User login
High-status occupation spells worse response to antidepressant therapy
VIENNA – Depressed patients with a high-status job appear to be significantly less responsive to antidepressant therapy than other workers, Joseph Zohar, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented a multicenter study of 654 Austrian, Israeli, Belgian, and Italian working adults with major depressive disorder. Those categorized as having a high occupational level were significantly less likely to have responded to the most recent trial of antidepressant medication for their current episode. They also had a significantly higher rate of treatment-resistant depression as defined by nonresponse to at least two previous adequate treatment trials, compared with the patients with mid- or low-level occupations.
“These results show that the need for precise prescribing is not only related to the symptoms and genetics but also to occupational level. One might need to prescribe different medication for the same disorder and need to take into account the occupational level in order to reach optimum effect,” he continued.
He and his coinvestigators categorized occupational level according to Hollingshead’s Occupational Scale, a tool widely used by sociologists. Occupational level is a different concept from socioeconomic status. It incorporates prestige, entry qualifications, job responsibilities, and skill requirements – as well as earnings. Examples of high occupational level jobs in this construct include physicians, engineers, architects, scientists, lawyers, CEOs, and professors.
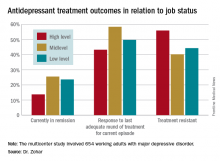
Fifty-one percent of the study participants were classified as having high-status occupations, with the rest evenly divided between mid- and low-level occupations. Only 43.2% of patients in the high-level group had responded to their last treatment episode based upon clinical judgment and a Hamilton Depression Rating Scale score of 17 or less, compared with 58.4% of patients with mid- and 49.7% with low-level jobs.
At this point the explanation for the observed relationship between occupation level and antidepressant treatment response remains speculative. This is the first large study of its kind, and the results require confirmation. If the findings hold up, the results could have important implications for employers, who might want to discourage patients with high-status jobs and a history of treatment-resistant depression from particularly high work stress environments in order to reduce depression-related disability in the workplace, according to the psychiatrist.
Potential explanations for the findings include the possibility that depressed patients in high-level occupations are more likely to have poor illness acceptance, be less adherent to prescribed medications, or possess certain personality, cognitive, or behavioral differences that predispose to poor treatment outcome.
The study was funded by the ECNP’s Expert Platform on Mental Health – Focus on Depression. Dr. Zohar reported having no relevant financial conflicts.
VIENNA – Depressed patients with a high-status job appear to be significantly less responsive to antidepressant therapy than other workers, Joseph Zohar, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented a multicenter study of 654 Austrian, Israeli, Belgian, and Italian working adults with major depressive disorder. Those categorized as having a high occupational level were significantly less likely to have responded to the most recent trial of antidepressant medication for their current episode. They also had a significantly higher rate of treatment-resistant depression as defined by nonresponse to at least two previous adequate treatment trials, compared with the patients with mid- or low-level occupations.
“These results show that the need for precise prescribing is not only related to the symptoms and genetics but also to occupational level. One might need to prescribe different medication for the same disorder and need to take into account the occupational level in order to reach optimum effect,” he continued.
He and his coinvestigators categorized occupational level according to Hollingshead’s Occupational Scale, a tool widely used by sociologists. Occupational level is a different concept from socioeconomic status. It incorporates prestige, entry qualifications, job responsibilities, and skill requirements – as well as earnings. Examples of high occupational level jobs in this construct include physicians, engineers, architects, scientists, lawyers, CEOs, and professors.
Fifty-one percent of the study participants were classified as having high-status occupations, with the rest evenly divided between mid- and low-level occupations. Only 43.2% of patients in the high-level group had responded to their last treatment episode based upon clinical judgment and a Hamilton Depression Rating Scale score of 17 or less, compared with 58.4% of patients with mid- and 49.7% with low-level jobs.
At this point the explanation for the observed relationship between occupation level and antidepressant treatment response remains speculative. This is the first large study of its kind, and the results require confirmation. If the findings hold up, the results could have important implications for employers, who might want to discourage patients with high-status jobs and a history of treatment-resistant depression from particularly high work stress environments in order to reduce depression-related disability in the workplace, according to the psychiatrist.
Potential explanations for the findings include the possibility that depressed patients in high-level occupations are more likely to have poor illness acceptance, be less adherent to prescribed medications, or possess certain personality, cognitive, or behavioral differences that predispose to poor treatment outcome.
The study was funded by the ECNP’s Expert Platform on Mental Health – Focus on Depression. Dr. Zohar reported having no relevant financial conflicts.
VIENNA – Depressed patients with a high-status job appear to be significantly less responsive to antidepressant therapy than other workers, Joseph Zohar, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented a multicenter study of 654 Austrian, Israeli, Belgian, and Italian working adults with major depressive disorder. Those categorized as having a high occupational level were significantly less likely to have responded to the most recent trial of antidepressant medication for their current episode. They also had a significantly higher rate of treatment-resistant depression as defined by nonresponse to at least two previous adequate treatment trials, compared with the patients with mid- or low-level occupations.
“These results show that the need for precise prescribing is not only related to the symptoms and genetics but also to occupational level. One might need to prescribe different medication for the same disorder and need to take into account the occupational level in order to reach optimum effect,” he continued.
He and his coinvestigators categorized occupational level according to Hollingshead’s Occupational Scale, a tool widely used by sociologists. Occupational level is a different concept from socioeconomic status. It incorporates prestige, entry qualifications, job responsibilities, and skill requirements – as well as earnings. Examples of high occupational level jobs in this construct include physicians, engineers, architects, scientists, lawyers, CEOs, and professors.
Fifty-one percent of the study participants were classified as having high-status occupations, with the rest evenly divided between mid- and low-level occupations. Only 43.2% of patients in the high-level group had responded to their last treatment episode based upon clinical judgment and a Hamilton Depression Rating Scale score of 17 or less, compared with 58.4% of patients with mid- and 49.7% with low-level jobs.
At this point the explanation for the observed relationship between occupation level and antidepressant treatment response remains speculative. This is the first large study of its kind, and the results require confirmation. If the findings hold up, the results could have important implications for employers, who might want to discourage patients with high-status jobs and a history of treatment-resistant depression from particularly high work stress environments in order to reduce depression-related disability in the workplace, according to the psychiatrist.
Potential explanations for the findings include the possibility that depressed patients in high-level occupations are more likely to have poor illness acceptance, be less adherent to prescribed medications, or possess certain personality, cognitive, or behavioral differences that predispose to poor treatment outcome.
The study was funded by the ECNP’s Expert Platform on Mental Health – Focus on Depression. Dr. Zohar reported having no relevant financial conflicts.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Fifty-six percent of patients with high-status occupations who were treated for major depression were classified as treatment resistant, compared with 40% of patients with midlevel occupations and 44% with low-level occupations.
Data source: A study of 654 working adults with major depression in four countries.
Disclosures: The study was funded by the Expert Platform on Mental Health Focus on Depression. The presenter reported having no relevant financial conflicts.
Children on antipsychotics show more early weight gain than adults
VIENNA – Antipsychotic-naive children and adolescents are at greater risk for clinically significant weight gain during their first 6 months on a second-generation antipsychotic agent than adults, Covadonga M. Diaz-Caneja, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
She presented what she believes to be the first prospective observational study comparing weight gain between antipsychotic-naive children and adults during their first 6 months on second-generation antipsychotics. Weight gains were significantly greater with olanzapine (Zyprexa) than risperidone (Risperdal) or quetiapine (Seroquel) in both age groups, but the magnitude and trajectory of olanzapine-related weight gain was significantly more impressive in the pediatric patients.
The study included 226 pediatric patients with a mean age of 15.2 years and 135 adults, average age 39 years. All were antipsychotic naive when placed on one of the three second-generation antipsychotics at clinician discretion. Fifty percent of the youths and 60% of adults had a psychosis diagnosis. Sixty-four percent of the pediatric group and 55% of the adults were male.
Weight gain during the first 6 months of therapy was expressed as change in body mass index z score. An increase in BMI z score of 0.5 or more is considered clinically significant.
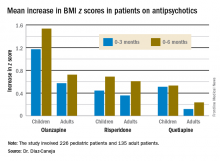
The least weight gain at 6 months occurred with quetiapine in both age groups. However, the BMI z score increased by 0.532 in the pediatric patients on the drug, more than twice the 0.236 increase in adults.
Risperidone was the only second-generation antipsychotic where the weight gain trajectories in pediatric and adult patients were not significantly different.
The study was funded by the Spanish Ministry of Economy and Competitiveness, and the European Commission. Dr. Diaz-Caneja reported having no financial conflicts of interest.
VIENNA – Antipsychotic-naive children and adolescents are at greater risk for clinically significant weight gain during their first 6 months on a second-generation antipsychotic agent than adults, Covadonga M. Diaz-Caneja, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
She presented what she believes to be the first prospective observational study comparing weight gain between antipsychotic-naive children and adults during their first 6 months on second-generation antipsychotics. Weight gains were significantly greater with olanzapine (Zyprexa) than risperidone (Risperdal) or quetiapine (Seroquel) in both age groups, but the magnitude and trajectory of olanzapine-related weight gain was significantly more impressive in the pediatric patients.
The study included 226 pediatric patients with a mean age of 15.2 years and 135 adults, average age 39 years. All were antipsychotic naive when placed on one of the three second-generation antipsychotics at clinician discretion. Fifty percent of the youths and 60% of adults had a psychosis diagnosis. Sixty-four percent of the pediatric group and 55% of the adults were male.
Weight gain during the first 6 months of therapy was expressed as change in body mass index z score. An increase in BMI z score of 0.5 or more is considered clinically significant.
The least weight gain at 6 months occurred with quetiapine in both age groups. However, the BMI z score increased by 0.532 in the pediatric patients on the drug, more than twice the 0.236 increase in adults.
Risperidone was the only second-generation antipsychotic where the weight gain trajectories in pediatric and adult patients were not significantly different.
The study was funded by the Spanish Ministry of Economy and Competitiveness, and the European Commission. Dr. Diaz-Caneja reported having no financial conflicts of interest.
VIENNA – Antipsychotic-naive children and adolescents are at greater risk for clinically significant weight gain during their first 6 months on a second-generation antipsychotic agent than adults, Covadonga M. Diaz-Caneja, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
She presented what she believes to be the first prospective observational study comparing weight gain between antipsychotic-naive children and adults during their first 6 months on second-generation antipsychotics. Weight gains were significantly greater with olanzapine (Zyprexa) than risperidone (Risperdal) or quetiapine (Seroquel) in both age groups, but the magnitude and trajectory of olanzapine-related weight gain was significantly more impressive in the pediatric patients.
The study included 226 pediatric patients with a mean age of 15.2 years and 135 adults, average age 39 years. All were antipsychotic naive when placed on one of the three second-generation antipsychotics at clinician discretion. Fifty percent of the youths and 60% of adults had a psychosis diagnosis. Sixty-four percent of the pediatric group and 55% of the adults were male.
Weight gain during the first 6 months of therapy was expressed as change in body mass index z score. An increase in BMI z score of 0.5 or more is considered clinically significant.
The least weight gain at 6 months occurred with quetiapine in both age groups. However, the BMI z score increased by 0.532 in the pediatric patients on the drug, more than twice the 0.236 increase in adults.
Risperidone was the only second-generation antipsychotic where the weight gain trajectories in pediatric and adult patients were not significantly different.
The study was funded by the Spanish Ministry of Economy and Competitiveness, and the European Commission. Dr. Diaz-Caneja reported having no financial conflicts of interest.
Key clinical point:
Major finding: The BMI z score increased by 1.535 in pediatric patients during their first 6 months on olanzapine – the most weight unfriendly of the second-generation antipsychotics – compared with 0.724 in adults.
Data source: This prospective, observational study examined weight gain in 361 antipsychotic-naive children, adolescents, and adults during their first 6 months on second-generation antipsychotic agents.
Disclosures: The study was funded by the Spanish Ministry of Economy and Competitiveness and the European Commission.
CEQUEL: Combo therapy a win in bipolar depression
VIENNA – Psychiatrists have received a welcome gift in the form of CEQUEL, a randomized trial that offers much needed guidance on how to proceed when quetiapine for bipolar depression isn’t achieving sufficient response, Eduard Vieta, MD, said at the annual congress of the European College of Neuropsychopharmacology.
The answer provided by CEQUEL is to add lamotrigine.
“Quetiapine is indicated for treatment of symptoms of bipolar depression, but many clinicians – including myself – are a bit underwhelmed about the practical results. In the clinical trials, quetiapine looks great, but when we use it in practice we’re not so happy with the results. The trials are consistently positive with a large effect size, whereas in clinical practice many patients respond only partially to quetiapine. The question has been what to do next,” observed Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Increasing the dose of quetiapine (Seroquel) above the recommended 300 mg/day in an effort to boost efficacy is problematic. Patients become extremely sedated. The CEQUEL (Comparative Evaluation of Quetiapine plus Lamotrigine) investigators decided to examine combination therapy with two drugs having completely different mechanisms of action.
A total of 202 patients with bipolar depression at 27 U.K. sites were placed on quetiapine and randomized double-blind to lamotrigine or placebo. Lamotrigine (Lamictal) was started at 25 mg/day and gradually increased to 200 mg daily. Because there is some limited evidence that folic acid is effective in unipolar depression, the investigators employed a 2x2 factorial design, further randomizing participants to 500 mcg/day of folic acid or placebo.
The primary endpoint was improvement in depressive symptoms at 12 weeks, with 52-week outcomes as a secondary endpoint. The group on combination therapy with quetiapine and lamotrigine showed significantly greater improvement both on mean depressive symptoms as measured with the Quick Inventory of Depressive Symptomatology-self report version 16 (QIDS-SR16), and on rates of remission at 12 and 52 weeks, compared with patients on quetiapine and placebo. The combination also reduced the risk of relapse in patients with bipolar I disorder with predominantly depressive symptoms.
Folic acid proved to be not only ineffective, it actually interfered with lamotrigine’s effectiveness. The mean reduction from baseline to 12 weeks on the QIDS-SR16 was 4.4 points in patients who received lamotrigine without folic acid, compared with a 0.12-point increase in those receiving lamotrigine plus folic acid (Lancet Psychiatry. 2016 Jan;3[1]:31-9).
Dr. Vieta wasn’t involved in CEQUEL, but he considers the study one of the highlights of the year in the field of affective disorders and urged his colleagues to consider lamotrigine/quetiapine combination therapy for bipolar depression.
“Quetiapine and lamotrigine are both now generic drugs. CEQUEL showed both are superior to quetiapine alone. It’s good news – a not-expensive treatment combining quetiapine with a drug, lamotrigine, that’s quite well tolerated,” he said.
CEQUEL was funded by the Medical Research Council. Dr. Vieta reported having no financial conflicts of interest.
VIENNA – Psychiatrists have received a welcome gift in the form of CEQUEL, a randomized trial that offers much needed guidance on how to proceed when quetiapine for bipolar depression isn’t achieving sufficient response, Eduard Vieta, MD, said at the annual congress of the European College of Neuropsychopharmacology.
The answer provided by CEQUEL is to add lamotrigine.
“Quetiapine is indicated for treatment of symptoms of bipolar depression, but many clinicians – including myself – are a bit underwhelmed about the practical results. In the clinical trials, quetiapine looks great, but when we use it in practice we’re not so happy with the results. The trials are consistently positive with a large effect size, whereas in clinical practice many patients respond only partially to quetiapine. The question has been what to do next,” observed Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Increasing the dose of quetiapine (Seroquel) above the recommended 300 mg/day in an effort to boost efficacy is problematic. Patients become extremely sedated. The CEQUEL (Comparative Evaluation of Quetiapine plus Lamotrigine) investigators decided to examine combination therapy with two drugs having completely different mechanisms of action.
A total of 202 patients with bipolar depression at 27 U.K. sites were placed on quetiapine and randomized double-blind to lamotrigine or placebo. Lamotrigine (Lamictal) was started at 25 mg/day and gradually increased to 200 mg daily. Because there is some limited evidence that folic acid is effective in unipolar depression, the investigators employed a 2x2 factorial design, further randomizing participants to 500 mcg/day of folic acid or placebo.
The primary endpoint was improvement in depressive symptoms at 12 weeks, with 52-week outcomes as a secondary endpoint. The group on combination therapy with quetiapine and lamotrigine showed significantly greater improvement both on mean depressive symptoms as measured with the Quick Inventory of Depressive Symptomatology-self report version 16 (QIDS-SR16), and on rates of remission at 12 and 52 weeks, compared with patients on quetiapine and placebo. The combination also reduced the risk of relapse in patients with bipolar I disorder with predominantly depressive symptoms.
Folic acid proved to be not only ineffective, it actually interfered with lamotrigine’s effectiveness. The mean reduction from baseline to 12 weeks on the QIDS-SR16 was 4.4 points in patients who received lamotrigine without folic acid, compared with a 0.12-point increase in those receiving lamotrigine plus folic acid (Lancet Psychiatry. 2016 Jan;3[1]:31-9).
Dr. Vieta wasn’t involved in CEQUEL, but he considers the study one of the highlights of the year in the field of affective disorders and urged his colleagues to consider lamotrigine/quetiapine combination therapy for bipolar depression.
“Quetiapine and lamotrigine are both now generic drugs. CEQUEL showed both are superior to quetiapine alone. It’s good news – a not-expensive treatment combining quetiapine with a drug, lamotrigine, that’s quite well tolerated,” he said.
CEQUEL was funded by the Medical Research Council. Dr. Vieta reported having no financial conflicts of interest.
VIENNA – Psychiatrists have received a welcome gift in the form of CEQUEL, a randomized trial that offers much needed guidance on how to proceed when quetiapine for bipolar depression isn’t achieving sufficient response, Eduard Vieta, MD, said at the annual congress of the European College of Neuropsychopharmacology.
The answer provided by CEQUEL is to add lamotrigine.
“Quetiapine is indicated for treatment of symptoms of bipolar depression, but many clinicians – including myself – are a bit underwhelmed about the practical results. In the clinical trials, quetiapine looks great, but when we use it in practice we’re not so happy with the results. The trials are consistently positive with a large effect size, whereas in clinical practice many patients respond only partially to quetiapine. The question has been what to do next,” observed Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Increasing the dose of quetiapine (Seroquel) above the recommended 300 mg/day in an effort to boost efficacy is problematic. Patients become extremely sedated. The CEQUEL (Comparative Evaluation of Quetiapine plus Lamotrigine) investigators decided to examine combination therapy with two drugs having completely different mechanisms of action.
A total of 202 patients with bipolar depression at 27 U.K. sites were placed on quetiapine and randomized double-blind to lamotrigine or placebo. Lamotrigine (Lamictal) was started at 25 mg/day and gradually increased to 200 mg daily. Because there is some limited evidence that folic acid is effective in unipolar depression, the investigators employed a 2x2 factorial design, further randomizing participants to 500 mcg/day of folic acid or placebo.
The primary endpoint was improvement in depressive symptoms at 12 weeks, with 52-week outcomes as a secondary endpoint. The group on combination therapy with quetiapine and lamotrigine showed significantly greater improvement both on mean depressive symptoms as measured with the Quick Inventory of Depressive Symptomatology-self report version 16 (QIDS-SR16), and on rates of remission at 12 and 52 weeks, compared with patients on quetiapine and placebo. The combination also reduced the risk of relapse in patients with bipolar I disorder with predominantly depressive symptoms.
Folic acid proved to be not only ineffective, it actually interfered with lamotrigine’s effectiveness. The mean reduction from baseline to 12 weeks on the QIDS-SR16 was 4.4 points in patients who received lamotrigine without folic acid, compared with a 0.12-point increase in those receiving lamotrigine plus folic acid (Lancet Psychiatry. 2016 Jan;3[1]:31-9).
Dr. Vieta wasn’t involved in CEQUEL, but he considers the study one of the highlights of the year in the field of affective disorders and urged his colleagues to consider lamotrigine/quetiapine combination therapy for bipolar depression.
“Quetiapine and lamotrigine are both now generic drugs. CEQUEL showed both are superior to quetiapine alone. It’s good news – a not-expensive treatment combining quetiapine with a drug, lamotrigine, that’s quite well tolerated,” he said.
CEQUEL was funded by the Medical Research Council. Dr. Vieta reported having no financial conflicts of interest.
Ketamine curbs suicidal ideation independent of antidepressant effect
VIENNA – Repeated infusions of ketamine appear to have a direct effect in quelling suicidal ideation independent of the drug’s impact on depressive symptoms, Pierre Blier, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“Our preliminary results indicate that ketamine reduced suicidal ideation even in patients who failed to show a reduction in depression severity with repeated infusions,” observed Dr. Blier, professor of psychiatry at the University of Ottawa.
If the findings from this proof-of-concept study are confirmed, it would be very good news indeed.
“Suicidal ideation often requires rapid intervention, but there are very few treatments that are effective in reducing suicidal ideation, and none are fast acting,” the psychiatrist noted.
Therapeutic interest in ketamine is running high because subanesthetic doses of the drug have been shown to reduce depressive symptoms within a matter of hours, albeit transiently, in patients with treatment-resistant depression. If the drug acts as quickly in decreasing suicidal ideation as it does for depression, it would have major implications for clinical practice. An estimated 1 million people die each year worldwide from suicide, and two-thirds of them have major depressive disorder.
Dr. Blier reported on 27 patients with treatment-resistant depression, defined as failure to respond to at least two antidepressant medications from different pharmacologic classes at adequate dosages for at least 6 weeks each, along with two augmentation strategies. Their mean baseline score on the Montgomery-Asberg Depression Rating Scale (MADRS) was roughly 35. Five of the 27 had made one or more prior suicide attempts. Twenty-six of the 27 had suicidal ideation at baseline as defined by a MADRS suicidal ideation score (MADRS-SI) of more than 0. The MADRS-SI is based upon item 10 on the MADRS and is scored 0-6. Eight patients reported marked suicidal ideation as defined by a baseline MADRS-SI score of 4 or higher.
Study participants received a total of seven intravenous infusions of ketamine over roughly 3 weeks. Patients were retested 2-3 days after the seventh infusion, at which time 16 patients were categorized as treatment responders on the basis of at least a 50% reduction in their total MADRS score. Indeed, they averaged a 20.7-point reduction in the non–suicide-related MADRS score – that is, items 1-9, which reflect symptoms of depression – compared with a mere 3.9-point decrease in the 11 ketamine nonresponders. This 59% response rate for depressive symptoms is consistent with reported results of other studies of ketamine in patients with treatment-resistant depression, according to Dr. Blier, also professor of cellular/molecular medicines at the university.
The baseline MADRS-SI score averaged 2.9 in treatment responders and was similar at 2.5 in the nonresponders. But after seven infusions of ketamine, 21 of 27 patients had a MADRS-SI score of 0 or 1. The key study finding was that both the ketamine responders and nonresponders in terms of depressive symptoms experienced significant reductions in suicidal ideation. The 16 responders averaged a 2.4-point decrease from baseline in MADRS-SI scores, while the 11 nonresponders averaged a 1.5-point reduction. The magnitude of the reduction in MADRS-SI was significantly greater in the antidepressant responders than the nonresponders, but the improvement in suicidal ideation was statistically significant and clinically meaningful in both groups.
Seven of the 8 patients with baseline marked suicidal ideation and a MADRS-SI of 4 or greater improved to a score of 2 or less after their seven ketamine infusions, meaning they were having at most only fleeting suicidal thoughts.
Dr. Blier and coinvestigators are now expanding the ketamine study, enrolling up to 40 additional patients with treatment-resistant depression and suicidality in order to confirm the preliminary findings.
Dr. Blier reported having no financial conflicts of interest regarding this study, which was funded by the Canadian Institutes of Health Research. He noted that he receives research grants from and/or serves on advisory boards for more than a dozen pharmaceutical companies.
VIENNA – Repeated infusions of ketamine appear to have a direct effect in quelling suicidal ideation independent of the drug’s impact on depressive symptoms, Pierre Blier, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“Our preliminary results indicate that ketamine reduced suicidal ideation even in patients who failed to show a reduction in depression severity with repeated infusions,” observed Dr. Blier, professor of psychiatry at the University of Ottawa.
If the findings from this proof-of-concept study are confirmed, it would be very good news indeed.
“Suicidal ideation often requires rapid intervention, but there are very few treatments that are effective in reducing suicidal ideation, and none are fast acting,” the psychiatrist noted.
Therapeutic interest in ketamine is running high because subanesthetic doses of the drug have been shown to reduce depressive symptoms within a matter of hours, albeit transiently, in patients with treatment-resistant depression. If the drug acts as quickly in decreasing suicidal ideation as it does for depression, it would have major implications for clinical practice. An estimated 1 million people die each year worldwide from suicide, and two-thirds of them have major depressive disorder.
Dr. Blier reported on 27 patients with treatment-resistant depression, defined as failure to respond to at least two antidepressant medications from different pharmacologic classes at adequate dosages for at least 6 weeks each, along with two augmentation strategies. Their mean baseline score on the Montgomery-Asberg Depression Rating Scale (MADRS) was roughly 35. Five of the 27 had made one or more prior suicide attempts. Twenty-six of the 27 had suicidal ideation at baseline as defined by a MADRS suicidal ideation score (MADRS-SI) of more than 0. The MADRS-SI is based upon item 10 on the MADRS and is scored 0-6. Eight patients reported marked suicidal ideation as defined by a baseline MADRS-SI score of 4 or higher.
Study participants received a total of seven intravenous infusions of ketamine over roughly 3 weeks. Patients were retested 2-3 days after the seventh infusion, at which time 16 patients were categorized as treatment responders on the basis of at least a 50% reduction in their total MADRS score. Indeed, they averaged a 20.7-point reduction in the non–suicide-related MADRS score – that is, items 1-9, which reflect symptoms of depression – compared with a mere 3.9-point decrease in the 11 ketamine nonresponders. This 59% response rate for depressive symptoms is consistent with reported results of other studies of ketamine in patients with treatment-resistant depression, according to Dr. Blier, also professor of cellular/molecular medicines at the university.
The baseline MADRS-SI score averaged 2.9 in treatment responders and was similar at 2.5 in the nonresponders. But after seven infusions of ketamine, 21 of 27 patients had a MADRS-SI score of 0 or 1. The key study finding was that both the ketamine responders and nonresponders in terms of depressive symptoms experienced significant reductions in suicidal ideation. The 16 responders averaged a 2.4-point decrease from baseline in MADRS-SI scores, while the 11 nonresponders averaged a 1.5-point reduction. The magnitude of the reduction in MADRS-SI was significantly greater in the antidepressant responders than the nonresponders, but the improvement in suicidal ideation was statistically significant and clinically meaningful in both groups.
Seven of the 8 patients with baseline marked suicidal ideation and a MADRS-SI of 4 or greater improved to a score of 2 or less after their seven ketamine infusions, meaning they were having at most only fleeting suicidal thoughts.
Dr. Blier and coinvestigators are now expanding the ketamine study, enrolling up to 40 additional patients with treatment-resistant depression and suicidality in order to confirm the preliminary findings.
Dr. Blier reported having no financial conflicts of interest regarding this study, which was funded by the Canadian Institutes of Health Research. He noted that he receives research grants from and/or serves on advisory boards for more than a dozen pharmaceutical companies.
VIENNA – Repeated infusions of ketamine appear to have a direct effect in quelling suicidal ideation independent of the drug’s impact on depressive symptoms, Pierre Blier, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“Our preliminary results indicate that ketamine reduced suicidal ideation even in patients who failed to show a reduction in depression severity with repeated infusions,” observed Dr. Blier, professor of psychiatry at the University of Ottawa.
If the findings from this proof-of-concept study are confirmed, it would be very good news indeed.
“Suicidal ideation often requires rapid intervention, but there are very few treatments that are effective in reducing suicidal ideation, and none are fast acting,” the psychiatrist noted.
Therapeutic interest in ketamine is running high because subanesthetic doses of the drug have been shown to reduce depressive symptoms within a matter of hours, albeit transiently, in patients with treatment-resistant depression. If the drug acts as quickly in decreasing suicidal ideation as it does for depression, it would have major implications for clinical practice. An estimated 1 million people die each year worldwide from suicide, and two-thirds of them have major depressive disorder.
Dr. Blier reported on 27 patients with treatment-resistant depression, defined as failure to respond to at least two antidepressant medications from different pharmacologic classes at adequate dosages for at least 6 weeks each, along with two augmentation strategies. Their mean baseline score on the Montgomery-Asberg Depression Rating Scale (MADRS) was roughly 35. Five of the 27 had made one or more prior suicide attempts. Twenty-six of the 27 had suicidal ideation at baseline as defined by a MADRS suicidal ideation score (MADRS-SI) of more than 0. The MADRS-SI is based upon item 10 on the MADRS and is scored 0-6. Eight patients reported marked suicidal ideation as defined by a baseline MADRS-SI score of 4 or higher.
Study participants received a total of seven intravenous infusions of ketamine over roughly 3 weeks. Patients were retested 2-3 days after the seventh infusion, at which time 16 patients were categorized as treatment responders on the basis of at least a 50% reduction in their total MADRS score. Indeed, they averaged a 20.7-point reduction in the non–suicide-related MADRS score – that is, items 1-9, which reflect symptoms of depression – compared with a mere 3.9-point decrease in the 11 ketamine nonresponders. This 59% response rate for depressive symptoms is consistent with reported results of other studies of ketamine in patients with treatment-resistant depression, according to Dr. Blier, also professor of cellular/molecular medicines at the university.
The baseline MADRS-SI score averaged 2.9 in treatment responders and was similar at 2.5 in the nonresponders. But after seven infusions of ketamine, 21 of 27 patients had a MADRS-SI score of 0 or 1. The key study finding was that both the ketamine responders and nonresponders in terms of depressive symptoms experienced significant reductions in suicidal ideation. The 16 responders averaged a 2.4-point decrease from baseline in MADRS-SI scores, while the 11 nonresponders averaged a 1.5-point reduction. The magnitude of the reduction in MADRS-SI was significantly greater in the antidepressant responders than the nonresponders, but the improvement in suicidal ideation was statistically significant and clinically meaningful in both groups.
Seven of the 8 patients with baseline marked suicidal ideation and a MADRS-SI of 4 or greater improved to a score of 2 or less after their seven ketamine infusions, meaning they were having at most only fleeting suicidal thoughts.
Dr. Blier and coinvestigators are now expanding the ketamine study, enrolling up to 40 additional patients with treatment-resistant depression and suicidality in order to confirm the preliminary findings.
Dr. Blier reported having no financial conflicts of interest regarding this study, which was funded by the Canadian Institutes of Health Research. He noted that he receives research grants from and/or serves on advisory boards for more than a dozen pharmaceutical companies.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Ketamine infusions significantly reduced suicidal ideation even in patients whose treatment-resistant depression did not respond to the drug.
Data source: This ongoing observational study included 27 patients with treatment-resistant depression who received seven intravenous infusions of ketamine over roughly a 3-week period.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study, which was funded by the Canadian Institutes of Health Research.
Therapy, methylphenidate combo proves best treatment of adult ADHD
VIENNA – One-on-one counseling and manualized cognitive-behavioral therapy are equally effective as adjuncts to methylphenidate in the treatment of adult ADHD, Jan K. Buitelaar, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
He highlighted this finding from what he considers a well-conducted German randomized, double-blind, multicenter clinical trial in his discussion of recent major developments in the field of adult attention-deficit/hyperactivity disorder. He singled out the study because it provides clinicians with an evidence-based approach to treatment of this common disorder: namely, psychotherapy plus medication is better than either alone, and it doesn’t matter whether the psychotherapy takes the form of individual clinical behavioral counseling or a structured group cognitive-behavioral therapy (CBT) program tailored specifically for adult ADHD.
Investigators in the German COMPAS (Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study) Consortium randomized 419 18- to 58-year-old outpatients with ADHD to one of four treatment arms: group CBT plus methylphenidate or placebo, or nonspecific individual counseling plus methylphenidate or placebo. Psychotherapy sessions were conducted once weekly for the first 3 months and monthly for the next 9 months. The group CBT emphasized building self-esteem, coping skills, and acceptance. Methylphenidate was started at 10 mg per day and titrated over 6 weeks to 60 mg per day or a maximum of 1.2 mg/kg. The medication portion of the trial was conducted double-blind.
The primary outcome was the change in the ADHD Index of the Conners’ Adult ADHD Rating Scale as assessed by blinded observers from baseline to the end of the initial 3-month intensive treatment. The ADHD Index improved significantly from a mean baseline score of 20.6 to 17.6 in the group therapy arms and 16.5 with individual counseling, with no significant difference between the groups. Methylphenidate proved superior to placebo: those in the groups that received the medication in addition to their psychological intervention had a mean 3-month ADHD Index score that was 1.7 points lower than those on placebo (JAMA Psychiatry. 2015 Dec;72[12]:1199-210).
Regarding secondary outcomes, the ADHD Index results remained stable at the study’s conclusion at 12 months. However, there were no significant changes in self-rated depression scores over time. Blinded observers rated the group CBT patients as showing significantly greater improvement on the Clinical Global Impression Scale of Effectiveness.
The COMPAS trial was funded by the German Federal Ministry of Research and Education.
“This study provides real guidance for those of us who treat adult ADHD. The critical question here, of course, is what is the longer-term effect,” Dr. Buitelaar said.
He reported having no financial conflicts of interest regarding his presentation.
VIENNA – One-on-one counseling and manualized cognitive-behavioral therapy are equally effective as adjuncts to methylphenidate in the treatment of adult ADHD, Jan K. Buitelaar, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
He highlighted this finding from what he considers a well-conducted German randomized, double-blind, multicenter clinical trial in his discussion of recent major developments in the field of adult attention-deficit/hyperactivity disorder. He singled out the study because it provides clinicians with an evidence-based approach to treatment of this common disorder: namely, psychotherapy plus medication is better than either alone, and it doesn’t matter whether the psychotherapy takes the form of individual clinical behavioral counseling or a structured group cognitive-behavioral therapy (CBT) program tailored specifically for adult ADHD.
Investigators in the German COMPAS (Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study) Consortium randomized 419 18- to 58-year-old outpatients with ADHD to one of four treatment arms: group CBT plus methylphenidate or placebo, or nonspecific individual counseling plus methylphenidate or placebo. Psychotherapy sessions were conducted once weekly for the first 3 months and monthly for the next 9 months. The group CBT emphasized building self-esteem, coping skills, and acceptance. Methylphenidate was started at 10 mg per day and titrated over 6 weeks to 60 mg per day or a maximum of 1.2 mg/kg. The medication portion of the trial was conducted double-blind.
The primary outcome was the change in the ADHD Index of the Conners’ Adult ADHD Rating Scale as assessed by blinded observers from baseline to the end of the initial 3-month intensive treatment. The ADHD Index improved significantly from a mean baseline score of 20.6 to 17.6 in the group therapy arms and 16.5 with individual counseling, with no significant difference between the groups. Methylphenidate proved superior to placebo: those in the groups that received the medication in addition to their psychological intervention had a mean 3-month ADHD Index score that was 1.7 points lower than those on placebo (JAMA Psychiatry. 2015 Dec;72[12]:1199-210).
Regarding secondary outcomes, the ADHD Index results remained stable at the study’s conclusion at 12 months. However, there were no significant changes in self-rated depression scores over time. Blinded observers rated the group CBT patients as showing significantly greater improvement on the Clinical Global Impression Scale of Effectiveness.
The COMPAS trial was funded by the German Federal Ministry of Research and Education.
“This study provides real guidance for those of us who treat adult ADHD. The critical question here, of course, is what is the longer-term effect,” Dr. Buitelaar said.
He reported having no financial conflicts of interest regarding his presentation.
VIENNA – One-on-one counseling and manualized cognitive-behavioral therapy are equally effective as adjuncts to methylphenidate in the treatment of adult ADHD, Jan K. Buitelaar, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
He highlighted this finding from what he considers a well-conducted German randomized, double-blind, multicenter clinical trial in his discussion of recent major developments in the field of adult attention-deficit/hyperactivity disorder. He singled out the study because it provides clinicians with an evidence-based approach to treatment of this common disorder: namely, psychotherapy plus medication is better than either alone, and it doesn’t matter whether the psychotherapy takes the form of individual clinical behavioral counseling or a structured group cognitive-behavioral therapy (CBT) program tailored specifically for adult ADHD.
Investigators in the German COMPAS (Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study) Consortium randomized 419 18- to 58-year-old outpatients with ADHD to one of four treatment arms: group CBT plus methylphenidate or placebo, or nonspecific individual counseling plus methylphenidate or placebo. Psychotherapy sessions were conducted once weekly for the first 3 months and monthly for the next 9 months. The group CBT emphasized building self-esteem, coping skills, and acceptance. Methylphenidate was started at 10 mg per day and titrated over 6 weeks to 60 mg per day or a maximum of 1.2 mg/kg. The medication portion of the trial was conducted double-blind.
The primary outcome was the change in the ADHD Index of the Conners’ Adult ADHD Rating Scale as assessed by blinded observers from baseline to the end of the initial 3-month intensive treatment. The ADHD Index improved significantly from a mean baseline score of 20.6 to 17.6 in the group therapy arms and 16.5 with individual counseling, with no significant difference between the groups. Methylphenidate proved superior to placebo: those in the groups that received the medication in addition to their psychological intervention had a mean 3-month ADHD Index score that was 1.7 points lower than those on placebo (JAMA Psychiatry. 2015 Dec;72[12]:1199-210).
Regarding secondary outcomes, the ADHD Index results remained stable at the study’s conclusion at 12 months. However, there were no significant changes in self-rated depression scores over time. Blinded observers rated the group CBT patients as showing significantly greater improvement on the Clinical Global Impression Scale of Effectiveness.
The COMPAS trial was funded by the German Federal Ministry of Research and Education.
“This study provides real guidance for those of us who treat adult ADHD. The critical question here, of course, is what is the longer-term effect,” Dr. Buitelaar said.
He reported having no financial conflicts of interest regarding his presentation.
AT THE ECNP
Key clinical point:
Major finding: ADHD Index scores in patients with adult ADHD improved to a similar extent in response to methylphenidate plus either nonspecific individual counseling or structured group cognitive-behavioral therapy.
Data source: A randomized, multicenter, 12-month, four-arm clinical trial of 419 patients with adult ADHD.
Disclosures: The presenter reported having no financial conflicts of interest.
Modafinil improves cognitive impairment in remitted depression
VIENNA – Modafinil shows potential for the treatment of episodic and working memory dysfunction in patients with remitted depression, Muzaffer Kaser, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented a randomized, double-blind, placebo-controlled proof-of-concept study in which 60 patients with remitted depression undertook a battery of cognitive tests before and 2 hours after either a single 200-mg dose of modafinil or placebo.
“For episodic memory we saw a medium-to-large effect size for modafinil’s effects over placebo, and a medium effect size for working memory. So for administration of a single dose we think these results are quite promising,” said Dr. Kaser, a psychiatrist and PhD candidate at the University of Cambridge, England.
Longer-term studies of modafinil are planned in light of the major unmet need for treatments to address cognitive dysfunction in depression, he added in an interview. Currently, there are none. Yet it is now recognized that cognitive dysfunction in the domains of memory, attention, and planning are a core feature of depression, and they tend to persist after mood symptoms have recovered.
“Because of the cognitive dysfunction, people have difficulty getting back to work at the same pre-illness level, which creates more stress and increases the potential for future relapse. So cognitive dysfunction in depression should be a target for treatment,” the psychiatrist said.
Modafinil’s well-established safety profile makes it an attractive candidate, he continued. In placebo-controlled studies of the drug as augmentation therapy for depression, modafinil’s side effects were at the placebo level.
In this proof-of-concept study, the payoff with a single dose of modafinil was confined to the domain of memory, where the effects were most evident at the most difficult stages of the tests. Most impressively, the modafinil group made half as many errors on the episodic memory test. However, the drug did not improve performance on tests of planning accuracy or sustained attention.
Study participants had a mean of 3.2 prior depressive episodes and were in their current remission for 8.2 months.
The tests were drawn from the Cambridge Neuropsychological Test Automated Battery. They included the Paired Associates Learning measure of episodic memory, Spatial Working Memory, the Stockings of Cambridge measure of planning and executive function, and the Rapid Visual Information Processing measure of sustained attention.
The study was funded by the U.K. Medical Research Council and the Wellcome Trust. Dr. Kaser reported having no financial conflicts of interest.
Correction, 12/15/16: An earlier version of this article misstated Dr. Kaser's name in the photo caption.
VIENNA – Modafinil shows potential for the treatment of episodic and working memory dysfunction in patients with remitted depression, Muzaffer Kaser, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented a randomized, double-blind, placebo-controlled proof-of-concept study in which 60 patients with remitted depression undertook a battery of cognitive tests before and 2 hours after either a single 200-mg dose of modafinil or placebo.
“For episodic memory we saw a medium-to-large effect size for modafinil’s effects over placebo, and a medium effect size for working memory. So for administration of a single dose we think these results are quite promising,” said Dr. Kaser, a psychiatrist and PhD candidate at the University of Cambridge, England.
Longer-term studies of modafinil are planned in light of the major unmet need for treatments to address cognitive dysfunction in depression, he added in an interview. Currently, there are none. Yet it is now recognized that cognitive dysfunction in the domains of memory, attention, and planning are a core feature of depression, and they tend to persist after mood symptoms have recovered.
“Because of the cognitive dysfunction, people have difficulty getting back to work at the same pre-illness level, which creates more stress and increases the potential for future relapse. So cognitive dysfunction in depression should be a target for treatment,” the psychiatrist said.
Modafinil’s well-established safety profile makes it an attractive candidate, he continued. In placebo-controlled studies of the drug as augmentation therapy for depression, modafinil’s side effects were at the placebo level.
In this proof-of-concept study, the payoff with a single dose of modafinil was confined to the domain of memory, where the effects were most evident at the most difficult stages of the tests. Most impressively, the modafinil group made half as many errors on the episodic memory test. However, the drug did not improve performance on tests of planning accuracy or sustained attention.
Study participants had a mean of 3.2 prior depressive episodes and were in their current remission for 8.2 months.
The tests were drawn from the Cambridge Neuropsychological Test Automated Battery. They included the Paired Associates Learning measure of episodic memory, Spatial Working Memory, the Stockings of Cambridge measure of planning and executive function, and the Rapid Visual Information Processing measure of sustained attention.
The study was funded by the U.K. Medical Research Council and the Wellcome Trust. Dr. Kaser reported having no financial conflicts of interest.
Correction, 12/15/16: An earlier version of this article misstated Dr. Kaser's name in the photo caption.
VIENNA – Modafinil shows potential for the treatment of episodic and working memory dysfunction in patients with remitted depression, Muzaffer Kaser, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented a randomized, double-blind, placebo-controlled proof-of-concept study in which 60 patients with remitted depression undertook a battery of cognitive tests before and 2 hours after either a single 200-mg dose of modafinil or placebo.
“For episodic memory we saw a medium-to-large effect size for modafinil’s effects over placebo, and a medium effect size for working memory. So for administration of a single dose we think these results are quite promising,” said Dr. Kaser, a psychiatrist and PhD candidate at the University of Cambridge, England.
Longer-term studies of modafinil are planned in light of the major unmet need for treatments to address cognitive dysfunction in depression, he added in an interview. Currently, there are none. Yet it is now recognized that cognitive dysfunction in the domains of memory, attention, and planning are a core feature of depression, and they tend to persist after mood symptoms have recovered.
“Because of the cognitive dysfunction, people have difficulty getting back to work at the same pre-illness level, which creates more stress and increases the potential for future relapse. So cognitive dysfunction in depression should be a target for treatment,” the psychiatrist said.
Modafinil’s well-established safety profile makes it an attractive candidate, he continued. In placebo-controlled studies of the drug as augmentation therapy for depression, modafinil’s side effects were at the placebo level.
In this proof-of-concept study, the payoff with a single dose of modafinil was confined to the domain of memory, where the effects were most evident at the most difficult stages of the tests. Most impressively, the modafinil group made half as many errors on the episodic memory test. However, the drug did not improve performance on tests of planning accuracy or sustained attention.
Study participants had a mean of 3.2 prior depressive episodes and were in their current remission for 8.2 months.
The tests were drawn from the Cambridge Neuropsychological Test Automated Battery. They included the Paired Associates Learning measure of episodic memory, Spatial Working Memory, the Stockings of Cambridge measure of planning and executive function, and the Rapid Visual Information Processing measure of sustained attention.
The study was funded by the U.K. Medical Research Council and the Wellcome Trust. Dr. Kaser reported having no financial conflicts of interest.
Correction, 12/15/16: An earlier version of this article misstated Dr. Kaser's name in the photo caption.
Key clinical point:
Major finding: Patients with remitted depression made half as many errors on a well-established measure of episodic memory 2 hours after taking a single dose of modafinil, compared with placebo.
Data source: This double-blind, randomized, placebo-controlled study included 60 adults with remitted depression who underwent cognitive testing before and 2 hours after taking a single 200-mg dose of modafinil or placebo.
Disclosures: The study was funded by the UK Medical Research Council and the Wellcome Trust. The presenter reported having no financial conflicts of interest.
Ketamine augmentation doesn’t boost ECT outcomes
VIENNA – Low-dose ketamine provided no benefit as adjunctive anesthesia for severely depressed patients undergoing electroconvulsive therapy in the randomized, multicenter U.K. Ketamine-ECT Study, Ian Anderson, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The hope was that ketamine would lessen the cognitive impairment that is a prominent side effect of ECT. It’s thought that this cognitive impairment results from treatment-induced excessive stimulation of glutamate receptors, and ketamine is a glutamate antagonist, explained Dr. Anderson of the University of Manchester (England).
He and his coinvestigators had also hypothesized that ketamine might result in more rapid improvement in depression in patients undergoing ECT, since a single intravenous infusion of the drug has been shown to produce an extremely rapid, albeit temporary, antidepressant effect. But this was not borne out in the Ketamine-ECT Study.
Dr. Anderson reported on 70 severely depressed patients who were randomized to ketamine at 0.5 mg/kg or saline as an adjunct to standard propofol anesthesia for their course of weekly ECT sessions at seven U.K. mental health centers.
The primary study endpoint was the delayed verbal recall score on the Hopkins Verbal Learning Test–Revised after four ECT sessions, which was midway through the full course of treatment. Blinded assessors found no significant difference between the ketamine and placebo groups then. Nor were significant differences evident at prespecified further assessments 1 and 4 months after conclusion of the treatment program.
Secondary outcomes comprised of cognitive measures of verbal fluency, and autobiographical, working, and visual memory also proved similar in the two study arms, as did assessments of quality of life, safety, and tolerability.
At the end of the full course of ECT, 39% of the ketamine group were categorized as being in remission based upon at least a 50% drop from their baseline score on the Montgomery-Åsberg Depression Rating Scale (MADRS) with a final score of 10 or less, as were 35% of controls. Forty-nine percent of the ketamine group and 60% of controls were categorized as treatment responders, meaning their MADRS score dropped by at least 50% but their final score was greater than 10.
No serious adverse reactions to ketamine occurred.
The study was funded by the United Kingdom's National Institute for Health Research and the Medical Research Council. Dr. Anderson reported having no relevant financial conflicts.
VIENNA – Low-dose ketamine provided no benefit as adjunctive anesthesia for severely depressed patients undergoing electroconvulsive therapy in the randomized, multicenter U.K. Ketamine-ECT Study, Ian Anderson, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The hope was that ketamine would lessen the cognitive impairment that is a prominent side effect of ECT. It’s thought that this cognitive impairment results from treatment-induced excessive stimulation of glutamate receptors, and ketamine is a glutamate antagonist, explained Dr. Anderson of the University of Manchester (England).
He and his coinvestigators had also hypothesized that ketamine might result in more rapid improvement in depression in patients undergoing ECT, since a single intravenous infusion of the drug has been shown to produce an extremely rapid, albeit temporary, antidepressant effect. But this was not borne out in the Ketamine-ECT Study.
Dr. Anderson reported on 70 severely depressed patients who were randomized to ketamine at 0.5 mg/kg or saline as an adjunct to standard propofol anesthesia for their course of weekly ECT sessions at seven U.K. mental health centers.
The primary study endpoint was the delayed verbal recall score on the Hopkins Verbal Learning Test–Revised after four ECT sessions, which was midway through the full course of treatment. Blinded assessors found no significant difference between the ketamine and placebo groups then. Nor were significant differences evident at prespecified further assessments 1 and 4 months after conclusion of the treatment program.
Secondary outcomes comprised of cognitive measures of verbal fluency, and autobiographical, working, and visual memory also proved similar in the two study arms, as did assessments of quality of life, safety, and tolerability.
At the end of the full course of ECT, 39% of the ketamine group were categorized as being in remission based upon at least a 50% drop from their baseline score on the Montgomery-Åsberg Depression Rating Scale (MADRS) with a final score of 10 or less, as were 35% of controls. Forty-nine percent of the ketamine group and 60% of controls were categorized as treatment responders, meaning their MADRS score dropped by at least 50% but their final score was greater than 10.
No serious adverse reactions to ketamine occurred.
The study was funded by the United Kingdom's National Institute for Health Research and the Medical Research Council. Dr. Anderson reported having no relevant financial conflicts.
VIENNA – Low-dose ketamine provided no benefit as adjunctive anesthesia for severely depressed patients undergoing electroconvulsive therapy in the randomized, multicenter U.K. Ketamine-ECT Study, Ian Anderson, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The hope was that ketamine would lessen the cognitive impairment that is a prominent side effect of ECT. It’s thought that this cognitive impairment results from treatment-induced excessive stimulation of glutamate receptors, and ketamine is a glutamate antagonist, explained Dr. Anderson of the University of Manchester (England).
He and his coinvestigators had also hypothesized that ketamine might result in more rapid improvement in depression in patients undergoing ECT, since a single intravenous infusion of the drug has been shown to produce an extremely rapid, albeit temporary, antidepressant effect. But this was not borne out in the Ketamine-ECT Study.
Dr. Anderson reported on 70 severely depressed patients who were randomized to ketamine at 0.5 mg/kg or saline as an adjunct to standard propofol anesthesia for their course of weekly ECT sessions at seven U.K. mental health centers.
The primary study endpoint was the delayed verbal recall score on the Hopkins Verbal Learning Test–Revised after four ECT sessions, which was midway through the full course of treatment. Blinded assessors found no significant difference between the ketamine and placebo groups then. Nor were significant differences evident at prespecified further assessments 1 and 4 months after conclusion of the treatment program.
Secondary outcomes comprised of cognitive measures of verbal fluency, and autobiographical, working, and visual memory also proved similar in the two study arms, as did assessments of quality of life, safety, and tolerability.
At the end of the full course of ECT, 39% of the ketamine group were categorized as being in remission based upon at least a 50% drop from their baseline score on the Montgomery-Åsberg Depression Rating Scale (MADRS) with a final score of 10 or less, as were 35% of controls. Forty-nine percent of the ketamine group and 60% of controls were categorized as treatment responders, meaning their MADRS score dropped by at least 50% but their final score was greater than 10.
No serious adverse reactions to ketamine occurred.
The study was funded by the United Kingdom's National Institute for Health Research and the Medical Research Council. Dr. Anderson reported having no relevant financial conflicts.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Cognitive impairment as measured by delayed verbal recall on the Hopkins Verbal Learning Task–Revised was not reduced by the use of ketamine as an adjunctive anesthetic agent for ECT.
Data source: This randomized, multicenter trial featuring blinded assessments included 70 severely depressed patients who received either ketamine or saline in addition to standard anesthesia during their ECT sessions.
Disclosures: The Ketamine-ECT Study was funded by the U.K. National Institute for Health Research and the Medical Research Council. The presenter reported having no conflicts of interest.
Cariprazine shows efficacy for schizophrenia’s negative symptoms
VIENNA – Cariprazine appears to improve predominant negative symptoms of schizophrenia as well as functional status in affected patients, István Bitter, MD, PhD, DSci reported at the annual congress of the European College of Neuropsychopharmacology.
Cariprazine, a dopamine D3/D2 receptor partial agonist, was approved in the United States in 2015 as Vraylar for adults with bipolar disorder or schizophrenia. The drug remains investigational in Europe.
“There is very clearly an unmet need for the treatment of deficit schizophrenia, or schizophrenia with predominant and persistent negative symptoms,” the psychiatrist observed.
The strong efficacy signal for cariprazine in patients with predominant negative symptoms was identified in a post hoc analysis of a randomized, double-blind, phase III head-to-head comparison of cariprazine versus risperidone (Risperdal) in 461 affected adults. Dr. Bitter was a coinvestigator in the 26-week multicenter European study, which was preceded by a 4-week lead-in phase in which participants were uptitrated to a target dose of cariprazine at 4.5 mg/day or risperidone at 4 mg/day.
The primary endpoint in the post hoc analysis was change from baseline on the Positive and Negative Symptoms Factor Score for Negative Symptoms (PANSS-FSNS). A significant difference in favor of cariprazine was seen by week 14, and the gap steadily enlarged thereafter through the remainder of the study. The difference was significant in five of the seven items on the PANSS-FSNS: blunted affect, emotional withdrawal, poor rapport, active social avoidance, and passive/apathetic social withdrawal. Cariprazine also outperformed risperidone on the other two elements of the scale – motor retardation, and lack of spontaneity and flow of conversation – but in those domains, the difference didn’t reach statistical significance.
Patients randomized to cariprazine also fared well on secondary endpoints. They displayed a significantly greater overall improvement in Marder factor scores over 26 weeks than the risperidone-treated group. This advantage was driven by a strong, statistically significant difference in the domain of difficulty in abstract thinking. However, the cariprazine group also showed nonsignificant trends for greater improvement in conceptual disorganization, mannerisms and posturing, disorientation, poor attention, and disturbed volition.
As evidence that cariprazine was exerting specific benefit on negative symptoms, Dr. Bitter cited the fact that the drug showed no significant difference from risperidone in change from baseline on the Marder factor scores for uncontrolled hostility/excitement and anxiety/depression, nor in the Calgary Depression Rating Scale for Schizophrenia.
Regulatory agencies in the United States and Europe have made it clear that, for a drug to obtain an indication for treatment of predominant negative symptoms of schizophrenia, it also has to show evidence of improved patient social function. The cariprazine group showed a significantly greater improvement on the PANSS-FSNS Personal and Social Performance Score. This advantage over risperidone-treated patients reached statistical significance at week 10 and broadened thereafter until study conclusion. Improvements in the subdomain scores for self-care, socially useful activities, and personal and social relationships led the way.
These positive results for cariprazine represent a sharp departure from the psychiatric field’s long-standing history of failed therapeutic attempts to improve negative symptoms. A comprehensive 2015 meta-analysis of all 168 randomized, placebo-controlled studies of various potential treatments for predominant negative symptoms of schizophrenia published through 2013 concluded that none of them provided evidence of clinically meaningful improvement (Schizophr Bull. 2015 Jul;41[4]:892-9).
Dr. Bitter observed that, since that discouraging meta-analysis, several additional novel agents for treatment of predominant negative symptoms of schizophrenia that showed “fantastic” promise in phase II studies subsequently went down in flames in advanced phase III trials. Among those were D-serine as an add-on to second-generation antipsychotics; encenicline, an alpha-7 nicotinic acetylcholine receptor antagonist; pomaglumetad methionil, a selective agonist for glutamate receptor subtypes mGluR2 and mGluR3; and bitopertin, a glycine reuptake inhibitor.
“This is basically a very negative message, that there is not much we can do about negative symptoms. But depending on who you ask, you can get a little bit more optimistic picture,” Dr. Bitter said.
That’s because there are considerable differences among the studies both in how negative symptoms are defined as well as in the measurement tools employed. For example, the Scale for Assessment of Negative Symptoms (SANS) includes items which aren’t strictly speaking part of the negative symptoms concept, so it yields skewed results. The Brief Psychiatric Rating Scale is a relic that has been abandoned by younger psychiatrists. So at present, the PANS-FSNS is the best available tool, and a reasonable common sense definition of predominant negative symptoms of schizophrenia is that, in an affected patient, the PANSS negative subscale score is higher than the positive one, he continued.
It’s noteworthy that none of the multitude of failed drugs for negative symptoms of schizophrenia target activity of dopamine-3 receptors. But cariprazine does.
“There is hope that dopamine-3 receptors might play a role in schizophrenia, especially in negative symptoms. According to experts, they are much older by a couple of million years than the D2 receptors. They don’t operate like D2. In fact, their function is not very clear. In animal studies, though, they help with cognition,” Dr. Bitter said.
“These cariprazine results open the door to some hope for the future,” commented Dr. Ferrara, professor of psychiatry at the University of Naples, Italy.
Even so, she added in an interview, most clinicians aren’t ready to handle a drug with an indication for treatment of negative symptoms. “We are not ready as clinicians to rate negative symptoms in our patients, or in many, many cases to even recognize them. In my opinion, several promising drugs failed because of the fact that the evaluations were not very well carried out in spite of training, but also because, so far, the assessment instruments have not been the best you could think of. Clinicians need training, and the assessment instruments need to be refined. We need instruments that provide more in-depth evaluation of the different aspects of negative symptoms and that are also more appealing to clinicians,” according to the psychiatrist.
She agreed with Dr. Bitter that, for now, the PANSS-FSNS is the best available assessment tool.
“The SANS is not a good tool for negative symptoms. We are all sure about that. We can and should do better,” Dr. Ferrara said.
The cariprazine study was funded by Gedeon Richter and Allergen. Dr. Bitter reported serving as an advisory board member and/or consultant to Gedeon Richter and eight other pharmaceutical companies.
VIENNA – Cariprazine appears to improve predominant negative symptoms of schizophrenia as well as functional status in affected patients, István Bitter, MD, PhD, DSci reported at the annual congress of the European College of Neuropsychopharmacology.
Cariprazine, a dopamine D3/D2 receptor partial agonist, was approved in the United States in 2015 as Vraylar for adults with bipolar disorder or schizophrenia. The drug remains investigational in Europe.
“There is very clearly an unmet need for the treatment of deficit schizophrenia, or schizophrenia with predominant and persistent negative symptoms,” the psychiatrist observed.
The strong efficacy signal for cariprazine in patients with predominant negative symptoms was identified in a post hoc analysis of a randomized, double-blind, phase III head-to-head comparison of cariprazine versus risperidone (Risperdal) in 461 affected adults. Dr. Bitter was a coinvestigator in the 26-week multicenter European study, which was preceded by a 4-week lead-in phase in which participants were uptitrated to a target dose of cariprazine at 4.5 mg/day or risperidone at 4 mg/day.
The primary endpoint in the post hoc analysis was change from baseline on the Positive and Negative Symptoms Factor Score for Negative Symptoms (PANSS-FSNS). A significant difference in favor of cariprazine was seen by week 14, and the gap steadily enlarged thereafter through the remainder of the study. The difference was significant in five of the seven items on the PANSS-FSNS: blunted affect, emotional withdrawal, poor rapport, active social avoidance, and passive/apathetic social withdrawal. Cariprazine also outperformed risperidone on the other two elements of the scale – motor retardation, and lack of spontaneity and flow of conversation – but in those domains, the difference didn’t reach statistical significance.
Patients randomized to cariprazine also fared well on secondary endpoints. They displayed a significantly greater overall improvement in Marder factor scores over 26 weeks than the risperidone-treated group. This advantage was driven by a strong, statistically significant difference in the domain of difficulty in abstract thinking. However, the cariprazine group also showed nonsignificant trends for greater improvement in conceptual disorganization, mannerisms and posturing, disorientation, poor attention, and disturbed volition.
As evidence that cariprazine was exerting specific benefit on negative symptoms, Dr. Bitter cited the fact that the drug showed no significant difference from risperidone in change from baseline on the Marder factor scores for uncontrolled hostility/excitement and anxiety/depression, nor in the Calgary Depression Rating Scale for Schizophrenia.
Regulatory agencies in the United States and Europe have made it clear that, for a drug to obtain an indication for treatment of predominant negative symptoms of schizophrenia, it also has to show evidence of improved patient social function. The cariprazine group showed a significantly greater improvement on the PANSS-FSNS Personal and Social Performance Score. This advantage over risperidone-treated patients reached statistical significance at week 10 and broadened thereafter until study conclusion. Improvements in the subdomain scores for self-care, socially useful activities, and personal and social relationships led the way.
These positive results for cariprazine represent a sharp departure from the psychiatric field’s long-standing history of failed therapeutic attempts to improve negative symptoms. A comprehensive 2015 meta-analysis of all 168 randomized, placebo-controlled studies of various potential treatments for predominant negative symptoms of schizophrenia published through 2013 concluded that none of them provided evidence of clinically meaningful improvement (Schizophr Bull. 2015 Jul;41[4]:892-9).
Dr. Bitter observed that, since that discouraging meta-analysis, several additional novel agents for treatment of predominant negative symptoms of schizophrenia that showed “fantastic” promise in phase II studies subsequently went down in flames in advanced phase III trials. Among those were D-serine as an add-on to second-generation antipsychotics; encenicline, an alpha-7 nicotinic acetylcholine receptor antagonist; pomaglumetad methionil, a selective agonist for glutamate receptor subtypes mGluR2 and mGluR3; and bitopertin, a glycine reuptake inhibitor.
“This is basically a very negative message, that there is not much we can do about negative symptoms. But depending on who you ask, you can get a little bit more optimistic picture,” Dr. Bitter said.
That’s because there are considerable differences among the studies both in how negative symptoms are defined as well as in the measurement tools employed. For example, the Scale for Assessment of Negative Symptoms (SANS) includes items which aren’t strictly speaking part of the negative symptoms concept, so it yields skewed results. The Brief Psychiatric Rating Scale is a relic that has been abandoned by younger psychiatrists. So at present, the PANS-FSNS is the best available tool, and a reasonable common sense definition of predominant negative symptoms of schizophrenia is that, in an affected patient, the PANSS negative subscale score is higher than the positive one, he continued.
It’s noteworthy that none of the multitude of failed drugs for negative symptoms of schizophrenia target activity of dopamine-3 receptors. But cariprazine does.
“There is hope that dopamine-3 receptors might play a role in schizophrenia, especially in negative symptoms. According to experts, they are much older by a couple of million years than the D2 receptors. They don’t operate like D2. In fact, their function is not very clear. In animal studies, though, they help with cognition,” Dr. Bitter said.
“These cariprazine results open the door to some hope for the future,” commented Dr. Ferrara, professor of psychiatry at the University of Naples, Italy.
Even so, she added in an interview, most clinicians aren’t ready to handle a drug with an indication for treatment of negative symptoms. “We are not ready as clinicians to rate negative symptoms in our patients, or in many, many cases to even recognize them. In my opinion, several promising drugs failed because of the fact that the evaluations were not very well carried out in spite of training, but also because, so far, the assessment instruments have not been the best you could think of. Clinicians need training, and the assessment instruments need to be refined. We need instruments that provide more in-depth evaluation of the different aspects of negative symptoms and that are also more appealing to clinicians,” according to the psychiatrist.
She agreed with Dr. Bitter that, for now, the PANSS-FSNS is the best available assessment tool.
“The SANS is not a good tool for negative symptoms. We are all sure about that. We can and should do better,” Dr. Ferrara said.
The cariprazine study was funded by Gedeon Richter and Allergen. Dr. Bitter reported serving as an advisory board member and/or consultant to Gedeon Richter and eight other pharmaceutical companies.
VIENNA – Cariprazine appears to improve predominant negative symptoms of schizophrenia as well as functional status in affected patients, István Bitter, MD, PhD, DSci reported at the annual congress of the European College of Neuropsychopharmacology.
Cariprazine, a dopamine D3/D2 receptor partial agonist, was approved in the United States in 2015 as Vraylar for adults with bipolar disorder or schizophrenia. The drug remains investigational in Europe.
“There is very clearly an unmet need for the treatment of deficit schizophrenia, or schizophrenia with predominant and persistent negative symptoms,” the psychiatrist observed.
The strong efficacy signal for cariprazine in patients with predominant negative symptoms was identified in a post hoc analysis of a randomized, double-blind, phase III head-to-head comparison of cariprazine versus risperidone (Risperdal) in 461 affected adults. Dr. Bitter was a coinvestigator in the 26-week multicenter European study, which was preceded by a 4-week lead-in phase in which participants were uptitrated to a target dose of cariprazine at 4.5 mg/day or risperidone at 4 mg/day.
The primary endpoint in the post hoc analysis was change from baseline on the Positive and Negative Symptoms Factor Score for Negative Symptoms (PANSS-FSNS). A significant difference in favor of cariprazine was seen by week 14, and the gap steadily enlarged thereafter through the remainder of the study. The difference was significant in five of the seven items on the PANSS-FSNS: blunted affect, emotional withdrawal, poor rapport, active social avoidance, and passive/apathetic social withdrawal. Cariprazine also outperformed risperidone on the other two elements of the scale – motor retardation, and lack of spontaneity and flow of conversation – but in those domains, the difference didn’t reach statistical significance.
Patients randomized to cariprazine also fared well on secondary endpoints. They displayed a significantly greater overall improvement in Marder factor scores over 26 weeks than the risperidone-treated group. This advantage was driven by a strong, statistically significant difference in the domain of difficulty in abstract thinking. However, the cariprazine group also showed nonsignificant trends for greater improvement in conceptual disorganization, mannerisms and posturing, disorientation, poor attention, and disturbed volition.
As evidence that cariprazine was exerting specific benefit on negative symptoms, Dr. Bitter cited the fact that the drug showed no significant difference from risperidone in change from baseline on the Marder factor scores for uncontrolled hostility/excitement and anxiety/depression, nor in the Calgary Depression Rating Scale for Schizophrenia.
Regulatory agencies in the United States and Europe have made it clear that, for a drug to obtain an indication for treatment of predominant negative symptoms of schizophrenia, it also has to show evidence of improved patient social function. The cariprazine group showed a significantly greater improvement on the PANSS-FSNS Personal and Social Performance Score. This advantage over risperidone-treated patients reached statistical significance at week 10 and broadened thereafter until study conclusion. Improvements in the subdomain scores for self-care, socially useful activities, and personal and social relationships led the way.
These positive results for cariprazine represent a sharp departure from the psychiatric field’s long-standing history of failed therapeutic attempts to improve negative symptoms. A comprehensive 2015 meta-analysis of all 168 randomized, placebo-controlled studies of various potential treatments for predominant negative symptoms of schizophrenia published through 2013 concluded that none of them provided evidence of clinically meaningful improvement (Schizophr Bull. 2015 Jul;41[4]:892-9).
Dr. Bitter observed that, since that discouraging meta-analysis, several additional novel agents for treatment of predominant negative symptoms of schizophrenia that showed “fantastic” promise in phase II studies subsequently went down in flames in advanced phase III trials. Among those were D-serine as an add-on to second-generation antipsychotics; encenicline, an alpha-7 nicotinic acetylcholine receptor antagonist; pomaglumetad methionil, a selective agonist for glutamate receptor subtypes mGluR2 and mGluR3; and bitopertin, a glycine reuptake inhibitor.
“This is basically a very negative message, that there is not much we can do about negative symptoms. But depending on who you ask, you can get a little bit more optimistic picture,” Dr. Bitter said.
That’s because there are considerable differences among the studies both in how negative symptoms are defined as well as in the measurement tools employed. For example, the Scale for Assessment of Negative Symptoms (SANS) includes items which aren’t strictly speaking part of the negative symptoms concept, so it yields skewed results. The Brief Psychiatric Rating Scale is a relic that has been abandoned by younger psychiatrists. So at present, the PANS-FSNS is the best available tool, and a reasonable common sense definition of predominant negative symptoms of schizophrenia is that, in an affected patient, the PANSS negative subscale score is higher than the positive one, he continued.
It’s noteworthy that none of the multitude of failed drugs for negative symptoms of schizophrenia target activity of dopamine-3 receptors. But cariprazine does.
“There is hope that dopamine-3 receptors might play a role in schizophrenia, especially in negative symptoms. According to experts, they are much older by a couple of million years than the D2 receptors. They don’t operate like D2. In fact, their function is not very clear. In animal studies, though, they help with cognition,” Dr. Bitter said.
“These cariprazine results open the door to some hope for the future,” commented Dr. Ferrara, professor of psychiatry at the University of Naples, Italy.
Even so, she added in an interview, most clinicians aren’t ready to handle a drug with an indication for treatment of negative symptoms. “We are not ready as clinicians to rate negative symptoms in our patients, or in many, many cases to even recognize them. In my opinion, several promising drugs failed because of the fact that the evaluations were not very well carried out in spite of training, but also because, so far, the assessment instruments have not been the best you could think of. Clinicians need training, and the assessment instruments need to be refined. We need instruments that provide more in-depth evaluation of the different aspects of negative symptoms and that are also more appealing to clinicians,” according to the psychiatrist.
She agreed with Dr. Bitter that, for now, the PANSS-FSNS is the best available assessment tool.
“The SANS is not a good tool for negative symptoms. We are all sure about that. We can and should do better,” Dr. Ferrara said.
The cariprazine study was funded by Gedeon Richter and Allergen. Dr. Bitter reported serving as an advisory board member and/or consultant to Gedeon Richter and eight other pharmaceutical companies.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Cariprazine resulted in significantly greater improvement than risperidone in negative symptoms of schizophrenia as well as in social functioning.
Data source: This was a post hoc analysis of a 28-week, randomized, multicenter, phase III head-to-head comparative trial of cariprazine versus risperidone in 461 schizophrenia patients with predominant negative symptoms.
Disclosures: The study was funded by Gedeon Richter and Allergen. The presenter reported serving as an advisory board member and/or consultant to Gedeon Richter and eight other pharmaceutical companies.
Early epilepsy increases risk of later comorbid ADHD in autism
VIENNA – Early-onset idiopathic epilepsy occurring before age 7 years nearly doubles the likelihood that a child with autism spectrum disorder will later develop comorbid attention-deficit/hyperactivity disorder, Johnny Downs, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Comorbid ADHD is common in the setting of autism spectrum disorder (ASD). In a search for risk factors for the comorbid condition, he and his coinvestigators reviewed the physical health records prior to age 7 years of 3,032 patients with ASD referred at ages 3-17 years to child and adolescent mental health services clinics serving South London.
“That’s information that often doesn’t make it into the clinical psychiatric record,” noted Dr. Downs, a child psychiatrist at King’s College London.
Half of the 3,032 subjects were diagnosed with ASD at age 6-12 years and another 39% at age 13-17. During 5 years of prospective follow-up after being diagnosed with ASD in this longitudinal observational study, 25.5% of patients were diagnosed with comorbid ADHD. Looking back through the early physical health records, 114 (3.76%) of study participants had experienced early-onset epilepsy before age 7 years.
This large sample size allowed for robust multivariate adjustment for potential confounders. In a multivariate analysis, ASD patients with a history of early-onset epilepsy were at a significant 1.75-fold increased risk for subsequent comorbid ADHD. The analysis was adjusted for family history of epilepsy, sociodemographic factors, intellectual disability, previous head injury, perinatal complications, central nervous system tumors, early meningitis, and other confounders.
“The take-home message would be if you’ve got social and communication difficulties in a young child appearing at the age of 5, 6, or 7 [years], and there’s a history of seizures, we are seeing from observational data that the child is at increased risk of ADHD over the age of 7,” Dr. Downs said in an interview.
Compared with white subjects with ASD, the risk of developing comorbid ADHD was reduced by 37% in black and by 52% in Asian patients with ASD.
He plans further studies aimed at determining whether conventional ADHD management strategies have the same risk/benefit ratios in children with ASD and comorbid ADHD as in those with ADHD alone.
Dr. Downs reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
VIENNA – Early-onset idiopathic epilepsy occurring before age 7 years nearly doubles the likelihood that a child with autism spectrum disorder will later develop comorbid attention-deficit/hyperactivity disorder, Johnny Downs, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Comorbid ADHD is common in the setting of autism spectrum disorder (ASD). In a search for risk factors for the comorbid condition, he and his coinvestigators reviewed the physical health records prior to age 7 years of 3,032 patients with ASD referred at ages 3-17 years to child and adolescent mental health services clinics serving South London.
“That’s information that often doesn’t make it into the clinical psychiatric record,” noted Dr. Downs, a child psychiatrist at King’s College London.
Half of the 3,032 subjects were diagnosed with ASD at age 6-12 years and another 39% at age 13-17. During 5 years of prospective follow-up after being diagnosed with ASD in this longitudinal observational study, 25.5% of patients were diagnosed with comorbid ADHD. Looking back through the early physical health records, 114 (3.76%) of study participants had experienced early-onset epilepsy before age 7 years.
This large sample size allowed for robust multivariate adjustment for potential confounders. In a multivariate analysis, ASD patients with a history of early-onset epilepsy were at a significant 1.75-fold increased risk for subsequent comorbid ADHD. The analysis was adjusted for family history of epilepsy, sociodemographic factors, intellectual disability, previous head injury, perinatal complications, central nervous system tumors, early meningitis, and other confounders.
“The take-home message would be if you’ve got social and communication difficulties in a young child appearing at the age of 5, 6, or 7 [years], and there’s a history of seizures, we are seeing from observational data that the child is at increased risk of ADHD over the age of 7,” Dr. Downs said in an interview.
Compared with white subjects with ASD, the risk of developing comorbid ADHD was reduced by 37% in black and by 52% in Asian patients with ASD.
He plans further studies aimed at determining whether conventional ADHD management strategies have the same risk/benefit ratios in children with ASD and comorbid ADHD as in those with ADHD alone.
Dr. Downs reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
VIENNA – Early-onset idiopathic epilepsy occurring before age 7 years nearly doubles the likelihood that a child with autism spectrum disorder will later develop comorbid attention-deficit/hyperactivity disorder, Johnny Downs, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Comorbid ADHD is common in the setting of autism spectrum disorder (ASD). In a search for risk factors for the comorbid condition, he and his coinvestigators reviewed the physical health records prior to age 7 years of 3,032 patients with ASD referred at ages 3-17 years to child and adolescent mental health services clinics serving South London.
“That’s information that often doesn’t make it into the clinical psychiatric record,” noted Dr. Downs, a child psychiatrist at King’s College London.
Half of the 3,032 subjects were diagnosed with ASD at age 6-12 years and another 39% at age 13-17. During 5 years of prospective follow-up after being diagnosed with ASD in this longitudinal observational study, 25.5% of patients were diagnosed with comorbid ADHD. Looking back through the early physical health records, 114 (3.76%) of study participants had experienced early-onset epilepsy before age 7 years.
This large sample size allowed for robust multivariate adjustment for potential confounders. In a multivariate analysis, ASD patients with a history of early-onset epilepsy were at a significant 1.75-fold increased risk for subsequent comorbid ADHD. The analysis was adjusted for family history of epilepsy, sociodemographic factors, intellectual disability, previous head injury, perinatal complications, central nervous system tumors, early meningitis, and other confounders.
“The take-home message would be if you’ve got social and communication difficulties in a young child appearing at the age of 5, 6, or 7 [years], and there’s a history of seizures, we are seeing from observational data that the child is at increased risk of ADHD over the age of 7,” Dr. Downs said in an interview.
Compared with white subjects with ASD, the risk of developing comorbid ADHD was reduced by 37% in black and by 52% in Asian patients with ASD.
He plans further studies aimed at determining whether conventional ADHD management strategies have the same risk/benefit ratios in children with ASD and comorbid ADHD as in those with ADHD alone.
Dr. Downs reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
AT THE ECNP CONGRESS
Key clinical point: Youths with autism spectrum disorder and a history of early-onset epilepsy before age 7 years are at an increased risk of subsequent comorbid ADHD.
Major finding: Youths with autism spectrum disorder who have a history of early-onset epilepsy before age 7 years are at 1.75-fold increased likelihood of subsequently developing comorbid ADHD.
Data source: This longitudinal study included 3,032 children and adolescents with autism spectrum disorder, 26% of whom developed ADHD during 5 years of prospective follow-up.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study, which was conducted free of commercial support.
Research yields fresh insights into ketamine for depression
VIENNA – The year 2016 has brought answers to two key questions regarding the off-label use of intravenous ketamine in patients with treatment-resistant depression: What’s the optimal dosing schedule? And what’s the likely mechanism of benefit?
Ketamine has generated enormous interest among psychiatrists and patients because the response is so dramatic, with marked improvement seen within hours in a much higher proportion of patients than respond to conventional antidepressants, which target the serotonergic system. But the benefits are not long lasting, and psychiatrists have wondered how often the treatment should be repeated. That question has been answered in a multicenter, double-blind U.S. randomized trial, Eduard Vieta, MD, PhD, noted at the annual congress of the European College of Neuropsychopharmacology.
Investigators randomized 67 patients with treatment-resistant depression to ketamine at 0.5 mg/kg of body weight at either two or three times per week, or to placebo. The mean reduction on the Montgomery-Åsberg Depression Rating Scale at day 15 was 18.4 points with twice-weekly therapy and similar at 17.7 with thrice-weekly therapy, both significantly better than with placebo (Am J Psychiatry. 2016 Aug 1;173[8]:816-26).
“It turns out that two and three times per week were equally effective, so obviously twice per week is enough,” said Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Ketamine’s approved indication is as an anesthetic agent. Its long-term safety as an antidepressant remains an open question. The drug has undesirable psychotropic side effects, including dissociation, but related compounds without those issues are speeding through the developmental pipeline. The Food and Drug Administration has granted Janssen Pharmaceuticals “fast track” and “breakthrough therapy” status for intranasal esketamine, the S(+) enantiomer of ketamine, which is now in phase III clinical trials for treatment-resistant depression as well as for depression with suicidal thoughts. The FDA reserves these designations for potential therapies addressing a major unmet need. Allergan has received the same designations from the FDA for its drug rapastinel, which also is now in phase III clinical trials.
“Ketamine is clearly not something to use as first-line therapy. I think there is a problem in certain places: I know in the U.S. there are now plenty of ketamine clinics administering the drug to first comers. That doesn’t make sense to me. But ketamine does open an important new avenue,” he said.
Dr. Vieta asserted that the future of new drug development for mood disorders lies in the glutamatergic system. However, a recent study by investigators at the National Institute of Mental Health – who pioneered the use of ketamine as an antidepressant – and colleagues at the University of Maryland, Baltimore, casts doubt upon the conventional wisdom that ketamine’s mechanism of benefit as an antidepressant involves N-methyl-d-aspartate receptor (NMDA) antagonism.
Instead, they reported, the antidepressant effect is actually exerted by a ketamine metabolite known as HNK, or (2S,6S;2R,6R)-hydroxynorketamine. And HNK’s antidepressant effect is not related to NMDA receptors, but is instead tied to activation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors. And in mice, at least, HNK lacks the unwelcome psychotomimetic side effects of ketamine (Nature. 2016 May 4;533[7604]:481-6).
“This is a very nice paper and very important. This opens up a new avenue in drug development, looking at agents that act on AMPA receptors to provide rapid relief of depressive symptoms in unipolar depression but probably also in bipolar depression,” said Dr. Vieta.
He reported receiving research grants from numerous pharmaceutical companies having an interest in treatments for mood disorders.
VIENNA – The year 2016 has brought answers to two key questions regarding the off-label use of intravenous ketamine in patients with treatment-resistant depression: What’s the optimal dosing schedule? And what’s the likely mechanism of benefit?
Ketamine has generated enormous interest among psychiatrists and patients because the response is so dramatic, with marked improvement seen within hours in a much higher proportion of patients than respond to conventional antidepressants, which target the serotonergic system. But the benefits are not long lasting, and psychiatrists have wondered how often the treatment should be repeated. That question has been answered in a multicenter, double-blind U.S. randomized trial, Eduard Vieta, MD, PhD, noted at the annual congress of the European College of Neuropsychopharmacology.
Investigators randomized 67 patients with treatment-resistant depression to ketamine at 0.5 mg/kg of body weight at either two or three times per week, or to placebo. The mean reduction on the Montgomery-Åsberg Depression Rating Scale at day 15 was 18.4 points with twice-weekly therapy and similar at 17.7 with thrice-weekly therapy, both significantly better than with placebo (Am J Psychiatry. 2016 Aug 1;173[8]:816-26).
“It turns out that two and three times per week were equally effective, so obviously twice per week is enough,” said Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Ketamine’s approved indication is as an anesthetic agent. Its long-term safety as an antidepressant remains an open question. The drug has undesirable psychotropic side effects, including dissociation, but related compounds without those issues are speeding through the developmental pipeline. The Food and Drug Administration has granted Janssen Pharmaceuticals “fast track” and “breakthrough therapy” status for intranasal esketamine, the S(+) enantiomer of ketamine, which is now in phase III clinical trials for treatment-resistant depression as well as for depression with suicidal thoughts. The FDA reserves these designations for potential therapies addressing a major unmet need. Allergan has received the same designations from the FDA for its drug rapastinel, which also is now in phase III clinical trials.
“Ketamine is clearly not something to use as first-line therapy. I think there is a problem in certain places: I know in the U.S. there are now plenty of ketamine clinics administering the drug to first comers. That doesn’t make sense to me. But ketamine does open an important new avenue,” he said.
Dr. Vieta asserted that the future of new drug development for mood disorders lies in the glutamatergic system. However, a recent study by investigators at the National Institute of Mental Health – who pioneered the use of ketamine as an antidepressant – and colleagues at the University of Maryland, Baltimore, casts doubt upon the conventional wisdom that ketamine’s mechanism of benefit as an antidepressant involves N-methyl-d-aspartate receptor (NMDA) antagonism.
Instead, they reported, the antidepressant effect is actually exerted by a ketamine metabolite known as HNK, or (2S,6S;2R,6R)-hydroxynorketamine. And HNK’s antidepressant effect is not related to NMDA receptors, but is instead tied to activation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors. And in mice, at least, HNK lacks the unwelcome psychotomimetic side effects of ketamine (Nature. 2016 May 4;533[7604]:481-6).
“This is a very nice paper and very important. This opens up a new avenue in drug development, looking at agents that act on AMPA receptors to provide rapid relief of depressive symptoms in unipolar depression but probably also in bipolar depression,” said Dr. Vieta.
He reported receiving research grants from numerous pharmaceutical companies having an interest in treatments for mood disorders.
VIENNA – The year 2016 has brought answers to two key questions regarding the off-label use of intravenous ketamine in patients with treatment-resistant depression: What’s the optimal dosing schedule? And what’s the likely mechanism of benefit?
Ketamine has generated enormous interest among psychiatrists and patients because the response is so dramatic, with marked improvement seen within hours in a much higher proportion of patients than respond to conventional antidepressants, which target the serotonergic system. But the benefits are not long lasting, and psychiatrists have wondered how often the treatment should be repeated. That question has been answered in a multicenter, double-blind U.S. randomized trial, Eduard Vieta, MD, PhD, noted at the annual congress of the European College of Neuropsychopharmacology.
Investigators randomized 67 patients with treatment-resistant depression to ketamine at 0.5 mg/kg of body weight at either two or three times per week, or to placebo. The mean reduction on the Montgomery-Åsberg Depression Rating Scale at day 15 was 18.4 points with twice-weekly therapy and similar at 17.7 with thrice-weekly therapy, both significantly better than with placebo (Am J Psychiatry. 2016 Aug 1;173[8]:816-26).
“It turns out that two and three times per week were equally effective, so obviously twice per week is enough,” said Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Ketamine’s approved indication is as an anesthetic agent. Its long-term safety as an antidepressant remains an open question. The drug has undesirable psychotropic side effects, including dissociation, but related compounds without those issues are speeding through the developmental pipeline. The Food and Drug Administration has granted Janssen Pharmaceuticals “fast track” and “breakthrough therapy” status for intranasal esketamine, the S(+) enantiomer of ketamine, which is now in phase III clinical trials for treatment-resistant depression as well as for depression with suicidal thoughts. The FDA reserves these designations for potential therapies addressing a major unmet need. Allergan has received the same designations from the FDA for its drug rapastinel, which also is now in phase III clinical trials.
“Ketamine is clearly not something to use as first-line therapy. I think there is a problem in certain places: I know in the U.S. there are now plenty of ketamine clinics administering the drug to first comers. That doesn’t make sense to me. But ketamine does open an important new avenue,” he said.
Dr. Vieta asserted that the future of new drug development for mood disorders lies in the glutamatergic system. However, a recent study by investigators at the National Institute of Mental Health – who pioneered the use of ketamine as an antidepressant – and colleagues at the University of Maryland, Baltimore, casts doubt upon the conventional wisdom that ketamine’s mechanism of benefit as an antidepressant involves N-methyl-d-aspartate receptor (NMDA) antagonism.
Instead, they reported, the antidepressant effect is actually exerted by a ketamine metabolite known as HNK, or (2S,6S;2R,6R)-hydroxynorketamine. And HNK’s antidepressant effect is not related to NMDA receptors, but is instead tied to activation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors. And in mice, at least, HNK lacks the unwelcome psychotomimetic side effects of ketamine (Nature. 2016 May 4;533[7604]:481-6).
“This is a very nice paper and very important. This opens up a new avenue in drug development, looking at agents that act on AMPA receptors to provide rapid relief of depressive symptoms in unipolar depression but probably also in bipolar depression,” said Dr. Vieta.
He reported receiving research grants from numerous pharmaceutical companies having an interest in treatments for mood disorders.
EXPERT ANALYSIS FROM THE ECNP CONGRESS