User login
European Association for the Study of Diabetes (EASD): Annual Meeting
EASD: Alirocumab lipid-lowering benefits extend to patients with high-risk diabetes
STOCKHOLM – The lipid-lowering drug alirocumab significantly lowered low-density lipoprotein cholesterol level to a similar extent in people with diabetes mellitus as in those without diabetes, based on a subanalysis of data from the ODYSSEY Long-Term study.
The calculated change in low-density lipoprotein cholesterol from baseline to 24 weeks was –60.1% with alirocumab vs. –0.9% with placebo, for an overall difference of –59.2% in those with diabetes. By comparison, the corresponding mean changes in LDL cholesterol were –61.5% and –1.8% (a difference of –59.7%) in nondiabetic individuals.
Improvements in other parameters – including triglycerides, high-density lipoprotein cholesterol, and lipoprotein(a) (Lp[a]) – were also similar in individuals with and without diabetes.
“The ODYSSEY Long-Term study is the longest study in the ODYSSEY phase III program,” said study investigator Dr. Helen Colhoun of the University of Dundee (Scotland). The primary endpoint was assessed at 24 weeks, but the trial ran for a further 54 weeks to gather as much lipid and general safety data as possible, she explained at the annual meeting of the European Association for the Study of Diabetes.
Alirocumab (Praulent) is a fully human monoclonal antibody that binds PCSK9 (proprotein convertase subtilisin/kexin type 9) that was recently approved by the Food and Drug Administration for use as an adjunct to diet and maximally tolerated statin therapy in people with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease who need additional LDL-cholesterol lowering.
The main results of the ODYSSEY Long-Term study were published in the New England Journal of Medicine in April (2015;372:1489-99 doi: 10.1056/NEJMoa1501031) and showed a –62% difference (P less than .001) in the mean percentage change from baseline in calculated LDL cholesterol between the alirocumab and placebo groups at 24 weeks. The difference remained consistent over the 78 weeks of follow-up.
Overall, the study included 2,341 individuals at high risk for cardiovascular disease with an LDL cholesterol level of more than 70 mg/dL despite being treated with maximally tolerated doses of statins with or without additional lipid-lowering therapy. Of these, just over one third of the population (35.7%, n = 838) had diabetes. Patients in the trial were randomized 2:1 to receive either alirocumab 150 mg or matching placebo as a 1-mL injection once every 2 weeks.
The present analysis looked at the long-term efficacy and safety of alirocumab in the diabetic subpopulation. Baseline demographic and lipid characteristics of the 838 patients with diabetes were broadly similar to the 1,503 patients in the trial who did not have diabetes, with mean ages of 61 and 60 years. Dr. Colhoun pointed out that virtually all patients (999; 100%) were taking a background statin, and well over one-third of those with diabetes and half of those without were taking a high-dose statin. Approximately 26% of the diabetic population were receiving insulin. Diabetic patients had slightly lower LDL cholesterol and higher triglycerides than their nondiabetic counterparts at baseline, as would be expected, she added.
Similarly high proportions of patients with (78.4% vs. 10.6%, P less than .0001) and without (79.7% vs. 6.6%, P less than .0001) diabetes who were treated with alirocumab vs. placebo achieved a target LDL cholesterol level of less than 70 mg/dL at 24 weeks.
Alirocumab treatment was associated with a 3.5% increase in HDL cholesterol in patients with diabetes and a 5.6% increase in those without, and triglycerides were reduced by a respective –18.5% and –16.7%. The adjusted mean differences in Lp(a) were –27.2% and –24.9%, an effect not seen with statins, Dr. Colhoun said.
“Overall the safety profile was excellent with this new class of drug, and there weren’t any major surprises or differences in safety profile between those with diabetes receiving alirocumab and those without diabetes,” she noted. Less than 10% of adverse events led to treatment discontinuation, at 8.3% vs. 5.0% for active and placebo treatment in the diabetic group and 6.5% vs. 6.3% in those without diabetes.
Data on the risk for new-onset or worsening diabetes in the ODYSSEY program will be presented at the scientific sessions of the American Heart Association in November, Dr. Colhoun said, giving a sneak peak of the findings from the ODYSSEY Long-Term study that indicated that there was no increased risk for either. Rates of new-onset diabetes were 1.8% in the alirocumab-treated group and 2% in the placebo group and of worsening diabetes, were 12.9% and 13.6%, respectively.
Treatment-emergent major cardiovascular events (MACE) were evaluated as a safety parameter in a post hoc analysis. Although not a main endpoint of the study, the results showed a lower rate of MACE in the overall population with alirocumab than placebo treatment (1.7% vs. 3.3%; hazard ratio, 0.52; 95% confidence interval, 0.31-0.90) and in both diabetic (2.5% vs. 4.3%; HR, 0.58; 95% CI, 0.27-1.25) and nondiabetic subjects (1.3% vs. 2.8%; HR, 0.47; 95% CI, 0.22-1.0). “This is reassuring, but it is not by any means definitive evidence of a [cardiovascular disease] benefit,” Dr. Colhoun stressed.
The potential for alirocumab to reduce cardiovascular events will be investigated in the large, phase III ODYSSEY Outcomes study. This trial is currently recruiting and aims to accrue around 18,000 high-risk patients including those with diabetes. The study is projected to complete by the end of 2017.
STOCKHOLM – The lipid-lowering drug alirocumab significantly lowered low-density lipoprotein cholesterol level to a similar extent in people with diabetes mellitus as in those without diabetes, based on a subanalysis of data from the ODYSSEY Long-Term study.
The calculated change in low-density lipoprotein cholesterol from baseline to 24 weeks was –60.1% with alirocumab vs. –0.9% with placebo, for an overall difference of –59.2% in those with diabetes. By comparison, the corresponding mean changes in LDL cholesterol were –61.5% and –1.8% (a difference of –59.7%) in nondiabetic individuals.
Improvements in other parameters – including triglycerides, high-density lipoprotein cholesterol, and lipoprotein(a) (Lp[a]) – were also similar in individuals with and without diabetes.
“The ODYSSEY Long-Term study is the longest study in the ODYSSEY phase III program,” said study investigator Dr. Helen Colhoun of the University of Dundee (Scotland). The primary endpoint was assessed at 24 weeks, but the trial ran for a further 54 weeks to gather as much lipid and general safety data as possible, she explained at the annual meeting of the European Association for the Study of Diabetes.
Alirocumab (Praulent) is a fully human monoclonal antibody that binds PCSK9 (proprotein convertase subtilisin/kexin type 9) that was recently approved by the Food and Drug Administration for use as an adjunct to diet and maximally tolerated statin therapy in people with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease who need additional LDL-cholesterol lowering.
The main results of the ODYSSEY Long-Term study were published in the New England Journal of Medicine in April (2015;372:1489-99 doi: 10.1056/NEJMoa1501031) and showed a –62% difference (P less than .001) in the mean percentage change from baseline in calculated LDL cholesterol between the alirocumab and placebo groups at 24 weeks. The difference remained consistent over the 78 weeks of follow-up.
Overall, the study included 2,341 individuals at high risk for cardiovascular disease with an LDL cholesterol level of more than 70 mg/dL despite being treated with maximally tolerated doses of statins with or without additional lipid-lowering therapy. Of these, just over one third of the population (35.7%, n = 838) had diabetes. Patients in the trial were randomized 2:1 to receive either alirocumab 150 mg or matching placebo as a 1-mL injection once every 2 weeks.
The present analysis looked at the long-term efficacy and safety of alirocumab in the diabetic subpopulation. Baseline demographic and lipid characteristics of the 838 patients with diabetes were broadly similar to the 1,503 patients in the trial who did not have diabetes, with mean ages of 61 and 60 years. Dr. Colhoun pointed out that virtually all patients (999; 100%) were taking a background statin, and well over one-third of those with diabetes and half of those without were taking a high-dose statin. Approximately 26% of the diabetic population were receiving insulin. Diabetic patients had slightly lower LDL cholesterol and higher triglycerides than their nondiabetic counterparts at baseline, as would be expected, she added.
Similarly high proportions of patients with (78.4% vs. 10.6%, P less than .0001) and without (79.7% vs. 6.6%, P less than .0001) diabetes who were treated with alirocumab vs. placebo achieved a target LDL cholesterol level of less than 70 mg/dL at 24 weeks.
Alirocumab treatment was associated with a 3.5% increase in HDL cholesterol in patients with diabetes and a 5.6% increase in those without, and triglycerides were reduced by a respective –18.5% and –16.7%. The adjusted mean differences in Lp(a) were –27.2% and –24.9%, an effect not seen with statins, Dr. Colhoun said.
“Overall the safety profile was excellent with this new class of drug, and there weren’t any major surprises or differences in safety profile between those with diabetes receiving alirocumab and those without diabetes,” she noted. Less than 10% of adverse events led to treatment discontinuation, at 8.3% vs. 5.0% for active and placebo treatment in the diabetic group and 6.5% vs. 6.3% in those without diabetes.
Data on the risk for new-onset or worsening diabetes in the ODYSSEY program will be presented at the scientific sessions of the American Heart Association in November, Dr. Colhoun said, giving a sneak peak of the findings from the ODYSSEY Long-Term study that indicated that there was no increased risk for either. Rates of new-onset diabetes were 1.8% in the alirocumab-treated group and 2% in the placebo group and of worsening diabetes, were 12.9% and 13.6%, respectively.
Treatment-emergent major cardiovascular events (MACE) were evaluated as a safety parameter in a post hoc analysis. Although not a main endpoint of the study, the results showed a lower rate of MACE in the overall population with alirocumab than placebo treatment (1.7% vs. 3.3%; hazard ratio, 0.52; 95% confidence interval, 0.31-0.90) and in both diabetic (2.5% vs. 4.3%; HR, 0.58; 95% CI, 0.27-1.25) and nondiabetic subjects (1.3% vs. 2.8%; HR, 0.47; 95% CI, 0.22-1.0). “This is reassuring, but it is not by any means definitive evidence of a [cardiovascular disease] benefit,” Dr. Colhoun stressed.
The potential for alirocumab to reduce cardiovascular events will be investigated in the large, phase III ODYSSEY Outcomes study. This trial is currently recruiting and aims to accrue around 18,000 high-risk patients including those with diabetes. The study is projected to complete by the end of 2017.
STOCKHOLM – The lipid-lowering drug alirocumab significantly lowered low-density lipoprotein cholesterol level to a similar extent in people with diabetes mellitus as in those without diabetes, based on a subanalysis of data from the ODYSSEY Long-Term study.
The calculated change in low-density lipoprotein cholesterol from baseline to 24 weeks was –60.1% with alirocumab vs. –0.9% with placebo, for an overall difference of –59.2% in those with diabetes. By comparison, the corresponding mean changes in LDL cholesterol were –61.5% and –1.8% (a difference of –59.7%) in nondiabetic individuals.
Improvements in other parameters – including triglycerides, high-density lipoprotein cholesterol, and lipoprotein(a) (Lp[a]) – were also similar in individuals with and without diabetes.
“The ODYSSEY Long-Term study is the longest study in the ODYSSEY phase III program,” said study investigator Dr. Helen Colhoun of the University of Dundee (Scotland). The primary endpoint was assessed at 24 weeks, but the trial ran for a further 54 weeks to gather as much lipid and general safety data as possible, she explained at the annual meeting of the European Association for the Study of Diabetes.
Alirocumab (Praulent) is a fully human monoclonal antibody that binds PCSK9 (proprotein convertase subtilisin/kexin type 9) that was recently approved by the Food and Drug Administration for use as an adjunct to diet and maximally tolerated statin therapy in people with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease who need additional LDL-cholesterol lowering.
The main results of the ODYSSEY Long-Term study were published in the New England Journal of Medicine in April (2015;372:1489-99 doi: 10.1056/NEJMoa1501031) and showed a –62% difference (P less than .001) in the mean percentage change from baseline in calculated LDL cholesterol between the alirocumab and placebo groups at 24 weeks. The difference remained consistent over the 78 weeks of follow-up.
Overall, the study included 2,341 individuals at high risk for cardiovascular disease with an LDL cholesterol level of more than 70 mg/dL despite being treated with maximally tolerated doses of statins with or without additional lipid-lowering therapy. Of these, just over one third of the population (35.7%, n = 838) had diabetes. Patients in the trial were randomized 2:1 to receive either alirocumab 150 mg or matching placebo as a 1-mL injection once every 2 weeks.
The present analysis looked at the long-term efficacy and safety of alirocumab in the diabetic subpopulation. Baseline demographic and lipid characteristics of the 838 patients with diabetes were broadly similar to the 1,503 patients in the trial who did not have diabetes, with mean ages of 61 and 60 years. Dr. Colhoun pointed out that virtually all patients (999; 100%) were taking a background statin, and well over one-third of those with diabetes and half of those without were taking a high-dose statin. Approximately 26% of the diabetic population were receiving insulin. Diabetic patients had slightly lower LDL cholesterol and higher triglycerides than their nondiabetic counterparts at baseline, as would be expected, she added.
Similarly high proportions of patients with (78.4% vs. 10.6%, P less than .0001) and without (79.7% vs. 6.6%, P less than .0001) diabetes who were treated with alirocumab vs. placebo achieved a target LDL cholesterol level of less than 70 mg/dL at 24 weeks.
Alirocumab treatment was associated with a 3.5% increase in HDL cholesterol in patients with diabetes and a 5.6% increase in those without, and triglycerides were reduced by a respective –18.5% and –16.7%. The adjusted mean differences in Lp(a) were –27.2% and –24.9%, an effect not seen with statins, Dr. Colhoun said.
“Overall the safety profile was excellent with this new class of drug, and there weren’t any major surprises or differences in safety profile between those with diabetes receiving alirocumab and those without diabetes,” she noted. Less than 10% of adverse events led to treatment discontinuation, at 8.3% vs. 5.0% for active and placebo treatment in the diabetic group and 6.5% vs. 6.3% in those without diabetes.
Data on the risk for new-onset or worsening diabetes in the ODYSSEY program will be presented at the scientific sessions of the American Heart Association in November, Dr. Colhoun said, giving a sneak peak of the findings from the ODYSSEY Long-Term study that indicated that there was no increased risk for either. Rates of new-onset diabetes were 1.8% in the alirocumab-treated group and 2% in the placebo group and of worsening diabetes, were 12.9% and 13.6%, respectively.
Treatment-emergent major cardiovascular events (MACE) were evaluated as a safety parameter in a post hoc analysis. Although not a main endpoint of the study, the results showed a lower rate of MACE in the overall population with alirocumab than placebo treatment (1.7% vs. 3.3%; hazard ratio, 0.52; 95% confidence interval, 0.31-0.90) and in both diabetic (2.5% vs. 4.3%; HR, 0.58; 95% CI, 0.27-1.25) and nondiabetic subjects (1.3% vs. 2.8%; HR, 0.47; 95% CI, 0.22-1.0). “This is reassuring, but it is not by any means definitive evidence of a [cardiovascular disease] benefit,” Dr. Colhoun stressed.
The potential for alirocumab to reduce cardiovascular events will be investigated in the large, phase III ODYSSEY Outcomes study. This trial is currently recruiting and aims to accrue around 18,000 high-risk patients including those with diabetes. The study is projected to complete by the end of 2017.
AT EASD 2015
Key clinical point: The benefits of alirocumab seen in the main study population extend to people with diabetes, with no risk for new onset or worsening of diabetes.
Major finding: Mean placebo-corrected reductions in LDL cholesterol level at 24 weeks were –59.2% in people with and –63.3% in people without diabetes (P = .1155).
Data source: A randomized, double-blind placebo-controlled study of alirocumab or placebo added to existing lipid-lowering treatment in 2,341 individuals at high cardiovascular risk and hyperlipidemia despite receiving statin therapy, including 838 with diabetes.
Disclosures: Sanofi and Regeneron Pharmaceuticals funded the study. Dr. Colhoun disclosed acting as an advisory panel member for both companies as well as several other companies including Roche, of which she was also a stockholder or shareholder.
EASD: Diabetes doubles death risk from many causes
STOCKHOLM – Having diabetes doubles the risk for death from a multitude of causes and shortens the life span by 5-7 years, judging from evidence from the United Kingdom Prospective Studies Collaboration presented at the annual meeting of the European Association for the Study of Diabetes.
Patients with diabetes were two and a half times more likely than those without it to die from ischemic heart disease (odds ratio, 2.37; 95% confidence interval, 2.23-2.52). They were also more likely to die from ischemic stroke (OR, 2.75; 95% CI, 2.29-3.31) and “a surprising association with” cardiac arrhythmia (OR, 2.47; 95% CI, 1.80-3.38) was found, said the presenting author, Dr. Louisa Gnatiuc of the clinical trial service unit and epidemiological studies unit at Oxford (England) University. She noted, however, that diabetes was more weakly associated with death from nonischemic causes (OR, 1.57; 95% CI, 1.34-1.85).
In addition, diabetic individuals were almost twice as likely as those with no diabetes to die from renal (OR, 1.90; 95% CI, 1.40-2.58) or hepatic (OR, 1.75; 95% CI, 1.36-2.26) causes, and there was a 13% increase in the overall risk for death from cancer (OR, 1.13; 95% CI, 1.06-1.21). The small increased risk for cancer death was largely due to deaths from oral (OR, 1.81; 95% CI, 1.17-2.81), liver (OR, 1.80; 95% CI, 1.26-2.58), and pancreatic (OR, 1.57; 95% CI, 1.21-2.03) cancers, Dr. Gnatiuc observed. Diabetic individuals were also more likely to die from respiratory causes than their nondiabetic counterparts (OR, 1.59; 95% CI, 1.37-1.83).
These data provide further insight into the risk for death from a multitude of causes in patients with diabetes, said Dr. Naveed Sattar, who was not involved in the analysis, in an interview. “While it is pretty much a replication of the Emerging Risk Factor Paper that was published in the New England Journal of Medicine, there is a little bit of new information here, such as the relative risks in women versus men and by age as well,” he observed.
The odds ratio for death from ischemic heart disease was higher among women aged 35-39 years with diabetes versus those with no diabetes than in men aged 35-39 years with diabetes versus no diabetes, at 5.61 (95% CI, 4.08-7.72) and 2.49 (95% CI, 2.16-2.85), respectively. Similar finding were seen at other age ranges, with around a twofold increase in men aged 60-69 years (OR, 2.26; 95% CI, 2.01-2.53) and 70-89 years (OR, 1.89; 95% CI, 1.70-2.11), but a two- to fourfold increase in women aged 60-69 years (OR, 4.15; 95% CI, 3.38-5.10) and 70-79 years (OR, 2.45, 95% CI, 2.11-2.84).
“So it looks like if you develop diabetes younger, your risk for mortality’s higher,” said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences, University of Glasgow (Scotland). “That makes sense,” he added, “because if you develop type 2 diabetes at the age of 30, it must be a much more malignant type of diabetes than, say, if you developed diabetes at age 80, and there are other data that now confirm that is the case as well.”
The analysis was based on data from 44 prospective studies that recorded 55,855 deaths in 690,700 adults who had no prior vascular or other chronic diseases at baseline. Men accounted for 60% of the study population, with a mean age of 48 years. During the postpresentation discussion, Dr. Gnatiuc noted that it was difficult to standardize the definition of diabetes used in the study and that all 25,000 cases of diabetes included in the study had been self-reported. It is likely that most cases were type 2. No information on the duration of diabetes was available, but she noted that this should not matter given the prospective nature of the analysis.
During 13 million patient-years of follow up the most common cause of death was ischemic heart disease, accounting for 17,218 deaths, followed by cancer (18,658 deaths). There were 5,466 strokes of which 1,353 were ischemic (OR, 2.75; 95% CI 2.29-3.31), 1,124 hemorrhagic (OR, 1.59; 95% CI 1.26-2.01), 2,563 that were unspecified (OR, 2.30; 95% CI 1.99-2.66), and 626 classed as subarachnoid hemorrhage (OR, 0.62; 95% CI, 0.36-1.08). Vascular causes of death included inflammatory heart disease, sudden death, and atherosclerosis, among others, which were all increased in diabetic versus nondiabetic subjects.
Dr. Gnatiuc noted that the risk for ischemic heart disease increased with increasing age, systolic blood pressure, and total cholesterol in diabetics and in nondiabetics, although it was much higher in those with diabetes than in those without. “Controlling blood pressure, cholesterol, and obesity are particularly important to reduce absolute mortality rates in those with diabetes, she suggested.
The differences between the sexes in terms of ischemic heart disease need further study, she suggested, noting that improved understanding of the sex differences may help better understand and thus reduce the excess mortality seen.
Premature mortality of up to 5 years in men and 7 years in women was observed. “At the age of 70 a man with diabetes would have about 10% less probability to survive up to the age of 90 as compared to their nondiabetic counterparts,” Dr. Gnatiuc said. Women would have about a 30% decrease in survival probability from age 70-90, she added.
STOCKHOLM – Having diabetes doubles the risk for death from a multitude of causes and shortens the life span by 5-7 years, judging from evidence from the United Kingdom Prospective Studies Collaboration presented at the annual meeting of the European Association for the Study of Diabetes.
Patients with diabetes were two and a half times more likely than those without it to die from ischemic heart disease (odds ratio, 2.37; 95% confidence interval, 2.23-2.52). They were also more likely to die from ischemic stroke (OR, 2.75; 95% CI, 2.29-3.31) and “a surprising association with” cardiac arrhythmia (OR, 2.47; 95% CI, 1.80-3.38) was found, said the presenting author, Dr. Louisa Gnatiuc of the clinical trial service unit and epidemiological studies unit at Oxford (England) University. She noted, however, that diabetes was more weakly associated with death from nonischemic causes (OR, 1.57; 95% CI, 1.34-1.85).
In addition, diabetic individuals were almost twice as likely as those with no diabetes to die from renal (OR, 1.90; 95% CI, 1.40-2.58) or hepatic (OR, 1.75; 95% CI, 1.36-2.26) causes, and there was a 13% increase in the overall risk for death from cancer (OR, 1.13; 95% CI, 1.06-1.21). The small increased risk for cancer death was largely due to deaths from oral (OR, 1.81; 95% CI, 1.17-2.81), liver (OR, 1.80; 95% CI, 1.26-2.58), and pancreatic (OR, 1.57; 95% CI, 1.21-2.03) cancers, Dr. Gnatiuc observed. Diabetic individuals were also more likely to die from respiratory causes than their nondiabetic counterparts (OR, 1.59; 95% CI, 1.37-1.83).
These data provide further insight into the risk for death from a multitude of causes in patients with diabetes, said Dr. Naveed Sattar, who was not involved in the analysis, in an interview. “While it is pretty much a replication of the Emerging Risk Factor Paper that was published in the New England Journal of Medicine, there is a little bit of new information here, such as the relative risks in women versus men and by age as well,” he observed.
The odds ratio for death from ischemic heart disease was higher among women aged 35-39 years with diabetes versus those with no diabetes than in men aged 35-39 years with diabetes versus no diabetes, at 5.61 (95% CI, 4.08-7.72) and 2.49 (95% CI, 2.16-2.85), respectively. Similar finding were seen at other age ranges, with around a twofold increase in men aged 60-69 years (OR, 2.26; 95% CI, 2.01-2.53) and 70-89 years (OR, 1.89; 95% CI, 1.70-2.11), but a two- to fourfold increase in women aged 60-69 years (OR, 4.15; 95% CI, 3.38-5.10) and 70-79 years (OR, 2.45, 95% CI, 2.11-2.84).
“So it looks like if you develop diabetes younger, your risk for mortality’s higher,” said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences, University of Glasgow (Scotland). “That makes sense,” he added, “because if you develop type 2 diabetes at the age of 30, it must be a much more malignant type of diabetes than, say, if you developed diabetes at age 80, and there are other data that now confirm that is the case as well.”
The analysis was based on data from 44 prospective studies that recorded 55,855 deaths in 690,700 adults who had no prior vascular or other chronic diseases at baseline. Men accounted for 60% of the study population, with a mean age of 48 years. During the postpresentation discussion, Dr. Gnatiuc noted that it was difficult to standardize the definition of diabetes used in the study and that all 25,000 cases of diabetes included in the study had been self-reported. It is likely that most cases were type 2. No information on the duration of diabetes was available, but she noted that this should not matter given the prospective nature of the analysis.
During 13 million patient-years of follow up the most common cause of death was ischemic heart disease, accounting for 17,218 deaths, followed by cancer (18,658 deaths). There were 5,466 strokes of which 1,353 were ischemic (OR, 2.75; 95% CI 2.29-3.31), 1,124 hemorrhagic (OR, 1.59; 95% CI 1.26-2.01), 2,563 that were unspecified (OR, 2.30; 95% CI 1.99-2.66), and 626 classed as subarachnoid hemorrhage (OR, 0.62; 95% CI, 0.36-1.08). Vascular causes of death included inflammatory heart disease, sudden death, and atherosclerosis, among others, which were all increased in diabetic versus nondiabetic subjects.
Dr. Gnatiuc noted that the risk for ischemic heart disease increased with increasing age, systolic blood pressure, and total cholesterol in diabetics and in nondiabetics, although it was much higher in those with diabetes than in those without. “Controlling blood pressure, cholesterol, and obesity are particularly important to reduce absolute mortality rates in those with diabetes, she suggested.
The differences between the sexes in terms of ischemic heart disease need further study, she suggested, noting that improved understanding of the sex differences may help better understand and thus reduce the excess mortality seen.
Premature mortality of up to 5 years in men and 7 years in women was observed. “At the age of 70 a man with diabetes would have about 10% less probability to survive up to the age of 90 as compared to their nondiabetic counterparts,” Dr. Gnatiuc said. Women would have about a 30% decrease in survival probability from age 70-90, she added.
STOCKHOLM – Having diabetes doubles the risk for death from a multitude of causes and shortens the life span by 5-7 years, judging from evidence from the United Kingdom Prospective Studies Collaboration presented at the annual meeting of the European Association for the Study of Diabetes.
Patients with diabetes were two and a half times more likely than those without it to die from ischemic heart disease (odds ratio, 2.37; 95% confidence interval, 2.23-2.52). They were also more likely to die from ischemic stroke (OR, 2.75; 95% CI, 2.29-3.31) and “a surprising association with” cardiac arrhythmia (OR, 2.47; 95% CI, 1.80-3.38) was found, said the presenting author, Dr. Louisa Gnatiuc of the clinical trial service unit and epidemiological studies unit at Oxford (England) University. She noted, however, that diabetes was more weakly associated with death from nonischemic causes (OR, 1.57; 95% CI, 1.34-1.85).
In addition, diabetic individuals were almost twice as likely as those with no diabetes to die from renal (OR, 1.90; 95% CI, 1.40-2.58) or hepatic (OR, 1.75; 95% CI, 1.36-2.26) causes, and there was a 13% increase in the overall risk for death from cancer (OR, 1.13; 95% CI, 1.06-1.21). The small increased risk for cancer death was largely due to deaths from oral (OR, 1.81; 95% CI, 1.17-2.81), liver (OR, 1.80; 95% CI, 1.26-2.58), and pancreatic (OR, 1.57; 95% CI, 1.21-2.03) cancers, Dr. Gnatiuc observed. Diabetic individuals were also more likely to die from respiratory causes than their nondiabetic counterparts (OR, 1.59; 95% CI, 1.37-1.83).
These data provide further insight into the risk for death from a multitude of causes in patients with diabetes, said Dr. Naveed Sattar, who was not involved in the analysis, in an interview. “While it is pretty much a replication of the Emerging Risk Factor Paper that was published in the New England Journal of Medicine, there is a little bit of new information here, such as the relative risks in women versus men and by age as well,” he observed.
The odds ratio for death from ischemic heart disease was higher among women aged 35-39 years with diabetes versus those with no diabetes than in men aged 35-39 years with diabetes versus no diabetes, at 5.61 (95% CI, 4.08-7.72) and 2.49 (95% CI, 2.16-2.85), respectively. Similar finding were seen at other age ranges, with around a twofold increase in men aged 60-69 years (OR, 2.26; 95% CI, 2.01-2.53) and 70-89 years (OR, 1.89; 95% CI, 1.70-2.11), but a two- to fourfold increase in women aged 60-69 years (OR, 4.15; 95% CI, 3.38-5.10) and 70-79 years (OR, 2.45, 95% CI, 2.11-2.84).
“So it looks like if you develop diabetes younger, your risk for mortality’s higher,” said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences, University of Glasgow (Scotland). “That makes sense,” he added, “because if you develop type 2 diabetes at the age of 30, it must be a much more malignant type of diabetes than, say, if you developed diabetes at age 80, and there are other data that now confirm that is the case as well.”
The analysis was based on data from 44 prospective studies that recorded 55,855 deaths in 690,700 adults who had no prior vascular or other chronic diseases at baseline. Men accounted for 60% of the study population, with a mean age of 48 years. During the postpresentation discussion, Dr. Gnatiuc noted that it was difficult to standardize the definition of diabetes used in the study and that all 25,000 cases of diabetes included in the study had been self-reported. It is likely that most cases were type 2. No information on the duration of diabetes was available, but she noted that this should not matter given the prospective nature of the analysis.
During 13 million patient-years of follow up the most common cause of death was ischemic heart disease, accounting for 17,218 deaths, followed by cancer (18,658 deaths). There were 5,466 strokes of which 1,353 were ischemic (OR, 2.75; 95% CI 2.29-3.31), 1,124 hemorrhagic (OR, 1.59; 95% CI 1.26-2.01), 2,563 that were unspecified (OR, 2.30; 95% CI 1.99-2.66), and 626 classed as subarachnoid hemorrhage (OR, 0.62; 95% CI, 0.36-1.08). Vascular causes of death included inflammatory heart disease, sudden death, and atherosclerosis, among others, which were all increased in diabetic versus nondiabetic subjects.
Dr. Gnatiuc noted that the risk for ischemic heart disease increased with increasing age, systolic blood pressure, and total cholesterol in diabetics and in nondiabetics, although it was much higher in those with diabetes than in those without. “Controlling blood pressure, cholesterol, and obesity are particularly important to reduce absolute mortality rates in those with diabetes, she suggested.
The differences between the sexes in terms of ischemic heart disease need further study, she suggested, noting that improved understanding of the sex differences may help better understand and thus reduce the excess mortality seen.
Premature mortality of up to 5 years in men and 7 years in women was observed. “At the age of 70 a man with diabetes would have about 10% less probability to survive up to the age of 90 as compared to their nondiabetic counterparts,” Dr. Gnatiuc said. Women would have about a 30% decrease in survival probability from age 70-90, she added.
AT EASD 2015
Key clinical point:Women with diabetes were more likely to die from ischemic heart disease than men with diabetes when compared to those without diabetes.
Major finding: Women with diabetes were more likely to die from ischemic heart disease than men with diabetes when compared to those without diabetes.
Data source: United Kingdom Prospective Studies Collaboration analysis of 44 prospective studies that recorded 55,855 deaths in 690,700 adults who had no prior vascular or other chronic diseases at baseline.
Disclosures: The study was funded by research grants from the British Heart Foundation, UK Medical Research Council, The National Institutes of Health (UK), Cancer Research UK, and the Clinical Trial Service Unit and Epidemiological Studies Unit at Oxford University, UK. Dr. Gnatiuc did not report any personal disclosures.
Metformin-induced B12 Deficiency Linked to Diabetic Neuropathy
STOCKHOLM – Clinicians might want to consider more routinely monitoring levels of vitamin B12 in their diabetic patients who are using metformin, according to new data from the HOME (Hyperinsulinaemia: the Outcome of Its Metabolic Effects) randomized controlled trial, which showed that B12 depletion was linked to neurotoxicity.
The new study findings, presented at the annual meeting of the European Association for the Study of Diabetes, showed that metformin increased levels of serum methylmalonic acid (MMA), the gold standard biomarker of tissue B12 deficiency, and that this in turn was linked to neuropathy measured using a validated score.
“We earlier showed that metformin causes B12 deficiency (<150 pmol/L) with a number needed to harm of 14 after 4.3 years,” said study investigator Dr. Mattijs Out of Bethesda Diabetes Research Center in Hoogeveen, the Netherlands, referring to the original results of the trial (BMJ. 2010;340:c2181).
“Today I showed you that metformin increases MMA, dose dependently and progressively over time,” he added. “Metformin has two opposite effects on neuropathy,” he continued, “a neuroprotective effect by improving glycemic control and a neurotoxic effect by inducing B12 depletion.”
Dr. Out noted that metformin remains the cornerstone of type 2 diabetes therapy with over 100 million prescriptions per year, putting many patients at risk for B12 deficiency and its consequences.
Current guidelines from the American Diabetes Association and EASD mention B12 deficiency as a potential downside of metformin use but do not go so far as giving specific recommendations because of lack of evidence on whether screening should be routinely performed or if vitamin B12 supplements should be given.
“Consequences of vitamin B12 deficiency, such as neuropathy or mental changes, may be profound,” Dr. Out said. Changes because of B12 deficits can be difficult to diagnose because they may be ascribed to old age or diabetes itself, and they may become irreversible if unchecked, he observed. Diagnosing and managing B12 deficiency is, in theory, “easy, cheap, and effective,” he said. A new trial would be needed, however, to determine whether screening for B12 deficiency or supplementation would be the better approach.
In the current study, structural equation monitoring (SEM) was used to determine the likely effects of metformin on the Valk neuropathy score (Diabetes Med. 1992;9:716-21) directly and via effects on hemoglobin A1c and MMA. The analysis involved 390 insulin-treated patients with type 2 diabetes who were also treated with 850 mg metformin or placebo up to three times daily for 52 months. Over a 4.3-year follow-up period, patients had HbA1c and neuropathy scores measured 17 times, and MMA was measured at six visits.
Compared with placebo, metformin use was associated with a significant (P= .001) 0.039 micromol/L (95% confidence interval, 0.019-0.055) increase in MMA over the course of the study.
While there was no significant difference in neuropathy scores between the placebo- and metformin-treated groups, SEM showed that metformin had a beneficial effect on lowering the neuropathy score via lowering HbA1c and an adverse effect on the neuropathy score by increasing MMA levels. Overall, metformin was associated with a 0.25-point increase in the neuropathy score, suggesting the net effect of the oral hypoglycemic drug is a negative one as higher scores mean worse neuropathy.
After the presentation of the findings, session chair Dr. Guntram Schernthaner commented: “In my center in Vienna, we have been using metformin for 30 years, but we have never used this high dosage [850 mg]. We have suspected several times the B12 deficiency and we measured it in many cases, but we never found any cases of severe B12 deficiency.”
Dr. Schernthaner, who is professor of medicine at the University of Vienna in Austria, suggested the next step would be to perform a large trial of maybe 2,000 metformin users to see how often B12 deficiency really occurs.
Dr. Out agreed a larger trial would be the ideal and conceded that the effect size was small. However, with such a large number of patients using the drug worldwide, potentially “many patients are at risk.”
He said a direct effect of metformin on neuropathy would perhaps not be found by general screening because of its protective effect via improved glycemic control, “so you may miss B12 deficiency–induced neuropathy.” Measuring MMA, where possible, may be a solution.
STOCKHOLM – Clinicians might want to consider more routinely monitoring levels of vitamin B12 in their diabetic patients who are using metformin, according to new data from the HOME (Hyperinsulinaemia: the Outcome of Its Metabolic Effects) randomized controlled trial, which showed that B12 depletion was linked to neurotoxicity.
The new study findings, presented at the annual meeting of the European Association for the Study of Diabetes, showed that metformin increased levels of serum methylmalonic acid (MMA), the gold standard biomarker of tissue B12 deficiency, and that this in turn was linked to neuropathy measured using a validated score.
“We earlier showed that metformin causes B12 deficiency (<150 pmol/L) with a number needed to harm of 14 after 4.3 years,” said study investigator Dr. Mattijs Out of Bethesda Diabetes Research Center in Hoogeveen, the Netherlands, referring to the original results of the trial (BMJ. 2010;340:c2181).
“Today I showed you that metformin increases MMA, dose dependently and progressively over time,” he added. “Metformin has two opposite effects on neuropathy,” he continued, “a neuroprotective effect by improving glycemic control and a neurotoxic effect by inducing B12 depletion.”
Dr. Out noted that metformin remains the cornerstone of type 2 diabetes therapy with over 100 million prescriptions per year, putting many patients at risk for B12 deficiency and its consequences.
Current guidelines from the American Diabetes Association and EASD mention B12 deficiency as a potential downside of metformin use but do not go so far as giving specific recommendations because of lack of evidence on whether screening should be routinely performed or if vitamin B12 supplements should be given.
“Consequences of vitamin B12 deficiency, such as neuropathy or mental changes, may be profound,” Dr. Out said. Changes because of B12 deficits can be difficult to diagnose because they may be ascribed to old age or diabetes itself, and they may become irreversible if unchecked, he observed. Diagnosing and managing B12 deficiency is, in theory, “easy, cheap, and effective,” he said. A new trial would be needed, however, to determine whether screening for B12 deficiency or supplementation would be the better approach.
In the current study, structural equation monitoring (SEM) was used to determine the likely effects of metformin on the Valk neuropathy score (Diabetes Med. 1992;9:716-21) directly and via effects on hemoglobin A1c and MMA. The analysis involved 390 insulin-treated patients with type 2 diabetes who were also treated with 850 mg metformin or placebo up to three times daily for 52 months. Over a 4.3-year follow-up period, patients had HbA1c and neuropathy scores measured 17 times, and MMA was measured at six visits.
Compared with placebo, metformin use was associated with a significant (P= .001) 0.039 micromol/L (95% confidence interval, 0.019-0.055) increase in MMA over the course of the study.
While there was no significant difference in neuropathy scores between the placebo- and metformin-treated groups, SEM showed that metformin had a beneficial effect on lowering the neuropathy score via lowering HbA1c and an adverse effect on the neuropathy score by increasing MMA levels. Overall, metformin was associated with a 0.25-point increase in the neuropathy score, suggesting the net effect of the oral hypoglycemic drug is a negative one as higher scores mean worse neuropathy.
After the presentation of the findings, session chair Dr. Guntram Schernthaner commented: “In my center in Vienna, we have been using metformin for 30 years, but we have never used this high dosage [850 mg]. We have suspected several times the B12 deficiency and we measured it in many cases, but we never found any cases of severe B12 deficiency.”
Dr. Schernthaner, who is professor of medicine at the University of Vienna in Austria, suggested the next step would be to perform a large trial of maybe 2,000 metformin users to see how often B12 deficiency really occurs.
Dr. Out agreed a larger trial would be the ideal and conceded that the effect size was small. However, with such a large number of patients using the drug worldwide, potentially “many patients are at risk.”
He said a direct effect of metformin on neuropathy would perhaps not be found by general screening because of its protective effect via improved glycemic control, “so you may miss B12 deficiency–induced neuropathy.” Measuring MMA, where possible, may be a solution.
STOCKHOLM – Clinicians might want to consider more routinely monitoring levels of vitamin B12 in their diabetic patients who are using metformin, according to new data from the HOME (Hyperinsulinaemia: the Outcome of Its Metabolic Effects) randomized controlled trial, which showed that B12 depletion was linked to neurotoxicity.
The new study findings, presented at the annual meeting of the European Association for the Study of Diabetes, showed that metformin increased levels of serum methylmalonic acid (MMA), the gold standard biomarker of tissue B12 deficiency, and that this in turn was linked to neuropathy measured using a validated score.
“We earlier showed that metformin causes B12 deficiency (<150 pmol/L) with a number needed to harm of 14 after 4.3 years,” said study investigator Dr. Mattijs Out of Bethesda Diabetes Research Center in Hoogeveen, the Netherlands, referring to the original results of the trial (BMJ. 2010;340:c2181).
“Today I showed you that metformin increases MMA, dose dependently and progressively over time,” he added. “Metformin has two opposite effects on neuropathy,” he continued, “a neuroprotective effect by improving glycemic control and a neurotoxic effect by inducing B12 depletion.”
Dr. Out noted that metformin remains the cornerstone of type 2 diabetes therapy with over 100 million prescriptions per year, putting many patients at risk for B12 deficiency and its consequences.
Current guidelines from the American Diabetes Association and EASD mention B12 deficiency as a potential downside of metformin use but do not go so far as giving specific recommendations because of lack of evidence on whether screening should be routinely performed or if vitamin B12 supplements should be given.
“Consequences of vitamin B12 deficiency, such as neuropathy or mental changes, may be profound,” Dr. Out said. Changes because of B12 deficits can be difficult to diagnose because they may be ascribed to old age or diabetes itself, and they may become irreversible if unchecked, he observed. Diagnosing and managing B12 deficiency is, in theory, “easy, cheap, and effective,” he said. A new trial would be needed, however, to determine whether screening for B12 deficiency or supplementation would be the better approach.
In the current study, structural equation monitoring (SEM) was used to determine the likely effects of metformin on the Valk neuropathy score (Diabetes Med. 1992;9:716-21) directly and via effects on hemoglobin A1c and MMA. The analysis involved 390 insulin-treated patients with type 2 diabetes who were also treated with 850 mg metformin or placebo up to three times daily for 52 months. Over a 4.3-year follow-up period, patients had HbA1c and neuropathy scores measured 17 times, and MMA was measured at six visits.
Compared with placebo, metformin use was associated with a significant (P= .001) 0.039 micromol/L (95% confidence interval, 0.019-0.055) increase in MMA over the course of the study.
While there was no significant difference in neuropathy scores between the placebo- and metformin-treated groups, SEM showed that metformin had a beneficial effect on lowering the neuropathy score via lowering HbA1c and an adverse effect on the neuropathy score by increasing MMA levels. Overall, metformin was associated with a 0.25-point increase in the neuropathy score, suggesting the net effect of the oral hypoglycemic drug is a negative one as higher scores mean worse neuropathy.
After the presentation of the findings, session chair Dr. Guntram Schernthaner commented: “In my center in Vienna, we have been using metformin for 30 years, but we have never used this high dosage [850 mg]. We have suspected several times the B12 deficiency and we measured it in many cases, but we never found any cases of severe B12 deficiency.”
Dr. Schernthaner, who is professor of medicine at the University of Vienna in Austria, suggested the next step would be to perform a large trial of maybe 2,000 metformin users to see how often B12 deficiency really occurs.
Dr. Out agreed a larger trial would be the ideal and conceded that the effect size was small. However, with such a large number of patients using the drug worldwide, potentially “many patients are at risk.”
He said a direct effect of metformin on neuropathy would perhaps not be found by general screening because of its protective effect via improved glycemic control, “so you may miss B12 deficiency–induced neuropathy.” Measuring MMA, where possible, may be a solution.
AT EASD 2015
EASD: Metformin-induced B12 deficiency linked to diabetic neuropathy
STOCKHOLM – Clinicians might want to consider more routinely monitoring levels of vitamin B12 in their diabetic patients who are using metformin, according to new data from the HOME (Hyperinsulinaemia: the Outcome of Its Metabolic Effects) randomized controlled trial, which showed that B12 depletion was linked to neurotoxicity.
The new study findings, presented at the annual meeting of the European Association for the Study of Diabetes, showed that metformin increased levels of serum methylmalonic acid (MMA), the gold standard biomarker of tissue B12 deficiency, and that this in turn was linked to neuropathy measured using a validated score.
“We earlier showed that metformin causes B12 deficiency (<150 pmol/L) with a number needed to harm of 14 after 4.3 years,” said study investigator Dr. Mattijs Out of Bethesda Diabetes Research Center in Hoogeveen, the Netherlands, referring to the original results of the trial (BMJ. 2010;340:c2181).
“Today I showed you that metformin increases MMA, dose dependently and progressively over time,” he added. “Metformin has two opposite effects on neuropathy,” he continued, “a neuroprotective effect by improving glycemic control and a neurotoxic effect by inducing B12 depletion.”
Dr. Out noted that metformin remains the cornerstone of type 2 diabetes therapy with over 100 million prescriptions per year, putting many patients at risk for B12 deficiency and its consequences.
Current guidelines from the American Diabetes Association and EASD mention B12 deficiency as a potential downside of metformin use but do not go so far as giving specific recommendations because of lack of evidence on whether screening should be routinely performed or if vitamin B12 supplements should be given.
“Consequences of vitamin B12 deficiency, such as neuropathy or mental changes, may be profound,” Dr. Out said. Changes because of B12 deficits can be difficult to diagnose because they may be ascribed to old age or diabetes itself, and they may become irreversible if unchecked, he observed. Diagnosing and managing B12 deficiency is, in theory, “easy, cheap, and effective,” he said. A new trial would be needed, however, to determine whether screening for B12 deficiency or supplementation would be the better approach.
In the current study, structural equation monitoring (SEM) was used to determine the likely effects of metformin on the Valk neuropathy score (Diabetes Med. 1992;9:716-21) directly and via effects on hemoglobin A1c and MMA. The analysis involved 390 insulin-treated patients with type 2 diabetes who were also treated with 850 mg metformin or placebo up to three times daily for 52 months. Over a 4.3-year follow-up period, patients had HbA1c and neuropathy scores measured 17 times, and MMA was measured at six visits.
Compared with placebo, metformin use was associated with a significant (P= .001) 0.039 micromol/L (95% confidence interval, 0.019-0.055) increase in MMA over the course of the study.
While there was no significant difference in neuropathy scores between the placebo- and metformin-treated groups, SEM showed that metformin had a beneficial effect on lowering the neuropathy score via lowering HbA1c and an adverse effect on the neuropathy score by increasing MMA levels. Overall, metformin was associated with a 0.25-point increase in the neuropathy score, suggesting the net effect of the oral hypoglycemic drug is a negative one as higher scores mean worse neuropathy.
After the presentation of the findings, session chair Dr. Guntram Schernthaner commented: “In my center in Vienna, we have been using metformin for 30 years, but we have never used this high dosage [850 mg]. We have suspected several times the B12 deficiency and we measured it in many cases, but we never found any cases of severe B12 deficiency.”
Dr. Schernthaner, who is professor of medicine at the University of Vienna in Austria, suggested the next step would be to perform a large trial of maybe 2,000 metformin users to see how often B12 deficiency really occurs.
Dr. Out agreed a larger trial would be the ideal and conceded that the effect size was small. However, with such a large number of patients using the drug worldwide, potentially “many patients are at risk.”
He said a direct effect of metformin on neuropathy would perhaps not be found by general screening because of its protective effect via improved glycemic control, “so you may miss B12 deficiency–induced neuropathy.” Measuring MMA, where possible, may be a solution.
STOCKHOLM – Clinicians might want to consider more routinely monitoring levels of vitamin B12 in their diabetic patients who are using metformin, according to new data from the HOME (Hyperinsulinaemia: the Outcome of Its Metabolic Effects) randomized controlled trial, which showed that B12 depletion was linked to neurotoxicity.
The new study findings, presented at the annual meeting of the European Association for the Study of Diabetes, showed that metformin increased levels of serum methylmalonic acid (MMA), the gold standard biomarker of tissue B12 deficiency, and that this in turn was linked to neuropathy measured using a validated score.
“We earlier showed that metformin causes B12 deficiency (<150 pmol/L) with a number needed to harm of 14 after 4.3 years,” said study investigator Dr. Mattijs Out of Bethesda Diabetes Research Center in Hoogeveen, the Netherlands, referring to the original results of the trial (BMJ. 2010;340:c2181).
“Today I showed you that metformin increases MMA, dose dependently and progressively over time,” he added. “Metformin has two opposite effects on neuropathy,” he continued, “a neuroprotective effect by improving glycemic control and a neurotoxic effect by inducing B12 depletion.”
Dr. Out noted that metformin remains the cornerstone of type 2 diabetes therapy with over 100 million prescriptions per year, putting many patients at risk for B12 deficiency and its consequences.
Current guidelines from the American Diabetes Association and EASD mention B12 deficiency as a potential downside of metformin use but do not go so far as giving specific recommendations because of lack of evidence on whether screening should be routinely performed or if vitamin B12 supplements should be given.
“Consequences of vitamin B12 deficiency, such as neuropathy or mental changes, may be profound,” Dr. Out said. Changes because of B12 deficits can be difficult to diagnose because they may be ascribed to old age or diabetes itself, and they may become irreversible if unchecked, he observed. Diagnosing and managing B12 deficiency is, in theory, “easy, cheap, and effective,” he said. A new trial would be needed, however, to determine whether screening for B12 deficiency or supplementation would be the better approach.
In the current study, structural equation monitoring (SEM) was used to determine the likely effects of metformin on the Valk neuropathy score (Diabetes Med. 1992;9:716-21) directly and via effects on hemoglobin A1c and MMA. The analysis involved 390 insulin-treated patients with type 2 diabetes who were also treated with 850 mg metformin or placebo up to three times daily for 52 months. Over a 4.3-year follow-up period, patients had HbA1c and neuropathy scores measured 17 times, and MMA was measured at six visits.
Compared with placebo, metformin use was associated with a significant (P= .001) 0.039 micromol/L (95% confidence interval, 0.019-0.055) increase in MMA over the course of the study.
While there was no significant difference in neuropathy scores between the placebo- and metformin-treated groups, SEM showed that metformin had a beneficial effect on lowering the neuropathy score via lowering HbA1c and an adverse effect on the neuropathy score by increasing MMA levels. Overall, metformin was associated with a 0.25-point increase in the neuropathy score, suggesting the net effect of the oral hypoglycemic drug is a negative one as higher scores mean worse neuropathy.
After the presentation of the findings, session chair Dr. Guntram Schernthaner commented: “In my center in Vienna, we have been using metformin for 30 years, but we have never used this high dosage [850 mg]. We have suspected several times the B12 deficiency and we measured it in many cases, but we never found any cases of severe B12 deficiency.”
Dr. Schernthaner, who is professor of medicine at the University of Vienna in Austria, suggested the next step would be to perform a large trial of maybe 2,000 metformin users to see how often B12 deficiency really occurs.
Dr. Out agreed a larger trial would be the ideal and conceded that the effect size was small. However, with such a large number of patients using the drug worldwide, potentially “many patients are at risk.”
He said a direct effect of metformin on neuropathy would perhaps not be found by general screening because of its protective effect via improved glycemic control, “so you may miss B12 deficiency–induced neuropathy.” Measuring MMA, where possible, may be a solution.
STOCKHOLM – Clinicians might want to consider more routinely monitoring levels of vitamin B12 in their diabetic patients who are using metformin, according to new data from the HOME (Hyperinsulinaemia: the Outcome of Its Metabolic Effects) randomized controlled trial, which showed that B12 depletion was linked to neurotoxicity.
The new study findings, presented at the annual meeting of the European Association for the Study of Diabetes, showed that metformin increased levels of serum methylmalonic acid (MMA), the gold standard biomarker of tissue B12 deficiency, and that this in turn was linked to neuropathy measured using a validated score.
“We earlier showed that metformin causes B12 deficiency (<150 pmol/L) with a number needed to harm of 14 after 4.3 years,” said study investigator Dr. Mattijs Out of Bethesda Diabetes Research Center in Hoogeveen, the Netherlands, referring to the original results of the trial (BMJ. 2010;340:c2181).
“Today I showed you that metformin increases MMA, dose dependently and progressively over time,” he added. “Metformin has two opposite effects on neuropathy,” he continued, “a neuroprotective effect by improving glycemic control and a neurotoxic effect by inducing B12 depletion.”
Dr. Out noted that metformin remains the cornerstone of type 2 diabetes therapy with over 100 million prescriptions per year, putting many patients at risk for B12 deficiency and its consequences.
Current guidelines from the American Diabetes Association and EASD mention B12 deficiency as a potential downside of metformin use but do not go so far as giving specific recommendations because of lack of evidence on whether screening should be routinely performed or if vitamin B12 supplements should be given.
“Consequences of vitamin B12 deficiency, such as neuropathy or mental changes, may be profound,” Dr. Out said. Changes because of B12 deficits can be difficult to diagnose because they may be ascribed to old age or diabetes itself, and they may become irreversible if unchecked, he observed. Diagnosing and managing B12 deficiency is, in theory, “easy, cheap, and effective,” he said. A new trial would be needed, however, to determine whether screening for B12 deficiency or supplementation would be the better approach.
In the current study, structural equation monitoring (SEM) was used to determine the likely effects of metformin on the Valk neuropathy score (Diabetes Med. 1992;9:716-21) directly and via effects on hemoglobin A1c and MMA. The analysis involved 390 insulin-treated patients with type 2 diabetes who were also treated with 850 mg metformin or placebo up to three times daily for 52 months. Over a 4.3-year follow-up period, patients had HbA1c and neuropathy scores measured 17 times, and MMA was measured at six visits.
Compared with placebo, metformin use was associated with a significant (P= .001) 0.039 micromol/L (95% confidence interval, 0.019-0.055) increase in MMA over the course of the study.
While there was no significant difference in neuropathy scores between the placebo- and metformin-treated groups, SEM showed that metformin had a beneficial effect on lowering the neuropathy score via lowering HbA1c and an adverse effect on the neuropathy score by increasing MMA levels. Overall, metformin was associated with a 0.25-point increase in the neuropathy score, suggesting the net effect of the oral hypoglycemic drug is a negative one as higher scores mean worse neuropathy.
After the presentation of the findings, session chair Dr. Guntram Schernthaner commented: “In my center in Vienna, we have been using metformin for 30 years, but we have never used this high dosage [850 mg]. We have suspected several times the B12 deficiency and we measured it in many cases, but we never found any cases of severe B12 deficiency.”
Dr. Schernthaner, who is professor of medicine at the University of Vienna in Austria, suggested the next step would be to perform a large trial of maybe 2,000 metformin users to see how often B12 deficiency really occurs.
Dr. Out agreed a larger trial would be the ideal and conceded that the effect size was small. However, with such a large number of patients using the drug worldwide, potentially “many patients are at risk.”
He said a direct effect of metformin on neuropathy would perhaps not be found by general screening because of its protective effect via improved glycemic control, “so you may miss B12 deficiency–induced neuropathy.” Measuring MMA, where possible, may be a solution.
AT EASD 2015
Key clinical point: Metformin has an overall detrimental effect on neuropathy mediated by its effect on MMA, a specific biomarker of B12 deficiency.
Major finding: Metformin use was associated with a significant (P = .001) 0.04 micromol/L increase in MMA over the course of the study when compared with placebo.
Data source: A prospective, randomized controlled trial of 390 insulin-treated patients with type 2 diabetes who were randomized to metformin or placebo for 4.3 years.
Disclosures: Dr. Out reported that he had no disclosures. The HOME study was supported by Takeda, Lifescan, Merck Sharpe & Dohme, and Novo Nordisk.
EASD: Liraglutide lowers HbA1c when added to insulin in longstanding type 2 diabetes
STOCKHOLM – Adding liraglutide to multiple daily insulin injections helped stabilize blood glucose in patients with type 2 diabetes, while also resulting in weight loss and reduction of daily insulin doses.
All this was accomplished without an increase in the risk of hypoglycemia, Dr. Marcus Lind said at the annual meeting of the European Association for the Study of Diabetes.
A randomized, placebo-controlled study showed that the approach was successful in patients with long-standing disease, said Dr. Lind of the University of Gothenburg, Sweden. “We used to think that incretin-based therapies were most helpful in early type 2 diabetes. This seems to confirm that they are effective during the entire disease process.”
The soon-to-be-published 24-week study examined the addition of liraglutide in 124 patients with type 2 diabetes of long duration (a mean of 17 years). They were overweight, with a mean body mass index of 33.5 (about 218 pounds). At baseline, their mean hemoglobin A1c was 9%; they were taking a mean of 105 units of insulin each day in about four injections.
The study’s primary endpoint was change in HbA1c at 24 weeks. This declined significantly more among those taking liraglutide than those taking a placebo (–1.6% vs. –0.4%). “This is a difference of 1.1%, which is very clinically significant,” Dr. Lind said.
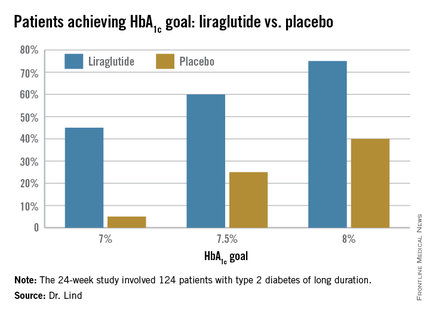
Dr. Lind assigned individualized HbA1c goals to patients, with the cutpoints of 7%, 7.5%, and 8%. Significantly more patients taking liraglutide were able to achieve those goals (see chart).
They also lost about 8 pounds more weight than did the placebo patients, and experienced a greater decrease in systolic blood pressure (–5.5 mm Hg more than placebo). There was no effect on diastolic blood pressure or lipids.
There were no severe hypoglycemic events in either group, nor any between-group difference in nonsevere events.
There were three serious adverse events among three patients taking liraglutide, and eight among four patients taking placebo; none of these were pancreatitis or pancreatic cancer.
Patients taking the study drug experienced significantly more nausea, especially at the beginning of the study, compared to the end of the study (22% vs. 5%).
The study was investigator initiated but received financial support from Novo Nordisk, which provided the drug. Dr. Lind disclosed financial relationships with numerous pharmaceutical companies, including honoraria from Novo Nordisk.
STOCKHOLM – Adding liraglutide to multiple daily insulin injections helped stabilize blood glucose in patients with type 2 diabetes, while also resulting in weight loss and reduction of daily insulin doses.
All this was accomplished without an increase in the risk of hypoglycemia, Dr. Marcus Lind said at the annual meeting of the European Association for the Study of Diabetes.
A randomized, placebo-controlled study showed that the approach was successful in patients with long-standing disease, said Dr. Lind of the University of Gothenburg, Sweden. “We used to think that incretin-based therapies were most helpful in early type 2 diabetes. This seems to confirm that they are effective during the entire disease process.”
The soon-to-be-published 24-week study examined the addition of liraglutide in 124 patients with type 2 diabetes of long duration (a mean of 17 years). They were overweight, with a mean body mass index of 33.5 (about 218 pounds). At baseline, their mean hemoglobin A1c was 9%; they were taking a mean of 105 units of insulin each day in about four injections.
The study’s primary endpoint was change in HbA1c at 24 weeks. This declined significantly more among those taking liraglutide than those taking a placebo (–1.6% vs. –0.4%). “This is a difference of 1.1%, which is very clinically significant,” Dr. Lind said.

Dr. Lind assigned individualized HbA1c goals to patients, with the cutpoints of 7%, 7.5%, and 8%. Significantly more patients taking liraglutide were able to achieve those goals (see chart).
They also lost about 8 pounds more weight than did the placebo patients, and experienced a greater decrease in systolic blood pressure (–5.5 mm Hg more than placebo). There was no effect on diastolic blood pressure or lipids.
There were no severe hypoglycemic events in either group, nor any between-group difference in nonsevere events.
There were three serious adverse events among three patients taking liraglutide, and eight among four patients taking placebo; none of these were pancreatitis or pancreatic cancer.
Patients taking the study drug experienced significantly more nausea, especially at the beginning of the study, compared to the end of the study (22% vs. 5%).
The study was investigator initiated but received financial support from Novo Nordisk, which provided the drug. Dr. Lind disclosed financial relationships with numerous pharmaceutical companies, including honoraria from Novo Nordisk.
STOCKHOLM – Adding liraglutide to multiple daily insulin injections helped stabilize blood glucose in patients with type 2 diabetes, while also resulting in weight loss and reduction of daily insulin doses.
All this was accomplished without an increase in the risk of hypoglycemia, Dr. Marcus Lind said at the annual meeting of the European Association for the Study of Diabetes.
A randomized, placebo-controlled study showed that the approach was successful in patients with long-standing disease, said Dr. Lind of the University of Gothenburg, Sweden. “We used to think that incretin-based therapies were most helpful in early type 2 diabetes. This seems to confirm that they are effective during the entire disease process.”
The soon-to-be-published 24-week study examined the addition of liraglutide in 124 patients with type 2 diabetes of long duration (a mean of 17 years). They were overweight, with a mean body mass index of 33.5 (about 218 pounds). At baseline, their mean hemoglobin A1c was 9%; they were taking a mean of 105 units of insulin each day in about four injections.
The study’s primary endpoint was change in HbA1c at 24 weeks. This declined significantly more among those taking liraglutide than those taking a placebo (–1.6% vs. –0.4%). “This is a difference of 1.1%, which is very clinically significant,” Dr. Lind said.

Dr. Lind assigned individualized HbA1c goals to patients, with the cutpoints of 7%, 7.5%, and 8%. Significantly more patients taking liraglutide were able to achieve those goals (see chart).
They also lost about 8 pounds more weight than did the placebo patients, and experienced a greater decrease in systolic blood pressure (–5.5 mm Hg more than placebo). There was no effect on diastolic blood pressure or lipids.
There were no severe hypoglycemic events in either group, nor any between-group difference in nonsevere events.
There were three serious adverse events among three patients taking liraglutide, and eight among four patients taking placebo; none of these were pancreatitis or pancreatic cancer.
Patients taking the study drug experienced significantly more nausea, especially at the beginning of the study, compared to the end of the study (22% vs. 5%).
The study was investigator initiated but received financial support from Novo Nordisk, which provided the drug. Dr. Lind disclosed financial relationships with numerous pharmaceutical companies, including honoraria from Novo Nordisk.
AT EASD 2015
Key clinical point: Liraglutide added to multiple daily insulin injections lowered HbA1c significantly more than did placebo in patients with type 2 diabetes.
Major finding: HbA1c at 24 weeks declined significantly more among those taking liraglutide than those taking a placebo (–1.6% vs. –0.4%).
Data source: A randomized, placebo-controlled study of 124 patients.
Disclosures: The study was investigator initiated but received financial support from Novo Nordisk, which provided the drug. Dr. Lind disclosed financial relationships with numerous pharmaceutical companies, including honoraria from Novo Nordisk.
EASD: Pre-pregnancy weight, not gestational gain, affects later diabetes risk
STOCKHOLM – Women had a more than six-fold increased risk of developing diabetes more than a decade after they gave birth if they were overweight or obese at the start of their pregnancy, according to the results of a large epidemiological study.
The odds ratio for the development of diabetes was 6.4 (95% confidence interval, 3.5-11.6, P < .001) comparing women who had a body mass index above 25 at the start of pregnancy with those who were of a lower body weight.
The heavier women also were more likely to be obese (OR, 21.9; 95% CI, 16.3-29.5; P< .001), have developed cardiac (OR, 2.7; 95% CI, 1.5-4.9; P= .001) or other endocrine (OR, 2.3; 95% CI, 1.5-3.4; P< .001) diseases 10-17 years later.
“A high pre-pregnancy BMI significantly increases the risk of several diseases later in life, including diabetes and cardiac disease,” Dr. Ulrika Moll of Lund University, Sweden, said at the annual meeting of the European Association for the Study of Diabetes.
The amount of weight gained during the pregnancy did not appear to matter, with no increase in the risk of cardiac or endocrine disease, even in women who gained more than the recommended weight set by the Institute of Medicine (IOM) guidelines. The IOM recommends that that women who are overweight should gain no more than 7–11.5 kg and those who are obese no more than 5-9 kg during their pregnancy. The mean maternal weight gain was 14.7 kg in the overweight group and 8.9 kg in the obese group.
Gaining more than 15 kg versus ≤15 kg during pregnancy did double the likelihood of women being overweight or obese in later life. A larger weight gain appeared to be protective against development of later psychiatric disease (OR, 0.6; 95% CI, 0.4-0.9; P= .03).
“A high weight gain during pregnancy did not have any implications on metabolic diseases within 10 years in our cohort,” Dr. Moll said. “These results have implications for the urgency of identification and care of the young women of childbearing age who struggle with being overweight or obese.”
Previous studies have shown that women who are overweight before they are pregnant and those that gain excessive amounts of weight during their pregnancies are more likely to experience poor outcomes, such as developing gestational diabetes or hypertension, needing a Cesarean section, giving birth prematurely, or having a larger baby. There was also some evidence that a high BMI at the start of pregnancy increases the risk form heart attack or stroke.
The aim of the current study was to see if a high maternal body weight at the start of pregnancy and a high or low gestational weight gain were associated with diseases later in life. Dr. Moll and associates used a population-based cohort of 23,524 women from southern Sweden and 30,559 records from the Swedish Medical Birth Register to find women who had at least one pregnancy and had completed a self-reported health questionnaire 10-17 years afterwards.
A total of 13,608 women were identified who had a mean pre-pregnancy BMI of 21.9, a follow-up BMI of 24.6 , and a mean gestational weight gain of 14.5 kg. Women were divided into groups based on their pre-pregnancy BMIt (≤25 or >25) and amount of they weight gained during their pregnancy (≤15 or >15 kg).
At follow up, the crude rates of diabetes, cardiac, and endocrine diseases were 1.6%, 3.3%, and 7.1%. There was a 0.4% rate of stroke and 2.3% rate of psychiatric disease; 37% of women were overweight, and 9.4% were obese.
In an interview, Dr. Naveed Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences, University of Glasgow, Scotland, provided an independent comment on the findings. He said the data “strongly reiterate that the BMI with which you enter pregnancy gives you not only pregnancy complications but means that you are much more likely to be obese later on.”
Dr. Sattar added: “The interesting thing that it showed beautifully was that weight gain in pregnancy is inversely associated with the BMI at the beginning of pregnancy, so women who actually gain the least weight in pregnancy are the most obese. That suggests to me that the IOM criteria are very crude and perhaps not that useful.”
The IOM criteria may need reevaluation, he suggested. “The most important fact is that your weight when entering pregnancy is much more important than any weight gain during pregnancy,” he noted.
Dr. Moll had no conflicts of interest to disclose. Dr. Sattar had no disclosures relevant to his comments.
STOCKHOLM – Women had a more than six-fold increased risk of developing diabetes more than a decade after they gave birth if they were overweight or obese at the start of their pregnancy, according to the results of a large epidemiological study.
The odds ratio for the development of diabetes was 6.4 (95% confidence interval, 3.5-11.6, P < .001) comparing women who had a body mass index above 25 at the start of pregnancy with those who were of a lower body weight.
The heavier women also were more likely to be obese (OR, 21.9; 95% CI, 16.3-29.5; P< .001), have developed cardiac (OR, 2.7; 95% CI, 1.5-4.9; P= .001) or other endocrine (OR, 2.3; 95% CI, 1.5-3.4; P< .001) diseases 10-17 years later.
“A high pre-pregnancy BMI significantly increases the risk of several diseases later in life, including diabetes and cardiac disease,” Dr. Ulrika Moll of Lund University, Sweden, said at the annual meeting of the European Association for the Study of Diabetes.
The amount of weight gained during the pregnancy did not appear to matter, with no increase in the risk of cardiac or endocrine disease, even in women who gained more than the recommended weight set by the Institute of Medicine (IOM) guidelines. The IOM recommends that that women who are overweight should gain no more than 7–11.5 kg and those who are obese no more than 5-9 kg during their pregnancy. The mean maternal weight gain was 14.7 kg in the overweight group and 8.9 kg in the obese group.
Gaining more than 15 kg versus ≤15 kg during pregnancy did double the likelihood of women being overweight or obese in later life. A larger weight gain appeared to be protective against development of later psychiatric disease (OR, 0.6; 95% CI, 0.4-0.9; P= .03).
“A high weight gain during pregnancy did not have any implications on metabolic diseases within 10 years in our cohort,” Dr. Moll said. “These results have implications for the urgency of identification and care of the young women of childbearing age who struggle with being overweight or obese.”
Previous studies have shown that women who are overweight before they are pregnant and those that gain excessive amounts of weight during their pregnancies are more likely to experience poor outcomes, such as developing gestational diabetes or hypertension, needing a Cesarean section, giving birth prematurely, or having a larger baby. There was also some evidence that a high BMI at the start of pregnancy increases the risk form heart attack or stroke.
The aim of the current study was to see if a high maternal body weight at the start of pregnancy and a high or low gestational weight gain were associated with diseases later in life. Dr. Moll and associates used a population-based cohort of 23,524 women from southern Sweden and 30,559 records from the Swedish Medical Birth Register to find women who had at least one pregnancy and had completed a self-reported health questionnaire 10-17 years afterwards.
A total of 13,608 women were identified who had a mean pre-pregnancy BMI of 21.9, a follow-up BMI of 24.6 , and a mean gestational weight gain of 14.5 kg. Women were divided into groups based on their pre-pregnancy BMIt (≤25 or >25) and amount of they weight gained during their pregnancy (≤15 or >15 kg).
At follow up, the crude rates of diabetes, cardiac, and endocrine diseases were 1.6%, 3.3%, and 7.1%. There was a 0.4% rate of stroke and 2.3% rate of psychiatric disease; 37% of women were overweight, and 9.4% were obese.
In an interview, Dr. Naveed Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences, University of Glasgow, Scotland, provided an independent comment on the findings. He said the data “strongly reiterate that the BMI with which you enter pregnancy gives you not only pregnancy complications but means that you are much more likely to be obese later on.”
Dr. Sattar added: “The interesting thing that it showed beautifully was that weight gain in pregnancy is inversely associated with the BMI at the beginning of pregnancy, so women who actually gain the least weight in pregnancy are the most obese. That suggests to me that the IOM criteria are very crude and perhaps not that useful.”
The IOM criteria may need reevaluation, he suggested. “The most important fact is that your weight when entering pregnancy is much more important than any weight gain during pregnancy,” he noted.
Dr. Moll had no conflicts of interest to disclose. Dr. Sattar had no disclosures relevant to his comments.
STOCKHOLM – Women had a more than six-fold increased risk of developing diabetes more than a decade after they gave birth if they were overweight or obese at the start of their pregnancy, according to the results of a large epidemiological study.
The odds ratio for the development of diabetes was 6.4 (95% confidence interval, 3.5-11.6, P < .001) comparing women who had a body mass index above 25 at the start of pregnancy with those who were of a lower body weight.
The heavier women also were more likely to be obese (OR, 21.9; 95% CI, 16.3-29.5; P< .001), have developed cardiac (OR, 2.7; 95% CI, 1.5-4.9; P= .001) or other endocrine (OR, 2.3; 95% CI, 1.5-3.4; P< .001) diseases 10-17 years later.
“A high pre-pregnancy BMI significantly increases the risk of several diseases later in life, including diabetes and cardiac disease,” Dr. Ulrika Moll of Lund University, Sweden, said at the annual meeting of the European Association for the Study of Diabetes.
The amount of weight gained during the pregnancy did not appear to matter, with no increase in the risk of cardiac or endocrine disease, even in women who gained more than the recommended weight set by the Institute of Medicine (IOM) guidelines. The IOM recommends that that women who are overweight should gain no more than 7–11.5 kg and those who are obese no more than 5-9 kg during their pregnancy. The mean maternal weight gain was 14.7 kg in the overweight group and 8.9 kg in the obese group.
Gaining more than 15 kg versus ≤15 kg during pregnancy did double the likelihood of women being overweight or obese in later life. A larger weight gain appeared to be protective against development of later psychiatric disease (OR, 0.6; 95% CI, 0.4-0.9; P= .03).
“A high weight gain during pregnancy did not have any implications on metabolic diseases within 10 years in our cohort,” Dr. Moll said. “These results have implications for the urgency of identification and care of the young women of childbearing age who struggle with being overweight or obese.”
Previous studies have shown that women who are overweight before they are pregnant and those that gain excessive amounts of weight during their pregnancies are more likely to experience poor outcomes, such as developing gestational diabetes or hypertension, needing a Cesarean section, giving birth prematurely, or having a larger baby. There was also some evidence that a high BMI at the start of pregnancy increases the risk form heart attack or stroke.
The aim of the current study was to see if a high maternal body weight at the start of pregnancy and a high or low gestational weight gain were associated with diseases later in life. Dr. Moll and associates used a population-based cohort of 23,524 women from southern Sweden and 30,559 records from the Swedish Medical Birth Register to find women who had at least one pregnancy and had completed a self-reported health questionnaire 10-17 years afterwards.
A total of 13,608 women were identified who had a mean pre-pregnancy BMI of 21.9, a follow-up BMI of 24.6 , and a mean gestational weight gain of 14.5 kg. Women were divided into groups based on their pre-pregnancy BMIt (≤25 or >25) and amount of they weight gained during their pregnancy (≤15 or >15 kg).
At follow up, the crude rates of diabetes, cardiac, and endocrine diseases were 1.6%, 3.3%, and 7.1%. There was a 0.4% rate of stroke and 2.3% rate of psychiatric disease; 37% of women were overweight, and 9.4% were obese.
In an interview, Dr. Naveed Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences, University of Glasgow, Scotland, provided an independent comment on the findings. He said the data “strongly reiterate that the BMI with which you enter pregnancy gives you not only pregnancy complications but means that you are much more likely to be obese later on.”
Dr. Sattar added: “The interesting thing that it showed beautifully was that weight gain in pregnancy is inversely associated with the BMI at the beginning of pregnancy, so women who actually gain the least weight in pregnancy are the most obese. That suggests to me that the IOM criteria are very crude and perhaps not that useful.”
The IOM criteria may need reevaluation, he suggested. “The most important fact is that your weight when entering pregnancy is much more important than any weight gain during pregnancy,” he noted.
Dr. Moll had no conflicts of interest to disclose. Dr. Sattar had no disclosures relevant to his comments.
AT EASD 2015
Key clinical point: Being overweight or obese at the start of pregnancy may determine the risk for developing type 2 diabetes a decade later.
Major finding: The odds ratio for the development of diabetes was 6.4 in women with a BMI of 25 or above at the start of pregnancy compared with leaner women.
Data source: 13,608 women identified from a Swedish population cohort of 23,524 who had at least one pregnancy follow up data 10-17 years after their pregnancy.
Disclosures: Dr. Moll had no conflicts of interest to disclose. Dr. Sattar had no disclosures relevant to his comments.
EASD: SGLT2 inhibitors show potential in 1DM despite DKA concern
STOCKHOLM – The use of sodium-glucose cotransporter 2 (SGLT2) inhibitors in patients with type 1 diabetes looks promising despite concerns over their potential to cause diabetic ketoacidosis (DKA), experts reported here at the annual meeting of the European Association for the Study of Diabetes.
Results of three trials, including the results of an 18-week phase II trial with canagliflozin, a 4-week phase II trial with sotagliflozin, and a 4-week phase II trial with empagliflozin showed that these drugs are effective when used in addition to insulin to lower hemoglobin A1c and have the added benefit of weight-reduction without increasing the risk for hypoglycemia.
“We must be very careful about using SGLT2 inhibitors in type 1 diabetes,” said Dr. David Matthews, professor of diabetes, emeritus founding chairman, Oxford (England) Centre for Diabetes, Endocrinology and Metabolism, in an interview. “These are preliminary studies, you’d have to use it off label, and you’d have to take responsibility for being very careful about the induction of DKA,” he observed.
Dr. Matthews, who chaired the session during which the canagliflozin results were presented, added: “DKA is clearly one of the risks, and we’ve learned a lot about why this might occur, for instance when there are the additional risks such as illness or with the use of anesthesia.”
DKA: Why the concern?
SGLT2 inhibitors are currently approved for use only in patients with type 2 diabetes when diet and exercise alone are not able to help patients achieve sufficient glycemic control. Recently, however, their potential to cause ketoacidosis even in patients with type 2 diabetes were the focus of a Drug Safety Communication issued by the Food and Drug Administration. The warning applied to all currently available SLGT2 preparations: canagliflozin (Invokana), canagliflozin/metformin (Invokamet), dapagliflozin (Farxiga), dapagliflozin/metformin extended release (Xigduo XR), empagliflozin (Jardiance), and empagliflozin/lingagliptin (Glyxambi); similar warnings followed from the European Medicines Agency.
Dr. Matthews observed that the risk of DKA in patients with type 2 diabetes may be clouded by patients with type 1 being misdiagnosed. “In type 2 diabetes, there is clearly a mix of people with type 1 whom you misdiagnose or who have got latent type 1 diabetes. Those are often the people on insulin, so they fall into the category of type 2 diabetes, and almost by mistake you are treating people with type 1 diabetes.”
Dr. Anne L. Peters of the University of Southern California, Los Angeles, and author of a recent article (Diabetes Care. 2015 Sep;38[9]:1687-93) highlighting the risk for DKA among type 2 diabetics treated with SGLT2 inhibitors, noted that use in type 1 diabetes had been shown to improve glucose control.
“Frankly, we know they work,” she said. “We know that they work in people with type 2 diabetes, and you are about to find out that they work in patients with type 1 diabetes, at least in a phase II trial,” Dr. Peters added.
“We know that the mechanism of action is insulin independent and, by design, [SGLT2 inhibitors] can work in both type 1 and type 2 diabetes,” she said. “But we also know that when we use [these agents] off label in diabetes that there is a signal for an increased risk for diabetic ketoacidosis.”
However, in her experience of using canagliflozin, every case of DKA involved “precipitating factors.” These included infections such as influenza or pneumonia that resulted in the patient not being well and not eating and thus reducing their insulin use or device or compliance issues resulting in reduced insulin dosing.
Dr. Peters suggested that should off-label use be considered outside of a clinical trial, patients must be adherent to routine glucose and ketone testing. She noted that she advised her patients to hold off using an SGLT2 inhibitor if they were going to be doing something different, such being more physically active, or if they had been ill and their insulin dosing had decreased.
Canagliflozin: Phase II study
Dr. Robert R. Henry, professor of medicine at the University of California, San Diego, presented the results of an 18-week, phase II study with canagliflozin, which was completed in June and accepted for publication in Diabetes Care. The double-blind, placebo-controlled, multicenter study involved 351 randomized patients with type 1 diabetes and a mean age of roughly 42 years treated with canagliflozin 100 mg (n = 117), canagliflozin 300 mg (n = 117), or placebo (n =1 17).
The primary efficacy endpoint was the proportion of patients with a decrease in HbA1c of 0.4% or more and no increase in body weight. This combined endpoint was met by 37% and 41% of the 100-mg and 300-mg canagliflozin-treated patients, respectively, and by 15% of those randomized to placebo (both P less than .001 vs. placebo). Considering each of the components separately showed that a higher percentage of patients achieved an HbA1c reduction of 0.4% or more (43%-45% vs. 23%) and reduced body weight (84%-96% vs. 49%).
Before randomization patients were advised to reduce their basal insulin doses by 20% if their HbA1c was below 8% and by 10% if it was above 8% to reduce the potential hypoglycemia risk. During treatment, they were instructed to titrate their basal and bolus insulin doses to achieve premeal and bedtime glucose levels between 80 and 120 mg/dL. Both doses of canagliflozin reduced the need for total daily insulin, with mean changes from baseline of –4.1 IU/day and –7.6 IU/day for the 100- and 300-mg doses, respectively. Reductions in basal and bolus insulin were –4.3 and –0.3 IU/day for the 100-mg dose and –0.3 and 03.2 for the 300-mg dose.
Patients with severe hypoglycemia or DKA within 6 months of randomization were excluded from the trial, with around 15% and 12%-13% of patients enrolled having had a prior history of either at any time. The rate of treatment-emergent hypoglycemia episodes were similar across the groups, at 97% for placebo and 98%-99% for canagliflozin/patient-year of exposure. Serious ketoacidosis events occurred in 4% and 6% of the canagliflozin 100- and 300-mg groups, but in all cases, there were precipitating factors that likely continued to the event, Dr. Henry said.
“Implementation of additional mitigation strategies in future studies may substantially reduce DKA risk in patients with type 1 diabetes treated with canagliflozin,” he concluded. These strategies included more-frequent monitoring for ketones, dose interruption when patients were ill, under stress, or more active, perhaps using lower doses of canagliflozin, and further education of physicians and patients about the potential risks. A paper on the specifics of the DKA cases seen in the trial is currently under review at Diabetes Care.
Sotagliflozin: Dual inhibition of SGLT1 and SGLT2
Dr. John Buse, professor of medicine and chief of the division of endocrinology at the Center for Diabetes Research at the University of North Carolina at Chapel Hill, presented the findings of a 4-week, randomized, double-blind, placebo-controlled phase II study with the investigational SGLT2 inhibitor sotagliflozin. Unlike other SGLT2 inhibitors already available for type 2 diabetes, sotagliflozin also inhibits SGLT1, meaning that it not only targets glucose reabsorption in the kidneys but also glucose and galactose reabsorption in the gastrointestinal tract. Although promising results have been seen in phase II trials involving patients with type 2 diabetes, the drug’s developer, Lexicon Pharmaceuticals, says it currently has no plans to pursue a license this indication.
The study involved 33 patients with type 1 diabetes aged between 18 and 55 years, 16 of whom were treated with sotagliflozin and 17 with placebo. Patients treated with sotagliflozin were older (median age, 45.5 years) than those randomized to placebo (median age, 34 years), but for a small trial the patients were otherwise “remarkably well matched” in terms of their other baseline characteristics Dr. Buse observed.
Given at a 400-mg daily oral dose, sotagliflozin was shown to improve glycemic control in intensively treated patients with type 1 diabetes after only 28 days vs. placebo, with a decrease in the primary endpoint of daily bolus insulin use (–32% vs. –6.4%; P =.007), but not in total daily basal insulin (–2.4 vs. +0.2%). “This is consistent with SGLT1 inhibition,” Dr. Buse noted, and “different than observed with selective SGLT2 inhibitors in type 1 diabetes.”
The insulin dose at mealtimes was lowered, statistically so at breakfast and numerically at other mealtimes. There was also a numerical reduction in postprandial glucose. A significant decrease in HbA1c was seen vs. placebo (–0.55% vs. –0.06%; P =.002) and there was no increase in daily hypoglycemic events (–0.7 vs. 0.4/patient per day) and there was the added benefit of weight loss (–1.7 kg vs. +0.5 kg; P =.005).
Results from the trial were also presented in a poster and showed that sotagliflozin significantly improved the time that patients blood glucose was within their target range and reduced glycemic variability versus placebo.
There were similar rates of adverse events between the active treatment and placebo groups with the exception of mild nausea (25% vs. 6%) and vomiting (13% vs. 6%) and two cases of ketoacidosis that occurred in the sotagliflozin group and creatinine phosphokinase elevation that occurred in two patients treated with placebo. Both DKA cases were considered to be down to the pump method of insulin administration rather than to be drug related. The safety profile of this particular dose, together with the efficacy seen “supports advancement to phase III studies”, said Dr. Buse.
Another phase II and three phase III trials with sotagliflozin in type 1 diabetes – inTandem1, inTandem2, and inTandem3 – are underway and have started to recruit patients. The phase II trial is expected to be complete by the start of next year, with results of the first two phase III trial to follow in the fall of 2016, and the results of the third by spring 2017.
Empagliflozin: EASE-1 study findings
Dr. Thomas Pieber of the Medical University of Graz in Austria presented the results of the 4-week phase II EASE-1 study, which included 75 patients with type 1 diabetes who were randomized to received empagliflozin once-daily at doses of 2.5 mg (n = 19), 10 mg (n = 19), or 25 mg (n = 18), or placebo (n = 19) for 28 days as adjunctive treatment to insulin.
The study findings (Diabetes Obes Metab. 2015 Jun 17. doi: 10.1111/dom.12494.) showed that empagliflozin used as an adjunct to insulin significantly increased urinary glucose excretion after 1, 7, and 29 days. Significant improvements in HbA1c were achieved with lower (12%-14% vs. baseline) total daily doses of insulin being used. Empagliflozin was also associated with weight reduction (–1.4 to –1.7 kg) and generally lower rates of moderate hypoglycemia and no episodes needed assistance, compared with placebo. There were no cases of DKA reported, but fasting beta-hydroxybutyrate was slightly raised at week 4 with all doses of empagliflozin.
One delegate commented after Dr. Pieber’s presentation that, although it was a well-conducted study and provided some evidence of an effect of SGLG2 inhibitors in patients with type 1 diabetes, he had “great reservation” about using this class of antidiabetic drug in this clinical situation. The delegate’s concern was around expanding the use of the drug to all patients, some of whom may not have regular eating patterns or may have abnormal eating behaviors who might be at higher risk for DKA. “Of course, in a controlled study, it is not seen, but you see already some small signal of increase of ketone bodies, so I would say it should be used with great reservation in all patients and can only be used in well-educated, well-monitored patients.”
Dr. Pieber responded: “I think we have to do clinical trials to see if there is a benefit or not for our patients. So this was a study performed as a dose-finding study that led to a phase II program and hopefully will lead to a phase III program.” He added that, bearing in mind the positive findings of the EMPA-REG OUTCOME study showing that empagliflozin reduced major cardiovascular outcomes in patients with type 2 diabetes to be presented later at the meeting, “If something works in type 2 we definitely should test it in type 1 diabetes.”
STOCKHOLM – The use of sodium-glucose cotransporter 2 (SGLT2) inhibitors in patients with type 1 diabetes looks promising despite concerns over their potential to cause diabetic ketoacidosis (DKA), experts reported here at the annual meeting of the European Association for the Study of Diabetes.
Results of three trials, including the results of an 18-week phase II trial with canagliflozin, a 4-week phase II trial with sotagliflozin, and a 4-week phase II trial with empagliflozin showed that these drugs are effective when used in addition to insulin to lower hemoglobin A1c and have the added benefit of weight-reduction without increasing the risk for hypoglycemia.
“We must be very careful about using SGLT2 inhibitors in type 1 diabetes,” said Dr. David Matthews, professor of diabetes, emeritus founding chairman, Oxford (England) Centre for Diabetes, Endocrinology and Metabolism, in an interview. “These are preliminary studies, you’d have to use it off label, and you’d have to take responsibility for being very careful about the induction of DKA,” he observed.
Dr. Matthews, who chaired the session during which the canagliflozin results were presented, added: “DKA is clearly one of the risks, and we’ve learned a lot about why this might occur, for instance when there are the additional risks such as illness or with the use of anesthesia.”
DKA: Why the concern?
SGLT2 inhibitors are currently approved for use only in patients with type 2 diabetes when diet and exercise alone are not able to help patients achieve sufficient glycemic control. Recently, however, their potential to cause ketoacidosis even in patients with type 2 diabetes were the focus of a Drug Safety Communication issued by the Food and Drug Administration. The warning applied to all currently available SLGT2 preparations: canagliflozin (Invokana), canagliflozin/metformin (Invokamet), dapagliflozin (Farxiga), dapagliflozin/metformin extended release (Xigduo XR), empagliflozin (Jardiance), and empagliflozin/lingagliptin (Glyxambi); similar warnings followed from the European Medicines Agency.
Dr. Matthews observed that the risk of DKA in patients with type 2 diabetes may be clouded by patients with type 1 being misdiagnosed. “In type 2 diabetes, there is clearly a mix of people with type 1 whom you misdiagnose or who have got latent type 1 diabetes. Those are often the people on insulin, so they fall into the category of type 2 diabetes, and almost by mistake you are treating people with type 1 diabetes.”
Dr. Anne L. Peters of the University of Southern California, Los Angeles, and author of a recent article (Diabetes Care. 2015 Sep;38[9]:1687-93) highlighting the risk for DKA among type 2 diabetics treated with SGLT2 inhibitors, noted that use in type 1 diabetes had been shown to improve glucose control.
“Frankly, we know they work,” she said. “We know that they work in people with type 2 diabetes, and you are about to find out that they work in patients with type 1 diabetes, at least in a phase II trial,” Dr. Peters added.
“We know that the mechanism of action is insulin independent and, by design, [SGLT2 inhibitors] can work in both type 1 and type 2 diabetes,” she said. “But we also know that when we use [these agents] off label in diabetes that there is a signal for an increased risk for diabetic ketoacidosis.”
However, in her experience of using canagliflozin, every case of DKA involved “precipitating factors.” These included infections such as influenza or pneumonia that resulted in the patient not being well and not eating and thus reducing their insulin use or device or compliance issues resulting in reduced insulin dosing.
Dr. Peters suggested that should off-label use be considered outside of a clinical trial, patients must be adherent to routine glucose and ketone testing. She noted that she advised her patients to hold off using an SGLT2 inhibitor if they were going to be doing something different, such being more physically active, or if they had been ill and their insulin dosing had decreased.
Canagliflozin: Phase II study
Dr. Robert R. Henry, professor of medicine at the University of California, San Diego, presented the results of an 18-week, phase II study with canagliflozin, which was completed in June and accepted for publication in Diabetes Care. The double-blind, placebo-controlled, multicenter study involved 351 randomized patients with type 1 diabetes and a mean age of roughly 42 years treated with canagliflozin 100 mg (n = 117), canagliflozin 300 mg (n = 117), or placebo (n =1 17).
The primary efficacy endpoint was the proportion of patients with a decrease in HbA1c of 0.4% or more and no increase in body weight. This combined endpoint was met by 37% and 41% of the 100-mg and 300-mg canagliflozin-treated patients, respectively, and by 15% of those randomized to placebo (both P less than .001 vs. placebo). Considering each of the components separately showed that a higher percentage of patients achieved an HbA1c reduction of 0.4% or more (43%-45% vs. 23%) and reduced body weight (84%-96% vs. 49%).
Before randomization patients were advised to reduce their basal insulin doses by 20% if their HbA1c was below 8% and by 10% if it was above 8% to reduce the potential hypoglycemia risk. During treatment, they were instructed to titrate their basal and bolus insulin doses to achieve premeal and bedtime glucose levels between 80 and 120 mg/dL. Both doses of canagliflozin reduced the need for total daily insulin, with mean changes from baseline of –4.1 IU/day and –7.6 IU/day for the 100- and 300-mg doses, respectively. Reductions in basal and bolus insulin were –4.3 and –0.3 IU/day for the 100-mg dose and –0.3 and 03.2 for the 300-mg dose.
Patients with severe hypoglycemia or DKA within 6 months of randomization were excluded from the trial, with around 15% and 12%-13% of patients enrolled having had a prior history of either at any time. The rate of treatment-emergent hypoglycemia episodes were similar across the groups, at 97% for placebo and 98%-99% for canagliflozin/patient-year of exposure. Serious ketoacidosis events occurred in 4% and 6% of the canagliflozin 100- and 300-mg groups, but in all cases, there were precipitating factors that likely continued to the event, Dr. Henry said.
“Implementation of additional mitigation strategies in future studies may substantially reduce DKA risk in patients with type 1 diabetes treated with canagliflozin,” he concluded. These strategies included more-frequent monitoring for ketones, dose interruption when patients were ill, under stress, or more active, perhaps using lower doses of canagliflozin, and further education of physicians and patients about the potential risks. A paper on the specifics of the DKA cases seen in the trial is currently under review at Diabetes Care.
Sotagliflozin: Dual inhibition of SGLT1 and SGLT2
Dr. John Buse, professor of medicine and chief of the division of endocrinology at the Center for Diabetes Research at the University of North Carolina at Chapel Hill, presented the findings of a 4-week, randomized, double-blind, placebo-controlled phase II study with the investigational SGLT2 inhibitor sotagliflozin. Unlike other SGLT2 inhibitors already available for type 2 diabetes, sotagliflozin also inhibits SGLT1, meaning that it not only targets glucose reabsorption in the kidneys but also glucose and galactose reabsorption in the gastrointestinal tract. Although promising results have been seen in phase II trials involving patients with type 2 diabetes, the drug’s developer, Lexicon Pharmaceuticals, says it currently has no plans to pursue a license this indication.
The study involved 33 patients with type 1 diabetes aged between 18 and 55 years, 16 of whom were treated with sotagliflozin and 17 with placebo. Patients treated with sotagliflozin were older (median age, 45.5 years) than those randomized to placebo (median age, 34 years), but for a small trial the patients were otherwise “remarkably well matched” in terms of their other baseline characteristics Dr. Buse observed.
Given at a 400-mg daily oral dose, sotagliflozin was shown to improve glycemic control in intensively treated patients with type 1 diabetes after only 28 days vs. placebo, with a decrease in the primary endpoint of daily bolus insulin use (–32% vs. –6.4%; P =.007), but not in total daily basal insulin (–2.4 vs. +0.2%). “This is consistent with SGLT1 inhibition,” Dr. Buse noted, and “different than observed with selective SGLT2 inhibitors in type 1 diabetes.”
The insulin dose at mealtimes was lowered, statistically so at breakfast and numerically at other mealtimes. There was also a numerical reduction in postprandial glucose. A significant decrease in HbA1c was seen vs. placebo (–0.55% vs. –0.06%; P =.002) and there was no increase in daily hypoglycemic events (–0.7 vs. 0.4/patient per day) and there was the added benefit of weight loss (–1.7 kg vs. +0.5 kg; P =.005).
Results from the trial were also presented in a poster and showed that sotagliflozin significantly improved the time that patients blood glucose was within their target range and reduced glycemic variability versus placebo.
There were similar rates of adverse events between the active treatment and placebo groups with the exception of mild nausea (25% vs. 6%) and vomiting (13% vs. 6%) and two cases of ketoacidosis that occurred in the sotagliflozin group and creatinine phosphokinase elevation that occurred in two patients treated with placebo. Both DKA cases were considered to be down to the pump method of insulin administration rather than to be drug related. The safety profile of this particular dose, together with the efficacy seen “supports advancement to phase III studies”, said Dr. Buse.
Another phase II and three phase III trials with sotagliflozin in type 1 diabetes – inTandem1, inTandem2, and inTandem3 – are underway and have started to recruit patients. The phase II trial is expected to be complete by the start of next year, with results of the first two phase III trial to follow in the fall of 2016, and the results of the third by spring 2017.
Empagliflozin: EASE-1 study findings
Dr. Thomas Pieber of the Medical University of Graz in Austria presented the results of the 4-week phase II EASE-1 study, which included 75 patients with type 1 diabetes who were randomized to received empagliflozin once-daily at doses of 2.5 mg (n = 19), 10 mg (n = 19), or 25 mg (n = 18), or placebo (n = 19) for 28 days as adjunctive treatment to insulin.
The study findings (Diabetes Obes Metab. 2015 Jun 17. doi: 10.1111/dom.12494.) showed that empagliflozin used as an adjunct to insulin significantly increased urinary glucose excretion after 1, 7, and 29 days. Significant improvements in HbA1c were achieved with lower (12%-14% vs. baseline) total daily doses of insulin being used. Empagliflozin was also associated with weight reduction (–1.4 to –1.7 kg) and generally lower rates of moderate hypoglycemia and no episodes needed assistance, compared with placebo. There were no cases of DKA reported, but fasting beta-hydroxybutyrate was slightly raised at week 4 with all doses of empagliflozin.
One delegate commented after Dr. Pieber’s presentation that, although it was a well-conducted study and provided some evidence of an effect of SGLG2 inhibitors in patients with type 1 diabetes, he had “great reservation” about using this class of antidiabetic drug in this clinical situation. The delegate’s concern was around expanding the use of the drug to all patients, some of whom may not have regular eating patterns or may have abnormal eating behaviors who might be at higher risk for DKA. “Of course, in a controlled study, it is not seen, but you see already some small signal of increase of ketone bodies, so I would say it should be used with great reservation in all patients and can only be used in well-educated, well-monitored patients.”
Dr. Pieber responded: “I think we have to do clinical trials to see if there is a benefit or not for our patients. So this was a study performed as a dose-finding study that led to a phase II program and hopefully will lead to a phase III program.” He added that, bearing in mind the positive findings of the EMPA-REG OUTCOME study showing that empagliflozin reduced major cardiovascular outcomes in patients with type 2 diabetes to be presented later at the meeting, “If something works in type 2 we definitely should test it in type 1 diabetes.”
STOCKHOLM – The use of sodium-glucose cotransporter 2 (SGLT2) inhibitors in patients with type 1 diabetes looks promising despite concerns over their potential to cause diabetic ketoacidosis (DKA), experts reported here at the annual meeting of the European Association for the Study of Diabetes.
Results of three trials, including the results of an 18-week phase II trial with canagliflozin, a 4-week phase II trial with sotagliflozin, and a 4-week phase II trial with empagliflozin showed that these drugs are effective when used in addition to insulin to lower hemoglobin A1c and have the added benefit of weight-reduction without increasing the risk for hypoglycemia.
“We must be very careful about using SGLT2 inhibitors in type 1 diabetes,” said Dr. David Matthews, professor of diabetes, emeritus founding chairman, Oxford (England) Centre for Diabetes, Endocrinology and Metabolism, in an interview. “These are preliminary studies, you’d have to use it off label, and you’d have to take responsibility for being very careful about the induction of DKA,” he observed.
Dr. Matthews, who chaired the session during which the canagliflozin results were presented, added: “DKA is clearly one of the risks, and we’ve learned a lot about why this might occur, for instance when there are the additional risks such as illness or with the use of anesthesia.”
DKA: Why the concern?
SGLT2 inhibitors are currently approved for use only in patients with type 2 diabetes when diet and exercise alone are not able to help patients achieve sufficient glycemic control. Recently, however, their potential to cause ketoacidosis even in patients with type 2 diabetes were the focus of a Drug Safety Communication issued by the Food and Drug Administration. The warning applied to all currently available SLGT2 preparations: canagliflozin (Invokana), canagliflozin/metformin (Invokamet), dapagliflozin (Farxiga), dapagliflozin/metformin extended release (Xigduo XR), empagliflozin (Jardiance), and empagliflozin/lingagliptin (Glyxambi); similar warnings followed from the European Medicines Agency.
Dr. Matthews observed that the risk of DKA in patients with type 2 diabetes may be clouded by patients with type 1 being misdiagnosed. “In type 2 diabetes, there is clearly a mix of people with type 1 whom you misdiagnose or who have got latent type 1 diabetes. Those are often the people on insulin, so they fall into the category of type 2 diabetes, and almost by mistake you are treating people with type 1 diabetes.”
Dr. Anne L. Peters of the University of Southern California, Los Angeles, and author of a recent article (Diabetes Care. 2015 Sep;38[9]:1687-93) highlighting the risk for DKA among type 2 diabetics treated with SGLT2 inhibitors, noted that use in type 1 diabetes had been shown to improve glucose control.
“Frankly, we know they work,” she said. “We know that they work in people with type 2 diabetes, and you are about to find out that they work in patients with type 1 diabetes, at least in a phase II trial,” Dr. Peters added.
“We know that the mechanism of action is insulin independent and, by design, [SGLT2 inhibitors] can work in both type 1 and type 2 diabetes,” she said. “But we also know that when we use [these agents] off label in diabetes that there is a signal for an increased risk for diabetic ketoacidosis.”
However, in her experience of using canagliflozin, every case of DKA involved “precipitating factors.” These included infections such as influenza or pneumonia that resulted in the patient not being well and not eating and thus reducing their insulin use or device or compliance issues resulting in reduced insulin dosing.
Dr. Peters suggested that should off-label use be considered outside of a clinical trial, patients must be adherent to routine glucose and ketone testing. She noted that she advised her patients to hold off using an SGLT2 inhibitor if they were going to be doing something different, such being more physically active, or if they had been ill and their insulin dosing had decreased.
Canagliflozin: Phase II study
Dr. Robert R. Henry, professor of medicine at the University of California, San Diego, presented the results of an 18-week, phase II study with canagliflozin, which was completed in June and accepted for publication in Diabetes Care. The double-blind, placebo-controlled, multicenter study involved 351 randomized patients with type 1 diabetes and a mean age of roughly 42 years treated with canagliflozin 100 mg (n = 117), canagliflozin 300 mg (n = 117), or placebo (n =1 17).
The primary efficacy endpoint was the proportion of patients with a decrease in HbA1c of 0.4% or more and no increase in body weight. This combined endpoint was met by 37% and 41% of the 100-mg and 300-mg canagliflozin-treated patients, respectively, and by 15% of those randomized to placebo (both P less than .001 vs. placebo). Considering each of the components separately showed that a higher percentage of patients achieved an HbA1c reduction of 0.4% or more (43%-45% vs. 23%) and reduced body weight (84%-96% vs. 49%).
Before randomization patients were advised to reduce their basal insulin doses by 20% if their HbA1c was below 8% and by 10% if it was above 8% to reduce the potential hypoglycemia risk. During treatment, they were instructed to titrate their basal and bolus insulin doses to achieve premeal and bedtime glucose levels between 80 and 120 mg/dL. Both doses of canagliflozin reduced the need for total daily insulin, with mean changes from baseline of –4.1 IU/day and –7.6 IU/day for the 100- and 300-mg doses, respectively. Reductions in basal and bolus insulin were –4.3 and –0.3 IU/day for the 100-mg dose and –0.3 and 03.2 for the 300-mg dose.
Patients with severe hypoglycemia or DKA within 6 months of randomization were excluded from the trial, with around 15% and 12%-13% of patients enrolled having had a prior history of either at any time. The rate of treatment-emergent hypoglycemia episodes were similar across the groups, at 97% for placebo and 98%-99% for canagliflozin/patient-year of exposure. Serious ketoacidosis events occurred in 4% and 6% of the canagliflozin 100- and 300-mg groups, but in all cases, there were precipitating factors that likely continued to the event, Dr. Henry said.
“Implementation of additional mitigation strategies in future studies may substantially reduce DKA risk in patients with type 1 diabetes treated with canagliflozin,” he concluded. These strategies included more-frequent monitoring for ketones, dose interruption when patients were ill, under stress, or more active, perhaps using lower doses of canagliflozin, and further education of physicians and patients about the potential risks. A paper on the specifics of the DKA cases seen in the trial is currently under review at Diabetes Care.
Sotagliflozin: Dual inhibition of SGLT1 and SGLT2
Dr. John Buse, professor of medicine and chief of the division of endocrinology at the Center for Diabetes Research at the University of North Carolina at Chapel Hill, presented the findings of a 4-week, randomized, double-blind, placebo-controlled phase II study with the investigational SGLT2 inhibitor sotagliflozin. Unlike other SGLT2 inhibitors already available for type 2 diabetes, sotagliflozin also inhibits SGLT1, meaning that it not only targets glucose reabsorption in the kidneys but also glucose and galactose reabsorption in the gastrointestinal tract. Although promising results have been seen in phase II trials involving patients with type 2 diabetes, the drug’s developer, Lexicon Pharmaceuticals, says it currently has no plans to pursue a license this indication.
The study involved 33 patients with type 1 diabetes aged between 18 and 55 years, 16 of whom were treated with sotagliflozin and 17 with placebo. Patients treated with sotagliflozin were older (median age, 45.5 years) than those randomized to placebo (median age, 34 years), but for a small trial the patients were otherwise “remarkably well matched” in terms of their other baseline characteristics Dr. Buse observed.
Given at a 400-mg daily oral dose, sotagliflozin was shown to improve glycemic control in intensively treated patients with type 1 diabetes after only 28 days vs. placebo, with a decrease in the primary endpoint of daily bolus insulin use (–32% vs. –6.4%; P =.007), but not in total daily basal insulin (–2.4 vs. +0.2%). “This is consistent with SGLT1 inhibition,” Dr. Buse noted, and “different than observed with selective SGLT2 inhibitors in type 1 diabetes.”
The insulin dose at mealtimes was lowered, statistically so at breakfast and numerically at other mealtimes. There was also a numerical reduction in postprandial glucose. A significant decrease in HbA1c was seen vs. placebo (–0.55% vs. –0.06%; P =.002) and there was no increase in daily hypoglycemic events (–0.7 vs. 0.4/patient per day) and there was the added benefit of weight loss (–1.7 kg vs. +0.5 kg; P =.005).
Results from the trial were also presented in a poster and showed that sotagliflozin significantly improved the time that patients blood glucose was within their target range and reduced glycemic variability versus placebo.
There were similar rates of adverse events between the active treatment and placebo groups with the exception of mild nausea (25% vs. 6%) and vomiting (13% vs. 6%) and two cases of ketoacidosis that occurred in the sotagliflozin group and creatinine phosphokinase elevation that occurred in two patients treated with placebo. Both DKA cases were considered to be down to the pump method of insulin administration rather than to be drug related. The safety profile of this particular dose, together with the efficacy seen “supports advancement to phase III studies”, said Dr. Buse.
Another phase II and three phase III trials with sotagliflozin in type 1 diabetes – inTandem1, inTandem2, and inTandem3 – are underway and have started to recruit patients. The phase II trial is expected to be complete by the start of next year, with results of the first two phase III trial to follow in the fall of 2016, and the results of the third by spring 2017.
Empagliflozin: EASE-1 study findings
Dr. Thomas Pieber of the Medical University of Graz in Austria presented the results of the 4-week phase II EASE-1 study, which included 75 patients with type 1 diabetes who were randomized to received empagliflozin once-daily at doses of 2.5 mg (n = 19), 10 mg (n = 19), or 25 mg (n = 18), or placebo (n = 19) for 28 days as adjunctive treatment to insulin.
The study findings (Diabetes Obes Metab. 2015 Jun 17. doi: 10.1111/dom.12494.) showed that empagliflozin used as an adjunct to insulin significantly increased urinary glucose excretion after 1, 7, and 29 days. Significant improvements in HbA1c were achieved with lower (12%-14% vs. baseline) total daily doses of insulin being used. Empagliflozin was also associated with weight reduction (–1.4 to –1.7 kg) and generally lower rates of moderate hypoglycemia and no episodes needed assistance, compared with placebo. There were no cases of DKA reported, but fasting beta-hydroxybutyrate was slightly raised at week 4 with all doses of empagliflozin.
One delegate commented after Dr. Pieber’s presentation that, although it was a well-conducted study and provided some evidence of an effect of SGLG2 inhibitors in patients with type 1 diabetes, he had “great reservation” about using this class of antidiabetic drug in this clinical situation. The delegate’s concern was around expanding the use of the drug to all patients, some of whom may not have regular eating patterns or may have abnormal eating behaviors who might be at higher risk for DKA. “Of course, in a controlled study, it is not seen, but you see already some small signal of increase of ketone bodies, so I would say it should be used with great reservation in all patients and can only be used in well-educated, well-monitored patients.”
Dr. Pieber responded: “I think we have to do clinical trials to see if there is a benefit or not for our patients. So this was a study performed as a dose-finding study that led to a phase II program and hopefully will lead to a phase III program.” He added that, bearing in mind the positive findings of the EMPA-REG OUTCOME study showing that empagliflozin reduced major cardiovascular outcomes in patients with type 2 diabetes to be presented later at the meeting, “If something works in type 2 we definitely should test it in type 1 diabetes.”
AT EASD 2015
Key clinical point: Glycemic control may be improved by using SGLT2 inhibitors in addition to insulin in type 1 diabetes, judging from early clinical data. Further clinical studies are needed to confirm the findings with these drugs, which are not approved for the treatment for type 1 diabetes.
Major finding: Canagliflozin, sotagliflozin, and empagliflozin used as an adjunct to insulin improved HbA1c, lowered insulin use, reduced weight, and did not increase the risk for hypoglycemia.
Data source: Three separate phase II studies assessing the use of SGLT2 inhibitors in patients with type 1 diabetes.
Disclosures: The studies were sponsored by Janssen (canagliflozin), Lexicon Pharmaceuticals (sotagliflozin), and Boehringer Ingelheim and Eli Lilly & Co. (empagliflozin/EASE-1). Dr. Henry and Dr. Peters were investigators for the canagliflozin trial. Dr. Henry disclosed receiving grants or acting as a consultant or advisory board member for numerous other pharmaceutical companies. Dr. Matthews is also a coinvestigator for a Janssen-sponsored canagliflozin trial and has received honoraria and support from Janssen. Dr. Buse was an investigator for the sotagliflozin trial and received research support from Lexicon Pharmaceuticals. He also disclosed acting as an advisory board member or consultant and receiving research support from multiple other companies and that he was a stock or shareholder in PhaseBio. Dr. Pieber disclosed acting as an adviser for Boehringer Ingelheim and Eli Lilly, among others. He has received research support from Novo Nordisk and is CSO of CBmed, the Center for Biomarker Research in Medicine.
In type 2 diabetes, pump therapy costs more but saves much
STOCKHOLM – Insulin pump therapy is considerably more expensive than daily insulin injections for patients with type 2 diabetes, but the benefits it confers offset about 30% of the price difference.
A cost-modeling study showed that patients who use the pump gain almost another year of life expectancy and experience a delay in the onset of major complications, such as end-stage renal disease and amputation, Stephane Roze said at the annual meeting of the European Association for the Study of Diabetes.
Pump therapy also conferred a significantly improved quality of life over multiple daily injections, a benefit that carries its own economic value, said Mr. Roze of HEVA-HEOR, a health economics evaluation company in Lyon, France.
“Even though these patients are initiating the pump at an older age than patients with type 1 diabetes, the therapy still looks to be cost effective,” he said. “It’s a good investment for payers.”
Mr. Roze used the CORE Diabetes Model to examine the cost/benefit ratio of insulin pump therapy, compared with multiple daily injections in patients with poorly controlled type 2 diabetes who lived in the Netherlands. The CORE model comprises 14 sub-Markov models that run in parallel; it examines all of the micro- and macrovascular complications related to diabetes. Model input data were the cohort characteristics and 6-month clinical outcomes of the OpT2mise study.
The trial examined glucose variability in 331 patients with type 2 diabetes who were randomized to either pump therapy or daily insulin injections.
It is an excellent study for cost-modeling initiatives, Mr. Roze said. It’s multicenter and multinational, conducted in 36 institutions in the United States, Canada, Europe, Israel, and South Africa. The cohort is large and diverse. Importantly, all of the patients had their treatment optimized with daily insulin analogue injections before being randomized. That initial step lent credence to their baseline glucose control; despite optimization, they still had a mean hemoglobin A1cof 9%. The OpT2mise patients were a mean of 56 years old, with mean disease duration of 15 years.
At 6 months, the study showed that HbA1c had decreased significantly more in the pump group than in the injection group (1.1% vs. 0.4%). Patients using the pump also needed significantly lower daily insulin doses (97 unit/kg per day vs. 122 unit/kg per day).
These results were inputted into the CORE model, along with the costs of diabetes-related complications in the Netherlands and the costs of both pump therapy and daily insulin injections. For the first year of treatment, pump therapy more than twice as expensive as daily injections (4,407 euros vs. 1,597 euros). This difference remained when costs were extrapolated to year 2 and beyond (4,365 euros vs. 1,555 euros).
The model, however, determined that pump therapy has the potential to extend life expectancy by almost 1 year, compared with injection therapy, by reducing and/or delaying life-threatening diabetes complications. These clinical benefits translated into an additional 9.38 quality-adjusted life-years (QALY) for patients using the pump, compared with 8.95 QALY for patients using injections.
Over a lifetime of treatment (13 years), pump therapy would cost a total of 58,024 euros, compared with 20,237 euros for injection therapy – a difference of 37,787 euros. Compared with injection therapy, though, pump therapy would save almost 10,000 euros in the cost of renal complications; 625 euros in the cost of ulcer, amputation, and neuropathy complications; 152 euros in the cost of eye complications; and 410 euros in complications due to hypoglycemia. These savings reduced the total cost difference between the two to 27,051 euros, Mr. Roze said.
The benefits held over time, despite the paradox of survivorship, he said. “The longer a patient with chronic disease lives, the more opportunity they have to develop a complication.”
The incremental cost-effectiveness ratio was 62,295 euros/QALY – an amount that falls well within the Netherlands’ “willingness to pay” threshold, which is a measure of what payers or society are generally willing to accept as a good value for health care expenditures.
The newest data from OpT2mise, also released at EASD, bolster Mr. Roze’s modeling. In phase II of the study, patients who had been on injections crossed over to pump therapy. Within 6 months of crossover, these patients experienced a mean HbA1c decrease of 0.8%. By 12 months from study start, when all patients had been on pump therapy for at least 6 months, the mean HbA1chad dropped to 7.8% from baseline.
“We were reassured to see the 12-month data, which confirm the sustainability of the effect,” Mr. Roze said. “This is one of the main questions when you do health care modeling.”
Mr. Roze is a co-owner of HEVA-HEOR.
STOCKHOLM – Insulin pump therapy is considerably more expensive than daily insulin injections for patients with type 2 diabetes, but the benefits it confers offset about 30% of the price difference.
A cost-modeling study showed that patients who use the pump gain almost another year of life expectancy and experience a delay in the onset of major complications, such as end-stage renal disease and amputation, Stephane Roze said at the annual meeting of the European Association for the Study of Diabetes.
Pump therapy also conferred a significantly improved quality of life over multiple daily injections, a benefit that carries its own economic value, said Mr. Roze of HEVA-HEOR, a health economics evaluation company in Lyon, France.
“Even though these patients are initiating the pump at an older age than patients with type 1 diabetes, the therapy still looks to be cost effective,” he said. “It’s a good investment for payers.”
Mr. Roze used the CORE Diabetes Model to examine the cost/benefit ratio of insulin pump therapy, compared with multiple daily injections in patients with poorly controlled type 2 diabetes who lived in the Netherlands. The CORE model comprises 14 sub-Markov models that run in parallel; it examines all of the micro- and macrovascular complications related to diabetes. Model input data were the cohort characteristics and 6-month clinical outcomes of the OpT2mise study.
The trial examined glucose variability in 331 patients with type 2 diabetes who were randomized to either pump therapy or daily insulin injections.
It is an excellent study for cost-modeling initiatives, Mr. Roze said. It’s multicenter and multinational, conducted in 36 institutions in the United States, Canada, Europe, Israel, and South Africa. The cohort is large and diverse. Importantly, all of the patients had their treatment optimized with daily insulin analogue injections before being randomized. That initial step lent credence to their baseline glucose control; despite optimization, they still had a mean hemoglobin A1cof 9%. The OpT2mise patients were a mean of 56 years old, with mean disease duration of 15 years.
At 6 months, the study showed that HbA1c had decreased significantly more in the pump group than in the injection group (1.1% vs. 0.4%). Patients using the pump also needed significantly lower daily insulin doses (97 unit/kg per day vs. 122 unit/kg per day).
These results were inputted into the CORE model, along with the costs of diabetes-related complications in the Netherlands and the costs of both pump therapy and daily insulin injections. For the first year of treatment, pump therapy more than twice as expensive as daily injections (4,407 euros vs. 1,597 euros). This difference remained when costs were extrapolated to year 2 and beyond (4,365 euros vs. 1,555 euros).
The model, however, determined that pump therapy has the potential to extend life expectancy by almost 1 year, compared with injection therapy, by reducing and/or delaying life-threatening diabetes complications. These clinical benefits translated into an additional 9.38 quality-adjusted life-years (QALY) for patients using the pump, compared with 8.95 QALY for patients using injections.
Over a lifetime of treatment (13 years), pump therapy would cost a total of 58,024 euros, compared with 20,237 euros for injection therapy – a difference of 37,787 euros. Compared with injection therapy, though, pump therapy would save almost 10,000 euros in the cost of renal complications; 625 euros in the cost of ulcer, amputation, and neuropathy complications; 152 euros in the cost of eye complications; and 410 euros in complications due to hypoglycemia. These savings reduced the total cost difference between the two to 27,051 euros, Mr. Roze said.
The benefits held over time, despite the paradox of survivorship, he said. “The longer a patient with chronic disease lives, the more opportunity they have to develop a complication.”
The incremental cost-effectiveness ratio was 62,295 euros/QALY – an amount that falls well within the Netherlands’ “willingness to pay” threshold, which is a measure of what payers or society are generally willing to accept as a good value for health care expenditures.
The newest data from OpT2mise, also released at EASD, bolster Mr. Roze’s modeling. In phase II of the study, patients who had been on injections crossed over to pump therapy. Within 6 months of crossover, these patients experienced a mean HbA1c decrease of 0.8%. By 12 months from study start, when all patients had been on pump therapy for at least 6 months, the mean HbA1chad dropped to 7.8% from baseline.
“We were reassured to see the 12-month data, which confirm the sustainability of the effect,” Mr. Roze said. “This is one of the main questions when you do health care modeling.”
Mr. Roze is a co-owner of HEVA-HEOR.
STOCKHOLM – Insulin pump therapy is considerably more expensive than daily insulin injections for patients with type 2 diabetes, but the benefits it confers offset about 30% of the price difference.
A cost-modeling study showed that patients who use the pump gain almost another year of life expectancy and experience a delay in the onset of major complications, such as end-stage renal disease and amputation, Stephane Roze said at the annual meeting of the European Association for the Study of Diabetes.
Pump therapy also conferred a significantly improved quality of life over multiple daily injections, a benefit that carries its own economic value, said Mr. Roze of HEVA-HEOR, a health economics evaluation company in Lyon, France.
“Even though these patients are initiating the pump at an older age than patients with type 1 diabetes, the therapy still looks to be cost effective,” he said. “It’s a good investment for payers.”
Mr. Roze used the CORE Diabetes Model to examine the cost/benefit ratio of insulin pump therapy, compared with multiple daily injections in patients with poorly controlled type 2 diabetes who lived in the Netherlands. The CORE model comprises 14 sub-Markov models that run in parallel; it examines all of the micro- and macrovascular complications related to diabetes. Model input data were the cohort characteristics and 6-month clinical outcomes of the OpT2mise study.
The trial examined glucose variability in 331 patients with type 2 diabetes who were randomized to either pump therapy or daily insulin injections.
It is an excellent study for cost-modeling initiatives, Mr. Roze said. It’s multicenter and multinational, conducted in 36 institutions in the United States, Canada, Europe, Israel, and South Africa. The cohort is large and diverse. Importantly, all of the patients had their treatment optimized with daily insulin analogue injections before being randomized. That initial step lent credence to their baseline glucose control; despite optimization, they still had a mean hemoglobin A1cof 9%. The OpT2mise patients were a mean of 56 years old, with mean disease duration of 15 years.
At 6 months, the study showed that HbA1c had decreased significantly more in the pump group than in the injection group (1.1% vs. 0.4%). Patients using the pump also needed significantly lower daily insulin doses (97 unit/kg per day vs. 122 unit/kg per day).
These results were inputted into the CORE model, along with the costs of diabetes-related complications in the Netherlands and the costs of both pump therapy and daily insulin injections. For the first year of treatment, pump therapy more than twice as expensive as daily injections (4,407 euros vs. 1,597 euros). This difference remained when costs were extrapolated to year 2 and beyond (4,365 euros vs. 1,555 euros).
The model, however, determined that pump therapy has the potential to extend life expectancy by almost 1 year, compared with injection therapy, by reducing and/or delaying life-threatening diabetes complications. These clinical benefits translated into an additional 9.38 quality-adjusted life-years (QALY) for patients using the pump, compared with 8.95 QALY for patients using injections.
Over a lifetime of treatment (13 years), pump therapy would cost a total of 58,024 euros, compared with 20,237 euros for injection therapy – a difference of 37,787 euros. Compared with injection therapy, though, pump therapy would save almost 10,000 euros in the cost of renal complications; 625 euros in the cost of ulcer, amputation, and neuropathy complications; 152 euros in the cost of eye complications; and 410 euros in complications due to hypoglycemia. These savings reduced the total cost difference between the two to 27,051 euros, Mr. Roze said.
The benefits held over time, despite the paradox of survivorship, he said. “The longer a patient with chronic disease lives, the more opportunity they have to develop a complication.”
The incremental cost-effectiveness ratio was 62,295 euros/QALY – an amount that falls well within the Netherlands’ “willingness to pay” threshold, which is a measure of what payers or society are generally willing to accept as a good value for health care expenditures.
The newest data from OpT2mise, also released at EASD, bolster Mr. Roze’s modeling. In phase II of the study, patients who had been on injections crossed over to pump therapy. Within 6 months of crossover, these patients experienced a mean HbA1c decrease of 0.8%. By 12 months from study start, when all patients had been on pump therapy for at least 6 months, the mean HbA1chad dropped to 7.8% from baseline.
“We were reassured to see the 12-month data, which confirm the sustainability of the effect,” Mr. Roze said. “This is one of the main questions when you do health care modeling.”
Mr. Roze is a co-owner of HEVA-HEOR.
AT EASD 2015
Key clinical point: The benefits of insulin pump therapy for patients with type 2 diabetes make up for about 30% of the increased cost compared with insulin injections.
Major finding: Insulin pump therapy saved money by adding almost a year of life expectancy for patients with type 2 diabetes and delaying the onset of micro- and macrovascular complications.
Data source: The cost-effectiveness model was based on OpT2mise, which randomized 331 patients with type 2 diabetes to pump therapy or daily insulin injections.
Disclosures: Mr. Roze is a co-owner of HEVA-HEOR.
‘Artificial beta-cell’ insulin systems post more positive data
STOCKHOLM – The artificial beta cell is moving from the lab bench to the bedside table.
These experimental closed-loop insulin systems, which deliver constantly adjusted pulses of insulin, continue to rack up research success. Tightly controlled studies have given way to more free-ranging trials conducted in diabetes camps and, now, even at home. The endpoints continue to align, providing a growing certainty that the systems regulate glucose much more efficiently than does a traditional insulin pump.
“We now have a large number of studies in patients of various types – adults, children, adolescents, and even pregnant women – and in various situations – at home, at camp, in clinics, and even in acute illness,” Dr. Lalantha Leelarathna said at the annual meeting of the European Association for the Study of Diabetes. “Compared with our current best therapy, these studies consistently show more time in target, lower mean glucose, less burden of hypoglycemia, less glucose variability, and an improvement in hemoglobin A1c.”
Not all the studies of the artificial beta-cell insulin system have been completely successful, he admitted. “We’ve had problems with sense accuracy, with device connectivity, and there is an urgent need to miniaturize these devices and integrate them so patients can use them easily. But there is no hiding the fact that there has been tremendous progress over the past 5-10 years in this field, and it’s becoming a realistic option for our patients to have these in the not-too-distant future.”
Dr. Leelarathna of the University of Manchester, England, discussed some of the newest data on the closed-loop systems, including two 12-week home-use trials, which were simultaneously published in the New England Journal of Medicine. They were conducted by the APCam Consortium and the AP@home Consortium. One examined the system’s 24-hour use in a population of adults; the other was an overnight study in children and teens. Both were randomized crossover trials that compared a closed-loop system with sensor-augmented pump therapy.
Both studies used the FlorenceD2A closed-loop system. The Florence system uses a smartphone or tablet to wirelessly communicate a model predictive algorithm to the pump. Every 12 minutes, the system calculates a new insulin infusion rate. The treat-to-target control algorithm aims to achieve glucose levels between 5.8 and 7.3 mmol/L and adjusts the actual level depending on fasting vs. postprandial status and the accuracy of model-based glucose predictions.
AP@home04
The study was conducted in the United Kingdom, Austria, and Germany; it comprised 33 adults with type 1 diabetes.
After a 4-week optimization period, the patients used either their control system or the closed-loop system day and night for 3 months. There was then a 4-week washout period, followed by 12 weeks’ use of the comparator system. The Florence system ran wirelessly off the patients’ smartphone, which was with them at all times.
The results consistently favored the closed-loop system, Dr. Leelarathna said. It significantly improved time spent in the target glucose range of 70-180 mg/dL (76% vs. 56% per 24 hours). It also reduced the time spent with glucose above 180 mg/dL (29% vs. 39%), as well as the time spent in the hypoglycemic range of less than 50 m/dL (0.3% vs. 0.4%). The mean glucose level was significantly lower (157 vs. 168 mg/dL). These improvements were achieved despite no increase in insulin (48.8 vs. 48.1 U/day).
HbA1cwas significantly improved from baseline on the closed-loop system, dropping from 7.6%-7.3%. While on the insulin pump, HbA1cwas unchanged (7.6% at both time points).
APCam08
The children’s study was conducted in three U.K. centers, and comprised 25 children and teens with type 1 diabetes. The mean HbA1c at baseline was 8.1%. The study protocol was the same, except that the children used the pumps only from midnight-8 a.m. The algorithm was transmitted to the unit via USB cable from a tablet on the bedside table.
The results were also quite similar. The closed-loop system significantly increased the time spent in the target range of 70-145 mg/dL (60% vs. 34% per 24 hours). Likewise, the time in hyperglycemia (above 145 mg/dL) was significantly reduced (37% vs. 61%). However, there was no difference in time spent in hypoglycemia.
Mean glucose level was significantly improved (146 vs. 176 mg/dL), and overnight insulin use slightly, but not significantly, reduced (7.6 vs. 7.7 U).
After the initial optimization period, the subjects’ mean HbA1c was 7.8%. This improved significantly on the closed loop system (7.6%), but rose slightly on the insulin pump (7.9%).
Although these two trials represent the longest free-living studies with the closed-loop system, they’re not the only ones, Dr. Leelarathna mentioned. A third was also presented at the meeting
Dr. Hans DeVries of the University of Amsterdam examined the closed-loop device for 2 months in 32 adults with type 1 diabetes. They used it overnight; the comparator was their own sensor-augmented pump, which they used during the day while at home.
Again, the closed-loop system improved time spent in target (66.7% vs. 58%); reduced time in hyperglycemia (31.6% vs. 38.5%); reduced time in hypoglycemia (1.6% vs. 3%)); and improved mean glucose (161.6 vs. 167.6 mg/dL). This study also showed a significant reduction in daily insulin (16.2 vs. 18.4 U/kg per day).
The devices are also being tested in a cohort of pregnant women who are enrolled at 8-24 weeks’ gestation. The planned sample size is 18, Dr. Leelarathna said. This is a randomized, crossover study that will use the closed-loop and insulin pump systems for 28 nights with a 2-4 week washout in between. At the end, the women will choose which system to continue with. Some have completed the study, and used the closed-loop system in labor and delivery. Preliminary results look good, Dr. Leelarathna said, although he didn’t release any data.
He had no financial disclosures.
STOCKHOLM – The artificial beta cell is moving from the lab bench to the bedside table.
These experimental closed-loop insulin systems, which deliver constantly adjusted pulses of insulin, continue to rack up research success. Tightly controlled studies have given way to more free-ranging trials conducted in diabetes camps and, now, even at home. The endpoints continue to align, providing a growing certainty that the systems regulate glucose much more efficiently than does a traditional insulin pump.
“We now have a large number of studies in patients of various types – adults, children, adolescents, and even pregnant women – and in various situations – at home, at camp, in clinics, and even in acute illness,” Dr. Lalantha Leelarathna said at the annual meeting of the European Association for the Study of Diabetes. “Compared with our current best therapy, these studies consistently show more time in target, lower mean glucose, less burden of hypoglycemia, less glucose variability, and an improvement in hemoglobin A1c.”
Not all the studies of the artificial beta-cell insulin system have been completely successful, he admitted. “We’ve had problems with sense accuracy, with device connectivity, and there is an urgent need to miniaturize these devices and integrate them so patients can use them easily. But there is no hiding the fact that there has been tremendous progress over the past 5-10 years in this field, and it’s becoming a realistic option for our patients to have these in the not-too-distant future.”
Dr. Leelarathna of the University of Manchester, England, discussed some of the newest data on the closed-loop systems, including two 12-week home-use trials, which were simultaneously published in the New England Journal of Medicine. They were conducted by the APCam Consortium and the AP@home Consortium. One examined the system’s 24-hour use in a population of adults; the other was an overnight study in children and teens. Both were randomized crossover trials that compared a closed-loop system with sensor-augmented pump therapy.
Both studies used the FlorenceD2A closed-loop system. The Florence system uses a smartphone or tablet to wirelessly communicate a model predictive algorithm to the pump. Every 12 minutes, the system calculates a new insulin infusion rate. The treat-to-target control algorithm aims to achieve glucose levels between 5.8 and 7.3 mmol/L and adjusts the actual level depending on fasting vs. postprandial status and the accuracy of model-based glucose predictions.
AP@home04
The study was conducted in the United Kingdom, Austria, and Germany; it comprised 33 adults with type 1 diabetes.
After a 4-week optimization period, the patients used either their control system or the closed-loop system day and night for 3 months. There was then a 4-week washout period, followed by 12 weeks’ use of the comparator system. The Florence system ran wirelessly off the patients’ smartphone, which was with them at all times.
The results consistently favored the closed-loop system, Dr. Leelarathna said. It significantly improved time spent in the target glucose range of 70-180 mg/dL (76% vs. 56% per 24 hours). It also reduced the time spent with glucose above 180 mg/dL (29% vs. 39%), as well as the time spent in the hypoglycemic range of less than 50 m/dL (0.3% vs. 0.4%). The mean glucose level was significantly lower (157 vs. 168 mg/dL). These improvements were achieved despite no increase in insulin (48.8 vs. 48.1 U/day).
HbA1cwas significantly improved from baseline on the closed-loop system, dropping from 7.6%-7.3%. While on the insulin pump, HbA1cwas unchanged (7.6% at both time points).
APCam08
The children’s study was conducted in three U.K. centers, and comprised 25 children and teens with type 1 diabetes. The mean HbA1c at baseline was 8.1%. The study protocol was the same, except that the children used the pumps only from midnight-8 a.m. The algorithm was transmitted to the unit via USB cable from a tablet on the bedside table.
The results were also quite similar. The closed-loop system significantly increased the time spent in the target range of 70-145 mg/dL (60% vs. 34% per 24 hours). Likewise, the time in hyperglycemia (above 145 mg/dL) was significantly reduced (37% vs. 61%). However, there was no difference in time spent in hypoglycemia.
Mean glucose level was significantly improved (146 vs. 176 mg/dL), and overnight insulin use slightly, but not significantly, reduced (7.6 vs. 7.7 U).
After the initial optimization period, the subjects’ mean HbA1c was 7.8%. This improved significantly on the closed loop system (7.6%), but rose slightly on the insulin pump (7.9%).
Although these two trials represent the longest free-living studies with the closed-loop system, they’re not the only ones, Dr. Leelarathna mentioned. A third was also presented at the meeting
Dr. Hans DeVries of the University of Amsterdam examined the closed-loop device for 2 months in 32 adults with type 1 diabetes. They used it overnight; the comparator was their own sensor-augmented pump, which they used during the day while at home.
Again, the closed-loop system improved time spent in target (66.7% vs. 58%); reduced time in hyperglycemia (31.6% vs. 38.5%); reduced time in hypoglycemia (1.6% vs. 3%)); and improved mean glucose (161.6 vs. 167.6 mg/dL). This study also showed a significant reduction in daily insulin (16.2 vs. 18.4 U/kg per day).
The devices are also being tested in a cohort of pregnant women who are enrolled at 8-24 weeks’ gestation. The planned sample size is 18, Dr. Leelarathna said. This is a randomized, crossover study that will use the closed-loop and insulin pump systems for 28 nights with a 2-4 week washout in between. At the end, the women will choose which system to continue with. Some have completed the study, and used the closed-loop system in labor and delivery. Preliminary results look good, Dr. Leelarathna said, although he didn’t release any data.
He had no financial disclosures.
STOCKHOLM – The artificial beta cell is moving from the lab bench to the bedside table.
These experimental closed-loop insulin systems, which deliver constantly adjusted pulses of insulin, continue to rack up research success. Tightly controlled studies have given way to more free-ranging trials conducted in diabetes camps and, now, even at home. The endpoints continue to align, providing a growing certainty that the systems regulate glucose much more efficiently than does a traditional insulin pump.
“We now have a large number of studies in patients of various types – adults, children, adolescents, and even pregnant women – and in various situations – at home, at camp, in clinics, and even in acute illness,” Dr. Lalantha Leelarathna said at the annual meeting of the European Association for the Study of Diabetes. “Compared with our current best therapy, these studies consistently show more time in target, lower mean glucose, less burden of hypoglycemia, less glucose variability, and an improvement in hemoglobin A1c.”
Not all the studies of the artificial beta-cell insulin system have been completely successful, he admitted. “We’ve had problems with sense accuracy, with device connectivity, and there is an urgent need to miniaturize these devices and integrate them so patients can use them easily. But there is no hiding the fact that there has been tremendous progress over the past 5-10 years in this field, and it’s becoming a realistic option for our patients to have these in the not-too-distant future.”
Dr. Leelarathna of the University of Manchester, England, discussed some of the newest data on the closed-loop systems, including two 12-week home-use trials, which were simultaneously published in the New England Journal of Medicine. They were conducted by the APCam Consortium and the AP@home Consortium. One examined the system’s 24-hour use in a population of adults; the other was an overnight study in children and teens. Both were randomized crossover trials that compared a closed-loop system with sensor-augmented pump therapy.
Both studies used the FlorenceD2A closed-loop system. The Florence system uses a smartphone or tablet to wirelessly communicate a model predictive algorithm to the pump. Every 12 minutes, the system calculates a new insulin infusion rate. The treat-to-target control algorithm aims to achieve glucose levels between 5.8 and 7.3 mmol/L and adjusts the actual level depending on fasting vs. postprandial status and the accuracy of model-based glucose predictions.
AP@home04
The study was conducted in the United Kingdom, Austria, and Germany; it comprised 33 adults with type 1 diabetes.
After a 4-week optimization period, the patients used either their control system or the closed-loop system day and night for 3 months. There was then a 4-week washout period, followed by 12 weeks’ use of the comparator system. The Florence system ran wirelessly off the patients’ smartphone, which was with them at all times.
The results consistently favored the closed-loop system, Dr. Leelarathna said. It significantly improved time spent in the target glucose range of 70-180 mg/dL (76% vs. 56% per 24 hours). It also reduced the time spent with glucose above 180 mg/dL (29% vs. 39%), as well as the time spent in the hypoglycemic range of less than 50 m/dL (0.3% vs. 0.4%). The mean glucose level was significantly lower (157 vs. 168 mg/dL). These improvements were achieved despite no increase in insulin (48.8 vs. 48.1 U/day).
HbA1cwas significantly improved from baseline on the closed-loop system, dropping from 7.6%-7.3%. While on the insulin pump, HbA1cwas unchanged (7.6% at both time points).
APCam08
The children’s study was conducted in three U.K. centers, and comprised 25 children and teens with type 1 diabetes. The mean HbA1c at baseline was 8.1%. The study protocol was the same, except that the children used the pumps only from midnight-8 a.m. The algorithm was transmitted to the unit via USB cable from a tablet on the bedside table.
The results were also quite similar. The closed-loop system significantly increased the time spent in the target range of 70-145 mg/dL (60% vs. 34% per 24 hours). Likewise, the time in hyperglycemia (above 145 mg/dL) was significantly reduced (37% vs. 61%). However, there was no difference in time spent in hypoglycemia.
Mean glucose level was significantly improved (146 vs. 176 mg/dL), and overnight insulin use slightly, but not significantly, reduced (7.6 vs. 7.7 U).
After the initial optimization period, the subjects’ mean HbA1c was 7.8%. This improved significantly on the closed loop system (7.6%), but rose slightly on the insulin pump (7.9%).
Although these two trials represent the longest free-living studies with the closed-loop system, they’re not the only ones, Dr. Leelarathna mentioned. A third was also presented at the meeting
Dr. Hans DeVries of the University of Amsterdam examined the closed-loop device for 2 months in 32 adults with type 1 diabetes. They used it overnight; the comparator was their own sensor-augmented pump, which they used during the day while at home.
Again, the closed-loop system improved time spent in target (66.7% vs. 58%); reduced time in hyperglycemia (31.6% vs. 38.5%); reduced time in hypoglycemia (1.6% vs. 3%)); and improved mean glucose (161.6 vs. 167.6 mg/dL). This study also showed a significant reduction in daily insulin (16.2 vs. 18.4 U/kg per day).
The devices are also being tested in a cohort of pregnant women who are enrolled at 8-24 weeks’ gestation. The planned sample size is 18, Dr. Leelarathna said. This is a randomized, crossover study that will use the closed-loop and insulin pump systems for 28 nights with a 2-4 week washout in between. At the end, the women will choose which system to continue with. Some have completed the study, and used the closed-loop system in labor and delivery. Preliminary results look good, Dr. Leelarathna said, although he didn’t release any data.
He had no financial disclosures.
AT EASD 2015
EASD: SGLT2 inhibitor empagliflozin cuts CV risk in patients with type 2 diabetes
STOCKHOLM – The combined risk for cardiovascular death, nonfatal MI, and nonfatal stroke was reduced by 14% when empagliflozin was added to standard care for type 2 diabetes in patients at high cardiovascular risk, compared with standard care alone, in the EMPA-REG OUTCOME study.
This primary composite endpoint was reached by 10.5% of the 4,687 patients treated with the antidiabetic drug versus 12.1% of the 2,333 patients given a placebo, with a hazard ratio (HR) of 0.86 and 95% confidence interval (CI) of 0.74 to 0.99 (P = .04 for superiority and P less than .001 for noninferiority).
The results of the EMPA-REG OUTCOME study, which were reported for the first time at the annual meeting of the European Association for the Study of Diabetes, were published simultaneously Sept. 17 online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1504720).
Dr. Silvio Inzucchi, professor of medicine at Yale University, New Haven, Conn., and the study investigator who presented the key outcome findings, said during the Q&A session that this was the first presentation of these data and that of course there had to be a little bit of caution before making any firm recommendations.
“It is really important that the diabetes and cardiology communities digest [these data], to try to understand them, and that we also have to be cautious here. This was a group of patients with 10 years’ duration of diabetes, all of whom had cardiovascular disease, so we can not necessarily ‘cut and paste’ these expectations to the entirety of the diabetes population.”
Putting the results into context ahead of their presentation in detail at the meeting, Dr. Naveed Sattar of the University of Glasgow, Scotland, said in an interview that there had been four other cardiovascular outcomes trials in this high-risk population of patients with type 2 diabetes and cardiovascular disease – three with DPP-4 inhibitors, namely the SAVOR-TIMI 53 trial, the EXAMINEtrial, and the recent TECOS. The fourth such trial involved a GLP-1 antagonist (ELIXA). All of these had shown modest or small additional reductions in blood glucose levels with the tested drugs versus standard of care or placebo control but had “shown unequivocally no benefit in [reducing] cardiovascular events.” Cardiovascular effects had simply been neutral or no cause for concern.
So to now have a trial with a drug that is potentially more potent at lowering blood glucose than the others and is showing a cardiovascular benefit is potentially game changing, said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Evidence suggests that empagliflozin does more than just lower blood glucose, said Dr. Sattar, who was not involved in the EMPA-REG trial. “Empagliflozin lowers blood pressure, it reduces weight, so is it these added effects that have conferred this benefit or is it something else?” He added: “Most people’s perception is that it is possibly a class effect, but the data need to be seen.”
Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, singled out the EMPA-REG study as one to watch. Speaking in an interview at the annual meeting of the European Society of Cardiology in London, he said that if the cardiovascular risk reduction touted were true it would make empagliflozin “the only modern glucose-lowering drug to have shown such a capacity.”
EMPA-REG OUTCOME was a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin, given at an oral dose of 10 mg/day or 25 mg/day compared to the best usual care in patients with type 2 diabetes who were at increased cardiovascular risk due to the presence of established cardiovascular disease. The study was done at 590 sites in 42 countries across six continents and involved more than 7,000 patients observed over a median of 3.1 years.
Looking at the individual components of the primary composite endpoint, there was no significant difference in terms of the relative risk reduction for MI or stroke. However, the unexpected results were that cardiovascular death, hospitalization for heart failure, and all-cause mortality were all reduced by more than one-third, with respective relative risk reductions of 38% (HR, 0.62; 95% CI, 0.49-0.77; P less than .001), 35% (HR, 0.68; 95% CI, 0.57-0.82; P less than .001), and 32% (HR, 0.65; 95% CI, 0.50-0.85; P = .002).
Even when the results were shown separating out the two doses of empagliflozin, the HR remained highly significant for these and the primary composite endpoint.
The key secondary outcome of the trial was a composite of the primary outcome plus hospitalization for unstable angina. However, no significant difference for superiority was seen between the groups (P = .008, P less than .001 for noninferiority).
“I am here because of the importance of this,” said Dr. Andrew Boulton outgoing president of the EASD and professor of medicine at the University of Manchester, England, who introduced the EASD-sponsored symposium where the results were revealed.
In an interview, Dr. Boulton said it “looks promising.”
The results were presented by the senior authors of the study and independently commented upon afterward by the EASD-invited discussant Dr. Hertzel Gerstein of McMaster University and Hamilton (Ont.) Health Sciences. “Without question, the EMPA-REG investigators have designed a good protocol, and they have answered a very important question,” he said. “This was a well done and very important study,” he noted.
Dr. Gerstein observed that empagliflozin “clearly reduces CV death and heart failure hospitalization,” an effect that starts to appear after just 3 months. This early effect is unlikely to be down to just glucose-lowering, antihypertensive effects of the background medications used, or weight loss because of this speed of onset. It could be due to the diuretic properties of the SLGT2 inhibitor or, maybe more likely, a combination of beneficial effects. “It is probably a class effect,” he said, “but you can never be certain.”
Based on the findings, he suggested that empagliflozin “may become first-line treatment for middle aged people with type 2 diabetes at risk for CV outcomes” and that the trial had “identified a treatment that could save many lives and reduce much suffering.” The results were unexpected and will open up new avenues for research.
Dr. Bernard Zinman of Mount Sinai Hospital, Toronto, lead author for the trial, said during the session that the number of patients needed to be treated with empagliflozin for 3 years to prevent one cardiovascular death was 39. By comparison, he said, the numbers needed to treat with simvastatin or ramipril for 5 years were 30 and 59, respectively.
Good safety was observed in the trial, reported Dr. David Fitchett, fellow author. Dr. Fitchett, a cardiologist at St Michael’s Hospital and associate professor at the University of Toronto, noted that overall rates of both any adverse events and serious adverse events were similar between the groups, with the exception of genital infections that were mainly mycoses and mostly in women.
Rates of confirmed hypoglycemic events were similar, he said, and there was no increase in diabetic ketoacidosis or bone fracture, which have been the focus of recent warnings issued by the Food and Drug Administration with regard to SLGT2 inhibitors.
Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
The EMPA-REG OUTCOME study results are spectacular in terms of the hard endpoints that were measured and the explicit reductions seen. It is exciting news because we have had quite a few negative trials with DPP4-inhibtiors where nothing much has happened and we don’t have cardiovascular outcomes. So that’s the upside, and that’s very exciting.
The downside has to be that the population studied included people who were post MI or were at higher cardiovascular risk than the general population with type 2 diabetes. So the study participants were people who are way into their diabetes pathology and are already having cardiovascular events. While it is of course good that these patients greatly benefit, one must not start to generalize and say that this shows that everyone with type 2 diabetes ought to be on an SGLT2 inhibitor.
In the future, I think one has to be very careful about the way that trials are set up and about who has gone into those trials. We still do not know very much about people who are newly onset with type 2 diabetes and if the SGLT2 inhibitors will be useful for them generally, but it is very nice to have positive outcomes in the group of patients studied.
These are big effects, which, as clinicians, we all like to see, and these are unlikely to be by chance because they are so big. The EMPA-REG OUTCOME findings clearly did not occur by chance. This is a really positive and exciting outcome for empagliflozin and it will affect the way people treat their patients. But the warning is that we must not generalize from the specifics of this trial in terms of who the patients treated were: they tended to be male, they were all people who had major events.
These remarks come from an interview with Dr. David Matthews, conducted onsite at EASD 2015 and exclusive to this news organization. Dr. Matthews is professor of diabetes and emeritus founding chairman, Oxford Centre for Diabetes, Endocrinology & Metabolism, Oxford, England. He is the coinvestigator for a Janssen-sponsored canagliflozin trial and has received honoraria and support from Janssen.
The EMPA-REG OUTCOME study results are spectacular in terms of the hard endpoints that were measured and the explicit reductions seen. It is exciting news because we have had quite a few negative trials with DPP4-inhibtiors where nothing much has happened and we don’t have cardiovascular outcomes. So that’s the upside, and that’s very exciting.
The downside has to be that the population studied included people who were post MI or were at higher cardiovascular risk than the general population with type 2 diabetes. So the study participants were people who are way into their diabetes pathology and are already having cardiovascular events. While it is of course good that these patients greatly benefit, one must not start to generalize and say that this shows that everyone with type 2 diabetes ought to be on an SGLT2 inhibitor.
In the future, I think one has to be very careful about the way that trials are set up and about who has gone into those trials. We still do not know very much about people who are newly onset with type 2 diabetes and if the SGLT2 inhibitors will be useful for them generally, but it is very nice to have positive outcomes in the group of patients studied.
These are big effects, which, as clinicians, we all like to see, and these are unlikely to be by chance because they are so big. The EMPA-REG OUTCOME findings clearly did not occur by chance. This is a really positive and exciting outcome for empagliflozin and it will affect the way people treat their patients. But the warning is that we must not generalize from the specifics of this trial in terms of who the patients treated were: they tended to be male, they were all people who had major events.
These remarks come from an interview with Dr. David Matthews, conducted onsite at EASD 2015 and exclusive to this news organization. Dr. Matthews is professor of diabetes and emeritus founding chairman, Oxford Centre for Diabetes, Endocrinology & Metabolism, Oxford, England. He is the coinvestigator for a Janssen-sponsored canagliflozin trial and has received honoraria and support from Janssen.
The EMPA-REG OUTCOME study results are spectacular in terms of the hard endpoints that were measured and the explicit reductions seen. It is exciting news because we have had quite a few negative trials with DPP4-inhibtiors where nothing much has happened and we don’t have cardiovascular outcomes. So that’s the upside, and that’s very exciting.
The downside has to be that the population studied included people who were post MI or were at higher cardiovascular risk than the general population with type 2 diabetes. So the study participants were people who are way into their diabetes pathology and are already having cardiovascular events. While it is of course good that these patients greatly benefit, one must not start to generalize and say that this shows that everyone with type 2 diabetes ought to be on an SGLT2 inhibitor.
In the future, I think one has to be very careful about the way that trials are set up and about who has gone into those trials. We still do not know very much about people who are newly onset with type 2 diabetes and if the SGLT2 inhibitors will be useful for them generally, but it is very nice to have positive outcomes in the group of patients studied.
These are big effects, which, as clinicians, we all like to see, and these are unlikely to be by chance because they are so big. The EMPA-REG OUTCOME findings clearly did not occur by chance. This is a really positive and exciting outcome for empagliflozin and it will affect the way people treat their patients. But the warning is that we must not generalize from the specifics of this trial in terms of who the patients treated were: they tended to be male, they were all people who had major events.
These remarks come from an interview with Dr. David Matthews, conducted onsite at EASD 2015 and exclusive to this news organization. Dr. Matthews is professor of diabetes and emeritus founding chairman, Oxford Centre for Diabetes, Endocrinology & Metabolism, Oxford, England. He is the coinvestigator for a Janssen-sponsored canagliflozin trial and has received honoraria and support from Janssen.
STOCKHOLM – The combined risk for cardiovascular death, nonfatal MI, and nonfatal stroke was reduced by 14% when empagliflozin was added to standard care for type 2 diabetes in patients at high cardiovascular risk, compared with standard care alone, in the EMPA-REG OUTCOME study.
This primary composite endpoint was reached by 10.5% of the 4,687 patients treated with the antidiabetic drug versus 12.1% of the 2,333 patients given a placebo, with a hazard ratio (HR) of 0.86 and 95% confidence interval (CI) of 0.74 to 0.99 (P = .04 for superiority and P less than .001 for noninferiority).
The results of the EMPA-REG OUTCOME study, which were reported for the first time at the annual meeting of the European Association for the Study of Diabetes, were published simultaneously Sept. 17 online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1504720).
Dr. Silvio Inzucchi, professor of medicine at Yale University, New Haven, Conn., and the study investigator who presented the key outcome findings, said during the Q&A session that this was the first presentation of these data and that of course there had to be a little bit of caution before making any firm recommendations.
“It is really important that the diabetes and cardiology communities digest [these data], to try to understand them, and that we also have to be cautious here. This was a group of patients with 10 years’ duration of diabetes, all of whom had cardiovascular disease, so we can not necessarily ‘cut and paste’ these expectations to the entirety of the diabetes population.”
Putting the results into context ahead of their presentation in detail at the meeting, Dr. Naveed Sattar of the University of Glasgow, Scotland, said in an interview that there had been four other cardiovascular outcomes trials in this high-risk population of patients with type 2 diabetes and cardiovascular disease – three with DPP-4 inhibitors, namely the SAVOR-TIMI 53 trial, the EXAMINEtrial, and the recent TECOS. The fourth such trial involved a GLP-1 antagonist (ELIXA). All of these had shown modest or small additional reductions in blood glucose levels with the tested drugs versus standard of care or placebo control but had “shown unequivocally no benefit in [reducing] cardiovascular events.” Cardiovascular effects had simply been neutral or no cause for concern.
So to now have a trial with a drug that is potentially more potent at lowering blood glucose than the others and is showing a cardiovascular benefit is potentially game changing, said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Evidence suggests that empagliflozin does more than just lower blood glucose, said Dr. Sattar, who was not involved in the EMPA-REG trial. “Empagliflozin lowers blood pressure, it reduces weight, so is it these added effects that have conferred this benefit or is it something else?” He added: “Most people’s perception is that it is possibly a class effect, but the data need to be seen.”
Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, singled out the EMPA-REG study as one to watch. Speaking in an interview at the annual meeting of the European Society of Cardiology in London, he said that if the cardiovascular risk reduction touted were true it would make empagliflozin “the only modern glucose-lowering drug to have shown such a capacity.”
EMPA-REG OUTCOME was a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin, given at an oral dose of 10 mg/day or 25 mg/day compared to the best usual care in patients with type 2 diabetes who were at increased cardiovascular risk due to the presence of established cardiovascular disease. The study was done at 590 sites in 42 countries across six continents and involved more than 7,000 patients observed over a median of 3.1 years.
Looking at the individual components of the primary composite endpoint, there was no significant difference in terms of the relative risk reduction for MI or stroke. However, the unexpected results were that cardiovascular death, hospitalization for heart failure, and all-cause mortality were all reduced by more than one-third, with respective relative risk reductions of 38% (HR, 0.62; 95% CI, 0.49-0.77; P less than .001), 35% (HR, 0.68; 95% CI, 0.57-0.82; P less than .001), and 32% (HR, 0.65; 95% CI, 0.50-0.85; P = .002).
Even when the results were shown separating out the two doses of empagliflozin, the HR remained highly significant for these and the primary composite endpoint.
The key secondary outcome of the trial was a composite of the primary outcome plus hospitalization for unstable angina. However, no significant difference for superiority was seen between the groups (P = .008, P less than .001 for noninferiority).
“I am here because of the importance of this,” said Dr. Andrew Boulton outgoing president of the EASD and professor of medicine at the University of Manchester, England, who introduced the EASD-sponsored symposium where the results were revealed.
In an interview, Dr. Boulton said it “looks promising.”
The results were presented by the senior authors of the study and independently commented upon afterward by the EASD-invited discussant Dr. Hertzel Gerstein of McMaster University and Hamilton (Ont.) Health Sciences. “Without question, the EMPA-REG investigators have designed a good protocol, and they have answered a very important question,” he said. “This was a well done and very important study,” he noted.
Dr. Gerstein observed that empagliflozin “clearly reduces CV death and heart failure hospitalization,” an effect that starts to appear after just 3 months. This early effect is unlikely to be down to just glucose-lowering, antihypertensive effects of the background medications used, or weight loss because of this speed of onset. It could be due to the diuretic properties of the SLGT2 inhibitor or, maybe more likely, a combination of beneficial effects. “It is probably a class effect,” he said, “but you can never be certain.”
Based on the findings, he suggested that empagliflozin “may become first-line treatment for middle aged people with type 2 diabetes at risk for CV outcomes” and that the trial had “identified a treatment that could save many lives and reduce much suffering.” The results were unexpected and will open up new avenues for research.
Dr. Bernard Zinman of Mount Sinai Hospital, Toronto, lead author for the trial, said during the session that the number of patients needed to be treated with empagliflozin for 3 years to prevent one cardiovascular death was 39. By comparison, he said, the numbers needed to treat with simvastatin or ramipril for 5 years were 30 and 59, respectively.
Good safety was observed in the trial, reported Dr. David Fitchett, fellow author. Dr. Fitchett, a cardiologist at St Michael’s Hospital and associate professor at the University of Toronto, noted that overall rates of both any adverse events and serious adverse events were similar between the groups, with the exception of genital infections that were mainly mycoses and mostly in women.
Rates of confirmed hypoglycemic events were similar, he said, and there was no increase in diabetic ketoacidosis or bone fracture, which have been the focus of recent warnings issued by the Food and Drug Administration with regard to SLGT2 inhibitors.
Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
STOCKHOLM – The combined risk for cardiovascular death, nonfatal MI, and nonfatal stroke was reduced by 14% when empagliflozin was added to standard care for type 2 diabetes in patients at high cardiovascular risk, compared with standard care alone, in the EMPA-REG OUTCOME study.
This primary composite endpoint was reached by 10.5% of the 4,687 patients treated with the antidiabetic drug versus 12.1% of the 2,333 patients given a placebo, with a hazard ratio (HR) of 0.86 and 95% confidence interval (CI) of 0.74 to 0.99 (P = .04 for superiority and P less than .001 for noninferiority).
The results of the EMPA-REG OUTCOME study, which were reported for the first time at the annual meeting of the European Association for the Study of Diabetes, were published simultaneously Sept. 17 online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1504720).
Dr. Silvio Inzucchi, professor of medicine at Yale University, New Haven, Conn., and the study investigator who presented the key outcome findings, said during the Q&A session that this was the first presentation of these data and that of course there had to be a little bit of caution before making any firm recommendations.
“It is really important that the diabetes and cardiology communities digest [these data], to try to understand them, and that we also have to be cautious here. This was a group of patients with 10 years’ duration of diabetes, all of whom had cardiovascular disease, so we can not necessarily ‘cut and paste’ these expectations to the entirety of the diabetes population.”
Putting the results into context ahead of their presentation in detail at the meeting, Dr. Naveed Sattar of the University of Glasgow, Scotland, said in an interview that there had been four other cardiovascular outcomes trials in this high-risk population of patients with type 2 diabetes and cardiovascular disease – three with DPP-4 inhibitors, namely the SAVOR-TIMI 53 trial, the EXAMINEtrial, and the recent TECOS. The fourth such trial involved a GLP-1 antagonist (ELIXA). All of these had shown modest or small additional reductions in blood glucose levels with the tested drugs versus standard of care or placebo control but had “shown unequivocally no benefit in [reducing] cardiovascular events.” Cardiovascular effects had simply been neutral or no cause for concern.
So to now have a trial with a drug that is potentially more potent at lowering blood glucose than the others and is showing a cardiovascular benefit is potentially game changing, said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Evidence suggests that empagliflozin does more than just lower blood glucose, said Dr. Sattar, who was not involved in the EMPA-REG trial. “Empagliflozin lowers blood pressure, it reduces weight, so is it these added effects that have conferred this benefit or is it something else?” He added: “Most people’s perception is that it is possibly a class effect, but the data need to be seen.”
Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, singled out the EMPA-REG study as one to watch. Speaking in an interview at the annual meeting of the European Society of Cardiology in London, he said that if the cardiovascular risk reduction touted were true it would make empagliflozin “the only modern glucose-lowering drug to have shown such a capacity.”
EMPA-REG OUTCOME was a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin, given at an oral dose of 10 mg/day or 25 mg/day compared to the best usual care in patients with type 2 diabetes who were at increased cardiovascular risk due to the presence of established cardiovascular disease. The study was done at 590 sites in 42 countries across six continents and involved more than 7,000 patients observed over a median of 3.1 years.
Looking at the individual components of the primary composite endpoint, there was no significant difference in terms of the relative risk reduction for MI or stroke. However, the unexpected results were that cardiovascular death, hospitalization for heart failure, and all-cause mortality were all reduced by more than one-third, with respective relative risk reductions of 38% (HR, 0.62; 95% CI, 0.49-0.77; P less than .001), 35% (HR, 0.68; 95% CI, 0.57-0.82; P less than .001), and 32% (HR, 0.65; 95% CI, 0.50-0.85; P = .002).
Even when the results were shown separating out the two doses of empagliflozin, the HR remained highly significant for these and the primary composite endpoint.
The key secondary outcome of the trial was a composite of the primary outcome plus hospitalization for unstable angina. However, no significant difference for superiority was seen between the groups (P = .008, P less than .001 for noninferiority).
“I am here because of the importance of this,” said Dr. Andrew Boulton outgoing president of the EASD and professor of medicine at the University of Manchester, England, who introduced the EASD-sponsored symposium where the results were revealed.
In an interview, Dr. Boulton said it “looks promising.”
The results were presented by the senior authors of the study and independently commented upon afterward by the EASD-invited discussant Dr. Hertzel Gerstein of McMaster University and Hamilton (Ont.) Health Sciences. “Without question, the EMPA-REG investigators have designed a good protocol, and they have answered a very important question,” he said. “This was a well done and very important study,” he noted.
Dr. Gerstein observed that empagliflozin “clearly reduces CV death and heart failure hospitalization,” an effect that starts to appear after just 3 months. This early effect is unlikely to be down to just glucose-lowering, antihypertensive effects of the background medications used, or weight loss because of this speed of onset. It could be due to the diuretic properties of the SLGT2 inhibitor or, maybe more likely, a combination of beneficial effects. “It is probably a class effect,” he said, “but you can never be certain.”
Based on the findings, he suggested that empagliflozin “may become first-line treatment for middle aged people with type 2 diabetes at risk for CV outcomes” and that the trial had “identified a treatment that could save many lives and reduce much suffering.” The results were unexpected and will open up new avenues for research.
Dr. Bernard Zinman of Mount Sinai Hospital, Toronto, lead author for the trial, said during the session that the number of patients needed to be treated with empagliflozin for 3 years to prevent one cardiovascular death was 39. By comparison, he said, the numbers needed to treat with simvastatin or ramipril for 5 years were 30 and 59, respectively.
Good safety was observed in the trial, reported Dr. David Fitchett, fellow author. Dr. Fitchett, a cardiologist at St Michael’s Hospital and associate professor at the University of Toronto, noted that overall rates of both any adverse events and serious adverse events were similar between the groups, with the exception of genital infections that were mainly mycoses and mostly in women.
Rates of confirmed hypoglycemic events were similar, he said, and there was no increase in diabetic ketoacidosis or bone fracture, which have been the focus of recent warnings issued by the Food and Drug Administration with regard to SLGT2 inhibitors.
Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
AT EASD 2015
Key clinical point: Empagliflozin reduced cardiovascular risk in patients with type 2 diabetes who were at high cardiovascular risk, making it the first antidiabetic drug to have been shown to have such an effect.
Major finding: This primary composite endpoint of cardiovascular death, nonfatal MI, and nonfatal stroke was reached by 10.5% (N = 4,687) of patients treated with empagliflozin versus 12.1% (N = 2,333) of those given placebo.
Data source: EMPA-REG OUTCOME: a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin added to usual care versus usual care alone in more than 7,000 patients with type 2 diabetes at high cardiovascular risk.
Disclosures: Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.