User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
BMI, age, and sex affect COVID-19 vaccine antibody response
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
JAMA podcast on racism in medicine faces backlash
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Docs become dog groomers and warehouse workers after COVID-19 work loss
One of the biggest conundrums of the COVID-19 pandemic has been the simultaneous panic-hiring of medical professionals in hot spots and significant downsizing of staff across the country. From huge hospital systems to private practices, the stoppage of breast reductions and knee replacements, not to mention the drops in motor vehicle accidents and bar fights, have quieted operating rooms and emergency departments and put doctors’ jobs on the chopping block. A widely cited survey suggests that 21% of doctors have had a work reduction due to COVID-19.
For many American doctors, this is their first extended period of unemployment. Unlike engineers or those with MBAs who might see their fortunes rise and fall with the whims of recessions and boom times, physicians are not exactly accustomed to being laid off. However, doctors were already smarting for years due to falling salaries and decreased autonomy, punctuated by endless clicks on electronic medical records software.
Stephanie Eschenbach Morgan, MD, a breast radiologist in North Carolina, trained for 10 years after college before earning a true physician’s salary.
“Being furloughed was awful. Initially, it was only going to be 2 weeks, and then it turned into 2 months with no pay,” she reflected.
Dr. Eschenbach Morgan and her surgeon husband, who lost a full quarter’s salary, had to ask for grace periods on their credit card and mortgage payments because they had paid a large tax bill right before the pandemic began. “We couldn’t get any stimulus help, so that added insult to injury,” she said.
With her time spent waiting in a holding pattern, Dr. Eschenbach Morgan homeschooled her two young children and started putting a home gym together. She went on a home organizing spree, started a garden, and, perhaps most impressively, caught up with 5 years of photo albums.
A bonus she noted: “I didn’t set an alarm for 2 months.”
Shella Farooki, MD, a radiologist in California, was also focused on homeschooling, itself a demanding job, and veered toward retirement. When one of her work contracts furloughed her (“at one point, I made $30K a month for [their business]”), she started saving money at home, teaching the kids, and applied for a Paycheck Protection Program loan. Her husband, a hospitalist, had had his shifts cut. Dr. Farooki tried a radiology artificial intelligence firm but backed out when she was asked to read 9,200 studies for them for $2,000 per month.
Now, she thinks about leaving medicine “every day.”
Some doctors are questioning whether they should be in medicine in the first place. Family medicine physician Jonathan Polak, MD, faced with his own pink slip, turned to pink T-shirts instead. His girlfriend manages an outlet of the teen fashion retailer Justice. Dr. Polak, who finished his residency just 2 years ago, didn’t hesitate to take a $10-an-hour gig as a stock doc, once even finding himself delivering a shelving unit from the shuttering store to a physician fleeing the city for rural New Hampshire to “escape.”
There’s no escape for him – yet. Saddled with “astronomical” student loans, he had considered grocery store work as well. Dr. Polak knows he can’t work part time or go into teaching long term, as he might like.
Even so, he’s doing everything he can to not be in patient care for the long haul – it’s just not what he thought it would be.
“The culture of medicine, bureaucracy, endless paperwork and charting, and threat of litigation sucks a lot of the joy out of it to the point that I don’t see myself doing it forever when imagining myself 5-10 years into it.”
Still, he recently took an 18-month hospital contract that will force him to move to Florida, but he’s also been turning himself into a veritable Renaissance man; composing music, training for an ultramarathon, studying the latest medical findings, roadtripping, and launching a podcast about dog grooming with a master groomer. “We found parallels between medicine and dog grooming,” he says, somewhat convincingly.
Also working the ruff life is Jen Tserng, MD, a former forensic pathologist who landed on news websites in recent years for becoming a professional dogwalker and housesitter without a permanent home. Dr. Tserng knows doctors were restless and unhappy before COVID-19, their thoughts wandering where the grass might be greener.
As her profile grew, she found her inbox gathering messages from disaffected medical minions: students with a fear of failing or staring down residency application season and employed doctors sick of the constant grind. As she recounted those de facto life coach conversations (“What do you really enjoy?” “Do you really like dogs?”) by phone from New York, she said matter-of-factly, “They don’t call because of COVID. They call because they hate their lives.”
Michelle Mudge-Riley, MD, a physician in Texas, has been seeing this shift for some time as well. She recently held a virtual version of her Physicians Helping Physicians conference, where doctors hear from their peers working successfully in fields like pharmaceuticals and real estate investing.
When COVID-19 hit, Dr. Mudge-Riley quickly pivoted to a virtual platform, where the MDs and DOs huddled in breakout rooms having honest chats about their fears and tentative hopes about their new careers.
“There has been increased interest in nonclinical exploration into full- and part-time careers, as well as side hustles, since COVID began,” she said. “Many physicians have had their hours or pay cut, and some have been laid off. Others are furloughed. Some just want out of an environment where they don’t feel safe.”
An ear, nose, and throat surgeon, Maansi Doshi, MD, from central California, didn’t feel safe – so she left. She had returned from India sick with a mystery virus right as the pandemic began (she said her COVID-19 tests were all negative) and was waiting to get well enough to go back to her private practice job. However, she said she clashed with Trump-supporting colleagues she feared might not be taking the pandemic seriously enough.
Finally getting over a relapse of her mystery virus, Dr. Doshi emailed her resignation in May. Her husband, family practice doctor Mark Mangiapane, MD, gave his job notice weeks later in solidarity because he worked in the same building. Together, they have embraced gardening, a Peloton splurge, and learning business skills to open private practices – solo primary care for him; ENT with a focus on her favorite surgery, rhinoplasty, for her.
Dr. Mangiapane had considered editing medical brochures and also tried to apply for a job as a county public health officer in rural California, but he received his own shock when he learned the county intended to open schools in the midst of the pandemic despite advisement to the contrary by the former health officer.
He retreated from job listings altogether after hearing his would-be peers were getting death threats – targeting their children.
Both doctors felt COVID-19 pushed them beyond their comfort zones. “If COVID hadn’t happened, I would be working. ... Be ‘owned.’ In a weird way, COVID made me more independent and take a risk with my career.”
Obstetrician Kwandaa Roberts, MD, certainly did; she took a budding interest in decorating dollhouses straight to Instagram and national news fame, and she is now a TV-show expert on “Sell This House.”
Like Dr. Doshi and Dr. Mangiapane, Dr. Polak wants to be more in control of his future – even if selling T-shirts at a mall means a certain loss of status along the way.
“Aside from my passion to learn and to have that connection with people, I went into medicine ... because of the job security I thought existed,” he said. “I would say that my getting furloughed has changed my view of the United States in a dramatic way. I do not feel as confident in the U.S. economy and general way of life as I did a year ago. And I am taking a number of steps to put myself in a more fluid, adaptable position in case another crisis like this occurs or if the current state of things worsens.”
A version of this article first appeared on Medscape.com.
One of the biggest conundrums of the COVID-19 pandemic has been the simultaneous panic-hiring of medical professionals in hot spots and significant downsizing of staff across the country. From huge hospital systems to private practices, the stoppage of breast reductions and knee replacements, not to mention the drops in motor vehicle accidents and bar fights, have quieted operating rooms and emergency departments and put doctors’ jobs on the chopping block. A widely cited survey suggests that 21% of doctors have had a work reduction due to COVID-19.
For many American doctors, this is their first extended period of unemployment. Unlike engineers or those with MBAs who might see their fortunes rise and fall with the whims of recessions and boom times, physicians are not exactly accustomed to being laid off. However, doctors were already smarting for years due to falling salaries and decreased autonomy, punctuated by endless clicks on electronic medical records software.
Stephanie Eschenbach Morgan, MD, a breast radiologist in North Carolina, trained for 10 years after college before earning a true physician’s salary.
“Being furloughed was awful. Initially, it was only going to be 2 weeks, and then it turned into 2 months with no pay,” she reflected.
Dr. Eschenbach Morgan and her surgeon husband, who lost a full quarter’s salary, had to ask for grace periods on their credit card and mortgage payments because they had paid a large tax bill right before the pandemic began. “We couldn’t get any stimulus help, so that added insult to injury,” she said.
With her time spent waiting in a holding pattern, Dr. Eschenbach Morgan homeschooled her two young children and started putting a home gym together. She went on a home organizing spree, started a garden, and, perhaps most impressively, caught up with 5 years of photo albums.
A bonus she noted: “I didn’t set an alarm for 2 months.”
Shella Farooki, MD, a radiologist in California, was also focused on homeschooling, itself a demanding job, and veered toward retirement. When one of her work contracts furloughed her (“at one point, I made $30K a month for [their business]”), she started saving money at home, teaching the kids, and applied for a Paycheck Protection Program loan. Her husband, a hospitalist, had had his shifts cut. Dr. Farooki tried a radiology artificial intelligence firm but backed out when she was asked to read 9,200 studies for them for $2,000 per month.
Now, she thinks about leaving medicine “every day.”
Some doctors are questioning whether they should be in medicine in the first place. Family medicine physician Jonathan Polak, MD, faced with his own pink slip, turned to pink T-shirts instead. His girlfriend manages an outlet of the teen fashion retailer Justice. Dr. Polak, who finished his residency just 2 years ago, didn’t hesitate to take a $10-an-hour gig as a stock doc, once even finding himself delivering a shelving unit from the shuttering store to a physician fleeing the city for rural New Hampshire to “escape.”
There’s no escape for him – yet. Saddled with “astronomical” student loans, he had considered grocery store work as well. Dr. Polak knows he can’t work part time or go into teaching long term, as he might like.
Even so, he’s doing everything he can to not be in patient care for the long haul – it’s just not what he thought it would be.
“The culture of medicine, bureaucracy, endless paperwork and charting, and threat of litigation sucks a lot of the joy out of it to the point that I don’t see myself doing it forever when imagining myself 5-10 years into it.”
Still, he recently took an 18-month hospital contract that will force him to move to Florida, but he’s also been turning himself into a veritable Renaissance man; composing music, training for an ultramarathon, studying the latest medical findings, roadtripping, and launching a podcast about dog grooming with a master groomer. “We found parallels between medicine and dog grooming,” he says, somewhat convincingly.
Also working the ruff life is Jen Tserng, MD, a former forensic pathologist who landed on news websites in recent years for becoming a professional dogwalker and housesitter without a permanent home. Dr. Tserng knows doctors were restless and unhappy before COVID-19, their thoughts wandering where the grass might be greener.
As her profile grew, she found her inbox gathering messages from disaffected medical minions: students with a fear of failing or staring down residency application season and employed doctors sick of the constant grind. As she recounted those de facto life coach conversations (“What do you really enjoy?” “Do you really like dogs?”) by phone from New York, she said matter-of-factly, “They don’t call because of COVID. They call because they hate their lives.”
Michelle Mudge-Riley, MD, a physician in Texas, has been seeing this shift for some time as well. She recently held a virtual version of her Physicians Helping Physicians conference, where doctors hear from their peers working successfully in fields like pharmaceuticals and real estate investing.
When COVID-19 hit, Dr. Mudge-Riley quickly pivoted to a virtual platform, where the MDs and DOs huddled in breakout rooms having honest chats about their fears and tentative hopes about their new careers.
“There has been increased interest in nonclinical exploration into full- and part-time careers, as well as side hustles, since COVID began,” she said. “Many physicians have had their hours or pay cut, and some have been laid off. Others are furloughed. Some just want out of an environment where they don’t feel safe.”
An ear, nose, and throat surgeon, Maansi Doshi, MD, from central California, didn’t feel safe – so she left. She had returned from India sick with a mystery virus right as the pandemic began (she said her COVID-19 tests were all negative) and was waiting to get well enough to go back to her private practice job. However, she said she clashed with Trump-supporting colleagues she feared might not be taking the pandemic seriously enough.
Finally getting over a relapse of her mystery virus, Dr. Doshi emailed her resignation in May. Her husband, family practice doctor Mark Mangiapane, MD, gave his job notice weeks later in solidarity because he worked in the same building. Together, they have embraced gardening, a Peloton splurge, and learning business skills to open private practices – solo primary care for him; ENT with a focus on her favorite surgery, rhinoplasty, for her.
Dr. Mangiapane had considered editing medical brochures and also tried to apply for a job as a county public health officer in rural California, but he received his own shock when he learned the county intended to open schools in the midst of the pandemic despite advisement to the contrary by the former health officer.
He retreated from job listings altogether after hearing his would-be peers were getting death threats – targeting their children.
Both doctors felt COVID-19 pushed them beyond their comfort zones. “If COVID hadn’t happened, I would be working. ... Be ‘owned.’ In a weird way, COVID made me more independent and take a risk with my career.”
Obstetrician Kwandaa Roberts, MD, certainly did; she took a budding interest in decorating dollhouses straight to Instagram and national news fame, and she is now a TV-show expert on “Sell This House.”
Like Dr. Doshi and Dr. Mangiapane, Dr. Polak wants to be more in control of his future – even if selling T-shirts at a mall means a certain loss of status along the way.
“Aside from my passion to learn and to have that connection with people, I went into medicine ... because of the job security I thought existed,” he said. “I would say that my getting furloughed has changed my view of the United States in a dramatic way. I do not feel as confident in the U.S. economy and general way of life as I did a year ago. And I am taking a number of steps to put myself in a more fluid, adaptable position in case another crisis like this occurs or if the current state of things worsens.”
A version of this article first appeared on Medscape.com.
One of the biggest conundrums of the COVID-19 pandemic has been the simultaneous panic-hiring of medical professionals in hot spots and significant downsizing of staff across the country. From huge hospital systems to private practices, the stoppage of breast reductions and knee replacements, not to mention the drops in motor vehicle accidents and bar fights, have quieted operating rooms and emergency departments and put doctors’ jobs on the chopping block. A widely cited survey suggests that 21% of doctors have had a work reduction due to COVID-19.
For many American doctors, this is their first extended period of unemployment. Unlike engineers or those with MBAs who might see their fortunes rise and fall with the whims of recessions and boom times, physicians are not exactly accustomed to being laid off. However, doctors were already smarting for years due to falling salaries and decreased autonomy, punctuated by endless clicks on electronic medical records software.
Stephanie Eschenbach Morgan, MD, a breast radiologist in North Carolina, trained for 10 years after college before earning a true physician’s salary.
“Being furloughed was awful. Initially, it was only going to be 2 weeks, and then it turned into 2 months with no pay,” she reflected.
Dr. Eschenbach Morgan and her surgeon husband, who lost a full quarter’s salary, had to ask for grace periods on their credit card and mortgage payments because they had paid a large tax bill right before the pandemic began. “We couldn’t get any stimulus help, so that added insult to injury,” she said.
With her time spent waiting in a holding pattern, Dr. Eschenbach Morgan homeschooled her two young children and started putting a home gym together. She went on a home organizing spree, started a garden, and, perhaps most impressively, caught up with 5 years of photo albums.
A bonus she noted: “I didn’t set an alarm for 2 months.”
Shella Farooki, MD, a radiologist in California, was also focused on homeschooling, itself a demanding job, and veered toward retirement. When one of her work contracts furloughed her (“at one point, I made $30K a month for [their business]”), she started saving money at home, teaching the kids, and applied for a Paycheck Protection Program loan. Her husband, a hospitalist, had had his shifts cut. Dr. Farooki tried a radiology artificial intelligence firm but backed out when she was asked to read 9,200 studies for them for $2,000 per month.
Now, she thinks about leaving medicine “every day.”
Some doctors are questioning whether they should be in medicine in the first place. Family medicine physician Jonathan Polak, MD, faced with his own pink slip, turned to pink T-shirts instead. His girlfriend manages an outlet of the teen fashion retailer Justice. Dr. Polak, who finished his residency just 2 years ago, didn’t hesitate to take a $10-an-hour gig as a stock doc, once even finding himself delivering a shelving unit from the shuttering store to a physician fleeing the city for rural New Hampshire to “escape.”
There’s no escape for him – yet. Saddled with “astronomical” student loans, he had considered grocery store work as well. Dr. Polak knows he can’t work part time or go into teaching long term, as he might like.
Even so, he’s doing everything he can to not be in patient care for the long haul – it’s just not what he thought it would be.
“The culture of medicine, bureaucracy, endless paperwork and charting, and threat of litigation sucks a lot of the joy out of it to the point that I don’t see myself doing it forever when imagining myself 5-10 years into it.”
Still, he recently took an 18-month hospital contract that will force him to move to Florida, but he’s also been turning himself into a veritable Renaissance man; composing music, training for an ultramarathon, studying the latest medical findings, roadtripping, and launching a podcast about dog grooming with a master groomer. “We found parallels between medicine and dog grooming,” he says, somewhat convincingly.
Also working the ruff life is Jen Tserng, MD, a former forensic pathologist who landed on news websites in recent years for becoming a professional dogwalker and housesitter without a permanent home. Dr. Tserng knows doctors were restless and unhappy before COVID-19, their thoughts wandering where the grass might be greener.
As her profile grew, she found her inbox gathering messages from disaffected medical minions: students with a fear of failing or staring down residency application season and employed doctors sick of the constant grind. As she recounted those de facto life coach conversations (“What do you really enjoy?” “Do you really like dogs?”) by phone from New York, she said matter-of-factly, “They don’t call because of COVID. They call because they hate their lives.”
Michelle Mudge-Riley, MD, a physician in Texas, has been seeing this shift for some time as well. She recently held a virtual version of her Physicians Helping Physicians conference, where doctors hear from their peers working successfully in fields like pharmaceuticals and real estate investing.
When COVID-19 hit, Dr. Mudge-Riley quickly pivoted to a virtual platform, where the MDs and DOs huddled in breakout rooms having honest chats about their fears and tentative hopes about their new careers.
“There has been increased interest in nonclinical exploration into full- and part-time careers, as well as side hustles, since COVID began,” she said. “Many physicians have had their hours or pay cut, and some have been laid off. Others are furloughed. Some just want out of an environment where they don’t feel safe.”
An ear, nose, and throat surgeon, Maansi Doshi, MD, from central California, didn’t feel safe – so she left. She had returned from India sick with a mystery virus right as the pandemic began (she said her COVID-19 tests were all negative) and was waiting to get well enough to go back to her private practice job. However, she said she clashed with Trump-supporting colleagues she feared might not be taking the pandemic seriously enough.
Finally getting over a relapse of her mystery virus, Dr. Doshi emailed her resignation in May. Her husband, family practice doctor Mark Mangiapane, MD, gave his job notice weeks later in solidarity because he worked in the same building. Together, they have embraced gardening, a Peloton splurge, and learning business skills to open private practices – solo primary care for him; ENT with a focus on her favorite surgery, rhinoplasty, for her.
Dr. Mangiapane had considered editing medical brochures and also tried to apply for a job as a county public health officer in rural California, but he received his own shock when he learned the county intended to open schools in the midst of the pandemic despite advisement to the contrary by the former health officer.
He retreated from job listings altogether after hearing his would-be peers were getting death threats – targeting their children.
Both doctors felt COVID-19 pushed them beyond their comfort zones. “If COVID hadn’t happened, I would be working. ... Be ‘owned.’ In a weird way, COVID made me more independent and take a risk with my career.”
Obstetrician Kwandaa Roberts, MD, certainly did; she took a budding interest in decorating dollhouses straight to Instagram and national news fame, and she is now a TV-show expert on “Sell This House.”
Like Dr. Doshi and Dr. Mangiapane, Dr. Polak wants to be more in control of his future – even if selling T-shirts at a mall means a certain loss of status along the way.
“Aside from my passion to learn and to have that connection with people, I went into medicine ... because of the job security I thought existed,” he said. “I would say that my getting furloughed has changed my view of the United States in a dramatic way. I do not feel as confident in the U.S. economy and general way of life as I did a year ago. And I am taking a number of steps to put myself in a more fluid, adaptable position in case another crisis like this occurs or if the current state of things worsens.”
A version of this article first appeared on Medscape.com.
Rural women receive antibiotics for longer than necessary for UTIs
Women living in rural areas were significantly more likely than were those in urban areas to receive inappropriate antibiotic prescriptions for urinary tract infections, based on data from an observational cohort study of more than 600,000 women.
Uncomplicated urinary tract infections (UTIs) are common among otherwise healthy women in the United States, and certain antibiotics are recommended as first-line therapy, wrote Abbye W. Clark, MD, of Washington University, St. Louis, and colleagues.
“However, the majority of antibiotic prescriptions for uncomplicated UTI are suboptimal because they are written for nonrecommended agents and durations,” they said.
Addressing rural health disparities has become a focus in the United States, and previous studies of respiratory tract infections have shown differences in antibiotic prescribing based on geographic region; “however, no large-scale studies have evaluated rural-urban differences in inappropriate outpatient prescribing for UTI,” they added.
In a study published in Infection Control & Hospital Epidemiology, the researchers identified 670,450 women aged 18-44 years who received oral antibiotics for uncomplicated UTIs between 2010 to 2015, using a commercial insurance database to determine diagnosis and antibiotic prescription information. Women were defined as urban if they lived in a metropolitan statistical area of at least 50,000 inhabitants (86.2%); all other women were defined as rural (13.8%). The median age was 30 years for both groups.
Overall, 46.7% of the women received prescriptions for inappropriate antibiotics, and 76.1% received antibiotics for inappropriate durations.
Antibiotics and durations were defined as appropriate or inappropriate based on current clinical guidelines. “We classified first-line agents (nitrofurantoin, TMP-SMX, fosfomycin) as appropriate and non–first-line agents (fluoroquinolones, beta-lactams) as inappropriate,” the researchers said.
The regimens classified as appropriate duration were “nitrofurantoin 5-day regimen, TMP-SMX (including TMP monotherapy) 3-day regimen, fosfomycin 1-day regimen, fluoroquinolones 3-day regimen, and beta-lactams 3- to 7-day regimen. All other regimens were classified as inappropriate duration,” they noted.
More rural women receive long-duration antibiotics
In a multivariate analysis, similar percentages of antibiotics for rural and urban women consisted of inappropriate agents (45.9% vs. 46.9%) including use of fluoroquinolones (41.0% vs. 41.7%) and beta-lactams (4.8% vs. 5.0%).
However, across all antibiotics, women in rural areas were more likely than were women in urban areas to receive prescriptions for inappropriately long durations (83.9% vs. 75.9%, adjusted risk ratio 1.10).
The percentage of women who received inappropriate antibiotic agents was not significantly different based on geographic region of the country.
From 2011 to 2015, the quarterly proportion of women overall who received inappropriate agents and antibiotics for inappropriate durations decreased slightly (48.5% to 43.7% and 78.3% to 73.4%, respectively), the researchers noted.
The study findings were limited by several factors including the potentially lenient definition of antibiotic duration, a study population that disproportionately oversampled from the South and undersampled from the West, use of ZIP codes to determine rural vs. urban status, lack of data on race and income, and lack of access to urine culture results, the researchers noted.
However, “our study identified rural-urban differences in antibiotic prescribing, including an actionable disparity in the duration of antibiotics that disproportionately affects women who live in rural locations,” they said.
“Given the large quantity of inappropriate prescriptions annually in the U.S., as well as the negative patient- and society-level consequences of unnecessary exposure to antibiotics, antimicrobial stewardship interventions are needed to improve outpatient UTI antibiotic prescribing, particularly in rural settings,” they concluded.
Data support need for education and stewardship
“This manuscript provides valuable information to all women’s health providers regarding the importance of antibiotic stewardship,” David M. Jaspan, DO, and Natasha Abdullah, MD, Einstein Medical Center, Philadelphia, said in an interview. Whether urban or rural, over 45% of the patients received inappropriate non–first-line treatment and 76% of the prescriptions were for an inappropriate duration (98.8% for longer than recommended), they emphasized.
“The potential negative impact of antibiotic resistance, coupled with the potential for increased side effects, should prompt providers to ensure that when treating uncomplicated UTIs in women, that the choice of treatment and the duration of treatment is tailored to the patient’s needs,” the Dr. Jaspan and Dr. Abdullah said.
To improve antibiotic prescribing, especially at the local and regional level, “We encourage providers to familiarize themselves with local information as it pertains to known resistance when prescribing empiric treatment regimens for uncomplicated UTIs,” they said.
The study was supported by the National Center for Advancing Translational Sciences at the National Institutes of Health. Lead author Dr. Clark, as well as Dr. Jaspan and Dr. Abdullah, had no financial conflicts to disclose.
Women living in rural areas were significantly more likely than were those in urban areas to receive inappropriate antibiotic prescriptions for urinary tract infections, based on data from an observational cohort study of more than 600,000 women.
Uncomplicated urinary tract infections (UTIs) are common among otherwise healthy women in the United States, and certain antibiotics are recommended as first-line therapy, wrote Abbye W. Clark, MD, of Washington University, St. Louis, and colleagues.
“However, the majority of antibiotic prescriptions for uncomplicated UTI are suboptimal because they are written for nonrecommended agents and durations,” they said.
Addressing rural health disparities has become a focus in the United States, and previous studies of respiratory tract infections have shown differences in antibiotic prescribing based on geographic region; “however, no large-scale studies have evaluated rural-urban differences in inappropriate outpatient prescribing for UTI,” they added.
In a study published in Infection Control & Hospital Epidemiology, the researchers identified 670,450 women aged 18-44 years who received oral antibiotics for uncomplicated UTIs between 2010 to 2015, using a commercial insurance database to determine diagnosis and antibiotic prescription information. Women were defined as urban if they lived in a metropolitan statistical area of at least 50,000 inhabitants (86.2%); all other women were defined as rural (13.8%). The median age was 30 years for both groups.
Overall, 46.7% of the women received prescriptions for inappropriate antibiotics, and 76.1% received antibiotics for inappropriate durations.
Antibiotics and durations were defined as appropriate or inappropriate based on current clinical guidelines. “We classified first-line agents (nitrofurantoin, TMP-SMX, fosfomycin) as appropriate and non–first-line agents (fluoroquinolones, beta-lactams) as inappropriate,” the researchers said.
The regimens classified as appropriate duration were “nitrofurantoin 5-day regimen, TMP-SMX (including TMP monotherapy) 3-day regimen, fosfomycin 1-day regimen, fluoroquinolones 3-day regimen, and beta-lactams 3- to 7-day regimen. All other regimens were classified as inappropriate duration,” they noted.
More rural women receive long-duration antibiotics
In a multivariate analysis, similar percentages of antibiotics for rural and urban women consisted of inappropriate agents (45.9% vs. 46.9%) including use of fluoroquinolones (41.0% vs. 41.7%) and beta-lactams (4.8% vs. 5.0%).
However, across all antibiotics, women in rural areas were more likely than were women in urban areas to receive prescriptions for inappropriately long durations (83.9% vs. 75.9%, adjusted risk ratio 1.10).
The percentage of women who received inappropriate antibiotic agents was not significantly different based on geographic region of the country.
From 2011 to 2015, the quarterly proportion of women overall who received inappropriate agents and antibiotics for inappropriate durations decreased slightly (48.5% to 43.7% and 78.3% to 73.4%, respectively), the researchers noted.
The study findings were limited by several factors including the potentially lenient definition of antibiotic duration, a study population that disproportionately oversampled from the South and undersampled from the West, use of ZIP codes to determine rural vs. urban status, lack of data on race and income, and lack of access to urine culture results, the researchers noted.
However, “our study identified rural-urban differences in antibiotic prescribing, including an actionable disparity in the duration of antibiotics that disproportionately affects women who live in rural locations,” they said.
“Given the large quantity of inappropriate prescriptions annually in the U.S., as well as the negative patient- and society-level consequences of unnecessary exposure to antibiotics, antimicrobial stewardship interventions are needed to improve outpatient UTI antibiotic prescribing, particularly in rural settings,” they concluded.
Data support need for education and stewardship
“This manuscript provides valuable information to all women’s health providers regarding the importance of antibiotic stewardship,” David M. Jaspan, DO, and Natasha Abdullah, MD, Einstein Medical Center, Philadelphia, said in an interview. Whether urban or rural, over 45% of the patients received inappropriate non–first-line treatment and 76% of the prescriptions were for an inappropriate duration (98.8% for longer than recommended), they emphasized.
“The potential negative impact of antibiotic resistance, coupled with the potential for increased side effects, should prompt providers to ensure that when treating uncomplicated UTIs in women, that the choice of treatment and the duration of treatment is tailored to the patient’s needs,” the Dr. Jaspan and Dr. Abdullah said.
To improve antibiotic prescribing, especially at the local and regional level, “We encourage providers to familiarize themselves with local information as it pertains to known resistance when prescribing empiric treatment regimens for uncomplicated UTIs,” they said.
The study was supported by the National Center for Advancing Translational Sciences at the National Institutes of Health. Lead author Dr. Clark, as well as Dr. Jaspan and Dr. Abdullah, had no financial conflicts to disclose.
Women living in rural areas were significantly more likely than were those in urban areas to receive inappropriate antibiotic prescriptions for urinary tract infections, based on data from an observational cohort study of more than 600,000 women.
Uncomplicated urinary tract infections (UTIs) are common among otherwise healthy women in the United States, and certain antibiotics are recommended as first-line therapy, wrote Abbye W. Clark, MD, of Washington University, St. Louis, and colleagues.
“However, the majority of antibiotic prescriptions for uncomplicated UTI are suboptimal because they are written for nonrecommended agents and durations,” they said.
Addressing rural health disparities has become a focus in the United States, and previous studies of respiratory tract infections have shown differences in antibiotic prescribing based on geographic region; “however, no large-scale studies have evaluated rural-urban differences in inappropriate outpatient prescribing for UTI,” they added.
In a study published in Infection Control & Hospital Epidemiology, the researchers identified 670,450 women aged 18-44 years who received oral antibiotics for uncomplicated UTIs between 2010 to 2015, using a commercial insurance database to determine diagnosis and antibiotic prescription information. Women were defined as urban if they lived in a metropolitan statistical area of at least 50,000 inhabitants (86.2%); all other women were defined as rural (13.8%). The median age was 30 years for both groups.
Overall, 46.7% of the women received prescriptions for inappropriate antibiotics, and 76.1% received antibiotics for inappropriate durations.
Antibiotics and durations were defined as appropriate or inappropriate based on current clinical guidelines. “We classified first-line agents (nitrofurantoin, TMP-SMX, fosfomycin) as appropriate and non–first-line agents (fluoroquinolones, beta-lactams) as inappropriate,” the researchers said.
The regimens classified as appropriate duration were “nitrofurantoin 5-day regimen, TMP-SMX (including TMP monotherapy) 3-day regimen, fosfomycin 1-day regimen, fluoroquinolones 3-day regimen, and beta-lactams 3- to 7-day regimen. All other regimens were classified as inappropriate duration,” they noted.
More rural women receive long-duration antibiotics
In a multivariate analysis, similar percentages of antibiotics for rural and urban women consisted of inappropriate agents (45.9% vs. 46.9%) including use of fluoroquinolones (41.0% vs. 41.7%) and beta-lactams (4.8% vs. 5.0%).
However, across all antibiotics, women in rural areas were more likely than were women in urban areas to receive prescriptions for inappropriately long durations (83.9% vs. 75.9%, adjusted risk ratio 1.10).
The percentage of women who received inappropriate antibiotic agents was not significantly different based on geographic region of the country.
From 2011 to 2015, the quarterly proportion of women overall who received inappropriate agents and antibiotics for inappropriate durations decreased slightly (48.5% to 43.7% and 78.3% to 73.4%, respectively), the researchers noted.
The study findings were limited by several factors including the potentially lenient definition of antibiotic duration, a study population that disproportionately oversampled from the South and undersampled from the West, use of ZIP codes to determine rural vs. urban status, lack of data on race and income, and lack of access to urine culture results, the researchers noted.
However, “our study identified rural-urban differences in antibiotic prescribing, including an actionable disparity in the duration of antibiotics that disproportionately affects women who live in rural locations,” they said.
“Given the large quantity of inappropriate prescriptions annually in the U.S., as well as the negative patient- and society-level consequences of unnecessary exposure to antibiotics, antimicrobial stewardship interventions are needed to improve outpatient UTI antibiotic prescribing, particularly in rural settings,” they concluded.
Data support need for education and stewardship
“This manuscript provides valuable information to all women’s health providers regarding the importance of antibiotic stewardship,” David M. Jaspan, DO, and Natasha Abdullah, MD, Einstein Medical Center, Philadelphia, said in an interview. Whether urban or rural, over 45% of the patients received inappropriate non–first-line treatment and 76% of the prescriptions were for an inappropriate duration (98.8% for longer than recommended), they emphasized.
“The potential negative impact of antibiotic resistance, coupled with the potential for increased side effects, should prompt providers to ensure that when treating uncomplicated UTIs in women, that the choice of treatment and the duration of treatment is tailored to the patient’s needs,” the Dr. Jaspan and Dr. Abdullah said.
To improve antibiotic prescribing, especially at the local and regional level, “We encourage providers to familiarize themselves with local information as it pertains to known resistance when prescribing empiric treatment regimens for uncomplicated UTIs,” they said.
The study was supported by the National Center for Advancing Translational Sciences at the National Institutes of Health. Lead author Dr. Clark, as well as Dr. Jaspan and Dr. Abdullah, had no financial conflicts to disclose.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY
Maribavir seen as superior to other antivirals for CMV clearance post transplant
Maribavir, an investigational antiviral agent with a novel mechanism of action, was superior to other antiviral strategies at clearing cytomegalovirus (CMV) viremia and controlling symptoms in hematopoietic cell or solid-organ transplant recipients, results of a phase 3 clinical trial showed.
CMV viremia clearance at study week 8 was seen in 55.7% of all patients randomized to receive maribavir, compared with 23.9% for patients assigned to receive investigator-assigned therapy (IAT), Francisco Marty, MD, from the Dana-Farber Cancer Institute in Boston reported at the Transplant & Cellular Therapies Meetings.
“Maribavir’s benefit was driven by lower incidence of treatment-limiting toxicities, compared with IAT,” he said a late-breaking abstract session during the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“Available anti-CMV antivirals are limited by development of resistance and toxicities, particularly myelosuppression with the use of valganciclovir and nephrotoxicity with the use of foscarnet and cidofovir. Alternative treatment options are required to address this unmet medical need,” he said.
Maribavir inhibits the CMV UL97 protein kinase and is thought to affect several critical processes in CMV replication, including viral DNA synthesis, viral gene expression, encapsidation, and egress of mature capsids from the nucleus.
Details of trial
In the phase 3 SHP620-30e trial (NCT02931539), Dr. Marty and colleagues enrolled patients with relapsed or refractory CMV infections after hematopoietic cell transplant (HCT) or solid-organ transplant (SOT) and after stratification by transplant type and screening CMV DNA level randomly assigned them on a 2:1 basis to receive either maribavir 400 mg twice daily (235 patients) or IAT (117 patients), consisting of either ganciclovir/valganciclovir, foscarnet, cidofovir, or combined foscarnet and val/ganciclovir.
The primary endpoint of viremia clearance at 8 weeks was defined as plasma CMV DNA less than 137 IU/mL in two consecutive tests at a central laboratory at least 5 days apart beginning at the end of week 8.
The trial met its primary endpoint, with a viremia clearance rate of 55.7% with maribavir versus 23.9% with IAT.
The viremia clearance rates were similar in each of the transplant groups: 55.9% versus 20.8%, respectively, in patients who underwent HCT, and 55.6% versus 26.1% in patients who underwent SOT (P < .001).
Clearance rates among patients with CMV DNA below 9,100 IU/mL at baseline were 62.1% with maribavir versus 24.7% with IAT. Among patients with baseline CMV DNA of 9100 IU/mL or above, the respective rates were 43.9% versus 21.9%.
CMV viremia clearance continued from week 8 to week 16 in 18.7% of patients assigned to maribavir and to 10.3% of patients randomized to IAT (P < .013).
The median time to first CMV viremia clearance as 22 days with maribavir versus 27 days with IAT (P = .039).
All-cause mortality was similar between the groups, at 11.5% versus 11.1%, respectively.
The incidences of serious and severe treatment-emergent adverse events (TEAE) were 38.5% and 32.1%, respectively, in the maribavir group, and 37.1% and 37.9% in the IAT group.
Any TEAE leading to study drug discontinuation was less common with maribavir, occurring in 13.2% of patients, compared with 31.9% of patients on IAT. Serious TEAEs leading to drug discontinuation occurred in 8.5% versus 14.7%, respectively.
Serious TEAEs leading to death occurred in 6.8% of patients on maribavir versus 5.2% of those on IAT.
Role of letermovir
In the question-and-answer session following the presentation, comoderator Monalisa Ghosh, MD, from the University of Michigan, Ann Arbor, asked whether any patients in the study were currently on letermovir (Prevymis) prophylaxis, and whether any patients had previously been treated with letermovir but had CMV reactivation and were then treated on study.
Dr. Marty noted that the trial was designed before letermovir was approved for CMV prophylaxis in adults who have undergone an allogeneic HCT.
“Nobody was on letermovir at the beginning of the trial,” he replied, but noted that some patients who were enrolled and had infections that were refractory or resistant to valganciclovir, foscarnet, or a combination of the two received letermovir as secondary prophylaxis.
“I haven’t got the data to tell you how often [letermovir] was used; I think part of the lack of mortality benefit [with maribavir] may be due to the fact that people jumped into secondary prophylaxis with letermovir to minimize the toxicities that we saw,” he said.
Although maribavir has not as of this writing received Food and Drug Administration approval, the drug may be available to some patients through a compassionate-use program from Takeda, Dr. Marty noted.
The study was funded by Shire ViroPharma. Dr. Marty disclosed research funding from Shire and from others. Dr. Ghosh had no relevant disclosures.
Maribavir, an investigational antiviral agent with a novel mechanism of action, was superior to other antiviral strategies at clearing cytomegalovirus (CMV) viremia and controlling symptoms in hematopoietic cell or solid-organ transplant recipients, results of a phase 3 clinical trial showed.
CMV viremia clearance at study week 8 was seen in 55.7% of all patients randomized to receive maribavir, compared with 23.9% for patients assigned to receive investigator-assigned therapy (IAT), Francisco Marty, MD, from the Dana-Farber Cancer Institute in Boston reported at the Transplant & Cellular Therapies Meetings.
“Maribavir’s benefit was driven by lower incidence of treatment-limiting toxicities, compared with IAT,” he said a late-breaking abstract session during the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“Available anti-CMV antivirals are limited by development of resistance and toxicities, particularly myelosuppression with the use of valganciclovir and nephrotoxicity with the use of foscarnet and cidofovir. Alternative treatment options are required to address this unmet medical need,” he said.
Maribavir inhibits the CMV UL97 protein kinase and is thought to affect several critical processes in CMV replication, including viral DNA synthesis, viral gene expression, encapsidation, and egress of mature capsids from the nucleus.
Details of trial
In the phase 3 SHP620-30e trial (NCT02931539), Dr. Marty and colleagues enrolled patients with relapsed or refractory CMV infections after hematopoietic cell transplant (HCT) or solid-organ transplant (SOT) and after stratification by transplant type and screening CMV DNA level randomly assigned them on a 2:1 basis to receive either maribavir 400 mg twice daily (235 patients) or IAT (117 patients), consisting of either ganciclovir/valganciclovir, foscarnet, cidofovir, or combined foscarnet and val/ganciclovir.
The primary endpoint of viremia clearance at 8 weeks was defined as plasma CMV DNA less than 137 IU/mL in two consecutive tests at a central laboratory at least 5 days apart beginning at the end of week 8.
The trial met its primary endpoint, with a viremia clearance rate of 55.7% with maribavir versus 23.9% with IAT.
The viremia clearance rates were similar in each of the transplant groups: 55.9% versus 20.8%, respectively, in patients who underwent HCT, and 55.6% versus 26.1% in patients who underwent SOT (P < .001).
Clearance rates among patients with CMV DNA below 9,100 IU/mL at baseline were 62.1% with maribavir versus 24.7% with IAT. Among patients with baseline CMV DNA of 9100 IU/mL or above, the respective rates were 43.9% versus 21.9%.
CMV viremia clearance continued from week 8 to week 16 in 18.7% of patients assigned to maribavir and to 10.3% of patients randomized to IAT (P < .013).
The median time to first CMV viremia clearance as 22 days with maribavir versus 27 days with IAT (P = .039).
All-cause mortality was similar between the groups, at 11.5% versus 11.1%, respectively.
The incidences of serious and severe treatment-emergent adverse events (TEAE) were 38.5% and 32.1%, respectively, in the maribavir group, and 37.1% and 37.9% in the IAT group.
Any TEAE leading to study drug discontinuation was less common with maribavir, occurring in 13.2% of patients, compared with 31.9% of patients on IAT. Serious TEAEs leading to drug discontinuation occurred in 8.5% versus 14.7%, respectively.
Serious TEAEs leading to death occurred in 6.8% of patients on maribavir versus 5.2% of those on IAT.
Role of letermovir
In the question-and-answer session following the presentation, comoderator Monalisa Ghosh, MD, from the University of Michigan, Ann Arbor, asked whether any patients in the study were currently on letermovir (Prevymis) prophylaxis, and whether any patients had previously been treated with letermovir but had CMV reactivation and were then treated on study.
Dr. Marty noted that the trial was designed before letermovir was approved for CMV prophylaxis in adults who have undergone an allogeneic HCT.
“Nobody was on letermovir at the beginning of the trial,” he replied, but noted that some patients who were enrolled and had infections that were refractory or resistant to valganciclovir, foscarnet, or a combination of the two received letermovir as secondary prophylaxis.
“I haven’t got the data to tell you how often [letermovir] was used; I think part of the lack of mortality benefit [with maribavir] may be due to the fact that people jumped into secondary prophylaxis with letermovir to minimize the toxicities that we saw,” he said.
Although maribavir has not as of this writing received Food and Drug Administration approval, the drug may be available to some patients through a compassionate-use program from Takeda, Dr. Marty noted.
The study was funded by Shire ViroPharma. Dr. Marty disclosed research funding from Shire and from others. Dr. Ghosh had no relevant disclosures.
Maribavir, an investigational antiviral agent with a novel mechanism of action, was superior to other antiviral strategies at clearing cytomegalovirus (CMV) viremia and controlling symptoms in hematopoietic cell or solid-organ transplant recipients, results of a phase 3 clinical trial showed.
CMV viremia clearance at study week 8 was seen in 55.7% of all patients randomized to receive maribavir, compared with 23.9% for patients assigned to receive investigator-assigned therapy (IAT), Francisco Marty, MD, from the Dana-Farber Cancer Institute in Boston reported at the Transplant & Cellular Therapies Meetings.
“Maribavir’s benefit was driven by lower incidence of treatment-limiting toxicities, compared with IAT,” he said a late-breaking abstract session during the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
“Available anti-CMV antivirals are limited by development of resistance and toxicities, particularly myelosuppression with the use of valganciclovir and nephrotoxicity with the use of foscarnet and cidofovir. Alternative treatment options are required to address this unmet medical need,” he said.
Maribavir inhibits the CMV UL97 protein kinase and is thought to affect several critical processes in CMV replication, including viral DNA synthesis, viral gene expression, encapsidation, and egress of mature capsids from the nucleus.
Details of trial
In the phase 3 SHP620-30e trial (NCT02931539), Dr. Marty and colleagues enrolled patients with relapsed or refractory CMV infections after hematopoietic cell transplant (HCT) or solid-organ transplant (SOT) and after stratification by transplant type and screening CMV DNA level randomly assigned them on a 2:1 basis to receive either maribavir 400 mg twice daily (235 patients) or IAT (117 patients), consisting of either ganciclovir/valganciclovir, foscarnet, cidofovir, or combined foscarnet and val/ganciclovir.
The primary endpoint of viremia clearance at 8 weeks was defined as plasma CMV DNA less than 137 IU/mL in two consecutive tests at a central laboratory at least 5 days apart beginning at the end of week 8.
The trial met its primary endpoint, with a viremia clearance rate of 55.7% with maribavir versus 23.9% with IAT.
The viremia clearance rates were similar in each of the transplant groups: 55.9% versus 20.8%, respectively, in patients who underwent HCT, and 55.6% versus 26.1% in patients who underwent SOT (P < .001).
Clearance rates among patients with CMV DNA below 9,100 IU/mL at baseline were 62.1% with maribavir versus 24.7% with IAT. Among patients with baseline CMV DNA of 9100 IU/mL or above, the respective rates were 43.9% versus 21.9%.
CMV viremia clearance continued from week 8 to week 16 in 18.7% of patients assigned to maribavir and to 10.3% of patients randomized to IAT (P < .013).
The median time to first CMV viremia clearance as 22 days with maribavir versus 27 days with IAT (P = .039).
All-cause mortality was similar between the groups, at 11.5% versus 11.1%, respectively.
The incidences of serious and severe treatment-emergent adverse events (TEAE) were 38.5% and 32.1%, respectively, in the maribavir group, and 37.1% and 37.9% in the IAT group.
Any TEAE leading to study drug discontinuation was less common with maribavir, occurring in 13.2% of patients, compared with 31.9% of patients on IAT. Serious TEAEs leading to drug discontinuation occurred in 8.5% versus 14.7%, respectively.
Serious TEAEs leading to death occurred in 6.8% of patients on maribavir versus 5.2% of those on IAT.
Role of letermovir
In the question-and-answer session following the presentation, comoderator Monalisa Ghosh, MD, from the University of Michigan, Ann Arbor, asked whether any patients in the study were currently on letermovir (Prevymis) prophylaxis, and whether any patients had previously been treated with letermovir but had CMV reactivation and were then treated on study.
Dr. Marty noted that the trial was designed before letermovir was approved for CMV prophylaxis in adults who have undergone an allogeneic HCT.
“Nobody was on letermovir at the beginning of the trial,” he replied, but noted that some patients who were enrolled and had infections that were refractory or resistant to valganciclovir, foscarnet, or a combination of the two received letermovir as secondary prophylaxis.
“I haven’t got the data to tell you how often [letermovir] was used; I think part of the lack of mortality benefit [with maribavir] may be due to the fact that people jumped into secondary prophylaxis with letermovir to minimize the toxicities that we saw,” he said.
Although maribavir has not as of this writing received Food and Drug Administration approval, the drug may be available to some patients through a compassionate-use program from Takeda, Dr. Marty noted.
The study was funded by Shire ViroPharma. Dr. Marty disclosed research funding from Shire and from others. Dr. Ghosh had no relevant disclosures.
FROM TCT 2021
Study clarifies who gets post–COVID-19 interstitial lung disease
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Decline in children’s COVID-19 cases slows
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
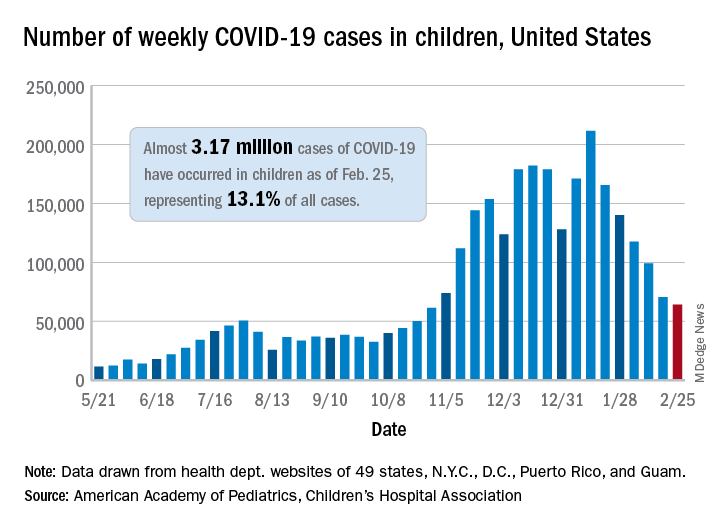
That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
Fired for good judgment a sign of physicians’ lost respect
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
COVID-19 vaccination linked to less mechanical ventilation
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
FDA grants emergency use authorization to Johnson & Johnson COVID-19 vaccine
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.