User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Simvastatin, atorvastatin cut mortality risk for sepsis patients
a large health care database review has determined.
Among almost 53,000 sepsis patients, those who had been taking simvastatin were 28% less likely to die within 30 days of a sepsis admission than were patients not taking a statin. Atorvastatin conferred a similar significant survival benefit, reducing the risk of death by 22%, Chien-Chang Lee, MD and his colleagues wrote in the April issue of the journal CHEST®.
The drugs also exert a direct antimicrobial effect, he asserted.
“Of note, simvastatin was shown by several reports to have the most potent antibacterial activity,” targeting both methicillin-resistant and -sensitive Staphylococcus aureus, as well as gram negative and positive bacteria.
Dr. Lee and his colleagues extracted mortality and statin prescription data from the Taiwan National Health Insurance Database from 2000-2011. They looked at 30- and 90-day mortality in 52,737 patients who developed sepsis; the statins of interest were atorvastatin, simvastatin, and rosuvastatin. Patients had to have been taking the medication for at least 30 days before sepsis onset to be included, and patients taking more than one statin were excluded from the analysis.
Patients were a mean of 69 years old. About half had a lower respiratory infection. The remainder had infections within the abdomen, the biliary or urinary tract, skin, or orthopedic infections. There were no significant differences in comorbidities or in other medications taken among the three statin groups or the nonusers.
Of the entire cohort, 17% died by 30 days and nearly 23% by 90 days. Compared with those who had never received a statin, the statin users were 12% less likely to die by 30 days (hazard ratio, 0.88). Mortality at 90 days was also decreased, when compared with nonusers (HR, 0.93).
Simvastatin demonstrated the greatest benefit, with a 28% decreased risk of 30-day mortality (HR, 0.72). Atorvastatin followed, with a 22% risk reduction (HR, 0.78). Rosuvastatin exerted a nonsignificant 13% benefit.
The authors then examined 90-day mortality risks for the patients with a propensity matching score using a subgroup comprising 536 simvastatin users, 536 atorvastatin users, and 536 rosuvastatin users. Simvastatin was associated with a 23% reduction in 30-day mortality risk (HR, 0.77) and atorvastatin with a 21% reduction (HR, 0.79), when compared with rosuvastatin.
Statins’ antimicrobial properties are probably partially caused by their inactivation of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway, Dr. Lee and his colleagues noted. In addition to being vital for cholesterol synthesis, this pathway “also contributes to the production of isoprenoids and lipid compounds that are essential for cell signaling and structure in the pathogen. Secondly, the chemical property of different types of statins may affect their targeting to bacteria. The lipophilic properties of simvastatin or atorvastatin may allow better binding to bacteria cell walls than the hydrophilic properties of rosuvastatin.”
The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
The statin-sepsis mortality link will probably never be definitively proven, but the study by Lee and colleagues gives us the best data so far on this intriguing connection, Steven Q. Simpson, MD and Joel D. Mermis, MD wrote in an accompanying editorial.
“It is unlikely that prospective randomized trials of statins for prevention of sepsis mortality will ever be undertaken, owing to the sheer number of patients that would require randomization in order to have adequate numbers who actually develop sepsis,” the colleagues wrote. “We believe that the next best thing to randomization and a prospective trial is exactly what the authors have done – identify a cohort, track them through time, even if nonconcurrently, and match cases to controls by propensity matching on important clinical characteristics.”
Nevertheless, the two said, “This brings us to one aspect of the study that leaves open a window for some doubt.”
Lee et al. extracted their data from a large national insurance claims database. These systems “are commonly believed to overestimate sepsis incidence,” Dr. Simpson and Dr. Mermis wrote. A 2009 U.S. study bore this out, they said. “That study showed that in the U.S in 2014, there were approximately 1.7 million cases of sepsis in a population of 330 million, for an annual incidence rate of five sepsis cases per 1,000 patient-years.”
However, a “quick calculation” of the Taiwan data suggests that the annual sepsis caseload is about 5,200 per year in a population of 23 million at risk – an annual incidence of only 0.2 cases per 1,000 patient-years.
“This represents an order of magnitude difference in sepsis incidence between the U.S. and Taiwan, providing some issues to ponder. Does Taiwan indeed have a lower incidence of sepsis by that much? If so, is the lower incidence related to genetics, environment, health care access, or other factors?
“Although Lee et al. have provided us with data of the highest quality that we can likely hope for, the book may not be quite closed, yet.”
Dr. Mermis and Dr. Simpson are pulmonologists at the University of Kansas, Kansas City. They made their comments in an editorial published in the April issue of CHEST® (Mermis JD and Simpson SQ. CHEST. 2018 April. doi: 10.1016/j.chest.2017.12.004.)
The statin-sepsis mortality link will probably never be definitively proven, but the study by Lee and colleagues gives us the best data so far on this intriguing connection, Steven Q. Simpson, MD and Joel D. Mermis, MD wrote in an accompanying editorial.
“It is unlikely that prospective randomized trials of statins for prevention of sepsis mortality will ever be undertaken, owing to the sheer number of patients that would require randomization in order to have adequate numbers who actually develop sepsis,” the colleagues wrote. “We believe that the next best thing to randomization and a prospective trial is exactly what the authors have done – identify a cohort, track them through time, even if nonconcurrently, and match cases to controls by propensity matching on important clinical characteristics.”
Nevertheless, the two said, “This brings us to one aspect of the study that leaves open a window for some doubt.”
Lee et al. extracted their data from a large national insurance claims database. These systems “are commonly believed to overestimate sepsis incidence,” Dr. Simpson and Dr. Mermis wrote. A 2009 U.S. study bore this out, they said. “That study showed that in the U.S in 2014, there were approximately 1.7 million cases of sepsis in a population of 330 million, for an annual incidence rate of five sepsis cases per 1,000 patient-years.”
However, a “quick calculation” of the Taiwan data suggests that the annual sepsis caseload is about 5,200 per year in a population of 23 million at risk – an annual incidence of only 0.2 cases per 1,000 patient-years.
“This represents an order of magnitude difference in sepsis incidence between the U.S. and Taiwan, providing some issues to ponder. Does Taiwan indeed have a lower incidence of sepsis by that much? If so, is the lower incidence related to genetics, environment, health care access, or other factors?
“Although Lee et al. have provided us with data of the highest quality that we can likely hope for, the book may not be quite closed, yet.”
Dr. Mermis and Dr. Simpson are pulmonologists at the University of Kansas, Kansas City. They made their comments in an editorial published in the April issue of CHEST® (Mermis JD and Simpson SQ. CHEST. 2018 April. doi: 10.1016/j.chest.2017.12.004.)
The statin-sepsis mortality link will probably never be definitively proven, but the study by Lee and colleagues gives us the best data so far on this intriguing connection, Steven Q. Simpson, MD and Joel D. Mermis, MD wrote in an accompanying editorial.
“It is unlikely that prospective randomized trials of statins for prevention of sepsis mortality will ever be undertaken, owing to the sheer number of patients that would require randomization in order to have adequate numbers who actually develop sepsis,” the colleagues wrote. “We believe that the next best thing to randomization and a prospective trial is exactly what the authors have done – identify a cohort, track them through time, even if nonconcurrently, and match cases to controls by propensity matching on important clinical characteristics.”
Nevertheless, the two said, “This brings us to one aspect of the study that leaves open a window for some doubt.”
Lee et al. extracted their data from a large national insurance claims database. These systems “are commonly believed to overestimate sepsis incidence,” Dr. Simpson and Dr. Mermis wrote. A 2009 U.S. study bore this out, they said. “That study showed that in the U.S in 2014, there were approximately 1.7 million cases of sepsis in a population of 330 million, for an annual incidence rate of five sepsis cases per 1,000 patient-years.”
However, a “quick calculation” of the Taiwan data suggests that the annual sepsis caseload is about 5,200 per year in a population of 23 million at risk – an annual incidence of only 0.2 cases per 1,000 patient-years.
“This represents an order of magnitude difference in sepsis incidence between the U.S. and Taiwan, providing some issues to ponder. Does Taiwan indeed have a lower incidence of sepsis by that much? If so, is the lower incidence related to genetics, environment, health care access, or other factors?
“Although Lee et al. have provided us with data of the highest quality that we can likely hope for, the book may not be quite closed, yet.”
Dr. Mermis and Dr. Simpson are pulmonologists at the University of Kansas, Kansas City. They made their comments in an editorial published in the April issue of CHEST® (Mermis JD and Simpson SQ. CHEST. 2018 April. doi: 10.1016/j.chest.2017.12.004.)
a large health care database review has determined.
Among almost 53,000 sepsis patients, those who had been taking simvastatin were 28% less likely to die within 30 days of a sepsis admission than were patients not taking a statin. Atorvastatin conferred a similar significant survival benefit, reducing the risk of death by 22%, Chien-Chang Lee, MD and his colleagues wrote in the April issue of the journal CHEST®.
The drugs also exert a direct antimicrobial effect, he asserted.
“Of note, simvastatin was shown by several reports to have the most potent antibacterial activity,” targeting both methicillin-resistant and -sensitive Staphylococcus aureus, as well as gram negative and positive bacteria.
Dr. Lee and his colleagues extracted mortality and statin prescription data from the Taiwan National Health Insurance Database from 2000-2011. They looked at 30- and 90-day mortality in 52,737 patients who developed sepsis; the statins of interest were atorvastatin, simvastatin, and rosuvastatin. Patients had to have been taking the medication for at least 30 days before sepsis onset to be included, and patients taking more than one statin were excluded from the analysis.
Patients were a mean of 69 years old. About half had a lower respiratory infection. The remainder had infections within the abdomen, the biliary or urinary tract, skin, or orthopedic infections. There were no significant differences in comorbidities or in other medications taken among the three statin groups or the nonusers.
Of the entire cohort, 17% died by 30 days and nearly 23% by 90 days. Compared with those who had never received a statin, the statin users were 12% less likely to die by 30 days (hazard ratio, 0.88). Mortality at 90 days was also decreased, when compared with nonusers (HR, 0.93).
Simvastatin demonstrated the greatest benefit, with a 28% decreased risk of 30-day mortality (HR, 0.72). Atorvastatin followed, with a 22% risk reduction (HR, 0.78). Rosuvastatin exerted a nonsignificant 13% benefit.
The authors then examined 90-day mortality risks for the patients with a propensity matching score using a subgroup comprising 536 simvastatin users, 536 atorvastatin users, and 536 rosuvastatin users. Simvastatin was associated with a 23% reduction in 30-day mortality risk (HR, 0.77) and atorvastatin with a 21% reduction (HR, 0.79), when compared with rosuvastatin.
Statins’ antimicrobial properties are probably partially caused by their inactivation of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway, Dr. Lee and his colleagues noted. In addition to being vital for cholesterol synthesis, this pathway “also contributes to the production of isoprenoids and lipid compounds that are essential for cell signaling and structure in the pathogen. Secondly, the chemical property of different types of statins may affect their targeting to bacteria. The lipophilic properties of simvastatin or atorvastatin may allow better binding to bacteria cell walls than the hydrophilic properties of rosuvastatin.”
The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
a large health care database review has determined.
Among almost 53,000 sepsis patients, those who had been taking simvastatin were 28% less likely to die within 30 days of a sepsis admission than were patients not taking a statin. Atorvastatin conferred a similar significant survival benefit, reducing the risk of death by 22%, Chien-Chang Lee, MD and his colleagues wrote in the April issue of the journal CHEST®.
The drugs also exert a direct antimicrobial effect, he asserted.
“Of note, simvastatin was shown by several reports to have the most potent antibacterial activity,” targeting both methicillin-resistant and -sensitive Staphylococcus aureus, as well as gram negative and positive bacteria.
Dr. Lee and his colleagues extracted mortality and statin prescription data from the Taiwan National Health Insurance Database from 2000-2011. They looked at 30- and 90-day mortality in 52,737 patients who developed sepsis; the statins of interest were atorvastatin, simvastatin, and rosuvastatin. Patients had to have been taking the medication for at least 30 days before sepsis onset to be included, and patients taking more than one statin were excluded from the analysis.
Patients were a mean of 69 years old. About half had a lower respiratory infection. The remainder had infections within the abdomen, the biliary or urinary tract, skin, or orthopedic infections. There were no significant differences in comorbidities or in other medications taken among the three statin groups or the nonusers.
Of the entire cohort, 17% died by 30 days and nearly 23% by 90 days. Compared with those who had never received a statin, the statin users were 12% less likely to die by 30 days (hazard ratio, 0.88). Mortality at 90 days was also decreased, when compared with nonusers (HR, 0.93).
Simvastatin demonstrated the greatest benefit, with a 28% decreased risk of 30-day mortality (HR, 0.72). Atorvastatin followed, with a 22% risk reduction (HR, 0.78). Rosuvastatin exerted a nonsignificant 13% benefit.
The authors then examined 90-day mortality risks for the patients with a propensity matching score using a subgroup comprising 536 simvastatin users, 536 atorvastatin users, and 536 rosuvastatin users. Simvastatin was associated with a 23% reduction in 30-day mortality risk (HR, 0.77) and atorvastatin with a 21% reduction (HR, 0.79), when compared with rosuvastatin.
Statins’ antimicrobial properties are probably partially caused by their inactivation of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway, Dr. Lee and his colleagues noted. In addition to being vital for cholesterol synthesis, this pathway “also contributes to the production of isoprenoids and lipid compounds that are essential for cell signaling and structure in the pathogen. Secondly, the chemical property of different types of statins may affect their targeting to bacteria. The lipophilic properties of simvastatin or atorvastatin may allow better binding to bacteria cell walls than the hydrophilic properties of rosuvastatin.”
The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
FROM CHEST
Key clinical point: Simvastatin and atorvastatin were associated with decreased mortality risk among sepsis patients.
Major finding: Compared with those not taking the drugs, those taking simvastatin were 28% less likely to die by 30 days, and those taking atorvastatin were 22% less likely.
Study details: The database study comprised almost 54,000 sepsis cases over 11 years.
Disclosures: The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
Source: Lee C-C et al. CHEST. 2018 April;153(4):769-70.
Use of a risk score may be able to identify high-risk patients presenting with acute heart failure
Clinical question: Can we use readily available data to risk stratify patients who present to the emergency department in acute heart failure (AHF)?
Background: Although cardiac biomarkers such as troponin and B-natriuretic peptide have general prognostic value in patients with AHF presenting to the emergency department, these values do not reliably aid in determining patients’ risk for unfavorable outcomes at the time of clinical decision making. Currently available published scores for risk-stratifying patients with AHF in the ED have limited applicability.
Setting: The registry included patients from 34 different hospitals in Spain.
Synopsis: This study analyzed clinical variables from a cohort of 4,897 AHF patients to determine predictors of patient outcomes. Thirteen clinical variables were identified as independent predictors of 30-day mortality and were incorporated into a risk score calculator (MEESSI-AHF). The risk score includes variables such as vital signs, age, labs values, and performance status. A second cohort of 3,229 patients were used to validate the risk score. The risk score effectively discriminates patients into low-, intermediate-, and high-risk patients. One important limitation is a high number of missing values in derivation cohort that required advanced statistics to overcome. The generalizability of the population studies (Spanish population) to other countries is still unclear. A risk score that can reasonably identify low-risk patients may be the most clinically useful in order to identify patients that either can be treated effectively in the emergency department and may not warrant inpatient admission.
Bottom line: The MEESSI-AHF risk score may be a helpful tool in identifying the risk of 30-day mortality in patients who present to the ED with AHF, but it is currently unclear if the score can be generalized to other populations.
Citation: Miro O et al. Predicting 30-day mortality for patients with acute heart failure in the emergency department: A cohort study. Ann Intern Med. 2017 Nov 21;167(10):698-705.
Dr. Maleque is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Clinical question: Can we use readily available data to risk stratify patients who present to the emergency department in acute heart failure (AHF)?
Background: Although cardiac biomarkers such as troponin and B-natriuretic peptide have general prognostic value in patients with AHF presenting to the emergency department, these values do not reliably aid in determining patients’ risk for unfavorable outcomes at the time of clinical decision making. Currently available published scores for risk-stratifying patients with AHF in the ED have limited applicability.
Setting: The registry included patients from 34 different hospitals in Spain.
Synopsis: This study analyzed clinical variables from a cohort of 4,897 AHF patients to determine predictors of patient outcomes. Thirteen clinical variables were identified as independent predictors of 30-day mortality and were incorporated into a risk score calculator (MEESSI-AHF). The risk score includes variables such as vital signs, age, labs values, and performance status. A second cohort of 3,229 patients were used to validate the risk score. The risk score effectively discriminates patients into low-, intermediate-, and high-risk patients. One important limitation is a high number of missing values in derivation cohort that required advanced statistics to overcome. The generalizability of the population studies (Spanish population) to other countries is still unclear. A risk score that can reasonably identify low-risk patients may be the most clinically useful in order to identify patients that either can be treated effectively in the emergency department and may not warrant inpatient admission.
Bottom line: The MEESSI-AHF risk score may be a helpful tool in identifying the risk of 30-day mortality in patients who present to the ED with AHF, but it is currently unclear if the score can be generalized to other populations.
Citation: Miro O et al. Predicting 30-day mortality for patients with acute heart failure in the emergency department: A cohort study. Ann Intern Med. 2017 Nov 21;167(10):698-705.
Dr. Maleque is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Clinical question: Can we use readily available data to risk stratify patients who present to the emergency department in acute heart failure (AHF)?
Background: Although cardiac biomarkers such as troponin and B-natriuretic peptide have general prognostic value in patients with AHF presenting to the emergency department, these values do not reliably aid in determining patients’ risk for unfavorable outcomes at the time of clinical decision making. Currently available published scores for risk-stratifying patients with AHF in the ED have limited applicability.
Setting: The registry included patients from 34 different hospitals in Spain.
Synopsis: This study analyzed clinical variables from a cohort of 4,897 AHF patients to determine predictors of patient outcomes. Thirteen clinical variables were identified as independent predictors of 30-day mortality and were incorporated into a risk score calculator (MEESSI-AHF). The risk score includes variables such as vital signs, age, labs values, and performance status. A second cohort of 3,229 patients were used to validate the risk score. The risk score effectively discriminates patients into low-, intermediate-, and high-risk patients. One important limitation is a high number of missing values in derivation cohort that required advanced statistics to overcome. The generalizability of the population studies (Spanish population) to other countries is still unclear. A risk score that can reasonably identify low-risk patients may be the most clinically useful in order to identify patients that either can be treated effectively in the emergency department and may not warrant inpatient admission.
Bottom line: The MEESSI-AHF risk score may be a helpful tool in identifying the risk of 30-day mortality in patients who present to the ED with AHF, but it is currently unclear if the score can be generalized to other populations.
Citation: Miro O et al. Predicting 30-day mortality for patients with acute heart failure in the emergency department: A cohort study. Ann Intern Med. 2017 Nov 21;167(10):698-705.
Dr. Maleque is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Video: SHM President Nasim Afsar seeks an “unrelenting focus on delivering value”
ORLANDO – In a video interview, Nasim Afsar, MD, SFHM, details the career road that led her to the “tremendous honor” of becoming president of the Society of Hospital Medicine.
Having been on the board of directors for 6 years was a profound experience, according to Dr. Afsar, and now as president she looks to take what she has learned and focus on the future of the field.
When asked about her overall vision for the coming year for the Society, Dr. Afsar said that she is committed to “an unrelenting focus on delivering value to our patients, our institutions, and society, and the way we do that is through population health management.”
ORLANDO – In a video interview, Nasim Afsar, MD, SFHM, details the career road that led her to the “tremendous honor” of becoming president of the Society of Hospital Medicine.
Having been on the board of directors for 6 years was a profound experience, according to Dr. Afsar, and now as president she looks to take what she has learned and focus on the future of the field.
When asked about her overall vision for the coming year for the Society, Dr. Afsar said that she is committed to “an unrelenting focus on delivering value to our patients, our institutions, and society, and the way we do that is through population health management.”
ORLANDO – In a video interview, Nasim Afsar, MD, SFHM, details the career road that led her to the “tremendous honor” of becoming president of the Society of Hospital Medicine.
Having been on the board of directors for 6 years was a profound experience, according to Dr. Afsar, and now as president she looks to take what she has learned and focus on the future of the field.
When asked about her overall vision for the coming year for the Society, Dr. Afsar said that she is committed to “an unrelenting focus on delivering value to our patients, our institutions, and society, and the way we do that is through population health management.”
REPORTING FROM HOSPITAL MEDICINE 2018
Two scoring systems helpful in diagnosing heparin-induced thrombocytopenia
SAN DIEGO – Both the 4Ts Score and the HIT Expert Probability (HEP) Score are useful in clinical practice for the diagnosis of heparin-induced thrombocytopenia, but the HEP score may have better operative characteristics in ICU patients, results from a “real world” analysis showed.
“The diagnosis of heparin-induced thrombocytopenia (HIT) is challenging,” Allyson M. Pishko, MD, one of the study authors, said at the biennial summit of the Thrombosis & Hemostasis Societies of North America. “The 4Ts Score is commonly used, but limitations include its low positive predictive value and significant interobserver variability.”
One external prospective study showed operating characteristics similar to those of 4Ts scores (Thromb Haemost 2015;113[3]:633-40).
The aim of the current study was to validate the HEP Score in a “real world” setting and to compare the performance of the HEP Score versus the 4Ts Score. The researchers enrolled 292 adults with suspected acute HIT who were hospitalized at the University of Pennsylvania or affiliated community hospitals, and who had HIT laboratory testing ordered.
The HEP Score and the 4Ts Score were calculated by a member of the clinical team and were completed prior to return of the HIT lab test result. The majority of scorers (62%) were hematology fellows, followed by attendings (35%), and residents/students (3%). All patients underwent testing with an HIT ELISA and serotonin-release assay (SRA). Patients in whom the optical density of the ELISA was less than 0.4 units were classified as not having HIT. The researchers used the Wilcoxon rank-sum test to compare HEP and 4Ts Scores in patients with and without HIT.
Of the 292 patients, 209 were HIT negative and 83 had their data reviewed by an expert panel. Of these 83 patients, 40 were found to be HIT negative and 43 were HIT positive, and their mean ages were 65 years and 63 years, respectively. Among the cases found to be positive for HIT, 93% had HIT ELISA optical density of 1 or greater and 69.7% were SRA positive. The median HEP Score in patients with and without HIT was 8 versus 5 (P less than .0001).
At the prespecified screening cut-off of 2 or more points, the HEP Score was 97.7% sensitive and 21.9% specific, with a positive predictive value of 17.7% and a negative predictive value of 98.2%. A cut-off of 5 or greater provided 90.7% sensitivity and 47.8% specificity with a positive predictive value of 23.1% and a negative predictive value of 96.8%. The mean time to calculate the HEP Score was 4.1 minutes.
The median 4Ts Score in patients with and without HIT was 5 versus 4 (P less than .0001), Dr. Pishko reported. A 4Ts Score of 4 or greater had a sensitivity of 97.7% and specificity of 32.9%, with a positive predictive value of 20.1% and a negative predictive value of 98.8%.
The area under the ROC curves for the HEP Score and 4Ts Score were similar (0.81 vs. 0.76; P = .121). Subset analysis revealed that compared with the 4Ts Score, the HEP Score had better operating characteristics in ICU patients (AUC 0.87 vs. 0.79; P= .029) and with trainee scorers (AUC 0.79 vs. 0.73; P = .032).
“Our data suggest that either the HEP Score or the 4Ts Score could be used in clinical practice,” Dr. Pishko said.
The National Institutes of Health funded the study. Dr. Pishko reported having no financial disclosures.
SOURCE: Pishko A et al. THSNA 2018.
SAN DIEGO – Both the 4Ts Score and the HIT Expert Probability (HEP) Score are useful in clinical practice for the diagnosis of heparin-induced thrombocytopenia, but the HEP score may have better operative characteristics in ICU patients, results from a “real world” analysis showed.
“The diagnosis of heparin-induced thrombocytopenia (HIT) is challenging,” Allyson M. Pishko, MD, one of the study authors, said at the biennial summit of the Thrombosis & Hemostasis Societies of North America. “The 4Ts Score is commonly used, but limitations include its low positive predictive value and significant interobserver variability.”
One external prospective study showed operating characteristics similar to those of 4Ts scores (Thromb Haemost 2015;113[3]:633-40).
The aim of the current study was to validate the HEP Score in a “real world” setting and to compare the performance of the HEP Score versus the 4Ts Score. The researchers enrolled 292 adults with suspected acute HIT who were hospitalized at the University of Pennsylvania or affiliated community hospitals, and who had HIT laboratory testing ordered.
The HEP Score and the 4Ts Score were calculated by a member of the clinical team and were completed prior to return of the HIT lab test result. The majority of scorers (62%) were hematology fellows, followed by attendings (35%), and residents/students (3%). All patients underwent testing with an HIT ELISA and serotonin-release assay (SRA). Patients in whom the optical density of the ELISA was less than 0.4 units were classified as not having HIT. The researchers used the Wilcoxon rank-sum test to compare HEP and 4Ts Scores in patients with and without HIT.
Of the 292 patients, 209 were HIT negative and 83 had their data reviewed by an expert panel. Of these 83 patients, 40 were found to be HIT negative and 43 were HIT positive, and their mean ages were 65 years and 63 years, respectively. Among the cases found to be positive for HIT, 93% had HIT ELISA optical density of 1 or greater and 69.7% were SRA positive. The median HEP Score in patients with and without HIT was 8 versus 5 (P less than .0001).
At the prespecified screening cut-off of 2 or more points, the HEP Score was 97.7% sensitive and 21.9% specific, with a positive predictive value of 17.7% and a negative predictive value of 98.2%. A cut-off of 5 or greater provided 90.7% sensitivity and 47.8% specificity with a positive predictive value of 23.1% and a negative predictive value of 96.8%. The mean time to calculate the HEP Score was 4.1 minutes.
The median 4Ts Score in patients with and without HIT was 5 versus 4 (P less than .0001), Dr. Pishko reported. A 4Ts Score of 4 or greater had a sensitivity of 97.7% and specificity of 32.9%, with a positive predictive value of 20.1% and a negative predictive value of 98.8%.
The area under the ROC curves for the HEP Score and 4Ts Score were similar (0.81 vs. 0.76; P = .121). Subset analysis revealed that compared with the 4Ts Score, the HEP Score had better operating characteristics in ICU patients (AUC 0.87 vs. 0.79; P= .029) and with trainee scorers (AUC 0.79 vs. 0.73; P = .032).
“Our data suggest that either the HEP Score or the 4Ts Score could be used in clinical practice,” Dr. Pishko said.
The National Institutes of Health funded the study. Dr. Pishko reported having no financial disclosures.
SOURCE: Pishko A et al. THSNA 2018.
SAN DIEGO – Both the 4Ts Score and the HIT Expert Probability (HEP) Score are useful in clinical practice for the diagnosis of heparin-induced thrombocytopenia, but the HEP score may have better operative characteristics in ICU patients, results from a “real world” analysis showed.
“The diagnosis of heparin-induced thrombocytopenia (HIT) is challenging,” Allyson M. Pishko, MD, one of the study authors, said at the biennial summit of the Thrombosis & Hemostasis Societies of North America. “The 4Ts Score is commonly used, but limitations include its low positive predictive value and significant interobserver variability.”
One external prospective study showed operating characteristics similar to those of 4Ts scores (Thromb Haemost 2015;113[3]:633-40).
The aim of the current study was to validate the HEP Score in a “real world” setting and to compare the performance of the HEP Score versus the 4Ts Score. The researchers enrolled 292 adults with suspected acute HIT who were hospitalized at the University of Pennsylvania or affiliated community hospitals, and who had HIT laboratory testing ordered.
The HEP Score and the 4Ts Score were calculated by a member of the clinical team and were completed prior to return of the HIT lab test result. The majority of scorers (62%) were hematology fellows, followed by attendings (35%), and residents/students (3%). All patients underwent testing with an HIT ELISA and serotonin-release assay (SRA). Patients in whom the optical density of the ELISA was less than 0.4 units were classified as not having HIT. The researchers used the Wilcoxon rank-sum test to compare HEP and 4Ts Scores in patients with and without HIT.
Of the 292 patients, 209 were HIT negative and 83 had their data reviewed by an expert panel. Of these 83 patients, 40 were found to be HIT negative and 43 were HIT positive, and their mean ages were 65 years and 63 years, respectively. Among the cases found to be positive for HIT, 93% had HIT ELISA optical density of 1 or greater and 69.7% were SRA positive. The median HEP Score in patients with and without HIT was 8 versus 5 (P less than .0001).
At the prespecified screening cut-off of 2 or more points, the HEP Score was 97.7% sensitive and 21.9% specific, with a positive predictive value of 17.7% and a negative predictive value of 98.2%. A cut-off of 5 or greater provided 90.7% sensitivity and 47.8% specificity with a positive predictive value of 23.1% and a negative predictive value of 96.8%. The mean time to calculate the HEP Score was 4.1 minutes.
The median 4Ts Score in patients with and without HIT was 5 versus 4 (P less than .0001), Dr. Pishko reported. A 4Ts Score of 4 or greater had a sensitivity of 97.7% and specificity of 32.9%, with a positive predictive value of 20.1% and a negative predictive value of 98.8%.
The area under the ROC curves for the HEP Score and 4Ts Score were similar (0.81 vs. 0.76; P = .121). Subset analysis revealed that compared with the 4Ts Score, the HEP Score had better operating characteristics in ICU patients (AUC 0.87 vs. 0.79; P= .029) and with trainee scorers (AUC 0.79 vs. 0.73; P = .032).
“Our data suggest that either the HEP Score or the 4Ts Score could be used in clinical practice,” Dr. Pishko said.
The National Institutes of Health funded the study. Dr. Pishko reported having no financial disclosures.
SOURCE: Pishko A et al. THSNA 2018.
REPORTING FROM THSNA 2018
Key clinical point:
Major finding: The area under the ROC curves for the HEP Score and 4Ts Score were similar (0.81 vs. 0.76; P = .121).
Study details: A prospective study of 292 adults with suspected acute HIT who were hospitalized at the University of Pennsylvania or affiliated community hospitals.
Disclosures: The National Institutes of Health funded the study. Dr. Pishko reported having no financial disclosures.
Source: Pishko A et al. THSNA 2018.
Boosting bedside skills in hands-on session
A low faculty-to-learner ratio helped HM18 attendees get the most from their learning experience in the Sunday pre-conference course “Bedside Procedures for the Hospitalist.”
The pre-course blended live didactic teaching and hands-on training with simulators so participants could not only learn but also review and demonstrate techniques for many common invasive procedures hospitalists encounter in practice.
“Our goal is to make the entire bedside procedures pre-course a unique experience,” course codirector Alyssa Burkhart, MD, of the Billings (Mont.) Clinic, said in an interview before the session.
“We carefully select the curriculum to create a program most relevant to the participants and their day-to-day work in patient care,” said Dr. Burkhart.
“The low faculty-to-learner ratio coupled with ample time to practice under expert guidance separates us from others. ... It’s a privilege to share our love of procedures with this year’s SHM participants,” said Dr. Burkhart, who comoderated the session with Joshua Lenchus, DO, SFHM, of the University of Miami.
An interactive focus on bedside procedures benefits novices and experienced clinicians, said Dr. Lenchus.
The simulation experience involved practice with ultrasound as well as anatomically representative training equipment.
“Our hope is that many hospitalists may once again find that spark of interest in performing more of their own procedures. The interactive sessions embedded within the pre-course are vital to the success of our program. Many other training sessions are didactics based. We strive to keep lecture time to a minimum so that small groups can learn from the expert facilitators,” Dr. Burkhart added.
“Ample hands-on practice time, interactive experience, and direct supervision separate our pre-course from other commercially available offerings,” Dr. Lenchus said.
The agenda kicked off with vascular and intraosseous access in the morning, followed by paracentesis, thoracentesis, lumbar puncture, and basic airway management, including the use of supraglottic devices.
Dr. Burkhart noted that the course included two separate practice sessions for vascular access because of the number of technical steps and potential complications. “Attendees typically wish to spend a considerable amount of time on vascular access,” she said. “The intraosseous access station and its exceptional trainers always receive very positive feedback.”
Dr. Burkhart and Dr. Lenchus had no financial conflicts to disclose.
A low faculty-to-learner ratio helped HM18 attendees get the most from their learning experience in the Sunday pre-conference course “Bedside Procedures for the Hospitalist.”
The pre-course blended live didactic teaching and hands-on training with simulators so participants could not only learn but also review and demonstrate techniques for many common invasive procedures hospitalists encounter in practice.
“Our goal is to make the entire bedside procedures pre-course a unique experience,” course codirector Alyssa Burkhart, MD, of the Billings (Mont.) Clinic, said in an interview before the session.
“We carefully select the curriculum to create a program most relevant to the participants and their day-to-day work in patient care,” said Dr. Burkhart.
“The low faculty-to-learner ratio coupled with ample time to practice under expert guidance separates us from others. ... It’s a privilege to share our love of procedures with this year’s SHM participants,” said Dr. Burkhart, who comoderated the session with Joshua Lenchus, DO, SFHM, of the University of Miami.
An interactive focus on bedside procedures benefits novices and experienced clinicians, said Dr. Lenchus.
The simulation experience involved practice with ultrasound as well as anatomically representative training equipment.
“Our hope is that many hospitalists may once again find that spark of interest in performing more of their own procedures. The interactive sessions embedded within the pre-course are vital to the success of our program. Many other training sessions are didactics based. We strive to keep lecture time to a minimum so that small groups can learn from the expert facilitators,” Dr. Burkhart added.
“Ample hands-on practice time, interactive experience, and direct supervision separate our pre-course from other commercially available offerings,” Dr. Lenchus said.
The agenda kicked off with vascular and intraosseous access in the morning, followed by paracentesis, thoracentesis, lumbar puncture, and basic airway management, including the use of supraglottic devices.
Dr. Burkhart noted that the course included two separate practice sessions for vascular access because of the number of technical steps and potential complications. “Attendees typically wish to spend a considerable amount of time on vascular access,” she said. “The intraosseous access station and its exceptional trainers always receive very positive feedback.”
Dr. Burkhart and Dr. Lenchus had no financial conflicts to disclose.
A low faculty-to-learner ratio helped HM18 attendees get the most from their learning experience in the Sunday pre-conference course “Bedside Procedures for the Hospitalist.”
The pre-course blended live didactic teaching and hands-on training with simulators so participants could not only learn but also review and demonstrate techniques for many common invasive procedures hospitalists encounter in practice.
“Our goal is to make the entire bedside procedures pre-course a unique experience,” course codirector Alyssa Burkhart, MD, of the Billings (Mont.) Clinic, said in an interview before the session.
“We carefully select the curriculum to create a program most relevant to the participants and their day-to-day work in patient care,” said Dr. Burkhart.
“The low faculty-to-learner ratio coupled with ample time to practice under expert guidance separates us from others. ... It’s a privilege to share our love of procedures with this year’s SHM participants,” said Dr. Burkhart, who comoderated the session with Joshua Lenchus, DO, SFHM, of the University of Miami.
An interactive focus on bedside procedures benefits novices and experienced clinicians, said Dr. Lenchus.
The simulation experience involved practice with ultrasound as well as anatomically representative training equipment.
“Our hope is that many hospitalists may once again find that spark of interest in performing more of their own procedures. The interactive sessions embedded within the pre-course are vital to the success of our program. Many other training sessions are didactics based. We strive to keep lecture time to a minimum so that small groups can learn from the expert facilitators,” Dr. Burkhart added.
“Ample hands-on practice time, interactive experience, and direct supervision separate our pre-course from other commercially available offerings,” Dr. Lenchus said.
The agenda kicked off with vascular and intraosseous access in the morning, followed by paracentesis, thoracentesis, lumbar puncture, and basic airway management, including the use of supraglottic devices.
Dr. Burkhart noted that the course included two separate practice sessions for vascular access because of the number of technical steps and potential complications. “Attendees typically wish to spend a considerable amount of time on vascular access,” she said. “The intraosseous access station and its exceptional trainers always receive very positive feedback.”
Dr. Burkhart and Dr. Lenchus had no financial conflicts to disclose.
Practical changes for improving practice management
An all-day HM18 pre-course – “Hospitalist Practice Management: How to Thrive in a Time of Intense Change” – for hospitalist leaders and practice administrators was all about practicality.
One of the goals of the session was to provide “quick, actionable interventions that attendees can implement right away, as well as alternatives for attendees to consider, which will require some work to employ,” said John Nelson, MD, MHM, a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif., the medical director of the Overlake Medical Center, Bellevue, Wash., and a course codirector and a faculty presenter.
Dr. Nelson pointed out that the hospitalist practice is a unique practice model. “We can’t effectively use the same approaches that other medical specialties use to ensure we have successful practices,” he said.
The pre-course, held Sunday before the official start of HM18, included more commentary and specifics than in past years about how to prosper in the rapidly changing health care landscape and how to reduce the chance of burnout.
Topics addressed included how to find, measure, and demonstrate value; how to incorporate different types of providers and clinical support staffing into a practice to support hospitalists; and how to recruit the right people and build a desirable culture.
The pre-course also covered effective roles for a variety of providers in a hospitalist group, including nurses, scribes, and coordinators, and delineated the benefits of providing telemedicine.
For group leaders and administrators in attendance, the session also shed light on how to interact with individual providers in their group and how to collaborate to build a healthy culture and practice, Ms. Flores said.
The day began with presentations that laid out valuable information and frameworks, including “A Tour of Survey Data: What It Does and Doesn’t Tell You” and “Defining and Measuring Value.” Sessions included six didactic lectures with a question-and-answer period, as well as what Dr. Nelson has dubbed “point/counterpoint” sessions in which faculty members debated particular issues, such as work scheduling models. During the last session, “Learning From Each Other,” participants shared with other attendees their own best practices in the areas covered.
Although there is no single best way to organize a hospitalist’s practice, the course provided lots of information and perspective to help listeners decide what is best for their practice.
“Even though we work in a stressful environment of constant change, hospitalists do have some control over their destiny, and there are things they can do to make hospitalist groups thrive in this challenging environment,” Ms. Flores concluded.
An all-day HM18 pre-course – “Hospitalist Practice Management: How to Thrive in a Time of Intense Change” – for hospitalist leaders and practice administrators was all about practicality.
One of the goals of the session was to provide “quick, actionable interventions that attendees can implement right away, as well as alternatives for attendees to consider, which will require some work to employ,” said John Nelson, MD, MHM, a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif., the medical director of the Overlake Medical Center, Bellevue, Wash., and a course codirector and a faculty presenter.
Dr. Nelson pointed out that the hospitalist practice is a unique practice model. “We can’t effectively use the same approaches that other medical specialties use to ensure we have successful practices,” he said.
The pre-course, held Sunday before the official start of HM18, included more commentary and specifics than in past years about how to prosper in the rapidly changing health care landscape and how to reduce the chance of burnout.
Topics addressed included how to find, measure, and demonstrate value; how to incorporate different types of providers and clinical support staffing into a practice to support hospitalists; and how to recruit the right people and build a desirable culture.
The pre-course also covered effective roles for a variety of providers in a hospitalist group, including nurses, scribes, and coordinators, and delineated the benefits of providing telemedicine.
For group leaders and administrators in attendance, the session also shed light on how to interact with individual providers in their group and how to collaborate to build a healthy culture and practice, Ms. Flores said.
The day began with presentations that laid out valuable information and frameworks, including “A Tour of Survey Data: What It Does and Doesn’t Tell You” and “Defining and Measuring Value.” Sessions included six didactic lectures with a question-and-answer period, as well as what Dr. Nelson has dubbed “point/counterpoint” sessions in which faculty members debated particular issues, such as work scheduling models. During the last session, “Learning From Each Other,” participants shared with other attendees their own best practices in the areas covered.
Although there is no single best way to organize a hospitalist’s practice, the course provided lots of information and perspective to help listeners decide what is best for their practice.
“Even though we work in a stressful environment of constant change, hospitalists do have some control over their destiny, and there are things they can do to make hospitalist groups thrive in this challenging environment,” Ms. Flores concluded.
An all-day HM18 pre-course – “Hospitalist Practice Management: How to Thrive in a Time of Intense Change” – for hospitalist leaders and practice administrators was all about practicality.
One of the goals of the session was to provide “quick, actionable interventions that attendees can implement right away, as well as alternatives for attendees to consider, which will require some work to employ,” said John Nelson, MD, MHM, a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif., the medical director of the Overlake Medical Center, Bellevue, Wash., and a course codirector and a faculty presenter.
Dr. Nelson pointed out that the hospitalist practice is a unique practice model. “We can’t effectively use the same approaches that other medical specialties use to ensure we have successful practices,” he said.
The pre-course, held Sunday before the official start of HM18, included more commentary and specifics than in past years about how to prosper in the rapidly changing health care landscape and how to reduce the chance of burnout.
Topics addressed included how to find, measure, and demonstrate value; how to incorporate different types of providers and clinical support staffing into a practice to support hospitalists; and how to recruit the right people and build a desirable culture.
The pre-course also covered effective roles for a variety of providers in a hospitalist group, including nurses, scribes, and coordinators, and delineated the benefits of providing telemedicine.
For group leaders and administrators in attendance, the session also shed light on how to interact with individual providers in their group and how to collaborate to build a healthy culture and practice, Ms. Flores said.
The day began with presentations that laid out valuable information and frameworks, including “A Tour of Survey Data: What It Does and Doesn’t Tell You” and “Defining and Measuring Value.” Sessions included six didactic lectures with a question-and-answer period, as well as what Dr. Nelson has dubbed “point/counterpoint” sessions in which faculty members debated particular issues, such as work scheduling models. During the last session, “Learning From Each Other,” participants shared with other attendees their own best practices in the areas covered.
Although there is no single best way to organize a hospitalist’s practice, the course provided lots of information and perspective to help listeners decide what is best for their practice.
“Even though we work in a stressful environment of constant change, hospitalists do have some control over their destiny, and there are things they can do to make hospitalist groups thrive in this challenging environment,” Ms. Flores concluded.
Journal of Hospital Medicine releases consensus statement on inpatient opioid prescribing
The Journal of Hospital Medicine, the official peer-reviewed journal of the Society of Hospital Medicine, has released a statement titled “Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement from the Society of Hospital Medicine” in response to the growing issue of managing opioid prescribing in the inpatient setting.
The statement offers 16 recommendations covering whether to use opioids in the hospital and how to improve the safety of opioid prescribing both during hospitalization and at discharge. The statement is available in the April 2018 issue of the journal.
SHM convened a working group to develop the consensus statement, intended for clinicians practicing in the inpatient setting. The development of the statement began with the working group conducting a systemic review of relevant guidelines and composing a draft based on extracted recommendations. The working group then obtained feedback from external experts in hospital-based opioid prescribing, SHM members, the SHM Patient-Family Advisory Council, other professional societies and peer reviewers.
The statement reads, “Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.”
“The journal is always pleased to be able to publish results of rigorous and innovative work, and the consensus document authored by Dr. Herzig and her team represents an outstanding example,” said Andrew Auerbach, MD, MPH, MHM, Professor of Medicine in Residence at the University of California, San Francisco, and editor in chief for the Journal of Hospital Medicine. “As we enter the ‘post-opioid’ era of pain management, papers like these will provide critical guidance for how to improve pain control among hospitalized patients; they are important first steps in the transition to new pain care strategies that are safer, more effective and patient-centered.”
Click here to access the consensus statement.
The Journal of Hospital Medicine, the official peer-reviewed journal of the Society of Hospital Medicine, has released a statement titled “Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement from the Society of Hospital Medicine” in response to the growing issue of managing opioid prescribing in the inpatient setting.
The statement offers 16 recommendations covering whether to use opioids in the hospital and how to improve the safety of opioid prescribing both during hospitalization and at discharge. The statement is available in the April 2018 issue of the journal.
SHM convened a working group to develop the consensus statement, intended for clinicians practicing in the inpatient setting. The development of the statement began with the working group conducting a systemic review of relevant guidelines and composing a draft based on extracted recommendations. The working group then obtained feedback from external experts in hospital-based opioid prescribing, SHM members, the SHM Patient-Family Advisory Council, other professional societies and peer reviewers.
The statement reads, “Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.”
“The journal is always pleased to be able to publish results of rigorous and innovative work, and the consensus document authored by Dr. Herzig and her team represents an outstanding example,” said Andrew Auerbach, MD, MPH, MHM, Professor of Medicine in Residence at the University of California, San Francisco, and editor in chief for the Journal of Hospital Medicine. “As we enter the ‘post-opioid’ era of pain management, papers like these will provide critical guidance for how to improve pain control among hospitalized patients; they are important first steps in the transition to new pain care strategies that are safer, more effective and patient-centered.”
Click here to access the consensus statement.
The Journal of Hospital Medicine, the official peer-reviewed journal of the Society of Hospital Medicine, has released a statement titled “Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement from the Society of Hospital Medicine” in response to the growing issue of managing opioid prescribing in the inpatient setting.
The statement offers 16 recommendations covering whether to use opioids in the hospital and how to improve the safety of opioid prescribing both during hospitalization and at discharge. The statement is available in the April 2018 issue of the journal.
SHM convened a working group to develop the consensus statement, intended for clinicians practicing in the inpatient setting. The development of the statement began with the working group conducting a systemic review of relevant guidelines and composing a draft based on extracted recommendations. The working group then obtained feedback from external experts in hospital-based opioid prescribing, SHM members, the SHM Patient-Family Advisory Council, other professional societies and peer reviewers.
The statement reads, “Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.”
“The journal is always pleased to be able to publish results of rigorous and innovative work, and the consensus document authored by Dr. Herzig and her team represents an outstanding example,” said Andrew Auerbach, MD, MPH, MHM, Professor of Medicine in Residence at the University of California, San Francisco, and editor in chief for the Journal of Hospital Medicine. “As we enter the ‘post-opioid’ era of pain management, papers like these will provide critical guidance for how to improve pain control among hospitalized patients; they are important first steps in the transition to new pain care strategies that are safer, more effective and patient-centered.”
Click here to access the consensus statement.
Sound and light levels are similarly disruptive in ICU and non-ICU wards
Clinical question: While it is generally thought that ICU wards are not conducive for sleep because of light and noise disruptions, are general wards any better?
Background: Hospitalized patients frequently report poor sleep, partly because of the inpatient environment. Sound level changes (SLCs), rather than the total volumes, are important in disrupting sleep. The World Health Organization recommends that nighttime baseline noise levels do not exceed 30 decibels (dB) and that nighttime noise peaks (i.e., loud noises) do not exceed 40 dB. The circadian rhythm system depends on ambient light to regulate the internal clock. Insufficient and inappropriately timed light exposure can desynchronize the biological clock, thereby negatively affecting sleep quality.
Setting: Tertiary care hospital in La Jolla, Calif.
Synopsis: For approximately 24-72 hours, recordings of sound and light were performed. ICU rooms were louder (hourly averages ranged from 56.1 dB to 60.3 dB) than were non-ICU wards (44.6-55.1 dB). However, SLCs of 17.5 dB or greater were not statistically different (ICU, 203.9 ± 28.8 times; non-ICU, 270.9 ± 39.5; P = .11). In both ICU and non-ICU wards, average daytime light levels were less than 250 lux and generally were brightest in the afternoon. This corresponds to low, office-level lighting, which may not be conducive for maintaining circadian rhythm.
Bottom line: While ICU wards are generally louder than non-ICU wards, sound level changes are equivalent and probably more important concerning sleep disruption. While no significant differences were seen in light levels, the amount and timing of lighting may not be optimal for keeping circadian rhythm.
Citation: Jaiswal SJ et al. Sound and light levels are similarly disruptive in ICU and non-ICU wards. J Hosp Med. 2017 Oct;12(10):798-804.
Dr. Kim is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Clinical question: While it is generally thought that ICU wards are not conducive for sleep because of light and noise disruptions, are general wards any better?
Background: Hospitalized patients frequently report poor sleep, partly because of the inpatient environment. Sound level changes (SLCs), rather than the total volumes, are important in disrupting sleep. The World Health Organization recommends that nighttime baseline noise levels do not exceed 30 decibels (dB) and that nighttime noise peaks (i.e., loud noises) do not exceed 40 dB. The circadian rhythm system depends on ambient light to regulate the internal clock. Insufficient and inappropriately timed light exposure can desynchronize the biological clock, thereby negatively affecting sleep quality.
Setting: Tertiary care hospital in La Jolla, Calif.
Synopsis: For approximately 24-72 hours, recordings of sound and light were performed. ICU rooms were louder (hourly averages ranged from 56.1 dB to 60.3 dB) than were non-ICU wards (44.6-55.1 dB). However, SLCs of 17.5 dB or greater were not statistically different (ICU, 203.9 ± 28.8 times; non-ICU, 270.9 ± 39.5; P = .11). In both ICU and non-ICU wards, average daytime light levels were less than 250 lux and generally were brightest in the afternoon. This corresponds to low, office-level lighting, which may not be conducive for maintaining circadian rhythm.
Bottom line: While ICU wards are generally louder than non-ICU wards, sound level changes are equivalent and probably more important concerning sleep disruption. While no significant differences were seen in light levels, the amount and timing of lighting may not be optimal for keeping circadian rhythm.
Citation: Jaiswal SJ et al. Sound and light levels are similarly disruptive in ICU and non-ICU wards. J Hosp Med. 2017 Oct;12(10):798-804.
Dr. Kim is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Clinical question: While it is generally thought that ICU wards are not conducive for sleep because of light and noise disruptions, are general wards any better?
Background: Hospitalized patients frequently report poor sleep, partly because of the inpatient environment. Sound level changes (SLCs), rather than the total volumes, are important in disrupting sleep. The World Health Organization recommends that nighttime baseline noise levels do not exceed 30 decibels (dB) and that nighttime noise peaks (i.e., loud noises) do not exceed 40 dB. The circadian rhythm system depends on ambient light to regulate the internal clock. Insufficient and inappropriately timed light exposure can desynchronize the biological clock, thereby negatively affecting sleep quality.
Setting: Tertiary care hospital in La Jolla, Calif.
Synopsis: For approximately 24-72 hours, recordings of sound and light were performed. ICU rooms were louder (hourly averages ranged from 56.1 dB to 60.3 dB) than were non-ICU wards (44.6-55.1 dB). However, SLCs of 17.5 dB or greater were not statistically different (ICU, 203.9 ± 28.8 times; non-ICU, 270.9 ± 39.5; P = .11). In both ICU and non-ICU wards, average daytime light levels were less than 250 lux and generally were brightest in the afternoon. This corresponds to low, office-level lighting, which may not be conducive for maintaining circadian rhythm.
Bottom line: While ICU wards are generally louder than non-ICU wards, sound level changes are equivalent and probably more important concerning sleep disruption. While no significant differences were seen in light levels, the amount and timing of lighting may not be optimal for keeping circadian rhythm.
Citation: Jaiswal SJ et al. Sound and light levels are similarly disruptive in ICU and non-ICU wards. J Hosp Med. 2017 Oct;12(10):798-804.
Dr. Kim is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
CMS finalizes measures to help combat opioid crisis
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.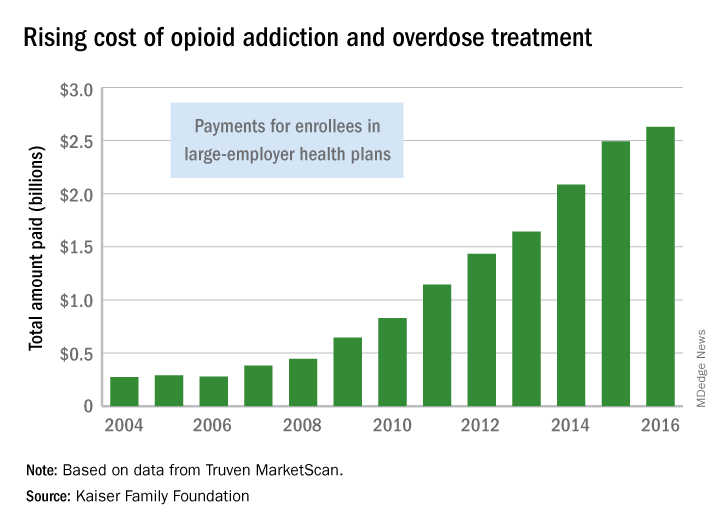
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.