User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Some infected patients could show COVID-19 symptoms after quarantine
Although a 14-day quarantine after exposure to novel coronavirus is “well supported” by evidence, some infected individuals will not become symptomatic until after that period, according to authors of a recent analysis published in Annals of Internal Medicine.
Most individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will develop symptoms by day 12 of the infection, which is within the 14-day period of active monitoring currently recommended by the Centers for Disease Control and Prevention, the authors wrote.
However, an estimated 101 out of 10,000 cases could become symptomatic after the end of that 14-day monitoring period, they cautioned.
“Our analyses do not preclude that estimate from being higher,” said the investigators, led by Stephen A. Lauer, PhD, MD, of Johns Hopkins Bloomberg School of Public Health, Baltimore.
The analysis, based on 181 confirmed cases of coronavirus disease 2019 (COVID-19) that were documented outside of the outbreak epicenter, Wuhan, China, makes “more conservative assumptions” about the window of symptom onset and potential for continued exposure, compared with analyses in previous studies, the researchers wrote.
The estimated incubation period for SARS-CoV-2 in the 181-patient study was a median of 5.1 days, which is comparable with previous estimates based on COVID-19 cases outside of Wuhan and consistent with other known human coronavirus diseases, such as SARS, which had a reported mean incubation period of 5 days, Dr. Lauer and colleagues noted.
Symptoms developed within 11.5 days for 97.5% of patients in the study.
Whether it’s acceptable to have 101 out of 10,000 cases becoming symptomatic beyond the recommended quarantine window depends on two factors, according to the authors. The first is the expected infection risk in the population that is being monitored, and the second is “judgment about the cost of missing cases,” wrote the authors.
In an interview, Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau, Oceanside, N.Y., said that in practical terms, the results suggest that the majority of patients with COVID-19 will be identified within 14 days, with an “outside chance” of an infected individual leaving quarantine and transmitting virus for a short period of time before becoming symptomatic.
“I think the proper message to give those patients [who are asymptomatic upon leaving quarantine] is, ‘after 14 days, we’re pretty sure you’re out of the woods, but should you get any symptoms, immediately requarantine yourself and seek medical care,” he said.
Study coauthor Kyra H. Grantz, a doctoral graduate student at the Johns Hopkins Bloomberg School of Public Health, said that extending a quarantine beyond 14 days might be considered in the highest-risk scenarios, though the benefits of doing so would have to be weighed against the costs to public health and to the individuals under quarantine.
“Our estimate of the incubation period definitely supports the 14-day recommendation that the CDC has been using,” she said in an interview.
Dr. Grantz emphasized that the estimate of 101 out of 10,000 cases developing symptoms after day 14 of active monitoring – representing the 99th percentile of cases – assumes the “most conservative, worst-case scenario” in a population that is fully infected.
“If you’re looking at a following a cohort of 1,000 people whom you think may have been exposed, only a certain percentage will be infected, and only a certain percentage of those will even develop symptoms – before we get to this idea of how many people would we miss,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, and the Alexander von Humboldt Foundation. Four authors reported disclosures related to those entities, and the remaining five reported no conflicts of interest.
SOURCE: Lauer SA et al. Ann Intern Med. 2020 Mar 9. doi:10.1101/2020.02.02.20020016.
Although a 14-day quarantine after exposure to novel coronavirus is “well supported” by evidence, some infected individuals will not become symptomatic until after that period, according to authors of a recent analysis published in Annals of Internal Medicine.
Most individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will develop symptoms by day 12 of the infection, which is within the 14-day period of active monitoring currently recommended by the Centers for Disease Control and Prevention, the authors wrote.
However, an estimated 101 out of 10,000 cases could become symptomatic after the end of that 14-day monitoring period, they cautioned.
“Our analyses do not preclude that estimate from being higher,” said the investigators, led by Stephen A. Lauer, PhD, MD, of Johns Hopkins Bloomberg School of Public Health, Baltimore.
The analysis, based on 181 confirmed cases of coronavirus disease 2019 (COVID-19) that were documented outside of the outbreak epicenter, Wuhan, China, makes “more conservative assumptions” about the window of symptom onset and potential for continued exposure, compared with analyses in previous studies, the researchers wrote.
The estimated incubation period for SARS-CoV-2 in the 181-patient study was a median of 5.1 days, which is comparable with previous estimates based on COVID-19 cases outside of Wuhan and consistent with other known human coronavirus diseases, such as SARS, which had a reported mean incubation period of 5 days, Dr. Lauer and colleagues noted.
Symptoms developed within 11.5 days for 97.5% of patients in the study.
Whether it’s acceptable to have 101 out of 10,000 cases becoming symptomatic beyond the recommended quarantine window depends on two factors, according to the authors. The first is the expected infection risk in the population that is being monitored, and the second is “judgment about the cost of missing cases,” wrote the authors.
In an interview, Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau, Oceanside, N.Y., said that in practical terms, the results suggest that the majority of patients with COVID-19 will be identified within 14 days, with an “outside chance” of an infected individual leaving quarantine and transmitting virus for a short period of time before becoming symptomatic.
“I think the proper message to give those patients [who are asymptomatic upon leaving quarantine] is, ‘after 14 days, we’re pretty sure you’re out of the woods, but should you get any symptoms, immediately requarantine yourself and seek medical care,” he said.
Study coauthor Kyra H. Grantz, a doctoral graduate student at the Johns Hopkins Bloomberg School of Public Health, said that extending a quarantine beyond 14 days might be considered in the highest-risk scenarios, though the benefits of doing so would have to be weighed against the costs to public health and to the individuals under quarantine.
“Our estimate of the incubation period definitely supports the 14-day recommendation that the CDC has been using,” she said in an interview.
Dr. Grantz emphasized that the estimate of 101 out of 10,000 cases developing symptoms after day 14 of active monitoring – representing the 99th percentile of cases – assumes the “most conservative, worst-case scenario” in a population that is fully infected.
“If you’re looking at a following a cohort of 1,000 people whom you think may have been exposed, only a certain percentage will be infected, and only a certain percentage of those will even develop symptoms – before we get to this idea of how many people would we miss,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, and the Alexander von Humboldt Foundation. Four authors reported disclosures related to those entities, and the remaining five reported no conflicts of interest.
SOURCE: Lauer SA et al. Ann Intern Med. 2020 Mar 9. doi:10.1101/2020.02.02.20020016.
Although a 14-day quarantine after exposure to novel coronavirus is “well supported” by evidence, some infected individuals will not become symptomatic until after that period, according to authors of a recent analysis published in Annals of Internal Medicine.
Most individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will develop symptoms by day 12 of the infection, which is within the 14-day period of active monitoring currently recommended by the Centers for Disease Control and Prevention, the authors wrote.
However, an estimated 101 out of 10,000 cases could become symptomatic after the end of that 14-day monitoring period, they cautioned.
“Our analyses do not preclude that estimate from being higher,” said the investigators, led by Stephen A. Lauer, PhD, MD, of Johns Hopkins Bloomberg School of Public Health, Baltimore.
The analysis, based on 181 confirmed cases of coronavirus disease 2019 (COVID-19) that were documented outside of the outbreak epicenter, Wuhan, China, makes “more conservative assumptions” about the window of symptom onset and potential for continued exposure, compared with analyses in previous studies, the researchers wrote.
The estimated incubation period for SARS-CoV-2 in the 181-patient study was a median of 5.1 days, which is comparable with previous estimates based on COVID-19 cases outside of Wuhan and consistent with other known human coronavirus diseases, such as SARS, which had a reported mean incubation period of 5 days, Dr. Lauer and colleagues noted.
Symptoms developed within 11.5 days for 97.5% of patients in the study.
Whether it’s acceptable to have 101 out of 10,000 cases becoming symptomatic beyond the recommended quarantine window depends on two factors, according to the authors. The first is the expected infection risk in the population that is being monitored, and the second is “judgment about the cost of missing cases,” wrote the authors.
In an interview, Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau, Oceanside, N.Y., said that in practical terms, the results suggest that the majority of patients with COVID-19 will be identified within 14 days, with an “outside chance” of an infected individual leaving quarantine and transmitting virus for a short period of time before becoming symptomatic.
“I think the proper message to give those patients [who are asymptomatic upon leaving quarantine] is, ‘after 14 days, we’re pretty sure you’re out of the woods, but should you get any symptoms, immediately requarantine yourself and seek medical care,” he said.
Study coauthor Kyra H. Grantz, a doctoral graduate student at the Johns Hopkins Bloomberg School of Public Health, said that extending a quarantine beyond 14 days might be considered in the highest-risk scenarios, though the benefits of doing so would have to be weighed against the costs to public health and to the individuals under quarantine.
“Our estimate of the incubation period definitely supports the 14-day recommendation that the CDC has been using,” she said in an interview.
Dr. Grantz emphasized that the estimate of 101 out of 10,000 cases developing symptoms after day 14 of active monitoring – representing the 99th percentile of cases – assumes the “most conservative, worst-case scenario” in a population that is fully infected.
“If you’re looking at a following a cohort of 1,000 people whom you think may have been exposed, only a certain percentage will be infected, and only a certain percentage of those will even develop symptoms – before we get to this idea of how many people would we miss,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, and the Alexander von Humboldt Foundation. Four authors reported disclosures related to those entities, and the remaining five reported no conflicts of interest.
SOURCE: Lauer SA et al. Ann Intern Med. 2020 Mar 9. doi:10.1101/2020.02.02.20020016.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Some individuals who are infected with the novel coronavirus could become symptomatic after the active 14-day quarantine period.
Major finding: The median incubation period was 5.1 days, with 97.5% of patients developing symptoms within 11.5 days, implying that 101 of every 10,000 cases (99th percentile) would develop symptoms beyond the quarantine period.
Study details: Analysis of 181 confirmed COVID-19 cases identified outside of the outbreak epicenter, Wuhan, China.
Disclosures: The study was supported by the U.S. Centers for Disease Control and Prevention, the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, and the Alexander von Humboldt Foundation. Four authors reported disclosures related to those entities, and the remaining five reported no conflicts of interest.
Source: Lauer SA et al. Ann Intern Med. 2020 Mar 9. doi: 10.1101/2020.02.02.20020016.
AUGUSTUS: Apixaban surpassed warfarin despite prior stroke or thromboembolism
LOS ANGELES – The edge that the direct-acting oral anticoagulant apixaban (Eliquis) has over warfarin for safely preventing ischemic events in patients with atrial fibrillation and either a recent acute coronary syndrome event or a recent percutaneous coronary intervention held up even in patients with a history of stroke, transient ischemic attack, or thromboembolic event, according to a prespecified secondary analysis of data collected in the AUGUSTUS trial.
The treatment advantages of apixaban, compared with warfarin, seen in the overall AUGUSTUS results, first reported in March 2019, “were consistent” with the benefits seen in the subgroup of enrolled patients with a prior stroke, transient ischemic attack (TIA), or thromboembolic (TE) event, M. Cecilia Bahit, MD, said at the International Stroke Conference sponsored by the American Heart Association.
All patients in AUGUSTUS received a P2Y12 inhibitor antiplatelet drug, which was clopidogrel for more than 90% of patients. The two-by-two factorial design of AUGUSTUS also assessed the safety and efficacy of either adding or withholding aspirin from the two-drug regimen that all patients in the study received with a P2Y12 inhibitor plus an anticoagulant (apixaban or warfarin). The most notable finding of the aspirin versus placebo analysis was that patients without a prior stroke, TIA, or TE event had a “more profound” increase in their rate of major or clinically relevant minor bleeds when also treated with aspirin, compared with patients who received aspirin and had a history of stroke, TIA, or TE event, reported Dr. Bahit, a chief of cardiology and director of clinical research at the INECO Foundation in Rosario, Argentina.
In general, the findings of the secondary analysis that took into account stroke, TIA, or TE history “confirmed” the main AUGUSTUS findings, Dr. Bahit said; an antithrombotic regimen of apixaban plus clopidogrel (or other P2Y12 inhibitor) without aspirin was superior for both efficacy and safety, compared with the alternative regimens that either substituted warfarin for apixaban or that added aspirin.
AUGUSTUS enrolled 4,614 atrial fibrillation (AFib) patients who either had a recent acute coronary syndrome (ACS) event or had recently undergone percutaneous coronary intervention (PCI) at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the incidence of major or clinically relevant minor bleeds after 6 months, which was significantly lower in the subgroups that received apixaban instead of warfarin and in patients who received placebo instead of aspirin. The secondary endpoint of death or hospitalization after 6 months was also significantly lower in the apixaban-treated patients, compared with those on warfarin, while the aspirin and placebo subgroups showed no difference in the incidence of these events (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
The results reported by Dr. Bahit also highlighted both the high risk faced by patients with AFib who also have had an ACS event or PCI, as well as a prior stroke, TIA, or TE event, noted Larry B. Goldstein, MD, professor and chairman of neurology at the University of Kentucky, Lexington. “It’s difficult, because these patients had an ACS event or PCI, and you don’t want a coronary too close up, but do these patients really need a P2Y12 inhibitor plus an anticoagulant? Could these patients do as well on apixaban only? I would have liked to see that treatment arm in the study,” Dr. Goldstein commented in an interview.
“These are challenging patients because they often require anticoagulation for the AFib as well as antiplatelet agents” for the recent PCI or ACS event, commented Mitchell S.V. Elkind, MD, professor of neurology at Columbia University, New York. “The question has always been: How many blood thinners should these patients be on? Potentially they could be on three different agents [an anticoagulant and two antiplatelet drugs], and we know that all of those drugs together pretty dramatically increase the risk of bleeding. About 15% of the patients in the overall AUGUSTUS trial had either cerebrovascular disease or systemic thromboembolism, so this was a small subgroup of the overall trial, but the overall trial was large so it’s a significant number of patients who met this criteria. The results confirmed that even in a group of patients who may be considered at high risk because they have a prior history of cerebrovascular disease use of apixaban instead of warfarin seemed safer, and that those patients did not need to be on aspirin as well as their other antiplatelet agent. Patients with a history of stroke, in fact, had a lower risk of bleeding than the other patients in this trial, so one could argue that they should be on an agent like apixaban as well as an antiplatelet agent like clopidogrel without addition of aspirin,” he said in a recorded statement.
In addition to implications for using prescription drugs like apixaban and clopidogrel, the findings also send a message about the need for very aggressive implementation of lifestyle measures that can reduce cardiovascular disease risk in these patients, added Dr. Goldstein. The AUGUSTUS outcome analyses that subdivided the study population into those with a prior stroke, TIA, or TE event – 633 patients or about 14% of the 4,581 patients eligible for this analysis – and those who did not have this history showed the extremely high, incrementally elevated risk faced by patients with these prior events.
A history of stroke, TIA, or TE event linked with a jump in the 90-day rate of major or clinically relevant minor bleeds from 13% without this history to 17%, which is a 31% relative increase; it boosted the 90-day rate of death or hospitalization from 25% to 31%, a 24% relative increase; and it jacked up the rate of death or ischemic events from 6% to 9%, a 50% relative increase, Dr. Bahit reported.
These substantial increases “suggest we need to be very aggressive” in managing these high-risk patients who combine a background of AFib, a prior stroke, TIA, or TE events, and a recent ACS event or PCI, Dr. Goldstein observed. In these patients, he suggested that clinicians make sure to address smoking cessation, obesity, exercise, diet, and statin use, and get each of these to an optimal level to further cut risk. If all five of these basic interventions were successfully administered to a patient they could collectively cut the patient’s event risk by about 80%, he added.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer, the companies that jointly market apixaban. Dr. Bahit has received honoraria from Pfizer, and from CSL Behring and Merck. Dr. Elkind and Dr. Goldstein had no relevant disclosures.
SOURCE: Bahit MC et al. ISC 2020, Abstract LB22.
LOS ANGELES – The edge that the direct-acting oral anticoagulant apixaban (Eliquis) has over warfarin for safely preventing ischemic events in patients with atrial fibrillation and either a recent acute coronary syndrome event or a recent percutaneous coronary intervention held up even in patients with a history of stroke, transient ischemic attack, or thromboembolic event, according to a prespecified secondary analysis of data collected in the AUGUSTUS trial.
The treatment advantages of apixaban, compared with warfarin, seen in the overall AUGUSTUS results, first reported in March 2019, “were consistent” with the benefits seen in the subgroup of enrolled patients with a prior stroke, transient ischemic attack (TIA), or thromboembolic (TE) event, M. Cecilia Bahit, MD, said at the International Stroke Conference sponsored by the American Heart Association.
All patients in AUGUSTUS received a P2Y12 inhibitor antiplatelet drug, which was clopidogrel for more than 90% of patients. The two-by-two factorial design of AUGUSTUS also assessed the safety and efficacy of either adding or withholding aspirin from the two-drug regimen that all patients in the study received with a P2Y12 inhibitor plus an anticoagulant (apixaban or warfarin). The most notable finding of the aspirin versus placebo analysis was that patients without a prior stroke, TIA, or TE event had a “more profound” increase in their rate of major or clinically relevant minor bleeds when also treated with aspirin, compared with patients who received aspirin and had a history of stroke, TIA, or TE event, reported Dr. Bahit, a chief of cardiology and director of clinical research at the INECO Foundation in Rosario, Argentina.
In general, the findings of the secondary analysis that took into account stroke, TIA, or TE history “confirmed” the main AUGUSTUS findings, Dr. Bahit said; an antithrombotic regimen of apixaban plus clopidogrel (or other P2Y12 inhibitor) without aspirin was superior for both efficacy and safety, compared with the alternative regimens that either substituted warfarin for apixaban or that added aspirin.
AUGUSTUS enrolled 4,614 atrial fibrillation (AFib) patients who either had a recent acute coronary syndrome (ACS) event or had recently undergone percutaneous coronary intervention (PCI) at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the incidence of major or clinically relevant minor bleeds after 6 months, which was significantly lower in the subgroups that received apixaban instead of warfarin and in patients who received placebo instead of aspirin. The secondary endpoint of death or hospitalization after 6 months was also significantly lower in the apixaban-treated patients, compared with those on warfarin, while the aspirin and placebo subgroups showed no difference in the incidence of these events (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
The results reported by Dr. Bahit also highlighted both the high risk faced by patients with AFib who also have had an ACS event or PCI, as well as a prior stroke, TIA, or TE event, noted Larry B. Goldstein, MD, professor and chairman of neurology at the University of Kentucky, Lexington. “It’s difficult, because these patients had an ACS event or PCI, and you don’t want a coronary too close up, but do these patients really need a P2Y12 inhibitor plus an anticoagulant? Could these patients do as well on apixaban only? I would have liked to see that treatment arm in the study,” Dr. Goldstein commented in an interview.
“These are challenging patients because they often require anticoagulation for the AFib as well as antiplatelet agents” for the recent PCI or ACS event, commented Mitchell S.V. Elkind, MD, professor of neurology at Columbia University, New York. “The question has always been: How many blood thinners should these patients be on? Potentially they could be on three different agents [an anticoagulant and two antiplatelet drugs], and we know that all of those drugs together pretty dramatically increase the risk of bleeding. About 15% of the patients in the overall AUGUSTUS trial had either cerebrovascular disease or systemic thromboembolism, so this was a small subgroup of the overall trial, but the overall trial was large so it’s a significant number of patients who met this criteria. The results confirmed that even in a group of patients who may be considered at high risk because they have a prior history of cerebrovascular disease use of apixaban instead of warfarin seemed safer, and that those patients did not need to be on aspirin as well as their other antiplatelet agent. Patients with a history of stroke, in fact, had a lower risk of bleeding than the other patients in this trial, so one could argue that they should be on an agent like apixaban as well as an antiplatelet agent like clopidogrel without addition of aspirin,” he said in a recorded statement.
In addition to implications for using prescription drugs like apixaban and clopidogrel, the findings also send a message about the need for very aggressive implementation of lifestyle measures that can reduce cardiovascular disease risk in these patients, added Dr. Goldstein. The AUGUSTUS outcome analyses that subdivided the study population into those with a prior stroke, TIA, or TE event – 633 patients or about 14% of the 4,581 patients eligible for this analysis – and those who did not have this history showed the extremely high, incrementally elevated risk faced by patients with these prior events.
A history of stroke, TIA, or TE event linked with a jump in the 90-day rate of major or clinically relevant minor bleeds from 13% without this history to 17%, which is a 31% relative increase; it boosted the 90-day rate of death or hospitalization from 25% to 31%, a 24% relative increase; and it jacked up the rate of death or ischemic events from 6% to 9%, a 50% relative increase, Dr. Bahit reported.
These substantial increases “suggest we need to be very aggressive” in managing these high-risk patients who combine a background of AFib, a prior stroke, TIA, or TE events, and a recent ACS event or PCI, Dr. Goldstein observed. In these patients, he suggested that clinicians make sure to address smoking cessation, obesity, exercise, diet, and statin use, and get each of these to an optimal level to further cut risk. If all five of these basic interventions were successfully administered to a patient they could collectively cut the patient’s event risk by about 80%, he added.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer, the companies that jointly market apixaban. Dr. Bahit has received honoraria from Pfizer, and from CSL Behring and Merck. Dr. Elkind and Dr. Goldstein had no relevant disclosures.
SOURCE: Bahit MC et al. ISC 2020, Abstract LB22.
LOS ANGELES – The edge that the direct-acting oral anticoagulant apixaban (Eliquis) has over warfarin for safely preventing ischemic events in patients with atrial fibrillation and either a recent acute coronary syndrome event or a recent percutaneous coronary intervention held up even in patients with a history of stroke, transient ischemic attack, or thromboembolic event, according to a prespecified secondary analysis of data collected in the AUGUSTUS trial.
The treatment advantages of apixaban, compared with warfarin, seen in the overall AUGUSTUS results, first reported in March 2019, “were consistent” with the benefits seen in the subgroup of enrolled patients with a prior stroke, transient ischemic attack (TIA), or thromboembolic (TE) event, M. Cecilia Bahit, MD, said at the International Stroke Conference sponsored by the American Heart Association.
All patients in AUGUSTUS received a P2Y12 inhibitor antiplatelet drug, which was clopidogrel for more than 90% of patients. The two-by-two factorial design of AUGUSTUS also assessed the safety and efficacy of either adding or withholding aspirin from the two-drug regimen that all patients in the study received with a P2Y12 inhibitor plus an anticoagulant (apixaban or warfarin). The most notable finding of the aspirin versus placebo analysis was that patients without a prior stroke, TIA, or TE event had a “more profound” increase in their rate of major or clinically relevant minor bleeds when also treated with aspirin, compared with patients who received aspirin and had a history of stroke, TIA, or TE event, reported Dr. Bahit, a chief of cardiology and director of clinical research at the INECO Foundation in Rosario, Argentina.
In general, the findings of the secondary analysis that took into account stroke, TIA, or TE history “confirmed” the main AUGUSTUS findings, Dr. Bahit said; an antithrombotic regimen of apixaban plus clopidogrel (or other P2Y12 inhibitor) without aspirin was superior for both efficacy and safety, compared with the alternative regimens that either substituted warfarin for apixaban or that added aspirin.
AUGUSTUS enrolled 4,614 atrial fibrillation (AFib) patients who either had a recent acute coronary syndrome (ACS) event or had recently undergone percutaneous coronary intervention (PCI) at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the incidence of major or clinically relevant minor bleeds after 6 months, which was significantly lower in the subgroups that received apixaban instead of warfarin and in patients who received placebo instead of aspirin. The secondary endpoint of death or hospitalization after 6 months was also significantly lower in the apixaban-treated patients, compared with those on warfarin, while the aspirin and placebo subgroups showed no difference in the incidence of these events (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
The results reported by Dr. Bahit also highlighted both the high risk faced by patients with AFib who also have had an ACS event or PCI, as well as a prior stroke, TIA, or TE event, noted Larry B. Goldstein, MD, professor and chairman of neurology at the University of Kentucky, Lexington. “It’s difficult, because these patients had an ACS event or PCI, and you don’t want a coronary too close up, but do these patients really need a P2Y12 inhibitor plus an anticoagulant? Could these patients do as well on apixaban only? I would have liked to see that treatment arm in the study,” Dr. Goldstein commented in an interview.
“These are challenging patients because they often require anticoagulation for the AFib as well as antiplatelet agents” for the recent PCI or ACS event, commented Mitchell S.V. Elkind, MD, professor of neurology at Columbia University, New York. “The question has always been: How many blood thinners should these patients be on? Potentially they could be on three different agents [an anticoagulant and two antiplatelet drugs], and we know that all of those drugs together pretty dramatically increase the risk of bleeding. About 15% of the patients in the overall AUGUSTUS trial had either cerebrovascular disease or systemic thromboembolism, so this was a small subgroup of the overall trial, but the overall trial was large so it’s a significant number of patients who met this criteria. The results confirmed that even in a group of patients who may be considered at high risk because they have a prior history of cerebrovascular disease use of apixaban instead of warfarin seemed safer, and that those patients did not need to be on aspirin as well as their other antiplatelet agent. Patients with a history of stroke, in fact, had a lower risk of bleeding than the other patients in this trial, so one could argue that they should be on an agent like apixaban as well as an antiplatelet agent like clopidogrel without addition of aspirin,” he said in a recorded statement.
In addition to implications for using prescription drugs like apixaban and clopidogrel, the findings also send a message about the need for very aggressive implementation of lifestyle measures that can reduce cardiovascular disease risk in these patients, added Dr. Goldstein. The AUGUSTUS outcome analyses that subdivided the study population into those with a prior stroke, TIA, or TE event – 633 patients or about 14% of the 4,581 patients eligible for this analysis – and those who did not have this history showed the extremely high, incrementally elevated risk faced by patients with these prior events.
A history of stroke, TIA, or TE event linked with a jump in the 90-day rate of major or clinically relevant minor bleeds from 13% without this history to 17%, which is a 31% relative increase; it boosted the 90-day rate of death or hospitalization from 25% to 31%, a 24% relative increase; and it jacked up the rate of death or ischemic events from 6% to 9%, a 50% relative increase, Dr. Bahit reported.
These substantial increases “suggest we need to be very aggressive” in managing these high-risk patients who combine a background of AFib, a prior stroke, TIA, or TE events, and a recent ACS event or PCI, Dr. Goldstein observed. In these patients, he suggested that clinicians make sure to address smoking cessation, obesity, exercise, diet, and statin use, and get each of these to an optimal level to further cut risk. If all five of these basic interventions were successfully administered to a patient they could collectively cut the patient’s event risk by about 80%, he added.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer, the companies that jointly market apixaban. Dr. Bahit has received honoraria from Pfizer, and from CSL Behring and Merck. Dr. Elkind and Dr. Goldstein had no relevant disclosures.
SOURCE: Bahit MC et al. ISC 2020, Abstract LB22.
REPORTING FROM ISC 2020
Flu activity declines again but remains high
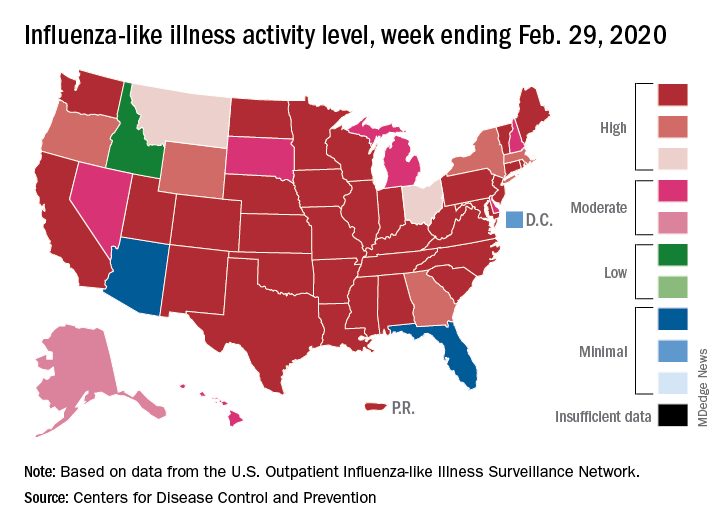
Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.

Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.

Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.
Novel coronavirus may cause environmental contamination through fecal shedding
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
FROM JAMA
Telehealth seen as a key tool to help fight COVID-19
Telehealth is increasingly being viewed as a key way to help fight the COVID-19 outbreak in the United States. Recognizing the potential of this technology to slow the spread of the disease, the House of Representatives included a provision in an $8.3 billion emergency response bill it approved today that would temporarily lift restrictions on Medicare telehealth coverage to assist in the efforts to contain the virus.
Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention (CDC), said that hospitals should be prepared to use telehealth as one of their tools in fighting the outbreak, according to a recent news release from the American Hospital Association (AHA).
Congress is responding to that need by including the service in the new coronavirus legislation now headed to the Senate, after the funding bill was approved in a 415-2 vote by the House.
The bill empowers the Secretary of Health and Human Services (HHS) to “waive or modify application of certain Medicare requirements with respect to telehealth services furnished during certain emergency periods.”
While the measure adds telehealth to the waiver authority that the HHS secretary currently has during national emergencies, it’s only for the coronavirus crisis in this case, Krista Drobac, executive director of the Alliance for Connected Care, told Medscape Medical News.
The waiver would apply to originating sites of telehealth visits, she noted. Thus Medicare coverage of telemedicine would be expanded beyond rural areas.
In addition, the waiver would allow coverage of virtual visits conducted on smartphones with audio and video capabilities. A “qualified provider,” as defined by the legislation, would be a practitioner who has an established relationship with the patient or who is in the same practice as the provider who has that relationship.
An advantage of telehealth, proponents say, is that it can enable people who believe they have COVID-19 to be seen at home rather than visit offices or emergency departments (EDs) where they might spread the disease or be in proximity to others who have it.
In an editorial published March 2 in Modern Healthcare, medical directors from Stanford Medicine, MedStar Health, and Intermountain Healthcare also noted that telehealth can give patients 24/7 access to care, allow surveillance of patients at risk while keeping them at home, ensure that treatment in hospitals is reserved for high-need patients, and enable providers to triage and screen more patients than can be handled in brick-and-mortar care settings.
However, telehealth screening would allow physicians only to judge whether a patient’s symptoms might be indicative of COVID-19, the Alliance for Connected Care, a telehealth advocacy group, noted in a letter to Congressional leaders. Patients would still have to be seen in person to be tested for the disease.
The group, which represents technology companies, health insurers, pharmacies, and other healthcare players, has been lobbying Congress to include telehealth in federal funds to combat the outbreak.
The American Telemedicine Association (ATA) also supports this goal, ATA President Joseph Kvedar, MD, told Medscape Medical News. And the authors of the Modern Healthcare editorial also advocated for this legislative solution. Because the fatality rate for COVID-19 is significantly higher for older people than for other age groups, they noted, telehealth should be an economically viable option for all seniors.
The Centers for Medicare and Medicaid Services (CMS) long covered telemedicine only in rural areas and only when initiated in healthcare settings. Recently, however, CMS loosened its approach to some extent. Virtual “check-in visits” can now be initiated from any location, including home, to determine whether a Medicare patient needs to be seen in the office. In addition, CMS allows Medicare Advantage plans to offer telemedicine as a core benefit.
Are healthcare systems prepared?
Some large healthcare systems such as Stanford, MedStar, and Intermountain are already using telehealth to diagnose and treat patients who have traditional influenza. Telehealth providers at Stanford estimate that almost 50% of these patients are being prescribed the antiviral drug Tamiflu.
It’s unclear whether other healthcare systems are this well prepared to offer telehealth on a large scale. But, according to an AHA survey, Kvedar noted, three quarters of AHA members are engaged in some form of telehealth.
Drobac said “it wouldn’t require too much effort” to ramp up a wide-scale telehealth program that could help reduce the impact of the outbreak. “The technology is there,” she noted. “You need a HIPAA-compliant telehealth platform, but there are so many out there.”
Kvedar agreed. To begin with, he said, hospitals might sequester patients who visit the ED with COVID-19 symptoms in a video-equipped “isolation room.” Staff members could then do the patient intake from a different location in the hospital.
He admitted that this approach would be infeasible if a lot of patients arrived in EDs with coronavirus symptoms. However, Kvedar noted, “All the tools are in place to go well beyond that. American Well, Teladoc, and others are all offering ways to get out in front of this. There are plenty of vendors out there, and most people have a connected cell phone that you can do a video call on.”
Hospital leaders would have to decide whether to embrace telehealth, which would mean less use of services in their institutions, he said. “But it would be for the greater good of the public.”
Kvedar recalled that there was some use of telehealth in the New York area after 9/11. Telehealth was also used in the aftermath of Hurricane Katrina in 2005. But the ATA president, who is also vice president of connected health at Partners HealthCare in Boston, noted that the COVID-19 outbreak is the first public health emergency to occur in the era of Skype and smartphones.
If Congress does ultimately authorize CMS to cover telehealth across the board during this emergency, might that lead to a permanent change in Medicare coverage policy? Kvedar wouldn’t venture an opinion. “However, the current CMS leadership has been incredibly telehealth friendly,” he said. “So it’s possible they would [embrace a lifting of restrictions]. As patients get a sense of this modality of care and how convenient it is for them, they’ll start asking for more.”
Meanwhile, he said, the telehealth opportunity goes beyond video visits with doctors to mitigate the outbreak. Telehealth data could also be used to track disease spread, similar to how researchers have studied Google searches to predict the spread of the flu, he noted.
Teladoc, a major telehealth vendor, recently told stock analysts it’s already working with the CDC on disease surveillance, according to a report in FierceHealthcare.
This article first appeared on Medscape.com.
Telehealth is increasingly being viewed as a key way to help fight the COVID-19 outbreak in the United States. Recognizing the potential of this technology to slow the spread of the disease, the House of Representatives included a provision in an $8.3 billion emergency response bill it approved today that would temporarily lift restrictions on Medicare telehealth coverage to assist in the efforts to contain the virus.
Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention (CDC), said that hospitals should be prepared to use telehealth as one of their tools in fighting the outbreak, according to a recent news release from the American Hospital Association (AHA).
Congress is responding to that need by including the service in the new coronavirus legislation now headed to the Senate, after the funding bill was approved in a 415-2 vote by the House.
The bill empowers the Secretary of Health and Human Services (HHS) to “waive or modify application of certain Medicare requirements with respect to telehealth services furnished during certain emergency periods.”
While the measure adds telehealth to the waiver authority that the HHS secretary currently has during national emergencies, it’s only for the coronavirus crisis in this case, Krista Drobac, executive director of the Alliance for Connected Care, told Medscape Medical News.
The waiver would apply to originating sites of telehealth visits, she noted. Thus Medicare coverage of telemedicine would be expanded beyond rural areas.
In addition, the waiver would allow coverage of virtual visits conducted on smartphones with audio and video capabilities. A “qualified provider,” as defined by the legislation, would be a practitioner who has an established relationship with the patient or who is in the same practice as the provider who has that relationship.
An advantage of telehealth, proponents say, is that it can enable people who believe they have COVID-19 to be seen at home rather than visit offices or emergency departments (EDs) where they might spread the disease or be in proximity to others who have it.
In an editorial published March 2 in Modern Healthcare, medical directors from Stanford Medicine, MedStar Health, and Intermountain Healthcare also noted that telehealth can give patients 24/7 access to care, allow surveillance of patients at risk while keeping them at home, ensure that treatment in hospitals is reserved for high-need patients, and enable providers to triage and screen more patients than can be handled in brick-and-mortar care settings.
However, telehealth screening would allow physicians only to judge whether a patient’s symptoms might be indicative of COVID-19, the Alliance for Connected Care, a telehealth advocacy group, noted in a letter to Congressional leaders. Patients would still have to be seen in person to be tested for the disease.
The group, which represents technology companies, health insurers, pharmacies, and other healthcare players, has been lobbying Congress to include telehealth in federal funds to combat the outbreak.
The American Telemedicine Association (ATA) also supports this goal, ATA President Joseph Kvedar, MD, told Medscape Medical News. And the authors of the Modern Healthcare editorial also advocated for this legislative solution. Because the fatality rate for COVID-19 is significantly higher for older people than for other age groups, they noted, telehealth should be an economically viable option for all seniors.
The Centers for Medicare and Medicaid Services (CMS) long covered telemedicine only in rural areas and only when initiated in healthcare settings. Recently, however, CMS loosened its approach to some extent. Virtual “check-in visits” can now be initiated from any location, including home, to determine whether a Medicare patient needs to be seen in the office. In addition, CMS allows Medicare Advantage plans to offer telemedicine as a core benefit.
Are healthcare systems prepared?
Some large healthcare systems such as Stanford, MedStar, and Intermountain are already using telehealth to diagnose and treat patients who have traditional influenza. Telehealth providers at Stanford estimate that almost 50% of these patients are being prescribed the antiviral drug Tamiflu.
It’s unclear whether other healthcare systems are this well prepared to offer telehealth on a large scale. But, according to an AHA survey, Kvedar noted, three quarters of AHA members are engaged in some form of telehealth.
Drobac said “it wouldn’t require too much effort” to ramp up a wide-scale telehealth program that could help reduce the impact of the outbreak. “The technology is there,” she noted. “You need a HIPAA-compliant telehealth platform, but there are so many out there.”
Kvedar agreed. To begin with, he said, hospitals might sequester patients who visit the ED with COVID-19 symptoms in a video-equipped “isolation room.” Staff members could then do the patient intake from a different location in the hospital.
He admitted that this approach would be infeasible if a lot of patients arrived in EDs with coronavirus symptoms. However, Kvedar noted, “All the tools are in place to go well beyond that. American Well, Teladoc, and others are all offering ways to get out in front of this. There are plenty of vendors out there, and most people have a connected cell phone that you can do a video call on.”
Hospital leaders would have to decide whether to embrace telehealth, which would mean less use of services in their institutions, he said. “But it would be for the greater good of the public.”
Kvedar recalled that there was some use of telehealth in the New York area after 9/11. Telehealth was also used in the aftermath of Hurricane Katrina in 2005. But the ATA president, who is also vice president of connected health at Partners HealthCare in Boston, noted that the COVID-19 outbreak is the first public health emergency to occur in the era of Skype and smartphones.
If Congress does ultimately authorize CMS to cover telehealth across the board during this emergency, might that lead to a permanent change in Medicare coverage policy? Kvedar wouldn’t venture an opinion. “However, the current CMS leadership has been incredibly telehealth friendly,” he said. “So it’s possible they would [embrace a lifting of restrictions]. As patients get a sense of this modality of care and how convenient it is for them, they’ll start asking for more.”
Meanwhile, he said, the telehealth opportunity goes beyond video visits with doctors to mitigate the outbreak. Telehealth data could also be used to track disease spread, similar to how researchers have studied Google searches to predict the spread of the flu, he noted.
Teladoc, a major telehealth vendor, recently told stock analysts it’s already working with the CDC on disease surveillance, according to a report in FierceHealthcare.
This article first appeared on Medscape.com.
Telehealth is increasingly being viewed as a key way to help fight the COVID-19 outbreak in the United States. Recognizing the potential of this technology to slow the spread of the disease, the House of Representatives included a provision in an $8.3 billion emergency response bill it approved today that would temporarily lift restrictions on Medicare telehealth coverage to assist in the efforts to contain the virus.
Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention (CDC), said that hospitals should be prepared to use telehealth as one of their tools in fighting the outbreak, according to a recent news release from the American Hospital Association (AHA).
Congress is responding to that need by including the service in the new coronavirus legislation now headed to the Senate, after the funding bill was approved in a 415-2 vote by the House.
The bill empowers the Secretary of Health and Human Services (HHS) to “waive or modify application of certain Medicare requirements with respect to telehealth services furnished during certain emergency periods.”
While the measure adds telehealth to the waiver authority that the HHS secretary currently has during national emergencies, it’s only for the coronavirus crisis in this case, Krista Drobac, executive director of the Alliance for Connected Care, told Medscape Medical News.
The waiver would apply to originating sites of telehealth visits, she noted. Thus Medicare coverage of telemedicine would be expanded beyond rural areas.
In addition, the waiver would allow coverage of virtual visits conducted on smartphones with audio and video capabilities. A “qualified provider,” as defined by the legislation, would be a practitioner who has an established relationship with the patient or who is in the same practice as the provider who has that relationship.
An advantage of telehealth, proponents say, is that it can enable people who believe they have COVID-19 to be seen at home rather than visit offices or emergency departments (EDs) where they might spread the disease or be in proximity to others who have it.
In an editorial published March 2 in Modern Healthcare, medical directors from Stanford Medicine, MedStar Health, and Intermountain Healthcare also noted that telehealth can give patients 24/7 access to care, allow surveillance of patients at risk while keeping them at home, ensure that treatment in hospitals is reserved for high-need patients, and enable providers to triage and screen more patients than can be handled in brick-and-mortar care settings.
However, telehealth screening would allow physicians only to judge whether a patient’s symptoms might be indicative of COVID-19, the Alliance for Connected Care, a telehealth advocacy group, noted in a letter to Congressional leaders. Patients would still have to be seen in person to be tested for the disease.
The group, which represents technology companies, health insurers, pharmacies, and other healthcare players, has been lobbying Congress to include telehealth in federal funds to combat the outbreak.
The American Telemedicine Association (ATA) also supports this goal, ATA President Joseph Kvedar, MD, told Medscape Medical News. And the authors of the Modern Healthcare editorial also advocated for this legislative solution. Because the fatality rate for COVID-19 is significantly higher for older people than for other age groups, they noted, telehealth should be an economically viable option for all seniors.
The Centers for Medicare and Medicaid Services (CMS) long covered telemedicine only in rural areas and only when initiated in healthcare settings. Recently, however, CMS loosened its approach to some extent. Virtual “check-in visits” can now be initiated from any location, including home, to determine whether a Medicare patient needs to be seen in the office. In addition, CMS allows Medicare Advantage plans to offer telemedicine as a core benefit.
Are healthcare systems prepared?
Some large healthcare systems such as Stanford, MedStar, and Intermountain are already using telehealth to diagnose and treat patients who have traditional influenza. Telehealth providers at Stanford estimate that almost 50% of these patients are being prescribed the antiviral drug Tamiflu.
It’s unclear whether other healthcare systems are this well prepared to offer telehealth on a large scale. But, according to an AHA survey, Kvedar noted, three quarters of AHA members are engaged in some form of telehealth.
Drobac said “it wouldn’t require too much effort” to ramp up a wide-scale telehealth program that could help reduce the impact of the outbreak. “The technology is there,” she noted. “You need a HIPAA-compliant telehealth platform, but there are so many out there.”
Kvedar agreed. To begin with, he said, hospitals might sequester patients who visit the ED with COVID-19 symptoms in a video-equipped “isolation room.” Staff members could then do the patient intake from a different location in the hospital.
He admitted that this approach would be infeasible if a lot of patients arrived in EDs with coronavirus symptoms. However, Kvedar noted, “All the tools are in place to go well beyond that. American Well, Teladoc, and others are all offering ways to get out in front of this. There are plenty of vendors out there, and most people have a connected cell phone that you can do a video call on.”
Hospital leaders would have to decide whether to embrace telehealth, which would mean less use of services in their institutions, he said. “But it would be for the greater good of the public.”
Kvedar recalled that there was some use of telehealth in the New York area after 9/11. Telehealth was also used in the aftermath of Hurricane Katrina in 2005. But the ATA president, who is also vice president of connected health at Partners HealthCare in Boston, noted that the COVID-19 outbreak is the first public health emergency to occur in the era of Skype and smartphones.
If Congress does ultimately authorize CMS to cover telehealth across the board during this emergency, might that lead to a permanent change in Medicare coverage policy? Kvedar wouldn’t venture an opinion. “However, the current CMS leadership has been incredibly telehealth friendly,” he said. “So it’s possible they would [embrace a lifting of restrictions]. As patients get a sense of this modality of care and how convenient it is for them, they’ll start asking for more.”
Meanwhile, he said, the telehealth opportunity goes beyond video visits with doctors to mitigate the outbreak. Telehealth data could also be used to track disease spread, similar to how researchers have studied Google searches to predict the spread of the flu, he noted.
Teladoc, a major telehealth vendor, recently told stock analysts it’s already working with the CDC on disease surveillance, according to a report in FierceHealthcare.
This article first appeared on Medscape.com.
COVID-19 and public health preparedness in the United States
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
SARS epidemiology provides clues to potential treatment for COVID-19
A team of researchers has discovered important commonalities between SARS-CoV-2 and SARS-CoV infection that could lead to a potential targets for antiviral intervention.
Markus Hoffmann, of the Leibniz Institute for Primate Research, Göttingen, Germany, and a team of investigators also found that antibody responses raised against SARS-S during infection or vaccination might offer some level of protection against SARS-CoV-2 infection. Their findings were published in Cell.
In order for coronaviruses to enter a cell, they must first bind their viral spike (S) proteins to cellular receptors and depend on S protein priming by host cell proteases. The study found that the SARS-CoV-2, causal agent for COVID-19, uses the same SARS-CoV receptor, ACE2, for entry and uses the serine protease TMPRSS2 for S protein priming as the original SARS-CoV-1 (SARS). Importantly, the researchers also found that the cellular serine protease TMPRSS2 primes SARS-CoV-2-S for entry and that a serine protease inhibitor blocks SARS-CoV-2 infection of lung cells, providing opportunities for potential therapeutic intervention.
The researchers performed a sequence analysis that showed SARS-CoV-2 clusters with SARS-CoV–related viruses from bats, of which some – but not all – can use ACE2 for host cell entry. Further analysis of the receptor binding motif known to make contact with ACE2 showed that most amino acid residues essential for ACE2 binding by SARS-S were conserved in SARS-2-S but were absent from S proteins of those SARS-related coronaviruses previously found not to use ACE2.
In addition, the researchers found that SARS-CoV-2–infected BHK-21 cells transfected to express ACE2 with high efficiency, but not the parental BHK-21 cells indicating that SARS-CoV-2-S, like the original SARS virus S protein, uses ACE2 for cellular entry.
Using cultured cells, the researchers found that the protease inhibitor, camostat mesylate, inhibited SARS-S and SARS-2-S entry into primary human lung cells, demonstrating that SARS-CoV-2 can use TMPRSS2 for S protein priming and that camostat mesylate can block SARS-CoV-2 infection of lung cells. Camostat mesylate has been used as a therapy for some forms of cancer and other viral infections.
In addition to their research on the protease inhibitor, the researchers also found that sera from convalescent SARS patients cross-neutralized SARS-2-S–driven entry. They found that four sera obtained from three convalescent SARS patients inhibited SARS-S entry into cell lines in a concentration dependent fashion.
“We demonstrate that SARS-CoV-2 uses the SARS55 CoV receptor, ACE2, for entry and the serine protease TMPRSS2 for S protein priming. A TMPRSS2 inhibitor approved for clinical use blocked entry and might constitute a treatment option. Finally, we show that the sera from convalescent SARS patients cross-neutralized SARS-2-S–driven entry,” the authors concluded.
The study was supported by BMBF (RAPID Consortium) and German Research Foundation (DFG). The authors reported that they had no conflicts.
SOURCE: Hoffmann M et al. Cell 2020. doi: 10.1016/j.cell.2020.02.052.
A team of researchers has discovered important commonalities between SARS-CoV-2 and SARS-CoV infection that could lead to a potential targets for antiviral intervention.
Markus Hoffmann, of the Leibniz Institute for Primate Research, Göttingen, Germany, and a team of investigators also found that antibody responses raised against SARS-S during infection or vaccination might offer some level of protection against SARS-CoV-2 infection. Their findings were published in Cell.
In order for coronaviruses to enter a cell, they must first bind their viral spike (S) proteins to cellular receptors and depend on S protein priming by host cell proteases. The study found that the SARS-CoV-2, causal agent for COVID-19, uses the same SARS-CoV receptor, ACE2, for entry and uses the serine protease TMPRSS2 for S protein priming as the original SARS-CoV-1 (SARS). Importantly, the researchers also found that the cellular serine protease TMPRSS2 primes SARS-CoV-2-S for entry and that a serine protease inhibitor blocks SARS-CoV-2 infection of lung cells, providing opportunities for potential therapeutic intervention.
The researchers performed a sequence analysis that showed SARS-CoV-2 clusters with SARS-CoV–related viruses from bats, of which some – but not all – can use ACE2 for host cell entry. Further analysis of the receptor binding motif known to make contact with ACE2 showed that most amino acid residues essential for ACE2 binding by SARS-S were conserved in SARS-2-S but were absent from S proteins of those SARS-related coronaviruses previously found not to use ACE2.
In addition, the researchers found that SARS-CoV-2–infected BHK-21 cells transfected to express ACE2 with high efficiency, but not the parental BHK-21 cells indicating that SARS-CoV-2-S, like the original SARS virus S protein, uses ACE2 for cellular entry.
Using cultured cells, the researchers found that the protease inhibitor, camostat mesylate, inhibited SARS-S and SARS-2-S entry into primary human lung cells, demonstrating that SARS-CoV-2 can use TMPRSS2 for S protein priming and that camostat mesylate can block SARS-CoV-2 infection of lung cells. Camostat mesylate has been used as a therapy for some forms of cancer and other viral infections.
In addition to their research on the protease inhibitor, the researchers also found that sera from convalescent SARS patients cross-neutralized SARS-2-S–driven entry. They found that four sera obtained from three convalescent SARS patients inhibited SARS-S entry into cell lines in a concentration dependent fashion.
“We demonstrate that SARS-CoV-2 uses the SARS55 CoV receptor, ACE2, for entry and the serine protease TMPRSS2 for S protein priming. A TMPRSS2 inhibitor approved for clinical use blocked entry and might constitute a treatment option. Finally, we show that the sera from convalescent SARS patients cross-neutralized SARS-2-S–driven entry,” the authors concluded.
The study was supported by BMBF (RAPID Consortium) and German Research Foundation (DFG). The authors reported that they had no conflicts.
SOURCE: Hoffmann M et al. Cell 2020. doi: 10.1016/j.cell.2020.02.052.
A team of researchers has discovered important commonalities between SARS-CoV-2 and SARS-CoV infection that could lead to a potential targets for antiviral intervention.
Markus Hoffmann, of the Leibniz Institute for Primate Research, Göttingen, Germany, and a team of investigators also found that antibody responses raised against SARS-S during infection or vaccination might offer some level of protection against SARS-CoV-2 infection. Their findings were published in Cell.
In order for coronaviruses to enter a cell, they must first bind their viral spike (S) proteins to cellular receptors and depend on S protein priming by host cell proteases. The study found that the SARS-CoV-2, causal agent for COVID-19, uses the same SARS-CoV receptor, ACE2, for entry and uses the serine protease TMPRSS2 for S protein priming as the original SARS-CoV-1 (SARS). Importantly, the researchers also found that the cellular serine protease TMPRSS2 primes SARS-CoV-2-S for entry and that a serine protease inhibitor blocks SARS-CoV-2 infection of lung cells, providing opportunities for potential therapeutic intervention.
The researchers performed a sequence analysis that showed SARS-CoV-2 clusters with SARS-CoV–related viruses from bats, of which some – but not all – can use ACE2 for host cell entry. Further analysis of the receptor binding motif known to make contact with ACE2 showed that most amino acid residues essential for ACE2 binding by SARS-S were conserved in SARS-2-S but were absent from S proteins of those SARS-related coronaviruses previously found not to use ACE2.
In addition, the researchers found that SARS-CoV-2–infected BHK-21 cells transfected to express ACE2 with high efficiency, but not the parental BHK-21 cells indicating that SARS-CoV-2-S, like the original SARS virus S protein, uses ACE2 for cellular entry.
Using cultured cells, the researchers found that the protease inhibitor, camostat mesylate, inhibited SARS-S and SARS-2-S entry into primary human lung cells, demonstrating that SARS-CoV-2 can use TMPRSS2 for S protein priming and that camostat mesylate can block SARS-CoV-2 infection of lung cells. Camostat mesylate has been used as a therapy for some forms of cancer and other viral infections.
In addition to their research on the protease inhibitor, the researchers also found that sera from convalescent SARS patients cross-neutralized SARS-2-S–driven entry. They found that four sera obtained from three convalescent SARS patients inhibited SARS-S entry into cell lines in a concentration dependent fashion.
“We demonstrate that SARS-CoV-2 uses the SARS55 CoV receptor, ACE2, for entry and the serine protease TMPRSS2 for S protein priming. A TMPRSS2 inhibitor approved for clinical use blocked entry and might constitute a treatment option. Finally, we show that the sera from convalescent SARS patients cross-neutralized SARS-2-S–driven entry,” the authors concluded.
The study was supported by BMBF (RAPID Consortium) and German Research Foundation (DFG). The authors reported that they had no conflicts.
SOURCE: Hoffmann M et al. Cell 2020. doi: 10.1016/j.cell.2020.02.052.
FROM CELL
Hospitalist profile: Charu Puri, MD
Charu Puri, MD, FHM, is a hospitalist and medical informaticist at Sutter East Bay Medical Group in Oakland, Calif. She also serves as medical director for onboarding, mentoring, and physician development.
Dr. Puri has been a member of the Society of Hospital Medicine since 2009, and attended the Society’s Leadership Academy, where she was inspired to create a mentorship program at her own institution. She is a member of the San Francisco Bay chapter of SHM and serves on the Performance Measurement and Reporting Committee.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
It was early on in my residency that it became clear to me that I wanted to pursue the hospitalist track. It was a natural fit, and I gravitated toward the hospitalist side of medicine. What appealed to me most was that we had the opportunity and privilege to provide care to patients in their most vulnerable state and experience the effects of that care in real time. I found that very gratifying.
There is also a sense of community and camaraderie that comes with working in a hospital setting. Everyone is working together, trying to help patients. The collegiality and the relationships that develop are very rewarding. I have been fortunate enough to have built strong friendships with the hospitalists in my group as well as colleagues from other disciplines in medicine that work in the hospital.
What is your current role at Sutter Health?
Alta Bates Summit Medical Center is part of the larger Sutter Health system. I have an administrative role with my medical group in addition to the clinical work I do at the medical center, although first and foremost I identify myself as a hospitalist. About 5 years ago I took on a role in clinical informatics, when our hospital implemented an EHR. Since then I have been working as an inpatient physician informaticist. Most recently I took on a new role as medical director for onboarding, mentoring, and physician development in my medical group.
How do you balance the different duties of your various roles?
I am full time in my administration role, between my informatics role and my onboarding role. I technically don’t have to do clinical shifts if I don’t want to, but it’s important to me to continue clinical practice and maintain my skills and connection to the hospital and colleagues. I do about four clinical shifts a month, and plan to continue doing that. In our group you must do 14 shifts a month to be considered full time, so what I do could be considered about one-third of that.
What are your favorite areas of clinical practice and/or research?
I haven’t had a lot of research experience. My residency program was a community-based program, and my current setting is a community hospital. I haven’t been involved much in the academic side of hospital medicine. As far as clinical practices goes, I think it’s the diversity of hospital medicine that appeals to me. You really get to be a jack of all trades, and experience all the different disciplines of medicine. I like the variety.
Both my informatics and onboarding roles came out of a need that I identified, and just began doing the work before there was an official role. When we implemented our EHR, it was essential to get our doctors organized to make sure they were ready to take care of patients that first day of go live. By the time our hospital went live on the EHR, I had a good understanding of how it worked, and so I was able to create a miniature curriculum for our physicians – templates, order sets, workflows, etc. – to help ensure everything went smoothly. A few months after we implemented the EHR, I was officially offered a physician informaticist role.
The onboarding role came about in an interesting way. I was participating in the leadership course offered by SHM and was lucky enough to be in the pilot for the Capstone course. That leadership course is focused around mentoring and sponsorship, and one of the faculty members was Nancy Spector, MD, the associate dean of faculty development at Drexel University, Philadelphia. She talked a lot about mentoring, and I was inspired to set up a mentoring program for our hospitalists. Dr. Spector graciously agreed to mentor me as I worked on my Capstone project, which was to create a mentoring program in a community-based hospitalist group. As I continued to work on the project, coincidentally our medical group decided to redesign our new physician onboarding process. Because I was already involved in the onboarding and training related to our EHR, I became very involved with our medical group's onboarding redesign.
My group's CEO decided to create a new directorship role for onboarding and mentoring, which I recently interviewed for and was offered about two months ago.
I think setting up systems to support our doctors is the common threat between the informatics and the onboarding roles. I want to implement systems that support our doctors, help them succeed, and hopefully make their jobs a little easier.
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
We practice in a very urban environment, with many low-income patients who have limited resources and access to health care. That can be very challenging. You always wonder if these patients have all the support they need after leaving the hospital. Sometimes I feel that I am just putting a band-aid on the medical problem, so to speak, but not solving the underlying issue. But it can be very rewarding during those times when the hospital and the broader community can bring our resources together to create interventions to help at-risk patients. It doesn’t happen as frequently as we would like, but when it does happen it feels good.
Another challenging aspect is related to perception. There are a lot of consultants in the hospital who view hospitalists as "house staff." That can be very frustrating, and it’s important to steer the conversations away from that perspective, and really try to establish ourselves as colleagues and peers.
How will hospital medicine change in the next decade or 2?
It’s a relatively young field, and we’re still figuring it out. I really don’t know how hospital medicine is going to change, but I do know that the field will continue to evolve, given the way U.S. health care is rapidly changing.
Do you have any advice for students and residents interested in hospital medicine?
It’s a fun way to practice medicine and I would encourage students to go into hospital medicine. It’s great for work/life balance. The advice I would give is that it is very important to get involved early in your career. Get involved in medical group or hospital committees. Stay away from the “shift mentality” – that I’m going to work my shifts and leave. That can lead to early burnout, which is a real concern in our field now. Early engagement is essential, so you can help lead these conversations at your hospital.
Charu Puri, MD, FHM, is a hospitalist and medical informaticist at Sutter East Bay Medical Group in Oakland, Calif. She also serves as medical director for onboarding, mentoring, and physician development.
Dr. Puri has been a member of the Society of Hospital Medicine since 2009, and attended the Society’s Leadership Academy, where she was inspired to create a mentorship program at her own institution. She is a member of the San Francisco Bay chapter of SHM and serves on the Performance Measurement and Reporting Committee.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
It was early on in my residency that it became clear to me that I wanted to pursue the hospitalist track. It was a natural fit, and I gravitated toward the hospitalist side of medicine. What appealed to me most was that we had the opportunity and privilege to provide care to patients in their most vulnerable state and experience the effects of that care in real time. I found that very gratifying.
There is also a sense of community and camaraderie that comes with working in a hospital setting. Everyone is working together, trying to help patients. The collegiality and the relationships that develop are very rewarding. I have been fortunate enough to have built strong friendships with the hospitalists in my group as well as colleagues from other disciplines in medicine that work in the hospital.
What is your current role at Sutter Health?
Alta Bates Summit Medical Center is part of the larger Sutter Health system. I have an administrative role with my medical group in addition to the clinical work I do at the medical center, although first and foremost I identify myself as a hospitalist. About 5 years ago I took on a role in clinical informatics, when our hospital implemented an EHR. Since then I have been working as an inpatient physician informaticist. Most recently I took on a new role as medical director for onboarding, mentoring, and physician development in my medical group.
How do you balance the different duties of your various roles?
I am full time in my administration role, between my informatics role and my onboarding role. I technically don’t have to do clinical shifts if I don’t want to, but it’s important to me to continue clinical practice and maintain my skills and connection to the hospital and colleagues. I do about four clinical shifts a month, and plan to continue doing that. In our group you must do 14 shifts a month to be considered full time, so what I do could be considered about one-third of that.
What are your favorite areas of clinical practice and/or research?
I haven’t had a lot of research experience. My residency program was a community-based program, and my current setting is a community hospital. I haven’t been involved much in the academic side of hospital medicine. As far as clinical practices goes, I think it’s the diversity of hospital medicine that appeals to me. You really get to be a jack of all trades, and experience all the different disciplines of medicine. I like the variety.
Both my informatics and onboarding roles came out of a need that I identified, and just began doing the work before there was an official role. When we implemented our EHR, it was essential to get our doctors organized to make sure they were ready to take care of patients that first day of go live. By the time our hospital went live on the EHR, I had a good understanding of how it worked, and so I was able to create a miniature curriculum for our physicians – templates, order sets, workflows, etc. – to help ensure everything went smoothly. A few months after we implemented the EHR, I was officially offered a physician informaticist role.
The onboarding role came about in an interesting way. I was participating in the leadership course offered by SHM and was lucky enough to be in the pilot for the Capstone course. That leadership course is focused around mentoring and sponsorship, and one of the faculty members was Nancy Spector, MD, the associate dean of faculty development at Drexel University, Philadelphia. She talked a lot about mentoring, and I was inspired to set up a mentoring program for our hospitalists. Dr. Spector graciously agreed to mentor me as I worked on my Capstone project, which was to create a mentoring program in a community-based hospitalist group. As I continued to work on the project, coincidentally our medical group decided to redesign our new physician onboarding process. Because I was already involved in the onboarding and training related to our EHR, I became very involved with our medical group's onboarding redesign.
My group's CEO decided to create a new directorship role for onboarding and mentoring, which I recently interviewed for and was offered about two months ago.
I think setting up systems to support our doctors is the common threat between the informatics and the onboarding roles. I want to implement systems that support our doctors, help them succeed, and hopefully make their jobs a little easier.
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
We practice in a very urban environment, with many low-income patients who have limited resources and access to health care. That can be very challenging. You always wonder if these patients have all the support they need after leaving the hospital. Sometimes I feel that I am just putting a band-aid on the medical problem, so to speak, but not solving the underlying issue. But it can be very rewarding during those times when the hospital and the broader community can bring our resources together to create interventions to help at-risk patients. It doesn’t happen as frequently as we would like, but when it does happen it feels good.
Another challenging aspect is related to perception. There are a lot of consultants in the hospital who view hospitalists as "house staff." That can be very frustrating, and it’s important to steer the conversations away from that perspective, and really try to establish ourselves as colleagues and peers.
How will hospital medicine change in the next decade or 2?
It’s a relatively young field, and we’re still figuring it out. I really don’t know how hospital medicine is going to change, but I do know that the field will continue to evolve, given the way U.S. health care is rapidly changing.
Do you have any advice for students and residents interested in hospital medicine?
It’s a fun way to practice medicine and I would encourage students to go into hospital medicine. It’s great for work/life balance. The advice I would give is that it is very important to get involved early in your career. Get involved in medical group or hospital committees. Stay away from the “shift mentality” – that I’m going to work my shifts and leave. That can lead to early burnout, which is a real concern in our field now. Early engagement is essential, so you can help lead these conversations at your hospital.
Charu Puri, MD, FHM, is a hospitalist and medical informaticist at Sutter East Bay Medical Group in Oakland, Calif. She also serves as medical director for onboarding, mentoring, and physician development.
Dr. Puri has been a member of the Society of Hospital Medicine since 2009, and attended the Society’s Leadership Academy, where she was inspired to create a mentorship program at her own institution. She is a member of the San Francisco Bay chapter of SHM and serves on the Performance Measurement and Reporting Committee.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
It was early on in my residency that it became clear to me that I wanted to pursue the hospitalist track. It was a natural fit, and I gravitated toward the hospitalist side of medicine. What appealed to me most was that we had the opportunity and privilege to provide care to patients in their most vulnerable state and experience the effects of that care in real time. I found that very gratifying.
There is also a sense of community and camaraderie that comes with working in a hospital setting. Everyone is working together, trying to help patients. The collegiality and the relationships that develop are very rewarding. I have been fortunate enough to have built strong friendships with the hospitalists in my group as well as colleagues from other disciplines in medicine that work in the hospital.
What is your current role at Sutter Health?
Alta Bates Summit Medical Center is part of the larger Sutter Health system. I have an administrative role with my medical group in addition to the clinical work I do at the medical center, although first and foremost I identify myself as a hospitalist. About 5 years ago I took on a role in clinical informatics, when our hospital implemented an EHR. Since then I have been working as an inpatient physician informaticist. Most recently I took on a new role as medical director for onboarding, mentoring, and physician development in my medical group.
How do you balance the different duties of your various roles?
I am full time in my administration role, between my informatics role and my onboarding role. I technically don’t have to do clinical shifts if I don’t want to, but it’s important to me to continue clinical practice and maintain my skills and connection to the hospital and colleagues. I do about four clinical shifts a month, and plan to continue doing that. In our group you must do 14 shifts a month to be considered full time, so what I do could be considered about one-third of that.
What are your favorite areas of clinical practice and/or research?
I haven’t had a lot of research experience. My residency program was a community-based program, and my current setting is a community hospital. I haven’t been involved much in the academic side of hospital medicine. As far as clinical practices goes, I think it’s the diversity of hospital medicine that appeals to me. You really get to be a jack of all trades, and experience all the different disciplines of medicine. I like the variety.
Both my informatics and onboarding roles came out of a need that I identified, and just began doing the work before there was an official role. When we implemented our EHR, it was essential to get our doctors organized to make sure they were ready to take care of patients that first day of go live. By the time our hospital went live on the EHR, I had a good understanding of how it worked, and so I was able to create a miniature curriculum for our physicians – templates, order sets, workflows, etc. – to help ensure everything went smoothly. A few months after we implemented the EHR, I was officially offered a physician informaticist role.
The onboarding role came about in an interesting way. I was participating in the leadership course offered by SHM and was lucky enough to be in the pilot for the Capstone course. That leadership course is focused around mentoring and sponsorship, and one of the faculty members was Nancy Spector, MD, the associate dean of faculty development at Drexel University, Philadelphia. She talked a lot about mentoring, and I was inspired to set up a mentoring program for our hospitalists. Dr. Spector graciously agreed to mentor me as I worked on my Capstone project, which was to create a mentoring program in a community-based hospitalist group. As I continued to work on the project, coincidentally our medical group decided to redesign our new physician onboarding process. Because I was already involved in the onboarding and training related to our EHR, I became very involved with our medical group's onboarding redesign.
My group's CEO decided to create a new directorship role for onboarding and mentoring, which I recently interviewed for and was offered about two months ago.
I think setting up systems to support our doctors is the common threat between the informatics and the onboarding roles. I want to implement systems that support our doctors, help them succeed, and hopefully make their jobs a little easier.
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
We practice in a very urban environment, with many low-income patients who have limited resources and access to health care. That can be very challenging. You always wonder if these patients have all the support they need after leaving the hospital. Sometimes I feel that I am just putting a band-aid on the medical problem, so to speak, but not solving the underlying issue. But it can be very rewarding during those times when the hospital and the broader community can bring our resources together to create interventions to help at-risk patients. It doesn’t happen as frequently as we would like, but when it does happen it feels good.
Another challenging aspect is related to perception. There are a lot of consultants in the hospital who view hospitalists as "house staff." That can be very frustrating, and it’s important to steer the conversations away from that perspective, and really try to establish ourselves as colleagues and peers.
How will hospital medicine change in the next decade or 2?
It’s a relatively young field, and we’re still figuring it out. I really don’t know how hospital medicine is going to change, but I do know that the field will continue to evolve, given the way U.S. health care is rapidly changing.
Do you have any advice for students and residents interested in hospital medicine?
It’s a fun way to practice medicine and I would encourage students to go into hospital medicine. It’s great for work/life balance. The advice I would give is that it is very important to get involved early in your career. Get involved in medical group or hospital committees. Stay away from the “shift mentality” – that I’m going to work my shifts and leave. That can lead to early burnout, which is a real concern in our field now. Early engagement is essential, so you can help lead these conversations at your hospital.
Infection control protects hospital staff from COVID-19, study shows
Hospital-related infections have been widely reported during the ongoing coronavirus outbreak, with healthcare professionals bearing a disproportionate risk. However, a proactive response in Hong Kong’s public hospital system appears to have bucked this trend and successfully protected both patients and staff from SARS-CoV-2, according to a study published online today in Infection Control & Hospital Epidemiology.
During the first 42 days of the outbreak, the 43 hospitals in the network tested 1275 suspected cases and treated 42 patients with confirmed COVID-19, the disease caused by SARS-CoV-2 infection. Yet, there were no nosocomial infections or infections among healthcare personnel, report Vincent C.C. Cheng, MD, FRCPath, the hospital’s infection control officer, and colleagues.
Cheng and colleagues note that 11 out of 413 healthcare workers who treat patients with confirmed infections had unprotected exposure and were in quarantine for 14 days, but none became ill.
In comparison, they note, the 2003 SARS outbreak saw almost 60% of nosocomial cases occurring in healthcare workers.
Proactive bundle
The Hong Kong success story may be due to a stepped-up proactive bundle of measures that included enhanced laboratory surveillance, early airborne infection isolation, and rapid-turnaround molecular diagnostics. Other strategies included staff forums and one-on-one discussions about infection control, employee training in protective equipment use, hand-hygiene compliance enforcement, and contact tracing for workers with unprotected exposure.
In addition, surgical masks were provided for all healthcare workers, patients, and visitors to clinical areas, a practice previously associated with reduced in-hospital transmission during influenza outbreaks, the authors note.
Hospitals also mandated use of personal protective equipment (PPE) for aerosol-generating procedures (AGPs), such as endotracheal intubation, open suctioning, and high-flow oxygen use, as AGPs had been linked to nosocomial transmission to healthcare workers during the 2003 SARS outbreak.
The infection control measures, which were part of a preparedness plan developed after the SARS outbreak, were initiated on December 31, when the first reports of a cluster of infections came from Wuhan, China.
As the outbreak evolved, the Hong Kong hospitals quickly widened the epidemiologic criteria for screening, from initially including only those who had been to a wet market in Wuhan within 14 days of symptom onset, to eventually including anyone who had been to Hubei province, been in a medical facility in mainland China, or in contact with a known case.
All suspected cases were sent to an airborne-infection isolation room (AIIR) or a ward with at least a meter of space between patients.
“Appropriate hospital infection control measures could prevent nosocomial transmission of SARS-CoV-2,” the authors write. “Vigilance in hand hygiene practice, wearing of surgical mask in the hospital, and appropriate use of PPE in patient care, especially [when] performing AGPs, are the key infection control measures to prevent nosocomial transmission of SARS-CoV-2 even before the availability of effective antiviral agents and vaccine.”
Asked for his perspective on the report, Aaron E. Glatt, MD, chairman of the department of medicine and chief of infectious diseases at Mount Sinai South Nassau in Oceanside, New York, said that apart from the widespread issuing of surgical masks to workers, patients, and visitors, the measures taken in Hong Kong are not different from standard infection-control practices in American hospitals. Glatt, who is also a hospital epidemiologist, said it was unclear how much impact the masks would have.
“Although the infection control was impressive, I don’t see any evidence of a difference in care,” he told Medscape Medical News.
Could zero infection transmission be achieved in the more far-flung and variable settings of hospitals across the United States? “The ability to get zero transmission is only possible if people adhere to the strictest infection-control guidelines,” Glatt said. “That is clearly the goal, and it will take time to see if our existing strict guidelines are sufficient to maintain zero or close to zero contamination and transmission rates in our hospitals.”
Rather than looking to change US practices, he stressed adherence to widely established tenets of care. “It’s critically important to keep paying close attention to the basics, to the simple blocking and tackling, and to identify which patients are at risk, and therefore, when workers need protective equipment,” he said.
“Follow the recommended standards,” continued Glatt, who is also a spokesperson for the Infectious Diseases Society of America and did not participate in this study.
In a finding from an ancillary pilot experiment, the Hong Kong researchers found exhaled air from a patient with a moderate coronavirus load showed no evidence of the virus, whether the patient was breathing normally or heavily, speaking, or coughing. And spot tests around the room detected the virus in just one location.
“We may not be able to make a definite conclusion based on the analysis of a single patient,” the authors write. “However, it may help to reassure our staff that the exhaled air may be rapidly diluted inside the AIIR with 12 air changes per hour, or probably the SARS-CoV-2 may not be predominantly transmitted by [the] airborne route.”
However, a recent Singapore study showed widespread environmental contamination by SARS-CoV-2 through respiratory droplets and fecal shedding, underlining the need for strict adherence to environmental and hand hygiene. Post-cleaning samples tested negative, suggesting that standard decontamination practices are effective.
This work was partly supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases of the Department of Health, Hong Kong Special Administrative Region; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Ministry of Education of China. The authors and Glatt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hospital-related infections have been widely reported during the ongoing coronavirus outbreak, with healthcare professionals bearing a disproportionate risk. However, a proactive response in Hong Kong’s public hospital system appears to have bucked this trend and successfully protected both patients and staff from SARS-CoV-2, according to a study published online today in Infection Control & Hospital Epidemiology.
During the first 42 days of the outbreak, the 43 hospitals in the network tested 1275 suspected cases and treated 42 patients with confirmed COVID-19, the disease caused by SARS-CoV-2 infection. Yet, there were no nosocomial infections or infections among healthcare personnel, report Vincent C.C. Cheng, MD, FRCPath, the hospital’s infection control officer, and colleagues.
Cheng and colleagues note that 11 out of 413 healthcare workers who treat patients with confirmed infections had unprotected exposure and were in quarantine for 14 days, but none became ill.
In comparison, they note, the 2003 SARS outbreak saw almost 60% of nosocomial cases occurring in healthcare workers.
Proactive bundle
The Hong Kong success story may be due to a stepped-up proactive bundle of measures that included enhanced laboratory surveillance, early airborne infection isolation, and rapid-turnaround molecular diagnostics. Other strategies included staff forums and one-on-one discussions about infection control, employee training in protective equipment use, hand-hygiene compliance enforcement, and contact tracing for workers with unprotected exposure.
In addition, surgical masks were provided for all healthcare workers, patients, and visitors to clinical areas, a practice previously associated with reduced in-hospital transmission during influenza outbreaks, the authors note.
Hospitals also mandated use of personal protective equipment (PPE) for aerosol-generating procedures (AGPs), such as endotracheal intubation, open suctioning, and high-flow oxygen use, as AGPs had been linked to nosocomial transmission to healthcare workers during the 2003 SARS outbreak.
The infection control measures, which were part of a preparedness plan developed after the SARS outbreak, were initiated on December 31, when the first reports of a cluster of infections came from Wuhan, China.
As the outbreak evolved, the Hong Kong hospitals quickly widened the epidemiologic criteria for screening, from initially including only those who had been to a wet market in Wuhan within 14 days of symptom onset, to eventually including anyone who had been to Hubei province, been in a medical facility in mainland China, or in contact with a known case.
All suspected cases were sent to an airborne-infection isolation room (AIIR) or a ward with at least a meter of space between patients.
“Appropriate hospital infection control measures could prevent nosocomial transmission of SARS-CoV-2,” the authors write. “Vigilance in hand hygiene practice, wearing of surgical mask in the hospital, and appropriate use of PPE in patient care, especially [when] performing AGPs, are the key infection control measures to prevent nosocomial transmission of SARS-CoV-2 even before the availability of effective antiviral agents and vaccine.”
Asked for his perspective on the report, Aaron E. Glatt, MD, chairman of the department of medicine and chief of infectious diseases at Mount Sinai South Nassau in Oceanside, New York, said that apart from the widespread issuing of surgical masks to workers, patients, and visitors, the measures taken in Hong Kong are not different from standard infection-control practices in American hospitals. Glatt, who is also a hospital epidemiologist, said it was unclear how much impact the masks would have.
“Although the infection control was impressive, I don’t see any evidence of a difference in care,” he told Medscape Medical News.
Could zero infection transmission be achieved in the more far-flung and variable settings of hospitals across the United States? “The ability to get zero transmission is only possible if people adhere to the strictest infection-control guidelines,” Glatt said. “That is clearly the goal, and it will take time to see if our existing strict guidelines are sufficient to maintain zero or close to zero contamination and transmission rates in our hospitals.”
Rather than looking to change US practices, he stressed adherence to widely established tenets of care. “It’s critically important to keep paying close attention to the basics, to the simple blocking and tackling, and to identify which patients are at risk, and therefore, when workers need protective equipment,” he said.
“Follow the recommended standards,” continued Glatt, who is also a spokesperson for the Infectious Diseases Society of America and did not participate in this study.
In a finding from an ancillary pilot experiment, the Hong Kong researchers found exhaled air from a patient with a moderate coronavirus load showed no evidence of the virus, whether the patient was breathing normally or heavily, speaking, or coughing. And spot tests around the room detected the virus in just one location.
“We may not be able to make a definite conclusion based on the analysis of a single patient,” the authors write. “However, it may help to reassure our staff that the exhaled air may be rapidly diluted inside the AIIR with 12 air changes per hour, or probably the SARS-CoV-2 may not be predominantly transmitted by [the] airborne route.”
However, a recent Singapore study showed widespread environmental contamination by SARS-CoV-2 through respiratory droplets and fecal shedding, underlining the need for strict adherence to environmental and hand hygiene. Post-cleaning samples tested negative, suggesting that standard decontamination practices are effective.
This work was partly supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases of the Department of Health, Hong Kong Special Administrative Region; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Ministry of Education of China. The authors and Glatt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hospital-related infections have been widely reported during the ongoing coronavirus outbreak, with healthcare professionals bearing a disproportionate risk. However, a proactive response in Hong Kong’s public hospital system appears to have bucked this trend and successfully protected both patients and staff from SARS-CoV-2, according to a study published online today in Infection Control & Hospital Epidemiology.
During the first 42 days of the outbreak, the 43 hospitals in the network tested 1275 suspected cases and treated 42 patients with confirmed COVID-19, the disease caused by SARS-CoV-2 infection. Yet, there were no nosocomial infections or infections among healthcare personnel, report Vincent C.C. Cheng, MD, FRCPath, the hospital’s infection control officer, and colleagues.
Cheng and colleagues note that 11 out of 413 healthcare workers who treat patients with confirmed infections had unprotected exposure and were in quarantine for 14 days, but none became ill.
In comparison, they note, the 2003 SARS outbreak saw almost 60% of nosocomial cases occurring in healthcare workers.
Proactive bundle
The Hong Kong success story may be due to a stepped-up proactive bundle of measures that included enhanced laboratory surveillance, early airborne infection isolation, and rapid-turnaround molecular diagnostics. Other strategies included staff forums and one-on-one discussions about infection control, employee training in protective equipment use, hand-hygiene compliance enforcement, and contact tracing for workers with unprotected exposure.
In addition, surgical masks were provided for all healthcare workers, patients, and visitors to clinical areas, a practice previously associated with reduced in-hospital transmission during influenza outbreaks, the authors note.
Hospitals also mandated use of personal protective equipment (PPE) for aerosol-generating procedures (AGPs), such as endotracheal intubation, open suctioning, and high-flow oxygen use, as AGPs had been linked to nosocomial transmission to healthcare workers during the 2003 SARS outbreak.
The infection control measures, which were part of a preparedness plan developed after the SARS outbreak, were initiated on December 31, when the first reports of a cluster of infections came from Wuhan, China.
As the outbreak evolved, the Hong Kong hospitals quickly widened the epidemiologic criteria for screening, from initially including only those who had been to a wet market in Wuhan within 14 days of symptom onset, to eventually including anyone who had been to Hubei province, been in a medical facility in mainland China, or in contact with a known case.
All suspected cases were sent to an airborne-infection isolation room (AIIR) or a ward with at least a meter of space between patients.
“Appropriate hospital infection control measures could prevent nosocomial transmission of SARS-CoV-2,” the authors write. “Vigilance in hand hygiene practice, wearing of surgical mask in the hospital, and appropriate use of PPE in patient care, especially [when] performing AGPs, are the key infection control measures to prevent nosocomial transmission of SARS-CoV-2 even before the availability of effective antiviral agents and vaccine.”
Asked for his perspective on the report, Aaron E. Glatt, MD, chairman of the department of medicine and chief of infectious diseases at Mount Sinai South Nassau in Oceanside, New York, said that apart from the widespread issuing of surgical masks to workers, patients, and visitors, the measures taken in Hong Kong are not different from standard infection-control practices in American hospitals. Glatt, who is also a hospital epidemiologist, said it was unclear how much impact the masks would have.
“Although the infection control was impressive, I don’t see any evidence of a difference in care,” he told Medscape Medical News.
Could zero infection transmission be achieved in the more far-flung and variable settings of hospitals across the United States? “The ability to get zero transmission is only possible if people adhere to the strictest infection-control guidelines,” Glatt said. “That is clearly the goal, and it will take time to see if our existing strict guidelines are sufficient to maintain zero or close to zero contamination and transmission rates in our hospitals.”
Rather than looking to change US practices, he stressed adherence to widely established tenets of care. “It’s critically important to keep paying close attention to the basics, to the simple blocking and tackling, and to identify which patients are at risk, and therefore, when workers need protective equipment,” he said.
“Follow the recommended standards,” continued Glatt, who is also a spokesperson for the Infectious Diseases Society of America and did not participate in this study.
In a finding from an ancillary pilot experiment, the Hong Kong researchers found exhaled air from a patient with a moderate coronavirus load showed no evidence of the virus, whether the patient was breathing normally or heavily, speaking, or coughing. And spot tests around the room detected the virus in just one location.
“We may not be able to make a definite conclusion based on the analysis of a single patient,” the authors write. “However, it may help to reassure our staff that the exhaled air may be rapidly diluted inside the AIIR with 12 air changes per hour, or probably the SARS-CoV-2 may not be predominantly transmitted by [the] airborne route.”
However, a recent Singapore study showed widespread environmental contamination by SARS-CoV-2 through respiratory droplets and fecal shedding, underlining the need for strict adherence to environmental and hand hygiene. Post-cleaning samples tested negative, suggesting that standard decontamination practices are effective.
This work was partly supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases of the Department of Health, Hong Kong Special Administrative Region; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Ministry of Education of China. The authors and Glatt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
CMS issues guidance on containing spread of coronavirus
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”