User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Assessing perinatal anxiety: What to ask
Emerging data demonstrate that untreated perinatal anxiety is associated with negative outcomes, including an increased risk for suicide.1 A 2017 systematic review and meta-analysis that included 102 studies with a total of 221,974 women from 34 countries found that the prevalence of self-reported anxiety symptoms and any anxiety disorder was 22.9% and 15.2%, respectively, across the 3 trimesters.1 During pregnancy, anxiety disorders (eg, generalized anxiety disorder) and anxiety-related disorders (eg, obsessive-compulsive disorder [OCD] and posttraumatic stress disorder [PTSD]) can present as new illnesses or as a reoccurrence of an existing illness. Patients with pre-existing OCD may notice that the nature of their obsessions is changing. Women with pre-existing PTSD may have their symptoms triggered by pregnancy or delivery or may develop PTSD as a result of a traumatic delivery. Anxiety is frequently comorbid with depression, and high anxiety during pregnancy is one of the strongest risk factors for depression.1,2
In light of this data, awareness and recognition of perinatal anxiety is critical. In this article, we describe how to accurately assess perinatal anxiety by avoiding assumptions and asking key questions during the clinical interview.
Avoid these common assumptions
Assessment begins with avoiding assumptions typically associated with maternal mental health. One common assumption is that pregnancy is a joyous occasion for all women. Pregnancy can be a stressful time that has its own unique difficulties, including the potential to develop or have a relapse of a mental illness. Another assumption is that the only concern is “postpartum depression.” In actuality, a significant percentage of women will experience depression during their pregnancy (not just in the postpartum period), and many other psychiatric illnesses are common during the perinatal period, including anxiety disorders.
Conduct a focused interview
Risk factors associated with antenatal anxiety include2:
- previous history of mental illness (particularly a history of anxiety and depression and a history of psychiatric treatment)
- lack of partner or social support
- history of abuse or domestic violence
- unplanned or unwanted pregnancy
- adverse events in life and high perceived stress
- present/past pregnancy complications
- pregnancy loss.
Symptoms of anxiety. The presence of anxiety or worrying does not necessarily mean a mother has an anxiety disorder. Using the DSM-5 as a guide, we should use the questions outlined in the following sections to inquire about all of the symptoms related to a particular illness, the pervasiveness of these symptoms, and to what extent these symptoms impair a woman’s ability to function and carry out her usual activities.3
Past psychiatric history. Ask your patient the following: Have you previously experienced anxiety and/or depressive symptoms? Were those symptoms limited only to times when you were pregnant or postpartum? Were your symptoms severe enough to disrupt your life (job, school, relationships, ability to complete daily tasks)? What treatments were effective for your symptoms? What treatments were ineffective?3
Social factors. Learn more about your patient’s support systems by asking: Who do you consider to be part of your social support? How is your relationship with your social support? Are there challenges in your relationship with your friends, family, or partner? If yes, what are those challenges? Are there other children in the home, and do you have support for them? Is your home environment safe? Do you feel that you have what you need for the baby? What stressors are you currently experiencing? Do you attend support groups for expectant mothers? Are you engaged in perinatal care?3
Continue to: Given the high prevalence...
Given the high prevalence of interpersonal violence in women of reproductive age, all patients should be screened for this. The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women recommends screening for interpersonal violence at the first visit during the perinatal period, during each trimester, and at the postpartum visit (at minimum).4 Potential screening questions include (but are not limited to): Have you and/or your children ever been threatened by or felt afraid of your partner? When you argue with your partner, do either of you get physical? Has your partner ever physically hurt you (eg, hit, choked)? Do you feel safe at home? Do you have a safe place to go with resources you and your children will need in case of an emergency?4-6
Feelings toward pregnancy, past/current pregnancy complications, and pregnancy loss. Ask your patient: Was this pregnancy planned? How do you feel about your pregnancy? How do you see yourself as a mother? Do you currently have pregnancy complications and/or have had them in the past, and, if so, what are/were they? Have you lost a pregnancy? If so, what was that like? Do you have fears related to childbirth, and, if so, what are they?3
Intrusive thoughts about harming the baby. Intrusive thoughts are common in postpartum women with anxiety disorders, including OCD.7 Merely asking patients if they’ve had thoughts of harming their baby is incomplete and insufficient to assess for intrusive thoughts. This question does not distinguish between intrusive thoughts and homicidal ideation; this distinction is absolutely necessary given the difference in potential risk to the infant.
Intrusive thoughts are generally associated with a low risk of mothers acting on their thoughts. These thoughts are typically ego dystonic and, in the most severe form, can be distressing to an extent that they cause behavioral changes, such as avoiding bathing the infant, avoiding diaper changes, avoiding knives, or separating themselves from the infant.7 On the contrary, having homicidal ideation carries a higher risk for harm to the infant. Homicidal ideation may be seen in patients with co-occurring psychosis, poor reality testing, and delusions.5,7
Questions such as “Do you worry about harm coming to your baby?” “Do you worry about you causing harm to your baby?” and “Have you had an upsetting thought about harming your baby?” are more likely to reveal intrusive thoughts and prompt further exploration. Statements such as “Some people tell me that they have distressing thoughts about harm coming to their baby” can gently open the door to a having a dialogue about such thoughts. This dialogue is significantly important in making informed assessments as we develop comprehensive treatment plans.
1. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. B J Psychiatry. 2017;210(5):315-323.
2. Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62-77.
3. Kirby N, Kilsby A, Walker R. Assessing low mood during pregnancy. BMJ. 2019;366:I4584. doi: 10.1136/bmj.I4584
4. American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee opinion: Intimate partner violence. Number 518. February 2012. Accessed March 23, 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/02/intimate-partner-violence
5. Massachusetts Child Psychiatry Access Program for Moms Provider Toolkit. Accessed March 18, 2020. https://www.mcpapformoms.org/Docs/AdultProviderToolkit12.09.2019.pdf
6. Ashur ML. Asking about domestic violence: SAFE questions. JAMA. 1993;269(18):2367.
7. Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7(2):99-110.
Emerging data demonstrate that untreated perinatal anxiety is associated with negative outcomes, including an increased risk for suicide.1 A 2017 systematic review and meta-analysis that included 102 studies with a total of 221,974 women from 34 countries found that the prevalence of self-reported anxiety symptoms and any anxiety disorder was 22.9% and 15.2%, respectively, across the 3 trimesters.1 During pregnancy, anxiety disorders (eg, generalized anxiety disorder) and anxiety-related disorders (eg, obsessive-compulsive disorder [OCD] and posttraumatic stress disorder [PTSD]) can present as new illnesses or as a reoccurrence of an existing illness. Patients with pre-existing OCD may notice that the nature of their obsessions is changing. Women with pre-existing PTSD may have their symptoms triggered by pregnancy or delivery or may develop PTSD as a result of a traumatic delivery. Anxiety is frequently comorbid with depression, and high anxiety during pregnancy is one of the strongest risk factors for depression.1,2
In light of this data, awareness and recognition of perinatal anxiety is critical. In this article, we describe how to accurately assess perinatal anxiety by avoiding assumptions and asking key questions during the clinical interview.
Avoid these common assumptions
Assessment begins with avoiding assumptions typically associated with maternal mental health. One common assumption is that pregnancy is a joyous occasion for all women. Pregnancy can be a stressful time that has its own unique difficulties, including the potential to develop or have a relapse of a mental illness. Another assumption is that the only concern is “postpartum depression.” In actuality, a significant percentage of women will experience depression during their pregnancy (not just in the postpartum period), and many other psychiatric illnesses are common during the perinatal period, including anxiety disorders.
Conduct a focused interview
Risk factors associated with antenatal anxiety include2:
- previous history of mental illness (particularly a history of anxiety and depression and a history of psychiatric treatment)
- lack of partner or social support
- history of abuse or domestic violence
- unplanned or unwanted pregnancy
- adverse events in life and high perceived stress
- present/past pregnancy complications
- pregnancy loss.
Symptoms of anxiety. The presence of anxiety or worrying does not necessarily mean a mother has an anxiety disorder. Using the DSM-5 as a guide, we should use the questions outlined in the following sections to inquire about all of the symptoms related to a particular illness, the pervasiveness of these symptoms, and to what extent these symptoms impair a woman’s ability to function and carry out her usual activities.3
Past psychiatric history. Ask your patient the following: Have you previously experienced anxiety and/or depressive symptoms? Were those symptoms limited only to times when you were pregnant or postpartum? Were your symptoms severe enough to disrupt your life (job, school, relationships, ability to complete daily tasks)? What treatments were effective for your symptoms? What treatments were ineffective?3
Social factors. Learn more about your patient’s support systems by asking: Who do you consider to be part of your social support? How is your relationship with your social support? Are there challenges in your relationship with your friends, family, or partner? If yes, what are those challenges? Are there other children in the home, and do you have support for them? Is your home environment safe? Do you feel that you have what you need for the baby? What stressors are you currently experiencing? Do you attend support groups for expectant mothers? Are you engaged in perinatal care?3
Continue to: Given the high prevalence...
Given the high prevalence of interpersonal violence in women of reproductive age, all patients should be screened for this. The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women recommends screening for interpersonal violence at the first visit during the perinatal period, during each trimester, and at the postpartum visit (at minimum).4 Potential screening questions include (but are not limited to): Have you and/or your children ever been threatened by or felt afraid of your partner? When you argue with your partner, do either of you get physical? Has your partner ever physically hurt you (eg, hit, choked)? Do you feel safe at home? Do you have a safe place to go with resources you and your children will need in case of an emergency?4-6
Feelings toward pregnancy, past/current pregnancy complications, and pregnancy loss. Ask your patient: Was this pregnancy planned? How do you feel about your pregnancy? How do you see yourself as a mother? Do you currently have pregnancy complications and/or have had them in the past, and, if so, what are/were they? Have you lost a pregnancy? If so, what was that like? Do you have fears related to childbirth, and, if so, what are they?3
Intrusive thoughts about harming the baby. Intrusive thoughts are common in postpartum women with anxiety disorders, including OCD.7 Merely asking patients if they’ve had thoughts of harming their baby is incomplete and insufficient to assess for intrusive thoughts. This question does not distinguish between intrusive thoughts and homicidal ideation; this distinction is absolutely necessary given the difference in potential risk to the infant.
Intrusive thoughts are generally associated with a low risk of mothers acting on their thoughts. These thoughts are typically ego dystonic and, in the most severe form, can be distressing to an extent that they cause behavioral changes, such as avoiding bathing the infant, avoiding diaper changes, avoiding knives, or separating themselves from the infant.7 On the contrary, having homicidal ideation carries a higher risk for harm to the infant. Homicidal ideation may be seen in patients with co-occurring psychosis, poor reality testing, and delusions.5,7
Questions such as “Do you worry about harm coming to your baby?” “Do you worry about you causing harm to your baby?” and “Have you had an upsetting thought about harming your baby?” are more likely to reveal intrusive thoughts and prompt further exploration. Statements such as “Some people tell me that they have distressing thoughts about harm coming to their baby” can gently open the door to a having a dialogue about such thoughts. This dialogue is significantly important in making informed assessments as we develop comprehensive treatment plans.
Emerging data demonstrate that untreated perinatal anxiety is associated with negative outcomes, including an increased risk for suicide.1 A 2017 systematic review and meta-analysis that included 102 studies with a total of 221,974 women from 34 countries found that the prevalence of self-reported anxiety symptoms and any anxiety disorder was 22.9% and 15.2%, respectively, across the 3 trimesters.1 During pregnancy, anxiety disorders (eg, generalized anxiety disorder) and anxiety-related disorders (eg, obsessive-compulsive disorder [OCD] and posttraumatic stress disorder [PTSD]) can present as new illnesses or as a reoccurrence of an existing illness. Patients with pre-existing OCD may notice that the nature of their obsessions is changing. Women with pre-existing PTSD may have their symptoms triggered by pregnancy or delivery or may develop PTSD as a result of a traumatic delivery. Anxiety is frequently comorbid with depression, and high anxiety during pregnancy is one of the strongest risk factors for depression.1,2
In light of this data, awareness and recognition of perinatal anxiety is critical. In this article, we describe how to accurately assess perinatal anxiety by avoiding assumptions and asking key questions during the clinical interview.
Avoid these common assumptions
Assessment begins with avoiding assumptions typically associated with maternal mental health. One common assumption is that pregnancy is a joyous occasion for all women. Pregnancy can be a stressful time that has its own unique difficulties, including the potential to develop or have a relapse of a mental illness. Another assumption is that the only concern is “postpartum depression.” In actuality, a significant percentage of women will experience depression during their pregnancy (not just in the postpartum period), and many other psychiatric illnesses are common during the perinatal period, including anxiety disorders.
Conduct a focused interview
Risk factors associated with antenatal anxiety include2:
- previous history of mental illness (particularly a history of anxiety and depression and a history of psychiatric treatment)
- lack of partner or social support
- history of abuse or domestic violence
- unplanned or unwanted pregnancy
- adverse events in life and high perceived stress
- present/past pregnancy complications
- pregnancy loss.
Symptoms of anxiety. The presence of anxiety or worrying does not necessarily mean a mother has an anxiety disorder. Using the DSM-5 as a guide, we should use the questions outlined in the following sections to inquire about all of the symptoms related to a particular illness, the pervasiveness of these symptoms, and to what extent these symptoms impair a woman’s ability to function and carry out her usual activities.3
Past psychiatric history. Ask your patient the following: Have you previously experienced anxiety and/or depressive symptoms? Were those symptoms limited only to times when you were pregnant or postpartum? Were your symptoms severe enough to disrupt your life (job, school, relationships, ability to complete daily tasks)? What treatments were effective for your symptoms? What treatments were ineffective?3
Social factors. Learn more about your patient’s support systems by asking: Who do you consider to be part of your social support? How is your relationship with your social support? Are there challenges in your relationship with your friends, family, or partner? If yes, what are those challenges? Are there other children in the home, and do you have support for them? Is your home environment safe? Do you feel that you have what you need for the baby? What stressors are you currently experiencing? Do you attend support groups for expectant mothers? Are you engaged in perinatal care?3
Continue to: Given the high prevalence...
Given the high prevalence of interpersonal violence in women of reproductive age, all patients should be screened for this. The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women recommends screening for interpersonal violence at the first visit during the perinatal period, during each trimester, and at the postpartum visit (at minimum).4 Potential screening questions include (but are not limited to): Have you and/or your children ever been threatened by or felt afraid of your partner? When you argue with your partner, do either of you get physical? Has your partner ever physically hurt you (eg, hit, choked)? Do you feel safe at home? Do you have a safe place to go with resources you and your children will need in case of an emergency?4-6
Feelings toward pregnancy, past/current pregnancy complications, and pregnancy loss. Ask your patient: Was this pregnancy planned? How do you feel about your pregnancy? How do you see yourself as a mother? Do you currently have pregnancy complications and/or have had them in the past, and, if so, what are/were they? Have you lost a pregnancy? If so, what was that like? Do you have fears related to childbirth, and, if so, what are they?3
Intrusive thoughts about harming the baby. Intrusive thoughts are common in postpartum women with anxiety disorders, including OCD.7 Merely asking patients if they’ve had thoughts of harming their baby is incomplete and insufficient to assess for intrusive thoughts. This question does not distinguish between intrusive thoughts and homicidal ideation; this distinction is absolutely necessary given the difference in potential risk to the infant.
Intrusive thoughts are generally associated with a low risk of mothers acting on their thoughts. These thoughts are typically ego dystonic and, in the most severe form, can be distressing to an extent that they cause behavioral changes, such as avoiding bathing the infant, avoiding diaper changes, avoiding knives, or separating themselves from the infant.7 On the contrary, having homicidal ideation carries a higher risk for harm to the infant. Homicidal ideation may be seen in patients with co-occurring psychosis, poor reality testing, and delusions.5,7
Questions such as “Do you worry about harm coming to your baby?” “Do you worry about you causing harm to your baby?” and “Have you had an upsetting thought about harming your baby?” are more likely to reveal intrusive thoughts and prompt further exploration. Statements such as “Some people tell me that they have distressing thoughts about harm coming to their baby” can gently open the door to a having a dialogue about such thoughts. This dialogue is significantly important in making informed assessments as we develop comprehensive treatment plans.
1. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. B J Psychiatry. 2017;210(5):315-323.
2. Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62-77.
3. Kirby N, Kilsby A, Walker R. Assessing low mood during pregnancy. BMJ. 2019;366:I4584. doi: 10.1136/bmj.I4584
4. American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee opinion: Intimate partner violence. Number 518. February 2012. Accessed March 23, 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/02/intimate-partner-violence
5. Massachusetts Child Psychiatry Access Program for Moms Provider Toolkit. Accessed March 18, 2020. https://www.mcpapformoms.org/Docs/AdultProviderToolkit12.09.2019.pdf
6. Ashur ML. Asking about domestic violence: SAFE questions. JAMA. 1993;269(18):2367.
7. Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7(2):99-110.
1. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. B J Psychiatry. 2017;210(5):315-323.
2. Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62-77.
3. Kirby N, Kilsby A, Walker R. Assessing low mood during pregnancy. BMJ. 2019;366:I4584. doi: 10.1136/bmj.I4584
4. American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee opinion: Intimate partner violence. Number 518. February 2012. Accessed March 23, 2020. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/02/intimate-partner-violence
5. Massachusetts Child Psychiatry Access Program for Moms Provider Toolkit. Accessed March 18, 2020. https://www.mcpapformoms.org/Docs/AdultProviderToolkit12.09.2019.pdf
6. Ashur ML. Asking about domestic violence: SAFE questions. JAMA. 1993;269(18):2367.
7. Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7(2):99-110.
Systemic trauma in the Black community: My perspective as an Asian American
Being a physician gives me great privilege. However, this privilege did not start the moment I donned the white coat, but when I was born Asian American, to parents who hold advanced education degrees. It grew when our family moved to a White neighborhood and I was accepted into an elite college. For medical school and residency, I chose an academic program embedded in an urban setting that serves underprivileged minority communities. I entered psychiatry to facilitate healing. Yet as I read the headlines about people of color who had died at the hands of law enforcement, I found myself feeling overwhelmingly hopeless and numb.
In these individuals, I saw people who looked and lived just like the patients I chose to serve. But during this time, I did not see myself as the healer, but part of the system that brought pain and distress. As an Asian American, I identified with Tou Thao—the Asian American police officer involved in George Floyd’s death. In the medical community with which I identified, I found that ever-rising cases of COVID-19 were disproportionately affecting lower-income minority communities. In a polarizing world, I felt my Asian American identity prevented me from experiencing the pain and suffering Black communities faced. This was not my fight, and if it was, I was more immersed in the side that brought trauma to my patients. From a purely rational perspective, I had no right to feel sad. Intellectually, I felt unqualified to share in their pain, yet here I was, crying in my room.
An evolving transformation
As much as I wanted to take a break, training did not stop. A transformation occurred from an emerging awareness of the unique environment within which I was training and the intersection of who I knew myself to be. Serving in an urban program, I was given the opportunity for candid conversations with health professionals of color. I was humbled when Black colleagues proactively reached out to educate me about the historical context of these events and help me process them. I asked hard questions of my fellow residents who were Black, and listened to their answers and personal stories, which was difficult.
With my patients, I began to listen more intently and think about the systemic issues I had previously written off. One patient missed their appointment because public transportation was closed due to COVID-19. Another patient who was homeless was helped immensely by assistance with housing when he could no longer sleep at his place of residence. Really listening to him revealed that his street had become a common route for protests. With my therapy patient who experienced panic attacks listening to the news, I simply sat and grieved with them. I chose these interactions not because I was uniquely qualified, intelligent, or had any ability to change the trajectory of our country, but because they grew from me simply working in the context I chose and seeking the relationships I naturally sought.
How I define myself
As doctors, we accept the burden of caring for society’s ailments with the ultimate hope of celebrating triumph over the adversity of psychiatric illness. However, superseding our profession is the social system in which we live. I am part of a system that has historically caused trauma to some while benefitting others. Thus, between the calling of my practice and the country I practice in, I found a divergence. Once I accepted the truth of this system and the very personal way it affects me, my colleagues, and patients I serve, I was able to internally reconcile and rediscover hope. While I cannot change my experiences, advantages, or privilege, these facts do not change the reality that I am a citizen of the globe and human first. This realization is the silver lining of these perilous times; training among people of color who graciously included me in their experiences, and my willingness to listen and self-reflect. I now choose to define myself by what makes me similar to my patients instead of what isolates me from them. The tangible results of this deliberate step toward authenticity are renewed inspiration and joy.
For those of you who may have found yourself with no “ethnic home team” (or a desire for a new one), I leave you with this simple charge: Let your emotional reactions guide you to truth, and challenge yourself to process them with someone who doesn’t look like you. Leave your title at the door and embrace humility. You might be pleasantly surprised at the human you find when you look in the mirror.
Being a physician gives me great privilege. However, this privilege did not start the moment I donned the white coat, but when I was born Asian American, to parents who hold advanced education degrees. It grew when our family moved to a White neighborhood and I was accepted into an elite college. For medical school and residency, I chose an academic program embedded in an urban setting that serves underprivileged minority communities. I entered psychiatry to facilitate healing. Yet as I read the headlines about people of color who had died at the hands of law enforcement, I found myself feeling overwhelmingly hopeless and numb.
In these individuals, I saw people who looked and lived just like the patients I chose to serve. But during this time, I did not see myself as the healer, but part of the system that brought pain and distress. As an Asian American, I identified with Tou Thao—the Asian American police officer involved in George Floyd’s death. In the medical community with which I identified, I found that ever-rising cases of COVID-19 were disproportionately affecting lower-income minority communities. In a polarizing world, I felt my Asian American identity prevented me from experiencing the pain and suffering Black communities faced. This was not my fight, and if it was, I was more immersed in the side that brought trauma to my patients. From a purely rational perspective, I had no right to feel sad. Intellectually, I felt unqualified to share in their pain, yet here I was, crying in my room.
An evolving transformation
As much as I wanted to take a break, training did not stop. A transformation occurred from an emerging awareness of the unique environment within which I was training and the intersection of who I knew myself to be. Serving in an urban program, I was given the opportunity for candid conversations with health professionals of color. I was humbled when Black colleagues proactively reached out to educate me about the historical context of these events and help me process them. I asked hard questions of my fellow residents who were Black, and listened to their answers and personal stories, which was difficult.
With my patients, I began to listen more intently and think about the systemic issues I had previously written off. One patient missed their appointment because public transportation was closed due to COVID-19. Another patient who was homeless was helped immensely by assistance with housing when he could no longer sleep at his place of residence. Really listening to him revealed that his street had become a common route for protests. With my therapy patient who experienced panic attacks listening to the news, I simply sat and grieved with them. I chose these interactions not because I was uniquely qualified, intelligent, or had any ability to change the trajectory of our country, but because they grew from me simply working in the context I chose and seeking the relationships I naturally sought.
How I define myself
As doctors, we accept the burden of caring for society’s ailments with the ultimate hope of celebrating triumph over the adversity of psychiatric illness. However, superseding our profession is the social system in which we live. I am part of a system that has historically caused trauma to some while benefitting others. Thus, between the calling of my practice and the country I practice in, I found a divergence. Once I accepted the truth of this system and the very personal way it affects me, my colleagues, and patients I serve, I was able to internally reconcile and rediscover hope. While I cannot change my experiences, advantages, or privilege, these facts do not change the reality that I am a citizen of the globe and human first. This realization is the silver lining of these perilous times; training among people of color who graciously included me in their experiences, and my willingness to listen and self-reflect. I now choose to define myself by what makes me similar to my patients instead of what isolates me from them. The tangible results of this deliberate step toward authenticity are renewed inspiration and joy.
For those of you who may have found yourself with no “ethnic home team” (or a desire for a new one), I leave you with this simple charge: Let your emotional reactions guide you to truth, and challenge yourself to process them with someone who doesn’t look like you. Leave your title at the door and embrace humility. You might be pleasantly surprised at the human you find when you look in the mirror.
Being a physician gives me great privilege. However, this privilege did not start the moment I donned the white coat, but when I was born Asian American, to parents who hold advanced education degrees. It grew when our family moved to a White neighborhood and I was accepted into an elite college. For medical school and residency, I chose an academic program embedded in an urban setting that serves underprivileged minority communities. I entered psychiatry to facilitate healing. Yet as I read the headlines about people of color who had died at the hands of law enforcement, I found myself feeling overwhelmingly hopeless and numb.
In these individuals, I saw people who looked and lived just like the patients I chose to serve. But during this time, I did not see myself as the healer, but part of the system that brought pain and distress. As an Asian American, I identified with Tou Thao—the Asian American police officer involved in George Floyd’s death. In the medical community with which I identified, I found that ever-rising cases of COVID-19 were disproportionately affecting lower-income minority communities. In a polarizing world, I felt my Asian American identity prevented me from experiencing the pain and suffering Black communities faced. This was not my fight, and if it was, I was more immersed in the side that brought trauma to my patients. From a purely rational perspective, I had no right to feel sad. Intellectually, I felt unqualified to share in their pain, yet here I was, crying in my room.
An evolving transformation
As much as I wanted to take a break, training did not stop. A transformation occurred from an emerging awareness of the unique environment within which I was training and the intersection of who I knew myself to be. Serving in an urban program, I was given the opportunity for candid conversations with health professionals of color. I was humbled when Black colleagues proactively reached out to educate me about the historical context of these events and help me process them. I asked hard questions of my fellow residents who were Black, and listened to their answers and personal stories, which was difficult.
With my patients, I began to listen more intently and think about the systemic issues I had previously written off. One patient missed their appointment because public transportation was closed due to COVID-19. Another patient who was homeless was helped immensely by assistance with housing when he could no longer sleep at his place of residence. Really listening to him revealed that his street had become a common route for protests. With my therapy patient who experienced panic attacks listening to the news, I simply sat and grieved with them. I chose these interactions not because I was uniquely qualified, intelligent, or had any ability to change the trajectory of our country, but because they grew from me simply working in the context I chose and seeking the relationships I naturally sought.
How I define myself
As doctors, we accept the burden of caring for society’s ailments with the ultimate hope of celebrating triumph over the adversity of psychiatric illness. However, superseding our profession is the social system in which we live. I am part of a system that has historically caused trauma to some while benefitting others. Thus, between the calling of my practice and the country I practice in, I found a divergence. Once I accepted the truth of this system and the very personal way it affects me, my colleagues, and patients I serve, I was able to internally reconcile and rediscover hope. While I cannot change my experiences, advantages, or privilege, these facts do not change the reality that I am a citizen of the globe and human first. This realization is the silver lining of these perilous times; training among people of color who graciously included me in their experiences, and my willingness to listen and self-reflect. I now choose to define myself by what makes me similar to my patients instead of what isolates me from them. The tangible results of this deliberate step toward authenticity are renewed inspiration and joy.
For those of you who may have found yourself with no “ethnic home team” (or a desire for a new one), I leave you with this simple charge: Let your emotional reactions guide you to truth, and challenge yourself to process them with someone who doesn’t look like you. Leave your title at the door and embrace humility. You might be pleasantly surprised at the human you find when you look in the mirror.
Evidence-based medicine: It’s not a cookbook!
The term evidence-based medicine (EBM) has been derided by some as “cookbook medicine.” To others, EBM conjures up the efforts of describing interventions in terms of comparative effectiveness, drowning us in a deluge of “evidence-based” publications. The moniker has also been hijacked by companies to name their Health Economics and Outcomes research divisions. The spirit behind EBM is getting lost. EBM is not just about the evidence; it is about how we use it.1
In this commentary, we describe the concept of EBM and discuss teaching EBM to medical students and residents, its role in continuing medical education, and how it may be applied to practice, using a case scenario as a guide.
What is evidence-based medicine?
Sackett et al2 summed it best in an editorial published in the BMJ in 1996, where he emphasized decision-making in the care of individual patients. When making clinical decisions, using the best evidence available makes sense, but so does integrating individual clinical expertise and considering the individual patient’s preferences. Sackett et al2 warns about practice becoming tyrannized by evidence: “even excellent external evidence may be inapplicable to or inappropriate for an individual patient.” Clearly, EBM is not cookbook medicine.
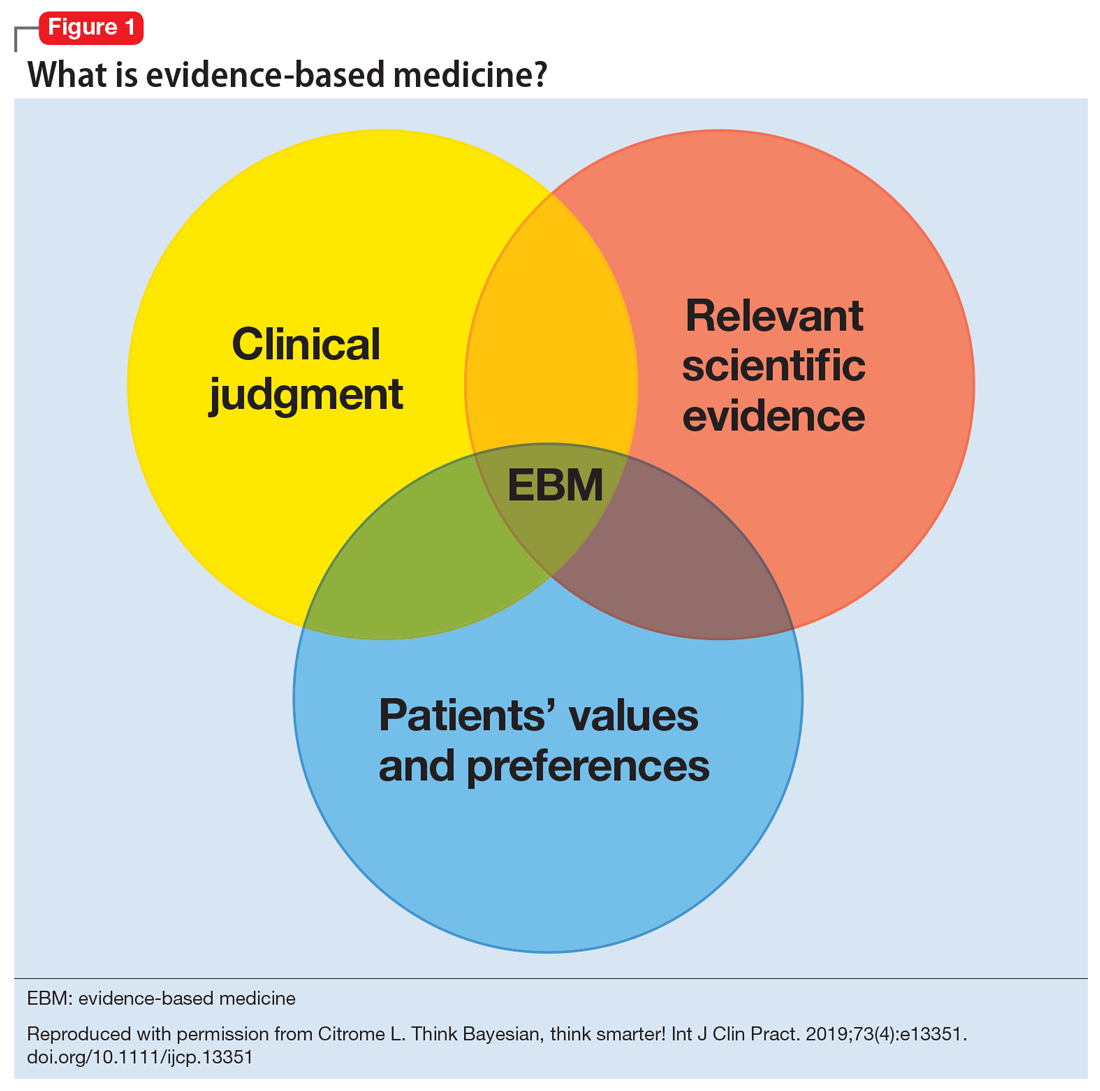
Figure 13 illustrates EBM as the confluence of clinical judgment, relevant scientific evidence, and patients’ values and preferences. The results from a clinical trial are only one part of the equation. As practitioners, we have the advantage of detailed knowledge about the patient, and our decisions are not “one size fits all.” Prior information about the patient dictates how we apply the evidence that supports potential interventions.
The concept of EBM was born out of necessity to bring scientific principles into the heart of medicine. As outlined by Sackett,4 the practice of EBM is a process of lifelong, self-directed learning in which caring for our own patients creates the need for clinically important information about diagnosis, prognosis, therapy, and other clinical and health care issues. Through EBM, we:
- convert these information needs into answerable questions
- track down, with maximum efficiency, the best evidence with which to answer questions (whether from clinical examination, diagnostic laboratory results, research evidence, or other sources)
- critically appraise that evidence for its validity (closeness to the truth) and usefulness (clinical applicability)
- integrate this appraisal with our clinical expertise and apply it in practice
- evaluate our performance.
Over the years, the original aim of EBM as a self-directed method for clinicians to practice high-quality medicine was morphed by some into a tool of enforced standardization and a boilerplate approach to managing costs across systems of care. As a result, the term EBM has been criticized because of:
- its reliance on empiricism
- a narrow definition of evidence
- a lack of evidence of efficacy
- its limited usefulness for individual patients
- threats to the autonomy of the doctor-patient relationship.
These 5 categories are associated with severe drawbacks when used for individual patient care.5 In addition to problems with applying standardized population research to a specific patient with a specific set of symptoms, medications, genetic variations, and unique environment, it can take years for clinicians to change their practices to incorporate new information.6
Continue to: Evidence that is too narrow...
Evidence that is too narrow in scope may not be useful. Single-molecule pharmaceutical clinical trials have erroneously become a synonym of EBM. Such studies do not reflect complex, real-life situations. Based on such studies, FDA product labeling can be inadequate in its guidance, particularly when faced with complex comorbidities. The standard comparison of active treatment to placebo is also seen as EBM, narrowing its scope and deflecting from clinical medicine when physicians measure one treatment’s success against another vs measuring real treatments against shams. Real-life treatment choice is frequently based on considering adverse effects as important to consider as therapeutic efficacy; however, this concept is outside of the common (mis)understanding of EBM.
Conflicting and ever-changing data and the push to replace clinical thinking with general dogmas trivializes medical practice and endangers treatment outcomes. This would not happen to the extent we see now if EBM was again seen as a guide and general direction rather than a blanket, distorted requirement to follow rigid recommendations for specific patients.
Insurance companies have driven a change in the understanding of EBM by using the FDA label as an excuse to deny, delay, and/or refuse to pay for treatments that are not explicitly and narrowly on-label. Dependence on on-label treatments is even more challenging in specialty medicine because primary care clinicians generally have tried the conventional approaches before referring patients to a specialist. However, insurance denials rarely differentiate between practice settings.
Medicolegal issues have cemented the present situation when clinically valid “off-label” treatments may be a reasonable consideration for patients but can place health care practitioners in jeopardy. The distorted EBM doctrine has become a justification for legal actions against clinicians who practice individualized medicine.
Concision bias (selectively focusing on information, losing nuance) and selection bias (patients in clinical trials who do not reflect real-life patients) have become an impediment to progress and EBM as originally intended.
Continue to: Training medical students and residents
Training medical students and residents
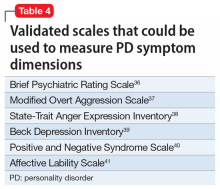
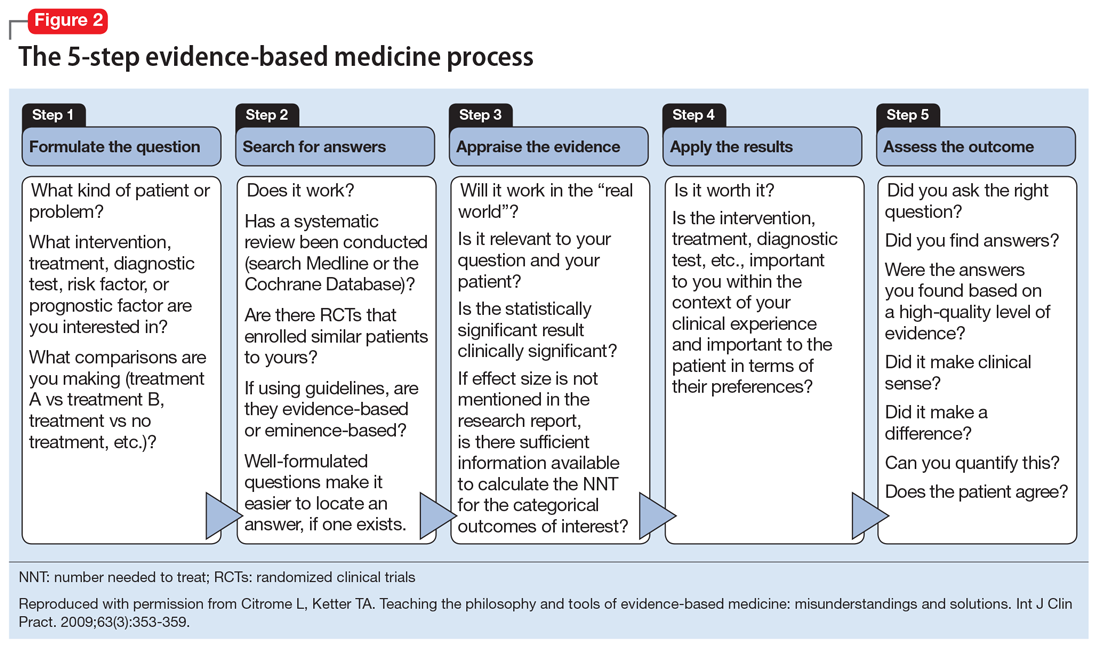
Although there is some variation in how EBM is taught to medical students and residents,7,8 the expectation is that such education occurs. The Accreditation Council for Graduate Medical Education requirements for a residency program state that “the program must advance residents’ knowledge and practice of the scholarly approach to evidence-based patient care.”9 The topic has been part of the American Society of Clinical Psychopharmacology Model Psychopharmacology Curriculum, but only in an optional lecture.10 The formal teaching of EBM includes how to find relevant biomedical publications for the clinical issues at hand, understand the different hierarchies of evidence, interpret results in terms of effect size, and apply this knowledge in the care of patients. This 5-step process is illustrated in Figure 28. See Related Resources for 3 books that provide a scholarly yet clinically relevant approach to EBM.
Continuing medical education
Most
Practical applications
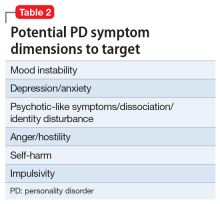
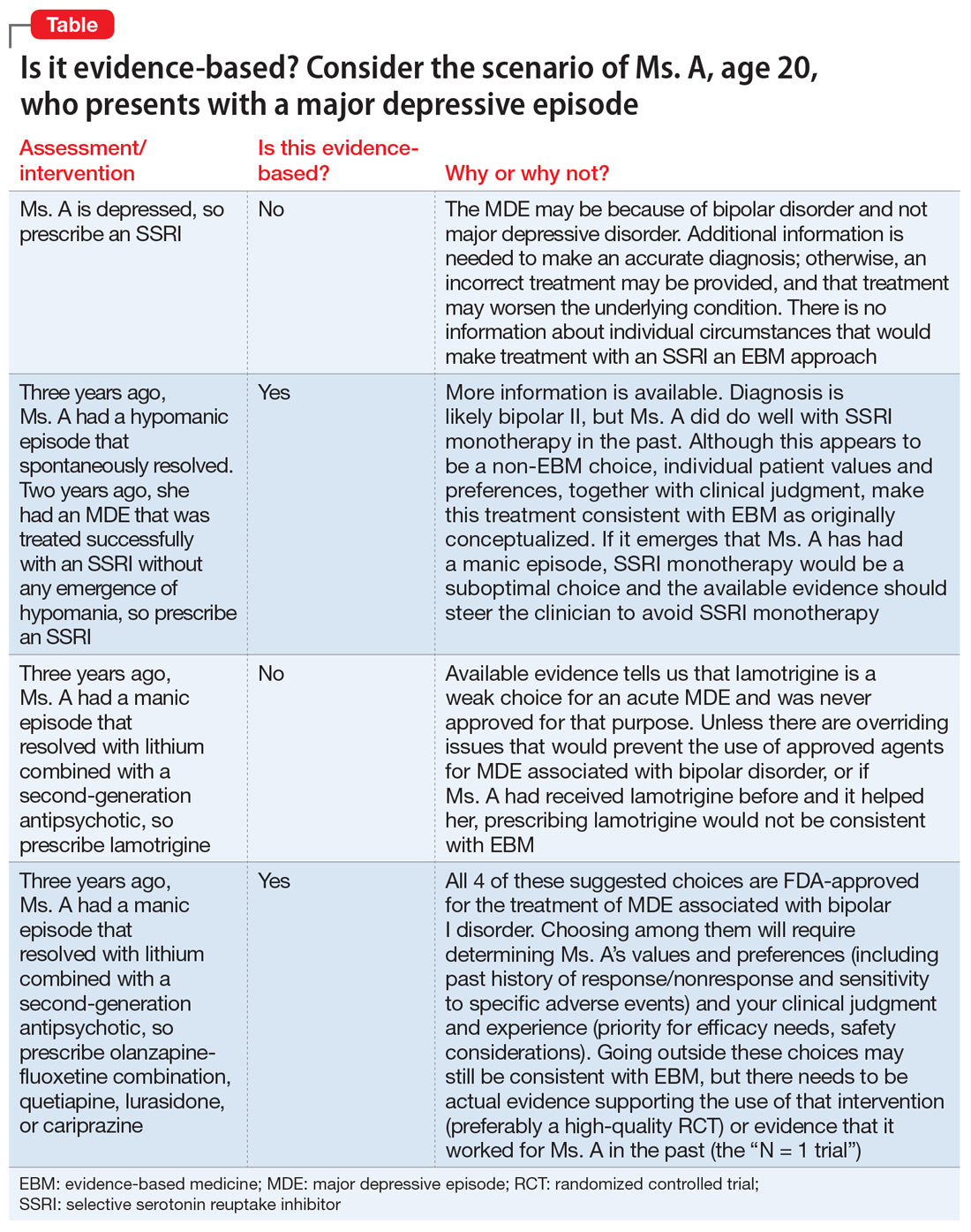
There are common clinical scenarios where evidence is ignored, or where it is overvalued. For example, the treatment of bipolar depression can be made worse with the use of antidepressants.14 Does this mean that antidepressants should never be used? What about patient history and preference? What if the approved agents fail to relieve symptoms or are not well tolerated? Available FDA-approved choices may not always be suitable.15 The Table illustrates some of these scenarios.
1. Citrome L. Evidence-based medicine: it’s not just about the evidence. Int J Clin Pract. 2011;65(6):634-635.
2. Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71.
3. Citrome L. Think Bayesian, think smarter! Int J Clin Pract. 2019;73(4):e13351. doi.org/10.1111/ijcp.13351
4. Sackett DL. Evidence-based medicine. Semin Perinatol. 1997;21(1):3-5.
5. Cohen AM, Stavri PZ, Hersh WR. A categorization and analysis of the criticisms of evidence-based medicine. Int J Med Inform. 2004;73(1):35-43.
6. Dutton DB. Worse than the disease: pitfalls of medical progress. Cambridge University Press; 1988.
7. Maggio LA. Educating physicians in evidence based medicine: current practices and curricular strategies. Perspect Med Educ. 2016;5(6):358-361.
8. Citrome L, Ketter TA. Teaching the philosophy and tools of evidence-based medicine: misunderstandings and solutions. Int J Clin Pract. 2009;63(3):353-359.
9. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Revised February 3, 2020. Accessed March 30, 2021. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRResidency2020.pdf
10. Citrome L, Ellison JM. Show me the evidence! Understanding the philosophy of evidence-based medicine and interpreting clinical trials. In: Glick ID, Macaluso M (Chair, Co-chair). ASCP model psychopharmacology curriculum for training directors and teachers of psychopharmacology in psychiatric residency programs, 10th ed. American Society of Clinical Psychopharmacology; 2019.
11. Citrome L. Interpreting and applying the CATIE results: with CATIE, context is key, when sorting out Phases 1, 1A, 1B, 2E, and 2T. Psychiatry (Edgmont). 2007;4(10):23-29.
12. Citrome L, Stroup TS. Schizophrenia, clinical antipsychotic trials of intervention effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract. 2006;60(8):933-940. doi: 10.1111/j.1742-1241.2006.01044.x
13. Citrome L. Dissecting clinical trials with ‘number needed to treat’. Current Psychiatry. 2007;6(3):66-71.
14. Goldberg JF, Freeman MP, Balon R, et al. The American Society of Clinical Psychopharmacology survey of psychopharmacologists’ practice patterns for the treatment of mood disorders. Depress Anxiety. 2015;32(8):605-613.
15. Citrome L. Food and Drug Administration-approved treatments for acute bipolar depression: what we have and what we need. J Clin Psychopharmacol. 2020;40(4):334-338.
The term evidence-based medicine (EBM) has been derided by some as “cookbook medicine.” To others, EBM conjures up the efforts of describing interventions in terms of comparative effectiveness, drowning us in a deluge of “evidence-based” publications. The moniker has also been hijacked by companies to name their Health Economics and Outcomes research divisions. The spirit behind EBM is getting lost. EBM is not just about the evidence; it is about how we use it.1
In this commentary, we describe the concept of EBM and discuss teaching EBM to medical students and residents, its role in continuing medical education, and how it may be applied to practice, using a case scenario as a guide.
What is evidence-based medicine?
Sackett et al2 summed it best in an editorial published in the BMJ in 1996, where he emphasized decision-making in the care of individual patients. When making clinical decisions, using the best evidence available makes sense, but so does integrating individual clinical expertise and considering the individual patient’s preferences. Sackett et al2 warns about practice becoming tyrannized by evidence: “even excellent external evidence may be inapplicable to or inappropriate for an individual patient.” Clearly, EBM is not cookbook medicine.
Figure 13 illustrates EBM as the confluence of clinical judgment, relevant scientific evidence, and patients’ values and preferences. The results from a clinical trial are only one part of the equation. As practitioners, we have the advantage of detailed knowledge about the patient, and our decisions are not “one size fits all.” Prior information about the patient dictates how we apply the evidence that supports potential interventions.

The concept of EBM was born out of necessity to bring scientific principles into the heart of medicine. As outlined by Sackett,4 the practice of EBM is a process of lifelong, self-directed learning in which caring for our own patients creates the need for clinically important information about diagnosis, prognosis, therapy, and other clinical and health care issues. Through EBM, we:
- convert these information needs into answerable questions
- track down, with maximum efficiency, the best evidence with which to answer questions (whether from clinical examination, diagnostic laboratory results, research evidence, or other sources)
- critically appraise that evidence for its validity (closeness to the truth) and usefulness (clinical applicability)
- integrate this appraisal with our clinical expertise and apply it in practice
- evaluate our performance.
Over the years, the original aim of EBM as a self-directed method for clinicians to practice high-quality medicine was morphed by some into a tool of enforced standardization and a boilerplate approach to managing costs across systems of care. As a result, the term EBM has been criticized because of:
- its reliance on empiricism
- a narrow definition of evidence
- a lack of evidence of efficacy
- its limited usefulness for individual patients
- threats to the autonomy of the doctor-patient relationship.
These 5 categories are associated with severe drawbacks when used for individual patient care.5 In addition to problems with applying standardized population research to a specific patient with a specific set of symptoms, medications, genetic variations, and unique environment, it can take years for clinicians to change their practices to incorporate new information.6
Continue to: Evidence that is too narrow...
Evidence that is too narrow in scope may not be useful. Single-molecule pharmaceutical clinical trials have erroneously become a synonym of EBM. Such studies do not reflect complex, real-life situations. Based on such studies, FDA product labeling can be inadequate in its guidance, particularly when faced with complex comorbidities. The standard comparison of active treatment to placebo is also seen as EBM, narrowing its scope and deflecting from clinical medicine when physicians measure one treatment’s success against another vs measuring real treatments against shams. Real-life treatment choice is frequently based on considering adverse effects as important to consider as therapeutic efficacy; however, this concept is outside of the common (mis)understanding of EBM.
Conflicting and ever-changing data and the push to replace clinical thinking with general dogmas trivializes medical practice and endangers treatment outcomes. This would not happen to the extent we see now if EBM was again seen as a guide and general direction rather than a blanket, distorted requirement to follow rigid recommendations for specific patients.
Insurance companies have driven a change in the understanding of EBM by using the FDA label as an excuse to deny, delay, and/or refuse to pay for treatments that are not explicitly and narrowly on-label. Dependence on on-label treatments is even more challenging in specialty medicine because primary care clinicians generally have tried the conventional approaches before referring patients to a specialist. However, insurance denials rarely differentiate between practice settings.
Medicolegal issues have cemented the present situation when clinically valid “off-label” treatments may be a reasonable consideration for patients but can place health care practitioners in jeopardy. The distorted EBM doctrine has become a justification for legal actions against clinicians who practice individualized medicine.
Concision bias (selectively focusing on information, losing nuance) and selection bias (patients in clinical trials who do not reflect real-life patients) have become an impediment to progress and EBM as originally intended.
Continue to: Training medical students and residents
Training medical students and residents
Although there is some variation in how EBM is taught to medical students and residents,7,8 the expectation is that such education occurs. The Accreditation Council for Graduate Medical Education requirements for a residency program state that “the program must advance residents’ knowledge and practice of the scholarly approach to evidence-based patient care.”9 The topic has been part of the American Society of Clinical Psychopharmacology Model Psychopharmacology Curriculum, but only in an optional lecture.10 The formal teaching of EBM includes how to find relevant biomedical publications for the clinical issues at hand, understand the different hierarchies of evidence, interpret results in terms of effect size, and apply this knowledge in the care of patients. This 5-step process is illustrated in Figure 28. See Related Resources for 3 books that provide a scholarly yet clinically relevant approach to EBM.

Continuing medical education
Most
Practical applications
There are common clinical scenarios where evidence is ignored, or where it is overvalued. For example, the treatment of bipolar depression can be made worse with the use of antidepressants.14 Does this mean that antidepressants should never be used? What about patient history and preference? What if the approved agents fail to relieve symptoms or are not well tolerated? Available FDA-approved choices may not always be suitable.15 The Table illustrates some of these scenarios.

The term evidence-based medicine (EBM) has been derided by some as “cookbook medicine.” To others, EBM conjures up the efforts of describing interventions in terms of comparative effectiveness, drowning us in a deluge of “evidence-based” publications. The moniker has also been hijacked by companies to name their Health Economics and Outcomes research divisions. The spirit behind EBM is getting lost. EBM is not just about the evidence; it is about how we use it.1
In this commentary, we describe the concept of EBM and discuss teaching EBM to medical students and residents, its role in continuing medical education, and how it may be applied to practice, using a case scenario as a guide.
What is evidence-based medicine?
Sackett et al2 summed it best in an editorial published in the BMJ in 1996, where he emphasized decision-making in the care of individual patients. When making clinical decisions, using the best evidence available makes sense, but so does integrating individual clinical expertise and considering the individual patient’s preferences. Sackett et al2 warns about practice becoming tyrannized by evidence: “even excellent external evidence may be inapplicable to or inappropriate for an individual patient.” Clearly, EBM is not cookbook medicine.
Figure 13 illustrates EBM as the confluence of clinical judgment, relevant scientific evidence, and patients’ values and preferences. The results from a clinical trial are only one part of the equation. As practitioners, we have the advantage of detailed knowledge about the patient, and our decisions are not “one size fits all.” Prior information about the patient dictates how we apply the evidence that supports potential interventions.

The concept of EBM was born out of necessity to bring scientific principles into the heart of medicine. As outlined by Sackett,4 the practice of EBM is a process of lifelong, self-directed learning in which caring for our own patients creates the need for clinically important information about diagnosis, prognosis, therapy, and other clinical and health care issues. Through EBM, we:
- convert these information needs into answerable questions
- track down, with maximum efficiency, the best evidence with which to answer questions (whether from clinical examination, diagnostic laboratory results, research evidence, or other sources)
- critically appraise that evidence for its validity (closeness to the truth) and usefulness (clinical applicability)
- integrate this appraisal with our clinical expertise and apply it in practice
- evaluate our performance.
Over the years, the original aim of EBM as a self-directed method for clinicians to practice high-quality medicine was morphed by some into a tool of enforced standardization and a boilerplate approach to managing costs across systems of care. As a result, the term EBM has been criticized because of:
- its reliance on empiricism
- a narrow definition of evidence
- a lack of evidence of efficacy
- its limited usefulness for individual patients
- threats to the autonomy of the doctor-patient relationship.
These 5 categories are associated with severe drawbacks when used for individual patient care.5 In addition to problems with applying standardized population research to a specific patient with a specific set of symptoms, medications, genetic variations, and unique environment, it can take years for clinicians to change their practices to incorporate new information.6
Continue to: Evidence that is too narrow...
Evidence that is too narrow in scope may not be useful. Single-molecule pharmaceutical clinical trials have erroneously become a synonym of EBM. Such studies do not reflect complex, real-life situations. Based on such studies, FDA product labeling can be inadequate in its guidance, particularly when faced with complex comorbidities. The standard comparison of active treatment to placebo is also seen as EBM, narrowing its scope and deflecting from clinical medicine when physicians measure one treatment’s success against another vs measuring real treatments against shams. Real-life treatment choice is frequently based on considering adverse effects as important to consider as therapeutic efficacy; however, this concept is outside of the common (mis)understanding of EBM.
Conflicting and ever-changing data and the push to replace clinical thinking with general dogmas trivializes medical practice and endangers treatment outcomes. This would not happen to the extent we see now if EBM was again seen as a guide and general direction rather than a blanket, distorted requirement to follow rigid recommendations for specific patients.
Insurance companies have driven a change in the understanding of EBM by using the FDA label as an excuse to deny, delay, and/or refuse to pay for treatments that are not explicitly and narrowly on-label. Dependence on on-label treatments is even more challenging in specialty medicine because primary care clinicians generally have tried the conventional approaches before referring patients to a specialist. However, insurance denials rarely differentiate between practice settings.
Medicolegal issues have cemented the present situation when clinically valid “off-label” treatments may be a reasonable consideration for patients but can place health care practitioners in jeopardy. The distorted EBM doctrine has become a justification for legal actions against clinicians who practice individualized medicine.
Concision bias (selectively focusing on information, losing nuance) and selection bias (patients in clinical trials who do not reflect real-life patients) have become an impediment to progress and EBM as originally intended.
Continue to: Training medical students and residents
Training medical students and residents
Although there is some variation in how EBM is taught to medical students and residents,7,8 the expectation is that such education occurs. The Accreditation Council for Graduate Medical Education requirements for a residency program state that “the program must advance residents’ knowledge and practice of the scholarly approach to evidence-based patient care.”9 The topic has been part of the American Society of Clinical Psychopharmacology Model Psychopharmacology Curriculum, but only in an optional lecture.10 The formal teaching of EBM includes how to find relevant biomedical publications for the clinical issues at hand, understand the different hierarchies of evidence, interpret results in terms of effect size, and apply this knowledge in the care of patients. This 5-step process is illustrated in Figure 28. See Related Resources for 3 books that provide a scholarly yet clinically relevant approach to EBM.

Continuing medical education
Most
Practical applications
There are common clinical scenarios where evidence is ignored, or where it is overvalued. For example, the treatment of bipolar depression can be made worse with the use of antidepressants.14 Does this mean that antidepressants should never be used? What about patient history and preference? What if the approved agents fail to relieve symptoms or are not well tolerated? Available FDA-approved choices may not always be suitable.15 The Table illustrates some of these scenarios.

1. Citrome L. Evidence-based medicine: it’s not just about the evidence. Int J Clin Pract. 2011;65(6):634-635.
2. Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71.
3. Citrome L. Think Bayesian, think smarter! Int J Clin Pract. 2019;73(4):e13351. doi.org/10.1111/ijcp.13351
4. Sackett DL. Evidence-based medicine. Semin Perinatol. 1997;21(1):3-5.
5. Cohen AM, Stavri PZ, Hersh WR. A categorization and analysis of the criticisms of evidence-based medicine. Int J Med Inform. 2004;73(1):35-43.
6. Dutton DB. Worse than the disease: pitfalls of medical progress. Cambridge University Press; 1988.
7. Maggio LA. Educating physicians in evidence based medicine: current practices and curricular strategies. Perspect Med Educ. 2016;5(6):358-361.
8. Citrome L, Ketter TA. Teaching the philosophy and tools of evidence-based medicine: misunderstandings and solutions. Int J Clin Pract. 2009;63(3):353-359.
9. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Revised February 3, 2020. Accessed March 30, 2021. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRResidency2020.pdf
10. Citrome L, Ellison JM. Show me the evidence! Understanding the philosophy of evidence-based medicine and interpreting clinical trials. In: Glick ID, Macaluso M (Chair, Co-chair). ASCP model psychopharmacology curriculum for training directors and teachers of psychopharmacology in psychiatric residency programs, 10th ed. American Society of Clinical Psychopharmacology; 2019.
11. Citrome L. Interpreting and applying the CATIE results: with CATIE, context is key, when sorting out Phases 1, 1A, 1B, 2E, and 2T. Psychiatry (Edgmont). 2007;4(10):23-29.
12. Citrome L, Stroup TS. Schizophrenia, clinical antipsychotic trials of intervention effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract. 2006;60(8):933-940. doi: 10.1111/j.1742-1241.2006.01044.x
13. Citrome L. Dissecting clinical trials with ‘number needed to treat’. Current Psychiatry. 2007;6(3):66-71.
14. Goldberg JF, Freeman MP, Balon R, et al. The American Society of Clinical Psychopharmacology survey of psychopharmacologists’ practice patterns for the treatment of mood disorders. Depress Anxiety. 2015;32(8):605-613.
15. Citrome L. Food and Drug Administration-approved treatments for acute bipolar depression: what we have and what we need. J Clin Psychopharmacol. 2020;40(4):334-338.
1. Citrome L. Evidence-based medicine: it’s not just about the evidence. Int J Clin Pract. 2011;65(6):634-635.
2. Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71.
3. Citrome L. Think Bayesian, think smarter! Int J Clin Pract. 2019;73(4):e13351. doi.org/10.1111/ijcp.13351
4. Sackett DL. Evidence-based medicine. Semin Perinatol. 1997;21(1):3-5.
5. Cohen AM, Stavri PZ, Hersh WR. A categorization and analysis of the criticisms of evidence-based medicine. Int J Med Inform. 2004;73(1):35-43.
6. Dutton DB. Worse than the disease: pitfalls of medical progress. Cambridge University Press; 1988.
7. Maggio LA. Educating physicians in evidence based medicine: current practices and curricular strategies. Perspect Med Educ. 2016;5(6):358-361.
8. Citrome L, Ketter TA. Teaching the philosophy and tools of evidence-based medicine: misunderstandings and solutions. Int J Clin Pract. 2009;63(3):353-359.
9. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Revised February 3, 2020. Accessed March 30, 2021. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRResidency2020.pdf
10. Citrome L, Ellison JM. Show me the evidence! Understanding the philosophy of evidence-based medicine and interpreting clinical trials. In: Glick ID, Macaluso M (Chair, Co-chair). ASCP model psychopharmacology curriculum for training directors and teachers of psychopharmacology in psychiatric residency programs, 10th ed. American Society of Clinical Psychopharmacology; 2019.
11. Citrome L. Interpreting and applying the CATIE results: with CATIE, context is key, when sorting out Phases 1, 1A, 1B, 2E, and 2T. Psychiatry (Edgmont). 2007;4(10):23-29.
12. Citrome L, Stroup TS. Schizophrenia, clinical antipsychotic trials of intervention effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract. 2006;60(8):933-940. doi: 10.1111/j.1742-1241.2006.01044.x
13. Citrome L. Dissecting clinical trials with ‘number needed to treat’. Current Psychiatry. 2007;6(3):66-71.
14. Goldberg JF, Freeman MP, Balon R, et al. The American Society of Clinical Psychopharmacology survey of psychopharmacologists’ practice patterns for the treatment of mood disorders. Depress Anxiety. 2015;32(8):605-613.
15. Citrome L. Food and Drug Administration-approved treatments for acute bipolar depression: what we have and what we need. J Clin Psychopharmacol. 2020;40(4):334-338.
10 devastating consequences of psychotic relapses
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
‘Canceling’ obsolete terms
I wanted to thank Dr. Nasrallah for his most important editorial, “Let’s ‘cancel’ these obsolete terms in DSM” (From the Editor,
Robert Barris, MD
Nassau University Medical Center
East Meadow, New York
How sad! This is my reaction to reading Dr. Nasrallah’s January 2021 editorial. Although biological psychiatry is synonymous with brain neurotransmitters and psychopharmacology, absent from this perspective is the visible biology of the human organism, specifically Sigmund Freud’s discovery of the psychosexual development of the infant and child and Wilhelm Reich’s discovery of characterological and muscular armor. Medicine, a natural science, is founded and grounded in observation. Psychiatry, having ignored and eliminated (“canceled”) recognition of these readily observable phenomena essential to understanding psychiatric disorders, including neurosis and schizophrenia, allows Dr. Nasrallah to suggest we “cancel” what should be at the heart of psychiatric diagnosis and treatment. Sadly, this heart has been lost for decades.
Howard Chavis, MD
New York, New York
Dr. Nasrallah responds
Psychiatry, like all medical and scientific disciplines, must go through an ongoing renewal, including the update of its terminology, with or without a change in its concepts or principles. Anxiety is a more accurate description of clinical symptoms than neurosis, and psychosis spectrum is more accurate than schizophrenia. Besides the accuracy issue, “neurotic” and “schizophrenic” have unfortunately devolved into pejorative and stigmatizing terms. The lexicon of psychiatry has gone through seismic changes over the past several decades, as I described in a previous editorial.1 Psychiatry is a vibrant, constantly evolving biopsychosocial/clinical neuroscience, not a static descriptive discipline.
Reference
1. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
I found myself having difficulty with Dr. Nasrallah’s editorial about canceling “obsolete” terms. I agree that making a diagnosis of borderline or narcissistic personality disorder can be pejorative if the clinician is using it to manage their own unprocessed countertransference. While all behavior is brain-mediated, human behavior is influenced by psychological events great and small. I am concerned that you seem to be reducing personality trait disturbances to biological abnormality, pure and simple. Losing psychological understanding of patients while overexplaining behavior as pathological brain dysfunction risks losing why patients see us in the first place.
Michael Friedman, DO
Cherry Hill, New Jersey
Dr. Nasrallah responds
The renaming I suggest goes beyond countertransference. It has to do with scientific validity of the diagnostic construct. And yes, personality traits are heavily genetic, but with some modulation by environmental factors. I suggest reading the seminal works of Thomas J. Bouchard Jr., PhD, and Kenneth S. Kendler, MD, on identical twins reared together or apart for more details about the genetics of personality traits.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
I wanted to thank Dr. Nasrallah for his most important editorial, “Let’s ‘cancel’ these obsolete terms in DSM” (From the Editor,
Robert Barris, MD
Nassau University Medical Center
East Meadow, New York
How sad! This is my reaction to reading Dr. Nasrallah’s January 2021 editorial. Although biological psychiatry is synonymous with brain neurotransmitters and psychopharmacology, absent from this perspective is the visible biology of the human organism, specifically Sigmund Freud’s discovery of the psychosexual development of the infant and child and Wilhelm Reich’s discovery of characterological and muscular armor. Medicine, a natural science, is founded and grounded in observation. Psychiatry, having ignored and eliminated (“canceled”) recognition of these readily observable phenomena essential to understanding psychiatric disorders, including neurosis and schizophrenia, allows Dr. Nasrallah to suggest we “cancel” what should be at the heart of psychiatric diagnosis and treatment. Sadly, this heart has been lost for decades.
Howard Chavis, MD
New York, New York
Dr. Nasrallah responds
Psychiatry, like all medical and scientific disciplines, must go through an ongoing renewal, including the update of its terminology, with or without a change in its concepts or principles. Anxiety is a more accurate description of clinical symptoms than neurosis, and psychosis spectrum is more accurate than schizophrenia. Besides the accuracy issue, “neurotic” and “schizophrenic” have unfortunately devolved into pejorative and stigmatizing terms. The lexicon of psychiatry has gone through seismic changes over the past several decades, as I described in a previous editorial.1 Psychiatry is a vibrant, constantly evolving biopsychosocial/clinical neuroscience, not a static descriptive discipline.
Reference
1. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
I found myself having difficulty with Dr. Nasrallah’s editorial about canceling “obsolete” terms. I agree that making a diagnosis of borderline or narcissistic personality disorder can be pejorative if the clinician is using it to manage their own unprocessed countertransference. While all behavior is brain-mediated, human behavior is influenced by psychological events great and small. I am concerned that you seem to be reducing personality trait disturbances to biological abnormality, pure and simple. Losing psychological understanding of patients while overexplaining behavior as pathological brain dysfunction risks losing why patients see us in the first place.
Michael Friedman, DO
Cherry Hill, New Jersey
Dr. Nasrallah responds
The renaming I suggest goes beyond countertransference. It has to do with scientific validity of the diagnostic construct. And yes, personality traits are heavily genetic, but with some modulation by environmental factors. I suggest reading the seminal works of Thomas J. Bouchard Jr., PhD, and Kenneth S. Kendler, MD, on identical twins reared together or apart for more details about the genetics of personality traits.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
I wanted to thank Dr. Nasrallah for his most important editorial, “Let’s ‘cancel’ these obsolete terms in DSM” (From the Editor,
Robert Barris, MD
Nassau University Medical Center
East Meadow, New York
How sad! This is my reaction to reading Dr. Nasrallah’s January 2021 editorial. Although biological psychiatry is synonymous with brain neurotransmitters and psychopharmacology, absent from this perspective is the visible biology of the human organism, specifically Sigmund Freud’s discovery of the psychosexual development of the infant and child and Wilhelm Reich’s discovery of characterological and muscular armor. Medicine, a natural science, is founded and grounded in observation. Psychiatry, having ignored and eliminated (“canceled”) recognition of these readily observable phenomena essential to understanding psychiatric disorders, including neurosis and schizophrenia, allows Dr. Nasrallah to suggest we “cancel” what should be at the heart of psychiatric diagnosis and treatment. Sadly, this heart has been lost for decades.
Howard Chavis, MD
New York, New York
Dr. Nasrallah responds
Psychiatry, like all medical and scientific disciplines, must go through an ongoing renewal, including the update of its terminology, with or without a change in its concepts or principles. Anxiety is a more accurate description of clinical symptoms than neurosis, and psychosis spectrum is more accurate than schizophrenia. Besides the accuracy issue, “neurotic” and “schizophrenic” have unfortunately devolved into pejorative and stigmatizing terms. The lexicon of psychiatry has gone through seismic changes over the past several decades, as I described in a previous editorial.1 Psychiatry is a vibrant, constantly evolving biopsychosocial/clinical neuroscience, not a static descriptive discipline.
Reference
1. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
I found myself having difficulty with Dr. Nasrallah’s editorial about canceling “obsolete” terms. I agree that making a diagnosis of borderline or narcissistic personality disorder can be pejorative if the clinician is using it to manage their own unprocessed countertransference. While all behavior is brain-mediated, human behavior is influenced by psychological events great and small. I am concerned that you seem to be reducing personality trait disturbances to biological abnormality, pure and simple. Losing psychological understanding of patients while overexplaining behavior as pathological brain dysfunction risks losing why patients see us in the first place.
Michael Friedman, DO
Cherry Hill, New Jersey
Dr. Nasrallah responds
The renaming I suggest goes beyond countertransference. It has to do with scientific validity of the diagnostic construct. And yes, personality traits are heavily genetic, but with some modulation by environmental factors. I suggest reading the seminal works of Thomas J. Bouchard Jr., PhD, and Kenneth S. Kendler, MD, on identical twins reared together or apart for more details about the genetics of personality traits.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
Bright light therapy for bipolar depression: A review of 6 studies
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).
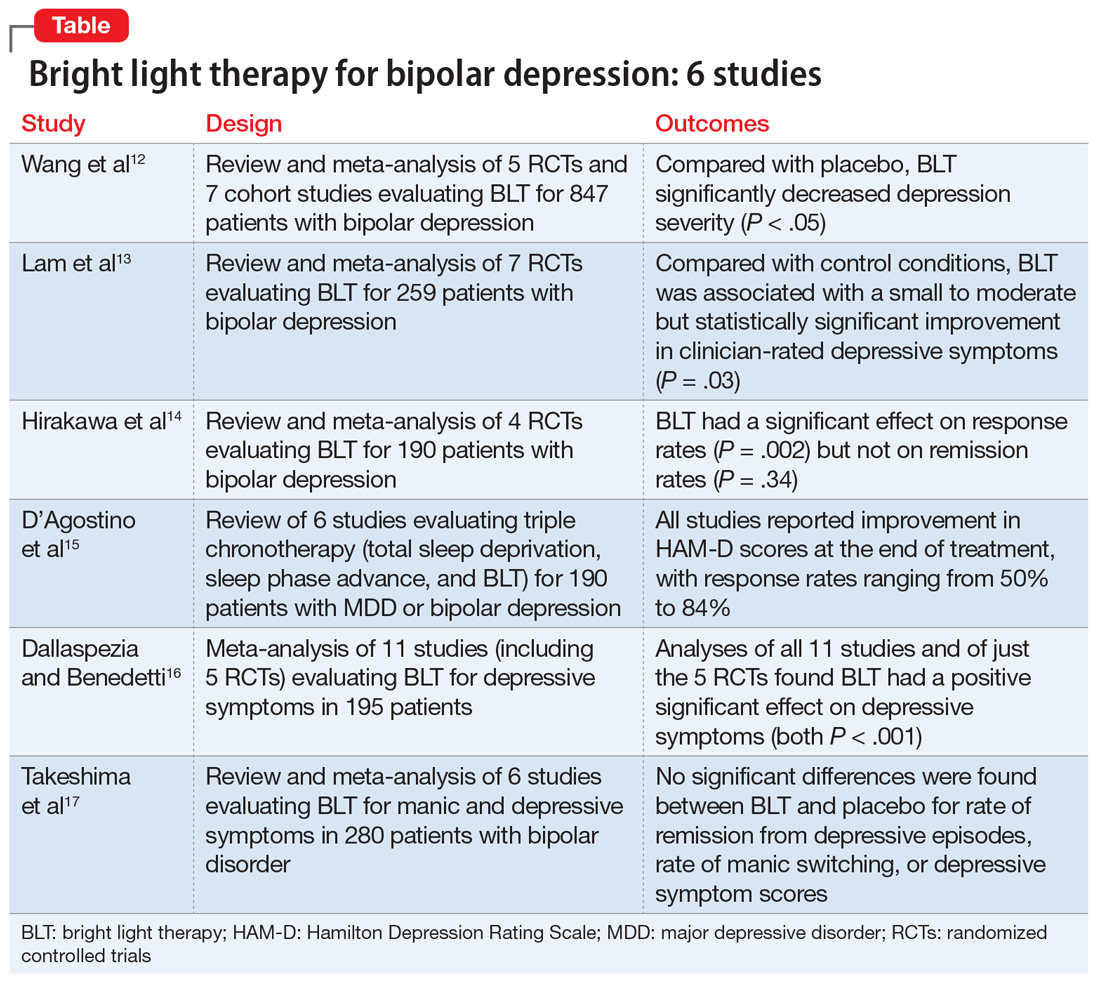
1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).

1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).

1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
ARISE to supportive psychotherapy
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
Supportive psychotherapy is a common type of therapy that often is used in combination with other modalities. By focusing on improving symptoms and accepting the patient’s limitations, it is particularly helpful for individuals who might have difficulty engaging in insight-oriented psychotherapies, such as those struggling with external stressors, including exposure to trauma, bereavement, physical disabilities, or socioeconomic challenges. Personal limitations, including severe personality disorder or intellectual disabilities, might also limit a patient’s ability to self-reflect on subconscious issues, which can lead to choosing a supportive modality.
While being supportive in the vernacular sense can be helpful, formal supportive psychotherapy employs well-defined goals and techniques.1 A therapist can facilitate progress by explicitly referring to these goals and techniques. The acronym ARISE can help therapists and other clinicians to use and appraise therapeutic progress toward these goals.
Alliance-building. The therapeutic alliance is an important predictor of the success of psychotherapy.2 Warmly encourage positive transference toward the therapist. The patient’s appreciation of the therapist’s empathic interactions can further the alliance. Paraphrasing the patient’s words can demonstrate and enhance empathy. Doing so allows clarification of the patient’s thoughts and helps the patient feel understood. Formulate and partner around shared therapeutic goals. Monitor the strength of the alliance and intervene if it is threatened. For example, if you misunderstand your patient and inadvertently offend them, apologizing may be helpful. In the face of disagreement between the patient and therapist, reorienting back to shared goals reinforces common ground.
Reduce anxiety and negative affect. In contrast to the caricature of the stiff psychoanalyst, the supportive therapist adopts an engaged conversational style to help the patient feel relaxed and to diminish the power differential between therapist and patient. If the patient appears uncomfortable with silence, maintaining the flow of conversation may reduce discomfort.1 Minimize your patient’s discomfort by approaching uncomfortable topics in manageable portions. Seek permission before introducing a subject that induces anxiety. Explain the reasoning behind approaching such topics.3 Reassurance and encouragement can further reduce anxiety.4 When not incongruous to the discussion, appropriate use of warm affect (eg, a smile) or even humor can elicit positive affect.
Increase awareness. Use psychoeducation and psychological interpretation (whether cognitive-behavioral or psychodynamic) to expand your patient’s awareness and help them understand their social contacts’ point of view. Clarification, gentle confrontation, and interpretation can make patients aware of biopsychosocial precipitants of distress.4
Strengthen coping mechanisms. Reinforce adaptive defense mechanisms, such as mature humor or suppression. Educating patients on practical organizational skills, problem-solving, relaxation techniques, and other relevant skills, can help them cope more effectively. Give advice only in limited circumstances, and when doing so, back up your advice with a rationale derived from your professional expertise. Because it is important for patients to realize that their life choices are their own, usually it is best to help the patient understand how they might come to their own decisions rather than to prescribe life choices in the form of advice.
Enhance self-esteem. Many patients in distress suffer from low self-esteem.5,6 Active encouragement and honest praise can nurture your patient’s ability to correct a distorted self-image and challenge self-reproach. Praise should not be false but reality-based. Praise can address preexisting strengths, highlight the patient’s willingness to express challenging material, or provide reinforcement on progress made toward treatment goals.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
1. Rothe, EM. Supportive psychotherapy in everyday clinical practice: it’s like riding a bicycle. Psychiatric Times. Published May 24, 2017. Accessed April 12, 2021. https://www.psychiatrictimes.com/view/supportive-psychotherapy-everyday-clinical-practice-its-riding-bicycle
2. Flückiger C, Del Re AC, Wampold BE, et al. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic). 2018;55(4):316-340.
3. Pine F. The interpretive moment. Variations on classical themes. Bull Menninger Clin. 1984;48(1), 54-71.
4. Grover S, Avasthi A, Jagiwala M. Clinical practice guidelines for practice of supportive psychotherapy. Indian J Psychiatry. 2020;62(Suppl 2):S173-S182.
5. Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14(3):297-314.
6. Zahn R, Lythe KE, Gethin JA, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. 2015;186:337-341.
A clinical approach to pharmacotherapy for personality disorders
DSM-5 defines personality disorders (PDs) as the presence of an enduring pattern of inner experience and behavior that “deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adulthood, is stable over time, and leads to distress or impairment.”1 As a general rule, PDs are not limited to episodes of illness, but reflect an individual’s long-term adjustment. These disorders occur in 10% to 15% of the general population; the rates are especially high in health care settings, in criminal offenders, and in those with a substance use disorder (SUD).2 PDs nearly always have an onset in adolescence or early adulthood and tend to diminish in severity with advancing age. They are associated with high rates of unemployment, homelessness, divorce and separation, domestic violence, substance misuse, and suicide.3
Psychotherapy is the first-line treatment for PDs, but there has been growing interest in using pharmacotherapy to treat PDs. While much of the PD treatment literature focuses on borderline PD,4-9 this article describes diagnosis, potential pharmacotherapy strategies, and methods to assess response to treatment for patients with all types of PDs.
Recognizing and diagnosing personality disorders
The diagnosis of a PD requires an understanding of DSM-5 criteria combined with a comprehensive psychiatric history and mental status examination. The patient’s history is the most important basis for diagnosing a PD.2 Collateral information from relatives or friends can help confirm the severity and pervasiveness of the individual’s personality problems. In some patients, long-term observation might be necessary to confirm the presence of a PD. Some clinicians are reluctant to diagnose PDs because of stigma, a problem common among patients with borderline PD.10,11
To screen for PDs, a clinician might ask the patient about problems with interpersonal relationships, sense of self, work, affect, impulse control, and reality testing. Table 112 lists general screening questions for the presence of a PD from the Iowa Personality Disorders Screen. Structured diagnostic interviews and self-report assessments could boost recognition of PDs, but these tools are rarely used outside of research settings.13,14
The PD clusters
DSM-5 divides 10 PDs into 3 clusters based on shared phenomenology and diagnostic criteria. Few patients have a “pure” case in which they meet criteria for only a single personality disorder.1
Cluster A. “Eccentric cluster” disorders are united by social aversion, a failure to form close attachments, or paranoia and suspiciousness.15 These include paranoid, schizoid, and schizotypal PD. Low self-awareness is typical. There are no treatment guidelines for these disorders, although there is some clinical trial data for schizotypal PD.
Cluster B. “Dramatic cluster” disorders share dramatic, emotional, and erratic characteristics.14 These include narcissistic, antisocial, borderline, and histrionic PD. Antisocial and narcissistic patients have low self-awareness. There are treatment guidelines for antisocial and borderline PD, and a variety of clinical trial data is available for the latter.15
Continue to: Cluster C
Cluster C. “Anxious cluster” disorders are united by anxiousness, fearfulness, and poor self-esteem. Many of these patients also display interpersonal rigidity.15 These disorders include avoidant, dependent, and obsessive-compulsive PD. There are no treatment guidelines or clinical trial data for these disorders.
Why consider pharmacotherapy for personality disorders?
The consensus among experts is that psychotherapy is the treatment of choice for PDs.15 Despite significant gaps in the evidence base, there has been a growing interest in using psychotropic medication to treat PDs. For example, research shows that >90% of patients with borderline PD are prescribed medication, most typically antidepressants, antipsychotics, mood stabilizers, stimulants, or sedative-hypnotics.16,17
Increased interest in pharmacotherapy for PDs could be related to research showing the importance of underlying neurobiology, particularly for antisocial and borderline PD.18,19 This work is complemented by genetic research showing the heritability of PD traits and disorders.20,21 Another factor could be renewed interest in dimensional approaches to the classification of PDs, as exemplified by DSM-5’s alternative model for PDs.1 This approach aligns with some expert recommendations to focus on treating PD symptom dimensions, rather than the syndrome itself.22
Importantly, no psychotropic medication is FDA-approved for the treatment of any PD. For that reason, prescribing medication for a PD is “off-label,” although prescribing a medication for a comorbid disorder for which the drug has an FDA-approved indication is not (eg, prescribing an antidepressant for major depressive disorder [MDD]).
Principles for prescribing
Despite gaps in research data, general principles for using medication to treat PDs have emerged from treatment guidelines for antisocial and borderline PD, clinical trial data, reviews and meta-analyses, and expert opinion. Clinicians should address the following considerations before prescribing medication to a patient with a PD.
Continue to: PD diagnosis
PD diagnosis. Has the patient been properly assessed and diagnosed? While history is the most important basis for diagnosis, the clinician should be familiar with the PDs and DSM-5 criteria. Has the patient been informed of the diagnosis and its implications for treatment?
Patient interest in medication. Is the patient interested in taking medication? Patients with borderline PD are often prescribed medication, but there are sparse data for the other PDs. The patient might have little interest in the PD diagnosis or its treatment.
Comorbidity. Has the patient been assessed for comorbid psychiatric disorders that could interfere with medication use (ie, an SUD) or might be a focus of treatment (eg, MDD)? Patients with PDs typically have significant comorbidity that a thorough evaluation will uncover.
PD symptom dimensions. Has the patient been assessed to determine cognitive or behavioral symptom dimensions of their PD? One or more symptom dimension(s) could be the focus of treatment. Table 2 lists examples of PD symptom dimensions.
Strategies to guide prescribing
Strategies to help guide prescribing include targeting any comorbid disorder(s), targeting important PD symptom dimensions (eg, impulsive aggression), choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself.
Continue to: Targeting comorbid disorders
Targeting comorbid disorders. National Institute for Health and Care Excellence guidelines for antisocial and borderline PD recommend that clinicians focus on treating comorbid disorders, a position echoed in Cochrane and other reviews.4,9,22-26 For example, a patient with borderline PD experiencing a major depressive episode could be treated with an antidepressant. Targeting the depressive symptoms could boost the patient’s mood, perhaps lessening the individual’s PD symptoms or reducing their severity.
Targeting important symptom dimensions. For patients with borderline PD, several guidelines and reviews have suggested that treatment should focus on emotional dysregulation and impulsive aggression (mood stabilizers, antipsychotics), or cognitive-perceptual symptoms (antipsychotics).4-6,15 There is some evidence that mood stabilizers or second-generation antipsychotics could help reduce impulsive aggression in patients with antisocial PD.27
Choosing medication based on similarity to another disorder known to respond to medication. Avoidant PD overlaps with social anxiety disorder and can be conceptualized as a chronic, pervasive social phobia. Avoidant PD might respond to a medication known to be effective for treating social anxiety disorder, such as a selective serotonin reuptake inhibitor (SSRI) or venlafaxine.28 Treating obsessive-compulsive PD with an SSRI is another example of this strategy, as 1 small study of fluvoxamine suggests.29 Obsessive-compulsive PD is common in persons with obsessive-compulsive disorder, and overlap includes preoccupation with orders, rules, and lists, and an inability to throw things out.
Targeting the PD syndrome. Another strategy is to target the PD itself. Clinical trial data suggest the antipsychotic risperidone can reduce the symptoms of schizotypal PD.30 Considering that this PD has a genetic association with schizophrenia, it is not surprising that the patient’s ideas of reference, odd communication, or transient paranoia might respond to an antipsychotic. Data from randomized controlled trials (RCTs) support the use of the second-generation antipsychotics aripiprazole and quetiapine to treat BPD.31,32 While older guidelines4,5 supported the use of the mood stabilizer lamotrigine, a recent RCT found that it was no more effective than placebo for borderline PD or its symptom dimensions.33
What to do before prescribing
Before writing a prescription, the clinician and patient should discuss the presence of a PD and the desirability of treatment. The patient should understand the limited evidence base and know that medication prescribed for a PD is off-label. The clinician should discuss medication selection and its rationale, and whether the medication is targeting a comorbid disorder, symptom dimension(s), or the PD itself. Additional considerations for prescribing for patients with PDs are listed in Table 3.34

Continue to: Avoid polypharmacy
Avoid polypharmacy. Many patients with borderline PD are prescribed multiple psychotropic medications.16,17 This approach leads to greater expense and more adverse effects, and is not evidence-based.
Avoid benzodiazepines. Many patients with borderline PD are prescribed benzodiazepines, often as part of a polypharmacy regimen. These drugs can cause disinhibition, thereby increasing acting-out behaviors and self-harm.35 Also, patients with PDs often have SUDs, which is a contraindication for benzodiazepine use.
Rate the patient’s improvement. Both the patient and clinician can benefit from monitoring symptomatic improvement. Several validated scales can be used to rate depression, anxiety, impulsivity, mood lability, anger, and aggression (Table 436-41).Some validated scales for borderline PD align with DSM-5 criteria. Two such widely used instruments are the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD)42 and the self-rated Borderline Evaluation of Severity Over Time (BEST).43 Each has questions that could be pulled to rate a symptom dimension of interest, such as affective instability, anger dyscontrol, or abandonment fears (Table 542,43).
A visual analog scale is easy to use and can target symptom dimensions of interest.44 For example, a clinician could use a visual analog scale to rate mood instability by asking a patient to rate their mood severity by making a mark along a 10-cm line (0 = “Most erratic emotions I have experienced,” 10 = “Most stable I have ever experienced my emotions to be”). This score can be recorded at baseline and subsequent visits.
Take-home points
PDs are common in the general population and health care settings. They are underrecognized by the general public and mental health professionals, often because of stigma. Clinicians could boost their recognition of these disorders by embedding simple screening questions in their patient assessments. Many patients with PDs will be interested in pharmacotherapy for their disorder or symptoms. Treatment strategies include targeting the comorbid disorder(s), targeting important PD symptom dimensions, choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself. Each strategy has its limitations and varying degrees of empirical support. Treatment response can be monitored using validated scales or a visual analog scale.
Continue to: Bottom Line
Bottom Line
Although psychotherapy is the first-line treatment and no medications are FDAapproved for treating personality disorders (PDs), there has been growing interest in using psychotropic medication to treat PDs. Strategies for pharmacotherapy include targeting comorbid disorders, PD symptom dimensions, or the PD itself. Choice of medication can be based on the similarity of the PD with another disorder known to respond to medication.
Related Resources
- Correa Da Costa S, Sanches M, Soares JC. Bipolar disorder or borderline personality disorder? Current Psychiatry. 2019;18(11):26-29,35-39.
- Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
Drug Brand Names
Aripiprazole • Abilify
Fluvoxamine • Luvox
Lamotrigine • Lamictal
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Black DW, Andreasen N. Personality disorders. In: Black DW, Andreasen N. Introductory textbook of psychiatry, 7th edition. American Psychiatric Publishing; 2020:410-423.
3. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord 2004;18(3):226-239.
4. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
5. Vita A, De Peri L, Sacchetti E. Antipsychotics, antidepressants, anticonvulsants, and placebo on the symptom dimensions of borderline personality disorder – a meta-analysis of randomized controlled and open-label trials. J Clin Psychopharmacol. 2011;31(5):613-624.
6. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep. 2015;17(1):534.
7. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31(5):345-356.
8. Black DW, Paris J, Schulz SC. Personality disorders: evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M, ed. Cognitive behavioral psychopharmacology: the clinical practice of evidence-based biopsychosocial integration. John Wiley & Sons; 2018:137-165.
9. Stoffers-Winterling J, Sorebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. 2020;22(8):37.
10. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
11. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
12. Langbehn DR, Pfohl BM, Reynolds S, et al. The Iowa Personality Disorder Screen: development and preliminary validation of a brief screening interview. J Pers Disord. 1999;13(1):75-89.
13. Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV). American Psychiatric Press; 1997.
14. First MB, Spitzer RL, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: multisite test-retest reliability study. J Pers Disord. 1995;9(2):92-104.
15. Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
16. Zanarini MC, Frankenburg FR, Reich DB, et al. Treatment rates for patients with borderline personality disorder and other personality disorders: a 16-year study. Psychiatr Serv. 2015;66(1):15-20.
17. Black DW, Allen J, McCormick B, et al. Treatment received by persons with BPD participating in a randomized clinical trial of the Systems Training for Emotional Predictability and Problem Solving programme. Person Ment Health. 2011;5(3):159-168.
18. Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behav Sci Law. 2008;26(1):65-83.
19. Ruocco AC, Amirthavasagam S, Choi-Kain LW, et al. Neural correlates of negative emotionality in BPD: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73(2):153-160.
20. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831.
21. Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158-162.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
23. National Institute for Health and Care Excellence (NICE). Borderline personality disorder: recognition and management. Clinical guideline [CG78]. Published January 2009. https://www.nice.org.uk/guidance/cg78
24. National Institute for Health and Care Excellence (NICE). Antisocial personality disorder: prevention and management. Clinical guideline [CG77]. Published January 2009. Updated March 27, 2013. https://www.nice.org.uk/guidance/cg77
25. Khalifa N, Duggan C, Stoffers J, et al. Pharmacologic interventions for antisocial personality disorder. Cochrane Database Syst Rep. 2010;(8):CD007667.
26. Stoffers JM, Völlm BA, Rücker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;2012(8):CD005652.
27. Black DW. The treatment of antisocial personality disorder. Current Treatment Options in Psychiatry. 2017. https://doi.org/10.1007/s40501-017-0123-z
28. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708-713.
29. Ansseau M. The obsessive-compulsive personality: diagnostic aspects and treatment possibilities. In: Den Boer JA, Westenberg HGM, eds. Focus on obsessive-compulsive spectrum disorders. Syn-Thesis; 1997:61-73.
30. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64(6):628-634.
31. Black DW, Zanarini MC, Romine A, et al. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2014;171(11):1174-1182.
32. Nickel MK, Muelbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2006;163(5):833-838.
33. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764.
34. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. 2004;65(12)1660-1665.
35. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder. Alprazolam, carbamazepine, trifluoperazine, and tranylcypromine. Arch Gen Psychiatry. 1988;45(2):111-119.
36. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812.
37. Ratey JJ, Gutheil CM. The measurement of aggressive behavior: reflections on the use of the Overt Aggression Scale and the Modified Overt Aggression Scale. J Neuropsychiatr Clin Neurosci. 1991;3(2):S57-S60.
38. Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers; 1999:993-1021.
39. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. Psychological Corp; 1996.
40. Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded Form. The University of Iowa; 1999.
41. Harvey D, Greenberg BR, Serper MR, et al. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45(5):786-793.
42. Zanarini MC, Vujanovic AA, Parachini EA, et al. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Person Disord. 2003:17(3):233-242.
43. Pfohl B, Blum N, St John D, et al. Reliability and validity of the Borderline Evaluation of Severity Over Time (BEST): a new scale to measure severity and change in borderline personality disorder. J Person Disord. 2009;23(3):281-293.
44. Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. 1997;31(5):569-579.
DSM-5 defines personality disorders (PDs) as the presence of an enduring pattern of inner experience and behavior that “deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adulthood, is stable over time, and leads to distress or impairment.”1 As a general rule, PDs are not limited to episodes of illness, but reflect an individual’s long-term adjustment. These disorders occur in 10% to 15% of the general population; the rates are especially high in health care settings, in criminal offenders, and in those with a substance use disorder (SUD).2 PDs nearly always have an onset in adolescence or early adulthood and tend to diminish in severity with advancing age. They are associated with high rates of unemployment, homelessness, divorce and separation, domestic violence, substance misuse, and suicide.3
Psychotherapy is the first-line treatment for PDs, but there has been growing interest in using pharmacotherapy to treat PDs. While much of the PD treatment literature focuses on borderline PD,4-9 this article describes diagnosis, potential pharmacotherapy strategies, and methods to assess response to treatment for patients with all types of PDs.
Recognizing and diagnosing personality disorders
The diagnosis of a PD requires an understanding of DSM-5 criteria combined with a comprehensive psychiatric history and mental status examination. The patient’s history is the most important basis for diagnosing a PD.2 Collateral information from relatives or friends can help confirm the severity and pervasiveness of the individual’s personality problems. In some patients, long-term observation might be necessary to confirm the presence of a PD. Some clinicians are reluctant to diagnose PDs because of stigma, a problem common among patients with borderline PD.10,11
To screen for PDs, a clinician might ask the patient about problems with interpersonal relationships, sense of self, work, affect, impulse control, and reality testing. Table 112 lists general screening questions for the presence of a PD from the Iowa Personality Disorders Screen. Structured diagnostic interviews and self-report assessments could boost recognition of PDs, but these tools are rarely used outside of research settings.13,14
The PD clusters
DSM-5 divides 10 PDs into 3 clusters based on shared phenomenology and diagnostic criteria. Few patients have a “pure” case in which they meet criteria for only a single personality disorder.1
Cluster A. “Eccentric cluster” disorders are united by social aversion, a failure to form close attachments, or paranoia and suspiciousness.15 These include paranoid, schizoid, and schizotypal PD. Low self-awareness is typical. There are no treatment guidelines for these disorders, although there is some clinical trial data for schizotypal PD.
Cluster B. “Dramatic cluster” disorders share dramatic, emotional, and erratic characteristics.14 These include narcissistic, antisocial, borderline, and histrionic PD. Antisocial and narcissistic patients have low self-awareness. There are treatment guidelines for antisocial and borderline PD, and a variety of clinical trial data is available for the latter.15
Continue to: Cluster C
Cluster C. “Anxious cluster” disorders are united by anxiousness, fearfulness, and poor self-esteem. Many of these patients also display interpersonal rigidity.15 These disorders include avoidant, dependent, and obsessive-compulsive PD. There are no treatment guidelines or clinical trial data for these disorders.
Why consider pharmacotherapy for personality disorders?
The consensus among experts is that psychotherapy is the treatment of choice for PDs.15 Despite significant gaps in the evidence base, there has been a growing interest in using psychotropic medication to treat PDs. For example, research shows that >90% of patients with borderline PD are prescribed medication, most typically antidepressants, antipsychotics, mood stabilizers, stimulants, or sedative-hypnotics.16,17
Increased interest in pharmacotherapy for PDs could be related to research showing the importance of underlying neurobiology, particularly for antisocial and borderline PD.18,19 This work is complemented by genetic research showing the heritability of PD traits and disorders.20,21 Another factor could be renewed interest in dimensional approaches to the classification of PDs, as exemplified by DSM-5’s alternative model for PDs.1 This approach aligns with some expert recommendations to focus on treating PD symptom dimensions, rather than the syndrome itself.22
Importantly, no psychotropic medication is FDA-approved for the treatment of any PD. For that reason, prescribing medication for a PD is “off-label,” although prescribing a medication for a comorbid disorder for which the drug has an FDA-approved indication is not (eg, prescribing an antidepressant for major depressive disorder [MDD]).
Principles for prescribing
Despite gaps in research data, general principles for using medication to treat PDs have emerged from treatment guidelines for antisocial and borderline PD, clinical trial data, reviews and meta-analyses, and expert opinion. Clinicians should address the following considerations before prescribing medication to a patient with a PD.
Continue to: PD diagnosis
PD diagnosis. Has the patient been properly assessed and diagnosed? While history is the most important basis for diagnosis, the clinician should be familiar with the PDs and DSM-5 criteria. Has the patient been informed of the diagnosis and its implications for treatment?
Patient interest in medication. Is the patient interested in taking medication? Patients with borderline PD are often prescribed medication, but there are sparse data for the other PDs. The patient might have little interest in the PD diagnosis or its treatment.
Comorbidity. Has the patient been assessed for comorbid psychiatric disorders that could interfere with medication use (ie, an SUD) or might be a focus of treatment (eg, MDD)? Patients with PDs typically have significant comorbidity that a thorough evaluation will uncover.
PD symptom dimensions. Has the patient been assessed to determine cognitive or behavioral symptom dimensions of their PD? One or more symptom dimension(s) could be the focus of treatment. Table 2 lists examples of PD symptom dimensions.
Strategies to guide prescribing
Strategies to help guide prescribing include targeting any comorbid disorder(s), targeting important PD symptom dimensions (eg, impulsive aggression), choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself.
Continue to: Targeting comorbid disorders
Targeting comorbid disorders. National Institute for Health and Care Excellence guidelines for antisocial and borderline PD recommend that clinicians focus on treating comorbid disorders, a position echoed in Cochrane and other reviews.4,9,22-26 For example, a patient with borderline PD experiencing a major depressive episode could be treated with an antidepressant. Targeting the depressive symptoms could boost the patient’s mood, perhaps lessening the individual’s PD symptoms or reducing their severity.
Targeting important symptom dimensions. For patients with borderline PD, several guidelines and reviews have suggested that treatment should focus on emotional dysregulation and impulsive aggression (mood stabilizers, antipsychotics), or cognitive-perceptual symptoms (antipsychotics).4-6,15 There is some evidence that mood stabilizers or second-generation antipsychotics could help reduce impulsive aggression in patients with antisocial PD.27
Choosing medication based on similarity to another disorder known to respond to medication. Avoidant PD overlaps with social anxiety disorder and can be conceptualized as a chronic, pervasive social phobia. Avoidant PD might respond to a medication known to be effective for treating social anxiety disorder, such as a selective serotonin reuptake inhibitor (SSRI) or venlafaxine.28 Treating obsessive-compulsive PD with an SSRI is another example of this strategy, as 1 small study of fluvoxamine suggests.29 Obsessive-compulsive PD is common in persons with obsessive-compulsive disorder, and overlap includes preoccupation with orders, rules, and lists, and an inability to throw things out.
Targeting the PD syndrome. Another strategy is to target the PD itself. Clinical trial data suggest the antipsychotic risperidone can reduce the symptoms of schizotypal PD.30 Considering that this PD has a genetic association with schizophrenia, it is not surprising that the patient’s ideas of reference, odd communication, or transient paranoia might respond to an antipsychotic. Data from randomized controlled trials (RCTs) support the use of the second-generation antipsychotics aripiprazole and quetiapine to treat BPD.31,32 While older guidelines4,5 supported the use of the mood stabilizer lamotrigine, a recent RCT found that it was no more effective than placebo for borderline PD or its symptom dimensions.33
What to do before prescribing
Before writing a prescription, the clinician and patient should discuss the presence of a PD and the desirability of treatment. The patient should understand the limited evidence base and know that medication prescribed for a PD is off-label. The clinician should discuss medication selection and its rationale, and whether the medication is targeting a comorbid disorder, symptom dimension(s), or the PD itself. Additional considerations for prescribing for patients with PDs are listed in Table 3.34

Continue to: Avoid polypharmacy
Avoid polypharmacy. Many patients with borderline PD are prescribed multiple psychotropic medications.16,17 This approach leads to greater expense and more adverse effects, and is not evidence-based.
Avoid benzodiazepines. Many patients with borderline PD are prescribed benzodiazepines, often as part of a polypharmacy regimen. These drugs can cause disinhibition, thereby increasing acting-out behaviors and self-harm.35 Also, patients with PDs often have SUDs, which is a contraindication for benzodiazepine use.
Rate the patient’s improvement. Both the patient and clinician can benefit from monitoring symptomatic improvement. Several validated scales can be used to rate depression, anxiety, impulsivity, mood lability, anger, and aggression (Table 436-41).Some validated scales for borderline PD align with DSM-5 criteria. Two such widely used instruments are the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD)42 and the self-rated Borderline Evaluation of Severity Over Time (BEST).43 Each has questions that could be pulled to rate a symptom dimension of interest, such as affective instability, anger dyscontrol, or abandonment fears (Table 542,43).
A visual analog scale is easy to use and can target symptom dimensions of interest.44 For example, a clinician could use a visual analog scale to rate mood instability by asking a patient to rate their mood severity by making a mark along a 10-cm line (0 = “Most erratic emotions I have experienced,” 10 = “Most stable I have ever experienced my emotions to be”). This score can be recorded at baseline and subsequent visits.
Take-home points
PDs are common in the general population and health care settings. They are underrecognized by the general public and mental health professionals, often because of stigma. Clinicians could boost their recognition of these disorders by embedding simple screening questions in their patient assessments. Many patients with PDs will be interested in pharmacotherapy for their disorder or symptoms. Treatment strategies include targeting the comorbid disorder(s), targeting important PD symptom dimensions, choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself. Each strategy has its limitations and varying degrees of empirical support. Treatment response can be monitored using validated scales or a visual analog scale.
Continue to: Bottom Line
Bottom Line
Although psychotherapy is the first-line treatment and no medications are FDAapproved for treating personality disorders (PDs), there has been growing interest in using psychotropic medication to treat PDs. Strategies for pharmacotherapy include targeting comorbid disorders, PD symptom dimensions, or the PD itself. Choice of medication can be based on the similarity of the PD with another disorder known to respond to medication.
Related Resources
- Correa Da Costa S, Sanches M, Soares JC. Bipolar disorder or borderline personality disorder? Current Psychiatry. 2019;18(11):26-29,35-39.
- Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
Drug Brand Names
Aripiprazole • Abilify
Fluvoxamine • Luvox
Lamotrigine • Lamictal
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
DSM-5 defines personality disorders (PDs) as the presence of an enduring pattern of inner experience and behavior that “deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adulthood, is stable over time, and leads to distress or impairment.”1 As a general rule, PDs are not limited to episodes of illness, but reflect an individual’s long-term adjustment. These disorders occur in 10% to 15% of the general population; the rates are especially high in health care settings, in criminal offenders, and in those with a substance use disorder (SUD).2 PDs nearly always have an onset in adolescence or early adulthood and tend to diminish in severity with advancing age. They are associated with high rates of unemployment, homelessness, divorce and separation, domestic violence, substance misuse, and suicide.3
Psychotherapy is the first-line treatment for PDs, but there has been growing interest in using pharmacotherapy to treat PDs. While much of the PD treatment literature focuses on borderline PD,4-9 this article describes diagnosis, potential pharmacotherapy strategies, and methods to assess response to treatment for patients with all types of PDs.
Recognizing and diagnosing personality disorders
The diagnosis of a PD requires an understanding of DSM-5 criteria combined with a comprehensive psychiatric history and mental status examination. The patient’s history is the most important basis for diagnosing a PD.2 Collateral information from relatives or friends can help confirm the severity and pervasiveness of the individual’s personality problems. In some patients, long-term observation might be necessary to confirm the presence of a PD. Some clinicians are reluctant to diagnose PDs because of stigma, a problem common among patients with borderline PD.10,11
To screen for PDs, a clinician might ask the patient about problems with interpersonal relationships, sense of self, work, affect, impulse control, and reality testing. Table 112 lists general screening questions for the presence of a PD from the Iowa Personality Disorders Screen. Structured diagnostic interviews and self-report assessments could boost recognition of PDs, but these tools are rarely used outside of research settings.13,14
The PD clusters
DSM-5 divides 10 PDs into 3 clusters based on shared phenomenology and diagnostic criteria. Few patients have a “pure” case in which they meet criteria for only a single personality disorder.1
Cluster A. “Eccentric cluster” disorders are united by social aversion, a failure to form close attachments, or paranoia and suspiciousness.15 These include paranoid, schizoid, and schizotypal PD. Low self-awareness is typical. There are no treatment guidelines for these disorders, although there is some clinical trial data for schizotypal PD.
Cluster B. “Dramatic cluster” disorders share dramatic, emotional, and erratic characteristics.14 These include narcissistic, antisocial, borderline, and histrionic PD. Antisocial and narcissistic patients have low self-awareness. There are treatment guidelines for antisocial and borderline PD, and a variety of clinical trial data is available for the latter.15
Continue to: Cluster C
Cluster C. “Anxious cluster” disorders are united by anxiousness, fearfulness, and poor self-esteem. Many of these patients also display interpersonal rigidity.15 These disorders include avoidant, dependent, and obsessive-compulsive PD. There are no treatment guidelines or clinical trial data for these disorders.
Why consider pharmacotherapy for personality disorders?
The consensus among experts is that psychotherapy is the treatment of choice for PDs.15 Despite significant gaps in the evidence base, there has been a growing interest in using psychotropic medication to treat PDs. For example, research shows that >90% of patients with borderline PD are prescribed medication, most typically antidepressants, antipsychotics, mood stabilizers, stimulants, or sedative-hypnotics.16,17
Increased interest in pharmacotherapy for PDs could be related to research showing the importance of underlying neurobiology, particularly for antisocial and borderline PD.18,19 This work is complemented by genetic research showing the heritability of PD traits and disorders.20,21 Another factor could be renewed interest in dimensional approaches to the classification of PDs, as exemplified by DSM-5’s alternative model for PDs.1 This approach aligns with some expert recommendations to focus on treating PD symptom dimensions, rather than the syndrome itself.22
Importantly, no psychotropic medication is FDA-approved for the treatment of any PD. For that reason, prescribing medication for a PD is “off-label,” although prescribing a medication for a comorbid disorder for which the drug has an FDA-approved indication is not (eg, prescribing an antidepressant for major depressive disorder [MDD]).
Principles for prescribing
Despite gaps in research data, general principles for using medication to treat PDs have emerged from treatment guidelines for antisocial and borderline PD, clinical trial data, reviews and meta-analyses, and expert opinion. Clinicians should address the following considerations before prescribing medication to a patient with a PD.
Continue to: PD diagnosis
PD diagnosis. Has the patient been properly assessed and diagnosed? While history is the most important basis for diagnosis, the clinician should be familiar with the PDs and DSM-5 criteria. Has the patient been informed of the diagnosis and its implications for treatment?
Patient interest in medication. Is the patient interested in taking medication? Patients with borderline PD are often prescribed medication, but there are sparse data for the other PDs. The patient might have little interest in the PD diagnosis or its treatment.
Comorbidity. Has the patient been assessed for comorbid psychiatric disorders that could interfere with medication use (ie, an SUD) or might be a focus of treatment (eg, MDD)? Patients with PDs typically have significant comorbidity that a thorough evaluation will uncover.
PD symptom dimensions. Has the patient been assessed to determine cognitive or behavioral symptom dimensions of their PD? One or more symptom dimension(s) could be the focus of treatment. Table 2 lists examples of PD symptom dimensions.
Strategies to guide prescribing
Strategies to help guide prescribing include targeting any comorbid disorder(s), targeting important PD symptom dimensions (eg, impulsive aggression), choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself.
Continue to: Targeting comorbid disorders
Targeting comorbid disorders. National Institute for Health and Care Excellence guidelines for antisocial and borderline PD recommend that clinicians focus on treating comorbid disorders, a position echoed in Cochrane and other reviews.4,9,22-26 For example, a patient with borderline PD experiencing a major depressive episode could be treated with an antidepressant. Targeting the depressive symptoms could boost the patient’s mood, perhaps lessening the individual’s PD symptoms or reducing their severity.
Targeting important symptom dimensions. For patients with borderline PD, several guidelines and reviews have suggested that treatment should focus on emotional dysregulation and impulsive aggression (mood stabilizers, antipsychotics), or cognitive-perceptual symptoms (antipsychotics).4-6,15 There is some evidence that mood stabilizers or second-generation antipsychotics could help reduce impulsive aggression in patients with antisocial PD.27
Choosing medication based on similarity to another disorder known to respond to medication. Avoidant PD overlaps with social anxiety disorder and can be conceptualized as a chronic, pervasive social phobia. Avoidant PD might respond to a medication known to be effective for treating social anxiety disorder, such as a selective serotonin reuptake inhibitor (SSRI) or venlafaxine.28 Treating obsessive-compulsive PD with an SSRI is another example of this strategy, as 1 small study of fluvoxamine suggests.29 Obsessive-compulsive PD is common in persons with obsessive-compulsive disorder, and overlap includes preoccupation with orders, rules, and lists, and an inability to throw things out.
Targeting the PD syndrome. Another strategy is to target the PD itself. Clinical trial data suggest the antipsychotic risperidone can reduce the symptoms of schizotypal PD.30 Considering that this PD has a genetic association with schizophrenia, it is not surprising that the patient’s ideas of reference, odd communication, or transient paranoia might respond to an antipsychotic. Data from randomized controlled trials (RCTs) support the use of the second-generation antipsychotics aripiprazole and quetiapine to treat BPD.31,32 While older guidelines4,5 supported the use of the mood stabilizer lamotrigine, a recent RCT found that it was no more effective than placebo for borderline PD or its symptom dimensions.33
What to do before prescribing
Before writing a prescription, the clinician and patient should discuss the presence of a PD and the desirability of treatment. The patient should understand the limited evidence base and know that medication prescribed for a PD is off-label. The clinician should discuss medication selection and its rationale, and whether the medication is targeting a comorbid disorder, symptom dimension(s), or the PD itself. Additional considerations for prescribing for patients with PDs are listed in Table 3.34

Continue to: Avoid polypharmacy
Avoid polypharmacy. Many patients with borderline PD are prescribed multiple psychotropic medications.16,17 This approach leads to greater expense and more adverse effects, and is not evidence-based.
Avoid benzodiazepines. Many patients with borderline PD are prescribed benzodiazepines, often as part of a polypharmacy regimen. These drugs can cause disinhibition, thereby increasing acting-out behaviors and self-harm.35 Also, patients with PDs often have SUDs, which is a contraindication for benzodiazepine use.
Rate the patient’s improvement. Both the patient and clinician can benefit from monitoring symptomatic improvement. Several validated scales can be used to rate depression, anxiety, impulsivity, mood lability, anger, and aggression (Table 436-41).Some validated scales for borderline PD align with DSM-5 criteria. Two such widely used instruments are the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD)42 and the self-rated Borderline Evaluation of Severity Over Time (BEST).43 Each has questions that could be pulled to rate a symptom dimension of interest, such as affective instability, anger dyscontrol, or abandonment fears (Table 542,43).
A visual analog scale is easy to use and can target symptom dimensions of interest.44 For example, a clinician could use a visual analog scale to rate mood instability by asking a patient to rate their mood severity by making a mark along a 10-cm line (0 = “Most erratic emotions I have experienced,” 10 = “Most stable I have ever experienced my emotions to be”). This score can be recorded at baseline and subsequent visits.
Take-home points
PDs are common in the general population and health care settings. They are underrecognized by the general public and mental health professionals, often because of stigma. Clinicians could boost their recognition of these disorders by embedding simple screening questions in their patient assessments. Many patients with PDs will be interested in pharmacotherapy for their disorder or symptoms. Treatment strategies include targeting the comorbid disorder(s), targeting important PD symptom dimensions, choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself. Each strategy has its limitations and varying degrees of empirical support. Treatment response can be monitored using validated scales or a visual analog scale.
Continue to: Bottom Line
Bottom Line
Although psychotherapy is the first-line treatment and no medications are FDAapproved for treating personality disorders (PDs), there has been growing interest in using psychotropic medication to treat PDs. Strategies for pharmacotherapy include targeting comorbid disorders, PD symptom dimensions, or the PD itself. Choice of medication can be based on the similarity of the PD with another disorder known to respond to medication.
Related Resources
- Correa Da Costa S, Sanches M, Soares JC. Bipolar disorder or borderline personality disorder? Current Psychiatry. 2019;18(11):26-29,35-39.
- Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
Drug Brand Names
Aripiprazole • Abilify
Fluvoxamine • Luvox
Lamotrigine • Lamictal
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Black DW, Andreasen N. Personality disorders. In: Black DW, Andreasen N. Introductory textbook of psychiatry, 7th edition. American Psychiatric Publishing; 2020:410-423.
3. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord 2004;18(3):226-239.
4. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
5. Vita A, De Peri L, Sacchetti E. Antipsychotics, antidepressants, anticonvulsants, and placebo on the symptom dimensions of borderline personality disorder – a meta-analysis of randomized controlled and open-label trials. J Clin Psychopharmacol. 2011;31(5):613-624.
6. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep. 2015;17(1):534.
7. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31(5):345-356.
8. Black DW, Paris J, Schulz SC. Personality disorders: evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M, ed. Cognitive behavioral psychopharmacology: the clinical practice of evidence-based biopsychosocial integration. John Wiley & Sons; 2018:137-165.
9. Stoffers-Winterling J, Sorebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. 2020;22(8):37.
10. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
11. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
12. Langbehn DR, Pfohl BM, Reynolds S, et al. The Iowa Personality Disorder Screen: development and preliminary validation of a brief screening interview. J Pers Disord. 1999;13(1):75-89.
13. Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV). American Psychiatric Press; 1997.
14. First MB, Spitzer RL, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: multisite test-retest reliability study. J Pers Disord. 1995;9(2):92-104.
15. Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
16. Zanarini MC, Frankenburg FR, Reich DB, et al. Treatment rates for patients with borderline personality disorder and other personality disorders: a 16-year study. Psychiatr Serv. 2015;66(1):15-20.
17. Black DW, Allen J, McCormick B, et al. Treatment received by persons with BPD participating in a randomized clinical trial of the Systems Training for Emotional Predictability and Problem Solving programme. Person Ment Health. 2011;5(3):159-168.
18. Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behav Sci Law. 2008;26(1):65-83.
19. Ruocco AC, Amirthavasagam S, Choi-Kain LW, et al. Neural correlates of negative emotionality in BPD: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73(2):153-160.
20. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831.
21. Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158-162.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
23. National Institute for Health and Care Excellence (NICE). Borderline personality disorder: recognition and management. Clinical guideline [CG78]. Published January 2009. https://www.nice.org.uk/guidance/cg78
24. National Institute for Health and Care Excellence (NICE). Antisocial personality disorder: prevention and management. Clinical guideline [CG77]. Published January 2009. Updated March 27, 2013. https://www.nice.org.uk/guidance/cg77
25. Khalifa N, Duggan C, Stoffers J, et al. Pharmacologic interventions for antisocial personality disorder. Cochrane Database Syst Rep. 2010;(8):CD007667.
26. Stoffers JM, Völlm BA, Rücker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;2012(8):CD005652.
27. Black DW. The treatment of antisocial personality disorder. Current Treatment Options in Psychiatry. 2017. https://doi.org/10.1007/s40501-017-0123-z
28. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708-713.
29. Ansseau M. The obsessive-compulsive personality: diagnostic aspects and treatment possibilities. In: Den Boer JA, Westenberg HGM, eds. Focus on obsessive-compulsive spectrum disorders. Syn-Thesis; 1997:61-73.
30. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64(6):628-634.
31. Black DW, Zanarini MC, Romine A, et al. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2014;171(11):1174-1182.
32. Nickel MK, Muelbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2006;163(5):833-838.
33. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764.
34. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. 2004;65(12)1660-1665.
35. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder. Alprazolam, carbamazepine, trifluoperazine, and tranylcypromine. Arch Gen Psychiatry. 1988;45(2):111-119.
36. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812.
37. Ratey JJ, Gutheil CM. The measurement of aggressive behavior: reflections on the use of the Overt Aggression Scale and the Modified Overt Aggression Scale. J Neuropsychiatr Clin Neurosci. 1991;3(2):S57-S60.
38. Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers; 1999:993-1021.
39. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. Psychological Corp; 1996.
40. Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded Form. The University of Iowa; 1999.
41. Harvey D, Greenberg BR, Serper MR, et al. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45(5):786-793.
42. Zanarini MC, Vujanovic AA, Parachini EA, et al. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Person Disord. 2003:17(3):233-242.
43. Pfohl B, Blum N, St John D, et al. Reliability and validity of the Borderline Evaluation of Severity Over Time (BEST): a new scale to measure severity and change in borderline personality disorder. J Person Disord. 2009;23(3):281-293.
44. Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. 1997;31(5):569-579.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Black DW, Andreasen N. Personality disorders. In: Black DW, Andreasen N. Introductory textbook of psychiatry, 7th edition. American Psychiatric Publishing; 2020:410-423.
3. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord 2004;18(3):226-239.
4. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
5. Vita A, De Peri L, Sacchetti E. Antipsychotics, antidepressants, anticonvulsants, and placebo on the symptom dimensions of borderline personality disorder – a meta-analysis of randomized controlled and open-label trials. J Clin Psychopharmacol. 2011;31(5):613-624.
6. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep. 2015;17(1):534.
7. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31(5):345-356.
8. Black DW, Paris J, Schulz SC. Personality disorders: evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M, ed. Cognitive behavioral psychopharmacology: the clinical practice of evidence-based biopsychosocial integration. John Wiley & Sons; 2018:137-165.
9. Stoffers-Winterling J, Sorebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. 2020;22(8):37.
10. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
11. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
12. Langbehn DR, Pfohl BM, Reynolds S, et al. The Iowa Personality Disorder Screen: development and preliminary validation of a brief screening interview. J Pers Disord. 1999;13(1):75-89.
13. Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV). American Psychiatric Press; 1997.
14. First MB, Spitzer RL, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: multisite test-retest reliability study. J Pers Disord. 1995;9(2):92-104.
15. Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
16. Zanarini MC, Frankenburg FR, Reich DB, et al. Treatment rates for patients with borderline personality disorder and other personality disorders: a 16-year study. Psychiatr Serv. 2015;66(1):15-20.
17. Black DW, Allen J, McCormick B, et al. Treatment received by persons with BPD participating in a randomized clinical trial of the Systems Training for Emotional Predictability and Problem Solving programme. Person Ment Health. 2011;5(3):159-168.
18. Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behav Sci Law. 2008;26(1):65-83.
19. Ruocco AC, Amirthavasagam S, Choi-Kain LW, et al. Neural correlates of negative emotionality in BPD: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73(2):153-160.
20. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831.
21. Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158-162.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
23. National Institute for Health and Care Excellence (NICE). Borderline personality disorder: recognition and management. Clinical guideline [CG78]. Published January 2009. https://www.nice.org.uk/guidance/cg78
24. National Institute for Health and Care Excellence (NICE). Antisocial personality disorder: prevention and management. Clinical guideline [CG77]. Published January 2009. Updated March 27, 2013. https://www.nice.org.uk/guidance/cg77
25. Khalifa N, Duggan C, Stoffers J, et al. Pharmacologic interventions for antisocial personality disorder. Cochrane Database Syst Rep. 2010;(8):CD007667.
26. Stoffers JM, Völlm BA, Rücker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;2012(8):CD005652.
27. Black DW. The treatment of antisocial personality disorder. Current Treatment Options in Psychiatry. 2017. https://doi.org/10.1007/s40501-017-0123-z
28. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708-713.
29. Ansseau M. The obsessive-compulsive personality: diagnostic aspects and treatment possibilities. In: Den Boer JA, Westenberg HGM, eds. Focus on obsessive-compulsive spectrum disorders. Syn-Thesis; 1997:61-73.
30. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64(6):628-634.
31. Black DW, Zanarini MC, Romine A, et al. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2014;171(11):1174-1182.
32. Nickel MK, Muelbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2006;163(5):833-838.
33. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764.
34. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. 2004;65(12)1660-1665.
35. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder. Alprazolam, carbamazepine, trifluoperazine, and tranylcypromine. Arch Gen Psychiatry. 1988;45(2):111-119.
36. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812.
37. Ratey JJ, Gutheil CM. The measurement of aggressive behavior: reflections on the use of the Overt Aggression Scale and the Modified Overt Aggression Scale. J Neuropsychiatr Clin Neurosci. 1991;3(2):S57-S60.
38. Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers; 1999:993-1021.
39. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. Psychological Corp; 1996.
40. Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded Form. The University of Iowa; 1999.
41. Harvey D, Greenberg BR, Serper MR, et al. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45(5):786-793.
42. Zanarini MC, Vujanovic AA, Parachini EA, et al. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Person Disord. 2003:17(3):233-242.
43. Pfohl B, Blum N, St John D, et al. Reliability and validity of the Borderline Evaluation of Severity Over Time (BEST): a new scale to measure severity and change in borderline personality disorder. J Person Disord. 2009;23(3):281-293.
44. Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. 1997;31(5):569-579.
Antidepressants, TMS, and the risk of affective switch in bipolar depression
Because treatment resistance is a pervasive problem in bipolar depression, the use of neuromodulation treatments such as transcranial magnetic stimulation (TMS) is increasing for patients with this disorder.1-7 Patients with bipolar disorder tend to spend the majority of the time with depressive symptoms, which underscores the importance of providing effective treatment for bipolar depression, especially given the chronicity of this disease.2,3,5 Only a few medications are FDA-approved for treating bipolar depression (Table).
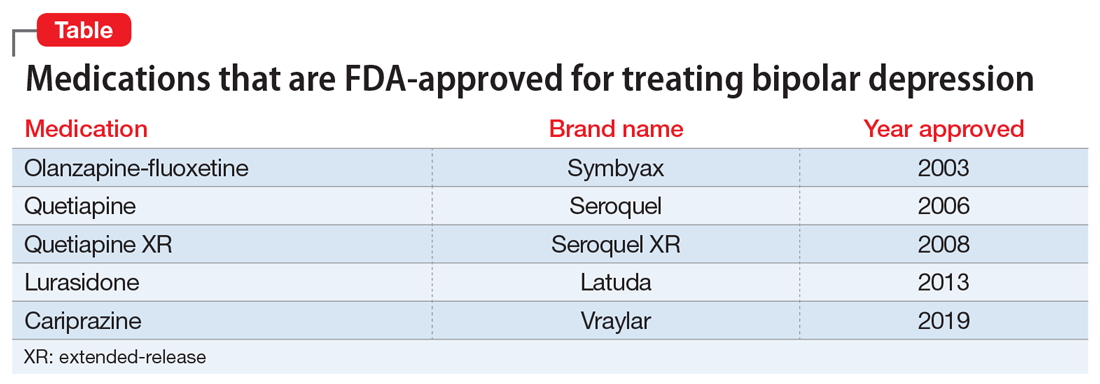
In this article, we describe the case of a patient with treatment-resistant bipolar depression undergoing adjunctive TMS treatment who experienced an affective switch from depression to mania. We also discuss evidence regarding the likelihood of treatment-emergent mania for antidepressants vs TMS in bipolar depression.
CASE
Ms. W, a 60-year-old White female with a history of bipolar I disorder and attention-deficit/hyperactivity disorder (ADHD), presented for TMS evaluation during a depressive episode. Throughout her life, she had experienced numerous manic episodes, but as she got older she noted an increasing frequency of depressive episodes. Over the course of her illness, she had completed adequate trials at therapeutic doses of many medications, including second-generation antipsychotics (SGAs) (aripiprazole, lurasidone, olanzapine, quetiapine), mood stabilizers (lamotrigine, lithium), and antidepressants (bupropion, venlafaxine, fluoxetine, mirtazapine, trazodone). A course of electroconvulsive therapy was not effective. Ms. W had a long-standing diagnosis of ADHD and had been treated with stimulants for >10 years, although it was unclear whether formal neuropsychological testing had been conducted to confirm this diagnosis. She had >10 suicide attempts and multiple psychiatric hospitalizations.
At her initial evaluation for TMS, Ms. W said she had depressive symptoms predominating for the past 2 years, including low mood, hopelessness, poor sleep, poor appetite, anhedonia, and suicidal ideation without a plan. At the time, she was taking clonazepam, 0.5 mg twice a day; lurasidone, 40 mg/d at bedtime; fluoxetine, 60 mg/d; trazodone, 50 mg/d at bedtime; and methylphenidate, 40 mg/d, and was participating in psychotherapy consistently.
After Ms. W and her clinicians discussed alternatives, risks, benefits, and adverse effects, she consented to adjunctive TMS treatment and provided written informed consent. The treatment plan was outlined as 6 weeks of daily TMS therapy (NeuroStar; Neuronetics, Malvern, PA), 1 treatment per day, 5 days a week. Her clinical status was assessed weekly using the Quick Inventory of Depressive Symptomatology (QIDS) for depression, Generalized Anxiety Disorder 7-item scale (GAD-7) for anxiety, and Young Mania Rating Scale (YMRS) for mania. The Figure shows the trends in Ms. W’s QIDS, GAD-7, and YMRS scores over the course of TMS treatment.
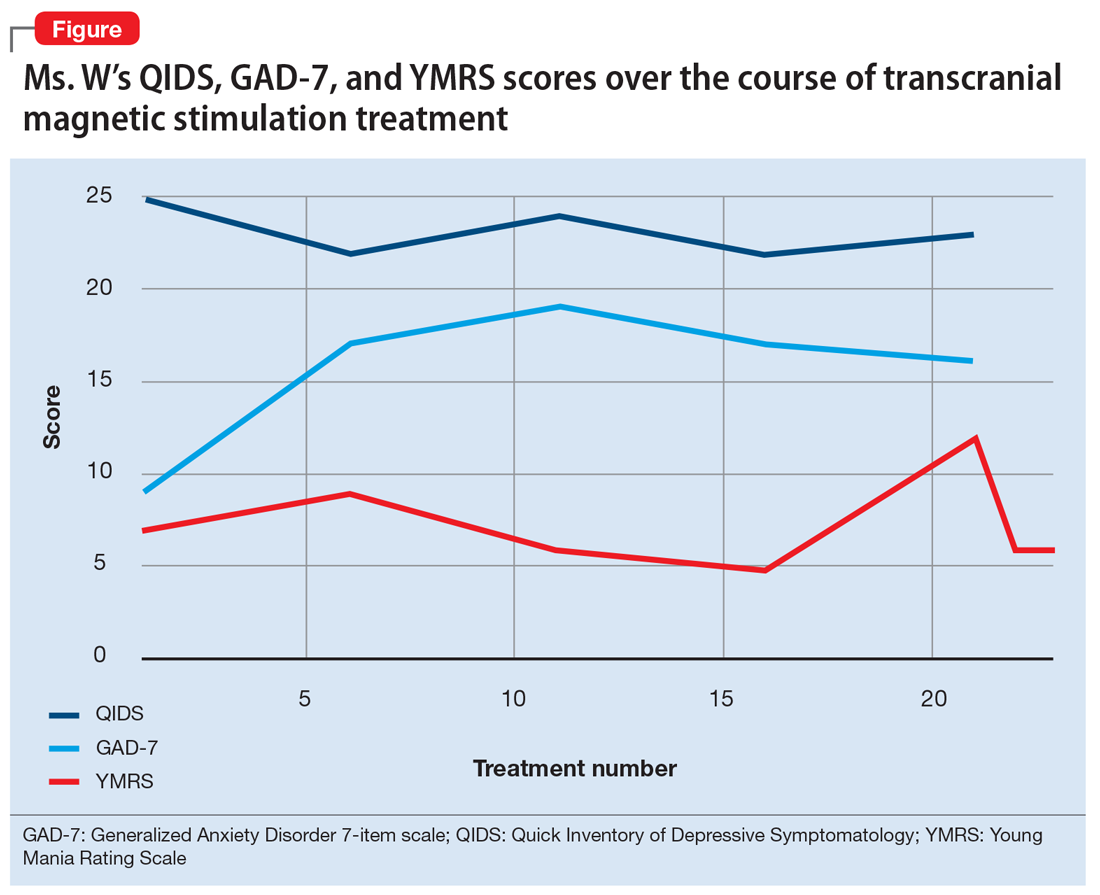
Prior to initiating TMS, her baseline scores were QIDS: 25, GAD-7: 9, and YMRS: 7, indicating very severe depression, mild anxiety, and the absence of mania. Ms. W’s psychotropic regimen remained unchanged throughout the course of her TMS treatment. After her motor threshold was determined, her TMS treatment began at 80% of motor threshold and was titrated up to 95% at the first treatment. By the second treatment, it was titrated up to 110%. By the third treatment, it was titrated up to 120% of motor threshold, which is the percentage used for the remaining treatments.
Initially, Ms. W reported some improvement in her depression, but this improvement was short-lived, and she continued to have elevated QIDS scores throughout treatment. By treatment #21, her QIDS and GAD-7 scores remained elevated, and her YMRS score had increased to 12. Due to this increase in YMRS score, the YMRS was repeated on the next 2 treatment days (#22 and #23), and her score was 6 on both days. When Ms. W presented for treatment #25, she was disorganized, irritable, and endorsed racing thoughts and decreased sleep. She was involuntarily hospitalized for mania, and TMS was discontinued. Unfortunately, she did not complete any clinical scales on that day. Upon admission to the hospital, Ms. W reported that at approximately the time of treatment #21, she had a fluctuation in her mood that consisted of increased goal-directed activity, decreased need for sleep, racing thoughts, and increased frivolous spending. She was treated with lithium, 300 mg twice a day. Lurasidone was increased to 80 mg/d at bedtime, and she continued clonazepam, trazodone, and methylphenidate at the previous doses. Over 14 days, Ms. W’s mania gradually resolved, and she was discharged home.
Continue to: Mixed evidence on the risk of switching
Mixed evidence on the risk of switching
Currently, several TMS devices are FDA-cleared for treating unipolar major depressive disorder, obsessive-compulsive disorder, and certain types of migraine. In March 2020, the FDA granted Breakthrough Device Designation for one TMS device, the NeuroStar Advanced Therapy System, for the treatment of bipolar depression.8 This designation created an expedited pathway for prioritized FDA review of the NeuroStar Advanced Therapy clinical trial program.
Few published clinical studies have evaluated using TMS to treat patients with bipolar depression.9-15 As with any antidepressant treatment for bipolar depression, there is a risk of affective switch from depression to mania when using TMS. Most of the literature available regarding the treatment of bipolar depression focuses on the risk of antidepressant medications to induce an affective switch. This risk depends on the class of the antidepressant,16 and there is a paucity of studies examining the risk of switch with TMS.
Interpretation of available literature is limited due to inconsistencies in the definition of an affective switch, the variable length of treatment with antidepressants, the use of concurrent medications such as mood stabilizers, and confounders such as the natural course of switching in bipolar disorder.17 Overall, the evidence for treatment-emergent mania related to antidepressant use is mixed, and the reported rate of treatment-emergent mania varies. In a systematic review and meta-analysis of >20 randomized controlled trials that included 1,316 patients with bipolar disorder who received antidepressants, Fornaro et al18 found that the incidence of treatment-emergent mania was 11.8%. It is generally recommended that if antidepressants are used to treat patients with bipolar disorder, they should be given with a traditional mood stabilizer to prevent affective switches, although whether mood stabilizers can prevent such switches is unproven.19
In a literature review by Xia et al,20 the affective switch rate in patients with bipolar depression who were treated with TMS was 3.1%, which was not statistically different from the affective switch rate with sham treatment.However, most of the patients included in this analysis were receiving other medications concurrently, and the length of treatment was 2 weeks, which is shorter than the average length of TMS treatment in clinical practice. In a recent literature review by Rachid,21 TMS was found to possibly induce manic episodes when used as monotherapy or in combination with antidepressants in patients with bipolar depression. To reduce the risk of treatment-emergent mania, current recommendations advise the use of a mood stabilizer for a minimum of 2 weeks before initiating TMS.1
In our case, Ms. W was receiving antidepressants (fluoxetine and trazodone), lurasidone (an SGA that is FDA-approved for bipolar depression), and methylphenidate before starting TMS treatment. Fluoxetine, trazodone, and methylphenidate may possibly contribute to an increased risk of an affective switch.1,22 Further studies are needed to clarify whether mood stabilizers or SGAs can prevent the development of mania in patients with bipolar depression who undergo TMS treatment.20
Continue to: Because bipolar depression poses...
Because bipolar depression poses a major clinical challenge,23,24 it is imperative to consider alternate treatments. When evaluating alternative treatment strategies, one may consider TMS in conjunction with a traditional mood stabilizer because this regimen may have a lower risk of treatment-emergent mania compared with antidepressants.1,25
Acknowledgment
The authors thank Dr. Sy Saeed for his expertise and guidance on this article.
Bottom Line
For patients with bipolar depression, treatment with transcranial magnetic stimulation in conjunction with a mood stabilizer may have lower rates of treatment-emergent mania than treatment with antidepressants.
Related Resources
- Transcranial magnetic stimulation: clinical applications for psychiatric practice. Bermudes RA, Lanocha K, Janicak PG, eds. American Psychiatric Association Publishing; 2017.
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9(10):e01419. doi: 10.1002/brb3.1419
Drug Brand Names
Aripiprazole • Abilify
Bupropion • Wellbutrin
Cariprazine • Vraylar
Clonazepam • Klonopin
Fluoxetine • Prozac
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin, Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Olanzapine-fluoxetine • Symbyax
Quetiapine • Seroquel
Trazodone • Desyrel
Venlafaxine • Effexor
1. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes RA, Lanocha K, Janicak PG, eds. Transcranial magnetic stimulation: clinical applications for psychiatric practice. American Psychiatric Association Publishing; 2017:127-156.
2. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
3. Gitlin M. Treatment-resistant bipolar disorder. Molecular Psychiatry. 2006;11(3):227-240.
4. Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41(1):18-30.
5. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241-251.
6. Myczkowski ML, Fernandes A, Moreno M, et al. Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J Affect Disord. 2018;235: 20-26.
7. Tavares DF, Myczkowski ML, Alberto RL, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593-2601.
8. Neuronetics. FDA grants NeuroStar® Advanced Therapy System Breakthrough Device Designation to treat bipolar depression. Accessed February 2, 2021. https://www.globenewswire.com/news-release/2020/03/06/1996447/0/en/FDA-Grants-NeuroStar-Advanced-Therapy-System-Breakthrough-Device-Designation-to-Treat-Bipolar-Depression.html
9. Cohen RB, Brunoni AR, Boggio PS, et al. Clinical predictors associated with duration of repetitive transcranial magnetic stimulation treatment for remission in bipolar depression: a naturalistic study. J Nerv Ment Dis. 2010;198(9):679-681.
10. Connolly KR, Helmer A, Cristancho MA, et al. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567-e573.
11. Dell’osso B, D’Urso N, Castellano F, et al. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: a 1-year follow-up study. J ECT. 2011;27(2):141-144.
12. Dell’Osso B, Mundo E, D’Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11(1):76-81.
13. Harel EV, Zangen A, Roth Y, et al. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119-126.
14. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5(1):40-47.
15. Tamas RL, Menkes D, El-Mallakh RS. Stimulating research: a prospective, randomized, double-blind, sham-controlled study of slow transcranial magnetic stimulation in depressed bipolar patients. J Neuropsychiatry Clin Neurosci. 2007;19(2):198-199.
16. Tundo A, Cavalieri P, Navari S, et al. Treating bipolar depression - antidepressants and alternatives: a critical review of the literature. Acta Neuropsychiatrica. 2011:23(3):94-105.
17. Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161(9):1537-1547.
18. Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant‐emergent mania in bipolar depression: a systematic review and meta‐analysis. Bipolar Disord. 2018;20(3):195-227.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
20. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119-130.
21. Rachid F. Repetitive transcranial magnetic stimulation and treatment-emergent mania and hypomania: a review of the literature. J Psychiatr Pract. 2017;23(2):150-159.
22. Victorin A, Rydén E, Thase M, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
23. Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27-35.
24. Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1.
25. Phillips AL, Burr RL, Dunner DL. Repetitive transcranial magnetic stimulation in the treatment of bipolar depression: Experience from a clinical setting. J Psychiatr Pract. 2020;26(1):37-45.
Because treatment resistance is a pervasive problem in bipolar depression, the use of neuromodulation treatments such as transcranial magnetic stimulation (TMS) is increasing for patients with this disorder.1-7 Patients with bipolar disorder tend to spend the majority of the time with depressive symptoms, which underscores the importance of providing effective treatment for bipolar depression, especially given the chronicity of this disease.2,3,5 Only a few medications are FDA-approved for treating bipolar depression (Table).

In this article, we describe the case of a patient with treatment-resistant bipolar depression undergoing adjunctive TMS treatment who experienced an affective switch from depression to mania. We also discuss evidence regarding the likelihood of treatment-emergent mania for antidepressants vs TMS in bipolar depression.
CASE
Ms. W, a 60-year-old White female with a history of bipolar I disorder and attention-deficit/hyperactivity disorder (ADHD), presented for TMS evaluation during a depressive episode. Throughout her life, she had experienced numerous manic episodes, but as she got older she noted an increasing frequency of depressive episodes. Over the course of her illness, she had completed adequate trials at therapeutic doses of many medications, including second-generation antipsychotics (SGAs) (aripiprazole, lurasidone, olanzapine, quetiapine), mood stabilizers (lamotrigine, lithium), and antidepressants (bupropion, venlafaxine, fluoxetine, mirtazapine, trazodone). A course of electroconvulsive therapy was not effective. Ms. W had a long-standing diagnosis of ADHD and had been treated with stimulants for >10 years, although it was unclear whether formal neuropsychological testing had been conducted to confirm this diagnosis. She had >10 suicide attempts and multiple psychiatric hospitalizations.
At her initial evaluation for TMS, Ms. W said she had depressive symptoms predominating for the past 2 years, including low mood, hopelessness, poor sleep, poor appetite, anhedonia, and suicidal ideation without a plan. At the time, she was taking clonazepam, 0.5 mg twice a day; lurasidone, 40 mg/d at bedtime; fluoxetine, 60 mg/d; trazodone, 50 mg/d at bedtime; and methylphenidate, 40 mg/d, and was participating in psychotherapy consistently.
After Ms. W and her clinicians discussed alternatives, risks, benefits, and adverse effects, she consented to adjunctive TMS treatment and provided written informed consent. The treatment plan was outlined as 6 weeks of daily TMS therapy (NeuroStar; Neuronetics, Malvern, PA), 1 treatment per day, 5 days a week. Her clinical status was assessed weekly using the Quick Inventory of Depressive Symptomatology (QIDS) for depression, Generalized Anxiety Disorder 7-item scale (GAD-7) for anxiety, and Young Mania Rating Scale (YMRS) for mania. The Figure shows the trends in Ms. W’s QIDS, GAD-7, and YMRS scores over the course of TMS treatment.

Prior to initiating TMS, her baseline scores were QIDS: 25, GAD-7: 9, and YMRS: 7, indicating very severe depression, mild anxiety, and the absence of mania. Ms. W’s psychotropic regimen remained unchanged throughout the course of her TMS treatment. After her motor threshold was determined, her TMS treatment began at 80% of motor threshold and was titrated up to 95% at the first treatment. By the second treatment, it was titrated up to 110%. By the third treatment, it was titrated up to 120% of motor threshold, which is the percentage used for the remaining treatments.
Initially, Ms. W reported some improvement in her depression, but this improvement was short-lived, and she continued to have elevated QIDS scores throughout treatment. By treatment #21, her QIDS and GAD-7 scores remained elevated, and her YMRS score had increased to 12. Due to this increase in YMRS score, the YMRS was repeated on the next 2 treatment days (#22 and #23), and her score was 6 on both days. When Ms. W presented for treatment #25, she was disorganized, irritable, and endorsed racing thoughts and decreased sleep. She was involuntarily hospitalized for mania, and TMS was discontinued. Unfortunately, she did not complete any clinical scales on that day. Upon admission to the hospital, Ms. W reported that at approximately the time of treatment #21, she had a fluctuation in her mood that consisted of increased goal-directed activity, decreased need for sleep, racing thoughts, and increased frivolous spending. She was treated with lithium, 300 mg twice a day. Lurasidone was increased to 80 mg/d at bedtime, and she continued clonazepam, trazodone, and methylphenidate at the previous doses. Over 14 days, Ms. W’s mania gradually resolved, and she was discharged home.
Continue to: Mixed evidence on the risk of switching
Mixed evidence on the risk of switching
Currently, several TMS devices are FDA-cleared for treating unipolar major depressive disorder, obsessive-compulsive disorder, and certain types of migraine. In March 2020, the FDA granted Breakthrough Device Designation for one TMS device, the NeuroStar Advanced Therapy System, for the treatment of bipolar depression.8 This designation created an expedited pathway for prioritized FDA review of the NeuroStar Advanced Therapy clinical trial program.
Few published clinical studies have evaluated using TMS to treat patients with bipolar depression.9-15 As with any antidepressant treatment for bipolar depression, there is a risk of affective switch from depression to mania when using TMS. Most of the literature available regarding the treatment of bipolar depression focuses on the risk of antidepressant medications to induce an affective switch. This risk depends on the class of the antidepressant,16 and there is a paucity of studies examining the risk of switch with TMS.
Interpretation of available literature is limited due to inconsistencies in the definition of an affective switch, the variable length of treatment with antidepressants, the use of concurrent medications such as mood stabilizers, and confounders such as the natural course of switching in bipolar disorder.17 Overall, the evidence for treatment-emergent mania related to antidepressant use is mixed, and the reported rate of treatment-emergent mania varies. In a systematic review and meta-analysis of >20 randomized controlled trials that included 1,316 patients with bipolar disorder who received antidepressants, Fornaro et al18 found that the incidence of treatment-emergent mania was 11.8%. It is generally recommended that if antidepressants are used to treat patients with bipolar disorder, they should be given with a traditional mood stabilizer to prevent affective switches, although whether mood stabilizers can prevent such switches is unproven.19
In a literature review by Xia et al,20 the affective switch rate in patients with bipolar depression who were treated with TMS was 3.1%, which was not statistically different from the affective switch rate with sham treatment.However, most of the patients included in this analysis were receiving other medications concurrently, and the length of treatment was 2 weeks, which is shorter than the average length of TMS treatment in clinical practice. In a recent literature review by Rachid,21 TMS was found to possibly induce manic episodes when used as monotherapy or in combination with antidepressants in patients with bipolar depression. To reduce the risk of treatment-emergent mania, current recommendations advise the use of a mood stabilizer for a minimum of 2 weeks before initiating TMS.1
In our case, Ms. W was receiving antidepressants (fluoxetine and trazodone), lurasidone (an SGA that is FDA-approved for bipolar depression), and methylphenidate before starting TMS treatment. Fluoxetine, trazodone, and methylphenidate may possibly contribute to an increased risk of an affective switch.1,22 Further studies are needed to clarify whether mood stabilizers or SGAs can prevent the development of mania in patients with bipolar depression who undergo TMS treatment.20
Continue to: Because bipolar depression poses...
Because bipolar depression poses a major clinical challenge,23,24 it is imperative to consider alternate treatments. When evaluating alternative treatment strategies, one may consider TMS in conjunction with a traditional mood stabilizer because this regimen may have a lower risk of treatment-emergent mania compared with antidepressants.1,25
Acknowledgment
The authors thank Dr. Sy Saeed for his expertise and guidance on this article.
Bottom Line
For patients with bipolar depression, treatment with transcranial magnetic stimulation in conjunction with a mood stabilizer may have lower rates of treatment-emergent mania than treatment with antidepressants.
Related Resources
- Transcranial magnetic stimulation: clinical applications for psychiatric practice. Bermudes RA, Lanocha K, Janicak PG, eds. American Psychiatric Association Publishing; 2017.
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9(10):e01419. doi: 10.1002/brb3.1419
Drug Brand Names
Aripiprazole • Abilify
Bupropion • Wellbutrin
Cariprazine • Vraylar
Clonazepam • Klonopin
Fluoxetine • Prozac
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin, Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Olanzapine-fluoxetine • Symbyax
Quetiapine • Seroquel
Trazodone • Desyrel
Venlafaxine • Effexor
Because treatment resistance is a pervasive problem in bipolar depression, the use of neuromodulation treatments such as transcranial magnetic stimulation (TMS) is increasing for patients with this disorder.1-7 Patients with bipolar disorder tend to spend the majority of the time with depressive symptoms, which underscores the importance of providing effective treatment for bipolar depression, especially given the chronicity of this disease.2,3,5 Only a few medications are FDA-approved for treating bipolar depression (Table).

In this article, we describe the case of a patient with treatment-resistant bipolar depression undergoing adjunctive TMS treatment who experienced an affective switch from depression to mania. We also discuss evidence regarding the likelihood of treatment-emergent mania for antidepressants vs TMS in bipolar depression.
CASE
Ms. W, a 60-year-old White female with a history of bipolar I disorder and attention-deficit/hyperactivity disorder (ADHD), presented for TMS evaluation during a depressive episode. Throughout her life, she had experienced numerous manic episodes, but as she got older she noted an increasing frequency of depressive episodes. Over the course of her illness, she had completed adequate trials at therapeutic doses of many medications, including second-generation antipsychotics (SGAs) (aripiprazole, lurasidone, olanzapine, quetiapine), mood stabilizers (lamotrigine, lithium), and antidepressants (bupropion, venlafaxine, fluoxetine, mirtazapine, trazodone). A course of electroconvulsive therapy was not effective. Ms. W had a long-standing diagnosis of ADHD and had been treated with stimulants for >10 years, although it was unclear whether formal neuropsychological testing had been conducted to confirm this diagnosis. She had >10 suicide attempts and multiple psychiatric hospitalizations.
At her initial evaluation for TMS, Ms. W said she had depressive symptoms predominating for the past 2 years, including low mood, hopelessness, poor sleep, poor appetite, anhedonia, and suicidal ideation without a plan. At the time, she was taking clonazepam, 0.5 mg twice a day; lurasidone, 40 mg/d at bedtime; fluoxetine, 60 mg/d; trazodone, 50 mg/d at bedtime; and methylphenidate, 40 mg/d, and was participating in psychotherapy consistently.
After Ms. W and her clinicians discussed alternatives, risks, benefits, and adverse effects, she consented to adjunctive TMS treatment and provided written informed consent. The treatment plan was outlined as 6 weeks of daily TMS therapy (NeuroStar; Neuronetics, Malvern, PA), 1 treatment per day, 5 days a week. Her clinical status was assessed weekly using the Quick Inventory of Depressive Symptomatology (QIDS) for depression, Generalized Anxiety Disorder 7-item scale (GAD-7) for anxiety, and Young Mania Rating Scale (YMRS) for mania. The Figure shows the trends in Ms. W’s QIDS, GAD-7, and YMRS scores over the course of TMS treatment.

Prior to initiating TMS, her baseline scores were QIDS: 25, GAD-7: 9, and YMRS: 7, indicating very severe depression, mild anxiety, and the absence of mania. Ms. W’s psychotropic regimen remained unchanged throughout the course of her TMS treatment. After her motor threshold was determined, her TMS treatment began at 80% of motor threshold and was titrated up to 95% at the first treatment. By the second treatment, it was titrated up to 110%. By the third treatment, it was titrated up to 120% of motor threshold, which is the percentage used for the remaining treatments.
Initially, Ms. W reported some improvement in her depression, but this improvement was short-lived, and she continued to have elevated QIDS scores throughout treatment. By treatment #21, her QIDS and GAD-7 scores remained elevated, and her YMRS score had increased to 12. Due to this increase in YMRS score, the YMRS was repeated on the next 2 treatment days (#22 and #23), and her score was 6 on both days. When Ms. W presented for treatment #25, she was disorganized, irritable, and endorsed racing thoughts and decreased sleep. She was involuntarily hospitalized for mania, and TMS was discontinued. Unfortunately, she did not complete any clinical scales on that day. Upon admission to the hospital, Ms. W reported that at approximately the time of treatment #21, she had a fluctuation in her mood that consisted of increased goal-directed activity, decreased need for sleep, racing thoughts, and increased frivolous spending. She was treated with lithium, 300 mg twice a day. Lurasidone was increased to 80 mg/d at bedtime, and she continued clonazepam, trazodone, and methylphenidate at the previous doses. Over 14 days, Ms. W’s mania gradually resolved, and she was discharged home.
Continue to: Mixed evidence on the risk of switching
Mixed evidence on the risk of switching
Currently, several TMS devices are FDA-cleared for treating unipolar major depressive disorder, obsessive-compulsive disorder, and certain types of migraine. In March 2020, the FDA granted Breakthrough Device Designation for one TMS device, the NeuroStar Advanced Therapy System, for the treatment of bipolar depression.8 This designation created an expedited pathway for prioritized FDA review of the NeuroStar Advanced Therapy clinical trial program.
Few published clinical studies have evaluated using TMS to treat patients with bipolar depression.9-15 As with any antidepressant treatment for bipolar depression, there is a risk of affective switch from depression to mania when using TMS. Most of the literature available regarding the treatment of bipolar depression focuses on the risk of antidepressant medications to induce an affective switch. This risk depends on the class of the antidepressant,16 and there is a paucity of studies examining the risk of switch with TMS.
Interpretation of available literature is limited due to inconsistencies in the definition of an affective switch, the variable length of treatment with antidepressants, the use of concurrent medications such as mood stabilizers, and confounders such as the natural course of switching in bipolar disorder.17 Overall, the evidence for treatment-emergent mania related to antidepressant use is mixed, and the reported rate of treatment-emergent mania varies. In a systematic review and meta-analysis of >20 randomized controlled trials that included 1,316 patients with bipolar disorder who received antidepressants, Fornaro et al18 found that the incidence of treatment-emergent mania was 11.8%. It is generally recommended that if antidepressants are used to treat patients with bipolar disorder, they should be given with a traditional mood stabilizer to prevent affective switches, although whether mood stabilizers can prevent such switches is unproven.19
In a literature review by Xia et al,20 the affective switch rate in patients with bipolar depression who were treated with TMS was 3.1%, which was not statistically different from the affective switch rate with sham treatment.However, most of the patients included in this analysis were receiving other medications concurrently, and the length of treatment was 2 weeks, which is shorter than the average length of TMS treatment in clinical practice. In a recent literature review by Rachid,21 TMS was found to possibly induce manic episodes when used as monotherapy or in combination with antidepressants in patients with bipolar depression. To reduce the risk of treatment-emergent mania, current recommendations advise the use of a mood stabilizer for a minimum of 2 weeks before initiating TMS.1
In our case, Ms. W was receiving antidepressants (fluoxetine and trazodone), lurasidone (an SGA that is FDA-approved for bipolar depression), and methylphenidate before starting TMS treatment. Fluoxetine, trazodone, and methylphenidate may possibly contribute to an increased risk of an affective switch.1,22 Further studies are needed to clarify whether mood stabilizers or SGAs can prevent the development of mania in patients with bipolar depression who undergo TMS treatment.20
Continue to: Because bipolar depression poses...
Because bipolar depression poses a major clinical challenge,23,24 it is imperative to consider alternate treatments. When evaluating alternative treatment strategies, one may consider TMS in conjunction with a traditional mood stabilizer because this regimen may have a lower risk of treatment-emergent mania compared with antidepressants.1,25
Acknowledgment
The authors thank Dr. Sy Saeed for his expertise and guidance on this article.
Bottom Line
For patients with bipolar depression, treatment with transcranial magnetic stimulation in conjunction with a mood stabilizer may have lower rates of treatment-emergent mania than treatment with antidepressants.
Related Resources
- Transcranial magnetic stimulation: clinical applications for psychiatric practice. Bermudes RA, Lanocha K, Janicak PG, eds. American Psychiatric Association Publishing; 2017.
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9(10):e01419. doi: 10.1002/brb3.1419
Drug Brand Names
Aripiprazole • Abilify
Bupropion • Wellbutrin
Cariprazine • Vraylar
Clonazepam • Klonopin
Fluoxetine • Prozac
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin, Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Olanzapine-fluoxetine • Symbyax
Quetiapine • Seroquel
Trazodone • Desyrel
Venlafaxine • Effexor
1. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes RA, Lanocha K, Janicak PG, eds. Transcranial magnetic stimulation: clinical applications for psychiatric practice. American Psychiatric Association Publishing; 2017:127-156.
2. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
3. Gitlin M. Treatment-resistant bipolar disorder. Molecular Psychiatry. 2006;11(3):227-240.
4. Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41(1):18-30.
5. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241-251.
6. Myczkowski ML, Fernandes A, Moreno M, et al. Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J Affect Disord. 2018;235: 20-26.
7. Tavares DF, Myczkowski ML, Alberto RL, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593-2601.
8. Neuronetics. FDA grants NeuroStar® Advanced Therapy System Breakthrough Device Designation to treat bipolar depression. Accessed February 2, 2021. https://www.globenewswire.com/news-release/2020/03/06/1996447/0/en/FDA-Grants-NeuroStar-Advanced-Therapy-System-Breakthrough-Device-Designation-to-Treat-Bipolar-Depression.html
9. Cohen RB, Brunoni AR, Boggio PS, et al. Clinical predictors associated with duration of repetitive transcranial magnetic stimulation treatment for remission in bipolar depression: a naturalistic study. J Nerv Ment Dis. 2010;198(9):679-681.
10. Connolly KR, Helmer A, Cristancho MA, et al. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567-e573.
11. Dell’osso B, D’Urso N, Castellano F, et al. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: a 1-year follow-up study. J ECT. 2011;27(2):141-144.
12. Dell’Osso B, Mundo E, D’Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11(1):76-81.
13. Harel EV, Zangen A, Roth Y, et al. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119-126.
14. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5(1):40-47.
15. Tamas RL, Menkes D, El-Mallakh RS. Stimulating research: a prospective, randomized, double-blind, sham-controlled study of slow transcranial magnetic stimulation in depressed bipolar patients. J Neuropsychiatry Clin Neurosci. 2007;19(2):198-199.
16. Tundo A, Cavalieri P, Navari S, et al. Treating bipolar depression - antidepressants and alternatives: a critical review of the literature. Acta Neuropsychiatrica. 2011:23(3):94-105.
17. Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161(9):1537-1547.
18. Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant‐emergent mania in bipolar depression: a systematic review and meta‐analysis. Bipolar Disord. 2018;20(3):195-227.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
20. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119-130.
21. Rachid F. Repetitive transcranial magnetic stimulation and treatment-emergent mania and hypomania: a review of the literature. J Psychiatr Pract. 2017;23(2):150-159.
22. Victorin A, Rydén E, Thase M, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
23. Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27-35.
24. Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1.
25. Phillips AL, Burr RL, Dunner DL. Repetitive transcranial magnetic stimulation in the treatment of bipolar depression: Experience from a clinical setting. J Psychiatr Pract. 2020;26(1):37-45.
1. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes RA, Lanocha K, Janicak PG, eds. Transcranial magnetic stimulation: clinical applications for psychiatric practice. American Psychiatric Association Publishing; 2017:127-156.
2. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
3. Gitlin M. Treatment-resistant bipolar disorder. Molecular Psychiatry. 2006;11(3):227-240.
4. Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41(1):18-30.
5. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241-251.
6. Myczkowski ML, Fernandes A, Moreno M, et al. Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J Affect Disord. 2018;235: 20-26.
7. Tavares DF, Myczkowski ML, Alberto RL, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593-2601.
8. Neuronetics. FDA grants NeuroStar® Advanced Therapy System Breakthrough Device Designation to treat bipolar depression. Accessed February 2, 2021. https://www.globenewswire.com/news-release/2020/03/06/1996447/0/en/FDA-Grants-NeuroStar-Advanced-Therapy-System-Breakthrough-Device-Designation-to-Treat-Bipolar-Depression.html
9. Cohen RB, Brunoni AR, Boggio PS, et al. Clinical predictors associated with duration of repetitive transcranial magnetic stimulation treatment for remission in bipolar depression: a naturalistic study. J Nerv Ment Dis. 2010;198(9):679-681.
10. Connolly KR, Helmer A, Cristancho MA, et al. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567-e573.
11. Dell’osso B, D’Urso N, Castellano F, et al. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: a 1-year follow-up study. J ECT. 2011;27(2):141-144.
12. Dell’Osso B, Mundo E, D’Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11(1):76-81.
13. Harel EV, Zangen A, Roth Y, et al. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119-126.
14. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5(1):40-47.
15. Tamas RL, Menkes D, El-Mallakh RS. Stimulating research: a prospective, randomized, double-blind, sham-controlled study of slow transcranial magnetic stimulation in depressed bipolar patients. J Neuropsychiatry Clin Neurosci. 2007;19(2):198-199.
16. Tundo A, Cavalieri P, Navari S, et al. Treating bipolar depression - antidepressants and alternatives: a critical review of the literature. Acta Neuropsychiatrica. 2011:23(3):94-105.
17. Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161(9):1537-1547.
18. Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant‐emergent mania in bipolar depression: a systematic review and meta‐analysis. Bipolar Disord. 2018;20(3):195-227.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
20. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119-130.
21. Rachid F. Repetitive transcranial magnetic stimulation and treatment-emergent mania and hypomania: a review of the literature. J Psychiatr Pract. 2017;23(2):150-159.
22. Victorin A, Rydén E, Thase M, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
23. Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27-35.
24. Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1.
25. Phillips AL, Burr RL, Dunner DL. Repetitive transcranial magnetic stimulation in the treatment of bipolar depression: Experience from a clinical setting. J Psychiatr Pract. 2020;26(1):37-45.