User login
European Association for the Study of Diabetes (EASD): Annual Meeting
Cannula pretreatment reduces hypoglycemia in insulin pump users
VIENNA – Pretreating cannulas with a recombinant human hyaluronidase reduces the risk of hypoglycemia in patients with type 1 diabetes using insulin pumps, according to the results of a phase IV study.
In CONSISTENT-1 (Continuous Subcutaneous Insulin Infusion Study Enrolling Type 1), there were 23% fewer hypoglycemic events, defined as a blood glucose level below 56 mg/dL, and 21% fewer nocturnal hypoglycemic events in patients who received the infusion site pretreatment, compared with those who did not.
Importantly, the primary endpoint of noninferiority for glycemic control after 6 months was achieved, with comparable final mean and mean change from baseline hemoglobin A1c results.
“Current rapid-acting analogue insulin preparations are too slow to mimic the physiologic insulin meal response,” Dr. Jay Skyler, a study investigator with the University of Miami, explained at the annual meeting of the European Association for the Study of Diabetes.
He noted that hyaluronidase has been used for more than 60 years and recombinant human hyaluronidase (rHuPH20) is approved by the Food and Drug Administration as a means of improving the dispersion and absorption of injected or infused drugs. “Previously, we have demonstrated that adding recombinant human hyaluronidase [Halozyme Therapeutics’ Hylex] to rapid-acting insulin preparations accelerates the absorption and action of insulin,” Dr. Skyler said.
In this study, rHuPH20 was given in a single injection into the cannula prior to the placement of a pump for continuous subcutaneous insulin infusion (CSII), but Dr. Skyler observed that it could also be coformulated with insulin for use in pens or vials.
The aim of CONSISTENT-1 was to assess the metabolic outcomes and safety of rHuPH20 after 6 months. The trial was open label, and 456 patients with type 1 diabetes were randomized, in a 3 to 1 ratio, to pretreatment with rHuPH20 or to standard CSII without rHuPH20.
The mean age of participants was 47 years in the rHuPH20 arm and 50 years in the CSII-alone arm. Around a quarter of patients in each group used continuous glucose monitors (CGMs), and 77% had used an insulin pump for more than 5 years. Patients had been using insulin pumps for a mean of about 10 years in both groups.
The number of hypoglycemic events per month was 13.8 in the rHuPH20-pretreated group and 12.1 in the CSII-only group when a blood glucose cutoff of 70 mg/dL or lower was used, representing a nonsignificant 12% reduction. However, when the lower threshold of 56 mg/dL or lower was used, there were fewer hypoglycemia events per month (4.0 vs. 3.1; P = .02).
While the difference in the number of nocturnal hypoglycemic events per month between the rHuPH20 and CSII-only groups was also significant (2.1 vs. 1.7; P = .02), the rate of severe hypoglycemic events per 100 subject-years was only numerically lower (19 vs. 7; P = .08) in the rHuPH20 group.
Subgroup analysis showed that the timing of bolus dosing was important, with fewer blood glucose excursions and hypoglycemic events if insulin was dosed within 15 minutes before a meal than if it was dosed within 15 minutes after a meal.
Looking at CGM profiles for breakfast, lunch, and dinner, there were fewer glucose excursions in the patients who had received rHuPH20, and, stating that it was the reason for performing the study, Dr. Skyler said there were fewer excursions on subsequent days.
In terms of safety, he noted that there was an increase in injection-site reactions in the rHuPH20-pretreated group, with pain, discomfort, or paresthesia in 15.5%, compared with 6.2% in the CSII-only patients. In addition, hematoma, bruising, or hemorrhage occurred in 3.8% of the rHuPH20 group vs. 0.0% of the standard CSII group. There was no “meaningful immunogenicity,” and adverse events were otherwise well balanced between the groups.
Dr. Skyler said that a 1.5-year extension study is in progress and that the current findings “demonstrate that rHuPH20 pretreatment eliminates the systemic variability in glucose control that naturally occurs as infusion sites age.
“This should allow for improved diabetes control since insulin responses will occur more predictably.”
Halozyme supported the study. Dr. Skyler has been an adviser for Halozyme and has consulted for other companies with an interest in insulin pump therapy.
VIENNA – Pretreating cannulas with a recombinant human hyaluronidase reduces the risk of hypoglycemia in patients with type 1 diabetes using insulin pumps, according to the results of a phase IV study.
In CONSISTENT-1 (Continuous Subcutaneous Insulin Infusion Study Enrolling Type 1), there were 23% fewer hypoglycemic events, defined as a blood glucose level below 56 mg/dL, and 21% fewer nocturnal hypoglycemic events in patients who received the infusion site pretreatment, compared with those who did not.
Importantly, the primary endpoint of noninferiority for glycemic control after 6 months was achieved, with comparable final mean and mean change from baseline hemoglobin A1c results.
“Current rapid-acting analogue insulin preparations are too slow to mimic the physiologic insulin meal response,” Dr. Jay Skyler, a study investigator with the University of Miami, explained at the annual meeting of the European Association for the Study of Diabetes.
He noted that hyaluronidase has been used for more than 60 years and recombinant human hyaluronidase (rHuPH20) is approved by the Food and Drug Administration as a means of improving the dispersion and absorption of injected or infused drugs. “Previously, we have demonstrated that adding recombinant human hyaluronidase [Halozyme Therapeutics’ Hylex] to rapid-acting insulin preparations accelerates the absorption and action of insulin,” Dr. Skyler said.
In this study, rHuPH20 was given in a single injection into the cannula prior to the placement of a pump for continuous subcutaneous insulin infusion (CSII), but Dr. Skyler observed that it could also be coformulated with insulin for use in pens or vials.
The aim of CONSISTENT-1 was to assess the metabolic outcomes and safety of rHuPH20 after 6 months. The trial was open label, and 456 patients with type 1 diabetes were randomized, in a 3 to 1 ratio, to pretreatment with rHuPH20 or to standard CSII without rHuPH20.
The mean age of participants was 47 years in the rHuPH20 arm and 50 years in the CSII-alone arm. Around a quarter of patients in each group used continuous glucose monitors (CGMs), and 77% had used an insulin pump for more than 5 years. Patients had been using insulin pumps for a mean of about 10 years in both groups.
The number of hypoglycemic events per month was 13.8 in the rHuPH20-pretreated group and 12.1 in the CSII-only group when a blood glucose cutoff of 70 mg/dL or lower was used, representing a nonsignificant 12% reduction. However, when the lower threshold of 56 mg/dL or lower was used, there were fewer hypoglycemia events per month (4.0 vs. 3.1; P = .02).
While the difference in the number of nocturnal hypoglycemic events per month between the rHuPH20 and CSII-only groups was also significant (2.1 vs. 1.7; P = .02), the rate of severe hypoglycemic events per 100 subject-years was only numerically lower (19 vs. 7; P = .08) in the rHuPH20 group.
Subgroup analysis showed that the timing of bolus dosing was important, with fewer blood glucose excursions and hypoglycemic events if insulin was dosed within 15 minutes before a meal than if it was dosed within 15 minutes after a meal.
Looking at CGM profiles for breakfast, lunch, and dinner, there were fewer glucose excursions in the patients who had received rHuPH20, and, stating that it was the reason for performing the study, Dr. Skyler said there were fewer excursions on subsequent days.
In terms of safety, he noted that there was an increase in injection-site reactions in the rHuPH20-pretreated group, with pain, discomfort, or paresthesia in 15.5%, compared with 6.2% in the CSII-only patients. In addition, hematoma, bruising, or hemorrhage occurred in 3.8% of the rHuPH20 group vs. 0.0% of the standard CSII group. There was no “meaningful immunogenicity,” and adverse events were otherwise well balanced between the groups.
Dr. Skyler said that a 1.5-year extension study is in progress and that the current findings “demonstrate that rHuPH20 pretreatment eliminates the systemic variability in glucose control that naturally occurs as infusion sites age.
“This should allow for improved diabetes control since insulin responses will occur more predictably.”
Halozyme supported the study. Dr. Skyler has been an adviser for Halozyme and has consulted for other companies with an interest in insulin pump therapy.
VIENNA – Pretreating cannulas with a recombinant human hyaluronidase reduces the risk of hypoglycemia in patients with type 1 diabetes using insulin pumps, according to the results of a phase IV study.
In CONSISTENT-1 (Continuous Subcutaneous Insulin Infusion Study Enrolling Type 1), there were 23% fewer hypoglycemic events, defined as a blood glucose level below 56 mg/dL, and 21% fewer nocturnal hypoglycemic events in patients who received the infusion site pretreatment, compared with those who did not.
Importantly, the primary endpoint of noninferiority for glycemic control after 6 months was achieved, with comparable final mean and mean change from baseline hemoglobin A1c results.
“Current rapid-acting analogue insulin preparations are too slow to mimic the physiologic insulin meal response,” Dr. Jay Skyler, a study investigator with the University of Miami, explained at the annual meeting of the European Association for the Study of Diabetes.
He noted that hyaluronidase has been used for more than 60 years and recombinant human hyaluronidase (rHuPH20) is approved by the Food and Drug Administration as a means of improving the dispersion and absorption of injected or infused drugs. “Previously, we have demonstrated that adding recombinant human hyaluronidase [Halozyme Therapeutics’ Hylex] to rapid-acting insulin preparations accelerates the absorption and action of insulin,” Dr. Skyler said.
In this study, rHuPH20 was given in a single injection into the cannula prior to the placement of a pump for continuous subcutaneous insulin infusion (CSII), but Dr. Skyler observed that it could also be coformulated with insulin for use in pens or vials.
The aim of CONSISTENT-1 was to assess the metabolic outcomes and safety of rHuPH20 after 6 months. The trial was open label, and 456 patients with type 1 diabetes were randomized, in a 3 to 1 ratio, to pretreatment with rHuPH20 or to standard CSII without rHuPH20.
The mean age of participants was 47 years in the rHuPH20 arm and 50 years in the CSII-alone arm. Around a quarter of patients in each group used continuous glucose monitors (CGMs), and 77% had used an insulin pump for more than 5 years. Patients had been using insulin pumps for a mean of about 10 years in both groups.
The number of hypoglycemic events per month was 13.8 in the rHuPH20-pretreated group and 12.1 in the CSII-only group when a blood glucose cutoff of 70 mg/dL or lower was used, representing a nonsignificant 12% reduction. However, when the lower threshold of 56 mg/dL or lower was used, there were fewer hypoglycemia events per month (4.0 vs. 3.1; P = .02).
While the difference in the number of nocturnal hypoglycemic events per month between the rHuPH20 and CSII-only groups was also significant (2.1 vs. 1.7; P = .02), the rate of severe hypoglycemic events per 100 subject-years was only numerically lower (19 vs. 7; P = .08) in the rHuPH20 group.
Subgroup analysis showed that the timing of bolus dosing was important, with fewer blood glucose excursions and hypoglycemic events if insulin was dosed within 15 minutes before a meal than if it was dosed within 15 minutes after a meal.
Looking at CGM profiles for breakfast, lunch, and dinner, there were fewer glucose excursions in the patients who had received rHuPH20, and, stating that it was the reason for performing the study, Dr. Skyler said there were fewer excursions on subsequent days.
In terms of safety, he noted that there was an increase in injection-site reactions in the rHuPH20-pretreated group, with pain, discomfort, or paresthesia in 15.5%, compared with 6.2% in the CSII-only patients. In addition, hematoma, bruising, or hemorrhage occurred in 3.8% of the rHuPH20 group vs. 0.0% of the standard CSII group. There was no “meaningful immunogenicity,” and adverse events were otherwise well balanced between the groups.
Dr. Skyler said that a 1.5-year extension study is in progress and that the current findings “demonstrate that rHuPH20 pretreatment eliminates the systemic variability in glucose control that naturally occurs as infusion sites age.
“This should allow for improved diabetes control since insulin responses will occur more predictably.”
Halozyme supported the study. Dr. Skyler has been an adviser for Halozyme and has consulted for other companies with an interest in insulin pump therapy.
AT EASD 2014
Key clinical point: Pretreating CSII sites with recombinant human hyaluronidase before unit changeover provides equivalent glycemic control but reduces the likelihood of hypoglycemia.
Major finding: Compared with no pretreatment of cannulas, pretreatment with rHuHP20 yielded a relative risk of 0.77 for any hypoglycemic event (blood glucose level less than 56 mg/dL) and 0.79 for nocturnal hypoglycemic events (both P = .02).
Data source: CONSISTENT-1, a 6-month, open-label study of 456 patients with type 1 diabetes using insulin pumps.
Disclosures: Halozyme supported the study. Dr. Skyler has been an adviser for Halozyme and has consulted for other companies with an interest in insulin pump therapy.
Intensive glycemic control safely cut end-stage renal disease
VIENNA – Five years of intensive glycemic control in patients with type 2 diabetes safely halved long-term rate of end-stage renal disease, compared with placebo, in a multicenter study with about 8,000 patients, a finding that refutes prior suggestions that more intensive glycemic control can harm patients.
Intensive glucose control that produced an average hemoglobin A1c of 6.5% “is important for preventing serious renal complications and does not cause harm in patients with established type 2 diabetes,” Dr. Sophia Zoungas said at the annual meeting of the European Association for the Study of Diabetes.
Following the report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial in 2008 (N. Engl. J. Med. 2008;358;2545-59), which showed increased mortality with intensive glycemic control, compared with standard treatment, many physicians became leery of treating patients to a HbA1c level below 7%. But 5-year follow-up results of patients originally enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial (N. Engl. J. Med. 2008;358:2560-72) has now shown that a prolonged period maintaining patients at a HbA1c of roughly 6.5% produced no excess rates of total mortality, major macrovascular events, or major clinical microvascular events when compared with patients on standard care with an average HbA1c of 7.2%, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
“Whether the excess mortality seen in ACCORD was a true effect or not, there has been concern [about tight glycemic control] that has translated into where HbA1c targets have been set,” Dr. Zoungas said in an interview. “We have been blinded by this signal of increased fatal myocardial infarctions [seen in ACCORD] that many have been a chance effect.”
She suggested that the safety that intense glycemic control showed during 10-year follow-up of patients in the ADVANCE posttrial Observational Study (ADVANCE ON) may be explained by the more gradual HbA1c reductions achieved in the ADVANCE patients, compared with patients in ACCORD. In ADVANCE, patients’ HbA1c levels came down to about 6.5% over the course of a year, compared with a 3-6 month period in ACCORD, Dr. Zoungas noted.
In addition to establishing safety, the new results reported by Dr. Zoungas showed a statistically significant, 46% reduction in the incidence of end-stage renal disease (defined as progression to dialysis or need for kidney transplant) during the entire, 10-year follow-up. Simultaneous with Dr. Zoungas’s report at the meeting the ADVANCE ON results were published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407963]).
“This is the first time study results have shown that maintaining a HbA1cof less than 7% is associated with a long-term reduction in end-stage kidney disease,” she said. “End-stage kidney disease has been hard to study [as an endpoint] because the overall incidence is low, so patients need to be followed for a long time to see enough events.”
ADVANCE ON initially included 8,494 of the roughly 10,000 patients who completed the 5-year ADVANCE intervention trial at 215 centers in 20 countries. ADVANCE tested the impact of an antihypertensive intervention as well as the effect of intensified glycemic control in patients aged 55 years or older with type 2 diabetes and at least one other cardiovascular risk factor. At the time ADVANCE ended, average HbA1c levels were 7.2% in the control patients and 6.5% in those who had been on a more intensive hypoglycemic regimen. However, at the time patients began ADVANCE ON, their average HbA1c was 7.5%, regardless of which arm of ADVANCE they had been in, and the average level remained there through a median of 5 more years of follow-up. A total of 5,131 patients remained under study for the entire 10 years of follow-up. Analysis of the ADVANCE ON findings also showed a significant long-term effect from 5 years of added antihypertensive treatment, results reported in early September at the annual congress of the European Society of Cardiology.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
On Twitter @mitchelzoler
The ADVANCE ON results change our perspective on the medical treatment of patients with type 2 diabetes.
The results provide more evidence for the safety of metformin and sulfonylureas, two of the major drugs used on patients in the study during the intensive-management phase. This is not level I evidence, but it is evidence of safety. The results from ADVANCE ON as well as from other trials also suggest that these drugs reduce microvascular and macrovascular complications. The long-term legacy effect of intensive glycemic control to reduce the incidence of end-stage kidney disease was impressive. The hemoglobin A1c target established by existing management guidelines can remain in place.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Joachim Spranger |
The results also highlight our desperate need for glucose-lowering drugs that can substantially reduce fatal cardiovascular disease.
New drugs being considered for treatment of type 2 diabetes need to outperform metformin, sulfonylureas, and insulin for reducing clinically relevant endpoints. It is not enough for trials to show that new drugs can reduce glucose while causing fewer adverse effects than the older agents. Proven impact on clinical endpoints is also needed. We also must remember that the safety of treatment with three oral agents in a single regimen remains unproven.
Dr. Joachim Spranger is a professor of endocrinology at Charité Medical University in Berlin. He had no disclosures. He made these comments as the invited discussant for ADVANCE ON.
The ADVANCE ON results change our perspective on the medical treatment of patients with type 2 diabetes.
The results provide more evidence for the safety of metformin and sulfonylureas, two of the major drugs used on patients in the study during the intensive-management phase. This is not level I evidence, but it is evidence of safety. The results from ADVANCE ON as well as from other trials also suggest that these drugs reduce microvascular and macrovascular complications. The long-term legacy effect of intensive glycemic control to reduce the incidence of end-stage kidney disease was impressive. The hemoglobin A1c target established by existing management guidelines can remain in place.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Joachim Spranger |
The results also highlight our desperate need for glucose-lowering drugs that can substantially reduce fatal cardiovascular disease.
New drugs being considered for treatment of type 2 diabetes need to outperform metformin, sulfonylureas, and insulin for reducing clinically relevant endpoints. It is not enough for trials to show that new drugs can reduce glucose while causing fewer adverse effects than the older agents. Proven impact on clinical endpoints is also needed. We also must remember that the safety of treatment with three oral agents in a single regimen remains unproven.
Dr. Joachim Spranger is a professor of endocrinology at Charité Medical University in Berlin. He had no disclosures. He made these comments as the invited discussant for ADVANCE ON.
The ADVANCE ON results change our perspective on the medical treatment of patients with type 2 diabetes.
The results provide more evidence for the safety of metformin and sulfonylureas, two of the major drugs used on patients in the study during the intensive-management phase. This is not level I evidence, but it is evidence of safety. The results from ADVANCE ON as well as from other trials also suggest that these drugs reduce microvascular and macrovascular complications. The long-term legacy effect of intensive glycemic control to reduce the incidence of end-stage kidney disease was impressive. The hemoglobin A1c target established by existing management guidelines can remain in place.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Joachim Spranger |
The results also highlight our desperate need for glucose-lowering drugs that can substantially reduce fatal cardiovascular disease.
New drugs being considered for treatment of type 2 diabetes need to outperform metformin, sulfonylureas, and insulin for reducing clinically relevant endpoints. It is not enough for trials to show that new drugs can reduce glucose while causing fewer adverse effects than the older agents. Proven impact on clinical endpoints is also needed. We also must remember that the safety of treatment with three oral agents in a single regimen remains unproven.
Dr. Joachim Spranger is a professor of endocrinology at Charité Medical University in Berlin. He had no disclosures. He made these comments as the invited discussant for ADVANCE ON.
VIENNA – Five years of intensive glycemic control in patients with type 2 diabetes safely halved long-term rate of end-stage renal disease, compared with placebo, in a multicenter study with about 8,000 patients, a finding that refutes prior suggestions that more intensive glycemic control can harm patients.
Intensive glucose control that produced an average hemoglobin A1c of 6.5% “is important for preventing serious renal complications and does not cause harm in patients with established type 2 diabetes,” Dr. Sophia Zoungas said at the annual meeting of the European Association for the Study of Diabetes.
Following the report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial in 2008 (N. Engl. J. Med. 2008;358;2545-59), which showed increased mortality with intensive glycemic control, compared with standard treatment, many physicians became leery of treating patients to a HbA1c level below 7%. But 5-year follow-up results of patients originally enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial (N. Engl. J. Med. 2008;358:2560-72) has now shown that a prolonged period maintaining patients at a HbA1c of roughly 6.5% produced no excess rates of total mortality, major macrovascular events, or major clinical microvascular events when compared with patients on standard care with an average HbA1c of 7.2%, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
“Whether the excess mortality seen in ACCORD was a true effect or not, there has been concern [about tight glycemic control] that has translated into where HbA1c targets have been set,” Dr. Zoungas said in an interview. “We have been blinded by this signal of increased fatal myocardial infarctions [seen in ACCORD] that many have been a chance effect.”
She suggested that the safety that intense glycemic control showed during 10-year follow-up of patients in the ADVANCE posttrial Observational Study (ADVANCE ON) may be explained by the more gradual HbA1c reductions achieved in the ADVANCE patients, compared with patients in ACCORD. In ADVANCE, patients’ HbA1c levels came down to about 6.5% over the course of a year, compared with a 3-6 month period in ACCORD, Dr. Zoungas noted.
In addition to establishing safety, the new results reported by Dr. Zoungas showed a statistically significant, 46% reduction in the incidence of end-stage renal disease (defined as progression to dialysis or need for kidney transplant) during the entire, 10-year follow-up. Simultaneous with Dr. Zoungas’s report at the meeting the ADVANCE ON results were published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407963]).
“This is the first time study results have shown that maintaining a HbA1cof less than 7% is associated with a long-term reduction in end-stage kidney disease,” she said. “End-stage kidney disease has been hard to study [as an endpoint] because the overall incidence is low, so patients need to be followed for a long time to see enough events.”
ADVANCE ON initially included 8,494 of the roughly 10,000 patients who completed the 5-year ADVANCE intervention trial at 215 centers in 20 countries. ADVANCE tested the impact of an antihypertensive intervention as well as the effect of intensified glycemic control in patients aged 55 years or older with type 2 diabetes and at least one other cardiovascular risk factor. At the time ADVANCE ended, average HbA1c levels were 7.2% in the control patients and 6.5% in those who had been on a more intensive hypoglycemic regimen. However, at the time patients began ADVANCE ON, their average HbA1c was 7.5%, regardless of which arm of ADVANCE they had been in, and the average level remained there through a median of 5 more years of follow-up. A total of 5,131 patients remained under study for the entire 10 years of follow-up. Analysis of the ADVANCE ON findings also showed a significant long-term effect from 5 years of added antihypertensive treatment, results reported in early September at the annual congress of the European Society of Cardiology.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
On Twitter @mitchelzoler
VIENNA – Five years of intensive glycemic control in patients with type 2 diabetes safely halved long-term rate of end-stage renal disease, compared with placebo, in a multicenter study with about 8,000 patients, a finding that refutes prior suggestions that more intensive glycemic control can harm patients.
Intensive glucose control that produced an average hemoglobin A1c of 6.5% “is important for preventing serious renal complications and does not cause harm in patients with established type 2 diabetes,” Dr. Sophia Zoungas said at the annual meeting of the European Association for the Study of Diabetes.
Following the report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial in 2008 (N. Engl. J. Med. 2008;358;2545-59), which showed increased mortality with intensive glycemic control, compared with standard treatment, many physicians became leery of treating patients to a HbA1c level below 7%. But 5-year follow-up results of patients originally enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial (N. Engl. J. Med. 2008;358:2560-72) has now shown that a prolonged period maintaining patients at a HbA1c of roughly 6.5% produced no excess rates of total mortality, major macrovascular events, or major clinical microvascular events when compared with patients on standard care with an average HbA1c of 7.2%, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
“Whether the excess mortality seen in ACCORD was a true effect or not, there has been concern [about tight glycemic control] that has translated into where HbA1c targets have been set,” Dr. Zoungas said in an interview. “We have been blinded by this signal of increased fatal myocardial infarctions [seen in ACCORD] that many have been a chance effect.”
She suggested that the safety that intense glycemic control showed during 10-year follow-up of patients in the ADVANCE posttrial Observational Study (ADVANCE ON) may be explained by the more gradual HbA1c reductions achieved in the ADVANCE patients, compared with patients in ACCORD. In ADVANCE, patients’ HbA1c levels came down to about 6.5% over the course of a year, compared with a 3-6 month period in ACCORD, Dr. Zoungas noted.
In addition to establishing safety, the new results reported by Dr. Zoungas showed a statistically significant, 46% reduction in the incidence of end-stage renal disease (defined as progression to dialysis or need for kidney transplant) during the entire, 10-year follow-up. Simultaneous with Dr. Zoungas’s report at the meeting the ADVANCE ON results were published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407963]).
“This is the first time study results have shown that maintaining a HbA1cof less than 7% is associated with a long-term reduction in end-stage kidney disease,” she said. “End-stage kidney disease has been hard to study [as an endpoint] because the overall incidence is low, so patients need to be followed for a long time to see enough events.”
ADVANCE ON initially included 8,494 of the roughly 10,000 patients who completed the 5-year ADVANCE intervention trial at 215 centers in 20 countries. ADVANCE tested the impact of an antihypertensive intervention as well as the effect of intensified glycemic control in patients aged 55 years or older with type 2 diabetes and at least one other cardiovascular risk factor. At the time ADVANCE ended, average HbA1c levels were 7.2% in the control patients and 6.5% in those who had been on a more intensive hypoglycemic regimen. However, at the time patients began ADVANCE ON, their average HbA1c was 7.5%, regardless of which arm of ADVANCE they had been in, and the average level remained there through a median of 5 more years of follow-up. A total of 5,131 patients remained under study for the entire 10 years of follow-up. Analysis of the ADVANCE ON findings also showed a significant long-term effect from 5 years of added antihypertensive treatment, results reported in early September at the annual congress of the European Society of Cardiology.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
On Twitter @mitchelzoler
AT EASD 2014
Key clinical point: Intensive glycemic control in patients with type 2 diabetes safely led to a long-term reduction in end-stage kidney disease, compared with standard management.
Major finding: Intensive glycemic-control patients had a 46% reduced rate of end-stage kidney disease, compared with standard-care patients.
Data source: The ADVANCE ON study, which followed 8,494 patients after their participation in an international glycemia-control trial.
Disclosures: ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
Saxagliptin reverses proteinuria in type 2 diabetes
VIENNA – Patients with type 2 diabetes treated with the oral hypoglycemic saxagliptin (Onglyza) benefited from stabilization and, in some cases, reversal of urinary protein levels in a prespecified, secondary analysis of data collected from more than 16,000 patients in a randomized, international trial.
Progression of patients with diabetes from normoalbuminuria to microalbuminuria and then proteinuria tracks along with their deteriorating renal function. Until now, no agent has been identified that could stop or reverse this process, although a similar effect had been documented in results from a few prior, much smaller studies using other drugs from the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which includes saxagliptin.
“It’s a very important finding that needs further investigation in longer-term studies so we can see whether the improvement in the [albumin to creatinine ratio (ACR)] will also mean less deterioration of the eGFR [estimated glomerular filtration rate] and thereby prevent end-stage renal disease,” Dr. Ofri Mosenzon said following her report at the annual meeting of the European Association for the Study of Diabetes.
The new analysis also showed that saxagliptin did this safely, without causing any renal damage, and that the effect on microalbuminuria and proteinuria was completely independent of the drug’s glycemic control effect measured by hemoglobin A1c levels. Dr. Mosenzon had no simple explanation for the mechanism by which saxagliptin and other DPP-4 inhibitors might exert this effect, though she suggested that an anti-inflammatory pleiotropic effect of the drug class might be involved.
“We have this clinical result. Now we need to look for an explanation,” said Dr. Mosenzon, a diabetologist at Hadassah Hospital in Jerusalem.
Her analysis used data collected in the the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which had the primary purpose of assessing the cardiovascular safety of saxagliptin in 16,492 patients with type 2 diabetes and a high risk for cardiovascular events (N. Engl. J. Med. 2013;369:1317-26). The study randomized patients to treatment with either 5 mg saxagliptin daily (2.5 mg daily in patients with impaired renal function at entry) or placebo in addition to whatever other standard medications their physicians prescribed. The secondary analysis of the impact of saxagliptin on proteinuria and renal function used prespecified definitions for evaluating the renal safety and renal-protective efficacy of treatment.
On the safety side, treatment with saxagliptin, compared with placebo linked with no statistically significant differences in the rate or magnitude of serum creatinine increases or in the rates of progression to dialysis or renal transplant.
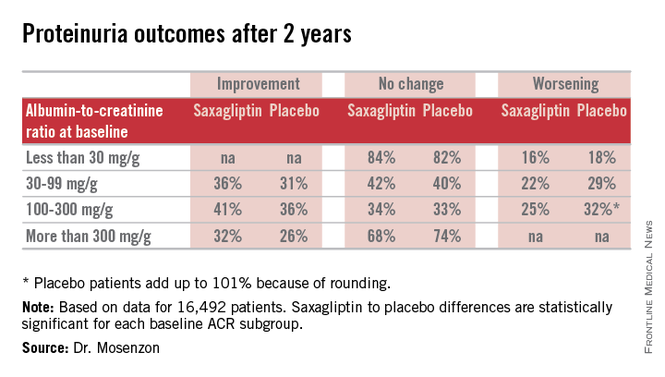
For efficacy, treatment with saxagliptin, compared with placebo led to a consistent pattern of stabilization and reversal of the severity of microalbuminuria and proteinuria that cut across patients at all baseline levels of urinary protein (see table). For example, among 1,638 categorized as having proteinuria at entry into the study, based on an ACR of more than 300 mg/g, 32% of the patients treated with saxagliptin had improved to daily urinary protein excretions of less than 300 mg/g after an average 2.1 years on treatment, compared with a 26% rate in the placebo-treated control patients, a statistically significant difference. The extent of reversals and progressions prevented were also statistically significant among the 9,696 patients who entered the study with normoalbuminuria and urinary protein levels of less than 30 mg/g and those who entered with microalbuminuria and daily urinary protein levels of 30-300 mg/g.
Expressed another way, when compared with placebo-treated patients those patients treated with saxagliptin for 2 years who entered the study with an eGFR of more than 50 mL/min/1.73 m2 had on average 19 mg less daily protein in their urine, those who entered with an eGFR of 30-50 mL/min/1.73 m2 averaged 105 mg less daily urine protein, and those who entered with an eGFR of less than 30 mL/min/1.73 m2 averaged 245 mg less daily urine protein.
SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.
VIENNA – Patients with type 2 diabetes treated with the oral hypoglycemic saxagliptin (Onglyza) benefited from stabilization and, in some cases, reversal of urinary protein levels in a prespecified, secondary analysis of data collected from more than 16,000 patients in a randomized, international trial.
Progression of patients with diabetes from normoalbuminuria to microalbuminuria and then proteinuria tracks along with their deteriorating renal function. Until now, no agent has been identified that could stop or reverse this process, although a similar effect had been documented in results from a few prior, much smaller studies using other drugs from the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which includes saxagliptin.

“It’s a very important finding that needs further investigation in longer-term studies so we can see whether the improvement in the [albumin to creatinine ratio (ACR)] will also mean less deterioration of the eGFR [estimated glomerular filtration rate] and thereby prevent end-stage renal disease,” Dr. Ofri Mosenzon said following her report at the annual meeting of the European Association for the Study of Diabetes.
The new analysis also showed that saxagliptin did this safely, without causing any renal damage, and that the effect on microalbuminuria and proteinuria was completely independent of the drug’s glycemic control effect measured by hemoglobin A1c levels. Dr. Mosenzon had no simple explanation for the mechanism by which saxagliptin and other DPP-4 inhibitors might exert this effect, though she suggested that an anti-inflammatory pleiotropic effect of the drug class might be involved.
“We have this clinical result. Now we need to look for an explanation,” said Dr. Mosenzon, a diabetologist at Hadassah Hospital in Jerusalem.
Her analysis used data collected in the the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which had the primary purpose of assessing the cardiovascular safety of saxagliptin in 16,492 patients with type 2 diabetes and a high risk for cardiovascular events (N. Engl. J. Med. 2013;369:1317-26). The study randomized patients to treatment with either 5 mg saxagliptin daily (2.5 mg daily in patients with impaired renal function at entry) or placebo in addition to whatever other standard medications their physicians prescribed. The secondary analysis of the impact of saxagliptin on proteinuria and renal function used prespecified definitions for evaluating the renal safety and renal-protective efficacy of treatment.
On the safety side, treatment with saxagliptin, compared with placebo linked with no statistically significant differences in the rate or magnitude of serum creatinine increases or in the rates of progression to dialysis or renal transplant.
For efficacy, treatment with saxagliptin, compared with placebo led to a consistent pattern of stabilization and reversal of the severity of microalbuminuria and proteinuria that cut across patients at all baseline levels of urinary protein (see table). For example, among 1,638 categorized as having proteinuria at entry into the study, based on an ACR of more than 300 mg/g, 32% of the patients treated with saxagliptin had improved to daily urinary protein excretions of less than 300 mg/g after an average 2.1 years on treatment, compared with a 26% rate in the placebo-treated control patients, a statistically significant difference. The extent of reversals and progressions prevented were also statistically significant among the 9,696 patients who entered the study with normoalbuminuria and urinary protein levels of less than 30 mg/g and those who entered with microalbuminuria and daily urinary protein levels of 30-300 mg/g.
Expressed another way, when compared with placebo-treated patients those patients treated with saxagliptin for 2 years who entered the study with an eGFR of more than 50 mL/min/1.73 m2 had on average 19 mg less daily protein in their urine, those who entered with an eGFR of 30-50 mL/min/1.73 m2 averaged 105 mg less daily urine protein, and those who entered with an eGFR of less than 30 mL/min/1.73 m2 averaged 245 mg less daily urine protein.
SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.
VIENNA – Patients with type 2 diabetes treated with the oral hypoglycemic saxagliptin (Onglyza) benefited from stabilization and, in some cases, reversal of urinary protein levels in a prespecified, secondary analysis of data collected from more than 16,000 patients in a randomized, international trial.
Progression of patients with diabetes from normoalbuminuria to microalbuminuria and then proteinuria tracks along with their deteriorating renal function. Until now, no agent has been identified that could stop or reverse this process, although a similar effect had been documented in results from a few prior, much smaller studies using other drugs from the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which includes saxagliptin.

“It’s a very important finding that needs further investigation in longer-term studies so we can see whether the improvement in the [albumin to creatinine ratio (ACR)] will also mean less deterioration of the eGFR [estimated glomerular filtration rate] and thereby prevent end-stage renal disease,” Dr. Ofri Mosenzon said following her report at the annual meeting of the European Association for the Study of Diabetes.
The new analysis also showed that saxagliptin did this safely, without causing any renal damage, and that the effect on microalbuminuria and proteinuria was completely independent of the drug’s glycemic control effect measured by hemoglobin A1c levels. Dr. Mosenzon had no simple explanation for the mechanism by which saxagliptin and other DPP-4 inhibitors might exert this effect, though she suggested that an anti-inflammatory pleiotropic effect of the drug class might be involved.
“We have this clinical result. Now we need to look for an explanation,” said Dr. Mosenzon, a diabetologist at Hadassah Hospital in Jerusalem.
Her analysis used data collected in the the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which had the primary purpose of assessing the cardiovascular safety of saxagliptin in 16,492 patients with type 2 diabetes and a high risk for cardiovascular events (N. Engl. J. Med. 2013;369:1317-26). The study randomized patients to treatment with either 5 mg saxagliptin daily (2.5 mg daily in patients with impaired renal function at entry) or placebo in addition to whatever other standard medications their physicians prescribed. The secondary analysis of the impact of saxagliptin on proteinuria and renal function used prespecified definitions for evaluating the renal safety and renal-protective efficacy of treatment.
On the safety side, treatment with saxagliptin, compared with placebo linked with no statistically significant differences in the rate or magnitude of serum creatinine increases or in the rates of progression to dialysis or renal transplant.
For efficacy, treatment with saxagliptin, compared with placebo led to a consistent pattern of stabilization and reversal of the severity of microalbuminuria and proteinuria that cut across patients at all baseline levels of urinary protein (see table). For example, among 1,638 categorized as having proteinuria at entry into the study, based on an ACR of more than 300 mg/g, 32% of the patients treated with saxagliptin had improved to daily urinary protein excretions of less than 300 mg/g after an average 2.1 years on treatment, compared with a 26% rate in the placebo-treated control patients, a statistically significant difference. The extent of reversals and progressions prevented were also statistically significant among the 9,696 patients who entered the study with normoalbuminuria and urinary protein levels of less than 30 mg/g and those who entered with microalbuminuria and daily urinary protein levels of 30-300 mg/g.
Expressed another way, when compared with placebo-treated patients those patients treated with saxagliptin for 2 years who entered the study with an eGFR of more than 50 mL/min/1.73 m2 had on average 19 mg less daily protein in their urine, those who entered with an eGFR of 30-50 mL/min/1.73 m2 averaged 105 mg less daily urine protein, and those who entered with an eGFR of less than 30 mL/min/1.73 m2 averaged 245 mg less daily urine protein.
SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.
AT EASD 2014
Key clinical point: Treatment with saxagliptin led to stabilization and in some patients reversal of microalbuminuria and proteinuria in patients with type 2 diabetes.
Major finding: Proteinuria reversed in 32% of patients treated with saxagliptin during 2.1 years, compared with a 26% rate in controls.
Data source: Prespecified, secondary analysis of data from SAVOR-TIMI 53, an international, randomized trial with 16,492 patients with type 2 diabetes..
Disclosures: SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.
Aflibercept’s diabetic retinopathy benefits independent of blood glucose
VIENNA – The beneficial effects of aflibercept on diabetic eye disease are independent of how well blood glucose is being controlled at the start of treatment, according to a post hoc analysis of two randomized, phase III studies.
Intravitreal injection of 2 mg of aflibercept every month or every other month had similar effects on the primary endpoint of best-corrected visual acuity (BCVA) in more than 800 subjects with diabetic macular edema (DME) with central involvement. BCVA was assessed by the Early Treatment Diabetic Retinopathy Study (ETDRS) chart.
The mean change in BCVA from baseline to 1-year follow-up was increased by around 11 letters with aflibercept treatment regardless of which quartile of hemoglobin A1c patients fit into at study entry. In contrast, patients treated with laser therapy appeared to have lessening benefit from treatment as baseline HbA1c increased, with an improvement of 4.1 letters in the first to –0.3 letters in the fourth HbA1c quartiles.
Aflibercept-treated patients also experienced a significant decrease in central retinal thickness (CRT) compared with laser therapy. The mean change in CRT from baseline to 1 year was –201 micrometers in aflibercept-treated patients with a baseline HbA1c of between 4.5% and less than 6.5% (quartile 1), and –195, –196, and –188 micrometers in patients with HbA1c levels of 6.7% to less than 7.4% (quartile 2), 7.4% to less than 8.6% (quartile 3), and 8.6% to 14.7% (quartile 4). Corresponding values for laser therapy were –102, –83, –69, and –43 micometers.
“The findings show that improvements achieved with intravitreal aflibercept over laser in anatomical and functional outcomes – best-corrected visual acuity and central retinal thickness – were robust and similar in patients even with variable systemic disease control at baseline,” said Dr. Oliver Zeitz of Bayer HealthCare, which sponsored the study and markets aflibercept (Eylea) in conjunction with Regeneron Pharmaceuticals.
“Furthermore, we could see in these trials, in the overall population, we could observe improvement in diabetic retinopathy severity scale, which may indicate that aflibercept is not only efficacious on treating DME but also has a potentially beneficial effect on the underlying diabetic retinopathy,” Dr. Zeitz added when presenting the findings at the annual meeting of the European Association for the Study of Diabetes.
VIVID-DME and VISTA-DME are two similarly designed, ongoing studies that are evaluating the efficacy and safety of intravitreal aflibercept injection for the treatment of DME versus laser photocoagulation. A total of 872 patients were randomized in both trials to receive aflibercept 2 mg every 4 or 8 weeks plus a sham laser treatment, or to macular laser photocoagulation plus sham intravitreal therapy.
Aflibercept is a vascular endothelial growth factor (VEGF) inhibitor especially formulated for injection into the eye. The rationale for using the drug is that it helps stop the growth of new blood vessels that will obscure visions if left unchecked. It also decreases vascular permeability in the eye by blocking VEGF-A and placental growth factor.
Intravitreal aflibercept is approved in the United States and the European Union and several other countries for the management of wet age-related macular degeneration and for macular edema after central retinal vein occlusion. The drug has also just received FDA approval for the treatment of DME, according to a Regeneron statement.
Safety is, of course, an important consideration, Dr. Zeitz said, adding that there was a similar overall incidence of ocular and nonocular adverse events. There was also no difference in ocular serious adverse events, including arterial thromboembolic events as defined by the Anti-Platelet Trialists’ Collaboration. There were no cases of endophthalmitis reported.
Dr. Zeitz and his coauthors are employees of Bayer HealthCare, which sponsored the VISTA- and VIVID-DME studies.
VIENNA – The beneficial effects of aflibercept on diabetic eye disease are independent of how well blood glucose is being controlled at the start of treatment, according to a post hoc analysis of two randomized, phase III studies.
Intravitreal injection of 2 mg of aflibercept every month or every other month had similar effects on the primary endpoint of best-corrected visual acuity (BCVA) in more than 800 subjects with diabetic macular edema (DME) with central involvement. BCVA was assessed by the Early Treatment Diabetic Retinopathy Study (ETDRS) chart.
The mean change in BCVA from baseline to 1-year follow-up was increased by around 11 letters with aflibercept treatment regardless of which quartile of hemoglobin A1c patients fit into at study entry. In contrast, patients treated with laser therapy appeared to have lessening benefit from treatment as baseline HbA1c increased, with an improvement of 4.1 letters in the first to –0.3 letters in the fourth HbA1c quartiles.
Aflibercept-treated patients also experienced a significant decrease in central retinal thickness (CRT) compared with laser therapy. The mean change in CRT from baseline to 1 year was –201 micrometers in aflibercept-treated patients with a baseline HbA1c of between 4.5% and less than 6.5% (quartile 1), and –195, –196, and –188 micrometers in patients with HbA1c levels of 6.7% to less than 7.4% (quartile 2), 7.4% to less than 8.6% (quartile 3), and 8.6% to 14.7% (quartile 4). Corresponding values for laser therapy were –102, –83, –69, and –43 micometers.
“The findings show that improvements achieved with intravitreal aflibercept over laser in anatomical and functional outcomes – best-corrected visual acuity and central retinal thickness – were robust and similar in patients even with variable systemic disease control at baseline,” said Dr. Oliver Zeitz of Bayer HealthCare, which sponsored the study and markets aflibercept (Eylea) in conjunction with Regeneron Pharmaceuticals.
“Furthermore, we could see in these trials, in the overall population, we could observe improvement in diabetic retinopathy severity scale, which may indicate that aflibercept is not only efficacious on treating DME but also has a potentially beneficial effect on the underlying diabetic retinopathy,” Dr. Zeitz added when presenting the findings at the annual meeting of the European Association for the Study of Diabetes.
VIVID-DME and VISTA-DME are two similarly designed, ongoing studies that are evaluating the efficacy and safety of intravitreal aflibercept injection for the treatment of DME versus laser photocoagulation. A total of 872 patients were randomized in both trials to receive aflibercept 2 mg every 4 or 8 weeks plus a sham laser treatment, or to macular laser photocoagulation plus sham intravitreal therapy.
Aflibercept is a vascular endothelial growth factor (VEGF) inhibitor especially formulated for injection into the eye. The rationale for using the drug is that it helps stop the growth of new blood vessels that will obscure visions if left unchecked. It also decreases vascular permeability in the eye by blocking VEGF-A and placental growth factor.
Intravitreal aflibercept is approved in the United States and the European Union and several other countries for the management of wet age-related macular degeneration and for macular edema after central retinal vein occlusion. The drug has also just received FDA approval for the treatment of DME, according to a Regeneron statement.
Safety is, of course, an important consideration, Dr. Zeitz said, adding that there was a similar overall incidence of ocular and nonocular adverse events. There was also no difference in ocular serious adverse events, including arterial thromboembolic events as defined by the Anti-Platelet Trialists’ Collaboration. There were no cases of endophthalmitis reported.
Dr. Zeitz and his coauthors are employees of Bayer HealthCare, which sponsored the VISTA- and VIVID-DME studies.
VIENNA – The beneficial effects of aflibercept on diabetic eye disease are independent of how well blood glucose is being controlled at the start of treatment, according to a post hoc analysis of two randomized, phase III studies.
Intravitreal injection of 2 mg of aflibercept every month or every other month had similar effects on the primary endpoint of best-corrected visual acuity (BCVA) in more than 800 subjects with diabetic macular edema (DME) with central involvement. BCVA was assessed by the Early Treatment Diabetic Retinopathy Study (ETDRS) chart.
The mean change in BCVA from baseline to 1-year follow-up was increased by around 11 letters with aflibercept treatment regardless of which quartile of hemoglobin A1c patients fit into at study entry. In contrast, patients treated with laser therapy appeared to have lessening benefit from treatment as baseline HbA1c increased, with an improvement of 4.1 letters in the first to –0.3 letters in the fourth HbA1c quartiles.
Aflibercept-treated patients also experienced a significant decrease in central retinal thickness (CRT) compared with laser therapy. The mean change in CRT from baseline to 1 year was –201 micrometers in aflibercept-treated patients with a baseline HbA1c of between 4.5% and less than 6.5% (quartile 1), and –195, –196, and –188 micrometers in patients with HbA1c levels of 6.7% to less than 7.4% (quartile 2), 7.4% to less than 8.6% (quartile 3), and 8.6% to 14.7% (quartile 4). Corresponding values for laser therapy were –102, –83, –69, and –43 micometers.
“The findings show that improvements achieved with intravitreal aflibercept over laser in anatomical and functional outcomes – best-corrected visual acuity and central retinal thickness – were robust and similar in patients even with variable systemic disease control at baseline,” said Dr. Oliver Zeitz of Bayer HealthCare, which sponsored the study and markets aflibercept (Eylea) in conjunction with Regeneron Pharmaceuticals.
“Furthermore, we could see in these trials, in the overall population, we could observe improvement in diabetic retinopathy severity scale, which may indicate that aflibercept is not only efficacious on treating DME but also has a potentially beneficial effect on the underlying diabetic retinopathy,” Dr. Zeitz added when presenting the findings at the annual meeting of the European Association for the Study of Diabetes.
VIVID-DME and VISTA-DME are two similarly designed, ongoing studies that are evaluating the efficacy and safety of intravitreal aflibercept injection for the treatment of DME versus laser photocoagulation. A total of 872 patients were randomized in both trials to receive aflibercept 2 mg every 4 or 8 weeks plus a sham laser treatment, or to macular laser photocoagulation plus sham intravitreal therapy.
Aflibercept is a vascular endothelial growth factor (VEGF) inhibitor especially formulated for injection into the eye. The rationale for using the drug is that it helps stop the growth of new blood vessels that will obscure visions if left unchecked. It also decreases vascular permeability in the eye by blocking VEGF-A and placental growth factor.
Intravitreal aflibercept is approved in the United States and the European Union and several other countries for the management of wet age-related macular degeneration and for macular edema after central retinal vein occlusion. The drug has also just received FDA approval for the treatment of DME, according to a Regeneron statement.
Safety is, of course, an important consideration, Dr. Zeitz said, adding that there was a similar overall incidence of ocular and nonocular adverse events. There was also no difference in ocular serious adverse events, including arterial thromboembolic events as defined by the Anti-Platelet Trialists’ Collaboration. There were no cases of endophthalmitis reported.
Dr. Zeitz and his coauthors are employees of Bayer HealthCare, which sponsored the VISTA- and VIVID-DME studies.
AT EASD 2014
Key clinical point: Intravitreal aflibercept improves anatomical and functional outcomes in patients with diabetic retinopathy regardless of how well their blood glucose is being controlled at the start of treatment.
Major finding: The mean changes in best-corrected visual acuity were 11.7, 11.9, 11.7, and 11.1 for aflibercept and 4.1, 1.9, 0.8 and –0.3 for laser-treated patients comparing the lowest to highest quartiles of baseline HbA1c.
Data source: Post hoc analysis of the VIVID-DME and VISTA-DME phase III studies involving more than 850 patients with diabetic macular edema treated with intravitreal aflibercept or laser photocoagulation.
Disclosures: Dr. Zeitz and his coauthors are employees of Bayer HealthCare, which funded the study.
1% Jump in Glucose Yields 25% Jump in Cardiovascular Risk
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
AT EASD 2014
1% jump in glucose yields 25% jump in cardiovascular risk
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
On Twitter @alz_gal
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
On Twitter @alz_gal
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
On Twitter @alz_gal
AT EASD 2014
Key clinical point: The risk of a major cardiovascular event increases as blood glucose rises.
Major finding: Every 1% increase in HbA1c conferred a 25% increase in the risk of a major cardiovascular event.
Data source: A 4-year, prospective, noninterventional study involving 2,999 patients.
Disclosures: CREDIT was sponsored by Sanofi. Dr. Freemantle has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
VIDEO: Gradual HbA1c reduction safely benefits T2DM
VIENNA – A “pragmatic and simple” approach to gradually lower the hemoglobin A1c level in patients with type 2 diabetes to 6.5% and then maintain it for an average of 5 years caused no suggestion of harm and led to an important halving of end-stage renal disease during 10-year follow-up in a controlled study with more than 8,000 patients, Dr. Sophia Zoungas said in an interview at the annual meeting of the European Association for the Study of Diabetes.
Intensive glucose control did not increase mortality or the rate of major macrovascular events in 10-year results from the ADVANCE ON (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation Post Trial Observational Study) trial. The results also showed for the first time that intensive glucose control produced a significant and large reduction in end-stage kidney disease, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck Sharp & Dohme, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIENNA – A “pragmatic and simple” approach to gradually lower the hemoglobin A1c level in patients with type 2 diabetes to 6.5% and then maintain it for an average of 5 years caused no suggestion of harm and led to an important halving of end-stage renal disease during 10-year follow-up in a controlled study with more than 8,000 patients, Dr. Sophia Zoungas said in an interview at the annual meeting of the European Association for the Study of Diabetes.
Intensive glucose control did not increase mortality or the rate of major macrovascular events in 10-year results from the ADVANCE ON (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation Post Trial Observational Study) trial. The results also showed for the first time that intensive glucose control produced a significant and large reduction in end-stage kidney disease, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck Sharp & Dohme, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIENNA – A “pragmatic and simple” approach to gradually lower the hemoglobin A1c level in patients with type 2 diabetes to 6.5% and then maintain it for an average of 5 years caused no suggestion of harm and led to an important halving of end-stage renal disease during 10-year follow-up in a controlled study with more than 8,000 patients, Dr. Sophia Zoungas said in an interview at the annual meeting of the European Association for the Study of Diabetes.
Intensive glucose control did not increase mortality or the rate of major macrovascular events in 10-year results from the ADVANCE ON (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation Post Trial Observational Study) trial. The results also showed for the first time that intensive glucose control produced a significant and large reduction in end-stage kidney disease, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck Sharp & Dohme, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT EASD 2014
Long-term insulin use had no heart effects in ORIGINALE
VIENNA – There is no increased risk for myocardial infarction or other vascular events from prolonged use of insulin in people with pre- or type 2 diabetes who are already at high risk for cardiovascular disease, according to additional follow-up data from the ORIGIN study.
The ORIGINALE (Origin and Legacy Effects) study provides an additional 2.5 years of observational follow-up of more than 8,000 of the 12,000-plus individuals who participated in the ORIGIN (Outcome Reduction with an Initial Glargine Intervention) study.
The results of the ORIGIN trial were that 6.2 years of treatment with insulin glargine (Sanofi’s Lantus) had a neutral effect on cardiovascular events and other serious health outcomes such as cancer, and possibly slowed diabetes progression (N. Engl. J. Med. 2012;367:319-28). In addition, omega-3 fatty acid supplementation was found to offer no benefit over placebo.
The results of the ORIGINALE study continue to support these findings, Dr. Hertzel C. Gerstein reported at the annual meeting of the European Society for the Study of Diabetes.
The key conclusion of ORIGIN and ORIGINALE with regard to insulin use is that “targeting a normal fasting plasma glucose with basal insulin glargine for more than 6 years improves metabolic status and has a neutral effect on serious health outcomes,” Dr. Gerstein of McMaster University, Hamilton, Ont., observed.
Two coprimary composite cardiovascular outcomes were assessed, neither of which showed any advantage or disadvantage of using insulin glargine versus standard care.
The hazard ratio for the first coprimary outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke in ORIGINALE was 1.01, and the HR for the second coprimary endpoint of any cardiovascular event, a revascularization procedure, or hospitalization for heart failure was 1.03.
There was also a “neutral effect” on the individual components of these composite endpoints, with HRs of 1.02, 1.05, 1.00, 0.90, and 1.04 for myocardial infarction, stroke, cardiovascular death, heart failure hospitalization, and revascularization, respectively.
Median hemoglobin A1c values at the end of ORIGIN and at the end of ORIGINALE were 6.3% and 6.6%, respectively, in the 2,351 patients treated with insulin glargine, and 6.52% and 6.70% in the 3,267 patients in the standard-care arm.
Likewise, analysis showed a neutral effect on cancer incidence and cancer death between the insulin and standard-care groups.
There was a trend toward fewer new cases of diabetes among insulin-treated patients than in the standard-care group (37.7% vs. 41.7%; P = .12), with fewer new and possible diabetes cases if insulin was received (41.2% vs. 47.7%; P = .014). The latter included patients who could not be confirmed as having diabetes by adjudication criteria.
In an interview, Dr. Gerstein commented that the findings are reassuring for people at high cardiovascular risk with impaired fasting glucose, impaired glucose tolerance, or early type 2 diabetes who are in need of insulin therapy.
“Insulin is a safe and effective drug, we can use it to lower patients’ glucose levels, and it’s not going to do anything else,” he said. “We have to make sure we use it correctly to avoid hypoglycemia, but there are no hidden perils, at least based on what we know now, and close to 10 years of follow-up.”
Regarding the value of omega-3 fatty acid supplementation, also studied in ORIGIN, the results of ORIGINALE showed no effect on any outcome. “All of the evidence around the world shows that if you take an omega-3 fatty acid capsule, you have no difference in your health outcomes than if you do not,” he said. “All you do is create expensive urine. You do not do anything else with respect to your health.”
Sanofi provided funding for the study, which was coordinated by the Population Health Research Institute in Hamilton. Dr. Gerstein has received research support and adviser fees from Sanofi, Novartis, and other companies.
VIENNA – There is no increased risk for myocardial infarction or other vascular events from prolonged use of insulin in people with pre- or type 2 diabetes who are already at high risk for cardiovascular disease, according to additional follow-up data from the ORIGIN study.
The ORIGINALE (Origin and Legacy Effects) study provides an additional 2.5 years of observational follow-up of more than 8,000 of the 12,000-plus individuals who participated in the ORIGIN (Outcome Reduction with an Initial Glargine Intervention) study.
The results of the ORIGIN trial were that 6.2 years of treatment with insulin glargine (Sanofi’s Lantus) had a neutral effect on cardiovascular events and other serious health outcomes such as cancer, and possibly slowed diabetes progression (N. Engl. J. Med. 2012;367:319-28). In addition, omega-3 fatty acid supplementation was found to offer no benefit over placebo.
The results of the ORIGINALE study continue to support these findings, Dr. Hertzel C. Gerstein reported at the annual meeting of the European Society for the Study of Diabetes.
The key conclusion of ORIGIN and ORIGINALE with regard to insulin use is that “targeting a normal fasting plasma glucose with basal insulin glargine for more than 6 years improves metabolic status and has a neutral effect on serious health outcomes,” Dr. Gerstein of McMaster University, Hamilton, Ont., observed.
Two coprimary composite cardiovascular outcomes were assessed, neither of which showed any advantage or disadvantage of using insulin glargine versus standard care.
The hazard ratio for the first coprimary outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke in ORIGINALE was 1.01, and the HR for the second coprimary endpoint of any cardiovascular event, a revascularization procedure, or hospitalization for heart failure was 1.03.
There was also a “neutral effect” on the individual components of these composite endpoints, with HRs of 1.02, 1.05, 1.00, 0.90, and 1.04 for myocardial infarction, stroke, cardiovascular death, heart failure hospitalization, and revascularization, respectively.
Median hemoglobin A1c values at the end of ORIGIN and at the end of ORIGINALE were 6.3% and 6.6%, respectively, in the 2,351 patients treated with insulin glargine, and 6.52% and 6.70% in the 3,267 patients in the standard-care arm.
Likewise, analysis showed a neutral effect on cancer incidence and cancer death between the insulin and standard-care groups.
There was a trend toward fewer new cases of diabetes among insulin-treated patients than in the standard-care group (37.7% vs. 41.7%; P = .12), with fewer new and possible diabetes cases if insulin was received (41.2% vs. 47.7%; P = .014). The latter included patients who could not be confirmed as having diabetes by adjudication criteria.
In an interview, Dr. Gerstein commented that the findings are reassuring for people at high cardiovascular risk with impaired fasting glucose, impaired glucose tolerance, or early type 2 diabetes who are in need of insulin therapy.
“Insulin is a safe and effective drug, we can use it to lower patients’ glucose levels, and it’s not going to do anything else,” he said. “We have to make sure we use it correctly to avoid hypoglycemia, but there are no hidden perils, at least based on what we know now, and close to 10 years of follow-up.”
Regarding the value of omega-3 fatty acid supplementation, also studied in ORIGIN, the results of ORIGINALE showed no effect on any outcome. “All of the evidence around the world shows that if you take an omega-3 fatty acid capsule, you have no difference in your health outcomes than if you do not,” he said. “All you do is create expensive urine. You do not do anything else with respect to your health.”
Sanofi provided funding for the study, which was coordinated by the Population Health Research Institute in Hamilton. Dr. Gerstein has received research support and adviser fees from Sanofi, Novartis, and other companies.
VIENNA – There is no increased risk for myocardial infarction or other vascular events from prolonged use of insulin in people with pre- or type 2 diabetes who are already at high risk for cardiovascular disease, according to additional follow-up data from the ORIGIN study.
The ORIGINALE (Origin and Legacy Effects) study provides an additional 2.5 years of observational follow-up of more than 8,000 of the 12,000-plus individuals who participated in the ORIGIN (Outcome Reduction with an Initial Glargine Intervention) study.
The results of the ORIGIN trial were that 6.2 years of treatment with insulin glargine (Sanofi’s Lantus) had a neutral effect on cardiovascular events and other serious health outcomes such as cancer, and possibly slowed diabetes progression (N. Engl. J. Med. 2012;367:319-28). In addition, omega-3 fatty acid supplementation was found to offer no benefit over placebo.
The results of the ORIGINALE study continue to support these findings, Dr. Hertzel C. Gerstein reported at the annual meeting of the European Society for the Study of Diabetes.
The key conclusion of ORIGIN and ORIGINALE with regard to insulin use is that “targeting a normal fasting plasma glucose with basal insulin glargine for more than 6 years improves metabolic status and has a neutral effect on serious health outcomes,” Dr. Gerstein of McMaster University, Hamilton, Ont., observed.
Two coprimary composite cardiovascular outcomes were assessed, neither of which showed any advantage or disadvantage of using insulin glargine versus standard care.
The hazard ratio for the first coprimary outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke in ORIGINALE was 1.01, and the HR for the second coprimary endpoint of any cardiovascular event, a revascularization procedure, or hospitalization for heart failure was 1.03.
There was also a “neutral effect” on the individual components of these composite endpoints, with HRs of 1.02, 1.05, 1.00, 0.90, and 1.04 for myocardial infarction, stroke, cardiovascular death, heart failure hospitalization, and revascularization, respectively.
Median hemoglobin A1c values at the end of ORIGIN and at the end of ORIGINALE were 6.3% and 6.6%, respectively, in the 2,351 patients treated with insulin glargine, and 6.52% and 6.70% in the 3,267 patients in the standard-care arm.
Likewise, analysis showed a neutral effect on cancer incidence and cancer death between the insulin and standard-care groups.
There was a trend toward fewer new cases of diabetes among insulin-treated patients than in the standard-care group (37.7% vs. 41.7%; P = .12), with fewer new and possible diabetes cases if insulin was received (41.2% vs. 47.7%; P = .014). The latter included patients who could not be confirmed as having diabetes by adjudication criteria.
In an interview, Dr. Gerstein commented that the findings are reassuring for people at high cardiovascular risk with impaired fasting glucose, impaired glucose tolerance, or early type 2 diabetes who are in need of insulin therapy.
“Insulin is a safe and effective drug, we can use it to lower patients’ glucose levels, and it’s not going to do anything else,” he said. “We have to make sure we use it correctly to avoid hypoglycemia, but there are no hidden perils, at least based on what we know now, and close to 10 years of follow-up.”
Regarding the value of omega-3 fatty acid supplementation, also studied in ORIGIN, the results of ORIGINALE showed no effect on any outcome. “All of the evidence around the world shows that if you take an omega-3 fatty acid capsule, you have no difference in your health outcomes than if you do not,” he said. “All you do is create expensive urine. You do not do anything else with respect to your health.”
Sanofi provided funding for the study, which was coordinated by the Population Health Research Institute in Hamilton. Dr. Gerstein has received research support and adviser fees from Sanofi, Novartis, and other companies.
AT EASD 2014
Key clinical point: People at high risk for cardiovascular disease with dysglycemia or type 2 diabetes can be reassured about the long-term safety of insulin use.
Major finding: The hazard ratio for the first coprimary composite cardiovascular endpoint was 1.01 in both the insulin glargine group the standard-care group, and the HR for the second coprimary endpoint was 1.03 in both groups.
Data source: The ORIGINALE study was a 2.5-year follow-up to the 6-year ORIGIN trial comparing insulin glargine versus standard care, and omega-3 fatty acid supplementation versus placebo, in more than 8,000 subjects with prediabetes or type 2 diabetes at risk of cardiovascular disease.
Disclosures: Sanofi provided funding for the study, which was coordinated by the Population Health Research Institute in Hamilton. Dr. Gerstein has received research support and adviser fees from Sanofi, Novartis, and other companies.
Novel insulin analogue combo misses noninferiority endpoint
VIENNA – A novel fixed-dose insulin analogue combination reduced blood glucose levels to a similar degree as a control basal-bolus regimen in patients with type 2 diabetes but just failed to show noninferiority for the primary endpoint in a phase IIIb randomized trial.
The percentage change in hemoglobin A1c from baseline to week 26 was –1.3% for IDegAsp (Ryzodeg, Novo Nordisk) and –1.5% for the basal-bolus regimen used as the comparator.
IDegAsp is a soluble combination of 70% insulin degludec (Tresiba, Novo Nordisk) and 30% insulin aspart (NovoLog, Novo Nordisk). Unlike other fixed-dose insulins, IDeg and IAsp exist separately in a coformulation.
Although noninferiority was not demonstrated for glycemic control, there was a significant benefit in terms of a lower dose of insulin used (1.11 vs. 1.34 U) and less weight gain, of about 1 kg, with IDegAsp than with the basal-bolus approach. There were also numerically lower rates of confirmed (rate ratio, 0.81) and nocturnal hypoglycemia (RR, 0.80). Both regimens were well tolerated.
“If we are very, very, strict, and as we did not reach noninferiority, we can’t really say anything about anything else,” study investigator Dr. John Cooper of Stavanger (Norway) University Hospital conceded in an interview at the annual meeting of the European Association for the Study of Diabetes. He added, however, “common sense says that it is very close and it looks like it is a useful insulin.”
Patients with type 2 diabetes of at least 6 months duration and who had an HbA1c of 7%-10% despite being treated with basal insulin with or without oral antidiabetic drugs for at least 3 months were eligible for inclusion in the study.
In all, 274 patients were included, with 138 randomized to twice-daily treatment with IDegAsp and 136 to IDeg once daily plus IAsp two to four times a day. The mean age of the participants was 60 years, the mean HbA1c at baseline was 8.3%, and the average duration of diabetes was 13 years. The majority (63%) of patients had been treated with insulin glargine prior to study entry.
“IDegAsp twice daily may represent a simple alternative to basal-bolus treatment in patients who require intensification of basal insulin regimens, especially when adherence to more complex regimens is challenging,” Dr. Cooper said at the conclusion of his presentation.
He noted during the discussion that quality of life had been assessed using the Short-Form 36 at the start and end of the trial, and while there were no differences between the regimens in terms of the physical scores, there was a benefit seen in the mental component with IDegAsp that was driven mainly by improvements in social functioning.
“I’ve mainly used basal plus meal-time insulin doses in type 2 diabetes patients, but I might reconsider using the combination, but that’s more on the basis that I know it gives less hypoglycemia, compared with the older, premixed insulins,” Dr. Cooper commented in the postpresentation interview.
The novel insulin formulation could also offer patients who need treatment intensification a more convenient approach to their insulin therapy, he said, rather than having to inject long-acting insulin once daily and short-acting insulin up to four times a day. With IDegAsp, patients could inject just twice a day at major meal times. “This will certainly be a useful addition for some patients,” Dr. Cooper suggested.
To date, IDegAsp has been approved for use in several European, Asian, and South American countries. The Food and Drug Administration, however, has requested additional cardiovascular outcomes data to be obtained before giving market authorization for its use.
The study was funded by Novo Nordisk. Dr. Cooper has received research support and speaker fees from Novo Nordisk, Sanofi, Eli Lilly and Company, Merck, and AstraZeneca, and is a member of a speakers bureau for Novartis.
VIENNA – A novel fixed-dose insulin analogue combination reduced blood glucose levels to a similar degree as a control basal-bolus regimen in patients with type 2 diabetes but just failed to show noninferiority for the primary endpoint in a phase IIIb randomized trial.
The percentage change in hemoglobin A1c from baseline to week 26 was –1.3% for IDegAsp (Ryzodeg, Novo Nordisk) and –1.5% for the basal-bolus regimen used as the comparator.
IDegAsp is a soluble combination of 70% insulin degludec (Tresiba, Novo Nordisk) and 30% insulin aspart (NovoLog, Novo Nordisk). Unlike other fixed-dose insulins, IDeg and IAsp exist separately in a coformulation.
Although noninferiority was not demonstrated for glycemic control, there was a significant benefit in terms of a lower dose of insulin used (1.11 vs. 1.34 U) and less weight gain, of about 1 kg, with IDegAsp than with the basal-bolus approach. There were also numerically lower rates of confirmed (rate ratio, 0.81) and nocturnal hypoglycemia (RR, 0.80). Both regimens were well tolerated.
“If we are very, very, strict, and as we did not reach noninferiority, we can’t really say anything about anything else,” study investigator Dr. John Cooper of Stavanger (Norway) University Hospital conceded in an interview at the annual meeting of the European Association for the Study of Diabetes. He added, however, “common sense says that it is very close and it looks like it is a useful insulin.”
Patients with type 2 diabetes of at least 6 months duration and who had an HbA1c of 7%-10% despite being treated with basal insulin with or without oral antidiabetic drugs for at least 3 months were eligible for inclusion in the study.
In all, 274 patients were included, with 138 randomized to twice-daily treatment with IDegAsp and 136 to IDeg once daily plus IAsp two to four times a day. The mean age of the participants was 60 years, the mean HbA1c at baseline was 8.3%, and the average duration of diabetes was 13 years. The majority (63%) of patients had been treated with insulin glargine prior to study entry.
“IDegAsp twice daily may represent a simple alternative to basal-bolus treatment in patients who require intensification of basal insulin regimens, especially when adherence to more complex regimens is challenging,” Dr. Cooper said at the conclusion of his presentation.
He noted during the discussion that quality of life had been assessed using the Short-Form 36 at the start and end of the trial, and while there were no differences between the regimens in terms of the physical scores, there was a benefit seen in the mental component with IDegAsp that was driven mainly by improvements in social functioning.
“I’ve mainly used basal plus meal-time insulin doses in type 2 diabetes patients, but I might reconsider using the combination, but that’s more on the basis that I know it gives less hypoglycemia, compared with the older, premixed insulins,” Dr. Cooper commented in the postpresentation interview.
The novel insulin formulation could also offer patients who need treatment intensification a more convenient approach to their insulin therapy, he said, rather than having to inject long-acting insulin once daily and short-acting insulin up to four times a day. With IDegAsp, patients could inject just twice a day at major meal times. “This will certainly be a useful addition for some patients,” Dr. Cooper suggested.
To date, IDegAsp has been approved for use in several European, Asian, and South American countries. The Food and Drug Administration, however, has requested additional cardiovascular outcomes data to be obtained before giving market authorization for its use.
The study was funded by Novo Nordisk. Dr. Cooper has received research support and speaker fees from Novo Nordisk, Sanofi, Eli Lilly and Company, Merck, and AstraZeneca, and is a member of a speakers bureau for Novartis.
VIENNA – A novel fixed-dose insulin analogue combination reduced blood glucose levels to a similar degree as a control basal-bolus regimen in patients with type 2 diabetes but just failed to show noninferiority for the primary endpoint in a phase IIIb randomized trial.
The percentage change in hemoglobin A1c from baseline to week 26 was –1.3% for IDegAsp (Ryzodeg, Novo Nordisk) and –1.5% for the basal-bolus regimen used as the comparator.
IDegAsp is a soluble combination of 70% insulin degludec (Tresiba, Novo Nordisk) and 30% insulin aspart (NovoLog, Novo Nordisk). Unlike other fixed-dose insulins, IDeg and IAsp exist separately in a coformulation.
Although noninferiority was not demonstrated for glycemic control, there was a significant benefit in terms of a lower dose of insulin used (1.11 vs. 1.34 U) and less weight gain, of about 1 kg, with IDegAsp than with the basal-bolus approach. There were also numerically lower rates of confirmed (rate ratio, 0.81) and nocturnal hypoglycemia (RR, 0.80). Both regimens were well tolerated.
“If we are very, very, strict, and as we did not reach noninferiority, we can’t really say anything about anything else,” study investigator Dr. John Cooper of Stavanger (Norway) University Hospital conceded in an interview at the annual meeting of the European Association for the Study of Diabetes. He added, however, “common sense says that it is very close and it looks like it is a useful insulin.”
Patients with type 2 diabetes of at least 6 months duration and who had an HbA1c of 7%-10% despite being treated with basal insulin with or without oral antidiabetic drugs for at least 3 months were eligible for inclusion in the study.
In all, 274 patients were included, with 138 randomized to twice-daily treatment with IDegAsp and 136 to IDeg once daily plus IAsp two to four times a day. The mean age of the participants was 60 years, the mean HbA1c at baseline was 8.3%, and the average duration of diabetes was 13 years. The majority (63%) of patients had been treated with insulin glargine prior to study entry.
“IDegAsp twice daily may represent a simple alternative to basal-bolus treatment in patients who require intensification of basal insulin regimens, especially when adherence to more complex regimens is challenging,” Dr. Cooper said at the conclusion of his presentation.
He noted during the discussion that quality of life had been assessed using the Short-Form 36 at the start and end of the trial, and while there were no differences between the regimens in terms of the physical scores, there was a benefit seen in the mental component with IDegAsp that was driven mainly by improvements in social functioning.
“I’ve mainly used basal plus meal-time insulin doses in type 2 diabetes patients, but I might reconsider using the combination, but that’s more on the basis that I know it gives less hypoglycemia, compared with the older, premixed insulins,” Dr. Cooper commented in the postpresentation interview.
The novel insulin formulation could also offer patients who need treatment intensification a more convenient approach to their insulin therapy, he said, rather than having to inject long-acting insulin once daily and short-acting insulin up to four times a day. With IDegAsp, patients could inject just twice a day at major meal times. “This will certainly be a useful addition for some patients,” Dr. Cooper suggested.
To date, IDegAsp has been approved for use in several European, Asian, and South American countries. The Food and Drug Administration, however, has requested additional cardiovascular outcomes data to be obtained before giving market authorization for its use.
The study was funded by Novo Nordisk. Dr. Cooper has received research support and speaker fees from Novo Nordisk, Sanofi, Eli Lilly and Company, Merck, and AstraZeneca, and is a member of a speakers bureau for Novartis.
AT EASD 2014
Major finding: The percentage change in HbA1cfrom baseline to week 26 was –1.3% for IDegAsp and –1.5% for the basal-bolus regimen used as the comparator.
Data source: Open-label, 26-week parallel group study of 274 patients with type 2 diabetes.
Disclosures: The study was funded by Novo Nordisk. Dr. Cooper has received research support and speaker fees from Novo Nordisk, Sanofi, Eli Lilly, Merck, and AstraZeneca, and is a member of a speaker’s bureau for Novartis.
Autism risks increase after in utero antidiabetic medication exposure
VIENNA – Children whose mothers had gestational diabetes and required antidiabetic medications may face a 22% increased risk of developing an autism spectrum disorder.
About a third of that increase was significantly associated with very-early diabetes diagnosis and treatment, suggesting that early first trimester hyperglycemia may affect fetal brain development, Anny H. Xiang, Ph.D., said at the annual meeting of the European Association for the Study of Diabetes.
“My speculation is that, since fetal brain development is critical in the first and early second trimesters, exposure to high glucose during that time might damage its development,” said Dr Xiang, a senior research scientist at the Kaiser Permanente Department of Research and Evaluation in Pasadena, Calif. “Later exposure, perhaps, is not as problematic,” for the developing brain.
Her longitudinal cohort study included 315,827 singleton infants who were born at 28-44 weeks’ gestation between 1995 and 2009. This included 290,792 who were not exposed to gestational diabetes (GDM), and 25,035 who were. Of these, 5,891 had been exposed to antidiabetic medications.
All of the mothers were enrolled in Kaiser Permanente Southern California at the time of the birth, and all the children were still in the health plan by ages 1-2 years. Women who had preexisting type 1 or 2 diabetes were excluded form the study.
In accordance with the literature, mothers with GDM had lower educational levels and lower household incomes. They were older and more of them were multiparous. They were more likely to have had preeclampsia or eclampsia with a prior pregnancy. Asians and Pacific Islanders were more likely to have GDM than were other ethnicities.
Women who required antidiabetic treatment were diagnosed a mean of 4 weeks earlier than those who did not (23 vs. 27 weeks). Gestational ages at delivery did not significantly differ between the groups (39 weeks), but the infants of mothers with GDM were significantly heavier than were those of mothers without it. Infants exposed to antidiabetic medications weighed a mean of 3,453 g, compared with 3,380 g in nonmedication exposed infants, and 3,309 g in infants not exposed to GDM.
In the Kaiser system, standard well-baby care includes neurodevelopmental assessments at 1 year, 18 months, 2 years, and annually thereafter. Any concerns prompt a referral to a pediatric specialty clinic. For this study, diagnosis of autism required two diagnostic codes, one of which had to be from such a clinic.
Overall, the incidence rate of an autism spectrum disorder was 1.8/1,000 person-years. It was highest among those who had been exposed to antidiabetic medications (2.75/1,000 person-years) – significantly higher than that of both the GDM nonexposed and the non-GDM groups (1.96 and 1.77 per 1,000 person-years, respectively).
A multivariate analysis adjusted for a number of maternal and fetal factors, including maternal age, parity, race/ethnicity, comorbidity, education, income, prior preeclampsia/eclampsia, the infant’s gender, and gestational age at GDM diagnosis.
In the unadjusted analysis, infants who were exposed to the medications were significantly more likely to develop an autism spectrum disorder than were GDM infants who were not exposed (hazard ratio, 1.45). Significance remained high when each of the other confounding factors were individually considered. The final adjusted analysis, which included all of these, found an increased risk of 22%; however, this just missed statistical significance with a P value of .06.
A second analysis examined only the medication-exposed infants. The unadjusted analysis found a 36% increased risk, compared with nonexposed GDM infants. Again, significance remained strong when the individual confounders were examined separately. But the 26% risk in the fully adjusted model just missed significance – again with P value of.06.
The final analysis was adjusted for all of the confounders, plus gestational age when the mother was diagnosed. The hazard ratio of 1.17 suggested that early diagnosis and treatment explained about one-third of the overall increased risk, Dr. Xiang said.
The study continues with an investigation of the possible effect of maternal obesity on the current findings, she added.
Kaiser Permanente sponsored the study. Dr. Xiang is an employee of the company.
On Twitter @alz_gal
About a third of that increase was significantly associated with very-early diabetes diagnosis and treatment, suggesting that early first trimester hyperglycemia may affect fetal brain development, Anny H. Xiang, Ph.D., said at the annual meeting of the European Association for the Study of Diabetes.
VIENNA – Children whose mothers had gestational diabetes and required antidiabetic medications may face a 22% increased risk of developing an autism spectrum disorder.
About a third of that increase was significantly associated with very-early diabetes diagnosis and treatment, suggesting that early first trimester hyperglycemia may affect fetal brain development, Anny H. Xiang, Ph.D., said at the annual meeting of the European Association for the Study of Diabetes.
“My speculation is that, since fetal brain development is critical in the first and early second trimesters, exposure to high glucose during that time might damage its development,” said Dr Xiang, a senior research scientist at the Kaiser Permanente Department of Research and Evaluation in Pasadena, Calif. “Later exposure, perhaps, is not as problematic,” for the developing brain.
Her longitudinal cohort study included 315,827 singleton infants who were born at 28-44 weeks’ gestation between 1995 and 2009. This included 290,792 who were not exposed to gestational diabetes (GDM), and 25,035 who were. Of these, 5,891 had been exposed to antidiabetic medications.
All of the mothers were enrolled in Kaiser Permanente Southern California at the time of the birth, and all the children were still in the health plan by ages 1-2 years. Women who had preexisting type 1 or 2 diabetes were excluded form the study.
In accordance with the literature, mothers with GDM had lower educational levels and lower household incomes. They were older and more of them were multiparous. They were more likely to have had preeclampsia or eclampsia with a prior pregnancy. Asians and Pacific Islanders were more likely to have GDM than were other ethnicities.
Women who required antidiabetic treatment were diagnosed a mean of 4 weeks earlier than those who did not (23 vs. 27 weeks). Gestational ages at delivery did not significantly differ between the groups (39 weeks), but the infants of mothers with GDM were significantly heavier than were those of mothers without it. Infants exposed to antidiabetic medications weighed a mean of 3,453 g, compared with 3,380 g in nonmedication exposed infants, and 3,309 g in infants not exposed to GDM.
In the Kaiser system, standard well-baby care includes neurodevelopmental assessments at 1 year, 18 months, 2 years, and annually thereafter. Any concerns prompt a referral to a pediatric specialty clinic. For this study, diagnosis of autism required two diagnostic codes, one of which had to be from such a clinic.
Overall, the incidence rate of an autism spectrum disorder was 1.8/1,000 person-years. It was highest among those who had been exposed to antidiabetic medications (2.75/1,000 person-years) – significantly higher than that of both the GDM nonexposed and the non-GDM groups (1.96 and 1.77 per 1,000 person-years, respectively).
A multivariate analysis adjusted for a number of maternal and fetal factors, including maternal age, parity, race/ethnicity, comorbidity, education, income, prior preeclampsia/eclampsia, the infant’s gender, and gestational age at GDM diagnosis.
In the unadjusted analysis, infants who were exposed to the medications were significantly more likely to develop an autism spectrum disorder than were GDM infants who were not exposed (hazard ratio, 1.45). Significance remained high when each of the other confounding factors were individually considered. The final adjusted analysis, which included all of these, found an increased risk of 22%; however, this just missed statistical significance with a P value of .06.
A second analysis examined only the medication-exposed infants. The unadjusted analysis found a 36% increased risk, compared with nonexposed GDM infants. Again, significance remained strong when the individual confounders were examined separately. But the 26% risk in the fully adjusted model just missed significance – again with P value of.06.
The final analysis was adjusted for all of the confounders, plus gestational age when the mother was diagnosed. The hazard ratio of 1.17 suggested that early diagnosis and treatment explained about one-third of the overall increased risk, Dr. Xiang said.
The study continues with an investigation of the possible effect of maternal obesity on the current findings, she added.
Kaiser Permanente sponsored the study. Dr. Xiang is an employee of the company.
On Twitter @alz_gal
VIENNA – Children whose mothers had gestational diabetes and required antidiabetic medications may face a 22% increased risk of developing an autism spectrum disorder.
About a third of that increase was significantly associated with very-early diabetes diagnosis and treatment, suggesting that early first trimester hyperglycemia may affect fetal brain development, Anny H. Xiang, Ph.D., said at the annual meeting of the European Association for the Study of Diabetes.
“My speculation is that, since fetal brain development is critical in the first and early second trimesters, exposure to high glucose during that time might damage its development,” said Dr Xiang, a senior research scientist at the Kaiser Permanente Department of Research and Evaluation in Pasadena, Calif. “Later exposure, perhaps, is not as problematic,” for the developing brain.
Her longitudinal cohort study included 315,827 singleton infants who were born at 28-44 weeks’ gestation between 1995 and 2009. This included 290,792 who were not exposed to gestational diabetes (GDM), and 25,035 who were. Of these, 5,891 had been exposed to antidiabetic medications.
All of the mothers were enrolled in Kaiser Permanente Southern California at the time of the birth, and all the children were still in the health plan by ages 1-2 years. Women who had preexisting type 1 or 2 diabetes were excluded form the study.
In accordance with the literature, mothers with GDM had lower educational levels and lower household incomes. They were older and more of them were multiparous. They were more likely to have had preeclampsia or eclampsia with a prior pregnancy. Asians and Pacific Islanders were more likely to have GDM than were other ethnicities.
Women who required antidiabetic treatment were diagnosed a mean of 4 weeks earlier than those who did not (23 vs. 27 weeks). Gestational ages at delivery did not significantly differ between the groups (39 weeks), but the infants of mothers with GDM were significantly heavier than were those of mothers without it. Infants exposed to antidiabetic medications weighed a mean of 3,453 g, compared with 3,380 g in nonmedication exposed infants, and 3,309 g in infants not exposed to GDM.
In the Kaiser system, standard well-baby care includes neurodevelopmental assessments at 1 year, 18 months, 2 years, and annually thereafter. Any concerns prompt a referral to a pediatric specialty clinic. For this study, diagnosis of autism required two diagnostic codes, one of which had to be from such a clinic.
Overall, the incidence rate of an autism spectrum disorder was 1.8/1,000 person-years. It was highest among those who had been exposed to antidiabetic medications (2.75/1,000 person-years) – significantly higher than that of both the GDM nonexposed and the non-GDM groups (1.96 and 1.77 per 1,000 person-years, respectively).
A multivariate analysis adjusted for a number of maternal and fetal factors, including maternal age, parity, race/ethnicity, comorbidity, education, income, prior preeclampsia/eclampsia, the infant’s gender, and gestational age at GDM diagnosis.
In the unadjusted analysis, infants who were exposed to the medications were significantly more likely to develop an autism spectrum disorder than were GDM infants who were not exposed (hazard ratio, 1.45). Significance remained high when each of the other confounding factors were individually considered. The final adjusted analysis, which included all of these, found an increased risk of 22%; however, this just missed statistical significance with a P value of .06.
A second analysis examined only the medication-exposed infants. The unadjusted analysis found a 36% increased risk, compared with nonexposed GDM infants. Again, significance remained strong when the individual confounders were examined separately. But the 26% risk in the fully adjusted model just missed significance – again with P value of.06.
The final analysis was adjusted for all of the confounders, plus gestational age when the mother was diagnosed. The hazard ratio of 1.17 suggested that early diagnosis and treatment explained about one-third of the overall increased risk, Dr. Xiang said.
The study continues with an investigation of the possible effect of maternal obesity on the current findings, she added.
Kaiser Permanente sponsored the study. Dr. Xiang is an employee of the company.
On Twitter @alz_gal
About a third of that increase was significantly associated with very-early diabetes diagnosis and treatment, suggesting that early first trimester hyperglycemia may affect fetal brain development, Anny H. Xiang, Ph.D., said at the annual meeting of the European Association for the Study of Diabetes.
About a third of that increase was significantly associated with very-early diabetes diagnosis and treatment, suggesting that early first trimester hyperglycemia may affect fetal brain development, Anny H. Xiang, Ph.D., said at the annual meeting of the European Association for the Study of Diabetes.
AT EASD 2014
Key clinical point: Infants of women with gestational diabetes may have an increased risk of autism, especially if antidiabetic medications were required.
Major finding: The incidence rate of autism among infants not affected by gestational diabetes was 1.77 per 1,000 person/years; the rate among infants whose mothers had GDM and were exposed to antidiabetic medications was 2.75 per 1,000 person/years.
Data source: The retrospective database study included more than 300,000 infants.
Disclosures: Kaiser Permanente sponsored the study. Dr. Xiang is an employee of the company.