User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Managing schizophrenia in a patient with cancer: A fine balance
CASE Stable with a new diagnosis
Ms. B, age 60, has a history of schizophrenia, which has been stable on clozapine, 500 mg/d, for more than 2 decades. After a series of hospitalizations in her 20s and 30s, clozapine was initiated and she has not required additional inpatient psychiatric care. She has been managed in the outpatient setting with standard biweekly absolute neutrophil count (ANC) monitoring. She lives independently and is an active member in her church.
After experiencing rectal bleeding, Ms. B is diagnosed with rectal carcinoma and is scheduled to undergo chemotherapy and radiation treatment.
[polldaddy:9754786]
The authors’ observations
Both clozapine and chemotherapy carry the risk of immunosuppression, presenting a clinical challenge when choosing an appropriate management strategy. However, the risks of stopping clozapine after a long period of symptom stability are substantial, with a relapse rate up to 50%.1 Among patients taking clozapine, the risk of agranulocytosis and neutropenia are approximately 0.8% and 3%, respectively, and >80% of agranulocyotis cases occur within the first 18 weeks of treatment.2,3 Although both clozapine and chemotherapy can lead to neutropenia and agranulocytosis, there currently is no evidence of a synergistic effect on bone marrow suppression with simultaneous use of these therapies2 nor is there evidence of the combination leading to sustained marrow suppression.4
Because of Ms. B’s positive response to clozapine, the risks associated with discontinuing the medication, and the relatively low risk of clozapine contributing to neutropenia after a long period of stabilization, her outpatient psychiatric providers decide to increase ANC monitoring to weekly while she undergoes cancer treatment.
TREATMENT Neutropenia, psychosis
Ms. B continues clozapine during radiation and chemotherapy, but develops leukopenia and neutropenia with a low of 1,220/μL white blood cells and an ANC of 610/μL. Clozapine is stopped, consistent with current recommendations to hold the drug if the neutrophil count is <1,000/μL in a patient without benign ethnic neutropenia, and her outpatient provider monitors her closely. The treatment team does not restart an antipsychotic immediately after discontinuing clozapine because of the risk that other antipsychotics can cause hematologic toxicity or prolong granulocytopenia associated with clozapine.5
Approximately 2 weeks later, Ms. B is admitted to a different hospital for altered mental status and is found to have hyponatremia and rectal bleeding. The workup suggests that her rectal carcinoma has not fully responded to initial therapies, and she likely will require further treatment. Her mental status improves after hyponatremia resolves, but she reports auditory hallucinations and paranoia. Risperidone, 4 mg/d, is initiated to target psychosis.
After discharge, Ms. B develops bilateral upper extremity tremor, which she finds intolerable and attributes to risperidone. She refuses to continue risperidone or try adjunctive medications to address the tremor, but is willing to consider a different antipsychotic. Olanzapine, 10 mg/d, is initiated and risperidone is slowly tapered. During this time, Ms. B experiences increased paranoia and believes that the Internal Revenue Service is calling her. She misses her next appointment.
Later, the fire department finds Ms. B wandering the streets and brings her to the psychiatric emergency room. During the examination, she is disheveled and withdrawn, and unable to reply to simple questions about diet and sleep. When asked why she was in the street, she says that she left her apartment because it was “too messy.” The treatment team learns that she had walked at least 10 miles from her apartment before sitting down by the side of the road and being picked up by the fire department. She reveals that she left her apartment and continued walking because “a man” told her to do so and threatened to harm her if she stopped.
When Ms. B is admitted to the psychiatric service, she is paranoid, disorganized, and guarded. She remains in her room for most of the day and either refuses to talk to providers or curses at them. She often is seen wearing soiled clothing with her hair mussed. She denies having rectal carcinoma, although she expressed understanding of her medical condition <2 months earlier.
[polldaddy:9754787]
The authors’ observations
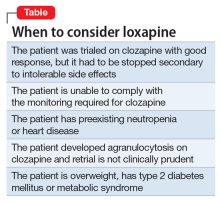
Clozapine is considered the most efficacious agent for treatment-resistant schizophrenia.6 Although non-compliance is the most common reason for discontinuing clozapine, >20% of patients stop clozapine because of adverse effects.7 Clozapine often is a drug of last resort because of the need for frequent monitoring and significant side effects; therefore deciding on a next step when clozapine fails or cannot be continued because of other factors can pose a challenge.
Ms. B’s treatment team gave serious consideration to restarting clozapine. However, because it was likely that Ms. B would undergo another round of chemotherapy and possibly radiation, the risk of neutropenia recurring was considered too high. Lithium has been used successfully to manage neutropenia in patients taking clozapine and, for some, adding lithium could help boost white cell count and allow a successful rechallenge with clozapine.3,8 However, because of Ms. B’s medical comorbidities, including cancer and chronic kidney disease, adding lithium was not thought to be clinically prudent at that time and the treatment team considered other options.
Olanzapine. Although research is limited, studies suggest olanzapine is the most commonly prescribed medication when a patient has to discontinue clozapine,7 with comparable response rates in those with refractory schizophrenia.9 Therefore, Ms. B was initially maintained on olanzapine, and the dosage increased to 30 mg over the course of 16 days in the hospital. However, she did not respond to the medication, remaining disorganized and paranoid without any notable improvement in her symptoms therefore other treatment options were explored.
Loxapine. Previous limited case reports have shown loxapine to be effective in treating individuals with refractory schizophrenia, either alone or in combination with other antipsychotics.10,11 FDA-approved in 1975, loxapine was among the last of the typical antipsychotics brought to the U.S. market before the introduction of clozapine, the first atypical.12 Loxapine is a dibenzoxazepine that has a molecular structure similar to clozapine.13 Unlike clozapine, however, loxapine is not known to cause agranulocytosis.14 Research suggests that although clozapine is oxidized to metabolites that are cytotoxic, loxapine is not, potentially accounting for their different effects on neutrophils.15
The efficacy of loxapine has shown to be similar to other typical and atypical antipsychotics, with approximately 70% of patients showing improvement.14 However, loxapine may be overlooked as an option, possibly because it was not included in the CATIE trial and was the last typical antipsychotic to be approved before atypicals were introduced.12 First available in oral and IM formulations, there has been increased interest in loxapine recently because of the approval of an inhaled formulation in 2012.16
Although classified as a typical antipsychotic, studies have suggested that loxapine acts as an atypical at low dosages.17,18 Previous work suggests, however, that the side effect profile of loxapine is similar to typical antipsychotics.14 At dosages <50 mg, it results in fewer cases of extrapyramidal side effects than expected with a typical antipsychotic.18
Loxapine’s binding profile seems to exist along this spectrum of typical to atypical. Tissue-based binding studies have shown a higher 5-HT2 affinity relative to D2, consistent with atypical antipsychotics.19 Positron emission tomography studies in humans show 5-HT2 saturation of loxapine to be close to equal to D2 binding in loxapine, thus a slightly lower ratio of 5-HT2 to D2 relative to atypicals, but more than that seen with typical antipsychotics.20 These differences between in vitro and in vivo studies may be secondary to the binding of loxapine’s active metabolites, particularly 7- and 8-hydroxyloxapine, which have more dopaminergic activity. In addition to increased 5-HT2A binding compared with typical antipsychotics, loxapine also has a high affinity for the D4 receptors, as well as interacting with other serotonin receptors 5-HT3, 5-HT6, and 5-HT7. Of note this is a similar pattern of binding affinity as seen in clozapine.19
It should be noted, however, that loxapine may not be an appropriate treatment in all forms of cancer. Similar to other first-generation antipsychotics, it increases prolactin levels, and thus may have a negative clinical impact on patients with prolactin receptor positive breast cancers.21,22 Finally, although clozapine can result in significant weight gain, dyslipidemia, and hyperglycemia, unlike many antipsychotics, loxapine has been shown to be weight neutral or result in weight loss,14 making it an option to consider for patients with type 2 diabetes mellitus, metabolic syndrome, dyslipidemia, or cardiovascular disease.
OUTCOME Improvement, stability
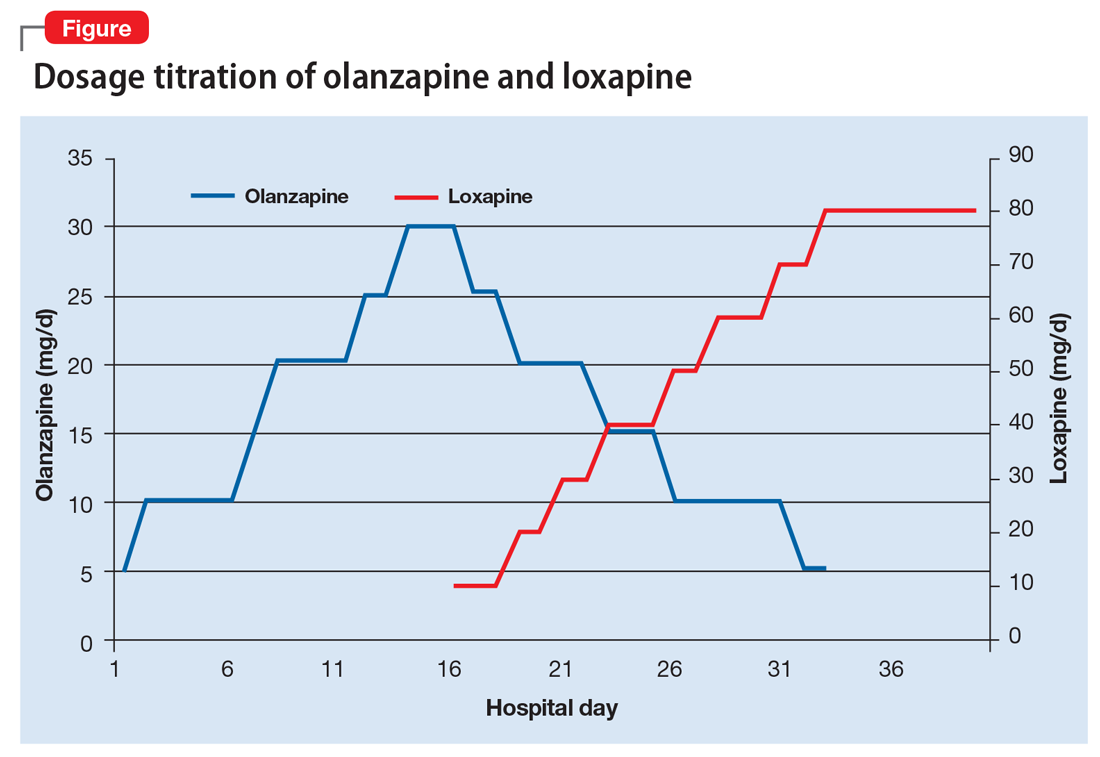
Ms. B begins taking loxapine, 10 mg/d, gradually cross-tapered with olanzapine, increasing loxapine by 10 mg every 2 to 3 days (Figure). After 8 days, when the dosage has reached 40 mg/d, Ms. B’s treatment team begins to observe a consistent change in her behavior. Ms. B comes into the interview room, where previously the team had to see her in her own room because she refused to come out. She also tolerates an extensive interview, even sharing parts of her history without prompting, and is able to discuss her treatment. Ms. B continues to express some paranoia regarding the treatment team. On day 12, receiving loxapine, 50 mg/d, Ms. B says that she likes the new medication and feels she is doing well with it. She becomes less reclusive and begins socializing with other patients. By day 19, receiving loxapine, 80 mg/d, a nurse, who knows Ms. B from the outpatient facility, visits the unit and reports that Ms. B is at her baseline.
At discharge, Ms. B is noted to be “bright,” well organized, neatly dressed, and wearing makeup. Her paranoia and auditory hallucinations have almost completely resolved. She is social, engages appropriately with the treatment team, and is able to describe a plan for self-care after discharge including following up with her oncologist. Her white blood cell counts were carefully monitored throughout her admission and are within normal limits when she is discharged.
One year later, Ms. B remains taking loxapine, 70 mg/d. Although she continues to report mild paranoia, she is living independently in her apartment and attends church regularly.
2. Usta NG, Poyraz CA, Aktan M, et al. Clozapine treatment of refractory schizophrenia during essential chemotherapy: a case study and mini review of a clinical dilemma. Ther Adv Psychopharmacol. 2014;4(6):276-281.
3. Meyer N, Gee S, Whiskey E, et al. Optimizing outcomes in clozapine rechallenge following neutropenia: a cohort analysis. J Clin Psychiatry. 2015;76(11):e1410-e1416.
4. Cunningham NT, Dennis N, Dattilo W, et al. Continuation of clozapine during chemotherapy: a case report and review of literature. Psychosomatics. 2014;55(6):673-679.
5. Co¸sar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18. J Clin Psychopharmacol. 2011;31(2):169-173.
6. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigation. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
7. Mustafa FA, Burke JG, Abukmeil SS, et al. “Schizophrenia past clozapine”: reasons for clozapine discontinuation, mortality, and alternative antipsychotic prescribing. Pharmacopsychiatry. 2015;48(1):11-14.
8. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
9. Bitter I, Dossenbach MR, Brook S, et al; Olanzapine HGCK Study Group. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):173-180.
10. Lehmann CR, Ereshefsky L, Saklad SR, et al. Very high dose loxapine in refractory schizophrenic patients. Am J Psychiatry. 1981;138(9):1212-1214.
11. Sokolski KN. Combination loxapine and aripiprazole for refractory hallucinations in schizophrenia. Ann Pharmacother. 2011;45(7-8):e36.
12. Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40(6):407-414.
13. Mazzola CD, Miron S, Jenkins AJ. Loxapine intoxication: case report and literature review. J Anal Toxicol. 2000;24(7):638-641.
14. Chakrabarti A, Bagnall A, Chue P, et al. Loxapine for schizophrenia. Cochrane Database Syst Rev. 2007(4):CD001943.
15. Jegouzo A, Gressier B, Frimat B, et al. Comparative oxidation of loxapine and clozapine by human neutrophils. Fundam Clin Pharmacol. 1999;13(1):113-119.
16. Keating GM. Loxapine inhalation powder: a review of its use in the acute treatment of agitation in patients with bipolar disorder or schizophrenia. CNS Drugs. 2013;27(6):479-489.
1 7. Glazer WM. Does loxapine have “atypical” properties? Clinical evidence. J Clin Psychiatry. 1999;60(suppl 10):42-46.
18. Hellings JA, Jadhav M, Jain S, et al. Low dose loxapine: neuromotor side effects and tolerability in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25(8):618-624.
19. Singh AN, Barlas C, Singh S, et al. A neurochemical basis for the antipsychotic activity of loxapine: interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes. J Psychiatry Neurosci. 1996;21(1):29-35.
20. Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156(2):286-293.
21. Robertson AG, Berry R, Meltzer HY. Prolactin stimulating effects of amoxapine and loxapine in psychiatric patients. Psychopharmacology (Berl). 1982;78(3):287-292.
22. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
CASE Stable with a new diagnosis
Ms. B, age 60, has a history of schizophrenia, which has been stable on clozapine, 500 mg/d, for more than 2 decades. After a series of hospitalizations in her 20s and 30s, clozapine was initiated and she has not required additional inpatient psychiatric care. She has been managed in the outpatient setting with standard biweekly absolute neutrophil count (ANC) monitoring. She lives independently and is an active member in her church.
After experiencing rectal bleeding, Ms. B is diagnosed with rectal carcinoma and is scheduled to undergo chemotherapy and radiation treatment.
[polldaddy:9754786]
The authors’ observations
Both clozapine and chemotherapy carry the risk of immunosuppression, presenting a clinical challenge when choosing an appropriate management strategy. However, the risks of stopping clozapine after a long period of symptom stability are substantial, with a relapse rate up to 50%.1 Among patients taking clozapine, the risk of agranulocytosis and neutropenia are approximately 0.8% and 3%, respectively, and >80% of agranulocyotis cases occur within the first 18 weeks of treatment.2,3 Although both clozapine and chemotherapy can lead to neutropenia and agranulocytosis, there currently is no evidence of a synergistic effect on bone marrow suppression with simultaneous use of these therapies2 nor is there evidence of the combination leading to sustained marrow suppression.4
Because of Ms. B’s positive response to clozapine, the risks associated with discontinuing the medication, and the relatively low risk of clozapine contributing to neutropenia after a long period of stabilization, her outpatient psychiatric providers decide to increase ANC monitoring to weekly while she undergoes cancer treatment.
TREATMENT Neutropenia, psychosis
Ms. B continues clozapine during radiation and chemotherapy, but develops leukopenia and neutropenia with a low of 1,220/μL white blood cells and an ANC of 610/μL. Clozapine is stopped, consistent with current recommendations to hold the drug if the neutrophil count is <1,000/μL in a patient without benign ethnic neutropenia, and her outpatient provider monitors her closely. The treatment team does not restart an antipsychotic immediately after discontinuing clozapine because of the risk that other antipsychotics can cause hematologic toxicity or prolong granulocytopenia associated with clozapine.5
Approximately 2 weeks later, Ms. B is admitted to a different hospital for altered mental status and is found to have hyponatremia and rectal bleeding. The workup suggests that her rectal carcinoma has not fully responded to initial therapies, and she likely will require further treatment. Her mental status improves after hyponatremia resolves, but she reports auditory hallucinations and paranoia. Risperidone, 4 mg/d, is initiated to target psychosis.
After discharge, Ms. B develops bilateral upper extremity tremor, which she finds intolerable and attributes to risperidone. She refuses to continue risperidone or try adjunctive medications to address the tremor, but is willing to consider a different antipsychotic. Olanzapine, 10 mg/d, is initiated and risperidone is slowly tapered. During this time, Ms. B experiences increased paranoia and believes that the Internal Revenue Service is calling her. She misses her next appointment.
Later, the fire department finds Ms. B wandering the streets and brings her to the psychiatric emergency room. During the examination, she is disheveled and withdrawn, and unable to reply to simple questions about diet and sleep. When asked why she was in the street, she says that she left her apartment because it was “too messy.” The treatment team learns that she had walked at least 10 miles from her apartment before sitting down by the side of the road and being picked up by the fire department. She reveals that she left her apartment and continued walking because “a man” told her to do so and threatened to harm her if she stopped.
When Ms. B is admitted to the psychiatric service, she is paranoid, disorganized, and guarded. She remains in her room for most of the day and either refuses to talk to providers or curses at them. She often is seen wearing soiled clothing with her hair mussed. She denies having rectal carcinoma, although she expressed understanding of her medical condition <2 months earlier.
[polldaddy:9754787]
The authors’ observations
Clozapine is considered the most efficacious agent for treatment-resistant schizophrenia.6 Although non-compliance is the most common reason for discontinuing clozapine, >20% of patients stop clozapine because of adverse effects.7 Clozapine often is a drug of last resort because of the need for frequent monitoring and significant side effects; therefore deciding on a next step when clozapine fails or cannot be continued because of other factors can pose a challenge.
Ms. B’s treatment team gave serious consideration to restarting clozapine. However, because it was likely that Ms. B would undergo another round of chemotherapy and possibly radiation, the risk of neutropenia recurring was considered too high. Lithium has been used successfully to manage neutropenia in patients taking clozapine and, for some, adding lithium could help boost white cell count and allow a successful rechallenge with clozapine.3,8 However, because of Ms. B’s medical comorbidities, including cancer and chronic kidney disease, adding lithium was not thought to be clinically prudent at that time and the treatment team considered other options.
Olanzapine. Although research is limited, studies suggest olanzapine is the most commonly prescribed medication when a patient has to discontinue clozapine,7 with comparable response rates in those with refractory schizophrenia.9 Therefore, Ms. B was initially maintained on olanzapine, and the dosage increased to 30 mg over the course of 16 days in the hospital. However, she did not respond to the medication, remaining disorganized and paranoid without any notable improvement in her symptoms therefore other treatment options were explored.
Loxapine. Previous limited case reports have shown loxapine to be effective in treating individuals with refractory schizophrenia, either alone or in combination with other antipsychotics.10,11 FDA-approved in 1975, loxapine was among the last of the typical antipsychotics brought to the U.S. market before the introduction of clozapine, the first atypical.12 Loxapine is a dibenzoxazepine that has a molecular structure similar to clozapine.13 Unlike clozapine, however, loxapine is not known to cause agranulocytosis.14 Research suggests that although clozapine is oxidized to metabolites that are cytotoxic, loxapine is not, potentially accounting for their different effects on neutrophils.15
The efficacy of loxapine has shown to be similar to other typical and atypical antipsychotics, with approximately 70% of patients showing improvement.14 However, loxapine may be overlooked as an option, possibly because it was not included in the CATIE trial and was the last typical antipsychotic to be approved before atypicals were introduced.12 First available in oral and IM formulations, there has been increased interest in loxapine recently because of the approval of an inhaled formulation in 2012.16
Although classified as a typical antipsychotic, studies have suggested that loxapine acts as an atypical at low dosages.17,18 Previous work suggests, however, that the side effect profile of loxapine is similar to typical antipsychotics.14 At dosages <50 mg, it results in fewer cases of extrapyramidal side effects than expected with a typical antipsychotic.18
Loxapine’s binding profile seems to exist along this spectrum of typical to atypical. Tissue-based binding studies have shown a higher 5-HT2 affinity relative to D2, consistent with atypical antipsychotics.19 Positron emission tomography studies in humans show 5-HT2 saturation of loxapine to be close to equal to D2 binding in loxapine, thus a slightly lower ratio of 5-HT2 to D2 relative to atypicals, but more than that seen with typical antipsychotics.20 These differences between in vitro and in vivo studies may be secondary to the binding of loxapine’s active metabolites, particularly 7- and 8-hydroxyloxapine, which have more dopaminergic activity. In addition to increased 5-HT2A binding compared with typical antipsychotics, loxapine also has a high affinity for the D4 receptors, as well as interacting with other serotonin receptors 5-HT3, 5-HT6, and 5-HT7. Of note this is a similar pattern of binding affinity as seen in clozapine.19
It should be noted, however, that loxapine may not be an appropriate treatment in all forms of cancer. Similar to other first-generation antipsychotics, it increases prolactin levels, and thus may have a negative clinical impact on patients with prolactin receptor positive breast cancers.21,22 Finally, although clozapine can result in significant weight gain, dyslipidemia, and hyperglycemia, unlike many antipsychotics, loxapine has been shown to be weight neutral or result in weight loss,14 making it an option to consider for patients with type 2 diabetes mellitus, metabolic syndrome, dyslipidemia, or cardiovascular disease.
OUTCOME Improvement, stability
Ms. B begins taking loxapine, 10 mg/d, gradually cross-tapered with olanzapine, increasing loxapine by 10 mg every 2 to 3 days (Figure). After 8 days, when the dosage has reached 40 mg/d, Ms. B’s treatment team begins to observe a consistent change in her behavior. Ms. B comes into the interview room, where previously the team had to see her in her own room because she refused to come out. She also tolerates an extensive interview, even sharing parts of her history without prompting, and is able to discuss her treatment. Ms. B continues to express some paranoia regarding the treatment team. On day 12, receiving loxapine, 50 mg/d, Ms. B says that she likes the new medication and feels she is doing well with it. She becomes less reclusive and begins socializing with other patients. By day 19, receiving loxapine, 80 mg/d, a nurse, who knows Ms. B from the outpatient facility, visits the unit and reports that Ms. B is at her baseline.

At discharge, Ms. B is noted to be “bright,” well organized, neatly dressed, and wearing makeup. Her paranoia and auditory hallucinations have almost completely resolved. She is social, engages appropriately with the treatment team, and is able to describe a plan for self-care after discharge including following up with her oncologist. Her white blood cell counts were carefully monitored throughout her admission and are within normal limits when she is discharged.
One year later, Ms. B remains taking loxapine, 70 mg/d. Although she continues to report mild paranoia, she is living independently in her apartment and attends church regularly.
CASE Stable with a new diagnosis
Ms. B, age 60, has a history of schizophrenia, which has been stable on clozapine, 500 mg/d, for more than 2 decades. After a series of hospitalizations in her 20s and 30s, clozapine was initiated and she has not required additional inpatient psychiatric care. She has been managed in the outpatient setting with standard biweekly absolute neutrophil count (ANC) monitoring. She lives independently and is an active member in her church.
After experiencing rectal bleeding, Ms. B is diagnosed with rectal carcinoma and is scheduled to undergo chemotherapy and radiation treatment.
[polldaddy:9754786]
The authors’ observations
Both clozapine and chemotherapy carry the risk of immunosuppression, presenting a clinical challenge when choosing an appropriate management strategy. However, the risks of stopping clozapine after a long period of symptom stability are substantial, with a relapse rate up to 50%.1 Among patients taking clozapine, the risk of agranulocytosis and neutropenia are approximately 0.8% and 3%, respectively, and >80% of agranulocyotis cases occur within the first 18 weeks of treatment.2,3 Although both clozapine and chemotherapy can lead to neutropenia and agranulocytosis, there currently is no evidence of a synergistic effect on bone marrow suppression with simultaneous use of these therapies2 nor is there evidence of the combination leading to sustained marrow suppression.4
Because of Ms. B’s positive response to clozapine, the risks associated with discontinuing the medication, and the relatively low risk of clozapine contributing to neutropenia after a long period of stabilization, her outpatient psychiatric providers decide to increase ANC monitoring to weekly while she undergoes cancer treatment.
TREATMENT Neutropenia, psychosis
Ms. B continues clozapine during radiation and chemotherapy, but develops leukopenia and neutropenia with a low of 1,220/μL white blood cells and an ANC of 610/μL. Clozapine is stopped, consistent with current recommendations to hold the drug if the neutrophil count is <1,000/μL in a patient without benign ethnic neutropenia, and her outpatient provider monitors her closely. The treatment team does not restart an antipsychotic immediately after discontinuing clozapine because of the risk that other antipsychotics can cause hematologic toxicity or prolong granulocytopenia associated with clozapine.5
Approximately 2 weeks later, Ms. B is admitted to a different hospital for altered mental status and is found to have hyponatremia and rectal bleeding. The workup suggests that her rectal carcinoma has not fully responded to initial therapies, and she likely will require further treatment. Her mental status improves after hyponatremia resolves, but she reports auditory hallucinations and paranoia. Risperidone, 4 mg/d, is initiated to target psychosis.
After discharge, Ms. B develops bilateral upper extremity tremor, which she finds intolerable and attributes to risperidone. She refuses to continue risperidone or try adjunctive medications to address the tremor, but is willing to consider a different antipsychotic. Olanzapine, 10 mg/d, is initiated and risperidone is slowly tapered. During this time, Ms. B experiences increased paranoia and believes that the Internal Revenue Service is calling her. She misses her next appointment.
Later, the fire department finds Ms. B wandering the streets and brings her to the psychiatric emergency room. During the examination, she is disheveled and withdrawn, and unable to reply to simple questions about diet and sleep. When asked why she was in the street, she says that she left her apartment because it was “too messy.” The treatment team learns that she had walked at least 10 miles from her apartment before sitting down by the side of the road and being picked up by the fire department. She reveals that she left her apartment and continued walking because “a man” told her to do so and threatened to harm her if she stopped.
When Ms. B is admitted to the psychiatric service, she is paranoid, disorganized, and guarded. She remains in her room for most of the day and either refuses to talk to providers or curses at them. She often is seen wearing soiled clothing with her hair mussed. She denies having rectal carcinoma, although she expressed understanding of her medical condition <2 months earlier.
[polldaddy:9754787]
The authors’ observations
Clozapine is considered the most efficacious agent for treatment-resistant schizophrenia.6 Although non-compliance is the most common reason for discontinuing clozapine, >20% of patients stop clozapine because of adverse effects.7 Clozapine often is a drug of last resort because of the need for frequent monitoring and significant side effects; therefore deciding on a next step when clozapine fails or cannot be continued because of other factors can pose a challenge.
Ms. B’s treatment team gave serious consideration to restarting clozapine. However, because it was likely that Ms. B would undergo another round of chemotherapy and possibly radiation, the risk of neutropenia recurring was considered too high. Lithium has been used successfully to manage neutropenia in patients taking clozapine and, for some, adding lithium could help boost white cell count and allow a successful rechallenge with clozapine.3,8 However, because of Ms. B’s medical comorbidities, including cancer and chronic kidney disease, adding lithium was not thought to be clinically prudent at that time and the treatment team considered other options.
Olanzapine. Although research is limited, studies suggest olanzapine is the most commonly prescribed medication when a patient has to discontinue clozapine,7 with comparable response rates in those with refractory schizophrenia.9 Therefore, Ms. B was initially maintained on olanzapine, and the dosage increased to 30 mg over the course of 16 days in the hospital. However, she did not respond to the medication, remaining disorganized and paranoid without any notable improvement in her symptoms therefore other treatment options were explored.
Loxapine. Previous limited case reports have shown loxapine to be effective in treating individuals with refractory schizophrenia, either alone or in combination with other antipsychotics.10,11 FDA-approved in 1975, loxapine was among the last of the typical antipsychotics brought to the U.S. market before the introduction of clozapine, the first atypical.12 Loxapine is a dibenzoxazepine that has a molecular structure similar to clozapine.13 Unlike clozapine, however, loxapine is not known to cause agranulocytosis.14 Research suggests that although clozapine is oxidized to metabolites that are cytotoxic, loxapine is not, potentially accounting for their different effects on neutrophils.15
The efficacy of loxapine has shown to be similar to other typical and atypical antipsychotics, with approximately 70% of patients showing improvement.14 However, loxapine may be overlooked as an option, possibly because it was not included in the CATIE trial and was the last typical antipsychotic to be approved before atypicals were introduced.12 First available in oral and IM formulations, there has been increased interest in loxapine recently because of the approval of an inhaled formulation in 2012.16
Although classified as a typical antipsychotic, studies have suggested that loxapine acts as an atypical at low dosages.17,18 Previous work suggests, however, that the side effect profile of loxapine is similar to typical antipsychotics.14 At dosages <50 mg, it results in fewer cases of extrapyramidal side effects than expected with a typical antipsychotic.18
Loxapine’s binding profile seems to exist along this spectrum of typical to atypical. Tissue-based binding studies have shown a higher 5-HT2 affinity relative to D2, consistent with atypical antipsychotics.19 Positron emission tomography studies in humans show 5-HT2 saturation of loxapine to be close to equal to D2 binding in loxapine, thus a slightly lower ratio of 5-HT2 to D2 relative to atypicals, but more than that seen with typical antipsychotics.20 These differences between in vitro and in vivo studies may be secondary to the binding of loxapine’s active metabolites, particularly 7- and 8-hydroxyloxapine, which have more dopaminergic activity. In addition to increased 5-HT2A binding compared with typical antipsychotics, loxapine also has a high affinity for the D4 receptors, as well as interacting with other serotonin receptors 5-HT3, 5-HT6, and 5-HT7. Of note this is a similar pattern of binding affinity as seen in clozapine.19
It should be noted, however, that loxapine may not be an appropriate treatment in all forms of cancer. Similar to other first-generation antipsychotics, it increases prolactin levels, and thus may have a negative clinical impact on patients with prolactin receptor positive breast cancers.21,22 Finally, although clozapine can result in significant weight gain, dyslipidemia, and hyperglycemia, unlike many antipsychotics, loxapine has been shown to be weight neutral or result in weight loss,14 making it an option to consider for patients with type 2 diabetes mellitus, metabolic syndrome, dyslipidemia, or cardiovascular disease.
OUTCOME Improvement, stability
Ms. B begins taking loxapine, 10 mg/d, gradually cross-tapered with olanzapine, increasing loxapine by 10 mg every 2 to 3 days (Figure). After 8 days, when the dosage has reached 40 mg/d, Ms. B’s treatment team begins to observe a consistent change in her behavior. Ms. B comes into the interview room, where previously the team had to see her in her own room because she refused to come out. She also tolerates an extensive interview, even sharing parts of her history without prompting, and is able to discuss her treatment. Ms. B continues to express some paranoia regarding the treatment team. On day 12, receiving loxapine, 50 mg/d, Ms. B says that she likes the new medication and feels she is doing well with it. She becomes less reclusive and begins socializing with other patients. By day 19, receiving loxapine, 80 mg/d, a nurse, who knows Ms. B from the outpatient facility, visits the unit and reports that Ms. B is at her baseline.

At discharge, Ms. B is noted to be “bright,” well organized, neatly dressed, and wearing makeup. Her paranoia and auditory hallucinations have almost completely resolved. She is social, engages appropriately with the treatment team, and is able to describe a plan for self-care after discharge including following up with her oncologist. Her white blood cell counts were carefully monitored throughout her admission and are within normal limits when she is discharged.
One year later, Ms. B remains taking loxapine, 70 mg/d. Although she continues to report mild paranoia, she is living independently in her apartment and attends church regularly.
2. Usta NG, Poyraz CA, Aktan M, et al. Clozapine treatment of refractory schizophrenia during essential chemotherapy: a case study and mini review of a clinical dilemma. Ther Adv Psychopharmacol. 2014;4(6):276-281.
3. Meyer N, Gee S, Whiskey E, et al. Optimizing outcomes in clozapine rechallenge following neutropenia: a cohort analysis. J Clin Psychiatry. 2015;76(11):e1410-e1416.
4. Cunningham NT, Dennis N, Dattilo W, et al. Continuation of clozapine during chemotherapy: a case report and review of literature. Psychosomatics. 2014;55(6):673-679.
5. Co¸sar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18. J Clin Psychopharmacol. 2011;31(2):169-173.
6. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigation. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
7. Mustafa FA, Burke JG, Abukmeil SS, et al. “Schizophrenia past clozapine”: reasons for clozapine discontinuation, mortality, and alternative antipsychotic prescribing. Pharmacopsychiatry. 2015;48(1):11-14.
8. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
9. Bitter I, Dossenbach MR, Brook S, et al; Olanzapine HGCK Study Group. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):173-180.
10. Lehmann CR, Ereshefsky L, Saklad SR, et al. Very high dose loxapine in refractory schizophrenic patients. Am J Psychiatry. 1981;138(9):1212-1214.
11. Sokolski KN. Combination loxapine and aripiprazole for refractory hallucinations in schizophrenia. Ann Pharmacother. 2011;45(7-8):e36.
12. Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40(6):407-414.
13. Mazzola CD, Miron S, Jenkins AJ. Loxapine intoxication: case report and literature review. J Anal Toxicol. 2000;24(7):638-641.
14. Chakrabarti A, Bagnall A, Chue P, et al. Loxapine for schizophrenia. Cochrane Database Syst Rev. 2007(4):CD001943.
15. Jegouzo A, Gressier B, Frimat B, et al. Comparative oxidation of loxapine and clozapine by human neutrophils. Fundam Clin Pharmacol. 1999;13(1):113-119.
16. Keating GM. Loxapine inhalation powder: a review of its use in the acute treatment of agitation in patients with bipolar disorder or schizophrenia. CNS Drugs. 2013;27(6):479-489.
1 7. Glazer WM. Does loxapine have “atypical” properties? Clinical evidence. J Clin Psychiatry. 1999;60(suppl 10):42-46.
18. Hellings JA, Jadhav M, Jain S, et al. Low dose loxapine: neuromotor side effects and tolerability in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25(8):618-624.
19. Singh AN, Barlas C, Singh S, et al. A neurochemical basis for the antipsychotic activity of loxapine: interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes. J Psychiatry Neurosci. 1996;21(1):29-35.
20. Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156(2):286-293.
21. Robertson AG, Berry R, Meltzer HY. Prolactin stimulating effects of amoxapine and loxapine in psychiatric patients. Psychopharmacology (Berl). 1982;78(3):287-292.
22. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
2. Usta NG, Poyraz CA, Aktan M, et al. Clozapine treatment of refractory schizophrenia during essential chemotherapy: a case study and mini review of a clinical dilemma. Ther Adv Psychopharmacol. 2014;4(6):276-281.
3. Meyer N, Gee S, Whiskey E, et al. Optimizing outcomes in clozapine rechallenge following neutropenia: a cohort analysis. J Clin Psychiatry. 2015;76(11):e1410-e1416.
4. Cunningham NT, Dennis N, Dattilo W, et al. Continuation of clozapine during chemotherapy: a case report and review of literature. Psychosomatics. 2014;55(6):673-679.
5. Co¸sar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18. J Clin Psychopharmacol. 2011;31(2):169-173.
6. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigation. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
7. Mustafa FA, Burke JG, Abukmeil SS, et al. “Schizophrenia past clozapine”: reasons for clozapine discontinuation, mortality, and alternative antipsychotic prescribing. Pharmacopsychiatry. 2015;48(1):11-14.
8. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
9. Bitter I, Dossenbach MR, Brook S, et al; Olanzapine HGCK Study Group. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):173-180.
10. Lehmann CR, Ereshefsky L, Saklad SR, et al. Very high dose loxapine in refractory schizophrenic patients. Am J Psychiatry. 1981;138(9):1212-1214.
11. Sokolski KN. Combination loxapine and aripiprazole for refractory hallucinations in schizophrenia. Ann Pharmacother. 2011;45(7-8):e36.
12. Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40(6):407-414.
13. Mazzola CD, Miron S, Jenkins AJ. Loxapine intoxication: case report and literature review. J Anal Toxicol. 2000;24(7):638-641.
14. Chakrabarti A, Bagnall A, Chue P, et al. Loxapine for schizophrenia. Cochrane Database Syst Rev. 2007(4):CD001943.
15. Jegouzo A, Gressier B, Frimat B, et al. Comparative oxidation of loxapine and clozapine by human neutrophils. Fundam Clin Pharmacol. 1999;13(1):113-119.
16. Keating GM. Loxapine inhalation powder: a review of its use in the acute treatment of agitation in patients with bipolar disorder or schizophrenia. CNS Drugs. 2013;27(6):479-489.
1 7. Glazer WM. Does loxapine have “atypical” properties? Clinical evidence. J Clin Psychiatry. 1999;60(suppl 10):42-46.
18. Hellings JA, Jadhav M, Jain S, et al. Low dose loxapine: neuromotor side effects and tolerability in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25(8):618-624.
19. Singh AN, Barlas C, Singh S, et al. A neurochemical basis for the antipsychotic activity of loxapine: interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes. J Psychiatry Neurosci. 1996;21(1):29-35.
20. Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156(2):286-293.
21. Robertson AG, Berry R, Meltzer HY. Prolactin stimulating effects of amoxapine and loxapine in psychiatric patients. Psychopharmacology (Berl). 1982;78(3):287-292.
22. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
Herb–drug interactions: Caution patients when changing supplements
Ms. X, age 41, has a history of bipolar disorder and presents with extreme sleepiness, constipation with mild abdominal cramping, occasional dizziness, and “palpitations.” Although usually she is quite articulate, Ms. X seems to have trouble describing her symptoms and reports that they have been worsening over 4 to 6 days. She is worried because she is making mistakes at work and repeatedly misunderstanding directions.
Ms. X has a family history of hyperlipidemia, heart disease, and diabetes, and she has been employing a healthy diet, exercise, and use of supplements for cardiovascular health since her early 20s. Her medication regimen includes lithium, 600 mg, twice a day, quetiapine, 1,200 mg/d, a multivitamin and mineral tablet once a day, a brand name garlic supplement (garlic powder, 300 mg, vitamin C, 80 mg, vitamin E, 20 IU, vitamin A, 2,640 IU) twice a day, and fish oil, 2 g/d, at bedtime. Lithium levels consistently have been 0.8 to 0.9 mEq/L for the last 3 years.
Factors of drug–supplement interactions
Because an interaction is possible doesn’t always mean that a drug and an offending botanical cannot be used together. With awareness and planning, possible interactions can be safely managed (Table 1). Such was the case of Ms. X, who was stable on a higher-than-usual dosage of quetiapine (average target is 600 mg/d for bipolar disorder) because of presumed moderate enzyme induction by the brand name garlic supplement. Ms. X did not want to stop taking this supplement when she started quetiapine. Although garlic is listed as a possible moderate cytochrome P450 (CYP) 3A4 inducer, there is conflicting evidence.1 Ms. X’s clinician advised her to avoid changes in dosage, because it could affect her quetiapine levels. However, the change in the botanical preparation from dried, powdered garlic to garlic oil likely removed the CYP3A4 enzyme induction, leading to a lower rate of metabolism and accumulation of the drug to toxic levels.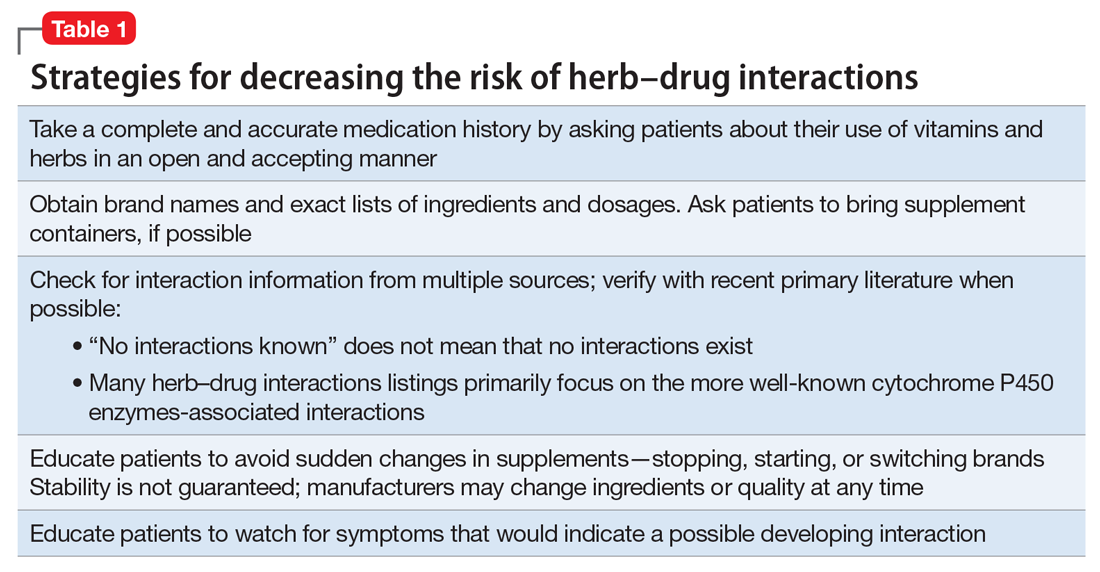
Drug metabolism. Practitioners are increasingly aware that St. John’s wort can significantly affect concomitantly administered drug levels by induction of the CYP isoenzyme 3A4 and more resources are listing this same possible induction for garlic.1 However, what is less understood is the extent to which different preparations of the same plant possess different chemical profiles (Table 2).
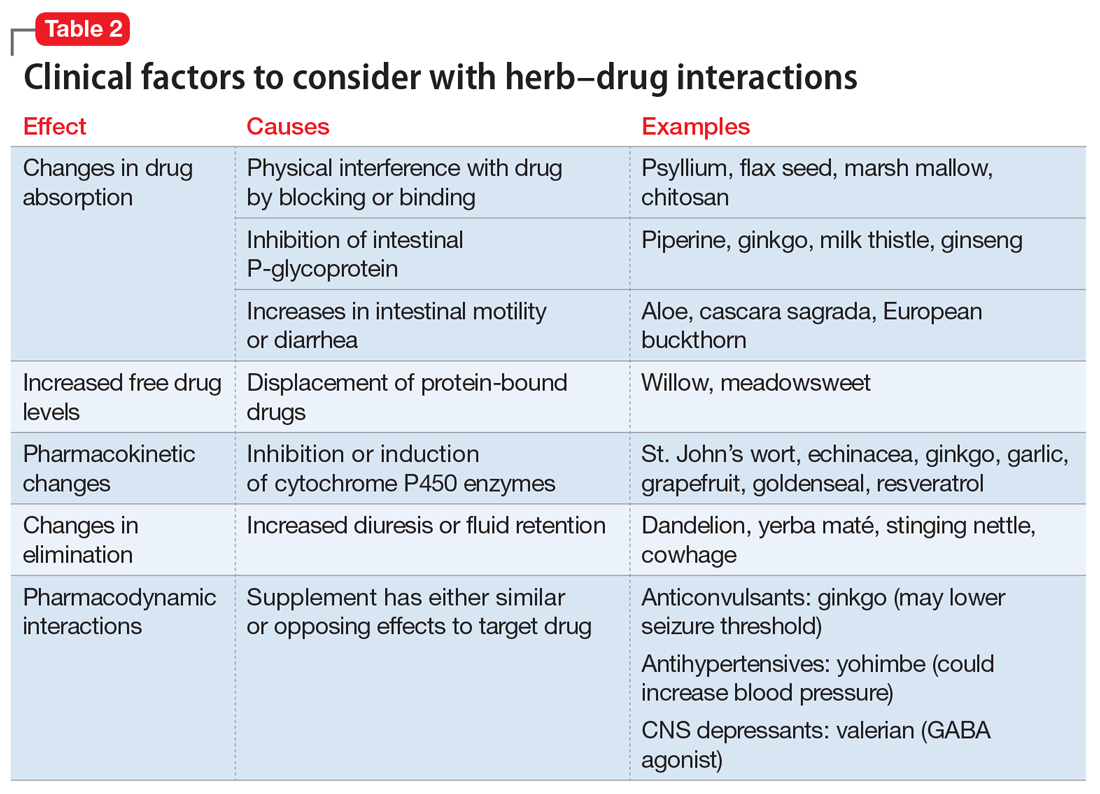
Clinical studies with different garlic preparations—dried powder, aqueous extracts, deodorized preparations, oils—have demonstrated diverse and highly variable results in tests of effects on CYP isoenzymes and other metabolism activities.
Drug absorption. Small differences in amounts of vitamins in the supplement are unlikely to be clinically significant, but the addition of piperine could be affecting quetiapine absorption. Piperine, a constituent of black pepper and long pepper, is used in Ayurvedic medicine for:
- pain
- influenza
- rheumatoid arthritis
- asthma
- loss of appetite
- stimulating peristalsis.6
Animal studies have demonstrated anti-inflammatory, anticonvulsant, anticarcinogenic, and antioxidant effects, as well as stimulation of digestion via digestive enzyme secretion and increased gastromotility.3,6
Because piperine is known to increase intestinal absorption by various mechanisms, it often is added to botanical medicines to increase bioavailability of active components. BioPerine is a 95% piperine extract marketed to be included in vitamin and herbal supplements for that purpose.3 This allows use of lower dosages to achieve outcomes, which, for expensive botanicals, could be a cost savings for the manufacturer. Studies examining piperine’s influence on drug absorption have demonstrated significant increases in carbamazepine, rifampin, phenytoin, nevirapine, and many other drugs.
In addition to increased absorption, piperine seems to be a non-specific general inhibitor of CYP isoenzymes; IV phenytoin levels also were higher among test participants.6,8 Piperine reduces intestinal glucuronidation via uridine 5’-diphospho-glucuronosyltransferase inhibition, and the small or moderate effects on lithium levels seem to be the result of diuretic activities.3,7
Patients often are motivated to control at least 1 aspect of their medical treatment, such as the supplements they choose to take. Being open to patient use of non-harmful or low-risk supplements, even when they are unlikely to have any medicinal benefit, helps preserve a relationship in which patients are more likely to consider your recommendation to avoid a harmful or high-risk supplement.
Related Resources
1. Natural Medicines Database. Garlic monograph. http://naturaldatabase.therapeuticresearch.com. Accessed May 1, 2017.
2. Wanwimolruk S, Prachayasittikul V. Cytochrome P450 enzyme mediated herbal drug interactions (part 1). EXCLI J. 2014;13:347-391.
3. Colalto C. Herbal interactions on absorption of drugs: mechanism of action and clinical risk assessment. Pharmacol Res. 2010;62(3):207-227.
4. Gurley BJ, Gardner SF, Hubbard MA, et al. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St. John’s wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging. 2005;22(6):525-539.
5. Gallicano K, Foster B, Choudhri S. Effect of short-term administration of garlic supplements on single-dose ritonavir pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2003;55(2):199-202.
6. Meghwal M, Goswami TK. Piper nigrum and piperine: an update. Phytother Res. 2013;27(8):1121-1130.
7. Natural Medicines Database. Black pepper monograph. https://www.naturalmedicines.therapeuticresearch.com. Accessed May 1, 2017.
8. Zhou S, Lim LY, Chowbay B. Herbal modulation of p-glycoprotein. Drug Metab Rev. 2004;36(1):57-104.
9. Chinta G, Syed B, Coumar MS, et al. Piperine: a comprehensive review of pre-clinical and clinical investigations. Curr Bioact Compd. 2015;11(3):156-169.
Ms. X, age 41, has a history of bipolar disorder and presents with extreme sleepiness, constipation with mild abdominal cramping, occasional dizziness, and “palpitations.” Although usually she is quite articulate, Ms. X seems to have trouble describing her symptoms and reports that they have been worsening over 4 to 6 days. She is worried because she is making mistakes at work and repeatedly misunderstanding directions.
Ms. X has a family history of hyperlipidemia, heart disease, and diabetes, and she has been employing a healthy diet, exercise, and use of supplements for cardiovascular health since her early 20s. Her medication regimen includes lithium, 600 mg, twice a day, quetiapine, 1,200 mg/d, a multivitamin and mineral tablet once a day, a brand name garlic supplement (garlic powder, 300 mg, vitamin C, 80 mg, vitamin E, 20 IU, vitamin A, 2,640 IU) twice a day, and fish oil, 2 g/d, at bedtime. Lithium levels consistently have been 0.8 to 0.9 mEq/L for the last 3 years.
Factors of drug–supplement interactions
Because an interaction is possible doesn’t always mean that a drug and an offending botanical cannot be used together. With awareness and planning, possible interactions can be safely managed (Table 1). Such was the case of Ms. X, who was stable on a higher-than-usual dosage of quetiapine (average target is 600 mg/d for bipolar disorder) because of presumed moderate enzyme induction by the brand name garlic supplement. Ms. X did not want to stop taking this supplement when she started quetiapine. Although garlic is listed as a possible moderate cytochrome P450 (CYP) 3A4 inducer, there is conflicting evidence.1 Ms. X’s clinician advised her to avoid changes in dosage, because it could affect her quetiapine levels. However, the change in the botanical preparation from dried, powdered garlic to garlic oil likely removed the CYP3A4 enzyme induction, leading to a lower rate of metabolism and accumulation of the drug to toxic levels.
Drug metabolism. Practitioners are increasingly aware that St. John’s wort can significantly affect concomitantly administered drug levels by induction of the CYP isoenzyme 3A4 and more resources are listing this same possible induction for garlic.1 However, what is less understood is the extent to which different preparations of the same plant possess different chemical profiles (Table 2).

Clinical studies with different garlic preparations—dried powder, aqueous extracts, deodorized preparations, oils—have demonstrated diverse and highly variable results in tests of effects on CYP isoenzymes and other metabolism activities.
Drug absorption. Small differences in amounts of vitamins in the supplement are unlikely to be clinically significant, but the addition of piperine could be affecting quetiapine absorption. Piperine, a constituent of black pepper and long pepper, is used in Ayurvedic medicine for:
- pain
- influenza
- rheumatoid arthritis
- asthma
- loss of appetite
- stimulating peristalsis.6
Animal studies have demonstrated anti-inflammatory, anticonvulsant, anticarcinogenic, and antioxidant effects, as well as stimulation of digestion via digestive enzyme secretion and increased gastromotility.3,6
Because piperine is known to increase intestinal absorption by various mechanisms, it often is added to botanical medicines to increase bioavailability of active components. BioPerine is a 95% piperine extract marketed to be included in vitamin and herbal supplements for that purpose.3 This allows use of lower dosages to achieve outcomes, which, for expensive botanicals, could be a cost savings for the manufacturer. Studies examining piperine’s influence on drug absorption have demonstrated significant increases in carbamazepine, rifampin, phenytoin, nevirapine, and many other drugs.
In addition to increased absorption, piperine seems to be a non-specific general inhibitor of CYP isoenzymes; IV phenytoin levels also were higher among test participants.6,8 Piperine reduces intestinal glucuronidation via uridine 5’-diphospho-glucuronosyltransferase inhibition, and the small or moderate effects on lithium levels seem to be the result of diuretic activities.3,7
Patients often are motivated to control at least 1 aspect of their medical treatment, such as the supplements they choose to take. Being open to patient use of non-harmful or low-risk supplements, even when they are unlikely to have any medicinal benefit, helps preserve a relationship in which patients are more likely to consider your recommendation to avoid a harmful or high-risk supplement.
Related Resources
Ms. X, age 41, has a history of bipolar disorder and presents with extreme sleepiness, constipation with mild abdominal cramping, occasional dizziness, and “palpitations.” Although usually she is quite articulate, Ms. X seems to have trouble describing her symptoms and reports that they have been worsening over 4 to 6 days. She is worried because she is making mistakes at work and repeatedly misunderstanding directions.
Ms. X has a family history of hyperlipidemia, heart disease, and diabetes, and she has been employing a healthy diet, exercise, and use of supplements for cardiovascular health since her early 20s. Her medication regimen includes lithium, 600 mg, twice a day, quetiapine, 1,200 mg/d, a multivitamin and mineral tablet once a day, a brand name garlic supplement (garlic powder, 300 mg, vitamin C, 80 mg, vitamin E, 20 IU, vitamin A, 2,640 IU) twice a day, and fish oil, 2 g/d, at bedtime. Lithium levels consistently have been 0.8 to 0.9 mEq/L for the last 3 years.
Factors of drug–supplement interactions
Because an interaction is possible doesn’t always mean that a drug and an offending botanical cannot be used together. With awareness and planning, possible interactions can be safely managed (Table 1). Such was the case of Ms. X, who was stable on a higher-than-usual dosage of quetiapine (average target is 600 mg/d for bipolar disorder) because of presumed moderate enzyme induction by the brand name garlic supplement. Ms. X did not want to stop taking this supplement when she started quetiapine. Although garlic is listed as a possible moderate cytochrome P450 (CYP) 3A4 inducer, there is conflicting evidence.1 Ms. X’s clinician advised her to avoid changes in dosage, because it could affect her quetiapine levels. However, the change in the botanical preparation from dried, powdered garlic to garlic oil likely removed the CYP3A4 enzyme induction, leading to a lower rate of metabolism and accumulation of the drug to toxic levels.
Drug metabolism. Practitioners are increasingly aware that St. John’s wort can significantly affect concomitantly administered drug levels by induction of the CYP isoenzyme 3A4 and more resources are listing this same possible induction for garlic.1 However, what is less understood is the extent to which different preparations of the same plant possess different chemical profiles (Table 2).

Clinical studies with different garlic preparations—dried powder, aqueous extracts, deodorized preparations, oils—have demonstrated diverse and highly variable results in tests of effects on CYP isoenzymes and other metabolism activities.
Drug absorption. Small differences in amounts of vitamins in the supplement are unlikely to be clinically significant, but the addition of piperine could be affecting quetiapine absorption. Piperine, a constituent of black pepper and long pepper, is used in Ayurvedic medicine for:
- pain
- influenza
- rheumatoid arthritis
- asthma
- loss of appetite
- stimulating peristalsis.6
Animal studies have demonstrated anti-inflammatory, anticonvulsant, anticarcinogenic, and antioxidant effects, as well as stimulation of digestion via digestive enzyme secretion and increased gastromotility.3,6
Because piperine is known to increase intestinal absorption by various mechanisms, it often is added to botanical medicines to increase bioavailability of active components. BioPerine is a 95% piperine extract marketed to be included in vitamin and herbal supplements for that purpose.3 This allows use of lower dosages to achieve outcomes, which, for expensive botanicals, could be a cost savings for the manufacturer. Studies examining piperine’s influence on drug absorption have demonstrated significant increases in carbamazepine, rifampin, phenytoin, nevirapine, and many other drugs.
In addition to increased absorption, piperine seems to be a non-specific general inhibitor of CYP isoenzymes; IV phenytoin levels also were higher among test participants.6,8 Piperine reduces intestinal glucuronidation via uridine 5’-diphospho-glucuronosyltransferase inhibition, and the small or moderate effects on lithium levels seem to be the result of diuretic activities.3,7
Patients often are motivated to control at least 1 aspect of their medical treatment, such as the supplements they choose to take. Being open to patient use of non-harmful or low-risk supplements, even when they are unlikely to have any medicinal benefit, helps preserve a relationship in which patients are more likely to consider your recommendation to avoid a harmful or high-risk supplement.
Related Resources
1. Natural Medicines Database. Garlic monograph. http://naturaldatabase.therapeuticresearch.com. Accessed May 1, 2017.
2. Wanwimolruk S, Prachayasittikul V. Cytochrome P450 enzyme mediated herbal drug interactions (part 1). EXCLI J. 2014;13:347-391.
3. Colalto C. Herbal interactions on absorption of drugs: mechanism of action and clinical risk assessment. Pharmacol Res. 2010;62(3):207-227.
4. Gurley BJ, Gardner SF, Hubbard MA, et al. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St. John’s wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging. 2005;22(6):525-539.
5. Gallicano K, Foster B, Choudhri S. Effect of short-term administration of garlic supplements on single-dose ritonavir pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2003;55(2):199-202.
6. Meghwal M, Goswami TK. Piper nigrum and piperine: an update. Phytother Res. 2013;27(8):1121-1130.
7. Natural Medicines Database. Black pepper monograph. https://www.naturalmedicines.therapeuticresearch.com. Accessed May 1, 2017.
8. Zhou S, Lim LY, Chowbay B. Herbal modulation of p-glycoprotein. Drug Metab Rev. 2004;36(1):57-104.
9. Chinta G, Syed B, Coumar MS, et al. Piperine: a comprehensive review of pre-clinical and clinical investigations. Curr Bioact Compd. 2015;11(3):156-169.
1. Natural Medicines Database. Garlic monograph. http://naturaldatabase.therapeuticresearch.com. Accessed May 1, 2017.
2. Wanwimolruk S, Prachayasittikul V. Cytochrome P450 enzyme mediated herbal drug interactions (part 1). EXCLI J. 2014;13:347-391.
3. Colalto C. Herbal interactions on absorption of drugs: mechanism of action and clinical risk assessment. Pharmacol Res. 2010;62(3):207-227.
4. Gurley BJ, Gardner SF, Hubbard MA, et al. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St. John’s wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging. 2005;22(6):525-539.
5. Gallicano K, Foster B, Choudhri S. Effect of short-term administration of garlic supplements on single-dose ritonavir pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2003;55(2):199-202.
6. Meghwal M, Goswami TK. Piper nigrum and piperine: an update. Phytother Res. 2013;27(8):1121-1130.
7. Natural Medicines Database. Black pepper monograph. https://www.naturalmedicines.therapeuticresearch.com. Accessed May 1, 2017.
8. Zhou S, Lim LY, Chowbay B. Herbal modulation of p-glycoprotein. Drug Metab Rev. 2004;36(1):57-104.
9. Chinta G, Syed B, Coumar MS, et al. Piperine: a comprehensive review of pre-clinical and clinical investigations. Curr Bioact Compd. 2015;11(3):156-169.
‘3 Strikes ‘n’ yer out’: Dismissing no-show patients
Dear Dr. Mossman,
The clinic where I work initiated a “3 misses and out” policy: If a patient doesn’t show for 3 appointments in a 12-month period, the clinic removes him from the patient rolls. I’ve heard such policies are common, but I worry: Is this abandonment?
Submitted by “Dr. C”
The short answer to Dr. C’s question is, “Handled properly, it’s not abandonment.” But if this response really was satisfactory, Dr. C probably would not have asked the question. Dealing with no-show patients has bothered psychiatrists, other mental health professionals, and other physicians for decades.1
Clinicians worry when patients miss important follow-up, and unreimbursed office time won’t help pay a clinician’s salary or office expenses.2 But a policy such as the one Dr. C describes may not be the best response—clinically or financially—for many patients who miss appointments repeatedly.
If no-show patients worry you or cause problems where you practice, read on as I cover:
- charging for missed appointments
- why patients miss appointments
- evidence-based methods to improve show-up rates
- when ending a treatment relationship unilaterally is not abandonment
- how to dismiss no-show patients from a practice properly.
Before the mid-1980s, most office-based psychiatrists worked in solo or small group and required patients to pay cash for treatment; approximately 40% of psychiatrists still practice this way.3 Often, private practice clinicians require payment for appointments missed without 24 hours’ notice. This well-accepted practice2,4,5 reinforces the notion that psychotherapy involves making a commitment to work on problems. It also protects clinicians’ financial interests and mitigates possible resentment that might arise if office time committed to a patient went unreimbursed.6 Clinicians who charge for missed appointments should inform patients of this at the beginning of treatment, explaining clearly that the patient—not the insurer—will be paying for unused treatment time.2,4
Since the 1980s, outpatient psychiatrists have increasingly worked in public agencies or other organizational practice settings7 where patients—whose care is funded by public monies or third-party payors—cannot afford to pay for missed appointments. If you work in a clinic such as the one where Dr. C provides services, you probably are paid an hourly wage whether your patients show up or not. To pay you and remain solvent, your clinic must find ways other than charging patients to address and reduce no-shows.
The literature abounds with research on why no-shows occur. But no-shows seem to be more common in psychiatry than in other medical specialties.8 The frequency of no-shows varies considerably, but it’s a big problem in some mental health treatment contexts, with reported rates ranging from 12% to 60%.9 A recent, comprehensive review reported that approximately 30% of patients refuse, drop out, or prematurely disengage from services after first-episode psychosis.10 No-shows and drop outs are linked to clinical deterioration11 and heightened risk of hospitalization.12
Jaeschke et al15 suggests that no-shows, dropping out of treatment, and other forms of what doctors call “noncompliance” or “nonadherence” might be better conceptualized as a lack of “concordance,” “mutuality,” or “shared ideology” about what ails patients and the role of their physicians. For this reason, striving for a “partnership between a physician and a patient,” with the patient “fully engaged in the two-way communication with a doctor … seems to be a much more suitable way of achieving therapeutic progress in the discipline of psychiatry.”15
Reducing no-shows: Evidence-based methods
Many medical and mental health articles describe evidence-based methods for lowering no-show rates. Studies document the value of assertive outreach, home visits, avoiding scheduling on religious holidays, scheduling appointments in the afternoon rather than the morning, providing assistance with transportation,8 sending reminder letters,16 or making telephone calls.17 Growing evidence suggests that text messages reduce missed appointments, even among patients with severe disorders (eg, schizophrenia) that compromise cognitive functioning.18
The measures I’ve described won’t prevent every patient from no-showing repeatedly. If you or your employer have tried some of these proven methods and they haven’t reduced a patient’s persistent no-shows, and if it makes sense from a clinical and financial standpoint, then it’s all right to dismiss the patient.
To understand why you are permitted to dismiss a patient from your practice, it helps to understand how the law views the doctor–patient relationship. A doctor has no legal duty to treat anyone—even someone who desperately needs care—unless the doctor has taken some action to establish a treatment relationship with that person. Having previously treated the patient establishes a treatment relationship, as could other actions such as giving specific advice or (in some cases) making an appointment for a person. Once you have a treatment relationship with someone, you usually must continue to provide necessary medical attention until either the treatment episode has concluded or you and the patient agree to end treatment.20
For many chronic mental illnesses, a treatment episode could last years. But this does not force you to continue caring for a patient indefinitely if your circumstances change or if the patient’s behavior causes you to want to withdraw from providing care.
To ethically end care of a patient while a treatment episode is ongoing, you must either transfer care to another competent physician, or give your patient adequate notice and opportunity to obtain appropriate treatment elsewhere.20 If you fail to do either, however, you are guilty of “abandonment” and potentially subject to discipline by state licensing authorities21 or, if harm results, a malpractice lawsuit.22
In many states, statutes or regulations describe what you must do to end a treatment relationship properly. Ohio’s rule is typical: You must send the patient a certified letter explaining that the treatment relationship is ending, that you will remain available to provide care for 30 days, and that you will send treatment records to another provider upon receiving the patient’s signed authorization.21
One note of caution, however: If you practice in hospitals or groups, or if you or the agency where you work has signed provider contracts, you may have agreed to terms of practice that make dismissing a patient more complicated.23 Whether you practice individually or in a large organization, it’s usually wise to get advice from an attorney and/or your malpractice carrier to make sure you’re handling a patient dismissal the right way.
1. Adler LM, Yamamoto J, Goin M. Failed psychiatric clinic appointments. Relationship to social class. Calif Med. 1963;99:388-392.
2. Buppert C. How to deal with missed appointments. Dermatol Nurs 2009;21(4):207-208.
3. National Council Medical Director Institute. The psychiatric shortage: causes and solutions. https://www.thenationalcouncil.org/wp-content/uploads/2017/03/Psychiatric-Shortage_National-Council-.pdf. Published March 28, 2017. Accessed April 6, 2017.
4. Legal & Regulatory Affairs staff. Practitioner pointer: how to handle late and missed appointments. http://www.apapracticecentral.org/update/2014/11-06/late-missed-appoitments.aspx. Published November 6, 2004. Accessed April 7, 2017.
5. Centers for Medicare & Medicaid Services. MLN Matters Number: MM5613. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5613.pdf. Updated November 12, 2014. Accessed April 7, 2017.
6. MacCutcheon M. Why I charge for late cancellations and no-shows to therapy. http://www.goodtherapy.org/blog/why-i-charge-for-late-cancellations-no-shows-to-therapy-0921164. Published September 21, 2016. Accessed April 6, 2017.
7. Kalman TP, Goldstein MA. Satisfaction of Manhattan psychiatrists with private practice: assessing the impact of managed care. http://www.medscape.com/viewarticle/430759_4. Accessed April 6, 2017.
8. Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Adv Psychiatr Treat. 2007;13(6):423-434.
9. Long J, Sakauye K, Chisty K, et al. The empty chair appointment. SAGE Open. 2016;6:1-5.
10. Doyle R, Turner N, Fanning F, et al. First-episode psychosis and disengagement from treatment: a systematic review. Psychiatr Serv. 2014;65(5):603-611.
11. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
12. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance. Characteristics and outcome. Br J Psychiatry. 2000;176:160-165.
13. Williamson AE, Ellis DA, Wilson P, et al. Understanding repeated non-attendance in health services: a pilot analysis of administrative data and full study protocol for a national retrospective cohort. BMJ Open. 2017;7(2):e014120. doi: 10.1136/bmjopen-2016-014120.
14. Binnie J, Boden Z. Non-attendance at psychological therapy appointments. Mental Health Rev J. 2016;21(3):231-248.
15. Jaeschke R, Siwek M, Dudek D. Various ways of understanding compliance: a psychiatrist’s view. Arch Psychiatr Psychother. 2011;13(3):49-55.
16. Boland B, Burnett F. Optimising outpatient efficiency – development of an innovative ‘Did Not Attend’ management approach. Int J Psychiatry Clin Pract. 2014;18(3):217-219.
17. Pennington D, Hodgson J. Non‐attendance and invitation methods within a CBT service. Mental Health Rev J. 2012;17(3):145-151.
18. Sims H, Sanghara H, Hayes D, et al. Text message reminders of appointments: a pilot intervention at four community mental health clinics in London. Psychiatr Serv. 2012;63(2):161-168.
19. Oldham M, Kellett S, Miles E, et al. Interventions to increase attendance at psychotherapy: a meta-analysis of randomized controlled trials. J Consult Clin Psychol. 2012;80(5):928-939.
20. Gore AG, Grossman EL, Martin L, et al. Physicians, surgeons, and other healers. In: American Jurisprudence. 2nd ed, §130. Eagan, MN: West Publishing; 2017:61.
21. Ohio Administrative Code §4731-27-02.
22. Lowery v Miller, 157 Wis 2d 503, 460 N.W. 2d 446 (Wis App 1990).
23. Brockway LH. Terminating patient relationships: how to dismiss without abandoning. TMLT. https://www.tmlt.org/tmlt/tmlt-resources/newscenter/blog/2009/Terminating-patient-relationships.html. Published June 19, 2009. Accessed April 3, 2017.
Dear Dr. Mossman,
The clinic where I work initiated a “3 misses and out” policy: If a patient doesn’t show for 3 appointments in a 12-month period, the clinic removes him from the patient rolls. I’ve heard such policies are common, but I worry: Is this abandonment?
Submitted by “Dr. C”
The short answer to Dr. C’s question is, “Handled properly, it’s not abandonment.” But if this response really was satisfactory, Dr. C probably would not have asked the question. Dealing with no-show patients has bothered psychiatrists, other mental health professionals, and other physicians for decades.1
Clinicians worry when patients miss important follow-up, and unreimbursed office time won’t help pay a clinician’s salary or office expenses.2 But a policy such as the one Dr. C describes may not be the best response—clinically or financially—for many patients who miss appointments repeatedly.
If no-show patients worry you or cause problems where you practice, read on as I cover:
- charging for missed appointments
- why patients miss appointments
- evidence-based methods to improve show-up rates
- when ending a treatment relationship unilaterally is not abandonment
- how to dismiss no-show patients from a practice properly.
Before the mid-1980s, most office-based psychiatrists worked in solo or small group and required patients to pay cash for treatment; approximately 40% of psychiatrists still practice this way.3 Often, private practice clinicians require payment for appointments missed without 24 hours’ notice. This well-accepted practice2,4,5 reinforces the notion that psychotherapy involves making a commitment to work on problems. It also protects clinicians’ financial interests and mitigates possible resentment that might arise if office time committed to a patient went unreimbursed.6 Clinicians who charge for missed appointments should inform patients of this at the beginning of treatment, explaining clearly that the patient—not the insurer—will be paying for unused treatment time.2,4
Since the 1980s, outpatient psychiatrists have increasingly worked in public agencies or other organizational practice settings7 where patients—whose care is funded by public monies or third-party payors—cannot afford to pay for missed appointments. If you work in a clinic such as the one where Dr. C provides services, you probably are paid an hourly wage whether your patients show up or not. To pay you and remain solvent, your clinic must find ways other than charging patients to address and reduce no-shows.
The literature abounds with research on why no-shows occur. But no-shows seem to be more common in psychiatry than in other medical specialties.8 The frequency of no-shows varies considerably, but it’s a big problem in some mental health treatment contexts, with reported rates ranging from 12% to 60%.9 A recent, comprehensive review reported that approximately 30% of patients refuse, drop out, or prematurely disengage from services after first-episode psychosis.10 No-shows and drop outs are linked to clinical deterioration11 and heightened risk of hospitalization.12
Jaeschke et al15 suggests that no-shows, dropping out of treatment, and other forms of what doctors call “noncompliance” or “nonadherence” might be better conceptualized as a lack of “concordance,” “mutuality,” or “shared ideology” about what ails patients and the role of their physicians. For this reason, striving for a “partnership between a physician and a patient,” with the patient “fully engaged in the two-way communication with a doctor … seems to be a much more suitable way of achieving therapeutic progress in the discipline of psychiatry.”15
Reducing no-shows: Evidence-based methods
Many medical and mental health articles describe evidence-based methods for lowering no-show rates. Studies document the value of assertive outreach, home visits, avoiding scheduling on religious holidays, scheduling appointments in the afternoon rather than the morning, providing assistance with transportation,8 sending reminder letters,16 or making telephone calls.17 Growing evidence suggests that text messages reduce missed appointments, even among patients with severe disorders (eg, schizophrenia) that compromise cognitive functioning.18
The measures I’ve described won’t prevent every patient from no-showing repeatedly. If you or your employer have tried some of these proven methods and they haven’t reduced a patient’s persistent no-shows, and if it makes sense from a clinical and financial standpoint, then it’s all right to dismiss the patient.
To understand why you are permitted to dismiss a patient from your practice, it helps to understand how the law views the doctor–patient relationship. A doctor has no legal duty to treat anyone—even someone who desperately needs care—unless the doctor has taken some action to establish a treatment relationship with that person. Having previously treated the patient establishes a treatment relationship, as could other actions such as giving specific advice or (in some cases) making an appointment for a person. Once you have a treatment relationship with someone, you usually must continue to provide necessary medical attention until either the treatment episode has concluded or you and the patient agree to end treatment.20
For many chronic mental illnesses, a treatment episode could last years. But this does not force you to continue caring for a patient indefinitely if your circumstances change or if the patient’s behavior causes you to want to withdraw from providing care.
To ethically end care of a patient while a treatment episode is ongoing, you must either transfer care to another competent physician, or give your patient adequate notice and opportunity to obtain appropriate treatment elsewhere.20 If you fail to do either, however, you are guilty of “abandonment” and potentially subject to discipline by state licensing authorities21 or, if harm results, a malpractice lawsuit.22
In many states, statutes or regulations describe what you must do to end a treatment relationship properly. Ohio’s rule is typical: You must send the patient a certified letter explaining that the treatment relationship is ending, that you will remain available to provide care for 30 days, and that you will send treatment records to another provider upon receiving the patient’s signed authorization.21
One note of caution, however: If you practice in hospitals or groups, or if you or the agency where you work has signed provider contracts, you may have agreed to terms of practice that make dismissing a patient more complicated.23 Whether you practice individually or in a large organization, it’s usually wise to get advice from an attorney and/or your malpractice carrier to make sure you’re handling a patient dismissal the right way.
Dear Dr. Mossman,
The clinic where I work initiated a “3 misses and out” policy: If a patient doesn’t show for 3 appointments in a 12-month period, the clinic removes him from the patient rolls. I’ve heard such policies are common, but I worry: Is this abandonment?
Submitted by “Dr. C”
The short answer to Dr. C’s question is, “Handled properly, it’s not abandonment.” But if this response really was satisfactory, Dr. C probably would not have asked the question. Dealing with no-show patients has bothered psychiatrists, other mental health professionals, and other physicians for decades.1
Clinicians worry when patients miss important follow-up, and unreimbursed office time won’t help pay a clinician’s salary or office expenses.2 But a policy such as the one Dr. C describes may not be the best response—clinically or financially—for many patients who miss appointments repeatedly.
If no-show patients worry you or cause problems where you practice, read on as I cover:
- charging for missed appointments
- why patients miss appointments
- evidence-based methods to improve show-up rates
- when ending a treatment relationship unilaterally is not abandonment
- how to dismiss no-show patients from a practice properly.
Before the mid-1980s, most office-based psychiatrists worked in solo or small group and required patients to pay cash for treatment; approximately 40% of psychiatrists still practice this way.3 Often, private practice clinicians require payment for appointments missed without 24 hours’ notice. This well-accepted practice2,4,5 reinforces the notion that psychotherapy involves making a commitment to work on problems. It also protects clinicians’ financial interests and mitigates possible resentment that might arise if office time committed to a patient went unreimbursed.6 Clinicians who charge for missed appointments should inform patients of this at the beginning of treatment, explaining clearly that the patient—not the insurer—will be paying for unused treatment time.2,4
Since the 1980s, outpatient psychiatrists have increasingly worked in public agencies or other organizational practice settings7 where patients—whose care is funded by public monies or third-party payors—cannot afford to pay for missed appointments. If you work in a clinic such as the one where Dr. C provides services, you probably are paid an hourly wage whether your patients show up or not. To pay you and remain solvent, your clinic must find ways other than charging patients to address and reduce no-shows.
The literature abounds with research on why no-shows occur. But no-shows seem to be more common in psychiatry than in other medical specialties.8 The frequency of no-shows varies considerably, but it’s a big problem in some mental health treatment contexts, with reported rates ranging from 12% to 60%.9 A recent, comprehensive review reported that approximately 30% of patients refuse, drop out, or prematurely disengage from services after first-episode psychosis.10 No-shows and drop outs are linked to clinical deterioration11 and heightened risk of hospitalization.12
Jaeschke et al15 suggests that no-shows, dropping out of treatment, and other forms of what doctors call “noncompliance” or “nonadherence” might be better conceptualized as a lack of “concordance,” “mutuality,” or “shared ideology” about what ails patients and the role of their physicians. For this reason, striving for a “partnership between a physician and a patient,” with the patient “fully engaged in the two-way communication with a doctor … seems to be a much more suitable way of achieving therapeutic progress in the discipline of psychiatry.”15
Reducing no-shows: Evidence-based methods
Many medical and mental health articles describe evidence-based methods for lowering no-show rates. Studies document the value of assertive outreach, home visits, avoiding scheduling on religious holidays, scheduling appointments in the afternoon rather than the morning, providing assistance with transportation,8 sending reminder letters,16 or making telephone calls.17 Growing evidence suggests that text messages reduce missed appointments, even among patients with severe disorders (eg, schizophrenia) that compromise cognitive functioning.18
The measures I’ve described won’t prevent every patient from no-showing repeatedly. If you or your employer have tried some of these proven methods and they haven’t reduced a patient’s persistent no-shows, and if it makes sense from a clinical and financial standpoint, then it’s all right to dismiss the patient.
To understand why you are permitted to dismiss a patient from your practice, it helps to understand how the law views the doctor–patient relationship. A doctor has no legal duty to treat anyone—even someone who desperately needs care—unless the doctor has taken some action to establish a treatment relationship with that person. Having previously treated the patient establishes a treatment relationship, as could other actions such as giving specific advice or (in some cases) making an appointment for a person. Once you have a treatment relationship with someone, you usually must continue to provide necessary medical attention until either the treatment episode has concluded or you and the patient agree to end treatment.20
For many chronic mental illnesses, a treatment episode could last years. But this does not force you to continue caring for a patient indefinitely if your circumstances change or if the patient’s behavior causes you to want to withdraw from providing care.
To ethically end care of a patient while a treatment episode is ongoing, you must either transfer care to another competent physician, or give your patient adequate notice and opportunity to obtain appropriate treatment elsewhere.20 If you fail to do either, however, you are guilty of “abandonment” and potentially subject to discipline by state licensing authorities21 or, if harm results, a malpractice lawsuit.22
In many states, statutes or regulations describe what you must do to end a treatment relationship properly. Ohio’s rule is typical: You must send the patient a certified letter explaining that the treatment relationship is ending, that you will remain available to provide care for 30 days, and that you will send treatment records to another provider upon receiving the patient’s signed authorization.21
One note of caution, however: If you practice in hospitals or groups, or if you or the agency where you work has signed provider contracts, you may have agreed to terms of practice that make dismissing a patient more complicated.23 Whether you practice individually or in a large organization, it’s usually wise to get advice from an attorney and/or your malpractice carrier to make sure you’re handling a patient dismissal the right way.
1. Adler LM, Yamamoto J, Goin M. Failed psychiatric clinic appointments. Relationship to social class. Calif Med. 1963;99:388-392.
2. Buppert C. How to deal with missed appointments. Dermatol Nurs 2009;21(4):207-208.
3. National Council Medical Director Institute. The psychiatric shortage: causes and solutions. https://www.thenationalcouncil.org/wp-content/uploads/2017/03/Psychiatric-Shortage_National-Council-.pdf. Published March 28, 2017. Accessed April 6, 2017.
4. Legal & Regulatory Affairs staff. Practitioner pointer: how to handle late and missed appointments. http://www.apapracticecentral.org/update/2014/11-06/late-missed-appoitments.aspx. Published November 6, 2004. Accessed April 7, 2017.
5. Centers for Medicare & Medicaid Services. MLN Matters Number: MM5613. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5613.pdf. Updated November 12, 2014. Accessed April 7, 2017.
6. MacCutcheon M. Why I charge for late cancellations and no-shows to therapy. http://www.goodtherapy.org/blog/why-i-charge-for-late-cancellations-no-shows-to-therapy-0921164. Published September 21, 2016. Accessed April 6, 2017.
7. Kalman TP, Goldstein MA. Satisfaction of Manhattan psychiatrists with private practice: assessing the impact of managed care. http://www.medscape.com/viewarticle/430759_4. Accessed April 6, 2017.
8. Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Adv Psychiatr Treat. 2007;13(6):423-434.
9. Long J, Sakauye K, Chisty K, et al. The empty chair appointment. SAGE Open. 2016;6:1-5.
10. Doyle R, Turner N, Fanning F, et al. First-episode psychosis and disengagement from treatment: a systematic review. Psychiatr Serv. 2014;65(5):603-611.
11. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
12. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance. Characteristics and outcome. Br J Psychiatry. 2000;176:160-165.
13. Williamson AE, Ellis DA, Wilson P, et al. Understanding repeated non-attendance in health services: a pilot analysis of administrative data and full study protocol for a national retrospective cohort. BMJ Open. 2017;7(2):e014120. doi: 10.1136/bmjopen-2016-014120.
14. Binnie J, Boden Z. Non-attendance at psychological therapy appointments. Mental Health Rev J. 2016;21(3):231-248.
15. Jaeschke R, Siwek M, Dudek D. Various ways of understanding compliance: a psychiatrist’s view. Arch Psychiatr Psychother. 2011;13(3):49-55.
16. Boland B, Burnett F. Optimising outpatient efficiency – development of an innovative ‘Did Not Attend’ management approach. Int J Psychiatry Clin Pract. 2014;18(3):217-219.
17. Pennington D, Hodgson J. Non‐attendance and invitation methods within a CBT service. Mental Health Rev J. 2012;17(3):145-151.
18. Sims H, Sanghara H, Hayes D, et al. Text message reminders of appointments: a pilot intervention at four community mental health clinics in London. Psychiatr Serv. 2012;63(2):161-168.
19. Oldham M, Kellett S, Miles E, et al. Interventions to increase attendance at psychotherapy: a meta-analysis of randomized controlled trials. J Consult Clin Psychol. 2012;80(5):928-939.
20. Gore AG, Grossman EL, Martin L, et al. Physicians, surgeons, and other healers. In: American Jurisprudence. 2nd ed, §130. Eagan, MN: West Publishing; 2017:61.
21. Ohio Administrative Code §4731-27-02.
22. Lowery v Miller, 157 Wis 2d 503, 460 N.W. 2d 446 (Wis App 1990).
23. Brockway LH. Terminating patient relationships: how to dismiss without abandoning. TMLT. https://www.tmlt.org/tmlt/tmlt-resources/newscenter/blog/2009/Terminating-patient-relationships.html. Published June 19, 2009. Accessed April 3, 2017.
1. Adler LM, Yamamoto J, Goin M. Failed psychiatric clinic appointments. Relationship to social class. Calif Med. 1963;99:388-392.
2. Buppert C. How to deal with missed appointments. Dermatol Nurs 2009;21(4):207-208.
3. National Council Medical Director Institute. The psychiatric shortage: causes and solutions. https://www.thenationalcouncil.org/wp-content/uploads/2017/03/Psychiatric-Shortage_National-Council-.pdf. Published March 28, 2017. Accessed April 6, 2017.
4. Legal & Regulatory Affairs staff. Practitioner pointer: how to handle late and missed appointments. http://www.apapracticecentral.org/update/2014/11-06/late-missed-appoitments.aspx. Published November 6, 2004. Accessed April 7, 2017.
5. Centers for Medicare & Medicaid Services. MLN Matters Number: MM5613. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5613.pdf. Updated November 12, 2014. Accessed April 7, 2017.
6. MacCutcheon M. Why I charge for late cancellations and no-shows to therapy. http://www.goodtherapy.org/blog/why-i-charge-for-late-cancellations-no-shows-to-therapy-0921164. Published September 21, 2016. Accessed April 6, 2017.
7. Kalman TP, Goldstein MA. Satisfaction of Manhattan psychiatrists with private practice: assessing the impact of managed care. http://www.medscape.com/viewarticle/430759_4. Accessed April 6, 2017.
8. Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Adv Psychiatr Treat. 2007;13(6):423-434.
9. Long J, Sakauye K, Chisty K, et al. The empty chair appointment. SAGE Open. 2016;6:1-5.
10. Doyle R, Turner N, Fanning F, et al. First-episode psychosis and disengagement from treatment: a systematic review. Psychiatr Serv. 2014;65(5):603-611.
11. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
12. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance. Characteristics and outcome. Br J Psychiatry. 2000;176:160-165.
13. Williamson AE, Ellis DA, Wilson P, et al. Understanding repeated non-attendance in health services: a pilot analysis of administrative data and full study protocol for a national retrospective cohort. BMJ Open. 2017;7(2):e014120. doi: 10.1136/bmjopen-2016-014120.
14. Binnie J, Boden Z. Non-attendance at psychological therapy appointments. Mental Health Rev J. 2016;21(3):231-248.
15. Jaeschke R, Siwek M, Dudek D. Various ways of understanding compliance: a psychiatrist’s view. Arch Psychiatr Psychother. 2011;13(3):49-55.
16. Boland B, Burnett F. Optimising outpatient efficiency – development of an innovative ‘Did Not Attend’ management approach. Int J Psychiatry Clin Pract. 2014;18(3):217-219.
17. Pennington D, Hodgson J. Non‐attendance and invitation methods within a CBT service. Mental Health Rev J. 2012;17(3):145-151.
18. Sims H, Sanghara H, Hayes D, et al. Text message reminders of appointments: a pilot intervention at four community mental health clinics in London. Psychiatr Serv. 2012;63(2):161-168.
19. Oldham M, Kellett S, Miles E, et al. Interventions to increase attendance at psychotherapy: a meta-analysis of randomized controlled trials. J Consult Clin Psychol. 2012;80(5):928-939.
20. Gore AG, Grossman EL, Martin L, et al. Physicians, surgeons, and other healers. In: American Jurisprudence. 2nd ed, §130. Eagan, MN: West Publishing; 2017:61.
21. Ohio Administrative Code §4731-27-02.
22. Lowery v Miller, 157 Wis 2d 503, 460 N.W. 2d 446 (Wis App 1990).
23. Brockway LH. Terminating patient relationships: how to dismiss without abandoning. TMLT. https://www.tmlt.org/tmlt/tmlt-resources/newscenter/blog/2009/Terminating-patient-relationships.html. Published June 19, 2009. Accessed April 3, 2017.
How to diagnose and manage hypertension in a psychiatric patient
Hypertension is a widespread, under-recognized, and undertreated cause of morbidity and mortality in the United States and is associated with several psychiatric illnesses. Left untreated, hypertension can have significant consequences, including increased risk of stroke, coronary heart disease, heart failure, chronic kidney failure, and death. Approximately 70 million adults in the United States have hypertension, but only 60% of them have been diagnosed, and of those only 50% have their blood pressure under control.1 In 2013, 360,000 deaths in the United States were attributed to hypertension.2
Hypertension is associated with major depressive disorder, generalized anxiety disorder, bipolar disorder, and schizophrenia.3-5 Additionally, impulsive eating disorders, substance abuse, anxiety, and depression are associated with a hypertension diagnosis, although patients with panic disorder develop hypertension at a younger age.6 A 2007 study found a 61% prevalence of hypertension in those with bipolar disorder compared with 41% among the general population.7 The strong link between bipolar disorder and hypertension might be because of a common disease mechanism; both are associated with hyperactive cellular calcium signaling and increased platelet intracellular calcium ion concentrations.8
Hypertension not only is common among patients with psychiatric illness, it likely contributes to worse clinical outcomes. Studies across different cultures have found higher mortality rates in individuals with mental illness.9-11 Persons with schizophrenia and other severe mental illnesses may lose ≥25 years of life expectancy, with the primary cause of death being cardiovascular disease, not suicide.12 Patients with depression have a 50% greater risk of cardiovascular disease, which is equivalent to the risk of smoking.13
Schizophrenia is strongly associated with numerous comorbidities and has been linked significantly to an elevated 10-year cardiac risk after controlling for body mass index.5 The high rate of non-treatment of hypertension for patients with schizophrenia (62.4%) is especially concerning.14
Because of the well-documented morbidity and mortality of hypertension and its increased prevalence and undertreatment in the psychiatric population, mental health providers are in an important position to recognize hypertension and evaluate its inherent risks to direct their patients toward proper treatment. This article reviews:
- the signs and symptoms of hypertension
- the mental health provider’s role in the evaluation and diagnosis
- how psychotropic drugs influence blood pressure and drug–drug interactions
- the management of hypertension in psychiatric patients, including strategies for counseling and lifestyle management.
Diagnosing hypertension
Hypertension is defined as a blood pressure >140/90 mm Hg, the average of ≥2 properly measured readings at ≥2 visits in a medical setting.15 The proper equipment, including a well-fitting blood pressure cuff, and technique to measure blood pressure are essential to avoid misdiagnosis. The patient should be at rest for ≥5 minutes, without active pain or emotional distress.
Most cases of hypertension (90% to 95%) are primary, commonly called essential hypertension. However, the differential diagnosis also should consider secondary causes, which may include:
- obesity
- medications
- chronic alcohol use
- methamphetamine or cocaine use
- primary kidney disease
- atherosclerotic renal artery stenosis
- obstructive sleep apnea
- hypothyroidism
- primary hyperaldosteronism
- narrowing of the aorta
- Cushing syndrome
- primary hyperparathyroidism
- polycythemia
- pheochromocytoma.
Medical evaluation. Once the diagnosis of hypertension is made, a medical evaluation is indicated to determine if the patient has end-organ damage from the elevated pressures, such as renal disease or heart disease, to identify other modifiable cardiovascular risk factors, such as hyperlipidemia, and to screen for secondary causes of hypertension. This evaluation includes15:
- a physical exam
- review of medications
- lipid profile
- urinalysis to screen for proteinuria
- serum electrolytes and creatinine
- electrocardiogram to screen for left ventricular hypertrophy or prior infarction
- fasting glucose or hemoglobin A1c to screen for type 2 diabetes mellitus.
Psychotropic drugs. In psychiatric patients, the evaluation must consider the potential impact psychotropic drug effects and drug–drug interactions can have on blood pressure (Table 2). For example, patients taking both diuretics and lithium are at increased risk for dehydration and increased serum lithium levels, which could cause severe neurologic symptoms and renal insufficiency.16 Several antihypertensives when taken with venlafaxine can increase blood pressure, but antihypertensives with α-1 blocking psychotropics can decrease blood pressure. Monoamine oxidase inhibitors can cause hypotension or hypertension with various classes of antihypertensives. Stimulants, such as methylphenidate, atomoxetine, dextroamphetamine, armodafinil, or modafinil, alone or combined with antihypertensives, can cause hypertension.17
Substance abuse, particularly alcohol, methamphetamine, and cocaine, can cause difficulty controlling blood pressure. Patients with refractory hypertension should have a reassessment of substance abuse as a potential cause.
Screening guidelines for mental health providers
For many patients with severe mental illness, visits to their mental health providers might be their only contact with the medical system. Therefore, screening in the mental health settings could detect cases that otherwise would be missed.
Screening recommendations. The U.S. Preventive Services Task Force recommends screening for hypertension in the general population beginning at age 18.18 Adults age 18 to 39 with normal blood pressure (<130/85 mm Hg) and no other risk factors (eg, overweight, obese, or African American) can be screened every 3 years. Those with risk factors or a blood pressure of 130/85 to 139/89 mm Hg and adults age ≥40 should have annual screenings.
Ideally, psychiatrists and other mental health providers should monitor blood pressure at each visit, especially in patients taking psychotropics because of their higher risk for hypertension.
Optimizing treatment. Once the diagnosis of essential hypertension is established, identifying psychiatric comorbidities and the severity of psychiatric symptoms are important to optimize treatment adherence. Patients with increased depressive symptoms are less likely to comply with antihypertensive medication,19 and patients with confirmed depression are 3 times more likely to not adhere to medical treatment recommendations than non-depressed patients.20
Physicians’ attitudes toward hypertension also can affect patients’ compliance and blood pressure control.21 Psychiatrists should be empathetic and motivational toward patients attempting to control their blood pressure. The Seventh Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure states, “Motivation improves when patients have positive experiences with, and trust in, the clinician. Empathy builds trust and is a potent motivator.”22
Treatment and management
Treatment of hypertension significantly reduces the risk of stroke, myocardial infarction, renal injury, heart failure, and premature death. Studies show that treatment that reduces systolic blood pressure by 12 mm Hg over 10 years will prevent 1 death for every 11 patients with essential hypertension. In those with concomitant cardiovascular disease or target organ damage, such a reduction would prevent death in 1 of every 9 patients treated.15Blood pressure goals. The 2014 Eighth Joint National Committee Guideline for Management of High Blood Pressure in Adults provides guidance on blood pressure goals depending on patients’ underlying medical history (Figure).23 Based on expert opinion and randomized controlled studies, blood pressure goals for patients without diabetes or chronic kidney disease (CKD)—an estimated or measured glomerular filtration rate (GFR) of ≤60 mL/min/1.73 m2—depend on age: <140/90 mm Hg for age 18 to 59 and <150/90 mm Hg for age ≥60. For patients with diabetes or CKD, the blood pressure goal is <140/90 mm Hg, regardless of age.
However, not all experts agree on these specific blood pressure goals. A major trial (SPRINT) published in 2015 found that intensive blood pressure goals do benefit higher-risk, non-diabetic patients.24 Specifically, the study randomized patients age ≥50 with systolic blood pressure of 130 to 180 mm Hg and increased cardiovascular risk to systolic blood pressure targets of <140 mm Hg (standard) or <120 mm Hg (intensive). Characteristics of increased cardiovascular risk were clinical or subclinical cardiovascular disease other than stroke, CKD with GFR of 20 to 60 mL/min/1.73 m2, age ≥75, or Framingham 10-year coronary heart disease risk score ≥15%. Intensive treatment significantly reduced overall mortality and the rate of acute coronary syndrome, myocardial infarction, heart failure, stroke, or cardiovascular death. However, the results of this study have not been assimilated into any recent guidelines. Therefore, consider a goal of <120 mm Hg for non-diabetic patients age ≥50 with any of these factors.
Lifestyle modifications. Psychiatrists are well equipped to motivate and encourage behavioral modification in patients with hypertension. Counseling and structured training courses could help to effectively lower blood pressure.25 Patients should receive education on lifestyle modifications including:
- weight reduction
- physical activity
- moderate alcohol consumption
- decreased sodium consumption
- implementation of the Dietary Approaches to Stop Hypertension (DASH) or Mediterranean diets.15
Maintaining a normal body weight is ideal, but weight reduction of 10 lb can reduce blood pressure in overweight patients. The DASH diet, consisting of fruits, vegetables, low-fat dairy products, high calcium and potassium intake, and reduced saturated and total fat intake can decrease systolic blood pressure from 8 to 14 mm Hg. Reduction of sodium intake to ≤2,400 mg/d can reduce systolic blood pressure from 2 to 8 mm Hg. Regular aerobic exercise of 30 minutes a day most days of the week can reduce systolic blood pressure up to 9 mm Hg. Patients also should be encouraged to quit smoking. Patients who implement ≥2 these modifications get better results.
Antihypertensive medications. Patients who do not reach their goals with lifestyle measures alone should receive antihypertensive medications. Most patients will require ≥2 agents to control their blood pressure. Clinical trials show that some patient subgroups have better outcomes with different first-line agents.
For example, in non-African American patients, thiazide diuretics, calcium channel blockers, angiotensin receptor blockers, and angiotensin-converting enzyme inhibitors are first-line treatments (Table 3). For African American patients without CKD, first-line treatments should be thiazide diuretics and calcium channel blockers, because angiotensin-converting enzyme inhibitors and angiotensin receptor blockers do not reduce cardiovascular events as effectively. African American patients with CKD and proteinuria, however, benefit from angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and are preferred first-line agents. However, blood pressure control is a more important factor in improving outcomes than the choice of medication.
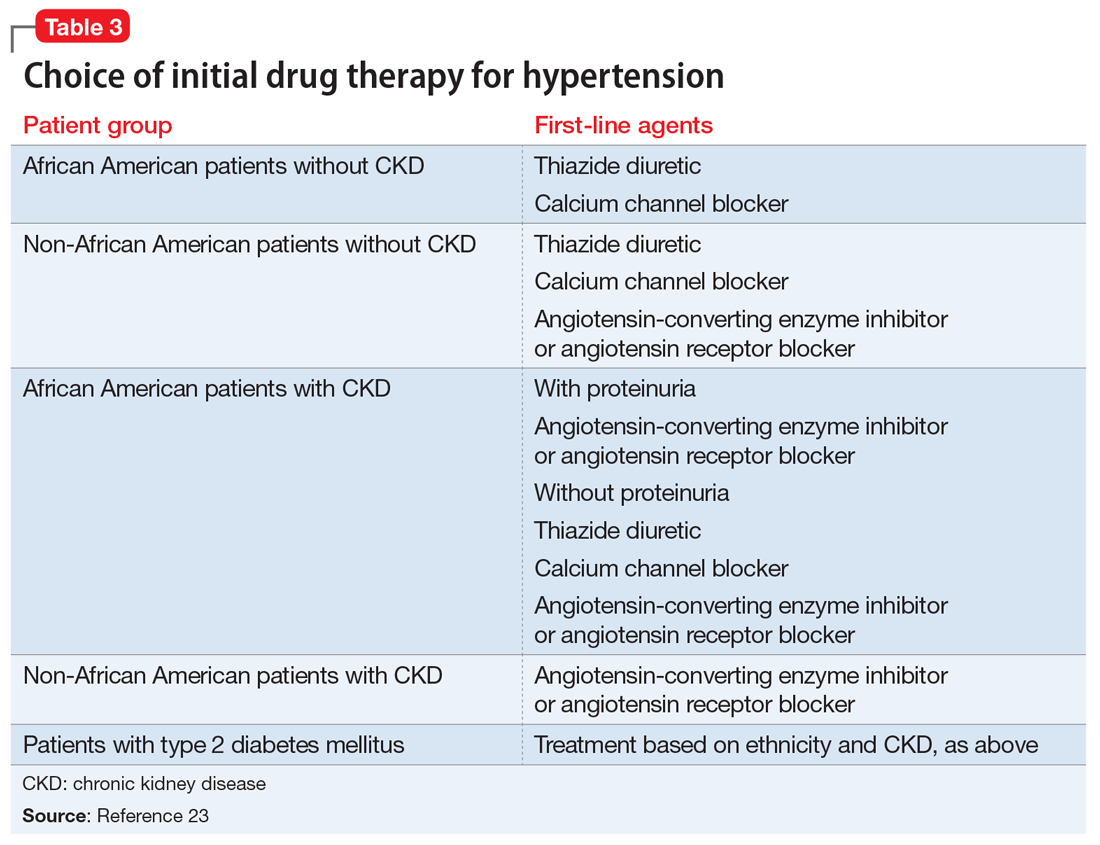
Psychiatrists’ role. Psychiatrists should aim to collaborate with the primary care provider when treating hypertension. However, when integrative care is not possible, they should start a first-line medication with follow-up in 1 month or sooner for patients with severe hypertension (>160/100 mm Hg) or significant comorbidities (eg, CKD, congestive heart failure, coronary disease). Patients with blood pressure >160/100 mm Hg often are started on a thiazide diuretic with one other medication because a single agent usually does not achieve goal blood pressure. Patients with CKD need close monitoring of potassium and creatinine when starting angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy, usually within 1 to 2 days of starting or adjusting their medication. Adjust or add medication dosages monthly until blood pressure goals are reached.
A general internist, cardiologist, or nephrologist who has expertise in managing complex cases should oversee care of a psychiatric patient in any of the following scenarios:
- suspected secondary cause of hypertension
- adverse reaction to antihypertensive medications
- complicated comorbid conditions (ie, creatinine >1.8 mg/dL, worsening renal failure, hyperkalemia, heart failure, coronary disease)
- blood pressure >180/120 mm Hg
- requires ≥3 antihypertensive medications.
Summing up
Hypertension is a significant comorbidity in many psychiatric patients, but usually is asymptomatic. Often the psychiatrist or other mental health provider will diagnose hypertension because of their frequent contact with these patients. Once the diagnosis is made, an initial evaluation can direct lifestyle modifications. Patients who continue to have significant elevation of blood pressure should start pharmacotherapy, either by the psychiatrist or by ensuring follow-up with a primary care physician. The psychiatrist may be able to manage cases of essential hypertension, but always must be vigilant for potential drug–disease or drug–drug interactions during treatment. A team-based approach may improve health outcomes in psychiatric patients.
1. Centers for Disease Control and Prevention (CDC). Vital signs: awareness and treatment of uncontrolled hypertension among adults—United States, 2003-2010. MMWR Morb Mortal Wkly Rep. 2012;61:703-709.
2. Mozzafarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322.
3. Carroll D, Phillips AC, Gale CR, et al. Generalized anxiety and major depressive disorders, their comorbidity and hypertension in middle-aged men. Psychosom Med. 2010;72(1):16-19.
4. Leboyer M, Soreca I, Scott J, et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord. 2012;141(1):1-10.
5. Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80(1):45-53.
6. Stein DJ, Aguilar-Gaxiola S, Alonso J, et al. Associations between mental disorders and subsequent onset of hypertension. Gen Hosp Psychiatry. 2014;36(2):142-149.
7. Birkenaes AB, Opjordsmoen S, Brunborg C, et al. The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study. J Clin Psychiatry. 2007;68(6):917-923.
8. Izzo JL, Black HR, Goodfriend TL. Hypertension primer: the essentials of high blood pressure. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
9. Osby U, Correia N, Brandt L, et al. Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophr Res. 2000;45(1-2):21-28.
10. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
11. Auquier P, Lançon C, Rouillon F, et al. Mortality in schizophrenia. Pharmacoepidemiol Drug Saf. 2007;16(12):1308-1312.
12. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
13. Bowis J, Parvanova A, McDaid D, et al. Mental and Physical Health Charter: bridging the gap between mental and physical health. https://www.idf.org/sites/default/files/Mental%2520and%2520Physical%2520Health%2520Charter%2520-%2520FINAL.pdf. Published October 7, 2009. Accessed March 6, 2017.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2571.
16. Handler J. Lithium and antihypertensive medication: a potentially dangerous interaction. J Clin Hypertens (Greenwich). 2009;11(12):738-742.
17. National Collaborating Centre for Mental Health (UK). Depression in adults with a chronic physical health problem: treatment and Management. Appendix 16: table of drug interactions. http://www.ncbi.nlm.nih.gov/books/NBK82914. Published 2010. Accessed March 6, 2017.
18. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015:163(10):778-786.
19. Wang PS, Bohn RL, Knight E, et al. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17(7):504-511.
20. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
21. Consoli SM, Lemogne C, Levy A, et al. Physicians’ degree of motivation regarding their perception of hypertension, and blood pressure control. J Hypertens. 2010;28(6):1330-1339.
22. National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Improving Hypertension Control. Bethesda, MD: U.S. Department of Health and Human Services; 2004:61-64.
23. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
24. The SPRINT Research Group; Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2016.
25. Boulware LE, Daumit GL, Frick KD, et al. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med. 2001;21(3):221-232.
Hypertension is a widespread, under-recognized, and undertreated cause of morbidity and mortality in the United States and is associated with several psychiatric illnesses. Left untreated, hypertension can have significant consequences, including increased risk of stroke, coronary heart disease, heart failure, chronic kidney failure, and death. Approximately 70 million adults in the United States have hypertension, but only 60% of them have been diagnosed, and of those only 50% have their blood pressure under control.1 In 2013, 360,000 deaths in the United States were attributed to hypertension.2
Hypertension is associated with major depressive disorder, generalized anxiety disorder, bipolar disorder, and schizophrenia.3-5 Additionally, impulsive eating disorders, substance abuse, anxiety, and depression are associated with a hypertension diagnosis, although patients with panic disorder develop hypertension at a younger age.6 A 2007 study found a 61% prevalence of hypertension in those with bipolar disorder compared with 41% among the general population.7 The strong link between bipolar disorder and hypertension might be because of a common disease mechanism; both are associated with hyperactive cellular calcium signaling and increased platelet intracellular calcium ion concentrations.8
Hypertension not only is common among patients with psychiatric illness, it likely contributes to worse clinical outcomes. Studies across different cultures have found higher mortality rates in individuals with mental illness.9-11 Persons with schizophrenia and other severe mental illnesses may lose ≥25 years of life expectancy, with the primary cause of death being cardiovascular disease, not suicide.12 Patients with depression have a 50% greater risk of cardiovascular disease, which is equivalent to the risk of smoking.13
Schizophrenia is strongly associated with numerous comorbidities and has been linked significantly to an elevated 10-year cardiac risk after controlling for body mass index.5 The high rate of non-treatment of hypertension for patients with schizophrenia (62.4%) is especially concerning.14
Because of the well-documented morbidity and mortality of hypertension and its increased prevalence and undertreatment in the psychiatric population, mental health providers are in an important position to recognize hypertension and evaluate its inherent risks to direct their patients toward proper treatment. This article reviews:
- the signs and symptoms of hypertension
- the mental health provider’s role in the evaluation and diagnosis
- how psychotropic drugs influence blood pressure and drug–drug interactions
- the management of hypertension in psychiatric patients, including strategies for counseling and lifestyle management.
Diagnosing hypertension
Hypertension is defined as a blood pressure >140/90 mm Hg, the average of ≥2 properly measured readings at ≥2 visits in a medical setting.15 The proper equipment, including a well-fitting blood pressure cuff, and technique to measure blood pressure are essential to avoid misdiagnosis. The patient should be at rest for ≥5 minutes, without active pain or emotional distress.
Most cases of hypertension (90% to 95%) are primary, commonly called essential hypertension. However, the differential diagnosis also should consider secondary causes, which may include:
- obesity
- medications
- chronic alcohol use
- methamphetamine or cocaine use
- primary kidney disease
- atherosclerotic renal artery stenosis
- obstructive sleep apnea
- hypothyroidism
- primary hyperaldosteronism
- narrowing of the aorta
- Cushing syndrome
- primary hyperparathyroidism
- polycythemia
- pheochromocytoma.
Medical evaluation. Once the diagnosis of hypertension is made, a medical evaluation is indicated to determine if the patient has end-organ damage from the elevated pressures, such as renal disease or heart disease, to identify other modifiable cardiovascular risk factors, such as hyperlipidemia, and to screen for secondary causes of hypertension. This evaluation includes15:
- a physical exam
- review of medications
- lipid profile
- urinalysis to screen for proteinuria
- serum electrolytes and creatinine
- electrocardiogram to screen for left ventricular hypertrophy or prior infarction
- fasting glucose or hemoglobin A1c to screen for type 2 diabetes mellitus.
Psychotropic drugs. In psychiatric patients, the evaluation must consider the potential impact psychotropic drug effects and drug–drug interactions can have on blood pressure (Table 2). For example, patients taking both diuretics and lithium are at increased risk for dehydration and increased serum lithium levels, which could cause severe neurologic symptoms and renal insufficiency.16 Several antihypertensives when taken with venlafaxine can increase blood pressure, but antihypertensives with α-1 blocking psychotropics can decrease blood pressure. Monoamine oxidase inhibitors can cause hypotension or hypertension with various classes of antihypertensives. Stimulants, such as methylphenidate, atomoxetine, dextroamphetamine, armodafinil, or modafinil, alone or combined with antihypertensives, can cause hypertension.17
Substance abuse, particularly alcohol, methamphetamine, and cocaine, can cause difficulty controlling blood pressure. Patients with refractory hypertension should have a reassessment of substance abuse as a potential cause.
Screening guidelines for mental health providers
For many patients with severe mental illness, visits to their mental health providers might be their only contact with the medical system. Therefore, screening in the mental health settings could detect cases that otherwise would be missed.
Screening recommendations. The U.S. Preventive Services Task Force recommends screening for hypertension in the general population beginning at age 18.18 Adults age 18 to 39 with normal blood pressure (<130/85 mm Hg) and no other risk factors (eg, overweight, obese, or African American) can be screened every 3 years. Those with risk factors or a blood pressure of 130/85 to 139/89 mm Hg and adults age ≥40 should have annual screenings.
Ideally, psychiatrists and other mental health providers should monitor blood pressure at each visit, especially in patients taking psychotropics because of their higher risk for hypertension.
Optimizing treatment. Once the diagnosis of essential hypertension is established, identifying psychiatric comorbidities and the severity of psychiatric symptoms are important to optimize treatment adherence. Patients with increased depressive symptoms are less likely to comply with antihypertensive medication,19 and patients with confirmed depression are 3 times more likely to not adhere to medical treatment recommendations than non-depressed patients.20
Physicians’ attitudes toward hypertension also can affect patients’ compliance and blood pressure control.21 Psychiatrists should be empathetic and motivational toward patients attempting to control their blood pressure. The Seventh Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure states, “Motivation improves when patients have positive experiences with, and trust in, the clinician. Empathy builds trust and is a potent motivator.”22
Treatment and management
Treatment of hypertension significantly reduces the risk of stroke, myocardial infarction, renal injury, heart failure, and premature death. Studies show that treatment that reduces systolic blood pressure by 12 mm Hg over 10 years will prevent 1 death for every 11 patients with essential hypertension. In those with concomitant cardiovascular disease or target organ damage, such a reduction would prevent death in 1 of every 9 patients treated.15Blood pressure goals. The 2014 Eighth Joint National Committee Guideline for Management of High Blood Pressure in Adults provides guidance on blood pressure goals depending on patients’ underlying medical history (Figure).23 Based on expert opinion and randomized controlled studies, blood pressure goals for patients without diabetes or chronic kidney disease (CKD)—an estimated or measured glomerular filtration rate (GFR) of ≤60 mL/min/1.73 m2—depend on age: <140/90 mm Hg for age 18 to 59 and <150/90 mm Hg for age ≥60. For patients with diabetes or CKD, the blood pressure goal is <140/90 mm Hg, regardless of age.

However, not all experts agree on these specific blood pressure goals. A major trial (SPRINT) published in 2015 found that intensive blood pressure goals do benefit higher-risk, non-diabetic patients.24 Specifically, the study randomized patients age ≥50 with systolic blood pressure of 130 to 180 mm Hg and increased cardiovascular risk to systolic blood pressure targets of <140 mm Hg (standard) or <120 mm Hg (intensive). Characteristics of increased cardiovascular risk were clinical or subclinical cardiovascular disease other than stroke, CKD with GFR of 20 to 60 mL/min/1.73 m2, age ≥75, or Framingham 10-year coronary heart disease risk score ≥15%. Intensive treatment significantly reduced overall mortality and the rate of acute coronary syndrome, myocardial infarction, heart failure, stroke, or cardiovascular death. However, the results of this study have not been assimilated into any recent guidelines. Therefore, consider a goal of <120 mm Hg for non-diabetic patients age ≥50 with any of these factors.
Lifestyle modifications. Psychiatrists are well equipped to motivate and encourage behavioral modification in patients with hypertension. Counseling and structured training courses could help to effectively lower blood pressure.25 Patients should receive education on lifestyle modifications including:
- weight reduction
- physical activity
- moderate alcohol consumption
- decreased sodium consumption
- implementation of the Dietary Approaches to Stop Hypertension (DASH) or Mediterranean diets.15
Maintaining a normal body weight is ideal, but weight reduction of 10 lb can reduce blood pressure in overweight patients. The DASH diet, consisting of fruits, vegetables, low-fat dairy products, high calcium and potassium intake, and reduced saturated and total fat intake can decrease systolic blood pressure from 8 to 14 mm Hg. Reduction of sodium intake to ≤2,400 mg/d can reduce systolic blood pressure from 2 to 8 mm Hg. Regular aerobic exercise of 30 minutes a day most days of the week can reduce systolic blood pressure up to 9 mm Hg. Patients also should be encouraged to quit smoking. Patients who implement ≥2 these modifications get better results.
Antihypertensive medications. Patients who do not reach their goals with lifestyle measures alone should receive antihypertensive medications. Most patients will require ≥2 agents to control their blood pressure. Clinical trials show that some patient subgroups have better outcomes with different first-line agents.
For example, in non-African American patients, thiazide diuretics, calcium channel blockers, angiotensin receptor blockers, and angiotensin-converting enzyme inhibitors are first-line treatments (Table 3). For African American patients without CKD, first-line treatments should be thiazide diuretics and calcium channel blockers, because angiotensin-converting enzyme inhibitors and angiotensin receptor blockers do not reduce cardiovascular events as effectively. African American patients with CKD and proteinuria, however, benefit from angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and are preferred first-line agents. However, blood pressure control is a more important factor in improving outcomes than the choice of medication.

Psychiatrists’ role. Psychiatrists should aim to collaborate with the primary care provider when treating hypertension. However, when integrative care is not possible, they should start a first-line medication with follow-up in 1 month or sooner for patients with severe hypertension (>160/100 mm Hg) or significant comorbidities (eg, CKD, congestive heart failure, coronary disease). Patients with blood pressure >160/100 mm Hg often are started on a thiazide diuretic with one other medication because a single agent usually does not achieve goal blood pressure. Patients with CKD need close monitoring of potassium and creatinine when starting angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy, usually within 1 to 2 days of starting or adjusting their medication. Adjust or add medication dosages monthly until blood pressure goals are reached.
A general internist, cardiologist, or nephrologist who has expertise in managing complex cases should oversee care of a psychiatric patient in any of the following scenarios:
- suspected secondary cause of hypertension
- adverse reaction to antihypertensive medications
- complicated comorbid conditions (ie, creatinine >1.8 mg/dL, worsening renal failure, hyperkalemia, heart failure, coronary disease)
- blood pressure >180/120 mm Hg
- requires ≥3 antihypertensive medications.
Summing up
Hypertension is a significant comorbidity in many psychiatric patients, but usually is asymptomatic. Often the psychiatrist or other mental health provider will diagnose hypertension because of their frequent contact with these patients. Once the diagnosis is made, an initial evaluation can direct lifestyle modifications. Patients who continue to have significant elevation of blood pressure should start pharmacotherapy, either by the psychiatrist or by ensuring follow-up with a primary care physician. The psychiatrist may be able to manage cases of essential hypertension, but always must be vigilant for potential drug–disease or drug–drug interactions during treatment. A team-based approach may improve health outcomes in psychiatric patients.
Hypertension is a widespread, under-recognized, and undertreated cause of morbidity and mortality in the United States and is associated with several psychiatric illnesses. Left untreated, hypertension can have significant consequences, including increased risk of stroke, coronary heart disease, heart failure, chronic kidney failure, and death. Approximately 70 million adults in the United States have hypertension, but only 60% of them have been diagnosed, and of those only 50% have their blood pressure under control.1 In 2013, 360,000 deaths in the United States were attributed to hypertension.2
Hypertension is associated with major depressive disorder, generalized anxiety disorder, bipolar disorder, and schizophrenia.3-5 Additionally, impulsive eating disorders, substance abuse, anxiety, and depression are associated with a hypertension diagnosis, although patients with panic disorder develop hypertension at a younger age.6 A 2007 study found a 61% prevalence of hypertension in those with bipolar disorder compared with 41% among the general population.7 The strong link between bipolar disorder and hypertension might be because of a common disease mechanism; both are associated with hyperactive cellular calcium signaling and increased platelet intracellular calcium ion concentrations.8
Hypertension not only is common among patients with psychiatric illness, it likely contributes to worse clinical outcomes. Studies across different cultures have found higher mortality rates in individuals with mental illness.9-11 Persons with schizophrenia and other severe mental illnesses may lose ≥25 years of life expectancy, with the primary cause of death being cardiovascular disease, not suicide.12 Patients with depression have a 50% greater risk of cardiovascular disease, which is equivalent to the risk of smoking.13
Schizophrenia is strongly associated with numerous comorbidities and has been linked significantly to an elevated 10-year cardiac risk after controlling for body mass index.5 The high rate of non-treatment of hypertension for patients with schizophrenia (62.4%) is especially concerning.14
Because of the well-documented morbidity and mortality of hypertension and its increased prevalence and undertreatment in the psychiatric population, mental health providers are in an important position to recognize hypertension and evaluate its inherent risks to direct their patients toward proper treatment. This article reviews:
- the signs and symptoms of hypertension
- the mental health provider’s role in the evaluation and diagnosis
- how psychotropic drugs influence blood pressure and drug–drug interactions
- the management of hypertension in psychiatric patients, including strategies for counseling and lifestyle management.
Diagnosing hypertension
Hypertension is defined as a blood pressure >140/90 mm Hg, the average of ≥2 properly measured readings at ≥2 visits in a medical setting.15 The proper equipment, including a well-fitting blood pressure cuff, and technique to measure blood pressure are essential to avoid misdiagnosis. The patient should be at rest for ≥5 minutes, without active pain or emotional distress.
Most cases of hypertension (90% to 95%) are primary, commonly called essential hypertension. However, the differential diagnosis also should consider secondary causes, which may include:
- obesity
- medications
- chronic alcohol use
- methamphetamine or cocaine use
- primary kidney disease
- atherosclerotic renal artery stenosis
- obstructive sleep apnea
- hypothyroidism
- primary hyperaldosteronism
- narrowing of the aorta
- Cushing syndrome
- primary hyperparathyroidism
- polycythemia
- pheochromocytoma.
Medical evaluation. Once the diagnosis of hypertension is made, a medical evaluation is indicated to determine if the patient has end-organ damage from the elevated pressures, such as renal disease or heart disease, to identify other modifiable cardiovascular risk factors, such as hyperlipidemia, and to screen for secondary causes of hypertension. This evaluation includes15:
- a physical exam
- review of medications
- lipid profile
- urinalysis to screen for proteinuria
- serum electrolytes and creatinine
- electrocardiogram to screen for left ventricular hypertrophy or prior infarction
- fasting glucose or hemoglobin A1c to screen for type 2 diabetes mellitus.
Psychotropic drugs. In psychiatric patients, the evaluation must consider the potential impact psychotropic drug effects and drug–drug interactions can have on blood pressure (Table 2). For example, patients taking both diuretics and lithium are at increased risk for dehydration and increased serum lithium levels, which could cause severe neurologic symptoms and renal insufficiency.16 Several antihypertensives when taken with venlafaxine can increase blood pressure, but antihypertensives with α-1 blocking psychotropics can decrease blood pressure. Monoamine oxidase inhibitors can cause hypotension or hypertension with various classes of antihypertensives. Stimulants, such as methylphenidate, atomoxetine, dextroamphetamine, armodafinil, or modafinil, alone or combined with antihypertensives, can cause hypertension.17
Substance abuse, particularly alcohol, methamphetamine, and cocaine, can cause difficulty controlling blood pressure. Patients with refractory hypertension should have a reassessment of substance abuse as a potential cause.
Screening guidelines for mental health providers
For many patients with severe mental illness, visits to their mental health providers might be their only contact with the medical system. Therefore, screening in the mental health settings could detect cases that otherwise would be missed.
Screening recommendations. The U.S. Preventive Services Task Force recommends screening for hypertension in the general population beginning at age 18.18 Adults age 18 to 39 with normal blood pressure (<130/85 mm Hg) and no other risk factors (eg, overweight, obese, or African American) can be screened every 3 years. Those with risk factors or a blood pressure of 130/85 to 139/89 mm Hg and adults age ≥40 should have annual screenings.
Ideally, psychiatrists and other mental health providers should monitor blood pressure at each visit, especially in patients taking psychotropics because of their higher risk for hypertension.
Optimizing treatment. Once the diagnosis of essential hypertension is established, identifying psychiatric comorbidities and the severity of psychiatric symptoms are important to optimize treatment adherence. Patients with increased depressive symptoms are less likely to comply with antihypertensive medication,19 and patients with confirmed depression are 3 times more likely to not adhere to medical treatment recommendations than non-depressed patients.20
Physicians’ attitudes toward hypertension also can affect patients’ compliance and blood pressure control.21 Psychiatrists should be empathetic and motivational toward patients attempting to control their blood pressure. The Seventh Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure states, “Motivation improves when patients have positive experiences with, and trust in, the clinician. Empathy builds trust and is a potent motivator.”22
Treatment and management
Treatment of hypertension significantly reduces the risk of stroke, myocardial infarction, renal injury, heart failure, and premature death. Studies show that treatment that reduces systolic blood pressure by 12 mm Hg over 10 years will prevent 1 death for every 11 patients with essential hypertension. In those with concomitant cardiovascular disease or target organ damage, such a reduction would prevent death in 1 of every 9 patients treated.15Blood pressure goals. The 2014 Eighth Joint National Committee Guideline for Management of High Blood Pressure in Adults provides guidance on blood pressure goals depending on patients’ underlying medical history (Figure).23 Based on expert opinion and randomized controlled studies, blood pressure goals for patients without diabetes or chronic kidney disease (CKD)—an estimated or measured glomerular filtration rate (GFR) of ≤60 mL/min/1.73 m2—depend on age: <140/90 mm Hg for age 18 to 59 and <150/90 mm Hg for age ≥60. For patients with diabetes or CKD, the blood pressure goal is <140/90 mm Hg, regardless of age.

However, not all experts agree on these specific blood pressure goals. A major trial (SPRINT) published in 2015 found that intensive blood pressure goals do benefit higher-risk, non-diabetic patients.24 Specifically, the study randomized patients age ≥50 with systolic blood pressure of 130 to 180 mm Hg and increased cardiovascular risk to systolic blood pressure targets of <140 mm Hg (standard) or <120 mm Hg (intensive). Characteristics of increased cardiovascular risk were clinical or subclinical cardiovascular disease other than stroke, CKD with GFR of 20 to 60 mL/min/1.73 m2, age ≥75, or Framingham 10-year coronary heart disease risk score ≥15%. Intensive treatment significantly reduced overall mortality and the rate of acute coronary syndrome, myocardial infarction, heart failure, stroke, or cardiovascular death. However, the results of this study have not been assimilated into any recent guidelines. Therefore, consider a goal of <120 mm Hg for non-diabetic patients age ≥50 with any of these factors.
Lifestyle modifications. Psychiatrists are well equipped to motivate and encourage behavioral modification in patients with hypertension. Counseling and structured training courses could help to effectively lower blood pressure.25 Patients should receive education on lifestyle modifications including:
- weight reduction
- physical activity
- moderate alcohol consumption
- decreased sodium consumption
- implementation of the Dietary Approaches to Stop Hypertension (DASH) or Mediterranean diets.15
Maintaining a normal body weight is ideal, but weight reduction of 10 lb can reduce blood pressure in overweight patients. The DASH diet, consisting of fruits, vegetables, low-fat dairy products, high calcium and potassium intake, and reduced saturated and total fat intake can decrease systolic blood pressure from 8 to 14 mm Hg. Reduction of sodium intake to ≤2,400 mg/d can reduce systolic blood pressure from 2 to 8 mm Hg. Regular aerobic exercise of 30 minutes a day most days of the week can reduce systolic blood pressure up to 9 mm Hg. Patients also should be encouraged to quit smoking. Patients who implement ≥2 these modifications get better results.
Antihypertensive medications. Patients who do not reach their goals with lifestyle measures alone should receive antihypertensive medications. Most patients will require ≥2 agents to control their blood pressure. Clinical trials show that some patient subgroups have better outcomes with different first-line agents.
For example, in non-African American patients, thiazide diuretics, calcium channel blockers, angiotensin receptor blockers, and angiotensin-converting enzyme inhibitors are first-line treatments (Table 3). For African American patients without CKD, first-line treatments should be thiazide diuretics and calcium channel blockers, because angiotensin-converting enzyme inhibitors and angiotensin receptor blockers do not reduce cardiovascular events as effectively. African American patients with CKD and proteinuria, however, benefit from angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and are preferred first-line agents. However, blood pressure control is a more important factor in improving outcomes than the choice of medication.

Psychiatrists’ role. Psychiatrists should aim to collaborate with the primary care provider when treating hypertension. However, when integrative care is not possible, they should start a first-line medication with follow-up in 1 month or sooner for patients with severe hypertension (>160/100 mm Hg) or significant comorbidities (eg, CKD, congestive heart failure, coronary disease). Patients with blood pressure >160/100 mm Hg often are started on a thiazide diuretic with one other medication because a single agent usually does not achieve goal blood pressure. Patients with CKD need close monitoring of potassium and creatinine when starting angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy, usually within 1 to 2 days of starting or adjusting their medication. Adjust or add medication dosages monthly until blood pressure goals are reached.
A general internist, cardiologist, or nephrologist who has expertise in managing complex cases should oversee care of a psychiatric patient in any of the following scenarios:
- suspected secondary cause of hypertension
- adverse reaction to antihypertensive medications
- complicated comorbid conditions (ie, creatinine >1.8 mg/dL, worsening renal failure, hyperkalemia, heart failure, coronary disease)
- blood pressure >180/120 mm Hg
- requires ≥3 antihypertensive medications.
Summing up
Hypertension is a significant comorbidity in many psychiatric patients, but usually is asymptomatic. Often the psychiatrist or other mental health provider will diagnose hypertension because of their frequent contact with these patients. Once the diagnosis is made, an initial evaluation can direct lifestyle modifications. Patients who continue to have significant elevation of blood pressure should start pharmacotherapy, either by the psychiatrist or by ensuring follow-up with a primary care physician. The psychiatrist may be able to manage cases of essential hypertension, but always must be vigilant for potential drug–disease or drug–drug interactions during treatment. A team-based approach may improve health outcomes in psychiatric patients.
1. Centers for Disease Control and Prevention (CDC). Vital signs: awareness and treatment of uncontrolled hypertension among adults—United States, 2003-2010. MMWR Morb Mortal Wkly Rep. 2012;61:703-709.
2. Mozzafarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322.
3. Carroll D, Phillips AC, Gale CR, et al. Generalized anxiety and major depressive disorders, their comorbidity and hypertension in middle-aged men. Psychosom Med. 2010;72(1):16-19.
4. Leboyer M, Soreca I, Scott J, et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord. 2012;141(1):1-10.
5. Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80(1):45-53.
6. Stein DJ, Aguilar-Gaxiola S, Alonso J, et al. Associations between mental disorders and subsequent onset of hypertension. Gen Hosp Psychiatry. 2014;36(2):142-149.
7. Birkenaes AB, Opjordsmoen S, Brunborg C, et al. The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study. J Clin Psychiatry. 2007;68(6):917-923.
8. Izzo JL, Black HR, Goodfriend TL. Hypertension primer: the essentials of high blood pressure. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
9. Osby U, Correia N, Brandt L, et al. Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophr Res. 2000;45(1-2):21-28.
10. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
11. Auquier P, Lançon C, Rouillon F, et al. Mortality in schizophrenia. Pharmacoepidemiol Drug Saf. 2007;16(12):1308-1312.
12. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
13. Bowis J, Parvanova A, McDaid D, et al. Mental and Physical Health Charter: bridging the gap between mental and physical health. https://www.idf.org/sites/default/files/Mental%2520and%2520Physical%2520Health%2520Charter%2520-%2520FINAL.pdf. Published October 7, 2009. Accessed March 6, 2017.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2571.
16. Handler J. Lithium and antihypertensive medication: a potentially dangerous interaction. J Clin Hypertens (Greenwich). 2009;11(12):738-742.
17. National Collaborating Centre for Mental Health (UK). Depression in adults with a chronic physical health problem: treatment and Management. Appendix 16: table of drug interactions. http://www.ncbi.nlm.nih.gov/books/NBK82914. Published 2010. Accessed March 6, 2017.
18. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015:163(10):778-786.
19. Wang PS, Bohn RL, Knight E, et al. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17(7):504-511.
20. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
21. Consoli SM, Lemogne C, Levy A, et al. Physicians’ degree of motivation regarding their perception of hypertension, and blood pressure control. J Hypertens. 2010;28(6):1330-1339.
22. National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Improving Hypertension Control. Bethesda, MD: U.S. Department of Health and Human Services; 2004:61-64.
23. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
24. The SPRINT Research Group; Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2016.
25. Boulware LE, Daumit GL, Frick KD, et al. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med. 2001;21(3):221-232.
1. Centers for Disease Control and Prevention (CDC). Vital signs: awareness and treatment of uncontrolled hypertension among adults—United States, 2003-2010. MMWR Morb Mortal Wkly Rep. 2012;61:703-709.
2. Mozzafarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322.
3. Carroll D, Phillips AC, Gale CR, et al. Generalized anxiety and major depressive disorders, their comorbidity and hypertension in middle-aged men. Psychosom Med. 2010;72(1):16-19.
4. Leboyer M, Soreca I, Scott J, et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord. 2012;141(1):1-10.
5. Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80(1):45-53.
6. Stein DJ, Aguilar-Gaxiola S, Alonso J, et al. Associations between mental disorders and subsequent onset of hypertension. Gen Hosp Psychiatry. 2014;36(2):142-149.
7. Birkenaes AB, Opjordsmoen S, Brunborg C, et al. The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study. J Clin Psychiatry. 2007;68(6):917-923.
8. Izzo JL, Black HR, Goodfriend TL. Hypertension primer: the essentials of high blood pressure. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
9. Osby U, Correia N, Brandt L, et al. Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophr Res. 2000;45(1-2):21-28.
10. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
11. Auquier P, Lançon C, Rouillon F, et al. Mortality in schizophrenia. Pharmacoepidemiol Drug Saf. 2007;16(12):1308-1312.
12. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
13. Bowis J, Parvanova A, McDaid D, et al. Mental and Physical Health Charter: bridging the gap between mental and physical health. https://www.idf.org/sites/default/files/Mental%2520and%2520Physical%2520Health%2520Charter%2520-%2520FINAL.pdf. Published October 7, 2009. Accessed March 6, 2017.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2571.
16. Handler J. Lithium and antihypertensive medication: a potentially dangerous interaction. J Clin Hypertens (Greenwich). 2009;11(12):738-742.
17. National Collaborating Centre for Mental Health (UK). Depression in adults with a chronic physical health problem: treatment and Management. Appendix 16: table of drug interactions. http://www.ncbi.nlm.nih.gov/books/NBK82914. Published 2010. Accessed March 6, 2017.
18. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015:163(10):778-786.
19. Wang PS, Bohn RL, Knight E, et al. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17(7):504-511.
20. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
21. Consoli SM, Lemogne C, Levy A, et al. Physicians’ degree of motivation regarding their perception of hypertension, and blood pressure control. J Hypertens. 2010;28(6):1330-1339.
22. National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Improving Hypertension Control. Bethesda, MD: U.S. Department of Health and Human Services; 2004:61-64.
23. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
24. The SPRINT Research Group; Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2016.
25. Boulware LE, Daumit GL, Frick KD, et al. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med. 2001;21(3):221-232.
Defining ‘remission’
The article “Residual symptoms of schizophrenia: What are realistic treatment goals?” (Evidence-Based Reviews,
Certainly most treatments for schizophrenia and other chronic psychiatric disorders leave some significant residual symptoms. However, the term “remission” should be reserved only for treatment that is sufficiently successful so that no significant residual symptoms remain.
Leonard Korn, MD
Psychiatrist
Portsmouth Regional Hospital
Portsmouth, New Hampshire
Reference
1. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
The authors respond
Dr. Korn makes a valid, relevant point about the correct use of the term “remission” with regard to the residual symptoms of schizophrenia. Although use of “remission” in the context of our article might seem to defy logic, our use of the term is predicated on the Consensus Criteria for remission, which allows for mild symptoms of core schizophrenia psychopathology.1
In 2005, the Remission in Schizophrenia Working Group published an operationally defined criterion for remission using a threshold of severity for core symptoms. The Consensus Criteria aimed to provide researchers and clinicians with a well- defined method to gauge outcomes and facilitate comparisons of the effectiveness of therapy in the long-term treatment of schizophrenia.2 The Consensus Criteria for remission is a highly debated and dynamic topic. Within 5 years after being published, 6 post hoc analyses tested these criteria against other remission criteria in schizophrenia.3
At the beginning of our article, we cited the naturalistic trial by Schennach et al,1 which investigated the discrepancy between remission status using the Consensus Criteria and remaining impairments or “residual symptoms” found in remitted patients. This trial also examined these remaining symptoms that persisted in 236 remitted patients. The symptoms most commonly found were: blunted affect, conceptual disorganization, passive/apathetic social withdrawal, emotional withdrawal, lack of judgment and insight, and poor attention.1
We agree with Dr. Korn’s comment, “Certainly most treatments of schizophrenia and other chronic psychiatric disorders leave some significant residual symptoms.” It is this reason why we find it difficult to eliminate symptoms of schizophrenia with currently available treatment options.
We also agree with his comment, “… the term ‘remission’ should be reserved only for treatment that is sufficiently successful so that no significant residual symptoms remain.” Defining remission as an absolute elimination of symptoms using currently available treatment options might not be practical for schizophrenia. This statement is based on the paucity of occurrences of complete remission or elimination of symptoms in our own patients. Therefore, in our article, we chose not to address the criteria or define the remission of schizophrenia. The primary focus was treating residual symptoms and providing realistic therapeutic goals.
In the future, we expect more effective treatment approaches, and advanced therapeutic goals will incite revisions to the remission criteria to meet even higher treatment expectations. We hope future research will focus on addressing residual symptoms, finding a potential cure for schizophrenia, and better defining the term “remission” and absolute nature for this most complex, chronic illness.
Ahsan Khan, MD, DFAPA, DABAM
Armor Correctional Health Services, Inc.
Oklahoma City, Oklahoma
Department of Psychiatry andBehavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Mona Ghavami, MD
Clinical Documentation Specialist
St. Joseph Medical Center
Kansas City, Missouri
George D. Ide, MD
Post-Graduate Research
CenterPointe Health System
St. Charles, Missouri
Rachna Kalia, MD
Clinical Assistant Professor
Department of Psychiatry and Behavioral Sciences
University of Kansas School ofMedicine-Wichita
Wichita, Kansas
1. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
2. Emsley R, Chiliza B, Asmal L, et al. The concepts of remission and recovery in schizophrenia. Curr Opin Psychiatry. 2011;24(2):114-121.
3. Lambert M, Karow A, Leucht S, et al. Remission in schizophrenia: validity, frequency, predictors, and patients’ perspective 5 years later. Dialogues Clin Neurosci. 2010;12(3):393-407.
The article “Residual symptoms of schizophrenia: What are realistic treatment goals?” (Evidence-Based Reviews,
Certainly most treatments for schizophrenia and other chronic psychiatric disorders leave some significant residual symptoms. However, the term “remission” should be reserved only for treatment that is sufficiently successful so that no significant residual symptoms remain.
Leonard Korn, MD
Psychiatrist
Portsmouth Regional Hospital
Portsmouth, New Hampshire
Reference
1. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
The authors respond
Dr. Korn makes a valid, relevant point about the correct use of the term “remission” with regard to the residual symptoms of schizophrenia. Although use of “remission” in the context of our article might seem to defy logic, our use of the term is predicated on the Consensus Criteria for remission, which allows for mild symptoms of core schizophrenia psychopathology.1
In 2005, the Remission in Schizophrenia Working Group published an operationally defined criterion for remission using a threshold of severity for core symptoms. The Consensus Criteria aimed to provide researchers and clinicians with a well- defined method to gauge outcomes and facilitate comparisons of the effectiveness of therapy in the long-term treatment of schizophrenia.2 The Consensus Criteria for remission is a highly debated and dynamic topic. Within 5 years after being published, 6 post hoc analyses tested these criteria against other remission criteria in schizophrenia.3
At the beginning of our article, we cited the naturalistic trial by Schennach et al,1 which investigated the discrepancy between remission status using the Consensus Criteria and remaining impairments or “residual symptoms” found in remitted patients. This trial also examined these remaining symptoms that persisted in 236 remitted patients. The symptoms most commonly found were: blunted affect, conceptual disorganization, passive/apathetic social withdrawal, emotional withdrawal, lack of judgment and insight, and poor attention.1
We agree with Dr. Korn’s comment, “Certainly most treatments of schizophrenia and other chronic psychiatric disorders leave some significant residual symptoms.” It is this reason why we find it difficult to eliminate symptoms of schizophrenia with currently available treatment options.
We also agree with his comment, “… the term ‘remission’ should be reserved only for treatment that is sufficiently successful so that no significant residual symptoms remain.” Defining remission as an absolute elimination of symptoms using currently available treatment options might not be practical for schizophrenia. This statement is based on the paucity of occurrences of complete remission or elimination of symptoms in our own patients. Therefore, in our article, we chose not to address the criteria or define the remission of schizophrenia. The primary focus was treating residual symptoms and providing realistic therapeutic goals.
In the future, we expect more effective treatment approaches, and advanced therapeutic goals will incite revisions to the remission criteria to meet even higher treatment expectations. We hope future research will focus on addressing residual symptoms, finding a potential cure for schizophrenia, and better defining the term “remission” and absolute nature for this most complex, chronic illness.
Ahsan Khan, MD, DFAPA, DABAM
Armor Correctional Health Services, Inc.
Oklahoma City, Oklahoma
Department of Psychiatry andBehavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Mona Ghavami, MD
Clinical Documentation Specialist
St. Joseph Medical Center
Kansas City, Missouri
George D. Ide, MD
Post-Graduate Research
CenterPointe Health System
St. Charles, Missouri
Rachna Kalia, MD
Clinical Assistant Professor
Department of Psychiatry and Behavioral Sciences
University of Kansas School ofMedicine-Wichita
Wichita, Kansas
The article “Residual symptoms of schizophrenia: What are realistic treatment goals?” (Evidence-Based Reviews,
Certainly most treatments for schizophrenia and other chronic psychiatric disorders leave some significant residual symptoms. However, the term “remission” should be reserved only for treatment that is sufficiently successful so that no significant residual symptoms remain.
Leonard Korn, MD
Psychiatrist
Portsmouth Regional Hospital
Portsmouth, New Hampshire
Reference
1. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
The authors respond
Dr. Korn makes a valid, relevant point about the correct use of the term “remission” with regard to the residual symptoms of schizophrenia. Although use of “remission” in the context of our article might seem to defy logic, our use of the term is predicated on the Consensus Criteria for remission, which allows for mild symptoms of core schizophrenia psychopathology.1
In 2005, the Remission in Schizophrenia Working Group published an operationally defined criterion for remission using a threshold of severity for core symptoms. The Consensus Criteria aimed to provide researchers and clinicians with a well- defined method to gauge outcomes and facilitate comparisons of the effectiveness of therapy in the long-term treatment of schizophrenia.2 The Consensus Criteria for remission is a highly debated and dynamic topic. Within 5 years after being published, 6 post hoc analyses tested these criteria against other remission criteria in schizophrenia.3
At the beginning of our article, we cited the naturalistic trial by Schennach et al,1 which investigated the discrepancy between remission status using the Consensus Criteria and remaining impairments or “residual symptoms” found in remitted patients. This trial also examined these remaining symptoms that persisted in 236 remitted patients. The symptoms most commonly found were: blunted affect, conceptual disorganization, passive/apathetic social withdrawal, emotional withdrawal, lack of judgment and insight, and poor attention.1
We agree with Dr. Korn’s comment, “Certainly most treatments of schizophrenia and other chronic psychiatric disorders leave some significant residual symptoms.” It is this reason why we find it difficult to eliminate symptoms of schizophrenia with currently available treatment options.
We also agree with his comment, “… the term ‘remission’ should be reserved only for treatment that is sufficiently successful so that no significant residual symptoms remain.” Defining remission as an absolute elimination of symptoms using currently available treatment options might not be practical for schizophrenia. This statement is based on the paucity of occurrences of complete remission or elimination of symptoms in our own patients. Therefore, in our article, we chose not to address the criteria or define the remission of schizophrenia. The primary focus was treating residual symptoms and providing realistic therapeutic goals.
In the future, we expect more effective treatment approaches, and advanced therapeutic goals will incite revisions to the remission criteria to meet even higher treatment expectations. We hope future research will focus on addressing residual symptoms, finding a potential cure for schizophrenia, and better defining the term “remission” and absolute nature for this most complex, chronic illness.
Ahsan Khan, MD, DFAPA, DABAM
Armor Correctional Health Services, Inc.
Oklahoma City, Oklahoma
Department of Psychiatry andBehavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Mona Ghavami, MD
Clinical Documentation Specialist
St. Joseph Medical Center
Kansas City, Missouri
George D. Ide, MD
Post-Graduate Research
CenterPointe Health System
St. Charles, Missouri
Rachna Kalia, MD
Clinical Assistant Professor
Department of Psychiatry and Behavioral Sciences
University of Kansas School ofMedicine-Wichita
Wichita, Kansas
1. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
2. Emsley R, Chiliza B, Asmal L, et al. The concepts of remission and recovery in schizophrenia. Curr Opin Psychiatry. 2011;24(2):114-121.
3. Lambert M, Karow A, Leucht S, et al. Remission in schizophrenia: validity, frequency, predictors, and patients’ perspective 5 years later. Dialogues Clin Neurosci. 2010;12(3):393-407.
1. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
2. Emsley R, Chiliza B, Asmal L, et al. The concepts of remission and recovery in schizophrenia. Curr Opin Psychiatry. 2011;24(2):114-121.
3. Lambert M, Karow A, Leucht S, et al. Remission in schizophrenia: validity, frequency, predictors, and patients’ perspective 5 years later. Dialogues Clin Neurosci. 2010;12(3):393-407.
How your body language affects patient care
Patient surveys reveal communication to be one of the most important competencies a physician should possess.1 However, communication is not only what is spoken. A physician’s nonverbal communication or “body language” sets the trajectory for treatment from the moment the patient first sees the physician. Body language includes all forms of communication other than words,2 such as vocal tone, posture, and facial and body movements. Being mindful of such behaviors can provide physicians with greater access to their patients. Effective nonverbal communication can have significant effects on patient engagement, compliance, and outcome.
First impressions
The physician’s nonverbal behavior is crucial to the patient’s impression of his (her) physician.3 Appropriate eye gaze, proper distance or forward lean, direct body orientation, uncrossed legs and arms, and arm symmetry also have been associated with patient reports of satisfaction.3,4 A physician who displays these affiliative nonverbal behaviors is more likely to engage with the patient and be rated higher for patient satisfaction.5,6 Once a patient has developed rapport and an alliance with the physician and is satisfied with care, you likely will see improvements in patient adherence.
Adherence to treatment
The physician’s ability to verbally and nonverbally communicate a safe, encouraging, and efficient relationship is crucial for patient adherence to treatment. Patients report greater alliance with their physicians when they perceive genuine engagement and concern.7 The physician showing interest impacts the patient’s rating of the relationship6 and provides confidence that the physician is sensitive and understanding.8 As a result, the patient is more trusting and communicative, which allows for greater progress in the patient’s care because it often leads to attending appointments as well as medication adherence.9
Medication nonadherence is a complex issue that is influenced by several factors,10 but it is widely accepted that lack of communication and patient education are important factors.11 Nonverbal communication can help the clinician to distinguish patients who are unwilling to take medication from those who are willing but unable to do so.11
Overall adherence with care also can be affected by nonverbal behaviors. Positive perception of the physician’s tone of voice has been associated with greater attendance at appointments,12 greater referral rates to alcohol abuse treatment clinics,13 and lower rates of malpractice among surgeons.14 Such trends demonstrate the influence that effective nonverbal communication could have on health care costs by reducing doctor shopping and malpractice rates and increasing effective care.
Outcomes
Physician’s positive nonverbal communication has been linked to positive patient outcomes. Physical therapists who smile, nod, and maintain eye contact compared with those who do not smile or look away from the patient, have been shown to achieve greater short- and long-term improvements in functioning of their patients.15 Perceptions of physicians as distant or detached are associated with poorer patient outcomes.5,6,16 Pain patients with high nonverbal support from their physicians show increased pain tolerance and reduction in the amount of pain expressed, compared with those interacting with low nonverbal support physicians.17 Patients respond more to care if they feel their physician is engaged and sensitive to their needs.
There is much to gain if a physician is mindful of his body language. As Henry A. Nasrallah, MD, Editor-in-Chief of
1. McBride CA, Shugars DA, DiMatteo MR, et al. The physician’s role. Views of the public and the profession on seven aspects of patient care. Arch Fam Med. 1994;3(11):948-953.
2. Knapp ML, Hall JA, Horgan TG. Nonverbal communication in human interaction. 8th ed. Boston, MA: Wadsworth, Cengage Learning; 2014.
3. Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract. 2002;15(1):25-38.
4. Bensing J. Doctor-patient communication and the quality of care. Soc Sci Med. 1991;32(11):1301-1310.
5. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318.
6. Larsen KM, Smith CK. Assessment of nonverbal communication in the patient-physician interview. J Fam Pract. 1981;12(3):481-488.
7. Pinto RZ, Ferreira ML, Oliveira VC, et al. Patient-centred communication is associated with positive therapeutic alliance: a systematic review. J Physiother. 2012;58(2):77-87.
8. DiMatteo MR, Taranta A, Friedman HS, et al. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387.
9. McCabe R, Bullenkamp J, Hansson L, et al. The therapeutic relationship and adherence to antipsychotic medication in schizophrenia. PLoS One. 2012;7(4):e36080.
10. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91.
11. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
12. Cruz M, Roter DL, Weiland M, et al. Appointment length, psychiatrists’ communication behaviors, and medication management appointment adherence. Psychiatr Serv. 2013;64(9):886-892.
13. Milmoe S, Rosenthal R, Blane HT, et al. The doctor’s voice: postdictor of successful referral of alcoholic patients. J Abnorm Psychol. 1967;72(1):78-84.
14. Ambady N, Laplante D, Nguyen T, et al. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9.
15. Ambady N, Koo J, Rosenthal R, et al. Physical therapists’ nonverbal communication predicts geriatric patients’ health outcomes. Psychol Aging. 2002;17(3):443-452.
16. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433.
17. Ruben MA, Blanch-Hartigan D, Hall JA. Nonverbal communication as a pain reliever: the impact of physician supportive nonverbal behavior on experimentally induced pain. Health Commun. 2016;1-7. doi: 10.1080/10410236.2016.1196418.
18. Nasrallah HA. The most powerful placebo is not a pill. Current Psychiatry. 2011;10(8):18-19.
Patient surveys reveal communication to be one of the most important competencies a physician should possess.1 However, communication is not only what is spoken. A physician’s nonverbal communication or “body language” sets the trajectory for treatment from the moment the patient first sees the physician. Body language includes all forms of communication other than words,2 such as vocal tone, posture, and facial and body movements. Being mindful of such behaviors can provide physicians with greater access to their patients. Effective nonverbal communication can have significant effects on patient engagement, compliance, and outcome.
First impressions
The physician’s nonverbal behavior is crucial to the patient’s impression of his (her) physician.3 Appropriate eye gaze, proper distance or forward lean, direct body orientation, uncrossed legs and arms, and arm symmetry also have been associated with patient reports of satisfaction.3,4 A physician who displays these affiliative nonverbal behaviors is more likely to engage with the patient and be rated higher for patient satisfaction.5,6 Once a patient has developed rapport and an alliance with the physician and is satisfied with care, you likely will see improvements in patient adherence.
Adherence to treatment
The physician’s ability to verbally and nonverbally communicate a safe, encouraging, and efficient relationship is crucial for patient adherence to treatment. Patients report greater alliance with their physicians when they perceive genuine engagement and concern.7 The physician showing interest impacts the patient’s rating of the relationship6 and provides confidence that the physician is sensitive and understanding.8 As a result, the patient is more trusting and communicative, which allows for greater progress in the patient’s care because it often leads to attending appointments as well as medication adherence.9
Medication nonadherence is a complex issue that is influenced by several factors,10 but it is widely accepted that lack of communication and patient education are important factors.11 Nonverbal communication can help the clinician to distinguish patients who are unwilling to take medication from those who are willing but unable to do so.11
Overall adherence with care also can be affected by nonverbal behaviors. Positive perception of the physician’s tone of voice has been associated with greater attendance at appointments,12 greater referral rates to alcohol abuse treatment clinics,13 and lower rates of malpractice among surgeons.14 Such trends demonstrate the influence that effective nonverbal communication could have on health care costs by reducing doctor shopping and malpractice rates and increasing effective care.
Outcomes
Physician’s positive nonverbal communication has been linked to positive patient outcomes. Physical therapists who smile, nod, and maintain eye contact compared with those who do not smile or look away from the patient, have been shown to achieve greater short- and long-term improvements in functioning of their patients.15 Perceptions of physicians as distant or detached are associated with poorer patient outcomes.5,6,16 Pain patients with high nonverbal support from their physicians show increased pain tolerance and reduction in the amount of pain expressed, compared with those interacting with low nonverbal support physicians.17 Patients respond more to care if they feel their physician is engaged and sensitive to their needs.
There is much to gain if a physician is mindful of his body language. As Henry A. Nasrallah, MD, Editor-in-Chief of
Patient surveys reveal communication to be one of the most important competencies a physician should possess.1 However, communication is not only what is spoken. A physician’s nonverbal communication or “body language” sets the trajectory for treatment from the moment the patient first sees the physician. Body language includes all forms of communication other than words,2 such as vocal tone, posture, and facial and body movements. Being mindful of such behaviors can provide physicians with greater access to their patients. Effective nonverbal communication can have significant effects on patient engagement, compliance, and outcome.
First impressions
The physician’s nonverbal behavior is crucial to the patient’s impression of his (her) physician.3 Appropriate eye gaze, proper distance or forward lean, direct body orientation, uncrossed legs and arms, and arm symmetry also have been associated with patient reports of satisfaction.3,4 A physician who displays these affiliative nonverbal behaviors is more likely to engage with the patient and be rated higher for patient satisfaction.5,6 Once a patient has developed rapport and an alliance with the physician and is satisfied with care, you likely will see improvements in patient adherence.
Adherence to treatment
The physician’s ability to verbally and nonverbally communicate a safe, encouraging, and efficient relationship is crucial for patient adherence to treatment. Patients report greater alliance with their physicians when they perceive genuine engagement and concern.7 The physician showing interest impacts the patient’s rating of the relationship6 and provides confidence that the physician is sensitive and understanding.8 As a result, the patient is more trusting and communicative, which allows for greater progress in the patient’s care because it often leads to attending appointments as well as medication adherence.9
Medication nonadherence is a complex issue that is influenced by several factors,10 but it is widely accepted that lack of communication and patient education are important factors.11 Nonverbal communication can help the clinician to distinguish patients who are unwilling to take medication from those who are willing but unable to do so.11
Overall adherence with care also can be affected by nonverbal behaviors. Positive perception of the physician’s tone of voice has been associated with greater attendance at appointments,12 greater referral rates to alcohol abuse treatment clinics,13 and lower rates of malpractice among surgeons.14 Such trends demonstrate the influence that effective nonverbal communication could have on health care costs by reducing doctor shopping and malpractice rates and increasing effective care.
Outcomes
Physician’s positive nonverbal communication has been linked to positive patient outcomes. Physical therapists who smile, nod, and maintain eye contact compared with those who do not smile or look away from the patient, have been shown to achieve greater short- and long-term improvements in functioning of their patients.15 Perceptions of physicians as distant or detached are associated with poorer patient outcomes.5,6,16 Pain patients with high nonverbal support from their physicians show increased pain tolerance and reduction in the amount of pain expressed, compared with those interacting with low nonverbal support physicians.17 Patients respond more to care if they feel their physician is engaged and sensitive to their needs.
There is much to gain if a physician is mindful of his body language. As Henry A. Nasrallah, MD, Editor-in-Chief of
1. McBride CA, Shugars DA, DiMatteo MR, et al. The physician’s role. Views of the public and the profession on seven aspects of patient care. Arch Fam Med. 1994;3(11):948-953.
2. Knapp ML, Hall JA, Horgan TG. Nonverbal communication in human interaction. 8th ed. Boston, MA: Wadsworth, Cengage Learning; 2014.
3. Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract. 2002;15(1):25-38.
4. Bensing J. Doctor-patient communication and the quality of care. Soc Sci Med. 1991;32(11):1301-1310.
5. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318.
6. Larsen KM, Smith CK. Assessment of nonverbal communication in the patient-physician interview. J Fam Pract. 1981;12(3):481-488.
7. Pinto RZ, Ferreira ML, Oliveira VC, et al. Patient-centred communication is associated with positive therapeutic alliance: a systematic review. J Physiother. 2012;58(2):77-87.
8. DiMatteo MR, Taranta A, Friedman HS, et al. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387.
9. McCabe R, Bullenkamp J, Hansson L, et al. The therapeutic relationship and adherence to antipsychotic medication in schizophrenia. PLoS One. 2012;7(4):e36080.
10. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91.
11. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
12. Cruz M, Roter DL, Weiland M, et al. Appointment length, psychiatrists’ communication behaviors, and medication management appointment adherence. Psychiatr Serv. 2013;64(9):886-892.
13. Milmoe S, Rosenthal R, Blane HT, et al. The doctor’s voice: postdictor of successful referral of alcoholic patients. J Abnorm Psychol. 1967;72(1):78-84.
14. Ambady N, Laplante D, Nguyen T, et al. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9.
15. Ambady N, Koo J, Rosenthal R, et al. Physical therapists’ nonverbal communication predicts geriatric patients’ health outcomes. Psychol Aging. 2002;17(3):443-452.
16. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433.
17. Ruben MA, Blanch-Hartigan D, Hall JA. Nonverbal communication as a pain reliever: the impact of physician supportive nonverbal behavior on experimentally induced pain. Health Commun. 2016;1-7. doi: 10.1080/10410236.2016.1196418.
18. Nasrallah HA. The most powerful placebo is not a pill. Current Psychiatry. 2011;10(8):18-19.
1. McBride CA, Shugars DA, DiMatteo MR, et al. The physician’s role. Views of the public and the profession on seven aspects of patient care. Arch Fam Med. 1994;3(11):948-953.
2. Knapp ML, Hall JA, Horgan TG. Nonverbal communication in human interaction. 8th ed. Boston, MA: Wadsworth, Cengage Learning; 2014.
3. Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract. 2002;15(1):25-38.
4. Bensing J. Doctor-patient communication and the quality of care. Soc Sci Med. 1991;32(11):1301-1310.
5. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318.
6. Larsen KM, Smith CK. Assessment of nonverbal communication in the patient-physician interview. J Fam Pract. 1981;12(3):481-488.
7. Pinto RZ, Ferreira ML, Oliveira VC, et al. Patient-centred communication is associated with positive therapeutic alliance: a systematic review. J Physiother. 2012;58(2):77-87.
8. DiMatteo MR, Taranta A, Friedman HS, et al. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387.
9. McCabe R, Bullenkamp J, Hansson L, et al. The therapeutic relationship and adherence to antipsychotic medication in schizophrenia. PLoS One. 2012;7(4):e36080.
10. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91.
11. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
12. Cruz M, Roter DL, Weiland M, et al. Appointment length, psychiatrists’ communication behaviors, and medication management appointment adherence. Psychiatr Serv. 2013;64(9):886-892.
13. Milmoe S, Rosenthal R, Blane HT, et al. The doctor’s voice: postdictor of successful referral of alcoholic patients. J Abnorm Psychol. 1967;72(1):78-84.
14. Ambady N, Laplante D, Nguyen T, et al. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9.
15. Ambady N, Koo J, Rosenthal R, et al. Physical therapists’ nonverbal communication predicts geriatric patients’ health outcomes. Psychol Aging. 2002;17(3):443-452.
16. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433.
17. Ruben MA, Blanch-Hartigan D, Hall JA. Nonverbal communication as a pain reliever: the impact of physician supportive nonverbal behavior on experimentally induced pain. Health Commun. 2016;1-7. doi: 10.1080/10410236.2016.1196418.
18. Nasrallah HA. The most powerful placebo is not a pill. Current Psychiatry. 2011;10(8):18-19.
Prescribing is the culmination of extensive medical training and psychologists don’t qualify
Practicing medicine without a license is a crime, but it seems to have become a hollow law. Politicians are now cynically legalizing it by granting prescribing privileges to individuals with no prior foundation of medical training. Perhaps it is because of serious ignorance of the difference between psychiatry and psychology or MD and PhD degrees. Or perhaps it is a quid pro quo to generous donors to their re-election campaigns who seek a convenient shortcut to the 28,000 hours it takes to become a psychiatrist in 8 years of medical school and psychiatric residency—and that comes after 4 years of college.
I recently consulted an attorney to discuss some legal documents. When he asked me what my line of work is, I then asked him if he knew the difference between a psychiatrist and a psychologist. He hesitated before admitting in an embarrassed tone that he did not really know and thought that they were all “shrinks” and very similar. I then informed him that both go through undergraduate college education, albeit taking very different courses, with pre-med scientific emphasis for future psychiatric physicians and predominately psychology emphasis for future psychologists.
However, psychiatrists then attend medical school for 4 years and rotate on multiple hospital-based medical specialties, such as internal medicine, surgery, pediatrics, obstetrics and gynecology, family medicine, neurology, pathology, psychiatry, ophthalmology, dermatology, anesthesia, radiology, otolaryngology, etc.
Psychologists, on the other hand, take additional advanced psychology courses in graduate school and write a dissertation that requires quite a bit of library time. After getting a MD, future psychiatrists spend 4 years in extensive training in residency programs across inpatient wards and outpatient clinics, assessing the physical and mental health of seriously sick patients with emphasis on both pharmacological and psychotherapeutic treatments for serious psychiatric disorders in patients, the majority of whom have comorbid medical conditions as well. Psychologists, on the other hand, spend 1 year of internship after getting their PhD or PsyD degree, essentially focused on developing counseling and psychotherapy skills. By the time they complete their training, psychologists and psychiatrists have disparate skills: heavily medical and pharmacological skills in psychiatrists and strong psychotherapeutic skills in psychologists.
After this long explanation, I asked the attorney what he thought about psychologists seeking prescription privileges. He was astounded that psychologists would attempt to expand this scope of practice through state legislations rather than going through medical training like all physicians. “That would be like practicing medicine without a license, which is a felony,” he said. He wryly added that his fellow malpractice and litigation lawyers will be the big winners while poorly treated patients will be the biggest losers. Being an avid runner, he also added that such a short-cut to prescribe without the requisite years of medial training reminded him of Rosie Ruiz, who snuck into the Boston marathon a couple of miles before the finish line and “won” the race, before she was caught and discredited.1
Psychology is a respected mental health discipline with strong psychotherapy training and orientation. For decades, psychologists have vigorously criticized the medical model of mental disorders that psychiatric physicians employ to diagnose and treat brain disorders that disrupt thinking, emotions, mood, cognition, and behavior. However, about 25 years ago, a small group of militant psychologists brazenly decided to lobby state legislatures to give them the right to prescribe psychotropics, although they have no formal medical training. Psychiatric physicians, represented by the American Psychiatric Association (APA), strongly opposed this initiative and regarded it as reckless disregard of the obvious need for extensive medical training to be able to prescribe drugs that affect every organ in the body, not only the brain. Psychiatric medications are associated with serious risks of morbidity and mortality.2 The ability to safely prescribe any medication represents the tip of a huge iceberg of 8 years of rigorous medical school education and specialty training. Yet, one of the early proponents of prescription privileges for psychologists, Patrick De Leon, sarcastically likened the ability to prescribe drugs to learning how to operate a desktop computer!
Not all psychologists agreed with the political campaign to lobby state legislatures to pass a law authorizing prescriptive privileges for psychologists.3-6 In fact, most academic psychologists oppose it.7 Most of the early supporters had a PsyD degree from professional schools of psychology, not a PhD degree in psychology, which is obtained from a university department of psychology. The National Alliance on Mental Illness is opposed to psychologists prescribing medications.8 Psychiatrists are outraged by this hazardous “solution” to the shortage of psychiatrists and point to the many potential dangers to patients. Some suggested that this is a quick way to enhance psychologists’ income and to generate more revenue for their professional journals and meetings with lucrative pharmaceutical ads and exhibit booths.
The campaign is ongoing, as Idaho became the fifth state to adopt such an ill-conceived “solution” to increasing access to mental health care, despite valiant effort by the APA to lobby against such laws. Although New Mexico (2002), Louisiana (2004), Illinois (2014), and Iowa (2016) have passed prescriptive authority for psychologists before Idaho, the APA has defeated such measures in numerous other states. But the painful truth is that this has been a lengthy political chess game in which psychologists have been gradually gaining ground and “capturing more pieces.”
Here is a brief, common sense rationale as to the need for full medical training necessary before safely and accurately prescribing medications, most of which are synthetic molecules, which are essentially foreign substances, with both benefits and risks detailed in the FDA-approved label of each drug that reaches the medical marketplace.
First: Making an accurate clinical diagnosis. If a patient presents with depression, the clinician must rule out other possible causes before diagnosing it as primary major depressive disorder for which an antidepressant can be prescribed. The panoply of secondary depressions, which are not treated with antidepressants, includes a variety of recreational drug-induced mood changes and dysphoria and depression induced by numerous prescription drugs (such as antihypertensives, hormonal contraceptives, steroids, interferon, proton pump inhibitors, H2 blockers, malaria drugs, etc.).
After drug-induced depression is ruled out, the clinician must rule out the possibility that an underlying medical condition might be causing the depression, which includes disorders such as hypothyroidism and other endocrinopathies, anemia, stroke, heart disease, hyperkalemia, lupus and other autoimmune disorders, cancer, Parkinsonism, etc. Therefore, a targeted exploration of past and current medical history, accompanied by a battery of lab tests (complete blood count, electrolytes, liver and kidney function tests, metabolic profile, thyroid-stimulating hormone, etc.) must be done to systematically arrive at the correct diagnosis. Only then can the proper treatment plan be determined, which may or may not include prescribing an antidepressant.
Conclusion: Medical training and psychiatric residency are required for an accurate diagnosis of a mental disorder. Even physicians with no psychiatric training might not have the full repertoire of knowledge needed to systematically rule out secondary depression.
Second: Drug selection. Psychiatric drugs can have various iatrogenic effects. Thus, the selection of an appropriate prescription medication from the available array of drugs approved for a given psychiatric indication must be safe and consistent with the patient’s medical history and must not potentially exacerbate ≥1 comorbid medical conditions.
Conclusion: Medical training and psychiatric residency are required.
Third: Knowledge of metabolic pathways of each psychiatric medication to be prescribed as well as the metabolic pathway of all other medications (psychiatric and non-psychiatric) the patient receives is essential to avoid adverse drug–drug interactions. This includes the hepatic enzymes (cytochromes), which often are responsible for metabolizing all the psychiatric and non-psychiatric drugs a patient is receiving. Knowledge of inhibitors and inducers of various cytochrome enzymes is vital for selecting a medication that does not cause a pharmacokinetic adverse reaction that can produce serious adverse effects (even death, such as with QTc prolongation) or can cause loss of efficacy of ≥1 medications that the patient is receiving, in addition to the antidepressant. Also, in addition to evaluating hepatic pathways, knowledge of renal excretion of the drug to be selected and the status of the patient’s kidney function or impairment must be evaluated.
Conclusion: Medical training is required.
Fourth: Laboratory ordering and monitoring. Ordering laboratory data during follow-up of a patient receiving a psychotropic drug is necessary to monitor serum concentrations and ensure a therapeutic range, or to check for serious adverse effects on various organ systems that could be affected by many psychiatric drugs (CNS, cardiovascular, gastrointestinal, sexual, endocrine, pulmonary, hepatic, renal, dermatologic, ophthalmologic, etc.).
Conclusion: Medical training is required.
Fifth: General medical treatment. Many patients might require combination drug therapy because of inadequate response to monotherapy. Clinicians must know what is rational and evidence-based polypharmacy and what is irrational, dangerous, or absurd polypharmacy.9 When possible, parsimonious pharmacotherapy should be employed to minimize the number of medications prescribed.10 A patient could experience severe drug–drug reactions that could lead to cardiopulmonary crises. The clinician must be able to examine, intervene, and manage the patient’s medical distress until help arrives.
Conclusion: Medical training is required.
Sixth: Pregnancy. Knowledge about the pharmacotherapeutic aspects of pregnant women with mental illness is critical. Full knowledge about what can or should not be prescribed during pregnancy (ie, avoiding teratogenic agents) is vital for physicians treating women with psychiatric illness who become pregnant.
Conclusion: Medical training is required.
Although I am against prescriptive privileges for psychologists, I want to emphasize how much I appreciate and respect what psychologists do for patients with mental illness. Their psychotherapy skills often are honed beyond those of psychiatrists who, by necessity, focus on medical diagnosis and pharmacotherapeutic management. Combination of pharmacotherapy and psychotherapy has been demonstrated to be superior to medications alone. In the 25 years since psychologists have been eagerly pursuing prescriptive privileges, neuroscience research has revealed the neurobiologic effects of psychotherapy. Many studies have shown that evidence-based psychotherapy can induce the same structural and functional brain changes as medications11,12 and can influence biomarkers that accompany psychiatric disorders just as medications do.13
Psychologists should reconsider the many potential hazards of prescription drugs compared with the relative safety and efficacy of psychotherapy. They should focus on their qualifications and main strength, which is psychotherapy, and collaborate with psychiatrists and nurse practitioners on a biopsychosocial approach to mental illness. They also should realize how physically ill most psychiatric patients are and the complex medical management they need for their myriad comorbidities.
Just as I began this editorial with an anecdote, I will end with an illustrative one as well. As an academic professor for the past 3 decades who has trained and supervised numerous psychiatric residents, I once closely supervised a former PhD psychologist who decided to become a psychiatrist by going to medical school, followed by a 4-year psychiatric residency. I asked her to compare her experience and functioning as a psychologist with her current work as a fourth-year psychiatric resident. Her response was enlightening: She said the 2 professions are vastly different in their knowledge base and in terms of how they conceptualize mental illness from a psychological vs medical model. As for prescribing medications, she added that even after 8 years of extensive medical training as a physician and a psychiatrist, she feels there is still much to learn about psychopharmacology to ensure not only efficacy but also safety, because a majority of psychiatric patients have ≥1 coexisting medical conditions and substance use as well. Based on her own experience as a psychologist who became a psychiatric physician, she was completely opposed to prescriptive privileges for psychologists unless they go to medical school and become eligible to prescribe safely.
This former resident is now a successful academic psychiatrist who continues to hone her psychopharmacology skills. State legislators should listen to professionals like her before they pass a law giving prescriptive authority to psychologists without having to go through the rigors of 28,000 hours of training in the 8 years of medical school and psychiatric residency. Legislators should also understand that like psychologists, social work counselors have hardly any medical training, yet they have never sought prescriptive privileges. That’s clearly rational and wise.
1. Rosie Ruiz tries to steal the Boston marathon. Runner’s World. http://www.runnersworld.com/running-times-info/rosie-ruiz-tries-to-steal-the-boston-marathon. Published July 1, 1980. Accessed May 15, 2017.
2. Nelson, JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: despite deficits in education and knowledge? J Clin Psychol Med Settings. 2003;10(3):211-221.
4. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: a looming health hazard? Clinical Psychology Science and Practice. 2002;9(3):231-248.
5. Kingsbury SJ. Some effects of prescribing privileges. Am Psychol. 1992;47(3):426-427.
6. Pollitt B. Fools gold: psychologists using disingenuous reasoning to mislead legislatures into granting psychologists prescriptive authority. Am J Law Med. 2003;29:489-524.
7. DeNelsky GY. The case against prescription privileges for psychologists. Am Psychol. 1996;51(3):207-212.
8. Walker K. An ethical dilemma: clinical psychologists prescribing psychotropic medications. Issues Ment Health Nurs. 2002;23(1):17-29.
9. Nasrallah HA. Polypharmacy subtypes: the necessary, the reasonable, the ridiculous and the hazardous. Current Psychiatry. 2011;10(4):10-12.
10. Nasrallah HA. Parsimonious pharmacotherapy. Current Psychiatry. 2011;10(5):12-16.
11. Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464-470.
12. Månsson KN, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2015;5:e727.
13. Redei EE, Andrus BM, Kwasny MJ, et al. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl Psychiatry. 2014;4:e442.
Practicing medicine without a license is a crime, but it seems to have become a hollow law. Politicians are now cynically legalizing it by granting prescribing privileges to individuals with no prior foundation of medical training. Perhaps it is because of serious ignorance of the difference between psychiatry and psychology or MD and PhD degrees. Or perhaps it is a quid pro quo to generous donors to their re-election campaigns who seek a convenient shortcut to the 28,000 hours it takes to become a psychiatrist in 8 years of medical school and psychiatric residency—and that comes after 4 years of college.
I recently consulted an attorney to discuss some legal documents. When he asked me what my line of work is, I then asked him if he knew the difference between a psychiatrist and a psychologist. He hesitated before admitting in an embarrassed tone that he did not really know and thought that they were all “shrinks” and very similar. I then informed him that both go through undergraduate college education, albeit taking very different courses, with pre-med scientific emphasis for future psychiatric physicians and predominately psychology emphasis for future psychologists.
However, psychiatrists then attend medical school for 4 years and rotate on multiple hospital-based medical specialties, such as internal medicine, surgery, pediatrics, obstetrics and gynecology, family medicine, neurology, pathology, psychiatry, ophthalmology, dermatology, anesthesia, radiology, otolaryngology, etc.
Psychologists, on the other hand, take additional advanced psychology courses in graduate school and write a dissertation that requires quite a bit of library time. After getting a MD, future psychiatrists spend 4 years in extensive training in residency programs across inpatient wards and outpatient clinics, assessing the physical and mental health of seriously sick patients with emphasis on both pharmacological and psychotherapeutic treatments for serious psychiatric disorders in patients, the majority of whom have comorbid medical conditions as well. Psychologists, on the other hand, spend 1 year of internship after getting their PhD or PsyD degree, essentially focused on developing counseling and psychotherapy skills. By the time they complete their training, psychologists and psychiatrists have disparate skills: heavily medical and pharmacological skills in psychiatrists and strong psychotherapeutic skills in psychologists.
After this long explanation, I asked the attorney what he thought about psychologists seeking prescription privileges. He was astounded that psychologists would attempt to expand this scope of practice through state legislations rather than going through medical training like all physicians. “That would be like practicing medicine without a license, which is a felony,” he said. He wryly added that his fellow malpractice and litigation lawyers will be the big winners while poorly treated patients will be the biggest losers. Being an avid runner, he also added that such a short-cut to prescribe without the requisite years of medial training reminded him of Rosie Ruiz, who snuck into the Boston marathon a couple of miles before the finish line and “won” the race, before she was caught and discredited.1
Psychology is a respected mental health discipline with strong psychotherapy training and orientation. For decades, psychologists have vigorously criticized the medical model of mental disorders that psychiatric physicians employ to diagnose and treat brain disorders that disrupt thinking, emotions, mood, cognition, and behavior. However, about 25 years ago, a small group of militant psychologists brazenly decided to lobby state legislatures to give them the right to prescribe psychotropics, although they have no formal medical training. Psychiatric physicians, represented by the American Psychiatric Association (APA), strongly opposed this initiative and regarded it as reckless disregard of the obvious need for extensive medical training to be able to prescribe drugs that affect every organ in the body, not only the brain. Psychiatric medications are associated with serious risks of morbidity and mortality.2 The ability to safely prescribe any medication represents the tip of a huge iceberg of 8 years of rigorous medical school education and specialty training. Yet, one of the early proponents of prescription privileges for psychologists, Patrick De Leon, sarcastically likened the ability to prescribe drugs to learning how to operate a desktop computer!
Not all psychologists agreed with the political campaign to lobby state legislatures to pass a law authorizing prescriptive privileges for psychologists.3-6 In fact, most academic psychologists oppose it.7 Most of the early supporters had a PsyD degree from professional schools of psychology, not a PhD degree in psychology, which is obtained from a university department of psychology. The National Alliance on Mental Illness is opposed to psychologists prescribing medications.8 Psychiatrists are outraged by this hazardous “solution” to the shortage of psychiatrists and point to the many potential dangers to patients. Some suggested that this is a quick way to enhance psychologists’ income and to generate more revenue for their professional journals and meetings with lucrative pharmaceutical ads and exhibit booths.
The campaign is ongoing, as Idaho became the fifth state to adopt such an ill-conceived “solution” to increasing access to mental health care, despite valiant effort by the APA to lobby against such laws. Although New Mexico (2002), Louisiana (2004), Illinois (2014), and Iowa (2016) have passed prescriptive authority for psychologists before Idaho, the APA has defeated such measures in numerous other states. But the painful truth is that this has been a lengthy political chess game in which psychologists have been gradually gaining ground and “capturing more pieces.”
Here is a brief, common sense rationale as to the need for full medical training necessary before safely and accurately prescribing medications, most of which are synthetic molecules, which are essentially foreign substances, with both benefits and risks detailed in the FDA-approved label of each drug that reaches the medical marketplace.
First: Making an accurate clinical diagnosis. If a patient presents with depression, the clinician must rule out other possible causes before diagnosing it as primary major depressive disorder for which an antidepressant can be prescribed. The panoply of secondary depressions, which are not treated with antidepressants, includes a variety of recreational drug-induced mood changes and dysphoria and depression induced by numerous prescription drugs (such as antihypertensives, hormonal contraceptives, steroids, interferon, proton pump inhibitors, H2 blockers, malaria drugs, etc.).
After drug-induced depression is ruled out, the clinician must rule out the possibility that an underlying medical condition might be causing the depression, which includes disorders such as hypothyroidism and other endocrinopathies, anemia, stroke, heart disease, hyperkalemia, lupus and other autoimmune disorders, cancer, Parkinsonism, etc. Therefore, a targeted exploration of past and current medical history, accompanied by a battery of lab tests (complete blood count, electrolytes, liver and kidney function tests, metabolic profile, thyroid-stimulating hormone, etc.) must be done to systematically arrive at the correct diagnosis. Only then can the proper treatment plan be determined, which may or may not include prescribing an antidepressant.
Conclusion: Medical training and psychiatric residency are required for an accurate diagnosis of a mental disorder. Even physicians with no psychiatric training might not have the full repertoire of knowledge needed to systematically rule out secondary depression.
Second: Drug selection. Psychiatric drugs can have various iatrogenic effects. Thus, the selection of an appropriate prescription medication from the available array of drugs approved for a given psychiatric indication must be safe and consistent with the patient’s medical history and must not potentially exacerbate ≥1 comorbid medical conditions.
Conclusion: Medical training and psychiatric residency are required.
Third: Knowledge of metabolic pathways of each psychiatric medication to be prescribed as well as the metabolic pathway of all other medications (psychiatric and non-psychiatric) the patient receives is essential to avoid adverse drug–drug interactions. This includes the hepatic enzymes (cytochromes), which often are responsible for metabolizing all the psychiatric and non-psychiatric drugs a patient is receiving. Knowledge of inhibitors and inducers of various cytochrome enzymes is vital for selecting a medication that does not cause a pharmacokinetic adverse reaction that can produce serious adverse effects (even death, such as with QTc prolongation) or can cause loss of efficacy of ≥1 medications that the patient is receiving, in addition to the antidepressant. Also, in addition to evaluating hepatic pathways, knowledge of renal excretion of the drug to be selected and the status of the patient’s kidney function or impairment must be evaluated.
Conclusion: Medical training is required.
Fourth: Laboratory ordering and monitoring. Ordering laboratory data during follow-up of a patient receiving a psychotropic drug is necessary to monitor serum concentrations and ensure a therapeutic range, or to check for serious adverse effects on various organ systems that could be affected by many psychiatric drugs (CNS, cardiovascular, gastrointestinal, sexual, endocrine, pulmonary, hepatic, renal, dermatologic, ophthalmologic, etc.).
Conclusion: Medical training is required.
Fifth: General medical treatment. Many patients might require combination drug therapy because of inadequate response to monotherapy. Clinicians must know what is rational and evidence-based polypharmacy and what is irrational, dangerous, or absurd polypharmacy.9 When possible, parsimonious pharmacotherapy should be employed to minimize the number of medications prescribed.10 A patient could experience severe drug–drug reactions that could lead to cardiopulmonary crises. The clinician must be able to examine, intervene, and manage the patient’s medical distress until help arrives.
Conclusion: Medical training is required.
Sixth: Pregnancy. Knowledge about the pharmacotherapeutic aspects of pregnant women with mental illness is critical. Full knowledge about what can or should not be prescribed during pregnancy (ie, avoiding teratogenic agents) is vital for physicians treating women with psychiatric illness who become pregnant.
Conclusion: Medical training is required.
Although I am against prescriptive privileges for psychologists, I want to emphasize how much I appreciate and respect what psychologists do for patients with mental illness. Their psychotherapy skills often are honed beyond those of psychiatrists who, by necessity, focus on medical diagnosis and pharmacotherapeutic management. Combination of pharmacotherapy and psychotherapy has been demonstrated to be superior to medications alone. In the 25 years since psychologists have been eagerly pursuing prescriptive privileges, neuroscience research has revealed the neurobiologic effects of psychotherapy. Many studies have shown that evidence-based psychotherapy can induce the same structural and functional brain changes as medications11,12 and can influence biomarkers that accompany psychiatric disorders just as medications do.13
Psychologists should reconsider the many potential hazards of prescription drugs compared with the relative safety and efficacy of psychotherapy. They should focus on their qualifications and main strength, which is psychotherapy, and collaborate with psychiatrists and nurse practitioners on a biopsychosocial approach to mental illness. They also should realize how physically ill most psychiatric patients are and the complex medical management they need for their myriad comorbidities.
Just as I began this editorial with an anecdote, I will end with an illustrative one as well. As an academic professor for the past 3 decades who has trained and supervised numerous psychiatric residents, I once closely supervised a former PhD psychologist who decided to become a psychiatrist by going to medical school, followed by a 4-year psychiatric residency. I asked her to compare her experience and functioning as a psychologist with her current work as a fourth-year psychiatric resident. Her response was enlightening: She said the 2 professions are vastly different in their knowledge base and in terms of how they conceptualize mental illness from a psychological vs medical model. As for prescribing medications, she added that even after 8 years of extensive medical training as a physician and a psychiatrist, she feels there is still much to learn about psychopharmacology to ensure not only efficacy but also safety, because a majority of psychiatric patients have ≥1 coexisting medical conditions and substance use as well. Based on her own experience as a psychologist who became a psychiatric physician, she was completely opposed to prescriptive privileges for psychologists unless they go to medical school and become eligible to prescribe safely.
This former resident is now a successful academic psychiatrist who continues to hone her psychopharmacology skills. State legislators should listen to professionals like her before they pass a law giving prescriptive authority to psychologists without having to go through the rigors of 28,000 hours of training in the 8 years of medical school and psychiatric residency. Legislators should also understand that like psychologists, social work counselors have hardly any medical training, yet they have never sought prescriptive privileges. That’s clearly rational and wise.
Practicing medicine without a license is a crime, but it seems to have become a hollow law. Politicians are now cynically legalizing it by granting prescribing privileges to individuals with no prior foundation of medical training. Perhaps it is because of serious ignorance of the difference between psychiatry and psychology or MD and PhD degrees. Or perhaps it is a quid pro quo to generous donors to their re-election campaigns who seek a convenient shortcut to the 28,000 hours it takes to become a psychiatrist in 8 years of medical school and psychiatric residency—and that comes after 4 years of college.
I recently consulted an attorney to discuss some legal documents. When he asked me what my line of work is, I then asked him if he knew the difference between a psychiatrist and a psychologist. He hesitated before admitting in an embarrassed tone that he did not really know and thought that they were all “shrinks” and very similar. I then informed him that both go through undergraduate college education, albeit taking very different courses, with pre-med scientific emphasis for future psychiatric physicians and predominately psychology emphasis for future psychologists.
However, psychiatrists then attend medical school for 4 years and rotate on multiple hospital-based medical specialties, such as internal medicine, surgery, pediatrics, obstetrics and gynecology, family medicine, neurology, pathology, psychiatry, ophthalmology, dermatology, anesthesia, radiology, otolaryngology, etc.
Psychologists, on the other hand, take additional advanced psychology courses in graduate school and write a dissertation that requires quite a bit of library time. After getting a MD, future psychiatrists spend 4 years in extensive training in residency programs across inpatient wards and outpatient clinics, assessing the physical and mental health of seriously sick patients with emphasis on both pharmacological and psychotherapeutic treatments for serious psychiatric disorders in patients, the majority of whom have comorbid medical conditions as well. Psychologists, on the other hand, spend 1 year of internship after getting their PhD or PsyD degree, essentially focused on developing counseling and psychotherapy skills. By the time they complete their training, psychologists and psychiatrists have disparate skills: heavily medical and pharmacological skills in psychiatrists and strong psychotherapeutic skills in psychologists.
After this long explanation, I asked the attorney what he thought about psychologists seeking prescription privileges. He was astounded that psychologists would attempt to expand this scope of practice through state legislations rather than going through medical training like all physicians. “That would be like practicing medicine without a license, which is a felony,” he said. He wryly added that his fellow malpractice and litigation lawyers will be the big winners while poorly treated patients will be the biggest losers. Being an avid runner, he also added that such a short-cut to prescribe without the requisite years of medial training reminded him of Rosie Ruiz, who snuck into the Boston marathon a couple of miles before the finish line and “won” the race, before she was caught and discredited.1
Psychology is a respected mental health discipline with strong psychotherapy training and orientation. For decades, psychologists have vigorously criticized the medical model of mental disorders that psychiatric physicians employ to diagnose and treat brain disorders that disrupt thinking, emotions, mood, cognition, and behavior. However, about 25 years ago, a small group of militant psychologists brazenly decided to lobby state legislatures to give them the right to prescribe psychotropics, although they have no formal medical training. Psychiatric physicians, represented by the American Psychiatric Association (APA), strongly opposed this initiative and regarded it as reckless disregard of the obvious need for extensive medical training to be able to prescribe drugs that affect every organ in the body, not only the brain. Psychiatric medications are associated with serious risks of morbidity and mortality.2 The ability to safely prescribe any medication represents the tip of a huge iceberg of 8 years of rigorous medical school education and specialty training. Yet, one of the early proponents of prescription privileges for psychologists, Patrick De Leon, sarcastically likened the ability to prescribe drugs to learning how to operate a desktop computer!
Not all psychologists agreed with the political campaign to lobby state legislatures to pass a law authorizing prescriptive privileges for psychologists.3-6 In fact, most academic psychologists oppose it.7 Most of the early supporters had a PsyD degree from professional schools of psychology, not a PhD degree in psychology, which is obtained from a university department of psychology. The National Alliance on Mental Illness is opposed to psychologists prescribing medications.8 Psychiatrists are outraged by this hazardous “solution” to the shortage of psychiatrists and point to the many potential dangers to patients. Some suggested that this is a quick way to enhance psychologists’ income and to generate more revenue for their professional journals and meetings with lucrative pharmaceutical ads and exhibit booths.
The campaign is ongoing, as Idaho became the fifth state to adopt such an ill-conceived “solution” to increasing access to mental health care, despite valiant effort by the APA to lobby against such laws. Although New Mexico (2002), Louisiana (2004), Illinois (2014), and Iowa (2016) have passed prescriptive authority for psychologists before Idaho, the APA has defeated such measures in numerous other states. But the painful truth is that this has been a lengthy political chess game in which psychologists have been gradually gaining ground and “capturing more pieces.”
Here is a brief, common sense rationale as to the need for full medical training necessary before safely and accurately prescribing medications, most of which are synthetic molecules, which are essentially foreign substances, with both benefits and risks detailed in the FDA-approved label of each drug that reaches the medical marketplace.
First: Making an accurate clinical diagnosis. If a patient presents with depression, the clinician must rule out other possible causes before diagnosing it as primary major depressive disorder for which an antidepressant can be prescribed. The panoply of secondary depressions, which are not treated with antidepressants, includes a variety of recreational drug-induced mood changes and dysphoria and depression induced by numerous prescription drugs (such as antihypertensives, hormonal contraceptives, steroids, interferon, proton pump inhibitors, H2 blockers, malaria drugs, etc.).
After drug-induced depression is ruled out, the clinician must rule out the possibility that an underlying medical condition might be causing the depression, which includes disorders such as hypothyroidism and other endocrinopathies, anemia, stroke, heart disease, hyperkalemia, lupus and other autoimmune disorders, cancer, Parkinsonism, etc. Therefore, a targeted exploration of past and current medical history, accompanied by a battery of lab tests (complete blood count, electrolytes, liver and kidney function tests, metabolic profile, thyroid-stimulating hormone, etc.) must be done to systematically arrive at the correct diagnosis. Only then can the proper treatment plan be determined, which may or may not include prescribing an antidepressant.
Conclusion: Medical training and psychiatric residency are required for an accurate diagnosis of a mental disorder. Even physicians with no psychiatric training might not have the full repertoire of knowledge needed to systematically rule out secondary depression.
Second: Drug selection. Psychiatric drugs can have various iatrogenic effects. Thus, the selection of an appropriate prescription medication from the available array of drugs approved for a given psychiatric indication must be safe and consistent with the patient’s medical history and must not potentially exacerbate ≥1 comorbid medical conditions.
Conclusion: Medical training and psychiatric residency are required.
Third: Knowledge of metabolic pathways of each psychiatric medication to be prescribed as well as the metabolic pathway of all other medications (psychiatric and non-psychiatric) the patient receives is essential to avoid adverse drug–drug interactions. This includes the hepatic enzymes (cytochromes), which often are responsible for metabolizing all the psychiatric and non-psychiatric drugs a patient is receiving. Knowledge of inhibitors and inducers of various cytochrome enzymes is vital for selecting a medication that does not cause a pharmacokinetic adverse reaction that can produce serious adverse effects (even death, such as with QTc prolongation) or can cause loss of efficacy of ≥1 medications that the patient is receiving, in addition to the antidepressant. Also, in addition to evaluating hepatic pathways, knowledge of renal excretion of the drug to be selected and the status of the patient’s kidney function or impairment must be evaluated.
Conclusion: Medical training is required.
Fourth: Laboratory ordering and monitoring. Ordering laboratory data during follow-up of a patient receiving a psychotropic drug is necessary to monitor serum concentrations and ensure a therapeutic range, or to check for serious adverse effects on various organ systems that could be affected by many psychiatric drugs (CNS, cardiovascular, gastrointestinal, sexual, endocrine, pulmonary, hepatic, renal, dermatologic, ophthalmologic, etc.).
Conclusion: Medical training is required.
Fifth: General medical treatment. Many patients might require combination drug therapy because of inadequate response to monotherapy. Clinicians must know what is rational and evidence-based polypharmacy and what is irrational, dangerous, or absurd polypharmacy.9 When possible, parsimonious pharmacotherapy should be employed to minimize the number of medications prescribed.10 A patient could experience severe drug–drug reactions that could lead to cardiopulmonary crises. The clinician must be able to examine, intervene, and manage the patient’s medical distress until help arrives.
Conclusion: Medical training is required.
Sixth: Pregnancy. Knowledge about the pharmacotherapeutic aspects of pregnant women with mental illness is critical. Full knowledge about what can or should not be prescribed during pregnancy (ie, avoiding teratogenic agents) is vital for physicians treating women with psychiatric illness who become pregnant.
Conclusion: Medical training is required.
Although I am against prescriptive privileges for psychologists, I want to emphasize how much I appreciate and respect what psychologists do for patients with mental illness. Their psychotherapy skills often are honed beyond those of psychiatrists who, by necessity, focus on medical diagnosis and pharmacotherapeutic management. Combination of pharmacotherapy and psychotherapy has been demonstrated to be superior to medications alone. In the 25 years since psychologists have been eagerly pursuing prescriptive privileges, neuroscience research has revealed the neurobiologic effects of psychotherapy. Many studies have shown that evidence-based psychotherapy can induce the same structural and functional brain changes as medications11,12 and can influence biomarkers that accompany psychiatric disorders just as medications do.13
Psychologists should reconsider the many potential hazards of prescription drugs compared with the relative safety and efficacy of psychotherapy. They should focus on their qualifications and main strength, which is psychotherapy, and collaborate with psychiatrists and nurse practitioners on a biopsychosocial approach to mental illness. They also should realize how physically ill most psychiatric patients are and the complex medical management they need for their myriad comorbidities.
Just as I began this editorial with an anecdote, I will end with an illustrative one as well. As an academic professor for the past 3 decades who has trained and supervised numerous psychiatric residents, I once closely supervised a former PhD psychologist who decided to become a psychiatrist by going to medical school, followed by a 4-year psychiatric residency. I asked her to compare her experience and functioning as a psychologist with her current work as a fourth-year psychiatric resident. Her response was enlightening: She said the 2 professions are vastly different in their knowledge base and in terms of how they conceptualize mental illness from a psychological vs medical model. As for prescribing medications, she added that even after 8 years of extensive medical training as a physician and a psychiatrist, she feels there is still much to learn about psychopharmacology to ensure not only efficacy but also safety, because a majority of psychiatric patients have ≥1 coexisting medical conditions and substance use as well. Based on her own experience as a psychologist who became a psychiatric physician, she was completely opposed to prescriptive privileges for psychologists unless they go to medical school and become eligible to prescribe safely.
This former resident is now a successful academic psychiatrist who continues to hone her psychopharmacology skills. State legislators should listen to professionals like her before they pass a law giving prescriptive authority to psychologists without having to go through the rigors of 28,000 hours of training in the 8 years of medical school and psychiatric residency. Legislators should also understand that like psychologists, social work counselors have hardly any medical training, yet they have never sought prescriptive privileges. That’s clearly rational and wise.
1. Rosie Ruiz tries to steal the Boston marathon. Runner’s World. http://www.runnersworld.com/running-times-info/rosie-ruiz-tries-to-steal-the-boston-marathon. Published July 1, 1980. Accessed May 15, 2017.
2. Nelson, JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: despite deficits in education and knowledge? J Clin Psychol Med Settings. 2003;10(3):211-221.
4. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: a looming health hazard? Clinical Psychology Science and Practice. 2002;9(3):231-248.
5. Kingsbury SJ. Some effects of prescribing privileges. Am Psychol. 1992;47(3):426-427.
6. Pollitt B. Fools gold: psychologists using disingenuous reasoning to mislead legislatures into granting psychologists prescriptive authority. Am J Law Med. 2003;29:489-524.
7. DeNelsky GY. The case against prescription privileges for psychologists. Am Psychol. 1996;51(3):207-212.
8. Walker K. An ethical dilemma: clinical psychologists prescribing psychotropic medications. Issues Ment Health Nurs. 2002;23(1):17-29.
9. Nasrallah HA. Polypharmacy subtypes: the necessary, the reasonable, the ridiculous and the hazardous. Current Psychiatry. 2011;10(4):10-12.
10. Nasrallah HA. Parsimonious pharmacotherapy. Current Psychiatry. 2011;10(5):12-16.
11. Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464-470.
12. Månsson KN, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2015;5:e727.
13. Redei EE, Andrus BM, Kwasny MJ, et al. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl Psychiatry. 2014;4:e442.
1. Rosie Ruiz tries to steal the Boston marathon. Runner’s World. http://www.runnersworld.com/running-times-info/rosie-ruiz-tries-to-steal-the-boston-marathon. Published July 1, 1980. Accessed May 15, 2017.
2. Nelson, JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: despite deficits in education and knowledge? J Clin Psychol Med Settings. 2003;10(3):211-221.
4. Robiner WN, Bearman DL, Berman M, et al. Prescriptive authority for psychologists: a looming health hazard? Clinical Psychology Science and Practice. 2002;9(3):231-248.
5. Kingsbury SJ. Some effects of prescribing privileges. Am Psychol. 1992;47(3):426-427.
6. Pollitt B. Fools gold: psychologists using disingenuous reasoning to mislead legislatures into granting psychologists prescriptive authority. Am J Law Med. 2003;29:489-524.
7. DeNelsky GY. The case against prescription privileges for psychologists. Am Psychol. 1996;51(3):207-212.
8. Walker K. An ethical dilemma: clinical psychologists prescribing psychotropic medications. Issues Ment Health Nurs. 2002;23(1):17-29.
9. Nasrallah HA. Polypharmacy subtypes: the necessary, the reasonable, the ridiculous and the hazardous. Current Psychiatry. 2011;10(4):10-12.
10. Nasrallah HA. Parsimonious pharmacotherapy. Current Psychiatry. 2011;10(5):12-16.
11. Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464-470.
12. Månsson KN, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2015;5:e727.
13. Redei EE, Andrus BM, Kwasny MJ, et al. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl Psychiatry. 2014;4:e442.
Are E-cigarettes effective for smoking cessation?
E-cigarettes and vapes: Do they work for smoking cessation and should we be recommending their use?
The popularity of electronic cigarettes (E-cigs) and “vapes” has grown dramatically, spawning a new industry of electronic nicotine delivery systems (ENDS). With the increasing use of E-cigs not only for smoking cessation, but also as a primary nicotine source, it is important for mental health professionals to be prepared to discuss use of these devices with patients. In this article, we will describe:
- the composition of E-cigs and their current use
- evidence for their use for smoking cessation
- adverse health effects
- recommendations of major regulatory agencies.
Finally, we will provide recommendations for E-cig use in clinical populations.
What is an electronic nicotine delivery system?
ENDS produce an aerosol with or without nicotine that is inhaled and is thought to mimic the use of combustible cigarettes. ENDS evolved from basic E-cigs into a less “cigarette-like” and more customizable product (Figure 1). ENDS include a range of designs and go by various names, including “personal vaporizers,” “e-cigars,” and “e-hookahs” (in this article, we will use the term “ENDS” to refer to these devices).
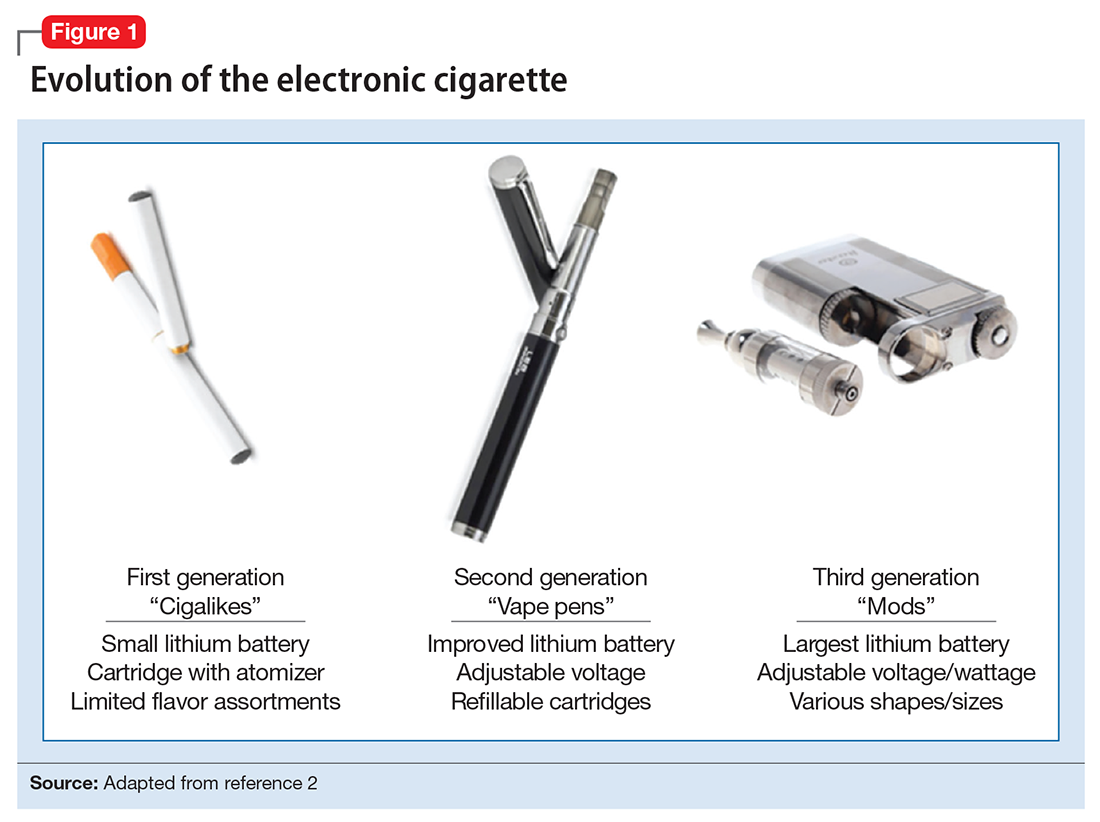
The general design of ENDS is a plastic tubing system that contains a mouthpiece, battery, electronic heating element (“vaporizer”), and a cartridge with liquid solvent with or without nicotine or flavoring (Figure 2). One draw on the mouthpiece or press of a button activates the device, heats the solution, and delivers a vapor in a similar manner to taking a puff of a cigarette. Although studies have shown that ENDS result in significant increases in plasma nicotine concentrations in 5 minutes,1 the plasma nicotine levels obtained with the first-generation “cigarette-like” ENDS are much lower than those caused by inhaling tobacco smoke.2 Over time nicotine delivery capability has improved as ENDS have evolved such that the rate of nicotine delivery and peak concentration obtained with newer models more closely mirror tobacco cigarettes.3 Whether the rapid delivery of larger amounts of nicotine helps or hinders one’s efforts to break nicotine addiction remains to be determined because of the reinforcing properties of the drug.
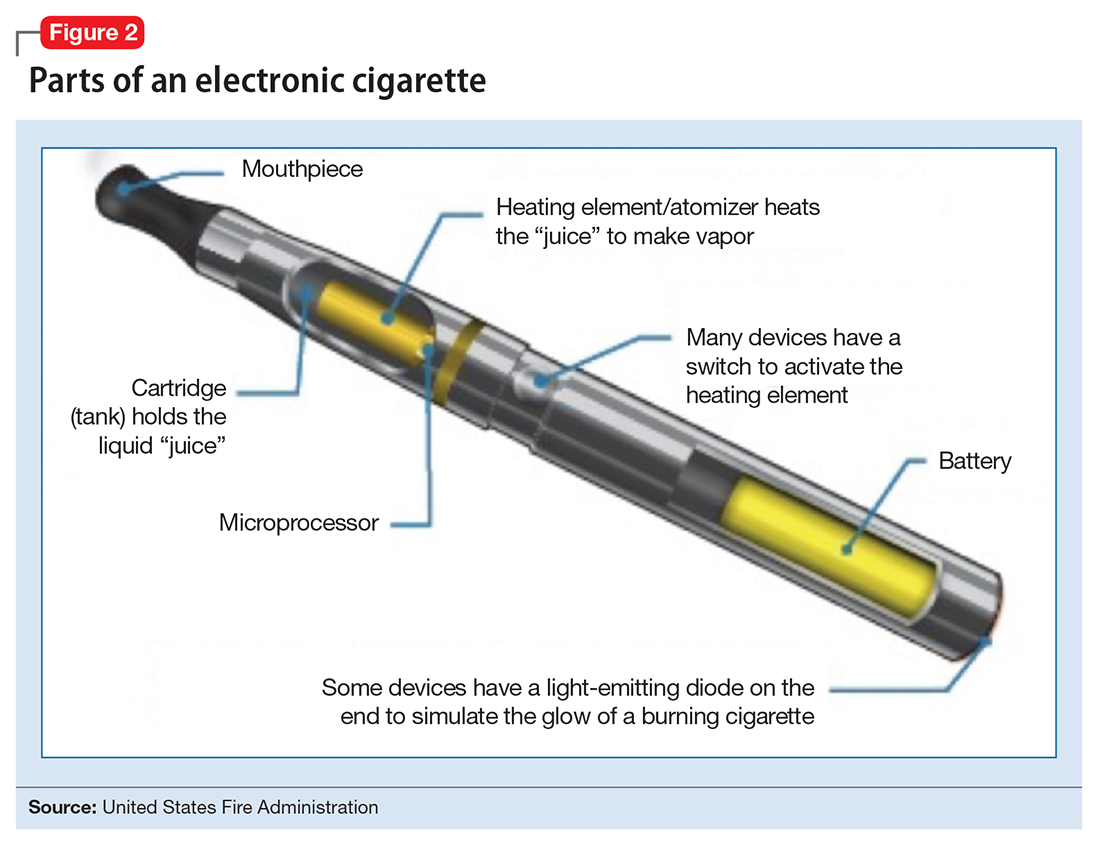
The liquid in the E-cig cartridge typically contains not only nicotine but a number of chemical compounds with potentially deleterious or unknown health risks. The 3 main ingredients include:
- a solvent of glycerin and/or propylene glycol
- nicotine in various concentrations
- flavorings.
The glycerin or propylene glycol forms the basis for the aerosol. Nicotine concentrations vary from 0 (denicotinized) to 35 mcg per puff.4 A study reported 7,700 unique flavors available for vaping liquid.5 The liquid also contains impurities, such as anabasine, which has effects on the α-7 nicotinic acetylcholine receptor and its principal use is as an insecticide and β-nicotyrine, which inhibits cytochrome P450 2A.
Epidemiology and end-user perspectives
In 2014, 12.4% of U.S. adults classified themselves as “ever users” of ENDS (used at least once) and 3.7% of adults classified themselves as current users, according to the National Health Interview Study.6 Importantly, among E-cig users who had not used combustible cigarettes, young adults (age 18 to 24) were more likely to have tried ENDS than older adults. ENDS are becoming more popular across the globe. A study in the European Union found that ever users of ENDS most commonly were current cigarette smokers (31%) followed by former (10.8%) and never smokers (2.3%).7
ENDS use is relevant for mental health professionals because of the high rate of comorbid tobacco use disorder in individuals with psychiatric conditions. For example, 2 U.S. population surveys8,9 revealed those with mental health conditions were 1.5 to 2 times more likely to have tried ENDS and 2 to 3 times more likely to be current users. Those with psychiatric illness reported similar reasons for ENDS use as other individuals, including “just because,” use as a smoking cessation aid, ease of use, and perceived safety vs combustible cigarettes.
A recent review that included 9 studies focusing on ENDS use in those with mental illness reported mixed findings on the utility of these devices to reduce or stop use of combustible cigarettes.10 Additionally, it is important to monitor the use of cigarettes and ENDS in patients with psychiatric illness because the byproducts of tobacco smoke can affect the metabolism of some psychotropic medications.11 Although reduced use of combustible cigarettes could lead to lower dosing of some psychotropics, an unreported decrease in combustible cigarette use could lead to supratherapeutic drug levels. There are no data on the effect of ENDS on the metabolism of psychotropics.
ENDS are increasingly popular among adolescents. In 2015, there were an estimated 4.6 million current tobacco users among middle/high school youths in the United States and 3 million current ENDS users, according to the National Youth Tobacco Surveys.12 The shift from combustible cigarettes to ENDS is notable, with an increase in the percentage of current E-cig users and a decrease in the percentage of exclusive combustible cigarette users. In addition, there has been no change in the prevalence of lifetime tobacco users.12 This is a global issue, as reports of ever use of ENDS by adolescents range from 6.5% to 31% in the United States, 14.6% in Canada, and 4.7% to 38.5% in Europe.13 Based on these trends, the U.S. Surgeon General released a statement warning against the use of ENDS in youth because of the lack of safety data and strong association with use of tobacco products.14
There are a number of possible reasons for the increasing popularity of ENDS, including the product’s novelty, lack of regulations regarding their sale, availability of flavorings, and the perception that ENDS are safe alternatives to cigarettes. E-cig–using youths have described ENDS as “not at all harmful” and “not at all addictive” and believe that ENDS with flavoring are less harmful than those without.15 Although studies in adults show some users reporting that ENDS are less satisfying, they are seen as useful in decreasing craving and a safer alternative to cigarettes.16,17
Are ENDS effective for smoking cessation?
The evidence for ENDS as aids to smoking cessation remains murky (Table 118-22). There is a paucity of randomized controlled clinical trials (RCTs) investigating ENDS for smoking cessation or reduction, and it is difficult to quantify the amount of nicotine used in ENDS because of the variety of delivery systems and cartridges. In a recent Cochrane review, those using ENDS to quit smoking were more likely to be abstinent from combustible cigarettes at 6 months vs those using nicotine-free ENDS (relative risk = 2.29; 95% CI, 1.05 to 4.96), but there was no significant difference in quit rates compared with nicotine patches.23 However, the confidence in this finding was rated as low because of the limited number of RCTs. Of note, the authors found 15 ongoing RCTs at the time of publication that might be eligible for later evaluation.
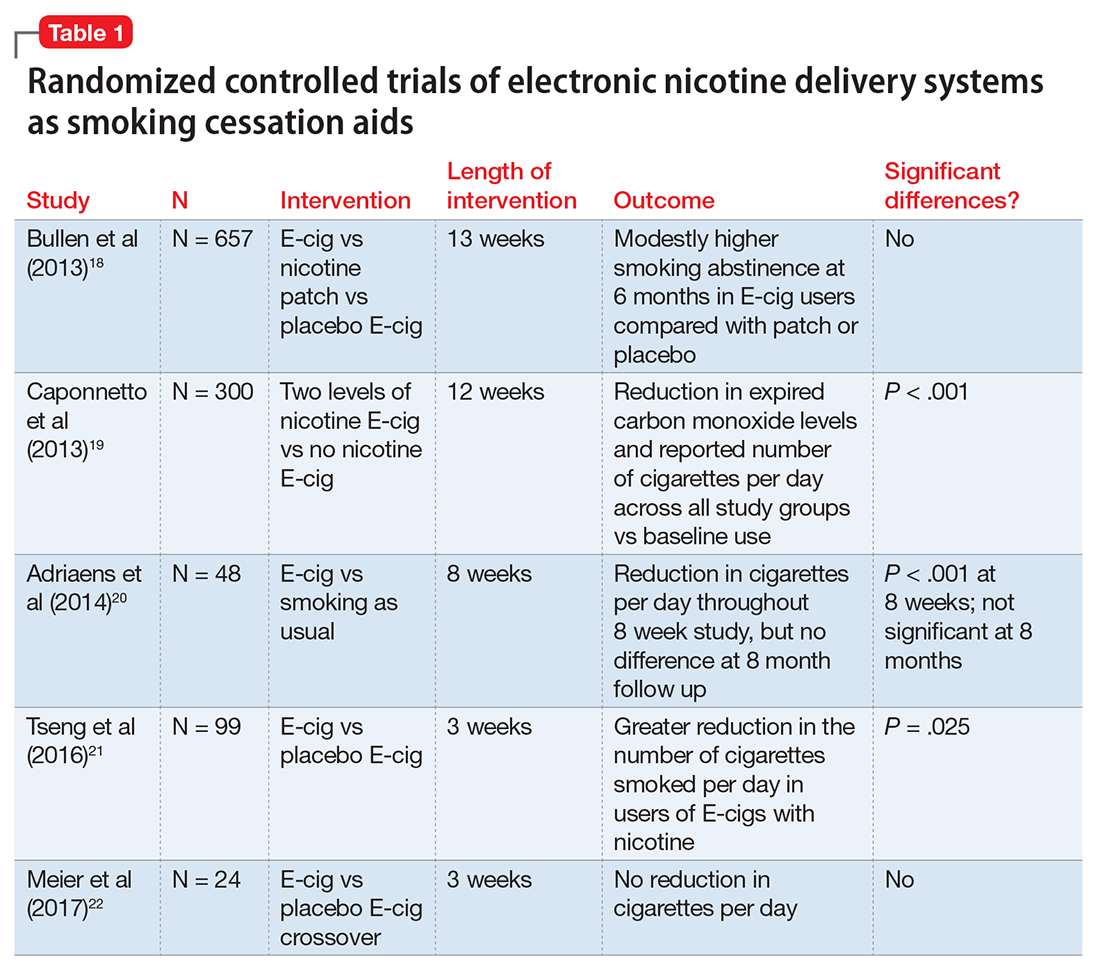
Non-RCTs reveal mixed data. Positive results include 1 study with an odds ratio of 6.07 to quit for intensive ENDS users vs non-users,24 and another with dual users of combustible and electronic cigarettes having a 46% quit rate at 1 year.25 Additionally, in a pilot study providing ENDS to 14 patients with schizophrenia who had no previous desire to quit smoking, authors noted a reduction in the number of cigarettes smoked per day by 50% in one-half of participants and abstinence in 14% of participants at 52 weeks.26 Studies with neutral or negative results include those showing ENDS users to be current combustible tobacco smokers, and use of ENDS not predicting smoking cessation.4,27 Data also are mixed regarding the use of ENDS as a harm reduction strategy. One study found that ENDS decreased cigarette consumption, but did not increase the likelihood of quitting,28 while another reported that daily use of ENDS increased the odds of reducing smoking by as much as 2.5 times compared with non-use of such aids.29 In a 24-month prospective cohort study following tobacco users, there was no difference in the number of cigarettes smoked per day in those who started the trial as users of combustible cigarettes alone vs combustible cigarettes plus ENDS users.30 Interestingly, those who started the study as combustible cigarette users and switched to ENDS and those who had continued dual use throughout the 24 months smoked fewer combustible cigarettes per day than those who never tried ENDS or quit during the study period.
Health effects


To better understand the adverse health effects of ENDS, one must consider potential short- and long-term consequences (Table 2). In the short-term, ENDS have been found to increase markers of inflammation and oxidative stress acutely as evidenced by in vivo laboratory studies.31,32 ENDS also have been linked to upper respiratory irritation, in part, because of the transformation of glycerin in the nicotine cartridge to acrolein upon combustion.33 Even 5 minutes of ad lib E-cig use has been found to significantly increase airflow resistance during pulmonary function tests34—changes that have been shown to precede more persistent alterations in peak expiratory flow, such as those seen in chronic obstructive pulmonary disease. The more common patient-reported side effects include:
- daytime cough (27%)
- phlegm production (25%)
- headache (21%)
- dry mouth/throat (20%)
- vertigo, headache, or nausea (9%).35,36
A RCT investigating efficacy of E-cigs vs nicotine patches vs denicotinized E-cigs found no difference among the groups in the number of reported adverse events.18 Interestingly, another RCT found a decrease in adverse events, such as dry cough, mouth irritation, throat irritation, shortness of breath, and headache, compared with baseline in combustible cigarette smokers who used regular or denicotinized E-cigs.19
Although no studies have directly investigated long-term health consequences of ENDS because of their relative novelty, one can extrapolate potential harmful long-term effects based on knowledge of the products’ chemical constituents. For example, propylene glycol can degrade into propylene oxide, a class 2B carcinogen.37 Other potential carcinogens in the aerosol include formaldehyde and acetaldehyde. On a broader scale, many of the particulates have been shown to cause systemic inflammation, which is thought to increase cardiovascular and respiratory disease and death.38 Flavorings in ENDS include a variety of components including, but not limited to, aldehydes, which are irritants, and other additives that have been associated with respiratory disease.39
Second-hand exposure. There are no long-term studies of second-hand vapor exposure, but similar to long-term health on primary users, one can glean some observations from the literature. It is promising that compared with cigarettes, ENDS lack sidestream smoke and the vapor has not been found to contain carbon monoxide.40 Some research has demonstrated that the size and spray of fine particles in the aerosol is as large or larger than combustible cigarettes.41 Formaldehyde, acetaldehyde, isoprene, and acetic acid have been found in ENDS vapor.40 Interestingly, a simulated café study found elevated nicotine, glycerine, hydrocarbon, and other materials classified as carcinogens in the air.42
Although it is popularly thought that ENDS are less toxic than tobacco cigarettes, there is not enough evidence to estimate precisely as to how much less toxic or the consequences of use. ENDS are increasingly popular and are being used by never smokers who should be educated on the potential harm that ENDS pose.
Recommendations from agencies and medical organizations
The World Health Organization (WHO) recommended prohibiting the use of ENDS in indoor spaces to minimize potential health risks to users and non-users. The WHO also aims to prevent dissemination of unproven health claims, including claims that ENDS are effective—or not—or that the devices are innocuous.36 In the United States, the FDA has stated that ENDS are not recommended for safe quitting (2009). In August 2016, the FDA introduced regulations banning the sale of ENDS to individuals age <18 and required manufacturers to submit documents detailing all ingredients for review and possible approval.
The American Lung Association has stated its concerns about the use of ENDS but has not made any direct recommendations. The American Heart Association reports a potential negative public health impact and provides clinical guideline recommendations.43 Prominent psychiatric organizations such as the American Psychiatric Association, American Academy of Addiction Psychiatry (AAAP), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institute of Drug Abuse do not have official statements supporting or rejecting the use of ENDS. However, they do note the potential harm and lack of substantial evidence for efficacy of ENDS as a smoking cessation tool, and the AAAP and SAMHSA state that they will work with regulatory agencies to reduce the use of toxic products with addictive potential including ENDS.44-46
Clinical recommendations
We do not recommend ENDS as a first-line treatment for smoking cessation because there is no evidence they are superior to the FDA-approved nicotine replacement therapies (NRTs), the paucity of research into the potential short- and long-term health risks of ENDS, and the fact that these products are not regulated for use as smoking cessation aids. It is, however, advisable to discuss ENDS use with patients by:
- asking if they are using the products
- assessing whether the user also is a smoker
- advising the patient to quit.
It also is important to assess the patient’s knowledge and attitudes regarding ENDS use and provide education about the products. Some patients firmly believe that ENDS are the lesser of 2 evils, and they are decreasing the harms of smoking by using these devices. While the debate over a potential harm reduction strategy unfolds,47 we think that because of the state of the evidence it is prudent to adopt a more precautionary stance and recommend that patients work toward abstinence from nicotine in any form.
For dual tobacco/ENDS users and for patients using ENDS who want to quit smoking, we recommend treatment with an approved pharmacotherapy (ie, NRTs, bupropion, and varenicline) combined with counseling. A 2013 Cochrane Review found that all pharamacotherapy options are more effective than placebo, and combination NRT and varenicline are superior to single NRT or bupropion (Box).23,48
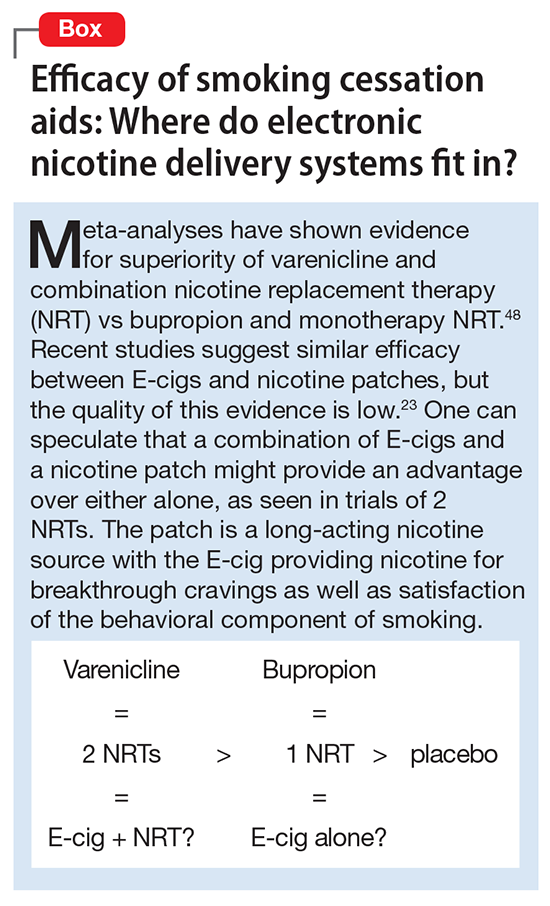
1. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175-179.
2. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86.
3. St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535-544.
4. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-1986.
5. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3-iii9. doi: 10.1136/tobaccocontrol-2014-051670.
6. Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015;(217):1-8.
7. Farsalinos KE, Poulas K, Voudris V, et al. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction. 2016;111(11):2032-2040.
8. Cummins SE, Zhu SH, Tedeschi GJ, et al. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2015;23(suppl 3):iii48-iii53. doi: 10.1136/tobaccocontrol-2013-051511.
9. Spears CA, Jones DM, Weaver SR, et al. Use of electronic nicotine delivery systems among adults with mental health conditions, 2015. Int J Environ Res Public Heal. 2017;14(1):10.
10. Hefner K, Valentine G, Sofuoglu M. Electronic cigarettes and mental illness: reviewing the evidence for help and harm among those with psychiatric and substance use disorders [published online February 2, 2017]. Am J Addict. doi: 10.1111/ajad.12504.
11. Anthenelli R. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
12. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361-367.
13. Greenhill R, Dawkins L, Notley C, et al. Adolescent awareness and use of electronic cigarettes: a review of emerging trends and findings. J Adolesc Heal. 2016;59(6):612-619.
14. U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016.
15. Cooper M, Harrell MB, Pérez A, et al. Flavorings and perceived harm and addictiveness of e-cigarettes among youth. Tob Regul Sci. 2016;2(3):278-289.
16. Kim H, Davis AH, Dohack JL, et al. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob Res. 2017;19(2):190-196.
17. Czoli CD, Fong GT, Mays D, et al. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control. 2017;26(e1):e49-e58.
18. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629-1637.
19. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317.
20. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220-11248.
21. Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937-1943.
22. Meier E, Wahlquist AE, Heckman BW, et al. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine Tob Res. 2017;19(2):176-182.
23. Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation [published online September 14, 2016]. Cochrane Database Syst Rev. 2016;9:CD010216.
24. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2014;17(2):127-133.
25. Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491-494.
26. Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446-461.
27. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923-930.
28. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: International Tobacco Control Four-Country Survey. Am J Prev Med. 2013;44(3):207-215.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160-1168.
30. Manzoli L, Flacco ME, Ferrante M, et al; ISLESE Working Group. Cohort study of electronic cigarette use: effectiveness and safety at 24 months [published online June 6, 2016]. Tob Control. doi: 10.1136/tobaccocontrol-2015-052822.
31. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732.
32. Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861.
33. US Environmental Protection Agency. Acrolein. https://www.epa.gov/sites/production/files/2016-08/documents/acrolein.pdf. Updated September 2009. Accessed April 7, 2017.
34. Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400-1406.
35. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231.
36. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32(2):133-140.
37. Laino T, Tuma C, Moor P, et al. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116(18):4602-4609.
38. Brook RD, Rajagopalan S, Pope CA 3rd, et al; American Heart Association Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331-2378.
39. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493-2494.
40. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25-31.
41. Fuoco FC, Buonanno G, Stabile L, et al. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523-529.
42. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628-637.
43. Bhatnagar A, Whitsel L, Ribisl K, et al; American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418-1436.
44. E-cigarettes pose risks. SAMHSA News. https://www.samhsa.gov/samhsaNewsLetter/Volume_22_Number_3/e_cigarettes. Published 2014. Accessed April 7, 2017.
45. National Institute on Drug Abuse. Electronic cigarettes (e-cigarettes). https://www.drugabuse.gov/publications/drugfacts/electronic-cigarettes-e-cigarettes. Revised May 2016. Accessed April 7, 2017.
46. American Academy of Addiction Psychiatry. Nicotine dependence. East Providence, RI: American Academy of Addition Psychiatry; 2015.
47. Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes — will England reframe the debate. N Engl J Med. 2016;374(14):1301-1303.
48. Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193-194.
The popularity of electronic cigarettes (E-cigs) and “vapes” has grown dramatically, spawning a new industry of electronic nicotine delivery systems (ENDS). With the increasing use of E-cigs not only for smoking cessation, but also as a primary nicotine source, it is important for mental health professionals to be prepared to discuss use of these devices with patients. In this article, we will describe:
- the composition of E-cigs and their current use
- evidence for their use for smoking cessation
- adverse health effects
- recommendations of major regulatory agencies.
Finally, we will provide recommendations for E-cig use in clinical populations.
What is an electronic nicotine delivery system?
ENDS produce an aerosol with or without nicotine that is inhaled and is thought to mimic the use of combustible cigarettes. ENDS evolved from basic E-cigs into a less “cigarette-like” and more customizable product (Figure 1). ENDS include a range of designs and go by various names, including “personal vaporizers,” “e-cigars,” and “e-hookahs” (in this article, we will use the term “ENDS” to refer to these devices).

The general design of ENDS is a plastic tubing system that contains a mouthpiece, battery, electronic heating element (“vaporizer”), and a cartridge with liquid solvent with or without nicotine or flavoring (Figure 2). One draw on the mouthpiece or press of a button activates the device, heats the solution, and delivers a vapor in a similar manner to taking a puff of a cigarette. Although studies have shown that ENDS result in significant increases in plasma nicotine concentrations in 5 minutes,1 the plasma nicotine levels obtained with the first-generation “cigarette-like” ENDS are much lower than those caused by inhaling tobacco smoke.2 Over time nicotine delivery capability has improved as ENDS have evolved such that the rate of nicotine delivery and peak concentration obtained with newer models more closely mirror tobacco cigarettes.3 Whether the rapid delivery of larger amounts of nicotine helps or hinders one’s efforts to break nicotine addiction remains to be determined because of the reinforcing properties of the drug.

The liquid in the E-cig cartridge typically contains not only nicotine but a number of chemical compounds with potentially deleterious or unknown health risks. The 3 main ingredients include:
- a solvent of glycerin and/or propylene glycol
- nicotine in various concentrations
- flavorings.
The glycerin or propylene glycol forms the basis for the aerosol. Nicotine concentrations vary from 0 (denicotinized) to 35 mcg per puff.4 A study reported 7,700 unique flavors available for vaping liquid.5 The liquid also contains impurities, such as anabasine, which has effects on the α-7 nicotinic acetylcholine receptor and its principal use is as an insecticide and β-nicotyrine, which inhibits cytochrome P450 2A.
Epidemiology and end-user perspectives
In 2014, 12.4% of U.S. adults classified themselves as “ever users” of ENDS (used at least once) and 3.7% of adults classified themselves as current users, according to the National Health Interview Study.6 Importantly, among E-cig users who had not used combustible cigarettes, young adults (age 18 to 24) were more likely to have tried ENDS than older adults. ENDS are becoming more popular across the globe. A study in the European Union found that ever users of ENDS most commonly were current cigarette smokers (31%) followed by former (10.8%) and never smokers (2.3%).7
ENDS use is relevant for mental health professionals because of the high rate of comorbid tobacco use disorder in individuals with psychiatric conditions. For example, 2 U.S. population surveys8,9 revealed those with mental health conditions were 1.5 to 2 times more likely to have tried ENDS and 2 to 3 times more likely to be current users. Those with psychiatric illness reported similar reasons for ENDS use as other individuals, including “just because,” use as a smoking cessation aid, ease of use, and perceived safety vs combustible cigarettes.
A recent review that included 9 studies focusing on ENDS use in those with mental illness reported mixed findings on the utility of these devices to reduce or stop use of combustible cigarettes.10 Additionally, it is important to monitor the use of cigarettes and ENDS in patients with psychiatric illness because the byproducts of tobacco smoke can affect the metabolism of some psychotropic medications.11 Although reduced use of combustible cigarettes could lead to lower dosing of some psychotropics, an unreported decrease in combustible cigarette use could lead to supratherapeutic drug levels. There are no data on the effect of ENDS on the metabolism of psychotropics.
ENDS are increasingly popular among adolescents. In 2015, there were an estimated 4.6 million current tobacco users among middle/high school youths in the United States and 3 million current ENDS users, according to the National Youth Tobacco Surveys.12 The shift from combustible cigarettes to ENDS is notable, with an increase in the percentage of current E-cig users and a decrease in the percentage of exclusive combustible cigarette users. In addition, there has been no change in the prevalence of lifetime tobacco users.12 This is a global issue, as reports of ever use of ENDS by adolescents range from 6.5% to 31% in the United States, 14.6% in Canada, and 4.7% to 38.5% in Europe.13 Based on these trends, the U.S. Surgeon General released a statement warning against the use of ENDS in youth because of the lack of safety data and strong association with use of tobacco products.14
There are a number of possible reasons for the increasing popularity of ENDS, including the product’s novelty, lack of regulations regarding their sale, availability of flavorings, and the perception that ENDS are safe alternatives to cigarettes. E-cig–using youths have described ENDS as “not at all harmful” and “not at all addictive” and believe that ENDS with flavoring are less harmful than those without.15 Although studies in adults show some users reporting that ENDS are less satisfying, they are seen as useful in decreasing craving and a safer alternative to cigarettes.16,17
Are ENDS effective for smoking cessation?
The evidence for ENDS as aids to smoking cessation remains murky (Table 118-22). There is a paucity of randomized controlled clinical trials (RCTs) investigating ENDS for smoking cessation or reduction, and it is difficult to quantify the amount of nicotine used in ENDS because of the variety of delivery systems and cartridges. In a recent Cochrane review, those using ENDS to quit smoking were more likely to be abstinent from combustible cigarettes at 6 months vs those using nicotine-free ENDS (relative risk = 2.29; 95% CI, 1.05 to 4.96), but there was no significant difference in quit rates compared with nicotine patches.23 However, the confidence in this finding was rated as low because of the limited number of RCTs. Of note, the authors found 15 ongoing RCTs at the time of publication that might be eligible for later evaluation.

Non-RCTs reveal mixed data. Positive results include 1 study with an odds ratio of 6.07 to quit for intensive ENDS users vs non-users,24 and another with dual users of combustible and electronic cigarettes having a 46% quit rate at 1 year.25 Additionally, in a pilot study providing ENDS to 14 patients with schizophrenia who had no previous desire to quit smoking, authors noted a reduction in the number of cigarettes smoked per day by 50% in one-half of participants and abstinence in 14% of participants at 52 weeks.26 Studies with neutral or negative results include those showing ENDS users to be current combustible tobacco smokers, and use of ENDS not predicting smoking cessation.4,27 Data also are mixed regarding the use of ENDS as a harm reduction strategy. One study found that ENDS decreased cigarette consumption, but did not increase the likelihood of quitting,28 while another reported that daily use of ENDS increased the odds of reducing smoking by as much as 2.5 times compared with non-use of such aids.29 In a 24-month prospective cohort study following tobacco users, there was no difference in the number of cigarettes smoked per day in those who started the trial as users of combustible cigarettes alone vs combustible cigarettes plus ENDS users.30 Interestingly, those who started the study as combustible cigarette users and switched to ENDS and those who had continued dual use throughout the 24 months smoked fewer combustible cigarettes per day than those who never tried ENDS or quit during the study period.
Health effects


To better understand the adverse health effects of ENDS, one must consider potential short- and long-term consequences (Table 2). In the short-term, ENDS have been found to increase markers of inflammation and oxidative stress acutely as evidenced by in vivo laboratory studies.31,32 ENDS also have been linked to upper respiratory irritation, in part, because of the transformation of glycerin in the nicotine cartridge to acrolein upon combustion.33 Even 5 minutes of ad lib E-cig use has been found to significantly increase airflow resistance during pulmonary function tests34—changes that have been shown to precede more persistent alterations in peak expiratory flow, such as those seen in chronic obstructive pulmonary disease. The more common patient-reported side effects include:
- daytime cough (27%)
- phlegm production (25%)
- headache (21%)
- dry mouth/throat (20%)
- vertigo, headache, or nausea (9%).35,36
A RCT investigating efficacy of E-cigs vs nicotine patches vs denicotinized E-cigs found no difference among the groups in the number of reported adverse events.18 Interestingly, another RCT found a decrease in adverse events, such as dry cough, mouth irritation, throat irritation, shortness of breath, and headache, compared with baseline in combustible cigarette smokers who used regular or denicotinized E-cigs.19
Although no studies have directly investigated long-term health consequences of ENDS because of their relative novelty, one can extrapolate potential harmful long-term effects based on knowledge of the products’ chemical constituents. For example, propylene glycol can degrade into propylene oxide, a class 2B carcinogen.37 Other potential carcinogens in the aerosol include formaldehyde and acetaldehyde. On a broader scale, many of the particulates have been shown to cause systemic inflammation, which is thought to increase cardiovascular and respiratory disease and death.38 Flavorings in ENDS include a variety of components including, but not limited to, aldehydes, which are irritants, and other additives that have been associated with respiratory disease.39
Second-hand exposure. There are no long-term studies of second-hand vapor exposure, but similar to long-term health on primary users, one can glean some observations from the literature. It is promising that compared with cigarettes, ENDS lack sidestream smoke and the vapor has not been found to contain carbon monoxide.40 Some research has demonstrated that the size and spray of fine particles in the aerosol is as large or larger than combustible cigarettes.41 Formaldehyde, acetaldehyde, isoprene, and acetic acid have been found in ENDS vapor.40 Interestingly, a simulated café study found elevated nicotine, glycerine, hydrocarbon, and other materials classified as carcinogens in the air.42
Although it is popularly thought that ENDS are less toxic than tobacco cigarettes, there is not enough evidence to estimate precisely as to how much less toxic or the consequences of use. ENDS are increasingly popular and are being used by never smokers who should be educated on the potential harm that ENDS pose.
Recommendations from agencies and medical organizations
The World Health Organization (WHO) recommended prohibiting the use of ENDS in indoor spaces to minimize potential health risks to users and non-users. The WHO also aims to prevent dissemination of unproven health claims, including claims that ENDS are effective—or not—or that the devices are innocuous.36 In the United States, the FDA has stated that ENDS are not recommended for safe quitting (2009). In August 2016, the FDA introduced regulations banning the sale of ENDS to individuals age <18 and required manufacturers to submit documents detailing all ingredients for review and possible approval.
The American Lung Association has stated its concerns about the use of ENDS but has not made any direct recommendations. The American Heart Association reports a potential negative public health impact and provides clinical guideline recommendations.43 Prominent psychiatric organizations such as the American Psychiatric Association, American Academy of Addiction Psychiatry (AAAP), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institute of Drug Abuse do not have official statements supporting or rejecting the use of ENDS. However, they do note the potential harm and lack of substantial evidence for efficacy of ENDS as a smoking cessation tool, and the AAAP and SAMHSA state that they will work with regulatory agencies to reduce the use of toxic products with addictive potential including ENDS.44-46
Clinical recommendations
We do not recommend ENDS as a first-line treatment for smoking cessation because there is no evidence they are superior to the FDA-approved nicotine replacement therapies (NRTs), the paucity of research into the potential short- and long-term health risks of ENDS, and the fact that these products are not regulated for use as smoking cessation aids. It is, however, advisable to discuss ENDS use with patients by:
- asking if they are using the products
- assessing whether the user also is a smoker
- advising the patient to quit.
It also is important to assess the patient’s knowledge and attitudes regarding ENDS use and provide education about the products. Some patients firmly believe that ENDS are the lesser of 2 evils, and they are decreasing the harms of smoking by using these devices. While the debate over a potential harm reduction strategy unfolds,47 we think that because of the state of the evidence it is prudent to adopt a more precautionary stance and recommend that patients work toward abstinence from nicotine in any form.
For dual tobacco/ENDS users and for patients using ENDS who want to quit smoking, we recommend treatment with an approved pharmacotherapy (ie, NRTs, bupropion, and varenicline) combined with counseling. A 2013 Cochrane Review found that all pharamacotherapy options are more effective than placebo, and combination NRT and varenicline are superior to single NRT or bupropion (Box).23,48

The popularity of electronic cigarettes (E-cigs) and “vapes” has grown dramatically, spawning a new industry of electronic nicotine delivery systems (ENDS). With the increasing use of E-cigs not only for smoking cessation, but also as a primary nicotine source, it is important for mental health professionals to be prepared to discuss use of these devices with patients. In this article, we will describe:
- the composition of E-cigs and their current use
- evidence for their use for smoking cessation
- adverse health effects
- recommendations of major regulatory agencies.
Finally, we will provide recommendations for E-cig use in clinical populations.
What is an electronic nicotine delivery system?
ENDS produce an aerosol with or without nicotine that is inhaled and is thought to mimic the use of combustible cigarettes. ENDS evolved from basic E-cigs into a less “cigarette-like” and more customizable product (Figure 1). ENDS include a range of designs and go by various names, including “personal vaporizers,” “e-cigars,” and “e-hookahs” (in this article, we will use the term “ENDS” to refer to these devices).

The general design of ENDS is a plastic tubing system that contains a mouthpiece, battery, electronic heating element (“vaporizer”), and a cartridge with liquid solvent with or without nicotine or flavoring (Figure 2). One draw on the mouthpiece or press of a button activates the device, heats the solution, and delivers a vapor in a similar manner to taking a puff of a cigarette. Although studies have shown that ENDS result in significant increases in plasma nicotine concentrations in 5 minutes,1 the plasma nicotine levels obtained with the first-generation “cigarette-like” ENDS are much lower than those caused by inhaling tobacco smoke.2 Over time nicotine delivery capability has improved as ENDS have evolved such that the rate of nicotine delivery and peak concentration obtained with newer models more closely mirror tobacco cigarettes.3 Whether the rapid delivery of larger amounts of nicotine helps or hinders one’s efforts to break nicotine addiction remains to be determined because of the reinforcing properties of the drug.

The liquid in the E-cig cartridge typically contains not only nicotine but a number of chemical compounds with potentially deleterious or unknown health risks. The 3 main ingredients include:
- a solvent of glycerin and/or propylene glycol
- nicotine in various concentrations
- flavorings.
The glycerin or propylene glycol forms the basis for the aerosol. Nicotine concentrations vary from 0 (denicotinized) to 35 mcg per puff.4 A study reported 7,700 unique flavors available for vaping liquid.5 The liquid also contains impurities, such as anabasine, which has effects on the α-7 nicotinic acetylcholine receptor and its principal use is as an insecticide and β-nicotyrine, which inhibits cytochrome P450 2A.
Epidemiology and end-user perspectives
In 2014, 12.4% of U.S. adults classified themselves as “ever users” of ENDS (used at least once) and 3.7% of adults classified themselves as current users, according to the National Health Interview Study.6 Importantly, among E-cig users who had not used combustible cigarettes, young adults (age 18 to 24) were more likely to have tried ENDS than older adults. ENDS are becoming more popular across the globe. A study in the European Union found that ever users of ENDS most commonly were current cigarette smokers (31%) followed by former (10.8%) and never smokers (2.3%).7
ENDS use is relevant for mental health professionals because of the high rate of comorbid tobacco use disorder in individuals with psychiatric conditions. For example, 2 U.S. population surveys8,9 revealed those with mental health conditions were 1.5 to 2 times more likely to have tried ENDS and 2 to 3 times more likely to be current users. Those with psychiatric illness reported similar reasons for ENDS use as other individuals, including “just because,” use as a smoking cessation aid, ease of use, and perceived safety vs combustible cigarettes.
A recent review that included 9 studies focusing on ENDS use in those with mental illness reported mixed findings on the utility of these devices to reduce or stop use of combustible cigarettes.10 Additionally, it is important to monitor the use of cigarettes and ENDS in patients with psychiatric illness because the byproducts of tobacco smoke can affect the metabolism of some psychotropic medications.11 Although reduced use of combustible cigarettes could lead to lower dosing of some psychotropics, an unreported decrease in combustible cigarette use could lead to supratherapeutic drug levels. There are no data on the effect of ENDS on the metabolism of psychotropics.
ENDS are increasingly popular among adolescents. In 2015, there were an estimated 4.6 million current tobacco users among middle/high school youths in the United States and 3 million current ENDS users, according to the National Youth Tobacco Surveys.12 The shift from combustible cigarettes to ENDS is notable, with an increase in the percentage of current E-cig users and a decrease in the percentage of exclusive combustible cigarette users. In addition, there has been no change in the prevalence of lifetime tobacco users.12 This is a global issue, as reports of ever use of ENDS by adolescents range from 6.5% to 31% in the United States, 14.6% in Canada, and 4.7% to 38.5% in Europe.13 Based on these trends, the U.S. Surgeon General released a statement warning against the use of ENDS in youth because of the lack of safety data and strong association with use of tobacco products.14
There are a number of possible reasons for the increasing popularity of ENDS, including the product’s novelty, lack of regulations regarding their sale, availability of flavorings, and the perception that ENDS are safe alternatives to cigarettes. E-cig–using youths have described ENDS as “not at all harmful” and “not at all addictive” and believe that ENDS with flavoring are less harmful than those without.15 Although studies in adults show some users reporting that ENDS are less satisfying, they are seen as useful in decreasing craving and a safer alternative to cigarettes.16,17
Are ENDS effective for smoking cessation?
The evidence for ENDS as aids to smoking cessation remains murky (Table 118-22). There is a paucity of randomized controlled clinical trials (RCTs) investigating ENDS for smoking cessation or reduction, and it is difficult to quantify the amount of nicotine used in ENDS because of the variety of delivery systems and cartridges. In a recent Cochrane review, those using ENDS to quit smoking were more likely to be abstinent from combustible cigarettes at 6 months vs those using nicotine-free ENDS (relative risk = 2.29; 95% CI, 1.05 to 4.96), but there was no significant difference in quit rates compared with nicotine patches.23 However, the confidence in this finding was rated as low because of the limited number of RCTs. Of note, the authors found 15 ongoing RCTs at the time of publication that might be eligible for later evaluation.

Non-RCTs reveal mixed data. Positive results include 1 study with an odds ratio of 6.07 to quit for intensive ENDS users vs non-users,24 and another with dual users of combustible and electronic cigarettes having a 46% quit rate at 1 year.25 Additionally, in a pilot study providing ENDS to 14 patients with schizophrenia who had no previous desire to quit smoking, authors noted a reduction in the number of cigarettes smoked per day by 50% in one-half of participants and abstinence in 14% of participants at 52 weeks.26 Studies with neutral or negative results include those showing ENDS users to be current combustible tobacco smokers, and use of ENDS not predicting smoking cessation.4,27 Data also are mixed regarding the use of ENDS as a harm reduction strategy. One study found that ENDS decreased cigarette consumption, but did not increase the likelihood of quitting,28 while another reported that daily use of ENDS increased the odds of reducing smoking by as much as 2.5 times compared with non-use of such aids.29 In a 24-month prospective cohort study following tobacco users, there was no difference in the number of cigarettes smoked per day in those who started the trial as users of combustible cigarettes alone vs combustible cigarettes plus ENDS users.30 Interestingly, those who started the study as combustible cigarette users and switched to ENDS and those who had continued dual use throughout the 24 months smoked fewer combustible cigarettes per day than those who never tried ENDS or quit during the study period.
Health effects


To better understand the adverse health effects of ENDS, one must consider potential short- and long-term consequences (Table 2). In the short-term, ENDS have been found to increase markers of inflammation and oxidative stress acutely as evidenced by in vivo laboratory studies.31,32 ENDS also have been linked to upper respiratory irritation, in part, because of the transformation of glycerin in the nicotine cartridge to acrolein upon combustion.33 Even 5 minutes of ad lib E-cig use has been found to significantly increase airflow resistance during pulmonary function tests34—changes that have been shown to precede more persistent alterations in peak expiratory flow, such as those seen in chronic obstructive pulmonary disease. The more common patient-reported side effects include:
- daytime cough (27%)
- phlegm production (25%)
- headache (21%)
- dry mouth/throat (20%)
- vertigo, headache, or nausea (9%).35,36
A RCT investigating efficacy of E-cigs vs nicotine patches vs denicotinized E-cigs found no difference among the groups in the number of reported adverse events.18 Interestingly, another RCT found a decrease in adverse events, such as dry cough, mouth irritation, throat irritation, shortness of breath, and headache, compared with baseline in combustible cigarette smokers who used regular or denicotinized E-cigs.19
Although no studies have directly investigated long-term health consequences of ENDS because of their relative novelty, one can extrapolate potential harmful long-term effects based on knowledge of the products’ chemical constituents. For example, propylene glycol can degrade into propylene oxide, a class 2B carcinogen.37 Other potential carcinogens in the aerosol include formaldehyde and acetaldehyde. On a broader scale, many of the particulates have been shown to cause systemic inflammation, which is thought to increase cardiovascular and respiratory disease and death.38 Flavorings in ENDS include a variety of components including, but not limited to, aldehydes, which are irritants, and other additives that have been associated with respiratory disease.39
Second-hand exposure. There are no long-term studies of second-hand vapor exposure, but similar to long-term health on primary users, one can glean some observations from the literature. It is promising that compared with cigarettes, ENDS lack sidestream smoke and the vapor has not been found to contain carbon monoxide.40 Some research has demonstrated that the size and spray of fine particles in the aerosol is as large or larger than combustible cigarettes.41 Formaldehyde, acetaldehyde, isoprene, and acetic acid have been found in ENDS vapor.40 Interestingly, a simulated café study found elevated nicotine, glycerine, hydrocarbon, and other materials classified as carcinogens in the air.42
Although it is popularly thought that ENDS are less toxic than tobacco cigarettes, there is not enough evidence to estimate precisely as to how much less toxic or the consequences of use. ENDS are increasingly popular and are being used by never smokers who should be educated on the potential harm that ENDS pose.
Recommendations from agencies and medical organizations
The World Health Organization (WHO) recommended prohibiting the use of ENDS in indoor spaces to minimize potential health risks to users and non-users. The WHO also aims to prevent dissemination of unproven health claims, including claims that ENDS are effective—or not—or that the devices are innocuous.36 In the United States, the FDA has stated that ENDS are not recommended for safe quitting (2009). In August 2016, the FDA introduced regulations banning the sale of ENDS to individuals age <18 and required manufacturers to submit documents detailing all ingredients for review and possible approval.
The American Lung Association has stated its concerns about the use of ENDS but has not made any direct recommendations. The American Heart Association reports a potential negative public health impact and provides clinical guideline recommendations.43 Prominent psychiatric organizations such as the American Psychiatric Association, American Academy of Addiction Psychiatry (AAAP), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institute of Drug Abuse do not have official statements supporting or rejecting the use of ENDS. However, they do note the potential harm and lack of substantial evidence for efficacy of ENDS as a smoking cessation tool, and the AAAP and SAMHSA state that they will work with regulatory agencies to reduce the use of toxic products with addictive potential including ENDS.44-46
Clinical recommendations
We do not recommend ENDS as a first-line treatment for smoking cessation because there is no evidence they are superior to the FDA-approved nicotine replacement therapies (NRTs), the paucity of research into the potential short- and long-term health risks of ENDS, and the fact that these products are not regulated for use as smoking cessation aids. It is, however, advisable to discuss ENDS use with patients by:
- asking if they are using the products
- assessing whether the user also is a smoker
- advising the patient to quit.
It also is important to assess the patient’s knowledge and attitudes regarding ENDS use and provide education about the products. Some patients firmly believe that ENDS are the lesser of 2 evils, and they are decreasing the harms of smoking by using these devices. While the debate over a potential harm reduction strategy unfolds,47 we think that because of the state of the evidence it is prudent to adopt a more precautionary stance and recommend that patients work toward abstinence from nicotine in any form.
For dual tobacco/ENDS users and for patients using ENDS who want to quit smoking, we recommend treatment with an approved pharmacotherapy (ie, NRTs, bupropion, and varenicline) combined with counseling. A 2013 Cochrane Review found that all pharamacotherapy options are more effective than placebo, and combination NRT and varenicline are superior to single NRT or bupropion (Box).23,48

1. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175-179.
2. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86.
3. St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535-544.
4. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-1986.
5. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3-iii9. doi: 10.1136/tobaccocontrol-2014-051670.
6. Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015;(217):1-8.
7. Farsalinos KE, Poulas K, Voudris V, et al. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction. 2016;111(11):2032-2040.
8. Cummins SE, Zhu SH, Tedeschi GJ, et al. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2015;23(suppl 3):iii48-iii53. doi: 10.1136/tobaccocontrol-2013-051511.
9. Spears CA, Jones DM, Weaver SR, et al. Use of electronic nicotine delivery systems among adults with mental health conditions, 2015. Int J Environ Res Public Heal. 2017;14(1):10.
10. Hefner K, Valentine G, Sofuoglu M. Electronic cigarettes and mental illness: reviewing the evidence for help and harm among those with psychiatric and substance use disorders [published online February 2, 2017]. Am J Addict. doi: 10.1111/ajad.12504.
11. Anthenelli R. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
12. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361-367.
13. Greenhill R, Dawkins L, Notley C, et al. Adolescent awareness and use of electronic cigarettes: a review of emerging trends and findings. J Adolesc Heal. 2016;59(6):612-619.
14. U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016.
15. Cooper M, Harrell MB, Pérez A, et al. Flavorings and perceived harm and addictiveness of e-cigarettes among youth. Tob Regul Sci. 2016;2(3):278-289.
16. Kim H, Davis AH, Dohack JL, et al. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob Res. 2017;19(2):190-196.
17. Czoli CD, Fong GT, Mays D, et al. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control. 2017;26(e1):e49-e58.
18. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629-1637.
19. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317.
20. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220-11248.
21. Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937-1943.
22. Meier E, Wahlquist AE, Heckman BW, et al. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine Tob Res. 2017;19(2):176-182.
23. Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation [published online September 14, 2016]. Cochrane Database Syst Rev. 2016;9:CD010216.
24. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2014;17(2):127-133.
25. Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491-494.
26. Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446-461.
27. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923-930.
28. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: International Tobacco Control Four-Country Survey. Am J Prev Med. 2013;44(3):207-215.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160-1168.
30. Manzoli L, Flacco ME, Ferrante M, et al; ISLESE Working Group. Cohort study of electronic cigarette use: effectiveness and safety at 24 months [published online June 6, 2016]. Tob Control. doi: 10.1136/tobaccocontrol-2015-052822.
31. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732.
32. Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861.
33. US Environmental Protection Agency. Acrolein. https://www.epa.gov/sites/production/files/2016-08/documents/acrolein.pdf. Updated September 2009. Accessed April 7, 2017.
34. Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400-1406.
35. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231.
36. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32(2):133-140.
37. Laino T, Tuma C, Moor P, et al. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116(18):4602-4609.
38. Brook RD, Rajagopalan S, Pope CA 3rd, et al; American Heart Association Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331-2378.
39. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493-2494.
40. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25-31.
41. Fuoco FC, Buonanno G, Stabile L, et al. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523-529.
42. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628-637.
43. Bhatnagar A, Whitsel L, Ribisl K, et al; American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418-1436.
44. E-cigarettes pose risks. SAMHSA News. https://www.samhsa.gov/samhsaNewsLetter/Volume_22_Number_3/e_cigarettes. Published 2014. Accessed April 7, 2017.
45. National Institute on Drug Abuse. Electronic cigarettes (e-cigarettes). https://www.drugabuse.gov/publications/drugfacts/electronic-cigarettes-e-cigarettes. Revised May 2016. Accessed April 7, 2017.
46. American Academy of Addiction Psychiatry. Nicotine dependence. East Providence, RI: American Academy of Addition Psychiatry; 2015.
47. Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes — will England reframe the debate. N Engl J Med. 2016;374(14):1301-1303.
48. Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193-194.
1. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175-179.
2. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86.
3. St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535-544.
4. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-1986.
5. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3-iii9. doi: 10.1136/tobaccocontrol-2014-051670.
6. Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015;(217):1-8.
7. Farsalinos KE, Poulas K, Voudris V, et al. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction. 2016;111(11):2032-2040.
8. Cummins SE, Zhu SH, Tedeschi GJ, et al. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2015;23(suppl 3):iii48-iii53. doi: 10.1136/tobaccocontrol-2013-051511.
9. Spears CA, Jones DM, Weaver SR, et al. Use of electronic nicotine delivery systems among adults with mental health conditions, 2015. Int J Environ Res Public Heal. 2017;14(1):10.
10. Hefner K, Valentine G, Sofuoglu M. Electronic cigarettes and mental illness: reviewing the evidence for help and harm among those with psychiatric and substance use disorders [published online February 2, 2017]. Am J Addict. doi: 10.1111/ajad.12504.
11. Anthenelli R. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
12. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361-367.
13. Greenhill R, Dawkins L, Notley C, et al. Adolescent awareness and use of electronic cigarettes: a review of emerging trends and findings. J Adolesc Heal. 2016;59(6):612-619.
14. U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016.
15. Cooper M, Harrell MB, Pérez A, et al. Flavorings and perceived harm and addictiveness of e-cigarettes among youth. Tob Regul Sci. 2016;2(3):278-289.
16. Kim H, Davis AH, Dohack JL, et al. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob Res. 2017;19(2):190-196.
17. Czoli CD, Fong GT, Mays D, et al. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control. 2017;26(e1):e49-e58.
18. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629-1637.
19. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317.
20. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220-11248.
21. Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937-1943.
22. Meier E, Wahlquist AE, Heckman BW, et al. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine Tob Res. 2017;19(2):176-182.
23. Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation [published online September 14, 2016]. Cochrane Database Syst Rev. 2016;9:CD010216.
24. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2014;17(2):127-133.
25. Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491-494.
26. Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446-461.
27. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923-930.
28. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: International Tobacco Control Four-Country Survey. Am J Prev Med. 2013;44(3):207-215.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160-1168.
30. Manzoli L, Flacco ME, Ferrante M, et al; ISLESE Working Group. Cohort study of electronic cigarette use: effectiveness and safety at 24 months [published online June 6, 2016]. Tob Control. doi: 10.1136/tobaccocontrol-2015-052822.
31. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732.
32. Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861.
33. US Environmental Protection Agency. Acrolein. https://www.epa.gov/sites/production/files/2016-08/documents/acrolein.pdf. Updated September 2009. Accessed April 7, 2017.
34. Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400-1406.
35. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231.
36. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32(2):133-140.
37. Laino T, Tuma C, Moor P, et al. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116(18):4602-4609.
38. Brook RD, Rajagopalan S, Pope CA 3rd, et al; American Heart Association Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331-2378.
39. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493-2494.
40. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25-31.
41. Fuoco FC, Buonanno G, Stabile L, et al. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523-529.
42. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628-637.
43. Bhatnagar A, Whitsel L, Ribisl K, et al; American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418-1436.
44. E-cigarettes pose risks. SAMHSA News. https://www.samhsa.gov/samhsaNewsLetter/Volume_22_Number_3/e_cigarettes. Published 2014. Accessed April 7, 2017.
45. National Institute on Drug Abuse. Electronic cigarettes (e-cigarettes). https://www.drugabuse.gov/publications/drugfacts/electronic-cigarettes-e-cigarettes. Revised May 2016. Accessed April 7, 2017.
46. American Academy of Addiction Psychiatry. Nicotine dependence. East Providence, RI: American Academy of Addition Psychiatry; 2015.
47. Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes — will England reframe the debate. N Engl J Med. 2016;374(14):1301-1303.
48. Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193-194.
Caution patients about common food–drug interactions
Many individuals read about the health benefits of certain foods, such as coffee, grapefruit, and red wine, but psychiatrists might neglect to inform their patients that these common foods could interact with drugs, thereby preventing certain psychotropics from achieving maximum benefit or causing toxicity. Educate your patients about food–drug interactions and to refrain from alcohol and specific foods when taking psychotropics. Although far from comprehensive, we present a discussion of the most frequently encountered and preventable food/nutrient–drug interactions.
Grapefruit juice may alter bioavailability of many psychotropics by inhibiting cytochrome P450 (CYP) 3A4 and 1A2 isoforms, interfering with prehepatic metabolism, and enteric absorption. Common medications affected by this interaction include alprazolam, buspirone, sertraline, carbamazepine, and methadone.1 Patients should be advised about eating grapefruit or drinking grapefruit juice as it could require dose adjustment to avoid drug toxicity.
Table salt. Lithium is a salt, and less table salt intake could cause lithium levels to rise and vice versa. Lithium and other salts compete for absorption and secretion in the renal tubules, which are responsible for this interaction. Therefore, it is advisable to keep a stable salt intake throughout treatment. Patients should be cautioned about eating salty foods during the summer because excessive sweating could lead to lithium intoxication.
Caffeine is a widely used stimulant; however, it can decrease blood lithium levels and block clozapine clearance via inhibition of the CYP1A2 enzyme. Excessive caffeine intake can lead to clozapine toxicity.2
Beef liver, aged sausage and cheese, and wine contain tyramine. Tyramine is broken down by monoamine oxidase (MAO) enzymes in the body, which are inhibited by MAO inhibitors (MAOI) such as phenelzine and selegiline. A patient taking a MAOI cannot catabolize tyramine and other amines. These exogenous amines could cause a life-threatening hyperadrenergic crisis. Physicians should educate their patients about the MAOI diet and monitor adherence to the food avoidance list.
St. John’s wort is a herb commonly used for treating mild depression. It is a strong inducer of the CYP3A4 enzyme and reduces plasma concentrations and could decrease clinical effectiveness of aripiprazole, quetiapine, alprazolam, and oxycodone.3 It could interact with serotonin reuptake inhibitors causing serotonin syndrome.
Full vs empty stomach. Food is known to affect bioavailability and enteral absorption of different psychotropics. Some medications are best taken on a full stomach and some on an empty one. For example, the antipsychotic ziprasidone should be taken with meals of at least 500 calories for optimal and consistent bioavailability. Benzodiazepines are rapidly absorbed when taken on an empty stomach.
Discuss dietary habits with patients to encourage a healthy lifestyle and provide valuable direction about potential food/nutrient–drug interactions.
1. Pawełczyk T, Kłoszewska I. Grapefruit juice interactions with psychotropic drugs: advantages and potential risk [in Polish]. Przegl Lek. 2008;62(2):92-95.
2. Hägg S, Spigset O, Mjörndal T, et al. Effect of caffeine on clozapine pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2000;49(1):59-63.
3. Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA. 2003;290(11):1500-1504.
Many individuals read about the health benefits of certain foods, such as coffee, grapefruit, and red wine, but psychiatrists might neglect to inform their patients that these common foods could interact with drugs, thereby preventing certain psychotropics from achieving maximum benefit or causing toxicity. Educate your patients about food–drug interactions and to refrain from alcohol and specific foods when taking psychotropics. Although far from comprehensive, we present a discussion of the most frequently encountered and preventable food/nutrient–drug interactions.
Grapefruit juice may alter bioavailability of many psychotropics by inhibiting cytochrome P450 (CYP) 3A4 and 1A2 isoforms, interfering with prehepatic metabolism, and enteric absorption. Common medications affected by this interaction include alprazolam, buspirone, sertraline, carbamazepine, and methadone.1 Patients should be advised about eating grapefruit or drinking grapefruit juice as it could require dose adjustment to avoid drug toxicity.
Table salt. Lithium is a salt, and less table salt intake could cause lithium levels to rise and vice versa. Lithium and other salts compete for absorption and secretion in the renal tubules, which are responsible for this interaction. Therefore, it is advisable to keep a stable salt intake throughout treatment. Patients should be cautioned about eating salty foods during the summer because excessive sweating could lead to lithium intoxication.
Caffeine is a widely used stimulant; however, it can decrease blood lithium levels and block clozapine clearance via inhibition of the CYP1A2 enzyme. Excessive caffeine intake can lead to clozapine toxicity.2
Beef liver, aged sausage and cheese, and wine contain tyramine. Tyramine is broken down by monoamine oxidase (MAO) enzymes in the body, which are inhibited by MAO inhibitors (MAOI) such as phenelzine and selegiline. A patient taking a MAOI cannot catabolize tyramine and other amines. These exogenous amines could cause a life-threatening hyperadrenergic crisis. Physicians should educate their patients about the MAOI diet and monitor adherence to the food avoidance list.
St. John’s wort is a herb commonly used for treating mild depression. It is a strong inducer of the CYP3A4 enzyme and reduces plasma concentrations and could decrease clinical effectiveness of aripiprazole, quetiapine, alprazolam, and oxycodone.3 It could interact with serotonin reuptake inhibitors causing serotonin syndrome.
Full vs empty stomach. Food is known to affect bioavailability and enteral absorption of different psychotropics. Some medications are best taken on a full stomach and some on an empty one. For example, the antipsychotic ziprasidone should be taken with meals of at least 500 calories for optimal and consistent bioavailability. Benzodiazepines are rapidly absorbed when taken on an empty stomach.
Discuss dietary habits with patients to encourage a healthy lifestyle and provide valuable direction about potential food/nutrient–drug interactions.
Many individuals read about the health benefits of certain foods, such as coffee, grapefruit, and red wine, but psychiatrists might neglect to inform their patients that these common foods could interact with drugs, thereby preventing certain psychotropics from achieving maximum benefit or causing toxicity. Educate your patients about food–drug interactions and to refrain from alcohol and specific foods when taking psychotropics. Although far from comprehensive, we present a discussion of the most frequently encountered and preventable food/nutrient–drug interactions.
Grapefruit juice may alter bioavailability of many psychotropics by inhibiting cytochrome P450 (CYP) 3A4 and 1A2 isoforms, interfering with prehepatic metabolism, and enteric absorption. Common medications affected by this interaction include alprazolam, buspirone, sertraline, carbamazepine, and methadone.1 Patients should be advised about eating grapefruit or drinking grapefruit juice as it could require dose adjustment to avoid drug toxicity.
Table salt. Lithium is a salt, and less table salt intake could cause lithium levels to rise and vice versa. Lithium and other salts compete for absorption and secretion in the renal tubules, which are responsible for this interaction. Therefore, it is advisable to keep a stable salt intake throughout treatment. Patients should be cautioned about eating salty foods during the summer because excessive sweating could lead to lithium intoxication.
Caffeine is a widely used stimulant; however, it can decrease blood lithium levels and block clozapine clearance via inhibition of the CYP1A2 enzyme. Excessive caffeine intake can lead to clozapine toxicity.2
Beef liver, aged sausage and cheese, and wine contain tyramine. Tyramine is broken down by monoamine oxidase (MAO) enzymes in the body, which are inhibited by MAO inhibitors (MAOI) such as phenelzine and selegiline. A patient taking a MAOI cannot catabolize tyramine and other amines. These exogenous amines could cause a life-threatening hyperadrenergic crisis. Physicians should educate their patients about the MAOI diet and monitor adherence to the food avoidance list.
St. John’s wort is a herb commonly used for treating mild depression. It is a strong inducer of the CYP3A4 enzyme and reduces plasma concentrations and could decrease clinical effectiveness of aripiprazole, quetiapine, alprazolam, and oxycodone.3 It could interact with serotonin reuptake inhibitors causing serotonin syndrome.
Full vs empty stomach. Food is known to affect bioavailability and enteral absorption of different psychotropics. Some medications are best taken on a full stomach and some on an empty one. For example, the antipsychotic ziprasidone should be taken with meals of at least 500 calories for optimal and consistent bioavailability. Benzodiazepines are rapidly absorbed when taken on an empty stomach.
Discuss dietary habits with patients to encourage a healthy lifestyle and provide valuable direction about potential food/nutrient–drug interactions.
1. Pawełczyk T, Kłoszewska I. Grapefruit juice interactions with psychotropic drugs: advantages and potential risk [in Polish]. Przegl Lek. 2008;62(2):92-95.
2. Hägg S, Spigset O, Mjörndal T, et al. Effect of caffeine on clozapine pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2000;49(1):59-63.
3. Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA. 2003;290(11):1500-1504.
1. Pawełczyk T, Kłoszewska I. Grapefruit juice interactions with psychotropic drugs: advantages and potential risk [in Polish]. Przegl Lek. 2008;62(2):92-95.
2. Hägg S, Spigset O, Mjörndal T, et al. Effect of caffeine on clozapine pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2000;49(1):59-63.
3. Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA. 2003;290(11):1500-1504.