User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Alternatives to 12-step groups
Persons addicted to drugs often are among the most marginalized psychiatric patients, but are in need of the most support.1 Many of these patients have comorbid medical and psychiatric problems, including difficult-to-treat pathologies that may have developed because of a traumatic experience or an attachment disorder that dominates their emotional lives.2 These patients value clinicians who engage them in an open, nonjudgmental, and empathetic way.
Eliciting a patient’s reasons for change and introducing him (her) to a variety of peer-led recovery group options that complement and support psychotherapy and pharmacotherapy can be valuable. Although most clinicians are aware of the traditional 12-step group model that embraces spirituality, many might know less about other groups that can play an instrumental role in engaging patients and placing them on the path to recovery.
SMART (Self-Management and Recovery Training) Recovery5 is a nonprofit organization that does not employ the 12-step model; instead, it uses evidence-based, non-confrontational, motivational, behavioral, and cognitive approaches to achieve abstinence.
Women for Sobriety6 helps women achieve abstinence.
LifeRing Secular Recovery7 works on empowering the “sober self” through groups that de-emphasize drug and alcohol use in personal histories.
Rational Recovery8 uses the Addictive Voice Recognition Technique to empower people overcoming addictions. This technique trains individuals to recognize the “addictive voice.” It does not support the theory of continuous recovery, or even recovery groups, but enables the user to achieve sobriety independently. This program greatly limits interaction between people overcoming addiction and physicians and counselors—save for periods of serious withdrawal.
The Community Reinforcement Approach (CRA)9 is an evidence-based program that focuses primarily on environmental and social factors influencing sobriety. This behavioral approach emphasizes the role of contingencies that can encourage or discourage sobriety. CRA has been studied in outpatients—predominantly homeless persons—and inpatients, and in a range of abused substances.
Click here for another Pearl on familiarizing yourself with Alcoholics Anonymous dictums.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kreek MJ. Extreme marginalization: addiction and other mental health disorders, stigma, and imprisonment. Ann N Y Acad Sci. 2011;1231:65-72.
2. Wu NS, Schairer LC, Dellor E, et al. Childhood trauma and health outcomes in adults with comorbid substance abuse and mental health disorders. Addict Behav. 2010;35(1):68-71.
3. Moderation Management. http://www.moderation.org. Accessed April 12, 2013.
4. Moderation Management. What is moderation management? http://www.moderation.org/whatisMM.shtml. Accessed August 6, 2013.
5. SMART (Self Management and Recovery Training) Recovery. http://www.smartrecovery.org. Accessed April 12, 2013.
6. Women for Sobriety. http://www.womenforsobriety.org. Accessed April 12, 2013.
7. LifeRing. http://lifering.org. Accessed April 12, 2013.
8. Rational Recovery. http://www.rational.org. Published October 25, 1995. Accessed April 12, 2013.
9. Miller WR, Meyers RJ, Hiller-Sturmhofel S. The community-reinforcement approach. http://pubs.niaaa.nih.gov/publications/arh23-2/116-121.pdf. Accessed August 6, 2013.
Persons addicted to drugs often are among the most marginalized psychiatric patients, but are in need of the most support.1 Many of these patients have comorbid medical and psychiatric problems, including difficult-to-treat pathologies that may have developed because of a traumatic experience or an attachment disorder that dominates their emotional lives.2 These patients value clinicians who engage them in an open, nonjudgmental, and empathetic way.
Eliciting a patient’s reasons for change and introducing him (her) to a variety of peer-led recovery group options that complement and support psychotherapy and pharmacotherapy can be valuable. Although most clinicians are aware of the traditional 12-step group model that embraces spirituality, many might know less about other groups that can play an instrumental role in engaging patients and placing them on the path to recovery.
SMART (Self-Management and Recovery Training) Recovery5 is a nonprofit organization that does not employ the 12-step model; instead, it uses evidence-based, non-confrontational, motivational, behavioral, and cognitive approaches to achieve abstinence.
Women for Sobriety6 helps women achieve abstinence.
LifeRing Secular Recovery7 works on empowering the “sober self” through groups that de-emphasize drug and alcohol use in personal histories.
Rational Recovery8 uses the Addictive Voice Recognition Technique to empower people overcoming addictions. This technique trains individuals to recognize the “addictive voice.” It does not support the theory of continuous recovery, or even recovery groups, but enables the user to achieve sobriety independently. This program greatly limits interaction between people overcoming addiction and physicians and counselors—save for periods of serious withdrawal.
The Community Reinforcement Approach (CRA)9 is an evidence-based program that focuses primarily on environmental and social factors influencing sobriety. This behavioral approach emphasizes the role of contingencies that can encourage or discourage sobriety. CRA has been studied in outpatients—predominantly homeless persons—and inpatients, and in a range of abused substances.
Click here for another Pearl on familiarizing yourself with Alcoholics Anonymous dictums.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Persons addicted to drugs often are among the most marginalized psychiatric patients, but are in need of the most support.1 Many of these patients have comorbid medical and psychiatric problems, including difficult-to-treat pathologies that may have developed because of a traumatic experience or an attachment disorder that dominates their emotional lives.2 These patients value clinicians who engage them in an open, nonjudgmental, and empathetic way.
Eliciting a patient’s reasons for change and introducing him (her) to a variety of peer-led recovery group options that complement and support psychotherapy and pharmacotherapy can be valuable. Although most clinicians are aware of the traditional 12-step group model that embraces spirituality, many might know less about other groups that can play an instrumental role in engaging patients and placing them on the path to recovery.
SMART (Self-Management and Recovery Training) Recovery5 is a nonprofit organization that does not employ the 12-step model; instead, it uses evidence-based, non-confrontational, motivational, behavioral, and cognitive approaches to achieve abstinence.
Women for Sobriety6 helps women achieve abstinence.
LifeRing Secular Recovery7 works on empowering the “sober self” through groups that de-emphasize drug and alcohol use in personal histories.
Rational Recovery8 uses the Addictive Voice Recognition Technique to empower people overcoming addictions. This technique trains individuals to recognize the “addictive voice.” It does not support the theory of continuous recovery, or even recovery groups, but enables the user to achieve sobriety independently. This program greatly limits interaction between people overcoming addiction and physicians and counselors—save for periods of serious withdrawal.
The Community Reinforcement Approach (CRA)9 is an evidence-based program that focuses primarily on environmental and social factors influencing sobriety. This behavioral approach emphasizes the role of contingencies that can encourage or discourage sobriety. CRA has been studied in outpatients—predominantly homeless persons—and inpatients, and in a range of abused substances.
Click here for another Pearl on familiarizing yourself with Alcoholics Anonymous dictums.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kreek MJ. Extreme marginalization: addiction and other mental health disorders, stigma, and imprisonment. Ann N Y Acad Sci. 2011;1231:65-72.
2. Wu NS, Schairer LC, Dellor E, et al. Childhood trauma and health outcomes in adults with comorbid substance abuse and mental health disorders. Addict Behav. 2010;35(1):68-71.
3. Moderation Management. http://www.moderation.org. Accessed April 12, 2013.
4. Moderation Management. What is moderation management? http://www.moderation.org/whatisMM.shtml. Accessed August 6, 2013.
5. SMART (Self Management and Recovery Training) Recovery. http://www.smartrecovery.org. Accessed April 12, 2013.
6. Women for Sobriety. http://www.womenforsobriety.org. Accessed April 12, 2013.
7. LifeRing. http://lifering.org. Accessed April 12, 2013.
8. Rational Recovery. http://www.rational.org. Published October 25, 1995. Accessed April 12, 2013.
9. Miller WR, Meyers RJ, Hiller-Sturmhofel S. The community-reinforcement approach. http://pubs.niaaa.nih.gov/publications/arh23-2/116-121.pdf. Accessed August 6, 2013.
1. Kreek MJ. Extreme marginalization: addiction and other mental health disorders, stigma, and imprisonment. Ann N Y Acad Sci. 2011;1231:65-72.
2. Wu NS, Schairer LC, Dellor E, et al. Childhood trauma and health outcomes in adults with comorbid substance abuse and mental health disorders. Addict Behav. 2010;35(1):68-71.
3. Moderation Management. http://www.moderation.org. Accessed April 12, 2013.
4. Moderation Management. What is moderation management? http://www.moderation.org/whatisMM.shtml. Accessed August 6, 2013.
5. SMART (Self Management and Recovery Training) Recovery. http://www.smartrecovery.org. Accessed April 12, 2013.
6. Women for Sobriety. http://www.womenforsobriety.org. Accessed April 12, 2013.
7. LifeRing. http://lifering.org. Accessed April 12, 2013.
8. Rational Recovery. http://www.rational.org. Published October 25, 1995. Accessed April 12, 2013.
9. Miller WR, Meyers RJ, Hiller-Sturmhofel S. The community-reinforcement approach. http://pubs.niaaa.nih.gov/publications/arh23-2/116-121.pdf. Accessed August 6, 2013.
Obsessed with Facebook
CASE: Paranoid and online
Mr. M, age 22, is brought to the emergency department by family because they are concerned about his paranoia and increasing agitation related to Facebook posts by friends and siblings. At age 8, Mr. M was diagnosed with depression, attention-deficit/hyperactivity disorder (ADHD), and anger management problems, which were well controlled with fluoxetine until last year, when he discontinued psychiatric follow-up. Mr. M’s girlfriend ended their relationship 1 month ago, although it is unclear whether the break-up was caused by his depressive symptoms or exacerbated them. In the last 2 days, his parents have noticed an increase in his delusional thoughts and aggressive behavior.
Family psychiatric history is not significant. Five years ago, Mr. M suffered a head injury in a motor vehicle collision, but completed high school without evidence of cognitive impairment or behavioral changes.
Mr. M appears disheveled and irritable. He reports his mood as “depressed,” but denies suicidal or homicidal ideations. He has no history of violence or antisocial behavior.
Mr. M is alert and oriented with clear speech, intact language, and grossly intact memory and concentration—although, he admits, “I just obsess over certain thoughts.” He endorses feelings of anxiety, insomnia, low energy, lack of sleep secondary to his paranoia, and claims that “something was said on Facebook about a girl and everyone is in on it.” He explains that his Facebook friends talk in “analogies” about him, and reports that, “I can just tell that’s what they are talking about even if they don’t say it directly.”
a) impulse control disorder
b) brief psychotic episode
c) psychotic depression
d) bipolar disorder
The authors’ observations
The last decade has seen a rise in the creation and use of social networking sites such as Facebook, Myspace, and Twitter. Facebook has 1.15 billion monthly active users.1 Seventy-five percent of teenagers own cell phones, and 25% report using their phones to access social media outlets.2 More than 50% of teenagers visit a social networking site daily, with 22% logging in to their favorite social media network more than 10 times a day.3 The easy accessibility of social media outlets has prompted study of the association of that accessibility with anxiety, depression, and self-esteem.3-7
Although not a DSM-5 or ICD-10 diagnosis, internet addiction has been correlated with depression.8 Similarly, O’Keefe and colleagues describe Facebook depression in teens who spend a large amount of time on social networking sites.4 The recently developed Bergen Facebook Addiction Scale (BFAS)9 evaluates the six core elements of addiction (salience, mood modification, tolerance, withdrawal, conflict, and relapse) in Facebook users.
Facebook certainly provides a valuable mechanism for friends to stay connected in an increasingly global society, and has acknowledged the potential it has to address mental illness. In 2011, Facebook partnered with the National Suicide Prevention Lifeline to allow users to report observed suicidal content, thereby utilizing the online community to facilitate delivery of mental health resources.10,11
HISTORY: Sibling rivalry
Mr. M had a romantic relationship with “Ms. B” in high school that he describes as “on and off,” beginning during his sophomore year. He describes himself as a “quick learner” who is task-oriented. He says he was outgoing in high school but became more introverted during his last year there. After high school, Mr. M worked as an electrician and discontinued psychiatric follow-up because he “felt fine.” He lives at home with his parents, two older sisters, and twin brother, who he identifies as being a lifelong “rival.”
After Ms. B ended her relationship with Mr. M, he began to suspect that she had become romantically involved with his twin brother. After Mr. M observed his brother leaving the house one night, he confronted his twin, who denied any involvement with Ms. B. After his brother left, Mr. M became enraged and punched a wall, fracturing his hand.
Two weeks before admission, Mr. M became increasingly preoccupied with suspicions of his brother’s involvement with Ms. B and looked for evidence on Facebook. Mr. M intensely monitored his Facebook news feed, which constantly updates to show public posts made by a user’s Facebook friends. He interpreted his friends’ posts as either directly relating to him or to a new relationship between Ms. B and his twin brother, stating that his friends were “talking in analogies” rather than directly using names.
Mr. M’s Facebook use rapidly increased to 3 or more hours a day. He can access Facebook from his laptop or cell phone, and reports logging in more than 10 times throughout the day. He says that, on Facebook, “it’s easier to talk trash” because people can say things they would not normally say face to face. He also states that Facebook is “ruining personal relationships,” and that it is “so easy to be in touch with everyone without really being in touch.”
The authors’ observations
In Mr. M’s case, Facebook served as a vehicle through which he could pursue a non-bizarre delusion. Mr. M openly admitted to viewing his twin brother as a rival; it is not surprising, therefore, that his delusions targeted his brother and ex-girlfriend.
Before social networks, the perseveration of this delusion might have been limited to internal thinking, or gathering corroborative information by means of stalking. Social media outlets have provided a means to perseverate and implicate others remotely, however, and Mr. M soon expanded his delusions to include more peers.
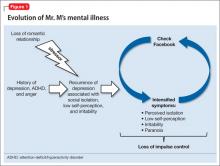
After beginning to suspect that friends and family are commenting on or criticizing him through Facebook, Mr. M experienced an irresistible impulse to repeatedly check the social network, which may have provided short-term relief of anticipatory anxiety, but that perpetuated the cycle. Constant access to the internet facilitated and intensified Mr. M’s cycle of paranoia, anxiety, and dysphoria. He called this process an “addiction.” A conceptual framework of the development of Mr. M’s maladaptive use of Facebook is illustrated in Figure 1.
Risk factors
Insecurity with one’s self-worth also may be a warning sign. Online social networking circumvents the need for physical interaction. A Facebook profile allows a person to selectively portray himself (herself) to the world, which may not be congruous with how his peers see him in everyday life. Patients who fear criticism or judgment may be more prone to maladaptive Facebook use, because they might feel empowered by the control they have over how others see them—online, at least.
Limited or, in Mr. M’s case, singular romantic experience may have influenced the course of his illness. Mr. M described his romantic involvement as a single, tumultuous relationship that lasted several years. Young patients with limited romantic experience may struggle to develop healthy protective mechanisms and may become preoccupied with the details of the situation, such that it interferes with functioning.
Mr. M’s history of ADHD might be a risk factor for abnormal patterns of internet use. Patients with ADHD have increased attentiveness with visually stimulating tasks—specifically, computers and video games.12
Last, it is unclear how, or if, Mr. M’s history of head injury contributed to his symptoms. There were no clear, temporal changes in cognition or emotion associated with the head injury, and he did not receive regular follow-up. Significant cognitive impairment does not appear to be a factor.
a) restart fluoxetine
b) begin an atypical antipsychotic
c) begin a mood stabilizer and atypical antipsychotic
d) encourage Mr. M to deactivate his Facebook account
TREATMENT: Observed use
Quetiapine is selected to target psychosis, agitation, and insomnia characterized by difficulty with sleep initiation. Risperidone is added as a short-term agent to boost antipsychotic effect during the day when Mr. M is not fully responsive to quetiapine alone. Valproic acid is added on admission as a mood stabilizer to target emotional lability, impulsiveness, and possible mania.
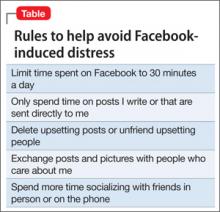
After several days of treatment, and without access to a computer, Mr. M is calmer. We begin to assess the challenges of self-limiting time spent on Facebook; Mr. M explains that, before hospitalization, he had deactivated his Facebook account several times to try to rid himself of what he describes as an “addiction to social media”; soon afterward, however, he experienced overwhelming anxiety that led him to reactivate his account.
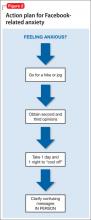
We sit with Mr. M as he logs in to Facebook and discuss the range of alternative explanations that specific public messages on his news feed could have. Explicitly listing alternative explanations is a technique used in cognitive-behavioral therapy. Mr. M begins to demonstrate increased insight regarding his paranoia and possible misinterpretation of information gleaned via Facebook; however, he still believes that masked references to him had existed. During his hospital stay he begins to acknowledge the problems that online interactions pose compared with face-to-face interactions, stating that, “There’s no emotion in [Facebook], so you can easily misinterpret what someone says.”
The authors’ observations
Mr. M was discharged after 7 days of treatment and has been seen weekly as an outpatient for 3 months without need for further hospitalization.
Bottom Line
Pervasive access to social media represents a vehicle for relapse of many psychiatric conditions. Younger patients may be especially at risk because they are more likely to use social media and are in the age range for onset of psychiatric illness. Although some degree of dependence on online networks can be considered normal, patients suffering from mental illness represent a vulnerable population for maladaptive online interactions.
Related Resources
• Sandler EP. If you’re in crisis, go online. Psychology Today. www.psychologytoday.com/blog/promoting-hope-preventing-suicide/201110/if-you-re-in-crisis-go-online. Published October 26, 2011.
• Nitzan U, Shoshan E, Lev-Ran S, et al. Internet-related psychosis−a sign of the times. Isr J Psychiatry Relat Sci. 2011;48(3):207-211.
• Martin EA, Bailey DH, Cicero DC, et al. Social networking profile correlates of schizotypy. Psychiatry Res. 2012;200(2-3):641-646.
Drug Brand Names
Fluoxetine • Prozac Quetiapine • Seroquel
Risperidone • Risperdal Valproic acid • Depakote
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Facebook. Facebook reports second quarter 2013 results. http://investor.fb.com/releasedetail.cfm?ReleaseID= 780093. Updated July 24, 2013. Accessed July 29, 2013.
2. Hinduja S, Patchin JW. Offline consequences of online victimization: school violence and delinquency. Journal of School Violence. 2007;6(3):89-112.
3. Pantic I, Damjanovic A, Todorovic J, et al. Associations between online social networking and depression in high school students: behavioral physiology viewpoint. Psychiatr Danub. 2012;24(1):90-93.
4. O’Keeffe GS, Clarke-Pearson K; Council on Communications and Media. The impact of social media on children, adolescents, and families. Pediatrics. 2011;127(4):800-804.
5. Gonzales AL, Hancock JT. Mirror, mirror on my Facebook wall: effects of exposure to Facebook on self-esteem. Cyberpsychol Behav Soc Netw. 2011;14(1-2):79-83.
6. Hinduja S, Patchin JW. Bullying, cyberbullying, and suicide. Arch Suicide Res. 2010;14(3):206-221.
7. Selfhout MH, Branje SJ, Delsing M, et al. Different types of Internet use, depression, and social anxiety: the role of perceived friendship quality. J Adolesc. 2009;32(4):819-833.
8. Morrison CM, Gore H. The relationship between excessive internet use and depression: a questionnaire-based study of 1,319 young people and adults. Psychopathology. 2010; 43:121-126.
9. Andreassen CS, Torsheim T, Brunborg GS, et al. Development of a Facebook Addiction Scale. Psychol Rep. 2012;110(2):501-517.
10. SAMHSA News. Suicide prevention: a national priority. vol 20, no 3. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
11. Facebook. New partnership between Facebook and the National Suicide Prevention Lifeline http://www.facebook.com/note.php?note_id=310287485658707. Accessed July 25, 2013.
12. Weinstein A, Weizman A. Emerging association between addictive gaming and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2012;14(5):590-597.
CASE: Paranoid and online
Mr. M, age 22, is brought to the emergency department by family because they are concerned about his paranoia and increasing agitation related to Facebook posts by friends and siblings. At age 8, Mr. M was diagnosed with depression, attention-deficit/hyperactivity disorder (ADHD), and anger management problems, which were well controlled with fluoxetine until last year, when he discontinued psychiatric follow-up. Mr. M’s girlfriend ended their relationship 1 month ago, although it is unclear whether the break-up was caused by his depressive symptoms or exacerbated them. In the last 2 days, his parents have noticed an increase in his delusional thoughts and aggressive behavior.
Family psychiatric history is not significant. Five years ago, Mr. M suffered a head injury in a motor vehicle collision, but completed high school without evidence of cognitive impairment or behavioral changes.
Mr. M appears disheveled and irritable. He reports his mood as “depressed,” but denies suicidal or homicidal ideations. He has no history of violence or antisocial behavior.
Mr. M is alert and oriented with clear speech, intact language, and grossly intact memory and concentration—although, he admits, “I just obsess over certain thoughts.” He endorses feelings of anxiety, insomnia, low energy, lack of sleep secondary to his paranoia, and claims that “something was said on Facebook about a girl and everyone is in on it.” He explains that his Facebook friends talk in “analogies” about him, and reports that, “I can just tell that’s what they are talking about even if they don’t say it directly.”
a) impulse control disorder
b) brief psychotic episode
c) psychotic depression
d) bipolar disorder
The authors’ observations
The last decade has seen a rise in the creation and use of social networking sites such as Facebook, Myspace, and Twitter. Facebook has 1.15 billion monthly active users.1 Seventy-five percent of teenagers own cell phones, and 25% report using their phones to access social media outlets.2 More than 50% of teenagers visit a social networking site daily, with 22% logging in to their favorite social media network more than 10 times a day.3 The easy accessibility of social media outlets has prompted study of the association of that accessibility with anxiety, depression, and self-esteem.3-7
Although not a DSM-5 or ICD-10 diagnosis, internet addiction has been correlated with depression.8 Similarly, O’Keefe and colleagues describe Facebook depression in teens who spend a large amount of time on social networking sites.4 The recently developed Bergen Facebook Addiction Scale (BFAS)9 evaluates the six core elements of addiction (salience, mood modification, tolerance, withdrawal, conflict, and relapse) in Facebook users.
Facebook certainly provides a valuable mechanism for friends to stay connected in an increasingly global society, and has acknowledged the potential it has to address mental illness. In 2011, Facebook partnered with the National Suicide Prevention Lifeline to allow users to report observed suicidal content, thereby utilizing the online community to facilitate delivery of mental health resources.10,11
HISTORY: Sibling rivalry
Mr. M had a romantic relationship with “Ms. B” in high school that he describes as “on and off,” beginning during his sophomore year. He describes himself as a “quick learner” who is task-oriented. He says he was outgoing in high school but became more introverted during his last year there. After high school, Mr. M worked as an electrician and discontinued psychiatric follow-up because he “felt fine.” He lives at home with his parents, two older sisters, and twin brother, who he identifies as being a lifelong “rival.”
After Ms. B ended her relationship with Mr. M, he began to suspect that she had become romantically involved with his twin brother. After Mr. M observed his brother leaving the house one night, he confronted his twin, who denied any involvement with Ms. B. After his brother left, Mr. M became enraged and punched a wall, fracturing his hand.
Two weeks before admission, Mr. M became increasingly preoccupied with suspicions of his brother’s involvement with Ms. B and looked for evidence on Facebook. Mr. M intensely monitored his Facebook news feed, which constantly updates to show public posts made by a user’s Facebook friends. He interpreted his friends’ posts as either directly relating to him or to a new relationship between Ms. B and his twin brother, stating that his friends were “talking in analogies” rather than directly using names.
Mr. M’s Facebook use rapidly increased to 3 or more hours a day. He can access Facebook from his laptop or cell phone, and reports logging in more than 10 times throughout the day. He says that, on Facebook, “it’s easier to talk trash” because people can say things they would not normally say face to face. He also states that Facebook is “ruining personal relationships,” and that it is “so easy to be in touch with everyone without really being in touch.”
The authors’ observations
In Mr. M’s case, Facebook served as a vehicle through which he could pursue a non-bizarre delusion. Mr. M openly admitted to viewing his twin brother as a rival; it is not surprising, therefore, that his delusions targeted his brother and ex-girlfriend.
Before social networks, the perseveration of this delusion might have been limited to internal thinking, or gathering corroborative information by means of stalking. Social media outlets have provided a means to perseverate and implicate others remotely, however, and Mr. M soon expanded his delusions to include more peers.
After beginning to suspect that friends and family are commenting on or criticizing him through Facebook, Mr. M experienced an irresistible impulse to repeatedly check the social network, which may have provided short-term relief of anticipatory anxiety, but that perpetuated the cycle. Constant access to the internet facilitated and intensified Mr. M’s cycle of paranoia, anxiety, and dysphoria. He called this process an “addiction.” A conceptual framework of the development of Mr. M’s maladaptive use of Facebook is illustrated in Figure 1.
Risk factors
Insecurity with one’s self-worth also may be a warning sign. Online social networking circumvents the need for physical interaction. A Facebook profile allows a person to selectively portray himself (herself) to the world, which may not be congruous with how his peers see him in everyday life. Patients who fear criticism or judgment may be more prone to maladaptive Facebook use, because they might feel empowered by the control they have over how others see them—online, at least.
Limited or, in Mr. M’s case, singular romantic experience may have influenced the course of his illness. Mr. M described his romantic involvement as a single, tumultuous relationship that lasted several years. Young patients with limited romantic experience may struggle to develop healthy protective mechanisms and may become preoccupied with the details of the situation, such that it interferes with functioning.
Mr. M’s history of ADHD might be a risk factor for abnormal patterns of internet use. Patients with ADHD have increased attentiveness with visually stimulating tasks—specifically, computers and video games.12
Last, it is unclear how, or if, Mr. M’s history of head injury contributed to his symptoms. There were no clear, temporal changes in cognition or emotion associated with the head injury, and he did not receive regular follow-up. Significant cognitive impairment does not appear to be a factor.
a) restart fluoxetine
b) begin an atypical antipsychotic
c) begin a mood stabilizer and atypical antipsychotic
d) encourage Mr. M to deactivate his Facebook account
TREATMENT: Observed use
Quetiapine is selected to target psychosis, agitation, and insomnia characterized by difficulty with sleep initiation. Risperidone is added as a short-term agent to boost antipsychotic effect during the day when Mr. M is not fully responsive to quetiapine alone. Valproic acid is added on admission as a mood stabilizer to target emotional lability, impulsiveness, and possible mania.
After several days of treatment, and without access to a computer, Mr. M is calmer. We begin to assess the challenges of self-limiting time spent on Facebook; Mr. M explains that, before hospitalization, he had deactivated his Facebook account several times to try to rid himself of what he describes as an “addiction to social media”; soon afterward, however, he experienced overwhelming anxiety that led him to reactivate his account.
We sit with Mr. M as he logs in to Facebook and discuss the range of alternative explanations that specific public messages on his news feed could have. Explicitly listing alternative explanations is a technique used in cognitive-behavioral therapy. Mr. M begins to demonstrate increased insight regarding his paranoia and possible misinterpretation of information gleaned via Facebook; however, he still believes that masked references to him had existed. During his hospital stay he begins to acknowledge the problems that online interactions pose compared with face-to-face interactions, stating that, “There’s no emotion in [Facebook], so you can easily misinterpret what someone says.”
The authors’ observations
Mr. M was discharged after 7 days of treatment and has been seen weekly as an outpatient for 3 months without need for further hospitalization.
Bottom Line
Pervasive access to social media represents a vehicle for relapse of many psychiatric conditions. Younger patients may be especially at risk because they are more likely to use social media and are in the age range for onset of psychiatric illness. Although some degree of dependence on online networks can be considered normal, patients suffering from mental illness represent a vulnerable population for maladaptive online interactions.
Related Resources
• Sandler EP. If you’re in crisis, go online. Psychology Today. www.psychologytoday.com/blog/promoting-hope-preventing-suicide/201110/if-you-re-in-crisis-go-online. Published October 26, 2011.
• Nitzan U, Shoshan E, Lev-Ran S, et al. Internet-related psychosis−a sign of the times. Isr J Psychiatry Relat Sci. 2011;48(3):207-211.
• Martin EA, Bailey DH, Cicero DC, et al. Social networking profile correlates of schizotypy. Psychiatry Res. 2012;200(2-3):641-646.
Drug Brand Names
Fluoxetine • Prozac Quetiapine • Seroquel
Risperidone • Risperdal Valproic acid • Depakote
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Paranoid and online
Mr. M, age 22, is brought to the emergency department by family because they are concerned about his paranoia and increasing agitation related to Facebook posts by friends and siblings. At age 8, Mr. M was diagnosed with depression, attention-deficit/hyperactivity disorder (ADHD), and anger management problems, which were well controlled with fluoxetine until last year, when he discontinued psychiatric follow-up. Mr. M’s girlfriend ended their relationship 1 month ago, although it is unclear whether the break-up was caused by his depressive symptoms or exacerbated them. In the last 2 days, his parents have noticed an increase in his delusional thoughts and aggressive behavior.
Family psychiatric history is not significant. Five years ago, Mr. M suffered a head injury in a motor vehicle collision, but completed high school without evidence of cognitive impairment or behavioral changes.
Mr. M appears disheveled and irritable. He reports his mood as “depressed,” but denies suicidal or homicidal ideations. He has no history of violence or antisocial behavior.
Mr. M is alert and oriented with clear speech, intact language, and grossly intact memory and concentration—although, he admits, “I just obsess over certain thoughts.” He endorses feelings of anxiety, insomnia, low energy, lack of sleep secondary to his paranoia, and claims that “something was said on Facebook about a girl and everyone is in on it.” He explains that his Facebook friends talk in “analogies” about him, and reports that, “I can just tell that’s what they are talking about even if they don’t say it directly.”
a) impulse control disorder
b) brief psychotic episode
c) psychotic depression
d) bipolar disorder
The authors’ observations
The last decade has seen a rise in the creation and use of social networking sites such as Facebook, Myspace, and Twitter. Facebook has 1.15 billion monthly active users.1 Seventy-five percent of teenagers own cell phones, and 25% report using their phones to access social media outlets.2 More than 50% of teenagers visit a social networking site daily, with 22% logging in to their favorite social media network more than 10 times a day.3 The easy accessibility of social media outlets has prompted study of the association of that accessibility with anxiety, depression, and self-esteem.3-7
Although not a DSM-5 or ICD-10 diagnosis, internet addiction has been correlated with depression.8 Similarly, O’Keefe and colleagues describe Facebook depression in teens who spend a large amount of time on social networking sites.4 The recently developed Bergen Facebook Addiction Scale (BFAS)9 evaluates the six core elements of addiction (salience, mood modification, tolerance, withdrawal, conflict, and relapse) in Facebook users.
Facebook certainly provides a valuable mechanism for friends to stay connected in an increasingly global society, and has acknowledged the potential it has to address mental illness. In 2011, Facebook partnered with the National Suicide Prevention Lifeline to allow users to report observed suicidal content, thereby utilizing the online community to facilitate delivery of mental health resources.10,11
HISTORY: Sibling rivalry
Mr. M had a romantic relationship with “Ms. B” in high school that he describes as “on and off,” beginning during his sophomore year. He describes himself as a “quick learner” who is task-oriented. He says he was outgoing in high school but became more introverted during his last year there. After high school, Mr. M worked as an electrician and discontinued psychiatric follow-up because he “felt fine.” He lives at home with his parents, two older sisters, and twin brother, who he identifies as being a lifelong “rival.”
After Ms. B ended her relationship with Mr. M, he began to suspect that she had become romantically involved with his twin brother. After Mr. M observed his brother leaving the house one night, he confronted his twin, who denied any involvement with Ms. B. After his brother left, Mr. M became enraged and punched a wall, fracturing his hand.
Two weeks before admission, Mr. M became increasingly preoccupied with suspicions of his brother’s involvement with Ms. B and looked for evidence on Facebook. Mr. M intensely monitored his Facebook news feed, which constantly updates to show public posts made by a user’s Facebook friends. He interpreted his friends’ posts as either directly relating to him or to a new relationship between Ms. B and his twin brother, stating that his friends were “talking in analogies” rather than directly using names.
Mr. M’s Facebook use rapidly increased to 3 or more hours a day. He can access Facebook from his laptop or cell phone, and reports logging in more than 10 times throughout the day. He says that, on Facebook, “it’s easier to talk trash” because people can say things they would not normally say face to face. He also states that Facebook is “ruining personal relationships,” and that it is “so easy to be in touch with everyone without really being in touch.”
The authors’ observations
In Mr. M’s case, Facebook served as a vehicle through which he could pursue a non-bizarre delusion. Mr. M openly admitted to viewing his twin brother as a rival; it is not surprising, therefore, that his delusions targeted his brother and ex-girlfriend.
Before social networks, the perseveration of this delusion might have been limited to internal thinking, or gathering corroborative information by means of stalking. Social media outlets have provided a means to perseverate and implicate others remotely, however, and Mr. M soon expanded his delusions to include more peers.
After beginning to suspect that friends and family are commenting on or criticizing him through Facebook, Mr. M experienced an irresistible impulse to repeatedly check the social network, which may have provided short-term relief of anticipatory anxiety, but that perpetuated the cycle. Constant access to the internet facilitated and intensified Mr. M’s cycle of paranoia, anxiety, and dysphoria. He called this process an “addiction.” A conceptual framework of the development of Mr. M’s maladaptive use of Facebook is illustrated in Figure 1.
Risk factors
Insecurity with one’s self-worth also may be a warning sign. Online social networking circumvents the need for physical interaction. A Facebook profile allows a person to selectively portray himself (herself) to the world, which may not be congruous with how his peers see him in everyday life. Patients who fear criticism or judgment may be more prone to maladaptive Facebook use, because they might feel empowered by the control they have over how others see them—online, at least.
Limited or, in Mr. M’s case, singular romantic experience may have influenced the course of his illness. Mr. M described his romantic involvement as a single, tumultuous relationship that lasted several years. Young patients with limited romantic experience may struggle to develop healthy protective mechanisms and may become preoccupied with the details of the situation, such that it interferes with functioning.
Mr. M’s history of ADHD might be a risk factor for abnormal patterns of internet use. Patients with ADHD have increased attentiveness with visually stimulating tasks—specifically, computers and video games.12
Last, it is unclear how, or if, Mr. M’s history of head injury contributed to his symptoms. There were no clear, temporal changes in cognition or emotion associated with the head injury, and he did not receive regular follow-up. Significant cognitive impairment does not appear to be a factor.
a) restart fluoxetine
b) begin an atypical antipsychotic
c) begin a mood stabilizer and atypical antipsychotic
d) encourage Mr. M to deactivate his Facebook account
TREATMENT: Observed use
Quetiapine is selected to target psychosis, agitation, and insomnia characterized by difficulty with sleep initiation. Risperidone is added as a short-term agent to boost antipsychotic effect during the day when Mr. M is not fully responsive to quetiapine alone. Valproic acid is added on admission as a mood stabilizer to target emotional lability, impulsiveness, and possible mania.
After several days of treatment, and without access to a computer, Mr. M is calmer. We begin to assess the challenges of self-limiting time spent on Facebook; Mr. M explains that, before hospitalization, he had deactivated his Facebook account several times to try to rid himself of what he describes as an “addiction to social media”; soon afterward, however, he experienced overwhelming anxiety that led him to reactivate his account.
We sit with Mr. M as he logs in to Facebook and discuss the range of alternative explanations that specific public messages on his news feed could have. Explicitly listing alternative explanations is a technique used in cognitive-behavioral therapy. Mr. M begins to demonstrate increased insight regarding his paranoia and possible misinterpretation of information gleaned via Facebook; however, he still believes that masked references to him had existed. During his hospital stay he begins to acknowledge the problems that online interactions pose compared with face-to-face interactions, stating that, “There’s no emotion in [Facebook], so you can easily misinterpret what someone says.”
The authors’ observations
Mr. M was discharged after 7 days of treatment and has been seen weekly as an outpatient for 3 months without need for further hospitalization.
Bottom Line
Pervasive access to social media represents a vehicle for relapse of many psychiatric conditions. Younger patients may be especially at risk because they are more likely to use social media and are in the age range for onset of psychiatric illness. Although some degree of dependence on online networks can be considered normal, patients suffering from mental illness represent a vulnerable population for maladaptive online interactions.
Related Resources
• Sandler EP. If you’re in crisis, go online. Psychology Today. www.psychologytoday.com/blog/promoting-hope-preventing-suicide/201110/if-you-re-in-crisis-go-online. Published October 26, 2011.
• Nitzan U, Shoshan E, Lev-Ran S, et al. Internet-related psychosis−a sign of the times. Isr J Psychiatry Relat Sci. 2011;48(3):207-211.
• Martin EA, Bailey DH, Cicero DC, et al. Social networking profile correlates of schizotypy. Psychiatry Res. 2012;200(2-3):641-646.
Drug Brand Names
Fluoxetine • Prozac Quetiapine • Seroquel
Risperidone • Risperdal Valproic acid • Depakote
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Facebook. Facebook reports second quarter 2013 results. http://investor.fb.com/releasedetail.cfm?ReleaseID= 780093. Updated July 24, 2013. Accessed July 29, 2013.
2. Hinduja S, Patchin JW. Offline consequences of online victimization: school violence and delinquency. Journal of School Violence. 2007;6(3):89-112.
3. Pantic I, Damjanovic A, Todorovic J, et al. Associations between online social networking and depression in high school students: behavioral physiology viewpoint. Psychiatr Danub. 2012;24(1):90-93.
4. O’Keeffe GS, Clarke-Pearson K; Council on Communications and Media. The impact of social media on children, adolescents, and families. Pediatrics. 2011;127(4):800-804.
5. Gonzales AL, Hancock JT. Mirror, mirror on my Facebook wall: effects of exposure to Facebook on self-esteem. Cyberpsychol Behav Soc Netw. 2011;14(1-2):79-83.
6. Hinduja S, Patchin JW. Bullying, cyberbullying, and suicide. Arch Suicide Res. 2010;14(3):206-221.
7. Selfhout MH, Branje SJ, Delsing M, et al. Different types of Internet use, depression, and social anxiety: the role of perceived friendship quality. J Adolesc. 2009;32(4):819-833.
8. Morrison CM, Gore H. The relationship between excessive internet use and depression: a questionnaire-based study of 1,319 young people and adults. Psychopathology. 2010; 43:121-126.
9. Andreassen CS, Torsheim T, Brunborg GS, et al. Development of a Facebook Addiction Scale. Psychol Rep. 2012;110(2):501-517.
10. SAMHSA News. Suicide prevention: a national priority. vol 20, no 3. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
11. Facebook. New partnership between Facebook and the National Suicide Prevention Lifeline http://www.facebook.com/note.php?note_id=310287485658707. Accessed July 25, 2013.
12. Weinstein A, Weizman A. Emerging association between addictive gaming and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2012;14(5):590-597.
1. Facebook. Facebook reports second quarter 2013 results. http://investor.fb.com/releasedetail.cfm?ReleaseID= 780093. Updated July 24, 2013. Accessed July 29, 2013.
2. Hinduja S, Patchin JW. Offline consequences of online victimization: school violence and delinquency. Journal of School Violence. 2007;6(3):89-112.
3. Pantic I, Damjanovic A, Todorovic J, et al. Associations between online social networking and depression in high school students: behavioral physiology viewpoint. Psychiatr Danub. 2012;24(1):90-93.
4. O’Keeffe GS, Clarke-Pearson K; Council on Communications and Media. The impact of social media on children, adolescents, and families. Pediatrics. 2011;127(4):800-804.
5. Gonzales AL, Hancock JT. Mirror, mirror on my Facebook wall: effects of exposure to Facebook on self-esteem. Cyberpsychol Behav Soc Netw. 2011;14(1-2):79-83.
6. Hinduja S, Patchin JW. Bullying, cyberbullying, and suicide. Arch Suicide Res. 2010;14(3):206-221.
7. Selfhout MH, Branje SJ, Delsing M, et al. Different types of Internet use, depression, and social anxiety: the role of perceived friendship quality. J Adolesc. 2009;32(4):819-833.
8. Morrison CM, Gore H. The relationship between excessive internet use and depression: a questionnaire-based study of 1,319 young people and adults. Psychopathology. 2010; 43:121-126.
9. Andreassen CS, Torsheim T, Brunborg GS, et al. Development of a Facebook Addiction Scale. Psychol Rep. 2012;110(2):501-517.
10. SAMHSA News. Suicide prevention: a national priority. vol 20, no 3. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
11. Facebook. New partnership between Facebook and the National Suicide Prevention Lifeline http://www.facebook.com/note.php?note_id=310287485658707. Accessed July 25, 2013.
12. Weinstein A, Weizman A. Emerging association between addictive gaming and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2012;14(5):590-597.
Consider this slow-taper program for benzodiazepines
Concerns about prescription medication abuse have led to the creation of remediation plans directed to reduce overuse, multiple prescribers, and diversion of prescribed drugs. One such plan from the United Kingdom, described below, has shown it is possible to taper a patient off of benzodiazepines.1,2
Before starting a tapering plan, inform the patient about the risks of withdrawal.3 Abrupt reductions from high-dose benzodiazepines can result in seizures, psychotic reactions, and agitation.1-3 Understanding the tapering regimen enhances compliance and outcomes. Stress the importance of careful adherence and provide close psychosocial monitoring and fail-safe means for patient contact if someone is experiencing difficulties. Supportive psychotherapy improves the prognosis.4 On a clinical basis, additional, adjunctive, symptomatic, or other medications may be required for safe illness management.
Managing comorbid medical conditions and psychopathologies—including addressing other substances of abuse—is important.1-4 Tapering one or more substances at a time—even nicotine—is not advised. Refer patients to a self-help group or substance abuse rehabilitation program.
Slow tapering is safer and better tolerated than more abrupt techniques.1-5 If the patient experiences overt clinical signs of withdrawal, such as tachycardia or other hyperadrenergia during dosage reduction, maintain the previous dosage until the next tapering date.
For persons who take a short-acting benzodiazepine—eg, alprazolam or lorazepam—convert the dosage into an equivalent dosage of a long-acting benzodiazepine—eg, diazepam.1,2 Metabolized slowly, with a long half-life, diazepam allows a consistent, slow decline in concentration while tapering the dosage. This helps avoid severe withdrawal.1-5 For patients who have been taking alprazolam or clonazepam, 1 mg, the equivalent diazepam dosage would be 20 mg; for temazepam, 30 mg, the diazepam dosage would be 15 mg; for lorazepam, 1 mg, oxazepam, 20 mg, or chlordiazepoxide, 25 mg, the diazepam dosage would be 10 mg.1,2
Prescribe the to-be-tapered benzodiazepine at five-sixths of that dose and prescribe one-sixth of the diazepam amount daily. Proceed with tapering
every 1 to 2 weeks by a one-sixth dose reduction of the tapered medication and a one-sixth increase in diazepam. Continue until diazepam is used alone and well-tolerated.
Once the patient is taking only diazepam, decrease the dosage by 2 mg every 2 weeks until the patient is doing well on a relatively small dosage of diazepam.1,2 Subsequent diazepam reductions are at 1 mg less every 1 to 2 weeks, until the patient is able to completely discontinue the medication.
Continue monitoring until clinical stability is achieved or otherwise indicated. Be aware that some people might switch to other substances of abuse.
1. Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J (Clin Res Ed). 1984;288(6424):1135-1140.
2. Benzodiazepines: how they work and how to withdraw (aka The Ashton Manual). http://www.benzo.org.uk/manual. Accessed March 27, 2013.
3. Lader M. Benzodiazepine harm: how can it be reduced? [published online August 10, 2012] Br J Clin Pharmacol. doi: 10.1111/j.1365-2125.2012.04418.x.
4. Morin CM, Bastien C, Guay B, et al. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161(2):332-342.
5. Lopez-Peig C, Mundet X, Casabella B, et al. Analysis of benzodiazepine withdrawal program managed by primary care nurses in Spain. BMC Res Notes. 2012;5:684. doi: 10.1186/1756-0500-5-684.
Concerns about prescription medication abuse have led to the creation of remediation plans directed to reduce overuse, multiple prescribers, and diversion of prescribed drugs. One such plan from the United Kingdom, described below, has shown it is possible to taper a patient off of benzodiazepines.1,2
Before starting a tapering plan, inform the patient about the risks of withdrawal.3 Abrupt reductions from high-dose benzodiazepines can result in seizures, psychotic reactions, and agitation.1-3 Understanding the tapering regimen enhances compliance and outcomes. Stress the importance of careful adherence and provide close psychosocial monitoring and fail-safe means for patient contact if someone is experiencing difficulties. Supportive psychotherapy improves the prognosis.4 On a clinical basis, additional, adjunctive, symptomatic, or other medications may be required for safe illness management.
Managing comorbid medical conditions and psychopathologies—including addressing other substances of abuse—is important.1-4 Tapering one or more substances at a time—even nicotine—is not advised. Refer patients to a self-help group or substance abuse rehabilitation program.
Slow tapering is safer and better tolerated than more abrupt techniques.1-5 If the patient experiences overt clinical signs of withdrawal, such as tachycardia or other hyperadrenergia during dosage reduction, maintain the previous dosage until the next tapering date.
For persons who take a short-acting benzodiazepine—eg, alprazolam or lorazepam—convert the dosage into an equivalent dosage of a long-acting benzodiazepine—eg, diazepam.1,2 Metabolized slowly, with a long half-life, diazepam allows a consistent, slow decline in concentration while tapering the dosage. This helps avoid severe withdrawal.1-5 For patients who have been taking alprazolam or clonazepam, 1 mg, the equivalent diazepam dosage would be 20 mg; for temazepam, 30 mg, the diazepam dosage would be 15 mg; for lorazepam, 1 mg, oxazepam, 20 mg, or chlordiazepoxide, 25 mg, the diazepam dosage would be 10 mg.1,2
Prescribe the to-be-tapered benzodiazepine at five-sixths of that dose and prescribe one-sixth of the diazepam amount daily. Proceed with tapering
every 1 to 2 weeks by a one-sixth dose reduction of the tapered medication and a one-sixth increase in diazepam. Continue until diazepam is used alone and well-tolerated.
Once the patient is taking only diazepam, decrease the dosage by 2 mg every 2 weeks until the patient is doing well on a relatively small dosage of diazepam.1,2 Subsequent diazepam reductions are at 1 mg less every 1 to 2 weeks, until the patient is able to completely discontinue the medication.
Continue monitoring until clinical stability is achieved or otherwise indicated. Be aware that some people might switch to other substances of abuse.
Concerns about prescription medication abuse have led to the creation of remediation plans directed to reduce overuse, multiple prescribers, and diversion of prescribed drugs. One such plan from the United Kingdom, described below, has shown it is possible to taper a patient off of benzodiazepines.1,2
Before starting a tapering plan, inform the patient about the risks of withdrawal.3 Abrupt reductions from high-dose benzodiazepines can result in seizures, psychotic reactions, and agitation.1-3 Understanding the tapering regimen enhances compliance and outcomes. Stress the importance of careful adherence and provide close psychosocial monitoring and fail-safe means for patient contact if someone is experiencing difficulties. Supportive psychotherapy improves the prognosis.4 On a clinical basis, additional, adjunctive, symptomatic, or other medications may be required for safe illness management.
Managing comorbid medical conditions and psychopathologies—including addressing other substances of abuse—is important.1-4 Tapering one or more substances at a time—even nicotine—is not advised. Refer patients to a self-help group or substance abuse rehabilitation program.
Slow tapering is safer and better tolerated than more abrupt techniques.1-5 If the patient experiences overt clinical signs of withdrawal, such as tachycardia or other hyperadrenergia during dosage reduction, maintain the previous dosage until the next tapering date.
For persons who take a short-acting benzodiazepine—eg, alprazolam or lorazepam—convert the dosage into an equivalent dosage of a long-acting benzodiazepine—eg, diazepam.1,2 Metabolized slowly, with a long half-life, diazepam allows a consistent, slow decline in concentration while tapering the dosage. This helps avoid severe withdrawal.1-5 For patients who have been taking alprazolam or clonazepam, 1 mg, the equivalent diazepam dosage would be 20 mg; for temazepam, 30 mg, the diazepam dosage would be 15 mg; for lorazepam, 1 mg, oxazepam, 20 mg, or chlordiazepoxide, 25 mg, the diazepam dosage would be 10 mg.1,2
Prescribe the to-be-tapered benzodiazepine at five-sixths of that dose and prescribe one-sixth of the diazepam amount daily. Proceed with tapering
every 1 to 2 weeks by a one-sixth dose reduction of the tapered medication and a one-sixth increase in diazepam. Continue until diazepam is used alone and well-tolerated.
Once the patient is taking only diazepam, decrease the dosage by 2 mg every 2 weeks until the patient is doing well on a relatively small dosage of diazepam.1,2 Subsequent diazepam reductions are at 1 mg less every 1 to 2 weeks, until the patient is able to completely discontinue the medication.
Continue monitoring until clinical stability is achieved or otherwise indicated. Be aware that some people might switch to other substances of abuse.
1. Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J (Clin Res Ed). 1984;288(6424):1135-1140.
2. Benzodiazepines: how they work and how to withdraw (aka The Ashton Manual). http://www.benzo.org.uk/manual. Accessed March 27, 2013.
3. Lader M. Benzodiazepine harm: how can it be reduced? [published online August 10, 2012] Br J Clin Pharmacol. doi: 10.1111/j.1365-2125.2012.04418.x.
4. Morin CM, Bastien C, Guay B, et al. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161(2):332-342.
5. Lopez-Peig C, Mundet X, Casabella B, et al. Analysis of benzodiazepine withdrawal program managed by primary care nurses in Spain. BMC Res Notes. 2012;5:684. doi: 10.1186/1756-0500-5-684.
1. Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J (Clin Res Ed). 1984;288(6424):1135-1140.
2. Benzodiazepines: how they work and how to withdraw (aka The Ashton Manual). http://www.benzo.org.uk/manual. Accessed March 27, 2013.
3. Lader M. Benzodiazepine harm: how can it be reduced? [published online August 10, 2012] Br J Clin Pharmacol. doi: 10.1111/j.1365-2125.2012.04418.x.
4. Morin CM, Bastien C, Guay B, et al. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161(2):332-342.
5. Lopez-Peig C, Mundet X, Casabella B, et al. Analysis of benzodiazepine withdrawal program managed by primary care nurses in Spain. BMC Res Notes. 2012;5:684. doi: 10.1186/1756-0500-5-684.
Do glucocorticoids hold promise as a treatment for PTSD?
As symptoms of posttraumatic stress disorder (PTSD) progress, the involved person’s physical and mental health deteriorates.1 This sparks lifestyle changes that allow them to avoid re-exposure to triggering stimuli; however, it also increases their risk of social isolation. Early clinical investigation has found that patients who experience hyperarousal symptoms of overt PTSD—difficulty sleeping, emotional dyscontrol, hypervigilance, and an enhanced startle response—could benefit from the stress-reducing capacity of glucocorticoids.
Decreased glucocorticoids
After a distressing situation, norepinephrine levels rise acutely.2,3 This contributes to a protective retention of potentially threatening memories, which is how people learn to avoid danger.
Glucocorticoid secretion enhances a patient’s coping mechanisms by helping them process information in a way that diminishes retrieval of fear-evoking memories.2,3 Glucocorticoid, also called cortisol, is referred to as a “stress hormone.” Cortisol promotes emotional adaptability following a traumatic event; this action diminishes future, inappropriate retrieval of frightening memories as a physiologic mechanism to help people cope with upsetting situations.3
PTSD pathogenesis involves altered hypothalamic-pituitary-adrenal axis function; sustained stress results in decreased levels of circulating glucocorticoid. This is a consequence of enhanced negative feedback and increased glucocorticoid receptor sensitivity, which is evidenced by results of abnormal dexamethasone suppression tests.1 Downregulation of corticotropin-releasing hormone (CRH) receptors in the pituitary glands and increased CRH levels have been documented in PTSD patients.1,4 An association between high CRH levels and an increase in startle response explains the exaggerated startle response observed in patients with PTSD. Higher circulating glucocorticoid has the opposite effect4; there is an inverse relationship between the daily level of glucocorticoid and startle amplitude. A low level of circulating glucocorticoid promotes recall of frightening events that results in persistent re-experiencing of traumatic memories.2,3
Glucocorticoids in PTSD
Glucocorticoid administration reduces psychological and physiological responses to stress.3 Exogenous glucocorticoid administration affects cognition by interacting with serotonin, dopamine, and ã-aminobutyric acid by actions on the amygdala, medial prefrontal cortex, and hippocampus.2,3 Research among veterans with and without PTSD recorded a decrease in startle response after administration of a single dose of 20 mg of hydrocortisone.4 Results of a large study documented that one dose of hydrocortisone administered at >35 mg can inhibit threatening memories and improve social function.3 Hydrocortisone is linked to anxiolytic effects in healthy persons and patients with social phobia or panic disorder.3,4 Because treatment of PTSD with antidepressants and benzodiazepines often is ineffective,5 glucocorticoids may offer a new pharmacotherapy option. Glucocorticoids have been prescribed as prophylactic agents shortly after an acutely stressful event to prevent development of PTSD.4 Hydrocortisone is not FDA-approved to treat PTSD; informed consent, physician discretion, and close monitoring are emphasized.
Glucocorticoid use in mitigating PTSD symptom emergence is under investigation. Research suggests that just one acute dose of hydrocortisone might benefit patients prone to PTSD.3,4 Further study is needed to establish whether prescribing hydrocortisone is efficacious.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones T, Moller MD. Implications of hypothalamic-pituitary-adrenal axis functioning in posttraumatic stress disorder. J Am Psychiatr Nurses Assoc. 2011;17(6):393-403.
2. Blundell J, Blaiss CA, Lagace DC, et al. Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiol Learn Mem. 2011;95(4):453-460.
3. Putman P, Roelofs K. Effects of single cortisol administrations on human affect reviewed: coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology. 2011;36(4):439-448.
4. Miller MW, McKinney AE, Kanter FS, et al. Hydrocortisone suppression of the fear-potentiated startle response and posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(7):970-980.
5. Nin MS, Martinez LA, Pibiri F, et al. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol (Lausanne). 2011;2(73):1-12.
As symptoms of posttraumatic stress disorder (PTSD) progress, the involved person’s physical and mental health deteriorates.1 This sparks lifestyle changes that allow them to avoid re-exposure to triggering stimuli; however, it also increases their risk of social isolation. Early clinical investigation has found that patients who experience hyperarousal symptoms of overt PTSD—difficulty sleeping, emotional dyscontrol, hypervigilance, and an enhanced startle response—could benefit from the stress-reducing capacity of glucocorticoids.
Decreased glucocorticoids
After a distressing situation, norepinephrine levels rise acutely.2,3 This contributes to a protective retention of potentially threatening memories, which is how people learn to avoid danger.
Glucocorticoid secretion enhances a patient’s coping mechanisms by helping them process information in a way that diminishes retrieval of fear-evoking memories.2,3 Glucocorticoid, also called cortisol, is referred to as a “stress hormone.” Cortisol promotes emotional adaptability following a traumatic event; this action diminishes future, inappropriate retrieval of frightening memories as a physiologic mechanism to help people cope with upsetting situations.3
PTSD pathogenesis involves altered hypothalamic-pituitary-adrenal axis function; sustained stress results in decreased levels of circulating glucocorticoid. This is a consequence of enhanced negative feedback and increased glucocorticoid receptor sensitivity, which is evidenced by results of abnormal dexamethasone suppression tests.1 Downregulation of corticotropin-releasing hormone (CRH) receptors in the pituitary glands and increased CRH levels have been documented in PTSD patients.1,4 An association between high CRH levels and an increase in startle response explains the exaggerated startle response observed in patients with PTSD. Higher circulating glucocorticoid has the opposite effect4; there is an inverse relationship between the daily level of glucocorticoid and startle amplitude. A low level of circulating glucocorticoid promotes recall of frightening events that results in persistent re-experiencing of traumatic memories.2,3
Glucocorticoids in PTSD
Glucocorticoid administration reduces psychological and physiological responses to stress.3 Exogenous glucocorticoid administration affects cognition by interacting with serotonin, dopamine, and ã-aminobutyric acid by actions on the amygdala, medial prefrontal cortex, and hippocampus.2,3 Research among veterans with and without PTSD recorded a decrease in startle response after administration of a single dose of 20 mg of hydrocortisone.4 Results of a large study documented that one dose of hydrocortisone administered at >35 mg can inhibit threatening memories and improve social function.3 Hydrocortisone is linked to anxiolytic effects in healthy persons and patients with social phobia or panic disorder.3,4 Because treatment of PTSD with antidepressants and benzodiazepines often is ineffective,5 glucocorticoids may offer a new pharmacotherapy option. Glucocorticoids have been prescribed as prophylactic agents shortly after an acutely stressful event to prevent development of PTSD.4 Hydrocortisone is not FDA-approved to treat PTSD; informed consent, physician discretion, and close monitoring are emphasized.
Glucocorticoid use in mitigating PTSD symptom emergence is under investigation. Research suggests that just one acute dose of hydrocortisone might benefit patients prone to PTSD.3,4 Further study is needed to establish whether prescribing hydrocortisone is efficacious.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
As symptoms of posttraumatic stress disorder (PTSD) progress, the involved person’s physical and mental health deteriorates.1 This sparks lifestyle changes that allow them to avoid re-exposure to triggering stimuli; however, it also increases their risk of social isolation. Early clinical investigation has found that patients who experience hyperarousal symptoms of overt PTSD—difficulty sleeping, emotional dyscontrol, hypervigilance, and an enhanced startle response—could benefit from the stress-reducing capacity of glucocorticoids.
Decreased glucocorticoids
After a distressing situation, norepinephrine levels rise acutely.2,3 This contributes to a protective retention of potentially threatening memories, which is how people learn to avoid danger.
Glucocorticoid secretion enhances a patient’s coping mechanisms by helping them process information in a way that diminishes retrieval of fear-evoking memories.2,3 Glucocorticoid, also called cortisol, is referred to as a “stress hormone.” Cortisol promotes emotional adaptability following a traumatic event; this action diminishes future, inappropriate retrieval of frightening memories as a physiologic mechanism to help people cope with upsetting situations.3
PTSD pathogenesis involves altered hypothalamic-pituitary-adrenal axis function; sustained stress results in decreased levels of circulating glucocorticoid. This is a consequence of enhanced negative feedback and increased glucocorticoid receptor sensitivity, which is evidenced by results of abnormal dexamethasone suppression tests.1 Downregulation of corticotropin-releasing hormone (CRH) receptors in the pituitary glands and increased CRH levels have been documented in PTSD patients.1,4 An association between high CRH levels and an increase in startle response explains the exaggerated startle response observed in patients with PTSD. Higher circulating glucocorticoid has the opposite effect4; there is an inverse relationship between the daily level of glucocorticoid and startle amplitude. A low level of circulating glucocorticoid promotes recall of frightening events that results in persistent re-experiencing of traumatic memories.2,3
Glucocorticoids in PTSD
Glucocorticoid administration reduces psychological and physiological responses to stress.3 Exogenous glucocorticoid administration affects cognition by interacting with serotonin, dopamine, and ã-aminobutyric acid by actions on the amygdala, medial prefrontal cortex, and hippocampus.2,3 Research among veterans with and without PTSD recorded a decrease in startle response after administration of a single dose of 20 mg of hydrocortisone.4 Results of a large study documented that one dose of hydrocortisone administered at >35 mg can inhibit threatening memories and improve social function.3 Hydrocortisone is linked to anxiolytic effects in healthy persons and patients with social phobia or panic disorder.3,4 Because treatment of PTSD with antidepressants and benzodiazepines often is ineffective,5 glucocorticoids may offer a new pharmacotherapy option. Glucocorticoids have been prescribed as prophylactic agents shortly after an acutely stressful event to prevent development of PTSD.4 Hydrocortisone is not FDA-approved to treat PTSD; informed consent, physician discretion, and close monitoring are emphasized.
Glucocorticoid use in mitigating PTSD symptom emergence is under investigation. Research suggests that just one acute dose of hydrocortisone might benefit patients prone to PTSD.3,4 Further study is needed to establish whether prescribing hydrocortisone is efficacious.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones T, Moller MD. Implications of hypothalamic-pituitary-adrenal axis functioning in posttraumatic stress disorder. J Am Psychiatr Nurses Assoc. 2011;17(6):393-403.
2. Blundell J, Blaiss CA, Lagace DC, et al. Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiol Learn Mem. 2011;95(4):453-460.
3. Putman P, Roelofs K. Effects of single cortisol administrations on human affect reviewed: coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology. 2011;36(4):439-448.
4. Miller MW, McKinney AE, Kanter FS, et al. Hydrocortisone suppression of the fear-potentiated startle response and posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(7):970-980.
5. Nin MS, Martinez LA, Pibiri F, et al. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol (Lausanne). 2011;2(73):1-12.
1. Jones T, Moller MD. Implications of hypothalamic-pituitary-adrenal axis functioning in posttraumatic stress disorder. J Am Psychiatr Nurses Assoc. 2011;17(6):393-403.
2. Blundell J, Blaiss CA, Lagace DC, et al. Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiol Learn Mem. 2011;95(4):453-460.
3. Putman P, Roelofs K. Effects of single cortisol administrations on human affect reviewed: coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology. 2011;36(4):439-448.
4. Miller MW, McKinney AE, Kanter FS, et al. Hydrocortisone suppression of the fear-potentiated startle response and posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(7):970-980.
5. Nin MS, Martinez LA, Pibiri F, et al. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol (Lausanne). 2011;2(73):1-12.
Using ECT
How best to engage patients in their psychiatric care
Providing patients and their families with information and education about their psychiatric illness is a central tenet of mental health care. Discussions about diagnostic impressions, treatment options, and the risks and benefits of interventions are customary. Additionally, patients and families often receive written material or referral to other information sources, including self-help books and a growing number of online resources. Although patient education remains a useful and expected element of good care, there is evidence that, alone, it is insufficient to change health behaviors.1
A growing body of literature and clinical experience suggests that self-management strategies complement patient education and improve treatment outcomes for patients with chronic illnesses, including psychiatric conditions.2,3 The Cochrane Collaboration describes patient education as “teaching or training of patients concerning their own health needs,” and self-management as “the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a long-term disorder.”4
In this article, we review:
• characteristics of long-term care models
• literature supporting the benefits of self-management programs
• clinical initiatives illustrating important elements of self-management support
• opportunities and challenges faced by clinicians, patients, families, clinics, and healthcare systems implementing self-management programs.
Principles of self-management
Chronic care models must account for conditions in which the clinical course can be variable, that are not amenable to a cure, and that demand long-term treatment. Optimal health outcomes rely on patients accurately monitoring, reporting, and responding to their symptoms, while engaging in critical health-related behaviors. In addition, clinicians must teach, partner with, and motivate patients to engage in crucial disease-management activities. Although typically not considered in this light, we believe most psychiatric disorders are best approached through a long-term care model, and benefit from self-management principles.
Basics of self-management include:
• patients must formulate goals and learn skills relevant to their disease
• problems are patient-selected and targeted with individualized, flexible treatment plans.
Corbin and Strauss believe effective “self-managers” achieve competency in three areas:
• role management, which entails healthy adjustments to changes in role
responsibilities, expectations, and self-identity
• emotional management, which often is particularly challenging in psychiatric conditions because of the emotional disruption inherent in living with a psychiatric illness.6
Proficiency in these three “self-manager” domains is enhanced by mastering five key self-management skills outlined in Table 1.7
Successful intervention programs vary widely with regard to individual vs group formats, communication interface, and involved health professionals. However, evidence indicates that problem solving, decision making, and action planning are key components.7 Successful planning includes:
• detailed descriptions of what, how, when, and where the activity will be accomplished
• assessment of patient confidence and adjustment of plans if confidence is limited
• continuous monitoring and self-tailoring of plans through collaborative discussions with providers, fostering a spirit of partnership and ownership.
Table 2 illustrates elements of successful action plans in our Action Planning Worksheet. Adapted from the work of Scharzer,8 Prochaska,9 and Clark,10 we developed this self-management tool for individual and group treatment settings. It serves as a vehicle for collaborative patient-provider discussion and planning. The nuts-and-bolts nature of the discussions inevitably leads to learning new, important, and often unexpected information about our patients’ daily lives and the challenges they face as they share their dominant priorities, fears, and insecurities.
Patients provide consistently positive feedback about action planning, and clinicians often find that the process reveals fruitful areas for further psychotherapeutic intervention. Examples include identifying a range of negative automatic thoughts or catastrophic thinking impeding initiation of important activation, and exposure activities for depressed or anxious patients.
Knowing what to do is different than actually doing it. Changing behavior is difficult in the best circumstances, let alone with the strain of a chronic illness. It is critical to recognize that the presence of depressive symptoms significantly reduces the likelihood that patients will employ self-management practices.11 When combined with anxiety and the impairment of motivation and executive functioning that is common in psychiatric conditions, it is not surprising that patients with a mental health condition struggle to embrace ownership of their illness and engage in critical health behaviors—which may include adhering to medication regimens; maintaining a healthy sleep cycle, nutrition, and exercise routines; vigilant symptom surveillance; and carrying out an agreed-upon action plan.
Interventions
Professionally-guided “light-touch” interventions and technology-assisted self-management interventions also can improve patient engagement in activities through individual encounters, group forums, and technology-mediated exchanges, including telephone, email, text message, tele-health, and web-based interventions.13 The DE-STRESS (Delivery of Self Training and Education for Stressful Situations) model illustrates these principles. This 8-week program combines elements of face-to-face, email, telephone, and web-based assignments and exchanges, and demonstrates a decline in posttraumatic stress disorder, depression, and anxiety scores.14 Our Michigan Depression Outreach and Collaborative Care program is another example of a self-management intervention (Box 1).
• modeling and social persuasion through having patients observe and engage others as they struggle to overcome similar obstacles
• re-interpretation of symptoms aimed at fostering the belief that symptoms generally are multi-determined with several potential explanations, and vary with daily routines.
Helping patients understand when common symptoms such as impaired concentration or dizziness should be “watched”—rather than responded to aggressively—is crucial for effective long-term management.
Challenges
Health care delivery and medical educational models have been slow to embrace this change to long-term care models because doing so involves what might be uncomfortable shifts in roles and responsibilities. Effective care for long-term illness necessitates that the patient become an expert on his (her) illness, and be an active participant and partner in their treatment. Preparing health professionals for this new role as teacher, mentor, and collaborator presents a challenge to health care systems and educational programs across disciplines.
When trying to facilitate effective patient-provider partnerships, it is important to recognize the variability in patient preference for what and how information is shared, how decisions are made, and the role patients are asked to play in their care. Patients differ in their desire for an active or collaborative shared-decision model; some prefer more directive provider communication and a passive role.18 Preferences are influenced by variables such as age, sex, race, anxiety level, and education.19 Open discussion of these matters between caregivers and patients is important; studies have shown that failure to address these issues of “fit” can
impede communication, healthy behavior, and positive outcomes.
Bottom Line
Emerging care models demand that health care providers become teachers and motivators to help patients develop and implement patterns of health surveillance and intervention that will optimize their well-being and functionality. As active collaborators in their care, patients form a partnership with their care teams, allowing for regular, reciprocal exchange of information and shared decision-making. This shift to a partnership creates new, exciting roles and responsibilities for all parties.
Related Resources
- Improving Chronic Illness Care. www.improvingchroniccare.org.
- Chronic disease self-management program (Better Choices, Better Health workshop). Stanford School of Medicine. http://patienteducation.stanford.edu/programs/cdsmp.html.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Thomas E. Fluent, MD, talks about addressing patient resistance to a self-management model of care. Dr. Fluent is Clinical Assistant Professor, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan.
1. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-2475.
2. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909-1914.
3. Druss BG, Zhao L, von Esenwein SA, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res. 2010;118(1-3):264-270.
4. Tomkins S, Collins A. Promoting optimal self-care: consultation techniques that improve quality of life for patients and clinicians. London, United Kingdom: National Health Service; 2005.
5. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511-544.
6. Corbin JM, Strauss AL. Unending work and care : managing chronic illness at home. 1st ed. San Francisco, CA: Jossey-Bass Publishers; 1988.
7. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1-7.
8. Schwarzer R. Social-cognitive factors in changing health-related behaviors. Current Directions in Psychological Science. 2001;10(2):47-51.
9. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psycholt. 1992;47(9):1102-1114.
10. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav. 2001; 28(6):769-782.
11. Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443-1463.
12. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):
1232-1245.
13. Tumur I, Kaltenthaler E, Ferriter M, et al. Computerised cognitive behaviour therapy for obsessive-compulsive disorder: a systematic review. Psychother Psychosom. 2007; 76(4):196-202.
14. Litz BT, Engel CC, Bryant RA, et al. A randomized, controlled proof-of-concept trial of an Internet-based, therapist-assisted self-management treatment for posttraumatic stress disorder. Am J Psychiatry. 2007;164(11):1676-1683.
15. Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239-243.
16. Williams A, Hagerty BM, Brasington SJ, et al. Stress Gym: feasibility of deploying a web-enhanced behavioral self-management program for stress in a military setting. Mil Med. 2010;175(7):487-493.
17. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
18. Davison BJ, Breckon E. Factors influencing treatment decision making and information p of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87(3):369-374.
19. Chewning B, Bylund CL, Shah B, et al. Patient p for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18.
Providing patients and their families with information and education about their psychiatric illness is a central tenet of mental health care. Discussions about diagnostic impressions, treatment options, and the risks and benefits of interventions are customary. Additionally, patients and families often receive written material or referral to other information sources, including self-help books and a growing number of online resources. Although patient education remains a useful and expected element of good care, there is evidence that, alone, it is insufficient to change health behaviors.1
A growing body of literature and clinical experience suggests that self-management strategies complement patient education and improve treatment outcomes for patients with chronic illnesses, including psychiatric conditions.2,3 The Cochrane Collaboration describes patient education as “teaching or training of patients concerning their own health needs,” and self-management as “the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a long-term disorder.”4
In this article, we review:
• characteristics of long-term care models
• literature supporting the benefits of self-management programs
• clinical initiatives illustrating important elements of self-management support
• opportunities and challenges faced by clinicians, patients, families, clinics, and healthcare systems implementing self-management programs.
Principles of self-management
Chronic care models must account for conditions in which the clinical course can be variable, that are not amenable to a cure, and that demand long-term treatment. Optimal health outcomes rely on patients accurately monitoring, reporting, and responding to their symptoms, while engaging in critical health-related behaviors. In addition, clinicians must teach, partner with, and motivate patients to engage in crucial disease-management activities. Although typically not considered in this light, we believe most psychiatric disorders are best approached through a long-term care model, and benefit from self-management principles.
Basics of self-management include:
• patients must formulate goals and learn skills relevant to their disease
• problems are patient-selected and targeted with individualized, flexible treatment plans.
Corbin and Strauss believe effective “self-managers” achieve competency in three areas:
• role management, which entails healthy adjustments to changes in role
responsibilities, expectations, and self-identity
• emotional management, which often is particularly challenging in psychiatric conditions because of the emotional disruption inherent in living with a psychiatric illness.6
Proficiency in these three “self-manager” domains is enhanced by mastering five key self-management skills outlined in Table 1.7
Successful intervention programs vary widely with regard to individual vs group formats, communication interface, and involved health professionals. However, evidence indicates that problem solving, decision making, and action planning are key components.7 Successful planning includes:
• detailed descriptions of what, how, when, and where the activity will be accomplished
• assessment of patient confidence and adjustment of plans if confidence is limited
• continuous monitoring and self-tailoring of plans through collaborative discussions with providers, fostering a spirit of partnership and ownership.
Table 2 illustrates elements of successful action plans in our Action Planning Worksheet. Adapted from the work of Scharzer,8 Prochaska,9 and Clark,10 we developed this self-management tool for individual and group treatment settings. It serves as a vehicle for collaborative patient-provider discussion and planning. The nuts-and-bolts nature of the discussions inevitably leads to learning new, important, and often unexpected information about our patients’ daily lives and the challenges they face as they share their dominant priorities, fears, and insecurities.
Patients provide consistently positive feedback about action planning, and clinicians often find that the process reveals fruitful areas for further psychotherapeutic intervention. Examples include identifying a range of negative automatic thoughts or catastrophic thinking impeding initiation of important activation, and exposure activities for depressed or anxious patients.
Knowing what to do is different than actually doing it. Changing behavior is difficult in the best circumstances, let alone with the strain of a chronic illness. It is critical to recognize that the presence of depressive symptoms significantly reduces the likelihood that patients will employ self-management practices.11 When combined with anxiety and the impairment of motivation and executive functioning that is common in psychiatric conditions, it is not surprising that patients with a mental health condition struggle to embrace ownership of their illness and engage in critical health behaviors—which may include adhering to medication regimens; maintaining a healthy sleep cycle, nutrition, and exercise routines; vigilant symptom surveillance; and carrying out an agreed-upon action plan.
Interventions
Professionally-guided “light-touch” interventions and technology-assisted self-management interventions also can improve patient engagement in activities through individual encounters, group forums, and technology-mediated exchanges, including telephone, email, text message, tele-health, and web-based interventions.13 The DE-STRESS (Delivery of Self Training and Education for Stressful Situations) model illustrates these principles. This 8-week program combines elements of face-to-face, email, telephone, and web-based assignments and exchanges, and demonstrates a decline in posttraumatic stress disorder, depression, and anxiety scores.14 Our Michigan Depression Outreach and Collaborative Care program is another example of a self-management intervention (Box 1).
• modeling and social persuasion through having patients observe and engage others as they struggle to overcome similar obstacles
• re-interpretation of symptoms aimed at fostering the belief that symptoms generally are multi-determined with several potential explanations, and vary with daily routines.
Helping patients understand when common symptoms such as impaired concentration or dizziness should be “watched”—rather than responded to aggressively—is crucial for effective long-term management.
Challenges
Health care delivery and medical educational models have been slow to embrace this change to long-term care models because doing so involves what might be uncomfortable shifts in roles and responsibilities. Effective care for long-term illness necessitates that the patient become an expert on his (her) illness, and be an active participant and partner in their treatment. Preparing health professionals for this new role as teacher, mentor, and collaborator presents a challenge to health care systems and educational programs across disciplines.
When trying to facilitate effective patient-provider partnerships, it is important to recognize the variability in patient preference for what and how information is shared, how decisions are made, and the role patients are asked to play in their care. Patients differ in their desire for an active or collaborative shared-decision model; some prefer more directive provider communication and a passive role.18 Preferences are influenced by variables such as age, sex, race, anxiety level, and education.19 Open discussion of these matters between caregivers and patients is important; studies have shown that failure to address these issues of “fit” can
impede communication, healthy behavior, and positive outcomes.
Bottom Line
Emerging care models demand that health care providers become teachers and motivators to help patients develop and implement patterns of health surveillance and intervention that will optimize their well-being and functionality. As active collaborators in their care, patients form a partnership with their care teams, allowing for regular, reciprocal exchange of information and shared decision-making. This shift to a partnership creates new, exciting roles and responsibilities for all parties.
Related Resources
- Improving Chronic Illness Care. www.improvingchroniccare.org.
- Chronic disease self-management program (Better Choices, Better Health workshop). Stanford School of Medicine. http://patienteducation.stanford.edu/programs/cdsmp.html.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Thomas E. Fluent, MD, talks about addressing patient resistance to a self-management model of care. Dr. Fluent is Clinical Assistant Professor, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan.
Providing patients and their families with information and education about their psychiatric illness is a central tenet of mental health care. Discussions about diagnostic impressions, treatment options, and the risks and benefits of interventions are customary. Additionally, patients and families often receive written material or referral to other information sources, including self-help books and a growing number of online resources. Although patient education remains a useful and expected element of good care, there is evidence that, alone, it is insufficient to change health behaviors.1
A growing body of literature and clinical experience suggests that self-management strategies complement patient education and improve treatment outcomes for patients with chronic illnesses, including psychiatric conditions.2,3 The Cochrane Collaboration describes patient education as “teaching or training of patients concerning their own health needs,” and self-management as “the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a long-term disorder.”4
In this article, we review:
• characteristics of long-term care models
• literature supporting the benefits of self-management programs
• clinical initiatives illustrating important elements of self-management support
• opportunities and challenges faced by clinicians, patients, families, clinics, and healthcare systems implementing self-management programs.
Principles of self-management
Chronic care models must account for conditions in which the clinical course can be variable, that are not amenable to a cure, and that demand long-term treatment. Optimal health outcomes rely on patients accurately monitoring, reporting, and responding to their symptoms, while engaging in critical health-related behaviors. In addition, clinicians must teach, partner with, and motivate patients to engage in crucial disease-management activities. Although typically not considered in this light, we believe most psychiatric disorders are best approached through a long-term care model, and benefit from self-management principles.
Basics of self-management include:
• patients must formulate goals and learn skills relevant to their disease
• problems are patient-selected and targeted with individualized, flexible treatment plans.
Corbin and Strauss believe effective “self-managers” achieve competency in three areas:
• role management, which entails healthy adjustments to changes in role
responsibilities, expectations, and self-identity
• emotional management, which often is particularly challenging in psychiatric conditions because of the emotional disruption inherent in living with a psychiatric illness.6
Proficiency in these three “self-manager” domains is enhanced by mastering five key self-management skills outlined in Table 1.7
Successful intervention programs vary widely with regard to individual vs group formats, communication interface, and involved health professionals. However, evidence indicates that problem solving, decision making, and action planning are key components.7 Successful planning includes:
• detailed descriptions of what, how, when, and where the activity will be accomplished
• assessment of patient confidence and adjustment of plans if confidence is limited
• continuous monitoring and self-tailoring of plans through collaborative discussions with providers, fostering a spirit of partnership and ownership.
Table 2 illustrates elements of successful action plans in our Action Planning Worksheet. Adapted from the work of Scharzer,8 Prochaska,9 and Clark,10 we developed this self-management tool for individual and group treatment settings. It serves as a vehicle for collaborative patient-provider discussion and planning. The nuts-and-bolts nature of the discussions inevitably leads to learning new, important, and often unexpected information about our patients’ daily lives and the challenges they face as they share their dominant priorities, fears, and insecurities.
Patients provide consistently positive feedback about action planning, and clinicians often find that the process reveals fruitful areas for further psychotherapeutic intervention. Examples include identifying a range of negative automatic thoughts or catastrophic thinking impeding initiation of important activation, and exposure activities for depressed or anxious patients.
Knowing what to do is different than actually doing it. Changing behavior is difficult in the best circumstances, let alone with the strain of a chronic illness. It is critical to recognize that the presence of depressive symptoms significantly reduces the likelihood that patients will employ self-management practices.11 When combined with anxiety and the impairment of motivation and executive functioning that is common in psychiatric conditions, it is not surprising that patients with a mental health condition struggle to embrace ownership of their illness and engage in critical health behaviors—which may include adhering to medication regimens; maintaining a healthy sleep cycle, nutrition, and exercise routines; vigilant symptom surveillance; and carrying out an agreed-upon action plan.
Interventions
Professionally-guided “light-touch” interventions and technology-assisted self-management interventions also can improve patient engagement in activities through individual encounters, group forums, and technology-mediated exchanges, including telephone, email, text message, tele-health, and web-based interventions.13 The DE-STRESS (Delivery of Self Training and Education for Stressful Situations) model illustrates these principles. This 8-week program combines elements of face-to-face, email, telephone, and web-based assignments and exchanges, and demonstrates a decline in posttraumatic stress disorder, depression, and anxiety scores.14 Our Michigan Depression Outreach and Collaborative Care program is another example of a self-management intervention (Box 1).
• modeling and social persuasion through having patients observe and engage others as they struggle to overcome similar obstacles
• re-interpretation of symptoms aimed at fostering the belief that symptoms generally are multi-determined with several potential explanations, and vary with daily routines.
Helping patients understand when common symptoms such as impaired concentration or dizziness should be “watched”—rather than responded to aggressively—is crucial for effective long-term management.
Challenges
Health care delivery and medical educational models have been slow to embrace this change to long-term care models because doing so involves what might be uncomfortable shifts in roles and responsibilities. Effective care for long-term illness necessitates that the patient become an expert on his (her) illness, and be an active participant and partner in their treatment. Preparing health professionals for this new role as teacher, mentor, and collaborator presents a challenge to health care systems and educational programs across disciplines.
When trying to facilitate effective patient-provider partnerships, it is important to recognize the variability in patient preference for what and how information is shared, how decisions are made, and the role patients are asked to play in their care. Patients differ in their desire for an active or collaborative shared-decision model; some prefer more directive provider communication and a passive role.18 Preferences are influenced by variables such as age, sex, race, anxiety level, and education.19 Open discussion of these matters between caregivers and patients is important; studies have shown that failure to address these issues of “fit” can
impede communication, healthy behavior, and positive outcomes.
Bottom Line
Emerging care models demand that health care providers become teachers and motivators to help patients develop and implement patterns of health surveillance and intervention that will optimize their well-being and functionality. As active collaborators in their care, patients form a partnership with their care teams, allowing for regular, reciprocal exchange of information and shared decision-making. This shift to a partnership creates new, exciting roles and responsibilities for all parties.
Related Resources
- Improving Chronic Illness Care. www.improvingchroniccare.org.
- Chronic disease self-management program (Better Choices, Better Health workshop). Stanford School of Medicine. http://patienteducation.stanford.edu/programs/cdsmp.html.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Thomas E. Fluent, MD, talks about addressing patient resistance to a self-management model of care. Dr. Fluent is Clinical Assistant Professor, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan.
1. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-2475.
2. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909-1914.
3. Druss BG, Zhao L, von Esenwein SA, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res. 2010;118(1-3):264-270.
4. Tomkins S, Collins A. Promoting optimal self-care: consultation techniques that improve quality of life for patients and clinicians. London, United Kingdom: National Health Service; 2005.
5. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511-544.
6. Corbin JM, Strauss AL. Unending work and care : managing chronic illness at home. 1st ed. San Francisco, CA: Jossey-Bass Publishers; 1988.
7. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1-7.
8. Schwarzer R. Social-cognitive factors in changing health-related behaviors. Current Directions in Psychological Science. 2001;10(2):47-51.
9. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psycholt. 1992;47(9):1102-1114.
10. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav. 2001; 28(6):769-782.
11. Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443-1463.
12. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):
1232-1245.
13. Tumur I, Kaltenthaler E, Ferriter M, et al. Computerised cognitive behaviour therapy for obsessive-compulsive disorder: a systematic review. Psychother Psychosom. 2007; 76(4):196-202.
14. Litz BT, Engel CC, Bryant RA, et al. A randomized, controlled proof-of-concept trial of an Internet-based, therapist-assisted self-management treatment for posttraumatic stress disorder. Am J Psychiatry. 2007;164(11):1676-1683.
15. Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239-243.
16. Williams A, Hagerty BM, Brasington SJ, et al. Stress Gym: feasibility of deploying a web-enhanced behavioral self-management program for stress in a military setting. Mil Med. 2010;175(7):487-493.
17. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
18. Davison BJ, Breckon E. Factors influencing treatment decision making and information p of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87(3):369-374.
19. Chewning B, Bylund CL, Shah B, et al. Patient p for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18.
1. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-2475.
2. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909-1914.
3. Druss BG, Zhao L, von Esenwein SA, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res. 2010;118(1-3):264-270.
4. Tomkins S, Collins A. Promoting optimal self-care: consultation techniques that improve quality of life for patients and clinicians. London, United Kingdom: National Health Service; 2005.
5. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511-544.
6. Corbin JM, Strauss AL. Unending work and care : managing chronic illness at home. 1st ed. San Francisco, CA: Jossey-Bass Publishers; 1988.
7. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1-7.
8. Schwarzer R. Social-cognitive factors in changing health-related behaviors. Current Directions in Psychological Science. 2001;10(2):47-51.
9. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psycholt. 1992;47(9):1102-1114.
10. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav. 2001; 28(6):769-782.
11. Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443-1463.
12. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):
1232-1245.
13. Tumur I, Kaltenthaler E, Ferriter M, et al. Computerised cognitive behaviour therapy for obsessive-compulsive disorder: a systematic review. Psychother Psychosom. 2007; 76(4):196-202.
14. Litz BT, Engel CC, Bryant RA, et al. A randomized, controlled proof-of-concept trial of an Internet-based, therapist-assisted self-management treatment for posttraumatic stress disorder. Am J Psychiatry. 2007;164(11):1676-1683.
15. Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239-243.
16. Williams A, Hagerty BM, Brasington SJ, et al. Stress Gym: feasibility of deploying a web-enhanced behavioral self-management program for stress in a military setting. Mil Med. 2010;175(7):487-493.
17. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
18. Davison BJ, Breckon E. Factors influencing treatment decision making and information p of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87(3):369-374.
19. Chewning B, Bylund CL, Shah B, et al. Patient p for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18.
Recommendations for lab monitoring of atypical antipsychotics
Mr. H, age 31, is admitted to an acute psychiatric unit with major depressive disorder, substance dependence, insomnia, and generalized anxiety. In the past, he was treated unsuccessfully with sertraline, fluoxetine, clonazepam, venlafaxine, and lithium. The treatment team starts Mr. H on quetiapine, titrated to 150 mg at bedtime, to address suspected bipolar II disorder.
At baseline, Mr. H is 68 inches tall and slightly overweight at 176 lbs (body mass index [BMI] 26.8 kg/m2). The laboratory reports his glycated hemoglobin (HbA1c) at 5.4%; low-density lipoprotein (LDL), 60 mg/dL; total cholesterol, 122 mg/dL; triglycerides, 141 mg/dL; and high-density lipoprotein (HDL), 34 mg/dL.
Within 1 month, Mr. H experiences a 16% increase in body weight. HbA1c increases to 5.6%; LDL, to 93 mg/dL. These metabolic changes are not addressed, and he continues quetiapine for another 5 months. At the end of 6 months, Mr. H weighs 223.8 lbs (BMI 34 kg/m2)—a 27% increase from baseline. HbA1c is in the prediabetic range, at 5.9%, and LDL is 120 mg/dL.1 The treatment team discusses the risks of further metabolic effects, cardiovascular disease, and diabetes with Mr. H. He agrees to a change in therapy.
An increase in weight is thought to be associated with the actions of antipsychotics on H1and 5-HT2c receptors.7 Clozapine and olanzapine pose the highest risk of weight gain. Quetiapine and risperidone are considered of intermediate risk; aripiprazole and ziprasidone present the lowest risk(Table 1).5,7
Patients taking an atypical antipsychotic may experience an elevation of blood glucose, serum triglyceride, and LDL levels, and a decrease in the HDL level.2 These effects may be seen without an increase in BMI, and should be considered a direct effect of the antipsychotic.5 Although the mechanism by which dyslipidemia occurs is poorly understood, an increase in the blood glucose level is thought to be, in part, mediated by antagonism of M3 muscarinic receptors on pancreatic â-cells.7 Clozapine and olanzapine pose the highest risk of dyslipidemia. Quetiapine and risperidone are considered of intermediate risk; the risk associated with quetiapine is closer to that of olanzpine.8,9 Aripiprazole and ziprasidone present a lower risk of dyslipidemia and glucose elevations.5
Newer atypical antipsychotics, such as asenapine, iloperidone, paliperidone, and lurasidone, seem to have a lower metabolic risk profile, similar to those seen with aripiprazole and ziprasidone.5 Patients enrolled in initial clinical trials might not be antipsychotic naïve, however, and may have been taking a high metabolic risk antipsychotic. When these patients are switched to an antipsychotic that carries less of a metabolic risk, it might appear that they are experiencing a decrease in metabolic adverse events.
Metabolic data on newer atypical antipsychotics are limited; most have not been subject to long-term study. Routine monitoring of metabolic side effects is recommended for all atypical antipsychotics, regardless of risk profile.
Recommended monitoring
Because of the known metabolic side effects that occur in patients taking an atypical antipsychotic, baseline and periodic monitoring is recommended (Table 2).2,10 BMI and waist circumference should be recorded at baseline and tracked throughout treatment. Ideally, obtain measurements monthly for the first 3 months of therapy, or after any medication adjustments, then at 6 months, and annually thereafter. Encourage patients to track their own weight.
HbA1c and fasting plasma glucose levels should be measured at baseline and throughout the course of treatment. Obtain another set of measurements at 3 months, then annually thereafter, unless the patient develops type 2 diabetes mellitus.2
Obtaining a fasting lipid panel at baseline and periodically throughout the course of treatment is recommended. After baseline measurement, another panel should be taken at 3 months and annually thereafter. Guidelines of the American Diabetes Association recommend a fasting lipid panel every 5 years—however, good clinical practice dictates obtaining a lipid panel annually.
Managing metabolic side effects
Assess whether the patient can benefit from a lower dosage of current medication, switching to an antipsychotic with less of a risk of metabolic disturbance, or from discontinuation of therapy. In most cases, aim to use monotherapy because polypharmacy contributes to an increased risk of side effects.10
Weight management. Recommend nutrition counseling and physical activity for all patients who are overweight. Referral to a health care professional or to a program with expertise in weight management also might be beneficial.2 Include family members and significant others in the patient’s education when possible.
Impaired fasting glucose. Encourage a low-carbohydrate, high-protein diet with high intake of vegetables. Patients should obtain at least 30 minutes of physical activity, five times a week. Referral to a diabetes self-management class also is appropriate. Consider referral to a primary care physician or a clinician with expertise in diabetes.2
Impaired fasting lipids. Encourage your patients to adhere to a heart-healthy diet that is low in saturated fats and to get adequate physical activity. Referral to a dietician and primary care provider for medical management of dyslipidemia might be appropriate.2
Related Resources
- American Diabetes Association. Guide to living with diabetes. www.diabetes.org/living-with-diabetes.
- MOVE! Weight Management Program for Veterans. www. move.va.gov.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Clonazepam • Klonopin
Clozapine • Clozaril
Fluoxetine • Prozac
Iloperidone • Fanapt
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosure
The authors report no financial relationships with any of the manufacturers mentioned in this article or with manufacturers of competing products.
1. American Diabetes Association. Executive summary: standards of medical care in diabetes—2010. Diabetes Care. 2010;33:
S4-S10.
2. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
3. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
4. Tarricone I, Ferrari Gozzi B, Serretti A, et al. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40(2):187-200.
5. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
6. De Hert M, Dobbelaere M, Sheridan EM, et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26(3):144-158.
7. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications. Oxford, United Kingdom: Cambridge University Press; 2008.
8. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):
1209-1223.
9. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773.
10. Gothefors D, Adolfsson R, Attvall S, et al; Swedish Psychiatric Association. Swedish clinical guidelines – prevention and management of metabolic risk in patients with severe psychiatric disorders. Nord J Psychiatry. 2010;64(5):294-302.
11. Schneiderhan ME, Batscha CL, Rosen C. Assessment of a point-of-care metabolic risk screening program in outpatients receiving antipsychotic agents. Pharmacotherapy. 2009;29(8): 975-987.
Mr. H, age 31, is admitted to an acute psychiatric unit with major depressive disorder, substance dependence, insomnia, and generalized anxiety. In the past, he was treated unsuccessfully with sertraline, fluoxetine, clonazepam, venlafaxine, and lithium. The treatment team starts Mr. H on quetiapine, titrated to 150 mg at bedtime, to address suspected bipolar II disorder.
At baseline, Mr. H is 68 inches tall and slightly overweight at 176 lbs (body mass index [BMI] 26.8 kg/m2). The laboratory reports his glycated hemoglobin (HbA1c) at 5.4%; low-density lipoprotein (LDL), 60 mg/dL; total cholesterol, 122 mg/dL; triglycerides, 141 mg/dL; and high-density lipoprotein (HDL), 34 mg/dL.
Within 1 month, Mr. H experiences a 16% increase in body weight. HbA1c increases to 5.6%; LDL, to 93 mg/dL. These metabolic changes are not addressed, and he continues quetiapine for another 5 months. At the end of 6 months, Mr. H weighs 223.8 lbs (BMI 34 kg/m2)—a 27% increase from baseline. HbA1c is in the prediabetic range, at 5.9%, and LDL is 120 mg/dL.1 The treatment team discusses the risks of further metabolic effects, cardiovascular disease, and diabetes with Mr. H. He agrees to a change in therapy.
An increase in weight is thought to be associated with the actions of antipsychotics on H1and 5-HT2c receptors.7 Clozapine and olanzapine pose the highest risk of weight gain. Quetiapine and risperidone are considered of intermediate risk; aripiprazole and ziprasidone present the lowest risk(Table 1).5,7
Patients taking an atypical antipsychotic may experience an elevation of blood glucose, serum triglyceride, and LDL levels, and a decrease in the HDL level.2 These effects may be seen without an increase in BMI, and should be considered a direct effect of the antipsychotic.5 Although the mechanism by which dyslipidemia occurs is poorly understood, an increase in the blood glucose level is thought to be, in part, mediated by antagonism of M3 muscarinic receptors on pancreatic â-cells.7 Clozapine and olanzapine pose the highest risk of dyslipidemia. Quetiapine and risperidone are considered of intermediate risk; the risk associated with quetiapine is closer to that of olanzpine.8,9 Aripiprazole and ziprasidone present a lower risk of dyslipidemia and glucose elevations.5
Newer atypical antipsychotics, such as asenapine, iloperidone, paliperidone, and lurasidone, seem to have a lower metabolic risk profile, similar to those seen with aripiprazole and ziprasidone.5 Patients enrolled in initial clinical trials might not be antipsychotic naïve, however, and may have been taking a high metabolic risk antipsychotic. When these patients are switched to an antipsychotic that carries less of a metabolic risk, it might appear that they are experiencing a decrease in metabolic adverse events.
Metabolic data on newer atypical antipsychotics are limited; most have not been subject to long-term study. Routine monitoring of metabolic side effects is recommended for all atypical antipsychotics, regardless of risk profile.
Recommended monitoring
Because of the known metabolic side effects that occur in patients taking an atypical antipsychotic, baseline and periodic monitoring is recommended (Table 2).2,10 BMI and waist circumference should be recorded at baseline and tracked throughout treatment. Ideally, obtain measurements monthly for the first 3 months of therapy, or after any medication adjustments, then at 6 months, and annually thereafter. Encourage patients to track their own weight.
HbA1c and fasting plasma glucose levels should be measured at baseline and throughout the course of treatment. Obtain another set of measurements at 3 months, then annually thereafter, unless the patient develops type 2 diabetes mellitus.2
Obtaining a fasting lipid panel at baseline and periodically throughout the course of treatment is recommended. After baseline measurement, another panel should be taken at 3 months and annually thereafter. Guidelines of the American Diabetes Association recommend a fasting lipid panel every 5 years—however, good clinical practice dictates obtaining a lipid panel annually.
Managing metabolic side effects
Assess whether the patient can benefit from a lower dosage of current medication, switching to an antipsychotic with less of a risk of metabolic disturbance, or from discontinuation of therapy. In most cases, aim to use monotherapy because polypharmacy contributes to an increased risk of side effects.10
Weight management. Recommend nutrition counseling and physical activity for all patients who are overweight. Referral to a health care professional or to a program with expertise in weight management also might be beneficial.2 Include family members and significant others in the patient’s education when possible.
Impaired fasting glucose. Encourage a low-carbohydrate, high-protein diet with high intake of vegetables. Patients should obtain at least 30 minutes of physical activity, five times a week. Referral to a diabetes self-management class also is appropriate. Consider referral to a primary care physician or a clinician with expertise in diabetes.2
Impaired fasting lipids. Encourage your patients to adhere to a heart-healthy diet that is low in saturated fats and to get adequate physical activity. Referral to a dietician and primary care provider for medical management of dyslipidemia might be appropriate.2
Related Resources
- American Diabetes Association. Guide to living with diabetes. www.diabetes.org/living-with-diabetes.
- MOVE! Weight Management Program for Veterans. www. move.va.gov.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Clonazepam • Klonopin
Clozapine • Clozaril
Fluoxetine • Prozac
Iloperidone • Fanapt
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosure
The authors report no financial relationships with any of the manufacturers mentioned in this article or with manufacturers of competing products.
Mr. H, age 31, is admitted to an acute psychiatric unit with major depressive disorder, substance dependence, insomnia, and generalized anxiety. In the past, he was treated unsuccessfully with sertraline, fluoxetine, clonazepam, venlafaxine, and lithium. The treatment team starts Mr. H on quetiapine, titrated to 150 mg at bedtime, to address suspected bipolar II disorder.
At baseline, Mr. H is 68 inches tall and slightly overweight at 176 lbs (body mass index [BMI] 26.8 kg/m2). The laboratory reports his glycated hemoglobin (HbA1c) at 5.4%; low-density lipoprotein (LDL), 60 mg/dL; total cholesterol, 122 mg/dL; triglycerides, 141 mg/dL; and high-density lipoprotein (HDL), 34 mg/dL.
Within 1 month, Mr. H experiences a 16% increase in body weight. HbA1c increases to 5.6%; LDL, to 93 mg/dL. These metabolic changes are not addressed, and he continues quetiapine for another 5 months. At the end of 6 months, Mr. H weighs 223.8 lbs (BMI 34 kg/m2)—a 27% increase from baseline. HbA1c is in the prediabetic range, at 5.9%, and LDL is 120 mg/dL.1 The treatment team discusses the risks of further metabolic effects, cardiovascular disease, and diabetes with Mr. H. He agrees to a change in therapy.
An increase in weight is thought to be associated with the actions of antipsychotics on H1and 5-HT2c receptors.7 Clozapine and olanzapine pose the highest risk of weight gain. Quetiapine and risperidone are considered of intermediate risk; aripiprazole and ziprasidone present the lowest risk(Table 1).5,7
Patients taking an atypical antipsychotic may experience an elevation of blood glucose, serum triglyceride, and LDL levels, and a decrease in the HDL level.2 These effects may be seen without an increase in BMI, and should be considered a direct effect of the antipsychotic.5 Although the mechanism by which dyslipidemia occurs is poorly understood, an increase in the blood glucose level is thought to be, in part, mediated by antagonism of M3 muscarinic receptors on pancreatic â-cells.7 Clozapine and olanzapine pose the highest risk of dyslipidemia. Quetiapine and risperidone are considered of intermediate risk; the risk associated with quetiapine is closer to that of olanzpine.8,9 Aripiprazole and ziprasidone present a lower risk of dyslipidemia and glucose elevations.5
Newer atypical antipsychotics, such as asenapine, iloperidone, paliperidone, and lurasidone, seem to have a lower metabolic risk profile, similar to those seen with aripiprazole and ziprasidone.5 Patients enrolled in initial clinical trials might not be antipsychotic naïve, however, and may have been taking a high metabolic risk antipsychotic. When these patients are switched to an antipsychotic that carries less of a metabolic risk, it might appear that they are experiencing a decrease in metabolic adverse events.
Metabolic data on newer atypical antipsychotics are limited; most have not been subject to long-term study. Routine monitoring of metabolic side effects is recommended for all atypical antipsychotics, regardless of risk profile.
Recommended monitoring
Because of the known metabolic side effects that occur in patients taking an atypical antipsychotic, baseline and periodic monitoring is recommended (Table 2).2,10 BMI and waist circumference should be recorded at baseline and tracked throughout treatment. Ideally, obtain measurements monthly for the first 3 months of therapy, or after any medication adjustments, then at 6 months, and annually thereafter. Encourage patients to track their own weight.
HbA1c and fasting plasma glucose levels should be measured at baseline and throughout the course of treatment. Obtain another set of measurements at 3 months, then annually thereafter, unless the patient develops type 2 diabetes mellitus.2
Obtaining a fasting lipid panel at baseline and periodically throughout the course of treatment is recommended. After baseline measurement, another panel should be taken at 3 months and annually thereafter. Guidelines of the American Diabetes Association recommend a fasting lipid panel every 5 years—however, good clinical practice dictates obtaining a lipid panel annually.
Managing metabolic side effects
Assess whether the patient can benefit from a lower dosage of current medication, switching to an antipsychotic with less of a risk of metabolic disturbance, or from discontinuation of therapy. In most cases, aim to use monotherapy because polypharmacy contributes to an increased risk of side effects.10
Weight management. Recommend nutrition counseling and physical activity for all patients who are overweight. Referral to a health care professional or to a program with expertise in weight management also might be beneficial.2 Include family members and significant others in the patient’s education when possible.
Impaired fasting glucose. Encourage a low-carbohydrate, high-protein diet with high intake of vegetables. Patients should obtain at least 30 minutes of physical activity, five times a week. Referral to a diabetes self-management class also is appropriate. Consider referral to a primary care physician or a clinician with expertise in diabetes.2
Impaired fasting lipids. Encourage your patients to adhere to a heart-healthy diet that is low in saturated fats and to get adequate physical activity. Referral to a dietician and primary care provider for medical management of dyslipidemia might be appropriate.2
Related Resources
- American Diabetes Association. Guide to living with diabetes. www.diabetes.org/living-with-diabetes.
- MOVE! Weight Management Program for Veterans. www. move.va.gov.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Clonazepam • Klonopin
Clozapine • Clozaril
Fluoxetine • Prozac
Iloperidone • Fanapt
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosure
The authors report no financial relationships with any of the manufacturers mentioned in this article or with manufacturers of competing products.
1. American Diabetes Association. Executive summary: standards of medical care in diabetes—2010. Diabetes Care. 2010;33:
S4-S10.
2. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
3. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
4. Tarricone I, Ferrari Gozzi B, Serretti A, et al. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40(2):187-200.
5. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
6. De Hert M, Dobbelaere M, Sheridan EM, et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26(3):144-158.
7. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications. Oxford, United Kingdom: Cambridge University Press; 2008.
8. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):
1209-1223.
9. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773.
10. Gothefors D, Adolfsson R, Attvall S, et al; Swedish Psychiatric Association. Swedish clinical guidelines – prevention and management of metabolic risk in patients with severe psychiatric disorders. Nord J Psychiatry. 2010;64(5):294-302.
11. Schneiderhan ME, Batscha CL, Rosen C. Assessment of a point-of-care metabolic risk screening program in outpatients receiving antipsychotic agents. Pharmacotherapy. 2009;29(8): 975-987.
1. American Diabetes Association. Executive summary: standards of medical care in diabetes—2010. Diabetes Care. 2010;33:
S4-S10.
2. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
3. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
4. Tarricone I, Ferrari Gozzi B, Serretti A, et al. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40(2):187-200.
5. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
6. De Hert M, Dobbelaere M, Sheridan EM, et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26(3):144-158.
7. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications. Oxford, United Kingdom: Cambridge University Press; 2008.
8. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):
1209-1223.
9. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773.
10. Gothefors D, Adolfsson R, Attvall S, et al; Swedish Psychiatric Association. Swedish clinical guidelines – prevention and management of metabolic risk in patients with severe psychiatric disorders. Nord J Psychiatry. 2010;64(5):294-302.
11. Schneiderhan ME, Batscha CL, Rosen C. Assessment of a point-of-care metabolic risk screening program in outpatients receiving antipsychotic agents. Pharmacotherapy. 2009;29(8): 975-987.
Assessing and treating depression in palliative care patients
Depression is highly prevalent in hospice and palliative care settings—especially among cancer patients, in whom the prevalence of depression may be 4 times that of the general population.1 Furthermore, suicide is a relatively common, unwanted consequence of depression among cancer patients.2 Whereas the risk of suicide among advanced cancer patients may be twice that of the general population,3 in specific cancer populations (male patients with pancreatic adenocarcinoma) the risk of suicide may be 11 times that of the general population.4
Mental health professionals often are consulted when treating depressed patients with advanced illness, especially when suicidal thoughts or wishes for a hastened death are expressed to oncologists or primary care physicians. To mitigate the effects of depression among seriously ill patients (Box),5,6 mental health professionals must be able to assess and manage depression in patients with progressive, incurable illnesses such as advanced malignancy.
Diagnostic challenges
Assessing depression in seriously ill patients can be a challenge for mental health professionals. Cardinal neurovegetative symptoms of depression, such as anergia, anorexia, impaired concentration, and sleep disturbances, also are common manifestations of advanced medical illness.7 Furthermore, it can be difficult to gauge the significance of psychological distress among cancer patients. Although depressive thoughts and symptoms may be present in 15% to 50% of cancer patients, only 5% to 20% will meet diagnostic criteria for major depressive disorder (MDD).8,9 You may find it challenging to determine whether to use pharmacotherapy for depressive symptoms or whether engaging in reflective listening and exploring the patient’s concerns is the appropriate therapeutic intervention.
Side effects from commonly used therapeutics for cancer patients—chemotherapeutic agents, opioids, benzodiazepines, glucocorticoids—can mimic depressive symptoms. Clinicians should include hypoactive delirium in the differential diagnosis of depressive symptoms in cancer patients. Delirium is an important consideration in the final days of life because the condition has been shown to occur in as many as 90% of these patients.10 A mistaken diagnosis of depression in a patient who has hypoactive delirium (see “Hospitalized, elderly, and delirious: What should you do for these patients?” page 10) might lead to a prescription for an antidepressant or a psychostimulant, which can exacerbate delirium rather than alleviate depressive symptoms.
Significant attitudinal barriers from both clinicians and patients can lead to under-
recognition and undertreatment of depression. Clinicians may believe the patient’s depression is an appropriate response to the dying process; indeed, feeling sad or depressed may be an appropriate response to bad news or a medical setback, but meeting MDD criteria should be viewed as a pathologic process that has adverse medical, psychological, and social consequences. Time constraints or personal discomfort with existential concerns may prevent a clinician from exploring a patient’s distress out of fear that such discussions may cause the patient to become more depressed.11 Patients may underreport or consciously disguise depressive symptoms in their final weeks of life.12
Responding to these challenges
The Science Committee of the Association of Palliative Medicine performed a thorough assessment of available screening tools and rating scales for depressive symptoms in palliative care. Although the committee found that commonly used tools such as the Edinburgh Depression Scale and the Hospital Anxiety and Depression Scale have validated cutoff thresholds for palliative care patients, the depression screening tool with the highest sensitivity, specificity, and positive predictive value was the question: “Are you feeling down, depressed, or hopeless most of the time over the last 2 weeks?”13,14
Other short screening algorithms have been validated among palliative care patients (Table 1).15 Endicott proposed a structured approach to help clinicians differentiate MDD from common physical ailments of progressive cancer in which physical criteria for an MDD diagnosis are substituted by affective symptoms (Table 2).16 The improved risk-benefit ratio of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), coupled with the potential significant morbidity associated with MDD and subsyndromal depressive symptoms, makes it necessary to recognize and treat those symptoms even when the cause of the depressive symptoms is unclear.
Psychotherapy in palliative care
Psychotherapeutic interventions such as dignity therapy, which invites patients to utilize a meaning-centered life review to address his (her) existential concerns, may help depressed palliative care patients.17 Evidence suggests a strong association between diminished dignity and depression in patients with advanced illness.18 Individualized psychotherapeutic interventions that provide a framework for addressing dignity-related issues and existential distress among terminally ill patients could help preserve a sense of purpose throughout the dying process. Surveys of dignity therapy have been encouraging: 91% of participants reported being satisfied with dignity therapy and more than two-thirds reported an improved sense of meaning.18
Other promising psychotherapeutic interventions include supportive-expressed group therapy, in which a group of advanced cancer patients meets with a mental health professional and discusses goals of building bonds, refining life’s priorities, and “detoxifying” the experience of death and dying.19 A primary purpose of this therapy is not just to foster improved relationships within a group of cancer patients, but also within their family and oncology team, with the aim of improving compliance with anticancer therapies. Nurse-delivered, one-on-one sessions focusing on depression education, problem-solving, coping techniques, and telecare management of pain and depression also improves outcomes among depressed cancer patients.20
Hospital-based inpatient and outpatient palliative care consultation teams are becoming more common. A randomized controlled trial of early palliative care outpatient consultation for patients with incurable lung cancer showed improved depression outcomes, better quality of life, and a modest improvement in survival.21 Although the most effective elements of a palliative care consult remain unspecified and require further research, improvement in outcomes may result from more effective symptom management, better acknowledgement of the burden of illness on the patient or family, or reduced need for hospitalization. Therefore, mental health professionals should consider palliative care consultation for advanced cancer patients with signs of psychological distress.
Pharmacotherapy options
Antidepressants. Patients with excessive guilt, anhedonia, hopelessness, or ruminative thinking along with a related impairment in quality of life may benefit from pharmacotherapy regardless of whether they meet diagnostic criteria for MDD. Although SSRIs and SNRIs have become a mainstay in managing depression, placebo-controlled trials have yielded mixed results in depressed cancer patients. Furthermore, differences in efficacy among these antidepressants may not be significant, according to a recent meta-analysis.22
Select an antidepressant based on the patient’s past treatment response, target symptoms, and potential for adverse events. Mirtazapine has relatively few drug interactions; the side effects of sedation and weight gain may be welcome among patients with insomnia and impaired appetite.23 Furthermore, mirtazapine is a 5-HT3 receptor antagonist,24 which suggests it might act as an effective antiemetic.25 Other SNRIs, such as venlafaxine and duloxetine, have demonstrated benefits in managing neuropathic pain in patients who do not have cancer.26
Psychostimulants. Patients with a prognosis of days or weeks might not have enough time for an antidepressant to achieve full effect. Open prospective trials and pilot studies have shown that psychostimulants can improve cancer-related fatigue and quality of life while also augmenting the action of antidepressants.27 Psychostimulants, such as methylphenidate, have been used for treating cancer-related fatigue and depressive symptoms in medically ill patients. Their rapid onset of action, coupled with minimal side effect profile, make them a good choice for seriously ill patients with significant neurovegetative symptoms of a depressive disorder. Note: Avoid psychostimulants in patients with delirium and use with caution in patients with heart disease.28
Novel agents. A growing body of preclinical research suggests that glutamate may be involved in the pathophysiology of MDD. Ketamine modulates glutamate neurotransmission as an N-methyl-d-aspartate receptor antagonist. A recent evaluation of a single dose IV of ketamine in a placebo-controlled, double-blind trial found that depressed patients receiving ketamine experienced significant improvement their depressive symptoms.29 Irwin and Iglewicz30 describe 2 hospice patients administered a single oral dose of ketamine, which provided rapid relief of depressive symptoms and was well tolerated.
Transdermal selegiline may help patients who have trouble taking oral medications, including antidepressants. Inability to tolerate or absorb medications may be related to several conditions such as head and neck cancer, severe mucositis, and dysphagia. The dose-related dietary requirements—tyramine restriction—and careful monitoring for drug interactions may limit the use of selegiline in medically ill patients.31Table 3 features a list of dosing recommendations for pharmacotherapeutic options.32
Use the strategy of “start low, go slow” when initiating and adjusting antidepressants because patients with cancer and other advanced illnesses often have concomitant organ failure and are at risk of drug interactions. Carefully review your patient’s medication list for agents that are no longer beneficial or possibly contributing to depressive symptoms to help reduce the risk of adverse pharmacokinetic and pharmaco-dynamic interactions.
Requests for a hastened death
As many as 8.5% of terminally ill patients have a sustained and pervasive wish for an early death.33 Although requests for a hastened death may evoke strong emotional reactions and compel many clinicians to recoil or harshly reject such requests, consider such requests as an opportunity to gain insight into the patient’s narrative of his (her) suffering. The clinician’s role in such cases is to identify suicidality and perform a thorough suicide risk assessment. Interventions to prevent suicide should attempt to balance the seriousness of self-harm threats with restrictions on the patient’s liberty.34
Clinicians also need to consider the patient’s prognosis in their decision-making. For example, an extremely depressed or suicidal patient may not benefit from psychiatric hospitalization if she (he) has progressive neurovegetative symptoms and a prognosis of only a few weeks to live. These situations often are challenging and require a careful, informed discussion of the risks and benefits of all proposed interventions.
Clinicians also should be familiar with distinctions among ethical issues in end-of-life care, including physician-assisted suicide, euthanasia, and palliative sedation (Table 4).35,36
In Oregon, requests for physician-assisted suicide and hastened death through the state’s Death with Dignity Act often are short lived, and may not persist when clinicians offer patients good symptom management and psychological support.37 Requests for a hastened death often are motivated by loss of control, inability to find meaning in death, indignity from being dependent, and concern for future suffering and burden on loved ones.37
Carefully evaluate requests for hastened death in a manner that balances your personal and professional integrity. To preserve personal integrity, clearly communicate therapeutic interventions that you can and cannot provide. To ensure the patient does not feel abandoned, identify factors that contribute to the patient’s suffering and express a desire to search for alternative care approaches that will be mutually acceptable to the patient and to you.
Advance care planning and palliative care consultations may help in these circumstances. A randomized trial comparing advance care planning vs standard care in hospitalized geriatric patients found that advance care planning was more likely to lead to end-of-life wishes that were recognized by clinicians, and was associated with less distress, anxiety, and depression as reported by bereaved family members.38
Clinicians can assist patients with advanced care planning by helping them fill out advance directives, such as durable health care power of attorney documents and a living will. Palliative care clinicians can offer specialty-level assistance in advance care planning, provide focused assessments of physical and psychosocial symptoms, develop appropriate clinical goals, and assist in coordinating individualized care plans for seriously ill patients.2
Bottom Line
Depression commonly is encountered in hospice and palliative care patients and is associated with morbidity and distress. Validated screening tools can help you distinguish major depressive disorder from depressive symptoms in this population. Several psychotherapeutic techniques have been shown to be beneficial. In addition to traditional antidepressants, psychostimulants or ketamine may help address acute depressive symptoms in patients who have days or weeks to live.
Related Resources
- American Academy of Hospice and Palliative Medicine. www.aahpm.org.
- Death with Dignity National Center. www.deathwithdignity.org.
- National Hospice and Palliative Care Organization. www.nhpco.org.
- Oregon Health Authority. Death with Dignity Act. http://public.health.oregon.gov/ProviderPartnerResources/Evaluationresearch/deathwithdignityact/Pages/index.aspx.
Drug Brand Names
Duloxetine • Cymbalta Modafinil • Provigil
Ketamine • Ketalar Selegiline (transdermal) • EMSAM
Methylphenidate • Concerta, Ritalin Mirtazipine • Remeron
Venlafaxine • Effexor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Irwin SA, Rao S, Bower K, et al. Psychiatric issues in palliative care: recognition of depression in patients enrolled in hospice care. J Palliat Med. 2008;11(2):158-163.
2. Misono S, Weiss NS, Fann JR, et al. Incidence of suicide in persons with cancer. J Clin Oncol. 2008;26(29):4731-4738.
3. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911.
4. Turaga KK, Malafa MP, Jacobsen PB, et al. Suicide in patients with pancreatic cancer. Cancer. 2011;117(3):642-647.
5. Rosenstein DL. Depression and end-of-life care for patients with cancer. Dialogues Clin Neurosci. 2011;13(1):101-108.
6. King DA, Heisel MJ, Lyness JM. Assessment and psychological treatment of depression in older adults with terminal or life-threatening illness. Clin Psychol (New York). 2005;12(3):339-353.
7. Block SD. Assessing and managing depression in the terminally ill patient. ACP-ASIM End-of-Life Care Consensus Panel. American College of Physicians - American Society of Internal Medicine. Ann Intern Med. 2000;132(3):209-218.
8. Chochinov HM, Wilson KG, Enns M, et al. Prevalence of depression in the terminally ill: effects of diagnostic criteria and symptom threshold judgments. Am J Psychiatry. 1994;151(4):537-540.
9. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71.
10. Spiller JA, Keen JC. Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliat Med. 2006;20(1):17-23.
11. Maguire P. Improving the detection of psychiatric problems in cancer patients. Soc Sci Med. 1985;20(8):819-823.
12. Hinton J. Can home care maintain an acceptable quality of life for patients with terminal cancer and their relatives? Palliat Med. 1994;8(3):183-196.
13. Lloyd-Williams M, Spiller J, Ward J. Which depression screening tools should be used in palliative care? Palliat Med. 2003;17(1):40-43.
14. Chochinov HM, Wilson KG, Enns M, et al. “Are you depressed?” Screening for depression in the terminally ill. Am J Psychiatry. 1997;154(5):674-676.
15. Robinson JA, Crawford GB. Identifying palliative care patients with symptoms of depression: an algorithm. Palliat Med. 2005;19(4):278-287.
16. Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53(10 suppl):2243-2249.
17. Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520-5525.
18. Chochinov HM. Dignity-conserving care-a new model for palliative care: helping the patient feel valued. JAMA. 2002;287(17):2253-2260.
19. Kissane DW, Grabsch B, Clarke DM, et al. Supportive-expressive group therapy: the transformation of existential ambivalence into creative living while enhancing adherence to anti-cancer therapies. Psychooncology. 2004;13(11):
755-768.
20. Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372(9632):40-48.
21. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
22. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155(11):772-785.
23. Kast RE, Foley KF. Cancer chemotherapy and cachexia: mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur J Cancer Care (Engl). 2007; 16(4):351-354.
24. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001; 7(3):249-264.
25. Pae CU. Low-dose mirtazapine may be successful treatment option for severe nausea and vomiting. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):
1143-1145.
26. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454.
27. Pereira J, Bruera E. Depression with psychomotor retardation: diagnostic challenges and the use of psychostimulants. J Palliat Med. 2001;4(1):15-21.
28. Jackson V, Block S. # 061 Use of Psycho-Stimulants in Palliative Care, 2nd ed. End of Life/Palliative Education Resource Center. Medical College of Wisconsin. http://www.eperc.mcw.edu/EPERC/FastFactsIndex/ff_061.htm. Accessed December 28, 2012.
29. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.
30. Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13(7):903-908.
31. Attard A, Ranjith G, Taylor D. Alternative routes to oral antidepressant therapy: case vignette and literature review. J Psychopharmacol. 2010;24(4):449-454.
32. Rozans M, Dreisbach A, Lertora JJ, et al. Palliative uses of methylphenidate in patients with cancer: a review. J Clin Oncol. 2002;20(1):335-339.
33. Chochinov HM, Wilson KG, Enns M, et al. Desire for death in the terminally ill. Am J Psychiatry. 1995;152(8):1185-1191.
34. Marks S, Heinrich TW, Rosielle D. Case report: are clinicians obligated to medically treat a suicide attempt in a patient with a prognosis of weeks? J Palliat Med. 2012;15(1):134-137.
35. Materstvedt LJ, Clark D, Ellershaw J, et al. Euthanasia and physician-assisted suicide: a view from an EAPC Ethics Task Force. Palliat Med. 2003;17(2):97-101; discussion 102-179.
36. Kirk TW, Mahon MM; Palliative Sedation Task Force of the National Hospice and Palliative Care Organization Ethics Committee. National Hospice and Palliative Care Organization (NHPCO) position statement and commentary on the use of palliative sedation in imminently dying terminally ill patients. J Pain Symptom Manage. 2010; 39(5):914-923.
37. Okie S. Physician-assisted suicide--Oregon and beyond. N Engl J Med. 2005;352(16):1627-1630.
38. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c134
Depression is highly prevalent in hospice and palliative care settings—especially among cancer patients, in whom the prevalence of depression may be 4 times that of the general population.1 Furthermore, suicide is a relatively common, unwanted consequence of depression among cancer patients.2 Whereas the risk of suicide among advanced cancer patients may be twice that of the general population,3 in specific cancer populations (male patients with pancreatic adenocarcinoma) the risk of suicide may be 11 times that of the general population.4
Mental health professionals often are consulted when treating depressed patients with advanced illness, especially when suicidal thoughts or wishes for a hastened death are expressed to oncologists or primary care physicians. To mitigate the effects of depression among seriously ill patients (Box),5,6 mental health professionals must be able to assess and manage depression in patients with progressive, incurable illnesses such as advanced malignancy.
Diagnostic challenges
Assessing depression in seriously ill patients can be a challenge for mental health professionals. Cardinal neurovegetative symptoms of depression, such as anergia, anorexia, impaired concentration, and sleep disturbances, also are common manifestations of advanced medical illness.7 Furthermore, it can be difficult to gauge the significance of psychological distress among cancer patients. Although depressive thoughts and symptoms may be present in 15% to 50% of cancer patients, only 5% to 20% will meet diagnostic criteria for major depressive disorder (MDD).8,9 You may find it challenging to determine whether to use pharmacotherapy for depressive symptoms or whether engaging in reflective listening and exploring the patient’s concerns is the appropriate therapeutic intervention.
Side effects from commonly used therapeutics for cancer patients—chemotherapeutic agents, opioids, benzodiazepines, glucocorticoids—can mimic depressive symptoms. Clinicians should include hypoactive delirium in the differential diagnosis of depressive symptoms in cancer patients. Delirium is an important consideration in the final days of life because the condition has been shown to occur in as many as 90% of these patients.10 A mistaken diagnosis of depression in a patient who has hypoactive delirium (see “Hospitalized, elderly, and delirious: What should you do for these patients?” page 10) might lead to a prescription for an antidepressant or a psychostimulant, which can exacerbate delirium rather than alleviate depressive symptoms.
Significant attitudinal barriers from both clinicians and patients can lead to under-
recognition and undertreatment of depression. Clinicians may believe the patient’s depression is an appropriate response to the dying process; indeed, feeling sad or depressed may be an appropriate response to bad news or a medical setback, but meeting MDD criteria should be viewed as a pathologic process that has adverse medical, psychological, and social consequences. Time constraints or personal discomfort with existential concerns may prevent a clinician from exploring a patient’s distress out of fear that such discussions may cause the patient to become more depressed.11 Patients may underreport or consciously disguise depressive symptoms in their final weeks of life.12
Responding to these challenges
The Science Committee of the Association of Palliative Medicine performed a thorough assessment of available screening tools and rating scales for depressive symptoms in palliative care. Although the committee found that commonly used tools such as the Edinburgh Depression Scale and the Hospital Anxiety and Depression Scale have validated cutoff thresholds for palliative care patients, the depression screening tool with the highest sensitivity, specificity, and positive predictive value was the question: “Are you feeling down, depressed, or hopeless most of the time over the last 2 weeks?”13,14
Other short screening algorithms have been validated among palliative care patients (Table 1).15 Endicott proposed a structured approach to help clinicians differentiate MDD from common physical ailments of progressive cancer in which physical criteria for an MDD diagnosis are substituted by affective symptoms (Table 2).16 The improved risk-benefit ratio of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), coupled with the potential significant morbidity associated with MDD and subsyndromal depressive symptoms, makes it necessary to recognize and treat those symptoms even when the cause of the depressive symptoms is unclear.
Psychotherapy in palliative care
Psychotherapeutic interventions such as dignity therapy, which invites patients to utilize a meaning-centered life review to address his (her) existential concerns, may help depressed palliative care patients.17 Evidence suggests a strong association between diminished dignity and depression in patients with advanced illness.18 Individualized psychotherapeutic interventions that provide a framework for addressing dignity-related issues and existential distress among terminally ill patients could help preserve a sense of purpose throughout the dying process. Surveys of dignity therapy have been encouraging: 91% of participants reported being satisfied with dignity therapy and more than two-thirds reported an improved sense of meaning.18
Other promising psychotherapeutic interventions include supportive-expressed group therapy, in which a group of advanced cancer patients meets with a mental health professional and discusses goals of building bonds, refining life’s priorities, and “detoxifying” the experience of death and dying.19 A primary purpose of this therapy is not just to foster improved relationships within a group of cancer patients, but also within their family and oncology team, with the aim of improving compliance with anticancer therapies. Nurse-delivered, one-on-one sessions focusing on depression education, problem-solving, coping techniques, and telecare management of pain and depression also improves outcomes among depressed cancer patients.20
Hospital-based inpatient and outpatient palliative care consultation teams are becoming more common. A randomized controlled trial of early palliative care outpatient consultation for patients with incurable lung cancer showed improved depression outcomes, better quality of life, and a modest improvement in survival.21 Although the most effective elements of a palliative care consult remain unspecified and require further research, improvement in outcomes may result from more effective symptom management, better acknowledgement of the burden of illness on the patient or family, or reduced need for hospitalization. Therefore, mental health professionals should consider palliative care consultation for advanced cancer patients with signs of psychological distress.
Pharmacotherapy options
Antidepressants. Patients with excessive guilt, anhedonia, hopelessness, or ruminative thinking along with a related impairment in quality of life may benefit from pharmacotherapy regardless of whether they meet diagnostic criteria for MDD. Although SSRIs and SNRIs have become a mainstay in managing depression, placebo-controlled trials have yielded mixed results in depressed cancer patients. Furthermore, differences in efficacy among these antidepressants may not be significant, according to a recent meta-analysis.22
Select an antidepressant based on the patient’s past treatment response, target symptoms, and potential for adverse events. Mirtazapine has relatively few drug interactions; the side effects of sedation and weight gain may be welcome among patients with insomnia and impaired appetite.23 Furthermore, mirtazapine is a 5-HT3 receptor antagonist,24 which suggests it might act as an effective antiemetic.25 Other SNRIs, such as venlafaxine and duloxetine, have demonstrated benefits in managing neuropathic pain in patients who do not have cancer.26
Psychostimulants. Patients with a prognosis of days or weeks might not have enough time for an antidepressant to achieve full effect. Open prospective trials and pilot studies have shown that psychostimulants can improve cancer-related fatigue and quality of life while also augmenting the action of antidepressants.27 Psychostimulants, such as methylphenidate, have been used for treating cancer-related fatigue and depressive symptoms in medically ill patients. Their rapid onset of action, coupled with minimal side effect profile, make them a good choice for seriously ill patients with significant neurovegetative symptoms of a depressive disorder. Note: Avoid psychostimulants in patients with delirium and use with caution in patients with heart disease.28
Novel agents. A growing body of preclinical research suggests that glutamate may be involved in the pathophysiology of MDD. Ketamine modulates glutamate neurotransmission as an N-methyl-d-aspartate receptor antagonist. A recent evaluation of a single dose IV of ketamine in a placebo-controlled, double-blind trial found that depressed patients receiving ketamine experienced significant improvement their depressive symptoms.29 Irwin and Iglewicz30 describe 2 hospice patients administered a single oral dose of ketamine, which provided rapid relief of depressive symptoms and was well tolerated.
Transdermal selegiline may help patients who have trouble taking oral medications, including antidepressants. Inability to tolerate or absorb medications may be related to several conditions such as head and neck cancer, severe mucositis, and dysphagia. The dose-related dietary requirements—tyramine restriction—and careful monitoring for drug interactions may limit the use of selegiline in medically ill patients.31Table 3 features a list of dosing recommendations for pharmacotherapeutic options.32
Use the strategy of “start low, go slow” when initiating and adjusting antidepressants because patients with cancer and other advanced illnesses often have concomitant organ failure and are at risk of drug interactions. Carefully review your patient’s medication list for agents that are no longer beneficial or possibly contributing to depressive symptoms to help reduce the risk of adverse pharmacokinetic and pharmaco-dynamic interactions.
Requests for a hastened death
As many as 8.5% of terminally ill patients have a sustained and pervasive wish for an early death.33 Although requests for a hastened death may evoke strong emotional reactions and compel many clinicians to recoil or harshly reject such requests, consider such requests as an opportunity to gain insight into the patient’s narrative of his (her) suffering. The clinician’s role in such cases is to identify suicidality and perform a thorough suicide risk assessment. Interventions to prevent suicide should attempt to balance the seriousness of self-harm threats with restrictions on the patient’s liberty.34
Clinicians also need to consider the patient’s prognosis in their decision-making. For example, an extremely depressed or suicidal patient may not benefit from psychiatric hospitalization if she (he) has progressive neurovegetative symptoms and a prognosis of only a few weeks to live. These situations often are challenging and require a careful, informed discussion of the risks and benefits of all proposed interventions.
Clinicians also should be familiar with distinctions among ethical issues in end-of-life care, including physician-assisted suicide, euthanasia, and palliative sedation (Table 4).35,36
In Oregon, requests for physician-assisted suicide and hastened death through the state’s Death with Dignity Act often are short lived, and may not persist when clinicians offer patients good symptom management and psychological support.37 Requests for a hastened death often are motivated by loss of control, inability to find meaning in death, indignity from being dependent, and concern for future suffering and burden on loved ones.37
Carefully evaluate requests for hastened death in a manner that balances your personal and professional integrity. To preserve personal integrity, clearly communicate therapeutic interventions that you can and cannot provide. To ensure the patient does not feel abandoned, identify factors that contribute to the patient’s suffering and express a desire to search for alternative care approaches that will be mutually acceptable to the patient and to you.
Advance care planning and palliative care consultations may help in these circumstances. A randomized trial comparing advance care planning vs standard care in hospitalized geriatric patients found that advance care planning was more likely to lead to end-of-life wishes that were recognized by clinicians, and was associated with less distress, anxiety, and depression as reported by bereaved family members.38
Clinicians can assist patients with advanced care planning by helping them fill out advance directives, such as durable health care power of attorney documents and a living will. Palliative care clinicians can offer specialty-level assistance in advance care planning, provide focused assessments of physical and psychosocial symptoms, develop appropriate clinical goals, and assist in coordinating individualized care plans for seriously ill patients.2
Bottom Line
Depression commonly is encountered in hospice and palliative care patients and is associated with morbidity and distress. Validated screening tools can help you distinguish major depressive disorder from depressive symptoms in this population. Several psychotherapeutic techniques have been shown to be beneficial. In addition to traditional antidepressants, psychostimulants or ketamine may help address acute depressive symptoms in patients who have days or weeks to live.
Related Resources
- American Academy of Hospice and Palliative Medicine. www.aahpm.org.
- Death with Dignity National Center. www.deathwithdignity.org.
- National Hospice and Palliative Care Organization. www.nhpco.org.
- Oregon Health Authority. Death with Dignity Act. http://public.health.oregon.gov/ProviderPartnerResources/Evaluationresearch/deathwithdignityact/Pages/index.aspx.
Drug Brand Names
Duloxetine • Cymbalta Modafinil • Provigil
Ketamine • Ketalar Selegiline (transdermal) • EMSAM
Methylphenidate • Concerta, Ritalin Mirtazipine • Remeron
Venlafaxine • Effexor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Depression is highly prevalent in hospice and palliative care settings—especially among cancer patients, in whom the prevalence of depression may be 4 times that of the general population.1 Furthermore, suicide is a relatively common, unwanted consequence of depression among cancer patients.2 Whereas the risk of suicide among advanced cancer patients may be twice that of the general population,3 in specific cancer populations (male patients with pancreatic adenocarcinoma) the risk of suicide may be 11 times that of the general population.4
Mental health professionals often are consulted when treating depressed patients with advanced illness, especially when suicidal thoughts or wishes for a hastened death are expressed to oncologists or primary care physicians. To mitigate the effects of depression among seriously ill patients (Box),5,6 mental health professionals must be able to assess and manage depression in patients with progressive, incurable illnesses such as advanced malignancy.
Diagnostic challenges
Assessing depression in seriously ill patients can be a challenge for mental health professionals. Cardinal neurovegetative symptoms of depression, such as anergia, anorexia, impaired concentration, and sleep disturbances, also are common manifestations of advanced medical illness.7 Furthermore, it can be difficult to gauge the significance of psychological distress among cancer patients. Although depressive thoughts and symptoms may be present in 15% to 50% of cancer patients, only 5% to 20% will meet diagnostic criteria for major depressive disorder (MDD).8,9 You may find it challenging to determine whether to use pharmacotherapy for depressive symptoms or whether engaging in reflective listening and exploring the patient’s concerns is the appropriate therapeutic intervention.
Side effects from commonly used therapeutics for cancer patients—chemotherapeutic agents, opioids, benzodiazepines, glucocorticoids—can mimic depressive symptoms. Clinicians should include hypoactive delirium in the differential diagnosis of depressive symptoms in cancer patients. Delirium is an important consideration in the final days of life because the condition has been shown to occur in as many as 90% of these patients.10 A mistaken diagnosis of depression in a patient who has hypoactive delirium (see “Hospitalized, elderly, and delirious: What should you do for these patients?” page 10) might lead to a prescription for an antidepressant or a psychostimulant, which can exacerbate delirium rather than alleviate depressive symptoms.
Significant attitudinal barriers from both clinicians and patients can lead to under-
recognition and undertreatment of depression. Clinicians may believe the patient’s depression is an appropriate response to the dying process; indeed, feeling sad or depressed may be an appropriate response to bad news or a medical setback, but meeting MDD criteria should be viewed as a pathologic process that has adverse medical, psychological, and social consequences. Time constraints or personal discomfort with existential concerns may prevent a clinician from exploring a patient’s distress out of fear that such discussions may cause the patient to become more depressed.11 Patients may underreport or consciously disguise depressive symptoms in their final weeks of life.12
Responding to these challenges
The Science Committee of the Association of Palliative Medicine performed a thorough assessment of available screening tools and rating scales for depressive symptoms in palliative care. Although the committee found that commonly used tools such as the Edinburgh Depression Scale and the Hospital Anxiety and Depression Scale have validated cutoff thresholds for palliative care patients, the depression screening tool with the highest sensitivity, specificity, and positive predictive value was the question: “Are you feeling down, depressed, or hopeless most of the time over the last 2 weeks?”13,14
Other short screening algorithms have been validated among palliative care patients (Table 1).15 Endicott proposed a structured approach to help clinicians differentiate MDD from common physical ailments of progressive cancer in which physical criteria for an MDD diagnosis are substituted by affective symptoms (Table 2).16 The improved risk-benefit ratio of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), coupled with the potential significant morbidity associated with MDD and subsyndromal depressive symptoms, makes it necessary to recognize and treat those symptoms even when the cause of the depressive symptoms is unclear.
Psychotherapy in palliative care
Psychotherapeutic interventions such as dignity therapy, which invites patients to utilize a meaning-centered life review to address his (her) existential concerns, may help depressed palliative care patients.17 Evidence suggests a strong association between diminished dignity and depression in patients with advanced illness.18 Individualized psychotherapeutic interventions that provide a framework for addressing dignity-related issues and existential distress among terminally ill patients could help preserve a sense of purpose throughout the dying process. Surveys of dignity therapy have been encouraging: 91% of participants reported being satisfied with dignity therapy and more than two-thirds reported an improved sense of meaning.18
Other promising psychotherapeutic interventions include supportive-expressed group therapy, in which a group of advanced cancer patients meets with a mental health professional and discusses goals of building bonds, refining life’s priorities, and “detoxifying” the experience of death and dying.19 A primary purpose of this therapy is not just to foster improved relationships within a group of cancer patients, but also within their family and oncology team, with the aim of improving compliance with anticancer therapies. Nurse-delivered, one-on-one sessions focusing on depression education, problem-solving, coping techniques, and telecare management of pain and depression also improves outcomes among depressed cancer patients.20
Hospital-based inpatient and outpatient palliative care consultation teams are becoming more common. A randomized controlled trial of early palliative care outpatient consultation for patients with incurable lung cancer showed improved depression outcomes, better quality of life, and a modest improvement in survival.21 Although the most effective elements of a palliative care consult remain unspecified and require further research, improvement in outcomes may result from more effective symptom management, better acknowledgement of the burden of illness on the patient or family, or reduced need for hospitalization. Therefore, mental health professionals should consider palliative care consultation for advanced cancer patients with signs of psychological distress.
Pharmacotherapy options
Antidepressants. Patients with excessive guilt, anhedonia, hopelessness, or ruminative thinking along with a related impairment in quality of life may benefit from pharmacotherapy regardless of whether they meet diagnostic criteria for MDD. Although SSRIs and SNRIs have become a mainstay in managing depression, placebo-controlled trials have yielded mixed results in depressed cancer patients. Furthermore, differences in efficacy among these antidepressants may not be significant, according to a recent meta-analysis.22
Select an antidepressant based on the patient’s past treatment response, target symptoms, and potential for adverse events. Mirtazapine has relatively few drug interactions; the side effects of sedation and weight gain may be welcome among patients with insomnia and impaired appetite.23 Furthermore, mirtazapine is a 5-HT3 receptor antagonist,24 which suggests it might act as an effective antiemetic.25 Other SNRIs, such as venlafaxine and duloxetine, have demonstrated benefits in managing neuropathic pain in patients who do not have cancer.26
Psychostimulants. Patients with a prognosis of days or weeks might not have enough time for an antidepressant to achieve full effect. Open prospective trials and pilot studies have shown that psychostimulants can improve cancer-related fatigue and quality of life while also augmenting the action of antidepressants.27 Psychostimulants, such as methylphenidate, have been used for treating cancer-related fatigue and depressive symptoms in medically ill patients. Their rapid onset of action, coupled with minimal side effect profile, make them a good choice for seriously ill patients with significant neurovegetative symptoms of a depressive disorder. Note: Avoid psychostimulants in patients with delirium and use with caution in patients with heart disease.28
Novel agents. A growing body of preclinical research suggests that glutamate may be involved in the pathophysiology of MDD. Ketamine modulates glutamate neurotransmission as an N-methyl-d-aspartate receptor antagonist. A recent evaluation of a single dose IV of ketamine in a placebo-controlled, double-blind trial found that depressed patients receiving ketamine experienced significant improvement their depressive symptoms.29 Irwin and Iglewicz30 describe 2 hospice patients administered a single oral dose of ketamine, which provided rapid relief of depressive symptoms and was well tolerated.
Transdermal selegiline may help patients who have trouble taking oral medications, including antidepressants. Inability to tolerate or absorb medications may be related to several conditions such as head and neck cancer, severe mucositis, and dysphagia. The dose-related dietary requirements—tyramine restriction—and careful monitoring for drug interactions may limit the use of selegiline in medically ill patients.31Table 3 features a list of dosing recommendations for pharmacotherapeutic options.32
Use the strategy of “start low, go slow” when initiating and adjusting antidepressants because patients with cancer and other advanced illnesses often have concomitant organ failure and are at risk of drug interactions. Carefully review your patient’s medication list for agents that are no longer beneficial or possibly contributing to depressive symptoms to help reduce the risk of adverse pharmacokinetic and pharmaco-dynamic interactions.
Requests for a hastened death
As many as 8.5% of terminally ill patients have a sustained and pervasive wish for an early death.33 Although requests for a hastened death may evoke strong emotional reactions and compel many clinicians to recoil or harshly reject such requests, consider such requests as an opportunity to gain insight into the patient’s narrative of his (her) suffering. The clinician’s role in such cases is to identify suicidality and perform a thorough suicide risk assessment. Interventions to prevent suicide should attempt to balance the seriousness of self-harm threats with restrictions on the patient’s liberty.34
Clinicians also need to consider the patient’s prognosis in their decision-making. For example, an extremely depressed or suicidal patient may not benefit from psychiatric hospitalization if she (he) has progressive neurovegetative symptoms and a prognosis of only a few weeks to live. These situations often are challenging and require a careful, informed discussion of the risks and benefits of all proposed interventions.
Clinicians also should be familiar with distinctions among ethical issues in end-of-life care, including physician-assisted suicide, euthanasia, and palliative sedation (Table 4).35,36
In Oregon, requests for physician-assisted suicide and hastened death through the state’s Death with Dignity Act often are short lived, and may not persist when clinicians offer patients good symptom management and psychological support.37 Requests for a hastened death often are motivated by loss of control, inability to find meaning in death, indignity from being dependent, and concern for future suffering and burden on loved ones.37
Carefully evaluate requests for hastened death in a manner that balances your personal and professional integrity. To preserve personal integrity, clearly communicate therapeutic interventions that you can and cannot provide. To ensure the patient does not feel abandoned, identify factors that contribute to the patient’s suffering and express a desire to search for alternative care approaches that will be mutually acceptable to the patient and to you.
Advance care planning and palliative care consultations may help in these circumstances. A randomized trial comparing advance care planning vs standard care in hospitalized geriatric patients found that advance care planning was more likely to lead to end-of-life wishes that were recognized by clinicians, and was associated with less distress, anxiety, and depression as reported by bereaved family members.38
Clinicians can assist patients with advanced care planning by helping them fill out advance directives, such as durable health care power of attorney documents and a living will. Palliative care clinicians can offer specialty-level assistance in advance care planning, provide focused assessments of physical and psychosocial symptoms, develop appropriate clinical goals, and assist in coordinating individualized care plans for seriously ill patients.2
Bottom Line
Depression commonly is encountered in hospice and palliative care patients and is associated with morbidity and distress. Validated screening tools can help you distinguish major depressive disorder from depressive symptoms in this population. Several psychotherapeutic techniques have been shown to be beneficial. In addition to traditional antidepressants, psychostimulants or ketamine may help address acute depressive symptoms in patients who have days or weeks to live.
Related Resources
- American Academy of Hospice and Palliative Medicine. www.aahpm.org.
- Death with Dignity National Center. www.deathwithdignity.org.
- National Hospice and Palliative Care Organization. www.nhpco.org.
- Oregon Health Authority. Death with Dignity Act. http://public.health.oregon.gov/ProviderPartnerResources/Evaluationresearch/deathwithdignityact/Pages/index.aspx.
Drug Brand Names
Duloxetine • Cymbalta Modafinil • Provigil
Ketamine • Ketalar Selegiline (transdermal) • EMSAM
Methylphenidate • Concerta, Ritalin Mirtazipine • Remeron
Venlafaxine • Effexor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Irwin SA, Rao S, Bower K, et al. Psychiatric issues in palliative care: recognition of depression in patients enrolled in hospice care. J Palliat Med. 2008;11(2):158-163.
2. Misono S, Weiss NS, Fann JR, et al. Incidence of suicide in persons with cancer. J Clin Oncol. 2008;26(29):4731-4738.
3. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911.
4. Turaga KK, Malafa MP, Jacobsen PB, et al. Suicide in patients with pancreatic cancer. Cancer. 2011;117(3):642-647.
5. Rosenstein DL. Depression and end-of-life care for patients with cancer. Dialogues Clin Neurosci. 2011;13(1):101-108.
6. King DA, Heisel MJ, Lyness JM. Assessment and psychological treatment of depression in older adults with terminal or life-threatening illness. Clin Psychol (New York). 2005;12(3):339-353.
7. Block SD. Assessing and managing depression in the terminally ill patient. ACP-ASIM End-of-Life Care Consensus Panel. American College of Physicians - American Society of Internal Medicine. Ann Intern Med. 2000;132(3):209-218.
8. Chochinov HM, Wilson KG, Enns M, et al. Prevalence of depression in the terminally ill: effects of diagnostic criteria and symptom threshold judgments. Am J Psychiatry. 1994;151(4):537-540.
9. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71.
10. Spiller JA, Keen JC. Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliat Med. 2006;20(1):17-23.
11. Maguire P. Improving the detection of psychiatric problems in cancer patients. Soc Sci Med. 1985;20(8):819-823.
12. Hinton J. Can home care maintain an acceptable quality of life for patients with terminal cancer and their relatives? Palliat Med. 1994;8(3):183-196.
13. Lloyd-Williams M, Spiller J, Ward J. Which depression screening tools should be used in palliative care? Palliat Med. 2003;17(1):40-43.
14. Chochinov HM, Wilson KG, Enns M, et al. “Are you depressed?” Screening for depression in the terminally ill. Am J Psychiatry. 1997;154(5):674-676.
15. Robinson JA, Crawford GB. Identifying palliative care patients with symptoms of depression: an algorithm. Palliat Med. 2005;19(4):278-287.
16. Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53(10 suppl):2243-2249.
17. Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520-5525.
18. Chochinov HM. Dignity-conserving care-a new model for palliative care: helping the patient feel valued. JAMA. 2002;287(17):2253-2260.
19. Kissane DW, Grabsch B, Clarke DM, et al. Supportive-expressive group therapy: the transformation of existential ambivalence into creative living while enhancing adherence to anti-cancer therapies. Psychooncology. 2004;13(11):
755-768.
20. Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372(9632):40-48.
21. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
22. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155(11):772-785.
23. Kast RE, Foley KF. Cancer chemotherapy and cachexia: mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur J Cancer Care (Engl). 2007; 16(4):351-354.
24. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001; 7(3):249-264.
25. Pae CU. Low-dose mirtazapine may be successful treatment option for severe nausea and vomiting. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):
1143-1145.
26. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454.
27. Pereira J, Bruera E. Depression with psychomotor retardation: diagnostic challenges and the use of psychostimulants. J Palliat Med. 2001;4(1):15-21.
28. Jackson V, Block S. # 061 Use of Psycho-Stimulants in Palliative Care, 2nd ed. End of Life/Palliative Education Resource Center. Medical College of Wisconsin. http://www.eperc.mcw.edu/EPERC/FastFactsIndex/ff_061.htm. Accessed December 28, 2012.
29. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.
30. Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13(7):903-908.
31. Attard A, Ranjith G, Taylor D. Alternative routes to oral antidepressant therapy: case vignette and literature review. J Psychopharmacol. 2010;24(4):449-454.
32. Rozans M, Dreisbach A, Lertora JJ, et al. Palliative uses of methylphenidate in patients with cancer: a review. J Clin Oncol. 2002;20(1):335-339.
33. Chochinov HM, Wilson KG, Enns M, et al. Desire for death in the terminally ill. Am J Psychiatry. 1995;152(8):1185-1191.
34. Marks S, Heinrich TW, Rosielle D. Case report: are clinicians obligated to medically treat a suicide attempt in a patient with a prognosis of weeks? J Palliat Med. 2012;15(1):134-137.
35. Materstvedt LJ, Clark D, Ellershaw J, et al. Euthanasia and physician-assisted suicide: a view from an EAPC Ethics Task Force. Palliat Med. 2003;17(2):97-101; discussion 102-179.
36. Kirk TW, Mahon MM; Palliative Sedation Task Force of the National Hospice and Palliative Care Organization Ethics Committee. National Hospice and Palliative Care Organization (NHPCO) position statement and commentary on the use of palliative sedation in imminently dying terminally ill patients. J Pain Symptom Manage. 2010; 39(5):914-923.
37. Okie S. Physician-assisted suicide--Oregon and beyond. N Engl J Med. 2005;352(16):1627-1630.
38. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c134
1. Irwin SA, Rao S, Bower K, et al. Psychiatric issues in palliative care: recognition of depression in patients enrolled in hospice care. J Palliat Med. 2008;11(2):158-163.
2. Misono S, Weiss NS, Fann JR, et al. Incidence of suicide in persons with cancer. J Clin Oncol. 2008;26(29):4731-4738.
3. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911.
4. Turaga KK, Malafa MP, Jacobsen PB, et al. Suicide in patients with pancreatic cancer. Cancer. 2011;117(3):642-647.
5. Rosenstein DL. Depression and end-of-life care for patients with cancer. Dialogues Clin Neurosci. 2011;13(1):101-108.
6. King DA, Heisel MJ, Lyness JM. Assessment and psychological treatment of depression in older adults with terminal or life-threatening illness. Clin Psychol (New York). 2005;12(3):339-353.
7. Block SD. Assessing and managing depression in the terminally ill patient. ACP-ASIM End-of-Life Care Consensus Panel. American College of Physicians - American Society of Internal Medicine. Ann Intern Med. 2000;132(3):209-218.
8. Chochinov HM, Wilson KG, Enns M, et al. Prevalence of depression in the terminally ill: effects of diagnostic criteria and symptom threshold judgments. Am J Psychiatry. 1994;151(4):537-540.
9. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71.
10. Spiller JA, Keen JC. Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliat Med. 2006;20(1):17-23.
11. Maguire P. Improving the detection of psychiatric problems in cancer patients. Soc Sci Med. 1985;20(8):819-823.
12. Hinton J. Can home care maintain an acceptable quality of life for patients with terminal cancer and their relatives? Palliat Med. 1994;8(3):183-196.
13. Lloyd-Williams M, Spiller J, Ward J. Which depression screening tools should be used in palliative care? Palliat Med. 2003;17(1):40-43.
14. Chochinov HM, Wilson KG, Enns M, et al. “Are you depressed?” Screening for depression in the terminally ill. Am J Psychiatry. 1997;154(5):674-676.
15. Robinson JA, Crawford GB. Identifying palliative care patients with symptoms of depression: an algorithm. Palliat Med. 2005;19(4):278-287.
16. Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53(10 suppl):2243-2249.
17. Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520-5525.
18. Chochinov HM. Dignity-conserving care-a new model for palliative care: helping the patient feel valued. JAMA. 2002;287(17):2253-2260.
19. Kissane DW, Grabsch B, Clarke DM, et al. Supportive-expressive group therapy: the transformation of existential ambivalence into creative living while enhancing adherence to anti-cancer therapies. Psychooncology. 2004;13(11):
755-768.
20. Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372(9632):40-48.
21. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
22. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155(11):772-785.
23. Kast RE, Foley KF. Cancer chemotherapy and cachexia: mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur J Cancer Care (Engl). 2007; 16(4):351-354.
24. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001; 7(3):249-264.
25. Pae CU. Low-dose mirtazapine may be successful treatment option for severe nausea and vomiting. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):
1143-1145.
26. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454.
27. Pereira J, Bruera E. Depression with psychomotor retardation: diagnostic challenges and the use of psychostimulants. J Palliat Med. 2001;4(1):15-21.
28. Jackson V, Block S. # 061 Use of Psycho-Stimulants in Palliative Care, 2nd ed. End of Life/Palliative Education Resource Center. Medical College of Wisconsin. http://www.eperc.mcw.edu/EPERC/FastFactsIndex/ff_061.htm. Accessed December 28, 2012.
29. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.
30. Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13(7):903-908.
31. Attard A, Ranjith G, Taylor D. Alternative routes to oral antidepressant therapy: case vignette and literature review. J Psychopharmacol. 2010;24(4):449-454.
32. Rozans M, Dreisbach A, Lertora JJ, et al. Palliative uses of methylphenidate in patients with cancer: a review. J Clin Oncol. 2002;20(1):335-339.
33. Chochinov HM, Wilson KG, Enns M, et al. Desire for death in the terminally ill. Am J Psychiatry. 1995;152(8):1185-1191.
34. Marks S, Heinrich TW, Rosielle D. Case report: are clinicians obligated to medically treat a suicide attempt in a patient with a prognosis of weeks? J Palliat Med. 2012;15(1):134-137.
35. Materstvedt LJ, Clark D, Ellershaw J, et al. Euthanasia and physician-assisted suicide: a view from an EAPC Ethics Task Force. Palliat Med. 2003;17(2):97-101; discussion 102-179.
36. Kirk TW, Mahon MM; Palliative Sedation Task Force of the National Hospice and Palliative Care Organization Ethics Committee. National Hospice and Palliative Care Organization (NHPCO) position statement and commentary on the use of palliative sedation in imminently dying terminally ill patients. J Pain Symptom Manage. 2010; 39(5):914-923.
37. Okie S. Physician-assisted suicide--Oregon and beyond. N Engl J Med. 2005;352(16):1627-1630.
38. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c134
Dihydropyridine calcium channel blockers in dementia and hypertension
Dementia affects 34 million people globally, with the most common cause of dementia, Alzheimer’s disease (AD), affecting 5.5 million Americans.1,2 The connection between cerebrovascular disorders and AD means that antihypertensive agents may play a role in dementia prophylaxis and management.1,2
Hypertension increases the risk of intellectual dysfunction by increasing susceptibility to heart disease, ischemic brain injury, and cerebrovascular pathology.1 In addition to senile plaques, ischemic brain lesions are observed in autopsies of AD patients,1 and brain infarctions are more common among AD patients than among controls.2 Brain pathology suggestive of AD was found in 30% to 50% of postmortem examinations of patients with vascular dementia.1
It is useful to note that dihydropyridines, a subgroup of calcium channel blockers, may inhibit amyloidogenesis.3
Hypertension and cognition
Hypertension-induced hyperdense lesions in cerebral white matter reflect pathology in small vessels, inflammatory change, and disruption of the blood-brain barrier, which may precede cognitive decline.1 Even subclinical ischemic changes may increase the probability of developing dementia.2 Hypertension also reduces cerebral perfusion, especially in the hippocampus, which may promote degeneration of memory function.1 Prolonged cerebral hypoxia increases amyloid precursor protein production and β-secretase activity.1,2 Patients who died of brain ischemia show prominent β-amyloid protein and apolipoprotein E in histopathologic analysis of the hippocampus.1 Compression of vessels by â-amyloid protein further augments this degenerative process.1
Inhibition of amyloidogenesis
Long-term administration of antihypertensive medications in patients age <75 decreases the probability of dementia by 8% each year.1 Calcium channel blockers protect neurons by lowering blood pressure and reversing cellular-level calcium channel dysfunction that occurs with age, cerebral infarction, and AD.
Select dihydropyridines may inhibit amyloidogenesis in apolipoprotein E carriers:
• amlodipine and nilvadipine reduce β-secretase activity and amyloid precursor protein-β production3
• nilvadipine and nitrendipine limit β-amyloid protein synthesis in the brain and promote their clearance through the blood-brain barrier3
• nilvadipine-treated apolipoprotein E carriers experience cognitive stabilization compared with cognitive decreases seen in non-treated subjects.
Dihydropyridines can produce therapeutic effects for both AD and cerebrovascular dementia patients, indicating the potential that certain agents in this class have for treating both conditions.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Valenzuela M, Esler M, Ritchie K, et al. Antihypertensives for combating dementia? A perspective on candidate molecular mechanisms and population-based prevention. Transl Psychiatry. 2012;2:e107.
2. Pimentel-Coelho PM, Rivest S. The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer’s disease. Eur J Neurosci. 2012;35(12):1917-1937.
3. Paris D, Bachmeier C, Patel N, et al. Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood-brain barrier. Mol Med. 2011;17(3-4):149-162.
Dementia affects 34 million people globally, with the most common cause of dementia, Alzheimer’s disease (AD), affecting 5.5 million Americans.1,2 The connection between cerebrovascular disorders and AD means that antihypertensive agents may play a role in dementia prophylaxis and management.1,2
Hypertension increases the risk of intellectual dysfunction by increasing susceptibility to heart disease, ischemic brain injury, and cerebrovascular pathology.1 In addition to senile plaques, ischemic brain lesions are observed in autopsies of AD patients,1 and brain infarctions are more common among AD patients than among controls.2 Brain pathology suggestive of AD was found in 30% to 50% of postmortem examinations of patients with vascular dementia.1
It is useful to note that dihydropyridines, a subgroup of calcium channel blockers, may inhibit amyloidogenesis.3
Hypertension and cognition
Hypertension-induced hyperdense lesions in cerebral white matter reflect pathology in small vessels, inflammatory change, and disruption of the blood-brain barrier, which may precede cognitive decline.1 Even subclinical ischemic changes may increase the probability of developing dementia.2 Hypertension also reduces cerebral perfusion, especially in the hippocampus, which may promote degeneration of memory function.1 Prolonged cerebral hypoxia increases amyloid precursor protein production and β-secretase activity.1,2 Patients who died of brain ischemia show prominent β-amyloid protein and apolipoprotein E in histopathologic analysis of the hippocampus.1 Compression of vessels by â-amyloid protein further augments this degenerative process.1
Inhibition of amyloidogenesis
Long-term administration of antihypertensive medications in patients age <75 decreases the probability of dementia by 8% each year.1 Calcium channel blockers protect neurons by lowering blood pressure and reversing cellular-level calcium channel dysfunction that occurs with age, cerebral infarction, and AD.
Select dihydropyridines may inhibit amyloidogenesis in apolipoprotein E carriers:
• amlodipine and nilvadipine reduce β-secretase activity and amyloid precursor protein-β production3
• nilvadipine and nitrendipine limit β-amyloid protein synthesis in the brain and promote their clearance through the blood-brain barrier3
• nilvadipine-treated apolipoprotein E carriers experience cognitive stabilization compared with cognitive decreases seen in non-treated subjects.
Dihydropyridines can produce therapeutic effects for both AD and cerebrovascular dementia patients, indicating the potential that certain agents in this class have for treating both conditions.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dementia affects 34 million people globally, with the most common cause of dementia, Alzheimer’s disease (AD), affecting 5.5 million Americans.1,2 The connection between cerebrovascular disorders and AD means that antihypertensive agents may play a role in dementia prophylaxis and management.1,2
Hypertension increases the risk of intellectual dysfunction by increasing susceptibility to heart disease, ischemic brain injury, and cerebrovascular pathology.1 In addition to senile plaques, ischemic brain lesions are observed in autopsies of AD patients,1 and brain infarctions are more common among AD patients than among controls.2 Brain pathology suggestive of AD was found in 30% to 50% of postmortem examinations of patients with vascular dementia.1
It is useful to note that dihydropyridines, a subgroup of calcium channel blockers, may inhibit amyloidogenesis.3
Hypertension and cognition
Hypertension-induced hyperdense lesions in cerebral white matter reflect pathology in small vessels, inflammatory change, and disruption of the blood-brain barrier, which may precede cognitive decline.1 Even subclinical ischemic changes may increase the probability of developing dementia.2 Hypertension also reduces cerebral perfusion, especially in the hippocampus, which may promote degeneration of memory function.1 Prolonged cerebral hypoxia increases amyloid precursor protein production and β-secretase activity.1,2 Patients who died of brain ischemia show prominent β-amyloid protein and apolipoprotein E in histopathologic analysis of the hippocampus.1 Compression of vessels by â-amyloid protein further augments this degenerative process.1
Inhibition of amyloidogenesis
Long-term administration of antihypertensive medications in patients age <75 decreases the probability of dementia by 8% each year.1 Calcium channel blockers protect neurons by lowering blood pressure and reversing cellular-level calcium channel dysfunction that occurs with age, cerebral infarction, and AD.
Select dihydropyridines may inhibit amyloidogenesis in apolipoprotein E carriers:
• amlodipine and nilvadipine reduce β-secretase activity and amyloid precursor protein-β production3
• nilvadipine and nitrendipine limit β-amyloid protein synthesis in the brain and promote their clearance through the blood-brain barrier3
• nilvadipine-treated apolipoprotein E carriers experience cognitive stabilization compared with cognitive decreases seen in non-treated subjects.
Dihydropyridines can produce therapeutic effects for both AD and cerebrovascular dementia patients, indicating the potential that certain agents in this class have for treating both conditions.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Valenzuela M, Esler M, Ritchie K, et al. Antihypertensives for combating dementia? A perspective on candidate molecular mechanisms and population-based prevention. Transl Psychiatry. 2012;2:e107.
2. Pimentel-Coelho PM, Rivest S. The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer’s disease. Eur J Neurosci. 2012;35(12):1917-1937.
3. Paris D, Bachmeier C, Patel N, et al. Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood-brain barrier. Mol Med. 2011;17(3-4):149-162.
1. Valenzuela M, Esler M, Ritchie K, et al. Antihypertensives for combating dementia? A perspective on candidate molecular mechanisms and population-based prevention. Transl Psychiatry. 2012;2:e107.
2. Pimentel-Coelho PM, Rivest S. The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer’s disease. Eur J Neurosci. 2012;35(12):1917-1937.
3. Paris D, Bachmeier C, Patel N, et al. Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood-brain barrier. Mol Med. 2011;17(3-4):149-162.
New ‘legal’ highs: Kratom and methoxetamine
The demand for “legal highs”—intoxicating natural or synthetic substances that are not prohibited by law—continues to increase. Young adults may use these substances, which are widely available on the internet, at “head shops,” and at gas stations. Such substances frequently cause adverse medical and psychiatric effects, exemplified by recent reports concerning the dangers of using synthetic cannabinoids (eg, “Spice,” “K2”) and synthetic cathinones (“bath salts”). Although these 2 substances now are illegal in many jurisdictions, other novel substances of misuse remain legal and widely available, including Kratom and methoxetamine.
Because these substances usually are not detectable on standard urine toxicology screens, clinicians need to be aware of them to be able to take an accurate substance use history, consider possible dangerous interactions with prescribed psychotropics, and address medical and psychiatric complications.
Kratom is an herbal product derived from Mitragyna speciosa, a plant native to Southeast Asia. Traditionally used as a medicinal herb, it increasingly is being used for recreational purposes and remains legal and widely available in the United States. Kratom’s leaves contain multiple alkaloids, including mitragynine and 7-hydroxymitragynine, which are believed to act as agonists at the μ-opioid receptor. Mitragynine also may have agonist activity at post-synaptic
α2-adrenergic receptors, as well as antagonist activity at 5-HT2A receptors.1 Mitragynine is 13 times more potent than morphine, and 7-hydroxymitragynine is 4 times more potent than mitragynine.2
Kratom is available as leaves, powdered leaves, or gum. It can be smoked, brewed into tea, or mixed with liquid and ingested. Effects are dose-dependent; lower doses tend to produce a stimulant effect and higher doses produce an opioid effect. A typical dose is 1 to 8 g.3 Users may take Kratom to experience euphoria or analgesia, or to self-treat opioid withdrawal symptoms.3 Kratom withdrawal syndrome shares many features of classic opioid withdrawal—diarrhea, rhinorrhea, cravings, anxiety, tremor, myalgia, sweating, and irritability—but has been reported to be less severe and shorter-lasting.1 Kratom withdrawal, like opioid withdrawal, may respond to supportive care in combination with opioid-replacement therapy. Airway management and naloxone treatment may be needed on an emergent basis if a user develops respiratory depression.2 There have been case reports of seizures occurring following Kratom use.2
Methoxetamine is a ketamine analog originally developed as an alternative to ketamine. It isn’t classified as a controlled substance in the United States and is available on the internet.2 Methoxetamine is a white powder typically snorted or taken sublingually, although it can be injected intramuscularly. Because methoxetamine’s structure is similar to ketamine, its mechanism of action is assumed to involve glutamate N-methyl-d-aspartate receptor antagonism and dopamine reuptake inhibition. Doses range from 20 to 100 mg orally and 10 to 50 mg when injected. Effects may not be apparent for 30 to 90 minutes after the drug is snorted, which may cause users to take another dose or ingest a different substance, possibly leading to synergistic adverse effects. Effects may emerge within 5 minutes when injected. The duration of effect generally is 5 to 7 hours—notably longer than ketamine—but as little as 1 hour when injected.
No clinical human or animal studies have been conducted on methoxetamine, which makes it difficult to ascertain the drug’s true clinical and toxic effects; instead, these effects must be surmised from user reports and case studies. Desired effects described by users are similar to those of ketamine: dissociation, short-term mood elevation, visual hallucinations, and alteration of sensory experiences. Reported adverse effects include catatonia, confusion, agitation, and depression.4 In addition, methoxetamine may induce sympathomimetic toxicity as evidenced by tachycardia and hypertension. Researchers have suggested that patients who experience methoxetamine toxicity and require emergency treatment be managed with supportive care and benzodiazepines.5
Staying current is key
New and potentially dangerous substances are being produced so quickly distributors are able to stay ahead of regulatory efforts. When one substance is declared illegal, another related substance quickly is available to take its place. To provide the best care for our patients, it is essential for psychiatrists to stay up-to-date about these novel substances.
Disclosure
Dr. Troy reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
2. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), Kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
3. Boyer EW, Babu KM, Macalino GE. Self-treatment of opioid withdrawal with a dietary supplement, Kratom. Am J Addict. 2007;16(5):352-356.
4. Corazza O, Schifano F, Simonato P, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27(2):
145-149.
5. Wood DM, Davies S, Puchnarewicz M, et al. Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol. 2012; 68(5):853-856.
The demand for “legal highs”—intoxicating natural or synthetic substances that are not prohibited by law—continues to increase. Young adults may use these substances, which are widely available on the internet, at “head shops,” and at gas stations. Such substances frequently cause adverse medical and psychiatric effects, exemplified by recent reports concerning the dangers of using synthetic cannabinoids (eg, “Spice,” “K2”) and synthetic cathinones (“bath salts”). Although these 2 substances now are illegal in many jurisdictions, other novel substances of misuse remain legal and widely available, including Kratom and methoxetamine.
Because these substances usually are not detectable on standard urine toxicology screens, clinicians need to be aware of them to be able to take an accurate substance use history, consider possible dangerous interactions with prescribed psychotropics, and address medical and psychiatric complications.
Kratom is an herbal product derived from Mitragyna speciosa, a plant native to Southeast Asia. Traditionally used as a medicinal herb, it increasingly is being used for recreational purposes and remains legal and widely available in the United States. Kratom’s leaves contain multiple alkaloids, including mitragynine and 7-hydroxymitragynine, which are believed to act as agonists at the μ-opioid receptor. Mitragynine also may have agonist activity at post-synaptic
α2-adrenergic receptors, as well as antagonist activity at 5-HT2A receptors.1 Mitragynine is 13 times more potent than morphine, and 7-hydroxymitragynine is 4 times more potent than mitragynine.2
Kratom is available as leaves, powdered leaves, or gum. It can be smoked, brewed into tea, or mixed with liquid and ingested. Effects are dose-dependent; lower doses tend to produce a stimulant effect and higher doses produce an opioid effect. A typical dose is 1 to 8 g.3 Users may take Kratom to experience euphoria or analgesia, or to self-treat opioid withdrawal symptoms.3 Kratom withdrawal syndrome shares many features of classic opioid withdrawal—diarrhea, rhinorrhea, cravings, anxiety, tremor, myalgia, sweating, and irritability—but has been reported to be less severe and shorter-lasting.1 Kratom withdrawal, like opioid withdrawal, may respond to supportive care in combination with opioid-replacement therapy. Airway management and naloxone treatment may be needed on an emergent basis if a user develops respiratory depression.2 There have been case reports of seizures occurring following Kratom use.2
Methoxetamine is a ketamine analog originally developed as an alternative to ketamine. It isn’t classified as a controlled substance in the United States and is available on the internet.2 Methoxetamine is a white powder typically snorted or taken sublingually, although it can be injected intramuscularly. Because methoxetamine’s structure is similar to ketamine, its mechanism of action is assumed to involve glutamate N-methyl-d-aspartate receptor antagonism and dopamine reuptake inhibition. Doses range from 20 to 100 mg orally and 10 to 50 mg when injected. Effects may not be apparent for 30 to 90 minutes after the drug is snorted, which may cause users to take another dose or ingest a different substance, possibly leading to synergistic adverse effects. Effects may emerge within 5 minutes when injected. The duration of effect generally is 5 to 7 hours—notably longer than ketamine—but as little as 1 hour when injected.
No clinical human or animal studies have been conducted on methoxetamine, which makes it difficult to ascertain the drug’s true clinical and toxic effects; instead, these effects must be surmised from user reports and case studies. Desired effects described by users are similar to those of ketamine: dissociation, short-term mood elevation, visual hallucinations, and alteration of sensory experiences. Reported adverse effects include catatonia, confusion, agitation, and depression.4 In addition, methoxetamine may induce sympathomimetic toxicity as evidenced by tachycardia and hypertension. Researchers have suggested that patients who experience methoxetamine toxicity and require emergency treatment be managed with supportive care and benzodiazepines.5
Staying current is key
New and potentially dangerous substances are being produced so quickly distributors are able to stay ahead of regulatory efforts. When one substance is declared illegal, another related substance quickly is available to take its place. To provide the best care for our patients, it is essential for psychiatrists to stay up-to-date about these novel substances.
Disclosure
Dr. Troy reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
The demand for “legal highs”—intoxicating natural or synthetic substances that are not prohibited by law—continues to increase. Young adults may use these substances, which are widely available on the internet, at “head shops,” and at gas stations. Such substances frequently cause adverse medical and psychiatric effects, exemplified by recent reports concerning the dangers of using synthetic cannabinoids (eg, “Spice,” “K2”) and synthetic cathinones (“bath salts”). Although these 2 substances now are illegal in many jurisdictions, other novel substances of misuse remain legal and widely available, including Kratom and methoxetamine.
Because these substances usually are not detectable on standard urine toxicology screens, clinicians need to be aware of them to be able to take an accurate substance use history, consider possible dangerous interactions with prescribed psychotropics, and address medical and psychiatric complications.
Kratom is an herbal product derived from Mitragyna speciosa, a plant native to Southeast Asia. Traditionally used as a medicinal herb, it increasingly is being used for recreational purposes and remains legal and widely available in the United States. Kratom’s leaves contain multiple alkaloids, including mitragynine and 7-hydroxymitragynine, which are believed to act as agonists at the μ-opioid receptor. Mitragynine also may have agonist activity at post-synaptic
α2-adrenergic receptors, as well as antagonist activity at 5-HT2A receptors.1 Mitragynine is 13 times more potent than morphine, and 7-hydroxymitragynine is 4 times more potent than mitragynine.2
Kratom is available as leaves, powdered leaves, or gum. It can be smoked, brewed into tea, or mixed with liquid and ingested. Effects are dose-dependent; lower doses tend to produce a stimulant effect and higher doses produce an opioid effect. A typical dose is 1 to 8 g.3 Users may take Kratom to experience euphoria or analgesia, or to self-treat opioid withdrawal symptoms.3 Kratom withdrawal syndrome shares many features of classic opioid withdrawal—diarrhea, rhinorrhea, cravings, anxiety, tremor, myalgia, sweating, and irritability—but has been reported to be less severe and shorter-lasting.1 Kratom withdrawal, like opioid withdrawal, may respond to supportive care in combination with opioid-replacement therapy. Airway management and naloxone treatment may be needed on an emergent basis if a user develops respiratory depression.2 There have been case reports of seizures occurring following Kratom use.2
Methoxetamine is a ketamine analog originally developed as an alternative to ketamine. It isn’t classified as a controlled substance in the United States and is available on the internet.2 Methoxetamine is a white powder typically snorted or taken sublingually, although it can be injected intramuscularly. Because methoxetamine’s structure is similar to ketamine, its mechanism of action is assumed to involve glutamate N-methyl-d-aspartate receptor antagonism and dopamine reuptake inhibition. Doses range from 20 to 100 mg orally and 10 to 50 mg when injected. Effects may not be apparent for 30 to 90 minutes after the drug is snorted, which may cause users to take another dose or ingest a different substance, possibly leading to synergistic adverse effects. Effects may emerge within 5 minutes when injected. The duration of effect generally is 5 to 7 hours—notably longer than ketamine—but as little as 1 hour when injected.
No clinical human or animal studies have been conducted on methoxetamine, which makes it difficult to ascertain the drug’s true clinical and toxic effects; instead, these effects must be surmised from user reports and case studies. Desired effects described by users are similar to those of ketamine: dissociation, short-term mood elevation, visual hallucinations, and alteration of sensory experiences. Reported adverse effects include catatonia, confusion, agitation, and depression.4 In addition, methoxetamine may induce sympathomimetic toxicity as evidenced by tachycardia and hypertension. Researchers have suggested that patients who experience methoxetamine toxicity and require emergency treatment be managed with supportive care and benzodiazepines.5
Staying current is key
New and potentially dangerous substances are being produced so quickly distributors are able to stay ahead of regulatory efforts. When one substance is declared illegal, another related substance quickly is available to take its place. To provide the best care for our patients, it is essential for psychiatrists to stay up-to-date about these novel substances.
Disclosure
Dr. Troy reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
2. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), Kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
3. Boyer EW, Babu KM, Macalino GE. Self-treatment of opioid withdrawal with a dietary supplement, Kratom. Am J Addict. 2007;16(5):352-356.
4. Corazza O, Schifano F, Simonato P, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27(2):
145-149.
5. Wood DM, Davies S, Puchnarewicz M, et al. Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol. 2012; 68(5):853-856.
1. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
2. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), Kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
3. Boyer EW, Babu KM, Macalino GE. Self-treatment of opioid withdrawal with a dietary supplement, Kratom. Am J Addict. 2007;16(5):352-356.
4. Corazza O, Schifano F, Simonato P, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27(2):
145-149.
5. Wood DM, Davies S, Puchnarewicz M, et al. Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol. 2012; 68(5):853-856.