User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Altha J. Stewart, MD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Altha J. Stewart, MD. Dr. Stewart is Senior Associate Dean for Community Health Engagement at the University of Tennessee Health Science Center (UTHSC)–Memphis. She also serves as Chief of the Division of Social and Community Psychiatry and Director, Center for Health in Justice Involved Youth at UTHSC, where she manages community-based programs serving children impacted by trauma and mental illness and their families. In 2018, she was elected President of the American Psychiatric Association, the first African American individual elected in the 175-year history of the organization.
Dr. Aftab: Structural racism in academic and organized psychiatry is an issue that is close to your heart. What is your perspective on the current state of structural racism in American psychiatry, and what do you think we can do about it?
Dr. Stewart: That’s a good question to start with because I think the conversations that we need to have in academia in general and in academic psychiatry specifically really do frame the current issues that we are facing, whether we’re talking about eliminating health disparities or achieving mental health equity. Historically, from the very beginning these discussions have been structured in a racist manner. The early days of American psychiatry were very clearly directed towards maintaining a system that excluded large segments of the population of the time, since a particularly violent form of chattel slavery was being practiced in this country.
The mental health care system was primarily designed for the landowning white men of some standing in society, and so there was never any intent to do much in the way of providing quality humane service to people who were not part of that group. What we have today is a system that was designed for a racist societal structure, that was intended to perpetuate certain behaviors, policies, and practices that had at their core a racist framework. We have to acknowledge and start from this beginning point. This is not to blame anyone currently alive. These are larger structural problems. Before we can begin setting up strategic plans and other actions, we have to go back and acknowledge how we got here. We have to accept the responsibility for being here, and then we have to allow the conversations that need to happen to happen in a safe way, without further alienating people, or maligning and demeaning people who are for the most part well-intentioned but perhaps operating on automatic pilot in a system that is structurally racist.
Dr. Aftab: Do you think that the conversations that need to happen are taking place?
Dr. Stewart: Yes, I think they are beginning to happen. I do a fair number of talks and grand rounds, and what I discover when I meet with different academic departments and different groups is that most places now have a diversity committee, or the residents and students have assigned themselves as diversity leaders. They are really pushing to have these conversations, to insert these conversations into the training and education curricula. The structures in power are so deeply entrenched that many people, particularly younger people, are easily frustrated by the lack of forward motion. One of the things that seasoned leaders in psychiatry have to do is to help everyone understand that the movement forward might be glacial in the beginning, but any movement forward is good when it comes to this. The psychiatrists of my generation talked about cultural competence in psychiatry, but generations of today talk about structural competence. These are similar concepts, except that cultural competency worked within the traditional model, while structural competency recognizes that the system itself needs to change. I find this development very encouraging.
Dr. Aftab: What do you see as some of the strengths of our profession?
Continue to: Dr. Stewart
Dr. Stewart: I am a hopeful optimist when it comes to psychiatry. I have dedicated my professional life to psychiatry and specifically to community psychiatry. Throughout the time that I have practiced psychiatry, I have been encouraged that what we do as a medical specialty really does improve the quality of life for the people we serve. Situationally right now, we’re in a unique position because the COVID pandemic has laid open and then laid bare the whole issue of how we deal with psychological distress, whether it’s diagnosed mental illness or a natural, normal response to a catastrophic event. We are the experts in this. This is our sweet spot, our wheelhouse, whatever analogy you prefer. This is the moment where we assert our expertise as the leaders—not as service add-ons, not as followers, not as adjuncts, but as the leaders.
I am so impressed with the next generation of psychiatrists. They have a wonderful blend of pride and privilege at what they have been able to accomplish to get to the point where they are doctors and psychiatrists, but they have aligned that with a strong core sense of social justice, and they are moved by their responsibility to the people in the society around them.
Another strength of our profession is what we consider to be the “art” of psychiatry. That is, the way we marry the relational aspects of psychiatry with the biological, technical, and digital aspects to arrive at a happy collaboration that benefits people. It is our great skill to engage people, to interact with them therapeutically, to recognize and acknowledge the nonverbal cues. This skill will be even more important in the age of online mental health services. I’m an “old-school” therapist. I like that face-to-face interaction. I think it’s important to preserve that aspect of our practice, even as we move towards online services.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Stewart: I don’t think we can afford to remain in status quo, because we need to constantly think and rethink, evaluate and re-evaluate, assess things in the light of new information. Particularly if we’re talking about people who rely on public funding to get even the bare minimum services, status quo doesn’t cut it. It’s not good enough. I had a teacher during my residency, a child psychiatrist, who used to say, “Good, better, best. Never let it rest, until your good is better and your better is best.” Something about that has stuck with me. As my career progressed, I heard variations of it, including one from former Surgeon General of the United States David Satcher, who was not a psychiatrist, but pulled together the group that published the first Surgeon General’s report on mental health, followed by the Surgeon General’s report on mental health, culture, race, and ethnicity. He had the penetrating insight that risk factors are not to be accepted as predictive factors due to protective factors. If I am at risk for mental illness or a chronic medical condition based on my race or ethnicity or socioeconomic status or employment status, this does not mean that I am destined to experience that illness. In fact, we are not doing our job if we accept these outcomes as inevitable and make no attempt to change them. So, for me, if we accept the status quo, we give up on the message of “Good, better, best. Never let it rest, until your good is better and your better is best.”
Continue to: Dr. Aftab
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Stewart: Well, this is going to sound harsh, and I do hope that the readers do not feel that I intend it to be harsh. We get in our own way. I work in the public sector, for example, and the reality is that there aren’t enough psychiatrists to provide all the necessary psychiatric services for the people who need them. So many mental health clinics and practices employ other mental health professionals, whether they are psychologists or nurse practitioners or physician assistants with special training in mental health to provide those services. To have a blanket concern about anyone who is not an MD practicing in what is considered “our area” just begs the question that if we can’t do it and we don’t have enough psychiatrists to do it, should people just not get mental health treatment? Is that the solution? I don’t think so. I don’t think that’s what people want, either, but because of the energy that gets aroused around these issues, we lose sight of that end goal. I think the answer is that we must take leadership for ensuring that our colleagues are well-trained, maybe not as well-trained as physicians, but well-trained enough to provide good care working under our supervision.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Stewart: I think we are moving naturally into the space of integrated or collaborative care. I think we’re going to have to acknowledge that going forward, the path to being a good psychiatrist means that we will also be consultants. Not just the consultation-liaison kind of consultant that we typically think of, but a consultant to the rest of medicine around shaping programs, addressing how we treat comorbid illness, looking at ways to minimize the morbidity and mortality associated with some of the chronic medical and mental diseases. We’re moving naturally in that direction. For some people, that must be frightening. All throughout medicine people are witnessing change, and we need to adapt. I would hope that the specialty that is designed to help others deal with change will figure out how to use those skills to help themselves deal with the changes that are coming!
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Altha J. Stewart, MD. Dr. Stewart is Senior Associate Dean for Community Health Engagement at the University of Tennessee Health Science Center (UTHSC)–Memphis. She also serves as Chief of the Division of Social and Community Psychiatry and Director, Center for Health in Justice Involved Youth at UTHSC, where she manages community-based programs serving children impacted by trauma and mental illness and their families. In 2018, she was elected President of the American Psychiatric Association, the first African American individual elected in the 175-year history of the organization.
Dr. Aftab: Structural racism in academic and organized psychiatry is an issue that is close to your heart. What is your perspective on the current state of structural racism in American psychiatry, and what do you think we can do about it?
Dr. Stewart: That’s a good question to start with because I think the conversations that we need to have in academia in general and in academic psychiatry specifically really do frame the current issues that we are facing, whether we’re talking about eliminating health disparities or achieving mental health equity. Historically, from the very beginning these discussions have been structured in a racist manner. The early days of American psychiatry were very clearly directed towards maintaining a system that excluded large segments of the population of the time, since a particularly violent form of chattel slavery was being practiced in this country.
The mental health care system was primarily designed for the landowning white men of some standing in society, and so there was never any intent to do much in the way of providing quality humane service to people who were not part of that group. What we have today is a system that was designed for a racist societal structure, that was intended to perpetuate certain behaviors, policies, and practices that had at their core a racist framework. We have to acknowledge and start from this beginning point. This is not to blame anyone currently alive. These are larger structural problems. Before we can begin setting up strategic plans and other actions, we have to go back and acknowledge how we got here. We have to accept the responsibility for being here, and then we have to allow the conversations that need to happen to happen in a safe way, without further alienating people, or maligning and demeaning people who are for the most part well-intentioned but perhaps operating on automatic pilot in a system that is structurally racist.
Dr. Aftab: Do you think that the conversations that need to happen are taking place?
Dr. Stewart: Yes, I think they are beginning to happen. I do a fair number of talks and grand rounds, and what I discover when I meet with different academic departments and different groups is that most places now have a diversity committee, or the residents and students have assigned themselves as diversity leaders. They are really pushing to have these conversations, to insert these conversations into the training and education curricula. The structures in power are so deeply entrenched that many people, particularly younger people, are easily frustrated by the lack of forward motion. One of the things that seasoned leaders in psychiatry have to do is to help everyone understand that the movement forward might be glacial in the beginning, but any movement forward is good when it comes to this. The psychiatrists of my generation talked about cultural competence in psychiatry, but generations of today talk about structural competence. These are similar concepts, except that cultural competency worked within the traditional model, while structural competency recognizes that the system itself needs to change. I find this development very encouraging.
Dr. Aftab: What do you see as some of the strengths of our profession?
Continue to: Dr. Stewart
Dr. Stewart: I am a hopeful optimist when it comes to psychiatry. I have dedicated my professional life to psychiatry and specifically to community psychiatry. Throughout the time that I have practiced psychiatry, I have been encouraged that what we do as a medical specialty really does improve the quality of life for the people we serve. Situationally right now, we’re in a unique position because the COVID pandemic has laid open and then laid bare the whole issue of how we deal with psychological distress, whether it’s diagnosed mental illness or a natural, normal response to a catastrophic event. We are the experts in this. This is our sweet spot, our wheelhouse, whatever analogy you prefer. This is the moment where we assert our expertise as the leaders—not as service add-ons, not as followers, not as adjuncts, but as the leaders.
I am so impressed with the next generation of psychiatrists. They have a wonderful blend of pride and privilege at what they have been able to accomplish to get to the point where they are doctors and psychiatrists, but they have aligned that with a strong core sense of social justice, and they are moved by their responsibility to the people in the society around them.
Another strength of our profession is what we consider to be the “art” of psychiatry. That is, the way we marry the relational aspects of psychiatry with the biological, technical, and digital aspects to arrive at a happy collaboration that benefits people. It is our great skill to engage people, to interact with them therapeutically, to recognize and acknowledge the nonverbal cues. This skill will be even more important in the age of online mental health services. I’m an “old-school” therapist. I like that face-to-face interaction. I think it’s important to preserve that aspect of our practice, even as we move towards online services.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Stewart: I don’t think we can afford to remain in status quo, because we need to constantly think and rethink, evaluate and re-evaluate, assess things in the light of new information. Particularly if we’re talking about people who rely on public funding to get even the bare minimum services, status quo doesn’t cut it. It’s not good enough. I had a teacher during my residency, a child psychiatrist, who used to say, “Good, better, best. Never let it rest, until your good is better and your better is best.” Something about that has stuck with me. As my career progressed, I heard variations of it, including one from former Surgeon General of the United States David Satcher, who was not a psychiatrist, but pulled together the group that published the first Surgeon General’s report on mental health, followed by the Surgeon General’s report on mental health, culture, race, and ethnicity. He had the penetrating insight that risk factors are not to be accepted as predictive factors due to protective factors. If I am at risk for mental illness or a chronic medical condition based on my race or ethnicity or socioeconomic status or employment status, this does not mean that I am destined to experience that illness. In fact, we are not doing our job if we accept these outcomes as inevitable and make no attempt to change them. So, for me, if we accept the status quo, we give up on the message of “Good, better, best. Never let it rest, until your good is better and your better is best.”
Continue to: Dr. Aftab
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Stewart: Well, this is going to sound harsh, and I do hope that the readers do not feel that I intend it to be harsh. We get in our own way. I work in the public sector, for example, and the reality is that there aren’t enough psychiatrists to provide all the necessary psychiatric services for the people who need them. So many mental health clinics and practices employ other mental health professionals, whether they are psychologists or nurse practitioners or physician assistants with special training in mental health to provide those services. To have a blanket concern about anyone who is not an MD practicing in what is considered “our area” just begs the question that if we can’t do it and we don’t have enough psychiatrists to do it, should people just not get mental health treatment? Is that the solution? I don’t think so. I don’t think that’s what people want, either, but because of the energy that gets aroused around these issues, we lose sight of that end goal. I think the answer is that we must take leadership for ensuring that our colleagues are well-trained, maybe not as well-trained as physicians, but well-trained enough to provide good care working under our supervision.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Stewart: I think we are moving naturally into the space of integrated or collaborative care. I think we’re going to have to acknowledge that going forward, the path to being a good psychiatrist means that we will also be consultants. Not just the consultation-liaison kind of consultant that we typically think of, but a consultant to the rest of medicine around shaping programs, addressing how we treat comorbid illness, looking at ways to minimize the morbidity and mortality associated with some of the chronic medical and mental diseases. We’re moving naturally in that direction. For some people, that must be frightening. All throughout medicine people are witnessing change, and we need to adapt. I would hope that the specialty that is designed to help others deal with change will figure out how to use those skills to help themselves deal with the changes that are coming!
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Altha J. Stewart, MD. Dr. Stewart is Senior Associate Dean for Community Health Engagement at the University of Tennessee Health Science Center (UTHSC)–Memphis. She also serves as Chief of the Division of Social and Community Psychiatry and Director, Center for Health in Justice Involved Youth at UTHSC, where she manages community-based programs serving children impacted by trauma and mental illness and their families. In 2018, she was elected President of the American Psychiatric Association, the first African American individual elected in the 175-year history of the organization.
Dr. Aftab: Structural racism in academic and organized psychiatry is an issue that is close to your heart. What is your perspective on the current state of structural racism in American psychiatry, and what do you think we can do about it?
Dr. Stewart: That’s a good question to start with because I think the conversations that we need to have in academia in general and in academic psychiatry specifically really do frame the current issues that we are facing, whether we’re talking about eliminating health disparities or achieving mental health equity. Historically, from the very beginning these discussions have been structured in a racist manner. The early days of American psychiatry were very clearly directed towards maintaining a system that excluded large segments of the population of the time, since a particularly violent form of chattel slavery was being practiced in this country.
The mental health care system was primarily designed for the landowning white men of some standing in society, and so there was never any intent to do much in the way of providing quality humane service to people who were not part of that group. What we have today is a system that was designed for a racist societal structure, that was intended to perpetuate certain behaviors, policies, and practices that had at their core a racist framework. We have to acknowledge and start from this beginning point. This is not to blame anyone currently alive. These are larger structural problems. Before we can begin setting up strategic plans and other actions, we have to go back and acknowledge how we got here. We have to accept the responsibility for being here, and then we have to allow the conversations that need to happen to happen in a safe way, without further alienating people, or maligning and demeaning people who are for the most part well-intentioned but perhaps operating on automatic pilot in a system that is structurally racist.
Dr. Aftab: Do you think that the conversations that need to happen are taking place?
Dr. Stewart: Yes, I think they are beginning to happen. I do a fair number of talks and grand rounds, and what I discover when I meet with different academic departments and different groups is that most places now have a diversity committee, or the residents and students have assigned themselves as diversity leaders. They are really pushing to have these conversations, to insert these conversations into the training and education curricula. The structures in power are so deeply entrenched that many people, particularly younger people, are easily frustrated by the lack of forward motion. One of the things that seasoned leaders in psychiatry have to do is to help everyone understand that the movement forward might be glacial in the beginning, but any movement forward is good when it comes to this. The psychiatrists of my generation talked about cultural competence in psychiatry, but generations of today talk about structural competence. These are similar concepts, except that cultural competency worked within the traditional model, while structural competency recognizes that the system itself needs to change. I find this development very encouraging.
Dr. Aftab: What do you see as some of the strengths of our profession?
Continue to: Dr. Stewart
Dr. Stewart: I am a hopeful optimist when it comes to psychiatry. I have dedicated my professional life to psychiatry and specifically to community psychiatry. Throughout the time that I have practiced psychiatry, I have been encouraged that what we do as a medical specialty really does improve the quality of life for the people we serve. Situationally right now, we’re in a unique position because the COVID pandemic has laid open and then laid bare the whole issue of how we deal with psychological distress, whether it’s diagnosed mental illness or a natural, normal response to a catastrophic event. We are the experts in this. This is our sweet spot, our wheelhouse, whatever analogy you prefer. This is the moment where we assert our expertise as the leaders—not as service add-ons, not as followers, not as adjuncts, but as the leaders.
I am so impressed with the next generation of psychiatrists. They have a wonderful blend of pride and privilege at what they have been able to accomplish to get to the point where they are doctors and psychiatrists, but they have aligned that with a strong core sense of social justice, and they are moved by their responsibility to the people in the society around them.
Another strength of our profession is what we consider to be the “art” of psychiatry. That is, the way we marry the relational aspects of psychiatry with the biological, technical, and digital aspects to arrive at a happy collaboration that benefits people. It is our great skill to engage people, to interact with them therapeutically, to recognize and acknowledge the nonverbal cues. This skill will be even more important in the age of online mental health services. I’m an “old-school” therapist. I like that face-to-face interaction. I think it’s important to preserve that aspect of our practice, even as we move towards online services.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Stewart: I don’t think we can afford to remain in status quo, because we need to constantly think and rethink, evaluate and re-evaluate, assess things in the light of new information. Particularly if we’re talking about people who rely on public funding to get even the bare minimum services, status quo doesn’t cut it. It’s not good enough. I had a teacher during my residency, a child psychiatrist, who used to say, “Good, better, best. Never let it rest, until your good is better and your better is best.” Something about that has stuck with me. As my career progressed, I heard variations of it, including one from former Surgeon General of the United States David Satcher, who was not a psychiatrist, but pulled together the group that published the first Surgeon General’s report on mental health, followed by the Surgeon General’s report on mental health, culture, race, and ethnicity. He had the penetrating insight that risk factors are not to be accepted as predictive factors due to protective factors. If I am at risk for mental illness or a chronic medical condition based on my race or ethnicity or socioeconomic status or employment status, this does not mean that I am destined to experience that illness. In fact, we are not doing our job if we accept these outcomes as inevitable and make no attempt to change them. So, for me, if we accept the status quo, we give up on the message of “Good, better, best. Never let it rest, until your good is better and your better is best.”
Continue to: Dr. Aftab
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Stewart: Well, this is going to sound harsh, and I do hope that the readers do not feel that I intend it to be harsh. We get in our own way. I work in the public sector, for example, and the reality is that there aren’t enough psychiatrists to provide all the necessary psychiatric services for the people who need them. So many mental health clinics and practices employ other mental health professionals, whether they are psychologists or nurse practitioners or physician assistants with special training in mental health to provide those services. To have a blanket concern about anyone who is not an MD practicing in what is considered “our area” just begs the question that if we can’t do it and we don’t have enough psychiatrists to do it, should people just not get mental health treatment? Is that the solution? I don’t think so. I don’t think that’s what people want, either, but because of the energy that gets aroused around these issues, we lose sight of that end goal. I think the answer is that we must take leadership for ensuring that our colleagues are well-trained, maybe not as well-trained as physicians, but well-trained enough to provide good care working under our supervision.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Stewart: I think we are moving naturally into the space of integrated or collaborative care. I think we’re going to have to acknowledge that going forward, the path to being a good psychiatrist means that we will also be consultants. Not just the consultation-liaison kind of consultant that we typically think of, but a consultant to the rest of medicine around shaping programs, addressing how we treat comorbid illness, looking at ways to minimize the morbidity and mortality associated with some of the chronic medical and mental diseases. We’re moving naturally in that direction. For some people, that must be frightening. All throughout medicine people are witnessing change, and we need to adapt. I would hope that the specialty that is designed to help others deal with change will figure out how to use those skills to help themselves deal with the changes that are coming!
Lithium and kidney disease: Understand the risks
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
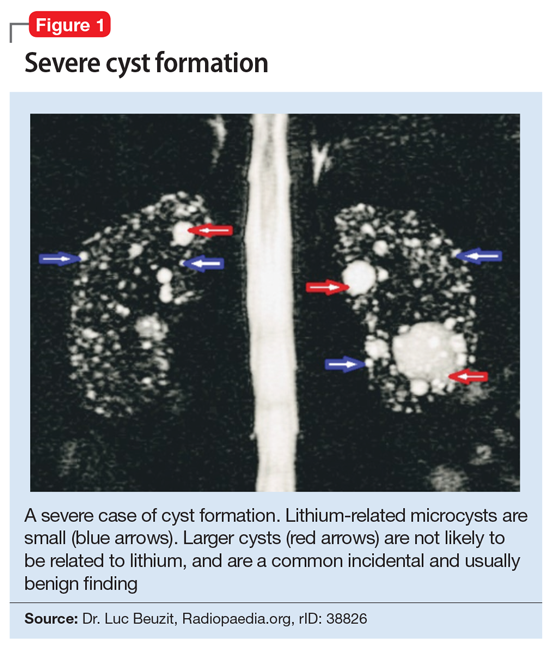
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
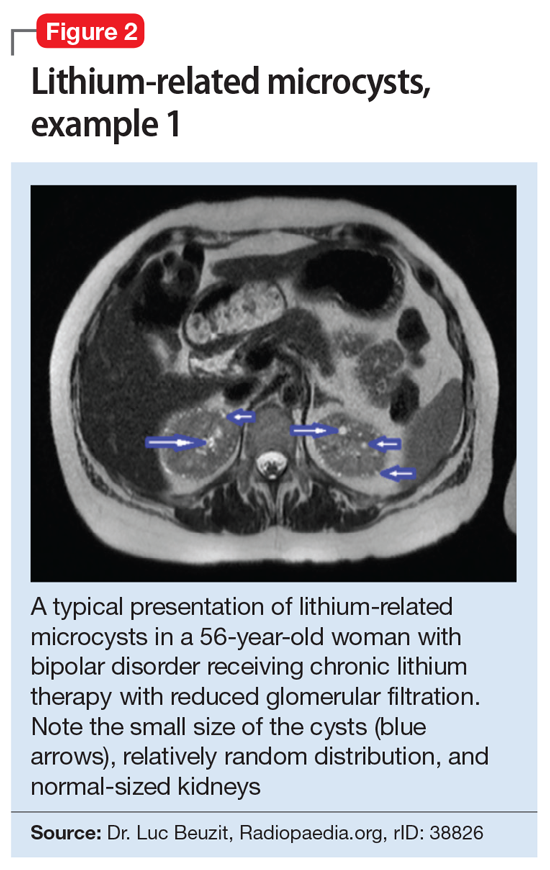
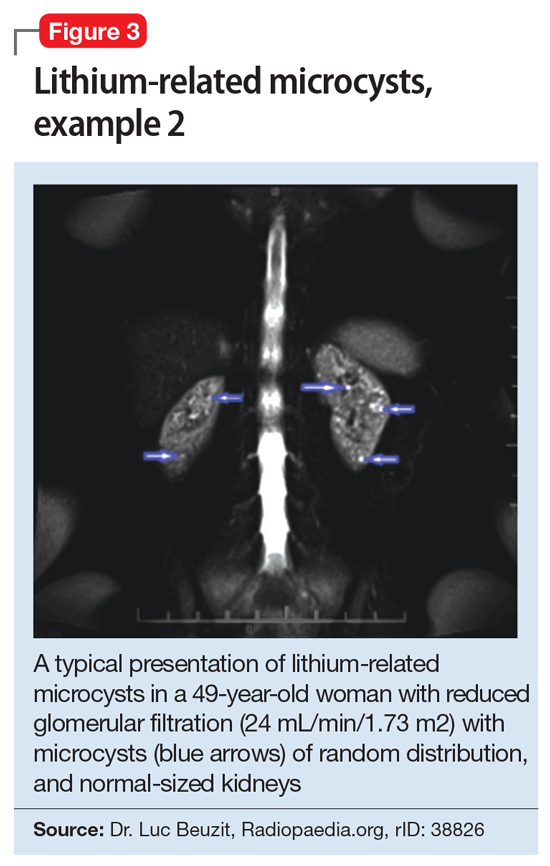
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.

Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37

This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).

Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.

Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37

This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).

Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
Storing patients’ credit card information: Keep it safe
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
Harassment of health care workers: A survey
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?
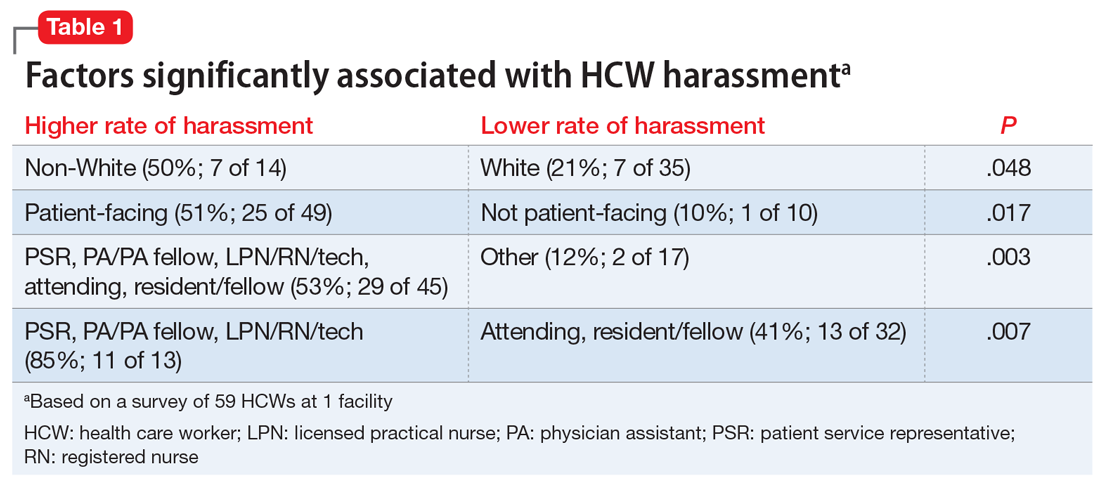
I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).
In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?

I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).

In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?

I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).

In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
Private practice: The basics for psychiatry trainees
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
Efficacy and safety of high-dose antipsychotic therapy
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).
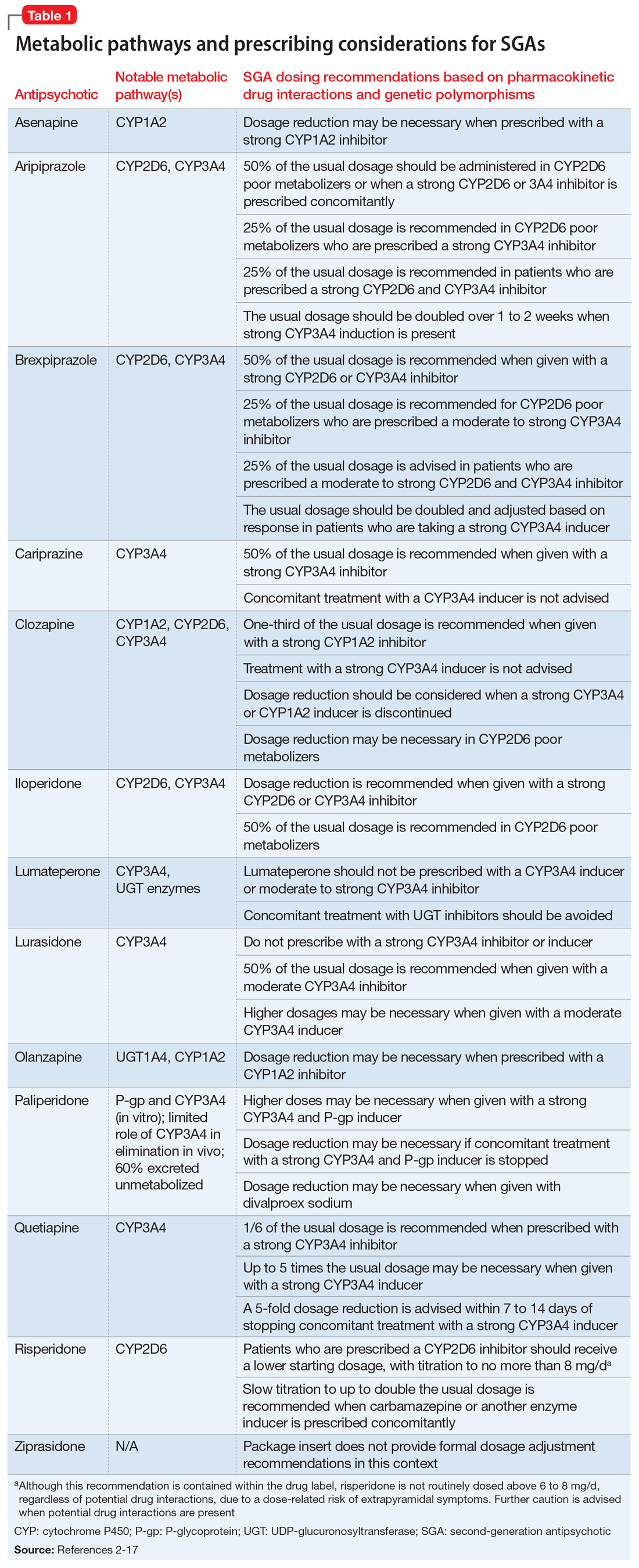
Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.
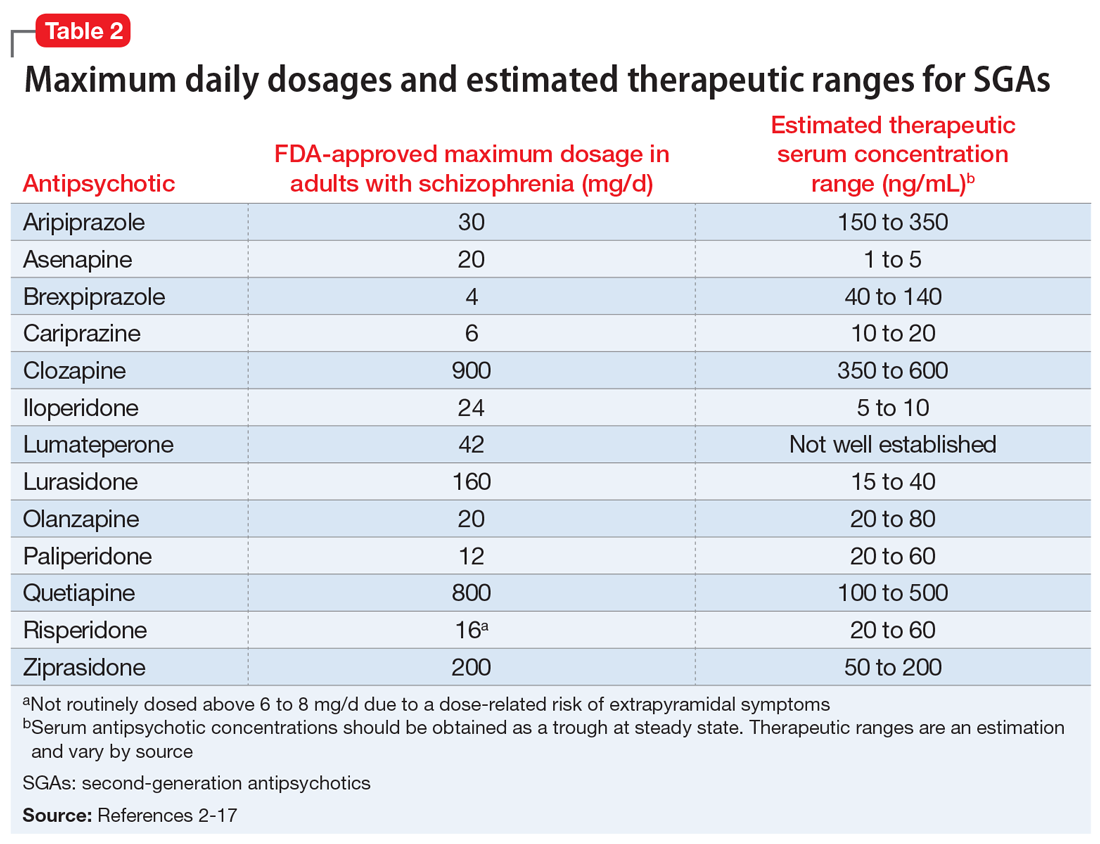
SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).

Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.

SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).

Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.

SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
Psychiatry is Neurology: White matter pathology permeates psychiatric disorders
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.
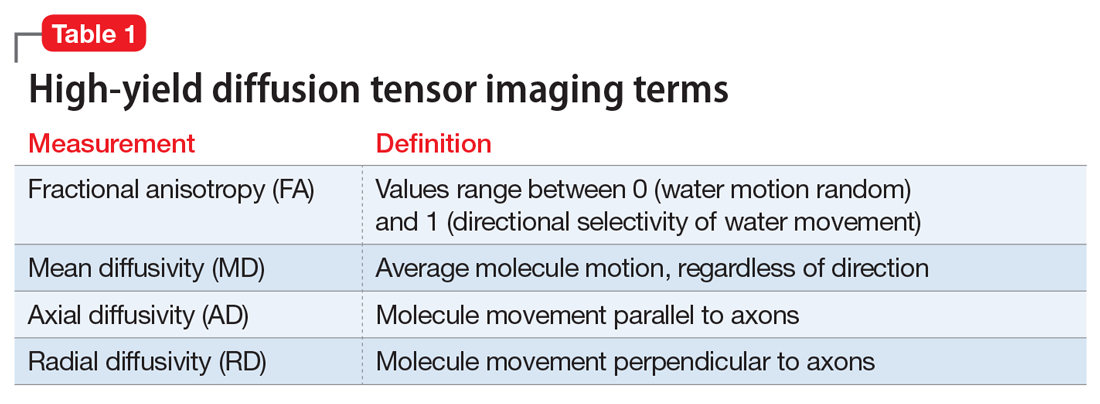
During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28
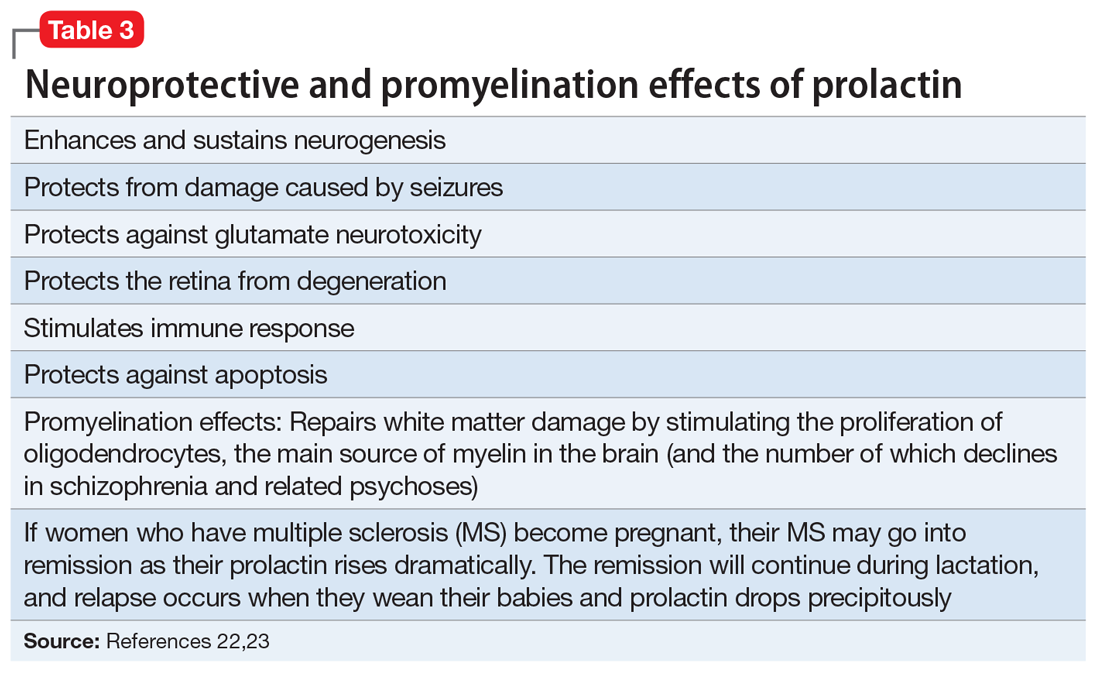
Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.

During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28

Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.

During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28

Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
Measuring cotinine to monitor tobacco use and smoking cessation
Cigarette smoking is common among patients with schizophrenia, mood disorders, anxiety disorders,1-3 substance use disorders (SUDs),4 and other psychiatric disorders. Research suggests that compared with the general population, patients with SUDs consume more nicotine products and are more vulnerable to the effects of smoking.5 Despite the availability of effective treatments, many mental health professionals are reluctant to identify and treat tobacco use disorder,6-8 or they prioritize other disorders over tobacco use. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illness.
Cotinine is a biomarker that can be used to detect tobacco use. It can be measured in routine clinical practice by collecting urinary, serum, or salivary specimens, and used to monitor psychiatric patients’ tobacco use. Monitoring cotinine levels is similar to using other biomarkers to assess medication adherence or identify illicit substance use. A growing body of evidence supports the utility of cotinine screening as a part of a comprehensive substance use disorder treatment plan,5,9,10 especially for:
- patients who have comorbid conditions that can be exacerbated by tobacco use, such as chronic obstructive pulmonary disease
- patients who are pregnant11,12
- patients who are less reliable in self-report or who require objective testing for validation.
Routine clinical screening of tobacco use is recommended for all patients and early detection may facilitate earlier treatment. Several FDA-approved medications are available for smoking cessation13; however, discussion of treatment options is beyond the scope of this review. In this article, we describe how cotinine is measured and analyzed, 3 case vignettes that illustrate its potential clinical utility, and limitations to its use as a biomarker of tobacco use.
Methods of measuring cotinine
Cigarette smoking is associated with the absorption of nicotine, which is mainly metabolized by cytochrome P450 (CYP) 2A6 to 6 primary metabolites: cotinine, hydroxycotinine, norcotinine, nornicotine, cotinine oxide, and nicotine oxide.14,15 Cotinine is the biomarker of choice for detecting use of tobacco/nicotine products due to its stability (it is not influenced by dietary or environmental factors), extended half-life (16 to 19 hours, compared with 2 hours for nicotine), and stable concentration throughout the day. Samples from saliva, urine, or blood can be analyzed through radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and gas/liquid chromatography.16 The specificity of cotinine for tobacco use is excellent, except for persons who are taking medications that contain nicotine.17
An advantage of cotinine over other biomarkers for smoking (such as carbon monoxide in expired air) is that the optimal cut-off points for cotinine are relatively uninfluenced by the prevalence of smoking in the population. The optimal cut-off levels used to detect current tobacco use may vary based on the sample or test used (saliva, urine, or plasma) and certain patient-specific factors (Box 111,16,18-21). However, for plasma or saliva cotinine, 16 ng/mL is the generally accepted cut-off level for detecting current tobacco use. A urinary cotinine cut-off level of 50 ng/mL is likely appropriate for most circumstances.17 Users of electronic nicotine delivery systems (electronic cigarettes) have been found to have cotinine levels similar to those of cigarette smokers.22
Box 1
Daily smokers typically have a serum/plasma cotinine concentration of ≥100 ng/mL. Individuals with heavy exposure to secondhand smoking may have plasma cotinine concentrations up to 25 ng/mL, and urine samples tend to be much more specific.16 However, serum cotinine has a wide cut-off range due to diverse racial/ethnic, gender, and pregnancy-related variations; the wide range is also associated with genetic polymorphisms of cytochrome P450 2A6 alleles and nicotine’s numerous metabolic pathways.11,18
Traditionally a serum/plasma cut-off point of approximately 15 ng/mL has been accepted to detect current tobacco use; however, recent studies21 recommend an average optimal cut-off point for US adults of 3 ng/mL. This possibly reflects differences in national cigarette smoking patterns and exposure.21 One study suggested optimal cut-off differences for men (1.78 ng/mL) and women (4.47 ng/mL).19 The same study also suggested different optimal cut-off levels for non-Hispanic White men (6.79 ng/ mL), non-Hispanic Black men (13.3 ng/mL), and Mexican-American men (0.79 ng/mL).19 These researchers also suggested different optimal cut-off levels for non-Hispanic White women (4.73 ng/mL), non-Hispanic Black women (5.91 ng/mL), and Mexican-American women (0.84 ng/mL).19 Genetic factors may also play a role in the progression of nicotine dependence and pose challenges that impact smoking persistence.20
Assessment of cotinine levels in saliva may be considered for outpatient monitoring due to its noninvasive nature, tolerability, and the ability to collect multiple samples over a limited period.23 Saliva cotinine levels correlate closely with blood concentrations. Urine cotinine levels offer some advantage because concentrations are 6 times higher in urine than in blood or saliva. For this reason, urine cotinine is the most widely used biomarker in individuals who use tobacco due to its high sensitivity, specificity, reliability, and noninvasive collection.23 By using a lower urinary cut-off of ≥2.47 ng/mL, ELISA kits detect the highest sensitivity and specificity, which is useful for monitoring daily tobacco use.24 This cut-off value was associated with 100% sensitivity and specificity, and these numbers declined with increases in the cut-off threshold.23
The following 3 clinical vignettes illustrate the impact of tobacco use disorder on patients, and how cotinine might help with their treatment.
Continue to: Vignette 1
Vignette 1
Mr. D, age 44, has a history of schizophrenia and has smoked 1 pack of cigarettes per day for the last 15 years. He was recently discharged from an inpatient psychiatric facility after his symptoms were stabilized. During his hospitalization, Mr. D used a nicotine-replacement product to comply with the hospital’s smoke-free policy. Unfortunately, since discharge, Mr. D reports worsening auditory hallucinations despite adherence with his antipsychotic medication, clozapine, 600 mg at bedtime. Collateral information gathered from Mr. D’s mother confirms that he has been adherent with the discharge medication regimen; however, Mr. D has resumed smoking 1 pack of cigarettes daily. The treatment team suspects that his worsening psychosis is related to the decrease of blood clozapine level due to CYP induction by cigarette smoke.
Cotinine and smoking-related drug interactions
Vignette 1 illustrates the significant impact tobacco smoke can have on the effectiveness of a psychotropic medication. This is caused by polycyclic aromatic hydrocarbons induction of hepatic CYP1A2 isoenzymes. Clinicians should routinely screen patients for smoking status due to the potential for drug interactions. Common major CYP1A2 substrates include
Vignette 2
Mr. B, age 34, has a history of cocaine use disorder and tobacco use disorder. He is referred to a treatment program and participates in a contingency management program for his substance use disorders. Biomarkers, including salivary cotinine, are used to assess Mr. B’s exposure to tobacco use. Mr. B and other participants in his program are eligible for prize draws if they are found to have samples that are negative for tobacco and other substances. There are other incentives in place for patients who show a reduced cotinine concentration.
Cotinine monitoring and contingency management
Clinicians can incorporate cotinine monitoring into existing SUD treatment. This is similar to the utilization of other biomarkers that are commonly used to identify recent illicit substance use or monitor adherence to treatment medications. For example, benzoylecgonine, a metabolite of cocaine, is frequently used to monitor abstinence from cocaine.
Treatments based on contingency management principles involve giving patients tangible rewards to reinforce desired (positive) behaviors. Smoking cessation can be confirmed by monitoring cotinine levels. Gayman et al9 found twice-weekly salivary testing was compatible with monitoring and promoting abstinence in a prize-based contingency management smoking cessation program. Most prior studies used urine cotinine measures to verify abstinence. Although highly reliable, urine samples require close monitoring to ensure sample validity, which can be a burden on staff and unpleasant for patients.9 It is also important to note that the rate of elimination of cotinine from saliva and urine are comparable. The half-life of cotinine is approximately 18 hours, and therefore the specificity of salivary test strips may be impacted during the first 4 to 5 days of abstinence. In the first few days of smoking cessation, a more intensive approach, such as quantifying urine cotinine levels and monitoring decline, may be appropriate.23
Continue to: Vignette 3
Vignette 3
Ms. C, age 34 and pregnant, is admitted to an outpatient treatment program for alcohol use disorder. She also has generalized anxiety disorder and tobacco use disorder. In addition to attending group therapy sessions and self-reporting any recent alcohol consumption, Ms. C also undergoes alcohol breathalyzer tests and urine studies of alcohol metabolites to monitor abstinence from alcohol. She says that the regular laboratory screening for alcohol use gives her a sense of accountability and tangible evidence of change that positively impacts her treatment. When the treating psychiatrist recommends that Ms. C also consider addressing her tobacco use disorder, she asks if there is some way to include laboratory testing to monitor her smoking cessation.
Cotinine as a predictor of smoking status
Smoking abstinence rates during pregnancy are lower than that for other substances, and pregnant women may not be aware of the impact of smoking on fetal development.30 Cotinine can be used to verify self-report of smoking status and severity.10,31,32
Salivary cotinine tests are commercially available, relatively economical, and convenient to use when frequent monitoring is required.32 In general, based on established cut-off values that are unique to the specimen collected, the overall high specificity and sensitivity of salivary testing allows clinicians to predict smoker vs nonsmoker status with confidence. For example, a 2008 study reported a salivary cotinine cut-off level of 12 ng/mL for smokers.21 The sensitivity and specificity of this cut-off value for distinguishing cigarette smokers from never smokers were 96.7% and 96.9%, respectively.21
Additionally, some studies suggest that cotinine levels may be predictive of treatment outcomes and retention in SUD treatment programs.33,34 One study of smoking cessation using nicotine replacement products found that compared with patients with lower baseline cotinine levels prior to treatment, patients with higher baseline cotinine plasma levels had lower smoking cessation success rates.34
A few caveats
There are several limitations to quantitative measures of cotinine (Box 221,23). These include (but are not limited to) potential errors related to sample collection, storage, shipping, and analysis.23 Compared with other methods, point-of-care cotinine measurement in saliva is noninvasive, simple, and requires less training to properly use.23
Box 2
Challenges in the collection of samples, storage, shipping, and instrumentation may limit cotinine consistency as a dependable biomarker in the clinical setting.23 Overall, quantitative measurements of cotinine have relative constructive utility in separating smokers from nonsmokers, because daily smokers typically have serum concentrations of 100 ng/mL or higher, in contrast to light/non-daily smokers, who have cotinine concentrations <10 ng/mL. Even heavy exposure to secondhand smoke typically yields plasma concentrations up to approximately 25 ng/mL. However, cotinine is a general metabolite found with the use of all nicotine products, which makes it extremely difficult to differentiate tobacco use from the use of nicotine replacement products, which are frequently used to treat tobacco use disorders.
One potential solution is to measure nicotine-derived nitrosamine ketone (NNK) and its metabolite 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanol (NNAL). Both NNK and NNAL are tobacco-specific lung carcinogens. NNAL can be measured in the urine. Although total NNAL represents only 15% of NNK dose intake, it has been quantified, with urine concentrations of ≥1,000 fmol/mL for daily smokers. NNAL also has an extremely high specificity to tobacco smoke, and thus allows differentiation of tobacco use from nicotine replacement treatment. Unfortunately, measurement for this biomarker requires specific chemical expertise and expensive equipment.
Another potential barrier to using cotinine in the clinical setting is the variable cut-off levels used in the United States, based on differences in race/ethnicity. This may be secondary to differences in smoking behaviors and/or differences in cotinine metabolism.21
Continue to: Confirmation of smoking cessation...
Confirmation of smoking cessation can be monitored reliably within the clinical setting using cotinine monitoring. However, this is not a routine test, and there are no guidelines or consensus on how or when it should be used. The clinical feasibility of cotinine monitoring for psychiatric patients will depend on the cost of testing, methods used, amount of reimbursement for performing the tests, and how clinicians value such testing.35
Bottom Line
Cotinine is a biomarker that can be used to detect tobacco use. Cotinine measurement can be used to monitor tobacco use and smoking cessation in psychiatric patients. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illnesses. However, cotinine measurement is not a routine test, and there are no guidelines on how or when this test should be used.
Related Resources
- Peckham E, Brabyn S, Cook L, et al. Smoking cessation in severe mental ill health: what works? An updated systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):252.
- Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065
Drug Brand Names
Asenapine • Saphris
Buprenorphine • Sublocade
Clozapine • Clozaril
Duloxetine • Cymbalta
Haloperidol • Haldol
Mirtazapine • Remeron
Olanzapine • Zyprexa
Ziprasidone • Geodon
Zolpidem • Ambien
1. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185.
2. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12.
3. Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106-123.
5. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
6. Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808-1814.
7. McHugh RK, Votaw VR, Fulciniti F, et al. Perceived barriers to smoking cessation among adults with substance use disorders. J Subst Abuse Treat. 2017;74:48-53.
8. Strong DR, Uebelacker L, Fokas K, et al. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. J Addict Med. 2014;8(2):77-83.
9. Gayman C, Anderson K, Pietras C. Saliva cotinine as a measure of smoking abstinence in contingency management – a feasibility study. The Psychological Record. 2017;67(2):261-272.
10. Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10(9):1495-1501.
11. Stragierowicz J, Mikolajewska K, Zawadzka-Stolarz M, et al. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int. 2013;2013:386784. doi.org/10.1155/2013/386784
12. Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi.org/10.1136/bmj.b4347
13. Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17(14):1343-1350.
14. McGuffey JE, Wei B, Bernert JT, et al. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers’ urine. PLoS One. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816
15. Duque A, Martinez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425-e431. doi: 10.4317/medoral.21292
16. Avila-Tang E, Elf JL, Cummings KM, et al. Assessing secondhand smoke exposure with reported measures. Tob Control. 2013;22(3):156-163.
17. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 Update. Nicotine Tob Res. 2020;22(7):1086-1097.
18. Nakajima M TY. Interindividual variability in nicotine metabolism: c-oxidation and glucuronidation. Drug Metab Pharmaokinet. 2005;20(4):227-235.
19. Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236-248.
20. Schnoll R, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psychiatry Rep. 2007;9(5):349-357.
21. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13(12):1236.
22. Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219-1220.
23. Raja M, Garg A, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04-06. doi: 10.7860/JCDR/2016/17360.7423
24. Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9(1):84-89.
25. Fankhauser M. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
26. Scheuermann TS, Richter KP, Rigotti NA, et al. Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227-2236.
27. Holtyn AF, Knealing TW, Jarvis BP, et al. Monitoring cocaine use and abstinence among cocaine users for contingency management interventions. Psychol Rec. 2017;67(2):253-259.
28. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51.
29. Sullivan M, Covey, LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4(5):388-396.
30. Forray A, Merry B, Lin H, et al. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147-155.
31. Parker DR, Lasater TM, Windsor R, et al. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2002;4(3):305-309.
32. Asha V, Dhanya M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J Cancer Prev. 2015;20(2):159-163.
33. Hall S, Herning RI, Jones RT, et al. Blood cotinine levels as indicators of smoking treatment outcome. Clin Pharmacol Ther. 1984;35(6):810-814.
34. Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9(4):643-651.
35. Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1858-1862.
Cigarette smoking is common among patients with schizophrenia, mood disorders, anxiety disorders,1-3 substance use disorders (SUDs),4 and other psychiatric disorders. Research suggests that compared with the general population, patients with SUDs consume more nicotine products and are more vulnerable to the effects of smoking.5 Despite the availability of effective treatments, many mental health professionals are reluctant to identify and treat tobacco use disorder,6-8 or they prioritize other disorders over tobacco use. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illness.
Cotinine is a biomarker that can be used to detect tobacco use. It can be measured in routine clinical practice by collecting urinary, serum, or salivary specimens, and used to monitor psychiatric patients’ tobacco use. Monitoring cotinine levels is similar to using other biomarkers to assess medication adherence or identify illicit substance use. A growing body of evidence supports the utility of cotinine screening as a part of a comprehensive substance use disorder treatment plan,5,9,10 especially for:
- patients who have comorbid conditions that can be exacerbated by tobacco use, such as chronic obstructive pulmonary disease
- patients who are pregnant11,12
- patients who are less reliable in self-report or who require objective testing for validation.
Routine clinical screening of tobacco use is recommended for all patients and early detection may facilitate earlier treatment. Several FDA-approved medications are available for smoking cessation13; however, discussion of treatment options is beyond the scope of this review. In this article, we describe how cotinine is measured and analyzed, 3 case vignettes that illustrate its potential clinical utility, and limitations to its use as a biomarker of tobacco use.
Methods of measuring cotinine
Cigarette smoking is associated with the absorption of nicotine, which is mainly metabolized by cytochrome P450 (CYP) 2A6 to 6 primary metabolites: cotinine, hydroxycotinine, norcotinine, nornicotine, cotinine oxide, and nicotine oxide.14,15 Cotinine is the biomarker of choice for detecting use of tobacco/nicotine products due to its stability (it is not influenced by dietary or environmental factors), extended half-life (16 to 19 hours, compared with 2 hours for nicotine), and stable concentration throughout the day. Samples from saliva, urine, or blood can be analyzed through radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and gas/liquid chromatography.16 The specificity of cotinine for tobacco use is excellent, except for persons who are taking medications that contain nicotine.17
An advantage of cotinine over other biomarkers for smoking (such as carbon monoxide in expired air) is that the optimal cut-off points for cotinine are relatively uninfluenced by the prevalence of smoking in the population. The optimal cut-off levels used to detect current tobacco use may vary based on the sample or test used (saliva, urine, or plasma) and certain patient-specific factors (Box 111,16,18-21). However, for plasma or saliva cotinine, 16 ng/mL is the generally accepted cut-off level for detecting current tobacco use. A urinary cotinine cut-off level of 50 ng/mL is likely appropriate for most circumstances.17 Users of electronic nicotine delivery systems (electronic cigarettes) have been found to have cotinine levels similar to those of cigarette smokers.22
Box 1
Daily smokers typically have a serum/plasma cotinine concentration of ≥100 ng/mL. Individuals with heavy exposure to secondhand smoking may have plasma cotinine concentrations up to 25 ng/mL, and urine samples tend to be much more specific.16 However, serum cotinine has a wide cut-off range due to diverse racial/ethnic, gender, and pregnancy-related variations; the wide range is also associated with genetic polymorphisms of cytochrome P450 2A6 alleles and nicotine’s numerous metabolic pathways.11,18
Traditionally a serum/plasma cut-off point of approximately 15 ng/mL has been accepted to detect current tobacco use; however, recent studies21 recommend an average optimal cut-off point for US adults of 3 ng/mL. This possibly reflects differences in national cigarette smoking patterns and exposure.21 One study suggested optimal cut-off differences for men (1.78 ng/mL) and women (4.47 ng/mL).19 The same study also suggested different optimal cut-off levels for non-Hispanic White men (6.79 ng/ mL), non-Hispanic Black men (13.3 ng/mL), and Mexican-American men (0.79 ng/mL).19 These researchers also suggested different optimal cut-off levels for non-Hispanic White women (4.73 ng/mL), non-Hispanic Black women (5.91 ng/mL), and Mexican-American women (0.84 ng/mL).19 Genetic factors may also play a role in the progression of nicotine dependence and pose challenges that impact smoking persistence.20
Assessment of cotinine levels in saliva may be considered for outpatient monitoring due to its noninvasive nature, tolerability, and the ability to collect multiple samples over a limited period.23 Saliva cotinine levels correlate closely with blood concentrations. Urine cotinine levels offer some advantage because concentrations are 6 times higher in urine than in blood or saliva. For this reason, urine cotinine is the most widely used biomarker in individuals who use tobacco due to its high sensitivity, specificity, reliability, and noninvasive collection.23 By using a lower urinary cut-off of ≥2.47 ng/mL, ELISA kits detect the highest sensitivity and specificity, which is useful for monitoring daily tobacco use.24 This cut-off value was associated with 100% sensitivity and specificity, and these numbers declined with increases in the cut-off threshold.23
The following 3 clinical vignettes illustrate the impact of tobacco use disorder on patients, and how cotinine might help with their treatment.
Continue to: Vignette 1
Vignette 1
Mr. D, age 44, has a history of schizophrenia and has smoked 1 pack of cigarettes per day for the last 15 years. He was recently discharged from an inpatient psychiatric facility after his symptoms were stabilized. During his hospitalization, Mr. D used a nicotine-replacement product to comply with the hospital’s smoke-free policy. Unfortunately, since discharge, Mr. D reports worsening auditory hallucinations despite adherence with his antipsychotic medication, clozapine, 600 mg at bedtime. Collateral information gathered from Mr. D’s mother confirms that he has been adherent with the discharge medication regimen; however, Mr. D has resumed smoking 1 pack of cigarettes daily. The treatment team suspects that his worsening psychosis is related to the decrease of blood clozapine level due to CYP induction by cigarette smoke.
Cotinine and smoking-related drug interactions
Vignette 1 illustrates the significant impact tobacco smoke can have on the effectiveness of a psychotropic medication. This is caused by polycyclic aromatic hydrocarbons induction of hepatic CYP1A2 isoenzymes. Clinicians should routinely screen patients for smoking status due to the potential for drug interactions. Common major CYP1A2 substrates include
Vignette 2
Mr. B, age 34, has a history of cocaine use disorder and tobacco use disorder. He is referred to a treatment program and participates in a contingency management program for his substance use disorders. Biomarkers, including salivary cotinine, are used to assess Mr. B’s exposure to tobacco use. Mr. B and other participants in his program are eligible for prize draws if they are found to have samples that are negative for tobacco and other substances. There are other incentives in place for patients who show a reduced cotinine concentration.
Cotinine monitoring and contingency management
Clinicians can incorporate cotinine monitoring into existing SUD treatment. This is similar to the utilization of other biomarkers that are commonly used to identify recent illicit substance use or monitor adherence to treatment medications. For example, benzoylecgonine, a metabolite of cocaine, is frequently used to monitor abstinence from cocaine.
Treatments based on contingency management principles involve giving patients tangible rewards to reinforce desired (positive) behaviors. Smoking cessation can be confirmed by monitoring cotinine levels. Gayman et al9 found twice-weekly salivary testing was compatible with monitoring and promoting abstinence in a prize-based contingency management smoking cessation program. Most prior studies used urine cotinine measures to verify abstinence. Although highly reliable, urine samples require close monitoring to ensure sample validity, which can be a burden on staff and unpleasant for patients.9 It is also important to note that the rate of elimination of cotinine from saliva and urine are comparable. The half-life of cotinine is approximately 18 hours, and therefore the specificity of salivary test strips may be impacted during the first 4 to 5 days of abstinence. In the first few days of smoking cessation, a more intensive approach, such as quantifying urine cotinine levels and monitoring decline, may be appropriate.23
Continue to: Vignette 3
Vignette 3
Ms. C, age 34 and pregnant, is admitted to an outpatient treatment program for alcohol use disorder. She also has generalized anxiety disorder and tobacco use disorder. In addition to attending group therapy sessions and self-reporting any recent alcohol consumption, Ms. C also undergoes alcohol breathalyzer tests and urine studies of alcohol metabolites to monitor abstinence from alcohol. She says that the regular laboratory screening for alcohol use gives her a sense of accountability and tangible evidence of change that positively impacts her treatment. When the treating psychiatrist recommends that Ms. C also consider addressing her tobacco use disorder, she asks if there is some way to include laboratory testing to monitor her smoking cessation.
Cotinine as a predictor of smoking status
Smoking abstinence rates during pregnancy are lower than that for other substances, and pregnant women may not be aware of the impact of smoking on fetal development.30 Cotinine can be used to verify self-report of smoking status and severity.10,31,32
Salivary cotinine tests are commercially available, relatively economical, and convenient to use when frequent monitoring is required.32 In general, based on established cut-off values that are unique to the specimen collected, the overall high specificity and sensitivity of salivary testing allows clinicians to predict smoker vs nonsmoker status with confidence. For example, a 2008 study reported a salivary cotinine cut-off level of 12 ng/mL for smokers.21 The sensitivity and specificity of this cut-off value for distinguishing cigarette smokers from never smokers were 96.7% and 96.9%, respectively.21
Additionally, some studies suggest that cotinine levels may be predictive of treatment outcomes and retention in SUD treatment programs.33,34 One study of smoking cessation using nicotine replacement products found that compared with patients with lower baseline cotinine levels prior to treatment, patients with higher baseline cotinine plasma levels had lower smoking cessation success rates.34
A few caveats
There are several limitations to quantitative measures of cotinine (Box 221,23). These include (but are not limited to) potential errors related to sample collection, storage, shipping, and analysis.23 Compared with other methods, point-of-care cotinine measurement in saliva is noninvasive, simple, and requires less training to properly use.23
Box 2
Challenges in the collection of samples, storage, shipping, and instrumentation may limit cotinine consistency as a dependable biomarker in the clinical setting.23 Overall, quantitative measurements of cotinine have relative constructive utility in separating smokers from nonsmokers, because daily smokers typically have serum concentrations of 100 ng/mL or higher, in contrast to light/non-daily smokers, who have cotinine concentrations <10 ng/mL. Even heavy exposure to secondhand smoke typically yields plasma concentrations up to approximately 25 ng/mL. However, cotinine is a general metabolite found with the use of all nicotine products, which makes it extremely difficult to differentiate tobacco use from the use of nicotine replacement products, which are frequently used to treat tobacco use disorders.
One potential solution is to measure nicotine-derived nitrosamine ketone (NNK) and its metabolite 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanol (NNAL). Both NNK and NNAL are tobacco-specific lung carcinogens. NNAL can be measured in the urine. Although total NNAL represents only 15% of NNK dose intake, it has been quantified, with urine concentrations of ≥1,000 fmol/mL for daily smokers. NNAL also has an extremely high specificity to tobacco smoke, and thus allows differentiation of tobacco use from nicotine replacement treatment. Unfortunately, measurement for this biomarker requires specific chemical expertise and expensive equipment.
Another potential barrier to using cotinine in the clinical setting is the variable cut-off levels used in the United States, based on differences in race/ethnicity. This may be secondary to differences in smoking behaviors and/or differences in cotinine metabolism.21
Continue to: Confirmation of smoking cessation...
Confirmation of smoking cessation can be monitored reliably within the clinical setting using cotinine monitoring. However, this is not a routine test, and there are no guidelines or consensus on how or when it should be used. The clinical feasibility of cotinine monitoring for psychiatric patients will depend on the cost of testing, methods used, amount of reimbursement for performing the tests, and how clinicians value such testing.35
Bottom Line
Cotinine is a biomarker that can be used to detect tobacco use. Cotinine measurement can be used to monitor tobacco use and smoking cessation in psychiatric patients. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illnesses. However, cotinine measurement is not a routine test, and there are no guidelines on how or when this test should be used.
Related Resources
- Peckham E, Brabyn S, Cook L, et al. Smoking cessation in severe mental ill health: what works? An updated systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):252.
- Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065
Drug Brand Names
Asenapine • Saphris
Buprenorphine • Sublocade
Clozapine • Clozaril
Duloxetine • Cymbalta
Haloperidol • Haldol
Mirtazapine • Remeron
Olanzapine • Zyprexa
Ziprasidone • Geodon
Zolpidem • Ambien
Cigarette smoking is common among patients with schizophrenia, mood disorders, anxiety disorders,1-3 substance use disorders (SUDs),4 and other psychiatric disorders. Research suggests that compared with the general population, patients with SUDs consume more nicotine products and are more vulnerable to the effects of smoking.5 Despite the availability of effective treatments, many mental health professionals are reluctant to identify and treat tobacco use disorder,6-8 or they prioritize other disorders over tobacco use. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illness.
Cotinine is a biomarker that can be used to detect tobacco use. It can be measured in routine clinical practice by collecting urinary, serum, or salivary specimens, and used to monitor psychiatric patients’ tobacco use. Monitoring cotinine levels is similar to using other biomarkers to assess medication adherence or identify illicit substance use. A growing body of evidence supports the utility of cotinine screening as a part of a comprehensive substance use disorder treatment plan,5,9,10 especially for:
- patients who have comorbid conditions that can be exacerbated by tobacco use, such as chronic obstructive pulmonary disease
- patients who are pregnant11,12
- patients who are less reliable in self-report or who require objective testing for validation.
Routine clinical screening of tobacco use is recommended for all patients and early detection may facilitate earlier treatment. Several FDA-approved medications are available for smoking cessation13; however, discussion of treatment options is beyond the scope of this review. In this article, we describe how cotinine is measured and analyzed, 3 case vignettes that illustrate its potential clinical utility, and limitations to its use as a biomarker of tobacco use.
Methods of measuring cotinine
Cigarette smoking is associated with the absorption of nicotine, which is mainly metabolized by cytochrome P450 (CYP) 2A6 to 6 primary metabolites: cotinine, hydroxycotinine, norcotinine, nornicotine, cotinine oxide, and nicotine oxide.14,15 Cotinine is the biomarker of choice for detecting use of tobacco/nicotine products due to its stability (it is not influenced by dietary or environmental factors), extended half-life (16 to 19 hours, compared with 2 hours for nicotine), and stable concentration throughout the day. Samples from saliva, urine, or blood can be analyzed through radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and gas/liquid chromatography.16 The specificity of cotinine for tobacco use is excellent, except for persons who are taking medications that contain nicotine.17
An advantage of cotinine over other biomarkers for smoking (such as carbon monoxide in expired air) is that the optimal cut-off points for cotinine are relatively uninfluenced by the prevalence of smoking in the population. The optimal cut-off levels used to detect current tobacco use may vary based on the sample or test used (saliva, urine, or plasma) and certain patient-specific factors (Box 111,16,18-21). However, for plasma or saliva cotinine, 16 ng/mL is the generally accepted cut-off level for detecting current tobacco use. A urinary cotinine cut-off level of 50 ng/mL is likely appropriate for most circumstances.17 Users of electronic nicotine delivery systems (electronic cigarettes) have been found to have cotinine levels similar to those of cigarette smokers.22
Box 1
Daily smokers typically have a serum/plasma cotinine concentration of ≥100 ng/mL. Individuals with heavy exposure to secondhand smoking may have plasma cotinine concentrations up to 25 ng/mL, and urine samples tend to be much more specific.16 However, serum cotinine has a wide cut-off range due to diverse racial/ethnic, gender, and pregnancy-related variations; the wide range is also associated with genetic polymorphisms of cytochrome P450 2A6 alleles and nicotine’s numerous metabolic pathways.11,18
Traditionally a serum/plasma cut-off point of approximately 15 ng/mL has been accepted to detect current tobacco use; however, recent studies21 recommend an average optimal cut-off point for US adults of 3 ng/mL. This possibly reflects differences in national cigarette smoking patterns and exposure.21 One study suggested optimal cut-off differences for men (1.78 ng/mL) and women (4.47 ng/mL).19 The same study also suggested different optimal cut-off levels for non-Hispanic White men (6.79 ng/ mL), non-Hispanic Black men (13.3 ng/mL), and Mexican-American men (0.79 ng/mL).19 These researchers also suggested different optimal cut-off levels for non-Hispanic White women (4.73 ng/mL), non-Hispanic Black women (5.91 ng/mL), and Mexican-American women (0.84 ng/mL).19 Genetic factors may also play a role in the progression of nicotine dependence and pose challenges that impact smoking persistence.20
Assessment of cotinine levels in saliva may be considered for outpatient monitoring due to its noninvasive nature, tolerability, and the ability to collect multiple samples over a limited period.23 Saliva cotinine levels correlate closely with blood concentrations. Urine cotinine levels offer some advantage because concentrations are 6 times higher in urine than in blood or saliva. For this reason, urine cotinine is the most widely used biomarker in individuals who use tobacco due to its high sensitivity, specificity, reliability, and noninvasive collection.23 By using a lower urinary cut-off of ≥2.47 ng/mL, ELISA kits detect the highest sensitivity and specificity, which is useful for monitoring daily tobacco use.24 This cut-off value was associated with 100% sensitivity and specificity, and these numbers declined with increases in the cut-off threshold.23
The following 3 clinical vignettes illustrate the impact of tobacco use disorder on patients, and how cotinine might help with their treatment.
Continue to: Vignette 1
Vignette 1
Mr. D, age 44, has a history of schizophrenia and has smoked 1 pack of cigarettes per day for the last 15 years. He was recently discharged from an inpatient psychiatric facility after his symptoms were stabilized. During his hospitalization, Mr. D used a nicotine-replacement product to comply with the hospital’s smoke-free policy. Unfortunately, since discharge, Mr. D reports worsening auditory hallucinations despite adherence with his antipsychotic medication, clozapine, 600 mg at bedtime. Collateral information gathered from Mr. D’s mother confirms that he has been adherent with the discharge medication regimen; however, Mr. D has resumed smoking 1 pack of cigarettes daily. The treatment team suspects that his worsening psychosis is related to the decrease of blood clozapine level due to CYP induction by cigarette smoke.
Cotinine and smoking-related drug interactions
Vignette 1 illustrates the significant impact tobacco smoke can have on the effectiveness of a psychotropic medication. This is caused by polycyclic aromatic hydrocarbons induction of hepatic CYP1A2 isoenzymes. Clinicians should routinely screen patients for smoking status due to the potential for drug interactions. Common major CYP1A2 substrates include
Vignette 2
Mr. B, age 34, has a history of cocaine use disorder and tobacco use disorder. He is referred to a treatment program and participates in a contingency management program for his substance use disorders. Biomarkers, including salivary cotinine, are used to assess Mr. B’s exposure to tobacco use. Mr. B and other participants in his program are eligible for prize draws if they are found to have samples that are negative for tobacco and other substances. There are other incentives in place for patients who show a reduced cotinine concentration.
Cotinine monitoring and contingency management
Clinicians can incorporate cotinine monitoring into existing SUD treatment. This is similar to the utilization of other biomarkers that are commonly used to identify recent illicit substance use or monitor adherence to treatment medications. For example, benzoylecgonine, a metabolite of cocaine, is frequently used to monitor abstinence from cocaine.
Treatments based on contingency management principles involve giving patients tangible rewards to reinforce desired (positive) behaviors. Smoking cessation can be confirmed by monitoring cotinine levels. Gayman et al9 found twice-weekly salivary testing was compatible with monitoring and promoting abstinence in a prize-based contingency management smoking cessation program. Most prior studies used urine cotinine measures to verify abstinence. Although highly reliable, urine samples require close monitoring to ensure sample validity, which can be a burden on staff and unpleasant for patients.9 It is also important to note that the rate of elimination of cotinine from saliva and urine are comparable. The half-life of cotinine is approximately 18 hours, and therefore the specificity of salivary test strips may be impacted during the first 4 to 5 days of abstinence. In the first few days of smoking cessation, a more intensive approach, such as quantifying urine cotinine levels and monitoring decline, may be appropriate.23
Continue to: Vignette 3
Vignette 3
Ms. C, age 34 and pregnant, is admitted to an outpatient treatment program for alcohol use disorder. She also has generalized anxiety disorder and tobacco use disorder. In addition to attending group therapy sessions and self-reporting any recent alcohol consumption, Ms. C also undergoes alcohol breathalyzer tests and urine studies of alcohol metabolites to monitor abstinence from alcohol. She says that the regular laboratory screening for alcohol use gives her a sense of accountability and tangible evidence of change that positively impacts her treatment. When the treating psychiatrist recommends that Ms. C also consider addressing her tobacco use disorder, she asks if there is some way to include laboratory testing to monitor her smoking cessation.
Cotinine as a predictor of smoking status
Smoking abstinence rates during pregnancy are lower than that for other substances, and pregnant women may not be aware of the impact of smoking on fetal development.30 Cotinine can be used to verify self-report of smoking status and severity.10,31,32
Salivary cotinine tests are commercially available, relatively economical, and convenient to use when frequent monitoring is required.32 In general, based on established cut-off values that are unique to the specimen collected, the overall high specificity and sensitivity of salivary testing allows clinicians to predict smoker vs nonsmoker status with confidence. For example, a 2008 study reported a salivary cotinine cut-off level of 12 ng/mL for smokers.21 The sensitivity and specificity of this cut-off value for distinguishing cigarette smokers from never smokers were 96.7% and 96.9%, respectively.21
Additionally, some studies suggest that cotinine levels may be predictive of treatment outcomes and retention in SUD treatment programs.33,34 One study of smoking cessation using nicotine replacement products found that compared with patients with lower baseline cotinine levels prior to treatment, patients with higher baseline cotinine plasma levels had lower smoking cessation success rates.34
A few caveats
There are several limitations to quantitative measures of cotinine (Box 221,23). These include (but are not limited to) potential errors related to sample collection, storage, shipping, and analysis.23 Compared with other methods, point-of-care cotinine measurement in saliva is noninvasive, simple, and requires less training to properly use.23
Box 2
Challenges in the collection of samples, storage, shipping, and instrumentation may limit cotinine consistency as a dependable biomarker in the clinical setting.23 Overall, quantitative measurements of cotinine have relative constructive utility in separating smokers from nonsmokers, because daily smokers typically have serum concentrations of 100 ng/mL or higher, in contrast to light/non-daily smokers, who have cotinine concentrations <10 ng/mL. Even heavy exposure to secondhand smoke typically yields plasma concentrations up to approximately 25 ng/mL. However, cotinine is a general metabolite found with the use of all nicotine products, which makes it extremely difficult to differentiate tobacco use from the use of nicotine replacement products, which are frequently used to treat tobacco use disorders.
One potential solution is to measure nicotine-derived nitrosamine ketone (NNK) and its metabolite 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanol (NNAL). Both NNK and NNAL are tobacco-specific lung carcinogens. NNAL can be measured in the urine. Although total NNAL represents only 15% of NNK dose intake, it has been quantified, with urine concentrations of ≥1,000 fmol/mL for daily smokers. NNAL also has an extremely high specificity to tobacco smoke, and thus allows differentiation of tobacco use from nicotine replacement treatment. Unfortunately, measurement for this biomarker requires specific chemical expertise and expensive equipment.
Another potential barrier to using cotinine in the clinical setting is the variable cut-off levels used in the United States, based on differences in race/ethnicity. This may be secondary to differences in smoking behaviors and/or differences in cotinine metabolism.21
Continue to: Confirmation of smoking cessation...
Confirmation of smoking cessation can be monitored reliably within the clinical setting using cotinine monitoring. However, this is not a routine test, and there are no guidelines or consensus on how or when it should be used. The clinical feasibility of cotinine monitoring for psychiatric patients will depend on the cost of testing, methods used, amount of reimbursement for performing the tests, and how clinicians value such testing.35
Bottom Line
Cotinine is a biomarker that can be used to detect tobacco use. Cotinine measurement can be used to monitor tobacco use and smoking cessation in psychiatric patients. Early detection and treatment of tobacco use disorder can improve patients’ health and reduce the incidence of acute and chronic illnesses. However, cotinine measurement is not a routine test, and there are no guidelines on how or when this test should be used.
Related Resources
- Peckham E, Brabyn S, Cook L, et al. Smoking cessation in severe mental ill health: what works? An updated systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):252.
- Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065
Drug Brand Names
Asenapine • Saphris
Buprenorphine • Sublocade
Clozapine • Clozaril
Duloxetine • Cymbalta
Haloperidol • Haldol
Mirtazapine • Remeron
Olanzapine • Zyprexa
Ziprasidone • Geodon
Zolpidem • Ambien
1. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185.
2. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12.
3. Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106-123.
5. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
6. Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808-1814.
7. McHugh RK, Votaw VR, Fulciniti F, et al. Perceived barriers to smoking cessation among adults with substance use disorders. J Subst Abuse Treat. 2017;74:48-53.
8. Strong DR, Uebelacker L, Fokas K, et al. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. J Addict Med. 2014;8(2):77-83.
9. Gayman C, Anderson K, Pietras C. Saliva cotinine as a measure of smoking abstinence in contingency management – a feasibility study. The Psychological Record. 2017;67(2):261-272.
10. Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10(9):1495-1501.
11. Stragierowicz J, Mikolajewska K, Zawadzka-Stolarz M, et al. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int. 2013;2013:386784. doi.org/10.1155/2013/386784
12. Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi.org/10.1136/bmj.b4347
13. Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17(14):1343-1350.
14. McGuffey JE, Wei B, Bernert JT, et al. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers’ urine. PLoS One. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816
15. Duque A, Martinez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425-e431. doi: 10.4317/medoral.21292
16. Avila-Tang E, Elf JL, Cummings KM, et al. Assessing secondhand smoke exposure with reported measures. Tob Control. 2013;22(3):156-163.
17. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 Update. Nicotine Tob Res. 2020;22(7):1086-1097.
18. Nakajima M TY. Interindividual variability in nicotine metabolism: c-oxidation and glucuronidation. Drug Metab Pharmaokinet. 2005;20(4):227-235.
19. Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236-248.
20. Schnoll R, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psychiatry Rep. 2007;9(5):349-357.
21. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13(12):1236.
22. Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219-1220.
23. Raja M, Garg A, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04-06. doi: 10.7860/JCDR/2016/17360.7423
24. Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9(1):84-89.
25. Fankhauser M. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
26. Scheuermann TS, Richter KP, Rigotti NA, et al. Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227-2236.
27. Holtyn AF, Knealing TW, Jarvis BP, et al. Monitoring cocaine use and abstinence among cocaine users for contingency management interventions. Psychol Rec. 2017;67(2):253-259.
28. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51.
29. Sullivan M, Covey, LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4(5):388-396.
30. Forray A, Merry B, Lin H, et al. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147-155.
31. Parker DR, Lasater TM, Windsor R, et al. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2002;4(3):305-309.
32. Asha V, Dhanya M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J Cancer Prev. 2015;20(2):159-163.
33. Hall S, Herning RI, Jones RT, et al. Blood cotinine levels as indicators of smoking treatment outcome. Clin Pharmacol Ther. 1984;35(6):810-814.
34. Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9(4):643-651.
35. Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1858-1862.
1. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185.
2. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12.
3. Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106-123.
5. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
6. Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808-1814.
7. McHugh RK, Votaw VR, Fulciniti F, et al. Perceived barriers to smoking cessation among adults with substance use disorders. J Subst Abuse Treat. 2017;74:48-53.
8. Strong DR, Uebelacker L, Fokas K, et al. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. J Addict Med. 2014;8(2):77-83.
9. Gayman C, Anderson K, Pietras C. Saliva cotinine as a measure of smoking abstinence in contingency management – a feasibility study. The Psychological Record. 2017;67(2):261-272.
10. Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10(9):1495-1501.
11. Stragierowicz J, Mikolajewska K, Zawadzka-Stolarz M, et al. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int. 2013;2013:386784. doi.org/10.1155/2013/386784
12. Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi.org/10.1136/bmj.b4347
13. Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17(14):1343-1350.
14. McGuffey JE, Wei B, Bernert JT, et al. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers’ urine. PLoS One. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816
15. Duque A, Martinez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425-e431. doi: 10.4317/medoral.21292
16. Avila-Tang E, Elf JL, Cummings KM, et al. Assessing secondhand smoke exposure with reported measures. Tob Control. 2013;22(3):156-163.
17. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 Update. Nicotine Tob Res. 2020;22(7):1086-1097.
18. Nakajima M TY. Interindividual variability in nicotine metabolism: c-oxidation and glucuronidation. Drug Metab Pharmaokinet. 2005;20(4):227-235.
19. Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236-248.
20. Schnoll R, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psychiatry Rep. 2007;9(5):349-357.
21. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13(12):1236.
22. Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219-1220.
23. Raja M, Garg A, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04-06. doi: 10.7860/JCDR/2016/17360.7423
24. Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9(1):84-89.
25. Fankhauser M. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
26. Scheuermann TS, Richter KP, Rigotti NA, et al. Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. 2017;112(12):2227-2236.
27. Holtyn AF, Knealing TW, Jarvis BP, et al. Monitoring cocaine use and abstinence among cocaine users for contingency management interventions. Psychol Rec. 2017;67(2):253-259.
28. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51.
29. Sullivan M, Covey, LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4(5):388-396.
30. Forray A, Merry B, Lin H, et al. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015;150:147-155.
31. Parker DR, Lasater TM, Windsor R, et al. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2002;4(3):305-309.
32. Asha V, Dhanya M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J Cancer Prev. 2015;20(2):159-163.
33. Hall S, Herning RI, Jones RT, et al. Blood cotinine levels as indicators of smoking treatment outcome. Clin Pharmacol Ther. 1984;35(6):810-814.
34. Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J. 1996;9(4):643-651.
35. Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1858-1862.
Cannabinoid-based medications for pain
Against the backdrop of an increasing opioid use epidemic and a marked acceleration of prescription opioid–related deaths,1,2 there has been an impetus to explore the usefulness of alternative and co-analgesic agents to assist patients with chronic pain. Preclinical studies employing animal-based models of human pain syndromes have demonstrated that cannabis and chemicals derived from cannabis extracts may mitigate several pain conditions.3
Because there are significant comorbidities between psychiatric disorders and chronic pain, psychiatrists are likely to care for patients with chronic pain. As the availability of and interest in cannabinoid-based medications (CBM) increases, psychiatrists will need to be apprised of the utility, adverse effects, and potential drug interactions of these agents.
The endocannabinoid system and cannabis receptors
The endogenous cannabinoid (endocannabinoid) system is abundantly present within the peripheral and central nervous systems. The first identified, and best studied, endocannabinoids are N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG).4 Unlike typical neurotransmitters, AEA and 2-AG are not stored within vesicles within presynaptic neuron axons. Instead, they are lipophilic molecules produced on demand, synthesized from phospholipids (ie, arachidonic acid derivatives) at the membranes of post-synaptic neurons, and released into the synapse directly.5
Acting as retrograde messengers, the endocannabinoids traverse the synapse, binding to receptors located on the axons of the presynaptic neuron. Two receptors—CB1 and CB2—have been most extensively studied and characterized.6,7 These receptors couple to Gi/o-proteins to inhibit adenylate cyclase, decreasing Ca2+ conductance and increasing K+ conductance.8 Once activated, cannabinoid receptors modulate neurotransmitter release from presynaptic axon terminals. Evidence points to a similar retrograde signaling between neurons and glial cells. Shortly after receptor activation, the endocannabinoids are deactivated by the actions of a transporter mechanism and enzyme degradation.9,10
The endocannabinoid system and pain transmission
Cannabinoid receptors are present in pain transmission circuits spanning from the peripheral sensory nerve endings (from which pain signals originate) to the spinal cord and supraspinal regions within the brain.11-14 CB1 receptors are abundantly present within the CNS, including regions involved in pain transmission. Binding to CB1 receptors, endocannabinoids modulate neurotransmission that impacts pain transmission centrally. Endocannabinoids can also indirectly modulate opiate and N-methyl-
By contrast, CB2 receptors are predominantly localized to peripheral tissues and immune cells, although there has been some discovery of their presence within the CNS (eg, on microglia). Endocannabinoid activation of CB2 receptors is thought to modulate the activity of peripheral afferent pain fibers and immune-mediated neuroinflammatory processes—such as inhibition of prostaglandin synthesis and mast cell degranulation—that can precipitate and maintain chronic pain states.16-18
Evidence garnered from preclinical (animal) studies points to the role of the endocannabinoid system in modulating normal pain transmission (see Manzanares et al3 for details). These studies offer a putative basis for understanding how exogenous cannabinoid congeners might serve to ameliorate pain transmission in pathophysiologic states, including chronic pain.
Continue to: Cannabinoid-based medications
Cannabinoid-based medications
Marijuana contains multiple components (cannabinoids). The most extensively studied are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Because it predominantly binds CB1 receptors centrally, THC is the major psychoactive component of cannabis; it promotes sleep and appetite, influences anxiety, and produces the “high” associated with cannabis use. By contrast, CBD weakly binds CB1 and thus exerts minimal or no psychoactive effects.19
Cannabinoid absorption, metabolism, bioavailability, and clinical effects vary depending on the formulation and method of administration (Table 1).20-22 THC and CBD content and potency in inhaled cannabis can vary significantly depending on the strains of the cannabis plant and manner of cultivation.23 To standardize approaches for administering cannabinoids in clinical trials and for clinical use, researchers have developed pharmaceutical analogs that contain extracted chemicals or synthetic chemicals similar to THC and/or CBD.
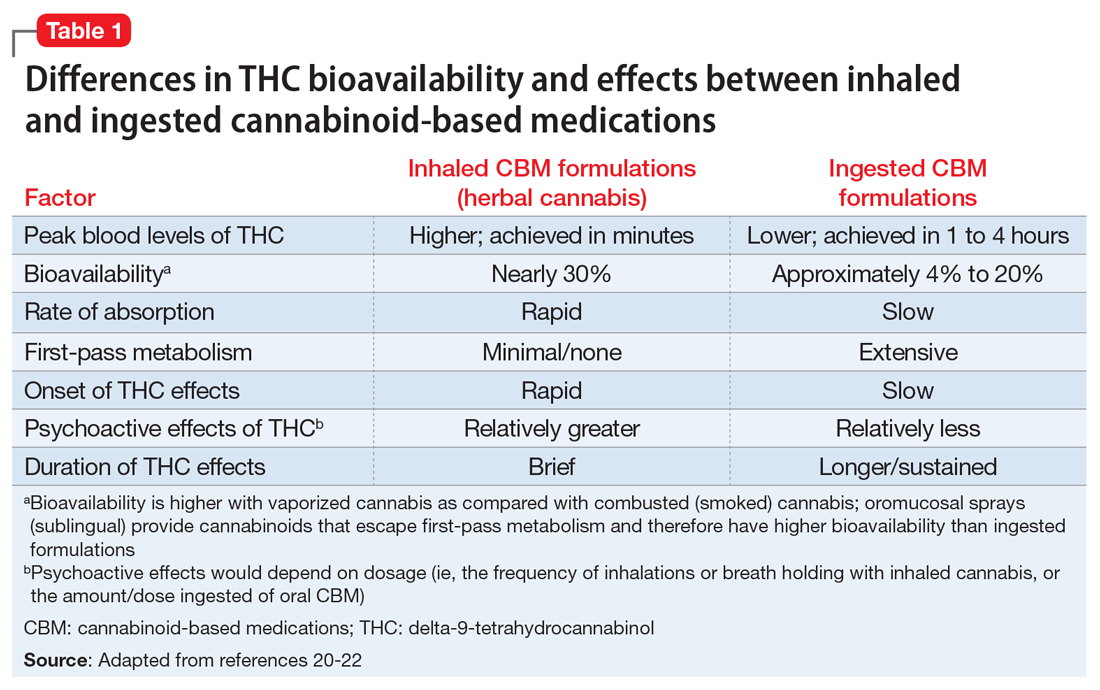
In this article, CBM refers to smoked/vaporized herbal cannabis as well as pharmaceutical cannabis analogs. Table 2 summarizes the characteristics of CBM commonly used in studies investigating their use for managing pain conditions.
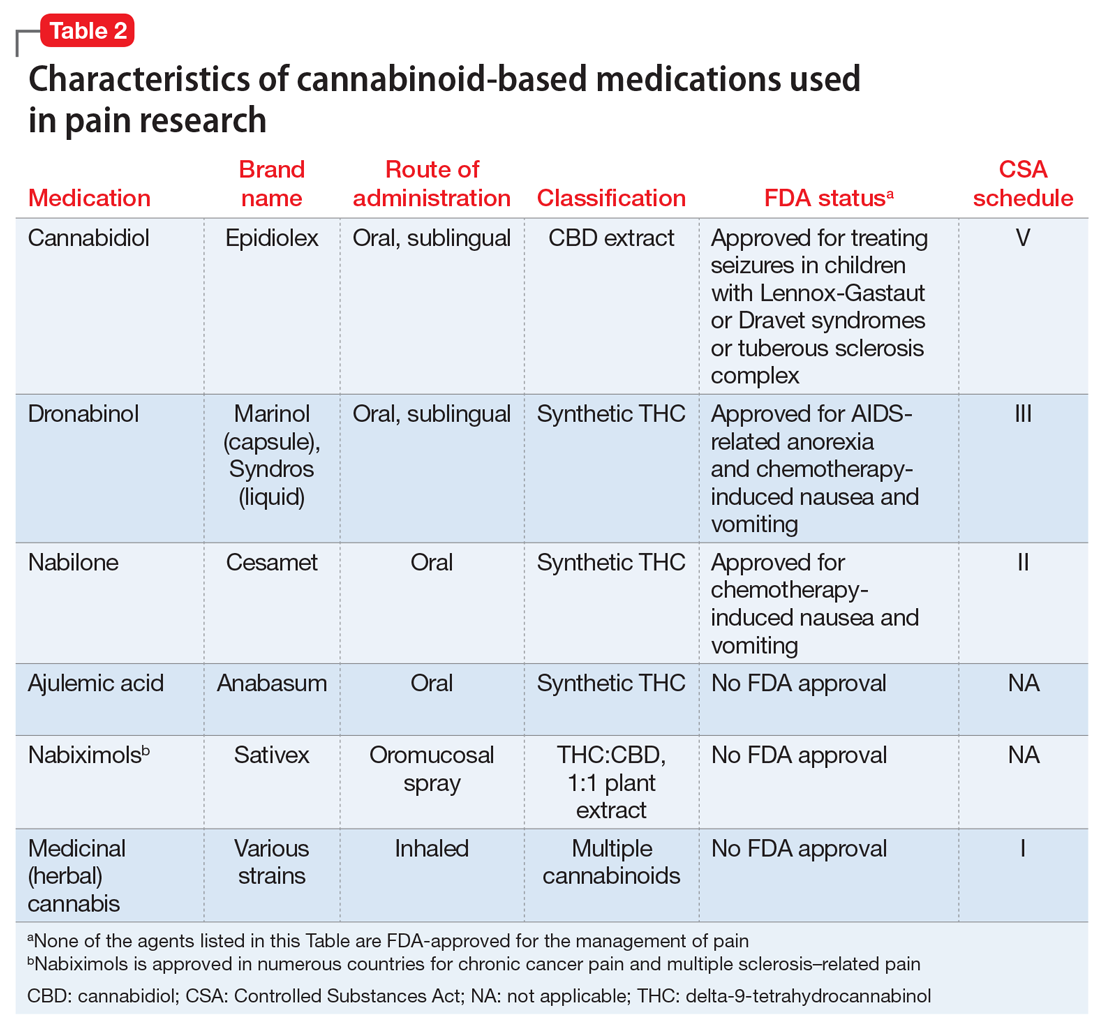
CBM for chronic pain
The literature base examining the role of CBM for managing chronic nonmalignant and malignant pain of varying etiologies is rapidly expanding. Randomized controlled trials (RCTs) have focused on inhaled/smoked products and related cannabinoid medications, some of which are FDA-approved (Table 2).
A multitude of other cannabinoid-based products are currently commercially available to consumers, including tincture and oil-based products; over-the-counter CBD products; and several other formulations of CBM (eg, edible and suppository products). Because such products are not standardized or quality-controlled,24 RCTs have not assessed their efficacy for mitigating pain. Consequently, the findings summarized in this article do not address the utility of these agents.
Continue to: CBM for non-cancer pain
CBM for non-cancer pain
Neuropathic pain. Randomized controlled trials have assessed the pain-mitigating effects of various CBM, including inhaled cannabis, synthetic THC, plant-extracted CBD, and a THC/CBD spray. Studies have shown that inhaled/vaporized cannabis can produce short-term pain reduction in patients with chronic neuropathic pain of diverse etiologies, including diabetes mellitus-, HIV-, trauma-, and medication-induced neuropathies.22,25,26 Similar beneficial effects have been observed with the use of cannabis analogues (eg, nabiximols).25,26-29
Meta-analyses and systematic reviews have determined that most of these RCTs were of low-to-moderate quality.26,30 Meta-analyses have revealed divergent and conflicting results because of differences in the inclusion and exclusion criteria used to select RCTs for analysis and differences in the standards with which the quality of evidence were determined.25,30
Overall, the benefit of CBM for mitigating neuropathic pain is promising, but the effectiveness may not be robust.30,31 Several noteworthy caveats limit the interpretation of the results of these RCTs:
- due to the small sample sizes and brief durations of study, questions remain regarding the extent to which effects are generalizable, whether the benefits are sustained, and whether adverse effects emerge over time with continued use
- most RCTs evaluated inhaled (herbal) cannabis and nabiximols; there is little data on the effectiveness of other CBM formulations25,26,30
- the pain-mitigating effects of CBM were usually compared with those of placebo; the comparative efficacy against agents commonly used to treat neuropathic pain remains largely unexamined
- these RCTs typically compared mean pain severity score differences between cannabis-treated and placebo groups using standard subjective rating scales of pain intensity, such as the Numerical Rating Scale or Visual Analogue Scale. Customarily, the pain literature has used a 30% or 50% reduction in pain severity from baseline as an indicator of significant clinical improvement.32,33 The RCTs of CBM for neuropathic pain rarely used this standard, which makes it unclear whether CBM results in clinically significant pain reductions30
- indirect measures of effectiveness (ie, whether using CBM reduces the need for opioids or other analgesics to manage pain) were seldom reported in these RCTs.
Due to these limitations, clinical guidelines and systematic reviews consider CBM as a third- or fourth-line therapy for patients experiencing chronic neuropathic pain for whom conventional agents such as anticonvulsants and antidepressants have failed.34,35
Spasticity in multiple sclerosis (MS). Several RCTs have assessed the use of CBM for MS-related spasticity, although few were deemed to be high quality. Nabiximols and synthetic THC were effective in managing spasticity and reducing pain severity associated with muscle spasms.36 Generally, investigations revealed that CBM were associated with improvements in subjective measures of spasticity, but these were not born out in clinical, objective measures.26,37 The efficacy of smoked cannabis was uncertain.37 The existing literature on CBM for MS-related spasticity does not address dosing, duration of effects, tolerability, or comparative effectiveness against conventional anti-spasm medications.36,37
Continue to: Other chronic pain conditions
Other chronic pain conditions. CBM have also been studied for their usefulness in several other noncancer chronic conditions, including Crohn’s disease, inflammatory bowel disease, fibromyalgia, and other rheumatologic pain conditions.22,31,38-40 However, a solid foundation of empirical work to inform their utility for managing pain in these conditions is lacking.
CBM for cancer pain
Anecdotal evidence suggests that inhaled cannabis has promising pain-mitigating effects in patients with advanced cancer.41-43 There is a dearth of high-quality RCTs assessing the utility of CBM in patients with cancer pain.43-45 The types of CBM used and dosing strategies varied across RCTs, which makes it difficult to infer how best to treat patients with cancer pain. The agents studied included nabiximols, THC spray, and synthetic THC capsules.43-45 Although some studies have demonstrated that synthetic THC and nabiximols have potential for reducing subjective pain ratings compared with placebo,46,47 these results were inconsistent.46,48 Oromucosal nabiximols did not appear to confer any additional analgesic benefit in patients who were already prescribed opioids.31,45
The benefit of CBM for mitigating cancer pain is promising, but it remains difficult to know how to position the use of CBM in managing cancer pain. Limitations in the cancer literature include:
- the RCTs addressing CBM use for cancer pain were often brief, which raises questions about the long-term effectiveness and adverse effects of these agents
- tolerability and dosing limits encountered due to adverse effects were seldom reported43,45
- the types of cancer pain that patients had were often quite diverse. The small sample sizes and the heterogeneity of conditions included in these RCTs limit the ability to determine whether pain-mitigating effects might vary according to type of cancer-related pain.31,45
Despite these limitations, some clinical guidelines and systematic reviews have suggested that CBM have some role in addressing refractory malignant pain conditions.49
Psychiatric considerations related to CBM
As of November 2020, 36 states had legalized the use of cannabis for medical purposes, typically for painful conditions, despite the fact that empirical evidence to support their efficacy is mixed.50 In light of recent changes in both the legal and popular attitudes regarding cannabis, the implications of legalizing CBM remains to be seen. For example, some research suggests that adults with pain are vulnerable to frequent nonmedical cannabis use and/or cannabis use disorder.51 Although well-intended, the legalization of CBM use might represent society’s next misstep in the quest to address the suffering of patients with chronic pain. Some evidence shows that cannabis use and cannabis use disorders increase in states that have legalized medical marijuana.52,53 Psychiatrists will be on the front lines of addressing any potential consequences arising from the use of CBM for treating pain.
Continue to: Psychiatric disorders and CBM
Psychiatric disorders and CBM. The psychological impact of CBM use among patients enduring chronic pain can include sedation, cognitive/attention disturbance, and fatigue. These adverse effects can limit the utility of such agents.22,29,45
Contraindications for CBM use, and conditions for which CBM ought to be used with caution, are listed in Table 354,55.The safety of CBM, particularly in patients with chronic pain and psychiatric disorders, has not been examined. Patients with psychiatric disorders may be poor candidates for medical cannabis. Epidemiologic data suggest that recreational cannabis use is positively associated both cross-sectionally and prospectively with psychotic spectrum disorders, depressive symptoms, and anxiety symptoms, including panic disorder.56 Psychotic reactions have also been associated with CBM (dronabinol and nabilone).57 Cannabis use also has been associated with an earlier onset of, and lower remission rates of, symptoms associated with bipolar disorder.58,59 Consequently, patients who have been diagnosed with or are at risk for developing any of the aforementioned conditions may not be suitable candidates for CBM. If CBM are used, patients should be closely monitored for the emergence/exacerbation of psychiatric symptoms. The frequency and extent of follow-up is not clear, however. Because of its reduced propensity to produce psychoactive effects, CBD may be safer than THC for managing pain in individuals who have or are vulnerable to developing psychiatric disorders.
There is a lack of evidence to support the use of CBM for treating primary depressive disorders, general anxiety disorder, posttraumatic stress disorder, or psychosis.60,61 Very low-quality evidence suggests that CBM could lead to a small improvement in anxiety among individuals with noncancer pain and MS.60 However, interpreting causality is complicated. It is plausible that, for some patients, subjective improvement in pain severity may be related to reduced anxiety.62 Conversely, it is equally plausible that reductions in emotional distress may reduce the propensity to attend to, and thus magnify, pain severity. In the latter case, the indirect impact of reducing pain by modifying emotional distress can be impacted by the type and dose of CBM used. For example, low concentrations of THC produce anxiolytic effects, but high concentrations may be anxiety-provoking.63,64
Several potential pharmacokinetic drug interactions may arise between herbal cannabis or CBM and other medications (Table 465,66). THC and CBD are both metabolized by cytochrome P450 (CYP) 2C19 and 3A4.65,66 In addition, THC is also metabolized by CYP2C9. Medications that inhibit or induce these enzymes can increase or decrease the bioavailability of THC and CBD.67
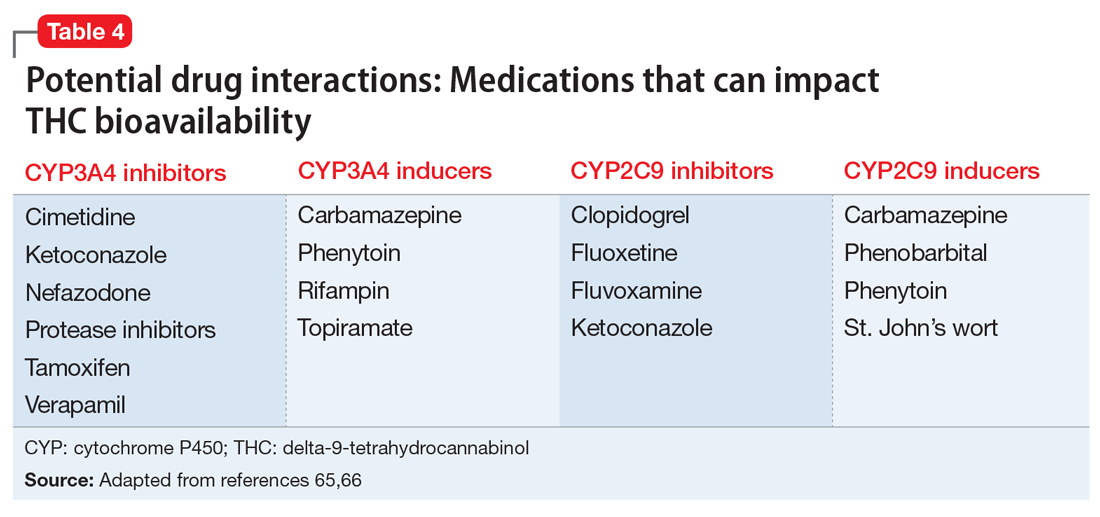
Simultaneously, cannabinoids can impact the bioavailability of co-prescribed medications (Table 566,68). Although such CYP enzyme interactions remain a theoretical possibility, it is uncertain whether significant perturbations in plasma concentrations (and clinical effects) have been encountered with prescription medications when co-administered with CBM.69 Nonetheless, patients receiving CBM should be closely monitored for their response to prescribed medications.70
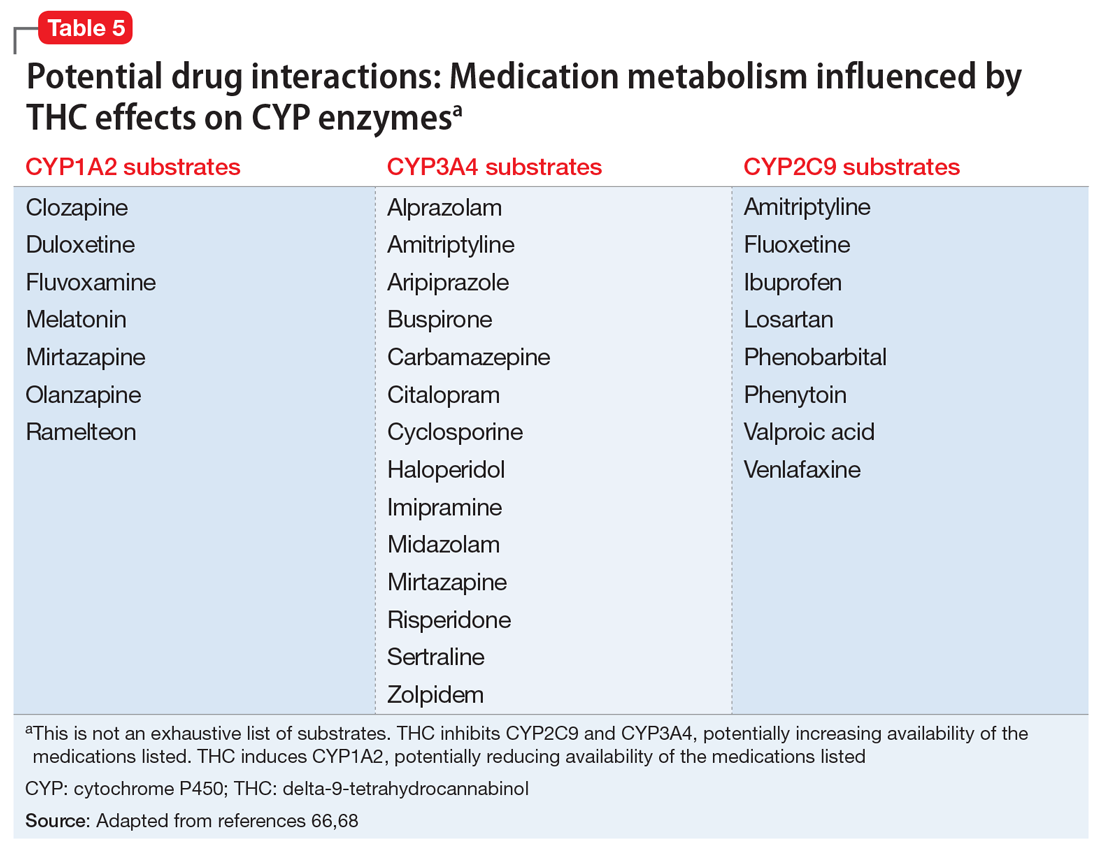
Continue to: Potential CYP enzyme interactions...
Potential CYP enzyme interactions aside, clinicians need to consider the additive effects that may occur when CBM are combined with sympathomimetic agents (eg, tachycardia, hypertension); CNS depressants such as alcohol, benzodiazepines, and opioids (eg, drowsiness, ataxia); or anticholinergics (eg, tachycardia, confusion).71 Inhaled herbal cannabis contains mutagens and can result in lung damage, exacerbations of chronic bronchitis, and certain types of cancer.54,72 Co-prescribing benzodiazepines may be contraindicated in light of their effects on respiratory rate and effort.
The THC contained in CBM produces hormonal effects (ie, significantly increases plasma levels of ghrelin and leptin and decreases peptide YY levels)73 that affect appetite and can produce weight gain. This may be problematic for patients receiving psychoactive medications associated with increased risk of weight gain and dyslipidemia. Because of the association between cannabis use and motor vehicle accidents, patients whose jobs require them to drive or operate industrial equipment may not be ideal candidates for CBM, especially if such patients also consume alcohol or are prescribed benzodiazepines and/or sedative hypnotics.74 Lastly, due to their lipophilicity, cannabinoids cross the placental barrier and can be found in breast milk75 and therefore can affect pregnancy outcomes and neurodevelopment.
Bottom Line
The popularity of cannabinoid-based medications (CBM) for the treatment of chronic pain conditions is growing, but the interest in their use may be outpacing the evidence supporting their analgesic benefits. High-quality, well-controlled randomized controlled trials are needed to decipher whether, and to what extent, these agents can be positioned in chronic pain management. Because psychiatrists are likely to encounter patients considering, or receiving, CBM, they must be aware of the potential benefits, risks, and adverse effects of such treatments.
Related Resources
- Joshi KG. Cannabis-derived compounds: what you need to know. Current Psychiatry. 2020;19(10):64-65. doi:10.12788/ cp.0050
- Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018; 17(1):34-41.
Drug Brand Names
Ajulemic acid • Anabasum
Alprazolam • Xanax
Amitriptyline • Elavil
Aripiprazole • Abilify, Abilify Maintena
Buspirone • BuSpar
Cannabidiol • Epidiolex
Carbamazepine • Tegretol, Equetro
Cimetidine • Tagamet HB
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Cyclosporine • Neoral, Sandimmune
Dronabinol • Marinol, Syndros
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Imipramine • Tofranil
Ketoconazole • Nizoral AD
Losartan • Cozaar
Midazolam • Versed
Mirtazapine • Remeron
Nabilone • Cesamet
Nabiximols • Sativex
Nefazodone • Serzone
Olanzapine • Zyprexa
Phenobarbital • Solfoton
Phenytoin • Dilantin
Ramelteon • Rozerem
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Topiramate • Topamax
Valproic acid • Depakote, Depakene
Venlafaxine • Effexor
Verapamil • Verelan
Zolpidem • Ambien
1. Okie S. A floor of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. doi:10.1056/NEJMp1011512
2. Powell D, Pacula RL, Taylor E. How increasing medical access to opioids contributes to the opioid epidemic: evidence from Medicare Part D. J Health Econ. 2020;71:102286. doi: 10.1016/j.jhealeco.2019.102286
3. Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257. doi: 10.2174/157015906778019527
4. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833
5. Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016;14(4):2899-2903. doi:10.3892/mmr.2016.5585
6. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83-90. doi:10.1016/0006-2952(95)00109-d
7. Walker JM, Krey JF, Chu CJ, et al. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids. 2002;121(1-2):159-172. doi: 10.1016/s0009-3084(02)00152-4
8. Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142(8):1209-1218. doi: 10.1038/sj.bjp.0705881
9. Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation, biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7-14.
10. Piomelli D, Beltramo M, Giuffrida A, et al. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5(6 Pt B):462-473. doi: 10.1006/nbdi.1998.0221
11. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175-191. doi: 10.1093/cercor/bhj136
12. Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534(Pt 3):805-812. doi: 10.1111/j.1469-7793.2001.00805.x
13. Vaughan CW, Connor M, Bagley EE, et al. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57(2):288-295.
14. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127(4):935-940. doi: 10.1038/sj.bjp.0702636
15. Raichlen DA, Foster AD, Gerdeman GI, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high.” J Exp Biol. 2012;215(Pt 8):1331-1336. doi: 10.1242/jeb.063677
16. Beltrano M. Cannabinoid type 2 receptor as a target for chronic pain. Mini Rev Chem. 2009;234:253-254.
17. Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529-10533. doi: 10.1073/pnas.1834309100
18. Valenzano KJ, Tafessem L, Lee G, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658-672.
19. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631. doi: 10.1124/pr.110.003004
20. Carter GT, Weydt P, Kyashna-Tocha M, et al. Medicinal cannabis: rational guidelines for dosing. Drugs. 2004;7(5):464-470.
21. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
22. Johal H, Devji T, Chang Y, et al. cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461. doi: 10.1177/1179544120906461
23. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91(6):966-975. doi: 10.3732/ajb.91.6.966
24. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199-210. doi: 10.1080/02791072.2013.805976
25. Andreae MH, Carter GM, Shaparin N, et al. inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221-1232. doi: 10.1016/j.jpain.2015.07.009
26. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi: 10.1001/jama.2015.6358
27. Boychuk DG, Goddard G, Mauro G, et al. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7-14. doi: 10.11607/ofph.1274
28. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735-744. doi: 10.1111/j.1365-2125.2011.03970.x
29. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932-1954. doi: 10.1097/j.pain.0000000000001293
30. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2
31. Häuser W, Fitzcharles MA, Radbruch L, et al. Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int. 2017;114(38):627-634. doi: 10.3238/arztebl.2017.0627
32. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005
33. Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. doi: 10.1016/j.clinthera.2008.05.011
34. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-335. doi: 10.1155/2014/754693
35. Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62-88. doi: 10.1007/s00482-015-0089-y
36. Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep. 2018;18(8):50. doi: 10.1007/s11910-018-0859-x
37. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563. doi: 10.1212/WNL.0000000000000363
38. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease and ulcerative colitis: evidence from Cochrane Reviews. Inflamm Bowel Dis. 2020;26(4):502-509. doi: 10.1093/ibd/izz233
39. Katz-Talmor D, Katz I, Porat-Katz BS, et al. Cannabinoids for the treatment of rheumatic diseases - where do we stand? Nat Rev Rheumatol. 2018;14(8):488-498. doi: 10.1038/s41584-018-0025-5
40. Walitt B, Klose P, Fitzcharles MA, et al. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2
41. Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37‐43. doi: 10.1016/j.ejim.2018.01.023
42. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705‐713. doi:10.1200/EDBK_180469
43. Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(Suppl 2):5215-5222. doi: 10.21037/apm.2017.08.05
44. Aviram J, Samuelly-Lechtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755-E796.
45. Häuser W, Welsch P, Klose P, et al. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424-436. doi: 10.1007/s00482-019-0373-3
46. Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010; 39:167-179.
47. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438-449. doi: 10.1016/j.jpain.2012.01.003
48. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166-173. doi: 10.1016/j.jpainsymman.2013.02.018
49. Kleckner AS, Kleckner IR, Kamen CS, et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019;11:1758835919866362. doi: 10.1177/1758835919866362
50. National Conference of State Legislatures (ncsl.org). State Medical Marijuana Laws. Accessed April 5, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
51. Hasin DS, Shmulewitz D, Cerda M, et al. US adults with pain, a group increasingly vulnerable to nonmedical cannabis use and cannabis use disorder: 2001-2002 and 2012-2013. Am J Psychiatry. 2020;177(7):611-618. doi: 10.1176/appi.ajp.2019.19030284
52. Hasin DS, Sarvet AL, Cerdá M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry. 2017;74(6):579-588. doi: 10.1001/jamapsychiatry.2017.0724
53. National Institute on Drug Abuse. Illicit cannabis use and use disorders increase in states with medical marijuana laws. April 26, 2017. Accessed October 24, 2020. https://archives.drugabuse.gov/news-events/news-releases/2017/04/illicit-cannabis-use-use-disorders-increase-in-states-medical-marijuana-laws
54. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press; 2017. https://doi.org/10.17226/24625
55. Stanford M. Physician recommended marijuana: contraindications & standards of care. A review of the literature. Accessed July 7, 2020. http://drneurosci.com/MedicalMarijuanaStandardsofCare.pdf
56. Repp K, Raich A. Marijuana and health: a comprehensive review of 20 years of research. Washington County Oregon Department of Health and Human Services. 2014. Accessed April 8, 2021. https://www.co.washington.or.us/CAO/upload/HHSmarijuana-review.pdf
57. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638-654. doi: 10.1016/j.sapharm.2015.09.002.
58. Leite RT, Nogueira Sde O, do Nascimento JP, et al. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast. 2015;2015:434127. doi: 10.1155/2015/43412
59. Kim SW, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349-355. doi: 10.4306/pi.2015.12.3.349
60. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995-1010.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. doi: 10.4088/JCP.15r10036.
62. Woolf CJ, American College of Physicians. American Physiological Society Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
63. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515‐523. doi: 10.1002/hup.1048
64. Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015;12(4):735‐746. doi: 10.1007/s13311-015-0380-8
65. Antoniou T, Bodkin J, Ho JMW. Drug interactions with cannabinoids. CMAJ. 2020;2;192:E206. doi: 10.1503/cmaj.191097
66. Brown JD. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug-drug interactions. J Clin Med. 2020;9(4):919. doi: 10.3390/jcm9040919.
67. Maida V, Daeninck P. A user’s guide to cannabinoid therapy in oncology. Curr Oncol. 2016;23(6):398-406. doi: http://dx.doi.org/10.3747/co.23.3487
68. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86-95. doi: 10.3109/03602532.2013.849268
69. Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol. 2016;23(52):S8-S14.
70. Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2018;6(1):3. doi: 10.3390/medicines6010003
71. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477-2482.
72. Ghasemiesfe M, Barrow B, Leonard S, et al. Association between marijuana use and risk of cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(11):e1916318. doi: 10.1001/jamanetworkopen.2019.16318
73. Riggs PK, Vaida F, Rossi SS, et al. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46-52. doi: 10.1016/j.brainres.2011.11.001
74. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536
75. Carlier J, Huestis MA, Zaami S, et al. Monitoring perinatal exposure to cannabis and synthetic cannabinoids. Ther Drug Monit. 2020;42(2):194-204.
Against the backdrop of an increasing opioid use epidemic and a marked acceleration of prescription opioid–related deaths,1,2 there has been an impetus to explore the usefulness of alternative and co-analgesic agents to assist patients with chronic pain. Preclinical studies employing animal-based models of human pain syndromes have demonstrated that cannabis and chemicals derived from cannabis extracts may mitigate several pain conditions.3
Because there are significant comorbidities between psychiatric disorders and chronic pain, psychiatrists are likely to care for patients with chronic pain. As the availability of and interest in cannabinoid-based medications (CBM) increases, psychiatrists will need to be apprised of the utility, adverse effects, and potential drug interactions of these agents.
The endocannabinoid system and cannabis receptors
The endogenous cannabinoid (endocannabinoid) system is abundantly present within the peripheral and central nervous systems. The first identified, and best studied, endocannabinoids are N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG).4 Unlike typical neurotransmitters, AEA and 2-AG are not stored within vesicles within presynaptic neuron axons. Instead, they are lipophilic molecules produced on demand, synthesized from phospholipids (ie, arachidonic acid derivatives) at the membranes of post-synaptic neurons, and released into the synapse directly.5
Acting as retrograde messengers, the endocannabinoids traverse the synapse, binding to receptors located on the axons of the presynaptic neuron. Two receptors—CB1 and CB2—have been most extensively studied and characterized.6,7 These receptors couple to Gi/o-proteins to inhibit adenylate cyclase, decreasing Ca2+ conductance and increasing K+ conductance.8 Once activated, cannabinoid receptors modulate neurotransmitter release from presynaptic axon terminals. Evidence points to a similar retrograde signaling between neurons and glial cells. Shortly after receptor activation, the endocannabinoids are deactivated by the actions of a transporter mechanism and enzyme degradation.9,10
The endocannabinoid system and pain transmission
Cannabinoid receptors are present in pain transmission circuits spanning from the peripheral sensory nerve endings (from which pain signals originate) to the spinal cord and supraspinal regions within the brain.11-14 CB1 receptors are abundantly present within the CNS, including regions involved in pain transmission. Binding to CB1 receptors, endocannabinoids modulate neurotransmission that impacts pain transmission centrally. Endocannabinoids can also indirectly modulate opiate and N-methyl-
By contrast, CB2 receptors are predominantly localized to peripheral tissues and immune cells, although there has been some discovery of their presence within the CNS (eg, on microglia). Endocannabinoid activation of CB2 receptors is thought to modulate the activity of peripheral afferent pain fibers and immune-mediated neuroinflammatory processes—such as inhibition of prostaglandin synthesis and mast cell degranulation—that can precipitate and maintain chronic pain states.16-18
Evidence garnered from preclinical (animal) studies points to the role of the endocannabinoid system in modulating normal pain transmission (see Manzanares et al3 for details). These studies offer a putative basis for understanding how exogenous cannabinoid congeners might serve to ameliorate pain transmission in pathophysiologic states, including chronic pain.
Continue to: Cannabinoid-based medications
Cannabinoid-based medications
Marijuana contains multiple components (cannabinoids). The most extensively studied are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Because it predominantly binds CB1 receptors centrally, THC is the major psychoactive component of cannabis; it promotes sleep and appetite, influences anxiety, and produces the “high” associated with cannabis use. By contrast, CBD weakly binds CB1 and thus exerts minimal or no psychoactive effects.19
Cannabinoid absorption, metabolism, bioavailability, and clinical effects vary depending on the formulation and method of administration (Table 1).20-22 THC and CBD content and potency in inhaled cannabis can vary significantly depending on the strains of the cannabis plant and manner of cultivation.23 To standardize approaches for administering cannabinoids in clinical trials and for clinical use, researchers have developed pharmaceutical analogs that contain extracted chemicals or synthetic chemicals similar to THC and/or CBD.

In this article, CBM refers to smoked/vaporized herbal cannabis as well as pharmaceutical cannabis analogs. Table 2 summarizes the characteristics of CBM commonly used in studies investigating their use for managing pain conditions.

CBM for chronic pain
The literature base examining the role of CBM for managing chronic nonmalignant and malignant pain of varying etiologies is rapidly expanding. Randomized controlled trials (RCTs) have focused on inhaled/smoked products and related cannabinoid medications, some of which are FDA-approved (Table 2).
A multitude of other cannabinoid-based products are currently commercially available to consumers, including tincture and oil-based products; over-the-counter CBD products; and several other formulations of CBM (eg, edible and suppository products). Because such products are not standardized or quality-controlled,24 RCTs have not assessed their efficacy for mitigating pain. Consequently, the findings summarized in this article do not address the utility of these agents.
Continue to: CBM for non-cancer pain
CBM for non-cancer pain
Neuropathic pain. Randomized controlled trials have assessed the pain-mitigating effects of various CBM, including inhaled cannabis, synthetic THC, plant-extracted CBD, and a THC/CBD spray. Studies have shown that inhaled/vaporized cannabis can produce short-term pain reduction in patients with chronic neuropathic pain of diverse etiologies, including diabetes mellitus-, HIV-, trauma-, and medication-induced neuropathies.22,25,26 Similar beneficial effects have been observed with the use of cannabis analogues (eg, nabiximols).25,26-29
Meta-analyses and systematic reviews have determined that most of these RCTs were of low-to-moderate quality.26,30 Meta-analyses have revealed divergent and conflicting results because of differences in the inclusion and exclusion criteria used to select RCTs for analysis and differences in the standards with which the quality of evidence were determined.25,30
Overall, the benefit of CBM for mitigating neuropathic pain is promising, but the effectiveness may not be robust.30,31 Several noteworthy caveats limit the interpretation of the results of these RCTs:
- due to the small sample sizes and brief durations of study, questions remain regarding the extent to which effects are generalizable, whether the benefits are sustained, and whether adverse effects emerge over time with continued use
- most RCTs evaluated inhaled (herbal) cannabis and nabiximols; there is little data on the effectiveness of other CBM formulations25,26,30
- the pain-mitigating effects of CBM were usually compared with those of placebo; the comparative efficacy against agents commonly used to treat neuropathic pain remains largely unexamined
- these RCTs typically compared mean pain severity score differences between cannabis-treated and placebo groups using standard subjective rating scales of pain intensity, such as the Numerical Rating Scale or Visual Analogue Scale. Customarily, the pain literature has used a 30% or 50% reduction in pain severity from baseline as an indicator of significant clinical improvement.32,33 The RCTs of CBM for neuropathic pain rarely used this standard, which makes it unclear whether CBM results in clinically significant pain reductions30
- indirect measures of effectiveness (ie, whether using CBM reduces the need for opioids or other analgesics to manage pain) were seldom reported in these RCTs.
Due to these limitations, clinical guidelines and systematic reviews consider CBM as a third- or fourth-line therapy for patients experiencing chronic neuropathic pain for whom conventional agents such as anticonvulsants and antidepressants have failed.34,35
Spasticity in multiple sclerosis (MS). Several RCTs have assessed the use of CBM for MS-related spasticity, although few were deemed to be high quality. Nabiximols and synthetic THC were effective in managing spasticity and reducing pain severity associated with muscle spasms.36 Generally, investigations revealed that CBM were associated with improvements in subjective measures of spasticity, but these were not born out in clinical, objective measures.26,37 The efficacy of smoked cannabis was uncertain.37 The existing literature on CBM for MS-related spasticity does not address dosing, duration of effects, tolerability, or comparative effectiveness against conventional anti-spasm medications.36,37
Continue to: Other chronic pain conditions
Other chronic pain conditions. CBM have also been studied for their usefulness in several other noncancer chronic conditions, including Crohn’s disease, inflammatory bowel disease, fibromyalgia, and other rheumatologic pain conditions.22,31,38-40 However, a solid foundation of empirical work to inform their utility for managing pain in these conditions is lacking.
CBM for cancer pain
Anecdotal evidence suggests that inhaled cannabis has promising pain-mitigating effects in patients with advanced cancer.41-43 There is a dearth of high-quality RCTs assessing the utility of CBM in patients with cancer pain.43-45 The types of CBM used and dosing strategies varied across RCTs, which makes it difficult to infer how best to treat patients with cancer pain. The agents studied included nabiximols, THC spray, and synthetic THC capsules.43-45 Although some studies have demonstrated that synthetic THC and nabiximols have potential for reducing subjective pain ratings compared with placebo,46,47 these results were inconsistent.46,48 Oromucosal nabiximols did not appear to confer any additional analgesic benefit in patients who were already prescribed opioids.31,45
The benefit of CBM for mitigating cancer pain is promising, but it remains difficult to know how to position the use of CBM in managing cancer pain. Limitations in the cancer literature include:
- the RCTs addressing CBM use for cancer pain were often brief, which raises questions about the long-term effectiveness and adverse effects of these agents
- tolerability and dosing limits encountered due to adverse effects were seldom reported43,45
- the types of cancer pain that patients had were often quite diverse. The small sample sizes and the heterogeneity of conditions included in these RCTs limit the ability to determine whether pain-mitigating effects might vary according to type of cancer-related pain.31,45
Despite these limitations, some clinical guidelines and systematic reviews have suggested that CBM have some role in addressing refractory malignant pain conditions.49
Psychiatric considerations related to CBM
As of November 2020, 36 states had legalized the use of cannabis for medical purposes, typically for painful conditions, despite the fact that empirical evidence to support their efficacy is mixed.50 In light of recent changes in both the legal and popular attitudes regarding cannabis, the implications of legalizing CBM remains to be seen. For example, some research suggests that adults with pain are vulnerable to frequent nonmedical cannabis use and/or cannabis use disorder.51 Although well-intended, the legalization of CBM use might represent society’s next misstep in the quest to address the suffering of patients with chronic pain. Some evidence shows that cannabis use and cannabis use disorders increase in states that have legalized medical marijuana.52,53 Psychiatrists will be on the front lines of addressing any potential consequences arising from the use of CBM for treating pain.
Continue to: Psychiatric disorders and CBM
Psychiatric disorders and CBM. The psychological impact of CBM use among patients enduring chronic pain can include sedation, cognitive/attention disturbance, and fatigue. These adverse effects can limit the utility of such agents.22,29,45
Contraindications for CBM use, and conditions for which CBM ought to be used with caution, are listed in Table 354,55.The safety of CBM, particularly in patients with chronic pain and psychiatric disorders, has not been examined. Patients with psychiatric disorders may be poor candidates for medical cannabis. Epidemiologic data suggest that recreational cannabis use is positively associated both cross-sectionally and prospectively with psychotic spectrum disorders, depressive symptoms, and anxiety symptoms, including panic disorder.56 Psychotic reactions have also been associated with CBM (dronabinol and nabilone).57 Cannabis use also has been associated with an earlier onset of, and lower remission rates of, symptoms associated with bipolar disorder.58,59 Consequently, patients who have been diagnosed with or are at risk for developing any of the aforementioned conditions may not be suitable candidates for CBM. If CBM are used, patients should be closely monitored for the emergence/exacerbation of psychiatric symptoms. The frequency and extent of follow-up is not clear, however. Because of its reduced propensity to produce psychoactive effects, CBD may be safer than THC for managing pain in individuals who have or are vulnerable to developing psychiatric disorders.
There is a lack of evidence to support the use of CBM for treating primary depressive disorders, general anxiety disorder, posttraumatic stress disorder, or psychosis.60,61 Very low-quality evidence suggests that CBM could lead to a small improvement in anxiety among individuals with noncancer pain and MS.60 However, interpreting causality is complicated. It is plausible that, for some patients, subjective improvement in pain severity may be related to reduced anxiety.62 Conversely, it is equally plausible that reductions in emotional distress may reduce the propensity to attend to, and thus magnify, pain severity. In the latter case, the indirect impact of reducing pain by modifying emotional distress can be impacted by the type and dose of CBM used. For example, low concentrations of THC produce anxiolytic effects, but high concentrations may be anxiety-provoking.63,64
Several potential pharmacokinetic drug interactions may arise between herbal cannabis or CBM and other medications (Table 465,66). THC and CBD are both metabolized by cytochrome P450 (CYP) 2C19 and 3A4.65,66 In addition, THC is also metabolized by CYP2C9. Medications that inhibit or induce these enzymes can increase or decrease the bioavailability of THC and CBD.67

Simultaneously, cannabinoids can impact the bioavailability of co-prescribed medications (Table 566,68). Although such CYP enzyme interactions remain a theoretical possibility, it is uncertain whether significant perturbations in plasma concentrations (and clinical effects) have been encountered with prescription medications when co-administered with CBM.69 Nonetheless, patients receiving CBM should be closely monitored for their response to prescribed medications.70

Continue to: Potential CYP enzyme interactions...
Potential CYP enzyme interactions aside, clinicians need to consider the additive effects that may occur when CBM are combined with sympathomimetic agents (eg, tachycardia, hypertension); CNS depressants such as alcohol, benzodiazepines, and opioids (eg, drowsiness, ataxia); or anticholinergics (eg, tachycardia, confusion).71 Inhaled herbal cannabis contains mutagens and can result in lung damage, exacerbations of chronic bronchitis, and certain types of cancer.54,72 Co-prescribing benzodiazepines may be contraindicated in light of their effects on respiratory rate and effort.
The THC contained in CBM produces hormonal effects (ie, significantly increases plasma levels of ghrelin and leptin and decreases peptide YY levels)73 that affect appetite and can produce weight gain. This may be problematic for patients receiving psychoactive medications associated with increased risk of weight gain and dyslipidemia. Because of the association between cannabis use and motor vehicle accidents, patients whose jobs require them to drive or operate industrial equipment may not be ideal candidates for CBM, especially if such patients also consume alcohol or are prescribed benzodiazepines and/or sedative hypnotics.74 Lastly, due to their lipophilicity, cannabinoids cross the placental barrier and can be found in breast milk75 and therefore can affect pregnancy outcomes and neurodevelopment.
Bottom Line
The popularity of cannabinoid-based medications (CBM) for the treatment of chronic pain conditions is growing, but the interest in their use may be outpacing the evidence supporting their analgesic benefits. High-quality, well-controlled randomized controlled trials are needed to decipher whether, and to what extent, these agents can be positioned in chronic pain management. Because psychiatrists are likely to encounter patients considering, or receiving, CBM, they must be aware of the potential benefits, risks, and adverse effects of such treatments.
Related Resources
- Joshi KG. Cannabis-derived compounds: what you need to know. Current Psychiatry. 2020;19(10):64-65. doi:10.12788/ cp.0050
- Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018; 17(1):34-41.
Drug Brand Names
Ajulemic acid • Anabasum
Alprazolam • Xanax
Amitriptyline • Elavil
Aripiprazole • Abilify, Abilify Maintena
Buspirone • BuSpar
Cannabidiol • Epidiolex
Carbamazepine • Tegretol, Equetro
Cimetidine • Tagamet HB
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Cyclosporine • Neoral, Sandimmune
Dronabinol • Marinol, Syndros
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Imipramine • Tofranil
Ketoconazole • Nizoral AD
Losartan • Cozaar
Midazolam • Versed
Mirtazapine • Remeron
Nabilone • Cesamet
Nabiximols • Sativex
Nefazodone • Serzone
Olanzapine • Zyprexa
Phenobarbital • Solfoton
Phenytoin • Dilantin
Ramelteon • Rozerem
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Topiramate • Topamax
Valproic acid • Depakote, Depakene
Venlafaxine • Effexor
Verapamil • Verelan
Zolpidem • Ambien
Against the backdrop of an increasing opioid use epidemic and a marked acceleration of prescription opioid–related deaths,1,2 there has been an impetus to explore the usefulness of alternative and co-analgesic agents to assist patients with chronic pain. Preclinical studies employing animal-based models of human pain syndromes have demonstrated that cannabis and chemicals derived from cannabis extracts may mitigate several pain conditions.3
Because there are significant comorbidities between psychiatric disorders and chronic pain, psychiatrists are likely to care for patients with chronic pain. As the availability of and interest in cannabinoid-based medications (CBM) increases, psychiatrists will need to be apprised of the utility, adverse effects, and potential drug interactions of these agents.
The endocannabinoid system and cannabis receptors
The endogenous cannabinoid (endocannabinoid) system is abundantly present within the peripheral and central nervous systems. The first identified, and best studied, endocannabinoids are N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG).4 Unlike typical neurotransmitters, AEA and 2-AG are not stored within vesicles within presynaptic neuron axons. Instead, they are lipophilic molecules produced on demand, synthesized from phospholipids (ie, arachidonic acid derivatives) at the membranes of post-synaptic neurons, and released into the synapse directly.5
Acting as retrograde messengers, the endocannabinoids traverse the synapse, binding to receptors located on the axons of the presynaptic neuron. Two receptors—CB1 and CB2—have been most extensively studied and characterized.6,7 These receptors couple to Gi/o-proteins to inhibit adenylate cyclase, decreasing Ca2+ conductance and increasing K+ conductance.8 Once activated, cannabinoid receptors modulate neurotransmitter release from presynaptic axon terminals. Evidence points to a similar retrograde signaling between neurons and glial cells. Shortly after receptor activation, the endocannabinoids are deactivated by the actions of a transporter mechanism and enzyme degradation.9,10
The endocannabinoid system and pain transmission
Cannabinoid receptors are present in pain transmission circuits spanning from the peripheral sensory nerve endings (from which pain signals originate) to the spinal cord and supraspinal regions within the brain.11-14 CB1 receptors are abundantly present within the CNS, including regions involved in pain transmission. Binding to CB1 receptors, endocannabinoids modulate neurotransmission that impacts pain transmission centrally. Endocannabinoids can also indirectly modulate opiate and N-methyl-
By contrast, CB2 receptors are predominantly localized to peripheral tissues and immune cells, although there has been some discovery of their presence within the CNS (eg, on microglia). Endocannabinoid activation of CB2 receptors is thought to modulate the activity of peripheral afferent pain fibers and immune-mediated neuroinflammatory processes—such as inhibition of prostaglandin synthesis and mast cell degranulation—that can precipitate and maintain chronic pain states.16-18
Evidence garnered from preclinical (animal) studies points to the role of the endocannabinoid system in modulating normal pain transmission (see Manzanares et al3 for details). These studies offer a putative basis for understanding how exogenous cannabinoid congeners might serve to ameliorate pain transmission in pathophysiologic states, including chronic pain.
Continue to: Cannabinoid-based medications
Cannabinoid-based medications
Marijuana contains multiple components (cannabinoids). The most extensively studied are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Because it predominantly binds CB1 receptors centrally, THC is the major psychoactive component of cannabis; it promotes sleep and appetite, influences anxiety, and produces the “high” associated with cannabis use. By contrast, CBD weakly binds CB1 and thus exerts minimal or no psychoactive effects.19
Cannabinoid absorption, metabolism, bioavailability, and clinical effects vary depending on the formulation and method of administration (Table 1).20-22 THC and CBD content and potency in inhaled cannabis can vary significantly depending on the strains of the cannabis plant and manner of cultivation.23 To standardize approaches for administering cannabinoids in clinical trials and for clinical use, researchers have developed pharmaceutical analogs that contain extracted chemicals or synthetic chemicals similar to THC and/or CBD.

In this article, CBM refers to smoked/vaporized herbal cannabis as well as pharmaceutical cannabis analogs. Table 2 summarizes the characteristics of CBM commonly used in studies investigating their use for managing pain conditions.

CBM for chronic pain
The literature base examining the role of CBM for managing chronic nonmalignant and malignant pain of varying etiologies is rapidly expanding. Randomized controlled trials (RCTs) have focused on inhaled/smoked products and related cannabinoid medications, some of which are FDA-approved (Table 2).
A multitude of other cannabinoid-based products are currently commercially available to consumers, including tincture and oil-based products; over-the-counter CBD products; and several other formulations of CBM (eg, edible and suppository products). Because such products are not standardized or quality-controlled,24 RCTs have not assessed their efficacy for mitigating pain. Consequently, the findings summarized in this article do not address the utility of these agents.
Continue to: CBM for non-cancer pain
CBM for non-cancer pain
Neuropathic pain. Randomized controlled trials have assessed the pain-mitigating effects of various CBM, including inhaled cannabis, synthetic THC, plant-extracted CBD, and a THC/CBD spray. Studies have shown that inhaled/vaporized cannabis can produce short-term pain reduction in patients with chronic neuropathic pain of diverse etiologies, including diabetes mellitus-, HIV-, trauma-, and medication-induced neuropathies.22,25,26 Similar beneficial effects have been observed with the use of cannabis analogues (eg, nabiximols).25,26-29
Meta-analyses and systematic reviews have determined that most of these RCTs were of low-to-moderate quality.26,30 Meta-analyses have revealed divergent and conflicting results because of differences in the inclusion and exclusion criteria used to select RCTs for analysis and differences in the standards with which the quality of evidence were determined.25,30
Overall, the benefit of CBM for mitigating neuropathic pain is promising, but the effectiveness may not be robust.30,31 Several noteworthy caveats limit the interpretation of the results of these RCTs:
- due to the small sample sizes and brief durations of study, questions remain regarding the extent to which effects are generalizable, whether the benefits are sustained, and whether adverse effects emerge over time with continued use
- most RCTs evaluated inhaled (herbal) cannabis and nabiximols; there is little data on the effectiveness of other CBM formulations25,26,30
- the pain-mitigating effects of CBM were usually compared with those of placebo; the comparative efficacy against agents commonly used to treat neuropathic pain remains largely unexamined
- these RCTs typically compared mean pain severity score differences between cannabis-treated and placebo groups using standard subjective rating scales of pain intensity, such as the Numerical Rating Scale or Visual Analogue Scale. Customarily, the pain literature has used a 30% or 50% reduction in pain severity from baseline as an indicator of significant clinical improvement.32,33 The RCTs of CBM for neuropathic pain rarely used this standard, which makes it unclear whether CBM results in clinically significant pain reductions30
- indirect measures of effectiveness (ie, whether using CBM reduces the need for opioids or other analgesics to manage pain) were seldom reported in these RCTs.
Due to these limitations, clinical guidelines and systematic reviews consider CBM as a third- or fourth-line therapy for patients experiencing chronic neuropathic pain for whom conventional agents such as anticonvulsants and antidepressants have failed.34,35
Spasticity in multiple sclerosis (MS). Several RCTs have assessed the use of CBM for MS-related spasticity, although few were deemed to be high quality. Nabiximols and synthetic THC were effective in managing spasticity and reducing pain severity associated with muscle spasms.36 Generally, investigations revealed that CBM were associated with improvements in subjective measures of spasticity, but these were not born out in clinical, objective measures.26,37 The efficacy of smoked cannabis was uncertain.37 The existing literature on CBM for MS-related spasticity does not address dosing, duration of effects, tolerability, or comparative effectiveness against conventional anti-spasm medications.36,37
Continue to: Other chronic pain conditions
Other chronic pain conditions. CBM have also been studied for their usefulness in several other noncancer chronic conditions, including Crohn’s disease, inflammatory bowel disease, fibromyalgia, and other rheumatologic pain conditions.22,31,38-40 However, a solid foundation of empirical work to inform their utility for managing pain in these conditions is lacking.
CBM for cancer pain
Anecdotal evidence suggests that inhaled cannabis has promising pain-mitigating effects in patients with advanced cancer.41-43 There is a dearth of high-quality RCTs assessing the utility of CBM in patients with cancer pain.43-45 The types of CBM used and dosing strategies varied across RCTs, which makes it difficult to infer how best to treat patients with cancer pain. The agents studied included nabiximols, THC spray, and synthetic THC capsules.43-45 Although some studies have demonstrated that synthetic THC and nabiximols have potential for reducing subjective pain ratings compared with placebo,46,47 these results were inconsistent.46,48 Oromucosal nabiximols did not appear to confer any additional analgesic benefit in patients who were already prescribed opioids.31,45
The benefit of CBM for mitigating cancer pain is promising, but it remains difficult to know how to position the use of CBM in managing cancer pain. Limitations in the cancer literature include:
- the RCTs addressing CBM use for cancer pain were often brief, which raises questions about the long-term effectiveness and adverse effects of these agents
- tolerability and dosing limits encountered due to adverse effects were seldom reported43,45
- the types of cancer pain that patients had were often quite diverse. The small sample sizes and the heterogeneity of conditions included in these RCTs limit the ability to determine whether pain-mitigating effects might vary according to type of cancer-related pain.31,45
Despite these limitations, some clinical guidelines and systematic reviews have suggested that CBM have some role in addressing refractory malignant pain conditions.49
Psychiatric considerations related to CBM
As of November 2020, 36 states had legalized the use of cannabis for medical purposes, typically for painful conditions, despite the fact that empirical evidence to support their efficacy is mixed.50 In light of recent changes in both the legal and popular attitudes regarding cannabis, the implications of legalizing CBM remains to be seen. For example, some research suggests that adults with pain are vulnerable to frequent nonmedical cannabis use and/or cannabis use disorder.51 Although well-intended, the legalization of CBM use might represent society’s next misstep in the quest to address the suffering of patients with chronic pain. Some evidence shows that cannabis use and cannabis use disorders increase in states that have legalized medical marijuana.52,53 Psychiatrists will be on the front lines of addressing any potential consequences arising from the use of CBM for treating pain.
Continue to: Psychiatric disorders and CBM
Psychiatric disorders and CBM. The psychological impact of CBM use among patients enduring chronic pain can include sedation, cognitive/attention disturbance, and fatigue. These adverse effects can limit the utility of such agents.22,29,45
Contraindications for CBM use, and conditions for which CBM ought to be used with caution, are listed in Table 354,55.The safety of CBM, particularly in patients with chronic pain and psychiatric disorders, has not been examined. Patients with psychiatric disorders may be poor candidates for medical cannabis. Epidemiologic data suggest that recreational cannabis use is positively associated both cross-sectionally and prospectively with psychotic spectrum disorders, depressive symptoms, and anxiety symptoms, including panic disorder.56 Psychotic reactions have also been associated with CBM (dronabinol and nabilone).57 Cannabis use also has been associated with an earlier onset of, and lower remission rates of, symptoms associated with bipolar disorder.58,59 Consequently, patients who have been diagnosed with or are at risk for developing any of the aforementioned conditions may not be suitable candidates for CBM. If CBM are used, patients should be closely monitored for the emergence/exacerbation of psychiatric symptoms. The frequency and extent of follow-up is not clear, however. Because of its reduced propensity to produce psychoactive effects, CBD may be safer than THC for managing pain in individuals who have or are vulnerable to developing psychiatric disorders.
There is a lack of evidence to support the use of CBM for treating primary depressive disorders, general anxiety disorder, posttraumatic stress disorder, or psychosis.60,61 Very low-quality evidence suggests that CBM could lead to a small improvement in anxiety among individuals with noncancer pain and MS.60 However, interpreting causality is complicated. It is plausible that, for some patients, subjective improvement in pain severity may be related to reduced anxiety.62 Conversely, it is equally plausible that reductions in emotional distress may reduce the propensity to attend to, and thus magnify, pain severity. In the latter case, the indirect impact of reducing pain by modifying emotional distress can be impacted by the type and dose of CBM used. For example, low concentrations of THC produce anxiolytic effects, but high concentrations may be anxiety-provoking.63,64
Several potential pharmacokinetic drug interactions may arise between herbal cannabis or CBM and other medications (Table 465,66). THC and CBD are both metabolized by cytochrome P450 (CYP) 2C19 and 3A4.65,66 In addition, THC is also metabolized by CYP2C9. Medications that inhibit or induce these enzymes can increase or decrease the bioavailability of THC and CBD.67

Simultaneously, cannabinoids can impact the bioavailability of co-prescribed medications (Table 566,68). Although such CYP enzyme interactions remain a theoretical possibility, it is uncertain whether significant perturbations in plasma concentrations (and clinical effects) have been encountered with prescription medications when co-administered with CBM.69 Nonetheless, patients receiving CBM should be closely monitored for their response to prescribed medications.70

Continue to: Potential CYP enzyme interactions...
Potential CYP enzyme interactions aside, clinicians need to consider the additive effects that may occur when CBM are combined with sympathomimetic agents (eg, tachycardia, hypertension); CNS depressants such as alcohol, benzodiazepines, and opioids (eg, drowsiness, ataxia); or anticholinergics (eg, tachycardia, confusion).71 Inhaled herbal cannabis contains mutagens and can result in lung damage, exacerbations of chronic bronchitis, and certain types of cancer.54,72 Co-prescribing benzodiazepines may be contraindicated in light of their effects on respiratory rate and effort.
The THC contained in CBM produces hormonal effects (ie, significantly increases plasma levels of ghrelin and leptin and decreases peptide YY levels)73 that affect appetite and can produce weight gain. This may be problematic for patients receiving psychoactive medications associated with increased risk of weight gain and dyslipidemia. Because of the association between cannabis use and motor vehicle accidents, patients whose jobs require them to drive or operate industrial equipment may not be ideal candidates for CBM, especially if such patients also consume alcohol or are prescribed benzodiazepines and/or sedative hypnotics.74 Lastly, due to their lipophilicity, cannabinoids cross the placental barrier and can be found in breast milk75 and therefore can affect pregnancy outcomes and neurodevelopment.
Bottom Line
The popularity of cannabinoid-based medications (CBM) for the treatment of chronic pain conditions is growing, but the interest in their use may be outpacing the evidence supporting their analgesic benefits. High-quality, well-controlled randomized controlled trials are needed to decipher whether, and to what extent, these agents can be positioned in chronic pain management. Because psychiatrists are likely to encounter patients considering, or receiving, CBM, they must be aware of the potential benefits, risks, and adverse effects of such treatments.
Related Resources
- Joshi KG. Cannabis-derived compounds: what you need to know. Current Psychiatry. 2020;19(10):64-65. doi:10.12788/ cp.0050
- Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018; 17(1):34-41.
Drug Brand Names
Ajulemic acid • Anabasum
Alprazolam • Xanax
Amitriptyline • Elavil
Aripiprazole • Abilify, Abilify Maintena
Buspirone • BuSpar
Cannabidiol • Epidiolex
Carbamazepine • Tegretol, Equetro
Cimetidine • Tagamet HB
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Cyclosporine • Neoral, Sandimmune
Dronabinol • Marinol, Syndros
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Imipramine • Tofranil
Ketoconazole • Nizoral AD
Losartan • Cozaar
Midazolam • Versed
Mirtazapine • Remeron
Nabilone • Cesamet
Nabiximols • Sativex
Nefazodone • Serzone
Olanzapine • Zyprexa
Phenobarbital • Solfoton
Phenytoin • Dilantin
Ramelteon • Rozerem
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Topiramate • Topamax
Valproic acid • Depakote, Depakene
Venlafaxine • Effexor
Verapamil • Verelan
Zolpidem • Ambien
1. Okie S. A floor of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. doi:10.1056/NEJMp1011512
2. Powell D, Pacula RL, Taylor E. How increasing medical access to opioids contributes to the opioid epidemic: evidence from Medicare Part D. J Health Econ. 2020;71:102286. doi: 10.1016/j.jhealeco.2019.102286
3. Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257. doi: 10.2174/157015906778019527
4. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833
5. Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016;14(4):2899-2903. doi:10.3892/mmr.2016.5585
6. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83-90. doi:10.1016/0006-2952(95)00109-d
7. Walker JM, Krey JF, Chu CJ, et al. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids. 2002;121(1-2):159-172. doi: 10.1016/s0009-3084(02)00152-4
8. Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142(8):1209-1218. doi: 10.1038/sj.bjp.0705881
9. Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation, biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7-14.
10. Piomelli D, Beltramo M, Giuffrida A, et al. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5(6 Pt B):462-473. doi: 10.1006/nbdi.1998.0221
11. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175-191. doi: 10.1093/cercor/bhj136
12. Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534(Pt 3):805-812. doi: 10.1111/j.1469-7793.2001.00805.x
13. Vaughan CW, Connor M, Bagley EE, et al. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57(2):288-295.
14. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127(4):935-940. doi: 10.1038/sj.bjp.0702636
15. Raichlen DA, Foster AD, Gerdeman GI, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high.” J Exp Biol. 2012;215(Pt 8):1331-1336. doi: 10.1242/jeb.063677
16. Beltrano M. Cannabinoid type 2 receptor as a target for chronic pain. Mini Rev Chem. 2009;234:253-254.
17. Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529-10533. doi: 10.1073/pnas.1834309100
18. Valenzano KJ, Tafessem L, Lee G, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658-672.
19. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631. doi: 10.1124/pr.110.003004
20. Carter GT, Weydt P, Kyashna-Tocha M, et al. Medicinal cannabis: rational guidelines for dosing. Drugs. 2004;7(5):464-470.
21. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
22. Johal H, Devji T, Chang Y, et al. cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461. doi: 10.1177/1179544120906461
23. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91(6):966-975. doi: 10.3732/ajb.91.6.966
24. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199-210. doi: 10.1080/02791072.2013.805976
25. Andreae MH, Carter GM, Shaparin N, et al. inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221-1232. doi: 10.1016/j.jpain.2015.07.009
26. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi: 10.1001/jama.2015.6358
27. Boychuk DG, Goddard G, Mauro G, et al. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7-14. doi: 10.11607/ofph.1274
28. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735-744. doi: 10.1111/j.1365-2125.2011.03970.x
29. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932-1954. doi: 10.1097/j.pain.0000000000001293
30. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2
31. Häuser W, Fitzcharles MA, Radbruch L, et al. Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int. 2017;114(38):627-634. doi: 10.3238/arztebl.2017.0627
32. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005
33. Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. doi: 10.1016/j.clinthera.2008.05.011
34. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-335. doi: 10.1155/2014/754693
35. Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62-88. doi: 10.1007/s00482-015-0089-y
36. Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep. 2018;18(8):50. doi: 10.1007/s11910-018-0859-x
37. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563. doi: 10.1212/WNL.0000000000000363
38. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease and ulcerative colitis: evidence from Cochrane Reviews. Inflamm Bowel Dis. 2020;26(4):502-509. doi: 10.1093/ibd/izz233
39. Katz-Talmor D, Katz I, Porat-Katz BS, et al. Cannabinoids for the treatment of rheumatic diseases - where do we stand? Nat Rev Rheumatol. 2018;14(8):488-498. doi: 10.1038/s41584-018-0025-5
40. Walitt B, Klose P, Fitzcharles MA, et al. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2
41. Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37‐43. doi: 10.1016/j.ejim.2018.01.023
42. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705‐713. doi:10.1200/EDBK_180469
43. Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(Suppl 2):5215-5222. doi: 10.21037/apm.2017.08.05
44. Aviram J, Samuelly-Lechtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755-E796.
45. Häuser W, Welsch P, Klose P, et al. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424-436. doi: 10.1007/s00482-019-0373-3
46. Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010; 39:167-179.
47. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438-449. doi: 10.1016/j.jpain.2012.01.003
48. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166-173. doi: 10.1016/j.jpainsymman.2013.02.018
49. Kleckner AS, Kleckner IR, Kamen CS, et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019;11:1758835919866362. doi: 10.1177/1758835919866362
50. National Conference of State Legislatures (ncsl.org). State Medical Marijuana Laws. Accessed April 5, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
51. Hasin DS, Shmulewitz D, Cerda M, et al. US adults with pain, a group increasingly vulnerable to nonmedical cannabis use and cannabis use disorder: 2001-2002 and 2012-2013. Am J Psychiatry. 2020;177(7):611-618. doi: 10.1176/appi.ajp.2019.19030284
52. Hasin DS, Sarvet AL, Cerdá M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry. 2017;74(6):579-588. doi: 10.1001/jamapsychiatry.2017.0724
53. National Institute on Drug Abuse. Illicit cannabis use and use disorders increase in states with medical marijuana laws. April 26, 2017. Accessed October 24, 2020. https://archives.drugabuse.gov/news-events/news-releases/2017/04/illicit-cannabis-use-use-disorders-increase-in-states-medical-marijuana-laws
54. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press; 2017. https://doi.org/10.17226/24625
55. Stanford M. Physician recommended marijuana: contraindications & standards of care. A review of the literature. Accessed July 7, 2020. http://drneurosci.com/MedicalMarijuanaStandardsofCare.pdf
56. Repp K, Raich A. Marijuana and health: a comprehensive review of 20 years of research. Washington County Oregon Department of Health and Human Services. 2014. Accessed April 8, 2021. https://www.co.washington.or.us/CAO/upload/HHSmarijuana-review.pdf
57. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638-654. doi: 10.1016/j.sapharm.2015.09.002.
58. Leite RT, Nogueira Sde O, do Nascimento JP, et al. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast. 2015;2015:434127. doi: 10.1155/2015/43412
59. Kim SW, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349-355. doi: 10.4306/pi.2015.12.3.349
60. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995-1010.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. doi: 10.4088/JCP.15r10036.
62. Woolf CJ, American College of Physicians. American Physiological Society Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
63. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515‐523. doi: 10.1002/hup.1048
64. Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015;12(4):735‐746. doi: 10.1007/s13311-015-0380-8
65. Antoniou T, Bodkin J, Ho JMW. Drug interactions with cannabinoids. CMAJ. 2020;2;192:E206. doi: 10.1503/cmaj.191097
66. Brown JD. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug-drug interactions. J Clin Med. 2020;9(4):919. doi: 10.3390/jcm9040919.
67. Maida V, Daeninck P. A user’s guide to cannabinoid therapy in oncology. Curr Oncol. 2016;23(6):398-406. doi: http://dx.doi.org/10.3747/co.23.3487
68. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86-95. doi: 10.3109/03602532.2013.849268
69. Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol. 2016;23(52):S8-S14.
70. Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2018;6(1):3. doi: 10.3390/medicines6010003
71. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477-2482.
72. Ghasemiesfe M, Barrow B, Leonard S, et al. Association between marijuana use and risk of cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(11):e1916318. doi: 10.1001/jamanetworkopen.2019.16318
73. Riggs PK, Vaida F, Rossi SS, et al. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46-52. doi: 10.1016/j.brainres.2011.11.001
74. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536
75. Carlier J, Huestis MA, Zaami S, et al. Monitoring perinatal exposure to cannabis and synthetic cannabinoids. Ther Drug Monit. 2020;42(2):194-204.
1. Okie S. A floor of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. doi:10.1056/NEJMp1011512
2. Powell D, Pacula RL, Taylor E. How increasing medical access to opioids contributes to the opioid epidemic: evidence from Medicare Part D. J Health Econ. 2020;71:102286. doi: 10.1016/j.jhealeco.2019.102286
3. Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257. doi: 10.2174/157015906778019527
4. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833
5. Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016;14(4):2899-2903. doi:10.3892/mmr.2016.5585
6. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83-90. doi:10.1016/0006-2952(95)00109-d
7. Walker JM, Krey JF, Chu CJ, et al. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids. 2002;121(1-2):159-172. doi: 10.1016/s0009-3084(02)00152-4
8. Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142(8):1209-1218. doi: 10.1038/sj.bjp.0705881
9. Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation, biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7-14.
10. Piomelli D, Beltramo M, Giuffrida A, et al. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5(6 Pt B):462-473. doi: 10.1006/nbdi.1998.0221
11. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175-191. doi: 10.1093/cercor/bhj136
12. Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534(Pt 3):805-812. doi: 10.1111/j.1469-7793.2001.00805.x
13. Vaughan CW, Connor M, Bagley EE, et al. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57(2):288-295.
14. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127(4):935-940. doi: 10.1038/sj.bjp.0702636
15. Raichlen DA, Foster AD, Gerdeman GI, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high.” J Exp Biol. 2012;215(Pt 8):1331-1336. doi: 10.1242/jeb.063677
16. Beltrano M. Cannabinoid type 2 receptor as a target for chronic pain. Mini Rev Chem. 2009;234:253-254.
17. Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529-10533. doi: 10.1073/pnas.1834309100
18. Valenzano KJ, Tafessem L, Lee G, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658-672.
19. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631. doi: 10.1124/pr.110.003004
20. Carter GT, Weydt P, Kyashna-Tocha M, et al. Medicinal cannabis: rational guidelines for dosing. Drugs. 2004;7(5):464-470.
21. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
22. Johal H, Devji T, Chang Y, et al. cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461. doi: 10.1177/1179544120906461
23. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91(6):966-975. doi: 10.3732/ajb.91.6.966
24. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199-210. doi: 10.1080/02791072.2013.805976
25. Andreae MH, Carter GM, Shaparin N, et al. inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221-1232. doi: 10.1016/j.jpain.2015.07.009
26. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi: 10.1001/jama.2015.6358
27. Boychuk DG, Goddard G, Mauro G, et al. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7-14. doi: 10.11607/ofph.1274
28. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735-744. doi: 10.1111/j.1365-2125.2011.03970.x
29. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932-1954. doi: 10.1097/j.pain.0000000000001293
30. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2
31. Häuser W, Fitzcharles MA, Radbruch L, et al. Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int. 2017;114(38):627-634. doi: 10.3238/arztebl.2017.0627
32. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005
33. Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. doi: 10.1016/j.clinthera.2008.05.011
34. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-335. doi: 10.1155/2014/754693
35. Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62-88. doi: 10.1007/s00482-015-0089-y
36. Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep. 2018;18(8):50. doi: 10.1007/s11910-018-0859-x
37. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563. doi: 10.1212/WNL.0000000000000363
38. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease and ulcerative colitis: evidence from Cochrane Reviews. Inflamm Bowel Dis. 2020;26(4):502-509. doi: 10.1093/ibd/izz233
39. Katz-Talmor D, Katz I, Porat-Katz BS, et al. Cannabinoids for the treatment of rheumatic diseases - where do we stand? Nat Rev Rheumatol. 2018;14(8):488-498. doi: 10.1038/s41584-018-0025-5
40. Walitt B, Klose P, Fitzcharles MA, et al. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2
41. Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37‐43. doi: 10.1016/j.ejim.2018.01.023
42. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705‐713. doi:10.1200/EDBK_180469
43. Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(Suppl 2):5215-5222. doi: 10.21037/apm.2017.08.05
44. Aviram J, Samuelly-Lechtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755-E796.
45. Häuser W, Welsch P, Klose P, et al. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424-436. doi: 10.1007/s00482-019-0373-3
46. Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010; 39:167-179.
47. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438-449. doi: 10.1016/j.jpain.2012.01.003
48. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166-173. doi: 10.1016/j.jpainsymman.2013.02.018
49. Kleckner AS, Kleckner IR, Kamen CS, et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019;11:1758835919866362. doi: 10.1177/1758835919866362
50. National Conference of State Legislatures (ncsl.org). State Medical Marijuana Laws. Accessed April 5, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
51. Hasin DS, Shmulewitz D, Cerda M, et al. US adults with pain, a group increasingly vulnerable to nonmedical cannabis use and cannabis use disorder: 2001-2002 and 2012-2013. Am J Psychiatry. 2020;177(7):611-618. doi: 10.1176/appi.ajp.2019.19030284
52. Hasin DS, Sarvet AL, Cerdá M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry. 2017;74(6):579-588. doi: 10.1001/jamapsychiatry.2017.0724
53. National Institute on Drug Abuse. Illicit cannabis use and use disorders increase in states with medical marijuana laws. April 26, 2017. Accessed October 24, 2020. https://archives.drugabuse.gov/news-events/news-releases/2017/04/illicit-cannabis-use-use-disorders-increase-in-states-medical-marijuana-laws
54. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press; 2017. https://doi.org/10.17226/24625
55. Stanford M. Physician recommended marijuana: contraindications & standards of care. A review of the literature. Accessed July 7, 2020. http://drneurosci.com/MedicalMarijuanaStandardsofCare.pdf
56. Repp K, Raich A. Marijuana and health: a comprehensive review of 20 years of research. Washington County Oregon Department of Health and Human Services. 2014. Accessed April 8, 2021. https://www.co.washington.or.us/CAO/upload/HHSmarijuana-review.pdf
57. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638-654. doi: 10.1016/j.sapharm.2015.09.002.
58. Leite RT, Nogueira Sde O, do Nascimento JP, et al. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast. 2015;2015:434127. doi: 10.1155/2015/43412
59. Kim SW, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349-355. doi: 10.4306/pi.2015.12.3.349
60. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995-1010.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. doi: 10.4088/JCP.15r10036.
62. Woolf CJ, American College of Physicians. American Physiological Society Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
63. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515‐523. doi: 10.1002/hup.1048
64. Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015;12(4):735‐746. doi: 10.1007/s13311-015-0380-8
65. Antoniou T, Bodkin J, Ho JMW. Drug interactions with cannabinoids. CMAJ. 2020;2;192:E206. doi: 10.1503/cmaj.191097
66. Brown JD. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug-drug interactions. J Clin Med. 2020;9(4):919. doi: 10.3390/jcm9040919.
67. Maida V, Daeninck P. A user’s guide to cannabinoid therapy in oncology. Curr Oncol. 2016;23(6):398-406. doi: http://dx.doi.org/10.3747/co.23.3487
68. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86-95. doi: 10.3109/03602532.2013.849268
69. Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol. 2016;23(52):S8-S14.
70. Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2018;6(1):3. doi: 10.3390/medicines6010003
71. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477-2482.
72. Ghasemiesfe M, Barrow B, Leonard S, et al. Association between marijuana use and risk of cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(11):e1916318. doi: 10.1001/jamanetworkopen.2019.16318
73. Riggs PK, Vaida F, Rossi SS, et al. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46-52. doi: 10.1016/j.brainres.2011.11.001
74. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536
75. Carlier J, Huestis MA, Zaami S, et al. Monitoring perinatal exposure to cannabis and synthetic cannabinoids. Ther Drug Monit. 2020;42(2):194-204.