User login
American Surgical Association (ASA): Annual Meeting
Better endografts mean fewer reinterventions for endovascular AAA
INDIANAPOLIS – Reintervention rates following endovascular repair of abdominal aortic aneurysms have fallen steadily with the introduction of each successive generation of endografts, while reintervention rates after open surgical repair remained stable during a recent 15-year period.
This was among the key findings from the first in-depth analysis of reinterventions occurring in contemporaneous cohorts of abdominal aortic aneurysm (AAA) patients undergoing endovascular aneurysm repair (EVAR) or open repair. The large single-center retrospective study demonstrated major differences between the two treatment strategies in terms of the incidence, nature, timing, and mortality associated with complications requiring reintervention, Dr. Mustafa Al-Jubouri said at the annual meeting of the American Surgical Association.
Dr. Al-Jubouri of Jobst Vascular Institute, Toledo, Ohio, reported on the 1,144 patients who underwent AAA repair there during 1996-2011. Forty-nine percent had EVAR, 51% open surgical repair. Beginning in 2003, more EVARs than open repairs were done annually at the Toledo institute, consistent with the experience at many major centers in the United States and elsewhere, where EVAR has become the first-line treatment based upon evidence that it offers lower operative mortality, less blood loss, and shorter ICU and hospital lengths of stay.
These advantages come at a cost, however: namely, a greater rate of secondary interventions, mainly due to device migration, failure, or endoleaks. The purpose of Dr. Al-Jubouri’s study was to evaluate the rates and reasons for reintervention over time in the two cohorts, as well as the impact of reintervention on long-term survival.
Reintervention was required in 13.6% of the EVAR group during a mean follow-up of 4.58 years, and in 5.1% of the open surgery group during 6.58 years. A single reintervention occurred in 7.9% of EVAR patients and 3.6% of the open repair group. More than one reintervention was required in 5.8% of EVAR patients compared to just 1.6% of the open repair group.
The types and timing of complications leading to reintervention were very different in the two groups. Sixty-eight percent of reinterventions in the EVAR group were for treatment of endoleaks. Another 11.5% were to address device migration, and an equal number were for occlusion.
In contrast, the three most frequent causes of reintervention in the open repair group were colonic ischemia, accounting for 30.4% of reintervention procedures; severe bleeding, 21.7%; and incisional hernia, which triggered another 21.7% of reinterventions.
Notably, 60% of all reinterventions in the open repair group occurred during the initial hospitalization, while less than 7% of reinterventions in the EVAR patients happened within 1 month of the index procedure and only one-third within the first year, the surgeon continued.
Thirty-day mortality in EVAR patients who underwent reintervention within the first month was zero, compared to a 23.3% mortality rate in open repair patients requiring reintervention within 1 month. However, when patients did not require early reintervention, 30-day mortality rates in the two groups did not differ significantly: 1.9% in EVAR group and in the open repair group. That means when patients in the open surgery group required early reintervention, their mortality rate shot up sevenfold.
After the first 30 days post-index procedure, long-term survival rates in the two groups were similar.
Need for reintervention in the open repair group was strongly related to larger aneurysm size. In contrast, reintervention rates were similar in the EVAR group regardless of aneurysm size.
A first reintervention after EVAR occurred in 23.7% of patients who received a first-generation endograft, such as the Ancure or Talent; in 16.2% of those who got the second-generation AneuRx endograft; and in 9.1% with a third-generation endograft, such as the Excluder, Endurant, Powerlink, or Zenith. The annualized rate of reintervention during the first 3 years of follow-up was 6.8% per year with first-generation devices, 7.2% per year with second-generation endografts, and significantly lower at 3.4% per year with the third-generation.
One major reason reintervention rates in EVAR patients have declined over time is that each newer generation of endograft is lower-profile, easier to deploy, and more durable. Also, many of the surgeons now putting in third-generation endografts were performing EVAR 15 years ago; they’re very experienced operators, Dr. Al-Jubouri noted.
Discussant Dr. James R. Debord proposed another explanation for the decrease in EVAR reinterventions over time.
"Isn’t it much more likely that it’s due to recognition of the fact that many of these type 2 endoleaks that we used to intervene on early on don’t require reintervention unless there’s sac enlargement?" commented Dr. Debord, professor of clinical surgery and chief of vascular surgery at the University of Illinois at Peoria.
Dr. Al-Jubouri concurred that this is an important factor in the declining rate of EVAR reinterventions.
"We saw a significant decrease in reinterventions for type 2 endoleaks between the first, second, and third generations," he said.
Asked how his study findings have changed the follow-up protocols at Jobst Vascular Institute, the surgeon replied that in the early years of the series EVAR patients got a CT scan at 6 weeks, 6 months, 1 year, and annually thereafter. This evaluation has evolved over time. Now EVAR patients get a CT scan at 6-12 weeks, and duplex ultrasounds at 6 months, 1 year, and annually thereafter.
"There is no standardized follow-up for open repair patients. However, most [patients] get an annual duplex ultrasound for their follow-up. A CT scan is not part of the follow-up of patients with open repair. But most if not all of the complications that developed in the open repair group were symptomatic," he explained.
He reported having no financial conflicts.
Dr. Mustafa Al-Jubouri and his colleagues assessed reinterventions and outcomes after EVAR and open AAA repair over a long time period, and found decreasing rates of reintervention after EVAR, which they attribute to improvements in technology from first to third and later-generation devices. I would concur with the one discussant, that some of the decrease may also be due to the understanding that not all type II endoleaks require repair. Further, much of the decrease may be due to physician experience – both with appropriate patient and device selection, and technical expertise, including with deployment. However, regardless of the underlying reason for the improvement in the reintervention rate, it is heartening that reintervention is decreasing as physicians become more facile, and industry provides technological improvements to the devices.
Dr. Linda Harris, FACS, is division chief and program director of vascular surgery at State University of New York, Buffalo. Dr. Harris has no disclosures
Dr. Mustafa Al-Jubouri and his colleagues assessed reinterventions and outcomes after EVAR and open AAA repair over a long time period, and found decreasing rates of reintervention after EVAR, which they attribute to improvements in technology from first to third and later-generation devices. I would concur with the one discussant, that some of the decrease may also be due to the understanding that not all type II endoleaks require repair. Further, much of the decrease may be due to physician experience – both with appropriate patient and device selection, and technical expertise, including with deployment. However, regardless of the underlying reason for the improvement in the reintervention rate, it is heartening that reintervention is decreasing as physicians become more facile, and industry provides technological improvements to the devices.
Dr. Linda Harris, FACS, is division chief and program director of vascular surgery at State University of New York, Buffalo. Dr. Harris has no disclosures
Dr. Mustafa Al-Jubouri and his colleagues assessed reinterventions and outcomes after EVAR and open AAA repair over a long time period, and found decreasing rates of reintervention after EVAR, which they attribute to improvements in technology from first to third and later-generation devices. I would concur with the one discussant, that some of the decrease may also be due to the understanding that not all type II endoleaks require repair. Further, much of the decrease may be due to physician experience – both with appropriate patient and device selection, and technical expertise, including with deployment. However, regardless of the underlying reason for the improvement in the reintervention rate, it is heartening that reintervention is decreasing as physicians become more facile, and industry provides technological improvements to the devices.
Dr. Linda Harris, FACS, is division chief and program director of vascular surgery at State University of New York, Buffalo. Dr. Harris has no disclosures
INDIANAPOLIS – Reintervention rates following endovascular repair of abdominal aortic aneurysms have fallen steadily with the introduction of each successive generation of endografts, while reintervention rates after open surgical repair remained stable during a recent 15-year period.
This was among the key findings from the first in-depth analysis of reinterventions occurring in contemporaneous cohorts of abdominal aortic aneurysm (AAA) patients undergoing endovascular aneurysm repair (EVAR) or open repair. The large single-center retrospective study demonstrated major differences between the two treatment strategies in terms of the incidence, nature, timing, and mortality associated with complications requiring reintervention, Dr. Mustafa Al-Jubouri said at the annual meeting of the American Surgical Association.
Dr. Al-Jubouri of Jobst Vascular Institute, Toledo, Ohio, reported on the 1,144 patients who underwent AAA repair there during 1996-2011. Forty-nine percent had EVAR, 51% open surgical repair. Beginning in 2003, more EVARs than open repairs were done annually at the Toledo institute, consistent with the experience at many major centers in the United States and elsewhere, where EVAR has become the first-line treatment based upon evidence that it offers lower operative mortality, less blood loss, and shorter ICU and hospital lengths of stay.
These advantages come at a cost, however: namely, a greater rate of secondary interventions, mainly due to device migration, failure, or endoleaks. The purpose of Dr. Al-Jubouri’s study was to evaluate the rates and reasons for reintervention over time in the two cohorts, as well as the impact of reintervention on long-term survival.
Reintervention was required in 13.6% of the EVAR group during a mean follow-up of 4.58 years, and in 5.1% of the open surgery group during 6.58 years. A single reintervention occurred in 7.9% of EVAR patients and 3.6% of the open repair group. More than one reintervention was required in 5.8% of EVAR patients compared to just 1.6% of the open repair group.
The types and timing of complications leading to reintervention were very different in the two groups. Sixty-eight percent of reinterventions in the EVAR group were for treatment of endoleaks. Another 11.5% were to address device migration, and an equal number were for occlusion.
In contrast, the three most frequent causes of reintervention in the open repair group were colonic ischemia, accounting for 30.4% of reintervention procedures; severe bleeding, 21.7%; and incisional hernia, which triggered another 21.7% of reinterventions.
Notably, 60% of all reinterventions in the open repair group occurred during the initial hospitalization, while less than 7% of reinterventions in the EVAR patients happened within 1 month of the index procedure and only one-third within the first year, the surgeon continued.
Thirty-day mortality in EVAR patients who underwent reintervention within the first month was zero, compared to a 23.3% mortality rate in open repair patients requiring reintervention within 1 month. However, when patients did not require early reintervention, 30-day mortality rates in the two groups did not differ significantly: 1.9% in EVAR group and in the open repair group. That means when patients in the open surgery group required early reintervention, their mortality rate shot up sevenfold.
After the first 30 days post-index procedure, long-term survival rates in the two groups were similar.
Need for reintervention in the open repair group was strongly related to larger aneurysm size. In contrast, reintervention rates were similar in the EVAR group regardless of aneurysm size.
A first reintervention after EVAR occurred in 23.7% of patients who received a first-generation endograft, such as the Ancure or Talent; in 16.2% of those who got the second-generation AneuRx endograft; and in 9.1% with a third-generation endograft, such as the Excluder, Endurant, Powerlink, or Zenith. The annualized rate of reintervention during the first 3 years of follow-up was 6.8% per year with first-generation devices, 7.2% per year with second-generation endografts, and significantly lower at 3.4% per year with the third-generation.
One major reason reintervention rates in EVAR patients have declined over time is that each newer generation of endograft is lower-profile, easier to deploy, and more durable. Also, many of the surgeons now putting in third-generation endografts were performing EVAR 15 years ago; they’re very experienced operators, Dr. Al-Jubouri noted.
Discussant Dr. James R. Debord proposed another explanation for the decrease in EVAR reinterventions over time.
"Isn’t it much more likely that it’s due to recognition of the fact that many of these type 2 endoleaks that we used to intervene on early on don’t require reintervention unless there’s sac enlargement?" commented Dr. Debord, professor of clinical surgery and chief of vascular surgery at the University of Illinois at Peoria.
Dr. Al-Jubouri concurred that this is an important factor in the declining rate of EVAR reinterventions.
"We saw a significant decrease in reinterventions for type 2 endoleaks between the first, second, and third generations," he said.
Asked how his study findings have changed the follow-up protocols at Jobst Vascular Institute, the surgeon replied that in the early years of the series EVAR patients got a CT scan at 6 weeks, 6 months, 1 year, and annually thereafter. This evaluation has evolved over time. Now EVAR patients get a CT scan at 6-12 weeks, and duplex ultrasounds at 6 months, 1 year, and annually thereafter.
"There is no standardized follow-up for open repair patients. However, most [patients] get an annual duplex ultrasound for their follow-up. A CT scan is not part of the follow-up of patients with open repair. But most if not all of the complications that developed in the open repair group were symptomatic," he explained.
He reported having no financial conflicts.
INDIANAPOLIS – Reintervention rates following endovascular repair of abdominal aortic aneurysms have fallen steadily with the introduction of each successive generation of endografts, while reintervention rates after open surgical repair remained stable during a recent 15-year period.
This was among the key findings from the first in-depth analysis of reinterventions occurring in contemporaneous cohorts of abdominal aortic aneurysm (AAA) patients undergoing endovascular aneurysm repair (EVAR) or open repair. The large single-center retrospective study demonstrated major differences between the two treatment strategies in terms of the incidence, nature, timing, and mortality associated with complications requiring reintervention, Dr. Mustafa Al-Jubouri said at the annual meeting of the American Surgical Association.
Dr. Al-Jubouri of Jobst Vascular Institute, Toledo, Ohio, reported on the 1,144 patients who underwent AAA repair there during 1996-2011. Forty-nine percent had EVAR, 51% open surgical repair. Beginning in 2003, more EVARs than open repairs were done annually at the Toledo institute, consistent with the experience at many major centers in the United States and elsewhere, where EVAR has become the first-line treatment based upon evidence that it offers lower operative mortality, less blood loss, and shorter ICU and hospital lengths of stay.
These advantages come at a cost, however: namely, a greater rate of secondary interventions, mainly due to device migration, failure, or endoleaks. The purpose of Dr. Al-Jubouri’s study was to evaluate the rates and reasons for reintervention over time in the two cohorts, as well as the impact of reintervention on long-term survival.
Reintervention was required in 13.6% of the EVAR group during a mean follow-up of 4.58 years, and in 5.1% of the open surgery group during 6.58 years. A single reintervention occurred in 7.9% of EVAR patients and 3.6% of the open repair group. More than one reintervention was required in 5.8% of EVAR patients compared to just 1.6% of the open repair group.
The types and timing of complications leading to reintervention were very different in the two groups. Sixty-eight percent of reinterventions in the EVAR group were for treatment of endoleaks. Another 11.5% were to address device migration, and an equal number were for occlusion.
In contrast, the three most frequent causes of reintervention in the open repair group were colonic ischemia, accounting for 30.4% of reintervention procedures; severe bleeding, 21.7%; and incisional hernia, which triggered another 21.7% of reinterventions.
Notably, 60% of all reinterventions in the open repair group occurred during the initial hospitalization, while less than 7% of reinterventions in the EVAR patients happened within 1 month of the index procedure and only one-third within the first year, the surgeon continued.
Thirty-day mortality in EVAR patients who underwent reintervention within the first month was zero, compared to a 23.3% mortality rate in open repair patients requiring reintervention within 1 month. However, when patients did not require early reintervention, 30-day mortality rates in the two groups did not differ significantly: 1.9% in EVAR group and in the open repair group. That means when patients in the open surgery group required early reintervention, their mortality rate shot up sevenfold.
After the first 30 days post-index procedure, long-term survival rates in the two groups were similar.
Need for reintervention in the open repair group was strongly related to larger aneurysm size. In contrast, reintervention rates were similar in the EVAR group regardless of aneurysm size.
A first reintervention after EVAR occurred in 23.7% of patients who received a first-generation endograft, such as the Ancure or Talent; in 16.2% of those who got the second-generation AneuRx endograft; and in 9.1% with a third-generation endograft, such as the Excluder, Endurant, Powerlink, or Zenith. The annualized rate of reintervention during the first 3 years of follow-up was 6.8% per year with first-generation devices, 7.2% per year with second-generation endografts, and significantly lower at 3.4% per year with the third-generation.
One major reason reintervention rates in EVAR patients have declined over time is that each newer generation of endograft is lower-profile, easier to deploy, and more durable. Also, many of the surgeons now putting in third-generation endografts were performing EVAR 15 years ago; they’re very experienced operators, Dr. Al-Jubouri noted.
Discussant Dr. James R. Debord proposed another explanation for the decrease in EVAR reinterventions over time.
"Isn’t it much more likely that it’s due to recognition of the fact that many of these type 2 endoleaks that we used to intervene on early on don’t require reintervention unless there’s sac enlargement?" commented Dr. Debord, professor of clinical surgery and chief of vascular surgery at the University of Illinois at Peoria.
Dr. Al-Jubouri concurred that this is an important factor in the declining rate of EVAR reinterventions.
"We saw a significant decrease in reinterventions for type 2 endoleaks between the first, second, and third generations," he said.
Asked how his study findings have changed the follow-up protocols at Jobst Vascular Institute, the surgeon replied that in the early years of the series EVAR patients got a CT scan at 6 weeks, 6 months, 1 year, and annually thereafter. This evaluation has evolved over time. Now EVAR patients get a CT scan at 6-12 weeks, and duplex ultrasounds at 6 months, 1 year, and annually thereafter.
"There is no standardized follow-up for open repair patients. However, most [patients] get an annual duplex ultrasound for their follow-up. A CT scan is not part of the follow-up of patients with open repair. But most if not all of the complications that developed in the open repair group were symptomatic," he explained.
He reported having no financial conflicts.
AT THE ASA ANNUAL MEETING
Major Finding: Reintervention rates were markedly higher following endovascular repair compared with open surgical repair of abdominal aortic aneurysms, but the adverse effects associated with reintervention after open repair were far more serious.
Data Source: A retrospective study of the 15-year experience at a large-volume vascular surgery. It encompassed 1,144 patients who underwent abdominal aortic aneurysm repair and their subsequent reintervention rates.
Disclosures: The presenter reported having no conflicts of interest.
Robotic pancreatic resection safe in 250-patient series
INDIANAPOLIS – Robotic-assisted major pancreatic resection is safe, feasible, reliable, and versatile, according to the findings of the largest reported single-center series of such procedures.
That being said, the next and absolutely critical step needs to be comparative effectiveness studies pitting robotic versus laparoscopic or open pancreatic resections, Dr. Herbert J. Zeh III reported at the annual meeting of the American Surgical Association.
He noted that there was a considerable learning curve with the procedure in this single-center series of 250 consecutive robotic-assisted major pancreatic resections. "If we had compared our first 30, 40, or even 60 cases, we would have been comparing an innovative procedure to one that’s been refined continuously since 1937," noted Dr. Zeh of the University of Pittsburgh.
Discussants praised Dr. Zeh and his coinvestigators as innovators who are taking a rigorously scientific and cautious approach in investigating the applicability of robotic techniques to major pancreatic surgery. But some discussants were concerned that the growing dissemination of robotic surgery is based largely upon what they consider to be marketing hype and competitive pressure.
Dr. Zeh explained that he and his coworkers have undertaken the study of robotic-assisted major pancreatic resections because they believe that a minimally invasive approach will reduce the substantial morbidity traditionally associated with open procedures, and that laparoscopic techniques aren’t the answer in these complex resections, which often require resuturing the pancreas to the GI tract.
"It was our perception as a group of dedicated pancreatic surgeons that we could not utilize the laparoscopic technology to adhere to the standard principles of open surgery that we thought were important for safe performance of pancreatic resections. These include meticulous dissection, safe control of major vascular structures, and precise suturing," he said.
The 250 consecutive robotic-assisted major pancreatic resections in this series included the full range of complex pancreatic operations. The two most common procedures were pancreaticoduodenectomy, also known as the Whipple procedure, in 132 patients and distal pancreatectomy in 83.
Overall 30- and 90-day mortality rates were 0.8% and 2.0%, respectively. All deaths were in pancreaticoduodenectomy patients, with 30- and 90-day mortality rates of 1.5% and 3.8%.
Clinically significant complications occurred in 21% of patients. The most common was intra-abdominal fluid collection requiring drainage via interventional radiology. Morbidity rates were similar to those reported in large series of open and laparoscopic pancreatic resections.
Estimated blood loss in pancreaticoduodenectomy averaged 499 mL in the first one-third of patients who had the robotic procedure; thereafter, the blood loss improved to 401 mL.
Rates of conversion from robotic to open surgery also improved over time, from 18.2% in the first third of the patient series to 3.4% in the latter two-thirds.
Mean operative time was 529 minutes for pancreaticoduodenectomy and 256 minutes for distal pancreatectomy. These times have dropped steadily with experience such that mean operative time in the last 50 pancreaticoduodenectomies was 444 minutes, while in the last 50 distal pancreatectomies it was 222 minutes, which approaches reported times for laparoscopic and open operations, Dr. Zeh noted.
The median length of stay was 10 days for pancreaticoduodenectomy patients and 6 days for those undergoing distal pancreatectomy. As a precautionary measure, surgeons kept patients treated early in the series in the hospital longer than was probably necessary. Length of stay has come down over time, although this trend hasn’t yet reached statistical significance.
The readmission rate was 24% in pancreaticoduodenectomy patients and 28% in distal pancreatectomy patients; 2% of patients required a reoperation.
As experience has grown, the group’s criteria for selecting patients for the robotic approach have loosened considerably. Many recent patients have been obese or superobese.
"Currently the only absolute contraindication is some sort of vascular involvement that would entail resecting a vein and reanastomosing it using a minimally invasive approach. That’s really the only frontier we haven’t crossed," said Dr. Zeh.
The potential advantages of the robotic platform that drew the researchers’ interest include greater range of motion for the robotic needle driver and other tools, compared with what is achievable laparoscopically; enhanced visualization with magnification; computerized smoothing of a surgeon’s tremor; and the ability to see structures in three dimensions, unlike in laparoscopy.
"I think the real advantage is the computer," he added. "Robotic surgery is probably misnamed; it’s really computer-assisted surgery. What this is going to allow us to do is to take the skill sets that we have to the next level. Pilots couldn’t control fighter jets without a computer between them and the plane. In the end, I think the addition of the computer between us and the patient is going to allow us to do things that we haven’t even thought about."
He reported having received an honorarium from Medtronic on a single occasion for participation in a symposium on minimally invasive pancreatic surgery.
When it comes to robotic surgery, the emperor is not wearing any clothes. I don’t believe there has ever been a series that has conclusively shown that the robot has made any difference in patient outcomes or quality of the procedure. I believe that it is a technology that enables surgeons who cannot otherwise perform the procedure to perform the procedure. That’s been shown in the urology literature, particularly.
What’s going on in my community and others throughout the country is a terrible abuse of this technology, where we have doctors in our local hospitals taking out ovaries with this technology, taking out a uterus, and who are doing single-site robotic cholecystectomies in 4 hours at $4,000 in cost. They’re using robotic technology to do simple procedures that could otherwise be done better and faster without this technology.
We need to be outspoken and realistic about the use of robotic surgery. We need to advance this technology, but carefully and with a caveat.
Dr. Jeffrey L. Ponsky is professor and chairman of the department of surgery at Case Western Reserve University, Cleveland. He made his remarks as a discussant at the meeting.
When it comes to robotic surgery, the emperor is not wearing any clothes. I don’t believe there has ever been a series that has conclusively shown that the robot has made any difference in patient outcomes or quality of the procedure. I believe that it is a technology that enables surgeons who cannot otherwise perform the procedure to perform the procedure. That’s been shown in the urology literature, particularly.
What’s going on in my community and others throughout the country is a terrible abuse of this technology, where we have doctors in our local hospitals taking out ovaries with this technology, taking out a uterus, and who are doing single-site robotic cholecystectomies in 4 hours at $4,000 in cost. They’re using robotic technology to do simple procedures that could otherwise be done better and faster without this technology.
We need to be outspoken and realistic about the use of robotic surgery. We need to advance this technology, but carefully and with a caveat.
Dr. Jeffrey L. Ponsky is professor and chairman of the department of surgery at Case Western Reserve University, Cleveland. He made his remarks as a discussant at the meeting.
When it comes to robotic surgery, the emperor is not wearing any clothes. I don’t believe there has ever been a series that has conclusively shown that the robot has made any difference in patient outcomes or quality of the procedure. I believe that it is a technology that enables surgeons who cannot otherwise perform the procedure to perform the procedure. That’s been shown in the urology literature, particularly.
What’s going on in my community and others throughout the country is a terrible abuse of this technology, where we have doctors in our local hospitals taking out ovaries with this technology, taking out a uterus, and who are doing single-site robotic cholecystectomies in 4 hours at $4,000 in cost. They’re using robotic technology to do simple procedures that could otherwise be done better and faster without this technology.
We need to be outspoken and realistic about the use of robotic surgery. We need to advance this technology, but carefully and with a caveat.
Dr. Jeffrey L. Ponsky is professor and chairman of the department of surgery at Case Western Reserve University, Cleveland. He made his remarks as a discussant at the meeting.
INDIANAPOLIS – Robotic-assisted major pancreatic resection is safe, feasible, reliable, and versatile, according to the findings of the largest reported single-center series of such procedures.
That being said, the next and absolutely critical step needs to be comparative effectiveness studies pitting robotic versus laparoscopic or open pancreatic resections, Dr. Herbert J. Zeh III reported at the annual meeting of the American Surgical Association.
He noted that there was a considerable learning curve with the procedure in this single-center series of 250 consecutive robotic-assisted major pancreatic resections. "If we had compared our first 30, 40, or even 60 cases, we would have been comparing an innovative procedure to one that’s been refined continuously since 1937," noted Dr. Zeh of the University of Pittsburgh.
Discussants praised Dr. Zeh and his coinvestigators as innovators who are taking a rigorously scientific and cautious approach in investigating the applicability of robotic techniques to major pancreatic surgery. But some discussants were concerned that the growing dissemination of robotic surgery is based largely upon what they consider to be marketing hype and competitive pressure.
Dr. Zeh explained that he and his coworkers have undertaken the study of robotic-assisted major pancreatic resections because they believe that a minimally invasive approach will reduce the substantial morbidity traditionally associated with open procedures, and that laparoscopic techniques aren’t the answer in these complex resections, which often require resuturing the pancreas to the GI tract.
"It was our perception as a group of dedicated pancreatic surgeons that we could not utilize the laparoscopic technology to adhere to the standard principles of open surgery that we thought were important for safe performance of pancreatic resections. These include meticulous dissection, safe control of major vascular structures, and precise suturing," he said.
The 250 consecutive robotic-assisted major pancreatic resections in this series included the full range of complex pancreatic operations. The two most common procedures were pancreaticoduodenectomy, also known as the Whipple procedure, in 132 patients and distal pancreatectomy in 83.
Overall 30- and 90-day mortality rates were 0.8% and 2.0%, respectively. All deaths were in pancreaticoduodenectomy patients, with 30- and 90-day mortality rates of 1.5% and 3.8%.
Clinically significant complications occurred in 21% of patients. The most common was intra-abdominal fluid collection requiring drainage via interventional radiology. Morbidity rates were similar to those reported in large series of open and laparoscopic pancreatic resections.
Estimated blood loss in pancreaticoduodenectomy averaged 499 mL in the first one-third of patients who had the robotic procedure; thereafter, the blood loss improved to 401 mL.
Rates of conversion from robotic to open surgery also improved over time, from 18.2% in the first third of the patient series to 3.4% in the latter two-thirds.
Mean operative time was 529 minutes for pancreaticoduodenectomy and 256 minutes for distal pancreatectomy. These times have dropped steadily with experience such that mean operative time in the last 50 pancreaticoduodenectomies was 444 minutes, while in the last 50 distal pancreatectomies it was 222 minutes, which approaches reported times for laparoscopic and open operations, Dr. Zeh noted.
The median length of stay was 10 days for pancreaticoduodenectomy patients and 6 days for those undergoing distal pancreatectomy. As a precautionary measure, surgeons kept patients treated early in the series in the hospital longer than was probably necessary. Length of stay has come down over time, although this trend hasn’t yet reached statistical significance.
The readmission rate was 24% in pancreaticoduodenectomy patients and 28% in distal pancreatectomy patients; 2% of patients required a reoperation.
As experience has grown, the group’s criteria for selecting patients for the robotic approach have loosened considerably. Many recent patients have been obese or superobese.
"Currently the only absolute contraindication is some sort of vascular involvement that would entail resecting a vein and reanastomosing it using a minimally invasive approach. That’s really the only frontier we haven’t crossed," said Dr. Zeh.
The potential advantages of the robotic platform that drew the researchers’ interest include greater range of motion for the robotic needle driver and other tools, compared with what is achievable laparoscopically; enhanced visualization with magnification; computerized smoothing of a surgeon’s tremor; and the ability to see structures in three dimensions, unlike in laparoscopy.
"I think the real advantage is the computer," he added. "Robotic surgery is probably misnamed; it’s really computer-assisted surgery. What this is going to allow us to do is to take the skill sets that we have to the next level. Pilots couldn’t control fighter jets without a computer between them and the plane. In the end, I think the addition of the computer between us and the patient is going to allow us to do things that we haven’t even thought about."
He reported having received an honorarium from Medtronic on a single occasion for participation in a symposium on minimally invasive pancreatic surgery.
INDIANAPOLIS – Robotic-assisted major pancreatic resection is safe, feasible, reliable, and versatile, according to the findings of the largest reported single-center series of such procedures.
That being said, the next and absolutely critical step needs to be comparative effectiveness studies pitting robotic versus laparoscopic or open pancreatic resections, Dr. Herbert J. Zeh III reported at the annual meeting of the American Surgical Association.
He noted that there was a considerable learning curve with the procedure in this single-center series of 250 consecutive robotic-assisted major pancreatic resections. "If we had compared our first 30, 40, or even 60 cases, we would have been comparing an innovative procedure to one that’s been refined continuously since 1937," noted Dr. Zeh of the University of Pittsburgh.
Discussants praised Dr. Zeh and his coinvestigators as innovators who are taking a rigorously scientific and cautious approach in investigating the applicability of robotic techniques to major pancreatic surgery. But some discussants were concerned that the growing dissemination of robotic surgery is based largely upon what they consider to be marketing hype and competitive pressure.
Dr. Zeh explained that he and his coworkers have undertaken the study of robotic-assisted major pancreatic resections because they believe that a minimally invasive approach will reduce the substantial morbidity traditionally associated with open procedures, and that laparoscopic techniques aren’t the answer in these complex resections, which often require resuturing the pancreas to the GI tract.
"It was our perception as a group of dedicated pancreatic surgeons that we could not utilize the laparoscopic technology to adhere to the standard principles of open surgery that we thought were important for safe performance of pancreatic resections. These include meticulous dissection, safe control of major vascular structures, and precise suturing," he said.
The 250 consecutive robotic-assisted major pancreatic resections in this series included the full range of complex pancreatic operations. The two most common procedures were pancreaticoduodenectomy, also known as the Whipple procedure, in 132 patients and distal pancreatectomy in 83.
Overall 30- and 90-day mortality rates were 0.8% and 2.0%, respectively. All deaths were in pancreaticoduodenectomy patients, with 30- and 90-day mortality rates of 1.5% and 3.8%.
Clinically significant complications occurred in 21% of patients. The most common was intra-abdominal fluid collection requiring drainage via interventional radiology. Morbidity rates were similar to those reported in large series of open and laparoscopic pancreatic resections.
Estimated blood loss in pancreaticoduodenectomy averaged 499 mL in the first one-third of patients who had the robotic procedure; thereafter, the blood loss improved to 401 mL.
Rates of conversion from robotic to open surgery also improved over time, from 18.2% in the first third of the patient series to 3.4% in the latter two-thirds.
Mean operative time was 529 minutes for pancreaticoduodenectomy and 256 minutes for distal pancreatectomy. These times have dropped steadily with experience such that mean operative time in the last 50 pancreaticoduodenectomies was 444 minutes, while in the last 50 distal pancreatectomies it was 222 minutes, which approaches reported times for laparoscopic and open operations, Dr. Zeh noted.
The median length of stay was 10 days for pancreaticoduodenectomy patients and 6 days for those undergoing distal pancreatectomy. As a precautionary measure, surgeons kept patients treated early in the series in the hospital longer than was probably necessary. Length of stay has come down over time, although this trend hasn’t yet reached statistical significance.
The readmission rate was 24% in pancreaticoduodenectomy patients and 28% in distal pancreatectomy patients; 2% of patients required a reoperation.
As experience has grown, the group’s criteria for selecting patients for the robotic approach have loosened considerably. Many recent patients have been obese or superobese.
"Currently the only absolute contraindication is some sort of vascular involvement that would entail resecting a vein and reanastomosing it using a minimally invasive approach. That’s really the only frontier we haven’t crossed," said Dr. Zeh.
The potential advantages of the robotic platform that drew the researchers’ interest include greater range of motion for the robotic needle driver and other tools, compared with what is achievable laparoscopically; enhanced visualization with magnification; computerized smoothing of a surgeon’s tremor; and the ability to see structures in three dimensions, unlike in laparoscopy.
"I think the real advantage is the computer," he added. "Robotic surgery is probably misnamed; it’s really computer-assisted surgery. What this is going to allow us to do is to take the skill sets that we have to the next level. Pilots couldn’t control fighter jets without a computer between them and the plane. In the end, I think the addition of the computer between us and the patient is going to allow us to do things that we haven’t even thought about."
He reported having received an honorarium from Medtronic on a single occasion for participation in a symposium on minimally invasive pancreatic surgery.
AT THE ASA ANNUAL MEETING
Major finding: Overall 30- and 90-day mortality rates were 0.8% and 2.0%, respectively, following various types of robotic-assisted major pancreatic resection, with deaths occurring only in the subset of patients undergoing pancreaticoduodenectomy.
Data source: A retrospective review of a prospectively maintained single-center database of 250 consecutive patients undergoing robotic-assisted major pancreatic resections.
Disclosures: The presenter reported having received an honorarium from Medtronic on a single occasion.
Endovascular AAA repair superior for kidney disease patients
INDIANAPOLIS – Contrary to conventional wisdom, endovascular aneurysm repair (EVAR) provides outcomes superior to those achieved with open surgical repair of abdominal aortic aneurysm in patients with chronic renal insufficiency, a large study indicates.
"EVAR should be the first-line therapy in the patient with chronic renal insufficiency when the patient has the appropriate anatomy. However, in patients with severe renal impairment, a higher threshold should be applied for repair because the risks of both open repair and EVAR are significantly higher," Dr. Bao-Ngoc H. Nguyen declared at the annual meeting of the American Surgical Association.
"Chronic renal failure is quite prevalent in patients with abdominal aortic aneurysm: up to 30%. It is quite worrisome because any further decline in renal function in these patients could push them toward dialysis. More than that, postoperative renal failure is a predictor for early and late mortality," noted Dr. Nguyen of George Washington University, Washington.
She presented a retrospective study in patients with abdominal aortic aneurysm and chronic kidney disease. The aim, she explained, was to answer a key question: "Which one of these two treatment modalities is the lesser of two evils?"
For answers, Dr. Nguyen and coinvestigators turned to the American College of Surgeons National Quality Improvement Program (NSQIP) database for 2005-2010. They identified 3,523 patients with moderate chronic renal insufficiency, defined as an estimated glomerular filtration rate (eGFR) of 30-60 mL/minute, who underwent EVAR for abdominal aortic aneurysm and 1,117 treated via open surgical repair. Another 363 EVAR patients had severe chronic renal insufficiency, with an eGFR of less than 30 mL/minute, as did 139 patients who underwent open repair. Vascular surgeons performed all procedures in this study.
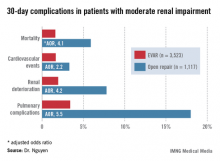
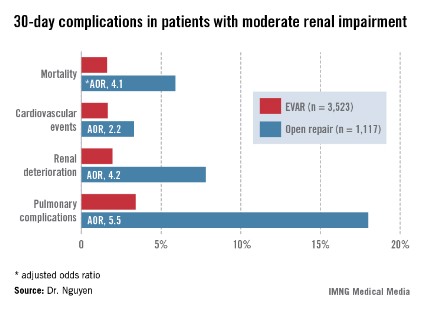
Patients with moderate renal insufficiency who underwent EVAR had markedly lower 30-day rates of mortality, pulmonary complications, cardiovascular events, and postoperative renal dysfunction, including acute kidney injury, than did those who had open surgical repair. One or more adverse events occurred in 6% of the EVAR group, compared with 24.1% of open repair patients. In a multivariate analysis controlled for preoperative differences in the patient groups, those undergoing open repair had an adjusted 4.1-fold greater risk of mortality as well as a 2.2-fold increased risk of cardiovascular events, a 4.2-fold increased risk of renal deterioration including a 5.2-fold greater risk of dialysis, and additional hazards.
In contrast, among the much smaller population of patients with baseline severe chronic renal insufficiency, there was no significant difference between the two treatment groups in terms of 30-day mortality, postoperative renal deterioration, or cardiovascular complications, although pulmonary complications were an adjusted fivefold more likely in the open surgery than among EVAR patients. Of note, rates of all adverse outcomes were markedly higher in both groups than in those with moderate chronic renal insufficiency, such that one or more adverse events occurred in 16.9% of EVAR patients and 42.5% of the open repair patients with severe chronic renal insufficiency.
Discussant Dr. Michael Watkins commented that this study has one glaring shortcoming resulting from a limitation of the NSQIP database.
While NSQIP contains only validated data entered by unbiased, well-trained professionals and NSQIP is "far superior" to the various administrative databases commonly used in evaluating outcomes, it doesn’t include key details about patients’ presenting anatomy, observed Dr. Watkins, director of the vascular research laboratory at Massachusetts General Hospital, Boston.
"Was the anatomy really similar in the two groups, or were patients who underwent open repair not candidates for EVAR?" he asked.
Dr. Nguyen conceded that this constitutes a major study limitation, adding that she agrees with Dr. Watkins that anatomy should be the first and foremost factor considered in deciding upon the surgical approach in abdominal aortic aneurysm repair.
She reported having no financial conflicts.
INDIANAPOLIS – Contrary to conventional wisdom, endovascular aneurysm repair (EVAR) provides outcomes superior to those achieved with open surgical repair of abdominal aortic aneurysm in patients with chronic renal insufficiency, a large study indicates.
"EVAR should be the first-line therapy in the patient with chronic renal insufficiency when the patient has the appropriate anatomy. However, in patients with severe renal impairment, a higher threshold should be applied for repair because the risks of both open repair and EVAR are significantly higher," Dr. Bao-Ngoc H. Nguyen declared at the annual meeting of the American Surgical Association.
"Chronic renal failure is quite prevalent in patients with abdominal aortic aneurysm: up to 30%. It is quite worrisome because any further decline in renal function in these patients could push them toward dialysis. More than that, postoperative renal failure is a predictor for early and late mortality," noted Dr. Nguyen of George Washington University, Washington.
She presented a retrospective study in patients with abdominal aortic aneurysm and chronic kidney disease. The aim, she explained, was to answer a key question: "Which one of these two treatment modalities is the lesser of two evils?"
For answers, Dr. Nguyen and coinvestigators turned to the American College of Surgeons National Quality Improvement Program (NSQIP) database for 2005-2010. They identified 3,523 patients with moderate chronic renal insufficiency, defined as an estimated glomerular filtration rate (eGFR) of 30-60 mL/minute, who underwent EVAR for abdominal aortic aneurysm and 1,117 treated via open surgical repair. Another 363 EVAR patients had severe chronic renal insufficiency, with an eGFR of less than 30 mL/minute, as did 139 patients who underwent open repair. Vascular surgeons performed all procedures in this study.
Patients with moderate renal insufficiency who underwent EVAR had markedly lower 30-day rates of mortality, pulmonary complications, cardiovascular events, and postoperative renal dysfunction, including acute kidney injury, than did those who had open surgical repair. One or more adverse events occurred in 6% of the EVAR group, compared with 24.1% of open repair patients. In a multivariate analysis controlled for preoperative differences in the patient groups, those undergoing open repair had an adjusted 4.1-fold greater risk of mortality as well as a 2.2-fold increased risk of cardiovascular events, a 4.2-fold increased risk of renal deterioration including a 5.2-fold greater risk of dialysis, and additional hazards.
In contrast, among the much smaller population of patients with baseline severe chronic renal insufficiency, there was no significant difference between the two treatment groups in terms of 30-day mortality, postoperative renal deterioration, or cardiovascular complications, although pulmonary complications were an adjusted fivefold more likely in the open surgery than among EVAR patients. Of note, rates of all adverse outcomes were markedly higher in both groups than in those with moderate chronic renal insufficiency, such that one or more adverse events occurred in 16.9% of EVAR patients and 42.5% of the open repair patients with severe chronic renal insufficiency.
Discussant Dr. Michael Watkins commented that this study has one glaring shortcoming resulting from a limitation of the NSQIP database.
While NSQIP contains only validated data entered by unbiased, well-trained professionals and NSQIP is "far superior" to the various administrative databases commonly used in evaluating outcomes, it doesn’t include key details about patients’ presenting anatomy, observed Dr. Watkins, director of the vascular research laboratory at Massachusetts General Hospital, Boston.
"Was the anatomy really similar in the two groups, or were patients who underwent open repair not candidates for EVAR?" he asked.
Dr. Nguyen conceded that this constitutes a major study limitation, adding that she agrees with Dr. Watkins that anatomy should be the first and foremost factor considered in deciding upon the surgical approach in abdominal aortic aneurysm repair.
She reported having no financial conflicts.
INDIANAPOLIS – Contrary to conventional wisdom, endovascular aneurysm repair (EVAR) provides outcomes superior to those achieved with open surgical repair of abdominal aortic aneurysm in patients with chronic renal insufficiency, a large study indicates.
"EVAR should be the first-line therapy in the patient with chronic renal insufficiency when the patient has the appropriate anatomy. However, in patients with severe renal impairment, a higher threshold should be applied for repair because the risks of both open repair and EVAR are significantly higher," Dr. Bao-Ngoc H. Nguyen declared at the annual meeting of the American Surgical Association.
"Chronic renal failure is quite prevalent in patients with abdominal aortic aneurysm: up to 30%. It is quite worrisome because any further decline in renal function in these patients could push them toward dialysis. More than that, postoperative renal failure is a predictor for early and late mortality," noted Dr. Nguyen of George Washington University, Washington.
She presented a retrospective study in patients with abdominal aortic aneurysm and chronic kidney disease. The aim, she explained, was to answer a key question: "Which one of these two treatment modalities is the lesser of two evils?"
For answers, Dr. Nguyen and coinvestigators turned to the American College of Surgeons National Quality Improvement Program (NSQIP) database for 2005-2010. They identified 3,523 patients with moderate chronic renal insufficiency, defined as an estimated glomerular filtration rate (eGFR) of 30-60 mL/minute, who underwent EVAR for abdominal aortic aneurysm and 1,117 treated via open surgical repair. Another 363 EVAR patients had severe chronic renal insufficiency, with an eGFR of less than 30 mL/minute, as did 139 patients who underwent open repair. Vascular surgeons performed all procedures in this study.
Patients with moderate renal insufficiency who underwent EVAR had markedly lower 30-day rates of mortality, pulmonary complications, cardiovascular events, and postoperative renal dysfunction, including acute kidney injury, than did those who had open surgical repair. One or more adverse events occurred in 6% of the EVAR group, compared with 24.1% of open repair patients. In a multivariate analysis controlled for preoperative differences in the patient groups, those undergoing open repair had an adjusted 4.1-fold greater risk of mortality as well as a 2.2-fold increased risk of cardiovascular events, a 4.2-fold increased risk of renal deterioration including a 5.2-fold greater risk of dialysis, and additional hazards.
In contrast, among the much smaller population of patients with baseline severe chronic renal insufficiency, there was no significant difference between the two treatment groups in terms of 30-day mortality, postoperative renal deterioration, or cardiovascular complications, although pulmonary complications were an adjusted fivefold more likely in the open surgery than among EVAR patients. Of note, rates of all adverse outcomes were markedly higher in both groups than in those with moderate chronic renal insufficiency, such that one or more adverse events occurred in 16.9% of EVAR patients and 42.5% of the open repair patients with severe chronic renal insufficiency.
Discussant Dr. Michael Watkins commented that this study has one glaring shortcoming resulting from a limitation of the NSQIP database.
While NSQIP contains only validated data entered by unbiased, well-trained professionals and NSQIP is "far superior" to the various administrative databases commonly used in evaluating outcomes, it doesn’t include key details about patients’ presenting anatomy, observed Dr. Watkins, director of the vascular research laboratory at Massachusetts General Hospital, Boston.
"Was the anatomy really similar in the two groups, or were patients who underwent open repair not candidates for EVAR?" he asked.
Dr. Nguyen conceded that this constitutes a major study limitation, adding that she agrees with Dr. Watkins that anatomy should be the first and foremost factor considered in deciding upon the surgical approach in abdominal aortic aneurysm repair.
She reported having no financial conflicts.
AT THE ASA ANNUAL MEETING
Major Finding: Patients with moderate chronic renal insufficiency who underwent open surgical repair of abdominal aortic aneurysm had a 4.2-fold greater risk of postoperative renal deterioration than did those who had an endovascular aneurysm repair.
Data Source: A retrospective study of a large national surgical database.
Disclosures: The presenter reported having no conflicts of interest.
Surgical educators flag training gaps
INDIANAPOLIS – The nation’s elite surgical educators are up in arms over reported widespread deficiencies in the skill set and judgment of recent graduates of 5-year general surgery residencies.
The source of their ire is a detailed new survey of the nation’s subspecialty fellowship program directors. Today 80% of graduating general surgery residents seek these year-long fellowships to obtain advanced training in bariatric, colorectal, thoracic, hepatobiliary, or other surgical areas. The surveyed program directors indicated many trainees arrive unprepared in essential areas.
"Many new fellows must gain basic and fundamental skills at the beginning of their fellowship before they can commence to benefit from the advanced skills that they originally came to obtain. The current high demand for fellowship training and the lack of readiness upon completion of general surgery residencies should be a call to action for all stakeholders in surgical training," Dr. Samer Mattar declared in presenting the survey results at the annual meeting of the American Surgical Association.
The survey was conducted by the Fellowship Council, an umbrella organization in charge of standardizing curricula, accrediting programs, and matching residents to fellowships. The group distributed the surveys to all 145 subspecialty fellowship program directors and drew a 63% response rate. That’s considered high for such a lengthy survey and is an indication of the importance educators place on the subject matter, said Dr. Mattar of Indiana University, Indianapolis.
The survey assessed five key educational domains: professionalism, independent practice, psychomotor skills, expertise in their chosen disease state, and scholarly focus.
"Incoming fellows exhibited high levels of professionalism, but there were deficiencies in autonomy and independence, psychomotor abilities, and – most profoundly – academics and scholarship," Dr. Mattar noted in summarizing the survey results.
The underlying theme of the responses is that many fellows are pursuing fellowship positions to make up for inadequacies in their residency rather than to push their skills to the next level. Among the key survey findings:
• Forty-three percent of program directors felt incoming fellows were unable to independently perform half an hour of a major procedure.
• Thirty percent of incoming fellows couldn’t independently perform basic operations such as laparoscopic cholecystectomy.
• Fifty-six percent were unable to laparoscopically suture and tie knots properly, and 26% couldn’t recognize anatomic planes through the laparoscope.
• One-quarter were deemed unable to recognize early signs of complications.
• Nearly 40% of program directors said new fellows display a lack of "patient ownership." "We promote patient ownership in our programs. We are somewhat disappointed and dismayed that the fellows feel that the patient is part of a service and not their own," Dr. Mattar commented.
• Only 51% of program directors indicated their incoming fellows demonstrated independence in the operating room and on call, although fellows did show marked improvement in these areas as the year went on.
• A large majority of program directors thought their fellows were disinterested in research and advancing the field, even though, as Dr. Mattar noted, "This is a mandate in our curriculum."
Discussant Dr. Michael G. Sarr was blunt: "This is a scary situation."
"There’s a clear message here from this study: We have a problem. I maintain that we have to stop being bullied by naive, public, politically driven agendas and by some of our own graybeard pundits – and I think we all know who those groups are – and once again take over the control of educating our successors," said Dr. Sarr, professor of surgery at the Mayo Medical School, Rochester, Minn.
He attributed the decline in graduating general surgery residents’ technical skills, patient ownership, and ability to function as trustworthy independent surgeons in large part to the mandated 80-hour maximum work week.
"We all admit and acknowledge that prior to the duty hours reduction of 2003, the expected duty hours most of us trained in were barbaric and often dangerous, and they involved too much scut work. But in the past the final product was superb," Dr. Sarr recalled.
He argued that while it would be folly to return to those days, some flexibility regarding the work hours limit would be beneficial.
"Should our politically driven ACGME [Accreditation Council for Graduate Medical Education] and our own RRC [Residency Review Committee] – yes, our own elected overseeing organization – liberalize its rigid, unbending, stringent rules to allow our residents to make more liberal decisions and to develop professionalism by exceeding their 80-hour work restriction when clinical situations demand their presence?" he asked.
Discussant Dr. Frank R. Lewis, executive director of the American Board of Surgery, said that even though the 80-hour work limit has effectively subtracted 6-12 months from the general surgery residency, he doesn’t believe this emotional and contentious issue is the main problem. He noted that at present the average number of operations done by a first-year resident is less than two per week, while second-year residents average only two to three per week.
"Our residents are spending 80 hours a week while doing two or three operations per week, which arguably could be done in half a day. It would be hard to imagine a less efficient educational process," Dr. Lewis complained.
He added that nobody should be surprised by the Fellowship Council survey results. During the past decade the failure rate on the American Board of Surgery’s oral exam has climbed steadily from 16% to 28%. At present the percentage of examinees who fail either the oral or written ABS exam the first time around is in the mid-30s.
"That’s arguably an absurd failure rate for a 5-year training program in a group of people who should have mastered the subject," the surgeon added.
He asserted that most of the factors responsible for the decline in the competence of graduating general surgery residents are beyond the control of academic surgeons. These factors include the gutting of surgical clerkship opportunities in the fourth year of medical school, along with changes in the surgical landscape that have caused once-popular operations to essentially go away due to technical advances or improved drug therapy.
Discussant Dr. Mark A. Malangoni, associate executive director of the ABS, noted that the more complex open surgery operations previously done by general surgery residents have in many cases been converted to complex laparoscopic procedures that have become the purview of the subspecialty fellowships. Why not abolish the fellowships and drive all those interesting cases and that dedicated training effort back into the residency years? he asked.
That’s not going to happen, Dr. Mattar replied, citing the huge market demand and need for these fellowships.
"They’re very rewarding to all stakeholders," he added.
But constructive changes are afoot, according to Dr. Mattar. Plans are well underway to change the fourth year of medical school so that students interested in a career in surgery can begin to prepare for it then. And there are also efforts to custom-tailor the final year of general surgery residency so that residents can prepare for their fellowship year. Toward that end the Fellowship Council has moved the fellowship match date up to June so residents who know they are fellowship bound can put their fifth year to the best use.
The survey was conducted by the Fellowship Council, an umbrella organization with oversight over surgical subspecialty fellowships. Dr. Mattar reported having no financial conflicts.
INDIANAPOLIS – The nation’s elite surgical educators are up in arms over reported widespread deficiencies in the skill set and judgment of recent graduates of 5-year general surgery residencies.
The source of their ire is a detailed new survey of the nation’s subspecialty fellowship program directors. Today 80% of graduating general surgery residents seek these year-long fellowships to obtain advanced training in bariatric, colorectal, thoracic, hepatobiliary, or other surgical areas. The surveyed program directors indicated many trainees arrive unprepared in essential areas.
"Many new fellows must gain basic and fundamental skills at the beginning of their fellowship before they can commence to benefit from the advanced skills that they originally came to obtain. The current high demand for fellowship training and the lack of readiness upon completion of general surgery residencies should be a call to action for all stakeholders in surgical training," Dr. Samer Mattar declared in presenting the survey results at the annual meeting of the American Surgical Association.
The survey was conducted by the Fellowship Council, an umbrella organization in charge of standardizing curricula, accrediting programs, and matching residents to fellowships. The group distributed the surveys to all 145 subspecialty fellowship program directors and drew a 63% response rate. That’s considered high for such a lengthy survey and is an indication of the importance educators place on the subject matter, said Dr. Mattar of Indiana University, Indianapolis.
The survey assessed five key educational domains: professionalism, independent practice, psychomotor skills, expertise in their chosen disease state, and scholarly focus.
"Incoming fellows exhibited high levels of professionalism, but there were deficiencies in autonomy and independence, psychomotor abilities, and – most profoundly – academics and scholarship," Dr. Mattar noted in summarizing the survey results.
The underlying theme of the responses is that many fellows are pursuing fellowship positions to make up for inadequacies in their residency rather than to push their skills to the next level. Among the key survey findings:
• Forty-three percent of program directors felt incoming fellows were unable to independently perform half an hour of a major procedure.
• Thirty percent of incoming fellows couldn’t independently perform basic operations such as laparoscopic cholecystectomy.
• Fifty-six percent were unable to laparoscopically suture and tie knots properly, and 26% couldn’t recognize anatomic planes through the laparoscope.
• One-quarter were deemed unable to recognize early signs of complications.
• Nearly 40% of program directors said new fellows display a lack of "patient ownership." "We promote patient ownership in our programs. We are somewhat disappointed and dismayed that the fellows feel that the patient is part of a service and not their own," Dr. Mattar commented.
• Only 51% of program directors indicated their incoming fellows demonstrated independence in the operating room and on call, although fellows did show marked improvement in these areas as the year went on.
• A large majority of program directors thought their fellows were disinterested in research and advancing the field, even though, as Dr. Mattar noted, "This is a mandate in our curriculum."
Discussant Dr. Michael G. Sarr was blunt: "This is a scary situation."
"There’s a clear message here from this study: We have a problem. I maintain that we have to stop being bullied by naive, public, politically driven agendas and by some of our own graybeard pundits – and I think we all know who those groups are – and once again take over the control of educating our successors," said Dr. Sarr, professor of surgery at the Mayo Medical School, Rochester, Minn.
He attributed the decline in graduating general surgery residents’ technical skills, patient ownership, and ability to function as trustworthy independent surgeons in large part to the mandated 80-hour maximum work week.
"We all admit and acknowledge that prior to the duty hours reduction of 2003, the expected duty hours most of us trained in were barbaric and often dangerous, and they involved too much scut work. But in the past the final product was superb," Dr. Sarr recalled.
He argued that while it would be folly to return to those days, some flexibility regarding the work hours limit would be beneficial.
"Should our politically driven ACGME [Accreditation Council for Graduate Medical Education] and our own RRC [Residency Review Committee] – yes, our own elected overseeing organization – liberalize its rigid, unbending, stringent rules to allow our residents to make more liberal decisions and to develop professionalism by exceeding their 80-hour work restriction when clinical situations demand their presence?" he asked.
Discussant Dr. Frank R. Lewis, executive director of the American Board of Surgery, said that even though the 80-hour work limit has effectively subtracted 6-12 months from the general surgery residency, he doesn’t believe this emotional and contentious issue is the main problem. He noted that at present the average number of operations done by a first-year resident is less than two per week, while second-year residents average only two to three per week.
"Our residents are spending 80 hours a week while doing two or three operations per week, which arguably could be done in half a day. It would be hard to imagine a less efficient educational process," Dr. Lewis complained.
He added that nobody should be surprised by the Fellowship Council survey results. During the past decade the failure rate on the American Board of Surgery’s oral exam has climbed steadily from 16% to 28%. At present the percentage of examinees who fail either the oral or written ABS exam the first time around is in the mid-30s.
"That’s arguably an absurd failure rate for a 5-year training program in a group of people who should have mastered the subject," the surgeon added.
He asserted that most of the factors responsible for the decline in the competence of graduating general surgery residents are beyond the control of academic surgeons. These factors include the gutting of surgical clerkship opportunities in the fourth year of medical school, along with changes in the surgical landscape that have caused once-popular operations to essentially go away due to technical advances or improved drug therapy.
Discussant Dr. Mark A. Malangoni, associate executive director of the ABS, noted that the more complex open surgery operations previously done by general surgery residents have in many cases been converted to complex laparoscopic procedures that have become the purview of the subspecialty fellowships. Why not abolish the fellowships and drive all those interesting cases and that dedicated training effort back into the residency years? he asked.
That’s not going to happen, Dr. Mattar replied, citing the huge market demand and need for these fellowships.
"They’re very rewarding to all stakeholders," he added.
But constructive changes are afoot, according to Dr. Mattar. Plans are well underway to change the fourth year of medical school so that students interested in a career in surgery can begin to prepare for it then. And there are also efforts to custom-tailor the final year of general surgery residency so that residents can prepare for their fellowship year. Toward that end the Fellowship Council has moved the fellowship match date up to June so residents who know they are fellowship bound can put their fifth year to the best use.
The survey was conducted by the Fellowship Council, an umbrella organization with oversight over surgical subspecialty fellowships. Dr. Mattar reported having no financial conflicts.
INDIANAPOLIS – The nation’s elite surgical educators are up in arms over reported widespread deficiencies in the skill set and judgment of recent graduates of 5-year general surgery residencies.
The source of their ire is a detailed new survey of the nation’s subspecialty fellowship program directors. Today 80% of graduating general surgery residents seek these year-long fellowships to obtain advanced training in bariatric, colorectal, thoracic, hepatobiliary, or other surgical areas. The surveyed program directors indicated many trainees arrive unprepared in essential areas.
"Many new fellows must gain basic and fundamental skills at the beginning of their fellowship before they can commence to benefit from the advanced skills that they originally came to obtain. The current high demand for fellowship training and the lack of readiness upon completion of general surgery residencies should be a call to action for all stakeholders in surgical training," Dr. Samer Mattar declared in presenting the survey results at the annual meeting of the American Surgical Association.
The survey was conducted by the Fellowship Council, an umbrella organization in charge of standardizing curricula, accrediting programs, and matching residents to fellowships. The group distributed the surveys to all 145 subspecialty fellowship program directors and drew a 63% response rate. That’s considered high for such a lengthy survey and is an indication of the importance educators place on the subject matter, said Dr. Mattar of Indiana University, Indianapolis.
The survey assessed five key educational domains: professionalism, independent practice, psychomotor skills, expertise in their chosen disease state, and scholarly focus.
"Incoming fellows exhibited high levels of professionalism, but there were deficiencies in autonomy and independence, psychomotor abilities, and – most profoundly – academics and scholarship," Dr. Mattar noted in summarizing the survey results.
The underlying theme of the responses is that many fellows are pursuing fellowship positions to make up for inadequacies in their residency rather than to push their skills to the next level. Among the key survey findings:
• Forty-three percent of program directors felt incoming fellows were unable to independently perform half an hour of a major procedure.
• Thirty percent of incoming fellows couldn’t independently perform basic operations such as laparoscopic cholecystectomy.
• Fifty-six percent were unable to laparoscopically suture and tie knots properly, and 26% couldn’t recognize anatomic planes through the laparoscope.
• One-quarter were deemed unable to recognize early signs of complications.
• Nearly 40% of program directors said new fellows display a lack of "patient ownership." "We promote patient ownership in our programs. We are somewhat disappointed and dismayed that the fellows feel that the patient is part of a service and not their own," Dr. Mattar commented.
• Only 51% of program directors indicated their incoming fellows demonstrated independence in the operating room and on call, although fellows did show marked improvement in these areas as the year went on.
• A large majority of program directors thought their fellows were disinterested in research and advancing the field, even though, as Dr. Mattar noted, "This is a mandate in our curriculum."
Discussant Dr. Michael G. Sarr was blunt: "This is a scary situation."
"There’s a clear message here from this study: We have a problem. I maintain that we have to stop being bullied by naive, public, politically driven agendas and by some of our own graybeard pundits – and I think we all know who those groups are – and once again take over the control of educating our successors," said Dr. Sarr, professor of surgery at the Mayo Medical School, Rochester, Minn.
He attributed the decline in graduating general surgery residents’ technical skills, patient ownership, and ability to function as trustworthy independent surgeons in large part to the mandated 80-hour maximum work week.
"We all admit and acknowledge that prior to the duty hours reduction of 2003, the expected duty hours most of us trained in were barbaric and often dangerous, and they involved too much scut work. But in the past the final product was superb," Dr. Sarr recalled.
He argued that while it would be folly to return to those days, some flexibility regarding the work hours limit would be beneficial.
"Should our politically driven ACGME [Accreditation Council for Graduate Medical Education] and our own RRC [Residency Review Committee] – yes, our own elected overseeing organization – liberalize its rigid, unbending, stringent rules to allow our residents to make more liberal decisions and to develop professionalism by exceeding their 80-hour work restriction when clinical situations demand their presence?" he asked.
Discussant Dr. Frank R. Lewis, executive director of the American Board of Surgery, said that even though the 80-hour work limit has effectively subtracted 6-12 months from the general surgery residency, he doesn’t believe this emotional and contentious issue is the main problem. He noted that at present the average number of operations done by a first-year resident is less than two per week, while second-year residents average only two to three per week.
"Our residents are spending 80 hours a week while doing two or three operations per week, which arguably could be done in half a day. It would be hard to imagine a less efficient educational process," Dr. Lewis complained.
He added that nobody should be surprised by the Fellowship Council survey results. During the past decade the failure rate on the American Board of Surgery’s oral exam has climbed steadily from 16% to 28%. At present the percentage of examinees who fail either the oral or written ABS exam the first time around is in the mid-30s.
"That’s arguably an absurd failure rate for a 5-year training program in a group of people who should have mastered the subject," the surgeon added.
He asserted that most of the factors responsible for the decline in the competence of graduating general surgery residents are beyond the control of academic surgeons. These factors include the gutting of surgical clerkship opportunities in the fourth year of medical school, along with changes in the surgical landscape that have caused once-popular operations to essentially go away due to technical advances or improved drug therapy.
Discussant Dr. Mark A. Malangoni, associate executive director of the ABS, noted that the more complex open surgery operations previously done by general surgery residents have in many cases been converted to complex laparoscopic procedures that have become the purview of the subspecialty fellowships. Why not abolish the fellowships and drive all those interesting cases and that dedicated training effort back into the residency years? he asked.
That’s not going to happen, Dr. Mattar replied, citing the huge market demand and need for these fellowships.
"They’re very rewarding to all stakeholders," he added.
But constructive changes are afoot, according to Dr. Mattar. Plans are well underway to change the fourth year of medical school so that students interested in a career in surgery can begin to prepare for it then. And there are also efforts to custom-tailor the final year of general surgery residency so that residents can prepare for their fellowship year. Toward that end the Fellowship Council has moved the fellowship match date up to June so residents who know they are fellowship bound can put their fifth year to the best use.
The survey was conducted by the Fellowship Council, an umbrella organization with oversight over surgical subspecialty fellowships. Dr. Mattar reported having no financial conflicts.
AT THE ASA ANNUAL MEETING
Major finding: Forty-three percent of incoming fellows in the nation’s surgical subspecialty programs were deemed by their program directors to be unable to independently perform half an hour of a major procedure.
Data source: A survey of the nation’s 145 surgical subspecialty program directors. It drew responses from 91 (63%).
Disclosures: The survey was conducted by the Fellowship Council, an umbrella organization with oversight over surgical subspecialty fellowships. Dr. Mattar reported having no financial conflicts.
Surgeons tackle readmission risk reduction
INDIANAPOLIS – Average 30-day readmission rates in a large national study varied widely by surgical specialty, ranging from 5% for general surgery patients to 12% for vascular surgery patients and 16% after hepato-pancreatic-biliary surgery.
This retrospective study was based upon American College of Surgeons National Surgical Quality Improvement Program (NSQIP) 2011 data on 240,125 patients discharged from 316 hospitals after these types of surgery. The results enabled investigators to identify the major risk factors for readmission. They used this information to generate a predictive model to identify patients at greatest risk with an eye toward introducing interventions to keep them out of the hospital, Dr. Timothy M. Pawlik said at the annual meeting of the American Surgical Association.
How effective such interventions will be in a surgical population is as yet unclear, he added.
The importance of this work lies in the growing emphasis health care payers are placing upon 30-day readmission as a quality-of-care indicator. In 2012, Medicare began cutting reimbursement by 1% to hospitals with above-average 30-day readmissions. Next year, this penalty is scheduled to increase to 3% under the Medicare Hospital Readmission Reduction Program. Prior research efforts to identify risk factors for readmission have focused chiefly on medical rather than surgical conditions, noted Dr. Pawlik, professor of surgery and chief of the division of surgical oncology at Johns Hopkins University, Baltimore.
The readmission risk predictive formula he and his coinvestigators developed is simple: American Society of Anesthesiologists physical status class + (length of stay/2). This number is rounded up to yield a risk score of 1-10. A patient with a readmission score of 1 has a 1% risk of readmission within 30 days of discharge. The risk climbs to 12% with a score of 5, 20% with a score of 8, and 40% with a score of 10.
"A score of 4 had an 8% readmission rate, with 77% sensitivity, 52% specificity, a low positive predictive value of 12%, but a high negative predictive value of 95%," the surgeon observed. "In essence, a score of 4 or higher was able to identify 80% of all readmissions, but it also included about half of all patients."
Because of a quirk in the NSQIP database – it records readmissions within 30 days of surgery instead of starting from discharge – the investigators had to limit the study population to surgery patients with a length of stay of 10 days or less and then apply statistical modeling. However, limiting the data set to patients with a maximum 10-day hospital stay only restricted the study cohort by 6%. Thus, the study results remain highly generalizable to U.S. surgery patients.
The area under the curve (AUC) of the receiver operating characteristic for the readmission risk formula was 0.70. Statisticians consider a test having an AUC of 0.50 to be worthless. An AUC of 1.0 would define a "perfect" test, while a test with an AUC in the 0.70-0.80 range is deemed of only "fair" accuracy.
The AUC for the readmission score varied considerably when applied to the various surgical subspecialties. For example, the AUC was 0.69 for general surgery patients but only 0.51 for thoracic surgery patients, 0.64 for vascular surgery patients, and 0.59 for colorectal surgery patients.
The fact that the predictive formula doesn’t perform any better than barely "fair" is testimony to the difficulty in identifying who will require readmission. That being said, the AUC for the readmission score after surgery compares favorably to published hospital readmission risk formulas developed for medical patients, which have similar and in many cases lower AUCs (JAMA 2011;306:1688-98), Dr. Pawlik observed.
At Johns Hopkins, the plan is to target surgery patients who have high readmission scores with interventions including more frequent follow-up phone calls and earlier scheduled postoperative clinic visits in an effort to keep them out of the hospital, he continued.
Discussant Dr. Keith D. Lillemoe was skeptical that this will result in reduced readmission rates. Indeed, he questioned whether the 30-day readmission rate is a legitimate quality measure for surgeons.
"I can look across the room and predict if a patient is going to come back in to the hospital. But what can we really do to keep such patients from being readmitted other than keeping them indeterminately long until we’ve passed that window? It seems like all the phone calls and early clinic visits in the world can’t stop the progression sometimes," reflected Dr. Lillemoe, professor and chairman of the department of surgery at Massachusetts General Hospital, Boston.
He noted with frustration that he had just stepped out of the lecture hall to arrange for readmission of a patient 1 week after discharge post pancreaticoduodenectomy.
"I made three phone calls myself to that guy trying to nurse him through his nausea and vomiting and other symptoms," the surgeon recalled.
Dr. Pawlik was sympathetic.
"It’s very hard to prevent readmissions. It’s a very complicated metric, and many would argue that it’s an inappropriate quality metric," he said. "Many things are outside our control before we even meet the patient, as are some things that occur in the hospital, and many things are beyond our control after discharge as far as where they live, their family structure, and their financial resources. In my opinion it’s a very problematic quality measure and I am not sure how we are going to tackle it."
He reported having no conflicts of interest.
INDIANAPOLIS – Average 30-day readmission rates in a large national study varied widely by surgical specialty, ranging from 5% for general surgery patients to 12% for vascular surgery patients and 16% after hepato-pancreatic-biliary surgery.
This retrospective study was based upon American College of Surgeons National Surgical Quality Improvement Program (NSQIP) 2011 data on 240,125 patients discharged from 316 hospitals after these types of surgery. The results enabled investigators to identify the major risk factors for readmission. They used this information to generate a predictive model to identify patients at greatest risk with an eye toward introducing interventions to keep them out of the hospital, Dr. Timothy M. Pawlik said at the annual meeting of the American Surgical Association.
How effective such interventions will be in a surgical population is as yet unclear, he added.
The importance of this work lies in the growing emphasis health care payers are placing upon 30-day readmission as a quality-of-care indicator. In 2012, Medicare began cutting reimbursement by 1% to hospitals with above-average 30-day readmissions. Next year, this penalty is scheduled to increase to 3% under the Medicare Hospital Readmission Reduction Program. Prior research efforts to identify risk factors for readmission have focused chiefly on medical rather than surgical conditions, noted Dr. Pawlik, professor of surgery and chief of the division of surgical oncology at Johns Hopkins University, Baltimore.
The readmission risk predictive formula he and his coinvestigators developed is simple: American Society of Anesthesiologists physical status class + (length of stay/2). This number is rounded up to yield a risk score of 1-10. A patient with a readmission score of 1 has a 1% risk of readmission within 30 days of discharge. The risk climbs to 12% with a score of 5, 20% with a score of 8, and 40% with a score of 10.
"A score of 4 had an 8% readmission rate, with 77% sensitivity, 52% specificity, a low positive predictive value of 12%, but a high negative predictive value of 95%," the surgeon observed. "In essence, a score of 4 or higher was able to identify 80% of all readmissions, but it also included about half of all patients."
Because of a quirk in the NSQIP database – it records readmissions within 30 days of surgery instead of starting from discharge – the investigators had to limit the study population to surgery patients with a length of stay of 10 days or less and then apply statistical modeling. However, limiting the data set to patients with a maximum 10-day hospital stay only restricted the study cohort by 6%. Thus, the study results remain highly generalizable to U.S. surgery patients.
The area under the curve (AUC) of the receiver operating characteristic for the readmission risk formula was 0.70. Statisticians consider a test having an AUC of 0.50 to be worthless. An AUC of 1.0 would define a "perfect" test, while a test with an AUC in the 0.70-0.80 range is deemed of only "fair" accuracy.
The AUC for the readmission score varied considerably when applied to the various surgical subspecialties. For example, the AUC was 0.69 for general surgery patients but only 0.51 for thoracic surgery patients, 0.64 for vascular surgery patients, and 0.59 for colorectal surgery patients.
The fact that the predictive formula doesn’t perform any better than barely "fair" is testimony to the difficulty in identifying who will require readmission. That being said, the AUC for the readmission score after surgery compares favorably to published hospital readmission risk formulas developed for medical patients, which have similar and in many cases lower AUCs (JAMA 2011;306:1688-98), Dr. Pawlik observed.
At Johns Hopkins, the plan is to target surgery patients who have high readmission scores with interventions including more frequent follow-up phone calls and earlier scheduled postoperative clinic visits in an effort to keep them out of the hospital, he continued.
Discussant Dr. Keith D. Lillemoe was skeptical that this will result in reduced readmission rates. Indeed, he questioned whether the 30-day readmission rate is a legitimate quality measure for surgeons.
"I can look across the room and predict if a patient is going to come back in to the hospital. But what can we really do to keep such patients from being readmitted other than keeping them indeterminately long until we’ve passed that window? It seems like all the phone calls and early clinic visits in the world can’t stop the progression sometimes," reflected Dr. Lillemoe, professor and chairman of the department of surgery at Massachusetts General Hospital, Boston.
He noted with frustration that he had just stepped out of the lecture hall to arrange for readmission of a patient 1 week after discharge post pancreaticoduodenectomy.
"I made three phone calls myself to that guy trying to nurse him through his nausea and vomiting and other symptoms," the surgeon recalled.
Dr. Pawlik was sympathetic.
"It’s very hard to prevent readmissions. It’s a very complicated metric, and many would argue that it’s an inappropriate quality metric," he said. "Many things are outside our control before we even meet the patient, as are some things that occur in the hospital, and many things are beyond our control after discharge as far as where they live, their family structure, and their financial resources. In my opinion it’s a very problematic quality measure and I am not sure how we are going to tackle it."
He reported having no conflicts of interest.
INDIANAPOLIS – Average 30-day readmission rates in a large national study varied widely by surgical specialty, ranging from 5% for general surgery patients to 12% for vascular surgery patients and 16% after hepato-pancreatic-biliary surgery.
This retrospective study was based upon American College of Surgeons National Surgical Quality Improvement Program (NSQIP) 2011 data on 240,125 patients discharged from 316 hospitals after these types of surgery. The results enabled investigators to identify the major risk factors for readmission. They used this information to generate a predictive model to identify patients at greatest risk with an eye toward introducing interventions to keep them out of the hospital, Dr. Timothy M. Pawlik said at the annual meeting of the American Surgical Association.
How effective such interventions will be in a surgical population is as yet unclear, he added.
The importance of this work lies in the growing emphasis health care payers are placing upon 30-day readmission as a quality-of-care indicator. In 2012, Medicare began cutting reimbursement by 1% to hospitals with above-average 30-day readmissions. Next year, this penalty is scheduled to increase to 3% under the Medicare Hospital Readmission Reduction Program. Prior research efforts to identify risk factors for readmission have focused chiefly on medical rather than surgical conditions, noted Dr. Pawlik, professor of surgery and chief of the division of surgical oncology at Johns Hopkins University, Baltimore.
The readmission risk predictive formula he and his coinvestigators developed is simple: American Society of Anesthesiologists physical status class + (length of stay/2). This number is rounded up to yield a risk score of 1-10. A patient with a readmission score of 1 has a 1% risk of readmission within 30 days of discharge. The risk climbs to 12% with a score of 5, 20% with a score of 8, and 40% with a score of 10.
"A score of 4 had an 8% readmission rate, with 77% sensitivity, 52% specificity, a low positive predictive value of 12%, but a high negative predictive value of 95%," the surgeon observed. "In essence, a score of 4 or higher was able to identify 80% of all readmissions, but it also included about half of all patients."
Because of a quirk in the NSQIP database – it records readmissions within 30 days of surgery instead of starting from discharge – the investigators had to limit the study population to surgery patients with a length of stay of 10 days or less and then apply statistical modeling. However, limiting the data set to patients with a maximum 10-day hospital stay only restricted the study cohort by 6%. Thus, the study results remain highly generalizable to U.S. surgery patients.
The area under the curve (AUC) of the receiver operating characteristic for the readmission risk formula was 0.70. Statisticians consider a test having an AUC of 0.50 to be worthless. An AUC of 1.0 would define a "perfect" test, while a test with an AUC in the 0.70-0.80 range is deemed of only "fair" accuracy.
The AUC for the readmission score varied considerably when applied to the various surgical subspecialties. For example, the AUC was 0.69 for general surgery patients but only 0.51 for thoracic surgery patients, 0.64 for vascular surgery patients, and 0.59 for colorectal surgery patients.
The fact that the predictive formula doesn’t perform any better than barely "fair" is testimony to the difficulty in identifying who will require readmission. That being said, the AUC for the readmission score after surgery compares favorably to published hospital readmission risk formulas developed for medical patients, which have similar and in many cases lower AUCs (JAMA 2011;306:1688-98), Dr. Pawlik observed.
At Johns Hopkins, the plan is to target surgery patients who have high readmission scores with interventions including more frequent follow-up phone calls and earlier scheduled postoperative clinic visits in an effort to keep them out of the hospital, he continued.
Discussant Dr. Keith D. Lillemoe was skeptical that this will result in reduced readmission rates. Indeed, he questioned whether the 30-day readmission rate is a legitimate quality measure for surgeons.
"I can look across the room and predict if a patient is going to come back in to the hospital. But what can we really do to keep such patients from being readmitted other than keeping them indeterminately long until we’ve passed that window? It seems like all the phone calls and early clinic visits in the world can’t stop the progression sometimes," reflected Dr. Lillemoe, professor and chairman of the department of surgery at Massachusetts General Hospital, Boston.
He noted with frustration that he had just stepped out of the lecture hall to arrange for readmission of a patient 1 week after discharge post pancreaticoduodenectomy.
"I made three phone calls myself to that guy trying to nurse him through his nausea and vomiting and other symptoms," the surgeon recalled.
Dr. Pawlik was sympathetic.
"It’s very hard to prevent readmissions. It’s a very complicated metric, and many would argue that it’s an inappropriate quality metric," he said. "Many things are outside our control before we even meet the patient, as are some things that occur in the hospital, and many things are beyond our control after discharge as far as where they live, their family structure, and their financial resources. In my opinion it’s a very problematic quality measure and I am not sure how we are going to tackle it."
He reported having no conflicts of interest.
AT THE ASA ANNUAL MEETING
Major Finding: The 30-day readmission rate following general, thoracic, and vascular surgery was 8%. It ranged from 5% to 16% depending upon the surgical subspecialty. The study led to generation of a simple readmission risk scoring system.
Data Source: A retrospective study of 240,125 patients in the American College of Surgeons National Surgical Quality Improvement Program who were discharged in 2011 following these types of surgery.
Disclosures: The presenter reported having no conflicts of interest.
Preop walking speed predicts postop morbidity
INDIANAPOLIS – Slower walking speed on the timed-up-and-go test in elderly patients scheduled for surgery is a significantly better forecaster of postoperative complications and 1-year mortality than are the considerably more complex patient risk calculators currently considered standard of care, Dr. Thomas N. Robinson reported at the annual meeting of the American Surgical Association.
"I think what walking speed reflects is global reduced physiologic reserve. It’s frailty. And by definition, an individual who’s frail will have adverse health care outcomes," explained Dr. Robinson, a general surgeon at the University of Colorado, Denver.
Use of preoperative walking speed to assess postoperative risk is a paradigm shift, he noted. Current surgical risk assessment strategies rely upon math-heavy patient risk calculators which evaluate single end-organ dysfunction, in some cases summing up the individual scores for heart, lung, liver, and other organ dysfunction in an attempt to define chronic disease burden. But in older patients, this approach is less effective than a simple frailty assessment based upon mobility: that is, walking speed, he continued.
Surgeons in the Veterans Affairs health care system use a risk calculator that involves input of 24 variables. Hitting the "compute risk" button then produces the patient’s estimated 30-day morbidity and mortality risks.
Dr. Robinson presented a prospective study in which the VA tool was compared to the timed-up-and-go (TUG) test in 272 patients aged older than 65 years who were followed for a minimum of 1 year after elective surgery. To see how the two tests performed across surgical specialties, the investigators included 174 patients with a cardiac operation and 98 who had colorectal surgery.
In the TUG test, a clinician starts a stopwatch as the patient rises from a chair, walks 10 feet, returns, and sits back down. The patient is instructed to walk at his or her normal pace and is free to use a walking aid. Dr. Robinson chose to study the TUG rather than a simple 5-meter gait speed test because he considers TUG more relevant to surgical patients.
"TUG combines lower extremity strength to stand up in addition to walking speed. And if you think about somebody who needs to make the transition from hospital to home, lower extremity strength is important," he said.
The investigators categorized a TUG time of 10 seconds or less as fast, 11-14 seconds as intermediate, and 15 seconds or longer as slow. Of note, the subjects’ TUG times were unrelated to common comorbid conditions in the elderly, including stroke, diabetes, heart failure, and hypertension.
In contrast, walking speed was strongly associated with classic indicators of frailty. For example, impaired cognition was present in 3% of subjects with a fast TUG time, 41% of those with an intermediate time, and 92% of slow performers. Another frailty indicator – a history of falling within the past 6 months – was present in 7% of the fast group, 21% of intermediate TUG walkers, and 85% of those with a TUG speed of 15 seconds or more.
In the cardiac surgery group, one or more postsurgical complications occurred in 11% of the 53 patients in the fast group, 25% of 88 patients with an intermediate TUG time, and 52% of 33 individuals in the slow group. The 1-year mortality rates were 2%, 3%, and 12%, respectively.
Similarly, in the colorectal surgery group, the complication rate was 12% among 30 fast walkers, 29% of 42 patients in the intermediate group, and 77% of 26 patients in the slow group. The 1-year mortality rates were 3%, 10%, and 31%, respectively.
The investigators judged comparative test performance in predicting postoperative morbidity and mortality on the basis of the receiver operating characteristic area under the curve, which was 77% with the TUG test compared to 55% with the VA risk calculator in the colorectal surgery patients. In the cardiac surgery group, the figures were 68% for TUG and 55% with the risk calculator.
Geriatricians typically measure TUG in seconds as a continuous variable. Dr. Robinson and coworkers decided the test would be more useful for surgeons if they created the three discrete categories of fast, intermediate, and slow.
Discussant Dr. Michael E. Zenilman praised the investigators for what he called "an outstanding study," and one that’s particularly welcome right now, as the wave of aging baby boomers swells.
"As we take care of more elderly patients, it’s important that we develop tools like this to quickly and objectively assess risk. The tools that we have now, such as the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) models and the VA risk calculator, are for me just too complicated," said Dr. Zenilman of Johns Hopkins University, Baltimore. Dr. Zenilman is the university’s vice chair and regional director of surgery for the Washington area. Noting that TUG, Mini-Mental Status scores, history of falling, and serum albumin levels all have been shown to serve as proxies for frailty, he asked Dr. Robinson to predict which one he thinks will win out as a postoperative risk predictor.
Dr. Robinson replied that TUG is a good frailty assessment tool for now, but he and others are trying to develop something better. The American College of Surgeons geriatric task force is collaborating with the NSQIP to identify variables present in patients’ charts that correlate with global frailty and can serve as reliable predictors of postoperative risk.
He reported having no financial conflicts.
INDIANAPOLIS – Slower walking speed on the timed-up-and-go test in elderly patients scheduled for surgery is a significantly better forecaster of postoperative complications and 1-year mortality than are the considerably more complex patient risk calculators currently considered standard of care, Dr. Thomas N. Robinson reported at the annual meeting of the American Surgical Association.
"I think what walking speed reflects is global reduced physiologic reserve. It’s frailty. And by definition, an individual who’s frail will have adverse health care outcomes," explained Dr. Robinson, a general surgeon at the University of Colorado, Denver.
Use of preoperative walking speed to assess postoperative risk is a paradigm shift, he noted. Current surgical risk assessment strategies rely upon math-heavy patient risk calculators which evaluate single end-organ dysfunction, in some cases summing up the individual scores for heart, lung, liver, and other organ dysfunction in an attempt to define chronic disease burden. But in older patients, this approach is less effective than a simple frailty assessment based upon mobility: that is, walking speed, he continued.
Surgeons in the Veterans Affairs health care system use a risk calculator that involves input of 24 variables. Hitting the "compute risk" button then produces the patient’s estimated 30-day morbidity and mortality risks.
Dr. Robinson presented a prospective study in which the VA tool was compared to the timed-up-and-go (TUG) test in 272 patients aged older than 65 years who were followed for a minimum of 1 year after elective surgery. To see how the two tests performed across surgical specialties, the investigators included 174 patients with a cardiac operation and 98 who had colorectal surgery.
In the TUG test, a clinician starts a stopwatch as the patient rises from a chair, walks 10 feet, returns, and sits back down. The patient is instructed to walk at his or her normal pace and is free to use a walking aid. Dr. Robinson chose to study the TUG rather than a simple 5-meter gait speed test because he considers TUG more relevant to surgical patients.
"TUG combines lower extremity strength to stand up in addition to walking speed. And if you think about somebody who needs to make the transition from hospital to home, lower extremity strength is important," he said.
The investigators categorized a TUG time of 10 seconds or less as fast, 11-14 seconds as intermediate, and 15 seconds or longer as slow. Of note, the subjects’ TUG times were unrelated to common comorbid conditions in the elderly, including stroke, diabetes, heart failure, and hypertension.
In contrast, walking speed was strongly associated with classic indicators of frailty. For example, impaired cognition was present in 3% of subjects with a fast TUG time, 41% of those with an intermediate time, and 92% of slow performers. Another frailty indicator – a history of falling within the past 6 months – was present in 7% of the fast group, 21% of intermediate TUG walkers, and 85% of those with a TUG speed of 15 seconds or more.
In the cardiac surgery group, one or more postsurgical complications occurred in 11% of the 53 patients in the fast group, 25% of 88 patients with an intermediate TUG time, and 52% of 33 individuals in the slow group. The 1-year mortality rates were 2%, 3%, and 12%, respectively.
Similarly, in the colorectal surgery group, the complication rate was 12% among 30 fast walkers, 29% of 42 patients in the intermediate group, and 77% of 26 patients in the slow group. The 1-year mortality rates were 3%, 10%, and 31%, respectively.
The investigators judged comparative test performance in predicting postoperative morbidity and mortality on the basis of the receiver operating characteristic area under the curve, which was 77% with the TUG test compared to 55% with the VA risk calculator in the colorectal surgery patients. In the cardiac surgery group, the figures were 68% for TUG and 55% with the risk calculator.
Geriatricians typically measure TUG in seconds as a continuous variable. Dr. Robinson and coworkers decided the test would be more useful for surgeons if they created the three discrete categories of fast, intermediate, and slow.
Discussant Dr. Michael E. Zenilman praised the investigators for what he called "an outstanding study," and one that’s particularly welcome right now, as the wave of aging baby boomers swells.
"As we take care of more elderly patients, it’s important that we develop tools like this to quickly and objectively assess risk. The tools that we have now, such as the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) models and the VA risk calculator, are for me just too complicated," said Dr. Zenilman of Johns Hopkins University, Baltimore. Dr. Zenilman is the university’s vice chair and regional director of surgery for the Washington area. Noting that TUG, Mini-Mental Status scores, history of falling, and serum albumin levels all have been shown to serve as proxies for frailty, he asked Dr. Robinson to predict which one he thinks will win out as a postoperative risk predictor.
Dr. Robinson replied that TUG is a good frailty assessment tool for now, but he and others are trying to develop something better. The American College of Surgeons geriatric task force is collaborating with the NSQIP to identify variables present in patients’ charts that correlate with global frailty and can serve as reliable predictors of postoperative risk.
He reported having no financial conflicts.
INDIANAPOLIS – Slower walking speed on the timed-up-and-go test in elderly patients scheduled for surgery is a significantly better forecaster of postoperative complications and 1-year mortality than are the considerably more complex patient risk calculators currently considered standard of care, Dr. Thomas N. Robinson reported at the annual meeting of the American Surgical Association.
"I think what walking speed reflects is global reduced physiologic reserve. It’s frailty. And by definition, an individual who’s frail will have adverse health care outcomes," explained Dr. Robinson, a general surgeon at the University of Colorado, Denver.
Use of preoperative walking speed to assess postoperative risk is a paradigm shift, he noted. Current surgical risk assessment strategies rely upon math-heavy patient risk calculators which evaluate single end-organ dysfunction, in some cases summing up the individual scores for heart, lung, liver, and other organ dysfunction in an attempt to define chronic disease burden. But in older patients, this approach is less effective than a simple frailty assessment based upon mobility: that is, walking speed, he continued.
Surgeons in the Veterans Affairs health care system use a risk calculator that involves input of 24 variables. Hitting the "compute risk" button then produces the patient’s estimated 30-day morbidity and mortality risks.
Dr. Robinson presented a prospective study in which the VA tool was compared to the timed-up-and-go (TUG) test in 272 patients aged older than 65 years who were followed for a minimum of 1 year after elective surgery. To see how the two tests performed across surgical specialties, the investigators included 174 patients with a cardiac operation and 98 who had colorectal surgery.
In the TUG test, a clinician starts a stopwatch as the patient rises from a chair, walks 10 feet, returns, and sits back down. The patient is instructed to walk at his or her normal pace and is free to use a walking aid. Dr. Robinson chose to study the TUG rather than a simple 5-meter gait speed test because he considers TUG more relevant to surgical patients.
"TUG combines lower extremity strength to stand up in addition to walking speed. And if you think about somebody who needs to make the transition from hospital to home, lower extremity strength is important," he said.
The investigators categorized a TUG time of 10 seconds or less as fast, 11-14 seconds as intermediate, and 15 seconds or longer as slow. Of note, the subjects’ TUG times were unrelated to common comorbid conditions in the elderly, including stroke, diabetes, heart failure, and hypertension.
In contrast, walking speed was strongly associated with classic indicators of frailty. For example, impaired cognition was present in 3% of subjects with a fast TUG time, 41% of those with an intermediate time, and 92% of slow performers. Another frailty indicator – a history of falling within the past 6 months – was present in 7% of the fast group, 21% of intermediate TUG walkers, and 85% of those with a TUG speed of 15 seconds or more.
In the cardiac surgery group, one or more postsurgical complications occurred in 11% of the 53 patients in the fast group, 25% of 88 patients with an intermediate TUG time, and 52% of 33 individuals in the slow group. The 1-year mortality rates were 2%, 3%, and 12%, respectively.
Similarly, in the colorectal surgery group, the complication rate was 12% among 30 fast walkers, 29% of 42 patients in the intermediate group, and 77% of 26 patients in the slow group. The 1-year mortality rates were 3%, 10%, and 31%, respectively.
The investigators judged comparative test performance in predicting postoperative morbidity and mortality on the basis of the receiver operating characteristic area under the curve, which was 77% with the TUG test compared to 55% with the VA risk calculator in the colorectal surgery patients. In the cardiac surgery group, the figures were 68% for TUG and 55% with the risk calculator.
Geriatricians typically measure TUG in seconds as a continuous variable. Dr. Robinson and coworkers decided the test would be more useful for surgeons if they created the three discrete categories of fast, intermediate, and slow.
Discussant Dr. Michael E. Zenilman praised the investigators for what he called "an outstanding study," and one that’s particularly welcome right now, as the wave of aging baby boomers swells.
"As we take care of more elderly patients, it’s important that we develop tools like this to quickly and objectively assess risk. The tools that we have now, such as the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) models and the VA risk calculator, are for me just too complicated," said Dr. Zenilman of Johns Hopkins University, Baltimore. Dr. Zenilman is the university’s vice chair and regional director of surgery for the Washington area. Noting that TUG, Mini-Mental Status scores, history of falling, and serum albumin levels all have been shown to serve as proxies for frailty, he asked Dr. Robinson to predict which one he thinks will win out as a postoperative risk predictor.
Dr. Robinson replied that TUG is a good frailty assessment tool for now, but he and others are trying to develop something better. The American College of Surgeons geriatric task force is collaborating with the NSQIP to identify variables present in patients’ charts that correlate with global frailty and can serve as reliable predictors of postoperative risk.
He reported having no financial conflicts.
AT THE ASA ANNUAL MEETING
Major Finding. One-year mortality rates for fast, intermediate, and slow cardiac patients were 2%, 3%, and 12%, respectively. In the colorectal surgery group, the 1-year mortality rates were 3%, 10%, and 31%, respectively.
Data Source: A prospective cohort study of postsurgical complications and 1-year mortality in 98 elderly patients undergoing elective colorectal surgery and 174 with elective cardiac surgery. All underwent a preoperative timed-up-and-go test as well as assessment via the Veterans Affairs surgical risk calculator.
Disclosures: The study presenter reported having no conflicts of interest.
Early surgery for adhesive bowel obstruction can save lives
INDIANAPOLIS – Patients requiring surgery for adhesive small bowel obstruction have markedly lower major morbidity and mortality rates if they’re operated on within 24 hours of hospital admission, according to an analysis of a large national database.
This finding is at odds with the conventional wisdom.
Both the World Society of Emergency Surgery and the Eastern Association for the Surgery of Trauma recommend in published guidelines an initial 3-5 days of nonoperative management to give the obstruction a chance to resolve on its own, Dr. Pedro G. Teixeira noted in presenting the study findings at the annual meeting of the American Surgical Association.
He and his coinvestigators identified 4,163 patients in the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for 2005-2010 who underwent emergency laparotomy for adhesive bowel obstruction. Thirty-day mortality was 3% in those operated upon within 24 hours of hospital admission. It rose in stepwise fashion thereafter: 4% mortality with surgery at 24-48 hours, 7% with surgery at 48-72 hours, and 9% a threefold increase – when surgery was delayed beyond 72 hours, according to Dr. Teixeira of the University of Southern California, Los Angeles.
Similarly, the incidence of systemic infectious complications, including pneumonia, urinary tract infections, and sepsis, climbed from 12% with early operation to 17% when surgery occurred at 24-48 hours, 21% at 48-72 hours, and 24% thereafter.
In a multivariate analysis adjusted for baseline comorbidities and other potential confounding variables, surgery delayed for 24 hours or more after admission was associated with a highly significant 58% increased risk of mortality, a 33% increase in surgical site infections, a 36% greater risk of pneumonia, and a 47% increased risk of septic shock, he continued.
Discussant Gregory J. Jurkovich commented that this study challenges current dogma and harkens back to a century-old adage that has since been cast aside, namely, "Never let the sun set on a bowel obstruction."
The trouble is, however, that having a low threshold for surgery within 24 hours would subject a massive number of patients to an unnecessary operation.
An analysis of Nationwide Inpatient Sample data for 2009 by other investigators concluded that bowel obstruction resolved on its own within 3 days in 60% of patients and within 5 days in 80%. Fewer than 20% of the patients who presented with adhesive small bowel obstruction without evidence of ischemia underwent surgery, noted Dr. Jurkovich, director of surgery at Denver Health Medical Center and professor of trauma surgery and vice chairman of the department of surgery at the University of Colorado at Denver.
Dr. Teixeira concurred that bowel obstruction will resolve on its own in most patients. The challenge for surgeons in light of his study findings, he stressed, is to expedite the identification of those patients who will fail the period of nonoperative management. The best tool for that, in his view, is a CT scan of the abdomen and pelvis with water-soluble contrast.
At the University of Southern California, he explained, a patient who presents with adhesive bowel obstruction without evidence of ischemia undergoes the CT scan and is admitted to the surgical observation unit for close monitoring.
"At our institution, failure to demonstrate contrast progression through the colon within 24 hours would be a very strong indication for surgical exploration," according to Dr. Teixeira.
He reported having no financial conflicts.
The study by Dr. Teixeira is intriguing in
that it suggests a return to practice patterns from a prior era.

|
| Dr. Chad Whelan |
The study does report increased risk in
complications including mortality with delays in surgery for small bowel
obstructions, even with risk adjustment. However, this is not a controlled
trial which limits our ability to reach definitive conclusions from it. Still,
hospitalists often are the primary physicians for patients admitted for small
bowel obstructions and should be aware of these findings so that they can
ensure that they have early surgical involvement.
Chad Whelan, M.D., is associate chief medical officer for
performance improvement and innovation and an associate professor of medicine
at the University
of Chicago Medical Center.
The study by Dr. Teixeira is intriguing in
that it suggests a return to practice patterns from a prior era.

|
| Dr. Chad Whelan |
The study does report increased risk in
complications including mortality with delays in surgery for small bowel
obstructions, even with risk adjustment. However, this is not a controlled
trial which limits our ability to reach definitive conclusions from it. Still,
hospitalists often are the primary physicians for patients admitted for small
bowel obstructions and should be aware of these findings so that they can
ensure that they have early surgical involvement.
Chad Whelan, M.D., is associate chief medical officer for
performance improvement and innovation and an associate professor of medicine
at the University
of Chicago Medical Center.
The study by Dr. Teixeira is intriguing in
that it suggests a return to practice patterns from a prior era.

|
| Dr. Chad Whelan |
The study does report increased risk in
complications including mortality with delays in surgery for small bowel
obstructions, even with risk adjustment. However, this is not a controlled
trial which limits our ability to reach definitive conclusions from it. Still,
hospitalists often are the primary physicians for patients admitted for small
bowel obstructions and should be aware of these findings so that they can
ensure that they have early surgical involvement.
Chad Whelan, M.D., is associate chief medical officer for
performance improvement and innovation and an associate professor of medicine
at the University
of Chicago Medical Center.
INDIANAPOLIS – Patients requiring surgery for adhesive small bowel obstruction have markedly lower major morbidity and mortality rates if they’re operated on within 24 hours of hospital admission, according to an analysis of a large national database.
This finding is at odds with the conventional wisdom.
Both the World Society of Emergency Surgery and the Eastern Association for the Surgery of Trauma recommend in published guidelines an initial 3-5 days of nonoperative management to give the obstruction a chance to resolve on its own, Dr. Pedro G. Teixeira noted in presenting the study findings at the annual meeting of the American Surgical Association.
He and his coinvestigators identified 4,163 patients in the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for 2005-2010 who underwent emergency laparotomy for adhesive bowel obstruction. Thirty-day mortality was 3% in those operated upon within 24 hours of hospital admission. It rose in stepwise fashion thereafter: 4% mortality with surgery at 24-48 hours, 7% with surgery at 48-72 hours, and 9% a threefold increase – when surgery was delayed beyond 72 hours, according to Dr. Teixeira of the University of Southern California, Los Angeles.
Similarly, the incidence of systemic infectious complications, including pneumonia, urinary tract infections, and sepsis, climbed from 12% with early operation to 17% when surgery occurred at 24-48 hours, 21% at 48-72 hours, and 24% thereafter.
In a multivariate analysis adjusted for baseline comorbidities and other potential confounding variables, surgery delayed for 24 hours or more after admission was associated with a highly significant 58% increased risk of mortality, a 33% increase in surgical site infections, a 36% greater risk of pneumonia, and a 47% increased risk of septic shock, he continued.
Discussant Gregory J. Jurkovich commented that this study challenges current dogma and harkens back to a century-old adage that has since been cast aside, namely, "Never let the sun set on a bowel obstruction."
The trouble is, however, that having a low threshold for surgery within 24 hours would subject a massive number of patients to an unnecessary operation.
An analysis of Nationwide Inpatient Sample data for 2009 by other investigators concluded that bowel obstruction resolved on its own within 3 days in 60% of patients and within 5 days in 80%. Fewer than 20% of the patients who presented with adhesive small bowel obstruction without evidence of ischemia underwent surgery, noted Dr. Jurkovich, director of surgery at Denver Health Medical Center and professor of trauma surgery and vice chairman of the department of surgery at the University of Colorado at Denver.
Dr. Teixeira concurred that bowel obstruction will resolve on its own in most patients. The challenge for surgeons in light of his study findings, he stressed, is to expedite the identification of those patients who will fail the period of nonoperative management. The best tool for that, in his view, is a CT scan of the abdomen and pelvis with water-soluble contrast.
At the University of Southern California, he explained, a patient who presents with adhesive bowel obstruction without evidence of ischemia undergoes the CT scan and is admitted to the surgical observation unit for close monitoring.
"At our institution, failure to demonstrate contrast progression through the colon within 24 hours would be a very strong indication for surgical exploration," according to Dr. Teixeira.
He reported having no financial conflicts.
INDIANAPOLIS – Patients requiring surgery for adhesive small bowel obstruction have markedly lower major morbidity and mortality rates if they’re operated on within 24 hours of hospital admission, according to an analysis of a large national database.
This finding is at odds with the conventional wisdom.
Both the World Society of Emergency Surgery and the Eastern Association for the Surgery of Trauma recommend in published guidelines an initial 3-5 days of nonoperative management to give the obstruction a chance to resolve on its own, Dr. Pedro G. Teixeira noted in presenting the study findings at the annual meeting of the American Surgical Association.
He and his coinvestigators identified 4,163 patients in the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for 2005-2010 who underwent emergency laparotomy for adhesive bowel obstruction. Thirty-day mortality was 3% in those operated upon within 24 hours of hospital admission. It rose in stepwise fashion thereafter: 4% mortality with surgery at 24-48 hours, 7% with surgery at 48-72 hours, and 9% a threefold increase – when surgery was delayed beyond 72 hours, according to Dr. Teixeira of the University of Southern California, Los Angeles.
Similarly, the incidence of systemic infectious complications, including pneumonia, urinary tract infections, and sepsis, climbed from 12% with early operation to 17% when surgery occurred at 24-48 hours, 21% at 48-72 hours, and 24% thereafter.
In a multivariate analysis adjusted for baseline comorbidities and other potential confounding variables, surgery delayed for 24 hours or more after admission was associated with a highly significant 58% increased risk of mortality, a 33% increase in surgical site infections, a 36% greater risk of pneumonia, and a 47% increased risk of septic shock, he continued.
Discussant Gregory J. Jurkovich commented that this study challenges current dogma and harkens back to a century-old adage that has since been cast aside, namely, "Never let the sun set on a bowel obstruction."
The trouble is, however, that having a low threshold for surgery within 24 hours would subject a massive number of patients to an unnecessary operation.
An analysis of Nationwide Inpatient Sample data for 2009 by other investigators concluded that bowel obstruction resolved on its own within 3 days in 60% of patients and within 5 days in 80%. Fewer than 20% of the patients who presented with adhesive small bowel obstruction without evidence of ischemia underwent surgery, noted Dr. Jurkovich, director of surgery at Denver Health Medical Center and professor of trauma surgery and vice chairman of the department of surgery at the University of Colorado at Denver.
Dr. Teixeira concurred that bowel obstruction will resolve on its own in most patients. The challenge for surgeons in light of his study findings, he stressed, is to expedite the identification of those patients who will fail the period of nonoperative management. The best tool for that, in his view, is a CT scan of the abdomen and pelvis with water-soluble contrast.
At the University of Southern California, he explained, a patient who presents with adhesive bowel obstruction without evidence of ischemia undergoes the CT scan and is admitted to the surgical observation unit for close monitoring.
"At our institution, failure to demonstrate contrast progression through the colon within 24 hours would be a very strong indication for surgical exploration," according to Dr. Teixeira.
He reported having no financial conflicts.
AT THE ASA ANNUAL MEETING
Major Finding: Surgery for adhesive small bowel obstruction had a 30-day mortality rate of 3% if performed within 24 hours of hospital admission, rising stepwise to 9% when the operation was delayed beyond 72 hours.
Data Source: This was a retrospective analysis of 4,163 patients in the American College of Surgeons National Quality Improvement Program database for 2005-2010 who underwent emergency laparotomy for adhesive bowel obstruction.
Disclosures: The presenter reported having no conflicts of interest.
Esophagectomy cases rising steadily
INDIANAPOLIS – Transthoracic esophagectomy for esophageal cancer provides significantly lower in-hospital mortality and major morbidity rates than does transhiatal esophagectomy, according to an analysis of a large multiyear national database.
Further, in-hospital outcomes of esophagectomy didn’t differ significantly between high-volume centers – in this study, defined as those doing 10 or more cases per year – and low-volume centers, Dr. Mehraneh D. Jafari reported at the annual meeting of the American Surgical Association.

That finding was met with skepticism, and discussants were quick to argue that study limitations make it difficult to draw any meaningful conclusions from the data. For one thing, speakers contended that defining a high-volume center based upon an institutional threshold of 10 or more cases per year sets the bar far too low given that a single dedicated esophageal surgery specialist might easily perform 50 or more esophagectomies annually.
Dr. Jafari presented an analysis of 11,473 transthoracic and 3,717 transhiatal esophagectomies performed for esophageal cancer. The data came from the Nationwide Inpatient Sample (NIS) during 2001-2010. The NIS records data on in-hospital outcomes for a nationally representative sample composed of roughly 20% of the country’s hospital discharges each year.
The number of esophagectomies rose steadily by an average of 4% annually during the study years, reflecting the substantial national increase in cases of esophageal cancer. The growing case count, expected to reach an estimated 18,000 cases of esophageal cancer nationwide in 2013, has been attributed to rising rates of gastroesophageal reflux disease, Barrett’s esophagus, and obesity. Transthoracic esophagectomy, used in 76% of cases, remained the preferred operative strategy throughout the study years.
In-hospital outcomes were markedly better in patients who had transthoracic esophagectomy. After adjustment for potential confounding variables in a multivariate analysis, transhiatal esophagectomy recipients had a 67% increased risk of in-hospital mortality and a 39% greater risk of serious complications, including a 37% increased risk of pulmonary complications. However, anastomotic leak rates were similar with both operations, according to Dr. Jafari of the University of California, Irvine.
Of note, the referral rate to high-volume esophagectomy centers climbed steadily over time, rising from 22% of all cases in 2001 to 58% in 2010.
The 35 high-volume centers performed an average of 16 cases per year. In contrast, the 484 low-volume centers averaged 2 cases per year. In-hospital mortality among the 9,386 patients treated in low-volume centers averaged 7.6% compared with 4.3% for patients in high-volume centers. Overall in-hospital serious morbidity rates were greater in the low-volume centers as well: 47% versus 41%. While these raw differences were statistically significant, a risk-adjusted multivariate analysis found no significant outcome differences between low- and high-volume centers.
Discussant Dr. Michael J. Zinner noted that in an earlier study he and his coworkers showed that an institutional threshold of roughly 30 esophagectomies per year is required to discriminate between low- and high-volume centers in terms of in-hospital mortality. So why define high-volume centers as those doing a mere 10 cases per year? asked Dr. Zinner, chairman of the department of surgery at Brigham and Women’s Hospital and professor of surgery at Harvard Medical School, Boston.
"The problem here is if you establish 30 cases per year as the threshold for a high-volume center, I can tell you there are probably less than 20 centers in the whole U.S. capable of doing that volume. That’s a real issue, because then how are patients who live in a remote region going to get care at one of those centers?" replied Dr. Jafari’s senior coauthor Dr. Ninh T. Nguyen, professor and vice-chair of surgery at UC Irvine.
"I think instead we should try to lift all boats: develop a national esophageal center network to identify the qualities reflective of better outcomes in the high-volume centers and introduce those factors at low-volume centers. This way we’re not impeding access to care for our patients," he continued.
Dr. Nguyen said that in-hospital surgical morbidity rates in the NIS need to be taken with a grain of salt, as the accuracy of coding for complications is "rather low." The development of minimally invasive techniques for intrathoracic anastomosis has transformed transthoracic esophagectomy into a procedure with an improved complication profile.
"I switched to transthoracic esophagectomy 5 years ago. One reason was development of the minimally invasive approach. As a result, we’re not scared of a chest anastomosis like we used to be. When patients undergoing transthoracic esophagectomy with thoracotomy had a leak in the chest they had a very high risk for mortality. That’s not the case anymore. We have not observed any mortality associated with a leak in the chest for many, many years now," he said.
The investigators reported having no conflicts of interest.
Dr. Luketich is professor of surgery and chief of the Heart, Lung, and Esophageal Surgery Institute at the University of Pittsburgh. He was the designated discussant of the study at the meeting.
I have major problems with this study, stemming from inherent limitations in the Nationwide Inpatient Sample. It’s an administrative database set up chiefly to track costs, utilization, and length of stay. It contains no information at all on key clinical outcomes such as 30- and 90-day mortality, discharge disposition, or 30-day readmission rates.

|
|
In addition, the accuracy of the quoted in-hospital morbidity rates is suspect, probably because data entry isn’t performed by trained researchers. For example, the 8% incidence of renal failure in esophagectomy patients cited in this study sounds too high to be right.
And there’s another major problem with this database: The superior outcomes reported for transthoracic esophagectomy recipients in this study fly in the face of earlier, well-conducted meta-analyses that reached the opposite conclusion. The most likely explanation for the discordant findings lies in the fact that the NIS doesn’t show whether a transthoracic esophagectomy was performed via open thoracotomy in the old-school manner or with an intrathoracic anastomosis created using contemporary minimally invasive techniques which, while complex, have been associated with better outcomes.
Dr. James D. Luketich is professor of surgery and chief of the Heart, Lung, and Esophageal Surgery Institute at the University of Pittsburgh. He was the designated discussant of the study at the meeting.
I have major problems with this study, stemming from inherent limitations in the Nationwide Inpatient Sample. It’s an administrative database set up chiefly to track costs, utilization, and length of stay. It contains no information at all on key clinical outcomes such as 30- and 90-day mortality, discharge disposition, or 30-day readmission rates.

|
|
In addition, the accuracy of the quoted in-hospital morbidity rates is suspect, probably because data entry isn’t performed by trained researchers. For example, the 8% incidence of renal failure in esophagectomy patients cited in this study sounds too high to be right.
And there’s another major problem with this database: The superior outcomes reported for transthoracic esophagectomy recipients in this study fly in the face of earlier, well-conducted meta-analyses that reached the opposite conclusion. The most likely explanation for the discordant findings lies in the fact that the NIS doesn’t show whether a transthoracic esophagectomy was performed via open thoracotomy in the old-school manner or with an intrathoracic anastomosis created using contemporary minimally invasive techniques which, while complex, have been associated with better outcomes.
Dr. James D. Luketich is professor of surgery and chief of the Heart, Lung, and Esophageal Surgery Institute at the University of Pittsburgh. He was the designated discussant of the study at the meeting.
I have major problems with this study, stemming from inherent limitations in the Nationwide Inpatient Sample. It’s an administrative database set up chiefly to track costs, utilization, and length of stay. It contains no information at all on key clinical outcomes such as 30- and 90-day mortality, discharge disposition, or 30-day readmission rates.

|
|
In addition, the accuracy of the quoted in-hospital morbidity rates is suspect, probably because data entry isn’t performed by trained researchers. For example, the 8% incidence of renal failure in esophagectomy patients cited in this study sounds too high to be right.
And there’s another major problem with this database: The superior outcomes reported for transthoracic esophagectomy recipients in this study fly in the face of earlier, well-conducted meta-analyses that reached the opposite conclusion. The most likely explanation for the discordant findings lies in the fact that the NIS doesn’t show whether a transthoracic esophagectomy was performed via open thoracotomy in the old-school manner or with an intrathoracic anastomosis created using contemporary minimally invasive techniques which, while complex, have been associated with better outcomes.
Dr. James D. Luketich is professor of surgery and chief of the Heart, Lung, and Esophageal Surgery Institute at the University of Pittsburgh. He was the designated discussant of the study at the meeting.
INDIANAPOLIS – Transthoracic esophagectomy for esophageal cancer provides significantly lower in-hospital mortality and major morbidity rates than does transhiatal esophagectomy, according to an analysis of a large multiyear national database.
Further, in-hospital outcomes of esophagectomy didn’t differ significantly between high-volume centers – in this study, defined as those doing 10 or more cases per year – and low-volume centers, Dr. Mehraneh D. Jafari reported at the annual meeting of the American Surgical Association.

That finding was met with skepticism, and discussants were quick to argue that study limitations make it difficult to draw any meaningful conclusions from the data. For one thing, speakers contended that defining a high-volume center based upon an institutional threshold of 10 or more cases per year sets the bar far too low given that a single dedicated esophageal surgery specialist might easily perform 50 or more esophagectomies annually.
Dr. Jafari presented an analysis of 11,473 transthoracic and 3,717 transhiatal esophagectomies performed for esophageal cancer. The data came from the Nationwide Inpatient Sample (NIS) during 2001-2010. The NIS records data on in-hospital outcomes for a nationally representative sample composed of roughly 20% of the country’s hospital discharges each year.
The number of esophagectomies rose steadily by an average of 4% annually during the study years, reflecting the substantial national increase in cases of esophageal cancer. The growing case count, expected to reach an estimated 18,000 cases of esophageal cancer nationwide in 2013, has been attributed to rising rates of gastroesophageal reflux disease, Barrett’s esophagus, and obesity. Transthoracic esophagectomy, used in 76% of cases, remained the preferred operative strategy throughout the study years.
In-hospital outcomes were markedly better in patients who had transthoracic esophagectomy. After adjustment for potential confounding variables in a multivariate analysis, transhiatal esophagectomy recipients had a 67% increased risk of in-hospital mortality and a 39% greater risk of serious complications, including a 37% increased risk of pulmonary complications. However, anastomotic leak rates were similar with both operations, according to Dr. Jafari of the University of California, Irvine.
Of note, the referral rate to high-volume esophagectomy centers climbed steadily over time, rising from 22% of all cases in 2001 to 58% in 2010.
The 35 high-volume centers performed an average of 16 cases per year. In contrast, the 484 low-volume centers averaged 2 cases per year. In-hospital mortality among the 9,386 patients treated in low-volume centers averaged 7.6% compared with 4.3% for patients in high-volume centers. Overall in-hospital serious morbidity rates were greater in the low-volume centers as well: 47% versus 41%. While these raw differences were statistically significant, a risk-adjusted multivariate analysis found no significant outcome differences between low- and high-volume centers.
Discussant Dr. Michael J. Zinner noted that in an earlier study he and his coworkers showed that an institutional threshold of roughly 30 esophagectomies per year is required to discriminate between low- and high-volume centers in terms of in-hospital mortality. So why define high-volume centers as those doing a mere 10 cases per year? asked Dr. Zinner, chairman of the department of surgery at Brigham and Women’s Hospital and professor of surgery at Harvard Medical School, Boston.
"The problem here is if you establish 30 cases per year as the threshold for a high-volume center, I can tell you there are probably less than 20 centers in the whole U.S. capable of doing that volume. That’s a real issue, because then how are patients who live in a remote region going to get care at one of those centers?" replied Dr. Jafari’s senior coauthor Dr. Ninh T. Nguyen, professor and vice-chair of surgery at UC Irvine.
"I think instead we should try to lift all boats: develop a national esophageal center network to identify the qualities reflective of better outcomes in the high-volume centers and introduce those factors at low-volume centers. This way we’re not impeding access to care for our patients," he continued.
Dr. Nguyen said that in-hospital surgical morbidity rates in the NIS need to be taken with a grain of salt, as the accuracy of coding for complications is "rather low." The development of minimally invasive techniques for intrathoracic anastomosis has transformed transthoracic esophagectomy into a procedure with an improved complication profile.
"I switched to transthoracic esophagectomy 5 years ago. One reason was development of the minimally invasive approach. As a result, we’re not scared of a chest anastomosis like we used to be. When patients undergoing transthoracic esophagectomy with thoracotomy had a leak in the chest they had a very high risk for mortality. That’s not the case anymore. We have not observed any mortality associated with a leak in the chest for many, many years now," he said.
The investigators reported having no conflicts of interest.
Dr. Luketich is professor of surgery and chief of the Heart, Lung, and Esophageal Surgery Institute at the University of Pittsburgh. He was the designated discussant of the study at the meeting.
INDIANAPOLIS – Transthoracic esophagectomy for esophageal cancer provides significantly lower in-hospital mortality and major morbidity rates than does transhiatal esophagectomy, according to an analysis of a large multiyear national database.
Further, in-hospital outcomes of esophagectomy didn’t differ significantly between high-volume centers – in this study, defined as those doing 10 or more cases per year – and low-volume centers, Dr. Mehraneh D. Jafari reported at the annual meeting of the American Surgical Association.

That finding was met with skepticism, and discussants were quick to argue that study limitations make it difficult to draw any meaningful conclusions from the data. For one thing, speakers contended that defining a high-volume center based upon an institutional threshold of 10 or more cases per year sets the bar far too low given that a single dedicated esophageal surgery specialist might easily perform 50 or more esophagectomies annually.
Dr. Jafari presented an analysis of 11,473 transthoracic and 3,717 transhiatal esophagectomies performed for esophageal cancer. The data came from the Nationwide Inpatient Sample (NIS) during 2001-2010. The NIS records data on in-hospital outcomes for a nationally representative sample composed of roughly 20% of the country’s hospital discharges each year.
The number of esophagectomies rose steadily by an average of 4% annually during the study years, reflecting the substantial national increase in cases of esophageal cancer. The growing case count, expected to reach an estimated 18,000 cases of esophageal cancer nationwide in 2013, has been attributed to rising rates of gastroesophageal reflux disease, Barrett’s esophagus, and obesity. Transthoracic esophagectomy, used in 76% of cases, remained the preferred operative strategy throughout the study years.
In-hospital outcomes were markedly better in patients who had transthoracic esophagectomy. After adjustment for potential confounding variables in a multivariate analysis, transhiatal esophagectomy recipients had a 67% increased risk of in-hospital mortality and a 39% greater risk of serious complications, including a 37% increased risk of pulmonary complications. However, anastomotic leak rates were similar with both operations, according to Dr. Jafari of the University of California, Irvine.
Of note, the referral rate to high-volume esophagectomy centers climbed steadily over time, rising from 22% of all cases in 2001 to 58% in 2010.
The 35 high-volume centers performed an average of 16 cases per year. In contrast, the 484 low-volume centers averaged 2 cases per year. In-hospital mortality among the 9,386 patients treated in low-volume centers averaged 7.6% compared with 4.3% for patients in high-volume centers. Overall in-hospital serious morbidity rates were greater in the low-volume centers as well: 47% versus 41%. While these raw differences were statistically significant, a risk-adjusted multivariate analysis found no significant outcome differences between low- and high-volume centers.
Discussant Dr. Michael J. Zinner noted that in an earlier study he and his coworkers showed that an institutional threshold of roughly 30 esophagectomies per year is required to discriminate between low- and high-volume centers in terms of in-hospital mortality. So why define high-volume centers as those doing a mere 10 cases per year? asked Dr. Zinner, chairman of the department of surgery at Brigham and Women’s Hospital and professor of surgery at Harvard Medical School, Boston.
"The problem here is if you establish 30 cases per year as the threshold for a high-volume center, I can tell you there are probably less than 20 centers in the whole U.S. capable of doing that volume. That’s a real issue, because then how are patients who live in a remote region going to get care at one of those centers?" replied Dr. Jafari’s senior coauthor Dr. Ninh T. Nguyen, professor and vice-chair of surgery at UC Irvine.
"I think instead we should try to lift all boats: develop a national esophageal center network to identify the qualities reflective of better outcomes in the high-volume centers and introduce those factors at low-volume centers. This way we’re not impeding access to care for our patients," he continued.
Dr. Nguyen said that in-hospital surgical morbidity rates in the NIS need to be taken with a grain of salt, as the accuracy of coding for complications is "rather low." The development of minimally invasive techniques for intrathoracic anastomosis has transformed transthoracic esophagectomy into a procedure with an improved complication profile.
"I switched to transthoracic esophagectomy 5 years ago. One reason was development of the minimally invasive approach. As a result, we’re not scared of a chest anastomosis like we used to be. When patients undergoing transthoracic esophagectomy with thoracotomy had a leak in the chest they had a very high risk for mortality. That’s not the case anymore. We have not observed any mortality associated with a leak in the chest for many, many years now," he said.
The investigators reported having no conflicts of interest.
Dr. Luketich is professor of surgery and chief of the Heart, Lung, and Esophageal Surgery Institute at the University of Pittsburgh. He was the designated discussant of the study at the meeting.
AT THE ASA ANNUAL MEETING
Major finding: In-hospital mortality occurred nationally in 5.8% of esophageal cancer patients who underwent transthoracic esophagectomy compared with 8.3% of transhiatal esophagectomy recipients.
Data source: A retrospective study of more than 15,000 patients who underwent esophagectomy for esophageal cancer during 2001-2010 and were Included in the Nationwide Inpatient Sample, a database sponsored by the Agency for Healthcare Research and Quality.
Disclosures: The study presenters reported having no financial conflicts.
Obesity epidemic's hidden cost: Hospital staff injuries
INDIANAPOLIS – A major hidden cost of the obesity epidemic is the physical toll it takes on hospital nurses and other employees in helping to move heavy patients. The cost of these workplace injuries is skyrocketing, not only in terms of direct medical care bills, but also in terms of work absenteeism, activity restrictions, retraining, and employee dissatisfaction and fear.
The solution at one large tertiary academic medical center in eastern North Carolina has been to create specially trained two-person lift teams available 24/7. The results of a pilot study have been impressive, particularly in light of the fact that nothing else hospital officials tried earlier – including spending more than $1.5 million for motorized patient ceiling lifts – had any significant impact, Dr. Walter J. Pories said at the annual meeting of the American Surgical Association.
Hospital employee injuries incurred in handling obese patients are an issue that has until now been largely beneath physicians’ radar.
"I don’t think anybody here would ask two nurses to pick up a washing machine, or a 400-lb calf, or an 800-lb lathe, and yet whether we like it or not we ask our staff to move patients with such weights daily," observed Dr. Pories, professor of surgery at East Carolina University in Greenville, N.C.
He practices at Vidant Medical Center, a 909-bed hospital where administrators noticed back in 2005 that employee workplace compensation claims for injuries during patient transport were rapidly escalating. In that year, 98 staff experienced patient-handling injuries, resulting in 670 lost workdays and 3,022 restricted workdays.
Hospital officials tried several interventions. First came an intensive education program on safe lifting and patient mobilization conducted by an ergonomist.
"It didn’t make a bit of difference," Dr. Pories recalled.
Indeed, during 2008 there were 2,141 lost workdays due to these types of injuries, more than triple the number in 2005.
Next came a big investment in ceiling-mounted motorized lifts. The impact was minimal: roughly a 10% reduction in staff injuries. Problems with the lifts abounded. They broke. Some staff had difficulty operating them. But the biggest issue was that the rooms with the lifts weren’t always available because the hospital is always fully occupied.
Dr. Pories credited the nursing staff with providing the leadership in developing what he calls the Vidant Medical Center lift team model. Coverage is available 24/7. There are three teams of two individuals on duty during the day and two teams of two at night. They work 12-hour shifts. There is one supervisor for 23 lift team technicians, including three women. They are paid an average of $10 per hour, which the surgeon called "a pretty good wage in eastern North Carolina."
Individuals must pass a rigorous physical assessment before they can join the lift team. Members take a 3-week orientation program in which they learn safe lift techniques, skin and wound care, and infection control. They have their own uniforms to foster esprit de corps.
Many other organizations had tried using lift teams, with disappointing results. That’s because they limited the teams to the day shift or scheduled transfers, according to Dr. Pories.
"The real problems happen late at night," he said.
Dr. Pories and his coinvestigators conducted a pilot study in which they utilized the lift teams in the five hospital units having the highest staff injury rates due to patient-handling mishaps. The lift team was called to those units for moving patients who weighed more than 200 lb, had pressure ulcers, or who were at risk for pressure ulcers by virtue of a Braden scale score of 18 or less.
During the pilot study, 8 employee injuries resulted from patient handling in the five test units, for a rate of 0.134 injuries per 1,000 patient-days, compared with 71 injuries and a rate of 0.319 injuries per 1,000 patient-days in control units.
Once use of the lift teams expanded hospital-wide, the result was a 39% drop in employee injuries due to patient handling, as well as a 43% reduction in hospital-acquired pressure ulcers. Multiple staff surveys have shown 90%-99% satisfaction rates with the lift teams as having improved the workplace while demonstrating that the hospital cares about employee safety.
The total direct and indirect cost of staff injuries due to patient handling was approximately $2.76 million per year prior to introduction of the lift teams. The lift teams provided an estimated $423,152 in savings due to fewer staff injuries and hospital-acquired pressure ulcers, even after factoring in team salaries and equipment, Dr. Pories reported.
The next step in this project will be to document whether the presence of the lift teams also has resulted in fewer patient injuries, he added.
Discussant Dr. Philip R. Schauer commented that if the favorable Vidant experience with dedicated lift teams can be confirmed elsewhere, this is the type of program that should be widely instituted all across the country.
"It’s quite extraordinary to improve patient care and employee health while at the same time reducing overall cost," noted Dr. Schauer, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
Discussant Dr. William B. Inabnet III said many malpractice insurance carriers are now offering hospitals incentives for reduced premiums for implementation of best-practices clinical pathways for management of obese patients.
"I think your work will support that type of mission. It’s a win-win for all parties involved," said Dr. Inabnet, professor of surgery at Mt. Sinai Hospital in New York.
Dr. Pories reported having no conflicts of interest.
INDIANAPOLIS – A major hidden cost of the obesity epidemic is the physical toll it takes on hospital nurses and other employees in helping to move heavy patients. The cost of these workplace injuries is skyrocketing, not only in terms of direct medical care bills, but also in terms of work absenteeism, activity restrictions, retraining, and employee dissatisfaction and fear.
The solution at one large tertiary academic medical center in eastern North Carolina has been to create specially trained two-person lift teams available 24/7. The results of a pilot study have been impressive, particularly in light of the fact that nothing else hospital officials tried earlier – including spending more than $1.5 million for motorized patient ceiling lifts – had any significant impact, Dr. Walter J. Pories said at the annual meeting of the American Surgical Association.
Hospital employee injuries incurred in handling obese patients are an issue that has until now been largely beneath physicians’ radar.
"I don’t think anybody here would ask two nurses to pick up a washing machine, or a 400-lb calf, or an 800-lb lathe, and yet whether we like it or not we ask our staff to move patients with such weights daily," observed Dr. Pories, professor of surgery at East Carolina University in Greenville, N.C.
He practices at Vidant Medical Center, a 909-bed hospital where administrators noticed back in 2005 that employee workplace compensation claims for injuries during patient transport were rapidly escalating. In that year, 98 staff experienced patient-handling injuries, resulting in 670 lost workdays and 3,022 restricted workdays.
Hospital officials tried several interventions. First came an intensive education program on safe lifting and patient mobilization conducted by an ergonomist.
"It didn’t make a bit of difference," Dr. Pories recalled.
Indeed, during 2008 there were 2,141 lost workdays due to these types of injuries, more than triple the number in 2005.
Next came a big investment in ceiling-mounted motorized lifts. The impact was minimal: roughly a 10% reduction in staff injuries. Problems with the lifts abounded. They broke. Some staff had difficulty operating them. But the biggest issue was that the rooms with the lifts weren’t always available because the hospital is always fully occupied.
Dr. Pories credited the nursing staff with providing the leadership in developing what he calls the Vidant Medical Center lift team model. Coverage is available 24/7. There are three teams of two individuals on duty during the day and two teams of two at night. They work 12-hour shifts. There is one supervisor for 23 lift team technicians, including three women. They are paid an average of $10 per hour, which the surgeon called "a pretty good wage in eastern North Carolina."
Individuals must pass a rigorous physical assessment before they can join the lift team. Members take a 3-week orientation program in which they learn safe lift techniques, skin and wound care, and infection control. They have their own uniforms to foster esprit de corps.
Many other organizations had tried using lift teams, with disappointing results. That’s because they limited the teams to the day shift or scheduled transfers, according to Dr. Pories.
"The real problems happen late at night," he said.
Dr. Pories and his coinvestigators conducted a pilot study in which they utilized the lift teams in the five hospital units having the highest staff injury rates due to patient-handling mishaps. The lift team was called to those units for moving patients who weighed more than 200 lb, had pressure ulcers, or who were at risk for pressure ulcers by virtue of a Braden scale score of 18 or less.
During the pilot study, 8 employee injuries resulted from patient handling in the five test units, for a rate of 0.134 injuries per 1,000 patient-days, compared with 71 injuries and a rate of 0.319 injuries per 1,000 patient-days in control units.
Once use of the lift teams expanded hospital-wide, the result was a 39% drop in employee injuries due to patient handling, as well as a 43% reduction in hospital-acquired pressure ulcers. Multiple staff surveys have shown 90%-99% satisfaction rates with the lift teams as having improved the workplace while demonstrating that the hospital cares about employee safety.
The total direct and indirect cost of staff injuries due to patient handling was approximately $2.76 million per year prior to introduction of the lift teams. The lift teams provided an estimated $423,152 in savings due to fewer staff injuries and hospital-acquired pressure ulcers, even after factoring in team salaries and equipment, Dr. Pories reported.
The next step in this project will be to document whether the presence of the lift teams also has resulted in fewer patient injuries, he added.
Discussant Dr. Philip R. Schauer commented that if the favorable Vidant experience with dedicated lift teams can be confirmed elsewhere, this is the type of program that should be widely instituted all across the country.
"It’s quite extraordinary to improve patient care and employee health while at the same time reducing overall cost," noted Dr. Schauer, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
Discussant Dr. William B. Inabnet III said many malpractice insurance carriers are now offering hospitals incentives for reduced premiums for implementation of best-practices clinical pathways for management of obese patients.
"I think your work will support that type of mission. It’s a win-win for all parties involved," said Dr. Inabnet, professor of surgery at Mt. Sinai Hospital in New York.
Dr. Pories reported having no conflicts of interest.
INDIANAPOLIS – A major hidden cost of the obesity epidemic is the physical toll it takes on hospital nurses and other employees in helping to move heavy patients. The cost of these workplace injuries is skyrocketing, not only in terms of direct medical care bills, but also in terms of work absenteeism, activity restrictions, retraining, and employee dissatisfaction and fear.
The solution at one large tertiary academic medical center in eastern North Carolina has been to create specially trained two-person lift teams available 24/7. The results of a pilot study have been impressive, particularly in light of the fact that nothing else hospital officials tried earlier – including spending more than $1.5 million for motorized patient ceiling lifts – had any significant impact, Dr. Walter J. Pories said at the annual meeting of the American Surgical Association.
Hospital employee injuries incurred in handling obese patients are an issue that has until now been largely beneath physicians’ radar.
"I don’t think anybody here would ask two nurses to pick up a washing machine, or a 400-lb calf, or an 800-lb lathe, and yet whether we like it or not we ask our staff to move patients with such weights daily," observed Dr. Pories, professor of surgery at East Carolina University in Greenville, N.C.
He practices at Vidant Medical Center, a 909-bed hospital where administrators noticed back in 2005 that employee workplace compensation claims for injuries during patient transport were rapidly escalating. In that year, 98 staff experienced patient-handling injuries, resulting in 670 lost workdays and 3,022 restricted workdays.
Hospital officials tried several interventions. First came an intensive education program on safe lifting and patient mobilization conducted by an ergonomist.
"It didn’t make a bit of difference," Dr. Pories recalled.
Indeed, during 2008 there were 2,141 lost workdays due to these types of injuries, more than triple the number in 2005.
Next came a big investment in ceiling-mounted motorized lifts. The impact was minimal: roughly a 10% reduction in staff injuries. Problems with the lifts abounded. They broke. Some staff had difficulty operating them. But the biggest issue was that the rooms with the lifts weren’t always available because the hospital is always fully occupied.
Dr. Pories credited the nursing staff with providing the leadership in developing what he calls the Vidant Medical Center lift team model. Coverage is available 24/7. There are three teams of two individuals on duty during the day and two teams of two at night. They work 12-hour shifts. There is one supervisor for 23 lift team technicians, including three women. They are paid an average of $10 per hour, which the surgeon called "a pretty good wage in eastern North Carolina."
Individuals must pass a rigorous physical assessment before they can join the lift team. Members take a 3-week orientation program in which they learn safe lift techniques, skin and wound care, and infection control. They have their own uniforms to foster esprit de corps.
Many other organizations had tried using lift teams, with disappointing results. That’s because they limited the teams to the day shift or scheduled transfers, according to Dr. Pories.
"The real problems happen late at night," he said.
Dr. Pories and his coinvestigators conducted a pilot study in which they utilized the lift teams in the five hospital units having the highest staff injury rates due to patient-handling mishaps. The lift team was called to those units for moving patients who weighed more than 200 lb, had pressure ulcers, or who were at risk for pressure ulcers by virtue of a Braden scale score of 18 or less.
During the pilot study, 8 employee injuries resulted from patient handling in the five test units, for a rate of 0.134 injuries per 1,000 patient-days, compared with 71 injuries and a rate of 0.319 injuries per 1,000 patient-days in control units.
Once use of the lift teams expanded hospital-wide, the result was a 39% drop in employee injuries due to patient handling, as well as a 43% reduction in hospital-acquired pressure ulcers. Multiple staff surveys have shown 90%-99% satisfaction rates with the lift teams as having improved the workplace while demonstrating that the hospital cares about employee safety.
The total direct and indirect cost of staff injuries due to patient handling was approximately $2.76 million per year prior to introduction of the lift teams. The lift teams provided an estimated $423,152 in savings due to fewer staff injuries and hospital-acquired pressure ulcers, even after factoring in team salaries and equipment, Dr. Pories reported.
The next step in this project will be to document whether the presence of the lift teams also has resulted in fewer patient injuries, he added.
Discussant Dr. Philip R. Schauer commented that if the favorable Vidant experience with dedicated lift teams can be confirmed elsewhere, this is the type of program that should be widely instituted all across the country.
"It’s quite extraordinary to improve patient care and employee health while at the same time reducing overall cost," noted Dr. Schauer, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
Discussant Dr. William B. Inabnet III said many malpractice insurance carriers are now offering hospitals incentives for reduced premiums for implementation of best-practices clinical pathways for management of obese patients.
"I think your work will support that type of mission. It’s a win-win for all parties involved," said Dr. Inabnet, professor of surgery at Mt. Sinai Hospital in New York.
Dr. Pories reported having no conflicts of interest.
AT THE ASA ANNUAL MEETING
Diabetes 'cure' holds up 6 years after bariatric surgery
INDIANAPOLIS – At 6 years of follow-up data, bariatric surgical cures of type 2 diabetes are holding steady in a single-center series of 217 patients.
"We see sustained weight loss, particularly in Roux-en-Y gastric bypass patients, 5-9 years after surgery." Diabetes remitted in 50% of patients, according to Dr. Stacy A. Brethauer of the Cleveland Clinic. "Bariatric surgery also achieved excellent long-term control of other cardiovascular risk factors; and diabetic nephropathy improved or stabilized."
Of 217 obese type 2 diabetic patients followed for a median of 6 years after bariatric surgery, 24% have been "cured." They have maintained a hemoglobin A1c level below 6% and fasting blood glucose values below 100 mg/dL for more than 5 years while off all antidiabetic medications, Dr. Brethauer reported at the annual meeting of the American Surgical Association.
Another 26% of patients have had partial remissions, meaning they maintained target HbA1c and fasting blood glucose levels off antidiabetic medications for more than 1 year but less than 5 years. In addition, 34% of patients were classified as "improved" based upon an absolute 1% or more reduction in HbA1c, a drop in fasting blood glucose in excess of 25 mg/dL, and a halving in the dose of antidiabetic medication for at least 1 year.
Diabetes recurred in 19% of subjects. Recurrence was defined as a return to an HbA1c of 6.5% or more or a fasting blood glucose level of at least 126 mg/dL.
The overall study population improved from a mean body mass index of 49 kg/m2 before surgery to 37 kg/m2 at 6 years after surgery. The mean HbA1c was 7.5% at baseline and 6.5% at 6 years. Fasting blood glucose at 6 years was a mean of 41.6 mg/dL lower than it was before surgery. LDL cholesterol levels were down by a mean of 10.1 mg/dL and HDL levels were up by 9.8 mg/dL. Systolic blood pressure was reduced by a mean of 10.9 mm Hg, with diastolic blood pressure was down by 3.2 mm Hg at 6 years after surgery.
Preoperatively, all but 5% of patients were on any antidiabetes medications; at 6 years after bariatric surgery, 54% were not on any antidiabetes drugs.
At baseline, 3% of subjects met all three key metabolic goals for diabetic patients as defined by the American Diabetes Association: an HbA1c below 7%, blood pressure below 130/80 mm Hg, and an LDL level below 100 mg/dL. At 6 years after surgery, 28% of patients met these goals. That result is particularly impressive in light of other studies that indicate 13% of the U.S. diabetic population as a whole meet all three goals.
The bariatric surgery group had a mean Framingham 10-year cardiovascular risk score of 28% preoperatively and of 22% at long-term followup.
The incidence of diabetic nephropathy among patients with type 2 diabetes is typically 2%-4% per year. Yet of 40 gastric bypass recipients known to have a normal urinary albumin-to-creatinine ratio preoperatively, only 1 developed macroalbuminuria and 1 microalbuminuria during a mean of 6 years postsurgical follow-up.
Also impressive, albuminuria regressed in 10 of the 19 gastric bypass recipients known to have the disorder at baseline. Albuminuria remained stable in the other 9 patients over the course of 6 years.
Mean excess weight loss at 1-2 years of follow-up was 61% in the 162 patients who underwent gastric bypass, 50% in the 23 with sleeve gastrectomy, and 30% in patients who had a gastric banding procedure.
In a multivariate analysis adjusted for baseline clinical characteristics, the significant predictors of diabetes remission following bariatric surgery were greater excess weight loss, preoperative diabetes duration of less than 5 years, and having a gastric bypass operation rather than sleeve gastrectomy or a gastric banding procedure.
Dr. Brethauer said this study, taken together with the findings of an earlier randomized clinical trial by the same investigators (N. Engl. J. Med. 2012;366:1567-76), conveys a clear message : "Bariatric surgery can induce a significant and sustainable remission of type 2 diabetes and other metabolic risk factors in obese patients and should be considered early in the course of the disease."
Discussant Dr. Walter J. Pories called Dr. Brethauer’s study "a really important contribution." He added that it’s high time for nonsurgeons to get on board.
"One in four adults over age 65 in this country has diabetes. One would have thought our medical colleagues would be ecstatic at the news that an operation on the gut – a safe procedure that can be done in about an hour – could produce full and durable remission of diabetes with complete prevention of amputations, blindness, and kidney failure. But that hasn’t been the case. All we’ve heard are cries for more and more evidence," said Dr. Pories, professor of surgery at East Carolina University, Greenville, N.C.
He added that in his view, Dr. Brethauer and his colleagues set the bar too high in requiring a sustained HbA1c below 6% as the definition of disease cure when the American Diabetes Association uses a figure of 7%. He noted that if the investigators had accepted the ADA metric, their combined cure/partial remission rate would have been considerably greater than the 50% figure they reported.
Dr. Brethauer replied that he and his coinvestigators chose a cutoff of 6% in order to make a point.
"When we raise this issue of ‘cure,’ which is still quite controversial and somewhat provocative, particularly with our endocrinology colleagues, I think we have to find the strictest and most conservative criteria that we can," he explained. "We continue to provide data in support of the concept that this is a surgically treated disease. It’s a major paradigm shift for our endocrinology colleagues to accept. I think it’s going to take a generation of endocrinologists before it’s embraced."
He reported that he serves as a consultant to Ethicon Endosurgery and Apollo Endosurgery.
INDIANAPOLIS – At 6 years of follow-up data, bariatric surgical cures of type 2 diabetes are holding steady in a single-center series of 217 patients.
"We see sustained weight loss, particularly in Roux-en-Y gastric bypass patients, 5-9 years after surgery." Diabetes remitted in 50% of patients, according to Dr. Stacy A. Brethauer of the Cleveland Clinic. "Bariatric surgery also achieved excellent long-term control of other cardiovascular risk factors; and diabetic nephropathy improved or stabilized."
Of 217 obese type 2 diabetic patients followed for a median of 6 years after bariatric surgery, 24% have been "cured." They have maintained a hemoglobin A1c level below 6% and fasting blood glucose values below 100 mg/dL for more than 5 years while off all antidiabetic medications, Dr. Brethauer reported at the annual meeting of the American Surgical Association.
Another 26% of patients have had partial remissions, meaning they maintained target HbA1c and fasting blood glucose levels off antidiabetic medications for more than 1 year but less than 5 years. In addition, 34% of patients were classified as "improved" based upon an absolute 1% or more reduction in HbA1c, a drop in fasting blood glucose in excess of 25 mg/dL, and a halving in the dose of antidiabetic medication for at least 1 year.
Diabetes recurred in 19% of subjects. Recurrence was defined as a return to an HbA1c of 6.5% or more or a fasting blood glucose level of at least 126 mg/dL.
The overall study population improved from a mean body mass index of 49 kg/m2 before surgery to 37 kg/m2 at 6 years after surgery. The mean HbA1c was 7.5% at baseline and 6.5% at 6 years. Fasting blood glucose at 6 years was a mean of 41.6 mg/dL lower than it was before surgery. LDL cholesterol levels were down by a mean of 10.1 mg/dL and HDL levels were up by 9.8 mg/dL. Systolic blood pressure was reduced by a mean of 10.9 mm Hg, with diastolic blood pressure was down by 3.2 mm Hg at 6 years after surgery.
Preoperatively, all but 5% of patients were on any antidiabetes medications; at 6 years after bariatric surgery, 54% were not on any antidiabetes drugs.
At baseline, 3% of subjects met all three key metabolic goals for diabetic patients as defined by the American Diabetes Association: an HbA1c below 7%, blood pressure below 130/80 mm Hg, and an LDL level below 100 mg/dL. At 6 years after surgery, 28% of patients met these goals. That result is particularly impressive in light of other studies that indicate 13% of the U.S. diabetic population as a whole meet all three goals.
The bariatric surgery group had a mean Framingham 10-year cardiovascular risk score of 28% preoperatively and of 22% at long-term followup.
The incidence of diabetic nephropathy among patients with type 2 diabetes is typically 2%-4% per year. Yet of 40 gastric bypass recipients known to have a normal urinary albumin-to-creatinine ratio preoperatively, only 1 developed macroalbuminuria and 1 microalbuminuria during a mean of 6 years postsurgical follow-up.
Also impressive, albuminuria regressed in 10 of the 19 gastric bypass recipients known to have the disorder at baseline. Albuminuria remained stable in the other 9 patients over the course of 6 years.
Mean excess weight loss at 1-2 years of follow-up was 61% in the 162 patients who underwent gastric bypass, 50% in the 23 with sleeve gastrectomy, and 30% in patients who had a gastric banding procedure.
In a multivariate analysis adjusted for baseline clinical characteristics, the significant predictors of diabetes remission following bariatric surgery were greater excess weight loss, preoperative diabetes duration of less than 5 years, and having a gastric bypass operation rather than sleeve gastrectomy or a gastric banding procedure.
Dr. Brethauer said this study, taken together with the findings of an earlier randomized clinical trial by the same investigators (N. Engl. J. Med. 2012;366:1567-76), conveys a clear message : "Bariatric surgery can induce a significant and sustainable remission of type 2 diabetes and other metabolic risk factors in obese patients and should be considered early in the course of the disease."
Discussant Dr. Walter J. Pories called Dr. Brethauer’s study "a really important contribution." He added that it’s high time for nonsurgeons to get on board.
"One in four adults over age 65 in this country has diabetes. One would have thought our medical colleagues would be ecstatic at the news that an operation on the gut – a safe procedure that can be done in about an hour – could produce full and durable remission of diabetes with complete prevention of amputations, blindness, and kidney failure. But that hasn’t been the case. All we’ve heard are cries for more and more evidence," said Dr. Pories, professor of surgery at East Carolina University, Greenville, N.C.
He added that in his view, Dr. Brethauer and his colleagues set the bar too high in requiring a sustained HbA1c below 6% as the definition of disease cure when the American Diabetes Association uses a figure of 7%. He noted that if the investigators had accepted the ADA metric, their combined cure/partial remission rate would have been considerably greater than the 50% figure they reported.
Dr. Brethauer replied that he and his coinvestigators chose a cutoff of 6% in order to make a point.
"When we raise this issue of ‘cure,’ which is still quite controversial and somewhat provocative, particularly with our endocrinology colleagues, I think we have to find the strictest and most conservative criteria that we can," he explained. "We continue to provide data in support of the concept that this is a surgically treated disease. It’s a major paradigm shift for our endocrinology colleagues to accept. I think it’s going to take a generation of endocrinologists before it’s embraced."
He reported that he serves as a consultant to Ethicon Endosurgery and Apollo Endosurgery.
INDIANAPOLIS – At 6 years of follow-up data, bariatric surgical cures of type 2 diabetes are holding steady in a single-center series of 217 patients.
"We see sustained weight loss, particularly in Roux-en-Y gastric bypass patients, 5-9 years after surgery." Diabetes remitted in 50% of patients, according to Dr. Stacy A. Brethauer of the Cleveland Clinic. "Bariatric surgery also achieved excellent long-term control of other cardiovascular risk factors; and diabetic nephropathy improved or stabilized."
Of 217 obese type 2 diabetic patients followed for a median of 6 years after bariatric surgery, 24% have been "cured." They have maintained a hemoglobin A1c level below 6% and fasting blood glucose values below 100 mg/dL for more than 5 years while off all antidiabetic medications, Dr. Brethauer reported at the annual meeting of the American Surgical Association.
Another 26% of patients have had partial remissions, meaning they maintained target HbA1c and fasting blood glucose levels off antidiabetic medications for more than 1 year but less than 5 years. In addition, 34% of patients were classified as "improved" based upon an absolute 1% or more reduction in HbA1c, a drop in fasting blood glucose in excess of 25 mg/dL, and a halving in the dose of antidiabetic medication for at least 1 year.
Diabetes recurred in 19% of subjects. Recurrence was defined as a return to an HbA1c of 6.5% or more or a fasting blood glucose level of at least 126 mg/dL.
The overall study population improved from a mean body mass index of 49 kg/m2 before surgery to 37 kg/m2 at 6 years after surgery. The mean HbA1c was 7.5% at baseline and 6.5% at 6 years. Fasting blood glucose at 6 years was a mean of 41.6 mg/dL lower than it was before surgery. LDL cholesterol levels were down by a mean of 10.1 mg/dL and HDL levels were up by 9.8 mg/dL. Systolic blood pressure was reduced by a mean of 10.9 mm Hg, with diastolic blood pressure was down by 3.2 mm Hg at 6 years after surgery.
Preoperatively, all but 5% of patients were on any antidiabetes medications; at 6 years after bariatric surgery, 54% were not on any antidiabetes drugs.
At baseline, 3% of subjects met all three key metabolic goals for diabetic patients as defined by the American Diabetes Association: an HbA1c below 7%, blood pressure below 130/80 mm Hg, and an LDL level below 100 mg/dL. At 6 years after surgery, 28% of patients met these goals. That result is particularly impressive in light of other studies that indicate 13% of the U.S. diabetic population as a whole meet all three goals.
The bariatric surgery group had a mean Framingham 10-year cardiovascular risk score of 28% preoperatively and of 22% at long-term followup.
The incidence of diabetic nephropathy among patients with type 2 diabetes is typically 2%-4% per year. Yet of 40 gastric bypass recipients known to have a normal urinary albumin-to-creatinine ratio preoperatively, only 1 developed macroalbuminuria and 1 microalbuminuria during a mean of 6 years postsurgical follow-up.
Also impressive, albuminuria regressed in 10 of the 19 gastric bypass recipients known to have the disorder at baseline. Albuminuria remained stable in the other 9 patients over the course of 6 years.
Mean excess weight loss at 1-2 years of follow-up was 61% in the 162 patients who underwent gastric bypass, 50% in the 23 with sleeve gastrectomy, and 30% in patients who had a gastric banding procedure.
In a multivariate analysis adjusted for baseline clinical characteristics, the significant predictors of diabetes remission following bariatric surgery were greater excess weight loss, preoperative diabetes duration of less than 5 years, and having a gastric bypass operation rather than sleeve gastrectomy or a gastric banding procedure.
Dr. Brethauer said this study, taken together with the findings of an earlier randomized clinical trial by the same investigators (N. Engl. J. Med. 2012;366:1567-76), conveys a clear message : "Bariatric surgery can induce a significant and sustainable remission of type 2 diabetes and other metabolic risk factors in obese patients and should be considered early in the course of the disease."
Discussant Dr. Walter J. Pories called Dr. Brethauer’s study "a really important contribution." He added that it’s high time for nonsurgeons to get on board.
"One in four adults over age 65 in this country has diabetes. One would have thought our medical colleagues would be ecstatic at the news that an operation on the gut – a safe procedure that can be done in about an hour – could produce full and durable remission of diabetes with complete prevention of amputations, blindness, and kidney failure. But that hasn’t been the case. All we’ve heard are cries for more and more evidence," said Dr. Pories, professor of surgery at East Carolina University, Greenville, N.C.
He added that in his view, Dr. Brethauer and his colleagues set the bar too high in requiring a sustained HbA1c below 6% as the definition of disease cure when the American Diabetes Association uses a figure of 7%. He noted that if the investigators had accepted the ADA metric, their combined cure/partial remission rate would have been considerably greater than the 50% figure they reported.
Dr. Brethauer replied that he and his coinvestigators chose a cutoff of 6% in order to make a point.
"When we raise this issue of ‘cure,’ which is still quite controversial and somewhat provocative, particularly with our endocrinology colleagues, I think we have to find the strictest and most conservative criteria that we can," he explained. "We continue to provide data in support of the concept that this is a surgically treated disease. It’s a major paradigm shift for our endocrinology colleagues to accept. I think it’s going to take a generation of endocrinologists before it’s embraced."
He reported that he serves as a consultant to Ethicon Endosurgery and Apollo Endosurgery.
AT THE ASA ANNUAL MEETING
Major finding: At a median of 6 years after bariatric surgery, 24% of patients have maintained an HbA1c below 6% and fasting blood glucose values below 100 mg/dL for more than 5 years while off all antidiabetic medications.
Data source: This is an ongoing retrospective single-center study in which 217 obese patients with type 2 diabetes have been followed for 5-9 years after bariatric surgery.
Disclosures: This study is sponsored by the Cleveland Clinic. The presenter reported serving as a consultant to Ethicon Endosurgery and Apollo Endosurgery.