User login
1% Jump in Glucose Yields 25% Jump in Cardiovascular Risk
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
AT EASD 2014
1% jump in glucose yields 25% jump in cardiovascular risk
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
On Twitter @alz_gal
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
On Twitter @alz_gal
VIENNA – Increasing hemoglobin A1c was directly tied to a significant increase in the risk of cardiovascular death and stroke in patients with type 2 diabetes.
For every 1% increase in HbA1c, the 4-year risk of a major cardiovascular event increased by 25%, a large prospective study has determined. The 1% increase also conferred significant increases in the risk of cardiovascular death and stroke, Dr. Nicholas Freemantle said at the annual meeting of the European Association for the Study of Diabetes.
The 4-year study, however, found no increase in the risk of fatal myocardial infarction – a surprise, said Dr. Freemantle, chair of clinical epidemiology and biostatistics at University College London. It was probably a chance finding, he added.
He reported results of the Cardiovascular Risk Evaluation in People With Type 2 Diabetes (CREDIT) study. CREDIT was noninterventional, designed to evaluate real-world clinical results in patients who are newly treated with insulin. There was no fixed visit schedule, although clinicians were required to record some data every 6 months.
The cohort comprised 2,999 patients with a mean age of 61 years and a mean baseline HbA1c of 9.3%. The mean diabetes duration was about 9 years. Most of the cohort (69%) had hypertension. About half engaged in regular physical activity; 19% were smokers. Roughly one-third had at least one macrovascular disease, and 70% at least one microvascular disease.
The insulin dose increased from a median of 0.2 units/kg at baseline to 0.4 units/kg at 1 year, and to 0.5 units/kg by the end of year 4. HbA1c decreased to around 7.4% by the end of year 1 and remained stable after that.
By the end of the study, 147 major cardiovascular events had occurred. These included 60 cardiovascular deaths, 44 nonfatal MIs, and 57 nonfatal strokes. There were 148 deaths from any cause.
A multivariate model adjusted for age when starting insulin, and for baseline macrovascular disease and hypertension. It found that, for every 1% increase in HbA1c, the risk of a major cardiovascular event increased by 25%. The same HbA1c increase boosted the risk of cardiovascular death by 31% and the risk of stroke by 36%. There was no increased risk of heart attack.
Severe or symptomatic hypoglycemic events had no significant impact on the risk of cardiovascular death, Dr. Freemantle said.
CREDIT was sponsored by Sanofi. Dr. Freemantle said that he has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
On Twitter @alz_gal
AT EASD 2014
Key clinical point: The risk of a major cardiovascular event increases as blood glucose rises.
Major finding: Every 1% increase in HbA1c conferred a 25% increase in the risk of a major cardiovascular event.
Data source: A 4-year, prospective, noninterventional study involving 2,999 patients.
Disclosures: CREDIT was sponsored by Sanofi. Dr. Freemantle has received financial support from the company, as well as from Pfizer, Novo Nordisk, Eli Lilly, and Medtronic.
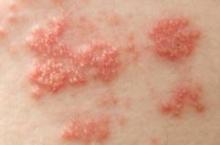
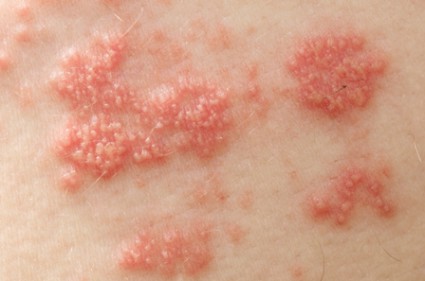
Shingles Vaccine Most Effective When Given to Patients in Their 60s
Because efficacy of the shingles vaccine appears to wane over time, it should not routinely be given to people in their 50s, according to information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
"At this time, there is insufficient evidence supporting long-term protection of the vaccine, so if people younger than 60 [years] are vaccinated, they might not be protected when chance of disease is highest," said Dr. Jeff Duchin, chair of the ACIP’s herpes zoster working group. "We will continue to monitor data as they becomes available, but at this point we are reaffirming that vaccination be done in those age 60 [years] and older."
The committee made its recommendation after reviewing newly released data from Merck’s Long-Term Persistence Study for shingles. It found that, 10 years after vaccination, the overall incidence rate of shingles had increased from about 4/1,000 person-years to 11/1,000 person-years. The observed incidence rate of shingles in historical controls is about 13 cases/1,000 person-years.
Dr. Janie Parrino, director of vaccine clinical research at Merck Research Laboratories, presented results of the company’s latest study of the vaccine’s extended efficacy. The shingles Long-Term Persistence Study is an 8-year follow-up of the Shingles Prevention Study, in which subjects were randomized to Merck’s Zostavax vaccine or placebo. It showed that the vaccine significantly reduced the incidence of herpes zoster and postherpetic neuralgia (PHN) as well as the shingles burden of illness, in adults older than 60 years.
A short-term follow-up study appeared last year; it added 4 more years of follow-up data on more than 14,000 of the original cohort.
It found a gradual decrease in vaccine efficacy by 6-7 years after vaccination. "Analysis of vaccine efficacy in each year after vaccination for all outcomes showed a decrease after year 1, with a further decline thereafter," the investigators said. "Vaccine efficacy was statistically significant for the incidence of herpes zoster and herpes zoster burden of illness through year 5 after vaccination, but vaccine efficacy is uncertain beyond that point."
The long-term study presented at the Atlanta meeting involved up to 12 years of data on almost 7,000 people in the original cohort. It demonstrated a continuous time-bound waning of efficacy.
In addition to the increasing annual incidence of shingles, the study found an increasing incidence of PHN, from less than 1/1,000 person-years during the year of vaccination to about 1/1,000 person years by year 10. However, the incidence among placebo subjects and historical controls was more than 2/1,000 person years by that time. The shingles burden of illness (a measure of acute pain) also increased as time passed, Dr. Parrino noted.
Taking into account both clinical efficacy and cost, Ismael Ortega-Sanchez, Ph.D., a health economist with the CDC, concluded that the shingles vaccine is most effective when given to people in their 60s and 70s.
Vaccinating from age 50-59 years would prevent about 20,000 shingles cases annually. But vaccinating in the 60s would prevent 26,000 and, in the 70s, 21,000. The trend was similar for cases of PHN: prevention of 1,000 cases for vaccinating in the 50s, 4,000 for vaccinating in the 60s, and 8,000 for vaccinating in the 70s.
Other outcomes – emergency visits, ambulatory visits, hospitalizations, length of hospital stay, and prescriptions – also increased significantly over the 3 decades.
Savings tracked clinical outcomes in a value assessment model of 6 million people: 1 million vaccinated and unvaccinated in each of the three age cohorts.
Compared with not vaccinating, vaccinating those in their 50s would save $16.8 million annually. Vaccinating during the 60s would save about $24.3 million, and during the 70s, $31.9 million.
And, because younger people are likely to have more systemic reactions, they cost more to vaccinate. A model that accounted for the cost of the vaccine, administration, and treating local and systemic reactions, put the total price tag at $193 million for those in their 50s, and $190 million for each of the two older groups.
Finally, the number needed to treat to prevent one case of shingles, PHN, or non-PHN complication case was higher in those in their 50s than in those in their 60s or 70s (one shingles case – 51, 38, and 47 respectively; one PHN case – 988, 247, and 124).
"This isn’t intended to tell people not to get the vaccine early, but to make them aware of the risks and benefits, given that the greatest risk of the disease is later in life," Dr. Duchin said. Since there are no data on booster shingles vaccines, he said "we only have one shot at this and we need to focus our efforts on those at the greatest risk."
Because efficacy of the shingles vaccine appears to wane over time, it should not routinely be given to people in their 50s, according to information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
"At this time, there is insufficient evidence supporting long-term protection of the vaccine, so if people younger than 60 [years] are vaccinated, they might not be protected when chance of disease is highest," said Dr. Jeff Duchin, chair of the ACIP’s herpes zoster working group. "We will continue to monitor data as they becomes available, but at this point we are reaffirming that vaccination be done in those age 60 [years] and older."
The committee made its recommendation after reviewing newly released data from Merck’s Long-Term Persistence Study for shingles. It found that, 10 years after vaccination, the overall incidence rate of shingles had increased from about 4/1,000 person-years to 11/1,000 person-years. The observed incidence rate of shingles in historical controls is about 13 cases/1,000 person-years.
Dr. Janie Parrino, director of vaccine clinical research at Merck Research Laboratories, presented results of the company’s latest study of the vaccine’s extended efficacy. The shingles Long-Term Persistence Study is an 8-year follow-up of the Shingles Prevention Study, in which subjects were randomized to Merck’s Zostavax vaccine or placebo. It showed that the vaccine significantly reduced the incidence of herpes zoster and postherpetic neuralgia (PHN) as well as the shingles burden of illness, in adults older than 60 years.
A short-term follow-up study appeared last year; it added 4 more years of follow-up data on more than 14,000 of the original cohort.
It found a gradual decrease in vaccine efficacy by 6-7 years after vaccination. "Analysis of vaccine efficacy in each year after vaccination for all outcomes showed a decrease after year 1, with a further decline thereafter," the investigators said. "Vaccine efficacy was statistically significant for the incidence of herpes zoster and herpes zoster burden of illness through year 5 after vaccination, but vaccine efficacy is uncertain beyond that point."
The long-term study presented at the Atlanta meeting involved up to 12 years of data on almost 7,000 people in the original cohort. It demonstrated a continuous time-bound waning of efficacy.
In addition to the increasing annual incidence of shingles, the study found an increasing incidence of PHN, from less than 1/1,000 person-years during the year of vaccination to about 1/1,000 person years by year 10. However, the incidence among placebo subjects and historical controls was more than 2/1,000 person years by that time. The shingles burden of illness (a measure of acute pain) also increased as time passed, Dr. Parrino noted.
Taking into account both clinical efficacy and cost, Ismael Ortega-Sanchez, Ph.D., a health economist with the CDC, concluded that the shingles vaccine is most effective when given to people in their 60s and 70s.
Vaccinating from age 50-59 years would prevent about 20,000 shingles cases annually. But vaccinating in the 60s would prevent 26,000 and, in the 70s, 21,000. The trend was similar for cases of PHN: prevention of 1,000 cases for vaccinating in the 50s, 4,000 for vaccinating in the 60s, and 8,000 for vaccinating in the 70s.
Other outcomes – emergency visits, ambulatory visits, hospitalizations, length of hospital stay, and prescriptions – also increased significantly over the 3 decades.
Savings tracked clinical outcomes in a value assessment model of 6 million people: 1 million vaccinated and unvaccinated in each of the three age cohorts.
Compared with not vaccinating, vaccinating those in their 50s would save $16.8 million annually. Vaccinating during the 60s would save about $24.3 million, and during the 70s, $31.9 million.
And, because younger people are likely to have more systemic reactions, they cost more to vaccinate. A model that accounted for the cost of the vaccine, administration, and treating local and systemic reactions, put the total price tag at $193 million for those in their 50s, and $190 million for each of the two older groups.
Finally, the number needed to treat to prevent one case of shingles, PHN, or non-PHN complication case was higher in those in their 50s than in those in their 60s or 70s (one shingles case – 51, 38, and 47 respectively; one PHN case – 988, 247, and 124).
"This isn’t intended to tell people not to get the vaccine early, but to make them aware of the risks and benefits, given that the greatest risk of the disease is later in life," Dr. Duchin said. Since there are no data on booster shingles vaccines, he said "we only have one shot at this and we need to focus our efforts on those at the greatest risk."
Because efficacy of the shingles vaccine appears to wane over time, it should not routinely be given to people in their 50s, according to information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
"At this time, there is insufficient evidence supporting long-term protection of the vaccine, so if people younger than 60 [years] are vaccinated, they might not be protected when chance of disease is highest," said Dr. Jeff Duchin, chair of the ACIP’s herpes zoster working group. "We will continue to monitor data as they becomes available, but at this point we are reaffirming that vaccination be done in those age 60 [years] and older."
The committee made its recommendation after reviewing newly released data from Merck’s Long-Term Persistence Study for shingles. It found that, 10 years after vaccination, the overall incidence rate of shingles had increased from about 4/1,000 person-years to 11/1,000 person-years. The observed incidence rate of shingles in historical controls is about 13 cases/1,000 person-years.
Dr. Janie Parrino, director of vaccine clinical research at Merck Research Laboratories, presented results of the company’s latest study of the vaccine’s extended efficacy. The shingles Long-Term Persistence Study is an 8-year follow-up of the Shingles Prevention Study, in which subjects were randomized to Merck’s Zostavax vaccine or placebo. It showed that the vaccine significantly reduced the incidence of herpes zoster and postherpetic neuralgia (PHN) as well as the shingles burden of illness, in adults older than 60 years.
A short-term follow-up study appeared last year; it added 4 more years of follow-up data on more than 14,000 of the original cohort.
It found a gradual decrease in vaccine efficacy by 6-7 years after vaccination. "Analysis of vaccine efficacy in each year after vaccination for all outcomes showed a decrease after year 1, with a further decline thereafter," the investigators said. "Vaccine efficacy was statistically significant for the incidence of herpes zoster and herpes zoster burden of illness through year 5 after vaccination, but vaccine efficacy is uncertain beyond that point."
The long-term study presented at the Atlanta meeting involved up to 12 years of data on almost 7,000 people in the original cohort. It demonstrated a continuous time-bound waning of efficacy.
In addition to the increasing annual incidence of shingles, the study found an increasing incidence of PHN, from less than 1/1,000 person-years during the year of vaccination to about 1/1,000 person years by year 10. However, the incidence among placebo subjects and historical controls was more than 2/1,000 person years by that time. The shingles burden of illness (a measure of acute pain) also increased as time passed, Dr. Parrino noted.
Taking into account both clinical efficacy and cost, Ismael Ortega-Sanchez, Ph.D., a health economist with the CDC, concluded that the shingles vaccine is most effective when given to people in their 60s and 70s.
Vaccinating from age 50-59 years would prevent about 20,000 shingles cases annually. But vaccinating in the 60s would prevent 26,000 and, in the 70s, 21,000. The trend was similar for cases of PHN: prevention of 1,000 cases for vaccinating in the 50s, 4,000 for vaccinating in the 60s, and 8,000 for vaccinating in the 70s.
Other outcomes – emergency visits, ambulatory visits, hospitalizations, length of hospital stay, and prescriptions – also increased significantly over the 3 decades.
Savings tracked clinical outcomes in a value assessment model of 6 million people: 1 million vaccinated and unvaccinated in each of the three age cohorts.
Compared with not vaccinating, vaccinating those in their 50s would save $16.8 million annually. Vaccinating during the 60s would save about $24.3 million, and during the 70s, $31.9 million.
And, because younger people are likely to have more systemic reactions, they cost more to vaccinate. A model that accounted for the cost of the vaccine, administration, and treating local and systemic reactions, put the total price tag at $193 million for those in their 50s, and $190 million for each of the two older groups.
Finally, the number needed to treat to prevent one case of shingles, PHN, or non-PHN complication case was higher in those in their 50s than in those in their 60s or 70s (one shingles case – 51, 38, and 47 respectively; one PHN case – 988, 247, and 124).
"This isn’t intended to tell people not to get the vaccine early, but to make them aware of the risks and benefits, given that the greatest risk of the disease is later in life," Dr. Duchin said. Since there are no data on booster shingles vaccines, he said "we only have one shot at this and we need to focus our efforts on those at the greatest risk."
FROM ACIP
Meningococcal Vaccine for At-risk Infants
The quadrivalent meningococcal vaccine MenACWY-CRM, or Menveo, may now be given to infants aged 2-23 months who are considered at high risk for meningococcal disease or who travel to areas where it is hyperendemic or epidemic, according to the recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
High-risk children are those with component complement deficiency, HIV infection, or anatomic or functional asplenia. Children in an outbreak of a vaccine-preventable meningitis serogroup also should receive the series. About 5,000 infants per year fit into these categories.
The Advisory Committee on Immunization Practices (ACIP) approved the decision by a vote of 13-1, with one abstention. The decision aligns ACIP’s recommendation with the recently expanded indication for the vaccine, which protects against serotypes A, C, W-135, and Y. In August, the Food and Drug Administration approved the vaccine for infants and toddlers older than 2 months.
However, because of the extremely low incidence of meningococcal disease in children in the United States, the committee did not recommend it for all healthy children. "Very few children are considered at increased risk, so a routine recommendation would prevent just a very low number of cases," said Jessica MacNeil, an epidemiologist with the CDC.
The FDA licensing of MenACWY-CRM and ACIP’s recommendation give pediatricians a third option for infant meningococcal vaccination. HibMenCY-TT (MenHibrix) and MenACWY-D (Menactra) are also approved for some of these same indications. However, Ms. MacNeil noted, Menactra cannot be used in children with asplenia, and MenHibrix can’t be used for travel or as a booster dose.
The committee based its decision on three phase III safety and efficacy trials. These studies comprised nearly 11,000 infants – 9,000 of whom had the four-dose series and 2,000 of whom had a two-dose series, said Dr. Peter Dull of Novartis Vaccines.
The studies confirmed an average 90% efficacy against all four serotypes after the 12-month dose. By 40 months of age, efficacy had waned somewhat: 10% of infants had a seroresponse to type A; 34%, to type C; 76%, to type W-135; and 67%, to type Y. Of the strains included in the vaccine, types C, Y, and W-135 cause 75% of meningococcal disease in those aged 11 years and older.
Dr. Dull said that Novartis is following a cohort that received the vaccine as infants; next year, 60-month immunogenicity data will be available.
After adjustment for some confounders, the vaccine did not significantly interact with the immunogenic potential of other recommended childhood vaccines. Novartis studies also found it safe. Up to 60% of children had mild to moderate injection site reactions. The incidence of systemic reactions was no different from that for routine vaccines alone.
There were 11 serious adverse events possibly related to the vaccine among the 5,000 children included in the safety analyses. These included acute encephalomyelitis, cellulitis, complex partial seizure, epilepsy, febrile seizure, and Kawasaki disease. Ten deaths occurred, but were not considered vaccine related.
The recommended dosing schedule will be:
• Aged 2-6 months: Four doses at 2, 4, 6, and 12 months.
• Aged 7-23 months: Two doses with the second dose given in the second year of life.
• Aged 2-11 years: One or two doses.
Boosters should be given to those who remain at risk, beginning at 3 years after the primary series and then every 5 years thereafter.
The committee also agreed, by a vote of 14 with one abstention, to include MenACWY-CRM in the Vaccines for Children program.
One member who voted disclosed that her institution receives research funding from drug companies. None of the other voting members had any relevant financial disclosures.
The quadrivalent meningococcal vaccine MenACWY-CRM, or Menveo, may now be given to infants aged 2-23 months who are considered at high risk for meningococcal disease or who travel to areas where it is hyperendemic or epidemic, according to the recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
High-risk children are those with component complement deficiency, HIV infection, or anatomic or functional asplenia. Children in an outbreak of a vaccine-preventable meningitis serogroup also should receive the series. About 5,000 infants per year fit into these categories.
The Advisory Committee on Immunization Practices (ACIP) approved the decision by a vote of 13-1, with one abstention. The decision aligns ACIP’s recommendation with the recently expanded indication for the vaccine, which protects against serotypes A, C, W-135, and Y. In August, the Food and Drug Administration approved the vaccine for infants and toddlers older than 2 months.
However, because of the extremely low incidence of meningococcal disease in children in the United States, the committee did not recommend it for all healthy children. "Very few children are considered at increased risk, so a routine recommendation would prevent just a very low number of cases," said Jessica MacNeil, an epidemiologist with the CDC.
The FDA licensing of MenACWY-CRM and ACIP’s recommendation give pediatricians a third option for infant meningococcal vaccination. HibMenCY-TT (MenHibrix) and MenACWY-D (Menactra) are also approved for some of these same indications. However, Ms. MacNeil noted, Menactra cannot be used in children with asplenia, and MenHibrix can’t be used for travel or as a booster dose.
The committee based its decision on three phase III safety and efficacy trials. These studies comprised nearly 11,000 infants – 9,000 of whom had the four-dose series and 2,000 of whom had a two-dose series, said Dr. Peter Dull of Novartis Vaccines.
The studies confirmed an average 90% efficacy against all four serotypes after the 12-month dose. By 40 months of age, efficacy had waned somewhat: 10% of infants had a seroresponse to type A; 34%, to type C; 76%, to type W-135; and 67%, to type Y. Of the strains included in the vaccine, types C, Y, and W-135 cause 75% of meningococcal disease in those aged 11 years and older.
Dr. Dull said that Novartis is following a cohort that received the vaccine as infants; next year, 60-month immunogenicity data will be available.
After adjustment for some confounders, the vaccine did not significantly interact with the immunogenic potential of other recommended childhood vaccines. Novartis studies also found it safe. Up to 60% of children had mild to moderate injection site reactions. The incidence of systemic reactions was no different from that for routine vaccines alone.
There were 11 serious adverse events possibly related to the vaccine among the 5,000 children included in the safety analyses. These included acute encephalomyelitis, cellulitis, complex partial seizure, epilepsy, febrile seizure, and Kawasaki disease. Ten deaths occurred, but were not considered vaccine related.
The recommended dosing schedule will be:
• Aged 2-6 months: Four doses at 2, 4, 6, and 12 months.
• Aged 7-23 months: Two doses with the second dose given in the second year of life.
• Aged 2-11 years: One or two doses.
Boosters should be given to those who remain at risk, beginning at 3 years after the primary series and then every 5 years thereafter.
The committee also agreed, by a vote of 14 with one abstention, to include MenACWY-CRM in the Vaccines for Children program.
One member who voted disclosed that her institution receives research funding from drug companies. None of the other voting members had any relevant financial disclosures.
The quadrivalent meningococcal vaccine MenACWY-CRM, or Menveo, may now be given to infants aged 2-23 months who are considered at high risk for meningococcal disease or who travel to areas where it is hyperendemic or epidemic, according to the recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
High-risk children are those with component complement deficiency, HIV infection, or anatomic or functional asplenia. Children in an outbreak of a vaccine-preventable meningitis serogroup also should receive the series. About 5,000 infants per year fit into these categories.
The Advisory Committee on Immunization Practices (ACIP) approved the decision by a vote of 13-1, with one abstention. The decision aligns ACIP’s recommendation with the recently expanded indication for the vaccine, which protects against serotypes A, C, W-135, and Y. In August, the Food and Drug Administration approved the vaccine for infants and toddlers older than 2 months.
However, because of the extremely low incidence of meningococcal disease in children in the United States, the committee did not recommend it for all healthy children. "Very few children are considered at increased risk, so a routine recommendation would prevent just a very low number of cases," said Jessica MacNeil, an epidemiologist with the CDC.
The FDA licensing of MenACWY-CRM and ACIP’s recommendation give pediatricians a third option for infant meningococcal vaccination. HibMenCY-TT (MenHibrix) and MenACWY-D (Menactra) are also approved for some of these same indications. However, Ms. MacNeil noted, Menactra cannot be used in children with asplenia, and MenHibrix can’t be used for travel or as a booster dose.
The committee based its decision on three phase III safety and efficacy trials. These studies comprised nearly 11,000 infants – 9,000 of whom had the four-dose series and 2,000 of whom had a two-dose series, said Dr. Peter Dull of Novartis Vaccines.
The studies confirmed an average 90% efficacy against all four serotypes after the 12-month dose. By 40 months of age, efficacy had waned somewhat: 10% of infants had a seroresponse to type A; 34%, to type C; 76%, to type W-135; and 67%, to type Y. Of the strains included in the vaccine, types C, Y, and W-135 cause 75% of meningococcal disease in those aged 11 years and older.
Dr. Dull said that Novartis is following a cohort that received the vaccine as infants; next year, 60-month immunogenicity data will be available.
After adjustment for some confounders, the vaccine did not significantly interact with the immunogenic potential of other recommended childhood vaccines. Novartis studies also found it safe. Up to 60% of children had mild to moderate injection site reactions. The incidence of systemic reactions was no different from that for routine vaccines alone.
There were 11 serious adverse events possibly related to the vaccine among the 5,000 children included in the safety analyses. These included acute encephalomyelitis, cellulitis, complex partial seizure, epilepsy, febrile seizure, and Kawasaki disease. Ten deaths occurred, but were not considered vaccine related.
The recommended dosing schedule will be:
• Aged 2-6 months: Four doses at 2, 4, 6, and 12 months.
• Aged 7-23 months: Two doses with the second dose given in the second year of life.
• Aged 2-11 years: One or two doses.
Boosters should be given to those who remain at risk, beginning at 3 years after the primary series and then every 5 years thereafter.
The committee also agreed, by a vote of 14 with one abstention, to include MenACWY-CRM in the Vaccines for Children program.
One member who voted disclosed that her institution receives research funding from drug companies. None of the other voting members had any relevant financial disclosures.
AT ACIP
In Hormone Therapy Fight, Bioidenticals Come Out Swinging
With seemingly ageless celebrities touting the benefits of bioidentical, compounded hormones, and the cloud of the Women’s Health Initiative still obscuring the view, both physicians and patients want to know the truth about natural hormone replacement.
But as in so many areas of life, the truth can be many things to many people, Dr. Jan L. Shifren shared at the annual meeting of the North American Menopause Society (NAMS).
"For a woman with bothersome menopausal symptoms and concerns about potential hormone therapy [HT] risks, ‘bioHT’ may mean a formulation of hormones with all of the benefits and no risks, as it’s ‘natural’ and contains no package insert with a black box warning," said Dr. Shifren, director of the menopause program at Massachusetts General Hospital, Boston. "For a bioHT practitioner, it may mean a way of helping menopausal women disenfranchised with the medical establishment, while providing a steady source of income. For a clinician or pharmacist practicing within the current standard of care, an FDA inspector, or a lawyer for a women with endometrial cancer associated with its use, bioHT may mean something quite different!"
Bioidentical hormones are typically plant-based compounds with a molecular structure identical to that of the corresponding human hormone. Dosing is individual and based on a woman’s salivary hormone levels. Prescriptions are pharmacist compounded, typically in a topical preparation, but sometimes in an oral tablet or injected pellet.
All of this may sound reasonable at first glance, Dr. Shifren said. But science and money just keep getting in the way of the celebrity endorsements.
"Hormone therapy should be guided by symptoms. There are no data to support the use of blood or saliva measurements to improve treatment efficacy," Dr. Shifren said. Serum hormone levels constantly fluctuate – not only within the menstrual cycle, but also within a single day, "so it’s impossible to ‘match’ any individual woman’s ‘ideal’ hormone levels."
Additionally, Dr. Shifren said, salivary levels don’t even correspond to serum levels.
Safety is one of the biggest problems with such compounds. Because their manufacture is in the hands of a single individual, there’s no quality oversight. Women can’t count on getting the correct prescribed dose of hormone – and sometimes not even close to it.
A recent study commissioned by MORE magazine examined the exact hormone content in 12 bioHT prescriptions, which were filled by 12 pharmacies. Two pharmacies were retail stores and 10 were online companies.
Flora Research Labs analyzed the medications with mass spectrometry. The values varied widely: 96%-260% of the prescribed estrogen dose and 60%-80% of the prescribed progesterone dose.
"None of these would have met FDA standards," Dr. Shifren said. "The purity, bioavailability, and dose-to-dose consistency of any given prescription is an unknown."
Concern about the safety of conventional HT frequently drives women to seek out what they consider a more natural alternative. Although much more data have emerged, the original furor over the Women’s Health Initiative still casts HT in a negative light for many, Dr. Shifren said.
For women in good health, who start combination estrogen/progesterone HT early in menopause, at the lowest effective dose, the increased risks of cardiovascular disease and cancer are negligible. A 2010 British study of 15,710 cases and almost 60,000 controls found that low-dose transdermal HT had no effect on stroke risk. High-dose patch estrogen increased the risk by 89%, and both low- and high-dose oral estrogen increased the risk by 69%; however, each of those increases translated to an absolute increase of only about 1 stroke per year (BMJ 2010;340:c2519 [doi: 10.1136/bmj.c2519]).
A 2008 French study of almost 81,000 women found no significant associations between breast cancer and route of HT administration. However, the study did conclude that the combination of estrogen and progesterone was probably the safest, with a risk ratio of 1.0. Estrogen plus dydrogesterone resulted in a nonsignificant risk increase (relative risk, 1.16). Estrogen in combination with other progestogens carried a significant 69% risk increase (Breast Cancer Res. Treat. 2008;107:103-11).
Finally, Dr. Shifren said, it’s just about impossible to take profit out of the picture. Compounding HT sales are reaching more than $2 billion per year now.
"Many practitioners who prescribe compounded hormones also sell them, or benefit financially from relationships with compounders. This poses a potential conflict of interest and violates standards of professional ethical conduct," she said.
Dr. Shifren provides research consulting for New England Research Institutes.
With seemingly ageless celebrities touting the benefits of bioidentical, compounded hormones, and the cloud of the Women’s Health Initiative still obscuring the view, both physicians and patients want to know the truth about natural hormone replacement.
But as in so many areas of life, the truth can be many things to many people, Dr. Jan L. Shifren shared at the annual meeting of the North American Menopause Society (NAMS).
"For a woman with bothersome menopausal symptoms and concerns about potential hormone therapy [HT] risks, ‘bioHT’ may mean a formulation of hormones with all of the benefits and no risks, as it’s ‘natural’ and contains no package insert with a black box warning," said Dr. Shifren, director of the menopause program at Massachusetts General Hospital, Boston. "For a bioHT practitioner, it may mean a way of helping menopausal women disenfranchised with the medical establishment, while providing a steady source of income. For a clinician or pharmacist practicing within the current standard of care, an FDA inspector, or a lawyer for a women with endometrial cancer associated with its use, bioHT may mean something quite different!"
Bioidentical hormones are typically plant-based compounds with a molecular structure identical to that of the corresponding human hormone. Dosing is individual and based on a woman’s salivary hormone levels. Prescriptions are pharmacist compounded, typically in a topical preparation, but sometimes in an oral tablet or injected pellet.
All of this may sound reasonable at first glance, Dr. Shifren said. But science and money just keep getting in the way of the celebrity endorsements.
"Hormone therapy should be guided by symptoms. There are no data to support the use of blood or saliva measurements to improve treatment efficacy," Dr. Shifren said. Serum hormone levels constantly fluctuate – not only within the menstrual cycle, but also within a single day, "so it’s impossible to ‘match’ any individual woman’s ‘ideal’ hormone levels."
Additionally, Dr. Shifren said, salivary levels don’t even correspond to serum levels.
Safety is one of the biggest problems with such compounds. Because their manufacture is in the hands of a single individual, there’s no quality oversight. Women can’t count on getting the correct prescribed dose of hormone – and sometimes not even close to it.
A recent study commissioned by MORE magazine examined the exact hormone content in 12 bioHT prescriptions, which were filled by 12 pharmacies. Two pharmacies were retail stores and 10 were online companies.
Flora Research Labs analyzed the medications with mass spectrometry. The values varied widely: 96%-260% of the prescribed estrogen dose and 60%-80% of the prescribed progesterone dose.
"None of these would have met FDA standards," Dr. Shifren said. "The purity, bioavailability, and dose-to-dose consistency of any given prescription is an unknown."
Concern about the safety of conventional HT frequently drives women to seek out what they consider a more natural alternative. Although much more data have emerged, the original furor over the Women’s Health Initiative still casts HT in a negative light for many, Dr. Shifren said.
For women in good health, who start combination estrogen/progesterone HT early in menopause, at the lowest effective dose, the increased risks of cardiovascular disease and cancer are negligible. A 2010 British study of 15,710 cases and almost 60,000 controls found that low-dose transdermal HT had no effect on stroke risk. High-dose patch estrogen increased the risk by 89%, and both low- and high-dose oral estrogen increased the risk by 69%; however, each of those increases translated to an absolute increase of only about 1 stroke per year (BMJ 2010;340:c2519 [doi: 10.1136/bmj.c2519]).
A 2008 French study of almost 81,000 women found no significant associations between breast cancer and route of HT administration. However, the study did conclude that the combination of estrogen and progesterone was probably the safest, with a risk ratio of 1.0. Estrogen plus dydrogesterone resulted in a nonsignificant risk increase (relative risk, 1.16). Estrogen in combination with other progestogens carried a significant 69% risk increase (Breast Cancer Res. Treat. 2008;107:103-11).
Finally, Dr. Shifren said, it’s just about impossible to take profit out of the picture. Compounding HT sales are reaching more than $2 billion per year now.
"Many practitioners who prescribe compounded hormones also sell them, or benefit financially from relationships with compounders. This poses a potential conflict of interest and violates standards of professional ethical conduct," she said.
Dr. Shifren provides research consulting for New England Research Institutes.
With seemingly ageless celebrities touting the benefits of bioidentical, compounded hormones, and the cloud of the Women’s Health Initiative still obscuring the view, both physicians and patients want to know the truth about natural hormone replacement.
But as in so many areas of life, the truth can be many things to many people, Dr. Jan L. Shifren shared at the annual meeting of the North American Menopause Society (NAMS).
"For a woman with bothersome menopausal symptoms and concerns about potential hormone therapy [HT] risks, ‘bioHT’ may mean a formulation of hormones with all of the benefits and no risks, as it’s ‘natural’ and contains no package insert with a black box warning," said Dr. Shifren, director of the menopause program at Massachusetts General Hospital, Boston. "For a bioHT practitioner, it may mean a way of helping menopausal women disenfranchised with the medical establishment, while providing a steady source of income. For a clinician or pharmacist practicing within the current standard of care, an FDA inspector, or a lawyer for a women with endometrial cancer associated with its use, bioHT may mean something quite different!"
Bioidentical hormones are typically plant-based compounds with a molecular structure identical to that of the corresponding human hormone. Dosing is individual and based on a woman’s salivary hormone levels. Prescriptions are pharmacist compounded, typically in a topical preparation, but sometimes in an oral tablet or injected pellet.
All of this may sound reasonable at first glance, Dr. Shifren said. But science and money just keep getting in the way of the celebrity endorsements.
"Hormone therapy should be guided by symptoms. There are no data to support the use of blood or saliva measurements to improve treatment efficacy," Dr. Shifren said. Serum hormone levels constantly fluctuate – not only within the menstrual cycle, but also within a single day, "so it’s impossible to ‘match’ any individual woman’s ‘ideal’ hormone levels."
Additionally, Dr. Shifren said, salivary levels don’t even correspond to serum levels.
Safety is one of the biggest problems with such compounds. Because their manufacture is in the hands of a single individual, there’s no quality oversight. Women can’t count on getting the correct prescribed dose of hormone – and sometimes not even close to it.
A recent study commissioned by MORE magazine examined the exact hormone content in 12 bioHT prescriptions, which were filled by 12 pharmacies. Two pharmacies were retail stores and 10 were online companies.
Flora Research Labs analyzed the medications with mass spectrometry. The values varied widely: 96%-260% of the prescribed estrogen dose and 60%-80% of the prescribed progesterone dose.
"None of these would have met FDA standards," Dr. Shifren said. "The purity, bioavailability, and dose-to-dose consistency of any given prescription is an unknown."
Concern about the safety of conventional HT frequently drives women to seek out what they consider a more natural alternative. Although much more data have emerged, the original furor over the Women’s Health Initiative still casts HT in a negative light for many, Dr. Shifren said.
For women in good health, who start combination estrogen/progesterone HT early in menopause, at the lowest effective dose, the increased risks of cardiovascular disease and cancer are negligible. A 2010 British study of 15,710 cases and almost 60,000 controls found that low-dose transdermal HT had no effect on stroke risk. High-dose patch estrogen increased the risk by 89%, and both low- and high-dose oral estrogen increased the risk by 69%; however, each of those increases translated to an absolute increase of only about 1 stroke per year (BMJ 2010;340:c2519 [doi: 10.1136/bmj.c2519]).
A 2008 French study of almost 81,000 women found no significant associations between breast cancer and route of HT administration. However, the study did conclude that the combination of estrogen and progesterone was probably the safest, with a risk ratio of 1.0. Estrogen plus dydrogesterone resulted in a nonsignificant risk increase (relative risk, 1.16). Estrogen in combination with other progestogens carried a significant 69% risk increase (Breast Cancer Res. Treat. 2008;107:103-11).
Finally, Dr. Shifren said, it’s just about impossible to take profit out of the picture. Compounding HT sales are reaching more than $2 billion per year now.
"Many practitioners who prescribe compounded hormones also sell them, or benefit financially from relationships with compounders. This poses a potential conflict of interest and violates standards of professional ethical conduct," she said.
Dr. Shifren provides research consulting for New England Research Institutes.
EXPERT ANALYSIS FROM THE NAMS 2013 ANNUAL MEETING
Self-Collected Swabs Okay for STDs in Men
CHICAGO — Patient-collected rectal swabs are just as accurate as provider-collected swabs for diagnosing chlamydia and gonorrhea infections in men, Dr. Christine Wigen reported at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention
Allowing men to collect their own specimens may help boost testing rates at STD clinics that lack appropriate staff, she said.
“Some STD testing sites don't have [the staff] to take samples from anatomical sites such as the rectum,” she said in an interview. “[They] may only have a phlebotomist to draw blood for HIV or syphilis tests and can only take urine specimens for genitourinary testing of gonorrhea and chlamydia. In the past, the provider had to collect the rectal specimens [so] the test wouldn't be done. With the self-collected method, the testing is possible even in the absence of a provider.”
In addition, she said, if the patient is asymptomatic, then the self-collected method fast-tracks him through the screening process, which would free up the provider to focus on symptomatic individuals and on those with positive tests.
Dr. Wigen examined the accuracy of 225 paired rectal swab samples collected from men who had experienced receptive anal sex in the previous year. Each provided both a self-collected swab and a swab collected by a provider during a visit to the Los Angeles Gay and Lesbian Center sexual health program, a community partner of the Los Angeles County Department of Public Health.
Their mean age was 34 years; 78% said they were gay, 11% said they were bisexual, 8% heterosexual, and 1% transgender.
Overall prevalence of rectal chlamydia was 19%. Provider- and self-collected swabs had a diagnostic agreement of 97%. Rectal chlamydia was found in 39 self-collected specimens and 40 provider-collected specimens. Overall prevalence of rectal gonorrhea was 16%. Provider- and self-collected swabs had a diagnostic agreement of 95%. Gonorrhea was found in 42 self-collected specimens and 37 provider-collected specimens. The sensitivity of self-collected swabs for both infections was 93%. Sensitivity of provider-collected swabs was 95% for chlamydia and 82% for gonorrhea.
CHICAGO — Patient-collected rectal swabs are just as accurate as provider-collected swabs for diagnosing chlamydia and gonorrhea infections in men, Dr. Christine Wigen reported at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention
Allowing men to collect their own specimens may help boost testing rates at STD clinics that lack appropriate staff, she said.
“Some STD testing sites don't have [the staff] to take samples from anatomical sites such as the rectum,” she said in an interview. “[They] may only have a phlebotomist to draw blood for HIV or syphilis tests and can only take urine specimens for genitourinary testing of gonorrhea and chlamydia. In the past, the provider had to collect the rectal specimens [so] the test wouldn't be done. With the self-collected method, the testing is possible even in the absence of a provider.”
In addition, she said, if the patient is asymptomatic, then the self-collected method fast-tracks him through the screening process, which would free up the provider to focus on symptomatic individuals and on those with positive tests.
Dr. Wigen examined the accuracy of 225 paired rectal swab samples collected from men who had experienced receptive anal sex in the previous year. Each provided both a self-collected swab and a swab collected by a provider during a visit to the Los Angeles Gay and Lesbian Center sexual health program, a community partner of the Los Angeles County Department of Public Health.
Their mean age was 34 years; 78% said they were gay, 11% said they were bisexual, 8% heterosexual, and 1% transgender.
Overall prevalence of rectal chlamydia was 19%. Provider- and self-collected swabs had a diagnostic agreement of 97%. Rectal chlamydia was found in 39 self-collected specimens and 40 provider-collected specimens. Overall prevalence of rectal gonorrhea was 16%. Provider- and self-collected swabs had a diagnostic agreement of 95%. Gonorrhea was found in 42 self-collected specimens and 37 provider-collected specimens. The sensitivity of self-collected swabs for both infections was 93%. Sensitivity of provider-collected swabs was 95% for chlamydia and 82% for gonorrhea.
CHICAGO — Patient-collected rectal swabs are just as accurate as provider-collected swabs for diagnosing chlamydia and gonorrhea infections in men, Dr. Christine Wigen reported at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention
Allowing men to collect their own specimens may help boost testing rates at STD clinics that lack appropriate staff, she said.
“Some STD testing sites don't have [the staff] to take samples from anatomical sites such as the rectum,” she said in an interview. “[They] may only have a phlebotomist to draw blood for HIV or syphilis tests and can only take urine specimens for genitourinary testing of gonorrhea and chlamydia. In the past, the provider had to collect the rectal specimens [so] the test wouldn't be done. With the self-collected method, the testing is possible even in the absence of a provider.”
In addition, she said, if the patient is asymptomatic, then the self-collected method fast-tracks him through the screening process, which would free up the provider to focus on symptomatic individuals and on those with positive tests.
Dr. Wigen examined the accuracy of 225 paired rectal swab samples collected from men who had experienced receptive anal sex in the previous year. Each provided both a self-collected swab and a swab collected by a provider during a visit to the Los Angeles Gay and Lesbian Center sexual health program, a community partner of the Los Angeles County Department of Public Health.
Their mean age was 34 years; 78% said they were gay, 11% said they were bisexual, 8% heterosexual, and 1% transgender.
Overall prevalence of rectal chlamydia was 19%. Provider- and self-collected swabs had a diagnostic agreement of 97%. Rectal chlamydia was found in 39 self-collected specimens and 40 provider-collected specimens. Overall prevalence of rectal gonorrhea was 16%. Provider- and self-collected swabs had a diagnostic agreement of 95%. Gonorrhea was found in 42 self-collected specimens and 37 provider-collected specimens. The sensitivity of self-collected swabs for both infections was 93%. Sensitivity of provider-collected swabs was 95% for chlamydia and 82% for gonorrhea.