User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Maintenance of certification and licensing: What you need to know
Discuss this article at www.facebook.com/CurrentPsychiatry
In 2000, the American Board of Medical Specialties (ABMS) made a commitment to develop a maintenance of certification (MOC) system for their 24 specialty boards. MOC aims to keep physicians up to date because medical knowledge and practice are rapidly evolving and health care systems expect greater accountability linked with performance and outcomes. Previously, board certification for most specialties was limited to a 1-time board exam; upon passing, a clinician was considered board certified for life. The American Board of Psychiatry and Neurology (ABPN) first issued time-limited certificates for board certification in 1994; 2007 was the first year of initial MOC enrollment for ABPN. Diplomates whose certificates were issued before October 1, 1994 are not required to participate in the MOC program.
The ABPN time-limited certificates are on 10-year cycles and require diplomates to fulfill 4 MOC program components: Professional Standing, Self-Assessment and Continuing Medical Education (CME), Cognitive Expertise, and Performance in Practice (PIP) (Table).1 Requirement details are available at www.abpn.com.
The ABMS MOC initiative is closely aligned with other initiatives, such as maintenance of licensure (MOL), that will impact all physicians, including those who are not board certified and those who were certified before October 1, 1994 and therefore not required to participate in MOC. Licensure, reimbursement, and institutional credentials are developing required measures based on self-assessment and performance.
Table
Maintenance of certification: 4 components
| Component | Description |
|---|---|
| Professional Standing | Diplomates must hold an active and unrestricted license to practice medicine in ≥1 state commonwealth territory or possession of the United States or province of Canada |
| Self-Assessment and CME | Self-assessment: Diplomates must participate in ≥2 major broad-based self-assessment activities that must cover new knowledge and/or current best practices and provide feedback to the diplomate that can be used as the basis for focused CME lifelong learning and/or career development |
| CME activities: Diplomates are required to complete an average of 30 specialty or subspecialty Category 1 CME credits per year over the 10-year MOC cycle. At least an average of 8 of the CME credits per year (averaged over 2 to 5 years) should involve self-assessment | |
| Cognitive Expertise | Diplomates must pass a cognitive examination before the expiration date of their certificates |
| Performance in Practice (PIP) | Diplomates will be required to complete 3 PIP units over the 10-year MOC cycle each consisting of both a clinical module (chart review) and a feedback module (patient/peer second-party external review) |
| CME: continuing medical education; MOC: maintenance of certification Source: Adapted from reference 1 | |
MOC requirements
The ABMS developed its MOC program around 6 general competencies identified by the Accreditation Council for Graduate Medical Education:
- professionalism
- patient care and procedural skills
- medical knowledge
- practice-based learning and improvement
- interpersonal and communications skills
- systems-based practice.
Physicians with “lifetime” certificates are not required to participate in MOC; there are no consequences for physicians who are not required to participate in MOC and choose not to participate, because MOC is a voluntary system. Physicians with time-limited certificates can choose not to participate, but would forfeit their certification. Physicians with certifications in multiple specialties may consider the value of maintaining all of their certifications because it would require them to participate in multiple MOC programs.
Two of the 4 parts of MOC (Parts I and III) are extensions of existing board certification requirements. Part I stipulates a diplomate hold a valid and unrestricted license in ≥1 states or jurisdictions in the United States, its territories, or Canada. Part III (Cognitive Expertise) requires that he or she must pass a cognitive examination every 10 years. To qualify to take the cognitive exam, a diplomate must meet all current MOC requirements.
Parts II and IV integrate continuing education, self-assessment, and the ability to apply both to practice improvements. Part II requires an average of 8 CME credit hours that include a self-assessment component; this likely would eliminate most traditional CME activities. The ABPN stipulates that feedback from the self-assessment must include a comparison with peers and specific literature recommendations for each question in the self-assessment. A small but growing number of accredited CME providers have developed self-directed CME activities that meet these criteria. As of 2014, only ABPN-approved self-assessment activities can be used to meet Part II requirements.
Part IV, the PIP activity, has raised the most concern. The PIP component focuses on quality improvement in 2 parts: a clinical module and a feedback module. This targets active clinicians, and both modules focus on quality improvement activities. The clinical module consists of a baseline chart review by the physician MOC applicant in which results are compared with best practices or practice guidelines. The practitioner-applicant repeats a second chart review after a period of time to determine if intervening practice improvements had a positive impact.
The feedback module consists of reviews of clinical performance by patients, peers, or other second parties such as other practice staff or administrators. These are repeated after a period of time to determine whether practice improvements have been effective.
The PIP model (assessment, practice improvement, reassessment) parallels requirements for Performance Improvement CME (PICME) activities. The American Medical Association (AMA) developed PICME at approximately the same time ABMS was creating MOC. PICME is aimed at changing physician behavior within the context of their clinical practice and is divided into 3 stages:
- Stage A: learning from current practice performance assessment
- Stage B: learning from the application of performance improvement to patient care
- Stage C: learning from the evaluation of the PICME effort.
For example, a coalition of academic, nonprofit, and business organizations—the NOW Coalition for Bipolar Disorder— developed an online quality improvement activity (see Related Resources), which the ABPN certified for assessment and PIP points. It also is certified for 20 points toward the Self-Evaluation of Practice Performance MOC requirement through the American Board of Internal Medicine’s Approved Quality Improvement Pathway, 20 AMA PRA Category 1 Credits™, and 20 Prescribed Credits by the AAFP. Many physicians hold multiple board certificates, and this kind of activity can simultaneously meet requirements for licensure and several MOC programs.
Merging requirements
- reflective self-assessment
- assessment of knowledge and skills
- PIP.
Effects on reimbursement
In 2012, the Centers for Medicare and Medicaid Services’ Physician Quality Reporting System MOC Program Incentive provided a 0.5% incentive payment to physicians participating in a qualified MOC program.5 Other insurers are examining similar reimbursement incentives tied to practice assessment and improvement. Public reporting of quality metrics also is becoming more prevalent in practice and reimbursement incentives.
- Pinals DA. Ready or not, here it comes: maintenance of certification. J Am Acad Psychiatry Law. 2011;39(3):294-296.
- American Board of Psychiatry and Neurology, Inc. www.abpn.com.
- Maintenance of certification. American Board of Psychiatry and Neurology, Inc. www.abpn.com/moc_products.asp.
- NOW coalition performance improvement (PI) CME activity. NOW Coalition for Bipolar Disorder. www.nowbipolar.org/pi-cme.php.
Dr. Kues reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Board of Psychiatry and Neurology. Inc. Maintenance of Certification (10YR-MOC). http://www.abpn.com/moc_10yrmoc.html. Accessed December 18, 2012.
2. American Board of Psychiatry and Neurology. Inc. Maintenance of certification (CP-MOC). http://www.abpn.com/moc_cpmoc.html. Accessed December 18, 2012.
3. American Medical Association. The Physician’s Recognition Award and credit system. http://www.ama-assn.org/resources/doc/cme/pra-booklet.pdf. Published 2012. Accessed December 18 2012.
4. Federation of State Medical Boards. Maintenance of licensure (MOL) information center. http://www.fsmb.org/mol.html. Published 2012. Accessed December 18, 2012.
5. Centers for Medicare and Medicaid Services. Physician quality reporting system. http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/index.html. Published September 27 2012. Accessed December 18, 2012.
Discuss this article at www.facebook.com/CurrentPsychiatry
In 2000, the American Board of Medical Specialties (ABMS) made a commitment to develop a maintenance of certification (MOC) system for their 24 specialty boards. MOC aims to keep physicians up to date because medical knowledge and practice are rapidly evolving and health care systems expect greater accountability linked with performance and outcomes. Previously, board certification for most specialties was limited to a 1-time board exam; upon passing, a clinician was considered board certified for life. The American Board of Psychiatry and Neurology (ABPN) first issued time-limited certificates for board certification in 1994; 2007 was the first year of initial MOC enrollment for ABPN. Diplomates whose certificates were issued before October 1, 1994 are not required to participate in the MOC program.
The ABPN time-limited certificates are on 10-year cycles and require diplomates to fulfill 4 MOC program components: Professional Standing, Self-Assessment and Continuing Medical Education (CME), Cognitive Expertise, and Performance in Practice (PIP) (Table).1 Requirement details are available at www.abpn.com.
The ABMS MOC initiative is closely aligned with other initiatives, such as maintenance of licensure (MOL), that will impact all physicians, including those who are not board certified and those who were certified before October 1, 1994 and therefore not required to participate in MOC. Licensure, reimbursement, and institutional credentials are developing required measures based on self-assessment and performance.
Table
Maintenance of certification: 4 components
| Component | Description |
|---|---|
| Professional Standing | Diplomates must hold an active and unrestricted license to practice medicine in ≥1 state commonwealth territory or possession of the United States or province of Canada |
| Self-Assessment and CME | Self-assessment: Diplomates must participate in ≥2 major broad-based self-assessment activities that must cover new knowledge and/or current best practices and provide feedback to the diplomate that can be used as the basis for focused CME lifelong learning and/or career development |
| CME activities: Diplomates are required to complete an average of 30 specialty or subspecialty Category 1 CME credits per year over the 10-year MOC cycle. At least an average of 8 of the CME credits per year (averaged over 2 to 5 years) should involve self-assessment | |
| Cognitive Expertise | Diplomates must pass a cognitive examination before the expiration date of their certificates |
| Performance in Practice (PIP) | Diplomates will be required to complete 3 PIP units over the 10-year MOC cycle each consisting of both a clinical module (chart review) and a feedback module (patient/peer second-party external review) |
| CME: continuing medical education; MOC: maintenance of certification Source: Adapted from reference 1 | |
MOC requirements
The ABMS developed its MOC program around 6 general competencies identified by the Accreditation Council for Graduate Medical Education:
- professionalism
- patient care and procedural skills
- medical knowledge
- practice-based learning and improvement
- interpersonal and communications skills
- systems-based practice.
Physicians with “lifetime” certificates are not required to participate in MOC; there are no consequences for physicians who are not required to participate in MOC and choose not to participate, because MOC is a voluntary system. Physicians with time-limited certificates can choose not to participate, but would forfeit their certification. Physicians with certifications in multiple specialties may consider the value of maintaining all of their certifications because it would require them to participate in multiple MOC programs.
Two of the 4 parts of MOC (Parts I and III) are extensions of existing board certification requirements. Part I stipulates a diplomate hold a valid and unrestricted license in ≥1 states or jurisdictions in the United States, its territories, or Canada. Part III (Cognitive Expertise) requires that he or she must pass a cognitive examination every 10 years. To qualify to take the cognitive exam, a diplomate must meet all current MOC requirements.
Parts II and IV integrate continuing education, self-assessment, and the ability to apply both to practice improvements. Part II requires an average of 8 CME credit hours that include a self-assessment component; this likely would eliminate most traditional CME activities. The ABPN stipulates that feedback from the self-assessment must include a comparison with peers and specific literature recommendations for each question in the self-assessment. A small but growing number of accredited CME providers have developed self-directed CME activities that meet these criteria. As of 2014, only ABPN-approved self-assessment activities can be used to meet Part II requirements.
Part IV, the PIP activity, has raised the most concern. The PIP component focuses on quality improvement in 2 parts: a clinical module and a feedback module. This targets active clinicians, and both modules focus on quality improvement activities. The clinical module consists of a baseline chart review by the physician MOC applicant in which results are compared with best practices or practice guidelines. The practitioner-applicant repeats a second chart review after a period of time to determine if intervening practice improvements had a positive impact.
The feedback module consists of reviews of clinical performance by patients, peers, or other second parties such as other practice staff or administrators. These are repeated after a period of time to determine whether practice improvements have been effective.
The PIP model (assessment, practice improvement, reassessment) parallels requirements for Performance Improvement CME (PICME) activities. The American Medical Association (AMA) developed PICME at approximately the same time ABMS was creating MOC. PICME is aimed at changing physician behavior within the context of their clinical practice and is divided into 3 stages:
- Stage A: learning from current practice performance assessment
- Stage B: learning from the application of performance improvement to patient care
- Stage C: learning from the evaluation of the PICME effort.
For example, a coalition of academic, nonprofit, and business organizations—the NOW Coalition for Bipolar Disorder— developed an online quality improvement activity (see Related Resources), which the ABPN certified for assessment and PIP points. It also is certified for 20 points toward the Self-Evaluation of Practice Performance MOC requirement through the American Board of Internal Medicine’s Approved Quality Improvement Pathway, 20 AMA PRA Category 1 Credits™, and 20 Prescribed Credits by the AAFP. Many physicians hold multiple board certificates, and this kind of activity can simultaneously meet requirements for licensure and several MOC programs.
Merging requirements
- reflective self-assessment
- assessment of knowledge and skills
- PIP.
Effects on reimbursement
In 2012, the Centers for Medicare and Medicaid Services’ Physician Quality Reporting System MOC Program Incentive provided a 0.5% incentive payment to physicians participating in a qualified MOC program.5 Other insurers are examining similar reimbursement incentives tied to practice assessment and improvement. Public reporting of quality metrics also is becoming more prevalent in practice and reimbursement incentives.
- Pinals DA. Ready or not, here it comes: maintenance of certification. J Am Acad Psychiatry Law. 2011;39(3):294-296.
- American Board of Psychiatry and Neurology, Inc. www.abpn.com.
- Maintenance of certification. American Board of Psychiatry and Neurology, Inc. www.abpn.com/moc_products.asp.
- NOW coalition performance improvement (PI) CME activity. NOW Coalition for Bipolar Disorder. www.nowbipolar.org/pi-cme.php.
Dr. Kues reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
In 2000, the American Board of Medical Specialties (ABMS) made a commitment to develop a maintenance of certification (MOC) system for their 24 specialty boards. MOC aims to keep physicians up to date because medical knowledge and practice are rapidly evolving and health care systems expect greater accountability linked with performance and outcomes. Previously, board certification for most specialties was limited to a 1-time board exam; upon passing, a clinician was considered board certified for life. The American Board of Psychiatry and Neurology (ABPN) first issued time-limited certificates for board certification in 1994; 2007 was the first year of initial MOC enrollment for ABPN. Diplomates whose certificates were issued before October 1, 1994 are not required to participate in the MOC program.
The ABPN time-limited certificates are on 10-year cycles and require diplomates to fulfill 4 MOC program components: Professional Standing, Self-Assessment and Continuing Medical Education (CME), Cognitive Expertise, and Performance in Practice (PIP) (Table).1 Requirement details are available at www.abpn.com.
The ABMS MOC initiative is closely aligned with other initiatives, such as maintenance of licensure (MOL), that will impact all physicians, including those who are not board certified and those who were certified before October 1, 1994 and therefore not required to participate in MOC. Licensure, reimbursement, and institutional credentials are developing required measures based on self-assessment and performance.
Table
Maintenance of certification: 4 components
| Component | Description |
|---|---|
| Professional Standing | Diplomates must hold an active and unrestricted license to practice medicine in ≥1 state commonwealth territory or possession of the United States or province of Canada |
| Self-Assessment and CME | Self-assessment: Diplomates must participate in ≥2 major broad-based self-assessment activities that must cover new knowledge and/or current best practices and provide feedback to the diplomate that can be used as the basis for focused CME lifelong learning and/or career development |
| CME activities: Diplomates are required to complete an average of 30 specialty or subspecialty Category 1 CME credits per year over the 10-year MOC cycle. At least an average of 8 of the CME credits per year (averaged over 2 to 5 years) should involve self-assessment | |
| Cognitive Expertise | Diplomates must pass a cognitive examination before the expiration date of their certificates |
| Performance in Practice (PIP) | Diplomates will be required to complete 3 PIP units over the 10-year MOC cycle each consisting of both a clinical module (chart review) and a feedback module (patient/peer second-party external review) |
| CME: continuing medical education; MOC: maintenance of certification Source: Adapted from reference 1 | |
MOC requirements
The ABMS developed its MOC program around 6 general competencies identified by the Accreditation Council for Graduate Medical Education:
- professionalism
- patient care and procedural skills
- medical knowledge
- practice-based learning and improvement
- interpersonal and communications skills
- systems-based practice.
Physicians with “lifetime” certificates are not required to participate in MOC; there are no consequences for physicians who are not required to participate in MOC and choose not to participate, because MOC is a voluntary system. Physicians with time-limited certificates can choose not to participate, but would forfeit their certification. Physicians with certifications in multiple specialties may consider the value of maintaining all of their certifications because it would require them to participate in multiple MOC programs.
Two of the 4 parts of MOC (Parts I and III) are extensions of existing board certification requirements. Part I stipulates a diplomate hold a valid and unrestricted license in ≥1 states or jurisdictions in the United States, its territories, or Canada. Part III (Cognitive Expertise) requires that he or she must pass a cognitive examination every 10 years. To qualify to take the cognitive exam, a diplomate must meet all current MOC requirements.
Parts II and IV integrate continuing education, self-assessment, and the ability to apply both to practice improvements. Part II requires an average of 8 CME credit hours that include a self-assessment component; this likely would eliminate most traditional CME activities. The ABPN stipulates that feedback from the self-assessment must include a comparison with peers and specific literature recommendations for each question in the self-assessment. A small but growing number of accredited CME providers have developed self-directed CME activities that meet these criteria. As of 2014, only ABPN-approved self-assessment activities can be used to meet Part II requirements.
Part IV, the PIP activity, has raised the most concern. The PIP component focuses on quality improvement in 2 parts: a clinical module and a feedback module. This targets active clinicians, and both modules focus on quality improvement activities. The clinical module consists of a baseline chart review by the physician MOC applicant in which results are compared with best practices or practice guidelines. The practitioner-applicant repeats a second chart review after a period of time to determine if intervening practice improvements had a positive impact.
The feedback module consists of reviews of clinical performance by patients, peers, or other second parties such as other practice staff or administrators. These are repeated after a period of time to determine whether practice improvements have been effective.
The PIP model (assessment, practice improvement, reassessment) parallels requirements for Performance Improvement CME (PICME) activities. The American Medical Association (AMA) developed PICME at approximately the same time ABMS was creating MOC. PICME is aimed at changing physician behavior within the context of their clinical practice and is divided into 3 stages:
- Stage A: learning from current practice performance assessment
- Stage B: learning from the application of performance improvement to patient care
- Stage C: learning from the evaluation of the PICME effort.
For example, a coalition of academic, nonprofit, and business organizations—the NOW Coalition for Bipolar Disorder— developed an online quality improvement activity (see Related Resources), which the ABPN certified for assessment and PIP points. It also is certified for 20 points toward the Self-Evaluation of Practice Performance MOC requirement through the American Board of Internal Medicine’s Approved Quality Improvement Pathway, 20 AMA PRA Category 1 Credits™, and 20 Prescribed Credits by the AAFP. Many physicians hold multiple board certificates, and this kind of activity can simultaneously meet requirements for licensure and several MOC programs.
Merging requirements
- reflective self-assessment
- assessment of knowledge and skills
- PIP.
Effects on reimbursement
In 2012, the Centers for Medicare and Medicaid Services’ Physician Quality Reporting System MOC Program Incentive provided a 0.5% incentive payment to physicians participating in a qualified MOC program.5 Other insurers are examining similar reimbursement incentives tied to practice assessment and improvement. Public reporting of quality metrics also is becoming more prevalent in practice and reimbursement incentives.
- Pinals DA. Ready or not, here it comes: maintenance of certification. J Am Acad Psychiatry Law. 2011;39(3):294-296.
- American Board of Psychiatry and Neurology, Inc. www.abpn.com.
- Maintenance of certification. American Board of Psychiatry and Neurology, Inc. www.abpn.com/moc_products.asp.
- NOW coalition performance improvement (PI) CME activity. NOW Coalition for Bipolar Disorder. www.nowbipolar.org/pi-cme.php.
Dr. Kues reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Board of Psychiatry and Neurology. Inc. Maintenance of Certification (10YR-MOC). http://www.abpn.com/moc_10yrmoc.html. Accessed December 18, 2012.
2. American Board of Psychiatry and Neurology. Inc. Maintenance of certification (CP-MOC). http://www.abpn.com/moc_cpmoc.html. Accessed December 18, 2012.
3. American Medical Association. The Physician’s Recognition Award and credit system. http://www.ama-assn.org/resources/doc/cme/pra-booklet.pdf. Published 2012. Accessed December 18 2012.
4. Federation of State Medical Boards. Maintenance of licensure (MOL) information center. http://www.fsmb.org/mol.html. Published 2012. Accessed December 18, 2012.
5. Centers for Medicare and Medicaid Services. Physician quality reporting system. http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/index.html. Published September 27 2012. Accessed December 18, 2012.
1. American Board of Psychiatry and Neurology. Inc. Maintenance of Certification (10YR-MOC). http://www.abpn.com/moc_10yrmoc.html. Accessed December 18, 2012.
2. American Board of Psychiatry and Neurology. Inc. Maintenance of certification (CP-MOC). http://www.abpn.com/moc_cpmoc.html. Accessed December 18, 2012.
3. American Medical Association. The Physician’s Recognition Award and credit system. http://www.ama-assn.org/resources/doc/cme/pra-booklet.pdf. Published 2012. Accessed December 18 2012.
4. Federation of State Medical Boards. Maintenance of licensure (MOL) information center. http://www.fsmb.org/mol.html. Published 2012. Accessed December 18, 2012.
5. Centers for Medicare and Medicaid Services. Physician quality reporting system. http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/index.html. Published September 27 2012. Accessed December 18, 2012.
Lab tests for psychiatric disorders: Few clinicians are aware of them
The lack of laboratory tests to validate the clinical diagnosis of schizophrenia is widely accepted and lamented by psychiatric practitioners. In a recent survey I conducted on CurrentPsychiatry.com, most respondents guessed there are 3 known biomarkers for schizophrenia and 4 for major depression.
The media’s view tends to be harsh, exploiting the ostensible absence of diagnostic biomarkers in psychiatry to cast unfair aspersions on the scientific validity of DSM-5 and its diagnostic guidelines.1 They seem to believe that lab tests for mental illness will never be feasible. Clearly, they have not done their homework.
Consider schizophrenia. It would come as a surprise to most people inside or outside the psychiatric community that 365 biomarkers for schizophrenia have been discovered, 273 of which are identifiable in plasma.2 Of these, 81 are diagnostic, 77 are markers of drug response, and 115 are for both. Some of these tests have been replicated at least 5 times (brain-derived neurotrophic factor, S100B, prolactin, interleukin (IL) 6, IL2, IN5, leptin, IL 1 receptor antagonist, IL8, and IL2 receptor α). The biologic functions of these 273 biomarkers include inflammatory disease or response, respiratory disease, cellular movement, lipid metabolism, molecular transport, immunologic disease, hematologic disease, renal and urologic disease, cell-to-cell signaling, cellular growth and proliferation, cardiovascular disease, genetic disorders, psychological disorders, metabolic disease, small molecule biochemistry, molecular transport, nutritional disease, endocrine system disorders, cell death, tissue morphology, organismal survival, lymphoid tissue structure and development, antigen presentation, tissue development, carbohydrate metabolism, organ morphology, embryonic development, behavior, and digestive system development and functions.2 Obviously, schizophrenia biomarkers overlap with multiple tissues and key biochemical and cellular processes in brain and body.
So why do none of these 273 blood tests appear in DSM-5, which had aspired to include objective methods in psychiatric diagnosis? The answer: heterogeneity. Schizophrenia and other major psychiatric illnesses are not 1 disorder but syndromes comprised of numerous clinically similar but biologically different disorders. There is extensive variability among the “schizophrenias” in genetic and nongenetic etiological factors and significant heterogeneity in neurobiology, treatment response, and clinical and functional outcomes. None of the individual 273 biomarkers alone can serve as a diagnostic tool for the schizophrenias because there will be high rates of false positives and false negatives. A lab test for a syndrome is impossible!
One company recently attempted to develop a blood test for schizophrenia. It used 51 biomarkers to comprise that test because none of them alone is a viable test (Table).3 The totality of the 51 biomarkers significantly increases the likelihood of diagnostic utility but still will be short of 100% specificity.
What is the point of identifying 273 blood tests if they have not been used to diagnose a heterogeneous syndrome? I believe there are many potentially useful applications for these biomarkers:
- To identify biologic subtypes of schizophrenia
- To shed light on the multiple pathophysiologies of schizophrenia, which may provide valuable clues for new treatments
- To help identify and characterize stages of schizophrenia. Some biomarkers have been found in the early stages, while others appear only in the chronic stages
- To help predict biologic predisposition to 1 of the schizophrenias. It is possible that the various susceptibility genes that have been identified in schizophrenia may be associated with certain biomarkers during fetal neurodevelopment, childhood, or the prodrome stage
- To explore the overlapping biologic features of psychotic disorders. For example, 21 biomarkers have been found to differentiate schizophrenia or bipolar disorder from healthy controls. Some biomarkers may point to the likelihood of psychiatric comorbidities such as depression or obsessive-compulsive disorder or medical comorbidities such as cardiovascular, immunologic, or gastrointestinal diseases
- Some biomarkers may identify state (ie, the psychotic phase only) vs trait (throughout life). Other biomarkers may be associated with the presence of a specific type of hallucination (auditory, visual, olfactory, or gustatory), delusion (bizarre vs simple), negative symptom (flat affect vs apathy vs avolition) or cognitive deficit (verbal memory vs learning deficit vs executive dysfunction)
- Biomarkers may assist in developing personalized medicine and designing customized evaluations and treatments for patients suffering from 1 of the many schizophrenias.
Lab tests for psychiatric disorders are indeed available but their use will not mirror traditional physical exam tests. The complex heterogeneity of most psychiatric syndromes means that biomarkers will help unravel the rich neurobiology of those disorders and help elucidate the multiple neurobiologic underpinnings of these syndromes. Psychiatrists should look forward with great optimism to a bright future for psychiatric diagnosis, combining a set of clinical signs and symptoms with a confirmatory cluster of lab tests. It may take time, but psychiatric clinicians will be using biomarkers in the future and the media and the public finally will perceive psychiatry as a “mature” medical discipline.
In the survey I mentioned at the beginning of this editorial, 60.5% of responders predicted that the DSM-6 (approximately a decade from now) will contain laboratory tests for psychiatric diagnosis. They may very well be right!
Table
Biomarkers for schizophrenia
| α-1 antitrypsin | IL-7 |
| Apolipoprotein A1 | IL-10 |
| Apolipoprotein A2 | IL-11 |
| Apolipoprotein B | IL-17 |
| Apolipoprotein CI | KIM-1 |
| Apolipoprotein H | LH |
| β-2 microglobulin | MCP-2 |
| Betacellulin | MDC |
| BDNF | MIF |
| CA | MIP-1 α |
| Calbindin | MMP-2 |
| Cancer antigen 125 | Prolactin |
| CD5L | Prostatic acid phosphatase |
| Complement 3 | PYY |
| Cortisol | Serum amyloid P |
| CTGF | Sortilin |
| EGFR | Testosterone |
| Endothelin 1 | Thrombopoietin |
| Ferritin | TIMP 1 |
| Fetuin A | TNF R 2 |
| FSH | Trail R3 |
| Haptoglobin | Transferrin |
| ICAM1 | TSH |
| IgA | VEGF |
| IgM | Vitronectin |
| IL-6 receptor | |
| BDNF: brain-derived neurotrophic factor; CD5L: CD5 molecule-like; CTGF: connective tissue growth factor; EGFR: epidermal growth factor receptor; FSH: follicle-stimulating hormone; ICAM1: intercellular adhesion molecule 1; IgA: immunoglobulin A; IgM: immunoglobulin M; IL-6 receptor: interleukin 6 receptor; IL-7: interleukin 7; IL-10: interleukin 10; IL-11: interleukin 11; IL-17: interleukin 17; KIM-1: kidney injury molecule-1; LH: luteinizing hormone; MCP-2: monocyte chemotactic protein 2; MDC: mature dendritic cell; MIF: macrophage migration inhibitory factor; MIP-1 α: macrophage inflammatory protein 1 α; MMP-2: matrix metalloproteinase 2; PYY: peptide YY; TIMP 1: TIMP metallopeptidase inhibitor 1; TNF R 2: tumor necrosis factor receptor 2; TSH: thyroid-stimulating hormone; VEGF: vascular endothelial growth factor Source: Reference 3 | |
1. Sepkowitz K. The DSM’s controversial update. Newsweek. December 10, 2012. http://www.thedailybeast.com/newsweek/2012/12/09/the-dsm-s-controversial-update.html. Accessed January 4, 2013.
2. Chan MK, Guest PC, Levin Y, et al. Converging evidence of blood-based biomarkers for schizophrenia: an update. Int Rev Neurobiol. 2011;101:95-144.
3. Schwarz E, Izmailov R, Spain M, et al. Validation of a blood-based laboratory test to aid in the confirmation of a diagnosis of schizophrenia. Biomark Insights. 2010;5:39-47.
The lack of laboratory tests to validate the clinical diagnosis of schizophrenia is widely accepted and lamented by psychiatric practitioners. In a recent survey I conducted on CurrentPsychiatry.com, most respondents guessed there are 3 known biomarkers for schizophrenia and 4 for major depression.
The media’s view tends to be harsh, exploiting the ostensible absence of diagnostic biomarkers in psychiatry to cast unfair aspersions on the scientific validity of DSM-5 and its diagnostic guidelines.1 They seem to believe that lab tests for mental illness will never be feasible. Clearly, they have not done their homework.
Consider schizophrenia. It would come as a surprise to most people inside or outside the psychiatric community that 365 biomarkers for schizophrenia have been discovered, 273 of which are identifiable in plasma.2 Of these, 81 are diagnostic, 77 are markers of drug response, and 115 are for both. Some of these tests have been replicated at least 5 times (brain-derived neurotrophic factor, S100B, prolactin, interleukin (IL) 6, IL2, IN5, leptin, IL 1 receptor antagonist, IL8, and IL2 receptor α). The biologic functions of these 273 biomarkers include inflammatory disease or response, respiratory disease, cellular movement, lipid metabolism, molecular transport, immunologic disease, hematologic disease, renal and urologic disease, cell-to-cell signaling, cellular growth and proliferation, cardiovascular disease, genetic disorders, psychological disorders, metabolic disease, small molecule biochemistry, molecular transport, nutritional disease, endocrine system disorders, cell death, tissue morphology, organismal survival, lymphoid tissue structure and development, antigen presentation, tissue development, carbohydrate metabolism, organ morphology, embryonic development, behavior, and digestive system development and functions.2 Obviously, schizophrenia biomarkers overlap with multiple tissues and key biochemical and cellular processes in brain and body.
So why do none of these 273 blood tests appear in DSM-5, which had aspired to include objective methods in psychiatric diagnosis? The answer: heterogeneity. Schizophrenia and other major psychiatric illnesses are not 1 disorder but syndromes comprised of numerous clinically similar but biologically different disorders. There is extensive variability among the “schizophrenias” in genetic and nongenetic etiological factors and significant heterogeneity in neurobiology, treatment response, and clinical and functional outcomes. None of the individual 273 biomarkers alone can serve as a diagnostic tool for the schizophrenias because there will be high rates of false positives and false negatives. A lab test for a syndrome is impossible!
One company recently attempted to develop a blood test for schizophrenia. It used 51 biomarkers to comprise that test because none of them alone is a viable test (Table).3 The totality of the 51 biomarkers significantly increases the likelihood of diagnostic utility but still will be short of 100% specificity.
What is the point of identifying 273 blood tests if they have not been used to diagnose a heterogeneous syndrome? I believe there are many potentially useful applications for these biomarkers:
- To identify biologic subtypes of schizophrenia
- To shed light on the multiple pathophysiologies of schizophrenia, which may provide valuable clues for new treatments
- To help identify and characterize stages of schizophrenia. Some biomarkers have been found in the early stages, while others appear only in the chronic stages
- To help predict biologic predisposition to 1 of the schizophrenias. It is possible that the various susceptibility genes that have been identified in schizophrenia may be associated with certain biomarkers during fetal neurodevelopment, childhood, or the prodrome stage
- To explore the overlapping biologic features of psychotic disorders. For example, 21 biomarkers have been found to differentiate schizophrenia or bipolar disorder from healthy controls. Some biomarkers may point to the likelihood of psychiatric comorbidities such as depression or obsessive-compulsive disorder or medical comorbidities such as cardiovascular, immunologic, or gastrointestinal diseases
- Some biomarkers may identify state (ie, the psychotic phase only) vs trait (throughout life). Other biomarkers may be associated with the presence of a specific type of hallucination (auditory, visual, olfactory, or gustatory), delusion (bizarre vs simple), negative symptom (flat affect vs apathy vs avolition) or cognitive deficit (verbal memory vs learning deficit vs executive dysfunction)
- Biomarkers may assist in developing personalized medicine and designing customized evaluations and treatments for patients suffering from 1 of the many schizophrenias.
Lab tests for psychiatric disorders are indeed available but their use will not mirror traditional physical exam tests. The complex heterogeneity of most psychiatric syndromes means that biomarkers will help unravel the rich neurobiology of those disorders and help elucidate the multiple neurobiologic underpinnings of these syndromes. Psychiatrists should look forward with great optimism to a bright future for psychiatric diagnosis, combining a set of clinical signs and symptoms with a confirmatory cluster of lab tests. It may take time, but psychiatric clinicians will be using biomarkers in the future and the media and the public finally will perceive psychiatry as a “mature” medical discipline.
In the survey I mentioned at the beginning of this editorial, 60.5% of responders predicted that the DSM-6 (approximately a decade from now) will contain laboratory tests for psychiatric diagnosis. They may very well be right!
Table
Biomarkers for schizophrenia
| α-1 antitrypsin | IL-7 |
| Apolipoprotein A1 | IL-10 |
| Apolipoprotein A2 | IL-11 |
| Apolipoprotein B | IL-17 |
| Apolipoprotein CI | KIM-1 |
| Apolipoprotein H | LH |
| β-2 microglobulin | MCP-2 |
| Betacellulin | MDC |
| BDNF | MIF |
| CA | MIP-1 α |
| Calbindin | MMP-2 |
| Cancer antigen 125 | Prolactin |
| CD5L | Prostatic acid phosphatase |
| Complement 3 | PYY |
| Cortisol | Serum amyloid P |
| CTGF | Sortilin |
| EGFR | Testosterone |
| Endothelin 1 | Thrombopoietin |
| Ferritin | TIMP 1 |
| Fetuin A | TNF R 2 |
| FSH | Trail R3 |
| Haptoglobin | Transferrin |
| ICAM1 | TSH |
| IgA | VEGF |
| IgM | Vitronectin |
| IL-6 receptor | |
| BDNF: brain-derived neurotrophic factor; CD5L: CD5 molecule-like; CTGF: connective tissue growth factor; EGFR: epidermal growth factor receptor; FSH: follicle-stimulating hormone; ICAM1: intercellular adhesion molecule 1; IgA: immunoglobulin A; IgM: immunoglobulin M; IL-6 receptor: interleukin 6 receptor; IL-7: interleukin 7; IL-10: interleukin 10; IL-11: interleukin 11; IL-17: interleukin 17; KIM-1: kidney injury molecule-1; LH: luteinizing hormone; MCP-2: monocyte chemotactic protein 2; MDC: mature dendritic cell; MIF: macrophage migration inhibitory factor; MIP-1 α: macrophage inflammatory protein 1 α; MMP-2: matrix metalloproteinase 2; PYY: peptide YY; TIMP 1: TIMP metallopeptidase inhibitor 1; TNF R 2: tumor necrosis factor receptor 2; TSH: thyroid-stimulating hormone; VEGF: vascular endothelial growth factor Source: Reference 3 | |
The lack of laboratory tests to validate the clinical diagnosis of schizophrenia is widely accepted and lamented by psychiatric practitioners. In a recent survey I conducted on CurrentPsychiatry.com, most respondents guessed there are 3 known biomarkers for schizophrenia and 4 for major depression.
The media’s view tends to be harsh, exploiting the ostensible absence of diagnostic biomarkers in psychiatry to cast unfair aspersions on the scientific validity of DSM-5 and its diagnostic guidelines.1 They seem to believe that lab tests for mental illness will never be feasible. Clearly, they have not done their homework.
Consider schizophrenia. It would come as a surprise to most people inside or outside the psychiatric community that 365 biomarkers for schizophrenia have been discovered, 273 of which are identifiable in plasma.2 Of these, 81 are diagnostic, 77 are markers of drug response, and 115 are for both. Some of these tests have been replicated at least 5 times (brain-derived neurotrophic factor, S100B, prolactin, interleukin (IL) 6, IL2, IN5, leptin, IL 1 receptor antagonist, IL8, and IL2 receptor α). The biologic functions of these 273 biomarkers include inflammatory disease or response, respiratory disease, cellular movement, lipid metabolism, molecular transport, immunologic disease, hematologic disease, renal and urologic disease, cell-to-cell signaling, cellular growth and proliferation, cardiovascular disease, genetic disorders, psychological disorders, metabolic disease, small molecule biochemistry, molecular transport, nutritional disease, endocrine system disorders, cell death, tissue morphology, organismal survival, lymphoid tissue structure and development, antigen presentation, tissue development, carbohydrate metabolism, organ morphology, embryonic development, behavior, and digestive system development and functions.2 Obviously, schizophrenia biomarkers overlap with multiple tissues and key biochemical and cellular processes in brain and body.
So why do none of these 273 blood tests appear in DSM-5, which had aspired to include objective methods in psychiatric diagnosis? The answer: heterogeneity. Schizophrenia and other major psychiatric illnesses are not 1 disorder but syndromes comprised of numerous clinically similar but biologically different disorders. There is extensive variability among the “schizophrenias” in genetic and nongenetic etiological factors and significant heterogeneity in neurobiology, treatment response, and clinical and functional outcomes. None of the individual 273 biomarkers alone can serve as a diagnostic tool for the schizophrenias because there will be high rates of false positives and false negatives. A lab test for a syndrome is impossible!
One company recently attempted to develop a blood test for schizophrenia. It used 51 biomarkers to comprise that test because none of them alone is a viable test (Table).3 The totality of the 51 biomarkers significantly increases the likelihood of diagnostic utility but still will be short of 100% specificity.
What is the point of identifying 273 blood tests if they have not been used to diagnose a heterogeneous syndrome? I believe there are many potentially useful applications for these biomarkers:
- To identify biologic subtypes of schizophrenia
- To shed light on the multiple pathophysiologies of schizophrenia, which may provide valuable clues for new treatments
- To help identify and characterize stages of schizophrenia. Some biomarkers have been found in the early stages, while others appear only in the chronic stages
- To help predict biologic predisposition to 1 of the schizophrenias. It is possible that the various susceptibility genes that have been identified in schizophrenia may be associated with certain biomarkers during fetal neurodevelopment, childhood, or the prodrome stage
- To explore the overlapping biologic features of psychotic disorders. For example, 21 biomarkers have been found to differentiate schizophrenia or bipolar disorder from healthy controls. Some biomarkers may point to the likelihood of psychiatric comorbidities such as depression or obsessive-compulsive disorder or medical comorbidities such as cardiovascular, immunologic, or gastrointestinal diseases
- Some biomarkers may identify state (ie, the psychotic phase only) vs trait (throughout life). Other biomarkers may be associated with the presence of a specific type of hallucination (auditory, visual, olfactory, or gustatory), delusion (bizarre vs simple), negative symptom (flat affect vs apathy vs avolition) or cognitive deficit (verbal memory vs learning deficit vs executive dysfunction)
- Biomarkers may assist in developing personalized medicine and designing customized evaluations and treatments for patients suffering from 1 of the many schizophrenias.
Lab tests for psychiatric disorders are indeed available but their use will not mirror traditional physical exam tests. The complex heterogeneity of most psychiatric syndromes means that biomarkers will help unravel the rich neurobiology of those disorders and help elucidate the multiple neurobiologic underpinnings of these syndromes. Psychiatrists should look forward with great optimism to a bright future for psychiatric diagnosis, combining a set of clinical signs and symptoms with a confirmatory cluster of lab tests. It may take time, but psychiatric clinicians will be using biomarkers in the future and the media and the public finally will perceive psychiatry as a “mature” medical discipline.
In the survey I mentioned at the beginning of this editorial, 60.5% of responders predicted that the DSM-6 (approximately a decade from now) will contain laboratory tests for psychiatric diagnosis. They may very well be right!
Table
Biomarkers for schizophrenia
| α-1 antitrypsin | IL-7 |
| Apolipoprotein A1 | IL-10 |
| Apolipoprotein A2 | IL-11 |
| Apolipoprotein B | IL-17 |
| Apolipoprotein CI | KIM-1 |
| Apolipoprotein H | LH |
| β-2 microglobulin | MCP-2 |
| Betacellulin | MDC |
| BDNF | MIF |
| CA | MIP-1 α |
| Calbindin | MMP-2 |
| Cancer antigen 125 | Prolactin |
| CD5L | Prostatic acid phosphatase |
| Complement 3 | PYY |
| Cortisol | Serum amyloid P |
| CTGF | Sortilin |
| EGFR | Testosterone |
| Endothelin 1 | Thrombopoietin |
| Ferritin | TIMP 1 |
| Fetuin A | TNF R 2 |
| FSH | Trail R3 |
| Haptoglobin | Transferrin |
| ICAM1 | TSH |
| IgA | VEGF |
| IgM | Vitronectin |
| IL-6 receptor | |
| BDNF: brain-derived neurotrophic factor; CD5L: CD5 molecule-like; CTGF: connective tissue growth factor; EGFR: epidermal growth factor receptor; FSH: follicle-stimulating hormone; ICAM1: intercellular adhesion molecule 1; IgA: immunoglobulin A; IgM: immunoglobulin M; IL-6 receptor: interleukin 6 receptor; IL-7: interleukin 7; IL-10: interleukin 10; IL-11: interleukin 11; IL-17: interleukin 17; KIM-1: kidney injury molecule-1; LH: luteinizing hormone; MCP-2: monocyte chemotactic protein 2; MDC: mature dendritic cell; MIF: macrophage migration inhibitory factor; MIP-1 α: macrophage inflammatory protein 1 α; MMP-2: matrix metalloproteinase 2; PYY: peptide YY; TIMP 1: TIMP metallopeptidase inhibitor 1; TNF R 2: tumor necrosis factor receptor 2; TSH: thyroid-stimulating hormone; VEGF: vascular endothelial growth factor Source: Reference 3 | |
1. Sepkowitz K. The DSM’s controversial update. Newsweek. December 10, 2012. http://www.thedailybeast.com/newsweek/2012/12/09/the-dsm-s-controversial-update.html. Accessed January 4, 2013.
2. Chan MK, Guest PC, Levin Y, et al. Converging evidence of blood-based biomarkers for schizophrenia: an update. Int Rev Neurobiol. 2011;101:95-144.
3. Schwarz E, Izmailov R, Spain M, et al. Validation of a blood-based laboratory test to aid in the confirmation of a diagnosis of schizophrenia. Biomark Insights. 2010;5:39-47.
1. Sepkowitz K. The DSM’s controversial update. Newsweek. December 10, 2012. http://www.thedailybeast.com/newsweek/2012/12/09/the-dsm-s-controversial-update.html. Accessed January 4, 2013.
2. Chan MK, Guest PC, Levin Y, et al. Converging evidence of blood-based biomarkers for schizophrenia: an update. Int Rev Neurobiol. 2011;101:95-144.
3. Schwarz E, Izmailov R, Spain M, et al. Validation of a blood-based laboratory test to aid in the confirmation of a diagnosis of schizophrenia. Biomark Insights. 2010;5:39-47.
Antidepressant use during pregnancy: How to avoid clinical and legal pitfalls
Discuss this article at www.facebook.com/CurrentPsychiatry
Recently there has been an increase in advertising soliciting participants for class-action lawsuits involving birth defects and antidepressants, particularly sertraline. Many psychiatrists are unsure why these ads are running in seemingly every medium because there has been no change in the FDA pregnancy classification for most selective serotonin reuptake inhibitors (SSRIs), except for paroxetine going from a C to a D rating in 2005.1 Some studies have found SSRIs increase the risk of adverse birth outcomes and others have not, which makes it difficult for clinicians to know what to discuss with patients regarding the risks and benefits of using antidepressants during pregnancy, as well as the risks of untreated major depressive disorder (MDD).
It can be hard to encourage some patients to take necessary medications in the best of circumstances, let alone suggest that a pregnant woman take a medication that has been labeled “dangerous.” This article seeks to alleviate physicians’ fears about being caught in a no-win situation by:
- explaining factors that may have led to this increase in class-action lawsuits
- clarifying the risks of using certain medications and not treating depression
- suggesting ways physicians can protect themselves and their patients.
The FDA’s position
In July 2006, the FDA issued a public health advisory regarding SSRI use during pregnancy and the possibility of persistent pulmonary hypertension (PPHN).2 This warning was based on a single study that found the risk of developing PPHN (baseline rate: 1 to 2 per 1,000 births) was 6 times greater for fetuses exposed to SSRIs in late pregnancy.3 Many legal websites highlight this 2006 warning as proof of SSRIs’ danger. However, because subsequent studies have had conflicting results, the FDA’s current position is that the risks of using SSRIs during pregnancy are “unknown” (Box).1-4
2006: In a warning about the risk of persistent pulmonary hypertension (PPHN) with antidepressant use during pregnancy, the FDA acknowledged “decisions about how to treat depression in pregnant women are increasingly complex.”2 The FDA issued the warning based on a study by Chambers et al,3 noting that the study was “too small” to look at individual medications.
This warning also cited a study by Cohen et al4 that found “women who stopped their [antidepressant] medicine were five times more likely to have a relapse of depression during their pregnancy than were the women who continued to take their antidepressant medicine while pregnant.”2 Although the warning identified a potential “rare” danger, the FDA guidance was that “women who are pregnant or thinking about becoming pregnant should not stop any antidepressant without first consulting their physician. The decision to continue medication or not should be made only after there has been careful consideration of the potential benefits and risks of the medication for each individual pregnant patient.”
2011: In this communication,1 the FDA stated “the initial Public Health Advisory in July 2006 on this potential risk was based on a single published study. Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of [selective serotonin reuptake inhibitors (SSRIs)] during pregnancy can cause PPHN.” The FDA also said that the “potential risk with SSRI use during pregnancy remains unknown.”
Risks of depression
Although most physicians know the risks of untreated MDD, they tend to minimize or forget these risks when a woman becomes pregnant. Pregnant women with MDD face not only the expected risks of their psychiatric illness but additionally face risks of pre-eclampsia, suicide (20% of deaths in the postpartum period are due to suicide), and infanticide.5-10 Risks to the fetus include poor prenatal care, increased risk of intrauterine exposure to drugs or alcohol, increased exposure to maternal cortisol with resulting neurodevelopmental changes, preterm delivery, low birth weight, and failure to thrive.6-8 Later difficulties for the child of a mother with untreated depression may include poor stress adaptation, decreased cognitive performance, and behavioral difficulties because of poor mother-child bonding and other factors.6
See Table 1 for key statistics regarding pregnancy and depression.
Table 1
Statistics on pregnancy and depression
| There are approximately 6 million pregnancies each year in the United Statesa |
| There are approximately 4 million live births each year in the United Statesa |
| Two percent to 3% of healthy pregnancies result in a birth defect or miscarriageb-d |
| Sixty percent to 70% of birth complications occur due to an unknown caused |
| Rates of depression during pregnancy are 7% to 25%b,e,f |
| Approximately 13% of pregnant women take an antidepressant during pregnancye |
| Fifteen percent of women with untreated depression in pregnancy attempt suicideb |
| Twenty percent of deaths in the postpartum period are due to suicidee |
| Women who discontinue antidepressants are 5 times more likely than women who continue medications in pregnancy to have a relapse of depressiong,h |
| SSRIs are the antidepressant class most frequently prescribed to pregnant womeni |
| Sertraline is one of the most frequently prescribed antidepressants perinatally and has low concentration in breast milk and infant serumj,k |
| SSRIs: selective serotonin reuptake inhibitors
|
Limitations of research
Because of ethical difficulties in studying MDD treatment during pregnancy, most data are retrospective and prone to detection and confounding biases, such as11-15:
- the risks associated with depression
- comorbid conditions such as obesity
- maternal age
- poor prenatal care
- how the baby was delivered (eg, Caesarean sections have higher rates of PPHN)13
- illicit substance use
- effects of other medications (80% of pregnant women use medications, including nonsteroidal anti-inflammatory drugs [NSAIDs], which are associated with PPHN).11,16
There are several potential adverse outcomes to consider when prescribing psychotropics to a pregnant woman, including miscarriage, malformation, preterm delivery, perinatal toxicity, and behavioral teratogenesis (Table 2).6,7 SSRIs have been implicated in adverse outcomes, but there is no strong evidence that they increase the miscarriage rate, and several studies found no increase in birth defects.6,13,18-20 Regarding teratogenesis, the FDA switched paroxetine from class C to class D because of a potential 1.5% to 2% risk of fetal cardiac malformation, compared with a 1% baseline rate in the general population.21 Drug toxicity or withdrawal in a neonate also is a risk; however, this condition is self-limited and managed supportively by neonatology.22 Behavioral teratogenesis—neurobehavioral problems that develop later in a child’s life—remains a hypothetical concern; research has been conflicting, and studies often used flawed methodology.
- these databases were not designed to answer these types of exposure questions (eg, limitations in data collected, such as other potential causes not recorded)
- they have many confounding biases (undocumented illicit substance use, possible minimization of smoking history, publication basis for positive findings, etc.)
- individuals who provided the data did not follow a standardized method (eg, variability among individual clinicians).
Not to case aspersions on this group’s work, it should be noted that this study had limitations, including that the researchers:
- did not take into account SSRI dosage
- did not measure depression severity or remittance
- were not able to fully account for potential exposures (eg, over-the-counter NSAIDs)
- were unable to confirm that patients took their medications because the variable measured was prescriptions filled
- did not interview participants about their medication use or symptoms.
In a more recent study,24 33 of 11,014 infants exposed to SSRIs after gestational week 20 developed PPHN (absolute risk: 3 per 1,000 births, compared with an incidence of 1.2 per 1,000 births in the general population), with an adjusted OR of 2.1 (95% CI 1.5 to 3.0). Although the authors warned that the results suggest a “class effect,” the rate of PPHN also was higher for mothers with a history of a psychiatric hospitalization within the last 10 years who were not taking medication (OR=1.3, 95% CI 1.0 to 1.6) and the OR for escitalopram (1.5, CI 0.2 to 10.5) was not statistically significant. This study did include a control group, but the 10-year window may have been too wide to represent a group with similar comorbid risks. Similar to the previously discussed study, mothers prescribed SSRIs were older, 1.7 times more likely to be smokers, and twice as likely to be prescribed NSAIDs. The study did not analyze the risk factors of smoking and body mass index because of an initial subset analysis (which was not reported) finding that these known risk factors for PPHN “did not confound the results.”24
Table 2
Potential concerns when treating pregnant women with psychotropics
| Miscarriage (spontaneous abortion) |
| Malformation (teratogenesis) |
| Preterm delivery |
| Perinatal syndrome (toxicity or withdrawal in neonate; usually self-limited and related to serotonin overstimulation or withdrawal; symptoms may include disrupted sleep irritability jitteriness or abnormal breathing) |
| Behavioral teratogenesis (later behavioral problems in child eg lower IQ developmental delays or autism) |
| Lactation compatibility or plans to bottle-feed |
| Source: References 6,7 |
The basis of class-action lawsuits
Interest in class-action lawsuits involving birth defects and antidepressants, particularly sertraline, appears to be increasing. Many websites advertising these lawsuits quote unnamed articles from reputable medical journals to support the claim that the medications are dangerous and cause a wide range of birth defects. Although some of the birth defects mentioned are specific, others (eg, “breathing problems” or “gastrointestinal side effects”) are so broad that any problem or complication could conceivably be attributed to the antidepressant. The degree of causation—if any at all—for many of these conditions has not been determined. A national advertising campaign looking for any problem may be occurring because the exact risks are “unknown.”1
The 2009 U.S. Supreme Court ruling in Wyeth v Levine25 allows individuals to sue manufacturers of branded medications in state and federal court for lack of proper labeling. However, the 2011 U.S. Supreme Court case of PLIVA, Inc. v Mensing26 prohibits state lawsuits against manufacturers of generic medications over labeling because by federal (superseding) law, generic manufacturers must use the same warnings as the branded medication. This may in part explain why many medications targeted in commercials and websites for class-action lawsuits are branded products, even though generics are available.
Protect your patient and yourself
An estimated 13% of pregnant women take antidepressants; SSRIs are the most commonly used antidepressant during and after pregnancy.9 Although not every depressed pregnant woman requires medication, those with moderate to severe depression often do. Rational medication decisions, informed consent, and good documentation are important when treating these women. Discuss the risks of untreated illness as well as the risks of medications to ensure that the patient understands that avoiding medication does not guarantee a safe pregnancy. Suggest psychotherapy and electroconvulsive therapy as options when appropriate. When possible, include the patient’s partner and family in the discussion to help improve compliance and potentially reduce strife.29 The psychiatrist or patient should discuss the medication plan with the patient’s obstetrician or family physician.
6,22
Many women become pregnant while being treated for depression. Approximately one-half of all pregnancies are unplanned, so women using antidepressants may unknowingly expose their fetus to medication.30 For this reason, it is important to discuss potential pregnancy and birth control concerns with all women of childbearing age before initiating pharmacotherapy.31 If an unintended pregnancy occurs, tell your patient to contact you before stopping any medications. Lawsuits also can occur because of wrongful death by suicide or infanticide because of lack of treatment; risk of untreated illness should not be treated lightly.
Related Resources
- Motherisk. www.motherisk.org.
- Organization of Teratology Information Specialists. www.otispregnancy.org.
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
- Escitalopram • Lexapro
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors appreciate suggestions on prior versions of the manuscript from Miriam Rosenthal, Jaina Amin, Sarah Nagle-Yang, Sonal Moratschek, J.P. Shand, and Scott R. Miller.
1. U.S. Food and Drug Administration. FDA drug safety communication: selective serotonin reuptake inhibitor (SSRI) antidepressant use during pregnancy and reports of a rare heart and lung condition in newborn babies. http://www.fda.gov/Drugs/DrugSafety/ucm283375.htm. Published December 14, 2011. Accessed December 20, 2012.
2. U.S. Food and Drug Administration. Public health advisory: treatment challenges of depression in pregnancy and the possibility of persistent pulmonary hypertension in newborns. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsand
Providers/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/
ucm124348.htm. Published July 19, 2006. Accessed December 20, 2012.
3. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579-587.
4. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
5. Muzik M, Hamilton S. Psychiatric illness during pregnancy. Current Psychiatry. 2012;11(2):23-32.
6. Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy. Current Psychiatry. 2006;5(4):31-40.
7. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
8. Friedman SH, Resnick PJ. Postpartum depression: an update. Women’s Health (Lond Engl). 2009;5(3):287-295.
9. Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
10. Friedman SH, Hall RCW. Treatment of mental illness in pregnancy and malpractice concerns. News Amer Acad Psychiatry Law. 2012;37(2):21-22.
11. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
12. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918-926.
13. Wilson KL, Zelig CM, Harvey JP. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol. 2011;28(1):19-24.
14. Silvani P, Camporesi A. Drug-induced pulmonary hypertension in newborns: a review. Curr Vasc Pharmacol. 2007;5(2):129-133.
15. Occhiogrosso M, Omran SS, Altemus M. Persistent pulmonary hypertension of the newborn and selective serotonin reuptake inhibitors: lessons from clinical and translational studies. Am J Psychiatry. 2012;169(2):134-140.
16. Delaney C, Cornfield D. Risk factors for persistent pulmonary hypertension of the newborn. Pulm Circ. 2012;2(1):15-20.
17. Centers for Disease Control and Prevention. Key findings: updated national birth prevalence estimates for selected birth defects in the United States 2004-2006. http://www.cdc.gov/ncbddd/features/birthdefects-keyfindings.html. Published September 28, 2010. Accessed December 20, 2012.
18. Einarson A, Choi J, Einarson TR, et al. Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. Can J Psychiatry. 2009;54(4):242-246.
19. Alwan S, Reefhuis J, Rasmussen SA, et al. National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684-2692.
20. Andrade SE, McPhillips H, Loren D. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009;18(3):246-252.
21. U.S. Food and Drug Administration. FDA advising of risk of birth defects with paxil. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108527.htm. Published December 8, 2005. Accessed December 20, 2012.
22. Koren G, Boucher N. Adverse effects in neonates exposed to SSRIs and SNRI in late gestation-Motherisk Update 2008. Can J Clin Pharmacol. 2009;16(1):e66-e67.
23. Pederson LH, Henriksen TB, Vestergaard M, et al. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339:b3569.-doi:10.1136/bmj.b3569.
24. Kieler H, Artama M, Engeland A, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ. 2012;344:d8012.-doi:10.1136/bmj.d801.
25. Wyeth v Levine, 555 US 555 (2009).
26. PLIVA, Inc. v Mensing, 588 F3d 603, 593 F3d 428 (2011).
27. Greenwood K. The mysteries of pregnancy: the role of law in solving the problem of unknown but knowable maternal–fetal medication risk. University of Cincinnati Law Review. 2011;79(1):267-322.
28. Lyam Kilker v SmithKline Beecham Corporation, Philadelphia Court of Common Pleas (2009).
29. Mulder E, Davis A, Gawley L, et al. Negative impact of non-evidence-based information received by women taking antidepressants during pregnancy from health care providers and others. J Obstet Gynaecol Can. 2012;34(1):66-71.
30. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30(1):24-29 46.
31. Altshuler L, Richards M, Yonkers K. Treating bipolar disorder during pregnancy. Current Psychiatry. 2003;2(7):14-26.
Discuss this article at www.facebook.com/CurrentPsychiatry
Recently there has been an increase in advertising soliciting participants for class-action lawsuits involving birth defects and antidepressants, particularly sertraline. Many psychiatrists are unsure why these ads are running in seemingly every medium because there has been no change in the FDA pregnancy classification for most selective serotonin reuptake inhibitors (SSRIs), except for paroxetine going from a C to a D rating in 2005.1 Some studies have found SSRIs increase the risk of adverse birth outcomes and others have not, which makes it difficult for clinicians to know what to discuss with patients regarding the risks and benefits of using antidepressants during pregnancy, as well as the risks of untreated major depressive disorder (MDD).
It can be hard to encourage some patients to take necessary medications in the best of circumstances, let alone suggest that a pregnant woman take a medication that has been labeled “dangerous.” This article seeks to alleviate physicians’ fears about being caught in a no-win situation by:
- explaining factors that may have led to this increase in class-action lawsuits
- clarifying the risks of using certain medications and not treating depression
- suggesting ways physicians can protect themselves and their patients.
The FDA’s position
In July 2006, the FDA issued a public health advisory regarding SSRI use during pregnancy and the possibility of persistent pulmonary hypertension (PPHN).2 This warning was based on a single study that found the risk of developing PPHN (baseline rate: 1 to 2 per 1,000 births) was 6 times greater for fetuses exposed to SSRIs in late pregnancy.3 Many legal websites highlight this 2006 warning as proof of SSRIs’ danger. However, because subsequent studies have had conflicting results, the FDA’s current position is that the risks of using SSRIs during pregnancy are “unknown” (Box).1-4
2006: In a warning about the risk of persistent pulmonary hypertension (PPHN) with antidepressant use during pregnancy, the FDA acknowledged “decisions about how to treat depression in pregnant women are increasingly complex.”2 The FDA issued the warning based on a study by Chambers et al,3 noting that the study was “too small” to look at individual medications.
This warning also cited a study by Cohen et al4 that found “women who stopped their [antidepressant] medicine were five times more likely to have a relapse of depression during their pregnancy than were the women who continued to take their antidepressant medicine while pregnant.”2 Although the warning identified a potential “rare” danger, the FDA guidance was that “women who are pregnant or thinking about becoming pregnant should not stop any antidepressant without first consulting their physician. The decision to continue medication or not should be made only after there has been careful consideration of the potential benefits and risks of the medication for each individual pregnant patient.”
2011: In this communication,1 the FDA stated “the initial Public Health Advisory in July 2006 on this potential risk was based on a single published study. Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of [selective serotonin reuptake inhibitors (SSRIs)] during pregnancy can cause PPHN.” The FDA also said that the “potential risk with SSRI use during pregnancy remains unknown.”
Risks of depression
Although most physicians know the risks of untreated MDD, they tend to minimize or forget these risks when a woman becomes pregnant. Pregnant women with MDD face not only the expected risks of their psychiatric illness but additionally face risks of pre-eclampsia, suicide (20% of deaths in the postpartum period are due to suicide), and infanticide.5-10 Risks to the fetus include poor prenatal care, increased risk of intrauterine exposure to drugs or alcohol, increased exposure to maternal cortisol with resulting neurodevelopmental changes, preterm delivery, low birth weight, and failure to thrive.6-8 Later difficulties for the child of a mother with untreated depression may include poor stress adaptation, decreased cognitive performance, and behavioral difficulties because of poor mother-child bonding and other factors.6
See Table 1 for key statistics regarding pregnancy and depression.
Table 1
Statistics on pregnancy and depression
| There are approximately 6 million pregnancies each year in the United Statesa |
| There are approximately 4 million live births each year in the United Statesa |
| Two percent to 3% of healthy pregnancies result in a birth defect or miscarriageb-d |
| Sixty percent to 70% of birth complications occur due to an unknown caused |
| Rates of depression during pregnancy are 7% to 25%b,e,f |
| Approximately 13% of pregnant women take an antidepressant during pregnancye |
| Fifteen percent of women with untreated depression in pregnancy attempt suicideb |
| Twenty percent of deaths in the postpartum period are due to suicidee |
| Women who discontinue antidepressants are 5 times more likely than women who continue medications in pregnancy to have a relapse of depressiong,h |
| SSRIs are the antidepressant class most frequently prescribed to pregnant womeni |
| Sertraline is one of the most frequently prescribed antidepressants perinatally and has low concentration in breast milk and infant serumj,k |
| SSRIs: selective serotonin reuptake inhibitors
|
Limitations of research
Because of ethical difficulties in studying MDD treatment during pregnancy, most data are retrospective and prone to detection and confounding biases, such as11-15:
- the risks associated with depression
- comorbid conditions such as obesity
- maternal age
- poor prenatal care
- how the baby was delivered (eg, Caesarean sections have higher rates of PPHN)13
- illicit substance use
- effects of other medications (80% of pregnant women use medications, including nonsteroidal anti-inflammatory drugs [NSAIDs], which are associated with PPHN).11,16
There are several potential adverse outcomes to consider when prescribing psychotropics to a pregnant woman, including miscarriage, malformation, preterm delivery, perinatal toxicity, and behavioral teratogenesis (Table 2).6,7 SSRIs have been implicated in adverse outcomes, but there is no strong evidence that they increase the miscarriage rate, and several studies found no increase in birth defects.6,13,18-20 Regarding teratogenesis, the FDA switched paroxetine from class C to class D because of a potential 1.5% to 2% risk of fetal cardiac malformation, compared with a 1% baseline rate in the general population.21 Drug toxicity or withdrawal in a neonate also is a risk; however, this condition is self-limited and managed supportively by neonatology.22 Behavioral teratogenesis—neurobehavioral problems that develop later in a child’s life—remains a hypothetical concern; research has been conflicting, and studies often used flawed methodology.
- these databases were not designed to answer these types of exposure questions (eg, limitations in data collected, such as other potential causes not recorded)
- they have many confounding biases (undocumented illicit substance use, possible minimization of smoking history, publication basis for positive findings, etc.)
- individuals who provided the data did not follow a standardized method (eg, variability among individual clinicians).
Not to case aspersions on this group’s work, it should be noted that this study had limitations, including that the researchers:
- did not take into account SSRI dosage
- did not measure depression severity or remittance
- were not able to fully account for potential exposures (eg, over-the-counter NSAIDs)
- were unable to confirm that patients took their medications because the variable measured was prescriptions filled
- did not interview participants about their medication use or symptoms.
In a more recent study,24 33 of 11,014 infants exposed to SSRIs after gestational week 20 developed PPHN (absolute risk: 3 per 1,000 births, compared with an incidence of 1.2 per 1,000 births in the general population), with an adjusted OR of 2.1 (95% CI 1.5 to 3.0). Although the authors warned that the results suggest a “class effect,” the rate of PPHN also was higher for mothers with a history of a psychiatric hospitalization within the last 10 years who were not taking medication (OR=1.3, 95% CI 1.0 to 1.6) and the OR for escitalopram (1.5, CI 0.2 to 10.5) was not statistically significant. This study did include a control group, but the 10-year window may have been too wide to represent a group with similar comorbid risks. Similar to the previously discussed study, mothers prescribed SSRIs were older, 1.7 times more likely to be smokers, and twice as likely to be prescribed NSAIDs. The study did not analyze the risk factors of smoking and body mass index because of an initial subset analysis (which was not reported) finding that these known risk factors for PPHN “did not confound the results.”24
Table 2
Potential concerns when treating pregnant women with psychotropics
| Miscarriage (spontaneous abortion) |
| Malformation (teratogenesis) |
| Preterm delivery |
| Perinatal syndrome (toxicity or withdrawal in neonate; usually self-limited and related to serotonin overstimulation or withdrawal; symptoms may include disrupted sleep irritability jitteriness or abnormal breathing) |
| Behavioral teratogenesis (later behavioral problems in child eg lower IQ developmental delays or autism) |
| Lactation compatibility or plans to bottle-feed |
| Source: References 6,7 |
The basis of class-action lawsuits
Interest in class-action lawsuits involving birth defects and antidepressants, particularly sertraline, appears to be increasing. Many websites advertising these lawsuits quote unnamed articles from reputable medical journals to support the claim that the medications are dangerous and cause a wide range of birth defects. Although some of the birth defects mentioned are specific, others (eg, “breathing problems” or “gastrointestinal side effects”) are so broad that any problem or complication could conceivably be attributed to the antidepressant. The degree of causation—if any at all—for many of these conditions has not been determined. A national advertising campaign looking for any problem may be occurring because the exact risks are “unknown.”1
The 2009 U.S. Supreme Court ruling in Wyeth v Levine25 allows individuals to sue manufacturers of branded medications in state and federal court for lack of proper labeling. However, the 2011 U.S. Supreme Court case of PLIVA, Inc. v Mensing26 prohibits state lawsuits against manufacturers of generic medications over labeling because by federal (superseding) law, generic manufacturers must use the same warnings as the branded medication. This may in part explain why many medications targeted in commercials and websites for class-action lawsuits are branded products, even though generics are available.
Protect your patient and yourself
An estimated 13% of pregnant women take antidepressants; SSRIs are the most commonly used antidepressant during and after pregnancy.9 Although not every depressed pregnant woman requires medication, those with moderate to severe depression often do. Rational medication decisions, informed consent, and good documentation are important when treating these women. Discuss the risks of untreated illness as well as the risks of medications to ensure that the patient understands that avoiding medication does not guarantee a safe pregnancy. Suggest psychotherapy and electroconvulsive therapy as options when appropriate. When possible, include the patient’s partner and family in the discussion to help improve compliance and potentially reduce strife.29 The psychiatrist or patient should discuss the medication plan with the patient’s obstetrician or family physician.
6,22
Many women become pregnant while being treated for depression. Approximately one-half of all pregnancies are unplanned, so women using antidepressants may unknowingly expose their fetus to medication.30 For this reason, it is important to discuss potential pregnancy and birth control concerns with all women of childbearing age before initiating pharmacotherapy.31 If an unintended pregnancy occurs, tell your patient to contact you before stopping any medications. Lawsuits also can occur because of wrongful death by suicide or infanticide because of lack of treatment; risk of untreated illness should not be treated lightly.
Related Resources
- Motherisk. www.motherisk.org.
- Organization of Teratology Information Specialists. www.otispregnancy.org.
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
- Escitalopram • Lexapro
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors appreciate suggestions on prior versions of the manuscript from Miriam Rosenthal, Jaina Amin, Sarah Nagle-Yang, Sonal Moratschek, J.P. Shand, and Scott R. Miller.
Discuss this article at www.facebook.com/CurrentPsychiatry
Recently there has been an increase in advertising soliciting participants for class-action lawsuits involving birth defects and antidepressants, particularly sertraline. Many psychiatrists are unsure why these ads are running in seemingly every medium because there has been no change in the FDA pregnancy classification for most selective serotonin reuptake inhibitors (SSRIs), except for paroxetine going from a C to a D rating in 2005.1 Some studies have found SSRIs increase the risk of adverse birth outcomes and others have not, which makes it difficult for clinicians to know what to discuss with patients regarding the risks and benefits of using antidepressants during pregnancy, as well as the risks of untreated major depressive disorder (MDD).
It can be hard to encourage some patients to take necessary medications in the best of circumstances, let alone suggest that a pregnant woman take a medication that has been labeled “dangerous.” This article seeks to alleviate physicians’ fears about being caught in a no-win situation by:
- explaining factors that may have led to this increase in class-action lawsuits
- clarifying the risks of using certain medications and not treating depression
- suggesting ways physicians can protect themselves and their patients.
The FDA’s position
In July 2006, the FDA issued a public health advisory regarding SSRI use during pregnancy and the possibility of persistent pulmonary hypertension (PPHN).2 This warning was based on a single study that found the risk of developing PPHN (baseline rate: 1 to 2 per 1,000 births) was 6 times greater for fetuses exposed to SSRIs in late pregnancy.3 Many legal websites highlight this 2006 warning as proof of SSRIs’ danger. However, because subsequent studies have had conflicting results, the FDA’s current position is that the risks of using SSRIs during pregnancy are “unknown” (Box).1-4
2006: In a warning about the risk of persistent pulmonary hypertension (PPHN) with antidepressant use during pregnancy, the FDA acknowledged “decisions about how to treat depression in pregnant women are increasingly complex.”2 The FDA issued the warning based on a study by Chambers et al,3 noting that the study was “too small” to look at individual medications.
This warning also cited a study by Cohen et al4 that found “women who stopped their [antidepressant] medicine were five times more likely to have a relapse of depression during their pregnancy than were the women who continued to take their antidepressant medicine while pregnant.”2 Although the warning identified a potential “rare” danger, the FDA guidance was that “women who are pregnant or thinking about becoming pregnant should not stop any antidepressant without first consulting their physician. The decision to continue medication or not should be made only after there has been careful consideration of the potential benefits and risks of the medication for each individual pregnant patient.”
2011: In this communication,1 the FDA stated “the initial Public Health Advisory in July 2006 on this potential risk was based on a single published study. Since then, there have been conflicting findings from new studies evaluating this potential risk, making it unclear whether use of [selective serotonin reuptake inhibitors (SSRIs)] during pregnancy can cause PPHN.” The FDA also said that the “potential risk with SSRI use during pregnancy remains unknown.”
Risks of depression
Although most physicians know the risks of untreated MDD, they tend to minimize or forget these risks when a woman becomes pregnant. Pregnant women with MDD face not only the expected risks of their psychiatric illness but additionally face risks of pre-eclampsia, suicide (20% of deaths in the postpartum period are due to suicide), and infanticide.5-10 Risks to the fetus include poor prenatal care, increased risk of intrauterine exposure to drugs or alcohol, increased exposure to maternal cortisol with resulting neurodevelopmental changes, preterm delivery, low birth weight, and failure to thrive.6-8 Later difficulties for the child of a mother with untreated depression may include poor stress adaptation, decreased cognitive performance, and behavioral difficulties because of poor mother-child bonding and other factors.6
See Table 1 for key statistics regarding pregnancy and depression.
Table 1
Statistics on pregnancy and depression
| There are approximately 6 million pregnancies each year in the United Statesa |
| There are approximately 4 million live births each year in the United Statesa |
| Two percent to 3% of healthy pregnancies result in a birth defect or miscarriageb-d |
| Sixty percent to 70% of birth complications occur due to an unknown caused |
| Rates of depression during pregnancy are 7% to 25%b,e,f |
| Approximately 13% of pregnant women take an antidepressant during pregnancye |
| Fifteen percent of women with untreated depression in pregnancy attempt suicideb |
| Twenty percent of deaths in the postpartum period are due to suicidee |
| Women who discontinue antidepressants are 5 times more likely than women who continue medications in pregnancy to have a relapse of depressiong,h |
| SSRIs are the antidepressant class most frequently prescribed to pregnant womeni |
| Sertraline is one of the most frequently prescribed antidepressants perinatally and has low concentration in breast milk and infant serumj,k |
| SSRIs: selective serotonin reuptake inhibitors
|
Limitations of research
Because of ethical difficulties in studying MDD treatment during pregnancy, most data are retrospective and prone to detection and confounding biases, such as11-15:
- the risks associated with depression
- comorbid conditions such as obesity
- maternal age
- poor prenatal care
- how the baby was delivered (eg, Caesarean sections have higher rates of PPHN)13
- illicit substance use
- effects of other medications (80% of pregnant women use medications, including nonsteroidal anti-inflammatory drugs [NSAIDs], which are associated with PPHN).11,16
There are several potential adverse outcomes to consider when prescribing psychotropics to a pregnant woman, including miscarriage, malformation, preterm delivery, perinatal toxicity, and behavioral teratogenesis (Table 2).6,7 SSRIs have been implicated in adverse outcomes, but there is no strong evidence that they increase the miscarriage rate, and several studies found no increase in birth defects.6,13,18-20 Regarding teratogenesis, the FDA switched paroxetine from class C to class D because of a potential 1.5% to 2% risk of fetal cardiac malformation, compared with a 1% baseline rate in the general population.21 Drug toxicity or withdrawal in a neonate also is a risk; however, this condition is self-limited and managed supportively by neonatology.22 Behavioral teratogenesis—neurobehavioral problems that develop later in a child’s life—remains a hypothetical concern; research has been conflicting, and studies often used flawed methodology.
- these databases were not designed to answer these types of exposure questions (eg, limitations in data collected, such as other potential causes not recorded)
- they have many confounding biases (undocumented illicit substance use, possible minimization of smoking history, publication basis for positive findings, etc.)
- individuals who provided the data did not follow a standardized method (eg, variability among individual clinicians).
Not to case aspersions on this group’s work, it should be noted that this study had limitations, including that the researchers:
- did not take into account SSRI dosage
- did not measure depression severity or remittance
- were not able to fully account for potential exposures (eg, over-the-counter NSAIDs)
- were unable to confirm that patients took their medications because the variable measured was prescriptions filled
- did not interview participants about their medication use or symptoms.
In a more recent study,24 33 of 11,014 infants exposed to SSRIs after gestational week 20 developed PPHN (absolute risk: 3 per 1,000 births, compared with an incidence of 1.2 per 1,000 births in the general population), with an adjusted OR of 2.1 (95% CI 1.5 to 3.0). Although the authors warned that the results suggest a “class effect,” the rate of PPHN also was higher for mothers with a history of a psychiatric hospitalization within the last 10 years who were not taking medication (OR=1.3, 95% CI 1.0 to 1.6) and the OR for escitalopram (1.5, CI 0.2 to 10.5) was not statistically significant. This study did include a control group, but the 10-year window may have been too wide to represent a group with similar comorbid risks. Similar to the previously discussed study, mothers prescribed SSRIs were older, 1.7 times more likely to be smokers, and twice as likely to be prescribed NSAIDs. The study did not analyze the risk factors of smoking and body mass index because of an initial subset analysis (which was not reported) finding that these known risk factors for PPHN “did not confound the results.”24
Table 2
Potential concerns when treating pregnant women with psychotropics
| Miscarriage (spontaneous abortion) |
| Malformation (teratogenesis) |
| Preterm delivery |
| Perinatal syndrome (toxicity or withdrawal in neonate; usually self-limited and related to serotonin overstimulation or withdrawal; symptoms may include disrupted sleep irritability jitteriness or abnormal breathing) |
| Behavioral teratogenesis (later behavioral problems in child eg lower IQ developmental delays or autism) |
| Lactation compatibility or plans to bottle-feed |
| Source: References 6,7 |
The basis of class-action lawsuits
Interest in class-action lawsuits involving birth defects and antidepressants, particularly sertraline, appears to be increasing. Many websites advertising these lawsuits quote unnamed articles from reputable medical journals to support the claim that the medications are dangerous and cause a wide range of birth defects. Although some of the birth defects mentioned are specific, others (eg, “breathing problems” or “gastrointestinal side effects”) are so broad that any problem or complication could conceivably be attributed to the antidepressant. The degree of causation—if any at all—for many of these conditions has not been determined. A national advertising campaign looking for any problem may be occurring because the exact risks are “unknown.”1
The 2009 U.S. Supreme Court ruling in Wyeth v Levine25 allows individuals to sue manufacturers of branded medications in state and federal court for lack of proper labeling. However, the 2011 U.S. Supreme Court case of PLIVA, Inc. v Mensing26 prohibits state lawsuits against manufacturers of generic medications over labeling because by federal (superseding) law, generic manufacturers must use the same warnings as the branded medication. This may in part explain why many medications targeted in commercials and websites for class-action lawsuits are branded products, even though generics are available.
Protect your patient and yourself
An estimated 13% of pregnant women take antidepressants; SSRIs are the most commonly used antidepressant during and after pregnancy.9 Although not every depressed pregnant woman requires medication, those with moderate to severe depression often do. Rational medication decisions, informed consent, and good documentation are important when treating these women. Discuss the risks of untreated illness as well as the risks of medications to ensure that the patient understands that avoiding medication does not guarantee a safe pregnancy. Suggest psychotherapy and electroconvulsive therapy as options when appropriate. When possible, include the patient’s partner and family in the discussion to help improve compliance and potentially reduce strife.29 The psychiatrist or patient should discuss the medication plan with the patient’s obstetrician or family physician.
6,22
Many women become pregnant while being treated for depression. Approximately one-half of all pregnancies are unplanned, so women using antidepressants may unknowingly expose their fetus to medication.30 For this reason, it is important to discuss potential pregnancy and birth control concerns with all women of childbearing age before initiating pharmacotherapy.31 If an unintended pregnancy occurs, tell your patient to contact you before stopping any medications. Lawsuits also can occur because of wrongful death by suicide or infanticide because of lack of treatment; risk of untreated illness should not be treated lightly.
Related Resources
- Motherisk. www.motherisk.org.
- Organization of Teratology Information Specialists. www.otispregnancy.org.
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org.
- Escitalopram • Lexapro
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors appreciate suggestions on prior versions of the manuscript from Miriam Rosenthal, Jaina Amin, Sarah Nagle-Yang, Sonal Moratschek, J.P. Shand, and Scott R. Miller.
1. U.S. Food and Drug Administration. FDA drug safety communication: selective serotonin reuptake inhibitor (SSRI) antidepressant use during pregnancy and reports of a rare heart and lung condition in newborn babies. http://www.fda.gov/Drugs/DrugSafety/ucm283375.htm. Published December 14, 2011. Accessed December 20, 2012.
2. U.S. Food and Drug Administration. Public health advisory: treatment challenges of depression in pregnancy and the possibility of persistent pulmonary hypertension in newborns. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsand
Providers/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/
ucm124348.htm. Published July 19, 2006. Accessed December 20, 2012.
3. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579-587.
4. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
5. Muzik M, Hamilton S. Psychiatric illness during pregnancy. Current Psychiatry. 2012;11(2):23-32.
6. Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy. Current Psychiatry. 2006;5(4):31-40.
7. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
8. Friedman SH, Resnick PJ. Postpartum depression: an update. Women’s Health (Lond Engl). 2009;5(3):287-295.
9. Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
10. Friedman SH, Hall RCW. Treatment of mental illness in pregnancy and malpractice concerns. News Amer Acad Psychiatry Law. 2012;37(2):21-22.
11. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
12. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918-926.
13. Wilson KL, Zelig CM, Harvey JP. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol. 2011;28(1):19-24.
14. Silvani P, Camporesi A. Drug-induced pulmonary hypertension in newborns: a review. Curr Vasc Pharmacol. 2007;5(2):129-133.
15. Occhiogrosso M, Omran SS, Altemus M. Persistent pulmonary hypertension of the newborn and selective serotonin reuptake inhibitors: lessons from clinical and translational studies. Am J Psychiatry. 2012;169(2):134-140.
16. Delaney C, Cornfield D. Risk factors for persistent pulmonary hypertension of the newborn. Pulm Circ. 2012;2(1):15-20.
17. Centers for Disease Control and Prevention. Key findings: updated national birth prevalence estimates for selected birth defects in the United States 2004-2006. http://www.cdc.gov/ncbddd/features/birthdefects-keyfindings.html. Published September 28, 2010. Accessed December 20, 2012.
18. Einarson A, Choi J, Einarson TR, et al. Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. Can J Psychiatry. 2009;54(4):242-246.
19. Alwan S, Reefhuis J, Rasmussen SA, et al. National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684-2692.
20. Andrade SE, McPhillips H, Loren D. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009;18(3):246-252.
21. U.S. Food and Drug Administration. FDA advising of risk of birth defects with paxil. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108527.htm. Published December 8, 2005. Accessed December 20, 2012.
22. Koren G, Boucher N. Adverse effects in neonates exposed to SSRIs and SNRI in late gestation-Motherisk Update 2008. Can J Clin Pharmacol. 2009;16(1):e66-e67.
23. Pederson LH, Henriksen TB, Vestergaard M, et al. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339:b3569.-doi:10.1136/bmj.b3569.
24. Kieler H, Artama M, Engeland A, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ. 2012;344:d8012.-doi:10.1136/bmj.d801.
25. Wyeth v Levine, 555 US 555 (2009).
26. PLIVA, Inc. v Mensing, 588 F3d 603, 593 F3d 428 (2011).
27. Greenwood K. The mysteries of pregnancy: the role of law in solving the problem of unknown but knowable maternal–fetal medication risk. University of Cincinnati Law Review. 2011;79(1):267-322.
28. Lyam Kilker v SmithKline Beecham Corporation, Philadelphia Court of Common Pleas (2009).
29. Mulder E, Davis A, Gawley L, et al. Negative impact of non-evidence-based information received by women taking antidepressants during pregnancy from health care providers and others. J Obstet Gynaecol Can. 2012;34(1):66-71.
30. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30(1):24-29 46.
31. Altshuler L, Richards M, Yonkers K. Treating bipolar disorder during pregnancy. Current Psychiatry. 2003;2(7):14-26.
1. U.S. Food and Drug Administration. FDA drug safety communication: selective serotonin reuptake inhibitor (SSRI) antidepressant use during pregnancy and reports of a rare heart and lung condition in newborn babies. http://www.fda.gov/Drugs/DrugSafety/ucm283375.htm. Published December 14, 2011. Accessed December 20, 2012.
2. U.S. Food and Drug Administration. Public health advisory: treatment challenges of depression in pregnancy and the possibility of persistent pulmonary hypertension in newborns. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsand
Providers/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/
ucm124348.htm. Published July 19, 2006. Accessed December 20, 2012.
3. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579-587.
4. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
5. Muzik M, Hamilton S. Psychiatric illness during pregnancy. Current Psychiatry. 2012;11(2):23-32.
6. Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy. Current Psychiatry. 2006;5(4):31-40.
7. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
8. Friedman SH, Resnick PJ. Postpartum depression: an update. Women’s Health (Lond Engl). 2009;5(3):287-295.
9. Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
10. Friedman SH, Hall RCW. Treatment of mental illness in pregnancy and malpractice concerns. News Amer Acad Psychiatry Law. 2012;37(2):21-22.
11. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
12. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918-926.
13. Wilson KL, Zelig CM, Harvey JP. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol. 2011;28(1):19-24.
14. Silvani P, Camporesi A. Drug-induced pulmonary hypertension in newborns: a review. Curr Vasc Pharmacol. 2007;5(2):129-133.
15. Occhiogrosso M, Omran SS, Altemus M. Persistent pulmonary hypertension of the newborn and selective serotonin reuptake inhibitors: lessons from clinical and translational studies. Am J Psychiatry. 2012;169(2):134-140.
16. Delaney C, Cornfield D. Risk factors for persistent pulmonary hypertension of the newborn. Pulm Circ. 2012;2(1):15-20.
17. Centers for Disease Control and Prevention. Key findings: updated national birth prevalence estimates for selected birth defects in the United States 2004-2006. http://www.cdc.gov/ncbddd/features/birthdefects-keyfindings.html. Published September 28, 2010. Accessed December 20, 2012.
18. Einarson A, Choi J, Einarson TR, et al. Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. Can J Psychiatry. 2009;54(4):242-246.
19. Alwan S, Reefhuis J, Rasmussen SA, et al. National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684-2692.
20. Andrade SE, McPhillips H, Loren D. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009;18(3):246-252.
21. U.S. Food and Drug Administration. FDA advising of risk of birth defects with paxil. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108527.htm. Published December 8, 2005. Accessed December 20, 2012.
22. Koren G, Boucher N. Adverse effects in neonates exposed to SSRIs and SNRI in late gestation-Motherisk Update 2008. Can J Clin Pharmacol. 2009;16(1):e66-e67.
23. Pederson LH, Henriksen TB, Vestergaard M, et al. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339:b3569.-doi:10.1136/bmj.b3569.
24. Kieler H, Artama M, Engeland A, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ. 2012;344:d8012.-doi:10.1136/bmj.d801.
25. Wyeth v Levine, 555 US 555 (2009).
26. PLIVA, Inc. v Mensing, 588 F3d 603, 593 F3d 428 (2011).
27. Greenwood K. The mysteries of pregnancy: the role of law in solving the problem of unknown but knowable maternal–fetal medication risk. University of Cincinnati Law Review. 2011;79(1):267-322.
28. Lyam Kilker v SmithKline Beecham Corporation, Philadelphia Court of Common Pleas (2009).
29. Mulder E, Davis A, Gawley L, et al. Negative impact of non-evidence-based information received by women taking antidepressants during pregnancy from health care providers and others. J Obstet Gynaecol Can. 2012;34(1):66-71.
30. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30(1):24-29 46.
31. Altshuler L, Richards M, Yonkers K. Treating bipolar disorder during pregnancy. Current Psychiatry. 2003;2(7):14-26.
Psychiatry and jail
Like Dr. Nasrallah wrote in his October editorial (“Psychiatry and the politics of incarceration,” Current Psychiatry, October 2012, p. 4-5; http://bit.ly/1JWYa87), I, too, was distressed to learn of Dorothea Dix Hospital’s closing.
I am employed at a large county jail and see the deplorable state of our mental health system. There are only 401 psychiatric beds in Nevada; at the Clark County Detention Center we have 500 inmates taking psychotropics. A Nevada resident with a mental illness is 10 times more likely to be incarcerated than cared for in a hospital. I found the funding and treatment of mentally ill individuals in Moldova (formerly part of the Soviet Union) to be superior to ours.
Too often mentally ill persons are brought to jail for minor charges, such as trespassing or jaywalking. They may have more charges added such as assaulting another inmate or an officer. As a result of their mental illness, not taking medications, and poor judgment, they have problems adjusting to incarceration. This vicious cycle means they will be in jail longer and may even receive a prison sentence. If you have never seen the heartbreaking effects on a seriously mentally ill (SMI) or mentally retarded person placed in a jail cell, this scenario may be hard to understand.
It saddens me that the only difference in the treatment of the mentally ill today from Dorothea Dix’s time is that we are not charging admission fees to see “crazy people” in jail. Why the entire country continues to cut mental health budgets is a mystery. It should be no surprise that with the decrease in psychiatric beds there is an increase in SMI individuals in jails. I do not understand why the politics of incarceration cannot be turned around to where we are treating, not punishing, the mentally ill.
It’s quite easy to write about the injustice; the difficult and the right action is to make the needed change happen.
Virginia Singer, DNP, PMHNP, BCClark County Detention CenterLas Vegas, NV
Like Dr. Nasrallah wrote in his October editorial (“Psychiatry and the politics of incarceration,” Current Psychiatry, October 2012, p. 4-5; http://bit.ly/1JWYa87), I, too, was distressed to learn of Dorothea Dix Hospital’s closing.
I am employed at a large county jail and see the deplorable state of our mental health system. There are only 401 psychiatric beds in Nevada; at the Clark County Detention Center we have 500 inmates taking psychotropics. A Nevada resident with a mental illness is 10 times more likely to be incarcerated than cared for in a hospital. I found the funding and treatment of mentally ill individuals in Moldova (formerly part of the Soviet Union) to be superior to ours.
Too often mentally ill persons are brought to jail for minor charges, such as trespassing or jaywalking. They may have more charges added such as assaulting another inmate or an officer. As a result of their mental illness, not taking medications, and poor judgment, they have problems adjusting to incarceration. This vicious cycle means they will be in jail longer and may even receive a prison sentence. If you have never seen the heartbreaking effects on a seriously mentally ill (SMI) or mentally retarded person placed in a jail cell, this scenario may be hard to understand.
It saddens me that the only difference in the treatment of the mentally ill today from Dorothea Dix’s time is that we are not charging admission fees to see “crazy people” in jail. Why the entire country continues to cut mental health budgets is a mystery. It should be no surprise that with the decrease in psychiatric beds there is an increase in SMI individuals in jails. I do not understand why the politics of incarceration cannot be turned around to where we are treating, not punishing, the mentally ill.
It’s quite easy to write about the injustice; the difficult and the right action is to make the needed change happen.
Virginia Singer, DNP, PMHNP, BCClark County Detention CenterLas Vegas, NV
Like Dr. Nasrallah wrote in his October editorial (“Psychiatry and the politics of incarceration,” Current Psychiatry, October 2012, p. 4-5; http://bit.ly/1JWYa87), I, too, was distressed to learn of Dorothea Dix Hospital’s closing.
I am employed at a large county jail and see the deplorable state of our mental health system. There are only 401 psychiatric beds in Nevada; at the Clark County Detention Center we have 500 inmates taking psychotropics. A Nevada resident with a mental illness is 10 times more likely to be incarcerated than cared for in a hospital. I found the funding and treatment of mentally ill individuals in Moldova (formerly part of the Soviet Union) to be superior to ours.
Too often mentally ill persons are brought to jail for minor charges, such as trespassing or jaywalking. They may have more charges added such as assaulting another inmate or an officer. As a result of their mental illness, not taking medications, and poor judgment, they have problems adjusting to incarceration. This vicious cycle means they will be in jail longer and may even receive a prison sentence. If you have never seen the heartbreaking effects on a seriously mentally ill (SMI) or mentally retarded person placed in a jail cell, this scenario may be hard to understand.
It saddens me that the only difference in the treatment of the mentally ill today from Dorothea Dix’s time is that we are not charging admission fees to see “crazy people” in jail. Why the entire country continues to cut mental health budgets is a mystery. It should be no surprise that with the decrease in psychiatric beds there is an increase in SMI individuals in jails. I do not understand why the politics of incarceration cannot be turned around to where we are treating, not punishing, the mentally ill.
It’s quite easy to write about the injustice; the difficult and the right action is to make the needed change happen.
Virginia Singer, DNP, PMHNP, BCClark County Detention CenterLas Vegas, NV
Misleading artwork?
I was surprised by the article, “How to collaborate effectively with psychiatric nurse practitioners” (Current Psychiatry, November 2012, p. 49-53; http://bit.ly/1QTTtkK). Although I found the article informative and appreciated the accurate description of psychiatric nurse practitioners’ education and scope of practice, I was confused by the article’s artwork that displayed a dominant male—presumably the physician—pointing a pen toward a somewhat cowering female. Because a picture is worth a thousand words, I’m hopeful this was not seen as a reasonable way to “collaborate” with psychiatric-mental health nurse practitioners.
Trish Canning, PMHNP-BCBrookings PsychiatryGold Beach, OR
I was surprised by the article, “How to collaborate effectively with psychiatric nurse practitioners” (Current Psychiatry, November 2012, p. 49-53; http://bit.ly/1QTTtkK). Although I found the article informative and appreciated the accurate description of psychiatric nurse practitioners’ education and scope of practice, I was confused by the article’s artwork that displayed a dominant male—presumably the physician—pointing a pen toward a somewhat cowering female. Because a picture is worth a thousand words, I’m hopeful this was not seen as a reasonable way to “collaborate” with psychiatric-mental health nurse practitioners.
Trish Canning, PMHNP-BCBrookings PsychiatryGold Beach, OR
I was surprised by the article, “How to collaborate effectively with psychiatric nurse practitioners” (Current Psychiatry, November 2012, p. 49-53; http://bit.ly/1QTTtkK). Although I found the article informative and appreciated the accurate description of psychiatric nurse practitioners’ education and scope of practice, I was confused by the article’s artwork that displayed a dominant male—presumably the physician—pointing a pen toward a somewhat cowering female. Because a picture is worth a thousand words, I’m hopeful this was not seen as a reasonable way to “collaborate” with psychiatric-mental health nurse practitioners.
Trish Canning, PMHNP-BCBrookings PsychiatryGold Beach, OR
Working with other disciplines
The overdose epidemic
VISUALS: Determining the cause of hallucinations in children and adolescents
Discuss this article at www.facebook.com/CurrentPsychiatry
Visual hallucinations in children and adolescents can be caused by many conditions other than psychosis. To prevent misdiagnosis and unnecessary antipsychotic use, it is important to rule out other causes of visual hallucinations. The mnemonic VISUALS reminds us of common causes.
Visions that are culturally sanctioned occur in non-Western societies—eg, images of fairy-like spirits are accepted and reinforced as part of the Filipino culture—and in several Christian denominations in the United States. Positive cultural connotations may increase the frequency of visual hallucinations as well as produce varied attitudes and emotional reactions to them.1
Imaginary friends often fulfill a child’s need for a relationship, although even social children may have these “friends.” Children often refer to imaginary friends in conversations and play with them. Usually they are also children. They may be extensions of people the child admires or be named after characters from stories, movies, or television. Children rarely are able to explain the imaginary friend’s appearance and more than half the time there is no trigger for the appearance of such friends.2,3
Stress and anxiety in preschool children may precipitate the onset of visual or tactile hallucinations. They often happen at night but also can occur when the child is awake. Typical visual hallucinations may include monsters, bugs, pets, or toys.2
Urine drug screens should be conducted for all adolescents and children. Cocaine, methamphetamine, and amphetamines—including high doses of prescribed stimulants—can cause visual hallucinations. Lysergic acid diethylamide (“LSD”), phencyclidine (“PCP”), 3,4-methylenedioxymethamphetamine (“ecstasy”), marijuana, nitrous oxide, and mescaline often cause visual hallucinations, although these substances may not be identified in a routine urine toxicology. Other considerations are withdrawal from benzodiazepines, sedative-hypnotics, or alcohol, and rare adverse reactions to antidepressants, antibiotics, or anticonvulsants.4,5
Age and developmental immaturity may make it difficult for children to distinguish between reality and non-reality, including dreams and shadows in the dark. Underdeveloped communication may make it difficult to interpret what the child is trying to communicate.2
Look into other medical explanations, such as migraines, seizures, tumors, ophthalmologic disease, delirium, or metabolic disorders (Table).4,5
Table
Medical causes of visual hallucinations in children and adolescents
| Medical condition | Symptoms |
|---|---|
| Neurologic | Migraine with aura; migraine coma; familial hemiplegic migraines; temporal or occipital lobe seizures; ictal, postictal, or interictal psychosis; tumors in occipital or temporal lobes |
| Ophthalmologic | Cataracts, retinal diseases, glaucoma |
| Inborn errors of metabolism | Homocysteine remethylation defects; urea cycle disorders; GM2 gangliosidoses; Niemann-Pick disease, type C; alpha-mannosidosis |
| Delirium | Metabolic disturbance, infection, intracranial process |
| Metabolic encephalopathy | Cardiopulmonary insufficiency, uremia, hepatic disease, vitamin deficiencies, inflammatory disease |
| Source:References 4,5 | |
Sleep-onset visual hallucinations (hypnagogic) and hallucinations upon awakening (hypnopompic) often are bizarre and dream-like. They may consist of geometric patterns, landscapes, faces, or figures. They mainly occur with narcolepsy but can be seen in insomnia or excessive daytime sleepiness.4,5
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. al-Issa I. The illusion of reality or the reality of illusion. Hallucinations and culture. Br J Psychiatry. 1995;166(3):368-373.
2. Tsai LY, Champine DJ. Schizophrenia and other psychotic disorders. In: Wiener JM Dulcan MK, eds. Textbook of child and adolescent psychiatry. 3rd ed. Washington, DC: American Psychiatric Publishing, Inc.; 2004:379–409.
3. Gleason TR, Sebanc AM, Hartup WW. Imaginary companions of preschool children. Dev Psychol. 2000;36(4):419-428.
4. Cummings JL, Miller BL. Visual hallucinations. Clinical occurrence and use in differential diagnosis. West J Med. 1987;146(1):46-51.
5. Teeple RC, Caplan JP, Stern TA. Visual hallucinations: differential diagnosis and treatment. Prim Care Companion J Clin Psychiatry. 2009;11(1):26-32.
Discuss this article at www.facebook.com/CurrentPsychiatry
Visual hallucinations in children and adolescents can be caused by many conditions other than psychosis. To prevent misdiagnosis and unnecessary antipsychotic use, it is important to rule out other causes of visual hallucinations. The mnemonic VISUALS reminds us of common causes.
Visions that are culturally sanctioned occur in non-Western societies—eg, images of fairy-like spirits are accepted and reinforced as part of the Filipino culture—and in several Christian denominations in the United States. Positive cultural connotations may increase the frequency of visual hallucinations as well as produce varied attitudes and emotional reactions to them.1
Imaginary friends often fulfill a child’s need for a relationship, although even social children may have these “friends.” Children often refer to imaginary friends in conversations and play with them. Usually they are also children. They may be extensions of people the child admires or be named after characters from stories, movies, or television. Children rarely are able to explain the imaginary friend’s appearance and more than half the time there is no trigger for the appearance of such friends.2,3
Stress and anxiety in preschool children may precipitate the onset of visual or tactile hallucinations. They often happen at night but also can occur when the child is awake. Typical visual hallucinations may include monsters, bugs, pets, or toys.2
Urine drug screens should be conducted for all adolescents and children. Cocaine, methamphetamine, and amphetamines—including high doses of prescribed stimulants—can cause visual hallucinations. Lysergic acid diethylamide (“LSD”), phencyclidine (“PCP”), 3,4-methylenedioxymethamphetamine (“ecstasy”), marijuana, nitrous oxide, and mescaline often cause visual hallucinations, although these substances may not be identified in a routine urine toxicology. Other considerations are withdrawal from benzodiazepines, sedative-hypnotics, or alcohol, and rare adverse reactions to antidepressants, antibiotics, or anticonvulsants.4,5
Age and developmental immaturity may make it difficult for children to distinguish between reality and non-reality, including dreams and shadows in the dark. Underdeveloped communication may make it difficult to interpret what the child is trying to communicate.2
Look into other medical explanations, such as migraines, seizures, tumors, ophthalmologic disease, delirium, or metabolic disorders (Table).4,5
Table
Medical causes of visual hallucinations in children and adolescents
| Medical condition | Symptoms |
|---|---|
| Neurologic | Migraine with aura; migraine coma; familial hemiplegic migraines; temporal or occipital lobe seizures; ictal, postictal, or interictal psychosis; tumors in occipital or temporal lobes |
| Ophthalmologic | Cataracts, retinal diseases, glaucoma |
| Inborn errors of metabolism | Homocysteine remethylation defects; urea cycle disorders; GM2 gangliosidoses; Niemann-Pick disease, type C; alpha-mannosidosis |
| Delirium | Metabolic disturbance, infection, intracranial process |
| Metabolic encephalopathy | Cardiopulmonary insufficiency, uremia, hepatic disease, vitamin deficiencies, inflammatory disease |
| Source:References 4,5 | |
Sleep-onset visual hallucinations (hypnagogic) and hallucinations upon awakening (hypnopompic) often are bizarre and dream-like. They may consist of geometric patterns, landscapes, faces, or figures. They mainly occur with narcolepsy but can be seen in insomnia or excessive daytime sleepiness.4,5
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Visual hallucinations in children and adolescents can be caused by many conditions other than psychosis. To prevent misdiagnosis and unnecessary antipsychotic use, it is important to rule out other causes of visual hallucinations. The mnemonic VISUALS reminds us of common causes.
Visions that are culturally sanctioned occur in non-Western societies—eg, images of fairy-like spirits are accepted and reinforced as part of the Filipino culture—and in several Christian denominations in the United States. Positive cultural connotations may increase the frequency of visual hallucinations as well as produce varied attitudes and emotional reactions to them.1
Imaginary friends often fulfill a child’s need for a relationship, although even social children may have these “friends.” Children often refer to imaginary friends in conversations and play with them. Usually they are also children. They may be extensions of people the child admires or be named after characters from stories, movies, or television. Children rarely are able to explain the imaginary friend’s appearance and more than half the time there is no trigger for the appearance of such friends.2,3
Stress and anxiety in preschool children may precipitate the onset of visual or tactile hallucinations. They often happen at night but also can occur when the child is awake. Typical visual hallucinations may include monsters, bugs, pets, or toys.2
Urine drug screens should be conducted for all adolescents and children. Cocaine, methamphetamine, and amphetamines—including high doses of prescribed stimulants—can cause visual hallucinations. Lysergic acid diethylamide (“LSD”), phencyclidine (“PCP”), 3,4-methylenedioxymethamphetamine (“ecstasy”), marijuana, nitrous oxide, and mescaline often cause visual hallucinations, although these substances may not be identified in a routine urine toxicology. Other considerations are withdrawal from benzodiazepines, sedative-hypnotics, or alcohol, and rare adverse reactions to antidepressants, antibiotics, or anticonvulsants.4,5
Age and developmental immaturity may make it difficult for children to distinguish between reality and non-reality, including dreams and shadows in the dark. Underdeveloped communication may make it difficult to interpret what the child is trying to communicate.2
Look into other medical explanations, such as migraines, seizures, tumors, ophthalmologic disease, delirium, or metabolic disorders (Table).4,5
Table
Medical causes of visual hallucinations in children and adolescents
| Medical condition | Symptoms |
|---|---|
| Neurologic | Migraine with aura; migraine coma; familial hemiplegic migraines; temporal or occipital lobe seizures; ictal, postictal, or interictal psychosis; tumors in occipital or temporal lobes |
| Ophthalmologic | Cataracts, retinal diseases, glaucoma |
| Inborn errors of metabolism | Homocysteine remethylation defects; urea cycle disorders; GM2 gangliosidoses; Niemann-Pick disease, type C; alpha-mannosidosis |
| Delirium | Metabolic disturbance, infection, intracranial process |
| Metabolic encephalopathy | Cardiopulmonary insufficiency, uremia, hepatic disease, vitamin deficiencies, inflammatory disease |
| Source:References 4,5 | |
Sleep-onset visual hallucinations (hypnagogic) and hallucinations upon awakening (hypnopompic) often are bizarre and dream-like. They may consist of geometric patterns, landscapes, faces, or figures. They mainly occur with narcolepsy but can be seen in insomnia or excessive daytime sleepiness.4,5
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. al-Issa I. The illusion of reality or the reality of illusion. Hallucinations and culture. Br J Psychiatry. 1995;166(3):368-373.
2. Tsai LY, Champine DJ. Schizophrenia and other psychotic disorders. In: Wiener JM Dulcan MK, eds. Textbook of child and adolescent psychiatry. 3rd ed. Washington, DC: American Psychiatric Publishing, Inc.; 2004:379–409.
3. Gleason TR, Sebanc AM, Hartup WW. Imaginary companions of preschool children. Dev Psychol. 2000;36(4):419-428.
4. Cummings JL, Miller BL. Visual hallucinations. Clinical occurrence and use in differential diagnosis. West J Med. 1987;146(1):46-51.
5. Teeple RC, Caplan JP, Stern TA. Visual hallucinations: differential diagnosis and treatment. Prim Care Companion J Clin Psychiatry. 2009;11(1):26-32.
1. al-Issa I. The illusion of reality or the reality of illusion. Hallucinations and culture. Br J Psychiatry. 1995;166(3):368-373.
2. Tsai LY, Champine DJ. Schizophrenia and other psychotic disorders. In: Wiener JM Dulcan MK, eds. Textbook of child and adolescent psychiatry. 3rd ed. Washington, DC: American Psychiatric Publishing, Inc.; 2004:379–409.
3. Gleason TR, Sebanc AM, Hartup WW. Imaginary companions of preschool children. Dev Psychol. 2000;36(4):419-428.
4. Cummings JL, Miller BL. Visual hallucinations. Clinical occurrence and use in differential diagnosis. West J Med. 1987;146(1):46-51.
5. Teeple RC, Caplan JP, Stern TA. Visual hallucinations: differential diagnosis and treatment. Prim Care Companion J Clin Psychiatry. 2009;11(1):26-32.
Pharmacotherapy for comorbid depression and alcohol dependence
Discuss this article at www.facebook.com/CurrentPsychiatry
With a lifetime prevalence of 30%, alcohol use disorders (AUDs)—which in DSM-IV-TR include alcohol abuse and alcohol dependence—are among the most common psychiatric disorders.1 Depressive disorders, including major depressive disorder (MDD) and dysthymia, frequently co-occur with AUDs.2-4 This pattern of comorbidity adversely affects the prognosis, course, and treatment of both MDD and AUDs.5 High severity in 1 of these disorders is associated with high severity in the other.2,6 Alcohol dependence appears to prolong the course of depression7,8 and increases the risk of suicidal symptoms and behaviors.9,10 Patients with depression and AUDs are at increased risk of relapse to heavy drinking.7,11
Whether the high comorbidity rate of depressive disorders and AUDs is a result of 1 disorder causing the other (ie, AUDs leading to depression or vice versa) or can be attributed to shared etiology is unknown. Clinicians need to consider this question when treating patients with this pattern of comorbidity because distinguishing primary depression from secondary depression influences treatment decisions.12
There is a great need for pharmacologic interventions that can concurrently treat both depression and AUDs. This article reviews the evidence for current treatments for dually diagnosed patients and highlights novel agents that are worthy of further study for this complex patient population.
Current treatment options
Pharmacotherapy for MDD and alcohol dependence is common when these conditions occur alone. FDA-approved medications for treating depression include monoamine oxidase inhibitors, tricyclic antidepressants (TCAs), tetracyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors.
SSRIs are the most widely used class of antidepressants. They gained FDA approval based on studies conducted in non-comorbid patients because patients with comorbid conditions usually are excluded from research studies.13 Few trials have evaluated patients with depression and AUDs; TCAs and SSRIs are best studied in these patients.
Serotonergic antidepressants
SSRIs are first-line medications for MDD because of their low abuse potential, favorable side effect profile, and relative safety in overdose.
Table 1
Serotonergic antidepressants for patients with AUDs and depression
| Study | Sample | Results |
|---|---|---|
| Cornelius et al, 199714 | Outpatients with severe major depression and AD 1. Fluoxetine (20 to 40 mg/d; n = 25) 2. Placebo (n = 26) | Greater reductions in depressive symptoms and drinking in patients treated with fluoxetine compared with placebo |
| Roy, 199815 | Inpatients with current major depression and AD who were abstinent for ≥2 weeks 1. Sertraline (100 mg/d; n = 18) 2. Placebo (n = 18) | Greater reductions in depressive symptoms in patients treated with sertraline compared with placebo. Drinking outcomes were not emphasized because 35 of 36 patients reported continuous abstinence throughout the trial |
| Kranzler et al, 199516 | Outpatients with AD. Fourteen percent had current major depression. All received weekly individual or group CBT focused on relapse prevention and skills building 1. Fluoxetine (mean daily dose: 48 mg; n = 51) 2. Placebo (n = 50) | Significant decrease in alcohol consumption for both groups during the trial. No significant differences in alcohol consumption between groups. Among those with current depression, patients treated with fluoxetine experienced greater reduction in depressive symptoms vs placebo |
| Moak et al, 200317 | Currently depressed, actively drinking, alcohol-dependent outpatients. All received individual CBT 1. Sertraline (mean daily dose: 186 mg; n = 38) 2. Placebo (n = 44) | Sertraline had an advantage over placebo in reducing depressive symptoms in women but not in men. Sertraline reduced drinks per drinking day but not other drinking-related outcomes |
| Cornelius et al, 200918 | Adolescents (age 15 to 20) with AA or AD and MDD. All received intensive manual-based therapy (CBT for MDD and AUD, MET for AUD) almost weekly 1. Fluoxetine (20 mg/d; n = 24) 2. Placebo (n = 26) | All improved during the course of trial. No significant differences between fluoxetine and placebo groups in depression- or drinking-related outcomes |
| Roy-Byrne et al, 200019 | Actively drinking alcohol-dependent outpatients with history of ≥1 depressive episode. All received weekly group therapy for alcoholism 1. Nefazodone (mean daily dose: 460 mg; n = 32) 2. Placebo (n = 32) | Greater reduction in depressive symptoms but not in drinking-related outcomes in patients treated with nefazodone |
| Hernandez-Avila et al, 200420 | Outpatients with AD and current major depression. All received supportive psychotherapy for 10 weeks 1. Nefazodone (mean daily dose: 413 mg; n = 21) 2. Placebo (n = 20) | Depressive and anxiety symptoms declined significantly over time, but no statistically significant differences in depressive or anxiety symptoms between nefazodone and placebo. Patients treated with nefazodone had significantly greater reductions in heavy drinking days and in total drinks compared with placebo-treated patients |
| AA: alcohol abuse; AD: alcohol dependence; AUDs: alcohol use disorders; CBT: cognitive-behavioral therapy; MDD: major depressive disorder; MET: motivational enhancement therapy | ||
In a study of adolescents, Cornelius et al18 failed to find any differences between fluoxetine and placebo in any depression or drinking-related outcomes. This study compared the efficacy of fluoxetine, 20 mg/d, with placebo in 50 adolescents with MDD and AUDs who also received intensive, manual-based cognitive-behavioral therapy and motivational enhancement therapy. All patients improved during the trial, but there were no significant differences between fluoxetine- and placebo-treated adolescents.
Other serotonergic medications. Two studies have evaluated nefazodone, a serotonin (5-HT2) antagonist, in dually diagnosed patients. In a 12-week trial, Roy-Byrne et al19 evaluated the efficacy of nefazodone (mean daily dose: 460 mg) vs placebo in 64 actively drinking alcohol-dependent patients who had ≥1 prior episode of depression; all participated in a weekly psychoeducation group on alcoholism. Nefazodone was associated with significantly greater reduction in depressive symptoms but no reductions in drinking compared with placebo. However, a 10-week study of nefazodone20 (mean daily dose: 413 mg) vs placebo in 41 alcohol-dependent patients with current major depression found that those who received nefazodone significantly reduced heavy drinking days compared with the placebo group. There were no significant differences in depressive symptoms between groups.
Conflicting evidence on TCAs
Although several studies suggest TCAs may help reduce depressive symptoms in patients with AUDs, results on their ability to reduce drinking are conflicting (Table 2).24-26 In 1 study, 6 months of desipramine (mean daily dose: 200 mg) reduced drinking in 28 alcohol-dependent individuals with secondary depression24; in another, 12 weeks of imipramine plus weekly relapse prevention psychotherapy did not affect drinking-related outcomes in 69 actively drinking alcoholic outpatients with current depressive disorders.25
Table 2
Limited evidence supports TCAs for comorbid depression and AUDs
| Study | Sample | Results |
|---|---|---|
| Mason et al, 199624 | Outpatients with AD and secondary depression. Part of larger study including non-depressed patients with AD (N = 71) 1. Desipramine (mean daily dose 200 mg; n = 15) 2. Placebo (n = 13) | Greater reduction in depressive symptoms and drinking in desipramine-treated patients compared with placebo-treated patients |
| McGrath et al, 199625 | Outpatients with AD or AA and major depression, dysthymia, or depressive disorder NOS 1. Imipramine (mean daily dose 260 mg; n = 36) 2. Placebo (n = 33) | Greater reduction in depressive symptoms for imipramine-treated patients compared with placebo-treated patients. Drinking-related outcomes were not directly affected by medication except improvements in mood led to reduced alcohol use |
| Altintoprak et al, 200826 | Inpatients with AD and MDD 1. Mirtazapine (30 mg/d; n = 24) 2. Amitriptyline (100 mg/d; n = 20) | Drinking-related outcomes were not emphasized because all patients were required to abstain from drinking during the study. Both treatments reduced depressive symptoms; there were no significant differences between groups |
| AA: alcohol abuse; AD: alcohol dependence; AUDs: alcohol use disorders; MDD: major depressive disorder; NOS: not otherwise specified; TCAs: tricyclic antidepressants | ||
Altintoprak et al26 compared the efficacy of the antidepressant mirtazapine, 30 mg/d, with the TCA amitriptyline, 100 mg/d, in 44 patients with comorbid alcohol dependence and MDD. All patients were required to abstain from drinking alcohol during the study. Both medications resulted in steady reductions in depressive symptoms and alcohol cravings; however, researchers found no significant differences between the 2 treatment groups.
Analyses of combined studies
Pettinati27 conducted a qualitative review of antidepressants for patients with depression and alcohol dependence that included 8 controlled clinical trials (2 on TCAs and 6 on serotonergic medications) conducted between 1994 and 2004. In this review, both TCAs and serotonergic medications were similarly effective in reducing depressive symptoms but not consistently effective in reducing drinking.
27 this review suggested that antidepressants can reduce depressive symptoms but not drinking. The authors also found evidence that the more the antidepressant reduced depressive symptoms, the more it reduced alcohol use. Studies published after these reviews have not substantially altered these findings.
Alcohol abuse medications
Four medications are FDA-approved for treating alcohol dependence:
- disulfiram
- naltrexone (in 2 formulations: oral and long-acting injectable)
- acamprosate.
Table 3
Can medications that target alcohol use also improve depression?
| Study | Sample | Results |
|---|---|---|
| Petrakis et al, 200729 | Outpatients with AD and an axis I disorder, including depression (secondary analysis of Petrakis et al, 200531) 1. Naltrexone (50 mg/d; n = 34) 2. Disulfiram (250 mg/d; n = 43) 3. Naltrexone + disulfiram (n = 28) 4. Placebo (n = 34) | Generally, medication was more effective than no medication. No advantage of 1 medication over the other. There was no relationship between depression diagnosis and medication condition, which suggests that for patients with depression, there was no advantage to medication |
| Pettinati et al, 201030 | Outpatients with AD and MDD 1. Sertraline (200 mg/d; n = 40) 2. Naltrexone (100 mg/d; n = 49) 3. Sertraline + naltrexone (n = 42) 4. Placebo (n = 39) | Greater proportion of patients in combined medication group abstained from alcohol and refrained from heavy alcohol use during the trial compared with those in sertraline-only or naltrexone-only groups. No significant differences among groups on depression-related outcomes |
| AD: alcohol dependence; MDD: major depressive disorder | ||
Nevertheless, these studies suggest that medications for treating depression or AUDs have, at best, only a modest effect in patients with both disorders.
Novel agents
Several novel medications have been evaluated as possible treatments for comorbid depression and AUDs because they target the underlying neurobiology of both disorders:
- agents that target the neurotransmitter glutamate, including the N-methyl-d-aspartate glutamate receptor antagonists memantine and ketamine
- dopaminergic agents such as quetiapine
- corticotropin-releasing factor (CRF) receptor 1 (CRF1) antagonists.
In a case study, a 55-year-old man with treatment-resistant major depression and co-occurring alcohol and benzodiazepine dependence who received a single dose of IV ketamine, 0.5 mg/kg over 50 minutes, experienced “significant improvements” in depressive symptoms that lasted throughout the 7-day follow-up.33 This study did not report on ketamine’s effects on his alcohol use.
The atypical antipsychotic quetiapine acts as a serotonin (5-HT1A and 5-HT2) and dopamine (D1 and D2) antagonist, and reports suggest it reduces alcohol craving and affective symptoms in patients with AUDs.34,35 In a 16-week, open-label study, quetiapine decreased alcohol consumption, alcohol craving, and intensity of some psychiatric symptoms in 28 alcohol-dependent patients with bipolar disorder, schizoaffective disorder, or borderline personality disorder.36
See the Box for a description of the role CRF1 antagonists may play in treating patients with concurrent MDD and AUDs. See Table 4 for studies of memantine and quetiapine in treating depression with AUDs.
Corticotropin-releasing factor (CRF) has a well-established role in stress and has been implicated for treating anxiety and depressive disorders. Evidence also suggests that CRF receptor 1 (CRF1) may be a treatment target for alcohol use disorders (AUDs). Acute alcohol withdrawal and prolonged alcohol use are associated with elevated levels of extrahypothalamic CRF and correlated anxiety. CRF antagonists can reduce the anxiogenic effects of alcohol withdrawal and reduce some symptoms of alcohol dependence, including excessive alcohol self-administration and stress-induced relapse to alcohol use in rats with alcohol dependence, but not in those without dependence. Therefore, CRF1 receptor antagonists may be especially helpful for individuals who use alcohol to reduce negative emotional states such as anxiety or dysphoria, including those with concurrent major depressive disorder and AUDs.
Bibliography
Funk CK, Zorrilla EP, Lee MJ, et al. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61(1):78-86.
Gehlert DR, Cippitelli A, Thorsell A, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27(10):2718-2726.
Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32(9):1535-1542.
Other agents may play a role in treating depression with AUDs
| Study | Sample | Results |
|---|---|---|
| Muhonen et al, 200832 | Outpatients with MDD and AD 1. Memantine (20 mg/d; n = 40) | Both treatments reduced depressive and anxiety symptoms. No significant differences between groups. Study did not examine alcohol-related outcomes |
| Martinotti et al, 200833 | Outpatients with comorbid AD and either bipolar disorder, schizoaffective disorder or borderline personality disorder. Open-label study 1. Quetiapine (300 to 800 mg/d; n = 28) | Quetiapine was associated with reduced alcohol consumption, alcohol craving, and intensity of psychiatric symptoms |
| AD: alcohol dependence; AUDs: alcohol use disorders; MDD: major depressive disorder | ||
Interpreting the evidence
These findings underscore the importance of thorough evaluations. SSRIs are a first-line treatment for depression and as such probably should be the first choice for patients with comorbid AUDs. Drinking should be monitored closely and abstinence encouraged. Using medications that target AUDs is safe and modestly effective in patients with comorbid depression. Evidence suggests that treating both disorders simultaneously is more effective than treating either alone. Medications should be prescribed as part of a comprehensive treatment plan that also includes psychotherapy.
Related Resources
- Pettinati H. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56(10):785-792.
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887-1896.
- Acamprosate • Campral
- Amitriptyline • Elavil
- Desipramine • Norpramin
- Disulfiram • Antabuse
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Ketamine • Ketalar
- Memantine • Namenda
- Mirtazapine • Remeron
- Naltrexone • Revia, Vivitrol
- Nefazodone • Serzone
- Quetiapine • Seroquel
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Petrakis receives research or grant support from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, the U.S. Department of Defense, and the U.S. Department of Veterans Affairs.
This work has been supported by a grant from the Veterans Affairs New England Mental Illness Research, Education, and Clinical Center and by the National Institute of Mental Health (T32MH062994-07).
Acknowledgements
The authors thank Elizabeth Guidone for her helpful comments and Diana Limoncelli for her assistance in manuscript preparation.
1. Hasin DS, Stinson FS, Ogburn E, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842.
2. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39(3):197-206.
3. Kessler RC, Crum RM, Warner LA, et al. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54(4):313-321.
4. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
5. Burns L, Teesson M, O’Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100(6):787-796.
6. Gilman SE, Abraham HD. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alcohol Depend. 2001;63(3):277-286.
7. Hasin DS, Tsai WY, Endicott J, et al. Five-year course of major depression: effects of comorbid alcoholism. J Affect Disord. 1996;41(1):63-70.
8. Mueller TI, Lavori PW, Keller MB, et al. Prognostic effect of the variable course of alcoholism on the 10-year course of depression. Am J Psychiatry. 1994;151(5):701-706.
9. Cornelius JR, Salloum IM, Mezzich J, et al. Disproportionate suicidality in patients with comorbid major depression and alcoholism. Am J Psychiatry. 1995;152(3):358-364.
10. Sher L, Oquendo MA, Galfalvy HC, et al. The relationship of aggression to suicidal behavior in depressed patients with a history of alcoholism. Addict Behav. 2005;30(6):1144-1153.
11. Greenfield SF, Weiss RD, Muenz LR, et al. The effect of depression on return to drinking: a prospective study. Arch Gen Psychiatry. 1998;55(3):259-265.
12. Brady KT, Verduin ML, Tolliver BK. Treatment of patients comorbid for addiction and other psychiatric disorders. Curr Psychiatry Rep. 2007;9(5):374-380.
13. Oslin DW. Treatment of late-life depression complicated by alcohol dependence. Am J Geriatr Psychiatry. 2005;13(6):491-500.
14. Cornelius JR, Salloum IM, Ehler JG, et al. Fluoxetine in depressed alcoholics. A double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1997;54(8):700-705.
15. Roy A. Placebo-controlled study of sertraline in depressed recently abstinent alcoholics. Biol Psychiatry. 1998;44(7):633-637.
16. Kranzler HR, Burleson JA, Korner P, et al. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995;152(3):391-397.
17. Moak DH, Anton RF, Latham PK, et al. Sertraline and cognitive behavioral therapy for depressed alcoholics: results of a placebo-controlled trial. J Clin Psychopharmacol. 2003;23(6):553-562.
18. Cornelius JR, Bukstein OG, Wood DS, et al. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905-909.
19. Roy-Byrne PP, Pages KP, Russo JE, et al. Nefazodone treatment of major depression in alcohol-dependent patients: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2000;20(2):129-136.
20. Hernandez-Avila CA, Modesto-Lowe V, Feinn R, et al. Nefazodone treatment of comorbid alcohol dependence and major depression. Alcohol Clin Exp Res. 2004;28(3):433-440.
21. Kranzler HR, Burleson JA, Brown J, et al. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20(9):1534-1541.
22. Naranjo CA, Bremner KE, Lanctôt KL. Effects of citalopram and a brief psycho-social intervention on alcohol intake dependence and problems. Addiction. 1995;90(1):87-99.
23. Pettinati HM, Volpicelli JR, Kranzler HR, et al. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24(7):1041-1049.
24. Mason BJ, Kocsis JH, Ritvo EC, et al. A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA. 1996;275(10):761-767.
25. McGrath PJ, Nunes EV, Stewart JW, et al. Imipramine treatment of alcoholics with primary depression: a placebo-controlled clinical trial. Arch Gen Psychiatry. 1996;53(3):232-240.
26. Altintoprak AE, Zorlu N, Coskunol H, et al. Effectiveness and tolerability of mirtazapine and amitriptyline in alcoholic patients with co-morbid depressive disorder: a randomized, double-blind study. Hum Psychopharmacol. 2008;23(4):313-319.
27. Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56(10):785-792.
28. Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887-1896.
29. Petrakis I, Ralevski E, Nich C, et al. Naltrexone and disulfiram in patients with alcohol dependence and current depression. J Clin Psychopharmacol. 2007;27(2):160-165.
30. Pettinati HM, Oslin DW, Kampman KM, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatry. 2010;167(6):668-675.
31. Petrakis IL, Poling J, Levinson C, et al. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
32. Muhonen LH, Lönnqvist J, Juva K, et al. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J Clin Psychiatry. 2008;69(3):392-399.
33. Liebrenz M, Borgeat A, Leisinger R, et al. Intravenous ketamine therapy in a patient with a treatment-resistant major depression. Swiss Med Wkly. 2007;137(15-16):234-236.
34. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol. 2007;27(4):344-351.
35. Croissant B, Klein O, Gehrlein L, et al. Quetiapine in relapse prevention in alcoholics suffering from craving and affective symptoms: a case series. Eur Psychiatry. 2006;21(8):570-573.
36. Martinotti G, Andreoli S, Di Nicola M, et al. Quetiapine decreases alcohol consumption, craving, and psychiatric symptoms in dually diagnosed alcoholics. Hum Psychopharmacol. 2008;23(5):417-424.
Discuss this article at www.facebook.com/CurrentPsychiatry
With a lifetime prevalence of 30%, alcohol use disorders (AUDs)—which in DSM-IV-TR include alcohol abuse and alcohol dependence—are among the most common psychiatric disorders.1 Depressive disorders, including major depressive disorder (MDD) and dysthymia, frequently co-occur with AUDs.2-4 This pattern of comorbidity adversely affects the prognosis, course, and treatment of both MDD and AUDs.5 High severity in 1 of these disorders is associated with high severity in the other.2,6 Alcohol dependence appears to prolong the course of depression7,8 and increases the risk of suicidal symptoms and behaviors.9,10 Patients with depression and AUDs are at increased risk of relapse to heavy drinking.7,11
Whether the high comorbidity rate of depressive disorders and AUDs is a result of 1 disorder causing the other (ie, AUDs leading to depression or vice versa) or can be attributed to shared etiology is unknown. Clinicians need to consider this question when treating patients with this pattern of comorbidity because distinguishing primary depression from secondary depression influences treatment decisions.12
There is a great need for pharmacologic interventions that can concurrently treat both depression and AUDs. This article reviews the evidence for current treatments for dually diagnosed patients and highlights novel agents that are worthy of further study for this complex patient population.
Current treatment options
Pharmacotherapy for MDD and alcohol dependence is common when these conditions occur alone. FDA-approved medications for treating depression include monoamine oxidase inhibitors, tricyclic antidepressants (TCAs), tetracyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors.
SSRIs are the most widely used class of antidepressants. They gained FDA approval based on studies conducted in non-comorbid patients because patients with comorbid conditions usually are excluded from research studies.13 Few trials have evaluated patients with depression and AUDs; TCAs and SSRIs are best studied in these patients.
Serotonergic antidepressants
SSRIs are first-line medications for MDD because of their low abuse potential, favorable side effect profile, and relative safety in overdose.
Table 1
Serotonergic antidepressants for patients with AUDs and depression
| Study | Sample | Results |
|---|---|---|
| Cornelius et al, 199714 | Outpatients with severe major depression and AD 1. Fluoxetine (20 to 40 mg/d; n = 25) 2. Placebo (n = 26) | Greater reductions in depressive symptoms and drinking in patients treated with fluoxetine compared with placebo |
| Roy, 199815 | Inpatients with current major depression and AD who were abstinent for ≥2 weeks 1. Sertraline (100 mg/d; n = 18) 2. Placebo (n = 18) | Greater reductions in depressive symptoms in patients treated with sertraline compared with placebo. Drinking outcomes were not emphasized because 35 of 36 patients reported continuous abstinence throughout the trial |
| Kranzler et al, 199516 | Outpatients with AD. Fourteen percent had current major depression. All received weekly individual or group CBT focused on relapse prevention and skills building 1. Fluoxetine (mean daily dose: 48 mg; n = 51) 2. Placebo (n = 50) | Significant decrease in alcohol consumption for both groups during the trial. No significant differences in alcohol consumption between groups. Among those with current depression, patients treated with fluoxetine experienced greater reduction in depressive symptoms vs placebo |
| Moak et al, 200317 | Currently depressed, actively drinking, alcohol-dependent outpatients. All received individual CBT 1. Sertraline (mean daily dose: 186 mg; n = 38) 2. Placebo (n = 44) | Sertraline had an advantage over placebo in reducing depressive symptoms in women but not in men. Sertraline reduced drinks per drinking day but not other drinking-related outcomes |
| Cornelius et al, 200918 | Adolescents (age 15 to 20) with AA or AD and MDD. All received intensive manual-based therapy (CBT for MDD and AUD, MET for AUD) almost weekly 1. Fluoxetine (20 mg/d; n = 24) 2. Placebo (n = 26) | All improved during the course of trial. No significant differences between fluoxetine and placebo groups in depression- or drinking-related outcomes |
| Roy-Byrne et al, 200019 | Actively drinking alcohol-dependent outpatients with history of ≥1 depressive episode. All received weekly group therapy for alcoholism 1. Nefazodone (mean daily dose: 460 mg; n = 32) 2. Placebo (n = 32) | Greater reduction in depressive symptoms but not in drinking-related outcomes in patients treated with nefazodone |
| Hernandez-Avila et al, 200420 | Outpatients with AD and current major depression. All received supportive psychotherapy for 10 weeks 1. Nefazodone (mean daily dose: 413 mg; n = 21) 2. Placebo (n = 20) | Depressive and anxiety symptoms declined significantly over time, but no statistically significant differences in depressive or anxiety symptoms between nefazodone and placebo. Patients treated with nefazodone had significantly greater reductions in heavy drinking days and in total drinks compared with placebo-treated patients |
| AA: alcohol abuse; AD: alcohol dependence; AUDs: alcohol use disorders; CBT: cognitive-behavioral therapy; MDD: major depressive disorder; MET: motivational enhancement therapy | ||
In a study of adolescents, Cornelius et al18 failed to find any differences between fluoxetine and placebo in any depression or drinking-related outcomes. This study compared the efficacy of fluoxetine, 20 mg/d, with placebo in 50 adolescents with MDD and AUDs who also received intensive, manual-based cognitive-behavioral therapy and motivational enhancement therapy. All patients improved during the trial, but there were no significant differences between fluoxetine- and placebo-treated adolescents.
Other serotonergic medications. Two studies have evaluated nefazodone, a serotonin (5-HT2) antagonist, in dually diagnosed patients. In a 12-week trial, Roy-Byrne et al19 evaluated the efficacy of nefazodone (mean daily dose: 460 mg) vs placebo in 64 actively drinking alcohol-dependent patients who had ≥1 prior episode of depression; all participated in a weekly psychoeducation group on alcoholism. Nefazodone was associated with significantly greater reduction in depressive symptoms but no reductions in drinking compared with placebo. However, a 10-week study of nefazodone20 (mean daily dose: 413 mg) vs placebo in 41 alcohol-dependent patients with current major depression found that those who received nefazodone significantly reduced heavy drinking days compared with the placebo group. There were no significant differences in depressive symptoms between groups.
Conflicting evidence on TCAs
Although several studies suggest TCAs may help reduce depressive symptoms in patients with AUDs, results on their ability to reduce drinking are conflicting (Table 2).24-26 In 1 study, 6 months of desipramine (mean daily dose: 200 mg) reduced drinking in 28 alcohol-dependent individuals with secondary depression24; in another, 12 weeks of imipramine plus weekly relapse prevention psychotherapy did not affect drinking-related outcomes in 69 actively drinking alcoholic outpatients with current depressive disorders.25
Table 2
Limited evidence supports TCAs for comorbid depression and AUDs
| Study | Sample | Results |
|---|---|---|
| Mason et al, 199624 | Outpatients with AD and secondary depression. Part of larger study including non-depressed patients with AD (N = 71) 1. Desipramine (mean daily dose 200 mg; n = 15) 2. Placebo (n = 13) | Greater reduction in depressive symptoms and drinking in desipramine-treated patients compared with placebo-treated patients |
| McGrath et al, 199625 | Outpatients with AD or AA and major depression, dysthymia, or depressive disorder NOS 1. Imipramine (mean daily dose 260 mg; n = 36) 2. Placebo (n = 33) | Greater reduction in depressive symptoms for imipramine-treated patients compared with placebo-treated patients. Drinking-related outcomes were not directly affected by medication except improvements in mood led to reduced alcohol use |
| Altintoprak et al, 200826 | Inpatients with AD and MDD 1. Mirtazapine (30 mg/d; n = 24) 2. Amitriptyline (100 mg/d; n = 20) | Drinking-related outcomes were not emphasized because all patients were required to abstain from drinking during the study. Both treatments reduced depressive symptoms; there were no significant differences between groups |
| AA: alcohol abuse; AD: alcohol dependence; AUDs: alcohol use disorders; MDD: major depressive disorder; NOS: not otherwise specified; TCAs: tricyclic antidepressants | ||
Altintoprak et al26 compared the efficacy of the antidepressant mirtazapine, 30 mg/d, with the TCA amitriptyline, 100 mg/d, in 44 patients with comorbid alcohol dependence and MDD. All patients were required to abstain from drinking alcohol during the study. Both medications resulted in steady reductions in depressive symptoms and alcohol cravings; however, researchers found no significant differences between the 2 treatment groups.
Analyses of combined studies
Pettinati27 conducted a qualitative review of antidepressants for patients with depression and alcohol dependence that included 8 controlled clinical trials (2 on TCAs and 6 on serotonergic medications) conducted between 1994 and 2004. In this review, both TCAs and serotonergic medications were similarly effective in reducing depressive symptoms but not consistently effective in reducing drinking.
27 this review suggested that antidepressants can reduce depressive symptoms but not drinking. The authors also found evidence that the more the antidepressant reduced depressive symptoms, the more it reduced alcohol use. Studies published after these reviews have not substantially altered these findings.
Alcohol abuse medications
Four medications are FDA-approved for treating alcohol dependence:
- disulfiram
- naltrexone (in 2 formulations: oral and long-acting injectable)
- acamprosate.
Table 3
Can medications that target alcohol use also improve depression?
| Study | Sample | Results |
|---|---|---|
| Petrakis et al, 200729 | Outpatients with AD and an axis I disorder, including depression (secondary analysis of Petrakis et al, 200531) 1. Naltrexone (50 mg/d; n = 34) 2. Disulfiram (250 mg/d; n = 43) 3. Naltrexone + disulfiram (n = 28) 4. Placebo (n = 34) | Generally, medication was more effective than no medication. No advantage of 1 medication over the other. There was no relationship between depression diagnosis and medication condition, which suggests that for patients with depression, there was no advantage to medication |
| Pettinati et al, 201030 | Outpatients with AD and MDD 1. Sertraline (200 mg/d; n = 40) 2. Naltrexone (100 mg/d; n = 49) 3. Sertraline + naltrexone (n = 42) 4. Placebo (n = 39) | Greater proportion of patients in combined medication group abstained from alcohol and refrained from heavy alcohol use during the trial compared with those in sertraline-only or naltrexone-only groups. No significant differences among groups on depression-related outcomes |
| AD: alcohol dependence; MDD: major depressive disorder | ||
Nevertheless, these studies suggest that medications for treating depression or AUDs have, at best, only a modest effect in patients with both disorders.
Novel agents
Several novel medications have been evaluated as possible treatments for comorbid depression and AUDs because they target the underlying neurobiology of both disorders:
- agents that target the neurotransmitter glutamate, including the N-methyl-d-aspartate glutamate receptor antagonists memantine and ketamine
- dopaminergic agents such as quetiapine
- corticotropin-releasing factor (CRF) receptor 1 (CRF1) antagonists.
In a case study, a 55-year-old man with treatment-resistant major depression and co-occurring alcohol and benzodiazepine dependence who received a single dose of IV ketamine, 0.5 mg/kg over 50 minutes, experienced “significant improvements” in depressive symptoms that lasted throughout the 7-day follow-up.33 This study did not report on ketamine’s effects on his alcohol use.
The atypical antipsychotic quetiapine acts as a serotonin (5-HT1A and 5-HT2) and dopamine (D1 and D2) antagonist, and reports suggest it reduces alcohol craving and affective symptoms in patients with AUDs.34,35 In a 16-week, open-label study, quetiapine decreased alcohol consumption, alcohol craving, and intensity of some psychiatric symptoms in 28 alcohol-dependent patients with bipolar disorder, schizoaffective disorder, or borderline personality disorder.36
See the Box for a description of the role CRF1 antagonists may play in treating patients with concurrent MDD and AUDs. See Table 4 for studies of memantine and quetiapine in treating depression with AUDs.
Corticotropin-releasing factor (CRF) has a well-established role in stress and has been implicated for treating anxiety and depressive disorders. Evidence also suggests that CRF receptor 1 (CRF1) may be a treatment target for alcohol use disorders (AUDs). Acute alcohol withdrawal and prolonged alcohol use are associated with elevated levels of extrahypothalamic CRF and correlated anxiety. CRF antagonists can reduce the anxiogenic effects of alcohol withdrawal and reduce some symptoms of alcohol dependence, including excessive alcohol self-administration and stress-induced relapse to alcohol use in rats with alcohol dependence, but not in those without dependence. Therefore, CRF1 receptor antagonists may be especially helpful for individuals who use alcohol to reduce negative emotional states such as anxiety or dysphoria, including those with concurrent major depressive disorder and AUDs.
Bibliography
Funk CK, Zorrilla EP, Lee MJ, et al. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61(1):78-86.
Gehlert DR, Cippitelli A, Thorsell A, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27(10):2718-2726.
Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32(9):1535-1542.
Other agents may play a role in treating depression with AUDs
| Study | Sample | Results |
|---|---|---|
| Muhonen et al, 200832 | Outpatients with MDD and AD 1. Memantine (20 mg/d; n = 40) | Both treatments reduced depressive and anxiety symptoms. No significant differences between groups. Study did not examine alcohol-related outcomes |
| Martinotti et al, 200833 | Outpatients with comorbid AD and either bipolar disorder, schizoaffective disorder or borderline personality disorder. Open-label study 1. Quetiapine (300 to 800 mg/d; n = 28) | Quetiapine was associated with reduced alcohol consumption, alcohol craving, and intensity of psychiatric symptoms |
| AD: alcohol dependence; AUDs: alcohol use disorders; MDD: major depressive disorder | ||
Interpreting the evidence
These findings underscore the importance of thorough evaluations. SSRIs are a first-line treatment for depression and as such probably should be the first choice for patients with comorbid AUDs. Drinking should be monitored closely and abstinence encouraged. Using medications that target AUDs is safe and modestly effective in patients with comorbid depression. Evidence suggests that treating both disorders simultaneously is more effective than treating either alone. Medications should be prescribed as part of a comprehensive treatment plan that also includes psychotherapy.
Related Resources
- Pettinati H. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56(10):785-792.
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887-1896.
- Acamprosate • Campral
- Amitriptyline • Elavil
- Desipramine • Norpramin
- Disulfiram • Antabuse
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Ketamine • Ketalar
- Memantine • Namenda
- Mirtazapine • Remeron
- Naltrexone • Revia, Vivitrol
- Nefazodone • Serzone
- Quetiapine • Seroquel
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Petrakis receives research or grant support from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, the U.S. Department of Defense, and the U.S. Department of Veterans Affairs.
This work has been supported by a grant from the Veterans Affairs New England Mental Illness Research, Education, and Clinical Center and by the National Institute of Mental Health (T32MH062994-07).
Acknowledgements
The authors thank Elizabeth Guidone for her helpful comments and Diana Limoncelli for her assistance in manuscript preparation.
Discuss this article at www.facebook.com/CurrentPsychiatry
With a lifetime prevalence of 30%, alcohol use disorders (AUDs)—which in DSM-IV-TR include alcohol abuse and alcohol dependence—are among the most common psychiatric disorders.1 Depressive disorders, including major depressive disorder (MDD) and dysthymia, frequently co-occur with AUDs.2-4 This pattern of comorbidity adversely affects the prognosis, course, and treatment of both MDD and AUDs.5 High severity in 1 of these disorders is associated with high severity in the other.2,6 Alcohol dependence appears to prolong the course of depression7,8 and increases the risk of suicidal symptoms and behaviors.9,10 Patients with depression and AUDs are at increased risk of relapse to heavy drinking.7,11
Whether the high comorbidity rate of depressive disorders and AUDs is a result of 1 disorder causing the other (ie, AUDs leading to depression or vice versa) or can be attributed to shared etiology is unknown. Clinicians need to consider this question when treating patients with this pattern of comorbidity because distinguishing primary depression from secondary depression influences treatment decisions.12
There is a great need for pharmacologic interventions that can concurrently treat both depression and AUDs. This article reviews the evidence for current treatments for dually diagnosed patients and highlights novel agents that are worthy of further study for this complex patient population.
Current treatment options
Pharmacotherapy for MDD and alcohol dependence is common when these conditions occur alone. FDA-approved medications for treating depression include monoamine oxidase inhibitors, tricyclic antidepressants (TCAs), tetracyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors.
SSRIs are the most widely used class of antidepressants. They gained FDA approval based on studies conducted in non-comorbid patients because patients with comorbid conditions usually are excluded from research studies.13 Few trials have evaluated patients with depression and AUDs; TCAs and SSRIs are best studied in these patients.
Serotonergic antidepressants
SSRIs are first-line medications for MDD because of their low abuse potential, favorable side effect profile, and relative safety in overdose.
Table 1
Serotonergic antidepressants for patients with AUDs and depression
| Study | Sample | Results |
|---|---|---|
| Cornelius et al, 199714 | Outpatients with severe major depression and AD 1. Fluoxetine (20 to 40 mg/d; n = 25) 2. Placebo (n = 26) | Greater reductions in depressive symptoms and drinking in patients treated with fluoxetine compared with placebo |
| Roy, 199815 | Inpatients with current major depression and AD who were abstinent for ≥2 weeks 1. Sertraline (100 mg/d; n = 18) 2. Placebo (n = 18) | Greater reductions in depressive symptoms in patients treated with sertraline compared with placebo. Drinking outcomes were not emphasized because 35 of 36 patients reported continuous abstinence throughout the trial |
| Kranzler et al, 199516 | Outpatients with AD. Fourteen percent had current major depression. All received weekly individual or group CBT focused on relapse prevention and skills building 1. Fluoxetine (mean daily dose: 48 mg; n = 51) 2. Placebo (n = 50) | Significant decrease in alcohol consumption for both groups during the trial. No significant differences in alcohol consumption between groups. Among those with current depression, patients treated with fluoxetine experienced greater reduction in depressive symptoms vs placebo |
| Moak et al, 200317 | Currently depressed, actively drinking, alcohol-dependent outpatients. All received individual CBT 1. Sertraline (mean daily dose: 186 mg; n = 38) 2. Placebo (n = 44) | Sertraline had an advantage over placebo in reducing depressive symptoms in women but not in men. Sertraline reduced drinks per drinking day but not other drinking-related outcomes |
| Cornelius et al, 200918 | Adolescents (age 15 to 20) with AA or AD and MDD. All received intensive manual-based therapy (CBT for MDD and AUD, MET for AUD) almost weekly 1. Fluoxetine (20 mg/d; n = 24) 2. Placebo (n = 26) | All improved during the course of trial. No significant differences between fluoxetine and placebo groups in depression- or drinking-related outcomes |
| Roy-Byrne et al, 200019 | Actively drinking alcohol-dependent outpatients with history of ≥1 depressive episode. All received weekly group therapy for alcoholism 1. Nefazodone (mean daily dose: 460 mg; n = 32) 2. Placebo (n = 32) | Greater reduction in depressive symptoms but not in drinking-related outcomes in patients treated with nefazodone |
| Hernandez-Avila et al, 200420 | Outpatients with AD and current major depression. All received supportive psychotherapy for 10 weeks 1. Nefazodone (mean daily dose: 413 mg; n = 21) 2. Placebo (n = 20) | Depressive and anxiety symptoms declined significantly over time, but no statistically significant differences in depressive or anxiety symptoms between nefazodone and placebo. Patients treated with nefazodone had significantly greater reductions in heavy drinking days and in total drinks compared with placebo-treated patients |
| AA: alcohol abuse; AD: alcohol dependence; AUDs: alcohol use disorders; CBT: cognitive-behavioral therapy; MDD: major depressive disorder; MET: motivational enhancement therapy | ||
In a study of adolescents, Cornelius et al18 failed to find any differences between fluoxetine and placebo in any depression or drinking-related outcomes. This study compared the efficacy of fluoxetine, 20 mg/d, with placebo in 50 adolescents with MDD and AUDs who also received intensive, manual-based cognitive-behavioral therapy and motivational enhancement therapy. All patients improved during the trial, but there were no significant differences between fluoxetine- and placebo-treated adolescents.
Other serotonergic medications. Two studies have evaluated nefazodone, a serotonin (5-HT2) antagonist, in dually diagnosed patients. In a 12-week trial, Roy-Byrne et al19 evaluated the efficacy of nefazodone (mean daily dose: 460 mg) vs placebo in 64 actively drinking alcohol-dependent patients who had ≥1 prior episode of depression; all participated in a weekly psychoeducation group on alcoholism. Nefazodone was associated with significantly greater reduction in depressive symptoms but no reductions in drinking compared with placebo. However, a 10-week study of nefazodone20 (mean daily dose: 413 mg) vs placebo in 41 alcohol-dependent patients with current major depression found that those who received nefazodone significantly reduced heavy drinking days compared with the placebo group. There were no significant differences in depressive symptoms between groups.
Conflicting evidence on TCAs
Although several studies suggest TCAs may help reduce depressive symptoms in patients with AUDs, results on their ability to reduce drinking are conflicting (Table 2).24-26 In 1 study, 6 months of desipramine (mean daily dose: 200 mg) reduced drinking in 28 alcohol-dependent individuals with secondary depression24; in another, 12 weeks of imipramine plus weekly relapse prevention psychotherapy did not affect drinking-related outcomes in 69 actively drinking alcoholic outpatients with current depressive disorders.25
Table 2
Limited evidence supports TCAs for comorbid depression and AUDs
| Study | Sample | Results |
|---|---|---|
| Mason et al, 199624 | Outpatients with AD and secondary depression. Part of larger study including non-depressed patients with AD (N = 71) 1. Desipramine (mean daily dose 200 mg; n = 15) 2. Placebo (n = 13) | Greater reduction in depressive symptoms and drinking in desipramine-treated patients compared with placebo-treated patients |
| McGrath et al, 199625 | Outpatients with AD or AA and major depression, dysthymia, or depressive disorder NOS 1. Imipramine (mean daily dose 260 mg; n = 36) 2. Placebo (n = 33) | Greater reduction in depressive symptoms for imipramine-treated patients compared with placebo-treated patients. Drinking-related outcomes were not directly affected by medication except improvements in mood led to reduced alcohol use |
| Altintoprak et al, 200826 | Inpatients with AD and MDD 1. Mirtazapine (30 mg/d; n = 24) 2. Amitriptyline (100 mg/d; n = 20) | Drinking-related outcomes were not emphasized because all patients were required to abstain from drinking during the study. Both treatments reduced depressive symptoms; there were no significant differences between groups |
| AA: alcohol abuse; AD: alcohol dependence; AUDs: alcohol use disorders; MDD: major depressive disorder; NOS: not otherwise specified; TCAs: tricyclic antidepressants | ||
Altintoprak et al26 compared the efficacy of the antidepressant mirtazapine, 30 mg/d, with the TCA amitriptyline, 100 mg/d, in 44 patients with comorbid alcohol dependence and MDD. All patients were required to abstain from drinking alcohol during the study. Both medications resulted in steady reductions in depressive symptoms and alcohol cravings; however, researchers found no significant differences between the 2 treatment groups.
Analyses of combined studies
Pettinati27 conducted a qualitative review of antidepressants for patients with depression and alcohol dependence that included 8 controlled clinical trials (2 on TCAs and 6 on serotonergic medications) conducted between 1994 and 2004. In this review, both TCAs and serotonergic medications were similarly effective in reducing depressive symptoms but not consistently effective in reducing drinking.
27 this review suggested that antidepressants can reduce depressive symptoms but not drinking. The authors also found evidence that the more the antidepressant reduced depressive symptoms, the more it reduced alcohol use. Studies published after these reviews have not substantially altered these findings.
Alcohol abuse medications
Four medications are FDA-approved for treating alcohol dependence:
- disulfiram
- naltrexone (in 2 formulations: oral and long-acting injectable)
- acamprosate.
Table 3
Can medications that target alcohol use also improve depression?
| Study | Sample | Results |
|---|---|---|
| Petrakis et al, 200729 | Outpatients with AD and an axis I disorder, including depression (secondary analysis of Petrakis et al, 200531) 1. Naltrexone (50 mg/d; n = 34) 2. Disulfiram (250 mg/d; n = 43) 3. Naltrexone + disulfiram (n = 28) 4. Placebo (n = 34) | Generally, medication was more effective than no medication. No advantage of 1 medication over the other. There was no relationship between depression diagnosis and medication condition, which suggests that for patients with depression, there was no advantage to medication |
| Pettinati et al, 201030 | Outpatients with AD and MDD 1. Sertraline (200 mg/d; n = 40) 2. Naltrexone (100 mg/d; n = 49) 3. Sertraline + naltrexone (n = 42) 4. Placebo (n = 39) | Greater proportion of patients in combined medication group abstained from alcohol and refrained from heavy alcohol use during the trial compared with those in sertraline-only or naltrexone-only groups. No significant differences among groups on depression-related outcomes |
| AD: alcohol dependence; MDD: major depressive disorder | ||
Nevertheless, these studies suggest that medications for treating depression or AUDs have, at best, only a modest effect in patients with both disorders.
Novel agents
Several novel medications have been evaluated as possible treatments for comorbid depression and AUDs because they target the underlying neurobiology of both disorders:
- agents that target the neurotransmitter glutamate, including the N-methyl-d-aspartate glutamate receptor antagonists memantine and ketamine
- dopaminergic agents such as quetiapine
- corticotropin-releasing factor (CRF) receptor 1 (CRF1) antagonists.
In a case study, a 55-year-old man with treatment-resistant major depression and co-occurring alcohol and benzodiazepine dependence who received a single dose of IV ketamine, 0.5 mg/kg over 50 minutes, experienced “significant improvements” in depressive symptoms that lasted throughout the 7-day follow-up.33 This study did not report on ketamine’s effects on his alcohol use.
The atypical antipsychotic quetiapine acts as a serotonin (5-HT1A and 5-HT2) and dopamine (D1 and D2) antagonist, and reports suggest it reduces alcohol craving and affective symptoms in patients with AUDs.34,35 In a 16-week, open-label study, quetiapine decreased alcohol consumption, alcohol craving, and intensity of some psychiatric symptoms in 28 alcohol-dependent patients with bipolar disorder, schizoaffective disorder, or borderline personality disorder.36
See the Box for a description of the role CRF1 antagonists may play in treating patients with concurrent MDD and AUDs. See Table 4 for studies of memantine and quetiapine in treating depression with AUDs.
Corticotropin-releasing factor (CRF) has a well-established role in stress and has been implicated for treating anxiety and depressive disorders. Evidence also suggests that CRF receptor 1 (CRF1) may be a treatment target for alcohol use disorders (AUDs). Acute alcohol withdrawal and prolonged alcohol use are associated with elevated levels of extrahypothalamic CRF and correlated anxiety. CRF antagonists can reduce the anxiogenic effects of alcohol withdrawal and reduce some symptoms of alcohol dependence, including excessive alcohol self-administration and stress-induced relapse to alcohol use in rats with alcohol dependence, but not in those without dependence. Therefore, CRF1 receptor antagonists may be especially helpful for individuals who use alcohol to reduce negative emotional states such as anxiety or dysphoria, including those with concurrent major depressive disorder and AUDs.
Bibliography
Funk CK, Zorrilla EP, Lee MJ, et al. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61(1):78-86.
Gehlert DR, Cippitelli A, Thorsell A, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27(10):2718-2726.
Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32(9):1535-1542.
Other agents may play a role in treating depression with AUDs
| Study | Sample | Results |
|---|---|---|
| Muhonen et al, 200832 | Outpatients with MDD and AD 1. Memantine (20 mg/d; n = 40) | Both treatments reduced depressive and anxiety symptoms. No significant differences between groups. Study did not examine alcohol-related outcomes |
| Martinotti et al, 200833 | Outpatients with comorbid AD and either bipolar disorder, schizoaffective disorder or borderline personality disorder. Open-label study 1. Quetiapine (300 to 800 mg/d; n = 28) | Quetiapine was associated with reduced alcohol consumption, alcohol craving, and intensity of psychiatric symptoms |
| AD: alcohol dependence; AUDs: alcohol use disorders; MDD: major depressive disorder | ||
Interpreting the evidence
These findings underscore the importance of thorough evaluations. SSRIs are a first-line treatment for depression and as such probably should be the first choice for patients with comorbid AUDs. Drinking should be monitored closely and abstinence encouraged. Using medications that target AUDs is safe and modestly effective in patients with comorbid depression. Evidence suggests that treating both disorders simultaneously is more effective than treating either alone. Medications should be prescribed as part of a comprehensive treatment plan that also includes psychotherapy.
Related Resources
- Pettinati H. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56(10):785-792.
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887-1896.
- Acamprosate • Campral
- Amitriptyline • Elavil
- Desipramine • Norpramin
- Disulfiram • Antabuse
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Ketamine • Ketalar
- Memantine • Namenda
- Mirtazapine • Remeron
- Naltrexone • Revia, Vivitrol
- Nefazodone • Serzone
- Quetiapine • Seroquel
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Petrakis receives research or grant support from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, the U.S. Department of Defense, and the U.S. Department of Veterans Affairs.
This work has been supported by a grant from the Veterans Affairs New England Mental Illness Research, Education, and Clinical Center and by the National Institute of Mental Health (T32MH062994-07).
Acknowledgements
The authors thank Elizabeth Guidone for her helpful comments and Diana Limoncelli for her assistance in manuscript preparation.
1. Hasin DS, Stinson FS, Ogburn E, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842.
2. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39(3):197-206.
3. Kessler RC, Crum RM, Warner LA, et al. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54(4):313-321.
4. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
5. Burns L, Teesson M, O’Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100(6):787-796.
6. Gilman SE, Abraham HD. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alcohol Depend. 2001;63(3):277-286.
7. Hasin DS, Tsai WY, Endicott J, et al. Five-year course of major depression: effects of comorbid alcoholism. J Affect Disord. 1996;41(1):63-70.
8. Mueller TI, Lavori PW, Keller MB, et al. Prognostic effect of the variable course of alcoholism on the 10-year course of depression. Am J Psychiatry. 1994;151(5):701-706.
9. Cornelius JR, Salloum IM, Mezzich J, et al. Disproportionate suicidality in patients with comorbid major depression and alcoholism. Am J Psychiatry. 1995;152(3):358-364.
10. Sher L, Oquendo MA, Galfalvy HC, et al. The relationship of aggression to suicidal behavior in depressed patients with a history of alcoholism. Addict Behav. 2005;30(6):1144-1153.
11. Greenfield SF, Weiss RD, Muenz LR, et al. The effect of depression on return to drinking: a prospective study. Arch Gen Psychiatry. 1998;55(3):259-265.
12. Brady KT, Verduin ML, Tolliver BK. Treatment of patients comorbid for addiction and other psychiatric disorders. Curr Psychiatry Rep. 2007;9(5):374-380.
13. Oslin DW. Treatment of late-life depression complicated by alcohol dependence. Am J Geriatr Psychiatry. 2005;13(6):491-500.
14. Cornelius JR, Salloum IM, Ehler JG, et al. Fluoxetine in depressed alcoholics. A double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1997;54(8):700-705.
15. Roy A. Placebo-controlled study of sertraline in depressed recently abstinent alcoholics. Biol Psychiatry. 1998;44(7):633-637.
16. Kranzler HR, Burleson JA, Korner P, et al. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995;152(3):391-397.
17. Moak DH, Anton RF, Latham PK, et al. Sertraline and cognitive behavioral therapy for depressed alcoholics: results of a placebo-controlled trial. J Clin Psychopharmacol. 2003;23(6):553-562.
18. Cornelius JR, Bukstein OG, Wood DS, et al. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905-909.
19. Roy-Byrne PP, Pages KP, Russo JE, et al. Nefazodone treatment of major depression in alcohol-dependent patients: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2000;20(2):129-136.
20. Hernandez-Avila CA, Modesto-Lowe V, Feinn R, et al. Nefazodone treatment of comorbid alcohol dependence and major depression. Alcohol Clin Exp Res. 2004;28(3):433-440.
21. Kranzler HR, Burleson JA, Brown J, et al. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20(9):1534-1541.
22. Naranjo CA, Bremner KE, Lanctôt KL. Effects of citalopram and a brief psycho-social intervention on alcohol intake dependence and problems. Addiction. 1995;90(1):87-99.
23. Pettinati HM, Volpicelli JR, Kranzler HR, et al. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24(7):1041-1049.
24. Mason BJ, Kocsis JH, Ritvo EC, et al. A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA. 1996;275(10):761-767.
25. McGrath PJ, Nunes EV, Stewart JW, et al. Imipramine treatment of alcoholics with primary depression: a placebo-controlled clinical trial. Arch Gen Psychiatry. 1996;53(3):232-240.
26. Altintoprak AE, Zorlu N, Coskunol H, et al. Effectiveness and tolerability of mirtazapine and amitriptyline in alcoholic patients with co-morbid depressive disorder: a randomized, double-blind study. Hum Psychopharmacol. 2008;23(4):313-319.
27. Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56(10):785-792.
28. Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887-1896.
29. Petrakis I, Ralevski E, Nich C, et al. Naltrexone and disulfiram in patients with alcohol dependence and current depression. J Clin Psychopharmacol. 2007;27(2):160-165.
30. Pettinati HM, Oslin DW, Kampman KM, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatry. 2010;167(6):668-675.
31. Petrakis IL, Poling J, Levinson C, et al. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
32. Muhonen LH, Lönnqvist J, Juva K, et al. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J Clin Psychiatry. 2008;69(3):392-399.
33. Liebrenz M, Borgeat A, Leisinger R, et al. Intravenous ketamine therapy in a patient with a treatment-resistant major depression. Swiss Med Wkly. 2007;137(15-16):234-236.
34. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol. 2007;27(4):344-351.
35. Croissant B, Klein O, Gehrlein L, et al. Quetiapine in relapse prevention in alcoholics suffering from craving and affective symptoms: a case series. Eur Psychiatry. 2006;21(8):570-573.
36. Martinotti G, Andreoli S, Di Nicola M, et al. Quetiapine decreases alcohol consumption, craving, and psychiatric symptoms in dually diagnosed alcoholics. Hum Psychopharmacol. 2008;23(5):417-424.
1. Hasin DS, Stinson FS, Ogburn E, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842.
2. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39(3):197-206.
3. Kessler RC, Crum RM, Warner LA, et al. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54(4):313-321.
4. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
5. Burns L, Teesson M, O’Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100(6):787-796.
6. Gilman SE, Abraham HD. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alcohol Depend. 2001;63(3):277-286.
7. Hasin DS, Tsai WY, Endicott J, et al. Five-year course of major depression: effects of comorbid alcoholism. J Affect Disord. 1996;41(1):63-70.
8. Mueller TI, Lavori PW, Keller MB, et al. Prognostic effect of the variable course of alcoholism on the 10-year course of depression. Am J Psychiatry. 1994;151(5):701-706.
9. Cornelius JR, Salloum IM, Mezzich J, et al. Disproportionate suicidality in patients with comorbid major depression and alcoholism. Am J Psychiatry. 1995;152(3):358-364.
10. Sher L, Oquendo MA, Galfalvy HC, et al. The relationship of aggression to suicidal behavior in depressed patients with a history of alcoholism. Addict Behav. 2005;30(6):1144-1153.
11. Greenfield SF, Weiss RD, Muenz LR, et al. The effect of depression on return to drinking: a prospective study. Arch Gen Psychiatry. 1998;55(3):259-265.
12. Brady KT, Verduin ML, Tolliver BK. Treatment of patients comorbid for addiction and other psychiatric disorders. Curr Psychiatry Rep. 2007;9(5):374-380.
13. Oslin DW. Treatment of late-life depression complicated by alcohol dependence. Am J Geriatr Psychiatry. 2005;13(6):491-500.
14. Cornelius JR, Salloum IM, Ehler JG, et al. Fluoxetine in depressed alcoholics. A double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1997;54(8):700-705.
15. Roy A. Placebo-controlled study of sertraline in depressed recently abstinent alcoholics. Biol Psychiatry. 1998;44(7):633-637.
16. Kranzler HR, Burleson JA, Korner P, et al. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995;152(3):391-397.
17. Moak DH, Anton RF, Latham PK, et al. Sertraline and cognitive behavioral therapy for depressed alcoholics: results of a placebo-controlled trial. J Clin Psychopharmacol. 2003;23(6):553-562.
18. Cornelius JR, Bukstein OG, Wood DS, et al. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905-909.
19. Roy-Byrne PP, Pages KP, Russo JE, et al. Nefazodone treatment of major depression in alcohol-dependent patients: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2000;20(2):129-136.
20. Hernandez-Avila CA, Modesto-Lowe V, Feinn R, et al. Nefazodone treatment of comorbid alcohol dependence and major depression. Alcohol Clin Exp Res. 2004;28(3):433-440.
21. Kranzler HR, Burleson JA, Brown J, et al. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20(9):1534-1541.
22. Naranjo CA, Bremner KE, Lanctôt KL. Effects of citalopram and a brief psycho-social intervention on alcohol intake dependence and problems. Addiction. 1995;90(1):87-99.
23. Pettinati HM, Volpicelli JR, Kranzler HR, et al. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24(7):1041-1049.
24. Mason BJ, Kocsis JH, Ritvo EC, et al. A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA. 1996;275(10):761-767.
25. McGrath PJ, Nunes EV, Stewart JW, et al. Imipramine treatment of alcoholics with primary depression: a placebo-controlled clinical trial. Arch Gen Psychiatry. 1996;53(3):232-240.
26. Altintoprak AE, Zorlu N, Coskunol H, et al. Effectiveness and tolerability of mirtazapine and amitriptyline in alcoholic patients with co-morbid depressive disorder: a randomized, double-blind study. Hum Psychopharmacol. 2008;23(4):313-319.
27. Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56(10):785-792.
28. Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887-1896.
29. Petrakis I, Ralevski E, Nich C, et al. Naltrexone and disulfiram in patients with alcohol dependence and current depression. J Clin Psychopharmacol. 2007;27(2):160-165.
30. Pettinati HM, Oslin DW, Kampman KM, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatry. 2010;167(6):668-675.
31. Petrakis IL, Poling J, Levinson C, et al. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
32. Muhonen LH, Lönnqvist J, Juva K, et al. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J Clin Psychiatry. 2008;69(3):392-399.
33. Liebrenz M, Borgeat A, Leisinger R, et al. Intravenous ketamine therapy in a patient with a treatment-resistant major depression. Swiss Med Wkly. 2007;137(15-16):234-236.
34. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol. 2007;27(4):344-351.
35. Croissant B, Klein O, Gehrlein L, et al. Quetiapine in relapse prevention in alcoholics suffering from craving and affective symptoms: a case series. Eur Psychiatry. 2006;21(8):570-573.
36. Martinotti G, Andreoli S, Di Nicola M, et al. Quetiapine decreases alcohol consumption, craving, and psychiatric symptoms in dually diagnosed alcoholics. Hum Psychopharmacol. 2008;23(5):417-424.
Paranoid, agitated, and manipulative
CASE: Agitation
Mrs. M, age 39, presents to the emergency department (ED) with altered mental status. She is escorted by her husband and the police. She has a history of severe alcohol dependence, bipolar disorder (BD), anxiety, borderline personality disorder (BPD), hypothyroidism, and bulimia, and had gastric bypass surgery 4 years ago. Her husband called 911 when he could no longer manage Mrs. M’s agitated state. The police found her to be extremely paranoid, restless, and disoriented. Her husband reports that she shouted “the world is going to end” before she escaped naked into her neighborhood streets.
On several occasions Mrs. M had been admitted to the same hospital for alcohol withdrawal and dependence with subsequent liver failure, leading to jaundice, coagulopathy, and ascites. During these hospitalizations, she exhibited poor behavioral tendencies, unhealthy psychological defenses, and chronic maladaptive coping and defense mechanisms congruent with her BPD diagnosis. Specifically, she engaged in splitting of hospital staff, ranging from extreme flattery to overt devaluation and hostility. Other defense mechanisms included denial, distortion, acting out, and passive-aggressive behavior. During these admissions, Mrs. M often displayed deficits in recall and attention on Mini-Mental State Examination (MMSE), but these deficits were associated with concurrent alcohol use and improved rapidly during her stay.
In her current presentation, Mrs. M’s mental status change is more pronounced and atypical compared with earlier admissions. Her outpatient medication regimen includes lamotrigine, 100 mg/d, levothyroxine, 88 mcg/d, venlafaxine extended release (XR), 75 mg/d, clonazepam, 3 mg/d, docusate as needed for constipation, and a daily multivitamin.
The authors’ observations
Delirium is a disturbance of consciousness manifested by a reduced clarity of awareness (impairment in attention) and change in cognition (impairment in orientation, memory, and language).1,2 The disturbance develops over a short time and tends to fluctuate during the day. Delirium is a direct physiological consequence of a general medical condition, substance use (intoxication or withdrawal), or both (Table).3
Delirium generally is a reversible mental disorder but can progress to irreversible brain damage. Prompt and accurate diagnosis of delirium is essential,4 although the condition often is underdiagnosed or misdiagnosed because of lack of recognition.
Table
DSM-IV-TR diagnostic criteria for delirium
|
| Source: Reference 3 |
Patients who have convoluted histories, such as Mrs. M, are common and difficult to manage and treat. These patients become substantially more complex when they are admitted to inpatient medical or surgical services. The need to clarify between delirium (primarily medical) and depression (primarily psychiatric) becomes paramount when administering treatment and evaluating decision-making capacity.5 In Mrs. M’s case, internal medicine, neurology, and psychiatry teams each had a different approach to altered mental status. Each team’s different terminology, assessment, and objectives further complicated an already challenging case.6
EVALUATION: Confounding results
The ED physicians offer a working diagnosis of acute mental status change, administer IV lorazepam, 4 mg, and order restraints for Mrs. M’s severe agitation. Her initial vital signs reveal slightly elevated blood pressure (140/90 mm Hg) and tachycardia (115 beats per minute). Internal medicine clinicians note that Mrs. M is not in acute distress, although she refuses to speak and has a small amount of dried blood on her lips, presumably from a struggle with the police before coming to the hospital, but this is not certain. Her abdomen is not tender; she has normal bowel sounds, and no asterixis is noted on neurologic exam. Physical exam is otherwise normal. A noncontrast head CT scan shows no acute process. Initial lab values show elevations in ammonia (277 μg/dL) and γ-glutamyl transpeptidase (68 U/L). Thyroid-stimulating hormone is 1.45 mlU/L, prothrombin time is 19.5 s, partial thromboplastin time is 40.3 s, and international normalized ratio is 1.67. The internal medicine team admits Mrs. M to the intensive care unit (ICU) for further management of her mental status change with alcohol withdrawal or hepatic encephalopathy as the most likely etiologies.
Mrs. M’s husband says that his wife has not consumed alcohol in the last 4 months in preparation for a possible liver transplant; however, past interactions with Mrs. M’s family suggest they are unreliable. The Clinical Institute Withdrawal Assessment (CIWA) protocol is implemented in case her symptoms are caused by alcohol withdrawal. Her vital signs are stable and IV lorazepam, 4 mg, is administered once for agitation. Mrs. M’s husband also reports that 1 month ago his wife underwent a transjugular intrahepatic portosystemic shunt (TIPS) procedure for portal hypertension. Outpatient psychotropics (lamotrigine, 100 mg/d, and venlafaxine XR, 75 mg/d) are restarted because withdrawal from these drugs may exacerbate her symptoms. In the ICU Mrs. M experiences a tonic-clonic seizure with fecal incontinence and bitten tongue, which results in a consultation from neurology and the psychiatry consultation-liaison service.
Psychiatry recommends withholding psychotropics, stopping CIWA, and using vital sign parameters along with objective signs of diaphoresis and tremors as indicators of alcohol withdrawal for lorazepam administration. Mrs. M receives IV haloperidol, 1 mg, once during her second day in the hospital for severe agitation, but this medication is discontinued because of concern about lowering her seizure threshold.7 After treatment with lactulose, her ammonia levels trend down to 33 μg/dL, but her altered mental state persists with significant deficits in attention and orientation.
The neurology service performs an EEG that shows no slow-wave, triphasic waves, or epileptiform activity, which likely would be present in delirium or seizures. See Figure 1 for an example of triphasic waves on an EEG and Figure 2 for Mrs. M's EEG results. Subsequent lumbar puncture, MRI, and a second EEG are unremarkable. By the fifth hospital day, Mrs. M is calm and her paranoia has subsided, but she still is confused and disoriented. Psychiatry orders a third EEG while she is in this confused state; it shows no pathologic process. Based on these examinations, neurology posits that Mrs. M is not encephalopathic.
Figure 1: Representative sample of triphasic waves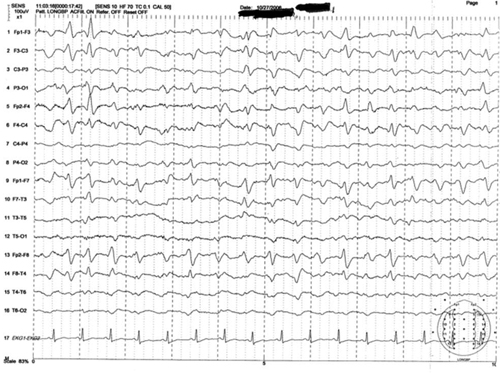
This EEG tracing is from a 54-year-old woman who underwent prolonged abdominal surgery for lysis of adhesions during which she suffered an intraoperative left subinsular stroke followed by nonconvulsive status epilepticus. The tracing demonstrates typical morphology with the positive sharp transient preceded and followed by smaller amplitude negative deflections. Symmetric, frontal predominance of findings seen is this tracing is common
Figure 2: Mrs. M’s EEG results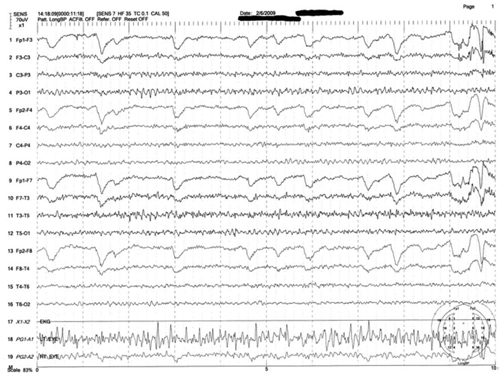
This is a representative tracing of Mrs. M’s 3 EEGs revealing an 8.5 to 9 Hz dominant alpha rhythm. There is superimposed frontally dominant beta fast activity, which is consistent with known administration of benzodiazepines
The authors’ observations
Mrs. M had repeated admissions for alcohol dependence and subsequent liver failure. Her recent hospitalization was complicated by a TIPS procedure done 1 month ago. The incidence of hepatic encephalopathy in patients undergoing TIPS is >30%, especially in the first month post-procedure, which raised suspicion that hepatic encephalopathy played a significant role in Mrs. M’s delirium.8
Because of frequent hospitalization, Mrs. M was well known to the internal medicine, neurology, and psychiatry teams, and each used different terms to describe her mental state. Internal medicine used the phrase “acute mental status change,” which covers a broad differential. Neurology used “encephalopathy,” which also is a general term. Psychiatry used “delirium,” which has narrower and more specific diagnostic criteria. Engel et al9 described the delirious patient as having “cerebral insufficiency” with universally abnormal EEG. Regardless of terminology, based on Mrs. M’s acute confusion, one would expect an abnormal EEG, but repeat EEGs were unremarkable.
Interpreting EEG
EEG is one of the few tools available for measuring acute changes in cerebral function, and an EEG slowing remains a hallmark in encephalopathic processes.10,11 Initially, the 3 specialties agreed that Mrs. M’s presentation likely was caused by underlying medical issues or substances (alcohol or others). EEG can help recognize delirium, and, in some cases, elucidate the underlying cause.10,12 It was surprising that Mrs. M’s EEGs were normal despite a clinical presentation of delirium. Because of the normal EEG findings, neurology leaned toward a primary psychiatric (“functional”) etiology as the cause of her delirium vs a general medical condition or alcohol withdrawal (“organic”).
A literature search in regards to sensitivity of EEG in delirium revealed conflicting statements and data. A standard textbook in neurology and psychiatry states that “a normal EEG virtually excludes a toxic-metabolic encephalopathy.”13 The American Psychiatric Association’s (APA) practice guidelines for delirium states: “The presence of EEG abnormalities has fairly good sensitivities for delirium (in one study, the sensitivity was found to be 75%), but the absence does not rule out the diagnosis; thus the EEG is no substitute for careful clinical observation.”6
At the beginning of Mrs. M’s care, in discussion with the neurology and internal medicine teams, we argued that Mrs. M was experiencing delirium despite her initial normal EEG. We did not expect that 2 subsequent EEGs would be normal, especially because the teams witnessed the final EEG being performed while Mrs. M was clinically evaluated and observed to be in a state of delirium.
OUTCOME: Cause still unknown
By the 6th day of hospitalization, Mrs. M’s vitals are normal and she remains hemodynamically stable. Differential diagnosis remains wide and unclear. The psychiatry team feels she could have atypical catatonia due to an underlying mood disorder. One hour after a trial of IV lorazepam, 1 mg, Mrs. M is more lucid and fully oriented, with MMSE of 28/30 (recall was 1/3), indicating normal cognition. During the exam, a psychiatry resident notes Mrs. M winks and feigns a yawn at the medical students and nurses in the room, displaying her boredom with the interview and simplicity of the mental status exam questions. Later that evening, Mrs. M exhibits bizarre sexual gestures toward male hospital staff, including licking a male nursing staff member’s hand.
Although Mrs. M’s initial confusion resolved, the severity of her comorbid psychiatric history warrants inpatient psychiatric hospitalization. She agrees to transfer to the psychiatric ward after she confesses anxiety regarding death, intense demoralization, and guilt related to her condition and her relationship with her 12-year-old daughter. She tearfully reports that she discontinued her psychotropic medications shortly after stopping alcohol 4 months ago. Shortly before her transfer, psychiatry is called back to the medicine floor because of Mrs. M’s disruptive behavior.
The team finds Mrs. M in her hospital gown, pursuing her husband in the hallway as he is leaving, yelling profanities and blaming him for her horrible experience in the hospital. Based on her demeanor, the team determines that she is back to her baseline mental state despite her mood disorder, and that her upcoming inpatient psychiatric stay likely would be too short to address her comorbid personality disorder. The next day she signs out of the hospital against medical advice.
The authors’ observations
We never clearly identified the specific etiology responsible for Mrs. M’s delirium. We assume at the initial presentation she had toxic-metabolic encephalopathy that rapidly resolved with lactulose treatment and lowering her ammonia. She then had a single tonic-clonic seizure, perhaps related to stopping and then restarting her psychotropics. Her subsequent confusion, bizarre sexual behavior, and demeanor on her final hospital days were more indicative of her psychiatric diagnoses. We now suspect that Mrs. M’s delirium was briefer than presumed and she returned to her baseline borderline personality, resulting in some factitious staging of delirium to confuse her 3 treating teams (a psychoanalyst may say this was a form of projective identification).
We felt that if Mrs. M truly was delirious due to metabolic or hepatic dysfunction or alcohol withdrawal, she would have had abnormal EEG findings. We discovered that the notion of “75% sensitivity” of EEG abnormalities cited in the APA guidelines comes from studies that include patients with “psychogenic” and “organic” delirium. Acute manias and agitated psychoses were termed “psychogenic delirium” and acute confusion due to medical conditions or substance issues was termed “organic delirium.”9,12,14-16
This poses a circular reasoning in the diagnostic criteria and clinical approach to delirium. The fallacy is that, according to DSM-IV-TR, delirium is supposed to be the result of a direct physiological consequence of a general medical condition or substance use (criterion D), and cannot be due to psychosis (eg, schizophrenia) or mania (eg, BD). We question the presumptive 75% sensitivity of EEG abnormalities in patients with delirium because it is possible that when some of these studies were conducted the definition of delirium was not solidified or fully understood. We suspect the sensitivity would be much higher if the correct definition of delirium according to DSM-IV-TR is used in future studies. To improve interdisciplinary communication and future research, it would be constructive if all disciplines could agree on a single term, with the same diagnostic criteria, when evaluating a patient with acute confusion.
Related Resources
- Meagher D. Delirium: the role of psychiatry. Advances in Psychiatric Treatment. 2001;7:433-442.
- Casey DA, DeFazio JV Jr, Vansickle K, et al. Delirium. Quick recognition, careful evaluation, and appropriate treatment. Postgrad Med. 1996;100(1):121-4, 128, 133-134.
Drug Brand Names
- Clonazepam • Klonopin
- Docusate • Surfak
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Levothyroxine • Levoxyl, Synthtoid
- Venlafaxine XR • Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the U.S. Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
1. Katz IR, Mossey J, Sussman N, et al. Bedside clinical and electrophysiological assessment: assessment of change in vulnerable patients. Int Psychogeriatr. 1991;3(2):289-300.
2. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. McPhee SJ, Papadakis M, Rabow MW. CURRENT medical diagnosis and treatment. New York NY: McGraw Hill Medical; 2012.
5. Brody B. Who has capacity? N Engl J Med. 2009;361(3):232-233.
6. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156(5 suppl):1-20.
7. Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165(7):803-812.
8. Sanyal AJ, Freedman AM, Shiffman ML, et al. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20(1 pt 1):46-55.
9. Engel GL, Romano J. Delirium a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. 2004;16(4):526-538.
10. Pro JD, Wells CE. The use of the electroencephalogram in the diagnosis of delirium. Dis Nerv Syst. 1977;38(10):804-808.
11. Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
12. Brenner RP. Utility of EEG in delirium: past views and current practice. Int Psychogeriatr. 1991;3(2):211-229.
13. Kaufman DM. Clinical neurology for psychiatrists. 5th ed. Philadelphia PA: Saunders; 2001: 230-232.
14. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
15. Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
16. Larson EW, Richelson E. Organic causes of mania. Mayo Clin Proc. 1988;63(9):906-912.
CASE: Agitation
Mrs. M, age 39, presents to the emergency department (ED) with altered mental status. She is escorted by her husband and the police. She has a history of severe alcohol dependence, bipolar disorder (BD), anxiety, borderline personality disorder (BPD), hypothyroidism, and bulimia, and had gastric bypass surgery 4 years ago. Her husband called 911 when he could no longer manage Mrs. M’s agitated state. The police found her to be extremely paranoid, restless, and disoriented. Her husband reports that she shouted “the world is going to end” before she escaped naked into her neighborhood streets.
On several occasions Mrs. M had been admitted to the same hospital for alcohol withdrawal and dependence with subsequent liver failure, leading to jaundice, coagulopathy, and ascites. During these hospitalizations, she exhibited poor behavioral tendencies, unhealthy psychological defenses, and chronic maladaptive coping and defense mechanisms congruent with her BPD diagnosis. Specifically, she engaged in splitting of hospital staff, ranging from extreme flattery to overt devaluation and hostility. Other defense mechanisms included denial, distortion, acting out, and passive-aggressive behavior. During these admissions, Mrs. M often displayed deficits in recall and attention on Mini-Mental State Examination (MMSE), but these deficits were associated with concurrent alcohol use and improved rapidly during her stay.
In her current presentation, Mrs. M’s mental status change is more pronounced and atypical compared with earlier admissions. Her outpatient medication regimen includes lamotrigine, 100 mg/d, levothyroxine, 88 mcg/d, venlafaxine extended release (XR), 75 mg/d, clonazepam, 3 mg/d, docusate as needed for constipation, and a daily multivitamin.
The authors’ observations
Delirium is a disturbance of consciousness manifested by a reduced clarity of awareness (impairment in attention) and change in cognition (impairment in orientation, memory, and language).1,2 The disturbance develops over a short time and tends to fluctuate during the day. Delirium is a direct physiological consequence of a general medical condition, substance use (intoxication or withdrawal), or both (Table).3
Delirium generally is a reversible mental disorder but can progress to irreversible brain damage. Prompt and accurate diagnosis of delirium is essential,4 although the condition often is underdiagnosed or misdiagnosed because of lack of recognition.
Table
DSM-IV-TR diagnostic criteria for delirium
|
| Source: Reference 3 |
Patients who have convoluted histories, such as Mrs. M, are common and difficult to manage and treat. These patients become substantially more complex when they are admitted to inpatient medical or surgical services. The need to clarify between delirium (primarily medical) and depression (primarily psychiatric) becomes paramount when administering treatment and evaluating decision-making capacity.5 In Mrs. M’s case, internal medicine, neurology, and psychiatry teams each had a different approach to altered mental status. Each team’s different terminology, assessment, and objectives further complicated an already challenging case.6
EVALUATION: Confounding results
The ED physicians offer a working diagnosis of acute mental status change, administer IV lorazepam, 4 mg, and order restraints for Mrs. M’s severe agitation. Her initial vital signs reveal slightly elevated blood pressure (140/90 mm Hg) and tachycardia (115 beats per minute). Internal medicine clinicians note that Mrs. M is not in acute distress, although she refuses to speak and has a small amount of dried blood on her lips, presumably from a struggle with the police before coming to the hospital, but this is not certain. Her abdomen is not tender; she has normal bowel sounds, and no asterixis is noted on neurologic exam. Physical exam is otherwise normal. A noncontrast head CT scan shows no acute process. Initial lab values show elevations in ammonia (277 μg/dL) and γ-glutamyl transpeptidase (68 U/L). Thyroid-stimulating hormone is 1.45 mlU/L, prothrombin time is 19.5 s, partial thromboplastin time is 40.3 s, and international normalized ratio is 1.67. The internal medicine team admits Mrs. M to the intensive care unit (ICU) for further management of her mental status change with alcohol withdrawal or hepatic encephalopathy as the most likely etiologies.
Mrs. M’s husband says that his wife has not consumed alcohol in the last 4 months in preparation for a possible liver transplant; however, past interactions with Mrs. M’s family suggest they are unreliable. The Clinical Institute Withdrawal Assessment (CIWA) protocol is implemented in case her symptoms are caused by alcohol withdrawal. Her vital signs are stable and IV lorazepam, 4 mg, is administered once for agitation. Mrs. M’s husband also reports that 1 month ago his wife underwent a transjugular intrahepatic portosystemic shunt (TIPS) procedure for portal hypertension. Outpatient psychotropics (lamotrigine, 100 mg/d, and venlafaxine XR, 75 mg/d) are restarted because withdrawal from these drugs may exacerbate her symptoms. In the ICU Mrs. M experiences a tonic-clonic seizure with fecal incontinence and bitten tongue, which results in a consultation from neurology and the psychiatry consultation-liaison service.
Psychiatry recommends withholding psychotropics, stopping CIWA, and using vital sign parameters along with objective signs of diaphoresis and tremors as indicators of alcohol withdrawal for lorazepam administration. Mrs. M receives IV haloperidol, 1 mg, once during her second day in the hospital for severe agitation, but this medication is discontinued because of concern about lowering her seizure threshold.7 After treatment with lactulose, her ammonia levels trend down to 33 μg/dL, but her altered mental state persists with significant deficits in attention and orientation.
The neurology service performs an EEG that shows no slow-wave, triphasic waves, or epileptiform activity, which likely would be present in delirium or seizures. See Figure 1 for an example of triphasic waves on an EEG and Figure 2 for Mrs. M's EEG results. Subsequent lumbar puncture, MRI, and a second EEG are unremarkable. By the fifth hospital day, Mrs. M is calm and her paranoia has subsided, but she still is confused and disoriented. Psychiatry orders a third EEG while she is in this confused state; it shows no pathologic process. Based on these examinations, neurology posits that Mrs. M is not encephalopathic.
Figure 1: Representative sample of triphasic waves
This EEG tracing is from a 54-year-old woman who underwent prolonged abdominal surgery for lysis of adhesions during which she suffered an intraoperative left subinsular stroke followed by nonconvulsive status epilepticus. The tracing demonstrates typical morphology with the positive sharp transient preceded and followed by smaller amplitude negative deflections. Symmetric, frontal predominance of findings seen is this tracing is common
Figure 2: Mrs. M’s EEG results
This is a representative tracing of Mrs. M’s 3 EEGs revealing an 8.5 to 9 Hz dominant alpha rhythm. There is superimposed frontally dominant beta fast activity, which is consistent with known administration of benzodiazepines
The authors’ observations
Mrs. M had repeated admissions for alcohol dependence and subsequent liver failure. Her recent hospitalization was complicated by a TIPS procedure done 1 month ago. The incidence of hepatic encephalopathy in patients undergoing TIPS is >30%, especially in the first month post-procedure, which raised suspicion that hepatic encephalopathy played a significant role in Mrs. M’s delirium.8
Because of frequent hospitalization, Mrs. M was well known to the internal medicine, neurology, and psychiatry teams, and each used different terms to describe her mental state. Internal medicine used the phrase “acute mental status change,” which covers a broad differential. Neurology used “encephalopathy,” which also is a general term. Psychiatry used “delirium,” which has narrower and more specific diagnostic criteria. Engel et al9 described the delirious patient as having “cerebral insufficiency” with universally abnormal EEG. Regardless of terminology, based on Mrs. M’s acute confusion, one would expect an abnormal EEG, but repeat EEGs were unremarkable.
Interpreting EEG
EEG is one of the few tools available for measuring acute changes in cerebral function, and an EEG slowing remains a hallmark in encephalopathic processes.10,11 Initially, the 3 specialties agreed that Mrs. M’s presentation likely was caused by underlying medical issues or substances (alcohol or others). EEG can help recognize delirium, and, in some cases, elucidate the underlying cause.10,12 It was surprising that Mrs. M’s EEGs were normal despite a clinical presentation of delirium. Because of the normal EEG findings, neurology leaned toward a primary psychiatric (“functional”) etiology as the cause of her delirium vs a general medical condition or alcohol withdrawal (“organic”).
A literature search in regards to sensitivity of EEG in delirium revealed conflicting statements and data. A standard textbook in neurology and psychiatry states that “a normal EEG virtually excludes a toxic-metabolic encephalopathy.”13 The American Psychiatric Association’s (APA) practice guidelines for delirium states: “The presence of EEG abnormalities has fairly good sensitivities for delirium (in one study, the sensitivity was found to be 75%), but the absence does not rule out the diagnosis; thus the EEG is no substitute for careful clinical observation.”6
At the beginning of Mrs. M’s care, in discussion with the neurology and internal medicine teams, we argued that Mrs. M was experiencing delirium despite her initial normal EEG. We did not expect that 2 subsequent EEGs would be normal, especially because the teams witnessed the final EEG being performed while Mrs. M was clinically evaluated and observed to be in a state of delirium.
OUTCOME: Cause still unknown
By the 6th day of hospitalization, Mrs. M’s vitals are normal and she remains hemodynamically stable. Differential diagnosis remains wide and unclear. The psychiatry team feels she could have atypical catatonia due to an underlying mood disorder. One hour after a trial of IV lorazepam, 1 mg, Mrs. M is more lucid and fully oriented, with MMSE of 28/30 (recall was 1/3), indicating normal cognition. During the exam, a psychiatry resident notes Mrs. M winks and feigns a yawn at the medical students and nurses in the room, displaying her boredom with the interview and simplicity of the mental status exam questions. Later that evening, Mrs. M exhibits bizarre sexual gestures toward male hospital staff, including licking a male nursing staff member’s hand.
Although Mrs. M’s initial confusion resolved, the severity of her comorbid psychiatric history warrants inpatient psychiatric hospitalization. She agrees to transfer to the psychiatric ward after she confesses anxiety regarding death, intense demoralization, and guilt related to her condition and her relationship with her 12-year-old daughter. She tearfully reports that she discontinued her psychotropic medications shortly after stopping alcohol 4 months ago. Shortly before her transfer, psychiatry is called back to the medicine floor because of Mrs. M’s disruptive behavior.
The team finds Mrs. M in her hospital gown, pursuing her husband in the hallway as he is leaving, yelling profanities and blaming him for her horrible experience in the hospital. Based on her demeanor, the team determines that she is back to her baseline mental state despite her mood disorder, and that her upcoming inpatient psychiatric stay likely would be too short to address her comorbid personality disorder. The next day she signs out of the hospital against medical advice.
The authors’ observations
We never clearly identified the specific etiology responsible for Mrs. M’s delirium. We assume at the initial presentation she had toxic-metabolic encephalopathy that rapidly resolved with lactulose treatment and lowering her ammonia. She then had a single tonic-clonic seizure, perhaps related to stopping and then restarting her psychotropics. Her subsequent confusion, bizarre sexual behavior, and demeanor on her final hospital days were more indicative of her psychiatric diagnoses. We now suspect that Mrs. M’s delirium was briefer than presumed and she returned to her baseline borderline personality, resulting in some factitious staging of delirium to confuse her 3 treating teams (a psychoanalyst may say this was a form of projective identification).
We felt that if Mrs. M truly was delirious due to metabolic or hepatic dysfunction or alcohol withdrawal, she would have had abnormal EEG findings. We discovered that the notion of “75% sensitivity” of EEG abnormalities cited in the APA guidelines comes from studies that include patients with “psychogenic” and “organic” delirium. Acute manias and agitated psychoses were termed “psychogenic delirium” and acute confusion due to medical conditions or substance issues was termed “organic delirium.”9,12,14-16
This poses a circular reasoning in the diagnostic criteria and clinical approach to delirium. The fallacy is that, according to DSM-IV-TR, delirium is supposed to be the result of a direct physiological consequence of a general medical condition or substance use (criterion D), and cannot be due to psychosis (eg, schizophrenia) or mania (eg, BD). We question the presumptive 75% sensitivity of EEG abnormalities in patients with delirium because it is possible that when some of these studies were conducted the definition of delirium was not solidified or fully understood. We suspect the sensitivity would be much higher if the correct definition of delirium according to DSM-IV-TR is used in future studies. To improve interdisciplinary communication and future research, it would be constructive if all disciplines could agree on a single term, with the same diagnostic criteria, when evaluating a patient with acute confusion.
Related Resources
- Meagher D. Delirium: the role of psychiatry. Advances in Psychiatric Treatment. 2001;7:433-442.
- Casey DA, DeFazio JV Jr, Vansickle K, et al. Delirium. Quick recognition, careful evaluation, and appropriate treatment. Postgrad Med. 1996;100(1):121-4, 128, 133-134.
Drug Brand Names
- Clonazepam • Klonopin
- Docusate • Surfak
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Levothyroxine • Levoxyl, Synthtoid
- Venlafaxine XR • Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the U.S. Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
CASE: Agitation
Mrs. M, age 39, presents to the emergency department (ED) with altered mental status. She is escorted by her husband and the police. She has a history of severe alcohol dependence, bipolar disorder (BD), anxiety, borderline personality disorder (BPD), hypothyroidism, and bulimia, and had gastric bypass surgery 4 years ago. Her husband called 911 when he could no longer manage Mrs. M’s agitated state. The police found her to be extremely paranoid, restless, and disoriented. Her husband reports that she shouted “the world is going to end” before she escaped naked into her neighborhood streets.
On several occasions Mrs. M had been admitted to the same hospital for alcohol withdrawal and dependence with subsequent liver failure, leading to jaundice, coagulopathy, and ascites. During these hospitalizations, she exhibited poor behavioral tendencies, unhealthy psychological defenses, and chronic maladaptive coping and defense mechanisms congruent with her BPD diagnosis. Specifically, she engaged in splitting of hospital staff, ranging from extreme flattery to overt devaluation and hostility. Other defense mechanisms included denial, distortion, acting out, and passive-aggressive behavior. During these admissions, Mrs. M often displayed deficits in recall and attention on Mini-Mental State Examination (MMSE), but these deficits were associated with concurrent alcohol use and improved rapidly during her stay.
In her current presentation, Mrs. M’s mental status change is more pronounced and atypical compared with earlier admissions. Her outpatient medication regimen includes lamotrigine, 100 mg/d, levothyroxine, 88 mcg/d, venlafaxine extended release (XR), 75 mg/d, clonazepam, 3 mg/d, docusate as needed for constipation, and a daily multivitamin.
The authors’ observations
Delirium is a disturbance of consciousness manifested by a reduced clarity of awareness (impairment in attention) and change in cognition (impairment in orientation, memory, and language).1,2 The disturbance develops over a short time and tends to fluctuate during the day. Delirium is a direct physiological consequence of a general medical condition, substance use (intoxication or withdrawal), or both (Table).3
Delirium generally is a reversible mental disorder but can progress to irreversible brain damage. Prompt and accurate diagnosis of delirium is essential,4 although the condition often is underdiagnosed or misdiagnosed because of lack of recognition.
Table
DSM-IV-TR diagnostic criteria for delirium
|
| Source: Reference 3 |
Patients who have convoluted histories, such as Mrs. M, are common and difficult to manage and treat. These patients become substantially more complex when they are admitted to inpatient medical or surgical services. The need to clarify between delirium (primarily medical) and depression (primarily psychiatric) becomes paramount when administering treatment and evaluating decision-making capacity.5 In Mrs. M’s case, internal medicine, neurology, and psychiatry teams each had a different approach to altered mental status. Each team’s different terminology, assessment, and objectives further complicated an already challenging case.6
EVALUATION: Confounding results
The ED physicians offer a working diagnosis of acute mental status change, administer IV lorazepam, 4 mg, and order restraints for Mrs. M’s severe agitation. Her initial vital signs reveal slightly elevated blood pressure (140/90 mm Hg) and tachycardia (115 beats per minute). Internal medicine clinicians note that Mrs. M is not in acute distress, although she refuses to speak and has a small amount of dried blood on her lips, presumably from a struggle with the police before coming to the hospital, but this is not certain. Her abdomen is not tender; she has normal bowel sounds, and no asterixis is noted on neurologic exam. Physical exam is otherwise normal. A noncontrast head CT scan shows no acute process. Initial lab values show elevations in ammonia (277 μg/dL) and γ-glutamyl transpeptidase (68 U/L). Thyroid-stimulating hormone is 1.45 mlU/L, prothrombin time is 19.5 s, partial thromboplastin time is 40.3 s, and international normalized ratio is 1.67. The internal medicine team admits Mrs. M to the intensive care unit (ICU) for further management of her mental status change with alcohol withdrawal or hepatic encephalopathy as the most likely etiologies.
Mrs. M’s husband says that his wife has not consumed alcohol in the last 4 months in preparation for a possible liver transplant; however, past interactions with Mrs. M’s family suggest they are unreliable. The Clinical Institute Withdrawal Assessment (CIWA) protocol is implemented in case her symptoms are caused by alcohol withdrawal. Her vital signs are stable and IV lorazepam, 4 mg, is administered once for agitation. Mrs. M’s husband also reports that 1 month ago his wife underwent a transjugular intrahepatic portosystemic shunt (TIPS) procedure for portal hypertension. Outpatient psychotropics (lamotrigine, 100 mg/d, and venlafaxine XR, 75 mg/d) are restarted because withdrawal from these drugs may exacerbate her symptoms. In the ICU Mrs. M experiences a tonic-clonic seizure with fecal incontinence and bitten tongue, which results in a consultation from neurology and the psychiatry consultation-liaison service.
Psychiatry recommends withholding psychotropics, stopping CIWA, and using vital sign parameters along with objective signs of diaphoresis and tremors as indicators of alcohol withdrawal for lorazepam administration. Mrs. M receives IV haloperidol, 1 mg, once during her second day in the hospital for severe agitation, but this medication is discontinued because of concern about lowering her seizure threshold.7 After treatment with lactulose, her ammonia levels trend down to 33 μg/dL, but her altered mental state persists with significant deficits in attention and orientation.
The neurology service performs an EEG that shows no slow-wave, triphasic waves, or epileptiform activity, which likely would be present in delirium or seizures. See Figure 1 for an example of triphasic waves on an EEG and Figure 2 for Mrs. M's EEG results. Subsequent lumbar puncture, MRI, and a second EEG are unremarkable. By the fifth hospital day, Mrs. M is calm and her paranoia has subsided, but she still is confused and disoriented. Psychiatry orders a third EEG while she is in this confused state; it shows no pathologic process. Based on these examinations, neurology posits that Mrs. M is not encephalopathic.
Figure 1: Representative sample of triphasic waves
This EEG tracing is from a 54-year-old woman who underwent prolonged abdominal surgery for lysis of adhesions during which she suffered an intraoperative left subinsular stroke followed by nonconvulsive status epilepticus. The tracing demonstrates typical morphology with the positive sharp transient preceded and followed by smaller amplitude negative deflections. Symmetric, frontal predominance of findings seen is this tracing is common
Figure 2: Mrs. M’s EEG results
This is a representative tracing of Mrs. M’s 3 EEGs revealing an 8.5 to 9 Hz dominant alpha rhythm. There is superimposed frontally dominant beta fast activity, which is consistent with known administration of benzodiazepines
The authors’ observations
Mrs. M had repeated admissions for alcohol dependence and subsequent liver failure. Her recent hospitalization was complicated by a TIPS procedure done 1 month ago. The incidence of hepatic encephalopathy in patients undergoing TIPS is >30%, especially in the first month post-procedure, which raised suspicion that hepatic encephalopathy played a significant role in Mrs. M’s delirium.8
Because of frequent hospitalization, Mrs. M was well known to the internal medicine, neurology, and psychiatry teams, and each used different terms to describe her mental state. Internal medicine used the phrase “acute mental status change,” which covers a broad differential. Neurology used “encephalopathy,” which also is a general term. Psychiatry used “delirium,” which has narrower and more specific diagnostic criteria. Engel et al9 described the delirious patient as having “cerebral insufficiency” with universally abnormal EEG. Regardless of terminology, based on Mrs. M’s acute confusion, one would expect an abnormal EEG, but repeat EEGs were unremarkable.
Interpreting EEG
EEG is one of the few tools available for measuring acute changes in cerebral function, and an EEG slowing remains a hallmark in encephalopathic processes.10,11 Initially, the 3 specialties agreed that Mrs. M’s presentation likely was caused by underlying medical issues or substances (alcohol or others). EEG can help recognize delirium, and, in some cases, elucidate the underlying cause.10,12 It was surprising that Mrs. M’s EEGs were normal despite a clinical presentation of delirium. Because of the normal EEG findings, neurology leaned toward a primary psychiatric (“functional”) etiology as the cause of her delirium vs a general medical condition or alcohol withdrawal (“organic”).
A literature search in regards to sensitivity of EEG in delirium revealed conflicting statements and data. A standard textbook in neurology and psychiatry states that “a normal EEG virtually excludes a toxic-metabolic encephalopathy.”13 The American Psychiatric Association’s (APA) practice guidelines for delirium states: “The presence of EEG abnormalities has fairly good sensitivities for delirium (in one study, the sensitivity was found to be 75%), but the absence does not rule out the diagnosis; thus the EEG is no substitute for careful clinical observation.”6
At the beginning of Mrs. M’s care, in discussion with the neurology and internal medicine teams, we argued that Mrs. M was experiencing delirium despite her initial normal EEG. We did not expect that 2 subsequent EEGs would be normal, especially because the teams witnessed the final EEG being performed while Mrs. M was clinically evaluated and observed to be in a state of delirium.
OUTCOME: Cause still unknown
By the 6th day of hospitalization, Mrs. M’s vitals are normal and she remains hemodynamically stable. Differential diagnosis remains wide and unclear. The psychiatry team feels she could have atypical catatonia due to an underlying mood disorder. One hour after a trial of IV lorazepam, 1 mg, Mrs. M is more lucid and fully oriented, with MMSE of 28/30 (recall was 1/3), indicating normal cognition. During the exam, a psychiatry resident notes Mrs. M winks and feigns a yawn at the medical students and nurses in the room, displaying her boredom with the interview and simplicity of the mental status exam questions. Later that evening, Mrs. M exhibits bizarre sexual gestures toward male hospital staff, including licking a male nursing staff member’s hand.
Although Mrs. M’s initial confusion resolved, the severity of her comorbid psychiatric history warrants inpatient psychiatric hospitalization. She agrees to transfer to the psychiatric ward after she confesses anxiety regarding death, intense demoralization, and guilt related to her condition and her relationship with her 12-year-old daughter. She tearfully reports that she discontinued her psychotropic medications shortly after stopping alcohol 4 months ago. Shortly before her transfer, psychiatry is called back to the medicine floor because of Mrs. M’s disruptive behavior.
The team finds Mrs. M in her hospital gown, pursuing her husband in the hallway as he is leaving, yelling profanities and blaming him for her horrible experience in the hospital. Based on her demeanor, the team determines that she is back to her baseline mental state despite her mood disorder, and that her upcoming inpatient psychiatric stay likely would be too short to address her comorbid personality disorder. The next day she signs out of the hospital against medical advice.
The authors’ observations
We never clearly identified the specific etiology responsible for Mrs. M’s delirium. We assume at the initial presentation she had toxic-metabolic encephalopathy that rapidly resolved with lactulose treatment and lowering her ammonia. She then had a single tonic-clonic seizure, perhaps related to stopping and then restarting her psychotropics. Her subsequent confusion, bizarre sexual behavior, and demeanor on her final hospital days were more indicative of her psychiatric diagnoses. We now suspect that Mrs. M’s delirium was briefer than presumed and she returned to her baseline borderline personality, resulting in some factitious staging of delirium to confuse her 3 treating teams (a psychoanalyst may say this was a form of projective identification).
We felt that if Mrs. M truly was delirious due to metabolic or hepatic dysfunction or alcohol withdrawal, she would have had abnormal EEG findings. We discovered that the notion of “75% sensitivity” of EEG abnormalities cited in the APA guidelines comes from studies that include patients with “psychogenic” and “organic” delirium. Acute manias and agitated psychoses were termed “psychogenic delirium” and acute confusion due to medical conditions or substance issues was termed “organic delirium.”9,12,14-16
This poses a circular reasoning in the diagnostic criteria and clinical approach to delirium. The fallacy is that, according to DSM-IV-TR, delirium is supposed to be the result of a direct physiological consequence of a general medical condition or substance use (criterion D), and cannot be due to psychosis (eg, schizophrenia) or mania (eg, BD). We question the presumptive 75% sensitivity of EEG abnormalities in patients with delirium because it is possible that when some of these studies were conducted the definition of delirium was not solidified or fully understood. We suspect the sensitivity would be much higher if the correct definition of delirium according to DSM-IV-TR is used in future studies. To improve interdisciplinary communication and future research, it would be constructive if all disciplines could agree on a single term, with the same diagnostic criteria, when evaluating a patient with acute confusion.
Related Resources
- Meagher D. Delirium: the role of psychiatry. Advances in Psychiatric Treatment. 2001;7:433-442.
- Casey DA, DeFazio JV Jr, Vansickle K, et al. Delirium. Quick recognition, careful evaluation, and appropriate treatment. Postgrad Med. 1996;100(1):121-4, 128, 133-134.
Drug Brand Names
- Clonazepam • Klonopin
- Docusate • Surfak
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Levothyroxine • Levoxyl, Synthtoid
- Venlafaxine XR • Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the U.S. Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
1. Katz IR, Mossey J, Sussman N, et al. Bedside clinical and electrophysiological assessment: assessment of change in vulnerable patients. Int Psychogeriatr. 1991;3(2):289-300.
2. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. McPhee SJ, Papadakis M, Rabow MW. CURRENT medical diagnosis and treatment. New York NY: McGraw Hill Medical; 2012.
5. Brody B. Who has capacity? N Engl J Med. 2009;361(3):232-233.
6. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156(5 suppl):1-20.
7. Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165(7):803-812.
8. Sanyal AJ, Freedman AM, Shiffman ML, et al. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20(1 pt 1):46-55.
9. Engel GL, Romano J. Delirium a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. 2004;16(4):526-538.
10. Pro JD, Wells CE. The use of the electroencephalogram in the diagnosis of delirium. Dis Nerv Syst. 1977;38(10):804-808.
11. Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
12. Brenner RP. Utility of EEG in delirium: past views and current practice. Int Psychogeriatr. 1991;3(2):211-229.
13. Kaufman DM. Clinical neurology for psychiatrists. 5th ed. Philadelphia PA: Saunders; 2001: 230-232.
14. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
15. Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
16. Larson EW, Richelson E. Organic causes of mania. Mayo Clin Proc. 1988;63(9):906-912.
1. Katz IR, Mossey J, Sussman N, et al. Bedside clinical and electrophysiological assessment: assessment of change in vulnerable patients. Int Psychogeriatr. 1991;3(2):289-300.
2. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. McPhee SJ, Papadakis M, Rabow MW. CURRENT medical diagnosis and treatment. New York NY: McGraw Hill Medical; 2012.
5. Brody B. Who has capacity? N Engl J Med. 2009;361(3):232-233.
6. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156(5 suppl):1-20.
7. Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165(7):803-812.
8. Sanyal AJ, Freedman AM, Shiffman ML, et al. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20(1 pt 1):46-55.
9. Engel GL, Romano J. Delirium a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. 2004;16(4):526-538.
10. Pro JD, Wells CE. The use of the electroencephalogram in the diagnosis of delirium. Dis Nerv Syst. 1977;38(10):804-808.
11. Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
12. Brenner RP. Utility of EEG in delirium: past views and current practice. Int Psychogeriatr. 1991;3(2):211-229.
13. Kaufman DM. Clinical neurology for psychiatrists. 5th ed. Philadelphia PA: Saunders; 2001: 230-232.
14. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
15. Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
16. Larson EW, Richelson E. Organic causes of mania. Mayo Clin Proc. 1988;63(9):906-912.

