mutism, and exhibit third-person auditory hallucinations and made phenomena (the feeling that some aspect of the individual is under the external control of others).19 Case reports of treatment of catatonic patients with ECT vs lorazepam are listed in Table 3.13-18
In pregnancy, ECT can be considered early in the course of illness. A review of the literature on ECT during pregnancy reported at least partial remission in 78% of studies reporting efficacy data.20 Among these 339 patients, there were 25 fetal or neonatal complications—only 11 of these were related to ECT—and 20 maternal complications, of which 18 were related to ECT. The authors of this review concluded that 1) ECT is an effective treatment for severe mental illness during pregnancy and 2) the risks to fetus and mother are low.
A 2007 study identified 1,979 infants whose mothers reported use of benzodiazepines or hypnotic benzodiazepine-receptor agonists during early pregnancy.21 An additional 401 infants born to mothers who were prescribed these medications during late pregnancy also were included in this study. Women who took these medications were at an increased risk for preterm birth and low birth weight. The rate of congenital malformations in this study was moderately increased among infants exposed in early pregnancy (adjusted odds ratio = 1.24 [95% confidence interval, 1.00 to 1.55]).
Because catatonic symptoms can appear during the course of schizophrenia, several antipsychotics have been used to treat this condition. The efficacy and safety of antipsychotics for treating catatonia remains largely unknown, however.1
OUTCOME: Recovery, baby girl
We begin oral haloperidol, 10 mg/d, for Mrs. K, which we then increase to 20 mg/d. Because she shows little response to haloperidol, we suggest a trial of ECT, but her husband refuses to consent. She is started on IM lorazepam, 6 mg/d.
Mrs. K gradually improves and increases her intake of food and liquids. After 10 days of lorazepam treatment, her BFCRS score decreases to 13. Mrs. K begins to speak and her gaze is less fixed. Negativistic behaviors are nearly absent.
Because we are concerned about Mrs. K’s pregnancy, lab tests are repeated. A complete metabolic panel shows an elevated glucose level (122 mg/dL); urinalysis reveals glycosuria (glucose, 1,000 mg/dL), proteinuria (protein, 10 mg/dL), and ketonuria, (ketones, 20 mg/dL). She is transferred to the obstetrics service for evaluation of gestational diabetes.
Psychotropics are continued while Mrs. K is on the obstetrics service; she returns to the inpatient psychiatric unit on an insulin regimen. IM lorazepam is increased to 8 mg/d, and haloperidol is decreased from 20 mg/d, to 10 mg/d, to prevent worsening of catatonia, which can occur when catatonic patients receive a psychotropic.11 Three days later, Mrs. K’s BFCRS score is 12 and she shows only mild rigidity. Mrs. K briefly interacts with staff, particularly when she wants something.
Lorazepam is decreased to 1 mg/d in anticipation of cesarean delivery. Mrs. K becomes more adherent with her medications; often, she takes the oral dose of haloperidol, rather than the IM formulation. On mental status examination she exhibits poor eye contact, rather than a fixed gaze, and her BFCRS score decreases to 7 by day 25.
By the end of lorazepam treatment, Mrs. K has fully recovered from her catatonic state. She interacts with staff, engages with the treatment team, and is excited to go home. At discharge, she is given a diagnosis of schizophrenia with catatonia, and is taking haloperidol, 5 mg, twice a day. She gives birth to a healthy girl.
The authors' observations
Mrs. K was treated initially with haloperidol for several reasons. Haloperidol is relatively safe during pregnancy (FDA pregnancy category C) as shown by a recent multicenter, prospective, cohort study in which babies exposed in utero to haloperidol showed a congenital malformation (limb defect) rate within the expected baseline risk for the general population.22 Lorazepam is FDA category D for use in pregnancy and can cause preterm delivery,23 floppy infant syndrome, and withdrawal syndromes.24 We did not use a second-generation antipsychotic (SGA) because it could have made Mrs. K’s hyperglycemia worse. SGAs can induce gestational diabetes and increase the incidence of large-for-gestational-age newborns, compared with first-generation antipsychotics.24 Last, Mrs. K’s family rejected ECT.
Because of Mrs. K’s poor response to haloperidol, the treatment team decided to start IM lorazepam, which eventually was increased to 8 mg/d. The haloperidol dose was reduced by half to avoid worsening of catatonia and reduce the risk of neuroleptic malignant syndrome.1,25 When clinical response was achieved, lorazepam was tapered and Mrs. K was discharged with only haloperidol.
In the absence of well-designed prospective follow-up studies, information on the potential impact of prenatal exposure to antipsychotics and benzodiazepines on a child’s cognitive development is limited.26 This case adds to the scant literature on the treatment of catatonia during pregnancy and illustrates how the BFCRS can be utilized during serial patient evaluations to monitor clinical improvement.
Bottom Line
Psychosis and catatonia during pregnancy are associated with complications to mother and child. The Bush-Francis Catatonia Rating Scale can be used to identify and track catatonic symptoms. Lorazepam and electroconvulsive therapy have been used safely and with good outcomes in mentally ill pregnant women when used appropriately.
Related Resources
- Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry. 2009;54(7):437-445.
- Seethalakshmi R, Dhavale S, Suggu K, et al. Catatonic syndrome: importance of detection and treatment with lorazepam. Ann Clin Psychiatry. 2008;20(1):5-8.
- Salam S, Kilzieh N. Lorazepam treatment of psychogenic catatonia: an update. J Clin Psychiatry, 1988;49(suppl):16-21.
Drug Brand Names
Ceftriaxone • Rocephin Olanzapine • Zyprexa
Haloperidol • Haldol Short-acting Insulin • Novolin, Humulin
Lorazepam • Ativan
Disclosures
Dr. Runyan receives grant support from Lippincott, Williams, & Wilkins. Drs. Durant, Prudent, and Sotelo report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Mute and unresponsive
Mrs. K, a 42-year-old Haitian who is 31 weeks pregnant, presents with a 4-week history of progressive mutism and psychomotor retardation. At inpatient admission, she is awake and alert but does not speak and resists the treatment team’s attempts to engage her. Mrs. K’s eyes are open, but she has a vacant stare and avoids eye contact. Her affect is flat and nonreactive and she appears internally preoccupied. Mrs. K exhibits motoric immobility, displays a rigid posture, and resists attempts to get her to move. Features of catatonic excitement, echo phenomena, posturing, stereotypies, and mannerisms are absent during the initial evaluation.
Mrs. K’s husband reports that they had been on vacation for 6 days before he brought her for psychiatric evaluation. He denies any recent evidence of psychosis or mood disturbance, stating that his wife was excited when she learned of the pregnancy, and attended all prenatal appointments. He reports that when this episode began, Mrs. K stopped talking to her 3-year-old daughter, did not respond to her name, and did not pay attention to those around her.
According to her husband, a similar episode occurred 2 years earlier, during which Mrs. K was selectively mute for approximately 1 month. She did not eat for 5 days and neglected the care of her daughter. There were 2 additional brief periods of prominent psychomotor retardation for which she was hospitalized in Haiti. According to the patient’s aunt, Mrs. K complained that her husband had cast a “voodoo spell” on her because he wanted sole custody of their daughter. Her husband recounted an episode, when they lived in Haiti, during which his wife became paranoid, left the house, and wandered the streets for 2 days.
The medical history is significant for a cervical polyp that was removed 2 years ago. Mrs. K has no history of substance abuse. She was born and raised in Haiti where she studied medicine. Her family reports that Mrs. K’s husband is “controlling,” which causes her distress.
a) major depressive disorder, severe with psychosis, with catatonia
b) schizophrenia, with catatonia
c) conversion disorder, with catatonia
d) bipolar I disorder, with psychosis and catatonia
The authors' observations
Catatonia is a neuropsychiatric syndrome that can occur in schizophrenia, mood disorders, mental retardation, neurologic disease, metabolic conditions, and drug intoxication.1 Catatonia can present in several ways, from catatonic stupor to extreme purposeless agitation; more than 60 catatonic signs and symptoms have been described.1 According to DSM-52 catatonia is characterized by 3 or more of the following symptoms:
• stupor
• catalepsy
• waxy flexibility
• mutism
• negativism
• posturing
• mannerism
• stereotypy
• agitation, not influenced by external stimuli
• grimacing
• echolalia
• echopraxia.
Mrs. K exhibited stupor, mutism, posturing, and grimacing (Table 1).2 We thought that her catatonic features were secondary to schizophrenia because she had paranoid delusions and displayed disorganized behavior while in Haiti. There was no evidence of past or current mood disorder, metabolic condition, neurologic illness, or substance abuse.
Catatonia and pregnancy
There is little available information to guide clinicians who are treating a pregnant woman who has a catatonic syndrome. Espinola-Nadurille and co-workers described a 22-year-old pregnant (21 weeks) woman from rural Mexico who was hospitalized with agitation, disorganized speech, restlessness, and hallucinations after several weeks of alternating agitation and withdrawal with mutism and refusal to eat or drink.3 This patient developed malignant catatonia with creatine phosphokinase elevation and leukocytosis and eventually responded to treatment with lorazepam and electroconvulsive therapy (ECT). She was given a diagnosis of schizophreniform disorder. Treating her catatonic symptoms did not result in any adverse effects on the pregnancy or the fetus.
Exacerbation of schizophrenia during pregnancy can lead to neglect of pregnancy and prenatal care,4 imminent harm to the fetus because of malnutrition and dehydration, and risk of preterm delivery and low weight at birth. Prolonged catatonia can cause medical complications such as decubitus ulcers, incontinence, recurrent urinary tract infections, aspiration pneumonia, increased risk of deep venous thrombosis, malnutrition, and ocular complications because of prolonged staring and reduced blinking (Table 25-10). For these reasons, it is important to treat this condition early and aggressively to improve pregnancy outcome and infant well-being.
of Mrs. K’s symptoms?
a) Positive and Negative Symptom Scale
b) The Northoff Catatonia Rating Scale
c) The Bush-Francis Catatonia Rating Scale (BFCRS)
d) The Rogers Catatonia Scale
EVALUATION: Flat affect
The mental status examination on admission describes a tall, black, Haitian woman with unkempt hair and fair hygiene. Mrs. K has a prominent abdomen, consistent with a 31-week pregnancy. She exhibits a blank stare without direct eye contact; she is mute, and exhibits flat affect. We cannot evaluate her thought processes and content because Mrs. K is mute, although she does appear internally preoccupied.
Physical examination on admission is unremarkable; vital signs are stable and within normal limits. Laboratory work-up reveals a urinary tract infection, which is treated with ceftriaxone. Mrs. K also has macrocytic anemia (hemoglobin, 11.7 g/dL; hematocrit, 34.7%; mean corpuscular volume, 99.2 μm3). Albumin is low at 2.6 g/dL. Urine drug toxicology screen is negative. Fingerstick glucose reading is 139 mg/dL. Mrs. K is given a presumptive diagnosis of schizophrenia with catatonia.
Mrs. K’s BFCRS11 score is 22 at admission. She is mute, holds postures for longer than a minute, and is resistant to repositioning. She also has extreme hypoactivity and does not interact with others. She has a fixed, blank stare, and exhibits mild grimacing.
The authors' observations
BFCRS defines each catatonic sign, rates its severity, and provides a standardized schema for clinical examination.11 The BFCRS is preferred for routine use because of its validity, reliability, and ease of administration.12 The treatment team rated Mrs. K at admission, during the course of treatment, and at discharge, showing a substantial improvement at the end of the hospitalization (Figure).
a) ECT
b) lorazepam
c) haloperidol
d) olanzapine
The authors' observations
Benzodiazepines (particularly lorazepam) and ECT are considered the treatment of choice for catatonic symptoms.11 More than 72% of patients with catatonic symptoms remit after a trial of a benzodiazepine.1 ECT is considered when patients do not respond to a benzodiazepine after 48 to 72 hours.3 Several cases of complete resolution of catatonic symptoms have been linked to ECT (Table 3).13-18
A recent retrospective review revealed that patients who do not respond to lorazepam are more likely to come from a rural setting, have a longer duration of illness, exhibit mutism, and exhibit third-person auditory hallucinations and made phenomena (the feeling that some aspect of the individual is under the external control of others).19 Case reports of treatment of catatonic patients with ECT vs lorazepam are listed in Table 3.13-18
In pregnancy, ECT can be considered early in the course of illness. A review of the literature on ECT during pregnancy reported at least partial remission in 78% of studies reporting efficacy data.20 Among these 339 patients, there were 25 fetal or neonatal complications—only 11 of these were related to ECT—and 20 maternal complications, of which 18 were related to ECT. The authors of this review concluded that 1) ECT is an effective treatment for severe mental illness during pregnancy and 2) the risks to fetus and mother are low.
A 2007 study identified 1,979 infants whose mothers reported use of benzodiazepines or hypnotic benzodiazepine-receptor agonists during early pregnancy.21 An additional 401 infants born to mothers who were prescribed these medications during late pregnancy also were included in this study. Women who took these medications were at an increased risk for preterm birth and low birth weight. The rate of congenital malformations in this study was moderately increased among infants exposed in early pregnancy (adjusted odds ratio = 1.24 [95% confidence interval, 1.00 to 1.55]).
Because catatonic symptoms can appear during the course of schizophrenia, several antipsychotics have been used to treat this condition. The efficacy and safety of antipsychotics for treating catatonia remains largely unknown, however.1
OUTCOME: Recovery, baby girl
We begin oral haloperidol, 10 mg/d, for Mrs. K, which we then increase to 20 mg/d. Because she shows little response to haloperidol, we suggest a trial of ECT, but her husband refuses to consent. She is started on IM lorazepam, 6 mg/d.
Mrs. K gradually improves and increases her intake of food and liquids. After 10 days of lorazepam treatment, her BFCRS score decreases to 13. Mrs. K begins to speak and her gaze is less fixed. Negativistic behaviors are nearly absent.
Because we are concerned about Mrs. K’s pregnancy, lab tests are repeated. A complete metabolic panel shows an elevated glucose level (122 mg/dL); urinalysis reveals glycosuria (glucose, 1,000 mg/dL), proteinuria (protein, 10 mg/dL), and ketonuria, (ketones, 20 mg/dL). She is transferred to the obstetrics service for evaluation of gestational diabetes.
Psychotropics are continued while Mrs. K is on the obstetrics service; she returns to the inpatient psychiatric unit on an insulin regimen. IM lorazepam is increased to 8 mg/d, and haloperidol is decreased from 20 mg/d, to 10 mg/d, to prevent worsening of catatonia, which can occur when catatonic patients receive a psychotropic.11 Three days later, Mrs. K’s BFCRS score is 12 and she shows only mild rigidity. Mrs. K briefly interacts with staff, particularly when she wants something.
Lorazepam is decreased to 1 mg/d in anticipation of cesarean delivery. Mrs. K becomes more adherent with her medications; often, she takes the oral dose of haloperidol, rather than the IM formulation. On mental status examination she exhibits poor eye contact, rather than a fixed gaze, and her BFCRS score decreases to 7 by day 25.
By the end of lorazepam treatment, Mrs. K has fully recovered from her catatonic state. She interacts with staff, engages with the treatment team, and is excited to go home. At discharge, she is given a diagnosis of schizophrenia with catatonia, and is taking haloperidol, 5 mg, twice a day. She gives birth to a healthy girl.
The authors' observations
Mrs. K was treated initially with haloperidol for several reasons. Haloperidol is relatively safe during pregnancy (FDA pregnancy category C) as shown by a recent multicenter, prospective, cohort study in which babies exposed in utero to haloperidol showed a congenital malformation (limb defect) rate within the expected baseline risk for the general population.22 Lorazepam is FDA category D for use in pregnancy and can cause preterm delivery,23 floppy infant syndrome, and withdrawal syndromes.24 We did not use a second-generation antipsychotic (SGA) because it could have made Mrs. K’s hyperglycemia worse. SGAs can induce gestational diabetes and increase the incidence of large-for-gestational-age newborns, compared with first-generation antipsychotics.24 Last, Mrs. K’s family rejected ECT.
Because of Mrs. K’s poor response to haloperidol, the treatment team decided to start IM lorazepam, which eventually was increased to 8 mg/d. The haloperidol dose was reduced by half to avoid worsening of catatonia and reduce the risk of neuroleptic malignant syndrome.1,25 When clinical response was achieved, lorazepam was tapered and Mrs. K was discharged with only haloperidol.
In the absence of well-designed prospective follow-up studies, information on the potential impact of prenatal exposure to antipsychotics and benzodiazepines on a child’s cognitive development is limited.26 This case adds to the scant literature on the treatment of catatonia during pregnancy and illustrates how the BFCRS can be utilized during serial patient evaluations to monitor clinical improvement.
Bottom Line
Psychosis and catatonia during pregnancy are associated with complications to mother and child. The Bush-Francis Catatonia Rating Scale can be used to identify and track catatonic symptoms. Lorazepam and electroconvulsive therapy have been used safely and with good outcomes in mentally ill pregnant women when used appropriately.
Related Resources
- Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry. 2009;54(7):437-445.
- Seethalakshmi R, Dhavale S, Suggu K, et al. Catatonic syndrome: importance of detection and treatment with lorazepam. Ann Clin Psychiatry. 2008;20(1):5-8.
- Salam S, Kilzieh N. Lorazepam treatment of psychogenic catatonia: an update. J Clin Psychiatry, 1988;49(suppl):16-21.
Drug Brand Names
Ceftriaxone • Rocephin Olanzapine • Zyprexa
Haloperidol • Haldol Short-acting Insulin • Novolin, Humulin
Lorazepam • Ativan
Disclosures
Dr. Runyan receives grant support from Lippincott, Williams, & Wilkins. Drs. Durant, Prudent, and Sotelo report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weder N, Muralee S, Penland H, et al. Catatonia: a review. Ann Clin Psychiatry. 2008;20(2):97-107.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
3. Espinola-Nadurille M, Ramirez-Bermudez J, Fricchione GL. Pregnancy and malignant catatonia. Gen Hosp Psychiatry. 2007;29(1):69-71.
4. Solari H, Dickson KE, Miller L. Understanding and treating women with schizophrenia during pregnancy and postpartum. Can J Clin Pharmacol. 2009;16(1):e23-e32.
5. Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry. 2008;10(12):
153-155.
6. Larsen HH, Ritchie JC, McNutt MD, et al. Pulmonary embolism in a patient with catatonia: an old disease, changing times. Psychosomatics. 2011;52(4):387-391.
7. Lachner C, Sandson NB. Medical complications of catatonia: a case of catatonia-induced deep venous thrombosis. Psychosomatics. 2003;44(6):512-4.
8. Morioka H, Nagatomo I, Yamada K, et al. Deep venous thrombosis of the leg due to psychiatric stupor. Psychiatry Clin Neurosci, 1997;51(5):323-326.
9. Nomoto H, Hatta K, Usui C, et al. Vitamin K deficiency due to prolongation of antibiotic treatment and decrease in food intake in a catatonia patient. Psychosomatics. 2011;52(5):
486-487.
10. Srivastava A, Gupta A, Murthy P, et al. Catatonia and multiple pressure ulcers: a rare complication in psychiatric setting. Indian J Psychiatry. 2009;51(3):206-208.
11. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185.
12. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1):1-9.
13. Zisselman MH, Jaffe RL. ECT in the treatment of a patient with catatonia: consent and complications. Am J Psychiatry. 2010;167(2):127-132.
14. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
15. Raveendranathan D, Narayanaswamy JC, Reddi SV. Response rate of catatonia to electroconvulsive therapy and its clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):425-430.
16. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to lorazepam. Indian J Psychiatry. 1999; 41(1):49-53.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Huang TL. Lorazepam and diazepam rapidly relieve catatonic signs in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(1):52-55.
19. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34:312-316.
20. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71:
235-242.
21. Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:
1203-1210.
22. Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:
518-544.
23. Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201:579e1-8.
24. Howland RH. Prescribing psychotropic medications during pregnancy and lactation: principles and guidelines. J Psychosoc Nurs Ment Health Serv. 2009;47(5):19-23.
25. White DA, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry. 1991; 158:419-421.
26. Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010; 27(7):675-686.
1. Weder N, Muralee S, Penland H, et al. Catatonia: a review. Ann Clin Psychiatry. 2008;20(2):97-107.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
3. Espinola-Nadurille M, Ramirez-Bermudez J, Fricchione GL. Pregnancy and malignant catatonia. Gen Hosp Psychiatry. 2007;29(1):69-71.
4. Solari H, Dickson KE, Miller L. Understanding and treating women with schizophrenia during pregnancy and postpartum. Can J Clin Pharmacol. 2009;16(1):e23-e32.
5. Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry. 2008;10(12):
153-155.
6. Larsen HH, Ritchie JC, McNutt MD, et al. Pulmonary embolism in a patient with catatonia: an old disease, changing times. Psychosomatics. 2011;52(4):387-391.
7. Lachner C, Sandson NB. Medical complications of catatonia: a case of catatonia-induced deep venous thrombosis. Psychosomatics. 2003;44(6):512-4.
8. Morioka H, Nagatomo I, Yamada K, et al. Deep venous thrombosis of the leg due to psychiatric stupor. Psychiatry Clin Neurosci, 1997;51(5):323-326.
9. Nomoto H, Hatta K, Usui C, et al. Vitamin K deficiency due to prolongation of antibiotic treatment and decrease in food intake in a catatonia patient. Psychosomatics. 2011;52(5):
486-487.
10. Srivastava A, Gupta A, Murthy P, et al. Catatonia and multiple pressure ulcers: a rare complication in psychiatric setting. Indian J Psychiatry. 2009;51(3):206-208.
11. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185.
12. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1):1-9.
13. Zisselman MH, Jaffe RL. ECT in the treatment of a patient with catatonia: consent and complications. Am J Psychiatry. 2010;167(2):127-132.
14. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
15. Raveendranathan D, Narayanaswamy JC, Reddi SV. Response rate of catatonia to electroconvulsive therapy and its clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):425-430.
16. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to lorazepam. Indian J Psychiatry. 1999; 41(1):49-53.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Huang TL. Lorazepam and diazepam rapidly relieve catatonic signs in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(1):52-55.
19. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34:312-316.
20. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71:
235-242.
21. Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:
1203-1210.
22. Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:
518-544.
23. Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201:579e1-8.
24. Howland RH. Prescribing psychotropic medications during pregnancy and lactation: principles and guidelines. J Psychosoc Nurs Ment Health Serv. 2009;47(5):19-23.
25. White DA, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry. 1991; 158:419-421.
26. Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010; 27(7):675-686.
Tips for discussing sexual dysfunction with oncology patients
Cancer therapy presents unique challenges to health care providers because of the evolving nature of managing a patient’s diagnosis, treatment, and recovery. Be conscientious about a patient’s mental health and physical health when considering treatment and support.
Sexual health is important
Sexual dysfunction is one of many variables a patient considers when deciding on a cancer treatment plan—particularly those who have a gynecological, gastrointestinal, or reproductive-tract cancer. Additionally, sexual dysfunction remains one of the major health complaints after many cancer therapies, which may be overlooked because of patients’ hesitancy to initiate discussion.
Many oncologic treatment options—surgery, chemotherapy, radiotherapy, and hormone therapy—are associated with sexual side effects, including radiation sequelae, erectile dysfunction, decreased lubrication, and vaginal atrophy.1 Because sexual dysfunction often is multifactorial, an approach that involves psychological assessment and treatment usually is optimal. A mental health provider can explore the interactions of such factors as decreased self-esteem, negative body image, altered interpersonal relationships, and change or loss of libido when assessing reported sexual dysfunction.2
The mnemonic SEMEN can help you address sexual health topics with oncology patients:
Take a Sexual history at diagnosis and before treatment begins.
Provide Educational materials to warn of potential adverse sexual side effects of various treatments.
Maintain an open dialogue during cancer therapy. Discuss any adverse sexual side effects the patient may be experiencing.
Educate and treat your patient. Offer information on medications, devices, and techniques that target sexual dysfunction.
For men with erectile dysfunction, recommend a phosphodiesterase type 5 (PDE5) inhibitor (sildenafil citrate, tadalafil, vardenafil), a vacuum pump, or intracavernosal penile injection, such as synthetic prostaglandin E1.
For men experiencing premature ejaculation, consider providing instruction on the “squeeze-pause” technique or prescribing a topical anesthetic cream such as lidocaine/prilocaine (available under the brand name EMLA), which is applied to the head of the penis and wiped off before intercourse. Some selective serotonin reuptake inhibitors, including fluoxetine, paroxetine, and sertraline, have been used off-label to treat premature ejaculation.
Women experiencing vaginal dryness or vaginal atrophy might benefit from vaginal estrogen (such as conjugated or estradiol estrogen tablets), an estradiol cream, or the estradiol vaginal ring. Other options include a vaginal moisturizing agent or lubricant.
Additional sexual education topics include:
• adjusting sexual positions
• enhancing foreplay
• seeking help from support organizations
• engaging a sexual therapist (recommend one who specializes in treating oncology patients).
Make Normality the goal after treatment or recovery. Encourage your patient to maintain a healthy sexual lifestyle by continuing discussions about sexual health, supporting healthy self-perception, and addressing possible future sexual dysfunction.
Being given a diagnosis of cancer, undergoing treatment, and surviving the experience are life-altering. Healthcare providers should be open to discussing patients’ past and current sexual practices; along with treating physical illness, you should attempt to maintain a sense of normality, which includes maintaining healthy sexuality.
1. Levenson JL. Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005.
2. National Institutes of Health. National Cancer Institute. Treatment of sexual problems in people with cancer. http://www.cancer.gov/cancertopics/pdq/supportivecare/sexuality/HealthProfessional/page5. Accessed March 26, 2013.
Cancer therapy presents unique challenges to health care providers because of the evolving nature of managing a patient’s diagnosis, treatment, and recovery. Be conscientious about a patient’s mental health and physical health when considering treatment and support.
Sexual health is important
Sexual dysfunction is one of many variables a patient considers when deciding on a cancer treatment plan—particularly those who have a gynecological, gastrointestinal, or reproductive-tract cancer. Additionally, sexual dysfunction remains one of the major health complaints after many cancer therapies, which may be overlooked because of patients’ hesitancy to initiate discussion.
Many oncologic treatment options—surgery, chemotherapy, radiotherapy, and hormone therapy—are associated with sexual side effects, including radiation sequelae, erectile dysfunction, decreased lubrication, and vaginal atrophy.1 Because sexual dysfunction often is multifactorial, an approach that involves psychological assessment and treatment usually is optimal. A mental health provider can explore the interactions of such factors as decreased self-esteem, negative body image, altered interpersonal relationships, and change or loss of libido when assessing reported sexual dysfunction.2
The mnemonic SEMEN can help you address sexual health topics with oncology patients:
Take a Sexual history at diagnosis and before treatment begins.
Provide Educational materials to warn of potential adverse sexual side effects of various treatments.
Maintain an open dialogue during cancer therapy. Discuss any adverse sexual side effects the patient may be experiencing.
Educate and treat your patient. Offer information on medications, devices, and techniques that target sexual dysfunction.
For men with erectile dysfunction, recommend a phosphodiesterase type 5 (PDE5) inhibitor (sildenafil citrate, tadalafil, vardenafil), a vacuum pump, or intracavernosal penile injection, such as synthetic prostaglandin E1.
For men experiencing premature ejaculation, consider providing instruction on the “squeeze-pause” technique or prescribing a topical anesthetic cream such as lidocaine/prilocaine (available under the brand name EMLA), which is applied to the head of the penis and wiped off before intercourse. Some selective serotonin reuptake inhibitors, including fluoxetine, paroxetine, and sertraline, have been used off-label to treat premature ejaculation.
Women experiencing vaginal dryness or vaginal atrophy might benefit from vaginal estrogen (such as conjugated or estradiol estrogen tablets), an estradiol cream, or the estradiol vaginal ring. Other options include a vaginal moisturizing agent or lubricant.
Additional sexual education topics include:
• adjusting sexual positions
• enhancing foreplay
• seeking help from support organizations
• engaging a sexual therapist (recommend one who specializes in treating oncology patients).
Make Normality the goal after treatment or recovery. Encourage your patient to maintain a healthy sexual lifestyle by continuing discussions about sexual health, supporting healthy self-perception, and addressing possible future sexual dysfunction.
Being given a diagnosis of cancer, undergoing treatment, and surviving the experience are life-altering. Healthcare providers should be open to discussing patients’ past and current sexual practices; along with treating physical illness, you should attempt to maintain a sense of normality, which includes maintaining healthy sexuality.
Cancer therapy presents unique challenges to health care providers because of the evolving nature of managing a patient’s diagnosis, treatment, and recovery. Be conscientious about a patient’s mental health and physical health when considering treatment and support.
Sexual health is important
Sexual dysfunction is one of many variables a patient considers when deciding on a cancer treatment plan—particularly those who have a gynecological, gastrointestinal, or reproductive-tract cancer. Additionally, sexual dysfunction remains one of the major health complaints after many cancer therapies, which may be overlooked because of patients’ hesitancy to initiate discussion.
Many oncologic treatment options—surgery, chemotherapy, radiotherapy, and hormone therapy—are associated with sexual side effects, including radiation sequelae, erectile dysfunction, decreased lubrication, and vaginal atrophy.1 Because sexual dysfunction often is multifactorial, an approach that involves psychological assessment and treatment usually is optimal. A mental health provider can explore the interactions of such factors as decreased self-esteem, negative body image, altered interpersonal relationships, and change or loss of libido when assessing reported sexual dysfunction.2
The mnemonic SEMEN can help you address sexual health topics with oncology patients:
Take a Sexual history at diagnosis and before treatment begins.
Provide Educational materials to warn of potential adverse sexual side effects of various treatments.
Maintain an open dialogue during cancer therapy. Discuss any adverse sexual side effects the patient may be experiencing.
Educate and treat your patient. Offer information on medications, devices, and techniques that target sexual dysfunction.
For men with erectile dysfunction, recommend a phosphodiesterase type 5 (PDE5) inhibitor (sildenafil citrate, tadalafil, vardenafil), a vacuum pump, or intracavernosal penile injection, such as synthetic prostaglandin E1.
For men experiencing premature ejaculation, consider providing instruction on the “squeeze-pause” technique or prescribing a topical anesthetic cream such as lidocaine/prilocaine (available under the brand name EMLA), which is applied to the head of the penis and wiped off before intercourse. Some selective serotonin reuptake inhibitors, including fluoxetine, paroxetine, and sertraline, have been used off-label to treat premature ejaculation.
Women experiencing vaginal dryness or vaginal atrophy might benefit from vaginal estrogen (such as conjugated or estradiol estrogen tablets), an estradiol cream, or the estradiol vaginal ring. Other options include a vaginal moisturizing agent or lubricant.
Additional sexual education topics include:
• adjusting sexual positions
• enhancing foreplay
• seeking help from support organizations
• engaging a sexual therapist (recommend one who specializes in treating oncology patients).
Make Normality the goal after treatment or recovery. Encourage your patient to maintain a healthy sexual lifestyle by continuing discussions about sexual health, supporting healthy self-perception, and addressing possible future sexual dysfunction.
Being given a diagnosis of cancer, undergoing treatment, and surviving the experience are life-altering. Healthcare providers should be open to discussing patients’ past and current sexual practices; along with treating physical illness, you should attempt to maintain a sense of normality, which includes maintaining healthy sexuality.
1. Levenson JL. Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005.
2. National Institutes of Health. National Cancer Institute. Treatment of sexual problems in people with cancer. http://www.cancer.gov/cancertopics/pdq/supportivecare/sexuality/HealthProfessional/page5. Accessed March 26, 2013.
1. Levenson JL. Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005.
2. National Institutes of Health. National Cancer Institute. Treatment of sexual problems in people with cancer. http://www.cancer.gov/cancertopics/pdq/supportivecare/sexuality/HealthProfessional/page5. Accessed March 26, 2013.
Tips for making the transition from inpatient to outpatient practice
Years of outpatient practice and supervising residents as they move from inpatient to outpatient rotations prompted me to examine the advice I give to clinicians transitioning to outpatient care.
1. Slow down! Keep in mind that your full assessment may take more than
1 session. Take advantage of follow-up appointments to add details or round out your sense of what is going on with your patient.
2. You don’t always have to ‘do something.’ We often feel that we need to “do something.” Perhaps it’s the difficulty of sitting with someone who’s suffering, or our own feelings of helplessness. Recognize this urge and evaluate whether your findings are something you must act on or if it’s your anxiety that is driving you.
3. Know the particulars of outpatient prescribing. Keep in mind that you should treat the whole person, not just her (his) symptoms. Sometimes it’s appropriate to treat individual symptoms but the justification for this and any other medical decisions needs to be documented.
- Be methodical. Often, this means making one medication change at a time. Although the urgency of inpatient hospitalization sometimes necessitates starting several medications simultaneously, outpatient psychiatry rarely requires that step. Most illnesses in outpatients are chronic;clinicians need to balance the need for treatment with the understanding that the patient may require psychiatric medication indefinitely. Starting several medications at once often leaves the patient and psychiatrist wondering which medications are helping and which may be causing adverse effects.
- Practice educated polypharmacy. Be careful and deliberate; maximize dosages before adding adjunctive therapy. Consider interactions with other medications (such as warfarin or omeprazole), their side effects, and alternative psychosocial treatments.
- Know the cost of medication. Consider generic drugs or medications on the $4 list available at some pharmacies. Be cognizant of less expensive dosing options and combinations. For example, one month of duloxetine, 90 mg/d, costs $587 if prescribed as 30-mg pills; the same dosage costs $390 when prescribed as 30-mg pills and 60-mg pills.1 Advise patients to shop around when purchasing prescriptions because cost can vary significantly among pharmacies.
- Often, patients should be weaned off medications.2 Most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can cause a discontinuation syndrome. Fluoxetine can be tapered faster; paroxetine and venlafaxine are notorious for causing issues. Abrupt discontinuation of mood stabilizers—especially lithium3—can cause rebound mania, and should be tapered cautiously.
Disclosure
Dr. Pheister reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. DRX. Drug compare. https://drugcompare.destinationrx.com/Home.aspx. Accessed October 17, 2012.
2. Baldessarini RJ, Viguera AC, Tondo L. Discontinuing psychotropic agents. J Psychopharmacol. 1999;13(3):292-293; discussion 299.
3. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
Years of outpatient practice and supervising residents as they move from inpatient to outpatient rotations prompted me to examine the advice I give to clinicians transitioning to outpatient care.
1. Slow down! Keep in mind that your full assessment may take more than
1 session. Take advantage of follow-up appointments to add details or round out your sense of what is going on with your patient.
2. You don’t always have to ‘do something.’ We often feel that we need to “do something.” Perhaps it’s the difficulty of sitting with someone who’s suffering, or our own feelings of helplessness. Recognize this urge and evaluate whether your findings are something you must act on or if it’s your anxiety that is driving you.
3. Know the particulars of outpatient prescribing. Keep in mind that you should treat the whole person, not just her (his) symptoms. Sometimes it’s appropriate to treat individual symptoms but the justification for this and any other medical decisions needs to be documented.
- Be methodical. Often, this means making one medication change at a time. Although the urgency of inpatient hospitalization sometimes necessitates starting several medications simultaneously, outpatient psychiatry rarely requires that step. Most illnesses in outpatients are chronic;clinicians need to balance the need for treatment with the understanding that the patient may require psychiatric medication indefinitely. Starting several medications at once often leaves the patient and psychiatrist wondering which medications are helping and which may be causing adverse effects.
- Practice educated polypharmacy. Be careful and deliberate; maximize dosages before adding adjunctive therapy. Consider interactions with other medications (such as warfarin or omeprazole), their side effects, and alternative psychosocial treatments.
- Know the cost of medication. Consider generic drugs or medications on the $4 list available at some pharmacies. Be cognizant of less expensive dosing options and combinations. For example, one month of duloxetine, 90 mg/d, costs $587 if prescribed as 30-mg pills; the same dosage costs $390 when prescribed as 30-mg pills and 60-mg pills.1 Advise patients to shop around when purchasing prescriptions because cost can vary significantly among pharmacies.
- Often, patients should be weaned off medications.2 Most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can cause a discontinuation syndrome. Fluoxetine can be tapered faster; paroxetine and venlafaxine are notorious for causing issues. Abrupt discontinuation of mood stabilizers—especially lithium3—can cause rebound mania, and should be tapered cautiously.
Disclosure
Dr. Pheister reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. DRX. Drug compare. https://drugcompare.destinationrx.com/Home.aspx. Accessed October 17, 2012.
2. Baldessarini RJ, Viguera AC, Tondo L. Discontinuing psychotropic agents. J Psychopharmacol. 1999;13(3):292-293; discussion 299.
3. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
Years of outpatient practice and supervising residents as they move from inpatient to outpatient rotations prompted me to examine the advice I give to clinicians transitioning to outpatient care.
1. Slow down! Keep in mind that your full assessment may take more than
1 session. Take advantage of follow-up appointments to add details or round out your sense of what is going on with your patient.
2. You don’t always have to ‘do something.’ We often feel that we need to “do something.” Perhaps it’s the difficulty of sitting with someone who’s suffering, or our own feelings of helplessness. Recognize this urge and evaluate whether your findings are something you must act on or if it’s your anxiety that is driving you.
3. Know the particulars of outpatient prescribing. Keep in mind that you should treat the whole person, not just her (his) symptoms. Sometimes it’s appropriate to treat individual symptoms but the justification for this and any other medical decisions needs to be documented.
- Be methodical. Often, this means making one medication change at a time. Although the urgency of inpatient hospitalization sometimes necessitates starting several medications simultaneously, outpatient psychiatry rarely requires that step. Most illnesses in outpatients are chronic;clinicians need to balance the need for treatment with the understanding that the patient may require psychiatric medication indefinitely. Starting several medications at once often leaves the patient and psychiatrist wondering which medications are helping and which may be causing adverse effects.
- Practice educated polypharmacy. Be careful and deliberate; maximize dosages before adding adjunctive therapy. Consider interactions with other medications (such as warfarin or omeprazole), their side effects, and alternative psychosocial treatments.
- Know the cost of medication. Consider generic drugs or medications on the $4 list available at some pharmacies. Be cognizant of less expensive dosing options and combinations. For example, one month of duloxetine, 90 mg/d, costs $587 if prescribed as 30-mg pills; the same dosage costs $390 when prescribed as 30-mg pills and 60-mg pills.1 Advise patients to shop around when purchasing prescriptions because cost can vary significantly among pharmacies.
- Often, patients should be weaned off medications.2 Most selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can cause a discontinuation syndrome. Fluoxetine can be tapered faster; paroxetine and venlafaxine are notorious for causing issues. Abrupt discontinuation of mood stabilizers—especially lithium3—can cause rebound mania, and should be tapered cautiously.
Disclosure
Dr. Pheister reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. DRX. Drug compare. https://drugcompare.destinationrx.com/Home.aspx. Accessed October 17, 2012.
2. Baldessarini RJ, Viguera AC, Tondo L. Discontinuing psychotropic agents. J Psychopharmacol. 1999;13(3):292-293; discussion 299.
3. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
Hospitalized, elderly, and delirious: What should you do for these patients?
Delirium is a common condition in hospitalized older patients. Often, a report of a “change in mental status” is the reason geriatric patients are sent to the emergency room for evaluation, although delirium also can develop after admission.
Delirium is a marker of underlying medical illness that needs careful workup and treatment. The condition can be iatrogenic, resulting from prescribed medication or a surgical procedure; most often, it is the consequence of multiple factors. Delirium can be expensive, because it increases hospital length of stay and overall costs—particularly if the patient is discharged to a nursing facility, not to home. Patients with delirium are at higher risk of death.
Delirium often goes unrecognized by physicians and nursing staff, and is not documented in medical records. Educating the medical staff on the identification and management of delirium is a key role for consulting psychiatrists.
CASE: Confused and agitated
Ms. T, a 93-year-old resident of an assisted living facility with a history of three
cerebral vascular accidents, atrial fibrillation, hypertension, multiple deep venous thromboses, blindness in her right eye, and deafness in her right ear without a hearing aid, is brought to the hospital after a syncopal episode lasting 10 minutes that was followed by slurred speech, confusion, and transient hypotension. Her dentist recently started her on azithromycin.
In the emergency room, Ms. T’s elevated blood pressure is managed with hydralazine and diltiazem. A CT scan of the head rules out hemorrhagic stroke. Complete blood count and tests of electrolytes, vitamin B12, and thyroid-stimulating hormone are within normal limits; urinalysis is negative for urinary tract infection.
Ms. T is noted to be in and out of sleep, with some confusion. She is maintained without oral food or fluids because of concerns about her ability to swallow. After 5 or 6 hours in the ER, Ms. T is transferred to a medical unit, where she becomes agitated and paranoid, with the delusion that her daughter is an impostor. She yells, is combative, and refuses medication.
Her confusion and behaviors become worse at night: She pulls out her IV line and telemetry leads. Blood pressure remains elevated, for which she receives additional doses of hydralazine.
For behavioral management, the medical team orders a one-time IM dose of haloperidol and starts her on risperidone, 0.5 mg every 4 hours as needed, which Ms. T refuses to take. She is incontinent and has foul-smelling urine.
Ms. T’s family is shocked at her condition; nursing staff is frustrated. With her worsening paranoia, delusions, and combative behaviors towards the nursing staff, psychiatry is consulted.
How to recognize and diagnose
The Box lists DSM-5 criteria for delirium.1 The key feature is a disturbance in attention—what was referred to in DSM-IV-TR as “disturbance in consciousness.” That finding contrasts with what is seen in dementia, with its hallmark memory impairment and chronic deterioration.
In a hospital setting, the question is often asked: Does this patient have dementia or delirium? In many cases, the answer is both, because preexisting cognitive impairment is an important risk factor for delirium.
In addition to the standard clinical interview, several screening instruments or delirium rating scales have been developed. The most commonly used (Table 1) is the Confusion Assessment Method developed by Inouye and colleagues.2
Subtypes of delirium have been described, largely based on motor activity. Patients can present as hyperactive, hypoactive, mixed, or neither.3 Psychiatrists are more likely to be consulted regarding patients with hyperactive delirium, because they are the ones who scream, pull out their IV line, hallucinate, and are delusional, insisting they “have to go home”—such as the patient described in the case above.
Patients with hypoactive delirium often, on the other hand, are difficult to recognize; they present with lethargy, drowsiness, apathy, and confusion. They become withdrawn and answer slowly4; often, psychiatry is consulted to assess them for depression.
Delirium can be difficult to diagnose in patients with underlying dementia, who are not able to provide information. In such cases, obtaining collateral information from a family member or primary caretaker is crucial. Knowing the patient’s baseline helps to determine whether there has been an acute change in mental status.
CASE CONTINUED: Acute mental status changes
Ms. T’s daughter reports that her mother has not been in this condition before. At baseline, Ms. T has had memory problems but no paranoia, delusions, or agitated behaviors. Her daughter also reports that Ms. T has visual and hearing impairments and is not wearing her hearing aid.
The acute change in mental status and the perceptual disturbances indicate that Ms. T has delirium, not dementia.
Who is likely to develop delirium?
Risk factors for delirium (Table 2) include preexisting cognitive impairment, older age, vision and hearing impairment, use of psychoactive drugs, severe illness, azotemia and dehydration, a metabolic abnormality, and infection. Male sex also seems to be a risk factor, perhaps because men are more likely to abuse alcohol before admission.
Many patients become delirious after starting a new medication. An experienced geriatrician teaches that the main causes of delirium are “drugs, drugs, drugs, infections, and everything else” (Kenneth Rockwood, MD, personal communication, 2012). At admission, urinary tract infection and pneumonia are common causes of delirium, especially in geriatric patients.
What is the clinical course?
The clinical course varies widely. Delirium often is the reason that a patient is brought to the hospital, presenting with the condition at admission or early in hospitalization. The highest incidence among surgical patients appears to be on the third postoperative day—in some cases because of alcohol or drug withdrawal.
As noted in the DSM-5 criteria, delirium often comes on acutely, over hours or days. Symptoms can persist for weeks after initial onset of episodes of delirium.5 Symptoms fluctuate over the course of the day; at times, they can be missed if a provider sees the patient only while she (he) is clearer and doesn’t review nursing notes from other shifts.
How does delirium affect outcome?
Delirium has been shown to be associated with prolonged hospital stay (21 days, compared with 11 days in the absence of delirium), functional decline during hospitalization, and increased admission to long-term care (36% compared with 13%).6 In a study by O’Keefe and Lavan,6 delirious patients were more likely to sustain falls and to develop urinary incontinence, pressure sores, and other complications during hospitalization.
Older patients with delirium superimposed on dementia had a more than twofold increased risk of mortality compared with patients with dementia alone or with neither dementia nor delirium.7 Rockwood found that an episode of delirium was associated with a much higher rate of subsequent dementia.8
Think of an acute medical illness as a “stress test” for the brain, such that, if the patient develops delirium, it suggests an underlying brain disease that was not evident before the acute episode. After hip fracture, for example, delirium was independently associated with poor functional recovery at 1 month9 and at 2 years.10
Older patients admitted to a skilled nursing facility with delirium are more likely to experience one or more complications (73% compared with 41%).11 In the study by Marcantonio and colleagues, patients with delirium were more than twice as likely to be hospitalized again within 30 days (30% and 13%), and less than half as likely to be discharged to the community (30% and 73%). Table 3 summarizes the impact of delirium on outcomes.
Appropriate management steps
Identifying and treating underlying medical illness is the definitive treatment for delirium; in a geriatric patient with multiple medical comorbidities the pathogenesis often is multifactorial or a definitive precipitant cannot always be identified.12
Managing a patient with delirium includes both non-pharmacotherapeutic interventions, which should be considered first-line, and pharmacotherapeutic interventions. Non-pharmacotherapeutic interventions include, but are not limited to:
• support and close observation by nursing staff
• placing a clock or calendar in the room
• frequent reorientation and reminders
• placing familiar possessions in the room
• putting the patient in an isolated room with a window
• regulating the sleep-wake cycle.4
Pharmacotherapeutic intervention in delirium should be used for behavioral symptoms, but only for the minimum duration necessary4 and preferably oral or IV. No drugs are FDA-approved for delirium, which means that use of any agent is off-label.13
Antipsychotics are the mainstay of pharmacotherapy for delirium in most settings. The use of antipsychotics relates to the dopamine excess-acetylcholine deficiency hypothesis of delirium pathophysiology.12 Haloperidol remains the first-line agent because it is available in multiple dosages and can be given by various routes. IV haloperidol appears to carry less risk of extrapyramidal symptoms than oral haloperidol does but, as with all antipsychotics, its use warrants monitoring for QTc prolongation.12
Studies have not shown that atypical antipsychotics are superior to typical antipsychotics for delirium. Multiple studies have shown that atypicals are as efficacious as haloperidol.
Benzodiazepines are the treatment of choice for delirium caused by alcohol withdrawal. A Cochrane review found no evidence that benzodiazepines were helpful in treating delirium unrelated to alcohol withdrawal.14 In some studies, benzodiazepines were associated with an increased risk of delirium, especially in patients in the intensive care unit.15
More recently, cholinesterase inhibitors have been used to treat delirium. The reasoning behind their use is the hypothesis of a central cholinergic deficiency in delirium.12 Regrettably, there have been few well-conducted studies of these agents in delirium, and a Cochrane review found no significant benefit for cholinesterase inhibitors.16 With the same hypothesis in mind, anticholinergic medications in patients with delirium should be avoided because these agents could exacerbate delirium by further decreasing the acetylcholine level.
Because delirium is common in the hospitalized population (especially older patients), a number of studies have examined strategies to prevent or reduce its development. Inouye and colleagues conducted a controlled clinical trial, in which they intervened to reduce six risk factors for delirium: cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration in hospitalized geriatric patients. The number and duration of events of delirium were significantly lower in the intervention group.17
Brummel et al reported that reducing modifiable risk factors in intensive care unit patients—including sedation management, minimizing deliriogenic medications (anticholinergics, antihistamines), minimizing sleep disruption, and encouraging early mobility—could prevent or reduce the incidence of delirium.15
CASE CONCLUDED: Return to baseline
Ms. T’s medications are minimized or discontinued, including azithromycin, based on case reports in the literature. She is stabilized hemodynamically.
Clinicians educate Ms. T’s family about delirium. To address Ms. T’s aggressive and paranoid behaviors, clinicians request that a family member is present to reassure Ms. T. She is continued on low-dose haloperidol. The family also is asked to bring Ms. T’s hearing aid and eyeglasses.
MRI is performed after Ms. T’s behavior is under control. The scan is negative for a new stroke.
Repeat blood tests the following day show an elevated white blood cell count; urinalysis is positive for a urinary tract infection. Ms. T is started on antibiotics. Subsequent urine culture shows no bacterial growth; the antibiotics are stopped after 3 days.
Ms. T slowly improves. According to her family, she is back at baseline in 3 or 4 days.
This case illustrates the complexity of trying to identify the precise cause of delirium among the many that could be involved. Often, no single cause can be found.18
Bottom Line
Delirium is a common and potentially life-threatening condition in hospitalized geriatric patients. General hospital psychiatrists should know how to recognize and treat the condition in collaboration with their medical colleagues.
Related Resources
- Treating delirium: a quick reference guide. Arlington, VA: American Psychiatric Association. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1662986.
- Cook IA. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952.
- Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens D, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:229-241.
- Ghandour A, Saab R, Mehr D. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60(12):726-734.
- Leentjens AF, Rundell J, Rummans T, et al. Delirium: an evidence-based medicine (EBM) monograph for psychosomatic medicine practice. J Psychosom Res. 2012;73:149-152.
- Liptzin B, Jacobson SA. Delirium. In: Sadock BJ, Sadock VA, Ruiz P, eds. Comprehensive textbook of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:4066-4073.
Drug Brand Names
Azithromycin • Zithromax Hydralazine • Apresoline
Diltiazem • Cardizem Risperidone • Risperdal
Haloperidol • Haldo
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Benjamin Liptzin, MD, describes the distinction between dementia and delirium. Dr. Liptzin is Chair of Psychiatry, Baystate Medical Center, Springfield, Massachusetts, and Professor and Deputy Chair, Department of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
3. Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843-845.
4. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.
5. Levkoff SE, Liptzin B, Evans D, et al. Progression and resolution of delirium in elderly patients hospitalized for acute care. Am J Geriatr Psychiatry. 1994;2:230-238.
6. O’Keefe S, Lavan J. The prognostic significance of delirium in older hospitalized patients. J Am Geriatr Soc. 1997;45:247-248.
7. Tsai MC, Weng HH, Chou SY, et al. One-year mortality of elderly inpatients with delirium, dementia or depression seen by a consultation-liaison service. Psychosomatics. 2012;53:433-438.
8. Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551-556.
9. Marcantonio E, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618-624.
10. Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M27-M34.
11. Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of elders admitted to post-acute facilities with delirium. J Am Geriatr Soc. 2005;53:963-969.
12. Bledowski J, Trutia A. A review of pharmacologic management and prevention strategies of delirium in the intensive care unit. Psychosomatics. 2012;53:203-211.
13. Breitbart W, Alici-Evcimen Y. Why off-label antipsychotics remain first-choice drugs for delirium. Current Psychiatry. 2007;6(9):49-63.
14. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. doi: 10.1002/14651858.CD006379.pub3.
15. Brummel NE, Girard TD. Preventing delirium in the ICU. Crit Care Clin. 2013;(29):51-65.
16. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008(1):CD005317. doi: 10.1002/14651858.CD005317.
17. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669-676.
18. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097-1101.
Delirium is a common condition in hospitalized older patients. Often, a report of a “change in mental status” is the reason geriatric patients are sent to the emergency room for evaluation, although delirium also can develop after admission.
Delirium is a marker of underlying medical illness that needs careful workup and treatment. The condition can be iatrogenic, resulting from prescribed medication or a surgical procedure; most often, it is the consequence of multiple factors. Delirium can be expensive, because it increases hospital length of stay and overall costs—particularly if the patient is discharged to a nursing facility, not to home. Patients with delirium are at higher risk of death.
Delirium often goes unrecognized by physicians and nursing staff, and is not documented in medical records. Educating the medical staff on the identification and management of delirium is a key role for consulting psychiatrists.
CASE: Confused and agitated
Ms. T, a 93-year-old resident of an assisted living facility with a history of three
cerebral vascular accidents, atrial fibrillation, hypertension, multiple deep venous thromboses, blindness in her right eye, and deafness in her right ear without a hearing aid, is brought to the hospital after a syncopal episode lasting 10 minutes that was followed by slurred speech, confusion, and transient hypotension. Her dentist recently started her on azithromycin.
In the emergency room, Ms. T’s elevated blood pressure is managed with hydralazine and diltiazem. A CT scan of the head rules out hemorrhagic stroke. Complete blood count and tests of electrolytes, vitamin B12, and thyroid-stimulating hormone are within normal limits; urinalysis is negative for urinary tract infection.
Ms. T is noted to be in and out of sleep, with some confusion. She is maintained without oral food or fluids because of concerns about her ability to swallow. After 5 or 6 hours in the ER, Ms. T is transferred to a medical unit, where she becomes agitated and paranoid, with the delusion that her daughter is an impostor. She yells, is combative, and refuses medication.
Her confusion and behaviors become worse at night: She pulls out her IV line and telemetry leads. Blood pressure remains elevated, for which she receives additional doses of hydralazine.
For behavioral management, the medical team orders a one-time IM dose of haloperidol and starts her on risperidone, 0.5 mg every 4 hours as needed, which Ms. T refuses to take. She is incontinent and has foul-smelling urine.
Ms. T’s family is shocked at her condition; nursing staff is frustrated. With her worsening paranoia, delusions, and combative behaviors towards the nursing staff, psychiatry is consulted.
How to recognize and diagnose
The Box lists DSM-5 criteria for delirium.1 The key feature is a disturbance in attention—what was referred to in DSM-IV-TR as “disturbance in consciousness.” That finding contrasts with what is seen in dementia, with its hallmark memory impairment and chronic deterioration.
In a hospital setting, the question is often asked: Does this patient have dementia or delirium? In many cases, the answer is both, because preexisting cognitive impairment is an important risk factor for delirium.
In addition to the standard clinical interview, several screening instruments or delirium rating scales have been developed. The most commonly used (Table 1) is the Confusion Assessment Method developed by Inouye and colleagues.2
Subtypes of delirium have been described, largely based on motor activity. Patients can present as hyperactive, hypoactive, mixed, or neither.3 Psychiatrists are more likely to be consulted regarding patients with hyperactive delirium, because they are the ones who scream, pull out their IV line, hallucinate, and are delusional, insisting they “have to go home”—such as the patient described in the case above.
Patients with hypoactive delirium often, on the other hand, are difficult to recognize; they present with lethargy, drowsiness, apathy, and confusion. They become withdrawn and answer slowly4; often, psychiatry is consulted to assess them for depression.
Delirium can be difficult to diagnose in patients with underlying dementia, who are not able to provide information. In such cases, obtaining collateral information from a family member or primary caretaker is crucial. Knowing the patient’s baseline helps to determine whether there has been an acute change in mental status.
CASE CONTINUED: Acute mental status changes
Ms. T’s daughter reports that her mother has not been in this condition before. At baseline, Ms. T has had memory problems but no paranoia, delusions, or agitated behaviors. Her daughter also reports that Ms. T has visual and hearing impairments and is not wearing her hearing aid.
The acute change in mental status and the perceptual disturbances indicate that Ms. T has delirium, not dementia.
Who is likely to develop delirium?
Risk factors for delirium (Table 2) include preexisting cognitive impairment, older age, vision and hearing impairment, use of psychoactive drugs, severe illness, azotemia and dehydration, a metabolic abnormality, and infection. Male sex also seems to be a risk factor, perhaps because men are more likely to abuse alcohol before admission.
Many patients become delirious after starting a new medication. An experienced geriatrician teaches that the main causes of delirium are “drugs, drugs, drugs, infections, and everything else” (Kenneth Rockwood, MD, personal communication, 2012). At admission, urinary tract infection and pneumonia are common causes of delirium, especially in geriatric patients.
What is the clinical course?
The clinical course varies widely. Delirium often is the reason that a patient is brought to the hospital, presenting with the condition at admission or early in hospitalization. The highest incidence among surgical patients appears to be on the third postoperative day—in some cases because of alcohol or drug withdrawal.
As noted in the DSM-5 criteria, delirium often comes on acutely, over hours or days. Symptoms can persist for weeks after initial onset of episodes of delirium.5 Symptoms fluctuate over the course of the day; at times, they can be missed if a provider sees the patient only while she (he) is clearer and doesn’t review nursing notes from other shifts.
How does delirium affect outcome?
Delirium has been shown to be associated with prolonged hospital stay (21 days, compared with 11 days in the absence of delirium), functional decline during hospitalization, and increased admission to long-term care (36% compared with 13%).6 In a study by O’Keefe and Lavan,6 delirious patients were more likely to sustain falls and to develop urinary incontinence, pressure sores, and other complications during hospitalization.
Older patients with delirium superimposed on dementia had a more than twofold increased risk of mortality compared with patients with dementia alone or with neither dementia nor delirium.7 Rockwood found that an episode of delirium was associated with a much higher rate of subsequent dementia.8
Think of an acute medical illness as a “stress test” for the brain, such that, if the patient develops delirium, it suggests an underlying brain disease that was not evident before the acute episode. After hip fracture, for example, delirium was independently associated with poor functional recovery at 1 month9 and at 2 years.10
Older patients admitted to a skilled nursing facility with delirium are more likely to experience one or more complications (73% compared with 41%).11 In the study by Marcantonio and colleagues, patients with delirium were more than twice as likely to be hospitalized again within 30 days (30% and 13%), and less than half as likely to be discharged to the community (30% and 73%). Table 3 summarizes the impact of delirium on outcomes.
Appropriate management steps
Identifying and treating underlying medical illness is the definitive treatment for delirium; in a geriatric patient with multiple medical comorbidities the pathogenesis often is multifactorial or a definitive precipitant cannot always be identified.12
Managing a patient with delirium includes both non-pharmacotherapeutic interventions, which should be considered first-line, and pharmacotherapeutic interventions. Non-pharmacotherapeutic interventions include, but are not limited to:
• support and close observation by nursing staff
• placing a clock or calendar in the room
• frequent reorientation and reminders
• placing familiar possessions in the room
• putting the patient in an isolated room with a window
• regulating the sleep-wake cycle.4
Pharmacotherapeutic intervention in delirium should be used for behavioral symptoms, but only for the minimum duration necessary4 and preferably oral or IV. No drugs are FDA-approved for delirium, which means that use of any agent is off-label.13
Antipsychotics are the mainstay of pharmacotherapy for delirium in most settings. The use of antipsychotics relates to the dopamine excess-acetylcholine deficiency hypothesis of delirium pathophysiology.12 Haloperidol remains the first-line agent because it is available in multiple dosages and can be given by various routes. IV haloperidol appears to carry less risk of extrapyramidal symptoms than oral haloperidol does but, as with all antipsychotics, its use warrants monitoring for QTc prolongation.12
Studies have not shown that atypical antipsychotics are superior to typical antipsychotics for delirium. Multiple studies have shown that atypicals are as efficacious as haloperidol.
Benzodiazepines are the treatment of choice for delirium caused by alcohol withdrawal. A Cochrane review found no evidence that benzodiazepines were helpful in treating delirium unrelated to alcohol withdrawal.14 In some studies, benzodiazepines were associated with an increased risk of delirium, especially in patients in the intensive care unit.15
More recently, cholinesterase inhibitors have been used to treat delirium. The reasoning behind their use is the hypothesis of a central cholinergic deficiency in delirium.12 Regrettably, there have been few well-conducted studies of these agents in delirium, and a Cochrane review found no significant benefit for cholinesterase inhibitors.16 With the same hypothesis in mind, anticholinergic medications in patients with delirium should be avoided because these agents could exacerbate delirium by further decreasing the acetylcholine level.
Because delirium is common in the hospitalized population (especially older patients), a number of studies have examined strategies to prevent or reduce its development. Inouye and colleagues conducted a controlled clinical trial, in which they intervened to reduce six risk factors for delirium: cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration in hospitalized geriatric patients. The number and duration of events of delirium were significantly lower in the intervention group.17
Brummel et al reported that reducing modifiable risk factors in intensive care unit patients—including sedation management, minimizing deliriogenic medications (anticholinergics, antihistamines), minimizing sleep disruption, and encouraging early mobility—could prevent or reduce the incidence of delirium.15
CASE CONCLUDED: Return to baseline
Ms. T’s medications are minimized or discontinued, including azithromycin, based on case reports in the literature. She is stabilized hemodynamically.
Clinicians educate Ms. T’s family about delirium. To address Ms. T’s aggressive and paranoid behaviors, clinicians request that a family member is present to reassure Ms. T. She is continued on low-dose haloperidol. The family also is asked to bring Ms. T’s hearing aid and eyeglasses.
MRI is performed after Ms. T’s behavior is under control. The scan is negative for a new stroke.
Repeat blood tests the following day show an elevated white blood cell count; urinalysis is positive for a urinary tract infection. Ms. T is started on antibiotics. Subsequent urine culture shows no bacterial growth; the antibiotics are stopped after 3 days.
Ms. T slowly improves. According to her family, she is back at baseline in 3 or 4 days.
This case illustrates the complexity of trying to identify the precise cause of delirium among the many that could be involved. Often, no single cause can be found.18
Bottom Line
Delirium is a common and potentially life-threatening condition in hospitalized geriatric patients. General hospital psychiatrists should know how to recognize and treat the condition in collaboration with their medical colleagues.
Related Resources
- Treating delirium: a quick reference guide. Arlington, VA: American Psychiatric Association. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1662986.
- Cook IA. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952.
- Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens D, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:229-241.
- Ghandour A, Saab R, Mehr D. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60(12):726-734.
- Leentjens AF, Rundell J, Rummans T, et al. Delirium: an evidence-based medicine (EBM) monograph for psychosomatic medicine practice. J Psychosom Res. 2012;73:149-152.
- Liptzin B, Jacobson SA. Delirium. In: Sadock BJ, Sadock VA, Ruiz P, eds. Comprehensive textbook of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:4066-4073.
Drug Brand Names
Azithromycin • Zithromax Hydralazine • Apresoline
Diltiazem • Cardizem Risperidone • Risperdal
Haloperidol • Haldo
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Benjamin Liptzin, MD, describes the distinction between dementia and delirium. Dr. Liptzin is Chair of Psychiatry, Baystate Medical Center, Springfield, Massachusetts, and Professor and Deputy Chair, Department of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts.
Delirium is a common condition in hospitalized older patients. Often, a report of a “change in mental status” is the reason geriatric patients are sent to the emergency room for evaluation, although delirium also can develop after admission.
Delirium is a marker of underlying medical illness that needs careful workup and treatment. The condition can be iatrogenic, resulting from prescribed medication or a surgical procedure; most often, it is the consequence of multiple factors. Delirium can be expensive, because it increases hospital length of stay and overall costs—particularly if the patient is discharged to a nursing facility, not to home. Patients with delirium are at higher risk of death.
Delirium often goes unrecognized by physicians and nursing staff, and is not documented in medical records. Educating the medical staff on the identification and management of delirium is a key role for consulting psychiatrists.
CASE: Confused and agitated
Ms. T, a 93-year-old resident of an assisted living facility with a history of three
cerebral vascular accidents, atrial fibrillation, hypertension, multiple deep venous thromboses, blindness in her right eye, and deafness in her right ear without a hearing aid, is brought to the hospital after a syncopal episode lasting 10 minutes that was followed by slurred speech, confusion, and transient hypotension. Her dentist recently started her on azithromycin.
In the emergency room, Ms. T’s elevated blood pressure is managed with hydralazine and diltiazem. A CT scan of the head rules out hemorrhagic stroke. Complete blood count and tests of electrolytes, vitamin B12, and thyroid-stimulating hormone are within normal limits; urinalysis is negative for urinary tract infection.
Ms. T is noted to be in and out of sleep, with some confusion. She is maintained without oral food or fluids because of concerns about her ability to swallow. After 5 or 6 hours in the ER, Ms. T is transferred to a medical unit, where she becomes agitated and paranoid, with the delusion that her daughter is an impostor. She yells, is combative, and refuses medication.
Her confusion and behaviors become worse at night: She pulls out her IV line and telemetry leads. Blood pressure remains elevated, for which she receives additional doses of hydralazine.
For behavioral management, the medical team orders a one-time IM dose of haloperidol and starts her on risperidone, 0.5 mg every 4 hours as needed, which Ms. T refuses to take. She is incontinent and has foul-smelling urine.
Ms. T’s family is shocked at her condition; nursing staff is frustrated. With her worsening paranoia, delusions, and combative behaviors towards the nursing staff, psychiatry is consulted.
How to recognize and diagnose
The Box lists DSM-5 criteria for delirium.1 The key feature is a disturbance in attention—what was referred to in DSM-IV-TR as “disturbance in consciousness.” That finding contrasts with what is seen in dementia, with its hallmark memory impairment and chronic deterioration.
In a hospital setting, the question is often asked: Does this patient have dementia or delirium? In many cases, the answer is both, because preexisting cognitive impairment is an important risk factor for delirium.
In addition to the standard clinical interview, several screening instruments or delirium rating scales have been developed. The most commonly used (Table 1) is the Confusion Assessment Method developed by Inouye and colleagues.2
Subtypes of delirium have been described, largely based on motor activity. Patients can present as hyperactive, hypoactive, mixed, or neither.3 Psychiatrists are more likely to be consulted regarding patients with hyperactive delirium, because they are the ones who scream, pull out their IV line, hallucinate, and are delusional, insisting they “have to go home”—such as the patient described in the case above.
Patients with hypoactive delirium often, on the other hand, are difficult to recognize; they present with lethargy, drowsiness, apathy, and confusion. They become withdrawn and answer slowly4; often, psychiatry is consulted to assess them for depression.
Delirium can be difficult to diagnose in patients with underlying dementia, who are not able to provide information. In such cases, obtaining collateral information from a family member or primary caretaker is crucial. Knowing the patient’s baseline helps to determine whether there has been an acute change in mental status.
CASE CONTINUED: Acute mental status changes
Ms. T’s daughter reports that her mother has not been in this condition before. At baseline, Ms. T has had memory problems but no paranoia, delusions, or agitated behaviors. Her daughter also reports that Ms. T has visual and hearing impairments and is not wearing her hearing aid.
The acute change in mental status and the perceptual disturbances indicate that Ms. T has delirium, not dementia.
Who is likely to develop delirium?
Risk factors for delirium (Table 2) include preexisting cognitive impairment, older age, vision and hearing impairment, use of psychoactive drugs, severe illness, azotemia and dehydration, a metabolic abnormality, and infection. Male sex also seems to be a risk factor, perhaps because men are more likely to abuse alcohol before admission.
Many patients become delirious after starting a new medication. An experienced geriatrician teaches that the main causes of delirium are “drugs, drugs, drugs, infections, and everything else” (Kenneth Rockwood, MD, personal communication, 2012). At admission, urinary tract infection and pneumonia are common causes of delirium, especially in geriatric patients.
What is the clinical course?
The clinical course varies widely. Delirium often is the reason that a patient is brought to the hospital, presenting with the condition at admission or early in hospitalization. The highest incidence among surgical patients appears to be on the third postoperative day—in some cases because of alcohol or drug withdrawal.
As noted in the DSM-5 criteria, delirium often comes on acutely, over hours or days. Symptoms can persist for weeks after initial onset of episodes of delirium.5 Symptoms fluctuate over the course of the day; at times, they can be missed if a provider sees the patient only while she (he) is clearer and doesn’t review nursing notes from other shifts.
How does delirium affect outcome?
Delirium has been shown to be associated with prolonged hospital stay (21 days, compared with 11 days in the absence of delirium), functional decline during hospitalization, and increased admission to long-term care (36% compared with 13%).6 In a study by O’Keefe and Lavan,6 delirious patients were more likely to sustain falls and to develop urinary incontinence, pressure sores, and other complications during hospitalization.
Older patients with delirium superimposed on dementia had a more than twofold increased risk of mortality compared with patients with dementia alone or with neither dementia nor delirium.7 Rockwood found that an episode of delirium was associated with a much higher rate of subsequent dementia.8
Think of an acute medical illness as a “stress test” for the brain, such that, if the patient develops delirium, it suggests an underlying brain disease that was not evident before the acute episode. After hip fracture, for example, delirium was independently associated with poor functional recovery at 1 month9 and at 2 years.10
Older patients admitted to a skilled nursing facility with delirium are more likely to experience one or more complications (73% compared with 41%).11 In the study by Marcantonio and colleagues, patients with delirium were more than twice as likely to be hospitalized again within 30 days (30% and 13%), and less than half as likely to be discharged to the community (30% and 73%). Table 3 summarizes the impact of delirium on outcomes.
Appropriate management steps
Identifying and treating underlying medical illness is the definitive treatment for delirium; in a geriatric patient with multiple medical comorbidities the pathogenesis often is multifactorial or a definitive precipitant cannot always be identified.12
Managing a patient with delirium includes both non-pharmacotherapeutic interventions, which should be considered first-line, and pharmacotherapeutic interventions. Non-pharmacotherapeutic interventions include, but are not limited to:
• support and close observation by nursing staff
• placing a clock or calendar in the room
• frequent reorientation and reminders
• placing familiar possessions in the room
• putting the patient in an isolated room with a window
• regulating the sleep-wake cycle.4
Pharmacotherapeutic intervention in delirium should be used for behavioral symptoms, but only for the minimum duration necessary4 and preferably oral or IV. No drugs are FDA-approved for delirium, which means that use of any agent is off-label.13
Antipsychotics are the mainstay of pharmacotherapy for delirium in most settings. The use of antipsychotics relates to the dopamine excess-acetylcholine deficiency hypothesis of delirium pathophysiology.12 Haloperidol remains the first-line agent because it is available in multiple dosages and can be given by various routes. IV haloperidol appears to carry less risk of extrapyramidal symptoms than oral haloperidol does but, as with all antipsychotics, its use warrants monitoring for QTc prolongation.12
Studies have not shown that atypical antipsychotics are superior to typical antipsychotics for delirium. Multiple studies have shown that atypicals are as efficacious as haloperidol.
Benzodiazepines are the treatment of choice for delirium caused by alcohol withdrawal. A Cochrane review found no evidence that benzodiazepines were helpful in treating delirium unrelated to alcohol withdrawal.14 In some studies, benzodiazepines were associated with an increased risk of delirium, especially in patients in the intensive care unit.15
More recently, cholinesterase inhibitors have been used to treat delirium. The reasoning behind their use is the hypothesis of a central cholinergic deficiency in delirium.12 Regrettably, there have been few well-conducted studies of these agents in delirium, and a Cochrane review found no significant benefit for cholinesterase inhibitors.16 With the same hypothesis in mind, anticholinergic medications in patients with delirium should be avoided because these agents could exacerbate delirium by further decreasing the acetylcholine level.
Because delirium is common in the hospitalized population (especially older patients), a number of studies have examined strategies to prevent or reduce its development. Inouye and colleagues conducted a controlled clinical trial, in which they intervened to reduce six risk factors for delirium: cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration in hospitalized geriatric patients. The number and duration of events of delirium were significantly lower in the intervention group.17
Brummel et al reported that reducing modifiable risk factors in intensive care unit patients—including sedation management, minimizing deliriogenic medications (anticholinergics, antihistamines), minimizing sleep disruption, and encouraging early mobility—could prevent or reduce the incidence of delirium.15
CASE CONCLUDED: Return to baseline
Ms. T’s medications are minimized or discontinued, including azithromycin, based on case reports in the literature. She is stabilized hemodynamically.
Clinicians educate Ms. T’s family about delirium. To address Ms. T’s aggressive and paranoid behaviors, clinicians request that a family member is present to reassure Ms. T. She is continued on low-dose haloperidol. The family also is asked to bring Ms. T’s hearing aid and eyeglasses.
MRI is performed after Ms. T’s behavior is under control. The scan is negative for a new stroke.
Repeat blood tests the following day show an elevated white blood cell count; urinalysis is positive for a urinary tract infection. Ms. T is started on antibiotics. Subsequent urine culture shows no bacterial growth; the antibiotics are stopped after 3 days.
Ms. T slowly improves. According to her family, she is back at baseline in 3 or 4 days.
This case illustrates the complexity of trying to identify the precise cause of delirium among the many that could be involved. Often, no single cause can be found.18
Bottom Line
Delirium is a common and potentially life-threatening condition in hospitalized geriatric patients. General hospital psychiatrists should know how to recognize and treat the condition in collaboration with their medical colleagues.
Related Resources
- Treating delirium: a quick reference guide. Arlington, VA: American Psychiatric Association. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1662986.
- Cook IA. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952.
- Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens D, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:229-241.
- Ghandour A, Saab R, Mehr D. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60(12):726-734.
- Leentjens AF, Rundell J, Rummans T, et al. Delirium: an evidence-based medicine (EBM) monograph for psychosomatic medicine practice. J Psychosom Res. 2012;73:149-152.
- Liptzin B, Jacobson SA. Delirium. In: Sadock BJ, Sadock VA, Ruiz P, eds. Comprehensive textbook of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:4066-4073.
Drug Brand Names
Azithromycin • Zithromax Hydralazine • Apresoline
Diltiazem • Cardizem Risperidone • Risperdal
Haloperidol • Haldo
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Benjamin Liptzin, MD, describes the distinction between dementia and delirium. Dr. Liptzin is Chair of Psychiatry, Baystate Medical Center, Springfield, Massachusetts, and Professor and Deputy Chair, Department of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
3. Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843-845.
4. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.
5. Levkoff SE, Liptzin B, Evans D, et al. Progression and resolution of delirium in elderly patients hospitalized for acute care. Am J Geriatr Psychiatry. 1994;2:230-238.
6. O’Keefe S, Lavan J. The prognostic significance of delirium in older hospitalized patients. J Am Geriatr Soc. 1997;45:247-248.
7. Tsai MC, Weng HH, Chou SY, et al. One-year mortality of elderly inpatients with delirium, dementia or depression seen by a consultation-liaison service. Psychosomatics. 2012;53:433-438.
8. Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551-556.
9. Marcantonio E, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618-624.
10. Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M27-M34.
11. Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of elders admitted to post-acute facilities with delirium. J Am Geriatr Soc. 2005;53:963-969.
12. Bledowski J, Trutia A. A review of pharmacologic management and prevention strategies of delirium in the intensive care unit. Psychosomatics. 2012;53:203-211.
13. Breitbart W, Alici-Evcimen Y. Why off-label antipsychotics remain first-choice drugs for delirium. Current Psychiatry. 2007;6(9):49-63.
14. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. doi: 10.1002/14651858.CD006379.pub3.
15. Brummel NE, Girard TD. Preventing delirium in the ICU. Crit Care Clin. 2013;(29):51-65.
16. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008(1):CD005317. doi: 10.1002/14651858.CD005317.
17. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669-676.
18. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097-1101.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
3. Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843-845.
4. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.
5. Levkoff SE, Liptzin B, Evans D, et al. Progression and resolution of delirium in elderly patients hospitalized for acute care. Am J Geriatr Psychiatry. 1994;2:230-238.
6. O’Keefe S, Lavan J. The prognostic significance of delirium in older hospitalized patients. J Am Geriatr Soc. 1997;45:247-248.
7. Tsai MC, Weng HH, Chou SY, et al. One-year mortality of elderly inpatients with delirium, dementia or depression seen by a consultation-liaison service. Psychosomatics. 2012;53:433-438.
8. Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551-556.
9. Marcantonio E, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618-624.
10. Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M27-M34.
11. Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of elders admitted to post-acute facilities with delirium. J Am Geriatr Soc. 2005;53:963-969.
12. Bledowski J, Trutia A. A review of pharmacologic management and prevention strategies of delirium in the intensive care unit. Psychosomatics. 2012;53:203-211.
13. Breitbart W, Alici-Evcimen Y. Why off-label antipsychotics remain first-choice drugs for delirium. Current Psychiatry. 2007;6(9):49-63.
14. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. doi: 10.1002/14651858.CD006379.pub3.
15. Brummel NE, Girard TD. Preventing delirium in the ICU. Crit Care Clin. 2013;(29):51-65.
16. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008(1):CD005317. doi: 10.1002/14651858.CD005317.
17. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669-676.
18. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097-1101.
Making the distinction between dementia and delirium
Let’s tear down the silos and reunify psychiatry and neurology!
A century ago, neurology and psychiatry were one specialty, with unified training, a unified journal (Archives of Neurology and Psychiatry), and a unified board exam through the American Board of Psychiatry and Neurology.1-3 Kraepelin, Alzheimer, Freud, and Meyer were all neuropsychiatrists who treated patients with all brain disorders—stroke, epilepsy, tertiary syphilis, psychosis, depression, and anxiety. Disorders of the “mind,” such as emotions, thought, or behavior, were rightfully regarded as manifestations of cerebral pathology, and research was based on that model.
That was the rational era, when disorders of the brain and its mind were integrated into one specialty.
Why did psychiatry and neurology drift apart?
Two answers to this question come to mind: 1) Freudian theory and psychoanalysis and 2) the inability to localize the brain “lesion” associated with psychiatric disorders.
Although Freud was a neurologist and psychiatrist, his psychodynamic formulation of human behavior was more speculative than empirical, and his psychoanalytic theory was not evidence-based (to be fair, almost all neurologic disorders lacked any treatment 100 years ago). Furthermore, neuropsychiatry did not have the sophisticated brain assessment tools that are available today to physically localize disorders of thought, affect, or mood in the brain. Knowledge of neurochemistry, receptors, neurotransmitters, and brain circuits was nonexistent—let alone an understanding of molecular and cellular neurobiology. The “mind” was therefore divorced, so to speak, from its physical foundation, the brain, and mental illness was erroneously re-conceptualized as “psychological,” not neurological!
In the 1950s, the American Medical Association’s Archives of Neurology and Psychiatry was split into Archives of Neurology and Archives of General Psychiatry. Since then, the two specialties have drifted apart and reduced to a minimum the overlap of their clinical, educational, and research emphases. Much has been lost over the past five decades because of the rupture of diseases of the human brain from diseases of the mind, which includes the most advanced functions of that brain.
The pendulum is swinging back
We are at a point at which advances in understanding of the neurological roots of mental disorders show that psychiatry is as much anchored in the brain as its sister specialty neurology is.4-7 See the box for a list of reasons that explain why the reconciliation and reintegration of neurology and psychiatry are accelerating.
Scientific progress has essentially nullified the reasons that led to the separation of psychiatry and neurology. The road to reintegration is littered with obstacles, however—not the least of which is the stubborn “turfishness” that accumulated over decades of alienation. Clinicians and academicians on both sides are entrenched in their habits and beliefs, and will resist changes to their practice and cherished conceptual models. Why? Bridging the chasm will require new clinical training and revisions to educational and residency curricula.
I believe that the majorities on both sides of the chasm understand the merits of abandoning the fallacious dualism of brain and mind and of merging the two disciplines into the neuropsychiatric specialty that our revered founders upheld and practiced. In fact, neuropsychiatry and behavioral neurology disciplines, which emerged in the 1980s, represent the bridges that recognize the cerebral basis of psychiatric disorders and the psychiatric consequences of neurologic lesions.
Just as all ophthalmologists train as ophthalmologists and then subspecialize into corneal specialists, cataract specialists, vitreoretinal specialists, or neuro-ophthalmologists, so can psychiatrists and neurologists train in neuropsychiatry and then subspecialize to become epileptologists, psychosis specialists, vascular neurologists/neurointensivists, mood disorders specialists, neuromuscular specialists, anxiety experts, and so on. Patients will benefit, because every psychiatric patient deserves a full neurological assessment and treatment and every neurologic patient deserves a full psychiatric assessment and treatment.
The unification of diseases of the brain and diseases of the mind will lead to a higher quality of care and will diminish the stigma of mental illness. In addition, novel strategies for brain repair will advance therapeutics for all brain disorders. Because neuropsychiatric disorders are the leading cause of burden of disease worldwide, early recognition and intervention, as well as prevention, are top public health priorities.
Call to action
The time has come to tear down the silos of neurology and psychiatry and reunify the disciplines, as they were 100 years ago.8 Forward-thinking medical schools should seriously consider this initiative and start moving to consolidate clinical brain disorders and mind disorders into one department. The American Board of Psychiatry and Neurology (which, fortunately, remained integrated during the decades of separation) would then revert to the days when board exam candidates were assessed on neurologic and psychiatric patients—as I was, when I sat for my oral boards.
The reintegration of psychiatry and neurology is good and necessary. It’s a no brainer!
Henry A. Nasrallah, MD
Editor-In-Chief
References
1. Yudofsky SC, Hales EH. Neuropsychiatry and the future of psychiatry and neurology. Am J Psychiatry. 2002;159:1261-1263.
2. Boller F, Dalla Barba G. The evolution of psychiatry and neurology: Two disciplines divided by a common goal? In: Jeste DV, Friedman JH, eds. Psychiatry for neurologists. Totowa, NJ: Human Press, Inc.; 2006:11-18.
3. Martin JB. The integration of neurology, psychiatry, and neuroscience in the 21st century. Am J Psychiatry. 2002;159:695-704.
4. Kandel ER. A new intellectual framework for psychiatry. Am J Psychiatry. 1998;155:457-469.
5. Schiffer RB, Bowen B, Hinderliter J, et al. Neuropsychiatry: a management model for academic medicine. J Neuropsychiatry Clin Neurosci. 2004; 16:336-341.
6. Lee TS, Ng BY, Lee WL. Neuropsychiatry: an emerging field. Ann Acad Med Singapore. 2008;37:601-605.
7. Reynolds CF, Lewis DA, Detre T, et al. The future of psychiatry as a nuclear neuroscience. Acad Med. 2009;84:446-450.
8. White PD, Richards H, Zeman HZ. Time to end the distinction between mental and neurological illnesses. BMJ. 2012;344(e3):4540.
A century ago, neurology and psychiatry were one specialty, with unified training, a unified journal (Archives of Neurology and Psychiatry), and a unified board exam through the American Board of Psychiatry and Neurology.1-3 Kraepelin, Alzheimer, Freud, and Meyer were all neuropsychiatrists who treated patients with all brain disorders—stroke, epilepsy, tertiary syphilis, psychosis, depression, and anxiety. Disorders of the “mind,” such as emotions, thought, or behavior, were rightfully regarded as manifestations of cerebral pathology, and research was based on that model.
That was the rational era, when disorders of the brain and its mind were integrated into one specialty.
Why did psychiatry and neurology drift apart?
Two answers to this question come to mind: 1) Freudian theory and psychoanalysis and 2) the inability to localize the brain “lesion” associated with psychiatric disorders.
Although Freud was a neurologist and psychiatrist, his psychodynamic formulation of human behavior was more speculative than empirical, and his psychoanalytic theory was not evidence-based (to be fair, almost all neurologic disorders lacked any treatment 100 years ago). Furthermore, neuropsychiatry did not have the sophisticated brain assessment tools that are available today to physically localize disorders of thought, affect, or mood in the brain. Knowledge of neurochemistry, receptors, neurotransmitters, and brain circuits was nonexistent—let alone an understanding of molecular and cellular neurobiology. The “mind” was therefore divorced, so to speak, from its physical foundation, the brain, and mental illness was erroneously re-conceptualized as “psychological,” not neurological!
In the 1950s, the American Medical Association’s Archives of Neurology and Psychiatry was split into Archives of Neurology and Archives of General Psychiatry. Since then, the two specialties have drifted apart and reduced to a minimum the overlap of their clinical, educational, and research emphases. Much has been lost over the past five decades because of the rupture of diseases of the human brain from diseases of the mind, which includes the most advanced functions of that brain.
The pendulum is swinging back
We are at a point at which advances in understanding of the neurological roots of mental disorders show that psychiatry is as much anchored in the brain as its sister specialty neurology is.4-7 See the box for a list of reasons that explain why the reconciliation and reintegration of neurology and psychiatry are accelerating.
Scientific progress has essentially nullified the reasons that led to the separation of psychiatry and neurology. The road to reintegration is littered with obstacles, however—not the least of which is the stubborn “turfishness” that accumulated over decades of alienation. Clinicians and academicians on both sides are entrenched in their habits and beliefs, and will resist changes to their practice and cherished conceptual models. Why? Bridging the chasm will require new clinical training and revisions to educational and residency curricula.
I believe that the majorities on both sides of the chasm understand the merits of abandoning the fallacious dualism of brain and mind and of merging the two disciplines into the neuropsychiatric specialty that our revered founders upheld and practiced. In fact, neuropsychiatry and behavioral neurology disciplines, which emerged in the 1980s, represent the bridges that recognize the cerebral basis of psychiatric disorders and the psychiatric consequences of neurologic lesions.
Just as all ophthalmologists train as ophthalmologists and then subspecialize into corneal specialists, cataract specialists, vitreoretinal specialists, or neuro-ophthalmologists, so can psychiatrists and neurologists train in neuropsychiatry and then subspecialize to become epileptologists, psychosis specialists, vascular neurologists/neurointensivists, mood disorders specialists, neuromuscular specialists, anxiety experts, and so on. Patients will benefit, because every psychiatric patient deserves a full neurological assessment and treatment and every neurologic patient deserves a full psychiatric assessment and treatment.
The unification of diseases of the brain and diseases of the mind will lead to a higher quality of care and will diminish the stigma of mental illness. In addition, novel strategies for brain repair will advance therapeutics for all brain disorders. Because neuropsychiatric disorders are the leading cause of burden of disease worldwide, early recognition and intervention, as well as prevention, are top public health priorities.
Call to action
The time has come to tear down the silos of neurology and psychiatry and reunify the disciplines, as they were 100 years ago.8 Forward-thinking medical schools should seriously consider this initiative and start moving to consolidate clinical brain disorders and mind disorders into one department. The American Board of Psychiatry and Neurology (which, fortunately, remained integrated during the decades of separation) would then revert to the days when board exam candidates were assessed on neurologic and psychiatric patients—as I was, when I sat for my oral boards.
The reintegration of psychiatry and neurology is good and necessary. It’s a no brainer!
Henry A. Nasrallah, MD
Editor-In-Chief
References
1. Yudofsky SC, Hales EH. Neuropsychiatry and the future of psychiatry and neurology. Am J Psychiatry. 2002;159:1261-1263.
2. Boller F, Dalla Barba G. The evolution of psychiatry and neurology: Two disciplines divided by a common goal? In: Jeste DV, Friedman JH, eds. Psychiatry for neurologists. Totowa, NJ: Human Press, Inc.; 2006:11-18.
3. Martin JB. The integration of neurology, psychiatry, and neuroscience in the 21st century. Am J Psychiatry. 2002;159:695-704.
4. Kandel ER. A new intellectual framework for psychiatry. Am J Psychiatry. 1998;155:457-469.
5. Schiffer RB, Bowen B, Hinderliter J, et al. Neuropsychiatry: a management model for academic medicine. J Neuropsychiatry Clin Neurosci. 2004; 16:336-341.
6. Lee TS, Ng BY, Lee WL. Neuropsychiatry: an emerging field. Ann Acad Med Singapore. 2008;37:601-605.
7. Reynolds CF, Lewis DA, Detre T, et al. The future of psychiatry as a nuclear neuroscience. Acad Med. 2009;84:446-450.
8. White PD, Richards H, Zeman HZ. Time to end the distinction between mental and neurological illnesses. BMJ. 2012;344(e3):4540.
A century ago, neurology and psychiatry were one specialty, with unified training, a unified journal (Archives of Neurology and Psychiatry), and a unified board exam through the American Board of Psychiatry and Neurology.1-3 Kraepelin, Alzheimer, Freud, and Meyer were all neuropsychiatrists who treated patients with all brain disorders—stroke, epilepsy, tertiary syphilis, psychosis, depression, and anxiety. Disorders of the “mind,” such as emotions, thought, or behavior, were rightfully regarded as manifestations of cerebral pathology, and research was based on that model.
That was the rational era, when disorders of the brain and its mind were integrated into one specialty.
Why did psychiatry and neurology drift apart?
Two answers to this question come to mind: 1) Freudian theory and psychoanalysis and 2) the inability to localize the brain “lesion” associated with psychiatric disorders.
Although Freud was a neurologist and psychiatrist, his psychodynamic formulation of human behavior was more speculative than empirical, and his psychoanalytic theory was not evidence-based (to be fair, almost all neurologic disorders lacked any treatment 100 years ago). Furthermore, neuropsychiatry did not have the sophisticated brain assessment tools that are available today to physically localize disorders of thought, affect, or mood in the brain. Knowledge of neurochemistry, receptors, neurotransmitters, and brain circuits was nonexistent—let alone an understanding of molecular and cellular neurobiology. The “mind” was therefore divorced, so to speak, from its physical foundation, the brain, and mental illness was erroneously re-conceptualized as “psychological,” not neurological!
In the 1950s, the American Medical Association’s Archives of Neurology and Psychiatry was split into Archives of Neurology and Archives of General Psychiatry. Since then, the two specialties have drifted apart and reduced to a minimum the overlap of their clinical, educational, and research emphases. Much has been lost over the past five decades because of the rupture of diseases of the human brain from diseases of the mind, which includes the most advanced functions of that brain.
The pendulum is swinging back
We are at a point at which advances in understanding of the neurological roots of mental disorders show that psychiatry is as much anchored in the brain as its sister specialty neurology is.4-7 See the box for a list of reasons that explain why the reconciliation and reintegration of neurology and psychiatry are accelerating.
Scientific progress has essentially nullified the reasons that led to the separation of psychiatry and neurology. The road to reintegration is littered with obstacles, however—not the least of which is the stubborn “turfishness” that accumulated over decades of alienation. Clinicians and academicians on both sides are entrenched in their habits and beliefs, and will resist changes to their practice and cherished conceptual models. Why? Bridging the chasm will require new clinical training and revisions to educational and residency curricula.
I believe that the majorities on both sides of the chasm understand the merits of abandoning the fallacious dualism of brain and mind and of merging the two disciplines into the neuropsychiatric specialty that our revered founders upheld and practiced. In fact, neuropsychiatry and behavioral neurology disciplines, which emerged in the 1980s, represent the bridges that recognize the cerebral basis of psychiatric disorders and the psychiatric consequences of neurologic lesions.
Just as all ophthalmologists train as ophthalmologists and then subspecialize into corneal specialists, cataract specialists, vitreoretinal specialists, or neuro-ophthalmologists, so can psychiatrists and neurologists train in neuropsychiatry and then subspecialize to become epileptologists, psychosis specialists, vascular neurologists/neurointensivists, mood disorders specialists, neuromuscular specialists, anxiety experts, and so on. Patients will benefit, because every psychiatric patient deserves a full neurological assessment and treatment and every neurologic patient deserves a full psychiatric assessment and treatment.
The unification of diseases of the brain and diseases of the mind will lead to a higher quality of care and will diminish the stigma of mental illness. In addition, novel strategies for brain repair will advance therapeutics for all brain disorders. Because neuropsychiatric disorders are the leading cause of burden of disease worldwide, early recognition and intervention, as well as prevention, are top public health priorities.
Call to action
The time has come to tear down the silos of neurology and psychiatry and reunify the disciplines, as they were 100 years ago.8 Forward-thinking medical schools should seriously consider this initiative and start moving to consolidate clinical brain disorders and mind disorders into one department. The American Board of Psychiatry and Neurology (which, fortunately, remained integrated during the decades of separation) would then revert to the days when board exam candidates were assessed on neurologic and psychiatric patients—as I was, when I sat for my oral boards.
The reintegration of psychiatry and neurology is good and necessary. It’s a no brainer!
Henry A. Nasrallah, MD
Editor-In-Chief
References
1. Yudofsky SC, Hales EH. Neuropsychiatry and the future of psychiatry and neurology. Am J Psychiatry. 2002;159:1261-1263.
2. Boller F, Dalla Barba G. The evolution of psychiatry and neurology: Two disciplines divided by a common goal? In: Jeste DV, Friedman JH, eds. Psychiatry for neurologists. Totowa, NJ: Human Press, Inc.; 2006:11-18.
3. Martin JB. The integration of neurology, psychiatry, and neuroscience in the 21st century. Am J Psychiatry. 2002;159:695-704.
4. Kandel ER. A new intellectual framework for psychiatry. Am J Psychiatry. 1998;155:457-469.
5. Schiffer RB, Bowen B, Hinderliter J, et al. Neuropsychiatry: a management model for academic medicine. J Neuropsychiatry Clin Neurosci. 2004; 16:336-341.
6. Lee TS, Ng BY, Lee WL. Neuropsychiatry: an emerging field. Ann Acad Med Singapore. 2008;37:601-605.
7. Reynolds CF, Lewis DA, Detre T, et al. The future of psychiatry as a nuclear neuroscience. Acad Med. 2009;84:446-450.
8. White PD, Richards H, Zeman HZ. Time to end the distinction between mental and neurological illnesses. BMJ. 2012;344(e3):4540.
Managing major depression
No laughing matter: Laughter is good psychiatric medicine
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
Lithium-induced diabetes insipidus: Prevention and management
Mr. H, age 33, was diagnosed with bipolar I disorder 9 years ago. For the past year, his mood symptoms have been well controlled with lithium 300 mg, 3 times a day, and olanzapine, 20 mg/d. He presents to the outpatient clinic for a routine visit complaining of insomnia, daytime sleepiness, and increased thirst. He also notes that his tremor has become more prominent over the last few weeks. Concerned about his symptoms, Mr. H’s clinician orders a comprehensive laboratory panel (Table).
Upon further questioning, Mr. H’s physician determines that his insomnia is caused by nocturnal urination, which is consistent with fluid and electrolyte imbalances seen in Mr. H’s laboratory panel. Mr. H is diagnosed with lithium-induced diabetes insipidus.
Although lithium’s exact mechanism of action is unknown, it is known that lithium can negatively affect the kidneys.1,2 Typically, antidiuretic hormone (ADH) regulates water permeability in the collecting duct of the nephron, allowing water to be reabsorbed through simple diffusion in the kidney’s collecting duct (Figure).3 Chronic lithium use reduces or desensitizes the kidney’s ability to respond to ADH. Resistance to ADH occurs when lithium accumulates in the cells of the collecting duct and inhibits ADH’s ability to increase water permeability. This inhibition can cause some of Mr. H’s symptoms, such as polydipsia and polyuria, and is estimated to occur in approximately 40% of patients receiving long-term lithium therapy.4,5
Diagnosis
Diagnosing lithium-induced nephrogenic diabetes insipidus (NDI) begins with a history of the patient’s symptoms and ordering lab tests.5 The next step involves a water restriction test, also known as a thirst test, to measure the patient’s ability to concentrate his or her urine. Baseline serum osmolality and electrolytes are compared with new values obtained after completing the water restriction test. Healthy people will have a 2-to-4-fold increase in urine osmolality compared with patients who have NDI. The last step includes administering desmopressin and differentiates between central diabetes insipidus and NDI.6
After desmopressin use, patients who have central diabetes insipidus will have a >50% increase in urine osmolality, whereas patients who have NDI will have <10% increase in urine osmolality. This distinction is important because patients with central diabetes insipidus might have more severe disease and might not benefit from measures commonly used for lithium-induced NDI.7
Prevention and management
Lithium-induced NDI is thought to be dose-dependent and may be prevented by using the lowest effective dose of lithium for an individual patient. It is important that patients taking lithium receive basic electrolyte, hematologic, liver function, renal function, and thyroid function tests at baseline and every 6 to 12 months after the lithium regimen is stable. Additionally, lithium levels should be monitored frequently. The frequency of these tests may range from twice weekly to every 3 to 4 months or longer, depending on the patient’s condition. This monitoring allows the prescriber to quickly identify emerging adverse effects.
Patients with impaired renal function and those with a urine output >3 liters a day are more susceptible to NDI and require monitoring every 3 months. Also, instruct patients to monitor their urine output and educate them about the dangers of fluid and electrolyte imbalances and the signs and symptoms of NDI, such as excessive thirst and urination.1,2
When a patient experiences lithium-induced NDI, re-evaluate treatment and dosage, including simplifying the dosing regimen or switching to once-daily dosing, usually at bedtime. Once-daily dosing results in a lower overall lithium trough, which might allow the kidneys more “drug-free” time.4,5 Additionally, 12-hour lithium levels are approximately 20% higher with once-daily monitoring; continued monitoring is needed during this switch. Patients who have a moderate or severe form of lithium-induced NDI may need to discontinue lithium altogether. There are several options for treating lithium-induced NDI in patients who need to take lithium. Closely monitor kidney function and lithium routinely with these strategies.
Amiloride. This potassium-sparing diuretic minimizes accumulation of lithium by inhibiting collecting duct sodium channels. Studies have shown that amiloride can decrease mean urine volume, increase urine osmolality, and improve the kidneys’ ability to respond to exogenous arginine vasopressin.8
Thiazide diuretics produce mild sodium depletion, which decreases the distal tubule delivery of sodium, therefore increasing water reabsorption in the collecting duct. Hydrochlorothiazide has been shown to reduce urine output by >50% in patients with NDI on a sodium-restricted diet. Hydrochlorothiazide use requires careful monitoring of potassium and lithium levels. Use of a thiazide diuretic also might warrant decreasing the lithium dose by as much as 50% to prevent toxicity.9,10
Low-sodium diet plus hydrochlorothiazide. This route provides another option to decrease urine output during lithium-induced NDI. A reduction in urine output has been shown to be directly proportional to a decrease in salt intake and excretion. Restricting sodium to <2.3 g/d is an appropriate goal for many patients to prevent reoccurring symptoms, which is more than the 3 g/d average that most Americans consume. Potassium and lithium levels must be monitored closely.9
Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs’ ability to inhibit prostaglandin synthesis prevents prostaglandins from antagonizing actions of ADH in the kidney. The result is increased urine concentration via the actions of ADH. Indomethacin has a greater effect than ibuprofen in increasing ADH’s actions on the kidney. Use of concomitant NSAIDs with lithium requires close monitoring of renal function tests.11
1. Ecelbarger CA. Lithium treatment and remodeling of the collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F37-38.
2. Christensen BM, Kim YH, Kwon TH, et al. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F39-48.
3. Francis SG, Gardner DG. Basic and clinical endocrinology. 7th ed. New York, NY: McGraw Hill; 2003:154-158.
4. Stone KA. Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract. 1999;12(1):43-47.
5. Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5(5):270-276.
6. Wesche D, Deen PM, Knoers NV. Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr Nephrol. 2012;27(12):2183-2204.
7. Rose BD, Post TW. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001:754-759,782-783.
8. Batlle DC, von Riotte AB, Gaviria M, et al. Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N Engl J Med. 1985;312(7):408-414.
9. Earley LE, Orloff J. The mechanism of antidiuresis associated with the administration of hydrochlorothiazide to patients with vasopressin-resistant diabetes insipidus. J Clin Invest. 1962;41(11):1988-1997.
10. Kim GH, Lee JW, Oh YK, et al. Antidiuretic effect of hydrochlorothiazide in lithium-induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin-2, Na-Cl co-transporter, and epithelial sodium channel. J Am Soc Nephrol. 2004;15(11):2836-2843.
11. Libber S, Harrison H, Spector D. Treatment of nephrogenic diabetes insipidus with prostaglandin synthesis inhibitors. J Pediatr. 1986;108(2):305-311.
Mr. H, age 33, was diagnosed with bipolar I disorder 9 years ago. For the past year, his mood symptoms have been well controlled with lithium 300 mg, 3 times a day, and olanzapine, 20 mg/d. He presents to the outpatient clinic for a routine visit complaining of insomnia, daytime sleepiness, and increased thirst. He also notes that his tremor has become more prominent over the last few weeks. Concerned about his symptoms, Mr. H’s clinician orders a comprehensive laboratory panel (Table).
Upon further questioning, Mr. H’s physician determines that his insomnia is caused by nocturnal urination, which is consistent with fluid and electrolyte imbalances seen in Mr. H’s laboratory panel. Mr. H is diagnosed with lithium-induced diabetes insipidus.
Although lithium’s exact mechanism of action is unknown, it is known that lithium can negatively affect the kidneys.1,2 Typically, antidiuretic hormone (ADH) regulates water permeability in the collecting duct of the nephron, allowing water to be reabsorbed through simple diffusion in the kidney’s collecting duct (Figure).3 Chronic lithium use reduces or desensitizes the kidney’s ability to respond to ADH. Resistance to ADH occurs when lithium accumulates in the cells of the collecting duct and inhibits ADH’s ability to increase water permeability. This inhibition can cause some of Mr. H’s symptoms, such as polydipsia and polyuria, and is estimated to occur in approximately 40% of patients receiving long-term lithium therapy.4,5
Diagnosis
Diagnosing lithium-induced nephrogenic diabetes insipidus (NDI) begins with a history of the patient’s symptoms and ordering lab tests.5 The next step involves a water restriction test, also known as a thirst test, to measure the patient’s ability to concentrate his or her urine. Baseline serum osmolality and electrolytes are compared with new values obtained after completing the water restriction test. Healthy people will have a 2-to-4-fold increase in urine osmolality compared with patients who have NDI. The last step includes administering desmopressin and differentiates between central diabetes insipidus and NDI.6
After desmopressin use, patients who have central diabetes insipidus will have a >50% increase in urine osmolality, whereas patients who have NDI will have <10% increase in urine osmolality. This distinction is important because patients with central diabetes insipidus might have more severe disease and might not benefit from measures commonly used for lithium-induced NDI.7
Prevention and management
Lithium-induced NDI is thought to be dose-dependent and may be prevented by using the lowest effective dose of lithium for an individual patient. It is important that patients taking lithium receive basic electrolyte, hematologic, liver function, renal function, and thyroid function tests at baseline and every 6 to 12 months after the lithium regimen is stable. Additionally, lithium levels should be monitored frequently. The frequency of these tests may range from twice weekly to every 3 to 4 months or longer, depending on the patient’s condition. This monitoring allows the prescriber to quickly identify emerging adverse effects.
Patients with impaired renal function and those with a urine output >3 liters a day are more susceptible to NDI and require monitoring every 3 months. Also, instruct patients to monitor their urine output and educate them about the dangers of fluid and electrolyte imbalances and the signs and symptoms of NDI, such as excessive thirst and urination.1,2
When a patient experiences lithium-induced NDI, re-evaluate treatment and dosage, including simplifying the dosing regimen or switching to once-daily dosing, usually at bedtime. Once-daily dosing results in a lower overall lithium trough, which might allow the kidneys more “drug-free” time.4,5 Additionally, 12-hour lithium levels are approximately 20% higher with once-daily monitoring; continued monitoring is needed during this switch. Patients who have a moderate or severe form of lithium-induced NDI may need to discontinue lithium altogether. There are several options for treating lithium-induced NDI in patients who need to take lithium. Closely monitor kidney function and lithium routinely with these strategies.
Amiloride. This potassium-sparing diuretic minimizes accumulation of lithium by inhibiting collecting duct sodium channels. Studies have shown that amiloride can decrease mean urine volume, increase urine osmolality, and improve the kidneys’ ability to respond to exogenous arginine vasopressin.8
Thiazide diuretics produce mild sodium depletion, which decreases the distal tubule delivery of sodium, therefore increasing water reabsorption in the collecting duct. Hydrochlorothiazide has been shown to reduce urine output by >50% in patients with NDI on a sodium-restricted diet. Hydrochlorothiazide use requires careful monitoring of potassium and lithium levels. Use of a thiazide diuretic also might warrant decreasing the lithium dose by as much as 50% to prevent toxicity.9,10
Low-sodium diet plus hydrochlorothiazide. This route provides another option to decrease urine output during lithium-induced NDI. A reduction in urine output has been shown to be directly proportional to a decrease in salt intake and excretion. Restricting sodium to <2.3 g/d is an appropriate goal for many patients to prevent reoccurring symptoms, which is more than the 3 g/d average that most Americans consume. Potassium and lithium levels must be monitored closely.9
Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs’ ability to inhibit prostaglandin synthesis prevents prostaglandins from antagonizing actions of ADH in the kidney. The result is increased urine concentration via the actions of ADH. Indomethacin has a greater effect than ibuprofen in increasing ADH’s actions on the kidney. Use of concomitant NSAIDs with lithium requires close monitoring of renal function tests.11
Mr. H, age 33, was diagnosed with bipolar I disorder 9 years ago. For the past year, his mood symptoms have been well controlled with lithium 300 mg, 3 times a day, and olanzapine, 20 mg/d. He presents to the outpatient clinic for a routine visit complaining of insomnia, daytime sleepiness, and increased thirst. He also notes that his tremor has become more prominent over the last few weeks. Concerned about his symptoms, Mr. H’s clinician orders a comprehensive laboratory panel (Table).
Upon further questioning, Mr. H’s physician determines that his insomnia is caused by nocturnal urination, which is consistent with fluid and electrolyte imbalances seen in Mr. H’s laboratory panel. Mr. H is diagnosed with lithium-induced diabetes insipidus.
Although lithium’s exact mechanism of action is unknown, it is known that lithium can negatively affect the kidneys.1,2 Typically, antidiuretic hormone (ADH) regulates water permeability in the collecting duct of the nephron, allowing water to be reabsorbed through simple diffusion in the kidney’s collecting duct (Figure).3 Chronic lithium use reduces or desensitizes the kidney’s ability to respond to ADH. Resistance to ADH occurs when lithium accumulates in the cells of the collecting duct and inhibits ADH’s ability to increase water permeability. This inhibition can cause some of Mr. H’s symptoms, such as polydipsia and polyuria, and is estimated to occur in approximately 40% of patients receiving long-term lithium therapy.4,5
Diagnosis
Diagnosing lithium-induced nephrogenic diabetes insipidus (NDI) begins with a history of the patient’s symptoms and ordering lab tests.5 The next step involves a water restriction test, also known as a thirst test, to measure the patient’s ability to concentrate his or her urine. Baseline serum osmolality and electrolytes are compared with new values obtained after completing the water restriction test. Healthy people will have a 2-to-4-fold increase in urine osmolality compared with patients who have NDI. The last step includes administering desmopressin and differentiates between central diabetes insipidus and NDI.6
After desmopressin use, patients who have central diabetes insipidus will have a >50% increase in urine osmolality, whereas patients who have NDI will have <10% increase in urine osmolality. This distinction is important because patients with central diabetes insipidus might have more severe disease and might not benefit from measures commonly used for lithium-induced NDI.7
Prevention and management
Lithium-induced NDI is thought to be dose-dependent and may be prevented by using the lowest effective dose of lithium for an individual patient. It is important that patients taking lithium receive basic electrolyte, hematologic, liver function, renal function, and thyroid function tests at baseline and every 6 to 12 months after the lithium regimen is stable. Additionally, lithium levels should be monitored frequently. The frequency of these tests may range from twice weekly to every 3 to 4 months or longer, depending on the patient’s condition. This monitoring allows the prescriber to quickly identify emerging adverse effects.
Patients with impaired renal function and those with a urine output >3 liters a day are more susceptible to NDI and require monitoring every 3 months. Also, instruct patients to monitor their urine output and educate them about the dangers of fluid and electrolyte imbalances and the signs and symptoms of NDI, such as excessive thirst and urination.1,2
When a patient experiences lithium-induced NDI, re-evaluate treatment and dosage, including simplifying the dosing regimen or switching to once-daily dosing, usually at bedtime. Once-daily dosing results in a lower overall lithium trough, which might allow the kidneys more “drug-free” time.4,5 Additionally, 12-hour lithium levels are approximately 20% higher with once-daily monitoring; continued monitoring is needed during this switch. Patients who have a moderate or severe form of lithium-induced NDI may need to discontinue lithium altogether. There are several options for treating lithium-induced NDI in patients who need to take lithium. Closely monitor kidney function and lithium routinely with these strategies.
Amiloride. This potassium-sparing diuretic minimizes accumulation of lithium by inhibiting collecting duct sodium channels. Studies have shown that amiloride can decrease mean urine volume, increase urine osmolality, and improve the kidneys’ ability to respond to exogenous arginine vasopressin.8
Thiazide diuretics produce mild sodium depletion, which decreases the distal tubule delivery of sodium, therefore increasing water reabsorption in the collecting duct. Hydrochlorothiazide has been shown to reduce urine output by >50% in patients with NDI on a sodium-restricted diet. Hydrochlorothiazide use requires careful monitoring of potassium and lithium levels. Use of a thiazide diuretic also might warrant decreasing the lithium dose by as much as 50% to prevent toxicity.9,10
Low-sodium diet plus hydrochlorothiazide. This route provides another option to decrease urine output during lithium-induced NDI. A reduction in urine output has been shown to be directly proportional to a decrease in salt intake and excretion. Restricting sodium to <2.3 g/d is an appropriate goal for many patients to prevent reoccurring symptoms, which is more than the 3 g/d average that most Americans consume. Potassium and lithium levels must be monitored closely.9
Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs’ ability to inhibit prostaglandin synthesis prevents prostaglandins from antagonizing actions of ADH in the kidney. The result is increased urine concentration via the actions of ADH. Indomethacin has a greater effect than ibuprofen in increasing ADH’s actions on the kidney. Use of concomitant NSAIDs with lithium requires close monitoring of renal function tests.11
1. Ecelbarger CA. Lithium treatment and remodeling of the collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F37-38.
2. Christensen BM, Kim YH, Kwon TH, et al. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F39-48.
3. Francis SG, Gardner DG. Basic and clinical endocrinology. 7th ed. New York, NY: McGraw Hill; 2003:154-158.
4. Stone KA. Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract. 1999;12(1):43-47.
5. Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5(5):270-276.
6. Wesche D, Deen PM, Knoers NV. Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr Nephrol. 2012;27(12):2183-2204.
7. Rose BD, Post TW. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001:754-759,782-783.
8. Batlle DC, von Riotte AB, Gaviria M, et al. Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N Engl J Med. 1985;312(7):408-414.
9. Earley LE, Orloff J. The mechanism of antidiuresis associated with the administration of hydrochlorothiazide to patients with vasopressin-resistant diabetes insipidus. J Clin Invest. 1962;41(11):1988-1997.
10. Kim GH, Lee JW, Oh YK, et al. Antidiuretic effect of hydrochlorothiazide in lithium-induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin-2, Na-Cl co-transporter, and epithelial sodium channel. J Am Soc Nephrol. 2004;15(11):2836-2843.
11. Libber S, Harrison H, Spector D. Treatment of nephrogenic diabetes insipidus with prostaglandin synthesis inhibitors. J Pediatr. 1986;108(2):305-311.
1. Ecelbarger CA. Lithium treatment and remodeling of the collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F37-38.
2. Christensen BM, Kim YH, Kwon TH, et al. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F39-48.
3. Francis SG, Gardner DG. Basic and clinical endocrinology. 7th ed. New York, NY: McGraw Hill; 2003:154-158.
4. Stone KA. Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract. 1999;12(1):43-47.
5. Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5(5):270-276.
6. Wesche D, Deen PM, Knoers NV. Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr Nephrol. 2012;27(12):2183-2204.
7. Rose BD, Post TW. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001:754-759,782-783.
8. Batlle DC, von Riotte AB, Gaviria M, et al. Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N Engl J Med. 1985;312(7):408-414.
9. Earley LE, Orloff J. The mechanism of antidiuresis associated with the administration of hydrochlorothiazide to patients with vasopressin-resistant diabetes insipidus. J Clin Invest. 1962;41(11):1988-1997.
10. Kim GH, Lee JW, Oh YK, et al. Antidiuretic effect of hydrochlorothiazide in lithium-induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin-2, Na-Cl co-transporter, and epithelial sodium channel. J Am Soc Nephrol. 2004;15(11):2836-2843.
11. Libber S, Harrison H, Spector D. Treatment of nephrogenic diabetes insipidus with prostaglandin synthesis inhibitors. J Pediatr. 1986;108(2):305-311.
Psychotic and needing prayer
CASE: Psychotic and assaultive
Mr. A, age 34, is involuntarily admitted to a psychiatric hospital after assaulting a family member and a police officer. He is charged with 2 counts of first-degree assault. He describes auditory hallucinations and believes God is telling him to refuse medication. One year earlier he was diagnosed with schizophrenia. Mr. A informs hospital staff that he is a Christian Scientist, and his religion precludes him from taking any medications. The local parish of the First Church of Christ, Scientist confirms that he is an active member. One day after admission, Mr. A is threatening and belligerent, and he continues to refuse any treatment.
How would you initially treat Mr. A?a) seek emergency guardianship
b) seek help from the Christian Science community
c) order the appropriate medication to effectively treat his symptoms
TREATMENT: Involuntary treatment
While in the hospital, Mr. A’s psychotic symptoms and aggressive behavior toward the staff and other patients lead to several psychiatric emergencies being declared and involuntary administration of antipsychotic medication. Because IM haloperidol, 5 mg/d, rapidly alleviates his symptoms, there is no need to pursue guardianship. Mr. A asks to meet with a member of the Christian Science community before his discharge, which is arranged. Upon being discharged, Mr. A schedules outpatient treatment at the community mental health center.
The author's observations
Mr. A’s case challenged staff to balance his clinical needs with his religious philosophy. Although psychotic, Mr. A provided a reason for refusing treatment—his belief in Christian Science—which would be considered a valid spiritual choice based on his values. However, his psychiatric symptoms created a dangerous situation for himself and others, which lead to emergency administration of antipsychotics against his will. Resolution of his symptoms did not warrant a petition for guardianship or a long-term involuntary hospitalization (Table 1). Allowing Mr. A to meet with a member of his church was crucial because it validated Mr. A’s religious practices and showed the staff’s willingness to respect his Christian Science beliefs.1,2
Honoring religious beliefs
Christian Science is based on the writings of Mary Baker Eddy and the Bible. Adherents believe that any form of evil, such as sin, disease, or death, is the opposite of God and is an illusion. Health care and treatment within the Christian Science community do not focus on what is wrong with the physical body, but rather what is wrong with the mind. Christian Scientists attempt healing through specific forms of prayer, not conventional methods such as medications or surgery.3 Christian Scientists believe there are no limits to the type of medical conditions that can be healed through prayer. Community members go to Christian Science practitioners for healing via prayer, focusing on the Bible and Mary Baker Eddy’s writings to alleviate their suffering.
The Christian Science church does not forbid its members from receiving conventional medical treatments, although prayer clearly is the preferred method of healing.4 Members can make their own choice about obtaining medical treatment. If they choose medical care, they cannot receive simultaneous treatment from Christian Science practitioners, but they can participate in other church activities. However, members compelled to get medical or psychiatric treatment via a guardianship or a court order can receive concurrent treatment from a Christian Science practitioner.
Other faith traditions generally do not draw such a clear line between medical treatment and religious healing. For example, Jehovah’s Witnesses have no prohibition against obtaining medical care, but they refuse blood transfusions, although they do accept medical alternatives to blood.5
ASSESSMENT: Remorse, reluctance
Mr. A stops taking his medication a few days after discharge, becomes psychotic, assaults his landlord, and is involuntarily readmitted to the hospital. His symptoms again are alleviated with IM haloperidol, 5 mg/d, and Mr. A is remorseful about his behavior while psychotic. He repeats his belief that his illness can be cured with prayer. The staff is reluctant to discharge Mr. A because of his history of nonadherence to treatment and assaultive episodes.
What are the next steps to consider in Mr. A’s treatment?a) seek guardianship because Mr. A does not appreciate the need for treatment
b) obtain a long-term commitment to the hospital with plans to conditionally release Mr. A when he is clinically stable
c) begin treatment with a long-acting injectable antipsychotic
EVALUATION: Next steps
The psychiatrist requests and receives a 3-year commitment for Mr. A from the probate court. The psychiatrist works with Mr. A and the community mental health center clinician to develop a conditional discharge plan in which Mr. A agrees to take medications as prescribed as a condition of his release. Mr. A initially is resistant to this plan. He is allowed to meet frequently with his Christian Science practitioner to discuss ways to continue treatment. Hospital staff supports these meetings, while explaining the importance of adhering to medication and how this will effectively treat his psychotic symptoms. Hospital staff does not negate or minimize Mr. A’s religious beliefs. The Christian Science practitioner allows Mr. A to continue his religious healing while receiving psychiatric care because he is a under court-ordered involuntary commitment. This leads Mr. A to find common ground between his religious beliefs and need for psychiatric treatment. Mr. A maintains his belief that he can be healed by prayer, but agrees to accept medications under the law of the probate commitment. To maximize adherence, he agrees to haloperidol decanoate, a long-acting injectable antipsychotic. He is conditionally discharged to continuing outpatient treatment at the community mental health center.
Mr. A adheres to treatment but begins to develop early signs of tardive dyskinesia (mild lip smacking and some tongue protrusion). Therefore, haloperidol decanoate is discontinued and replaced with oral olanzapine, 20 mg/d. Mr. A is no longer psychotic, and his psychotic symptoms are in remission. He continues to hold fast to his Christian Science beliefs.
One month before the end of his 3-year commitment, Mr. A informs his psychiatrist that he plans to stop his antipsychotic when the commitment ends and to pursue treatment with his Christian Science practitioner via prayer. He wants to prove to everyone that medications are no longer necessary.
What should Mr. A’s treating psychiatrist do?a) immediately readmit Mr. A involuntarily because of his potential dangerousness and impending treatment nonadherence
b) pursue guardianship because Mr. A is incapable of understanding that he has a serious mental illness
c) not pursue legal action but continue to treat Mr. A with antipsychotics and encourage compliance
d) readmit Mr. A to the hospital, request an extension of the commitment order, and consider a medication holiday in a safe setting to address Mr. A’s religious beliefs
OUTCOME: Court-ordered treatment
Mr. A agrees to hospitalization and at a court hearing is committed to the hospital for a period not to exceed 5 years. The judge also orders that Mr. A undergo a period of reducing or stopping his antipsychotic to see if he decompensates. The judicial order states that if it is determined that Mr. A no longer needs medication, the judge may reconsider the terms of the long-term commitment.
Mr. A, his inpatient and outpatient psychiatrists, and a Christian Science practitioner work together to develop a plan to taper his medication. Over 2 weeks, olanzapine is tapered from 20 mg/d to 10 mg/d. Two weeks into the taper, Mr. A becomes increasingly irritable, paranoid, and vigilant. The staff gives him prompt feedback about his apparent decompensation. Mr. A accepts this. He resumes taking olanzapine, 20 mg/d, and his symptoms resolve. He feels discouraged because taking medication is against his religious values. Nevertheless, he accepts the 5-year commitment as a court-mandated treatment that he must abide by. He is conditionally discharged from the hospital. For a summary of Mr. A’s clinical course, see Table 2.
Mr. A continues to do well in the community. New Hampshire’s law allowing up to a 5-year commitment to the hospital has been effective in maximizing Mr. A’s treatment adherence (Table 3).6 He has not been rehospitalized and his psychotic symptoms are in remission. Mr. A still believes his symptoms can be best treated with Christian Science prayer, but sees the state-imposed conditional discharge as a necessary “evil” that he must adhere to. He continues to be an active member of his church.
The author's observations
With the support of his outpatient and inpatient psychiatrists, treatment teams, and Christian Science practitioner, Mr. A has successfully integrated 2 seemingly opposing views regarding treatment, allowing him to live successfully in the community.
From this case, we learned that clinicians:
- need to understand patients’ religious beliefs and how these beliefs can impact their care
- must be aware that caring for patients from different religious traditions may present unique treatment challenges
- need to put their personal views regarding a patient’s religious beliefs aside and work with the patient to alleviate suffering
- must give patients ample opportunity to meet with their faith community, allowing adequate time for discussion and problem solving
Bottom Line
Balancing a patient’s clinical and spiritual needs can be challenging when those needs seem mutually exclusive. Clear communication, legal guidance, careful planning, and a strong therapeutic alliance can create opportunities for the patient to make both needs work to his advantage.
Related Resources
- Christian Science. www.christianscience.com.
- de Nesnera A, Vidaver RM. New Hampshire’s commitment law: treatment implications. New Hampshire Bar Journal. 2007;48(2):68-73.
- Ehman J. Religious diversity: practical points for health care
providers: www.uphs.upenn.edu/pastoral/resed/diversity_points.html.
Drug Brand Names
Haloperidol • Haldol
Olanzapine • Zyprexa
Disclosure
Dr. de Nesnera reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pavlo AM, Bursztajn H, Gutheil T. Christian Science and competence to make treatment choices: clinical challenges in assessing values. Int J Law Psych. 1987;10(4):395-401.
2. Pavlo AM, Bursztajn H, Gutheil T, et al. Weighing religious beliefs in determining competence. Hosp Comm Psych. 1987;38(4):350-352.
3. Eddy MB. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875:1-17.
4. Eddy M. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875: 167.
5. The growing demand for bloodless medicine and surgery. Awake! January 8, 2000:4-6. http://wol.jw.org/en/wol/d/r1/lp-e/102000003. Accessed June 4, 2013.
6. NH Rev Stat Ann § 135-C:27-46.
CASE: Psychotic and assaultive
Mr. A, age 34, is involuntarily admitted to a psychiatric hospital after assaulting a family member and a police officer. He is charged with 2 counts of first-degree assault. He describes auditory hallucinations and believes God is telling him to refuse medication. One year earlier he was diagnosed with schizophrenia. Mr. A informs hospital staff that he is a Christian Scientist, and his religion precludes him from taking any medications. The local parish of the First Church of Christ, Scientist confirms that he is an active member. One day after admission, Mr. A is threatening and belligerent, and he continues to refuse any treatment.
How would you initially treat Mr. A?a) seek emergency guardianship
b) seek help from the Christian Science community
c) order the appropriate medication to effectively treat his symptoms
TREATMENT: Involuntary treatment
While in the hospital, Mr. A’s psychotic symptoms and aggressive behavior toward the staff and other patients lead to several psychiatric emergencies being declared and involuntary administration of antipsychotic medication. Because IM haloperidol, 5 mg/d, rapidly alleviates his symptoms, there is no need to pursue guardianship. Mr. A asks to meet with a member of the Christian Science community before his discharge, which is arranged. Upon being discharged, Mr. A schedules outpatient treatment at the community mental health center.
The author's observations
Mr. A’s case challenged staff to balance his clinical needs with his religious philosophy. Although psychotic, Mr. A provided a reason for refusing treatment—his belief in Christian Science—which would be considered a valid spiritual choice based on his values. However, his psychiatric symptoms created a dangerous situation for himself and others, which lead to emergency administration of antipsychotics against his will. Resolution of his symptoms did not warrant a petition for guardianship or a long-term involuntary hospitalization (Table 1). Allowing Mr. A to meet with a member of his church was crucial because it validated Mr. A’s religious practices and showed the staff’s willingness to respect his Christian Science beliefs.1,2
Honoring religious beliefs
Christian Science is based on the writings of Mary Baker Eddy and the Bible. Adherents believe that any form of evil, such as sin, disease, or death, is the opposite of God and is an illusion. Health care and treatment within the Christian Science community do not focus on what is wrong with the physical body, but rather what is wrong with the mind. Christian Scientists attempt healing through specific forms of prayer, not conventional methods such as medications or surgery.3 Christian Scientists believe there are no limits to the type of medical conditions that can be healed through prayer. Community members go to Christian Science practitioners for healing via prayer, focusing on the Bible and Mary Baker Eddy’s writings to alleviate their suffering.
The Christian Science church does not forbid its members from receiving conventional medical treatments, although prayer clearly is the preferred method of healing.4 Members can make their own choice about obtaining medical treatment. If they choose medical care, they cannot receive simultaneous treatment from Christian Science practitioners, but they can participate in other church activities. However, members compelled to get medical or psychiatric treatment via a guardianship or a court order can receive concurrent treatment from a Christian Science practitioner.
Other faith traditions generally do not draw such a clear line between medical treatment and religious healing. For example, Jehovah’s Witnesses have no prohibition against obtaining medical care, but they refuse blood transfusions, although they do accept medical alternatives to blood.5
ASSESSMENT: Remorse, reluctance
Mr. A stops taking his medication a few days after discharge, becomes psychotic, assaults his landlord, and is involuntarily readmitted to the hospital. His symptoms again are alleviated with IM haloperidol, 5 mg/d, and Mr. A is remorseful about his behavior while psychotic. He repeats his belief that his illness can be cured with prayer. The staff is reluctant to discharge Mr. A because of his history of nonadherence to treatment and assaultive episodes.
What are the next steps to consider in Mr. A’s treatment?a) seek guardianship because Mr. A does not appreciate the need for treatment
b) obtain a long-term commitment to the hospital with plans to conditionally release Mr. A when he is clinically stable
c) begin treatment with a long-acting injectable antipsychotic
EVALUATION: Next steps
The psychiatrist requests and receives a 3-year commitment for Mr. A from the probate court. The psychiatrist works with Mr. A and the community mental health center clinician to develop a conditional discharge plan in which Mr. A agrees to take medications as prescribed as a condition of his release. Mr. A initially is resistant to this plan. He is allowed to meet frequently with his Christian Science practitioner to discuss ways to continue treatment. Hospital staff supports these meetings, while explaining the importance of adhering to medication and how this will effectively treat his psychotic symptoms. Hospital staff does not negate or minimize Mr. A’s religious beliefs. The Christian Science practitioner allows Mr. A to continue his religious healing while receiving psychiatric care because he is a under court-ordered involuntary commitment. This leads Mr. A to find common ground between his religious beliefs and need for psychiatric treatment. Mr. A maintains his belief that he can be healed by prayer, but agrees to accept medications under the law of the probate commitment. To maximize adherence, he agrees to haloperidol decanoate, a long-acting injectable antipsychotic. He is conditionally discharged to continuing outpatient treatment at the community mental health center.
Mr. A adheres to treatment but begins to develop early signs of tardive dyskinesia (mild lip smacking and some tongue protrusion). Therefore, haloperidol decanoate is discontinued and replaced with oral olanzapine, 20 mg/d. Mr. A is no longer psychotic, and his psychotic symptoms are in remission. He continues to hold fast to his Christian Science beliefs.
One month before the end of his 3-year commitment, Mr. A informs his psychiatrist that he plans to stop his antipsychotic when the commitment ends and to pursue treatment with his Christian Science practitioner via prayer. He wants to prove to everyone that medications are no longer necessary.
What should Mr. A’s treating psychiatrist do?a) immediately readmit Mr. A involuntarily because of his potential dangerousness and impending treatment nonadherence
b) pursue guardianship because Mr. A is incapable of understanding that he has a serious mental illness
c) not pursue legal action but continue to treat Mr. A with antipsychotics and encourage compliance
d) readmit Mr. A to the hospital, request an extension of the commitment order, and consider a medication holiday in a safe setting to address Mr. A’s religious beliefs
OUTCOME: Court-ordered treatment
Mr. A agrees to hospitalization and at a court hearing is committed to the hospital for a period not to exceed 5 years. The judge also orders that Mr. A undergo a period of reducing or stopping his antipsychotic to see if he decompensates. The judicial order states that if it is determined that Mr. A no longer needs medication, the judge may reconsider the terms of the long-term commitment.
Mr. A, his inpatient and outpatient psychiatrists, and a Christian Science practitioner work together to develop a plan to taper his medication. Over 2 weeks, olanzapine is tapered from 20 mg/d to 10 mg/d. Two weeks into the taper, Mr. A becomes increasingly irritable, paranoid, and vigilant. The staff gives him prompt feedback about his apparent decompensation. Mr. A accepts this. He resumes taking olanzapine, 20 mg/d, and his symptoms resolve. He feels discouraged because taking medication is against his religious values. Nevertheless, he accepts the 5-year commitment as a court-mandated treatment that he must abide by. He is conditionally discharged from the hospital. For a summary of Mr. A’s clinical course, see Table 2.
Mr. A continues to do well in the community. New Hampshire’s law allowing up to a 5-year commitment to the hospital has been effective in maximizing Mr. A’s treatment adherence (Table 3).6 He has not been rehospitalized and his psychotic symptoms are in remission. Mr. A still believes his symptoms can be best treated with Christian Science prayer, but sees the state-imposed conditional discharge as a necessary “evil” that he must adhere to. He continues to be an active member of his church.
The author's observations
With the support of his outpatient and inpatient psychiatrists, treatment teams, and Christian Science practitioner, Mr. A has successfully integrated 2 seemingly opposing views regarding treatment, allowing him to live successfully in the community.
From this case, we learned that clinicians:
- need to understand patients’ religious beliefs and how these beliefs can impact their care
- must be aware that caring for patients from different religious traditions may present unique treatment challenges
- need to put their personal views regarding a patient’s religious beliefs aside and work with the patient to alleviate suffering
- must give patients ample opportunity to meet with their faith community, allowing adequate time for discussion and problem solving
Bottom Line
Balancing a patient’s clinical and spiritual needs can be challenging when those needs seem mutually exclusive. Clear communication, legal guidance, careful planning, and a strong therapeutic alliance can create opportunities for the patient to make both needs work to his advantage.
Related Resources
- Christian Science. www.christianscience.com.
- de Nesnera A, Vidaver RM. New Hampshire’s commitment law: treatment implications. New Hampshire Bar Journal. 2007;48(2):68-73.
- Ehman J. Religious diversity: practical points for health care
providers: www.uphs.upenn.edu/pastoral/resed/diversity_points.html.
Drug Brand Names
Haloperidol • Haldol
Olanzapine • Zyprexa
Disclosure
Dr. de Nesnera reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Psychotic and assaultive
Mr. A, age 34, is involuntarily admitted to a psychiatric hospital after assaulting a family member and a police officer. He is charged with 2 counts of first-degree assault. He describes auditory hallucinations and believes God is telling him to refuse medication. One year earlier he was diagnosed with schizophrenia. Mr. A informs hospital staff that he is a Christian Scientist, and his religion precludes him from taking any medications. The local parish of the First Church of Christ, Scientist confirms that he is an active member. One day after admission, Mr. A is threatening and belligerent, and he continues to refuse any treatment.
How would you initially treat Mr. A?a) seek emergency guardianship
b) seek help from the Christian Science community
c) order the appropriate medication to effectively treat his symptoms
TREATMENT: Involuntary treatment
While in the hospital, Mr. A’s psychotic symptoms and aggressive behavior toward the staff and other patients lead to several psychiatric emergencies being declared and involuntary administration of antipsychotic medication. Because IM haloperidol, 5 mg/d, rapidly alleviates his symptoms, there is no need to pursue guardianship. Mr. A asks to meet with a member of the Christian Science community before his discharge, which is arranged. Upon being discharged, Mr. A schedules outpatient treatment at the community mental health center.
The author's observations
Mr. A’s case challenged staff to balance his clinical needs with his religious philosophy. Although psychotic, Mr. A provided a reason for refusing treatment—his belief in Christian Science—which would be considered a valid spiritual choice based on his values. However, his psychiatric symptoms created a dangerous situation for himself and others, which lead to emergency administration of antipsychotics against his will. Resolution of his symptoms did not warrant a petition for guardianship or a long-term involuntary hospitalization (Table 1). Allowing Mr. A to meet with a member of his church was crucial because it validated Mr. A’s religious practices and showed the staff’s willingness to respect his Christian Science beliefs.1,2
Honoring religious beliefs
Christian Science is based on the writings of Mary Baker Eddy and the Bible. Adherents believe that any form of evil, such as sin, disease, or death, is the opposite of God and is an illusion. Health care and treatment within the Christian Science community do not focus on what is wrong with the physical body, but rather what is wrong with the mind. Christian Scientists attempt healing through specific forms of prayer, not conventional methods such as medications or surgery.3 Christian Scientists believe there are no limits to the type of medical conditions that can be healed through prayer. Community members go to Christian Science practitioners for healing via prayer, focusing on the Bible and Mary Baker Eddy’s writings to alleviate their suffering.
The Christian Science church does not forbid its members from receiving conventional medical treatments, although prayer clearly is the preferred method of healing.4 Members can make their own choice about obtaining medical treatment. If they choose medical care, they cannot receive simultaneous treatment from Christian Science practitioners, but they can participate in other church activities. However, members compelled to get medical or psychiatric treatment via a guardianship or a court order can receive concurrent treatment from a Christian Science practitioner.
Other faith traditions generally do not draw such a clear line between medical treatment and religious healing. For example, Jehovah’s Witnesses have no prohibition against obtaining medical care, but they refuse blood transfusions, although they do accept medical alternatives to blood.5
ASSESSMENT: Remorse, reluctance
Mr. A stops taking his medication a few days after discharge, becomes psychotic, assaults his landlord, and is involuntarily readmitted to the hospital. His symptoms again are alleviated with IM haloperidol, 5 mg/d, and Mr. A is remorseful about his behavior while psychotic. He repeats his belief that his illness can be cured with prayer. The staff is reluctant to discharge Mr. A because of his history of nonadherence to treatment and assaultive episodes.
What are the next steps to consider in Mr. A’s treatment?a) seek guardianship because Mr. A does not appreciate the need for treatment
b) obtain a long-term commitment to the hospital with plans to conditionally release Mr. A when he is clinically stable
c) begin treatment with a long-acting injectable antipsychotic
EVALUATION: Next steps
The psychiatrist requests and receives a 3-year commitment for Mr. A from the probate court. The psychiatrist works with Mr. A and the community mental health center clinician to develop a conditional discharge plan in which Mr. A agrees to take medications as prescribed as a condition of his release. Mr. A initially is resistant to this plan. He is allowed to meet frequently with his Christian Science practitioner to discuss ways to continue treatment. Hospital staff supports these meetings, while explaining the importance of adhering to medication and how this will effectively treat his psychotic symptoms. Hospital staff does not negate or minimize Mr. A’s religious beliefs. The Christian Science practitioner allows Mr. A to continue his religious healing while receiving psychiatric care because he is a under court-ordered involuntary commitment. This leads Mr. A to find common ground between his religious beliefs and need for psychiatric treatment. Mr. A maintains his belief that he can be healed by prayer, but agrees to accept medications under the law of the probate commitment. To maximize adherence, he agrees to haloperidol decanoate, a long-acting injectable antipsychotic. He is conditionally discharged to continuing outpatient treatment at the community mental health center.
Mr. A adheres to treatment but begins to develop early signs of tardive dyskinesia (mild lip smacking and some tongue protrusion). Therefore, haloperidol decanoate is discontinued and replaced with oral olanzapine, 20 mg/d. Mr. A is no longer psychotic, and his psychotic symptoms are in remission. He continues to hold fast to his Christian Science beliefs.
One month before the end of his 3-year commitment, Mr. A informs his psychiatrist that he plans to stop his antipsychotic when the commitment ends and to pursue treatment with his Christian Science practitioner via prayer. He wants to prove to everyone that medications are no longer necessary.
What should Mr. A’s treating psychiatrist do?a) immediately readmit Mr. A involuntarily because of his potential dangerousness and impending treatment nonadherence
b) pursue guardianship because Mr. A is incapable of understanding that he has a serious mental illness
c) not pursue legal action but continue to treat Mr. A with antipsychotics and encourage compliance
d) readmit Mr. A to the hospital, request an extension of the commitment order, and consider a medication holiday in a safe setting to address Mr. A’s religious beliefs
OUTCOME: Court-ordered treatment
Mr. A agrees to hospitalization and at a court hearing is committed to the hospital for a period not to exceed 5 years. The judge also orders that Mr. A undergo a period of reducing or stopping his antipsychotic to see if he decompensates. The judicial order states that if it is determined that Mr. A no longer needs medication, the judge may reconsider the terms of the long-term commitment.
Mr. A, his inpatient and outpatient psychiatrists, and a Christian Science practitioner work together to develop a plan to taper his medication. Over 2 weeks, olanzapine is tapered from 20 mg/d to 10 mg/d. Two weeks into the taper, Mr. A becomes increasingly irritable, paranoid, and vigilant. The staff gives him prompt feedback about his apparent decompensation. Mr. A accepts this. He resumes taking olanzapine, 20 mg/d, and his symptoms resolve. He feels discouraged because taking medication is against his religious values. Nevertheless, he accepts the 5-year commitment as a court-mandated treatment that he must abide by. He is conditionally discharged from the hospital. For a summary of Mr. A’s clinical course, see Table 2.
Mr. A continues to do well in the community. New Hampshire’s law allowing up to a 5-year commitment to the hospital has been effective in maximizing Mr. A’s treatment adherence (Table 3).6 He has not been rehospitalized and his psychotic symptoms are in remission. Mr. A still believes his symptoms can be best treated with Christian Science prayer, but sees the state-imposed conditional discharge as a necessary “evil” that he must adhere to. He continues to be an active member of his church.
The author's observations
With the support of his outpatient and inpatient psychiatrists, treatment teams, and Christian Science practitioner, Mr. A has successfully integrated 2 seemingly opposing views regarding treatment, allowing him to live successfully in the community.
From this case, we learned that clinicians:
- need to understand patients’ religious beliefs and how these beliefs can impact their care
- must be aware that caring for patients from different religious traditions may present unique treatment challenges
- need to put their personal views regarding a patient’s religious beliefs aside and work with the patient to alleviate suffering
- must give patients ample opportunity to meet with their faith community, allowing adequate time for discussion and problem solving
Bottom Line
Balancing a patient’s clinical and spiritual needs can be challenging when those needs seem mutually exclusive. Clear communication, legal guidance, careful planning, and a strong therapeutic alliance can create opportunities for the patient to make both needs work to his advantage.
Related Resources
- Christian Science. www.christianscience.com.
- de Nesnera A, Vidaver RM. New Hampshire’s commitment law: treatment implications. New Hampshire Bar Journal. 2007;48(2):68-73.
- Ehman J. Religious diversity: practical points for health care
providers: www.uphs.upenn.edu/pastoral/resed/diversity_points.html.
Drug Brand Names
Haloperidol • Haldol
Olanzapine • Zyprexa
Disclosure
Dr. de Nesnera reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pavlo AM, Bursztajn H, Gutheil T. Christian Science and competence to make treatment choices: clinical challenges in assessing values. Int J Law Psych. 1987;10(4):395-401.
2. Pavlo AM, Bursztajn H, Gutheil T, et al. Weighing religious beliefs in determining competence. Hosp Comm Psych. 1987;38(4):350-352.
3. Eddy MB. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875:1-17.
4. Eddy M. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875: 167.
5. The growing demand for bloodless medicine and surgery. Awake! January 8, 2000:4-6. http://wol.jw.org/en/wol/d/r1/lp-e/102000003. Accessed June 4, 2013.
6. NH Rev Stat Ann § 135-C:27-46.
1. Pavlo AM, Bursztajn H, Gutheil T. Christian Science and competence to make treatment choices: clinical challenges in assessing values. Int J Law Psych. 1987;10(4):395-401.
2. Pavlo AM, Bursztajn H, Gutheil T, et al. Weighing religious beliefs in determining competence. Hosp Comm Psych. 1987;38(4):350-352.
3. Eddy MB. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875:1-17.
4. Eddy M. Science and health with key to the scriptures. Boston, MA: Christian Science Publishing Company; 1875: 167.
5. The growing demand for bloodless medicine and surgery. Awake! January 8, 2000:4-6. http://wol.jw.org/en/wol/d/r1/lp-e/102000003. Accessed June 4, 2013.
6. NH Rev Stat Ann § 135-C:27-46.