User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
A girl repeatedly jabs her finger up her nose: Compulsion or self-injury?
CASE Anxious and self-injurious
A, age 6, is forcibly inserting her finger into her nose repeatedly until she bleeds profusely, as many as 20 times per day. She is not nose-picking but is jabbing her finger into her nose as far as possible in a repetitive ramming motion. Less frequently, she inserts her finger into her vagina, resulting in chronic urinary tract infections (UTIs). She has bedtime checking rituals; worries that her parents will die; has a fear of vomiting to the point where she stopped eating normally and lost 5 lb in 6 months; intense fear of storms; refusal to use public bathrooms; and involuntary throat clearing, facial grimacing, and lip twitches.
A’s symptoms began at age 3. There is no history of physical or sexual abuse. She does well in school, but these behaviors have had a significant impact on her social functioning. She is not taking any medications and has been in weekly cognitive-behavioral therapy (CBT) for the last year. A has had several UTIs but otherwise is physically healthy.
Which diagnosis best describes A’s condition?
a) non-suicidal self-injury (NSSI)
b) generalized anxiety disorder (GAD)
c) obsessive-compulsive disorder (OCD)
d) Tourette’s disorder (TD)
The authors’ observations
A is causing herself to bleed and says she wants to stop this behavior. Onset of NSSI typically is age 12 to 14 and could be accompanied by traits of cluster B personality disorders.1 In A’s case, her age and absence of any stated desire to relieve stress or intense negative affective states rules out NSSI.
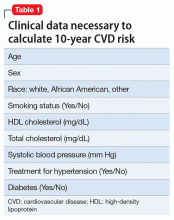
Because A has multiple and frequent fears, worries, and anxieties that have been present for years and have caused significant functional impairment, a diagnosis of GAD is warranted. Because she has had both motor and vocal tics for more than 1 year, she also meets diagnostic criteria for TD (Table 1).
In young children, OCD manifests primarily with compulsive behavior, such as excessive hand washing, counting, and ordering, that interferes with functioning. Although A has bedtime checking rituals, she has no significant functional impairment from these rituals alone. A’s finger-insertion behavior could be interpreted as a complex motor tic or as a compulsion, in which case impairment was significant enough to justify a diagnosis of OCD.
Many individuals with OCD report the need to engage in compulsive behavior to decrease anxiety or until they experience a “just right” feeling.2 However, neither A nor her mother reported the need for the “just right” feeling. The child recognized the urge to put her finger in her nose and did experience relief of anxiety after drawing blood. Although A said that she was unable to control her hands, she was observed frequently touching the side of her nose in an attempt to avoid inserting her finger in her nose.
Compulsive behavior that results in self-injury typically is not seen in OCD except in children with severe neurologic complications, low intellectual functioning, psychosis, or autism.3
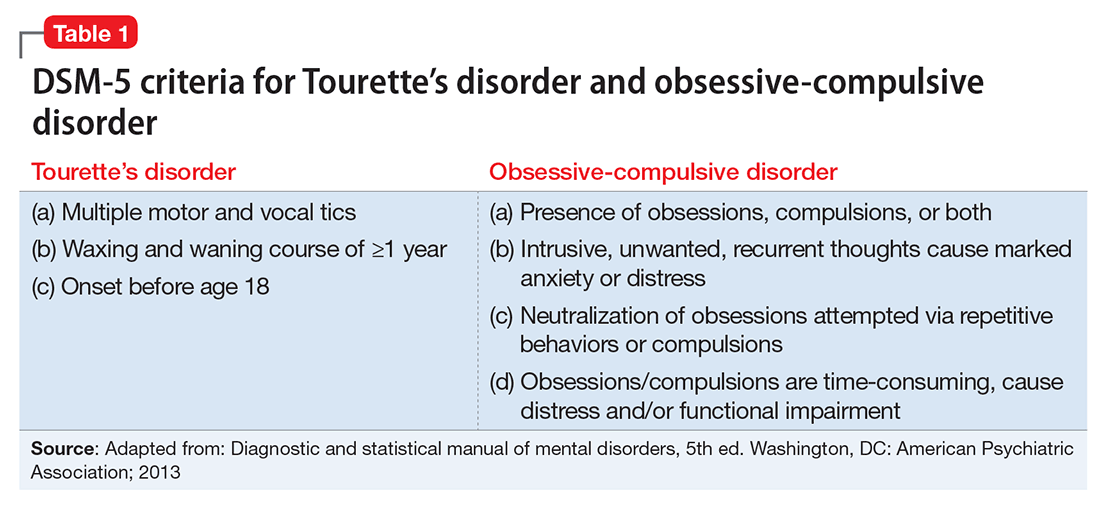
It often is difficult to determine if complex motor or vocal tics are compulsions (Table 2). Indeed, the same biologic mechanisms are thought to be implicated in TD and OCD.4 A significant percentage of children with OCD have tics, and patients often report that they are unable to distinguish between compulsions and complex tics.5 Therefore, we thought that a reasonable differential included both TD and OCD, but more careful assessment over time was required.
Treatment options
A has been receiving CBT for more than 1 year but her symptoms were worsening, which prompted her parents to seek evaluation in our clinic. Because of the level of interference with daily functioning and significant distress, our priority was developing a treatment plan that has the best chance of quickly reducing symptom severity and frequency. The results of the large-scale Pediatric OCD Treatment Study (POTS), which evaluated children age 7 to 17, and the Child/Adolescent Multimodal Anxiety Study, which evaluated children age <12, indicated that the combination of CBT with a selective serotonin reuptake inhibitor (SSRI) reduced OCD symptoms more than either modality alone.6,7 Considerations for using SSRIs in this age group include:
- the risk of behavioral activation
- poor tolerability
- lack of an evidence base for dosage optimization.
The American Academy of Child and Adolescent Psychiatry’s Preschool Psychopharmacology Working Group’s guidelines for treating anxiety in preschoolers state that pharmacotherapeutic intervention can be considered when symptoms are intolerable and adequate psychotherapy interventions have been tried.8 In A’s case, she had been receiving CBT for a year without improvement in symptoms; therefore, initiating medication was indicated, as well as an examination of therapeutic modalities being used.
Treatment Next steps
A is started on liquid fluoxetine, 20 mg/5 mL, 1 mL (4 mg) daily, because of her inability to swallow pills and her young age. According to her mother, a week later A is sleeping better and seems happier. The entire family seems less stressed. During the third week, A successfully goes on a camping trip with her family and is starting to eat better. Her finger-in-nose insertions still are occurring but, according to her mother, she is not putting her finger in her vagina. In session, she is not observed putting her finger in her nose or touching her nose, which she had done frequently during the initial evaluation. Fluoxetine seems to be well tolerated and the dosage is increased to 2 mL (8 mg) per day.
Although A has weekly scheduled appointments, she is not brought in again until a month later. At that time her mother reports an approximately 40% improvement in overall symptoms, including less frequent nose-insertion behaviors.
What type of psychotherapy would you employ for A?
a) CBT
b) behavioral therapy
c) habit reversal training (HRT)
d) pharmacotherapy alone
The authors’ observations
The treatment team planned to begin psychotherapy after A showed a decrease in anxiety and frequency of problem behaviors to a point where she could benefit. Evidence-based treatment for compulsions and tics is CBT and/or HRT.9 However, clinicians frequently encounter special challenges in helping young children (age 5 to 8) who have OCD. Factors such as family functioning, parental accommodation to the child’s symptoms, and the child’s ability to understand symptoms, exposure and response prevention, and willingness to tolerate discomfort should be considered if treatment is to be effective.
Research has shown that including parents when treating anxious children—especially young children—can facilitate gains and hasten positive outcomes.10,11 The POTS Jr study showed the relative efficacy of a family-based CBT model for young children with OCD that emphasizes consistent involvement of parents in all phases of treatment.12 In this case, A and her mother were seen together for psychotherapy, with an initial focus on learning more about the antecedents and consequences of the child’s behaviors.
OUTCOME Inconsistencies
Treatment was initiated during the summer. With the upcoming start of the school year, A begins to complain of daily headache, stomachache, and anxiety related to the start of school. Fluoxetine is increased to 3 mL/d (12 mg/d). After school starts, her mother stops going to work and begins attending school daily with A to relieve both her and the child’s anxiety.
The following week, the mother pages the psychiatrist, hysterical and crying because she thought the child was “pulling her hair out so much she looks like a cancer survivor.” Both parents blame the increase in fluoxetine for the heightened anxiety. At the next visit, the treatment team does not notice any evidence of unusual hair loss on the child. A has not attended school for several weeks, and her mother has not returned to work. Her parents report that the finger-to-nose behavior has increased, although it is not observed during the session, and fluoxetine is tapered as her parents requested.
At the next session, her mother notes a significant increase in finger-to-nose behavior and requests that the child be put back on fluoxetine, saying, “I would give anything to have the child I had on Prozac back.”
How would you proceed?
a) confront the mother’s inconsistencies
b) restart fluoxetine and continue psychotherapy
c) refer A to another clinic or therapist
d) refer A to inpatient care
The authors’ observations
The treatment team identified several barriers to successful treatment in our clinic. The level of functional interference caused by A’s symptoms indicated sessions more often than once a week, but the parents felt that the distance from our clinic to their home made this too difficult. The mother’s anxiety and obvious distress over her daughter’s symptoms precluded working closely with child. Parental anxiety is correlated with the child’s anxiety and can moderate treatment outcome.11 In response to the suffering of their anxious children, especially young ones, parents often will become anxious and accommodate to the child’s symptoms, which we strongly suspected was happening with A’s mother.
Parents’ concerns about A’s symptoms and response to treatment were addressed during a family meeting. Recognizing that the level of care needed by this family was higher than could be provided in our clinic, we recommended referral to a specialty clinic. A was brought to another clinic, and treatment at our facility was terminated.
1. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27(2):226-239.
2. Miguel EC, do Rosário-Campos MC, Prado HS, et al. Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry. 2000;61(2):150-156.
3. Nock MK, Favazza A. Non-suicidal self-injury: definition and classification. In: Nock MK, ed. Understanding nonsuicidal self-injury: origins, assessment, and treatment. Washington, DC: American Psychological Association; 2009:9-18.
4. Goodman WK, Storch EA, Geffken GR, et al. Obsessive-compulsive disorder in Tourette syndrome. J Child Neurol. 2006;21(8):704-714.
5. Garcia AM, Freeman JB, Himle MB, et al. Phenomenology of early childhood onset obsessive-compulsive disorder. J Psychopathol Behav Assess. 2009;31(2):104-111.
6. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
7. Piacentini JC, Bennett S, Compton SN, et al. 24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS). J Am Acad Child Adolesc Psychiatry. 2014;53(3):297-310.
8. Gleason MM, Egger HL, Emslie GJ, et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1532-1572.
9. Abramowitz JS, Whiteside SP, Deacon BJ. The effectiveness of treatment for pediatric obsessive-compulsive disorder: a meta-analysis. Behavior Therapy. 2005;36(1):55-63.
10. Barmish AJ, Kendall PC. Should parents be co-clients in cognitive-behavioral therapy for anxious youth. J Clin Child Adolesc Psychol. 2005;34(3):569-581.
11. Drake KL, Ginsburg GS. Family factors in the development, treatment, and prevention of childhood anxiety disorders. Clin Child Fam Psychol Rev. 2012;15(2):144-162.
12. Freeman J, Sapyta J, Garcia A, et al. Family-based treatment of early childhood obsessive-compulsive disorder: the Pediatric Obsessive-Compulsive Disorder Treatment Study for Young Children (POTS Jr)—a randomized clinical trial. JAMA Psychiatry. 2014;71(6):689-698.
CASE Anxious and self-injurious
A, age 6, is forcibly inserting her finger into her nose repeatedly until she bleeds profusely, as many as 20 times per day. She is not nose-picking but is jabbing her finger into her nose as far as possible in a repetitive ramming motion. Less frequently, she inserts her finger into her vagina, resulting in chronic urinary tract infections (UTIs). She has bedtime checking rituals; worries that her parents will die; has a fear of vomiting to the point where she stopped eating normally and lost 5 lb in 6 months; intense fear of storms; refusal to use public bathrooms; and involuntary throat clearing, facial grimacing, and lip twitches.
A’s symptoms began at age 3. There is no history of physical or sexual abuse. She does well in school, but these behaviors have had a significant impact on her social functioning. She is not taking any medications and has been in weekly cognitive-behavioral therapy (CBT) for the last year. A has had several UTIs but otherwise is physically healthy.
Which diagnosis best describes A’s condition?
a) non-suicidal self-injury (NSSI)
b) generalized anxiety disorder (GAD)
c) obsessive-compulsive disorder (OCD)
d) Tourette’s disorder (TD)
The authors’ observations
A is causing herself to bleed and says she wants to stop this behavior. Onset of NSSI typically is age 12 to 14 and could be accompanied by traits of cluster B personality disorders.1 In A’s case, her age and absence of any stated desire to relieve stress or intense negative affective states rules out NSSI.
Because A has multiple and frequent fears, worries, and anxieties that have been present for years and have caused significant functional impairment, a diagnosis of GAD is warranted. Because she has had both motor and vocal tics for more than 1 year, she also meets diagnostic criteria for TD (Table 1).

In young children, OCD manifests primarily with compulsive behavior, such as excessive hand washing, counting, and ordering, that interferes with functioning. Although A has bedtime checking rituals, she has no significant functional impairment from these rituals alone. A’s finger-insertion behavior could be interpreted as a complex motor tic or as a compulsion, in which case impairment was significant enough to justify a diagnosis of OCD.
Many individuals with OCD report the need to engage in compulsive behavior to decrease anxiety or until they experience a “just right” feeling.2 However, neither A nor her mother reported the need for the “just right” feeling. The child recognized the urge to put her finger in her nose and did experience relief of anxiety after drawing blood. Although A said that she was unable to control her hands, she was observed frequently touching the side of her nose in an attempt to avoid inserting her finger in her nose.
Compulsive behavior that results in self-injury typically is not seen in OCD except in children with severe neurologic complications, low intellectual functioning, psychosis, or autism.3
It often is difficult to determine if complex motor or vocal tics are compulsions (Table 2). Indeed, the same biologic mechanisms are thought to be implicated in TD and OCD.4 A significant percentage of children with OCD have tics, and patients often report that they are unable to distinguish between compulsions and complex tics.5 Therefore, we thought that a reasonable differential included both TD and OCD, but more careful assessment over time was required.

Treatment options
A has been receiving CBT for more than 1 year but her symptoms were worsening, which prompted her parents to seek evaluation in our clinic. Because of the level of interference with daily functioning and significant distress, our priority was developing a treatment plan that has the best chance of quickly reducing symptom severity and frequency. The results of the large-scale Pediatric OCD Treatment Study (POTS), which evaluated children age 7 to 17, and the Child/Adolescent Multimodal Anxiety Study, which evaluated children age <12, indicated that the combination of CBT with a selective serotonin reuptake inhibitor (SSRI) reduced OCD symptoms more than either modality alone.6,7 Considerations for using SSRIs in this age group include:
- the risk of behavioral activation
- poor tolerability
- lack of an evidence base for dosage optimization.
The American Academy of Child and Adolescent Psychiatry’s Preschool Psychopharmacology Working Group’s guidelines for treating anxiety in preschoolers state that pharmacotherapeutic intervention can be considered when symptoms are intolerable and adequate psychotherapy interventions have been tried.8 In A’s case, she had been receiving CBT for a year without improvement in symptoms; therefore, initiating medication was indicated, as well as an examination of therapeutic modalities being used.
Treatment Next steps
A is started on liquid fluoxetine, 20 mg/5 mL, 1 mL (4 mg) daily, because of her inability to swallow pills and her young age. According to her mother, a week later A is sleeping better and seems happier. The entire family seems less stressed. During the third week, A successfully goes on a camping trip with her family and is starting to eat better. Her finger-in-nose insertions still are occurring but, according to her mother, she is not putting her finger in her vagina. In session, she is not observed putting her finger in her nose or touching her nose, which she had done frequently during the initial evaluation. Fluoxetine seems to be well tolerated and the dosage is increased to 2 mL (8 mg) per day.
Although A has weekly scheduled appointments, she is not brought in again until a month later. At that time her mother reports an approximately 40% improvement in overall symptoms, including less frequent nose-insertion behaviors.
What type of psychotherapy would you employ for A?
a) CBT
b) behavioral therapy
c) habit reversal training (HRT)
d) pharmacotherapy alone
The authors’ observations
The treatment team planned to begin psychotherapy after A showed a decrease in anxiety and frequency of problem behaviors to a point where she could benefit. Evidence-based treatment for compulsions and tics is CBT and/or HRT.9 However, clinicians frequently encounter special challenges in helping young children (age 5 to 8) who have OCD. Factors such as family functioning, parental accommodation to the child’s symptoms, and the child’s ability to understand symptoms, exposure and response prevention, and willingness to tolerate discomfort should be considered if treatment is to be effective.
Research has shown that including parents when treating anxious children—especially young children—can facilitate gains and hasten positive outcomes.10,11 The POTS Jr study showed the relative efficacy of a family-based CBT model for young children with OCD that emphasizes consistent involvement of parents in all phases of treatment.12 In this case, A and her mother were seen together for psychotherapy, with an initial focus on learning more about the antecedents and consequences of the child’s behaviors.
OUTCOME Inconsistencies
Treatment was initiated during the summer. With the upcoming start of the school year, A begins to complain of daily headache, stomachache, and anxiety related to the start of school. Fluoxetine is increased to 3 mL/d (12 mg/d). After school starts, her mother stops going to work and begins attending school daily with A to relieve both her and the child’s anxiety.
The following week, the mother pages the psychiatrist, hysterical and crying because she thought the child was “pulling her hair out so much she looks like a cancer survivor.” Both parents blame the increase in fluoxetine for the heightened anxiety. At the next visit, the treatment team does not notice any evidence of unusual hair loss on the child. A has not attended school for several weeks, and her mother has not returned to work. Her parents report that the finger-to-nose behavior has increased, although it is not observed during the session, and fluoxetine is tapered as her parents requested.
At the next session, her mother notes a significant increase in finger-to-nose behavior and requests that the child be put back on fluoxetine, saying, “I would give anything to have the child I had on Prozac back.”
How would you proceed?
a) confront the mother’s inconsistencies
b) restart fluoxetine and continue psychotherapy
c) refer A to another clinic or therapist
d) refer A to inpatient care
The authors’ observations
The treatment team identified several barriers to successful treatment in our clinic. The level of functional interference caused by A’s symptoms indicated sessions more often than once a week, but the parents felt that the distance from our clinic to their home made this too difficult. The mother’s anxiety and obvious distress over her daughter’s symptoms precluded working closely with child. Parental anxiety is correlated with the child’s anxiety and can moderate treatment outcome.11 In response to the suffering of their anxious children, especially young ones, parents often will become anxious and accommodate to the child’s symptoms, which we strongly suspected was happening with A’s mother.
Parents’ concerns about A’s symptoms and response to treatment were addressed during a family meeting. Recognizing that the level of care needed by this family was higher than could be provided in our clinic, we recommended referral to a specialty clinic. A was brought to another clinic, and treatment at our facility was terminated.
CASE Anxious and self-injurious
A, age 6, is forcibly inserting her finger into her nose repeatedly until she bleeds profusely, as many as 20 times per day. She is not nose-picking but is jabbing her finger into her nose as far as possible in a repetitive ramming motion. Less frequently, she inserts her finger into her vagina, resulting in chronic urinary tract infections (UTIs). She has bedtime checking rituals; worries that her parents will die; has a fear of vomiting to the point where she stopped eating normally and lost 5 lb in 6 months; intense fear of storms; refusal to use public bathrooms; and involuntary throat clearing, facial grimacing, and lip twitches.
A’s symptoms began at age 3. There is no history of physical or sexual abuse. She does well in school, but these behaviors have had a significant impact on her social functioning. She is not taking any medications and has been in weekly cognitive-behavioral therapy (CBT) for the last year. A has had several UTIs but otherwise is physically healthy.
Which diagnosis best describes A’s condition?
a) non-suicidal self-injury (NSSI)
b) generalized anxiety disorder (GAD)
c) obsessive-compulsive disorder (OCD)
d) Tourette’s disorder (TD)
The authors’ observations
A is causing herself to bleed and says she wants to stop this behavior. Onset of NSSI typically is age 12 to 14 and could be accompanied by traits of cluster B personality disorders.1 In A’s case, her age and absence of any stated desire to relieve stress or intense negative affective states rules out NSSI.
Because A has multiple and frequent fears, worries, and anxieties that have been present for years and have caused significant functional impairment, a diagnosis of GAD is warranted. Because she has had both motor and vocal tics for more than 1 year, she also meets diagnostic criteria for TD (Table 1).

In young children, OCD manifests primarily with compulsive behavior, such as excessive hand washing, counting, and ordering, that interferes with functioning. Although A has bedtime checking rituals, she has no significant functional impairment from these rituals alone. A’s finger-insertion behavior could be interpreted as a complex motor tic or as a compulsion, in which case impairment was significant enough to justify a diagnosis of OCD.
Many individuals with OCD report the need to engage in compulsive behavior to decrease anxiety or until they experience a “just right” feeling.2 However, neither A nor her mother reported the need for the “just right” feeling. The child recognized the urge to put her finger in her nose and did experience relief of anxiety after drawing blood. Although A said that she was unable to control her hands, she was observed frequently touching the side of her nose in an attempt to avoid inserting her finger in her nose.
Compulsive behavior that results in self-injury typically is not seen in OCD except in children with severe neurologic complications, low intellectual functioning, psychosis, or autism.3
It often is difficult to determine if complex motor or vocal tics are compulsions (Table 2). Indeed, the same biologic mechanisms are thought to be implicated in TD and OCD.4 A significant percentage of children with OCD have tics, and patients often report that they are unable to distinguish between compulsions and complex tics.5 Therefore, we thought that a reasonable differential included both TD and OCD, but more careful assessment over time was required.

Treatment options
A has been receiving CBT for more than 1 year but her symptoms were worsening, which prompted her parents to seek evaluation in our clinic. Because of the level of interference with daily functioning and significant distress, our priority was developing a treatment plan that has the best chance of quickly reducing symptom severity and frequency. The results of the large-scale Pediatric OCD Treatment Study (POTS), which evaluated children age 7 to 17, and the Child/Adolescent Multimodal Anxiety Study, which evaluated children age <12, indicated that the combination of CBT with a selective serotonin reuptake inhibitor (SSRI) reduced OCD symptoms more than either modality alone.6,7 Considerations for using SSRIs in this age group include:
- the risk of behavioral activation
- poor tolerability
- lack of an evidence base for dosage optimization.
The American Academy of Child and Adolescent Psychiatry’s Preschool Psychopharmacology Working Group’s guidelines for treating anxiety in preschoolers state that pharmacotherapeutic intervention can be considered when symptoms are intolerable and adequate psychotherapy interventions have been tried.8 In A’s case, she had been receiving CBT for a year without improvement in symptoms; therefore, initiating medication was indicated, as well as an examination of therapeutic modalities being used.
Treatment Next steps
A is started on liquid fluoxetine, 20 mg/5 mL, 1 mL (4 mg) daily, because of her inability to swallow pills and her young age. According to her mother, a week later A is sleeping better and seems happier. The entire family seems less stressed. During the third week, A successfully goes on a camping trip with her family and is starting to eat better. Her finger-in-nose insertions still are occurring but, according to her mother, she is not putting her finger in her vagina. In session, she is not observed putting her finger in her nose or touching her nose, which she had done frequently during the initial evaluation. Fluoxetine seems to be well tolerated and the dosage is increased to 2 mL (8 mg) per day.
Although A has weekly scheduled appointments, she is not brought in again until a month later. At that time her mother reports an approximately 40% improvement in overall symptoms, including less frequent nose-insertion behaviors.
What type of psychotherapy would you employ for A?
a) CBT
b) behavioral therapy
c) habit reversal training (HRT)
d) pharmacotherapy alone
The authors’ observations
The treatment team planned to begin psychotherapy after A showed a decrease in anxiety and frequency of problem behaviors to a point where she could benefit. Evidence-based treatment for compulsions and tics is CBT and/or HRT.9 However, clinicians frequently encounter special challenges in helping young children (age 5 to 8) who have OCD. Factors such as family functioning, parental accommodation to the child’s symptoms, and the child’s ability to understand symptoms, exposure and response prevention, and willingness to tolerate discomfort should be considered if treatment is to be effective.
Research has shown that including parents when treating anxious children—especially young children—can facilitate gains and hasten positive outcomes.10,11 The POTS Jr study showed the relative efficacy of a family-based CBT model for young children with OCD that emphasizes consistent involvement of parents in all phases of treatment.12 In this case, A and her mother were seen together for psychotherapy, with an initial focus on learning more about the antecedents and consequences of the child’s behaviors.
OUTCOME Inconsistencies
Treatment was initiated during the summer. With the upcoming start of the school year, A begins to complain of daily headache, stomachache, and anxiety related to the start of school. Fluoxetine is increased to 3 mL/d (12 mg/d). After school starts, her mother stops going to work and begins attending school daily with A to relieve both her and the child’s anxiety.
The following week, the mother pages the psychiatrist, hysterical and crying because she thought the child was “pulling her hair out so much she looks like a cancer survivor.” Both parents blame the increase in fluoxetine for the heightened anxiety. At the next visit, the treatment team does not notice any evidence of unusual hair loss on the child. A has not attended school for several weeks, and her mother has not returned to work. Her parents report that the finger-to-nose behavior has increased, although it is not observed during the session, and fluoxetine is tapered as her parents requested.
At the next session, her mother notes a significant increase in finger-to-nose behavior and requests that the child be put back on fluoxetine, saying, “I would give anything to have the child I had on Prozac back.”
How would you proceed?
a) confront the mother’s inconsistencies
b) restart fluoxetine and continue psychotherapy
c) refer A to another clinic or therapist
d) refer A to inpatient care
The authors’ observations
The treatment team identified several barriers to successful treatment in our clinic. The level of functional interference caused by A’s symptoms indicated sessions more often than once a week, but the parents felt that the distance from our clinic to their home made this too difficult. The mother’s anxiety and obvious distress over her daughter’s symptoms precluded working closely with child. Parental anxiety is correlated with the child’s anxiety and can moderate treatment outcome.11 In response to the suffering of their anxious children, especially young ones, parents often will become anxious and accommodate to the child’s symptoms, which we strongly suspected was happening with A’s mother.
Parents’ concerns about A’s symptoms and response to treatment were addressed during a family meeting. Recognizing that the level of care needed by this family was higher than could be provided in our clinic, we recommended referral to a specialty clinic. A was brought to another clinic, and treatment at our facility was terminated.
1. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27(2):226-239.
2. Miguel EC, do Rosário-Campos MC, Prado HS, et al. Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry. 2000;61(2):150-156.
3. Nock MK, Favazza A. Non-suicidal self-injury: definition and classification. In: Nock MK, ed. Understanding nonsuicidal self-injury: origins, assessment, and treatment. Washington, DC: American Psychological Association; 2009:9-18.
4. Goodman WK, Storch EA, Geffken GR, et al. Obsessive-compulsive disorder in Tourette syndrome. J Child Neurol. 2006;21(8):704-714.
5. Garcia AM, Freeman JB, Himle MB, et al. Phenomenology of early childhood onset obsessive-compulsive disorder. J Psychopathol Behav Assess. 2009;31(2):104-111.
6. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
7. Piacentini JC, Bennett S, Compton SN, et al. 24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS). J Am Acad Child Adolesc Psychiatry. 2014;53(3):297-310.
8. Gleason MM, Egger HL, Emslie GJ, et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1532-1572.
9. Abramowitz JS, Whiteside SP, Deacon BJ. The effectiveness of treatment for pediatric obsessive-compulsive disorder: a meta-analysis. Behavior Therapy. 2005;36(1):55-63.
10. Barmish AJ, Kendall PC. Should parents be co-clients in cognitive-behavioral therapy for anxious youth. J Clin Child Adolesc Psychol. 2005;34(3):569-581.
11. Drake KL, Ginsburg GS. Family factors in the development, treatment, and prevention of childhood anxiety disorders. Clin Child Fam Psychol Rev. 2012;15(2):144-162.
12. Freeman J, Sapyta J, Garcia A, et al. Family-based treatment of early childhood obsessive-compulsive disorder: the Pediatric Obsessive-Compulsive Disorder Treatment Study for Young Children (POTS Jr)—a randomized clinical trial. JAMA Psychiatry. 2014;71(6):689-698.
1. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27(2):226-239.
2. Miguel EC, do Rosário-Campos MC, Prado HS, et al. Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry. 2000;61(2):150-156.
3. Nock MK, Favazza A. Non-suicidal self-injury: definition and classification. In: Nock MK, ed. Understanding nonsuicidal self-injury: origins, assessment, and treatment. Washington, DC: American Psychological Association; 2009:9-18.
4. Goodman WK, Storch EA, Geffken GR, et al. Obsessive-compulsive disorder in Tourette syndrome. J Child Neurol. 2006;21(8):704-714.
5. Garcia AM, Freeman JB, Himle MB, et al. Phenomenology of early childhood onset obsessive-compulsive disorder. J Psychopathol Behav Assess. 2009;31(2):104-111.
6. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
7. Piacentini JC, Bennett S, Compton SN, et al. 24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS). J Am Acad Child Adolesc Psychiatry. 2014;53(3):297-310.
8. Gleason MM, Egger HL, Emslie GJ, et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1532-1572.
9. Abramowitz JS, Whiteside SP, Deacon BJ. The effectiveness of treatment for pediatric obsessive-compulsive disorder: a meta-analysis. Behavior Therapy. 2005;36(1):55-63.
10. Barmish AJ, Kendall PC. Should parents be co-clients in cognitive-behavioral therapy for anxious youth. J Clin Child Adolesc Psychol. 2005;34(3):569-581.
11. Drake KL, Ginsburg GS. Family factors in the development, treatment, and prevention of childhood anxiety disorders. Clin Child Fam Psychol Rev. 2012;15(2):144-162.
12. Freeman J, Sapyta J, Garcia A, et al. Family-based treatment of early childhood obsessive-compulsive disorder: the Pediatric Obsessive-Compulsive Disorder Treatment Study for Young Children (POTS Jr)—a randomized clinical trial. JAMA Psychiatry. 2014;71(6):689-698.
Treating agitation in schizophrenia
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
New bipolar disorder algorithm changes ranking of first-line therapies
Psychiatric consultations in long-term care: An evidence-based practical guide
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
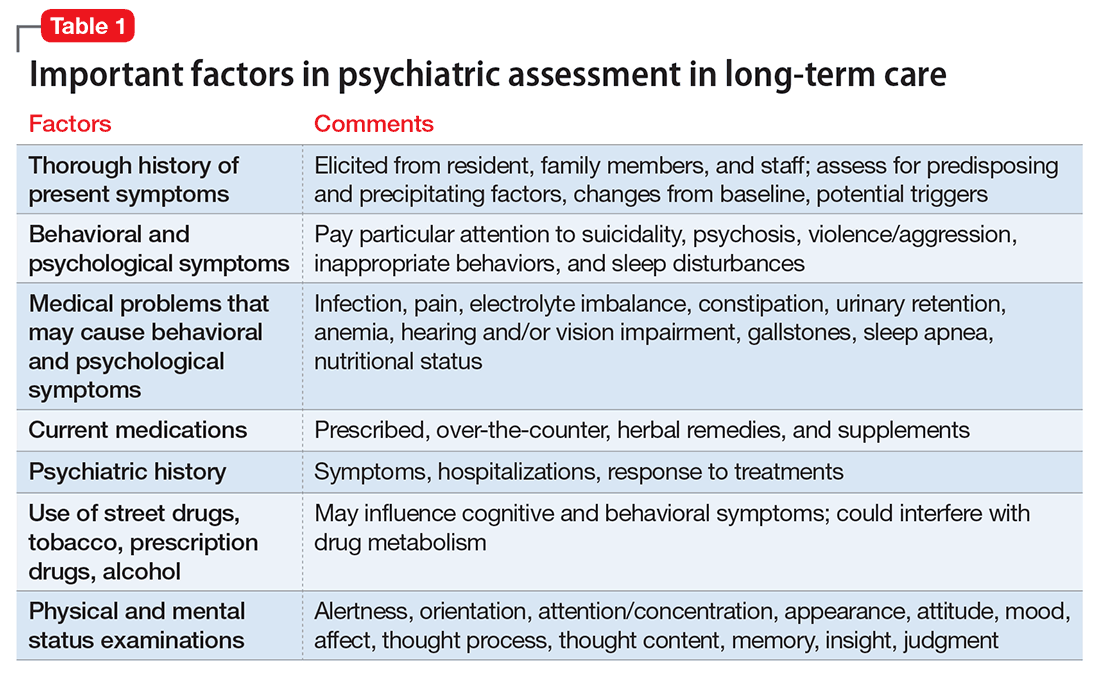
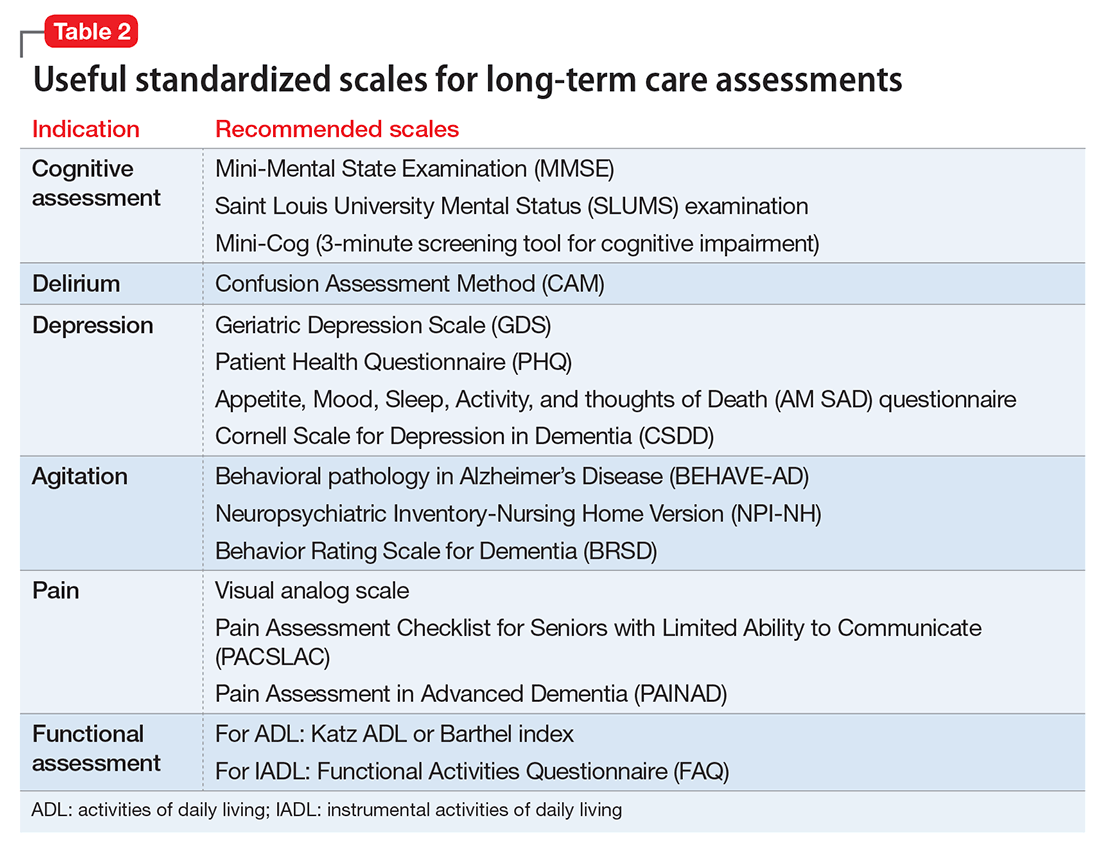
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).
This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
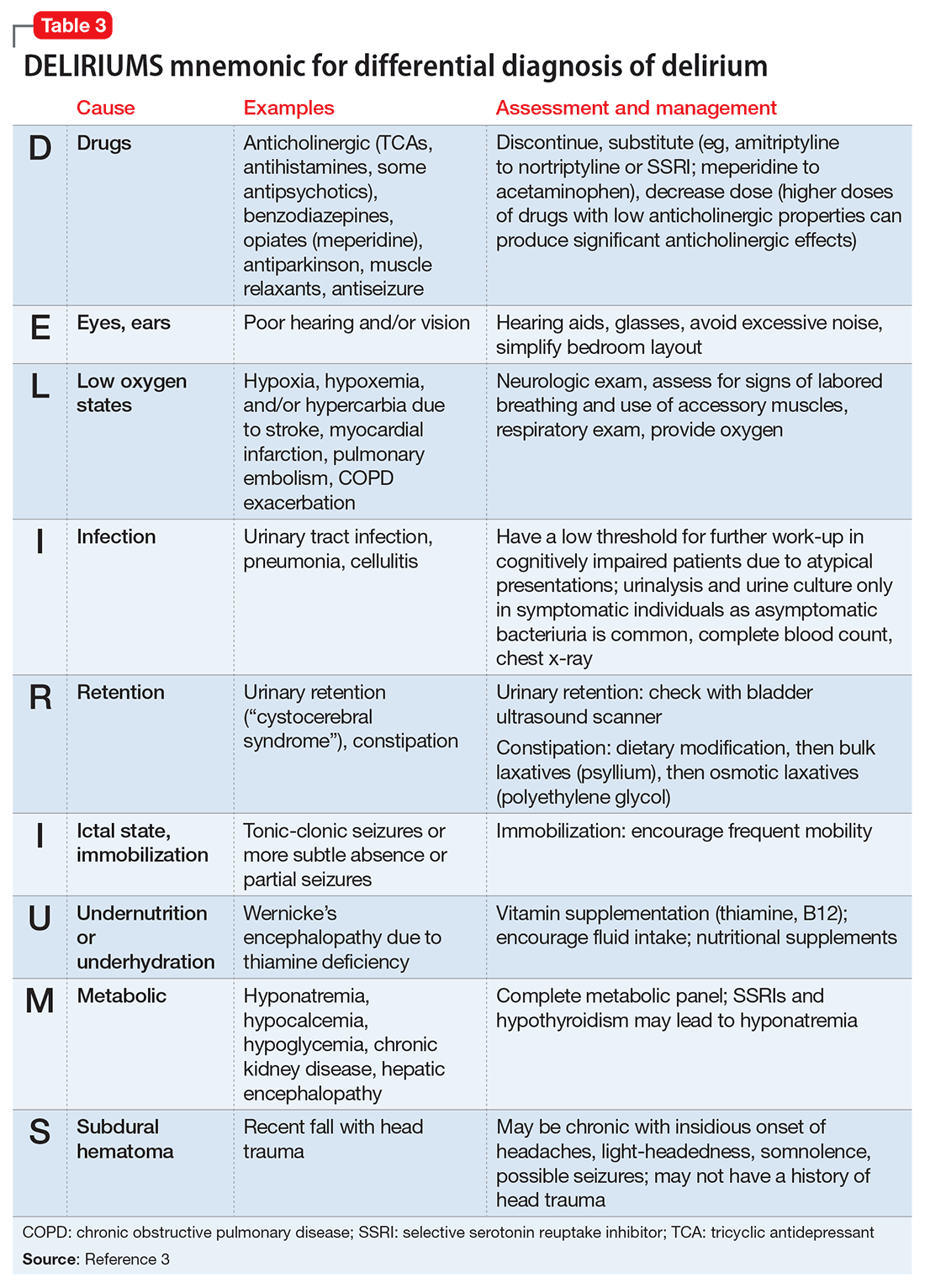
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5
The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14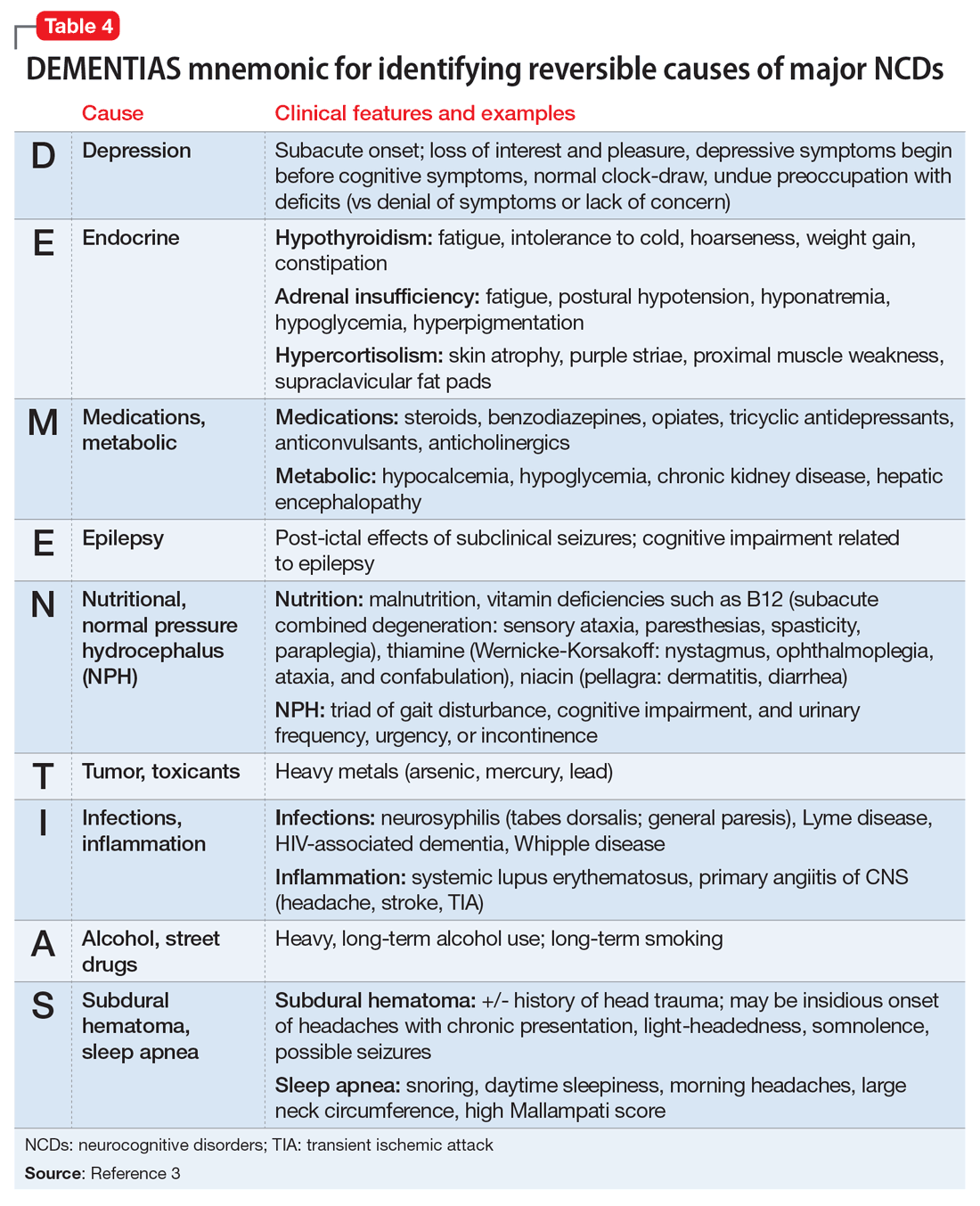
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).


This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5

The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).


This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5

The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
How to assess and manage high cholesterol in patients with mental illness
High serum cholesterol is a leading cause of heart attack and stroke,1,2 yet remains one of the most under-screened and undertreated modifiable risk factors in persons with mental illness. Well tolerated and effective treatments can considerably lower the risk of cardiovascular events, and should be offered to psychiatric patients who are at high risk, while considering possible adverse effects and potential interactions between psychotropics and medications used to lower cholesterol.
Systematic lowering of total cholesterol and, particularly, atherogenic low-density lipoprotein (LDL) and non-high density lipoprotein (HDL) cholesterol, results in consistent and significant reduction in risk of cardiovascular events in persons at risk for developing cardiovascular disease (CVD) and in preventing reoccurrence of these events.1,3,4 Even individuals who have relatively lower levels of total cholesterol but are at high risk (such as if a cardiovascular event has occurred) could reduce their CVD risk (known as secondary prevention) through lipid lowering therapies.5,6
Adults with psychiatric illness shoulder a disproportionate burden of CVD morbidity and mortality, especially those with severe mental illness (SMI, schizophrenia, schizoaffective disorder, bipolar disorder, treatment-resistant depression).7-9 Among modifiable CVD risk factors, dyslipidemia has the highest rates of missed screenings and treatment within psychiatric populations. In one analysis, up to 90% of adults with SMI and identified lipid disorders did not receive treatment.10 Persons with SMI generally do not receive guideline-concordant, systematic quality preventive care, which contributes to a widening mortality gap for this population.11,12
This review aims to provide clinicians with practical guidance on the assessment and management of high cholesterol to improve recognition and treatment, lower CVD risk, and reduce this observed mortality gap.
Screening and diagnosis
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) released updated guidelines on diagnosing and managing high cholesterol to reduce CVD risk.1 These guidelines focus on updated 10-year CVD risk assessment models with treatment goals reliant on adherence to statin therapy rather than pre-specified cholesterol targets listed in previous guidelines.13
Updates to assessment and treatment guidelines have removed some barriers to screening and diagnosing high cholesterol—namely, fasting lipid panels are no longer required to determine 10-year CVD risk and initiate treatment.14 For adults taking a second-generation antipsychotic that is associated with weight gain and metabolic syndrome, experts generally recommend yearly non-fasting lipid panels.6,14
The United States Preventive Services Task Force recommends screening:
- men age ≥35 at average risk for CVD every 5 years
- women age ≥45 every 5 years15
- adults as young as age 20 who have accelerated risk factors, such as cigarette smoking and hypertension
- adults with a family history of heart attack or stroke in male first-degree relative age ≥50 and female first-degree relatives age ≥60.
Many adults receiving care in behavioral health settings, regardless of their medication regimen, qualify for screening at least every 5 years, if not more frequently. Although statin treatment before age 40 is less beneficial and likely not necessary for primary prevention, monitoring could help identify alternative therapies and prioritize more intensive diet and lifestyle modifications.
At a routine office visit, clinicians can collect vital signs, record smoking status, and reconcile all medications, which provides the data needed to calculate a patient’s 10-year CVD risk (Table 1). Coupled with laboratory testing, which includes a non-fasting total cholesterol, HDL, and hemoglobin A1c (representative of a 3-month blood sugar average, ≥6.5% is diagnostic of type 2 diabetes mellitus [T2DM]), all data points can be entered into online risk calculators (search “ASCVD risk calculator” or visit http://tools.acc.org/ASCVD-Risk-Estimator to access the ACC/AHA risk calculator). Persons scoring >20% 10-year risk are considered at extremely high risk, and are in the same risk category as adults with existing CVD or who have had a cardiovascular event. Persons at <5% 10-year risk generally are considered low risk, and primary prevention with a statin medication is not indicated.
Treatment and management
Dietary modification and lifestyle changes (exercise, quitting smoking), lowering high cholesterol with medications, and switching from highly metabolically active drugs to less metabolically active ones can help lower total cholesterol in patients at risk of CVD.
Statins
HMG-CoA reductase inhibitors (statins) consistently reduce total cholesterol and non-HDL cholesterol by 30% to 50%, depending on drug and dosage (potency, listed as low, medium, and high). Not all statins are equally effective at lowering cholesterol; some are more potent than others (Table 2).16
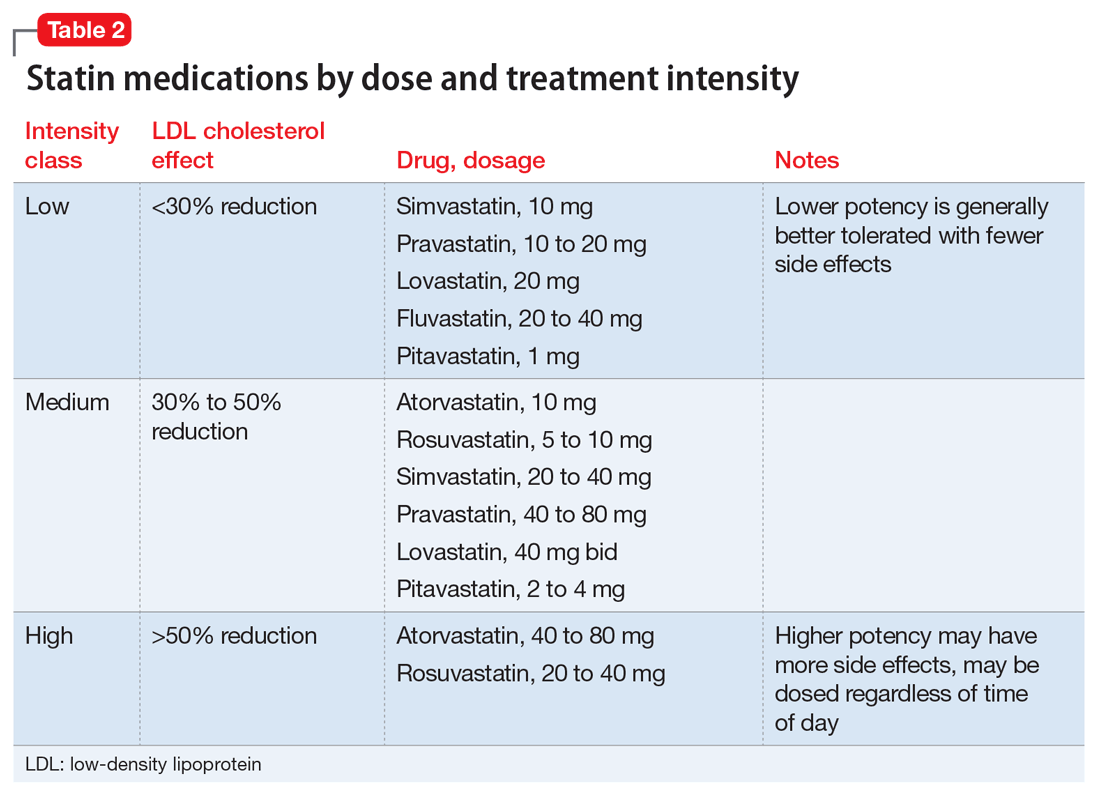
Individuals are eligible for statin therapy based on their level of CVD risk. Persons at higher risk generally benefit from greater intensity statin treatment and cholesterol reduction; highest intensity statin regimens can lower total cholesterol by approximately 50%.
There are 4 statin eligibility classes (Table 3). Most adults fall into category 4: 10-year risk of >7.5% and needing primary prevention. In addition to removing specific LDL targets as therapy goals, calculation of this risk percentage and the specific cut-off values have been the most controversial aspects of the new cholesterol guidelines. Most experts agree that, in adults age 40 to 75, 10-year risk >10% indicates daily statin use as tolerated for primary prevention, and 10-year risk <5% does not warrant statin use. Recent large studies have validated these new techniques for calculating risk, and found them to be beneficial in potential for cost savings and risk classification.17,18
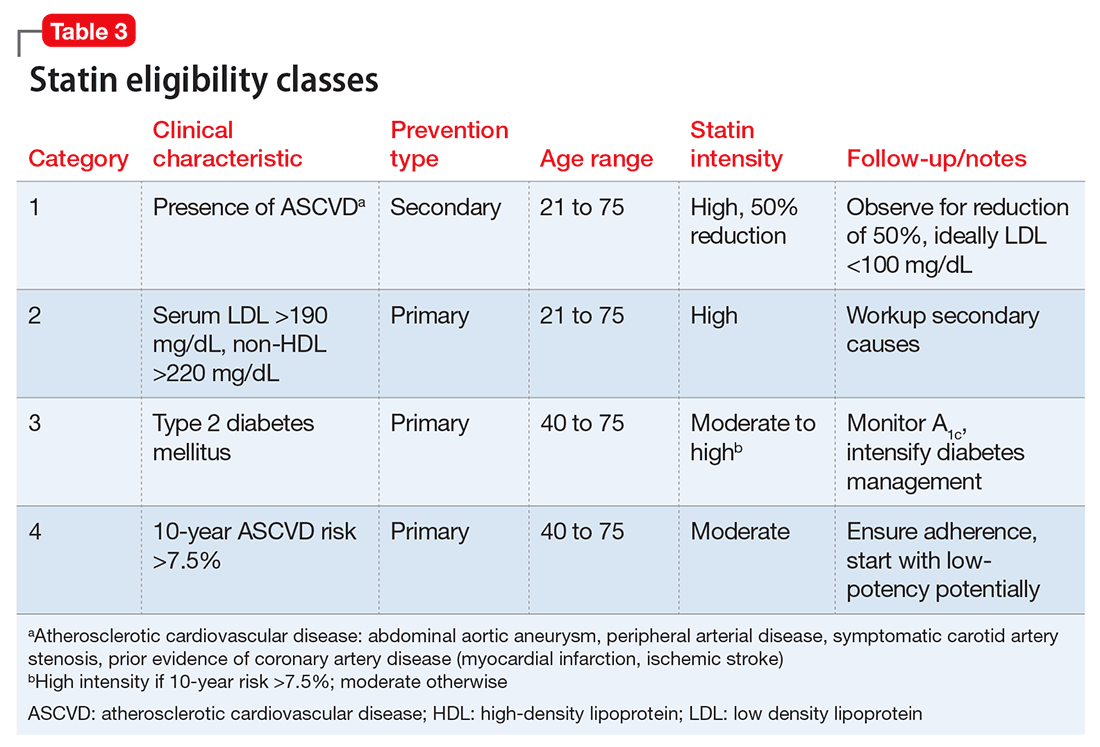
Considerations in psychiatric patients. Statins have been associated with depression in case series, but larger analyses have not confirmed this association.19 Emerging evidence has identified a potential correlation between statin use and accelerated onset of T2DM, but the absolute risk is relatively low and most experts continue to recommend statin therapy despite this potential risk.13 Many statins, including atorvastatin, are available as a generic and can be taken once daily. Some, such as simvastatin, have notable interactions with commonly prescribed psychotropics including risperidone and quetiapine. Pravastatin is dually excreted by the liver and kidneys and may have fewer drug-drug interactions in patients with psychiatric illness taking common psychotropic therapies, but is not considered a high-potency statin and might not confer adequate benefits in CVD risk reduction.
Contraindications. Statins are pregnancy category X, and generally should not be prescribed for women of childbearing age without intensive counseling. The most notable adverse effects for statins include muscle aches and cramps (myalgia), but generally are not severe. If encountered, consider checking a serum creatinine kinase (CK) level, and if significantly elevated above 10 times the upper limit, stopping statin therapy would be advised. If the CK is only mildly elevated, consider lowering the dosage or switching to a lower potency agent. Lovastatin and pravastatin generally are better tolerated than atorvastatin and are considered lower potency (Table 2).
Statins can be safely used in the presence of liver conditions, such as hepatitis C and alcohol use, although periodic monitoring of transaminase levels is recommended. For adults in the general population without liver disease, regular monitoring of transaminase levels is not necessary.
Alternate lipid-lowering pharmacotherapies unfortunately have fallen out of favor. Fibrates, niacin, ezetimibe, and omega-3 fatty acids once were recommended to lower triglycerides or raise HDL cholesterol levels, but since have been shown to have little effect on cardiovascular morbidity or mortality. Adding further medications, other than statins, to lower cholesterol values to pre-defined targets is not the current standard of care.
High triglyceride concentrations traditionally have been addressed directly, but failure to improve CVD mortality or morbidity by treating triglycerides alone has resulted in refocusing clinical efforts in dyslipidemia management on atherogenic cholesterol, including LDL and non-HDL fractions.20 Non-fasting triglycerides >500 mg/dL should be retested when fasting, and levels that remain >500 mg/dL could place the patient at risk for pancreatitis and might warrant intervention with fibrates at that time. This scenario is not common, and referral to a primary care physician or endocrinologist may be warranted.
Lifestyle changes
With or without statin therapies, diet and lifestyle changes are the cornerstone of healthy living and should be encouraged in all patients. Most overweight or obese patients will benefit from exercise and dietary modifications. Such interventions have shown potential for reducing total cholesterol and non-HDL and HDL cholesterol, but rarely are these interventions sustained long enough to produce meaningful reduction in CVD risk through lipid lowering. Diets rich in isocaloric tree nuts and red-yeast rice extract—a form of a statin—have shown promise in reducing cholesterol, but typically take excessive personal resources and are not sustained to the degree necessary to reduce CVD risk over time.21 Similarly, regular exercise routines can help lower overall cholesterol numbers, but rarely reduce total cholesterol by >10%.
Because individuals with SMI smoke at a higher rate than the general population, it should be noted that smoking cessation is associated with a reduction in total cholesterol and a trial of smoking cessation therapy is warranted before initiating a statin medication for primary prevention of CVD. Many patients would discover that their 10-year ASCVD risk would fall under the level needed for statin therapy if they could successfully stop smoking.
Switching pharmacotherapies
Switching antipsychotic agents from highly metabolically risky compounds, such as risperidone and olanzapine, to less metabolically active compounds, such as aripiprazole, ziprasidone, or haloperidol, have been associated with improvements in lipid profiles.22-24 Clinicians must weigh the potential benefits of switching therapies against the risk of psychiatric destabilization and long-term adherence, keeping in mind that changes in lipids seen with switching could be mild (approximately 10% reduction in total cholesterol).
Summing up
Cholesterol management is considered part of a program to systematically lower CVD risk. Statin therapy usually is indicated for life, or until the age of 75, at which point treatment risks and benefits change because of life expectancy. Other components of CVD risk reduction include a focus on blood pressure control, smoking cessation, T2DM management, and weight loss. Tracking lipid profiles over time to ensure broad targets of 30% to 50% reduction in total cholesterol, approximately 3 months after initiation and yearly thereafter, can help ensure adherence to therapy. With systematic lowering of modifiable CVD risk factors, we can hope to gradually improve the quality of life for our patients with mental illnesses (see the Box for a case example illustrating successful use of these strategies).
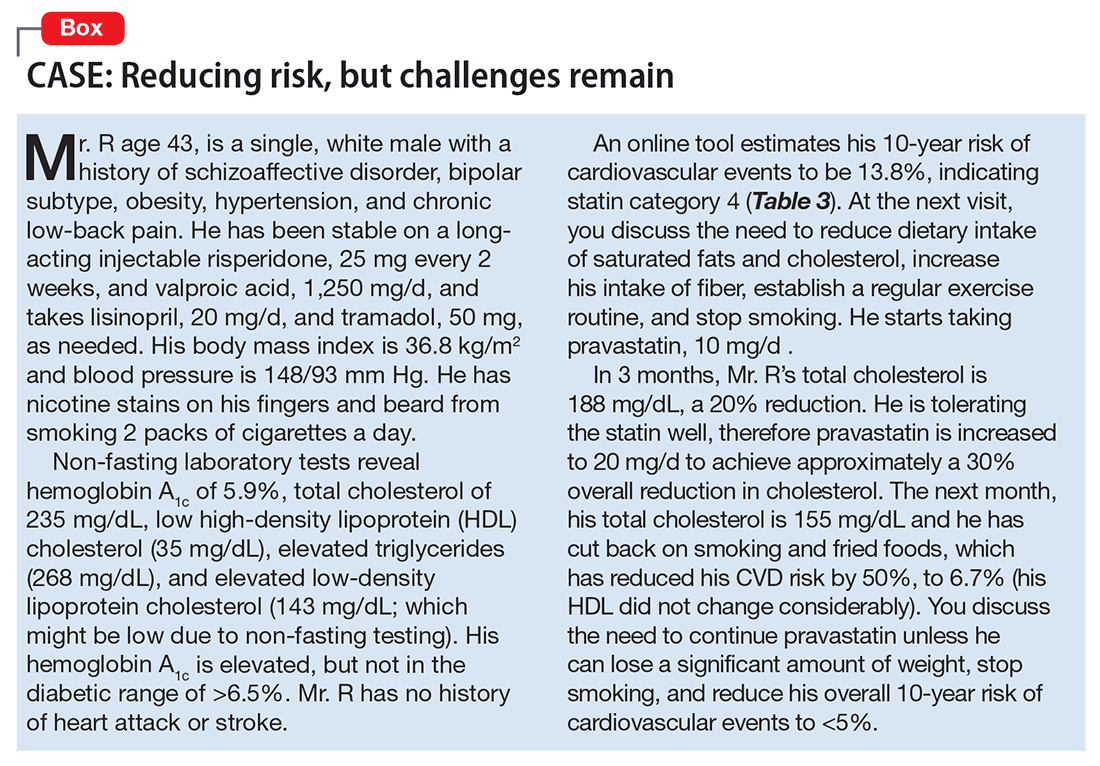
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 suppl B):S1-S45.
2. LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81(5):1721-1733.
3. Albert MA, Glynn RJ, Fonseca FA, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Am Heart J. 2011;162(1):106-114.e2.
4. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858.CD004816.pub5.
5. Gaziano JM, Gaziano TA. What’s new with measuring cholesterol? JAMA. 2013;310(19):2043-2044.
6. Emerging Risk Factors Collaboration; Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
7. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
8. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324-333.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states [published online March 15, 2006]. Prev Chronic Dis. 2006;3(2):A42.
10. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
11. Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321(7259):483-484.
12. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(suppl 4):69-80.
13. Ganda OP. Deciphering cholesterol treatment guidelines: a clinician’s perspective. JAMA. 2015;313(10):1009-1010.
14. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv. 2014;65(5):573-576.
15. U.S. Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia). http://www.uspreventiveservicestaskforce.org/uspstf/uspschol.htm. Published June 2008. Accessed October 12, 2016.
16. Cupp M. Characteristics of the various statins. Pharmacist’s Letter. 2012;28(6):280606.
17. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314(2):134-141.
18. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142-150.
19. You H, Lu W, Zhao S, et al. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14(11):1467-1476.
20. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81(9):1097-1102.
22. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205(1):23-32.
23. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
24. Stroup TS, McEvoy JP, Ring KD, et al; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947-956.
High serum cholesterol is a leading cause of heart attack and stroke,1,2 yet remains one of the most under-screened and undertreated modifiable risk factors in persons with mental illness. Well tolerated and effective treatments can considerably lower the risk of cardiovascular events, and should be offered to psychiatric patients who are at high risk, while considering possible adverse effects and potential interactions between psychotropics and medications used to lower cholesterol.
Systematic lowering of total cholesterol and, particularly, atherogenic low-density lipoprotein (LDL) and non-high density lipoprotein (HDL) cholesterol, results in consistent and significant reduction in risk of cardiovascular events in persons at risk for developing cardiovascular disease (CVD) and in preventing reoccurrence of these events.1,3,4 Even individuals who have relatively lower levels of total cholesterol but are at high risk (such as if a cardiovascular event has occurred) could reduce their CVD risk (known as secondary prevention) through lipid lowering therapies.5,6
Adults with psychiatric illness shoulder a disproportionate burden of CVD morbidity and mortality, especially those with severe mental illness (SMI, schizophrenia, schizoaffective disorder, bipolar disorder, treatment-resistant depression).7-9 Among modifiable CVD risk factors, dyslipidemia has the highest rates of missed screenings and treatment within psychiatric populations. In one analysis, up to 90% of adults with SMI and identified lipid disorders did not receive treatment.10 Persons with SMI generally do not receive guideline-concordant, systematic quality preventive care, which contributes to a widening mortality gap for this population.11,12
This review aims to provide clinicians with practical guidance on the assessment and management of high cholesterol to improve recognition and treatment, lower CVD risk, and reduce this observed mortality gap.
Screening and diagnosis
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) released updated guidelines on diagnosing and managing high cholesterol to reduce CVD risk.1 These guidelines focus on updated 10-year CVD risk assessment models with treatment goals reliant on adherence to statin therapy rather than pre-specified cholesterol targets listed in previous guidelines.13
Updates to assessment and treatment guidelines have removed some barriers to screening and diagnosing high cholesterol—namely, fasting lipid panels are no longer required to determine 10-year CVD risk and initiate treatment.14 For adults taking a second-generation antipsychotic that is associated with weight gain and metabolic syndrome, experts generally recommend yearly non-fasting lipid panels.6,14
The United States Preventive Services Task Force recommends screening:
- men age ≥35 at average risk for CVD every 5 years
- women age ≥45 every 5 years15
- adults as young as age 20 who have accelerated risk factors, such as cigarette smoking and hypertension
- adults with a family history of heart attack or stroke in male first-degree relative age ≥50 and female first-degree relatives age ≥60.
Many adults receiving care in behavioral health settings, regardless of their medication regimen, qualify for screening at least every 5 years, if not more frequently. Although statin treatment before age 40 is less beneficial and likely not necessary for primary prevention, monitoring could help identify alternative therapies and prioritize more intensive diet and lifestyle modifications.
At a routine office visit, clinicians can collect vital signs, record smoking status, and reconcile all medications, which provides the data needed to calculate a patient’s 10-year CVD risk (Table 1). Coupled with laboratory testing, which includes a non-fasting total cholesterol, HDL, and hemoglobin A1c (representative of a 3-month blood sugar average, ≥6.5% is diagnostic of type 2 diabetes mellitus [T2DM]), all data points can be entered into online risk calculators (search “ASCVD risk calculator” or visit http://tools.acc.org/ASCVD-Risk-Estimator to access the ACC/AHA risk calculator). Persons scoring >20% 10-year risk are considered at extremely high risk, and are in the same risk category as adults with existing CVD or who have had a cardiovascular event. Persons at <5% 10-year risk generally are considered low risk, and primary prevention with a statin medication is not indicated.
Treatment and management
Dietary modification and lifestyle changes (exercise, quitting smoking), lowering high cholesterol with medications, and switching from highly metabolically active drugs to less metabolically active ones can help lower total cholesterol in patients at risk of CVD.
Statins
HMG-CoA reductase inhibitors (statins) consistently reduce total cholesterol and non-HDL cholesterol by 30% to 50%, depending on drug and dosage (potency, listed as low, medium, and high). Not all statins are equally effective at lowering cholesterol; some are more potent than others (Table 2).16

Individuals are eligible for statin therapy based on their level of CVD risk. Persons at higher risk generally benefit from greater intensity statin treatment and cholesterol reduction; highest intensity statin regimens can lower total cholesterol by approximately 50%.
There are 4 statin eligibility classes (Table 3). Most adults fall into category 4: 10-year risk of >7.5% and needing primary prevention. In addition to removing specific LDL targets as therapy goals, calculation of this risk percentage and the specific cut-off values have been the most controversial aspects of the new cholesterol guidelines. Most experts agree that, in adults age 40 to 75, 10-year risk >10% indicates daily statin use as tolerated for primary prevention, and 10-year risk <5% does not warrant statin use. Recent large studies have validated these new techniques for calculating risk, and found them to be beneficial in potential for cost savings and risk classification.17,18

Considerations in psychiatric patients. Statins have been associated with depression in case series, but larger analyses have not confirmed this association.19 Emerging evidence has identified a potential correlation between statin use and accelerated onset of T2DM, but the absolute risk is relatively low and most experts continue to recommend statin therapy despite this potential risk.13 Many statins, including atorvastatin, are available as a generic and can be taken once daily. Some, such as simvastatin, have notable interactions with commonly prescribed psychotropics including risperidone and quetiapine. Pravastatin is dually excreted by the liver and kidneys and may have fewer drug-drug interactions in patients with psychiatric illness taking common psychotropic therapies, but is not considered a high-potency statin and might not confer adequate benefits in CVD risk reduction.
Contraindications. Statins are pregnancy category X, and generally should not be prescribed for women of childbearing age without intensive counseling. The most notable adverse effects for statins include muscle aches and cramps (myalgia), but generally are not severe. If encountered, consider checking a serum creatinine kinase (CK) level, and if significantly elevated above 10 times the upper limit, stopping statin therapy would be advised. If the CK is only mildly elevated, consider lowering the dosage or switching to a lower potency agent. Lovastatin and pravastatin generally are better tolerated than atorvastatin and are considered lower potency (Table 2).
Statins can be safely used in the presence of liver conditions, such as hepatitis C and alcohol use, although periodic monitoring of transaminase levels is recommended. For adults in the general population without liver disease, regular monitoring of transaminase levels is not necessary.
Alternate lipid-lowering pharmacotherapies unfortunately have fallen out of favor. Fibrates, niacin, ezetimibe, and omega-3 fatty acids once were recommended to lower triglycerides or raise HDL cholesterol levels, but since have been shown to have little effect on cardiovascular morbidity or mortality. Adding further medications, other than statins, to lower cholesterol values to pre-defined targets is not the current standard of care.
High triglyceride concentrations traditionally have been addressed directly, but failure to improve CVD mortality or morbidity by treating triglycerides alone has resulted in refocusing clinical efforts in dyslipidemia management on atherogenic cholesterol, including LDL and non-HDL fractions.20 Non-fasting triglycerides >500 mg/dL should be retested when fasting, and levels that remain >500 mg/dL could place the patient at risk for pancreatitis and might warrant intervention with fibrates at that time. This scenario is not common, and referral to a primary care physician or endocrinologist may be warranted.
Lifestyle changes
With or without statin therapies, diet and lifestyle changes are the cornerstone of healthy living and should be encouraged in all patients. Most overweight or obese patients will benefit from exercise and dietary modifications. Such interventions have shown potential for reducing total cholesterol and non-HDL and HDL cholesterol, but rarely are these interventions sustained long enough to produce meaningful reduction in CVD risk through lipid lowering. Diets rich in isocaloric tree nuts and red-yeast rice extract—a form of a statin—have shown promise in reducing cholesterol, but typically take excessive personal resources and are not sustained to the degree necessary to reduce CVD risk over time.21 Similarly, regular exercise routines can help lower overall cholesterol numbers, but rarely reduce total cholesterol by >10%.
Because individuals with SMI smoke at a higher rate than the general population, it should be noted that smoking cessation is associated with a reduction in total cholesterol and a trial of smoking cessation therapy is warranted before initiating a statin medication for primary prevention of CVD. Many patients would discover that their 10-year ASCVD risk would fall under the level needed for statin therapy if they could successfully stop smoking.
Switching pharmacotherapies
Switching antipsychotic agents from highly metabolically risky compounds, such as risperidone and olanzapine, to less metabolically active compounds, such as aripiprazole, ziprasidone, or haloperidol, have been associated with improvements in lipid profiles.22-24 Clinicians must weigh the potential benefits of switching therapies against the risk of psychiatric destabilization and long-term adherence, keeping in mind that changes in lipids seen with switching could be mild (approximately 10% reduction in total cholesterol).
Summing up
Cholesterol management is considered part of a program to systematically lower CVD risk. Statin therapy usually is indicated for life, or until the age of 75, at which point treatment risks and benefits change because of life expectancy. Other components of CVD risk reduction include a focus on blood pressure control, smoking cessation, T2DM management, and weight loss. Tracking lipid profiles over time to ensure broad targets of 30% to 50% reduction in total cholesterol, approximately 3 months after initiation and yearly thereafter, can help ensure adherence to therapy. With systematic lowering of modifiable CVD risk factors, we can hope to gradually improve the quality of life for our patients with mental illnesses (see the Box for a case example illustrating successful use of these strategies).

High serum cholesterol is a leading cause of heart attack and stroke,1,2 yet remains one of the most under-screened and undertreated modifiable risk factors in persons with mental illness. Well tolerated and effective treatments can considerably lower the risk of cardiovascular events, and should be offered to psychiatric patients who are at high risk, while considering possible adverse effects and potential interactions between psychotropics and medications used to lower cholesterol.
Systematic lowering of total cholesterol and, particularly, atherogenic low-density lipoprotein (LDL) and non-high density lipoprotein (HDL) cholesterol, results in consistent and significant reduction in risk of cardiovascular events in persons at risk for developing cardiovascular disease (CVD) and in preventing reoccurrence of these events.1,3,4 Even individuals who have relatively lower levels of total cholesterol but are at high risk (such as if a cardiovascular event has occurred) could reduce their CVD risk (known as secondary prevention) through lipid lowering therapies.5,6
Adults with psychiatric illness shoulder a disproportionate burden of CVD morbidity and mortality, especially those with severe mental illness (SMI, schizophrenia, schizoaffective disorder, bipolar disorder, treatment-resistant depression).7-9 Among modifiable CVD risk factors, dyslipidemia has the highest rates of missed screenings and treatment within psychiatric populations. In one analysis, up to 90% of adults with SMI and identified lipid disorders did not receive treatment.10 Persons with SMI generally do not receive guideline-concordant, systematic quality preventive care, which contributes to a widening mortality gap for this population.11,12
This review aims to provide clinicians with practical guidance on the assessment and management of high cholesterol to improve recognition and treatment, lower CVD risk, and reduce this observed mortality gap.
Screening and diagnosis
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) released updated guidelines on diagnosing and managing high cholesterol to reduce CVD risk.1 These guidelines focus on updated 10-year CVD risk assessment models with treatment goals reliant on adherence to statin therapy rather than pre-specified cholesterol targets listed in previous guidelines.13
Updates to assessment and treatment guidelines have removed some barriers to screening and diagnosing high cholesterol—namely, fasting lipid panels are no longer required to determine 10-year CVD risk and initiate treatment.14 For adults taking a second-generation antipsychotic that is associated with weight gain and metabolic syndrome, experts generally recommend yearly non-fasting lipid panels.6,14
The United States Preventive Services Task Force recommends screening:
- men age ≥35 at average risk for CVD every 5 years
- women age ≥45 every 5 years15
- adults as young as age 20 who have accelerated risk factors, such as cigarette smoking and hypertension
- adults with a family history of heart attack or stroke in male first-degree relative age ≥50 and female first-degree relatives age ≥60.
Many adults receiving care in behavioral health settings, regardless of their medication regimen, qualify for screening at least every 5 years, if not more frequently. Although statin treatment before age 40 is less beneficial and likely not necessary for primary prevention, monitoring could help identify alternative therapies and prioritize more intensive diet and lifestyle modifications.
At a routine office visit, clinicians can collect vital signs, record smoking status, and reconcile all medications, which provides the data needed to calculate a patient’s 10-year CVD risk (Table 1). Coupled with laboratory testing, which includes a non-fasting total cholesterol, HDL, and hemoglobin A1c (representative of a 3-month blood sugar average, ≥6.5% is diagnostic of type 2 diabetes mellitus [T2DM]), all data points can be entered into online risk calculators (search “ASCVD risk calculator” or visit http://tools.acc.org/ASCVD-Risk-Estimator to access the ACC/AHA risk calculator). Persons scoring >20% 10-year risk are considered at extremely high risk, and are in the same risk category as adults with existing CVD or who have had a cardiovascular event. Persons at <5% 10-year risk generally are considered low risk, and primary prevention with a statin medication is not indicated.
Treatment and management
Dietary modification and lifestyle changes (exercise, quitting smoking), lowering high cholesterol with medications, and switching from highly metabolically active drugs to less metabolically active ones can help lower total cholesterol in patients at risk of CVD.
Statins
HMG-CoA reductase inhibitors (statins) consistently reduce total cholesterol and non-HDL cholesterol by 30% to 50%, depending on drug and dosage (potency, listed as low, medium, and high). Not all statins are equally effective at lowering cholesterol; some are more potent than others (Table 2).16

Individuals are eligible for statin therapy based on their level of CVD risk. Persons at higher risk generally benefit from greater intensity statin treatment and cholesterol reduction; highest intensity statin regimens can lower total cholesterol by approximately 50%.
There are 4 statin eligibility classes (Table 3). Most adults fall into category 4: 10-year risk of >7.5% and needing primary prevention. In addition to removing specific LDL targets as therapy goals, calculation of this risk percentage and the specific cut-off values have been the most controversial aspects of the new cholesterol guidelines. Most experts agree that, in adults age 40 to 75, 10-year risk >10% indicates daily statin use as tolerated for primary prevention, and 10-year risk <5% does not warrant statin use. Recent large studies have validated these new techniques for calculating risk, and found them to be beneficial in potential for cost savings and risk classification.17,18

Considerations in psychiatric patients. Statins have been associated with depression in case series, but larger analyses have not confirmed this association.19 Emerging evidence has identified a potential correlation between statin use and accelerated onset of T2DM, but the absolute risk is relatively low and most experts continue to recommend statin therapy despite this potential risk.13 Many statins, including atorvastatin, are available as a generic and can be taken once daily. Some, such as simvastatin, have notable interactions with commonly prescribed psychotropics including risperidone and quetiapine. Pravastatin is dually excreted by the liver and kidneys and may have fewer drug-drug interactions in patients with psychiatric illness taking common psychotropic therapies, but is not considered a high-potency statin and might not confer adequate benefits in CVD risk reduction.
Contraindications. Statins are pregnancy category X, and generally should not be prescribed for women of childbearing age without intensive counseling. The most notable adverse effects for statins include muscle aches and cramps (myalgia), but generally are not severe. If encountered, consider checking a serum creatinine kinase (CK) level, and if significantly elevated above 10 times the upper limit, stopping statin therapy would be advised. If the CK is only mildly elevated, consider lowering the dosage or switching to a lower potency agent. Lovastatin and pravastatin generally are better tolerated than atorvastatin and are considered lower potency (Table 2).
Statins can be safely used in the presence of liver conditions, such as hepatitis C and alcohol use, although periodic monitoring of transaminase levels is recommended. For adults in the general population without liver disease, regular monitoring of transaminase levels is not necessary.
Alternate lipid-lowering pharmacotherapies unfortunately have fallen out of favor. Fibrates, niacin, ezetimibe, and omega-3 fatty acids once were recommended to lower triglycerides or raise HDL cholesterol levels, but since have been shown to have little effect on cardiovascular morbidity or mortality. Adding further medications, other than statins, to lower cholesterol values to pre-defined targets is not the current standard of care.
High triglyceride concentrations traditionally have been addressed directly, but failure to improve CVD mortality or morbidity by treating triglycerides alone has resulted in refocusing clinical efforts in dyslipidemia management on atherogenic cholesterol, including LDL and non-HDL fractions.20 Non-fasting triglycerides >500 mg/dL should be retested when fasting, and levels that remain >500 mg/dL could place the patient at risk for pancreatitis and might warrant intervention with fibrates at that time. This scenario is not common, and referral to a primary care physician or endocrinologist may be warranted.
Lifestyle changes
With or without statin therapies, diet and lifestyle changes are the cornerstone of healthy living and should be encouraged in all patients. Most overweight or obese patients will benefit from exercise and dietary modifications. Such interventions have shown potential for reducing total cholesterol and non-HDL and HDL cholesterol, but rarely are these interventions sustained long enough to produce meaningful reduction in CVD risk through lipid lowering. Diets rich in isocaloric tree nuts and red-yeast rice extract—a form of a statin—have shown promise in reducing cholesterol, but typically take excessive personal resources and are not sustained to the degree necessary to reduce CVD risk over time.21 Similarly, regular exercise routines can help lower overall cholesterol numbers, but rarely reduce total cholesterol by >10%.
Because individuals with SMI smoke at a higher rate than the general population, it should be noted that smoking cessation is associated with a reduction in total cholesterol and a trial of smoking cessation therapy is warranted before initiating a statin medication for primary prevention of CVD. Many patients would discover that their 10-year ASCVD risk would fall under the level needed for statin therapy if they could successfully stop smoking.
Switching pharmacotherapies
Switching antipsychotic agents from highly metabolically risky compounds, such as risperidone and olanzapine, to less metabolically active compounds, such as aripiprazole, ziprasidone, or haloperidol, have been associated with improvements in lipid profiles.22-24 Clinicians must weigh the potential benefits of switching therapies against the risk of psychiatric destabilization and long-term adherence, keeping in mind that changes in lipids seen with switching could be mild (approximately 10% reduction in total cholesterol).
Summing up
Cholesterol management is considered part of a program to systematically lower CVD risk. Statin therapy usually is indicated for life, or until the age of 75, at which point treatment risks and benefits change because of life expectancy. Other components of CVD risk reduction include a focus on blood pressure control, smoking cessation, T2DM management, and weight loss. Tracking lipid profiles over time to ensure broad targets of 30% to 50% reduction in total cholesterol, approximately 3 months after initiation and yearly thereafter, can help ensure adherence to therapy. With systematic lowering of modifiable CVD risk factors, we can hope to gradually improve the quality of life for our patients with mental illnesses (see the Box for a case example illustrating successful use of these strategies).

1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 suppl B):S1-S45.
2. LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81(5):1721-1733.
3. Albert MA, Glynn RJ, Fonseca FA, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Am Heart J. 2011;162(1):106-114.e2.
4. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858.CD004816.pub5.
5. Gaziano JM, Gaziano TA. What’s new with measuring cholesterol? JAMA. 2013;310(19):2043-2044.
6. Emerging Risk Factors Collaboration; Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
7. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
8. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324-333.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states [published online March 15, 2006]. Prev Chronic Dis. 2006;3(2):A42.
10. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
11. Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321(7259):483-484.
12. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(suppl 4):69-80.
13. Ganda OP. Deciphering cholesterol treatment guidelines: a clinician’s perspective. JAMA. 2015;313(10):1009-1010.
14. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv. 2014;65(5):573-576.
15. U.S. Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia). http://www.uspreventiveservicestaskforce.org/uspstf/uspschol.htm. Published June 2008. Accessed October 12, 2016.
16. Cupp M. Characteristics of the various statins. Pharmacist’s Letter. 2012;28(6):280606.
17. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314(2):134-141.
18. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142-150.
19. You H, Lu W, Zhao S, et al. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14(11):1467-1476.
20. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81(9):1097-1102.
22. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205(1):23-32.
23. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
24. Stroup TS, McEvoy JP, Ring KD, et al; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947-956.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 suppl B):S1-S45.
2. LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81(5):1721-1733.
3. Albert MA, Glynn RJ, Fonseca FA, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Am Heart J. 2011;162(1):106-114.e2.
4. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858.CD004816.pub5.
5. Gaziano JM, Gaziano TA. What’s new with measuring cholesterol? JAMA. 2013;310(19):2043-2044.
6. Emerging Risk Factors Collaboration; Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
7. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
8. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324-333.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states [published online March 15, 2006]. Prev Chronic Dis. 2006;3(2):A42.
10. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
11. Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321(7259):483-484.
12. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(suppl 4):69-80.
13. Ganda OP. Deciphering cholesterol treatment guidelines: a clinician’s perspective. JAMA. 2015;313(10):1009-1010.
14. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv. 2014;65(5):573-576.
15. U.S. Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia). http://www.uspreventiveservicestaskforce.org/uspstf/uspschol.htm. Published June 2008. Accessed October 12, 2016.
16. Cupp M. Characteristics of the various statins. Pharmacist’s Letter. 2012;28(6):280606.
17. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314(2):134-141.
18. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142-150.
19. You H, Lu W, Zhao S, et al. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14(11):1467-1476.
20. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81(9):1097-1102.
22. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205(1):23-32.
23. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
24. Stroup TS, McEvoy JP, Ring KD, et al; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947-956.
National Initiative to Prevent Suicide: A new proposal to improve the understanding and prevention of suicide
Suicide is a staggering, tragic, and growing cause of death in the United States. One out of every 62 Americans will die from suicide, based on the national lifetime prevalence rate.1 More than 42,000 Americans died from suicide in 2014, making suicide the second leading cause of death in individuals age 15 to 34, the fourth leading cause among those age 35 to 54, and the tenth leading cause of death in the country overall.2 The incidence of suicide in the general population of the United States increased by 24% between 1999 and 2014.3 This tragedy obviously is not solving itself.
The proposal
U.S. Centers for Disease Control and Prevention (CDC) publishes statistics about the number of suicides, as well as demographic information, collected from coroners and medical examiners across the country. However, these sources do not provide a biological sample that could be used to gather data concerning DNA, RNA, and other potential blood markers, including those reflecting inflammatory and epigenetic processes. However, such biological samples are commonly collected by the U.S. medicolegal death investigation system. In 2003, this system investigated 450,000 unnatural and/or unexplained deaths (ie, approximately 20% of the 2.4 million deaths in the United States that year).4
Each unnatural or unexplained death is examined, often extensively, by a coroner or medical examiner. This examination system costs more than $600 million annually. Yet the data that are collected are handled on a case-by-case and often county-by-county basis, rather than in aggregate. The essence of the proposal presented here is to take the information and biological samples collected in this process and put them into a National Suicide Database (NSD), which then can serve as a resource for scientists to increase our understanding of the genetic, epigenetic, and other factors underlying death due to suicide. This increased understanding will result in the development more effective tools to detect to those at risk for suicide (ie, risk factor tests), to monitor treatment, and to develop new treatments based on a better understanding of the underlying pathophysiology and pathogenesis of suicide. These tools will reduce:
- the number of lives lost to suicide
- the pain and suffering of loved ones
- lost productivity to society, especially when one considers that suicide disproportionately affects individuals during the most productive period of their lives (ie, age 15 to 54).
The NSD will be organized as a government–private partnership, with the government represented by the National Institutes of Health (NIH) and/or the CDC. The goal will be to take the information that is currently being collected by the nation’s medicolegal death investigation system, including the biological samples, systematize it, enter it into a common database, and make it available to qualified researchers across the country. The administrative arm of the system will be responsible for ensuring systematic data collection, storage in a searchable and integrated database housed within the NIH and/or the CDC, and vetting researchers who will have access to the data, including those with expertise in genomics, molecular biology, suicide, epidemiology, and data-mining. (Currently, the CDC’s National Violent Death Reporting System, which is a state-based surveillance system, pools data on violent deaths from multiple sources into a usable, anonymous database. These sources include state and local medical examiners, coroners, law enforcement, crime labs, and vital statistics records, but they do not include any biological material even though it is collected [personal correspondence with the CDC, July 2016].)
Because information on suicides currently are handled primarily on a county-by-county basis, data concerning these deaths are not facilitating a better understanding of the causes and strategies for preventing suicide. Correcting this situation is the goal of this proposal, as modeled by the National Cancer Institute’s War on Cancer, which has transformed the treatment and the outcomes of cancer. If this proposal is enacted, the same type of transformation will occur and result in a reduction in the suicide rate and better outcomes for the psychiatric illnesses that underlie most instances of suicide.
The proposed NSD will address a major and common problem for researchers in this area—small sample sizes. When considered from the perspective of the size of samples feasible for most independent research teams to collect and study, suicide on an annual basis is rare—however, that is not the case when the incidence of suicide in the nation as a whole is considered. In contrast to the data concerning suicides that individual research teams can collect, the proposed genomic database will grow by approximately 40,000 individuals every year, until a meaningful reduction in deaths due to suicide is achieved.
From a research perspective, suicide, although tragic, is one of the few binary outcomes in psychiatry—that is, life or death. Although there may be >1 genetic and/or epigenetic contributor to suicide, within a relatively short period of time, the proposed database will amass—and continue to amass on an ongoing basis—data from a large population of suicide victims. Researchers then can compare the findings from this database with the normative human genome, looking for variants that are over-represented in the population of those who have died by suicide.
Environmental factors undoubtedly also contribute to the risk of suicide, given that the incidence of suicide increases with age, particularly among white males, and with the addition of psychiatric and medical comorbidities. Inflammatory processes also have been implicated in the pathophysiology of a number of psychiatric disorders, including major depression, which is the primary psychiatric risk factor for suicide. Therefore, consideration should be given to collecting whole blood samples if the time between death and autopsy is within an appropriate limit to obtain interpretable data concerning RNA (ie, gene expression) and even biomarkers of inflammatory and other processes at the time of the suicide. This approach has been used by Niculescu et al5,6 for whole blood gene expression. The rationale for using samples of whole blood is that this strategy could be more easily adapted to clinical practice in contrast to using samples from the target organ (ie, brain) or cerebrospinal fluid.
Roadblocks to progress. In the absence of this proposed NSD, progress in this area has been stymied despite concerted governmental efforts (Box7-10). One reason for the lack of progress has been that governmental efforts have focused on a public health model rather than also including a basic science model aimed at exploring the biological mechanisms underlying the risk of death from suicide. In the current decentralized system, individual researchers and even teams of researchers cannot easily collect data from a sufficiently large population of suicide victims to make inroads in gaining the needed understanding.

Because of the relatively small samples that individual research teams can collect in a reasonable period of time (ie, in terms of grant cycles), many investigators have studied suicide attempts as a surrogate for suicide itself, undoubtedly because suicide attempts are more numerous than suicides themselves, making it easier to collect data. However, there is evidence that these 2 populations—suicide attempters vs those who die by suicide—only partially overlap.
First, the frequency of suicide attempts is 10 to 20 times higher than actual suicides. Second, suicide attempters are 3 times more likely to be female whereas those who die by suicide are 4 times more likely to be male. Third, most individuals who die by suicide do so on their first or second attempt, whereas individuals who have made ≥4 attempts have an increased risk of future attempts rather than for completed suicide compared with the general population. Fourth, certain psychiatric illnesses are more often associated with death by suicide (particularly major depressive disorder, bipolar disorder, and schizophrenia in the first 5 years of an illness) whereas multiple suicide attempts are more often associated with other psychiatric diagnoses such as antisocial and borderline personality disorders.
Finally, in a study in men with a psychiatric disorder, Niculescu et al5 started with 412 candidate genes and found that 208 were associated with suicidal ideation but not suicide itself, whereas 76 genes were associated with both suicidal ideation and completion. Taken together, this evidence suggests that findings concerning suicide attempters, especially those who have made multiple (ie, >3) attempts, might not be extrapolatable to the population of actual suicides.
Is there evidence that this proposal could work?
Yes, research supports the potential utility of the proposed NSD, and this section highlights some of the major findings from these studies, although this review is not intended to be exhaustive.
First, considerable evidence exists for a biological basis for the risk of death due to suicide. The concordance rates for suicide are 10 times higher in monozygotic (“identical”) vs dizygotic (“fraternal”) twins (24.1% vs 2.8%) and 2 to 5 times higher in relatives of those who die by suicide than in the general population. Heritability estimates of fatal suicides and nonfatal suicide attempts in biological relatives of adoptees who die from suicide range from 17% to 45%.11
Second, studies using information from small samples that was arduously collected by individual research groups have yielded important positive data. Most recently, in 2015, a multidisciplinary group led by Niculescu et al5 at Indiana University and other institutions described a test that could predict suicidality in men. This test was developed on the basis of a within-participant discovery approach to identify genes that change in expression between states of no suicidal ideation and high suicidal ideation, which was combined with clinical information assessed by 2 scales, the Convergent Functional Information for Suicidality and the Simplified Affective State Scale. Gene expression was measured in whole blood collected postmortem unless the method of suicide involved a medication overdose that could affect gene expression. These researchers identified 76 genes that likely were involved in suicidal ideation and suicide.
This report had a number of limitations.5 All of the individuals in these studies were being treated for psychiatric illness, were being closely followed by the investigators, and all were male. In addition, as noted above, suicides by overdose were eliminated from the analysis.
In a subsequent study published in 2016, the Niculescu group6 extended their work to women and identified 50 genes contributing to suicide risk in women. Underscoring the need for larger samples, only 3 of the top contributing genes were seen in both men and women, suggesting that there are likely significant sex differences in the biology of suicide completion. This important work needs to be replicated and extended.
In addition to these remarkable advances made in genetic understanding of the risk of suicide, recent research also has demonstrated a role for epigenetic and inflammatory processes as contributors to suicide risk.12-15
There are likely many contributors, including genetic, epigenetic, and environmental factors such as inflammatory processes, that increase the risk of suicide. The goal of this article is not to provide an exhaustive or integrative review of research in this area but rather to argue for the establishment of a national initiative to study all of these factors and to begin that process by establishing the NSD.
What will be the foreseeable outcome of this initiative?
The establishment of the NSD is expected to lead to better identification of those who are genetically at increased risk of suicide as well as biological factors (eg, inflammatory or other processes) and environmental factors (eg, drug abuse), which can turn that genetic risk into reality. Using research results made possible by the implementation of this proposal, objective testing can be developed to monitor risk more effectively than is currently possible using clinical assessment alone.
Furthermore, this work also can provide targets for developing new treatments. For example, there is convergence between the work of Niculescu et al,5,6 who identified genetic biomarkers for mechanistic target of rapamycin (mTOR) signaling as a risk factor in individuals who died by suicide and the work of Li et al and other researchers,16-18 whose findings have implicated mTOR-dependent synapse formation as a mechanism underlying the rapid (ie, within hours to a couple of days) antidepressant effects of N-methyl-
In aggregate, establishment of this proposed database will facilitate identification of biological (and therefore pharmaceutical) mechanisms beyond those involving biogenic amines, which have been the exclusive biological targets for antidepressants for the past 50 years.22 The likely consequences of the findings generated from research made possible by the proposed NSD will open completely new vistas for helping people at risk for suicide and psychiatric illnesses.
What foreseeable obstacles will need to be addressed?
Of course, obstacles and problems will arise but these will not exceed those encountered by the War on Cancer and they can similarly be overcome with sufficient public support and cooperation. Potential obstacles include:
- need for incremental funding
- obtaining the cooperation of the offices of each county medical examiner or coroner in a process that includes uniform systematic data collection
- determining the situations (eg, time after death and means of death) that will allow for meaningful collection of data such as RNA and inflammatory biomarkers
- establishing how data and particularly biological samples will be transported and stored
- issues related to privacy of health information particularly for relatives of suicide victims
- ensuring the reliability, validity, and comparability of the data received from different medical examiners and coroners.
With regard to the last issue, because stigma is associated with death by suicide, some true suicides could be missed, which would compromise sensitivity but simultaneously increase specificity. Other obstacles or problems may arise; however, I am certain that all such issues are surmountable and that the resulting NSD will be much better than what we have now and will propel our understanding of the biological underpinnings of the loss of life to suicide. (The author proposed a similar but even more ambitious plan 25 years ago,23 but he believes that this is an idea whose time has come.)
Acknowledgments
The author thanks Wayne C. Drevets, MD, Alexander Niculescu, MD, PhD, John Oldman, MD, and John Savitz, PhD, David Sheehan, MD, and Matthew Macaluso, DO for their review and suggestions concerning this proposal/manuscript, and Kaylee Hervey, MPH, from the Sedgwick County Health Department, Wichita, Kansas, for her input. The author also thanks Ruth Ross, as always, for her excellent editing and general assistance.
1. Pompili M, Gonda X, Serafini G, et al. Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar Disord. 2013;15(5):457-490.
2. National Vital Statistics System; National Center for Health Statistics; Centers for Disease Control and Prevention. Ten leading causes of death by age group, United States–2014. Centers for Disease Control and Prevention. http://www.cdc.gov/injury/images/lc-charts/leading_causes_of_death_age_group_2014_1050w760h.gif. Accessed October 17, 2016.
3. Curtin SC, Warner M, Hedegaard H, et al. Increase in suicide in the United States, 1999-2014. National Center for Health Statistics Data Brief No. 241. Atlanta GA: National Center for Health Statistics, U.S. Department of Health and Human Services. http://www.cdc.gov/nchs/products/databriefs/db241.htm. Published April 2016. Accessed June 30, 2016.
4. Committee for the Workshop on the Medicolegal Death Investigation System; Board on Health Promotion and Disease Prevention. Medicolegal death investigation system: workshop summary. Washington, DC: National Academies Press; 2003.
5. Niculescu AB, Levey DF, Phalen PL, et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015;20(11):1266-1285.
6. Levey DF, Niculescu EM, Le-Niculescu H, et al. Towards understanding and predicting suicidality in women: biomarkers and clinical risk assessment. Mol Psychiatry. 2016;21(6):768-785.
7. World Health Organization. Prevention of suicide: guidelines for the formulation and implementation of national strategies. Geneva, Switzerland: World Health Organization; 1996.
8. U.S. Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: U.S. Public Health Service; 1999.
9. U.S. Department of Health and Human Services (HHS). National Strategy for Suicide Prevention: goals and objectives for action. Rockville, MD: U.S. Department of Health and Human Services; 2001.
10. U.S. Department of Health and Human Services (HHS). National Strategy for Suicide Prevention: goals and objectives for action. Rockville, MD; U.S. Department of Health and Human Services; 2012.
11. Brent DA, Melham N. Familial transmission of suicidal behavior. Psychiatr Clin North Am. 2008;31(2):157-177.
12. Guintivano J, Brown T, Newcomer A, et al. Identification and replication of a combined epigenetic and genetic biomarker predicting suicide and suicidal behaviors. Am J Psychiatry. 2014;171(12):1287-1296.
13. Bay-Richter C, Linderholm KR, Lim CK, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110-117.
14. Brundin L, Bryleva EY, Thirtamara Rajamani K. Role of inflammation in suicide: from mechanisms to treatment [published online July 27, 2016]. Neuropsychopharmacology. doi: 10.1038/npp.2016.116.
15. Steiner J, Walter M, Gos T, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94.
16. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959-964.
17. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
18. Preskorn SH, Baker B, Kolluri S, et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631-637.
19. Canuso C, Singh J, Fedgchin M, et al. PeRSEVERe: a study of esketamine for the rapid reduction of the symptoms of major depressive disorder, including suicidal ideation, in subjects assessed to be at imminent risk for suicide. Presentation at the Annual Meeting of the American Society of Clinical Psychopharmacology, Scottsdale AZ, May 30-June 3, 2016.
20. Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756-758.
21. Moore PA, Rosen CA, Carter KC. Assignment of the human FKBP12-rapamycin-associated protein (FRAP) gene to chromosome 1p36 by fluorescence in situ hybridization. Genomics. 1996;33(2):331-332.
22. Ha
23. Preskorn SH. The future and psychopharmacology: potentials and needs. Psychiatr Ann. 1990;20(11):625-633.
Suicide is a staggering, tragic, and growing cause of death in the United States. One out of every 62 Americans will die from suicide, based on the national lifetime prevalence rate.1 More than 42,000 Americans died from suicide in 2014, making suicide the second leading cause of death in individuals age 15 to 34, the fourth leading cause among those age 35 to 54, and the tenth leading cause of death in the country overall.2 The incidence of suicide in the general population of the United States increased by 24% between 1999 and 2014.3 This tragedy obviously is not solving itself.
The proposal
U.S. Centers for Disease Control and Prevention (CDC) publishes statistics about the number of suicides, as well as demographic information, collected from coroners and medical examiners across the country. However, these sources do not provide a biological sample that could be used to gather data concerning DNA, RNA, and other potential blood markers, including those reflecting inflammatory and epigenetic processes. However, such biological samples are commonly collected by the U.S. medicolegal death investigation system. In 2003, this system investigated 450,000 unnatural and/or unexplained deaths (ie, approximately 20% of the 2.4 million deaths in the United States that year).4
Each unnatural or unexplained death is examined, often extensively, by a coroner or medical examiner. This examination system costs more than $600 million annually. Yet the data that are collected are handled on a case-by-case and often county-by-county basis, rather than in aggregate. The essence of the proposal presented here is to take the information and biological samples collected in this process and put them into a National Suicide Database (NSD), which then can serve as a resource for scientists to increase our understanding of the genetic, epigenetic, and other factors underlying death due to suicide. This increased understanding will result in the development more effective tools to detect to those at risk for suicide (ie, risk factor tests), to monitor treatment, and to develop new treatments based on a better understanding of the underlying pathophysiology and pathogenesis of suicide. These tools will reduce:
- the number of lives lost to suicide
- the pain and suffering of loved ones
- lost productivity to society, especially when one considers that suicide disproportionately affects individuals during the most productive period of their lives (ie, age 15 to 54).
The NSD will be organized as a government–private partnership, with the government represented by the National Institutes of Health (NIH) and/or the CDC. The goal will be to take the information that is currently being collected by the nation’s medicolegal death investigation system, including the biological samples, systematize it, enter it into a common database, and make it available to qualified researchers across the country. The administrative arm of the system will be responsible for ensuring systematic data collection, storage in a searchable and integrated database housed within the NIH and/or the CDC, and vetting researchers who will have access to the data, including those with expertise in genomics, molecular biology, suicide, epidemiology, and data-mining. (Currently, the CDC’s National Violent Death Reporting System, which is a state-based surveillance system, pools data on violent deaths from multiple sources into a usable, anonymous database. These sources include state and local medical examiners, coroners, law enforcement, crime labs, and vital statistics records, but they do not include any biological material even though it is collected [personal correspondence with the CDC, July 2016].)
Because information on suicides currently are handled primarily on a county-by-county basis, data concerning these deaths are not facilitating a better understanding of the causes and strategies for preventing suicide. Correcting this situation is the goal of this proposal, as modeled by the National Cancer Institute’s War on Cancer, which has transformed the treatment and the outcomes of cancer. If this proposal is enacted, the same type of transformation will occur and result in a reduction in the suicide rate and better outcomes for the psychiatric illnesses that underlie most instances of suicide.
The proposed NSD will address a major and common problem for researchers in this area—small sample sizes. When considered from the perspective of the size of samples feasible for most independent research teams to collect and study, suicide on an annual basis is rare—however, that is not the case when the incidence of suicide in the nation as a whole is considered. In contrast to the data concerning suicides that individual research teams can collect, the proposed genomic database will grow by approximately 40,000 individuals every year, until a meaningful reduction in deaths due to suicide is achieved.
From a research perspective, suicide, although tragic, is one of the few binary outcomes in psychiatry—that is, life or death. Although there may be >1 genetic and/or epigenetic contributor to suicide, within a relatively short period of time, the proposed database will amass—and continue to amass on an ongoing basis—data from a large population of suicide victims. Researchers then can compare the findings from this database with the normative human genome, looking for variants that are over-represented in the population of those who have died by suicide.
Environmental factors undoubtedly also contribute to the risk of suicide, given that the incidence of suicide increases with age, particularly among white males, and with the addition of psychiatric and medical comorbidities. Inflammatory processes also have been implicated in the pathophysiology of a number of psychiatric disorders, including major depression, which is the primary psychiatric risk factor for suicide. Therefore, consideration should be given to collecting whole blood samples if the time between death and autopsy is within an appropriate limit to obtain interpretable data concerning RNA (ie, gene expression) and even biomarkers of inflammatory and other processes at the time of the suicide. This approach has been used by Niculescu et al5,6 for whole blood gene expression. The rationale for using samples of whole blood is that this strategy could be more easily adapted to clinical practice in contrast to using samples from the target organ (ie, brain) or cerebrospinal fluid.
Roadblocks to progress. In the absence of this proposed NSD, progress in this area has been stymied despite concerted governmental efforts (Box7-10). One reason for the lack of progress has been that governmental efforts have focused on a public health model rather than also including a basic science model aimed at exploring the biological mechanisms underlying the risk of death from suicide. In the current decentralized system, individual researchers and even teams of researchers cannot easily collect data from a sufficiently large population of suicide victims to make inroads in gaining the needed understanding.

Because of the relatively small samples that individual research teams can collect in a reasonable period of time (ie, in terms of grant cycles), many investigators have studied suicide attempts as a surrogate for suicide itself, undoubtedly because suicide attempts are more numerous than suicides themselves, making it easier to collect data. However, there is evidence that these 2 populations—suicide attempters vs those who die by suicide—only partially overlap.
First, the frequency of suicide attempts is 10 to 20 times higher than actual suicides. Second, suicide attempters are 3 times more likely to be female whereas those who die by suicide are 4 times more likely to be male. Third, most individuals who die by suicide do so on their first or second attempt, whereas individuals who have made ≥4 attempts have an increased risk of future attempts rather than for completed suicide compared with the general population. Fourth, certain psychiatric illnesses are more often associated with death by suicide (particularly major depressive disorder, bipolar disorder, and schizophrenia in the first 5 years of an illness) whereas multiple suicide attempts are more often associated with other psychiatric diagnoses such as antisocial and borderline personality disorders.
Finally, in a study in men with a psychiatric disorder, Niculescu et al5 started with 412 candidate genes and found that 208 were associated with suicidal ideation but not suicide itself, whereas 76 genes were associated with both suicidal ideation and completion. Taken together, this evidence suggests that findings concerning suicide attempters, especially those who have made multiple (ie, >3) attempts, might not be extrapolatable to the population of actual suicides.
Is there evidence that this proposal could work?
Yes, research supports the potential utility of the proposed NSD, and this section highlights some of the major findings from these studies, although this review is not intended to be exhaustive.
First, considerable evidence exists for a biological basis for the risk of death due to suicide. The concordance rates for suicide are 10 times higher in monozygotic (“identical”) vs dizygotic (“fraternal”) twins (24.1% vs 2.8%) and 2 to 5 times higher in relatives of those who die by suicide than in the general population. Heritability estimates of fatal suicides and nonfatal suicide attempts in biological relatives of adoptees who die from suicide range from 17% to 45%.11
Second, studies using information from small samples that was arduously collected by individual research groups have yielded important positive data. Most recently, in 2015, a multidisciplinary group led by Niculescu et al5 at Indiana University and other institutions described a test that could predict suicidality in men. This test was developed on the basis of a within-participant discovery approach to identify genes that change in expression between states of no suicidal ideation and high suicidal ideation, which was combined with clinical information assessed by 2 scales, the Convergent Functional Information for Suicidality and the Simplified Affective State Scale. Gene expression was measured in whole blood collected postmortem unless the method of suicide involved a medication overdose that could affect gene expression. These researchers identified 76 genes that likely were involved in suicidal ideation and suicide.
This report had a number of limitations.5 All of the individuals in these studies were being treated for psychiatric illness, were being closely followed by the investigators, and all were male. In addition, as noted above, suicides by overdose were eliminated from the analysis.
In a subsequent study published in 2016, the Niculescu group6 extended their work to women and identified 50 genes contributing to suicide risk in women. Underscoring the need for larger samples, only 3 of the top contributing genes were seen in both men and women, suggesting that there are likely significant sex differences in the biology of suicide completion. This important work needs to be replicated and extended.
In addition to these remarkable advances made in genetic understanding of the risk of suicide, recent research also has demonstrated a role for epigenetic and inflammatory processes as contributors to suicide risk.12-15
There are likely many contributors, including genetic, epigenetic, and environmental factors such as inflammatory processes, that increase the risk of suicide. The goal of this article is not to provide an exhaustive or integrative review of research in this area but rather to argue for the establishment of a national initiative to study all of these factors and to begin that process by establishing the NSD.
What will be the foreseeable outcome of this initiative?
The establishment of the NSD is expected to lead to better identification of those who are genetically at increased risk of suicide as well as biological factors (eg, inflammatory or other processes) and environmental factors (eg, drug abuse), which can turn that genetic risk into reality. Using research results made possible by the implementation of this proposal, objective testing can be developed to monitor risk more effectively than is currently possible using clinical assessment alone.
Furthermore, this work also can provide targets for developing new treatments. For example, there is convergence between the work of Niculescu et al,5,6 who identified genetic biomarkers for mechanistic target of rapamycin (mTOR) signaling as a risk factor in individuals who died by suicide and the work of Li et al and other researchers,16-18 whose findings have implicated mTOR-dependent synapse formation as a mechanism underlying the rapid (ie, within hours to a couple of days) antidepressant effects of N-methyl-
In aggregate, establishment of this proposed database will facilitate identification of biological (and therefore pharmaceutical) mechanisms beyond those involving biogenic amines, which have been the exclusive biological targets for antidepressants for the past 50 years.22 The likely consequences of the findings generated from research made possible by the proposed NSD will open completely new vistas for helping people at risk for suicide and psychiatric illnesses.
What foreseeable obstacles will need to be addressed?
Of course, obstacles and problems will arise but these will not exceed those encountered by the War on Cancer and they can similarly be overcome with sufficient public support and cooperation. Potential obstacles include:
- need for incremental funding
- obtaining the cooperation of the offices of each county medical examiner or coroner in a process that includes uniform systematic data collection
- determining the situations (eg, time after death and means of death) that will allow for meaningful collection of data such as RNA and inflammatory biomarkers
- establishing how data and particularly biological samples will be transported and stored
- issues related to privacy of health information particularly for relatives of suicide victims
- ensuring the reliability, validity, and comparability of the data received from different medical examiners and coroners.
With regard to the last issue, because stigma is associated with death by suicide, some true suicides could be missed, which would compromise sensitivity but simultaneously increase specificity. Other obstacles or problems may arise; however, I am certain that all such issues are surmountable and that the resulting NSD will be much better than what we have now and will propel our understanding of the biological underpinnings of the loss of life to suicide. (The author proposed a similar but even more ambitious plan 25 years ago,23 but he believes that this is an idea whose time has come.)
Acknowledgments
The author thanks Wayne C. Drevets, MD, Alexander Niculescu, MD, PhD, John Oldman, MD, and John Savitz, PhD, David Sheehan, MD, and Matthew Macaluso, DO for their review and suggestions concerning this proposal/manuscript, and Kaylee Hervey, MPH, from the Sedgwick County Health Department, Wichita, Kansas, for her input. The author also thanks Ruth Ross, as always, for her excellent editing and general assistance.
Suicide is a staggering, tragic, and growing cause of death in the United States. One out of every 62 Americans will die from suicide, based on the national lifetime prevalence rate.1 More than 42,000 Americans died from suicide in 2014, making suicide the second leading cause of death in individuals age 15 to 34, the fourth leading cause among those age 35 to 54, and the tenth leading cause of death in the country overall.2 The incidence of suicide in the general population of the United States increased by 24% between 1999 and 2014.3 This tragedy obviously is not solving itself.
The proposal
U.S. Centers for Disease Control and Prevention (CDC) publishes statistics about the number of suicides, as well as demographic information, collected from coroners and medical examiners across the country. However, these sources do not provide a biological sample that could be used to gather data concerning DNA, RNA, and other potential blood markers, including those reflecting inflammatory and epigenetic processes. However, such biological samples are commonly collected by the U.S. medicolegal death investigation system. In 2003, this system investigated 450,000 unnatural and/or unexplained deaths (ie, approximately 20% of the 2.4 million deaths in the United States that year).4
Each unnatural or unexplained death is examined, often extensively, by a coroner or medical examiner. This examination system costs more than $600 million annually. Yet the data that are collected are handled on a case-by-case and often county-by-county basis, rather than in aggregate. The essence of the proposal presented here is to take the information and biological samples collected in this process and put them into a National Suicide Database (NSD), which then can serve as a resource for scientists to increase our understanding of the genetic, epigenetic, and other factors underlying death due to suicide. This increased understanding will result in the development more effective tools to detect to those at risk for suicide (ie, risk factor tests), to monitor treatment, and to develop new treatments based on a better understanding of the underlying pathophysiology and pathogenesis of suicide. These tools will reduce:
- the number of lives lost to suicide
- the pain and suffering of loved ones
- lost productivity to society, especially when one considers that suicide disproportionately affects individuals during the most productive period of their lives (ie, age 15 to 54).
The NSD will be organized as a government–private partnership, with the government represented by the National Institutes of Health (NIH) and/or the CDC. The goal will be to take the information that is currently being collected by the nation’s medicolegal death investigation system, including the biological samples, systematize it, enter it into a common database, and make it available to qualified researchers across the country. The administrative arm of the system will be responsible for ensuring systematic data collection, storage in a searchable and integrated database housed within the NIH and/or the CDC, and vetting researchers who will have access to the data, including those with expertise in genomics, molecular biology, suicide, epidemiology, and data-mining. (Currently, the CDC’s National Violent Death Reporting System, which is a state-based surveillance system, pools data on violent deaths from multiple sources into a usable, anonymous database. These sources include state and local medical examiners, coroners, law enforcement, crime labs, and vital statistics records, but they do not include any biological material even though it is collected [personal correspondence with the CDC, July 2016].)
Because information on suicides currently are handled primarily on a county-by-county basis, data concerning these deaths are not facilitating a better understanding of the causes and strategies for preventing suicide. Correcting this situation is the goal of this proposal, as modeled by the National Cancer Institute’s War on Cancer, which has transformed the treatment and the outcomes of cancer. If this proposal is enacted, the same type of transformation will occur and result in a reduction in the suicide rate and better outcomes for the psychiatric illnesses that underlie most instances of suicide.
The proposed NSD will address a major and common problem for researchers in this area—small sample sizes. When considered from the perspective of the size of samples feasible for most independent research teams to collect and study, suicide on an annual basis is rare—however, that is not the case when the incidence of suicide in the nation as a whole is considered. In contrast to the data concerning suicides that individual research teams can collect, the proposed genomic database will grow by approximately 40,000 individuals every year, until a meaningful reduction in deaths due to suicide is achieved.
From a research perspective, suicide, although tragic, is one of the few binary outcomes in psychiatry—that is, life or death. Although there may be >1 genetic and/or epigenetic contributor to suicide, within a relatively short period of time, the proposed database will amass—and continue to amass on an ongoing basis—data from a large population of suicide victims. Researchers then can compare the findings from this database with the normative human genome, looking for variants that are over-represented in the population of those who have died by suicide.
Environmental factors undoubtedly also contribute to the risk of suicide, given that the incidence of suicide increases with age, particularly among white males, and with the addition of psychiatric and medical comorbidities. Inflammatory processes also have been implicated in the pathophysiology of a number of psychiatric disorders, including major depression, which is the primary psychiatric risk factor for suicide. Therefore, consideration should be given to collecting whole blood samples if the time between death and autopsy is within an appropriate limit to obtain interpretable data concerning RNA (ie, gene expression) and even biomarkers of inflammatory and other processes at the time of the suicide. This approach has been used by Niculescu et al5,6 for whole blood gene expression. The rationale for using samples of whole blood is that this strategy could be more easily adapted to clinical practice in contrast to using samples from the target organ (ie, brain) or cerebrospinal fluid.
Roadblocks to progress. In the absence of this proposed NSD, progress in this area has been stymied despite concerted governmental efforts (Box7-10). One reason for the lack of progress has been that governmental efforts have focused on a public health model rather than also including a basic science model aimed at exploring the biological mechanisms underlying the risk of death from suicide. In the current decentralized system, individual researchers and even teams of researchers cannot easily collect data from a sufficiently large population of suicide victims to make inroads in gaining the needed understanding.

Because of the relatively small samples that individual research teams can collect in a reasonable period of time (ie, in terms of grant cycles), many investigators have studied suicide attempts as a surrogate for suicide itself, undoubtedly because suicide attempts are more numerous than suicides themselves, making it easier to collect data. However, there is evidence that these 2 populations—suicide attempters vs those who die by suicide—only partially overlap.
First, the frequency of suicide attempts is 10 to 20 times higher than actual suicides. Second, suicide attempters are 3 times more likely to be female whereas those who die by suicide are 4 times more likely to be male. Third, most individuals who die by suicide do so on their first or second attempt, whereas individuals who have made ≥4 attempts have an increased risk of future attempts rather than for completed suicide compared with the general population. Fourth, certain psychiatric illnesses are more often associated with death by suicide (particularly major depressive disorder, bipolar disorder, and schizophrenia in the first 5 years of an illness) whereas multiple suicide attempts are more often associated with other psychiatric diagnoses such as antisocial and borderline personality disorders.
Finally, in a study in men with a psychiatric disorder, Niculescu et al5 started with 412 candidate genes and found that 208 were associated with suicidal ideation but not suicide itself, whereas 76 genes were associated with both suicidal ideation and completion. Taken together, this evidence suggests that findings concerning suicide attempters, especially those who have made multiple (ie, >3) attempts, might not be extrapolatable to the population of actual suicides.
Is there evidence that this proposal could work?
Yes, research supports the potential utility of the proposed NSD, and this section highlights some of the major findings from these studies, although this review is not intended to be exhaustive.
First, considerable evidence exists for a biological basis for the risk of death due to suicide. The concordance rates for suicide are 10 times higher in monozygotic (“identical”) vs dizygotic (“fraternal”) twins (24.1% vs 2.8%) and 2 to 5 times higher in relatives of those who die by suicide than in the general population. Heritability estimates of fatal suicides and nonfatal suicide attempts in biological relatives of adoptees who die from suicide range from 17% to 45%.11
Second, studies using information from small samples that was arduously collected by individual research groups have yielded important positive data. Most recently, in 2015, a multidisciplinary group led by Niculescu et al5 at Indiana University and other institutions described a test that could predict suicidality in men. This test was developed on the basis of a within-participant discovery approach to identify genes that change in expression between states of no suicidal ideation and high suicidal ideation, which was combined with clinical information assessed by 2 scales, the Convergent Functional Information for Suicidality and the Simplified Affective State Scale. Gene expression was measured in whole blood collected postmortem unless the method of suicide involved a medication overdose that could affect gene expression. These researchers identified 76 genes that likely were involved in suicidal ideation and suicide.
This report had a number of limitations.5 All of the individuals in these studies were being treated for psychiatric illness, were being closely followed by the investigators, and all were male. In addition, as noted above, suicides by overdose were eliminated from the analysis.
In a subsequent study published in 2016, the Niculescu group6 extended their work to women and identified 50 genes contributing to suicide risk in women. Underscoring the need for larger samples, only 3 of the top contributing genes were seen in both men and women, suggesting that there are likely significant sex differences in the biology of suicide completion. This important work needs to be replicated and extended.
In addition to these remarkable advances made in genetic understanding of the risk of suicide, recent research also has demonstrated a role for epigenetic and inflammatory processes as contributors to suicide risk.12-15
There are likely many contributors, including genetic, epigenetic, and environmental factors such as inflammatory processes, that increase the risk of suicide. The goal of this article is not to provide an exhaustive or integrative review of research in this area but rather to argue for the establishment of a national initiative to study all of these factors and to begin that process by establishing the NSD.
What will be the foreseeable outcome of this initiative?
The establishment of the NSD is expected to lead to better identification of those who are genetically at increased risk of suicide as well as biological factors (eg, inflammatory or other processes) and environmental factors (eg, drug abuse), which can turn that genetic risk into reality. Using research results made possible by the implementation of this proposal, objective testing can be developed to monitor risk more effectively than is currently possible using clinical assessment alone.
Furthermore, this work also can provide targets for developing new treatments. For example, there is convergence between the work of Niculescu et al,5,6 who identified genetic biomarkers for mechanistic target of rapamycin (mTOR) signaling as a risk factor in individuals who died by suicide and the work of Li et al and other researchers,16-18 whose findings have implicated mTOR-dependent synapse formation as a mechanism underlying the rapid (ie, within hours to a couple of days) antidepressant effects of N-methyl-
In aggregate, establishment of this proposed database will facilitate identification of biological (and therefore pharmaceutical) mechanisms beyond those involving biogenic amines, which have been the exclusive biological targets for antidepressants for the past 50 years.22 The likely consequences of the findings generated from research made possible by the proposed NSD will open completely new vistas for helping people at risk for suicide and psychiatric illnesses.
What foreseeable obstacles will need to be addressed?
Of course, obstacles and problems will arise but these will not exceed those encountered by the War on Cancer and they can similarly be overcome with sufficient public support and cooperation. Potential obstacles include:
- need for incremental funding
- obtaining the cooperation of the offices of each county medical examiner or coroner in a process that includes uniform systematic data collection
- determining the situations (eg, time after death and means of death) that will allow for meaningful collection of data such as RNA and inflammatory biomarkers
- establishing how data and particularly biological samples will be transported and stored
- issues related to privacy of health information particularly for relatives of suicide victims
- ensuring the reliability, validity, and comparability of the data received from different medical examiners and coroners.
With regard to the last issue, because stigma is associated with death by suicide, some true suicides could be missed, which would compromise sensitivity but simultaneously increase specificity. Other obstacles or problems may arise; however, I am certain that all such issues are surmountable and that the resulting NSD will be much better than what we have now and will propel our understanding of the biological underpinnings of the loss of life to suicide. (The author proposed a similar but even more ambitious plan 25 years ago,23 but he believes that this is an idea whose time has come.)
Acknowledgments
The author thanks Wayne C. Drevets, MD, Alexander Niculescu, MD, PhD, John Oldman, MD, and John Savitz, PhD, David Sheehan, MD, and Matthew Macaluso, DO for their review and suggestions concerning this proposal/manuscript, and Kaylee Hervey, MPH, from the Sedgwick County Health Department, Wichita, Kansas, for her input. The author also thanks Ruth Ross, as always, for her excellent editing and general assistance.
1. Pompili M, Gonda X, Serafini G, et al. Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar Disord. 2013;15(5):457-490.
2. National Vital Statistics System; National Center for Health Statistics; Centers for Disease Control and Prevention. Ten leading causes of death by age group, United States–2014. Centers for Disease Control and Prevention. http://www.cdc.gov/injury/images/lc-charts/leading_causes_of_death_age_group_2014_1050w760h.gif. Accessed October 17, 2016.
3. Curtin SC, Warner M, Hedegaard H, et al. Increase in suicide in the United States, 1999-2014. National Center for Health Statistics Data Brief No. 241. Atlanta GA: National Center for Health Statistics, U.S. Department of Health and Human Services. http://www.cdc.gov/nchs/products/databriefs/db241.htm. Published April 2016. Accessed June 30, 2016.
4. Committee for the Workshop on the Medicolegal Death Investigation System; Board on Health Promotion and Disease Prevention. Medicolegal death investigation system: workshop summary. Washington, DC: National Academies Press; 2003.
5. Niculescu AB, Levey DF, Phalen PL, et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015;20(11):1266-1285.
6. Levey DF, Niculescu EM, Le-Niculescu H, et al. Towards understanding and predicting suicidality in women: biomarkers and clinical risk assessment. Mol Psychiatry. 2016;21(6):768-785.
7. World Health Organization. Prevention of suicide: guidelines for the formulation and implementation of national strategies. Geneva, Switzerland: World Health Organization; 1996.
8. U.S. Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: U.S. Public Health Service; 1999.
9. U.S. Department of Health and Human Services (HHS). National Strategy for Suicide Prevention: goals and objectives for action. Rockville, MD: U.S. Department of Health and Human Services; 2001.
10. U.S. Department of Health and Human Services (HHS). National Strategy for Suicide Prevention: goals and objectives for action. Rockville, MD; U.S. Department of Health and Human Services; 2012.
11. Brent DA, Melham N. Familial transmission of suicidal behavior. Psychiatr Clin North Am. 2008;31(2):157-177.
12. Guintivano J, Brown T, Newcomer A, et al. Identification and replication of a combined epigenetic and genetic biomarker predicting suicide and suicidal behaviors. Am J Psychiatry. 2014;171(12):1287-1296.
13. Bay-Richter C, Linderholm KR, Lim CK, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110-117.
14. Brundin L, Bryleva EY, Thirtamara Rajamani K. Role of inflammation in suicide: from mechanisms to treatment [published online July 27, 2016]. Neuropsychopharmacology. doi: 10.1038/npp.2016.116.
15. Steiner J, Walter M, Gos T, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94.
16. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959-964.
17. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
18. Preskorn SH, Baker B, Kolluri S, et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631-637.
19. Canuso C, Singh J, Fedgchin M, et al. PeRSEVERe: a study of esketamine for the rapid reduction of the symptoms of major depressive disorder, including suicidal ideation, in subjects assessed to be at imminent risk for suicide. Presentation at the Annual Meeting of the American Society of Clinical Psychopharmacology, Scottsdale AZ, May 30-June 3, 2016.
20. Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756-758.
21. Moore PA, Rosen CA, Carter KC. Assignment of the human FKBP12-rapamycin-associated protein (FRAP) gene to chromosome 1p36 by fluorescence in situ hybridization. Genomics. 1996;33(2):331-332.
22. Ha
23. Preskorn SH. The future and psychopharmacology: potentials and needs. Psychiatr Ann. 1990;20(11):625-633.
1. Pompili M, Gonda X, Serafini G, et al. Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar Disord. 2013;15(5):457-490.
2. National Vital Statistics System; National Center for Health Statistics; Centers for Disease Control and Prevention. Ten leading causes of death by age group, United States–2014. Centers for Disease Control and Prevention. http://www.cdc.gov/injury/images/lc-charts/leading_causes_of_death_age_group_2014_1050w760h.gif. Accessed October 17, 2016.
3. Curtin SC, Warner M, Hedegaard H, et al. Increase in suicide in the United States, 1999-2014. National Center for Health Statistics Data Brief No. 241. Atlanta GA: National Center for Health Statistics, U.S. Department of Health and Human Services. http://www.cdc.gov/nchs/products/databriefs/db241.htm. Published April 2016. Accessed June 30, 2016.
4. Committee for the Workshop on the Medicolegal Death Investigation System; Board on Health Promotion and Disease Prevention. Medicolegal death investigation system: workshop summary. Washington, DC: National Academies Press; 2003.
5. Niculescu AB, Levey DF, Phalen PL, et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015;20(11):1266-1285.
6. Levey DF, Niculescu EM, Le-Niculescu H, et al. Towards understanding and predicting suicidality in women: biomarkers and clinical risk assessment. Mol Psychiatry. 2016;21(6):768-785.
7. World Health Organization. Prevention of suicide: guidelines for the formulation and implementation of national strategies. Geneva, Switzerland: World Health Organization; 1996.
8. U.S. Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: U.S. Public Health Service; 1999.
9. U.S. Department of Health and Human Services (HHS). National Strategy for Suicide Prevention: goals and objectives for action. Rockville, MD: U.S. Department of Health and Human Services; 2001.
10. U.S. Department of Health and Human Services (HHS). National Strategy for Suicide Prevention: goals and objectives for action. Rockville, MD; U.S. Department of Health and Human Services; 2012.
11. Brent DA, Melham N. Familial transmission of suicidal behavior. Psychiatr Clin North Am. 2008;31(2):157-177.
12. Guintivano J, Brown T, Newcomer A, et al. Identification and replication of a combined epigenetic and genetic biomarker predicting suicide and suicidal behaviors. Am J Psychiatry. 2014;171(12):1287-1296.
13. Bay-Richter C, Linderholm KR, Lim CK, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110-117.
14. Brundin L, Bryleva EY, Thirtamara Rajamani K. Role of inflammation in suicide: from mechanisms to treatment [published online July 27, 2016]. Neuropsychopharmacology. doi: 10.1038/npp.2016.116.
15. Steiner J, Walter M, Gos T, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94.
16. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959-964.
17. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
18. Preskorn SH, Baker B, Kolluri S, et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631-637.
19. Canuso C, Singh J, Fedgchin M, et al. PeRSEVERe: a study of esketamine for the rapid reduction of the symptoms of major depressive disorder, including suicidal ideation, in subjects assessed to be at imminent risk for suicide. Presentation at the Annual Meeting of the American Society of Clinical Psychopharmacology, Scottsdale AZ, May 30-June 3, 2016.
20. Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756-758.
21. Moore PA, Rosen CA, Carter KC. Assignment of the human FKBP12-rapamycin-associated protein (FRAP) gene to chromosome 1p36 by fluorescence in situ hybridization. Genomics. 1996;33(2):331-332.
22. Ha
23. Preskorn SH. The future and psychopharmacology: potentials and needs. Psychiatr Ann. 1990;20(11):625-633.
Accelerated aging in schizophrenia: Shortened telomeres, mitochondrial dysfunction, inflammation, and oxidative stress
This implies early senescence, segmental aging, and, in young adult patients, premature onset of multi-system medical illnesses associated with aging, including cardiovascular disease, cancer, brain atrophy, and cognitive decline. This might be the real reason why persons with schizophrenia die 25 to 30 years too early, not only because of an unhealthy lifestyle and iatrogenic cardio-metabolic adverse effects.
One of the most consistent observations pointing to accelerated aging in schizophrenia is shortened telomeres.2,3 Telomeres are the terminal part of chromosomes (similar to the plastic aglets of shoelaces), which are known to shorten with each cell division because of “end replication losses.” Telomeres are measured in lymphocytes, which researchers regard as “windows to the brain” because they reflect brain aging.4 One study of lymphocytes in patients with schizophrenia found that they appear to be approximately 25 years older than the lymphocytes of healthy individuals!4
Possible causes of accelerated aging
Inflammation. The leading hypothesis for accelerated aging in schizophrenia is based on the inflammatory theory of aging. Schizophrenia has been strongly linked to immune dysregulation and neuroinflammation.5 Other components of the accelerated aging hypothesis include oxidative and nitrosative stress, which is associated with high levels of free radicals, and, importantly, mitochondrial dysfunction that fails to generate antioxidants (glutathione peroxidase, superoxide dismutase, and catalase) that can neutralize free radicals and reverse oxidative stress, as numerous studies have shown.
Clinically, and at a relatively young age, persons with schizophrenia show many physical features consistent with aging,6 including the following system changes:
- CNS: dilated ventricles, reduced brain volume and gray matter volume; hypofrontality, neurocognitive deficits such as executive functioning, working memory, and attention; neurophysiologic (low amplitudes on evoked potentials)
- Musculoskeletal system: abnormalities in muscle fibers; altered nerve conduction velocity; reduced bone density
- Skin: aging skin
- Eyes: increased rate of cataracts (not caused by medications); degradation in motion discrimination
- Endocrine system: abnormal gonadal hormones; low estrogen; low androgen; thyroid dysfunction, elevated cortisol
- Metabolism: increased rates of obesity; glucose dysregulation even before antipsychotic treatment; increased insulin resistance; abnormal glucose tolerance; reduced insulin-like growth factor-1 levels
- Immune system: increased pro-inflammatory cytokines (interleukin [IL]-1B, IL-6, IL-3, IL-4, IL-10, IL-13, tumor necrosis factor-Symbol Stdα) and decrease in anti-inflammatory cytokines (IL-2, interferon [INF]-Symbol Stdα, INF-Symbol Stdγ) and vitamin D
- Cardiovascular: systolic hypertension, increased pulse pressure
- Oxidative stress and mitochondrial dysfunction: increase in reactive oxygen species in brain tissue and increased DNA and RNA oxidation markers
- Telomere dynamics: significantly higher rates of telomere loss.
The mitochondrial theory of aging.7 The origin of this theory dates back to the landmark work of Denham Harman more than 2 decades ago in which he proposed a connection between free radicals and aging, which is associated with cell mutations and cancer.8 He suggested that because mitochondrial DNA is not protected by histones as DNA in the nucleus is, it might be the main target for free radicals, making the mitochondria more susceptible to oxidative damage. Therefore, it is possible that the high oxidative stress of schizophrenia could contribute to mitochondrial dysfunction, which leads to further telomere erosion.9 Perhaps reducing oxidative stress in schizophrenia with a powerful antioxidant, such as the supplement N-acetylcysteine,10 could help repair the dysfunctional mitochondria found in patients with schizophrenia and might mitigate accelerated aging.
I would like to propose a bolder, even radical, “out-of-the-box” therapeutic strategy for accelerated aging: mitochondrial transplantation. In fact, “mitochondrial donation” and transplant has been performed on fetuses with genetically defective mitochondria, which has prevented rapid death after birth.11
Similarities with progeria. The accumulating evidence for accelerated aging in schizophrenia has promoted some researchers to consider it a form of progeria12 because of the accelerated aging features that patients with schizophrenia manifest. Patients with schizophrenia share some risk factors with patients with progeria, including high paternal age, prenatal stress, prenatal famine, low birth weight, and premature cognitive decline. Both progeria and schizophrenia are associated with increased apoptosis and cell senescence, which could reduce the risk of cancer but result in premature aging along with age-related medical disorders that lead to mortality in the elderly.
This is why collaborative care between psychiatrists and primary care providers is so vital for patients with schizophrenia from the onset of the illness during the teens and young adulthood, not after years of treatment and unhealthy lifestyle habits (smoking, sedentary living, high-fat and high-calorie diet), which add insult to injury, culminating in loss of 25 to 30 years of potential life. Preventative medical care starting when schizophrenia is first diagnosed is vital, in addition to comprehensive psychiatric care, because premature mortality is the worst outcome in medicine.
Henry A. Nasrallah, MD
Editor-in-Chief
1. Kirkpatrick B, Messias E, Harvey PD, et al. Schizophrenia as a syndrome of accelerated aging? Schizophr Bull. 2005;34(6):1024-1032.
2. Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557-579.
3. Kao HT, Cawthon RM, Delisi LE, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13(2):118-119.
4. Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probes: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):559-576.
5. Horváth S, Mirnics K. Immune system disturbance in schizophrenia. Biol Psychiatry. 2014;75(4):316-323.
6. Shirakumar V, Kalmady SV, Venkatasubramanian G, et al. Do schizophrenia patients age early? Asian J Psychiatr. 2014;10:3-9.
7. Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp Gerontol. 2005;40(6):466-472.
8. Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1992;20(4):145-147.
9. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2000;27(7):339-344.
10. Chen AT, Chibnall JT, Nasrallah HA. A systematic review of placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
11. Three’s company. The Economist. July 9, 2016:88.
12. Papanastasiov E, Gaughran F, Smith S. Schizophrenia as segmental progeria. J R Soc Med. 2011;104(11):475-484.
This implies early senescence, segmental aging, and, in young adult patients, premature onset of multi-system medical illnesses associated with aging, including cardiovascular disease, cancer, brain atrophy, and cognitive decline. This might be the real reason why persons with schizophrenia die 25 to 30 years too early, not only because of an unhealthy lifestyle and iatrogenic cardio-metabolic adverse effects.
One of the most consistent observations pointing to accelerated aging in schizophrenia is shortened telomeres.2,3 Telomeres are the terminal part of chromosomes (similar to the plastic aglets of shoelaces), which are known to shorten with each cell division because of “end replication losses.” Telomeres are measured in lymphocytes, which researchers regard as “windows to the brain” because they reflect brain aging.4 One study of lymphocytes in patients with schizophrenia found that they appear to be approximately 25 years older than the lymphocytes of healthy individuals!4
Possible causes of accelerated aging
Inflammation. The leading hypothesis for accelerated aging in schizophrenia is based on the inflammatory theory of aging. Schizophrenia has been strongly linked to immune dysregulation and neuroinflammation.5 Other components of the accelerated aging hypothesis include oxidative and nitrosative stress, which is associated with high levels of free radicals, and, importantly, mitochondrial dysfunction that fails to generate antioxidants (glutathione peroxidase, superoxide dismutase, and catalase) that can neutralize free radicals and reverse oxidative stress, as numerous studies have shown.
Clinically, and at a relatively young age, persons with schizophrenia show many physical features consistent with aging,6 including the following system changes:
- CNS: dilated ventricles, reduced brain volume and gray matter volume; hypofrontality, neurocognitive deficits such as executive functioning, working memory, and attention; neurophysiologic (low amplitudes on evoked potentials)
- Musculoskeletal system: abnormalities in muscle fibers; altered nerve conduction velocity; reduced bone density
- Skin: aging skin
- Eyes: increased rate of cataracts (not caused by medications); degradation in motion discrimination
- Endocrine system: abnormal gonadal hormones; low estrogen; low androgen; thyroid dysfunction, elevated cortisol
- Metabolism: increased rates of obesity; glucose dysregulation even before antipsychotic treatment; increased insulin resistance; abnormal glucose tolerance; reduced insulin-like growth factor-1 levels
- Immune system: increased pro-inflammatory cytokines (interleukin [IL]-1B, IL-6, IL-3, IL-4, IL-10, IL-13, tumor necrosis factor-Symbol Stdα) and decrease in anti-inflammatory cytokines (IL-2, interferon [INF]-Symbol Stdα, INF-Symbol Stdγ) and vitamin D
- Cardiovascular: systolic hypertension, increased pulse pressure
- Oxidative stress and mitochondrial dysfunction: increase in reactive oxygen species in brain tissue and increased DNA and RNA oxidation markers
- Telomere dynamics: significantly higher rates of telomere loss.
The mitochondrial theory of aging.7 The origin of this theory dates back to the landmark work of Denham Harman more than 2 decades ago in which he proposed a connection between free radicals and aging, which is associated with cell mutations and cancer.8 He suggested that because mitochondrial DNA is not protected by histones as DNA in the nucleus is, it might be the main target for free radicals, making the mitochondria more susceptible to oxidative damage. Therefore, it is possible that the high oxidative stress of schizophrenia could contribute to mitochondrial dysfunction, which leads to further telomere erosion.9 Perhaps reducing oxidative stress in schizophrenia with a powerful antioxidant, such as the supplement N-acetylcysteine,10 could help repair the dysfunctional mitochondria found in patients with schizophrenia and might mitigate accelerated aging.
I would like to propose a bolder, even radical, “out-of-the-box” therapeutic strategy for accelerated aging: mitochondrial transplantation. In fact, “mitochondrial donation” and transplant has been performed on fetuses with genetically defective mitochondria, which has prevented rapid death after birth.11
Similarities with progeria. The accumulating evidence for accelerated aging in schizophrenia has promoted some researchers to consider it a form of progeria12 because of the accelerated aging features that patients with schizophrenia manifest. Patients with schizophrenia share some risk factors with patients with progeria, including high paternal age, prenatal stress, prenatal famine, low birth weight, and premature cognitive decline. Both progeria and schizophrenia are associated with increased apoptosis and cell senescence, which could reduce the risk of cancer but result in premature aging along with age-related medical disorders that lead to mortality in the elderly.
This is why collaborative care between psychiatrists and primary care providers is so vital for patients with schizophrenia from the onset of the illness during the teens and young adulthood, not after years of treatment and unhealthy lifestyle habits (smoking, sedentary living, high-fat and high-calorie diet), which add insult to injury, culminating in loss of 25 to 30 years of potential life. Preventative medical care starting when schizophrenia is first diagnosed is vital, in addition to comprehensive psychiatric care, because premature mortality is the worst outcome in medicine.
Henry A. Nasrallah, MD
Editor-in-Chief
This implies early senescence, segmental aging, and, in young adult patients, premature onset of multi-system medical illnesses associated with aging, including cardiovascular disease, cancer, brain atrophy, and cognitive decline. This might be the real reason why persons with schizophrenia die 25 to 30 years too early, not only because of an unhealthy lifestyle and iatrogenic cardio-metabolic adverse effects.
One of the most consistent observations pointing to accelerated aging in schizophrenia is shortened telomeres.2,3 Telomeres are the terminal part of chromosomes (similar to the plastic aglets of shoelaces), which are known to shorten with each cell division because of “end replication losses.” Telomeres are measured in lymphocytes, which researchers regard as “windows to the brain” because they reflect brain aging.4 One study of lymphocytes in patients with schizophrenia found that they appear to be approximately 25 years older than the lymphocytes of healthy individuals!4
Possible causes of accelerated aging
Inflammation. The leading hypothesis for accelerated aging in schizophrenia is based on the inflammatory theory of aging. Schizophrenia has been strongly linked to immune dysregulation and neuroinflammation.5 Other components of the accelerated aging hypothesis include oxidative and nitrosative stress, which is associated with high levels of free radicals, and, importantly, mitochondrial dysfunction that fails to generate antioxidants (glutathione peroxidase, superoxide dismutase, and catalase) that can neutralize free radicals and reverse oxidative stress, as numerous studies have shown.
Clinically, and at a relatively young age, persons with schizophrenia show many physical features consistent with aging,6 including the following system changes:
- CNS: dilated ventricles, reduced brain volume and gray matter volume; hypofrontality, neurocognitive deficits such as executive functioning, working memory, and attention; neurophysiologic (low amplitudes on evoked potentials)
- Musculoskeletal system: abnormalities in muscle fibers; altered nerve conduction velocity; reduced bone density
- Skin: aging skin
- Eyes: increased rate of cataracts (not caused by medications); degradation in motion discrimination
- Endocrine system: abnormal gonadal hormones; low estrogen; low androgen; thyroid dysfunction, elevated cortisol
- Metabolism: increased rates of obesity; glucose dysregulation even before antipsychotic treatment; increased insulin resistance; abnormal glucose tolerance; reduced insulin-like growth factor-1 levels
- Immune system: increased pro-inflammatory cytokines (interleukin [IL]-1B, IL-6, IL-3, IL-4, IL-10, IL-13, tumor necrosis factor-Symbol Stdα) and decrease in anti-inflammatory cytokines (IL-2, interferon [INF]-Symbol Stdα, INF-Symbol Stdγ) and vitamin D
- Cardiovascular: systolic hypertension, increased pulse pressure
- Oxidative stress and mitochondrial dysfunction: increase in reactive oxygen species in brain tissue and increased DNA and RNA oxidation markers
- Telomere dynamics: significantly higher rates of telomere loss.
The mitochondrial theory of aging.7 The origin of this theory dates back to the landmark work of Denham Harman more than 2 decades ago in which he proposed a connection between free radicals and aging, which is associated with cell mutations and cancer.8 He suggested that because mitochondrial DNA is not protected by histones as DNA in the nucleus is, it might be the main target for free radicals, making the mitochondria more susceptible to oxidative damage. Therefore, it is possible that the high oxidative stress of schizophrenia could contribute to mitochondrial dysfunction, which leads to further telomere erosion.9 Perhaps reducing oxidative stress in schizophrenia with a powerful antioxidant, such as the supplement N-acetylcysteine,10 could help repair the dysfunctional mitochondria found in patients with schizophrenia and might mitigate accelerated aging.
I would like to propose a bolder, even radical, “out-of-the-box” therapeutic strategy for accelerated aging: mitochondrial transplantation. In fact, “mitochondrial donation” and transplant has been performed on fetuses with genetically defective mitochondria, which has prevented rapid death after birth.11
Similarities with progeria. The accumulating evidence for accelerated aging in schizophrenia has promoted some researchers to consider it a form of progeria12 because of the accelerated aging features that patients with schizophrenia manifest. Patients with schizophrenia share some risk factors with patients with progeria, including high paternal age, prenatal stress, prenatal famine, low birth weight, and premature cognitive decline. Both progeria and schizophrenia are associated with increased apoptosis and cell senescence, which could reduce the risk of cancer but result in premature aging along with age-related medical disorders that lead to mortality in the elderly.
This is why collaborative care between psychiatrists and primary care providers is so vital for patients with schizophrenia from the onset of the illness during the teens and young adulthood, not after years of treatment and unhealthy lifestyle habits (smoking, sedentary living, high-fat and high-calorie diet), which add insult to injury, culminating in loss of 25 to 30 years of potential life. Preventative medical care starting when schizophrenia is first diagnosed is vital, in addition to comprehensive psychiatric care, because premature mortality is the worst outcome in medicine.
Henry A. Nasrallah, MD
Editor-in-Chief
1. Kirkpatrick B, Messias E, Harvey PD, et al. Schizophrenia as a syndrome of accelerated aging? Schizophr Bull. 2005;34(6):1024-1032.
2. Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557-579.
3. Kao HT, Cawthon RM, Delisi LE, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13(2):118-119.
4. Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probes: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):559-576.
5. Horváth S, Mirnics K. Immune system disturbance in schizophrenia. Biol Psychiatry. 2014;75(4):316-323.
6. Shirakumar V, Kalmady SV, Venkatasubramanian G, et al. Do schizophrenia patients age early? Asian J Psychiatr. 2014;10:3-9.
7. Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp Gerontol. 2005;40(6):466-472.
8. Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1992;20(4):145-147.
9. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2000;27(7):339-344.
10. Chen AT, Chibnall JT, Nasrallah HA. A systematic review of placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
11. Three’s company. The Economist. July 9, 2016:88.
12. Papanastasiov E, Gaughran F, Smith S. Schizophrenia as segmental progeria. J R Soc Med. 2011;104(11):475-484.
1. Kirkpatrick B, Messias E, Harvey PD, et al. Schizophrenia as a syndrome of accelerated aging? Schizophr Bull. 2005;34(6):1024-1032.
2. Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557-579.
3. Kao HT, Cawthon RM, Delisi LE, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13(2):118-119.
4. Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probes: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):559-576.
5. Horváth S, Mirnics K. Immune system disturbance in schizophrenia. Biol Psychiatry. 2014;75(4):316-323.
6. Shirakumar V, Kalmady SV, Venkatasubramanian G, et al. Do schizophrenia patients age early? Asian J Psychiatr. 2014;10:3-9.
7. Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp Gerontol. 2005;40(6):466-472.
8. Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1992;20(4):145-147.
9. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2000;27(7):339-344.
10. Chen AT, Chibnall JT, Nasrallah HA. A systematic review of placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
11. Three’s company. The Economist. July 9, 2016:88.
12. Papanastasiov E, Gaughran F, Smith S. Schizophrenia as segmental progeria. J R Soc Med. 2011;104(11):475-484.
Assess and treat catatonia using this systematic approach
Catatonia is a neuropsychiatric condition with varying presentations that involve behavioral, motoric, cognitive, affective, and, occasionally, autonomic disturbances. Underlying causes of the syndrome include:
- mood disorders
- psychotic disorders
- neurologic disease
- general medical conditions
- metabolic abnormalities
- drug intoxication or withdrawal.
- deep vein thrombosis and pulmonary embolism
- pressure sores or ulcers
- muscle contractures
- nutritional deficiencies and dehydration from decreased oral intake.1
Prompt recognition, assessment, and treatment are vital.
We recommend the following systematic approach to evaluate and treat catatonia (Table).
Assess
Appropriate assessment of catatonia requires recognition of the array of potential underlying causes of the syndrome.
Obtain a complete history, including:
- recent changes in behavior
- past psychiatric illness and hospitalization
- past or current neurologic or medical disease
- prescription and illicit drug use.
Collateral informants, such as family members and caregivers, could provide valuable information. This history could reveal causative factors and identify appropriate targets for treatment.
Physical and mental status examinations can help characterize the type and severity of motoric and behavioral symptoms, such as rigidity, waxy flexibility, negativism, automatic obedience, ambitendency, and perseveration. Monitoring vital signs is crucial because of the risk of medical complications and malignant catatonia, which can be lethal if not treated.
Laboratory testing and imaging might be indicated to rule out medical causes, such as infection, metabolic disturbances, drug intoxication and withdrawal, and acute neurologic etiologies.
Rate
Identify and rate symptom severity. After determining that a patient has catatonia, consider using a standardized instrument, such as the Bush Francis Catatonia Rating Scale (BFCRS),2 to assess the patient’s type of symptoms and degree of impairment. Scores obtained on such instruments can be tracked as the patient receives treatment. Although the BFCRS is imperfect because of ambiguous symptom descriptions and because symptoms can remain after effective treatment, it is the most widely researched catatonia scale.
Treat and monitor
Although there are no published data from large-scale, randomized, controlled trials, clinical experience shows that the mainstays of treatment still are benzodiazepines and electroconvulsive therapy (ECT). A benzodiazepine challenge of IV lorazepam, 2 mg, can lead to rapid, substantial symptomatic relief with relatively low risk of harm. An estimated 50% to 70% of patients with catatonia respond within 5 days to IV lorazepam, 2 mg, every 3 to 8 hours.3
When patients do not respond to benzodiazepines, consider ECT. For patients with medical, neurologic, and toxic metabolic causes of catatonia, treat the underlying disturbance first.
1. Clinebell K, Azzam PN, Gopalan P, et al. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry. 2014;75(6):644-651.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Fink M. Catatonia: syndrome or schizophrenia subtype? Recognition and treatment. J Neural Transmission (Vienna). 2001;108(6):637-644.
Catatonia is a neuropsychiatric condition with varying presentations that involve behavioral, motoric, cognitive, affective, and, occasionally, autonomic disturbances. Underlying causes of the syndrome include:
- mood disorders
- psychotic disorders
- neurologic disease
- general medical conditions
- metabolic abnormalities
- drug intoxication or withdrawal.
- deep vein thrombosis and pulmonary embolism
- pressure sores or ulcers
- muscle contractures
- nutritional deficiencies and dehydration from decreased oral intake.1
Prompt recognition, assessment, and treatment are vital.
We recommend the following systematic approach to evaluate and treat catatonia (Table).
Assess
Appropriate assessment of catatonia requires recognition of the array of potential underlying causes of the syndrome.
Obtain a complete history, including:
- recent changes in behavior
- past psychiatric illness and hospitalization
- past or current neurologic or medical disease
- prescription and illicit drug use.
Collateral informants, such as family members and caregivers, could provide valuable information. This history could reveal causative factors and identify appropriate targets for treatment.
Physical and mental status examinations can help characterize the type and severity of motoric and behavioral symptoms, such as rigidity, waxy flexibility, negativism, automatic obedience, ambitendency, and perseveration. Monitoring vital signs is crucial because of the risk of medical complications and malignant catatonia, which can be lethal if not treated.
Laboratory testing and imaging might be indicated to rule out medical causes, such as infection, metabolic disturbances, drug intoxication and withdrawal, and acute neurologic etiologies.
Rate
Identify and rate symptom severity. After determining that a patient has catatonia, consider using a standardized instrument, such as the Bush Francis Catatonia Rating Scale (BFCRS),2 to assess the patient’s type of symptoms and degree of impairment. Scores obtained on such instruments can be tracked as the patient receives treatment. Although the BFCRS is imperfect because of ambiguous symptom descriptions and because symptoms can remain after effective treatment, it is the most widely researched catatonia scale.
Treat and monitor
Although there are no published data from large-scale, randomized, controlled trials, clinical experience shows that the mainstays of treatment still are benzodiazepines and electroconvulsive therapy (ECT). A benzodiazepine challenge of IV lorazepam, 2 mg, can lead to rapid, substantial symptomatic relief with relatively low risk of harm. An estimated 50% to 70% of patients with catatonia respond within 5 days to IV lorazepam, 2 mg, every 3 to 8 hours.3
When patients do not respond to benzodiazepines, consider ECT. For patients with medical, neurologic, and toxic metabolic causes of catatonia, treat the underlying disturbance first.
Catatonia is a neuropsychiatric condition with varying presentations that involve behavioral, motoric, cognitive, affective, and, occasionally, autonomic disturbances. Underlying causes of the syndrome include:
- mood disorders
- psychotic disorders
- neurologic disease
- general medical conditions
- metabolic abnormalities
- drug intoxication or withdrawal.
- deep vein thrombosis and pulmonary embolism
- pressure sores or ulcers
- muscle contractures
- nutritional deficiencies and dehydration from decreased oral intake.1
Prompt recognition, assessment, and treatment are vital.
We recommend the following systematic approach to evaluate and treat catatonia (Table).
Assess
Appropriate assessment of catatonia requires recognition of the array of potential underlying causes of the syndrome.
Obtain a complete history, including:
- recent changes in behavior
- past psychiatric illness and hospitalization
- past or current neurologic or medical disease
- prescription and illicit drug use.
Collateral informants, such as family members and caregivers, could provide valuable information. This history could reveal causative factors and identify appropriate targets for treatment.
Physical and mental status examinations can help characterize the type and severity of motoric and behavioral symptoms, such as rigidity, waxy flexibility, negativism, automatic obedience, ambitendency, and perseveration. Monitoring vital signs is crucial because of the risk of medical complications and malignant catatonia, which can be lethal if not treated.
Laboratory testing and imaging might be indicated to rule out medical causes, such as infection, metabolic disturbances, drug intoxication and withdrawal, and acute neurologic etiologies.
Rate
Identify and rate symptom severity. After determining that a patient has catatonia, consider using a standardized instrument, such as the Bush Francis Catatonia Rating Scale (BFCRS),2 to assess the patient’s type of symptoms and degree of impairment. Scores obtained on such instruments can be tracked as the patient receives treatment. Although the BFCRS is imperfect because of ambiguous symptom descriptions and because symptoms can remain after effective treatment, it is the most widely researched catatonia scale.
Treat and monitor
Although there are no published data from large-scale, randomized, controlled trials, clinical experience shows that the mainstays of treatment still are benzodiazepines and electroconvulsive therapy (ECT). A benzodiazepine challenge of IV lorazepam, 2 mg, can lead to rapid, substantial symptomatic relief with relatively low risk of harm. An estimated 50% to 70% of patients with catatonia respond within 5 days to IV lorazepam, 2 mg, every 3 to 8 hours.3
When patients do not respond to benzodiazepines, consider ECT. For patients with medical, neurologic, and toxic metabolic causes of catatonia, treat the underlying disturbance first.
1. Clinebell K, Azzam PN, Gopalan P, et al. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry. 2014;75(6):644-651.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Fink M. Catatonia: syndrome or schizophrenia subtype? Recognition and treatment. J Neural Transmission (Vienna). 2001;108(6):637-644.
1. Clinebell K, Azzam PN, Gopalan P, et al. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry. 2014;75(6):644-651.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Fink M. Catatonia: syndrome or schizophrenia subtype? Recognition and treatment. J Neural Transmission (Vienna). 2001;108(6):637-644.
Consider Rx metformin to prevent metabolic syndrome
Many atypical antipsychotics, particularly clozapine and olanzapine, are associated with weight gain, insulin resistance, and metabolic syndrome. Metabolic syndrome is associated with type 2 diabetes mellitus (T2DM) and cardiovascular disease, which are among the leading causes of morbidity and mortality in persons with severe mental illness.1
Clinicians should take measures to prevent T2DM and weight gain in individuals taking antipsychotics before these conditions develop. Metformin re-sensitizes the body to insulin and is a first-line treatment for T2DM. Adding metformin when patients start metabolically high-risk antipsychotics or shortly after they begin gaining weight is an evidence-based strategy to prevent metabolic syndrome.
Evaluate the evidence
In randomized controlled trials, metformin was associated with modest weight loss and improvement in metabolic parameters (eg, fasting blood glucose, serum triglycerides, and total cholesterol) in patients with schizophrenia receiving antipsychotics.1,2 Metformin is effective for preventing metabolic syndrome and as a treatment intervention; therefore, it may prove most beneficial early in treatment before weight gain or insulin resistance develop.
Importantly, weight gain and metabolic syndrome are risk factors for cardiovascular disease, but the number needed to treat for metformin to prevent cardiovascular outcomes, such as myocardial infarction, is not known. Also, metformin is not FDA-approved for this indication. Clinicians should discuss with the patient the risks and benefits of prophylactic metformin, and consider his (her) treatment preferences.
Tolerability and adverse effects
Metformin generally is well-tolerated. Gastrointestinal (GI) symptoms, including nausea and vomiting (14%) and diarrhea (7%), are the most common adverse effects.2 Lactic acidosis is rare and is associated with alcohol use disorders and impaired renal, hepatic, or cardiopulmonary function.3 Because metformin is excreted renally, toxicity could occur in patients with impaired renal function.
Before initiating prophylactic metformin, confirm that the patient does not have T2DM (eg, hemoglobin A1c <6.5%). A thorough medical history, including alcohol use and kidney and liver function tests, are needed to reduce the risk of lactic acidosis.3
Dosing
Although metformin has been studied at many dosages,2 we recommend gradual titration to 1,000 mg, twice daily, taken with meals to reduce the risk of GI effects.
Additional interventions
Metformin alone is not sufficient to mitigate metabolic risk. Providers should address dietary interventions, exercise, and smoking cessation at each visit, and communicate actively with other providers to create a comprehensive treatment plan.
1. Jarskog LF, Hamer RF, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight patients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
2. Zheng W, Li X-B, Tang Y-L, et al. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509.
3. Wang M, Tong J-H, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
Many atypical antipsychotics, particularly clozapine and olanzapine, are associated with weight gain, insulin resistance, and metabolic syndrome. Metabolic syndrome is associated with type 2 diabetes mellitus (T2DM) and cardiovascular disease, which are among the leading causes of morbidity and mortality in persons with severe mental illness.1
Clinicians should take measures to prevent T2DM and weight gain in individuals taking antipsychotics before these conditions develop. Metformin re-sensitizes the body to insulin and is a first-line treatment for T2DM. Adding metformin when patients start metabolically high-risk antipsychotics or shortly after they begin gaining weight is an evidence-based strategy to prevent metabolic syndrome.
Evaluate the evidence
In randomized controlled trials, metformin was associated with modest weight loss and improvement in metabolic parameters (eg, fasting blood glucose, serum triglycerides, and total cholesterol) in patients with schizophrenia receiving antipsychotics.1,2 Metformin is effective for preventing metabolic syndrome and as a treatment intervention; therefore, it may prove most beneficial early in treatment before weight gain or insulin resistance develop.
Importantly, weight gain and metabolic syndrome are risk factors for cardiovascular disease, but the number needed to treat for metformin to prevent cardiovascular outcomes, such as myocardial infarction, is not known. Also, metformin is not FDA-approved for this indication. Clinicians should discuss with the patient the risks and benefits of prophylactic metformin, and consider his (her) treatment preferences.
Tolerability and adverse effects
Metformin generally is well-tolerated. Gastrointestinal (GI) symptoms, including nausea and vomiting (14%) and diarrhea (7%), are the most common adverse effects.2 Lactic acidosis is rare and is associated with alcohol use disorders and impaired renal, hepatic, or cardiopulmonary function.3 Because metformin is excreted renally, toxicity could occur in patients with impaired renal function.
Before initiating prophylactic metformin, confirm that the patient does not have T2DM (eg, hemoglobin A1c <6.5%). A thorough medical history, including alcohol use and kidney and liver function tests, are needed to reduce the risk of lactic acidosis.3
Dosing
Although metformin has been studied at many dosages,2 we recommend gradual titration to 1,000 mg, twice daily, taken with meals to reduce the risk of GI effects.
Additional interventions
Metformin alone is not sufficient to mitigate metabolic risk. Providers should address dietary interventions, exercise, and smoking cessation at each visit, and communicate actively with other providers to create a comprehensive treatment plan.
Many atypical antipsychotics, particularly clozapine and olanzapine, are associated with weight gain, insulin resistance, and metabolic syndrome. Metabolic syndrome is associated with type 2 diabetes mellitus (T2DM) and cardiovascular disease, which are among the leading causes of morbidity and mortality in persons with severe mental illness.1
Clinicians should take measures to prevent T2DM and weight gain in individuals taking antipsychotics before these conditions develop. Metformin re-sensitizes the body to insulin and is a first-line treatment for T2DM. Adding metformin when patients start metabolically high-risk antipsychotics or shortly after they begin gaining weight is an evidence-based strategy to prevent metabolic syndrome.
Evaluate the evidence
In randomized controlled trials, metformin was associated with modest weight loss and improvement in metabolic parameters (eg, fasting blood glucose, serum triglycerides, and total cholesterol) in patients with schizophrenia receiving antipsychotics.1,2 Metformin is effective for preventing metabolic syndrome and as a treatment intervention; therefore, it may prove most beneficial early in treatment before weight gain or insulin resistance develop.
Importantly, weight gain and metabolic syndrome are risk factors for cardiovascular disease, but the number needed to treat for metformin to prevent cardiovascular outcomes, such as myocardial infarction, is not known. Also, metformin is not FDA-approved for this indication. Clinicians should discuss with the patient the risks and benefits of prophylactic metformin, and consider his (her) treatment preferences.
Tolerability and adverse effects
Metformin generally is well-tolerated. Gastrointestinal (GI) symptoms, including nausea and vomiting (14%) and diarrhea (7%), are the most common adverse effects.2 Lactic acidosis is rare and is associated with alcohol use disorders and impaired renal, hepatic, or cardiopulmonary function.3 Because metformin is excreted renally, toxicity could occur in patients with impaired renal function.
Before initiating prophylactic metformin, confirm that the patient does not have T2DM (eg, hemoglobin A1c <6.5%). A thorough medical history, including alcohol use and kidney and liver function tests, are needed to reduce the risk of lactic acidosis.3
Dosing
Although metformin has been studied at many dosages,2 we recommend gradual titration to 1,000 mg, twice daily, taken with meals to reduce the risk of GI effects.
Additional interventions
Metformin alone is not sufficient to mitigate metabolic risk. Providers should address dietary interventions, exercise, and smoking cessation at each visit, and communicate actively with other providers to create a comprehensive treatment plan.
1. Jarskog LF, Hamer RF, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight patients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
2. Zheng W, Li X-B, Tang Y-L, et al. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509.
3. Wang M, Tong J-H, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
1. Jarskog LF, Hamer RF, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight patients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
2. Zheng W, Li X-B, Tang Y-L, et al. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509.
3. Wang M, Tong J-H, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.