User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Thick chart syndrome: Treatment resistance is our greatest challenge
We all have patients with thick charts, the mentally ill individuals who push our clinical skills to the limit. They respond poorly to the entire algorithm of approved medications for depression, anxiety, or psychosis. Their symptoms hardly budge despite multiple psychotherapeutic interventions. They lead lives of quiet desperation and suffer through many hospitalizations and outpatient visits. They are perennially at high risk for harm to self or others. They get many side effects yet meager benefits from pharmacotherapy. Their social and vocational functions often are minimal to nil. Their life has little meaning beyond doleful patienthood.
Too complicated to be managed by primary care providers and most mental health practitioners, treatment-resistant patients often have several psychiatric comorbidities—both axis I and II. They frequently suffer from axis III disorders as well. Their lack of tangible response (let alone remission) frustrates us. Their poor treatment course and outcomes eventually tempt us to resort to unapproved polypharmacy and other non evidence-based practices in a desperate effort to help them.
We worry about our persistently unimproved patients; they haunt our thoughts after work. They are a constant reminder of how critical it is for our field to conduct aggressive, relentless research to unravel the underlying biology of chronic nonresponsive, disabling psychiatric brain disorders that rob children, adults, and elderly persons of their potential or even the ability to pursue happiness. We long for treatment breakthroughs that may reverse the downward spiral of their tortured lives.
Treatment resistance in my long-suffering patients incites me to ask important questions that beg for answers, such as:
- Are treatment-resistant patients afflicted by a categorically different subtype of illness, or do they suffer from a more severe form of the illness (ie, a dimensional difference)?
- Are some treatment-resistant patients victims of misdiagnosis? Do they have a psychiatric illness secondary to an unrecognized general medical condition that fails to respond to standard psychiatric treatments (such as a lack of response to several antidepressants in a patient with hypothyroid-induced depression or lack of efficacy of neuroleptics in psychosis secondary to a porphyria or Niemann-Pick disease)?
- Why isn’t the pharmaceutical industry conducting trials that target treatment-resistant patients? Controlled research trials in all clinical drug development programs for psychotropic medications explicitly exclude patients with a history of nonresponse. Thus, if a drug proves to be superior to placebo in FDA trials, it is likely to have efficacy in responsive patients but not in patients who have a history of nonresponse to prior medications
- Why are there no FDA studies of combination therapy—using drugs with different mechanisms of action—jointly sponsored (where necessary) by 2 or more pharmaceutical companies? Evidence-based, FDA -approved combinations are common for severe hypertension, diabetes, and cardiovascular disease; why not for severe psychiatric disorders?
- Why are personality disorders and psychiatric comorbidities more likely in treatment-resistant patients, and is this a neurobiologic clue for our nosologic/diagnostic framework and an impetus for better and innovative drug development?
- Why isn’t more funding from the National Institute of Mental Health targeting treatment-resistant diagnostic groups? Effective solutions for treatment-resistant populations, whose care often is very expensive, can be extremely beneficial for the affected individuals as well as substantially cost-effective for society at large.
Until my questions can be answered—and better treatment options emerge for my treatment-resistant patients—I will continue to do my best to relieve their agony and anguish. I will then write more progress notes and add yet more sheets of paper to their already thick charts.
We all have patients with thick charts, the mentally ill individuals who push our clinical skills to the limit. They respond poorly to the entire algorithm of approved medications for depression, anxiety, or psychosis. Their symptoms hardly budge despite multiple psychotherapeutic interventions. They lead lives of quiet desperation and suffer through many hospitalizations and outpatient visits. They are perennially at high risk for harm to self or others. They get many side effects yet meager benefits from pharmacotherapy. Their social and vocational functions often are minimal to nil. Their life has little meaning beyond doleful patienthood.
Too complicated to be managed by primary care providers and most mental health practitioners, treatment-resistant patients often have several psychiatric comorbidities—both axis I and II. They frequently suffer from axis III disorders as well. Their lack of tangible response (let alone remission) frustrates us. Their poor treatment course and outcomes eventually tempt us to resort to unapproved polypharmacy and other non evidence-based practices in a desperate effort to help them.
We worry about our persistently unimproved patients; they haunt our thoughts after work. They are a constant reminder of how critical it is for our field to conduct aggressive, relentless research to unravel the underlying biology of chronic nonresponsive, disabling psychiatric brain disorders that rob children, adults, and elderly persons of their potential or even the ability to pursue happiness. We long for treatment breakthroughs that may reverse the downward spiral of their tortured lives.
Treatment resistance in my long-suffering patients incites me to ask important questions that beg for answers, such as:
- Are treatment-resistant patients afflicted by a categorically different subtype of illness, or do they suffer from a more severe form of the illness (ie, a dimensional difference)?
- Are some treatment-resistant patients victims of misdiagnosis? Do they have a psychiatric illness secondary to an unrecognized general medical condition that fails to respond to standard psychiatric treatments (such as a lack of response to several antidepressants in a patient with hypothyroid-induced depression or lack of efficacy of neuroleptics in psychosis secondary to a porphyria or Niemann-Pick disease)?
- Why isn’t the pharmaceutical industry conducting trials that target treatment-resistant patients? Controlled research trials in all clinical drug development programs for psychotropic medications explicitly exclude patients with a history of nonresponse. Thus, if a drug proves to be superior to placebo in FDA trials, it is likely to have efficacy in responsive patients but not in patients who have a history of nonresponse to prior medications
- Why are there no FDA studies of combination therapy—using drugs with different mechanisms of action—jointly sponsored (where necessary) by 2 or more pharmaceutical companies? Evidence-based, FDA -approved combinations are common for severe hypertension, diabetes, and cardiovascular disease; why not for severe psychiatric disorders?
- Why are personality disorders and psychiatric comorbidities more likely in treatment-resistant patients, and is this a neurobiologic clue for our nosologic/diagnostic framework and an impetus for better and innovative drug development?
- Why isn’t more funding from the National Institute of Mental Health targeting treatment-resistant diagnostic groups? Effective solutions for treatment-resistant populations, whose care often is very expensive, can be extremely beneficial for the affected individuals as well as substantially cost-effective for society at large.
Until my questions can be answered—and better treatment options emerge for my treatment-resistant patients—I will continue to do my best to relieve their agony and anguish. I will then write more progress notes and add yet more sheets of paper to their already thick charts.
We all have patients with thick charts, the mentally ill individuals who push our clinical skills to the limit. They respond poorly to the entire algorithm of approved medications for depression, anxiety, or psychosis. Their symptoms hardly budge despite multiple psychotherapeutic interventions. They lead lives of quiet desperation and suffer through many hospitalizations and outpatient visits. They are perennially at high risk for harm to self or others. They get many side effects yet meager benefits from pharmacotherapy. Their social and vocational functions often are minimal to nil. Their life has little meaning beyond doleful patienthood.
Too complicated to be managed by primary care providers and most mental health practitioners, treatment-resistant patients often have several psychiatric comorbidities—both axis I and II. They frequently suffer from axis III disorders as well. Their lack of tangible response (let alone remission) frustrates us. Their poor treatment course and outcomes eventually tempt us to resort to unapproved polypharmacy and other non evidence-based practices in a desperate effort to help them.
We worry about our persistently unimproved patients; they haunt our thoughts after work. They are a constant reminder of how critical it is for our field to conduct aggressive, relentless research to unravel the underlying biology of chronic nonresponsive, disabling psychiatric brain disorders that rob children, adults, and elderly persons of their potential or even the ability to pursue happiness. We long for treatment breakthroughs that may reverse the downward spiral of their tortured lives.
Treatment resistance in my long-suffering patients incites me to ask important questions that beg for answers, such as:
- Are treatment-resistant patients afflicted by a categorically different subtype of illness, or do they suffer from a more severe form of the illness (ie, a dimensional difference)?
- Are some treatment-resistant patients victims of misdiagnosis? Do they have a psychiatric illness secondary to an unrecognized general medical condition that fails to respond to standard psychiatric treatments (such as a lack of response to several antidepressants in a patient with hypothyroid-induced depression or lack of efficacy of neuroleptics in psychosis secondary to a porphyria or Niemann-Pick disease)?
- Why isn’t the pharmaceutical industry conducting trials that target treatment-resistant patients? Controlled research trials in all clinical drug development programs for psychotropic medications explicitly exclude patients with a history of nonresponse. Thus, if a drug proves to be superior to placebo in FDA trials, it is likely to have efficacy in responsive patients but not in patients who have a history of nonresponse to prior medications
- Why are there no FDA studies of combination therapy—using drugs with different mechanisms of action—jointly sponsored (where necessary) by 2 or more pharmaceutical companies? Evidence-based, FDA -approved combinations are common for severe hypertension, diabetes, and cardiovascular disease; why not for severe psychiatric disorders?
- Why are personality disorders and psychiatric comorbidities more likely in treatment-resistant patients, and is this a neurobiologic clue for our nosologic/diagnostic framework and an impetus for better and innovative drug development?
- Why isn’t more funding from the National Institute of Mental Health targeting treatment-resistant diagnostic groups? Effective solutions for treatment-resistant populations, whose care often is very expensive, can be extremely beneficial for the affected individuals as well as substantially cost-effective for society at large.
Until my questions can be answered—and better treatment options emerge for my treatment-resistant patients—I will continue to do my best to relieve their agony and anguish. I will then write more progress notes and add yet more sheets of paper to their already thick charts.
Borderline or bipolar? Don't skimp on the life story
Guanfacine extended release for ADHD
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants
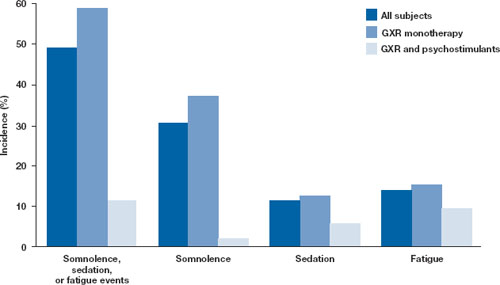
In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants

In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants

In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
Sad Dad: Identify depression in new fathers
After the birth of a child, family changes can put fathers at risk for postpartum depression. Long recognized as a problem affecting some new mothers, postpartum depression also can grip men. Ten percent of new fathers and 14% of new mothers are affected by depression.1 Still, most men and their partners fail to recognize postpartum depression—characterized by mood changes after a baby is born—when it arises.
Different causes, similar symptoms
Symptoms of postpartum depression are similar in both sexes, but the causes may be different. Hormonal changes contribute to women’s suffering, whereas sudden and unexpected lifestyle changes are thought to trigger fathers’ depression.
After the birth of a child, a father might not get the same attention from his partner and sexual activity may be reduced. His sleep is affected, and he may feel pressure to work longer hours to provide for the family economically.2 Some fathers may believe the child is a binding force in an unsatisfactory marriage.3
Depressed new dads—like depressed men in general—are more likely than depressed women to engage in destructive behaviors, including alcohol or drug abuse, angry outbursts, or taking unnecessary risks such as reckless driving or extramarital sex. Other signs to look for include depressed mood, loss of interest or pleasure, weight gain or loss, oversleeping or insomnia, restlessness, fatigue, feelings of worthlessness or guilt, impaired concentration, and thoughts of suicide or death.
Treatment
Postpartum depression can began within days or weeks of a child’s delivery and can last one year or more. In both sexes, it can be successfully treated with psycho therapy, medication, or both. The family’s involvement is critical to identifying depression in a new father. Often, the woman will be the first to notice her partner’s depression. A history of depression or mental illness and having a spouse with postpartum depression increases a father’s risk of depression.
1. Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics. 2006;118(2):659-668.
2. Scarton D. Post-partum depression strikes new dads, too. US News and World Report. May 21, 2008. Available at: http://health.usnews.com/articles/health/sexual-reproductive/2008/05/21/postpartum-depression-strikes-new-fathers-too.html. Accessed October 23, 2009.
3. Sadock BJ, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:869.
After the birth of a child, family changes can put fathers at risk for postpartum depression. Long recognized as a problem affecting some new mothers, postpartum depression also can grip men. Ten percent of new fathers and 14% of new mothers are affected by depression.1 Still, most men and their partners fail to recognize postpartum depression—characterized by mood changes after a baby is born—when it arises.
Different causes, similar symptoms
Symptoms of postpartum depression are similar in both sexes, but the causes may be different. Hormonal changes contribute to women’s suffering, whereas sudden and unexpected lifestyle changes are thought to trigger fathers’ depression.
After the birth of a child, a father might not get the same attention from his partner and sexual activity may be reduced. His sleep is affected, and he may feel pressure to work longer hours to provide for the family economically.2 Some fathers may believe the child is a binding force in an unsatisfactory marriage.3
Depressed new dads—like depressed men in general—are more likely than depressed women to engage in destructive behaviors, including alcohol or drug abuse, angry outbursts, or taking unnecessary risks such as reckless driving or extramarital sex. Other signs to look for include depressed mood, loss of interest or pleasure, weight gain or loss, oversleeping or insomnia, restlessness, fatigue, feelings of worthlessness or guilt, impaired concentration, and thoughts of suicide or death.
Treatment
Postpartum depression can began within days or weeks of a child’s delivery and can last one year or more. In both sexes, it can be successfully treated with psycho therapy, medication, or both. The family’s involvement is critical to identifying depression in a new father. Often, the woman will be the first to notice her partner’s depression. A history of depression or mental illness and having a spouse with postpartum depression increases a father’s risk of depression.
After the birth of a child, family changes can put fathers at risk for postpartum depression. Long recognized as a problem affecting some new mothers, postpartum depression also can grip men. Ten percent of new fathers and 14% of new mothers are affected by depression.1 Still, most men and their partners fail to recognize postpartum depression—characterized by mood changes after a baby is born—when it arises.
Different causes, similar symptoms
Symptoms of postpartum depression are similar in both sexes, but the causes may be different. Hormonal changes contribute to women’s suffering, whereas sudden and unexpected lifestyle changes are thought to trigger fathers’ depression.
After the birth of a child, a father might not get the same attention from his partner and sexual activity may be reduced. His sleep is affected, and he may feel pressure to work longer hours to provide for the family economically.2 Some fathers may believe the child is a binding force in an unsatisfactory marriage.3
Depressed new dads—like depressed men in general—are more likely than depressed women to engage in destructive behaviors, including alcohol or drug abuse, angry outbursts, or taking unnecessary risks such as reckless driving or extramarital sex. Other signs to look for include depressed mood, loss of interest or pleasure, weight gain or loss, oversleeping or insomnia, restlessness, fatigue, feelings of worthlessness or guilt, impaired concentration, and thoughts of suicide or death.
Treatment
Postpartum depression can began within days or weeks of a child’s delivery and can last one year or more. In both sexes, it can be successfully treated with psycho therapy, medication, or both. The family’s involvement is critical to identifying depression in a new father. Often, the woman will be the first to notice her partner’s depression. A history of depression or mental illness and having a spouse with postpartum depression increases a father’s risk of depression.
1. Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics. 2006;118(2):659-668.
2. Scarton D. Post-partum depression strikes new dads, too. US News and World Report. May 21, 2008. Available at: http://health.usnews.com/articles/health/sexual-reproductive/2008/05/21/postpartum-depression-strikes-new-fathers-too.html. Accessed October 23, 2009.
3. Sadock BJ, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:869.
1. Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics. 2006;118(2):659-668.
2. Scarton D. Post-partum depression strikes new dads, too. US News and World Report. May 21, 2008. Available at: http://health.usnews.com/articles/health/sexual-reproductive/2008/05/21/postpartum-depression-strikes-new-fathers-too.html. Accessed October 23, 2009.
3. Sadock BJ, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:869.
Terrifying visions
CASE: Seeing things
Family members bring Mrs. L, age 82, to the emergency room (ER) because she is agitated, nervous, and carries a knife “for protection.” In the past few months, she has been seeing things her family could not, such as bugs in her food and people trying to break into her house. Mrs. L becomes increasingly frightened and angry because her family denies seeing these things. Her family is concerned she might hurt herself or others.
Despite some hearing loss, Mrs. L had been relatively healthy and independent until a few years ago, when her vision decreased secondary to age-related macular degeneration and diabetic retinopathy. In addition to sensory impairment and diabetes mellitus, her medical history includes mild hypothyroidism and intervertebral disc herniation. She has no history of liver disease or alcohol or substance abuse. A few weeks ago Mrs. L’s primary care physician began treating her with donepezil, 5 mg/d, because he suspected dementia was causing her hallucinations. Otherwise, she has no psychiatric history.
On exam, Mrs. L is easily directable and cooperative. She seems angry because no one believes her; she reports seeing a cat that nobody else could see in the ER immediately before being evaluated. She is frightened because she believes her hallucinations are real, although she is unable to explain them. Mrs. L reports feeling anxious most of the time and having difficulty sleeping because of her fears. She also feels sad and occasionally worthless because she cannot see or hear as well as when she was younger.
A mental status examination shows partial impairment of concentration and short-term memory, but Mrs. L is alert and oriented. No theme of delusions is detected. She has no physical complaints, and physical examination is unremarkable.
The authors’ observations
Mrs. L presented with new-onset agitation, visual hallucinations, and mildly decreased concentration and short-term memory. Our next step after history and examination was to perform laboratory testing to narrow the diagnosis ( Table 1 ).
A basic electrolyte panel including kidney function can point toward electrolyte imbalance or uremia as a cause of delirium. Mrs. L’s basic metabolic panel and liver function were normal. Urinalysis ruled out urinary tract infection.
Mrs. L’s thyroid-stimulating hormone (TSH) level was mildly elevated at 5.6 mU/L (in our laboratory, the upper normal limit is 5.2 mU/L). Hypothyroidism and hyperthyroidism are not associated with hallucinations, but hyperthyroidism is an important medical cause of anxiety and hypothyroidism can cause a dementia-like presentation. CT of the head to rule out a space-occupying lesion or acute process—such as cerebrovascular accident—shows only chronic vascular changes.
Based on Mrs. L’s history, physical examination, and lab results, we provisionally diagnose dementia, Alzheimer’s type with psychotic features, and prescribe quetiapine, 25 mg at bedtime. We offer to admit Mrs. L, but she and her family prefer close outpatient follow-up. After discussing safety concerns and pharmacotherapy with the patient and her family, we discharge Mrs. L home and advise her to follow up with the psychiatric clinic.
Table 1
Suggested workup for elderly patients with hallucinations
| History and physical exam |
| History of dementia, mood disorder, Parkinson’s disease, or drug abuse |
| Presence of delusions or mood/anxiety symptoms |
| Detailed medication history |
| Level of consciousness, alertness, and cognitive function assessment (eg, MMSE) |
| Vital signs (instability may reflect delirium, meningitis/encephalitis, or intoxication) |
| Physical exam (may confirm acute medical illness causing delirium) |
| Neurologic exam (may show focal neurologic signs reflecting space-occupying lesion, signs of Parkinson’s disease, vitamin B12 deficiency) |
| Ophthalmologic history/exam |
| Investigations |
| Electrolyte imbalance, especially calcium |
| Glucose level |
| Uremia, impaired liver function, and increased ammonia |
| CBC |
| Urine drug screen |
| Urinalysis, culture, and sensitivity |
| Additional tests |
| VDRL |
| Arterial blood gas |
| ECG and cardiac enzymes |
| Chest radiography |
| Vitamin B12/folate |
| TSH |
| EEG |
| Serum drug levels |
| CT/MRI of the head |
| Lumbar puncture and cerebrospinal fluid analysis |
| Heavy metal screen |
| HIV screen |
| CBC: complete blood count; CT: computed tomography; ECG: electrocardiography; EEG: electroencephalography; HIV: human immunodeficiency virus; MMSE: Mini-Mental State Exam; MRI: magnetic resonance imaging; TSH: thyroid-stimulating hormone; VDRL: venereal disease research laboratory |
EVALUATION: Lasting hallucinations
Quetiapine improves Mrs. L’s sleep and agitation but does not reduce her hallucinations. She sees a helicopter planting wires on a tree next to her house, a snake in the house, children in her room (some are “beautiful”), fire, and creatures with scary faces. These hallucinations occur mostly when she is alone. She denies hearing or touching the things she sees but continues to feel fear and anxiety when she sees them, although this diminishes with education and reassurance.
The authors’ observations
In Mrs. L’s subsequent psychiatry clinic visits, we gather additional information that helped us rule out several differential diagnoses ( Table 2 ).
The most common causes of new-onset psychosis in later life are:
- dementia-related syndromes with psychosis, delirium, or drug-induced psychosis
- primary psychiatric disorders, most commonly depression.1
Alzheimer’s disease has been associated with up to a 60% incidence of psychotic symptoms at some point in the disease course.2 Although Mrs. L’s short-term memory had declined in recent years, she does not have aphasia, apraxia, agnosia, or decrease in mental executive functioning to meet Alzheimer’s dementia criteria.
Perceptual disturbance is a feature of delirium that can cause agitation and hallucinations in elderly patients. However, Mrs. L did not have a decreased level of consciousness or an acute medical illness that would explain delirium.
Despite Mrs. L’s symptoms and progressive hearing and vision loss with resultant disability, she generally was organized in terms of basic self-care, hygiene, and activities of daily living. She was able to have a conversation when she could hear the physician. Surprisingly, her Mini-Mental State Examination (MMSE) score was within normal limits or only mildly impaired at office visits. This was not compatible with the initial diagnosis of dementia, although it may suggest mild cognitive impairment.
Mrs. L took donepezil as prescribed by her primary care physician for only a few weeks before we stopped it. We attributed Mrs. L’s slightly impaired concentration and short-term memory in the ER to the anxiety and stress of oscillating visual hallucinations.
Schizophrenia is another cause of psychosis, but Mrs. L had no history of negative symptoms, delusions, disorganized speech/behavior, or family history of psychotic disorders. In addition, schizophrenia is most likely to appear in a patient’s third decade. Although more common in women than men, late-onset schizophrenia—defined as onset after age 40—has a 1-year prevalence rate of 0.6%3 and therefore is an unlikely cause of Mrs. L’s symptoms.
Mrs. L had no history of neurologic deficit to suggest cerebrovascular disease or space-occupying brain lesion. Her TSH, which was slightly increased when she presented in the ER, was normal on subsequent testing. Folic acid and vitamin B12 were normal. We ordered brain MRI to rule out organic causes not seen with CT, but Mrs. L felt claustrophobic in the machine and could not finish the test.
EEG was ordered to rule out epilepsy. Hallucinations can be a prominent component of seizures and are more common when the seizure focus is in the left temporal lobe.4 However, the development of psychotic symptoms often follows the onset of seizures by approximately 14 to 17 years.5 Although Mrs. L never obtained the ordered EEG, the absence of a history of clinical seizures or focal neurologic signs makes it unlikely that epilepsy accounted for her hallucinations. The normal workup ruled out most possible medical/organic causes of Mrs. L’s visual hallucinations.
We considered depression with psychotic features because Mrs. L had occasional depressed mood, feelings of worthlessness, and low self-esteem. These symptoms started only after she began losing her vision and hearing and she did not experience them most of the time. Furthermore, her predominant negative feeling was anxiety related to the hallucinations. Mrs. L had no other depressive symptoms such as guilt or loss of appetite.
Table 2
Differential diagnosis of hallucinations in elderly patients
| Diagnosis | Comments |
|---|---|
| Delirium | Secondary to a generalized medical condition, substance-induced, or substance withdrawal |
| Dementia | Alzheimer’s, vascular, Lewy body, or less common types |
| Parkinson’s disease | Medications can induce visual hallucinations |
| Brain tumor/mass/CVA | Usually accompanied by other neurologic symptoms and signs |
| Schizophrenia | Usually starts in early adulthood |
| Mood disorder with psychotic features | Depression can present as pseudodementia in elderly patients |
| Drug abuse/withdrawal or side effect | Numerous medications are known to worsen delirium in elderly patients |
| Other causes | HIV, tertiary syphilis, Charles Bonnet syndrome |
| CVA: cerebrovascular accident; HIV: human immunodeficiency virus | |
A diagnosis of exclusion
We began to suspect that Mrs. L had Charles Bonnet syndrome (CBS), a condition in which visually impaired persons experience visual hallucinations without other known mental illnesses. These hallucinations tend to be complex, vivid, and elaborate, lasting from a few seconds to most of the day.6,7 CBS occurs in 10% to 15% of patients with visual impairment, including up to 3.5% of elderly patients referred to psychiatrists for visual hallucinations.7,8 CBS is most common among the elderly because of the high prevalence of visual impairment in this population.
Many patients with CBS are aware that their hallucinations are not real.7 Mrs. L’s presentation was atypical because she believed what she was seeing was real and because most images were terrifying, which also is not usually the case in CBS.
CBS frequently goes unrecognized in clinical practice.9 Patients who admit to experiencing hallucinations often are labeled demented or psychotic.10 The course of CBS is categorized in 3 patterns:
- episodic (in this least common pattern, hallucinations occur over days to months and then resolve)
- periodic (hallucinatory activity alternates with phases of remission)
- continuous (patients experience no hallucination-free intervals).7,11
The pathophysiology of CBS is not fully understood. The deafferentation hypothesis suggests that reduced or absent visual system stimulation leads to increased excitability of areas of the cerebral cortex associated with vision, resulting in phantom vision.6,7,12
CBS has no universally accepted diagnostic criteria; it is a diagnosis of exclusion. Because we ruled out medical and organic causes, dementia, delirium, schizophrenia, and depression with psychotic features—and because Mrs. L had advanced macular degeneration and retinopathy—we believed CBS was a likely diagnosis. We referred her to an ophthalmologist, who confirmed the CBS diagnosis.
TREATMENT: Temporary improvement
We prescribe low-dose lorazepam, 0.5 to 1 mg every 8 hours as needed for agitation, and increase quetiapine to 50 mg up to twice a day as needed. These approaches fail because of excessive sedation and delayed onset of action in relation to the fast onset of Mrs. L’s hallucinations.
Based on a published report, we prescribe gabapentin, 100 mg bid, which seems to help Mrs. L. For several months, her hallucinations are reduced, and she occasionally experiences a hallucination-free day. After several months, however, the frequency of her hallucinations increases. Mrs. L refuses to take a higher dosage of gabapentin because she doesn’t like “a lot of medicine.”
Her cognitive function remains mostly stable over the next few months, with an MMSE score of 23+/-1, which is equal to 25.6 +/- 1 when corrected for unperformed tasks secondary to severe visual impairment. She develops no aphasia, apraxia, or agnosia.
Educating Mrs. L about her illness—reassuring her that she is not “crazy”—helped to decrease her anxiety, as did teaching her family to acknowledge the hallucinations and react appropriately. Mrs. L’s hallucinations are less frequent when she interacts with other people and more frequent when she is alone with less sensory stimulation. Although Mrs. L has not yet recovered, a low dose of gabapentin temporarily decreased hallucinations and anxiety.
The authors’ observations
CBS treatment is based mostly on case reports. No pharmacologic treatment is universally effective, but anticonvulsants may help reduce hallucinations.7 Low-dose gabapentin is reported to have produced permanent remission.13
Patients may benefit from using magnifiers and other low-vision devices to maximize residual sight. Increased social interaction and brighter lighting also may help.7 Reassuring the patient that the hallucinations are not real and do not indicate mental illness can be strongly therapeutic.7 Hallucinations may resolve spontaneously, with improved vision, or with increased social interaction.7
Related Resource
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48:58-72.
Drug Brand Names
- Donepezil • Aricept
- Gabapentin • Neurontin
- Lorazepam • Ativan
- Quetiapine • Seroquel
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Brown FW. Late-life psychosis: making the diagnosis and controlling symptoms. Geriatrics. 1998;53:26-42.
2. Lautenschlager NT, Forstl H. Organic psychosis. Curr Psychiatry Rep. 2001;3:319-325.
3. Mortensen PB, Pedersen CB, Westergaard T, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603-608.
4. Roberts GW, Done DJ, Bruton C, et al. A “mock up” of schizophrenia: temporal lobe epilepsy and schizophrenia-like psychosis. Biol Psychiatry. 1990;28:127-143.
5. Bruton CJ, Stevens JR, Frith CD. Epilepsy, psychosis, and schizophrenia: clinical and neuropathologic correlations. Neurology. 1994;44:34-42.
6. Jacob A, Prasad S, Boggild M, et al. Charles Bonnet syndrome—elderly people and visual hallucinations. BMJ. 2004;328(7455):1552-1554.
7. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48:58-72.
8. O’Reilly R, Chamberlaine C. Charles Bonnet syndrome: incidence and demographic and clinical features. Can J Psychiatry. 1996;41(4):259-260.
9. Brown GC, Murphy RP. Visual symptoms associated with choroidal neovascularization. Photopsias and the Charles Bonnet syndrome. Arch Ophthalmol. 1992;110(9):1251-1256.
10. Hart J. Phantom visions: real enough to touch. Elder Care. 1997;9(1):30-32.
11. de Morsier G. Le Syndrome de Charles Bonnet: hallucinations visuelles des viellards sans deficience mentale. Ann Med Psychol. 1967;125:677-702.
12. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121:1819-1840.
13. Paulig M, Mentrup H. Charles Bonnet’s syndrome: complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
CASE: Seeing things
Family members bring Mrs. L, age 82, to the emergency room (ER) because she is agitated, nervous, and carries a knife “for protection.” In the past few months, she has been seeing things her family could not, such as bugs in her food and people trying to break into her house. Mrs. L becomes increasingly frightened and angry because her family denies seeing these things. Her family is concerned she might hurt herself or others.
Despite some hearing loss, Mrs. L had been relatively healthy and independent until a few years ago, when her vision decreased secondary to age-related macular degeneration and diabetic retinopathy. In addition to sensory impairment and diabetes mellitus, her medical history includes mild hypothyroidism and intervertebral disc herniation. She has no history of liver disease or alcohol or substance abuse. A few weeks ago Mrs. L’s primary care physician began treating her with donepezil, 5 mg/d, because he suspected dementia was causing her hallucinations. Otherwise, she has no psychiatric history.
On exam, Mrs. L is easily directable and cooperative. She seems angry because no one believes her; she reports seeing a cat that nobody else could see in the ER immediately before being evaluated. She is frightened because she believes her hallucinations are real, although she is unable to explain them. Mrs. L reports feeling anxious most of the time and having difficulty sleeping because of her fears. She also feels sad and occasionally worthless because she cannot see or hear as well as when she was younger.
A mental status examination shows partial impairment of concentration and short-term memory, but Mrs. L is alert and oriented. No theme of delusions is detected. She has no physical complaints, and physical examination is unremarkable.
The authors’ observations
Mrs. L presented with new-onset agitation, visual hallucinations, and mildly decreased concentration and short-term memory. Our next step after history and examination was to perform laboratory testing to narrow the diagnosis ( Table 1 ).
A basic electrolyte panel including kidney function can point toward electrolyte imbalance or uremia as a cause of delirium. Mrs. L’s basic metabolic panel and liver function were normal. Urinalysis ruled out urinary tract infection.
Mrs. L’s thyroid-stimulating hormone (TSH) level was mildly elevated at 5.6 mU/L (in our laboratory, the upper normal limit is 5.2 mU/L). Hypothyroidism and hyperthyroidism are not associated with hallucinations, but hyperthyroidism is an important medical cause of anxiety and hypothyroidism can cause a dementia-like presentation. CT of the head to rule out a space-occupying lesion or acute process—such as cerebrovascular accident—shows only chronic vascular changes.
Based on Mrs. L’s history, physical examination, and lab results, we provisionally diagnose dementia, Alzheimer’s type with psychotic features, and prescribe quetiapine, 25 mg at bedtime. We offer to admit Mrs. L, but she and her family prefer close outpatient follow-up. After discussing safety concerns and pharmacotherapy with the patient and her family, we discharge Mrs. L home and advise her to follow up with the psychiatric clinic.
Table 1
Suggested workup for elderly patients with hallucinations
| History and physical exam |
| History of dementia, mood disorder, Parkinson’s disease, or drug abuse |
| Presence of delusions or mood/anxiety symptoms |
| Detailed medication history |
| Level of consciousness, alertness, and cognitive function assessment (eg, MMSE) |
| Vital signs (instability may reflect delirium, meningitis/encephalitis, or intoxication) |
| Physical exam (may confirm acute medical illness causing delirium) |
| Neurologic exam (may show focal neurologic signs reflecting space-occupying lesion, signs of Parkinson’s disease, vitamin B12 deficiency) |
| Ophthalmologic history/exam |
| Investigations |
| Electrolyte imbalance, especially calcium |
| Glucose level |
| Uremia, impaired liver function, and increased ammonia |
| CBC |
| Urine drug screen |
| Urinalysis, culture, and sensitivity |
| Additional tests |
| VDRL |
| Arterial blood gas |
| ECG and cardiac enzymes |
| Chest radiography |
| Vitamin B12/folate |
| TSH |
| EEG |
| Serum drug levels |
| CT/MRI of the head |
| Lumbar puncture and cerebrospinal fluid analysis |
| Heavy metal screen |
| HIV screen |
| CBC: complete blood count; CT: computed tomography; ECG: electrocardiography; EEG: electroencephalography; HIV: human immunodeficiency virus; MMSE: Mini-Mental State Exam; MRI: magnetic resonance imaging; TSH: thyroid-stimulating hormone; VDRL: venereal disease research laboratory |
EVALUATION: Lasting hallucinations
Quetiapine improves Mrs. L’s sleep and agitation but does not reduce her hallucinations. She sees a helicopter planting wires on a tree next to her house, a snake in the house, children in her room (some are “beautiful”), fire, and creatures with scary faces. These hallucinations occur mostly when she is alone. She denies hearing or touching the things she sees but continues to feel fear and anxiety when she sees them, although this diminishes with education and reassurance.
The authors’ observations
In Mrs. L’s subsequent psychiatry clinic visits, we gather additional information that helped us rule out several differential diagnoses ( Table 2 ).
The most common causes of new-onset psychosis in later life are:
- dementia-related syndromes with psychosis, delirium, or drug-induced psychosis
- primary psychiatric disorders, most commonly depression.1
Alzheimer’s disease has been associated with up to a 60% incidence of psychotic symptoms at some point in the disease course.2 Although Mrs. L’s short-term memory had declined in recent years, she does not have aphasia, apraxia, agnosia, or decrease in mental executive functioning to meet Alzheimer’s dementia criteria.
Perceptual disturbance is a feature of delirium that can cause agitation and hallucinations in elderly patients. However, Mrs. L did not have a decreased level of consciousness or an acute medical illness that would explain delirium.
Despite Mrs. L’s symptoms and progressive hearing and vision loss with resultant disability, she generally was organized in terms of basic self-care, hygiene, and activities of daily living. She was able to have a conversation when she could hear the physician. Surprisingly, her Mini-Mental State Examination (MMSE) score was within normal limits or only mildly impaired at office visits. This was not compatible with the initial diagnosis of dementia, although it may suggest mild cognitive impairment.
Mrs. L took donepezil as prescribed by her primary care physician for only a few weeks before we stopped it. We attributed Mrs. L’s slightly impaired concentration and short-term memory in the ER to the anxiety and stress of oscillating visual hallucinations.
Schizophrenia is another cause of psychosis, but Mrs. L had no history of negative symptoms, delusions, disorganized speech/behavior, or family history of psychotic disorders. In addition, schizophrenia is most likely to appear in a patient’s third decade. Although more common in women than men, late-onset schizophrenia—defined as onset after age 40—has a 1-year prevalence rate of 0.6%3 and therefore is an unlikely cause of Mrs. L’s symptoms.
Mrs. L had no history of neurologic deficit to suggest cerebrovascular disease or space-occupying brain lesion. Her TSH, which was slightly increased when she presented in the ER, was normal on subsequent testing. Folic acid and vitamin B12 were normal. We ordered brain MRI to rule out organic causes not seen with CT, but Mrs. L felt claustrophobic in the machine and could not finish the test.
EEG was ordered to rule out epilepsy. Hallucinations can be a prominent component of seizures and are more common when the seizure focus is in the left temporal lobe.4 However, the development of psychotic symptoms often follows the onset of seizures by approximately 14 to 17 years.5 Although Mrs. L never obtained the ordered EEG, the absence of a history of clinical seizures or focal neurologic signs makes it unlikely that epilepsy accounted for her hallucinations. The normal workup ruled out most possible medical/organic causes of Mrs. L’s visual hallucinations.
We considered depression with psychotic features because Mrs. L had occasional depressed mood, feelings of worthlessness, and low self-esteem. These symptoms started only after she began losing her vision and hearing and she did not experience them most of the time. Furthermore, her predominant negative feeling was anxiety related to the hallucinations. Mrs. L had no other depressive symptoms such as guilt or loss of appetite.
Table 2
Differential diagnosis of hallucinations in elderly patients
| Diagnosis | Comments |
|---|---|
| Delirium | Secondary to a generalized medical condition, substance-induced, or substance withdrawal |
| Dementia | Alzheimer’s, vascular, Lewy body, or less common types |
| Parkinson’s disease | Medications can induce visual hallucinations |
| Brain tumor/mass/CVA | Usually accompanied by other neurologic symptoms and signs |
| Schizophrenia | Usually starts in early adulthood |
| Mood disorder with psychotic features | Depression can present as pseudodementia in elderly patients |
| Drug abuse/withdrawal or side effect | Numerous medications are known to worsen delirium in elderly patients |
| Other causes | HIV, tertiary syphilis, Charles Bonnet syndrome |
| CVA: cerebrovascular accident; HIV: human immunodeficiency virus | |
A diagnosis of exclusion
We began to suspect that Mrs. L had Charles Bonnet syndrome (CBS), a condition in which visually impaired persons experience visual hallucinations without other known mental illnesses. These hallucinations tend to be complex, vivid, and elaborate, lasting from a few seconds to most of the day.6,7 CBS occurs in 10% to 15% of patients with visual impairment, including up to 3.5% of elderly patients referred to psychiatrists for visual hallucinations.7,8 CBS is most common among the elderly because of the high prevalence of visual impairment in this population.
Many patients with CBS are aware that their hallucinations are not real.7 Mrs. L’s presentation was atypical because she believed what she was seeing was real and because most images were terrifying, which also is not usually the case in CBS.
CBS frequently goes unrecognized in clinical practice.9 Patients who admit to experiencing hallucinations often are labeled demented or psychotic.10 The course of CBS is categorized in 3 patterns:
- episodic (in this least common pattern, hallucinations occur over days to months and then resolve)
- periodic (hallucinatory activity alternates with phases of remission)
- continuous (patients experience no hallucination-free intervals).7,11
The pathophysiology of CBS is not fully understood. The deafferentation hypothesis suggests that reduced or absent visual system stimulation leads to increased excitability of areas of the cerebral cortex associated with vision, resulting in phantom vision.6,7,12
CBS has no universally accepted diagnostic criteria; it is a diagnosis of exclusion. Because we ruled out medical and organic causes, dementia, delirium, schizophrenia, and depression with psychotic features—and because Mrs. L had advanced macular degeneration and retinopathy—we believed CBS was a likely diagnosis. We referred her to an ophthalmologist, who confirmed the CBS diagnosis.
TREATMENT: Temporary improvement
We prescribe low-dose lorazepam, 0.5 to 1 mg every 8 hours as needed for agitation, and increase quetiapine to 50 mg up to twice a day as needed. These approaches fail because of excessive sedation and delayed onset of action in relation to the fast onset of Mrs. L’s hallucinations.
Based on a published report, we prescribe gabapentin, 100 mg bid, which seems to help Mrs. L. For several months, her hallucinations are reduced, and she occasionally experiences a hallucination-free day. After several months, however, the frequency of her hallucinations increases. Mrs. L refuses to take a higher dosage of gabapentin because she doesn’t like “a lot of medicine.”
Her cognitive function remains mostly stable over the next few months, with an MMSE score of 23+/-1, which is equal to 25.6 +/- 1 when corrected for unperformed tasks secondary to severe visual impairment. She develops no aphasia, apraxia, or agnosia.
Educating Mrs. L about her illness—reassuring her that she is not “crazy”—helped to decrease her anxiety, as did teaching her family to acknowledge the hallucinations and react appropriately. Mrs. L’s hallucinations are less frequent when she interacts with other people and more frequent when she is alone with less sensory stimulation. Although Mrs. L has not yet recovered, a low dose of gabapentin temporarily decreased hallucinations and anxiety.
The authors’ observations
CBS treatment is based mostly on case reports. No pharmacologic treatment is universally effective, but anticonvulsants may help reduce hallucinations.7 Low-dose gabapentin is reported to have produced permanent remission.13
Patients may benefit from using magnifiers and other low-vision devices to maximize residual sight. Increased social interaction and brighter lighting also may help.7 Reassuring the patient that the hallucinations are not real and do not indicate mental illness can be strongly therapeutic.7 Hallucinations may resolve spontaneously, with improved vision, or with increased social interaction.7
Related Resource
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48:58-72.
Drug Brand Names
- Donepezil • Aricept
- Gabapentin • Neurontin
- Lorazepam • Ativan
- Quetiapine • Seroquel
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Seeing things
Family members bring Mrs. L, age 82, to the emergency room (ER) because she is agitated, nervous, and carries a knife “for protection.” In the past few months, she has been seeing things her family could not, such as bugs in her food and people trying to break into her house. Mrs. L becomes increasingly frightened and angry because her family denies seeing these things. Her family is concerned she might hurt herself or others.
Despite some hearing loss, Mrs. L had been relatively healthy and independent until a few years ago, when her vision decreased secondary to age-related macular degeneration and diabetic retinopathy. In addition to sensory impairment and diabetes mellitus, her medical history includes mild hypothyroidism and intervertebral disc herniation. She has no history of liver disease or alcohol or substance abuse. A few weeks ago Mrs. L’s primary care physician began treating her with donepezil, 5 mg/d, because he suspected dementia was causing her hallucinations. Otherwise, she has no psychiatric history.
On exam, Mrs. L is easily directable and cooperative. She seems angry because no one believes her; she reports seeing a cat that nobody else could see in the ER immediately before being evaluated. She is frightened because she believes her hallucinations are real, although she is unable to explain them. Mrs. L reports feeling anxious most of the time and having difficulty sleeping because of her fears. She also feels sad and occasionally worthless because she cannot see or hear as well as when she was younger.
A mental status examination shows partial impairment of concentration and short-term memory, but Mrs. L is alert and oriented. No theme of delusions is detected. She has no physical complaints, and physical examination is unremarkable.
The authors’ observations
Mrs. L presented with new-onset agitation, visual hallucinations, and mildly decreased concentration and short-term memory. Our next step after history and examination was to perform laboratory testing to narrow the diagnosis ( Table 1 ).
A basic electrolyte panel including kidney function can point toward electrolyte imbalance or uremia as a cause of delirium. Mrs. L’s basic metabolic panel and liver function were normal. Urinalysis ruled out urinary tract infection.
Mrs. L’s thyroid-stimulating hormone (TSH) level was mildly elevated at 5.6 mU/L (in our laboratory, the upper normal limit is 5.2 mU/L). Hypothyroidism and hyperthyroidism are not associated with hallucinations, but hyperthyroidism is an important medical cause of anxiety and hypothyroidism can cause a dementia-like presentation. CT of the head to rule out a space-occupying lesion or acute process—such as cerebrovascular accident—shows only chronic vascular changes.
Based on Mrs. L’s history, physical examination, and lab results, we provisionally diagnose dementia, Alzheimer’s type with psychotic features, and prescribe quetiapine, 25 mg at bedtime. We offer to admit Mrs. L, but she and her family prefer close outpatient follow-up. After discussing safety concerns and pharmacotherapy with the patient and her family, we discharge Mrs. L home and advise her to follow up with the psychiatric clinic.
Table 1
Suggested workup for elderly patients with hallucinations
| History and physical exam |
| History of dementia, mood disorder, Parkinson’s disease, or drug abuse |
| Presence of delusions or mood/anxiety symptoms |
| Detailed medication history |
| Level of consciousness, alertness, and cognitive function assessment (eg, MMSE) |
| Vital signs (instability may reflect delirium, meningitis/encephalitis, or intoxication) |
| Physical exam (may confirm acute medical illness causing delirium) |
| Neurologic exam (may show focal neurologic signs reflecting space-occupying lesion, signs of Parkinson’s disease, vitamin B12 deficiency) |
| Ophthalmologic history/exam |
| Investigations |
| Electrolyte imbalance, especially calcium |
| Glucose level |
| Uremia, impaired liver function, and increased ammonia |
| CBC |
| Urine drug screen |
| Urinalysis, culture, and sensitivity |
| Additional tests |
| VDRL |
| Arterial blood gas |
| ECG and cardiac enzymes |
| Chest radiography |
| Vitamin B12/folate |
| TSH |
| EEG |
| Serum drug levels |
| CT/MRI of the head |
| Lumbar puncture and cerebrospinal fluid analysis |
| Heavy metal screen |
| HIV screen |
| CBC: complete blood count; CT: computed tomography; ECG: electrocardiography; EEG: electroencephalography; HIV: human immunodeficiency virus; MMSE: Mini-Mental State Exam; MRI: magnetic resonance imaging; TSH: thyroid-stimulating hormone; VDRL: venereal disease research laboratory |
EVALUATION: Lasting hallucinations
Quetiapine improves Mrs. L’s sleep and agitation but does not reduce her hallucinations. She sees a helicopter planting wires on a tree next to her house, a snake in the house, children in her room (some are “beautiful”), fire, and creatures with scary faces. These hallucinations occur mostly when she is alone. She denies hearing or touching the things she sees but continues to feel fear and anxiety when she sees them, although this diminishes with education and reassurance.
The authors’ observations
In Mrs. L’s subsequent psychiatry clinic visits, we gather additional information that helped us rule out several differential diagnoses ( Table 2 ).
The most common causes of new-onset psychosis in later life are:
- dementia-related syndromes with psychosis, delirium, or drug-induced psychosis
- primary psychiatric disorders, most commonly depression.1
Alzheimer’s disease has been associated with up to a 60% incidence of psychotic symptoms at some point in the disease course.2 Although Mrs. L’s short-term memory had declined in recent years, she does not have aphasia, apraxia, agnosia, or decrease in mental executive functioning to meet Alzheimer’s dementia criteria.
Perceptual disturbance is a feature of delirium that can cause agitation and hallucinations in elderly patients. However, Mrs. L did not have a decreased level of consciousness or an acute medical illness that would explain delirium.
Despite Mrs. L’s symptoms and progressive hearing and vision loss with resultant disability, she generally was organized in terms of basic self-care, hygiene, and activities of daily living. She was able to have a conversation when she could hear the physician. Surprisingly, her Mini-Mental State Examination (MMSE) score was within normal limits or only mildly impaired at office visits. This was not compatible with the initial diagnosis of dementia, although it may suggest mild cognitive impairment.
Mrs. L took donepezil as prescribed by her primary care physician for only a few weeks before we stopped it. We attributed Mrs. L’s slightly impaired concentration and short-term memory in the ER to the anxiety and stress of oscillating visual hallucinations.
Schizophrenia is another cause of psychosis, but Mrs. L had no history of negative symptoms, delusions, disorganized speech/behavior, or family history of psychotic disorders. In addition, schizophrenia is most likely to appear in a patient’s third decade. Although more common in women than men, late-onset schizophrenia—defined as onset after age 40—has a 1-year prevalence rate of 0.6%3 and therefore is an unlikely cause of Mrs. L’s symptoms.
Mrs. L had no history of neurologic deficit to suggest cerebrovascular disease or space-occupying brain lesion. Her TSH, which was slightly increased when she presented in the ER, was normal on subsequent testing. Folic acid and vitamin B12 were normal. We ordered brain MRI to rule out organic causes not seen with CT, but Mrs. L felt claustrophobic in the machine and could not finish the test.
EEG was ordered to rule out epilepsy. Hallucinations can be a prominent component of seizures and are more common when the seizure focus is in the left temporal lobe.4 However, the development of psychotic symptoms often follows the onset of seizures by approximately 14 to 17 years.5 Although Mrs. L never obtained the ordered EEG, the absence of a history of clinical seizures or focal neurologic signs makes it unlikely that epilepsy accounted for her hallucinations. The normal workup ruled out most possible medical/organic causes of Mrs. L’s visual hallucinations.
We considered depression with psychotic features because Mrs. L had occasional depressed mood, feelings of worthlessness, and low self-esteem. These symptoms started only after she began losing her vision and hearing and she did not experience them most of the time. Furthermore, her predominant negative feeling was anxiety related to the hallucinations. Mrs. L had no other depressive symptoms such as guilt or loss of appetite.
Table 2
Differential diagnosis of hallucinations in elderly patients
| Diagnosis | Comments |
|---|---|
| Delirium | Secondary to a generalized medical condition, substance-induced, or substance withdrawal |
| Dementia | Alzheimer’s, vascular, Lewy body, or less common types |
| Parkinson’s disease | Medications can induce visual hallucinations |
| Brain tumor/mass/CVA | Usually accompanied by other neurologic symptoms and signs |
| Schizophrenia | Usually starts in early adulthood |
| Mood disorder with psychotic features | Depression can present as pseudodementia in elderly patients |
| Drug abuse/withdrawal or side effect | Numerous medications are known to worsen delirium in elderly patients |
| Other causes | HIV, tertiary syphilis, Charles Bonnet syndrome |
| CVA: cerebrovascular accident; HIV: human immunodeficiency virus | |
A diagnosis of exclusion
We began to suspect that Mrs. L had Charles Bonnet syndrome (CBS), a condition in which visually impaired persons experience visual hallucinations without other known mental illnesses. These hallucinations tend to be complex, vivid, and elaborate, lasting from a few seconds to most of the day.6,7 CBS occurs in 10% to 15% of patients with visual impairment, including up to 3.5% of elderly patients referred to psychiatrists for visual hallucinations.7,8 CBS is most common among the elderly because of the high prevalence of visual impairment in this population.
Many patients with CBS are aware that their hallucinations are not real.7 Mrs. L’s presentation was atypical because she believed what she was seeing was real and because most images were terrifying, which also is not usually the case in CBS.
CBS frequently goes unrecognized in clinical practice.9 Patients who admit to experiencing hallucinations often are labeled demented or psychotic.10 The course of CBS is categorized in 3 patterns:
- episodic (in this least common pattern, hallucinations occur over days to months and then resolve)
- periodic (hallucinatory activity alternates with phases of remission)
- continuous (patients experience no hallucination-free intervals).7,11
The pathophysiology of CBS is not fully understood. The deafferentation hypothesis suggests that reduced or absent visual system stimulation leads to increased excitability of areas of the cerebral cortex associated with vision, resulting in phantom vision.6,7,12
CBS has no universally accepted diagnostic criteria; it is a diagnosis of exclusion. Because we ruled out medical and organic causes, dementia, delirium, schizophrenia, and depression with psychotic features—and because Mrs. L had advanced macular degeneration and retinopathy—we believed CBS was a likely diagnosis. We referred her to an ophthalmologist, who confirmed the CBS diagnosis.
TREATMENT: Temporary improvement
We prescribe low-dose lorazepam, 0.5 to 1 mg every 8 hours as needed for agitation, and increase quetiapine to 50 mg up to twice a day as needed. These approaches fail because of excessive sedation and delayed onset of action in relation to the fast onset of Mrs. L’s hallucinations.
Based on a published report, we prescribe gabapentin, 100 mg bid, which seems to help Mrs. L. For several months, her hallucinations are reduced, and she occasionally experiences a hallucination-free day. After several months, however, the frequency of her hallucinations increases. Mrs. L refuses to take a higher dosage of gabapentin because she doesn’t like “a lot of medicine.”
Her cognitive function remains mostly stable over the next few months, with an MMSE score of 23+/-1, which is equal to 25.6 +/- 1 when corrected for unperformed tasks secondary to severe visual impairment. She develops no aphasia, apraxia, or agnosia.
Educating Mrs. L about her illness—reassuring her that she is not “crazy”—helped to decrease her anxiety, as did teaching her family to acknowledge the hallucinations and react appropriately. Mrs. L’s hallucinations are less frequent when she interacts with other people and more frequent when she is alone with less sensory stimulation. Although Mrs. L has not yet recovered, a low dose of gabapentin temporarily decreased hallucinations and anxiety.
The authors’ observations
CBS treatment is based mostly on case reports. No pharmacologic treatment is universally effective, but anticonvulsants may help reduce hallucinations.7 Low-dose gabapentin is reported to have produced permanent remission.13
Patients may benefit from using magnifiers and other low-vision devices to maximize residual sight. Increased social interaction and brighter lighting also may help.7 Reassuring the patient that the hallucinations are not real and do not indicate mental illness can be strongly therapeutic.7 Hallucinations may resolve spontaneously, with improved vision, or with increased social interaction.7
Related Resource
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48:58-72.
Drug Brand Names
- Donepezil • Aricept
- Gabapentin • Neurontin
- Lorazepam • Ativan
- Quetiapine • Seroquel
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Brown FW. Late-life psychosis: making the diagnosis and controlling symptoms. Geriatrics. 1998;53:26-42.
2. Lautenschlager NT, Forstl H. Organic psychosis. Curr Psychiatry Rep. 2001;3:319-325.
3. Mortensen PB, Pedersen CB, Westergaard T, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603-608.
4. Roberts GW, Done DJ, Bruton C, et al. A “mock up” of schizophrenia: temporal lobe epilepsy and schizophrenia-like psychosis. Biol Psychiatry. 1990;28:127-143.
5. Bruton CJ, Stevens JR, Frith CD. Epilepsy, psychosis, and schizophrenia: clinical and neuropathologic correlations. Neurology. 1994;44:34-42.
6. Jacob A, Prasad S, Boggild M, et al. Charles Bonnet syndrome—elderly people and visual hallucinations. BMJ. 2004;328(7455):1552-1554.
7. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48:58-72.
8. O’Reilly R, Chamberlaine C. Charles Bonnet syndrome: incidence and demographic and clinical features. Can J Psychiatry. 1996;41(4):259-260.
9. Brown GC, Murphy RP. Visual symptoms associated with choroidal neovascularization. Photopsias and the Charles Bonnet syndrome. Arch Ophthalmol. 1992;110(9):1251-1256.
10. Hart J. Phantom visions: real enough to touch. Elder Care. 1997;9(1):30-32.
11. de Morsier G. Le Syndrome de Charles Bonnet: hallucinations visuelles des viellards sans deficience mentale. Ann Med Psychol. 1967;125:677-702.
12. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121:1819-1840.
13. Paulig M, Mentrup H. Charles Bonnet’s syndrome: complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
1. Brown FW. Late-life psychosis: making the diagnosis and controlling symptoms. Geriatrics. 1998;53:26-42.
2. Lautenschlager NT, Forstl H. Organic psychosis. Curr Psychiatry Rep. 2001;3:319-325.
3. Mortensen PB, Pedersen CB, Westergaard T, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603-608.
4. Roberts GW, Done DJ, Bruton C, et al. A “mock up” of schizophrenia: temporal lobe epilepsy and schizophrenia-like psychosis. Biol Psychiatry. 1990;28:127-143.
5. Bruton CJ, Stevens JR, Frith CD. Epilepsy, psychosis, and schizophrenia: clinical and neuropathologic correlations. Neurology. 1994;44:34-42.
6. Jacob A, Prasad S, Boggild M, et al. Charles Bonnet syndrome—elderly people and visual hallucinations. BMJ. 2004;328(7455):1552-1554.
7. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48:58-72.
8. O’Reilly R, Chamberlaine C. Charles Bonnet syndrome: incidence and demographic and clinical features. Can J Psychiatry. 1996;41(4):259-260.
9. Brown GC, Murphy RP. Visual symptoms associated with choroidal neovascularization. Photopsias and the Charles Bonnet syndrome. Arch Ophthalmol. 1992;110(9):1251-1256.
10. Hart J. Phantom visions: real enough to touch. Elder Care. 1997;9(1):30-32.
11. de Morsier G. Le Syndrome de Charles Bonnet: hallucinations visuelles des viellards sans deficience mentale. Ann Med Psychol. 1967;125:677-702.
12. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121:1819-1840.
13. Paulig M, Mentrup H. Charles Bonnet’s syndrome: complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
Adolescents in crisis: When to admit for self-harm or aggressive behavior
Ms. R, age 17, has a history of major depression, obsessive-compulsive disorder, and self-harm through superficial cutting of her arms and inguinal region. She reports that 10 days ago she ingested 7 times her prescribed fluoxetine dosage of 20 mg/d and aripiprazole dosage of 2 mg/d because she no longer wanted to feel emotional pain. She did not tell anyone she did this or seek medical attention.
Ms. R complains of chronic difficulties with her stepfather, who she describes as alcoholic. She feels her depression is worsening and support from her mother has deteriorated. Ms. R’s parents say they are trying to respond to their daughter, but she will not talk with them and some nights she does not return home. Ms. R admits to staying overnight in local mall parking lots to be alone. Her psychiatrist recommends acute inpatient care for Ms. R’s safety.
Admitting an adolescent such as Ms. R to a psychiatric inpatient facility may be necessary to address a crisis. Interdependent links among the patient, family, and support network complicate the determination of whether an adolescent requires inpatient care. To make the best decision, a psychiatrist needs to understand the youth’s difficulties within family, school, and community.
Who needs inpatient care?
Inpatient treatment remains an important part of the continuum of care for adolescent psychiatric treatment.1 Inpatient treatment typically is reserved for patients whose psychiatric disorder impairs multiple areas of functioning or poses a significant danger to self or others and for whom less-restrictive treatment resources are not appropriate or available.2 The number of psychiatric hospitalizations for adolescents is increasing, although lengths of stay are decreasing.3,4
Psychiatric inpatient care is appropriate for patients who require 24-hour nursing care and psychiatric monitoring to stabilize symptoms when they are in acute crisis and have a high risk of harm, and for initiation of treatments required for stabilization and integration into a less-restrictive setting.5 The decision to admit an adolescent rests on:
- the clinician’s ability to evaluate the risk of harm and functional status
- how much support the family and/or caregivers can provide
- the clinician’s knowledge of treatment resources available to the adolescent and family.6
Exploring suicide risk
Understanding potential lethality of suicidal thought and intent is complex and requires assessing suicidal behavior, the patient’s past and current intent, the risk of engaging in or repeating a suicide act, the underlying diagnosis, and protective factors. To quantify imminent suicide risk, directly address suicidality when interviewing an adolescent, progressing from past thoughts to current intent, plan, and ability to carry out such a plan (Table 1) .7
Planning and lethality. Also examine the patient’s degree of planning for a suicide attempt, efforts to avoid discovery and rescue, and his or her perceived lethality of a suicide attempt or plan. Patients who develop a coherent plan that would successfully avoid discovery clearly are at highest risk. Lethality of method is frequently misunderstood—especially among younger individuals—and thus their perception of the dangerousness of an attempt is more important than reality. Previous suicide attempts and chronic suicidality with recent escalation imply greater risk.
Motivation. Exploring the feelings that motivate a suicide attempt, intent, or ideation will help assess risk. Common motivations include:
- escaping from stress or hopelessness from perceived intolerable circumstances
- rejoining a dead loved one
- getting notice or attention from a parent, romantic interest, or other important individual
- injuring others around them.
Serious suicide risk may persist if the motivating feelings are not addressed satisfactorily.7
Unclear signals. An adolescent who expresses a clear intent to die, has a plausible plan, and is unable to work with or rejects caregivers’ attempts to help is at high risk and requires a secure setting, such as hospitalization. Typically, however, patients do not give such clear indicators; in these cases, consider other factors.
Unstable and unpredictable behavior implies serious short-term risk. Factors that indicate difficulties in a patient’s ability to maintain a safety plan include:
- a history of multiple suicide attempts or escalating seriousness of ideation
- inability to be truthful and form an alliance with the clinician
- difficulties in expressing and regulating emotions
- presence or likelihood of intoxication.
Psychosis, command hallucinations, high impulsivity, cycling associated with bipolar disorder, and substance abuse also are associated with high suicide risk.8
The clinician must determine whether an adolescent can form an alliance to report suicidal ideation, intent, or plan to a family member or other responsible adult, and if the family/caregivers are willing and capable of providing support, supervision, and compliance with future treatment recommendations that will ensure safety. If the answers are no, the patient requires hospitalization.
Table 1
Suggested questions for assessing adolescent suicidality
| Have you had thoughts of hurting yourself? |
| Have you ever tried to hurt yourself? |
| Have you ever wished you were not alive? |
| Have you had thoughts of taking your life? |
| Have you done things that are so dangerous that you knew you might get hurt or die? |
| Have you ever tried to kill yourself? |
| Have you had recent thoughts of killing yourself? |
| Do you have a plan to kill yourself? |
| Are the methods to kill yourself available to you? |
| Do you have access to guns? |
| Source: Adapted from reference 7 |
CASE CONTINUED: Unsafe at home
Ms. R feels she cannot be safe at home and cannot reliably form an alliance with her mother and stepfather to discuss whether her self-harm behaviors would escalate to serious injury or death. As a result, she is admitted to a psychiatric hospital. Inpatient care includes family intervention and a plan to intensify outpatient therapy. When Ms. R is discharged after 6 days, she reports improved mood and ability to contract with her family.
Aggressive behaviors
Besides suicidality, aggressive and combative behaviors in adolescents may lead to psychiatric referral.9–11 Overt homicidal ideation is not common; typically, patients exhibit escalating, disruptive, aggressive episodes in the home, school, or community that pose risk to themselves or others. Families seek clinical help because they feel unable to keep their child safe at home.
Aggressive behavior is linked to multiple patient factors, such as male gender, history of abuse and neglect, out-of-home placement in community systems, developmental disorders, mental retardation, disruptive behavior disorders, and learning disabilities. Aggressive behavior may include planned proactive situational-reactive or impulsive aggression, or it can stem from an altered mental status caused by illicit drug intoxication, medications, psychosis, or severe mood disorders.9-12
Psychiatric hospitalization of aggressive adolescents raises safety concerns, and some practitioners perceive that treatment is ineffective for these patients. However, high rates of psychiatric comorbidity and indications that positive outcomes are possible suggest that many aggressive youth can benefit from intervention.1,11
Because of the crisis nature of acute aggression and the often conflicted, hidden, and stressful situations these patients and families or caregivers are experiencing, hospitalization often is needed to stabilize the adolescent.
Assessment work with family/caregivers is vital because patients typically minimize the intensity of their aggressive behavior. Use a structured scale—such as the modified Overt Aggression Scale—to help quantify the severity of aggressive episodes, determine dangerousness, and establish a common language and measurement among caregivers, patients, and clinicians.13
The family/caregivers’ capacity and willingness to provide a safe environment, to avoid triggering events, and to provide support to de-escalate a potential crisis also determine if safety can be maintained in the home or if hospitalization is required. Hospitalization may be appropriate if the adolescent’s aggressive behavior substantially endangers the patient or others, is increasing in intensity, exceeds the ability to be managed in the home or living environment, and cannot be maintained in available less-restrictive settings.
In addition to the patient’s potential for suicidal or aggressive behavior, consider other aspects of potential harm, such as:
- unintentional harm associated with altered mental status from psychosis or intoxication
- the adolescent’s impulsivity or judgment in situations he or she is likely to encounter
- the patient’s ability to recognize potential threats and take appropriate action for safety
- severely impaired self-care.14
The Child and Adolescent Service Intensity Instrument can be used to help determine the level of care an adolescent patient requires ( Box ).14
CASII can help determine appropriate care for teens
The Child and Adolescent Service Intensity Instrument (CASII) can help you determine what level of care is most appropriate for your adolescent patient. This scale—developed by a work group of the American Academy of Child and Adolescent Psychiatry (AACAP)—links clinical assessment with standardized levels of care. It includes scoring in 6 dimensions:
- risk and harm
- functional status
- co-occurrence of conditions
- recovery environment
- resiliency and response to services
- primary caretaker involvement in services.
Scores are combined to generate a recommend level of service intensity from 0 (basic services) to 7 (24-hour psychiatric management—admission to a hospital or locked residential unit).
The AACAP strongly encourages clinicians to receive training to use the CASII and provides 1-and 2-day courses.
Source: Reference 14
Comorbid conditions
Comorbid medical illness, substance use disorders, and cognitive disability are common complications in determining the level of care for an adolescent in crisis. Active or passive noncompliance with treatment for medical conditions can pose an immediate or chronic threat to the individual and may represent a method of self-harm. Medical comorbidities and care requirements frequently preclude quick access to services such as group homes, therapeutic foster programs, and residential treatment. Hospitalization often is required to stabilize psychiatric conditions and medical illness.
CASE REPORT: Multiple comorbidities
Ms. P, age 16, has type 1 diabetes mellitus, posttraumatic stress disorder from early physical and sexual abuse, and an IQ of 49. She presents after repeated arguments and physical confrontations with her mother, with whom she lives. She has been caught hoarding high-sugar foods.
The most recent fight is over Ms. P wanting to consume large amounts of candy. She has been hospitalized twice for diabetic ketoacidosis in the last 6 months. Her most recent blood sugar levels ranged from 250 to 500 mg/dL. Ms. P states she is angry at her mother and will hit her if she tries to control her diet. She says she doesn’t care if she gets sick, but her recognition of medical complications is limited.
Developmental delays may complicate treatment for psychiatric illness or impair an adolescent’s ability to understand the dangerousness of his or her behaviors.15 Communication barriers make it challenging to assess risk or the patient’s ability to comply with a safety plan. In patients with developmental delay who live in the community, external structure, monitoring, and the ability to manage crises depends on the family/caregivers. Strongly consider hospitalization if an adolescent’s developmental delay has a serious adverse effect on managing the psychiatric condition, causing increased risk of harm to self or others.
Substance use frequently accompanies adolescent psychiatric illness and may pose severe risk by disinhibiting impulse control, exacerbating mood symptoms, altering mental status, or causing intoxication or withdrawal syndromes. Substance use also carries inherent risks, such as contracting human immunodeficiency virus or other blood-borne infections.
Substance use is well-documented as a severe risk factor for suicide and suicide attempts7,8 and frequently is associated with violence.16 Hospitalization may be the safest way to manage an adolescent who exhibits escalating substance use that complicates management of the psychiatric illness or indicates progressive endangering behavior.
Functional assessment
In addition to exploring risk of self-harm, aggressive behaviors, and medical comorbidities, evaluate the adolescent’s ability to function in interpersonal relationships, self-care, and school. A pattern of severe or worsening functional impairment often indicates illness progression or that management or supports are not meeting the patient’s needs.
Strongly consider hospitalizing patients who demonstrate serious deterioration in interpersonal relationships with peers, adults, or family, as evidenced by escalating threats, episodic violence, or disorganized communication. Additional concerns include severe social withdrawal, neglect of self-care appropriate to developmental level, and inability to perform academically despite appropriate accommodations.
Identify impaired physical functions. When severe medical complications accompany anorexia nervosa or other psychiatric illness, hospitalization is needed to ensure the patient’s safety and to begin appropriate assessment and treatment ( Table 2 ).17
Table 2
Adolescents with eating disorders: Admission criteria
| Heart rate near 40 bpm |
| Orthostasis (pulse change >20 bpm or blood pressure drop of >10 to 20 mm Hg from sitting to standing) |
| Hypotension (blood pressure <80/50 mm Hg) |
| Electrolyte imbalance (hypokalemia, hypophosphatemia, hypomagnesemia) |
| Weight <85% of ideal body weight |
| Acute weight decline with food refusal |
| Suicidal ideation |
| Needs supervision during and after all meals and in bathrooms because of disabling purging |
| Suitability of pediatric vs psychiatric unit depends on level of medical care required and respective units’ ability to manage eating disorders |
| Source: Adapted from reference 17 |
Family and environmental factors
The decision to admit an adolescent to a psychiatric hospital or provide a home treatment plan often hinges on the ability and willingness of the patient’s family/caregivers and support systems to meet the patient’s needs. Consider whether family functioning has been disrupted by a parent’s illness, death, divorce, medical problems, psychiatric illness, substance abuse, or financial stress. If you suspect abuse or violence in the home, observe reporting laws in your jurisdiction and intervene with the family to ensure the adolescent’s safety. Hospitalization may be the best means of providing safety during an investigation.
Determine if the family or primary caregivers are able to meet the adolescent’s developmental, material, and emotional requirements, and if help from treatment or support services or community resources could provide these needs. If not, hospitalization likely is required.
CASE CONTINUED: Risk of physical harm
Ms. P is admitted to the psychiatric hospital because her mother reports that in the past week she and her daughter have had 2 physical altercations—resulting from arguments about her daughter’s dietary intake—that caused injuries. She does not feel she can keep her daughter safe. Ms. P’s mother states she feels she is poorly trained in diabetic care and cannot provide the medical intervention her daughter needs.
Know your system
Child and adolescent psychiatric services are in great demand but often fall far short in meeting these needs across ethnic and socioeconomic groups. Availability of resources differs by geographic location and payer source.18,19 Community-funded mental health varies considerably. The best organizations offer a complete system of care, including outpatient therapy, medication management, case management, wraparound services, respite care, group homes, residential programs, and crisis programs.
Be familiar with local and regional programs and methods of accessing them. For patients who have access to a system of care, timely mobilization of appropriate resources often can avoid a hospital admission and place a patient in a less restrictive setting. Such options, however, frequently are not available to patients covered by commercial payers. For them, the decision typically is reduced to whether the family can manage the patient at home; if the family is unable to ensure safety, the adolescent is hospitalized.
Knowing the capability of available inpatient programs is essential to making an appropriate referral. Consider the level of medical care the psychiatric unit can provide and the accessibility of medical consultation services—both primary and medical subspecialty. Specialized programs for young people with comorbid severe cognitive delays, eating disorders, or forensic difficulties also assist with effective management. The inpatient unit’s collaboration and communication with outpatient providers frequently determines the success of the patient’s transition to less restrictive care.
- Child and Adolescent Service Intensity Instrument (CASII). www.aacap.org/cs/root/member_information/practice_information/casii.
Drug brand names
- Aripiprazole • Abilify
- Fluoxetine • Prozac
Disclosure
Dr. Sorter reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Green J, Jacobs B, Beecham J, et al. Inpatient treatment in child and adolescent psychiatry—a prospective study of health gain and costs. J Child Psychol Psychiatry. 2007;48(12):1259-1267.
2. American Academy of Child and Adolescent Psychiatry. Inpatient hospital treatment of children and adolescents. Policy statement. June 1989. Available at: http://www.aacap.org/cs/root/policy_statements/inpatient_hospital_treatment_of_children_
and_adolescents. Accessed November 19, 2009.
3. Case BG, Olfson M, Marcus SC, et al. Trends in the inpatient mental health treatment of children and adolescents in U.S. community hospitals between 1990 and 2000. Arch Gen Psychiatry. 2007;64:89-96.
4. Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996-2004. Biol Psychiatry. 2007;62:107-114.
5. Sharfstein SS. Goals of inpatient treatment for psychiatric disorders. Annu Rev Med. 2009;60:393-403.
6. Thienhaus OJ. The decision to admit. In: Thienhaus OJ, ed. Manual of clinical hospital psychiatry. Washington, DC: American Psychiatric Press, Inc; 1995:3–16.
7. Shaffer D, Pfeffer CR. Practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7):24S-51S.
8. Spirito A, Espposito-Smythers C. Attempted and completed suicide in adolescence. Ann Rev Clin Psychol. 2005;2:237-266.
9. Jenson PS, Youngstrom EA, Steiner H, et al. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. J Am Acad Child Adolesc Psychiatry. 2007;46(3):309-322.
10. Connor DF, Carlson GA, Chang KD, et al. Juvenile maladaptive aggression: a review of prevention, treatment, and service configuration and a proposed research agenda. J Clin Psychiatry. 2006;67(5):808-820.
11. Dean AJ, Duke SG, Scott J, et al. Physical aggression during admission to a child and adolescent inpatient unit: predictors and impact on clinical outcomes. Aust N Z J Psychiatry. 2008;42(6):536-543.
12. Masters KJ, Bellonci C. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2):4S-25S.
13. Collett BR, Ohan JL, Myers KM. Ten-year review of rating scales. VI: scales assessing externalizing behaviors. J Am Acad Child Adolesc Psychiatry. 2003;42(10):1143-1170.
14. Child and adolescent service intensity instrument user’s manual. Version 3.0. Washington, DC: American Academy of Child and Adolescent Psychiatry; 2007.
15. Lee P, Friedlander R. Attention-deficit and disruptive behavior disorders. In: Fletcher R, Loschen E, Stavrakaki C, et al, eds. Diagnostic manual-intellectual disability: a textbook of diagnosis of mental disorders in persons with intellectual disability. Kingston, NY: National Association for the Dually Diagnosed; 2007:127–144.
16. Turgay A. Aggression and disruptive behavior disorders in children and adolescents. Expert Rev Neurother. 2004;4(4):623-632.
17. American Psychiatric Association. Treatment of patients with eating disorders, third edition. Am J Psychiatry. 2006;163 (7 suppl):4-54.
18. Sturm R, Ringel JS, Andreyeva T. Geographic disparities in children’s mental health care. Pediatrics. 2003;112(4):e308-e315.
19. Katoaka SM, Zhang L, Wells KB. Unmet need for mental health care among U.S. children: variation by ethnicity and insurance status. Am J Psychiatry. 2002;159:1548-1555.
Ms. R, age 17, has a history of major depression, obsessive-compulsive disorder, and self-harm through superficial cutting of her arms and inguinal region. She reports that 10 days ago she ingested 7 times her prescribed fluoxetine dosage of 20 mg/d and aripiprazole dosage of 2 mg/d because she no longer wanted to feel emotional pain. She did not tell anyone she did this or seek medical attention.
Ms. R complains of chronic difficulties with her stepfather, who she describes as alcoholic. She feels her depression is worsening and support from her mother has deteriorated. Ms. R’s parents say they are trying to respond to their daughter, but she will not talk with them and some nights she does not return home. Ms. R admits to staying overnight in local mall parking lots to be alone. Her psychiatrist recommends acute inpatient care for Ms. R’s safety.
Admitting an adolescent such as Ms. R to a psychiatric inpatient facility may be necessary to address a crisis. Interdependent links among the patient, family, and support network complicate the determination of whether an adolescent requires inpatient care. To make the best decision, a psychiatrist needs to understand the youth’s difficulties within family, school, and community.
Who needs inpatient care?
Inpatient treatment remains an important part of the continuum of care for adolescent psychiatric treatment.1 Inpatient treatment typically is reserved for patients whose psychiatric disorder impairs multiple areas of functioning or poses a significant danger to self or others and for whom less-restrictive treatment resources are not appropriate or available.2 The number of psychiatric hospitalizations for adolescents is increasing, although lengths of stay are decreasing.3,4
Psychiatric inpatient care is appropriate for patients who require 24-hour nursing care and psychiatric monitoring to stabilize symptoms when they are in acute crisis and have a high risk of harm, and for initiation of treatments required for stabilization and integration into a less-restrictive setting.5 The decision to admit an adolescent rests on:
- the clinician’s ability to evaluate the risk of harm and functional status
- how much support the family and/or caregivers can provide
- the clinician’s knowledge of treatment resources available to the adolescent and family.6
Exploring suicide risk
Understanding potential lethality of suicidal thought and intent is complex and requires assessing suicidal behavior, the patient’s past and current intent, the risk of engaging in or repeating a suicide act, the underlying diagnosis, and protective factors. To quantify imminent suicide risk, directly address suicidality when interviewing an adolescent, progressing from past thoughts to current intent, plan, and ability to carry out such a plan (Table 1) .7
Planning and lethality. Also examine the patient’s degree of planning for a suicide attempt, efforts to avoid discovery and rescue, and his or her perceived lethality of a suicide attempt or plan. Patients who develop a coherent plan that would successfully avoid discovery clearly are at highest risk. Lethality of method is frequently misunderstood—especially among younger individuals—and thus their perception of the dangerousness of an attempt is more important than reality. Previous suicide attempts and chronic suicidality with recent escalation imply greater risk.
Motivation. Exploring the feelings that motivate a suicide attempt, intent, or ideation will help assess risk. Common motivations include:
- escaping from stress or hopelessness from perceived intolerable circumstances
- rejoining a dead loved one
- getting notice or attention from a parent, romantic interest, or other important individual
- injuring others around them.
Serious suicide risk may persist if the motivating feelings are not addressed satisfactorily.7
Unclear signals. An adolescent who expresses a clear intent to die, has a plausible plan, and is unable to work with or rejects caregivers’ attempts to help is at high risk and requires a secure setting, such as hospitalization. Typically, however, patients do not give such clear indicators; in these cases, consider other factors.
Unstable and unpredictable behavior implies serious short-term risk. Factors that indicate difficulties in a patient’s ability to maintain a safety plan include:
- a history of multiple suicide attempts or escalating seriousness of ideation
- inability to be truthful and form an alliance with the clinician
- difficulties in expressing and regulating emotions
- presence or likelihood of intoxication.
Psychosis, command hallucinations, high impulsivity, cycling associated with bipolar disorder, and substance abuse also are associated with high suicide risk.8
The clinician must determine whether an adolescent can form an alliance to report suicidal ideation, intent, or plan to a family member or other responsible adult, and if the family/caregivers are willing and capable of providing support, supervision, and compliance with future treatment recommendations that will ensure safety. If the answers are no, the patient requires hospitalization.
Table 1
Suggested questions for assessing adolescent suicidality
| Have you had thoughts of hurting yourself? |
| Have you ever tried to hurt yourself? |
| Have you ever wished you were not alive? |
| Have you had thoughts of taking your life? |
| Have you done things that are so dangerous that you knew you might get hurt or die? |
| Have you ever tried to kill yourself? |
| Have you had recent thoughts of killing yourself? |
| Do you have a plan to kill yourself? |
| Are the methods to kill yourself available to you? |
| Do you have access to guns? |
| Source: Adapted from reference 7 |
CASE CONTINUED: Unsafe at home
Ms. R feels she cannot be safe at home and cannot reliably form an alliance with her mother and stepfather to discuss whether her self-harm behaviors would escalate to serious injury or death. As a result, she is admitted to a psychiatric hospital. Inpatient care includes family intervention and a plan to intensify outpatient therapy. When Ms. R is discharged after 6 days, she reports improved mood and ability to contract with her family.
Aggressive behaviors
Besides suicidality, aggressive and combative behaviors in adolescents may lead to psychiatric referral.9–11 Overt homicidal ideation is not common; typically, patients exhibit escalating, disruptive, aggressive episodes in the home, school, or community that pose risk to themselves or others. Families seek clinical help because they feel unable to keep their child safe at home.
Aggressive behavior is linked to multiple patient factors, such as male gender, history of abuse and neglect, out-of-home placement in community systems, developmental disorders, mental retardation, disruptive behavior disorders, and learning disabilities. Aggressive behavior may include planned proactive situational-reactive or impulsive aggression, or it can stem from an altered mental status caused by illicit drug intoxication, medications, psychosis, or severe mood disorders.9-12
Psychiatric hospitalization of aggressive adolescents raises safety concerns, and some practitioners perceive that treatment is ineffective for these patients. However, high rates of psychiatric comorbidity and indications that positive outcomes are possible suggest that many aggressive youth can benefit from intervention.1,11
Because of the crisis nature of acute aggression and the often conflicted, hidden, and stressful situations these patients and families or caregivers are experiencing, hospitalization often is needed to stabilize the adolescent.
Assessment work with family/caregivers is vital because patients typically minimize the intensity of their aggressive behavior. Use a structured scale—such as the modified Overt Aggression Scale—to help quantify the severity of aggressive episodes, determine dangerousness, and establish a common language and measurement among caregivers, patients, and clinicians.13
The family/caregivers’ capacity and willingness to provide a safe environment, to avoid triggering events, and to provide support to de-escalate a potential crisis also determine if safety can be maintained in the home or if hospitalization is required. Hospitalization may be appropriate if the adolescent’s aggressive behavior substantially endangers the patient or others, is increasing in intensity, exceeds the ability to be managed in the home or living environment, and cannot be maintained in available less-restrictive settings.
In addition to the patient’s potential for suicidal or aggressive behavior, consider other aspects of potential harm, such as:
- unintentional harm associated with altered mental status from psychosis or intoxication
- the adolescent’s impulsivity or judgment in situations he or she is likely to encounter
- the patient’s ability to recognize potential threats and take appropriate action for safety
- severely impaired self-care.14
The Child and Adolescent Service Intensity Instrument can be used to help determine the level of care an adolescent patient requires ( Box ).14
CASII can help determine appropriate care for teens
The Child and Adolescent Service Intensity Instrument (CASII) can help you determine what level of care is most appropriate for your adolescent patient. This scale—developed by a work group of the American Academy of Child and Adolescent Psychiatry (AACAP)—links clinical assessment with standardized levels of care. It includes scoring in 6 dimensions:
- risk and harm
- functional status
- co-occurrence of conditions
- recovery environment
- resiliency and response to services
- primary caretaker involvement in services.
Scores are combined to generate a recommend level of service intensity from 0 (basic services) to 7 (24-hour psychiatric management—admission to a hospital or locked residential unit).
The AACAP strongly encourages clinicians to receive training to use the CASII and provides 1-and 2-day courses.
Source: Reference 14
Comorbid conditions
Comorbid medical illness, substance use disorders, and cognitive disability are common complications in determining the level of care for an adolescent in crisis. Active or passive noncompliance with treatment for medical conditions can pose an immediate or chronic threat to the individual and may represent a method of self-harm. Medical comorbidities and care requirements frequently preclude quick access to services such as group homes, therapeutic foster programs, and residential treatment. Hospitalization often is required to stabilize psychiatric conditions and medical illness.
CASE REPORT: Multiple comorbidities
Ms. P, age 16, has type 1 diabetes mellitus, posttraumatic stress disorder from early physical and sexual abuse, and an IQ of 49. She presents after repeated arguments and physical confrontations with her mother, with whom she lives. She has been caught hoarding high-sugar foods.
The most recent fight is over Ms. P wanting to consume large amounts of candy. She has been hospitalized twice for diabetic ketoacidosis in the last 6 months. Her most recent blood sugar levels ranged from 250 to 500 mg/dL. Ms. P states she is angry at her mother and will hit her if she tries to control her diet. She says she doesn’t care if she gets sick, but her recognition of medical complications is limited.
Developmental delays may complicate treatment for psychiatric illness or impair an adolescent’s ability to understand the dangerousness of his or her behaviors.15 Communication barriers make it challenging to assess risk or the patient’s ability to comply with a safety plan. In patients with developmental delay who live in the community, external structure, monitoring, and the ability to manage crises depends on the family/caregivers. Strongly consider hospitalization if an adolescent’s developmental delay has a serious adverse effect on managing the psychiatric condition, causing increased risk of harm to self or others.
Substance use frequently accompanies adolescent psychiatric illness and may pose severe risk by disinhibiting impulse control, exacerbating mood symptoms, altering mental status, or causing intoxication or withdrawal syndromes. Substance use also carries inherent risks, such as contracting human immunodeficiency virus or other blood-borne infections.
Substance use is well-documented as a severe risk factor for suicide and suicide attempts7,8 and frequently is associated with violence.16 Hospitalization may be the safest way to manage an adolescent who exhibits escalating substance use that complicates management of the psychiatric illness or indicates progressive endangering behavior.
Functional assessment
In addition to exploring risk of self-harm, aggressive behaviors, and medical comorbidities, evaluate the adolescent’s ability to function in interpersonal relationships, self-care, and school. A pattern of severe or worsening functional impairment often indicates illness progression or that management or supports are not meeting the patient’s needs.
Strongly consider hospitalizing patients who demonstrate serious deterioration in interpersonal relationships with peers, adults, or family, as evidenced by escalating threats, episodic violence, or disorganized communication. Additional concerns include severe social withdrawal, neglect of self-care appropriate to developmental level, and inability to perform academically despite appropriate accommodations.
Identify impaired physical functions. When severe medical complications accompany anorexia nervosa or other psychiatric illness, hospitalization is needed to ensure the patient’s safety and to begin appropriate assessment and treatment ( Table 2 ).17
Table 2
Adolescents with eating disorders: Admission criteria
| Heart rate near 40 bpm |
| Orthostasis (pulse change >20 bpm or blood pressure drop of >10 to 20 mm Hg from sitting to standing) |
| Hypotension (blood pressure <80/50 mm Hg) |
| Electrolyte imbalance (hypokalemia, hypophosphatemia, hypomagnesemia) |
| Weight <85% of ideal body weight |
| Acute weight decline with food refusal |
| Suicidal ideation |
| Needs supervision during and after all meals and in bathrooms because of disabling purging |
| Suitability of pediatric vs psychiatric unit depends on level of medical care required and respective units’ ability to manage eating disorders |
| Source: Adapted from reference 17 |
Family and environmental factors
The decision to admit an adolescent to a psychiatric hospital or provide a home treatment plan often hinges on the ability and willingness of the patient’s family/caregivers and support systems to meet the patient’s needs. Consider whether family functioning has been disrupted by a parent’s illness, death, divorce, medical problems, psychiatric illness, substance abuse, or financial stress. If you suspect abuse or violence in the home, observe reporting laws in your jurisdiction and intervene with the family to ensure the adolescent’s safety. Hospitalization may be the best means of providing safety during an investigation.
Determine if the family or primary caregivers are able to meet the adolescent’s developmental, material, and emotional requirements, and if help from treatment or support services or community resources could provide these needs. If not, hospitalization likely is required.
CASE CONTINUED: Risk of physical harm
Ms. P is admitted to the psychiatric hospital because her mother reports that in the past week she and her daughter have had 2 physical altercations—resulting from arguments about her daughter’s dietary intake—that caused injuries. She does not feel she can keep her daughter safe. Ms. P’s mother states she feels she is poorly trained in diabetic care and cannot provide the medical intervention her daughter needs.
Know your system
Child and adolescent psychiatric services are in great demand but often fall far short in meeting these needs across ethnic and socioeconomic groups. Availability of resources differs by geographic location and payer source.18,19 Community-funded mental health varies considerably. The best organizations offer a complete system of care, including outpatient therapy, medication management, case management, wraparound services, respite care, group homes, residential programs, and crisis programs.
Be familiar with local and regional programs and methods of accessing them. For patients who have access to a system of care, timely mobilization of appropriate resources often can avoid a hospital admission and place a patient in a less restrictive setting. Such options, however, frequently are not available to patients covered by commercial payers. For them, the decision typically is reduced to whether the family can manage the patient at home; if the family is unable to ensure safety, the adolescent is hospitalized.
Knowing the capability of available inpatient programs is essential to making an appropriate referral. Consider the level of medical care the psychiatric unit can provide and the accessibility of medical consultation services—both primary and medical subspecialty. Specialized programs for young people with comorbid severe cognitive delays, eating disorders, or forensic difficulties also assist with effective management. The inpatient unit’s collaboration and communication with outpatient providers frequently determines the success of the patient’s transition to less restrictive care.
- Child and Adolescent Service Intensity Instrument (CASII). www.aacap.org/cs/root/member_information/practice_information/casii.
Drug brand names
- Aripiprazole • Abilify
- Fluoxetine • Prozac
Disclosure
Dr. Sorter reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. R, age 17, has a history of major depression, obsessive-compulsive disorder, and self-harm through superficial cutting of her arms and inguinal region. She reports that 10 days ago she ingested 7 times her prescribed fluoxetine dosage of 20 mg/d and aripiprazole dosage of 2 mg/d because she no longer wanted to feel emotional pain. She did not tell anyone she did this or seek medical attention.
Ms. R complains of chronic difficulties with her stepfather, who she describes as alcoholic. She feels her depression is worsening and support from her mother has deteriorated. Ms. R’s parents say they are trying to respond to their daughter, but she will not talk with them and some nights she does not return home. Ms. R admits to staying overnight in local mall parking lots to be alone. Her psychiatrist recommends acute inpatient care for Ms. R’s safety.
Admitting an adolescent such as Ms. R to a psychiatric inpatient facility may be necessary to address a crisis. Interdependent links among the patient, family, and support network complicate the determination of whether an adolescent requires inpatient care. To make the best decision, a psychiatrist needs to understand the youth’s difficulties within family, school, and community.
Who needs inpatient care?
Inpatient treatment remains an important part of the continuum of care for adolescent psychiatric treatment.1 Inpatient treatment typically is reserved for patients whose psychiatric disorder impairs multiple areas of functioning or poses a significant danger to self or others and for whom less-restrictive treatment resources are not appropriate or available.2 The number of psychiatric hospitalizations for adolescents is increasing, although lengths of stay are decreasing.3,4
Psychiatric inpatient care is appropriate for patients who require 24-hour nursing care and psychiatric monitoring to stabilize symptoms when they are in acute crisis and have a high risk of harm, and for initiation of treatments required for stabilization and integration into a less-restrictive setting.5 The decision to admit an adolescent rests on:
- the clinician’s ability to evaluate the risk of harm and functional status
- how much support the family and/or caregivers can provide
- the clinician’s knowledge of treatment resources available to the adolescent and family.6
Exploring suicide risk
Understanding potential lethality of suicidal thought and intent is complex and requires assessing suicidal behavior, the patient’s past and current intent, the risk of engaging in or repeating a suicide act, the underlying diagnosis, and protective factors. To quantify imminent suicide risk, directly address suicidality when interviewing an adolescent, progressing from past thoughts to current intent, plan, and ability to carry out such a plan (Table 1) .7
Planning and lethality. Also examine the patient’s degree of planning for a suicide attempt, efforts to avoid discovery and rescue, and his or her perceived lethality of a suicide attempt or plan. Patients who develop a coherent plan that would successfully avoid discovery clearly are at highest risk. Lethality of method is frequently misunderstood—especially among younger individuals—and thus their perception of the dangerousness of an attempt is more important than reality. Previous suicide attempts and chronic suicidality with recent escalation imply greater risk.
Motivation. Exploring the feelings that motivate a suicide attempt, intent, or ideation will help assess risk. Common motivations include:
- escaping from stress or hopelessness from perceived intolerable circumstances
- rejoining a dead loved one
- getting notice or attention from a parent, romantic interest, or other important individual
- injuring others around them.
Serious suicide risk may persist if the motivating feelings are not addressed satisfactorily.7
Unclear signals. An adolescent who expresses a clear intent to die, has a plausible plan, and is unable to work with or rejects caregivers’ attempts to help is at high risk and requires a secure setting, such as hospitalization. Typically, however, patients do not give such clear indicators; in these cases, consider other factors.
Unstable and unpredictable behavior implies serious short-term risk. Factors that indicate difficulties in a patient’s ability to maintain a safety plan include:
- a history of multiple suicide attempts or escalating seriousness of ideation
- inability to be truthful and form an alliance with the clinician
- difficulties in expressing and regulating emotions
- presence or likelihood of intoxication.
Psychosis, command hallucinations, high impulsivity, cycling associated with bipolar disorder, and substance abuse also are associated with high suicide risk.8
The clinician must determine whether an adolescent can form an alliance to report suicidal ideation, intent, or plan to a family member or other responsible adult, and if the family/caregivers are willing and capable of providing support, supervision, and compliance with future treatment recommendations that will ensure safety. If the answers are no, the patient requires hospitalization.
Table 1
Suggested questions for assessing adolescent suicidality
| Have you had thoughts of hurting yourself? |
| Have you ever tried to hurt yourself? |
| Have you ever wished you were not alive? |
| Have you had thoughts of taking your life? |
| Have you done things that are so dangerous that you knew you might get hurt or die? |
| Have you ever tried to kill yourself? |
| Have you had recent thoughts of killing yourself? |
| Do you have a plan to kill yourself? |
| Are the methods to kill yourself available to you? |
| Do you have access to guns? |
| Source: Adapted from reference 7 |
CASE CONTINUED: Unsafe at home
Ms. R feels she cannot be safe at home and cannot reliably form an alliance with her mother and stepfather to discuss whether her self-harm behaviors would escalate to serious injury or death. As a result, she is admitted to a psychiatric hospital. Inpatient care includes family intervention and a plan to intensify outpatient therapy. When Ms. R is discharged after 6 days, she reports improved mood and ability to contract with her family.
Aggressive behaviors
Besides suicidality, aggressive and combative behaviors in adolescents may lead to psychiatric referral.9–11 Overt homicidal ideation is not common; typically, patients exhibit escalating, disruptive, aggressive episodes in the home, school, or community that pose risk to themselves or others. Families seek clinical help because they feel unable to keep their child safe at home.
Aggressive behavior is linked to multiple patient factors, such as male gender, history of abuse and neglect, out-of-home placement in community systems, developmental disorders, mental retardation, disruptive behavior disorders, and learning disabilities. Aggressive behavior may include planned proactive situational-reactive or impulsive aggression, or it can stem from an altered mental status caused by illicit drug intoxication, medications, psychosis, or severe mood disorders.9-12
Psychiatric hospitalization of aggressive adolescents raises safety concerns, and some practitioners perceive that treatment is ineffective for these patients. However, high rates of psychiatric comorbidity and indications that positive outcomes are possible suggest that many aggressive youth can benefit from intervention.1,11
Because of the crisis nature of acute aggression and the often conflicted, hidden, and stressful situations these patients and families or caregivers are experiencing, hospitalization often is needed to stabilize the adolescent.
Assessment work with family/caregivers is vital because patients typically minimize the intensity of their aggressive behavior. Use a structured scale—such as the modified Overt Aggression Scale—to help quantify the severity of aggressive episodes, determine dangerousness, and establish a common language and measurement among caregivers, patients, and clinicians.13
The family/caregivers’ capacity and willingness to provide a safe environment, to avoid triggering events, and to provide support to de-escalate a potential crisis also determine if safety can be maintained in the home or if hospitalization is required. Hospitalization may be appropriate if the adolescent’s aggressive behavior substantially endangers the patient or others, is increasing in intensity, exceeds the ability to be managed in the home or living environment, and cannot be maintained in available less-restrictive settings.
In addition to the patient’s potential for suicidal or aggressive behavior, consider other aspects of potential harm, such as:
- unintentional harm associated with altered mental status from psychosis or intoxication
- the adolescent’s impulsivity or judgment in situations he or she is likely to encounter
- the patient’s ability to recognize potential threats and take appropriate action for safety
- severely impaired self-care.14
The Child and Adolescent Service Intensity Instrument can be used to help determine the level of care an adolescent patient requires ( Box ).14
CASII can help determine appropriate care for teens
The Child and Adolescent Service Intensity Instrument (CASII) can help you determine what level of care is most appropriate for your adolescent patient. This scale—developed by a work group of the American Academy of Child and Adolescent Psychiatry (AACAP)—links clinical assessment with standardized levels of care. It includes scoring in 6 dimensions:
- risk and harm
- functional status
- co-occurrence of conditions
- recovery environment
- resiliency and response to services
- primary caretaker involvement in services.
Scores are combined to generate a recommend level of service intensity from 0 (basic services) to 7 (24-hour psychiatric management—admission to a hospital or locked residential unit).
The AACAP strongly encourages clinicians to receive training to use the CASII and provides 1-and 2-day courses.
Source: Reference 14
Comorbid conditions
Comorbid medical illness, substance use disorders, and cognitive disability are common complications in determining the level of care for an adolescent in crisis. Active or passive noncompliance with treatment for medical conditions can pose an immediate or chronic threat to the individual and may represent a method of self-harm. Medical comorbidities and care requirements frequently preclude quick access to services such as group homes, therapeutic foster programs, and residential treatment. Hospitalization often is required to stabilize psychiatric conditions and medical illness.
CASE REPORT: Multiple comorbidities
Ms. P, age 16, has type 1 diabetes mellitus, posttraumatic stress disorder from early physical and sexual abuse, and an IQ of 49. She presents after repeated arguments and physical confrontations with her mother, with whom she lives. She has been caught hoarding high-sugar foods.
The most recent fight is over Ms. P wanting to consume large amounts of candy. She has been hospitalized twice for diabetic ketoacidosis in the last 6 months. Her most recent blood sugar levels ranged from 250 to 500 mg/dL. Ms. P states she is angry at her mother and will hit her if she tries to control her diet. She says she doesn’t care if she gets sick, but her recognition of medical complications is limited.
Developmental delays may complicate treatment for psychiatric illness or impair an adolescent’s ability to understand the dangerousness of his or her behaviors.15 Communication barriers make it challenging to assess risk or the patient’s ability to comply with a safety plan. In patients with developmental delay who live in the community, external structure, monitoring, and the ability to manage crises depends on the family/caregivers. Strongly consider hospitalization if an adolescent’s developmental delay has a serious adverse effect on managing the psychiatric condition, causing increased risk of harm to self or others.
Substance use frequently accompanies adolescent psychiatric illness and may pose severe risk by disinhibiting impulse control, exacerbating mood symptoms, altering mental status, or causing intoxication or withdrawal syndromes. Substance use also carries inherent risks, such as contracting human immunodeficiency virus or other blood-borne infections.
Substance use is well-documented as a severe risk factor for suicide and suicide attempts7,8 and frequently is associated with violence.16 Hospitalization may be the safest way to manage an adolescent who exhibits escalating substance use that complicates management of the psychiatric illness or indicates progressive endangering behavior.
Functional assessment
In addition to exploring risk of self-harm, aggressive behaviors, and medical comorbidities, evaluate the adolescent’s ability to function in interpersonal relationships, self-care, and school. A pattern of severe or worsening functional impairment often indicates illness progression or that management or supports are not meeting the patient’s needs.
Strongly consider hospitalizing patients who demonstrate serious deterioration in interpersonal relationships with peers, adults, or family, as evidenced by escalating threats, episodic violence, or disorganized communication. Additional concerns include severe social withdrawal, neglect of self-care appropriate to developmental level, and inability to perform academically despite appropriate accommodations.
Identify impaired physical functions. When severe medical complications accompany anorexia nervosa or other psychiatric illness, hospitalization is needed to ensure the patient’s safety and to begin appropriate assessment and treatment ( Table 2 ).17
Table 2
Adolescents with eating disorders: Admission criteria
| Heart rate near 40 bpm |
| Orthostasis (pulse change >20 bpm or blood pressure drop of >10 to 20 mm Hg from sitting to standing) |
| Hypotension (blood pressure <80/50 mm Hg) |
| Electrolyte imbalance (hypokalemia, hypophosphatemia, hypomagnesemia) |
| Weight <85% of ideal body weight |
| Acute weight decline with food refusal |
| Suicidal ideation |
| Needs supervision during and after all meals and in bathrooms because of disabling purging |
| Suitability of pediatric vs psychiatric unit depends on level of medical care required and respective units’ ability to manage eating disorders |
| Source: Adapted from reference 17 |
Family and environmental factors
The decision to admit an adolescent to a psychiatric hospital or provide a home treatment plan often hinges on the ability and willingness of the patient’s family/caregivers and support systems to meet the patient’s needs. Consider whether family functioning has been disrupted by a parent’s illness, death, divorce, medical problems, psychiatric illness, substance abuse, or financial stress. If you suspect abuse or violence in the home, observe reporting laws in your jurisdiction and intervene with the family to ensure the adolescent’s safety. Hospitalization may be the best means of providing safety during an investigation.
Determine if the family or primary caregivers are able to meet the adolescent’s developmental, material, and emotional requirements, and if help from treatment or support services or community resources could provide these needs. If not, hospitalization likely is required.
CASE CONTINUED: Risk of physical harm
Ms. P is admitted to the psychiatric hospital because her mother reports that in the past week she and her daughter have had 2 physical altercations—resulting from arguments about her daughter’s dietary intake—that caused injuries. She does not feel she can keep her daughter safe. Ms. P’s mother states she feels she is poorly trained in diabetic care and cannot provide the medical intervention her daughter needs.
Know your system
Child and adolescent psychiatric services are in great demand but often fall far short in meeting these needs across ethnic and socioeconomic groups. Availability of resources differs by geographic location and payer source.18,19 Community-funded mental health varies considerably. The best organizations offer a complete system of care, including outpatient therapy, medication management, case management, wraparound services, respite care, group homes, residential programs, and crisis programs.
Be familiar with local and regional programs and methods of accessing them. For patients who have access to a system of care, timely mobilization of appropriate resources often can avoid a hospital admission and place a patient in a less restrictive setting. Such options, however, frequently are not available to patients covered by commercial payers. For them, the decision typically is reduced to whether the family can manage the patient at home; if the family is unable to ensure safety, the adolescent is hospitalized.
Knowing the capability of available inpatient programs is essential to making an appropriate referral. Consider the level of medical care the psychiatric unit can provide and the accessibility of medical consultation services—both primary and medical subspecialty. Specialized programs for young people with comorbid severe cognitive delays, eating disorders, or forensic difficulties also assist with effective management. The inpatient unit’s collaboration and communication with outpatient providers frequently determines the success of the patient’s transition to less restrictive care.
- Child and Adolescent Service Intensity Instrument (CASII). www.aacap.org/cs/root/member_information/practice_information/casii.
Drug brand names
- Aripiprazole • Abilify
- Fluoxetine • Prozac
Disclosure
Dr. Sorter reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Green J, Jacobs B, Beecham J, et al. Inpatient treatment in child and adolescent psychiatry—a prospective study of health gain and costs. J Child Psychol Psychiatry. 2007;48(12):1259-1267.
2. American Academy of Child and Adolescent Psychiatry. Inpatient hospital treatment of children and adolescents. Policy statement. June 1989. Available at: http://www.aacap.org/cs/root/policy_statements/inpatient_hospital_treatment_of_children_
and_adolescents. Accessed November 19, 2009.
3. Case BG, Olfson M, Marcus SC, et al. Trends in the inpatient mental health treatment of children and adolescents in U.S. community hospitals between 1990 and 2000. Arch Gen Psychiatry. 2007;64:89-96.
4. Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996-2004. Biol Psychiatry. 2007;62:107-114.
5. Sharfstein SS. Goals of inpatient treatment for psychiatric disorders. Annu Rev Med. 2009;60:393-403.
6. Thienhaus OJ. The decision to admit. In: Thienhaus OJ, ed. Manual of clinical hospital psychiatry. Washington, DC: American Psychiatric Press, Inc; 1995:3–16.
7. Shaffer D, Pfeffer CR. Practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7):24S-51S.
8. Spirito A, Espposito-Smythers C. Attempted and completed suicide in adolescence. Ann Rev Clin Psychol. 2005;2:237-266.
9. Jenson PS, Youngstrom EA, Steiner H, et al. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. J Am Acad Child Adolesc Psychiatry. 2007;46(3):309-322.
10. Connor DF, Carlson GA, Chang KD, et al. Juvenile maladaptive aggression: a review of prevention, treatment, and service configuration and a proposed research agenda. J Clin Psychiatry. 2006;67(5):808-820.
11. Dean AJ, Duke SG, Scott J, et al. Physical aggression during admission to a child and adolescent inpatient unit: predictors and impact on clinical outcomes. Aust N Z J Psychiatry. 2008;42(6):536-543.
12. Masters KJ, Bellonci C. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2):4S-25S.
13. Collett BR, Ohan JL, Myers KM. Ten-year review of rating scales. VI: scales assessing externalizing behaviors. J Am Acad Child Adolesc Psychiatry. 2003;42(10):1143-1170.
14. Child and adolescent service intensity instrument user’s manual. Version 3.0. Washington, DC: American Academy of Child and Adolescent Psychiatry; 2007.
15. Lee P, Friedlander R. Attention-deficit and disruptive behavior disorders. In: Fletcher R, Loschen E, Stavrakaki C, et al, eds. Diagnostic manual-intellectual disability: a textbook of diagnosis of mental disorders in persons with intellectual disability. Kingston, NY: National Association for the Dually Diagnosed; 2007:127–144.
16. Turgay A. Aggression and disruptive behavior disorders in children and adolescents. Expert Rev Neurother. 2004;4(4):623-632.
17. American Psychiatric Association. Treatment of patients with eating disorders, third edition. Am J Psychiatry. 2006;163 (7 suppl):4-54.
18. Sturm R, Ringel JS, Andreyeva T. Geographic disparities in children’s mental health care. Pediatrics. 2003;112(4):e308-e315.
19. Katoaka SM, Zhang L, Wells KB. Unmet need for mental health care among U.S. children: variation by ethnicity and insurance status. Am J Psychiatry. 2002;159:1548-1555.
1. Green J, Jacobs B, Beecham J, et al. Inpatient treatment in child and adolescent psychiatry—a prospective study of health gain and costs. J Child Psychol Psychiatry. 2007;48(12):1259-1267.
2. American Academy of Child and Adolescent Psychiatry. Inpatient hospital treatment of children and adolescents. Policy statement. June 1989. Available at: http://www.aacap.org/cs/root/policy_statements/inpatient_hospital_treatment_of_children_
and_adolescents. Accessed November 19, 2009.
3. Case BG, Olfson M, Marcus SC, et al. Trends in the inpatient mental health treatment of children and adolescents in U.S. community hospitals between 1990 and 2000. Arch Gen Psychiatry. 2007;64:89-96.
4. Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996-2004. Biol Psychiatry. 2007;62:107-114.
5. Sharfstein SS. Goals of inpatient treatment for psychiatric disorders. Annu Rev Med. 2009;60:393-403.
6. Thienhaus OJ. The decision to admit. In: Thienhaus OJ, ed. Manual of clinical hospital psychiatry. Washington, DC: American Psychiatric Press, Inc; 1995:3–16.
7. Shaffer D, Pfeffer CR. Practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7):24S-51S.
8. Spirito A, Espposito-Smythers C. Attempted and completed suicide in adolescence. Ann Rev Clin Psychol. 2005;2:237-266.
9. Jenson PS, Youngstrom EA, Steiner H, et al. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. J Am Acad Child Adolesc Psychiatry. 2007;46(3):309-322.
10. Connor DF, Carlson GA, Chang KD, et al. Juvenile maladaptive aggression: a review of prevention, treatment, and service configuration and a proposed research agenda. J Clin Psychiatry. 2006;67(5):808-820.
11. Dean AJ, Duke SG, Scott J, et al. Physical aggression during admission to a child and adolescent inpatient unit: predictors and impact on clinical outcomes. Aust N Z J Psychiatry. 2008;42(6):536-543.
12. Masters KJ, Bellonci C. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2):4S-25S.
13. Collett BR, Ohan JL, Myers KM. Ten-year review of rating scales. VI: scales assessing externalizing behaviors. J Am Acad Child Adolesc Psychiatry. 2003;42(10):1143-1170.
14. Child and adolescent service intensity instrument user’s manual. Version 3.0. Washington, DC: American Academy of Child and Adolescent Psychiatry; 2007.
15. Lee P, Friedlander R. Attention-deficit and disruptive behavior disorders. In: Fletcher R, Loschen E, Stavrakaki C, et al, eds. Diagnostic manual-intellectual disability: a textbook of diagnosis of mental disorders in persons with intellectual disability. Kingston, NY: National Association for the Dually Diagnosed; 2007:127–144.
16. Turgay A. Aggression and disruptive behavior disorders in children and adolescents. Expert Rev Neurother. 2004;4(4):623-632.
17. American Psychiatric Association. Treatment of patients with eating disorders, third edition. Am J Psychiatry. 2006;163 (7 suppl):4-54.
18. Sturm R, Ringel JS, Andreyeva T. Geographic disparities in children’s mental health care. Pediatrics. 2003;112(4):e308-e315.
19. Katoaka SM, Zhang L, Wells KB. Unmet need for mental health care among U.S. children: variation by ethnicity and insurance status. Am J Psychiatry. 2002;159:1548-1555.
Borderline, bipolar, or both? Frame your diagnosis on the patient history
Borderline personality disorder (BPD) and bipolar disorder are frequently confused with each other, in part because of their considerable symptomatic overlap. This redundancy occurs despite the different ways these disorders are conceptualized: BPD as a personality disorder and bipolar disorder as a brain disease among Axis I clinical disorders.
BPD and bipolar disorder—especially bipolar II—often co-occur ( Box ) and are frequently misidentified, as shown by clinical and epidemiologic studies. Misdiagnosis creates problems for clinicians and patients. When diagnosed with BPD, patients with bipolar disorder may be deprived of potentially effective pharmacologic treatments.1 Conversely, the stigma that BPD carries—particularly in the mental health community—may lead clinicians to:
- not even disclose the BPD diagnosis to patients2
- lean in the direction of diagnosing BPD as bipolar disorder, potentially resulting in treatments that have little relevance or failure to refer for more appropriate psychosocial treatments.
To help you avoid confusion and the pitfalls of misdiagnosis, this article clarifies the distinctions between bipolar disorder and BPD. We discuss symptom overlap, highlight key differences between the constructs, outline diagnostic differences, and provide useful suggestions to discern the differential diagnosis.
between BPD and bipolar disorder*
1. Inability of current nosology to separate 2 distinct conditions
Relatively indistinct diagnostic boundaries confuse the differentiation of borderline personality disorder (BPD) and bipolar disorder (5 of 9 BPD criteria may occur with mania or hypomania). In this model, the person has 1 disorder but because of symptom overlap receives a diagnosis of both. Because structured interviews do not allow for subjective judgment or expert opinion, the result is the generation of 2 diagnoses when 1 may provide a more parsimonious and valid explanation.
2. BPD exists on a spectrum with bipolar disorder
The mood lability of BPD may be viewed as not unlike that seen with bipolar disorder.1 Behaviors displayed by patients with BPD are subsequently conceptualized as arising from their unstable mood. Supporting arguments cite family study data and evidence from pharmacotherapy trials of anticonvulsants, including divalproex, for rapid cycling bipolar disorder and BPD.2 Family studies have been notable for their failure to directly characterize family members, however, and clinical trials have been quite small. Further, treatment response may have very limited nosologic implications.
3. Bipolar disorder is a risk factor for BPD
4. BPD is a risk factor for bipolar disorder
Early emergence of a bipolar disorder (in preadolescent or adolescent patients) has been proposed to disrupt psychological development, leading to BPD. This adverse impact on personality development—the “scar hypothesis”3 —is supported by data showing greater risk of co-occurring BPD with earlier onset bipolar disorder.4 More important, prospective studies of patients with bipolar disorder show a greater risk for developing BPD.5
BPD also may be a risk factor for the development of bipolar disorder—the “vulnerability hypothesis.”3 Patients with BPD are more likely to develop bipolar disorder, even compared to patients with other personality disorders.5
5. Shared risk factors
BPD and bipolar disorder may be linked by shared risk factors, such as shared genes or trait neuroticism.3
*Some evidence supports each potential explanation, and they are not necessarily mutually exclusive
References
a. Akiskal HS. Demystifying borderline personality: critique of the concept and unorthodox reflections on its natural kinship with the bipolar spectrum. Acta Psychiatr Scand. 2004;110(6):401-407.
b. Mackinnon DF, Pies R. Affective instability as rapid cycling: theoretical and clinical implications for borderline personality and bipolar spectrum disorders. Bipolar Disord. 2006;8(1):1-14.
c. Christensen MV, Kessing LV. Do personality traits predict first onset in depressive and bipolar disorder? Nord J Psychiatry. 2006;60(2):79-88.
d. Goldberg JF, Garno JL. Age at onset of bipolar disorder and risk for comorbid borderline personality disorder. Bipolar Disord. 2009;11(2):205-208.
e. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
Overlapping symptoms
Bipolar disorder is generally considered a clinical disorder or brain disease that can be understood as a broken mood “thermostat.” The lifetime prevalence of bipolar types I and II is approximately 2%.3 Approximately one-half of patients have a family history of illness, and multiple genes are believed to influence inheritance. Mania is the disorder’s hallmark,4 although overactivity has alternatively been proposed as a core feature.5 Most patients with mania ultimately experience depression6 ( Table 1 ).
No dimensional personality correlates have been consistently demonstrated in bipolar disorder, although co-occurring personality disorders—often the “dramatic” Cluster B type—are common4,7 and may adversely affect treatment response and suicide risk.8,9
Both bipolar disorder and BPD are associated with considerable risk of suicide or suicide attempts.10,11 Self-mutilation or self-injurious behavior without suicidal intent are particularly common in BPD.12 Threats of suicide—which may be manipulative or help-seeking—also are common in BPD and tend to be acute rather than chronic.13
Borderline personality disorder is characterized by an enduring and inflexible pattern of thoughts, feelings, and behaviors that impairs an individual’s psychosocial or vocational function. Its estimated prevalence is approximately 1%,14 although recent community estimates approach 6%.15 Genetic influences play a lesser etiologic role in BPD than in bipolar disorder.
Several of BPD’s common features ( Table 2 )—impulsivity, mood instability, inappropriate anger, suicidal behavior, and unstable relationships—are shared with bipolar disorder, but patients with BPD tend to show higher levels of impulsiveness and hostility than patients with bipolar disorder.16 Dimensional assessments of personality traits suggest that BPD is characterized by high neuroticism and low agreeableness.17 BPD also has been more strongly associated with a childhood history of abuse, even when compared with control groups having other personality disorders or major depression.18 The male-to-female ratio for bipolar disorder approximates 1:1;3 in BPD this ratio has been estimated at 1:4 in clinical samples19 and near 1:1 in community samples.15
BPD and bipolar disorder often co-occur. Evidence indicates ≤20% of patients with BPD have comorbid bipolar disorder20 and 15% of patients with bipolar disorder have comorbid BPD.21 Co-occurrence happens much more often than would be expected by chance. These similar bidirectional comorbidity estimates (15% to 20%) would not be expected for conditions of such differing prevalence (<1% vs 2% or more). This suggests:
- the estimated prevalence of bipolar disorder in BPD is too low
- the estimated prevalence of BPD in bipolar disorder samples is too high
- borderline personality disorder is present in >1% of the population
- bipolar disorder is less common
- some combination of the above.
Among these possibilities, the prevalence estimates of bipolar disorder are the most consistent. Several studies suggest that BPD may be much more common, with some estimates exceeding 5%.15
Table 1
Common signs and symptoms
associated with mania and depression in bipolar disorder
| (Hypo)mania | Depression |
|---|---|
| Elevated mood | Decreased mood |
| Irritability | Irritability |
| Decreased need for sleep | Anhedonia |
| Grandiosity | Decreased self-attitude |
| Talkativeness | Insomnia/hypersomnia |
| Racing thoughts | Change in appetite/weight |
| Increased motor activity | Fatigue |
| Increased sex drive | Hopelessness |
| Religiosity | Suicidal thoughts |
| Distractibility | Impaired concentration |
Table 2
Borderline personality disorder: Commonly reported features
| Impulsivity |
| Unstable relationships |
| Unstable self-image |
| Affective instability |
| Fear of abandonment |
| Recurrent self-injurious or suicidal behavior |
| Feelings of emptiness |
| Intense anger or hostility |
| Transient paranoia or dissociative symptoms |
Roots of misdiagnosis
The presence of bipolar disorder or BPD may increase the risk that the other will be misdiagnosed. When symptoms of both are present, those suggesting 1 diagnosis may reflect the consequences of the other. A diagnosis of BPD could represent a partially treated or treatment-resistant bipolar disorder, or a BPD diagnosis could be the result of several years of disruption by a mood disorder.
Characteristics of bipolar disorder have contributed to clinician bias in favor of that diagnosis rather than BPD ( Table 3 ).22,23 Bipolar disorder also may be misdiagnosed as BPD. This error may most likely occur when the history focuses excessively on cross-sectional symptoms, such as when a patient with bipolar disorder shows prominent mood lability or interpersonal sensitivity during a mood episode but not when euthymic.
Bipolar II disorder. The confusion between bipolar disorder and BPD may be particularly problematic for patients with bipolar II disorder or subthreshold bipolar disorders. The manias of bipolar I disorder are much more readily distinguishable from the mood instability or reactivity of BPD. The manic symptoms of bipolar I are more florid, more pronounced, and lead to more obvious impairment.
The milder highs of bipolar II may resemble the mood fluctuations seen in BPD. Further, bipolar II is characterized by a greater chronicity and affective morbidity than bipolar I, and episodes of illness may be characterized by irritability, anger, and racing thoughts.24 Whereas impulsivity or aggression are more characteristic of BPD, bipolar II is similar to BPD on dimensions of affective instability.24,25
When present in BPD, affective instability or lability is conceptualized as ultra-rapid or ultradian, with a frequency of hours to days. BPD is less likely than bipolar II to show affective lability between depression and euthymia or elation and more likely to show fluctuations into anger and anxiety.26
Nonetheless, because of the increased prominence of shared features and reduced distinguishing features, bipolar II and BPD are prone to misdiagnosis and commonly co-occur.
Table 3
Clinician biases that may favor a bipolar disorder diagnosis, rather than BPD
| Bipolar disorder is supported by decades of research |
| Patients with bipolar disorder are often considered more “likeable” than those with BPD |
| Bipolar disorder is more treatable and has a better long-term outcome than BPD (although BPD is generally characterized by clinical improvement, whereas bipolar disorder is more stable with perhaps some increase in depressive symptom burden) |
| Widely thought to have a biologic basis, the bipolar diagnosis conveys less stigma than BPD, which often is less empathically attributed to the patient’s own failings |
| A bipolar diagnosis is easier to explain to patients than BPD; many psychiatrists have difficulty explaining personality disorders in terms patients understand |
| BPD: borderline personality disorder |
| Source: References 22,23 |
History, the diagnostic key
A thorough and rigorous psychiatric history is essential to distinguish BPD from bipolar disorder. Supplementing the patient’s history with an informant interview is often helpful.
Because personality disorders are considered a chronic and enduring pattern of maladaptive behavior, focus the history on longitudinal course and not simply cross-sectional symptoms. Thus, symptoms suggestive of BPD that are confined only to clearly defined episodes of mood disturbance and are absent during euthymia would not warrant a BPD diagnosis.
Temporal relationship. A detailed chronologic history can help determine the temporal relationship between any borderline features and mood episodes. When the patient’s life story is used as a scaffold for the phenomenologic portions of the psychiatric history, one can determine whether any such functional impairment is confined to episodes of mood disorder or appears as an enduring pattern of thinking, acting, and relating. Exploring what happened at notable life transitions—leaving school, loss of job, divorce/separation—may be similarly helpful.
Family history of psychiatric illness may provide a clue to an individual’s genetic predisposition but, of course, does not determine diagnosis. A detailed family and social history that provides evidence of an individual’s function in school, work, and interpersonal relationships is more relevant.
Abandonment and identity issues. Essential to BPD is fear of abandonment, often an undue fear that those important to patients will leave them. Patients may go to extremes to avoid being “abandoned,” even when this threat is not genuine.27,28 Their insecure attachments often lead them to fear being alone. The patient with BPD may:
- make frantic phone calls or send text messages to a friend or lover seeking reassurance
- take extreme measures such as refusing to leave the person’s home or pleading with them not to leave.
Patients with BPD often struggle with identity disturbance, leading them to wonder who they are and what their beliefs and core values are.29 Although occasionally patients with bipolar disorder may have these symptoms, they are not characteristic of bipolar disorder.
Mood lability. The time course of changes in affect or mood swings also may help distinguish BPD from bipolar disorder.
- With bipolar disorder the shift typically is from depression to elation or the reverse, and moods are sustained. Manias or hypomanias are often immediately followed by a “crash” into depression.
- With BPD, “roller-coaster moods” are typical, mood shifts are nonsustained, and the poles often are anxiety, anger, or desperation.
Patients with BPD often report moods shifting rapidly over minutes or hours, but they rarely describe moods sustained for days or weeks on end—other than perhaps depression. Mood lability of BPD often is produced by interpersonal sensitivity, whereas mood lability in bipolar disorder tends to be autonomous and persistent.
Young patients. Assessment can be particularly challenging in young adults and adolescents because symptoms of an emerging bipolar disorder can be more difficult to distinguish from BPD.30 Patients this young also may have less longitudinal history to distinguish an enduring pattern of thinking and relating from a mood disorder. For these cases, it may be particularly important to classify the frequency and pattern of mood symptoms.
Affective dysregulation is a core feature of BPD and is variably defined as a mood reactivity, typically of short duration (often hours). Cycling in bipolar disorder classically involves a periodicity of weeks to months. Even the broadest definitions include a minimum duration of 2 days for hypomania.5
Mood reactivity can occur within episodes of bipolar disorder, although episodes may occur spontaneously and without an obvious precipitant or stressor. Impulsivity may represent more of an essential feature of BPD than affective instability or mood reactivity and may be of particular diagnostic relevance.
Treatment implications
When you are unable to make a clear diagnosis, describe your clinical reasoning and differential diagnosis in the assessment or formulation. With close follow-up, the longitudinal history and course of illness may eventually lead you to an accurate diagnosis.
There are good reasons to acknowledge both conditions when bipolar disorder and BPD are present. Proper recognition of bipolar disorder is a prerequisite to taking full advantage of proven pharmacologic treatments. The evidence base for pharmacologic management of BPD remains limited,31 but recognizing this disorder may help the patient understand his or her psychiatric history and encourage the use of effective psychosocial treatments.
Psychosocial treatments for bipolar disorder may target demoralization and circadian rhythms with sleep hygiene or social rhythms therapy. Acknowledging BPD:
- helps both clinician and patient to better understand the condition
- facilitates setting realistic treatment goals because BPD tends to respond to medication less robustly than bipolar disorder.
Recognizing BPD also allows for referral to targeted psychosocial treatments, including dialectical behavior therapy, mentalization-based treatment, or Systems Training for Emotional Predictability and Problem Solving (STEPPS).32-34
Related resources
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
- National Institute of Mental Health. Bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml.
- Systems Training for Emotional Predictability and Problem Solving (STEPPS). www.uihealthcare.com/topics/medicaldepartments/psychiatry/stepps/index.html.
Drug brand name
- Divalproex • Depakote, Depakene, others
Disclosures
Dr. Fiedorowicz reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Acknowledgment
The authors would like to thank Nancee Blum, MSW, and Nancy Hale, RN, for their assistance and expertise in the preparation of this article.
1. John H, Sharma V. Misdiagnosis of bipolar disorder as borderline personality disorder: clinical and economic consequences. World J Biol Psychiatry. 2007;1-4[epub ahead of print].
2. Lequesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004;10(3):170-176.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351(5):476-486.
5. Benazzi F. Testing new diagnostic criteria for hypomania. Ann Clin Psychiatry. 2007;19(2):99-104.
6. Solomon DA, Leon AC, Endicott J, et al. Unipolar mania over the course of a 20-year follow-up study. Am J Psychiatry. 2003;160(11):2049-2051.
7. Schiavone P, Dorz S, Conforti D, et al. Comorbidity of DSM-IV personality disorders in unipolar and bipolar affective disorders: a comparative study. Psychol Rep. 2004;95(1):121-128.
8. Fan AH, Hassell J. Bipolar disorder and comorbid personality psychopathology: a review of the literature. J Clin Psychiatry. 2008;69(11):1794-1803.
9. Garno JL, Goldberg JF, Ramirez PM, et al. Bipolar disorder with comorbid cluster B personality disorder features: impact on suicidality. J Clin Psychiatry. 2005;66(3):339-345.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Fiedorowicz JG, Leon AC, Keller MB, et al. Do risk factors for suicidal behavior differ by affective disorder polarity? Psychol Med. 2009;39(5):763-771.
12. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13(3):179-185.
13. Zanarini MC, Frankenburg FR, Hennen J, et al. The McLean Study of Adult Development (MSAD): overview and implications of the first six years of prospective follow-up. J Pers Disord. 2005;19(5):505-523.
14. Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Arch Gen Psychiatry. 2001;58(6):590-596.
15. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
16. Wilson ST, Stanley B, Oquendo MA, et al. Comparing impulsiveness, hostility, and depression in borderline personality disorder and bipolar II disorder. J Clin Psychiatry. 2007;68(10):1533-1539.
17. Zweig-Frank H, Paris J. The five-factor model of personality in borderline and nonborderline personality disorders. Can J Psychiatry. 1995;40(9):523-526.
18. Zanarini MC, Frankenburg FR, Reich DB, et al. Adult experiences of abuse reported by borderline patients and Axis II comparison subjects over six years of prospective follow-up. J Nerv Ment Dis. 2005;193(6):412-416.
19. Zanarini MC, Frankenburg FR, Reich DB, et al. Violence in the lives of adult borderline patients. J Nerv Ment Dis. 1999;187(2):65-71.
20. McCormick B, Blum N, Hansel R, et al. Relationship of sex to symptom severity, psychiatric comorbidity, and health care utilization in 163 subjects with borderline personality disorder. Compr Psychiatry. 2007;48(5):406-412.
21. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
22. Paris J, Zweig-Frank H. A 27-year follow-up of patients with borderline personality disorder. Compr Psychiatry. 2001;42(6):482-487.
23. Coryell WH, Fiedorowicz JG, Solomon D, et al. Age transitions in the course of bipolar I disorder. Psychol Med. 2009;39(8):1247-1252.
24. Benazzi F. Borderline personality-bipolar spectrum relationship. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(1):68-74.
25. Critchfield KL, Levy KN, Clarkin JF. The relationship between impulsivity, aggression, and impulsive-aggression in borderline personality disorder: an empirical analysis of self-report measures. J Pers Disord. 2004;18(6):555-570.
26. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatr Res. 2001;35(6):307-312.
27. Bray A. The extended mind and borderline personality disorder. Australas Psychiatry. 2008;16(1):8-12.
28. Gunderson JG. The borderline patient’s intolerance of aloneness: insecure attachments and therapist availability. Am J Psychiatry. 1996;153(6):752-758.
29. Jorgensen CR. Disturbed sense of identity in borderline personality disorder. J Pers Disord. 2006;20(6):618-644.
30. Smith DJ, Muir WJ, Blackwood DH. Borderline personality disorder characteristics in young adults with recurrent mood disorders: a comparison of bipolar and unipolar depression. J Affect Disord. 2005;87(1):17-23.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006(1);CD005653.-
32. Lynch TR, Trost WT, Salsman N, et al. Dialectical behavior therapy for borderline personality disorder. Annu Rev Clin Psychol. 2007;3:181-205.
33. Bateman AW, Fonagy P. Mentalization-based treatment of BPD. J Pers Disord. 2004;18(1):36-51.
34. Blum N, St John D, Pfohl B, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165(4):468-478.
Borderline personality disorder (BPD) and bipolar disorder are frequently confused with each other, in part because of their considerable symptomatic overlap. This redundancy occurs despite the different ways these disorders are conceptualized: BPD as a personality disorder and bipolar disorder as a brain disease among Axis I clinical disorders.
BPD and bipolar disorder—especially bipolar II—often co-occur ( Box ) and are frequently misidentified, as shown by clinical and epidemiologic studies. Misdiagnosis creates problems for clinicians and patients. When diagnosed with BPD, patients with bipolar disorder may be deprived of potentially effective pharmacologic treatments.1 Conversely, the stigma that BPD carries—particularly in the mental health community—may lead clinicians to:
- not even disclose the BPD diagnosis to patients2
- lean in the direction of diagnosing BPD as bipolar disorder, potentially resulting in treatments that have little relevance or failure to refer for more appropriate psychosocial treatments.
To help you avoid confusion and the pitfalls of misdiagnosis, this article clarifies the distinctions between bipolar disorder and BPD. We discuss symptom overlap, highlight key differences between the constructs, outline diagnostic differences, and provide useful suggestions to discern the differential diagnosis.
between BPD and bipolar disorder*
1. Inability of current nosology to separate 2 distinct conditions
Relatively indistinct diagnostic boundaries confuse the differentiation of borderline personality disorder (BPD) and bipolar disorder (5 of 9 BPD criteria may occur with mania or hypomania). In this model, the person has 1 disorder but because of symptom overlap receives a diagnosis of both. Because structured interviews do not allow for subjective judgment or expert opinion, the result is the generation of 2 diagnoses when 1 may provide a more parsimonious and valid explanation.
2. BPD exists on a spectrum with bipolar disorder
The mood lability of BPD may be viewed as not unlike that seen with bipolar disorder.1 Behaviors displayed by patients with BPD are subsequently conceptualized as arising from their unstable mood. Supporting arguments cite family study data and evidence from pharmacotherapy trials of anticonvulsants, including divalproex, for rapid cycling bipolar disorder and BPD.2 Family studies have been notable for their failure to directly characterize family members, however, and clinical trials have been quite small. Further, treatment response may have very limited nosologic implications.
3. Bipolar disorder is a risk factor for BPD
4. BPD is a risk factor for bipolar disorder
Early emergence of a bipolar disorder (in preadolescent or adolescent patients) has been proposed to disrupt psychological development, leading to BPD. This adverse impact on personality development—the “scar hypothesis”3 —is supported by data showing greater risk of co-occurring BPD with earlier onset bipolar disorder.4 More important, prospective studies of patients with bipolar disorder show a greater risk for developing BPD.5
BPD also may be a risk factor for the development of bipolar disorder—the “vulnerability hypothesis.”3 Patients with BPD are more likely to develop bipolar disorder, even compared to patients with other personality disorders.5
5. Shared risk factors
BPD and bipolar disorder may be linked by shared risk factors, such as shared genes or trait neuroticism.3
*Some evidence supports each potential explanation, and they are not necessarily mutually exclusive
References
a. Akiskal HS. Demystifying borderline personality: critique of the concept and unorthodox reflections on its natural kinship with the bipolar spectrum. Acta Psychiatr Scand. 2004;110(6):401-407.
b. Mackinnon DF, Pies R. Affective instability as rapid cycling: theoretical and clinical implications for borderline personality and bipolar spectrum disorders. Bipolar Disord. 2006;8(1):1-14.
c. Christensen MV, Kessing LV. Do personality traits predict first onset in depressive and bipolar disorder? Nord J Psychiatry. 2006;60(2):79-88.
d. Goldberg JF, Garno JL. Age at onset of bipolar disorder and risk for comorbid borderline personality disorder. Bipolar Disord. 2009;11(2):205-208.
e. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
Overlapping symptoms
Bipolar disorder is generally considered a clinical disorder or brain disease that can be understood as a broken mood “thermostat.” The lifetime prevalence of bipolar types I and II is approximately 2%.3 Approximately one-half of patients have a family history of illness, and multiple genes are believed to influence inheritance. Mania is the disorder’s hallmark,4 although overactivity has alternatively been proposed as a core feature.5 Most patients with mania ultimately experience depression6 ( Table 1 ).
No dimensional personality correlates have been consistently demonstrated in bipolar disorder, although co-occurring personality disorders—often the “dramatic” Cluster B type—are common4,7 and may adversely affect treatment response and suicide risk.8,9
Both bipolar disorder and BPD are associated with considerable risk of suicide or suicide attempts.10,11 Self-mutilation or self-injurious behavior without suicidal intent are particularly common in BPD.12 Threats of suicide—which may be manipulative or help-seeking—also are common in BPD and tend to be acute rather than chronic.13
Borderline personality disorder is characterized by an enduring and inflexible pattern of thoughts, feelings, and behaviors that impairs an individual’s psychosocial or vocational function. Its estimated prevalence is approximately 1%,14 although recent community estimates approach 6%.15 Genetic influences play a lesser etiologic role in BPD than in bipolar disorder.
Several of BPD’s common features ( Table 2 )—impulsivity, mood instability, inappropriate anger, suicidal behavior, and unstable relationships—are shared with bipolar disorder, but patients with BPD tend to show higher levels of impulsiveness and hostility than patients with bipolar disorder.16 Dimensional assessments of personality traits suggest that BPD is characterized by high neuroticism and low agreeableness.17 BPD also has been more strongly associated with a childhood history of abuse, even when compared with control groups having other personality disorders or major depression.18 The male-to-female ratio for bipolar disorder approximates 1:1;3 in BPD this ratio has been estimated at 1:4 in clinical samples19 and near 1:1 in community samples.15
BPD and bipolar disorder often co-occur. Evidence indicates ≤20% of patients with BPD have comorbid bipolar disorder20 and 15% of patients with bipolar disorder have comorbid BPD.21 Co-occurrence happens much more often than would be expected by chance. These similar bidirectional comorbidity estimates (15% to 20%) would not be expected for conditions of such differing prevalence (<1% vs 2% or more). This suggests:
- the estimated prevalence of bipolar disorder in BPD is too low
- the estimated prevalence of BPD in bipolar disorder samples is too high
- borderline personality disorder is present in >1% of the population
- bipolar disorder is less common
- some combination of the above.
Among these possibilities, the prevalence estimates of bipolar disorder are the most consistent. Several studies suggest that BPD may be much more common, with some estimates exceeding 5%.15
Table 1
Common signs and symptoms
associated with mania and depression in bipolar disorder
| (Hypo)mania | Depression |
|---|---|
| Elevated mood | Decreased mood |
| Irritability | Irritability |
| Decreased need for sleep | Anhedonia |
| Grandiosity | Decreased self-attitude |
| Talkativeness | Insomnia/hypersomnia |
| Racing thoughts | Change in appetite/weight |
| Increased motor activity | Fatigue |
| Increased sex drive | Hopelessness |
| Religiosity | Suicidal thoughts |
| Distractibility | Impaired concentration |
Table 2
Borderline personality disorder: Commonly reported features
| Impulsivity |
| Unstable relationships |
| Unstable self-image |
| Affective instability |
| Fear of abandonment |
| Recurrent self-injurious or suicidal behavior |
| Feelings of emptiness |
| Intense anger or hostility |
| Transient paranoia or dissociative symptoms |
Roots of misdiagnosis
The presence of bipolar disorder or BPD may increase the risk that the other will be misdiagnosed. When symptoms of both are present, those suggesting 1 diagnosis may reflect the consequences of the other. A diagnosis of BPD could represent a partially treated or treatment-resistant bipolar disorder, or a BPD diagnosis could be the result of several years of disruption by a mood disorder.
Characteristics of bipolar disorder have contributed to clinician bias in favor of that diagnosis rather than BPD ( Table 3 ).22,23 Bipolar disorder also may be misdiagnosed as BPD. This error may most likely occur when the history focuses excessively on cross-sectional symptoms, such as when a patient with bipolar disorder shows prominent mood lability or interpersonal sensitivity during a mood episode but not when euthymic.
Bipolar II disorder. The confusion between bipolar disorder and BPD may be particularly problematic for patients with bipolar II disorder or subthreshold bipolar disorders. The manias of bipolar I disorder are much more readily distinguishable from the mood instability or reactivity of BPD. The manic symptoms of bipolar I are more florid, more pronounced, and lead to more obvious impairment.
The milder highs of bipolar II may resemble the mood fluctuations seen in BPD. Further, bipolar II is characterized by a greater chronicity and affective morbidity than bipolar I, and episodes of illness may be characterized by irritability, anger, and racing thoughts.24 Whereas impulsivity or aggression are more characteristic of BPD, bipolar II is similar to BPD on dimensions of affective instability.24,25
When present in BPD, affective instability or lability is conceptualized as ultra-rapid or ultradian, with a frequency of hours to days. BPD is less likely than bipolar II to show affective lability between depression and euthymia or elation and more likely to show fluctuations into anger and anxiety.26
Nonetheless, because of the increased prominence of shared features and reduced distinguishing features, bipolar II and BPD are prone to misdiagnosis and commonly co-occur.
Table 3
Clinician biases that may favor a bipolar disorder diagnosis, rather than BPD
| Bipolar disorder is supported by decades of research |
| Patients with bipolar disorder are often considered more “likeable” than those with BPD |
| Bipolar disorder is more treatable and has a better long-term outcome than BPD (although BPD is generally characterized by clinical improvement, whereas bipolar disorder is more stable with perhaps some increase in depressive symptom burden) |
| Widely thought to have a biologic basis, the bipolar diagnosis conveys less stigma than BPD, which often is less empathically attributed to the patient’s own failings |
| A bipolar diagnosis is easier to explain to patients than BPD; many psychiatrists have difficulty explaining personality disorders in terms patients understand |
| BPD: borderline personality disorder |
| Source: References 22,23 |
History, the diagnostic key
A thorough and rigorous psychiatric history is essential to distinguish BPD from bipolar disorder. Supplementing the patient’s history with an informant interview is often helpful.
Because personality disorders are considered a chronic and enduring pattern of maladaptive behavior, focus the history on longitudinal course and not simply cross-sectional symptoms. Thus, symptoms suggestive of BPD that are confined only to clearly defined episodes of mood disturbance and are absent during euthymia would not warrant a BPD diagnosis.
Temporal relationship. A detailed chronologic history can help determine the temporal relationship between any borderline features and mood episodes. When the patient’s life story is used as a scaffold for the phenomenologic portions of the psychiatric history, one can determine whether any such functional impairment is confined to episodes of mood disorder or appears as an enduring pattern of thinking, acting, and relating. Exploring what happened at notable life transitions—leaving school, loss of job, divorce/separation—may be similarly helpful.
Family history of psychiatric illness may provide a clue to an individual’s genetic predisposition but, of course, does not determine diagnosis. A detailed family and social history that provides evidence of an individual’s function in school, work, and interpersonal relationships is more relevant.
Abandonment and identity issues. Essential to BPD is fear of abandonment, often an undue fear that those important to patients will leave them. Patients may go to extremes to avoid being “abandoned,” even when this threat is not genuine.27,28 Their insecure attachments often lead them to fear being alone. The patient with BPD may:
- make frantic phone calls or send text messages to a friend or lover seeking reassurance
- take extreme measures such as refusing to leave the person’s home or pleading with them not to leave.
Patients with BPD often struggle with identity disturbance, leading them to wonder who they are and what their beliefs and core values are.29 Although occasionally patients with bipolar disorder may have these symptoms, they are not characteristic of bipolar disorder.
Mood lability. The time course of changes in affect or mood swings also may help distinguish BPD from bipolar disorder.
- With bipolar disorder the shift typically is from depression to elation or the reverse, and moods are sustained. Manias or hypomanias are often immediately followed by a “crash” into depression.
- With BPD, “roller-coaster moods” are typical, mood shifts are nonsustained, and the poles often are anxiety, anger, or desperation.
Patients with BPD often report moods shifting rapidly over minutes or hours, but they rarely describe moods sustained for days or weeks on end—other than perhaps depression. Mood lability of BPD often is produced by interpersonal sensitivity, whereas mood lability in bipolar disorder tends to be autonomous and persistent.
Young patients. Assessment can be particularly challenging in young adults and adolescents because symptoms of an emerging bipolar disorder can be more difficult to distinguish from BPD.30 Patients this young also may have less longitudinal history to distinguish an enduring pattern of thinking and relating from a mood disorder. For these cases, it may be particularly important to classify the frequency and pattern of mood symptoms.
Affective dysregulation is a core feature of BPD and is variably defined as a mood reactivity, typically of short duration (often hours). Cycling in bipolar disorder classically involves a periodicity of weeks to months. Even the broadest definitions include a minimum duration of 2 days for hypomania.5
Mood reactivity can occur within episodes of bipolar disorder, although episodes may occur spontaneously and without an obvious precipitant or stressor. Impulsivity may represent more of an essential feature of BPD than affective instability or mood reactivity and may be of particular diagnostic relevance.
Treatment implications
When you are unable to make a clear diagnosis, describe your clinical reasoning and differential diagnosis in the assessment or formulation. With close follow-up, the longitudinal history and course of illness may eventually lead you to an accurate diagnosis.
There are good reasons to acknowledge both conditions when bipolar disorder and BPD are present. Proper recognition of bipolar disorder is a prerequisite to taking full advantage of proven pharmacologic treatments. The evidence base for pharmacologic management of BPD remains limited,31 but recognizing this disorder may help the patient understand his or her psychiatric history and encourage the use of effective psychosocial treatments.
Psychosocial treatments for bipolar disorder may target demoralization and circadian rhythms with sleep hygiene or social rhythms therapy. Acknowledging BPD:
- helps both clinician and patient to better understand the condition
- facilitates setting realistic treatment goals because BPD tends to respond to medication less robustly than bipolar disorder.
Recognizing BPD also allows for referral to targeted psychosocial treatments, including dialectical behavior therapy, mentalization-based treatment, or Systems Training for Emotional Predictability and Problem Solving (STEPPS).32-34
Related resources
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
- National Institute of Mental Health. Bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml.
- Systems Training for Emotional Predictability and Problem Solving (STEPPS). www.uihealthcare.com/topics/medicaldepartments/psychiatry/stepps/index.html.
Drug brand name
- Divalproex • Depakote, Depakene, others
Disclosures
Dr. Fiedorowicz reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Acknowledgment
The authors would like to thank Nancee Blum, MSW, and Nancy Hale, RN, for their assistance and expertise in the preparation of this article.
Borderline personality disorder (BPD) and bipolar disorder are frequently confused with each other, in part because of their considerable symptomatic overlap. This redundancy occurs despite the different ways these disorders are conceptualized: BPD as a personality disorder and bipolar disorder as a brain disease among Axis I clinical disorders.
BPD and bipolar disorder—especially bipolar II—often co-occur ( Box ) and are frequently misidentified, as shown by clinical and epidemiologic studies. Misdiagnosis creates problems for clinicians and patients. When diagnosed with BPD, patients with bipolar disorder may be deprived of potentially effective pharmacologic treatments.1 Conversely, the stigma that BPD carries—particularly in the mental health community—may lead clinicians to:
- not even disclose the BPD diagnosis to patients2
- lean in the direction of diagnosing BPD as bipolar disorder, potentially resulting in treatments that have little relevance or failure to refer for more appropriate psychosocial treatments.
To help you avoid confusion and the pitfalls of misdiagnosis, this article clarifies the distinctions between bipolar disorder and BPD. We discuss symptom overlap, highlight key differences between the constructs, outline diagnostic differences, and provide useful suggestions to discern the differential diagnosis.
between BPD and bipolar disorder*
1. Inability of current nosology to separate 2 distinct conditions
Relatively indistinct diagnostic boundaries confuse the differentiation of borderline personality disorder (BPD) and bipolar disorder (5 of 9 BPD criteria may occur with mania or hypomania). In this model, the person has 1 disorder but because of symptom overlap receives a diagnosis of both. Because structured interviews do not allow for subjective judgment or expert opinion, the result is the generation of 2 diagnoses when 1 may provide a more parsimonious and valid explanation.
2. BPD exists on a spectrum with bipolar disorder
The mood lability of BPD may be viewed as not unlike that seen with bipolar disorder.1 Behaviors displayed by patients with BPD are subsequently conceptualized as arising from their unstable mood. Supporting arguments cite family study data and evidence from pharmacotherapy trials of anticonvulsants, including divalproex, for rapid cycling bipolar disorder and BPD.2 Family studies have been notable for their failure to directly characterize family members, however, and clinical trials have been quite small. Further, treatment response may have very limited nosologic implications.
3. Bipolar disorder is a risk factor for BPD
4. BPD is a risk factor for bipolar disorder
Early emergence of a bipolar disorder (in preadolescent or adolescent patients) has been proposed to disrupt psychological development, leading to BPD. This adverse impact on personality development—the “scar hypothesis”3 —is supported by data showing greater risk of co-occurring BPD with earlier onset bipolar disorder.4 More important, prospective studies of patients with bipolar disorder show a greater risk for developing BPD.5
BPD also may be a risk factor for the development of bipolar disorder—the “vulnerability hypothesis.”3 Patients with BPD are more likely to develop bipolar disorder, even compared to patients with other personality disorders.5
5. Shared risk factors
BPD and bipolar disorder may be linked by shared risk factors, such as shared genes or trait neuroticism.3
*Some evidence supports each potential explanation, and they are not necessarily mutually exclusive
References
a. Akiskal HS. Demystifying borderline personality: critique of the concept and unorthodox reflections on its natural kinship with the bipolar spectrum. Acta Psychiatr Scand. 2004;110(6):401-407.
b. Mackinnon DF, Pies R. Affective instability as rapid cycling: theoretical and clinical implications for borderline personality and bipolar spectrum disorders. Bipolar Disord. 2006;8(1):1-14.
c. Christensen MV, Kessing LV. Do personality traits predict first onset in depressive and bipolar disorder? Nord J Psychiatry. 2006;60(2):79-88.
d. Goldberg JF, Garno JL. Age at onset of bipolar disorder and risk for comorbid borderline personality disorder. Bipolar Disord. 2009;11(2):205-208.
e. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
Overlapping symptoms
Bipolar disorder is generally considered a clinical disorder or brain disease that can be understood as a broken mood “thermostat.” The lifetime prevalence of bipolar types I and II is approximately 2%.3 Approximately one-half of patients have a family history of illness, and multiple genes are believed to influence inheritance. Mania is the disorder’s hallmark,4 although overactivity has alternatively been proposed as a core feature.5 Most patients with mania ultimately experience depression6 ( Table 1 ).
No dimensional personality correlates have been consistently demonstrated in bipolar disorder, although co-occurring personality disorders—often the “dramatic” Cluster B type—are common4,7 and may adversely affect treatment response and suicide risk.8,9
Both bipolar disorder and BPD are associated with considerable risk of suicide or suicide attempts.10,11 Self-mutilation or self-injurious behavior without suicidal intent are particularly common in BPD.12 Threats of suicide—which may be manipulative or help-seeking—also are common in BPD and tend to be acute rather than chronic.13
Borderline personality disorder is characterized by an enduring and inflexible pattern of thoughts, feelings, and behaviors that impairs an individual’s psychosocial or vocational function. Its estimated prevalence is approximately 1%,14 although recent community estimates approach 6%.15 Genetic influences play a lesser etiologic role in BPD than in bipolar disorder.
Several of BPD’s common features ( Table 2 )—impulsivity, mood instability, inappropriate anger, suicidal behavior, and unstable relationships—are shared with bipolar disorder, but patients with BPD tend to show higher levels of impulsiveness and hostility than patients with bipolar disorder.16 Dimensional assessments of personality traits suggest that BPD is characterized by high neuroticism and low agreeableness.17 BPD also has been more strongly associated with a childhood history of abuse, even when compared with control groups having other personality disorders or major depression.18 The male-to-female ratio for bipolar disorder approximates 1:1;3 in BPD this ratio has been estimated at 1:4 in clinical samples19 and near 1:1 in community samples.15
BPD and bipolar disorder often co-occur. Evidence indicates ≤20% of patients with BPD have comorbid bipolar disorder20 and 15% of patients with bipolar disorder have comorbid BPD.21 Co-occurrence happens much more often than would be expected by chance. These similar bidirectional comorbidity estimates (15% to 20%) would not be expected for conditions of such differing prevalence (<1% vs 2% or more). This suggests:
- the estimated prevalence of bipolar disorder in BPD is too low
- the estimated prevalence of BPD in bipolar disorder samples is too high
- borderline personality disorder is present in >1% of the population
- bipolar disorder is less common
- some combination of the above.
Among these possibilities, the prevalence estimates of bipolar disorder are the most consistent. Several studies suggest that BPD may be much more common, with some estimates exceeding 5%.15
Table 1
Common signs and symptoms
associated with mania and depression in bipolar disorder
| (Hypo)mania | Depression |
|---|---|
| Elevated mood | Decreased mood |
| Irritability | Irritability |
| Decreased need for sleep | Anhedonia |
| Grandiosity | Decreased self-attitude |
| Talkativeness | Insomnia/hypersomnia |
| Racing thoughts | Change in appetite/weight |
| Increased motor activity | Fatigue |
| Increased sex drive | Hopelessness |
| Religiosity | Suicidal thoughts |
| Distractibility | Impaired concentration |
Table 2
Borderline personality disorder: Commonly reported features
| Impulsivity |
| Unstable relationships |
| Unstable self-image |
| Affective instability |
| Fear of abandonment |
| Recurrent self-injurious or suicidal behavior |
| Feelings of emptiness |
| Intense anger or hostility |
| Transient paranoia or dissociative symptoms |
Roots of misdiagnosis
The presence of bipolar disorder or BPD may increase the risk that the other will be misdiagnosed. When symptoms of both are present, those suggesting 1 diagnosis may reflect the consequences of the other. A diagnosis of BPD could represent a partially treated or treatment-resistant bipolar disorder, or a BPD diagnosis could be the result of several years of disruption by a mood disorder.
Characteristics of bipolar disorder have contributed to clinician bias in favor of that diagnosis rather than BPD ( Table 3 ).22,23 Bipolar disorder also may be misdiagnosed as BPD. This error may most likely occur when the history focuses excessively on cross-sectional symptoms, such as when a patient with bipolar disorder shows prominent mood lability or interpersonal sensitivity during a mood episode but not when euthymic.
Bipolar II disorder. The confusion between bipolar disorder and BPD may be particularly problematic for patients with bipolar II disorder or subthreshold bipolar disorders. The manias of bipolar I disorder are much more readily distinguishable from the mood instability or reactivity of BPD. The manic symptoms of bipolar I are more florid, more pronounced, and lead to more obvious impairment.
The milder highs of bipolar II may resemble the mood fluctuations seen in BPD. Further, bipolar II is characterized by a greater chronicity and affective morbidity than bipolar I, and episodes of illness may be characterized by irritability, anger, and racing thoughts.24 Whereas impulsivity or aggression are more characteristic of BPD, bipolar II is similar to BPD on dimensions of affective instability.24,25
When present in BPD, affective instability or lability is conceptualized as ultra-rapid or ultradian, with a frequency of hours to days. BPD is less likely than bipolar II to show affective lability between depression and euthymia or elation and more likely to show fluctuations into anger and anxiety.26
Nonetheless, because of the increased prominence of shared features and reduced distinguishing features, bipolar II and BPD are prone to misdiagnosis and commonly co-occur.
Table 3
Clinician biases that may favor a bipolar disorder diagnosis, rather than BPD
| Bipolar disorder is supported by decades of research |
| Patients with bipolar disorder are often considered more “likeable” than those with BPD |
| Bipolar disorder is more treatable and has a better long-term outcome than BPD (although BPD is generally characterized by clinical improvement, whereas bipolar disorder is more stable with perhaps some increase in depressive symptom burden) |
| Widely thought to have a biologic basis, the bipolar diagnosis conveys less stigma than BPD, which often is less empathically attributed to the patient’s own failings |
| A bipolar diagnosis is easier to explain to patients than BPD; many psychiatrists have difficulty explaining personality disorders in terms patients understand |
| BPD: borderline personality disorder |
| Source: References 22,23 |
History, the diagnostic key
A thorough and rigorous psychiatric history is essential to distinguish BPD from bipolar disorder. Supplementing the patient’s history with an informant interview is often helpful.
Because personality disorders are considered a chronic and enduring pattern of maladaptive behavior, focus the history on longitudinal course and not simply cross-sectional symptoms. Thus, symptoms suggestive of BPD that are confined only to clearly defined episodes of mood disturbance and are absent during euthymia would not warrant a BPD diagnosis.
Temporal relationship. A detailed chronologic history can help determine the temporal relationship between any borderline features and mood episodes. When the patient’s life story is used as a scaffold for the phenomenologic portions of the psychiatric history, one can determine whether any such functional impairment is confined to episodes of mood disorder or appears as an enduring pattern of thinking, acting, and relating. Exploring what happened at notable life transitions—leaving school, loss of job, divorce/separation—may be similarly helpful.
Family history of psychiatric illness may provide a clue to an individual’s genetic predisposition but, of course, does not determine diagnosis. A detailed family and social history that provides evidence of an individual’s function in school, work, and interpersonal relationships is more relevant.
Abandonment and identity issues. Essential to BPD is fear of abandonment, often an undue fear that those important to patients will leave them. Patients may go to extremes to avoid being “abandoned,” even when this threat is not genuine.27,28 Their insecure attachments often lead them to fear being alone. The patient with BPD may:
- make frantic phone calls or send text messages to a friend or lover seeking reassurance
- take extreme measures such as refusing to leave the person’s home or pleading with them not to leave.
Patients with BPD often struggle with identity disturbance, leading them to wonder who they are and what their beliefs and core values are.29 Although occasionally patients with bipolar disorder may have these symptoms, they are not characteristic of bipolar disorder.
Mood lability. The time course of changes in affect or mood swings also may help distinguish BPD from bipolar disorder.
- With bipolar disorder the shift typically is from depression to elation or the reverse, and moods are sustained. Manias or hypomanias are often immediately followed by a “crash” into depression.
- With BPD, “roller-coaster moods” are typical, mood shifts are nonsustained, and the poles often are anxiety, anger, or desperation.
Patients with BPD often report moods shifting rapidly over minutes or hours, but they rarely describe moods sustained for days or weeks on end—other than perhaps depression. Mood lability of BPD often is produced by interpersonal sensitivity, whereas mood lability in bipolar disorder tends to be autonomous and persistent.
Young patients. Assessment can be particularly challenging in young adults and adolescents because symptoms of an emerging bipolar disorder can be more difficult to distinguish from BPD.30 Patients this young also may have less longitudinal history to distinguish an enduring pattern of thinking and relating from a mood disorder. For these cases, it may be particularly important to classify the frequency and pattern of mood symptoms.
Affective dysregulation is a core feature of BPD and is variably defined as a mood reactivity, typically of short duration (often hours). Cycling in bipolar disorder classically involves a periodicity of weeks to months. Even the broadest definitions include a minimum duration of 2 days for hypomania.5
Mood reactivity can occur within episodes of bipolar disorder, although episodes may occur spontaneously and without an obvious precipitant or stressor. Impulsivity may represent more of an essential feature of BPD than affective instability or mood reactivity and may be of particular diagnostic relevance.
Treatment implications
When you are unable to make a clear diagnosis, describe your clinical reasoning and differential diagnosis in the assessment or formulation. With close follow-up, the longitudinal history and course of illness may eventually lead you to an accurate diagnosis.
There are good reasons to acknowledge both conditions when bipolar disorder and BPD are present. Proper recognition of bipolar disorder is a prerequisite to taking full advantage of proven pharmacologic treatments. The evidence base for pharmacologic management of BPD remains limited,31 but recognizing this disorder may help the patient understand his or her psychiatric history and encourage the use of effective psychosocial treatments.
Psychosocial treatments for bipolar disorder may target demoralization and circadian rhythms with sleep hygiene or social rhythms therapy. Acknowledging BPD:
- helps both clinician and patient to better understand the condition
- facilitates setting realistic treatment goals because BPD tends to respond to medication less robustly than bipolar disorder.
Recognizing BPD also allows for referral to targeted psychosocial treatments, including dialectical behavior therapy, mentalization-based treatment, or Systems Training for Emotional Predictability and Problem Solving (STEPPS).32-34
Related resources
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
- National Institute of Mental Health. Bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml.
- Systems Training for Emotional Predictability and Problem Solving (STEPPS). www.uihealthcare.com/topics/medicaldepartments/psychiatry/stepps/index.html.
Drug brand name
- Divalproex • Depakote, Depakene, others
Disclosures
Dr. Fiedorowicz reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Acknowledgment
The authors would like to thank Nancee Blum, MSW, and Nancy Hale, RN, for their assistance and expertise in the preparation of this article.
1. John H, Sharma V. Misdiagnosis of bipolar disorder as borderline personality disorder: clinical and economic consequences. World J Biol Psychiatry. 2007;1-4[epub ahead of print].
2. Lequesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004;10(3):170-176.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351(5):476-486.
5. Benazzi F. Testing new diagnostic criteria for hypomania. Ann Clin Psychiatry. 2007;19(2):99-104.
6. Solomon DA, Leon AC, Endicott J, et al. Unipolar mania over the course of a 20-year follow-up study. Am J Psychiatry. 2003;160(11):2049-2051.
7. Schiavone P, Dorz S, Conforti D, et al. Comorbidity of DSM-IV personality disorders in unipolar and bipolar affective disorders: a comparative study. Psychol Rep. 2004;95(1):121-128.
8. Fan AH, Hassell J. Bipolar disorder and comorbid personality psychopathology: a review of the literature. J Clin Psychiatry. 2008;69(11):1794-1803.
9. Garno JL, Goldberg JF, Ramirez PM, et al. Bipolar disorder with comorbid cluster B personality disorder features: impact on suicidality. J Clin Psychiatry. 2005;66(3):339-345.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Fiedorowicz JG, Leon AC, Keller MB, et al. Do risk factors for suicidal behavior differ by affective disorder polarity? Psychol Med. 2009;39(5):763-771.
12. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13(3):179-185.
13. Zanarini MC, Frankenburg FR, Hennen J, et al. The McLean Study of Adult Development (MSAD): overview and implications of the first six years of prospective follow-up. J Pers Disord. 2005;19(5):505-523.
14. Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Arch Gen Psychiatry. 2001;58(6):590-596.
15. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
16. Wilson ST, Stanley B, Oquendo MA, et al. Comparing impulsiveness, hostility, and depression in borderline personality disorder and bipolar II disorder. J Clin Psychiatry. 2007;68(10):1533-1539.
17. Zweig-Frank H, Paris J. The five-factor model of personality in borderline and nonborderline personality disorders. Can J Psychiatry. 1995;40(9):523-526.
18. Zanarini MC, Frankenburg FR, Reich DB, et al. Adult experiences of abuse reported by borderline patients and Axis II comparison subjects over six years of prospective follow-up. J Nerv Ment Dis. 2005;193(6):412-416.
19. Zanarini MC, Frankenburg FR, Reich DB, et al. Violence in the lives of adult borderline patients. J Nerv Ment Dis. 1999;187(2):65-71.
20. McCormick B, Blum N, Hansel R, et al. Relationship of sex to symptom severity, psychiatric comorbidity, and health care utilization in 163 subjects with borderline personality disorder. Compr Psychiatry. 2007;48(5):406-412.
21. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
22. Paris J, Zweig-Frank H. A 27-year follow-up of patients with borderline personality disorder. Compr Psychiatry. 2001;42(6):482-487.
23. Coryell WH, Fiedorowicz JG, Solomon D, et al. Age transitions in the course of bipolar I disorder. Psychol Med. 2009;39(8):1247-1252.
24. Benazzi F. Borderline personality-bipolar spectrum relationship. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(1):68-74.
25. Critchfield KL, Levy KN, Clarkin JF. The relationship between impulsivity, aggression, and impulsive-aggression in borderline personality disorder: an empirical analysis of self-report measures. J Pers Disord. 2004;18(6):555-570.
26. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatr Res. 2001;35(6):307-312.
27. Bray A. The extended mind and borderline personality disorder. Australas Psychiatry. 2008;16(1):8-12.
28. Gunderson JG. The borderline patient’s intolerance of aloneness: insecure attachments and therapist availability. Am J Psychiatry. 1996;153(6):752-758.
29. Jorgensen CR. Disturbed sense of identity in borderline personality disorder. J Pers Disord. 2006;20(6):618-644.
30. Smith DJ, Muir WJ, Blackwood DH. Borderline personality disorder characteristics in young adults with recurrent mood disorders: a comparison of bipolar and unipolar depression. J Affect Disord. 2005;87(1):17-23.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006(1);CD005653.-
32. Lynch TR, Trost WT, Salsman N, et al. Dialectical behavior therapy for borderline personality disorder. Annu Rev Clin Psychol. 2007;3:181-205.
33. Bateman AW, Fonagy P. Mentalization-based treatment of BPD. J Pers Disord. 2004;18(1):36-51.
34. Blum N, St John D, Pfohl B, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165(4):468-478.
1. John H, Sharma V. Misdiagnosis of bipolar disorder as borderline personality disorder: clinical and economic consequences. World J Biol Psychiatry. 2007;1-4[epub ahead of print].
2. Lequesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004;10(3):170-176.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351(5):476-486.
5. Benazzi F. Testing new diagnostic criteria for hypomania. Ann Clin Psychiatry. 2007;19(2):99-104.
6. Solomon DA, Leon AC, Endicott J, et al. Unipolar mania over the course of a 20-year follow-up study. Am J Psychiatry. 2003;160(11):2049-2051.
7. Schiavone P, Dorz S, Conforti D, et al. Comorbidity of DSM-IV personality disorders in unipolar and bipolar affective disorders: a comparative study. Psychol Rep. 2004;95(1):121-128.
8. Fan AH, Hassell J. Bipolar disorder and comorbid personality psychopathology: a review of the literature. J Clin Psychiatry. 2008;69(11):1794-1803.
9. Garno JL, Goldberg JF, Ramirez PM, et al. Bipolar disorder with comorbid cluster B personality disorder features: impact on suicidality. J Clin Psychiatry. 2005;66(3):339-345.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Fiedorowicz JG, Leon AC, Keller MB, et al. Do risk factors for suicidal behavior differ by affective disorder polarity? Psychol Med. 2009;39(5):763-771.
12. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13(3):179-185.
13. Zanarini MC, Frankenburg FR, Hennen J, et al. The McLean Study of Adult Development (MSAD): overview and implications of the first six years of prospective follow-up. J Pers Disord. 2005;19(5):505-523.
14. Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Arch Gen Psychiatry. 2001;58(6):590-596.
15. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
16. Wilson ST, Stanley B, Oquendo MA, et al. Comparing impulsiveness, hostility, and depression in borderline personality disorder and bipolar II disorder. J Clin Psychiatry. 2007;68(10):1533-1539.
17. Zweig-Frank H, Paris J. The five-factor model of personality in borderline and nonborderline personality disorders. Can J Psychiatry. 1995;40(9):523-526.
18. Zanarini MC, Frankenburg FR, Reich DB, et al. Adult experiences of abuse reported by borderline patients and Axis II comparison subjects over six years of prospective follow-up. J Nerv Ment Dis. 2005;193(6):412-416.
19. Zanarini MC, Frankenburg FR, Reich DB, et al. Violence in the lives of adult borderline patients. J Nerv Ment Dis. 1999;187(2):65-71.
20. McCormick B, Blum N, Hansel R, et al. Relationship of sex to symptom severity, psychiatric comorbidity, and health care utilization in 163 subjects with borderline personality disorder. Compr Psychiatry. 2007;48(5):406-412.
21. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
22. Paris J, Zweig-Frank H. A 27-year follow-up of patients with borderline personality disorder. Compr Psychiatry. 2001;42(6):482-487.
23. Coryell WH, Fiedorowicz JG, Solomon D, et al. Age transitions in the course of bipolar I disorder. Psychol Med. 2009;39(8):1247-1252.
24. Benazzi F. Borderline personality-bipolar spectrum relationship. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(1):68-74.
25. Critchfield KL, Levy KN, Clarkin JF. The relationship between impulsivity, aggression, and impulsive-aggression in borderline personality disorder: an empirical analysis of self-report measures. J Pers Disord. 2004;18(6):555-570.
26. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatr Res. 2001;35(6):307-312.
27. Bray A. The extended mind and borderline personality disorder. Australas Psychiatry. 2008;16(1):8-12.
28. Gunderson JG. The borderline patient’s intolerance of aloneness: insecure attachments and therapist availability. Am J Psychiatry. 1996;153(6):752-758.
29. Jorgensen CR. Disturbed sense of identity in borderline personality disorder. J Pers Disord. 2006;20(6):618-644.
30. Smith DJ, Muir WJ, Blackwood DH. Borderline personality disorder characteristics in young adults with recurrent mood disorders: a comparison of bipolar and unipolar depression. J Affect Disord. 2005;87(1):17-23.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006(1);CD005653.-
32. Lynch TR, Trost WT, Salsman N, et al. Dialectical behavior therapy for borderline personality disorder. Annu Rev Clin Psychol. 2007;3:181-205.
33. Bateman AW, Fonagy P. Mentalization-based treatment of BPD. J Pers Disord. 2004;18(1):36-51.
34. Blum N, St John D, Pfohl B, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165(4):468-478.
Corticosteroid psychosis: Stop therapy or add psychotropics?
Mrs. E, age 31, develops rapid, pressured speech and insomnia for 4 consecutive nights, but reports a normal energy level after receiving high-dose methylprednisolone for an acute flare of systemic lupus erythematosus (SLE).
Her medical history indicates an overlap syndrome between SLE and systemic sclerosis for the last 5 years, migraine headaches, and 4 spontaneous miscarriages, but she has no psychiatric history. Her family history is negative for psychiatric illness and positive for diabetes mellitus, hypertension, and coronary artery disease.
Mrs. E lives with her husband and 10-year-old son. She admits to multiple stressors, including her health problems and financial dificulties, which recently led to the family’s decision to move to her mother-in-law’s house. Mrs. E denies using illicit drugs, cigarettes, or alcohol.
Mrs. E is admitted to the hospital, and her corticosteroid dosage is reduced with a switch to prednisone, 60 mg/d. She is started on risperidone, 1 mg at bedtime, which is titrated without adverse effect. Her psychotic symptoms improve over 4 days, and she is discharged on prednisone, 60 mg/d, and risperidone, 0.5 mg in the morning and 2 mg at night.
After completing her corticosteroid course, Mrs. E experiences complete resolution of psychiatric symptoms and is tapered off risperidone after 6 months.
Corticosteroid use can cause a variety of psychiatric syndromes, including mania, psychosis, depression, and delirium. A meta-analysis reports severe psychotic reactions in 5.7% of patients taking corticosteroids and mild-to-moderate reactions in 28% of patients.1 Hypomania, mania, and psychosis are the most common psychiatric reactions to acute corticosteroid therapy.2 This article reviews case reports and open-label trials of antipsychotics, mood stabilizers, and anticonvulsants to treat corticosteroid-induced mania and psychosis and outlines treatment options.
Symptoms
Corticosteroid-induced psychosis represents a spectrum of psychological changes that can occur at any time during treatment. Mild-to-moderate symptoms include agitation, anxiety, insomnia, irritability, and restlessness, whereas severe symptoms include mania, depression, and psychosis.3 Case reports reveal:
- mania and hypomania in 35% of patients with corticosteroid-induced psychosis
- acute psychotic disorder in 24% of patients, with hallucinations reported in one-half of these cases
- depression, which is more common with chronic corticosteroid therapy, in 28% of patients.4
Delirium and cognitive deficits also have been reported, although these symptoms generally subside with corticosteroid reduction or withdrawal.4,5
Psychiatric symptoms often develop after 4 days of corticosteroid therapy, although they can occur late in therapy or after treatment ends.6 Delirium often resolves within a few days, psychosis within 7 days, and mania within 2 to 3 weeks, whereas depression can last for more than 3 weeks.4 A 3-level grading system can gauge severity of corticosteroid-induced psychosis; grade 2 or 3 warrants treatment (Table 1).4
Table 1
Grading scale for corticosteroid-induced psychiatric symptoms
| Grade | Symptoms |
|---|---|
| Grade 1 | Mild, nonpathologic, and subclinical euphoria |
| Grade 2 | Reversible acute or subacute mania and/or depression |
| Grade 3 | Bipolar disorder with relapses possible without steroids |
| Source: Reference 4 | |
Risk factors
High corticosteroid dose is the primary risk factor for psychosis. The Boston Collaborative Drug Surveillance Program reported that among individuals taking prednisone, psychiatric disturbances are seen in:
- 1.3% of patients taking <40 mg/d
- 4.6% of patients taking 40 to 80 mg/d
- 18.4% of patients taking >80 mg/d.7
However, the corticosteroid dosage does not predict onset, severity, type of reaction, or duration.3,7 Female patients are at higher risk of corticosteroid-induced psychosis, even after one controls for medical conditions diagnosed more often in women, such as SLE and rheumatoid arthritis.3 Previous episodes of corticosteroid-induced psychosis, history of psychiatric illness, and age are not associated with corticosteroid-induced psychosis.3
Treatment
Management includes tapering corticosteroids, with or without adding medications to treat the acute state. Decreasing corticosteroids to the lowest dose possible—<40 mg/d—or gradually discontinuing therapy to prevent triggering adrenal insufficiency may improve psychotic symptoms and avoids the risk of adverse effects from adjunctive medications.
Psychopharmacologic treatment may be necessary, depending on the severity of psychosis or the underlying disease, particularly if corticosteroids cannot be tapered or discontinued. Evidence from open-label trials (Table 2)8-12 and case reports indicates that psychotic symptoms could be prevented and treated with off-label antipsychotics, mood stabilizers, and anticonvulsants.
Consider your patient’s underlying medical condition when selecting psychotropics. For example, try to avoid prescribing:
- antipsychotics to patients with cardiac conduction abnormalities
- lithium to patients who need diuretic or angiotensin-converting enzyme inhibitor therapy or those with underlying renal insufficiency.
When appropriate, collaborate with the provider who prescribed the corticosteroids because tapering or discontinuation might not be possible.
Table 2
Corticosteroid-induced psychosis: Adjunctive treatment studies
| Medication and source | Patient population | Results |
|---|---|---|
| Olanzapine (Brown et al, 20058) | 12 outpatients experiencing manic or mixed symptoms received olanzapine, mean 8.5 mg/d | Reductions on YMRS, HRSD, and BPRS with no change in extrapyramidal symptom side-effect scales, weight, or glucose measurements |
| Lithium (Falk et al, 19799) | 27 patients diagnosed with multiple sclerosis or retrobulbar neuritis treated with corticotropin received lithium | 38% of lithium patients developed psychiatric symptoms compared with 62% of controls |
| Phenytoin (Brown et al, 200510) | 39 patients received phenytoin, 300 mg/d, or placebo at prednisone therapy initiation | Patients receiving phenytoin reported a smaller increase in ACT score compared with controls |
| Levetiracetam (Brown et al, 200711) | 30 outpatients receiving corticosteroids randomized to levetiracetam, 1500 mg/d, or placebo | No significant change in HRSD, YMRS, or ACT scores |
| Lamotrigine (Brown et al, 200312) | 5 patients on chronic corticosteroid treatment received open-label lamotrigine, mean dose 340 mg/d | No significant difference in HRSD, YMRS, or the depression subscale of the Internal State Scale |
| ACT: Internal State Scale Activation subscale; BPRS: Brief Psychiatric Rating Scale; HRSD: Hamilton Rating Scale for Depression; YMRS: Young Mania Rating Scale | ||
Antipsychotics
Open-label trial. Olanzapine reduced psychiatric symptoms in a 5-week, open-label trial of 12 outpatients experiencing manic or mixed symptoms secondary to corticosteroids.8 At baseline, patients had a mean score of 15.25 on the Young Mania Rating Scale (YMRS) on a mean prednisone dose of 14.4 mg/d. After receiving olanzapine, 2.5 mg/d titrated to a maximum 20 mg/d (mean 8.5 mg/d), subjects demonstrated a significant decrease on the YMRS (P=.002), Hamilton Rating Scale for Depression (HRSD) (P=.005), and Brief Psychiatric Rating Scale (BPRS) (P=.006) with no change in extrapyramidal side-effect scales, weight, or glucose measurements.
Case reports. Among antipsychotics, olanzapine has the greatest number of case reports for treating corticosteroid-induced psychosis, mainly for mania.13-15 Benefit with olanzapine was demonstrated at dosages from 2.5 to 15 mg/d and improvement occurred within days to weeks. Several patients remained symptom-free with olanzapine and continued corticosteroid therapy.
Other reports describe benefit with risperidone for a variety of psychiatric symptoms—including hypomania, hallucinations, and delusions—associated with corticosteroid therapy.16-19 Risperidone dosing ranged from 1 to 4 mg/d, and symptoms improved within days to weeks.
One case report describes quetiapine for the treatment of corticosteroid-induced mania.20 The patient’s symptoms improved within 10 hours of initiating quetiapine, 25 mg/d, and YMRS score decreased from 31 before therapy to 5 at discharge. No case reports exist for ziprasidone or aripiprazole.
Mood stabilizers
Cohort study. One study suggests that lithium may be effective for preventing and treating corticosteroid-induced psychosis. A retrospective cohort study examined records of patients diagnosed with multiple sclerosis or retrobulbar neuritis who were treated with corticotropin.9 Corticotropin has been reported to cause psychotic reactions in up to 11% of patients through a mechanism thought to mirror corticosteroid-induced psychosis (Box).21-23 Psychiatric symptoms developed in 38% of patients treated with lithium compared with 62% of controls. No patients pretreated with lithium maintained at 0.8 to 1.2 mEq/L reported mood disturbances or psychotic reactions.
Case reports. Among mood stabilizers, lithium has the greatest number of case reports on its use for prevention and treatment of corticosteroid-induced psychosis. In these reports, patients pretreated with lithium did not experience a relapse of psychosis related to chronic corticosteroid therapy.24-27 Case reports also describe benefit with valproic acid and carbamazepine.28-30
Anticonvulsants
Trials. In a 1-week trial, 39 patients without previous psychiatric diagnosis or psychotropic use were randomly assigned to phenytoin, 300 mg/d, or placebo as prednisone therapy was initiated.10 Compared with placebo, the phenytoin group reported a smaller increase on the Internal State Scale Activation subscale (ACT), a self-report measure of mania symptom severity. No significant differences were found on the YMRS or HRSD scales. Based on the ACT scale finding, the authors concluded that phenytoin attenuated manic or hypomanic effects of prednisone.
A study of levetiracetam, 1500 mg/d, showed no significant change in HRSD, YMRS, or ACT scores from baseline to end point for either levetiracetam or placebo.11
A 12-week, open-label trial of lamotrigine in 5 patients receiving corticosteroids continuously for 6 months showed no significant difference in mood changes as measured by the HRSD, YMRS, or the depression sub-scale of the Internal State Scale.12
Case reports show that lamotrigine and gabapentin have been used effectively to prevent manic symptoms in patients receiving corticosteroid therapy.31,32
Treatment recommendations
Establishing a treatment algorithm for corticosteroid-induced psychosis is hampered by the lack of prospective placebo-controlled trials. However, most case reports describe benefit from administrating atypical antipsychotics and lithium.
How corticosteroids cause psychosis is not well understood. One theory suggests that corticosteroids act at steroid-specific receptors and suppress filtering by the hippocampus of irrelevant stimuli.21
Supporting this theory of hippocampal change, a study of 17 patients receiving corticosteroid therapy for >6 months found decreased hippocampal volume compared with a control group.22 Other possible causes include suppressed hypothalamus-pituitary axis and enhanced dopamine neurotransmission.23
Consider adding a low-dose atypical antipsychotic with which case studies report quick symptom resolution and patients tolerating these agents. Monitor carefully for metabolic changes, a risk associated with antipsychotics and corticosteroids. Lithium would be a good second-line therapy because of its demonstrated benefit for both prophylaxis and treatment of psychiatric disturbances.
Lithium use can be complicated and dangerous in patients who have underlying diseases associated with renal dysfunction, however—such as nephrotic syndromes and SLE—leading some authors to suggest valproic acid or carbamazepine instead.33 In addition, corticosteroid-induced changes in sodium balance could increase the risk of lithium toxicity.34
When patients cannot tolerate atypical antipsychotics or lithium, case reports support the use of valproic acid, carbamazepine, lamotrigine, or gabapentin to treat symptoms of corticosteroid-induced psychosis.
Related resources
- Cerullo MA. Expect psychiatric side effects from corticosteroid use in the elderly. Geriatrics. 2008;63(1):15-18.
- Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25:27-33.
- Patten SB, Neutel CI. Corticosteroid-induced adverse psychiatric effects: incidence, diagnosis, and management. Drug Safety. 2000;22(2):111-122.
- Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Corticotropin • Acthar
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium • Lithobid, Eskalith, others
- Methylprednisolone • Medrol
- Olanzapine • Zyprexa
- Phenytoin • Dilantin
- Prednisone • Deltasone
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproic acid • Depakene
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lewis DA, Smith RE. Steroid-induced psychiatric syndromes: a report of 14 cases and a review of the literature. J Affect Disord. 1983;5:319-332.
2. Bolanos SH, Khan DA, Hanczyc M, et al. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol. 2004;92:500-505.
3. Warrington TP, Bostwick JM. Psychiatric adverse effect of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
4. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25:27-33.
5. Newcomer JW, Craft S, Hershey T, et al. Glucocorticoid-induced impairments in declarative memory performance in adult humans. J Neurosci. 1991;14:2047-2053.
6. Hall RC, Popkin MK, Stickney SK, et al. Presentation of the steroid induced psychosis. J Nerv Ment Dis. 1979;167:229-236.
7. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13:694-698.
8. Brown ES, Chamberlain W, Dhanani N, et al. An open-label trial of olanzapine for corticosteroid-induced mood symptoms. J Affect Disord. 2004;83:277-281.
9. Falk WE, Mahnke MW, Poskanzer DC. Lithium prophylaxis of corticotropin-induced psychosis. JAMA. 1979;241:1011-1012.
10. Brown ES, Stuard G, Liggin JD, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Bio Psychiatry. 2005;57:543-548.
11. Brown ES, Frol AB, Khan DA, et al. Impact of levetiracetam on mood and cognition during prednisone therapy. Eur Psychiatry. 2007;22:448-452.
12. Brown ES, Frol A, Bobadilla L, et al. Effect of lamotrigine on mood and cognition in patients receiving chronic exogenous corticosteroids. Psychosomatics. 2003;44(3):204-208.
13. Goldman LS, Goveas J. Olanzapine treatment of corticosteroid-induced mood disorders. Psychosomatics. 2002;43(6):495-497.
14. Brown ES, Khan DA, Suppes T. Treatment of corticosteroid-induced mood changes with olanzapine. Am J Psychiatry. 1999;156(6):968.-
15. Budur K, Pozuelo L. Olanzapine for corticosteroid-induced mood disorders. Psychosomatics. 2003;44(4):353.-
16. Herguner S, Bilge I, Yavuz Yilmaz A, et al. Steroid-induced psychosis in an adolescent: treatment and prophylaxis with risperidone. Turk J Pediatr. 2006;48:244-247.
17. DeSilva CC, Nurse MC, Vokey K. Steroid-induced psychosis treated with risperidone. Can J Psychiatry. 2002;47:388-389.
18. Kato O, Misawa H. Steroid-induced psychosis treated with valproic acid and risperidone in a patient with systemic lupus erythematosus. Prim Care Companion J Clin Psychiatry. 2005;7(6):312.-
19. Kramer TM, Cottingham EM. Risperidone in the treatment of steroid-induced psychosis. J Child Adolec Psychopharmacol. 1999;9:315-316.
20. Siddiqui Z, Ramaswamy S, Petty F. Quetiapine therapy for coricosteroid-induced mania. Can J Psychiatry. 2005;50(1):77-78.
21. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days’ corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31.
22. Brown ES, Woolston DJ, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry. 2004;55:538-545.
23. Schatzberg AF, Rothschild AJ, Langlais PJ, et al. A corticosteroid/dopamine hypothesis for psychotic depression and related states. J Psychiat Res. 1985;19(1):57-64.
24. Sabet-Sharghi F, Hutzler JC. Prophylaxis of steroid-induced psychiatric syndromes. Psychosomatics. 1990;31(1):113-114.
25. Siegal FP. Lithium for steroid-induced psychosis. N Engl J Med. 1978;299(3):155-156.
26. Goggans FC, Weisberg LJ, Koran LM. Lithium prophylaxis of prednisone psychosis: a case report. J Clin Psychiatry. 1983;44(3):111-112.
27. Merrill W. Case 35-1998: use of lithium to prevent corticosteroid-induced mania. N Engl J Med. 1999;340(14):1123.-
28. Himelhoch S, Haller E. Extreme mood lability associated with systemic lupus erythematosus and stroke successfully treated with valproic acid. J Clin Psychopharmacol. 1996;16(6):469-470.
29. Kahn D, Stevenson E, Douglas CJ. Effect of sodium valproate in three patients with organic brain syndromes. Am J Psychiatry. 1998;145(8):1010-1011.
30. Abbas A, Styra R. Valproate prophylaxis against steroid induced psychosis. Can J Psychiatry. 1994;39(3):188-189.
31. Preda A, Fazeli A, McKay BG, et al. Lamotrigine as prophylaxis against steroid-induced mania. J Clin Psychiatry. 1999;60(10):708-709.
32. Ginsberg DL, Sussman N. Gabapentin as prophylaxis against corticosteroid-induced mania. Can J Psychiatry. 2001;44:455-456.
33. Wada K, Yamada N, Yamauchi Y, et al. Carbamazepine treatment of corticosteroid-induced mood disorder. J Affect Disord. 2001;65:315-317.
34. Saklad S. Management of corticosteroid-induced psychosis with lithium. Clin Pharm. 1987;6(3):186.-
Mrs. E, age 31, develops rapid, pressured speech and insomnia for 4 consecutive nights, but reports a normal energy level after receiving high-dose methylprednisolone for an acute flare of systemic lupus erythematosus (SLE).
Her medical history indicates an overlap syndrome between SLE and systemic sclerosis for the last 5 years, migraine headaches, and 4 spontaneous miscarriages, but she has no psychiatric history. Her family history is negative for psychiatric illness and positive for diabetes mellitus, hypertension, and coronary artery disease.
Mrs. E lives with her husband and 10-year-old son. She admits to multiple stressors, including her health problems and financial dificulties, which recently led to the family’s decision to move to her mother-in-law’s house. Mrs. E denies using illicit drugs, cigarettes, or alcohol.
Mrs. E is admitted to the hospital, and her corticosteroid dosage is reduced with a switch to prednisone, 60 mg/d. She is started on risperidone, 1 mg at bedtime, which is titrated without adverse effect. Her psychotic symptoms improve over 4 days, and she is discharged on prednisone, 60 mg/d, and risperidone, 0.5 mg in the morning and 2 mg at night.
After completing her corticosteroid course, Mrs. E experiences complete resolution of psychiatric symptoms and is tapered off risperidone after 6 months.
Corticosteroid use can cause a variety of psychiatric syndromes, including mania, psychosis, depression, and delirium. A meta-analysis reports severe psychotic reactions in 5.7% of patients taking corticosteroids and mild-to-moderate reactions in 28% of patients.1 Hypomania, mania, and psychosis are the most common psychiatric reactions to acute corticosteroid therapy.2 This article reviews case reports and open-label trials of antipsychotics, mood stabilizers, and anticonvulsants to treat corticosteroid-induced mania and psychosis and outlines treatment options.
Symptoms
Corticosteroid-induced psychosis represents a spectrum of psychological changes that can occur at any time during treatment. Mild-to-moderate symptoms include agitation, anxiety, insomnia, irritability, and restlessness, whereas severe symptoms include mania, depression, and psychosis.3 Case reports reveal:
- mania and hypomania in 35% of patients with corticosteroid-induced psychosis
- acute psychotic disorder in 24% of patients, with hallucinations reported in one-half of these cases
- depression, which is more common with chronic corticosteroid therapy, in 28% of patients.4
Delirium and cognitive deficits also have been reported, although these symptoms generally subside with corticosteroid reduction or withdrawal.4,5
Psychiatric symptoms often develop after 4 days of corticosteroid therapy, although they can occur late in therapy or after treatment ends.6 Delirium often resolves within a few days, psychosis within 7 days, and mania within 2 to 3 weeks, whereas depression can last for more than 3 weeks.4 A 3-level grading system can gauge severity of corticosteroid-induced psychosis; grade 2 or 3 warrants treatment (Table 1).4
Table 1
Grading scale for corticosteroid-induced psychiatric symptoms
| Grade | Symptoms |
|---|---|
| Grade 1 | Mild, nonpathologic, and subclinical euphoria |
| Grade 2 | Reversible acute or subacute mania and/or depression |
| Grade 3 | Bipolar disorder with relapses possible without steroids |
| Source: Reference 4 | |
Risk factors
High corticosteroid dose is the primary risk factor for psychosis. The Boston Collaborative Drug Surveillance Program reported that among individuals taking prednisone, psychiatric disturbances are seen in:
- 1.3% of patients taking <40 mg/d
- 4.6% of patients taking 40 to 80 mg/d
- 18.4% of patients taking >80 mg/d.7
However, the corticosteroid dosage does not predict onset, severity, type of reaction, or duration.3,7 Female patients are at higher risk of corticosteroid-induced psychosis, even after one controls for medical conditions diagnosed more often in women, such as SLE and rheumatoid arthritis.3 Previous episodes of corticosteroid-induced psychosis, history of psychiatric illness, and age are not associated with corticosteroid-induced psychosis.3
Treatment
Management includes tapering corticosteroids, with or without adding medications to treat the acute state. Decreasing corticosteroids to the lowest dose possible—<40 mg/d—or gradually discontinuing therapy to prevent triggering adrenal insufficiency may improve psychotic symptoms and avoids the risk of adverse effects from adjunctive medications.
Psychopharmacologic treatment may be necessary, depending on the severity of psychosis or the underlying disease, particularly if corticosteroids cannot be tapered or discontinued. Evidence from open-label trials (Table 2)8-12 and case reports indicates that psychotic symptoms could be prevented and treated with off-label antipsychotics, mood stabilizers, and anticonvulsants.
Consider your patient’s underlying medical condition when selecting psychotropics. For example, try to avoid prescribing:
- antipsychotics to patients with cardiac conduction abnormalities
- lithium to patients who need diuretic or angiotensin-converting enzyme inhibitor therapy or those with underlying renal insufficiency.
When appropriate, collaborate with the provider who prescribed the corticosteroids because tapering or discontinuation might not be possible.
Table 2
Corticosteroid-induced psychosis: Adjunctive treatment studies
| Medication and source | Patient population | Results |
|---|---|---|
| Olanzapine (Brown et al, 20058) | 12 outpatients experiencing manic or mixed symptoms received olanzapine, mean 8.5 mg/d | Reductions on YMRS, HRSD, and BPRS with no change in extrapyramidal symptom side-effect scales, weight, or glucose measurements |
| Lithium (Falk et al, 19799) | 27 patients diagnosed with multiple sclerosis or retrobulbar neuritis treated with corticotropin received lithium | 38% of lithium patients developed psychiatric symptoms compared with 62% of controls |
| Phenytoin (Brown et al, 200510) | 39 patients received phenytoin, 300 mg/d, or placebo at prednisone therapy initiation | Patients receiving phenytoin reported a smaller increase in ACT score compared with controls |
| Levetiracetam (Brown et al, 200711) | 30 outpatients receiving corticosteroids randomized to levetiracetam, 1500 mg/d, or placebo | No significant change in HRSD, YMRS, or ACT scores |
| Lamotrigine (Brown et al, 200312) | 5 patients on chronic corticosteroid treatment received open-label lamotrigine, mean dose 340 mg/d | No significant difference in HRSD, YMRS, or the depression subscale of the Internal State Scale |
| ACT: Internal State Scale Activation subscale; BPRS: Brief Psychiatric Rating Scale; HRSD: Hamilton Rating Scale for Depression; YMRS: Young Mania Rating Scale | ||
Antipsychotics
Open-label trial. Olanzapine reduced psychiatric symptoms in a 5-week, open-label trial of 12 outpatients experiencing manic or mixed symptoms secondary to corticosteroids.8 At baseline, patients had a mean score of 15.25 on the Young Mania Rating Scale (YMRS) on a mean prednisone dose of 14.4 mg/d. After receiving olanzapine, 2.5 mg/d titrated to a maximum 20 mg/d (mean 8.5 mg/d), subjects demonstrated a significant decrease on the YMRS (P=.002), Hamilton Rating Scale for Depression (HRSD) (P=.005), and Brief Psychiatric Rating Scale (BPRS) (P=.006) with no change in extrapyramidal side-effect scales, weight, or glucose measurements.
Case reports. Among antipsychotics, olanzapine has the greatest number of case reports for treating corticosteroid-induced psychosis, mainly for mania.13-15 Benefit with olanzapine was demonstrated at dosages from 2.5 to 15 mg/d and improvement occurred within days to weeks. Several patients remained symptom-free with olanzapine and continued corticosteroid therapy.
Other reports describe benefit with risperidone for a variety of psychiatric symptoms—including hypomania, hallucinations, and delusions—associated with corticosteroid therapy.16-19 Risperidone dosing ranged from 1 to 4 mg/d, and symptoms improved within days to weeks.
One case report describes quetiapine for the treatment of corticosteroid-induced mania.20 The patient’s symptoms improved within 10 hours of initiating quetiapine, 25 mg/d, and YMRS score decreased from 31 before therapy to 5 at discharge. No case reports exist for ziprasidone or aripiprazole.
Mood stabilizers
Cohort study. One study suggests that lithium may be effective for preventing and treating corticosteroid-induced psychosis. A retrospective cohort study examined records of patients diagnosed with multiple sclerosis or retrobulbar neuritis who were treated with corticotropin.9 Corticotropin has been reported to cause psychotic reactions in up to 11% of patients through a mechanism thought to mirror corticosteroid-induced psychosis (Box).21-23 Psychiatric symptoms developed in 38% of patients treated with lithium compared with 62% of controls. No patients pretreated with lithium maintained at 0.8 to 1.2 mEq/L reported mood disturbances or psychotic reactions.
Case reports. Among mood stabilizers, lithium has the greatest number of case reports on its use for prevention and treatment of corticosteroid-induced psychosis. In these reports, patients pretreated with lithium did not experience a relapse of psychosis related to chronic corticosteroid therapy.24-27 Case reports also describe benefit with valproic acid and carbamazepine.28-30
Anticonvulsants
Trials. In a 1-week trial, 39 patients without previous psychiatric diagnosis or psychotropic use were randomly assigned to phenytoin, 300 mg/d, or placebo as prednisone therapy was initiated.10 Compared with placebo, the phenytoin group reported a smaller increase on the Internal State Scale Activation subscale (ACT), a self-report measure of mania symptom severity. No significant differences were found on the YMRS or HRSD scales. Based on the ACT scale finding, the authors concluded that phenytoin attenuated manic or hypomanic effects of prednisone.
A study of levetiracetam, 1500 mg/d, showed no significant change in HRSD, YMRS, or ACT scores from baseline to end point for either levetiracetam or placebo.11
A 12-week, open-label trial of lamotrigine in 5 patients receiving corticosteroids continuously for 6 months showed no significant difference in mood changes as measured by the HRSD, YMRS, or the depression sub-scale of the Internal State Scale.12
Case reports show that lamotrigine and gabapentin have been used effectively to prevent manic symptoms in patients receiving corticosteroid therapy.31,32
Treatment recommendations
Establishing a treatment algorithm for corticosteroid-induced psychosis is hampered by the lack of prospective placebo-controlled trials. However, most case reports describe benefit from administrating atypical antipsychotics and lithium.
How corticosteroids cause psychosis is not well understood. One theory suggests that corticosteroids act at steroid-specific receptors and suppress filtering by the hippocampus of irrelevant stimuli.21
Supporting this theory of hippocampal change, a study of 17 patients receiving corticosteroid therapy for >6 months found decreased hippocampal volume compared with a control group.22 Other possible causes include suppressed hypothalamus-pituitary axis and enhanced dopamine neurotransmission.23
Consider adding a low-dose atypical antipsychotic with which case studies report quick symptom resolution and patients tolerating these agents. Monitor carefully for metabolic changes, a risk associated with antipsychotics and corticosteroids. Lithium would be a good second-line therapy because of its demonstrated benefit for both prophylaxis and treatment of psychiatric disturbances.
Lithium use can be complicated and dangerous in patients who have underlying diseases associated with renal dysfunction, however—such as nephrotic syndromes and SLE—leading some authors to suggest valproic acid or carbamazepine instead.33 In addition, corticosteroid-induced changes in sodium balance could increase the risk of lithium toxicity.34
When patients cannot tolerate atypical antipsychotics or lithium, case reports support the use of valproic acid, carbamazepine, lamotrigine, or gabapentin to treat symptoms of corticosteroid-induced psychosis.
Related resources
- Cerullo MA. Expect psychiatric side effects from corticosteroid use in the elderly. Geriatrics. 2008;63(1):15-18.
- Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25:27-33.
- Patten SB, Neutel CI. Corticosteroid-induced adverse psychiatric effects: incidence, diagnosis, and management. Drug Safety. 2000;22(2):111-122.
- Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Corticotropin • Acthar
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium • Lithobid, Eskalith, others
- Methylprednisolone • Medrol
- Olanzapine • Zyprexa
- Phenytoin • Dilantin
- Prednisone • Deltasone
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproic acid • Depakene
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mrs. E, age 31, develops rapid, pressured speech and insomnia for 4 consecutive nights, but reports a normal energy level after receiving high-dose methylprednisolone for an acute flare of systemic lupus erythematosus (SLE).
Her medical history indicates an overlap syndrome between SLE and systemic sclerosis for the last 5 years, migraine headaches, and 4 spontaneous miscarriages, but she has no psychiatric history. Her family history is negative for psychiatric illness and positive for diabetes mellitus, hypertension, and coronary artery disease.
Mrs. E lives with her husband and 10-year-old son. She admits to multiple stressors, including her health problems and financial dificulties, which recently led to the family’s decision to move to her mother-in-law’s house. Mrs. E denies using illicit drugs, cigarettes, or alcohol.
Mrs. E is admitted to the hospital, and her corticosteroid dosage is reduced with a switch to prednisone, 60 mg/d. She is started on risperidone, 1 mg at bedtime, which is titrated without adverse effect. Her psychotic symptoms improve over 4 days, and she is discharged on prednisone, 60 mg/d, and risperidone, 0.5 mg in the morning and 2 mg at night.
After completing her corticosteroid course, Mrs. E experiences complete resolution of psychiatric symptoms and is tapered off risperidone after 6 months.
Corticosteroid use can cause a variety of psychiatric syndromes, including mania, psychosis, depression, and delirium. A meta-analysis reports severe psychotic reactions in 5.7% of patients taking corticosteroids and mild-to-moderate reactions in 28% of patients.1 Hypomania, mania, and psychosis are the most common psychiatric reactions to acute corticosteroid therapy.2 This article reviews case reports and open-label trials of antipsychotics, mood stabilizers, and anticonvulsants to treat corticosteroid-induced mania and psychosis and outlines treatment options.
Symptoms
Corticosteroid-induced psychosis represents a spectrum of psychological changes that can occur at any time during treatment. Mild-to-moderate symptoms include agitation, anxiety, insomnia, irritability, and restlessness, whereas severe symptoms include mania, depression, and psychosis.3 Case reports reveal:
- mania and hypomania in 35% of patients with corticosteroid-induced psychosis
- acute psychotic disorder in 24% of patients, with hallucinations reported in one-half of these cases
- depression, which is more common with chronic corticosteroid therapy, in 28% of patients.4
Delirium and cognitive deficits also have been reported, although these symptoms generally subside with corticosteroid reduction or withdrawal.4,5
Psychiatric symptoms often develop after 4 days of corticosteroid therapy, although they can occur late in therapy or after treatment ends.6 Delirium often resolves within a few days, psychosis within 7 days, and mania within 2 to 3 weeks, whereas depression can last for more than 3 weeks.4 A 3-level grading system can gauge severity of corticosteroid-induced psychosis; grade 2 or 3 warrants treatment (Table 1).4
Table 1
Grading scale for corticosteroid-induced psychiatric symptoms
| Grade | Symptoms |
|---|---|
| Grade 1 | Mild, nonpathologic, and subclinical euphoria |
| Grade 2 | Reversible acute or subacute mania and/or depression |
| Grade 3 | Bipolar disorder with relapses possible without steroids |
| Source: Reference 4 | |
Risk factors
High corticosteroid dose is the primary risk factor for psychosis. The Boston Collaborative Drug Surveillance Program reported that among individuals taking prednisone, psychiatric disturbances are seen in:
- 1.3% of patients taking <40 mg/d
- 4.6% of patients taking 40 to 80 mg/d
- 18.4% of patients taking >80 mg/d.7
However, the corticosteroid dosage does not predict onset, severity, type of reaction, or duration.3,7 Female patients are at higher risk of corticosteroid-induced psychosis, even after one controls for medical conditions diagnosed more often in women, such as SLE and rheumatoid arthritis.3 Previous episodes of corticosteroid-induced psychosis, history of psychiatric illness, and age are not associated with corticosteroid-induced psychosis.3
Treatment
Management includes tapering corticosteroids, with or without adding medications to treat the acute state. Decreasing corticosteroids to the lowest dose possible—<40 mg/d—or gradually discontinuing therapy to prevent triggering adrenal insufficiency may improve psychotic symptoms and avoids the risk of adverse effects from adjunctive medications.
Psychopharmacologic treatment may be necessary, depending on the severity of psychosis or the underlying disease, particularly if corticosteroids cannot be tapered or discontinued. Evidence from open-label trials (Table 2)8-12 and case reports indicates that psychotic symptoms could be prevented and treated with off-label antipsychotics, mood stabilizers, and anticonvulsants.
Consider your patient’s underlying medical condition when selecting psychotropics. For example, try to avoid prescribing:
- antipsychotics to patients with cardiac conduction abnormalities
- lithium to patients who need diuretic or angiotensin-converting enzyme inhibitor therapy or those with underlying renal insufficiency.
When appropriate, collaborate with the provider who prescribed the corticosteroids because tapering or discontinuation might not be possible.
Table 2
Corticosteroid-induced psychosis: Adjunctive treatment studies
| Medication and source | Patient population | Results |
|---|---|---|
| Olanzapine (Brown et al, 20058) | 12 outpatients experiencing manic or mixed symptoms received olanzapine, mean 8.5 mg/d | Reductions on YMRS, HRSD, and BPRS with no change in extrapyramidal symptom side-effect scales, weight, or glucose measurements |
| Lithium (Falk et al, 19799) | 27 patients diagnosed with multiple sclerosis or retrobulbar neuritis treated with corticotropin received lithium | 38% of lithium patients developed psychiatric symptoms compared with 62% of controls |
| Phenytoin (Brown et al, 200510) | 39 patients received phenytoin, 300 mg/d, or placebo at prednisone therapy initiation | Patients receiving phenytoin reported a smaller increase in ACT score compared with controls |
| Levetiracetam (Brown et al, 200711) | 30 outpatients receiving corticosteroids randomized to levetiracetam, 1500 mg/d, or placebo | No significant change in HRSD, YMRS, or ACT scores |
| Lamotrigine (Brown et al, 200312) | 5 patients on chronic corticosteroid treatment received open-label lamotrigine, mean dose 340 mg/d | No significant difference in HRSD, YMRS, or the depression subscale of the Internal State Scale |
| ACT: Internal State Scale Activation subscale; BPRS: Brief Psychiatric Rating Scale; HRSD: Hamilton Rating Scale for Depression; YMRS: Young Mania Rating Scale | ||
Antipsychotics
Open-label trial. Olanzapine reduced psychiatric symptoms in a 5-week, open-label trial of 12 outpatients experiencing manic or mixed symptoms secondary to corticosteroids.8 At baseline, patients had a mean score of 15.25 on the Young Mania Rating Scale (YMRS) on a mean prednisone dose of 14.4 mg/d. After receiving olanzapine, 2.5 mg/d titrated to a maximum 20 mg/d (mean 8.5 mg/d), subjects demonstrated a significant decrease on the YMRS (P=.002), Hamilton Rating Scale for Depression (HRSD) (P=.005), and Brief Psychiatric Rating Scale (BPRS) (P=.006) with no change in extrapyramidal side-effect scales, weight, or glucose measurements.
Case reports. Among antipsychotics, olanzapine has the greatest number of case reports for treating corticosteroid-induced psychosis, mainly for mania.13-15 Benefit with olanzapine was demonstrated at dosages from 2.5 to 15 mg/d and improvement occurred within days to weeks. Several patients remained symptom-free with olanzapine and continued corticosteroid therapy.
Other reports describe benefit with risperidone for a variety of psychiatric symptoms—including hypomania, hallucinations, and delusions—associated with corticosteroid therapy.16-19 Risperidone dosing ranged from 1 to 4 mg/d, and symptoms improved within days to weeks.
One case report describes quetiapine for the treatment of corticosteroid-induced mania.20 The patient’s symptoms improved within 10 hours of initiating quetiapine, 25 mg/d, and YMRS score decreased from 31 before therapy to 5 at discharge. No case reports exist for ziprasidone or aripiprazole.
Mood stabilizers
Cohort study. One study suggests that lithium may be effective for preventing and treating corticosteroid-induced psychosis. A retrospective cohort study examined records of patients diagnosed with multiple sclerosis or retrobulbar neuritis who were treated with corticotropin.9 Corticotropin has been reported to cause psychotic reactions in up to 11% of patients through a mechanism thought to mirror corticosteroid-induced psychosis (Box).21-23 Psychiatric symptoms developed in 38% of patients treated with lithium compared with 62% of controls. No patients pretreated with lithium maintained at 0.8 to 1.2 mEq/L reported mood disturbances or psychotic reactions.
Case reports. Among mood stabilizers, lithium has the greatest number of case reports on its use for prevention and treatment of corticosteroid-induced psychosis. In these reports, patients pretreated with lithium did not experience a relapse of psychosis related to chronic corticosteroid therapy.24-27 Case reports also describe benefit with valproic acid and carbamazepine.28-30
Anticonvulsants
Trials. In a 1-week trial, 39 patients without previous psychiatric diagnosis or psychotropic use were randomly assigned to phenytoin, 300 mg/d, or placebo as prednisone therapy was initiated.10 Compared with placebo, the phenytoin group reported a smaller increase on the Internal State Scale Activation subscale (ACT), a self-report measure of mania symptom severity. No significant differences were found on the YMRS or HRSD scales. Based on the ACT scale finding, the authors concluded that phenytoin attenuated manic or hypomanic effects of prednisone.
A study of levetiracetam, 1500 mg/d, showed no significant change in HRSD, YMRS, or ACT scores from baseline to end point for either levetiracetam or placebo.11
A 12-week, open-label trial of lamotrigine in 5 patients receiving corticosteroids continuously for 6 months showed no significant difference in mood changes as measured by the HRSD, YMRS, or the depression sub-scale of the Internal State Scale.12
Case reports show that lamotrigine and gabapentin have been used effectively to prevent manic symptoms in patients receiving corticosteroid therapy.31,32
Treatment recommendations
Establishing a treatment algorithm for corticosteroid-induced psychosis is hampered by the lack of prospective placebo-controlled trials. However, most case reports describe benefit from administrating atypical antipsychotics and lithium.
How corticosteroids cause psychosis is not well understood. One theory suggests that corticosteroids act at steroid-specific receptors and suppress filtering by the hippocampus of irrelevant stimuli.21
Supporting this theory of hippocampal change, a study of 17 patients receiving corticosteroid therapy for >6 months found decreased hippocampal volume compared with a control group.22 Other possible causes include suppressed hypothalamus-pituitary axis and enhanced dopamine neurotransmission.23
Consider adding a low-dose atypical antipsychotic with which case studies report quick symptom resolution and patients tolerating these agents. Monitor carefully for metabolic changes, a risk associated with antipsychotics and corticosteroids. Lithium would be a good second-line therapy because of its demonstrated benefit for both prophylaxis and treatment of psychiatric disturbances.
Lithium use can be complicated and dangerous in patients who have underlying diseases associated with renal dysfunction, however—such as nephrotic syndromes and SLE—leading some authors to suggest valproic acid or carbamazepine instead.33 In addition, corticosteroid-induced changes in sodium balance could increase the risk of lithium toxicity.34
When patients cannot tolerate atypical antipsychotics or lithium, case reports support the use of valproic acid, carbamazepine, lamotrigine, or gabapentin to treat symptoms of corticosteroid-induced psychosis.
Related resources
- Cerullo MA. Expect psychiatric side effects from corticosteroid use in the elderly. Geriatrics. 2008;63(1):15-18.
- Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25:27-33.
- Patten SB, Neutel CI. Corticosteroid-induced adverse psychiatric effects: incidence, diagnosis, and management. Drug Safety. 2000;22(2):111-122.
- Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Corticotropin • Acthar
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Lithium • Lithobid, Eskalith, others
- Methylprednisolone • Medrol
- Olanzapine • Zyprexa
- Phenytoin • Dilantin
- Prednisone • Deltasone
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproic acid • Depakene
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lewis DA, Smith RE. Steroid-induced psychiatric syndromes: a report of 14 cases and a review of the literature. J Affect Disord. 1983;5:319-332.
2. Bolanos SH, Khan DA, Hanczyc M, et al. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol. 2004;92:500-505.
3. Warrington TP, Bostwick JM. Psychiatric adverse effect of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
4. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25:27-33.
5. Newcomer JW, Craft S, Hershey T, et al. Glucocorticoid-induced impairments in declarative memory performance in adult humans. J Neurosci. 1991;14:2047-2053.
6. Hall RC, Popkin MK, Stickney SK, et al. Presentation of the steroid induced psychosis. J Nerv Ment Dis. 1979;167:229-236.
7. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13:694-698.
8. Brown ES, Chamberlain W, Dhanani N, et al. An open-label trial of olanzapine for corticosteroid-induced mood symptoms. J Affect Disord. 2004;83:277-281.
9. Falk WE, Mahnke MW, Poskanzer DC. Lithium prophylaxis of corticotropin-induced psychosis. JAMA. 1979;241:1011-1012.
10. Brown ES, Stuard G, Liggin JD, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Bio Psychiatry. 2005;57:543-548.
11. Brown ES, Frol AB, Khan DA, et al. Impact of levetiracetam on mood and cognition during prednisone therapy. Eur Psychiatry. 2007;22:448-452.
12. Brown ES, Frol A, Bobadilla L, et al. Effect of lamotrigine on mood and cognition in patients receiving chronic exogenous corticosteroids. Psychosomatics. 2003;44(3):204-208.
13. Goldman LS, Goveas J. Olanzapine treatment of corticosteroid-induced mood disorders. Psychosomatics. 2002;43(6):495-497.
14. Brown ES, Khan DA, Suppes T. Treatment of corticosteroid-induced mood changes with olanzapine. Am J Psychiatry. 1999;156(6):968.-
15. Budur K, Pozuelo L. Olanzapine for corticosteroid-induced mood disorders. Psychosomatics. 2003;44(4):353.-
16. Herguner S, Bilge I, Yavuz Yilmaz A, et al. Steroid-induced psychosis in an adolescent: treatment and prophylaxis with risperidone. Turk J Pediatr. 2006;48:244-247.
17. DeSilva CC, Nurse MC, Vokey K. Steroid-induced psychosis treated with risperidone. Can J Psychiatry. 2002;47:388-389.
18. Kato O, Misawa H. Steroid-induced psychosis treated with valproic acid and risperidone in a patient with systemic lupus erythematosus. Prim Care Companion J Clin Psychiatry. 2005;7(6):312.-
19. Kramer TM, Cottingham EM. Risperidone in the treatment of steroid-induced psychosis. J Child Adolec Psychopharmacol. 1999;9:315-316.
20. Siddiqui Z, Ramaswamy S, Petty F. Quetiapine therapy for coricosteroid-induced mania. Can J Psychiatry. 2005;50(1):77-78.
21. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days’ corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31.
22. Brown ES, Woolston DJ, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry. 2004;55:538-545.
23. Schatzberg AF, Rothschild AJ, Langlais PJ, et al. A corticosteroid/dopamine hypothesis for psychotic depression and related states. J Psychiat Res. 1985;19(1):57-64.
24. Sabet-Sharghi F, Hutzler JC. Prophylaxis of steroid-induced psychiatric syndromes. Psychosomatics. 1990;31(1):113-114.
25. Siegal FP. Lithium for steroid-induced psychosis. N Engl J Med. 1978;299(3):155-156.
26. Goggans FC, Weisberg LJ, Koran LM. Lithium prophylaxis of prednisone psychosis: a case report. J Clin Psychiatry. 1983;44(3):111-112.
27. Merrill W. Case 35-1998: use of lithium to prevent corticosteroid-induced mania. N Engl J Med. 1999;340(14):1123.-
28. Himelhoch S, Haller E. Extreme mood lability associated with systemic lupus erythematosus and stroke successfully treated with valproic acid. J Clin Psychopharmacol. 1996;16(6):469-470.
29. Kahn D, Stevenson E, Douglas CJ. Effect of sodium valproate in three patients with organic brain syndromes. Am J Psychiatry. 1998;145(8):1010-1011.
30. Abbas A, Styra R. Valproate prophylaxis against steroid induced psychosis. Can J Psychiatry. 1994;39(3):188-189.
31. Preda A, Fazeli A, McKay BG, et al. Lamotrigine as prophylaxis against steroid-induced mania. J Clin Psychiatry. 1999;60(10):708-709.
32. Ginsberg DL, Sussman N. Gabapentin as prophylaxis against corticosteroid-induced mania. Can J Psychiatry. 2001;44:455-456.
33. Wada K, Yamada N, Yamauchi Y, et al. Carbamazepine treatment of corticosteroid-induced mood disorder. J Affect Disord. 2001;65:315-317.
34. Saklad S. Management of corticosteroid-induced psychosis with lithium. Clin Pharm. 1987;6(3):186.-
1. Lewis DA, Smith RE. Steroid-induced psychiatric syndromes: a report of 14 cases and a review of the literature. J Affect Disord. 1983;5:319-332.
2. Bolanos SH, Khan DA, Hanczyc M, et al. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol. 2004;92:500-505.
3. Warrington TP, Bostwick JM. Psychiatric adverse effect of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
4. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25:27-33.
5. Newcomer JW, Craft S, Hershey T, et al. Glucocorticoid-induced impairments in declarative memory performance in adult humans. J Neurosci. 1991;14:2047-2053.
6. Hall RC, Popkin MK, Stickney SK, et al. Presentation of the steroid induced psychosis. J Nerv Ment Dis. 1979;167:229-236.
7. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13:694-698.
8. Brown ES, Chamberlain W, Dhanani N, et al. An open-label trial of olanzapine for corticosteroid-induced mood symptoms. J Affect Disord. 2004;83:277-281.
9. Falk WE, Mahnke MW, Poskanzer DC. Lithium prophylaxis of corticotropin-induced psychosis. JAMA. 1979;241:1011-1012.
10. Brown ES, Stuard G, Liggin JD, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Bio Psychiatry. 2005;57:543-548.
11. Brown ES, Frol AB, Khan DA, et al. Impact of levetiracetam on mood and cognition during prednisone therapy. Eur Psychiatry. 2007;22:448-452.
12. Brown ES, Frol A, Bobadilla L, et al. Effect of lamotrigine on mood and cognition in patients receiving chronic exogenous corticosteroids. Psychosomatics. 2003;44(3):204-208.
13. Goldman LS, Goveas J. Olanzapine treatment of corticosteroid-induced mood disorders. Psychosomatics. 2002;43(6):495-497.
14. Brown ES, Khan DA, Suppes T. Treatment of corticosteroid-induced mood changes with olanzapine. Am J Psychiatry. 1999;156(6):968.-
15. Budur K, Pozuelo L. Olanzapine for corticosteroid-induced mood disorders. Psychosomatics. 2003;44(4):353.-
16. Herguner S, Bilge I, Yavuz Yilmaz A, et al. Steroid-induced psychosis in an adolescent: treatment and prophylaxis with risperidone. Turk J Pediatr. 2006;48:244-247.
17. DeSilva CC, Nurse MC, Vokey K. Steroid-induced psychosis treated with risperidone. Can J Psychiatry. 2002;47:388-389.
18. Kato O, Misawa H. Steroid-induced psychosis treated with valproic acid and risperidone in a patient with systemic lupus erythematosus. Prim Care Companion J Clin Psychiatry. 2005;7(6):312.-
19. Kramer TM, Cottingham EM. Risperidone in the treatment of steroid-induced psychosis. J Child Adolec Psychopharmacol. 1999;9:315-316.
20. Siddiqui Z, Ramaswamy S, Petty F. Quetiapine therapy for coricosteroid-induced mania. Can J Psychiatry. 2005;50(1):77-78.
21. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days’ corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31.
22. Brown ES, Woolston DJ, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry. 2004;55:538-545.
23. Schatzberg AF, Rothschild AJ, Langlais PJ, et al. A corticosteroid/dopamine hypothesis for psychotic depression and related states. J Psychiat Res. 1985;19(1):57-64.
24. Sabet-Sharghi F, Hutzler JC. Prophylaxis of steroid-induced psychiatric syndromes. Psychosomatics. 1990;31(1):113-114.
25. Siegal FP. Lithium for steroid-induced psychosis. N Engl J Med. 1978;299(3):155-156.
26. Goggans FC, Weisberg LJ, Koran LM. Lithium prophylaxis of prednisone psychosis: a case report. J Clin Psychiatry. 1983;44(3):111-112.
27. Merrill W. Case 35-1998: use of lithium to prevent corticosteroid-induced mania. N Engl J Med. 1999;340(14):1123.-
28. Himelhoch S, Haller E. Extreme mood lability associated with systemic lupus erythematosus and stroke successfully treated with valproic acid. J Clin Psychopharmacol. 1996;16(6):469-470.
29. Kahn D, Stevenson E, Douglas CJ. Effect of sodium valproate in three patients with organic brain syndromes. Am J Psychiatry. 1998;145(8):1010-1011.
30. Abbas A, Styra R. Valproate prophylaxis against steroid induced psychosis. Can J Psychiatry. 1994;39(3):188-189.
31. Preda A, Fazeli A, McKay BG, et al. Lamotrigine as prophylaxis against steroid-induced mania. J Clin Psychiatry. 1999;60(10):708-709.
32. Ginsberg DL, Sussman N. Gabapentin as prophylaxis against corticosteroid-induced mania. Can J Psychiatry. 2001;44:455-456.
33. Wada K, Yamada N, Yamauchi Y, et al. Carbamazepine treatment of corticosteroid-induced mood disorder. J Affect Disord. 2001;65:315-317.
34. Saklad S. Management of corticosteroid-induced psychosis with lithium. Clin Pharm. 1987;6(3):186.-
Opioid use could cause mania
In “A mysterious case of mania” (Cases That Test Your Skills, Current Psychiatry), we concur with the authors’ diagnosis of a substance-induced mood disorder (secondary mania) in Mrs. P, who presented with an abrupt onset of a mania with no known history of a similar episode. The authors list substances that could have provoked this patient’s mania: antidepressants, phenylephrine in an over-the-counter medication, and sudden withdrawal of methadone. We propose another possible contributing factor: the direct mood-elevating effect of opioids. We previously reported this clinically important potential adverse effect in a study of patients with bipolar disorder.1
A clue to preexisting bipolar disorder in Mrs. P is that she had been taking sertraline and desipramine prescribed for “unknown reasons.” Mrs. P reportedly accidentally overdosed on maintenance opioid pain medications (methadone, hydrocodone, and tramadol) 2 weeks before the onset of her manic episode. This overdose probably caused a sudden increase in opioid blood levels, which could have caused the manic episode. Psychiatrists should be alert for this possible reaction in patients with bipolar disorder who take opioids prescribed by other physicians.
Charles B. Schaffer, MD
Clinical professor of psychiatry
Thomas E. Nordahl, MD, PhD
Professor of psychiatry
University of California Davis School of Medicine
Sacramento, CA
Reference
1. Schaffer C, Nordahl T, Schaffer L, et al. Mood-elevating effects of opioid analgesics in patients with bipolar disorder. J Neuropsychiatry Clin Neurosci. 2007;19(4):449-452.
The authors respond
The question of whether opioid pain medications could induce mania is not without some controversy. A literature search revealed only 2 case reports on tramadol-induced mania.1,2 Our case presentation discussed a manic syndrome that was most likely cause by multiple substances. With Mrs. P’s complex polypharmacy, we were unable to pinpoint the exact cause of her symptoms. If we hypothesize that she had mania induced by tramadol, we still have to include other factors that were present on the day of admission, such as antidepressants, phenylephrine in an over-the-counter medicine, and sudden withdrawal of methadone.
Gold et al1 present data that support the hypothesis that opioids have antidepressant, antimanic, and antipanic effects. Interestingly, case reports suggest that opioids may have an antidepressant effect in patients with affective disorders.1,2 A few case reports have noted hypomanic or manic symptoms associated with opioid use.3-5 Specific opioids suggested to have this property include the μ-opioid agonists tramadol and codeine and the partial agonist buprenorphine.
To our knowledge, there are no case reports commenting on possible manic effects from the use of oxycodone, hydrocodone, methadone, or morphine. Sleep loss also may trigger mania and plays an important role in the condition.
Magdalena Romanowicz, MD
Psychiatry resident
Timothy W. Lineberry, MD
Assistant professor
Mayo Clinic
Rochester, MN
1. Gold MS, Pottash AC, Sweeney D, et al. Antimanic, antidepressant, and antipanic effects of opiates: clinical, neuroanatomical, and biochemical evidence. Ann N Y Acad Sci. 1982;398:140-150.
2. Emrich HM, Vogt P, Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Ann N Y Acad Sci. 1982;398:108-112.
3. Gonzalez-Pinto A, Imaz H, De Heredia JL, et al. Mania and tramadol-fluoxetine combination. Am J Psychiatry. 2001;158(6):964-965.
4. Watts BV, Grady TA. Tramadol-induced mania. Am J Psychiatry. 1997;154(11):1624.-
5. Orr KG, Mostert J, Castle DJ. Mania associated with codeine and paracetamol. Aust N Z J Psychiatry. 1998;32:586-588.
In “A mysterious case of mania” (Cases That Test Your Skills, Current Psychiatry), we concur with the authors’ diagnosis of a substance-induced mood disorder (secondary mania) in Mrs. P, who presented with an abrupt onset of a mania with no known history of a similar episode. The authors list substances that could have provoked this patient’s mania: antidepressants, phenylephrine in an over-the-counter medication, and sudden withdrawal of methadone. We propose another possible contributing factor: the direct mood-elevating effect of opioids. We previously reported this clinically important potential adverse effect in a study of patients with bipolar disorder.1
A clue to preexisting bipolar disorder in Mrs. P is that she had been taking sertraline and desipramine prescribed for “unknown reasons.” Mrs. P reportedly accidentally overdosed on maintenance opioid pain medications (methadone, hydrocodone, and tramadol) 2 weeks before the onset of her manic episode. This overdose probably caused a sudden increase in opioid blood levels, which could have caused the manic episode. Psychiatrists should be alert for this possible reaction in patients with bipolar disorder who take opioids prescribed by other physicians.
Charles B. Schaffer, MD
Clinical professor of psychiatry
Thomas E. Nordahl, MD, PhD
Professor of psychiatry
University of California Davis School of Medicine
Sacramento, CA
Reference
1. Schaffer C, Nordahl T, Schaffer L, et al. Mood-elevating effects of opioid analgesics in patients with bipolar disorder. J Neuropsychiatry Clin Neurosci. 2007;19(4):449-452.
The authors respond
The question of whether opioid pain medications could induce mania is not without some controversy. A literature search revealed only 2 case reports on tramadol-induced mania.1,2 Our case presentation discussed a manic syndrome that was most likely cause by multiple substances. With Mrs. P’s complex polypharmacy, we were unable to pinpoint the exact cause of her symptoms. If we hypothesize that she had mania induced by tramadol, we still have to include other factors that were present on the day of admission, such as antidepressants, phenylephrine in an over-the-counter medicine, and sudden withdrawal of methadone.
Gold et al1 present data that support the hypothesis that opioids have antidepressant, antimanic, and antipanic effects. Interestingly, case reports suggest that opioids may have an antidepressant effect in patients with affective disorders.1,2 A few case reports have noted hypomanic or manic symptoms associated with opioid use.3-5 Specific opioids suggested to have this property include the μ-opioid agonists tramadol and codeine and the partial agonist buprenorphine.
To our knowledge, there are no case reports commenting on possible manic effects from the use of oxycodone, hydrocodone, methadone, or morphine. Sleep loss also may trigger mania and plays an important role in the condition.
Magdalena Romanowicz, MD
Psychiatry resident
Timothy W. Lineberry, MD
Assistant professor
Mayo Clinic
Rochester, MN
In “A mysterious case of mania” (Cases That Test Your Skills, Current Psychiatry), we concur with the authors’ diagnosis of a substance-induced mood disorder (secondary mania) in Mrs. P, who presented with an abrupt onset of a mania with no known history of a similar episode. The authors list substances that could have provoked this patient’s mania: antidepressants, phenylephrine in an over-the-counter medication, and sudden withdrawal of methadone. We propose another possible contributing factor: the direct mood-elevating effect of opioids. We previously reported this clinically important potential adverse effect in a study of patients with bipolar disorder.1
A clue to preexisting bipolar disorder in Mrs. P is that she had been taking sertraline and desipramine prescribed for “unknown reasons.” Mrs. P reportedly accidentally overdosed on maintenance opioid pain medications (methadone, hydrocodone, and tramadol) 2 weeks before the onset of her manic episode. This overdose probably caused a sudden increase in opioid blood levels, which could have caused the manic episode. Psychiatrists should be alert for this possible reaction in patients with bipolar disorder who take opioids prescribed by other physicians.
Charles B. Schaffer, MD
Clinical professor of psychiatry
Thomas E. Nordahl, MD, PhD
Professor of psychiatry
University of California Davis School of Medicine
Sacramento, CA
Reference
1. Schaffer C, Nordahl T, Schaffer L, et al. Mood-elevating effects of opioid analgesics in patients with bipolar disorder. J Neuropsychiatry Clin Neurosci. 2007;19(4):449-452.
The authors respond
The question of whether opioid pain medications could induce mania is not without some controversy. A literature search revealed only 2 case reports on tramadol-induced mania.1,2 Our case presentation discussed a manic syndrome that was most likely cause by multiple substances. With Mrs. P’s complex polypharmacy, we were unable to pinpoint the exact cause of her symptoms. If we hypothesize that she had mania induced by tramadol, we still have to include other factors that were present on the day of admission, such as antidepressants, phenylephrine in an over-the-counter medicine, and sudden withdrawal of methadone.
Gold et al1 present data that support the hypothesis that opioids have antidepressant, antimanic, and antipanic effects. Interestingly, case reports suggest that opioids may have an antidepressant effect in patients with affective disorders.1,2 A few case reports have noted hypomanic or manic symptoms associated with opioid use.3-5 Specific opioids suggested to have this property include the μ-opioid agonists tramadol and codeine and the partial agonist buprenorphine.
To our knowledge, there are no case reports commenting on possible manic effects from the use of oxycodone, hydrocodone, methadone, or morphine. Sleep loss also may trigger mania and plays an important role in the condition.
Magdalena Romanowicz, MD
Psychiatry resident
Timothy W. Lineberry, MD
Assistant professor
Mayo Clinic
Rochester, MN
1. Gold MS, Pottash AC, Sweeney D, et al. Antimanic, antidepressant, and antipanic effects of opiates: clinical, neuroanatomical, and biochemical evidence. Ann N Y Acad Sci. 1982;398:140-150.
2. Emrich HM, Vogt P, Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Ann N Y Acad Sci. 1982;398:108-112.
3. Gonzalez-Pinto A, Imaz H, De Heredia JL, et al. Mania and tramadol-fluoxetine combination. Am J Psychiatry. 2001;158(6):964-965.
4. Watts BV, Grady TA. Tramadol-induced mania. Am J Psychiatry. 1997;154(11):1624.-
5. Orr KG, Mostert J, Castle DJ. Mania associated with codeine and paracetamol. Aust N Z J Psychiatry. 1998;32:586-588.
1. Gold MS, Pottash AC, Sweeney D, et al. Antimanic, antidepressant, and antipanic effects of opiates: clinical, neuroanatomical, and biochemical evidence. Ann N Y Acad Sci. 1982;398:140-150.
2. Emrich HM, Vogt P, Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Ann N Y Acad Sci. 1982;398:108-112.
3. Gonzalez-Pinto A, Imaz H, De Heredia JL, et al. Mania and tramadol-fluoxetine combination. Am J Psychiatry. 2001;158(6):964-965.
4. Watts BV, Grady TA. Tramadol-induced mania. Am J Psychiatry. 1997;154(11):1624.-
5. Orr KG, Mostert J, Castle DJ. Mania associated with codeine and paracetamol. Aust N Z J Psychiatry. 1998;32:586-588.
Consider cost and efficacy
After reading “A case of sudden psychosis” (Cases That Test Your Skills, Current Psychiatry), I wondered why the authors administered ziprasidone IM and olanzapine IM when haloperidol and lorazepam IM would have been effective for a lower cost. I have seen clinicians use ziprasidone and lorazepam instead of haloperidol and lorazepam for acute agitation, but there is little evidence that it is better.
In a naturalistic study, Preval et al1 reported that ziprasidone is as effective as haloperidol plus lorazepam, and observed that ziprasidone patients wake up sooner and therefore can be triaged more quickly, but this statement may be premature.
I think there are few, if any, reasons to use ziprasidone or olanzapine IM instead of haloperidol, 5 mg, and lorazepam IM, 2 mg every 6 hours as needed, in an emergency setting. I am curious if anyone has a different opinion based on scientific evidence.
Corey Yilmaz, MD
Southwest Behavioral Health Services
Tolleson, AZ
Reference
1. Preval H, Klotz SG, Southard R, et al. Rapid-acting IM ziprasidone in a psychiatric emergency service: a naturalistic study. Gen Hosp Psychiatry. 2005;27(2):140-144.
The authors respond
At 2 of our local hospital pharmacies, the cost of haloperidol combined with lorazepam is approximately $9 less than ziprasidone per administration: $1.51 vs $10.29 and $3.08 vs $12.23, respectively. Certainly, imagining this cost difference on a larger scale highlights the importance of cost efficiency, but herein enters the fear of sacrificing optimal patient care for the sake of budgeting.
With respect to efficacy, ziprasidone IM has been found to be superior to haloperidol IM in the acute setting.1 In a recent study of severe agitation in adolescent patients, ziprasidone monotherapy was as effective as haloperidol plus lorazepam.2 Regarding side effects, ziprasidone has demonstrated greater safety in terms of extrapyramidal symptoms and akathisia when objectively measured with the Extrapyramidal Symptom Rating Scale and Barnes Akathisia Scale.3 Change in QTc generally was comparable between the 2 medications; however, a more substantial change with haloperidol was seen at 24 hours, given its longer half-life.
Finally, as Dr. Yilmaz mentions, less sedation with ziprasidone vs haloperidol is a marked advantage in the emergency room, which is where our patient initially received these medications. In the case of our previously healthy patient presenting with new-onset psychiatric symptoms, return to a lucid state was important to elucidate an accurate diagnosis and implement appropriate treatment promptly.
Cathy Southammakosane, MD
Pediatrics/psychiatry/child psychiatry resident
Cincinnati Children’s Hospital Medical Center
Anthony Cavalieri, MD
General psychiatry resident
Christopher White, MD, JD, FCLM
Assistant professor of psychiatry and family medicine
University of Cincinnati
Cincinnati, OH
1. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. J Clin Psychiatry. 2000;61(12):933-941.
2. Jangro WC, Preval H, Southard R, et al. Conventional intramuscular sedatives versus ziprasidone for severe agitation in adolescents: case-control study. Child Adolesc Psychiatry Ment Health. 2009;3(1):9.-
3. Brook S, Walden J, Benattia I, et al. Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment study. Psychopharmacology (Berl). 2005;178(4):514-523.
After reading “A case of sudden psychosis” (Cases That Test Your Skills, Current Psychiatry), I wondered why the authors administered ziprasidone IM and olanzapine IM when haloperidol and lorazepam IM would have been effective for a lower cost. I have seen clinicians use ziprasidone and lorazepam instead of haloperidol and lorazepam for acute agitation, but there is little evidence that it is better.
In a naturalistic study, Preval et al1 reported that ziprasidone is as effective as haloperidol plus lorazepam, and observed that ziprasidone patients wake up sooner and therefore can be triaged more quickly, but this statement may be premature.
I think there are few, if any, reasons to use ziprasidone or olanzapine IM instead of haloperidol, 5 mg, and lorazepam IM, 2 mg every 6 hours as needed, in an emergency setting. I am curious if anyone has a different opinion based on scientific evidence.
Corey Yilmaz, MD
Southwest Behavioral Health Services
Tolleson, AZ
Reference
1. Preval H, Klotz SG, Southard R, et al. Rapid-acting IM ziprasidone in a psychiatric emergency service: a naturalistic study. Gen Hosp Psychiatry. 2005;27(2):140-144.
The authors respond
At 2 of our local hospital pharmacies, the cost of haloperidol combined with lorazepam is approximately $9 less than ziprasidone per administration: $1.51 vs $10.29 and $3.08 vs $12.23, respectively. Certainly, imagining this cost difference on a larger scale highlights the importance of cost efficiency, but herein enters the fear of sacrificing optimal patient care for the sake of budgeting.
With respect to efficacy, ziprasidone IM has been found to be superior to haloperidol IM in the acute setting.1 In a recent study of severe agitation in adolescent patients, ziprasidone monotherapy was as effective as haloperidol plus lorazepam.2 Regarding side effects, ziprasidone has demonstrated greater safety in terms of extrapyramidal symptoms and akathisia when objectively measured with the Extrapyramidal Symptom Rating Scale and Barnes Akathisia Scale.3 Change in QTc generally was comparable between the 2 medications; however, a more substantial change with haloperidol was seen at 24 hours, given its longer half-life.
Finally, as Dr. Yilmaz mentions, less sedation with ziprasidone vs haloperidol is a marked advantage in the emergency room, which is where our patient initially received these medications. In the case of our previously healthy patient presenting with new-onset psychiatric symptoms, return to a lucid state was important to elucidate an accurate diagnosis and implement appropriate treatment promptly.
Cathy Southammakosane, MD
Pediatrics/psychiatry/child psychiatry resident
Cincinnati Children’s Hospital Medical Center
Anthony Cavalieri, MD
General psychiatry resident
Christopher White, MD, JD, FCLM
Assistant professor of psychiatry and family medicine
University of Cincinnati
Cincinnati, OH
After reading “A case of sudden psychosis” (Cases That Test Your Skills, Current Psychiatry), I wondered why the authors administered ziprasidone IM and olanzapine IM when haloperidol and lorazepam IM would have been effective for a lower cost. I have seen clinicians use ziprasidone and lorazepam instead of haloperidol and lorazepam for acute agitation, but there is little evidence that it is better.
In a naturalistic study, Preval et al1 reported that ziprasidone is as effective as haloperidol plus lorazepam, and observed that ziprasidone patients wake up sooner and therefore can be triaged more quickly, but this statement may be premature.
I think there are few, if any, reasons to use ziprasidone or olanzapine IM instead of haloperidol, 5 mg, and lorazepam IM, 2 mg every 6 hours as needed, in an emergency setting. I am curious if anyone has a different opinion based on scientific evidence.
Corey Yilmaz, MD
Southwest Behavioral Health Services
Tolleson, AZ
Reference
1. Preval H, Klotz SG, Southard R, et al. Rapid-acting IM ziprasidone in a psychiatric emergency service: a naturalistic study. Gen Hosp Psychiatry. 2005;27(2):140-144.
The authors respond
At 2 of our local hospital pharmacies, the cost of haloperidol combined with lorazepam is approximately $9 less than ziprasidone per administration: $1.51 vs $10.29 and $3.08 vs $12.23, respectively. Certainly, imagining this cost difference on a larger scale highlights the importance of cost efficiency, but herein enters the fear of sacrificing optimal patient care for the sake of budgeting.
With respect to efficacy, ziprasidone IM has been found to be superior to haloperidol IM in the acute setting.1 In a recent study of severe agitation in adolescent patients, ziprasidone monotherapy was as effective as haloperidol plus lorazepam.2 Regarding side effects, ziprasidone has demonstrated greater safety in terms of extrapyramidal symptoms and akathisia when objectively measured with the Extrapyramidal Symptom Rating Scale and Barnes Akathisia Scale.3 Change in QTc generally was comparable between the 2 medications; however, a more substantial change with haloperidol was seen at 24 hours, given its longer half-life.
Finally, as Dr. Yilmaz mentions, less sedation with ziprasidone vs haloperidol is a marked advantage in the emergency room, which is where our patient initially received these medications. In the case of our previously healthy patient presenting with new-onset psychiatric symptoms, return to a lucid state was important to elucidate an accurate diagnosis and implement appropriate treatment promptly.
Cathy Southammakosane, MD
Pediatrics/psychiatry/child psychiatry resident
Cincinnati Children’s Hospital Medical Center
Anthony Cavalieri, MD
General psychiatry resident
Christopher White, MD, JD, FCLM
Assistant professor of psychiatry and family medicine
University of Cincinnati
Cincinnati, OH
1. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. J Clin Psychiatry. 2000;61(12):933-941.
2. Jangro WC, Preval H, Southard R, et al. Conventional intramuscular sedatives versus ziprasidone for severe agitation in adolescents: case-control study. Child Adolesc Psychiatry Ment Health. 2009;3(1):9.-
3. Brook S, Walden J, Benattia I, et al. Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment study. Psychopharmacology (Berl). 2005;178(4):514-523.
1. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. J Clin Psychiatry. 2000;61(12):933-941.
2. Jangro WC, Preval H, Southard R, et al. Conventional intramuscular sedatives versus ziprasidone for severe agitation in adolescents: case-control study. Child Adolesc Psychiatry Ment Health. 2009;3(1):9.-
3. Brook S, Walden J, Benattia I, et al. Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment study. Psychopharmacology (Berl). 2005;178(4):514-523.