User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Aripiprazole safety
In “Did antismoking therapy make him sick?” (Cases That Test Your Skills, Current Psychiatry, February 2007), Drs. Steven G. Sugden and James A. Bourgeois describe a complex case of neuroleptic malignant syndrome (NMS). After resolution of an acute NMS episode, they started aripiprazole, 15 mg/d, and the patient improved. The authors state that, “Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS.” While theoretically interesting, there is no evidence suggesting that this pharmacodynamic property makes aripiprazole less likely to precipitate NMS, particularly in susceptible patients. In fact, recent reports indicate that NMS may occur in patients treated with aripiprazole.1
In patients with prior NMS episodes, antipsychotic rechallenge may be associated with a 30% chance of developing NMS.2 Nevertheless, most patients requiring antipsychotics can be treated safely, provided precautions include gradual titration after a test dose and monitoring for early signs of NMS.3,4 In the absence of controlled trials, low doses of first- or second-generation antipsychotics may be preferred.4 However, it is interesting that the patient described in this article did not develop NMS, even when aripiprazole—a D2 receptor antagonist—was initiated at 15 mg/d.
Jeffrey Strawn, MD
Clinical instructor in psychiatry
University of Cincinnati College of Medicine
Cincinnati, Ohio
1. Strawn JR. Aripiprazole and the neuroleptic malignant syndrome. Schizophr Res 2006;85:298-9.
2. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Psychopharmacol Bull 1988;24:25-9.
3. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin N Am 1993;77:185-202.
4. Strawn JR, Keck PE, Jr., Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry (in press).
In “Did antismoking therapy make him sick?” (Cases That Test Your Skills, Current Psychiatry, February 2007), Drs. Steven G. Sugden and James A. Bourgeois describe a complex case of neuroleptic malignant syndrome (NMS). After resolution of an acute NMS episode, they started aripiprazole, 15 mg/d, and the patient improved. The authors state that, “Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS.” While theoretically interesting, there is no evidence suggesting that this pharmacodynamic property makes aripiprazole less likely to precipitate NMS, particularly in susceptible patients. In fact, recent reports indicate that NMS may occur in patients treated with aripiprazole.1
In patients with prior NMS episodes, antipsychotic rechallenge may be associated with a 30% chance of developing NMS.2 Nevertheless, most patients requiring antipsychotics can be treated safely, provided precautions include gradual titration after a test dose and monitoring for early signs of NMS.3,4 In the absence of controlled trials, low doses of first- or second-generation antipsychotics may be preferred.4 However, it is interesting that the patient described in this article did not develop NMS, even when aripiprazole—a D2 receptor antagonist—was initiated at 15 mg/d.
Jeffrey Strawn, MD
Clinical instructor in psychiatry
University of Cincinnati College of Medicine
Cincinnati, Ohio
In “Did antismoking therapy make him sick?” (Cases That Test Your Skills, Current Psychiatry, February 2007), Drs. Steven G. Sugden and James A. Bourgeois describe a complex case of neuroleptic malignant syndrome (NMS). After resolution of an acute NMS episode, they started aripiprazole, 15 mg/d, and the patient improved. The authors state that, “Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS.” While theoretically interesting, there is no evidence suggesting that this pharmacodynamic property makes aripiprazole less likely to precipitate NMS, particularly in susceptible patients. In fact, recent reports indicate that NMS may occur in patients treated with aripiprazole.1
In patients with prior NMS episodes, antipsychotic rechallenge may be associated with a 30% chance of developing NMS.2 Nevertheless, most patients requiring antipsychotics can be treated safely, provided precautions include gradual titration after a test dose and monitoring for early signs of NMS.3,4 In the absence of controlled trials, low doses of first- or second-generation antipsychotics may be preferred.4 However, it is interesting that the patient described in this article did not develop NMS, even when aripiprazole—a D2 receptor antagonist—was initiated at 15 mg/d.
Jeffrey Strawn, MD
Clinical instructor in psychiatry
University of Cincinnati College of Medicine
Cincinnati, Ohio
1. Strawn JR. Aripiprazole and the neuroleptic malignant syndrome. Schizophr Res 2006;85:298-9.
2. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Psychopharmacol Bull 1988;24:25-9.
3. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin N Am 1993;77:185-202.
4. Strawn JR, Keck PE, Jr., Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry (in press).
1. Strawn JR. Aripiprazole and the neuroleptic malignant syndrome. Schizophr Res 2006;85:298-9.
2. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Psychopharmacol Bull 1988;24:25-9.
3. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin N Am 1993;77:185-202.
4. Strawn JR, Keck PE, Jr., Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry (in press).
Delirium debate
I would like to address Dr. Mitchell Levy’s comments regarding the staggering percentage of delirium cases seen in consultation-liaison settings and physicians’ astonishing lack of understanding of the condition (10 delirium myths debunked,” Pearls, Current Psychiatry, October 2006) salient aspects of delirium diagnosis and management that could be useful for a range of medical providers. I agree that few comprehensive teaching resources exist for nonspecialists, and methods of addressing delirium often are late, nonstandardized, and desultory.
Dr. Pistone stresses the existing gaps in resources for comprehensive and evidence-based management of delirium. Collaborations such as the one he suggests may help update our treatment guidelines at the national level. My group is developing a hospital-wide protocol for identifying at-risk patients and directing intervention. I hope that these and other efforts will help physicians address a problem that may occur more frequently as patients age and medical procedures increase in intensity and severity.
Mitchell Levy, MD
Assistant professor in psychiatry
University of Washington, Seattle
I would like to address Dr. Mitchell Levy’s comments regarding the staggering percentage of delirium cases seen in consultation-liaison settings and physicians’ astonishing lack of understanding of the condition (10 delirium myths debunked,” Pearls, Current Psychiatry, October 2006) salient aspects of delirium diagnosis and management that could be useful for a range of medical providers. I agree that few comprehensive teaching resources exist for nonspecialists, and methods of addressing delirium often are late, nonstandardized, and desultory.
Dr. Pistone stresses the existing gaps in resources for comprehensive and evidence-based management of delirium. Collaborations such as the one he suggests may help update our treatment guidelines at the national level. My group is developing a hospital-wide protocol for identifying at-risk patients and directing intervention. I hope that these and other efforts will help physicians address a problem that may occur more frequently as patients age and medical procedures increase in intensity and severity.
Mitchell Levy, MD
Assistant professor in psychiatry
University of Washington, Seattle
I would like to address Dr. Mitchell Levy’s comments regarding the staggering percentage of delirium cases seen in consultation-liaison settings and physicians’ astonishing lack of understanding of the condition (10 delirium myths debunked,” Pearls, Current Psychiatry, October 2006) salient aspects of delirium diagnosis and management that could be useful for a range of medical providers. I agree that few comprehensive teaching resources exist for nonspecialists, and methods of addressing delirium often are late, nonstandardized, and desultory.
Dr. Pistone stresses the existing gaps in resources for comprehensive and evidence-based management of delirium. Collaborations such as the one he suggests may help update our treatment guidelines at the national level. My group is developing a hospital-wide protocol for identifying at-risk patients and directing intervention. I hope that these and other efforts will help physicians address a problem that may occur more frequently as patients age and medical procedures increase in intensity and severity.
Mitchell Levy, MD
Assistant professor in psychiatry
University of Washington, Seattle
3 types of ‘EBM’: Which do you practice?
Psychiatric practitioners often are urged to practice evidence-based medicine (EBM), but some clinicians prefer to follow expert consensus guidelines—Eminence-Based Medicine. Still others uphold their own practice observations—Experience-Based Medicine. Which form of EBM do you practice?
Pros and cons of each. The most scientifically credible EBM is based on evidence from double-blind, randomized controlled clinical trials, such as those conducted by pharmaceutical companies seeking FDA approval of a new drug or indication. Critics point out, however, that this form of EBM does not reflect real-world practice because patients in FDA pivotal trials often are “too clean”—they’re frequently treatment-responsive and not drug-dependent, medically ill, or receiving other medications.
Eminence-based medicine—usually disseminated in practice guidelines—is respected because it reflects recommendations of some 30 to 50 experts on a set of psychiatric disorders (usually prominent clinical researchers with a critical approach to data). However, many practice guideline algorithms are based on educated opinions and extrapolations from narrow evidence-based data that are extended to various manifestations of a specific disorder.
Experience-based medicine, which combines evidence-based principles with a hefty dose of personal clinical observations in a heterogeneous patient population over time, is a prevalent source of information for clinical practitioners. Research purists often brush aside this form of EBM as too subjective, or because they feel using it can lead to risky conclusions about how to use a particular therapy. A common criticism of experience-based medicine is that a placebo response, which can occur in up to one-third of psychiatric patients (as can be seen in most FDA registration trials) may masquerade as a positive outcome.
A role for all three. In my opinion, research-driven, evidence-based medicine is the indispensable foundation for medical decision-making, but expert opinion and personal experience legitimately belong in a clinician’s toolbox as well. Treatments for psychiatric disorders have been evaluated in randomized controlled trials (first-tier evidence) for only a small proportion of DSM-IV diagnoses. What’s a clinician to do when faced with a disorder for which evidence-based medicine has proven no treatment to be effective? This is where the art of medicine comes into play.
Combining art and science. A clinician can try an intervention that may be supported by weaker evidence, such as from single-blind studies (second-tier evidence) or several published case series or reports. When nothing else has worked, such as in treatment-resistant patients or those with complex comorbidities, a clinician may boldly go where no one has gone before and try a novel but untested combination. Such a therapeutic foray is high-risk exploration that may fail dismally—or it may serendipitously usher in a radical yet effective new approach to alleviating the symptoms of a serious disease.
Clinicians who stumble upon a new approach should publish their observations in a letter to the editor or case report to stimulate replications, rebuttals, or additional personal observations. Subjecting unexpected findings to critique and refinement in the dynamic market of ideas can increase their value.
Eminence-based practice guidelines—through a reasonably calibrated amalgam of evidence and experience—provide clinicians with a series of steps and an acceptable risk–to-benefit ratio to manage patients who do not respond adequately to evidence-based treatment. Consensus-driven expert opinion integrates the art and science of medicine and commands greater credibility than the opinion of a single clinician.
Using every tool. Each of us implements all three types of EBM when managing our patients. We need to, and we have to. That is the reality of the medical practice of psychiatry.
Psychiatric practitioners often are urged to practice evidence-based medicine (EBM), but some clinicians prefer to follow expert consensus guidelines—Eminence-Based Medicine. Still others uphold their own practice observations—Experience-Based Medicine. Which form of EBM do you practice?
Pros and cons of each. The most scientifically credible EBM is based on evidence from double-blind, randomized controlled clinical trials, such as those conducted by pharmaceutical companies seeking FDA approval of a new drug or indication. Critics point out, however, that this form of EBM does not reflect real-world practice because patients in FDA pivotal trials often are “too clean”—they’re frequently treatment-responsive and not drug-dependent, medically ill, or receiving other medications.
Eminence-based medicine—usually disseminated in practice guidelines—is respected because it reflects recommendations of some 30 to 50 experts on a set of psychiatric disorders (usually prominent clinical researchers with a critical approach to data). However, many practice guideline algorithms are based on educated opinions and extrapolations from narrow evidence-based data that are extended to various manifestations of a specific disorder.
Experience-based medicine, which combines evidence-based principles with a hefty dose of personal clinical observations in a heterogeneous patient population over time, is a prevalent source of information for clinical practitioners. Research purists often brush aside this form of EBM as too subjective, or because they feel using it can lead to risky conclusions about how to use a particular therapy. A common criticism of experience-based medicine is that a placebo response, which can occur in up to one-third of psychiatric patients (as can be seen in most FDA registration trials) may masquerade as a positive outcome.
A role for all three. In my opinion, research-driven, evidence-based medicine is the indispensable foundation for medical decision-making, but expert opinion and personal experience legitimately belong in a clinician’s toolbox as well. Treatments for psychiatric disorders have been evaluated in randomized controlled trials (first-tier evidence) for only a small proportion of DSM-IV diagnoses. What’s a clinician to do when faced with a disorder for which evidence-based medicine has proven no treatment to be effective? This is where the art of medicine comes into play.
Combining art and science. A clinician can try an intervention that may be supported by weaker evidence, such as from single-blind studies (second-tier evidence) or several published case series or reports. When nothing else has worked, such as in treatment-resistant patients or those with complex comorbidities, a clinician may boldly go where no one has gone before and try a novel but untested combination. Such a therapeutic foray is high-risk exploration that may fail dismally—or it may serendipitously usher in a radical yet effective new approach to alleviating the symptoms of a serious disease.
Clinicians who stumble upon a new approach should publish their observations in a letter to the editor or case report to stimulate replications, rebuttals, or additional personal observations. Subjecting unexpected findings to critique and refinement in the dynamic market of ideas can increase their value.
Eminence-based practice guidelines—through a reasonably calibrated amalgam of evidence and experience—provide clinicians with a series of steps and an acceptable risk–to-benefit ratio to manage patients who do not respond adequately to evidence-based treatment. Consensus-driven expert opinion integrates the art and science of medicine and commands greater credibility than the opinion of a single clinician.
Using every tool. Each of us implements all three types of EBM when managing our patients. We need to, and we have to. That is the reality of the medical practice of psychiatry.
Psychiatric practitioners often are urged to practice evidence-based medicine (EBM), but some clinicians prefer to follow expert consensus guidelines—Eminence-Based Medicine. Still others uphold their own practice observations—Experience-Based Medicine. Which form of EBM do you practice?
Pros and cons of each. The most scientifically credible EBM is based on evidence from double-blind, randomized controlled clinical trials, such as those conducted by pharmaceutical companies seeking FDA approval of a new drug or indication. Critics point out, however, that this form of EBM does not reflect real-world practice because patients in FDA pivotal trials often are “too clean”—they’re frequently treatment-responsive and not drug-dependent, medically ill, or receiving other medications.
Eminence-based medicine—usually disseminated in practice guidelines—is respected because it reflects recommendations of some 30 to 50 experts on a set of psychiatric disorders (usually prominent clinical researchers with a critical approach to data). However, many practice guideline algorithms are based on educated opinions and extrapolations from narrow evidence-based data that are extended to various manifestations of a specific disorder.
Experience-based medicine, which combines evidence-based principles with a hefty dose of personal clinical observations in a heterogeneous patient population over time, is a prevalent source of information for clinical practitioners. Research purists often brush aside this form of EBM as too subjective, or because they feel using it can lead to risky conclusions about how to use a particular therapy. A common criticism of experience-based medicine is that a placebo response, which can occur in up to one-third of psychiatric patients (as can be seen in most FDA registration trials) may masquerade as a positive outcome.
A role for all three. In my opinion, research-driven, evidence-based medicine is the indispensable foundation for medical decision-making, but expert opinion and personal experience legitimately belong in a clinician’s toolbox as well. Treatments for psychiatric disorders have been evaluated in randomized controlled trials (first-tier evidence) for only a small proportion of DSM-IV diagnoses. What’s a clinician to do when faced with a disorder for which evidence-based medicine has proven no treatment to be effective? This is where the art of medicine comes into play.
Combining art and science. A clinician can try an intervention that may be supported by weaker evidence, such as from single-blind studies (second-tier evidence) or several published case series or reports. When nothing else has worked, such as in treatment-resistant patients or those with complex comorbidities, a clinician may boldly go where no one has gone before and try a novel but untested combination. Such a therapeutic foray is high-risk exploration that may fail dismally—or it may serendipitously usher in a radical yet effective new approach to alleviating the symptoms of a serious disease.
Clinicians who stumble upon a new approach should publish their observations in a letter to the editor or case report to stimulate replications, rebuttals, or additional personal observations. Subjecting unexpected findings to critique and refinement in the dynamic market of ideas can increase their value.
Eminence-based practice guidelines—through a reasonably calibrated amalgam of evidence and experience—provide clinicians with a series of steps and an acceptable risk–to-benefit ratio to manage patients who do not respond adequately to evidence-based treatment. Consensus-driven expert opinion integrates the art and science of medicine and commands greater credibility than the opinion of a single clinician.
Using every tool. Each of us implements all three types of EBM when managing our patients. We need to, and we have to. That is the reality of the medical practice of psychiatry.
After 3 months, she’s still ‘mad’
History: ‘They want to kill me’
Police and security agents arrest Ms. A, age 64, at a metropolitan airport. She is extremely agitated and behaving bizarrely, yelling that “the Mafia” is trying to kill her. She has spent 3 days hiding in area hotels, fleeing her “assailants.”
Police arrange Ms. A’s return home; under court order, she is hospitalized in a psychiatric facility. She is diagnosed with paranoid schizophrenia and receives IM haloperidol, 2 mg bid, but shows minimal improvement after 2½ weeks. Her psychotic symptoms improve slightly after the psychiatrist switches her to risperidone, 2 mg bid, but she still cannot function normally. Three weeks after admission, she is transferred to a nursing home for long-term care. She continues risperidone but remains paranoid and delusional.
Three months later, Ms. A is rehospitalized. She is anxious, delusional, confused, and hallucinating at admission. The patient is verbally and physically combative, fearful that medical staff will harm her. She is too violent to be examined, but staff notice that her skin appears thickened, her eyes puffy, and her hair coarse. Her voice sounds low and raspy.
I speak with Ms. A’s son, who reports that before his mother’s arrest he found her in the kitchen wielding a knife, exclaiming she wanted to kill herself. He says she heard a “whoosh” or “ringing” in her right ear while a male voice in her left ear told her, “End it, end it.”
Ms. A is severely obese (weight 325 lbs, body mass index 49 kg/m2). Blood pressure is 140/90 mm Hg, and she is taking captopril, 50 mg bid, for hypertension. Pulse rate and temperature are normal.
Dr. Lachover’s observations
Ms. A’s hallucinatory experiences are atypical, and her psychotic symptoms show little response after 2 months of aggressive inpatient treatment. Three months after discharge, she is rehospitalized in a florid paranoid psychotic state.
The patient’s weight poses an additional obstacle. I avoided second-generation antipsychotics (SGAs) that can cause weight gain, such as clozapine or olanzapine. I tried the SGA risperidone after IM haloperidol, a first-generation antipsychotic, produced minimal response.
Ms. A’s physical symptoms (thickened skin, coarse hair, puffiness under her eyes, and vocal raspiness) suggest an underlying organic process that might be causing her psychosis.
TESTING: Telling results
I order laboratory and other tests to check for an underlying organic disorder:
- Brain MRI is normal, as are CBC, renal and liver function, and serum copper, ceruloplasmin, vitamin B12, and heavy metal levels.
- Slit lamp eye exam reveals no Kayser-Fleischer ring, which would have indicated Wilson’s disease.
- EEG shows a diffuse, nonspecific, abnormal pattern of slowing and decreased amplitude, suggesting diffuse cerebral dysfunction.
- ECG shows sinus bradycardia and a significantly prolonged corrected QT (QTc) interval, indicating delayed ventricular repolarization.
- Thyroid panel is abnormal with markedly elevated thyrotropin (31.07 mIU/L).
Across 3 weeks, Ms. A’s delusional perceptions and hallucination intensity decrease, and her reality testing and socialization skills improve. She is discharged, after which the internist and I see her weekly to monitor thyroid function and psychiatric symptoms, respectively. Thyroid function gradually returns to normal over 4 to 6 months, and she is maintained on levothyroxine, 0.025 mg/d. Her weight gradually decreases over 12 months to 229 lbs.
Six months after discharge, Ms. A is notably more adept at activities of daily living. Mental status exam shows progressively improved reality testing and decreased paranoia. She is more active, and her mood and affect have brightened. Risperidone is stopped 10 months after discharge, and she has not been rehospitalized for psychiatric problems.
Table 1
Ms. A’s thyroid panel values
| Component | Ms. A’s readings | Normal values |
| Serum cholesterol | 310 mg/dL | 100 to 199 mg/dL |
| TSH (thyrotropin) | 31.07 mIU/L | 0.25 to 4.30 mIU/L |
| Free T4 | 0.34 ng/dL | 0.80 to 1.80 ng/dL |
| Total T4 (serum thyroxine) | 1.5 µg/dL | 4.6 to 12 µg/dL |
| Total T3 (serum triiodothyronine) | 67 ng/dL | 70 to 180 ng/dL |
Dr. Lachover’s observations
Erroneously diagnosed with paranoid schizophrenia, Ms. A endured 2 extended hospitalizations. Her psychosis and mental state—both of which improved with thyroid replacement therapy—appear to have been a psychiatric manifestation of severe hypothyroidism, or “myxedema madness” (Box).1-3
Myxedema prevalence in the general public has been reported at 0.5% to 18%. It is roughly 10 times more common in women than in men,4 and 5% to 15% of patients with myxedema might develop signs of psychosis.4 Myxedema-induced psychosis usually occurs during middle age but has been reported between ages 18 and 73. Prevalence increases with age.4
Recognizing ‘myxedema madness’
Detecting and treating myxedema in patients with treatment-resistant psychosis can resolve psychiatric and medical symptoms and restore quality of life. Left untreated, it can impair cognitive function and cause lethargy, dysarthria, myopathy, neuropathy, status epilepticus, and coma.5-7
Myxedema can impair perception and intellectual functioning,9 and acute mania has been reported in some cases.10 Increasing delirium reduces integration of perceptual input, leading to misidentification and disorientation. Cognitive functioning may be impaired, and abnormal thyroid hormone levels might delay event-related brain potential.11
Physical signs also can be telling. The patient might show general psychomotor retardation and slowed speech. The tongue might be swollen, the voice hoarse and croaking. Hair is often coarse and brittle, with hair loss along the sides of the eyebrows. Body temperature often dips below normal.4
Dr. Lachover’s observations
Detecting Ms. A’s hypothyroidism early could have prevented needless hospitalizations and failed treatment. Order a baseline thyroid panel for every patient who presents with psychotic symptoms or depression, which is the primary affective disturbance seen in myxedema.
Researchers have proposed many potential causes for the psychotic and depressive symptoms seen in myxedema.
Psychotic symptoms. Tonks1 has attributed psychosis in myxedema to decreases in cerebral oxygenation and glucose metabolism, resulting in a relative cerebral hypoxia. Among patients with myxedema, Sheinberg et al2 reported markedly reduced cardiac output and found that:
- cerebral blood flow was reduced 38%
- oxygen and glucose absorption were decreased approximately 30%
- cerebrovascular resistance was notably increased.
Depressive symptoms. Catecholamine deficiency at the neuronal receptor sites might cause depression in hypothyroidism. Evidence suggests that thyroid hormone influences catecholamine function at the neuronal level.3
Monoamine oxidase, which is increased in myxedema, has also been implicated. This enzyme might lead to depression by helping to break down catecholamines at the neuronal axon-dendrite levels.3
Diffuse slowing of background activity is the most common EEG change found in myxedema.13 ECG might show slow, regular sinus rhythm or bradycardia, low voltage, prolonged QTc interval, and flattened T waves.14 Prolonged QRS complexes on ECG indicate delayed ventricular repolarization.11,15 Torsades de pointes, the potentially fatal ventricular tachycardia, can result from a prolonged QTc interval in rare myxedema cases.16
Table 2
Is it myxedema? Check the lab findings
| Component | Values that suggest myxedema |
| Serum cholesterol | >200 mg/dL |
| Free T4 | |
| Total T4 (serum thyroxine) | |
| Total T3 (serum triiodothyronine) | |
| TSH (thyrotropin) | >4.5 mIU/L |
| EEG | Diffuse slowing |
| EKG | Prolonged QTc interval |
Treating 2 sets of symptoms
Prescribe concomitant dessicated thyroid and low-dose antipsychotics over 4 to 6 months to treat both the thyroid dysfunction and psychosis. Because weight gain is common in myxedema, choose an antipsychotic that carries a relatively low risk of weight gain, such as risperidone, 2 mg bid, or aripiprazole, 5 to 10 mg/d.
Many patients reach euthyroidism and their psychosis improves gradually but notably over weeks or months after starting thyroid hormone replacement. Psychosis could recur if desiccated thyroid is stopped; restarting it will improve the patient’s mental state.17 Recovery takes about 3 months on average.4
Continue the SGA until delusion perception is gone and reality testing improves, then taper the medication until all psychotic symptoms have abated. Monitor thyroid function monthly.
For patients with myxedema-induced depression, supplement thyroid hormone replacement with a selective serotonin reuptake inhibitor such as sertraline at regular starting dosages.
Dr. Lachover’s observations
Consider contributing medical illness in any patient with psychosis, particularly with psychotic symptom onset after age 40 and lack of response to weeks of adequate antipsychotic therapy.
A meticulous search to rule out medical disorders in all patients with psychosis and/or depression is essential to planning treatment. Testing is especially urgent for elderly patients, as multiple medical comorbidities or medication side effects can mask hypothyroidism’s signs and symptoms and delay diagnosis.18
Check complete blood count, electrolytes, thyroid panel, urinalysis, urine drug screen, blood urea nitrogen, and creatinine to rule out an underlying metabolic or endocrinologic cause for psychosis. Watch for signs of anticholinergic syndrome during physical examination.
If any of the above results suggest a medical problem, test for the following as clinical suspicion warrants:
- serum copper/ceruloplasmin and liver function to rule out Wilson’s disease, a genetic disorder that causes copper to accumulate in the liver and brain
- systemic lupus erythematosus
- lead, magnesium, mercury, or manganese to rule out metal poisoning.
- Cronin AJ. The Citadel. Boston: Little, Brown & Co.;1937:399.
- Asher R. Myxoedamatous madness. BMJ 1949;2:555-62.
- Aripiprazole • Abilify
- Captopril • Capoten
- Clozapine • Clozaril
- Haloperidol • Haldol
- Levothyroxine • Synthroid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tonks CM. Mental illness and hypothyroid patients. Br J Psychiatry 1964;110:706-10.
2. Scheinberg P, et al. Cerebral metabolism and cardiac output in myxedema. J Clin Invest 1950;29:1139-46.
3. Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry 1969;20:48-63.
4. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry 2003;5:260-6.
5. Jansen HJ, Doebe SR, Louwerse ES, et al. Status epilepticus caused by a myxoedema coma. Neth J Med 2006;64:202-5.
6. Pimental L, Hansen KN. Thyroid disease in the emergency department: a clinical and laboratory review. J Emerg Med 2005;28:201-9.
7. Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am 2006;35:687-98.
8. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Int Med 2006;145:573-81.
9. Adams CW. Electrocardiographic changes in hypothyroidism. Chest 1964;46:87-8.
10. Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics 2005;46:259-61.
11. Strachan SR, Afolabi O, Brown N, Gray D. Chest pain, enzymes, and hypothyroidism. Postgrad Med J 2000;76:168-9.
12. Lolas F, de la Parra G, Gramegna G. Event-related slow potential (ERSP) correlates of thyroid gland function levels. Psychosom Med 1978;40:226-35.
13. Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc 2002;133:849-58.
14. Khedr EM, El Toony LF, Tarkhan MN, Abdella G. Peripheral and central nervous system alterations in hypothyroidism; electrophysiological findings. Neuropsychobiology 2000;41:88-94.
15. Bosch R, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 1999;277(1 Pt 2):H211-20.
16. Schenck JB, Rizvi AA, Lin T. Severe primary hypothyroidism manifesting with torsades de pointes. Am J Med Sci 2006;331:154-6.
17. McGaffee J, Barnes MA, Lippmann S. Psychiatric presentations of hypothyroidism. Am Fam Physicia 1981;23:129-33.
18. Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J 2005;98:543-9.
History: ‘They want to kill me’
Police and security agents arrest Ms. A, age 64, at a metropolitan airport. She is extremely agitated and behaving bizarrely, yelling that “the Mafia” is trying to kill her. She has spent 3 days hiding in area hotels, fleeing her “assailants.”
Police arrange Ms. A’s return home; under court order, she is hospitalized in a psychiatric facility. She is diagnosed with paranoid schizophrenia and receives IM haloperidol, 2 mg bid, but shows minimal improvement after 2½ weeks. Her psychotic symptoms improve slightly after the psychiatrist switches her to risperidone, 2 mg bid, but she still cannot function normally. Three weeks after admission, she is transferred to a nursing home for long-term care. She continues risperidone but remains paranoid and delusional.
Three months later, Ms. A is rehospitalized. She is anxious, delusional, confused, and hallucinating at admission. The patient is verbally and physically combative, fearful that medical staff will harm her. She is too violent to be examined, but staff notice that her skin appears thickened, her eyes puffy, and her hair coarse. Her voice sounds low and raspy.
I speak with Ms. A’s son, who reports that before his mother’s arrest he found her in the kitchen wielding a knife, exclaiming she wanted to kill herself. He says she heard a “whoosh” or “ringing” in her right ear while a male voice in her left ear told her, “End it, end it.”
Ms. A is severely obese (weight 325 lbs, body mass index 49 kg/m2). Blood pressure is 140/90 mm Hg, and she is taking captopril, 50 mg bid, for hypertension. Pulse rate and temperature are normal.
Dr. Lachover’s observations
Ms. A’s hallucinatory experiences are atypical, and her psychotic symptoms show little response after 2 months of aggressive inpatient treatment. Three months after discharge, she is rehospitalized in a florid paranoid psychotic state.
The patient’s weight poses an additional obstacle. I avoided second-generation antipsychotics (SGAs) that can cause weight gain, such as clozapine or olanzapine. I tried the SGA risperidone after IM haloperidol, a first-generation antipsychotic, produced minimal response.
Ms. A’s physical symptoms (thickened skin, coarse hair, puffiness under her eyes, and vocal raspiness) suggest an underlying organic process that might be causing her psychosis.
TESTING: Telling results
I order laboratory and other tests to check for an underlying organic disorder:
- Brain MRI is normal, as are CBC, renal and liver function, and serum copper, ceruloplasmin, vitamin B12, and heavy metal levels.
- Slit lamp eye exam reveals no Kayser-Fleischer ring, which would have indicated Wilson’s disease.
- EEG shows a diffuse, nonspecific, abnormal pattern of slowing and decreased amplitude, suggesting diffuse cerebral dysfunction.
- ECG shows sinus bradycardia and a significantly prolonged corrected QT (QTc) interval, indicating delayed ventricular repolarization.
- Thyroid panel is abnormal with markedly elevated thyrotropin (31.07 mIU/L).
Across 3 weeks, Ms. A’s delusional perceptions and hallucination intensity decrease, and her reality testing and socialization skills improve. She is discharged, after which the internist and I see her weekly to monitor thyroid function and psychiatric symptoms, respectively. Thyroid function gradually returns to normal over 4 to 6 months, and she is maintained on levothyroxine, 0.025 mg/d. Her weight gradually decreases over 12 months to 229 lbs.
Six months after discharge, Ms. A is notably more adept at activities of daily living. Mental status exam shows progressively improved reality testing and decreased paranoia. She is more active, and her mood and affect have brightened. Risperidone is stopped 10 months after discharge, and she has not been rehospitalized for psychiatric problems.
Table 1
Ms. A’s thyroid panel values
| Component | Ms. A’s readings | Normal values |
| Serum cholesterol | 310 mg/dL | 100 to 199 mg/dL |
| TSH (thyrotropin) | 31.07 mIU/L | 0.25 to 4.30 mIU/L |
| Free T4 | 0.34 ng/dL | 0.80 to 1.80 ng/dL |
| Total T4 (serum thyroxine) | 1.5 µg/dL | 4.6 to 12 µg/dL |
| Total T3 (serum triiodothyronine) | 67 ng/dL | 70 to 180 ng/dL |
Dr. Lachover’s observations
Erroneously diagnosed with paranoid schizophrenia, Ms. A endured 2 extended hospitalizations. Her psychosis and mental state—both of which improved with thyroid replacement therapy—appear to have been a psychiatric manifestation of severe hypothyroidism, or “myxedema madness” (Box).1-3
Myxedema prevalence in the general public has been reported at 0.5% to 18%. It is roughly 10 times more common in women than in men,4 and 5% to 15% of patients with myxedema might develop signs of psychosis.4 Myxedema-induced psychosis usually occurs during middle age but has been reported between ages 18 and 73. Prevalence increases with age.4
Recognizing ‘myxedema madness’
Detecting and treating myxedema in patients with treatment-resistant psychosis can resolve psychiatric and medical symptoms and restore quality of life. Left untreated, it can impair cognitive function and cause lethargy, dysarthria, myopathy, neuropathy, status epilepticus, and coma.5-7
Myxedema can impair perception and intellectual functioning,9 and acute mania has been reported in some cases.10 Increasing delirium reduces integration of perceptual input, leading to misidentification and disorientation. Cognitive functioning may be impaired, and abnormal thyroid hormone levels might delay event-related brain potential.11
Physical signs also can be telling. The patient might show general psychomotor retardation and slowed speech. The tongue might be swollen, the voice hoarse and croaking. Hair is often coarse and brittle, with hair loss along the sides of the eyebrows. Body temperature often dips below normal.4
Dr. Lachover’s observations
Detecting Ms. A’s hypothyroidism early could have prevented needless hospitalizations and failed treatment. Order a baseline thyroid panel for every patient who presents with psychotic symptoms or depression, which is the primary affective disturbance seen in myxedema.
Researchers have proposed many potential causes for the psychotic and depressive symptoms seen in myxedema.
Psychotic symptoms. Tonks1 has attributed psychosis in myxedema to decreases in cerebral oxygenation and glucose metabolism, resulting in a relative cerebral hypoxia. Among patients with myxedema, Sheinberg et al2 reported markedly reduced cardiac output and found that:
- cerebral blood flow was reduced 38%
- oxygen and glucose absorption were decreased approximately 30%
- cerebrovascular resistance was notably increased.
Depressive symptoms. Catecholamine deficiency at the neuronal receptor sites might cause depression in hypothyroidism. Evidence suggests that thyroid hormone influences catecholamine function at the neuronal level.3
Monoamine oxidase, which is increased in myxedema, has also been implicated. This enzyme might lead to depression by helping to break down catecholamines at the neuronal axon-dendrite levels.3
Diffuse slowing of background activity is the most common EEG change found in myxedema.13 ECG might show slow, regular sinus rhythm or bradycardia, low voltage, prolonged QTc interval, and flattened T waves.14 Prolonged QRS complexes on ECG indicate delayed ventricular repolarization.11,15 Torsades de pointes, the potentially fatal ventricular tachycardia, can result from a prolonged QTc interval in rare myxedema cases.16
Table 2
Is it myxedema? Check the lab findings
| Component | Values that suggest myxedema |
| Serum cholesterol | >200 mg/dL |
| Free T4 | |
| Total T4 (serum thyroxine) | |
| Total T3 (serum triiodothyronine) | |
| TSH (thyrotropin) | >4.5 mIU/L |
| EEG | Diffuse slowing |
| EKG | Prolonged QTc interval |
Treating 2 sets of symptoms
Prescribe concomitant dessicated thyroid and low-dose antipsychotics over 4 to 6 months to treat both the thyroid dysfunction and psychosis. Because weight gain is common in myxedema, choose an antipsychotic that carries a relatively low risk of weight gain, such as risperidone, 2 mg bid, or aripiprazole, 5 to 10 mg/d.
Many patients reach euthyroidism and their psychosis improves gradually but notably over weeks or months after starting thyroid hormone replacement. Psychosis could recur if desiccated thyroid is stopped; restarting it will improve the patient’s mental state.17 Recovery takes about 3 months on average.4
Continue the SGA until delusion perception is gone and reality testing improves, then taper the medication until all psychotic symptoms have abated. Monitor thyroid function monthly.
For patients with myxedema-induced depression, supplement thyroid hormone replacement with a selective serotonin reuptake inhibitor such as sertraline at regular starting dosages.
Dr. Lachover’s observations
Consider contributing medical illness in any patient with psychosis, particularly with psychotic symptom onset after age 40 and lack of response to weeks of adequate antipsychotic therapy.
A meticulous search to rule out medical disorders in all patients with psychosis and/or depression is essential to planning treatment. Testing is especially urgent for elderly patients, as multiple medical comorbidities or medication side effects can mask hypothyroidism’s signs and symptoms and delay diagnosis.18
Check complete blood count, electrolytes, thyroid panel, urinalysis, urine drug screen, blood urea nitrogen, and creatinine to rule out an underlying metabolic or endocrinologic cause for psychosis. Watch for signs of anticholinergic syndrome during physical examination.
If any of the above results suggest a medical problem, test for the following as clinical suspicion warrants:
- serum copper/ceruloplasmin and liver function to rule out Wilson’s disease, a genetic disorder that causes copper to accumulate in the liver and brain
- systemic lupus erythematosus
- lead, magnesium, mercury, or manganese to rule out metal poisoning.
- Cronin AJ. The Citadel. Boston: Little, Brown & Co.;1937:399.
- Asher R. Myxoedamatous madness. BMJ 1949;2:555-62.
- Aripiprazole • Abilify
- Captopril • Capoten
- Clozapine • Clozaril
- Haloperidol • Haldol
- Levothyroxine • Synthroid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
History: ‘They want to kill me’
Police and security agents arrest Ms. A, age 64, at a metropolitan airport. She is extremely agitated and behaving bizarrely, yelling that “the Mafia” is trying to kill her. She has spent 3 days hiding in area hotels, fleeing her “assailants.”
Police arrange Ms. A’s return home; under court order, she is hospitalized in a psychiatric facility. She is diagnosed with paranoid schizophrenia and receives IM haloperidol, 2 mg bid, but shows minimal improvement after 2½ weeks. Her psychotic symptoms improve slightly after the psychiatrist switches her to risperidone, 2 mg bid, but she still cannot function normally. Three weeks after admission, she is transferred to a nursing home for long-term care. She continues risperidone but remains paranoid and delusional.
Three months later, Ms. A is rehospitalized. She is anxious, delusional, confused, and hallucinating at admission. The patient is verbally and physically combative, fearful that medical staff will harm her. She is too violent to be examined, but staff notice that her skin appears thickened, her eyes puffy, and her hair coarse. Her voice sounds low and raspy.
I speak with Ms. A’s son, who reports that before his mother’s arrest he found her in the kitchen wielding a knife, exclaiming she wanted to kill herself. He says she heard a “whoosh” or “ringing” in her right ear while a male voice in her left ear told her, “End it, end it.”
Ms. A is severely obese (weight 325 lbs, body mass index 49 kg/m2). Blood pressure is 140/90 mm Hg, and she is taking captopril, 50 mg bid, for hypertension. Pulse rate and temperature are normal.
Dr. Lachover’s observations
Ms. A’s hallucinatory experiences are atypical, and her psychotic symptoms show little response after 2 months of aggressive inpatient treatment. Three months after discharge, she is rehospitalized in a florid paranoid psychotic state.
The patient’s weight poses an additional obstacle. I avoided second-generation antipsychotics (SGAs) that can cause weight gain, such as clozapine or olanzapine. I tried the SGA risperidone after IM haloperidol, a first-generation antipsychotic, produced minimal response.
Ms. A’s physical symptoms (thickened skin, coarse hair, puffiness under her eyes, and vocal raspiness) suggest an underlying organic process that might be causing her psychosis.
TESTING: Telling results
I order laboratory and other tests to check for an underlying organic disorder:
- Brain MRI is normal, as are CBC, renal and liver function, and serum copper, ceruloplasmin, vitamin B12, and heavy metal levels.
- Slit lamp eye exam reveals no Kayser-Fleischer ring, which would have indicated Wilson’s disease.
- EEG shows a diffuse, nonspecific, abnormal pattern of slowing and decreased amplitude, suggesting diffuse cerebral dysfunction.
- ECG shows sinus bradycardia and a significantly prolonged corrected QT (QTc) interval, indicating delayed ventricular repolarization.
- Thyroid panel is abnormal with markedly elevated thyrotropin (31.07 mIU/L).
Across 3 weeks, Ms. A’s delusional perceptions and hallucination intensity decrease, and her reality testing and socialization skills improve. She is discharged, after which the internist and I see her weekly to monitor thyroid function and psychiatric symptoms, respectively. Thyroid function gradually returns to normal over 4 to 6 months, and she is maintained on levothyroxine, 0.025 mg/d. Her weight gradually decreases over 12 months to 229 lbs.
Six months after discharge, Ms. A is notably more adept at activities of daily living. Mental status exam shows progressively improved reality testing and decreased paranoia. She is more active, and her mood and affect have brightened. Risperidone is stopped 10 months after discharge, and she has not been rehospitalized for psychiatric problems.
Table 1
Ms. A’s thyroid panel values
| Component | Ms. A’s readings | Normal values |
| Serum cholesterol | 310 mg/dL | 100 to 199 mg/dL |
| TSH (thyrotropin) | 31.07 mIU/L | 0.25 to 4.30 mIU/L |
| Free T4 | 0.34 ng/dL | 0.80 to 1.80 ng/dL |
| Total T4 (serum thyroxine) | 1.5 µg/dL | 4.6 to 12 µg/dL |
| Total T3 (serum triiodothyronine) | 67 ng/dL | 70 to 180 ng/dL |
Dr. Lachover’s observations
Erroneously diagnosed with paranoid schizophrenia, Ms. A endured 2 extended hospitalizations. Her psychosis and mental state—both of which improved with thyroid replacement therapy—appear to have been a psychiatric manifestation of severe hypothyroidism, or “myxedema madness” (Box).1-3
Myxedema prevalence in the general public has been reported at 0.5% to 18%. It is roughly 10 times more common in women than in men,4 and 5% to 15% of patients with myxedema might develop signs of psychosis.4 Myxedema-induced psychosis usually occurs during middle age but has been reported between ages 18 and 73. Prevalence increases with age.4
Recognizing ‘myxedema madness’
Detecting and treating myxedema in patients with treatment-resistant psychosis can resolve psychiatric and medical symptoms and restore quality of life. Left untreated, it can impair cognitive function and cause lethargy, dysarthria, myopathy, neuropathy, status epilepticus, and coma.5-7
Myxedema can impair perception and intellectual functioning,9 and acute mania has been reported in some cases.10 Increasing delirium reduces integration of perceptual input, leading to misidentification and disorientation. Cognitive functioning may be impaired, and abnormal thyroid hormone levels might delay event-related brain potential.11
Physical signs also can be telling. The patient might show general psychomotor retardation and slowed speech. The tongue might be swollen, the voice hoarse and croaking. Hair is often coarse and brittle, with hair loss along the sides of the eyebrows. Body temperature often dips below normal.4
Dr. Lachover’s observations
Detecting Ms. A’s hypothyroidism early could have prevented needless hospitalizations and failed treatment. Order a baseline thyroid panel for every patient who presents with psychotic symptoms or depression, which is the primary affective disturbance seen in myxedema.
Researchers have proposed many potential causes for the psychotic and depressive symptoms seen in myxedema.
Psychotic symptoms. Tonks1 has attributed psychosis in myxedema to decreases in cerebral oxygenation and glucose metabolism, resulting in a relative cerebral hypoxia. Among patients with myxedema, Sheinberg et al2 reported markedly reduced cardiac output and found that:
- cerebral blood flow was reduced 38%
- oxygen and glucose absorption were decreased approximately 30%
- cerebrovascular resistance was notably increased.
Depressive symptoms. Catecholamine deficiency at the neuronal receptor sites might cause depression in hypothyroidism. Evidence suggests that thyroid hormone influences catecholamine function at the neuronal level.3
Monoamine oxidase, which is increased in myxedema, has also been implicated. This enzyme might lead to depression by helping to break down catecholamines at the neuronal axon-dendrite levels.3
Diffuse slowing of background activity is the most common EEG change found in myxedema.13 ECG might show slow, regular sinus rhythm or bradycardia, low voltage, prolonged QTc interval, and flattened T waves.14 Prolonged QRS complexes on ECG indicate delayed ventricular repolarization.11,15 Torsades de pointes, the potentially fatal ventricular tachycardia, can result from a prolonged QTc interval in rare myxedema cases.16
Table 2
Is it myxedema? Check the lab findings
| Component | Values that suggest myxedema |
| Serum cholesterol | >200 mg/dL |
| Free T4 | |
| Total T4 (serum thyroxine) | |
| Total T3 (serum triiodothyronine) | |
| TSH (thyrotropin) | >4.5 mIU/L |
| EEG | Diffuse slowing |
| EKG | Prolonged QTc interval |
Treating 2 sets of symptoms
Prescribe concomitant dessicated thyroid and low-dose antipsychotics over 4 to 6 months to treat both the thyroid dysfunction and psychosis. Because weight gain is common in myxedema, choose an antipsychotic that carries a relatively low risk of weight gain, such as risperidone, 2 mg bid, or aripiprazole, 5 to 10 mg/d.
Many patients reach euthyroidism and their psychosis improves gradually but notably over weeks or months after starting thyroid hormone replacement. Psychosis could recur if desiccated thyroid is stopped; restarting it will improve the patient’s mental state.17 Recovery takes about 3 months on average.4
Continue the SGA until delusion perception is gone and reality testing improves, then taper the medication until all psychotic symptoms have abated. Monitor thyroid function monthly.
For patients with myxedema-induced depression, supplement thyroid hormone replacement with a selective serotonin reuptake inhibitor such as sertraline at regular starting dosages.
Dr. Lachover’s observations
Consider contributing medical illness in any patient with psychosis, particularly with psychotic symptom onset after age 40 and lack of response to weeks of adequate antipsychotic therapy.
A meticulous search to rule out medical disorders in all patients with psychosis and/or depression is essential to planning treatment. Testing is especially urgent for elderly patients, as multiple medical comorbidities or medication side effects can mask hypothyroidism’s signs and symptoms and delay diagnosis.18
Check complete blood count, electrolytes, thyroid panel, urinalysis, urine drug screen, blood urea nitrogen, and creatinine to rule out an underlying metabolic or endocrinologic cause for psychosis. Watch for signs of anticholinergic syndrome during physical examination.
If any of the above results suggest a medical problem, test for the following as clinical suspicion warrants:
- serum copper/ceruloplasmin and liver function to rule out Wilson’s disease, a genetic disorder that causes copper to accumulate in the liver and brain
- systemic lupus erythematosus
- lead, magnesium, mercury, or manganese to rule out metal poisoning.
- Cronin AJ. The Citadel. Boston: Little, Brown & Co.;1937:399.
- Asher R. Myxoedamatous madness. BMJ 1949;2:555-62.
- Aripiprazole • Abilify
- Captopril • Capoten
- Clozapine • Clozaril
- Haloperidol • Haldol
- Levothyroxine • Synthroid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tonks CM. Mental illness and hypothyroid patients. Br J Psychiatry 1964;110:706-10.
2. Scheinberg P, et al. Cerebral metabolism and cardiac output in myxedema. J Clin Invest 1950;29:1139-46.
3. Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry 1969;20:48-63.
4. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry 2003;5:260-6.
5. Jansen HJ, Doebe SR, Louwerse ES, et al. Status epilepticus caused by a myxoedema coma. Neth J Med 2006;64:202-5.
6. Pimental L, Hansen KN. Thyroid disease in the emergency department: a clinical and laboratory review. J Emerg Med 2005;28:201-9.
7. Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am 2006;35:687-98.
8. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Int Med 2006;145:573-81.
9. Adams CW. Electrocardiographic changes in hypothyroidism. Chest 1964;46:87-8.
10. Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics 2005;46:259-61.
11. Strachan SR, Afolabi O, Brown N, Gray D. Chest pain, enzymes, and hypothyroidism. Postgrad Med J 2000;76:168-9.
12. Lolas F, de la Parra G, Gramegna G. Event-related slow potential (ERSP) correlates of thyroid gland function levels. Psychosom Med 1978;40:226-35.
13. Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc 2002;133:849-58.
14. Khedr EM, El Toony LF, Tarkhan MN, Abdella G. Peripheral and central nervous system alterations in hypothyroidism; electrophysiological findings. Neuropsychobiology 2000;41:88-94.
15. Bosch R, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 1999;277(1 Pt 2):H211-20.
16. Schenck JB, Rizvi AA, Lin T. Severe primary hypothyroidism manifesting with torsades de pointes. Am J Med Sci 2006;331:154-6.
17. McGaffee J, Barnes MA, Lippmann S. Psychiatric presentations of hypothyroidism. Am Fam Physicia 1981;23:129-33.
18. Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J 2005;98:543-9.
1. Tonks CM. Mental illness and hypothyroid patients. Br J Psychiatry 1964;110:706-10.
2. Scheinberg P, et al. Cerebral metabolism and cardiac output in myxedema. J Clin Invest 1950;29:1139-46.
3. Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry 1969;20:48-63.
4. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry 2003;5:260-6.
5. Jansen HJ, Doebe SR, Louwerse ES, et al. Status epilepticus caused by a myxoedema coma. Neth J Med 2006;64:202-5.
6. Pimental L, Hansen KN. Thyroid disease in the emergency department: a clinical and laboratory review. J Emerg Med 2005;28:201-9.
7. Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am 2006;35:687-98.
8. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Int Med 2006;145:573-81.
9. Adams CW. Electrocardiographic changes in hypothyroidism. Chest 1964;46:87-8.
10. Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics 2005;46:259-61.
11. Strachan SR, Afolabi O, Brown N, Gray D. Chest pain, enzymes, and hypothyroidism. Postgrad Med J 2000;76:168-9.
12. Lolas F, de la Parra G, Gramegna G. Event-related slow potential (ERSP) correlates of thyroid gland function levels. Psychosom Med 1978;40:226-35.
13. Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc 2002;133:849-58.
14. Khedr EM, El Toony LF, Tarkhan MN, Abdella G. Peripheral and central nervous system alterations in hypothyroidism; electrophysiological findings. Neuropsychobiology 2000;41:88-94.
15. Bosch R, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 1999;277(1 Pt 2):H211-20.
16. Schenck JB, Rizvi AA, Lin T. Severe primary hypothyroidism manifesting with torsades de pointes. Am J Med Sci 2006;331:154-6.
17. McGaffee J, Barnes MA, Lippmann S. Psychiatric presentations of hypothyroidism. Am Fam Physicia 1981;23:129-33.
18. Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J 2005;98:543-9.
Violent behavior: Choosing antipsychotics and other agents
When a patient with major psychiatric illness exhibits violent behavior, consider the course of violence in relation to his or her fixed and changing symptoms and deficits.1,2 Although most patients with schizophrenia, major depression, or bipolar disorder are not violent, effectively treating those who are calls for:
- differentiating between transient and persistent violent behavior
- providing medications and nonpharmacologic interventions shown to reduce each behavior
- addressing substance abuse and violent behavior concurrently.
Is violence transient or persistent?
Violent behavior is a common reason for psychiatric admission and prolonged hospital stays3 and a barrier to appropriate community placement4 and successful community reintegration.
Transient violence is limited to an acute psychotic episode; as psychotic symptoms abate, the violence resolves. Delusions, hallucinations, and conceptual disorganization are key triggers of transient violence.5 Excitement, anger, and agitation are its prominent symptoms.6
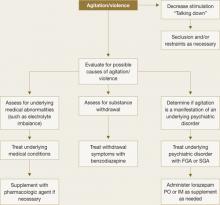
Treat a patient experiencing an acute violent episode with an oral first- or second-generation antipsychotic. For acute agitation, intramuscular (IM) delivery provides more rapid symptom resolution (Table 1). IM ziprasidone is approved for agitation associated with schizophrenia and IM olanzapine for use in agitation associated with schizophrenia or bipolar mania. Try talking calmly to the patient and explaining the need for medication (Figure 1). If this is not possible, a show of force might induce the patient’s cooperation.7
For sedation, antipsychotic medication can be supplemented by lorazepam, the only benzodiazepine that is reliably absorbed when administered IM. Lorazepam has a relatively short half-life, and the usual dosage of 1 to 2 mg can be administered orally, sublingually, intramuscularly, or intravenously every 1 to 6 hours. Exercise caution, however, when respiratory depression is a possibility.
Table 1
Medications used to treat violence on an emergency basis
| Drug | Route of administration* | Recommended dosage† |
|---|---|---|
| Benzodiazepines | ||
| Lorazepam | IM or PO | 1 to 2 mg IM or PO |
| Midazolam | IM | 5 mg |
| First-generation antipsychotic‡ | ||
| Haloperidol | IM or PO | 2 to 7.5 mg IM or PO |
| Second-generation antipsychotics‡ | ||
| Olanzapine | IM or PO | 10 mg IM or PO |
| Risperidone | PO | 2 to 6 mg |
| Ziprasidone | IM | 10 to 20 mg |
| * Use oral medication if patient is cooperative; otherwise use an intramuscular injection. | ||
| † Lower dosages are used for elderly patients or those with dementia. | ||
| ‡ Antipsychotics are not recommended for aggressive patients without a psychotic disorder or bipolar mania diagnosis. | ||
Figure 1 Managing patients who present with acute violence or agitation
FGA: First-generation antipsychotic; SGA: Second-generation antipsychoticPersistent violence. Emotional turmoil is usually less pronounced in patients whose violence is persistent.8 Neurocognitive impairments, antisocial traits, and specific psychotic symptoms may exist singly or in combination in patients prone to persistent violence.
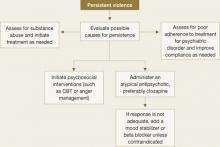
When a patient continues to be violent, consider poor treatment adherence or substance abuse, especially with outpatients (Figure 2).
Figure 2 Managing patients who exhibit persistent violent behavior
Neurological and neurocognitive impairments are associated with persistent violence. Fairly broad impairments in various domains are seen on a variety of tests. Patients with neurocognitive impairment often present with impulsivity9 and deficits in behavioral adaptability.
In general, the consequences of a behavior determine its course; assaultive behavior usually decreases rapidly when strongly discouraged. Violent behavior that persists, therefore, suggests that neurocognitive impairment is causing a failure in behavioral adaptability.
Psychopathy, antisocial traits, and antisocial personality disorder (APD) also can result in persistent violence.
Antisocial personality is defined primarily by behavioral symptoms such as irresponsibility and criminal activities. Psychopathy also includes these symptoms but adds interpersonal and affective impairments such as callousness, grandiosity, and lack of remorse.
Psychopathy was the strongest clinical predictor of violence in a large trial of outpatients with major psychiatric disorders.10 APD, on the other hand, was the most significant clinical predictor of violent recidivism among offenders with mental illness in a meta-analysis of predictive longitudinal studies from 1959 to 1995.11 In another study, psychopaths were about 5 times more likely than nonpsychopaths to engage in violent recidivism.12
In addition to recidivism, the violence associated with psychopathy is characterized by its severity. Violence in these patients is often premeditated, deliberate, and goal-driven.
Persistent psychotic symptoms also play an important role in persistent violence and may represent treatment resistance. Some specific delusions, for example, are more likely to lead to violence and may persist despite overall improvement in psychotic symptoms.
Link et al13 have proposed that violence is more likely when a person has “threat/control override” delusions—if he believes people are seeking to harm him or outside forces are controlling his mind.
Junginger stressed that violent themes in a patient’s delusions are important predictors of violence.14 Delusions associated with violence are often chronic and well-circumscribed.
The role of antipsychotics
Pharmacologic intervention for violent behavior targets the underlying disorder, such as schizophrenia or bipolar disorder. Usual regimens used to treat patients with these disorders may need to be modified, however, for persistently violent patients (Table 2).
Second-generation antipsychotics (SGAs)—particularly clozapine—have superior antiaggressive properties beyond their antipsychotic or sedative effects, compared with first-generation antipsychotics. Retrospective studies have shown clozapine can significantly decrease the number of violent incidents and episodes of seclusion and restraint.15,16 Evidence for efficacy of other SGAs in reducing physical assaults is more limited:
- Risperidone had a greater effect than haloperidol on hostility in a large, multicenter comparison trial.17
- Clozapine was more effective than haloperidol or risperidone in reducing hostility in a double-blind study of schizophrenia patients.18 This finding was independent of clozapine’s antipsychotic effect.
Clozapine also was more effective than haloperidol in reducing the number and severity of aggressive incidents.19 The patients in this study, however, were not selected on the basis of aggressive behavior.
One large federally funded, double-blind, randomized trial compared clozapine, olanzapine, and haloperidol in 110 assaultive patients with schizophrenia or schizoaffective disorder. Patients had documented episodes of recent physical assaults and persistent aggressive behaviors during a 2-week period. Clozapine showed greater efficacy than olanzapine—and olanzapine greater efficacy than haloperidol—in reducing aggressive behavior.20 This effect was independent of the drugs’ antipsychotic and sedative actions.
Table 2
Medications used to treat persistent violence
| Drug | Initial dosage | Target dosage |
|---|---|---|
| Second-generation antipsychotics | ||
| Clozapine | 12.5 to 50 mg/d | 300 to 450 mg/d* |
| Olanzapine | 5 to 10 mg/d | 15 to 30 mg/d |
| Quetiapine | 50 to 100 mg/d | 400 to 700 mg/d |
| Risperidone | 1 to 3 mg/d | 4 to 6 mg/d |
| First-generation antipsychotic | ||
| Haloperidol | 5 to 10 mg/d | 10 to 20 mg/d |
| Mood stabilizers | ||
| Carbamazepine | 200 to 400 mg/d | 1,000 to 1,400 mg/d* |
| Lithium | 300 mg bid | 300 mg tid* |
| Valproate | 500 to 1,000 mg/d | 1,000 to 1,500 mg/d* |
| Beta blockers† | ||
| Nadolol | 40 mg/d | 80 to 140 mg/d |
| Propranolol | 20 mg tid | 200 mg to 600 (delayed onset of action) |
| * Serum levels should be obtained. | ||
| † Contraindicated for patients with cardiovascular disease, asthma, or diabetes. | ||
Dual-diagnosis patients. Clozapine may be beneficial for patients with concurrent substance abuse because in addition to reducing aggression, it also may prevent relapse to substance abuse. In addition to intoxication, drug and alcohol abuse has disruptive effects on prefrontal function. These impairments play an important role in substance use-related aggression.
Substance abuse also can exacerbate psychotic symptoms, both directly and indirectly through poor treatment compliance. Patients with psychopathy are much more likely to abuse drugs. The association between drug abuse and violence can then be due in part to the higher percentage of psychopaths in the group of drug abusers.
Fortunately, patients with dual diagnosis who receive extensive substance abuse treatment show greater clinical improvement and better outcomes.21 Several studies found that clozapine was associated with decreased substance use. In one trial, schizophrenic patients with a history of drug abuse who received clozapine were much less likely to use substances over the next year than patients taking other antipsychotic medications.22
Thus, clozapine has clear antiaggressive effects, but its use as a first-choice treatment for aggression is limited by the risk of side effects, in particular agranulocytosis. With careful blood monitoring, this complication is very rare, but persistently violent patients might not cooperate fully with the required monitoring.
Other medications
Other agents used to treat violent patients with mental disorders include mood stabilizers, beta blockers, and antidepressants.23
Mood stabilizers such as lithium, carbamazepine, or valproate might be useful as adjuncts to antipsychotic medications in managing assaultive patients with schizophrenia or other major psychiatric disorders. These medications might decrease violence by enhancing serotonergic activity.
Most evidence for mood stabilizers’ anti-aggressive effect comes from studies of patients with personality disorders. Divalproex, for example, was more effective than placebo in reducing impulsive aggression in patients with Cluster B personality disorders.24
Lithium reduces aggression and irritability in bipolar mania, while stabilizing the underlying disorder. Lithium can decrease aggression in other populations as well, including:
- the developmentally disabled
- prisoners with no apparent psychiatric diagnoses
- aggressive children and adolescents with conduct disorder
- adults with borderline personality disorder.
Beta blockers such as nadolol, pindolol, and propranolol have been reported to reduce aggression. Their usefulness is limited, however, because they are contraindicated in patients with cardiovascular disease, asthma, or diabetes.
Antidepressants. Selective serotonin reuptake inhibitors may reduce impulsive aggression in nondepressed patients with personality disorders.25
Nonpharmacologic treatments
To provide proper treatment, the clinician must understand the patient as a whole person, including his perception of his aggressive behavior. Nonpharmacologic interventions should be implemented with this in mind.
Compared with standard care, for example, intensive case management reduces the incidence of violence.26
Behavioral techniques can decrease violence by addressing specific impairments underlying the violence. For example, improving a patient’s cognitive functioning can counter impaired processing of feedback that is associated with neurological dysfunction.
Specific interventions, such as cueing to exaggerate the link between stimulus and response, could be beneficial.27 Similarly, these patients might respond to a high degree of structure, supervision, and specific environmental modifications, such as transfer to a unit that specializes in treating violent patients.28
In cognitive-behavioral therapy, patients can learn ways they can satisfy their needs without being violent. They also can be trained in problem-solving skills and in understanding the consequences of their actions. Such therapy might be useful for diminishing antisocial traits. Interventions aimed at preventing, decreasing, or counteracting arousal are important in addressing acute violence.
Anger management programs can help patients respond to interpersonal provocations in a more adaptive way.29 These programs include:
- education about aggression
- self-monitoring of anger frequency, intensity, and situational triggers
- relaxation to reduce arousal and enable guided imagery training
- training in behavioral coping, communication, and assertiveness through role play
- practicing new anger-coping skills.30
Tailor treatments to the dominant mechanisms underlying persistent violence.
Related resources
- Citrome L, Volavka J. Aggression. eMedicine from WebMD. www.emedicine.com/med/topic3005.htm.
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 2000;289:591-94.
Drug brand names
- Carbamazepine • Carbatrol, Equetro, Tegretol
- Clozapine • Clozaril
- Divalproex • Depakote
- Haloperidol • Haldol
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Midazolam • Versed
- Nadolol • Corgard
- Olanzapine • Zyprexa
- Pindolol • Visken
- Propranolol • Inderal, Inderide
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosure
Dr. Krakowski receives research support from Eli Lilly and Co. and GlaxoSmithKline.
1. Krakowski M, Czobor P. Violence in psychiatric patients: the role of psychosis, frontal lobe impairment and ward turmoil. Compr Psychiatry 1997;38:230-36.
2. Krakowski M, Convit A, Jaeger J, et al. Neurological impairment in violent schizophrenic inpatients. Am J Psychiatry 1989;146:849-53.
3. Lelliott P, Wing J, Clifford P. A national audit of new longstay psychiatric patients. I: Method and description of the cohort. Br J Psychiatry 1994;165:1609.-
4. Bigelow DA, Cutler DL, Moore LJ, et al. Characteristics of state hospital patients who are hard to place. Hosp Community Psychiatry 1988;39:1815.-
5. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry 1994;45:133-7.
6. Craig TJ. An epidemiologic study of problems associated with violence among psychiatric inpatients. Am J Psychiatry 1982;139:1262-6.
7. Allen MH. Managing the agitated psychiatric patient: a reappraisal of the evidence. J Clin Psychiatry 2000;61(suppl 4):11-20.
8. Krakowski M, Czobor P, Chou J. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull 1999;25:505-17.
9. Stein DJ, Hollander E, Cohen L, et al. Neuropsychiatric impairment in impulsive personality disorders. Psychiatry Res 1993;48:257-66.
10. Skeem JL, Mulvey EP. Psychopathy and community violence among civil psychiatric patients: results from the MacArthur Violence Risk Assessment Study. J Consult Clin Psychol 2001;69:358-74.
11. Bonta J, Hanson K, Law M. The prediction of criminal and violent recidivism among mentally disordered offenders: a meta-analysis. Psychol Bull 1998;123(2):123-42.
12. Serin RC, Amos NL. The role of psychopathy in the assessment of dangerousness. Int J Law Psychiatry 1995;18(2):231-8.
13. Link BG, Andrews H, Cullen FT. The violent and illegal behavior of mental patients reconsidered. Am Sociol Rev 1992;57(3):275-92.
14. Junginger J. Command hallucinations and the prediction of dangerousness. Psychiatr Serv 1995;46(9):911-14.
15. Wilson WH. Clinical review of clozapine treatment in a state hospital. Hosp Community Psychiatry 1992;43:700-3.
16. Volavka J, Zito JM, Vitrai J, Czobor P. Clozapine effects on hostility and aggression in schizophrenia. J Clin Psychopharmacol 1993;13:287-9.
17. Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. J Clin Psychopharmacol 1995;15:243-9.
18. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility in treatment-resistant patients with schizophrenia and schizoaffective disorder. Psychiatr Serv 2001;52:1510-14.
19. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol 2004;24:225-8.
20. Krakowski M, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 2006;63:622-9.
21. Gonzalez G, Rosenheck RA. Outcomes and service use among homeless persons with serious mental illness and substance abuse. Psychiatr Serv 2002;53:437-46.
22. Brunette MF, Drake RE, Xie H, et al. Clozapine use and relapses of substance use disorder among patients with co-occurring schizophrenia and substance use disorders. Schizophr Bull 2006;32:637-43.
23. Citrome L, Volavka J. Psychopharmacology of violence, I: assessment and acute treatment. Psychiatr Ann 1997;27:691-5.
24. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology 2003;28:1186-97.
25. Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry 1997;54:1081-8.
26. Walsh E, Gilvarry C, Samele C, et al. Reducing violence in severe mental illness: randomised controlled trial of intensive case management compared with standard care. BMJ 2001;323:1093-6.
27. Becker ME, Vakil E. Behavioural psychotherapy of the frontal-lobe-injured patient in an outpatient setting. Brain Inj 1993;7:515-23.
28. Goldney R, Bowes J, Spence N, et al. The psychiatric intensive care unit. Br J Psychiatry 1985;146:50-4
29. Beck R, Fernandez E. Cognitive-behavioral therapy in the treatment of anger: a meta-analysis. Cognit Ther Res 1998;22:63-74.
30. Novaco RW. Anger control: the development and evaluation of an experimental treatment. Lexington, MA: D.C. Heath, 1975.
When a patient with major psychiatric illness exhibits violent behavior, consider the course of violence in relation to his or her fixed and changing symptoms and deficits.1,2 Although most patients with schizophrenia, major depression, or bipolar disorder are not violent, effectively treating those who are calls for:
- differentiating between transient and persistent violent behavior
- providing medications and nonpharmacologic interventions shown to reduce each behavior
- addressing substance abuse and violent behavior concurrently.
Is violence transient or persistent?
Violent behavior is a common reason for psychiatric admission and prolonged hospital stays3 and a barrier to appropriate community placement4 and successful community reintegration.
Transient violence is limited to an acute psychotic episode; as psychotic symptoms abate, the violence resolves. Delusions, hallucinations, and conceptual disorganization are key triggers of transient violence.5 Excitement, anger, and agitation are its prominent symptoms.6
Treat a patient experiencing an acute violent episode with an oral first- or second-generation antipsychotic. For acute agitation, intramuscular (IM) delivery provides more rapid symptom resolution (Table 1). IM ziprasidone is approved for agitation associated with schizophrenia and IM olanzapine for use in agitation associated with schizophrenia or bipolar mania. Try talking calmly to the patient and explaining the need for medication (Figure 1). If this is not possible, a show of force might induce the patient’s cooperation.7
For sedation, antipsychotic medication can be supplemented by lorazepam, the only benzodiazepine that is reliably absorbed when administered IM. Lorazepam has a relatively short half-life, and the usual dosage of 1 to 2 mg can be administered orally, sublingually, intramuscularly, or intravenously every 1 to 6 hours. Exercise caution, however, when respiratory depression is a possibility.
Table 1
Medications used to treat violence on an emergency basis
| Drug | Route of administration* | Recommended dosage† |
|---|---|---|
| Benzodiazepines | ||
| Lorazepam | IM or PO | 1 to 2 mg IM or PO |
| Midazolam | IM | 5 mg |
| First-generation antipsychotic‡ | ||
| Haloperidol | IM or PO | 2 to 7.5 mg IM or PO |
| Second-generation antipsychotics‡ | ||
| Olanzapine | IM or PO | 10 mg IM or PO |
| Risperidone | PO | 2 to 6 mg |
| Ziprasidone | IM | 10 to 20 mg |
| * Use oral medication if patient is cooperative; otherwise use an intramuscular injection. | ||
| † Lower dosages are used for elderly patients or those with dementia. | ||
| ‡ Antipsychotics are not recommended for aggressive patients without a psychotic disorder or bipolar mania diagnosis. | ||
Figure 1 Managing patients who present with acute violence or agitation
FGA: First-generation antipsychotic; SGA: Second-generation antipsychoticPersistent violence. Emotional turmoil is usually less pronounced in patients whose violence is persistent.8 Neurocognitive impairments, antisocial traits, and specific psychotic symptoms may exist singly or in combination in patients prone to persistent violence.
When a patient continues to be violent, consider poor treatment adherence or substance abuse, especially with outpatients (Figure 2).
Figure 2 Managing patients who exhibit persistent violent behavior
Neurological and neurocognitive impairments are associated with persistent violence. Fairly broad impairments in various domains are seen on a variety of tests. Patients with neurocognitive impairment often present with impulsivity9 and deficits in behavioral adaptability.
In general, the consequences of a behavior determine its course; assaultive behavior usually decreases rapidly when strongly discouraged. Violent behavior that persists, therefore, suggests that neurocognitive impairment is causing a failure in behavioral adaptability.
Psychopathy, antisocial traits, and antisocial personality disorder (APD) also can result in persistent violence.
Antisocial personality is defined primarily by behavioral symptoms such as irresponsibility and criminal activities. Psychopathy also includes these symptoms but adds interpersonal and affective impairments such as callousness, grandiosity, and lack of remorse.
Psychopathy was the strongest clinical predictor of violence in a large trial of outpatients with major psychiatric disorders.10 APD, on the other hand, was the most significant clinical predictor of violent recidivism among offenders with mental illness in a meta-analysis of predictive longitudinal studies from 1959 to 1995.11 In another study, psychopaths were about 5 times more likely than nonpsychopaths to engage in violent recidivism.12
In addition to recidivism, the violence associated with psychopathy is characterized by its severity. Violence in these patients is often premeditated, deliberate, and goal-driven.
Persistent psychotic symptoms also play an important role in persistent violence and may represent treatment resistance. Some specific delusions, for example, are more likely to lead to violence and may persist despite overall improvement in psychotic symptoms.
Link et al13 have proposed that violence is more likely when a person has “threat/control override” delusions—if he believes people are seeking to harm him or outside forces are controlling his mind.
Junginger stressed that violent themes in a patient’s delusions are important predictors of violence.14 Delusions associated with violence are often chronic and well-circumscribed.
The role of antipsychotics
Pharmacologic intervention for violent behavior targets the underlying disorder, such as schizophrenia or bipolar disorder. Usual regimens used to treat patients with these disorders may need to be modified, however, for persistently violent patients (Table 2).
Second-generation antipsychotics (SGAs)—particularly clozapine—have superior antiaggressive properties beyond their antipsychotic or sedative effects, compared with first-generation antipsychotics. Retrospective studies have shown clozapine can significantly decrease the number of violent incidents and episodes of seclusion and restraint.15,16 Evidence for efficacy of other SGAs in reducing physical assaults is more limited:
- Risperidone had a greater effect than haloperidol on hostility in a large, multicenter comparison trial.17
- Clozapine was more effective than haloperidol or risperidone in reducing hostility in a double-blind study of schizophrenia patients.18 This finding was independent of clozapine’s antipsychotic effect.
Clozapine also was more effective than haloperidol in reducing the number and severity of aggressive incidents.19 The patients in this study, however, were not selected on the basis of aggressive behavior.
One large federally funded, double-blind, randomized trial compared clozapine, olanzapine, and haloperidol in 110 assaultive patients with schizophrenia or schizoaffective disorder. Patients had documented episodes of recent physical assaults and persistent aggressive behaviors during a 2-week period. Clozapine showed greater efficacy than olanzapine—and olanzapine greater efficacy than haloperidol—in reducing aggressive behavior.20 This effect was independent of the drugs’ antipsychotic and sedative actions.
Table 2
Medications used to treat persistent violence
| Drug | Initial dosage | Target dosage |
|---|---|---|
| Second-generation antipsychotics | ||
| Clozapine | 12.5 to 50 mg/d | 300 to 450 mg/d* |
| Olanzapine | 5 to 10 mg/d | 15 to 30 mg/d |
| Quetiapine | 50 to 100 mg/d | 400 to 700 mg/d |
| Risperidone | 1 to 3 mg/d | 4 to 6 mg/d |
| First-generation antipsychotic | ||
| Haloperidol | 5 to 10 mg/d | 10 to 20 mg/d |
| Mood stabilizers | ||
| Carbamazepine | 200 to 400 mg/d | 1,000 to 1,400 mg/d* |
| Lithium | 300 mg bid | 300 mg tid* |
| Valproate | 500 to 1,000 mg/d | 1,000 to 1,500 mg/d* |
| Beta blockers† | ||
| Nadolol | 40 mg/d | 80 to 140 mg/d |
| Propranolol | 20 mg tid | 200 mg to 600 (delayed onset of action) |
| * Serum levels should be obtained. | ||
| † Contraindicated for patients with cardiovascular disease, asthma, or diabetes. | ||
Dual-diagnosis patients. Clozapine may be beneficial for patients with concurrent substance abuse because in addition to reducing aggression, it also may prevent relapse to substance abuse. In addition to intoxication, drug and alcohol abuse has disruptive effects on prefrontal function. These impairments play an important role in substance use-related aggression.
Substance abuse also can exacerbate psychotic symptoms, both directly and indirectly through poor treatment compliance. Patients with psychopathy are much more likely to abuse drugs. The association between drug abuse and violence can then be due in part to the higher percentage of psychopaths in the group of drug abusers.
Fortunately, patients with dual diagnosis who receive extensive substance abuse treatment show greater clinical improvement and better outcomes.21 Several studies found that clozapine was associated with decreased substance use. In one trial, schizophrenic patients with a history of drug abuse who received clozapine were much less likely to use substances over the next year than patients taking other antipsychotic medications.22
Thus, clozapine has clear antiaggressive effects, but its use as a first-choice treatment for aggression is limited by the risk of side effects, in particular agranulocytosis. With careful blood monitoring, this complication is very rare, but persistently violent patients might not cooperate fully with the required monitoring.
Other medications
Other agents used to treat violent patients with mental disorders include mood stabilizers, beta blockers, and antidepressants.23
Mood stabilizers such as lithium, carbamazepine, or valproate might be useful as adjuncts to antipsychotic medications in managing assaultive patients with schizophrenia or other major psychiatric disorders. These medications might decrease violence by enhancing serotonergic activity.
Most evidence for mood stabilizers’ anti-aggressive effect comes from studies of patients with personality disorders. Divalproex, for example, was more effective than placebo in reducing impulsive aggression in patients with Cluster B personality disorders.24
Lithium reduces aggression and irritability in bipolar mania, while stabilizing the underlying disorder. Lithium can decrease aggression in other populations as well, including:
- the developmentally disabled
- prisoners with no apparent psychiatric diagnoses
- aggressive children and adolescents with conduct disorder
- adults with borderline personality disorder.
Beta blockers such as nadolol, pindolol, and propranolol have been reported to reduce aggression. Their usefulness is limited, however, because they are contraindicated in patients with cardiovascular disease, asthma, or diabetes.
Antidepressants. Selective serotonin reuptake inhibitors may reduce impulsive aggression in nondepressed patients with personality disorders.25
Nonpharmacologic treatments
To provide proper treatment, the clinician must understand the patient as a whole person, including his perception of his aggressive behavior. Nonpharmacologic interventions should be implemented with this in mind.
Compared with standard care, for example, intensive case management reduces the incidence of violence.26
Behavioral techniques can decrease violence by addressing specific impairments underlying the violence. For example, improving a patient’s cognitive functioning can counter impaired processing of feedback that is associated with neurological dysfunction.
Specific interventions, such as cueing to exaggerate the link between stimulus and response, could be beneficial.27 Similarly, these patients might respond to a high degree of structure, supervision, and specific environmental modifications, such as transfer to a unit that specializes in treating violent patients.28
In cognitive-behavioral therapy, patients can learn ways they can satisfy their needs without being violent. They also can be trained in problem-solving skills and in understanding the consequences of their actions. Such therapy might be useful for diminishing antisocial traits. Interventions aimed at preventing, decreasing, or counteracting arousal are important in addressing acute violence.
Anger management programs can help patients respond to interpersonal provocations in a more adaptive way.29 These programs include:
- education about aggression
- self-monitoring of anger frequency, intensity, and situational triggers
- relaxation to reduce arousal and enable guided imagery training
- training in behavioral coping, communication, and assertiveness through role play
- practicing new anger-coping skills.30
Tailor treatments to the dominant mechanisms underlying persistent violence.
Related resources
- Citrome L, Volavka J. Aggression. eMedicine from WebMD. www.emedicine.com/med/topic3005.htm.
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 2000;289:591-94.
Drug brand names
- Carbamazepine • Carbatrol, Equetro, Tegretol
- Clozapine • Clozaril
- Divalproex • Depakote
- Haloperidol • Haldol
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Midazolam • Versed
- Nadolol • Corgard
- Olanzapine • Zyprexa
- Pindolol • Visken
- Propranolol • Inderal, Inderide
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosure
Dr. Krakowski receives research support from Eli Lilly and Co. and GlaxoSmithKline.
When a patient with major psychiatric illness exhibits violent behavior, consider the course of violence in relation to his or her fixed and changing symptoms and deficits.1,2 Although most patients with schizophrenia, major depression, or bipolar disorder are not violent, effectively treating those who are calls for:
- differentiating between transient and persistent violent behavior
- providing medications and nonpharmacologic interventions shown to reduce each behavior
- addressing substance abuse and violent behavior concurrently.
Is violence transient or persistent?
Violent behavior is a common reason for psychiatric admission and prolonged hospital stays3 and a barrier to appropriate community placement4 and successful community reintegration.
Transient violence is limited to an acute psychotic episode; as psychotic symptoms abate, the violence resolves. Delusions, hallucinations, and conceptual disorganization are key triggers of transient violence.5 Excitement, anger, and agitation are its prominent symptoms.6
Treat a patient experiencing an acute violent episode with an oral first- or second-generation antipsychotic. For acute agitation, intramuscular (IM) delivery provides more rapid symptom resolution (Table 1). IM ziprasidone is approved for agitation associated with schizophrenia and IM olanzapine for use in agitation associated with schizophrenia or bipolar mania. Try talking calmly to the patient and explaining the need for medication (Figure 1). If this is not possible, a show of force might induce the patient’s cooperation.7
For sedation, antipsychotic medication can be supplemented by lorazepam, the only benzodiazepine that is reliably absorbed when administered IM. Lorazepam has a relatively short half-life, and the usual dosage of 1 to 2 mg can be administered orally, sublingually, intramuscularly, or intravenously every 1 to 6 hours. Exercise caution, however, when respiratory depression is a possibility.
Table 1
Medications used to treat violence on an emergency basis
| Drug | Route of administration* | Recommended dosage† |
|---|---|---|
| Benzodiazepines | ||
| Lorazepam | IM or PO | 1 to 2 mg IM or PO |
| Midazolam | IM | 5 mg |
| First-generation antipsychotic‡ | ||
| Haloperidol | IM or PO | 2 to 7.5 mg IM or PO |
| Second-generation antipsychotics‡ | ||
| Olanzapine | IM or PO | 10 mg IM or PO |
| Risperidone | PO | 2 to 6 mg |
| Ziprasidone | IM | 10 to 20 mg |
| * Use oral medication if patient is cooperative; otherwise use an intramuscular injection. | ||
| † Lower dosages are used for elderly patients or those with dementia. | ||
| ‡ Antipsychotics are not recommended for aggressive patients without a psychotic disorder or bipolar mania diagnosis. | ||
Figure 1 Managing patients who present with acute violence or agitation
FGA: First-generation antipsychotic; SGA: Second-generation antipsychoticPersistent violence. Emotional turmoil is usually less pronounced in patients whose violence is persistent.8 Neurocognitive impairments, antisocial traits, and specific psychotic symptoms may exist singly or in combination in patients prone to persistent violence.
When a patient continues to be violent, consider poor treatment adherence or substance abuse, especially with outpatients (Figure 2).
Figure 2 Managing patients who exhibit persistent violent behavior
Neurological and neurocognitive impairments are associated with persistent violence. Fairly broad impairments in various domains are seen on a variety of tests. Patients with neurocognitive impairment often present with impulsivity9 and deficits in behavioral adaptability.
In general, the consequences of a behavior determine its course; assaultive behavior usually decreases rapidly when strongly discouraged. Violent behavior that persists, therefore, suggests that neurocognitive impairment is causing a failure in behavioral adaptability.
Psychopathy, antisocial traits, and antisocial personality disorder (APD) also can result in persistent violence.
Antisocial personality is defined primarily by behavioral symptoms such as irresponsibility and criminal activities. Psychopathy also includes these symptoms but adds interpersonal and affective impairments such as callousness, grandiosity, and lack of remorse.
Psychopathy was the strongest clinical predictor of violence in a large trial of outpatients with major psychiatric disorders.10 APD, on the other hand, was the most significant clinical predictor of violent recidivism among offenders with mental illness in a meta-analysis of predictive longitudinal studies from 1959 to 1995.11 In another study, psychopaths were about 5 times more likely than nonpsychopaths to engage in violent recidivism.12
In addition to recidivism, the violence associated with psychopathy is characterized by its severity. Violence in these patients is often premeditated, deliberate, and goal-driven.
Persistent psychotic symptoms also play an important role in persistent violence and may represent treatment resistance. Some specific delusions, for example, are more likely to lead to violence and may persist despite overall improvement in psychotic symptoms.
Link et al13 have proposed that violence is more likely when a person has “threat/control override” delusions—if he believes people are seeking to harm him or outside forces are controlling his mind.
Junginger stressed that violent themes in a patient’s delusions are important predictors of violence.14 Delusions associated with violence are often chronic and well-circumscribed.
The role of antipsychotics
Pharmacologic intervention for violent behavior targets the underlying disorder, such as schizophrenia or bipolar disorder. Usual regimens used to treat patients with these disorders may need to be modified, however, for persistently violent patients (Table 2).
Second-generation antipsychotics (SGAs)—particularly clozapine—have superior antiaggressive properties beyond their antipsychotic or sedative effects, compared with first-generation antipsychotics. Retrospective studies have shown clozapine can significantly decrease the number of violent incidents and episodes of seclusion and restraint.15,16 Evidence for efficacy of other SGAs in reducing physical assaults is more limited:
- Risperidone had a greater effect than haloperidol on hostility in a large, multicenter comparison trial.17
- Clozapine was more effective than haloperidol or risperidone in reducing hostility in a double-blind study of schizophrenia patients.18 This finding was independent of clozapine’s antipsychotic effect.
Clozapine also was more effective than haloperidol in reducing the number and severity of aggressive incidents.19 The patients in this study, however, were not selected on the basis of aggressive behavior.
One large federally funded, double-blind, randomized trial compared clozapine, olanzapine, and haloperidol in 110 assaultive patients with schizophrenia or schizoaffective disorder. Patients had documented episodes of recent physical assaults and persistent aggressive behaviors during a 2-week period. Clozapine showed greater efficacy than olanzapine—and olanzapine greater efficacy than haloperidol—in reducing aggressive behavior.20 This effect was independent of the drugs’ antipsychotic and sedative actions.
Table 2
Medications used to treat persistent violence
| Drug | Initial dosage | Target dosage |
|---|---|---|
| Second-generation antipsychotics | ||
| Clozapine | 12.5 to 50 mg/d | 300 to 450 mg/d* |
| Olanzapine | 5 to 10 mg/d | 15 to 30 mg/d |
| Quetiapine | 50 to 100 mg/d | 400 to 700 mg/d |
| Risperidone | 1 to 3 mg/d | 4 to 6 mg/d |
| First-generation antipsychotic | ||
| Haloperidol | 5 to 10 mg/d | 10 to 20 mg/d |
| Mood stabilizers | ||
| Carbamazepine | 200 to 400 mg/d | 1,000 to 1,400 mg/d* |
| Lithium | 300 mg bid | 300 mg tid* |
| Valproate | 500 to 1,000 mg/d | 1,000 to 1,500 mg/d* |
| Beta blockers† | ||
| Nadolol | 40 mg/d | 80 to 140 mg/d |
| Propranolol | 20 mg tid | 200 mg to 600 (delayed onset of action) |
| * Serum levels should be obtained. | ||
| † Contraindicated for patients with cardiovascular disease, asthma, or diabetes. | ||
Dual-diagnosis patients. Clozapine may be beneficial for patients with concurrent substance abuse because in addition to reducing aggression, it also may prevent relapse to substance abuse. In addition to intoxication, drug and alcohol abuse has disruptive effects on prefrontal function. These impairments play an important role in substance use-related aggression.
Substance abuse also can exacerbate psychotic symptoms, both directly and indirectly through poor treatment compliance. Patients with psychopathy are much more likely to abuse drugs. The association between drug abuse and violence can then be due in part to the higher percentage of psychopaths in the group of drug abusers.
Fortunately, patients with dual diagnosis who receive extensive substance abuse treatment show greater clinical improvement and better outcomes.21 Several studies found that clozapine was associated with decreased substance use. In one trial, schizophrenic patients with a history of drug abuse who received clozapine were much less likely to use substances over the next year than patients taking other antipsychotic medications.22
Thus, clozapine has clear antiaggressive effects, but its use as a first-choice treatment for aggression is limited by the risk of side effects, in particular agranulocytosis. With careful blood monitoring, this complication is very rare, but persistently violent patients might not cooperate fully with the required monitoring.
Other medications
Other agents used to treat violent patients with mental disorders include mood stabilizers, beta blockers, and antidepressants.23
Mood stabilizers such as lithium, carbamazepine, or valproate might be useful as adjuncts to antipsychotic medications in managing assaultive patients with schizophrenia or other major psychiatric disorders. These medications might decrease violence by enhancing serotonergic activity.
Most evidence for mood stabilizers’ anti-aggressive effect comes from studies of patients with personality disorders. Divalproex, for example, was more effective than placebo in reducing impulsive aggression in patients with Cluster B personality disorders.24
Lithium reduces aggression and irritability in bipolar mania, while stabilizing the underlying disorder. Lithium can decrease aggression in other populations as well, including:
- the developmentally disabled
- prisoners with no apparent psychiatric diagnoses
- aggressive children and adolescents with conduct disorder
- adults with borderline personality disorder.
Beta blockers such as nadolol, pindolol, and propranolol have been reported to reduce aggression. Their usefulness is limited, however, because they are contraindicated in patients with cardiovascular disease, asthma, or diabetes.
Antidepressants. Selective serotonin reuptake inhibitors may reduce impulsive aggression in nondepressed patients with personality disorders.25
Nonpharmacologic treatments
To provide proper treatment, the clinician must understand the patient as a whole person, including his perception of his aggressive behavior. Nonpharmacologic interventions should be implemented with this in mind.
Compared with standard care, for example, intensive case management reduces the incidence of violence.26
Behavioral techniques can decrease violence by addressing specific impairments underlying the violence. For example, improving a patient’s cognitive functioning can counter impaired processing of feedback that is associated with neurological dysfunction.
Specific interventions, such as cueing to exaggerate the link between stimulus and response, could be beneficial.27 Similarly, these patients might respond to a high degree of structure, supervision, and specific environmental modifications, such as transfer to a unit that specializes in treating violent patients.28
In cognitive-behavioral therapy, patients can learn ways they can satisfy their needs without being violent. They also can be trained in problem-solving skills and in understanding the consequences of their actions. Such therapy might be useful for diminishing antisocial traits. Interventions aimed at preventing, decreasing, or counteracting arousal are important in addressing acute violence.
Anger management programs can help patients respond to interpersonal provocations in a more adaptive way.29 These programs include:
- education about aggression
- self-monitoring of anger frequency, intensity, and situational triggers
- relaxation to reduce arousal and enable guided imagery training
- training in behavioral coping, communication, and assertiveness through role play
- practicing new anger-coping skills.30
Tailor treatments to the dominant mechanisms underlying persistent violence.
Related resources
- Citrome L, Volavka J. Aggression. eMedicine from WebMD. www.emedicine.com/med/topic3005.htm.
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 2000;289:591-94.
Drug brand names
- Carbamazepine • Carbatrol, Equetro, Tegretol
- Clozapine • Clozaril
- Divalproex • Depakote
- Haloperidol • Haldol
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Midazolam • Versed
- Nadolol • Corgard
- Olanzapine • Zyprexa
- Pindolol • Visken
- Propranolol • Inderal, Inderide
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosure
Dr. Krakowski receives research support from Eli Lilly and Co. and GlaxoSmithKline.
1. Krakowski M, Czobor P. Violence in psychiatric patients: the role of psychosis, frontal lobe impairment and ward turmoil. Compr Psychiatry 1997;38:230-36.
2. Krakowski M, Convit A, Jaeger J, et al. Neurological impairment in violent schizophrenic inpatients. Am J Psychiatry 1989;146:849-53.
3. Lelliott P, Wing J, Clifford P. A national audit of new longstay psychiatric patients. I: Method and description of the cohort. Br J Psychiatry 1994;165:1609.-
4. Bigelow DA, Cutler DL, Moore LJ, et al. Characteristics of state hospital patients who are hard to place. Hosp Community Psychiatry 1988;39:1815.-
5. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry 1994;45:133-7.
6. Craig TJ. An epidemiologic study of problems associated with violence among psychiatric inpatients. Am J Psychiatry 1982;139:1262-6.
7. Allen MH. Managing the agitated psychiatric patient: a reappraisal of the evidence. J Clin Psychiatry 2000;61(suppl 4):11-20.
8. Krakowski M, Czobor P, Chou J. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull 1999;25:505-17.
9. Stein DJ, Hollander E, Cohen L, et al. Neuropsychiatric impairment in impulsive personality disorders. Psychiatry Res 1993;48:257-66.
10. Skeem JL, Mulvey EP. Psychopathy and community violence among civil psychiatric patients: results from the MacArthur Violence Risk Assessment Study. J Consult Clin Psychol 2001;69:358-74.
11. Bonta J, Hanson K, Law M. The prediction of criminal and violent recidivism among mentally disordered offenders: a meta-analysis. Psychol Bull 1998;123(2):123-42.
12. Serin RC, Amos NL. The role of psychopathy in the assessment of dangerousness. Int J Law Psychiatry 1995;18(2):231-8.
13. Link BG, Andrews H, Cullen FT. The violent and illegal behavior of mental patients reconsidered. Am Sociol Rev 1992;57(3):275-92.
14. Junginger J. Command hallucinations and the prediction of dangerousness. Psychiatr Serv 1995;46(9):911-14.
15. Wilson WH. Clinical review of clozapine treatment in a state hospital. Hosp Community Psychiatry 1992;43:700-3.
16. Volavka J, Zito JM, Vitrai J, Czobor P. Clozapine effects on hostility and aggression in schizophrenia. J Clin Psychopharmacol 1993;13:287-9.
17. Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. J Clin Psychopharmacol 1995;15:243-9.
18. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility in treatment-resistant patients with schizophrenia and schizoaffective disorder. Psychiatr Serv 2001;52:1510-14.
19. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol 2004;24:225-8.
20. Krakowski M, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 2006;63:622-9.
21. Gonzalez G, Rosenheck RA. Outcomes and service use among homeless persons with serious mental illness and substance abuse. Psychiatr Serv 2002;53:437-46.
22. Brunette MF, Drake RE, Xie H, et al. Clozapine use and relapses of substance use disorder among patients with co-occurring schizophrenia and substance use disorders. Schizophr Bull 2006;32:637-43.
23. Citrome L, Volavka J. Psychopharmacology of violence, I: assessment and acute treatment. Psychiatr Ann 1997;27:691-5.
24. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology 2003;28:1186-97.
25. Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry 1997;54:1081-8.
26. Walsh E, Gilvarry C, Samele C, et al. Reducing violence in severe mental illness: randomised controlled trial of intensive case management compared with standard care. BMJ 2001;323:1093-6.
27. Becker ME, Vakil E. Behavioural psychotherapy of the frontal-lobe-injured patient in an outpatient setting. Brain Inj 1993;7:515-23.
28. Goldney R, Bowes J, Spence N, et al. The psychiatric intensive care unit. Br J Psychiatry 1985;146:50-4
29. Beck R, Fernandez E. Cognitive-behavioral therapy in the treatment of anger: a meta-analysis. Cognit Ther Res 1998;22:63-74.
30. Novaco RW. Anger control: the development and evaluation of an experimental treatment. Lexington, MA: D.C. Heath, 1975.
1. Krakowski M, Czobor P. Violence in psychiatric patients: the role of psychosis, frontal lobe impairment and ward turmoil. Compr Psychiatry 1997;38:230-36.
2. Krakowski M, Convit A, Jaeger J, et al. Neurological impairment in violent schizophrenic inpatients. Am J Psychiatry 1989;146:849-53.
3. Lelliott P, Wing J, Clifford P. A national audit of new longstay psychiatric patients. I: Method and description of the cohort. Br J Psychiatry 1994;165:1609.-
4. Bigelow DA, Cutler DL, Moore LJ, et al. Characteristics of state hospital patients who are hard to place. Hosp Community Psychiatry 1988;39:1815.-
5. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry 1994;45:133-7.
6. Craig TJ. An epidemiologic study of problems associated with violence among psychiatric inpatients. Am J Psychiatry 1982;139:1262-6.
7. Allen MH. Managing the agitated psychiatric patient: a reappraisal of the evidence. J Clin Psychiatry 2000;61(suppl 4):11-20.
8. Krakowski M, Czobor P, Chou J. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull 1999;25:505-17.
9. Stein DJ, Hollander E, Cohen L, et al. Neuropsychiatric impairment in impulsive personality disorders. Psychiatry Res 1993;48:257-66.
10. Skeem JL, Mulvey EP. Psychopathy and community violence among civil psychiatric patients: results from the MacArthur Violence Risk Assessment Study. J Consult Clin Psychol 2001;69:358-74.
11. Bonta J, Hanson K, Law M. The prediction of criminal and violent recidivism among mentally disordered offenders: a meta-analysis. Psychol Bull 1998;123(2):123-42.
12. Serin RC, Amos NL. The role of psychopathy in the assessment of dangerousness. Int J Law Psychiatry 1995;18(2):231-8.
13. Link BG, Andrews H, Cullen FT. The violent and illegal behavior of mental patients reconsidered. Am Sociol Rev 1992;57(3):275-92.
14. Junginger J. Command hallucinations and the prediction of dangerousness. Psychiatr Serv 1995;46(9):911-14.
15. Wilson WH. Clinical review of clozapine treatment in a state hospital. Hosp Community Psychiatry 1992;43:700-3.
16. Volavka J, Zito JM, Vitrai J, Czobor P. Clozapine effects on hostility and aggression in schizophrenia. J Clin Psychopharmacol 1993;13:287-9.
17. Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. J Clin Psychopharmacol 1995;15:243-9.
18. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility in treatment-resistant patients with schizophrenia and schizoaffective disorder. Psychiatr Serv 2001;52:1510-14.
19. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol 2004;24:225-8.
20. Krakowski M, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 2006;63:622-9.
21. Gonzalez G, Rosenheck RA. Outcomes and service use among homeless persons with serious mental illness and substance abuse. Psychiatr Serv 2002;53:437-46.
22. Brunette MF, Drake RE, Xie H, et al. Clozapine use and relapses of substance use disorder among patients with co-occurring schizophrenia and substance use disorders. Schizophr Bull 2006;32:637-43.
23. Citrome L, Volavka J. Psychopharmacology of violence, I: assessment and acute treatment. Psychiatr Ann 1997;27:691-5.
24. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology 2003;28:1186-97.
25. Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry 1997;54:1081-8.
26. Walsh E, Gilvarry C, Samele C, et al. Reducing violence in severe mental illness: randomised controlled trial of intensive case management compared with standard care. BMJ 2001;323:1093-6.
27. Becker ME, Vakil E. Behavioural psychotherapy of the frontal-lobe-injured patient in an outpatient setting. Brain Inj 1993;7:515-23.
28. Goldney R, Bowes J, Spence N, et al. The psychiatric intensive care unit. Br J Psychiatry 1985;146:50-4
29. Beck R, Fernandez E. Cognitive-behavioral therapy in the treatment of anger: a meta-analysis. Cognit Ther Res 1998;22:63-74.
30. Novaco RW. Anger control: the development and evaluation of an experimental treatment. Lexington, MA: D.C. Heath, 1975.
Factitious illness: A 3-step consultation-liaison approach
Ms. J, age 33, arrives at the emergency department (ED) complaining of chest pain and shortness of breath—symptoms she says are similar to those she had during episodes of pulmonary embolism. Routine laboratory workup, including chest CT and ultrasound of the lower extremities, indicate a very low likelihood of PE, but she insists that she be admitted.
Hear Dr. Stern's insights on recognizing and treating Munchausen's syndrome. Click here.
On the medical floor, nursing staff note that Ms. J appears short of breath only when directly observed. Medical records reveal multiple visits to other hospitals with repeated requests for admission. When gently confronted, she maintains she will die
if she is not treated.
Has your hospital’s medical staff ever been puzzled by a patient’s inconsistent presentation or unsettled by a concern that he or she was not being straightforward with them? Have they suspected that a patient such as Ms. J may be voluntarily producing his or her symptoms?
This article suggests a 3-step approach by which the consultation-liaison psychiatrist can help medical staff identify and manage patients with factitious illness.
Cardinal features
In factitious illness, the patient’s symptoms are:
- under voluntary control and consciously produced
- not a direct result of a medical or psychiatric condition
- produced to assume the sick role (not to accrue secondary gain—a core feature of malingering).
CASE: Self-inflicted injury
Ms. H, age 50, surprises even the most seasoned clinicians when she presents to the ED with brain parenchyma herniating from an open wound in her skull. She denies having picked at her scalp and does not endorse a history of obsessive-compulsive disorder or trichotillomania.
On the medical floor, however, she is seen picking at the wound, which leaves blood on her protective mittens. Surgical repair is repeatedly attempted, and her case is complicated by chronic infections and a nonhealing wound.
Clinical presentation
Factitious disorder presents 3 diagnostic and treatment challenges for a hospital’s medical staff:
- To recognize and treat (even self-inflicted) serious medical conditions that can be life-threatening.
- To orchestrate appropriate diagnostic evaluation. (Remember that factitious illness is a diagnosis of exclusion.)
- To handle countertransference reactions to patients that can be intense; physicians may experience anger, frustration, resignation, and hatred.
CASE: ‘Suicidal’ but not depressed
Mr. B, age 48, presents to the ED with thoughts of suicide and profoundly depressed mood. On examination, however, he does not appear depressed. He repeatedly requests food, cigarettes, and assistance in finding shelter, which lead to concern that his main goal is secondary gain. However, because Mr. B has a history of serious suicide attempts—including some while an inpatient—the ED physician is reluctant to dismiss his complaints and unsure about how to proceed.
3-step diagnostic approach
Treating factitious illness is predicated upon making the correct diagnosis, which requires the medical team to investigate and gather data from collateral sources, such as outside hospital medical records and other providers. The diagnostic process can be summarized in 3 steps:
Step 1. Determine whether the patient has an identifiable medical or psychiatric problem that could explain the symptoms.
Step 2. Determine whether the symptoms are consciously or unconsciously produced. Somatoform disorders—such as conversion disorder and somatization disorder, for example—are thought to result from processes outside the patient’s control.
Step 3. Distinguish if the motivation is to obtain the sick role (consider factitious illness) or if material benefits are the goal (consider malingering). Both motivations may be operative in a given patient.
Table 1
Medical clues to a patient with factitious illness
| Vague symptom history that frays upon examination |
| Irritability and evasiveness with continued questioning |
| Familiarity with hospital procedures and protocols (some patients have received medical training) |
| Multiple scars as evidence of past procedures and hospitalizations |
| Acceptance of painful medical procedures without complaint |
| Itinerant lives devoid of close personal relationships |
| Failure to accurately identify themselves |
| Lack of a verifiable history |
| Source: Reference 1 |
Psychiatric evaluation. Physicians should think of factitious psychiatric illness when:
- a patient’s behavior is notably different when he believes he is being directly observed and when he believes he is alone3
- psychiatric symptoms do not readily fit into diagnostic categories (such as a vague mix of memory loss, suicidal thoughts, and psychosis)
- the patient is suggestible or provides a diffusely positive review of systems (for example, he may report additional symptoms after having observed other patients).
The psychiatric presentations of Munchausen syndrome can be especially complicated, as they are usually associated with less objective evidence than are medical presentations (Box).4-10 Clarity of the history and diagnosis may be in the eye of the beholder.
Admission characteristics
Somatic complaints. Chaos often surrounds the hospitalized patient with factitious illness. The ED commonly is their gateway, and they tend to arrive in the evening or on weekends when less experienced staff are on call.11
Presentation severity ranges up to Munchausen syndrome
Munchausen syndrome—a particularly severe factitious illness—is characterized by peregrination, recurrent presentations, and pseudologia fantastica (stories that seem outrageously exaggerated).4 In 1951, Asher named this syndrome for Baron von Münchhausen, an 18th century Prussian officer who wandered from city to city creating tall tales about his life.5
Munchausen by proxy, in which a parent is responsible for producing illness in a child, may lead to extensive medical evaluations and treatment.
After more than 50 years, factitious illness continues to draw scientific and clinical attention. A search of PubMed over the last 10 years found nearly 500 citations. Presentations included:
- symptomatic bradycardia caused by beta-blocker ingestion6
- refractory hypoglycemia caused by surreptitious insulin injections7
- false reports of aortic dissection8
- recurrent episodes of self-harm including bilateral blindness from ocular trauma9
- fabricated sweat chloride test results in a patient claiming to have cystic fibrosis.10
Escalating demands. During the hospital stay, patients with factitious illness may make repeated requests for care, which may escalate into demands if their needs are not met.13 At this point, staff often start to experience negative countertransference reactions. As medical tests reveal little to no evidence of an organic basis for their symptoms and no cohesive psychiatric diagnosis is reached, patients may complain of misdiagnosis and mistreatment.13
Patients usually leave before psychiatric consultation can be obtained, and the underlying suffering that led to their factitious complaints remains unaddressed. Typically, patients are lost to follow-up until the next presentation at another hospital, where the process begins again.
What motivates patients?
The motivation behind factitious presentations can be bewildering. Asher’s paper on Munchausen syndrome described several possible reasons for patients’ behavior, such as desire to be the center of attention, holding a grudge against the medical profession, drug seeking, looking for shelter, and running from police.5 This list, however, includes correlates of secondary gain, which with today’s psychiatric nomenclature would lead to a diagnosis of malingering.
Psychological factors. Some clinicians have tried to address underlying psychiatric factors, but data on evaluation and management are limited because these patients usually eschew psychiatric examination. Although the patient is voluntarily producing the symptoms, unconscious psychological factors are at play and are an essential part of the picture.14
When assessed, patients appear to have lived rootless lives with few attachments, which may have been the result of sadistic and unsatisfying relationships with authority figures of their youth.15,16 Their grandiosity and distortion of the truth suggest a narcissistic need to overcome feelings of incompetence or impotence.17 Their ambivalent relationship to hospitals and physicians may reflect a need for caretaking, arising from early relationships and past caretakers.
Lastly, there is a component of masochism; this makes some individuals (erroneously) believe that if you don’t inflict pain you don’t care about them.13
Treatment challenges
Because patients with factitious disorder are not easily studied, no particular treatment is well-supported in the literature. Approaches that have been reported include preventing patients from being re-admitted to medical facilities, admitting patients for psychiatric treatment, and providing outpatient therapies such as individual psychodynamic psychotherapy, behavioral modification, and group psychotherapy.18
Other management strategies suggested in the literature include:
- reframing cognitive distortions
- drawing up a set of realistic hospitalization goals (with a written contract)
- maximizing the therapeutic alliance
- avoiding team splitting
- minimizing iatrogenic harm.19
Table 2
Recommended care for a patient with factitious illness
| Fully investigate all medical and psychiatric complaints, especially if physical safety is threatened |
| Maintain a healthy skepticism about unusual or illogical presentations while attempting to preserve an empathic connection with the patient |
| Be aware of countertransference reactions, as they may provide valuable insight about the underlying cause of the patient’s symptoms |
| Realize that psychiatric symptoms and medical presentations fall on a continuum from conscious to unconscious; at times there may be a mix of motivations |
| Report all findings nonjudgmentally, both to the patient and in medical documentation |
Nevertheless, prepare the physician for the patient to respond to confrontation with denial and resistance because he or she feels exposed and humiliated. If the physician makes it clear that ongoing medical care will still be available—even if the symptoms are fabricated—the patient may be more willing to accept psychiatric treatment.13
Related resources
- Barsky AJ, Stern TA, Greenberg DB, Cassem NH. Functional somatic symptoms and somatoform disorders. In: Stern TA, Fricchione GL, Cassem NH, et al, eds. The Massachusetts General Hospital handbook of general hospital psychiatry 5th ed. Philadelphia: Mosby/Elsevier; 2004:269-91.
- Elwyn TS, Ahmed I. Factitious disorder. EMedicine from WebMD. Last updated April 13, 2006. www.emedicine.com/med/topic3125.htm.
1. Stern T. Malingering, factitious illness, and somatization. In: Hyman S, ed. Manual of psychiatric emergencies. Boston: Little, Brown, and Co; 1988;23:217-25.-
2. Turner J, Reid S. Munchausen’s syndrome. Lancet. 2002;359:346-9.
3. Popli A, Prakash S, Dewan M. Factitious disorders with psychological symptoms. J Clin Psychiatry 1992;53:9.-
4. Huffman J, Stern T. The diagnosis and treatment of Munchausen’s syndrome. Gen Hosp Psychiatry 2003;25:358-63.
5. Asher R. Munchausen’s syndrome. Lancet 1951;1:339-41.
6. Steinwender C, Hofmann R, Kypta A, Leisch F. Recurrent symptomatic bradycardia due to secret ingestion of beta-blockers—a rare manifestation of cardiac Munchausen syndrome. Wien Klon Wochenschr 2005;117(18):647-50.
7. Bretz S, Richards J. Munchausen syndrome presenting acutely in the emergency department. J Emerg Med 2000;18(4):417-20.
8. Hopkins R, Harrington C, Poppas A. Munchausen syndrome simulating acute aortic dissection. Ann Thorac Surg 2006;81(4):1497-99.
9. Salvo M, Pinna A, Milia P, Carta F. Ocular Munchausen syndrome resulting in bilateral blindness. Eur J Ophthalmol 2006;16(4):654-56.
10. Highland K, Flume P. A “story” of a woman with cystic fibrosis. Chest 2002;121(5):1704-7.
11. Stretton J. Munchausen syndrome. Lancet 1951;1:474.-
12. Stern T. Munchausen’s syndrome revisited. Psychosomatics 1980;21(4):329-36.
13. Stern T. Factitious disorders. In: Hyman S, Jenike M, eds. Manual of clinical problems in psychiatry Boston: Little, Brown, and Co; 1990;21:190-4.
14. Greenacre P. The imposter. Psychoanal Q 1958;27:359-82.
15. Cramer B, Gershberg M, Stern M. Munchausen syndrome. Arch Gen Psychiatry 1971;24:573-8.
16. Ford C. The Munchausen syndrome: a report of four new cases and a review of psychodynamic considerations. Psychiatry Med 1973;4:31-45.
17. Bursten B. On Munchausen’s syndrome. Arch Gen Psychiatry 1965;13:261-8.
18. Yassa R. Munchausen’s syndrome: a successfully treated case. Psychosomatics 1978;19:242.-
19. Gregory RJ, Jindal S. Factitious disorder on an inpatient psychiatry ward. Am J Orthopsychiatry 2006;76(1):31-6.
Ms. J, age 33, arrives at the emergency department (ED) complaining of chest pain and shortness of breath—symptoms she says are similar to those she had during episodes of pulmonary embolism. Routine laboratory workup, including chest CT and ultrasound of the lower extremities, indicate a very low likelihood of PE, but she insists that she be admitted.
Hear Dr. Stern's insights on recognizing and treating Munchausen's syndrome. Click here.
On the medical floor, nursing staff note that Ms. J appears short of breath only when directly observed. Medical records reveal multiple visits to other hospitals with repeated requests for admission. When gently confronted, she maintains she will die
if she is not treated.
Has your hospital’s medical staff ever been puzzled by a patient’s inconsistent presentation or unsettled by a concern that he or she was not being straightforward with them? Have they suspected that a patient such as Ms. J may be voluntarily producing his or her symptoms?
This article suggests a 3-step approach by which the consultation-liaison psychiatrist can help medical staff identify and manage patients with factitious illness.
Cardinal features
In factitious illness, the patient’s symptoms are:
- under voluntary control and consciously produced
- not a direct result of a medical or psychiatric condition
- produced to assume the sick role (not to accrue secondary gain—a core feature of malingering).
CASE: Self-inflicted injury
Ms. H, age 50, surprises even the most seasoned clinicians when she presents to the ED with brain parenchyma herniating from an open wound in her skull. She denies having picked at her scalp and does not endorse a history of obsessive-compulsive disorder or trichotillomania.
On the medical floor, however, she is seen picking at the wound, which leaves blood on her protective mittens. Surgical repair is repeatedly attempted, and her case is complicated by chronic infections and a nonhealing wound.
Clinical presentation
Factitious disorder presents 3 diagnostic and treatment challenges for a hospital’s medical staff:
- To recognize and treat (even self-inflicted) serious medical conditions that can be life-threatening.
- To orchestrate appropriate diagnostic evaluation. (Remember that factitious illness is a diagnosis of exclusion.)
- To handle countertransference reactions to patients that can be intense; physicians may experience anger, frustration, resignation, and hatred.
CASE: ‘Suicidal’ but not depressed
Mr. B, age 48, presents to the ED with thoughts of suicide and profoundly depressed mood. On examination, however, he does not appear depressed. He repeatedly requests food, cigarettes, and assistance in finding shelter, which lead to concern that his main goal is secondary gain. However, because Mr. B has a history of serious suicide attempts—including some while an inpatient—the ED physician is reluctant to dismiss his complaints and unsure about how to proceed.
3-step diagnostic approach
Treating factitious illness is predicated upon making the correct diagnosis, which requires the medical team to investigate and gather data from collateral sources, such as outside hospital medical records and other providers. The diagnostic process can be summarized in 3 steps:
Step 1. Determine whether the patient has an identifiable medical or psychiatric problem that could explain the symptoms.
Step 2. Determine whether the symptoms are consciously or unconsciously produced. Somatoform disorders—such as conversion disorder and somatization disorder, for example—are thought to result from processes outside the patient’s control.
Step 3. Distinguish if the motivation is to obtain the sick role (consider factitious illness) or if material benefits are the goal (consider malingering). Both motivations may be operative in a given patient.
Table 1
Medical clues to a patient with factitious illness
| Vague symptom history that frays upon examination |
| Irritability and evasiveness with continued questioning |
| Familiarity with hospital procedures and protocols (some patients have received medical training) |
| Multiple scars as evidence of past procedures and hospitalizations |
| Acceptance of painful medical procedures without complaint |
| Itinerant lives devoid of close personal relationships |
| Failure to accurately identify themselves |
| Lack of a verifiable history |
| Source: Reference 1 |
Psychiatric evaluation. Physicians should think of factitious psychiatric illness when:
- a patient’s behavior is notably different when he believes he is being directly observed and when he believes he is alone3
- psychiatric symptoms do not readily fit into diagnostic categories (such as a vague mix of memory loss, suicidal thoughts, and psychosis)
- the patient is suggestible or provides a diffusely positive review of systems (for example, he may report additional symptoms after having observed other patients).
The psychiatric presentations of Munchausen syndrome can be especially complicated, as they are usually associated with less objective evidence than are medical presentations (Box).4-10 Clarity of the history and diagnosis may be in the eye of the beholder.
Admission characteristics
Somatic complaints. Chaos often surrounds the hospitalized patient with factitious illness. The ED commonly is their gateway, and they tend to arrive in the evening or on weekends when less experienced staff are on call.11
Presentation severity ranges up to Munchausen syndrome
Munchausen syndrome—a particularly severe factitious illness—is characterized by peregrination, recurrent presentations, and pseudologia fantastica (stories that seem outrageously exaggerated).4 In 1951, Asher named this syndrome for Baron von Münchhausen, an 18th century Prussian officer who wandered from city to city creating tall tales about his life.5
Munchausen by proxy, in which a parent is responsible for producing illness in a child, may lead to extensive medical evaluations and treatment.
After more than 50 years, factitious illness continues to draw scientific and clinical attention. A search of PubMed over the last 10 years found nearly 500 citations. Presentations included:
- symptomatic bradycardia caused by beta-blocker ingestion6
- refractory hypoglycemia caused by surreptitious insulin injections7
- false reports of aortic dissection8
- recurrent episodes of self-harm including bilateral blindness from ocular trauma9
- fabricated sweat chloride test results in a patient claiming to have cystic fibrosis.10
Escalating demands. During the hospital stay, patients with factitious illness may make repeated requests for care, which may escalate into demands if their needs are not met.13 At this point, staff often start to experience negative countertransference reactions. As medical tests reveal little to no evidence of an organic basis for their symptoms and no cohesive psychiatric diagnosis is reached, patients may complain of misdiagnosis and mistreatment.13
Patients usually leave before psychiatric consultation can be obtained, and the underlying suffering that led to their factitious complaints remains unaddressed. Typically, patients are lost to follow-up until the next presentation at another hospital, where the process begins again.
What motivates patients?
The motivation behind factitious presentations can be bewildering. Asher’s paper on Munchausen syndrome described several possible reasons for patients’ behavior, such as desire to be the center of attention, holding a grudge against the medical profession, drug seeking, looking for shelter, and running from police.5 This list, however, includes correlates of secondary gain, which with today’s psychiatric nomenclature would lead to a diagnosis of malingering.
Psychological factors. Some clinicians have tried to address underlying psychiatric factors, but data on evaluation and management are limited because these patients usually eschew psychiatric examination. Although the patient is voluntarily producing the symptoms, unconscious psychological factors are at play and are an essential part of the picture.14
When assessed, patients appear to have lived rootless lives with few attachments, which may have been the result of sadistic and unsatisfying relationships with authority figures of their youth.15,16 Their grandiosity and distortion of the truth suggest a narcissistic need to overcome feelings of incompetence or impotence.17 Their ambivalent relationship to hospitals and physicians may reflect a need for caretaking, arising from early relationships and past caretakers.
Lastly, there is a component of masochism; this makes some individuals (erroneously) believe that if you don’t inflict pain you don’t care about them.13
Treatment challenges
Because patients with factitious disorder are not easily studied, no particular treatment is well-supported in the literature. Approaches that have been reported include preventing patients from being re-admitted to medical facilities, admitting patients for psychiatric treatment, and providing outpatient therapies such as individual psychodynamic psychotherapy, behavioral modification, and group psychotherapy.18
Other management strategies suggested in the literature include:
- reframing cognitive distortions
- drawing up a set of realistic hospitalization goals (with a written contract)
- maximizing the therapeutic alliance
- avoiding team splitting
- minimizing iatrogenic harm.19
Table 2
Recommended care for a patient with factitious illness
| Fully investigate all medical and psychiatric complaints, especially if physical safety is threatened |
| Maintain a healthy skepticism about unusual or illogical presentations while attempting to preserve an empathic connection with the patient |
| Be aware of countertransference reactions, as they may provide valuable insight about the underlying cause of the patient’s symptoms |
| Realize that psychiatric symptoms and medical presentations fall on a continuum from conscious to unconscious; at times there may be a mix of motivations |
| Report all findings nonjudgmentally, both to the patient and in medical documentation |
Nevertheless, prepare the physician for the patient to respond to confrontation with denial and resistance because he or she feels exposed and humiliated. If the physician makes it clear that ongoing medical care will still be available—even if the symptoms are fabricated—the patient may be more willing to accept psychiatric treatment.13
Related resources
- Barsky AJ, Stern TA, Greenberg DB, Cassem NH. Functional somatic symptoms and somatoform disorders. In: Stern TA, Fricchione GL, Cassem NH, et al, eds. The Massachusetts General Hospital handbook of general hospital psychiatry 5th ed. Philadelphia: Mosby/Elsevier; 2004:269-91.
- Elwyn TS, Ahmed I. Factitious disorder. EMedicine from WebMD. Last updated April 13, 2006. www.emedicine.com/med/topic3125.htm.
Ms. J, age 33, arrives at the emergency department (ED) complaining of chest pain and shortness of breath—symptoms she says are similar to those she had during episodes of pulmonary embolism. Routine laboratory workup, including chest CT and ultrasound of the lower extremities, indicate a very low likelihood of PE, but she insists that she be admitted.
Hear Dr. Stern's insights on recognizing and treating Munchausen's syndrome. Click here.
On the medical floor, nursing staff note that Ms. J appears short of breath only when directly observed. Medical records reveal multiple visits to other hospitals with repeated requests for admission. When gently confronted, she maintains she will die
if she is not treated.
Has your hospital’s medical staff ever been puzzled by a patient’s inconsistent presentation or unsettled by a concern that he or she was not being straightforward with them? Have they suspected that a patient such as Ms. J may be voluntarily producing his or her symptoms?
This article suggests a 3-step approach by which the consultation-liaison psychiatrist can help medical staff identify and manage patients with factitious illness.
Cardinal features
In factitious illness, the patient’s symptoms are:
- under voluntary control and consciously produced
- not a direct result of a medical or psychiatric condition
- produced to assume the sick role (not to accrue secondary gain—a core feature of malingering).
CASE: Self-inflicted injury
Ms. H, age 50, surprises even the most seasoned clinicians when she presents to the ED with brain parenchyma herniating from an open wound in her skull. She denies having picked at her scalp and does not endorse a history of obsessive-compulsive disorder or trichotillomania.
On the medical floor, however, she is seen picking at the wound, which leaves blood on her protective mittens. Surgical repair is repeatedly attempted, and her case is complicated by chronic infections and a nonhealing wound.
Clinical presentation
Factitious disorder presents 3 diagnostic and treatment challenges for a hospital’s medical staff:
- To recognize and treat (even self-inflicted) serious medical conditions that can be life-threatening.
- To orchestrate appropriate diagnostic evaluation. (Remember that factitious illness is a diagnosis of exclusion.)
- To handle countertransference reactions to patients that can be intense; physicians may experience anger, frustration, resignation, and hatred.
CASE: ‘Suicidal’ but not depressed
Mr. B, age 48, presents to the ED with thoughts of suicide and profoundly depressed mood. On examination, however, he does not appear depressed. He repeatedly requests food, cigarettes, and assistance in finding shelter, which lead to concern that his main goal is secondary gain. However, because Mr. B has a history of serious suicide attempts—including some while an inpatient—the ED physician is reluctant to dismiss his complaints and unsure about how to proceed.
3-step diagnostic approach
Treating factitious illness is predicated upon making the correct diagnosis, which requires the medical team to investigate and gather data from collateral sources, such as outside hospital medical records and other providers. The diagnostic process can be summarized in 3 steps:
Step 1. Determine whether the patient has an identifiable medical or psychiatric problem that could explain the symptoms.
Step 2. Determine whether the symptoms are consciously or unconsciously produced. Somatoform disorders—such as conversion disorder and somatization disorder, for example—are thought to result from processes outside the patient’s control.
Step 3. Distinguish if the motivation is to obtain the sick role (consider factitious illness) or if material benefits are the goal (consider malingering). Both motivations may be operative in a given patient.
Table 1
Medical clues to a patient with factitious illness
| Vague symptom history that frays upon examination |
| Irritability and evasiveness with continued questioning |
| Familiarity with hospital procedures and protocols (some patients have received medical training) |
| Multiple scars as evidence of past procedures and hospitalizations |
| Acceptance of painful medical procedures without complaint |
| Itinerant lives devoid of close personal relationships |
| Failure to accurately identify themselves |
| Lack of a verifiable history |
| Source: Reference 1 |
Psychiatric evaluation. Physicians should think of factitious psychiatric illness when:
- a patient’s behavior is notably different when he believes he is being directly observed and when he believes he is alone3
- psychiatric symptoms do not readily fit into diagnostic categories (such as a vague mix of memory loss, suicidal thoughts, and psychosis)
- the patient is suggestible or provides a diffusely positive review of systems (for example, he may report additional symptoms after having observed other patients).
The psychiatric presentations of Munchausen syndrome can be especially complicated, as they are usually associated with less objective evidence than are medical presentations (Box).4-10 Clarity of the history and diagnosis may be in the eye of the beholder.
Admission characteristics
Somatic complaints. Chaos often surrounds the hospitalized patient with factitious illness. The ED commonly is their gateway, and they tend to arrive in the evening or on weekends when less experienced staff are on call.11
Presentation severity ranges up to Munchausen syndrome
Munchausen syndrome—a particularly severe factitious illness—is characterized by peregrination, recurrent presentations, and pseudologia fantastica (stories that seem outrageously exaggerated).4 In 1951, Asher named this syndrome for Baron von Münchhausen, an 18th century Prussian officer who wandered from city to city creating tall tales about his life.5
Munchausen by proxy, in which a parent is responsible for producing illness in a child, may lead to extensive medical evaluations and treatment.
After more than 50 years, factitious illness continues to draw scientific and clinical attention. A search of PubMed over the last 10 years found nearly 500 citations. Presentations included:
- symptomatic bradycardia caused by beta-blocker ingestion6
- refractory hypoglycemia caused by surreptitious insulin injections7
- false reports of aortic dissection8
- recurrent episodes of self-harm including bilateral blindness from ocular trauma9
- fabricated sweat chloride test results in a patient claiming to have cystic fibrosis.10
Escalating demands. During the hospital stay, patients with factitious illness may make repeated requests for care, which may escalate into demands if their needs are not met.13 At this point, staff often start to experience negative countertransference reactions. As medical tests reveal little to no evidence of an organic basis for their symptoms and no cohesive psychiatric diagnosis is reached, patients may complain of misdiagnosis and mistreatment.13
Patients usually leave before psychiatric consultation can be obtained, and the underlying suffering that led to their factitious complaints remains unaddressed. Typically, patients are lost to follow-up until the next presentation at another hospital, where the process begins again.
What motivates patients?
The motivation behind factitious presentations can be bewildering. Asher’s paper on Munchausen syndrome described several possible reasons for patients’ behavior, such as desire to be the center of attention, holding a grudge against the medical profession, drug seeking, looking for shelter, and running from police.5 This list, however, includes correlates of secondary gain, which with today’s psychiatric nomenclature would lead to a diagnosis of malingering.
Psychological factors. Some clinicians have tried to address underlying psychiatric factors, but data on evaluation and management are limited because these patients usually eschew psychiatric examination. Although the patient is voluntarily producing the symptoms, unconscious psychological factors are at play and are an essential part of the picture.14
When assessed, patients appear to have lived rootless lives with few attachments, which may have been the result of sadistic and unsatisfying relationships with authority figures of their youth.15,16 Their grandiosity and distortion of the truth suggest a narcissistic need to overcome feelings of incompetence or impotence.17 Their ambivalent relationship to hospitals and physicians may reflect a need for caretaking, arising from early relationships and past caretakers.
Lastly, there is a component of masochism; this makes some individuals (erroneously) believe that if you don’t inflict pain you don’t care about them.13
Treatment challenges
Because patients with factitious disorder are not easily studied, no particular treatment is well-supported in the literature. Approaches that have been reported include preventing patients from being re-admitted to medical facilities, admitting patients for psychiatric treatment, and providing outpatient therapies such as individual psychodynamic psychotherapy, behavioral modification, and group psychotherapy.18
Other management strategies suggested in the literature include:
- reframing cognitive distortions
- drawing up a set of realistic hospitalization goals (with a written contract)
- maximizing the therapeutic alliance
- avoiding team splitting
- minimizing iatrogenic harm.19
Table 2
Recommended care for a patient with factitious illness
| Fully investigate all medical and psychiatric complaints, especially if physical safety is threatened |
| Maintain a healthy skepticism about unusual or illogical presentations while attempting to preserve an empathic connection with the patient |
| Be aware of countertransference reactions, as they may provide valuable insight about the underlying cause of the patient’s symptoms |
| Realize that psychiatric symptoms and medical presentations fall on a continuum from conscious to unconscious; at times there may be a mix of motivations |
| Report all findings nonjudgmentally, both to the patient and in medical documentation |
Nevertheless, prepare the physician for the patient to respond to confrontation with denial and resistance because he or she feels exposed and humiliated. If the physician makes it clear that ongoing medical care will still be available—even if the symptoms are fabricated—the patient may be more willing to accept psychiatric treatment.13
Related resources
- Barsky AJ, Stern TA, Greenberg DB, Cassem NH. Functional somatic symptoms and somatoform disorders. In: Stern TA, Fricchione GL, Cassem NH, et al, eds. The Massachusetts General Hospital handbook of general hospital psychiatry 5th ed. Philadelphia: Mosby/Elsevier; 2004:269-91.
- Elwyn TS, Ahmed I. Factitious disorder. EMedicine from WebMD. Last updated April 13, 2006. www.emedicine.com/med/topic3125.htm.
1. Stern T. Malingering, factitious illness, and somatization. In: Hyman S, ed. Manual of psychiatric emergencies. Boston: Little, Brown, and Co; 1988;23:217-25.-
2. Turner J, Reid S. Munchausen’s syndrome. Lancet. 2002;359:346-9.
3. Popli A, Prakash S, Dewan M. Factitious disorders with psychological symptoms. J Clin Psychiatry 1992;53:9.-
4. Huffman J, Stern T. The diagnosis and treatment of Munchausen’s syndrome. Gen Hosp Psychiatry 2003;25:358-63.
5. Asher R. Munchausen’s syndrome. Lancet 1951;1:339-41.
6. Steinwender C, Hofmann R, Kypta A, Leisch F. Recurrent symptomatic bradycardia due to secret ingestion of beta-blockers—a rare manifestation of cardiac Munchausen syndrome. Wien Klon Wochenschr 2005;117(18):647-50.
7. Bretz S, Richards J. Munchausen syndrome presenting acutely in the emergency department. J Emerg Med 2000;18(4):417-20.
8. Hopkins R, Harrington C, Poppas A. Munchausen syndrome simulating acute aortic dissection. Ann Thorac Surg 2006;81(4):1497-99.
9. Salvo M, Pinna A, Milia P, Carta F. Ocular Munchausen syndrome resulting in bilateral blindness. Eur J Ophthalmol 2006;16(4):654-56.
10. Highland K, Flume P. A “story” of a woman with cystic fibrosis. Chest 2002;121(5):1704-7.
11. Stretton J. Munchausen syndrome. Lancet 1951;1:474.-
12. Stern T. Munchausen’s syndrome revisited. Psychosomatics 1980;21(4):329-36.
13. Stern T. Factitious disorders. In: Hyman S, Jenike M, eds. Manual of clinical problems in psychiatry Boston: Little, Brown, and Co; 1990;21:190-4.
14. Greenacre P. The imposter. Psychoanal Q 1958;27:359-82.
15. Cramer B, Gershberg M, Stern M. Munchausen syndrome. Arch Gen Psychiatry 1971;24:573-8.
16. Ford C. The Munchausen syndrome: a report of four new cases and a review of psychodynamic considerations. Psychiatry Med 1973;4:31-45.
17. Bursten B. On Munchausen’s syndrome. Arch Gen Psychiatry 1965;13:261-8.
18. Yassa R. Munchausen’s syndrome: a successfully treated case. Psychosomatics 1978;19:242.-
19. Gregory RJ, Jindal S. Factitious disorder on an inpatient psychiatry ward. Am J Orthopsychiatry 2006;76(1):31-6.
1. Stern T. Malingering, factitious illness, and somatization. In: Hyman S, ed. Manual of psychiatric emergencies. Boston: Little, Brown, and Co; 1988;23:217-25.-
2. Turner J, Reid S. Munchausen’s syndrome. Lancet. 2002;359:346-9.
3. Popli A, Prakash S, Dewan M. Factitious disorders with psychological symptoms. J Clin Psychiatry 1992;53:9.-
4. Huffman J, Stern T. The diagnosis and treatment of Munchausen’s syndrome. Gen Hosp Psychiatry 2003;25:358-63.
5. Asher R. Munchausen’s syndrome. Lancet 1951;1:339-41.
6. Steinwender C, Hofmann R, Kypta A, Leisch F. Recurrent symptomatic bradycardia due to secret ingestion of beta-blockers—a rare manifestation of cardiac Munchausen syndrome. Wien Klon Wochenschr 2005;117(18):647-50.
7. Bretz S, Richards J. Munchausen syndrome presenting acutely in the emergency department. J Emerg Med 2000;18(4):417-20.
8. Hopkins R, Harrington C, Poppas A. Munchausen syndrome simulating acute aortic dissection. Ann Thorac Surg 2006;81(4):1497-99.
9. Salvo M, Pinna A, Milia P, Carta F. Ocular Munchausen syndrome resulting in bilateral blindness. Eur J Ophthalmol 2006;16(4):654-56.
10. Highland K, Flume P. A “story” of a woman with cystic fibrosis. Chest 2002;121(5):1704-7.
11. Stretton J. Munchausen syndrome. Lancet 1951;1:474.-
12. Stern T. Munchausen’s syndrome revisited. Psychosomatics 1980;21(4):329-36.
13. Stern T. Factitious disorders. In: Hyman S, Jenike M, eds. Manual of clinical problems in psychiatry Boston: Little, Brown, and Co; 1990;21:190-4.
14. Greenacre P. The imposter. Psychoanal Q 1958;27:359-82.
15. Cramer B, Gershberg M, Stern M. Munchausen syndrome. Arch Gen Psychiatry 1971;24:573-8.
16. Ford C. The Munchausen syndrome: a report of four new cases and a review of psychodynamic considerations. Psychiatry Med 1973;4:31-45.
17. Bursten B. On Munchausen’s syndrome. Arch Gen Psychiatry 1965;13:261-8.
18. Yassa R. Munchausen’s syndrome: a successfully treated case. Psychosomatics 1978;19:242.-
19. Gregory RJ, Jindal S. Factitious disorder on an inpatient psychiatry ward. Am J Orthopsychiatry 2006;76(1):31-6.
Hypnotics and driving: FDA action, clinical trials show need for precautions
“Sleep driving” blamed on the hypnotic zolpidem was used as a defense last year in Virginia in a criminal case involving impaired driving. The defendant’s attorney argued that the defendant should not be held criminally liable because he was “essentially unconscious” and the accident therefore was involuntary.
The “sleep driving” defense failed when testimony revealed the defendant had taken 5 times the recommended zolpidem dose before the accident. The judge found him guilty of a felony charge of driving under the influence of a sleep medication.1
Sedative-hypnotics are increasingly being used to treat insomnia2-4 and as a result some patients try to drive while under the drugs’ sedating effects. Also, new FDA-ordered labeling for all 13 available prescription sleep aids warns of potential risks of “complex sleep-related behaviors,” including driving, phoning, and eating while asleep (Box 1).
Hypnotics can improve quality of life and well-being by addressing insomnia’s complications—hypertension, diabetes, coronary artery disease, depression, and anxiety5-7—but they also have been associated with impaired motor coordination and somnambulism. To help you and your patients weigh sleep medications’ relative risks and benefits, we report on clinical studies and court cases in the literature. Most of the data focus on zolpidem, by far the most prescribed hypnotic (Box 2).8,9
Labeling of all sedative-hypnotic drugs now carries FDA-ordered precautions about “sleep-driving and other complex behaviors” that may occur without the patient being fully awake. FDA cited reports of patients preparing and eating food, making phone calls, and having sex after taking a sedative-hypnotic, usually without memory of the event. A warning also was added about rare, potentially fatal anaphylactic reactions in patients taking first or later doses of sleep medications.
Steven Galson, MD, MPH, director of FDA’s Center for Drug Evaluation and Research, said the labeling changes were needed to inform patients and prescribers about the risks of sleep aids that “are well-tolerated and effective for many people.”
Source: Walsh S, Rawlings K. FDA requests label change for all sleep disorder drug products. Available at www.fda.gov/bbs/topics/NEWS/2007/NEW01587.html.
Zolpidem incidents and cases
In 2005, Americans filled 43 million prescriptions for sedative-hypnotics—26.5 million for zolpidem alone—compared with 29 million prescriptions in 2001.4 In addition to the new the FDA-requested warnings about sleep-related behaviors, zolpidem’s labeling cautions patients about operating heavy machinery, driving, or engaging in hazardous occupations after taking the drug. The manufacturer tells patients:
- to ingest zolpidem only before going to bed
- that they may experience residual sedation the following day.
Impaired driving. Besides the “sleep driving” case in Virginia, a highly publicized zolpidem-related driving incident occurred May 4, 2006, when U.S. Representative Patrick Kennedy was involved in an accident after having taken zolpidem in combination with an antinausea medication. Another driving-related case has used zolpidem as a defense for impairment, but the court decided that the medication was not at fault because the defendant also had ingested alcohol.10
Other litigation. Although zolpidem-related impairment apparently has not been used successfully as a defense for a driving incident, class action suits alleging failure to disclose potentially harmful side effects have been filed against the manufacturer.
In Janet Makinen and others v. sanofi-aventis,11 at least 500 plaintiffs claim zolpidem is related to sleep-driving, sleep-eating, and other somnambulistic behaviors. Plaintiffs allege negligence, breach of implied warranties, fraud, unfair trade practices, express warranty violations, strict liability, and consumer fraud violations. Other suits claim dangerous sleep-related side effects with zolpidem use.12
What clinical evidence shows
Driving impairment. Clinical studies have shown conflicting results about driving impairment associated with zolpidem. The literature falls into 2 categories, based on treatment duration:
- Zolpidem affects performance and memory within the first 4 to 5 hours of administration (Table 1).
- Beyond 5 hours, no residual effects on performance have been identified (Table 2), and repeat nightly dosing has not caused impairment or tolerance.
- All sedative hypnotic benzodiazepines had statistically significant residual effects 10 to 11 hours after ingestion, with longer periods of impairment corresponding to medications with longer half-lives.
- Zopiclone was associated with significant residual impairment for up to 10 hours after ingestion.
- Zolpidem and zaleplon showed no significant impairment in driving 10 to 11 hours after ingestion. Impairment was found, however, when zolpidem was taken within 5 hours of driving.14-18
Table 1
Studies of zolpidem-associated driving skills impairment
(
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Wilkinson, 199514 Blinded; 29 subjects | Zolpidem, 10 mg, 15 mg, and placebo in combination with an alcoholic drink (to reach a BAC of 0.08%) or placebo drink; testing 45 min, 130 min, and 230 min after administration | Visual backward masking test (approximates driving performance) and attention tests | Zolpidem produced significant impairment in combination with alcohol and when administered alone during peak effect assessment; alcohol did not potentiate zolpidem’s effects; additive effects of alcohol seen with 10-mg dose but not 15-mg dose of zolpidem |
| Rush et al, 199815 Double-blind, crossover; 9 subjects | Zolpidem, 7.5, 15, and 22.5 mg; quazepam, 15, 30, and 45 mg; triazolam, 0.1875, 0.375, and 0.5625 mg; testing ½, 1, 1½, 2, 2½, 3, 4, 5, and 6 hours after administration | Subject- and observer-rated questionnaires; tests of recall and delayed recognition | Performance-impairing effects of zolpidem were virtually indistinguishable from those of classic benzodiazepines, such as triazolam |
| Mattila et al, 199816 Randomized, placebo-controlled, double-blind, crossover; 12 subjects | Zolpidem, 15 mg; diazepam, 15 mg; oxazepam, 30 mg; zopiclone, 7.5 mg; alcohol testing before and 1, 3½, and 5 hours after administration | Simulated driving and other measures | Zolpidem impaired coordination, reaction, and cognition at 1 and 3½ hours; tracking remained impaired at 5 hours; all agents (especially zolpidem) impaired learning and memory |
| Mintzer et al, 199917 Double-blind, placebo-controlled; 16 subjects | Zolpidem, 15 mg/70 kg (dosed by subject weight); testing ½, 1, 2, and 3 hours after administration | Memory tasks (recall, fragment completion, recognition) | Zolpidem interfered with explicit but not implicit memory after administration; zolpidem produced a specific deficit in acquisition of contextual information |
| Verster et al, 200418 2-step randomized, placebo-controlled, double-blind, crossover; 30 subjects | Zolpidem, 10 mg and 20 mg; zaleplon, 10 mg and 20 mg; middle-of-the-night dosing; testing 4 hours after dosing | On-the-road driving and other tests of attention, learning, and thinking | Zolpidem, 10 mg and 20 mg, significantly impaired driving function; zolpidem, 20 mg, produced significant impairment on all psychomotor and memory tests; zaleplon, 10 mg and 20 mg, did not differ significantly from placebo |
| BAC: Blood alcohol concentration | |||
Studies of zolpidem-associated driving skills impairment
(>5 hours after dosing)
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Fairweather et al, 199223 Randomized, placebo-controlled; 24 older volunteers taking no other medications | Zolpidem, 5 mg or 10 mg, or placebo taken before bedtime; testing 8.5 hours after administration | Numerous, including reactive time, memory, word recognition | Zolpidem consistently helped with sleep latency, with no residual performance deficits; no tolerance seen with repeated dosing |
| Bocca et al, 199924 Double-blind, crossover; 16 volunteers | Zolpidem, 10 mg; zopiclone, 7.5 mg; flunitrazepam,* 1 mg; and placebo given at 11 PM, with testing at 9 AM | Driving simulation and real time test drive; eye movements measured after driving tests | No residual effects with zolpidem; zopiclone impaired driving ability and increased saccadic latency; flunitrazepam impaired early morning driving and saccadic eye movements longer than zopiclone |
| Partinen et al, 200320 Randomized, placebo-controlled, double-blind, 3-period crossover; 18 women with insomnia | Zolpidem, 10 mg; temazepam, 20 mg; dosing at 2 AM, testing 5.5 hours after dosing | Driving simulation; delayed word recall and memory testing (FePsy test) | No statistically significant effects on driving ability with either drug; no significant differences in FePsy results compared with baseline or placebo |
| Staner et al, 200525 Randomized, placebo-controlled, double-blind, four-way crossover; 23 subjects with DSM-IV-TR diagnosis of insomnia | Zolpidem, 10 mg; zopiclone, 7.5 mg; lormetazepam,* 1 mg; 7 days of dosing; tests given 9 to 11 hours post-dosing | Driving simulation; EEG at rest and while driving | Zolpidem showed no impairment of driving ability and no EEG changes compared with placebo; driving impairment and EEG alterations were found with zopiclone and lormetazepam |
| * Hypnotics not approved in the United States but available elsewhere. | |||
Acute effects (
Combined with alcohol. Wilkinson14 conducted a randomized, 6-way crossover study in which subjects received 10- or 15-mg doses of zolpidem or placebo plus an alcoholic beverage (enough to obtain a blood alcohol concentration [BAC] of ~0.08%) or placebo beverage. Tests given shortly after patients took the study medications showed that zolpidem caused statistically significant impairment both in combination with alcohol and alone during peak drug effect—identified as 45 minutes after ingestion. Alcohol did not potentiate the impairment associated with zolpidem.
Using a similar design, Mattila et al16 compared acute performance impairment associated with zolpidem, diazepam, oxazepam, and zopiclone. In this randomized, double-blinded, crossover study, all comparison medications impaired antecedent learning and memory, but zolpidem given at 15 mg had the greatest effect. Zolpidem impaired coordination, reactive functioning, and cognitive skills at 1 and 3.5 hours after administration, and simulated driving test performance remained impaired at 5 hours (approximately two half-lives of the medication). Of note is that the 15-mg zolpidem dose used in this study was shown by Wilkinson et al14 to be more impairing than the recommended maximum 10-mg dose.
A study from the University of Toronto19 that did not include zolpidem examined potential psychomotor performance deficits and sleepiness in a comparison of time-released melatonin, 6 mg; zaleplon, 10 mg; zopiclone, 7.5 mg; temazepam, 15 mg, and placebo. Tests were given to 9 men and 14 women, ages 21 to 53, just before drug administration and 7 hours later.
Zaleplon had the greatest effect on psychomotor performance, followed by temazepam and zopiclone. Aside from prolonged perceived sleepiness, melatonin and placebo did not interfere with performance testing.
Zolpidem, a benzodiazepine receptor agonist, was the 7th most prescribed drug in the United States in 2005 (2006 data not available).8 It is FDA-approved for short-term treatment of insomnia, although “short-term” is not defined. Package labeling states:
This nonbenzodiazepine hypnotic has been shown to decrease sleep latency and increase sleep duration for up to 35 days in controlled clinical trials. Patients should be evaluated for a primary psychiatric or medical illness if insomnia does not remit after 7 to 10 days of treatment.
An imidazopyridine that acts as an agonist of GABA A1, zolpidem produces sedation while avoiding anticonvulsant, anxiolytic, and muscle relaxation effects. Available in 5- and 10-mg tablets, the drug is rapidly absorbed in the GI tract and excreted primarily through the kidneys. Its half-life is approximately 2.5 hours (approximately 3 hours in elderly patients). The most common side effects are daytime drowsiness, dizziness, and diarrhea; others include asthenia, hiccup, and diplopia.9
- driving ability 4 hours after administration
- memory and psychomotor performance 6 hours after administration.
Partinen et al20 used the recommended zolpidem dose in a similar study of after-midnight use by women with insomnia. The double-blind, randomized, controlled trial evaluated performance with a driving simulator and neuropsychological testing 5.5 hours after medication dosing. Patients taking zolpidem, 10 mg, showed no significant impairment when compared with those taking placebo. Some patients scored poorly on the driving tests alone, and the authors concluded that this group was more susceptible to zolpidem’s effect.
Memory. In a double-blind, placebo-controlled trial by Mintzner et al,17 zolpidem dosed by patient weight at 15 mg/70 kg:
- significantly impaired explicit memory (requires conscious recollection for recall)
- did not affect implicit memory (lack of conscious awareness in the act of recollection).
These findings support complaints of zolpidem-related anterograde amnestic episodes, which also occur with some benzodiazepines (such as midazolam).
Similar to benzodiazepines? Rush et al’s results21 support Mintzer’s assertion17 that zolpidem shares many side effects with benzodiazepines. Performance impairment associated with zolpidem—as rated by subjects and observers—is virtually indistinguishable from a benzodiazepine effect, except that the duration is shorter with zolpidem (5 hours), compared with up to 10 hours for benzodiazepines.
Logan and Couper22 reviewed police reports and toxicology profiles of individuals suspected of driving while impaired. Zolpidem was found in 29 subjects, 5 of whom showed no other substances. In those 5, zolpidem blood levels ranged from 0.08 to 1.40 mg/L and did not appear to correlate with the degree of impairment.
Residual effects (>5 hours)
Older patients. In a randomized, placebo-controlled trial by Fairweather et al,23 zolpidem improved sleep latency in 24 subjects ages 63 to 80. No evidence of impairment in reactive time, memory, or word recognition was found 8.5 hours after nighttime dosing, and tolerance was not seen after 1 week of repeated dosing.
Driving impairment. Bocca et al24 compared degree of driving impairment by zolpidem, zopiclone, flunitrazepam (not approved in the United States), and placebo. The 16 subjects received each medication at 11 pm, with a 2-week washout between medications. One group of 8 was tested at 9 am and the other 8 subjects at 11 am. Those taking zolpidem showed no residual performance impairment, as measured by simulated driving, a test drive, and saccadic eye movements.
Staner et al25 reported similar results when comparing zolpidem, zopiclone, lormetazepam (not approved in the United States), and placebo. Using a driving simulator and electroencephalography (EEG), they evaluated 23 subjects diagnosed with insomnia at 9 and 11 hours post-dose. Zolpidem did not significantly impair driving ability and did not differ from placebo on EEG analysis (resting or driving). The study showed driving impairment with zopiclone and lormetazepam, along with characteristic benzodiazepine EEG changes. This study further supports evidence of limited impairment on driving after appropriate use of zolpidem.
Informed consent
In the informed consent process, failing to warn a patient about medication side effects can lead to legal claims against both manufacturers and prescribers. With any medication, patients have the right to know about a drug’s risks, benefits, and alternate therapies—including no therapy.
Two standards are associated with informed consent and negligence:
- The “reasonable practitioner” standard outlined in Natanson v. Kline (1960)26 mandates that the prescribing physician has revealed all that an “average, reasonable practitioner” would disclose in similar circumstances.
- The “reasonable patient” standard set in Canterbury v. Spence (1972)27 mandates that the prescribing physician has informed the patient about the proposed treatment, its side effects, and alternatives to the proposed treatment that a reasonable patient would consider material to the decision of whether or not to undergo treatment.
The vaccine was licensed as a prescription drug but administered through county health departments. In 1970, a nurse in a Texas Department of Health clinic administered the vaccine to 8-month-old Anita Reyes without telling the girl’s parents of warnings in the package circular. Holding Wyeth Laboratories to a reasonableness standard, the court found that the company knew or should have known how the vaccine would be distributed.
The package insert was not shown to have given inadequate warning, and the vaccine was not shown to be defective (it was a trivalent live-virus Sabin oral polio vaccine, as intended).
Vioxx cases. Similarly, some plaintiffs have been awarded millions of dollars (as in Ernst v. Merck & Co., Inc.29) in rulings that Merck & Co. failed to disclose the risk of cardiotoxicity with the arthritis drug rofecoxib (Vioxx) and thus failed to provide physicians with information needed when prescribing the drug. In Humeston v. Merck & Co.,30 a Texas court in 2005 held that Vioxx’s warning labels were adequate. In a retrial, however, the New Jersey Superior Court awarded the plaintiff $47.5 million.31
As with the polio vaccine and Vioxx litigations, courts are being asked to decide if patients were adequately informed about sleep-driving and other risks associated with the use of sedative-hypnotics.
Clinical recommendations
Zolpidem—like many other medications—carries a substantial risk of side effects, even when used appropriately. However, given the medical and mental health risks of untreated insomnia, the benefits of a medication such as zolpidem will likely outweigh its risks.
Numerous studies have shown that zolpidem is effective for improving sleep latency and that there are mild, if any, residual side effects beyond what would normally be a restful night’s sleep. Impairments are evident, however, during the hours following the drug’s administration, with some effects lasting >5 hours depending on the dose.
Risk management. When prescribing nonbenzodiazepine hypnotics such as zolpidem, you may want to adopt a risk management approach as you would with other medications that can have serious side effects. An approach to benzodiazepine prescribing proposed by Bursztajn et al31 advocates:
- using the informed-consent process to build an alliance with patients
- not prescribing the medication in isolation of other beneficial therapies
- being aware of and always documenting your decision-making process.
When you make patients aware of all risks, benefits, alternate therapies, and possible outcomes with no treatment, you have informed them effectively. Patients are then left to decide whether or not to agree to the treatment. You also are responsible for monitoring the patient, addressing the patient’s questions, and relaying important safety information.
When prescribing zolpidem, discuss safety information with the patient, such as:
- Do not drive or operate heavy equipment for at least 5 to 6 hours after administration.
- Have a safety plan in place for transportation during those hours.
- Do not use this medication with alcohol or other sedative/hypnotics.
- Contact the prescriber about any suspected adverse effects.
- MedlinePlus information on sleep disorders. National Institutes of Health and National Library of Medicine. www.nlm.nih.gov/medlineplus/sleepdisorders.html.
- Zolpidem (systemic). Mayoclinic.com: Tools for healthier lives. www.mayoclinic.com/health/drug-information/DR202707.
- Diazepam • Valium
- Eszopiclone • Lunesta
- Midazolam • Versed
- Oxazepam • Serax
- Quazepam • Doral
- Rofecoxib • Vioxx
- Temazepam • Restoril
- Triazolam • Halcion
- Zolpidem • Ambien, Ambien CR
- Zaleplon • Sonata
- Zopiclone • Imovane (in Europe)
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors acknowledge the assistance and guidance of Linda T. Moore, JD, in preparing this manuscript.
1. Markon J. Sleeping Va. driver convicted in crash; man had taken too much Ambien. The Washington Post, August 2, 2006. Accessed August 26, 2006 from LexisNexis Academic Database.
2. Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: an unmet public health problem. Available at: http://www.iom.edu/CMS/3740/23160/33668.aspx. Accessed February 21, 2007.
3. Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment; prevalence and correlates. Arch Gen Psychiatry 1985;42:225-32.
4. Barclay L. Driving, other erratic behaviors reported after taking zolpidem. Available at http://www.medscape.com/viewarticle/528415. Accessed February 21, 2007.
5. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep 2006;29(8):1009-14.
6. Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005;165(8):863-7.
7. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med 2006;4(2):104-13.
8. Verispan VONA. Top 200 brand name drugs by units in 2005. Drug Topics 2006. Available at: http://www.drugtopics.com/drugtopics/data/articlestandard/drugtopics/102006/311294/article.pdf. Accessed February 22, 2007.
9. sanofi-aventis Ambien prescribing information. Available at: http://products.sanofi-aventis.us/ambien/ambien.html. Accessed February 21, 2007.
10. Pear R. Patrick Kennedy crashes car into a Capitol Hill barrier. The New York Times, May 5, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
11. Janet Makinen and others v. Sanofi-Synthelabo & Sanofi-Synthelabo, Inc. Class action suit filed March 6, 2006 in U.S. District Court for the Southern District of New York, NY.
12. Tooher NL. Ambien users are filing lawsuits. Kansas City Daily Record, April 12, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
13. Verster JC, Veldhuijzen DS, Volkerts ER. Residual effects of sleep medication on driving ability. Sleep Med Rev 2004;8(4):309-25.
14. Wilkinson CJ. The acute effects of zolpidem, administered alone and with alcohol, on cognitive and psychomotor function. J Clin Psychiatry 1995;56(7):309-18.
15. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
16. Mattila MJ, Vanakoski J, Kalska H, Seppala T. Effects of alcohol, zolpidem, and some other sedatives and hypnotics on human performance and memory. Pharmacol Biochem Behav 1998;59(4):917-23.
17. Mintzer MZ, Griffiths RR. Selective effects of zolpidem on human memory functions. J Psychopharmacol 1999;13(1):18-31.
18. Verster JC, Volkerts ER, Schreuder AH, et al. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. J Clin Psychopharmacol 2002;22(6):576-83.
19. Paul MA, Gray G, Kenny G, Pigeau RA. Impact of melatonin, zaleplon, zopiclone, and temazepam on psychomotor performance. Aviat Space Environ Med 2003;74(12):1263-70.
20. Partinen M, Hirvonen K, Hublin C, et al. Effects of after-midnight intake of zolpidem and temazepam on driving ability in women with non-organic insomnia. Sleep Med 2003;4(6):553-61.
21. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
22. Logan BK, Couper FJ. Zolpidem and driving impairment. J Forensic Sci 2001;46(1):105-10.
23. Fairweather DB, Kerr JS, Hindmarch I. The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. Eur J Clin Pharmacol 1992;43(6):597-601.
24. Bocca ML, Le Doze F, Etard O, et al. Residual effect of zolpidem 10 mg and zopiclone 7.5 mg versus flunitrazepam 1 mg and placebo on driving performance and ocular saccades. Psychopharmacology (Berl) 1999;143(4):373-9.
25. Staner L, Ertle S, Boeijinga P, et al. Next-day residual effects of hypnotics in DSM-IV primary insomnia: a driving simulator study with simultaneous electroencephalogram monitoring. Psychopharmacology (Berl) 2005;181(4):790-8.
26. Natanson v. Kline 300 P.2d 1093 (1960).
27. Canterbury v. Spence 464 F.2d 772 (1972).
28. Reyes v. Wyeth Laboratories. 498 F.2d 1264 (1974).
29. Ernst v. Merck & Co. 24 PLLR 149 (2005).
30. Humeston v. Merck & Co., No. ATL-L-2272-03-MT, Super. Ct., Atlantic County, NJ, November 3, 2005.
31. Johnson LA. Jury blames Vioxx for man’s heart attack, awards $47.5 million. Available at http://news.findlaw.com/ap/o/51/03-12-2007/85f9000f67cd576a.html. Accessed March 16, 2007.
32. Bursztajn HJ, Brodsky A. Ethical and legal dimensions of benzodiazepine prescription: a commentary. Psychiatr Ann 1998;28(3):121-7.
“Sleep driving” blamed on the hypnotic zolpidem was used as a defense last year in Virginia in a criminal case involving impaired driving. The defendant’s attorney argued that the defendant should not be held criminally liable because he was “essentially unconscious” and the accident therefore was involuntary.
The “sleep driving” defense failed when testimony revealed the defendant had taken 5 times the recommended zolpidem dose before the accident. The judge found him guilty of a felony charge of driving under the influence of a sleep medication.1
Sedative-hypnotics are increasingly being used to treat insomnia2-4 and as a result some patients try to drive while under the drugs’ sedating effects. Also, new FDA-ordered labeling for all 13 available prescription sleep aids warns of potential risks of “complex sleep-related behaviors,” including driving, phoning, and eating while asleep (Box 1).
Hypnotics can improve quality of life and well-being by addressing insomnia’s complications—hypertension, diabetes, coronary artery disease, depression, and anxiety5-7—but they also have been associated with impaired motor coordination and somnambulism. To help you and your patients weigh sleep medications’ relative risks and benefits, we report on clinical studies and court cases in the literature. Most of the data focus on zolpidem, by far the most prescribed hypnotic (Box 2).8,9
Labeling of all sedative-hypnotic drugs now carries FDA-ordered precautions about “sleep-driving and other complex behaviors” that may occur without the patient being fully awake. FDA cited reports of patients preparing and eating food, making phone calls, and having sex after taking a sedative-hypnotic, usually without memory of the event. A warning also was added about rare, potentially fatal anaphylactic reactions in patients taking first or later doses of sleep medications.
Steven Galson, MD, MPH, director of FDA’s Center for Drug Evaluation and Research, said the labeling changes were needed to inform patients and prescribers about the risks of sleep aids that “are well-tolerated and effective for many people.”
Source: Walsh S, Rawlings K. FDA requests label change for all sleep disorder drug products. Available at www.fda.gov/bbs/topics/NEWS/2007/NEW01587.html.
Zolpidem incidents and cases
In 2005, Americans filled 43 million prescriptions for sedative-hypnotics—26.5 million for zolpidem alone—compared with 29 million prescriptions in 2001.4 In addition to the new the FDA-requested warnings about sleep-related behaviors, zolpidem’s labeling cautions patients about operating heavy machinery, driving, or engaging in hazardous occupations after taking the drug. The manufacturer tells patients:
- to ingest zolpidem only before going to bed
- that they may experience residual sedation the following day.
Impaired driving. Besides the “sleep driving” case in Virginia, a highly publicized zolpidem-related driving incident occurred May 4, 2006, when U.S. Representative Patrick Kennedy was involved in an accident after having taken zolpidem in combination with an antinausea medication. Another driving-related case has used zolpidem as a defense for impairment, but the court decided that the medication was not at fault because the defendant also had ingested alcohol.10
Other litigation. Although zolpidem-related impairment apparently has not been used successfully as a defense for a driving incident, class action suits alleging failure to disclose potentially harmful side effects have been filed against the manufacturer.
In Janet Makinen and others v. sanofi-aventis,11 at least 500 plaintiffs claim zolpidem is related to sleep-driving, sleep-eating, and other somnambulistic behaviors. Plaintiffs allege negligence, breach of implied warranties, fraud, unfair trade practices, express warranty violations, strict liability, and consumer fraud violations. Other suits claim dangerous sleep-related side effects with zolpidem use.12
What clinical evidence shows
Driving impairment. Clinical studies have shown conflicting results about driving impairment associated with zolpidem. The literature falls into 2 categories, based on treatment duration:
- Zolpidem affects performance and memory within the first 4 to 5 hours of administration (Table 1).
- Beyond 5 hours, no residual effects on performance have been identified (Table 2), and repeat nightly dosing has not caused impairment or tolerance.
- All sedative hypnotic benzodiazepines had statistically significant residual effects 10 to 11 hours after ingestion, with longer periods of impairment corresponding to medications with longer half-lives.
- Zopiclone was associated with significant residual impairment for up to 10 hours after ingestion.
- Zolpidem and zaleplon showed no significant impairment in driving 10 to 11 hours after ingestion. Impairment was found, however, when zolpidem was taken within 5 hours of driving.14-18
Table 1
Studies of zolpidem-associated driving skills impairment
(
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Wilkinson, 199514 Blinded; 29 subjects | Zolpidem, 10 mg, 15 mg, and placebo in combination with an alcoholic drink (to reach a BAC of 0.08%) or placebo drink; testing 45 min, 130 min, and 230 min after administration | Visual backward masking test (approximates driving performance) and attention tests | Zolpidem produced significant impairment in combination with alcohol and when administered alone during peak effect assessment; alcohol did not potentiate zolpidem’s effects; additive effects of alcohol seen with 10-mg dose but not 15-mg dose of zolpidem |
| Rush et al, 199815 Double-blind, crossover; 9 subjects | Zolpidem, 7.5, 15, and 22.5 mg; quazepam, 15, 30, and 45 mg; triazolam, 0.1875, 0.375, and 0.5625 mg; testing ½, 1, 1½, 2, 2½, 3, 4, 5, and 6 hours after administration | Subject- and observer-rated questionnaires; tests of recall and delayed recognition | Performance-impairing effects of zolpidem were virtually indistinguishable from those of classic benzodiazepines, such as triazolam |
| Mattila et al, 199816 Randomized, placebo-controlled, double-blind, crossover; 12 subjects | Zolpidem, 15 mg; diazepam, 15 mg; oxazepam, 30 mg; zopiclone, 7.5 mg; alcohol testing before and 1, 3½, and 5 hours after administration | Simulated driving and other measures | Zolpidem impaired coordination, reaction, and cognition at 1 and 3½ hours; tracking remained impaired at 5 hours; all agents (especially zolpidem) impaired learning and memory |
| Mintzer et al, 199917 Double-blind, placebo-controlled; 16 subjects | Zolpidem, 15 mg/70 kg (dosed by subject weight); testing ½, 1, 2, and 3 hours after administration | Memory tasks (recall, fragment completion, recognition) | Zolpidem interfered with explicit but not implicit memory after administration; zolpidem produced a specific deficit in acquisition of contextual information |
| Verster et al, 200418 2-step randomized, placebo-controlled, double-blind, crossover; 30 subjects | Zolpidem, 10 mg and 20 mg; zaleplon, 10 mg and 20 mg; middle-of-the-night dosing; testing 4 hours after dosing | On-the-road driving and other tests of attention, learning, and thinking | Zolpidem, 10 mg and 20 mg, significantly impaired driving function; zolpidem, 20 mg, produced significant impairment on all psychomotor and memory tests; zaleplon, 10 mg and 20 mg, did not differ significantly from placebo |
| BAC: Blood alcohol concentration | |||
Studies of zolpidem-associated driving skills impairment
(>5 hours after dosing)
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Fairweather et al, 199223 Randomized, placebo-controlled; 24 older volunteers taking no other medications | Zolpidem, 5 mg or 10 mg, or placebo taken before bedtime; testing 8.5 hours after administration | Numerous, including reactive time, memory, word recognition | Zolpidem consistently helped with sleep latency, with no residual performance deficits; no tolerance seen with repeated dosing |
| Bocca et al, 199924 Double-blind, crossover; 16 volunteers | Zolpidem, 10 mg; zopiclone, 7.5 mg; flunitrazepam,* 1 mg; and placebo given at 11 PM, with testing at 9 AM | Driving simulation and real time test drive; eye movements measured after driving tests | No residual effects with zolpidem; zopiclone impaired driving ability and increased saccadic latency; flunitrazepam impaired early morning driving and saccadic eye movements longer than zopiclone |
| Partinen et al, 200320 Randomized, placebo-controlled, double-blind, 3-period crossover; 18 women with insomnia | Zolpidem, 10 mg; temazepam, 20 mg; dosing at 2 AM, testing 5.5 hours after dosing | Driving simulation; delayed word recall and memory testing (FePsy test) | No statistically significant effects on driving ability with either drug; no significant differences in FePsy results compared with baseline or placebo |
| Staner et al, 200525 Randomized, placebo-controlled, double-blind, four-way crossover; 23 subjects with DSM-IV-TR diagnosis of insomnia | Zolpidem, 10 mg; zopiclone, 7.5 mg; lormetazepam,* 1 mg; 7 days of dosing; tests given 9 to 11 hours post-dosing | Driving simulation; EEG at rest and while driving | Zolpidem showed no impairment of driving ability and no EEG changes compared with placebo; driving impairment and EEG alterations were found with zopiclone and lormetazepam |
| * Hypnotics not approved in the United States but available elsewhere. | |||
Acute effects (
Combined with alcohol. Wilkinson14 conducted a randomized, 6-way crossover study in which subjects received 10- or 15-mg doses of zolpidem or placebo plus an alcoholic beverage (enough to obtain a blood alcohol concentration [BAC] of ~0.08%) or placebo beverage. Tests given shortly after patients took the study medications showed that zolpidem caused statistically significant impairment both in combination with alcohol and alone during peak drug effect—identified as 45 minutes after ingestion. Alcohol did not potentiate the impairment associated with zolpidem.
Using a similar design, Mattila et al16 compared acute performance impairment associated with zolpidem, diazepam, oxazepam, and zopiclone. In this randomized, double-blinded, crossover study, all comparison medications impaired antecedent learning and memory, but zolpidem given at 15 mg had the greatest effect. Zolpidem impaired coordination, reactive functioning, and cognitive skills at 1 and 3.5 hours after administration, and simulated driving test performance remained impaired at 5 hours (approximately two half-lives of the medication). Of note is that the 15-mg zolpidem dose used in this study was shown by Wilkinson et al14 to be more impairing than the recommended maximum 10-mg dose.
A study from the University of Toronto19 that did not include zolpidem examined potential psychomotor performance deficits and sleepiness in a comparison of time-released melatonin, 6 mg; zaleplon, 10 mg; zopiclone, 7.5 mg; temazepam, 15 mg, and placebo. Tests were given to 9 men and 14 women, ages 21 to 53, just before drug administration and 7 hours later.
Zaleplon had the greatest effect on psychomotor performance, followed by temazepam and zopiclone. Aside from prolonged perceived sleepiness, melatonin and placebo did not interfere with performance testing.
Zolpidem, a benzodiazepine receptor agonist, was the 7th most prescribed drug in the United States in 2005 (2006 data not available).8 It is FDA-approved for short-term treatment of insomnia, although “short-term” is not defined. Package labeling states:
This nonbenzodiazepine hypnotic has been shown to decrease sleep latency and increase sleep duration for up to 35 days in controlled clinical trials. Patients should be evaluated for a primary psychiatric or medical illness if insomnia does not remit after 7 to 10 days of treatment.
An imidazopyridine that acts as an agonist of GABA A1, zolpidem produces sedation while avoiding anticonvulsant, anxiolytic, and muscle relaxation effects. Available in 5- and 10-mg tablets, the drug is rapidly absorbed in the GI tract and excreted primarily through the kidneys. Its half-life is approximately 2.5 hours (approximately 3 hours in elderly patients). The most common side effects are daytime drowsiness, dizziness, and diarrhea; others include asthenia, hiccup, and diplopia.9
- driving ability 4 hours after administration
- memory and psychomotor performance 6 hours after administration.
Partinen et al20 used the recommended zolpidem dose in a similar study of after-midnight use by women with insomnia. The double-blind, randomized, controlled trial evaluated performance with a driving simulator and neuropsychological testing 5.5 hours after medication dosing. Patients taking zolpidem, 10 mg, showed no significant impairment when compared with those taking placebo. Some patients scored poorly on the driving tests alone, and the authors concluded that this group was more susceptible to zolpidem’s effect.
Memory. In a double-blind, placebo-controlled trial by Mintzner et al,17 zolpidem dosed by patient weight at 15 mg/70 kg:
- significantly impaired explicit memory (requires conscious recollection for recall)
- did not affect implicit memory (lack of conscious awareness in the act of recollection).
These findings support complaints of zolpidem-related anterograde amnestic episodes, which also occur with some benzodiazepines (such as midazolam).
Similar to benzodiazepines? Rush et al’s results21 support Mintzer’s assertion17 that zolpidem shares many side effects with benzodiazepines. Performance impairment associated with zolpidem—as rated by subjects and observers—is virtually indistinguishable from a benzodiazepine effect, except that the duration is shorter with zolpidem (5 hours), compared with up to 10 hours for benzodiazepines.
Logan and Couper22 reviewed police reports and toxicology profiles of individuals suspected of driving while impaired. Zolpidem was found in 29 subjects, 5 of whom showed no other substances. In those 5, zolpidem blood levels ranged from 0.08 to 1.40 mg/L and did not appear to correlate with the degree of impairment.
Residual effects (>5 hours)
Older patients. In a randomized, placebo-controlled trial by Fairweather et al,23 zolpidem improved sleep latency in 24 subjects ages 63 to 80. No evidence of impairment in reactive time, memory, or word recognition was found 8.5 hours after nighttime dosing, and tolerance was not seen after 1 week of repeated dosing.
Driving impairment. Bocca et al24 compared degree of driving impairment by zolpidem, zopiclone, flunitrazepam (not approved in the United States), and placebo. The 16 subjects received each medication at 11 pm, with a 2-week washout between medications. One group of 8 was tested at 9 am and the other 8 subjects at 11 am. Those taking zolpidem showed no residual performance impairment, as measured by simulated driving, a test drive, and saccadic eye movements.
Staner et al25 reported similar results when comparing zolpidem, zopiclone, lormetazepam (not approved in the United States), and placebo. Using a driving simulator and electroencephalography (EEG), they evaluated 23 subjects diagnosed with insomnia at 9 and 11 hours post-dose. Zolpidem did not significantly impair driving ability and did not differ from placebo on EEG analysis (resting or driving). The study showed driving impairment with zopiclone and lormetazepam, along with characteristic benzodiazepine EEG changes. This study further supports evidence of limited impairment on driving after appropriate use of zolpidem.
Informed consent
In the informed consent process, failing to warn a patient about medication side effects can lead to legal claims against both manufacturers and prescribers. With any medication, patients have the right to know about a drug’s risks, benefits, and alternate therapies—including no therapy.
Two standards are associated with informed consent and negligence:
- The “reasonable practitioner” standard outlined in Natanson v. Kline (1960)26 mandates that the prescribing physician has revealed all that an “average, reasonable practitioner” would disclose in similar circumstances.
- The “reasonable patient” standard set in Canterbury v. Spence (1972)27 mandates that the prescribing physician has informed the patient about the proposed treatment, its side effects, and alternatives to the proposed treatment that a reasonable patient would consider material to the decision of whether or not to undergo treatment.
The vaccine was licensed as a prescription drug but administered through county health departments. In 1970, a nurse in a Texas Department of Health clinic administered the vaccine to 8-month-old Anita Reyes without telling the girl’s parents of warnings in the package circular. Holding Wyeth Laboratories to a reasonableness standard, the court found that the company knew or should have known how the vaccine would be distributed.
The package insert was not shown to have given inadequate warning, and the vaccine was not shown to be defective (it was a trivalent live-virus Sabin oral polio vaccine, as intended).
Vioxx cases. Similarly, some plaintiffs have been awarded millions of dollars (as in Ernst v. Merck & Co., Inc.29) in rulings that Merck & Co. failed to disclose the risk of cardiotoxicity with the arthritis drug rofecoxib (Vioxx) and thus failed to provide physicians with information needed when prescribing the drug. In Humeston v. Merck & Co.,30 a Texas court in 2005 held that Vioxx’s warning labels were adequate. In a retrial, however, the New Jersey Superior Court awarded the plaintiff $47.5 million.31
As with the polio vaccine and Vioxx litigations, courts are being asked to decide if patients were adequately informed about sleep-driving and other risks associated with the use of sedative-hypnotics.
Clinical recommendations
Zolpidem—like many other medications—carries a substantial risk of side effects, even when used appropriately. However, given the medical and mental health risks of untreated insomnia, the benefits of a medication such as zolpidem will likely outweigh its risks.
Numerous studies have shown that zolpidem is effective for improving sleep latency and that there are mild, if any, residual side effects beyond what would normally be a restful night’s sleep. Impairments are evident, however, during the hours following the drug’s administration, with some effects lasting >5 hours depending on the dose.
Risk management. When prescribing nonbenzodiazepine hypnotics such as zolpidem, you may want to adopt a risk management approach as you would with other medications that can have serious side effects. An approach to benzodiazepine prescribing proposed by Bursztajn et al31 advocates:
- using the informed-consent process to build an alliance with patients
- not prescribing the medication in isolation of other beneficial therapies
- being aware of and always documenting your decision-making process.
When you make patients aware of all risks, benefits, alternate therapies, and possible outcomes with no treatment, you have informed them effectively. Patients are then left to decide whether or not to agree to the treatment. You also are responsible for monitoring the patient, addressing the patient’s questions, and relaying important safety information.
When prescribing zolpidem, discuss safety information with the patient, such as:
- Do not drive or operate heavy equipment for at least 5 to 6 hours after administration.
- Have a safety plan in place for transportation during those hours.
- Do not use this medication with alcohol or other sedative/hypnotics.
- Contact the prescriber about any suspected adverse effects.
- MedlinePlus information on sleep disorders. National Institutes of Health and National Library of Medicine. www.nlm.nih.gov/medlineplus/sleepdisorders.html.
- Zolpidem (systemic). Mayoclinic.com: Tools for healthier lives. www.mayoclinic.com/health/drug-information/DR202707.
- Diazepam • Valium
- Eszopiclone • Lunesta
- Midazolam • Versed
- Oxazepam • Serax
- Quazepam • Doral
- Rofecoxib • Vioxx
- Temazepam • Restoril
- Triazolam • Halcion
- Zolpidem • Ambien, Ambien CR
- Zaleplon • Sonata
- Zopiclone • Imovane (in Europe)
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors acknowledge the assistance and guidance of Linda T. Moore, JD, in preparing this manuscript.
“Sleep driving” blamed on the hypnotic zolpidem was used as a defense last year in Virginia in a criminal case involving impaired driving. The defendant’s attorney argued that the defendant should not be held criminally liable because he was “essentially unconscious” and the accident therefore was involuntary.
The “sleep driving” defense failed when testimony revealed the defendant had taken 5 times the recommended zolpidem dose before the accident. The judge found him guilty of a felony charge of driving under the influence of a sleep medication.1
Sedative-hypnotics are increasingly being used to treat insomnia2-4 and as a result some patients try to drive while under the drugs’ sedating effects. Also, new FDA-ordered labeling for all 13 available prescription sleep aids warns of potential risks of “complex sleep-related behaviors,” including driving, phoning, and eating while asleep (Box 1).
Hypnotics can improve quality of life and well-being by addressing insomnia’s complications—hypertension, diabetes, coronary artery disease, depression, and anxiety5-7—but they also have been associated with impaired motor coordination and somnambulism. To help you and your patients weigh sleep medications’ relative risks and benefits, we report on clinical studies and court cases in the literature. Most of the data focus on zolpidem, by far the most prescribed hypnotic (Box 2).8,9
Labeling of all sedative-hypnotic drugs now carries FDA-ordered precautions about “sleep-driving and other complex behaviors” that may occur without the patient being fully awake. FDA cited reports of patients preparing and eating food, making phone calls, and having sex after taking a sedative-hypnotic, usually without memory of the event. A warning also was added about rare, potentially fatal anaphylactic reactions in patients taking first or later doses of sleep medications.
Steven Galson, MD, MPH, director of FDA’s Center for Drug Evaluation and Research, said the labeling changes were needed to inform patients and prescribers about the risks of sleep aids that “are well-tolerated and effective for many people.”
Source: Walsh S, Rawlings K. FDA requests label change for all sleep disorder drug products. Available at www.fda.gov/bbs/topics/NEWS/2007/NEW01587.html.
Zolpidem incidents and cases
In 2005, Americans filled 43 million prescriptions for sedative-hypnotics—26.5 million for zolpidem alone—compared with 29 million prescriptions in 2001.4 In addition to the new the FDA-requested warnings about sleep-related behaviors, zolpidem’s labeling cautions patients about operating heavy machinery, driving, or engaging in hazardous occupations after taking the drug. The manufacturer tells patients:
- to ingest zolpidem only before going to bed
- that they may experience residual sedation the following day.
Impaired driving. Besides the “sleep driving” case in Virginia, a highly publicized zolpidem-related driving incident occurred May 4, 2006, when U.S. Representative Patrick Kennedy was involved in an accident after having taken zolpidem in combination with an antinausea medication. Another driving-related case has used zolpidem as a defense for impairment, but the court decided that the medication was not at fault because the defendant also had ingested alcohol.10
Other litigation. Although zolpidem-related impairment apparently has not been used successfully as a defense for a driving incident, class action suits alleging failure to disclose potentially harmful side effects have been filed against the manufacturer.
In Janet Makinen and others v. sanofi-aventis,11 at least 500 plaintiffs claim zolpidem is related to sleep-driving, sleep-eating, and other somnambulistic behaviors. Plaintiffs allege negligence, breach of implied warranties, fraud, unfair trade practices, express warranty violations, strict liability, and consumer fraud violations. Other suits claim dangerous sleep-related side effects with zolpidem use.12
What clinical evidence shows
Driving impairment. Clinical studies have shown conflicting results about driving impairment associated with zolpidem. The literature falls into 2 categories, based on treatment duration:
- Zolpidem affects performance and memory within the first 4 to 5 hours of administration (Table 1).
- Beyond 5 hours, no residual effects on performance have been identified (Table 2), and repeat nightly dosing has not caused impairment or tolerance.
- All sedative hypnotic benzodiazepines had statistically significant residual effects 10 to 11 hours after ingestion, with longer periods of impairment corresponding to medications with longer half-lives.
- Zopiclone was associated with significant residual impairment for up to 10 hours after ingestion.
- Zolpidem and zaleplon showed no significant impairment in driving 10 to 11 hours after ingestion. Impairment was found, however, when zolpidem was taken within 5 hours of driving.14-18
Table 1
Studies of zolpidem-associated driving skills impairment
(
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Wilkinson, 199514 Blinded; 29 subjects | Zolpidem, 10 mg, 15 mg, and placebo in combination with an alcoholic drink (to reach a BAC of 0.08%) or placebo drink; testing 45 min, 130 min, and 230 min after administration | Visual backward masking test (approximates driving performance) and attention tests | Zolpidem produced significant impairment in combination with alcohol and when administered alone during peak effect assessment; alcohol did not potentiate zolpidem’s effects; additive effects of alcohol seen with 10-mg dose but not 15-mg dose of zolpidem |
| Rush et al, 199815 Double-blind, crossover; 9 subjects | Zolpidem, 7.5, 15, and 22.5 mg; quazepam, 15, 30, and 45 mg; triazolam, 0.1875, 0.375, and 0.5625 mg; testing ½, 1, 1½, 2, 2½, 3, 4, 5, and 6 hours after administration | Subject- and observer-rated questionnaires; tests of recall and delayed recognition | Performance-impairing effects of zolpidem were virtually indistinguishable from those of classic benzodiazepines, such as triazolam |
| Mattila et al, 199816 Randomized, placebo-controlled, double-blind, crossover; 12 subjects | Zolpidem, 15 mg; diazepam, 15 mg; oxazepam, 30 mg; zopiclone, 7.5 mg; alcohol testing before and 1, 3½, and 5 hours after administration | Simulated driving and other measures | Zolpidem impaired coordination, reaction, and cognition at 1 and 3½ hours; tracking remained impaired at 5 hours; all agents (especially zolpidem) impaired learning and memory |
| Mintzer et al, 199917 Double-blind, placebo-controlled; 16 subjects | Zolpidem, 15 mg/70 kg (dosed by subject weight); testing ½, 1, 2, and 3 hours after administration | Memory tasks (recall, fragment completion, recognition) | Zolpidem interfered with explicit but not implicit memory after administration; zolpidem produced a specific deficit in acquisition of contextual information |
| Verster et al, 200418 2-step randomized, placebo-controlled, double-blind, crossover; 30 subjects | Zolpidem, 10 mg and 20 mg; zaleplon, 10 mg and 20 mg; middle-of-the-night dosing; testing 4 hours after dosing | On-the-road driving and other tests of attention, learning, and thinking | Zolpidem, 10 mg and 20 mg, significantly impaired driving function; zolpidem, 20 mg, produced significant impairment on all psychomotor and memory tests; zaleplon, 10 mg and 20 mg, did not differ significantly from placebo |
| BAC: Blood alcohol concentration | |||
Studies of zolpidem-associated driving skills impairment
(>5 hours after dosing)
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Fairweather et al, 199223 Randomized, placebo-controlled; 24 older volunteers taking no other medications | Zolpidem, 5 mg or 10 mg, or placebo taken before bedtime; testing 8.5 hours after administration | Numerous, including reactive time, memory, word recognition | Zolpidem consistently helped with sleep latency, with no residual performance deficits; no tolerance seen with repeated dosing |
| Bocca et al, 199924 Double-blind, crossover; 16 volunteers | Zolpidem, 10 mg; zopiclone, 7.5 mg; flunitrazepam,* 1 mg; and placebo given at 11 PM, with testing at 9 AM | Driving simulation and real time test drive; eye movements measured after driving tests | No residual effects with zolpidem; zopiclone impaired driving ability and increased saccadic latency; flunitrazepam impaired early morning driving and saccadic eye movements longer than zopiclone |
| Partinen et al, 200320 Randomized, placebo-controlled, double-blind, 3-period crossover; 18 women with insomnia | Zolpidem, 10 mg; temazepam, 20 mg; dosing at 2 AM, testing 5.5 hours after dosing | Driving simulation; delayed word recall and memory testing (FePsy test) | No statistically significant effects on driving ability with either drug; no significant differences in FePsy results compared with baseline or placebo |
| Staner et al, 200525 Randomized, placebo-controlled, double-blind, four-way crossover; 23 subjects with DSM-IV-TR diagnosis of insomnia | Zolpidem, 10 mg; zopiclone, 7.5 mg; lormetazepam,* 1 mg; 7 days of dosing; tests given 9 to 11 hours post-dosing | Driving simulation; EEG at rest and while driving | Zolpidem showed no impairment of driving ability and no EEG changes compared with placebo; driving impairment and EEG alterations were found with zopiclone and lormetazepam |
| * Hypnotics not approved in the United States but available elsewhere. | |||
Acute effects (
Combined with alcohol. Wilkinson14 conducted a randomized, 6-way crossover study in which subjects received 10- or 15-mg doses of zolpidem or placebo plus an alcoholic beverage (enough to obtain a blood alcohol concentration [BAC] of ~0.08%) or placebo beverage. Tests given shortly after patients took the study medications showed that zolpidem caused statistically significant impairment both in combination with alcohol and alone during peak drug effect—identified as 45 minutes after ingestion. Alcohol did not potentiate the impairment associated with zolpidem.
Using a similar design, Mattila et al16 compared acute performance impairment associated with zolpidem, diazepam, oxazepam, and zopiclone. In this randomized, double-blinded, crossover study, all comparison medications impaired antecedent learning and memory, but zolpidem given at 15 mg had the greatest effect. Zolpidem impaired coordination, reactive functioning, and cognitive skills at 1 and 3.5 hours after administration, and simulated driving test performance remained impaired at 5 hours (approximately two half-lives of the medication). Of note is that the 15-mg zolpidem dose used in this study was shown by Wilkinson et al14 to be more impairing than the recommended maximum 10-mg dose.
A study from the University of Toronto19 that did not include zolpidem examined potential psychomotor performance deficits and sleepiness in a comparison of time-released melatonin, 6 mg; zaleplon, 10 mg; zopiclone, 7.5 mg; temazepam, 15 mg, and placebo. Tests were given to 9 men and 14 women, ages 21 to 53, just before drug administration and 7 hours later.
Zaleplon had the greatest effect on psychomotor performance, followed by temazepam and zopiclone. Aside from prolonged perceived sleepiness, melatonin and placebo did not interfere with performance testing.
Zolpidem, a benzodiazepine receptor agonist, was the 7th most prescribed drug in the United States in 2005 (2006 data not available).8 It is FDA-approved for short-term treatment of insomnia, although “short-term” is not defined. Package labeling states:
This nonbenzodiazepine hypnotic has been shown to decrease sleep latency and increase sleep duration for up to 35 days in controlled clinical trials. Patients should be evaluated for a primary psychiatric or medical illness if insomnia does not remit after 7 to 10 days of treatment.
An imidazopyridine that acts as an agonist of GABA A1, zolpidem produces sedation while avoiding anticonvulsant, anxiolytic, and muscle relaxation effects. Available in 5- and 10-mg tablets, the drug is rapidly absorbed in the GI tract and excreted primarily through the kidneys. Its half-life is approximately 2.5 hours (approximately 3 hours in elderly patients). The most common side effects are daytime drowsiness, dizziness, and diarrhea; others include asthenia, hiccup, and diplopia.9
- driving ability 4 hours after administration
- memory and psychomotor performance 6 hours after administration.
Partinen et al20 used the recommended zolpidem dose in a similar study of after-midnight use by women with insomnia. The double-blind, randomized, controlled trial evaluated performance with a driving simulator and neuropsychological testing 5.5 hours after medication dosing. Patients taking zolpidem, 10 mg, showed no significant impairment when compared with those taking placebo. Some patients scored poorly on the driving tests alone, and the authors concluded that this group was more susceptible to zolpidem’s effect.
Memory. In a double-blind, placebo-controlled trial by Mintzner et al,17 zolpidem dosed by patient weight at 15 mg/70 kg:
- significantly impaired explicit memory (requires conscious recollection for recall)
- did not affect implicit memory (lack of conscious awareness in the act of recollection).
These findings support complaints of zolpidem-related anterograde amnestic episodes, which also occur with some benzodiazepines (such as midazolam).
Similar to benzodiazepines? Rush et al’s results21 support Mintzer’s assertion17 that zolpidem shares many side effects with benzodiazepines. Performance impairment associated with zolpidem—as rated by subjects and observers—is virtually indistinguishable from a benzodiazepine effect, except that the duration is shorter with zolpidem (5 hours), compared with up to 10 hours for benzodiazepines.
Logan and Couper22 reviewed police reports and toxicology profiles of individuals suspected of driving while impaired. Zolpidem was found in 29 subjects, 5 of whom showed no other substances. In those 5, zolpidem blood levels ranged from 0.08 to 1.40 mg/L and did not appear to correlate with the degree of impairment.
Residual effects (>5 hours)
Older patients. In a randomized, placebo-controlled trial by Fairweather et al,23 zolpidem improved sleep latency in 24 subjects ages 63 to 80. No evidence of impairment in reactive time, memory, or word recognition was found 8.5 hours after nighttime dosing, and tolerance was not seen after 1 week of repeated dosing.
Driving impairment. Bocca et al24 compared degree of driving impairment by zolpidem, zopiclone, flunitrazepam (not approved in the United States), and placebo. The 16 subjects received each medication at 11 pm, with a 2-week washout between medications. One group of 8 was tested at 9 am and the other 8 subjects at 11 am. Those taking zolpidem showed no residual performance impairment, as measured by simulated driving, a test drive, and saccadic eye movements.
Staner et al25 reported similar results when comparing zolpidem, zopiclone, lormetazepam (not approved in the United States), and placebo. Using a driving simulator and electroencephalography (EEG), they evaluated 23 subjects diagnosed with insomnia at 9 and 11 hours post-dose. Zolpidem did not significantly impair driving ability and did not differ from placebo on EEG analysis (resting or driving). The study showed driving impairment with zopiclone and lormetazepam, along with characteristic benzodiazepine EEG changes. This study further supports evidence of limited impairment on driving after appropriate use of zolpidem.
Informed consent
In the informed consent process, failing to warn a patient about medication side effects can lead to legal claims against both manufacturers and prescribers. With any medication, patients have the right to know about a drug’s risks, benefits, and alternate therapies—including no therapy.
Two standards are associated with informed consent and negligence:
- The “reasonable practitioner” standard outlined in Natanson v. Kline (1960)26 mandates that the prescribing physician has revealed all that an “average, reasonable practitioner” would disclose in similar circumstances.
- The “reasonable patient” standard set in Canterbury v. Spence (1972)27 mandates that the prescribing physician has informed the patient about the proposed treatment, its side effects, and alternatives to the proposed treatment that a reasonable patient would consider material to the decision of whether or not to undergo treatment.
The vaccine was licensed as a prescription drug but administered through county health departments. In 1970, a nurse in a Texas Department of Health clinic administered the vaccine to 8-month-old Anita Reyes without telling the girl’s parents of warnings in the package circular. Holding Wyeth Laboratories to a reasonableness standard, the court found that the company knew or should have known how the vaccine would be distributed.
The package insert was not shown to have given inadequate warning, and the vaccine was not shown to be defective (it was a trivalent live-virus Sabin oral polio vaccine, as intended).
Vioxx cases. Similarly, some plaintiffs have been awarded millions of dollars (as in Ernst v. Merck & Co., Inc.29) in rulings that Merck & Co. failed to disclose the risk of cardiotoxicity with the arthritis drug rofecoxib (Vioxx) and thus failed to provide physicians with information needed when prescribing the drug. In Humeston v. Merck & Co.,30 a Texas court in 2005 held that Vioxx’s warning labels were adequate. In a retrial, however, the New Jersey Superior Court awarded the plaintiff $47.5 million.31
As with the polio vaccine and Vioxx litigations, courts are being asked to decide if patients were adequately informed about sleep-driving and other risks associated with the use of sedative-hypnotics.
Clinical recommendations
Zolpidem—like many other medications—carries a substantial risk of side effects, even when used appropriately. However, given the medical and mental health risks of untreated insomnia, the benefits of a medication such as zolpidem will likely outweigh its risks.
Numerous studies have shown that zolpidem is effective for improving sleep latency and that there are mild, if any, residual side effects beyond what would normally be a restful night’s sleep. Impairments are evident, however, during the hours following the drug’s administration, with some effects lasting >5 hours depending on the dose.
Risk management. When prescribing nonbenzodiazepine hypnotics such as zolpidem, you may want to adopt a risk management approach as you would with other medications that can have serious side effects. An approach to benzodiazepine prescribing proposed by Bursztajn et al31 advocates:
- using the informed-consent process to build an alliance with patients
- not prescribing the medication in isolation of other beneficial therapies
- being aware of and always documenting your decision-making process.
When you make patients aware of all risks, benefits, alternate therapies, and possible outcomes with no treatment, you have informed them effectively. Patients are then left to decide whether or not to agree to the treatment. You also are responsible for monitoring the patient, addressing the patient’s questions, and relaying important safety information.
When prescribing zolpidem, discuss safety information with the patient, such as:
- Do not drive or operate heavy equipment for at least 5 to 6 hours after administration.
- Have a safety plan in place for transportation during those hours.
- Do not use this medication with alcohol or other sedative/hypnotics.
- Contact the prescriber about any suspected adverse effects.
- MedlinePlus information on sleep disorders. National Institutes of Health and National Library of Medicine. www.nlm.nih.gov/medlineplus/sleepdisorders.html.
- Zolpidem (systemic). Mayoclinic.com: Tools for healthier lives. www.mayoclinic.com/health/drug-information/DR202707.
- Diazepam • Valium
- Eszopiclone • Lunesta
- Midazolam • Versed
- Oxazepam • Serax
- Quazepam • Doral
- Rofecoxib • Vioxx
- Temazepam • Restoril
- Triazolam • Halcion
- Zolpidem • Ambien, Ambien CR
- Zaleplon • Sonata
- Zopiclone • Imovane (in Europe)
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors acknowledge the assistance and guidance of Linda T. Moore, JD, in preparing this manuscript.
1. Markon J. Sleeping Va. driver convicted in crash; man had taken too much Ambien. The Washington Post, August 2, 2006. Accessed August 26, 2006 from LexisNexis Academic Database.
2. Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: an unmet public health problem. Available at: http://www.iom.edu/CMS/3740/23160/33668.aspx. Accessed February 21, 2007.
3. Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment; prevalence and correlates. Arch Gen Psychiatry 1985;42:225-32.
4. Barclay L. Driving, other erratic behaviors reported after taking zolpidem. Available at http://www.medscape.com/viewarticle/528415. Accessed February 21, 2007.
5. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep 2006;29(8):1009-14.
6. Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005;165(8):863-7.
7. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med 2006;4(2):104-13.
8. Verispan VONA. Top 200 brand name drugs by units in 2005. Drug Topics 2006. Available at: http://www.drugtopics.com/drugtopics/data/articlestandard/drugtopics/102006/311294/article.pdf. Accessed February 22, 2007.
9. sanofi-aventis Ambien prescribing information. Available at: http://products.sanofi-aventis.us/ambien/ambien.html. Accessed February 21, 2007.
10. Pear R. Patrick Kennedy crashes car into a Capitol Hill barrier. The New York Times, May 5, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
11. Janet Makinen and others v. Sanofi-Synthelabo & Sanofi-Synthelabo, Inc. Class action suit filed March 6, 2006 in U.S. District Court for the Southern District of New York, NY.
12. Tooher NL. Ambien users are filing lawsuits. Kansas City Daily Record, April 12, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
13. Verster JC, Veldhuijzen DS, Volkerts ER. Residual effects of sleep medication on driving ability. Sleep Med Rev 2004;8(4):309-25.
14. Wilkinson CJ. The acute effects of zolpidem, administered alone and with alcohol, on cognitive and psychomotor function. J Clin Psychiatry 1995;56(7):309-18.
15. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
16. Mattila MJ, Vanakoski J, Kalska H, Seppala T. Effects of alcohol, zolpidem, and some other sedatives and hypnotics on human performance and memory. Pharmacol Biochem Behav 1998;59(4):917-23.
17. Mintzer MZ, Griffiths RR. Selective effects of zolpidem on human memory functions. J Psychopharmacol 1999;13(1):18-31.
18. Verster JC, Volkerts ER, Schreuder AH, et al. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. J Clin Psychopharmacol 2002;22(6):576-83.
19. Paul MA, Gray G, Kenny G, Pigeau RA. Impact of melatonin, zaleplon, zopiclone, and temazepam on psychomotor performance. Aviat Space Environ Med 2003;74(12):1263-70.
20. Partinen M, Hirvonen K, Hublin C, et al. Effects of after-midnight intake of zolpidem and temazepam on driving ability in women with non-organic insomnia. Sleep Med 2003;4(6):553-61.
21. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
22. Logan BK, Couper FJ. Zolpidem and driving impairment. J Forensic Sci 2001;46(1):105-10.
23. Fairweather DB, Kerr JS, Hindmarch I. The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. Eur J Clin Pharmacol 1992;43(6):597-601.
24. Bocca ML, Le Doze F, Etard O, et al. Residual effect of zolpidem 10 mg and zopiclone 7.5 mg versus flunitrazepam 1 mg and placebo on driving performance and ocular saccades. Psychopharmacology (Berl) 1999;143(4):373-9.
25. Staner L, Ertle S, Boeijinga P, et al. Next-day residual effects of hypnotics in DSM-IV primary insomnia: a driving simulator study with simultaneous electroencephalogram monitoring. Psychopharmacology (Berl) 2005;181(4):790-8.
26. Natanson v. Kline 300 P.2d 1093 (1960).
27. Canterbury v. Spence 464 F.2d 772 (1972).
28. Reyes v. Wyeth Laboratories. 498 F.2d 1264 (1974).
29. Ernst v. Merck & Co. 24 PLLR 149 (2005).
30. Humeston v. Merck & Co., No. ATL-L-2272-03-MT, Super. Ct., Atlantic County, NJ, November 3, 2005.
31. Johnson LA. Jury blames Vioxx for man’s heart attack, awards $47.5 million. Available at http://news.findlaw.com/ap/o/51/03-12-2007/85f9000f67cd576a.html. Accessed March 16, 2007.
32. Bursztajn HJ, Brodsky A. Ethical and legal dimensions of benzodiazepine prescription: a commentary. Psychiatr Ann 1998;28(3):121-7.
1. Markon J. Sleeping Va. driver convicted in crash; man had taken too much Ambien. The Washington Post, August 2, 2006. Accessed August 26, 2006 from LexisNexis Academic Database.
2. Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: an unmet public health problem. Available at: http://www.iom.edu/CMS/3740/23160/33668.aspx. Accessed February 21, 2007.
3. Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment; prevalence and correlates. Arch Gen Psychiatry 1985;42:225-32.
4. Barclay L. Driving, other erratic behaviors reported after taking zolpidem. Available at http://www.medscape.com/viewarticle/528415. Accessed February 21, 2007.
5. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep 2006;29(8):1009-14.
6. Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005;165(8):863-7.
7. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med 2006;4(2):104-13.
8. Verispan VONA. Top 200 brand name drugs by units in 2005. Drug Topics 2006. Available at: http://www.drugtopics.com/drugtopics/data/articlestandard/drugtopics/102006/311294/article.pdf. Accessed February 22, 2007.
9. sanofi-aventis Ambien prescribing information. Available at: http://products.sanofi-aventis.us/ambien/ambien.html. Accessed February 21, 2007.
10. Pear R. Patrick Kennedy crashes car into a Capitol Hill barrier. The New York Times, May 5, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
11. Janet Makinen and others v. Sanofi-Synthelabo & Sanofi-Synthelabo, Inc. Class action suit filed March 6, 2006 in U.S. District Court for the Southern District of New York, NY.
12. Tooher NL. Ambien users are filing lawsuits. Kansas City Daily Record, April 12, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
13. Verster JC, Veldhuijzen DS, Volkerts ER. Residual effects of sleep medication on driving ability. Sleep Med Rev 2004;8(4):309-25.
14. Wilkinson CJ. The acute effects of zolpidem, administered alone and with alcohol, on cognitive and psychomotor function. J Clin Psychiatry 1995;56(7):309-18.
15. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
16. Mattila MJ, Vanakoski J, Kalska H, Seppala T. Effects of alcohol, zolpidem, and some other sedatives and hypnotics on human performance and memory. Pharmacol Biochem Behav 1998;59(4):917-23.
17. Mintzer MZ, Griffiths RR. Selective effects of zolpidem on human memory functions. J Psychopharmacol 1999;13(1):18-31.
18. Verster JC, Volkerts ER, Schreuder AH, et al. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. J Clin Psychopharmacol 2002;22(6):576-83.
19. Paul MA, Gray G, Kenny G, Pigeau RA. Impact of melatonin, zaleplon, zopiclone, and temazepam on psychomotor performance. Aviat Space Environ Med 2003;74(12):1263-70.
20. Partinen M, Hirvonen K, Hublin C, et al. Effects of after-midnight intake of zolpidem and temazepam on driving ability in women with non-organic insomnia. Sleep Med 2003;4(6):553-61.
21. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
22. Logan BK, Couper FJ. Zolpidem and driving impairment. J Forensic Sci 2001;46(1):105-10.
23. Fairweather DB, Kerr JS, Hindmarch I. The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. Eur J Clin Pharmacol 1992;43(6):597-601.
24. Bocca ML, Le Doze F, Etard O, et al. Residual effect of zolpidem 10 mg and zopiclone 7.5 mg versus flunitrazepam 1 mg and placebo on driving performance and ocular saccades. Psychopharmacology (Berl) 1999;143(4):373-9.
25. Staner L, Ertle S, Boeijinga P, et al. Next-day residual effects of hypnotics in DSM-IV primary insomnia: a driving simulator study with simultaneous electroencephalogram monitoring. Psychopharmacology (Berl) 2005;181(4):790-8.
26. Natanson v. Kline 300 P.2d 1093 (1960).
27. Canterbury v. Spence 464 F.2d 772 (1972).
28. Reyes v. Wyeth Laboratories. 498 F.2d 1264 (1974).
29. Ernst v. Merck & Co. 24 PLLR 149 (2005).
30. Humeston v. Merck & Co., No. ATL-L-2272-03-MT, Super. Ct., Atlantic County, NJ, November 3, 2005.
31. Johnson LA. Jury blames Vioxx for man’s heart attack, awards $47.5 million. Available at http://news.findlaw.com/ap/o/51/03-12-2007/85f9000f67cd576a.html. Accessed March 16, 2007.
32. Bursztajn HJ, Brodsky A. Ethical and legal dimensions of benzodiazepine prescription: a commentary. Psychiatr Ann 1998;28(3):121-7.
6 keys to resilience for PTSD and everyday stress
Ms. M, age 24, works as a magazine editor in New York City. On a December evening, she walks out of the subway and heads to her boyfriend’s apartment, looking forward to unloading her heavy bag and checking her e-mail. Out of nowhere, a man runs up behind her and smashes a huge rock into her head.
She feels momentarily disconnected from her body and surroundings but manages to scream. As the assailant runs away, 2 girls rush to her aid.
Ms. M hurts everywhere. Her glasses have been knocked off, and her orbit is fractured; her eye will require multiple surgeries. She reaches for her cell phone, but it’s slippery with blood. A bystander dials 911, and paramedics arrive within minutes.
Most persons experience trauma during their lives,1 but not usually an attack as severe as Ms. M’s. Post-traumatic stress disorder (PTSD) and other psychopathologies are not inevitable or even common, however, developing in 8% to 12% of trauma survivors.2 Why are some individuals more resilient to trauma than others?
Resilience to stress is associated consistently with at least 6 psychosocial factors: active coping styles, regular physical exercise, a positive outlook, a moral compass, social support, and cognitive flexibility (Table 1). This article describes how motivated persons can enhance these “resilience factors” to become more resistant to everyday stressors and unexpected traumas.
Table 1
6 psychosocial factors that protect against
and aid recovery from posttraumatic stress
| Factor | Definition |
|---|---|
| Active coping style | Problem-solving and managing emotions that accompany stress; learning to face fears |
| Physical exercise | Engaging in physical activity to improve mood and health |
| Positive outlook | Using cognitive-behavioral strategies to enhance optimism and decrease pessimism; embracing humor |
| Moral compass | Developing and living by meaningful principles; putting them into action through altruism |
| Social support | Developing and nurturing friendships; seeking resilient role models and learning from them |
| Cognitive flexibility | Finding good in adverse situations; remaining flexible in one’s approach to solving problems |
1. Active coping style
Resilience is the process of adapting well to stress or trauma (Box 1).3-5 Learning to manage stressful situations requires active coping, which can be conceptualized as 2 types:
- “problem-focused” (working to solve the problem)
- “emotion-focused” (accepting and dealing with emotions caused by the stressor).
Undertaking and mastering difficult tasks appears to be effective in increasing resilience to stress. The “stress inoculation” hypothesis (Box 2)8-11 provides a plausible explanation for the observation that children who learn to cope with stress tend to become hardy adults. Successfully overcoming challenges improves self-confidence and also may alter the neurobiology of the stress response.
Prolonged-exposure therapy. PTSD development and maintenance depend in part on fear conditioning. By avoiding exposure to reminders of their trauma, survivors unwittingly solidify associations between traumatic triggers (people, places, or things that are reminders) and fear. Actively facing fears is necessary to break these associations.
Prolonged-exposure therapy was designed to help patients face their fears.12 As part of therapy, participants retell their trauma stories and engage in avoided activities in a safe environment. This treatment has been found to be highly effective in reducing PTSD symptoms, and its benefits often last longer than those conferred by pharmacologic interventions.13
CASE CONTINUED: Feeling ‘out of sync’
Ms. M remains frightened and angry after 2 months and is referred for psychological evaluation. She is diagnosed with PTSD based on her debilitating symptoms, including flashbacks, frightening nightmares, avoiding the subway, and feeling emotionally numb (which she describes as “being out of sync” with loved ones). Ms. M also complains of difficulty sleeping and irritability.
The therapist initiates prolonged-exposure treatment, including imaginal and in vivo exposure. In imaginal exposure, Ms. M tells and retells her trauma story in the safety of the therapist’s office. To desensitize herself to the memory, she listens to her recorded voice recounting her trauma. In vivo exposure involves homework, such as visiting the attack site during the day with a companion and talking with loved ones about the event. These assignments allow Ms. M to reclaim the life she lost because of severe anxiety and fear associated with anything related to the attack.
Within 3 months, Ms. M’s symptoms have improved and no longer meet DSM-IV-TR criteria for PTSD. She continues to struggle with insomnia, affective constriction, and a sense of social isolation—symptoms that often remit slowly, if at all, in trauma victims despite good treatment. She stays in therapy to work on confronting her fears and finding meaning in her experience.
2. Physical exercise
Exercise is a type of active coping that diminishes negative emotions caused by stress. Regular exercisers report less-frequent depression,14 and exercise has been shown to improve clinical depression in adults.15 Exercise builds physical and emotional hardiness, lifts mood, and improves memory. It produces these health benefits by:
- releasing endorphins and serotonin precursors
- attenuating basal hypothalamic-pituitary-adrenal axis activity
- promoting expression of neurotrophic and neuroprotective factors.16
CASE CONTINUED: Learning to self-soothe
Ms. M learns to read children’s stories to help her fall asleep at night and stave off nightmares. She takes up yoga to combat residual anxiety. She also resumes singing in her local chorus, which includes riding the subway home from rehearsals at 10 pm.
Resilience is the ability to maintain normal functioning despite adversity. It can be viewed as the successful operation of “basic human adaptational systems.” Conversely, depression and posttraumatic stress disorder (PTSD) may be understood, in part, as failure to adapt to stress.
Risk factors for PTSD. Traumas with the highest risk for psychopathology are severe, unpredictable, or uncontrollable and those that involve loss of property or (especially) a loved one, danger to self, or physical injury. PTSD risk also is increased by the cumulative effect of multiple, severe, uncontrollable traumas and personal factors such as:
3. A Positive outlook
Depressed individuals tend to view their problems as permanent and pervasive, whereas those who are resilient see adversity as temporary and limited in scope.
Role of dopamine. Humor and positive emotions have been linked to the dopaminergic reward mechanism in the mesolimbic circuitry. Dopaminergic neurons in the ventral tegmental area fire when a reward is received (Table 2); firing increases when a reward is unexpected or greater than expected. These same neurons release less dopamine when rewards are smaller than expected or not received at all.
Optimists are thought to have a robust dopaminergic response to reward, which is either hypersensitive to rewards and/or resistant to dysregulation under stressful (unrewarding) conditions.20
Undertaking and mastering difficult tasks appears to be an effective way to increase resilience to stress. The “stress inoculation” hypothesis provides a plausible explanation for the observation that children who learn to cope with stress become hardy adults.
Men and women who successfully managed stressful situations in childhood—including death or illness of a parent or sibling, family relocation, and loss of friendship—are more resistant to adulthood stressors, such as divorce, death or major illness of a loved one, and job loss.8 Conversely, individuals who experienced extreme childhood stress that they could not control or master—such as physical and/or sexual abuse—may be more vulnerable to future stressors.
Like vaccination? Organisms develop immunity after exposure to a pathogen’s attenuated form; similarly, they may develop resistance to stress after being exposed to and overcoming mild stressors.9 Immunity to stress is not specific to the type of stressor first encountered; early exposure to manageable stress appears to enhance resilience to many adverse experiences.
Neurobiology of resilience. In a series of studies, Special Forces soldiers had higher blood levels of 2 stress-protective hormones—neuropeptide Y (NPY) and dehydroepiandrosterone (DHEA)—immediately after high-stress interrogations than did soldiers who received lessintensive training.10 These hormones also correlated with better performance under stress.
NPY and DHEA help keep the stress response in check by inhibiting release of norepinephrine, cortisol, and other stress-related hormones under high-stress conditions.11 To what degree genetics, development, and/or training enhance NPY and DHEA release is not clear.
Table 2
Neurobiology of resilience:
Factors that influence physiologic stress response
| Selected neurobiological factors | Effect on stress response |
|---|---|
| Up-regulators | |
| Norepinephrine | Neurohormone and neurotransmitter released by the locus ceruleus in response to stress; sympathetic nervous system mediator; increases autonomic arousal (elevates blood pressure, heart rate); facilitates fear memory formation |
| Cortisol | Glucocorticoid released by adrenals in response to HPA axis activation by locus ceruleus; increases arousal, attention, and fear memory formation; initially adaptive, but prolonged/excess release has harmful systemic effects (hypertension, osteoporosis, immune suppression) |
| Down-regulators | |
| DHEA | Steroid released by adrenal cortex under stress; down-regulates stress response; has antiglucocorticoid activity; may protect against PTSD |
| NPY | Neuropeptide that counters locus ceruleus activity; blocks release of cortisol; anxiolytic |
| Galanin | Neuropeptide that counters locus ceruleus activity; anxiolytic |
| Other neurotransmitters | |
| Dopamine | Optimal levels enable reward system functioning; excess or deficit linked to learned helplessness and stress |
| Serotonin | Mixed effects, but high activity at 5HT1A receptors is linked to resilience |
| DHEA: dehydroepiandrosterone; HPA: hypothalamic-pituitary-adrenal; NPY: neuropeptide Y; PTSD: posttraumatic stress disorder | |
CASE CONTINUED: ‘I’m not bitter’
Ms. M can make an occasional joke about her attack and the massive stacks of paperwork she must sort through to pay medical bills and get reimbursed by insurance. She says, “I’m not bitter. I don’t want to carry that anger around for the rest of my life, so I won’t.”
4. A moral compass
Religious faith is associated with lower rates of depression in many populations, including college students, bereaved adults, and elderly hospitalized patients.21 Religious faith is not essential to a strong moral compass, however.
Morality appears to have a neural basis—a hypothesis supported by the observation that brain injury can damage one’s moral sense. “Acquired sociopathy” can result from trauma to certain brain areas, including the anterior prefrontal cortex and anterior temporal lobes.
‘Required helpfulness.’ Altruism—putting one’s moral compass into action—benefits the person who practices it and the person who receives it. Persons who help others perceive themselves as necessary and derive fulfillment. This phenomenon known as “required helpfulness” was first described during World War II, when those who cared for others after bombardments suffered less posttraumatic psychopathology than those who did not.22
Some individuals find healing in a “survivor mission” after personal tragedy, helping others cope with the same problem they faced. Mothers Against Drunk Driving—founded by mothers who lost children in car accidents—is one example.23
CASE CONTINUED: Altruism in action
Ms. M hopes to prevent attacks on other women. She participates in an organization that teaches women self-defense. She also speaks publicly for women’s safety and works with a local board to help defray crime survivors’ medical costs.
5. Social support
Individuals with strong social support tend to be more resilient than those without.24 Social support can reduce risk-taking behavior, encourage active coping, decrease loneliness, increase feelings of self-worth, and help a person put problems into perspective. A lack of social support correlates with depression, stress, and increased morbidity and mortality during medical illness.
Role models. People can learn to manage stress by mimicking the behavior of someone they respect. Many resilient adults credit a parent, grandparent, or other role model for teaching them to act honestly and inspiring them to be strong. In a study of 770 teenagers, those who had a strong nonparental mentor (such as a neighbor, teacher, or coach) reported less drug use and delinquency and a greater belief in the importance of school than those without such a mentor.25
CASE CONTINUED: Dad’s her role model
When she has bad days, Ms. M draws strength by thinking about her father, who has suffered much and whom she respects.
6. Cognitive flexibility
Being able to positively reframe negative events (“cognitive reappraisal”) is crucial to resilience. Individuals who successfully overcome adverse events usually manage to find some meaning in their tragedy.
Psychiatrist and Holocaust survivor Viktor Frankl26 wrote of the importance of “meaning making.” Despite suffering for years in Nazi concentration camps, Frankl wrote that he gained the opportunity to exercise inner strength and be “brave, dignified and unselfish.” He struggled to survive because he came to believe that his suffering had a purpose: to live to teach others about his experiences.
Neuroimaging studies indicate that individuals who use cognitive reappraisal to deal with adversity have strong “top-down control” of emotions. They can modify their reaction to stress or trauma by activating the prefrontal cortex, which then modulates amygdalar response to the situation.27
CASE CONTINUED: Reappraisal
Although Ms. M wishes she had never been attacked and can find no rational explanation for it, she is weaving the event into the fabric of her life. She insists she has become stronger, wiser, and safer and wants to share her story with others.
Related resources
- National Center for Posttraumatic Stress Disorder. U.S. Department of Veterans Affairs. www.ncptsd.va.gov.
- The road to resilience. American Psychological Association Help Center. www.apahelpcenter.org/featuredtopics/feature.php?id=6.
- Positive Psychology Center. University of Pennsylvania. www.ppc.sas.upenn.edu.
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Frans O, Rimmo PA, Aberg L, Fredrikson M. Trauma exposure and post-traumatic stress disorder in the general population. Acta Psychiatr Scand 2005;111(4):291-9.
2. Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry 1991;48(3):216-22.
3. Fauerbach JA, Lawrence JW, Schmidt CW, Jr, et al. Personality predictors of injury-related posttraumatic stress disorder. J Nerv Ment Dis 2000;188(8):510-7.
4. Masten AS. Ordinary magic. Resilience processes in development. Am Psychol 2001;56(3):227-38.
5. Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res 2006;40(1):1-21.
6. Park CL, Adler NE. Coping style as a predictor of health and well-being across the first year of medical school. Health Psychol 2003;22(6):627-31.
7. Muris P, Schmidt H, Lambrichs R, Meesters C. Protective and vulnerability factors of depression in normal adolescents. Behav Res Ther 2001;39(5):555-65.
8. Khoshaba DM, Maddi SR. Early experiences in hardiness development. Consulting Psychology Journal: Practice and Research 1999;51(2):106-16.
9. Rutter M. Resilience: some conceptual considerations. J Adolesc Health 1993;14(8):626-31,690-6.
10. Morgan CA, 3rd, Wang S, Southwick SM, et al. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry 2000;47(10):902-9.
11. Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci 1994;17(2):80-5.
12. Foa EB, Rothbaum BO. Treating the trauma of rape: cognitive behavioral therapy for PTSD. New York: Guilford Press; 1998.
13. Foa EB, Dancu CV, Hembree EA, et al. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol 1999;67(2):194-200.
14. Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002;32(12):741-60.
15. Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci 2001;56(8):M497-504.
16. Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002;25(6):295-301.
17. Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345-56.
18. Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol 2001;56(3):218-26.
19. Folkman S. Positive psychological states and coping with severe stress. Soc Sci Med 1997;45(8):1207-21.
20. Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 2004;161(2):195-216.
21. Koenig HG, George LK, Peterson BL. Religiosity and remission of depression in medically ill older patients. Am J Psychiatry 1998;155(4):536-42.
22. Rachman S. The concept of required helpfulness. Behav Res Ther 1979;17(1):1-6.
23. Southwick SM, Vythilingham M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annual Review of Clinical Psychology 2005;1:255-91.
24. Resick PA. Clinical psychology: a modular course. Philadelphia: Taylor & Francis Group; 2001.
25. Rhodes JE, Roffman J, Grossman JB. The rhetoric and reality of youth mentoring. In: Rhodes JE, ed. New directions in youth development: theory, practice, and research—a critical view of youth mentoring. San Francisco: Jossey-Bass; 2002: 9-20.
26. Frankl VE. Man’s search for meaning. Boston: Beacon Press; 1959:75-7.
27. Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 2004;23(2):483-99.
Ms. M, age 24, works as a magazine editor in New York City. On a December evening, she walks out of the subway and heads to her boyfriend’s apartment, looking forward to unloading her heavy bag and checking her e-mail. Out of nowhere, a man runs up behind her and smashes a huge rock into her head.
She feels momentarily disconnected from her body and surroundings but manages to scream. As the assailant runs away, 2 girls rush to her aid.
Ms. M hurts everywhere. Her glasses have been knocked off, and her orbit is fractured; her eye will require multiple surgeries. She reaches for her cell phone, but it’s slippery with blood. A bystander dials 911, and paramedics arrive within minutes.
Most persons experience trauma during their lives,1 but not usually an attack as severe as Ms. M’s. Post-traumatic stress disorder (PTSD) and other psychopathologies are not inevitable or even common, however, developing in 8% to 12% of trauma survivors.2 Why are some individuals more resilient to trauma than others?
Resilience to stress is associated consistently with at least 6 psychosocial factors: active coping styles, regular physical exercise, a positive outlook, a moral compass, social support, and cognitive flexibility (Table 1). This article describes how motivated persons can enhance these “resilience factors” to become more resistant to everyday stressors and unexpected traumas.
Table 1
6 psychosocial factors that protect against
and aid recovery from posttraumatic stress
| Factor | Definition |
|---|---|
| Active coping style | Problem-solving and managing emotions that accompany stress; learning to face fears |
| Physical exercise | Engaging in physical activity to improve mood and health |
| Positive outlook | Using cognitive-behavioral strategies to enhance optimism and decrease pessimism; embracing humor |
| Moral compass | Developing and living by meaningful principles; putting them into action through altruism |
| Social support | Developing and nurturing friendships; seeking resilient role models and learning from them |
| Cognitive flexibility | Finding good in adverse situations; remaining flexible in one’s approach to solving problems |
1. Active coping style
Resilience is the process of adapting well to stress or trauma (Box 1).3-5 Learning to manage stressful situations requires active coping, which can be conceptualized as 2 types:
- “problem-focused” (working to solve the problem)
- “emotion-focused” (accepting and dealing with emotions caused by the stressor).
Undertaking and mastering difficult tasks appears to be effective in increasing resilience to stress. The “stress inoculation” hypothesis (Box 2)8-11 provides a plausible explanation for the observation that children who learn to cope with stress tend to become hardy adults. Successfully overcoming challenges improves self-confidence and also may alter the neurobiology of the stress response.
Prolonged-exposure therapy. PTSD development and maintenance depend in part on fear conditioning. By avoiding exposure to reminders of their trauma, survivors unwittingly solidify associations between traumatic triggers (people, places, or things that are reminders) and fear. Actively facing fears is necessary to break these associations.
Prolonged-exposure therapy was designed to help patients face their fears.12 As part of therapy, participants retell their trauma stories and engage in avoided activities in a safe environment. This treatment has been found to be highly effective in reducing PTSD symptoms, and its benefits often last longer than those conferred by pharmacologic interventions.13
CASE CONTINUED: Feeling ‘out of sync’
Ms. M remains frightened and angry after 2 months and is referred for psychological evaluation. She is diagnosed with PTSD based on her debilitating symptoms, including flashbacks, frightening nightmares, avoiding the subway, and feeling emotionally numb (which she describes as “being out of sync” with loved ones). Ms. M also complains of difficulty sleeping and irritability.
The therapist initiates prolonged-exposure treatment, including imaginal and in vivo exposure. In imaginal exposure, Ms. M tells and retells her trauma story in the safety of the therapist’s office. To desensitize herself to the memory, she listens to her recorded voice recounting her trauma. In vivo exposure involves homework, such as visiting the attack site during the day with a companion and talking with loved ones about the event. These assignments allow Ms. M to reclaim the life she lost because of severe anxiety and fear associated with anything related to the attack.
Within 3 months, Ms. M’s symptoms have improved and no longer meet DSM-IV-TR criteria for PTSD. She continues to struggle with insomnia, affective constriction, and a sense of social isolation—symptoms that often remit slowly, if at all, in trauma victims despite good treatment. She stays in therapy to work on confronting her fears and finding meaning in her experience.
2. Physical exercise
Exercise is a type of active coping that diminishes negative emotions caused by stress. Regular exercisers report less-frequent depression,14 and exercise has been shown to improve clinical depression in adults.15 Exercise builds physical and emotional hardiness, lifts mood, and improves memory. It produces these health benefits by:
- releasing endorphins and serotonin precursors
- attenuating basal hypothalamic-pituitary-adrenal axis activity
- promoting expression of neurotrophic and neuroprotective factors.16
CASE CONTINUED: Learning to self-soothe
Ms. M learns to read children’s stories to help her fall asleep at night and stave off nightmares. She takes up yoga to combat residual anxiety. She also resumes singing in her local chorus, which includes riding the subway home from rehearsals at 10 pm.
Resilience is the ability to maintain normal functioning despite adversity. It can be viewed as the successful operation of “basic human adaptational systems.” Conversely, depression and posttraumatic stress disorder (PTSD) may be understood, in part, as failure to adapt to stress.
Risk factors for PTSD. Traumas with the highest risk for psychopathology are severe, unpredictable, or uncontrollable and those that involve loss of property or (especially) a loved one, danger to self, or physical injury. PTSD risk also is increased by the cumulative effect of multiple, severe, uncontrollable traumas and personal factors such as:
3. A Positive outlook
Depressed individuals tend to view their problems as permanent and pervasive, whereas those who are resilient see adversity as temporary and limited in scope.
Role of dopamine. Humor and positive emotions have been linked to the dopaminergic reward mechanism in the mesolimbic circuitry. Dopaminergic neurons in the ventral tegmental area fire when a reward is received (Table 2); firing increases when a reward is unexpected or greater than expected. These same neurons release less dopamine when rewards are smaller than expected or not received at all.
Optimists are thought to have a robust dopaminergic response to reward, which is either hypersensitive to rewards and/or resistant to dysregulation under stressful (unrewarding) conditions.20
Undertaking and mastering difficult tasks appears to be an effective way to increase resilience to stress. The “stress inoculation” hypothesis provides a plausible explanation for the observation that children who learn to cope with stress become hardy adults.
Men and women who successfully managed stressful situations in childhood—including death or illness of a parent or sibling, family relocation, and loss of friendship—are more resistant to adulthood stressors, such as divorce, death or major illness of a loved one, and job loss.8 Conversely, individuals who experienced extreme childhood stress that they could not control or master—such as physical and/or sexual abuse—may be more vulnerable to future stressors.
Like vaccination? Organisms develop immunity after exposure to a pathogen’s attenuated form; similarly, they may develop resistance to stress after being exposed to and overcoming mild stressors.9 Immunity to stress is not specific to the type of stressor first encountered; early exposure to manageable stress appears to enhance resilience to many adverse experiences.
Neurobiology of resilience. In a series of studies, Special Forces soldiers had higher blood levels of 2 stress-protective hormones—neuropeptide Y (NPY) and dehydroepiandrosterone (DHEA)—immediately after high-stress interrogations than did soldiers who received lessintensive training.10 These hormones also correlated with better performance under stress.
NPY and DHEA help keep the stress response in check by inhibiting release of norepinephrine, cortisol, and other stress-related hormones under high-stress conditions.11 To what degree genetics, development, and/or training enhance NPY and DHEA release is not clear.
Table 2
Neurobiology of resilience:
Factors that influence physiologic stress response
| Selected neurobiological factors | Effect on stress response |
|---|---|
| Up-regulators | |
| Norepinephrine | Neurohormone and neurotransmitter released by the locus ceruleus in response to stress; sympathetic nervous system mediator; increases autonomic arousal (elevates blood pressure, heart rate); facilitates fear memory formation |
| Cortisol | Glucocorticoid released by adrenals in response to HPA axis activation by locus ceruleus; increases arousal, attention, and fear memory formation; initially adaptive, but prolonged/excess release has harmful systemic effects (hypertension, osteoporosis, immune suppression) |
| Down-regulators | |
| DHEA | Steroid released by adrenal cortex under stress; down-regulates stress response; has antiglucocorticoid activity; may protect against PTSD |
| NPY | Neuropeptide that counters locus ceruleus activity; blocks release of cortisol; anxiolytic |
| Galanin | Neuropeptide that counters locus ceruleus activity; anxiolytic |
| Other neurotransmitters | |
| Dopamine | Optimal levels enable reward system functioning; excess or deficit linked to learned helplessness and stress |
| Serotonin | Mixed effects, but high activity at 5HT1A receptors is linked to resilience |
| DHEA: dehydroepiandrosterone; HPA: hypothalamic-pituitary-adrenal; NPY: neuropeptide Y; PTSD: posttraumatic stress disorder | |
CASE CONTINUED: ‘I’m not bitter’
Ms. M can make an occasional joke about her attack and the massive stacks of paperwork she must sort through to pay medical bills and get reimbursed by insurance. She says, “I’m not bitter. I don’t want to carry that anger around for the rest of my life, so I won’t.”
4. A moral compass
Religious faith is associated with lower rates of depression in many populations, including college students, bereaved adults, and elderly hospitalized patients.21 Religious faith is not essential to a strong moral compass, however.
Morality appears to have a neural basis—a hypothesis supported by the observation that brain injury can damage one’s moral sense. “Acquired sociopathy” can result from trauma to certain brain areas, including the anterior prefrontal cortex and anterior temporal lobes.
‘Required helpfulness.’ Altruism—putting one’s moral compass into action—benefits the person who practices it and the person who receives it. Persons who help others perceive themselves as necessary and derive fulfillment. This phenomenon known as “required helpfulness” was first described during World War II, when those who cared for others after bombardments suffered less posttraumatic psychopathology than those who did not.22
Some individuals find healing in a “survivor mission” after personal tragedy, helping others cope with the same problem they faced. Mothers Against Drunk Driving—founded by mothers who lost children in car accidents—is one example.23
CASE CONTINUED: Altruism in action
Ms. M hopes to prevent attacks on other women. She participates in an organization that teaches women self-defense. She also speaks publicly for women’s safety and works with a local board to help defray crime survivors’ medical costs.
5. Social support
Individuals with strong social support tend to be more resilient than those without.24 Social support can reduce risk-taking behavior, encourage active coping, decrease loneliness, increase feelings of self-worth, and help a person put problems into perspective. A lack of social support correlates with depression, stress, and increased morbidity and mortality during medical illness.
Role models. People can learn to manage stress by mimicking the behavior of someone they respect. Many resilient adults credit a parent, grandparent, or other role model for teaching them to act honestly and inspiring them to be strong. In a study of 770 teenagers, those who had a strong nonparental mentor (such as a neighbor, teacher, or coach) reported less drug use and delinquency and a greater belief in the importance of school than those without such a mentor.25
CASE CONTINUED: Dad’s her role model
When she has bad days, Ms. M draws strength by thinking about her father, who has suffered much and whom she respects.
6. Cognitive flexibility
Being able to positively reframe negative events (“cognitive reappraisal”) is crucial to resilience. Individuals who successfully overcome adverse events usually manage to find some meaning in their tragedy.
Psychiatrist and Holocaust survivor Viktor Frankl26 wrote of the importance of “meaning making.” Despite suffering for years in Nazi concentration camps, Frankl wrote that he gained the opportunity to exercise inner strength and be “brave, dignified and unselfish.” He struggled to survive because he came to believe that his suffering had a purpose: to live to teach others about his experiences.
Neuroimaging studies indicate that individuals who use cognitive reappraisal to deal with adversity have strong “top-down control” of emotions. They can modify their reaction to stress or trauma by activating the prefrontal cortex, which then modulates amygdalar response to the situation.27
CASE CONTINUED: Reappraisal
Although Ms. M wishes she had never been attacked and can find no rational explanation for it, she is weaving the event into the fabric of her life. She insists she has become stronger, wiser, and safer and wants to share her story with others.
Related resources
- National Center for Posttraumatic Stress Disorder. U.S. Department of Veterans Affairs. www.ncptsd.va.gov.
- The road to resilience. American Psychological Association Help Center. www.apahelpcenter.org/featuredtopics/feature.php?id=6.
- Positive Psychology Center. University of Pennsylvania. www.ppc.sas.upenn.edu.
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. M, age 24, works as a magazine editor in New York City. On a December evening, she walks out of the subway and heads to her boyfriend’s apartment, looking forward to unloading her heavy bag and checking her e-mail. Out of nowhere, a man runs up behind her and smashes a huge rock into her head.
She feels momentarily disconnected from her body and surroundings but manages to scream. As the assailant runs away, 2 girls rush to her aid.
Ms. M hurts everywhere. Her glasses have been knocked off, and her orbit is fractured; her eye will require multiple surgeries. She reaches for her cell phone, but it’s slippery with blood. A bystander dials 911, and paramedics arrive within minutes.
Most persons experience trauma during their lives,1 but not usually an attack as severe as Ms. M’s. Post-traumatic stress disorder (PTSD) and other psychopathologies are not inevitable or even common, however, developing in 8% to 12% of trauma survivors.2 Why are some individuals more resilient to trauma than others?
Resilience to stress is associated consistently with at least 6 psychosocial factors: active coping styles, regular physical exercise, a positive outlook, a moral compass, social support, and cognitive flexibility (Table 1). This article describes how motivated persons can enhance these “resilience factors” to become more resistant to everyday stressors and unexpected traumas.
Table 1
6 psychosocial factors that protect against
and aid recovery from posttraumatic stress
| Factor | Definition |
|---|---|
| Active coping style | Problem-solving and managing emotions that accompany stress; learning to face fears |
| Physical exercise | Engaging in physical activity to improve mood and health |
| Positive outlook | Using cognitive-behavioral strategies to enhance optimism and decrease pessimism; embracing humor |
| Moral compass | Developing and living by meaningful principles; putting them into action through altruism |
| Social support | Developing and nurturing friendships; seeking resilient role models and learning from them |
| Cognitive flexibility | Finding good in adverse situations; remaining flexible in one’s approach to solving problems |
1. Active coping style
Resilience is the process of adapting well to stress or trauma (Box 1).3-5 Learning to manage stressful situations requires active coping, which can be conceptualized as 2 types:
- “problem-focused” (working to solve the problem)
- “emotion-focused” (accepting and dealing with emotions caused by the stressor).
Undertaking and mastering difficult tasks appears to be effective in increasing resilience to stress. The “stress inoculation” hypothesis (Box 2)8-11 provides a plausible explanation for the observation that children who learn to cope with stress tend to become hardy adults. Successfully overcoming challenges improves self-confidence and also may alter the neurobiology of the stress response.
Prolonged-exposure therapy. PTSD development and maintenance depend in part on fear conditioning. By avoiding exposure to reminders of their trauma, survivors unwittingly solidify associations between traumatic triggers (people, places, or things that are reminders) and fear. Actively facing fears is necessary to break these associations.
Prolonged-exposure therapy was designed to help patients face their fears.12 As part of therapy, participants retell their trauma stories and engage in avoided activities in a safe environment. This treatment has been found to be highly effective in reducing PTSD symptoms, and its benefits often last longer than those conferred by pharmacologic interventions.13
CASE CONTINUED: Feeling ‘out of sync’
Ms. M remains frightened and angry after 2 months and is referred for psychological evaluation. She is diagnosed with PTSD based on her debilitating symptoms, including flashbacks, frightening nightmares, avoiding the subway, and feeling emotionally numb (which she describes as “being out of sync” with loved ones). Ms. M also complains of difficulty sleeping and irritability.
The therapist initiates prolonged-exposure treatment, including imaginal and in vivo exposure. In imaginal exposure, Ms. M tells and retells her trauma story in the safety of the therapist’s office. To desensitize herself to the memory, she listens to her recorded voice recounting her trauma. In vivo exposure involves homework, such as visiting the attack site during the day with a companion and talking with loved ones about the event. These assignments allow Ms. M to reclaim the life she lost because of severe anxiety and fear associated with anything related to the attack.
Within 3 months, Ms. M’s symptoms have improved and no longer meet DSM-IV-TR criteria for PTSD. She continues to struggle with insomnia, affective constriction, and a sense of social isolation—symptoms that often remit slowly, if at all, in trauma victims despite good treatment. She stays in therapy to work on confronting her fears and finding meaning in her experience.
2. Physical exercise
Exercise is a type of active coping that diminishes negative emotions caused by stress. Regular exercisers report less-frequent depression,14 and exercise has been shown to improve clinical depression in adults.15 Exercise builds physical and emotional hardiness, lifts mood, and improves memory. It produces these health benefits by:
- releasing endorphins and serotonin precursors
- attenuating basal hypothalamic-pituitary-adrenal axis activity
- promoting expression of neurotrophic and neuroprotective factors.16
CASE CONTINUED: Learning to self-soothe
Ms. M learns to read children’s stories to help her fall asleep at night and stave off nightmares. She takes up yoga to combat residual anxiety. She also resumes singing in her local chorus, which includes riding the subway home from rehearsals at 10 pm.
Resilience is the ability to maintain normal functioning despite adversity. It can be viewed as the successful operation of “basic human adaptational systems.” Conversely, depression and posttraumatic stress disorder (PTSD) may be understood, in part, as failure to adapt to stress.
Risk factors for PTSD. Traumas with the highest risk for psychopathology are severe, unpredictable, or uncontrollable and those that involve loss of property or (especially) a loved one, danger to self, or physical injury. PTSD risk also is increased by the cumulative effect of multiple, severe, uncontrollable traumas and personal factors such as:
3. A Positive outlook
Depressed individuals tend to view their problems as permanent and pervasive, whereas those who are resilient see adversity as temporary and limited in scope.
Role of dopamine. Humor and positive emotions have been linked to the dopaminergic reward mechanism in the mesolimbic circuitry. Dopaminergic neurons in the ventral tegmental area fire when a reward is received (Table 2); firing increases when a reward is unexpected or greater than expected. These same neurons release less dopamine when rewards are smaller than expected or not received at all.
Optimists are thought to have a robust dopaminergic response to reward, which is either hypersensitive to rewards and/or resistant to dysregulation under stressful (unrewarding) conditions.20
Undertaking and mastering difficult tasks appears to be an effective way to increase resilience to stress. The “stress inoculation” hypothesis provides a plausible explanation for the observation that children who learn to cope with stress become hardy adults.
Men and women who successfully managed stressful situations in childhood—including death or illness of a parent or sibling, family relocation, and loss of friendship—are more resistant to adulthood stressors, such as divorce, death or major illness of a loved one, and job loss.8 Conversely, individuals who experienced extreme childhood stress that they could not control or master—such as physical and/or sexual abuse—may be more vulnerable to future stressors.
Like vaccination? Organisms develop immunity after exposure to a pathogen’s attenuated form; similarly, they may develop resistance to stress after being exposed to and overcoming mild stressors.9 Immunity to stress is not specific to the type of stressor first encountered; early exposure to manageable stress appears to enhance resilience to many adverse experiences.
Neurobiology of resilience. In a series of studies, Special Forces soldiers had higher blood levels of 2 stress-protective hormones—neuropeptide Y (NPY) and dehydroepiandrosterone (DHEA)—immediately after high-stress interrogations than did soldiers who received lessintensive training.10 These hormones also correlated with better performance under stress.
NPY and DHEA help keep the stress response in check by inhibiting release of norepinephrine, cortisol, and other stress-related hormones under high-stress conditions.11 To what degree genetics, development, and/or training enhance NPY and DHEA release is not clear.
Table 2
Neurobiology of resilience:
Factors that influence physiologic stress response
| Selected neurobiological factors | Effect on stress response |
|---|---|
| Up-regulators | |
| Norepinephrine | Neurohormone and neurotransmitter released by the locus ceruleus in response to stress; sympathetic nervous system mediator; increases autonomic arousal (elevates blood pressure, heart rate); facilitates fear memory formation |
| Cortisol | Glucocorticoid released by adrenals in response to HPA axis activation by locus ceruleus; increases arousal, attention, and fear memory formation; initially adaptive, but prolonged/excess release has harmful systemic effects (hypertension, osteoporosis, immune suppression) |
| Down-regulators | |
| DHEA | Steroid released by adrenal cortex under stress; down-regulates stress response; has antiglucocorticoid activity; may protect against PTSD |
| NPY | Neuropeptide that counters locus ceruleus activity; blocks release of cortisol; anxiolytic |
| Galanin | Neuropeptide that counters locus ceruleus activity; anxiolytic |
| Other neurotransmitters | |
| Dopamine | Optimal levels enable reward system functioning; excess or deficit linked to learned helplessness and stress |
| Serotonin | Mixed effects, but high activity at 5HT1A receptors is linked to resilience |
| DHEA: dehydroepiandrosterone; HPA: hypothalamic-pituitary-adrenal; NPY: neuropeptide Y; PTSD: posttraumatic stress disorder | |
CASE CONTINUED: ‘I’m not bitter’
Ms. M can make an occasional joke about her attack and the massive stacks of paperwork she must sort through to pay medical bills and get reimbursed by insurance. She says, “I’m not bitter. I don’t want to carry that anger around for the rest of my life, so I won’t.”
4. A moral compass
Religious faith is associated with lower rates of depression in many populations, including college students, bereaved adults, and elderly hospitalized patients.21 Religious faith is not essential to a strong moral compass, however.
Morality appears to have a neural basis—a hypothesis supported by the observation that brain injury can damage one’s moral sense. “Acquired sociopathy” can result from trauma to certain brain areas, including the anterior prefrontal cortex and anterior temporal lobes.
‘Required helpfulness.’ Altruism—putting one’s moral compass into action—benefits the person who practices it and the person who receives it. Persons who help others perceive themselves as necessary and derive fulfillment. This phenomenon known as “required helpfulness” was first described during World War II, when those who cared for others after bombardments suffered less posttraumatic psychopathology than those who did not.22
Some individuals find healing in a “survivor mission” after personal tragedy, helping others cope with the same problem they faced. Mothers Against Drunk Driving—founded by mothers who lost children in car accidents—is one example.23
CASE CONTINUED: Altruism in action
Ms. M hopes to prevent attacks on other women. She participates in an organization that teaches women self-defense. She also speaks publicly for women’s safety and works with a local board to help defray crime survivors’ medical costs.
5. Social support
Individuals with strong social support tend to be more resilient than those without.24 Social support can reduce risk-taking behavior, encourage active coping, decrease loneliness, increase feelings of self-worth, and help a person put problems into perspective. A lack of social support correlates with depression, stress, and increased morbidity and mortality during medical illness.
Role models. People can learn to manage stress by mimicking the behavior of someone they respect. Many resilient adults credit a parent, grandparent, or other role model for teaching them to act honestly and inspiring them to be strong. In a study of 770 teenagers, those who had a strong nonparental mentor (such as a neighbor, teacher, or coach) reported less drug use and delinquency and a greater belief in the importance of school than those without such a mentor.25
CASE CONTINUED: Dad’s her role model
When she has bad days, Ms. M draws strength by thinking about her father, who has suffered much and whom she respects.
6. Cognitive flexibility
Being able to positively reframe negative events (“cognitive reappraisal”) is crucial to resilience. Individuals who successfully overcome adverse events usually manage to find some meaning in their tragedy.
Psychiatrist and Holocaust survivor Viktor Frankl26 wrote of the importance of “meaning making.” Despite suffering for years in Nazi concentration camps, Frankl wrote that he gained the opportunity to exercise inner strength and be “brave, dignified and unselfish.” He struggled to survive because he came to believe that his suffering had a purpose: to live to teach others about his experiences.
Neuroimaging studies indicate that individuals who use cognitive reappraisal to deal with adversity have strong “top-down control” of emotions. They can modify their reaction to stress or trauma by activating the prefrontal cortex, which then modulates amygdalar response to the situation.27
CASE CONTINUED: Reappraisal
Although Ms. M wishes she had never been attacked and can find no rational explanation for it, she is weaving the event into the fabric of her life. She insists she has become stronger, wiser, and safer and wants to share her story with others.
Related resources
- National Center for Posttraumatic Stress Disorder. U.S. Department of Veterans Affairs. www.ncptsd.va.gov.
- The road to resilience. American Psychological Association Help Center. www.apahelpcenter.org/featuredtopics/feature.php?id=6.
- Positive Psychology Center. University of Pennsylvania. www.ppc.sas.upenn.edu.
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Frans O, Rimmo PA, Aberg L, Fredrikson M. Trauma exposure and post-traumatic stress disorder in the general population. Acta Psychiatr Scand 2005;111(4):291-9.
2. Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry 1991;48(3):216-22.
3. Fauerbach JA, Lawrence JW, Schmidt CW, Jr, et al. Personality predictors of injury-related posttraumatic stress disorder. J Nerv Ment Dis 2000;188(8):510-7.
4. Masten AS. Ordinary magic. Resilience processes in development. Am Psychol 2001;56(3):227-38.
5. Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res 2006;40(1):1-21.
6. Park CL, Adler NE. Coping style as a predictor of health and well-being across the first year of medical school. Health Psychol 2003;22(6):627-31.
7. Muris P, Schmidt H, Lambrichs R, Meesters C. Protective and vulnerability factors of depression in normal adolescents. Behav Res Ther 2001;39(5):555-65.
8. Khoshaba DM, Maddi SR. Early experiences in hardiness development. Consulting Psychology Journal: Practice and Research 1999;51(2):106-16.
9. Rutter M. Resilience: some conceptual considerations. J Adolesc Health 1993;14(8):626-31,690-6.
10. Morgan CA, 3rd, Wang S, Southwick SM, et al. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry 2000;47(10):902-9.
11. Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci 1994;17(2):80-5.
12. Foa EB, Rothbaum BO. Treating the trauma of rape: cognitive behavioral therapy for PTSD. New York: Guilford Press; 1998.
13. Foa EB, Dancu CV, Hembree EA, et al. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol 1999;67(2):194-200.
14. Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002;32(12):741-60.
15. Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci 2001;56(8):M497-504.
16. Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002;25(6):295-301.
17. Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345-56.
18. Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol 2001;56(3):218-26.
19. Folkman S. Positive psychological states and coping with severe stress. Soc Sci Med 1997;45(8):1207-21.
20. Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 2004;161(2):195-216.
21. Koenig HG, George LK, Peterson BL. Religiosity and remission of depression in medically ill older patients. Am J Psychiatry 1998;155(4):536-42.
22. Rachman S. The concept of required helpfulness. Behav Res Ther 1979;17(1):1-6.
23. Southwick SM, Vythilingham M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annual Review of Clinical Psychology 2005;1:255-91.
24. Resick PA. Clinical psychology: a modular course. Philadelphia: Taylor & Francis Group; 2001.
25. Rhodes JE, Roffman J, Grossman JB. The rhetoric and reality of youth mentoring. In: Rhodes JE, ed. New directions in youth development: theory, practice, and research—a critical view of youth mentoring. San Francisco: Jossey-Bass; 2002: 9-20.
26. Frankl VE. Man’s search for meaning. Boston: Beacon Press; 1959:75-7.
27. Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 2004;23(2):483-99.
1. Frans O, Rimmo PA, Aberg L, Fredrikson M. Trauma exposure and post-traumatic stress disorder in the general population. Acta Psychiatr Scand 2005;111(4):291-9.
2. Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry 1991;48(3):216-22.
3. Fauerbach JA, Lawrence JW, Schmidt CW, Jr, et al. Personality predictors of injury-related posttraumatic stress disorder. J Nerv Ment Dis 2000;188(8):510-7.
4. Masten AS. Ordinary magic. Resilience processes in development. Am Psychol 2001;56(3):227-38.
5. Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res 2006;40(1):1-21.
6. Park CL, Adler NE. Coping style as a predictor of health and well-being across the first year of medical school. Health Psychol 2003;22(6):627-31.
7. Muris P, Schmidt H, Lambrichs R, Meesters C. Protective and vulnerability factors of depression in normal adolescents. Behav Res Ther 2001;39(5):555-65.
8. Khoshaba DM, Maddi SR. Early experiences in hardiness development. Consulting Psychology Journal: Practice and Research 1999;51(2):106-16.
9. Rutter M. Resilience: some conceptual considerations. J Adolesc Health 1993;14(8):626-31,690-6.
10. Morgan CA, 3rd, Wang S, Southwick SM, et al. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry 2000;47(10):902-9.
11. Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci 1994;17(2):80-5.
12. Foa EB, Rothbaum BO. Treating the trauma of rape: cognitive behavioral therapy for PTSD. New York: Guilford Press; 1998.
13. Foa EB, Dancu CV, Hembree EA, et al. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol 1999;67(2):194-200.
14. Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002;32(12):741-60.
15. Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci 2001;56(8):M497-504.
16. Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002;25(6):295-301.
17. Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345-56.
18. Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol 2001;56(3):218-26.
19. Folkman S. Positive psychological states and coping with severe stress. Soc Sci Med 1997;45(8):1207-21.
20. Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 2004;161(2):195-216.
21. Koenig HG, George LK, Peterson BL. Religiosity and remission of depression in medically ill older patients. Am J Psychiatry 1998;155(4):536-42.
22. Rachman S. The concept of required helpfulness. Behav Res Ther 1979;17(1):1-6.
23. Southwick SM, Vythilingham M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annual Review of Clinical Psychology 2005;1:255-91.
24. Resick PA. Clinical psychology: a modular course. Philadelphia: Taylor & Francis Group; 2001.
25. Rhodes JE, Roffman J, Grossman JB. The rhetoric and reality of youth mentoring. In: Rhodes JE, ed. New directions in youth development: theory, practice, and research—a critical view of youth mentoring. San Francisco: Jossey-Bass; 2002: 9-20.
26. Frankl VE. Man’s search for meaning. Boston: Beacon Press; 1959:75-7.
27. Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 2004;23(2):483-99.
Online clinical resources: To pay or not to pay?
Why pay for diagnostic, treatment, and research information when the Internet offers scores of free resources? Which free references are best, and when are they more clinically beneficial than paid information?
This article reviews select free online references and—where applicable—compares them with fee-based resources.
Diagnostic resources
We often don’t look past DSM-IV-TR guidelines when forming a diagnosis. In such cases, BehaveNet’s simple yet comprehensive list of DSM-IV-TR criteria is handy (www.behavenet.com, click on “Diagnoses and criteria by category”).
For in-depth diagnostic guidelines, American Psychiatric Publishing (www.appi.org) offers its DSM-IV-TR Quick Reference for $29, and Skyscape (www.skyscape.com) offers the full DSM-IV-TR at $77. Both personal digital assistant (PDA)-based programs come in Palm OS and Pocket PC versions.
Treatment decision aids
Free drug information references such as PDR.net (www.pdr.net) and Epocrates (www.epocrates.com) can help with medication choices. Both services let you check interactions on several drugs at once, which is important when treating patients who are taking multiple medications.
PDR.net is free for U.S.-based physicians and prescribers and requires registration. Epocrates can be used without registering, but going through the free registration process will provide additional features such as medication cost estimates. For a fee, Epocrates will throw in premium features such as pill identification, clinical tables and guidelines, and medical calculators.
Both PDR.net and Epocrates offer free PDA versions (Epocrates started as a PDA-based service). Other PDA-based drug references charge for access, but in some cases the information is more comprehensive and accurate.
For cytochrome P-450 metabolism information, you could reference the metabolism sections of each drug information sheet. Alternatively:
- Indiana University offers a good free drug interaction table showing substrates, inhibitors, and inducers of CYP-450 isoenzymes (http://medicine.iupui.edu/flockhart).
- http://mhc.com/Cytochromes offers similar information and links to more CYP-450 resources.
Clinical trials. Numerous Web sites offer free access to evidence-based findings and randomized clinical trials. The National Institute of Mental Health, for example, offers free online information on:
- Sequenced Treatment Alternatives to Relieve Depression (STAR*D, www.nimh.nih.gov/healthinformation/stard.cfm)
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE, www.nimh.nih.gov/healthinformation/catie.cfm).
The sites describe these medication trials in a lucid question-and-answer format. Clinicians can use the study results to guide medication choices. The STAR*D site also lists percentages of treatment success and describes duration-of-treatment trials.
Online algorithms. Free drug treatment algorithms and guidelines based on literature reviews and expert consensus are available online:
- The Texas Medication Algorithm Project (www.dshs.state.tx.us/mhprograms/TMAPtoc.shtm) addresses depression, bipolar disorder, and schizophrenia treatment.
- The Harvard Psychopharmacology Algorithm Project (http://mhc.com/Algorithms) covers depression, schizophrenia, and anxiety in patients with substance abuse.
- The International Psychopharmacology Algorithm Project (www.ipap.org) covers schizophrenia, posttraumatic stress disorder, and generalized anxiety disorder.
Free psychiatric rating scales—such as the Patient Health Questionnaire-9 (PHQ-9, www.depression-primarycare.org/clinicians/toolkits/materials/forms/phq9)—can help you efficiently monitor the progress of patients who complete the self-report forms.
The Psychiatric Rating Scales Index (www.neurotransmitter.net/ratingscales.html) lists links to other rating scales and descriptions, and notes which scales are free and which must be purchased.
Access to clinical articles
Online abstracts. By subscribing to Medline services, academic institutions provide students and faculty access to journal articles.
If you don’t have Medline access, use the free National Institutes of Health PubMed database (www.pubmed.gov) to search for abstracts on a given topic. You can view abstracts to all types of articles or click on the “review” tab on the search results page to view only reviews.
Although abstracts are free, full-text access usually is not unless your institution has a site license for that journal or you have purchased online access to that publication. By reading the abstract, you often can tell whether the full article contains information relevant to your practice.
Evidence-based medicine. The Centre for Evidence Based Mental Health (www.cebmh.com, click on “Research”) lists links to reviews, clinical trial information, and resources for learning about evidence-based medicine. CEBMH posts references to articles but does not offer full-text access.
The Cochrane Collaboration (www.cochrane.org/reviews/en/topics/index.html) offers free online abstracts of systematic reviews of psychiatric treatments, but you need a paid subscription to access full articles.
News sites such as Psychiatric News (http://pn.psychiatryonline.org), Psychiatric Times (www.psychiatrictimes.com), Clinical Psychiatry News (www.clinicalpsychiatrynews.com), and Medscape Psychiatry (www.medscape.com/psychiatry) offer free full-text access to news updates and summaries of recent major papers and presentations. These summaries help you stay abreast of the literature, but they are not as detailed as the original sources.
You don’t need a subscription to request free electronic tables of contents (e-TOCs) from selected journals. Some e-TOCs list links to abstracts of all articles in the current issue.
Online clinical textbooks and journals. American Psychiatric Publishing’s online DSM Premium package (www.psychiatryonline.com) includes access to the Textbook of Clinical Psychiatry, 5 psychiatry journals, American Psychiatric Association (APA) practice guidelines, and the DSM Library. DSM Premium Plus also includes access to 3 PDA-based eBooks (DSM-IV-TR Quick Reference, DSM-IV-TR Differential Diagnosis, and APA Practice Guidelines Quick Reference). Packages cost $229 to $399 annually, depending on which package you choose and whether you are an APA member.
Open-source book technology allows users to contribute to and edit an online volume. Wikipedia (http://en.wikipedia.org), the prototypical open-source site, hosts a free online encyclopedia.
Giles1 in 2005 found the accuracy of science entries in Wikipedia comparable to similar entries in Encyclopaedia Britannica. Among 42 entries from both encyclopedias, researchers found on average 4 errors per entry with Wikipedia and 3 with Encyclopaedia Britannica.
One sister Wiki project, Wikibooks (http://en.wikibooks.org), is designed to encourage production of open-source textbooks. This has led to one fledgling psychiatry textbook (http://en.wikibooks.org/wiki/Psychiatry) and others soon could follow. The clinical accuracy of this Wiki-based psychiatry textbook is variable, though this could improve if more psychiatrists become involved with Wikibook peer review.
Reference
1. Giles J. Internet encyclopaedias go head to head. Nature 2005;438(7070):900-1. Available at: http://www.nature.com/news/2005/051212/full/438900a.html. Accessed February 14, 2007.
Why pay for diagnostic, treatment, and research information when the Internet offers scores of free resources? Which free references are best, and when are they more clinically beneficial than paid information?
This article reviews select free online references and—where applicable—compares them with fee-based resources.
Diagnostic resources
We often don’t look past DSM-IV-TR guidelines when forming a diagnosis. In such cases, BehaveNet’s simple yet comprehensive list of DSM-IV-TR criteria is handy (www.behavenet.com, click on “Diagnoses and criteria by category”).
For in-depth diagnostic guidelines, American Psychiatric Publishing (www.appi.org) offers its DSM-IV-TR Quick Reference for $29, and Skyscape (www.skyscape.com) offers the full DSM-IV-TR at $77. Both personal digital assistant (PDA)-based programs come in Palm OS and Pocket PC versions.
Treatment decision aids
Free drug information references such as PDR.net (www.pdr.net) and Epocrates (www.epocrates.com) can help with medication choices. Both services let you check interactions on several drugs at once, which is important when treating patients who are taking multiple medications.
PDR.net is free for U.S.-based physicians and prescribers and requires registration. Epocrates can be used without registering, but going through the free registration process will provide additional features such as medication cost estimates. For a fee, Epocrates will throw in premium features such as pill identification, clinical tables and guidelines, and medical calculators.
Both PDR.net and Epocrates offer free PDA versions (Epocrates started as a PDA-based service). Other PDA-based drug references charge for access, but in some cases the information is more comprehensive and accurate.
For cytochrome P-450 metabolism information, you could reference the metabolism sections of each drug information sheet. Alternatively:
- Indiana University offers a good free drug interaction table showing substrates, inhibitors, and inducers of CYP-450 isoenzymes (http://medicine.iupui.edu/flockhart).
- http://mhc.com/Cytochromes offers similar information and links to more CYP-450 resources.
Clinical trials. Numerous Web sites offer free access to evidence-based findings and randomized clinical trials. The National Institute of Mental Health, for example, offers free online information on:
- Sequenced Treatment Alternatives to Relieve Depression (STAR*D, www.nimh.nih.gov/healthinformation/stard.cfm)
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE, www.nimh.nih.gov/healthinformation/catie.cfm).
The sites describe these medication trials in a lucid question-and-answer format. Clinicians can use the study results to guide medication choices. The STAR*D site also lists percentages of treatment success and describes duration-of-treatment trials.
Online algorithms. Free drug treatment algorithms and guidelines based on literature reviews and expert consensus are available online:
- The Texas Medication Algorithm Project (www.dshs.state.tx.us/mhprograms/TMAPtoc.shtm) addresses depression, bipolar disorder, and schizophrenia treatment.
- The Harvard Psychopharmacology Algorithm Project (http://mhc.com/Algorithms) covers depression, schizophrenia, and anxiety in patients with substance abuse.
- The International Psychopharmacology Algorithm Project (www.ipap.org) covers schizophrenia, posttraumatic stress disorder, and generalized anxiety disorder.
Free psychiatric rating scales—such as the Patient Health Questionnaire-9 (PHQ-9, www.depression-primarycare.org/clinicians/toolkits/materials/forms/phq9)—can help you efficiently monitor the progress of patients who complete the self-report forms.
The Psychiatric Rating Scales Index (www.neurotransmitter.net/ratingscales.html) lists links to other rating scales and descriptions, and notes which scales are free and which must be purchased.
Access to clinical articles
Online abstracts. By subscribing to Medline services, academic institutions provide students and faculty access to journal articles.
If you don’t have Medline access, use the free National Institutes of Health PubMed database (www.pubmed.gov) to search for abstracts on a given topic. You can view abstracts to all types of articles or click on the “review” tab on the search results page to view only reviews.
Although abstracts are free, full-text access usually is not unless your institution has a site license for that journal or you have purchased online access to that publication. By reading the abstract, you often can tell whether the full article contains information relevant to your practice.
Evidence-based medicine. The Centre for Evidence Based Mental Health (www.cebmh.com, click on “Research”) lists links to reviews, clinical trial information, and resources for learning about evidence-based medicine. CEBMH posts references to articles but does not offer full-text access.
The Cochrane Collaboration (www.cochrane.org/reviews/en/topics/index.html) offers free online abstracts of systematic reviews of psychiatric treatments, but you need a paid subscription to access full articles.
News sites such as Psychiatric News (http://pn.psychiatryonline.org), Psychiatric Times (www.psychiatrictimes.com), Clinical Psychiatry News (www.clinicalpsychiatrynews.com), and Medscape Psychiatry (www.medscape.com/psychiatry) offer free full-text access to news updates and summaries of recent major papers and presentations. These summaries help you stay abreast of the literature, but they are not as detailed as the original sources.
You don’t need a subscription to request free electronic tables of contents (e-TOCs) from selected journals. Some e-TOCs list links to abstracts of all articles in the current issue.
Online clinical textbooks and journals. American Psychiatric Publishing’s online DSM Premium package (www.psychiatryonline.com) includes access to the Textbook of Clinical Psychiatry, 5 psychiatry journals, American Psychiatric Association (APA) practice guidelines, and the DSM Library. DSM Premium Plus also includes access to 3 PDA-based eBooks (DSM-IV-TR Quick Reference, DSM-IV-TR Differential Diagnosis, and APA Practice Guidelines Quick Reference). Packages cost $229 to $399 annually, depending on which package you choose and whether you are an APA member.
Open-source book technology allows users to contribute to and edit an online volume. Wikipedia (http://en.wikipedia.org), the prototypical open-source site, hosts a free online encyclopedia.
Giles1 in 2005 found the accuracy of science entries in Wikipedia comparable to similar entries in Encyclopaedia Britannica. Among 42 entries from both encyclopedias, researchers found on average 4 errors per entry with Wikipedia and 3 with Encyclopaedia Britannica.
One sister Wiki project, Wikibooks (http://en.wikibooks.org), is designed to encourage production of open-source textbooks. This has led to one fledgling psychiatry textbook (http://en.wikibooks.org/wiki/Psychiatry) and others soon could follow. The clinical accuracy of this Wiki-based psychiatry textbook is variable, though this could improve if more psychiatrists become involved with Wikibook peer review.
Why pay for diagnostic, treatment, and research information when the Internet offers scores of free resources? Which free references are best, and when are they more clinically beneficial than paid information?
This article reviews select free online references and—where applicable—compares them with fee-based resources.
Diagnostic resources
We often don’t look past DSM-IV-TR guidelines when forming a diagnosis. In such cases, BehaveNet’s simple yet comprehensive list of DSM-IV-TR criteria is handy (www.behavenet.com, click on “Diagnoses and criteria by category”).
For in-depth diagnostic guidelines, American Psychiatric Publishing (www.appi.org) offers its DSM-IV-TR Quick Reference for $29, and Skyscape (www.skyscape.com) offers the full DSM-IV-TR at $77. Both personal digital assistant (PDA)-based programs come in Palm OS and Pocket PC versions.
Treatment decision aids
Free drug information references such as PDR.net (www.pdr.net) and Epocrates (www.epocrates.com) can help with medication choices. Both services let you check interactions on several drugs at once, which is important when treating patients who are taking multiple medications.
PDR.net is free for U.S.-based physicians and prescribers and requires registration. Epocrates can be used without registering, but going through the free registration process will provide additional features such as medication cost estimates. For a fee, Epocrates will throw in premium features such as pill identification, clinical tables and guidelines, and medical calculators.
Both PDR.net and Epocrates offer free PDA versions (Epocrates started as a PDA-based service). Other PDA-based drug references charge for access, but in some cases the information is more comprehensive and accurate.
For cytochrome P-450 metabolism information, you could reference the metabolism sections of each drug information sheet. Alternatively:
- Indiana University offers a good free drug interaction table showing substrates, inhibitors, and inducers of CYP-450 isoenzymes (http://medicine.iupui.edu/flockhart).
- http://mhc.com/Cytochromes offers similar information and links to more CYP-450 resources.
Clinical trials. Numerous Web sites offer free access to evidence-based findings and randomized clinical trials. The National Institute of Mental Health, for example, offers free online information on:
- Sequenced Treatment Alternatives to Relieve Depression (STAR*D, www.nimh.nih.gov/healthinformation/stard.cfm)
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE, www.nimh.nih.gov/healthinformation/catie.cfm).
The sites describe these medication trials in a lucid question-and-answer format. Clinicians can use the study results to guide medication choices. The STAR*D site also lists percentages of treatment success and describes duration-of-treatment trials.
Online algorithms. Free drug treatment algorithms and guidelines based on literature reviews and expert consensus are available online:
- The Texas Medication Algorithm Project (www.dshs.state.tx.us/mhprograms/TMAPtoc.shtm) addresses depression, bipolar disorder, and schizophrenia treatment.
- The Harvard Psychopharmacology Algorithm Project (http://mhc.com/Algorithms) covers depression, schizophrenia, and anxiety in patients with substance abuse.
- The International Psychopharmacology Algorithm Project (www.ipap.org) covers schizophrenia, posttraumatic stress disorder, and generalized anxiety disorder.
Free psychiatric rating scales—such as the Patient Health Questionnaire-9 (PHQ-9, www.depression-primarycare.org/clinicians/toolkits/materials/forms/phq9)—can help you efficiently monitor the progress of patients who complete the self-report forms.
The Psychiatric Rating Scales Index (www.neurotransmitter.net/ratingscales.html) lists links to other rating scales and descriptions, and notes which scales are free and which must be purchased.
Access to clinical articles
Online abstracts. By subscribing to Medline services, academic institutions provide students and faculty access to journal articles.
If you don’t have Medline access, use the free National Institutes of Health PubMed database (www.pubmed.gov) to search for abstracts on a given topic. You can view abstracts to all types of articles or click on the “review” tab on the search results page to view only reviews.
Although abstracts are free, full-text access usually is not unless your institution has a site license for that journal or you have purchased online access to that publication. By reading the abstract, you often can tell whether the full article contains information relevant to your practice.
Evidence-based medicine. The Centre for Evidence Based Mental Health (www.cebmh.com, click on “Research”) lists links to reviews, clinical trial information, and resources for learning about evidence-based medicine. CEBMH posts references to articles but does not offer full-text access.
The Cochrane Collaboration (www.cochrane.org/reviews/en/topics/index.html) offers free online abstracts of systematic reviews of psychiatric treatments, but you need a paid subscription to access full articles.
News sites such as Psychiatric News (http://pn.psychiatryonline.org), Psychiatric Times (www.psychiatrictimes.com), Clinical Psychiatry News (www.clinicalpsychiatrynews.com), and Medscape Psychiatry (www.medscape.com/psychiatry) offer free full-text access to news updates and summaries of recent major papers and presentations. These summaries help you stay abreast of the literature, but they are not as detailed as the original sources.
You don’t need a subscription to request free electronic tables of contents (e-TOCs) from selected journals. Some e-TOCs list links to abstracts of all articles in the current issue.
Online clinical textbooks and journals. American Psychiatric Publishing’s online DSM Premium package (www.psychiatryonline.com) includes access to the Textbook of Clinical Psychiatry, 5 psychiatry journals, American Psychiatric Association (APA) practice guidelines, and the DSM Library. DSM Premium Plus also includes access to 3 PDA-based eBooks (DSM-IV-TR Quick Reference, DSM-IV-TR Differential Diagnosis, and APA Practice Guidelines Quick Reference). Packages cost $229 to $399 annually, depending on which package you choose and whether you are an APA member.
Open-source book technology allows users to contribute to and edit an online volume. Wikipedia (http://en.wikipedia.org), the prototypical open-source site, hosts a free online encyclopedia.
Giles1 in 2005 found the accuracy of science entries in Wikipedia comparable to similar entries in Encyclopaedia Britannica. Among 42 entries from both encyclopedias, researchers found on average 4 errors per entry with Wikipedia and 3 with Encyclopaedia Britannica.
One sister Wiki project, Wikibooks (http://en.wikibooks.org), is designed to encourage production of open-source textbooks. This has led to one fledgling psychiatry textbook (http://en.wikibooks.org/wiki/Psychiatry) and others soon could follow. The clinical accuracy of this Wiki-based psychiatry textbook is variable, though this could improve if more psychiatrists become involved with Wikibook peer review.
Reference
1. Giles J. Internet encyclopaedias go head to head. Nature 2005;438(7070):900-1. Available at: http://www.nature.com/news/2005/051212/full/438900a.html. Accessed February 14, 2007.
Reference
1. Giles J. Internet encyclopaedias go head to head. Nature 2005;438(7070):900-1. Available at: http://www.nature.com/news/2005/051212/full/438900a.html. Accessed February 14, 2007.
Thoughtful diagnoses: Not ‘checklist’ psychiatry
In our experience, psychiatry residents often are encouraged to present rich psychodynamic or biopsychosocial formulations,1 while diagnostic assessments are relegated to robotic statements about whether patients meet DSM-IV-TR criteria. This practice can lead to “checklist psychiatry.”2
However, thoughtfully invoking DSM criteria can enhance clinical acumen if the following conclusions are chosen and justified during patient assessments.
“This person meets diagnostic criteria, and I believe this is the correct diagnosis.”
Ask the resident to back up his or her conclusion that symptoms are “not due to another condition” and cause “significant distress or impairment” as required by DSM. Emphasize differential diagnosis and understanding illness impact and illness behaviors. Also ask the resident to explain why the patient is considered a reliable reporter of his or her experience.
“This person seems to meet criteria, but I do not believe the diagnosis is correct.”
Seeming to meet criteria is not the same as “having” a psychiatric diagnosis. Ask the resident to discuss alternate diagnoses and confounding factors in the patient’s presentation. Some patients overreport psychological distress to pursue secondary gain or because of idiosyncratic ways of experiencing distress. Likewise, some clinicians interpret too narrowly patients’ endorsements of symptoms and assume that patients share their definitions of terms such as depression and panic.3
“This person does not meet criteria, but I believe the disorder is present.”
This scenario often leads to a rapid “not otherwise specified” (NOS) diagnosis. However, if a patient has an incomplete yet longitudinally consistent and sufficiently severe version of a known syndrome, an NOS diagnosis is not clinically useful (research settings are a different story). Encourage the trainee to justify the diagnosis that he or she plans to treat.
“This person does not meet criteria, and I believe no disorder is present.”
Some people are not mentally ill; in fact, most are not. Yet most residents we supervise cannot recall the last time they diagnosed “no mental illness” or saw a supervisor do so. Adopt this practice, and give trainees overt permission to make this assessment.
1. Kassaw K, Gabbard GO. Creating a psychodynamic formulation from a clinical evaluation. Am J Psychiatry 2002;5:721-6.
2. Freudenreich O, Querques J, Kontos N. Checklist psychiatry’s effect on psychiatric education [letter]. Am J Psychiatry 2004;161(5):930.-
3. Kontos N, Freudenreich O, Querques J, Norris E. The consultation psychiatrist as effective physician. Gen Hosp Psychiatry 2003;25:20-3.
Dr. Kontos is associate director, consultation-liaison psychiatry, Cambridge Health Alliance, Cambridge, MA.
Dr. Freudenreich is director, first episode and early psychosis program, Massachusetts General Hospital, Boston, MA.
Dr. Querques is an assistant in psychiatry, Massachusetts General Hospital, Boston, MA.
In our experience, psychiatry residents often are encouraged to present rich psychodynamic or biopsychosocial formulations,1 while diagnostic assessments are relegated to robotic statements about whether patients meet DSM-IV-TR criteria. This practice can lead to “checklist psychiatry.”2
However, thoughtfully invoking DSM criteria can enhance clinical acumen if the following conclusions are chosen and justified during patient assessments.
“This person meets diagnostic criteria, and I believe this is the correct diagnosis.”
Ask the resident to back up his or her conclusion that symptoms are “not due to another condition” and cause “significant distress or impairment” as required by DSM. Emphasize differential diagnosis and understanding illness impact and illness behaviors. Also ask the resident to explain why the patient is considered a reliable reporter of his or her experience.
“This person seems to meet criteria, but I do not believe the diagnosis is correct.”
Seeming to meet criteria is not the same as “having” a psychiatric diagnosis. Ask the resident to discuss alternate diagnoses and confounding factors in the patient’s presentation. Some patients overreport psychological distress to pursue secondary gain or because of idiosyncratic ways of experiencing distress. Likewise, some clinicians interpret too narrowly patients’ endorsements of symptoms and assume that patients share their definitions of terms such as depression and panic.3
“This person does not meet criteria, but I believe the disorder is present.”
This scenario often leads to a rapid “not otherwise specified” (NOS) diagnosis. However, if a patient has an incomplete yet longitudinally consistent and sufficiently severe version of a known syndrome, an NOS diagnosis is not clinically useful (research settings are a different story). Encourage the trainee to justify the diagnosis that he or she plans to treat.
“This person does not meet criteria, and I believe no disorder is present.”
Some people are not mentally ill; in fact, most are not. Yet most residents we supervise cannot recall the last time they diagnosed “no mental illness” or saw a supervisor do so. Adopt this practice, and give trainees overt permission to make this assessment.
In our experience, psychiatry residents often are encouraged to present rich psychodynamic or biopsychosocial formulations,1 while diagnostic assessments are relegated to robotic statements about whether patients meet DSM-IV-TR criteria. This practice can lead to “checklist psychiatry.”2
However, thoughtfully invoking DSM criteria can enhance clinical acumen if the following conclusions are chosen and justified during patient assessments.
“This person meets diagnostic criteria, and I believe this is the correct diagnosis.”
Ask the resident to back up his or her conclusion that symptoms are “not due to another condition” and cause “significant distress or impairment” as required by DSM. Emphasize differential diagnosis and understanding illness impact and illness behaviors. Also ask the resident to explain why the patient is considered a reliable reporter of his or her experience.
“This person seems to meet criteria, but I do not believe the diagnosis is correct.”
Seeming to meet criteria is not the same as “having” a psychiatric diagnosis. Ask the resident to discuss alternate diagnoses and confounding factors in the patient’s presentation. Some patients overreport psychological distress to pursue secondary gain or because of idiosyncratic ways of experiencing distress. Likewise, some clinicians interpret too narrowly patients’ endorsements of symptoms and assume that patients share their definitions of terms such as depression and panic.3
“This person does not meet criteria, but I believe the disorder is present.”
This scenario often leads to a rapid “not otherwise specified” (NOS) diagnosis. However, if a patient has an incomplete yet longitudinally consistent and sufficiently severe version of a known syndrome, an NOS diagnosis is not clinically useful (research settings are a different story). Encourage the trainee to justify the diagnosis that he or she plans to treat.
“This person does not meet criteria, and I believe no disorder is present.”
Some people are not mentally ill; in fact, most are not. Yet most residents we supervise cannot recall the last time they diagnosed “no mental illness” or saw a supervisor do so. Adopt this practice, and give trainees overt permission to make this assessment.
1. Kassaw K, Gabbard GO. Creating a psychodynamic formulation from a clinical evaluation. Am J Psychiatry 2002;5:721-6.
2. Freudenreich O, Querques J, Kontos N. Checklist psychiatry’s effect on psychiatric education [letter]. Am J Psychiatry 2004;161(5):930.-
3. Kontos N, Freudenreich O, Querques J, Norris E. The consultation psychiatrist as effective physician. Gen Hosp Psychiatry 2003;25:20-3.
Dr. Kontos is associate director, consultation-liaison psychiatry, Cambridge Health Alliance, Cambridge, MA.
Dr. Freudenreich is director, first episode and early psychosis program, Massachusetts General Hospital, Boston, MA.
Dr. Querques is an assistant in psychiatry, Massachusetts General Hospital, Boston, MA.
1. Kassaw K, Gabbard GO. Creating a psychodynamic formulation from a clinical evaluation. Am J Psychiatry 2002;5:721-6.
2. Freudenreich O, Querques J, Kontos N. Checklist psychiatry’s effect on psychiatric education [letter]. Am J Psychiatry 2004;161(5):930.-
3. Kontos N, Freudenreich O, Querques J, Norris E. The consultation psychiatrist as effective physician. Gen Hosp Psychiatry 2003;25:20-3.
Dr. Kontos is associate director, consultation-liaison psychiatry, Cambridge Health Alliance, Cambridge, MA.
Dr. Freudenreich is director, first episode and early psychosis program, Massachusetts General Hospital, Boston, MA.
Dr. Querques is an assistant in psychiatry, Massachusetts General Hospital, Boston, MA.