User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Lower-dose FOLFIRINOX effective, safer for pancreatic cancer
TOPLINE:
Although practice patterns vary widely, and it is less likely to cause febrile neutropenia.
METHODOLOGY:
- No randomized controlled trials have directly compared modified FOLFIRINOX to standard FOLFIRINOX; this meta-analysis aims to fill the evidence gap.
- The investigators winnowed hundreds of first-line FOLFIRINOX studies down to 37 – 11 prospective and 26 retrospective analyses – to assess practice patterns and clinical outcomes.
- Dose information was grouped into four categories: planned dose in the standard FOLFIRINOX group; actual administered dose in the standard group; planned dose in the modified group; actual administered dose in the modified group.
TAKEAWAY:
- There were 12 types of “planned” dose reductions in FOLFIRINOX: 75%-100% oxaliplatin, 75%-100% irinotecan, 0%-100% 5-fluorouracil (5-FU) bolus, and 75%-133% 5-FU continuous injection.
- Doses actually delivered fell further to 54%-96% for oxaliplatin, 61%-88% for irinotecan, 0%-92% for 5-FU bolus, and 63%-98% 5-FU continuous injection.
- Despite the variations in dosing, reduced doses of FOLFIRINOX were associated with a slightly but not significantly higher objective response rate: 33.8% versus 28.2% for standard dosing (P = .1).
- The incidence of febrile neutropenia was significantly lower in the reduced-dose groups: 5.5% with modified FOLFIRINOX versus 11.6% with standard (P = .03).
IN PRACTICE:
Although the study supports reduced-dose regimens, it also shows that there is “still no consensus” on appropriate dose modification, the authors said. “The best dose modification protocol” remains to be determined and standardized for metastatic pancreatic cancer.
SOURCE:
The study was led by Kwangrok Jung at Seoul (South Korea) National University, and was published June 29 in Therapeutic Advances in Medical Oncology.
LIMITATIONS:
- Only 11 of the 37 studies were prospective.
- The studies often lacked key information, including the reason for dose reductions or detailed dose reduction protocols.
- Studies were also inconsistent in how they reported FOLFIRINOX dose modifications.
DISCLOSURES:
There was no funding for the study, and the investigators had no disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Although practice patterns vary widely, and it is less likely to cause febrile neutropenia.
METHODOLOGY:
- No randomized controlled trials have directly compared modified FOLFIRINOX to standard FOLFIRINOX; this meta-analysis aims to fill the evidence gap.
- The investigators winnowed hundreds of first-line FOLFIRINOX studies down to 37 – 11 prospective and 26 retrospective analyses – to assess practice patterns and clinical outcomes.
- Dose information was grouped into four categories: planned dose in the standard FOLFIRINOX group; actual administered dose in the standard group; planned dose in the modified group; actual administered dose in the modified group.
TAKEAWAY:
- There were 12 types of “planned” dose reductions in FOLFIRINOX: 75%-100% oxaliplatin, 75%-100% irinotecan, 0%-100% 5-fluorouracil (5-FU) bolus, and 75%-133% 5-FU continuous injection.
- Doses actually delivered fell further to 54%-96% for oxaliplatin, 61%-88% for irinotecan, 0%-92% for 5-FU bolus, and 63%-98% 5-FU continuous injection.
- Despite the variations in dosing, reduced doses of FOLFIRINOX were associated with a slightly but not significantly higher objective response rate: 33.8% versus 28.2% for standard dosing (P = .1).
- The incidence of febrile neutropenia was significantly lower in the reduced-dose groups: 5.5% with modified FOLFIRINOX versus 11.6% with standard (P = .03).
IN PRACTICE:
Although the study supports reduced-dose regimens, it also shows that there is “still no consensus” on appropriate dose modification, the authors said. “The best dose modification protocol” remains to be determined and standardized for metastatic pancreatic cancer.
SOURCE:
The study was led by Kwangrok Jung at Seoul (South Korea) National University, and was published June 29 in Therapeutic Advances in Medical Oncology.
LIMITATIONS:
- Only 11 of the 37 studies were prospective.
- The studies often lacked key information, including the reason for dose reductions or detailed dose reduction protocols.
- Studies were also inconsistent in how they reported FOLFIRINOX dose modifications.
DISCLOSURES:
There was no funding for the study, and the investigators had no disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Although practice patterns vary widely, and it is less likely to cause febrile neutropenia.
METHODOLOGY:
- No randomized controlled trials have directly compared modified FOLFIRINOX to standard FOLFIRINOX; this meta-analysis aims to fill the evidence gap.
- The investigators winnowed hundreds of first-line FOLFIRINOX studies down to 37 – 11 prospective and 26 retrospective analyses – to assess practice patterns and clinical outcomes.
- Dose information was grouped into four categories: planned dose in the standard FOLFIRINOX group; actual administered dose in the standard group; planned dose in the modified group; actual administered dose in the modified group.
TAKEAWAY:
- There were 12 types of “planned” dose reductions in FOLFIRINOX: 75%-100% oxaliplatin, 75%-100% irinotecan, 0%-100% 5-fluorouracil (5-FU) bolus, and 75%-133% 5-FU continuous injection.
- Doses actually delivered fell further to 54%-96% for oxaliplatin, 61%-88% for irinotecan, 0%-92% for 5-FU bolus, and 63%-98% 5-FU continuous injection.
- Despite the variations in dosing, reduced doses of FOLFIRINOX were associated with a slightly but not significantly higher objective response rate: 33.8% versus 28.2% for standard dosing (P = .1).
- The incidence of febrile neutropenia was significantly lower in the reduced-dose groups: 5.5% with modified FOLFIRINOX versus 11.6% with standard (P = .03).
IN PRACTICE:
Although the study supports reduced-dose regimens, it also shows that there is “still no consensus” on appropriate dose modification, the authors said. “The best dose modification protocol” remains to be determined and standardized for metastatic pancreatic cancer.
SOURCE:
The study was led by Kwangrok Jung at Seoul (South Korea) National University, and was published June 29 in Therapeutic Advances in Medical Oncology.
LIMITATIONS:
- Only 11 of the 37 studies were prospective.
- The studies often lacked key information, including the reason for dose reductions or detailed dose reduction protocols.
- Studies were also inconsistent in how they reported FOLFIRINOX dose modifications.
DISCLOSURES:
There was no funding for the study, and the investigators had no disclosures.
A version of this article first appeared on Medscape.com.
No benefit to adding limited radiation in advanced cancer
TOPLINE:
METHODOLOGY:
- In the phase 2 CHEERS trial, 52 patients with advanced solid tumors were randomized to anti-PD-1/PD-L1 monotherapy and 47 patients to the same treatment plus stereotactic body radiotherapy (3 x 8 Gy) to a maximum of three lesions before the second or third cycle of an immune checkpoint inhibitor.
- Patients had locally advanced or metastatic melanoma, renal cell carcinoma, urothelial carcinoma, non-small cell lung carcinoma, or head and neck squamous cell carcinoma and were treated at five Belgian hospitals.
- Most patients had more than three lesions.
- Seven patients in the experimental group did not complete radiotherapy because of early progression or intercurrent illness.
TAKEAWAY:
- Over a median follow-up of 12.5 months, median progression-free survival was 4.4 months in the radiotherapy group versus 2.8 months in the control group (hazard ratio, 0.95; P = .82).
- Median overall survival was not significantly better with radiotherapy, compared with the control group (14.3 vs. 11 months; HR, 0.82; P = .47), nor was the objective response rate (27% vs. 22%; P = .56).
- However, a post hoc analysis demonstrated a significant association between the number of irradiated lesions and overall survival among patients receiving radiotherapy (HR, 0.31; P = .002).
- The incidence of grade 3 or worse treatment-related adverse events was 18% in both groups.
IN PRACTICE:
Although the study was negative overall, the post hoc analysis coupled with “recent evidence suggests that treating all active disease sites with higher radiation doses ... may be a more promising strategy to optimize systemic disease control,” the authors concluded.
SOURCE:
The study was led by Mathieu Spaas, MD, department of radiation oncology, Ghent (Bellgium) University, and published online in JAMA Oncology.
LIMITATIONS:
- There was insufficient power to detect if certain cancers benefited more from add-on radiation because of the small sample size.
- More than half of patients in the control group had already received some form of radiotherapy before study inclusion, which may mean the study underestimated the benefit of radiotherapy.
DISCLOSURES:
The work was funded by Kom Op Tegen Kanker and Varian Medical Systems.
Investigators disclosed numerous industry ties, including Merck, Novartis, and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In the phase 2 CHEERS trial, 52 patients with advanced solid tumors were randomized to anti-PD-1/PD-L1 monotherapy and 47 patients to the same treatment plus stereotactic body radiotherapy (3 x 8 Gy) to a maximum of three lesions before the second or third cycle of an immune checkpoint inhibitor.
- Patients had locally advanced or metastatic melanoma, renal cell carcinoma, urothelial carcinoma, non-small cell lung carcinoma, or head and neck squamous cell carcinoma and were treated at five Belgian hospitals.
- Most patients had more than three lesions.
- Seven patients in the experimental group did not complete radiotherapy because of early progression or intercurrent illness.
TAKEAWAY:
- Over a median follow-up of 12.5 months, median progression-free survival was 4.4 months in the radiotherapy group versus 2.8 months in the control group (hazard ratio, 0.95; P = .82).
- Median overall survival was not significantly better with radiotherapy, compared with the control group (14.3 vs. 11 months; HR, 0.82; P = .47), nor was the objective response rate (27% vs. 22%; P = .56).
- However, a post hoc analysis demonstrated a significant association between the number of irradiated lesions and overall survival among patients receiving radiotherapy (HR, 0.31; P = .002).
- The incidence of grade 3 or worse treatment-related adverse events was 18% in both groups.
IN PRACTICE:
Although the study was negative overall, the post hoc analysis coupled with “recent evidence suggests that treating all active disease sites with higher radiation doses ... may be a more promising strategy to optimize systemic disease control,” the authors concluded.
SOURCE:
The study was led by Mathieu Spaas, MD, department of radiation oncology, Ghent (Bellgium) University, and published online in JAMA Oncology.
LIMITATIONS:
- There was insufficient power to detect if certain cancers benefited more from add-on radiation because of the small sample size.
- More than half of patients in the control group had already received some form of radiotherapy before study inclusion, which may mean the study underestimated the benefit of radiotherapy.
DISCLOSURES:
The work was funded by Kom Op Tegen Kanker and Varian Medical Systems.
Investigators disclosed numerous industry ties, including Merck, Novartis, and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In the phase 2 CHEERS trial, 52 patients with advanced solid tumors were randomized to anti-PD-1/PD-L1 monotherapy and 47 patients to the same treatment plus stereotactic body radiotherapy (3 x 8 Gy) to a maximum of three lesions before the second or third cycle of an immune checkpoint inhibitor.
- Patients had locally advanced or metastatic melanoma, renal cell carcinoma, urothelial carcinoma, non-small cell lung carcinoma, or head and neck squamous cell carcinoma and were treated at five Belgian hospitals.
- Most patients had more than three lesions.
- Seven patients in the experimental group did not complete radiotherapy because of early progression or intercurrent illness.
TAKEAWAY:
- Over a median follow-up of 12.5 months, median progression-free survival was 4.4 months in the radiotherapy group versus 2.8 months in the control group (hazard ratio, 0.95; P = .82).
- Median overall survival was not significantly better with radiotherapy, compared with the control group (14.3 vs. 11 months; HR, 0.82; P = .47), nor was the objective response rate (27% vs. 22%; P = .56).
- However, a post hoc analysis demonstrated a significant association between the number of irradiated lesions and overall survival among patients receiving radiotherapy (HR, 0.31; P = .002).
- The incidence of grade 3 or worse treatment-related adverse events was 18% in both groups.
IN PRACTICE:
Although the study was negative overall, the post hoc analysis coupled with “recent evidence suggests that treating all active disease sites with higher radiation doses ... may be a more promising strategy to optimize systemic disease control,” the authors concluded.
SOURCE:
The study was led by Mathieu Spaas, MD, department of radiation oncology, Ghent (Bellgium) University, and published online in JAMA Oncology.
LIMITATIONS:
- There was insufficient power to detect if certain cancers benefited more from add-on radiation because of the small sample size.
- More than half of patients in the control group had already received some form of radiotherapy before study inclusion, which may mean the study underestimated the benefit of radiotherapy.
DISCLOSURES:
The work was funded by Kom Op Tegen Kanker and Varian Medical Systems.
Investigators disclosed numerous industry ties, including Merck, Novartis, and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
A teenage girl refuses more cancer treatment; her father disagrees
This transcript has been edited for clarity.
Hi. I’m Art Caplan, PhD. I’m director of the division of medical ethics at the New York University Grossman School of Medicine.
Every once in a while at my school, I get referrals about interesting or difficult clinical cases where doctors would like some input or advice that they can consider in managing a patient. Sometimes those requests come from other hospitals to me. I’ve been doing that kind of ethics consulting, both as a member of various ethics committees and sometimes individually, when, for various reasons, doctors don’t want to go to the Ethics Committee as a first stop.
There was a very interesting case recently involving a young woman I’m going to call Tinslee. She was 17 years old and she suffered, sadly, from recurrent metastatic osteogenic sarcoma. She had bone cancer. It had first been diagnosed at the age of 9. She had received chemotherapy and been under that treatment for a while.
If osteosarcoma is treated before it spreads outside the area where it began, the 5-year survival rate for people like her is about 75%. If the cancer spreads outside of the bones and gets into surrounding tissues, organs, or – worse – into the lymph nodes and starts traveling around, the 5-year survival rate drops to about 60%. The two approaches are chemotherapy and amputation. That’s what we have to offer patients like Tinslee.
Initially, her chemotherapy worked. She went to school and enjoyed sports. She was a real fan of softball and tried to manage the team and be involved. At the time I learned about her, she was planning to go to college. Her love of softball remained, but given the recurrence of the cancer, she had no chance to pursue her athletic interests, not only as a player, but also as a manager or even as a coach for younger players. That was all off the table.
She’d been very compliant up until this time with her chemotherapy. When the recommendation came in that she undergo nonstandard chemotherapy because of the reoccurrence, with experimental drugs using an experimental protocol, she said to her family and the doctors that she didn’t want to do it. She would rather die. She couldn’t take any more chemotherapy and she certainly didn’t want to do it if it was experimental, with the outcomes of this intervention being uncertain.
Her mother said, “Her input matters. I want to listen to her.” Her mom wasn’t as adamant about doing it or not, but she really felt that Tinslee should be heard loudly because she felt she was mature enough or old enough, even though a minor, to really have a position about what it is to undergo chemotherapy.
Time matters in trying to control the spread, and the doctors were pushing for experimental intervention. I should add, by the way, that although it didn’t really drive the decision about whether to do it or not do it, experimental care like this is not covered by most insurance, and it wasn’t covered by their insurance, so they were facing a big bill if the experimental intervention was administered.
There was some money in a grant to cover some of it, but they were going to face some big financial costs. It never came up in my discussions with the doctors about what to do. I’m not sure whether it ever came up with the family’s discussion with the doctors about what to do, or even whether Tinslee was worrying and didn’t want her family to face a financial burden.
I suggested that we bring the family in. We did some counseling. We had a social worker and we brought in a pastor because these people were fairly religious. We talked about all scenarios, including accepting death, knowing that this disease was not likely to go into remission with the experimental effort; maybe it would, but the doctors were not optimistic.
We tried to talk about how much we should listen to what this young woman wanted. We knew there was the possibility of going to court and having a judge decide this, but in my experience, I do not like going to judges and courts because I know what they’re going to say. They almost always say “administer the intervention.” They don’t want to be in a position of saying don’t do something. They’re a little less willing to do that if something is experimental, but generally speaking, if you’re headed to court, it’s because you’ve decided that you want this to happen.
I felt, in all honesty, that this young woman should have some real respect of her position because the treatment was experimental. She is approaching the age of competency and consent, and she’s been through many interventions. She knows what’s involved. I think you really have to listen hard to what she’s saying.
By the way, after this case, I looked and there have been some surveys of residents in pediatrics. A large number of them said that they hadn’t received any training about what to do when mature minors refuse experimental treatments. The study I saw said that 30% had not undergone any training about this, so we certainly want to introduce that into the appropriate areas of medicine and talk about this with residents and fellows.
Long story short, we had the family meeting, we had another meeting with dad and mom and Tinslee, and the dad began to come around and he began to listen hard. Tinslee said what she wanted was to go to her prom. She wanted to get to her sister’s junior high school softball championship game. If you will, setting some smaller goals that seemed to make her very, very happy began to satisfy mom and dad and they could accept her refusal.
Ultimately, an agreement was reached that she would not undergo the experimental intervention. We agreed on a course of palliative care, recommended that as what the doctors follow, and they decided to do so. Sadly, Tinslee died. She died at home. She did make it to her prom.
I think the outcome, while difficult, sad, tragic, and a close call, was correct. Mature minors who have been through a rough life of interventions and know the price to pay – and for those who have recurrent disease and now face only experimental options – if they say no, that’s something we really have to listen to very hard.
Dr. Kaplan is director, division of medical ethics, New York University Langone Medical Center, New York. He reported a conflict of interest with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan, PhD. I’m director of the division of medical ethics at the New York University Grossman School of Medicine.
Every once in a while at my school, I get referrals about interesting or difficult clinical cases where doctors would like some input or advice that they can consider in managing a patient. Sometimes those requests come from other hospitals to me. I’ve been doing that kind of ethics consulting, both as a member of various ethics committees and sometimes individually, when, for various reasons, doctors don’t want to go to the Ethics Committee as a first stop.
There was a very interesting case recently involving a young woman I’m going to call Tinslee. She was 17 years old and she suffered, sadly, from recurrent metastatic osteogenic sarcoma. She had bone cancer. It had first been diagnosed at the age of 9. She had received chemotherapy and been under that treatment for a while.
If osteosarcoma is treated before it spreads outside the area where it began, the 5-year survival rate for people like her is about 75%. If the cancer spreads outside of the bones and gets into surrounding tissues, organs, or – worse – into the lymph nodes and starts traveling around, the 5-year survival rate drops to about 60%. The two approaches are chemotherapy and amputation. That’s what we have to offer patients like Tinslee.
Initially, her chemotherapy worked. She went to school and enjoyed sports. She was a real fan of softball and tried to manage the team and be involved. At the time I learned about her, she was planning to go to college. Her love of softball remained, but given the recurrence of the cancer, she had no chance to pursue her athletic interests, not only as a player, but also as a manager or even as a coach for younger players. That was all off the table.
She’d been very compliant up until this time with her chemotherapy. When the recommendation came in that she undergo nonstandard chemotherapy because of the reoccurrence, with experimental drugs using an experimental protocol, she said to her family and the doctors that she didn’t want to do it. She would rather die. She couldn’t take any more chemotherapy and she certainly didn’t want to do it if it was experimental, with the outcomes of this intervention being uncertain.
Her mother said, “Her input matters. I want to listen to her.” Her mom wasn’t as adamant about doing it or not, but she really felt that Tinslee should be heard loudly because she felt she was mature enough or old enough, even though a minor, to really have a position about what it is to undergo chemotherapy.
Time matters in trying to control the spread, and the doctors were pushing for experimental intervention. I should add, by the way, that although it didn’t really drive the decision about whether to do it or not do it, experimental care like this is not covered by most insurance, and it wasn’t covered by their insurance, so they were facing a big bill if the experimental intervention was administered.
There was some money in a grant to cover some of it, but they were going to face some big financial costs. It never came up in my discussions with the doctors about what to do. I’m not sure whether it ever came up with the family’s discussion with the doctors about what to do, or even whether Tinslee was worrying and didn’t want her family to face a financial burden.
I suggested that we bring the family in. We did some counseling. We had a social worker and we brought in a pastor because these people were fairly religious. We talked about all scenarios, including accepting death, knowing that this disease was not likely to go into remission with the experimental effort; maybe it would, but the doctors were not optimistic.
We tried to talk about how much we should listen to what this young woman wanted. We knew there was the possibility of going to court and having a judge decide this, but in my experience, I do not like going to judges and courts because I know what they’re going to say. They almost always say “administer the intervention.” They don’t want to be in a position of saying don’t do something. They’re a little less willing to do that if something is experimental, but generally speaking, if you’re headed to court, it’s because you’ve decided that you want this to happen.
I felt, in all honesty, that this young woman should have some real respect of her position because the treatment was experimental. She is approaching the age of competency and consent, and she’s been through many interventions. She knows what’s involved. I think you really have to listen hard to what she’s saying.
By the way, after this case, I looked and there have been some surveys of residents in pediatrics. A large number of them said that they hadn’t received any training about what to do when mature minors refuse experimental treatments. The study I saw said that 30% had not undergone any training about this, so we certainly want to introduce that into the appropriate areas of medicine and talk about this with residents and fellows.
Long story short, we had the family meeting, we had another meeting with dad and mom and Tinslee, and the dad began to come around and he began to listen hard. Tinslee said what she wanted was to go to her prom. She wanted to get to her sister’s junior high school softball championship game. If you will, setting some smaller goals that seemed to make her very, very happy began to satisfy mom and dad and they could accept her refusal.
Ultimately, an agreement was reached that she would not undergo the experimental intervention. We agreed on a course of palliative care, recommended that as what the doctors follow, and they decided to do so. Sadly, Tinslee died. She died at home. She did make it to her prom.
I think the outcome, while difficult, sad, tragic, and a close call, was correct. Mature minors who have been through a rough life of interventions and know the price to pay – and for those who have recurrent disease and now face only experimental options – if they say no, that’s something we really have to listen to very hard.
Dr. Kaplan is director, division of medical ethics, New York University Langone Medical Center, New York. He reported a conflict of interest with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan, PhD. I’m director of the division of medical ethics at the New York University Grossman School of Medicine.
Every once in a while at my school, I get referrals about interesting or difficult clinical cases where doctors would like some input or advice that they can consider in managing a patient. Sometimes those requests come from other hospitals to me. I’ve been doing that kind of ethics consulting, both as a member of various ethics committees and sometimes individually, when, for various reasons, doctors don’t want to go to the Ethics Committee as a first stop.
There was a very interesting case recently involving a young woman I’m going to call Tinslee. She was 17 years old and she suffered, sadly, from recurrent metastatic osteogenic sarcoma. She had bone cancer. It had first been diagnosed at the age of 9. She had received chemotherapy and been under that treatment for a while.
If osteosarcoma is treated before it spreads outside the area where it began, the 5-year survival rate for people like her is about 75%. If the cancer spreads outside of the bones and gets into surrounding tissues, organs, or – worse – into the lymph nodes and starts traveling around, the 5-year survival rate drops to about 60%. The two approaches are chemotherapy and amputation. That’s what we have to offer patients like Tinslee.
Initially, her chemotherapy worked. She went to school and enjoyed sports. She was a real fan of softball and tried to manage the team and be involved. At the time I learned about her, she was planning to go to college. Her love of softball remained, but given the recurrence of the cancer, she had no chance to pursue her athletic interests, not only as a player, but also as a manager or even as a coach for younger players. That was all off the table.
She’d been very compliant up until this time with her chemotherapy. When the recommendation came in that she undergo nonstandard chemotherapy because of the reoccurrence, with experimental drugs using an experimental protocol, she said to her family and the doctors that she didn’t want to do it. She would rather die. She couldn’t take any more chemotherapy and she certainly didn’t want to do it if it was experimental, with the outcomes of this intervention being uncertain.
Her mother said, “Her input matters. I want to listen to her.” Her mom wasn’t as adamant about doing it or not, but she really felt that Tinslee should be heard loudly because she felt she was mature enough or old enough, even though a minor, to really have a position about what it is to undergo chemotherapy.
Time matters in trying to control the spread, and the doctors were pushing for experimental intervention. I should add, by the way, that although it didn’t really drive the decision about whether to do it or not do it, experimental care like this is not covered by most insurance, and it wasn’t covered by their insurance, so they were facing a big bill if the experimental intervention was administered.
There was some money in a grant to cover some of it, but they were going to face some big financial costs. It never came up in my discussions with the doctors about what to do. I’m not sure whether it ever came up with the family’s discussion with the doctors about what to do, or even whether Tinslee was worrying and didn’t want her family to face a financial burden.
I suggested that we bring the family in. We did some counseling. We had a social worker and we brought in a pastor because these people were fairly religious. We talked about all scenarios, including accepting death, knowing that this disease was not likely to go into remission with the experimental effort; maybe it would, but the doctors were not optimistic.
We tried to talk about how much we should listen to what this young woman wanted. We knew there was the possibility of going to court and having a judge decide this, but in my experience, I do not like going to judges and courts because I know what they’re going to say. They almost always say “administer the intervention.” They don’t want to be in a position of saying don’t do something. They’re a little less willing to do that if something is experimental, but generally speaking, if you’re headed to court, it’s because you’ve decided that you want this to happen.
I felt, in all honesty, that this young woman should have some real respect of her position because the treatment was experimental. She is approaching the age of competency and consent, and she’s been through many interventions. She knows what’s involved. I think you really have to listen hard to what she’s saying.
By the way, after this case, I looked and there have been some surveys of residents in pediatrics. A large number of them said that they hadn’t received any training about what to do when mature minors refuse experimental treatments. The study I saw said that 30% had not undergone any training about this, so we certainly want to introduce that into the appropriate areas of medicine and talk about this with residents and fellows.
Long story short, we had the family meeting, we had another meeting with dad and mom and Tinslee, and the dad began to come around and he began to listen hard. Tinslee said what she wanted was to go to her prom. She wanted to get to her sister’s junior high school softball championship game. If you will, setting some smaller goals that seemed to make her very, very happy began to satisfy mom and dad and they could accept her refusal.
Ultimately, an agreement was reached that she would not undergo the experimental intervention. We agreed on a course of palliative care, recommended that as what the doctors follow, and they decided to do so. Sadly, Tinslee died. She died at home. She did make it to her prom.
I think the outcome, while difficult, sad, tragic, and a close call, was correct. Mature minors who have been through a rough life of interventions and know the price to pay – and for those who have recurrent disease and now face only experimental options – if they say no, that’s something we really have to listen to very hard.
Dr. Kaplan is director, division of medical ethics, New York University Langone Medical Center, New York. He reported a conflict of interest with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
Can a biodegradable brain implant deliver lifesaving cancer meds?
It’s the latest advance in a rapidly growing field using ultrasound – high-frequency sound waves undetectable to humans – to fight cancer and other diseases.
The problem addressed by the researchers is the blood-brain barrier, a nearly impenetrable blood vessel lining that keeps harmful molecules from passing into the brain from the blood. But this lining can also block chemo drugs from reaching cancer cells.
So the scientists implanted 1-cm2 devices into the skulls of mice, directly behind the tumor site. The implants generate ultrasound waves, loosening the barrier and allowing the drugs to reach the tumor. The sound waves leave healthy tissue undamaged.
“You inject the drug into the body and turn on the ultrasound at the same time. You’re going to hit precisely at the tumor area every single time you use it,” said lead study author Thanh Nguyen, PhD, an associate professor of mechanical engineering at the University of Connecticut, Storrs.
The drug used in the study was paclitaxel, which normally struggles to get through the blood-brain barrier. The tumors shrank, and the mice doubled their lifetime, compared with untreated mice. The mice showed no bad health effects 6 months later.
Breaking through the blood-brain barrier
The biodegradable implant is made of glycine, an amino acid that’s also strongly piezoelectric, meaning it vibrates when subjected to an electrical current. To make it, researchers cultivated glycine crystals, shattered them into pieces, and finally used a process called electrospinning, which applies a high electrical voltage to the nanocrystals.
Voltage flows to the implant via an external device. The resulting ultrasound causes the tightly adhered cells of the blood-brain barrier to vibrate, stretching them out and creating space for pores to form.
“That allows in very tiny particles, including chemo drugs,” said Dr. Nguyen.
His earlier biodegradable implant broke apart from the force, but the new glycine implant is more flexible, stable, and highly piezoelectric. It could be implanted after a patient has surgery to remove a brain tumor, to continue treating residual cancer cells. The implant dissolves harmlessly in the body over time, and doctors can control its lifespan.
A new wave of uses for ultrasound
Dr. Nguyen’s study builds on similar efforts, including a recent clinical trial of a nonbiodegradable implant for treating brain tumors. Ultrasound can focus energy on precise targets in the body.
It’s like “using a magnifying glass to focus multiple beams of light on a point and burn a hole in a leaf,” said Neal Kassell, MD, founder and chairman of the Focused Ultrasound Foundation. This approach spares adjacent normal tissue.
Doctors now understand more than 30 ways that ultrasound interacts with tissue – from destroying abnormal tissue to delivering drugs more effectively to stimulating an immune response. A decade ago, only five such interactions were known.
This opens the door for treating “a wide spectrum of medical disorders,” from neurodegenerative diseases like Alzheimer’s and Parkinson’s to difficult-to-treat cancers of the prostate and pancreas, and even addiction, said Dr. Kassell.
Dr. Kassell envisions using focused ultrasound to treat brain tumors as an alternative (or complement) to surgery, chemotherapy, immunotherapy, or radiation therapy. In the meantime, implants have helped show “the effectiveness of opening the blood-brain barrier.”
Dr. Nguyen’s team plans on testing the safety and efficacy of their implant in pigs next. Eventually, Dr. Nguyen hopes to develop a patch with an array of implants to target different areas of the brain.
One study coauthor is cofounder of PiezoBioMembrane and SingleTimeMicroneedles. The other study authors reported no conflicts of interest.
A version of this article originally appeared on WebMD.com.
It’s the latest advance in a rapidly growing field using ultrasound – high-frequency sound waves undetectable to humans – to fight cancer and other diseases.
The problem addressed by the researchers is the blood-brain barrier, a nearly impenetrable blood vessel lining that keeps harmful molecules from passing into the brain from the blood. But this lining can also block chemo drugs from reaching cancer cells.
So the scientists implanted 1-cm2 devices into the skulls of mice, directly behind the tumor site. The implants generate ultrasound waves, loosening the barrier and allowing the drugs to reach the tumor. The sound waves leave healthy tissue undamaged.
“You inject the drug into the body and turn on the ultrasound at the same time. You’re going to hit precisely at the tumor area every single time you use it,” said lead study author Thanh Nguyen, PhD, an associate professor of mechanical engineering at the University of Connecticut, Storrs.
The drug used in the study was paclitaxel, which normally struggles to get through the blood-brain barrier. The tumors shrank, and the mice doubled their lifetime, compared with untreated mice. The mice showed no bad health effects 6 months later.
Breaking through the blood-brain barrier
The biodegradable implant is made of glycine, an amino acid that’s also strongly piezoelectric, meaning it vibrates when subjected to an electrical current. To make it, researchers cultivated glycine crystals, shattered them into pieces, and finally used a process called electrospinning, which applies a high electrical voltage to the nanocrystals.
Voltage flows to the implant via an external device. The resulting ultrasound causes the tightly adhered cells of the blood-brain barrier to vibrate, stretching them out and creating space for pores to form.
“That allows in very tiny particles, including chemo drugs,” said Dr. Nguyen.
His earlier biodegradable implant broke apart from the force, but the new glycine implant is more flexible, stable, and highly piezoelectric. It could be implanted after a patient has surgery to remove a brain tumor, to continue treating residual cancer cells. The implant dissolves harmlessly in the body over time, and doctors can control its lifespan.
A new wave of uses for ultrasound
Dr. Nguyen’s study builds on similar efforts, including a recent clinical trial of a nonbiodegradable implant for treating brain tumors. Ultrasound can focus energy on precise targets in the body.
It’s like “using a magnifying glass to focus multiple beams of light on a point and burn a hole in a leaf,” said Neal Kassell, MD, founder and chairman of the Focused Ultrasound Foundation. This approach spares adjacent normal tissue.
Doctors now understand more than 30 ways that ultrasound interacts with tissue – from destroying abnormal tissue to delivering drugs more effectively to stimulating an immune response. A decade ago, only five such interactions were known.
This opens the door for treating “a wide spectrum of medical disorders,” from neurodegenerative diseases like Alzheimer’s and Parkinson’s to difficult-to-treat cancers of the prostate and pancreas, and even addiction, said Dr. Kassell.
Dr. Kassell envisions using focused ultrasound to treat brain tumors as an alternative (or complement) to surgery, chemotherapy, immunotherapy, or radiation therapy. In the meantime, implants have helped show “the effectiveness of opening the blood-brain barrier.”
Dr. Nguyen’s team plans on testing the safety and efficacy of their implant in pigs next. Eventually, Dr. Nguyen hopes to develop a patch with an array of implants to target different areas of the brain.
One study coauthor is cofounder of PiezoBioMembrane and SingleTimeMicroneedles. The other study authors reported no conflicts of interest.
A version of this article originally appeared on WebMD.com.
It’s the latest advance in a rapidly growing field using ultrasound – high-frequency sound waves undetectable to humans – to fight cancer and other diseases.
The problem addressed by the researchers is the blood-brain barrier, a nearly impenetrable blood vessel lining that keeps harmful molecules from passing into the brain from the blood. But this lining can also block chemo drugs from reaching cancer cells.
So the scientists implanted 1-cm2 devices into the skulls of mice, directly behind the tumor site. The implants generate ultrasound waves, loosening the barrier and allowing the drugs to reach the tumor. The sound waves leave healthy tissue undamaged.
“You inject the drug into the body and turn on the ultrasound at the same time. You’re going to hit precisely at the tumor area every single time you use it,” said lead study author Thanh Nguyen, PhD, an associate professor of mechanical engineering at the University of Connecticut, Storrs.
The drug used in the study was paclitaxel, which normally struggles to get through the blood-brain barrier. The tumors shrank, and the mice doubled their lifetime, compared with untreated mice. The mice showed no bad health effects 6 months later.
Breaking through the blood-brain barrier
The biodegradable implant is made of glycine, an amino acid that’s also strongly piezoelectric, meaning it vibrates when subjected to an electrical current. To make it, researchers cultivated glycine crystals, shattered them into pieces, and finally used a process called electrospinning, which applies a high electrical voltage to the nanocrystals.
Voltage flows to the implant via an external device. The resulting ultrasound causes the tightly adhered cells of the blood-brain barrier to vibrate, stretching them out and creating space for pores to form.
“That allows in very tiny particles, including chemo drugs,” said Dr. Nguyen.
His earlier biodegradable implant broke apart from the force, but the new glycine implant is more flexible, stable, and highly piezoelectric. It could be implanted after a patient has surgery to remove a brain tumor, to continue treating residual cancer cells. The implant dissolves harmlessly in the body over time, and doctors can control its lifespan.
A new wave of uses for ultrasound
Dr. Nguyen’s study builds on similar efforts, including a recent clinical trial of a nonbiodegradable implant for treating brain tumors. Ultrasound can focus energy on precise targets in the body.
It’s like “using a magnifying glass to focus multiple beams of light on a point and burn a hole in a leaf,” said Neal Kassell, MD, founder and chairman of the Focused Ultrasound Foundation. This approach spares adjacent normal tissue.
Doctors now understand more than 30 ways that ultrasound interacts with tissue – from destroying abnormal tissue to delivering drugs more effectively to stimulating an immune response. A decade ago, only five such interactions were known.
This opens the door for treating “a wide spectrum of medical disorders,” from neurodegenerative diseases like Alzheimer’s and Parkinson’s to difficult-to-treat cancers of the prostate and pancreas, and even addiction, said Dr. Kassell.
Dr. Kassell envisions using focused ultrasound to treat brain tumors as an alternative (or complement) to surgery, chemotherapy, immunotherapy, or radiation therapy. In the meantime, implants have helped show “the effectiveness of opening the blood-brain barrier.”
Dr. Nguyen’s team plans on testing the safety and efficacy of their implant in pigs next. Eventually, Dr. Nguyen hopes to develop a patch with an array of implants to target different areas of the brain.
One study coauthor is cofounder of PiezoBioMembrane and SingleTimeMicroneedles. The other study authors reported no conflicts of interest.
A version of this article originally appeared on WebMD.com.
FROM SCIENCE ADVANCES
The surprising occupations with higher-than-expected ovarian cancer rates
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.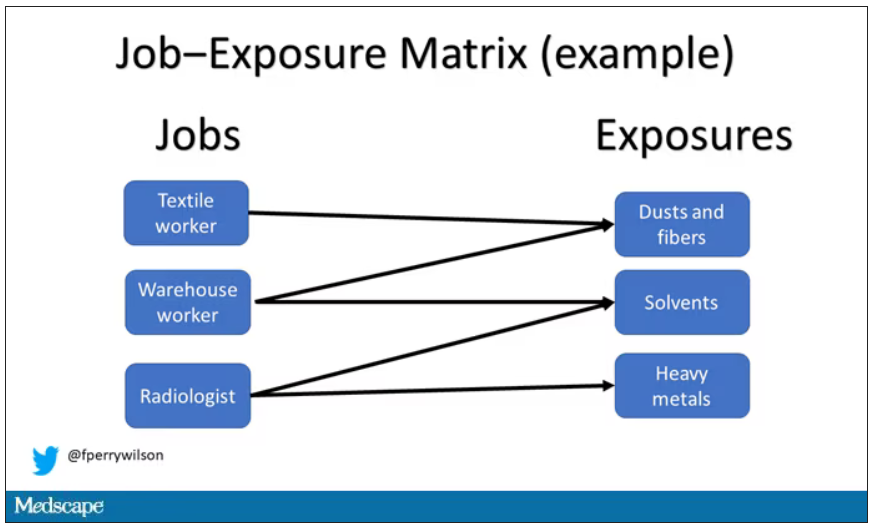
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.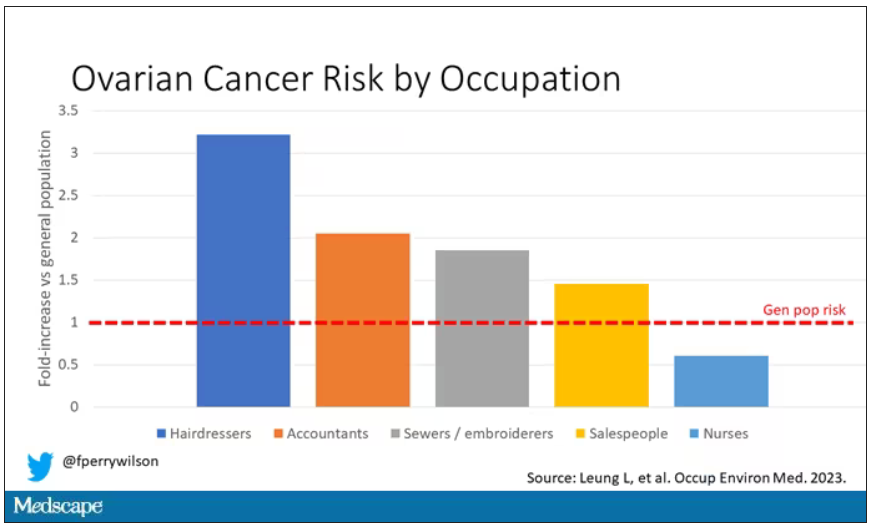
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Higher alcohol consumption linked to early-onset CRC
TOPLINE:
Higher levels of alcohol consumption appear to increase an individual’s risk of early-onset colorectal cancer (CRC), particularly distal colon and rectal cancers, according to a population-based study from South Korea.
METHODOLOGY:
- The investigators retrospectively compared average daily alcohol consumption with early-onset CRC risk among nearly 5.7 million adults younger than 50 years, using data from the Korean National Health Insurance Service.
- Alcohol consumption levels were defined as nondrinker, light (< 10 g/day or < 0.7 U.S. drinks/day), moderate (10-30 g/day for men, 10-20 g/day for women), and heavy (≥ 30 g/day or ≥ 2.1 drinks/day for men, ≥ 20 g/day or ≥ 1.4 drinks/day for women).
- The primary outcome was incidence of early-onset CRC diagnosed before age 50. Models were adjusted for age, sex, smoking status, exercise, and income, as well as for comorbidities.
TAKEAWAY:
- Overall, 8,314 incident early-onset CRC cases occurred during the mean follow-up period of 7.4 years.
- Compared with light drinking, moderate and heavy drinking were associated with a significantly elevated risk of early-onset CRC (adjusted hazard ratio, 1.09 and 1.20, respectively); by sex, significant associations were found only among men.
- Among men, heavy drinking vs. light drinking was associated with a 26% increased risk of distal colon cancer, a 17% higher risk of rectal cancer, and a 29% higher risk of unspecified colon cancer (but not proximal colon cancer).
- Among women, moderate drinking was associated with a 47% increased risk of distal colon cancer. Among nondrinkers, there was a 14% reduced risk of rectal cancer, compared with light drinkers.
IN PRACTICE:
“This population-based study provides evidence that higher levels of alcohol consumption may increase the risk of early-onset CRC,” the investigators concluded. “[E]ffective interventions are required to discourage alcohol consumption among young people and to tailor CRC screening approaches for high-risk individuals.”
SOURCE:
The study was led by researchers at Seoul National University, South Korea. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Study limitations include self-reported alcohol consumption. Data were missing for a higher number of male participants and younger participants, and there was a potential problem related to multiple comparisons and confounders. Only Korean individuals were included in the study, so larger studies involving various races are needed.
DISCLOSURES:
Funding was provided by grants from the Korea Health Technology R&D Project and the Ministry of Health and Welfare, Republic of Korea. No potential conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
Higher levels of alcohol consumption appear to increase an individual’s risk of early-onset colorectal cancer (CRC), particularly distal colon and rectal cancers, according to a population-based study from South Korea.
METHODOLOGY:
- The investigators retrospectively compared average daily alcohol consumption with early-onset CRC risk among nearly 5.7 million adults younger than 50 years, using data from the Korean National Health Insurance Service.
- Alcohol consumption levels were defined as nondrinker, light (< 10 g/day or < 0.7 U.S. drinks/day), moderate (10-30 g/day for men, 10-20 g/day for women), and heavy (≥ 30 g/day or ≥ 2.1 drinks/day for men, ≥ 20 g/day or ≥ 1.4 drinks/day for women).
- The primary outcome was incidence of early-onset CRC diagnosed before age 50. Models were adjusted for age, sex, smoking status, exercise, and income, as well as for comorbidities.
TAKEAWAY:
- Overall, 8,314 incident early-onset CRC cases occurred during the mean follow-up period of 7.4 years.
- Compared with light drinking, moderate and heavy drinking were associated with a significantly elevated risk of early-onset CRC (adjusted hazard ratio, 1.09 and 1.20, respectively); by sex, significant associations were found only among men.
- Among men, heavy drinking vs. light drinking was associated with a 26% increased risk of distal colon cancer, a 17% higher risk of rectal cancer, and a 29% higher risk of unspecified colon cancer (but not proximal colon cancer).
- Among women, moderate drinking was associated with a 47% increased risk of distal colon cancer. Among nondrinkers, there was a 14% reduced risk of rectal cancer, compared with light drinkers.
IN PRACTICE:
“This population-based study provides evidence that higher levels of alcohol consumption may increase the risk of early-onset CRC,” the investigators concluded. “[E]ffective interventions are required to discourage alcohol consumption among young people and to tailor CRC screening approaches for high-risk individuals.”
SOURCE:
The study was led by researchers at Seoul National University, South Korea. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Study limitations include self-reported alcohol consumption. Data were missing for a higher number of male participants and younger participants, and there was a potential problem related to multiple comparisons and confounders. Only Korean individuals were included in the study, so larger studies involving various races are needed.
DISCLOSURES:
Funding was provided by grants from the Korea Health Technology R&D Project and the Ministry of Health and Welfare, Republic of Korea. No potential conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
Higher levels of alcohol consumption appear to increase an individual’s risk of early-onset colorectal cancer (CRC), particularly distal colon and rectal cancers, according to a population-based study from South Korea.
METHODOLOGY:
- The investigators retrospectively compared average daily alcohol consumption with early-onset CRC risk among nearly 5.7 million adults younger than 50 years, using data from the Korean National Health Insurance Service.
- Alcohol consumption levels were defined as nondrinker, light (< 10 g/day or < 0.7 U.S. drinks/day), moderate (10-30 g/day for men, 10-20 g/day for women), and heavy (≥ 30 g/day or ≥ 2.1 drinks/day for men, ≥ 20 g/day or ≥ 1.4 drinks/day for women).
- The primary outcome was incidence of early-onset CRC diagnosed before age 50. Models were adjusted for age, sex, smoking status, exercise, and income, as well as for comorbidities.
TAKEAWAY:
- Overall, 8,314 incident early-onset CRC cases occurred during the mean follow-up period of 7.4 years.
- Compared with light drinking, moderate and heavy drinking were associated with a significantly elevated risk of early-onset CRC (adjusted hazard ratio, 1.09 and 1.20, respectively); by sex, significant associations were found only among men.
- Among men, heavy drinking vs. light drinking was associated with a 26% increased risk of distal colon cancer, a 17% higher risk of rectal cancer, and a 29% higher risk of unspecified colon cancer (but not proximal colon cancer).
- Among women, moderate drinking was associated with a 47% increased risk of distal colon cancer. Among nondrinkers, there was a 14% reduced risk of rectal cancer, compared with light drinkers.
IN PRACTICE:
“This population-based study provides evidence that higher levels of alcohol consumption may increase the risk of early-onset CRC,” the investigators concluded. “[E]ffective interventions are required to discourage alcohol consumption among young people and to tailor CRC screening approaches for high-risk individuals.”
SOURCE:
The study was led by researchers at Seoul National University, South Korea. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Study limitations include self-reported alcohol consumption. Data were missing for a higher number of male participants and younger participants, and there was a potential problem related to multiple comparisons and confounders. Only Korean individuals were included in the study, so larger studies involving various races are needed.
DISCLOSURES:
Funding was provided by grants from the Korea Health Technology R&D Project and the Ministry of Health and Welfare, Republic of Korea. No potential conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Cardiorespiratory fitness linked to cancer risk, mortality?
TOPLINE:
a large Swedish cohort study suggests.
METHODOLOGY:
- A prospective cohort study included 177,709 Swedish men (mean age, 42; mean body mass index, 26 kg/m2) who completed an occupational health profile assessment and were followed for a mean of 9.6 years.
- CRF was assessed by determining maximal oxygen consumption during an aerobic fitness test, known as a submaximal Åstrand cycle ergometer test.
- Participants reported physical activity habits, lifestyle, and perceived health.
- Data on prostate, colon, and lung cancer incidence and mortality were derived from national registers.
- Outcomes from three higher CRF groups (low, > 25-35; moderate, > 35-45; high, > 45 mL/min per kg) were compared with those from the very low CRF group (25 mL/min per kg or less). Models were adjusted for various factors, including age, BMI, education, dietary habits, comorbidity, and smoking.
TAKEAWAY:
- During follow-up, investigators identified 1,918 prostate, 499 colon, and 283 lung cancer cases as well as 141 prostate, 207 lung, and 152 colon cancer deaths.
- In the fully adjusted model, higher CRF levels were associated with a significantly lower risk for colon cancer (hazard ratio, 0.72 for moderate; HR, 0.63 for high).
- In this model, higher CRF was also associated with a lower risk of death from prostate cancer (HR, 0.67 for low; HR, 0.57 for moderate; HR, 0.29 for high).
- For lung cancer mortality, only high CRF was associated with a significantly lower risk of death (HR, 0.41).
- An association between CRF and lung cancer incidence (HR, 0.99) and death (HR, 0.99) was only evident among adults aged 60 and older.
IN PRACTICE:
“The clinical implications of these findings further emphasize the importance of CRF for possibly reducing cancer incidence and mortality,” the authors concluded. “It is important for the general public to understand that higher-intensity [physical activity] has greater effects on CRF and is likely to be more protective against the risk of developing and dying from certain cancers.”
SOURCE:
The study was led by Elin Ekblom-Bak, PhD, from the Swedish School of Sport and Health Sciences, Stockholm. It was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by voluntary participation, inclusion of only employed individuals, and estimations of CRF via submaximal tests. Data on smoking status were not optimal and there was a small number of cancer cases and deaths.
DISCLOSURES:
Funding was provided by the Swedish Cancer Society. The authors have reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a large Swedish cohort study suggests.
METHODOLOGY:
- A prospective cohort study included 177,709 Swedish men (mean age, 42; mean body mass index, 26 kg/m2) who completed an occupational health profile assessment and were followed for a mean of 9.6 years.
- CRF was assessed by determining maximal oxygen consumption during an aerobic fitness test, known as a submaximal Åstrand cycle ergometer test.
- Participants reported physical activity habits, lifestyle, and perceived health.
- Data on prostate, colon, and lung cancer incidence and mortality were derived from national registers.
- Outcomes from three higher CRF groups (low, > 25-35; moderate, > 35-45; high, > 45 mL/min per kg) were compared with those from the very low CRF group (25 mL/min per kg or less). Models were adjusted for various factors, including age, BMI, education, dietary habits, comorbidity, and smoking.
TAKEAWAY:
- During follow-up, investigators identified 1,918 prostate, 499 colon, and 283 lung cancer cases as well as 141 prostate, 207 lung, and 152 colon cancer deaths.
- In the fully adjusted model, higher CRF levels were associated with a significantly lower risk for colon cancer (hazard ratio, 0.72 for moderate; HR, 0.63 for high).
- In this model, higher CRF was also associated with a lower risk of death from prostate cancer (HR, 0.67 for low; HR, 0.57 for moderate; HR, 0.29 for high).
- For lung cancer mortality, only high CRF was associated with a significantly lower risk of death (HR, 0.41).
- An association between CRF and lung cancer incidence (HR, 0.99) and death (HR, 0.99) was only evident among adults aged 60 and older.
IN PRACTICE:
“The clinical implications of these findings further emphasize the importance of CRF for possibly reducing cancer incidence and mortality,” the authors concluded. “It is important for the general public to understand that higher-intensity [physical activity] has greater effects on CRF and is likely to be more protective against the risk of developing and dying from certain cancers.”
SOURCE:
The study was led by Elin Ekblom-Bak, PhD, from the Swedish School of Sport and Health Sciences, Stockholm. It was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by voluntary participation, inclusion of only employed individuals, and estimations of CRF via submaximal tests. Data on smoking status were not optimal and there was a small number of cancer cases and deaths.
DISCLOSURES:
Funding was provided by the Swedish Cancer Society. The authors have reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a large Swedish cohort study suggests.
METHODOLOGY:
- A prospective cohort study included 177,709 Swedish men (mean age, 42; mean body mass index, 26 kg/m2) who completed an occupational health profile assessment and were followed for a mean of 9.6 years.
- CRF was assessed by determining maximal oxygen consumption during an aerobic fitness test, known as a submaximal Åstrand cycle ergometer test.
- Participants reported physical activity habits, lifestyle, and perceived health.
- Data on prostate, colon, and lung cancer incidence and mortality were derived from national registers.
- Outcomes from three higher CRF groups (low, > 25-35; moderate, > 35-45; high, > 45 mL/min per kg) were compared with those from the very low CRF group (25 mL/min per kg or less). Models were adjusted for various factors, including age, BMI, education, dietary habits, comorbidity, and smoking.
TAKEAWAY:
- During follow-up, investigators identified 1,918 prostate, 499 colon, and 283 lung cancer cases as well as 141 prostate, 207 lung, and 152 colon cancer deaths.
- In the fully adjusted model, higher CRF levels were associated with a significantly lower risk for colon cancer (hazard ratio, 0.72 for moderate; HR, 0.63 for high).
- In this model, higher CRF was also associated with a lower risk of death from prostate cancer (HR, 0.67 for low; HR, 0.57 for moderate; HR, 0.29 for high).
- For lung cancer mortality, only high CRF was associated with a significantly lower risk of death (HR, 0.41).
- An association between CRF and lung cancer incidence (HR, 0.99) and death (HR, 0.99) was only evident among adults aged 60 and older.
IN PRACTICE:
“The clinical implications of these findings further emphasize the importance of CRF for possibly reducing cancer incidence and mortality,” the authors concluded. “It is important for the general public to understand that higher-intensity [physical activity] has greater effects on CRF and is likely to be more protective against the risk of developing and dying from certain cancers.”
SOURCE:
The study was led by Elin Ekblom-Bak, PhD, from the Swedish School of Sport and Health Sciences, Stockholm. It was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by voluntary participation, inclusion of only employed individuals, and estimations of CRF via submaximal tests. Data on smoking status were not optimal and there was a small number of cancer cases and deaths.
DISCLOSURES:
Funding was provided by the Swedish Cancer Society. The authors have reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Diabetes may short circuit pembrolizumab benefits in NSCLC
TOPLINE:
METHODOLOGY:
- Investigators reviewed the medical records of 203 consecutive patients with metastatic NSCLC who received first-line pembrolizumab either alone or in combination with chemotherapy at a single tertiary center in Israel.
- Overall, 1 in 4 patients (n = 51) had diabetes mellitus; most (n = 42) were being treated with oral hypoglycemic agents, frequently metformin, and 7 were taking insulin.
- Rates of tumors with PD‐L1 expression above 50% were not significantly different among patients with diabetes and those without.
TAKEAWAY:
- Overall, among patients with diabetes, median progression-free survival (PFS) was significantly shorter than among patients without diabetes (5.9 vs. 7.1 months), as was overall survival (12 vs. 21 months).
- Shorter overall survival was more pronounced among those with diabetes who received pembrolizumab alone (12 vs. 27 months) in comparison with patients who received pembrolizumab plus chemotherapy (14.3 vs. 19.4 months).
- After adjusting for potential confounders, multivariate analysis confirmed that diabetes was an independent risk factor for shorter PFS (hazard ratio, 1.67) and shorter overall survival (HR, 1.73) for patients with NSCLC.
- In a validation cohort of 452 patients with metastatic NSCLC, only 19.6% of those with diabetes continued to take pembrolizumab at 12 months versus 31.7% of those without diabetes.
IN PRACTICE:
“As NSCLC patients with [diabetes] constitute a significant subgroup, there is an urgent need to validate our findings and explore whether outcomes in these patients can be improved by better glycemic control,” the authors said, adding that “chemotherapy may offset some of the deleterious effects” of diabetes.
SOURCE:
The study was led by Yasmin Leshem, MD, PhD, of the Tel Aviv Sourasky Medical Center, and was published in Cancer.
LIMITATIONS:
- Without access to blood test results outside the hospital, the researchers could not determine whether better glycemic control might have improved outcomes.
- The incidence of type 1 or 2 diabetes was not well documented.
DISCLOSURES:
- No funding source was reported.
- Two investigators reported receiving consulting and/or other fees from Bristol-Myers Squibb, Roche, Merck, Novartis, and Merck Sharp and Dohme.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators reviewed the medical records of 203 consecutive patients with metastatic NSCLC who received first-line pembrolizumab either alone or in combination with chemotherapy at a single tertiary center in Israel.
- Overall, 1 in 4 patients (n = 51) had diabetes mellitus; most (n = 42) were being treated with oral hypoglycemic agents, frequently metformin, and 7 were taking insulin.
- Rates of tumors with PD‐L1 expression above 50% were not significantly different among patients with diabetes and those without.
TAKEAWAY:
- Overall, among patients with diabetes, median progression-free survival (PFS) was significantly shorter than among patients without diabetes (5.9 vs. 7.1 months), as was overall survival (12 vs. 21 months).
- Shorter overall survival was more pronounced among those with diabetes who received pembrolizumab alone (12 vs. 27 months) in comparison with patients who received pembrolizumab plus chemotherapy (14.3 vs. 19.4 months).
- After adjusting for potential confounders, multivariate analysis confirmed that diabetes was an independent risk factor for shorter PFS (hazard ratio, 1.67) and shorter overall survival (HR, 1.73) for patients with NSCLC.
- In a validation cohort of 452 patients with metastatic NSCLC, only 19.6% of those with diabetes continued to take pembrolizumab at 12 months versus 31.7% of those without diabetes.
IN PRACTICE:
“As NSCLC patients with [diabetes] constitute a significant subgroup, there is an urgent need to validate our findings and explore whether outcomes in these patients can be improved by better glycemic control,” the authors said, adding that “chemotherapy may offset some of the deleterious effects” of diabetes.
SOURCE:
The study was led by Yasmin Leshem, MD, PhD, of the Tel Aviv Sourasky Medical Center, and was published in Cancer.
LIMITATIONS:
- Without access to blood test results outside the hospital, the researchers could not determine whether better glycemic control might have improved outcomes.
- The incidence of type 1 or 2 diabetes was not well documented.
DISCLOSURES:
- No funding source was reported.
- Two investigators reported receiving consulting and/or other fees from Bristol-Myers Squibb, Roche, Merck, Novartis, and Merck Sharp and Dohme.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators reviewed the medical records of 203 consecutive patients with metastatic NSCLC who received first-line pembrolizumab either alone or in combination with chemotherapy at a single tertiary center in Israel.
- Overall, 1 in 4 patients (n = 51) had diabetes mellitus; most (n = 42) were being treated with oral hypoglycemic agents, frequently metformin, and 7 were taking insulin.
- Rates of tumors with PD‐L1 expression above 50% were not significantly different among patients with diabetes and those without.
TAKEAWAY:
- Overall, among patients with diabetes, median progression-free survival (PFS) was significantly shorter than among patients without diabetes (5.9 vs. 7.1 months), as was overall survival (12 vs. 21 months).
- Shorter overall survival was more pronounced among those with diabetes who received pembrolizumab alone (12 vs. 27 months) in comparison with patients who received pembrolizumab plus chemotherapy (14.3 vs. 19.4 months).
- After adjusting for potential confounders, multivariate analysis confirmed that diabetes was an independent risk factor for shorter PFS (hazard ratio, 1.67) and shorter overall survival (HR, 1.73) for patients with NSCLC.
- In a validation cohort of 452 patients with metastatic NSCLC, only 19.6% of those with diabetes continued to take pembrolizumab at 12 months versus 31.7% of those without diabetes.
IN PRACTICE:
“As NSCLC patients with [diabetes] constitute a significant subgroup, there is an urgent need to validate our findings and explore whether outcomes in these patients can be improved by better glycemic control,” the authors said, adding that “chemotherapy may offset some of the deleterious effects” of diabetes.
SOURCE:
The study was led by Yasmin Leshem, MD, PhD, of the Tel Aviv Sourasky Medical Center, and was published in Cancer.
LIMITATIONS:
- Without access to blood test results outside the hospital, the researchers could not determine whether better glycemic control might have improved outcomes.
- The incidence of type 1 or 2 diabetes was not well documented.
DISCLOSURES:
- No funding source was reported.
- Two investigators reported receiving consulting and/or other fees from Bristol-Myers Squibb, Roche, Merck, Novartis, and Merck Sharp and Dohme.
A version of this article first appeared on Medscape.com.
Cannabis for cancer symptoms: Perceived or real benefit?
TOPLINE:
METHODOLOGY:
- Participants included 267 adults (mean age, 58 years; 70% women; 88% White) undergoing treatment for cancer, most commonly breast (47%) and ovarian (29%).
- Participants completed online surveys to characterize cannabis use, reasons for using it, perceived benefits and harms, and physical/psychological symptoms.
- Participants who had used cannabis for more than 1 day during the previous 30 days were compared with those who had not.
TAKEAWAY:
- Overall, 26% of respondents reported cannabis use in the past 30 days, most often edibles (65%) or smoked cannabis (51%).
- Cannabis users were more likely to be younger, male, Black, to have lower income, worse physical/psychological symptoms, and to be disabled or unable to work in comparison with nonusers.
- Cannabis was used to treat pain, cancer, sleep problems, anxiety, nausea, and poor appetite; perceived benefits were greatest with respect to sleep, nausea, pain, muscle spasms, and anxiety.
- Despite perceived benefits, cannabis users reported worse overall distress, anxiety, sleep disturbances, appetite, nausea, fatigue, and pain.
IN PRACTICE:
“The study findings indicate that patients with cancer perceived benefits to using cannabis for many symptoms” but also revealed that “those who used cannabis in the past 30 days had significantly worse symptom profiles overall than those who did not use cannabis,” the authors wrote.
SOURCE:
The study, led by Desiree R. Azizoddin, PsyD, University of Oklahoma Health Science Center, Oklahoma City, was published online in Cancer.
LIMITATIONS:
It’s not known whether adults who used cannabis had significantly worse symptoms at the outset, which may have prompted cannabis use, or whether cannabis use may have exacerbated their symptoms.
DISCLOSURES:
Funding for the study was provided by grants from the National Cancer Institute and the Oklahoma Tobacco Settlement Endowment Trust. Nine of the 10 authors have disclosed no relevant conflicts of interest. One author has relationships with various pharmaceutical companies involved in oncology.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Participants included 267 adults (mean age, 58 years; 70% women; 88% White) undergoing treatment for cancer, most commonly breast (47%) and ovarian (29%).
- Participants completed online surveys to characterize cannabis use, reasons for using it, perceived benefits and harms, and physical/psychological symptoms.
- Participants who had used cannabis for more than 1 day during the previous 30 days were compared with those who had not.
TAKEAWAY:
- Overall, 26% of respondents reported cannabis use in the past 30 days, most often edibles (65%) or smoked cannabis (51%).
- Cannabis users were more likely to be younger, male, Black, to have lower income, worse physical/psychological symptoms, and to be disabled or unable to work in comparison with nonusers.
- Cannabis was used to treat pain, cancer, sleep problems, anxiety, nausea, and poor appetite; perceived benefits were greatest with respect to sleep, nausea, pain, muscle spasms, and anxiety.
- Despite perceived benefits, cannabis users reported worse overall distress, anxiety, sleep disturbances, appetite, nausea, fatigue, and pain.
IN PRACTICE:
“The study findings indicate that patients with cancer perceived benefits to using cannabis for many symptoms” but also revealed that “those who used cannabis in the past 30 days had significantly worse symptom profiles overall than those who did not use cannabis,” the authors wrote.
SOURCE:
The study, led by Desiree R. Azizoddin, PsyD, University of Oklahoma Health Science Center, Oklahoma City, was published online in Cancer.
LIMITATIONS:
It’s not known whether adults who used cannabis had significantly worse symptoms at the outset, which may have prompted cannabis use, or whether cannabis use may have exacerbated their symptoms.
DISCLOSURES:
Funding for the study was provided by grants from the National Cancer Institute and the Oklahoma Tobacco Settlement Endowment Trust. Nine of the 10 authors have disclosed no relevant conflicts of interest. One author has relationships with various pharmaceutical companies involved in oncology.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Participants included 267 adults (mean age, 58 years; 70% women; 88% White) undergoing treatment for cancer, most commonly breast (47%) and ovarian (29%).
- Participants completed online surveys to characterize cannabis use, reasons for using it, perceived benefits and harms, and physical/psychological symptoms.
- Participants who had used cannabis for more than 1 day during the previous 30 days were compared with those who had not.
TAKEAWAY:
- Overall, 26% of respondents reported cannabis use in the past 30 days, most often edibles (65%) or smoked cannabis (51%).
- Cannabis users were more likely to be younger, male, Black, to have lower income, worse physical/psychological symptoms, and to be disabled or unable to work in comparison with nonusers.
- Cannabis was used to treat pain, cancer, sleep problems, anxiety, nausea, and poor appetite; perceived benefits were greatest with respect to sleep, nausea, pain, muscle spasms, and anxiety.
- Despite perceived benefits, cannabis users reported worse overall distress, anxiety, sleep disturbances, appetite, nausea, fatigue, and pain.
IN PRACTICE:
“The study findings indicate that patients with cancer perceived benefits to using cannabis for many symptoms” but also revealed that “those who used cannabis in the past 30 days had significantly worse symptom profiles overall than those who did not use cannabis,” the authors wrote.
SOURCE:
The study, led by Desiree R. Azizoddin, PsyD, University of Oklahoma Health Science Center, Oklahoma City, was published online in Cancer.
LIMITATIONS:
It’s not known whether adults who used cannabis had significantly worse symptoms at the outset, which may have prompted cannabis use, or whether cannabis use may have exacerbated their symptoms.
DISCLOSURES:
Funding for the study was provided by grants from the National Cancer Institute and the Oklahoma Tobacco Settlement Endowment Trust. Nine of the 10 authors have disclosed no relevant conflicts of interest. One author has relationships with various pharmaceutical companies involved in oncology.
A version of this article first appeared on Medscape.com.
Cancer Data Trends 2023
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment

