User login
Early Behavior After Head Trauma Predicts Postconcussional Syndrome
PARIS – Patients with mild traumatic brain injury who reported all or nothing behavior were significantly more likely to have postconcussional syndrome 3 months later, data from a prospective study of 107 adults have shown.
Approximately 15%-30% of mild traumatic brain injury (MTBI) patients are at risk of developing postconcussional syndrome (PCS), Dr. Ruihua Hou of the University of Southampton (England) explained at the annual meeting of the European Congress of Neuropsychopharmacology.
PCS is a symptom cluster that includes physical, cognitive, emotional, and behavioral symptoms. To investigate the etiology of PCS, the researchers developed a cognitive-behavioral model that compared MTBI patients who did and did not develop the syndrome.
Patients were assessed for cognitive, behavioral, emotional, and social variables at baseline using the Brief Illness Perception Questionnaire, the Behavioral Responses to Illness Questionnaire, the Impact of Events Scale, the Hospital Anxiety and Depression Scale, the Anxiety Sensitivity Index, and the Brief Social Support Questionnaire.
At the 3- and 6-month follow-up visits, the researchers used the Rivermead Post-Concussion Symptoms Questionnaire to assess the severity of PCS symptoms and the ICD-10 code F07.2 to diagnose PCS.
All or nothing behavior shortly after MTBI was a significant predictor for the onset of PCS at 3 months (odds ratio, 1.141).
However, all or nothing behavior was not a significant predictor of PCS after 6 months, the researchers noted. Instead, patients’ early negative injury perception was a significant predictor of the onset of PCS after 6 months (odds ratio, 1.053).
At 3 months and 6 months, no significant differences were observed between PCS cases and noncases with regard to demographics, including age, sex, education level, and occupation.
Although the findings were limited by the prospective nature of the study, they provide support for the cognitive model that was used, Dr. Hou said.
In addition, "the findings provide research evidence for the role of illness perception and coping behavior shortly after MTBI in the development of PCS and indicate that they may be important early-intervention targets," Dr. Hou emphasized.
The study was funded by the Faculty of Medicine Research Management Committee at the University of Southampton. The researchers said they had no relevant financial disclosures.
PARIS – Patients with mild traumatic brain injury who reported all or nothing behavior were significantly more likely to have postconcussional syndrome 3 months later, data from a prospective study of 107 adults have shown.
Approximately 15%-30% of mild traumatic brain injury (MTBI) patients are at risk of developing postconcussional syndrome (PCS), Dr. Ruihua Hou of the University of Southampton (England) explained at the annual meeting of the European Congress of Neuropsychopharmacology.
PCS is a symptom cluster that includes physical, cognitive, emotional, and behavioral symptoms. To investigate the etiology of PCS, the researchers developed a cognitive-behavioral model that compared MTBI patients who did and did not develop the syndrome.
Patients were assessed for cognitive, behavioral, emotional, and social variables at baseline using the Brief Illness Perception Questionnaire, the Behavioral Responses to Illness Questionnaire, the Impact of Events Scale, the Hospital Anxiety and Depression Scale, the Anxiety Sensitivity Index, and the Brief Social Support Questionnaire.
At the 3- and 6-month follow-up visits, the researchers used the Rivermead Post-Concussion Symptoms Questionnaire to assess the severity of PCS symptoms and the ICD-10 code F07.2 to diagnose PCS.
All or nothing behavior shortly after MTBI was a significant predictor for the onset of PCS at 3 months (odds ratio, 1.141).
However, all or nothing behavior was not a significant predictor of PCS after 6 months, the researchers noted. Instead, patients’ early negative injury perception was a significant predictor of the onset of PCS after 6 months (odds ratio, 1.053).
At 3 months and 6 months, no significant differences were observed between PCS cases and noncases with regard to demographics, including age, sex, education level, and occupation.
Although the findings were limited by the prospective nature of the study, they provide support for the cognitive model that was used, Dr. Hou said.
In addition, "the findings provide research evidence for the role of illness perception and coping behavior shortly after MTBI in the development of PCS and indicate that they may be important early-intervention targets," Dr. Hou emphasized.
The study was funded by the Faculty of Medicine Research Management Committee at the University of Southampton. The researchers said they had no relevant financial disclosures.
PARIS – Patients with mild traumatic brain injury who reported all or nothing behavior were significantly more likely to have postconcussional syndrome 3 months later, data from a prospective study of 107 adults have shown.
Approximately 15%-30% of mild traumatic brain injury (MTBI) patients are at risk of developing postconcussional syndrome (PCS), Dr. Ruihua Hou of the University of Southampton (England) explained at the annual meeting of the European Congress of Neuropsychopharmacology.
PCS is a symptom cluster that includes physical, cognitive, emotional, and behavioral symptoms. To investigate the etiology of PCS, the researchers developed a cognitive-behavioral model that compared MTBI patients who did and did not develop the syndrome.
Patients were assessed for cognitive, behavioral, emotional, and social variables at baseline using the Brief Illness Perception Questionnaire, the Behavioral Responses to Illness Questionnaire, the Impact of Events Scale, the Hospital Anxiety and Depression Scale, the Anxiety Sensitivity Index, and the Brief Social Support Questionnaire.
At the 3- and 6-month follow-up visits, the researchers used the Rivermead Post-Concussion Symptoms Questionnaire to assess the severity of PCS symptoms and the ICD-10 code F07.2 to diagnose PCS.
All or nothing behavior shortly after MTBI was a significant predictor for the onset of PCS at 3 months (odds ratio, 1.141).
However, all or nothing behavior was not a significant predictor of PCS after 6 months, the researchers noted. Instead, patients’ early negative injury perception was a significant predictor of the onset of PCS after 6 months (odds ratio, 1.053).
At 3 months and 6 months, no significant differences were observed between PCS cases and noncases with regard to demographics, including age, sex, education level, and occupation.
Although the findings were limited by the prospective nature of the study, they provide support for the cognitive model that was used, Dr. Hou said.
In addition, "the findings provide research evidence for the role of illness perception and coping behavior shortly after MTBI in the development of PCS and indicate that they may be important early-intervention targets," Dr. Hou emphasized.
The study was funded by the Faculty of Medicine Research Management Committee at the University of Southampton. The researchers said they had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE EUROPEAN CONGRESS OF NEURO-
PSYCHOPHARMACOLOGY
Major Finding: All or nothing behavior shortly after mild traumatic brain injury was a significant predictor for the onset of postconcussional syndrome at 3 months (odds ratio, 1.141), but early negative injury perception was a significant predictor of the onset of PCS after 6 months (odds ratio, 1.053).
Data Source: A prospective study of 107 adults with mild traumatic brain injury.
Disclosures: The study was funded by the Faculty of Medicine Research Management Committee at the University of Southampton. The researchers said they had no relevant financial disclosures.
More Nuanced Directions for Deep Brain Stimulation
PARIS – Deep brain stimulation could help a majority of patients with treatment-resistant major depression, preliminary studies suggest, but it’s only a start to dealing with patients’ problems.
The conceptual framework of deep brain stimulation is shifting from the idea of a global treatment for depression to targeted treatment of individual aspects of depression. There’s also a growing recognition that deep brain stimulation should be part of a holistic treatment plan, after two patients who achieved remission in early trials later committed suicide.
"These were people who had reached remission, who had actually done quite well, but were having trouble over time with other issues in their life," Dr. Helen S. Mayberg said at the annual congress of the European College of Neuropsychopharmacology. "This is not a cure, this is not a panacea, and we still have to deal with these elements of these patients."
Part of the evolving concepts of deep brain stimulation involves reframing expectations. When patients are severely depressed, what they most want is to make the psychic pain go away. Deep brain stimulation may lift that pain, but then the patient has other needs – a job, a friend, a direction in life.
"Recovery takes more than a stimulator," said Dr. Mayberg, professor of psychiatry and neurology at Emory University, Atlanta. As one patient described it, "we reset the system. We took the parking brake off the car that’s not moving," but then rehabilitation strategies are needed to help the patient drive to full recovery.
Another evolving concept involves the goal of deep brain stimulation. Dr. Mayberg likened it to the treatment of Parkinson’s disease. Physicians don’t try to treat the whole Parkinson’s syndrome; they fairly successfully treat tremor or rigidity and less successfully target gait and other aspects of the disease. "And they make no apologies about it," she said.
For major depression, it’s unclear which of the following characteristics deep brain stimulation should target: anhedonia, psychic pain, sleep disturbance, or suicidality. It’s probably unrealistic to think that deep brain stimulation can "get all of it," she said. "Having an idea in mind about which things are primary and which things are secondary might be important for this."
Preliminary studies targeting the subcallosal cingulate for stimulation hoped to affect negative mood, with a 60% response rate. Stimulation of the nucleus accumbens in other early studies focused on anhedonia, with a 50% response rate. Case reports of deep brain stimulation of the lateral habenula hoped to affect negative reward signals. Other brain sites have been targeted using different logical rationales.
Dr. Mayberg said she became preoccupied with the subcallosal cingulate in her first experiments with deep brain stimulation for depression, as evidence converged to support the hypothesis that resistance to conventional treatments was attributable to an inability to regulate this region. If the subcallosal cingulate couldn’t be talked into cooperation, drugged into cooperation, or shocked into cooperation, perhaps it could be targeted strategically with brain stimulation to modulate it.
In her pilot study in six patients who’d had treatment-resistant depression for nearly 6 years, deep brain stimulation produced a response in four patients and remission in three after 6 months (Neuron 2005;45:651-60).
She and her associates then implanted devices in 14 more patients and followed the total cohort of 20 for 1 year, at which time 55% were responding to the treatment (Biol. Psych. 2008;64:461-6). Long-term follow-up for 3-6 years showed that response and remission rates seemed to improve and stabilize over time, with an average response rate of 75% at 3 years and an overall 64% response rate at an average follow-up of 42 months. Remission rates averaged 50% at 3 years and 42% in the average 42 months of total follow-up (Am. J. Psych. 2011;168:502-10).
No late-developing side effects were seen, "so there doesn’t seem to be a price to pay for brain stimulation in this region," she said. Most meaningful was that many of the responders returned to work and meaningful activities after 5-6 years of severe illness.
Three devices were explanted because patients did not improve and because two patients in the original cohort developed infections. In addition, two patients committed suicide 3 years and 6.5 years after implantation, and not because their depression did not get better.
"Recovery takes more than a stimulator."
More recent, unpublished work by Dr. Mayberg and her associates replicated results in a 1-month, sham-controlled trial in 17 patients that also showed a difference in efficacy in patients with unipolar or bipolar depression. Average remission rates were 18% at 6 months, 36% at 1 year, and 58% at 2 years. The treatment did not induce mania or hypomania in any patients.
That study included preplanned periods in which the stimulation was turned off in a blinded fashion in patients who had responded to treatment. That led to a slow, steady relapse over approximately a 2-week period. Improvements returned after stimulation was resumed, but recovery was not immediate.
Dr. Mayberg said she imagines a day when further research will identify the critical brain circuits to target with deep brain stimulation, imaging will guide electrode placement to effective sites for stimulation, and new devices will tune the current flow to get into the tracts that mediate the acute effects of depression.
Dr. David J. Nutt, chair of neuropsychopharmacology at Imperial College London, said at the meeting that Dr. Mayberg’s research "does tell us something very fundamental about the way we should think about this. You can, with a small number of people, well studied, well characterized with brain imaging, make huge insights. You may actually be showing us a new way of doing other kinds of interventions, not just deep brain stimulation."
Dr. Mayberg discussed the off-label, experimental use of two devices for deep brain stimulation. She has an interest in related patents and has been a consultant for St. Jude Medical, which donated devices for some of the research. Her research has been funded by grants from the National Institute of Mental Health, the Brain and Behavior Research Foundation (formerly known as the National Alliance for Research on Schizophrenia and Depression, or NARSAD), the Dana Foundation, the Woodruff Foundation, and the Stanley Medical Research Institute.
PARIS – Deep brain stimulation could help a majority of patients with treatment-resistant major depression, preliminary studies suggest, but it’s only a start to dealing with patients’ problems.
The conceptual framework of deep brain stimulation is shifting from the idea of a global treatment for depression to targeted treatment of individual aspects of depression. There’s also a growing recognition that deep brain stimulation should be part of a holistic treatment plan, after two patients who achieved remission in early trials later committed suicide.
"These were people who had reached remission, who had actually done quite well, but were having trouble over time with other issues in their life," Dr. Helen S. Mayberg said at the annual congress of the European College of Neuropsychopharmacology. "This is not a cure, this is not a panacea, and we still have to deal with these elements of these patients."
Part of the evolving concepts of deep brain stimulation involves reframing expectations. When patients are severely depressed, what they most want is to make the psychic pain go away. Deep brain stimulation may lift that pain, but then the patient has other needs – a job, a friend, a direction in life.
"Recovery takes more than a stimulator," said Dr. Mayberg, professor of psychiatry and neurology at Emory University, Atlanta. As one patient described it, "we reset the system. We took the parking brake off the car that’s not moving," but then rehabilitation strategies are needed to help the patient drive to full recovery.
Another evolving concept involves the goal of deep brain stimulation. Dr. Mayberg likened it to the treatment of Parkinson’s disease. Physicians don’t try to treat the whole Parkinson’s syndrome; they fairly successfully treat tremor or rigidity and less successfully target gait and other aspects of the disease. "And they make no apologies about it," she said.
For major depression, it’s unclear which of the following characteristics deep brain stimulation should target: anhedonia, psychic pain, sleep disturbance, or suicidality. It’s probably unrealistic to think that deep brain stimulation can "get all of it," she said. "Having an idea in mind about which things are primary and which things are secondary might be important for this."
Preliminary studies targeting the subcallosal cingulate for stimulation hoped to affect negative mood, with a 60% response rate. Stimulation of the nucleus accumbens in other early studies focused on anhedonia, with a 50% response rate. Case reports of deep brain stimulation of the lateral habenula hoped to affect negative reward signals. Other brain sites have been targeted using different logical rationales.
Dr. Mayberg said she became preoccupied with the subcallosal cingulate in her first experiments with deep brain stimulation for depression, as evidence converged to support the hypothesis that resistance to conventional treatments was attributable to an inability to regulate this region. If the subcallosal cingulate couldn’t be talked into cooperation, drugged into cooperation, or shocked into cooperation, perhaps it could be targeted strategically with brain stimulation to modulate it.
In her pilot study in six patients who’d had treatment-resistant depression for nearly 6 years, deep brain stimulation produced a response in four patients and remission in three after 6 months (Neuron 2005;45:651-60).
She and her associates then implanted devices in 14 more patients and followed the total cohort of 20 for 1 year, at which time 55% were responding to the treatment (Biol. Psych. 2008;64:461-6). Long-term follow-up for 3-6 years showed that response and remission rates seemed to improve and stabilize over time, with an average response rate of 75% at 3 years and an overall 64% response rate at an average follow-up of 42 months. Remission rates averaged 50% at 3 years and 42% in the average 42 months of total follow-up (Am. J. Psych. 2011;168:502-10).
No late-developing side effects were seen, "so there doesn’t seem to be a price to pay for brain stimulation in this region," she said. Most meaningful was that many of the responders returned to work and meaningful activities after 5-6 years of severe illness.
Three devices were explanted because patients did not improve and because two patients in the original cohort developed infections. In addition, two patients committed suicide 3 years and 6.5 years after implantation, and not because their depression did not get better.
"Recovery takes more than a stimulator."
More recent, unpublished work by Dr. Mayberg and her associates replicated results in a 1-month, sham-controlled trial in 17 patients that also showed a difference in efficacy in patients with unipolar or bipolar depression. Average remission rates were 18% at 6 months, 36% at 1 year, and 58% at 2 years. The treatment did not induce mania or hypomania in any patients.
That study included preplanned periods in which the stimulation was turned off in a blinded fashion in patients who had responded to treatment. That led to a slow, steady relapse over approximately a 2-week period. Improvements returned after stimulation was resumed, but recovery was not immediate.
Dr. Mayberg said she imagines a day when further research will identify the critical brain circuits to target with deep brain stimulation, imaging will guide electrode placement to effective sites for stimulation, and new devices will tune the current flow to get into the tracts that mediate the acute effects of depression.
Dr. David J. Nutt, chair of neuropsychopharmacology at Imperial College London, said at the meeting that Dr. Mayberg’s research "does tell us something very fundamental about the way we should think about this. You can, with a small number of people, well studied, well characterized with brain imaging, make huge insights. You may actually be showing us a new way of doing other kinds of interventions, not just deep brain stimulation."
Dr. Mayberg discussed the off-label, experimental use of two devices for deep brain stimulation. She has an interest in related patents and has been a consultant for St. Jude Medical, which donated devices for some of the research. Her research has been funded by grants from the National Institute of Mental Health, the Brain and Behavior Research Foundation (formerly known as the National Alliance for Research on Schizophrenia and Depression, or NARSAD), the Dana Foundation, the Woodruff Foundation, and the Stanley Medical Research Institute.
PARIS – Deep brain stimulation could help a majority of patients with treatment-resistant major depression, preliminary studies suggest, but it’s only a start to dealing with patients’ problems.
The conceptual framework of deep brain stimulation is shifting from the idea of a global treatment for depression to targeted treatment of individual aspects of depression. There’s also a growing recognition that deep brain stimulation should be part of a holistic treatment plan, after two patients who achieved remission in early trials later committed suicide.
"These were people who had reached remission, who had actually done quite well, but were having trouble over time with other issues in their life," Dr. Helen S. Mayberg said at the annual congress of the European College of Neuropsychopharmacology. "This is not a cure, this is not a panacea, and we still have to deal with these elements of these patients."
Part of the evolving concepts of deep brain stimulation involves reframing expectations. When patients are severely depressed, what they most want is to make the psychic pain go away. Deep brain stimulation may lift that pain, but then the patient has other needs – a job, a friend, a direction in life.
"Recovery takes more than a stimulator," said Dr. Mayberg, professor of psychiatry and neurology at Emory University, Atlanta. As one patient described it, "we reset the system. We took the parking brake off the car that’s not moving," but then rehabilitation strategies are needed to help the patient drive to full recovery.
Another evolving concept involves the goal of deep brain stimulation. Dr. Mayberg likened it to the treatment of Parkinson’s disease. Physicians don’t try to treat the whole Parkinson’s syndrome; they fairly successfully treat tremor or rigidity and less successfully target gait and other aspects of the disease. "And they make no apologies about it," she said.
For major depression, it’s unclear which of the following characteristics deep brain stimulation should target: anhedonia, psychic pain, sleep disturbance, or suicidality. It’s probably unrealistic to think that deep brain stimulation can "get all of it," she said. "Having an idea in mind about which things are primary and which things are secondary might be important for this."
Preliminary studies targeting the subcallosal cingulate for stimulation hoped to affect negative mood, with a 60% response rate. Stimulation of the nucleus accumbens in other early studies focused on anhedonia, with a 50% response rate. Case reports of deep brain stimulation of the lateral habenula hoped to affect negative reward signals. Other brain sites have been targeted using different logical rationales.
Dr. Mayberg said she became preoccupied with the subcallosal cingulate in her first experiments with deep brain stimulation for depression, as evidence converged to support the hypothesis that resistance to conventional treatments was attributable to an inability to regulate this region. If the subcallosal cingulate couldn’t be talked into cooperation, drugged into cooperation, or shocked into cooperation, perhaps it could be targeted strategically with brain stimulation to modulate it.
In her pilot study in six patients who’d had treatment-resistant depression for nearly 6 years, deep brain stimulation produced a response in four patients and remission in three after 6 months (Neuron 2005;45:651-60).
She and her associates then implanted devices in 14 more patients and followed the total cohort of 20 for 1 year, at which time 55% were responding to the treatment (Biol. Psych. 2008;64:461-6). Long-term follow-up for 3-6 years showed that response and remission rates seemed to improve and stabilize over time, with an average response rate of 75% at 3 years and an overall 64% response rate at an average follow-up of 42 months. Remission rates averaged 50% at 3 years and 42% in the average 42 months of total follow-up (Am. J. Psych. 2011;168:502-10).
No late-developing side effects were seen, "so there doesn’t seem to be a price to pay for brain stimulation in this region," she said. Most meaningful was that many of the responders returned to work and meaningful activities after 5-6 years of severe illness.
Three devices were explanted because patients did not improve and because two patients in the original cohort developed infections. In addition, two patients committed suicide 3 years and 6.5 years after implantation, and not because their depression did not get better.
"Recovery takes more than a stimulator."
More recent, unpublished work by Dr. Mayberg and her associates replicated results in a 1-month, sham-controlled trial in 17 patients that also showed a difference in efficacy in patients with unipolar or bipolar depression. Average remission rates were 18% at 6 months, 36% at 1 year, and 58% at 2 years. The treatment did not induce mania or hypomania in any patients.
That study included preplanned periods in which the stimulation was turned off in a blinded fashion in patients who had responded to treatment. That led to a slow, steady relapse over approximately a 2-week period. Improvements returned after stimulation was resumed, but recovery was not immediate.
Dr. Mayberg said she imagines a day when further research will identify the critical brain circuits to target with deep brain stimulation, imaging will guide electrode placement to effective sites for stimulation, and new devices will tune the current flow to get into the tracts that mediate the acute effects of depression.
Dr. David J. Nutt, chair of neuropsychopharmacology at Imperial College London, said at the meeting that Dr. Mayberg’s research "does tell us something very fundamental about the way we should think about this. You can, with a small number of people, well studied, well characterized with brain imaging, make huge insights. You may actually be showing us a new way of doing other kinds of interventions, not just deep brain stimulation."
Dr. Mayberg discussed the off-label, experimental use of two devices for deep brain stimulation. She has an interest in related patents and has been a consultant for St. Jude Medical, which donated devices for some of the research. Her research has been funded by grants from the National Institute of Mental Health, the Brain and Behavior Research Foundation (formerly known as the National Alliance for Research on Schizophrenia and Depression, or NARSAD), the Dana Foundation, the Woodruff Foundation, and the Stanley Medical Research Institute.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN COLLEGE OF NEUROPSYCHO-PHARMACOLOGY
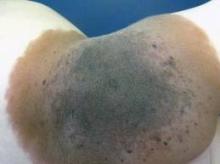
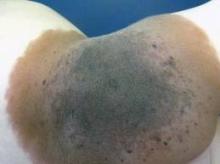
Definition of Giant Congenital Nevus Refined
SAN FRANCISCO – A new definition of giant congenital nevi should help physicians identify infants with supersized moles that are at risk for melanoma, neurocutaneous melanocytosis, liposarcoma, and other tumors.
For many years pediatric dermatologists sized congenital nevi as small, medium, or large, with "large" comprising nevi that are estimated to be 20 cm or greater in diameter when the patient has grown into adulthood. Then, physicians started referring to some of the large nevi as "giant" congenital nevi, with no universal agreement on what defines a giant nevus, Dr. Sheila Fallon Friedlander said at the Women’s & Pediatric Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
"Here’s our new definition that you’re going to be seeing in the literature," said Dr. Friedlander of the University of California, San Diego. Congenital nevi with diameters less than 1.5 cm after the patient is fully grown are considered small, nevi with diameters of at least 1.5 cm but less than 20 cm are medium size, and large nevi are at least 20 cm but less than 40 cm in diameter. Giant congenital nevi are those with diameters of 40 cm or greater when the patient is fully grown.
"When you are examining a baby in a nursery, how does that help you? That’s an adult-type figure," she noted. To estimate the grown size of a congenital nevus, measure the nevus on the baby and multiply by a scaling factor that’s based on different growth rates for different parts of the body. Lower extremities grow the most, so multiply the size of nevi located there by 3.3. Multiply the size of a nevus on the torso or an upper extremity by 2.8, and a nevus on the head by 1.7.
For example, a congenital nevus 7 cm in diameter on a baby’s leg qualifies as a large congenital nevus (7 x 3.3 = 23.1 cm), "so you need to worry more" about risks for that baby, she said.
Estimates of risk have been evolving with the terminology. Most studies have lumped large and giant categories together and referred to them as large congenital nevi. The true risks in studies of registry data are clouded by the fact that many families choose to excise or partially excise large congenital nevi.
Between 1% and 11% of patients with large congenital nevi will develop malignant melanoma or neurocutaneous melanocytosis, a 52-1,000 times greater risk than for someone without the lesion, according to the results of a New York University study of 160 patients (Pediatrics 2000;106:736-41). Large congenital nevi also are associated with increased risks for liposarcoma, rhabdomyosarcoma, and (in some body locations) tethered spinal cord syndrome.
In the New York University study, 14 of 15 patients who developed melanoma or neurocutaneous melanocytosis had lesions greater than 50 cm in diameter, which now would be considered giant. The risk is highest for lesions on the torso. The melanoma often can occur deep in the dermal-epidermal junction, making them hard to find, Dr. Friedlander said. The melanoma also can occur underneath the lesion in the CNS or mesentery.
Although melanomas in the general population are unlikely to develop until adolescence or adulthood, in children with large congenital nevi who develop melanoma, 70% of the cancers appear by age 10 years, and 50% appear by age 5. "So this is something that you can’t put off. You really need to deal with it," she said.
Studies report that 7% of patients with large congenital nevi will develop neurocutaneous melanocytosis, an aberrant migration of melanocytes in leptomeninges that creates a risk for ventricular obstruction. The presence of more than 20 satellite lesions around a large congenital nevus increases the risk for neurocutaneous melanocytosis five-fold and warrants an MRI evaluation, she said.
"Five years ago I was taught that posterior axial location may have a higher risk for neurocutaneous melanocytosis, but recent data shows that it’s the satellite lesions rather than the location" that matters, Dr. Friedlander said.
A posterior axial location is, however, a risk factor for cutaneous melanoma in patients with large congenital nevi. Nevus size is a risk factor for both melanoma and neurocutaneous melanocytosis, said Dr. Friedlander; "20 cm is bad, and 40 cm is worse."
Dr. Friedlander disclosed that she has been a speaker, consultant, or researcher for Pierre Fabre, Onset Therapeutics, Johnson & Johnson, and Galderma.
SDEF and this news organization are owned by Elsevier.
SAN FRANCISCO – A new definition of giant congenital nevi should help physicians identify infants with supersized moles that are at risk for melanoma, neurocutaneous melanocytosis, liposarcoma, and other tumors.
For many years pediatric dermatologists sized congenital nevi as small, medium, or large, with "large" comprising nevi that are estimated to be 20 cm or greater in diameter when the patient has grown into adulthood. Then, physicians started referring to some of the large nevi as "giant" congenital nevi, with no universal agreement on what defines a giant nevus, Dr. Sheila Fallon Friedlander said at the Women’s & Pediatric Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
"Here’s our new definition that you’re going to be seeing in the literature," said Dr. Friedlander of the University of California, San Diego. Congenital nevi with diameters less than 1.5 cm after the patient is fully grown are considered small, nevi with diameters of at least 1.5 cm but less than 20 cm are medium size, and large nevi are at least 20 cm but less than 40 cm in diameter. Giant congenital nevi are those with diameters of 40 cm or greater when the patient is fully grown.
"When you are examining a baby in a nursery, how does that help you? That’s an adult-type figure," she noted. To estimate the grown size of a congenital nevus, measure the nevus on the baby and multiply by a scaling factor that’s based on different growth rates for different parts of the body. Lower extremities grow the most, so multiply the size of nevi located there by 3.3. Multiply the size of a nevus on the torso or an upper extremity by 2.8, and a nevus on the head by 1.7.
For example, a congenital nevus 7 cm in diameter on a baby’s leg qualifies as a large congenital nevus (7 x 3.3 = 23.1 cm), "so you need to worry more" about risks for that baby, she said.
Estimates of risk have been evolving with the terminology. Most studies have lumped large and giant categories together and referred to them as large congenital nevi. The true risks in studies of registry data are clouded by the fact that many families choose to excise or partially excise large congenital nevi.
Between 1% and 11% of patients with large congenital nevi will develop malignant melanoma or neurocutaneous melanocytosis, a 52-1,000 times greater risk than for someone without the lesion, according to the results of a New York University study of 160 patients (Pediatrics 2000;106:736-41). Large congenital nevi also are associated with increased risks for liposarcoma, rhabdomyosarcoma, and (in some body locations) tethered spinal cord syndrome.
In the New York University study, 14 of 15 patients who developed melanoma or neurocutaneous melanocytosis had lesions greater than 50 cm in diameter, which now would be considered giant. The risk is highest for lesions on the torso. The melanoma often can occur deep in the dermal-epidermal junction, making them hard to find, Dr. Friedlander said. The melanoma also can occur underneath the lesion in the CNS or mesentery.
Although melanomas in the general population are unlikely to develop until adolescence or adulthood, in children with large congenital nevi who develop melanoma, 70% of the cancers appear by age 10 years, and 50% appear by age 5. "So this is something that you can’t put off. You really need to deal with it," she said.
Studies report that 7% of patients with large congenital nevi will develop neurocutaneous melanocytosis, an aberrant migration of melanocytes in leptomeninges that creates a risk for ventricular obstruction. The presence of more than 20 satellite lesions around a large congenital nevus increases the risk for neurocutaneous melanocytosis five-fold and warrants an MRI evaluation, she said.
"Five years ago I was taught that posterior axial location may have a higher risk for neurocutaneous melanocytosis, but recent data shows that it’s the satellite lesions rather than the location" that matters, Dr. Friedlander said.
A posterior axial location is, however, a risk factor for cutaneous melanoma in patients with large congenital nevi. Nevus size is a risk factor for both melanoma and neurocutaneous melanocytosis, said Dr. Friedlander; "20 cm is bad, and 40 cm is worse."
Dr. Friedlander disclosed that she has been a speaker, consultant, or researcher for Pierre Fabre, Onset Therapeutics, Johnson & Johnson, and Galderma.
SDEF and this news organization are owned by Elsevier.
SAN FRANCISCO – A new definition of giant congenital nevi should help physicians identify infants with supersized moles that are at risk for melanoma, neurocutaneous melanocytosis, liposarcoma, and other tumors.
For many years pediatric dermatologists sized congenital nevi as small, medium, or large, with "large" comprising nevi that are estimated to be 20 cm or greater in diameter when the patient has grown into adulthood. Then, physicians started referring to some of the large nevi as "giant" congenital nevi, with no universal agreement on what defines a giant nevus, Dr. Sheila Fallon Friedlander said at the Women’s & Pediatric Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
"Here’s our new definition that you’re going to be seeing in the literature," said Dr. Friedlander of the University of California, San Diego. Congenital nevi with diameters less than 1.5 cm after the patient is fully grown are considered small, nevi with diameters of at least 1.5 cm but less than 20 cm are medium size, and large nevi are at least 20 cm but less than 40 cm in diameter. Giant congenital nevi are those with diameters of 40 cm or greater when the patient is fully grown.
"When you are examining a baby in a nursery, how does that help you? That’s an adult-type figure," she noted. To estimate the grown size of a congenital nevus, measure the nevus on the baby and multiply by a scaling factor that’s based on different growth rates for different parts of the body. Lower extremities grow the most, so multiply the size of nevi located there by 3.3. Multiply the size of a nevus on the torso or an upper extremity by 2.8, and a nevus on the head by 1.7.
For example, a congenital nevus 7 cm in diameter on a baby’s leg qualifies as a large congenital nevus (7 x 3.3 = 23.1 cm), "so you need to worry more" about risks for that baby, she said.
Estimates of risk have been evolving with the terminology. Most studies have lumped large and giant categories together and referred to them as large congenital nevi. The true risks in studies of registry data are clouded by the fact that many families choose to excise or partially excise large congenital nevi.
Between 1% and 11% of patients with large congenital nevi will develop malignant melanoma or neurocutaneous melanocytosis, a 52-1,000 times greater risk than for someone without the lesion, according to the results of a New York University study of 160 patients (Pediatrics 2000;106:736-41). Large congenital nevi also are associated with increased risks for liposarcoma, rhabdomyosarcoma, and (in some body locations) tethered spinal cord syndrome.
In the New York University study, 14 of 15 patients who developed melanoma or neurocutaneous melanocytosis had lesions greater than 50 cm in diameter, which now would be considered giant. The risk is highest for lesions on the torso. The melanoma often can occur deep in the dermal-epidermal junction, making them hard to find, Dr. Friedlander said. The melanoma also can occur underneath the lesion in the CNS or mesentery.
Although melanomas in the general population are unlikely to develop until adolescence or adulthood, in children with large congenital nevi who develop melanoma, 70% of the cancers appear by age 10 years, and 50% appear by age 5. "So this is something that you can’t put off. You really need to deal with it," she said.
Studies report that 7% of patients with large congenital nevi will develop neurocutaneous melanocytosis, an aberrant migration of melanocytes in leptomeninges that creates a risk for ventricular obstruction. The presence of more than 20 satellite lesions around a large congenital nevus increases the risk for neurocutaneous melanocytosis five-fold and warrants an MRI evaluation, she said.
"Five years ago I was taught that posterior axial location may have a higher risk for neurocutaneous melanocytosis, but recent data shows that it’s the satellite lesions rather than the location" that matters, Dr. Friedlander said.
A posterior axial location is, however, a risk factor for cutaneous melanoma in patients with large congenital nevi. Nevus size is a risk factor for both melanoma and neurocutaneous melanocytosis, said Dr. Friedlander; "20 cm is bad, and 40 cm is worse."
Dr. Friedlander disclosed that she has been a speaker, consultant, or researcher for Pierre Fabre, Onset Therapeutics, Johnson & Johnson, and Galderma.
SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF WOMEN'S & PEDIATRIC DERMATOLOGY SEMINAR
Definition of Giant Congenital Nevus Refined
SAN FRANCISCO – A new definition of giant congenital nevi should help physicians identify infants with supersized moles that are at risk for melanoma, neurocutaneous melanocytosis, liposarcoma, and other tumors.
For many years pediatric dermatologists sized congenital nevi as small, medium, or large, with "large" comprising nevi that are estimated to be 20 cm or greater in diameter when the patient has grown into adulthood. Then, physicians started referring to some of the large nevi as "giant" congenital nevi, with no universal agreement on what defines a giant nevus, Dr. Sheila Fallon Friedlander said at the Women’s & Pediatric Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
"Here’s our new definition that you’re going to be seeing in the literature," said Dr. Friedlander of the University of California, San Diego. Congenital nevi with diameters less than 1.5 cm after the patient is fully grown are considered small, nevi with diameters of at least 1.5 cm but less than 20 cm are medium size, and large nevi are at least 20 cm but less than 40 cm in diameter. Giant congenital nevi are those with diameters of 40 cm or greater when the patient is fully grown.
"When you are examining a baby in a nursery, how does that help you? That’s an adult-type figure," she noted. To estimate the grown size of a congenital nevus, measure the nevus on the baby and multiply by a scaling factor that’s based on different growth rates for different parts of the body. Lower extremities grow the most, so multiply the size of nevi located there by 3.3. Multiply the size of a nevus on the torso or an upper extremity by 2.8, and a nevus on the head by 1.7.
For example, a congenital nevus 7 cm in diameter on a baby’s leg qualifies as a large congenital nevus (7 x 3.3 = 23.1 cm), "so you need to worry more" about risks for that baby, she said.
Estimates of risk have been evolving with the terminology. Most studies have lumped large and giant categories together and referred to them as large congenital nevi. The true risks in studies of registry data are clouded by the fact that many families choose to excise or partially excise large congenital nevi.
Between 1% and 11% of patients with large congenital nevi will develop malignant melanoma or neurocutaneous melanocytosis, a 52-1,000 times greater risk than for someone without the lesion, according to the results of a New York University study of 160 patients (Pediatrics 2000;106:736-41). Large congenital nevi also are associated with increased risks for liposarcoma, rhabdomyosarcoma, and (in some body locations) tethered spinal cord syndrome.
In the New York University study, 14 of 15 patients who developed melanoma or neurocutaneous melanocytosis had lesions greater than 50 cm in diameter, which now would be considered giant. The risk is highest for lesions on the torso. The melanoma often can occur deep in the dermal-epidermal junction, making them hard to find, Dr. Friedlander said. The melanoma also can occur underneath the lesion in the CNS or mesentery.
Although melanomas in the general population are unlikely to develop until adolescence or adulthood, in children with large congenital nevi who develop melanoma, 70% of the cancers appear by age 10 years, and 50% appear by age 5. "So this is something that you can’t put off. You really need to deal with it," she said.
Studies report that 7% of patients with large congenital nevi will develop neurocutaneous melanocytosis, an aberrant migration of melanocytes in leptomeninges that creates a risk for ventricular obstruction. The presence of more than 20 satellite lesions around a large congenital nevus increases the risk for neurocutaneous melanocytosis five-fold and warrants an MRI evaluation, she said.
"Five years ago I was taught that posterior axial location may have a higher risk for neurocutaneous melanocytosis, but recent data shows that it’s the satellite lesions rather than the location" that matters, Dr. Friedlander said.
A posterior axial location is, however, a risk factor for cutaneous melanoma in patients with large congenital nevi. Nevus size is a risk factor for both melanoma and neurocutaneous melanocytosis, said Dr. Friedlander; "20 cm is bad, and 40 cm is worse."
Dr. Friedlander disclosed that she has been a speaker, consultant, or researcher for Pierre Fabre, Onset Therapeutics, Johnson & Johnson, and Galderma.
SDEF and this news organization are owned by Elsevier.
SAN FRANCISCO – A new definition of giant congenital nevi should help physicians identify infants with supersized moles that are at risk for melanoma, neurocutaneous melanocytosis, liposarcoma, and other tumors.
For many years pediatric dermatologists sized congenital nevi as small, medium, or large, with "large" comprising nevi that are estimated to be 20 cm or greater in diameter when the patient has grown into adulthood. Then, physicians started referring to some of the large nevi as "giant" congenital nevi, with no universal agreement on what defines a giant nevus, Dr. Sheila Fallon Friedlander said at the Women’s & Pediatric Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
"Here’s our new definition that you’re going to be seeing in the literature," said Dr. Friedlander of the University of California, San Diego. Congenital nevi with diameters less than 1.5 cm after the patient is fully grown are considered small, nevi with diameters of at least 1.5 cm but less than 20 cm are medium size, and large nevi are at least 20 cm but less than 40 cm in diameter. Giant congenital nevi are those with diameters of 40 cm or greater when the patient is fully grown.
"When you are examining a baby in a nursery, how does that help you? That’s an adult-type figure," she noted. To estimate the grown size of a congenital nevus, measure the nevus on the baby and multiply by a scaling factor that’s based on different growth rates for different parts of the body. Lower extremities grow the most, so multiply the size of nevi located there by 3.3. Multiply the size of a nevus on the torso or an upper extremity by 2.8, and a nevus on the head by 1.7.
For example, a congenital nevus 7 cm in diameter on a baby’s leg qualifies as a large congenital nevus (7 x 3.3 = 23.1 cm), "so you need to worry more" about risks for that baby, she said.
Estimates of risk have been evolving with the terminology. Most studies have lumped large and giant categories together and referred to them as large congenital nevi. The true risks in studies of registry data are clouded by the fact that many families choose to excise or partially excise large congenital nevi.
Between 1% and 11% of patients with large congenital nevi will develop malignant melanoma or neurocutaneous melanocytosis, a 52-1,000 times greater risk than for someone without the lesion, according to the results of a New York University study of 160 patients (Pediatrics 2000;106:736-41). Large congenital nevi also are associated with increased risks for liposarcoma, rhabdomyosarcoma, and (in some body locations) tethered spinal cord syndrome.
In the New York University study, 14 of 15 patients who developed melanoma or neurocutaneous melanocytosis had lesions greater than 50 cm in diameter, which now would be considered giant. The risk is highest for lesions on the torso. The melanoma often can occur deep in the dermal-epidermal junction, making them hard to find, Dr. Friedlander said. The melanoma also can occur underneath the lesion in the CNS or mesentery.
Although melanomas in the general population are unlikely to develop until adolescence or adulthood, in children with large congenital nevi who develop melanoma, 70% of the cancers appear by age 10 years, and 50% appear by age 5. "So this is something that you can’t put off. You really need to deal with it," she said.
Studies report that 7% of patients with large congenital nevi will develop neurocutaneous melanocytosis, an aberrant migration of melanocytes in leptomeninges that creates a risk for ventricular obstruction. The presence of more than 20 satellite lesions around a large congenital nevus increases the risk for neurocutaneous melanocytosis five-fold and warrants an MRI evaluation, she said.
"Five years ago I was taught that posterior axial location may have a higher risk for neurocutaneous melanocytosis, but recent data shows that it’s the satellite lesions rather than the location" that matters, Dr. Friedlander said.
A posterior axial location is, however, a risk factor for cutaneous melanoma in patients with large congenital nevi. Nevus size is a risk factor for both melanoma and neurocutaneous melanocytosis, said Dr. Friedlander; "20 cm is bad, and 40 cm is worse."
Dr. Friedlander disclosed that she has been a speaker, consultant, or researcher for Pierre Fabre, Onset Therapeutics, Johnson & Johnson, and Galderma.
SDEF and this news organization are owned by Elsevier.
SAN FRANCISCO – A new definition of giant congenital nevi should help physicians identify infants with supersized moles that are at risk for melanoma, neurocutaneous melanocytosis, liposarcoma, and other tumors.
For many years pediatric dermatologists sized congenital nevi as small, medium, or large, with "large" comprising nevi that are estimated to be 20 cm or greater in diameter when the patient has grown into adulthood. Then, physicians started referring to some of the large nevi as "giant" congenital nevi, with no universal agreement on what defines a giant nevus, Dr. Sheila Fallon Friedlander said at the Women’s & Pediatric Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
"Here’s our new definition that you’re going to be seeing in the literature," said Dr. Friedlander of the University of California, San Diego. Congenital nevi with diameters less than 1.5 cm after the patient is fully grown are considered small, nevi with diameters of at least 1.5 cm but less than 20 cm are medium size, and large nevi are at least 20 cm but less than 40 cm in diameter. Giant congenital nevi are those with diameters of 40 cm or greater when the patient is fully grown.
"When you are examining a baby in a nursery, how does that help you? That’s an adult-type figure," she noted. To estimate the grown size of a congenital nevus, measure the nevus on the baby and multiply by a scaling factor that’s based on different growth rates for different parts of the body. Lower extremities grow the most, so multiply the size of nevi located there by 3.3. Multiply the size of a nevus on the torso or an upper extremity by 2.8, and a nevus on the head by 1.7.
For example, a congenital nevus 7 cm in diameter on a baby’s leg qualifies as a large congenital nevus (7 x 3.3 = 23.1 cm), "so you need to worry more" about risks for that baby, she said.
Estimates of risk have been evolving with the terminology. Most studies have lumped large and giant categories together and referred to them as large congenital nevi. The true risks in studies of registry data are clouded by the fact that many families choose to excise or partially excise large congenital nevi.
Between 1% and 11% of patients with large congenital nevi will develop malignant melanoma or neurocutaneous melanocytosis, a 52-1,000 times greater risk than for someone without the lesion, according to the results of a New York University study of 160 patients (Pediatrics 2000;106:736-41). Large congenital nevi also are associated with increased risks for liposarcoma, rhabdomyosarcoma, and (in some body locations) tethered spinal cord syndrome.
In the New York University study, 14 of 15 patients who developed melanoma or neurocutaneous melanocytosis had lesions greater than 50 cm in diameter, which now would be considered giant. The risk is highest for lesions on the torso. The melanoma often can occur deep in the dermal-epidermal junction, making them hard to find, Dr. Friedlander said. The melanoma also can occur underneath the lesion in the CNS or mesentery.
Although melanomas in the general population are unlikely to develop until adolescence or adulthood, in children with large congenital nevi who develop melanoma, 70% of the cancers appear by age 10 years, and 50% appear by age 5. "So this is something that you can’t put off. You really need to deal with it," she said.
Studies report that 7% of patients with large congenital nevi will develop neurocutaneous melanocytosis, an aberrant migration of melanocytes in leptomeninges that creates a risk for ventricular obstruction. The presence of more than 20 satellite lesions around a large congenital nevus increases the risk for neurocutaneous melanocytosis five-fold and warrants an MRI evaluation, she said.
"Five years ago I was taught that posterior axial location may have a higher risk for neurocutaneous melanocytosis, but recent data shows that it’s the satellite lesions rather than the location" that matters, Dr. Friedlander said.
A posterior axial location is, however, a risk factor for cutaneous melanoma in patients with large congenital nevi. Nevus size is a risk factor for both melanoma and neurocutaneous melanocytosis, said Dr. Friedlander; "20 cm is bad, and 40 cm is worse."
Dr. Friedlander disclosed that she has been a speaker, consultant, or researcher for Pierre Fabre, Onset Therapeutics, Johnson & Johnson, and Galderma.
SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF WOMEN'S & PEDIATRIC DERMATOLOGY SEMINAR
Guided TMS Not Effective for Hallucinations
PARIS – Transcranial magnetic stimulation guided by functional MRI reduced auditory hallucinations in patients with schizophrenia, but so did a sham treatment in a randomized, controlled study of 62 patients.
The findings were "an unpleasant surprise," Dr. Iris E.C. Sommer reported at the annual congress of the European College of Neuropsychopharmacology.
Even when the fMRI-guided group and the standard TMS group were pooled, results were not significantly better than with placebo
The severity of auditory hallucinations decreased by about 40% after 15 sessions of 20-minute treatments in each of three groups. One group received 1 Hz of transcranial magnetic stimulation (TMS) at 90% of the individual motor threshold, guided by functional MRI (fMRI) to the area of brain activity during hallucinations. A second group underwent nonguided fMRI aimed at the left temporoparietal cortex, and the third group had a placebo TMS coil placed perpendicular to the head.
The results contradict a meta-analysis of previous studies of TMS for hallucinations that reported a mean halving of hallucinations using 1 Hz TMS treatments (J. Clin. Psychiatry 2010;71:873-84). The earlier studies in the meta-analysis might have had a positive bias toward new technology, suggested Dr. Sommer of the department of psychiatry at University Medical Center Utrecht (the Netherlands).
The current study’s results are supported by three other studies that were not included in the meta-analysis, including one Canadian study with more than 100 patients, she added.
Dr. Sommer and her associates had hoped that guiding TMS via fMRI would make the treatment more effective. First, they used fMRI to localize brain activity during hallucinations and used the results to calculate hallucination "hot spots" in the brain. They repeated these steps in 33 patients because "fMRI is notorious for having low test-retest reliability," she said. The mean distance between hot spots in the first and second scans was less than 2 cm for all brain regions, averaging 1.4 cm, "which is large but still doable for TMS," she said.
The results suggest that fMRI guidance for hallucinations in single patients is feasible for TMS and possibly other symptoms with on/off states, such as obsessions, compulsions, tics, dystonia, or tardive dyskinesia.
However, the 1-HzTMS stimulation protocol was not effective enough for hallucinations, Dr. Sommer said. Even when the fMRI-guided group and the standard TMS group were pooled, results were not significantly better than with placebo (Biol. Psychiatry 2011;69:450-6).
It’s possible that results might differ with more effective TMS coils or other TMS stimulation paradigms such as burst frequencies.
Auditory hallucinations affect 70%-90% of patients with schizophrenia, and about 8% will have treatment-resistant auditory hallucinations. This represents 0.08% of the general population and can greatly affect quality of life and patients’ risks if they are hearing commands like, "You must burn down the library," she said.
Dr. Sommer said she has no relevant conflicts of interest.
PARIS – Transcranial magnetic stimulation guided by functional MRI reduced auditory hallucinations in patients with schizophrenia, but so did a sham treatment in a randomized, controlled study of 62 patients.
The findings were "an unpleasant surprise," Dr. Iris E.C. Sommer reported at the annual congress of the European College of Neuropsychopharmacology.
Even when the fMRI-guided group and the standard TMS group were pooled, results were not significantly better than with placebo
The severity of auditory hallucinations decreased by about 40% after 15 sessions of 20-minute treatments in each of three groups. One group received 1 Hz of transcranial magnetic stimulation (TMS) at 90% of the individual motor threshold, guided by functional MRI (fMRI) to the area of brain activity during hallucinations. A second group underwent nonguided fMRI aimed at the left temporoparietal cortex, and the third group had a placebo TMS coil placed perpendicular to the head.
The results contradict a meta-analysis of previous studies of TMS for hallucinations that reported a mean halving of hallucinations using 1 Hz TMS treatments (J. Clin. Psychiatry 2010;71:873-84). The earlier studies in the meta-analysis might have had a positive bias toward new technology, suggested Dr. Sommer of the department of psychiatry at University Medical Center Utrecht (the Netherlands).
The current study’s results are supported by three other studies that were not included in the meta-analysis, including one Canadian study with more than 100 patients, she added.
Dr. Sommer and her associates had hoped that guiding TMS via fMRI would make the treatment more effective. First, they used fMRI to localize brain activity during hallucinations and used the results to calculate hallucination "hot spots" in the brain. They repeated these steps in 33 patients because "fMRI is notorious for having low test-retest reliability," she said. The mean distance between hot spots in the first and second scans was less than 2 cm for all brain regions, averaging 1.4 cm, "which is large but still doable for TMS," she said.
The results suggest that fMRI guidance for hallucinations in single patients is feasible for TMS and possibly other symptoms with on/off states, such as obsessions, compulsions, tics, dystonia, or tardive dyskinesia.
However, the 1-HzTMS stimulation protocol was not effective enough for hallucinations, Dr. Sommer said. Even when the fMRI-guided group and the standard TMS group were pooled, results were not significantly better than with placebo (Biol. Psychiatry 2011;69:450-6).
It’s possible that results might differ with more effective TMS coils or other TMS stimulation paradigms such as burst frequencies.
Auditory hallucinations affect 70%-90% of patients with schizophrenia, and about 8% will have treatment-resistant auditory hallucinations. This represents 0.08% of the general population and can greatly affect quality of life and patients’ risks if they are hearing commands like, "You must burn down the library," she said.
Dr. Sommer said she has no relevant conflicts of interest.
PARIS – Transcranial magnetic stimulation guided by functional MRI reduced auditory hallucinations in patients with schizophrenia, but so did a sham treatment in a randomized, controlled study of 62 patients.
The findings were "an unpleasant surprise," Dr. Iris E.C. Sommer reported at the annual congress of the European College of Neuropsychopharmacology.
Even when the fMRI-guided group and the standard TMS group were pooled, results were not significantly better than with placebo
The severity of auditory hallucinations decreased by about 40% after 15 sessions of 20-minute treatments in each of three groups. One group received 1 Hz of transcranial magnetic stimulation (TMS) at 90% of the individual motor threshold, guided by functional MRI (fMRI) to the area of brain activity during hallucinations. A second group underwent nonguided fMRI aimed at the left temporoparietal cortex, and the third group had a placebo TMS coil placed perpendicular to the head.
The results contradict a meta-analysis of previous studies of TMS for hallucinations that reported a mean halving of hallucinations using 1 Hz TMS treatments (J. Clin. Psychiatry 2010;71:873-84). The earlier studies in the meta-analysis might have had a positive bias toward new technology, suggested Dr. Sommer of the department of psychiatry at University Medical Center Utrecht (the Netherlands).
The current study’s results are supported by three other studies that were not included in the meta-analysis, including one Canadian study with more than 100 patients, she added.
Dr. Sommer and her associates had hoped that guiding TMS via fMRI would make the treatment more effective. First, they used fMRI to localize brain activity during hallucinations and used the results to calculate hallucination "hot spots" in the brain. They repeated these steps in 33 patients because "fMRI is notorious for having low test-retest reliability," she said. The mean distance between hot spots in the first and second scans was less than 2 cm for all brain regions, averaging 1.4 cm, "which is large but still doable for TMS," she said.
The results suggest that fMRI guidance for hallucinations in single patients is feasible for TMS and possibly other symptoms with on/off states, such as obsessions, compulsions, tics, dystonia, or tardive dyskinesia.
However, the 1-HzTMS stimulation protocol was not effective enough for hallucinations, Dr. Sommer said. Even when the fMRI-guided group and the standard TMS group were pooled, results were not significantly better than with placebo (Biol. Psychiatry 2011;69:450-6).
It’s possible that results might differ with more effective TMS coils or other TMS stimulation paradigms such as burst frequencies.
Auditory hallucinations affect 70%-90% of patients with schizophrenia, and about 8% will have treatment-resistant auditory hallucinations. This represents 0.08% of the general population and can greatly affect quality of life and patients’ risks if they are hearing commands like, "You must burn down the library," she said.
Dr. Sommer said she has no relevant conflicts of interest.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN COLLEGE OF NEUROPSYCHO- PHARMACOLOGY
Major Finding: Transcranial magnetic stimulation guided by fMRI was no more effective than conventional TMS or sham TMS in reducing auditory hallucinations.
Data Source: Randomized, controlled trial in 62 patients with schizophrenia and auditory hallucinations.
Disclosures: Dr. Sommer said she has no relevant conflicts of interest.
Treating Bipolar Depression Depends on Evidence, Judgment
PARIS – Existing evidence on the best ways to treat bipolar depression is limited, and must balance against what works in the real world based on empirical experience. When treating bipolar depression "we are in the land of the uncertain, where [psychiatrists] must improvise and do their best with what’s available," Dr. Andrew A. Nierenberg said at the annual congress of the European College of Neuropsychopharmacology.
The trials that get drugs approved by the Food and Drug Administration often do not inform routine care. Patients in trials often differ from "real" patients, generally have milder disease, and are less susceptible to the worst outcomes. The published evidence base for treating bipolar depression should not be viewed as synonymous with best practice. Treatment is not a cookbook, and evidence-based medicine is very difficult to actually implement, said Dr. Nierenberg, medical director of the bipolar clinic and research program at Massachusetts General Hospital, and professor of psychiatry at Harvard Medical School, both in Boston.
Treatment of patients with bipolar disorder in actual practice requires personalization, including prescribing agents that work and expecting patients to continue to take them despite possible adverse effects. Published evidence leaves it unclear how best to treat any specific patient, he said. That requires not only evidence but also clinical experience and knowledge of the results obtained by others, followed by feedback and outcomes assessment to guide future changes in treatment.
Perhaps the biggest question in the treatment of bipolar depression today is whether antidepressants work. A 2004 meta-analysis said they did (Am. J. Psychiatry 2004;161:1537-47), but this was "a terrible" meta-analysis because the overall numbers of patients were small, and the results from a single study heavily influenced the overall meta-analysis results, Dr. Nierenberg said. In addition, results from the STEP-BD (Systematic Treatment Enhancement Program for Bipolar Disorder) trial showed that adding an antidepressant to the mood stabilizer lithium gave no significant added benefit (N. Engl. J. Med. 2007;356:1711-22).
Taken together, the literature provides insufficient evidence to document a beneficial role for antidepressants in the treatment of bipolar depression, along with some evidence against any efficacy, yet Dr. Nierenberg said he prescribes an antidepressant as monotherapy or as an add-on treatment to many patients, as do many other psychiatrists. Psychiatrists generally feel that the data against adding an antidepressant are unconvincing, because they have seen antidepressants work on many prior patients. "The data are inconsistent with their clinical experience," he noted.
The roles of several other drugs or drug combinations also feature conflicts between the evidence base and their use in everyday practice.
The combination of olanzapine and fluoxetine showed efficacy in a highly influential 2003 study (Arch. Gen. Psychiatry 2003;60:1079-88). But the olanzapine and fluoxetine pairing is seldom used today because of concerns that it causes weight gain and tends to trigger metabolic syndrome. It’s ironic that olanzapine and fluoxetine is an FDA-approved combination that few patients actually receive, he said. Patients don’t like to gain weight, and when they do, they often stop taking the drugs.
Lamotrigine showed efficacy for treating bipolar depression in a 1999 study (J. Clin. Psychiatry 1999;60:79-88), but failed to show substantial efficacy in five other studies. In addition, lamotrigine failed to receive FDA approval for treating acute depression. Despite that, U.S. physicians appear to have embraced lamotrigine for treating depression in patients with bipolar disorder, "a discrepancy between what the literature says and what people actually do," Dr. Nierenberg noted. Results from a 2009 study showed that adding lamotrigine to lithium led to an improved decrease in depression, compared with lithium plus placebo (J. Clin. Psychiatry 2009;70:223-31), but physicians also prescribe lamotrigine monotherapy, even though published findings do not support that approach.
Perhaps the drug with the best clinical performance on paper is quetiapine, which has produced the largest effect size seen in depression (Am. J. Psychiatry 2005;162:1351-60), both monopolar and bipolar, and has had efficacy for mania, anxiety, insomnia, and psychosis. Based on this evidence, Dr. Nierenberg said that he would expect the extensive prescribing of quetiapine, but in reality, few patients receive it. Again, it’s a drug with significant side effects, including sedation and weight gain.
Other approaches to treating bipolar depression that are often used as first-line therapy, despite a poor evidence base, include optimization of a regimen of lithium, valproate, or carbamazepine that the patient might already take. Psychotherapy also can help, but less so once a patient has had 10-20 depressive or manic episodes. And electroconvulsive therapy is an important option for treatment-refractory bipolar depression. But the right treatment in many other clinical situations might involve unclear options, such as how to handle patients who are already on antimania treatment, and how to continue an antidepressant that produced partial improvement. Common complications of bipolar depression that can influence treatment decisions include attention-deficit/hyperactivity disorder, substance abuse, a chaotic life, and poor drug compliance. These and other factors make it impossible to rely just on evidence when choosing a patient’s treatment, Dr. Nierenberg said.
Dr. Nierenberg said he has a patent pending on combined treatment of mood disorders with buspirone, bupropion, and melatonin, and he co-owns a copyright on a structured clinical interview for the Montgomery-Åsberg Depression Rating Scale. He said he has received honoraria from Eli Lilly, AstraZeneca, Forest, and Janssen, and that he has received research grants from Pam Labs, Pfizer, and Shire.
PARIS – Existing evidence on the best ways to treat bipolar depression is limited, and must balance against what works in the real world based on empirical experience. When treating bipolar depression "we are in the land of the uncertain, where [psychiatrists] must improvise and do their best with what’s available," Dr. Andrew A. Nierenberg said at the annual congress of the European College of Neuropsychopharmacology.
The trials that get drugs approved by the Food and Drug Administration often do not inform routine care. Patients in trials often differ from "real" patients, generally have milder disease, and are less susceptible to the worst outcomes. The published evidence base for treating bipolar depression should not be viewed as synonymous with best practice. Treatment is not a cookbook, and evidence-based medicine is very difficult to actually implement, said Dr. Nierenberg, medical director of the bipolar clinic and research program at Massachusetts General Hospital, and professor of psychiatry at Harvard Medical School, both in Boston.
Treatment of patients with bipolar disorder in actual practice requires personalization, including prescribing agents that work and expecting patients to continue to take them despite possible adverse effects. Published evidence leaves it unclear how best to treat any specific patient, he said. That requires not only evidence but also clinical experience and knowledge of the results obtained by others, followed by feedback and outcomes assessment to guide future changes in treatment.
Perhaps the biggest question in the treatment of bipolar depression today is whether antidepressants work. A 2004 meta-analysis said they did (Am. J. Psychiatry 2004;161:1537-47), but this was "a terrible" meta-analysis because the overall numbers of patients were small, and the results from a single study heavily influenced the overall meta-analysis results, Dr. Nierenberg said. In addition, results from the STEP-BD (Systematic Treatment Enhancement Program for Bipolar Disorder) trial showed that adding an antidepressant to the mood stabilizer lithium gave no significant added benefit (N. Engl. J. Med. 2007;356:1711-22).
Taken together, the literature provides insufficient evidence to document a beneficial role for antidepressants in the treatment of bipolar depression, along with some evidence against any efficacy, yet Dr. Nierenberg said he prescribes an antidepressant as monotherapy or as an add-on treatment to many patients, as do many other psychiatrists. Psychiatrists generally feel that the data against adding an antidepressant are unconvincing, because they have seen antidepressants work on many prior patients. "The data are inconsistent with their clinical experience," he noted.
The roles of several other drugs or drug combinations also feature conflicts between the evidence base and their use in everyday practice.
The combination of olanzapine and fluoxetine showed efficacy in a highly influential 2003 study (Arch. Gen. Psychiatry 2003;60:1079-88). But the olanzapine and fluoxetine pairing is seldom used today because of concerns that it causes weight gain and tends to trigger metabolic syndrome. It’s ironic that olanzapine and fluoxetine is an FDA-approved combination that few patients actually receive, he said. Patients don’t like to gain weight, and when they do, they often stop taking the drugs.
Lamotrigine showed efficacy for treating bipolar depression in a 1999 study (J. Clin. Psychiatry 1999;60:79-88), but failed to show substantial efficacy in five other studies. In addition, lamotrigine failed to receive FDA approval for treating acute depression. Despite that, U.S. physicians appear to have embraced lamotrigine for treating depression in patients with bipolar disorder, "a discrepancy between what the literature says and what people actually do," Dr. Nierenberg noted. Results from a 2009 study showed that adding lamotrigine to lithium led to an improved decrease in depression, compared with lithium plus placebo (J. Clin. Psychiatry 2009;70:223-31), but physicians also prescribe lamotrigine monotherapy, even though published findings do not support that approach.
Perhaps the drug with the best clinical performance on paper is quetiapine, which has produced the largest effect size seen in depression (Am. J. Psychiatry 2005;162:1351-60), both monopolar and bipolar, and has had efficacy for mania, anxiety, insomnia, and psychosis. Based on this evidence, Dr. Nierenberg said that he would expect the extensive prescribing of quetiapine, but in reality, few patients receive it. Again, it’s a drug with significant side effects, including sedation and weight gain.
Other approaches to treating bipolar depression that are often used as first-line therapy, despite a poor evidence base, include optimization of a regimen of lithium, valproate, or carbamazepine that the patient might already take. Psychotherapy also can help, but less so once a patient has had 10-20 depressive or manic episodes. And electroconvulsive therapy is an important option for treatment-refractory bipolar depression. But the right treatment in many other clinical situations might involve unclear options, such as how to handle patients who are already on antimania treatment, and how to continue an antidepressant that produced partial improvement. Common complications of bipolar depression that can influence treatment decisions include attention-deficit/hyperactivity disorder, substance abuse, a chaotic life, and poor drug compliance. These and other factors make it impossible to rely just on evidence when choosing a patient’s treatment, Dr. Nierenberg said.
Dr. Nierenberg said he has a patent pending on combined treatment of mood disorders with buspirone, bupropion, and melatonin, and he co-owns a copyright on a structured clinical interview for the Montgomery-Åsberg Depression Rating Scale. He said he has received honoraria from Eli Lilly, AstraZeneca, Forest, and Janssen, and that he has received research grants from Pam Labs, Pfizer, and Shire.
PARIS – Existing evidence on the best ways to treat bipolar depression is limited, and must balance against what works in the real world based on empirical experience. When treating bipolar depression "we are in the land of the uncertain, where [psychiatrists] must improvise and do their best with what’s available," Dr. Andrew A. Nierenberg said at the annual congress of the European College of Neuropsychopharmacology.
The trials that get drugs approved by the Food and Drug Administration often do not inform routine care. Patients in trials often differ from "real" patients, generally have milder disease, and are less susceptible to the worst outcomes. The published evidence base for treating bipolar depression should not be viewed as synonymous with best practice. Treatment is not a cookbook, and evidence-based medicine is very difficult to actually implement, said Dr. Nierenberg, medical director of the bipolar clinic and research program at Massachusetts General Hospital, and professor of psychiatry at Harvard Medical School, both in Boston.
Treatment of patients with bipolar disorder in actual practice requires personalization, including prescribing agents that work and expecting patients to continue to take them despite possible adverse effects. Published evidence leaves it unclear how best to treat any specific patient, he said. That requires not only evidence but also clinical experience and knowledge of the results obtained by others, followed by feedback and outcomes assessment to guide future changes in treatment.
Perhaps the biggest question in the treatment of bipolar depression today is whether antidepressants work. A 2004 meta-analysis said they did (Am. J. Psychiatry 2004;161:1537-47), but this was "a terrible" meta-analysis because the overall numbers of patients were small, and the results from a single study heavily influenced the overall meta-analysis results, Dr. Nierenberg said. In addition, results from the STEP-BD (Systematic Treatment Enhancement Program for Bipolar Disorder) trial showed that adding an antidepressant to the mood stabilizer lithium gave no significant added benefit (N. Engl. J. Med. 2007;356:1711-22).
Taken together, the literature provides insufficient evidence to document a beneficial role for antidepressants in the treatment of bipolar depression, along with some evidence against any efficacy, yet Dr. Nierenberg said he prescribes an antidepressant as monotherapy or as an add-on treatment to many patients, as do many other psychiatrists. Psychiatrists generally feel that the data against adding an antidepressant are unconvincing, because they have seen antidepressants work on many prior patients. "The data are inconsistent with their clinical experience," he noted.
The roles of several other drugs or drug combinations also feature conflicts between the evidence base and their use in everyday practice.
The combination of olanzapine and fluoxetine showed efficacy in a highly influential 2003 study (Arch. Gen. Psychiatry 2003;60:1079-88). But the olanzapine and fluoxetine pairing is seldom used today because of concerns that it causes weight gain and tends to trigger metabolic syndrome. It’s ironic that olanzapine and fluoxetine is an FDA-approved combination that few patients actually receive, he said. Patients don’t like to gain weight, and when they do, they often stop taking the drugs.
Lamotrigine showed efficacy for treating bipolar depression in a 1999 study (J. Clin. Psychiatry 1999;60:79-88), but failed to show substantial efficacy in five other studies. In addition, lamotrigine failed to receive FDA approval for treating acute depression. Despite that, U.S. physicians appear to have embraced lamotrigine for treating depression in patients with bipolar disorder, "a discrepancy between what the literature says and what people actually do," Dr. Nierenberg noted. Results from a 2009 study showed that adding lamotrigine to lithium led to an improved decrease in depression, compared with lithium plus placebo (J. Clin. Psychiatry 2009;70:223-31), but physicians also prescribe lamotrigine monotherapy, even though published findings do not support that approach.
Perhaps the drug with the best clinical performance on paper is quetiapine, which has produced the largest effect size seen in depression (Am. J. Psychiatry 2005;162:1351-60), both monopolar and bipolar, and has had efficacy for mania, anxiety, insomnia, and psychosis. Based on this evidence, Dr. Nierenberg said that he would expect the extensive prescribing of quetiapine, but in reality, few patients receive it. Again, it’s a drug with significant side effects, including sedation and weight gain.
Other approaches to treating bipolar depression that are often used as first-line therapy, despite a poor evidence base, include optimization of a regimen of lithium, valproate, or carbamazepine that the patient might already take. Psychotherapy also can help, but less so once a patient has had 10-20 depressive or manic episodes. And electroconvulsive therapy is an important option for treatment-refractory bipolar depression. But the right treatment in many other clinical situations might involve unclear options, such as how to handle patients who are already on antimania treatment, and how to continue an antidepressant that produced partial improvement. Common complications of bipolar depression that can influence treatment decisions include attention-deficit/hyperactivity disorder, substance abuse, a chaotic life, and poor drug compliance. These and other factors make it impossible to rely just on evidence when choosing a patient’s treatment, Dr. Nierenberg said.
Dr. Nierenberg said he has a patent pending on combined treatment of mood disorders with buspirone, bupropion, and melatonin, and he co-owns a copyright on a structured clinical interview for the Montgomery-Åsberg Depression Rating Scale. He said he has received honoraria from Eli Lilly, AstraZeneca, Forest, and Janssen, and that he has received research grants from Pam Labs, Pfizer, and Shire.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN COLLEGE OF NEURO-
PSYCHOPHARMACOLOGY
Injectable Naltrexone Enhances Recovery From Opioid Dependence
PARIS – Patients recovering from opioid dependence who received monthly injections of 380 mg of extended-release naltrexone reported significant improvements in weeks of abstinence, cravings, and opioid-free days, compared with a placebo group. The findings were published in the Lancet (2011; 377:1506-13) and subsequently presented at the annual meeting of the European Congress of Neuropsychopharmacology.
Naltrexone, an opioid receptor antagonist, has been used to support recovery in opioid-addicted patients, but adherence to the oral medication tends to be poor, which limits effectiveness, said Dr. Evgeny M. Krupitsky of St. Petersburg State Pavlov Medical University, Russia, and his colleagues.
A once-monthly, extended-release naltrexone (XR-NTX) injection has been approved in Russia and in the United States to treat alcohol dependence. Dr. Krupitsky and his colleagues conducted a randomized, placebo-controlled trial to test the safety and effectiveness of the injectable drug in patients with opioid dependence.
The study population included 250 patients aged 18 years and older who were within 30 days or less of completing an inpatient opioid detoxification program. The majority of the patients were young, white men who had been suffering from heroin addiction for about 10 years. Patients were excluded if they were on parole or probation, were pregnant or breastfeeding, or had significant medical conditions such as renal failure, hepatic failure, or histories of AIDS-indicator disease.
Patients were randomized to 380 mg XR-NTX or placebo starting within 1 week after completing detoxification and continuing with one injection every 4 weeks, for a total of six injections over a 24-week period. Patients also were offered counseling sessions.
"XR-NTX offers a new treatment option without the risk of physical dependence or illegal diversion."
After 24 weeks, the median proportion of weeks of confirmed abstinence (the primary end point, based on urine tests) was significantly higher in the treatment group, compared with the placebo group (90% vs. 35%). This translated to 45 patients with confirmed abstinence in the treatment group, compared with 28 patients in the placebo group.
The median retention rate was more than 168 days in the treatment group, compared with 96 days in the placebo group. Relapses were confirmed in 17 placebo patients and 1 XR-NTX patient using a naloxone challenge.
In addition, the mean change in cravings, compared with baseline, was significantly greater in the XR-NTX group, compared with the placebo group (–10.1 vs. 0.7) and significantly more patients in the XR-NTX group reported opioid-free days, compared with the placebo group (99% vs. 60%).
Overall, 63 of the XR-NTX patients (50%) and 40 of the placebo patients (32%) experienced at least one adverse event, the most common of which were nasopharyngitis and insomnia.
Two patients in each group discontinued the study because of adverse events, but none of the XR-NTX patients died, overdosed, or discontinued treatment because of severe adverse events. Three patients in the XR-NTX group reported four serious adverse events, including infectious processes such as AIDS or HIV. Four patients in the placebo group reported five serious adverse events, including infections, psychotic disorder, and a peptic ulcer.
The study was limited by a lack of comparison with oral naltrexone, but other studies comparing oral naltrexone with placebo have failed to show an effect on craving reduction or relapse prevention, the researchers noted.
"The extent of patient interest in XR-NTX when opioid substitution treatments are available remains a topic for future health services research," the investigators said. However, "XR-NTX offers a new treatment option without the risk of physical dependence or illegal diversion," and the injectable treatment approach might improve acceptance of pharmacotherapy for opioid addiction, they said.
The study was funded by Alkermes. Dr. Krupitsky had no financial conflicts to disclose.
PARIS – Patients recovering from opioid dependence who received monthly injections of 380 mg of extended-release naltrexone reported significant improvements in weeks of abstinence, cravings, and opioid-free days, compared with a placebo group. The findings were published in the Lancet (2011; 377:1506-13) and subsequently presented at the annual meeting of the European Congress of Neuropsychopharmacology.
Naltrexone, an opioid receptor antagonist, has been used to support recovery in opioid-addicted patients, but adherence to the oral medication tends to be poor, which limits effectiveness, said Dr. Evgeny M. Krupitsky of St. Petersburg State Pavlov Medical University, Russia, and his colleagues.
A once-monthly, extended-release naltrexone (XR-NTX) injection has been approved in Russia and in the United States to treat alcohol dependence. Dr. Krupitsky and his colleagues conducted a randomized, placebo-controlled trial to test the safety and effectiveness of the injectable drug in patients with opioid dependence.
The study population included 250 patients aged 18 years and older who were within 30 days or less of completing an inpatient opioid detoxification program. The majority of the patients were young, white men who had been suffering from heroin addiction for about 10 years. Patients were excluded if they were on parole or probation, were pregnant or breastfeeding, or had significant medical conditions such as renal failure, hepatic failure, or histories of AIDS-indicator disease.
Patients were randomized to 380 mg XR-NTX or placebo starting within 1 week after completing detoxification and continuing with one injection every 4 weeks, for a total of six injections over a 24-week period. Patients also were offered counseling sessions.
"XR-NTX offers a new treatment option without the risk of physical dependence or illegal diversion."
After 24 weeks, the median proportion of weeks of confirmed abstinence (the primary end point, based on urine tests) was significantly higher in the treatment group, compared with the placebo group (90% vs. 35%). This translated to 45 patients with confirmed abstinence in the treatment group, compared with 28 patients in the placebo group.
The median retention rate was more than 168 days in the treatment group, compared with 96 days in the placebo group. Relapses were confirmed in 17 placebo patients and 1 XR-NTX patient using a naloxone challenge.
In addition, the mean change in cravings, compared with baseline, was significantly greater in the XR-NTX group, compared with the placebo group (–10.1 vs. 0.7) and significantly more patients in the XR-NTX group reported opioid-free days, compared with the placebo group (99% vs. 60%).
Overall, 63 of the XR-NTX patients (50%) and 40 of the placebo patients (32%) experienced at least one adverse event, the most common of which were nasopharyngitis and insomnia.
Two patients in each group discontinued the study because of adverse events, but none of the XR-NTX patients died, overdosed, or discontinued treatment because of severe adverse events. Three patients in the XR-NTX group reported four serious adverse events, including infectious processes such as AIDS or HIV. Four patients in the placebo group reported five serious adverse events, including infections, psychotic disorder, and a peptic ulcer.
The study was limited by a lack of comparison with oral naltrexone, but other studies comparing oral naltrexone with placebo have failed to show an effect on craving reduction or relapse prevention, the researchers noted.
"The extent of patient interest in XR-NTX when opioid substitution treatments are available remains a topic for future health services research," the investigators said. However, "XR-NTX offers a new treatment option without the risk of physical dependence or illegal diversion," and the injectable treatment approach might improve acceptance of pharmacotherapy for opioid addiction, they said.
The study was funded by Alkermes. Dr. Krupitsky had no financial conflicts to disclose.
PARIS – Patients recovering from opioid dependence who received monthly injections of 380 mg of extended-release naltrexone reported significant improvements in weeks of abstinence, cravings, and opioid-free days, compared with a placebo group. The findings were published in the Lancet (2011; 377:1506-13) and subsequently presented at the annual meeting of the European Congress of Neuropsychopharmacology.
Naltrexone, an opioid receptor antagonist, has been used to support recovery in opioid-addicted patients, but adherence to the oral medication tends to be poor, which limits effectiveness, said Dr. Evgeny M. Krupitsky of St. Petersburg State Pavlov Medical University, Russia, and his colleagues.
A once-monthly, extended-release naltrexone (XR-NTX) injection has been approved in Russia and in the United States to treat alcohol dependence. Dr. Krupitsky and his colleagues conducted a randomized, placebo-controlled trial to test the safety and effectiveness of the injectable drug in patients with opioid dependence.
The study population included 250 patients aged 18 years and older who were within 30 days or less of completing an inpatient opioid detoxification program. The majority of the patients were young, white men who had been suffering from heroin addiction for about 10 years. Patients were excluded if they were on parole or probation, were pregnant or breastfeeding, or had significant medical conditions such as renal failure, hepatic failure, or histories of AIDS-indicator disease.
Patients were randomized to 380 mg XR-NTX or placebo starting within 1 week after completing detoxification and continuing with one injection every 4 weeks, for a total of six injections over a 24-week period. Patients also were offered counseling sessions.
"XR-NTX offers a new treatment option without the risk of physical dependence or illegal diversion."
After 24 weeks, the median proportion of weeks of confirmed abstinence (the primary end point, based on urine tests) was significantly higher in the treatment group, compared with the placebo group (90% vs. 35%). This translated to 45 patients with confirmed abstinence in the treatment group, compared with 28 patients in the placebo group.
The median retention rate was more than 168 days in the treatment group, compared with 96 days in the placebo group. Relapses were confirmed in 17 placebo patients and 1 XR-NTX patient using a naloxone challenge.
In addition, the mean change in cravings, compared with baseline, was significantly greater in the XR-NTX group, compared with the placebo group (–10.1 vs. 0.7) and significantly more patients in the XR-NTX group reported opioid-free days, compared with the placebo group (99% vs. 60%).
Overall, 63 of the XR-NTX patients (50%) and 40 of the placebo patients (32%) experienced at least one adverse event, the most common of which were nasopharyngitis and insomnia.
Two patients in each group discontinued the study because of adverse events, but none of the XR-NTX patients died, overdosed, or discontinued treatment because of severe adverse events. Three patients in the XR-NTX group reported four serious adverse events, including infectious processes such as AIDS or HIV. Four patients in the placebo group reported five serious adverse events, including infections, psychotic disorder, and a peptic ulcer.
The study was limited by a lack of comparison with oral naltrexone, but other studies comparing oral naltrexone with placebo have failed to show an effect on craving reduction or relapse prevention, the researchers noted.
"The extent of patient interest in XR-NTX when opioid substitution treatments are available remains a topic for future health services research," the investigators said. However, "XR-NTX offers a new treatment option without the risk of physical dependence or illegal diversion," and the injectable treatment approach might improve acceptance of pharmacotherapy for opioid addiction, they said.
The study was funded by Alkermes. Dr. Krupitsky had no financial conflicts to disclose.
FROM THE ANNUAL MEETING OF THE EUROPEAN CONGRESS OF NEUROPSYCHO-PHARMACOLOGY
Major Finding: Monthly injections of 380 mg of extended-release naltrexone, an opioid receptor antagonist, significantly improved ongoing abstinence in opioid-dependent patients.
Data Source: A 24-week randomized trial including 250 patients aged 18 years and older who were completing an inpatient detoxification program.
Disclosures: The study was funded by Alkermes. Dr. Krupitsky had no financial conflicts to disclose.
Inflammatory Cause of Bipolar Disorder Suggests New Treatments
PARIS – Recently reported evidence implicating inflammatory mediators in the pathophysiology of bipolar disorder and major depression have opened the door to testing new agents for treating these psychiatric disorders or slowing their progression.
"Bipolar disorder is associated with neuroprogression, and oxidative stress, neurotrophins, and inflammation may underpin this process, Dr. Michael Berk said at the annual Congress of the European College of Neuropsychopharmacology. "Early interventions can potentially improve the outcome" of bipolar disorder, and the new findings give new opportunities to find effective neuroprotective agents, said Dr. Berk, professor and chairman of psychiatry at Deakin University in Geelong, Australia.
"We increasingly think there is a systemic biology that underpins" bipolar disorder and potentially other inflammatory diseases. "The brain does not exist in isolation, and we need to understand that pathways similar to those that underpin risks for cardiovascular disorders, stroke, and osteoporosis might also underpin the risk for psychiatric disorders, and that other treatments might be helpful," he said in an interview.
Based on this concept, and supported by suggestive results from epidemiologic studies, Dr. Berk said he and his associates are running a prospective, randomized study assessing a role for aspirin in treating bipolar disorder, and that they are seeking funding for a second study to test combined treatment with a statin and aspirin in bipolar disorder patients.
"We are only starting to understand the core elements of neuroprogression" in bipolar disorder patients, including the roles of neurogenesis, apoptosis, oxidative stress, and mitochondrial energy generation. "If we accept that these are determining long-term outcomes, it gives us a diversity of novel treatments we can begin to look at that might have a potential impact on these underlying processes. I think this is one of the most hopeful areas of psychiatry."
Evidence of a role for inflammation in bipolar disorder and major depression includes the finding by Dr. Berk and his associates that women who received statin treatment had about a 2% incidence of newly diagnosed major depressive disorder during 10 years of follow-up, significantly less than the 10% incidence rate among women who did not receive a statin, in a nonrandomized study that controlled for age (Psychother. Psychosom. 2010;79:323-5).
In another study, Dr. Berk and his associates found a link between elevated serum levels of high sensitivity C-reactive protein (hsCRP) and the incidence of major depressive disorder. They followed 644 randomly selected women aged 20-84 and with no history of depression at baseline for 10 years. During follow-up, the rate of new-onset major depressive disorder rose by a statistically significant 44% for each one standard deviation increase in the log-transformed level of serum hsCRP, after adjustment for baseline differences in weight, smoking history, and use of nonsteroidal anti-inflammatory drugs (Br. J. Psychiatry 2010;197:372-7).
Also, a 2006 report from Irish researchers had results from a study of the plasma levels of five different cytokines in 42 people aged 1-68, including nine with bipolar affective disorder in the depressive phase, 12 with bipolar affective disorder in the manic phase, and 21 control people with no personal or family history of a mood disorder. The results showed significantly elevated plasma levels of interleukin-8 and tumor necrosis factor-α in both subgroups of bipolar disorder patients studied compared with the controls (J. Affective Disorders 2006;90:263-7).
Dr. Berk also reported as yet unpublished results from his group that further supported a possible causal role for inflammation or autoimmunity in major depression. Results from one study showed significantly lower plasma levels of tumor necrosis factor-α in control subjects, compared with patients with melancholia or recurrent major depressive disorder. Results from a second study showed a significantly higher level of IgM that reacted against an oxidatively modified form of tryptophan in patients with new-onset major depressive disorder. "This may be a major cause of neuroprogression and a factor in chronicity and treatment resistance," he said.
In addition, recent findings on the trajectory of major depression and bipolar disorder also suggest a critical role for early treatment. Although Dr. Berk cautioned that "we need prospective data, and we need to be cautious in interpreting the data" now available, recent findings suggest that "the earlier on in illness the simpler treatment can be and the more likely is response to treatment," he said. Conversely, more advanced bipolar disorder appears to be more treatment resistant and only treatable with more complex regimens. The basis for these associations seems to be the progressive neuropathologic processes that underlie these disorders. "Mania appears to be the most neurotoxic phase of [bipolar disorder] illness," he said. "Prevention of mania may be most effective for preventing neuroprogression."
He cited clinical suggestions of progression to more treatment-resistant forms of disease. "You often see the history that initial treatment with antidepressant drugs helped, but later they did not." For example, in a recent meta-analysis of 12 treatment studies involving more than 4,000 total patients with bipolar disorder, Dr. Berk and his associates found patients in the earliest stages of their disease consistently had better responses to treatment, compared with patients who had experienced more episodes of mania or depression. "Vigorous attention needs to be paid to early diagnosis," they wrote in their report published earlier this year (Bipolar Dis. 2011;13:87-98). "There is every possibility that early and appropriate treatment and the prevention of new episodes may prevent this cascade," they wrote, but also added "this remains to be established."
Dr. Berk said he had been a speaker for, a consultant to, and has received research grants from Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, and Servier; he has been a speaker for and consultant to AstraZeneca, Janssen Cilag, and Lundbeck; he has been a speaker for Merck, Sanofi Synthélabo, and Solvay and Wyeth, and he has received research grants from Organon, Novartis, and Mayne.
PARIS – Recently reported evidence implicating inflammatory mediators in the pathophysiology of bipolar disorder and major depression have opened the door to testing new agents for treating these psychiatric disorders or slowing their progression.
"Bipolar disorder is associated with neuroprogression, and oxidative stress, neurotrophins, and inflammation may underpin this process, Dr. Michael Berk said at the annual Congress of the European College of Neuropsychopharmacology. "Early interventions can potentially improve the outcome" of bipolar disorder, and the new findings give new opportunities to find effective neuroprotective agents, said Dr. Berk, professor and chairman of psychiatry at Deakin University in Geelong, Australia.
"We increasingly think there is a systemic biology that underpins" bipolar disorder and potentially other inflammatory diseases. "The brain does not exist in isolation, and we need to understand that pathways similar to those that underpin risks for cardiovascular disorders, stroke, and osteoporosis might also underpin the risk for psychiatric disorders, and that other treatments might be helpful," he said in an interview.
Based on this concept, and supported by suggestive results from epidemiologic studies, Dr. Berk said he and his associates are running a prospective, randomized study assessing a role for aspirin in treating bipolar disorder, and that they are seeking funding for a second study to test combined treatment with a statin and aspirin in bipolar disorder patients.
"We are only starting to understand the core elements of neuroprogression" in bipolar disorder patients, including the roles of neurogenesis, apoptosis, oxidative stress, and mitochondrial energy generation. "If we accept that these are determining long-term outcomes, it gives us a diversity of novel treatments we can begin to look at that might have a potential impact on these underlying processes. I think this is one of the most hopeful areas of psychiatry."
Evidence of a role for inflammation in bipolar disorder and major depression includes the finding by Dr. Berk and his associates that women who received statin treatment had about a 2% incidence of newly diagnosed major depressive disorder during 10 years of follow-up, significantly less than the 10% incidence rate among women who did not receive a statin, in a nonrandomized study that controlled for age (Psychother. Psychosom. 2010;79:323-5).
In another study, Dr. Berk and his associates found a link between elevated serum levels of high sensitivity C-reactive protein (hsCRP) and the incidence of major depressive disorder. They followed 644 randomly selected women aged 20-84 and with no history of depression at baseline for 10 years. During follow-up, the rate of new-onset major depressive disorder rose by a statistically significant 44% for each one standard deviation increase in the log-transformed level of serum hsCRP, after adjustment for baseline differences in weight, smoking history, and use of nonsteroidal anti-inflammatory drugs (Br. J. Psychiatry 2010;197:372-7).
Also, a 2006 report from Irish researchers had results from a study of the plasma levels of five different cytokines in 42 people aged 1-68, including nine with bipolar affective disorder in the depressive phase, 12 with bipolar affective disorder in the manic phase, and 21 control people with no personal or family history of a mood disorder. The results showed significantly elevated plasma levels of interleukin-8 and tumor necrosis factor-α in both subgroups of bipolar disorder patients studied compared with the controls (J. Affective Disorders 2006;90:263-7).
Dr. Berk also reported as yet unpublished results from his group that further supported a possible causal role for inflammation or autoimmunity in major depression. Results from one study showed significantly lower plasma levels of tumor necrosis factor-α in control subjects, compared with patients with melancholia or recurrent major depressive disorder. Results from a second study showed a significantly higher level of IgM that reacted against an oxidatively modified form of tryptophan in patients with new-onset major depressive disorder. "This may be a major cause of neuroprogression and a factor in chronicity and treatment resistance," he said.
In addition, recent findings on the trajectory of major depression and bipolar disorder also suggest a critical role for early treatment. Although Dr. Berk cautioned that "we need prospective data, and we need to be cautious in interpreting the data" now available, recent findings suggest that "the earlier on in illness the simpler treatment can be and the more likely is response to treatment," he said. Conversely, more advanced bipolar disorder appears to be more treatment resistant and only treatable with more complex regimens. The basis for these associations seems to be the progressive neuropathologic processes that underlie these disorders. "Mania appears to be the most neurotoxic phase of [bipolar disorder] illness," he said. "Prevention of mania may be most effective for preventing neuroprogression."
He cited clinical suggestions of progression to more treatment-resistant forms of disease. "You often see the history that initial treatment with antidepressant drugs helped, but later they did not." For example, in a recent meta-analysis of 12 treatment studies involving more than 4,000 total patients with bipolar disorder, Dr. Berk and his associates found patients in the earliest stages of their disease consistently had better responses to treatment, compared with patients who had experienced more episodes of mania or depression. "Vigorous attention needs to be paid to early diagnosis," they wrote in their report published earlier this year (Bipolar Dis. 2011;13:87-98). "There is every possibility that early and appropriate treatment and the prevention of new episodes may prevent this cascade," they wrote, but also added "this remains to be established."
Dr. Berk said he had been a speaker for, a consultant to, and has received research grants from Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, and Servier; he has been a speaker for and consultant to AstraZeneca, Janssen Cilag, and Lundbeck; he has been a speaker for Merck, Sanofi Synthélabo, and Solvay and Wyeth, and he has received research grants from Organon, Novartis, and Mayne.
PARIS – Recently reported evidence implicating inflammatory mediators in the pathophysiology of bipolar disorder and major depression have opened the door to testing new agents for treating these psychiatric disorders or slowing their progression.
"Bipolar disorder is associated with neuroprogression, and oxidative stress, neurotrophins, and inflammation may underpin this process, Dr. Michael Berk said at the annual Congress of the European College of Neuropsychopharmacology. "Early interventions can potentially improve the outcome" of bipolar disorder, and the new findings give new opportunities to find effective neuroprotective agents, said Dr. Berk, professor and chairman of psychiatry at Deakin University in Geelong, Australia.
"We increasingly think there is a systemic biology that underpins" bipolar disorder and potentially other inflammatory diseases. "The brain does not exist in isolation, and we need to understand that pathways similar to those that underpin risks for cardiovascular disorders, stroke, and osteoporosis might also underpin the risk for psychiatric disorders, and that other treatments might be helpful," he said in an interview.
Based on this concept, and supported by suggestive results from epidemiologic studies, Dr. Berk said he and his associates are running a prospective, randomized study assessing a role for aspirin in treating bipolar disorder, and that they are seeking funding for a second study to test combined treatment with a statin and aspirin in bipolar disorder patients.
"We are only starting to understand the core elements of neuroprogression" in bipolar disorder patients, including the roles of neurogenesis, apoptosis, oxidative stress, and mitochondrial energy generation. "If we accept that these are determining long-term outcomes, it gives us a diversity of novel treatments we can begin to look at that might have a potential impact on these underlying processes. I think this is one of the most hopeful areas of psychiatry."
Evidence of a role for inflammation in bipolar disorder and major depression includes the finding by Dr. Berk and his associates that women who received statin treatment had about a 2% incidence of newly diagnosed major depressive disorder during 10 years of follow-up, significantly less than the 10% incidence rate among women who did not receive a statin, in a nonrandomized study that controlled for age (Psychother. Psychosom. 2010;79:323-5).
In another study, Dr. Berk and his associates found a link between elevated serum levels of high sensitivity C-reactive protein (hsCRP) and the incidence of major depressive disorder. They followed 644 randomly selected women aged 20-84 and with no history of depression at baseline for 10 years. During follow-up, the rate of new-onset major depressive disorder rose by a statistically significant 44% for each one standard deviation increase in the log-transformed level of serum hsCRP, after adjustment for baseline differences in weight, smoking history, and use of nonsteroidal anti-inflammatory drugs (Br. J. Psychiatry 2010;197:372-7).
Also, a 2006 report from Irish researchers had results from a study of the plasma levels of five different cytokines in 42 people aged 1-68, including nine with bipolar affective disorder in the depressive phase, 12 with bipolar affective disorder in the manic phase, and 21 control people with no personal or family history of a mood disorder. The results showed significantly elevated plasma levels of interleukin-8 and tumor necrosis factor-α in both subgroups of bipolar disorder patients studied compared with the controls (J. Affective Disorders 2006;90:263-7).
Dr. Berk also reported as yet unpublished results from his group that further supported a possible causal role for inflammation or autoimmunity in major depression. Results from one study showed significantly lower plasma levels of tumor necrosis factor-α in control subjects, compared with patients with melancholia or recurrent major depressive disorder. Results from a second study showed a significantly higher level of IgM that reacted against an oxidatively modified form of tryptophan in patients with new-onset major depressive disorder. "This may be a major cause of neuroprogression and a factor in chronicity and treatment resistance," he said.
In addition, recent findings on the trajectory of major depression and bipolar disorder also suggest a critical role for early treatment. Although Dr. Berk cautioned that "we need prospective data, and we need to be cautious in interpreting the data" now available, recent findings suggest that "the earlier on in illness the simpler treatment can be and the more likely is response to treatment," he said. Conversely, more advanced bipolar disorder appears to be more treatment resistant and only treatable with more complex regimens. The basis for these associations seems to be the progressive neuropathologic processes that underlie these disorders. "Mania appears to be the most neurotoxic phase of [bipolar disorder] illness," he said. "Prevention of mania may be most effective for preventing neuroprogression."
He cited clinical suggestions of progression to more treatment-resistant forms of disease. "You often see the history that initial treatment with antidepressant drugs helped, but later they did not." For example, in a recent meta-analysis of 12 treatment studies involving more than 4,000 total patients with bipolar disorder, Dr. Berk and his associates found patients in the earliest stages of their disease consistently had better responses to treatment, compared with patients who had experienced more episodes of mania or depression. "Vigorous attention needs to be paid to early diagnosis," they wrote in their report published earlier this year (Bipolar Dis. 2011;13:87-98). "There is every possibility that early and appropriate treatment and the prevention of new episodes may prevent this cascade," they wrote, but also added "this remains to be established."
Dr. Berk said he had been a speaker for, a consultant to, and has received research grants from Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, and Servier; he has been a speaker for and consultant to AstraZeneca, Janssen Cilag, and Lundbeck; he has been a speaker for Merck, Sanofi Synthélabo, and Solvay and Wyeth, and he has received research grants from Organon, Novartis, and Mayne.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN COLLEGE OF NEUROPSYCHO-
PHARMACOLOGY
Sertraline Added to Therapy May Fall Short in Postpartum Depression
PARIS – Postpartum depression is such a common problem that it’s surprising there are so few randomized, controlled studies comparing drug therapy versus nondrug treatment options. Many women with postpartum depression don’t seek treatment, some are treated only with psychotherapy because of concerns regarding pharmacotherapy, and others are treated with antidepressants alone.
Israeli investigators conducted a small, randomized, controlled study in 40 women with mild to moderate postpartum depression lasting 6 months or longer. They expected psychotherapy plus the antidepressant sertraline to be more effective than psychotherapy alone, but they were surprised to find no additional benefit from the drug.
Response rates were 70% in women who received both psychotherapy and sertraline and 55% in those who got psychotherapy and a placebo. This was not a statistically significant difference at a P value of 0.33, Dr. Miki Bloch and his associates reported at the annual congress of the European College of Neuropsychopharmacology. The rates of remission after 8 weeks of treatment were 65% in the combination therapy group and 50% in the psychotherapy plus placebo group, which also was not a significant difference at a P value of 0.34, said Dr. Bloch of Tel Aviv (Israel) University.
All patients started 12 consecutive weekly sessions of Malan-based brief focused dynamic psychotherapy by expert psychotherapists, Dr. Bloch said in an interview. At the same time, the women were randomized to receive 50 mg sertraline or a placebo pill for 4 weeks, after which the psychiatrist could choose to increase the dose to 100 mg. After 8 weeks, the drug groups were unblinded and patients could continue open-label drug therapy for 4 weeks if desired.
Two women in the sertraline group developed hypomanic symptoms, but treatment was otherwise well tolerated. Initially, 42 women were enrolled but 2 dropped out during the first week and were not included in the intent-to-treat analysis.
Adding sertraline does not seem to add any benefit to psychotherapy alone, based on this small trial. "While a type 2 error cannot be ruled out, any difference between the groups does not appear to be of clinical significance," Dr. Bloch said.
Prior to this study, Dr. Bloch could find only four randomized and two placebo-controlled studies in the medical literature on antidepressant therapy for postpartum depression. Data comparing pharmacotherapy and psychotherapy for postpartum depression are "virtually lacking," as are studies of the potential efficacy of combining these treatment modalities, he said.
There was no placebo-only comparison in the current study, because it was considered unethical not to offer treatment to women with postpartum depression. Because of this, the investigators could not say that psychotherapy was better than placebo, but the results do suggest that adding sertraline to psychotherapy does not improve upon any effects that psychotherapy might have.
Dr. Bloch said he has no relevant conflicts of interest.
PARIS – Postpartum depression is such a common problem that it’s surprising there are so few randomized, controlled studies comparing drug therapy versus nondrug treatment options. Many women with postpartum depression don’t seek treatment, some are treated only with psychotherapy because of concerns regarding pharmacotherapy, and others are treated with antidepressants alone.
Israeli investigators conducted a small, randomized, controlled study in 40 women with mild to moderate postpartum depression lasting 6 months or longer. They expected psychotherapy plus the antidepressant sertraline to be more effective than psychotherapy alone, but they were surprised to find no additional benefit from the drug.
Response rates were 70% in women who received both psychotherapy and sertraline and 55% in those who got psychotherapy and a placebo. This was not a statistically significant difference at a P value of 0.33, Dr. Miki Bloch and his associates reported at the annual congress of the European College of Neuropsychopharmacology. The rates of remission after 8 weeks of treatment were 65% in the combination therapy group and 50% in the psychotherapy plus placebo group, which also was not a significant difference at a P value of 0.34, said Dr. Bloch of Tel Aviv (Israel) University.
All patients started 12 consecutive weekly sessions of Malan-based brief focused dynamic psychotherapy by expert psychotherapists, Dr. Bloch said in an interview. At the same time, the women were randomized to receive 50 mg sertraline or a placebo pill for 4 weeks, after which the psychiatrist could choose to increase the dose to 100 mg. After 8 weeks, the drug groups were unblinded and patients could continue open-label drug therapy for 4 weeks if desired.
Two women in the sertraline group developed hypomanic symptoms, but treatment was otherwise well tolerated. Initially, 42 women were enrolled but 2 dropped out during the first week and were not included in the intent-to-treat analysis.
Adding sertraline does not seem to add any benefit to psychotherapy alone, based on this small trial. "While a type 2 error cannot be ruled out, any difference between the groups does not appear to be of clinical significance," Dr. Bloch said.
Prior to this study, Dr. Bloch could find only four randomized and two placebo-controlled studies in the medical literature on antidepressant therapy for postpartum depression. Data comparing pharmacotherapy and psychotherapy for postpartum depression are "virtually lacking," as are studies of the potential efficacy of combining these treatment modalities, he said.
There was no placebo-only comparison in the current study, because it was considered unethical not to offer treatment to women with postpartum depression. Because of this, the investigators could not say that psychotherapy was better than placebo, but the results do suggest that adding sertraline to psychotherapy does not improve upon any effects that psychotherapy might have.
Dr. Bloch said he has no relevant conflicts of interest.
PARIS – Postpartum depression is such a common problem that it’s surprising there are so few randomized, controlled studies comparing drug therapy versus nondrug treatment options. Many women with postpartum depression don’t seek treatment, some are treated only with psychotherapy because of concerns regarding pharmacotherapy, and others are treated with antidepressants alone.
Israeli investigators conducted a small, randomized, controlled study in 40 women with mild to moderate postpartum depression lasting 6 months or longer. They expected psychotherapy plus the antidepressant sertraline to be more effective than psychotherapy alone, but they were surprised to find no additional benefit from the drug.
Response rates were 70% in women who received both psychotherapy and sertraline and 55% in those who got psychotherapy and a placebo. This was not a statistically significant difference at a P value of 0.33, Dr. Miki Bloch and his associates reported at the annual congress of the European College of Neuropsychopharmacology. The rates of remission after 8 weeks of treatment were 65% in the combination therapy group and 50% in the psychotherapy plus placebo group, which also was not a significant difference at a P value of 0.34, said Dr. Bloch of Tel Aviv (Israel) University.
All patients started 12 consecutive weekly sessions of Malan-based brief focused dynamic psychotherapy by expert psychotherapists, Dr. Bloch said in an interview. At the same time, the women were randomized to receive 50 mg sertraline or a placebo pill for 4 weeks, after which the psychiatrist could choose to increase the dose to 100 mg. After 8 weeks, the drug groups were unblinded and patients could continue open-label drug therapy for 4 weeks if desired.
Two women in the sertraline group developed hypomanic symptoms, but treatment was otherwise well tolerated. Initially, 42 women were enrolled but 2 dropped out during the first week and were not included in the intent-to-treat analysis.
Adding sertraline does not seem to add any benefit to psychotherapy alone, based on this small trial. "While a type 2 error cannot be ruled out, any difference between the groups does not appear to be of clinical significance," Dr. Bloch said.
Prior to this study, Dr. Bloch could find only four randomized and two placebo-controlled studies in the medical literature on antidepressant therapy for postpartum depression. Data comparing pharmacotherapy and psychotherapy for postpartum depression are "virtually lacking," as are studies of the potential efficacy of combining these treatment modalities, he said.
There was no placebo-only comparison in the current study, because it was considered unethical not to offer treatment to women with postpartum depression. Because of this, the investigators could not say that psychotherapy was better than placebo, but the results do suggest that adding sertraline to psychotherapy does not improve upon any effects that psychotherapy might have.
Dr. Bloch said he has no relevant conflicts of interest.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN COLLEGE OF NEUROPSYCHO-PHARMACOLOGY
Depression Derails Truthful Talk to Physicians
PARIS – Eliciting truthful dialogue from patients with depression may be as elusive as a simple cure for the condition.
A large Internet-based survey involving 2,020 patients who had received treatment for depression within the past year found that a full 70.2% had lied, at least once, during treatment.
More than two-thirds, or 69%, fibbed about daily activities such as work, while 52.6% failed, on purpose, to accurately report their symptoms, Dr. Norifusa Sawada reported at the annual meeting of the European College of Neuropsychopharmacology.
"The fact that more than 70% of patients with depression do not disclose the truth emphasizes the need for clinical suspicion, as well as more objective assessment of their symptoms and medication adherence," he suggested.
Men were significantly more likely than women to be dishonest about daily activities and intake of alcohol and illicit drugs.
In contrast, women were more likely to be dishonest about adherence to prescribed medication and data such as body temperature and weight.
The most frequent (49%) reason why patients didn’t tell the truth was because they found it difficult to talk to their doctor, reported Dr. Sawada, a psychiatrist at Keio University in Toyko. In all, 30% of Japanese patients said they were embarrassed to tell the truth. A disconcerting 36.5%, however, failed to tell the truth because "I thought my doctor would not take it seriously, even if I told him or her."
Female patients were significantly more likely than males to cite this reason, but also said they were less than frank about the truth because they could not trust their doctor or their doctor looked busy.
In contrast, men were significantly more likely to report they didn’t tell the truth because of concerns that their physician would recommend they take sick leave or quit their job.
Logistic regression analysis found significant associations between not telling the truth and lower satisfaction in communication with their physicians (odds ratio, 1.98 for not satisfied and OR, 2.78 for dissatisfied; P value less than .001 for both).
Other significant predictors were female sex (OR, 1.54, P less than .001) and younger age (20-29 years vs. 50-59 years OR, 0.65, P = .004; 20-29 years vs. 60-69 years, OR, 0.29, P = .027). Physician age and gender, and patient income had no effect.
All patients, aged 20-69 years, had received treatment within the past year for a diagnosis of major depressive disorder by a psychiatrist. None had bipolar, and all were registered with Macromill, one of the largest online research companies in Japan.
In all, 32% of patients reported quitting treatment for depression without consulting their physician, and 45% discontinued antidepressants without consultation, Dr. Sawada noted.
The researchers previously reported that only 44% of 367 outpatients with major depressive disorder continued their antidepressants for 6 months (BMC Psychiatry 2009;9:38).
In the current analysis, the most common reason patients gave for discontinuing treatment was that their symptoms got better, followed by, "I did not get along with my doctor," and that they could not or did not want to go out.
"When communication between patients and their physician is suboptimal, patient status and behavior should be monitored with more objective vigilance in an effort to avoid undesirable premature withdrawal from antidepressants." This also underscores the importance of a good patient-physician alliance to gather relevant information toward a successful treatment, Dr. Sawada said.
Pfizer sponsored the study. The authors report no conflicts.
PARIS – Eliciting truthful dialogue from patients with depression may be as elusive as a simple cure for the condition.
A large Internet-based survey involving 2,020 patients who had received treatment for depression within the past year found that a full 70.2% had lied, at least once, during treatment.
More than two-thirds, or 69%, fibbed about daily activities such as work, while 52.6% failed, on purpose, to accurately report their symptoms, Dr. Norifusa Sawada reported at the annual meeting of the European College of Neuropsychopharmacology.
"The fact that more than 70% of patients with depression do not disclose the truth emphasizes the need for clinical suspicion, as well as more objective assessment of their symptoms and medication adherence," he suggested.
Men were significantly more likely than women to be dishonest about daily activities and intake of alcohol and illicit drugs.
In contrast, women were more likely to be dishonest about adherence to prescribed medication and data such as body temperature and weight.
The most frequent (49%) reason why patients didn’t tell the truth was because they found it difficult to talk to their doctor, reported Dr. Sawada, a psychiatrist at Keio University in Toyko. In all, 30% of Japanese patients said they were embarrassed to tell the truth. A disconcerting 36.5%, however, failed to tell the truth because "I thought my doctor would not take it seriously, even if I told him or her."
Female patients were significantly more likely than males to cite this reason, but also said they were less than frank about the truth because they could not trust their doctor or their doctor looked busy.
In contrast, men were significantly more likely to report they didn’t tell the truth because of concerns that their physician would recommend they take sick leave or quit their job.
Logistic regression analysis found significant associations between not telling the truth and lower satisfaction in communication with their physicians (odds ratio, 1.98 for not satisfied and OR, 2.78 for dissatisfied; P value less than .001 for both).
Other significant predictors were female sex (OR, 1.54, P less than .001) and younger age (20-29 years vs. 50-59 years OR, 0.65, P = .004; 20-29 years vs. 60-69 years, OR, 0.29, P = .027). Physician age and gender, and patient income had no effect.
All patients, aged 20-69 years, had received treatment within the past year for a diagnosis of major depressive disorder by a psychiatrist. None had bipolar, and all were registered with Macromill, one of the largest online research companies in Japan.
In all, 32% of patients reported quitting treatment for depression without consulting their physician, and 45% discontinued antidepressants without consultation, Dr. Sawada noted.
The researchers previously reported that only 44% of 367 outpatients with major depressive disorder continued their antidepressants for 6 months (BMC Psychiatry 2009;9:38).
In the current analysis, the most common reason patients gave for discontinuing treatment was that their symptoms got better, followed by, "I did not get along with my doctor," and that they could not or did not want to go out.
"When communication between patients and their physician is suboptimal, patient status and behavior should be monitored with more objective vigilance in an effort to avoid undesirable premature withdrawal from antidepressants." This also underscores the importance of a good patient-physician alliance to gather relevant information toward a successful treatment, Dr. Sawada said.
Pfizer sponsored the study. The authors report no conflicts.
PARIS – Eliciting truthful dialogue from patients with depression may be as elusive as a simple cure for the condition.
A large Internet-based survey involving 2,020 patients who had received treatment for depression within the past year found that a full 70.2% had lied, at least once, during treatment.
More than two-thirds, or 69%, fibbed about daily activities such as work, while 52.6% failed, on purpose, to accurately report their symptoms, Dr. Norifusa Sawada reported at the annual meeting of the European College of Neuropsychopharmacology.
"The fact that more than 70% of patients with depression do not disclose the truth emphasizes the need for clinical suspicion, as well as more objective assessment of their symptoms and medication adherence," he suggested.
Men were significantly more likely than women to be dishonest about daily activities and intake of alcohol and illicit drugs.
In contrast, women were more likely to be dishonest about adherence to prescribed medication and data such as body temperature and weight.
The most frequent (49%) reason why patients didn’t tell the truth was because they found it difficult to talk to their doctor, reported Dr. Sawada, a psychiatrist at Keio University in Toyko. In all, 30% of Japanese patients said they were embarrassed to tell the truth. A disconcerting 36.5%, however, failed to tell the truth because "I thought my doctor would not take it seriously, even if I told him or her."
Female patients were significantly more likely than males to cite this reason, but also said they were less than frank about the truth because they could not trust their doctor or their doctor looked busy.
In contrast, men were significantly more likely to report they didn’t tell the truth because of concerns that their physician would recommend they take sick leave or quit their job.
Logistic regression analysis found significant associations between not telling the truth and lower satisfaction in communication with their physicians (odds ratio, 1.98 for not satisfied and OR, 2.78 for dissatisfied; P value less than .001 for both).
Other significant predictors were female sex (OR, 1.54, P less than .001) and younger age (20-29 years vs. 50-59 years OR, 0.65, P = .004; 20-29 years vs. 60-69 years, OR, 0.29, P = .027). Physician age and gender, and patient income had no effect.
All patients, aged 20-69 years, had received treatment within the past year for a diagnosis of major depressive disorder by a psychiatrist. None had bipolar, and all were registered with Macromill, one of the largest online research companies in Japan.
In all, 32% of patients reported quitting treatment for depression without consulting their physician, and 45% discontinued antidepressants without consultation, Dr. Sawada noted.
The researchers previously reported that only 44% of 367 outpatients with major depressive disorder continued their antidepressants for 6 months (BMC Psychiatry 2009;9:38).
In the current analysis, the most common reason patients gave for discontinuing treatment was that their symptoms got better, followed by, "I did not get along with my doctor," and that they could not or did not want to go out.
"When communication between patients and their physician is suboptimal, patient status and behavior should be monitored with more objective vigilance in an effort to avoid undesirable premature withdrawal from antidepressants." This also underscores the importance of a good patient-physician alliance to gather relevant information toward a successful treatment, Dr. Sawada said.
Pfizer sponsored the study. The authors report no conflicts.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN COLLEGE OF NEUROPSYCHO- PHARMACOLOGY
Major Finding: A full 70.2% of patients with depression reported they had, at least once, not told the truth to their physician.
Data Source: Internet-based survey of 2,020 patients who received treatment for major depressive disorder within the past year.
Disclosures: Pfizer sponsored the study. The authors report no conflicts.