User login
Infectious Diseases Society of America (IDSA)/ Society for Healthcare Epidemiology of America (SHEA)/ HIV Medicine Association (HIVMA)/ Pediatric Infectious Diseases Society (PIDS): IDWeek 2015
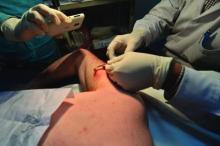
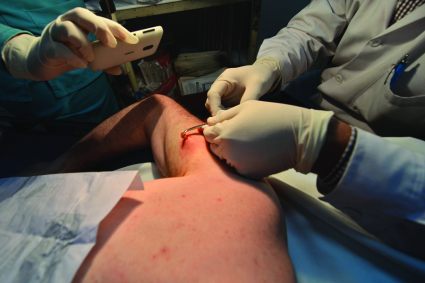
IDWEEK: Smother and pull, but don’t chop – how to identify and remove botfly larvae
SAN DIEGO – Identifying a botfly infestation can be the toughest part of treating it. It’s not uncommon for travelers to return from Costa Rica, Belize, and other Central and South American countries with Dermatobia hominis infestations, but clinicians in North America are not necessarily comfortable diagnosing them or removing the larvae,, said Dr. Susan McLellan, who explained how to do so at an annual scientific meeting on infectious diseases.
Clinicians here may confuse botfly infestations with infected sebaceous cysts, staphylococcal infections, cellulitis, or even cutaneous leismaniasis. To make the diagnosis, ask about travel history in patients who present with painful furuncular lesions, said Dr. McLellan, associate professor of clinical medicine at Tulane University, New Orleans.
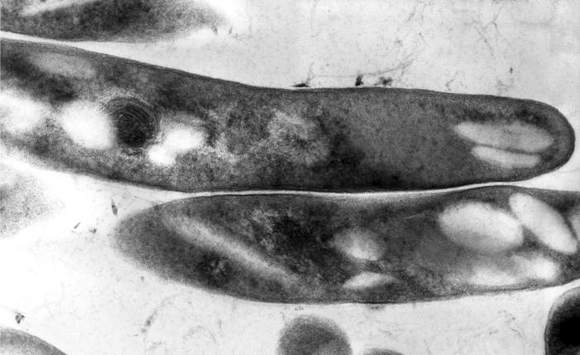
Closer inspection of these lesions may reveal the two dark spiracles, or breathing holes, of the larvae, which are key to removing them, Dr. Mclellan added. “The larvae have to stick their little spiracles out to breathe,” she said. “You can get them to come further out by covering up these breathing holes with anything occlusive. It can be peanut butter. It can be petroleum jelly. Just cover them up until they stick themselves out more, and then you can grab them and pull them out.”
The “first smother, then pull” approach does not always work with botfly larvae because they have spines on their bodies that can hook into subepidermal tissue, Dr. McLellan noted. “Sometimes you have to make a very, very careful incision,” she said. “Do not incise the larva at all, though. Just pop it out. These lesions do not get superinfected unless you chop up the larva while they are still inside.”
Also do not bother asking patients if they were bitten by the black adult botfly, said Dr. McLellan. Adult D. hominis do not bite, but instead lay their eggs on mosquitos. “Any biting insect that flies could potentially be a carrier,” Dr. McLellan noted. “The mother botfly catches the other flying insect, dumps her eggs on it, and that other insect starts delivering them.” A single mosquito bite can transfer several botfly eggs to a human host. When the larvae hatch and burrow into the skin, they cause pain, redness, and swelling until removed.
Dr. McLellan spoke at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. She reported having no conflicts of interest.
SAN DIEGO – Identifying a botfly infestation can be the toughest part of treating it. It’s not uncommon for travelers to return from Costa Rica, Belize, and other Central and South American countries with Dermatobia hominis infestations, but clinicians in North America are not necessarily comfortable diagnosing them or removing the larvae,, said Dr. Susan McLellan, who explained how to do so at an annual scientific meeting on infectious diseases.
Clinicians here may confuse botfly infestations with infected sebaceous cysts, staphylococcal infections, cellulitis, or even cutaneous leismaniasis. To make the diagnosis, ask about travel history in patients who present with painful furuncular lesions, said Dr. McLellan, associate professor of clinical medicine at Tulane University, New Orleans.
Closer inspection of these lesions may reveal the two dark spiracles, or breathing holes, of the larvae, which are key to removing them, Dr. Mclellan added. “The larvae have to stick their little spiracles out to breathe,” she said. “You can get them to come further out by covering up these breathing holes with anything occlusive. It can be peanut butter. It can be petroleum jelly. Just cover them up until they stick themselves out more, and then you can grab them and pull them out.”
The “first smother, then pull” approach does not always work with botfly larvae because they have spines on their bodies that can hook into subepidermal tissue, Dr. McLellan noted. “Sometimes you have to make a very, very careful incision,” she said. “Do not incise the larva at all, though. Just pop it out. These lesions do not get superinfected unless you chop up the larva while they are still inside.”
Also do not bother asking patients if they were bitten by the black adult botfly, said Dr. McLellan. Adult D. hominis do not bite, but instead lay their eggs on mosquitos. “Any biting insect that flies could potentially be a carrier,” Dr. McLellan noted. “The mother botfly catches the other flying insect, dumps her eggs on it, and that other insect starts delivering them.” A single mosquito bite can transfer several botfly eggs to a human host. When the larvae hatch and burrow into the skin, they cause pain, redness, and swelling until removed.
Dr. McLellan spoke at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. She reported having no conflicts of interest.
SAN DIEGO – Identifying a botfly infestation can be the toughest part of treating it. It’s not uncommon for travelers to return from Costa Rica, Belize, and other Central and South American countries with Dermatobia hominis infestations, but clinicians in North America are not necessarily comfortable diagnosing them or removing the larvae,, said Dr. Susan McLellan, who explained how to do so at an annual scientific meeting on infectious diseases.
Clinicians here may confuse botfly infestations with infected sebaceous cysts, staphylococcal infections, cellulitis, or even cutaneous leismaniasis. To make the diagnosis, ask about travel history in patients who present with painful furuncular lesions, said Dr. McLellan, associate professor of clinical medicine at Tulane University, New Orleans.
Closer inspection of these lesions may reveal the two dark spiracles, or breathing holes, of the larvae, which are key to removing them, Dr. Mclellan added. “The larvae have to stick their little spiracles out to breathe,” she said. “You can get them to come further out by covering up these breathing holes with anything occlusive. It can be peanut butter. It can be petroleum jelly. Just cover them up until they stick themselves out more, and then you can grab them and pull them out.”
The “first smother, then pull” approach does not always work with botfly larvae because they have spines on their bodies that can hook into subepidermal tissue, Dr. McLellan noted. “Sometimes you have to make a very, very careful incision,” she said. “Do not incise the larva at all, though. Just pop it out. These lesions do not get superinfected unless you chop up the larva while they are still inside.”
Also do not bother asking patients if they were bitten by the black adult botfly, said Dr. McLellan. Adult D. hominis do not bite, but instead lay their eggs on mosquitos. “Any biting insect that flies could potentially be a carrier,” Dr. McLellan noted. “The mother botfly catches the other flying insect, dumps her eggs on it, and that other insect starts delivering them.” A single mosquito bite can transfer several botfly eggs to a human host. When the larvae hatch and burrow into the skin, they cause pain, redness, and swelling until removed.
Dr. McLellan spoke at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. She reported having no conflicts of interest.
EXPERT ANALYSIS FROM IDWEEK 2015
Palivizumab cut odds of RSV hospitalization, belying AAP recommendations
SAN DIEGO – Prophylactic palivizumab cut the odds of hospitalization for severe respiratory syncytial virus (RSV) infection by about 75% among preterm infants born at more than 29 weeks’ gestational age – even those without congenital heart disease or chronic lung disease, investigators reported.
The finding belies the American Academy of Pediatrics’ recommendation to limit use of the humanized monoclonal antibody to infants born before 29 weeks’ gestation and to children who have other risk factors for severe RSV infection, Dr. Ram Yogev of Ann and Robert H. Lurie Children’s Hospital, Chicago, said in an interview.
“Our results validate the older studies, except this was done in real life,” added lead investigator Dr. Eric Simões of Children’s Hospital Colorado, Aurora, who presented the findings at an annual scientific meeting on infectious diseases.
RSV usually causes mild upper respiratory tract infections, but premature infants and children who have comorbid cardiac or pulmonary disease can develop severe infections of the lower respiratory tract. Weekly palivizumab dosing was 45%-80% effective in preventing RSV-related hospitalizations in clinical trials of these high-risk patients, noted Dr. Simões and his associates.
But in 2014, the American Academy of Pediatrics reviewed the literature and revised its guidance to limit palivizumab to preterm infants born before 29 weeks’ gestation and to infants with comorbid risk conditions. The biologic “has been shown to have a limited effect on reducing RSV hospitalization,” the academy concluded. The update drew criticism from some pediatric infectious disease experts, who contended that AAP cited observational studies that actually contradicted its conclusions.
To further examine the issue, Dr. Simões and associates analyzed data from a multicenter study of high-risk infants and children under the age of 2 years who had been hospitalized with lower respiratory tract infections. Between 2002 and 2006, 849 of these patients had a nasopharyngeal wash or endotracheal aspirate tested for RSV, and 403 were positive. The investigators determined that the odds of a positive RSV test were 58% lower for patients who had received prophylactic palivizumab, compared with patients who had not (95% confidence interval for efficacy, 43%-69%; P less than .0001).
Furthermore, palivizumab was 75% effective against severe RSV disease in preterm patients born at 29-35 weeks’ gestation who were chronologically younger than 6 months and had no congenital heart disease or chronic lung disease, said the investigators. Based on that finding, AAP should reconsider its recommendations on palivizumab, said Dr. Yogev.
“This study should answer some of the issues raised in the AAP recommendations,” added Dr. Simões.
Palivizumab did not prevent hospitalizations for human metapneumovirus (hMPV) infection, which further validated the results on RSV, said Dr. Simões. The test-negative case-control design of the study “cuts out the whole issue of [bias due to] care-seeking behavior,” he added. “But children on palivizumab would be more seriously ill, so how do you correct for that? Traditional methods are based on multivariate analysis, but we used propensity scores.” A goodness-of-fit test showed that this approach controlled for all variables except palivizumab exposure and age greater than 6 months, meaning that only these two factors were significantly protective against RSV infection, he said.
IDWeek marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
MedImmune sponsored the original study of hMPV tract infections, from which these data were obtained. Dr. Simões reported having received funding support and consulting fees from MedImmune.
SAN DIEGO – Prophylactic palivizumab cut the odds of hospitalization for severe respiratory syncytial virus (RSV) infection by about 75% among preterm infants born at more than 29 weeks’ gestational age – even those without congenital heart disease or chronic lung disease, investigators reported.
The finding belies the American Academy of Pediatrics’ recommendation to limit use of the humanized monoclonal antibody to infants born before 29 weeks’ gestation and to children who have other risk factors for severe RSV infection, Dr. Ram Yogev of Ann and Robert H. Lurie Children’s Hospital, Chicago, said in an interview.
“Our results validate the older studies, except this was done in real life,” added lead investigator Dr. Eric Simões of Children’s Hospital Colorado, Aurora, who presented the findings at an annual scientific meeting on infectious diseases.
RSV usually causes mild upper respiratory tract infections, but premature infants and children who have comorbid cardiac or pulmonary disease can develop severe infections of the lower respiratory tract. Weekly palivizumab dosing was 45%-80% effective in preventing RSV-related hospitalizations in clinical trials of these high-risk patients, noted Dr. Simões and his associates.
But in 2014, the American Academy of Pediatrics reviewed the literature and revised its guidance to limit palivizumab to preterm infants born before 29 weeks’ gestation and to infants with comorbid risk conditions. The biologic “has been shown to have a limited effect on reducing RSV hospitalization,” the academy concluded. The update drew criticism from some pediatric infectious disease experts, who contended that AAP cited observational studies that actually contradicted its conclusions.
To further examine the issue, Dr. Simões and associates analyzed data from a multicenter study of high-risk infants and children under the age of 2 years who had been hospitalized with lower respiratory tract infections. Between 2002 and 2006, 849 of these patients had a nasopharyngeal wash or endotracheal aspirate tested for RSV, and 403 were positive. The investigators determined that the odds of a positive RSV test were 58% lower for patients who had received prophylactic palivizumab, compared with patients who had not (95% confidence interval for efficacy, 43%-69%; P less than .0001).
Furthermore, palivizumab was 75% effective against severe RSV disease in preterm patients born at 29-35 weeks’ gestation who were chronologically younger than 6 months and had no congenital heart disease or chronic lung disease, said the investigators. Based on that finding, AAP should reconsider its recommendations on palivizumab, said Dr. Yogev.
“This study should answer some of the issues raised in the AAP recommendations,” added Dr. Simões.
Palivizumab did not prevent hospitalizations for human metapneumovirus (hMPV) infection, which further validated the results on RSV, said Dr. Simões. The test-negative case-control design of the study “cuts out the whole issue of [bias due to] care-seeking behavior,” he added. “But children on palivizumab would be more seriously ill, so how do you correct for that? Traditional methods are based on multivariate analysis, but we used propensity scores.” A goodness-of-fit test showed that this approach controlled for all variables except palivizumab exposure and age greater than 6 months, meaning that only these two factors were significantly protective against RSV infection, he said.
IDWeek marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
MedImmune sponsored the original study of hMPV tract infections, from which these data were obtained. Dr. Simões reported having received funding support and consulting fees from MedImmune.
SAN DIEGO – Prophylactic palivizumab cut the odds of hospitalization for severe respiratory syncytial virus (RSV) infection by about 75% among preterm infants born at more than 29 weeks’ gestational age – even those without congenital heart disease or chronic lung disease, investigators reported.
The finding belies the American Academy of Pediatrics’ recommendation to limit use of the humanized monoclonal antibody to infants born before 29 weeks’ gestation and to children who have other risk factors for severe RSV infection, Dr. Ram Yogev of Ann and Robert H. Lurie Children’s Hospital, Chicago, said in an interview.
“Our results validate the older studies, except this was done in real life,” added lead investigator Dr. Eric Simões of Children’s Hospital Colorado, Aurora, who presented the findings at an annual scientific meeting on infectious diseases.
RSV usually causes mild upper respiratory tract infections, but premature infants and children who have comorbid cardiac or pulmonary disease can develop severe infections of the lower respiratory tract. Weekly palivizumab dosing was 45%-80% effective in preventing RSV-related hospitalizations in clinical trials of these high-risk patients, noted Dr. Simões and his associates.
But in 2014, the American Academy of Pediatrics reviewed the literature and revised its guidance to limit palivizumab to preterm infants born before 29 weeks’ gestation and to infants with comorbid risk conditions. The biologic “has been shown to have a limited effect on reducing RSV hospitalization,” the academy concluded. The update drew criticism from some pediatric infectious disease experts, who contended that AAP cited observational studies that actually contradicted its conclusions.
To further examine the issue, Dr. Simões and associates analyzed data from a multicenter study of high-risk infants and children under the age of 2 years who had been hospitalized with lower respiratory tract infections. Between 2002 and 2006, 849 of these patients had a nasopharyngeal wash or endotracheal aspirate tested for RSV, and 403 were positive. The investigators determined that the odds of a positive RSV test were 58% lower for patients who had received prophylactic palivizumab, compared with patients who had not (95% confidence interval for efficacy, 43%-69%; P less than .0001).
Furthermore, palivizumab was 75% effective against severe RSV disease in preterm patients born at 29-35 weeks’ gestation who were chronologically younger than 6 months and had no congenital heart disease or chronic lung disease, said the investigators. Based on that finding, AAP should reconsider its recommendations on palivizumab, said Dr. Yogev.
“This study should answer some of the issues raised in the AAP recommendations,” added Dr. Simões.
Palivizumab did not prevent hospitalizations for human metapneumovirus (hMPV) infection, which further validated the results on RSV, said Dr. Simões. The test-negative case-control design of the study “cuts out the whole issue of [bias due to] care-seeking behavior,” he added. “But children on palivizumab would be more seriously ill, so how do you correct for that? Traditional methods are based on multivariate analysis, but we used propensity scores.” A goodness-of-fit test showed that this approach controlled for all variables except palivizumab exposure and age greater than 6 months, meaning that only these two factors were significantly protective against RSV infection, he said.
IDWeek marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
MedImmune sponsored the original study of hMPV tract infections, from which these data were obtained. Dr. Simões reported having received funding support and consulting fees from MedImmune.
AT IDWEEK 2015
Key clinical point: Palivizumab cut the odds of severe RSV infection by nearly 75% among patients who AAP has recommended not routinely receive the prophylactic biologic.
Major finding: The odds of confirmed RSV infection were 74% lower among treated patients, compared with untreated patients (P less than .0001).
Data source: Multicenter case-control study of 849 infants and children who had been hospitalized with severe lower respiratory tract infections.
Disclosures: MedImmune sponsored the original study of human metapneumovirus respiratory tract infections, from which these data were obtained. Dr. Simões reported funding support and consulting fees from MedImmune.
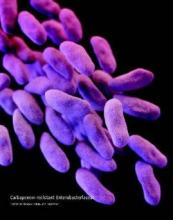
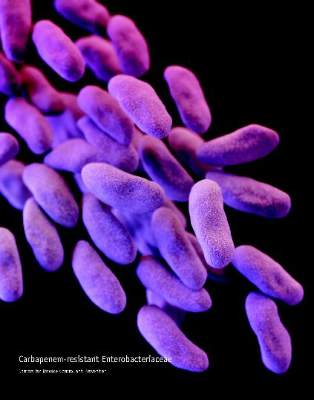
IDWeek: VA found no evidence of CRE transmission through duodenoscopes
SAN DIEGO – Researchers found “no compelling evidence” that carbapenem-resistant enterobacteriaceae were transmitted to Veterans Affairs patients during more than 55,000 endoscopic retrograde cholangiopancreatography (ERCP) and ultrasonography (EUS) procedures, Dr. Russell Ryono said at an annual meeting on infectious diseases.
The few clusters of cases uncovered by the 5-year retrospective analysis were separated not only in time, but by culture-negative patients treated with the same duodenoscopes at the same facilities, said Dr. Ryono of the public health surveillance and research office at the Department of Veterans Affairs in Palo Alto, Calif. “Our findings do not provide evidence of CRE transmission related to ERCP or EUS in Veterans Affairs,” Dr. Ryono and his associates concluded.
Contaminated duodenoscopes made headlines across the country this year after they caused outbreaks of fatal CRE infections in Los Angeles County. The Food and Drug Administration later acknowledged that the “complex design of the devices makes it difficult to remove contaminants compared to other types of endoscopes,” and both the Centers for Disease Control and Prevention and FDA recommended surveillance and reprocessing practices to help keep the scopes from transmitting serious infections.
In response to these concerns, the VA mined its data warehouses for ERCP and EUS procedures and CRE isolates recovered between January 2010 and the end of February 2015. Investigators looked for patients who were culture positive for the same type of CRE, underwent ERCP/EUS at the same facility within 6 months of each other, and were treated with the same scope or with an unidentified scope. Within this group, researchers honed in on “pairs” – or clusters – of patients in which the second patient exposed to the scope was CRE negative before the procedure and CRE positive afterward.
The initial query yielded more than 55,600 procedures among 40,329 veterans at 38 VA centers, as well as nearly 5,000 CRE isolates, more than half of which were cultured from urine samples, said Dr. Ryono. However, only 81 ERCP/EUS patients had CRE cultured from any anatomical site at any time. Among them, 57 patients were CRE negative before their procedure but CRE positive afterward, Dr. Ryono said.
There were 10 pairs of culture-positive patients treated at four facilities, one of which did not track serial numbers of duodenoscopes, according to Dr. Ryono. At that facility, procedure dates for pairs were 68-143 days apart, he said. Procedure dates for pairs at the other three facilities were spaced at least 82 days apart. Based on such extensive temporal spacing and the fact that the same scopes were used on culture-negative patients during intervening times, the investigators concluded that duodenoscopes were unlikely to have caused CRE infection or colonization.
Dr. Ryono noted several study limitations, however. Not only were CRE isolates unavailable for epidemiologic testing, but researchers also could not determine the extent to which facilities had implemented the 2010 guidelines for CLSI breakpoints. “Certainly, those facilities that have implemented those guidelines are more likely to catch CRE,” he said. Also, VA does not routinely screen patients for CRE before they undergo ERCP or EUS, “so it is very possible that some of our patients might have been colonized with CRE before the procedure, and we would not have been able to capture that,” he added. “Given the limitations of this review, the possibility that there was some transmission certainly cannot be completely excluded.”
Dr. Ryono reported these findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The researchers declared no funding sources or conflicts of interest.
SAN DIEGO – Researchers found “no compelling evidence” that carbapenem-resistant enterobacteriaceae were transmitted to Veterans Affairs patients during more than 55,000 endoscopic retrograde cholangiopancreatography (ERCP) and ultrasonography (EUS) procedures, Dr. Russell Ryono said at an annual meeting on infectious diseases.
The few clusters of cases uncovered by the 5-year retrospective analysis were separated not only in time, but by culture-negative patients treated with the same duodenoscopes at the same facilities, said Dr. Ryono of the public health surveillance and research office at the Department of Veterans Affairs in Palo Alto, Calif. “Our findings do not provide evidence of CRE transmission related to ERCP or EUS in Veterans Affairs,” Dr. Ryono and his associates concluded.
Contaminated duodenoscopes made headlines across the country this year after they caused outbreaks of fatal CRE infections in Los Angeles County. The Food and Drug Administration later acknowledged that the “complex design of the devices makes it difficult to remove contaminants compared to other types of endoscopes,” and both the Centers for Disease Control and Prevention and FDA recommended surveillance and reprocessing practices to help keep the scopes from transmitting serious infections.
In response to these concerns, the VA mined its data warehouses for ERCP and EUS procedures and CRE isolates recovered between January 2010 and the end of February 2015. Investigators looked for patients who were culture positive for the same type of CRE, underwent ERCP/EUS at the same facility within 6 months of each other, and were treated with the same scope or with an unidentified scope. Within this group, researchers honed in on “pairs” – or clusters – of patients in which the second patient exposed to the scope was CRE negative before the procedure and CRE positive afterward.
The initial query yielded more than 55,600 procedures among 40,329 veterans at 38 VA centers, as well as nearly 5,000 CRE isolates, more than half of which were cultured from urine samples, said Dr. Ryono. However, only 81 ERCP/EUS patients had CRE cultured from any anatomical site at any time. Among them, 57 patients were CRE negative before their procedure but CRE positive afterward, Dr. Ryono said.
There were 10 pairs of culture-positive patients treated at four facilities, one of which did not track serial numbers of duodenoscopes, according to Dr. Ryono. At that facility, procedure dates for pairs were 68-143 days apart, he said. Procedure dates for pairs at the other three facilities were spaced at least 82 days apart. Based on such extensive temporal spacing and the fact that the same scopes were used on culture-negative patients during intervening times, the investigators concluded that duodenoscopes were unlikely to have caused CRE infection or colonization.
Dr. Ryono noted several study limitations, however. Not only were CRE isolates unavailable for epidemiologic testing, but researchers also could not determine the extent to which facilities had implemented the 2010 guidelines for CLSI breakpoints. “Certainly, those facilities that have implemented those guidelines are more likely to catch CRE,” he said. Also, VA does not routinely screen patients for CRE before they undergo ERCP or EUS, “so it is very possible that some of our patients might have been colonized with CRE before the procedure, and we would not have been able to capture that,” he added. “Given the limitations of this review, the possibility that there was some transmission certainly cannot be completely excluded.”
Dr. Ryono reported these findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The researchers declared no funding sources or conflicts of interest.
SAN DIEGO – Researchers found “no compelling evidence” that carbapenem-resistant enterobacteriaceae were transmitted to Veterans Affairs patients during more than 55,000 endoscopic retrograde cholangiopancreatography (ERCP) and ultrasonography (EUS) procedures, Dr. Russell Ryono said at an annual meeting on infectious diseases.
The few clusters of cases uncovered by the 5-year retrospective analysis were separated not only in time, but by culture-negative patients treated with the same duodenoscopes at the same facilities, said Dr. Ryono of the public health surveillance and research office at the Department of Veterans Affairs in Palo Alto, Calif. “Our findings do not provide evidence of CRE transmission related to ERCP or EUS in Veterans Affairs,” Dr. Ryono and his associates concluded.
Contaminated duodenoscopes made headlines across the country this year after they caused outbreaks of fatal CRE infections in Los Angeles County. The Food and Drug Administration later acknowledged that the “complex design of the devices makes it difficult to remove contaminants compared to other types of endoscopes,” and both the Centers for Disease Control and Prevention and FDA recommended surveillance and reprocessing practices to help keep the scopes from transmitting serious infections.
In response to these concerns, the VA mined its data warehouses for ERCP and EUS procedures and CRE isolates recovered between January 2010 and the end of February 2015. Investigators looked for patients who were culture positive for the same type of CRE, underwent ERCP/EUS at the same facility within 6 months of each other, and were treated with the same scope or with an unidentified scope. Within this group, researchers honed in on “pairs” – or clusters – of patients in which the second patient exposed to the scope was CRE negative before the procedure and CRE positive afterward.
The initial query yielded more than 55,600 procedures among 40,329 veterans at 38 VA centers, as well as nearly 5,000 CRE isolates, more than half of which were cultured from urine samples, said Dr. Ryono. However, only 81 ERCP/EUS patients had CRE cultured from any anatomical site at any time. Among them, 57 patients were CRE negative before their procedure but CRE positive afterward, Dr. Ryono said.
There were 10 pairs of culture-positive patients treated at four facilities, one of which did not track serial numbers of duodenoscopes, according to Dr. Ryono. At that facility, procedure dates for pairs were 68-143 days apart, he said. Procedure dates for pairs at the other three facilities were spaced at least 82 days apart. Based on such extensive temporal spacing and the fact that the same scopes were used on culture-negative patients during intervening times, the investigators concluded that duodenoscopes were unlikely to have caused CRE infection or colonization.
Dr. Ryono noted several study limitations, however. Not only were CRE isolates unavailable for epidemiologic testing, but researchers also could not determine the extent to which facilities had implemented the 2010 guidelines for CLSI breakpoints. “Certainly, those facilities that have implemented those guidelines are more likely to catch CRE,” he said. Also, VA does not routinely screen patients for CRE before they undergo ERCP or EUS, “so it is very possible that some of our patients might have been colonized with CRE before the procedure, and we would not have been able to capture that,” he added. “Given the limitations of this review, the possibility that there was some transmission certainly cannot be completely excluded.”
Dr. Ryono reported these findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The researchers declared no funding sources or conflicts of interest.
AT IDWEEK 2015
Key clinical point: In a large retrospective review, the Department of Veterans Affairs found no evidence of transmission of carbapenem-resistant enterobacteriaceae through ERCP or EUS.
Major finding: The review found no compelling evidence of CRE transmission.
Data source: Retrospective study of 55,676 ERCP/EUS procedures in 40,329 veterans and 4,914 CRE isolates from 2,383 patients.
Disclosures:. The researchers reported no funding sources or conflicts of interest.
IDWeek: Despite better drugs, HCV deaths keep rising
SAN DIEGO – Despite better therapies, deaths from hepatitis C virus (HCV) infection continue to rise, indicating poor penetrance of medications and care to patients who need them, Dr. Scott Holmberg said at an annual scientific meeting on infectious diseases.
“Deaths in chronic HCV–infected persons, even when grossly under-enumerated on death certificates, far outstrip deaths from 60 other infectious conditions reportable to CDC,” said Dr. Holmberg of the Centers for Disease Control and Prevention Division of Viral Hepatitis in Atlanta.
Drugs for chronic HCV infection have vastly improved in the past several years, yielding far better rates of sustained viral response (SVR) and high chances of cure after 8-24 weeks of treatment. To see if better antiviral therapies have affected HCV mortality rates, Dr. Holmberg and his associates studied ICD-9 data from Multiple Cause of Death records for all U.S. death certificates between 2003 and 2013. They divided deaths that were linked to HCV or 60 other nationally notifiable infectious diseases by U.S. Census numbers for the same year. They also examined data from the Chronic Hepatitis Cohort Study (Clin Infect Dis. 2014;58:1055-61), which includes patients presumed to have adequate access to HCV treatment.
Chronic HCV-related deaths climbed from about 12,000 annually in 2003 to more than 19,000 in 2013, said Dr. Holmberg. In contrast, deaths from the 60 other reportable infectious diseases dropped from about 25,000 annually to below 20,000 per year. Annual deaths tied to HIV infection ranked second behind HCV at about 8,800, followed by Staphylococcus aureus (including MRSA), hepatitis B virus, tuberculosis, and pneumococcal disease. “This does not include 4,444 adult influenza deaths, but does include 165 childhood influenza deaths in 2013,” Dr. Holmberg noted.
The analysis of the Chronic Hepatitis Cohort Study revealed a doubling in mortality from chronic HCV infection among patients who should have had adequate access to treatment, according to Dr. Holmberg. For every 100 person-years of observation, about 2.5 people died from consequences of chronic HCV infection in 2007, compared with about 5.5 in 2013, he said. “Hidden mortality from HCV is considerable,” he added. “Only 19% of HCV patients who died had their infection noted anywhere on their death certificates, despite the fact that more than 75% had premortem evidence of liver disease.”
Uptake of sofosbuvir-based regimens more than quintupled in the second quarter of 2015, compared with a year earlier, according to data from Gilead Sciences presented by Dr. Holmberg. But high drug costs have spurred state Medicaid programs and private payers to stipulate many preapproval requirements, he noted. Patients must be drug and alcohol free for at least 6 months, and in many states, must provide evidence of liver scarring from a recent biopsy or FibroScan, which is not always easy to access. “This is often a barrier,” Dr. Holmberg said. “For those in more rural areas, finding a specialist, as required by many state Medicaid offices, can be very difficult.”
And there are even more obstacles. Many clinicians still see HCV as a “benign condition,” and patients often have other urgent health, social, or financial problems, Dr. Holmberg said. The public, for its part, may not prioritize infectious diseases. “These patients lack a strong advocacy group,” he added. “Most are former injection drug users, and the public is often reluctant to help them.”
So what are the measurable results of these barriers? Among about 3.2 million individuals in the United States with chronic HCV infection, only half were ever tested for HCV, 38% received some sort of care related to their infection, 11% were treated, and 6% achieved SVR, Dr. Holmberg and his associates noted in a perspective piece (N Engl J Med. 2013;368:1859-861).
At the same time, the United States faces an emerging epidemic of new HCV infections in nonurban areas among young persons who inject drugs (MMWR. 64;453-8). “This is really a tale of two epidemics,” he added. “Control of the chronic and the acute outbreaks will require a multipronged approach, with interventions along a testing to cure continuum of care.”
Dr. Holmberg and his associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported no funding sources and had no financial disclosures.
SAN DIEGO – Despite better therapies, deaths from hepatitis C virus (HCV) infection continue to rise, indicating poor penetrance of medications and care to patients who need them, Dr. Scott Holmberg said at an annual scientific meeting on infectious diseases.
“Deaths in chronic HCV–infected persons, even when grossly under-enumerated on death certificates, far outstrip deaths from 60 other infectious conditions reportable to CDC,” said Dr. Holmberg of the Centers for Disease Control and Prevention Division of Viral Hepatitis in Atlanta.
Drugs for chronic HCV infection have vastly improved in the past several years, yielding far better rates of sustained viral response (SVR) and high chances of cure after 8-24 weeks of treatment. To see if better antiviral therapies have affected HCV mortality rates, Dr. Holmberg and his associates studied ICD-9 data from Multiple Cause of Death records for all U.S. death certificates between 2003 and 2013. They divided deaths that were linked to HCV or 60 other nationally notifiable infectious diseases by U.S. Census numbers for the same year. They also examined data from the Chronic Hepatitis Cohort Study (Clin Infect Dis. 2014;58:1055-61), which includes patients presumed to have adequate access to HCV treatment.
Chronic HCV-related deaths climbed from about 12,000 annually in 2003 to more than 19,000 in 2013, said Dr. Holmberg. In contrast, deaths from the 60 other reportable infectious diseases dropped from about 25,000 annually to below 20,000 per year. Annual deaths tied to HIV infection ranked second behind HCV at about 8,800, followed by Staphylococcus aureus (including MRSA), hepatitis B virus, tuberculosis, and pneumococcal disease. “This does not include 4,444 adult influenza deaths, but does include 165 childhood influenza deaths in 2013,” Dr. Holmberg noted.
The analysis of the Chronic Hepatitis Cohort Study revealed a doubling in mortality from chronic HCV infection among patients who should have had adequate access to treatment, according to Dr. Holmberg. For every 100 person-years of observation, about 2.5 people died from consequences of chronic HCV infection in 2007, compared with about 5.5 in 2013, he said. “Hidden mortality from HCV is considerable,” he added. “Only 19% of HCV patients who died had their infection noted anywhere on their death certificates, despite the fact that more than 75% had premortem evidence of liver disease.”
Uptake of sofosbuvir-based regimens more than quintupled in the second quarter of 2015, compared with a year earlier, according to data from Gilead Sciences presented by Dr. Holmberg. But high drug costs have spurred state Medicaid programs and private payers to stipulate many preapproval requirements, he noted. Patients must be drug and alcohol free for at least 6 months, and in many states, must provide evidence of liver scarring from a recent biopsy or FibroScan, which is not always easy to access. “This is often a barrier,” Dr. Holmberg said. “For those in more rural areas, finding a specialist, as required by many state Medicaid offices, can be very difficult.”
And there are even more obstacles. Many clinicians still see HCV as a “benign condition,” and patients often have other urgent health, social, or financial problems, Dr. Holmberg said. The public, for its part, may not prioritize infectious diseases. “These patients lack a strong advocacy group,” he added. “Most are former injection drug users, and the public is often reluctant to help them.”
So what are the measurable results of these barriers? Among about 3.2 million individuals in the United States with chronic HCV infection, only half were ever tested for HCV, 38% received some sort of care related to their infection, 11% were treated, and 6% achieved SVR, Dr. Holmberg and his associates noted in a perspective piece (N Engl J Med. 2013;368:1859-861).
At the same time, the United States faces an emerging epidemic of new HCV infections in nonurban areas among young persons who inject drugs (MMWR. 64;453-8). “This is really a tale of two epidemics,” he added. “Control of the chronic and the acute outbreaks will require a multipronged approach, with interventions along a testing to cure continuum of care.”
Dr. Holmberg and his associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported no funding sources and had no financial disclosures.
SAN DIEGO – Despite better therapies, deaths from hepatitis C virus (HCV) infection continue to rise, indicating poor penetrance of medications and care to patients who need them, Dr. Scott Holmberg said at an annual scientific meeting on infectious diseases.
“Deaths in chronic HCV–infected persons, even when grossly under-enumerated on death certificates, far outstrip deaths from 60 other infectious conditions reportable to CDC,” said Dr. Holmberg of the Centers for Disease Control and Prevention Division of Viral Hepatitis in Atlanta.
Drugs for chronic HCV infection have vastly improved in the past several years, yielding far better rates of sustained viral response (SVR) and high chances of cure after 8-24 weeks of treatment. To see if better antiviral therapies have affected HCV mortality rates, Dr. Holmberg and his associates studied ICD-9 data from Multiple Cause of Death records for all U.S. death certificates between 2003 and 2013. They divided deaths that were linked to HCV or 60 other nationally notifiable infectious diseases by U.S. Census numbers for the same year. They also examined data from the Chronic Hepatitis Cohort Study (Clin Infect Dis. 2014;58:1055-61), which includes patients presumed to have adequate access to HCV treatment.
Chronic HCV-related deaths climbed from about 12,000 annually in 2003 to more than 19,000 in 2013, said Dr. Holmberg. In contrast, deaths from the 60 other reportable infectious diseases dropped from about 25,000 annually to below 20,000 per year. Annual deaths tied to HIV infection ranked second behind HCV at about 8,800, followed by Staphylococcus aureus (including MRSA), hepatitis B virus, tuberculosis, and pneumococcal disease. “This does not include 4,444 adult influenza deaths, but does include 165 childhood influenza deaths in 2013,” Dr. Holmberg noted.
The analysis of the Chronic Hepatitis Cohort Study revealed a doubling in mortality from chronic HCV infection among patients who should have had adequate access to treatment, according to Dr. Holmberg. For every 100 person-years of observation, about 2.5 people died from consequences of chronic HCV infection in 2007, compared with about 5.5 in 2013, he said. “Hidden mortality from HCV is considerable,” he added. “Only 19% of HCV patients who died had their infection noted anywhere on their death certificates, despite the fact that more than 75% had premortem evidence of liver disease.”
Uptake of sofosbuvir-based regimens more than quintupled in the second quarter of 2015, compared with a year earlier, according to data from Gilead Sciences presented by Dr. Holmberg. But high drug costs have spurred state Medicaid programs and private payers to stipulate many preapproval requirements, he noted. Patients must be drug and alcohol free for at least 6 months, and in many states, must provide evidence of liver scarring from a recent biopsy or FibroScan, which is not always easy to access. “This is often a barrier,” Dr. Holmberg said. “For those in more rural areas, finding a specialist, as required by many state Medicaid offices, can be very difficult.”
And there are even more obstacles. Many clinicians still see HCV as a “benign condition,” and patients often have other urgent health, social, or financial problems, Dr. Holmberg said. The public, for its part, may not prioritize infectious diseases. “These patients lack a strong advocacy group,” he added. “Most are former injection drug users, and the public is often reluctant to help them.”
So what are the measurable results of these barriers? Among about 3.2 million individuals in the United States with chronic HCV infection, only half were ever tested for HCV, 38% received some sort of care related to their infection, 11% were treated, and 6% achieved SVR, Dr. Holmberg and his associates noted in a perspective piece (N Engl J Med. 2013;368:1859-861).
At the same time, the United States faces an emerging epidemic of new HCV infections in nonurban areas among young persons who inject drugs (MMWR. 64;453-8). “This is really a tale of two epidemics,” he added. “Control of the chronic and the acute outbreaks will require a multipronged approach, with interventions along a testing to cure continuum of care.”
Dr. Holmberg and his associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported no funding sources and had no financial disclosures.
AT IDWEEK 2015
Key clinical point: Mortality from chronic hepatitis C virus infection continues to rise, despite significant improvements in antiviral therapies.
Major finding: Even with substantial underreporting, in 2013, deaths tied to chronic HCV infection exceeded mortality from 60 other reportable infectious diseases.
Data source: Analysis of 10 years of national death certificate data and 7 years of data from the Chronic Hepatitis Cohort Study.
Disclosures: The researchers reported no funding sources and made no financial disclosures.
IDWeek: Testing delays linked to misclassification of hospital-onset C. difficile
SAN DIEGO – Delays in laboratory testing led a hospital to misclassify the origin of nearly a quarter of Clostridium difficile infections, Dr. Christopher Polage said at an annual scientific meeting on infectious diseases.
“Many patients with symptoms and risk factors are not being tested within 3 days of admission, leading to overreporting of hospital-onset [Clostridium difficile infections] and underreporting of community-onset CDI,” said Dr. Polage of the UC Davis (Calif.) Health System. By testing patients who have diarrhea and risk factors for CDI sooner after admission, hospitals can prevent outbreaks and improve their standardized infection ratio for CDI, he added.
Clostridium difficile is implicated in about 29,000 deaths every year in the United States. The vast majority of such cases are classified as hospital onset, based on the “3-day rule,” meaning that patients were tested more than 3 days after admission. “This is the preventable hospital outcome that we’re all trying to bring down,” Dr. Polage said.
As part of that effort, he and his colleagues studied 11 hospital units that were considered high risk for CDI. To identify toxigenic C. difficile, they performed culture and polymerase chain reaction testing on perianal swabs collected from all adult patients admitted to these units. They also analyzed toxin immunoassays of stool samples when physicians requested them for patients with diarrhea.
“The question was, how often was misclassification happening?” said Dr. Polage. Among 48 cases that the laboratory reported as hospital onset, based on the “3-day rule,” close to half (44%) actually had CD-positive perianal swabs when admitted, he said. And half of these swab-positive patients waited more than 3 days for a CD stool test even though they had current or recent diarrhea, he added. In fact, swab-positive patients with diarrhea went a median of 6 days without a CD stool test, and some went untested for 10 days, Dr. Polage said.
“Anyone who has tried to determine if a hospitalized patient is having diarrhea knows that this can be very hard to pin down,” Dr. Polage noted. But some patients with positive swabs on admission met three different definitions for diarrhea, “making it pretty clear that they had community-onset CDI,” he said. This most conservative approach found that 23% of “hospital-onset” cases were actually community onset, he said.
Thus far, UC Davis Health System seems not to have had an increase in antibiotic prescriptions in response to greater detection of community-onset CDI, Dr. Polage said. “This is not something we take lightly,” he added. “We put together a lot of educational materials for patients, physicians, and providers, and work with our antibiotic stewardship team. We found that we might be able to focus on patients who had CDI at time of admission, and intervene with them individually to more carefully monitor what antibiotic they were using.”
Dr. Polage reported these findings at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The Gordon and Betty Moore Foundation helped fund the study. Dr. Polage reported having received research materials or honoraria from Cepheid, TechLab, Alere, Meridian, and ACEA Biosciences.
SAN DIEGO – Delays in laboratory testing led a hospital to misclassify the origin of nearly a quarter of Clostridium difficile infections, Dr. Christopher Polage said at an annual scientific meeting on infectious diseases.
“Many patients with symptoms and risk factors are not being tested within 3 days of admission, leading to overreporting of hospital-onset [Clostridium difficile infections] and underreporting of community-onset CDI,” said Dr. Polage of the UC Davis (Calif.) Health System. By testing patients who have diarrhea and risk factors for CDI sooner after admission, hospitals can prevent outbreaks and improve their standardized infection ratio for CDI, he added.
Clostridium difficile is implicated in about 29,000 deaths every year in the United States. The vast majority of such cases are classified as hospital onset, based on the “3-day rule,” meaning that patients were tested more than 3 days after admission. “This is the preventable hospital outcome that we’re all trying to bring down,” Dr. Polage said.
As part of that effort, he and his colleagues studied 11 hospital units that were considered high risk for CDI. To identify toxigenic C. difficile, they performed culture and polymerase chain reaction testing on perianal swabs collected from all adult patients admitted to these units. They also analyzed toxin immunoassays of stool samples when physicians requested them for patients with diarrhea.
“The question was, how often was misclassification happening?” said Dr. Polage. Among 48 cases that the laboratory reported as hospital onset, based on the “3-day rule,” close to half (44%) actually had CD-positive perianal swabs when admitted, he said. And half of these swab-positive patients waited more than 3 days for a CD stool test even though they had current or recent diarrhea, he added. In fact, swab-positive patients with diarrhea went a median of 6 days without a CD stool test, and some went untested for 10 days, Dr. Polage said.
“Anyone who has tried to determine if a hospitalized patient is having diarrhea knows that this can be very hard to pin down,” Dr. Polage noted. But some patients with positive swabs on admission met three different definitions for diarrhea, “making it pretty clear that they had community-onset CDI,” he said. This most conservative approach found that 23% of “hospital-onset” cases were actually community onset, he said.
Thus far, UC Davis Health System seems not to have had an increase in antibiotic prescriptions in response to greater detection of community-onset CDI, Dr. Polage said. “This is not something we take lightly,” he added. “We put together a lot of educational materials for patients, physicians, and providers, and work with our antibiotic stewardship team. We found that we might be able to focus on patients who had CDI at time of admission, and intervene with them individually to more carefully monitor what antibiotic they were using.”
Dr. Polage reported these findings at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The Gordon and Betty Moore Foundation helped fund the study. Dr. Polage reported having received research materials or honoraria from Cepheid, TechLab, Alere, Meridian, and ACEA Biosciences.
SAN DIEGO – Delays in laboratory testing led a hospital to misclassify the origin of nearly a quarter of Clostridium difficile infections, Dr. Christopher Polage said at an annual scientific meeting on infectious diseases.
“Many patients with symptoms and risk factors are not being tested within 3 days of admission, leading to overreporting of hospital-onset [Clostridium difficile infections] and underreporting of community-onset CDI,” said Dr. Polage of the UC Davis (Calif.) Health System. By testing patients who have diarrhea and risk factors for CDI sooner after admission, hospitals can prevent outbreaks and improve their standardized infection ratio for CDI, he added.
Clostridium difficile is implicated in about 29,000 deaths every year in the United States. The vast majority of such cases are classified as hospital onset, based on the “3-day rule,” meaning that patients were tested more than 3 days after admission. “This is the preventable hospital outcome that we’re all trying to bring down,” Dr. Polage said.
As part of that effort, he and his colleagues studied 11 hospital units that were considered high risk for CDI. To identify toxigenic C. difficile, they performed culture and polymerase chain reaction testing on perianal swabs collected from all adult patients admitted to these units. They also analyzed toxin immunoassays of stool samples when physicians requested them for patients with diarrhea.
“The question was, how often was misclassification happening?” said Dr. Polage. Among 48 cases that the laboratory reported as hospital onset, based on the “3-day rule,” close to half (44%) actually had CD-positive perianal swabs when admitted, he said. And half of these swab-positive patients waited more than 3 days for a CD stool test even though they had current or recent diarrhea, he added. In fact, swab-positive patients with diarrhea went a median of 6 days without a CD stool test, and some went untested for 10 days, Dr. Polage said.
“Anyone who has tried to determine if a hospitalized patient is having diarrhea knows that this can be very hard to pin down,” Dr. Polage noted. But some patients with positive swabs on admission met three different definitions for diarrhea, “making it pretty clear that they had community-onset CDI,” he said. This most conservative approach found that 23% of “hospital-onset” cases were actually community onset, he said.
Thus far, UC Davis Health System seems not to have had an increase in antibiotic prescriptions in response to greater detection of community-onset CDI, Dr. Polage said. “This is not something we take lightly,” he added. “We put together a lot of educational materials for patients, physicians, and providers, and work with our antibiotic stewardship team. We found that we might be able to focus on patients who had CDI at time of admission, and intervene with them individually to more carefully monitor what antibiotic they were using.”
Dr. Polage reported these findings at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The Gordon and Betty Moore Foundation helped fund the study. Dr. Polage reported having received research materials or honoraria from Cepheid, TechLab, Alere, Meridian, and ACEA Biosciences.
AT IDWEEK 2015
Key clinical point: Delays in laboratory testing led to misclassification of Clostridium difficile infections.
Major finding: At least 23% of cases that were reported as hospital onset were actually community onset.
Data source: An analysis of 12 months of C. difficile infection surveillance data from one academic medical center.
Disclosures: The Gordon and Betty Moore Foundation helped fund the study. Dr. Polage reported having received research materials or honoraria from Cepheid, TechLab, Alere, Meridian, and ACEA Biosciences.
IDWEEK: Antibiotic ‘time-out’ cut vancomycin use
SAN DIEGO – A “time-out” to review antibiotic therapy 72-96 hours into treatment increased appropriate discontinuations of vancomycin by 31%, researchers said at an annual scientific meeting on infectious diseases.
The practice fit into hospital work flow, streamlined other antibiotic stewardship practices, and respected provider autonomy, said Dr. Christopher Graber of VA Greater Los Angeles Healthcare System and the University of California, Los Angeles. It also was durable, persisting into the second 6 months of the program even though active research support had ended, he and his associates said.
The Centers for Disease Control and Prevention endorses the antibiotic time-out as a way to encourage providers to switch from empirical treatment with broad-spectrum antibiotics to a more tailored plan after culture and other laboratory results become available. The Veterans Affairs teaching hospital in greater Los Angeles created a self-directed time-out program to encourage reconsideration of broad-spectrum therapy with vancomycin and piperacillin-tazobactam after the first 3 days of empirical treatment. The program included an antimicrobial “dashboard” report, an electronic template to document time-outs, and a marketing program consisting of educational documents, lectures, “clinical champions,” and reminder notes and fliers posted near computers.
To evaluate early and late responses to the program, Dr. Graber and his associates tracked discontinuations of vancomycin and piperacillin-tazobactam, as well as decisions to continue these antibiotics through day 5 when doing so contradicted guidelines. Among 276 vancomycin episodes during the program, clinicians discontinued 175 (63%) – significantly more than before the program started (96 of 199; 48%; P = .001). They documented vancomycin time-outs 46% of the time, continued patients on vancomycin through day 5 without a time-out in 7.6% of cases, and allowed vancomycin to expire without a time-out 46% of the time.
The rate of inappropriate discontinuations of vancomycin during the program exceeded baseline (4.7% vs. none; P = .001), but this trend was balanced out by the overall rise in vancomycin discontinuations, the researchers said. Furthermore, providers documented vancomycin time-outs more often during the second 6 months, compared with the first (54% vs. 37%; P = .005). The increase in vancomycin discontinuations also was durable (about 63% throughout the two 6-month periods), and inappropriate continuations remained stable at about 4.5%.
“Piperacillin-tazobactam did not see any change in discontinuations between the two study periods,” said the researchers. The discontinuation rate was about 62% before and during the program. Clinicians performed time-outs about half the time, and inappropriately continued the antibiotics beyond day 5 in 10% of cases, compared with 2% before the program started (P = .02). Clinicians continued performing piperacillin-tazobactam time-outs at the same rate in the second 6 months of the program as during the beginning, and rates of discontinuation and inappropriate continuations also remained similar.
The investigators reported these results at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
CDC funded the research with a grant to the University of Utah. The researchers reported no conflicts of interest.
SAN DIEGO – A “time-out” to review antibiotic therapy 72-96 hours into treatment increased appropriate discontinuations of vancomycin by 31%, researchers said at an annual scientific meeting on infectious diseases.
The practice fit into hospital work flow, streamlined other antibiotic stewardship practices, and respected provider autonomy, said Dr. Christopher Graber of VA Greater Los Angeles Healthcare System and the University of California, Los Angeles. It also was durable, persisting into the second 6 months of the program even though active research support had ended, he and his associates said.
The Centers for Disease Control and Prevention endorses the antibiotic time-out as a way to encourage providers to switch from empirical treatment with broad-spectrum antibiotics to a more tailored plan after culture and other laboratory results become available. The Veterans Affairs teaching hospital in greater Los Angeles created a self-directed time-out program to encourage reconsideration of broad-spectrum therapy with vancomycin and piperacillin-tazobactam after the first 3 days of empirical treatment. The program included an antimicrobial “dashboard” report, an electronic template to document time-outs, and a marketing program consisting of educational documents, lectures, “clinical champions,” and reminder notes and fliers posted near computers.
To evaluate early and late responses to the program, Dr. Graber and his associates tracked discontinuations of vancomycin and piperacillin-tazobactam, as well as decisions to continue these antibiotics through day 5 when doing so contradicted guidelines. Among 276 vancomycin episodes during the program, clinicians discontinued 175 (63%) – significantly more than before the program started (96 of 199; 48%; P = .001). They documented vancomycin time-outs 46% of the time, continued patients on vancomycin through day 5 without a time-out in 7.6% of cases, and allowed vancomycin to expire without a time-out 46% of the time.
The rate of inappropriate discontinuations of vancomycin during the program exceeded baseline (4.7% vs. none; P = .001), but this trend was balanced out by the overall rise in vancomycin discontinuations, the researchers said. Furthermore, providers documented vancomycin time-outs more often during the second 6 months, compared with the first (54% vs. 37%; P = .005). The increase in vancomycin discontinuations also was durable (about 63% throughout the two 6-month periods), and inappropriate continuations remained stable at about 4.5%.
“Piperacillin-tazobactam did not see any change in discontinuations between the two study periods,” said the researchers. The discontinuation rate was about 62% before and during the program. Clinicians performed time-outs about half the time, and inappropriately continued the antibiotics beyond day 5 in 10% of cases, compared with 2% before the program started (P = .02). Clinicians continued performing piperacillin-tazobactam time-outs at the same rate in the second 6 months of the program as during the beginning, and rates of discontinuation and inappropriate continuations also remained similar.
The investigators reported these results at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
CDC funded the research with a grant to the University of Utah. The researchers reported no conflicts of interest.
SAN DIEGO – A “time-out” to review antibiotic therapy 72-96 hours into treatment increased appropriate discontinuations of vancomycin by 31%, researchers said at an annual scientific meeting on infectious diseases.
The practice fit into hospital work flow, streamlined other antibiotic stewardship practices, and respected provider autonomy, said Dr. Christopher Graber of VA Greater Los Angeles Healthcare System and the University of California, Los Angeles. It also was durable, persisting into the second 6 months of the program even though active research support had ended, he and his associates said.
The Centers for Disease Control and Prevention endorses the antibiotic time-out as a way to encourage providers to switch from empirical treatment with broad-spectrum antibiotics to a more tailored plan after culture and other laboratory results become available. The Veterans Affairs teaching hospital in greater Los Angeles created a self-directed time-out program to encourage reconsideration of broad-spectrum therapy with vancomycin and piperacillin-tazobactam after the first 3 days of empirical treatment. The program included an antimicrobial “dashboard” report, an electronic template to document time-outs, and a marketing program consisting of educational documents, lectures, “clinical champions,” and reminder notes and fliers posted near computers.
To evaluate early and late responses to the program, Dr. Graber and his associates tracked discontinuations of vancomycin and piperacillin-tazobactam, as well as decisions to continue these antibiotics through day 5 when doing so contradicted guidelines. Among 276 vancomycin episodes during the program, clinicians discontinued 175 (63%) – significantly more than before the program started (96 of 199; 48%; P = .001). They documented vancomycin time-outs 46% of the time, continued patients on vancomycin through day 5 without a time-out in 7.6% of cases, and allowed vancomycin to expire without a time-out 46% of the time.
The rate of inappropriate discontinuations of vancomycin during the program exceeded baseline (4.7% vs. none; P = .001), but this trend was balanced out by the overall rise in vancomycin discontinuations, the researchers said. Furthermore, providers documented vancomycin time-outs more often during the second 6 months, compared with the first (54% vs. 37%; P = .005). The increase in vancomycin discontinuations also was durable (about 63% throughout the two 6-month periods), and inappropriate continuations remained stable at about 4.5%.
“Piperacillin-tazobactam did not see any change in discontinuations between the two study periods,” said the researchers. The discontinuation rate was about 62% before and during the program. Clinicians performed time-outs about half the time, and inappropriately continued the antibiotics beyond day 5 in 10% of cases, compared with 2% before the program started (P = .02). Clinicians continued performing piperacillin-tazobactam time-outs at the same rate in the second 6 months of the program as during the beginning, and rates of discontinuation and inappropriate continuations also remained similar.
The investigators reported these results at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
CDC funded the research with a grant to the University of Utah. The researchers reported no conflicts of interest.
AT IDWEEK 2015
Key clinical point: A self-directed antibiotic time-out performed 72-96 hours into treatment was tied to increases in appropriate discontinuations of vancomycin.
Major finding: Discontinuation of vancomycin was higher than before the intervention (63% vs. 48%; P = .001).
Data source: Prospective single-center study.
Disclosures: CDC helped fund the study with a grant to the University of Utah. The researchers reported no conflicts of interest.
IDWeek: Rifapentine had best completion rates for health care workers with latent TB
SAN DIEGO – Health care workers with latent tuberculosis infection (LTBI) were significantly more likely to continue a shorter course of weekly rifapentine plus isoniazid (INH) than daily INH monotherapy, researchers reported at an annual scientific meeting on infectious diseases.
“Consideration should be given to no longer routinely recommending INH for the treatment of LTBI among health care workers,” said Dr. Esther Arguello Perez of Memorial Sloan Kettering Cancer Center, New York.
Health care workers face a greater risk of TB infection than the general population, regardless of the income level in the country where they live; patients with undiagnosed laryngeal or pulmonary TB usually pose the greatest risk, especially during procedures that cause coughing, such as sputum induction and bronchoscopy (Int J Tuberc Lung Dis. 2007;11[6]:593-605).
Although occupational TB testing is routine in U.S. health care organizations, more than half of health care workers who start treatment for LTBI historically have failed to finish (Chest. 2010;137[2]:401-9. doi: 10.1378/chest.09-0394). The standard LTBI regimen – 300 mg INH daily for 9 months – has been linked to potentially intolerable adverse effects such as hepatotoxicity, persistent gastrointestinal symptoms, rash, and neuropsychiatric problems (Drug Healthc Patient Saf. 2014;6:145-9. doi: 10.2147/DHPS.S68837).
In a 2011 multicenter trial, investigators reported a significantly higher completion rate for weekly rifapentine plus INH (900 mg each; 82% vs. 69% for daily INH; P < .001). Rates of adverse effects were significantly lower with weekly rifapentine plus INH, although grade 3-4 events and risk of death did not differ between the groups (N Engl J Med. 2011;365:2155-66. doi: 10.1056/NEJMoa1104875). The results of that trial quickly transformed recommendations for LTBI treatment (MMWR. 2011:60(48);1650-53).
Memorial Sloan Kettering implemented weekly rifapentine plus INH for its LTBI personnel in 2011. By 2014, about three-quarters of personnel with LTBI received rifapentine plus INH, while the rest were evenly split between rifampin and INH monotherapy, Dr. Arguello Perez reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
To understand how health care workers’ attitudes and treatment acceptance shifted along with practice, the investigators reviewed records from all health care workers at Memorial Sloan Kettering who were diagnosed with LTBI for 2005-2014. Among 930 patients, only 357 (38%) accepted treatment, although 76% of these individuals finished the regimen they started, she noted. Rifapentine plus INH had the highest completion rate (88%), significantly exceeding rates for a 4-month course of daily rifampin (84%) and for 9 months of INH monotherapy (70%; P < .01 for both differences). In contrast, completion rates for rifampin and INH did not significantly differ, Dr. Arguello Perez said.
Notably, LTBI treatment completion rates among health care workers rose by 26% between 2013, when most prescriptions were for rifampin or INH monotherapy, and 2014, when most were for rifapentine plus INH. “Health care workers might be more likely to accept treatment for LTBI if they know about alternatives to INH,” she concluded.
Dr. Arguello Perez and her associates reported no funding sources and had no financial disclosures.
SAN DIEGO – Health care workers with latent tuberculosis infection (LTBI) were significantly more likely to continue a shorter course of weekly rifapentine plus isoniazid (INH) than daily INH monotherapy, researchers reported at an annual scientific meeting on infectious diseases.
“Consideration should be given to no longer routinely recommending INH for the treatment of LTBI among health care workers,” said Dr. Esther Arguello Perez of Memorial Sloan Kettering Cancer Center, New York.
Health care workers face a greater risk of TB infection than the general population, regardless of the income level in the country where they live; patients with undiagnosed laryngeal or pulmonary TB usually pose the greatest risk, especially during procedures that cause coughing, such as sputum induction and bronchoscopy (Int J Tuberc Lung Dis. 2007;11[6]:593-605).
Although occupational TB testing is routine in U.S. health care organizations, more than half of health care workers who start treatment for LTBI historically have failed to finish (Chest. 2010;137[2]:401-9. doi: 10.1378/chest.09-0394). The standard LTBI regimen – 300 mg INH daily for 9 months – has been linked to potentially intolerable adverse effects such as hepatotoxicity, persistent gastrointestinal symptoms, rash, and neuropsychiatric problems (Drug Healthc Patient Saf. 2014;6:145-9. doi: 10.2147/DHPS.S68837).
In a 2011 multicenter trial, investigators reported a significantly higher completion rate for weekly rifapentine plus INH (900 mg each; 82% vs. 69% for daily INH; P < .001). Rates of adverse effects were significantly lower with weekly rifapentine plus INH, although grade 3-4 events and risk of death did not differ between the groups (N Engl J Med. 2011;365:2155-66. doi: 10.1056/NEJMoa1104875). The results of that trial quickly transformed recommendations for LTBI treatment (MMWR. 2011:60(48);1650-53).
Memorial Sloan Kettering implemented weekly rifapentine plus INH for its LTBI personnel in 2011. By 2014, about three-quarters of personnel with LTBI received rifapentine plus INH, while the rest were evenly split between rifampin and INH monotherapy, Dr. Arguello Perez reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
To understand how health care workers’ attitudes and treatment acceptance shifted along with practice, the investigators reviewed records from all health care workers at Memorial Sloan Kettering who were diagnosed with LTBI for 2005-2014. Among 930 patients, only 357 (38%) accepted treatment, although 76% of these individuals finished the regimen they started, she noted. Rifapentine plus INH had the highest completion rate (88%), significantly exceeding rates for a 4-month course of daily rifampin (84%) and for 9 months of INH monotherapy (70%; P < .01 for both differences). In contrast, completion rates for rifampin and INH did not significantly differ, Dr. Arguello Perez said.
Notably, LTBI treatment completion rates among health care workers rose by 26% between 2013, when most prescriptions were for rifampin or INH monotherapy, and 2014, when most were for rifapentine plus INH. “Health care workers might be more likely to accept treatment for LTBI if they know about alternatives to INH,” she concluded.
Dr. Arguello Perez and her associates reported no funding sources and had no financial disclosures.
SAN DIEGO – Health care workers with latent tuberculosis infection (LTBI) were significantly more likely to continue a shorter course of weekly rifapentine plus isoniazid (INH) than daily INH monotherapy, researchers reported at an annual scientific meeting on infectious diseases.
“Consideration should be given to no longer routinely recommending INH for the treatment of LTBI among health care workers,” said Dr. Esther Arguello Perez of Memorial Sloan Kettering Cancer Center, New York.
Health care workers face a greater risk of TB infection than the general population, regardless of the income level in the country where they live; patients with undiagnosed laryngeal or pulmonary TB usually pose the greatest risk, especially during procedures that cause coughing, such as sputum induction and bronchoscopy (Int J Tuberc Lung Dis. 2007;11[6]:593-605).
Although occupational TB testing is routine in U.S. health care organizations, more than half of health care workers who start treatment for LTBI historically have failed to finish (Chest. 2010;137[2]:401-9. doi: 10.1378/chest.09-0394). The standard LTBI regimen – 300 mg INH daily for 9 months – has been linked to potentially intolerable adverse effects such as hepatotoxicity, persistent gastrointestinal symptoms, rash, and neuropsychiatric problems (Drug Healthc Patient Saf. 2014;6:145-9. doi: 10.2147/DHPS.S68837).
In a 2011 multicenter trial, investigators reported a significantly higher completion rate for weekly rifapentine plus INH (900 mg each; 82% vs. 69% for daily INH; P < .001). Rates of adverse effects were significantly lower with weekly rifapentine plus INH, although grade 3-4 events and risk of death did not differ between the groups (N Engl J Med. 2011;365:2155-66. doi: 10.1056/NEJMoa1104875). The results of that trial quickly transformed recommendations for LTBI treatment (MMWR. 2011:60(48);1650-53).
Memorial Sloan Kettering implemented weekly rifapentine plus INH for its LTBI personnel in 2011. By 2014, about three-quarters of personnel with LTBI received rifapentine plus INH, while the rest were evenly split between rifampin and INH monotherapy, Dr. Arguello Perez reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
To understand how health care workers’ attitudes and treatment acceptance shifted along with practice, the investigators reviewed records from all health care workers at Memorial Sloan Kettering who were diagnosed with LTBI for 2005-2014. Among 930 patients, only 357 (38%) accepted treatment, although 76% of these individuals finished the regimen they started, she noted. Rifapentine plus INH had the highest completion rate (88%), significantly exceeding rates for a 4-month course of daily rifampin (84%) and for 9 months of INH monotherapy (70%; P < .01 for both differences). In contrast, completion rates for rifampin and INH did not significantly differ, Dr. Arguello Perez said.
Notably, LTBI treatment completion rates among health care workers rose by 26% between 2013, when most prescriptions were for rifampin or INH monotherapy, and 2014, when most were for rifapentine plus INH. “Health care workers might be more likely to accept treatment for LTBI if they know about alternatives to INH,” she concluded.
Dr. Arguello Perez and her associates reported no funding sources and had no financial disclosures.
AT IDweek 2015
Key clinical point: Isoniazid monotherapy should not be recommended routinely for health care workers with latent tuberculosis infections.
Major finding: Rates of treatment completion were significantly higher for weekly rifapentine plus isoniazid than for daily isoniazid monotherapy (P < .01).
Data source: Retrospective analysis of all health care workers with LTBI at Memorial Sloan Kettering Cancer Center during 2005-2014.
Disclosures: The researchers reported no funding sources and made no financial disclosures.
Some household pets found to be colonized with S. aureus
SAN DIEGO – In households of children with methicillin-resistant Staphylococcus aureus (MRSA) infection, pet dogs and cats often were colonized with S. aureus. In addition, the S. aureus strains colonizing the pets were likely to be concordant with those found on humans and/or their environmental surfaces within the household.
Those are key findings from a study that set out to determine the molecular epidemiology of S. aureus colonization of pets in the context of their human contacts and household environments, in households of children with community-associated MRSA infections.
“S. aureus is a significant pathogen in both health care and community settings and causes a spectrum of infections ranging from superficial skin and soft tissue infections (SSTIs) to invasive, life-threatening infections,” Ryley M. Thompson said at an annual scientific meeting on infectious diseases. “Due to the enormous clinical and economic burden posed by S. aureus, transmission prevention is essential.”
According to traditional dogma, “humans are the source of S. aureus for their pets and … pets are not a natural reservoir for S. aureus,” said Mr. Thompson, a clinical research study assistant in the Clinical and Translational Research Laboratory of Dr. Stephanie Fritz in the department of pediatrics at Washington University, St. Louis. “This is supported by the fact that pets often clear colonization without antimicrobial treatment. Risk factors for pet colonization include veterinary health care contact, contact with children, and using their mouths to interact with their environment. To date, directionality of S. aureus transmission between humans and pets is unclear.”
Between 2012 and 2015, the researchers enrolled 100 households of children with active or recent community-associated MRSA SSTIs who had been treated at St. Louis Children’s Hospital or other pediatric practices in the area. Over the course of 1 year, five study visits were conducted in each of the patient’s homes. Every 3 months, cultures were obtained from index patients and their household contacts, indoor dogs and cats, and 21 household environmental surfaces. The index patients and household contacts were swabbed at their axillae, nares, and inguinal folds; indoor dogs and cats were swabbed at their nares and dorsal fur; and household surfaces thought to be frequently touched by multiple household members were swabbed, such as TV remote controls, refrigerator door handles, and toilet seats. Researchers also administered a detailed survey to evaluate health, hygiene, and activities that may be associated with S. aureus infection transmission.
Molecular typing of all S. aureus strains was performed by repetitive-sequence polymerase chain reaction to determine strain relatedness, and staphylococcal cassette chromosome mec (SCCmec) characterization was performed by multiplex PCR.
Of 100 households, 49 had a total of 89 pets: 63 dogs and 26 cats. Of the 63 dogs, 13 (21%) were colonized with S. aureus (9 with MRSA) and 2 of 26 cats (8%) were colonized with MRSA. Eleven isolates were SCCmec type IV (MRSA), one was type II (MRSA), and two were type III (MRSA). At baseline, the researchers recovered 16 S. aureus isolates from 15 pets: 13 from the nares and 3 from pet dorsal fur.
One dog was colonized at both sites with concordant strains. In the three households that had two colonized pets, one household had two colonized dogs with matching strain types, the second had two dogs with nonmatching strain types, and the third had a dog and a cat with nonmatching strain types, Mr. Thompson reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Pet characteristics significantly associated with S. aureus colonization at study enrollment were older age (P = .04) and advanced number of years living in the home (P = .03), but sleeping in the same bed as a household member was not (P =. 96).
Molecular analysis revealed that the primary caretaker for 10 of the 15 colonized pets (67%) also was colonized with S. aureus, and 70% of these strains were concordant with the pet strain. In addition, seven of eight humans (88%) who shared a bed with a colonized pet also were colonized with S. aureus, and 43% of these strains were concordant with the pet strain.
Mr. Thompson also presented the longitudinal molecular epidemiology results in pets. In this analysis, 37 of the 89 pets were colonized with S. aureus at some point over the period of 12 months. Of these, 24 were colonized just once, while 13 were colonized at more than one of the samplings over time. Among these 13, two (15%) had concordant strains at all samplings, five (39%) had concordant and discordant strains, and six (46%) had discordant strains over the longitudinal study period.
Mr. Thompson and his associates intend to complete enrollment and analysis of 150 households in a 2-year longitudinal study. After this, he said, “we will be able to determine the directionality of human-pet S. aureus transmission as well as define the role of pets in S. aureus household transmission dynamics.”
The study was funded by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, the Agency for Healthcare Research and Quality, and the National Institutes of Health. The researchers reported having no financial disclosures.
SAN DIEGO – In households of children with methicillin-resistant Staphylococcus aureus (MRSA) infection, pet dogs and cats often were colonized with S. aureus. In addition, the S. aureus strains colonizing the pets were likely to be concordant with those found on humans and/or their environmental surfaces within the household.
Those are key findings from a study that set out to determine the molecular epidemiology of S. aureus colonization of pets in the context of their human contacts and household environments, in households of children with community-associated MRSA infections.
“S. aureus is a significant pathogen in both health care and community settings and causes a spectrum of infections ranging from superficial skin and soft tissue infections (SSTIs) to invasive, life-threatening infections,” Ryley M. Thompson said at an annual scientific meeting on infectious diseases. “Due to the enormous clinical and economic burden posed by S. aureus, transmission prevention is essential.”
According to traditional dogma, “humans are the source of S. aureus for their pets and … pets are not a natural reservoir for S. aureus,” said Mr. Thompson, a clinical research study assistant in the Clinical and Translational Research Laboratory of Dr. Stephanie Fritz in the department of pediatrics at Washington University, St. Louis. “This is supported by the fact that pets often clear colonization without antimicrobial treatment. Risk factors for pet colonization include veterinary health care contact, contact with children, and using their mouths to interact with their environment. To date, directionality of S. aureus transmission between humans and pets is unclear.”
Between 2012 and 2015, the researchers enrolled 100 households of children with active or recent community-associated MRSA SSTIs who had been treated at St. Louis Children’s Hospital or other pediatric practices in the area. Over the course of 1 year, five study visits were conducted in each of the patient’s homes. Every 3 months, cultures were obtained from index patients and their household contacts, indoor dogs and cats, and 21 household environmental surfaces. The index patients and household contacts were swabbed at their axillae, nares, and inguinal folds; indoor dogs and cats were swabbed at their nares and dorsal fur; and household surfaces thought to be frequently touched by multiple household members were swabbed, such as TV remote controls, refrigerator door handles, and toilet seats. Researchers also administered a detailed survey to evaluate health, hygiene, and activities that may be associated with S. aureus infection transmission.
Molecular typing of all S. aureus strains was performed by repetitive-sequence polymerase chain reaction to determine strain relatedness, and staphylococcal cassette chromosome mec (SCCmec) characterization was performed by multiplex PCR.
Of 100 households, 49 had a total of 89 pets: 63 dogs and 26 cats. Of the 63 dogs, 13 (21%) were colonized with S. aureus (9 with MRSA) and 2 of 26 cats (8%) were colonized with MRSA. Eleven isolates were SCCmec type IV (MRSA), one was type II (MRSA), and two were type III (MRSA). At baseline, the researchers recovered 16 S. aureus isolates from 15 pets: 13 from the nares and 3 from pet dorsal fur.
One dog was colonized at both sites with concordant strains. In the three households that had two colonized pets, one household had two colonized dogs with matching strain types, the second had two dogs with nonmatching strain types, and the third had a dog and a cat with nonmatching strain types, Mr. Thompson reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Pet characteristics significantly associated with S. aureus colonization at study enrollment were older age (P = .04) and advanced number of years living in the home (P = .03), but sleeping in the same bed as a household member was not (P =. 96).
Molecular analysis revealed that the primary caretaker for 10 of the 15 colonized pets (67%) also was colonized with S. aureus, and 70% of these strains were concordant with the pet strain. In addition, seven of eight humans (88%) who shared a bed with a colonized pet also were colonized with S. aureus, and 43% of these strains were concordant with the pet strain.
Mr. Thompson also presented the longitudinal molecular epidemiology results in pets. In this analysis, 37 of the 89 pets were colonized with S. aureus at some point over the period of 12 months. Of these, 24 were colonized just once, while 13 were colonized at more than one of the samplings over time. Among these 13, two (15%) had concordant strains at all samplings, five (39%) had concordant and discordant strains, and six (46%) had discordant strains over the longitudinal study period.
Mr. Thompson and his associates intend to complete enrollment and analysis of 150 households in a 2-year longitudinal study. After this, he said, “we will be able to determine the directionality of human-pet S. aureus transmission as well as define the role of pets in S. aureus household transmission dynamics.”
The study was funded by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, the Agency for Healthcare Research and Quality, and the National Institutes of Health. The researchers reported having no financial disclosures.
SAN DIEGO – In households of children with methicillin-resistant Staphylococcus aureus (MRSA) infection, pet dogs and cats often were colonized with S. aureus. In addition, the S. aureus strains colonizing the pets were likely to be concordant with those found on humans and/or their environmental surfaces within the household.
Those are key findings from a study that set out to determine the molecular epidemiology of S. aureus colonization of pets in the context of their human contacts and household environments, in households of children with community-associated MRSA infections.
“S. aureus is a significant pathogen in both health care and community settings and causes a spectrum of infections ranging from superficial skin and soft tissue infections (SSTIs) to invasive, life-threatening infections,” Ryley M. Thompson said at an annual scientific meeting on infectious diseases. “Due to the enormous clinical and economic burden posed by S. aureus, transmission prevention is essential.”
According to traditional dogma, “humans are the source of S. aureus for their pets and … pets are not a natural reservoir for S. aureus,” said Mr. Thompson, a clinical research study assistant in the Clinical and Translational Research Laboratory of Dr. Stephanie Fritz in the department of pediatrics at Washington University, St. Louis. “This is supported by the fact that pets often clear colonization without antimicrobial treatment. Risk factors for pet colonization include veterinary health care contact, contact with children, and using their mouths to interact with their environment. To date, directionality of S. aureus transmission between humans and pets is unclear.”
Between 2012 and 2015, the researchers enrolled 100 households of children with active or recent community-associated MRSA SSTIs who had been treated at St. Louis Children’s Hospital or other pediatric practices in the area. Over the course of 1 year, five study visits were conducted in each of the patient’s homes. Every 3 months, cultures were obtained from index patients and their household contacts, indoor dogs and cats, and 21 household environmental surfaces. The index patients and household contacts were swabbed at their axillae, nares, and inguinal folds; indoor dogs and cats were swabbed at their nares and dorsal fur; and household surfaces thought to be frequently touched by multiple household members were swabbed, such as TV remote controls, refrigerator door handles, and toilet seats. Researchers also administered a detailed survey to evaluate health, hygiene, and activities that may be associated with S. aureus infection transmission.
Molecular typing of all S. aureus strains was performed by repetitive-sequence polymerase chain reaction to determine strain relatedness, and staphylococcal cassette chromosome mec (SCCmec) characterization was performed by multiplex PCR.
Of 100 households, 49 had a total of 89 pets: 63 dogs and 26 cats. Of the 63 dogs, 13 (21%) were colonized with S. aureus (9 with MRSA) and 2 of 26 cats (8%) were colonized with MRSA. Eleven isolates were SCCmec type IV (MRSA), one was type II (MRSA), and two were type III (MRSA). At baseline, the researchers recovered 16 S. aureus isolates from 15 pets: 13 from the nares and 3 from pet dorsal fur.
One dog was colonized at both sites with concordant strains. In the three households that had two colonized pets, one household had two colonized dogs with matching strain types, the second had two dogs with nonmatching strain types, and the third had a dog and a cat with nonmatching strain types, Mr. Thompson reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Pet characteristics significantly associated with S. aureus colonization at study enrollment were older age (P = .04) and advanced number of years living in the home (P = .03), but sleeping in the same bed as a household member was not (P =. 96).
Molecular analysis revealed that the primary caretaker for 10 of the 15 colonized pets (67%) also was colonized with S. aureus, and 70% of these strains were concordant with the pet strain. In addition, seven of eight humans (88%) who shared a bed with a colonized pet also were colonized with S. aureus, and 43% of these strains were concordant with the pet strain.
Mr. Thompson also presented the longitudinal molecular epidemiology results in pets. In this analysis, 37 of the 89 pets were colonized with S. aureus at some point over the period of 12 months. Of these, 24 were colonized just once, while 13 were colonized at more than one of the samplings over time. Among these 13, two (15%) had concordant strains at all samplings, five (39%) had concordant and discordant strains, and six (46%) had discordant strains over the longitudinal study period.
Mr. Thompson and his associates intend to complete enrollment and analysis of 150 households in a 2-year longitudinal study. After this, he said, “we will be able to determine the directionality of human-pet S. aureus transmission as well as define the role of pets in S. aureus household transmission dynamics.”
The study was funded by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, the Agency for Healthcare Research and Quality, and the National Institutes of Health. The researchers reported having no financial disclosures.
AT IDWEEK 2015
Key clinical point: In homes of children with MRSA infection, pet dogs and cats were often colonized with S. aureus.
Major finding: Of the 63 dogs, 13 (21%) were colonized with S. aureus (9 with MRSA) and 2 of 26 cats (8%) were colonized with MRSA.
Data source: An analysis of 100 households of children with active or recent community-associated MRSA superficial skin and soft tissue infections who had been treated at St. Louis Children’s Hospital or other pediatric practices in the area between 2012 and 2015.
Disclosures: The study was funded by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, the Agency for Healthcare Research and Quality, and the National Institutes of Health. The researchers reported having no financial disclosures.
Software picked up HAI clusters faster than hospitals
SAN DIEGO – An automated outbreak detection system was popular among infection preventionists and detected pathogenic clusters about 5 days sooner than did hospitals themselves, researchers said at an annual scientific meeting on infectious diseases.
“The vast majority of hospitals were very pleased to expand surveillance beyond multidrug-resistant organisms, and most thought this system would improve their ability to detect outbreaks and streamline their work,” said Dr. Meghan Baker of Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
Patients in acute-care hospitals acquired almost 782,000 healthcare-associated infections (HAIs) in 2011, according to the Centers for Disease Control and Prevention.
Traditionally, infection preventionists at these hospitals look for HAIs by identifying temporal or spatial clusters of “a limited number of prespecified pathogens,” Dr. Baker said. But some real clusters do not meet these empirical rules, leading to more cases and potentially severe consequences for patients. Automated HAI outbreak detection systems can help, but not if they constantly trigger false alarms or, conversely, are so specific that they miss outbreaks.
Using the Premier SafetySurveillor infection control tool and free statistical software, Dr. Baker and her associates analyzed 83 years of historical microbiology data from 44 hospitals. The system signaled for any cluster involving at least three cases, even if cases occurred by chance less than once a year, she said. The researchers compared the results with outbreak data submitted by a convenience sample of hospitals that used their usual surveillance methods.
The automated approach identified 230 clusters. Most were detected based on antimicrobial resistance, but others shared the same ward or specialty service and some produced more than one signal. Clusters most often involved Staphylococcus aureus, Enterococcus, Pseudomonas, Escherichia coli, and Klebsiella, but most organisms were not under routine surveillance. As a result, 89% of clusters detected by the automated tool were not detected by hospitals using their usual methods, she emphasized. “Some were not real breaks, as determined later by genetic typing,” she added. “Most real clusters were [methicillin-resistant S. aureus], Clostridium difficile, and some were resistant gram-negative rods.”
When surveyed, 76% of infection preventionists said the automated tool moderately or greatly improved their ability to detect outbreaks, Dr. Baker reported. They would have wanted notification about 81% of the clusters, and considered 47% to be moderately or highly concerning. Notably, 51% of clusters expanded after detection.
“If these had been detected in real time, it would have been possible to intervene and possibly curtail the outbreak,” and 237 (42%) of 559 infections might have been avoided if these interventions were successful, she said.
Dr. Baker and her associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Baker reported no competing interests. Two of her associates reported financial and relationships with Premier, Sage, Molnycke, and 3M.
SAN DIEGO – An automated outbreak detection system was popular among infection preventionists and detected pathogenic clusters about 5 days sooner than did hospitals themselves, researchers said at an annual scientific meeting on infectious diseases.
“The vast majority of hospitals were very pleased to expand surveillance beyond multidrug-resistant organisms, and most thought this system would improve their ability to detect outbreaks and streamline their work,” said Dr. Meghan Baker of Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
Patients in acute-care hospitals acquired almost 782,000 healthcare-associated infections (HAIs) in 2011, according to the Centers for Disease Control and Prevention.
Traditionally, infection preventionists at these hospitals look for HAIs by identifying temporal or spatial clusters of “a limited number of prespecified pathogens,” Dr. Baker said. But some real clusters do not meet these empirical rules, leading to more cases and potentially severe consequences for patients. Automated HAI outbreak detection systems can help, but not if they constantly trigger false alarms or, conversely, are so specific that they miss outbreaks.
Using the Premier SafetySurveillor infection control tool and free statistical software, Dr. Baker and her associates analyzed 83 years of historical microbiology data from 44 hospitals. The system signaled for any cluster involving at least three cases, even if cases occurred by chance less than once a year, she said. The researchers compared the results with outbreak data submitted by a convenience sample of hospitals that used their usual surveillance methods.
The automated approach identified 230 clusters. Most were detected based on antimicrobial resistance, but others shared the same ward or specialty service and some produced more than one signal. Clusters most often involved Staphylococcus aureus, Enterococcus, Pseudomonas, Escherichia coli, and Klebsiella, but most organisms were not under routine surveillance. As a result, 89% of clusters detected by the automated tool were not detected by hospitals using their usual methods, she emphasized. “Some were not real breaks, as determined later by genetic typing,” she added. “Most real clusters were [methicillin-resistant S. aureus], Clostridium difficile, and some were resistant gram-negative rods.”
When surveyed, 76% of infection preventionists said the automated tool moderately or greatly improved their ability to detect outbreaks, Dr. Baker reported. They would have wanted notification about 81% of the clusters, and considered 47% to be moderately or highly concerning. Notably, 51% of clusters expanded after detection.
“If these had been detected in real time, it would have been possible to intervene and possibly curtail the outbreak,” and 237 (42%) of 559 infections might have been avoided if these interventions were successful, she said.
Dr. Baker and her associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Baker reported no competing interests. Two of her associates reported financial and relationships with Premier, Sage, Molnycke, and 3M.
SAN DIEGO – An automated outbreak detection system was popular among infection preventionists and detected pathogenic clusters about 5 days sooner than did hospitals themselves, researchers said at an annual scientific meeting on infectious diseases.
“The vast majority of hospitals were very pleased to expand surveillance beyond multidrug-resistant organisms, and most thought this system would improve their ability to detect outbreaks and streamline their work,” said Dr. Meghan Baker of Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
Patients in acute-care hospitals acquired almost 782,000 healthcare-associated infections (HAIs) in 2011, according to the Centers for Disease Control and Prevention.
Traditionally, infection preventionists at these hospitals look for HAIs by identifying temporal or spatial clusters of “a limited number of prespecified pathogens,” Dr. Baker said. But some real clusters do not meet these empirical rules, leading to more cases and potentially severe consequences for patients. Automated HAI outbreak detection systems can help, but not if they constantly trigger false alarms or, conversely, are so specific that they miss outbreaks.
Using the Premier SafetySurveillor infection control tool and free statistical software, Dr. Baker and her associates analyzed 83 years of historical microbiology data from 44 hospitals. The system signaled for any cluster involving at least three cases, even if cases occurred by chance less than once a year, she said. The researchers compared the results with outbreak data submitted by a convenience sample of hospitals that used their usual surveillance methods.
The automated approach identified 230 clusters. Most were detected based on antimicrobial resistance, but others shared the same ward or specialty service and some produced more than one signal. Clusters most often involved Staphylococcus aureus, Enterococcus, Pseudomonas, Escherichia coli, and Klebsiella, but most organisms were not under routine surveillance. As a result, 89% of clusters detected by the automated tool were not detected by hospitals using their usual methods, she emphasized. “Some were not real breaks, as determined later by genetic typing,” she added. “Most real clusters were [methicillin-resistant S. aureus], Clostridium difficile, and some were resistant gram-negative rods.”
When surveyed, 76% of infection preventionists said the automated tool moderately or greatly improved their ability to detect outbreaks, Dr. Baker reported. They would have wanted notification about 81% of the clusters, and considered 47% to be moderately or highly concerning. Notably, 51% of clusters expanded after detection.
“If these had been detected in real time, it would have been possible to intervene and possibly curtail the outbreak,” and 237 (42%) of 559 infections might have been avoided if these interventions were successful, she said.
Dr. Baker and her associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Baker reported no competing interests. Two of her associates reported financial and relationships with Premier, Sage, Molnycke, and 3M.
AT IDWEEK 2015
Key clinical point: An automated outbreak detection system can augment traditional methods for detecting pathogenic clusters.
Major finding: The software tool identified clusters about 5 days sooner than did hospitals themselves.
Data source: Comparison of an automated outbreak detection tool with usual hospital surveillance methods, and surveys of infection preventionists.
Disclosures: Dr. Baker reported no competing interests. Two of her associates reported financial and relationships with Premier, Sage, Molnycke, and 3M.
HIV testing low among patients admitted for pneumonia
SAN DIEGO – Only 39% of patients hospitalized for pneumonia underwent HIV testing, even though federal recommendations for universal HIV screening in all health care settings have been in place since 2006, according to the results of a retrospective, single-center study.
“Despite universal recommendations for HIV screening in all health care settings, HIV testing rates remain low among patients hospitalized with pneumonia,” Dr. Dana C. Clifton said at an annual scientific meeting on infectious diseases. “A number of patients were subsequently diagnosed with HIV after a prolonged delay.”
Of patients newly diagnosed with HIV in the United States, 41% report no prior HIV testing and an estimated 14%-25% of those living with HIV are undiagnosed, said Dr. Clifton, an internist at Duke University Medical Center, Durham, N.C. In 2006, the Centers for Disease Control and Prevention recommended routine HIV screening in all health care settings for all patients aged between 13 and 64 years old. “Multiple studies have shown that routine screening is cost effective, compared with screening tests for colon cancer, diabetes, and breast cancer,” Dr. Clifton said. In addition, bacterial pneumonia “is a predictor of HIV infection, and the clinical manifestations of bacterial pneumonia are similar whether one has HIV or not. So the question is, how do you decide whom to screen for HIV at hospital admission for pneumonia?”
Dr. Clifton and her associates retrospectively evaluated patients admitted to Duke University Health System between Jan. 1, 1996 and Dec. 31, 2014 with a first primary diagnosis of pneumonia. They used ICD-9 codes for primary diagnosis of pneumonia at time of hospital admission, reviewed a subset of charts to validate the diagnosis, and conducted a random sample of those without prior HIV diagnosis to evaluate HIV testing. The primary outcome was HIV testing during pneumonia admission. Secondary outcomes were documented prior HIV testing in the electronic medical record and subsequent new HIV diagnosis following pneumonia admission.
During the time period studied, 6,858 patients were admitted with a primary diagnosis of pneumonia. Their median age was 50 years, 49% were male, 53% were white, 41% were African American, and the rest were from other ethnic groups. In all, 5,133 (75%) were discharged by general medicine or pulmonary service.
Of the 6,858 patients, 6,513 (95%) were not previously known to be HIV positive (95%), while 345 (5%) were previously known to be HIV positive. Of the 6,513 not previously known to be HIV positive, 19 (0.3%) were diagnosed with HIV during hospital admission and 46 (0.7%) were diagnosed with HIV a median of 807 days after admission.
When the researchers evaluated a random sample of 207 patients not previously known to be HIV positive, the researchers found that only 69 (33%) had an HIV test result ever documented before or during admission, while 16 (8%) were tested for HIV sometime after discharge.
The researchers noted a slight but nonsignificant improvement in the proportion of patients with pneumonia who were ever tested for HIV before or during admission, before and after implementation of the CDC guidelines in 2006 (from 28% to 39%; P = .09). Dr. Clifton pointed out that the 5% prevalence of HIV observed in patients admitted with pneumonia is 10 times higher than the prevalence of HIV in the general population (.47%).
Limitations of the study, she said, include its retrospective, single-center design and the fact that it relied on an administrative database. “There’s also potential for coding bias using ICD-9 codes,” she said. “However, prior studies using ICD-9 codes for diagnosis of pneumonia show reasonably good specificity.”
She concluded her presentation by calling for “more studies to evaluate HIV testing and diagnosis in this higher-risk population of patients admitted with pneumonia. Opt-out HIV testing among pneumonia inpatients should be implemented for earlier HIV diagnosis and improved outcomes.”
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported having no financial disclosures.
The researchers reported having no financial disclosures.
SAN DIEGO – Only 39% of patients hospitalized for pneumonia underwent HIV testing, even though federal recommendations for universal HIV screening in all health care settings have been in place since 2006, according to the results of a retrospective, single-center study.
“Despite universal recommendations for HIV screening in all health care settings, HIV testing rates remain low among patients hospitalized with pneumonia,” Dr. Dana C. Clifton said at an annual scientific meeting on infectious diseases. “A number of patients were subsequently diagnosed with HIV after a prolonged delay.”
Of patients newly diagnosed with HIV in the United States, 41% report no prior HIV testing and an estimated 14%-25% of those living with HIV are undiagnosed, said Dr. Clifton, an internist at Duke University Medical Center, Durham, N.C. In 2006, the Centers for Disease Control and Prevention recommended routine HIV screening in all health care settings for all patients aged between 13 and 64 years old. “Multiple studies have shown that routine screening is cost effective, compared with screening tests for colon cancer, diabetes, and breast cancer,” Dr. Clifton said. In addition, bacterial pneumonia “is a predictor of HIV infection, and the clinical manifestations of bacterial pneumonia are similar whether one has HIV or not. So the question is, how do you decide whom to screen for HIV at hospital admission for pneumonia?”
Dr. Clifton and her associates retrospectively evaluated patients admitted to Duke University Health System between Jan. 1, 1996 and Dec. 31, 2014 with a first primary diagnosis of pneumonia. They used ICD-9 codes for primary diagnosis of pneumonia at time of hospital admission, reviewed a subset of charts to validate the diagnosis, and conducted a random sample of those without prior HIV diagnosis to evaluate HIV testing. The primary outcome was HIV testing during pneumonia admission. Secondary outcomes were documented prior HIV testing in the electronic medical record and subsequent new HIV diagnosis following pneumonia admission.
During the time period studied, 6,858 patients were admitted with a primary diagnosis of pneumonia. Their median age was 50 years, 49% were male, 53% were white, 41% were African American, and the rest were from other ethnic groups. In all, 5,133 (75%) were discharged by general medicine or pulmonary service.
Of the 6,858 patients, 6,513 (95%) were not previously known to be HIV positive (95%), while 345 (5%) were previously known to be HIV positive. Of the 6,513 not previously known to be HIV positive, 19 (0.3%) were diagnosed with HIV during hospital admission and 46 (0.7%) were diagnosed with HIV a median of 807 days after admission.
When the researchers evaluated a random sample of 207 patients not previously known to be HIV positive, the researchers found that only 69 (33%) had an HIV test result ever documented before or during admission, while 16 (8%) were tested for HIV sometime after discharge.
The researchers noted a slight but nonsignificant improvement in the proportion of patients with pneumonia who were ever tested for HIV before or during admission, before and after implementation of the CDC guidelines in 2006 (from 28% to 39%; P = .09). Dr. Clifton pointed out that the 5% prevalence of HIV observed in patients admitted with pneumonia is 10 times higher than the prevalence of HIV in the general population (.47%).
Limitations of the study, she said, include its retrospective, single-center design and the fact that it relied on an administrative database. “There’s also potential for coding bias using ICD-9 codes,” she said. “However, prior studies using ICD-9 codes for diagnosis of pneumonia show reasonably good specificity.”
She concluded her presentation by calling for “more studies to evaluate HIV testing and diagnosis in this higher-risk population of patients admitted with pneumonia. Opt-out HIV testing among pneumonia inpatients should be implemented for earlier HIV diagnosis and improved outcomes.”
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported having no financial disclosures.
The researchers reported having no financial disclosures.
SAN DIEGO – Only 39% of patients hospitalized for pneumonia underwent HIV testing, even though federal recommendations for universal HIV screening in all health care settings have been in place since 2006, according to the results of a retrospective, single-center study.
“Despite universal recommendations for HIV screening in all health care settings, HIV testing rates remain low among patients hospitalized with pneumonia,” Dr. Dana C. Clifton said at an annual scientific meeting on infectious diseases. “A number of patients were subsequently diagnosed with HIV after a prolonged delay.”
Of patients newly diagnosed with HIV in the United States, 41% report no prior HIV testing and an estimated 14%-25% of those living with HIV are undiagnosed, said Dr. Clifton, an internist at Duke University Medical Center, Durham, N.C. In 2006, the Centers for Disease Control and Prevention recommended routine HIV screening in all health care settings for all patients aged between 13 and 64 years old. “Multiple studies have shown that routine screening is cost effective, compared with screening tests for colon cancer, diabetes, and breast cancer,” Dr. Clifton said. In addition, bacterial pneumonia “is a predictor of HIV infection, and the clinical manifestations of bacterial pneumonia are similar whether one has HIV or not. So the question is, how do you decide whom to screen for HIV at hospital admission for pneumonia?”
Dr. Clifton and her associates retrospectively evaluated patients admitted to Duke University Health System between Jan. 1, 1996 and Dec. 31, 2014 with a first primary diagnosis of pneumonia. They used ICD-9 codes for primary diagnosis of pneumonia at time of hospital admission, reviewed a subset of charts to validate the diagnosis, and conducted a random sample of those without prior HIV diagnosis to evaluate HIV testing. The primary outcome was HIV testing during pneumonia admission. Secondary outcomes were documented prior HIV testing in the electronic medical record and subsequent new HIV diagnosis following pneumonia admission.
During the time period studied, 6,858 patients were admitted with a primary diagnosis of pneumonia. Their median age was 50 years, 49% were male, 53% were white, 41% were African American, and the rest were from other ethnic groups. In all, 5,133 (75%) were discharged by general medicine or pulmonary service.
Of the 6,858 patients, 6,513 (95%) were not previously known to be HIV positive (95%), while 345 (5%) were previously known to be HIV positive. Of the 6,513 not previously known to be HIV positive, 19 (0.3%) were diagnosed with HIV during hospital admission and 46 (0.7%) were diagnosed with HIV a median of 807 days after admission.
When the researchers evaluated a random sample of 207 patients not previously known to be HIV positive, the researchers found that only 69 (33%) had an HIV test result ever documented before or during admission, while 16 (8%) were tested for HIV sometime after discharge.
The researchers noted a slight but nonsignificant improvement in the proportion of patients with pneumonia who were ever tested for HIV before or during admission, before and after implementation of the CDC guidelines in 2006 (from 28% to 39%; P = .09). Dr. Clifton pointed out that the 5% prevalence of HIV observed in patients admitted with pneumonia is 10 times higher than the prevalence of HIV in the general population (.47%).
Limitations of the study, she said, include its retrospective, single-center design and the fact that it relied on an administrative database. “There’s also potential for coding bias using ICD-9 codes,” she said. “However, prior studies using ICD-9 codes for diagnosis of pneumonia show reasonably good specificity.”
She concluded her presentation by calling for “more studies to evaluate HIV testing and diagnosis in this higher-risk population of patients admitted with pneumonia. Opt-out HIV testing among pneumonia inpatients should be implemented for earlier HIV diagnosis and improved outcomes.”
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported having no financial disclosures.
The researchers reported having no financial disclosures.
AT IDWEEK 2015
Key clinical point: The proportion of adults hospitalized with pneumonia who undergo HIV testing is low.
Major finding: The proportion of patients with pneumonia who were ever tested for HIV before or during hospital admission improved slightly following implementation of CDC guidelines in 2006 (from 28% to 39%; P =. 09).
Data source: A retrospective study of 6,858 adults admitted to Duke University Health System between Jan. 1, 1996 and Dec. 31, 2014 with a first primary diagnosis of pneumonia.
Disclosures: The researchers reported having no financial disclosures.