User login
Can Safety of Filler Injections Be Improved? Yes, They Cannula
ORLANDO – Short, fixed cannulas can deliver filler products to augment multiple areas of the face, including nasolabial folds, the dorsum of the nose, and under the eyes, according to Dr. Doris Hexsel.
The small caliber, short cannulas also offer greater precision for filler placement, compared with the longer, flexible cannulas currently on the market, said Dr. Hexsel of the department of dermatology at Pontificia Universidade Católica do Rio Grande (Brazil) do Sul.
Cannulas are disposable, can be attached to different syringe types, and can replace needles for most facial filler indications, Dr. Hexsel said. One notable exception is in the treatment of superficial lines or defects, where she said she still recommends the use of a needle.
Cannulas can deliver a wide range of filler products. "Anything we inject with needles we can also inject with cannulas," Dr. Hexsel said at the annual meeting of the Florida Society of Dermatologic Surgeons.
All cannulas with a rounded end typically cause less bruising and trauma, compared with needles. Cannulas also help to avoid other needle-related adverse events, Dr. Hexsel said. Perforation of the veins or arteries and accidental injection of fillers into vessels are the most serious examples.
Facial augmentation via cannula is "particularly useful for patients taking anticoagulants or who cannot bruise because they have a social event," Dr. Hexsel said. Reduction of the risk of a sharps injury is a plus for physicians, she added.
Cannulas cannot puncture the skin, so a needle stick is still required to make an entry hole. Proponents of long cannulas will point to a need for only one entry point, Dr. Hexsel said, but "a single orifice and use of a long cannula cannot reach all indications." Nasolabial folds, for example, require at least two entry points to treat.
Dr. Hexsel designed a cannula that she and her colleagues compared with a standard needle in a prospective, randomized, phase II bilateral study of 25 women (Dermatol. Surg. 2011 Oct. 19 [doi:10.1111/j.1524-4725.2011.02195.x]). The metallic cannula safely and effectively delivered hyaluronic acid for nasolabial fold augmentation and was associated with less pain, edema, hematoma and redness at the site, compared with the side treated with a needle. At day 3, the mean Modified Fitzpatrick Wrinkle Scale was comparable for both treated sides of the face (from 2.40 at baseline to 1.46 on the cannula-injected side and from 2.40 to 1.48 on the needle-injected side).
Another use for cannulas is to deliver filler products to correct any defects of the nasal dorsum after rhinoplasty, she said.
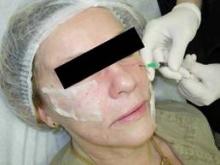
Cannulas can also deliver fillers to help improve the appearance of dark circles under the eyes. After Dr. Hexsel cleans and marks the area, she said she injects a small amount of lidocaine anesthetic. She said that she then makes a small hole with a regular needle and introduces the cannula. Only inject a small amount of filler product at a time, she said. "You can ask patients to participate. I can put in a little Restylane under the eyes, give the patient a mirror, and ask them where they want more." The patient leaves with nothing visible; a small micropore dressing can be placed over the entry points.
Dr. Hexsel disclosed holding design patents on cannula devices not yet available in the United States.
ORLANDO – Short, fixed cannulas can deliver filler products to augment multiple areas of the face, including nasolabial folds, the dorsum of the nose, and under the eyes, according to Dr. Doris Hexsel.
The small caliber, short cannulas also offer greater precision for filler placement, compared with the longer, flexible cannulas currently on the market, said Dr. Hexsel of the department of dermatology at Pontificia Universidade Católica do Rio Grande (Brazil) do Sul.
Cannulas are disposable, can be attached to different syringe types, and can replace needles for most facial filler indications, Dr. Hexsel said. One notable exception is in the treatment of superficial lines or defects, where she said she still recommends the use of a needle.
Cannulas can deliver a wide range of filler products. "Anything we inject with needles we can also inject with cannulas," Dr. Hexsel said at the annual meeting of the Florida Society of Dermatologic Surgeons.
All cannulas with a rounded end typically cause less bruising and trauma, compared with needles. Cannulas also help to avoid other needle-related adverse events, Dr. Hexsel said. Perforation of the veins or arteries and accidental injection of fillers into vessels are the most serious examples.
Facial augmentation via cannula is "particularly useful for patients taking anticoagulants or who cannot bruise because they have a social event," Dr. Hexsel said. Reduction of the risk of a sharps injury is a plus for physicians, she added.
Cannulas cannot puncture the skin, so a needle stick is still required to make an entry hole. Proponents of long cannulas will point to a need for only one entry point, Dr. Hexsel said, but "a single orifice and use of a long cannula cannot reach all indications." Nasolabial folds, for example, require at least two entry points to treat.
Dr. Hexsel designed a cannula that she and her colleagues compared with a standard needle in a prospective, randomized, phase II bilateral study of 25 women (Dermatol. Surg. 2011 Oct. 19 [doi:10.1111/j.1524-4725.2011.02195.x]). The metallic cannula safely and effectively delivered hyaluronic acid for nasolabial fold augmentation and was associated with less pain, edema, hematoma and redness at the site, compared with the side treated with a needle. At day 3, the mean Modified Fitzpatrick Wrinkle Scale was comparable for both treated sides of the face (from 2.40 at baseline to 1.46 on the cannula-injected side and from 2.40 to 1.48 on the needle-injected side).
Another use for cannulas is to deliver filler products to correct any defects of the nasal dorsum after rhinoplasty, she said.
Cannulas can also deliver fillers to help improve the appearance of dark circles under the eyes. After Dr. Hexsel cleans and marks the area, she said she injects a small amount of lidocaine anesthetic. She said that she then makes a small hole with a regular needle and introduces the cannula. Only inject a small amount of filler product at a time, she said. "You can ask patients to participate. I can put in a little Restylane under the eyes, give the patient a mirror, and ask them where they want more." The patient leaves with nothing visible; a small micropore dressing can be placed over the entry points.
Dr. Hexsel disclosed holding design patents on cannula devices not yet available in the United States.
ORLANDO – Short, fixed cannulas can deliver filler products to augment multiple areas of the face, including nasolabial folds, the dorsum of the nose, and under the eyes, according to Dr. Doris Hexsel.
The small caliber, short cannulas also offer greater precision for filler placement, compared with the longer, flexible cannulas currently on the market, said Dr. Hexsel of the department of dermatology at Pontificia Universidade Católica do Rio Grande (Brazil) do Sul.
Cannulas are disposable, can be attached to different syringe types, and can replace needles for most facial filler indications, Dr. Hexsel said. One notable exception is in the treatment of superficial lines or defects, where she said she still recommends the use of a needle.
Cannulas can deliver a wide range of filler products. "Anything we inject with needles we can also inject with cannulas," Dr. Hexsel said at the annual meeting of the Florida Society of Dermatologic Surgeons.
All cannulas with a rounded end typically cause less bruising and trauma, compared with needles. Cannulas also help to avoid other needle-related adverse events, Dr. Hexsel said. Perforation of the veins or arteries and accidental injection of fillers into vessels are the most serious examples.
Facial augmentation via cannula is "particularly useful for patients taking anticoagulants or who cannot bruise because they have a social event," Dr. Hexsel said. Reduction of the risk of a sharps injury is a plus for physicians, she added.
Cannulas cannot puncture the skin, so a needle stick is still required to make an entry hole. Proponents of long cannulas will point to a need for only one entry point, Dr. Hexsel said, but "a single orifice and use of a long cannula cannot reach all indications." Nasolabial folds, for example, require at least two entry points to treat.
Dr. Hexsel designed a cannula that she and her colleagues compared with a standard needle in a prospective, randomized, phase II bilateral study of 25 women (Dermatol. Surg. 2011 Oct. 19 [doi:10.1111/j.1524-4725.2011.02195.x]). The metallic cannula safely and effectively delivered hyaluronic acid for nasolabial fold augmentation and was associated with less pain, edema, hematoma and redness at the site, compared with the side treated with a needle. At day 3, the mean Modified Fitzpatrick Wrinkle Scale was comparable for both treated sides of the face (from 2.40 at baseline to 1.46 on the cannula-injected side and from 2.40 to 1.48 on the needle-injected side).
Another use for cannulas is to deliver filler products to correct any defects of the nasal dorsum after rhinoplasty, she said.
Cannulas can also deliver fillers to help improve the appearance of dark circles under the eyes. After Dr. Hexsel cleans and marks the area, she said she injects a small amount of lidocaine anesthetic. She said that she then makes a small hole with a regular needle and introduces the cannula. Only inject a small amount of filler product at a time, she said. "You can ask patients to participate. I can put in a little Restylane under the eyes, give the patient a mirror, and ask them where they want more." The patient leaves with nothing visible; a small micropore dressing can be placed over the entry points.
Dr. Hexsel disclosed holding design patents on cannula devices not yet available in the United States.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE FLORIDA SOCIETY OF DERMATOLOGIC SURGEONS
'Pinch Bleph' Within Cosmetic Dermatologists' Realm of Expertise
ORLANDO – Cosmetic dermatologists can easily perform a skin pinch blepharoplasty to remove excess skin from a patient’s lower eyelid, according to N. Fred Eaglstein, D.O.
"This is a very simple technique, especially for derm surgeons used to doing large skin flaps and grafts," said Dr. Eaglstein at the annual meeting of the Florida Society of Dermatologic surgeons. "I do all these procedures in my office."
The "pinch bleph" can be performed alone or in conjunction with laser resurfacing to improve the appearance of dermatochalasis and thin, wrinkled, sun-damaged lower eyelid skin, Dr. Eaglstein said. Not all patients are candidates, however. Because the technique removes only excess skin, it is not indicated for patients with lower, orbital, fat-pad protrusion.
Following a baseline ophthalmology examination, instruct the patient to discontinue aspirin, NSAIDs, and any herbal products that could prolong bleeding. Exclude or get clearance for patients with significant medical problems such as thyroid disease, Dr. Eaglstein said.
To determine how much skin to remove, pinch the lower eyelid skin together using blunt forceps until the eyelid margins start to evert. Then, mark the area with a fine tip gentian violet marker. He said that he crushes the excess skin using a curved hemostat and excises the tissue with Westcott or sharp iris scissors. He recommends 6-0 nylon sutures or 6-0 fast absorbing gut sutures to close the wound. "I use 6-0 fast absorbing."
"Don’t take too much skin if you plan to do laser resurfacing. You don’t want to get too much tightening [if you also plan to do] erbium laser resurfacing," said Dr. Eaglstein, a private practice dermatologist in Orange Park, Fla.
Expected complications include ecchymosis and edema. Less commonly, patients can experience hematoma, infection, scleral show, or ectropion.
"The pinch blepharoplasty is a simple, safe, and effective surgical procedure for the derm surgeon interested in providing cosmetic rejuvenation of the lower eyelid," Dr. Eaglstein said.
For more information, Dr. Eaglstein recommended a report by Joesph Niamtu III, D.M.D. on his lower eyelid blepharoplasty technique and experience (Cosmetic Derm. 2008;21:652-7).
He also recommended a report on a series of 77 candidates for traditional lower blepharoplasty who underwent a pinch blepharoplasty (Plast. Reconstr. Surg. 2005;115:1405-12). The author reported no significant scleral show or ectropion adverse events.
Dr. Eaglstein reported having no relevant conflicts of interest.
ORLANDO – Cosmetic dermatologists can easily perform a skin pinch blepharoplasty to remove excess skin from a patient’s lower eyelid, according to N. Fred Eaglstein, D.O.
"This is a very simple technique, especially for derm surgeons used to doing large skin flaps and grafts," said Dr. Eaglstein at the annual meeting of the Florida Society of Dermatologic surgeons. "I do all these procedures in my office."
The "pinch bleph" can be performed alone or in conjunction with laser resurfacing to improve the appearance of dermatochalasis and thin, wrinkled, sun-damaged lower eyelid skin, Dr. Eaglstein said. Not all patients are candidates, however. Because the technique removes only excess skin, it is not indicated for patients with lower, orbital, fat-pad protrusion.
Following a baseline ophthalmology examination, instruct the patient to discontinue aspirin, NSAIDs, and any herbal products that could prolong bleeding. Exclude or get clearance for patients with significant medical problems such as thyroid disease, Dr. Eaglstein said.
To determine how much skin to remove, pinch the lower eyelid skin together using blunt forceps until the eyelid margins start to evert. Then, mark the area with a fine tip gentian violet marker. He said that he crushes the excess skin using a curved hemostat and excises the tissue with Westcott or sharp iris scissors. He recommends 6-0 nylon sutures or 6-0 fast absorbing gut sutures to close the wound. "I use 6-0 fast absorbing."
"Don’t take too much skin if you plan to do laser resurfacing. You don’t want to get too much tightening [if you also plan to do] erbium laser resurfacing," said Dr. Eaglstein, a private practice dermatologist in Orange Park, Fla.
Expected complications include ecchymosis and edema. Less commonly, patients can experience hematoma, infection, scleral show, or ectropion.
"The pinch blepharoplasty is a simple, safe, and effective surgical procedure for the derm surgeon interested in providing cosmetic rejuvenation of the lower eyelid," Dr. Eaglstein said.
For more information, Dr. Eaglstein recommended a report by Joesph Niamtu III, D.M.D. on his lower eyelid blepharoplasty technique and experience (Cosmetic Derm. 2008;21:652-7).
He also recommended a report on a series of 77 candidates for traditional lower blepharoplasty who underwent a pinch blepharoplasty (Plast. Reconstr. Surg. 2005;115:1405-12). The author reported no significant scleral show or ectropion adverse events.
Dr. Eaglstein reported having no relevant conflicts of interest.
ORLANDO – Cosmetic dermatologists can easily perform a skin pinch blepharoplasty to remove excess skin from a patient’s lower eyelid, according to N. Fred Eaglstein, D.O.
"This is a very simple technique, especially for derm surgeons used to doing large skin flaps and grafts," said Dr. Eaglstein at the annual meeting of the Florida Society of Dermatologic surgeons. "I do all these procedures in my office."
The "pinch bleph" can be performed alone or in conjunction with laser resurfacing to improve the appearance of dermatochalasis and thin, wrinkled, sun-damaged lower eyelid skin, Dr. Eaglstein said. Not all patients are candidates, however. Because the technique removes only excess skin, it is not indicated for patients with lower, orbital, fat-pad protrusion.
Following a baseline ophthalmology examination, instruct the patient to discontinue aspirin, NSAIDs, and any herbal products that could prolong bleeding. Exclude or get clearance for patients with significant medical problems such as thyroid disease, Dr. Eaglstein said.
To determine how much skin to remove, pinch the lower eyelid skin together using blunt forceps until the eyelid margins start to evert. Then, mark the area with a fine tip gentian violet marker. He said that he crushes the excess skin using a curved hemostat and excises the tissue with Westcott or sharp iris scissors. He recommends 6-0 nylon sutures or 6-0 fast absorbing gut sutures to close the wound. "I use 6-0 fast absorbing."
"Don’t take too much skin if you plan to do laser resurfacing. You don’t want to get too much tightening [if you also plan to do] erbium laser resurfacing," said Dr. Eaglstein, a private practice dermatologist in Orange Park, Fla.
Expected complications include ecchymosis and edema. Less commonly, patients can experience hematoma, infection, scleral show, or ectropion.
"The pinch blepharoplasty is a simple, safe, and effective surgical procedure for the derm surgeon interested in providing cosmetic rejuvenation of the lower eyelid," Dr. Eaglstein said.
For more information, Dr. Eaglstein recommended a report by Joesph Niamtu III, D.M.D. on his lower eyelid blepharoplasty technique and experience (Cosmetic Derm. 2008;21:652-7).
He also recommended a report on a series of 77 candidates for traditional lower blepharoplasty who underwent a pinch blepharoplasty (Plast. Reconstr. Surg. 2005;115:1405-12). The author reported no significant scleral show or ectropion adverse events.
Dr. Eaglstein reported having no relevant conflicts of interest.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE FLORIDA SOCIETY OF DERMATOLOGIC SURGEONS
Skin Cancer Tops Malpractice Claims in Florida
ORLANDO – A higher than expected number of malpractice claims related to dermatologic surgery and treatment of psoriasis – as well as relatively few related to cosmetic dermatology were among the surprises revealed in a review of closed malpractice claims in Florida.
"There is a significant risk of malpractice actions in dermatology and dermatologic surgery," Dr. Ferdinand F. Becker said at the meeting. "Dermatologic surgeons would be well advised to be vigilant in diagnosis and appropriate treatment with the goal of avoiding complications at all cost."
General Dermatology Claims
Of 180 claims against dermatologists and dermatologic surgeons over a decade, 43 claims or 24% involved a general dermatology treatment. Of these, "44% were adjudicated or settled in favor of the plaintiff and 56% in favor of the defendant, so we came out better there," Dr. Becker said.
A total of 18 cases were adjudicated or settled in favor of the plaintiff – including 2 settled for an unknown amount. The largest settlement, $1 million, went to a patient who complained of meningoencephalitis and cerebral palsy secondary to failure to diagnose herpes simplex virus (conjunctival herpes simplex virus was the initial diagnosis). "This was the biggest claim in the whole shooting match," Dr. Becker said.
Another 25 of the general dermatology cases were decided or settled for the defendant physician, including 22 suits dropped by the plaintiff. Of the three remaining cases, two were summary judgments for the defendant and one judgment awarded the physician $50,000. In this case, the plaintiff had claimed avascular necrosis from treatment of chronic dermatitis with long-term steroid therapy.
Of note, a failure to diagnose Lyme disease when a patient presented with a rash of the axilla and groin resulted in a judgment for the plaintiff for $20,000, Dr. Becker said.
Psoriasis Claims
Dr. Becker identified seven claims involving psoriasis when he culled through the closed claims data from Florida’s Office of Insurance Regulation from January 2000 to December 2009. "I made a separate category for psoriasis because ... treatment of psoriasis is particularly problematic in general dermatology."
Four psoriasis treatment claims were settled in favor of the plaintiff from $500 to $250,000. The largest settlement involved a complaint of Stevens-Johnson syndrome with skin sloughing, oozing, and weeping sores resulting from methotrexate treatment. The defendant physician prevailed in three other cases – two dropped lawsuits and one summary judgment in which the patient had claimed steroids used to treat psoriasis had caused osteoporosis.
Cosmetic Dermatology Claims
A total of 28 claims or 16% involved cosmetic dermatology procedures. Outcomes were approximately split, with 54% adjudicated or settled in favor of the plaintiff and 46% in favor of the defendant.
"The majority were cases of laser hair removal," said Dr. Becker, a facial plastic surgeon and otolaryngologist in private practice in Vero Beach, Fla. Twelve of the 17 claims for laser hair removal were settled for the plaintiff for $2,500-$90,000. The biggest settlement followed a complaint of depigmentation and scarring related to laser hair removal. The remaining five laser cases involved complaints of burning, scarring, and/or pigmentary changes and were subsequently dropped by the plaintiff.
Dr, Becker found five suits involving Botox and filler treatments, each dropped by the plaintiff in favor of the defendant. Three plaintiffs claimed adverse reactions, one was unhappy with results, and one "patient left unattended after treatment, fell to the floor and broke three teeth, injured jaw, and cut lip."
Based on this lower number of malpractice claims, Botox and filler treatment "appears to be quite safe," Dr. Becker said.
The cosmetic dermatology category also included three claims involving liposuction, two settled in favor of the plaintiff and the other – a patient unhappy with abdominal liposuction results – dropped.
There was also a case involving sclerotherapy settled for $13,195 in favor of the plaintiff. The patient in this case claimed chronic ulceration resulting from treatment of spider veins.
A claim of pain, suffering, and a need for reconstructive surgery associated with a blepharoplasty resulted in a settlement of $100,000 for the plaintiff. Another suit, filed after a chemical facial peel, alleged facial burns and scarring ensued when the physician’s aesthetician acted outside the scope of her job.
Skin Cancer Claims
The highest percentage of claims in Florida (57%) involved skin cancer diagnosis and treatment. Of these, 57% were settled or adjudicated in favor of the plaintiff, 35% in favor of the defendant, and 8% were settled out of court for an unknown amount.
The greatest amount paid for non-melanoma skin cancer, $500,000, involved a patient treated with a biopsy and excision of a basal cell carcinoma on the upper lip. The patient filed suit, claiming they had to be referred for Mohs surgery and then experienced extensive scarring.
This and 19 other non-melanoma skin cancer malpractice claims were settled in favor of the plaintiff; 3 resulted in summary judgments for the defendant; 8 were settled out of court; and 15 suits were dropped by the plaintiff in favor of the physician defendant.
Melanoma diagnosis and/or treatment were cited in 17 malpractice cases. The second largest settlement to a plaintiff (out of the 180 cases reported) was $900,000 to a patient with malignant melanoma who had a biopsy but no pathology results or other follow-up. This and six other melanoma cases were settled in favor of the plaintiff. One case went to court and the plaintiff received $679,000 for severe scarring of his/her back related to malignant melanoma.
Another four melanoma cases were settled for an unknown amount and five claims were dropped by the plaintiff in favor of the defendant.
Mohs Surgery Claims
Mohs surgery comprised another major category with 29 malpractice claims. The largest settlement for a plaintiff was $875,000, stemming from Mohs surgery to remove a tumor from the arm. The patient lost the arm and claimed the dermatologic surgeon failed to diagnose malignant fibrous histiocytoma.
Two Mohs surgery claims were adjudicated as summary judgments for the defendant. Another ten cases were suits dropped by the plaintiff in favor of the defendant physician.
"This is the opposite of what I expected. I thought there would be more cases in the cosmetic derm area and less in derm surgery," Dr. Terry Cronin Jr., a private practice dermatologist in Melbourne, Fla., said during a Q and A session.
"I was surprised about this, too," Dr. Becker replied.
Overall, only 11 of the 180 closed claims actually went to court. Dr. Becker said, "The large majority [eight of these] were settled by the court with a summary judgment. This is the best news."
A meeting attendee asked if it is better to be direct with the patient or to call a lawyer if something does not go well. Dr. Becker replied: "Talking to your patient directly is a good idea and talking to your lawyer is also a good idea."
Dr. Becker said he had no relevant financial disclosures.
ORLANDO – A higher than expected number of malpractice claims related to dermatologic surgery and treatment of psoriasis – as well as relatively few related to cosmetic dermatology were among the surprises revealed in a review of closed malpractice claims in Florida.
"There is a significant risk of malpractice actions in dermatology and dermatologic surgery," Dr. Ferdinand F. Becker said at the meeting. "Dermatologic surgeons would be well advised to be vigilant in diagnosis and appropriate treatment with the goal of avoiding complications at all cost."
General Dermatology Claims
Of 180 claims against dermatologists and dermatologic surgeons over a decade, 43 claims or 24% involved a general dermatology treatment. Of these, "44% were adjudicated or settled in favor of the plaintiff and 56% in favor of the defendant, so we came out better there," Dr. Becker said.
A total of 18 cases were adjudicated or settled in favor of the plaintiff – including 2 settled for an unknown amount. The largest settlement, $1 million, went to a patient who complained of meningoencephalitis and cerebral palsy secondary to failure to diagnose herpes simplex virus (conjunctival herpes simplex virus was the initial diagnosis). "This was the biggest claim in the whole shooting match," Dr. Becker said.
Another 25 of the general dermatology cases were decided or settled for the defendant physician, including 22 suits dropped by the plaintiff. Of the three remaining cases, two were summary judgments for the defendant and one judgment awarded the physician $50,000. In this case, the plaintiff had claimed avascular necrosis from treatment of chronic dermatitis with long-term steroid therapy.
Of note, a failure to diagnose Lyme disease when a patient presented with a rash of the axilla and groin resulted in a judgment for the plaintiff for $20,000, Dr. Becker said.
Psoriasis Claims
Dr. Becker identified seven claims involving psoriasis when he culled through the closed claims data from Florida’s Office of Insurance Regulation from January 2000 to December 2009. "I made a separate category for psoriasis because ... treatment of psoriasis is particularly problematic in general dermatology."
Four psoriasis treatment claims were settled in favor of the plaintiff from $500 to $250,000. The largest settlement involved a complaint of Stevens-Johnson syndrome with skin sloughing, oozing, and weeping sores resulting from methotrexate treatment. The defendant physician prevailed in three other cases – two dropped lawsuits and one summary judgment in which the patient had claimed steroids used to treat psoriasis had caused osteoporosis.
Cosmetic Dermatology Claims
A total of 28 claims or 16% involved cosmetic dermatology procedures. Outcomes were approximately split, with 54% adjudicated or settled in favor of the plaintiff and 46% in favor of the defendant.
"The majority were cases of laser hair removal," said Dr. Becker, a facial plastic surgeon and otolaryngologist in private practice in Vero Beach, Fla. Twelve of the 17 claims for laser hair removal were settled for the plaintiff for $2,500-$90,000. The biggest settlement followed a complaint of depigmentation and scarring related to laser hair removal. The remaining five laser cases involved complaints of burning, scarring, and/or pigmentary changes and were subsequently dropped by the plaintiff.
Dr, Becker found five suits involving Botox and filler treatments, each dropped by the plaintiff in favor of the defendant. Three plaintiffs claimed adverse reactions, one was unhappy with results, and one "patient left unattended after treatment, fell to the floor and broke three teeth, injured jaw, and cut lip."
Based on this lower number of malpractice claims, Botox and filler treatment "appears to be quite safe," Dr. Becker said.
The cosmetic dermatology category also included three claims involving liposuction, two settled in favor of the plaintiff and the other – a patient unhappy with abdominal liposuction results – dropped.
There was also a case involving sclerotherapy settled for $13,195 in favor of the plaintiff. The patient in this case claimed chronic ulceration resulting from treatment of spider veins.
A claim of pain, suffering, and a need for reconstructive surgery associated with a blepharoplasty resulted in a settlement of $100,000 for the plaintiff. Another suit, filed after a chemical facial peel, alleged facial burns and scarring ensued when the physician’s aesthetician acted outside the scope of her job.
Skin Cancer Claims
The highest percentage of claims in Florida (57%) involved skin cancer diagnosis and treatment. Of these, 57% were settled or adjudicated in favor of the plaintiff, 35% in favor of the defendant, and 8% were settled out of court for an unknown amount.
The greatest amount paid for non-melanoma skin cancer, $500,000, involved a patient treated with a biopsy and excision of a basal cell carcinoma on the upper lip. The patient filed suit, claiming they had to be referred for Mohs surgery and then experienced extensive scarring.
This and 19 other non-melanoma skin cancer malpractice claims were settled in favor of the plaintiff; 3 resulted in summary judgments for the defendant; 8 were settled out of court; and 15 suits were dropped by the plaintiff in favor of the physician defendant.
Melanoma diagnosis and/or treatment were cited in 17 malpractice cases. The second largest settlement to a plaintiff (out of the 180 cases reported) was $900,000 to a patient with malignant melanoma who had a biopsy but no pathology results or other follow-up. This and six other melanoma cases were settled in favor of the plaintiff. One case went to court and the plaintiff received $679,000 for severe scarring of his/her back related to malignant melanoma.
Another four melanoma cases were settled for an unknown amount and five claims were dropped by the plaintiff in favor of the defendant.
Mohs Surgery Claims
Mohs surgery comprised another major category with 29 malpractice claims. The largest settlement for a plaintiff was $875,000, stemming from Mohs surgery to remove a tumor from the arm. The patient lost the arm and claimed the dermatologic surgeon failed to diagnose malignant fibrous histiocytoma.
Two Mohs surgery claims were adjudicated as summary judgments for the defendant. Another ten cases were suits dropped by the plaintiff in favor of the defendant physician.
"This is the opposite of what I expected. I thought there would be more cases in the cosmetic derm area and less in derm surgery," Dr. Terry Cronin Jr., a private practice dermatologist in Melbourne, Fla., said during a Q and A session.
"I was surprised about this, too," Dr. Becker replied.
Overall, only 11 of the 180 closed claims actually went to court. Dr. Becker said, "The large majority [eight of these] were settled by the court with a summary judgment. This is the best news."
A meeting attendee asked if it is better to be direct with the patient or to call a lawyer if something does not go well. Dr. Becker replied: "Talking to your patient directly is a good idea and talking to your lawyer is also a good idea."
Dr. Becker said he had no relevant financial disclosures.
ORLANDO – A higher than expected number of malpractice claims related to dermatologic surgery and treatment of psoriasis – as well as relatively few related to cosmetic dermatology were among the surprises revealed in a review of closed malpractice claims in Florida.
"There is a significant risk of malpractice actions in dermatology and dermatologic surgery," Dr. Ferdinand F. Becker said at the meeting. "Dermatologic surgeons would be well advised to be vigilant in diagnosis and appropriate treatment with the goal of avoiding complications at all cost."
General Dermatology Claims
Of 180 claims against dermatologists and dermatologic surgeons over a decade, 43 claims or 24% involved a general dermatology treatment. Of these, "44% were adjudicated or settled in favor of the plaintiff and 56% in favor of the defendant, so we came out better there," Dr. Becker said.
A total of 18 cases were adjudicated or settled in favor of the plaintiff – including 2 settled for an unknown amount. The largest settlement, $1 million, went to a patient who complained of meningoencephalitis and cerebral palsy secondary to failure to diagnose herpes simplex virus (conjunctival herpes simplex virus was the initial diagnosis). "This was the biggest claim in the whole shooting match," Dr. Becker said.
Another 25 of the general dermatology cases were decided or settled for the defendant physician, including 22 suits dropped by the plaintiff. Of the three remaining cases, two were summary judgments for the defendant and one judgment awarded the physician $50,000. In this case, the plaintiff had claimed avascular necrosis from treatment of chronic dermatitis with long-term steroid therapy.
Of note, a failure to diagnose Lyme disease when a patient presented with a rash of the axilla and groin resulted in a judgment for the plaintiff for $20,000, Dr. Becker said.
Psoriasis Claims
Dr. Becker identified seven claims involving psoriasis when he culled through the closed claims data from Florida’s Office of Insurance Regulation from January 2000 to December 2009. "I made a separate category for psoriasis because ... treatment of psoriasis is particularly problematic in general dermatology."
Four psoriasis treatment claims were settled in favor of the plaintiff from $500 to $250,000. The largest settlement involved a complaint of Stevens-Johnson syndrome with skin sloughing, oozing, and weeping sores resulting from methotrexate treatment. The defendant physician prevailed in three other cases – two dropped lawsuits and one summary judgment in which the patient had claimed steroids used to treat psoriasis had caused osteoporosis.
Cosmetic Dermatology Claims
A total of 28 claims or 16% involved cosmetic dermatology procedures. Outcomes were approximately split, with 54% adjudicated or settled in favor of the plaintiff and 46% in favor of the defendant.
"The majority were cases of laser hair removal," said Dr. Becker, a facial plastic surgeon and otolaryngologist in private practice in Vero Beach, Fla. Twelve of the 17 claims for laser hair removal were settled for the plaintiff for $2,500-$90,000. The biggest settlement followed a complaint of depigmentation and scarring related to laser hair removal. The remaining five laser cases involved complaints of burning, scarring, and/or pigmentary changes and were subsequently dropped by the plaintiff.
Dr, Becker found five suits involving Botox and filler treatments, each dropped by the plaintiff in favor of the defendant. Three plaintiffs claimed adverse reactions, one was unhappy with results, and one "patient left unattended after treatment, fell to the floor and broke three teeth, injured jaw, and cut lip."
Based on this lower number of malpractice claims, Botox and filler treatment "appears to be quite safe," Dr. Becker said.
The cosmetic dermatology category also included three claims involving liposuction, two settled in favor of the plaintiff and the other – a patient unhappy with abdominal liposuction results – dropped.
There was also a case involving sclerotherapy settled for $13,195 in favor of the plaintiff. The patient in this case claimed chronic ulceration resulting from treatment of spider veins.
A claim of pain, suffering, and a need for reconstructive surgery associated with a blepharoplasty resulted in a settlement of $100,000 for the plaintiff. Another suit, filed after a chemical facial peel, alleged facial burns and scarring ensued when the physician’s aesthetician acted outside the scope of her job.
Skin Cancer Claims
The highest percentage of claims in Florida (57%) involved skin cancer diagnosis and treatment. Of these, 57% were settled or adjudicated in favor of the plaintiff, 35% in favor of the defendant, and 8% were settled out of court for an unknown amount.
The greatest amount paid for non-melanoma skin cancer, $500,000, involved a patient treated with a biopsy and excision of a basal cell carcinoma on the upper lip. The patient filed suit, claiming they had to be referred for Mohs surgery and then experienced extensive scarring.
This and 19 other non-melanoma skin cancer malpractice claims were settled in favor of the plaintiff; 3 resulted in summary judgments for the defendant; 8 were settled out of court; and 15 suits were dropped by the plaintiff in favor of the physician defendant.
Melanoma diagnosis and/or treatment were cited in 17 malpractice cases. The second largest settlement to a plaintiff (out of the 180 cases reported) was $900,000 to a patient with malignant melanoma who had a biopsy but no pathology results or other follow-up. This and six other melanoma cases were settled in favor of the plaintiff. One case went to court and the plaintiff received $679,000 for severe scarring of his/her back related to malignant melanoma.
Another four melanoma cases were settled for an unknown amount and five claims were dropped by the plaintiff in favor of the defendant.
Mohs Surgery Claims
Mohs surgery comprised another major category with 29 malpractice claims. The largest settlement for a plaintiff was $875,000, stemming from Mohs surgery to remove a tumor from the arm. The patient lost the arm and claimed the dermatologic surgeon failed to diagnose malignant fibrous histiocytoma.
Two Mohs surgery claims were adjudicated as summary judgments for the defendant. Another ten cases were suits dropped by the plaintiff in favor of the defendant physician.
"This is the opposite of what I expected. I thought there would be more cases in the cosmetic derm area and less in derm surgery," Dr. Terry Cronin Jr., a private practice dermatologist in Melbourne, Fla., said during a Q and A session.
"I was surprised about this, too," Dr. Becker replied.
Overall, only 11 of the 180 closed claims actually went to court. Dr. Becker said, "The large majority [eight of these] were settled by the court with a summary judgment. This is the best news."
A meeting attendee asked if it is better to be direct with the patient or to call a lawyer if something does not go well. Dr. Becker replied: "Talking to your patient directly is a good idea and talking to your lawyer is also a good idea."
Dr. Becker said he had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE FLORIDA SOCIETY OF DERMATOLOGIC SURGEONS
Major Finding: Skin cancer diagnosis and treatment led malpractice claims against dermatologists in Florida, accounting for 57% of 180 lawsuits.
Data Source: Review of malpractice claims reported to Florida’s Office of Insurance Regulation from January 2000 to December 2009.
Disclosures: Dr. Becker said that he had no relevant disclosures.
Botulinum Toxin: Less Is More in Lower Face
ORLANDO – Dose is the most important consideration when injecting botulinum toxin in the lower face – even more important than during treatment of the upper face, according to Dr. Doris Hexsel.
"You should always use the lowest effective dose in the lower face," said Dr. Hexsel. This strategy reduces the risk for asymmetry, muscle dysfunction, and temporary oral paralysis. "These are dose-related and technique-related side effects."
Botulinum toxin can treat perioral wrinkles and marionette lines, as well as improve the appearance of a patient’s chin or gummy smile. However, only treat one or two areas in the lower face during the same session to minimize the risk of "sum of effect," she said. In other words, the effects of multiple, simultaneous injections around the mouth can be cumulative.
Also consider a combination of the botulinum toxin and filler injections, Dr. Hexsel said at the annual meeting of the Florida Society of Dermatologic Surgeons.
Dr. Hexsel shared her expertise with botulinum toxin for the following indications:
• Perioral wrinkles. Injections should be superficial and at least 1.5 cm from the corners of the mouth. Any closer and you increase the risk of undesirable relaxation of the depressor anguli oris muscle, the zygomaticus major muscle, and the risorius muscle, said Dr. Hexsel of the department of dermatology at Pontificia Universidade Católica do Rio Grande do Sul, Brazil.
She said that she typically injects 1.25 U-2.5 U abobotulinumtoxinA (Dysport, Ipsen/Medicis) per point, or 0.5 U-1.0 U onabotulinumtoxinA (Botox, Allergan). With two to six injection points, the total dose varies from 4 U-18 U for abobotulinum toxin or 4 U-10 U for onabotulinumtoxinA.
As a bonus, she said, "We observe a slight increase in the volume of the lips due to just the relaxation."
• "Cellulitic chin." Loss of collagen and subcutaneous fat, along with action of the jaw muscles, can cause a chin to have a cellulitic or "peau d’orange" appearance. Botulinum toxin can treat this area as well, said Dr. Hexsel. "I prefer two point injections – bilaterally at the most distal point of the mentalis muscle.
Again, keep the injections superficial and avoid high doses to minimize undesirable relaxation of the depressor labii inferioris muscle, she said. The total dose for a dimpled chin ranges from 15 U to 20 U of abobotulinumtoxinA and from 5 U to 10 U of onabotulinumtoxinA.
"I touch up patients 15 to 30 days later, if necessary," she added.
• Marionette lines. Botulinum toxin can improve the appearance of patients when the corners of their mouth appear permanently turned down. Better results may be obtained by combining toxin with fillers, she said.
For mild presentations, treat the mentalis muscle first, she advised. Treatment of this muscle also recruits the depressor anguli oris (DAO) muscle less (the mentalis is the agonist muscle to the DAO). Dr. Hexsel said she generally uses a total dose of 10 U-20 U of abobotulinumtoxinA or 3 U-6 U of onabotulinumtoxinA for this indication.
• Gummy smile. Consider botulinum toxin when a patient complains of a gummy smile. To foster a more natural look, also treat any natural asymmetries or posterior gummy smile, Dr. Hexsel said.
To treat posterior gingival exposure, inject two points on either side of the malar region. Inject in the nasolabial fold at the point of greatest lateral contraction during a smile.
Total doses vary from 5 U to 15 U of abobotulinumtoxinA or from 4 U to 10 U of onabotulinumtoxinA.
A final tip is to take before and after clinical photos, both during movement and at rest, when injecting the lower face.
Dr. Hexsel reported receiving grants and research support from Allergan, Galderma, and Ipsen. She also is a consultant for Allergan and Ipsen.
ORLANDO – Dose is the most important consideration when injecting botulinum toxin in the lower face – even more important than during treatment of the upper face, according to Dr. Doris Hexsel.
"You should always use the lowest effective dose in the lower face," said Dr. Hexsel. This strategy reduces the risk for asymmetry, muscle dysfunction, and temporary oral paralysis. "These are dose-related and technique-related side effects."
Botulinum toxin can treat perioral wrinkles and marionette lines, as well as improve the appearance of a patient’s chin or gummy smile. However, only treat one or two areas in the lower face during the same session to minimize the risk of "sum of effect," she said. In other words, the effects of multiple, simultaneous injections around the mouth can be cumulative.
Also consider a combination of the botulinum toxin and filler injections, Dr. Hexsel said at the annual meeting of the Florida Society of Dermatologic Surgeons.
Dr. Hexsel shared her expertise with botulinum toxin for the following indications:
• Perioral wrinkles. Injections should be superficial and at least 1.5 cm from the corners of the mouth. Any closer and you increase the risk of undesirable relaxation of the depressor anguli oris muscle, the zygomaticus major muscle, and the risorius muscle, said Dr. Hexsel of the department of dermatology at Pontificia Universidade Católica do Rio Grande do Sul, Brazil.
She said that she typically injects 1.25 U-2.5 U abobotulinumtoxinA (Dysport, Ipsen/Medicis) per point, or 0.5 U-1.0 U onabotulinumtoxinA (Botox, Allergan). With two to six injection points, the total dose varies from 4 U-18 U for abobotulinum toxin or 4 U-10 U for onabotulinumtoxinA.
As a bonus, she said, "We observe a slight increase in the volume of the lips due to just the relaxation."
• "Cellulitic chin." Loss of collagen and subcutaneous fat, along with action of the jaw muscles, can cause a chin to have a cellulitic or "peau d’orange" appearance. Botulinum toxin can treat this area as well, said Dr. Hexsel. "I prefer two point injections – bilaterally at the most distal point of the mentalis muscle.
Again, keep the injections superficial and avoid high doses to minimize undesirable relaxation of the depressor labii inferioris muscle, she said. The total dose for a dimpled chin ranges from 15 U to 20 U of abobotulinumtoxinA and from 5 U to 10 U of onabotulinumtoxinA.
"I touch up patients 15 to 30 days later, if necessary," she added.
• Marionette lines. Botulinum toxin can improve the appearance of patients when the corners of their mouth appear permanently turned down. Better results may be obtained by combining toxin with fillers, she said.
For mild presentations, treat the mentalis muscle first, she advised. Treatment of this muscle also recruits the depressor anguli oris (DAO) muscle less (the mentalis is the agonist muscle to the DAO). Dr. Hexsel said she generally uses a total dose of 10 U-20 U of abobotulinumtoxinA or 3 U-6 U of onabotulinumtoxinA for this indication.
• Gummy smile. Consider botulinum toxin when a patient complains of a gummy smile. To foster a more natural look, also treat any natural asymmetries or posterior gummy smile, Dr. Hexsel said.
To treat posterior gingival exposure, inject two points on either side of the malar region. Inject in the nasolabial fold at the point of greatest lateral contraction during a smile.
Total doses vary from 5 U to 15 U of abobotulinumtoxinA or from 4 U to 10 U of onabotulinumtoxinA.
A final tip is to take before and after clinical photos, both during movement and at rest, when injecting the lower face.
Dr. Hexsel reported receiving grants and research support from Allergan, Galderma, and Ipsen. She also is a consultant for Allergan and Ipsen.
ORLANDO – Dose is the most important consideration when injecting botulinum toxin in the lower face – even more important than during treatment of the upper face, according to Dr. Doris Hexsel.
"You should always use the lowest effective dose in the lower face," said Dr. Hexsel. This strategy reduces the risk for asymmetry, muscle dysfunction, and temporary oral paralysis. "These are dose-related and technique-related side effects."
Botulinum toxin can treat perioral wrinkles and marionette lines, as well as improve the appearance of a patient’s chin or gummy smile. However, only treat one or two areas in the lower face during the same session to minimize the risk of "sum of effect," she said. In other words, the effects of multiple, simultaneous injections around the mouth can be cumulative.
Also consider a combination of the botulinum toxin and filler injections, Dr. Hexsel said at the annual meeting of the Florida Society of Dermatologic Surgeons.
Dr. Hexsel shared her expertise with botulinum toxin for the following indications:
• Perioral wrinkles. Injections should be superficial and at least 1.5 cm from the corners of the mouth. Any closer and you increase the risk of undesirable relaxation of the depressor anguli oris muscle, the zygomaticus major muscle, and the risorius muscle, said Dr. Hexsel of the department of dermatology at Pontificia Universidade Católica do Rio Grande do Sul, Brazil.
She said that she typically injects 1.25 U-2.5 U abobotulinumtoxinA (Dysport, Ipsen/Medicis) per point, or 0.5 U-1.0 U onabotulinumtoxinA (Botox, Allergan). With two to six injection points, the total dose varies from 4 U-18 U for abobotulinum toxin or 4 U-10 U for onabotulinumtoxinA.
As a bonus, she said, "We observe a slight increase in the volume of the lips due to just the relaxation."
• "Cellulitic chin." Loss of collagen and subcutaneous fat, along with action of the jaw muscles, can cause a chin to have a cellulitic or "peau d’orange" appearance. Botulinum toxin can treat this area as well, said Dr. Hexsel. "I prefer two point injections – bilaterally at the most distal point of the mentalis muscle.
Again, keep the injections superficial and avoid high doses to minimize undesirable relaxation of the depressor labii inferioris muscle, she said. The total dose for a dimpled chin ranges from 15 U to 20 U of abobotulinumtoxinA and from 5 U to 10 U of onabotulinumtoxinA.
"I touch up patients 15 to 30 days later, if necessary," she added.
• Marionette lines. Botulinum toxin can improve the appearance of patients when the corners of their mouth appear permanently turned down. Better results may be obtained by combining toxin with fillers, she said.
For mild presentations, treat the mentalis muscle first, she advised. Treatment of this muscle also recruits the depressor anguli oris (DAO) muscle less (the mentalis is the agonist muscle to the DAO). Dr. Hexsel said she generally uses a total dose of 10 U-20 U of abobotulinumtoxinA or 3 U-6 U of onabotulinumtoxinA for this indication.
• Gummy smile. Consider botulinum toxin when a patient complains of a gummy smile. To foster a more natural look, also treat any natural asymmetries or posterior gummy smile, Dr. Hexsel said.
To treat posterior gingival exposure, inject two points on either side of the malar region. Inject in the nasolabial fold at the point of greatest lateral contraction during a smile.
Total doses vary from 5 U to 15 U of abobotulinumtoxinA or from 4 U to 10 U of onabotulinumtoxinA.
A final tip is to take before and after clinical photos, both during movement and at rest, when injecting the lower face.
Dr. Hexsel reported receiving grants and research support from Allergan, Galderma, and Ipsen. She also is a consultant for Allergan and Ipsen.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE FLORIDA SOCIETY OF DERMATOLOGIC SURGEONS
Lasers Have Role in Pediatric Care, Expert Says
ORLANDO – Lasers can effectively target and treat several well-known pediatric skin conditions and, thanks to advances in technology, potentially treat more in the near future, according to Dr. Jan Izakovic.
The right laser in skilled hands can improve port wine stains, superficial hemangiomas, and nevi, he said at the annual meeting of the Florida Society of Dermatologic Surgeons. Children with vitiligo, likewise, can experience repigmentation with appropriate laser treatment, based on evidence in the literature and personal experience, he added.
In contrast, the use of lasers for striae, café au lait spots, and other potential indications is supported only by smaller case report–type studies, said Dr. Izakovic, codirector of pediatric dermatology at the University of Miami.
Port Wine Stains
The pulsed dye laser (PDL) is a frequent player in Dr. Izakovic’s armamentarium. When a child presents with a port wine stain, for example, he said he treats them with 585-nm or 595-nm PDLs. Nd:YAG and intense pulsed light (IPL), as well as a combination approach, are other options.
"I usually do a small test area first, and eventually increase the energy in small increments if there is no or too little effect," Dr. Izakovic said. Treatments should be scheduled at 4- to 6-week intervals. The total number of sessions will vary. "You can have great success after one or two treatments, but I wouldn’t tell parents that up front," he said.
About 70%-80% lightening of the port wine stain is realistic. Ultimately, success depends on the color, site, and thickness of the lesion. "The whiter the skin and the redder the port wine stain, the better your outcome will be," Dr. Izakovic said.
"Pain is usually minimal and momentary," but counsel patients on possible effects so they know what to expect, he advised. Purpura associated with PDL treatment generally resolves in 10-12 days, for example. Also, "you can expect to see the white dots fully formed 3-4 weeks post treatment. Tell patients you will address the leftovers at the next session," Dr. Izakovic said.
A comparison study found lightening of port wine stains to be similar with either PDL or IPL (Br. J. Dermatol. 2009;160:359-64). However, PDL was associated with better overall efficacy and was preferred by patients.
IPL has the advantage of only rarely causing purpura, Dr. Izakovic said.
Although he has not used combination therapy, Dr. Izakovic said better outcomes might be possible when PDL treatment is combined with photodynamic therapy, imiquimod, or rapamycin.
Hemangioma Treatment
PDL 585-nm or 595-nm devices can also treat superficial hemangiomas or ulcerated hemangiomas, or improve residual erythema or telangiectasia following involution of a hemangioma, he said. Although some physicians might be reluctant to treat a superficial eyelid hemangioma with PDL, there is some support for this as well in the literature (Dermatol. Surg. 2010;36:590-7).
Lasers can be used in a combination approach with other therapies such as oral propranolol or steroids, Dr. Izakovic said. "[With lasers] you are helping with the superficial part of it." PDL energy at the highest setting penetrates to a maximum of 1.5 mm.
Immediate results with a laser can be beneficial, which provides great relief for parents. "I tell them the goal is to stop the growth, stop the darkening, and stimulate the involution, " he said.
Children With Nevi
Lasers also can treat nevi of Ota, Dr. Izakovic said, but there is some variability in outcome; however, satisfying results can be achieved.
The issue surrounds complete ablation of a regular nevus with laser energy. He said he prefers to reserve laser treatment for children who first had a biopsy to rule out malignancy. Another option is to remove the nevus surgically and test the sample for any relevant pathology. "Always check what you remove histologically when you remove nevi," he said.
Several Q-switched lasers, such as a ruby 694 nm, an alexandrite 755 nm, and an Nd:YAG 1,064 nm, are appropriate for nevi treatment. Remember that anesthesia might be indicated because treatment with a Q-switched laser can be painful, he said.
Pediatric Vitiligo
Children with vitiligo can likewise see improvement following laser treatment, Dr. Izakovic said. Among the newer options is the use of an excimer laser 308 nm by itself or in combination with topical therapies. The use of this device is increasing for the treatment of vitiligo, especially on isolated small areas of the face, neck, or head, he said.
"There are some good responses after a couple of treatments. You can see signs of repigmentation," he noted.
Other Uses
Rarer instances include PDL 585-nm treatment of striae. "This only treats the erythematous part and it’s not an overwhelming effect," Dr. Izakovic said. Café au lait spot lightening is another marginal indication for lasers that is not yet fully supported in the literature.
There have been case reports of laser treatment of molluscum contagiosum, atopic dermatitis, and lichen sclerosus et atrophicus. Laser treatment for such marginal indications, where there are other well-established treatment options, remains to be supported by more rigorous research, he said.
Dr. Izakovic said that he had no relevant financial disclosures.
ORLANDO – Lasers can effectively target and treat several well-known pediatric skin conditions and, thanks to advances in technology, potentially treat more in the near future, according to Dr. Jan Izakovic.
The right laser in skilled hands can improve port wine stains, superficial hemangiomas, and nevi, he said at the annual meeting of the Florida Society of Dermatologic Surgeons. Children with vitiligo, likewise, can experience repigmentation with appropriate laser treatment, based on evidence in the literature and personal experience, he added.
In contrast, the use of lasers for striae, café au lait spots, and other potential indications is supported only by smaller case report–type studies, said Dr. Izakovic, codirector of pediatric dermatology at the University of Miami.
Port Wine Stains
The pulsed dye laser (PDL) is a frequent player in Dr. Izakovic’s armamentarium. When a child presents with a port wine stain, for example, he said he treats them with 585-nm or 595-nm PDLs. Nd:YAG and intense pulsed light (IPL), as well as a combination approach, are other options.
"I usually do a small test area first, and eventually increase the energy in small increments if there is no or too little effect," Dr. Izakovic said. Treatments should be scheduled at 4- to 6-week intervals. The total number of sessions will vary. "You can have great success after one or two treatments, but I wouldn’t tell parents that up front," he said.
About 70%-80% lightening of the port wine stain is realistic. Ultimately, success depends on the color, site, and thickness of the lesion. "The whiter the skin and the redder the port wine stain, the better your outcome will be," Dr. Izakovic said.
"Pain is usually minimal and momentary," but counsel patients on possible effects so they know what to expect, he advised. Purpura associated with PDL treatment generally resolves in 10-12 days, for example. Also, "you can expect to see the white dots fully formed 3-4 weeks post treatment. Tell patients you will address the leftovers at the next session," Dr. Izakovic said.
A comparison study found lightening of port wine stains to be similar with either PDL or IPL (Br. J. Dermatol. 2009;160:359-64). However, PDL was associated with better overall efficacy and was preferred by patients.
IPL has the advantage of only rarely causing purpura, Dr. Izakovic said.
Although he has not used combination therapy, Dr. Izakovic said better outcomes might be possible when PDL treatment is combined with photodynamic therapy, imiquimod, or rapamycin.
Hemangioma Treatment
PDL 585-nm or 595-nm devices can also treat superficial hemangiomas or ulcerated hemangiomas, or improve residual erythema or telangiectasia following involution of a hemangioma, he said. Although some physicians might be reluctant to treat a superficial eyelid hemangioma with PDL, there is some support for this as well in the literature (Dermatol. Surg. 2010;36:590-7).
Lasers can be used in a combination approach with other therapies such as oral propranolol or steroids, Dr. Izakovic said. "[With lasers] you are helping with the superficial part of it." PDL energy at the highest setting penetrates to a maximum of 1.5 mm.
Immediate results with a laser can be beneficial, which provides great relief for parents. "I tell them the goal is to stop the growth, stop the darkening, and stimulate the involution, " he said.
Children With Nevi
Lasers also can treat nevi of Ota, Dr. Izakovic said, but there is some variability in outcome; however, satisfying results can be achieved.
The issue surrounds complete ablation of a regular nevus with laser energy. He said he prefers to reserve laser treatment for children who first had a biopsy to rule out malignancy. Another option is to remove the nevus surgically and test the sample for any relevant pathology. "Always check what you remove histologically when you remove nevi," he said.
Several Q-switched lasers, such as a ruby 694 nm, an alexandrite 755 nm, and an Nd:YAG 1,064 nm, are appropriate for nevi treatment. Remember that anesthesia might be indicated because treatment with a Q-switched laser can be painful, he said.
Pediatric Vitiligo
Children with vitiligo can likewise see improvement following laser treatment, Dr. Izakovic said. Among the newer options is the use of an excimer laser 308 nm by itself or in combination with topical therapies. The use of this device is increasing for the treatment of vitiligo, especially on isolated small areas of the face, neck, or head, he said.
"There are some good responses after a couple of treatments. You can see signs of repigmentation," he noted.
Other Uses
Rarer instances include PDL 585-nm treatment of striae. "This only treats the erythematous part and it’s not an overwhelming effect," Dr. Izakovic said. Café au lait spot lightening is another marginal indication for lasers that is not yet fully supported in the literature.
There have been case reports of laser treatment of molluscum contagiosum, atopic dermatitis, and lichen sclerosus et atrophicus. Laser treatment for such marginal indications, where there are other well-established treatment options, remains to be supported by more rigorous research, he said.
Dr. Izakovic said that he had no relevant financial disclosures.
ORLANDO – Lasers can effectively target and treat several well-known pediatric skin conditions and, thanks to advances in technology, potentially treat more in the near future, according to Dr. Jan Izakovic.
The right laser in skilled hands can improve port wine stains, superficial hemangiomas, and nevi, he said at the annual meeting of the Florida Society of Dermatologic Surgeons. Children with vitiligo, likewise, can experience repigmentation with appropriate laser treatment, based on evidence in the literature and personal experience, he added.
In contrast, the use of lasers for striae, café au lait spots, and other potential indications is supported only by smaller case report–type studies, said Dr. Izakovic, codirector of pediatric dermatology at the University of Miami.
Port Wine Stains
The pulsed dye laser (PDL) is a frequent player in Dr. Izakovic’s armamentarium. When a child presents with a port wine stain, for example, he said he treats them with 585-nm or 595-nm PDLs. Nd:YAG and intense pulsed light (IPL), as well as a combination approach, are other options.
"I usually do a small test area first, and eventually increase the energy in small increments if there is no or too little effect," Dr. Izakovic said. Treatments should be scheduled at 4- to 6-week intervals. The total number of sessions will vary. "You can have great success after one or two treatments, but I wouldn’t tell parents that up front," he said.
About 70%-80% lightening of the port wine stain is realistic. Ultimately, success depends on the color, site, and thickness of the lesion. "The whiter the skin and the redder the port wine stain, the better your outcome will be," Dr. Izakovic said.
"Pain is usually minimal and momentary," but counsel patients on possible effects so they know what to expect, he advised. Purpura associated with PDL treatment generally resolves in 10-12 days, for example. Also, "you can expect to see the white dots fully formed 3-4 weeks post treatment. Tell patients you will address the leftovers at the next session," Dr. Izakovic said.
A comparison study found lightening of port wine stains to be similar with either PDL or IPL (Br. J. Dermatol. 2009;160:359-64). However, PDL was associated with better overall efficacy and was preferred by patients.
IPL has the advantage of only rarely causing purpura, Dr. Izakovic said.
Although he has not used combination therapy, Dr. Izakovic said better outcomes might be possible when PDL treatment is combined with photodynamic therapy, imiquimod, or rapamycin.
Hemangioma Treatment
PDL 585-nm or 595-nm devices can also treat superficial hemangiomas or ulcerated hemangiomas, or improve residual erythema or telangiectasia following involution of a hemangioma, he said. Although some physicians might be reluctant to treat a superficial eyelid hemangioma with PDL, there is some support for this as well in the literature (Dermatol. Surg. 2010;36:590-7).
Lasers can be used in a combination approach with other therapies such as oral propranolol or steroids, Dr. Izakovic said. "[With lasers] you are helping with the superficial part of it." PDL energy at the highest setting penetrates to a maximum of 1.5 mm.
Immediate results with a laser can be beneficial, which provides great relief for parents. "I tell them the goal is to stop the growth, stop the darkening, and stimulate the involution, " he said.
Children With Nevi
Lasers also can treat nevi of Ota, Dr. Izakovic said, but there is some variability in outcome; however, satisfying results can be achieved.
The issue surrounds complete ablation of a regular nevus with laser energy. He said he prefers to reserve laser treatment for children who first had a biopsy to rule out malignancy. Another option is to remove the nevus surgically and test the sample for any relevant pathology. "Always check what you remove histologically when you remove nevi," he said.
Several Q-switched lasers, such as a ruby 694 nm, an alexandrite 755 nm, and an Nd:YAG 1,064 nm, are appropriate for nevi treatment. Remember that anesthesia might be indicated because treatment with a Q-switched laser can be painful, he said.
Pediatric Vitiligo
Children with vitiligo can likewise see improvement following laser treatment, Dr. Izakovic said. Among the newer options is the use of an excimer laser 308 nm by itself or in combination with topical therapies. The use of this device is increasing for the treatment of vitiligo, especially on isolated small areas of the face, neck, or head, he said.
"There are some good responses after a couple of treatments. You can see signs of repigmentation," he noted.
Other Uses
Rarer instances include PDL 585-nm treatment of striae. "This only treats the erythematous part and it’s not an overwhelming effect," Dr. Izakovic said. Café au lait spot lightening is another marginal indication for lasers that is not yet fully supported in the literature.
There have been case reports of laser treatment of molluscum contagiosum, atopic dermatitis, and lichen sclerosus et atrophicus. Laser treatment for such marginal indications, where there are other well-established treatment options, remains to be supported by more rigorous research, he said.
Dr. Izakovic said that he had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE FLORIDA SOCIETY OF DERMATOLOGIC SURGEONS
Studies Back Azficel-T for Nasolabial Fold Wrinkles
ORLANDO – One number keeps coming up with the new autologous fibroblast biologic product that enhances a patient’s nasolabial wrinkles: three.
"There are a lot of threes: three biopsies 3 mm in size, a 3 month’s wait, and three treatment sessions," Dr. Robert Weiss said at the annual meeting of the Florida Society of Dermatology Surgeons.
The Food and Drug Administration cleared the marketing of Fibrocell Science’s azficel-T (LaVív) in June 2011, and the product is now reaching the market. "It’s very exciting to be right at the launch of this," said Dr .Weiss, a dermatologist in private practice in Hunt Valley, Md., and with the dermatology department at Johns Hopkins University, Baltimore. "I’ve been working on this for close to a decade, so it was a personal triumph to see this finally cleared by the FDA."
The FDA based its clearance on two identically designed, multicenter, double-blind, vehicle-controlled studies. A total of 421 patients with moderate to severe nasolabial fold wrinkles were assessed, with 210 being randomized to azficel-T and 211 to vehicle injections. The average age was 55 years, and 90% were women.
A 2-point improvement in the appearance of the nasolabial fold wrinkles was the primary outcome. Physicians at the study sites who were not injectors blindly rated results.
At the 6-month follow-up, doctors observed the 2-point improvement in 33% of the azficel-T group, compared with 7% of controls in the first study. In the second study, 19% of the azficel-T group had at least a 2-point improvement, compared with 7% of controls. These differences were on the borderline of statistical significance, Dr. Weiss said.
"The FDA was convinced that this was effective. We were certainly very convinced by what we observed with the patients," Dr. Weiss said.
Patient reported outcomes were more statistically significant, with 57% reporting a 2-point improvement, compared with 30% in the vehicle group in an intent-to-treat analysis of the first study. In the second study, 45% of the treated patients reported this outcome, compared with 18% of the vehicle group.
The most common adverse effects were injection site reactions lasting approximately 2 hours, Dr. Weiss said. These events included erythema, bruising, and swelling. Facial or eyelid edema, hypersensitivity or decreased skin sensation at the injection site, and postprocedural discomfort were among the adverse events reported by 1% of patients or fewer.
"One of the first questions patients ask me is: ‘How are you going to make sure I’m getting my own cells?’ " Dr. Weiss explained there are strict controls. Each patient is assigned a lot number. Vials for injection are labeled with each patient’s initials, date of birth, and a unique identification number. "All the critical steps are verified twice."
The treatment protocol involves extraction, purification, culturing, and injection. Dr. Weiss said he injects 1 cc to 2 cc of lidocaine in the postauricular area before taking the biopsies. The tissue is sent to Fibrocell for purification and culturing of the fibroblasts.
After 3 months, a vial containing 15 million to 20 million fibroblasts is shipped back to arrive just prior to the patient appointment. "Advise your patients that advance notice of at least 2 business days is required to reschedule treatment. By the FDA guidelines, you cannot inject the cells the next day because the viability count goes down." Treatment at 3- to 6-week intervals is recommended.
Because the product is a biologic, the FDA requires physician certification prior to use of azficel-T. "If you are interested in offering this to your patients, the company will train your staff. There are webinars," Dr. Weiss said. Staff training reinforces proper packing, handling, and shipping.
The product has to be handled gingerly and injected superficially in the papillary dermis, he said. "This is for superficial rhytids; this is not a deep volume filler." The needle will be visible under the skin if you are injecting in the right plane.
Resuspend the cells by inverting the vial slowly a few times. Be careful putting the vial on the tray because it can fall over. Apply light pressure to the plunger and inject slowly along the wrinkle line.
Instruct the patient not to wash the site for 24 hours and not to scrub, rub, massage, or apply any pressure for 72 hours. An NSAID can be taken for pain.
Also, instruct patients to call if they experience any adverse reactions. Fibrocell plans to launch a patient registry in July 2012 that will track hypersensitivity reactions.
Dr. Weiss participated in one of the clinical trials as early as 2003. "What we’ve noted on long-term follow-up is that patients maintain this improvement, often for many, many years, which is interesting."
"I’ve seen some phenomenal results, particularly in studies of acne scars, which is another potential application the company is applying for," he added.
Dr. Weiss has been an investigator for Fibrocell Science, which sponsored his presentation.
ORLANDO – One number keeps coming up with the new autologous fibroblast biologic product that enhances a patient’s nasolabial wrinkles: three.
"There are a lot of threes: three biopsies 3 mm in size, a 3 month’s wait, and three treatment sessions," Dr. Robert Weiss said at the annual meeting of the Florida Society of Dermatology Surgeons.
The Food and Drug Administration cleared the marketing of Fibrocell Science’s azficel-T (LaVív) in June 2011, and the product is now reaching the market. "It’s very exciting to be right at the launch of this," said Dr .Weiss, a dermatologist in private practice in Hunt Valley, Md., and with the dermatology department at Johns Hopkins University, Baltimore. "I’ve been working on this for close to a decade, so it was a personal triumph to see this finally cleared by the FDA."
The FDA based its clearance on two identically designed, multicenter, double-blind, vehicle-controlled studies. A total of 421 patients with moderate to severe nasolabial fold wrinkles were assessed, with 210 being randomized to azficel-T and 211 to vehicle injections. The average age was 55 years, and 90% were women.
A 2-point improvement in the appearance of the nasolabial fold wrinkles was the primary outcome. Physicians at the study sites who were not injectors blindly rated results.
At the 6-month follow-up, doctors observed the 2-point improvement in 33% of the azficel-T group, compared with 7% of controls in the first study. In the second study, 19% of the azficel-T group had at least a 2-point improvement, compared with 7% of controls. These differences were on the borderline of statistical significance, Dr. Weiss said.
"The FDA was convinced that this was effective. We were certainly very convinced by what we observed with the patients," Dr. Weiss said.
Patient reported outcomes were more statistically significant, with 57% reporting a 2-point improvement, compared with 30% in the vehicle group in an intent-to-treat analysis of the first study. In the second study, 45% of the treated patients reported this outcome, compared with 18% of the vehicle group.
The most common adverse effects were injection site reactions lasting approximately 2 hours, Dr. Weiss said. These events included erythema, bruising, and swelling. Facial or eyelid edema, hypersensitivity or decreased skin sensation at the injection site, and postprocedural discomfort were among the adverse events reported by 1% of patients or fewer.
"One of the first questions patients ask me is: ‘How are you going to make sure I’m getting my own cells?’ " Dr. Weiss explained there are strict controls. Each patient is assigned a lot number. Vials for injection are labeled with each patient’s initials, date of birth, and a unique identification number. "All the critical steps are verified twice."
The treatment protocol involves extraction, purification, culturing, and injection. Dr. Weiss said he injects 1 cc to 2 cc of lidocaine in the postauricular area before taking the biopsies. The tissue is sent to Fibrocell for purification and culturing of the fibroblasts.
After 3 months, a vial containing 15 million to 20 million fibroblasts is shipped back to arrive just prior to the patient appointment. "Advise your patients that advance notice of at least 2 business days is required to reschedule treatment. By the FDA guidelines, you cannot inject the cells the next day because the viability count goes down." Treatment at 3- to 6-week intervals is recommended.
Because the product is a biologic, the FDA requires physician certification prior to use of azficel-T. "If you are interested in offering this to your patients, the company will train your staff. There are webinars," Dr. Weiss said. Staff training reinforces proper packing, handling, and shipping.
The product has to be handled gingerly and injected superficially in the papillary dermis, he said. "This is for superficial rhytids; this is not a deep volume filler." The needle will be visible under the skin if you are injecting in the right plane.
Resuspend the cells by inverting the vial slowly a few times. Be careful putting the vial on the tray because it can fall over. Apply light pressure to the plunger and inject slowly along the wrinkle line.
Instruct the patient not to wash the site for 24 hours and not to scrub, rub, massage, or apply any pressure for 72 hours. An NSAID can be taken for pain.
Also, instruct patients to call if they experience any adverse reactions. Fibrocell plans to launch a patient registry in July 2012 that will track hypersensitivity reactions.
Dr. Weiss participated in one of the clinical trials as early as 2003. "What we’ve noted on long-term follow-up is that patients maintain this improvement, often for many, many years, which is interesting."
"I’ve seen some phenomenal results, particularly in studies of acne scars, which is another potential application the company is applying for," he added.
Dr. Weiss has been an investigator for Fibrocell Science, which sponsored his presentation.
ORLANDO – One number keeps coming up with the new autologous fibroblast biologic product that enhances a patient’s nasolabial wrinkles: three.
"There are a lot of threes: three biopsies 3 mm in size, a 3 month’s wait, and three treatment sessions," Dr. Robert Weiss said at the annual meeting of the Florida Society of Dermatology Surgeons.
The Food and Drug Administration cleared the marketing of Fibrocell Science’s azficel-T (LaVív) in June 2011, and the product is now reaching the market. "It’s very exciting to be right at the launch of this," said Dr .Weiss, a dermatologist in private practice in Hunt Valley, Md., and with the dermatology department at Johns Hopkins University, Baltimore. "I’ve been working on this for close to a decade, so it was a personal triumph to see this finally cleared by the FDA."
The FDA based its clearance on two identically designed, multicenter, double-blind, vehicle-controlled studies. A total of 421 patients with moderate to severe nasolabial fold wrinkles were assessed, with 210 being randomized to azficel-T and 211 to vehicle injections. The average age was 55 years, and 90% were women.
A 2-point improvement in the appearance of the nasolabial fold wrinkles was the primary outcome. Physicians at the study sites who were not injectors blindly rated results.
At the 6-month follow-up, doctors observed the 2-point improvement in 33% of the azficel-T group, compared with 7% of controls in the first study. In the second study, 19% of the azficel-T group had at least a 2-point improvement, compared with 7% of controls. These differences were on the borderline of statistical significance, Dr. Weiss said.
"The FDA was convinced that this was effective. We were certainly very convinced by what we observed with the patients," Dr. Weiss said.
Patient reported outcomes were more statistically significant, with 57% reporting a 2-point improvement, compared with 30% in the vehicle group in an intent-to-treat analysis of the first study. In the second study, 45% of the treated patients reported this outcome, compared with 18% of the vehicle group.
The most common adverse effects were injection site reactions lasting approximately 2 hours, Dr. Weiss said. These events included erythema, bruising, and swelling. Facial or eyelid edema, hypersensitivity or decreased skin sensation at the injection site, and postprocedural discomfort were among the adverse events reported by 1% of patients or fewer.
"One of the first questions patients ask me is: ‘How are you going to make sure I’m getting my own cells?’ " Dr. Weiss explained there are strict controls. Each patient is assigned a lot number. Vials for injection are labeled with each patient’s initials, date of birth, and a unique identification number. "All the critical steps are verified twice."
The treatment protocol involves extraction, purification, culturing, and injection. Dr. Weiss said he injects 1 cc to 2 cc of lidocaine in the postauricular area before taking the biopsies. The tissue is sent to Fibrocell for purification and culturing of the fibroblasts.
After 3 months, a vial containing 15 million to 20 million fibroblasts is shipped back to arrive just prior to the patient appointment. "Advise your patients that advance notice of at least 2 business days is required to reschedule treatment. By the FDA guidelines, you cannot inject the cells the next day because the viability count goes down." Treatment at 3- to 6-week intervals is recommended.
Because the product is a biologic, the FDA requires physician certification prior to use of azficel-T. "If you are interested in offering this to your patients, the company will train your staff. There are webinars," Dr. Weiss said. Staff training reinforces proper packing, handling, and shipping.
The product has to be handled gingerly and injected superficially in the papillary dermis, he said. "This is for superficial rhytids; this is not a deep volume filler." The needle will be visible under the skin if you are injecting in the right plane.
Resuspend the cells by inverting the vial slowly a few times. Be careful putting the vial on the tray because it can fall over. Apply light pressure to the plunger and inject slowly along the wrinkle line.
Instruct the patient not to wash the site for 24 hours and not to scrub, rub, massage, or apply any pressure for 72 hours. An NSAID can be taken for pain.
Also, instruct patients to call if they experience any adverse reactions. Fibrocell plans to launch a patient registry in July 2012 that will track hypersensitivity reactions.
Dr. Weiss participated in one of the clinical trials as early as 2003. "What we’ve noted on long-term follow-up is that patients maintain this improvement, often for many, many years, which is interesting."
"I’ve seen some phenomenal results, particularly in studies of acne scars, which is another potential application the company is applying for," he added.
Dr. Weiss has been an investigator for Fibrocell Science, which sponsored his presentation.
FROM THE ANNUAL MEEETING OF THE FLORIDA SOCIETY OF DERMATOLOGIC SURGEONS
Major Finding: Nasolabial fold wrinkles improved 2 points on a physician rating scale for 33% of patients in one study and 19% in another.
Data Source: Two trials of 421 patients with moderate to severe nasolabial fold wrinkles.
Disclosures: Dr. Weiss has been an investigator for Fibrocell Science, which sponsored his presentation.