User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Decline in weekly child COVID-19 cases has almost stopped
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.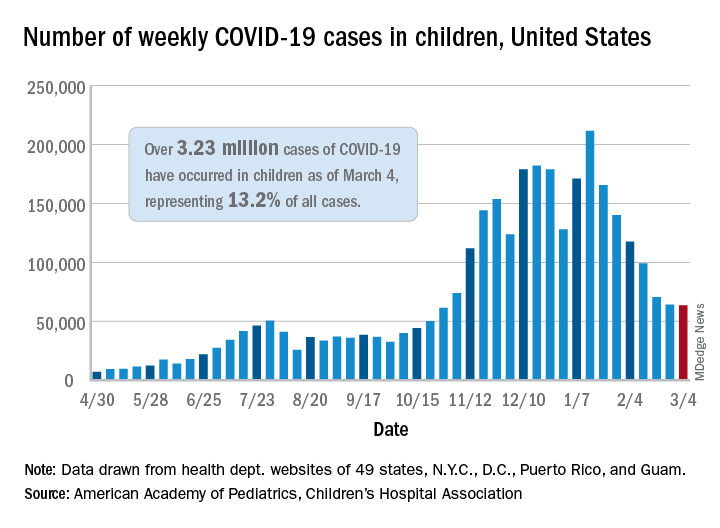
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
Call to action on obesity amid COVID-19 pandemic
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Pediatric TB – more work needed, especially with HIV-coinfection
Despite recent advances in the diagnosis, treatment, and prevention of pediatric tuberculosis in children living with HIV (CLHIV) and HIV-exposed uninfected children (HEU), several unmet needs remain, including studies evaluating the feasibility of shortened TB treatment regimens.
“Children living with HIV contribute disproportionately to pediatric TB mortality rates, accounting for 16% of child TB deaths, and many cases are underdiagnosed and underreported,” said Nicole Salazar-Austin, MD, of Johns Hopkins University in Baltimore. She provided an update on pediatric TB prevention and treatment during an educational symposium at this year’s virtual Conference on Retroviruses & Opportunistic Infections.
Dr. Salazar-Austin summarized current diagnostics for pediatric TB and reviewed options for the prevention and treatment of TB in CLHIV and HEU.
TB and CLHIV
Presently, TB is the most common opportunistic infection among CLHIV, and those with severe immune suppression have a fivefold greater risk of TB disease. While antiretroviral therapy (ART) is highly protective against TB disease in CLHIV, only about 50% of eligible children receive ART.
Dr. Salazar-Austin explained that many individuals with TB/HIV coinfection are unaware of their coinfection and not receiving treatment. Despite recommendations, TB preventive therapy is poorly implemented in CLHIV, especially in high-burden settings.
Pediatric TB diagnosis
Smear microscopy, culture, and Xpert MTB/RIF Ultra are the main diagnostic modalities for pediatric TB. The Xpert MTB/RIF test is an automated PCR-based assay that simultaneously and rapidly detects Mycobacterium tuberculosis complex and resistance to rifampin. The test is currently recommended by the World Health Organization as the initial diagnostic method for presumptive TB cases in both adults and children.
However, under optimal conditions, only 40% of TB cases will be detected. This is in part due to limited implementation of sputum collection procedures, but recent evidence has shown that collection of multiple specimens improves sensitivity for both culture and Xpert MTB/RIF Ultra across all specimen types, Dr. Salazar-Austin explained.
In 2020, the WHO endorsed the use of stool samples for the diagnosis of pediatric pulmonary TB. Stool Xpert is an emerging alternative, noninvasive method for ruling in pediatric TB disease, and has shown sensitivity and specificity similar to that of Xpert MTB/RIF Ultra.
“TB diagnostics have limited sensitivity in children, and efforts are ongoing to maximize current diagnostics, but new diagnostics are needed,” said Dr. Salazar-Austin.
Pediatric TB treatment
Despite the high frequency of TB as an opportunistic infection in CLHIV, current data on co-treatment strategies are limited.
Dolutegravir-based regimens are the preferred first-line regimen for CLHIV. In June 2020, the Food and Drug Administration approved the dispersible dolutegravir tablet, and it is expected to become widely available in 2021.
In children with TB/HIV coinfection who receive dolutegravir and rifampicin, dolutegravir is typically dosed twice daily because of a known drug interaction, based on data from the ODYSSEY study. The WHO recommendations for treatment of pediatric TB/HIV coinfection were recently updated to reflect twice-daily dosing of dolutegravir.
Despite these new recommendations, data are currently limited, and observational pharmacokinetic studies evaluating twice daily dolutegravir with TB treatment in young children are needed.
“More work is needed to evaluate the drug-drug interactions and proper dosing of rifamycins with dolutegravir for the treatment and prevention of TB in CLHIV,” Dr. Salazar-Austin said.
Based on data from TBTC Study 31/ACTG A5349, high-dose rifapentine (a rifamycin) with moxifloxacin (a fluoroquinolone) was noninferior to rifapentine alone in newly diagnosed, culture positive, drug-susceptible TB in children 12 years and older.
Whether rifapentine and moxifloxacin (RPT-Mox) can be used in children under 12 years remains unknown, but future studies may help answer this question, Dr. Salazar-Austin noted. The FDA has restricted the use of fluoroquinolones in children because of a possible effect on cartilage development, she explained.
Furthermore, recent data from the SHINE trial suggested that shortened treatment regimens may hold promise for children with TB.
“While shortened TB treatment regimens hold promise, much work needs to be done in children to implement RPT-Mox, but the results from SHINE can be implemented rapidly,” Dr. Salazar-Austin said.
Dr. Salazar-Austin disclosed no conflicts of interest. The presentation was funded by NICHD, UNITAID, Fogarty Institute, and the IMPAACT network.
Despite recent advances in the diagnosis, treatment, and prevention of pediatric tuberculosis in children living with HIV (CLHIV) and HIV-exposed uninfected children (HEU), several unmet needs remain, including studies evaluating the feasibility of shortened TB treatment regimens.
“Children living with HIV contribute disproportionately to pediatric TB mortality rates, accounting for 16% of child TB deaths, and many cases are underdiagnosed and underreported,” said Nicole Salazar-Austin, MD, of Johns Hopkins University in Baltimore. She provided an update on pediatric TB prevention and treatment during an educational symposium at this year’s virtual Conference on Retroviruses & Opportunistic Infections.
Dr. Salazar-Austin summarized current diagnostics for pediatric TB and reviewed options for the prevention and treatment of TB in CLHIV and HEU.
TB and CLHIV
Presently, TB is the most common opportunistic infection among CLHIV, and those with severe immune suppression have a fivefold greater risk of TB disease. While antiretroviral therapy (ART) is highly protective against TB disease in CLHIV, only about 50% of eligible children receive ART.
Dr. Salazar-Austin explained that many individuals with TB/HIV coinfection are unaware of their coinfection and not receiving treatment. Despite recommendations, TB preventive therapy is poorly implemented in CLHIV, especially in high-burden settings.
Pediatric TB diagnosis
Smear microscopy, culture, and Xpert MTB/RIF Ultra are the main diagnostic modalities for pediatric TB. The Xpert MTB/RIF test is an automated PCR-based assay that simultaneously and rapidly detects Mycobacterium tuberculosis complex and resistance to rifampin. The test is currently recommended by the World Health Organization as the initial diagnostic method for presumptive TB cases in both adults and children.
However, under optimal conditions, only 40% of TB cases will be detected. This is in part due to limited implementation of sputum collection procedures, but recent evidence has shown that collection of multiple specimens improves sensitivity for both culture and Xpert MTB/RIF Ultra across all specimen types, Dr. Salazar-Austin explained.
In 2020, the WHO endorsed the use of stool samples for the diagnosis of pediatric pulmonary TB. Stool Xpert is an emerging alternative, noninvasive method for ruling in pediatric TB disease, and has shown sensitivity and specificity similar to that of Xpert MTB/RIF Ultra.
“TB diagnostics have limited sensitivity in children, and efforts are ongoing to maximize current diagnostics, but new diagnostics are needed,” said Dr. Salazar-Austin.
Pediatric TB treatment
Despite the high frequency of TB as an opportunistic infection in CLHIV, current data on co-treatment strategies are limited.
Dolutegravir-based regimens are the preferred first-line regimen for CLHIV. In June 2020, the Food and Drug Administration approved the dispersible dolutegravir tablet, and it is expected to become widely available in 2021.
In children with TB/HIV coinfection who receive dolutegravir and rifampicin, dolutegravir is typically dosed twice daily because of a known drug interaction, based on data from the ODYSSEY study. The WHO recommendations for treatment of pediatric TB/HIV coinfection were recently updated to reflect twice-daily dosing of dolutegravir.
Despite these new recommendations, data are currently limited, and observational pharmacokinetic studies evaluating twice daily dolutegravir with TB treatment in young children are needed.
“More work is needed to evaluate the drug-drug interactions and proper dosing of rifamycins with dolutegravir for the treatment and prevention of TB in CLHIV,” Dr. Salazar-Austin said.
Based on data from TBTC Study 31/ACTG A5349, high-dose rifapentine (a rifamycin) with moxifloxacin (a fluoroquinolone) was noninferior to rifapentine alone in newly diagnosed, culture positive, drug-susceptible TB in children 12 years and older.
Whether rifapentine and moxifloxacin (RPT-Mox) can be used in children under 12 years remains unknown, but future studies may help answer this question, Dr. Salazar-Austin noted. The FDA has restricted the use of fluoroquinolones in children because of a possible effect on cartilage development, she explained.
Furthermore, recent data from the SHINE trial suggested that shortened treatment regimens may hold promise for children with TB.
“While shortened TB treatment regimens hold promise, much work needs to be done in children to implement RPT-Mox, but the results from SHINE can be implemented rapidly,” Dr. Salazar-Austin said.
Dr. Salazar-Austin disclosed no conflicts of interest. The presentation was funded by NICHD, UNITAID, Fogarty Institute, and the IMPAACT network.
Despite recent advances in the diagnosis, treatment, and prevention of pediatric tuberculosis in children living with HIV (CLHIV) and HIV-exposed uninfected children (HEU), several unmet needs remain, including studies evaluating the feasibility of shortened TB treatment regimens.
“Children living with HIV contribute disproportionately to pediatric TB mortality rates, accounting for 16% of child TB deaths, and many cases are underdiagnosed and underreported,” said Nicole Salazar-Austin, MD, of Johns Hopkins University in Baltimore. She provided an update on pediatric TB prevention and treatment during an educational symposium at this year’s virtual Conference on Retroviruses & Opportunistic Infections.
Dr. Salazar-Austin summarized current diagnostics for pediatric TB and reviewed options for the prevention and treatment of TB in CLHIV and HEU.
TB and CLHIV
Presently, TB is the most common opportunistic infection among CLHIV, and those with severe immune suppression have a fivefold greater risk of TB disease. While antiretroviral therapy (ART) is highly protective against TB disease in CLHIV, only about 50% of eligible children receive ART.
Dr. Salazar-Austin explained that many individuals with TB/HIV coinfection are unaware of their coinfection and not receiving treatment. Despite recommendations, TB preventive therapy is poorly implemented in CLHIV, especially in high-burden settings.
Pediatric TB diagnosis
Smear microscopy, culture, and Xpert MTB/RIF Ultra are the main diagnostic modalities for pediatric TB. The Xpert MTB/RIF test is an automated PCR-based assay that simultaneously and rapidly detects Mycobacterium tuberculosis complex and resistance to rifampin. The test is currently recommended by the World Health Organization as the initial diagnostic method for presumptive TB cases in both adults and children.
However, under optimal conditions, only 40% of TB cases will be detected. This is in part due to limited implementation of sputum collection procedures, but recent evidence has shown that collection of multiple specimens improves sensitivity for both culture and Xpert MTB/RIF Ultra across all specimen types, Dr. Salazar-Austin explained.
In 2020, the WHO endorsed the use of stool samples for the diagnosis of pediatric pulmonary TB. Stool Xpert is an emerging alternative, noninvasive method for ruling in pediatric TB disease, and has shown sensitivity and specificity similar to that of Xpert MTB/RIF Ultra.
“TB diagnostics have limited sensitivity in children, and efforts are ongoing to maximize current diagnostics, but new diagnostics are needed,” said Dr. Salazar-Austin.
Pediatric TB treatment
Despite the high frequency of TB as an opportunistic infection in CLHIV, current data on co-treatment strategies are limited.
Dolutegravir-based regimens are the preferred first-line regimen for CLHIV. In June 2020, the Food and Drug Administration approved the dispersible dolutegravir tablet, and it is expected to become widely available in 2021.
In children with TB/HIV coinfection who receive dolutegravir and rifampicin, dolutegravir is typically dosed twice daily because of a known drug interaction, based on data from the ODYSSEY study. The WHO recommendations for treatment of pediatric TB/HIV coinfection were recently updated to reflect twice-daily dosing of dolutegravir.
Despite these new recommendations, data are currently limited, and observational pharmacokinetic studies evaluating twice daily dolutegravir with TB treatment in young children are needed.
“More work is needed to evaluate the drug-drug interactions and proper dosing of rifamycins with dolutegravir for the treatment and prevention of TB in CLHIV,” Dr. Salazar-Austin said.
Based on data from TBTC Study 31/ACTG A5349, high-dose rifapentine (a rifamycin) with moxifloxacin (a fluoroquinolone) was noninferior to rifapentine alone in newly diagnosed, culture positive, drug-susceptible TB in children 12 years and older.
Whether rifapentine and moxifloxacin (RPT-Mox) can be used in children under 12 years remains unknown, but future studies may help answer this question, Dr. Salazar-Austin noted. The FDA has restricted the use of fluoroquinolones in children because of a possible effect on cartilage development, she explained.
Furthermore, recent data from the SHINE trial suggested that shortened treatment regimens may hold promise for children with TB.
“While shortened TB treatment regimens hold promise, much work needs to be done in children to implement RPT-Mox, but the results from SHINE can be implemented rapidly,” Dr. Salazar-Austin said.
Dr. Salazar-Austin disclosed no conflicts of interest. The presentation was funded by NICHD, UNITAID, Fogarty Institute, and the IMPAACT network.
FROM CROI 2021
Are long-acting injectables the future of TB treatment?
Long-acting injectable (LAI) drug formulations represent a promising new strategy for the prevention and treatment of tuberculosis in women and children, according to an online presentation at the Conference on Retroviruses & Opportunistic Infections, held virtually.
“As a delivery strategy, LAIs hold the potential to unlock a vast chemical space of lipophilic compounds with very potent anti-TB activity that would otherwise not be developed due to poor predicted oral bioavailability,” explained presenter Eric Nuermberger, MD.
He summarized current preventive treatment options for TB and reviewed the potential impact of LAI formulations on TB therapy. In addition, he identified key challenges for future LAI development and proposed a new development path for clinical implementation.
Current TB preventive therapies
Despite widespread availability, the uptake of TB preventive therapy is poor and currently lags behind global targets. One key barrier to widespread uptake is the long duration of treatment, which may hinder patient adherence to therapy.
While shorter preventive regimens, such as 1 month of daily isoniazid plus rifapentine, show similar efficacy and higher completion rates, further shortening of therapy and reducing clinic visits are the most direct methods to increase adherence and treatment completion rates, Dr. Nuermberger said.
LAI drugs
LAI drug formulations allow for slow release of suitable drugs from a depot injected subcutaneously or intramuscularly.
The goal of LAI formulations is to free patients from the daily burden of oral administration. Other potential benefits include better adherence and efficacy, drug exposure, and the potential to overcome intrinsic poor oral bioavailability by bypassing the GI tract entirely.
Potential indications for LAIs include treatment of latent tuberculosis infection (LTBI), and as continuous therapy in people living with HIV in high-burden settings. There is also potential for treating younger children, such as household contacts, who have difficulty taking oral medications.
“We’ve already seen LAIs revolutionize other areas, such as psychiatry and contraception, and we appear to have another revolution in HIV prevention and treatment,” Dr. Nuermberger explained.
Not all existing TB drugs are suitable for LAI formulations, but drugs such as rifapentine, rifabutin, delamanid, and bedaquiline, show more promise than isoniazid or rifampin because of their physiochemical composition. Of all, bedaquiline may offer the best profile for LAI formulation, Dr. Nuermberger said.
Early proof-of-concept in vivo studies have shown potential use of LAI bedaquiline for TB prevention in both drug-sensitive and drug-resistant TB contacts. Translational PK modeling and simulation predicted that a 1-g intramuscular injection of LAI bedaquiline could maintain therapeutic plasma concentrations in humans for greater than 1 month.
Dr. Nuermberger noted that novel diarylquinoline-based therapies, currently in phase 1 studies, may be even better candidates for LAI-based TB preventive therapy. Early data suggests these compounds may be 10-20 times more potent and have a lower CV risk profile than that of bedaquiline.
Considerations for development and implementation
“Despite the promising potential of long-acting injectables for TB, we are still in the very early stages,” said Dr. Nuermberger.
Ensuring and optimizing acceptance of LAI formulations, especially in at-risk populations, will be very important, he explained. Early involvement of children and pregnant women in studies of who may benefit most from LAI drugs will also be essential.
Other important considerations include cost-effectiveness, particularly in at-risk and vulnerable populations. Furthermore, new dedicated research and development programs are needed to continue to develop more drug candidates suitable for LAI.
“Long-acting formulations hold enormous promise to be transformative for combating TB, through simplification of delivery and overcoming issues of adherence that can compromise success of current interventions,” said Andrew Owen, PhD, of the University of Liverpool (England).
“The ability to deliver an entire course of drug in a single visit promises to ensure missed doses don’t compromise outcomes or place unnecessary selective pressure in favor of drug resistance,” Dr. Owen said.
“Recent studies showing the value of one-month oral treatment regimens for LTBI make long-acting formulations seem more realistic and drugs such as long-acting bedaquiline put a one-shot regimen within reach,” Charles W. Flexner, MD, of Johns Hopkins University, Baltimore, said in an interview.
While no LAIs have been approved for TB, Dr. Nuermberger was optimistic that the recent success of LAI formulations for HIV treatment and prevention will catalyze further efforts in the TB landscape.
Dr. Nuermberger disclosed research support from Janssen Pharmaceuticals, TB Alliance, and the Gates Medical Research Institute. The presentation was sponsored by Janssen Pharmaceuticals, Johns Hopkins CFAR, NIH, Unitaid, and the TB Alliance.
Long-acting injectable (LAI) drug formulations represent a promising new strategy for the prevention and treatment of tuberculosis in women and children, according to an online presentation at the Conference on Retroviruses & Opportunistic Infections, held virtually.
“As a delivery strategy, LAIs hold the potential to unlock a vast chemical space of lipophilic compounds with very potent anti-TB activity that would otherwise not be developed due to poor predicted oral bioavailability,” explained presenter Eric Nuermberger, MD.
He summarized current preventive treatment options for TB and reviewed the potential impact of LAI formulations on TB therapy. In addition, he identified key challenges for future LAI development and proposed a new development path for clinical implementation.
Current TB preventive therapies
Despite widespread availability, the uptake of TB preventive therapy is poor and currently lags behind global targets. One key barrier to widespread uptake is the long duration of treatment, which may hinder patient adherence to therapy.
While shorter preventive regimens, such as 1 month of daily isoniazid plus rifapentine, show similar efficacy and higher completion rates, further shortening of therapy and reducing clinic visits are the most direct methods to increase adherence and treatment completion rates, Dr. Nuermberger said.
LAI drugs
LAI drug formulations allow for slow release of suitable drugs from a depot injected subcutaneously or intramuscularly.
The goal of LAI formulations is to free patients from the daily burden of oral administration. Other potential benefits include better adherence and efficacy, drug exposure, and the potential to overcome intrinsic poor oral bioavailability by bypassing the GI tract entirely.
Potential indications for LAIs include treatment of latent tuberculosis infection (LTBI), and as continuous therapy in people living with HIV in high-burden settings. There is also potential for treating younger children, such as household contacts, who have difficulty taking oral medications.
“We’ve already seen LAIs revolutionize other areas, such as psychiatry and contraception, and we appear to have another revolution in HIV prevention and treatment,” Dr. Nuermberger explained.
Not all existing TB drugs are suitable for LAI formulations, but drugs such as rifapentine, rifabutin, delamanid, and bedaquiline, show more promise than isoniazid or rifampin because of their physiochemical composition. Of all, bedaquiline may offer the best profile for LAI formulation, Dr. Nuermberger said.
Early proof-of-concept in vivo studies have shown potential use of LAI bedaquiline for TB prevention in both drug-sensitive and drug-resistant TB contacts. Translational PK modeling and simulation predicted that a 1-g intramuscular injection of LAI bedaquiline could maintain therapeutic plasma concentrations in humans for greater than 1 month.
Dr. Nuermberger noted that novel diarylquinoline-based therapies, currently in phase 1 studies, may be even better candidates for LAI-based TB preventive therapy. Early data suggests these compounds may be 10-20 times more potent and have a lower CV risk profile than that of bedaquiline.
Considerations for development and implementation
“Despite the promising potential of long-acting injectables for TB, we are still in the very early stages,” said Dr. Nuermberger.
Ensuring and optimizing acceptance of LAI formulations, especially in at-risk populations, will be very important, he explained. Early involvement of children and pregnant women in studies of who may benefit most from LAI drugs will also be essential.
Other important considerations include cost-effectiveness, particularly in at-risk and vulnerable populations. Furthermore, new dedicated research and development programs are needed to continue to develop more drug candidates suitable for LAI.
“Long-acting formulations hold enormous promise to be transformative for combating TB, through simplification of delivery and overcoming issues of adherence that can compromise success of current interventions,” said Andrew Owen, PhD, of the University of Liverpool (England).
“The ability to deliver an entire course of drug in a single visit promises to ensure missed doses don’t compromise outcomes or place unnecessary selective pressure in favor of drug resistance,” Dr. Owen said.
“Recent studies showing the value of one-month oral treatment regimens for LTBI make long-acting formulations seem more realistic and drugs such as long-acting bedaquiline put a one-shot regimen within reach,” Charles W. Flexner, MD, of Johns Hopkins University, Baltimore, said in an interview.
While no LAIs have been approved for TB, Dr. Nuermberger was optimistic that the recent success of LAI formulations for HIV treatment and prevention will catalyze further efforts in the TB landscape.
Dr. Nuermberger disclosed research support from Janssen Pharmaceuticals, TB Alliance, and the Gates Medical Research Institute. The presentation was sponsored by Janssen Pharmaceuticals, Johns Hopkins CFAR, NIH, Unitaid, and the TB Alliance.
Long-acting injectable (LAI) drug formulations represent a promising new strategy for the prevention and treatment of tuberculosis in women and children, according to an online presentation at the Conference on Retroviruses & Opportunistic Infections, held virtually.
“As a delivery strategy, LAIs hold the potential to unlock a vast chemical space of lipophilic compounds with very potent anti-TB activity that would otherwise not be developed due to poor predicted oral bioavailability,” explained presenter Eric Nuermberger, MD.
He summarized current preventive treatment options for TB and reviewed the potential impact of LAI formulations on TB therapy. In addition, he identified key challenges for future LAI development and proposed a new development path for clinical implementation.
Current TB preventive therapies
Despite widespread availability, the uptake of TB preventive therapy is poor and currently lags behind global targets. One key barrier to widespread uptake is the long duration of treatment, which may hinder patient adherence to therapy.
While shorter preventive regimens, such as 1 month of daily isoniazid plus rifapentine, show similar efficacy and higher completion rates, further shortening of therapy and reducing clinic visits are the most direct methods to increase adherence and treatment completion rates, Dr. Nuermberger said.
LAI drugs
LAI drug formulations allow for slow release of suitable drugs from a depot injected subcutaneously or intramuscularly.
The goal of LAI formulations is to free patients from the daily burden of oral administration. Other potential benefits include better adherence and efficacy, drug exposure, and the potential to overcome intrinsic poor oral bioavailability by bypassing the GI tract entirely.
Potential indications for LAIs include treatment of latent tuberculosis infection (LTBI), and as continuous therapy in people living with HIV in high-burden settings. There is also potential for treating younger children, such as household contacts, who have difficulty taking oral medications.
“We’ve already seen LAIs revolutionize other areas, such as psychiatry and contraception, and we appear to have another revolution in HIV prevention and treatment,” Dr. Nuermberger explained.
Not all existing TB drugs are suitable for LAI formulations, but drugs such as rifapentine, rifabutin, delamanid, and bedaquiline, show more promise than isoniazid or rifampin because of their physiochemical composition. Of all, bedaquiline may offer the best profile for LAI formulation, Dr. Nuermberger said.
Early proof-of-concept in vivo studies have shown potential use of LAI bedaquiline for TB prevention in both drug-sensitive and drug-resistant TB contacts. Translational PK modeling and simulation predicted that a 1-g intramuscular injection of LAI bedaquiline could maintain therapeutic plasma concentrations in humans for greater than 1 month.
Dr. Nuermberger noted that novel diarylquinoline-based therapies, currently in phase 1 studies, may be even better candidates for LAI-based TB preventive therapy. Early data suggests these compounds may be 10-20 times more potent and have a lower CV risk profile than that of bedaquiline.
Considerations for development and implementation
“Despite the promising potential of long-acting injectables for TB, we are still in the very early stages,” said Dr. Nuermberger.
Ensuring and optimizing acceptance of LAI formulations, especially in at-risk populations, will be very important, he explained. Early involvement of children and pregnant women in studies of who may benefit most from LAI drugs will also be essential.
Other important considerations include cost-effectiveness, particularly in at-risk and vulnerable populations. Furthermore, new dedicated research and development programs are needed to continue to develop more drug candidates suitable for LAI.
“Long-acting formulations hold enormous promise to be transformative for combating TB, through simplification of delivery and overcoming issues of adherence that can compromise success of current interventions,” said Andrew Owen, PhD, of the University of Liverpool (England).
“The ability to deliver an entire course of drug in a single visit promises to ensure missed doses don’t compromise outcomes or place unnecessary selective pressure in favor of drug resistance,” Dr. Owen said.
“Recent studies showing the value of one-month oral treatment regimens for LTBI make long-acting formulations seem more realistic and drugs such as long-acting bedaquiline put a one-shot regimen within reach,” Charles W. Flexner, MD, of Johns Hopkins University, Baltimore, said in an interview.
While no LAIs have been approved for TB, Dr. Nuermberger was optimistic that the recent success of LAI formulations for HIV treatment and prevention will catalyze further efforts in the TB landscape.
Dr. Nuermberger disclosed research support from Janssen Pharmaceuticals, TB Alliance, and the Gates Medical Research Institute. The presentation was sponsored by Janssen Pharmaceuticals, Johns Hopkins CFAR, NIH, Unitaid, and the TB Alliance.
FROM CROI 2021
Potential COVID-19 variant surge looms over U.S.
Another coronavirus surge may be on the way in the United States as daily COVID-19 cases continue to plateau around 60,000, states begin to lift restrictions, and people embark on spring break trips this week, according to CNN.
Outbreaks will likely stem from the B.1.1.7 variant, which was first identified in the United Kingdom, and gain momentum during the next 6-14 weeks.
“Four weeks ago, the B.1.1.7 variant made up about 1%-4% of the virus that we were seeing in communities across the country. Today it’s up to 30%-40%,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told NBC’s Meet the Press on March 7.
Dr. Osterholm compared the current situation with the “eye of the hurricane,” where the skies appear clear but more storms are on the way. Across Europe, 27 countries are seeing significant B.1.1.7 case increases, and 10 are getting hit hard, he said.
“What we’ve seen in Europe, when we hit that 50% mark, you see cases surge,” he said. “So right now, we do have to keep America as safe as we can from this virus by not letting up on any of the public health measures we’ve taken.”
In January, the CDC warned that B.1.1.7 variant cases would increase in 2021 and become the dominant variant in the country by this month. The United States has now reported more than 3,000 cases across 46 states, according to the latest CDC tally updated on March 7. More than 600 cases have been found in Florida, followed by more than 400 in Michigan.
The CDC has said the tally doesn’t represent the total number of B.1.1.7 cases in the United States, only the ones that have been identified by analyzing samples through genomic sequencing.
“Where it has hit in the U.K. and now elsewhere in Europe, it has been catastrophic,” Celine Gounder, MD, an infectious disease specialist with New York University Langone Health, told CNN on March 7.
The variant is more transmissible than the original novel coronavirus, and the cases in the United States are “increasing exponentially,” she said.
“It has driven up rates of hospitalizations and deaths and it’s very difficult to control,” Dr. Gounder said.
Vaccination numbers aren’t yet high enough to stop the predicted surge, she added. The United States has shipped more than 116 million vaccine doses, according to the latest CDC update on March 7. Nearly 59 million people have received at least one dose, and 30.6 million people have received two vaccine doses. About 9% of the U.S. population has been fully vaccinated.
States shouldn’t ease restrictions until the vaccination numbers are much higher and daily COVID-19 cases fall below 10,000 – and maybe “considerably less than that,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNN on March 4.
Several states have already begun to lift COVID-19 safety protocols, with Texas and Mississippi removing mask mandates last week. Businesses in Texas will be able to reopen at full capacity on March 10. For now, public health officials are urging Americans to continue to wear masks, avoid crowds, and follow social distancing guidelines as vaccines roll out across the country.
“This is sort of like we’ve been running this really long marathon, and we’re 100 yards from the finish line and we sit down and we give up,” Dr. Gounder told CNN on Sunday. ‘We’re almost there, we just need to give ourselves a bit more time to get a larger proportion of the population covered with vaccines.”
A version of this article first appeared on WebMD.com.
Another coronavirus surge may be on the way in the United States as daily COVID-19 cases continue to plateau around 60,000, states begin to lift restrictions, and people embark on spring break trips this week, according to CNN.
Outbreaks will likely stem from the B.1.1.7 variant, which was first identified in the United Kingdom, and gain momentum during the next 6-14 weeks.
“Four weeks ago, the B.1.1.7 variant made up about 1%-4% of the virus that we were seeing in communities across the country. Today it’s up to 30%-40%,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told NBC’s Meet the Press on March 7.
Dr. Osterholm compared the current situation with the “eye of the hurricane,” where the skies appear clear but more storms are on the way. Across Europe, 27 countries are seeing significant B.1.1.7 case increases, and 10 are getting hit hard, he said.
“What we’ve seen in Europe, when we hit that 50% mark, you see cases surge,” he said. “So right now, we do have to keep America as safe as we can from this virus by not letting up on any of the public health measures we’ve taken.”
In January, the CDC warned that B.1.1.7 variant cases would increase in 2021 and become the dominant variant in the country by this month. The United States has now reported more than 3,000 cases across 46 states, according to the latest CDC tally updated on March 7. More than 600 cases have been found in Florida, followed by more than 400 in Michigan.
The CDC has said the tally doesn’t represent the total number of B.1.1.7 cases in the United States, only the ones that have been identified by analyzing samples through genomic sequencing.
“Where it has hit in the U.K. and now elsewhere in Europe, it has been catastrophic,” Celine Gounder, MD, an infectious disease specialist with New York University Langone Health, told CNN on March 7.
The variant is more transmissible than the original novel coronavirus, and the cases in the United States are “increasing exponentially,” she said.
“It has driven up rates of hospitalizations and deaths and it’s very difficult to control,” Dr. Gounder said.
Vaccination numbers aren’t yet high enough to stop the predicted surge, she added. The United States has shipped more than 116 million vaccine doses, according to the latest CDC update on March 7. Nearly 59 million people have received at least one dose, and 30.6 million people have received two vaccine doses. About 9% of the U.S. population has been fully vaccinated.
States shouldn’t ease restrictions until the vaccination numbers are much higher and daily COVID-19 cases fall below 10,000 – and maybe “considerably less than that,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNN on March 4.
Several states have already begun to lift COVID-19 safety protocols, with Texas and Mississippi removing mask mandates last week. Businesses in Texas will be able to reopen at full capacity on March 10. For now, public health officials are urging Americans to continue to wear masks, avoid crowds, and follow social distancing guidelines as vaccines roll out across the country.
“This is sort of like we’ve been running this really long marathon, and we’re 100 yards from the finish line and we sit down and we give up,” Dr. Gounder told CNN on Sunday. ‘We’re almost there, we just need to give ourselves a bit more time to get a larger proportion of the population covered with vaccines.”
A version of this article first appeared on WebMD.com.
Another coronavirus surge may be on the way in the United States as daily COVID-19 cases continue to plateau around 60,000, states begin to lift restrictions, and people embark on spring break trips this week, according to CNN.
Outbreaks will likely stem from the B.1.1.7 variant, which was first identified in the United Kingdom, and gain momentum during the next 6-14 weeks.
“Four weeks ago, the B.1.1.7 variant made up about 1%-4% of the virus that we were seeing in communities across the country. Today it’s up to 30%-40%,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told NBC’s Meet the Press on March 7.
Dr. Osterholm compared the current situation with the “eye of the hurricane,” where the skies appear clear but more storms are on the way. Across Europe, 27 countries are seeing significant B.1.1.7 case increases, and 10 are getting hit hard, he said.
“What we’ve seen in Europe, when we hit that 50% mark, you see cases surge,” he said. “So right now, we do have to keep America as safe as we can from this virus by not letting up on any of the public health measures we’ve taken.”
In January, the CDC warned that B.1.1.7 variant cases would increase in 2021 and become the dominant variant in the country by this month. The United States has now reported more than 3,000 cases across 46 states, according to the latest CDC tally updated on March 7. More than 600 cases have been found in Florida, followed by more than 400 in Michigan.
The CDC has said the tally doesn’t represent the total number of B.1.1.7 cases in the United States, only the ones that have been identified by analyzing samples through genomic sequencing.
“Where it has hit in the U.K. and now elsewhere in Europe, it has been catastrophic,” Celine Gounder, MD, an infectious disease specialist with New York University Langone Health, told CNN on March 7.
The variant is more transmissible than the original novel coronavirus, and the cases in the United States are “increasing exponentially,” she said.
“It has driven up rates of hospitalizations and deaths and it’s very difficult to control,” Dr. Gounder said.
Vaccination numbers aren’t yet high enough to stop the predicted surge, she added. The United States has shipped more than 116 million vaccine doses, according to the latest CDC update on March 7. Nearly 59 million people have received at least one dose, and 30.6 million people have received two vaccine doses. About 9% of the U.S. population has been fully vaccinated.
States shouldn’t ease restrictions until the vaccination numbers are much higher and daily COVID-19 cases fall below 10,000 – and maybe “considerably less than that,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNN on March 4.
Several states have already begun to lift COVID-19 safety protocols, with Texas and Mississippi removing mask mandates last week. Businesses in Texas will be able to reopen at full capacity on March 10. For now, public health officials are urging Americans to continue to wear masks, avoid crowds, and follow social distancing guidelines as vaccines roll out across the country.
“This is sort of like we’ve been running this really long marathon, and we’re 100 yards from the finish line and we sit down and we give up,” Dr. Gounder told CNN on Sunday. ‘We’re almost there, we just need to give ourselves a bit more time to get a larger proportion of the population covered with vaccines.”
A version of this article first appeared on WebMD.com.
Five-day course of oral antiviral appears to stop SARS-CoV-2 in its tracks
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
CDC: Vaccinated people can gather indoors without masks
People who are fully vaccinated against COVID-19 can safely gather unmasked and inside with nonvulnerable people who are not yet immunized, according to long-awaited guidance released by the CDC.
“Today’s action represents an important first step. It is not our final destination,” CDC Director Rochelle Walensky, MD, said March 8 at a White House briefing. “As more people get vaccinated, levels of COVID-19 infection decline in communities, and as our understanding of COVID immunity improves, we look forward to updating these recommendations to the public.”
According to the new guidance, people who are at least 2 weeks out from their last dose can:
- Visit with other fully vaccinated people indoors without wearing masks or physical distancing.
- Visit with unvaccinated people from a single household who are at low risk for severe COVID-19 disease indoors without wearing masks or physical distancing
- Avoid quarantine and testing following exposure to someone if they remain asymptomatic.
However, there are still restrictions that will remain until further data are collected. Those who are fully vaccinated must still:
- Wear masks and physically distance in public settings and around people at high risk for severe disease.
- Wear masks and physically distance when visiting unvaccinated people from more than one household.
- Avoid medium- and large-sized gatherings.
- Avoid travel.
People considered at high risk for severe disease include older adults and those with cancer, chronic kidney disease, COPD, Down syndrome, heart disease, heart failure, a weakened immune system, obesity, sickle cell disease, and type 2 diabetes. The category also includes pregnant women and smokers.
“In public spaces, fully vaccinated people should continue to follow guidance to protect themselves and others, including wearing a well-fitted mask, physical distancing (at least 6 feet), avoiding crowds, avoiding poorly ventilated spaces, covering coughs and sneezes, washing hands often, and following any applicable workplace or school guidance,” the guidance says. “Fully vaccinated people should still watch for symptoms of COVID-19, especially following an exposure to someone with suspected or confirmed COVID-19.”
Respecting travel restrictions is still crucial, Dr. Walensky said, given past surges and variants that have emerged after periods of increased travel.
"We would like to give the opportunity for vaccinated grandparents to visit children and grandchildren who are healthy and local,” Dr. Walensky said.
But, she said, “It’s important to realize as we’re working through this that over 90% of the population is not yet vaccinated.”
For now, there are not enough data on transmission rates from those who are vaccinated to the rest of the public. However, Anthony Fauci, MD, said at a briefing last month that preliminary data are “pointing in a very favorable direction.”
Studies from Spain and Israel published last month showed the amount of viral load – or the amount of the COVID-19 virus in someone’s body – is significantly lower if someone gets infected after they’ve been vaccinated, compared with people who get infected and didn’t have the vaccine. Lower viral load means much lower chances of passing the virus to someone else, Dr. Fauci said.
“The science of COVID-19 is complex,” Dr. Walensky said, “and our understanding of it continues to evolve.”
A version of this article first appeared on WebMD.com.
People who are fully vaccinated against COVID-19 can safely gather unmasked and inside with nonvulnerable people who are not yet immunized, according to long-awaited guidance released by the CDC.
“Today’s action represents an important first step. It is not our final destination,” CDC Director Rochelle Walensky, MD, said March 8 at a White House briefing. “As more people get vaccinated, levels of COVID-19 infection decline in communities, and as our understanding of COVID immunity improves, we look forward to updating these recommendations to the public.”
According to the new guidance, people who are at least 2 weeks out from their last dose can:
- Visit with other fully vaccinated people indoors without wearing masks or physical distancing.
- Visit with unvaccinated people from a single household who are at low risk for severe COVID-19 disease indoors without wearing masks or physical distancing
- Avoid quarantine and testing following exposure to someone if they remain asymptomatic.
However, there are still restrictions that will remain until further data are collected. Those who are fully vaccinated must still:
- Wear masks and physically distance in public settings and around people at high risk for severe disease.
- Wear masks and physically distance when visiting unvaccinated people from more than one household.
- Avoid medium- and large-sized gatherings.
- Avoid travel.
People considered at high risk for severe disease include older adults and those with cancer, chronic kidney disease, COPD, Down syndrome, heart disease, heart failure, a weakened immune system, obesity, sickle cell disease, and type 2 diabetes. The category also includes pregnant women and smokers.
“In public spaces, fully vaccinated people should continue to follow guidance to protect themselves and others, including wearing a well-fitted mask, physical distancing (at least 6 feet), avoiding crowds, avoiding poorly ventilated spaces, covering coughs and sneezes, washing hands often, and following any applicable workplace or school guidance,” the guidance says. “Fully vaccinated people should still watch for symptoms of COVID-19, especially following an exposure to someone with suspected or confirmed COVID-19.”
Respecting travel restrictions is still crucial, Dr. Walensky said, given past surges and variants that have emerged after periods of increased travel.
"We would like to give the opportunity for vaccinated grandparents to visit children and grandchildren who are healthy and local,” Dr. Walensky said.
But, she said, “It’s important to realize as we’re working through this that over 90% of the population is not yet vaccinated.”
For now, there are not enough data on transmission rates from those who are vaccinated to the rest of the public. However, Anthony Fauci, MD, said at a briefing last month that preliminary data are “pointing in a very favorable direction.”
Studies from Spain and Israel published last month showed the amount of viral load – or the amount of the COVID-19 virus in someone’s body – is significantly lower if someone gets infected after they’ve been vaccinated, compared with people who get infected and didn’t have the vaccine. Lower viral load means much lower chances of passing the virus to someone else, Dr. Fauci said.
“The science of COVID-19 is complex,” Dr. Walensky said, “and our understanding of it continues to evolve.”
A version of this article first appeared on WebMD.com.
People who are fully vaccinated against COVID-19 can safely gather unmasked and inside with nonvulnerable people who are not yet immunized, according to long-awaited guidance released by the CDC.
“Today’s action represents an important first step. It is not our final destination,” CDC Director Rochelle Walensky, MD, said March 8 at a White House briefing. “As more people get vaccinated, levels of COVID-19 infection decline in communities, and as our understanding of COVID immunity improves, we look forward to updating these recommendations to the public.”
According to the new guidance, people who are at least 2 weeks out from their last dose can:
- Visit with other fully vaccinated people indoors without wearing masks or physical distancing.
- Visit with unvaccinated people from a single household who are at low risk for severe COVID-19 disease indoors without wearing masks or physical distancing
- Avoid quarantine and testing following exposure to someone if they remain asymptomatic.
However, there are still restrictions that will remain until further data are collected. Those who are fully vaccinated must still:
- Wear masks and physically distance in public settings and around people at high risk for severe disease.
- Wear masks and physically distance when visiting unvaccinated people from more than one household.
- Avoid medium- and large-sized gatherings.
- Avoid travel.
People considered at high risk for severe disease include older adults and those with cancer, chronic kidney disease, COPD, Down syndrome, heart disease, heart failure, a weakened immune system, obesity, sickle cell disease, and type 2 diabetes. The category also includes pregnant women and smokers.
“In public spaces, fully vaccinated people should continue to follow guidance to protect themselves and others, including wearing a well-fitted mask, physical distancing (at least 6 feet), avoiding crowds, avoiding poorly ventilated spaces, covering coughs and sneezes, washing hands often, and following any applicable workplace or school guidance,” the guidance says. “Fully vaccinated people should still watch for symptoms of COVID-19, especially following an exposure to someone with suspected or confirmed COVID-19.”
Respecting travel restrictions is still crucial, Dr. Walensky said, given past surges and variants that have emerged after periods of increased travel.
"We would like to give the opportunity for vaccinated grandparents to visit children and grandchildren who are healthy and local,” Dr. Walensky said.
But, she said, “It’s important to realize as we’re working through this that over 90% of the population is not yet vaccinated.”
For now, there are not enough data on transmission rates from those who are vaccinated to the rest of the public. However, Anthony Fauci, MD, said at a briefing last month that preliminary data are “pointing in a very favorable direction.”
Studies from Spain and Israel published last month showed the amount of viral load – or the amount of the COVID-19 virus in someone’s body – is significantly lower if someone gets infected after they’ve been vaccinated, compared with people who get infected and didn’t have the vaccine. Lower viral load means much lower chances of passing the virus to someone else, Dr. Fauci said.
“The science of COVID-19 is complex,” Dr. Walensky said, “and our understanding of it continues to evolve.”
A version of this article first appeared on WebMD.com.
RECOVERY trial of COVID-19 treatments stops colchicine arm
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
MIS-C follow-up proves challenging across pediatric hospitals
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
Dining restrictions, mask mandates tied to less illness, death, CDC reaffirms
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.








