User login
In Case You Missed It: COVID
COVID-19: Peginterferon lambda may prevent clinical deterioration, shorten viral shedding
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
FROM THE LANCET RESPIRATORY MEDICINE
Is COVID-19 accelerating progress toward high-value care?
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
Teenagers get in the queue for COVID-19 vaccines
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some COVID-19 vaccine reactions could be pseudoallergic, experts say
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
‘We can do better’: COVID-19 vaccination in patients with cancer
Every day, around 1.5 million doses of the COVID-19 vaccine are being delivered across the United States, but oncologists and patient advocates say that patients with cancer are missing out.
While official bodies recommend that patients with cancer are given priority, only 16 states currently prioritize them in the vaccine rollout. The other 34 states have thus far not singled out patients with cancer for earlier vaccination.
This flies in the face of recommendations from heavy hitters such as the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the National Comprehensive Cancer Network, and the American Association for Cancer Research.
All are in agreement: Patients on active cancer treatment should be prioritized for available vaccine because of their greater risk of death or complications from SARS-CoV-2 infection.
“All municipalities, states, cities, and even individual hospitals have so far been left to their own devices to try to figure out what the best way to do this is and that often conflicts with other recommendations or guidelines,” said E. John Wherry, PhD, chair of the department of systems pharmacology and translational therapeutics at the University of Pennsylvania, Philadelphia.
Dr. Wherry was on a panel at an AACR conference last week that discussed the failings of vaccine delivery to cancer patients.
During the meeting, lung cancer advocate Jill Feldman commented on the situation in Chicago, one of the jurisdictions that has not prioritized patients with cancer: “People don’t know what to do. ‘Do I need to sign up myself somewhere? Is my doctor’s office going to contact me?’ ”
Ms. Feldman said many people have called their cancer centers, “but cancer centers aren’t really providing updates directly to us. And they aren’t because they don’t have the information [either].”
Even in the 16 states that have ushered patients with cancer to the front of the line, the process for flagging these individuals is often unclear or nonexistent.
“Everyone that registers is basically on the same playing field ... because there’s no verification process. That’s very unfortunate,” said patient advocate Grace Cordovano, PhD, describing the vaccine sign-up process in New Jersey.
“It’s an easy fix,” said Dr. Cordovano. “Adding a few more fields [in the form] could really make a difference.”
COVID-19 fatality rates are twice as high in people with cancer than in people without cancer, according to a review published in December 2020 by the AACR’s COVID-19 and Cancer Task Force in the journal Cancer Discovery. Hematologic malignancies conferred an especially high risk.
“Any delay in vaccine access will result in loss of life that could be prevented with earlier access to vaccination,” AACR President Antoni Ribas, MD, told this news organization at the time.
There are also sound epidemiologic reasons to prioritize high-risk cancer patients for the COVID-19 vaccine, Dr. Wherry said in an interview. “What we do in infectious disease is to think about where your transmission and your risks are highest,” he said, citing cancer treatment centers as examples.
People with hematologic malignancies also tend to be long-term viral shedders, he said, putting caregivers and health care staff at increased risk. “There’s a big, big impact [in vaccinating cancer patients] and the numbers are not small.”
The CDC’s Jan. 1 recommendation is that patients with cancer should be assigned to priority group 1c, along with other “persons aged 16-64 with other high-risk medical conditions.”
However, more recent guidance from the NCCN hastened the urgency, advising that “patients with cancer should be assigned to the [CDC] priority group 1b/c.”
Out of 16 states that currently prioritize patients with cancer, 3 states have exceeded official advice, placing patients with cancer in priority group 1a. They opened their first batches of vaccine to everyone “deemed extremely vulnerable to COVID-19 by hospital providers” (Florida), or to “16-64 years old with a chronic health condition” (Mississippi) and to “persons aged 16-64 with high-risk conditions” (Pennsylvania, some jurisdictions).
However, despite these heroic intentions, no jurisdiction appears to have specifically tackled the thorny issue of subgroups of cancer that are more urgent than others, and this worries oncologists.
“Not all cancer patients are the same,” said Marina Garassino, MD, a medical oncologist at the National Tumor Institute of Milan. She shared registry data with the AACR panelists indicating that COVID-19 mortality in thoracic and hematologic malignancies rises to 30%-40%, compared with 13% for cancer overall.
At the AACR meeting, Dr. Ribas summed up his feelings on the issue: “It’s clear to me that patients with cancer should be prioritized. We have to then start defining this population and it should be the patient with an active cancer diagnosis undergoing treatment, in particular patients with lung cancers or hematologic malignancies.”
Since patients with cancer as a whole have problems getting timely vaccination – let alone someone with lung cancer or leukemia – the AACR meeting panelists grappled with solutions.
Dr. Cordovano said it was a “no brainer” to start with cancer centers. “Patients there are already registered, they have an account in the electronic health record system, they have insurance information, the care team knows them.”
Vaccination referrals sent directly from oncology centers would eliminate the need for the patient to provide verification or any additional documentation, she pointed out.
However, in New Jersey, cancer centers “have been completely excluded from the process,” she said.
Florida and New Hampshire have somewhat adopted the mechanism suggested by Dr. Cordovano. These states require health care providers to verify that a patient is “especially vulnerable” (Florida) or “medically vulnerable” (New Hampshire) in order for the patient to receive priority vaccine access. In New Hampshire, patients must have at least one other medical condition in addition to cancer to get on the list.
Jia Luo, MD, a medical oncology fellow at Memorial Sloan Kettering Cancer Center, New York, told the meeting that MSKCC has set up a proactive task force that sends “daily emails” to clinic staff highlighting patients eligible for the vaccine. “My sense is, it’s being prioritized to active cancer treatment,” said Dr. Luo. “All of our physicians are currently discussing [it] at each appointment and ... all of our nurses and staff have been talking to our patients on the phone.”
Dr. Cordovano, while advocating hard for cancer patients today, retained optimism about tomorrow: “This isn’t a long-term thing. This is just until things catch up. We knew we were going to have this problem.” Her hope is that, within 6 months, COVID-19 vaccination will become a standard of care in cancer.
Dr. Wherry agreed: “It’s going to take time to catch up with how far behind we are on certain things. ... What we’re seeing is a healthy debate rather than something that we should be concerned about – as long as that debate leads to rapid action.”
“We have to follow the science,” concluded Cordovano. “We can do better than this.”
Dr. Cordovano, Ms. Feldman, and Dr. Wherry have disclosed no relevant financial relationships. Dr. Luo declared a financial relationship with Targeted Oncology. Dr. Ribas declared financial relationships with 4C Biomed, Advaxis, Agilent, Amgen, AstraZeneca, Arcus, Bristol-Myers Squibb, and Kite-Gilead.
A version of this article first appeared on Medscape.com.
Every day, around 1.5 million doses of the COVID-19 vaccine are being delivered across the United States, but oncologists and patient advocates say that patients with cancer are missing out.
While official bodies recommend that patients with cancer are given priority, only 16 states currently prioritize them in the vaccine rollout. The other 34 states have thus far not singled out patients with cancer for earlier vaccination.
This flies in the face of recommendations from heavy hitters such as the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the National Comprehensive Cancer Network, and the American Association for Cancer Research.
All are in agreement: Patients on active cancer treatment should be prioritized for available vaccine because of their greater risk of death or complications from SARS-CoV-2 infection.
“All municipalities, states, cities, and even individual hospitals have so far been left to their own devices to try to figure out what the best way to do this is and that often conflicts with other recommendations or guidelines,” said E. John Wherry, PhD, chair of the department of systems pharmacology and translational therapeutics at the University of Pennsylvania, Philadelphia.
Dr. Wherry was on a panel at an AACR conference last week that discussed the failings of vaccine delivery to cancer patients.
During the meeting, lung cancer advocate Jill Feldman commented on the situation in Chicago, one of the jurisdictions that has not prioritized patients with cancer: “People don’t know what to do. ‘Do I need to sign up myself somewhere? Is my doctor’s office going to contact me?’ ”
Ms. Feldman said many people have called their cancer centers, “but cancer centers aren’t really providing updates directly to us. And they aren’t because they don’t have the information [either].”
Even in the 16 states that have ushered patients with cancer to the front of the line, the process for flagging these individuals is often unclear or nonexistent.
“Everyone that registers is basically on the same playing field ... because there’s no verification process. That’s very unfortunate,” said patient advocate Grace Cordovano, PhD, describing the vaccine sign-up process in New Jersey.
“It’s an easy fix,” said Dr. Cordovano. “Adding a few more fields [in the form] could really make a difference.”
COVID-19 fatality rates are twice as high in people with cancer than in people without cancer, according to a review published in December 2020 by the AACR’s COVID-19 and Cancer Task Force in the journal Cancer Discovery. Hematologic malignancies conferred an especially high risk.
“Any delay in vaccine access will result in loss of life that could be prevented with earlier access to vaccination,” AACR President Antoni Ribas, MD, told this news organization at the time.
There are also sound epidemiologic reasons to prioritize high-risk cancer patients for the COVID-19 vaccine, Dr. Wherry said in an interview. “What we do in infectious disease is to think about where your transmission and your risks are highest,” he said, citing cancer treatment centers as examples.
People with hematologic malignancies also tend to be long-term viral shedders, he said, putting caregivers and health care staff at increased risk. “There’s a big, big impact [in vaccinating cancer patients] and the numbers are not small.”
The CDC’s Jan. 1 recommendation is that patients with cancer should be assigned to priority group 1c, along with other “persons aged 16-64 with other high-risk medical conditions.”
However, more recent guidance from the NCCN hastened the urgency, advising that “patients with cancer should be assigned to the [CDC] priority group 1b/c.”
Out of 16 states that currently prioritize patients with cancer, 3 states have exceeded official advice, placing patients with cancer in priority group 1a. They opened their first batches of vaccine to everyone “deemed extremely vulnerable to COVID-19 by hospital providers” (Florida), or to “16-64 years old with a chronic health condition” (Mississippi) and to “persons aged 16-64 with high-risk conditions” (Pennsylvania, some jurisdictions).
However, despite these heroic intentions, no jurisdiction appears to have specifically tackled the thorny issue of subgroups of cancer that are more urgent than others, and this worries oncologists.
“Not all cancer patients are the same,” said Marina Garassino, MD, a medical oncologist at the National Tumor Institute of Milan. She shared registry data with the AACR panelists indicating that COVID-19 mortality in thoracic and hematologic malignancies rises to 30%-40%, compared with 13% for cancer overall.
At the AACR meeting, Dr. Ribas summed up his feelings on the issue: “It’s clear to me that patients with cancer should be prioritized. We have to then start defining this population and it should be the patient with an active cancer diagnosis undergoing treatment, in particular patients with lung cancers or hematologic malignancies.”
Since patients with cancer as a whole have problems getting timely vaccination – let alone someone with lung cancer or leukemia – the AACR meeting panelists grappled with solutions.
Dr. Cordovano said it was a “no brainer” to start with cancer centers. “Patients there are already registered, they have an account in the electronic health record system, they have insurance information, the care team knows them.”
Vaccination referrals sent directly from oncology centers would eliminate the need for the patient to provide verification or any additional documentation, she pointed out.
However, in New Jersey, cancer centers “have been completely excluded from the process,” she said.
Florida and New Hampshire have somewhat adopted the mechanism suggested by Dr. Cordovano. These states require health care providers to verify that a patient is “especially vulnerable” (Florida) or “medically vulnerable” (New Hampshire) in order for the patient to receive priority vaccine access. In New Hampshire, patients must have at least one other medical condition in addition to cancer to get on the list.
Jia Luo, MD, a medical oncology fellow at Memorial Sloan Kettering Cancer Center, New York, told the meeting that MSKCC has set up a proactive task force that sends “daily emails” to clinic staff highlighting patients eligible for the vaccine. “My sense is, it’s being prioritized to active cancer treatment,” said Dr. Luo. “All of our physicians are currently discussing [it] at each appointment and ... all of our nurses and staff have been talking to our patients on the phone.”
Dr. Cordovano, while advocating hard for cancer patients today, retained optimism about tomorrow: “This isn’t a long-term thing. This is just until things catch up. We knew we were going to have this problem.” Her hope is that, within 6 months, COVID-19 vaccination will become a standard of care in cancer.
Dr. Wherry agreed: “It’s going to take time to catch up with how far behind we are on certain things. ... What we’re seeing is a healthy debate rather than something that we should be concerned about – as long as that debate leads to rapid action.”
“We have to follow the science,” concluded Cordovano. “We can do better than this.”
Dr. Cordovano, Ms. Feldman, and Dr. Wherry have disclosed no relevant financial relationships. Dr. Luo declared a financial relationship with Targeted Oncology. Dr. Ribas declared financial relationships with 4C Biomed, Advaxis, Agilent, Amgen, AstraZeneca, Arcus, Bristol-Myers Squibb, and Kite-Gilead.
A version of this article first appeared on Medscape.com.
Every day, around 1.5 million doses of the COVID-19 vaccine are being delivered across the United States, but oncologists and patient advocates say that patients with cancer are missing out.
While official bodies recommend that patients with cancer are given priority, only 16 states currently prioritize them in the vaccine rollout. The other 34 states have thus far not singled out patients with cancer for earlier vaccination.
This flies in the face of recommendations from heavy hitters such as the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the National Comprehensive Cancer Network, and the American Association for Cancer Research.
All are in agreement: Patients on active cancer treatment should be prioritized for available vaccine because of their greater risk of death or complications from SARS-CoV-2 infection.
“All municipalities, states, cities, and even individual hospitals have so far been left to their own devices to try to figure out what the best way to do this is and that often conflicts with other recommendations or guidelines,” said E. John Wherry, PhD, chair of the department of systems pharmacology and translational therapeutics at the University of Pennsylvania, Philadelphia.
Dr. Wherry was on a panel at an AACR conference last week that discussed the failings of vaccine delivery to cancer patients.
During the meeting, lung cancer advocate Jill Feldman commented on the situation in Chicago, one of the jurisdictions that has not prioritized patients with cancer: “People don’t know what to do. ‘Do I need to sign up myself somewhere? Is my doctor’s office going to contact me?’ ”
Ms. Feldman said many people have called their cancer centers, “but cancer centers aren’t really providing updates directly to us. And they aren’t because they don’t have the information [either].”
Even in the 16 states that have ushered patients with cancer to the front of the line, the process for flagging these individuals is often unclear or nonexistent.
“Everyone that registers is basically on the same playing field ... because there’s no verification process. That’s very unfortunate,” said patient advocate Grace Cordovano, PhD, describing the vaccine sign-up process in New Jersey.
“It’s an easy fix,” said Dr. Cordovano. “Adding a few more fields [in the form] could really make a difference.”
COVID-19 fatality rates are twice as high in people with cancer than in people without cancer, according to a review published in December 2020 by the AACR’s COVID-19 and Cancer Task Force in the journal Cancer Discovery. Hematologic malignancies conferred an especially high risk.
“Any delay in vaccine access will result in loss of life that could be prevented with earlier access to vaccination,” AACR President Antoni Ribas, MD, told this news organization at the time.
There are also sound epidemiologic reasons to prioritize high-risk cancer patients for the COVID-19 vaccine, Dr. Wherry said in an interview. “What we do in infectious disease is to think about where your transmission and your risks are highest,” he said, citing cancer treatment centers as examples.
People with hematologic malignancies also tend to be long-term viral shedders, he said, putting caregivers and health care staff at increased risk. “There’s a big, big impact [in vaccinating cancer patients] and the numbers are not small.”
The CDC’s Jan. 1 recommendation is that patients with cancer should be assigned to priority group 1c, along with other “persons aged 16-64 with other high-risk medical conditions.”
However, more recent guidance from the NCCN hastened the urgency, advising that “patients with cancer should be assigned to the [CDC] priority group 1b/c.”
Out of 16 states that currently prioritize patients with cancer, 3 states have exceeded official advice, placing patients with cancer in priority group 1a. They opened their first batches of vaccine to everyone “deemed extremely vulnerable to COVID-19 by hospital providers” (Florida), or to “16-64 years old with a chronic health condition” (Mississippi) and to “persons aged 16-64 with high-risk conditions” (Pennsylvania, some jurisdictions).
However, despite these heroic intentions, no jurisdiction appears to have specifically tackled the thorny issue of subgroups of cancer that are more urgent than others, and this worries oncologists.
“Not all cancer patients are the same,” said Marina Garassino, MD, a medical oncologist at the National Tumor Institute of Milan. She shared registry data with the AACR panelists indicating that COVID-19 mortality in thoracic and hematologic malignancies rises to 30%-40%, compared with 13% for cancer overall.
At the AACR meeting, Dr. Ribas summed up his feelings on the issue: “It’s clear to me that patients with cancer should be prioritized. We have to then start defining this population and it should be the patient with an active cancer diagnosis undergoing treatment, in particular patients with lung cancers or hematologic malignancies.”
Since patients with cancer as a whole have problems getting timely vaccination – let alone someone with lung cancer or leukemia – the AACR meeting panelists grappled with solutions.
Dr. Cordovano said it was a “no brainer” to start with cancer centers. “Patients there are already registered, they have an account in the electronic health record system, they have insurance information, the care team knows them.”
Vaccination referrals sent directly from oncology centers would eliminate the need for the patient to provide verification or any additional documentation, she pointed out.
However, in New Jersey, cancer centers “have been completely excluded from the process,” she said.
Florida and New Hampshire have somewhat adopted the mechanism suggested by Dr. Cordovano. These states require health care providers to verify that a patient is “especially vulnerable” (Florida) or “medically vulnerable” (New Hampshire) in order for the patient to receive priority vaccine access. In New Hampshire, patients must have at least one other medical condition in addition to cancer to get on the list.
Jia Luo, MD, a medical oncology fellow at Memorial Sloan Kettering Cancer Center, New York, told the meeting that MSKCC has set up a proactive task force that sends “daily emails” to clinic staff highlighting patients eligible for the vaccine. “My sense is, it’s being prioritized to active cancer treatment,” said Dr. Luo. “All of our physicians are currently discussing [it] at each appointment and ... all of our nurses and staff have been talking to our patients on the phone.”
Dr. Cordovano, while advocating hard for cancer patients today, retained optimism about tomorrow: “This isn’t a long-term thing. This is just until things catch up. We knew we were going to have this problem.” Her hope is that, within 6 months, COVID-19 vaccination will become a standard of care in cancer.
Dr. Wherry agreed: “It’s going to take time to catch up with how far behind we are on certain things. ... What we’re seeing is a healthy debate rather than something that we should be concerned about – as long as that debate leads to rapid action.”
“We have to follow the science,” concluded Cordovano. “We can do better than this.”
Dr. Cordovano, Ms. Feldman, and Dr. Wherry have disclosed no relevant financial relationships. Dr. Luo declared a financial relationship with Targeted Oncology. Dr. Ribas declared financial relationships with 4C Biomed, Advaxis, Agilent, Amgen, AstraZeneca, Arcus, Bristol-Myers Squibb, and Kite-Gilead.
A version of this article first appeared on Medscape.com.
As demand for mental health care spikes, budget ax set to strike
When the pandemic hit, health officials in Montana’s Beaverhead County had barely begun to fill a hole left by the 2017 closure of the local public assistance office, mental health clinic, chemical dependency center and job placement office after the state’s last budget shortfall.
Now, those health officials worry more cuts are coming, even as they brace for a spike in demand for substance abuse and mental health services. That would be no small challenge in a poor farming and ranching region where stigma often prevents people from admitting they need help, said Katherine Buckley-Patton, who chairs the county’s Mental Health Local Advisory Council.
“I find it very challenging to find the words that will not make one of my hard-nosed cowboys turn around and walk away,” Ms. Buckley-Patton said.
States across the U.S. are still stinging after businesses closed and millions of people lost jobs because of COVID-related shutdowns and restrictions. Meanwhile, the pandemic has led to a dramatic increase in the number of people who say their mental health has suffered, rising from one in three people in March to more than half of people polled by KFF in July. (KHN is an editorially independent program of KFF.)
The full extent of the mental health crisis and the demand for behavioral health services may not be known until after the pandemic is over, mental health experts said. That could add costs that budget writers haven’t anticipated.
“It usually takes a while before people feel comfortable seeking care from a specialty behavioral health organization,” said Chuck Ingoglia, president and CEO of the nonprofit National Council for Behavioral Health in Washington, D.C. “We are not likely to see the results of that either in terms of people seeking care – or suicide rates going up – until we’re on the other side of the pandemic.”
Last year, states slashed agency budgets, froze pay, furloughed workers, borrowed money, and tapped into rainy-day funds to make ends meet. Health programs, often among the most expensive part of a state’s budget, were targeted for cuts in several states even as health officials led efforts to stem the spread of the coronavirus.
This year, the outlook doesn’t seem quite so bleak, partly because of relief packages passed by Congress last spring and in December that buoyed state economies. Another major advantage was that income increased or held steady for people with well-paying jobs and investment income, which boosted states’ tax revenues even as millions of lower-income workers were laid off.
“It has turned out to be not as bad as it might have been in terms of state budgets,” said Mike Leachman, vice president for state fiscal policy for the nonpartisan Center on Budget and Policy Priorities.
But many states still face cash shortfalls that will be made worse if additional federal aid doesn’t come, Mr. Leachman said. President Joe Biden has pledged to push through Congress a $1.9 billion relief package that includes aid to states, while congressional Republicans are proposing a package worth about a third of that amount. States are banking on federal help.
New York Gov. Andrew Cuomo, a Democrat, predicted his state would have to plug a $15 billion deficit with spending cuts and tax increases if a fresh round of aid doesn’t materialize. Some states, such as New Jersey, borrowed to make their budgets whole, and they’re going to have to start paying that money back. Tourism states such as Hawaii and energy-producing states such as Alaska and Wyoming continue to face grim economic outlooks with oil, gas, and coal prices down and tourists cutting back on travel, Mr. Leachman said.
Even states with a relatively rosy economic outlook are being cautious. In Colorado, for example, Democratic Gov. Jared Polis proposed a budget that restores the cuts made last year to Medicaid and substance abuse programs. But health providers are doubtful the legislature will approve any significant spending increases in this economy.
“Everybody right now is just trying to protect and make sure we don’t have additional cuts,” said Doyle Forrestal, CEO of the Colorado Behavioral Healthcare Council.
That’s also what Ms. Buckley-Patton wants for Montana’s Beaverhead County, where most of the 9,400 residents live in poverty or earn low incomes.
She led the county’s effort to recover from the loss in 2017 of a wide range of behavioral health services, along with offices to help poor people receive Medicaid health services, plus cash and food assistance.
Through persuasive grant writing and donations coaxed from elected officials, Ms. Buckley-Patton and her team secured office space, equipment, and a part-time employee for a resource center that’s open once a week in the county in the southwestern corner of the state, she said. They also convinced the state health department to send two people every other week on a 120-mile round trip from the Butte office to help county residents with their Medicaid and public assistance applications.
But now Ms. Buckley-Patton worries even those modest gains will be threatened in this year’s budget. Montana is one of the few states with a budget on a 2-year cycle, so this is the first time lawmakers have had to craft a spending plan since the pandemic began.
Revenue forecasts predict healthy tax collections over the next 2 years.
In January, at the start of the legislative session, the panel in charge of building the state health department’s budget proposed starting with nearly $1 billion in cuts. The panel’s chairperson, Republican Rep. Matt Regier, pledged to add back programs and services on their merits during the months-long budget process.
It’s a strategy Ms. Buckley-Patton worries will lead to a net loss of funding for Beaverhead County, which covers more land than Connecticut.
“I have grave concerns about this legislative session,” she said. “We’re not digging out of the hole; we’re only going deeper.”
Republicans, who are in control of the Montana House, Senate, and governor’s office for the first time in 16 years, are considering reducing the income tax level for the state’s top earners. Such a measure that could affect state revenue in an uncertain economy has some observers concerned, particularly when an increased need for health services is expected.
“Are legislators committed to building back up that budget in a way that works for communities and for health providers, or are we going to see tax cuts that reduce revenue that put us yet again in another really tight budget?” asked Heather O’Loughlin, codirector of the Montana Budget and Policy Center.
Mary Windecker, executive director of the Behavioral Health Alliance of Montana, said that health providers across the state are still clawing back from more than $100 million in budget cuts in 2017, and that she worries more cuts are on the horizon.
But one bright spot, she said, is a proposal by new Gov. Greg Gianforte to create a fund that would put $23 million a year toward community substance abuse prevention and treatment programs. It would be partially funded by tax revenue the state will receive from recreational marijuana, which voters approved in November, with sales to begin next year.
Ms. Windecker cautioned, though, that mental health and substance use are linked, and the governor and lawmakers should plan with that in mind.
“In the public’s mind, there’s drug addicts and there’s the mentally ill,” she said. “Quite often, the same people who have a substance use disorder are using it to treat a mental health issue that is underlying that substance use. So, you can never split the two out.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
When the pandemic hit, health officials in Montana’s Beaverhead County had barely begun to fill a hole left by the 2017 closure of the local public assistance office, mental health clinic, chemical dependency center and job placement office after the state’s last budget shortfall.
Now, those health officials worry more cuts are coming, even as they brace for a spike in demand for substance abuse and mental health services. That would be no small challenge in a poor farming and ranching region where stigma often prevents people from admitting they need help, said Katherine Buckley-Patton, who chairs the county’s Mental Health Local Advisory Council.
“I find it very challenging to find the words that will not make one of my hard-nosed cowboys turn around and walk away,” Ms. Buckley-Patton said.
States across the U.S. are still stinging after businesses closed and millions of people lost jobs because of COVID-related shutdowns and restrictions. Meanwhile, the pandemic has led to a dramatic increase in the number of people who say their mental health has suffered, rising from one in three people in March to more than half of people polled by KFF in July. (KHN is an editorially independent program of KFF.)
The full extent of the mental health crisis and the demand for behavioral health services may not be known until after the pandemic is over, mental health experts said. That could add costs that budget writers haven’t anticipated.
“It usually takes a while before people feel comfortable seeking care from a specialty behavioral health organization,” said Chuck Ingoglia, president and CEO of the nonprofit National Council for Behavioral Health in Washington, D.C. “We are not likely to see the results of that either in terms of people seeking care – or suicide rates going up – until we’re on the other side of the pandemic.”
Last year, states slashed agency budgets, froze pay, furloughed workers, borrowed money, and tapped into rainy-day funds to make ends meet. Health programs, often among the most expensive part of a state’s budget, were targeted for cuts in several states even as health officials led efforts to stem the spread of the coronavirus.
This year, the outlook doesn’t seem quite so bleak, partly because of relief packages passed by Congress last spring and in December that buoyed state economies. Another major advantage was that income increased or held steady for people with well-paying jobs and investment income, which boosted states’ tax revenues even as millions of lower-income workers were laid off.
“It has turned out to be not as bad as it might have been in terms of state budgets,” said Mike Leachman, vice president for state fiscal policy for the nonpartisan Center on Budget and Policy Priorities.
But many states still face cash shortfalls that will be made worse if additional federal aid doesn’t come, Mr. Leachman said. President Joe Biden has pledged to push through Congress a $1.9 billion relief package that includes aid to states, while congressional Republicans are proposing a package worth about a third of that amount. States are banking on federal help.
New York Gov. Andrew Cuomo, a Democrat, predicted his state would have to plug a $15 billion deficit with spending cuts and tax increases if a fresh round of aid doesn’t materialize. Some states, such as New Jersey, borrowed to make their budgets whole, and they’re going to have to start paying that money back. Tourism states such as Hawaii and energy-producing states such as Alaska and Wyoming continue to face grim economic outlooks with oil, gas, and coal prices down and tourists cutting back on travel, Mr. Leachman said.
Even states with a relatively rosy economic outlook are being cautious. In Colorado, for example, Democratic Gov. Jared Polis proposed a budget that restores the cuts made last year to Medicaid and substance abuse programs. But health providers are doubtful the legislature will approve any significant spending increases in this economy.
“Everybody right now is just trying to protect and make sure we don’t have additional cuts,” said Doyle Forrestal, CEO of the Colorado Behavioral Healthcare Council.
That’s also what Ms. Buckley-Patton wants for Montana’s Beaverhead County, where most of the 9,400 residents live in poverty or earn low incomes.
She led the county’s effort to recover from the loss in 2017 of a wide range of behavioral health services, along with offices to help poor people receive Medicaid health services, plus cash and food assistance.
Through persuasive grant writing and donations coaxed from elected officials, Ms. Buckley-Patton and her team secured office space, equipment, and a part-time employee for a resource center that’s open once a week in the county in the southwestern corner of the state, she said. They also convinced the state health department to send two people every other week on a 120-mile round trip from the Butte office to help county residents with their Medicaid and public assistance applications.
But now Ms. Buckley-Patton worries even those modest gains will be threatened in this year’s budget. Montana is one of the few states with a budget on a 2-year cycle, so this is the first time lawmakers have had to craft a spending plan since the pandemic began.
Revenue forecasts predict healthy tax collections over the next 2 years.
In January, at the start of the legislative session, the panel in charge of building the state health department’s budget proposed starting with nearly $1 billion in cuts. The panel’s chairperson, Republican Rep. Matt Regier, pledged to add back programs and services on their merits during the months-long budget process.
It’s a strategy Ms. Buckley-Patton worries will lead to a net loss of funding for Beaverhead County, which covers more land than Connecticut.
“I have grave concerns about this legislative session,” she said. “We’re not digging out of the hole; we’re only going deeper.”
Republicans, who are in control of the Montana House, Senate, and governor’s office for the first time in 16 years, are considering reducing the income tax level for the state’s top earners. Such a measure that could affect state revenue in an uncertain economy has some observers concerned, particularly when an increased need for health services is expected.
“Are legislators committed to building back up that budget in a way that works for communities and for health providers, or are we going to see tax cuts that reduce revenue that put us yet again in another really tight budget?” asked Heather O’Loughlin, codirector of the Montana Budget and Policy Center.
Mary Windecker, executive director of the Behavioral Health Alliance of Montana, said that health providers across the state are still clawing back from more than $100 million in budget cuts in 2017, and that she worries more cuts are on the horizon.
But one bright spot, she said, is a proposal by new Gov. Greg Gianforte to create a fund that would put $23 million a year toward community substance abuse prevention and treatment programs. It would be partially funded by tax revenue the state will receive from recreational marijuana, which voters approved in November, with sales to begin next year.
Ms. Windecker cautioned, though, that mental health and substance use are linked, and the governor and lawmakers should plan with that in mind.
“In the public’s mind, there’s drug addicts and there’s the mentally ill,” she said. “Quite often, the same people who have a substance use disorder are using it to treat a mental health issue that is underlying that substance use. So, you can never split the two out.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
When the pandemic hit, health officials in Montana’s Beaverhead County had barely begun to fill a hole left by the 2017 closure of the local public assistance office, mental health clinic, chemical dependency center and job placement office after the state’s last budget shortfall.
Now, those health officials worry more cuts are coming, even as they brace for a spike in demand for substance abuse and mental health services. That would be no small challenge in a poor farming and ranching region where stigma often prevents people from admitting they need help, said Katherine Buckley-Patton, who chairs the county’s Mental Health Local Advisory Council.
“I find it very challenging to find the words that will not make one of my hard-nosed cowboys turn around and walk away,” Ms. Buckley-Patton said.
States across the U.S. are still stinging after businesses closed and millions of people lost jobs because of COVID-related shutdowns and restrictions. Meanwhile, the pandemic has led to a dramatic increase in the number of people who say their mental health has suffered, rising from one in three people in March to more than half of people polled by KFF in July. (KHN is an editorially independent program of KFF.)
The full extent of the mental health crisis and the demand for behavioral health services may not be known until after the pandemic is over, mental health experts said. That could add costs that budget writers haven’t anticipated.
“It usually takes a while before people feel comfortable seeking care from a specialty behavioral health organization,” said Chuck Ingoglia, president and CEO of the nonprofit National Council for Behavioral Health in Washington, D.C. “We are not likely to see the results of that either in terms of people seeking care – or suicide rates going up – until we’re on the other side of the pandemic.”
Last year, states slashed agency budgets, froze pay, furloughed workers, borrowed money, and tapped into rainy-day funds to make ends meet. Health programs, often among the most expensive part of a state’s budget, were targeted for cuts in several states even as health officials led efforts to stem the spread of the coronavirus.
This year, the outlook doesn’t seem quite so bleak, partly because of relief packages passed by Congress last spring and in December that buoyed state economies. Another major advantage was that income increased or held steady for people with well-paying jobs and investment income, which boosted states’ tax revenues even as millions of lower-income workers were laid off.
“It has turned out to be not as bad as it might have been in terms of state budgets,” said Mike Leachman, vice president for state fiscal policy for the nonpartisan Center on Budget and Policy Priorities.
But many states still face cash shortfalls that will be made worse if additional federal aid doesn’t come, Mr. Leachman said. President Joe Biden has pledged to push through Congress a $1.9 billion relief package that includes aid to states, while congressional Republicans are proposing a package worth about a third of that amount. States are banking on federal help.
New York Gov. Andrew Cuomo, a Democrat, predicted his state would have to plug a $15 billion deficit with spending cuts and tax increases if a fresh round of aid doesn’t materialize. Some states, such as New Jersey, borrowed to make their budgets whole, and they’re going to have to start paying that money back. Tourism states such as Hawaii and energy-producing states such as Alaska and Wyoming continue to face grim economic outlooks with oil, gas, and coal prices down and tourists cutting back on travel, Mr. Leachman said.
Even states with a relatively rosy economic outlook are being cautious. In Colorado, for example, Democratic Gov. Jared Polis proposed a budget that restores the cuts made last year to Medicaid and substance abuse programs. But health providers are doubtful the legislature will approve any significant spending increases in this economy.
“Everybody right now is just trying to protect and make sure we don’t have additional cuts,” said Doyle Forrestal, CEO of the Colorado Behavioral Healthcare Council.
That’s also what Ms. Buckley-Patton wants for Montana’s Beaverhead County, where most of the 9,400 residents live in poverty or earn low incomes.
She led the county’s effort to recover from the loss in 2017 of a wide range of behavioral health services, along with offices to help poor people receive Medicaid health services, plus cash and food assistance.
Through persuasive grant writing and donations coaxed from elected officials, Ms. Buckley-Patton and her team secured office space, equipment, and a part-time employee for a resource center that’s open once a week in the county in the southwestern corner of the state, she said. They also convinced the state health department to send two people every other week on a 120-mile round trip from the Butte office to help county residents with their Medicaid and public assistance applications.
But now Ms. Buckley-Patton worries even those modest gains will be threatened in this year’s budget. Montana is one of the few states with a budget on a 2-year cycle, so this is the first time lawmakers have had to craft a spending plan since the pandemic began.
Revenue forecasts predict healthy tax collections over the next 2 years.
In January, at the start of the legislative session, the panel in charge of building the state health department’s budget proposed starting with nearly $1 billion in cuts. The panel’s chairperson, Republican Rep. Matt Regier, pledged to add back programs and services on their merits during the months-long budget process.
It’s a strategy Ms. Buckley-Patton worries will lead to a net loss of funding for Beaverhead County, which covers more land than Connecticut.
“I have grave concerns about this legislative session,” she said. “We’re not digging out of the hole; we’re only going deeper.”
Republicans, who are in control of the Montana House, Senate, and governor’s office for the first time in 16 years, are considering reducing the income tax level for the state’s top earners. Such a measure that could affect state revenue in an uncertain economy has some observers concerned, particularly when an increased need for health services is expected.
“Are legislators committed to building back up that budget in a way that works for communities and for health providers, or are we going to see tax cuts that reduce revenue that put us yet again in another really tight budget?” asked Heather O’Loughlin, codirector of the Montana Budget and Policy Center.
Mary Windecker, executive director of the Behavioral Health Alliance of Montana, said that health providers across the state are still clawing back from more than $100 million in budget cuts in 2017, and that she worries more cuts are on the horizon.
But one bright spot, she said, is a proposal by new Gov. Greg Gianforte to create a fund that would put $23 million a year toward community substance abuse prevention and treatment programs. It would be partially funded by tax revenue the state will receive from recreational marijuana, which voters approved in November, with sales to begin next year.
Ms. Windecker cautioned, though, that mental health and substance use are linked, and the governor and lawmakers should plan with that in mind.
“In the public’s mind, there’s drug addicts and there’s the mentally ill,” she said. “Quite often, the same people who have a substance use disorder are using it to treat a mental health issue that is underlying that substance use. So, you can never split the two out.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
COVID-19 in children: New cases down for third straight week
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
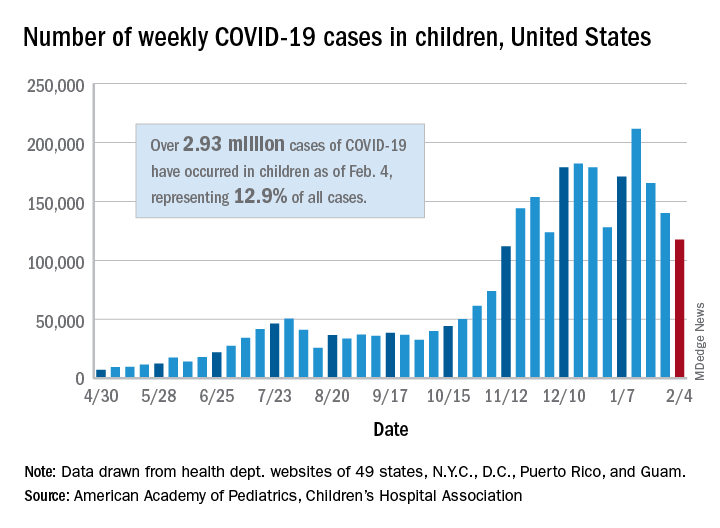
New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
CXR-Net: An AI-based diagnostic tool for COVID-19
The system, called CXR-Net, was trained to differentiate SARS-CoV-2 chest x-rays (CXRs) from CXRs that are either normal or non–COVID-19 lung pathologies, explained Abdulah Haikal, an MD candidate at Wayne State University, Detroit.
Mr. Haikal described CXR-Net at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S11-04).
CXR-Net is a two-module pipeline, Mr. Haikal explained. Module I is based on Res-CR-Net, a type of neural network originally designed for the semantic segmentation of microscopy images, with the ability to retain the original resolution of the input images in the feature maps of all layers and in the final output.
Module II is a hybrid convolutional neural network in which the first convolutional layer with learned coefficients is replaced by a layer with fixed coefficients provided by the Wavelet Scattering Transform. Module II inputs patients’ CXRs and corresponding lung masks quantified by Module I, and generates as outputs a class assignment (COVID-19 or non–COVID-19) and high-resolution heat maps that detect the severe acute respiratory syndrome–-associated lung regions.
“The system is trained to differentiate COVID and non-COVID pathologies and produces a highly discriminative heat map to point to lung regions where COVID is suspected,” Mr. Haikal said. “The Wavelet Scattering Transform allows for fast determination of COVID versus non-COVID CXRs.”
Preliminary results and implications
CXR-Net was piloted on a small dataset of CXRs from non–COVID-19 and polymerase chain reaction–confirmed COVID-19 patients acquired at a single center in Detroit.
Upon fivefold cross validation of the training set with 2,265 images, 90% accuracy was observed when the training set was tested against the validation set. However, once 1,532 new images were introduced, a 76% accuracy rate was observed.
The F1 scores were 0.81 and 0.70 for the training and test sets, respectively.
“I’m really excited about this new approach, and I think AI will allow us to do more with less, which is exciting,” said Ross L. Levine, MD, of Memorial Sloan Kettering Cancer Center in New York, who led a discussion session with Mr. Haikal about CXR-Net.
One question raised during the discussion was whether the technology will help health care providers be more thoughtful about when and how they image COVID-19 patients.
“The more data you feed into the system, the stronger and more accurate it becomes,” Mr. Haikal said. “However, until we have data sharing from multiple centers, we won’t see improved accuracy results.”
Another question was whether this technology could be integrated with more clinical parameters.
“Some individuals are afraid that AI will replace the job of a professional, but it will only make it better for us,” Mr. Haikal said. “We don’t rely on current imaging techniques to make a definitive diagnosis, but rather have a specificity and sensitivity to establish a diagnosis, and AI can be used in the same way as a diagnostic tool.”
Mr. Haikal and Dr. Levine disclosed no conflicts of interest. No funding sources were reported in the presentation.
The system, called CXR-Net, was trained to differentiate SARS-CoV-2 chest x-rays (CXRs) from CXRs that are either normal or non–COVID-19 lung pathologies, explained Abdulah Haikal, an MD candidate at Wayne State University, Detroit.
Mr. Haikal described CXR-Net at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S11-04).
CXR-Net is a two-module pipeline, Mr. Haikal explained. Module I is based on Res-CR-Net, a type of neural network originally designed for the semantic segmentation of microscopy images, with the ability to retain the original resolution of the input images in the feature maps of all layers and in the final output.
Module II is a hybrid convolutional neural network in which the first convolutional layer with learned coefficients is replaced by a layer with fixed coefficients provided by the Wavelet Scattering Transform. Module II inputs patients’ CXRs and corresponding lung masks quantified by Module I, and generates as outputs a class assignment (COVID-19 or non–COVID-19) and high-resolution heat maps that detect the severe acute respiratory syndrome–-associated lung regions.
“The system is trained to differentiate COVID and non-COVID pathologies and produces a highly discriminative heat map to point to lung regions where COVID is suspected,” Mr. Haikal said. “The Wavelet Scattering Transform allows for fast determination of COVID versus non-COVID CXRs.”
Preliminary results and implications
CXR-Net was piloted on a small dataset of CXRs from non–COVID-19 and polymerase chain reaction–confirmed COVID-19 patients acquired at a single center in Detroit.
Upon fivefold cross validation of the training set with 2,265 images, 90% accuracy was observed when the training set was tested against the validation set. However, once 1,532 new images were introduced, a 76% accuracy rate was observed.
The F1 scores were 0.81 and 0.70 for the training and test sets, respectively.
“I’m really excited about this new approach, and I think AI will allow us to do more with less, which is exciting,” said Ross L. Levine, MD, of Memorial Sloan Kettering Cancer Center in New York, who led a discussion session with Mr. Haikal about CXR-Net.
One question raised during the discussion was whether the technology will help health care providers be more thoughtful about when and how they image COVID-19 patients.
“The more data you feed into the system, the stronger and more accurate it becomes,” Mr. Haikal said. “However, until we have data sharing from multiple centers, we won’t see improved accuracy results.”
Another question was whether this technology could be integrated with more clinical parameters.
“Some individuals are afraid that AI will replace the job of a professional, but it will only make it better for us,” Mr. Haikal said. “We don’t rely on current imaging techniques to make a definitive diagnosis, but rather have a specificity and sensitivity to establish a diagnosis, and AI can be used in the same way as a diagnostic tool.”
Mr. Haikal and Dr. Levine disclosed no conflicts of interest. No funding sources were reported in the presentation.
The system, called CXR-Net, was trained to differentiate SARS-CoV-2 chest x-rays (CXRs) from CXRs that are either normal or non–COVID-19 lung pathologies, explained Abdulah Haikal, an MD candidate at Wayne State University, Detroit.
Mr. Haikal described CXR-Net at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S11-04).
CXR-Net is a two-module pipeline, Mr. Haikal explained. Module I is based on Res-CR-Net, a type of neural network originally designed for the semantic segmentation of microscopy images, with the ability to retain the original resolution of the input images in the feature maps of all layers and in the final output.
Module II is a hybrid convolutional neural network in which the first convolutional layer with learned coefficients is replaced by a layer with fixed coefficients provided by the Wavelet Scattering Transform. Module II inputs patients’ CXRs and corresponding lung masks quantified by Module I, and generates as outputs a class assignment (COVID-19 or non–COVID-19) and high-resolution heat maps that detect the severe acute respiratory syndrome–-associated lung regions.
“The system is trained to differentiate COVID and non-COVID pathologies and produces a highly discriminative heat map to point to lung regions where COVID is suspected,” Mr. Haikal said. “The Wavelet Scattering Transform allows for fast determination of COVID versus non-COVID CXRs.”
Preliminary results and implications
CXR-Net was piloted on a small dataset of CXRs from non–COVID-19 and polymerase chain reaction–confirmed COVID-19 patients acquired at a single center in Detroit.
Upon fivefold cross validation of the training set with 2,265 images, 90% accuracy was observed when the training set was tested against the validation set. However, once 1,532 new images were introduced, a 76% accuracy rate was observed.
The F1 scores were 0.81 and 0.70 for the training and test sets, respectively.
“I’m really excited about this new approach, and I think AI will allow us to do more with less, which is exciting,” said Ross L. Levine, MD, of Memorial Sloan Kettering Cancer Center in New York, who led a discussion session with Mr. Haikal about CXR-Net.
One question raised during the discussion was whether the technology will help health care providers be more thoughtful about when and how they image COVID-19 patients.
“The more data you feed into the system, the stronger and more accurate it becomes,” Mr. Haikal said. “However, until we have data sharing from multiple centers, we won’t see improved accuracy results.”
Another question was whether this technology could be integrated with more clinical parameters.
“Some individuals are afraid that AI will replace the job of a professional, but it will only make it better for us,” Mr. Haikal said. “We don’t rely on current imaging techniques to make a definitive diagnosis, but rather have a specificity and sensitivity to establish a diagnosis, and AI can be used in the same way as a diagnostic tool.”
Mr. Haikal and Dr. Levine disclosed no conflicts of interest. No funding sources were reported in the presentation.
FROM AACR: COVID-19 AND CANCER 2021
U.K. COVID-19 variant doubling every 10 days in the U.S.: Study
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
Are diagnosticians chasing COVID-linked zebras and missing horses?
The emergence of multiple inflammatory syndrome in children (MIS-C) in association with COVID-19 may be complicating the investigation and diagnosis of more common viral and bacterial infections, potentially delaying treatment and prolonging hospital stays.
Two recent articles published online in Hospital Pediatrics provide evidence of this phenomenon. The articles outlined case studies of children who underwent extensive investigation for MIS-C when in fact they had less severe and more common infections. MIS-C is a severe but rare syndrome that involves systemic hyperinflammation with fever and multisystem organ dysfunction similar to that of Kawasaki disease (KD).
In one of the articles, Matthew Molloy, MD, MPH, of the division of pediatric hospital medicine at Cincinnati Children’s Hospital Medical Center, and colleagues aptly asked: “What are we missing in our search for MIS-C?”
E. coli, not SARS-CoV-2
That question arose from a case involving a 3-year-old boy who had a 6-day history of fever and fatigue. Three days earlier, he had tested negative for strep antigen and COVID-19. He had a persistent, high fever, reduced appetite, and reduced urine output and was taken to the ED. On physical examination, there was no rash, skin peeling, redness of the eye or oral mucosa, congestion, rhinorrhea, cough, shortness of breath, chest pain, abdominal pain, nausea, vomiting, or diarrhea.
Urinalysis results and exam findings were suspicious for pyelonephritis. Other findings from an extensive laboratory workup raised the alarm that the boy was suffering from MIS-C as opposed to incomplete KD. After admission to hospital medicine, the cardiology, rheumatology, and infectious disease teams were called in to consult.
Repeat labs were planned for the following day before initiating therapy. On day 2, the child’s urine culture was positive for gram-negative rods, later identified as Escherichia coli. The boy was started on ceftriaxone. Left renal scarring was apparent on ultrasound. The patient’s condition resolved after 36 hours, and he was discharged home with antibiotics.
‘Diagnosis derailed’
Calling this a case of “diagnosis derailed,” the authors noted that, in the pre-COVID era, this child’s signs and symptoms would likely have triggered a more targeted and less costly evaluation for more common infectious and noninfectious causes, including pyelonephritis, absent any physical exam findings consistent with KD.
“However, the patient presented in the midst of the COVID-19 pandemic with growing awareness of a new clinical entity,” Dr. Molloy and colleagues wrote. “Anchored to the patient’s persistent fever, the medical team initiated an extensive, costly, and ultimately unnecessary workup to avoid missing the diagnosis of MIS-C; a not yet well-described diagnosis with potentially severe morbidity.”
Confirmation bias and diagnostic momentum likely contributed to the early focus on MIS-C rather than more common alternatives, the authors acknowledged. The addition of mildly abnormal laboratory data not typically obtained in the evaluation of fever led the team astray. “The diagnosis and definitive treatment may have been made earlier had the focus on concern for MIS-C not been present,” Dr. Molloy said in an interview.
Keeping value in care
The authors recognized that their initial approach to evaluating for MIS-C provided low-value care. “In our desire to not ‘miss’ MIS-C, we were performing costly evaluations that at times produced mildly abnormal, nonspecific results,” they wrote. That triggered a cascade of specialty consultations, follow-up testing, and an unwarranted diagnostic preoccupation with MIS-C.
Determining the extra price tag for the child’s workup would be complex and difficult because there is a difference in the cost to the hospital and the cost to the family, Dr. Molloy said. “However, there are potential cost savings that would be related to making a correct diagnosis in a timely manner in terms of preventing downstream effects from delayed diagnoses.”
Even as clinicians struggle with the challenging SARS-CoV-2 learning curve, Dr. Molloy and associates urged them to continue to strive for high-value care, with an unwavering focus on using only necessary resources, a stewardship the pandemic has shown to be critical.
“The COVID-19 pandemic has been an incredibly stressful time for physicians and for families,” Dr. Molloy said. “COVID-19 and related conditions like MIS-C are new, and we are learning more and more about them every week. These diagnoses are understandably on the minds of physicians and families when children present with fever.” Notwithstanding, the boy’s case underscores the need for clinicians to consider alternate diagnoses and the value of the care provided.
Impact of bias
Dr. Molloy’s group brings home the cognitive biases practitioners often suffer from, including anchoring and confirmation bias and diagnostic momentum, according to J. Howard Smart, MD, chief of pediatrics at Sharp Mary Birch Hospital for Women and Newborns, San Diego, and an assistant clinical professor of pediatrics at University of California, San Diego.
“But it is one thing to recognize these in retrospect and quite another to consider whether they may be happening to you yourself in real time,” he said in an interview. “It is almost as if we need to have a ‘time out,’ where we stop and ask ourselves whether there is something else that could be explaining our patient’s presentation, something that would be more common and more likely to be occurring.”
According to Dr. Smart, who was not involved in Dr. Molloy’s study, the team’s premature diagnostic focus on MIS-C was almost the inverse of what typically happens with KD. “It is usually the case that Kawasaki disease does not enter the differential diagnosis until late in the course of the fever, typically on day 5 or later, when it may have been better to think of it earlier,” he said.
In the second article, Andrea Dean, MD, of the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, and colleagues outlined the cases of five patients aged 8-17 years who were hospitalized in May 2020 for suspected MIS-C. They exhibited inflammatory and other concerning indicators but were eventually discharged with a diagnosis of murine typhus.
This flea-borne infection, most commonly reported in the United States in the southeastern Gulf Coast region, Hawaii, and California, is often associated with a triad of fever, rash, and headache.
Cases have been rising in southern Texas, and Dr. Dean and colleagues postulated that school closures and social distancing may have increased exposure as a result of children spending more time outdoors or with pets. “Alternatively, parental concern for SARS-CoV-2 infection could mean children with symptoms are presenting to care and being referred or admitted to the hospital more frequently due to provider concern for MIS-C,” they wrote.
Cardiac involvement
The most concerning of the five cases in terms of possible MIS-C, Dr. Dean said in an interview, was that of a 12-year-old boy who had fever for 6 days in association with headache, eczematous rash, dry lips, and conjunctivitis. Laboratory tests showed a mildly elevated C-reactive protein level, hyponatremia, and thrombocytopenia, as well as sterile pyuria and mildly elevated prothrombin time. He was treated empirically with doxycycline, and his fever resolved over the next 24 hours.
An echocardiogram at initial evaluation, however, revealed mild dilation of the left anterior descending and right coronary arteries, which led to the administration of intravenous immunoglobulin and aspirin for atypical KD, in contrast to MIS-C. The authors postulated that mild cardiac involvement in disorders other than MIS-C and KD may be underrecognized.
The lesson from these cases, Dr. Dean and associates concluded, is that hospitalists must maintain a wide differential diagnosis when assessing a child with prolonged fever and evidence of systemic inflammation. The CDC stipulates that a diagnosis of MIS-C requires the absence of a plausible alternative diagnosis.
In addition to common viral, bacterial, and noninfectious disorders, a range of regional endemic rickettsial and parasitic infections must be considered as alternative diagnoses to MIS-C. “Many of these diseases cannot be reliably differentiated from MIS-C on presentation, and as community exposure to SARS-CoV-2 grows, hospitalists should be prepared to admit febrile children with evidence of systemic inflammation for brief observation periods to evaluate for MIS-C,” Dr. Dean’s group wrote. In this context, however, empiric treatment for common or even uncommon infectious diseases may avoid overdiagnosis and overtreatment of MIS-C as well as improve patient outcomes.
“We do have specific MIS-C guidelines at our institution,” Dr. Dean said, “but like all institutions, we are dealing with the broad definition of MIS-C according to the World Health Organization and the CDC, which is really the takeaway from this paper.”
More difficult differentiation
Both groups of authors pointed out that, as SARS-CoV-2 spreads throughout a community, a higher percentage of the population will have positive results on antibody testing, and such results will become less useful for differentiating between MIS-C and other conditions.
Despite these series’ cautionary lessons, other experts point to the critical importance of including MIS-C early on in the interest of efficient diagnosis and therapy. “In the cases cited, other pathologies were evaluated for and treated accordingly,” said Kara Gross Margolis, MD, AGAF, an associate professor of pediatrics in the division of pediatric gastroenterology, hepatology, and nutrition at Morgan Stanley Children’s Hospital,New York. “These papers stress the need for a balance that is important, and all potential diagnoses need to be considered, but MIS-C, due to its potential severe consequences, also needs to be on our differential now.”
In her view, as this new high-morbidity entity becomes more widespread during the pandemic, it will be increasingly important to keep this condition on the diagnostic radar.
Interestingly, in a converse example of diagnostic clouding, Dr. Gross Margolis’s group reported (Gastroenterology. 2020 Oct;159[4]:1571-4.e2) last year on a pediatric case series in which the presence of gastrointestinal symptoms in children with COVID-19–related MIS-C muddied the diagnosis by confusing this potentially severe syndrome with more common and less toxic gastrointestinal infections.
According to Dr. Smart, although the two reports don’t offer evidence for a particular diagnostic practice, they can inform the decision-making process. “It may be that we will have enough evidence shortly to say what the best practice is regarding diagnostic evaluation of possible MIS-C cases,” he said. “Until then, we must remember that common things occur commonly, even during a global pandemic.”
Neither of the two reports received any specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emergence of multiple inflammatory syndrome in children (MIS-C) in association with COVID-19 may be complicating the investigation and diagnosis of more common viral and bacterial infections, potentially delaying treatment and prolonging hospital stays.
Two recent articles published online in Hospital Pediatrics provide evidence of this phenomenon. The articles outlined case studies of children who underwent extensive investigation for MIS-C when in fact they had less severe and more common infections. MIS-C is a severe but rare syndrome that involves systemic hyperinflammation with fever and multisystem organ dysfunction similar to that of Kawasaki disease (KD).
In one of the articles, Matthew Molloy, MD, MPH, of the division of pediatric hospital medicine at Cincinnati Children’s Hospital Medical Center, and colleagues aptly asked: “What are we missing in our search for MIS-C?”
E. coli, not SARS-CoV-2
That question arose from a case involving a 3-year-old boy who had a 6-day history of fever and fatigue. Three days earlier, he had tested negative for strep antigen and COVID-19. He had a persistent, high fever, reduced appetite, and reduced urine output and was taken to the ED. On physical examination, there was no rash, skin peeling, redness of the eye or oral mucosa, congestion, rhinorrhea, cough, shortness of breath, chest pain, abdominal pain, nausea, vomiting, or diarrhea.
Urinalysis results and exam findings were suspicious for pyelonephritis. Other findings from an extensive laboratory workup raised the alarm that the boy was suffering from MIS-C as opposed to incomplete KD. After admission to hospital medicine, the cardiology, rheumatology, and infectious disease teams were called in to consult.
Repeat labs were planned for the following day before initiating therapy. On day 2, the child’s urine culture was positive for gram-negative rods, later identified as Escherichia coli. The boy was started on ceftriaxone. Left renal scarring was apparent on ultrasound. The patient’s condition resolved after 36 hours, and he was discharged home with antibiotics.
‘Diagnosis derailed’
Calling this a case of “diagnosis derailed,” the authors noted that, in the pre-COVID era, this child’s signs and symptoms would likely have triggered a more targeted and less costly evaluation for more common infectious and noninfectious causes, including pyelonephritis, absent any physical exam findings consistent with KD.
“However, the patient presented in the midst of the COVID-19 pandemic with growing awareness of a new clinical entity,” Dr. Molloy and colleagues wrote. “Anchored to the patient’s persistent fever, the medical team initiated an extensive, costly, and ultimately unnecessary workup to avoid missing the diagnosis of MIS-C; a not yet well-described diagnosis with potentially severe morbidity.”
Confirmation bias and diagnostic momentum likely contributed to the early focus on MIS-C rather than more common alternatives, the authors acknowledged. The addition of mildly abnormal laboratory data not typically obtained in the evaluation of fever led the team astray. “The diagnosis and definitive treatment may have been made earlier had the focus on concern for MIS-C not been present,” Dr. Molloy said in an interview.
Keeping value in care
The authors recognized that their initial approach to evaluating for MIS-C provided low-value care. “In our desire to not ‘miss’ MIS-C, we were performing costly evaluations that at times produced mildly abnormal, nonspecific results,” they wrote. That triggered a cascade of specialty consultations, follow-up testing, and an unwarranted diagnostic preoccupation with MIS-C.
Determining the extra price tag for the child’s workup would be complex and difficult because there is a difference in the cost to the hospital and the cost to the family, Dr. Molloy said. “However, there are potential cost savings that would be related to making a correct diagnosis in a timely manner in terms of preventing downstream effects from delayed diagnoses.”
Even as clinicians struggle with the challenging SARS-CoV-2 learning curve, Dr. Molloy and associates urged them to continue to strive for high-value care, with an unwavering focus on using only necessary resources, a stewardship the pandemic has shown to be critical.
“The COVID-19 pandemic has been an incredibly stressful time for physicians and for families,” Dr. Molloy said. “COVID-19 and related conditions like MIS-C are new, and we are learning more and more about them every week. These diagnoses are understandably on the minds of physicians and families when children present with fever.” Notwithstanding, the boy’s case underscores the need for clinicians to consider alternate diagnoses and the value of the care provided.
Impact of bias
Dr. Molloy’s group brings home the cognitive biases practitioners often suffer from, including anchoring and confirmation bias and diagnostic momentum, according to J. Howard Smart, MD, chief of pediatrics at Sharp Mary Birch Hospital for Women and Newborns, San Diego, and an assistant clinical professor of pediatrics at University of California, San Diego.
“But it is one thing to recognize these in retrospect and quite another to consider whether they may be happening to you yourself in real time,” he said in an interview. “It is almost as if we need to have a ‘time out,’ where we stop and ask ourselves whether there is something else that could be explaining our patient’s presentation, something that would be more common and more likely to be occurring.”
According to Dr. Smart, who was not involved in Dr. Molloy’s study, the team’s premature diagnostic focus on MIS-C was almost the inverse of what typically happens with KD. “It is usually the case that Kawasaki disease does not enter the differential diagnosis until late in the course of the fever, typically on day 5 or later, when it may have been better to think of it earlier,” he said.
In the second article, Andrea Dean, MD, of the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, and colleagues outlined the cases of five patients aged 8-17 years who were hospitalized in May 2020 for suspected MIS-C. They exhibited inflammatory and other concerning indicators but were eventually discharged with a diagnosis of murine typhus.
This flea-borne infection, most commonly reported in the United States in the southeastern Gulf Coast region, Hawaii, and California, is often associated with a triad of fever, rash, and headache.
Cases have been rising in southern Texas, and Dr. Dean and colleagues postulated that school closures and social distancing may have increased exposure as a result of children spending more time outdoors or with pets. “Alternatively, parental concern for SARS-CoV-2 infection could mean children with symptoms are presenting to care and being referred or admitted to the hospital more frequently due to provider concern for MIS-C,” they wrote.
Cardiac involvement
The most concerning of the five cases in terms of possible MIS-C, Dr. Dean said in an interview, was that of a 12-year-old boy who had fever for 6 days in association with headache, eczematous rash, dry lips, and conjunctivitis. Laboratory tests showed a mildly elevated C-reactive protein level, hyponatremia, and thrombocytopenia, as well as sterile pyuria and mildly elevated prothrombin time. He was treated empirically with doxycycline, and his fever resolved over the next 24 hours.
An echocardiogram at initial evaluation, however, revealed mild dilation of the left anterior descending and right coronary arteries, which led to the administration of intravenous immunoglobulin and aspirin for atypical KD, in contrast to MIS-C. The authors postulated that mild cardiac involvement in disorders other than MIS-C and KD may be underrecognized.
The lesson from these cases, Dr. Dean and associates concluded, is that hospitalists must maintain a wide differential diagnosis when assessing a child with prolonged fever and evidence of systemic inflammation. The CDC stipulates that a diagnosis of MIS-C requires the absence of a plausible alternative diagnosis.
In addition to common viral, bacterial, and noninfectious disorders, a range of regional endemic rickettsial and parasitic infections must be considered as alternative diagnoses to MIS-C. “Many of these diseases cannot be reliably differentiated from MIS-C on presentation, and as community exposure to SARS-CoV-2 grows, hospitalists should be prepared to admit febrile children with evidence of systemic inflammation for brief observation periods to evaluate for MIS-C,” Dr. Dean’s group wrote. In this context, however, empiric treatment for common or even uncommon infectious diseases may avoid overdiagnosis and overtreatment of MIS-C as well as improve patient outcomes.
“We do have specific MIS-C guidelines at our institution,” Dr. Dean said, “but like all institutions, we are dealing with the broad definition of MIS-C according to the World Health Organization and the CDC, which is really the takeaway from this paper.”
More difficult differentiation
Both groups of authors pointed out that, as SARS-CoV-2 spreads throughout a community, a higher percentage of the population will have positive results on antibody testing, and such results will become less useful for differentiating between MIS-C and other conditions.
Despite these series’ cautionary lessons, other experts point to the critical importance of including MIS-C early on in the interest of efficient diagnosis and therapy. “In the cases cited, other pathologies were evaluated for and treated accordingly,” said Kara Gross Margolis, MD, AGAF, an associate professor of pediatrics in the division of pediatric gastroenterology, hepatology, and nutrition at Morgan Stanley Children’s Hospital,New York. “These papers stress the need for a balance that is important, and all potential diagnoses need to be considered, but MIS-C, due to its potential severe consequences, also needs to be on our differential now.”
In her view, as this new high-morbidity entity becomes more widespread during the pandemic, it will be increasingly important to keep this condition on the diagnostic radar.
Interestingly, in a converse example of diagnostic clouding, Dr. Gross Margolis’s group reported (Gastroenterology. 2020 Oct;159[4]:1571-4.e2) last year on a pediatric case series in which the presence of gastrointestinal symptoms in children with COVID-19–related MIS-C muddied the diagnosis by confusing this potentially severe syndrome with more common and less toxic gastrointestinal infections.
According to Dr. Smart, although the two reports don’t offer evidence for a particular diagnostic practice, they can inform the decision-making process. “It may be that we will have enough evidence shortly to say what the best practice is regarding diagnostic evaluation of possible MIS-C cases,” he said. “Until then, we must remember that common things occur commonly, even during a global pandemic.”
Neither of the two reports received any specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emergence of multiple inflammatory syndrome in children (MIS-C) in association with COVID-19 may be complicating the investigation and diagnosis of more common viral and bacterial infections, potentially delaying treatment and prolonging hospital stays.
Two recent articles published online in Hospital Pediatrics provide evidence of this phenomenon. The articles outlined case studies of children who underwent extensive investigation for MIS-C when in fact they had less severe and more common infections. MIS-C is a severe but rare syndrome that involves systemic hyperinflammation with fever and multisystem organ dysfunction similar to that of Kawasaki disease (KD).
In one of the articles, Matthew Molloy, MD, MPH, of the division of pediatric hospital medicine at Cincinnati Children’s Hospital Medical Center, and colleagues aptly asked: “What are we missing in our search for MIS-C?”
E. coli, not SARS-CoV-2
That question arose from a case involving a 3-year-old boy who had a 6-day history of fever and fatigue. Three days earlier, he had tested negative for strep antigen and COVID-19. He had a persistent, high fever, reduced appetite, and reduced urine output and was taken to the ED. On physical examination, there was no rash, skin peeling, redness of the eye or oral mucosa, congestion, rhinorrhea, cough, shortness of breath, chest pain, abdominal pain, nausea, vomiting, or diarrhea.
Urinalysis results and exam findings were suspicious for pyelonephritis. Other findings from an extensive laboratory workup raised the alarm that the boy was suffering from MIS-C as opposed to incomplete KD. After admission to hospital medicine, the cardiology, rheumatology, and infectious disease teams were called in to consult.
Repeat labs were planned for the following day before initiating therapy. On day 2, the child’s urine culture was positive for gram-negative rods, later identified as Escherichia coli. The boy was started on ceftriaxone. Left renal scarring was apparent on ultrasound. The patient’s condition resolved after 36 hours, and he was discharged home with antibiotics.
‘Diagnosis derailed’
Calling this a case of “diagnosis derailed,” the authors noted that, in the pre-COVID era, this child’s signs and symptoms would likely have triggered a more targeted and less costly evaluation for more common infectious and noninfectious causes, including pyelonephritis, absent any physical exam findings consistent with KD.
“However, the patient presented in the midst of the COVID-19 pandemic with growing awareness of a new clinical entity,” Dr. Molloy and colleagues wrote. “Anchored to the patient’s persistent fever, the medical team initiated an extensive, costly, and ultimately unnecessary workup to avoid missing the diagnosis of MIS-C; a not yet well-described diagnosis with potentially severe morbidity.”
Confirmation bias and diagnostic momentum likely contributed to the early focus on MIS-C rather than more common alternatives, the authors acknowledged. The addition of mildly abnormal laboratory data not typically obtained in the evaluation of fever led the team astray. “The diagnosis and definitive treatment may have been made earlier had the focus on concern for MIS-C not been present,” Dr. Molloy said in an interview.
Keeping value in care
The authors recognized that their initial approach to evaluating for MIS-C provided low-value care. “In our desire to not ‘miss’ MIS-C, we were performing costly evaluations that at times produced mildly abnormal, nonspecific results,” they wrote. That triggered a cascade of specialty consultations, follow-up testing, and an unwarranted diagnostic preoccupation with MIS-C.
Determining the extra price tag for the child’s workup would be complex and difficult because there is a difference in the cost to the hospital and the cost to the family, Dr. Molloy said. “However, there are potential cost savings that would be related to making a correct diagnosis in a timely manner in terms of preventing downstream effects from delayed diagnoses.”
Even as clinicians struggle with the challenging SARS-CoV-2 learning curve, Dr. Molloy and associates urged them to continue to strive for high-value care, with an unwavering focus on using only necessary resources, a stewardship the pandemic has shown to be critical.
“The COVID-19 pandemic has been an incredibly stressful time for physicians and for families,” Dr. Molloy said. “COVID-19 and related conditions like MIS-C are new, and we are learning more and more about them every week. These diagnoses are understandably on the minds of physicians and families when children present with fever.” Notwithstanding, the boy’s case underscores the need for clinicians to consider alternate diagnoses and the value of the care provided.
Impact of bias
Dr. Molloy’s group brings home the cognitive biases practitioners often suffer from, including anchoring and confirmation bias and diagnostic momentum, according to J. Howard Smart, MD, chief of pediatrics at Sharp Mary Birch Hospital for Women and Newborns, San Diego, and an assistant clinical professor of pediatrics at University of California, San Diego.
“But it is one thing to recognize these in retrospect and quite another to consider whether they may be happening to you yourself in real time,” he said in an interview. “It is almost as if we need to have a ‘time out,’ where we stop and ask ourselves whether there is something else that could be explaining our patient’s presentation, something that would be more common and more likely to be occurring.”
According to Dr. Smart, who was not involved in Dr. Molloy’s study, the team’s premature diagnostic focus on MIS-C was almost the inverse of what typically happens with KD. “It is usually the case that Kawasaki disease does not enter the differential diagnosis until late in the course of the fever, typically on day 5 or later, when it may have been better to think of it earlier,” he said.
In the second article, Andrea Dean, MD, of the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, and colleagues outlined the cases of five patients aged 8-17 years who were hospitalized in May 2020 for suspected MIS-C. They exhibited inflammatory and other concerning indicators but were eventually discharged with a diagnosis of murine typhus.
This flea-borne infection, most commonly reported in the United States in the southeastern Gulf Coast region, Hawaii, and California, is often associated with a triad of fever, rash, and headache.
Cases have been rising in southern Texas, and Dr. Dean and colleagues postulated that school closures and social distancing may have increased exposure as a result of children spending more time outdoors or with pets. “Alternatively, parental concern for SARS-CoV-2 infection could mean children with symptoms are presenting to care and being referred or admitted to the hospital more frequently due to provider concern for MIS-C,” they wrote.
Cardiac involvement
The most concerning of the five cases in terms of possible MIS-C, Dr. Dean said in an interview, was that of a 12-year-old boy who had fever for 6 days in association with headache, eczematous rash, dry lips, and conjunctivitis. Laboratory tests showed a mildly elevated C-reactive protein level, hyponatremia, and thrombocytopenia, as well as sterile pyuria and mildly elevated prothrombin time. He was treated empirically with doxycycline, and his fever resolved over the next 24 hours.
An echocardiogram at initial evaluation, however, revealed mild dilation of the left anterior descending and right coronary arteries, which led to the administration of intravenous immunoglobulin and aspirin for atypical KD, in contrast to MIS-C. The authors postulated that mild cardiac involvement in disorders other than MIS-C and KD may be underrecognized.
The lesson from these cases, Dr. Dean and associates concluded, is that hospitalists must maintain a wide differential diagnosis when assessing a child with prolonged fever and evidence of systemic inflammation. The CDC stipulates that a diagnosis of MIS-C requires the absence of a plausible alternative diagnosis.
In addition to common viral, bacterial, and noninfectious disorders, a range of regional endemic rickettsial and parasitic infections must be considered as alternative diagnoses to MIS-C. “Many of these diseases cannot be reliably differentiated from MIS-C on presentation, and as community exposure to SARS-CoV-2 grows, hospitalists should be prepared to admit febrile children with evidence of systemic inflammation for brief observation periods to evaluate for MIS-C,” Dr. Dean’s group wrote. In this context, however, empiric treatment for common or even uncommon infectious diseases may avoid overdiagnosis and overtreatment of MIS-C as well as improve patient outcomes.
“We do have specific MIS-C guidelines at our institution,” Dr. Dean said, “but like all institutions, we are dealing with the broad definition of MIS-C according to the World Health Organization and the CDC, which is really the takeaway from this paper.”
More difficult differentiation
Both groups of authors pointed out that, as SARS-CoV-2 spreads throughout a community, a higher percentage of the population will have positive results on antibody testing, and such results will become less useful for differentiating between MIS-C and other conditions.
Despite these series’ cautionary lessons, other experts point to the critical importance of including MIS-C early on in the interest of efficient diagnosis and therapy. “In the cases cited, other pathologies were evaluated for and treated accordingly,” said Kara Gross Margolis, MD, AGAF, an associate professor of pediatrics in the division of pediatric gastroenterology, hepatology, and nutrition at Morgan Stanley Children’s Hospital,New York. “These papers stress the need for a balance that is important, and all potential diagnoses need to be considered, but MIS-C, due to its potential severe consequences, also needs to be on our differential now.”
In her view, as this new high-morbidity entity becomes more widespread during the pandemic, it will be increasingly important to keep this condition on the diagnostic radar.
Interestingly, in a converse example of diagnostic clouding, Dr. Gross Margolis’s group reported (Gastroenterology. 2020 Oct;159[4]:1571-4.e2) last year on a pediatric case series in which the presence of gastrointestinal symptoms in children with COVID-19–related MIS-C muddied the diagnosis by confusing this potentially severe syndrome with more common and less toxic gastrointestinal infections.
According to Dr. Smart, although the two reports don’t offer evidence for a particular diagnostic practice, they can inform the decision-making process. “It may be that we will have enough evidence shortly to say what the best practice is regarding diagnostic evaluation of possible MIS-C cases,” he said. “Until then, we must remember that common things occur commonly, even during a global pandemic.”
Neither of the two reports received any specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.









