User login
VIDEO: Beware of over-relying on MRI findings in axSpA
SAN DIEGO – Healthy individuals can show signs of spinal and pelvic inflammation on MRI, but these scans can be misleading if relied on to make a diagnosis of axial spondyloarthritis, according to findings from three separate studies at the annual meeting of the American College of Rheumatology.
“Don’t rely on MRI alone is our message,” said Robert Landewé, MD, PhD, of the University of Amsterdam, who was a coauthor of one of the three studies. “A positive MRI may occur in individuals that are completely healthy. We need to make sure that not too many patients with chronic lower back pain are diagnosed with a disease they don’t have.”
The axial form of spondyloarthritis (axSpA) affects the spinal and pelvic joints of an estimated 1.4% of the U.S. population, and the term encompasses the diagnosis of ankylosing spondylitis (0.5% of U.S. population) in which advanced sacroiliitis is seen on conventional radiography, according to the ACR. Axial SpA is particularly common in young people, especially males, in their teens and 20s.
Researchers believe that MRI scans can misleadingly suggest that patients have the condition. “We know that MRI is a sensitive method, but there’s a lack of data regarding its specificity,” Thomas Renson, MD, of Ghent (Belgium) University, said at a press conference during the meeting.
Dr. Landewé led a study that compared MRIs of sacroiliac joints in 47 healthy people, 47 axSpA patients matched for gender and age, 47 chronic back pain patients, 7 women with postpartum back pain, and 24 frequent runners. Positive MRIs were common in the axSpA patients (43 of 47), but they were also found in healthy people (11 of 47), chronic back pain patients (3 of 47), frequent runners (3 of 24), and women with postpartum back pain (4 of 7).
In another study, Dr. Renson and his colleagues sought to understand whether a sustained period of intense physical activity affected spinal findings in 22 healthy military recruits who did not have SpA.
However, there was a statistically significant increase of combined structural and inflammatory lesions (P = .038) from baseline to post training.
The findings underscore “the importance of interpretation of imaging in the right clinical context,” Dr. Renson said, since they point to the possibility of an incorrect diagnosis “even in a young, active population.”
Another study, led by Ulrich Weber, MD, of King Christian 10th Hospital for Rheumatic Diseases, Gråsten, Denmark, sought to understand levels of normal low-grade BME in 20 amateur runners (8 men) and 22 professional Danish hockey players (all men). On average, the researchers found signs of BME in 3.1 sacroiliac joint quadrants in the runners before and after they ran a race. Hockey players were scanned at the end of the competitive season and showed signs of BME in an average of 3.6 sacroiliac joint quadrants.
In an interview, Dr. Landewé said the studies point to how common positive MRIs are in healthy people. “It was far higher than we would have thought 10 years ago,” he said.
Are MRIs still useful then? Dr. Weber said MRI scans are still helpful in axSpA diagnoses even though they have major limitations. “The imaging method is the only one that’s halfway reliable,” he said. “These joints are deep in the body, so we have virtually no clinical ways to diagnose this.”
However, Dr. Landewé said, “you should do it only when you have sufficient suspicion of spondyloarthritis” – due to accompanying conditions such as positive family history, acute anterior uveitis, psoriasis, or peripheral arthritis – and not just when a patient has chronic back pain.
Dr. Renson reported having no relevant disclosures; two of his coauthors reported extensive disclosures. Dr. Weber and his coauthors reported having no relevant disclosures. Dr. Landewé reported having no relevant disclosures; several of his coauthors reported various disclosures. Funding for the studies was not reported.
SAN DIEGO – Healthy individuals can show signs of spinal and pelvic inflammation on MRI, but these scans can be misleading if relied on to make a diagnosis of axial spondyloarthritis, according to findings from three separate studies at the annual meeting of the American College of Rheumatology.
“Don’t rely on MRI alone is our message,” said Robert Landewé, MD, PhD, of the University of Amsterdam, who was a coauthor of one of the three studies. “A positive MRI may occur in individuals that are completely healthy. We need to make sure that not too many patients with chronic lower back pain are diagnosed with a disease they don’t have.”
The axial form of spondyloarthritis (axSpA) affects the spinal and pelvic joints of an estimated 1.4% of the U.S. population, and the term encompasses the diagnosis of ankylosing spondylitis (0.5% of U.S. population) in which advanced sacroiliitis is seen on conventional radiography, according to the ACR. Axial SpA is particularly common in young people, especially males, in their teens and 20s.
Researchers believe that MRI scans can misleadingly suggest that patients have the condition. “We know that MRI is a sensitive method, but there’s a lack of data regarding its specificity,” Thomas Renson, MD, of Ghent (Belgium) University, said at a press conference during the meeting.
Dr. Landewé led a study that compared MRIs of sacroiliac joints in 47 healthy people, 47 axSpA patients matched for gender and age, 47 chronic back pain patients, 7 women with postpartum back pain, and 24 frequent runners. Positive MRIs were common in the axSpA patients (43 of 47), but they were also found in healthy people (11 of 47), chronic back pain patients (3 of 47), frequent runners (3 of 24), and women with postpartum back pain (4 of 7).
In another study, Dr. Renson and his colleagues sought to understand whether a sustained period of intense physical activity affected spinal findings in 22 healthy military recruits who did not have SpA.
However, there was a statistically significant increase of combined structural and inflammatory lesions (P = .038) from baseline to post training.
The findings underscore “the importance of interpretation of imaging in the right clinical context,” Dr. Renson said, since they point to the possibility of an incorrect diagnosis “even in a young, active population.”
Another study, led by Ulrich Weber, MD, of King Christian 10th Hospital for Rheumatic Diseases, Gråsten, Denmark, sought to understand levels of normal low-grade BME in 20 amateur runners (8 men) and 22 professional Danish hockey players (all men). On average, the researchers found signs of BME in 3.1 sacroiliac joint quadrants in the runners before and after they ran a race. Hockey players were scanned at the end of the competitive season and showed signs of BME in an average of 3.6 sacroiliac joint quadrants.
In an interview, Dr. Landewé said the studies point to how common positive MRIs are in healthy people. “It was far higher than we would have thought 10 years ago,” he said.
Are MRIs still useful then? Dr. Weber said MRI scans are still helpful in axSpA diagnoses even though they have major limitations. “The imaging method is the only one that’s halfway reliable,” he said. “These joints are deep in the body, so we have virtually no clinical ways to diagnose this.”
However, Dr. Landewé said, “you should do it only when you have sufficient suspicion of spondyloarthritis” – due to accompanying conditions such as positive family history, acute anterior uveitis, psoriasis, or peripheral arthritis – and not just when a patient has chronic back pain.
Dr. Renson reported having no relevant disclosures; two of his coauthors reported extensive disclosures. Dr. Weber and his coauthors reported having no relevant disclosures. Dr. Landewé reported having no relevant disclosures; several of his coauthors reported various disclosures. Funding for the studies was not reported.
SAN DIEGO – Healthy individuals can show signs of spinal and pelvic inflammation on MRI, but these scans can be misleading if relied on to make a diagnosis of axial spondyloarthritis, according to findings from three separate studies at the annual meeting of the American College of Rheumatology.
“Don’t rely on MRI alone is our message,” said Robert Landewé, MD, PhD, of the University of Amsterdam, who was a coauthor of one of the three studies. “A positive MRI may occur in individuals that are completely healthy. We need to make sure that not too many patients with chronic lower back pain are diagnosed with a disease they don’t have.”
The axial form of spondyloarthritis (axSpA) affects the spinal and pelvic joints of an estimated 1.4% of the U.S. population, and the term encompasses the diagnosis of ankylosing spondylitis (0.5% of U.S. population) in which advanced sacroiliitis is seen on conventional radiography, according to the ACR. Axial SpA is particularly common in young people, especially males, in their teens and 20s.
Researchers believe that MRI scans can misleadingly suggest that patients have the condition. “We know that MRI is a sensitive method, but there’s a lack of data regarding its specificity,” Thomas Renson, MD, of Ghent (Belgium) University, said at a press conference during the meeting.
Dr. Landewé led a study that compared MRIs of sacroiliac joints in 47 healthy people, 47 axSpA patients matched for gender and age, 47 chronic back pain patients, 7 women with postpartum back pain, and 24 frequent runners. Positive MRIs were common in the axSpA patients (43 of 47), but they were also found in healthy people (11 of 47), chronic back pain patients (3 of 47), frequent runners (3 of 24), and women with postpartum back pain (4 of 7).
In another study, Dr. Renson and his colleagues sought to understand whether a sustained period of intense physical activity affected spinal findings in 22 healthy military recruits who did not have SpA.
However, there was a statistically significant increase of combined structural and inflammatory lesions (P = .038) from baseline to post training.
The findings underscore “the importance of interpretation of imaging in the right clinical context,” Dr. Renson said, since they point to the possibility of an incorrect diagnosis “even in a young, active population.”
Another study, led by Ulrich Weber, MD, of King Christian 10th Hospital for Rheumatic Diseases, Gråsten, Denmark, sought to understand levels of normal low-grade BME in 20 amateur runners (8 men) and 22 professional Danish hockey players (all men). On average, the researchers found signs of BME in 3.1 sacroiliac joint quadrants in the runners before and after they ran a race. Hockey players were scanned at the end of the competitive season and showed signs of BME in an average of 3.6 sacroiliac joint quadrants.
In an interview, Dr. Landewé said the studies point to how common positive MRIs are in healthy people. “It was far higher than we would have thought 10 years ago,” he said.
Are MRIs still useful then? Dr. Weber said MRI scans are still helpful in axSpA diagnoses even though they have major limitations. “The imaging method is the only one that’s halfway reliable,” he said. “These joints are deep in the body, so we have virtually no clinical ways to diagnose this.”
However, Dr. Landewé said, “you should do it only when you have sufficient suspicion of spondyloarthritis” – due to accompanying conditions such as positive family history, acute anterior uveitis, psoriasis, or peripheral arthritis – and not just when a patient has chronic back pain.
Dr. Renson reported having no relevant disclosures; two of his coauthors reported extensive disclosures. Dr. Weber and his coauthors reported having no relevant disclosures. Dr. Landewé reported having no relevant disclosures; several of his coauthors reported various disclosures. Funding for the studies was not reported.
AT ACR 2017
Blindness linked to HCQ use rare in rheumatic patients
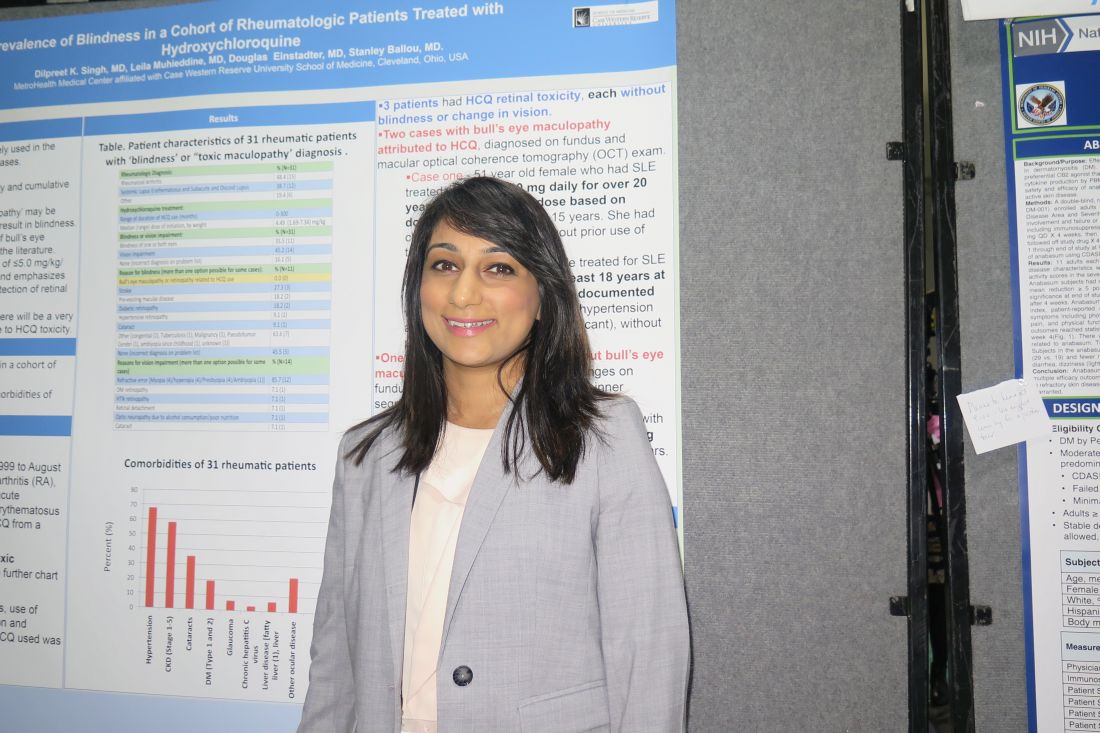
SAN DIEGO – Out of a cohort of nearly 2,900 rheumatic patients, none developed blindness attributable to toxic maculopathy from using hydroxychloroquine, in a single-center retrospective study.
“That’s very reassuring,” lead study author Dilpreet K. Singh, MD, said in an interview at the annual meeting of the American College of Rheumatology. “It’s still important to be vigilant with our screening and also to take note of the hydroxychloroquine dose that they’re on, because a lot of our patients have been on hydroxychloroquine for 10 or 15 years and there may not have been a dose adjustment. It’s also important to focus on the patients’ comorbidities and get them under better control.”
In an effort to assess the prevalence of blindness in a cohort of rheumatic patients and to identify the characteristics and comorbidities of those with HCQ retinal toxicity, Dr. Singh and her associates retrospectively evaluated 2,898 patients at MetroHealth Medical Center between January 1999 and August of 2017 with diagnoses of rheumatoid arthritis, inflammatory polyarthritis, systemic lupus erythematosus, subacute cutaneous lupus, and discoid lupus erythematosus who had a prescription written for HCQ.
In all, 31 had a diagnosis of “blindness” or “toxic maculopathy,” and these cases were further assessed for patient demographics, comorbidities, use of tamoxifen, weight and dose at initiation and discontinuation of HCQ, and duration of HCQ. Nearly 70% of these patients had hypertension, about 60% had chronic kidney disease, and 35% had cataracts. The researchers confirmed that in each of the cases the blindness was not caused by HCQ ocular toxicity, but instead by stroke, preexisting macular disease, diabetic retinopathy, hypertensive retinopathy, or cataracts.
Only 3 of the 31 patients were found to have HCQ retinal toxicity, each without blindness or change in vision. Two patients had bull’s-eye maculopathy and one had HCQ toxic maculopathy. Each of the three patients had received HCQ for more than 18 years at doses that ranged from 6.3 to 8.2 mg/kg based on documented weight, and none had functional vision loss at diagnosis.
“It’s reassuring to know that there’s a very small percentage of patients that will have HCQ-related toxicity,” Dr. Singh concluded. “We should also be focusing on the comorbidities [such as] diabetes, hypertension, and stroke-related vision loss that are common in our population of rheumatic patients, because these are contributing to visual impairment and blindness.”
She reported having no disclosures.
[email protected]
SAN DIEGO – Out of a cohort of nearly 2,900 rheumatic patients, none developed blindness attributable to toxic maculopathy from using hydroxychloroquine, in a single-center retrospective study.
“That’s very reassuring,” lead study author Dilpreet K. Singh, MD, said in an interview at the annual meeting of the American College of Rheumatology. “It’s still important to be vigilant with our screening and also to take note of the hydroxychloroquine dose that they’re on, because a lot of our patients have been on hydroxychloroquine for 10 or 15 years and there may not have been a dose adjustment. It’s also important to focus on the patients’ comorbidities and get them under better control.”
In an effort to assess the prevalence of blindness in a cohort of rheumatic patients and to identify the characteristics and comorbidities of those with HCQ retinal toxicity, Dr. Singh and her associates retrospectively evaluated 2,898 patients at MetroHealth Medical Center between January 1999 and August of 2017 with diagnoses of rheumatoid arthritis, inflammatory polyarthritis, systemic lupus erythematosus, subacute cutaneous lupus, and discoid lupus erythematosus who had a prescription written for HCQ.
In all, 31 had a diagnosis of “blindness” or “toxic maculopathy,” and these cases were further assessed for patient demographics, comorbidities, use of tamoxifen, weight and dose at initiation and discontinuation of HCQ, and duration of HCQ. Nearly 70% of these patients had hypertension, about 60% had chronic kidney disease, and 35% had cataracts. The researchers confirmed that in each of the cases the blindness was not caused by HCQ ocular toxicity, but instead by stroke, preexisting macular disease, diabetic retinopathy, hypertensive retinopathy, or cataracts.
Only 3 of the 31 patients were found to have HCQ retinal toxicity, each without blindness or change in vision. Two patients had bull’s-eye maculopathy and one had HCQ toxic maculopathy. Each of the three patients had received HCQ for more than 18 years at doses that ranged from 6.3 to 8.2 mg/kg based on documented weight, and none had functional vision loss at diagnosis.
“It’s reassuring to know that there’s a very small percentage of patients that will have HCQ-related toxicity,” Dr. Singh concluded. “We should also be focusing on the comorbidities [such as] diabetes, hypertension, and stroke-related vision loss that are common in our population of rheumatic patients, because these are contributing to visual impairment and blindness.”
She reported having no disclosures.
[email protected]
SAN DIEGO – Out of a cohort of nearly 2,900 rheumatic patients, none developed blindness attributable to toxic maculopathy from using hydroxychloroquine, in a single-center retrospective study.
“That’s very reassuring,” lead study author Dilpreet K. Singh, MD, said in an interview at the annual meeting of the American College of Rheumatology. “It’s still important to be vigilant with our screening and also to take note of the hydroxychloroquine dose that they’re on, because a lot of our patients have been on hydroxychloroquine for 10 or 15 years and there may not have been a dose adjustment. It’s also important to focus on the patients’ comorbidities and get them under better control.”
In an effort to assess the prevalence of blindness in a cohort of rheumatic patients and to identify the characteristics and comorbidities of those with HCQ retinal toxicity, Dr. Singh and her associates retrospectively evaluated 2,898 patients at MetroHealth Medical Center between January 1999 and August of 2017 with diagnoses of rheumatoid arthritis, inflammatory polyarthritis, systemic lupus erythematosus, subacute cutaneous lupus, and discoid lupus erythematosus who had a prescription written for HCQ.
In all, 31 had a diagnosis of “blindness” or “toxic maculopathy,” and these cases were further assessed for patient demographics, comorbidities, use of tamoxifen, weight and dose at initiation and discontinuation of HCQ, and duration of HCQ. Nearly 70% of these patients had hypertension, about 60% had chronic kidney disease, and 35% had cataracts. The researchers confirmed that in each of the cases the blindness was not caused by HCQ ocular toxicity, but instead by stroke, preexisting macular disease, diabetic retinopathy, hypertensive retinopathy, or cataracts.
Only 3 of the 31 patients were found to have HCQ retinal toxicity, each without blindness or change in vision. Two patients had bull’s-eye maculopathy and one had HCQ toxic maculopathy. Each of the three patients had received HCQ for more than 18 years at doses that ranged from 6.3 to 8.2 mg/kg based on documented weight, and none had functional vision loss at diagnosis.
“It’s reassuring to know that there’s a very small percentage of patients that will have HCQ-related toxicity,” Dr. Singh concluded. “We should also be focusing on the comorbidities [such as] diabetes, hypertension, and stroke-related vision loss that are common in our population of rheumatic patients, because these are contributing to visual impairment and blindness.”
She reported having no disclosures.
[email protected]
AT ACR 2017
Key clinical point: The incidence of blindness due to hydroxychloroquine toxicity is very low.
Major finding: No patients developed blindness attributable to toxic maculopathy from using hydroxychloroquine.
Study details: A single-center retrospective study of 2,898 rheumatic patients.
Disclosures: Dr. Singh reported having no disclosures.
VIDEO: New PsA guideline expected in 2018
SAN DIEGO – For the first time, a forthcoming evidence-based guideline for the management of psoriatic arthritis recommends tumor necrosis factor inhibitor biologics as first-line therapy.
“Guidelines that have been around for the last several years have been skirting around the fact that there’s really no evidence that methotrexate works for PsA,” Dafna D. Gladman, MD, said during a press briefing at the annual meeting of the American College of Rheumatology. “So it’s refreshing and reassuring that when you do an appropriate, evidence-based approach, you finally find the truth in front of you, and you have TNF inhibitors as the first-line treatment. Obviously, they’re not for everybody. There are patients in whom we cannot use TNF inhibitors, either because they don’t like needles, or because they have contraindications to getting these particular needles, but at least we have a recommendation for the use of these drugs as a first-line treatment.”

“At first, I wasn’t a big fan of the idea of the GRADE guidelines because the number of questions blows up so fast, [but] it really makes you focus on what the most common [clinical] settings are,” said another core oversight team member, Alexis Ogdie, MD, a rheumatologist at the University of Pennsylvania, Philadelphia. “These guidelines also reveal the major gap of no head-to-head studies. I think we’ve known that, but this really called that out as important. When we’re making a treatment decision between [drugs] A and B, we need those studies to be able to better understand how to treat our patients, rather than using the data from one trial to make a decision. ... For my patients, I’m excited that I can now use a TNF inhibitor as a first-line agent. When we have patients come in with very severe disease, occasionally they also have severe psoriasis, so we’ve been able to use TNF inhibitors as first-line treatment in some of our patients in Pennsylvania. This differs state by state. But the exciting thing is that they get better so fast and you don’t have to tell them to wait 12 weeks for methotrexate to work.”
The ACR/NPF guideline is currently under peer review and is expected to be published in Arthritis & Rheumatology, Arthritis Care & Research, and the Journal of Psoriasis and Psoriatic Arthritis in the spring or summer of 2018. It focuses on common PsA patients, not exceptional cases. It includes recommendations on the management of patients with active PsA that is defined by the patients’ self-report and judged by the examining clinician to be caused by PsA, based on the on the presence of at least one of the following: actively inflamed joints; dactylitis; enthesitis; axial disease; active skin and/or nail involvement; and/or extra-articular manifestations such as uveitis or inflammatory bowel disease. Authors of the guideline considered cost as one of many possible factors affecting the use of the recommendations, but explicit cost-effectiveness analyses were not conducted. Also, since the NPF and the American Academy of Dermatology are concurrently developing a psoriasis treatment guideline, the treatment of skin psoriasis was not included in the guideline.
According to the guideline’s principal investigator Jasvinder Singh, MD, professor of medicine and epidemiology at the University of Alabama at Birmingham, the guideline will include 80 recommendations, 75 (94%) that are rated as “conditional,” and 5 (6%) that are rated as “strong,” based on the quality of evidence in the existing medical literature. “Most of our treatment guidelines rely on very low-to-moderate quality evidence, which means that there needs to be an active discussion between the physician and the patient with regard to which treatment to choose,” said Dr. Singh, who is also a staff rheumatologist at the Birmingham Veterans Affairs Medical Center and who led development of the 2012 and 2015 ACR treatment guidelines for RA. “When you’re not choosing the preferred treatment, there are defined specific recommendations under which that second treatment may be preferred over the first treatment.”
During a separate session at the meeting, Dr. Singh unveiled a few of the draft recommendations. One calls for using a treat-to-target strategy over not using one. In the setting of immunizing patients who are receiving a biologic, another recommendation calls for clinicians to start the indicated biologic and administer killed vaccines (as indicated) in patients with active PsA rather than delaying the biologic to give the killed vaccines. In addition, delaying the start of the indicated biologic is recommended over not delaying in order to administer a live attenuated vaccine in patients with active PsA. When patients continue to have with active PsA despite being on a TNF inhibitor, the draft guideline recommends switching to a different TNF inhibitor rather than an IL-17 inhibitor, an IL-12/IL-23 inhibitor, abatacept (Orencia), tofacitinib (Xeljanz), or adding methotrexate. If PsA is still active, the guideline recommends switching to an IL-17 inhibitor instead of an IL-12/IL-23 inhibitor, abatacept, or tofacitinib. If PsA is still active, the guideline recommends switching to an IL-12/IL-23 inhibitor over abatacept or tofacitinib.
The guideline also includes suggestions for nonpharmacologic treatments, including recommending low-impact exercise over high-impact exercise, occupational therapy, physical therapy, and weight loss. It also includes a strong recommendation to provide smoking cessation advice to patients.
Dr. Singh acknowledged significant research gaps in the current PsA medical literature, including no head-to-head comparisons of treatments. He said that the field also could benefit from specific studies for enthesitis, axial disease, and arthritis mutilans; randomized trials of nonpharmacologic interventions; more trials of monotherapy vs. combination therapy; vaccination trials for live attenuated vaccines; trials and registry studies of patients with common comorbidities, and studies of NSAIDs and glucocorticoids, to define their role.
Possible topics for future PsA guidelines, he continued, include treatment options for patients for whom biologic medication is not an option; use of therapies in pregnancy and conception; incorporation of high-quality cost or cost-effectiveness analysis into recommendations; and the role of other comorbidities, such as fibromyalgia, hepatitis, depression/anxiety, malignancy, and cardiovascular disease.
“Evidence-based medicine needs to be practiced, even in situations where it’s difficult to get a drug,” Dr. Gladman said. “One of the things we hope will happen in the near future is that companies will start doing head-to-head studies, to help us support evidence-based recommendations in the future.”
None of the speakers reported having relevant financial disclosures.
SAN DIEGO – For the first time, a forthcoming evidence-based guideline for the management of psoriatic arthritis recommends tumor necrosis factor inhibitor biologics as first-line therapy.
“Guidelines that have been around for the last several years have been skirting around the fact that there’s really no evidence that methotrexate works for PsA,” Dafna D. Gladman, MD, said during a press briefing at the annual meeting of the American College of Rheumatology. “So it’s refreshing and reassuring that when you do an appropriate, evidence-based approach, you finally find the truth in front of you, and you have TNF inhibitors as the first-line treatment. Obviously, they’re not for everybody. There are patients in whom we cannot use TNF inhibitors, either because they don’t like needles, or because they have contraindications to getting these particular needles, but at least we have a recommendation for the use of these drugs as a first-line treatment.”

“At first, I wasn’t a big fan of the idea of the GRADE guidelines because the number of questions blows up so fast, [but] it really makes you focus on what the most common [clinical] settings are,” said another core oversight team member, Alexis Ogdie, MD, a rheumatologist at the University of Pennsylvania, Philadelphia. “These guidelines also reveal the major gap of no head-to-head studies. I think we’ve known that, but this really called that out as important. When we’re making a treatment decision between [drugs] A and B, we need those studies to be able to better understand how to treat our patients, rather than using the data from one trial to make a decision. ... For my patients, I’m excited that I can now use a TNF inhibitor as a first-line agent. When we have patients come in with very severe disease, occasionally they also have severe psoriasis, so we’ve been able to use TNF inhibitors as first-line treatment in some of our patients in Pennsylvania. This differs state by state. But the exciting thing is that they get better so fast and you don’t have to tell them to wait 12 weeks for methotrexate to work.”
The ACR/NPF guideline is currently under peer review and is expected to be published in Arthritis & Rheumatology, Arthritis Care & Research, and the Journal of Psoriasis and Psoriatic Arthritis in the spring or summer of 2018. It focuses on common PsA patients, not exceptional cases. It includes recommendations on the management of patients with active PsA that is defined by the patients’ self-report and judged by the examining clinician to be caused by PsA, based on the on the presence of at least one of the following: actively inflamed joints; dactylitis; enthesitis; axial disease; active skin and/or nail involvement; and/or extra-articular manifestations such as uveitis or inflammatory bowel disease. Authors of the guideline considered cost as one of many possible factors affecting the use of the recommendations, but explicit cost-effectiveness analyses were not conducted. Also, since the NPF and the American Academy of Dermatology are concurrently developing a psoriasis treatment guideline, the treatment of skin psoriasis was not included in the guideline.
According to the guideline’s principal investigator Jasvinder Singh, MD, professor of medicine and epidemiology at the University of Alabama at Birmingham, the guideline will include 80 recommendations, 75 (94%) that are rated as “conditional,” and 5 (6%) that are rated as “strong,” based on the quality of evidence in the existing medical literature. “Most of our treatment guidelines rely on very low-to-moderate quality evidence, which means that there needs to be an active discussion between the physician and the patient with regard to which treatment to choose,” said Dr. Singh, who is also a staff rheumatologist at the Birmingham Veterans Affairs Medical Center and who led development of the 2012 and 2015 ACR treatment guidelines for RA. “When you’re not choosing the preferred treatment, there are defined specific recommendations under which that second treatment may be preferred over the first treatment.”
During a separate session at the meeting, Dr. Singh unveiled a few of the draft recommendations. One calls for using a treat-to-target strategy over not using one. In the setting of immunizing patients who are receiving a biologic, another recommendation calls for clinicians to start the indicated biologic and administer killed vaccines (as indicated) in patients with active PsA rather than delaying the biologic to give the killed vaccines. In addition, delaying the start of the indicated biologic is recommended over not delaying in order to administer a live attenuated vaccine in patients with active PsA. When patients continue to have with active PsA despite being on a TNF inhibitor, the draft guideline recommends switching to a different TNF inhibitor rather than an IL-17 inhibitor, an IL-12/IL-23 inhibitor, abatacept (Orencia), tofacitinib (Xeljanz), or adding methotrexate. If PsA is still active, the guideline recommends switching to an IL-17 inhibitor instead of an IL-12/IL-23 inhibitor, abatacept, or tofacitinib. If PsA is still active, the guideline recommends switching to an IL-12/IL-23 inhibitor over abatacept or tofacitinib.
The guideline also includes suggestions for nonpharmacologic treatments, including recommending low-impact exercise over high-impact exercise, occupational therapy, physical therapy, and weight loss. It also includes a strong recommendation to provide smoking cessation advice to patients.
Dr. Singh acknowledged significant research gaps in the current PsA medical literature, including no head-to-head comparisons of treatments. He said that the field also could benefit from specific studies for enthesitis, axial disease, and arthritis mutilans; randomized trials of nonpharmacologic interventions; more trials of monotherapy vs. combination therapy; vaccination trials for live attenuated vaccines; trials and registry studies of patients with common comorbidities, and studies of NSAIDs and glucocorticoids, to define their role.
Possible topics for future PsA guidelines, he continued, include treatment options for patients for whom biologic medication is not an option; use of therapies in pregnancy and conception; incorporation of high-quality cost or cost-effectiveness analysis into recommendations; and the role of other comorbidities, such as fibromyalgia, hepatitis, depression/anxiety, malignancy, and cardiovascular disease.
“Evidence-based medicine needs to be practiced, even in situations where it’s difficult to get a drug,” Dr. Gladman said. “One of the things we hope will happen in the near future is that companies will start doing head-to-head studies, to help us support evidence-based recommendations in the future.”
None of the speakers reported having relevant financial disclosures.
SAN DIEGO – For the first time, a forthcoming evidence-based guideline for the management of psoriatic arthritis recommends tumor necrosis factor inhibitor biologics as first-line therapy.
“Guidelines that have been around for the last several years have been skirting around the fact that there’s really no evidence that methotrexate works for PsA,” Dafna D. Gladman, MD, said during a press briefing at the annual meeting of the American College of Rheumatology. “So it’s refreshing and reassuring that when you do an appropriate, evidence-based approach, you finally find the truth in front of you, and you have TNF inhibitors as the first-line treatment. Obviously, they’re not for everybody. There are patients in whom we cannot use TNF inhibitors, either because they don’t like needles, or because they have contraindications to getting these particular needles, but at least we have a recommendation for the use of these drugs as a first-line treatment.”

“At first, I wasn’t a big fan of the idea of the GRADE guidelines because the number of questions blows up so fast, [but] it really makes you focus on what the most common [clinical] settings are,” said another core oversight team member, Alexis Ogdie, MD, a rheumatologist at the University of Pennsylvania, Philadelphia. “These guidelines also reveal the major gap of no head-to-head studies. I think we’ve known that, but this really called that out as important. When we’re making a treatment decision between [drugs] A and B, we need those studies to be able to better understand how to treat our patients, rather than using the data from one trial to make a decision. ... For my patients, I’m excited that I can now use a TNF inhibitor as a first-line agent. When we have patients come in with very severe disease, occasionally they also have severe psoriasis, so we’ve been able to use TNF inhibitors as first-line treatment in some of our patients in Pennsylvania. This differs state by state. But the exciting thing is that they get better so fast and you don’t have to tell them to wait 12 weeks for methotrexate to work.”
The ACR/NPF guideline is currently under peer review and is expected to be published in Arthritis & Rheumatology, Arthritis Care & Research, and the Journal of Psoriasis and Psoriatic Arthritis in the spring or summer of 2018. It focuses on common PsA patients, not exceptional cases. It includes recommendations on the management of patients with active PsA that is defined by the patients’ self-report and judged by the examining clinician to be caused by PsA, based on the on the presence of at least one of the following: actively inflamed joints; dactylitis; enthesitis; axial disease; active skin and/or nail involvement; and/or extra-articular manifestations such as uveitis or inflammatory bowel disease. Authors of the guideline considered cost as one of many possible factors affecting the use of the recommendations, but explicit cost-effectiveness analyses were not conducted. Also, since the NPF and the American Academy of Dermatology are concurrently developing a psoriasis treatment guideline, the treatment of skin psoriasis was not included in the guideline.
According to the guideline’s principal investigator Jasvinder Singh, MD, professor of medicine and epidemiology at the University of Alabama at Birmingham, the guideline will include 80 recommendations, 75 (94%) that are rated as “conditional,” and 5 (6%) that are rated as “strong,” based on the quality of evidence in the existing medical literature. “Most of our treatment guidelines rely on very low-to-moderate quality evidence, which means that there needs to be an active discussion between the physician and the patient with regard to which treatment to choose,” said Dr. Singh, who is also a staff rheumatologist at the Birmingham Veterans Affairs Medical Center and who led development of the 2012 and 2015 ACR treatment guidelines for RA. “When you’re not choosing the preferred treatment, there are defined specific recommendations under which that second treatment may be preferred over the first treatment.”
During a separate session at the meeting, Dr. Singh unveiled a few of the draft recommendations. One calls for using a treat-to-target strategy over not using one. In the setting of immunizing patients who are receiving a biologic, another recommendation calls for clinicians to start the indicated biologic and administer killed vaccines (as indicated) in patients with active PsA rather than delaying the biologic to give the killed vaccines. In addition, delaying the start of the indicated biologic is recommended over not delaying in order to administer a live attenuated vaccine in patients with active PsA. When patients continue to have with active PsA despite being on a TNF inhibitor, the draft guideline recommends switching to a different TNF inhibitor rather than an IL-17 inhibitor, an IL-12/IL-23 inhibitor, abatacept (Orencia), tofacitinib (Xeljanz), or adding methotrexate. If PsA is still active, the guideline recommends switching to an IL-17 inhibitor instead of an IL-12/IL-23 inhibitor, abatacept, or tofacitinib. If PsA is still active, the guideline recommends switching to an IL-12/IL-23 inhibitor over abatacept or tofacitinib.
The guideline also includes suggestions for nonpharmacologic treatments, including recommending low-impact exercise over high-impact exercise, occupational therapy, physical therapy, and weight loss. It also includes a strong recommendation to provide smoking cessation advice to patients.
Dr. Singh acknowledged significant research gaps in the current PsA medical literature, including no head-to-head comparisons of treatments. He said that the field also could benefit from specific studies for enthesitis, axial disease, and arthritis mutilans; randomized trials of nonpharmacologic interventions; more trials of monotherapy vs. combination therapy; vaccination trials for live attenuated vaccines; trials and registry studies of patients with common comorbidities, and studies of NSAIDs and glucocorticoids, to define their role.
Possible topics for future PsA guidelines, he continued, include treatment options for patients for whom biologic medication is not an option; use of therapies in pregnancy and conception; incorporation of high-quality cost or cost-effectiveness analysis into recommendations; and the role of other comorbidities, such as fibromyalgia, hepatitis, depression/anxiety, malignancy, and cardiovascular disease.
“Evidence-based medicine needs to be practiced, even in situations where it’s difficult to get a drug,” Dr. Gladman said. “One of the things we hope will happen in the near future is that companies will start doing head-to-head studies, to help us support evidence-based recommendations in the future.”
None of the speakers reported having relevant financial disclosures.
AT ACR 2017
Obesity linked to pain, fatigue in SLE
SAN DIEGO – A new study offers a double message about the potential impact of obesity on systemic lupus erythematosus (SLE) in women: Excess pounds are linked to a higher risk of patient-reported outcomes such as pain and fatigue, and body mass index may be an appropriate tool to study weight issues in this population.
Researchers found “a strong relationship between body composition and worse outcomes,” Sarah Patterson, MD, a fellow in rheumatology at the University of California, San Francisco, and the lead study author, said at the annual meeting of the American College of Rheumatology.
For the new study, Dr. Patterson and her colleagues analyzed findings from surveys of 148 participants in the Arthritis Body Composition and Disability study. All participants were women with a verified SLE diagnosis.
About two-thirds of the sample were white, 14% were Asian, and 13% were African American. The average age was 48 years, the average disease duration was 16 years, and 45% took glucocorticoids.
Researchers used two measurements of obesity: BMI of 30 kg/m2 or greater and fat mass index (FMI) of 13 kg/m2 or greater.
They calculated FMI with data collected via whole dual x-ray absorptiometry. Of the participants, 32% and 30% met criteria for obesity under FMI and BMI definitions, respectively.
Researchers also collected survey data regarding measurements of disease activity, depressive symptoms, pain and fatigue.
The study authors controlled their results to account for factors such as age, race, and prednisone use. They found that those defined as obese via FMI had more disease activity and depression than did nonobese women: 14.8 versus 11.5, P = .010, on the Systemic Lupus Activity Questionnaire scale, and 19.8 versus 13.1, P = .004, on the Center for Epidemiologic Studies Depression scale.
On two other scales of pain and fatigue, obese patients scored lower – a sign of worse status – compared with nonobese women: 38.7 versus 44.2, P = .004, on the Short Form 36 (SF-36) Health Survey pain subscale and 39.6 versus 45.2, P = .010, on the SF-36 vitality subscale. The researchers reported similar findings when using BMI to assess obesity.
It’s not clear why obesity and lupus may be linked, Dr. Patterson said, though she noted that inflammation is a shared factor. “People with lupus have arthritis and chronic pain, so there may be this vicious feedback cycle with hindrances to be able to live healthy lifestyles,” she added.
The study has limitations, including that the sample is largely white, while lupus is more common among minority women. In addition, the study does not include underweight patients or track patients over time. “It will be important to look at obesity and patient-reported outcomes to determine whether weight loss results in better outcomes,” Dr. Patterson said.
The study does provide an extra benefit by suggesting that BMI is not an inferior tool to measure the effects of obesity in the SLE population, Dr. Patterson said. BMI has been criticized as a misleading measurement of obesity. But the BMI and FMI measures produced similar results in this study. “That’s really good news in a way for the practicalities of using this information,” she said.
But FMI may still be a better measurement of obesity in the general population, where BMI may be more likely to be thrown off by high muscle mass.
It may seem obvious that obesity is linked to worse lupus outcomes, but rheumatologist Bryant England, MD, of the University of Nebraska, Omaha, said that this research is noteworthy because it highlights the importance of focusing on obesity in the clinic.
Rheumatologists shouldn’t leave obesity to primary care physicians but instead confront it themselves, said Dr. England, who moderated a discussion of new research at an ACR annual meeting press conference. But he cautioned that prudence is especially important when talking about obesity with lupus patients because they may be sensitive about medication-related weight gain.
Dr. Patterson and the other study authors reported having no relevant disclosures. Dr. England also reported no relevant disclosures. The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
SAN DIEGO – A new study offers a double message about the potential impact of obesity on systemic lupus erythematosus (SLE) in women: Excess pounds are linked to a higher risk of patient-reported outcomes such as pain and fatigue, and body mass index may be an appropriate tool to study weight issues in this population.
Researchers found “a strong relationship between body composition and worse outcomes,” Sarah Patterson, MD, a fellow in rheumatology at the University of California, San Francisco, and the lead study author, said at the annual meeting of the American College of Rheumatology.
For the new study, Dr. Patterson and her colleagues analyzed findings from surveys of 148 participants in the Arthritis Body Composition and Disability study. All participants were women with a verified SLE diagnosis.
About two-thirds of the sample were white, 14% were Asian, and 13% were African American. The average age was 48 years, the average disease duration was 16 years, and 45% took glucocorticoids.
Researchers used two measurements of obesity: BMI of 30 kg/m2 or greater and fat mass index (FMI) of 13 kg/m2 or greater.
They calculated FMI with data collected via whole dual x-ray absorptiometry. Of the participants, 32% and 30% met criteria for obesity under FMI and BMI definitions, respectively.
Researchers also collected survey data regarding measurements of disease activity, depressive symptoms, pain and fatigue.
The study authors controlled their results to account for factors such as age, race, and prednisone use. They found that those defined as obese via FMI had more disease activity and depression than did nonobese women: 14.8 versus 11.5, P = .010, on the Systemic Lupus Activity Questionnaire scale, and 19.8 versus 13.1, P = .004, on the Center for Epidemiologic Studies Depression scale.
On two other scales of pain and fatigue, obese patients scored lower – a sign of worse status – compared with nonobese women: 38.7 versus 44.2, P = .004, on the Short Form 36 (SF-36) Health Survey pain subscale and 39.6 versus 45.2, P = .010, on the SF-36 vitality subscale. The researchers reported similar findings when using BMI to assess obesity.
It’s not clear why obesity and lupus may be linked, Dr. Patterson said, though she noted that inflammation is a shared factor. “People with lupus have arthritis and chronic pain, so there may be this vicious feedback cycle with hindrances to be able to live healthy lifestyles,” she added.
The study has limitations, including that the sample is largely white, while lupus is more common among minority women. In addition, the study does not include underweight patients or track patients over time. “It will be important to look at obesity and patient-reported outcomes to determine whether weight loss results in better outcomes,” Dr. Patterson said.
The study does provide an extra benefit by suggesting that BMI is not an inferior tool to measure the effects of obesity in the SLE population, Dr. Patterson said. BMI has been criticized as a misleading measurement of obesity. But the BMI and FMI measures produced similar results in this study. “That’s really good news in a way for the practicalities of using this information,” she said.
But FMI may still be a better measurement of obesity in the general population, where BMI may be more likely to be thrown off by high muscle mass.
It may seem obvious that obesity is linked to worse lupus outcomes, but rheumatologist Bryant England, MD, of the University of Nebraska, Omaha, said that this research is noteworthy because it highlights the importance of focusing on obesity in the clinic.
Rheumatologists shouldn’t leave obesity to primary care physicians but instead confront it themselves, said Dr. England, who moderated a discussion of new research at an ACR annual meeting press conference. But he cautioned that prudence is especially important when talking about obesity with lupus patients because they may be sensitive about medication-related weight gain.
Dr. Patterson and the other study authors reported having no relevant disclosures. Dr. England also reported no relevant disclosures. The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
SAN DIEGO – A new study offers a double message about the potential impact of obesity on systemic lupus erythematosus (SLE) in women: Excess pounds are linked to a higher risk of patient-reported outcomes such as pain and fatigue, and body mass index may be an appropriate tool to study weight issues in this population.
Researchers found “a strong relationship between body composition and worse outcomes,” Sarah Patterson, MD, a fellow in rheumatology at the University of California, San Francisco, and the lead study author, said at the annual meeting of the American College of Rheumatology.
For the new study, Dr. Patterson and her colleagues analyzed findings from surveys of 148 participants in the Arthritis Body Composition and Disability study. All participants were women with a verified SLE diagnosis.
About two-thirds of the sample were white, 14% were Asian, and 13% were African American. The average age was 48 years, the average disease duration was 16 years, and 45% took glucocorticoids.
Researchers used two measurements of obesity: BMI of 30 kg/m2 or greater and fat mass index (FMI) of 13 kg/m2 or greater.
They calculated FMI with data collected via whole dual x-ray absorptiometry. Of the participants, 32% and 30% met criteria for obesity under FMI and BMI definitions, respectively.
Researchers also collected survey data regarding measurements of disease activity, depressive symptoms, pain and fatigue.
The study authors controlled their results to account for factors such as age, race, and prednisone use. They found that those defined as obese via FMI had more disease activity and depression than did nonobese women: 14.8 versus 11.5, P = .010, on the Systemic Lupus Activity Questionnaire scale, and 19.8 versus 13.1, P = .004, on the Center for Epidemiologic Studies Depression scale.
On two other scales of pain and fatigue, obese patients scored lower – a sign of worse status – compared with nonobese women: 38.7 versus 44.2, P = .004, on the Short Form 36 (SF-36) Health Survey pain subscale and 39.6 versus 45.2, P = .010, on the SF-36 vitality subscale. The researchers reported similar findings when using BMI to assess obesity.
It’s not clear why obesity and lupus may be linked, Dr. Patterson said, though she noted that inflammation is a shared factor. “People with lupus have arthritis and chronic pain, so there may be this vicious feedback cycle with hindrances to be able to live healthy lifestyles,” she added.
The study has limitations, including that the sample is largely white, while lupus is more common among minority women. In addition, the study does not include underweight patients or track patients over time. “It will be important to look at obesity and patient-reported outcomes to determine whether weight loss results in better outcomes,” Dr. Patterson said.
The study does provide an extra benefit by suggesting that BMI is not an inferior tool to measure the effects of obesity in the SLE population, Dr. Patterson said. BMI has been criticized as a misleading measurement of obesity. But the BMI and FMI measures produced similar results in this study. “That’s really good news in a way for the practicalities of using this information,” she said.
But FMI may still be a better measurement of obesity in the general population, where BMI may be more likely to be thrown off by high muscle mass.
It may seem obvious that obesity is linked to worse lupus outcomes, but rheumatologist Bryant England, MD, of the University of Nebraska, Omaha, said that this research is noteworthy because it highlights the importance of focusing on obesity in the clinic.
Rheumatologists shouldn’t leave obesity to primary care physicians but instead confront it themselves, said Dr. England, who moderated a discussion of new research at an ACR annual meeting press conference. But he cautioned that prudence is especially important when talking about obesity with lupus patients because they may be sensitive about medication-related weight gain.
Dr. Patterson and the other study authors reported having no relevant disclosures. Dr. England also reported no relevant disclosures. The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
AT ACR 2017
Key clinical point:
Major finding: Obese women with SLE had more disease activity than did nonobese women (14.8 versus 11.5, P = .010).
Data source: An analysis of 148 SLE patients (65% white, mean age 48, about 31% obese) with obesity measured by body mass index or fat mass index.
Disclosures: The study authors reported having no relevant disclosures. The National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the study.
Gout incidence is intertwined with serum urate, but only up to a point
SAN DIEGO – The incidence of gout is strongly linked to patients’ concentration of serum uric acid over time, but even so, less than half of patients with levels of 10 mg/dL or above develop the condition by 15 years, according to the largest individual person-level analysis to examine the relationship.
The incidence of gout rose from about 1% after 15 years in patients with a serum uric acid (sUA) level of less than 6 mg/dL to almost 49% in those with 10 mg/dL or higher in the study, which implies “a long period of hyperuricemia preceding the onset of clinical gout” and also “supports a role for additional factors in the pathogenesis of gout,” Nicola Dalbeth, MD, said in her presentation of the results at the annual meeting of the American College of Rheumatology.
Dr. Dalbeth and her associates found four studies in a search of PubMed and the Database of Genotype and Phenotype from Jan. 1, 1980, to June 11, 2016, that met the inclusion criteria of containing publicly available participant level data, recorded incident gout (via classification criteria, doctor’s diagnosis, or self report of disease), and had a minimum of 3 years of follow-up. The four studies were the Atherosclerosis Risk in Communities study, the Coronary Artery Risk Development in Young Adults study, the original cohort of the Framingham Heart Study, and the offspring cohort of the Framingham Heart Study, comprising 18,889 individuals who were gout free at the beginning of follow-up, which lasted a mean of 11.2 years.
In all studies combined, the incidence of gout at an sUA level of less than 6 mg/dL steadily increased from 0.21% at 3 years of follow-up to 1.12% at 15 years. In contrast, sUA at 10 mg/dL or higher led to gout in 10.00% at 3 years and in 48.57% at 15 years.
The same general pattern held for the incidence of gout in both men and women, although men had a higher incidence across nearly all sUA concentration ranges.
Female sex provided a 30% reduced risk of gout, and European ethnicity nearly halved the risk for gout, compared with non-Europeans, The risk for gout rose across decades of age, starting at 40-49 years, and also increased significantly for each 1-mg/dL interval of sUA starting at 6 mg/dL.
The study’s conclusions are limited by the use of variable definitions of gout and how it was ascertained. In addition, the study did not analyze other endpoints that are associated with hyperuricemia and may be relevant to discuss in counseling people with elevated sUA levels, such as hypertension, chronic kidney disease, and cardiovascular disease, Dr. Dalbeth said.
Audience member Daniel H. Solomon, MD, of Brigham and Women’s Hospital, Boston, said after the presentation that it is possible that age might not be independent of sUA level because it’s unknown when patients first had hyperuricemia, and so it could just serve as a marker of the duration of the effect of hyperuricemia. “You showed us that the longer you wait for people who have higher [sUA] levels, the more likely you are to observe gout. So it’s probably some mixture of duration [of hyperuricemia] and age,” he said.
Dr. Dalbeth agreed, saying that it could also help to explain why the incidence of gout is lower at younger ages in women but then subsequently becomes higher.
The Health Research Council of New Zealand supported the research. Dr. Dalbeth reported receiving consulting fees, grants, or speaking fees from Takeda, Horizon, Menarini, AstraZeneca, Ardea Biosciences, Pfizer, and Cymabay, but none are related to this study. Two other authors also had several financial disclosures, but none of the others did.
SAN DIEGO – The incidence of gout is strongly linked to patients’ concentration of serum uric acid over time, but even so, less than half of patients with levels of 10 mg/dL or above develop the condition by 15 years, according to the largest individual person-level analysis to examine the relationship.
The incidence of gout rose from about 1% after 15 years in patients with a serum uric acid (sUA) level of less than 6 mg/dL to almost 49% in those with 10 mg/dL or higher in the study, which implies “a long period of hyperuricemia preceding the onset of clinical gout” and also “supports a role for additional factors in the pathogenesis of gout,” Nicola Dalbeth, MD, said in her presentation of the results at the annual meeting of the American College of Rheumatology.
Dr. Dalbeth and her associates found four studies in a search of PubMed and the Database of Genotype and Phenotype from Jan. 1, 1980, to June 11, 2016, that met the inclusion criteria of containing publicly available participant level data, recorded incident gout (via classification criteria, doctor’s diagnosis, or self report of disease), and had a minimum of 3 years of follow-up. The four studies were the Atherosclerosis Risk in Communities study, the Coronary Artery Risk Development in Young Adults study, the original cohort of the Framingham Heart Study, and the offspring cohort of the Framingham Heart Study, comprising 18,889 individuals who were gout free at the beginning of follow-up, which lasted a mean of 11.2 years.
In all studies combined, the incidence of gout at an sUA level of less than 6 mg/dL steadily increased from 0.21% at 3 years of follow-up to 1.12% at 15 years. In contrast, sUA at 10 mg/dL or higher led to gout in 10.00% at 3 years and in 48.57% at 15 years.
The same general pattern held for the incidence of gout in both men and women, although men had a higher incidence across nearly all sUA concentration ranges.
Female sex provided a 30% reduced risk of gout, and European ethnicity nearly halved the risk for gout, compared with non-Europeans, The risk for gout rose across decades of age, starting at 40-49 years, and also increased significantly for each 1-mg/dL interval of sUA starting at 6 mg/dL.
The study’s conclusions are limited by the use of variable definitions of gout and how it was ascertained. In addition, the study did not analyze other endpoints that are associated with hyperuricemia and may be relevant to discuss in counseling people with elevated sUA levels, such as hypertension, chronic kidney disease, and cardiovascular disease, Dr. Dalbeth said.
Audience member Daniel H. Solomon, MD, of Brigham and Women’s Hospital, Boston, said after the presentation that it is possible that age might not be independent of sUA level because it’s unknown when patients first had hyperuricemia, and so it could just serve as a marker of the duration of the effect of hyperuricemia. “You showed us that the longer you wait for people who have higher [sUA] levels, the more likely you are to observe gout. So it’s probably some mixture of duration [of hyperuricemia] and age,” he said.
Dr. Dalbeth agreed, saying that it could also help to explain why the incidence of gout is lower at younger ages in women but then subsequently becomes higher.
The Health Research Council of New Zealand supported the research. Dr. Dalbeth reported receiving consulting fees, grants, or speaking fees from Takeda, Horizon, Menarini, AstraZeneca, Ardea Biosciences, Pfizer, and Cymabay, but none are related to this study. Two other authors also had several financial disclosures, but none of the others did.
SAN DIEGO – The incidence of gout is strongly linked to patients’ concentration of serum uric acid over time, but even so, less than half of patients with levels of 10 mg/dL or above develop the condition by 15 years, according to the largest individual person-level analysis to examine the relationship.
The incidence of gout rose from about 1% after 15 years in patients with a serum uric acid (sUA) level of less than 6 mg/dL to almost 49% in those with 10 mg/dL or higher in the study, which implies “a long period of hyperuricemia preceding the onset of clinical gout” and also “supports a role for additional factors in the pathogenesis of gout,” Nicola Dalbeth, MD, said in her presentation of the results at the annual meeting of the American College of Rheumatology.
Dr. Dalbeth and her associates found four studies in a search of PubMed and the Database of Genotype and Phenotype from Jan. 1, 1980, to June 11, 2016, that met the inclusion criteria of containing publicly available participant level data, recorded incident gout (via classification criteria, doctor’s diagnosis, or self report of disease), and had a minimum of 3 years of follow-up. The four studies were the Atherosclerosis Risk in Communities study, the Coronary Artery Risk Development in Young Adults study, the original cohort of the Framingham Heart Study, and the offspring cohort of the Framingham Heart Study, comprising 18,889 individuals who were gout free at the beginning of follow-up, which lasted a mean of 11.2 years.
In all studies combined, the incidence of gout at an sUA level of less than 6 mg/dL steadily increased from 0.21% at 3 years of follow-up to 1.12% at 15 years. In contrast, sUA at 10 mg/dL or higher led to gout in 10.00% at 3 years and in 48.57% at 15 years.
The same general pattern held for the incidence of gout in both men and women, although men had a higher incidence across nearly all sUA concentration ranges.
Female sex provided a 30% reduced risk of gout, and European ethnicity nearly halved the risk for gout, compared with non-Europeans, The risk for gout rose across decades of age, starting at 40-49 years, and also increased significantly for each 1-mg/dL interval of sUA starting at 6 mg/dL.
The study’s conclusions are limited by the use of variable definitions of gout and how it was ascertained. In addition, the study did not analyze other endpoints that are associated with hyperuricemia and may be relevant to discuss in counseling people with elevated sUA levels, such as hypertension, chronic kidney disease, and cardiovascular disease, Dr. Dalbeth said.
Audience member Daniel H. Solomon, MD, of Brigham and Women’s Hospital, Boston, said after the presentation that it is possible that age might not be independent of sUA level because it’s unknown when patients first had hyperuricemia, and so it could just serve as a marker of the duration of the effect of hyperuricemia. “You showed us that the longer you wait for people who have higher [sUA] levels, the more likely you are to observe gout. So it’s probably some mixture of duration [of hyperuricemia] and age,” he said.
Dr. Dalbeth agreed, saying that it could also help to explain why the incidence of gout is lower at younger ages in women but then subsequently becomes higher.
The Health Research Council of New Zealand supported the research. Dr. Dalbeth reported receiving consulting fees, grants, or speaking fees from Takeda, Horizon, Menarini, AstraZeneca, Ardea Biosciences, Pfizer, and Cymabay, but none are related to this study. Two other authors also had several financial disclosures, but none of the others did.
AT ACR 2017
Key clinical point:
Major finding: The incidence of gout rose to about 1% after 15 years in patients with a serum uric acid (sUA) level of less than 6 mg/dL to almost 49% in those with 10 mg/dL or higher.
Data source: An analysis of 18,889 participants in four longitudinal observational cohort studies for whom baseline serum uric acid levels were available.
Disclosures: The Health Research Council of New Zealand supported the research. Dr. Dalbeth reported receiving consulting fees, grants, or speaking fees from Takeda, Horizon, Menarini, AstraZeneca, Ardea Biosciences, Pfizer, and Cymabay, but none are related to this study. Two other authors also had several financial disclosures, but none of the others did.
Use of opioids, SSRIs linked to increased fracture risk in RA
SAN DIEGO – The use of selective serotonin reuptake inhibitors and opioids was associated with an increased osteoporotic fracture risk in patients with rheumatoid arthritis, results from an analysis of national data showed.
“Osteoporotic fractures are one of the important causes of disability, health-related costs, and mortality in RA, with substantially higher complication and mortality rates than the general population,” study author Gulsen Ozen, MD, said in an interview prior to the annual meeting of the American College of Rheumatology. “Given the burden of osteoporotic fractures and the suboptimal osteoporosis care, identifying the factors associated with fracture risk in RA patients is of paramount importance.”
During a median follow-up of nearly 6 years, 863 patients (7.8%) sustained osteoporotic fractures. Compared with patients who did not develop fractures, those who did were significantly older, had higher disease duration and activity, glucocorticoid use, comorbidity and FRAX, a fracture risk assessment tool, scores at baseline. After adjusting for sociodemographics, comorbidities, body mass index, fracture risk by FRAX, and RA severity measures, the researchers found a significant risk of osteoporotic fractures with use of opioids of any strength (weak agents, hazard ratio, 1.45; strong agents, HR, 1.79; P less than .001 for both), SSRI use (HR, 1.35; P = .003), and glucocorticoid use of 3 months or longer at a dose of at least 7.5 mg per day (HR, 1.74; P less than .05). Osteoporotic fracture risk increase started even after 1-30 days of opioid use (HR, 1.96; P less than .001), whereas SSRI-associated risk increase started after 3 months of use (HR, 1.42; P = .054). No significant association with fracture risk was observed with the use of other disease-modifying antirheumatic drugs, statins, antidepressants, proton pump inhibitors, NSAIDs, anticonvulsants, and antipsychotics.
“One of the first surprising findings was that almost 40% of the RA patients older than 40 years of age were at least once exposed to opioid analgesics,” said Dr. Ozen, who is a research fellow in the division of immunology and rheumatology at the medical center. “Another surprising finding was that even very short-term (1-30 days) use of opioids was associated with increased fracture risk.” She went on to note that careful and regular reviewing of patient medications “is an essential part of the RA patient care, as the use of medications not indicated anymore brings harm rather than a benefit. The most well-known example for this is glucocorticoid use. This is valid for all medications, too. Therefore, we hope that our findings provide more awareness about osteoporotic fractures and associated risk factors in RA patients.”
She acknowledged certain limitations of the study, including its observational design. “Additionally, fracture and the level of the trauma in our cohort were reported by patients,” she said. “Therefore, there might be some misclassification of fractures as osteoporotic fractures. Lastly, we did not have detailed data regarding fall risk, which might explain the associations we observed with opioids and potentially, SSRIs.”
Dr. Ozen reported having no disclosures.
SAN DIEGO – The use of selective serotonin reuptake inhibitors and opioids was associated with an increased osteoporotic fracture risk in patients with rheumatoid arthritis, results from an analysis of national data showed.
“Osteoporotic fractures are one of the important causes of disability, health-related costs, and mortality in RA, with substantially higher complication and mortality rates than the general population,” study author Gulsen Ozen, MD, said in an interview prior to the annual meeting of the American College of Rheumatology. “Given the burden of osteoporotic fractures and the suboptimal osteoporosis care, identifying the factors associated with fracture risk in RA patients is of paramount importance.”
During a median follow-up of nearly 6 years, 863 patients (7.8%) sustained osteoporotic fractures. Compared with patients who did not develop fractures, those who did were significantly older, had higher disease duration and activity, glucocorticoid use, comorbidity and FRAX, a fracture risk assessment tool, scores at baseline. After adjusting for sociodemographics, comorbidities, body mass index, fracture risk by FRAX, and RA severity measures, the researchers found a significant risk of osteoporotic fractures with use of opioids of any strength (weak agents, hazard ratio, 1.45; strong agents, HR, 1.79; P less than .001 for both), SSRI use (HR, 1.35; P = .003), and glucocorticoid use of 3 months or longer at a dose of at least 7.5 mg per day (HR, 1.74; P less than .05). Osteoporotic fracture risk increase started even after 1-30 days of opioid use (HR, 1.96; P less than .001), whereas SSRI-associated risk increase started after 3 months of use (HR, 1.42; P = .054). No significant association with fracture risk was observed with the use of other disease-modifying antirheumatic drugs, statins, antidepressants, proton pump inhibitors, NSAIDs, anticonvulsants, and antipsychotics.
“One of the first surprising findings was that almost 40% of the RA patients older than 40 years of age were at least once exposed to opioid analgesics,” said Dr. Ozen, who is a research fellow in the division of immunology and rheumatology at the medical center. “Another surprising finding was that even very short-term (1-30 days) use of opioids was associated with increased fracture risk.” She went on to note that careful and regular reviewing of patient medications “is an essential part of the RA patient care, as the use of medications not indicated anymore brings harm rather than a benefit. The most well-known example for this is glucocorticoid use. This is valid for all medications, too. Therefore, we hope that our findings provide more awareness about osteoporotic fractures and associated risk factors in RA patients.”
She acknowledged certain limitations of the study, including its observational design. “Additionally, fracture and the level of the trauma in our cohort were reported by patients,” she said. “Therefore, there might be some misclassification of fractures as osteoporotic fractures. Lastly, we did not have detailed data regarding fall risk, which might explain the associations we observed with opioids and potentially, SSRIs.”
Dr. Ozen reported having no disclosures.
SAN DIEGO – The use of selective serotonin reuptake inhibitors and opioids was associated with an increased osteoporotic fracture risk in patients with rheumatoid arthritis, results from an analysis of national data showed.
“Osteoporotic fractures are one of the important causes of disability, health-related costs, and mortality in RA, with substantially higher complication and mortality rates than the general population,” study author Gulsen Ozen, MD, said in an interview prior to the annual meeting of the American College of Rheumatology. “Given the burden of osteoporotic fractures and the suboptimal osteoporosis care, identifying the factors associated with fracture risk in RA patients is of paramount importance.”
During a median follow-up of nearly 6 years, 863 patients (7.8%) sustained osteoporotic fractures. Compared with patients who did not develop fractures, those who did were significantly older, had higher disease duration and activity, glucocorticoid use, comorbidity and FRAX, a fracture risk assessment tool, scores at baseline. After adjusting for sociodemographics, comorbidities, body mass index, fracture risk by FRAX, and RA severity measures, the researchers found a significant risk of osteoporotic fractures with use of opioids of any strength (weak agents, hazard ratio, 1.45; strong agents, HR, 1.79; P less than .001 for both), SSRI use (HR, 1.35; P = .003), and glucocorticoid use of 3 months or longer at a dose of at least 7.5 mg per day (HR, 1.74; P less than .05). Osteoporotic fracture risk increase started even after 1-30 days of opioid use (HR, 1.96; P less than .001), whereas SSRI-associated risk increase started after 3 months of use (HR, 1.42; P = .054). No significant association with fracture risk was observed with the use of other disease-modifying antirheumatic drugs, statins, antidepressants, proton pump inhibitors, NSAIDs, anticonvulsants, and antipsychotics.
“One of the first surprising findings was that almost 40% of the RA patients older than 40 years of age were at least once exposed to opioid analgesics,” said Dr. Ozen, who is a research fellow in the division of immunology and rheumatology at the medical center. “Another surprising finding was that even very short-term (1-30 days) use of opioids was associated with increased fracture risk.” She went on to note that careful and regular reviewing of patient medications “is an essential part of the RA patient care, as the use of medications not indicated anymore brings harm rather than a benefit. The most well-known example for this is glucocorticoid use. This is valid for all medications, too. Therefore, we hope that our findings provide more awareness about osteoporotic fractures and associated risk factors in RA patients.”
She acknowledged certain limitations of the study, including its observational design. “Additionally, fracture and the level of the trauma in our cohort were reported by patients,” she said. “Therefore, there might be some misclassification of fractures as osteoporotic fractures. Lastly, we did not have detailed data regarding fall risk, which might explain the associations we observed with opioids and potentially, SSRIs.”
Dr. Ozen reported having no disclosures.
AT ACR 2017
Key clinical point: When managing with opioids, even in the short-term, clinicians should be aware of the fracture risk.
Major finding: In patients with RA, concomitant use of selective serotonin reuptake inhibitors was associated with an increased risk of osteoporotic fracture (HR, 1.35; P = .003), as was opioid use (HR, 1.45 and HR, 1.79) for weak and strong agents, respectively; P less than .001 for both).
Study details: An observational study of 11,049 patients from the National Data Bank for Rheumatic Diseases.
Disclosures: Dr. Ozen reported having no disclosures.
Legislative landscape affecting rheumatology has potential wins but many challenges
SAN DIEGO – , but ongoing efforts to advocate for the specialty and patients are showing signs of paying off in some areas, Angus Worthing, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Worthing, who is chair of the ACR’s Government Affairs Committee and a practicing rheumatologist in the Washington area, encouraged rheumatologists to become involved in advocacy efforts and asked members of the audience at the meeting to visit the ACR’s advocacy website to learn how to help.
The ACR supports a group of bills that have been introduced in either the House or Senate that should have an effect on alleviating the projected shortage of rheumatologists across the United States through 2030. These bills will help, although much of the effort to address the shortage and maldistribution of rheumatologists across the United States will “probably be solved at the local level. It’s not going to be a federal solution. It will be relationships and treatment programs between primary care and rheumatology care that are very local,” Dr. Worthing said.
The Conrad State 30 and Physician Access Reauthorization Act (H.R. 2141, S. 898) aims to streamline visas for foreign physicians to practice in underserved areas.
The Resident Physician Shortage Reduction Act of 2017 (H.R. 2267) would increase for the first time since 1997 the number of graduate medical education residency slots in the United States.
The Ensuring Children’s Access to Specialty Care Act of 2017 (S. 989) allows pediatric subspecialists, including pediatric rheumatologists, to get access to the National Health Service Corps loan repayment program when they work in underserved areas.
More recently, in spring 2017 the American Medical Association played a big role in getting the Trump administration to reverse its stance on not allowing premium processing of H1-B visas for professionals such as physicians. If this had gone into effect, all the rheumatology fellows in training who were going to be practicing – some in underserved areas – might have been forced to return to their home country because of a lack of time to get their H1-B visa processed before finishing their fellowship, Dr. Worthing said.
Affordable Care Act (ACA)
- Provide sufficient, affordable, continuous coverage that encourages access to high-quality care for all.
- Prohibit exclusions based on preexisting conditions.
- Allow children to remain on parent’s insurance until age 26 years.
- Remove excessive administrative burdens that take focus away from patient care.
- Cap annual out-of-pocket costs and ban lifetime limits.
- Have affordable premiums, deductibles, and cost sharing.
- Continue the 10 essential health benefits that are required for ACA marketplace plans.
Alliance for Transparent & Affordable Prescriptions (ATAP)
The ACR convened this alliance along with the Coalition of State Rheumatology Organizations, the Global Healthy Living Foundation, the Association of Women in Rheumatology, the Rheumatology Nurses Society, and others to try to bring transparency to how pharmacy benefit managers (PBMs) operate in getting certain drugs on the formularies of payers. The ATAP recently had some success in making lawmakers aware of the PBM’s role in influencing drug prices via rebates to drug manufacturers. At a Congressional hearing in Oct. 2017, after many visits from rheumatologists and members of ATAP, the members of the Senate Committee on Health, Education, Labor, and Pensions “held the feet of these PBMs to the fire a little bit asking them about these rebates,” Dr. Worthing said, where at one point committee chair Sen. Lamar Alexander (R-Tenn.) asked, “ ‘Do we really need these rebates?’ ”
National Institutes of Health budget
After the National Institutes of Health received a $2 billion increase in funding for fiscal year 2017, the Trump administration proposed last summer to cut the NIH budget by 22%. Since then, however, bills to increase the NIH budget by $1.1 billion from the House and by $2 billion from the Senate have made their way through committees. But a budget must be passed by Congress and then signed by the president to make a potential budget increase a reality. Otherwise, a continuing resolution would leave the current level of funding in place through fiscal year 2018, Dr. Worthing noted.
Patients’ Access to Treatments Act of 2017 (H.R. 2999)
This bill has been raised for a fourth time after not making it past committees in previous Congresses, but the prospects for it passing appear somewhat better this time around, Dr. Worthing said. It would prevent insurance companies from putting drugs in specialty tiers that require patients to pay increasingly higher rates of coinsurance for the drugs on different tiers.
“It has been gathering momentum. We hope to get it across the finish line. And if we don’t get this across, then we’ll join with the coalition that rheumatology has formed around this issue of access to specialty treatments some other way, because this is a burning issue for us and our patients,” he said.
Medicare Access to Rehabilitation Services Act of 2017 (H.R. 807 and S. 253)
This bill would repeal the annual cap that was placed on rehabilitation services for patients covered by Medicare in 1997. The bill has bipartisan, majority support and has been gaining momentum for the past 4 years, Dr. Worthing said. It was advanced from both Senate and House committees in Oct. 2017.
SAN DIEGO – , but ongoing efforts to advocate for the specialty and patients are showing signs of paying off in some areas, Angus Worthing, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Worthing, who is chair of the ACR’s Government Affairs Committee and a practicing rheumatologist in the Washington area, encouraged rheumatologists to become involved in advocacy efforts and asked members of the audience at the meeting to visit the ACR’s advocacy website to learn how to help.
The ACR supports a group of bills that have been introduced in either the House or Senate that should have an effect on alleviating the projected shortage of rheumatologists across the United States through 2030. These bills will help, although much of the effort to address the shortage and maldistribution of rheumatologists across the United States will “probably be solved at the local level. It’s not going to be a federal solution. It will be relationships and treatment programs between primary care and rheumatology care that are very local,” Dr. Worthing said.
The Conrad State 30 and Physician Access Reauthorization Act (H.R. 2141, S. 898) aims to streamline visas for foreign physicians to practice in underserved areas.
The Resident Physician Shortage Reduction Act of 2017 (H.R. 2267) would increase for the first time since 1997 the number of graduate medical education residency slots in the United States.
The Ensuring Children’s Access to Specialty Care Act of 2017 (S. 989) allows pediatric subspecialists, including pediatric rheumatologists, to get access to the National Health Service Corps loan repayment program when they work in underserved areas.
More recently, in spring 2017 the American Medical Association played a big role in getting the Trump administration to reverse its stance on not allowing premium processing of H1-B visas for professionals such as physicians. If this had gone into effect, all the rheumatology fellows in training who were going to be practicing – some in underserved areas – might have been forced to return to their home country because of a lack of time to get their H1-B visa processed before finishing their fellowship, Dr. Worthing said.
Affordable Care Act (ACA)
- Provide sufficient, affordable, continuous coverage that encourages access to high-quality care for all.
- Prohibit exclusions based on preexisting conditions.
- Allow children to remain on parent’s insurance until age 26 years.
- Remove excessive administrative burdens that take focus away from patient care.
- Cap annual out-of-pocket costs and ban lifetime limits.
- Have affordable premiums, deductibles, and cost sharing.
- Continue the 10 essential health benefits that are required for ACA marketplace plans.
Alliance for Transparent & Affordable Prescriptions (ATAP)
The ACR convened this alliance along with the Coalition of State Rheumatology Organizations, the Global Healthy Living Foundation, the Association of Women in Rheumatology, the Rheumatology Nurses Society, and others to try to bring transparency to how pharmacy benefit managers (PBMs) operate in getting certain drugs on the formularies of payers. The ATAP recently had some success in making lawmakers aware of the PBM’s role in influencing drug prices via rebates to drug manufacturers. At a Congressional hearing in Oct. 2017, after many visits from rheumatologists and members of ATAP, the members of the Senate Committee on Health, Education, Labor, and Pensions “held the feet of these PBMs to the fire a little bit asking them about these rebates,” Dr. Worthing said, where at one point committee chair Sen. Lamar Alexander (R-Tenn.) asked, “ ‘Do we really need these rebates?’ ”
National Institutes of Health budget
After the National Institutes of Health received a $2 billion increase in funding for fiscal year 2017, the Trump administration proposed last summer to cut the NIH budget by 22%. Since then, however, bills to increase the NIH budget by $1.1 billion from the House and by $2 billion from the Senate have made their way through committees. But a budget must be passed by Congress and then signed by the president to make a potential budget increase a reality. Otherwise, a continuing resolution would leave the current level of funding in place through fiscal year 2018, Dr. Worthing noted.
Patients’ Access to Treatments Act of 2017 (H.R. 2999)
This bill has been raised for a fourth time after not making it past committees in previous Congresses, but the prospects for it passing appear somewhat better this time around, Dr. Worthing said. It would prevent insurance companies from putting drugs in specialty tiers that require patients to pay increasingly higher rates of coinsurance for the drugs on different tiers.
“It has been gathering momentum. We hope to get it across the finish line. And if we don’t get this across, then we’ll join with the coalition that rheumatology has formed around this issue of access to specialty treatments some other way, because this is a burning issue for us and our patients,” he said.
Medicare Access to Rehabilitation Services Act of 2017 (H.R. 807 and S. 253)
This bill would repeal the annual cap that was placed on rehabilitation services for patients covered by Medicare in 1997. The bill has bipartisan, majority support and has been gaining momentum for the past 4 years, Dr. Worthing said. It was advanced from both Senate and House committees in Oct. 2017.
SAN DIEGO – , but ongoing efforts to advocate for the specialty and patients are showing signs of paying off in some areas, Angus Worthing, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Worthing, who is chair of the ACR’s Government Affairs Committee and a practicing rheumatologist in the Washington area, encouraged rheumatologists to become involved in advocacy efforts and asked members of the audience at the meeting to visit the ACR’s advocacy website to learn how to help.
The ACR supports a group of bills that have been introduced in either the House or Senate that should have an effect on alleviating the projected shortage of rheumatologists across the United States through 2030. These bills will help, although much of the effort to address the shortage and maldistribution of rheumatologists across the United States will “probably be solved at the local level. It’s not going to be a federal solution. It will be relationships and treatment programs between primary care and rheumatology care that are very local,” Dr. Worthing said.
The Conrad State 30 and Physician Access Reauthorization Act (H.R. 2141, S. 898) aims to streamline visas for foreign physicians to practice in underserved areas.
The Resident Physician Shortage Reduction Act of 2017 (H.R. 2267) would increase for the first time since 1997 the number of graduate medical education residency slots in the United States.
The Ensuring Children’s Access to Specialty Care Act of 2017 (S. 989) allows pediatric subspecialists, including pediatric rheumatologists, to get access to the National Health Service Corps loan repayment program when they work in underserved areas.
More recently, in spring 2017 the American Medical Association played a big role in getting the Trump administration to reverse its stance on not allowing premium processing of H1-B visas for professionals such as physicians. If this had gone into effect, all the rheumatology fellows in training who were going to be practicing – some in underserved areas – might have been forced to return to their home country because of a lack of time to get their H1-B visa processed before finishing their fellowship, Dr. Worthing said.
Affordable Care Act (ACA)
- Provide sufficient, affordable, continuous coverage that encourages access to high-quality care for all.
- Prohibit exclusions based on preexisting conditions.
- Allow children to remain on parent’s insurance until age 26 years.
- Remove excessive administrative burdens that take focus away from patient care.
- Cap annual out-of-pocket costs and ban lifetime limits.
- Have affordable premiums, deductibles, and cost sharing.
- Continue the 10 essential health benefits that are required for ACA marketplace plans.
Alliance for Transparent & Affordable Prescriptions (ATAP)
The ACR convened this alliance along with the Coalition of State Rheumatology Organizations, the Global Healthy Living Foundation, the Association of Women in Rheumatology, the Rheumatology Nurses Society, and others to try to bring transparency to how pharmacy benefit managers (PBMs) operate in getting certain drugs on the formularies of payers. The ATAP recently had some success in making lawmakers aware of the PBM’s role in influencing drug prices via rebates to drug manufacturers. At a Congressional hearing in Oct. 2017, after many visits from rheumatologists and members of ATAP, the members of the Senate Committee on Health, Education, Labor, and Pensions “held the feet of these PBMs to the fire a little bit asking them about these rebates,” Dr. Worthing said, where at one point committee chair Sen. Lamar Alexander (R-Tenn.) asked, “ ‘Do we really need these rebates?’ ”
National Institutes of Health budget
After the National Institutes of Health received a $2 billion increase in funding for fiscal year 2017, the Trump administration proposed last summer to cut the NIH budget by 22%. Since then, however, bills to increase the NIH budget by $1.1 billion from the House and by $2 billion from the Senate have made their way through committees. But a budget must be passed by Congress and then signed by the president to make a potential budget increase a reality. Otherwise, a continuing resolution would leave the current level of funding in place through fiscal year 2018, Dr. Worthing noted.
Patients’ Access to Treatments Act of 2017 (H.R. 2999)
This bill has been raised for a fourth time after not making it past committees in previous Congresses, but the prospects for it passing appear somewhat better this time around, Dr. Worthing said. It would prevent insurance companies from putting drugs in specialty tiers that require patients to pay increasingly higher rates of coinsurance for the drugs on different tiers.
“It has been gathering momentum. We hope to get it across the finish line. And if we don’t get this across, then we’ll join with the coalition that rheumatology has formed around this issue of access to specialty treatments some other way, because this is a burning issue for us and our patients,” he said.
Medicare Access to Rehabilitation Services Act of 2017 (H.R. 807 and S. 253)
This bill would repeal the annual cap that was placed on rehabilitation services for patients covered by Medicare in 1997. The bill has bipartisan, majority support and has been gaining momentum for the past 4 years, Dr. Worthing said. It was advanced from both Senate and House committees in Oct. 2017.
AT ACR 2017
Obesity linked to RA disease activity, disability
SAN DIEGO – In what may be the largest study of its kind, British researchers have linked obesity to significantly higher odds of rheumatoid arthritis disease activity and disability.
“This study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis,” says Elena Nikiphorou, MD, of King’s College London, who presented the study findings at the annual meeting of the American College of Rheumatology. “Obesity reduced the odds of achieving remission or low disease activity by around 30%. And the odds of having disability were increased by 63%. This confirms what’s been shown in other, smaller studies.”
Despite obesity having been tied to decreased joint damage in established RA, Eric L. Matteson, MD, noted in an interview, that“the biomechanical effect of [being] overweight, especially on the weight-bearing joints” is one of the two “especially important” mechanisms explaining the link between RA and obesity. “The other is that fat cells produce inflammatory proteins, which contribute to the disease process and make it more difficult to treat,” said Dr. Matteson, a rheumatologist at the Mayo Clinic, Rochester, Minn.
“In my view the mechanical risk to the joint outweighs any possible ‘protective’ effect of RA,” Dr. Matteson added in an interview.
For the new study, Dr. Nikiphorou and colleagues compiled statistics from two consecutive United Kingdom RA inception cohorts. One tracked 1,465 patients for up to 25 years (median follow-up, 10 years), and the other tracked 1,236 patients for as many as 10 years (median follow-up, 6 years).
At baseline, 37.2% of 2,420 patients (90% of total) were overweight, and 21.3% were obese. Average body mass index (BMI) rose between the two consecutive studies from 25.5 to 27.6.
The researchers found that obesity was linked to lower likelihoods of remission and low disease activity status (odds ratio, 0.71; 95% confidence interval, 0.55-0.93 and OR, 0.69; 95% CI, 0.55-0.87, respectively.) After controlling for factors such as age and gender, they also saw slightly lower odds of remission in those with higher BMI (OR, 0.97; 95% CI, 0.95-0.99). But there was no statistically significant link between higher BMI and low disease activity status.
The study also connected obesity to higher odds of disability (OR, 1.63; 95% CI, 1.20-2.23). Furthermore, higher BMI was linked to higher odds of disability (OR, 1.04; 95% CI, 1.01-1.06).
Study lead author Dr. Nikiphorou, who spoke in an interview and in comments at an ACR press conference, said “this study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis. It creates a strong case for addressing BMI and addressing obesity, flagging it up to primary care.”
She added that rheumatologists too often focus on only rheumatoid conditions. “We place so much evidence on disease activity scores,” she said. “How often do we really address the patient in terms of other things going on, including obesity? What we can do is include discussion of BMI, exercise, nutrition.”
Dr. Nikiphorou and Dr. Matteson report no relevant disclosures. No specific study funding is reported.
This article was updated 11/10/17.
SAN DIEGO – In what may be the largest study of its kind, British researchers have linked obesity to significantly higher odds of rheumatoid arthritis disease activity and disability.
“This study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis,” says Elena Nikiphorou, MD, of King’s College London, who presented the study findings at the annual meeting of the American College of Rheumatology. “Obesity reduced the odds of achieving remission or low disease activity by around 30%. And the odds of having disability were increased by 63%. This confirms what’s been shown in other, smaller studies.”
Despite obesity having been tied to decreased joint damage in established RA, Eric L. Matteson, MD, noted in an interview, that“the biomechanical effect of [being] overweight, especially on the weight-bearing joints” is one of the two “especially important” mechanisms explaining the link between RA and obesity. “The other is that fat cells produce inflammatory proteins, which contribute to the disease process and make it more difficult to treat,” said Dr. Matteson, a rheumatologist at the Mayo Clinic, Rochester, Minn.
“In my view the mechanical risk to the joint outweighs any possible ‘protective’ effect of RA,” Dr. Matteson added in an interview.
For the new study, Dr. Nikiphorou and colleagues compiled statistics from two consecutive United Kingdom RA inception cohorts. One tracked 1,465 patients for up to 25 years (median follow-up, 10 years), and the other tracked 1,236 patients for as many as 10 years (median follow-up, 6 years).
At baseline, 37.2% of 2,420 patients (90% of total) were overweight, and 21.3% were obese. Average body mass index (BMI) rose between the two consecutive studies from 25.5 to 27.6.
The researchers found that obesity was linked to lower likelihoods of remission and low disease activity status (odds ratio, 0.71; 95% confidence interval, 0.55-0.93 and OR, 0.69; 95% CI, 0.55-0.87, respectively.) After controlling for factors such as age and gender, they also saw slightly lower odds of remission in those with higher BMI (OR, 0.97; 95% CI, 0.95-0.99). But there was no statistically significant link between higher BMI and low disease activity status.
The study also connected obesity to higher odds of disability (OR, 1.63; 95% CI, 1.20-2.23). Furthermore, higher BMI was linked to higher odds of disability (OR, 1.04; 95% CI, 1.01-1.06).
Study lead author Dr. Nikiphorou, who spoke in an interview and in comments at an ACR press conference, said “this study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis. It creates a strong case for addressing BMI and addressing obesity, flagging it up to primary care.”
She added that rheumatologists too often focus on only rheumatoid conditions. “We place so much evidence on disease activity scores,” she said. “How often do we really address the patient in terms of other things going on, including obesity? What we can do is include discussion of BMI, exercise, nutrition.”
Dr. Nikiphorou and Dr. Matteson report no relevant disclosures. No specific study funding is reported.
This article was updated 11/10/17.
SAN DIEGO – In what may be the largest study of its kind, British researchers have linked obesity to significantly higher odds of rheumatoid arthritis disease activity and disability.
“This study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis,” says Elena Nikiphorou, MD, of King’s College London, who presented the study findings at the annual meeting of the American College of Rheumatology. “Obesity reduced the odds of achieving remission or low disease activity by around 30%. And the odds of having disability were increased by 63%. This confirms what’s been shown in other, smaller studies.”
Despite obesity having been tied to decreased joint damage in established RA, Eric L. Matteson, MD, noted in an interview, that“the biomechanical effect of [being] overweight, especially on the weight-bearing joints” is one of the two “especially important” mechanisms explaining the link between RA and obesity. “The other is that fat cells produce inflammatory proteins, which contribute to the disease process and make it more difficult to treat,” said Dr. Matteson, a rheumatologist at the Mayo Clinic, Rochester, Minn.
“In my view the mechanical risk to the joint outweighs any possible ‘protective’ effect of RA,” Dr. Matteson added in an interview.
For the new study, Dr. Nikiphorou and colleagues compiled statistics from two consecutive United Kingdom RA inception cohorts. One tracked 1,465 patients for up to 25 years (median follow-up, 10 years), and the other tracked 1,236 patients for as many as 10 years (median follow-up, 6 years).
At baseline, 37.2% of 2,420 patients (90% of total) were overweight, and 21.3% were obese. Average body mass index (BMI) rose between the two consecutive studies from 25.5 to 27.6.
The researchers found that obesity was linked to lower likelihoods of remission and low disease activity status (odds ratio, 0.71; 95% confidence interval, 0.55-0.93 and OR, 0.69; 95% CI, 0.55-0.87, respectively.) After controlling for factors such as age and gender, they also saw slightly lower odds of remission in those with higher BMI (OR, 0.97; 95% CI, 0.95-0.99). But there was no statistically significant link between higher BMI and low disease activity status.
The study also connected obesity to higher odds of disability (OR, 1.63; 95% CI, 1.20-2.23). Furthermore, higher BMI was linked to higher odds of disability (OR, 1.04; 95% CI, 1.01-1.06).
Study lead author Dr. Nikiphorou, who spoke in an interview and in comments at an ACR press conference, said “this study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis. It creates a strong case for addressing BMI and addressing obesity, flagging it up to primary care.”
She added that rheumatologists too often focus on only rheumatoid conditions. “We place so much evidence on disease activity scores,” she said. “How often do we really address the patient in terms of other things going on, including obesity? What we can do is include discussion of BMI, exercise, nutrition.”
Dr. Nikiphorou and Dr. Matteson report no relevant disclosures. No specific study funding is reported.
This article was updated 11/10/17.
AT ACR 2017
Key clinical point: Obesity may worsen the risk of disease activity and disability in rheumatoid arthritis.
Major finding: In an adjusted analysis, obese patients with RA were less likely to reach remission and low disease activity status (OR, 0.71; 95% CI, 0.55-0.93 and OR, 0.69; 95% CI, 0.55-0.87, respectively).
Data source: Two consecutive inception cohorts with a total of 1,236 RA patients followed for up to 25 years.
Disclosures: The lead study author reports no disclosures, and no other disclosures are reported. No specific study funding is reported.
Low vitamin D levels linked to increased ESRD risk in SLE patients
SAN DIEGO – , results from a single-center cohort study showed.
“We had previously proved that vitamin D supplementation helped lupus activity,” lead study author Michelle Petri, MD, MPH, said in an interview in advance of the annual meeting of the American College of Rheumatology. “Now, we prove that it specifically helps renal activity as measured by the urine protein. By helping to reduce urine protein, it helps to prevent permanent renal damage and end-stage renal disease.”
The first measure of vitamin D typically occurred in late 2009 or 2010 for existing patients and at the first visit of new patients after that. The researchers categorized patients based on their first measure of vitamin D as less than 20 ng/mL or 20 ng/mL or higher. At the first visit when vitamin D was measured, 27.3% had levels of 25-hydroxyvitamin D less than 20 ng/mL. The mean age of patients was 47.3 years, 92% were female, 50% were white, and 41% were African American.
In the study, Dr. Petri and her associates used the Systemic Lupus International Collaborative Clinics/American College of Rheumatology Damage Index to calculate the risk of lifetime organ damage. After adjusting for age, gender, and ethnicity, low levels of vitamin D were significantly associated with increased risk of renal damage (RR, 1.66; P = .0206) and total organ damage (RR, 1.17; P = .0245), they found.
Skin damage was another concern, with an adjusted relative risk of 1.22, though it was not statistically significant (P = .3561). The investigators observed no association between low vitamin D and musculoskeletal damage, including osteoporotic fractures.
“There is a lot of interest in lupus right now, due to [singer Selena] Gomez’s kidney transplant for lupus nephritis,” said Dr. Petri, who also directs the Johns Hopkins Lupus Center. “So, I think there is interest in how to prevent the need for kidney transplant. Vitamin D helps kidney lupus – and we only need to achieve a level of 40 ng/mL, [which is] safe and easy to do.” She acknowledged the study’s single-center design as a limitation but underscored its large sample size as a strength.
The Hopkins Lupus Cohort is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Petri disclosed having received research support from Anthera, GlaxoSmithKline, EMD Serono, Eli Lilly, Bristol-Myers Squibb, Amgen, United Rheumatology, Global Academy, and Exagen.
SAN DIEGO – , results from a single-center cohort study showed.
“We had previously proved that vitamin D supplementation helped lupus activity,” lead study author Michelle Petri, MD, MPH, said in an interview in advance of the annual meeting of the American College of Rheumatology. “Now, we prove that it specifically helps renal activity as measured by the urine protein. By helping to reduce urine protein, it helps to prevent permanent renal damage and end-stage renal disease.”
The first measure of vitamin D typically occurred in late 2009 or 2010 for existing patients and at the first visit of new patients after that. The researchers categorized patients based on their first measure of vitamin D as less than 20 ng/mL or 20 ng/mL or higher. At the first visit when vitamin D was measured, 27.3% had levels of 25-hydroxyvitamin D less than 20 ng/mL. The mean age of patients was 47.3 years, 92% were female, 50% were white, and 41% were African American.
In the study, Dr. Petri and her associates used the Systemic Lupus International Collaborative Clinics/American College of Rheumatology Damage Index to calculate the risk of lifetime organ damage. After adjusting for age, gender, and ethnicity, low levels of vitamin D were significantly associated with increased risk of renal damage (RR, 1.66; P = .0206) and total organ damage (RR, 1.17; P = .0245), they found.
Skin damage was another concern, with an adjusted relative risk of 1.22, though it was not statistically significant (P = .3561). The investigators observed no association between low vitamin D and musculoskeletal damage, including osteoporotic fractures.
“There is a lot of interest in lupus right now, due to [singer Selena] Gomez’s kidney transplant for lupus nephritis,” said Dr. Petri, who also directs the Johns Hopkins Lupus Center. “So, I think there is interest in how to prevent the need for kidney transplant. Vitamin D helps kidney lupus – and we only need to achieve a level of 40 ng/mL, [which is] safe and easy to do.” She acknowledged the study’s single-center design as a limitation but underscored its large sample size as a strength.
The Hopkins Lupus Cohort is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Petri disclosed having received research support from Anthera, GlaxoSmithKline, EMD Serono, Eli Lilly, Bristol-Myers Squibb, Amgen, United Rheumatology, Global Academy, and Exagen.
SAN DIEGO – , results from a single-center cohort study showed.
“We had previously proved that vitamin D supplementation helped lupus activity,” lead study author Michelle Petri, MD, MPH, said in an interview in advance of the annual meeting of the American College of Rheumatology. “Now, we prove that it specifically helps renal activity as measured by the urine protein. By helping to reduce urine protein, it helps to prevent permanent renal damage and end-stage renal disease.”
The first measure of vitamin D typically occurred in late 2009 or 2010 for existing patients and at the first visit of new patients after that. The researchers categorized patients based on their first measure of vitamin D as less than 20 ng/mL or 20 ng/mL or higher. At the first visit when vitamin D was measured, 27.3% had levels of 25-hydroxyvitamin D less than 20 ng/mL. The mean age of patients was 47.3 years, 92% were female, 50% were white, and 41% were African American.
In the study, Dr. Petri and her associates used the Systemic Lupus International Collaborative Clinics/American College of Rheumatology Damage Index to calculate the risk of lifetime organ damage. After adjusting for age, gender, and ethnicity, low levels of vitamin D were significantly associated with increased risk of renal damage (RR, 1.66; P = .0206) and total organ damage (RR, 1.17; P = .0245), they found.
Skin damage was another concern, with an adjusted relative risk of 1.22, though it was not statistically significant (P = .3561). The investigators observed no association between low vitamin D and musculoskeletal damage, including osteoporotic fractures.
“There is a lot of interest in lupus right now, due to [singer Selena] Gomez’s kidney transplant for lupus nephritis,” said Dr. Petri, who also directs the Johns Hopkins Lupus Center. “So, I think there is interest in how to prevent the need for kidney transplant. Vitamin D helps kidney lupus – and we only need to achieve a level of 40 ng/mL, [which is] safe and easy to do.” She acknowledged the study’s single-center design as a limitation but underscored its large sample size as a strength.
The Hopkins Lupus Cohort is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Petri disclosed having received research support from Anthera, GlaxoSmithKline, EMD Serono, Eli Lilly, Bristol-Myers Squibb, Amgen, United Rheumatology, Global Academy, and Exagen.
AT ACR 2017
Key clinical point: Supplemental vitamin D should be part of the treatment plan for patients with systemic lupus erythematosus (SLE).
Major finding: SLE patients with low vitamin D levels face a significantly increased risk of renal damage (relatve risk, 1.66; P = .0206) and total organ damage (RR, 1.17; P = .0245).
Study details: A single-center cohort study of 1,392 patients with SLE.
Disclosures: The Hopkins Lupus Cohort is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Petri disclosed having received research support from Anthera, GlaxoSmithKline, EMD Serono, Eli Lilly, Bristol-Myers Squibb, Amgen, United Rheumatology, Global Academy, and Exagen.
Methotrexate holiday linked to better flu vaccine immunogenicity
SAN DIEGO – Patients with well-controlled rheumatoid arthritis (RA) fared well during a 2-week holiday from methotrexate after flu vaccination and later showed signs of boosted immunity against the flu in comparison with patients who had not stopped the drug, according to results from a randomized controlled trial.
The research doesn’t confirm that vaccinated patients who take a break from methotrexate actually have lower rates of flu. Still, the findings suggest that brief holidays from methotrexate could be feasible in a variety of situations, such as after vaccinations and prior to surgery, said Jin Kyun Park, MD, of Seoul (South Korea) National University Hospital, lead author of the study presented at the annual meeting of the American College of Rheumatology.
The study notes that RA patients are especially prone to infections for two reasons: dysfunctional immune systems and immunity-weakening treatments. According to Dr. Park, methotrexate reduces the effectiveness of flu vaccines by 15%-20%.
In a previous study, Dr. Park and his colleagues found no statistically significant sign of increased flares in patients who went without methotrexate for 2 weeks before and 2 weeks after vaccination, 4 weeks after vaccination, and 4 weeks before vaccination (Ann Rheum Dis. 2017 Sep;76[9]:1559-65).
The earlier findings also suggested that flu vaccine uptake is highest in those who stop methotrexate after vaccination.
For the new study, a randomized controlled trial, researchers recruited patients with well-controlled RA. They assigned 159 to continue weekly doses of methotrexate after flu vaccination and 161 to stop it for 2 weeks.
The groups in the final analysis (156 and 160 subjects, respectively) were similar – about 85% women, average age of 52-53 years, and about half took glucocorticoids. Their methotrexate dose per week was about 13 mg.
At 4 weeks, just over three-quarters of the patients who had briefly stopped methotrexate showed at least a fourfold increase in hemagglutination inhibition antibody titer against two or more vaccine strains. Of those who continued the medication, just 54.5% showed this level of response, which the researchers considered to be satisfactory.
The researchers reported that there was no appreciable increase in RA disease activity.
Dr. Park cautioned that vaccine titers don’t directly reflect immunoprotection levels. Patients who took a break from methotrexate were less likely to develop a flulike illness, but the difference wasn’t statistically significant.
The research raises questions about whether methotrexate could be stopped a week or two before surgery to lower the risk of infections, Dr. Park said.
Dr. Park said that future research should focus on whether stopping methotrexate briefly affects whether patients go on to develop the flu. He would also like to look at whether a break from the medication will boost the immune response in RA patients who get herpes zoster (shingles) vaccines.
Paul Sufka, MD, of HealthPartners and Regions Hospital in St. Paul, Minn., praised the research. The 2-week break from methotrexate is “a fairly pragmatic approach,” said Dr. Sufka, who moderated a press conference where Dr. Park presented his research.
“You can actually pull this off,” he said, versus telling patients to stop the medication for the 2 weeks before they get vaccinated. He cautioned, however, that “these people have a fairly low disease activity. You may not be able to pull this off with those who have high disease activity.”
Dr. Park and Dr. Sufka reported no relevant disclosures. A study author reported consulting for Pfizer and receiving research grants from Green Cross Corp. and Hanmi Pharmaceutical. The study was funded by Green Cross.
SAN DIEGO – Patients with well-controlled rheumatoid arthritis (RA) fared well during a 2-week holiday from methotrexate after flu vaccination and later showed signs of boosted immunity against the flu in comparison with patients who had not stopped the drug, according to results from a randomized controlled trial.
The research doesn’t confirm that vaccinated patients who take a break from methotrexate actually have lower rates of flu. Still, the findings suggest that brief holidays from methotrexate could be feasible in a variety of situations, such as after vaccinations and prior to surgery, said Jin Kyun Park, MD, of Seoul (South Korea) National University Hospital, lead author of the study presented at the annual meeting of the American College of Rheumatology.
The study notes that RA patients are especially prone to infections for two reasons: dysfunctional immune systems and immunity-weakening treatments. According to Dr. Park, methotrexate reduces the effectiveness of flu vaccines by 15%-20%.
In a previous study, Dr. Park and his colleagues found no statistically significant sign of increased flares in patients who went without methotrexate for 2 weeks before and 2 weeks after vaccination, 4 weeks after vaccination, and 4 weeks before vaccination (Ann Rheum Dis. 2017 Sep;76[9]:1559-65).
The earlier findings also suggested that flu vaccine uptake is highest in those who stop methotrexate after vaccination.
For the new study, a randomized controlled trial, researchers recruited patients with well-controlled RA. They assigned 159 to continue weekly doses of methotrexate after flu vaccination and 161 to stop it for 2 weeks.
The groups in the final analysis (156 and 160 subjects, respectively) were similar – about 85% women, average age of 52-53 years, and about half took glucocorticoids. Their methotrexate dose per week was about 13 mg.
At 4 weeks, just over three-quarters of the patients who had briefly stopped methotrexate showed at least a fourfold increase in hemagglutination inhibition antibody titer against two or more vaccine strains. Of those who continued the medication, just 54.5% showed this level of response, which the researchers considered to be satisfactory.
The researchers reported that there was no appreciable increase in RA disease activity.
Dr. Park cautioned that vaccine titers don’t directly reflect immunoprotection levels. Patients who took a break from methotrexate were less likely to develop a flulike illness, but the difference wasn’t statistically significant.
The research raises questions about whether methotrexate could be stopped a week or two before surgery to lower the risk of infections, Dr. Park said.
Dr. Park said that future research should focus on whether stopping methotrexate briefly affects whether patients go on to develop the flu. He would also like to look at whether a break from the medication will boost the immune response in RA patients who get herpes zoster (shingles) vaccines.
Paul Sufka, MD, of HealthPartners and Regions Hospital in St. Paul, Minn., praised the research. The 2-week break from methotrexate is “a fairly pragmatic approach,” said Dr. Sufka, who moderated a press conference where Dr. Park presented his research.
“You can actually pull this off,” he said, versus telling patients to stop the medication for the 2 weeks before they get vaccinated. He cautioned, however, that “these people have a fairly low disease activity. You may not be able to pull this off with those who have high disease activity.”
Dr. Park and Dr. Sufka reported no relevant disclosures. A study author reported consulting for Pfizer and receiving research grants from Green Cross Corp. and Hanmi Pharmaceutical. The study was funded by Green Cross.
SAN DIEGO – Patients with well-controlled rheumatoid arthritis (RA) fared well during a 2-week holiday from methotrexate after flu vaccination and later showed signs of boosted immunity against the flu in comparison with patients who had not stopped the drug, according to results from a randomized controlled trial.
The research doesn’t confirm that vaccinated patients who take a break from methotrexate actually have lower rates of flu. Still, the findings suggest that brief holidays from methotrexate could be feasible in a variety of situations, such as after vaccinations and prior to surgery, said Jin Kyun Park, MD, of Seoul (South Korea) National University Hospital, lead author of the study presented at the annual meeting of the American College of Rheumatology.
The study notes that RA patients are especially prone to infections for two reasons: dysfunctional immune systems and immunity-weakening treatments. According to Dr. Park, methotrexate reduces the effectiveness of flu vaccines by 15%-20%.
In a previous study, Dr. Park and his colleagues found no statistically significant sign of increased flares in patients who went without methotrexate for 2 weeks before and 2 weeks after vaccination, 4 weeks after vaccination, and 4 weeks before vaccination (Ann Rheum Dis. 2017 Sep;76[9]:1559-65).
The earlier findings also suggested that flu vaccine uptake is highest in those who stop methotrexate after vaccination.
For the new study, a randomized controlled trial, researchers recruited patients with well-controlled RA. They assigned 159 to continue weekly doses of methotrexate after flu vaccination and 161 to stop it for 2 weeks.
The groups in the final analysis (156 and 160 subjects, respectively) were similar – about 85% women, average age of 52-53 years, and about half took glucocorticoids. Their methotrexate dose per week was about 13 mg.
At 4 weeks, just over three-quarters of the patients who had briefly stopped methotrexate showed at least a fourfold increase in hemagglutination inhibition antibody titer against two or more vaccine strains. Of those who continued the medication, just 54.5% showed this level of response, which the researchers considered to be satisfactory.
The researchers reported that there was no appreciable increase in RA disease activity.
Dr. Park cautioned that vaccine titers don’t directly reflect immunoprotection levels. Patients who took a break from methotrexate were less likely to develop a flulike illness, but the difference wasn’t statistically significant.
The research raises questions about whether methotrexate could be stopped a week or two before surgery to lower the risk of infections, Dr. Park said.
Dr. Park said that future research should focus on whether stopping methotrexate briefly affects whether patients go on to develop the flu. He would also like to look at whether a break from the medication will boost the immune response in RA patients who get herpes zoster (shingles) vaccines.
Paul Sufka, MD, of HealthPartners and Regions Hospital in St. Paul, Minn., praised the research. The 2-week break from methotrexate is “a fairly pragmatic approach,” said Dr. Sufka, who moderated a press conference where Dr. Park presented his research.
“You can actually pull this off,” he said, versus telling patients to stop the medication for the 2 weeks before they get vaccinated. He cautioned, however, that “these people have a fairly low disease activity. You may not be able to pull this off with those who have high disease activity.”
Dr. Park and Dr. Sufka reported no relevant disclosures. A study author reported consulting for Pfizer and receiving research grants from Green Cross Corp. and Hanmi Pharmaceutical. The study was funded by Green Cross.
AT ACR 2017
Key clinical point:
Major finding: More than three-quarters of patients who had briefly stopped methotrexate and 54.5% of patients who kept using methotrexate showed at least a fourfold increase in hemagglutination inhibition antibody titer against two or more vaccine strains at 4 weeks.
Data source: A randomized controlled trial of 320 patients with RA who were taking methotrexate.
Disclosures: The study was funded by Green Cross Corp. The presenter reported no relevant disclosures. A study author reported consulting for Pfizer and receiving research grants from Green Cross and Hanmi Pharmaceutical.