User login
American Diabetes Association (ADA): Annual Scientific Sessions
Would patients open wallets for fewer diabetes drug doses?
CHICAGO – Conventional wisdom holds that, given the option, patients prefer the convenience of less-frequent dosing of oral drugs, but just how much more money are patients with type 2 diabetes willing to pay out of pocket to change from daily to once-weekly dosing of their oral antidiabetic medications?
Not so much, apparently. Specifically, they’d be prepared to fork over amounts ranging from no more than an extra $3.64 per month to change from one pill twice daily to one pill once a day, up to $13.88 to switch from two pills once per day to one pill once a week, according to a new survey.
To reduce their pill burden from one pill per day to one per week, patients with type 2 diabetes would be willing to spend an extra $5.86 out of pocket per month, A. Brett Hauber, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
"Reducing the frequency of dosing has monetary value to patients. The value isn’t huge. It’s modest but real," declared Dr. Hauber, senior economist and vice president at RTI Health Solutions, a medical industry research and consulting firm based in Research Triangle Park, N.C.
RTI was hired by Merck to evaluate patient preferences for reducing the pill burden associated with oral antidiabetic agents relative to other patient priorities. To do so, Dr. Hauber and his coworkers employed a technique social scientists call a discrete-choice experiment.
They presented a large group of type 2 diabetes patients with a detailed survey involving a series of pairs of hypothetical oral antidiabetic drug profiles and had participants choose their preferred option for each pair. The profiles addressed reductions in average blood glucose, hypoglycemia risk, frequency of mild-to-moderate GI side effects, out-of-pocket cost, dosing frequency, weight change, and incremental risk of chronic heart failure.
Each of these attributes generated a relative importance score that enabled researchers to see what was most important to patients with type 2 diabetes. For example, the data indicated that patients considered a 13% reduction in the risk of GI side effects to be about as important as an absolute 2% reduction in the risk of developing heart failure, from 3% to 1%, Dr. Hauber explained.
The study population consisted of 923 patients with type 2 diabetes who were not on injectable therapy. A total of 197 were treatment-naive, while the rest were taking a single oral antidiabetic drug. Participants were recruited by Knowledge Networks, an online research company with access to a nationally representative sample of U.S. households. Knowledge Networks knows its recruits very, very well: The company tracks 4,500 variables on each of them.
The two strongest drivers of patient choice regarding oral antidiabetic therapy to emerge from the study were out-of-pocket cost and glucose control. Cost was hands down the most important factor. Assigning it a standardized relative importance rate of 10, glucose control came in at 5.3. This was followed by hypoglycemia at 3.1, GI side effects at 2.6, weight change at 2.5, heart failure risk at 2.0, and, lastly, daily dosing schedule at 0.8.
"What’s most interesting is every one of those relative importance scores is statistically significantly different from zero. What that means is every one of these attributes was important, at least to some extent, to patients. Some were more important than others, but everything we assessed influenced their choices," Dr. Hauber said.
Sixty-seven percent of patients preferred weekly drug dosing, but 33% actually favored daily dosing. Younger patients – those under age 45 years – particularly preferred weekly dosing over daily, by a margin of 78% to 22%. Patients not currently on treatment also expressed a stronger than average preference for weekly over daily dosing, by a margin of 75% to 25%.
"It is likely that, if we can offer more convenient, once-weekly dosing to younger patients and treatment-naive patients, it may be that they would be more likely to initiate therapy and potentially to adhere to therapy. But this is just my speculation based on the results that we have. We haven’t actually proven that here," Dr. Hauber observed.
The study was sponsored by Merck. The presenter is an executive at RTI Health Solutions, the research firm hired to conduct the investigation.
CHICAGO – Conventional wisdom holds that, given the option, patients prefer the convenience of less-frequent dosing of oral drugs, but just how much more money are patients with type 2 diabetes willing to pay out of pocket to change from daily to once-weekly dosing of their oral antidiabetic medications?
Not so much, apparently. Specifically, they’d be prepared to fork over amounts ranging from no more than an extra $3.64 per month to change from one pill twice daily to one pill once a day, up to $13.88 to switch from two pills once per day to one pill once a week, according to a new survey.
To reduce their pill burden from one pill per day to one per week, patients with type 2 diabetes would be willing to spend an extra $5.86 out of pocket per month, A. Brett Hauber, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
"Reducing the frequency of dosing has monetary value to patients. The value isn’t huge. It’s modest but real," declared Dr. Hauber, senior economist and vice president at RTI Health Solutions, a medical industry research and consulting firm based in Research Triangle Park, N.C.
RTI was hired by Merck to evaluate patient preferences for reducing the pill burden associated with oral antidiabetic agents relative to other patient priorities. To do so, Dr. Hauber and his coworkers employed a technique social scientists call a discrete-choice experiment.
They presented a large group of type 2 diabetes patients with a detailed survey involving a series of pairs of hypothetical oral antidiabetic drug profiles and had participants choose their preferred option for each pair. The profiles addressed reductions in average blood glucose, hypoglycemia risk, frequency of mild-to-moderate GI side effects, out-of-pocket cost, dosing frequency, weight change, and incremental risk of chronic heart failure.
Each of these attributes generated a relative importance score that enabled researchers to see what was most important to patients with type 2 diabetes. For example, the data indicated that patients considered a 13% reduction in the risk of GI side effects to be about as important as an absolute 2% reduction in the risk of developing heart failure, from 3% to 1%, Dr. Hauber explained.
The study population consisted of 923 patients with type 2 diabetes who were not on injectable therapy. A total of 197 were treatment-naive, while the rest were taking a single oral antidiabetic drug. Participants were recruited by Knowledge Networks, an online research company with access to a nationally representative sample of U.S. households. Knowledge Networks knows its recruits very, very well: The company tracks 4,500 variables on each of them.
The two strongest drivers of patient choice regarding oral antidiabetic therapy to emerge from the study were out-of-pocket cost and glucose control. Cost was hands down the most important factor. Assigning it a standardized relative importance rate of 10, glucose control came in at 5.3. This was followed by hypoglycemia at 3.1, GI side effects at 2.6, weight change at 2.5, heart failure risk at 2.0, and, lastly, daily dosing schedule at 0.8.
"What’s most interesting is every one of those relative importance scores is statistically significantly different from zero. What that means is every one of these attributes was important, at least to some extent, to patients. Some were more important than others, but everything we assessed influenced their choices," Dr. Hauber said.
Sixty-seven percent of patients preferred weekly drug dosing, but 33% actually favored daily dosing. Younger patients – those under age 45 years – particularly preferred weekly dosing over daily, by a margin of 78% to 22%. Patients not currently on treatment also expressed a stronger than average preference for weekly over daily dosing, by a margin of 75% to 25%.
"It is likely that, if we can offer more convenient, once-weekly dosing to younger patients and treatment-naive patients, it may be that they would be more likely to initiate therapy and potentially to adhere to therapy. But this is just my speculation based on the results that we have. We haven’t actually proven that here," Dr. Hauber observed.
The study was sponsored by Merck. The presenter is an executive at RTI Health Solutions, the research firm hired to conduct the investigation.
CHICAGO – Conventional wisdom holds that, given the option, patients prefer the convenience of less-frequent dosing of oral drugs, but just how much more money are patients with type 2 diabetes willing to pay out of pocket to change from daily to once-weekly dosing of their oral antidiabetic medications?
Not so much, apparently. Specifically, they’d be prepared to fork over amounts ranging from no more than an extra $3.64 per month to change from one pill twice daily to one pill once a day, up to $13.88 to switch from two pills once per day to one pill once a week, according to a new survey.
To reduce their pill burden from one pill per day to one per week, patients with type 2 diabetes would be willing to spend an extra $5.86 out of pocket per month, A. Brett Hauber, Ph.D., reported at the annual scientific sessions of the American Diabetes Association.
"Reducing the frequency of dosing has monetary value to patients. The value isn’t huge. It’s modest but real," declared Dr. Hauber, senior economist and vice president at RTI Health Solutions, a medical industry research and consulting firm based in Research Triangle Park, N.C.
RTI was hired by Merck to evaluate patient preferences for reducing the pill burden associated with oral antidiabetic agents relative to other patient priorities. To do so, Dr. Hauber and his coworkers employed a technique social scientists call a discrete-choice experiment.
They presented a large group of type 2 diabetes patients with a detailed survey involving a series of pairs of hypothetical oral antidiabetic drug profiles and had participants choose their preferred option for each pair. The profiles addressed reductions in average blood glucose, hypoglycemia risk, frequency of mild-to-moderate GI side effects, out-of-pocket cost, dosing frequency, weight change, and incremental risk of chronic heart failure.
Each of these attributes generated a relative importance score that enabled researchers to see what was most important to patients with type 2 diabetes. For example, the data indicated that patients considered a 13% reduction in the risk of GI side effects to be about as important as an absolute 2% reduction in the risk of developing heart failure, from 3% to 1%, Dr. Hauber explained.
The study population consisted of 923 patients with type 2 diabetes who were not on injectable therapy. A total of 197 were treatment-naive, while the rest were taking a single oral antidiabetic drug. Participants were recruited by Knowledge Networks, an online research company with access to a nationally representative sample of U.S. households. Knowledge Networks knows its recruits very, very well: The company tracks 4,500 variables on each of them.
The two strongest drivers of patient choice regarding oral antidiabetic therapy to emerge from the study were out-of-pocket cost and glucose control. Cost was hands down the most important factor. Assigning it a standardized relative importance rate of 10, glucose control came in at 5.3. This was followed by hypoglycemia at 3.1, GI side effects at 2.6, weight change at 2.5, heart failure risk at 2.0, and, lastly, daily dosing schedule at 0.8.
"What’s most interesting is every one of those relative importance scores is statistically significantly different from zero. What that means is every one of these attributes was important, at least to some extent, to patients. Some were more important than others, but everything we assessed influenced their choices," Dr. Hauber said.
Sixty-seven percent of patients preferred weekly drug dosing, but 33% actually favored daily dosing. Younger patients – those under age 45 years – particularly preferred weekly dosing over daily, by a margin of 78% to 22%. Patients not currently on treatment also expressed a stronger than average preference for weekly over daily dosing, by a margin of 75% to 25%.
"It is likely that, if we can offer more convenient, once-weekly dosing to younger patients and treatment-naive patients, it may be that they would be more likely to initiate therapy and potentially to adhere to therapy. But this is just my speculation based on the results that we have. We haven’t actually proven that here," Dr. Hauber observed.
The study was sponsored by Merck. The presenter is an executive at RTI Health Solutions, the research firm hired to conduct the investigation.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
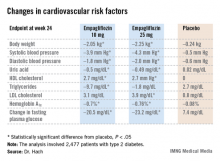
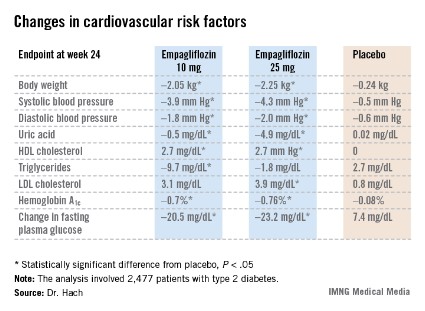
Major Finding: Patients with type 2 diabetes would be willing to spend an extra $5.86 out of pocket per month on their oral antidiabetic medication to change from one pill per day to one per week.
Data Source: An economic discrete-choice experiment involving a detailed survey completed by 923 patients with type 2 diabetes who were not on injectable therapy.
Disclosures: The study was sponsored by Merck. The presenter is an executive at RTI Health Solutions, the research firm hired to conduct the investigation.
Enteral nutrition linked to in-hospital mortality
CHICAGO – Hyperglycemia is common in patients receiving enteral nutrition and is a significant risk factor for hospital mortality, according to a retrospective study involving 157 patients.
Nearly 60% of patients had capillary blood glucose (CBG) values that exceeded 140 mg/dL, and nearly one-third exceeded 180 mg/dL.
More important, almost 40% of patients had a mean CBG of more than 180 mg/dL for the entire duration of their tube feedings.
At the threshold glucose of 180 mg/dL or more, the odds of dying in the hospital during tube feeding are approximately three times higher than for patients who had better glycemic control, Dr. Michael Jakoby said at the annual scientific sessions of the American Diabetes Association.
"We look at this body of work as just a stepping point to the next logical extension, which is to investigate ways that we can improve glycemic control for patients receiving any artificial nutrition, whether it’s parenteral or enteral," said Dr. Jakoby, chief of endocrinology at St. John’s Hospital in Springfield, Ill.
He previously reported that using an insulin protocol that determined insulin doses based on carbohydrate delivery and CBG was superior to ad hoc insulin dosing in the management of parenteral nutrition–induced hyperglycemia (JPEN J. Parenter. Enteral. Nutr. 2012;36:183-8).
Malnutrition among nonsurgical patients is high, and more than 40% of general surgery service patients have been reported to be malnourished (Am. J. Clin. Nutr. 1997;66:1232-9). Five studies since 2005 have established a link between parenteral nutrition and increased morbidity and in-hospital mortality, but little is available on outcomes with enteral nutrition, Dr. Jakoby explained.
The current analysis involved 157 patients receiving enteral nutrition in 2011 at St. John’s, a 350-bed hospital where each year an estimated 11,000 patients receive artificial nutrition for 2 weeks.
Of those patients, 58 were diagnosed with hyperglycemia (37%), defined as a mean CBG of 180 mg/dL or more for the duration of tube feedings.
Hyperglycemic patients were significantly older than controls (75 vs. 67), twice as likely to have a preexisting diagnosis of diabetes (81% vs. 41%), had 2 fewer days of enteral nutrition (7 vs. 9), and were less likely to be in the ICU during their tube feedings (62% vs. 83%), he said.
The two groups were well matched with regard to the amount of protein, fat, and total energy they received; however, hyperglycemic patients received significantly fewer carbohydrates than did controls (2.2 g/kg per day vs. 2.7 g/kg per day).
"With the documentation available to us, it seemed that this was an adaptive response to help combat the hyperglycemia that was happening during pure enteral nutrition," Dr. Jakoby said.
Cardiac complications – a composite of myocardial infarction, arrhythmia, and cardiac arrest – were increased in hyperglycemic patients (34% vs. 22%), but this difference was not significant in univariate analysis (odds ratio, 1.84; P = .13).
The risk of hospital mortality, however, was significantly higher in hyperglycemic patients in univariate analysis (36% vs. 16%; OR, 2.94), and remained significant in multivariate analysis after adjustment for age, sex, and preexisting diagnosis of diabetes (OR, 3.28), he noted.
During a discussion of the results, Dr. Jakoby said analyses are forthcoming looking at the impact of glycemic variability during hospitalization, observing that in a prior study he conducted, glycemic variability in the noncritical care setting strongly correlated with hospital stay and a greater likelihood of going from hospital to nursing home or the morgue.
Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
CHICAGO – Hyperglycemia is common in patients receiving enteral nutrition and is a significant risk factor for hospital mortality, according to a retrospective study involving 157 patients.
Nearly 60% of patients had capillary blood glucose (CBG) values that exceeded 140 mg/dL, and nearly one-third exceeded 180 mg/dL.
More important, almost 40% of patients had a mean CBG of more than 180 mg/dL for the entire duration of their tube feedings.
At the threshold glucose of 180 mg/dL or more, the odds of dying in the hospital during tube feeding are approximately three times higher than for patients who had better glycemic control, Dr. Michael Jakoby said at the annual scientific sessions of the American Diabetes Association.
"We look at this body of work as just a stepping point to the next logical extension, which is to investigate ways that we can improve glycemic control for patients receiving any artificial nutrition, whether it’s parenteral or enteral," said Dr. Jakoby, chief of endocrinology at St. John’s Hospital in Springfield, Ill.
He previously reported that using an insulin protocol that determined insulin doses based on carbohydrate delivery and CBG was superior to ad hoc insulin dosing in the management of parenteral nutrition–induced hyperglycemia (JPEN J. Parenter. Enteral. Nutr. 2012;36:183-8).
Malnutrition among nonsurgical patients is high, and more than 40% of general surgery service patients have been reported to be malnourished (Am. J. Clin. Nutr. 1997;66:1232-9). Five studies since 2005 have established a link between parenteral nutrition and increased morbidity and in-hospital mortality, but little is available on outcomes with enteral nutrition, Dr. Jakoby explained.
The current analysis involved 157 patients receiving enteral nutrition in 2011 at St. John’s, a 350-bed hospital where each year an estimated 11,000 patients receive artificial nutrition for 2 weeks.
Of those patients, 58 were diagnosed with hyperglycemia (37%), defined as a mean CBG of 180 mg/dL or more for the duration of tube feedings.
Hyperglycemic patients were significantly older than controls (75 vs. 67), twice as likely to have a preexisting diagnosis of diabetes (81% vs. 41%), had 2 fewer days of enteral nutrition (7 vs. 9), and were less likely to be in the ICU during their tube feedings (62% vs. 83%), he said.
The two groups were well matched with regard to the amount of protein, fat, and total energy they received; however, hyperglycemic patients received significantly fewer carbohydrates than did controls (2.2 g/kg per day vs. 2.7 g/kg per day).
"With the documentation available to us, it seemed that this was an adaptive response to help combat the hyperglycemia that was happening during pure enteral nutrition," Dr. Jakoby said.
Cardiac complications – a composite of myocardial infarction, arrhythmia, and cardiac arrest – were increased in hyperglycemic patients (34% vs. 22%), but this difference was not significant in univariate analysis (odds ratio, 1.84; P = .13).
The risk of hospital mortality, however, was significantly higher in hyperglycemic patients in univariate analysis (36% vs. 16%; OR, 2.94), and remained significant in multivariate analysis after adjustment for age, sex, and preexisting diagnosis of diabetes (OR, 3.28), he noted.
During a discussion of the results, Dr. Jakoby said analyses are forthcoming looking at the impact of glycemic variability during hospitalization, observing that in a prior study he conducted, glycemic variability in the noncritical care setting strongly correlated with hospital stay and a greater likelihood of going from hospital to nursing home or the morgue.
Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
CHICAGO – Hyperglycemia is common in patients receiving enteral nutrition and is a significant risk factor for hospital mortality, according to a retrospective study involving 157 patients.
Nearly 60% of patients had capillary blood glucose (CBG) values that exceeded 140 mg/dL, and nearly one-third exceeded 180 mg/dL.
More important, almost 40% of patients had a mean CBG of more than 180 mg/dL for the entire duration of their tube feedings.
At the threshold glucose of 180 mg/dL or more, the odds of dying in the hospital during tube feeding are approximately three times higher than for patients who had better glycemic control, Dr. Michael Jakoby said at the annual scientific sessions of the American Diabetes Association.
"We look at this body of work as just a stepping point to the next logical extension, which is to investigate ways that we can improve glycemic control for patients receiving any artificial nutrition, whether it’s parenteral or enteral," said Dr. Jakoby, chief of endocrinology at St. John’s Hospital in Springfield, Ill.
He previously reported that using an insulin protocol that determined insulin doses based on carbohydrate delivery and CBG was superior to ad hoc insulin dosing in the management of parenteral nutrition–induced hyperglycemia (JPEN J. Parenter. Enteral. Nutr. 2012;36:183-8).
Malnutrition among nonsurgical patients is high, and more than 40% of general surgery service patients have been reported to be malnourished (Am. J. Clin. Nutr. 1997;66:1232-9). Five studies since 2005 have established a link between parenteral nutrition and increased morbidity and in-hospital mortality, but little is available on outcomes with enteral nutrition, Dr. Jakoby explained.
The current analysis involved 157 patients receiving enteral nutrition in 2011 at St. John’s, a 350-bed hospital where each year an estimated 11,000 patients receive artificial nutrition for 2 weeks.
Of those patients, 58 were diagnosed with hyperglycemia (37%), defined as a mean CBG of 180 mg/dL or more for the duration of tube feedings.
Hyperglycemic patients were significantly older than controls (75 vs. 67), twice as likely to have a preexisting diagnosis of diabetes (81% vs. 41%), had 2 fewer days of enteral nutrition (7 vs. 9), and were less likely to be in the ICU during their tube feedings (62% vs. 83%), he said.
The two groups were well matched with regard to the amount of protein, fat, and total energy they received; however, hyperglycemic patients received significantly fewer carbohydrates than did controls (2.2 g/kg per day vs. 2.7 g/kg per day).
"With the documentation available to us, it seemed that this was an adaptive response to help combat the hyperglycemia that was happening during pure enteral nutrition," Dr. Jakoby said.
Cardiac complications – a composite of myocardial infarction, arrhythmia, and cardiac arrest – were increased in hyperglycemic patients (34% vs. 22%), but this difference was not significant in univariate analysis (odds ratio, 1.84; P = .13).
The risk of hospital mortality, however, was significantly higher in hyperglycemic patients in univariate analysis (36% vs. 16%; OR, 2.94), and remained significant in multivariate analysis after adjustment for age, sex, and preexisting diagnosis of diabetes (OR, 3.28), he noted.
During a discussion of the results, Dr. Jakoby said analyses are forthcoming looking at the impact of glycemic variability during hospitalization, observing that in a prior study he conducted, glycemic variability in the noncritical care setting strongly correlated with hospital stay and a greater likelihood of going from hospital to nursing home or the morgue.
Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
AT THE ANNUAL ADA SCIENTIFIC SESSIONS
Major finding: The mortality rate was 36% in hyperglycemic patients vs. 16% in controls (unadjusted OR 2.94; adjusted 3.28), a significant difference.
Data source: Retrospective analysis of 157 hospitalized patients receiving enteral nutrition.
Disclosures: Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
Oral sitagliptin promising for inpatient glycemic management in type 2 diabetes
CHICAGO – Once-daily oral sitagliptin appears to be safe and effective for the management of hyperglycemia in patients with type 2 diabetes hospitalized on general medicine or surgery wards, according to an industry-sponsored pilot study.
"Our results suggest that many hospitalized patients with type 2 diabetes could be treated with sitagliptin plus corrective rapid-acting insulin as needed. Patients with persistent hyperglycemia can be treated with sitagliptin and a low dose of glargine once daily or with basal-bolus insulin," Dr. Roma Gianchandani said at the annual scientific sessions of the American Diabetes Association.
Sitagliptin (Januvia) is an attractive alternative to current guideline-recommended basal-bolus insulin regimens, which are labor intensive, require multiple daily injections, and entail a significant risk of hypoglycemia, said Dr. Gianchandani of the University of Michigan, Ann Arbor.
She presented a randomized, open-label study of 82 noncritically ill patients with type 2 diabetes hospitalized on general medicine or surgery wards. Their mean hospital length of stay was 6.5 days. Because there have been no previous studies on the use of a dipeptidyl peptidase–4 (DPP-4) inhibitor such as sitagliptin in this context, entry criteria were stringent. Patients were enrolled if they had early-stage type 2 diabetes as reflected in a prehospital treatment regimen involving diet and oral antidiabetic agents or a low total daily insulin dose of up to 0.4 units/kg per day.
Participants were randomized to one of three treatment arms: sitagliptin at 100 mg once daily provided their creatinine clearance exceeded 50 mL/min, or 50 mg/day if their creatinine clearance was 30-50 mL/min; sitagliptin plus once-daily insulin glargine at 0.2 units/kg per day if their admission blood glucose value was 140-200 mg/dL, and glargine at 0.25 units/kg per day if it was 201-400 mg/dL; or a standard basal-bolus insulin regimen at a total daily insulin dose of 0.44 units/kg per day if their admission blood glucose was 140-200 mg/dL and 0.5 units/kg per day if it was 201-400 mg/dL. In the basal-bolus group, half of the total daily dose was given as glargine once daily and half as lispro in three equal doses before meals or every 6 hours.
Patients in all three study arms received a supplemental dose of lispro before meals if their premeal blood glucose exceeded 140 mg/dL.
A key study finding was that glycemic control improved similarly in all three study groups. After the first day of treatment there were no significant differences in mean daily blood glucose. Nor did the percentage of blood glucose readings falling within the target range of 70-140 mg/dL differ significantly: It was 36% in the sitagliptin-only group and 43% in the other two treatment arms. The percentage of blood glucose readings above the cutoffs of 140 and 200 mg/dL was likewise similar in all three treatment groups. The rate of treatment failure, defined as three or more consecutive blood glucose readings greater than 240 mg/dL or a mean daily blood glucose above that level, was 11% in the sitagliptin-alone group, 10% with sitagliptin plus basal insulin, and 8% with basal-bolus therapy.
The rate of hypoglycemia below 70 mg/dL was statistically similar in all three groups: 4% with sitagliptin alone, 7% with sitagliptin and basal insulin, and 8% with basal-bolus insulin.
Patients in the sitagliptin group averaged 1.8 insulin injections per day after day 1 of their hospital stay, significantly less than the 2.5 per day in the basal-bolus therapy group. Patients on sitagliptin plus basal insulin averaged 1.3 injections per day. Total insulin per day averaged 11.5 units in the sitagliptin group, 28.2 units in the sitagliptin plus basal insulin–treated patients, and 39.6 units in the basal-bolus group.
There were no between-group differences in complication rates or length of stay, Dr. Gianchandani continued.
One audience member said the generalizability of the study findings is limited by the small number of patients and stringent entry criteria. He estimated that 80% of his own type 2 diabetic patients hospitalized on general medicine or surgery wards wouldn’t have qualified for the trial.
Dr. Gianchandani responded that based on the favorable results of this pilot study, a larger study with a broader range of patients is being planned.
Another audience member, Dr. Stanley Schwartz of Ardmore, Pa., rose to enthusiastically declare, "I think this is actually a seminal study." He noted that nearly half of the patients in the sitagliptin-alone group never required any supplemental insulin injections during their hospital stay.
"Think about it: 40%-50% of these patients with early diabetes who may have otherwise required low doses of insulin didn’t need insulin because they were on sitagliptin alone. I think this is a superb study and should be pursued further," he said.
"We’re very excited about it, too," Dr. Gianchandani replied.
The study was sponsored by Merck, which markets sitagliptin. Dr. Gianchandani reported having no financial conflicts.
CHICAGO – Once-daily oral sitagliptin appears to be safe and effective for the management of hyperglycemia in patients with type 2 diabetes hospitalized on general medicine or surgery wards, according to an industry-sponsored pilot study.
"Our results suggest that many hospitalized patients with type 2 diabetes could be treated with sitagliptin plus corrective rapid-acting insulin as needed. Patients with persistent hyperglycemia can be treated with sitagliptin and a low dose of glargine once daily or with basal-bolus insulin," Dr. Roma Gianchandani said at the annual scientific sessions of the American Diabetes Association.
Sitagliptin (Januvia) is an attractive alternative to current guideline-recommended basal-bolus insulin regimens, which are labor intensive, require multiple daily injections, and entail a significant risk of hypoglycemia, said Dr. Gianchandani of the University of Michigan, Ann Arbor.
She presented a randomized, open-label study of 82 noncritically ill patients with type 2 diabetes hospitalized on general medicine or surgery wards. Their mean hospital length of stay was 6.5 days. Because there have been no previous studies on the use of a dipeptidyl peptidase–4 (DPP-4) inhibitor such as sitagliptin in this context, entry criteria were stringent. Patients were enrolled if they had early-stage type 2 diabetes as reflected in a prehospital treatment regimen involving diet and oral antidiabetic agents or a low total daily insulin dose of up to 0.4 units/kg per day.
Participants were randomized to one of three treatment arms: sitagliptin at 100 mg once daily provided their creatinine clearance exceeded 50 mL/min, or 50 mg/day if their creatinine clearance was 30-50 mL/min; sitagliptin plus once-daily insulin glargine at 0.2 units/kg per day if their admission blood glucose value was 140-200 mg/dL, and glargine at 0.25 units/kg per day if it was 201-400 mg/dL; or a standard basal-bolus insulin regimen at a total daily insulin dose of 0.44 units/kg per day if their admission blood glucose was 140-200 mg/dL and 0.5 units/kg per day if it was 201-400 mg/dL. In the basal-bolus group, half of the total daily dose was given as glargine once daily and half as lispro in three equal doses before meals or every 6 hours.
Patients in all three study arms received a supplemental dose of lispro before meals if their premeal blood glucose exceeded 140 mg/dL.
A key study finding was that glycemic control improved similarly in all three study groups. After the first day of treatment there were no significant differences in mean daily blood glucose. Nor did the percentage of blood glucose readings falling within the target range of 70-140 mg/dL differ significantly: It was 36% in the sitagliptin-only group and 43% in the other two treatment arms. The percentage of blood glucose readings above the cutoffs of 140 and 200 mg/dL was likewise similar in all three treatment groups. The rate of treatment failure, defined as three or more consecutive blood glucose readings greater than 240 mg/dL or a mean daily blood glucose above that level, was 11% in the sitagliptin-alone group, 10% with sitagliptin plus basal insulin, and 8% with basal-bolus therapy.
The rate of hypoglycemia below 70 mg/dL was statistically similar in all three groups: 4% with sitagliptin alone, 7% with sitagliptin and basal insulin, and 8% with basal-bolus insulin.
Patients in the sitagliptin group averaged 1.8 insulin injections per day after day 1 of their hospital stay, significantly less than the 2.5 per day in the basal-bolus therapy group. Patients on sitagliptin plus basal insulin averaged 1.3 injections per day. Total insulin per day averaged 11.5 units in the sitagliptin group, 28.2 units in the sitagliptin plus basal insulin–treated patients, and 39.6 units in the basal-bolus group.
There were no between-group differences in complication rates or length of stay, Dr. Gianchandani continued.
One audience member said the generalizability of the study findings is limited by the small number of patients and stringent entry criteria. He estimated that 80% of his own type 2 diabetic patients hospitalized on general medicine or surgery wards wouldn’t have qualified for the trial.
Dr. Gianchandani responded that based on the favorable results of this pilot study, a larger study with a broader range of patients is being planned.
Another audience member, Dr. Stanley Schwartz of Ardmore, Pa., rose to enthusiastically declare, "I think this is actually a seminal study." He noted that nearly half of the patients in the sitagliptin-alone group never required any supplemental insulin injections during their hospital stay.
"Think about it: 40%-50% of these patients with early diabetes who may have otherwise required low doses of insulin didn’t need insulin because they were on sitagliptin alone. I think this is a superb study and should be pursued further," he said.
"We’re very excited about it, too," Dr. Gianchandani replied.
The study was sponsored by Merck, which markets sitagliptin. Dr. Gianchandani reported having no financial conflicts.
CHICAGO – Once-daily oral sitagliptin appears to be safe and effective for the management of hyperglycemia in patients with type 2 diabetes hospitalized on general medicine or surgery wards, according to an industry-sponsored pilot study.
"Our results suggest that many hospitalized patients with type 2 diabetes could be treated with sitagliptin plus corrective rapid-acting insulin as needed. Patients with persistent hyperglycemia can be treated with sitagliptin and a low dose of glargine once daily or with basal-bolus insulin," Dr. Roma Gianchandani said at the annual scientific sessions of the American Diabetes Association.
Sitagliptin (Januvia) is an attractive alternative to current guideline-recommended basal-bolus insulin regimens, which are labor intensive, require multiple daily injections, and entail a significant risk of hypoglycemia, said Dr. Gianchandani of the University of Michigan, Ann Arbor.
She presented a randomized, open-label study of 82 noncritically ill patients with type 2 diabetes hospitalized on general medicine or surgery wards. Their mean hospital length of stay was 6.5 days. Because there have been no previous studies on the use of a dipeptidyl peptidase–4 (DPP-4) inhibitor such as sitagliptin in this context, entry criteria were stringent. Patients were enrolled if they had early-stage type 2 diabetes as reflected in a prehospital treatment regimen involving diet and oral antidiabetic agents or a low total daily insulin dose of up to 0.4 units/kg per day.
Participants were randomized to one of three treatment arms: sitagliptin at 100 mg once daily provided their creatinine clearance exceeded 50 mL/min, or 50 mg/day if their creatinine clearance was 30-50 mL/min; sitagliptin plus once-daily insulin glargine at 0.2 units/kg per day if their admission blood glucose value was 140-200 mg/dL, and glargine at 0.25 units/kg per day if it was 201-400 mg/dL; or a standard basal-bolus insulin regimen at a total daily insulin dose of 0.44 units/kg per day if their admission blood glucose was 140-200 mg/dL and 0.5 units/kg per day if it was 201-400 mg/dL. In the basal-bolus group, half of the total daily dose was given as glargine once daily and half as lispro in three equal doses before meals or every 6 hours.
Patients in all three study arms received a supplemental dose of lispro before meals if their premeal blood glucose exceeded 140 mg/dL.
A key study finding was that glycemic control improved similarly in all three study groups. After the first day of treatment there were no significant differences in mean daily blood glucose. Nor did the percentage of blood glucose readings falling within the target range of 70-140 mg/dL differ significantly: It was 36% in the sitagliptin-only group and 43% in the other two treatment arms. The percentage of blood glucose readings above the cutoffs of 140 and 200 mg/dL was likewise similar in all three treatment groups. The rate of treatment failure, defined as three or more consecutive blood glucose readings greater than 240 mg/dL or a mean daily blood glucose above that level, was 11% in the sitagliptin-alone group, 10% with sitagliptin plus basal insulin, and 8% with basal-bolus therapy.
The rate of hypoglycemia below 70 mg/dL was statistically similar in all three groups: 4% with sitagliptin alone, 7% with sitagliptin and basal insulin, and 8% with basal-bolus insulin.
Patients in the sitagliptin group averaged 1.8 insulin injections per day after day 1 of their hospital stay, significantly less than the 2.5 per day in the basal-bolus therapy group. Patients on sitagliptin plus basal insulin averaged 1.3 injections per day. Total insulin per day averaged 11.5 units in the sitagliptin group, 28.2 units in the sitagliptin plus basal insulin–treated patients, and 39.6 units in the basal-bolus group.
There were no between-group differences in complication rates or length of stay, Dr. Gianchandani continued.
One audience member said the generalizability of the study findings is limited by the small number of patients and stringent entry criteria. He estimated that 80% of his own type 2 diabetic patients hospitalized on general medicine or surgery wards wouldn’t have qualified for the trial.
Dr. Gianchandani responded that based on the favorable results of this pilot study, a larger study with a broader range of patients is being planned.
Another audience member, Dr. Stanley Schwartz of Ardmore, Pa., rose to enthusiastically declare, "I think this is actually a seminal study." He noted that nearly half of the patients in the sitagliptin-alone group never required any supplemental insulin injections during their hospital stay.
"Think about it: 40%-50% of these patients with early diabetes who may have otherwise required low doses of insulin didn’t need insulin because they were on sitagliptin alone. I think this is a superb study and should be pursued further," he said.
"We’re very excited about it, too," Dr. Gianchandani replied.
The study was sponsored by Merck, which markets sitagliptin. Dr. Gianchandani reported having no financial conflicts.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Glycemic control was statistically as good with once-daily oral sitagliptin as with guideline-recommended multi-injection basal-bolus insulin (36% and 43%, respectively).
Data source: A randomized, prospective, open-label pilot study involving 82 type 2 diabetic patients hospitalized on general medicine or surgery wards.
Disclosures: The study was sponsored by Merck, which markets sitagliptin. Dr. Gianchandani reported having no financial conflicts.
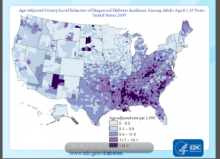
Hypoglycemia admissions tops among Medicare patients
CHICAGO – Hospital admissions for hypoglycemia exceed those for hyperglycemia among Medicare beneficiaries, with significant disparities existing between subgroups.
Blacks had a fourfold higher admission rate for hyperglycemia and hypoglycemia than did whites during 1999-2011, while those aged 85 years and older had twofold higher admissions for hypoglycemia than did younger beneficiaries.
"Continued efforts are needed to reduce hypoglycemia, particularly among blacks and oldest patients," Dr. Kasia J. Lipska said at the annual scientific sessions of the American Diabetes Association. "We suggest that assessment of adverse effects of treatment, such as hypoglycemia, is important to fully understand the impact of treatment strategies."
More intensive glycemic control and increased availability of glucose-lowering drugs in the past 2 decades has helped drive down hospitalizations for hyperglycemia, but as an unintended consequence, admissions for hypoglycemia may have risen, particularly in older patients.
The investigators evaluated changes in admission rates for principal discharge diagnoses of hyperglycemia or hypoglycemia among nearly 34 million Medicare fee-for-service patients, aged 65 and older, from 1999 to 2011. Hyperglycemia diagnoses included uncontrolled diabetes, diabetic ketoacidosis, diabetes with hyperosmolarity, and diabetes with other coma. Hypoglycemia diagnoses were hypoglycemic coma; diabetic hypoglycemia not otherwise specified or hypoglycemic shock; specified and unspecified hypoglycemia; and poisoning by insulin or antidiabetic agents.
During the study period, 302,095 patients were hospitalized for hyperglycemia and 429,850 for hypoglycemia, reported Dr. Lipska, instructor in medicine (endocrinology) at Yale University, New Haven, Conn.
Hyperglycemia admissions fell 39% from 114/100,000 patient-years in 1999 to 70/100,000 patient-years in 2011.
On the other hand, hypoglycemia admissions increased steadily from 94/100,000 patient-years in 1999 to their peak of 130/100,000 in 2007, declining modestly since then to 105/100,000 in 2011.
"There has been a flip, with hypoglycemia now clearly exceeding hyperglycemia by 2011," Dr. Lipska said.
After adjustment for diabetes prevalence, hyperglycemia admissions came down even more dramatically, falling by 55% from 1999 to 2011 (820 to 367/100,000 patient-years), while hypoglycemia admissions appear more stable, with a slight decline overall (676 to 612/100,000 patient-years), she added.
Session comoderator Dr. Hermes Florez, with the University of Miami Health System, said in an interview, "Clearly, with the change in practice and a growing of the elderly population, we’re doing a better job reducing significantly the hyperglycemic events, but maybe being too aggressive at some point, especially with the elderly population, may have triggered a higher incidence of hypoglycemia."
He observed that admission trends began to shift in 2007, possibly reflecting the reduction in rosiglitazone (Avandia) use, after the drug was found to increase the risk of heart attacks. At the same time, enthusiasm for intensive glycemic control began to wane as a result of the excess mortality reported in the pivotal ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial (N. Engl. J. Med. 2008;358;2545-59).
"The tasks for us are to reduce, hopefully, the rates of hypoglycemia by better educating providers, and tailoring this education to the different subgroups of older adults," Dr. Florez said. "You might have individuals that are quite functional, and you can be more aggressive in these patients, but you also have more vulnerable individuals who are more prone to hypoglycemia."
During a discussion of the analysis, an audience member asked whether paramedic data were included as the results could represent a great underestimate because many patients receive treatment for diabetic-related emergencies outside the hospital setting.
Dr. Lipska said these data were not available, but agreed that this may be only the tip of the iceberg. "Yes, there are lots of patients who are seen by paramedics and never see a doctor in the emergency room," she remarked.
The study was supported by the National Heart, Lung, and Blood Institute and the National Institute on Aging. Dr. Lipska reported having no financial disclosures.
CHICAGO – Hospital admissions for hypoglycemia exceed those for hyperglycemia among Medicare beneficiaries, with significant disparities existing between subgroups.
Blacks had a fourfold higher admission rate for hyperglycemia and hypoglycemia than did whites during 1999-2011, while those aged 85 years and older had twofold higher admissions for hypoglycemia than did younger beneficiaries.
"Continued efforts are needed to reduce hypoglycemia, particularly among blacks and oldest patients," Dr. Kasia J. Lipska said at the annual scientific sessions of the American Diabetes Association. "We suggest that assessment of adverse effects of treatment, such as hypoglycemia, is important to fully understand the impact of treatment strategies."
More intensive glycemic control and increased availability of glucose-lowering drugs in the past 2 decades has helped drive down hospitalizations for hyperglycemia, but as an unintended consequence, admissions for hypoglycemia may have risen, particularly in older patients.
The investigators evaluated changes in admission rates for principal discharge diagnoses of hyperglycemia or hypoglycemia among nearly 34 million Medicare fee-for-service patients, aged 65 and older, from 1999 to 2011. Hyperglycemia diagnoses included uncontrolled diabetes, diabetic ketoacidosis, diabetes with hyperosmolarity, and diabetes with other coma. Hypoglycemia diagnoses were hypoglycemic coma; diabetic hypoglycemia not otherwise specified or hypoglycemic shock; specified and unspecified hypoglycemia; and poisoning by insulin or antidiabetic agents.
During the study period, 302,095 patients were hospitalized for hyperglycemia and 429,850 for hypoglycemia, reported Dr. Lipska, instructor in medicine (endocrinology) at Yale University, New Haven, Conn.
Hyperglycemia admissions fell 39% from 114/100,000 patient-years in 1999 to 70/100,000 patient-years in 2011.
On the other hand, hypoglycemia admissions increased steadily from 94/100,000 patient-years in 1999 to their peak of 130/100,000 in 2007, declining modestly since then to 105/100,000 in 2011.
"There has been a flip, with hypoglycemia now clearly exceeding hyperglycemia by 2011," Dr. Lipska said.
After adjustment for diabetes prevalence, hyperglycemia admissions came down even more dramatically, falling by 55% from 1999 to 2011 (820 to 367/100,000 patient-years), while hypoglycemia admissions appear more stable, with a slight decline overall (676 to 612/100,000 patient-years), she added.
Session comoderator Dr. Hermes Florez, with the University of Miami Health System, said in an interview, "Clearly, with the change in practice and a growing of the elderly population, we’re doing a better job reducing significantly the hyperglycemic events, but maybe being too aggressive at some point, especially with the elderly population, may have triggered a higher incidence of hypoglycemia."
He observed that admission trends began to shift in 2007, possibly reflecting the reduction in rosiglitazone (Avandia) use, after the drug was found to increase the risk of heart attacks. At the same time, enthusiasm for intensive glycemic control began to wane as a result of the excess mortality reported in the pivotal ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial (N. Engl. J. Med. 2008;358;2545-59).
"The tasks for us are to reduce, hopefully, the rates of hypoglycemia by better educating providers, and tailoring this education to the different subgroups of older adults," Dr. Florez said. "You might have individuals that are quite functional, and you can be more aggressive in these patients, but you also have more vulnerable individuals who are more prone to hypoglycemia."
During a discussion of the analysis, an audience member asked whether paramedic data were included as the results could represent a great underestimate because many patients receive treatment for diabetic-related emergencies outside the hospital setting.
Dr. Lipska said these data were not available, but agreed that this may be only the tip of the iceberg. "Yes, there are lots of patients who are seen by paramedics and never see a doctor in the emergency room," she remarked.
The study was supported by the National Heart, Lung, and Blood Institute and the National Institute on Aging. Dr. Lipska reported having no financial disclosures.
CHICAGO – Hospital admissions for hypoglycemia exceed those for hyperglycemia among Medicare beneficiaries, with significant disparities existing between subgroups.
Blacks had a fourfold higher admission rate for hyperglycemia and hypoglycemia than did whites during 1999-2011, while those aged 85 years and older had twofold higher admissions for hypoglycemia than did younger beneficiaries.
"Continued efforts are needed to reduce hypoglycemia, particularly among blacks and oldest patients," Dr. Kasia J. Lipska said at the annual scientific sessions of the American Diabetes Association. "We suggest that assessment of adverse effects of treatment, such as hypoglycemia, is important to fully understand the impact of treatment strategies."
More intensive glycemic control and increased availability of glucose-lowering drugs in the past 2 decades has helped drive down hospitalizations for hyperglycemia, but as an unintended consequence, admissions for hypoglycemia may have risen, particularly in older patients.
The investigators evaluated changes in admission rates for principal discharge diagnoses of hyperglycemia or hypoglycemia among nearly 34 million Medicare fee-for-service patients, aged 65 and older, from 1999 to 2011. Hyperglycemia diagnoses included uncontrolled diabetes, diabetic ketoacidosis, diabetes with hyperosmolarity, and diabetes with other coma. Hypoglycemia diagnoses were hypoglycemic coma; diabetic hypoglycemia not otherwise specified or hypoglycemic shock; specified and unspecified hypoglycemia; and poisoning by insulin or antidiabetic agents.
During the study period, 302,095 patients were hospitalized for hyperglycemia and 429,850 for hypoglycemia, reported Dr. Lipska, instructor in medicine (endocrinology) at Yale University, New Haven, Conn.
Hyperglycemia admissions fell 39% from 114/100,000 patient-years in 1999 to 70/100,000 patient-years in 2011.
On the other hand, hypoglycemia admissions increased steadily from 94/100,000 patient-years in 1999 to their peak of 130/100,000 in 2007, declining modestly since then to 105/100,000 in 2011.
"There has been a flip, with hypoglycemia now clearly exceeding hyperglycemia by 2011," Dr. Lipska said.
After adjustment for diabetes prevalence, hyperglycemia admissions came down even more dramatically, falling by 55% from 1999 to 2011 (820 to 367/100,000 patient-years), while hypoglycemia admissions appear more stable, with a slight decline overall (676 to 612/100,000 patient-years), she added.
Session comoderator Dr. Hermes Florez, with the University of Miami Health System, said in an interview, "Clearly, with the change in practice and a growing of the elderly population, we’re doing a better job reducing significantly the hyperglycemic events, but maybe being too aggressive at some point, especially with the elderly population, may have triggered a higher incidence of hypoglycemia."
He observed that admission trends began to shift in 2007, possibly reflecting the reduction in rosiglitazone (Avandia) use, after the drug was found to increase the risk of heart attacks. At the same time, enthusiasm for intensive glycemic control began to wane as a result of the excess mortality reported in the pivotal ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial (N. Engl. J. Med. 2008;358;2545-59).
"The tasks for us are to reduce, hopefully, the rates of hypoglycemia by better educating providers, and tailoring this education to the different subgroups of older adults," Dr. Florez said. "You might have individuals that are quite functional, and you can be more aggressive in these patients, but you also have more vulnerable individuals who are more prone to hypoglycemia."
During a discussion of the analysis, an audience member asked whether paramedic data were included as the results could represent a great underestimate because many patients receive treatment for diabetic-related emergencies outside the hospital setting.
Dr. Lipska said these data were not available, but agreed that this may be only the tip of the iceberg. "Yes, there are lots of patients who are seen by paramedics and never see a doctor in the emergency room," she remarked.
The study was supported by the National Heart, Lung, and Blood Institute and the National Institute on Aging. Dr. Lipska reported having no financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Hypoglycemia admissions were 94/100,000 patient-years in 1999, peaked at 130/100,000 in 2007, and declined modestly to 105/100,000 in 2011.
Data source: Retrospective analysis of hypo- and hyperglycemia admissions among nearly 34 million Medicare fee-for-service patients.
Disclosures: The study was supported by the National Heart, Lung, and Blood Institute and the National Institute on Aging. Dr. Lipska reported having no financial disclosures.
Acute antipsychotics do not worsen glycemia in psychiatric inpatients
CHICAGO – Treatment with antipsychotic agents in acute psychiatric inpatient units is not associated with increased blood glucose levels, according to a multicenter study.
"This finding is contrary to what many of us may have previously considered," Dr. Dawn D. Smiley observed in presenting the study findings at the annual scientific sessions of the American Diabetes Association.
A well-recognized association exists between the use of antipsychotic agents and an increased prevalence of diabetes and hyperglycemia, although it remains unclear whether the relationship is causal. The association is especially strong for the atypical or second-generation antipsychotics, which account for more than 90% of all antipsychotic use and are among the top-selling prescription drugs in the United States. Indeed, the Food and Drug Administration has for a decade required the product labeling for all atypical antipsychotics to include a warning about the risks of hyperglycemia and diabetes.
But most data on antipsychotic-associated diabetes come from long-term studies, which show that diabetes, when it occurs, most often does so within the first 6 months on the medication. Little is known about the acute impact of antipsychotic agents on blood glucose levels and clinical outcomes in patients admitted to acute psychiatric units for stays averaging a few weeks. This information gap provided the rationale for Dr. Smiley’s study.
She presented a chart review of 1,212 patients admitted to three acute inpatient psychiatric units in inner-city Atlanta during 2008. The mean age of the patients was 57 years, they had a mean body mass index of 27 kg/m2, and the mean daily blood glucose concentration was 122 mg/dL during their stay. Twenty-one percent of the patients had a history of diabetes, and 6% had new hyperglycemia at admission. The most common primary diagnoses on admission were suicidal ideation in 28% of patients, a primary psychotic disorder in 27%, and depression in 16%.
Thirty-seven percent of patients had no prior experience with antipsychotic agents but were newly started on an antipsychotic during their acute inpatient stay. An additional 38% had been on antipsychotic therapy prior to admission and continued on it while hospitalized. Twenty-five percent of patients had never been treated with antipsychotic agents and were not during their hospitalization. Fifty-two percent of patients were treated with atypical antipsychotic agents during their inpatient stay, 17% received both atypical and typical antipsychotics, and 6% got typical antipsychotics only, explained Dr. Smiley, an endocrinologist at Emory University, Atlanta.
She sought answers to three research questions:
• Does prior exposure to antipsychotic therapy affect inpatient blood glucose levels or clinical outcome measures? Mean psychiatric inpatient length of stay was significantly shorter for patients never exposed to antipsychotics, at 9.5 days, compared with 14.2 days in patients who continued on their previous antipsychotic regimen and 13.7 days in antipsychotic-naive patients started on an antipsychotic for the first time. The most likely explanation for the longer stays in patients on antipsychotic therapy was the need to monitor them for side effects, according to Dr. Smiley.
Importantly, mean daily blood glucose concentrations during psychiatric hospitalization did not differ between patients never exposed to antipsychotic agents, those started on an antipsychotic for the first time during their hospitalization, and patients continued on their previous antipsychotics.
• Does the type of antipsychotic agent used during the acute inpatient admission affect blood glucose levels or clinical outcomes?
Mean daily blood glucose levels were similar regardless of whether patients were on atypical antipsychotics, typical antipsychotics, or both. The mean hemoglobin A1c level was 7.3% in all three groups of antipsychotic-treated patients. However, the rate of medical complications during psychiatric hospitalization was significantly higher among patients on atypical agents, at 23%, compared with 17% in patients on typical antipsychotics and 13% in those on both.
Three percent of patients on atypical antipsychotics-only required transfer to a medical unit for treatment of a complication that arose during psychiatric hospitalization. That was also true for 3% of patients on combined atypical/typical antipsychotic therapy. In contrast, none of the relatively few patients on typical antipsychotics-only required transfer to a medical unit. It’s worth noting that all atypical antipsychotics are not alike in terms of the strength of their association with diabetes. Most antipsychotic-treated patients in this study got olanzapine (Zyprexa) or clozapine (Clozaril). Newer, less diabetogenic atypical agents were less frequently used in this largely indigent population.
• Does a history of diabetes or new hyperglycemia at admission affect outcome measures? Not unexpectedly, patients with a history of diabetes prior to admission had a significantly higher rate of medical complications during their psychiatric inpatient stay: a 28% rate, compared with 22% in patients with no history of diabetes but with hyperglycemia at admission and 20% in patients with neither metabolic abnormality. The most common complication in patients with a history of diabetes was urinary tract infection.
Again unsurprisingly, patients with a history of diabetes had a significantly higher rate of transfer to a medical unit because of medical complications: 5%, compared with no transfers in patients with new hyperglycemia at admission and a 1% transfer rate in patients with neither metabolic abnormality.
Dr. Smiley reported that her research funding came from the National Institutes of Health, Sanofi-Aventis, and Merck.
CHICAGO – Treatment with antipsychotic agents in acute psychiatric inpatient units is not associated with increased blood glucose levels, according to a multicenter study.
"This finding is contrary to what many of us may have previously considered," Dr. Dawn D. Smiley observed in presenting the study findings at the annual scientific sessions of the American Diabetes Association.
A well-recognized association exists between the use of antipsychotic agents and an increased prevalence of diabetes and hyperglycemia, although it remains unclear whether the relationship is causal. The association is especially strong for the atypical or second-generation antipsychotics, which account for more than 90% of all antipsychotic use and are among the top-selling prescription drugs in the United States. Indeed, the Food and Drug Administration has for a decade required the product labeling for all atypical antipsychotics to include a warning about the risks of hyperglycemia and diabetes.
But most data on antipsychotic-associated diabetes come from long-term studies, which show that diabetes, when it occurs, most often does so within the first 6 months on the medication. Little is known about the acute impact of antipsychotic agents on blood glucose levels and clinical outcomes in patients admitted to acute psychiatric units for stays averaging a few weeks. This information gap provided the rationale for Dr. Smiley’s study.
She presented a chart review of 1,212 patients admitted to three acute inpatient psychiatric units in inner-city Atlanta during 2008. The mean age of the patients was 57 years, they had a mean body mass index of 27 kg/m2, and the mean daily blood glucose concentration was 122 mg/dL during their stay. Twenty-one percent of the patients had a history of diabetes, and 6% had new hyperglycemia at admission. The most common primary diagnoses on admission were suicidal ideation in 28% of patients, a primary psychotic disorder in 27%, and depression in 16%.
Thirty-seven percent of patients had no prior experience with antipsychotic agents but were newly started on an antipsychotic during their acute inpatient stay. An additional 38% had been on antipsychotic therapy prior to admission and continued on it while hospitalized. Twenty-five percent of patients had never been treated with antipsychotic agents and were not during their hospitalization. Fifty-two percent of patients were treated with atypical antipsychotic agents during their inpatient stay, 17% received both atypical and typical antipsychotics, and 6% got typical antipsychotics only, explained Dr. Smiley, an endocrinologist at Emory University, Atlanta.
She sought answers to three research questions:
• Does prior exposure to antipsychotic therapy affect inpatient blood glucose levels or clinical outcome measures? Mean psychiatric inpatient length of stay was significantly shorter for patients never exposed to antipsychotics, at 9.5 days, compared with 14.2 days in patients who continued on their previous antipsychotic regimen and 13.7 days in antipsychotic-naive patients started on an antipsychotic for the first time. The most likely explanation for the longer stays in patients on antipsychotic therapy was the need to monitor them for side effects, according to Dr. Smiley.
Importantly, mean daily blood glucose concentrations during psychiatric hospitalization did not differ between patients never exposed to antipsychotic agents, those started on an antipsychotic for the first time during their hospitalization, and patients continued on their previous antipsychotics.
• Does the type of antipsychotic agent used during the acute inpatient admission affect blood glucose levels or clinical outcomes?
Mean daily blood glucose levels were similar regardless of whether patients were on atypical antipsychotics, typical antipsychotics, or both. The mean hemoglobin A1c level was 7.3% in all three groups of antipsychotic-treated patients. However, the rate of medical complications during psychiatric hospitalization was significantly higher among patients on atypical agents, at 23%, compared with 17% in patients on typical antipsychotics and 13% in those on both.
Three percent of patients on atypical antipsychotics-only required transfer to a medical unit for treatment of a complication that arose during psychiatric hospitalization. That was also true for 3% of patients on combined atypical/typical antipsychotic therapy. In contrast, none of the relatively few patients on typical antipsychotics-only required transfer to a medical unit. It’s worth noting that all atypical antipsychotics are not alike in terms of the strength of their association with diabetes. Most antipsychotic-treated patients in this study got olanzapine (Zyprexa) or clozapine (Clozaril). Newer, less diabetogenic atypical agents were less frequently used in this largely indigent population.
• Does a history of diabetes or new hyperglycemia at admission affect outcome measures? Not unexpectedly, patients with a history of diabetes prior to admission had a significantly higher rate of medical complications during their psychiatric inpatient stay: a 28% rate, compared with 22% in patients with no history of diabetes but with hyperglycemia at admission and 20% in patients with neither metabolic abnormality. The most common complication in patients with a history of diabetes was urinary tract infection.
Again unsurprisingly, patients with a history of diabetes had a significantly higher rate of transfer to a medical unit because of medical complications: 5%, compared with no transfers in patients with new hyperglycemia at admission and a 1% transfer rate in patients with neither metabolic abnormality.
Dr. Smiley reported that her research funding came from the National Institutes of Health, Sanofi-Aventis, and Merck.
CHICAGO – Treatment with antipsychotic agents in acute psychiatric inpatient units is not associated with increased blood glucose levels, according to a multicenter study.
"This finding is contrary to what many of us may have previously considered," Dr. Dawn D. Smiley observed in presenting the study findings at the annual scientific sessions of the American Diabetes Association.
A well-recognized association exists between the use of antipsychotic agents and an increased prevalence of diabetes and hyperglycemia, although it remains unclear whether the relationship is causal. The association is especially strong for the atypical or second-generation antipsychotics, which account for more than 90% of all antipsychotic use and are among the top-selling prescription drugs in the United States. Indeed, the Food and Drug Administration has for a decade required the product labeling for all atypical antipsychotics to include a warning about the risks of hyperglycemia and diabetes.
But most data on antipsychotic-associated diabetes come from long-term studies, which show that diabetes, when it occurs, most often does so within the first 6 months on the medication. Little is known about the acute impact of antipsychotic agents on blood glucose levels and clinical outcomes in patients admitted to acute psychiatric units for stays averaging a few weeks. This information gap provided the rationale for Dr. Smiley’s study.
She presented a chart review of 1,212 patients admitted to three acute inpatient psychiatric units in inner-city Atlanta during 2008. The mean age of the patients was 57 years, they had a mean body mass index of 27 kg/m2, and the mean daily blood glucose concentration was 122 mg/dL during their stay. Twenty-one percent of the patients had a history of diabetes, and 6% had new hyperglycemia at admission. The most common primary diagnoses on admission were suicidal ideation in 28% of patients, a primary psychotic disorder in 27%, and depression in 16%.
Thirty-seven percent of patients had no prior experience with antipsychotic agents but were newly started on an antipsychotic during their acute inpatient stay. An additional 38% had been on antipsychotic therapy prior to admission and continued on it while hospitalized. Twenty-five percent of patients had never been treated with antipsychotic agents and were not during their hospitalization. Fifty-two percent of patients were treated with atypical antipsychotic agents during their inpatient stay, 17% received both atypical and typical antipsychotics, and 6% got typical antipsychotics only, explained Dr. Smiley, an endocrinologist at Emory University, Atlanta.
She sought answers to three research questions:
• Does prior exposure to antipsychotic therapy affect inpatient blood glucose levels or clinical outcome measures? Mean psychiatric inpatient length of stay was significantly shorter for patients never exposed to antipsychotics, at 9.5 days, compared with 14.2 days in patients who continued on their previous antipsychotic regimen and 13.7 days in antipsychotic-naive patients started on an antipsychotic for the first time. The most likely explanation for the longer stays in patients on antipsychotic therapy was the need to monitor them for side effects, according to Dr. Smiley.
Importantly, mean daily blood glucose concentrations during psychiatric hospitalization did not differ between patients never exposed to antipsychotic agents, those started on an antipsychotic for the first time during their hospitalization, and patients continued on their previous antipsychotics.
• Does the type of antipsychotic agent used during the acute inpatient admission affect blood glucose levels or clinical outcomes?
Mean daily blood glucose levels were similar regardless of whether patients were on atypical antipsychotics, typical antipsychotics, or both. The mean hemoglobin A1c level was 7.3% in all three groups of antipsychotic-treated patients. However, the rate of medical complications during psychiatric hospitalization was significantly higher among patients on atypical agents, at 23%, compared with 17% in patients on typical antipsychotics and 13% in those on both.
Three percent of patients on atypical antipsychotics-only required transfer to a medical unit for treatment of a complication that arose during psychiatric hospitalization. That was also true for 3% of patients on combined atypical/typical antipsychotic therapy. In contrast, none of the relatively few patients on typical antipsychotics-only required transfer to a medical unit. It’s worth noting that all atypical antipsychotics are not alike in terms of the strength of their association with diabetes. Most antipsychotic-treated patients in this study got olanzapine (Zyprexa) or clozapine (Clozaril). Newer, less diabetogenic atypical agents were less frequently used in this largely indigent population.
• Does a history of diabetes or new hyperglycemia at admission affect outcome measures? Not unexpectedly, patients with a history of diabetes prior to admission had a significantly higher rate of medical complications during their psychiatric inpatient stay: a 28% rate, compared with 22% in patients with no history of diabetes but with hyperglycemia at admission and 20% in patients with neither metabolic abnormality. The most common complication in patients with a history of diabetes was urinary tract infection.
Again unsurprisingly, patients with a history of diabetes had a significantly higher rate of transfer to a medical unit because of medical complications: 5%, compared with no transfers in patients with new hyperglycemia at admission and a 1% transfer rate in patients with neither metabolic abnormality.
Dr. Smiley reported that her research funding came from the National Institutes of Health, Sanofi-Aventis, and Merck.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Blood glucose levels during acute psychiatric hospitalizations did not differ between antipsychotic-naive patients newly placed on antipsychotic therapy during their inpatient stay, and patients never exposed to antipsychotic agents.
Data source: This was a chart review of 1,212 patients admitted to three acute inpatient psychiatric units located in Atlanta.
Disclosures: Dr. Smiley reported that her research funding came from the National Institutes of Health, Sanofi-Aventis, and Merck.
New metformin formulation broadens candidate population
CHICAGO – A novel delayed-release formulation of oral metformin holds the promise of making the drug work for the many patients with type 2 diabetes who are unable to take generic metformin because of renal insufficiency or GI intolerance.
The key to this development was the discovery that metformin’s primary site of action isn’t the circulation, it’s the lower bowel – specifically, the gut enteroendocrine L-cells. Metformin plasma exposure is not required for the drug’s glucose-lowering effects, Dr. Ralph A. DeFronzo asserted at the annual scientific sessions of the American Diabetes Association.
The investigational proprietary delayed-release formulation of metformin, known as NewMet, is designed to minimize the drug’s bioavailability. The delayed-release tablet bypasses the highly absorptive upper bowel, instead delivering the medication to the distal small intestine, where metformin is poorly absorbed into the circulation and enteroendocrine L-cells are concentrated. Metformin’s glucose-lowering effects are now thought to result from the drug’s stimulation of the L-cells to release gut hormones, including glucagon-like peptide-1 (GLP-1) and peptide YY, said Dr. DeFronzo, professor of medicine and director of the diabetes division at University of Texas Health Science Center at San Antonio.
He presented a proof-of-concept study demonstrating that metformin’s glucose-lowering effect is dissociated from plasma drug levels. The randomized, double-blind, crossover study involved 24 patients with type 2 diabetes. After being withdrawn from oral diabetes therapy for 2 weeks, they received in random order 5 days of commercially available, immediate-release metformin at 1,000 mg twice daily; delayed-release metformin at 1,000 mg twice daily; and delayed-release metformin at 500 mg twice daily. Each 5-day treatment period was separated by a 9- to 12-day washout.
Each of the two dosing regimens of delayed-release metformin proved as effective as conventional metformin. They reduced fasting and postprandial plasma glucose levels as well as the 10-hour glucose area under the curve to the same extent as that of immediate-release metformin, without changing the insulin area under the curve. In other words, 1,000 mg/day of delayed-release metformin was as effective as was standard full-dose conventional metformin at 2,000 mg/day. Yet plasma metformin exposure was reduced by 45% with delayed-release metformin at 1,000 mg twice daily and by 57% by the investigational agent at 500 mg twice daily. Both the conventional and delayed-release formulations of metformin increased GLP-1 and peptide YY levels to a similar extent.
Delayed-release metformin was better tolerated. Unlike conventional metformin, it did not cause any nausea or vomiting, since it was not yet released in the upper bowel. However, both immediate- and delayed-release metformin were associated with a 10%-15% incidence of diarrhea, a side effect of metformin in the lower bowel.
It appears that when a patient is on full-dose conventional metformin, about half of the dose is absorbed into the circulation from the stomach, while the other half continues downstream to the lower bowel, where it actually exerts its therapeutic effects, according to Dr. DeFronzo.
Conventional metformin is contraindicated in patients with renal insufficiency. That’s because once metformin is absorbed into the circulation, it gets cleared by the kidney. If renal function is impaired, the drug accumulates and can cause lactic acidosis due to metformin-induced liver toxicity.
Delayed-release metformin should sidestep this problem because the plasma levels are so much lower than with the conventional drug. That was suggested in a separate study presented at the ADA meeting by Mark Fineman, Ph.D., senior vice president for research and development at San Diego–based Elcelyx Therapeutics Inc., which is developing NewMet. He presented a model designed to predict plasma metformin concentrations that would result if type 2 diabetes patients with varying degrees of renal impairment were to take delayed-release metformin. The results suggested no increased risk of lactic acidosis at 1,000 or 2,000 mg/day. And a study is now underway to see if 800 or 600 mg once daily can provide the full effect seen at 500 mg twice daily in Dr. DeFronzo’s study, Dr. Fineman said in an interview.
Metformin is recommended in ADA treatment guidelines as the initial drug of choice for patients with type 2 diabetes. It is the most commonly prescribed medication for type 2 diabetes in the world. Yet roughly 40% of patients don’t take it due to renal insufficiency or GI intolerance. Moreover, many patients on metformin are unable to take full-dose therapy because of limited tolerability. These are the patient populations where delayed-release metformin could be a boon.
Dr. Fineman said Elcelyx is now in discussions with the FDA about the requirements for marketing approval, which are likely to be abbreviated since NewMet is a new formulation of an approved drug.
"We’ll need to do a phase III program to show that in non–renally impaired people delayed-release metformin works just as well as regular metformin, maybe better, and then we’ll do studies in renally impaired patients to show what the efficacy is," he said.
Dr. DeFronzo is a clinical adviser to Elcelyx.
CHICAGO – A novel delayed-release formulation of oral metformin holds the promise of making the drug work for the many patients with type 2 diabetes who are unable to take generic metformin because of renal insufficiency or GI intolerance.
The key to this development was the discovery that metformin’s primary site of action isn’t the circulation, it’s the lower bowel – specifically, the gut enteroendocrine L-cells. Metformin plasma exposure is not required for the drug’s glucose-lowering effects, Dr. Ralph A. DeFronzo asserted at the annual scientific sessions of the American Diabetes Association.
The investigational proprietary delayed-release formulation of metformin, known as NewMet, is designed to minimize the drug’s bioavailability. The delayed-release tablet bypasses the highly absorptive upper bowel, instead delivering the medication to the distal small intestine, where metformin is poorly absorbed into the circulation and enteroendocrine L-cells are concentrated. Metformin’s glucose-lowering effects are now thought to result from the drug’s stimulation of the L-cells to release gut hormones, including glucagon-like peptide-1 (GLP-1) and peptide YY, said Dr. DeFronzo, professor of medicine and director of the diabetes division at University of Texas Health Science Center at San Antonio.
He presented a proof-of-concept study demonstrating that metformin’s glucose-lowering effect is dissociated from plasma drug levels. The randomized, double-blind, crossover study involved 24 patients with type 2 diabetes. After being withdrawn from oral diabetes therapy for 2 weeks, they received in random order 5 days of commercially available, immediate-release metformin at 1,000 mg twice daily; delayed-release metformin at 1,000 mg twice daily; and delayed-release metformin at 500 mg twice daily. Each 5-day treatment period was separated by a 9- to 12-day washout.
Each of the two dosing regimens of delayed-release metformin proved as effective as conventional metformin. They reduced fasting and postprandial plasma glucose levels as well as the 10-hour glucose area under the curve to the same extent as that of immediate-release metformin, without changing the insulin area under the curve. In other words, 1,000 mg/day of delayed-release metformin was as effective as was standard full-dose conventional metformin at 2,000 mg/day. Yet plasma metformin exposure was reduced by 45% with delayed-release metformin at 1,000 mg twice daily and by 57% by the investigational agent at 500 mg twice daily. Both the conventional and delayed-release formulations of metformin increased GLP-1 and peptide YY levels to a similar extent.
Delayed-release metformin was better tolerated. Unlike conventional metformin, it did not cause any nausea or vomiting, since it was not yet released in the upper bowel. However, both immediate- and delayed-release metformin were associated with a 10%-15% incidence of diarrhea, a side effect of metformin in the lower bowel.
It appears that when a patient is on full-dose conventional metformin, about half of the dose is absorbed into the circulation from the stomach, while the other half continues downstream to the lower bowel, where it actually exerts its therapeutic effects, according to Dr. DeFronzo.
Conventional metformin is contraindicated in patients with renal insufficiency. That’s because once metformin is absorbed into the circulation, it gets cleared by the kidney. If renal function is impaired, the drug accumulates and can cause lactic acidosis due to metformin-induced liver toxicity.
Delayed-release metformin should sidestep this problem because the plasma levels are so much lower than with the conventional drug. That was suggested in a separate study presented at the ADA meeting by Mark Fineman, Ph.D., senior vice president for research and development at San Diego–based Elcelyx Therapeutics Inc., which is developing NewMet. He presented a model designed to predict plasma metformin concentrations that would result if type 2 diabetes patients with varying degrees of renal impairment were to take delayed-release metformin. The results suggested no increased risk of lactic acidosis at 1,000 or 2,000 mg/day. And a study is now underway to see if 800 or 600 mg once daily can provide the full effect seen at 500 mg twice daily in Dr. DeFronzo’s study, Dr. Fineman said in an interview.
Metformin is recommended in ADA treatment guidelines as the initial drug of choice for patients with type 2 diabetes. It is the most commonly prescribed medication for type 2 diabetes in the world. Yet roughly 40% of patients don’t take it due to renal insufficiency or GI intolerance. Moreover, many patients on metformin are unable to take full-dose therapy because of limited tolerability. These are the patient populations where delayed-release metformin could be a boon.
Dr. Fineman said Elcelyx is now in discussions with the FDA about the requirements for marketing approval, which are likely to be abbreviated since NewMet is a new formulation of an approved drug.
"We’ll need to do a phase III program to show that in non–renally impaired people delayed-release metformin works just as well as regular metformin, maybe better, and then we’ll do studies in renally impaired patients to show what the efficacy is," he said.
Dr. DeFronzo is a clinical adviser to Elcelyx.
CHICAGO – A novel delayed-release formulation of oral metformin holds the promise of making the drug work for the many patients with type 2 diabetes who are unable to take generic metformin because of renal insufficiency or GI intolerance.
The key to this development was the discovery that metformin’s primary site of action isn’t the circulation, it’s the lower bowel – specifically, the gut enteroendocrine L-cells. Metformin plasma exposure is not required for the drug’s glucose-lowering effects, Dr. Ralph A. DeFronzo asserted at the annual scientific sessions of the American Diabetes Association.
The investigational proprietary delayed-release formulation of metformin, known as NewMet, is designed to minimize the drug’s bioavailability. The delayed-release tablet bypasses the highly absorptive upper bowel, instead delivering the medication to the distal small intestine, where metformin is poorly absorbed into the circulation and enteroendocrine L-cells are concentrated. Metformin’s glucose-lowering effects are now thought to result from the drug’s stimulation of the L-cells to release gut hormones, including glucagon-like peptide-1 (GLP-1) and peptide YY, said Dr. DeFronzo, professor of medicine and director of the diabetes division at University of Texas Health Science Center at San Antonio.
He presented a proof-of-concept study demonstrating that metformin’s glucose-lowering effect is dissociated from plasma drug levels. The randomized, double-blind, crossover study involved 24 patients with type 2 diabetes. After being withdrawn from oral diabetes therapy for 2 weeks, they received in random order 5 days of commercially available, immediate-release metformin at 1,000 mg twice daily; delayed-release metformin at 1,000 mg twice daily; and delayed-release metformin at 500 mg twice daily. Each 5-day treatment period was separated by a 9- to 12-day washout.
Each of the two dosing regimens of delayed-release metformin proved as effective as conventional metformin. They reduced fasting and postprandial plasma glucose levels as well as the 10-hour glucose area under the curve to the same extent as that of immediate-release metformin, without changing the insulin area under the curve. In other words, 1,000 mg/day of delayed-release metformin was as effective as was standard full-dose conventional metformin at 2,000 mg/day. Yet plasma metformin exposure was reduced by 45% with delayed-release metformin at 1,000 mg twice daily and by 57% by the investigational agent at 500 mg twice daily. Both the conventional and delayed-release formulations of metformin increased GLP-1 and peptide YY levels to a similar extent.
Delayed-release metformin was better tolerated. Unlike conventional metformin, it did not cause any nausea or vomiting, since it was not yet released in the upper bowel. However, both immediate- and delayed-release metformin were associated with a 10%-15% incidence of diarrhea, a side effect of metformin in the lower bowel.
It appears that when a patient is on full-dose conventional metformin, about half of the dose is absorbed into the circulation from the stomach, while the other half continues downstream to the lower bowel, where it actually exerts its therapeutic effects, according to Dr. DeFronzo.
Conventional metformin is contraindicated in patients with renal insufficiency. That’s because once metformin is absorbed into the circulation, it gets cleared by the kidney. If renal function is impaired, the drug accumulates and can cause lactic acidosis due to metformin-induced liver toxicity.
Delayed-release metformin should sidestep this problem because the plasma levels are so much lower than with the conventional drug. That was suggested in a separate study presented at the ADA meeting by Mark Fineman, Ph.D., senior vice president for research and development at San Diego–based Elcelyx Therapeutics Inc., which is developing NewMet. He presented a model designed to predict plasma metformin concentrations that would result if type 2 diabetes patients with varying degrees of renal impairment were to take delayed-release metformin. The results suggested no increased risk of lactic acidosis at 1,000 or 2,000 mg/day. And a study is now underway to see if 800 or 600 mg once daily can provide the full effect seen at 500 mg twice daily in Dr. DeFronzo’s study, Dr. Fineman said in an interview.
Metformin is recommended in ADA treatment guidelines as the initial drug of choice for patients with type 2 diabetes. It is the most commonly prescribed medication for type 2 diabetes in the world. Yet roughly 40% of patients don’t take it due to renal insufficiency or GI intolerance. Moreover, many patients on metformin are unable to take full-dose therapy because of limited tolerability. These are the patient populations where delayed-release metformin could be a boon.
Dr. Fineman said Elcelyx is now in discussions with the FDA about the requirements for marketing approval, which are likely to be abbreviated since NewMet is a new formulation of an approved drug.
"We’ll need to do a phase III program to show that in non–renally impaired people delayed-release metformin works just as well as regular metformin, maybe better, and then we’ll do studies in renally impaired patients to show what the efficacy is," he said.
Dr. DeFronzo is a clinical adviser to Elcelyx.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major Finding: A novel delayed-release formulation of metformin reduced fasting plasma glucose in patients with type 2 diabetes by 19.9 mg/dL at 1,000 mg twice daily and by 16.4 mg/dL at 500 mg twice daily, effect sizes similar to what was seen with commercially available immediate-release metformin at 1,000 mg twice daily.
Data Source: A proof-of-concept study demonstrating that metformin’s mechanism of lowering glucose is mediated by the gut rather than the circulation.
Disclosures: The study was sponsored by Elcelyx Therapeutics, which is developing the proprietary delayed-release metformin. Dr. DeFronzo is a clinical adviser to the company.
Empagliflozin improves cardiovascular risk factors in T2DM
CHICAGO – Twenty-four weeks of treatment with empagliflozin in patients with type 2 diabetes achieved clinically meaningful improvements in cardiovascular risk factors as well as in glycemic control, according to pooled data from four pivotal phase III clinical trials.
Empagliflozin is a sodium glucose cotransporter 2 (SGLT2) inhibitor. This is a novel emerging class of oral diabetic medications that work by boosting urinary glucose excretion. Canagliflozin (Invokana), the first-in-class SGLT2 inhibitor, received Food and Drug Administration marketing approval in March for treatment of type 2 diabetes. Empagliflozin’s application for marketing approval is now under review by the FDA and the European Medicines Agency, Dr. Thomas Hach noted at the annual scientific sessions of the American Diabetes Association.
Empagliflozin resulted in significant, clinically meaningful improvements in blood pressure, body weight, uric acid, HDL cholesterol, hemoglobin A1c, and fasting plasma glucose, along with a modest, albeit unwelcome, increase in LDL cholesterol.
The improvement in blood pressure was particularly noteworthy. Among patients with uncontrolled blood pressure at baseline – that is, blood pressures of 130/80 mm Hg or higher – 33.3% of those who received empagliflozin at 10 mg/day and 32.2% on empagliflozin at 25 mg/day had controlled blood pressure at week 24. That was true of only 18.6% of placebo-treated controls, according to Dr. Hach of Boehringer Ingelheim Pharma in Ingelheim, Germany.
The impact of empagliflozin on cardiovascular events in patients with type 2 diabetes is under study in the ongoing EMPA-REG OUTCOME cardiovascular outcomes trial.
Empagliflozin is being jointly developed by Boehringer Ingelheim and Eli Lilly.
*Correction, 7/10/2013: An earlier version of the graphic accompanying this story incorrectly reported the change in uric acid.
CHICAGO – Twenty-four weeks of treatment with empagliflozin in patients with type 2 diabetes achieved clinically meaningful improvements in cardiovascular risk factors as well as in glycemic control, according to pooled data from four pivotal phase III clinical trials.
Empagliflozin is a sodium glucose cotransporter 2 (SGLT2) inhibitor. This is a novel emerging class of oral diabetic medications that work by boosting urinary glucose excretion. Canagliflozin (Invokana), the first-in-class SGLT2 inhibitor, received Food and Drug Administration marketing approval in March for treatment of type 2 diabetes. Empagliflozin’s application for marketing approval is now under review by the FDA and the European Medicines Agency, Dr. Thomas Hach noted at the annual scientific sessions of the American Diabetes Association.
Empagliflozin resulted in significant, clinically meaningful improvements in blood pressure, body weight, uric acid, HDL cholesterol, hemoglobin A1c, and fasting plasma glucose, along with a modest, albeit unwelcome, increase in LDL cholesterol.
The improvement in blood pressure was particularly noteworthy. Among patients with uncontrolled blood pressure at baseline – that is, blood pressures of 130/80 mm Hg or higher – 33.3% of those who received empagliflozin at 10 mg/day and 32.2% on empagliflozin at 25 mg/day had controlled blood pressure at week 24. That was true of only 18.6% of placebo-treated controls, according to Dr. Hach of Boehringer Ingelheim Pharma in Ingelheim, Germany.
The impact of empagliflozin on cardiovascular events in patients with type 2 diabetes is under study in the ongoing EMPA-REG OUTCOME cardiovascular outcomes trial.
Empagliflozin is being jointly developed by Boehringer Ingelheim and Eli Lilly.
*Correction, 7/10/2013: An earlier version of the graphic accompanying this story incorrectly reported the change in uric acid.
CHICAGO – Twenty-four weeks of treatment with empagliflozin in patients with type 2 diabetes achieved clinically meaningful improvements in cardiovascular risk factors as well as in glycemic control, according to pooled data from four pivotal phase III clinical trials.
Empagliflozin is a sodium glucose cotransporter 2 (SGLT2) inhibitor. This is a novel emerging class of oral diabetic medications that work by boosting urinary glucose excretion. Canagliflozin (Invokana), the first-in-class SGLT2 inhibitor, received Food and Drug Administration marketing approval in March for treatment of type 2 diabetes. Empagliflozin’s application for marketing approval is now under review by the FDA and the European Medicines Agency, Dr. Thomas Hach noted at the annual scientific sessions of the American Diabetes Association.
Empagliflozin resulted in significant, clinically meaningful improvements in blood pressure, body weight, uric acid, HDL cholesterol, hemoglobin A1c, and fasting plasma glucose, along with a modest, albeit unwelcome, increase in LDL cholesterol.
The improvement in blood pressure was particularly noteworthy. Among patients with uncontrolled blood pressure at baseline – that is, blood pressures of 130/80 mm Hg or higher – 33.3% of those who received empagliflozin at 10 mg/day and 32.2% on empagliflozin at 25 mg/day had controlled blood pressure at week 24. That was true of only 18.6% of placebo-treated controls, according to Dr. Hach of Boehringer Ingelheim Pharma in Ingelheim, Germany.
The impact of empagliflozin on cardiovascular events in patients with type 2 diabetes is under study in the ongoing EMPA-REG OUTCOME cardiovascular outcomes trial.
Empagliflozin is being jointly developed by Boehringer Ingelheim and Eli Lilly.
*Correction, 7/10/2013: An earlier version of the graphic accompanying this story incorrectly reported the change in uric acid.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major Finding: One-third of type 2 diabetes patients with uncontrolled hypertension at baseline lowered their blood pressure to below 130/80 mm Hg after 24 weeks on empagliflozin.
Data Source: A pooled analysis of data from four randomized, placebo-controlled pivotal phase III clinical trials of empagliflozin in 2,477 patients with type 2 diabetes.
Disclosures: Empagliflozin is being jointly developed by Boehringer Ingelheim and Eli Lilly. Dr. Hach is an employee of Boehringer Ingelheim.
Canagliflozin bests sitagliptin for type 2 DM in comparative trial
CHICAGO – Canagliflozin outperformed sitagliptin for the treatment of patients with type 2 diabetes in the first randomized head-to-head comparison of drugs representing the two different classes of oral antidiabetic medications.
Canagliflozin (Invokana) received approval from the Food and Drug Administration in March as the first in a new class of medications for type 2 diabetes known as sodium glucose co-transporter 2 (SGLT2) inhibitors. SGLT2 is responsible for most glucose reabsorption in the kidney, so its inhibition boosts urinary glucose excretion. Other SGLT2 inhibitors are coming through the developmental pipeline.
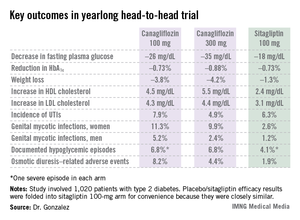
In a phase III, double-blind, 52-week clinical trial, canagliflozin improved glycemic control, lowered body weight, and reduced blood pressure to a significantly greater extent than did sitagliptin (Januvia), a dipeptidyl peptidase-4 (DPP-4) inhibitor, Dr. Fernando Lavalle Gonzalez reported at the annual scientific sessions of the American Diabetes Association.
The multicenter four-armed study included 1,020 type 2 patients with diabetes who had inadequate glycemic control on metformin monotherapy. They were randomized to canagliflozin at 100 or 300 mg/day, sitagliptin at 100 mg/day, or 26 weeks of placebo followed by 26 weeks on sitagliptin at 100 mg/day. All participants continued on metformin.
Reductions in fasting plasma glucose at 52 weeks averaged 26.2 mg/dL in the lower-dose canagliflozin group and 35.2 mg/dL in the higher-dose group, both significantly greater than the 17.7-mg/dL decrease in patients on sitagliptin.
In addition to the better outcomes seen with canagliflozin in terms of the major efficacy endpoints involving glycemic control, weight loss, and blood pressure, the SGLT2 inhibitor resulted in a bigger boost in HDL cholesterol (see chart). On the downside, both canagliflozin and sitagliptin resulted in modest increases in low-density lipoprotein (LDL), noted Dr. Gonzalez, an endocrinologist at the Autonomous University of Nuevo Leon in Monterrey, Mexico.
Canagliflozin was associated with significantly higher rates of genital mycotic infections in both men and women, as well as more adverse events related to osmotic diuresis. The infections were symptomatic, easily diagnosed, and readily treated with topical or oral antifungals. The adverse events related to increased urination were typically mild. These side effects led to very few study discontinuations, according to Dr. Gonzalez.
He said mechanistic studies have identified two factors as being responsible for the clinically meaningful reductions in blood pressure seen with canagliflozin in this study, which amounted to a mean 3.5 mm Hg decrease in systolic blood pressure at 100 mg/day and a 4.7 mm Hg reduction at 300 mg/day. It appears that about half of the blood pressure reduction is due to the negative salt and water balance, on the order of 750-1,000 cc, occurring in the first 3-4 days of treatment, and the other half is related to the weight loss accompanying canagliflozin therapy.
Session chair Dr. Ralph A. DeFronzo, who is involved in SGLT2 inhibitor research, said a widespread misconception exists that the medications are associated with an increased risk of urinary tract infections.
"It’s very commonly stated that this class of drugs is associated with an increase in UTIs [urinary tract infections]. In fact, if you look at the data rather than what’s said, this really in my opinion doesn’t hold up. You can see it in this study, where there’s no significant difference between the groups," said Dr. DeFronzo, professor of medicine and director of the diabetes division at the University of Texas Health Science Center, San Antonio.
He also put into perspective the increase in LDL seen with canagliflozin.
"Looking across the studies, with canagliflozin at the 100-mg dose the rise in LDL is about 4 mg/dL, and it’s about 8 mg/dL at the 300-mg dose. So if you’re truly treating your patients to goal and they’re at an LDL of 70 mg/dL, in the worst case scenario you’d be going from 70 to 78 mg/dL. I think many of us here in the audience probably wouldn’t even increase the dose of a statin, but if you did up it by one dose, you’d get the LDL back down to 70," he said.
Dr. DeFronzo reported serving on advisory panels for Janssen, which markets canagliflozin, as well as for Amylin Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Takeda. Dr. Gonzalez is on speakers bureaus and advisory panels for Janssen and numerous other companies.
Invokana, Food and Drug Administration, FDA approval, SGLT2,
CHICAGO – Canagliflozin outperformed sitagliptin for the treatment of patients with type 2 diabetes in the first randomized head-to-head comparison of drugs representing the two different classes of oral antidiabetic medications.
Canagliflozin (Invokana) received approval from the Food and Drug Administration in March as the first in a new class of medications for type 2 diabetes known as sodium glucose co-transporter 2 (SGLT2) inhibitors. SGLT2 is responsible for most glucose reabsorption in the kidney, so its inhibition boosts urinary glucose excretion. Other SGLT2 inhibitors are coming through the developmental pipeline.

In a phase III, double-blind, 52-week clinical trial, canagliflozin improved glycemic control, lowered body weight, and reduced blood pressure to a significantly greater extent than did sitagliptin (Januvia), a dipeptidyl peptidase-4 (DPP-4) inhibitor, Dr. Fernando Lavalle Gonzalez reported at the annual scientific sessions of the American Diabetes Association.
The multicenter four-armed study included 1,020 type 2 patients with diabetes who had inadequate glycemic control on metformin monotherapy. They were randomized to canagliflozin at 100 or 300 mg/day, sitagliptin at 100 mg/day, or 26 weeks of placebo followed by 26 weeks on sitagliptin at 100 mg/day. All participants continued on metformin.
Reductions in fasting plasma glucose at 52 weeks averaged 26.2 mg/dL in the lower-dose canagliflozin group and 35.2 mg/dL in the higher-dose group, both significantly greater than the 17.7-mg/dL decrease in patients on sitagliptin.
In addition to the better outcomes seen with canagliflozin in terms of the major efficacy endpoints involving glycemic control, weight loss, and blood pressure, the SGLT2 inhibitor resulted in a bigger boost in HDL cholesterol (see chart). On the downside, both canagliflozin and sitagliptin resulted in modest increases in low-density lipoprotein (LDL), noted Dr. Gonzalez, an endocrinologist at the Autonomous University of Nuevo Leon in Monterrey, Mexico.
Canagliflozin was associated with significantly higher rates of genital mycotic infections in both men and women, as well as more adverse events related to osmotic diuresis. The infections were symptomatic, easily diagnosed, and readily treated with topical or oral antifungals. The adverse events related to increased urination were typically mild. These side effects led to very few study discontinuations, according to Dr. Gonzalez.
He said mechanistic studies have identified two factors as being responsible for the clinically meaningful reductions in blood pressure seen with canagliflozin in this study, which amounted to a mean 3.5 mm Hg decrease in systolic blood pressure at 100 mg/day and a 4.7 mm Hg reduction at 300 mg/day. It appears that about half of the blood pressure reduction is due to the negative salt and water balance, on the order of 750-1,000 cc, occurring in the first 3-4 days of treatment, and the other half is related to the weight loss accompanying canagliflozin therapy.
Session chair Dr. Ralph A. DeFronzo, who is involved in SGLT2 inhibitor research, said a widespread misconception exists that the medications are associated with an increased risk of urinary tract infections.
"It’s very commonly stated that this class of drugs is associated with an increase in UTIs [urinary tract infections]. In fact, if you look at the data rather than what’s said, this really in my opinion doesn’t hold up. You can see it in this study, where there’s no significant difference between the groups," said Dr. DeFronzo, professor of medicine and director of the diabetes division at the University of Texas Health Science Center, San Antonio.
He also put into perspective the increase in LDL seen with canagliflozin.
"Looking across the studies, with canagliflozin at the 100-mg dose the rise in LDL is about 4 mg/dL, and it’s about 8 mg/dL at the 300-mg dose. So if you’re truly treating your patients to goal and they’re at an LDL of 70 mg/dL, in the worst case scenario you’d be going from 70 to 78 mg/dL. I think many of us here in the audience probably wouldn’t even increase the dose of a statin, but if you did up it by one dose, you’d get the LDL back down to 70," he said.
Dr. DeFronzo reported serving on advisory panels for Janssen, which markets canagliflozin, as well as for Amylin Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Takeda. Dr. Gonzalez is on speakers bureaus and advisory panels for Janssen and numerous other companies.
CHICAGO – Canagliflozin outperformed sitagliptin for the treatment of patients with type 2 diabetes in the first randomized head-to-head comparison of drugs representing the two different classes of oral antidiabetic medications.
Canagliflozin (Invokana) received approval from the Food and Drug Administration in March as the first in a new class of medications for type 2 diabetes known as sodium glucose co-transporter 2 (SGLT2) inhibitors. SGLT2 is responsible for most glucose reabsorption in the kidney, so its inhibition boosts urinary glucose excretion. Other SGLT2 inhibitors are coming through the developmental pipeline.

In a phase III, double-blind, 52-week clinical trial, canagliflozin improved glycemic control, lowered body weight, and reduced blood pressure to a significantly greater extent than did sitagliptin (Januvia), a dipeptidyl peptidase-4 (DPP-4) inhibitor, Dr. Fernando Lavalle Gonzalez reported at the annual scientific sessions of the American Diabetes Association.
The multicenter four-armed study included 1,020 type 2 patients with diabetes who had inadequate glycemic control on metformin monotherapy. They were randomized to canagliflozin at 100 or 300 mg/day, sitagliptin at 100 mg/day, or 26 weeks of placebo followed by 26 weeks on sitagliptin at 100 mg/day. All participants continued on metformin.
Reductions in fasting plasma glucose at 52 weeks averaged 26.2 mg/dL in the lower-dose canagliflozin group and 35.2 mg/dL in the higher-dose group, both significantly greater than the 17.7-mg/dL decrease in patients on sitagliptin.
In addition to the better outcomes seen with canagliflozin in terms of the major efficacy endpoints involving glycemic control, weight loss, and blood pressure, the SGLT2 inhibitor resulted in a bigger boost in HDL cholesterol (see chart). On the downside, both canagliflozin and sitagliptin resulted in modest increases in low-density lipoprotein (LDL), noted Dr. Gonzalez, an endocrinologist at the Autonomous University of Nuevo Leon in Monterrey, Mexico.
Canagliflozin was associated with significantly higher rates of genital mycotic infections in both men and women, as well as more adverse events related to osmotic diuresis. The infections were symptomatic, easily diagnosed, and readily treated with topical or oral antifungals. The adverse events related to increased urination were typically mild. These side effects led to very few study discontinuations, according to Dr. Gonzalez.
He said mechanistic studies have identified two factors as being responsible for the clinically meaningful reductions in blood pressure seen with canagliflozin in this study, which amounted to a mean 3.5 mm Hg decrease in systolic blood pressure at 100 mg/day and a 4.7 mm Hg reduction at 300 mg/day. It appears that about half of the blood pressure reduction is due to the negative salt and water balance, on the order of 750-1,000 cc, occurring in the first 3-4 days of treatment, and the other half is related to the weight loss accompanying canagliflozin therapy.
Session chair Dr. Ralph A. DeFronzo, who is involved in SGLT2 inhibitor research, said a widespread misconception exists that the medications are associated with an increased risk of urinary tract infections.
"It’s very commonly stated that this class of drugs is associated with an increase in UTIs [urinary tract infections]. In fact, if you look at the data rather than what’s said, this really in my opinion doesn’t hold up. You can see it in this study, where there’s no significant difference between the groups," said Dr. DeFronzo, professor of medicine and director of the diabetes division at the University of Texas Health Science Center, San Antonio.
He also put into perspective the increase in LDL seen with canagliflozin.
"Looking across the studies, with canagliflozin at the 100-mg dose the rise in LDL is about 4 mg/dL, and it’s about 8 mg/dL at the 300-mg dose. So if you’re truly treating your patients to goal and they’re at an LDL of 70 mg/dL, in the worst case scenario you’d be going from 70 to 78 mg/dL. I think many of us here in the audience probably wouldn’t even increase the dose of a statin, but if you did up it by one dose, you’d get the LDL back down to 70," he said.
Dr. DeFronzo reported serving on advisory panels for Janssen, which markets canagliflozin, as well as for Amylin Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Takeda. Dr. Gonzalez is on speakers bureaus and advisory panels for Janssen and numerous other companies.
Invokana, Food and Drug Administration, FDA approval, SGLT2,
Invokana, Food and Drug Administration, FDA approval, SGLT2,
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Patients with type 2 diabetes averaged a 26.2-mg/dL reduction from baseline in fasting plasma glucose after 52 weeks on canagliflozin at 100 mg/day and a 35.2-mg/dL decrease on the sodium glucose transporter 2 inhibitor at 300 mg/day, both superior to the 17.7-mg/dL reduction seen with sitagliptin.
Data source: A 52-week, double-blind, multicenter, phase III randomized trial involving 1,020 patients with type 2 diabetes who had inadequate glycemic control with metformin monotherapy.
Disclosures: Dr. DeFronzo reported serving on advisory panels for Janssen, which markets canagliflozin, as well as for Amylin Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Takeda. Dr. Gonzalez is on speakers bureaus and advisory panels for Janssen and numerous other companies.
Investigational albiglutide bests sitagliptin in head-to-head trial
CHICAGO – Once-weekly, fixed-dose albiglutide achieved superior glycemic control compared with dose-adjusted sitagliptin in patients with type 2 diabetes who had inadequate glycemic control and varying degrees of renal impairment in a phase III trial.
Albiglutide is an investigational glucagonlike peptide–1 (GLP-1) agonist now under review at both the U.S. Food and Drug Administration and the European Medicines Agency as a potential new treatment for type 2 diabetes. It is a large molecule cleared by the reticuloendothelial system rather than by the kidney, so its metabolism is not affected by the renal dysfunction that is common in patients with type 2 diabetes.
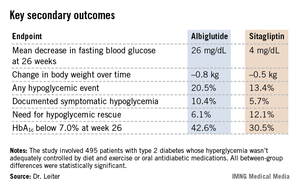
"Albiglutide will be a useful treatment addition for patients with renal impairment and type 2 diabetes. Current treatments for such patients are limited. Several antidiabetic medications are contraindicated in this population, and many others require dose adjustment," Dr. Lawrence A. Leiter observed in presenting the trial results at the annual scientific sessions of the American Diabetes Association.
The 52-week double-blind study involved 495 patients with type 2 diabetes whose hyperglycemia wasn’t adequately controlled by diet and exercise or oral antidiabetic medications. Renal impairment was mild in slightly over half of the participants, moderate in 41%, and severe in the remainder.
Patients were randomized to self-administered subcutaneous injection of albiglutide at 30 mg once a week or the oral dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin, dose-adjusted on the basis of the degree of renal impairment as specified in the product labeling. Thirty-five percent of patients in the albiglutide group had their daily dose uptitrated from 30 mg to 50 mg owing to insufficient response to the initial dose.
The primary endpoint was the change in mean hemoglobin A1c from baseline to week 26. The HbA1c dropped by 0.8% in the albiglutide group, significantly better than the 0.5% decrease with sitagliptin (P = .0003). The advantage favoring the investigational agent was seen in all three renal impairment severity groups, reported Dr. Leiter, professor of medicine and nutritional sciences at the University of Toronto and president of the Canadian Society of Endocrinology and Metabolism.
Albiglutide-treated patients also fared significantly better in terms of multiple key secondary endpoints (see chart). For example, 42.6% of them achieved a clinically meaningful HbA1c response by 26 weeks, defined as an HbA1c below 7.0%, compared with 30.5% of patients on sitagliptin.
Both drugs were well tolerated. The rate of adverse events leading to study withdrawal was 6.4% with albiglutide and 8.1% with sitagliptin. Both drugs had impressively low rates of nausea and vomiting; typically, less than 1% of patients per week reported either symptom.
Session chair Dr. Julio Rosenstock called this study noteworthy for several reasons. It is the first study of a GLP-1 agonist in patients with renal insufficiency; head-to-head comparative randomized trials in diabetes are rare. And the low-single-digit rates of nausea and vomiting seen with albiglutide in this study are "remarkable." Other GLP-1 agonists are typically associated with nausea and vomiting rates in the 15%-20% range or even higher, noted Dr. Rosenstock, director of the Dallas Diabetes and Endocrine Center at Medical City.
Dr. Leiter agreed, offering two potential explanations for the rock-bottom nausea and vomiting rates. One is that the once-weekly injection results in a very gradual increase in drug levels. Also, as a large molecule, albiglutide probably has less central action, including less activity at the central nervous system’s nausea and vomiting centers.
Other large, completed phase III trials have shown benefits for albiglutide in additional, common clinical scenarios, including yearlong studies of albiglutide as add-on therapy in patients with type 2 diabetes not controlled on pioglitazone and metformin, albiglutide monotherapy in drug-naive type 2 diabetic patients, a comparison of the novel GLP-1 agonist to insulin glargine in patients with type 2 diabetes, and albiglutide versus pioglitazone as add-on therapy in patients on background metformin and glimepiride.
Albiglutide is being developed by GlaxoSmithKline. Dr. Leiter has received research grants from and served as a consultant to GSK and other companies.
CHICAGO – Once-weekly, fixed-dose albiglutide achieved superior glycemic control compared with dose-adjusted sitagliptin in patients with type 2 diabetes who had inadequate glycemic control and varying degrees of renal impairment in a phase III trial.
Albiglutide is an investigational glucagonlike peptide–1 (GLP-1) agonist now under review at both the U.S. Food and Drug Administration and the European Medicines Agency as a potential new treatment for type 2 diabetes. It is a large molecule cleared by the reticuloendothelial system rather than by the kidney, so its metabolism is not affected by the renal dysfunction that is common in patients with type 2 diabetes.

"Albiglutide will be a useful treatment addition for patients with renal impairment and type 2 diabetes. Current treatments for such patients are limited. Several antidiabetic medications are contraindicated in this population, and many others require dose adjustment," Dr. Lawrence A. Leiter observed in presenting the trial results at the annual scientific sessions of the American Diabetes Association.
The 52-week double-blind study involved 495 patients with type 2 diabetes whose hyperglycemia wasn’t adequately controlled by diet and exercise or oral antidiabetic medications. Renal impairment was mild in slightly over half of the participants, moderate in 41%, and severe in the remainder.
Patients were randomized to self-administered subcutaneous injection of albiglutide at 30 mg once a week or the oral dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin, dose-adjusted on the basis of the degree of renal impairment as specified in the product labeling. Thirty-five percent of patients in the albiglutide group had their daily dose uptitrated from 30 mg to 50 mg owing to insufficient response to the initial dose.
The primary endpoint was the change in mean hemoglobin A1c from baseline to week 26. The HbA1c dropped by 0.8% in the albiglutide group, significantly better than the 0.5% decrease with sitagliptin (P = .0003). The advantage favoring the investigational agent was seen in all three renal impairment severity groups, reported Dr. Leiter, professor of medicine and nutritional sciences at the University of Toronto and president of the Canadian Society of Endocrinology and Metabolism.
Albiglutide-treated patients also fared significantly better in terms of multiple key secondary endpoints (see chart). For example, 42.6% of them achieved a clinically meaningful HbA1c response by 26 weeks, defined as an HbA1c below 7.0%, compared with 30.5% of patients on sitagliptin.
Both drugs were well tolerated. The rate of adverse events leading to study withdrawal was 6.4% with albiglutide and 8.1% with sitagliptin. Both drugs had impressively low rates of nausea and vomiting; typically, less than 1% of patients per week reported either symptom.
Session chair Dr. Julio Rosenstock called this study noteworthy for several reasons. It is the first study of a GLP-1 agonist in patients with renal insufficiency; head-to-head comparative randomized trials in diabetes are rare. And the low-single-digit rates of nausea and vomiting seen with albiglutide in this study are "remarkable." Other GLP-1 agonists are typically associated with nausea and vomiting rates in the 15%-20% range or even higher, noted Dr. Rosenstock, director of the Dallas Diabetes and Endocrine Center at Medical City.
Dr. Leiter agreed, offering two potential explanations for the rock-bottom nausea and vomiting rates. One is that the once-weekly injection results in a very gradual increase in drug levels. Also, as a large molecule, albiglutide probably has less central action, including less activity at the central nervous system’s nausea and vomiting centers.
Other large, completed phase III trials have shown benefits for albiglutide in additional, common clinical scenarios, including yearlong studies of albiglutide as add-on therapy in patients with type 2 diabetes not controlled on pioglitazone and metformin, albiglutide monotherapy in drug-naive type 2 diabetic patients, a comparison of the novel GLP-1 agonist to insulin glargine in patients with type 2 diabetes, and albiglutide versus pioglitazone as add-on therapy in patients on background metformin and glimepiride.
Albiglutide is being developed by GlaxoSmithKline. Dr. Leiter has received research grants from and served as a consultant to GSK and other companies.
CHICAGO – Once-weekly, fixed-dose albiglutide achieved superior glycemic control compared with dose-adjusted sitagliptin in patients with type 2 diabetes who had inadequate glycemic control and varying degrees of renal impairment in a phase III trial.
Albiglutide is an investigational glucagonlike peptide–1 (GLP-1) agonist now under review at both the U.S. Food and Drug Administration and the European Medicines Agency as a potential new treatment for type 2 diabetes. It is a large molecule cleared by the reticuloendothelial system rather than by the kidney, so its metabolism is not affected by the renal dysfunction that is common in patients with type 2 diabetes.

"Albiglutide will be a useful treatment addition for patients with renal impairment and type 2 diabetes. Current treatments for such patients are limited. Several antidiabetic medications are contraindicated in this population, and many others require dose adjustment," Dr. Lawrence A. Leiter observed in presenting the trial results at the annual scientific sessions of the American Diabetes Association.
The 52-week double-blind study involved 495 patients with type 2 diabetes whose hyperglycemia wasn’t adequately controlled by diet and exercise or oral antidiabetic medications. Renal impairment was mild in slightly over half of the participants, moderate in 41%, and severe in the remainder.
Patients were randomized to self-administered subcutaneous injection of albiglutide at 30 mg once a week or the oral dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin, dose-adjusted on the basis of the degree of renal impairment as specified in the product labeling. Thirty-five percent of patients in the albiglutide group had their daily dose uptitrated from 30 mg to 50 mg owing to insufficient response to the initial dose.
The primary endpoint was the change in mean hemoglobin A1c from baseline to week 26. The HbA1c dropped by 0.8% in the albiglutide group, significantly better than the 0.5% decrease with sitagliptin (P = .0003). The advantage favoring the investigational agent was seen in all three renal impairment severity groups, reported Dr. Leiter, professor of medicine and nutritional sciences at the University of Toronto and president of the Canadian Society of Endocrinology and Metabolism.
Albiglutide-treated patients also fared significantly better in terms of multiple key secondary endpoints (see chart). For example, 42.6% of them achieved a clinically meaningful HbA1c response by 26 weeks, defined as an HbA1c below 7.0%, compared with 30.5% of patients on sitagliptin.
Both drugs were well tolerated. The rate of adverse events leading to study withdrawal was 6.4% with albiglutide and 8.1% with sitagliptin. Both drugs had impressively low rates of nausea and vomiting; typically, less than 1% of patients per week reported either symptom.
Session chair Dr. Julio Rosenstock called this study noteworthy for several reasons. It is the first study of a GLP-1 agonist in patients with renal insufficiency; head-to-head comparative randomized trials in diabetes are rare. And the low-single-digit rates of nausea and vomiting seen with albiglutide in this study are "remarkable." Other GLP-1 agonists are typically associated with nausea and vomiting rates in the 15%-20% range or even higher, noted Dr. Rosenstock, director of the Dallas Diabetes and Endocrine Center at Medical City.
Dr. Leiter agreed, offering two potential explanations for the rock-bottom nausea and vomiting rates. One is that the once-weekly injection results in a very gradual increase in drug levels. Also, as a large molecule, albiglutide probably has less central action, including less activity at the central nervous system’s nausea and vomiting centers.
Other large, completed phase III trials have shown benefits for albiglutide in additional, common clinical scenarios, including yearlong studies of albiglutide as add-on therapy in patients with type 2 diabetes not controlled on pioglitazone and metformin, albiglutide monotherapy in drug-naive type 2 diabetic patients, a comparison of the novel GLP-1 agonist to insulin glargine in patients with type 2 diabetes, and albiglutide versus pioglitazone as add-on therapy in patients on background metformin and glimepiride.
Albiglutide is being developed by GlaxoSmithKline. Dr. Leiter has received research grants from and served as a consultant to GSK and other companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Mean HbA1c levels dropped by 0.8% after 26 weeks on albiglutide in renally impaired type 2 diabetic patients, a significantly better result than the mean 0.5% decrease with sitagliptin. Patients on albiglutide also fared significantly better in terms of numerous secondary endpoints.
Data source: A phase III, randomized, double-blind trial in 495 patients with type 2 diabetes and varying degrees of renal insufficiency and baseline inadequate glycemic control assigned to either the novel once-weekly GLP-1 agonist albiglutide or sitagliptin.
Disclosures: Albiglutide is being developed by GlaxoSmithKline. Dr. Leiter has received research grants from and served as a consultant to GSK and other companies.
High diabetes incidence in Southern and Appalachian states' counties
CHICAGO – Counties in Southern and Appalachian states have some of the highest incidence rates of diabetes, while counties in large metropolitan areas reported some of the highest numbers of new cases, a government study looking at diabetes incidence rates across the United States revealed.
"We’re hoping that people can use these data to identify high-risk areas, and use it as a tool in implementing and developing diabetes prevention interventions," said Nilka R. Burrows, an epidemiologist at the Centers for Disease Control and Prevention, who presented her abstract at the annual scientific sessions of the American Diabetes Association.
Because changes in incidence rates are one of the first indicators of whether diabetes prevention efforts have been successful, the county-level estimates can help local policy makers, she said.
The interactive maps of diabetes incidence can be found at www.cdc.gov/diabetes/atlas.
Although the study is one of the first of its kind, "the findings don’t tell us anything we didn’t know," said Mercedes R. Carnethon, Ph.D., associate professor at Northwestern University, Chicago, who was not involved in the study. "But [the study] really serves to reemphasize how substantial the burden of the problem is in the high-risk areas," she said.
In 2010, nearly 26 million Americans had diabetes, and 2 million were diagnosed within the past year, said Ms. Burrows.
The authors looked at data for the period of 2004-2009 for adults aged 20 years or older in 3,143 U.S. counties or county equivalents, to identify geographic patterns and high-risk areas.
The county-level incidence estimates incorporated data from the Behavioral Risk Factor Surveillance System (BRFSS) and the U.S. Census.
The sample was age-adjusted to the 2000 U.S. standard population, Ms. Burrows said.
In 2009, the age-adjusted diabetes rate ranged from 4 per 1000 in Colorado to 21 per 1000 in Alabama. Areas that had high diabetes incidence rates (11.7 and higher) were mostly concentrated in Southern and Appalachian states, and in some counties in Arizona, New Mexico, Oklahoma, South Dakota, and Maine, Ms. Burrows reported.
The authors also looked for areas where there were more than 9,000 new diabetes cases in 2009. They identified several major metropolitan areas, including Los Angeles’ LA County with 50,000 new cases, Chicago’s Cook County with 30,000 new cases, Harris County in Houston, and Maricopa County in Phoenix, with 20,000 new cases each.
Urban counties with higher numbers of cases didn’t coincide with counties in rural areas that had the highest incidence rates, since absolute numbers are a function of population size, said Ms. Burrows.
The data "highlighted where we have the highest burden of the problem," said Dr. Carnethon. "So when you think about resources to manage diabetes, including clinical resources, such as people being in those locations, clinicians in particular, we obviously have a higher need for practitioners to deal with diabetes in those parts of the U.S."
The study also showed that the number of counties with a diabetes incidence rate of 11.7 or higher increased from 500 (16%) in 2004 to 870 (28%) in 2009. The median incidence rate was 9.7 in 2004, and 10.1 in 2009.
When calculating the 2009-to-2004 ratio of county-level diabetes incidence rates, the ratios ranged from 0.65 in Indiana to 1.79 in Pennsylvania.
Using a liberal confidence level of 0.1, the 2009-to-2004 incidence rates didn’t change in most of the counties during the 5-year period.
The analysis has some limitations. The diabetes incidence may be underestimated because it was self-reported. Also, the estimates are subject to sampling variability. In addition, during a short time period of 5 years, incidence is not likely to change dramatically, and more years of data are needed.
Ms. Burrows said that surveillance of the information should continue to gauge diabetes prevention efforts.
Ms. Burrows and Dr. Carnethon had no disclosures.
[email protected] On Twitter @NaseemSMiller
CHICAGO – Counties in Southern and Appalachian states have some of the highest incidence rates of diabetes, while counties in large metropolitan areas reported some of the highest numbers of new cases, a government study looking at diabetes incidence rates across the United States revealed.
"We’re hoping that people can use these data to identify high-risk areas, and use it as a tool in implementing and developing diabetes prevention interventions," said Nilka R. Burrows, an epidemiologist at the Centers for Disease Control and Prevention, who presented her abstract at the annual scientific sessions of the American Diabetes Association.
Because changes in incidence rates are one of the first indicators of whether diabetes prevention efforts have been successful, the county-level estimates can help local policy makers, she said.
The interactive maps of diabetes incidence can be found at www.cdc.gov/diabetes/atlas.
Although the study is one of the first of its kind, "the findings don’t tell us anything we didn’t know," said Mercedes R. Carnethon, Ph.D., associate professor at Northwestern University, Chicago, who was not involved in the study. "But [the study] really serves to reemphasize how substantial the burden of the problem is in the high-risk areas," she said.
In 2010, nearly 26 million Americans had diabetes, and 2 million were diagnosed within the past year, said Ms. Burrows.
The authors looked at data for the period of 2004-2009 for adults aged 20 years or older in 3,143 U.S. counties or county equivalents, to identify geographic patterns and high-risk areas.
The county-level incidence estimates incorporated data from the Behavioral Risk Factor Surveillance System (BRFSS) and the U.S. Census.
The sample was age-adjusted to the 2000 U.S. standard population, Ms. Burrows said.
In 2009, the age-adjusted diabetes rate ranged from 4 per 1000 in Colorado to 21 per 1000 in Alabama. Areas that had high diabetes incidence rates (11.7 and higher) were mostly concentrated in Southern and Appalachian states, and in some counties in Arizona, New Mexico, Oklahoma, South Dakota, and Maine, Ms. Burrows reported.
The authors also looked for areas where there were more than 9,000 new diabetes cases in 2009. They identified several major metropolitan areas, including Los Angeles’ LA County with 50,000 new cases, Chicago’s Cook County with 30,000 new cases, Harris County in Houston, and Maricopa County in Phoenix, with 20,000 new cases each.
Urban counties with higher numbers of cases didn’t coincide with counties in rural areas that had the highest incidence rates, since absolute numbers are a function of population size, said Ms. Burrows.
The data "highlighted where we have the highest burden of the problem," said Dr. Carnethon. "So when you think about resources to manage diabetes, including clinical resources, such as people being in those locations, clinicians in particular, we obviously have a higher need for practitioners to deal with diabetes in those parts of the U.S."
The study also showed that the number of counties with a diabetes incidence rate of 11.7 or higher increased from 500 (16%) in 2004 to 870 (28%) in 2009. The median incidence rate was 9.7 in 2004, and 10.1 in 2009.
When calculating the 2009-to-2004 ratio of county-level diabetes incidence rates, the ratios ranged from 0.65 in Indiana to 1.79 in Pennsylvania.
Using a liberal confidence level of 0.1, the 2009-to-2004 incidence rates didn’t change in most of the counties during the 5-year period.
The analysis has some limitations. The diabetes incidence may be underestimated because it was self-reported. Also, the estimates are subject to sampling variability. In addition, during a short time period of 5 years, incidence is not likely to change dramatically, and more years of data are needed.
Ms. Burrows said that surveillance of the information should continue to gauge diabetes prevention efforts.
Ms. Burrows and Dr. Carnethon had no disclosures.
[email protected] On Twitter @NaseemSMiller
CHICAGO – Counties in Southern and Appalachian states have some of the highest incidence rates of diabetes, while counties in large metropolitan areas reported some of the highest numbers of new cases, a government study looking at diabetes incidence rates across the United States revealed.
"We’re hoping that people can use these data to identify high-risk areas, and use it as a tool in implementing and developing diabetes prevention interventions," said Nilka R. Burrows, an epidemiologist at the Centers for Disease Control and Prevention, who presented her abstract at the annual scientific sessions of the American Diabetes Association.
Because changes in incidence rates are one of the first indicators of whether diabetes prevention efforts have been successful, the county-level estimates can help local policy makers, she said.
The interactive maps of diabetes incidence can be found at www.cdc.gov/diabetes/atlas.
Although the study is one of the first of its kind, "the findings don’t tell us anything we didn’t know," said Mercedes R. Carnethon, Ph.D., associate professor at Northwestern University, Chicago, who was not involved in the study. "But [the study] really serves to reemphasize how substantial the burden of the problem is in the high-risk areas," she said.
In 2010, nearly 26 million Americans had diabetes, and 2 million were diagnosed within the past year, said Ms. Burrows.
The authors looked at data for the period of 2004-2009 for adults aged 20 years or older in 3,143 U.S. counties or county equivalents, to identify geographic patterns and high-risk areas.
The county-level incidence estimates incorporated data from the Behavioral Risk Factor Surveillance System (BRFSS) and the U.S. Census.
The sample was age-adjusted to the 2000 U.S. standard population, Ms. Burrows said.
In 2009, the age-adjusted diabetes rate ranged from 4 per 1000 in Colorado to 21 per 1000 in Alabama. Areas that had high diabetes incidence rates (11.7 and higher) were mostly concentrated in Southern and Appalachian states, and in some counties in Arizona, New Mexico, Oklahoma, South Dakota, and Maine, Ms. Burrows reported.
The authors also looked for areas where there were more than 9,000 new diabetes cases in 2009. They identified several major metropolitan areas, including Los Angeles’ LA County with 50,000 new cases, Chicago’s Cook County with 30,000 new cases, Harris County in Houston, and Maricopa County in Phoenix, with 20,000 new cases each.
Urban counties with higher numbers of cases didn’t coincide with counties in rural areas that had the highest incidence rates, since absolute numbers are a function of population size, said Ms. Burrows.
The data "highlighted where we have the highest burden of the problem," said Dr. Carnethon. "So when you think about resources to manage diabetes, including clinical resources, such as people being in those locations, clinicians in particular, we obviously have a higher need for practitioners to deal with diabetes in those parts of the U.S."
The study also showed that the number of counties with a diabetes incidence rate of 11.7 or higher increased from 500 (16%) in 2004 to 870 (28%) in 2009. The median incidence rate was 9.7 in 2004, and 10.1 in 2009.
When calculating the 2009-to-2004 ratio of county-level diabetes incidence rates, the ratios ranged from 0.65 in Indiana to 1.79 in Pennsylvania.
Using a liberal confidence level of 0.1, the 2009-to-2004 incidence rates didn’t change in most of the counties during the 5-year period.
The analysis has some limitations. The diabetes incidence may be underestimated because it was self-reported. Also, the estimates are subject to sampling variability. In addition, during a short time period of 5 years, incidence is not likely to change dramatically, and more years of data are needed.
Ms. Burrows said that surveillance of the information should continue to gauge diabetes prevention efforts.
Ms. Burrows and Dr. Carnethon had no disclosures.
[email protected] On Twitter @NaseemSMiller
AT ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Counties with high incidence rates of diabetes (11.7 and higher) were mostly concentrated in Southern and Appalachian states, and in some counties in Arizona, New Mexico, Oklahoma, South Dakota, and Maine.
Data source: Centers for Disease Control and Prevention’s county-level data between 2004 and 2009 for adults aged 20 years or older, in 3,143 U.S. counties or county equivalents.
Disclosures: Ms. Burrows and Dr. Carnethon had no disclosures.