User login
American Academy of Allergy, Asthma, & Immunology (AAAAI): Annual Meeting
VIDEO: Penicillin skin testing improves inpatient antibiotic stewardship
HOUSTON– Dr. Megan S. Motosue of the Mayo Clinic in Rochester, Minn., discusses the value of penicillin skin testing as a potent tool for improving inpatient antibiotic stewardship. By routinely performing penicillin skin testing using standard methods in a consecutive series of inpatients with a self-reported history of penicillin allergy, roughly 80% were found to not in fact be allergic. And this information changed their physicians’ behavior: prior to testing, vancomycin was the most commonly used antibiotic in the inpatients, but its use was reduced by more than half based upon the skin test results, with a commensurate increase in prescriptions for semi-synthetic penicillins.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON– Dr. Megan S. Motosue of the Mayo Clinic in Rochester, Minn., discusses the value of penicillin skin testing as a potent tool for improving inpatient antibiotic stewardship. By routinely performing penicillin skin testing using standard methods in a consecutive series of inpatients with a self-reported history of penicillin allergy, roughly 80% were found to not in fact be allergic. And this information changed their physicians’ behavior: prior to testing, vancomycin was the most commonly used antibiotic in the inpatients, but its use was reduced by more than half based upon the skin test results, with a commensurate increase in prescriptions for semi-synthetic penicillins.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON– Dr. Megan S. Motosue of the Mayo Clinic in Rochester, Minn., discusses the value of penicillin skin testing as a potent tool for improving inpatient antibiotic stewardship. By routinely performing penicillin skin testing using standard methods in a consecutive series of inpatients with a self-reported history of penicillin allergy, roughly 80% were found to not in fact be allergic. And this information changed their physicians’ behavior: prior to testing, vancomycin was the most commonly used antibiotic in the inpatients, but its use was reduced by more than half based upon the skin test results, with a commensurate increase in prescriptions for semi-synthetic penicillins.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE 2015 AAAAI ANNUAL MEETING
When to use SLIT and SCID in atopic dermatitis
HOUSTON – Whether atopic dermatitis should be treated with allergen immunotherapy is still a matter of debate, as high-quality trial evidence remains scant, but clinical practice is starting to recognize the potential use of subcutaneous and sublingual immunotherapy for certain presentations of the disease.
For patients with allergy to house-dust mites, evidence is leaning toward a role for subcutaneous immunotherapy (SCIT) in severe forms of atopic dermatitis (AD), and to sublingual immunotherapy (SLIT) for milder forms, researchers said.
At the annual meeting of the American Academy of Allergy, Asthma, and Immunotherapy, Dr. Linda Cox, an allergist in private practice in Fort Lauderdale, Fla., said that poor trial evidence and a lack of standardization in outcome measures have hindered a better understanding of when SLIT and SCIT might be indicated in atopic dermatitis. Systematic reviews have failed to offer compelling evidence for its efficacy.
This is why current practice guidelines, published in 2012, say only that clinicians “might consider” allergen immunotherapy in selected patients with AD with aeroallergen sensitivity, and the research community has been striving to standardize outcome measures using two published eczema scoring systems, EASI (Eczema Area and Severity Index) and SCORAD (Severity Scoring of Atopic Dermatitis). Dr. Cox, part of the AAAAI/American College of Asthma, Allergy, and Immunology joint task force on allergen immunotherapies, said new clinical guidelines in progress would likely attempt to better define the role of SLIT and SCIT in atopic dermatitis.
Dr. Cox and another speaker at the conference, Dr. Moises Calderon, both pointed to house-dust mite allergy with AD as the most promising indication for immunotherapy in AD. A closer look at the few well-designed trials that have been conducted so far reveal the potential for a better-defined role for SLIT and SCIT, they agreed.
Dr. Calderon of Imperial College London identified four important trials dealing with this issue. The first was a German study of adults with AD aggravated by house-dust mites (n = 168) randomized to SCID or placebo and treated for 18 months. The study revealed no statistically significant differences for the treatment group as a whole, but a subgroup of patients with severe AD showed a statistically significant reduction of SCORAD disease scores by 18% (P = .02), compared with placebo (J Allergy Clin Immunol. 2012;130:925-31).
An Italian trial enrolling house-dust mite–sensitive children with AD (n = 56, randomized 1:1 to treatment with SLIT or placebo) found a significant difference in SCORAD from baseline (P = .25) between the children treated with SLIT and the placebo group starting 9 months after treatment. However, the difference was seen in patients with mild or moderate disease only; minimal benefit was found for children with severe disease (J. Allergy Clin. Immunol. 2007;120:164-70).
Dr. Calderon pointed to two additional studies published that also suggested benefit. The first was an open-label Colombian study enrolling children and young adults (n = 60). Of the 31 patients randomized to treatment with SCIT and topical therapies (controls received only topical treatment), the treatment group had significant SCORAD-measured improvement, compared with the control group (P = .03). At 1 year the active group also used topical steroids and tacrolimus significantly less (P = .02), and also had a significant increase in mite-specific IgG4 (doi:10.5402/2012/183983).
Dr. Calderon also mentioned a recent observational study from Poland that looked at long-term follow-up of a group of 15 AD patients treated with SCIT between 1995 and 2001 and followed for 12 years. The results suggested that SCIT may be associated with lasting improvements even after treatment termination (Eur. Ann. Allergy Clin. Immunol. 2015;47:5-9).
Both Dr. Cox and Dr. Calderon agreed that more and better evidence was needed to understand when and how SLIT and SCIT can work in atopic dermatitis. “AD is a heterogeneous disease, and immunotherapy cannot be a treatment for all types of atopic dermatitis – no one treatment is probably effective for all types of AD,” Dr. Cox cautioned. But “the direction of therapy is headed toward some acceptance.”
Dr, Calderon concurred. “We are on the way to acceptance. But new studies will be key,” he said.
Dr. Cox disclosed financial relationships with Greer and Circassia. Dr. Calderon disclosed no conflicts of interest.
HOUSTON – Whether atopic dermatitis should be treated with allergen immunotherapy is still a matter of debate, as high-quality trial evidence remains scant, but clinical practice is starting to recognize the potential use of subcutaneous and sublingual immunotherapy for certain presentations of the disease.
For patients with allergy to house-dust mites, evidence is leaning toward a role for subcutaneous immunotherapy (SCIT) in severe forms of atopic dermatitis (AD), and to sublingual immunotherapy (SLIT) for milder forms, researchers said.
At the annual meeting of the American Academy of Allergy, Asthma, and Immunotherapy, Dr. Linda Cox, an allergist in private practice in Fort Lauderdale, Fla., said that poor trial evidence and a lack of standardization in outcome measures have hindered a better understanding of when SLIT and SCIT might be indicated in atopic dermatitis. Systematic reviews have failed to offer compelling evidence for its efficacy.
This is why current practice guidelines, published in 2012, say only that clinicians “might consider” allergen immunotherapy in selected patients with AD with aeroallergen sensitivity, and the research community has been striving to standardize outcome measures using two published eczema scoring systems, EASI (Eczema Area and Severity Index) and SCORAD (Severity Scoring of Atopic Dermatitis). Dr. Cox, part of the AAAAI/American College of Asthma, Allergy, and Immunology joint task force on allergen immunotherapies, said new clinical guidelines in progress would likely attempt to better define the role of SLIT and SCIT in atopic dermatitis.
Dr. Cox and another speaker at the conference, Dr. Moises Calderon, both pointed to house-dust mite allergy with AD as the most promising indication for immunotherapy in AD. A closer look at the few well-designed trials that have been conducted so far reveal the potential for a better-defined role for SLIT and SCIT, they agreed.
Dr. Calderon of Imperial College London identified four important trials dealing with this issue. The first was a German study of adults with AD aggravated by house-dust mites (n = 168) randomized to SCID or placebo and treated for 18 months. The study revealed no statistically significant differences for the treatment group as a whole, but a subgroup of patients with severe AD showed a statistically significant reduction of SCORAD disease scores by 18% (P = .02), compared with placebo (J Allergy Clin Immunol. 2012;130:925-31).
An Italian trial enrolling house-dust mite–sensitive children with AD (n = 56, randomized 1:1 to treatment with SLIT or placebo) found a significant difference in SCORAD from baseline (P = .25) between the children treated with SLIT and the placebo group starting 9 months after treatment. However, the difference was seen in patients with mild or moderate disease only; minimal benefit was found for children with severe disease (J. Allergy Clin. Immunol. 2007;120:164-70).
Dr. Calderon pointed to two additional studies published that also suggested benefit. The first was an open-label Colombian study enrolling children and young adults (n = 60). Of the 31 patients randomized to treatment with SCIT and topical therapies (controls received only topical treatment), the treatment group had significant SCORAD-measured improvement, compared with the control group (P = .03). At 1 year the active group also used topical steroids and tacrolimus significantly less (P = .02), and also had a significant increase in mite-specific IgG4 (doi:10.5402/2012/183983).
Dr. Calderon also mentioned a recent observational study from Poland that looked at long-term follow-up of a group of 15 AD patients treated with SCIT between 1995 and 2001 and followed for 12 years. The results suggested that SCIT may be associated with lasting improvements even after treatment termination (Eur. Ann. Allergy Clin. Immunol. 2015;47:5-9).
Both Dr. Cox and Dr. Calderon agreed that more and better evidence was needed to understand when and how SLIT and SCIT can work in atopic dermatitis. “AD is a heterogeneous disease, and immunotherapy cannot be a treatment for all types of atopic dermatitis – no one treatment is probably effective for all types of AD,” Dr. Cox cautioned. But “the direction of therapy is headed toward some acceptance.”
Dr, Calderon concurred. “We are on the way to acceptance. But new studies will be key,” he said.
Dr. Cox disclosed financial relationships with Greer and Circassia. Dr. Calderon disclosed no conflicts of interest.
HOUSTON – Whether atopic dermatitis should be treated with allergen immunotherapy is still a matter of debate, as high-quality trial evidence remains scant, but clinical practice is starting to recognize the potential use of subcutaneous and sublingual immunotherapy for certain presentations of the disease.
For patients with allergy to house-dust mites, evidence is leaning toward a role for subcutaneous immunotherapy (SCIT) in severe forms of atopic dermatitis (AD), and to sublingual immunotherapy (SLIT) for milder forms, researchers said.
At the annual meeting of the American Academy of Allergy, Asthma, and Immunotherapy, Dr. Linda Cox, an allergist in private practice in Fort Lauderdale, Fla., said that poor trial evidence and a lack of standardization in outcome measures have hindered a better understanding of when SLIT and SCIT might be indicated in atopic dermatitis. Systematic reviews have failed to offer compelling evidence for its efficacy.
This is why current practice guidelines, published in 2012, say only that clinicians “might consider” allergen immunotherapy in selected patients with AD with aeroallergen sensitivity, and the research community has been striving to standardize outcome measures using two published eczema scoring systems, EASI (Eczema Area and Severity Index) and SCORAD (Severity Scoring of Atopic Dermatitis). Dr. Cox, part of the AAAAI/American College of Asthma, Allergy, and Immunology joint task force on allergen immunotherapies, said new clinical guidelines in progress would likely attempt to better define the role of SLIT and SCIT in atopic dermatitis.
Dr. Cox and another speaker at the conference, Dr. Moises Calderon, both pointed to house-dust mite allergy with AD as the most promising indication for immunotherapy in AD. A closer look at the few well-designed trials that have been conducted so far reveal the potential for a better-defined role for SLIT and SCIT, they agreed.
Dr. Calderon of Imperial College London identified four important trials dealing with this issue. The first was a German study of adults with AD aggravated by house-dust mites (n = 168) randomized to SCID or placebo and treated for 18 months. The study revealed no statistically significant differences for the treatment group as a whole, but a subgroup of patients with severe AD showed a statistically significant reduction of SCORAD disease scores by 18% (P = .02), compared with placebo (J Allergy Clin Immunol. 2012;130:925-31).
An Italian trial enrolling house-dust mite–sensitive children with AD (n = 56, randomized 1:1 to treatment with SLIT or placebo) found a significant difference in SCORAD from baseline (P = .25) between the children treated with SLIT and the placebo group starting 9 months after treatment. However, the difference was seen in patients with mild or moderate disease only; minimal benefit was found for children with severe disease (J. Allergy Clin. Immunol. 2007;120:164-70).
Dr. Calderon pointed to two additional studies published that also suggested benefit. The first was an open-label Colombian study enrolling children and young adults (n = 60). Of the 31 patients randomized to treatment with SCIT and topical therapies (controls received only topical treatment), the treatment group had significant SCORAD-measured improvement, compared with the control group (P = .03). At 1 year the active group also used topical steroids and tacrolimus significantly less (P = .02), and also had a significant increase in mite-specific IgG4 (doi:10.5402/2012/183983).
Dr. Calderon also mentioned a recent observational study from Poland that looked at long-term follow-up of a group of 15 AD patients treated with SCIT between 1995 and 2001 and followed for 12 years. The results suggested that SCIT may be associated with lasting improvements even after treatment termination (Eur. Ann. Allergy Clin. Immunol. 2015;47:5-9).
Both Dr. Cox and Dr. Calderon agreed that more and better evidence was needed to understand when and how SLIT and SCIT can work in atopic dermatitis. “AD is a heterogeneous disease, and immunotherapy cannot be a treatment for all types of atopic dermatitis – no one treatment is probably effective for all types of AD,” Dr. Cox cautioned. But “the direction of therapy is headed toward some acceptance.”
Dr, Calderon concurred. “We are on the way to acceptance. But new studies will be key,” he said.
Dr. Cox disclosed financial relationships with Greer and Circassia. Dr. Calderon disclosed no conflicts of interest.
AT 2015 AAAAI ANNUAL MEETING
SLIT: Guidelines in progress and practical concerns
HOUSTON – Sublingual immunotherapy, or SLIT, long used by clinicians worldwide, is newly approved for use in the United States, and practice guidelines have yet to be published for U.S. clinicians.
A joint task force of the American Academy of Allergy, Asthma, and Immunology (AAAAI) and the American College of Allergy, Asthma, and Immunology (ACAAI) is drafting the guidelines. While clinicians wait, here is a look at the evidence for efficacy and safety of SLIT – particularly when compared with subcutaneous immunotherapy – as well as at the progress of the guidelines, offered by Dr. Linda Cox, an allergist in private practice in Fort Lauderdale, Fla. Dr. Cox spoke at the annual meeting of the AAAAI.
The guidelines will closely follow package labeling for the three sublingual pollen-allergy medications approved last year by the Food and Drug Administration, Dr. Cox said. These are the multipollen allergen tablet Oralair (Stallergenes), the Timothy grass pollen tablet Grastek (Merck), and the ragweed tablet Ragwitek (Merck). The medicines’ indications differ in terms of minimum age for prescription, ranging from 5 to 18 years, and the timing of treatment, with Grastek indicated as a year-round treatment, and the others as seasonal treatments.
FDA labeling on all three medications recommends coprescription of epinephrine pens in case of systemic reactions, and Dr. Cox said that the AAAAI/ACAAI guidelines would reflect this. Nonetheless, coprescription of epi pens “has never been the standard in Europe or other parts of the globe. We don’t have guidance from the broader community in terms of when to take the epinephrine in terms of a SLIT reaction,” she noted. Physicians should “be aware and think about what sort of instructions you’re going to give your patients” on the use of epi pens.
The guidelines in progress advise physicians to counsel patients that site application symptoms such as itching and swelling are very common during the first week of SLIT treatment, and that the majority of SLIT reactions are local (oral, pharyngeal, or abdominal). These local reactions usually disappear within days to weeks without treatment or dose modification, though some can be severe or bothersome enough to discontinue treatment. Systemic allergic reactions are very uncommon.
The guidelines are also likely to caution physicians that there are insufficient studies comparing SLIT and subcutaneous immunotherapy, or SCIT, to make a definitive statement about efficacy, Dr. Cox said. They will note that available studies suggest SCIT is more effective in the first year of treatment than SLIT, and comparative long-term efficacy studies have not been conducted, she added.
Finally, the guidelines are likely to caution that SLIT is contraindicated in patients who are not likely to survive a systemic adverse reaction or the resultant treatment.
Dr. Cox did not say when the guidelines were likely to be published, just that “we are frantically trying to get this document together,” noting the short time that SLIT has been commercially available in the United States.
At the same session on SLIT, Dr. Desiree Larenas Linneman, an allergist in private practice in Mexico City, spoke at the meeting about practical concerns related to SLIT and how to choose whether a patient is better suited to shots or sublingual therapy.
“When you bring sublingual to the public there are several issues you are going to have to be very careful with,” Dr. Larenas Linneman said. Among these are prescriptions of highly concentrated medications by nonallergists, adherence, and patient preference. “And we still do not have data for SLIT on a vast array of comorbidities,” she said.
“When we only had SCIT, these patients came into our offices and the only decision was, would we go for shots or not?” Now the choices are more complicated. A multiallergic 8-year-old, ordinarily a good candidate for SCIT, might be better treated with tablets if the parent is adamant that the child not get shots, she said. An adult who travels constantly for work might find tablets more convenient.
But for multiallergic and highly allergic patients, SCIT is generally the more flexible and reliable option, Dr. Larenas Linneman said. Allergists have access to a great variety of different allergens they can combine to make SCIT, while the approved tablets cover only pollen. And a tablet is a fixed dose, she said, “so there’s no adjustment for highly allergic patients.”
Dr. Larenas Linneman noted that adherence and patient preference are closely related, something that physicians must keep in mind when choosing the form of immunotherapy to offer. “Adherence is a very important issue because we know adherence is quite poor both with SLIT and SCIT,” she said.
“Physicians think that what’s most important for the patient is efficacy, cost, and side effects,” but patients’ ability to comply is also key, she said, and clinicians should let patient preference guide their decisions when possible.
Dr. Cox disclosed ongoing consulting relationships with Greer and Circassia. Dr. Larenas Linnemann disclosed financial support from AstraZeneca, Pfizer, Novartis, MEDA, Sanofi, and Senosiain.
HOUSTON – Sublingual immunotherapy, or SLIT, long used by clinicians worldwide, is newly approved for use in the United States, and practice guidelines have yet to be published for U.S. clinicians.
A joint task force of the American Academy of Allergy, Asthma, and Immunology (AAAAI) and the American College of Allergy, Asthma, and Immunology (ACAAI) is drafting the guidelines. While clinicians wait, here is a look at the evidence for efficacy and safety of SLIT – particularly when compared with subcutaneous immunotherapy – as well as at the progress of the guidelines, offered by Dr. Linda Cox, an allergist in private practice in Fort Lauderdale, Fla. Dr. Cox spoke at the annual meeting of the AAAAI.
The guidelines will closely follow package labeling for the three sublingual pollen-allergy medications approved last year by the Food and Drug Administration, Dr. Cox said. These are the multipollen allergen tablet Oralair (Stallergenes), the Timothy grass pollen tablet Grastek (Merck), and the ragweed tablet Ragwitek (Merck). The medicines’ indications differ in terms of minimum age for prescription, ranging from 5 to 18 years, and the timing of treatment, with Grastek indicated as a year-round treatment, and the others as seasonal treatments.
FDA labeling on all three medications recommends coprescription of epinephrine pens in case of systemic reactions, and Dr. Cox said that the AAAAI/ACAAI guidelines would reflect this. Nonetheless, coprescription of epi pens “has never been the standard in Europe or other parts of the globe. We don’t have guidance from the broader community in terms of when to take the epinephrine in terms of a SLIT reaction,” she noted. Physicians should “be aware and think about what sort of instructions you’re going to give your patients” on the use of epi pens.
The guidelines in progress advise physicians to counsel patients that site application symptoms such as itching and swelling are very common during the first week of SLIT treatment, and that the majority of SLIT reactions are local (oral, pharyngeal, or abdominal). These local reactions usually disappear within days to weeks without treatment or dose modification, though some can be severe or bothersome enough to discontinue treatment. Systemic allergic reactions are very uncommon.
The guidelines are also likely to caution physicians that there are insufficient studies comparing SLIT and subcutaneous immunotherapy, or SCIT, to make a definitive statement about efficacy, Dr. Cox said. They will note that available studies suggest SCIT is more effective in the first year of treatment than SLIT, and comparative long-term efficacy studies have not been conducted, she added.
Finally, the guidelines are likely to caution that SLIT is contraindicated in patients who are not likely to survive a systemic adverse reaction or the resultant treatment.
Dr. Cox did not say when the guidelines were likely to be published, just that “we are frantically trying to get this document together,” noting the short time that SLIT has been commercially available in the United States.
At the same session on SLIT, Dr. Desiree Larenas Linneman, an allergist in private practice in Mexico City, spoke at the meeting about practical concerns related to SLIT and how to choose whether a patient is better suited to shots or sublingual therapy.
“When you bring sublingual to the public there are several issues you are going to have to be very careful with,” Dr. Larenas Linneman said. Among these are prescriptions of highly concentrated medications by nonallergists, adherence, and patient preference. “And we still do not have data for SLIT on a vast array of comorbidities,” she said.
“When we only had SCIT, these patients came into our offices and the only decision was, would we go for shots or not?” Now the choices are more complicated. A multiallergic 8-year-old, ordinarily a good candidate for SCIT, might be better treated with tablets if the parent is adamant that the child not get shots, she said. An adult who travels constantly for work might find tablets more convenient.
But for multiallergic and highly allergic patients, SCIT is generally the more flexible and reliable option, Dr. Larenas Linneman said. Allergists have access to a great variety of different allergens they can combine to make SCIT, while the approved tablets cover only pollen. And a tablet is a fixed dose, she said, “so there’s no adjustment for highly allergic patients.”
Dr. Larenas Linneman noted that adherence and patient preference are closely related, something that physicians must keep in mind when choosing the form of immunotherapy to offer. “Adherence is a very important issue because we know adherence is quite poor both with SLIT and SCIT,” she said.
“Physicians think that what’s most important for the patient is efficacy, cost, and side effects,” but patients’ ability to comply is also key, she said, and clinicians should let patient preference guide their decisions when possible.
Dr. Cox disclosed ongoing consulting relationships with Greer and Circassia. Dr. Larenas Linnemann disclosed financial support from AstraZeneca, Pfizer, Novartis, MEDA, Sanofi, and Senosiain.
HOUSTON – Sublingual immunotherapy, or SLIT, long used by clinicians worldwide, is newly approved for use in the United States, and practice guidelines have yet to be published for U.S. clinicians.
A joint task force of the American Academy of Allergy, Asthma, and Immunology (AAAAI) and the American College of Allergy, Asthma, and Immunology (ACAAI) is drafting the guidelines. While clinicians wait, here is a look at the evidence for efficacy and safety of SLIT – particularly when compared with subcutaneous immunotherapy – as well as at the progress of the guidelines, offered by Dr. Linda Cox, an allergist in private practice in Fort Lauderdale, Fla. Dr. Cox spoke at the annual meeting of the AAAAI.
The guidelines will closely follow package labeling for the three sublingual pollen-allergy medications approved last year by the Food and Drug Administration, Dr. Cox said. These are the multipollen allergen tablet Oralair (Stallergenes), the Timothy grass pollen tablet Grastek (Merck), and the ragweed tablet Ragwitek (Merck). The medicines’ indications differ in terms of minimum age for prescription, ranging from 5 to 18 years, and the timing of treatment, with Grastek indicated as a year-round treatment, and the others as seasonal treatments.
FDA labeling on all three medications recommends coprescription of epinephrine pens in case of systemic reactions, and Dr. Cox said that the AAAAI/ACAAI guidelines would reflect this. Nonetheless, coprescription of epi pens “has never been the standard in Europe or other parts of the globe. We don’t have guidance from the broader community in terms of when to take the epinephrine in terms of a SLIT reaction,” she noted. Physicians should “be aware and think about what sort of instructions you’re going to give your patients” on the use of epi pens.
The guidelines in progress advise physicians to counsel patients that site application symptoms such as itching and swelling are very common during the first week of SLIT treatment, and that the majority of SLIT reactions are local (oral, pharyngeal, or abdominal). These local reactions usually disappear within days to weeks without treatment or dose modification, though some can be severe or bothersome enough to discontinue treatment. Systemic allergic reactions are very uncommon.
The guidelines are also likely to caution physicians that there are insufficient studies comparing SLIT and subcutaneous immunotherapy, or SCIT, to make a definitive statement about efficacy, Dr. Cox said. They will note that available studies suggest SCIT is more effective in the first year of treatment than SLIT, and comparative long-term efficacy studies have not been conducted, she added.
Finally, the guidelines are likely to caution that SLIT is contraindicated in patients who are not likely to survive a systemic adverse reaction or the resultant treatment.
Dr. Cox did not say when the guidelines were likely to be published, just that “we are frantically trying to get this document together,” noting the short time that SLIT has been commercially available in the United States.
At the same session on SLIT, Dr. Desiree Larenas Linneman, an allergist in private practice in Mexico City, spoke at the meeting about practical concerns related to SLIT and how to choose whether a patient is better suited to shots or sublingual therapy.
“When you bring sublingual to the public there are several issues you are going to have to be very careful with,” Dr. Larenas Linneman said. Among these are prescriptions of highly concentrated medications by nonallergists, adherence, and patient preference. “And we still do not have data for SLIT on a vast array of comorbidities,” she said.
“When we only had SCIT, these patients came into our offices and the only decision was, would we go for shots or not?” Now the choices are more complicated. A multiallergic 8-year-old, ordinarily a good candidate for SCIT, might be better treated with tablets if the parent is adamant that the child not get shots, she said. An adult who travels constantly for work might find tablets more convenient.
But for multiallergic and highly allergic patients, SCIT is generally the more flexible and reliable option, Dr. Larenas Linneman said. Allergists have access to a great variety of different allergens they can combine to make SCIT, while the approved tablets cover only pollen. And a tablet is a fixed dose, she said, “so there’s no adjustment for highly allergic patients.”
Dr. Larenas Linneman noted that adherence and patient preference are closely related, something that physicians must keep in mind when choosing the form of immunotherapy to offer. “Adherence is a very important issue because we know adherence is quite poor both with SLIT and SCIT,” she said.
“Physicians think that what’s most important for the patient is efficacy, cost, and side effects,” but patients’ ability to comply is also key, she said, and clinicians should let patient preference guide their decisions when possible.
Dr. Cox disclosed ongoing consulting relationships with Greer and Circassia. Dr. Larenas Linnemann disclosed financial support from AstraZeneca, Pfizer, Novartis, MEDA, Sanofi, and Senosiain.
AT 2015 AAAAI ANNUAL MEETING
Beware common management pitfalls in severe refractory pediatric AD
HOUSTON – Dermatologists, allergists, and other physicians treating children with extremely refractory forms of atopic dermatitis (AD) must be aware of common treatment and management pitfalls before jumping to immunosuppressant or biologic therapies, says pediatric eczema researcher Dr. Donald Y. M. Leung, particularly as this is a patient group for whom no systemic therapies have been approved.
In a presentation at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, Dr. Leung shared insights from his clinical experience at National Jewish Health in Denver, whose pediatric eczema program represents a national referral center for patients with severely refractory disease and their families.
“As managed care continues to march on, you will be faced with taking care of the more difficult patients,” Dr. Leung told clinicians.“You will see all forms of AD in clinical practice, therefore one size does not fit all, and a stepwise approach is needed to [treat] the different forms of eczema.”
Clinicians first need to determine what step, or grade, of eczema a patient has. For example, step 2 and 3 eczema is characterized by moderate disease not controlled by intermittent use of topical steroids or calcineurin inhibitors.
National Jewish Health’s day-based program aims to first clear up children presenting with severe eczema before clinicians attempt diagnostic testing. This is achieved through the use of wet wraps, until skin has healed enough for testing to begin.
With wet wraps and day hospitalization, “you can go from severe to mild within a few days,” Dr. Leung said. For children who do not improve after the wet wraps, ruling out immune deficiency or other diagnoses is key. Dr. Leung and colleagues look for Dock 8 (dedicator of cytokinesis 8) protein deficiency in children who fail to respond to wet-wrap treatment, especially if they have presented with recurrent herpes infections or persistent warts.
Another important function of the day program is to observe how parents manage children with eczema. Many, Dr. Leung said, turn out to be noncompliant with recommendations on bathing and medications, often because of the discomfort it causes the child. The monitoring is essential to reveal errors in care. “You can’t tell this in a 10-minute office visit,” he said.
A top reason children fail therapy, he said, is because parents misinterpret recommendations on applying medicine after bathing. Many think that after applying a topical medication, “you can get better absorption if you put the moisturizer on top of the topical steroid. That really just dilutes the medication and you get ineffective therapy.”
Another upshot of the monitoring is that clinicians can identify parents suffering from depression, stress, or financial problems that prevent them from complying. “As with asthma, psychosocial factors loom big,” he said. Parents are extensively counseled and referred as needed, he said. Children also can be trained not to scratch their skin, and children over 5 years in the eczema program are offered hypnosis and biofeedback training to learn how to control their response to itching.
When taking a history of suspected food reactions, Dr. Leung said, it’s important to note that only hives and anaphylaxis are clearly linked to food allergy. “Skin testing and blood work is what we all do, but that’s helpful only if negative. If it’s positive, it still doesn’t tell you that they have food-induced eczema, and like it or not, some form of the food challenge is necessary.”
Dr. Leung said it is appropriate to conduct some oral food challenges unblinded after a negative test. “If a test is positive, and the parent insists that this food may cause eczema, it’s often necessary to do a double-blind, placebo-controlled test,” he said, which is the gold standard.
In discussing systemic therapies, Dr. Leung noted that general immunosuppressants were an approach favored by dermatologists, but urged caution with regard to interferons, mycophenolate, methotrexate, azathioprine, and cyclosporin A. “These are expensive therapies not approved in children, and you really need very good documentation that they’ve failed other forms of therapy before you’d move to that,” Dr. Leung said.
Oral steroids should be avoided. “I have seen thousands of cases of severe eczema, and I’ve never put somebody on oral steroids unless they happened to have an asthma exacerbation concurrent with AD,” he said. “This is because they often have rebound within a week of stopping oral steroids, and that rebound can be worse than the disease you started with.”
If oral steroids must be used, “you should taper slowly and increase the intensity of skin care, so they don’t have a severe rebound.”
Dr. Leung said that while omalizumab should work in any disease with an elevated serum IgE level, “more often than not it doesn’t,” and it probably should be reserved for patients with a very clear history of allergen-induced eczema, underlying urticaria, or other forms of respiratory allergy that may be triggering asthma.
Two potential approaches in which allergists and dermatologists can work together, Dr. Leung said, are phototherapy and allergy immunotherapy. The latter is controversial in AD, he acknowledged, “but if somebody has mainly dust mite allergy or is monosensitized, it’s more likely you will get good benefit. If they’re polysensitized, it is unlikely because it’s mainly a barrier problem.”
Dr. Leung did not recommend antibiotics except in the case of overt Staphylococcus aureus infection, so as not to select for methicillin-resistant S. aureus (MRSA). “If you’re going to treat with some regimen, keep in mind that staph comes from the nose; that’s the body’s reservoir. You should always use an intranasal Bactroban [mupirocin] along with a systemic antibiotic.” Effective eradication of MRSA infection requires more drastic measures, including treatment of other family members and pets.
Dr. Leung disclosed doing consulting work for Celgene, Novartis, Regeneron, and Sanofi-Aventis, and a research grant from Horizon Pharma.
HOUSTON – Dermatologists, allergists, and other physicians treating children with extremely refractory forms of atopic dermatitis (AD) must be aware of common treatment and management pitfalls before jumping to immunosuppressant or biologic therapies, says pediatric eczema researcher Dr. Donald Y. M. Leung, particularly as this is a patient group for whom no systemic therapies have been approved.
In a presentation at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, Dr. Leung shared insights from his clinical experience at National Jewish Health in Denver, whose pediatric eczema program represents a national referral center for patients with severely refractory disease and their families.
“As managed care continues to march on, you will be faced with taking care of the more difficult patients,” Dr. Leung told clinicians.“You will see all forms of AD in clinical practice, therefore one size does not fit all, and a stepwise approach is needed to [treat] the different forms of eczema.”
Clinicians first need to determine what step, or grade, of eczema a patient has. For example, step 2 and 3 eczema is characterized by moderate disease not controlled by intermittent use of topical steroids or calcineurin inhibitors.
National Jewish Health’s day-based program aims to first clear up children presenting with severe eczema before clinicians attempt diagnostic testing. This is achieved through the use of wet wraps, until skin has healed enough for testing to begin.
With wet wraps and day hospitalization, “you can go from severe to mild within a few days,” Dr. Leung said. For children who do not improve after the wet wraps, ruling out immune deficiency or other diagnoses is key. Dr. Leung and colleagues look for Dock 8 (dedicator of cytokinesis 8) protein deficiency in children who fail to respond to wet-wrap treatment, especially if they have presented with recurrent herpes infections or persistent warts.
Another important function of the day program is to observe how parents manage children with eczema. Many, Dr. Leung said, turn out to be noncompliant with recommendations on bathing and medications, often because of the discomfort it causes the child. The monitoring is essential to reveal errors in care. “You can’t tell this in a 10-minute office visit,” he said.
A top reason children fail therapy, he said, is because parents misinterpret recommendations on applying medicine after bathing. Many think that after applying a topical medication, “you can get better absorption if you put the moisturizer on top of the topical steroid. That really just dilutes the medication and you get ineffective therapy.”
Another upshot of the monitoring is that clinicians can identify parents suffering from depression, stress, or financial problems that prevent them from complying. “As with asthma, psychosocial factors loom big,” he said. Parents are extensively counseled and referred as needed, he said. Children also can be trained not to scratch their skin, and children over 5 years in the eczema program are offered hypnosis and biofeedback training to learn how to control their response to itching.
When taking a history of suspected food reactions, Dr. Leung said, it’s important to note that only hives and anaphylaxis are clearly linked to food allergy. “Skin testing and blood work is what we all do, but that’s helpful only if negative. If it’s positive, it still doesn’t tell you that they have food-induced eczema, and like it or not, some form of the food challenge is necessary.”
Dr. Leung said it is appropriate to conduct some oral food challenges unblinded after a negative test. “If a test is positive, and the parent insists that this food may cause eczema, it’s often necessary to do a double-blind, placebo-controlled test,” he said, which is the gold standard.
In discussing systemic therapies, Dr. Leung noted that general immunosuppressants were an approach favored by dermatologists, but urged caution with regard to interferons, mycophenolate, methotrexate, azathioprine, and cyclosporin A. “These are expensive therapies not approved in children, and you really need very good documentation that they’ve failed other forms of therapy before you’d move to that,” Dr. Leung said.
Oral steroids should be avoided. “I have seen thousands of cases of severe eczema, and I’ve never put somebody on oral steroids unless they happened to have an asthma exacerbation concurrent with AD,” he said. “This is because they often have rebound within a week of stopping oral steroids, and that rebound can be worse than the disease you started with.”
If oral steroids must be used, “you should taper slowly and increase the intensity of skin care, so they don’t have a severe rebound.”
Dr. Leung said that while omalizumab should work in any disease with an elevated serum IgE level, “more often than not it doesn’t,” and it probably should be reserved for patients with a very clear history of allergen-induced eczema, underlying urticaria, or other forms of respiratory allergy that may be triggering asthma.
Two potential approaches in which allergists and dermatologists can work together, Dr. Leung said, are phototherapy and allergy immunotherapy. The latter is controversial in AD, he acknowledged, “but if somebody has mainly dust mite allergy or is monosensitized, it’s more likely you will get good benefit. If they’re polysensitized, it is unlikely because it’s mainly a barrier problem.”
Dr. Leung did not recommend antibiotics except in the case of overt Staphylococcus aureus infection, so as not to select for methicillin-resistant S. aureus (MRSA). “If you’re going to treat with some regimen, keep in mind that staph comes from the nose; that’s the body’s reservoir. You should always use an intranasal Bactroban [mupirocin] along with a systemic antibiotic.” Effective eradication of MRSA infection requires more drastic measures, including treatment of other family members and pets.
Dr. Leung disclosed doing consulting work for Celgene, Novartis, Regeneron, and Sanofi-Aventis, and a research grant from Horizon Pharma.
HOUSTON – Dermatologists, allergists, and other physicians treating children with extremely refractory forms of atopic dermatitis (AD) must be aware of common treatment and management pitfalls before jumping to immunosuppressant or biologic therapies, says pediatric eczema researcher Dr. Donald Y. M. Leung, particularly as this is a patient group for whom no systemic therapies have been approved.
In a presentation at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, Dr. Leung shared insights from his clinical experience at National Jewish Health in Denver, whose pediatric eczema program represents a national referral center for patients with severely refractory disease and their families.
“As managed care continues to march on, you will be faced with taking care of the more difficult patients,” Dr. Leung told clinicians.“You will see all forms of AD in clinical practice, therefore one size does not fit all, and a stepwise approach is needed to [treat] the different forms of eczema.”
Clinicians first need to determine what step, or grade, of eczema a patient has. For example, step 2 and 3 eczema is characterized by moderate disease not controlled by intermittent use of topical steroids or calcineurin inhibitors.
National Jewish Health’s day-based program aims to first clear up children presenting with severe eczema before clinicians attempt diagnostic testing. This is achieved through the use of wet wraps, until skin has healed enough for testing to begin.
With wet wraps and day hospitalization, “you can go from severe to mild within a few days,” Dr. Leung said. For children who do not improve after the wet wraps, ruling out immune deficiency or other diagnoses is key. Dr. Leung and colleagues look for Dock 8 (dedicator of cytokinesis 8) protein deficiency in children who fail to respond to wet-wrap treatment, especially if they have presented with recurrent herpes infections or persistent warts.
Another important function of the day program is to observe how parents manage children with eczema. Many, Dr. Leung said, turn out to be noncompliant with recommendations on bathing and medications, often because of the discomfort it causes the child. The monitoring is essential to reveal errors in care. “You can’t tell this in a 10-minute office visit,” he said.
A top reason children fail therapy, he said, is because parents misinterpret recommendations on applying medicine after bathing. Many think that after applying a topical medication, “you can get better absorption if you put the moisturizer on top of the topical steroid. That really just dilutes the medication and you get ineffective therapy.”
Another upshot of the monitoring is that clinicians can identify parents suffering from depression, stress, or financial problems that prevent them from complying. “As with asthma, psychosocial factors loom big,” he said. Parents are extensively counseled and referred as needed, he said. Children also can be trained not to scratch their skin, and children over 5 years in the eczema program are offered hypnosis and biofeedback training to learn how to control their response to itching.
When taking a history of suspected food reactions, Dr. Leung said, it’s important to note that only hives and anaphylaxis are clearly linked to food allergy. “Skin testing and blood work is what we all do, but that’s helpful only if negative. If it’s positive, it still doesn’t tell you that they have food-induced eczema, and like it or not, some form of the food challenge is necessary.”
Dr. Leung said it is appropriate to conduct some oral food challenges unblinded after a negative test. “If a test is positive, and the parent insists that this food may cause eczema, it’s often necessary to do a double-blind, placebo-controlled test,” he said, which is the gold standard.
In discussing systemic therapies, Dr. Leung noted that general immunosuppressants were an approach favored by dermatologists, but urged caution with regard to interferons, mycophenolate, methotrexate, azathioprine, and cyclosporin A. “These are expensive therapies not approved in children, and you really need very good documentation that they’ve failed other forms of therapy before you’d move to that,” Dr. Leung said.
Oral steroids should be avoided. “I have seen thousands of cases of severe eczema, and I’ve never put somebody on oral steroids unless they happened to have an asthma exacerbation concurrent with AD,” he said. “This is because they often have rebound within a week of stopping oral steroids, and that rebound can be worse than the disease you started with.”
If oral steroids must be used, “you should taper slowly and increase the intensity of skin care, so they don’t have a severe rebound.”
Dr. Leung said that while omalizumab should work in any disease with an elevated serum IgE level, “more often than not it doesn’t,” and it probably should be reserved for patients with a very clear history of allergen-induced eczema, underlying urticaria, or other forms of respiratory allergy that may be triggering asthma.
Two potential approaches in which allergists and dermatologists can work together, Dr. Leung said, are phototherapy and allergy immunotherapy. The latter is controversial in AD, he acknowledged, “but if somebody has mainly dust mite allergy or is monosensitized, it’s more likely you will get good benefit. If they’re polysensitized, it is unlikely because it’s mainly a barrier problem.”
Dr. Leung did not recommend antibiotics except in the case of overt Staphylococcus aureus infection, so as not to select for methicillin-resistant S. aureus (MRSA). “If you’re going to treat with some regimen, keep in mind that staph comes from the nose; that’s the body’s reservoir. You should always use an intranasal Bactroban [mupirocin] along with a systemic antibiotic.” Effective eradication of MRSA infection requires more drastic measures, including treatment of other family members and pets.
Dr. Leung disclosed doing consulting work for Celgene, Novartis, Regeneron, and Sanofi-Aventis, and a research grant from Horizon Pharma.
EXPERT ANALYSIS FROM 2015 AAAAI ANNUAL MEETING
Epinephrine use for anaphylaxis in schools: First national survey
HOUSTON – Fully 22% of the anaphylactic events occurring in students while attending U.S. schools, grades kindergarten through high school, are first episodes with no previous known allergy.
That was just one of several surprising findings in the first-ever national study of anaphylactic events and the use of epinephrine autoinjectors in U.S. schools.
“The big surprise for me was how frequently there was no known allergy. For those kids, having epinephrine stocked in the school was potentially life saving. And it underscores the importance that the teaching staff and aides and nurses know how to recognize anaphylaxis, because they wouldn’t know that that child was even at risk,” Dr. Martha V. White said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Dr. White, an allergist in private practice in Wheaton, Md., was lead investigator in the survey of schools participating in the national EPIPEN4SCHOOLS program. The program, conducted by Mylan, provides free EpiPen epinephrine autoinjectors to schools that can document that they have staff trained in their use. The epinephrine autoinjectors (EAIs) are stored at school for use in anyone experiencing anaphylaxis who doesn’t have their own.
The survey provides the clearest picture yet of anaphylaxis in school settings. It included all reported anaphylactic events occurring during the 2013-2014 school year at 6,019 responding U.S. schools. A total of 919 anaphylactic events were reported by 11% of the schools. Eighty-nine percent of the episodes occurred in students, the rest in school staff or visitors.
Twenty percent of anaphylactic events in students were associated with unknown triggers. When a trigger was identified, it was food in 62% of cases, an insect bite or sting in 10%, a medication or environmental trigger in 7%, and latex in 1%.
Among the surprising findings:
Epinephrine wasn’t used in 25% of anaphylactic events. Most of those events were treated with an oral antihistamine, a finding that caused Dr. White to bristle.
“That’s an educational opportunity, because antihistamines won’t stop allergic reactions,” she said in an interview. “They might make you feel like you’re doing something – they might help with the itching – but antihistamines aren’t going to actually stop an allergic reaction.”
Twenty percent of individuals with anaphylaxis weren’t sent to a hospital emergency department. “That’s another opportunity for education because you can have a recurrence of the anaphylaxis,” the allergist noted.
Indeed, roughly 20% of patients who experience anaphylaxis have a biphasic reaction, with recurrence of symptoms up to 38 hours after the first reaction. For this reason, the product labeling for EIAs states that emergency medical follow-up treatment is recommended after treatment of an anaphylactic event.
High school students appear to be at increased risk for in-school anaphylactic events. High schoolers accounted for 18% of the total K-12 student population, but they experienced 49% of all anaphylactic events.
School staff varied widely in their preparedness to recognize anaphylaxis and treat it with an EAI. At 36% of schools, only the school nurse and select staff were trained to recognize anaphylaxis. Twenty-nine percent of schools trained most staff, and 31% trained all staff, including coaches, athletic trainers, and office staff. More adults on campus were trained to recognize anaphylaxis than to actually administer epinephrine.
Mylan is continuing to operate the EPIPEN4SCHOOLS program. That’s good news, Dr. White said, because some major health insurance companies refuse to pay for two sets of EAIs – one for home and another for school – forcing parents to decide in which setting to leave their child unprotected.
“Remember, the autoinjector needs to be where the child is,” Dr. White said.
The survey was supported by Mylan. Dr. White reported serving on Mylan’s scientific advisory board and receiving research grants from most pharmaceutical companies with an interest in asthma medications.
HOUSTON – Fully 22% of the anaphylactic events occurring in students while attending U.S. schools, grades kindergarten through high school, are first episodes with no previous known allergy.
That was just one of several surprising findings in the first-ever national study of anaphylactic events and the use of epinephrine autoinjectors in U.S. schools.
“The big surprise for me was how frequently there was no known allergy. For those kids, having epinephrine stocked in the school was potentially life saving. And it underscores the importance that the teaching staff and aides and nurses know how to recognize anaphylaxis, because they wouldn’t know that that child was even at risk,” Dr. Martha V. White said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Dr. White, an allergist in private practice in Wheaton, Md., was lead investigator in the survey of schools participating in the national EPIPEN4SCHOOLS program. The program, conducted by Mylan, provides free EpiPen epinephrine autoinjectors to schools that can document that they have staff trained in their use. The epinephrine autoinjectors (EAIs) are stored at school for use in anyone experiencing anaphylaxis who doesn’t have their own.
The survey provides the clearest picture yet of anaphylaxis in school settings. It included all reported anaphylactic events occurring during the 2013-2014 school year at 6,019 responding U.S. schools. A total of 919 anaphylactic events were reported by 11% of the schools. Eighty-nine percent of the episodes occurred in students, the rest in school staff or visitors.
Twenty percent of anaphylactic events in students were associated with unknown triggers. When a trigger was identified, it was food in 62% of cases, an insect bite or sting in 10%, a medication or environmental trigger in 7%, and latex in 1%.
Among the surprising findings:
Epinephrine wasn’t used in 25% of anaphylactic events. Most of those events were treated with an oral antihistamine, a finding that caused Dr. White to bristle.
“That’s an educational opportunity, because antihistamines won’t stop allergic reactions,” she said in an interview. “They might make you feel like you’re doing something – they might help with the itching – but antihistamines aren’t going to actually stop an allergic reaction.”
Twenty percent of individuals with anaphylaxis weren’t sent to a hospital emergency department. “That’s another opportunity for education because you can have a recurrence of the anaphylaxis,” the allergist noted.
Indeed, roughly 20% of patients who experience anaphylaxis have a biphasic reaction, with recurrence of symptoms up to 38 hours after the first reaction. For this reason, the product labeling for EIAs states that emergency medical follow-up treatment is recommended after treatment of an anaphylactic event.
High school students appear to be at increased risk for in-school anaphylactic events. High schoolers accounted for 18% of the total K-12 student population, but they experienced 49% of all anaphylactic events.
School staff varied widely in their preparedness to recognize anaphylaxis and treat it with an EAI. At 36% of schools, only the school nurse and select staff were trained to recognize anaphylaxis. Twenty-nine percent of schools trained most staff, and 31% trained all staff, including coaches, athletic trainers, and office staff. More adults on campus were trained to recognize anaphylaxis than to actually administer epinephrine.
Mylan is continuing to operate the EPIPEN4SCHOOLS program. That’s good news, Dr. White said, because some major health insurance companies refuse to pay for two sets of EAIs – one for home and another for school – forcing parents to decide in which setting to leave their child unprotected.
“Remember, the autoinjector needs to be where the child is,” Dr. White said.
The survey was supported by Mylan. Dr. White reported serving on Mylan’s scientific advisory board and receiving research grants from most pharmaceutical companies with an interest in asthma medications.
HOUSTON – Fully 22% of the anaphylactic events occurring in students while attending U.S. schools, grades kindergarten through high school, are first episodes with no previous known allergy.
That was just one of several surprising findings in the first-ever national study of anaphylactic events and the use of epinephrine autoinjectors in U.S. schools.
“The big surprise for me was how frequently there was no known allergy. For those kids, having epinephrine stocked in the school was potentially life saving. And it underscores the importance that the teaching staff and aides and nurses know how to recognize anaphylaxis, because they wouldn’t know that that child was even at risk,” Dr. Martha V. White said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Dr. White, an allergist in private practice in Wheaton, Md., was lead investigator in the survey of schools participating in the national EPIPEN4SCHOOLS program. The program, conducted by Mylan, provides free EpiPen epinephrine autoinjectors to schools that can document that they have staff trained in their use. The epinephrine autoinjectors (EAIs) are stored at school for use in anyone experiencing anaphylaxis who doesn’t have their own.
The survey provides the clearest picture yet of anaphylaxis in school settings. It included all reported anaphylactic events occurring during the 2013-2014 school year at 6,019 responding U.S. schools. A total of 919 anaphylactic events were reported by 11% of the schools. Eighty-nine percent of the episodes occurred in students, the rest in school staff or visitors.
Twenty percent of anaphylactic events in students were associated with unknown triggers. When a trigger was identified, it was food in 62% of cases, an insect bite or sting in 10%, a medication or environmental trigger in 7%, and latex in 1%.
Among the surprising findings:
Epinephrine wasn’t used in 25% of anaphylactic events. Most of those events were treated with an oral antihistamine, a finding that caused Dr. White to bristle.
“That’s an educational opportunity, because antihistamines won’t stop allergic reactions,” she said in an interview. “They might make you feel like you’re doing something – they might help with the itching – but antihistamines aren’t going to actually stop an allergic reaction.”
Twenty percent of individuals with anaphylaxis weren’t sent to a hospital emergency department. “That’s another opportunity for education because you can have a recurrence of the anaphylaxis,” the allergist noted.
Indeed, roughly 20% of patients who experience anaphylaxis have a biphasic reaction, with recurrence of symptoms up to 38 hours after the first reaction. For this reason, the product labeling for EIAs states that emergency medical follow-up treatment is recommended after treatment of an anaphylactic event.
High school students appear to be at increased risk for in-school anaphylactic events. High schoolers accounted for 18% of the total K-12 student population, but they experienced 49% of all anaphylactic events.
School staff varied widely in their preparedness to recognize anaphylaxis and treat it with an EAI. At 36% of schools, only the school nurse and select staff were trained to recognize anaphylaxis. Twenty-nine percent of schools trained most staff, and 31% trained all staff, including coaches, athletic trainers, and office staff. More adults on campus were trained to recognize anaphylaxis than to actually administer epinephrine.
Mylan is continuing to operate the EPIPEN4SCHOOLS program. That’s good news, Dr. White said, because some major health insurance companies refuse to pay for two sets of EAIs – one for home and another for school – forcing parents to decide in which setting to leave their child unprotected.
“Remember, the autoinjector needs to be where the child is,” Dr. White said.
The survey was supported by Mylan. Dr. White reported serving on Mylan’s scientific advisory board and receiving research grants from most pharmaceutical companies with an interest in asthma medications.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: 11% of U.S. schools reported that one or more anaphylactic events occurred on site during the 2013-2014 school year.
Major finding: 20% of students treated with an epinephrine autoinjector while at school weren’t sent to the emergency department afterwards, even though the product labeling recommends doing so.
Data source: This national survey of anaphylactic episodes included 6,019 U.S. schools, kindergarten through high school.
Disclosures: The survey was funded by Mylan. The presenter reported serving on Mylan’s scientific advisory board and receiving research grants from most pharmaceutical companies with an interest in asthma medications.
Annual Recurrence Rate of Anaphylaxis in Kids Is Nearly 30%
HOUSTON – The annual incidence of recurrent anaphylaxis in children was 29% in the first prospective study to examine the issue.
“That rate is higher than previously reported in retrospective studies,” Dr. Andrew O’Keefe said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a study conducted as part of the Cross-Canada Anaphylaxis Registry (C-CARE). In the prospective study, the parents of 266 children who presented with anaphylaxis to two Montreal hospitals were contacted annually thereafter and asked about subsequent allergic reactions.
The parents of 96 children participated. Twenty-five of these 96 children experienced a total of 42 recurrent episodes of anaphylaxis, with an annual recurrence rate of 29%. Three-quarters of recurrences were categorized by investigators as moderate in severity, meaning they entailed crampy abdominal pain, recurrent vomiting, diarrhea, a barky cough, stridor, hoarseness, difficulty swallowing, shortness of breath, and/or moderate wheezing.
A striking study finding was that an epinephrine autoinjector was utilized prior to arrival at the hospital in only 52% of recurrences. That’s serious underutilization, commented Dr. O’Keefe, an allergist at Memorial University in St. Johns, Newfoundland.
“Physicians need to educate patients as to how to use the injectable epinephrine devices and encourage them to do so early during an episode of anaphylaxis, when they’re most effective,” he stressed in an interview.
Continue for principal triggers >>
Food was the principal trigger for 91% of recurrent episodes. Interestingly, children with recurrent anaphylaxis were 71% less likely to have peanut as a trigger, most likely because of extra vigilance regarding this notorious allergen, according to Dr. O’Keefe.
He noted a couple of significant study limitations. One is the small sample size. However, the study is being expanded to other academic medical centers across Canada, which will strengthen the findings.
The other limitation is the potential for bias introduced because parents whose child had severe anaphylaxis as the first episode were more than threefold more likely to participate in the prospective study. Still, the finding of a 29% annual recurrence rate among children in the Canadian study is not far afield from the results of some retrospective studies. For example, investigators at the Mayo Clinic reported as part of the Rochester Epidemiology Project a 21% incidence of a second anaphylactic event occurring at a median of 395 days after the first event in both child and adult residents of Olmsted County, Minn. (J. Allergy Clin. Immunol. 2008;122:1161-5).
That study and others also suggest that the incidence of anaphylaxis is increasing. In Olmsted County, the rate rose from 46.9 cases/100,000 persons in 1990 to 58.9 cases/100,000 in 2000.
Asked why the prehospital use of injectable epinephrine was so low in the Montreal children, Dr. O’Keefe said there are several possible reasons.
“We know that the rates among physicians as well as parents and children are lower than what they should be. Some of the reasons include failure to identify anaphylaxis, not having the device with you, and, finally, patients have to know how to use it and be willing to. There’s a psychological hurdle involving fear of misusing the device or hurting their child or using it inappropriately that prevents people from using it,” he said.
The study was supported by research grants from AllerGen, Health Canada, and Sanofi.
HOUSTON – The annual incidence of recurrent anaphylaxis in children was 29% in the first prospective study to examine the issue.
“That rate is higher than previously reported in retrospective studies,” Dr. Andrew O’Keefe said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a study conducted as part of the Cross-Canada Anaphylaxis Registry (C-CARE). In the prospective study, the parents of 266 children who presented with anaphylaxis to two Montreal hospitals were contacted annually thereafter and asked about subsequent allergic reactions.
The parents of 96 children participated. Twenty-five of these 96 children experienced a total of 42 recurrent episodes of anaphylaxis, with an annual recurrence rate of 29%. Three-quarters of recurrences were categorized by investigators as moderate in severity, meaning they entailed crampy abdominal pain, recurrent vomiting, diarrhea, a barky cough, stridor, hoarseness, difficulty swallowing, shortness of breath, and/or moderate wheezing.
A striking study finding was that an epinephrine autoinjector was utilized prior to arrival at the hospital in only 52% of recurrences. That’s serious underutilization, commented Dr. O’Keefe, an allergist at Memorial University in St. Johns, Newfoundland.
“Physicians need to educate patients as to how to use the injectable epinephrine devices and encourage them to do so early during an episode of anaphylaxis, when they’re most effective,” he stressed in an interview.
Continue for principal triggers >>
Food was the principal trigger for 91% of recurrent episodes. Interestingly, children with recurrent anaphylaxis were 71% less likely to have peanut as a trigger, most likely because of extra vigilance regarding this notorious allergen, according to Dr. O’Keefe.
He noted a couple of significant study limitations. One is the small sample size. However, the study is being expanded to other academic medical centers across Canada, which will strengthen the findings.
The other limitation is the potential for bias introduced because parents whose child had severe anaphylaxis as the first episode were more than threefold more likely to participate in the prospective study. Still, the finding of a 29% annual recurrence rate among children in the Canadian study is not far afield from the results of some retrospective studies. For example, investigators at the Mayo Clinic reported as part of the Rochester Epidemiology Project a 21% incidence of a second anaphylactic event occurring at a median of 395 days after the first event in both child and adult residents of Olmsted County, Minn. (J. Allergy Clin. Immunol. 2008;122:1161-5).
That study and others also suggest that the incidence of anaphylaxis is increasing. In Olmsted County, the rate rose from 46.9 cases/100,000 persons in 1990 to 58.9 cases/100,000 in 2000.
Asked why the prehospital use of injectable epinephrine was so low in the Montreal children, Dr. O’Keefe said there are several possible reasons.
“We know that the rates among physicians as well as parents and children are lower than what they should be. Some of the reasons include failure to identify anaphylaxis, not having the device with you, and, finally, patients have to know how to use it and be willing to. There’s a psychological hurdle involving fear of misusing the device or hurting their child or using it inappropriately that prevents people from using it,” he said.
The study was supported by research grants from AllerGen, Health Canada, and Sanofi.
HOUSTON – The annual incidence of recurrent anaphylaxis in children was 29% in the first prospective study to examine the issue.
“That rate is higher than previously reported in retrospective studies,” Dr. Andrew O’Keefe said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a study conducted as part of the Cross-Canada Anaphylaxis Registry (C-CARE). In the prospective study, the parents of 266 children who presented with anaphylaxis to two Montreal hospitals were contacted annually thereafter and asked about subsequent allergic reactions.
The parents of 96 children participated. Twenty-five of these 96 children experienced a total of 42 recurrent episodes of anaphylaxis, with an annual recurrence rate of 29%. Three-quarters of recurrences were categorized by investigators as moderate in severity, meaning they entailed crampy abdominal pain, recurrent vomiting, diarrhea, a barky cough, stridor, hoarseness, difficulty swallowing, shortness of breath, and/or moderate wheezing.
A striking study finding was that an epinephrine autoinjector was utilized prior to arrival at the hospital in only 52% of recurrences. That’s serious underutilization, commented Dr. O’Keefe, an allergist at Memorial University in St. Johns, Newfoundland.
“Physicians need to educate patients as to how to use the injectable epinephrine devices and encourage them to do so early during an episode of anaphylaxis, when they’re most effective,” he stressed in an interview.
Continue for principal triggers >>
Food was the principal trigger for 91% of recurrent episodes. Interestingly, children with recurrent anaphylaxis were 71% less likely to have peanut as a trigger, most likely because of extra vigilance regarding this notorious allergen, according to Dr. O’Keefe.
He noted a couple of significant study limitations. One is the small sample size. However, the study is being expanded to other academic medical centers across Canada, which will strengthen the findings.
The other limitation is the potential for bias introduced because parents whose child had severe anaphylaxis as the first episode were more than threefold more likely to participate in the prospective study. Still, the finding of a 29% annual recurrence rate among children in the Canadian study is not far afield from the results of some retrospective studies. For example, investigators at the Mayo Clinic reported as part of the Rochester Epidemiology Project a 21% incidence of a second anaphylactic event occurring at a median of 395 days after the first event in both child and adult residents of Olmsted County, Minn. (J. Allergy Clin. Immunol. 2008;122:1161-5).
That study and others also suggest that the incidence of anaphylaxis is increasing. In Olmsted County, the rate rose from 46.9 cases/100,000 persons in 1990 to 58.9 cases/100,000 in 2000.
Asked why the prehospital use of injectable epinephrine was so low in the Montreal children, Dr. O’Keefe said there are several possible reasons.
“We know that the rates among physicians as well as parents and children are lower than what they should be. Some of the reasons include failure to identify anaphylaxis, not having the device with you, and, finally, patients have to know how to use it and be willing to. There’s a psychological hurdle involving fear of misusing the device or hurting their child or using it inappropriately that prevents people from using it,” he said.
The study was supported by research grants from AllerGen, Health Canada, and Sanofi.
AT 2015 AAAAI ANNUAL MEETING
Annual recurrence rate of anaphylaxis in kids is nearly 30%
HOUSTON – The annual incidence of recurrent anaphylaxis in children was 29% in the first prospective study to examine the issue.
“That rate is higher than previously reported in retrospective studies,” Dr. Andrew O’Keefe said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a study conducted as part of the Cross-Canada Anaphylaxis Registry (C-CARE). In the prospective study, the parents of 266 children who presented with anaphylaxis to two Montreal hospitals were contacted annually thereafter and asked about subsequent allergic reactions.
The parents of 96 children participated. Twenty-five of these 96 children experienced a total of 42 recurrent episodes of anaphylaxis, with an annual recurrence rate of 29%. Three-quarters of recurrences were categorized by investigators as moderate in severity, meaning they entailed crampy abdominal pain, recurrent vomiting, diarrhea, a barky cough, stridor, hoarseness, difficulty swallowing, shortness of breath, and/or moderate wheezing.
A striking study finding was that an epinephrine autoinjector was utilized prior to arrival at the hospital in only 52% of recurrences. That’s serious underutilization, commented Dr. O’Keefe, an allergist at Memorial University in St. Johns, Newfoundland.
“Physicians need to educate patients as to how to use the injectable epinephrine devices and encourage them to do so early during an episode of anaphylaxis, when they’re most effective,” he stressed in an interview.
Food was the principal trigger for 91% of recurrent episodes. Interestingly, children with recurrent anaphylaxis were 71% less likely to have peanut as a trigger, most likely because of extra vigilance regarding this notorious allergen, according to Dr. O’Keefe.
He noted a couple of significant study limitations. One is the small sample size. However, the study is being expanded to other academic medical centers across Canada, which will strengthen the findings.
The other limitation is the potential for bias introduced because parents whose child had severe anaphylaxis as the first episode were more than threefold more likely to participate in the prospective study. Still, the finding of a 29% annual recurrence rate among children in the Canadian study is not far afield from the results of some retrospective studies. For example, investigators at the Mayo Clinic reported as part of the Rochester Epidemiology Project a 21% incidence of a second anaphylactic event occurring at a median of 395 days after the first event in both child and adult residents of Olmsted County, Minn. (J. Allergy Clin. Immunol. 2008;122:1161-5).
That study and others also suggest that the incidence of anaphylaxis is increasing. In Olmsted County, the rate rose from 46.9 cases/100,000 persons in 1990 to 58.9 cases/100,000 in 2000.
Asked why the prehospital use of injectable epinephrine was so low in the Montreal children, Dr. O’Keefe said there are several possible reasons.
“We know that the rates among physicians as well as parents and children are lower than what they should be. Some of the reasons include failure to identify anaphylaxis, not having the device with you, and, finally, patients have to know how to use it and be willing to. There’s a psychological hurdle involving fear of misusing the device or hurting their child or using it inappropriately that prevents people from using it,” he said.
The study was supported by research grants from AllerGen, Health Canada, and Sanofi.
HOUSTON – The annual incidence of recurrent anaphylaxis in children was 29% in the first prospective study to examine the issue.
“That rate is higher than previously reported in retrospective studies,” Dr. Andrew O’Keefe said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a study conducted as part of the Cross-Canada Anaphylaxis Registry (C-CARE). In the prospective study, the parents of 266 children who presented with anaphylaxis to two Montreal hospitals were contacted annually thereafter and asked about subsequent allergic reactions.
The parents of 96 children participated. Twenty-five of these 96 children experienced a total of 42 recurrent episodes of anaphylaxis, with an annual recurrence rate of 29%. Three-quarters of recurrences were categorized by investigators as moderate in severity, meaning they entailed crampy abdominal pain, recurrent vomiting, diarrhea, a barky cough, stridor, hoarseness, difficulty swallowing, shortness of breath, and/or moderate wheezing.
A striking study finding was that an epinephrine autoinjector was utilized prior to arrival at the hospital in only 52% of recurrences. That’s serious underutilization, commented Dr. O’Keefe, an allergist at Memorial University in St. Johns, Newfoundland.
“Physicians need to educate patients as to how to use the injectable epinephrine devices and encourage them to do so early during an episode of anaphylaxis, when they’re most effective,” he stressed in an interview.
Food was the principal trigger for 91% of recurrent episodes. Interestingly, children with recurrent anaphylaxis were 71% less likely to have peanut as a trigger, most likely because of extra vigilance regarding this notorious allergen, according to Dr. O’Keefe.
He noted a couple of significant study limitations. One is the small sample size. However, the study is being expanded to other academic medical centers across Canada, which will strengthen the findings.
The other limitation is the potential for bias introduced because parents whose child had severe anaphylaxis as the first episode were more than threefold more likely to participate in the prospective study. Still, the finding of a 29% annual recurrence rate among children in the Canadian study is not far afield from the results of some retrospective studies. For example, investigators at the Mayo Clinic reported as part of the Rochester Epidemiology Project a 21% incidence of a second anaphylactic event occurring at a median of 395 days after the first event in both child and adult residents of Olmsted County, Minn. (J. Allergy Clin. Immunol. 2008;122:1161-5).
That study and others also suggest that the incidence of anaphylaxis is increasing. In Olmsted County, the rate rose from 46.9 cases/100,000 persons in 1990 to 58.9 cases/100,000 in 2000.
Asked why the prehospital use of injectable epinephrine was so low in the Montreal children, Dr. O’Keefe said there are several possible reasons.
“We know that the rates among physicians as well as parents and children are lower than what they should be. Some of the reasons include failure to identify anaphylaxis, not having the device with you, and, finally, patients have to know how to use it and be willing to. There’s a psychological hurdle involving fear of misusing the device or hurting their child or using it inappropriately that prevents people from using it,” he said.
The study was supported by research grants from AllerGen, Health Canada, and Sanofi.
HOUSTON – The annual incidence of recurrent anaphylaxis in children was 29% in the first prospective study to examine the issue.
“That rate is higher than previously reported in retrospective studies,” Dr. Andrew O’Keefe said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a study conducted as part of the Cross-Canada Anaphylaxis Registry (C-CARE). In the prospective study, the parents of 266 children who presented with anaphylaxis to two Montreal hospitals were contacted annually thereafter and asked about subsequent allergic reactions.
The parents of 96 children participated. Twenty-five of these 96 children experienced a total of 42 recurrent episodes of anaphylaxis, with an annual recurrence rate of 29%. Three-quarters of recurrences were categorized by investigators as moderate in severity, meaning they entailed crampy abdominal pain, recurrent vomiting, diarrhea, a barky cough, stridor, hoarseness, difficulty swallowing, shortness of breath, and/or moderate wheezing.
A striking study finding was that an epinephrine autoinjector was utilized prior to arrival at the hospital in only 52% of recurrences. That’s serious underutilization, commented Dr. O’Keefe, an allergist at Memorial University in St. Johns, Newfoundland.
“Physicians need to educate patients as to how to use the injectable epinephrine devices and encourage them to do so early during an episode of anaphylaxis, when they’re most effective,” he stressed in an interview.
Food was the principal trigger for 91% of recurrent episodes. Interestingly, children with recurrent anaphylaxis were 71% less likely to have peanut as a trigger, most likely because of extra vigilance regarding this notorious allergen, according to Dr. O’Keefe.
He noted a couple of significant study limitations. One is the small sample size. However, the study is being expanded to other academic medical centers across Canada, which will strengthen the findings.
The other limitation is the potential for bias introduced because parents whose child had severe anaphylaxis as the first episode were more than threefold more likely to participate in the prospective study. Still, the finding of a 29% annual recurrence rate among children in the Canadian study is not far afield from the results of some retrospective studies. For example, investigators at the Mayo Clinic reported as part of the Rochester Epidemiology Project a 21% incidence of a second anaphylactic event occurring at a median of 395 days after the first event in both child and adult residents of Olmsted County, Minn. (J. Allergy Clin. Immunol. 2008;122:1161-5).
That study and others also suggest that the incidence of anaphylaxis is increasing. In Olmsted County, the rate rose from 46.9 cases/100,000 persons in 1990 to 58.9 cases/100,000 in 2000.
Asked why the prehospital use of injectable epinephrine was so low in the Montreal children, Dr. O’Keefe said there are several possible reasons.
“We know that the rates among physicians as well as parents and children are lower than what they should be. Some of the reasons include failure to identify anaphylaxis, not having the device with you, and, finally, patients have to know how to use it and be willing to. There’s a psychological hurdle involving fear of misusing the device or hurting their child or using it inappropriately that prevents people from using it,” he said.
The study was supported by research grants from AllerGen, Health Canada, and Sanofi.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: The risk of recurrent anaphylaxis in children who’ve experienced a first episode may be higher than previously recognized.
Major finding: The annual anaphylaxis recurrence rate was 29% in a group of prospectively followed Montreal children.
Data source: A prospective study of 96 children.
Disclosures: The study was supported by research grants from AllerGen, Health Canada, and Sanofi. The presenter reported having no financial conflicts of interest.
Reslizumab aces pivotal trials in asthma with eosinophilia
HOUSTON – Reslizumab, a next-generation molecular-based asthma therapy, achieved its primary and secondary endpoints and demonstrated favorable safety in patients with moderate to severe asthma and eosinophilia in two pivotal clinical trials.
“We believe that reslizumab is an effective therapy for controlling asthma in patients with elevated blood eosinophils who are inadequately controlled on medium to high dose inhaled corticosteroid–based regimens,” Dr. Mario Castro concluded in presenting the two phase III results at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The frequency of clinical asthma exacerbations was reduced by more than half in reslizumab-treated patients, compared with controls in the two year-long studies. In addition, the reslizumab group experienced an early improvement in lung function as expressed in forced expiratory volume in 1 second (FEV1) that was sustained throughout the year-long trials, as well as improvements in other measures of asthma control, including quality of life, reported Dr. Castro, professor of pulmonary and critical care medicine and pediatrics at Washington University in St. Louis.
Elevated blood and sputum levels of eosinophils define an asthma phenotype at increased risk for serious asthma exacerbations. Reslizumab is a humanized monoclonal antibody that binds circulating interleukin-5 and prevents binding to the IL-5 receptor, thereby disrupting eosinophil production and function.
Dr. Castro presented two identically designed phase III, double-blind, placebo-controlled, 12-month studies totaling 953 adolescents and adults. They were randomized to intravenous reslizumab at 3 mg/kg or placebo every 4 weeks for a year.
The primary endpoint was frequency of clinical asthma exacerbations (CAEs), an independently adjudicated composite outcome which required an episode featuring an increase in corticosteroids, an asthma-related ER visit or unscheduled office visit, evidence of asthma worsening in the form of at least a 20% drop from baseline in FEV1 or a 30% reduction in peak expiratory flow rate on 2 consecutive days, and worsening clinical symptoms.
Participants averaged two CAEs during the year prior to enrollment. The placebo-treated controls maintained that event rate during the two year-long studies, while the reslizumab-treated patients experienced 50% and 59% reductions relative to controls (P < .0001).
Reslizumab also increased the time to first CAE. In the two trials, 61% and 73% of reslizumab-treated patients didn’t develop a single CAE during 52 weeks, compared with 44% and 52% of controls.
The more CAEs a patient had in the year prior to enrollment, the greater the magnitude of benefit with reslizumab. While the relative risk reduction was 54%, compared with placebo in the combined studies, it climbed to 64% in patients with four or more CAEs in the previous year.
FEV1 improved after the first dose of reslizumab. The benefit – placebo-subtracted gains of 0.126 L in one trial and 0.09 L in the other – was sustained throughout the 52 weeks.
The reslizumab group also outperformed controls in terms of Asthma Control Questionnaire scores and Asthma Quality of Life Questionnaire scores. For example, 74% and 73% of reslizumab-treated patients in the two studies experienced at least a 0.5-point improvement in the AQLQ, which is considered the minimal clinically important difference, compared with 65% and 62% of controls, Dr. Castro continued.
Study discontinuation due to adverse events occurred in 2% of patients on reslizumab, with worsening asthma the No. 1 reason. Two patients on reslizumab experienced anaphylactoid reactions, neither requiring epinephrine. Three percent of reslizumab-treated patients developed low-titer, generally transient antidrug antibodies that didn’t affect eosinophil levels, which plunged with the first dose of reslizumab and stayed low throughout.
Reslizumab is one of a cluster of novel agents in development for severe or treatment-resistant asthma. These are targeted therapies directed at specific patient phenotypes. Biomarkers such as eosinophilia provide guidance as to the specific asthmatic inflammatory pathways involved.
Recent European Respiratory Society/American Thoracic Society guidelines stress the major unmet need for new treatments for severe asthma (Eur. Respir. J. 2014;43:343-73).
In an interview, Dr. James E. Gern, who wasn’t involved in the reslizumab studies, said the various severe asthma phenotypes account for a relatively small proportion of the total asthma population, but a tremendously disproportionate amount of health care utilization.
“These new medications that are coming out are probably going to be indicated for a relatively small number of people. But for those people, it’ll make a huge difference because of the huge burden that severe asthma has on quality of life,” said Dr. Gern, professor of pediatrics at the University of Wisconsin, Madison.
The two pivotal reslizumab studies were sponsored by Teva. Dr. Castro is on the company’s speakers’ bureau and receives research grants from more than a dozen pharmaceutical and device companies as well as from the National Institutes of Health and Centers for Disease Control and Prevention. Simultaneously with Dr. Castro’s presentation at AAAAI, the study results were published online (Lancet Respir. Med. 2015 [doi.org/10.1016/S2213-2600(15)00042-9]).
HOUSTON – Reslizumab, a next-generation molecular-based asthma therapy, achieved its primary and secondary endpoints and demonstrated favorable safety in patients with moderate to severe asthma and eosinophilia in two pivotal clinical trials.
“We believe that reslizumab is an effective therapy for controlling asthma in patients with elevated blood eosinophils who are inadequately controlled on medium to high dose inhaled corticosteroid–based regimens,” Dr. Mario Castro concluded in presenting the two phase III results at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The frequency of clinical asthma exacerbations was reduced by more than half in reslizumab-treated patients, compared with controls in the two year-long studies. In addition, the reslizumab group experienced an early improvement in lung function as expressed in forced expiratory volume in 1 second (FEV1) that was sustained throughout the year-long trials, as well as improvements in other measures of asthma control, including quality of life, reported Dr. Castro, professor of pulmonary and critical care medicine and pediatrics at Washington University in St. Louis.
Elevated blood and sputum levels of eosinophils define an asthma phenotype at increased risk for serious asthma exacerbations. Reslizumab is a humanized monoclonal antibody that binds circulating interleukin-5 and prevents binding to the IL-5 receptor, thereby disrupting eosinophil production and function.
Dr. Castro presented two identically designed phase III, double-blind, placebo-controlled, 12-month studies totaling 953 adolescents and adults. They were randomized to intravenous reslizumab at 3 mg/kg or placebo every 4 weeks for a year.
The primary endpoint was frequency of clinical asthma exacerbations (CAEs), an independently adjudicated composite outcome which required an episode featuring an increase in corticosteroids, an asthma-related ER visit or unscheduled office visit, evidence of asthma worsening in the form of at least a 20% drop from baseline in FEV1 or a 30% reduction in peak expiratory flow rate on 2 consecutive days, and worsening clinical symptoms.
Participants averaged two CAEs during the year prior to enrollment. The placebo-treated controls maintained that event rate during the two year-long studies, while the reslizumab-treated patients experienced 50% and 59% reductions relative to controls (P < .0001).
Reslizumab also increased the time to first CAE. In the two trials, 61% and 73% of reslizumab-treated patients didn’t develop a single CAE during 52 weeks, compared with 44% and 52% of controls.
The more CAEs a patient had in the year prior to enrollment, the greater the magnitude of benefit with reslizumab. While the relative risk reduction was 54%, compared with placebo in the combined studies, it climbed to 64% in patients with four or more CAEs in the previous year.
FEV1 improved after the first dose of reslizumab. The benefit – placebo-subtracted gains of 0.126 L in one trial and 0.09 L in the other – was sustained throughout the 52 weeks.
The reslizumab group also outperformed controls in terms of Asthma Control Questionnaire scores and Asthma Quality of Life Questionnaire scores. For example, 74% and 73% of reslizumab-treated patients in the two studies experienced at least a 0.5-point improvement in the AQLQ, which is considered the minimal clinically important difference, compared with 65% and 62% of controls, Dr. Castro continued.
Study discontinuation due to adverse events occurred in 2% of patients on reslizumab, with worsening asthma the No. 1 reason. Two patients on reslizumab experienced anaphylactoid reactions, neither requiring epinephrine. Three percent of reslizumab-treated patients developed low-titer, generally transient antidrug antibodies that didn’t affect eosinophil levels, which plunged with the first dose of reslizumab and stayed low throughout.
Reslizumab is one of a cluster of novel agents in development for severe or treatment-resistant asthma. These are targeted therapies directed at specific patient phenotypes. Biomarkers such as eosinophilia provide guidance as to the specific asthmatic inflammatory pathways involved.
Recent European Respiratory Society/American Thoracic Society guidelines stress the major unmet need for new treatments for severe asthma (Eur. Respir. J. 2014;43:343-73).
In an interview, Dr. James E. Gern, who wasn’t involved in the reslizumab studies, said the various severe asthma phenotypes account for a relatively small proportion of the total asthma population, but a tremendously disproportionate amount of health care utilization.
“These new medications that are coming out are probably going to be indicated for a relatively small number of people. But for those people, it’ll make a huge difference because of the huge burden that severe asthma has on quality of life,” said Dr. Gern, professor of pediatrics at the University of Wisconsin, Madison.
The two pivotal reslizumab studies were sponsored by Teva. Dr. Castro is on the company’s speakers’ bureau and receives research grants from more than a dozen pharmaceutical and device companies as well as from the National Institutes of Health and Centers for Disease Control and Prevention. Simultaneously with Dr. Castro’s presentation at AAAAI, the study results were published online (Lancet Respir. Med. 2015 [doi.org/10.1016/S2213-2600(15)00042-9]).
HOUSTON – Reslizumab, a next-generation molecular-based asthma therapy, achieved its primary and secondary endpoints and demonstrated favorable safety in patients with moderate to severe asthma and eosinophilia in two pivotal clinical trials.
“We believe that reslizumab is an effective therapy for controlling asthma in patients with elevated blood eosinophils who are inadequately controlled on medium to high dose inhaled corticosteroid–based regimens,” Dr. Mario Castro concluded in presenting the two phase III results at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The frequency of clinical asthma exacerbations was reduced by more than half in reslizumab-treated patients, compared with controls in the two year-long studies. In addition, the reslizumab group experienced an early improvement in lung function as expressed in forced expiratory volume in 1 second (FEV1) that was sustained throughout the year-long trials, as well as improvements in other measures of asthma control, including quality of life, reported Dr. Castro, professor of pulmonary and critical care medicine and pediatrics at Washington University in St. Louis.
Elevated blood and sputum levels of eosinophils define an asthma phenotype at increased risk for serious asthma exacerbations. Reslizumab is a humanized monoclonal antibody that binds circulating interleukin-5 and prevents binding to the IL-5 receptor, thereby disrupting eosinophil production and function.
Dr. Castro presented two identically designed phase III, double-blind, placebo-controlled, 12-month studies totaling 953 adolescents and adults. They were randomized to intravenous reslizumab at 3 mg/kg or placebo every 4 weeks for a year.
The primary endpoint was frequency of clinical asthma exacerbations (CAEs), an independently adjudicated composite outcome which required an episode featuring an increase in corticosteroids, an asthma-related ER visit or unscheduled office visit, evidence of asthma worsening in the form of at least a 20% drop from baseline in FEV1 or a 30% reduction in peak expiratory flow rate on 2 consecutive days, and worsening clinical symptoms.
Participants averaged two CAEs during the year prior to enrollment. The placebo-treated controls maintained that event rate during the two year-long studies, while the reslizumab-treated patients experienced 50% and 59% reductions relative to controls (P < .0001).
Reslizumab also increased the time to first CAE. In the two trials, 61% and 73% of reslizumab-treated patients didn’t develop a single CAE during 52 weeks, compared with 44% and 52% of controls.
The more CAEs a patient had in the year prior to enrollment, the greater the magnitude of benefit with reslizumab. While the relative risk reduction was 54%, compared with placebo in the combined studies, it climbed to 64% in patients with four or more CAEs in the previous year.
FEV1 improved after the first dose of reslizumab. The benefit – placebo-subtracted gains of 0.126 L in one trial and 0.09 L in the other – was sustained throughout the 52 weeks.
The reslizumab group also outperformed controls in terms of Asthma Control Questionnaire scores and Asthma Quality of Life Questionnaire scores. For example, 74% and 73% of reslizumab-treated patients in the two studies experienced at least a 0.5-point improvement in the AQLQ, which is considered the minimal clinically important difference, compared with 65% and 62% of controls, Dr. Castro continued.
Study discontinuation due to adverse events occurred in 2% of patients on reslizumab, with worsening asthma the No. 1 reason. Two patients on reslizumab experienced anaphylactoid reactions, neither requiring epinephrine. Three percent of reslizumab-treated patients developed low-titer, generally transient antidrug antibodies that didn’t affect eosinophil levels, which plunged with the first dose of reslizumab and stayed low throughout.
Reslizumab is one of a cluster of novel agents in development for severe or treatment-resistant asthma. These are targeted therapies directed at specific patient phenotypes. Biomarkers such as eosinophilia provide guidance as to the specific asthmatic inflammatory pathways involved.
Recent European Respiratory Society/American Thoracic Society guidelines stress the major unmet need for new treatments for severe asthma (Eur. Respir. J. 2014;43:343-73).
In an interview, Dr. James E. Gern, who wasn’t involved in the reslizumab studies, said the various severe asthma phenotypes account for a relatively small proportion of the total asthma population, but a tremendously disproportionate amount of health care utilization.
“These new medications that are coming out are probably going to be indicated for a relatively small number of people. But for those people, it’ll make a huge difference because of the huge burden that severe asthma has on quality of life,” said Dr. Gern, professor of pediatrics at the University of Wisconsin, Madison.
The two pivotal reslizumab studies were sponsored by Teva. Dr. Castro is on the company’s speakers’ bureau and receives research grants from more than a dozen pharmaceutical and device companies as well as from the National Institutes of Health and Centers for Disease Control and Prevention. Simultaneously with Dr. Castro’s presentation at AAAAI, the study results were published online (Lancet Respir. Med. 2015 [doi.org/10.1016/S2213-2600(15)00042-9]).
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: A monoclonal antibody against interleukin-5 significantly reduced the frequency of clinical asthma exacerbations in an important subset of asthma patients.
Major finding: Intravenous reslizumab given every 4 weeks reduced the annual rate of clinical asthma exacerbations by 50% and 59%, compared with placebo in two phase III trials.
Data source: The two randomized, double-blind, placebo-controlled, 1-year pivotal trials totaled 953 patients with moderate to severe asthma with elevated blood eosinophils inadequately controlled using inhaled corticosteroid–based regimens.
Disclosures: The studies were sponsored by Teva. The presenter is on the company’s speakers’ bureau and receives research grants from more than a dozen pharmaceutical and device companies.
Respiratory harm reversal seen in asthmatic smokers on e-cigarettes
HOUSTON – Asthmatic smokers who switched to electronic cigarettes showed evidence suggestive of respiratory harm reversal in a retrospective pilot study.
“Electronic cigarette use improves respiratory physiology and subjective asthma outcomes in asthmatic smokers. E-cigarettes are a safer alternative to conventional cigarettes in this vulnerable population,” Dr. Cristina Russo declared at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
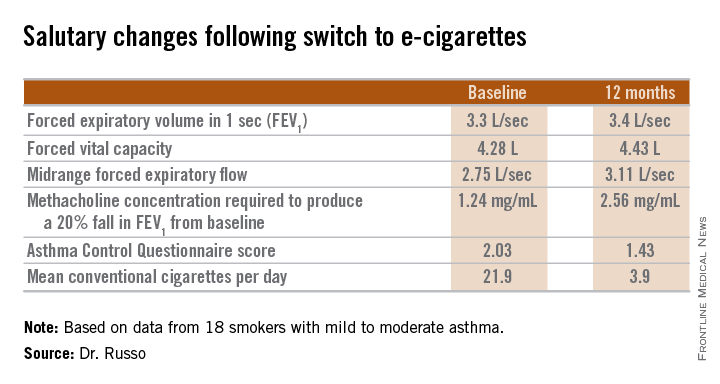
She said that her small retrospective study is the first to examine the respiratory health impact of a switch to e-cigarettes by asthmatic smokers.
Every one of the objective and subjective measures of asthma status evaluated in the study showed statistically significant improvement 1 year after patients adopted e-cigarettes, and the e-cigarette users’ consumption of conventional cigarettes dropped precipitously, reported Dr. Russo of the University of Catania (Italy).
She and her colleagues in the university asthma clinic have taken to suggesting the use of battery-powered e-cigarettes to their asthmatic smokers who haven’t benefited from or aren’t interested in trying the more conventional approaches to smoking cessation or reduction, including medications. While abstinence from cigarette smoking is best, the available evidence indicates e-cigarettes are at least 95% less harmful than conventional cigarettes in the general population, she said.
The study included 18 smokers with mild to moderate asthma who switched to e-cigarettes and underwent spirometry and other testing at baseline and 6 and 12 months of follow-up. Ten patients switched over to e-cigarettes exclusively, while the other 8 used both conventional and e-cigarettes.
Among the highlights: The mid-range forced expiratory flow (25%-75%) showed a major, clinically important improvement, increasing from 2.75 L/sec to 3.11 L/sec. And patients’ mean self-reported conventional cigarette consumption dropped from 21.9 per day at baseline to 5 at 6 months and 3.9 per day at 12 months.
Among the group at large, no significant change was seen in the frequency of asthma exacerbations resulting in hospitalization. However, among the frequent exacerbators – the six patients with two or more exacerbations during the 6 months prior to baseline – exacerbation frequency was cut in half both 6 and 12 months following the switch to e-cigarettes.
Dr. Russo’s presentation sparked vigorous audience discussion. Several physicians cited a Centers for Disease Control and Prevention warning about the unknowns regarding e-cigarette safety, and one allergist declared he didn’t think physicians should ever encourage patients to smoke anything. But others defended the “lesser of two evils” approach adopted by Dr. Russo and coworkers.
Dr. Russo noted that the prevalence of smoking among asthma patients is similar to that of the general population. She called smoking and asthma “a dangerous liaison.” Smoking accelerates asthma patients’ decline in lung function, worsens persistent airways obstruction, and increases insensitivity to corticosteroids.
Her study was supported by a university grant and the Italian League Against Smoking. She reported having no financial conflicts.
HOUSTON – Asthmatic smokers who switched to electronic cigarettes showed evidence suggestive of respiratory harm reversal in a retrospective pilot study.
“Electronic cigarette use improves respiratory physiology and subjective asthma outcomes in asthmatic smokers. E-cigarettes are a safer alternative to conventional cigarettes in this vulnerable population,” Dr. Cristina Russo declared at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.

She said that her small retrospective study is the first to examine the respiratory health impact of a switch to e-cigarettes by asthmatic smokers.
Every one of the objective and subjective measures of asthma status evaluated in the study showed statistically significant improvement 1 year after patients adopted e-cigarettes, and the e-cigarette users’ consumption of conventional cigarettes dropped precipitously, reported Dr. Russo of the University of Catania (Italy).
She and her colleagues in the university asthma clinic have taken to suggesting the use of battery-powered e-cigarettes to their asthmatic smokers who haven’t benefited from or aren’t interested in trying the more conventional approaches to smoking cessation or reduction, including medications. While abstinence from cigarette smoking is best, the available evidence indicates e-cigarettes are at least 95% less harmful than conventional cigarettes in the general population, she said.
The study included 18 smokers with mild to moderate asthma who switched to e-cigarettes and underwent spirometry and other testing at baseline and 6 and 12 months of follow-up. Ten patients switched over to e-cigarettes exclusively, while the other 8 used both conventional and e-cigarettes.
Among the highlights: The mid-range forced expiratory flow (25%-75%) showed a major, clinically important improvement, increasing from 2.75 L/sec to 3.11 L/sec. And patients’ mean self-reported conventional cigarette consumption dropped from 21.9 per day at baseline to 5 at 6 months and 3.9 per day at 12 months.
Among the group at large, no significant change was seen in the frequency of asthma exacerbations resulting in hospitalization. However, among the frequent exacerbators – the six patients with two or more exacerbations during the 6 months prior to baseline – exacerbation frequency was cut in half both 6 and 12 months following the switch to e-cigarettes.
Dr. Russo’s presentation sparked vigorous audience discussion. Several physicians cited a Centers for Disease Control and Prevention warning about the unknowns regarding e-cigarette safety, and one allergist declared he didn’t think physicians should ever encourage patients to smoke anything. But others defended the “lesser of two evils” approach adopted by Dr. Russo and coworkers.
Dr. Russo noted that the prevalence of smoking among asthma patients is similar to that of the general population. She called smoking and asthma “a dangerous liaison.” Smoking accelerates asthma patients’ decline in lung function, worsens persistent airways obstruction, and increases insensitivity to corticosteroids.
Her study was supported by a university grant and the Italian League Against Smoking. She reported having no financial conflicts.
HOUSTON – Asthmatic smokers who switched to electronic cigarettes showed evidence suggestive of respiratory harm reversal in a retrospective pilot study.
“Electronic cigarette use improves respiratory physiology and subjective asthma outcomes in asthmatic smokers. E-cigarettes are a safer alternative to conventional cigarettes in this vulnerable population,” Dr. Cristina Russo declared at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.

She said that her small retrospective study is the first to examine the respiratory health impact of a switch to e-cigarettes by asthmatic smokers.
Every one of the objective and subjective measures of asthma status evaluated in the study showed statistically significant improvement 1 year after patients adopted e-cigarettes, and the e-cigarette users’ consumption of conventional cigarettes dropped precipitously, reported Dr. Russo of the University of Catania (Italy).
She and her colleagues in the university asthma clinic have taken to suggesting the use of battery-powered e-cigarettes to their asthmatic smokers who haven’t benefited from or aren’t interested in trying the more conventional approaches to smoking cessation or reduction, including medications. While abstinence from cigarette smoking is best, the available evidence indicates e-cigarettes are at least 95% less harmful than conventional cigarettes in the general population, she said.
The study included 18 smokers with mild to moderate asthma who switched to e-cigarettes and underwent spirometry and other testing at baseline and 6 and 12 months of follow-up. Ten patients switched over to e-cigarettes exclusively, while the other 8 used both conventional and e-cigarettes.
Among the highlights: The mid-range forced expiratory flow (25%-75%) showed a major, clinically important improvement, increasing from 2.75 L/sec to 3.11 L/sec. And patients’ mean self-reported conventional cigarette consumption dropped from 21.9 per day at baseline to 5 at 6 months and 3.9 per day at 12 months.
Among the group at large, no significant change was seen in the frequency of asthma exacerbations resulting in hospitalization. However, among the frequent exacerbators – the six patients with two or more exacerbations during the 6 months prior to baseline – exacerbation frequency was cut in half both 6 and 12 months following the switch to e-cigarettes.
Dr. Russo’s presentation sparked vigorous audience discussion. Several physicians cited a Centers for Disease Control and Prevention warning about the unknowns regarding e-cigarette safety, and one allergist declared he didn’t think physicians should ever encourage patients to smoke anything. But others defended the “lesser of two evils” approach adopted by Dr. Russo and coworkers.
Dr. Russo noted that the prevalence of smoking among asthma patients is similar to that of the general population. She called smoking and asthma “a dangerous liaison.” Smoking accelerates asthma patients’ decline in lung function, worsens persistent airways obstruction, and increases insensitivity to corticosteroids.
Her study was supported by a university grant and the Italian League Against Smoking. She reported having no financial conflicts.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: Asthmatic smokers who adopted physician advice to switch to electronic cigarettes showed significant 1-year improvements in lung function, methacholine-provoked airway hyperresponsiveness, and asthma-related quality of life.
Major finding: Self-reported daily consumption of conventional cigarettes by asthmatic smokers who switched to e-cigarettes fell from a mean of 21.9 per day at baseline to 5.0 at 6 months and 3.9 at 12 months of follow-up.
Data source: This retrospective pilot study included 18 asthmatic smokers who switched to e-cigarettes.
Disclosures: The study was supported by a university grant and the Italian League Against Smoking. The presenter reported having no financial conflicts.
Early consumption of peanuts can induce tolerance in high-risk children
HOUSTON – The introduction of peanuts at an early age to children who are more highly predisposed to having a peanut allergy can induce tolerance of peanuts and thereby significantly decrease the likelihood of developing a sustained allergy.
This finding from the Learning Early about Peanut Allergy (LEAP) study was presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology and simultaneously published by the New England Journal of Medicine. Investigators enrolled 640 infants aged 4-11 months between December 2006 and May 6, 2009, all of whom were given skin-prick tests at baseline to determine existing peanut allergies, and randomized them into cohorts that would either consume or avoid peanuts until they reached 60 months of age (N. Engl. J. Med. 2015 Feb. 23 [doi:10.1056/NEJMoa1414850]).
Of the 530 children who had negative skin-prick test results, the prevalence of peanut allergy after 60 months was 13.7% in the avoidance cohort and 1.9% in the consumption cohort (P < .001). Of 98 children who had positive skin-prick test results, 35% of the avoidance cohort had peanut allergy after 60 months, while only 11% of the consumption cohort had the allergy (P = .004). Several children who were initially enrolled and randomized either dropped out or were excluded because of inadequate adherence to treatment or missing data.
Outcomes were measured by placing children in a double-blind, placebo-controlled study that had them consume a cumulative total of 9.4 g of peanut protein to determine whether or not tolerance to peanuts had been effectively induced. All children consumed peanuts by eating peanut butter or a peanut-based snack called Bamba, which was provided for the study at a discounted rate. Children whose skin-prick tests showed wheals of larger than 4 mm in diameter were excluded for presumed existing peanut allergy.
“If you enroll children after 11 months of age, you get about twice the rate of [already allergic] children, with a steady decline as [age] goes down,” corresponding author Dr. Gideon Lack, head of the department of pediatric allergy at King’s College, London, said in a press conference following presentation of the study. “But of children younger than 5 months of age, none of them were peanut allergic, so that would suggest that timing here is key: There is a narrow window of opportunity to intervene if you want to be effective.”
Investigators also noted that children who consumed peanuts had increased levels of IgG4 antibody, while those told to avoid peanuts had higher levels of IgE antibody. They also found that development of peanut allergy was generally associated with subjects who both displayed larger wheals on the skin-prick test and had a lower ratio of IgG4:IgE antibodies. Skin-prick tests that yielded wheals of 1-2 mm in diameter were considered indicative of early risk for peanut allergy.
There was no significant difference in the rates of adverse events or hospitalizations between the consumption and avoidance cohort, but 99% of subjects in each group did experience at least one adverse event: 4,527 in the consumption cohort and 4,287 in the avoidance cohort (P = .02). Infants who consumed peanuts had higher instances of upper respiratory tract infection, viral skin infection, gastroenteritis, urticaria, and conjunctivitis, but events mostly ranged from mild to moderate and their severity was not significantly different from similar incidents reported in the avoidance cohort.
Hospitalization rates also were low, with 52 children in the avoidance cohort and 50 children who consumed peanuts being admitted over the study interval, or 16.2% and 15.7%, respectively (P = .86). Rates of serious adverse effects also were not significantly different between the two cohorts, with 101 children who avoided peanuts and 89 who consumed peanuts experiencing such events (P = .41).
Adequate adherence to treatment for the children randomized into the peanut-avoidance group was defined as eating less than 0.2 g of peanut protein on any single occasion and no more than 0.5 g over the course of a single week in the first 24 months of life. For children in the consumption cohort, adequate adherence meant consuming at least 2 g of peanut protein on at least one occasion in both the first and second years of life, and at least 3 g of peanut protein weekly for at least half the number of weeks for which data was collected.
“Whether or not these benefits can be sustained, we will find out in 1 year,” Dr. Lack said, adding that “all the children who had been consuming peanut have stopped [for] 1 year, and we will see if peanut allergy persists after 1 year of cessation of consumption.” He predicted that the findings would most likely produce a mixed response, with some children relapsing while others maintain sustained tolerance.
The LEAP study was supported by grants from the National Institute of Allergy and Infectious Diseases, Food Allergy and Research Education, the Medical Research Council and Asthma UK, the UK Department of Health’s National Institute for Health Research, the National Peanut Board, and the UK Food Standards Agency. Dr. Lack disclosed financial affiliations with DBV Technologies, and coauthor Dr. Helen Brough disclosed receiving financial support from Fare and Action Medical Research, along with study materials from Stallergenes, Thermo Scientific, and Meridian Foods.
HOUSTON – The introduction of peanuts at an early age to children who are more highly predisposed to having a peanut allergy can induce tolerance of peanuts and thereby significantly decrease the likelihood of developing a sustained allergy.
This finding from the Learning Early about Peanut Allergy (LEAP) study was presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology and simultaneously published by the New England Journal of Medicine. Investigators enrolled 640 infants aged 4-11 months between December 2006 and May 6, 2009, all of whom were given skin-prick tests at baseline to determine existing peanut allergies, and randomized them into cohorts that would either consume or avoid peanuts until they reached 60 months of age (N. Engl. J. Med. 2015 Feb. 23 [doi:10.1056/NEJMoa1414850]).
Of the 530 children who had negative skin-prick test results, the prevalence of peanut allergy after 60 months was 13.7% in the avoidance cohort and 1.9% in the consumption cohort (P < .001). Of 98 children who had positive skin-prick test results, 35% of the avoidance cohort had peanut allergy after 60 months, while only 11% of the consumption cohort had the allergy (P = .004). Several children who were initially enrolled and randomized either dropped out or were excluded because of inadequate adherence to treatment or missing data.
Outcomes were measured by placing children in a double-blind, placebo-controlled study that had them consume a cumulative total of 9.4 g of peanut protein to determine whether or not tolerance to peanuts had been effectively induced. All children consumed peanuts by eating peanut butter or a peanut-based snack called Bamba, which was provided for the study at a discounted rate. Children whose skin-prick tests showed wheals of larger than 4 mm in diameter were excluded for presumed existing peanut allergy.
“If you enroll children after 11 months of age, you get about twice the rate of [already allergic] children, with a steady decline as [age] goes down,” corresponding author Dr. Gideon Lack, head of the department of pediatric allergy at King’s College, London, said in a press conference following presentation of the study. “But of children younger than 5 months of age, none of them were peanut allergic, so that would suggest that timing here is key: There is a narrow window of opportunity to intervene if you want to be effective.”
Investigators also noted that children who consumed peanuts had increased levels of IgG4 antibody, while those told to avoid peanuts had higher levels of IgE antibody. They also found that development of peanut allergy was generally associated with subjects who both displayed larger wheals on the skin-prick test and had a lower ratio of IgG4:IgE antibodies. Skin-prick tests that yielded wheals of 1-2 mm in diameter were considered indicative of early risk for peanut allergy.
There was no significant difference in the rates of adverse events or hospitalizations between the consumption and avoidance cohort, but 99% of subjects in each group did experience at least one adverse event: 4,527 in the consumption cohort and 4,287 in the avoidance cohort (P = .02). Infants who consumed peanuts had higher instances of upper respiratory tract infection, viral skin infection, gastroenteritis, urticaria, and conjunctivitis, but events mostly ranged from mild to moderate and their severity was not significantly different from similar incidents reported in the avoidance cohort.
Hospitalization rates also were low, with 52 children in the avoidance cohort and 50 children who consumed peanuts being admitted over the study interval, or 16.2% and 15.7%, respectively (P = .86). Rates of serious adverse effects also were not significantly different between the two cohorts, with 101 children who avoided peanuts and 89 who consumed peanuts experiencing such events (P = .41).
Adequate adherence to treatment for the children randomized into the peanut-avoidance group was defined as eating less than 0.2 g of peanut protein on any single occasion and no more than 0.5 g over the course of a single week in the first 24 months of life. For children in the consumption cohort, adequate adherence meant consuming at least 2 g of peanut protein on at least one occasion in both the first and second years of life, and at least 3 g of peanut protein weekly for at least half the number of weeks for which data was collected.
“Whether or not these benefits can be sustained, we will find out in 1 year,” Dr. Lack said, adding that “all the children who had been consuming peanut have stopped [for] 1 year, and we will see if peanut allergy persists after 1 year of cessation of consumption.” He predicted that the findings would most likely produce a mixed response, with some children relapsing while others maintain sustained tolerance.
The LEAP study was supported by grants from the National Institute of Allergy and Infectious Diseases, Food Allergy and Research Education, the Medical Research Council and Asthma UK, the UK Department of Health’s National Institute for Health Research, the National Peanut Board, and the UK Food Standards Agency. Dr. Lack disclosed financial affiliations with DBV Technologies, and coauthor Dr. Helen Brough disclosed receiving financial support from Fare and Action Medical Research, along with study materials from Stallergenes, Thermo Scientific, and Meridian Foods.
HOUSTON – The introduction of peanuts at an early age to children who are more highly predisposed to having a peanut allergy can induce tolerance of peanuts and thereby significantly decrease the likelihood of developing a sustained allergy.
This finding from the Learning Early about Peanut Allergy (LEAP) study was presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology and simultaneously published by the New England Journal of Medicine. Investigators enrolled 640 infants aged 4-11 months between December 2006 and May 6, 2009, all of whom were given skin-prick tests at baseline to determine existing peanut allergies, and randomized them into cohorts that would either consume or avoid peanuts until they reached 60 months of age (N. Engl. J. Med. 2015 Feb. 23 [doi:10.1056/NEJMoa1414850]).
Of the 530 children who had negative skin-prick test results, the prevalence of peanut allergy after 60 months was 13.7% in the avoidance cohort and 1.9% in the consumption cohort (P < .001). Of 98 children who had positive skin-prick test results, 35% of the avoidance cohort had peanut allergy after 60 months, while only 11% of the consumption cohort had the allergy (P = .004). Several children who were initially enrolled and randomized either dropped out or were excluded because of inadequate adherence to treatment or missing data.
Outcomes were measured by placing children in a double-blind, placebo-controlled study that had them consume a cumulative total of 9.4 g of peanut protein to determine whether or not tolerance to peanuts had been effectively induced. All children consumed peanuts by eating peanut butter or a peanut-based snack called Bamba, which was provided for the study at a discounted rate. Children whose skin-prick tests showed wheals of larger than 4 mm in diameter were excluded for presumed existing peanut allergy.
“If you enroll children after 11 months of age, you get about twice the rate of [already allergic] children, with a steady decline as [age] goes down,” corresponding author Dr. Gideon Lack, head of the department of pediatric allergy at King’s College, London, said in a press conference following presentation of the study. “But of children younger than 5 months of age, none of them were peanut allergic, so that would suggest that timing here is key: There is a narrow window of opportunity to intervene if you want to be effective.”
Investigators also noted that children who consumed peanuts had increased levels of IgG4 antibody, while those told to avoid peanuts had higher levels of IgE antibody. They also found that development of peanut allergy was generally associated with subjects who both displayed larger wheals on the skin-prick test and had a lower ratio of IgG4:IgE antibodies. Skin-prick tests that yielded wheals of 1-2 mm in diameter were considered indicative of early risk for peanut allergy.
There was no significant difference in the rates of adverse events or hospitalizations between the consumption and avoidance cohort, but 99% of subjects in each group did experience at least one adverse event: 4,527 in the consumption cohort and 4,287 in the avoidance cohort (P = .02). Infants who consumed peanuts had higher instances of upper respiratory tract infection, viral skin infection, gastroenteritis, urticaria, and conjunctivitis, but events mostly ranged from mild to moderate and their severity was not significantly different from similar incidents reported in the avoidance cohort.
Hospitalization rates also were low, with 52 children in the avoidance cohort and 50 children who consumed peanuts being admitted over the study interval, or 16.2% and 15.7%, respectively (P = .86). Rates of serious adverse effects also were not significantly different between the two cohorts, with 101 children who avoided peanuts and 89 who consumed peanuts experiencing such events (P = .41).
Adequate adherence to treatment for the children randomized into the peanut-avoidance group was defined as eating less than 0.2 g of peanut protein on any single occasion and no more than 0.5 g over the course of a single week in the first 24 months of life. For children in the consumption cohort, adequate adherence meant consuming at least 2 g of peanut protein on at least one occasion in both the first and second years of life, and at least 3 g of peanut protein weekly for at least half the number of weeks for which data was collected.
“Whether or not these benefits can be sustained, we will find out in 1 year,” Dr. Lack said, adding that “all the children who had been consuming peanut have stopped [for] 1 year, and we will see if peanut allergy persists after 1 year of cessation of consumption.” He predicted that the findings would most likely produce a mixed response, with some children relapsing while others maintain sustained tolerance.
The LEAP study was supported by grants from the National Institute of Allergy and Infectious Diseases, Food Allergy and Research Education, the Medical Research Council and Asthma UK, the UK Department of Health’s National Institute for Health Research, the National Peanut Board, and the UK Food Standards Agency. Dr. Lack disclosed financial affiliations with DBV Technologies, and coauthor Dr. Helen Brough disclosed receiving financial support from Fare and Action Medical Research, along with study materials from Stallergenes, Thermo Scientific, and Meridian Foods.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: Early introduction of peanuts into the diets of children at high risk for peanut allergy can significantly decrease the possibility of peanut allergy development.
Major finding: Of 530 infants who initially tested negative for peanut allergy, prevalence of said allergy after 60 months was 13.7% in the avoidance cohort and 1.9% in the consumption cohort (P < .001); of the 98 infants who initially tested positive, prevalence after 60 months was 35% in the avoidance cohort and 11% in the consumption cohort (P = .004).
Data source: Randomized cohort study of 640 children enrolled at ages 4-11 months between December 2006 and May 2009.
Disclosures: The LEAP study was funded by several health care agencies in the United States and United Kingdom. Corresponding author Dr. Gideon Lack disclosed financial affiliations with DBV Technologies, and coauthor Dr. Helen Brough disclosed receiving financial support from Food Allergy and Research Education and Action Medical Research, along with study materials from Stallergenes, Thermo Scientific, and Meridian Foods.