User login
Get ready for cancer immunotherapy-induced rheumatic diseases
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Seven shortcuts help with diagnosis of CNS vasculitis
SNOWMASS, COLO. – A lumbar puncture is indispensable when entertaining the diagnosis of primary angiitis of the CNS, Leonard H. Calabrese, DO, declared at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“There’s never an excuse short of an absolute surgical contraindication for not doing a lumbar puncture. It’s amazing to me how often this heuristic is overlooked. Virtually all patients with biopsy-proven CNS vasculitis have an inflammatory spinal fluid. This gets you into the club. I always have great unease when I’m seeing a patient – no matter what else I’m seeing that suggests this condition – if the spinal fluid is totally pristine. This does not happen very often at all,” said Dr. Calabrese, professor of medicine and vice chairman of the department of rheumatic and immunological diseases at the Cleveland Clinic in Ohio.
“Not many of us in rheumatology take care of these patients on a regular basis, but the question of whether a patient has CNS vasculitis is actually pretty common. If you do any kind of hospital consultation work, you’ll get called onto the neurology unit to evaluate an obtunded patient with multiple strokes,” he observed.
Neurologists know a lot more about the brain, yet they often seek input from rheumatologists, who are typically much more familiar with the heavy-hitting drugs used in treating PACNS.
In an influential paper published nearly 3 decades ago, Dr. Calabrese and a colleague proposed diagnostic criteria for PACNS which still hold up today. They defined the disorder as a neurologic deficit that remains unexplained after a vigorous diagnostic work-up accompanied by either a high-probability angiogram for vasculitis or biopsy evidence of CNS vasculitis along with exclusion of all conditions capable of either mimicking the angiographic features of arteritis or producing secondary arteritis (Medicine [Baltimore]. 1988 Jan;67[1]:20-39).
Mimickers of PACNS include systemic inflammatory conditions such as Sjögren’s, systemic vasculitis, sarcoidosis, and paraneoplastic conditions, all of which a rheumatologist can typically rule out at the bedside. Other mimickers include coagulation disorders, infections, demyelinating disorders, CNS lymphoma, reversible cerebral vasoconstriction syndromes, and an ever-expanding list of genetic disorders.
“There is no one in the world who’s an expert on all these diseases, so it’s very important for us to work interprofessionally,” the rheumatologist stressed.
At the Snowmass meeting, Dr. Calabrese presented his seven heuristics – that is, loosely defined rules or mental shortcuts – for getting the diagnosis right.
• PACNS can never be securely diagnosed based solely on clinical findings
The findings with the highest pretest probability of PACNS are chronic meningitis for more than 3 weeks, multiple strokes, or unexplained strokes with poststroke cognitive impairment.
Less specific findings in patients with PACNS may include headaches, behavioral changes, encephalopathy, focal sensorimotor abnormalities, ataxia, scotoma and other visual changes, radiculopathy, and myopathy.
• Don’t skip the lumbar puncture
The cerebrospinal fluid (CSF) is abnormal in more than 95% of patients with PACNS. The findings usually are consistent with aseptic meningitis, with modest pleocytosis, elevated protein levels, and a normal glucose.
• Nonvascular imaging is highly sensitive but has low specificity for PACNS
Thus, nonvascular imaging can’t confirm the diagnosis.
“I don’t believe patients can have this diagnosis with a normal MRI with gadolinium enhancement and diffusion-weighted imaging. With true vascular inflammation and parenchymal destruction, something will be seen. So in a patient who has a headache and can’t think properly but has a pristine MRI, it’s probably not this disease, and it’s time to move along,” according to the rheumatologist.
• No angiographic study has 100% specificity for the diagnosis of PACNS
“No one can tell you ‘This is vasculitis’ from an angiogram, just like no one can tell you an abnormal chest x-ray is always pneumonia. While an angiogram can be very, very suggestive, the specificity drops off in small-vessel disease,” Dr. Calabrese said.
• Don’t fear brain biopsy
Brain biopsy is clearly underutilized. It’s a valuable yet imperfect diagnostic tool.
“A well-done biopsy by a good neurosurgeon who’s interested in CNS vasculitis and works interprofessionally probably has greater than 80% sensitivity and 90%-100% specificity for PACNS,” according to Dr. Calabrese.
The brain biopsy is not only helpful in ruling in the diagnosis of PACNS, it’s also an excellent tool for identifying rule outs. In one study, mimickers of PACNS were identified by brain biopsy in 39% of patients.
“You find something else about 40% of the time – and that’s a good thing,” he said.
Physicians who have difficulty getting a patient or family to okay a brain biopsy are generally going about it wrong, Dr. Calabrese continued.
“I can’t think of a single instance in all my years of practice where a patient has refused a brain biopsy after we’ve engaged in a shared decision-making process. Why? Because I tell them I think it’s important. This is a grave diagnosis with a tremendous impact for the patient. It involves serious therapies and a guarded prognosis,” he explained.
“Often the prospect of brain biopsy is presented to the patient as just the worst thing in the world that could happen to them: ‘They’ll take a piece of your brain. You’ll lose your piano lessons.’ When actually there’s good evidence that biopsies taken in the absence of brain edema involve minimal morbidity and virtually no mortality,” he noted.
• Mind the must-rule-outs
“Ask yourself,” Dr. Calabrese said, “‘What’s the worst thing that could happen here if I goof up this diagnosis?’”
The answer is the worst that can happen is that a CNS infection or malignancy gets misdiagnosed as PACNS. Those are the two must-rule-outs: infection – be it viral, tuberculosis, fungal, syphilis, bacteria, parasites, or Rickettsia – and malignancy.
“Malignancies can be insanely complex. Five percent of solid tumors will have leptomeningeal metastasis and present with chronic meningitis; that’s always goofing us up,” Dr. Calabrese said.
Intravascular CNS lymphoma is an important mimicker of PACNS. The affected patient may have headaches, punctate infarctions upon imaging, an abnormal CSF, and a mildly abnormal angiogram. The only way to distinguish it from PACNS is by brain biopsy.
“CNS lymphomas are always angiocentric, so unless you’ve got a really good pathologist and a really good biopsy specimen you may goof this up,” Dr. Calabrese cautioned.
• Failure to respond to cytotoxic agents and glucocorticoids suggests an alternative diagnosis, not refractory disease
It’s very unusual for a patient with PACNS to fail a robust course of cyclophosphamide or methotrexate plus steroids. This is a red flag situation warranting a pause to reconsider the diagnosis.
Other red flags commonly encountered by a consulting rheumatologists are that a neurologist diagnosed PACNS in the absence of a lumbar puncture, or on the basis of angiographic findings with a normal CSF.
Dr. Calabrese reported having no financial conflicts of interest.
SNOWMASS, COLO. – A lumbar puncture is indispensable when entertaining the diagnosis of primary angiitis of the CNS, Leonard H. Calabrese, DO, declared at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“There’s never an excuse short of an absolute surgical contraindication for not doing a lumbar puncture. It’s amazing to me how often this heuristic is overlooked. Virtually all patients with biopsy-proven CNS vasculitis have an inflammatory spinal fluid. This gets you into the club. I always have great unease when I’m seeing a patient – no matter what else I’m seeing that suggests this condition – if the spinal fluid is totally pristine. This does not happen very often at all,” said Dr. Calabrese, professor of medicine and vice chairman of the department of rheumatic and immunological diseases at the Cleveland Clinic in Ohio.
“Not many of us in rheumatology take care of these patients on a regular basis, but the question of whether a patient has CNS vasculitis is actually pretty common. If you do any kind of hospital consultation work, you’ll get called onto the neurology unit to evaluate an obtunded patient with multiple strokes,” he observed.
Neurologists know a lot more about the brain, yet they often seek input from rheumatologists, who are typically much more familiar with the heavy-hitting drugs used in treating PACNS.
In an influential paper published nearly 3 decades ago, Dr. Calabrese and a colleague proposed diagnostic criteria for PACNS which still hold up today. They defined the disorder as a neurologic deficit that remains unexplained after a vigorous diagnostic work-up accompanied by either a high-probability angiogram for vasculitis or biopsy evidence of CNS vasculitis along with exclusion of all conditions capable of either mimicking the angiographic features of arteritis or producing secondary arteritis (Medicine [Baltimore]. 1988 Jan;67[1]:20-39).
Mimickers of PACNS include systemic inflammatory conditions such as Sjögren’s, systemic vasculitis, sarcoidosis, and paraneoplastic conditions, all of which a rheumatologist can typically rule out at the bedside. Other mimickers include coagulation disorders, infections, demyelinating disorders, CNS lymphoma, reversible cerebral vasoconstriction syndromes, and an ever-expanding list of genetic disorders.
“There is no one in the world who’s an expert on all these diseases, so it’s very important for us to work interprofessionally,” the rheumatologist stressed.
At the Snowmass meeting, Dr. Calabrese presented his seven heuristics – that is, loosely defined rules or mental shortcuts – for getting the diagnosis right.
• PACNS can never be securely diagnosed based solely on clinical findings
The findings with the highest pretest probability of PACNS are chronic meningitis for more than 3 weeks, multiple strokes, or unexplained strokes with poststroke cognitive impairment.
Less specific findings in patients with PACNS may include headaches, behavioral changes, encephalopathy, focal sensorimotor abnormalities, ataxia, scotoma and other visual changes, radiculopathy, and myopathy.
• Don’t skip the lumbar puncture
The cerebrospinal fluid (CSF) is abnormal in more than 95% of patients with PACNS. The findings usually are consistent with aseptic meningitis, with modest pleocytosis, elevated protein levels, and a normal glucose.
• Nonvascular imaging is highly sensitive but has low specificity for PACNS
Thus, nonvascular imaging can’t confirm the diagnosis.
“I don’t believe patients can have this diagnosis with a normal MRI with gadolinium enhancement and diffusion-weighted imaging. With true vascular inflammation and parenchymal destruction, something will be seen. So in a patient who has a headache and can’t think properly but has a pristine MRI, it’s probably not this disease, and it’s time to move along,” according to the rheumatologist.
• No angiographic study has 100% specificity for the diagnosis of PACNS
“No one can tell you ‘This is vasculitis’ from an angiogram, just like no one can tell you an abnormal chest x-ray is always pneumonia. While an angiogram can be very, very suggestive, the specificity drops off in small-vessel disease,” Dr. Calabrese said.
• Don’t fear brain biopsy
Brain biopsy is clearly underutilized. It’s a valuable yet imperfect diagnostic tool.
“A well-done biopsy by a good neurosurgeon who’s interested in CNS vasculitis and works interprofessionally probably has greater than 80% sensitivity and 90%-100% specificity for PACNS,” according to Dr. Calabrese.
The brain biopsy is not only helpful in ruling in the diagnosis of PACNS, it’s also an excellent tool for identifying rule outs. In one study, mimickers of PACNS were identified by brain biopsy in 39% of patients.
“You find something else about 40% of the time – and that’s a good thing,” he said.
Physicians who have difficulty getting a patient or family to okay a brain biopsy are generally going about it wrong, Dr. Calabrese continued.
“I can’t think of a single instance in all my years of practice where a patient has refused a brain biopsy after we’ve engaged in a shared decision-making process. Why? Because I tell them I think it’s important. This is a grave diagnosis with a tremendous impact for the patient. It involves serious therapies and a guarded prognosis,” he explained.
“Often the prospect of brain biopsy is presented to the patient as just the worst thing in the world that could happen to them: ‘They’ll take a piece of your brain. You’ll lose your piano lessons.’ When actually there’s good evidence that biopsies taken in the absence of brain edema involve minimal morbidity and virtually no mortality,” he noted.
• Mind the must-rule-outs
“Ask yourself,” Dr. Calabrese said, “‘What’s the worst thing that could happen here if I goof up this diagnosis?’”
The answer is the worst that can happen is that a CNS infection or malignancy gets misdiagnosed as PACNS. Those are the two must-rule-outs: infection – be it viral, tuberculosis, fungal, syphilis, bacteria, parasites, or Rickettsia – and malignancy.
“Malignancies can be insanely complex. Five percent of solid tumors will have leptomeningeal metastasis and present with chronic meningitis; that’s always goofing us up,” Dr. Calabrese said.
Intravascular CNS lymphoma is an important mimicker of PACNS. The affected patient may have headaches, punctate infarctions upon imaging, an abnormal CSF, and a mildly abnormal angiogram. The only way to distinguish it from PACNS is by brain biopsy.
“CNS lymphomas are always angiocentric, so unless you’ve got a really good pathologist and a really good biopsy specimen you may goof this up,” Dr. Calabrese cautioned.
• Failure to respond to cytotoxic agents and glucocorticoids suggests an alternative diagnosis, not refractory disease
It’s very unusual for a patient with PACNS to fail a robust course of cyclophosphamide or methotrexate plus steroids. This is a red flag situation warranting a pause to reconsider the diagnosis.
Other red flags commonly encountered by a consulting rheumatologists are that a neurologist diagnosed PACNS in the absence of a lumbar puncture, or on the basis of angiographic findings with a normal CSF.
Dr. Calabrese reported having no financial conflicts of interest.
SNOWMASS, COLO. – A lumbar puncture is indispensable when entertaining the diagnosis of primary angiitis of the CNS, Leonard H. Calabrese, DO, declared at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“There’s never an excuse short of an absolute surgical contraindication for not doing a lumbar puncture. It’s amazing to me how often this heuristic is overlooked. Virtually all patients with biopsy-proven CNS vasculitis have an inflammatory spinal fluid. This gets you into the club. I always have great unease when I’m seeing a patient – no matter what else I’m seeing that suggests this condition – if the spinal fluid is totally pristine. This does not happen very often at all,” said Dr. Calabrese, professor of medicine and vice chairman of the department of rheumatic and immunological diseases at the Cleveland Clinic in Ohio.
“Not many of us in rheumatology take care of these patients on a regular basis, but the question of whether a patient has CNS vasculitis is actually pretty common. If you do any kind of hospital consultation work, you’ll get called onto the neurology unit to evaluate an obtunded patient with multiple strokes,” he observed.
Neurologists know a lot more about the brain, yet they often seek input from rheumatologists, who are typically much more familiar with the heavy-hitting drugs used in treating PACNS.
In an influential paper published nearly 3 decades ago, Dr. Calabrese and a colleague proposed diagnostic criteria for PACNS which still hold up today. They defined the disorder as a neurologic deficit that remains unexplained after a vigorous diagnostic work-up accompanied by either a high-probability angiogram for vasculitis or biopsy evidence of CNS vasculitis along with exclusion of all conditions capable of either mimicking the angiographic features of arteritis or producing secondary arteritis (Medicine [Baltimore]. 1988 Jan;67[1]:20-39).
Mimickers of PACNS include systemic inflammatory conditions such as Sjögren’s, systemic vasculitis, sarcoidosis, and paraneoplastic conditions, all of which a rheumatologist can typically rule out at the bedside. Other mimickers include coagulation disorders, infections, demyelinating disorders, CNS lymphoma, reversible cerebral vasoconstriction syndromes, and an ever-expanding list of genetic disorders.
“There is no one in the world who’s an expert on all these diseases, so it’s very important for us to work interprofessionally,” the rheumatologist stressed.
At the Snowmass meeting, Dr. Calabrese presented his seven heuristics – that is, loosely defined rules or mental shortcuts – for getting the diagnosis right.
• PACNS can never be securely diagnosed based solely on clinical findings
The findings with the highest pretest probability of PACNS are chronic meningitis for more than 3 weeks, multiple strokes, or unexplained strokes with poststroke cognitive impairment.
Less specific findings in patients with PACNS may include headaches, behavioral changes, encephalopathy, focal sensorimotor abnormalities, ataxia, scotoma and other visual changes, radiculopathy, and myopathy.
• Don’t skip the lumbar puncture
The cerebrospinal fluid (CSF) is abnormal in more than 95% of patients with PACNS. The findings usually are consistent with aseptic meningitis, with modest pleocytosis, elevated protein levels, and a normal glucose.
• Nonvascular imaging is highly sensitive but has low specificity for PACNS
Thus, nonvascular imaging can’t confirm the diagnosis.
“I don’t believe patients can have this diagnosis with a normal MRI with gadolinium enhancement and diffusion-weighted imaging. With true vascular inflammation and parenchymal destruction, something will be seen. So in a patient who has a headache and can’t think properly but has a pristine MRI, it’s probably not this disease, and it’s time to move along,” according to the rheumatologist.
• No angiographic study has 100% specificity for the diagnosis of PACNS
“No one can tell you ‘This is vasculitis’ from an angiogram, just like no one can tell you an abnormal chest x-ray is always pneumonia. While an angiogram can be very, very suggestive, the specificity drops off in small-vessel disease,” Dr. Calabrese said.
• Don’t fear brain biopsy
Brain biopsy is clearly underutilized. It’s a valuable yet imperfect diagnostic tool.
“A well-done biopsy by a good neurosurgeon who’s interested in CNS vasculitis and works interprofessionally probably has greater than 80% sensitivity and 90%-100% specificity for PACNS,” according to Dr. Calabrese.
The brain biopsy is not only helpful in ruling in the diagnosis of PACNS, it’s also an excellent tool for identifying rule outs. In one study, mimickers of PACNS were identified by brain biopsy in 39% of patients.
“You find something else about 40% of the time – and that’s a good thing,” he said.
Physicians who have difficulty getting a patient or family to okay a brain biopsy are generally going about it wrong, Dr. Calabrese continued.
“I can’t think of a single instance in all my years of practice where a patient has refused a brain biopsy after we’ve engaged in a shared decision-making process. Why? Because I tell them I think it’s important. This is a grave diagnosis with a tremendous impact for the patient. It involves serious therapies and a guarded prognosis,” he explained.
“Often the prospect of brain biopsy is presented to the patient as just the worst thing in the world that could happen to them: ‘They’ll take a piece of your brain. You’ll lose your piano lessons.’ When actually there’s good evidence that biopsies taken in the absence of brain edema involve minimal morbidity and virtually no mortality,” he noted.
• Mind the must-rule-outs
“Ask yourself,” Dr. Calabrese said, “‘What’s the worst thing that could happen here if I goof up this diagnosis?’”
The answer is the worst that can happen is that a CNS infection or malignancy gets misdiagnosed as PACNS. Those are the two must-rule-outs: infection – be it viral, tuberculosis, fungal, syphilis, bacteria, parasites, or Rickettsia – and malignancy.
“Malignancies can be insanely complex. Five percent of solid tumors will have leptomeningeal metastasis and present with chronic meningitis; that’s always goofing us up,” Dr. Calabrese said.
Intravascular CNS lymphoma is an important mimicker of PACNS. The affected patient may have headaches, punctate infarctions upon imaging, an abnormal CSF, and a mildly abnormal angiogram. The only way to distinguish it from PACNS is by brain biopsy.
“CNS lymphomas are always angiocentric, so unless you’ve got a really good pathologist and a really good biopsy specimen you may goof this up,” Dr. Calabrese cautioned.
• Failure to respond to cytotoxic agents and glucocorticoids suggests an alternative diagnosis, not refractory disease
It’s very unusual for a patient with PACNS to fail a robust course of cyclophosphamide or methotrexate plus steroids. This is a red flag situation warranting a pause to reconsider the diagnosis.
Other red flags commonly encountered by a consulting rheumatologists are that a neurologist diagnosed PACNS in the absence of a lumbar puncture, or on the basis of angiographic findings with a normal CSF.
Dr. Calabrese reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
U.S. chikungunya epidemic would likely put rheumatologists on front line
SNOWMASS, COLO. – The continental United States is vulnerable to an epidemic of chikungunya virus disease, an event which would have profound consequences for rheumatologists, Robert T. Schoen, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We certainly have all the factors in place where we could have a major epidemic of chikungunya, particularly in Florida, Texas, and neighboring states. As rheumatologists, I think we can own this infection if we want to because it causes an arthritis that is a true arthritis in a significant percentage of patients,” said Dr. Schoen, a rheumatologist at Yale University in New Haven, Conn.
“That’s a major impact when you think that these epidemics affect tens of thousands of individuals. If 25% of them develop long-term arthritis disability, that’s a whole new world for us,” the rheumatologist observed.
Chikungunya is a single-stranded RNA virus in the togaviridae family, which also contains rubella. The infection is transmitted by Aedes aegypti and A. albopictus, also known as the yellow fever and Asian tiger mosquitoes, respectively. Both mosquitoes are established inhabitants of much of the United States.
“This infection is a one-bite deal. These are very aggressive mosquitoes,” Dr. Schoen observed. “If you haven’t seen a case of chikungunya yet, you will soon,” he added.
In 2014, at the height of a massive Caribbean epidemic which included half a million cases in Puerto Rico, roughly 2,800 cases of chikungunya were imported into 46 U.S. states. In 2015, however, as the Caribbean epidemic waned and herd immunity developed, that figure fell to 653 imported cases. But the epidemic in India, which began in 2008, remains ongoing with no end in sight. Several of Dr. Schoen’s patients with chikungunya had recently returned from India when they first became ill.
“India provides an unlimited reservoir of immunologically naive patients to perpetuate the infection,” he observed.
Course of illness
The rate of asymptomatic infection has been variously estimated at 3%-25%. Symptomatic chikungunya is a biphasic illness. After a 2- to 6-day incubation period, patients develop rapid-onset fever with severe joint pain and muscle aches. Indeed, chikungunya means “bent over” in Makonde, an African Bantu language.
Roughly 60% of patients develop a rash during the acute febrile phase of the illness, which lasts about a week. The dermatitis is most often a maculopapular rash on the trunk which is “absolutely indistinguishable” from the rash caused by Zika virus infection, transmitted by the same vectors, Dr. Schoen noted.
During this acute phase, almost all patients develop a severe polyarthritis which can last for weeks or, less commonly, for months or years. This polyarthritis mimics seronegative rheumatoid arthritis. It is usually symmetric and often affects the hands, wrists, and feet.
The acute febrile phase is characterized by high levels of viremia, with up to 1 billion viral particles per milliliter of blood. During this period, definitive diagnosis of chikungunya can be made through reverse transcriptase–polymerase chain reaction testing or viral culture.
Typically, though, patients make their way to a rheumatologist only well after the viremic period is over. In that situation, the diagnostic mainstay is serologic testing. Antichikungunya virus Immunoglobulin M is detectable starting on about day 5 after symptom onset, and it persists for the next 1-3 months. Immunoglobulin G (IgG) antibodies become detectable in the same time frame and remain elevated for years.
Dr. Schoen highlighted a small but intriguing study from Singapore that suggests that the early appearance of chikungunya-specific neutralizing IgG3–antibodies may constitute a marker for favorable long-term prognosis. The observational study involved 30 patients hospitalized for severe acute chikungunya infection. The investigators classified them into two groups: 14 patients had low levels of acute viremia, a less severe acute illness, and late development of IgG3 antibodies; the other 16 had a high initial viral load, a more severe acute phase, and a rapid IgG3 and interleukin-6 response to infection.
The patients with a robust early IgG3 response cleared the virus faster and none of them developed chronic arthritis. In contrast, those without early, chikungunya-specific IgG3 had a high rate of persistent arthralgia (J Infect Dis. 2012 Apr 1;205[7]:1147-54).
Treatment
No evidence-based treatment for chikungunya exists. Treatment in the acute phase is primarily supportive care. Rheumatologists on the Caribbean island of Martinique have reported a favorable experience using methotrexate at doses up to 25 mg/week or with standard doses of anti–tumor necrosis factor agents in chikungunya patients with severe bilateral symmetric chronic inflammatory joint disease arising during the 2013-2015 epidemic. The treatment response and tolerability were comparable with the results typically obtained in rheumatoid arthritis patients, according to the rheumatologists (Arthritis Rheumatol. 2016 Nov;68[11]:2817-24).
Dr. Schoen found the report from Martinique to be reassuring. He too has resorted to familiar rheumatologic medications in his severely affected patients. Anecdotally, his greatest success involved a patient with a 5-year history of disabling chikungunya polyarthritis who showed marked improvement after several months on hydroxychloroquine.
The Martinique investigators also reported on 22 patients who developed chikungunya while on biologic agents for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, or systemic lupus erythematosus. The biologics weren’t protective against the viral disease in these patients. All 22 of them developed severe acute chikungunya polyarthritis with a mean swollen joint count of 9.6 (Joint Bone Spine. 2016 Mar;83[2]:245-6).
Infection during pregnancy
There is a legitimate concern about chikungunya infection occurring during pregnancy, but, based on the experiences documented on Reunion Island, the risk to the baby is confined to intrapartum exposure in a viremic mother.
Reunion Island is a French region in the Indian Ocean off the eastern coast of Africa. It has first-world medical care. Much of the best documented early work on chikungunya outbreaks grew out of the 2005-2006 epidemic there, which affected more than one-third of the island’s 800,000-plus residents.
In a prospective study of 7,504 deliveries on the island during a 22-month period, mother-to-child transmission of chikungunya occurred only in the context of intrapartum viremia, with 19 cases of vertical transmission occurring among 39 affected mothers who delivered at a median 38 weeks of gestation. Cesarean section had no protective effect. All 19 infected neonates were asymptomatic at birth, with median onset of pain, fever, and thrombocytopenia on day 4. Nine of the 19 infected neonates developed encephalopathy with pathologic MRI findings, including cerebral hemorrhage in two cases (PLoS Med. 2008 Mar 18;5[3]:e60).
The case fatality rate for chikungunya is low at 0.1%. Most deaths occur in young children or elderly individuals with major comorbid conditions. Despite this low mortality, interest in chikungunya vaccine development is being driven by the infection’s impact on tourism, its spread to temperate climates, the economic impact of the considerable time lost from work, and military need. A promising attenuated, virus-like, particle-based vaccine is currently in phase II testing in the Caribbean.
Dr. Schoen reported having no financial conflicts.
SNOWMASS, COLO. – The continental United States is vulnerable to an epidemic of chikungunya virus disease, an event which would have profound consequences for rheumatologists, Robert T. Schoen, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We certainly have all the factors in place where we could have a major epidemic of chikungunya, particularly in Florida, Texas, and neighboring states. As rheumatologists, I think we can own this infection if we want to because it causes an arthritis that is a true arthritis in a significant percentage of patients,” said Dr. Schoen, a rheumatologist at Yale University in New Haven, Conn.
“That’s a major impact when you think that these epidemics affect tens of thousands of individuals. If 25% of them develop long-term arthritis disability, that’s a whole new world for us,” the rheumatologist observed.
Chikungunya is a single-stranded RNA virus in the togaviridae family, which also contains rubella. The infection is transmitted by Aedes aegypti and A. albopictus, also known as the yellow fever and Asian tiger mosquitoes, respectively. Both mosquitoes are established inhabitants of much of the United States.
“This infection is a one-bite deal. These are very aggressive mosquitoes,” Dr. Schoen observed. “If you haven’t seen a case of chikungunya yet, you will soon,” he added.
In 2014, at the height of a massive Caribbean epidemic which included half a million cases in Puerto Rico, roughly 2,800 cases of chikungunya were imported into 46 U.S. states. In 2015, however, as the Caribbean epidemic waned and herd immunity developed, that figure fell to 653 imported cases. But the epidemic in India, which began in 2008, remains ongoing with no end in sight. Several of Dr. Schoen’s patients with chikungunya had recently returned from India when they first became ill.
“India provides an unlimited reservoir of immunologically naive patients to perpetuate the infection,” he observed.
Course of illness
The rate of asymptomatic infection has been variously estimated at 3%-25%. Symptomatic chikungunya is a biphasic illness. After a 2- to 6-day incubation period, patients develop rapid-onset fever with severe joint pain and muscle aches. Indeed, chikungunya means “bent over” in Makonde, an African Bantu language.
Roughly 60% of patients develop a rash during the acute febrile phase of the illness, which lasts about a week. The dermatitis is most often a maculopapular rash on the trunk which is “absolutely indistinguishable” from the rash caused by Zika virus infection, transmitted by the same vectors, Dr. Schoen noted.
During this acute phase, almost all patients develop a severe polyarthritis which can last for weeks or, less commonly, for months or years. This polyarthritis mimics seronegative rheumatoid arthritis. It is usually symmetric and often affects the hands, wrists, and feet.
The acute febrile phase is characterized by high levels of viremia, with up to 1 billion viral particles per milliliter of blood. During this period, definitive diagnosis of chikungunya can be made through reverse transcriptase–polymerase chain reaction testing or viral culture.
Typically, though, patients make their way to a rheumatologist only well after the viremic period is over. In that situation, the diagnostic mainstay is serologic testing. Antichikungunya virus Immunoglobulin M is detectable starting on about day 5 after symptom onset, and it persists for the next 1-3 months. Immunoglobulin G (IgG) antibodies become detectable in the same time frame and remain elevated for years.
Dr. Schoen highlighted a small but intriguing study from Singapore that suggests that the early appearance of chikungunya-specific neutralizing IgG3–antibodies may constitute a marker for favorable long-term prognosis. The observational study involved 30 patients hospitalized for severe acute chikungunya infection. The investigators classified them into two groups: 14 patients had low levels of acute viremia, a less severe acute illness, and late development of IgG3 antibodies; the other 16 had a high initial viral load, a more severe acute phase, and a rapid IgG3 and interleukin-6 response to infection.
The patients with a robust early IgG3 response cleared the virus faster and none of them developed chronic arthritis. In contrast, those without early, chikungunya-specific IgG3 had a high rate of persistent arthralgia (J Infect Dis. 2012 Apr 1;205[7]:1147-54).
Treatment
No evidence-based treatment for chikungunya exists. Treatment in the acute phase is primarily supportive care. Rheumatologists on the Caribbean island of Martinique have reported a favorable experience using methotrexate at doses up to 25 mg/week or with standard doses of anti–tumor necrosis factor agents in chikungunya patients with severe bilateral symmetric chronic inflammatory joint disease arising during the 2013-2015 epidemic. The treatment response and tolerability were comparable with the results typically obtained in rheumatoid arthritis patients, according to the rheumatologists (Arthritis Rheumatol. 2016 Nov;68[11]:2817-24).
Dr. Schoen found the report from Martinique to be reassuring. He too has resorted to familiar rheumatologic medications in his severely affected patients. Anecdotally, his greatest success involved a patient with a 5-year history of disabling chikungunya polyarthritis who showed marked improvement after several months on hydroxychloroquine.
The Martinique investigators also reported on 22 patients who developed chikungunya while on biologic agents for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, or systemic lupus erythematosus. The biologics weren’t protective against the viral disease in these patients. All 22 of them developed severe acute chikungunya polyarthritis with a mean swollen joint count of 9.6 (Joint Bone Spine. 2016 Mar;83[2]:245-6).
Infection during pregnancy
There is a legitimate concern about chikungunya infection occurring during pregnancy, but, based on the experiences documented on Reunion Island, the risk to the baby is confined to intrapartum exposure in a viremic mother.
Reunion Island is a French region in the Indian Ocean off the eastern coast of Africa. It has first-world medical care. Much of the best documented early work on chikungunya outbreaks grew out of the 2005-2006 epidemic there, which affected more than one-third of the island’s 800,000-plus residents.
In a prospective study of 7,504 deliveries on the island during a 22-month period, mother-to-child transmission of chikungunya occurred only in the context of intrapartum viremia, with 19 cases of vertical transmission occurring among 39 affected mothers who delivered at a median 38 weeks of gestation. Cesarean section had no protective effect. All 19 infected neonates were asymptomatic at birth, with median onset of pain, fever, and thrombocytopenia on day 4. Nine of the 19 infected neonates developed encephalopathy with pathologic MRI findings, including cerebral hemorrhage in two cases (PLoS Med. 2008 Mar 18;5[3]:e60).
The case fatality rate for chikungunya is low at 0.1%. Most deaths occur in young children or elderly individuals with major comorbid conditions. Despite this low mortality, interest in chikungunya vaccine development is being driven by the infection’s impact on tourism, its spread to temperate climates, the economic impact of the considerable time lost from work, and military need. A promising attenuated, virus-like, particle-based vaccine is currently in phase II testing in the Caribbean.
Dr. Schoen reported having no financial conflicts.
SNOWMASS, COLO. – The continental United States is vulnerable to an epidemic of chikungunya virus disease, an event which would have profound consequences for rheumatologists, Robert T. Schoen, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We certainly have all the factors in place where we could have a major epidemic of chikungunya, particularly in Florida, Texas, and neighboring states. As rheumatologists, I think we can own this infection if we want to because it causes an arthritis that is a true arthritis in a significant percentage of patients,” said Dr. Schoen, a rheumatologist at Yale University in New Haven, Conn.
“That’s a major impact when you think that these epidemics affect tens of thousands of individuals. If 25% of them develop long-term arthritis disability, that’s a whole new world for us,” the rheumatologist observed.
Chikungunya is a single-stranded RNA virus in the togaviridae family, which also contains rubella. The infection is transmitted by Aedes aegypti and A. albopictus, also known as the yellow fever and Asian tiger mosquitoes, respectively. Both mosquitoes are established inhabitants of much of the United States.
“This infection is a one-bite deal. These are very aggressive mosquitoes,” Dr. Schoen observed. “If you haven’t seen a case of chikungunya yet, you will soon,” he added.
In 2014, at the height of a massive Caribbean epidemic which included half a million cases in Puerto Rico, roughly 2,800 cases of chikungunya were imported into 46 U.S. states. In 2015, however, as the Caribbean epidemic waned and herd immunity developed, that figure fell to 653 imported cases. But the epidemic in India, which began in 2008, remains ongoing with no end in sight. Several of Dr. Schoen’s patients with chikungunya had recently returned from India when they first became ill.
“India provides an unlimited reservoir of immunologically naive patients to perpetuate the infection,” he observed.
Course of illness
The rate of asymptomatic infection has been variously estimated at 3%-25%. Symptomatic chikungunya is a biphasic illness. After a 2- to 6-day incubation period, patients develop rapid-onset fever with severe joint pain and muscle aches. Indeed, chikungunya means “bent over” in Makonde, an African Bantu language.
Roughly 60% of patients develop a rash during the acute febrile phase of the illness, which lasts about a week. The dermatitis is most often a maculopapular rash on the trunk which is “absolutely indistinguishable” from the rash caused by Zika virus infection, transmitted by the same vectors, Dr. Schoen noted.
During this acute phase, almost all patients develop a severe polyarthritis which can last for weeks or, less commonly, for months or years. This polyarthritis mimics seronegative rheumatoid arthritis. It is usually symmetric and often affects the hands, wrists, and feet.
The acute febrile phase is characterized by high levels of viremia, with up to 1 billion viral particles per milliliter of blood. During this period, definitive diagnosis of chikungunya can be made through reverse transcriptase–polymerase chain reaction testing or viral culture.
Typically, though, patients make their way to a rheumatologist only well after the viremic period is over. In that situation, the diagnostic mainstay is serologic testing. Antichikungunya virus Immunoglobulin M is detectable starting on about day 5 after symptom onset, and it persists for the next 1-3 months. Immunoglobulin G (IgG) antibodies become detectable in the same time frame and remain elevated for years.
Dr. Schoen highlighted a small but intriguing study from Singapore that suggests that the early appearance of chikungunya-specific neutralizing IgG3–antibodies may constitute a marker for favorable long-term prognosis. The observational study involved 30 patients hospitalized for severe acute chikungunya infection. The investigators classified them into two groups: 14 patients had low levels of acute viremia, a less severe acute illness, and late development of IgG3 antibodies; the other 16 had a high initial viral load, a more severe acute phase, and a rapid IgG3 and interleukin-6 response to infection.
The patients with a robust early IgG3 response cleared the virus faster and none of them developed chronic arthritis. In contrast, those without early, chikungunya-specific IgG3 had a high rate of persistent arthralgia (J Infect Dis. 2012 Apr 1;205[7]:1147-54).
Treatment
No evidence-based treatment for chikungunya exists. Treatment in the acute phase is primarily supportive care. Rheumatologists on the Caribbean island of Martinique have reported a favorable experience using methotrexate at doses up to 25 mg/week or with standard doses of anti–tumor necrosis factor agents in chikungunya patients with severe bilateral symmetric chronic inflammatory joint disease arising during the 2013-2015 epidemic. The treatment response and tolerability were comparable with the results typically obtained in rheumatoid arthritis patients, according to the rheumatologists (Arthritis Rheumatol. 2016 Nov;68[11]:2817-24).
Dr. Schoen found the report from Martinique to be reassuring. He too has resorted to familiar rheumatologic medications in his severely affected patients. Anecdotally, his greatest success involved a patient with a 5-year history of disabling chikungunya polyarthritis who showed marked improvement after several months on hydroxychloroquine.
The Martinique investigators also reported on 22 patients who developed chikungunya while on biologic agents for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, or systemic lupus erythematosus. The biologics weren’t protective against the viral disease in these patients. All 22 of them developed severe acute chikungunya polyarthritis with a mean swollen joint count of 9.6 (Joint Bone Spine. 2016 Mar;83[2]:245-6).
Infection during pregnancy
There is a legitimate concern about chikungunya infection occurring during pregnancy, but, based on the experiences documented on Reunion Island, the risk to the baby is confined to intrapartum exposure in a viremic mother.
Reunion Island is a French region in the Indian Ocean off the eastern coast of Africa. It has first-world medical care. Much of the best documented early work on chikungunya outbreaks grew out of the 2005-2006 epidemic there, which affected more than one-third of the island’s 800,000-plus residents.
In a prospective study of 7,504 deliveries on the island during a 22-month period, mother-to-child transmission of chikungunya occurred only in the context of intrapartum viremia, with 19 cases of vertical transmission occurring among 39 affected mothers who delivered at a median 38 weeks of gestation. Cesarean section had no protective effect. All 19 infected neonates were asymptomatic at birth, with median onset of pain, fever, and thrombocytopenia on day 4. Nine of the 19 infected neonates developed encephalopathy with pathologic MRI findings, including cerebral hemorrhage in two cases (PLoS Med. 2008 Mar 18;5[3]:e60).
The case fatality rate for chikungunya is low at 0.1%. Most deaths occur in young children or elderly individuals with major comorbid conditions. Despite this low mortality, interest in chikungunya vaccine development is being driven by the infection’s impact on tourism, its spread to temperate climates, the economic impact of the considerable time lost from work, and military need. A promising attenuated, virus-like, particle-based vaccine is currently in phase II testing in the Caribbean.
Dr. Schoen reported having no financial conflicts.
Expert provides deeper insight into tocilizumab for giant cell arteritis
SNOWMASS, COLO. – The sustained remission rate in patients with giant cell arteritis was three times better with subcutaneous tocilizumab than with long-term prednisone in the landmark GiACTA trial, John H. Stone, MD, reported at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Moreover, the serious adverse event rate was significantly lower with the interleukin-6 receptor inhibitor tocilizumab (Actemra). This finding serves to underscore the pronounced, yet sometimes stealthy, toxicity of long-term, high-dose prednisone. Many rheumatologists have underplayed these side effects for lack of a better treatment option, because until the randomized, double-blind GiACTA trial, corticosteroids were the standard of care and only known effective therapy for giant cell arteritis (GCA), observed Dr. Stone, professor of medicine at Harvard Medical School, Boston.
“There’s finally something new in giant cell arteritis,” the rheumatologist declared. “The era of unending steroid therapy with no viable alternative is over.”
Dr. Stone was global principal investigator in GiACTA, a 14-country study including 251 GCA patients, roughly evenly divided between newly diagnosed patients and those with previously treated relapsing disease. GiACTA, he noted, was a study of several firsts: the first-ever clinical trial in any disease to utilize a blinded variable-dose steroid regimen, the first to feature a double-blind steroid-tapering regimen, and the first prospective study of 1 full year of tapered-dose prednisone.
The primary outcome was sustained remission as defined by absence of flare during study weeks 12-52 coupled with normalization of the C-reactive protein level to less than 1 mg/dL. This sustained remission endpoint was achieved in 14% of patients on 26 weeks of prednisone plus placebo and 17.6% of those on 52 weeks of prednisone and placebo.
“The results in the two steroid arms are lousy,” the rheumatologist pointed out. “This is I think one of the most astounding things about the trial.”
In sharp contrast, the sustained remission rate was 56% with 162 mg of subcutaneous tocilizumab once weekly plus 26 weeks of prednisone, and 53.1% with 162 mg of the humanized monoclonal antibody every 2 weeks plus 26 weeks of prednisone.
Tocilizumab’s steroid-sparing capability was impressive. The mean cumulative prednisone dose was 4,199 mg in patients on a 52-week prednisone taper plus placebo – a steroid regimen that most closely mimics what most rheumatologists have until now considered standard practice – versus only 2,098 mg in patients on once-weekly tocilizumab plus a 26-week prednisone taper.
Serious adverse events occurred in 24% of patients on prednisone-only, compared with less than 15% of those on tocilizumab. Quality of life scores as measured on the physical component score of the Short Form–36 were significantly better in the tocilizumab recipients.
“I think this speaks again to corticosteroid toxicity,” Dr. Stone said.
Another corticosteroid toxicity underscored in GiACTA was weight gain. At enrollment, patients with newly diagnosed GCA weighed significantly less than did those with previously diagnosed, relapsing disease for which they’d already been on long-term steroids. This body weight disparity was particularly pronounced in men. Men with relapsing GCA weighed a mean of 18.6 pounds more at baseline – nearly three additional body mass index points – than did newly diagnosed men who hadn’t been on aggressive steroid therapy.
“This is a steroid toxicity that I think sneaks up on us,” Dr. Stone observed.
The ongoing GiACTA study is continuing for an additional 2 years of open-label tocilizumab restricted to participants who flared during the initial double-blind phase or thereafter.
Joel M. Kremer, MD, rose from the audience to pronounce GiACTA “a home run.”
“I thought this was the most important study presented at the 2016 ACR annual meeting,” added Dr. Kremer, professor of medicine at the University of Albany (N.Y.) and president and founder of CORRONA, the Consortium of Rheumatology Researchers of North America.
The Snowmass symposium afforded Dr. Stone the opportunity to delve into aspects of GiACTA that time didn’t permit him to address at the 2016 ACR annual meeting:
• What’s the best dose of tocilizumab?
Weekly therapy appears to be preferable, although that’s a decision for the regulatory agencies. Time to first relapse post steroid taper was significantly longer with weekly than with biweekly tocilizumab, a result driven primarily by a markedly better result in the baseline relapser group. The pharmacokinetic data also support weekly dosing: Although patients randomized to weekly tocilizumab got twice as much of the biologic over the course of 52 weeks, their serum trough levels were actually six times higher than in those on biweekly therapy.
“This has implications for control of acute phase reactants,” the rheumatologist noted.
• Which patients with GCA should receive tocilizumab, and for how long?
The GiACTA finding that patients on 52 weeks of prednisone were only one-third as likely to be in continuous remission at 1 year is a deal breaker for the traditional therapy so far as Dr. Stone is concerned, he said in response to an audience question.
“I would treat almost everyone with tocilizumab right out of the gate, and I think the goal is to get them off concomitant prednisone fast. I think we can probably achieve that in less than the 6 months we used in GiACTA,” he said.
The ongoing second, open-label phase of the study will be informative regarding optimal treatment duration. In the meantime, the rheumatologist said, “I would treat patients for 12 months and then follow them to see what they need. We treat ANCA-associated vasculitis with rituximab and steroids, and some of them go into remission that lasts 5 years. I think some giant cell arteritis patients will be the same way, and others will flare right after you stop tocilizumab or maybe even while they’re still on it. Tocilizumab is not a cure, and these patients will still need to be followed closely.”
• What about intravenous tocilizumab, the far less costly option under Medicare?
The Food and Drug Administration will consider approval only for subcutaneous tocilizumab in GCA, since that what was used in GiACTA, although Dr. Stone is convinced based upon personal experience that the subcutaneous and intravenous formulations act identically. Roche/Genentech officials have told him they will pursue a separate approval process for the intravenous formulation.
• How about Takayasu’s arteritis?
It looks like a separate approval process, including a new study, will be required for this form of large-vessel vasculitis, Dr. Stone said.
• Intriguing baseline clinical features in GiACTA:
Mouth pain/jaw claudication was present in 34% of the GCA patients at enrollment.
“Jaw claudication is, I think, vastly underestimated as a very important symptom in giant cell arteritis. Of course, patients don’t say, ‘I have jaw claudication today, doc.’ You have to ask about it specifically. It’s not part of the ACR criteria, and I think it should be,” Dr. Stone said.
Also, cranial symptoms were present in only 79% of patients at enrollment.
“I think that’s important for physicians to know: One-fifth of patients had no cranial symptoms when they were enrolled in the study,” he continued.
Another key finding: Only 67% of GCA patients had a new-onset headache at enrollment.
“We think of headache as being so crucial in this disease, and it is important, but only two-thirds of patients in this trial had it,” the rheumatologist said.
Also, 62% of GCA patients had polymyalgia rheumatica at enrollment. More interestingly, 21% of patients with confirmed GCA had only polymyalgia rheumatica, with no cranial symptoms at all.
Dr. Stone reported receiving research grants from and serving as a consultant to Roche/Genentech, which sponsored GiACTA and markets tocilizumab for the treatment of rheumatoid arthritis and juvenile idiopathic arthritis.
SNOWMASS, COLO. – The sustained remission rate in patients with giant cell arteritis was three times better with subcutaneous tocilizumab than with long-term prednisone in the landmark GiACTA trial, John H. Stone, MD, reported at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Moreover, the serious adverse event rate was significantly lower with the interleukin-6 receptor inhibitor tocilizumab (Actemra). This finding serves to underscore the pronounced, yet sometimes stealthy, toxicity of long-term, high-dose prednisone. Many rheumatologists have underplayed these side effects for lack of a better treatment option, because until the randomized, double-blind GiACTA trial, corticosteroids were the standard of care and only known effective therapy for giant cell arteritis (GCA), observed Dr. Stone, professor of medicine at Harvard Medical School, Boston.
“There’s finally something new in giant cell arteritis,” the rheumatologist declared. “The era of unending steroid therapy with no viable alternative is over.”
Dr. Stone was global principal investigator in GiACTA, a 14-country study including 251 GCA patients, roughly evenly divided between newly diagnosed patients and those with previously treated relapsing disease. GiACTA, he noted, was a study of several firsts: the first-ever clinical trial in any disease to utilize a blinded variable-dose steroid regimen, the first to feature a double-blind steroid-tapering regimen, and the first prospective study of 1 full year of tapered-dose prednisone.
The primary outcome was sustained remission as defined by absence of flare during study weeks 12-52 coupled with normalization of the C-reactive protein level to less than 1 mg/dL. This sustained remission endpoint was achieved in 14% of patients on 26 weeks of prednisone plus placebo and 17.6% of those on 52 weeks of prednisone and placebo.
“The results in the two steroid arms are lousy,” the rheumatologist pointed out. “This is I think one of the most astounding things about the trial.”
In sharp contrast, the sustained remission rate was 56% with 162 mg of subcutaneous tocilizumab once weekly plus 26 weeks of prednisone, and 53.1% with 162 mg of the humanized monoclonal antibody every 2 weeks plus 26 weeks of prednisone.
Tocilizumab’s steroid-sparing capability was impressive. The mean cumulative prednisone dose was 4,199 mg in patients on a 52-week prednisone taper plus placebo – a steroid regimen that most closely mimics what most rheumatologists have until now considered standard practice – versus only 2,098 mg in patients on once-weekly tocilizumab plus a 26-week prednisone taper.
Serious adverse events occurred in 24% of patients on prednisone-only, compared with less than 15% of those on tocilizumab. Quality of life scores as measured on the physical component score of the Short Form–36 were significantly better in the tocilizumab recipients.
“I think this speaks again to corticosteroid toxicity,” Dr. Stone said.
Another corticosteroid toxicity underscored in GiACTA was weight gain. At enrollment, patients with newly diagnosed GCA weighed significantly less than did those with previously diagnosed, relapsing disease for which they’d already been on long-term steroids. This body weight disparity was particularly pronounced in men. Men with relapsing GCA weighed a mean of 18.6 pounds more at baseline – nearly three additional body mass index points – than did newly diagnosed men who hadn’t been on aggressive steroid therapy.
“This is a steroid toxicity that I think sneaks up on us,” Dr. Stone observed.
The ongoing GiACTA study is continuing for an additional 2 years of open-label tocilizumab restricted to participants who flared during the initial double-blind phase or thereafter.
Joel M. Kremer, MD, rose from the audience to pronounce GiACTA “a home run.”
“I thought this was the most important study presented at the 2016 ACR annual meeting,” added Dr. Kremer, professor of medicine at the University of Albany (N.Y.) and president and founder of CORRONA, the Consortium of Rheumatology Researchers of North America.
The Snowmass symposium afforded Dr. Stone the opportunity to delve into aspects of GiACTA that time didn’t permit him to address at the 2016 ACR annual meeting:
• What’s the best dose of tocilizumab?
Weekly therapy appears to be preferable, although that’s a decision for the regulatory agencies. Time to first relapse post steroid taper was significantly longer with weekly than with biweekly tocilizumab, a result driven primarily by a markedly better result in the baseline relapser group. The pharmacokinetic data also support weekly dosing: Although patients randomized to weekly tocilizumab got twice as much of the biologic over the course of 52 weeks, their serum trough levels were actually six times higher than in those on biweekly therapy.
“This has implications for control of acute phase reactants,” the rheumatologist noted.
• Which patients with GCA should receive tocilizumab, and for how long?
The GiACTA finding that patients on 52 weeks of prednisone were only one-third as likely to be in continuous remission at 1 year is a deal breaker for the traditional therapy so far as Dr. Stone is concerned, he said in response to an audience question.
“I would treat almost everyone with tocilizumab right out of the gate, and I think the goal is to get them off concomitant prednisone fast. I think we can probably achieve that in less than the 6 months we used in GiACTA,” he said.
The ongoing second, open-label phase of the study will be informative regarding optimal treatment duration. In the meantime, the rheumatologist said, “I would treat patients for 12 months and then follow them to see what they need. We treat ANCA-associated vasculitis with rituximab and steroids, and some of them go into remission that lasts 5 years. I think some giant cell arteritis patients will be the same way, and others will flare right after you stop tocilizumab or maybe even while they’re still on it. Tocilizumab is not a cure, and these patients will still need to be followed closely.”
• What about intravenous tocilizumab, the far less costly option under Medicare?
The Food and Drug Administration will consider approval only for subcutaneous tocilizumab in GCA, since that what was used in GiACTA, although Dr. Stone is convinced based upon personal experience that the subcutaneous and intravenous formulations act identically. Roche/Genentech officials have told him they will pursue a separate approval process for the intravenous formulation.
• How about Takayasu’s arteritis?
It looks like a separate approval process, including a new study, will be required for this form of large-vessel vasculitis, Dr. Stone said.
• Intriguing baseline clinical features in GiACTA:
Mouth pain/jaw claudication was present in 34% of the GCA patients at enrollment.
“Jaw claudication is, I think, vastly underestimated as a very important symptom in giant cell arteritis. Of course, patients don’t say, ‘I have jaw claudication today, doc.’ You have to ask about it specifically. It’s not part of the ACR criteria, and I think it should be,” Dr. Stone said.
Also, cranial symptoms were present in only 79% of patients at enrollment.
“I think that’s important for physicians to know: One-fifth of patients had no cranial symptoms when they were enrolled in the study,” he continued.
Another key finding: Only 67% of GCA patients had a new-onset headache at enrollment.
“We think of headache as being so crucial in this disease, and it is important, but only two-thirds of patients in this trial had it,” the rheumatologist said.
Also, 62% of GCA patients had polymyalgia rheumatica at enrollment. More interestingly, 21% of patients with confirmed GCA had only polymyalgia rheumatica, with no cranial symptoms at all.
Dr. Stone reported receiving research grants from and serving as a consultant to Roche/Genentech, which sponsored GiACTA and markets tocilizumab for the treatment of rheumatoid arthritis and juvenile idiopathic arthritis.
SNOWMASS, COLO. – The sustained remission rate in patients with giant cell arteritis was three times better with subcutaneous tocilizumab than with long-term prednisone in the landmark GiACTA trial, John H. Stone, MD, reported at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Moreover, the serious adverse event rate was significantly lower with the interleukin-6 receptor inhibitor tocilizumab (Actemra). This finding serves to underscore the pronounced, yet sometimes stealthy, toxicity of long-term, high-dose prednisone. Many rheumatologists have underplayed these side effects for lack of a better treatment option, because until the randomized, double-blind GiACTA trial, corticosteroids were the standard of care and only known effective therapy for giant cell arteritis (GCA), observed Dr. Stone, professor of medicine at Harvard Medical School, Boston.
“There’s finally something new in giant cell arteritis,” the rheumatologist declared. “The era of unending steroid therapy with no viable alternative is over.”
Dr. Stone was global principal investigator in GiACTA, a 14-country study including 251 GCA patients, roughly evenly divided between newly diagnosed patients and those with previously treated relapsing disease. GiACTA, he noted, was a study of several firsts: the first-ever clinical trial in any disease to utilize a blinded variable-dose steroid regimen, the first to feature a double-blind steroid-tapering regimen, and the first prospective study of 1 full year of tapered-dose prednisone.
The primary outcome was sustained remission as defined by absence of flare during study weeks 12-52 coupled with normalization of the C-reactive protein level to less than 1 mg/dL. This sustained remission endpoint was achieved in 14% of patients on 26 weeks of prednisone plus placebo and 17.6% of those on 52 weeks of prednisone and placebo.
“The results in the two steroid arms are lousy,” the rheumatologist pointed out. “This is I think one of the most astounding things about the trial.”
In sharp contrast, the sustained remission rate was 56% with 162 mg of subcutaneous tocilizumab once weekly plus 26 weeks of prednisone, and 53.1% with 162 mg of the humanized monoclonal antibody every 2 weeks plus 26 weeks of prednisone.
Tocilizumab’s steroid-sparing capability was impressive. The mean cumulative prednisone dose was 4,199 mg in patients on a 52-week prednisone taper plus placebo – a steroid regimen that most closely mimics what most rheumatologists have until now considered standard practice – versus only 2,098 mg in patients on once-weekly tocilizumab plus a 26-week prednisone taper.
Serious adverse events occurred in 24% of patients on prednisone-only, compared with less than 15% of those on tocilizumab. Quality of life scores as measured on the physical component score of the Short Form–36 were significantly better in the tocilizumab recipients.
“I think this speaks again to corticosteroid toxicity,” Dr. Stone said.
Another corticosteroid toxicity underscored in GiACTA was weight gain. At enrollment, patients with newly diagnosed GCA weighed significantly less than did those with previously diagnosed, relapsing disease for which they’d already been on long-term steroids. This body weight disparity was particularly pronounced in men. Men with relapsing GCA weighed a mean of 18.6 pounds more at baseline – nearly three additional body mass index points – than did newly diagnosed men who hadn’t been on aggressive steroid therapy.
“This is a steroid toxicity that I think sneaks up on us,” Dr. Stone observed.
The ongoing GiACTA study is continuing for an additional 2 years of open-label tocilizumab restricted to participants who flared during the initial double-blind phase or thereafter.
Joel M. Kremer, MD, rose from the audience to pronounce GiACTA “a home run.”
“I thought this was the most important study presented at the 2016 ACR annual meeting,” added Dr. Kremer, professor of medicine at the University of Albany (N.Y.) and president and founder of CORRONA, the Consortium of Rheumatology Researchers of North America.
The Snowmass symposium afforded Dr. Stone the opportunity to delve into aspects of GiACTA that time didn’t permit him to address at the 2016 ACR annual meeting:
• What’s the best dose of tocilizumab?
Weekly therapy appears to be preferable, although that’s a decision for the regulatory agencies. Time to first relapse post steroid taper was significantly longer with weekly than with biweekly tocilizumab, a result driven primarily by a markedly better result in the baseline relapser group. The pharmacokinetic data also support weekly dosing: Although patients randomized to weekly tocilizumab got twice as much of the biologic over the course of 52 weeks, their serum trough levels were actually six times higher than in those on biweekly therapy.
“This has implications for control of acute phase reactants,” the rheumatologist noted.
• Which patients with GCA should receive tocilizumab, and for how long?
The GiACTA finding that patients on 52 weeks of prednisone were only one-third as likely to be in continuous remission at 1 year is a deal breaker for the traditional therapy so far as Dr. Stone is concerned, he said in response to an audience question.
“I would treat almost everyone with tocilizumab right out of the gate, and I think the goal is to get them off concomitant prednisone fast. I think we can probably achieve that in less than the 6 months we used in GiACTA,” he said.
The ongoing second, open-label phase of the study will be informative regarding optimal treatment duration. In the meantime, the rheumatologist said, “I would treat patients for 12 months and then follow them to see what they need. We treat ANCA-associated vasculitis with rituximab and steroids, and some of them go into remission that lasts 5 years. I think some giant cell arteritis patients will be the same way, and others will flare right after you stop tocilizumab or maybe even while they’re still on it. Tocilizumab is not a cure, and these patients will still need to be followed closely.”
• What about intravenous tocilizumab, the far less costly option under Medicare?
The Food and Drug Administration will consider approval only for subcutaneous tocilizumab in GCA, since that what was used in GiACTA, although Dr. Stone is convinced based upon personal experience that the subcutaneous and intravenous formulations act identically. Roche/Genentech officials have told him they will pursue a separate approval process for the intravenous formulation.
• How about Takayasu’s arteritis?
It looks like a separate approval process, including a new study, will be required for this form of large-vessel vasculitis, Dr. Stone said.
• Intriguing baseline clinical features in GiACTA:
Mouth pain/jaw claudication was present in 34% of the GCA patients at enrollment.
“Jaw claudication is, I think, vastly underestimated as a very important symptom in giant cell arteritis. Of course, patients don’t say, ‘I have jaw claudication today, doc.’ You have to ask about it specifically. It’s not part of the ACR criteria, and I think it should be,” Dr. Stone said.
Also, cranial symptoms were present in only 79% of patients at enrollment.
“I think that’s important for physicians to know: One-fifth of patients had no cranial symptoms when they were enrolled in the study,” he continued.
Another key finding: Only 67% of GCA patients had a new-onset headache at enrollment.
“We think of headache as being so crucial in this disease, and it is important, but only two-thirds of patients in this trial had it,” the rheumatologist said.
Also, 62% of GCA patients had polymyalgia rheumatica at enrollment. More interestingly, 21% of patients with confirmed GCA had only polymyalgia rheumatica, with no cranial symptoms at all.
Dr. Stone reported receiving research grants from and serving as a consultant to Roche/Genentech, which sponsored GiACTA and markets tocilizumab for the treatment of rheumatoid arthritis and juvenile idiopathic arthritis.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Biosimilars: No big dollar savings, but are clinically ‘dead on’
SNOWMASS, COLO. – If you thought biosimilars would bring sharply reduced pricing compared with their parent agents, with resultant greater patient access to highly effective therapies for rheumatic diseases ... think again.
“The promise to our patients of biosimilars – greater access to treatments – is something I think we’re just not going to see, at least not here in the U.S.,” Michael E. Weinblatt, MD, declared at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In contrast, the safety and efficacy of the biosimilars, as well as their interchangeability with their reference products, appear to be as hoped for. At the 2016 annual meeting of the American College of Rheumatology, Dr. Weinblatt presented the week 24 results of a phase III, randomized trial involving rheumatoid arthritis patients on background methotrexate plus either adalimumab (Humira) or its biosimilar SB5.
“Essentially, they’re dead on in clinical response, they’re dead on in antibody levels, and they’re dead on in toxicity. And, you can put any of the biosimilars up there and the results are the same. If they get approved, this is what you’re going to see,” the rheumatologist said.
Also at the 2016 ACR annual meeting, he noted, Danish investigators presented reassuring 1-year follow-up data on 802 Danes with inflammatory rheumatic diseases who switched from infliximab (Remicade) to its biosimilar Remsima. Disease activity and flare rates in the year following the switch were similar to those in the year before. The 1-year rate of adherence to Remsima was 84%, similar to the historical 86% 1-year rate with infliximab.
“So, I’m pretty comfortable with the biosimilars,” Dr. Weinblatt continued.
He observed that, of all the systemic rheumatic diseases, the greatest progress has occurred in the treatment of rheumatoid arthritis.
“We have made great advances in the treatment of this disease, unlike many of our other diseases. Methotrexate and combination therapies with small molecules and biologics has dramatically changed the course of the disease,” he noted. “The greatest challenge we have now as rheumatologists is access barriers for our patients.”
Dr. Weinblatt reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – If you thought biosimilars would bring sharply reduced pricing compared with their parent agents, with resultant greater patient access to highly effective therapies for rheumatic diseases ... think again.
“The promise to our patients of biosimilars – greater access to treatments – is something I think we’re just not going to see, at least not here in the U.S.,” Michael E. Weinblatt, MD, declared at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In contrast, the safety and efficacy of the biosimilars, as well as their interchangeability with their reference products, appear to be as hoped for. At the 2016 annual meeting of the American College of Rheumatology, Dr. Weinblatt presented the week 24 results of a phase III, randomized trial involving rheumatoid arthritis patients on background methotrexate plus either adalimumab (Humira) or its biosimilar SB5.
“Essentially, they’re dead on in clinical response, they’re dead on in antibody levels, and they’re dead on in toxicity. And, you can put any of the biosimilars up there and the results are the same. If they get approved, this is what you’re going to see,” the rheumatologist said.
Also at the 2016 ACR annual meeting, he noted, Danish investigators presented reassuring 1-year follow-up data on 802 Danes with inflammatory rheumatic diseases who switched from infliximab (Remicade) to its biosimilar Remsima. Disease activity and flare rates in the year following the switch were similar to those in the year before. The 1-year rate of adherence to Remsima was 84%, similar to the historical 86% 1-year rate with infliximab.
“So, I’m pretty comfortable with the biosimilars,” Dr. Weinblatt continued.
He observed that, of all the systemic rheumatic diseases, the greatest progress has occurred in the treatment of rheumatoid arthritis.
“We have made great advances in the treatment of this disease, unlike many of our other diseases. Methotrexate and combination therapies with small molecules and biologics has dramatically changed the course of the disease,” he noted. “The greatest challenge we have now as rheumatologists is access barriers for our patients.”
Dr. Weinblatt reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – If you thought biosimilars would bring sharply reduced pricing compared with their parent agents, with resultant greater patient access to highly effective therapies for rheumatic diseases ... think again.
“The promise to our patients of biosimilars – greater access to treatments – is something I think we’re just not going to see, at least not here in the U.S.,” Michael E. Weinblatt, MD, declared at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In contrast, the safety and efficacy of the biosimilars, as well as their interchangeability with their reference products, appear to be as hoped for. At the 2016 annual meeting of the American College of Rheumatology, Dr. Weinblatt presented the week 24 results of a phase III, randomized trial involving rheumatoid arthritis patients on background methotrexate plus either adalimumab (Humira) or its biosimilar SB5.
“Essentially, they’re dead on in clinical response, they’re dead on in antibody levels, and they’re dead on in toxicity. And, you can put any of the biosimilars up there and the results are the same. If they get approved, this is what you’re going to see,” the rheumatologist said.
Also at the 2016 ACR annual meeting, he noted, Danish investigators presented reassuring 1-year follow-up data on 802 Danes with inflammatory rheumatic diseases who switched from infliximab (Remicade) to its biosimilar Remsima. Disease activity and flare rates in the year following the switch were similar to those in the year before. The 1-year rate of adherence to Remsima was 84%, similar to the historical 86% 1-year rate with infliximab.
“So, I’m pretty comfortable with the biosimilars,” Dr. Weinblatt continued.
He observed that, of all the systemic rheumatic diseases, the greatest progress has occurred in the treatment of rheumatoid arthritis.
“We have made great advances in the treatment of this disease, unlike many of our other diseases. Methotrexate and combination therapies with small molecules and biologics has dramatically changed the course of the disease,” he noted. “The greatest challenge we have now as rheumatologists is access barriers for our patients.”
Dr. Weinblatt reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Dactylitis signals more severe psoriatic arthritis
SNOWMASS, COLO. – Dactylitis is a common and painful extra-articular manifestation of psoriatic arthritis that takes on added clinical significance because it’s also a marker of greater disease severity, Christopher T. Ritchlin, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Indeed, psoriatic arthritis (PsA) patients with dactylitis are more likely to have polyarticular disease and radiologic evidence of bony damage, noted Dr. Ritchlin, professor of medicine and chief of the allergy, immunology, and rheumatology division at the University of Rochester (N.Y.).
“We have no idea why this is,” confessed Dr. Ritchlin, who is also director of the Clinical Immunology Research Center at the university.
The differential diagnosis for dactylitis includes psoriatic arthritis, other spondyloarthropathies, sickle cell disease, tuberculosis, sarcoidosis, and pyogenic flexor tenosynovitis, a closed-space infection that is the major issue in the differential. Dr. Ritchlin sees many more cases of dactylitis due to PsA that get misdiagnosed as a flexor tendon sheath infection and inappropriately subjected to surgery and/or intravenous antibiotics than vice versa.
Pyogenic flexor tenosynovitis can be identified using the four Kanavel signs: diffuse swelling of a digit, often with discoloration; intense pain over the whole length of the tendon sheath, but limited to the sheath; the involved digit being held in a semiflexed posture; and exquisite pain upon passive extension of the digit, with the pain being worst at the proximal end.
University of Toronto investigators have demonstrated that, in their large longitudinal database of PsA patients, the prevalence of radiologic damage in participants with acute dactylitis of the hands is twice as great as in PsA patients without dactylitis.
“I’ve been struck over the years by how often I see psoriatic arthritis patients with dactylitic digits who not only have erosions but who actually have a complete fusion or ankylosis of the joint. The point is, when you have a joint with diffuse inflammation, in many patients it’s associated with activation of both osteoclasts and osteoblasts,” according to the rheumatologist.
Enthesitis
Enthesitis, another cardinal extra-articular manifestation of PsA, is defined by inflammation at the sites where tendons, ligaments, and joint capsules attach into bone. The most commonly involved sites are the Achilles tendon and plantar fascia.
“It can also involve a lot of other areas and can lead to misdiagnosis as a result. Many of these patients end up in rheumatologists’ offices with previous diagnoses ranging from fibromyalgia or other chronic pain syndromes to malingering,” Dr. Ritchlin said.
Sites to examine for enthesitis, in addition to the foot and Achilles tendon, include the patellar and quadriceps tendons, iliac crest, greater trochanter, lateral epicondyle, the small joints of the hands, and the supraspinatus tendon.
“We have a registry of several hundred psoriatic arthritis patients, and I’ve been struck by the amount of enthesopathy when we examine these points,” the rheumatologist observed.
Enthesitis is a prominent feature of both early and established PsA. Power Doppler ultrasound is more sensitive than radiographs at identifying it. Italian investigators have shown ultrasound to be useful in the differential diagnosis between early rheumatoid arthritis and early PsA in patients with hand involvement. They assessed 52 clinically involved joints in 26 patients with early PsA and 68 involved joints in 34 early-RA patients. Synovitis was detected in 91% of the joints of the RA patients, compared with only 60% of the PsA patients’ joints.
In contrast, soft tissue edema was present in 42% of the most clinically involved fingers of the early PsA patients, compared with just 3% in those with early RA. Central slip enthesitis was seen in 21% of the clinically involved proximal interphalangeal joints of the PsA patients but in none of those belonging to patients with early RA. Peritendon inflammation of the extensor digitorum tendon was noted in 54% of the joints of the PsA group, compared with less than 3% of the early RA group (Clin Exp Rheumatol. 2016 May-Jun;34[3]:459-65).
“Basically, if you do ultrasound, you see there is significantly more enthesitis in early psoriatic arthritis than early rheumatoid arthritis, which has certainly been our experience as well,” Dr. Ritchlin commented.
Enthesitis is not as simple a disease process as most physicians were taught in training. Dr. Ritchlin credits Dennis McGonagle, MD, of the University of Leeds (England) with introducing the now-accepted concept of a synovio-entheseal complex as being a key player in the expression of PsA (Arthritis Rheum. 2007 Aug;56[8]:2482-91).
“The old idea is that the enthesis inserts onto bone and that’s where the pathology is. But it’s more complicated than that,” Dr. Ritchlin explained.
Dr. McGonagle and his coworkers showed that fibrocartilagenous entheses attach to bone much more deeply than previously recognized, like a tree with deep roots. That makes for lots of intimate contact between bony cells and vascular channels. And key structures are located near the intersection of enthesis and bone, including bursae and synovial membrane. For example, the Achilles tendon synovio-entheseal complex includes sesamoid fibrocartilage, periosteal fibrocartilage, the retrocalcaneal bursa, subchondral bone, and enthesis fibrocartilage, as well as the tendon itself.
Dr. McGonagle and coworkers argued that the pathogenesis of tissue inflammation and damage in PsA involves biomechanical stress, with resultant synovial inflammation accompanied by the release of inflammatory cytokines, which in turn leads to diffuse inflammation in and around the area where the enthesis inserts.
“The purpose of the enthesis is to distribute force away from the area where the tendon inserts into bone. So when biomechanical stress pulls on that tendon, other adjacent areas are also affected. What’s come out from imaging studies is that there’s synovial inflammation, bursitis, and also inflammation in and around the fibrocartilage in areas of enthesitis,” Dr. Ritchlin said.
He reported serving as a consultant to half a dozen pharmaceutical companies.
SNOWMASS, COLO. – Dactylitis is a common and painful extra-articular manifestation of psoriatic arthritis that takes on added clinical significance because it’s also a marker of greater disease severity, Christopher T. Ritchlin, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Indeed, psoriatic arthritis (PsA) patients with dactylitis are more likely to have polyarticular disease and radiologic evidence of bony damage, noted Dr. Ritchlin, professor of medicine and chief of the allergy, immunology, and rheumatology division at the University of Rochester (N.Y.).
“We have no idea why this is,” confessed Dr. Ritchlin, who is also director of the Clinical Immunology Research Center at the university.
The differential diagnosis for dactylitis includes psoriatic arthritis, other spondyloarthropathies, sickle cell disease, tuberculosis, sarcoidosis, and pyogenic flexor tenosynovitis, a closed-space infection that is the major issue in the differential. Dr. Ritchlin sees many more cases of dactylitis due to PsA that get misdiagnosed as a flexor tendon sheath infection and inappropriately subjected to surgery and/or intravenous antibiotics than vice versa.
Pyogenic flexor tenosynovitis can be identified using the four Kanavel signs: diffuse swelling of a digit, often with discoloration; intense pain over the whole length of the tendon sheath, but limited to the sheath; the involved digit being held in a semiflexed posture; and exquisite pain upon passive extension of the digit, with the pain being worst at the proximal end.
University of Toronto investigators have demonstrated that, in their large longitudinal database of PsA patients, the prevalence of radiologic damage in participants with acute dactylitis of the hands is twice as great as in PsA patients without dactylitis.
“I’ve been struck over the years by how often I see psoriatic arthritis patients with dactylitic digits who not only have erosions but who actually have a complete fusion or ankylosis of the joint. The point is, when you have a joint with diffuse inflammation, in many patients it’s associated with activation of both osteoclasts and osteoblasts,” according to the rheumatologist.
Enthesitis
Enthesitis, another cardinal extra-articular manifestation of PsA, is defined by inflammation at the sites where tendons, ligaments, and joint capsules attach into bone. The most commonly involved sites are the Achilles tendon and plantar fascia.
“It can also involve a lot of other areas and can lead to misdiagnosis as a result. Many of these patients end up in rheumatologists’ offices with previous diagnoses ranging from fibromyalgia or other chronic pain syndromes to malingering,” Dr. Ritchlin said.
Sites to examine for enthesitis, in addition to the foot and Achilles tendon, include the patellar and quadriceps tendons, iliac crest, greater trochanter, lateral epicondyle, the small joints of the hands, and the supraspinatus tendon.
“We have a registry of several hundred psoriatic arthritis patients, and I’ve been struck by the amount of enthesopathy when we examine these points,” the rheumatologist observed.
Enthesitis is a prominent feature of both early and established PsA. Power Doppler ultrasound is more sensitive than radiographs at identifying it. Italian investigators have shown ultrasound to be useful in the differential diagnosis between early rheumatoid arthritis and early PsA in patients with hand involvement. They assessed 52 clinically involved joints in 26 patients with early PsA and 68 involved joints in 34 early-RA patients. Synovitis was detected in 91% of the joints of the RA patients, compared with only 60% of the PsA patients’ joints.
In contrast, soft tissue edema was present in 42% of the most clinically involved fingers of the early PsA patients, compared with just 3% in those with early RA. Central slip enthesitis was seen in 21% of the clinically involved proximal interphalangeal joints of the PsA patients but in none of those belonging to patients with early RA. Peritendon inflammation of the extensor digitorum tendon was noted in 54% of the joints of the PsA group, compared with less than 3% of the early RA group (Clin Exp Rheumatol. 2016 May-Jun;34[3]:459-65).
“Basically, if you do ultrasound, you see there is significantly more enthesitis in early psoriatic arthritis than early rheumatoid arthritis, which has certainly been our experience as well,” Dr. Ritchlin commented.
Enthesitis is not as simple a disease process as most physicians were taught in training. Dr. Ritchlin credits Dennis McGonagle, MD, of the University of Leeds (England) with introducing the now-accepted concept of a synovio-entheseal complex as being a key player in the expression of PsA (Arthritis Rheum. 2007 Aug;56[8]:2482-91).
“The old idea is that the enthesis inserts onto bone and that’s where the pathology is. But it’s more complicated than that,” Dr. Ritchlin explained.
Dr. McGonagle and his coworkers showed that fibrocartilagenous entheses attach to bone much more deeply than previously recognized, like a tree with deep roots. That makes for lots of intimate contact between bony cells and vascular channels. And key structures are located near the intersection of enthesis and bone, including bursae and synovial membrane. For example, the Achilles tendon synovio-entheseal complex includes sesamoid fibrocartilage, periosteal fibrocartilage, the retrocalcaneal bursa, subchondral bone, and enthesis fibrocartilage, as well as the tendon itself.
Dr. McGonagle and coworkers argued that the pathogenesis of tissue inflammation and damage in PsA involves biomechanical stress, with resultant synovial inflammation accompanied by the release of inflammatory cytokines, which in turn leads to diffuse inflammation in and around the area where the enthesis inserts.
“The purpose of the enthesis is to distribute force away from the area where the tendon inserts into bone. So when biomechanical stress pulls on that tendon, other adjacent areas are also affected. What’s come out from imaging studies is that there’s synovial inflammation, bursitis, and also inflammation in and around the fibrocartilage in areas of enthesitis,” Dr. Ritchlin said.
He reported serving as a consultant to half a dozen pharmaceutical companies.
SNOWMASS, COLO. – Dactylitis is a common and painful extra-articular manifestation of psoriatic arthritis that takes on added clinical significance because it’s also a marker of greater disease severity, Christopher T. Ritchlin, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Indeed, psoriatic arthritis (PsA) patients with dactylitis are more likely to have polyarticular disease and radiologic evidence of bony damage, noted Dr. Ritchlin, professor of medicine and chief of the allergy, immunology, and rheumatology division at the University of Rochester (N.Y.).
“We have no idea why this is,” confessed Dr. Ritchlin, who is also director of the Clinical Immunology Research Center at the university.
The differential diagnosis for dactylitis includes psoriatic arthritis, other spondyloarthropathies, sickle cell disease, tuberculosis, sarcoidosis, and pyogenic flexor tenosynovitis, a closed-space infection that is the major issue in the differential. Dr. Ritchlin sees many more cases of dactylitis due to PsA that get misdiagnosed as a flexor tendon sheath infection and inappropriately subjected to surgery and/or intravenous antibiotics than vice versa.
Pyogenic flexor tenosynovitis can be identified using the four Kanavel signs: diffuse swelling of a digit, often with discoloration; intense pain over the whole length of the tendon sheath, but limited to the sheath; the involved digit being held in a semiflexed posture; and exquisite pain upon passive extension of the digit, with the pain being worst at the proximal end.
University of Toronto investigators have demonstrated that, in their large longitudinal database of PsA patients, the prevalence of radiologic damage in participants with acute dactylitis of the hands is twice as great as in PsA patients without dactylitis.
“I’ve been struck over the years by how often I see psoriatic arthritis patients with dactylitic digits who not only have erosions but who actually have a complete fusion or ankylosis of the joint. The point is, when you have a joint with diffuse inflammation, in many patients it’s associated with activation of both osteoclasts and osteoblasts,” according to the rheumatologist.
Enthesitis
Enthesitis, another cardinal extra-articular manifestation of PsA, is defined by inflammation at the sites where tendons, ligaments, and joint capsules attach into bone. The most commonly involved sites are the Achilles tendon and plantar fascia.
“It can also involve a lot of other areas and can lead to misdiagnosis as a result. Many of these patients end up in rheumatologists’ offices with previous diagnoses ranging from fibromyalgia or other chronic pain syndromes to malingering,” Dr. Ritchlin said.
Sites to examine for enthesitis, in addition to the foot and Achilles tendon, include the patellar and quadriceps tendons, iliac crest, greater trochanter, lateral epicondyle, the small joints of the hands, and the supraspinatus tendon.
“We have a registry of several hundred psoriatic arthritis patients, and I’ve been struck by the amount of enthesopathy when we examine these points,” the rheumatologist observed.
Enthesitis is a prominent feature of both early and established PsA. Power Doppler ultrasound is more sensitive than radiographs at identifying it. Italian investigators have shown ultrasound to be useful in the differential diagnosis between early rheumatoid arthritis and early PsA in patients with hand involvement. They assessed 52 clinically involved joints in 26 patients with early PsA and 68 involved joints in 34 early-RA patients. Synovitis was detected in 91% of the joints of the RA patients, compared with only 60% of the PsA patients’ joints.
In contrast, soft tissue edema was present in 42% of the most clinically involved fingers of the early PsA patients, compared with just 3% in those with early RA. Central slip enthesitis was seen in 21% of the clinically involved proximal interphalangeal joints of the PsA patients but in none of those belonging to patients with early RA. Peritendon inflammation of the extensor digitorum tendon was noted in 54% of the joints of the PsA group, compared with less than 3% of the early RA group (Clin Exp Rheumatol. 2016 May-Jun;34[3]:459-65).
“Basically, if you do ultrasound, you see there is significantly more enthesitis in early psoriatic arthritis than early rheumatoid arthritis, which has certainly been our experience as well,” Dr. Ritchlin commented.
Enthesitis is not as simple a disease process as most physicians were taught in training. Dr. Ritchlin credits Dennis McGonagle, MD, of the University of Leeds (England) with introducing the now-accepted concept of a synovio-entheseal complex as being a key player in the expression of PsA (Arthritis Rheum. 2007 Aug;56[8]:2482-91).
“The old idea is that the enthesis inserts onto bone and that’s where the pathology is. But it’s more complicated than that,” Dr. Ritchlin explained.
Dr. McGonagle and his coworkers showed that fibrocartilagenous entheses attach to bone much more deeply than previously recognized, like a tree with deep roots. That makes for lots of intimate contact between bony cells and vascular channels. And key structures are located near the intersection of enthesis and bone, including bursae and synovial membrane. For example, the Achilles tendon synovio-entheseal complex includes sesamoid fibrocartilage, periosteal fibrocartilage, the retrocalcaneal bursa, subchondral bone, and enthesis fibrocartilage, as well as the tendon itself.
Dr. McGonagle and coworkers argued that the pathogenesis of tissue inflammation and damage in PsA involves biomechanical stress, with resultant synovial inflammation accompanied by the release of inflammatory cytokines, which in turn leads to diffuse inflammation in and around the area where the enthesis inserts.
“The purpose of the enthesis is to distribute force away from the area where the tendon inserts into bone. So when biomechanical stress pulls on that tendon, other adjacent areas are also affected. What’s come out from imaging studies is that there’s synovial inflammation, bursitis, and also inflammation in and around the fibrocartilage in areas of enthesitis,” Dr. Ritchlin said.
He reported serving as a consultant to half a dozen pharmaceutical companies.
IgG4-related disease can strike any organ system
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swath the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Treatment with rituxumab
Glucocorticoids remain the first-line therapy in IgG4-related disease, but it’s essential to bear in mind that their long-term efficacy in this immune-mediated fibroinflammatory disease is the exception rather than the rule, Dr. Stone said at the symposium.
Dr. Stone was a coauthor of an international expert consensus statement on the treatment of IgG4-related disease (IgG4-RD), which emphasized that point (Arthritis Rheumatol. 2015 Jul;67[7]:1688-99).
“I typically start with prednisone at 40 mg/day, and there’s a dramatic response in these patients. Then I taper them off after 2-3 months. If 2-3 months doesn’t put them into a long-term sustained remission, it’s time to go to something else,” said Dr. Stone.
So what’s the next move, then, after steroids fail? Dr. Stone was a pioneer in the strikingly successful use of B cell depletion via rituximab (Rituxan) in patients with IgG4-RD. First he and his coinvestigators demonstrated that this off-label use of rituximab led to rapid clinical and histologic improvement (Ann Rheum Dis. 2015 Jun; 74[6]:1171-7), then they showed it also causes levels of circulating plasmablasts, serum IgG4, and biomarkers of fibrosis to plunge, suggesting B cell depletion may halt the destructive process of collagen deposition that characterizes this immune-related disease (Ann Rheum Dis. 2015 Dec;74[12]:2236-43). They have also reported that patients with very elevated baseline serum IgG4 levels are at more than sixfold increased risk of relapse at a median of 244 days from their first rituximab infusion (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
The success with rituximab is just one example of how improved understanding of the pathophysiology of IgG4-RD has opened the door to novel treatments. Dr. Stone is the lead investigator in an ongoing phase II, open-label study in which 15 patients with active IgG4-RD will receive intravenous XmAb5871 every 2 weeks for 6 months to evaluate the effect on the IgG4-RD Responder Index. XmAb5871 is a monoclonal antibody that binds to CD19 and FCgammaRIIb in order to downregulate activated B cells and plasmablasts. It is also being developed for treatment of systemic lupus erythematosus.
Dr. Stone and his coinvestigators are working on a therapeutic approach to IgG4-RD that targets antigen presentation by activated B cells to CD4+ cytotoxic T cells at sites of disease. These CD4+ cytotoxic T cells contain signaling lymphocyte activation molecule F7 (SLAMF7) as a surface marker. Elotuzumab (Empliciti), an immunostimulatory humanized monoclonal antibody targeting SLAMF7, is already on the market for treatment of multiple myeloma, he noted.
Dr. Stone reported receiving IgG4-RD-related research funding from and serving as a consultant to Genentech and Xencor, which is developing XmAb5871.
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swath the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Treatment with rituxumab
Glucocorticoids remain the first-line therapy in IgG4-related disease, but it’s essential to bear in mind that their long-term efficacy in this immune-mediated fibroinflammatory disease is the exception rather than the rule, Dr. Stone said at the symposium.
Dr. Stone was a coauthor of an international expert consensus statement on the treatment of IgG4-related disease (IgG4-RD), which emphasized that point (Arthritis Rheumatol. 2015 Jul;67[7]:1688-99).
“I typically start with prednisone at 40 mg/day, and there’s a dramatic response in these patients. Then I taper them off after 2-3 months. If 2-3 months doesn’t put them into a long-term sustained remission, it’s time to go to something else,” said Dr. Stone.
So what’s the next move, then, after steroids fail? Dr. Stone was a pioneer in the strikingly successful use of B cell depletion via rituximab (Rituxan) in patients with IgG4-RD. First he and his coinvestigators demonstrated that this off-label use of rituximab led to rapid clinical and histologic improvement (Ann Rheum Dis. 2015 Jun; 74[6]:1171-7), then they showed it also causes levels of circulating plasmablasts, serum IgG4, and biomarkers of fibrosis to plunge, suggesting B cell depletion may halt the destructive process of collagen deposition that characterizes this immune-related disease (Ann Rheum Dis. 2015 Dec;74[12]:2236-43). They have also reported that patients with very elevated baseline serum IgG4 levels are at more than sixfold increased risk of relapse at a median of 244 days from their first rituximab infusion (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
The success with rituximab is just one example of how improved understanding of the pathophysiology of IgG4-RD has opened the door to novel treatments. Dr. Stone is the lead investigator in an ongoing phase II, open-label study in which 15 patients with active IgG4-RD will receive intravenous XmAb5871 every 2 weeks for 6 months to evaluate the effect on the IgG4-RD Responder Index. XmAb5871 is a monoclonal antibody that binds to CD19 and FCgammaRIIb in order to downregulate activated B cells and plasmablasts. It is also being developed for treatment of systemic lupus erythematosus.
Dr. Stone and his coinvestigators are working on a therapeutic approach to IgG4-RD that targets antigen presentation by activated B cells to CD4+ cytotoxic T cells at sites of disease. These CD4+ cytotoxic T cells contain signaling lymphocyte activation molecule F7 (SLAMF7) as a surface marker. Elotuzumab (Empliciti), an immunostimulatory humanized monoclonal antibody targeting SLAMF7, is already on the market for treatment of multiple myeloma, he noted.
Dr. Stone reported receiving IgG4-RD-related research funding from and serving as a consultant to Genentech and Xencor, which is developing XmAb5871.
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swath the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Treatment with rituxumab
Glucocorticoids remain the first-line therapy in IgG4-related disease, but it’s essential to bear in mind that their long-term efficacy in this immune-mediated fibroinflammatory disease is the exception rather than the rule, Dr. Stone said at the symposium.
Dr. Stone was a coauthor of an international expert consensus statement on the treatment of IgG4-related disease (IgG4-RD), which emphasized that point (Arthritis Rheumatol. 2015 Jul;67[7]:1688-99).
“I typically start with prednisone at 40 mg/day, and there’s a dramatic response in these patients. Then I taper them off after 2-3 months. If 2-3 months doesn’t put them into a long-term sustained remission, it’s time to go to something else,” said Dr. Stone.
So what’s the next move, then, after steroids fail? Dr. Stone was a pioneer in the strikingly successful use of B cell depletion via rituximab (Rituxan) in patients with IgG4-RD. First he and his coinvestigators demonstrated that this off-label use of rituximab led to rapid clinical and histologic improvement (Ann Rheum Dis. 2015 Jun; 74[6]:1171-7), then they showed it also causes levels of circulating plasmablasts, serum IgG4, and biomarkers of fibrosis to plunge, suggesting B cell depletion may halt the destructive process of collagen deposition that characterizes this immune-related disease (Ann Rheum Dis. 2015 Dec;74[12]:2236-43). They have also reported that patients with very elevated baseline serum IgG4 levels are at more than sixfold increased risk of relapse at a median of 244 days from their first rituximab infusion (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
The success with rituximab is just one example of how improved understanding of the pathophysiology of IgG4-RD has opened the door to novel treatments. Dr. Stone is the lead investigator in an ongoing phase II, open-label study in which 15 patients with active IgG4-RD will receive intravenous XmAb5871 every 2 weeks for 6 months to evaluate the effect on the IgG4-RD Responder Index. XmAb5871 is a monoclonal antibody that binds to CD19 and FCgammaRIIb in order to downregulate activated B cells and plasmablasts. It is also being developed for treatment of systemic lupus erythematosus.
Dr. Stone and his coinvestigators are working on a therapeutic approach to IgG4-RD that targets antigen presentation by activated B cells to CD4+ cytotoxic T cells at sites of disease. These CD4+ cytotoxic T cells contain signaling lymphocyte activation molecule F7 (SLAMF7) as a surface marker. Elotuzumab (Empliciti), an immunostimulatory humanized monoclonal antibody targeting SLAMF7, is already on the market for treatment of multiple myeloma, he noted.
Dr. Stone reported receiving IgG4-RD-related research funding from and serving as a consultant to Genentech and Xencor, which is developing XmAb5871.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Methotrexate for RA: A 'fascinating drug’
SNOWMASS, COLO. – “When I started working with methotrexate in 1982, I never would have predicted that methotrexate would become the standard of care in treating rheumatoid arthritis. There’s just no way,” Michael E. Weinblatt, MD, recalled at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Even now, 35 years later, we continue to learn more about this fascinating drug,” added Dr. Weinblatt, professor of medicine at Harvard Medical School, Boston.
He highlighted recent developments in this ongoing story and presented some tricks of the trade gained in 35 years of up-close experience with the drug.
Enhancing effectiveness of biologics
One of the hottest topics in methotrexate research is the drug’s ability to enhance the effectiveness of many, but not all, biologic agents. All of the anti–tumor necrosis factor (anti-TNF) biologics as well as rituximab (Rituxan) are demonstrably more effective when used in combination with methotrexate. Dr. Weinblatt considers the widespread underutilization of this combination strategy scandalous.
“This is an incredibly important point: All of you use biologics and all of you use methotrexate, but I’ve been depressed by the fact that up to 30%-40% of patients – no matter which data set you look at – are on monotherapy biological therapy,” he said.
He cited data from the ongoing BRASS Registry (Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Registry) to underscore his point that good things happen when biologics and methotrexate are used together. Of 1,395 BRASS Registry participants prospectively followed since 2003, the proportion on biologic therapy has climbed steadily from 41% at the outset to 68% in 2016. Remarkably, 82% of patients on biologic therapy remain on their first biologic agent. Fewer than 4% have switched biologics more than twice. That’s very unlike the experiences reported elsewhere.
“I think one of the reasons we have such positive data is that we have a high percentage of patients staying on their background methotrexate,” said Dr. Weinblatt, codirector of clinical rheumatology and associate director of the Center for Arthritis and Joint Diseases at Brigham and Women’s Hospital, Boston.
He noted that Dutch investigators reported at the 2016 annual meeting of the American College of Rheumatology that among 1,230 consecutive rheumatoid arthritis patients started on etanercept (Enbrel) or adalimumab (Humira), 28% in the etanercept group were on concomitant methotrexate, as were 64% of those who started on adalimumab. The patients spent a median of 1.3-1.6 years and a maximum of 9.2-9.3 years on their biologic agent. Patients on adalimumab monotherapy were 2.61-fold more likely to drop out than were those on dual therapy with methotrexate. Patients on etanercept monotherapy were 1.2-fold more likely to drop out, a difference that didn’t achieve statistical significance.
Although the investigators did not study the mechanism of prolonged on-treatment survival, they speculated that it probably involved methotrexate’s documented ability to prevent formation of anti-adalimumab antibodies. In contrast, patients on etanercept don’t develop blocking antibodies, Dr. Weinblatt observed.
The randomized, double-blind CONCERTO trial conducted in 395 methotrexate- and biologic-naive RA patients demonstrated that methotrexate reduces the immunogenicity of adalimumab in dose-dependent fashion. Participants were randomized to open-label adalimumab at 40 mg every 2 weeks plus weekly double-blind methotrexate at 2.5, 5, 10, or 20 mg. Clinical outcomes at 26 weeks as reflected in 28-joint count disease activity score and the Clinical Disease Activity Index were significantly better in patients on 10 or 20 mg/week of methotrexate than in those on 2.5 or 5 mg/week. Serum adalimumab levels were higher in patients on the two higher doses of methotrexate as well (Ann Rheum Dis. 2015 Jun;74[6]:1037-44).
“It ends up that if you don’t use methotrexate or you use a very low dose you increase the risk of developing antibodies against adalimumab and decrease the efficacy of the drug. So in clinical practice, if you’re going to be working with dose titration of methotrexate and your patient is on adalimumab, there’s a threshold below which you probably shouldn’t go. In this study, doses of 10 mg/week or more induced a greater clinical response,” he said.
With infliximab (Remicade), based upon 20-year-old studies, the threshold is 7.5 mg of methotrexate per week.
“With etanercept, we don’t know what the threshold is. You don’t develop blocking anti-drug antibodies with etanercept, but we do know that methotrexate enhances the efficacy of etanercept, and it doesn’t do it by changing the biologic’s pharmacokinetics and there’s no increase in methotrexate blood levels,” the rheumatologist continued.
Unlike the anti-TNF biologics and rituximab, the efficacy of the Janus kinase inhibitors tofacitinib (Xeljanz) and baricitinib is not enhanced when the drugs are used in combination with methotrexate, studies indicate.
The efficacy of certolizumab pegol (Cimzia) wasn’t affected by methotrexate dose category in a prespecified pooled subgroup analysis of the phase III RAPID 1 and RAPID 2 clinical trials. In the 1,273 certolizumab-treated patients, the week-24 treatment response was similar regardless of whether patients were on methotrexate at 10 mg/week or less, 10-15 mg/week, or more than 15 mg/week. The investigators concluded that to minimize treatment-emergent adverse events, physicians can tailor background methotrexate dosing based upon individual patient tolerance without affecting certolizumab’s efficacy (Arthritis Care Res [Hoboken]. 2016 Mar;68[3]:299-307).
An important aspect of this analysis was that among the 325 subjects randomized to placebo rather than certolizumab, the treatment response at week 24 was significantly better in those on more than 15 mg/week of methotrexate than with lower doses of the drug.
“Most patients on methotrexate need more than 15 mg/week. So it astonishes me that such a high percentage of patients enrolled in clinical trials around the world are on, like, 14 mg/week. I mean, most patients need somewhere between 15 and 25 mg/week for a response, although over time you might be able to decrease that dose,” Dr. Weinblatt said.
Side effects of methotrexate
“The biggest issue with methotrexate is the tolerability problem, since serious adverse events are incredibly rare with this molecule,” he said.
Hepatotoxicity is a concern, but Dr. Weinblatt emphasized that elevated liver function tests do not equal cirrhosis.
“Historically, during the first 6 months on methotrexate 20%-25% of patients increase their transaminases in every clinical trial where that’s been looked at. Over time, the liver compensates for the drug. But 5%-6% of patients experience repeated moderate elevations more than 1.5 times the upper limit of normal,” he said.
Key risk factors for methotrexate-related hepatotoxicity were identified in a national observational cohort study of 659 military veterans over age 65 when they started methotrexate for rheumatic diseases. The investigators found a 6% incidence of moderately elevated liver enzymes during a mean follow-up period of 7 months. Obesity was associated with a 1.9-fold increased risk, a serum total cholesterol greater than 240 mg/dL conferred a 5.8-fold elevated risk, and abnormal liver function tests at baseline were associated with a 3.2-fold increased risk (Arthritis Care Res [Hoboken]. 2014 Aug;66[8]:1159-66).
“No surprise: It’s patients who weigh more who are at increased risk for methotrexate-related transaminase increases. I actually think the biggest factor with regard to methotrexate liver disease is the patient’s [body mass index]. Patients in North America aren’t getting any slimmer, so you need to look at this with your patients. If you have a morbidly obese patient on methotrexate whose transaminases suddenly start going up, that’s the patient who’s at greatest risk for methotrexate hepatotoxicity,” he cautioned.
The 3.2-fold increased risk of repeated elevated transaminases associated with abnormal baseline liver function tests in the Veterans Affairs study should be a red flag for rheumatologists.
“I personally think patients shouldn’t start on methotrexate if they have elevated transaminases. They ought to be normal at the start. There are too many other good options now to treat our patients,” Dr. Weinblatt said.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – “When I started working with methotrexate in 1982, I never would have predicted that methotrexate would become the standard of care in treating rheumatoid arthritis. There’s just no way,” Michael E. Weinblatt, MD, recalled at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Even now, 35 years later, we continue to learn more about this fascinating drug,” added Dr. Weinblatt, professor of medicine at Harvard Medical School, Boston.
He highlighted recent developments in this ongoing story and presented some tricks of the trade gained in 35 years of up-close experience with the drug.
Enhancing effectiveness of biologics
One of the hottest topics in methotrexate research is the drug’s ability to enhance the effectiveness of many, but not all, biologic agents. All of the anti–tumor necrosis factor (anti-TNF) biologics as well as rituximab (Rituxan) are demonstrably more effective when used in combination with methotrexate. Dr. Weinblatt considers the widespread underutilization of this combination strategy scandalous.
“This is an incredibly important point: All of you use biologics and all of you use methotrexate, but I’ve been depressed by the fact that up to 30%-40% of patients – no matter which data set you look at – are on monotherapy biological therapy,” he said.
He cited data from the ongoing BRASS Registry (Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Registry) to underscore his point that good things happen when biologics and methotrexate are used together. Of 1,395 BRASS Registry participants prospectively followed since 2003, the proportion on biologic therapy has climbed steadily from 41% at the outset to 68% in 2016. Remarkably, 82% of patients on biologic therapy remain on their first biologic agent. Fewer than 4% have switched biologics more than twice. That’s very unlike the experiences reported elsewhere.
“I think one of the reasons we have such positive data is that we have a high percentage of patients staying on their background methotrexate,” said Dr. Weinblatt, codirector of clinical rheumatology and associate director of the Center for Arthritis and Joint Diseases at Brigham and Women’s Hospital, Boston.
He noted that Dutch investigators reported at the 2016 annual meeting of the American College of Rheumatology that among 1,230 consecutive rheumatoid arthritis patients started on etanercept (Enbrel) or adalimumab (Humira), 28% in the etanercept group were on concomitant methotrexate, as were 64% of those who started on adalimumab. The patients spent a median of 1.3-1.6 years and a maximum of 9.2-9.3 years on their biologic agent. Patients on adalimumab monotherapy were 2.61-fold more likely to drop out than were those on dual therapy with methotrexate. Patients on etanercept monotherapy were 1.2-fold more likely to drop out, a difference that didn’t achieve statistical significance.
Although the investigators did not study the mechanism of prolonged on-treatment survival, they speculated that it probably involved methotrexate’s documented ability to prevent formation of anti-adalimumab antibodies. In contrast, patients on etanercept don’t develop blocking antibodies, Dr. Weinblatt observed.
The randomized, double-blind CONCERTO trial conducted in 395 methotrexate- and biologic-naive RA patients demonstrated that methotrexate reduces the immunogenicity of adalimumab in dose-dependent fashion. Participants were randomized to open-label adalimumab at 40 mg every 2 weeks plus weekly double-blind methotrexate at 2.5, 5, 10, or 20 mg. Clinical outcomes at 26 weeks as reflected in 28-joint count disease activity score and the Clinical Disease Activity Index were significantly better in patients on 10 or 20 mg/week of methotrexate than in those on 2.5 or 5 mg/week. Serum adalimumab levels were higher in patients on the two higher doses of methotrexate as well (Ann Rheum Dis. 2015 Jun;74[6]:1037-44).
“It ends up that if you don’t use methotrexate or you use a very low dose you increase the risk of developing antibodies against adalimumab and decrease the efficacy of the drug. So in clinical practice, if you’re going to be working with dose titration of methotrexate and your patient is on adalimumab, there’s a threshold below which you probably shouldn’t go. In this study, doses of 10 mg/week or more induced a greater clinical response,” he said.
With infliximab (Remicade), based upon 20-year-old studies, the threshold is 7.5 mg of methotrexate per week.
“With etanercept, we don’t know what the threshold is. You don’t develop blocking anti-drug antibodies with etanercept, but we do know that methotrexate enhances the efficacy of etanercept, and it doesn’t do it by changing the biologic’s pharmacokinetics and there’s no increase in methotrexate blood levels,” the rheumatologist continued.
Unlike the anti-TNF biologics and rituximab, the efficacy of the Janus kinase inhibitors tofacitinib (Xeljanz) and baricitinib is not enhanced when the drugs are used in combination with methotrexate, studies indicate.
The efficacy of certolizumab pegol (Cimzia) wasn’t affected by methotrexate dose category in a prespecified pooled subgroup analysis of the phase III RAPID 1 and RAPID 2 clinical trials. In the 1,273 certolizumab-treated patients, the week-24 treatment response was similar regardless of whether patients were on methotrexate at 10 mg/week or less, 10-15 mg/week, or more than 15 mg/week. The investigators concluded that to minimize treatment-emergent adverse events, physicians can tailor background methotrexate dosing based upon individual patient tolerance without affecting certolizumab’s efficacy (Arthritis Care Res [Hoboken]. 2016 Mar;68[3]:299-307).
An important aspect of this analysis was that among the 325 subjects randomized to placebo rather than certolizumab, the treatment response at week 24 was significantly better in those on more than 15 mg/week of methotrexate than with lower doses of the drug.
“Most patients on methotrexate need more than 15 mg/week. So it astonishes me that such a high percentage of patients enrolled in clinical trials around the world are on, like, 14 mg/week. I mean, most patients need somewhere between 15 and 25 mg/week for a response, although over time you might be able to decrease that dose,” Dr. Weinblatt said.
Side effects of methotrexate
“The biggest issue with methotrexate is the tolerability problem, since serious adverse events are incredibly rare with this molecule,” he said.
Hepatotoxicity is a concern, but Dr. Weinblatt emphasized that elevated liver function tests do not equal cirrhosis.
“Historically, during the first 6 months on methotrexate 20%-25% of patients increase their transaminases in every clinical trial where that’s been looked at. Over time, the liver compensates for the drug. But 5%-6% of patients experience repeated moderate elevations more than 1.5 times the upper limit of normal,” he said.
Key risk factors for methotrexate-related hepatotoxicity were identified in a national observational cohort study of 659 military veterans over age 65 when they started methotrexate for rheumatic diseases. The investigators found a 6% incidence of moderately elevated liver enzymes during a mean follow-up period of 7 months. Obesity was associated with a 1.9-fold increased risk, a serum total cholesterol greater than 240 mg/dL conferred a 5.8-fold elevated risk, and abnormal liver function tests at baseline were associated with a 3.2-fold increased risk (Arthritis Care Res [Hoboken]. 2014 Aug;66[8]:1159-66).
“No surprise: It’s patients who weigh more who are at increased risk for methotrexate-related transaminase increases. I actually think the biggest factor with regard to methotrexate liver disease is the patient’s [body mass index]. Patients in North America aren’t getting any slimmer, so you need to look at this with your patients. If you have a morbidly obese patient on methotrexate whose transaminases suddenly start going up, that’s the patient who’s at greatest risk for methotrexate hepatotoxicity,” he cautioned.
The 3.2-fold increased risk of repeated elevated transaminases associated with abnormal baseline liver function tests in the Veterans Affairs study should be a red flag for rheumatologists.
“I personally think patients shouldn’t start on methotrexate if they have elevated transaminases. They ought to be normal at the start. There are too many other good options now to treat our patients,” Dr. Weinblatt said.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – “When I started working with methotrexate in 1982, I never would have predicted that methotrexate would become the standard of care in treating rheumatoid arthritis. There’s just no way,” Michael E. Weinblatt, MD, recalled at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Even now, 35 years later, we continue to learn more about this fascinating drug,” added Dr. Weinblatt, professor of medicine at Harvard Medical School, Boston.
He highlighted recent developments in this ongoing story and presented some tricks of the trade gained in 35 years of up-close experience with the drug.
Enhancing effectiveness of biologics
One of the hottest topics in methotrexate research is the drug’s ability to enhance the effectiveness of many, but not all, biologic agents. All of the anti–tumor necrosis factor (anti-TNF) biologics as well as rituximab (Rituxan) are demonstrably more effective when used in combination with methotrexate. Dr. Weinblatt considers the widespread underutilization of this combination strategy scandalous.
“This is an incredibly important point: All of you use biologics and all of you use methotrexate, but I’ve been depressed by the fact that up to 30%-40% of patients – no matter which data set you look at – are on monotherapy biological therapy,” he said.
He cited data from the ongoing BRASS Registry (Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Registry) to underscore his point that good things happen when biologics and methotrexate are used together. Of 1,395 BRASS Registry participants prospectively followed since 2003, the proportion on biologic therapy has climbed steadily from 41% at the outset to 68% in 2016. Remarkably, 82% of patients on biologic therapy remain on their first biologic agent. Fewer than 4% have switched biologics more than twice. That’s very unlike the experiences reported elsewhere.
“I think one of the reasons we have such positive data is that we have a high percentage of patients staying on their background methotrexate,” said Dr. Weinblatt, codirector of clinical rheumatology and associate director of the Center for Arthritis and Joint Diseases at Brigham and Women’s Hospital, Boston.
He noted that Dutch investigators reported at the 2016 annual meeting of the American College of Rheumatology that among 1,230 consecutive rheumatoid arthritis patients started on etanercept (Enbrel) or adalimumab (Humira), 28% in the etanercept group were on concomitant methotrexate, as were 64% of those who started on adalimumab. The patients spent a median of 1.3-1.6 years and a maximum of 9.2-9.3 years on their biologic agent. Patients on adalimumab monotherapy were 2.61-fold more likely to drop out than were those on dual therapy with methotrexate. Patients on etanercept monotherapy were 1.2-fold more likely to drop out, a difference that didn’t achieve statistical significance.
Although the investigators did not study the mechanism of prolonged on-treatment survival, they speculated that it probably involved methotrexate’s documented ability to prevent formation of anti-adalimumab antibodies. In contrast, patients on etanercept don’t develop blocking antibodies, Dr. Weinblatt observed.
The randomized, double-blind CONCERTO trial conducted in 395 methotrexate- and biologic-naive RA patients demonstrated that methotrexate reduces the immunogenicity of adalimumab in dose-dependent fashion. Participants were randomized to open-label adalimumab at 40 mg every 2 weeks plus weekly double-blind methotrexate at 2.5, 5, 10, or 20 mg. Clinical outcomes at 26 weeks as reflected in 28-joint count disease activity score and the Clinical Disease Activity Index were significantly better in patients on 10 or 20 mg/week of methotrexate than in those on 2.5 or 5 mg/week. Serum adalimumab levels were higher in patients on the two higher doses of methotrexate as well (Ann Rheum Dis. 2015 Jun;74[6]:1037-44).
“It ends up that if you don’t use methotrexate or you use a very low dose you increase the risk of developing antibodies against adalimumab and decrease the efficacy of the drug. So in clinical practice, if you’re going to be working with dose titration of methotrexate and your patient is on adalimumab, there’s a threshold below which you probably shouldn’t go. In this study, doses of 10 mg/week or more induced a greater clinical response,” he said.
With infliximab (Remicade), based upon 20-year-old studies, the threshold is 7.5 mg of methotrexate per week.
“With etanercept, we don’t know what the threshold is. You don’t develop blocking anti-drug antibodies with etanercept, but we do know that methotrexate enhances the efficacy of etanercept, and it doesn’t do it by changing the biologic’s pharmacokinetics and there’s no increase in methotrexate blood levels,” the rheumatologist continued.
Unlike the anti-TNF biologics and rituximab, the efficacy of the Janus kinase inhibitors tofacitinib (Xeljanz) and baricitinib is not enhanced when the drugs are used in combination with methotrexate, studies indicate.
The efficacy of certolizumab pegol (Cimzia) wasn’t affected by methotrexate dose category in a prespecified pooled subgroup analysis of the phase III RAPID 1 and RAPID 2 clinical trials. In the 1,273 certolizumab-treated patients, the week-24 treatment response was similar regardless of whether patients were on methotrexate at 10 mg/week or less, 10-15 mg/week, or more than 15 mg/week. The investigators concluded that to minimize treatment-emergent adverse events, physicians can tailor background methotrexate dosing based upon individual patient tolerance without affecting certolizumab’s efficacy (Arthritis Care Res [Hoboken]. 2016 Mar;68[3]:299-307).
An important aspect of this analysis was that among the 325 subjects randomized to placebo rather than certolizumab, the treatment response at week 24 was significantly better in those on more than 15 mg/week of methotrexate than with lower doses of the drug.
“Most patients on methotrexate need more than 15 mg/week. So it astonishes me that such a high percentage of patients enrolled in clinical trials around the world are on, like, 14 mg/week. I mean, most patients need somewhere between 15 and 25 mg/week for a response, although over time you might be able to decrease that dose,” Dr. Weinblatt said.
Side effects of methotrexate
“The biggest issue with methotrexate is the tolerability problem, since serious adverse events are incredibly rare with this molecule,” he said.
Hepatotoxicity is a concern, but Dr. Weinblatt emphasized that elevated liver function tests do not equal cirrhosis.
“Historically, during the first 6 months on methotrexate 20%-25% of patients increase their transaminases in every clinical trial where that’s been looked at. Over time, the liver compensates for the drug. But 5%-6% of patients experience repeated moderate elevations more than 1.5 times the upper limit of normal,” he said.
Key risk factors for methotrexate-related hepatotoxicity were identified in a national observational cohort study of 659 military veterans over age 65 when they started methotrexate for rheumatic diseases. The investigators found a 6% incidence of moderately elevated liver enzymes during a mean follow-up period of 7 months. Obesity was associated with a 1.9-fold increased risk, a serum total cholesterol greater than 240 mg/dL conferred a 5.8-fold elevated risk, and abnormal liver function tests at baseline were associated with a 3.2-fold increased risk (Arthritis Care Res [Hoboken]. 2014 Aug;66[8]:1159-66).
“No surprise: It’s patients who weigh more who are at increased risk for methotrexate-related transaminase increases. I actually think the biggest factor with regard to methotrexate liver disease is the patient’s [body mass index]. Patients in North America aren’t getting any slimmer, so you need to look at this with your patients. If you have a morbidly obese patient on methotrexate whose transaminases suddenly start going up, that’s the patient who’s at greatest risk for methotrexate hepatotoxicity,” he cautioned.
The 3.2-fold increased risk of repeated elevated transaminases associated with abnormal baseline liver function tests in the Veterans Affairs study should be a red flag for rheumatologists.
“I personally think patients shouldn’t start on methotrexate if they have elevated transaminases. They ought to be normal at the start. There are too many other good options now to treat our patients,” Dr. Weinblatt said.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Hypertension in SLE pregnancy: Is it lupus flare or preeclampsia?
SNOWMASS, COLO. – No hard and fast test exists that would enable a physician to tell a flare of systemic lupus erythematosus from preeclampsia in a pregnant lupus patient who becomes hypertensive and ill, but there are highly useful clues, Bonnie L. Bermas, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“There is no perfect way of distinguishing between a lupus flare and preeclampsia. I’ve never walked away from the labor floor and said, ‘This is great – I know this is a lupus flare,’ or ‘I know this is preeclampsia.’ But you make your best guess as to which one it is, and that will inform your management,” explained Dr. Bermas, a rheumatologist and director of the clinical lupus program at Brigham and Women’s Hospital in Boston.
“Why do we care? Because if it’s preeclampsia the mother needs to be delivered immediately for her safety, while if it’s an SLE flare sometimes you can treat it and get the fetus to a more viable age. A 23-week-old baby isn’t at all likely to make it, but a 27-week-old could,” Dr. Bermas said.
The fact that the patient has thrombocytopenia isn’t helpful in making the distinction, since that feature is shared in common by SLE flares and preeclampsia. But the uric acid level is a useful clue.
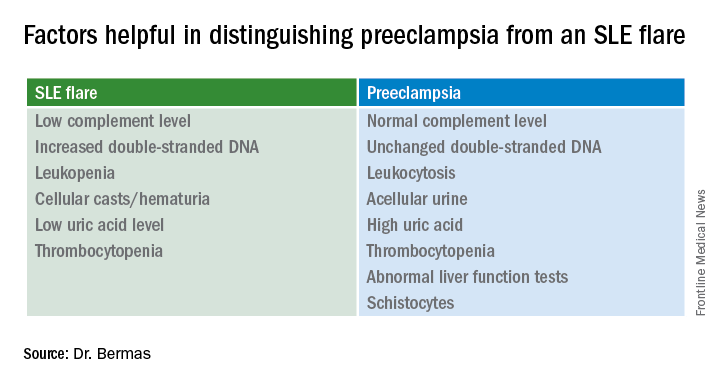
It’s quite possible that much of the current guesswork in predicting preeclampsia and other adverse pregnancy outcomes in lupus patients will give way to reliable risk testing within the next several years. Investigators in the U.S. multicenter prospective PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in APL Syndrome and SLE) study have reported that circulating levels of the angiogenic factors soluble fms-like tyrosine kinase-1 and placental growth factor were abnormal as early as gestational weeks 12-15 in patients who went on to develop preeclampsia or other adverse pregnancy outcomes.
Indeed, monthly testing demonstrated that SLE patients in the top quartile for soluble fms-like tyrosine kinase-1 at weeks 12-15 had an adjusted 17.3-fold greater likelihood of experiencing a severe adverse pregnancy outcome than did those in the lowest quartile. A high level had a positive predictive value of 61% and a negative predictive value of 93% (Am J Obstet Gynecol. 2016 Jan;214[1]:108.e1-14). These findings are being further explored in ongoing studies.
“Hopefully, in another few years we’re going to be able to say early in pregnancy, ‘This person is set up to get preeclampsia.’ Maybe that will lead to better treatment as well,” Dr. Bermas said.
The risk of preeclampsia has been shown to be threefold higher in women with SLE than in the general population of pregnant women in a study of more than 16.7 million admissions for childbirth in the United States during a 4-year period. The SLE patients were also at 2.4-fold increased risk for preterm labor. Their risks of infection, thrombosis, thrombocytopenia, and transfusion were each three- to seven-fold higher as well (Am J Obstet Gynecol. 2008 Aug;199[2]:127.e1-16).
Dr. Bermas reported serving as a consultant to UCB.
SNOWMASS, COLO. – No hard and fast test exists that would enable a physician to tell a flare of systemic lupus erythematosus from preeclampsia in a pregnant lupus patient who becomes hypertensive and ill, but there are highly useful clues, Bonnie L. Bermas, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“There is no perfect way of distinguishing between a lupus flare and preeclampsia. I’ve never walked away from the labor floor and said, ‘This is great – I know this is a lupus flare,’ or ‘I know this is preeclampsia.’ But you make your best guess as to which one it is, and that will inform your management,” explained Dr. Bermas, a rheumatologist and director of the clinical lupus program at Brigham and Women’s Hospital in Boston.
“Why do we care? Because if it’s preeclampsia the mother needs to be delivered immediately for her safety, while if it’s an SLE flare sometimes you can treat it and get the fetus to a more viable age. A 23-week-old baby isn’t at all likely to make it, but a 27-week-old could,” Dr. Bermas said.
The fact that the patient has thrombocytopenia isn’t helpful in making the distinction, since that feature is shared in common by SLE flares and preeclampsia. But the uric acid level is a useful clue.

It’s quite possible that much of the current guesswork in predicting preeclampsia and other adverse pregnancy outcomes in lupus patients will give way to reliable risk testing within the next several years. Investigators in the U.S. multicenter prospective PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in APL Syndrome and SLE) study have reported that circulating levels of the angiogenic factors soluble fms-like tyrosine kinase-1 and placental growth factor were abnormal as early as gestational weeks 12-15 in patients who went on to develop preeclampsia or other adverse pregnancy outcomes.
Indeed, monthly testing demonstrated that SLE patients in the top quartile for soluble fms-like tyrosine kinase-1 at weeks 12-15 had an adjusted 17.3-fold greater likelihood of experiencing a severe adverse pregnancy outcome than did those in the lowest quartile. A high level had a positive predictive value of 61% and a negative predictive value of 93% (Am J Obstet Gynecol. 2016 Jan;214[1]:108.e1-14). These findings are being further explored in ongoing studies.
“Hopefully, in another few years we’re going to be able to say early in pregnancy, ‘This person is set up to get preeclampsia.’ Maybe that will lead to better treatment as well,” Dr. Bermas said.
The risk of preeclampsia has been shown to be threefold higher in women with SLE than in the general population of pregnant women in a study of more than 16.7 million admissions for childbirth in the United States during a 4-year period. The SLE patients were also at 2.4-fold increased risk for preterm labor. Their risks of infection, thrombosis, thrombocytopenia, and transfusion were each three- to seven-fold higher as well (Am J Obstet Gynecol. 2008 Aug;199[2]:127.e1-16).
Dr. Bermas reported serving as a consultant to UCB.
SNOWMASS, COLO. – No hard and fast test exists that would enable a physician to tell a flare of systemic lupus erythematosus from preeclampsia in a pregnant lupus patient who becomes hypertensive and ill, but there are highly useful clues, Bonnie L. Bermas, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“There is no perfect way of distinguishing between a lupus flare and preeclampsia. I’ve never walked away from the labor floor and said, ‘This is great – I know this is a lupus flare,’ or ‘I know this is preeclampsia.’ But you make your best guess as to which one it is, and that will inform your management,” explained Dr. Bermas, a rheumatologist and director of the clinical lupus program at Brigham and Women’s Hospital in Boston.
“Why do we care? Because if it’s preeclampsia the mother needs to be delivered immediately for her safety, while if it’s an SLE flare sometimes you can treat it and get the fetus to a more viable age. A 23-week-old baby isn’t at all likely to make it, but a 27-week-old could,” Dr. Bermas said.
The fact that the patient has thrombocytopenia isn’t helpful in making the distinction, since that feature is shared in common by SLE flares and preeclampsia. But the uric acid level is a useful clue.

It’s quite possible that much of the current guesswork in predicting preeclampsia and other adverse pregnancy outcomes in lupus patients will give way to reliable risk testing within the next several years. Investigators in the U.S. multicenter prospective PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in APL Syndrome and SLE) study have reported that circulating levels of the angiogenic factors soluble fms-like tyrosine kinase-1 and placental growth factor were abnormal as early as gestational weeks 12-15 in patients who went on to develop preeclampsia or other adverse pregnancy outcomes.
Indeed, monthly testing demonstrated that SLE patients in the top quartile for soluble fms-like tyrosine kinase-1 at weeks 12-15 had an adjusted 17.3-fold greater likelihood of experiencing a severe adverse pregnancy outcome than did those in the lowest quartile. A high level had a positive predictive value of 61% and a negative predictive value of 93% (Am J Obstet Gynecol. 2016 Jan;214[1]:108.e1-14). These findings are being further explored in ongoing studies.
“Hopefully, in another few years we’re going to be able to say early in pregnancy, ‘This person is set up to get preeclampsia.’ Maybe that will lead to better treatment as well,” Dr. Bermas said.
The risk of preeclampsia has been shown to be threefold higher in women with SLE than in the general population of pregnant women in a study of more than 16.7 million admissions for childbirth in the United States during a 4-year period. The SLE patients were also at 2.4-fold increased risk for preterm labor. Their risks of infection, thrombosis, thrombocytopenia, and transfusion were each three- to seven-fold higher as well (Am J Obstet Gynecol. 2008 Aug;199[2]:127.e1-16).
Dr. Bermas reported serving as a consultant to UCB.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Ultrasound guidance of early RA therapy doesn’t improve outcomes
SNOWMASS, COLO. – Rheumatologists who use musculoskeletal ultrasound to help guide a treat-to-target strategy in early rheumatoid arthritis may want to reconsider that practice in light of the results of a recent randomized trial known as the TaSER study, Michael E. Weinblatt, MD, observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In TaSER, Scottish investigators randomized 111 newly diagnosed early rheumatoid arthritis patients to an intensive treat-to-target strategy guided by 28-joint Disease Activity Score with erythrocyte sedimentation rate (DAS28-ESR) alone or in conjunction with musculoskeletal ultrasound assessment of disease activity in a limited joint set.
The stepped treatment escalation regimen began with methotrexate monotherapy, followed by the addition of sulfasalazine and hydroxychloroquine to methotrexate as triple therapy, then etanercept plus triple therapy.
The primary outcome was improvement in DAS44 at 18 months. Both groups experienced similarly robust improvements: a mean 2.69-point reduction in the ultrasound group and a 2.59-point decrease in the control arm. Nor were there any between-group differences in radiographic or MRI erosions. This was the case even though the ultrasound group received more intensive therapy (Ann Rheum Dis. 2016 Jun;75[6]:1043-50).
“This doesn’t mean that there’s not great value in ultrasound to evaluate disease activity, but I’m not convinced that using ultrasound to guide your treatment per se shows any advantages over a good clinical evaluation of the patient,” concluded Dr. Weinblatt, professor of medicine at Harvard Medical School in Boston.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – Rheumatologists who use musculoskeletal ultrasound to help guide a treat-to-target strategy in early rheumatoid arthritis may want to reconsider that practice in light of the results of a recent randomized trial known as the TaSER study, Michael E. Weinblatt, MD, observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In TaSER, Scottish investigators randomized 111 newly diagnosed early rheumatoid arthritis patients to an intensive treat-to-target strategy guided by 28-joint Disease Activity Score with erythrocyte sedimentation rate (DAS28-ESR) alone or in conjunction with musculoskeletal ultrasound assessment of disease activity in a limited joint set.
The stepped treatment escalation regimen began with methotrexate monotherapy, followed by the addition of sulfasalazine and hydroxychloroquine to methotrexate as triple therapy, then etanercept plus triple therapy.
The primary outcome was improvement in DAS44 at 18 months. Both groups experienced similarly robust improvements: a mean 2.69-point reduction in the ultrasound group and a 2.59-point decrease in the control arm. Nor were there any between-group differences in radiographic or MRI erosions. This was the case even though the ultrasound group received more intensive therapy (Ann Rheum Dis. 2016 Jun;75[6]:1043-50).
“This doesn’t mean that there’s not great value in ultrasound to evaluate disease activity, but I’m not convinced that using ultrasound to guide your treatment per se shows any advantages over a good clinical evaluation of the patient,” concluded Dr. Weinblatt, professor of medicine at Harvard Medical School in Boston.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – Rheumatologists who use musculoskeletal ultrasound to help guide a treat-to-target strategy in early rheumatoid arthritis may want to reconsider that practice in light of the results of a recent randomized trial known as the TaSER study, Michael E. Weinblatt, MD, observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In TaSER, Scottish investigators randomized 111 newly diagnosed early rheumatoid arthritis patients to an intensive treat-to-target strategy guided by 28-joint Disease Activity Score with erythrocyte sedimentation rate (DAS28-ESR) alone or in conjunction with musculoskeletal ultrasound assessment of disease activity in a limited joint set.
The stepped treatment escalation regimen began with methotrexate monotherapy, followed by the addition of sulfasalazine and hydroxychloroquine to methotrexate as triple therapy, then etanercept plus triple therapy.
The primary outcome was improvement in DAS44 at 18 months. Both groups experienced similarly robust improvements: a mean 2.69-point reduction in the ultrasound group and a 2.59-point decrease in the control arm. Nor were there any between-group differences in radiographic or MRI erosions. This was the case even though the ultrasound group received more intensive therapy (Ann Rheum Dis. 2016 Jun;75[6]:1043-50).
“This doesn’t mean that there’s not great value in ultrasound to evaluate disease activity, but I’m not convinced that using ultrasound to guide your treatment per se shows any advantages over a good clinical evaluation of the patient,” concluded Dr. Weinblatt, professor of medicine at Harvard Medical School in Boston.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM













