User login
Pediatric Academic Societies (PAS): Annual Meeting
PAS: Low-dose hydrocortisone improves outcomes in extreme preemies
SAN DIEGO – Early prophylactic very-low-dose hydrocortisone improved survival free of bronchopulmonary dysplasia in extremely preterm neonates in a large French randomized trial.
The intervention also brought positive results on multiple secondary endpoints, including rates of extubation by day 10 and patent ductus arteriosus closure without resort to ligation, Dr. Olivier Baud reported at the annual meeting of the Pediatric Academic Societies.
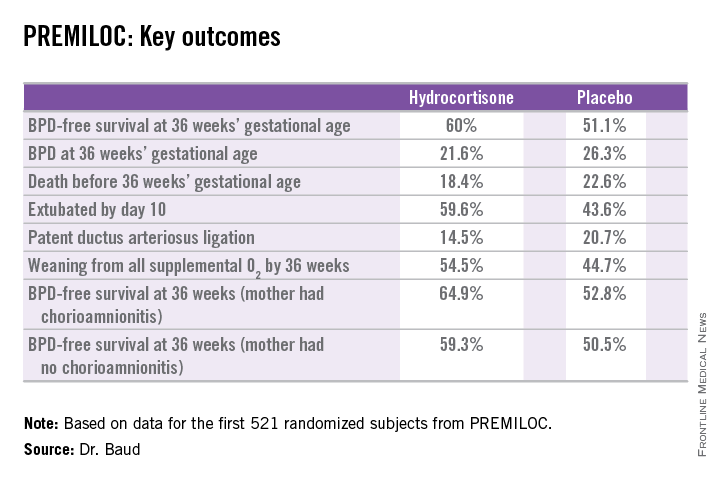
He presented the findings of PREMILOC, a double-blind, randomized, placebo-controlled, 21-center French study. The study was halted by the data safety monitoring board for ethical reasons, based upon compelling evidence of superiority after scrutinizing results in the first 521 randomized subjects.
The primary outcome – survival free of bronchopulmonary dysplasia (BPD) at 36 weeks of gestational age – occurred in 60% of the hydrocortisone group, compared with 51% of placebo-treated controls, for an adjusted 48% increased likelihood of favorable outcome. The number-needed-to-treat was 11 patients, said Dr. Baud, professor of pediatrics and chief of the neonatal medicine unit at Robert Debré Hospital in Paris and Paris Diderot University.
The intervention involved administration of intravenous hydrocortisone sodium succinate for the first 10 postnatal days. The dose was 0.5 mg/kg/12 hours for 7 days followed by 0.5 mg/kg/24 hours for 3 days. Treatment began as soon as possible after birth and always within 24 hours. The total cumulative dose of hydrocortisone was 8.5 mg/kg. That’s 15-30 times less corticosteroid than employed in earlier studies of higher-dose dexamethasone, where gastrointestinal bleeding, intestinal perforation, and other serious adverse events were a major problem.
“This is the lowest hydrocortisone dose ever studied in a randomized controlled trial,” according to the neonatologist.
All study participants were born at 24-27 weeks’ gestational age. In order to minimize the risk of treatment-related serious adverse events, especially GI perforation – patients with intrauterine growth retardation or who were on nonsteroidal anti-inflammatory agents were ineligible for the trial.
The rationale for the study, Dr. Baud explained, comes from the landmark work of Dr. Kristi L. Watterberg, professor of pediatrics at the University of New Mexico, Albuquerque, who has argued that early adrenal insufficiency in extremely small-for-gestational-age infants is linked to BPD, patent ductus arteriosus, persistent lung inflammation, and poor enteral nutrition. She introduced the concept that administration of very-low-dose corticosteroids at a dose comparable to normal endogenous steroid production could serve as a global therapy addressing these multiple health problems.
Two prior studies of low-dose hydrocortisone in extremely preterm babies were halted due to an increase in GI perforation, Dr. Baud noted, which is why the French investigators excluded neonates at increased risk for this complication.
In a prespecified PREMILOC subgroup analysis stratified by gestational age, the rate of survival free of BPD at 36 weeks among neonates born at 24-25 weeks was 33.7% with hydrocortisone, compared with 23.3% with placebo, for an adjusted 67% increased likelihood of a positive outcome with active treatment. In babies born at 26-27 weeks, the rates were 72.7% and 65.3%, respectively. On the other hand, hydrocortisone was associated with a highly significant 55% relative risk reduction for neonatal mortality in infants born at 26-27 weeks.
Post hoc analysis spotlighted several factors which were unexpectedly associated with differential impacts on outcome. For example, hydrocortisone achieved a significant benefit in terms of survival free of BPD at 36 weeks only in females, where the rates were 69.4% versus 53%, for an adjusted 2.25-fold increased rate. In males, the rate was 51.1% with hydrocortisone, compared with 49.7% in controls.
There were no differences between the hydrocortisone and placebo groups in rates of any serious adverse events, including GI perforation, necrotizing enterocolitis, air leaks, severe sepsis, or persistent pulmonary hypertension.
Dr. Baud said the next stage of the PREMILOC study will be to report 2-year outcomes; a greater than 90% follow-up rate is anticipated. Also, he and his coinvestigators are planning ancillary studies examining placental findings, the impact of pretreatment serum cortisol levels, thyroid function, and other issues.
“Our goal is to identify a targeted population that could strongly benefit from prophylactic hydrocortisone,” the neonatologist said.
He reported having no financial conflicts regarding the study, supported by INSERM and other French national research organizations.
SAN DIEGO – Early prophylactic very-low-dose hydrocortisone improved survival free of bronchopulmonary dysplasia in extremely preterm neonates in a large French randomized trial.
The intervention also brought positive results on multiple secondary endpoints, including rates of extubation by day 10 and patent ductus arteriosus closure without resort to ligation, Dr. Olivier Baud reported at the annual meeting of the Pediatric Academic Societies.

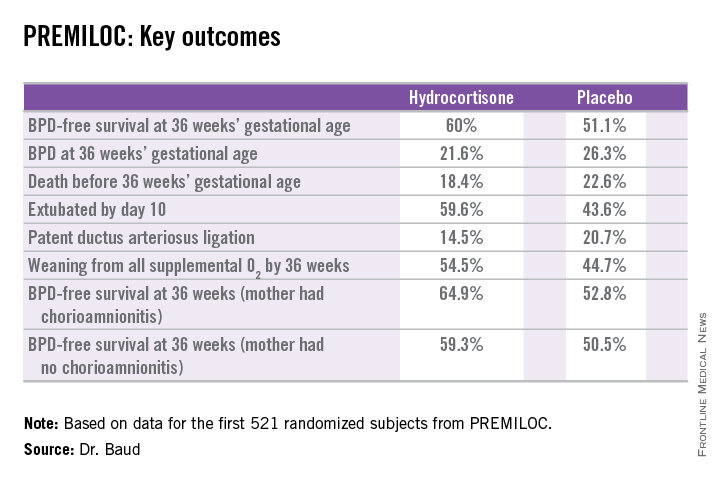
He presented the findings of PREMILOC, a double-blind, randomized, placebo-controlled, 21-center French study. The study was halted by the data safety monitoring board for ethical reasons, based upon compelling evidence of superiority after scrutinizing results in the first 521 randomized subjects.
The primary outcome – survival free of bronchopulmonary dysplasia (BPD) at 36 weeks of gestational age – occurred in 60% of the hydrocortisone group, compared with 51% of placebo-treated controls, for an adjusted 48% increased likelihood of favorable outcome. The number-needed-to-treat was 11 patients, said Dr. Baud, professor of pediatrics and chief of the neonatal medicine unit at Robert Debré Hospital in Paris and Paris Diderot University.
The intervention involved administration of intravenous hydrocortisone sodium succinate for the first 10 postnatal days. The dose was 0.5 mg/kg/12 hours for 7 days followed by 0.5 mg/kg/24 hours for 3 days. Treatment began as soon as possible after birth and always within 24 hours. The total cumulative dose of hydrocortisone was 8.5 mg/kg. That’s 15-30 times less corticosteroid than employed in earlier studies of higher-dose dexamethasone, where gastrointestinal bleeding, intestinal perforation, and other serious adverse events were a major problem.
“This is the lowest hydrocortisone dose ever studied in a randomized controlled trial,” according to the neonatologist.
All study participants were born at 24-27 weeks’ gestational age. In order to minimize the risk of treatment-related serious adverse events, especially GI perforation – patients with intrauterine growth retardation or who were on nonsteroidal anti-inflammatory agents were ineligible for the trial.
The rationale for the study, Dr. Baud explained, comes from the landmark work of Dr. Kristi L. Watterberg, professor of pediatrics at the University of New Mexico, Albuquerque, who has argued that early adrenal insufficiency in extremely small-for-gestational-age infants is linked to BPD, patent ductus arteriosus, persistent lung inflammation, and poor enteral nutrition. She introduced the concept that administration of very-low-dose corticosteroids at a dose comparable to normal endogenous steroid production could serve as a global therapy addressing these multiple health problems.
Two prior studies of low-dose hydrocortisone in extremely preterm babies were halted due to an increase in GI perforation, Dr. Baud noted, which is why the French investigators excluded neonates at increased risk for this complication.
In a prespecified PREMILOC subgroup analysis stratified by gestational age, the rate of survival free of BPD at 36 weeks among neonates born at 24-25 weeks was 33.7% with hydrocortisone, compared with 23.3% with placebo, for an adjusted 67% increased likelihood of a positive outcome with active treatment. In babies born at 26-27 weeks, the rates were 72.7% and 65.3%, respectively. On the other hand, hydrocortisone was associated with a highly significant 55% relative risk reduction for neonatal mortality in infants born at 26-27 weeks.
Post hoc analysis spotlighted several factors which were unexpectedly associated with differential impacts on outcome. For example, hydrocortisone achieved a significant benefit in terms of survival free of BPD at 36 weeks only in females, where the rates were 69.4% versus 53%, for an adjusted 2.25-fold increased rate. In males, the rate was 51.1% with hydrocortisone, compared with 49.7% in controls.
There were no differences between the hydrocortisone and placebo groups in rates of any serious adverse events, including GI perforation, necrotizing enterocolitis, air leaks, severe sepsis, or persistent pulmonary hypertension.
Dr. Baud said the next stage of the PREMILOC study will be to report 2-year outcomes; a greater than 90% follow-up rate is anticipated. Also, he and his coinvestigators are planning ancillary studies examining placental findings, the impact of pretreatment serum cortisol levels, thyroid function, and other issues.
“Our goal is to identify a targeted population that could strongly benefit from prophylactic hydrocortisone,” the neonatologist said.
He reported having no financial conflicts regarding the study, supported by INSERM and other French national research organizations.
SAN DIEGO – Early prophylactic very-low-dose hydrocortisone improved survival free of bronchopulmonary dysplasia in extremely preterm neonates in a large French randomized trial.
The intervention also brought positive results on multiple secondary endpoints, including rates of extubation by day 10 and patent ductus arteriosus closure without resort to ligation, Dr. Olivier Baud reported at the annual meeting of the Pediatric Academic Societies.

He presented the findings of PREMILOC, a double-blind, randomized, placebo-controlled, 21-center French study. The study was halted by the data safety monitoring board for ethical reasons, based upon compelling evidence of superiority after scrutinizing results in the first 521 randomized subjects.
The primary outcome – survival free of bronchopulmonary dysplasia (BPD) at 36 weeks of gestational age – occurred in 60% of the hydrocortisone group, compared with 51% of placebo-treated controls, for an adjusted 48% increased likelihood of favorable outcome. The number-needed-to-treat was 11 patients, said Dr. Baud, professor of pediatrics and chief of the neonatal medicine unit at Robert Debré Hospital in Paris and Paris Diderot University.
The intervention involved administration of intravenous hydrocortisone sodium succinate for the first 10 postnatal days. The dose was 0.5 mg/kg/12 hours for 7 days followed by 0.5 mg/kg/24 hours for 3 days. Treatment began as soon as possible after birth and always within 24 hours. The total cumulative dose of hydrocortisone was 8.5 mg/kg. That’s 15-30 times less corticosteroid than employed in earlier studies of higher-dose dexamethasone, where gastrointestinal bleeding, intestinal perforation, and other serious adverse events were a major problem.
“This is the lowest hydrocortisone dose ever studied in a randomized controlled trial,” according to the neonatologist.
All study participants were born at 24-27 weeks’ gestational age. In order to minimize the risk of treatment-related serious adverse events, especially GI perforation – patients with intrauterine growth retardation or who were on nonsteroidal anti-inflammatory agents were ineligible for the trial.
The rationale for the study, Dr. Baud explained, comes from the landmark work of Dr. Kristi L. Watterberg, professor of pediatrics at the University of New Mexico, Albuquerque, who has argued that early adrenal insufficiency in extremely small-for-gestational-age infants is linked to BPD, patent ductus arteriosus, persistent lung inflammation, and poor enteral nutrition. She introduced the concept that administration of very-low-dose corticosteroids at a dose comparable to normal endogenous steroid production could serve as a global therapy addressing these multiple health problems.
Two prior studies of low-dose hydrocortisone in extremely preterm babies were halted due to an increase in GI perforation, Dr. Baud noted, which is why the French investigators excluded neonates at increased risk for this complication.
In a prespecified PREMILOC subgroup analysis stratified by gestational age, the rate of survival free of BPD at 36 weeks among neonates born at 24-25 weeks was 33.7% with hydrocortisone, compared with 23.3% with placebo, for an adjusted 67% increased likelihood of a positive outcome with active treatment. In babies born at 26-27 weeks, the rates were 72.7% and 65.3%, respectively. On the other hand, hydrocortisone was associated with a highly significant 55% relative risk reduction for neonatal mortality in infants born at 26-27 weeks.
Post hoc analysis spotlighted several factors which were unexpectedly associated with differential impacts on outcome. For example, hydrocortisone achieved a significant benefit in terms of survival free of BPD at 36 weeks only in females, where the rates were 69.4% versus 53%, for an adjusted 2.25-fold increased rate. In males, the rate was 51.1% with hydrocortisone, compared with 49.7% in controls.
There were no differences between the hydrocortisone and placebo groups in rates of any serious adverse events, including GI perforation, necrotizing enterocolitis, air leaks, severe sepsis, or persistent pulmonary hypertension.
Dr. Baud said the next stage of the PREMILOC study will be to report 2-year outcomes; a greater than 90% follow-up rate is anticipated. Also, he and his coinvestigators are planning ancillary studies examining placental findings, the impact of pretreatment serum cortisol levels, thyroid function, and other issues.
“Our goal is to identify a targeted population that could strongly benefit from prophylactic hydrocortisone,” the neonatologist said.
He reported having no financial conflicts regarding the study, supported by INSERM and other French national research organizations.
AT THE PAS ANNUAL MEETING
Key clinical point: In extremely premature neonates, prophylactic very-low-dose hydrocortisone improved the likelihood of survival free of bronchopulmonary dysplasia at 36 weeks’ gestational age by roughly 50%.
Major finding: The number needed to treat to achieve one additional survival without bronchopulmonary dysplasia at 36 weeks’ gestational age was 11.
Data source: This was a randomized, double-blind, placebo-controlled, multicenter French study involving 521 patients born at 24-27 weeks’ gestational age.
Disclosures: The PREMILOC study was supported by INSERM and other French scientific research organizations. The presenter reported having no financial conflicts.
PAS: Screen for postpartum depression during infant hospitalization
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
AT THE PAS ANNUAL MEETING
Key clinical point: Screening for maternal postpartum depression during an infant’s hospitalization captures large numbers of previously unscreened women.
Major finding: Twenty-eight percent of women screened during their infant’s hospital admission were deemed at risk for postpartum depression.
Data source: The study included 310 mothers, only 18% of whom had been screened for postpartum depression during their infant’s routine office visits as guidelines recommend.
Disclosures: This study was conducted free of commercial support. The presenter reported having no financial conflicts.
PAS: Screen for postpartum depression during infant hospitalization
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
The American Academy of Pediatrics recommends screening mothers for postpartum depression during an infant’s routine office visits. Realistically, that often doesn’t happen, because of time constraints, because of physician discomfort with diagnosing the illness, or because an infant with prolonged hospitalization for severe illness misses the scheduled office visits, she said at the annual meeting of the Pediatric Academic Societies.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
The American Academy of Pediatrics recommends screening mothers for postpartum depression during an infant’s routine office visits. Realistically, that often doesn’t happen, because of time constraints, because of physician discomfort with diagnosing the illness, or because an infant with prolonged hospitalization for severe illness misses the scheduled office visits, she said at the annual meeting of the Pediatric Academic Societies.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
SAN DIEGO – Infant hospitalizations provide an untapped opportunity to screen for maternal postpartum depression, according to Dr. Margaret Trost.
“A hospital setting places a mother at the bedside with her infant for much of the day, which may provide time for screening and counseling,” noted Dr. Trost, a pediatric hospitalist at Children’s Hospital Los Angeles and the University of Southern California in Los Angeles.
The American Academy of Pediatrics recommends screening mothers for postpartum depression during an infant’s routine office visits. Realistically, that often doesn’t happen, because of time constraints, because of physician discomfort with diagnosing the illness, or because an infant with prolonged hospitalization for severe illness misses the scheduled office visits, she said at the annual meeting of the Pediatric Academic Societies.
Dr. Trost presented what she believes is the first-ever formal study of screening for maternal postpartum depression during infant hospitalization. The results demonstrated that this approach is readily accomplished and captures large numbers of previously unscreened mothers. Moreover, women who initially screened positive and followed staff advice to discuss postpartum depression with their own physician or a recommended mental health referral resource had lower postpartum depression scores upon repeat screening at 3 and/or 6 months of follow-up.
Maternal postpartum depression is a depressive episode occurring within 1 year of childbirth. It affects an estimated 10% of mothers, with markedly higher rates in high-risk populations, including teenage or low-income mothers. In addition to the harmful effects on the mother, postpartum depression can harm an infant’s cognitive development and contribute to behavioral and emotional problems, Dr. Trost noted.
She reported on 310 mothers screened for postpartum depression 24-48 hours after their infant was admitted to Children’s Hospital Los Angeles. All infants had to be at least 2 weeks of age, because maternal depressive symptoms within the first 2 weeks after childbirth are classified as “baby blues” rather than postpartum depression. Study eligibility was restricted to the mothers of infants who were not admitted to an intensive care unit.
The screening tool utilized in the study was the Edinburgh Postpartum Depression Scale (EPDS), a validated 10-question instrument that probes a mother’s feelings, including energy, mood, and suicidality, within the past 7 days. A score of 10 or more out of a possible 30 identifies an at-risk mother.
All participants also were assessed using the Maternal-Infant Bonding Tool. In addition, information was collected on maternal demographics, social isolation, and history of previous psychiatric illness, as well as the infants’ comorbid conditions.
Based upon EPDS results, 28% of the mothers were deemed at risk for postpartum depression. They received counseling from both a physician and a social worker, got a handout on local and national mental health resources, and were advised to discuss their screening results with their personal physician or someone on the mental health referral list.
Of note, a mere 18% of study participants reported that they had been screened for postpartum depression since their most recent childbirth, underscoring the point that this important task doesn’t consistently get done in the outpatient setting, Dr. Trost said.
In this study, the higher a mother’s score on the EPDS, the worse her maternal-infant bonding score.
In a logistic regression analysis, Hispanic mothers were significantly less likely to have postpartum depression symptoms than non-Hispanics, with an odds ratio of 0.46.
Several risk factors for postpartum depression were identified in the study. Women who reported having low or no social support were 3.5 times more likely to have an at-risk EPDS score than those with self-described good social support. Those with a history of psychiatric illness were at 5.1-fold increased risk.
Those maternal risk factors for postpartum depression were consistent with the results of previous studies. A novel finding in this study was the identification of an infant risk factor: neurodevelopmental disorders. The 22 mothers of infants with a neurodevelopmental abnormality – cerebral palsy, mental retardation, hydrocephalus, seizures, a ventriculoperitoneal shunt, or craniosynostosis – had a 3.4-fold increased risk of a positive screen for postpartum depression. Moreover, in a multivariate logistic regression analysis controlled for race, social support, and history of psychiatric diagnosis, having an infant with a neurodevelopmental problem still remained associated with a threefold increased risk of maternal postpartum depression.
Attempts to follow up with all study participants by phone 3 and 6 months later met with only limited success. Of 87 mothers who screened positive by the EPDS, 21 completed follow-up phone interviews involving repeat screening, at which point 11 women still screened positive while 10 screened negative.
Follow-up interviews also were completed with 76 of the 223 mothers who initially screened negative. Of note, 6 women, or 8%, screened positive at follow-up.
Among the 21 mothers with an at-risk EPDS score at the initial screening during their infant’s hospitalization, 8 (38%) women took the advice to have a follow-up discussion about their risk for postpartum depression. This was typically with their personal physician, presumably because they felt more comfortable talking about the problem with a professional with whom there was already an established relationship, Dr. Trost said. On follow-up EPDS screening, those women showed a significant reduction from their baseline scores down to levels below the threshold for concern. In contrast, the change over time in EPDS scores in the 13 women who didn’t seek help was unimpressive, although the small sample size – just 21 mothers – must be noted, she said.
One audience member suggested that the intervention part of the program would be much more effective – and the follow-up rate higher – if the mental health referral resources could be incorporated into the initial infant hospitalization. Dr. Trost agreed, adding that she is looking into bringing in on-site small-group sessions.
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
AT THE PAS ANNUAL MEETING
Key clinical point: Screening for maternal postpartum depression during an infant’s hospitalization captures large numbers of previously unscreened women.
Major finding: Twenty-eight percent of women screened during their infant’s hospital admission were deemed at risk for postpartum depression.
Data source: The study included 310 mothers, only 18% of whom had been screened for postpartum depression during their infant’s routine office visits as guidelines recommend.
Disclosures: This study was conducted free of commercial support. The presenter reported having no financial conflicts.
PAS: Intervention eases transition from pediatric to adult care
SAN DIEGO – A dedicated care coordination intervention had a positive impact on facilitating the transition from pediatric to adult care among youth with special health care needs, a single-center randomized trial showed.
In an interview in advance of the annual meeting of the Pediatric Academic Societies, Dr. Lisa K. Tuchman said that coordination interventions in the existing literature have had mixed results. The current study is unique, she said, because it focuses on urban-based, publicly insured youth aged 16-22 years with special health care needs. “Other studies focus primarily on either pediatric or adult patients, not this in-between age group who is very vulnerable to dropping out of care,” explained Dr. Tuchman of the division of adolescent and young adult medicine at Children’s National Health System, Washington.
“Young adulthood is a time of many transitions; in this study we are focusing on transition between the pediatric and adult health care system – or when youth ‘age out’ of pediatric primary care and need to establish a new adult-oriented medical home – often in a different health system. This gap in care is known as a high-risk time for all youth, but especially for those youth with complex chronic conditions who may have adherence challenges, are navigating taking on more responsibility for their medical care, and often have a high rate of emergency department usage without a stable medical home.”
The current study also serves as a proof-of-effectiveness study. The health care transition recommendations published in Pediatrics in July 2011 are based on expert consensus, as evidence and data have been slow to evolve in this area (Pediatrics 2011;128:182-200). According to Dr. Tuchman, the current study is the first to demonstrate that these recommended guidelines improve quality and perception of care coordination, compared with a control group.
She and her colleagues recruited 210 patients aged 16-22 years from Children’s National Health System and randomly assigned them to a health care transition care coordination intervention (n = 105) or to a control group (n = 105).
The transition care coordination intervention consisted of implementing the Six Core Elements of Health Care Transition 2.0: transition policy, tracking and monitoring, transition readiness, planning, transfer of care, and completion. The last steps include communicating with the adult practice prior to planned transfer, confirming completion of transfer, and offering consultation assistance as needed.
The control group underwent usual care.
At baseline, and at 6 and 12 months, patients in both groups completed the Patient Assessment of Care for Chronic Conditions (PACIC), which assesses five domains (patient activation, delivery system design, goal setting, problem solving, and coordination/follow-up), as well as the Client Perceptions of Coordination Questionnaire (CPCQ), which assesses perception of patient-centered care and care coordination.
The study participants also completed a self-rating Likert scale (ranging from 1 to 10), a measurement of how ready they feel to transfer to adult care.
Dr. Tuchman reported that there were no statistically significant differences in PACIC, CPCQ, or readiness scores between the two groups at baseline. At 6 months, however, patients in the care coordination intervention group, compared with their counterparts in the control group, rated the quality of their chronic illness higher (P = .065) and reported receiving less conflicting advice from care providers (P = .018).
At 12 months, patients in the care coordination intervention group rated the following PACIC domains higher, compared with those in the control group: patient activation (P = .015), goal setting (P = .034), problem solving (P = .009), and coordination/follow-up (P = .016). Patients in the care coordination intervention group also reported more often receiving services they thought they needed (P = .03), were less confused about the role of providers (P = .012), and reported having more frequent discussions with providers about future care (P = .05).
The researchers observed no differences between the two groups in self-rating of transition readiness throughout the study period.
“[It was] surprising that this perceived higher quality care coordination and quality of care did not change perception of readiness to transfer from pediatrics to adult care,” Dr. Tuchman said. “This likely means that readiness is difficult to measure, and not related to care quality.”
She acknowledged certain limitations of the analysis, including the fact it “was a predominately African American, urban, publicly insured population of adolescents with special health care needs, and therefore may not be generalizable to other populations. But [it’s] still very important as Washington, D.C., has the fourth highest rate of unmet transition needs nationally.”
Maternal and Child Health, a bureau of the Department of Health and Human Services’ Health Resources and Services Administration, funded the study. Dr. Tuchman reported having no relevant financial disclosures.
On Twitter @dougbrunk
This article was updated 4/30/2015.
SAN DIEGO – A dedicated care coordination intervention had a positive impact on facilitating the transition from pediatric to adult care among youth with special health care needs, a single-center randomized trial showed.
In an interview in advance of the annual meeting of the Pediatric Academic Societies, Dr. Lisa K. Tuchman said that coordination interventions in the existing literature have had mixed results. The current study is unique, she said, because it focuses on urban-based, publicly insured youth aged 16-22 years with special health care needs. “Other studies focus primarily on either pediatric or adult patients, not this in-between age group who is very vulnerable to dropping out of care,” explained Dr. Tuchman of the division of adolescent and young adult medicine at Children’s National Health System, Washington.
“Young adulthood is a time of many transitions; in this study we are focusing on transition between the pediatric and adult health care system – or when youth ‘age out’ of pediatric primary care and need to establish a new adult-oriented medical home – often in a different health system. This gap in care is known as a high-risk time for all youth, but especially for those youth with complex chronic conditions who may have adherence challenges, are navigating taking on more responsibility for their medical care, and often have a high rate of emergency department usage without a stable medical home.”
The current study also serves as a proof-of-effectiveness study. The health care transition recommendations published in Pediatrics in July 2011 are based on expert consensus, as evidence and data have been slow to evolve in this area (Pediatrics 2011;128:182-200). According to Dr. Tuchman, the current study is the first to demonstrate that these recommended guidelines improve quality and perception of care coordination, compared with a control group.
She and her colleagues recruited 210 patients aged 16-22 years from Children’s National Health System and randomly assigned them to a health care transition care coordination intervention (n = 105) or to a control group (n = 105).
The transition care coordination intervention consisted of implementing the Six Core Elements of Health Care Transition 2.0: transition policy, tracking and monitoring, transition readiness, planning, transfer of care, and completion. The last steps include communicating with the adult practice prior to planned transfer, confirming completion of transfer, and offering consultation assistance as needed.
The control group underwent usual care.
At baseline, and at 6 and 12 months, patients in both groups completed the Patient Assessment of Care for Chronic Conditions (PACIC), which assesses five domains (patient activation, delivery system design, goal setting, problem solving, and coordination/follow-up), as well as the Client Perceptions of Coordination Questionnaire (CPCQ), which assesses perception of patient-centered care and care coordination.
The study participants also completed a self-rating Likert scale (ranging from 1 to 10), a measurement of how ready they feel to transfer to adult care.
Dr. Tuchman reported that there were no statistically significant differences in PACIC, CPCQ, or readiness scores between the two groups at baseline. At 6 months, however, patients in the care coordination intervention group, compared with their counterparts in the control group, rated the quality of their chronic illness higher (P = .065) and reported receiving less conflicting advice from care providers (P = .018).
At 12 months, patients in the care coordination intervention group rated the following PACIC domains higher, compared with those in the control group: patient activation (P = .015), goal setting (P = .034), problem solving (P = .009), and coordination/follow-up (P = .016). Patients in the care coordination intervention group also reported more often receiving services they thought they needed (P = .03), were less confused about the role of providers (P = .012), and reported having more frequent discussions with providers about future care (P = .05).
The researchers observed no differences between the two groups in self-rating of transition readiness throughout the study period.
“[It was] surprising that this perceived higher quality care coordination and quality of care did not change perception of readiness to transfer from pediatrics to adult care,” Dr. Tuchman said. “This likely means that readiness is difficult to measure, and not related to care quality.”
She acknowledged certain limitations of the analysis, including the fact it “was a predominately African American, urban, publicly insured population of adolescents with special health care needs, and therefore may not be generalizable to other populations. But [it’s] still very important as Washington, D.C., has the fourth highest rate of unmet transition needs nationally.”
Maternal and Child Health, a bureau of the Department of Health and Human Services’ Health Resources and Services Administration, funded the study. Dr. Tuchman reported having no relevant financial disclosures.
On Twitter @dougbrunk
This article was updated 4/30/2015.
SAN DIEGO – A dedicated care coordination intervention had a positive impact on facilitating the transition from pediatric to adult care among youth with special health care needs, a single-center randomized trial showed.
In an interview in advance of the annual meeting of the Pediatric Academic Societies, Dr. Lisa K. Tuchman said that coordination interventions in the existing literature have had mixed results. The current study is unique, she said, because it focuses on urban-based, publicly insured youth aged 16-22 years with special health care needs. “Other studies focus primarily on either pediatric or adult patients, not this in-between age group who is very vulnerable to dropping out of care,” explained Dr. Tuchman of the division of adolescent and young adult medicine at Children’s National Health System, Washington.
“Young adulthood is a time of many transitions; in this study we are focusing on transition between the pediatric and adult health care system – or when youth ‘age out’ of pediatric primary care and need to establish a new adult-oriented medical home – often in a different health system. This gap in care is known as a high-risk time for all youth, but especially for those youth with complex chronic conditions who may have adherence challenges, are navigating taking on more responsibility for their medical care, and often have a high rate of emergency department usage without a stable medical home.”
The current study also serves as a proof-of-effectiveness study. The health care transition recommendations published in Pediatrics in July 2011 are based on expert consensus, as evidence and data have been slow to evolve in this area (Pediatrics 2011;128:182-200). According to Dr. Tuchman, the current study is the first to demonstrate that these recommended guidelines improve quality and perception of care coordination, compared with a control group.
She and her colleagues recruited 210 patients aged 16-22 years from Children’s National Health System and randomly assigned them to a health care transition care coordination intervention (n = 105) or to a control group (n = 105).
The transition care coordination intervention consisted of implementing the Six Core Elements of Health Care Transition 2.0: transition policy, tracking and monitoring, transition readiness, planning, transfer of care, and completion. The last steps include communicating with the adult practice prior to planned transfer, confirming completion of transfer, and offering consultation assistance as needed.
The control group underwent usual care.
At baseline, and at 6 and 12 months, patients in both groups completed the Patient Assessment of Care for Chronic Conditions (PACIC), which assesses five domains (patient activation, delivery system design, goal setting, problem solving, and coordination/follow-up), as well as the Client Perceptions of Coordination Questionnaire (CPCQ), which assesses perception of patient-centered care and care coordination.
The study participants also completed a self-rating Likert scale (ranging from 1 to 10), a measurement of how ready they feel to transfer to adult care.
Dr. Tuchman reported that there were no statistically significant differences in PACIC, CPCQ, or readiness scores between the two groups at baseline. At 6 months, however, patients in the care coordination intervention group, compared with their counterparts in the control group, rated the quality of their chronic illness higher (P = .065) and reported receiving less conflicting advice from care providers (P = .018).
At 12 months, patients in the care coordination intervention group rated the following PACIC domains higher, compared with those in the control group: patient activation (P = .015), goal setting (P = .034), problem solving (P = .009), and coordination/follow-up (P = .016). Patients in the care coordination intervention group also reported more often receiving services they thought they needed (P = .03), were less confused about the role of providers (P = .012), and reported having more frequent discussions with providers about future care (P = .05).
The researchers observed no differences between the two groups in self-rating of transition readiness throughout the study period.
“[It was] surprising that this perceived higher quality care coordination and quality of care did not change perception of readiness to transfer from pediatrics to adult care,” Dr. Tuchman said. “This likely means that readiness is difficult to measure, and not related to care quality.”
She acknowledged certain limitations of the analysis, including the fact it “was a predominately African American, urban, publicly insured population of adolescents with special health care needs, and therefore may not be generalizable to other populations. But [it’s] still very important as Washington, D.C., has the fourth highest rate of unmet transition needs nationally.”
Maternal and Child Health, a bureau of the Department of Health and Human Services’ Health Resources and Services Administration, funded the study. Dr. Tuchman reported having no relevant financial disclosures.
On Twitter @dougbrunk
This article was updated 4/30/2015.
AT THE PAS ANNUAL MEETING
Key clinical point: A coordinated health care transition intervention improved many aspects of quality care for youth with chronic illnesses.
Major finding: At 12 months, compared with the control group, patients in the transition care coordination intervention group reported more often receiving services they thought they needed (P = .03), were less confused about the role of providers (P = .012), and reported having more frequent discussions with providers about future care (P = .05).
Data source: A study of 210 patients aged 16-22 years with special health care needs who were randomly assigned to a health care transition care coordination intervention or a control group.
Disclosures: Maternal and Child Health, a bureau of the Department of Health and Human Services’ Health Resources and Services Administration, funded the study. Dr. Tuchman reported having no relevant financial disclosures.
PAS: Early antibiotics linked to later overweight
SAN DIEGO – The use of oral antibiotics before 4 years of age was associated with a significantly increased risk of later overweight in a large cohort study.
“Antibiotics may provide a physician-modifiable risk factor for obesity prevention in early childhood,” Dr. Elizabeth Dawson-Hahn said at the annual meeting of the Pediatric Academic Societies.
The study included 4,938 children born at Group Health Cooperative, the Seattle-based integrated health care system. Their electronic medical records indicated that 3,533 of them, or 72%, filled one or more prescriptions for oral antibiotics at ages 0-47 months. In this group, 53% were less than 12 months old at the time of their first oral antibiotic exposure, while the rest were 12-47 months old.
At ages 48-59 months, roughly 12% of all study participants were overweight, as defined by a body mass index at or above the 85th percentile. The prevalence was similar regardless of whether a child was exposed to early oral antibiotics. However, the groups with and without early antibiotic exposure differed in key ways, including a more than threefold higher prevalence of childhood asthma in those with early antibiotic exposure.
In a prespecified logistic regression analysis adjusted for sex, Medicaid status, childhood asthma, race, maternal antibiotic exposure during pregnancy, delivery type, and birth weight, each course of oral antibiotics a child received up to 47 months of age was associated with a 3% increased likelihood of being overweight at 48-59 months – and children who got early oral antibiotics received a mean of 3.7 courses by age 47 months. Thus, early antibiotic exposure was associated on average with a significant 11% increased risk of later overweight (P = .005), reported Dr. Dawson-Hahn, a general pediatrics fellow at the University of Washington, Seattle.
The increased risk of overweight related to early antibiotics was concentrated in the children who received the medications in infancy. In another logistic regression analysis, children who got oral antibiotics prior to age 12 months were at an adjusted 20% increased risk of being overweight at ages 48-59 months, compared with children who didn’t receive oral antibiotics before 48 months of age. In contrast, the risk of overweight in kids who received oral antibiotics at ages 12-47 months wasn’t significantly different from the risk in the unexposed group.
Future studies should explore the mechanism of the observed relationship between early antibiotic exposure and later overweight, Dr. Dawson-Hahn said. One leading hypothesis is that the early exposure alters the composition of the developing gut microbiome. Antibiotics have been used for decades as a means of boosting the weight of livestock, she noted.
Dr. Dawson-Hahn said she is planning to reanalyze the data set to see if weight gain differed depending upon the type of antibiotic involved in an early exposure. More than 80% of children with early antibiotic exposure received amoxicillin.
One audience member noted that just because the health plan’s electronic pharmacy records show a prescription for oral antibiotics was filled doesn’t mean the child took the full course. Dr. Dawson-Hahn agreed, but noted that her study findings are consistent with the results of prior studies by other investigators in the past 2 years, which typically relied upon parental recall of antibiotic exposure.
“We thought documented prescription fill would be an improvement over that,” she added.
Dr. Dawson-Hahn’s study was funded by a Hearst Foundation Fellowship Award and a Ruth L. Kirschstein National Research Service Award. She reported having no relevant financial conflicts.
SAN DIEGO – The use of oral antibiotics before 4 years of age was associated with a significantly increased risk of later overweight in a large cohort study.
“Antibiotics may provide a physician-modifiable risk factor for obesity prevention in early childhood,” Dr. Elizabeth Dawson-Hahn said at the annual meeting of the Pediatric Academic Societies.
The study included 4,938 children born at Group Health Cooperative, the Seattle-based integrated health care system. Their electronic medical records indicated that 3,533 of them, or 72%, filled one or more prescriptions for oral antibiotics at ages 0-47 months. In this group, 53% were less than 12 months old at the time of their first oral antibiotic exposure, while the rest were 12-47 months old.
At ages 48-59 months, roughly 12% of all study participants were overweight, as defined by a body mass index at or above the 85th percentile. The prevalence was similar regardless of whether a child was exposed to early oral antibiotics. However, the groups with and without early antibiotic exposure differed in key ways, including a more than threefold higher prevalence of childhood asthma in those with early antibiotic exposure.
In a prespecified logistic regression analysis adjusted for sex, Medicaid status, childhood asthma, race, maternal antibiotic exposure during pregnancy, delivery type, and birth weight, each course of oral antibiotics a child received up to 47 months of age was associated with a 3% increased likelihood of being overweight at 48-59 months – and children who got early oral antibiotics received a mean of 3.7 courses by age 47 months. Thus, early antibiotic exposure was associated on average with a significant 11% increased risk of later overweight (P = .005), reported Dr. Dawson-Hahn, a general pediatrics fellow at the University of Washington, Seattle.
The increased risk of overweight related to early antibiotics was concentrated in the children who received the medications in infancy. In another logistic regression analysis, children who got oral antibiotics prior to age 12 months were at an adjusted 20% increased risk of being overweight at ages 48-59 months, compared with children who didn’t receive oral antibiotics before 48 months of age. In contrast, the risk of overweight in kids who received oral antibiotics at ages 12-47 months wasn’t significantly different from the risk in the unexposed group.
Future studies should explore the mechanism of the observed relationship between early antibiotic exposure and later overweight, Dr. Dawson-Hahn said. One leading hypothesis is that the early exposure alters the composition of the developing gut microbiome. Antibiotics have been used for decades as a means of boosting the weight of livestock, she noted.
Dr. Dawson-Hahn said she is planning to reanalyze the data set to see if weight gain differed depending upon the type of antibiotic involved in an early exposure. More than 80% of children with early antibiotic exposure received amoxicillin.
One audience member noted that just because the health plan’s electronic pharmacy records show a prescription for oral antibiotics was filled doesn’t mean the child took the full course. Dr. Dawson-Hahn agreed, but noted that her study findings are consistent with the results of prior studies by other investigators in the past 2 years, which typically relied upon parental recall of antibiotic exposure.
“We thought documented prescription fill would be an improvement over that,” she added.
Dr. Dawson-Hahn’s study was funded by a Hearst Foundation Fellowship Award and a Ruth L. Kirschstein National Research Service Award. She reported having no relevant financial conflicts.
SAN DIEGO – The use of oral antibiotics before 4 years of age was associated with a significantly increased risk of later overweight in a large cohort study.
“Antibiotics may provide a physician-modifiable risk factor for obesity prevention in early childhood,” Dr. Elizabeth Dawson-Hahn said at the annual meeting of the Pediatric Academic Societies.
The study included 4,938 children born at Group Health Cooperative, the Seattle-based integrated health care system. Their electronic medical records indicated that 3,533 of them, or 72%, filled one or more prescriptions for oral antibiotics at ages 0-47 months. In this group, 53% were less than 12 months old at the time of their first oral antibiotic exposure, while the rest were 12-47 months old.
At ages 48-59 months, roughly 12% of all study participants were overweight, as defined by a body mass index at or above the 85th percentile. The prevalence was similar regardless of whether a child was exposed to early oral antibiotics. However, the groups with and without early antibiotic exposure differed in key ways, including a more than threefold higher prevalence of childhood asthma in those with early antibiotic exposure.
In a prespecified logistic regression analysis adjusted for sex, Medicaid status, childhood asthma, race, maternal antibiotic exposure during pregnancy, delivery type, and birth weight, each course of oral antibiotics a child received up to 47 months of age was associated with a 3% increased likelihood of being overweight at 48-59 months – and children who got early oral antibiotics received a mean of 3.7 courses by age 47 months. Thus, early antibiotic exposure was associated on average with a significant 11% increased risk of later overweight (P = .005), reported Dr. Dawson-Hahn, a general pediatrics fellow at the University of Washington, Seattle.
The increased risk of overweight related to early antibiotics was concentrated in the children who received the medications in infancy. In another logistic regression analysis, children who got oral antibiotics prior to age 12 months were at an adjusted 20% increased risk of being overweight at ages 48-59 months, compared with children who didn’t receive oral antibiotics before 48 months of age. In contrast, the risk of overweight in kids who received oral antibiotics at ages 12-47 months wasn’t significantly different from the risk in the unexposed group.
Future studies should explore the mechanism of the observed relationship between early antibiotic exposure and later overweight, Dr. Dawson-Hahn said. One leading hypothesis is that the early exposure alters the composition of the developing gut microbiome. Antibiotics have been used for decades as a means of boosting the weight of livestock, she noted.
Dr. Dawson-Hahn said she is planning to reanalyze the data set to see if weight gain differed depending upon the type of antibiotic involved in an early exposure. More than 80% of children with early antibiotic exposure received amoxicillin.
One audience member noted that just because the health plan’s electronic pharmacy records show a prescription for oral antibiotics was filled doesn’t mean the child took the full course. Dr. Dawson-Hahn agreed, but noted that her study findings are consistent with the results of prior studies by other investigators in the past 2 years, which typically relied upon parental recall of antibiotic exposure.
“We thought documented prescription fill would be an improvement over that,” she added.
Dr. Dawson-Hahn’s study was funded by a Hearst Foundation Fellowship Award and a Ruth L. Kirschstein National Research Service Award. She reported having no relevant financial conflicts.
AT THE PAS ANNUAL MEETING
Key clinical point: Children who received oral antibiotics prior to 48 months of age were more likely to be overweight at ages 48-59 months.
Major finding: For each course of oral antibiotics a child received during 0-47 months of age, the odds of being at or above the 85th percentile for body mass index at 48-59 months increased by 3%.
Data source: A retrospective cohort study including 4,938 children whose families belonged to a large integrated health plan.
Disclosures: Dr. Dawson-Hahn’s study was funded by a Hearst Foundation Fellowship Award and a Ruth L. Kirschstein National Research Service Award. She reported having no relevant financial conflicts.
PAS: Device decreases hospitalization costs for bronchiolitis
SAN DIEGO – Implementation of guidelines for the use of the high-flow nasal cannula in general pediatric wards for infants admitted with bronchiolitis can lead to significant decreases in length of stay, need for ICU level of care, and overall hospitalization costs, a retrospective chart study showed.
In the nonrandomized, pre- and postintervention chart analysis, the investigators reviewed the data for 2,446 infants under the age of 2 years who were admitted to Hasbro Children’s Hospital with a diagnosis for bronchiolitis in the 24 months before and after March 2012, when the hospital initiated high-flow nasal cannula (HFNC) protocols in its general pediatric wards.
“Admissions for bronchiolitis are extremely common for children under the age of 1 [year], and the costs associated with this are obviously quite high, but although centers around the country are now using [HFNC] for bronchiolitis, there’s little data at this point regarding the use of it on the general wards,” said Dr. Jamie Fierce of Hasbro Children’s Hospital in Providence, R.I., adding that the 2014 American Academy of Pediatrics guidelines on bronchiolitis called for more research on the efficacy of HFNC (Pediatrics 2014;134:e1474-502).
In total, 533 infants were selected for inclusion in the study, and were divided into groups based on whether they were admitted and discharged before or after the March 2012 implementation of HFNC protocols. The primary outcome measured for the study was the length of hospital stay; the median length before implementation was 4 days, while after implementation, the median length of stay decreased to 3 days (P < .001). In addition, the number of patients who required an ICU level of care decreased from the mandated 100% – because every subject who received HFNC would have to be admitted to the ICU before the new protocols were in place – to 70% of subjects after the new protocols were put in place (P < .001).
The cost of hospitalization also decreased significantly; prior to HFNC use on general wards, the median cost per patient was $12,865, but that amount decreased to $8,952 after March 2012, a difference of almost $4,000. Furthermore, there was no increase in intubation rates, nor in 30-day readmission rates from before to after March 2012. The average number of days spent on HFNC dropped from 2.5 days to 2 days, and the mean maximum HFNC rate also decreased from 9 L/min to 7 L/min, Dr. Fierce reported at the annual meeting of the Pediatric Academic Societies.
“One important limitation to this study is that it’s difficult to assess bronchiolitis severity in each of our groups, so there could have been seasonal variations that may have affected our outcomes,” he said. “Our after-implementation group is larger than our before-implementation group, and it’s hard to tell if that’s due to a seasonal increase in bronchiolitis cases, or if there was just higher use of HFNC on patients once it was allowed in the general wards.”
Demographic information was collected from the hospital billing database on subjects’ age, race, sex, and secondhand smoke exposure and whether they had public or private insurance. Although there was a statistically significant difference in age between the groups – 3 months before March 2012, 5 months after – the other demographic data were largely consistent from before implementation to after.
Dr. Fierce did not report any relevant financial disclosures.
SAN DIEGO – Implementation of guidelines for the use of the high-flow nasal cannula in general pediatric wards for infants admitted with bronchiolitis can lead to significant decreases in length of stay, need for ICU level of care, and overall hospitalization costs, a retrospective chart study showed.
In the nonrandomized, pre- and postintervention chart analysis, the investigators reviewed the data for 2,446 infants under the age of 2 years who were admitted to Hasbro Children’s Hospital with a diagnosis for bronchiolitis in the 24 months before and after March 2012, when the hospital initiated high-flow nasal cannula (HFNC) protocols in its general pediatric wards.
“Admissions for bronchiolitis are extremely common for children under the age of 1 [year], and the costs associated with this are obviously quite high, but although centers around the country are now using [HFNC] for bronchiolitis, there’s little data at this point regarding the use of it on the general wards,” said Dr. Jamie Fierce of Hasbro Children’s Hospital in Providence, R.I., adding that the 2014 American Academy of Pediatrics guidelines on bronchiolitis called for more research on the efficacy of HFNC (Pediatrics 2014;134:e1474-502).
In total, 533 infants were selected for inclusion in the study, and were divided into groups based on whether they were admitted and discharged before or after the March 2012 implementation of HFNC protocols. The primary outcome measured for the study was the length of hospital stay; the median length before implementation was 4 days, while after implementation, the median length of stay decreased to 3 days (P < .001). In addition, the number of patients who required an ICU level of care decreased from the mandated 100% – because every subject who received HFNC would have to be admitted to the ICU before the new protocols were in place – to 70% of subjects after the new protocols were put in place (P < .001).
The cost of hospitalization also decreased significantly; prior to HFNC use on general wards, the median cost per patient was $12,865, but that amount decreased to $8,952 after March 2012, a difference of almost $4,000. Furthermore, there was no increase in intubation rates, nor in 30-day readmission rates from before to after March 2012. The average number of days spent on HFNC dropped from 2.5 days to 2 days, and the mean maximum HFNC rate also decreased from 9 L/min to 7 L/min, Dr. Fierce reported at the annual meeting of the Pediatric Academic Societies.
“One important limitation to this study is that it’s difficult to assess bronchiolitis severity in each of our groups, so there could have been seasonal variations that may have affected our outcomes,” he said. “Our after-implementation group is larger than our before-implementation group, and it’s hard to tell if that’s due to a seasonal increase in bronchiolitis cases, or if there was just higher use of HFNC on patients once it was allowed in the general wards.”
Demographic information was collected from the hospital billing database on subjects’ age, race, sex, and secondhand smoke exposure and whether they had public or private insurance. Although there was a statistically significant difference in age between the groups – 3 months before March 2012, 5 months after – the other demographic data were largely consistent from before implementation to after.
Dr. Fierce did not report any relevant financial disclosures.
SAN DIEGO – Implementation of guidelines for the use of the high-flow nasal cannula in general pediatric wards for infants admitted with bronchiolitis can lead to significant decreases in length of stay, need for ICU level of care, and overall hospitalization costs, a retrospective chart study showed.
In the nonrandomized, pre- and postintervention chart analysis, the investigators reviewed the data for 2,446 infants under the age of 2 years who were admitted to Hasbro Children’s Hospital with a diagnosis for bronchiolitis in the 24 months before and after March 2012, when the hospital initiated high-flow nasal cannula (HFNC) protocols in its general pediatric wards.
“Admissions for bronchiolitis are extremely common for children under the age of 1 [year], and the costs associated with this are obviously quite high, but although centers around the country are now using [HFNC] for bronchiolitis, there’s little data at this point regarding the use of it on the general wards,” said Dr. Jamie Fierce of Hasbro Children’s Hospital in Providence, R.I., adding that the 2014 American Academy of Pediatrics guidelines on bronchiolitis called for more research on the efficacy of HFNC (Pediatrics 2014;134:e1474-502).
In total, 533 infants were selected for inclusion in the study, and were divided into groups based on whether they were admitted and discharged before or after the March 2012 implementation of HFNC protocols. The primary outcome measured for the study was the length of hospital stay; the median length before implementation was 4 days, while after implementation, the median length of stay decreased to 3 days (P < .001). In addition, the number of patients who required an ICU level of care decreased from the mandated 100% – because every subject who received HFNC would have to be admitted to the ICU before the new protocols were in place – to 70% of subjects after the new protocols were put in place (P < .001).
The cost of hospitalization also decreased significantly; prior to HFNC use on general wards, the median cost per patient was $12,865, but that amount decreased to $8,952 after March 2012, a difference of almost $4,000. Furthermore, there was no increase in intubation rates, nor in 30-day readmission rates from before to after March 2012. The average number of days spent on HFNC dropped from 2.5 days to 2 days, and the mean maximum HFNC rate also decreased from 9 L/min to 7 L/min, Dr. Fierce reported at the annual meeting of the Pediatric Academic Societies.
“One important limitation to this study is that it’s difficult to assess bronchiolitis severity in each of our groups, so there could have been seasonal variations that may have affected our outcomes,” he said. “Our after-implementation group is larger than our before-implementation group, and it’s hard to tell if that’s due to a seasonal increase in bronchiolitis cases, or if there was just higher use of HFNC on patients once it was allowed in the general wards.”
Demographic information was collected from the hospital billing database on subjects’ age, race, sex, and secondhand smoke exposure and whether they had public or private insurance. Although there was a statistically significant difference in age between the groups – 3 months before March 2012, 5 months after – the other demographic data were largely consistent from before implementation to after.
Dr. Fierce did not report any relevant financial disclosures.
AT THE PAS ANNUAL MEETING
Key clinical point: Implementation of a high-flow nasal cannula in a general pediatric hospital ward can lead to decreases in length of stay, need for admittance to an ICU, and hospitalization costs for children with bronchiolitis.
Major finding: After HFNC implementation, median length of stay decreased from 4 days to 3 days (P < .001), the proportion of bronchiolitis patients who spent any time in the ICU decreased from 100% to 70% (P < .001), and the median hospitalization costs decreased from $12,865 to $8,952 (P < .001).
Data source: A retrospective chart study of 533 children under the age of 2 years, for 24 months before and after implementation of HFNC in general pediatric wards at Hasbro Children’s Hospital.
Disclosures: Dr. Fierce did not report any relevant financial disclosures.
PAS: Even slight preterm/low-weight births increase infection risk
SAN DIEGO – Infants born even slightly preterm or of marginally low birth weight are at significantly increased risk of being readmitted to the hospital with infection throughout childhood and adolescence, results from a large, long-term Australian study demonstrated.
“These children are a large and previously unrecognized group at increased risk of infection, who may benefit from targeted prevention,” co-lead study author David Burgner, Ph.D., a pediatric infectious diseases expert at Murdoch Children’s Research Institute in Melbourne, said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “This may be particularly important in resource-limited populations, where low-birth-weight and late-preterm birth is particularly common.”
In an effort to determine the effect of gestational age and birth weight on rehospitalization with infection, Dr. Tobias Strunk, who co-led the study with Dr. Burgner, said they retrospectively evaluated 719,311 non-Aboriginal singleton births in Western Australia from 1980-2010.
“We focused on readmission to hospital after discharge from the initial birth-related admission, so the focus was more on post neonatal infections,” said Dr. Strunk, a neonatologist with King Edward Memorial Hospital for Women and Princess Margaret Hospital for Children, which are both in Subiaco, Western Australia. “Infants were followed to the age of 18 years; that is over 6.5 million person-years of follow-up. We were therefore able to look at the risk of infection in those born very close to term and close to normal birth weight, as well as the most preterm and lowest-birth-weight infants, and to look at this risk all the way through childhood and adolescence.”
After reviewing 30 years of data the researchers observed significantly increased rates of rehospitalization with infection, even among infants born late preterm (defined as 34-36 weeks’ gestational age) and early term (defined as 37-38 weeks’ gestational age), and in those born close to normal birth weight (defined as 2.5-2.9 kg). “Although the risk was less than in the extremely preterm infants and very-low-birth-weight infants, it was still increased, right up until the age of 18 years,” said Dr. Strunk, who is also a faculty member at the University of Western Australia Centre for Research Excellence for Improving Outcomes for Preterm Infants. “In fact, for every week decrease in gestational age less than 40 weeks, there was a 10.5% increased risk in hospitalization with infection. And for every 500 g reduction in birth weight below 3 kg, there was a 19.7% increased risk.”
Rates of rehospitalization with infection were highest for the most preterm infants (defined as those less than 28 weeks’ gestational age) and those with the lowest birth weights (defined as less those weighing less than 1,000 g). Specifically, these infants faced a 13-fold increased risk of lower respiratory tract infection, an 8.5-fold increased risk of viral infection, and a 4.5-fold increased risk of invasive bacterial infection, compared with infants born at term and those born at normal birth weight.
The researchers were surprised to find that the increased risk of hospitalization with infection persisted in all groups up until age 18 years. “So overall, even in infants born near term or near normal birth weight, previously considered at low or no risk, there is an unrecognized increased risk of severe infection right through childhood and the teenage years,” Dr. Strunk said. “The risk is increased for all common types of infection, including lower respiratory tract infections, bacterial infections, and viral infections.”
The researchers acknowledged certain limitations of the study, including the fact that the researchers were unable to assess individual admissions, “so it relies on statutory data from all hospitalizations. These data are less biased by differences in health-seeking behavior and individual physician practice than emergency department visits and office visits, but the study cannot capture the total burden of infection that is managed outside hospital.” Dr. Strunk also noted that the study did not include data from Aboriginal children, “who have higher rates of infectious diseases and in whom social disadvantage contributes to poor health. The study should be repeated in other populations, where we would expect similar results. We are currently collaborating to identify datasets in resource-poor settings globally to assess the possible impact of low birth weight and prematurity on childhood infection in these populations.”
The researchers reported having no relevant financial conflicts of interest.
On Twitter @dougbrunk
SAN DIEGO – Infants born even slightly preterm or of marginally low birth weight are at significantly increased risk of being readmitted to the hospital with infection throughout childhood and adolescence, results from a large, long-term Australian study demonstrated.
“These children are a large and previously unrecognized group at increased risk of infection, who may benefit from targeted prevention,” co-lead study author David Burgner, Ph.D., a pediatric infectious diseases expert at Murdoch Children’s Research Institute in Melbourne, said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “This may be particularly important in resource-limited populations, where low-birth-weight and late-preterm birth is particularly common.”
In an effort to determine the effect of gestational age and birth weight on rehospitalization with infection, Dr. Tobias Strunk, who co-led the study with Dr. Burgner, said they retrospectively evaluated 719,311 non-Aboriginal singleton births in Western Australia from 1980-2010.
“We focused on readmission to hospital after discharge from the initial birth-related admission, so the focus was more on post neonatal infections,” said Dr. Strunk, a neonatologist with King Edward Memorial Hospital for Women and Princess Margaret Hospital for Children, which are both in Subiaco, Western Australia. “Infants were followed to the age of 18 years; that is over 6.5 million person-years of follow-up. We were therefore able to look at the risk of infection in those born very close to term and close to normal birth weight, as well as the most preterm and lowest-birth-weight infants, and to look at this risk all the way through childhood and adolescence.”
After reviewing 30 years of data the researchers observed significantly increased rates of rehospitalization with infection, even among infants born late preterm (defined as 34-36 weeks’ gestational age) and early term (defined as 37-38 weeks’ gestational age), and in those born close to normal birth weight (defined as 2.5-2.9 kg). “Although the risk was less than in the extremely preterm infants and very-low-birth-weight infants, it was still increased, right up until the age of 18 years,” said Dr. Strunk, who is also a faculty member at the University of Western Australia Centre for Research Excellence for Improving Outcomes for Preterm Infants. “In fact, for every week decrease in gestational age less than 40 weeks, there was a 10.5% increased risk in hospitalization with infection. And for every 500 g reduction in birth weight below 3 kg, there was a 19.7% increased risk.”
Rates of rehospitalization with infection were highest for the most preterm infants (defined as those less than 28 weeks’ gestational age) and those with the lowest birth weights (defined as less those weighing less than 1,000 g). Specifically, these infants faced a 13-fold increased risk of lower respiratory tract infection, an 8.5-fold increased risk of viral infection, and a 4.5-fold increased risk of invasive bacterial infection, compared with infants born at term and those born at normal birth weight.
The researchers were surprised to find that the increased risk of hospitalization with infection persisted in all groups up until age 18 years. “So overall, even in infants born near term or near normal birth weight, previously considered at low or no risk, there is an unrecognized increased risk of severe infection right through childhood and the teenage years,” Dr. Strunk said. “The risk is increased for all common types of infection, including lower respiratory tract infections, bacterial infections, and viral infections.”
The researchers acknowledged certain limitations of the study, including the fact that the researchers were unable to assess individual admissions, “so it relies on statutory data from all hospitalizations. These data are less biased by differences in health-seeking behavior and individual physician practice than emergency department visits and office visits, but the study cannot capture the total burden of infection that is managed outside hospital.” Dr. Strunk also noted that the study did not include data from Aboriginal children, “who have higher rates of infectious diseases and in whom social disadvantage contributes to poor health. The study should be repeated in other populations, where we would expect similar results. We are currently collaborating to identify datasets in resource-poor settings globally to assess the possible impact of low birth weight and prematurity on childhood infection in these populations.”
The researchers reported having no relevant financial conflicts of interest.
On Twitter @dougbrunk
SAN DIEGO – Infants born even slightly preterm or of marginally low birth weight are at significantly increased risk of being readmitted to the hospital with infection throughout childhood and adolescence, results from a large, long-term Australian study demonstrated.
“These children are a large and previously unrecognized group at increased risk of infection, who may benefit from targeted prevention,” co-lead study author David Burgner, Ph.D., a pediatric infectious diseases expert at Murdoch Children’s Research Institute in Melbourne, said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “This may be particularly important in resource-limited populations, where low-birth-weight and late-preterm birth is particularly common.”
In an effort to determine the effect of gestational age and birth weight on rehospitalization with infection, Dr. Tobias Strunk, who co-led the study with Dr. Burgner, said they retrospectively evaluated 719,311 non-Aboriginal singleton births in Western Australia from 1980-2010.
“We focused on readmission to hospital after discharge from the initial birth-related admission, so the focus was more on post neonatal infections,” said Dr. Strunk, a neonatologist with King Edward Memorial Hospital for Women and Princess Margaret Hospital for Children, which are both in Subiaco, Western Australia. “Infants were followed to the age of 18 years; that is over 6.5 million person-years of follow-up. We were therefore able to look at the risk of infection in those born very close to term and close to normal birth weight, as well as the most preterm and lowest-birth-weight infants, and to look at this risk all the way through childhood and adolescence.”
After reviewing 30 years of data the researchers observed significantly increased rates of rehospitalization with infection, even among infants born late preterm (defined as 34-36 weeks’ gestational age) and early term (defined as 37-38 weeks’ gestational age), and in those born close to normal birth weight (defined as 2.5-2.9 kg). “Although the risk was less than in the extremely preterm infants and very-low-birth-weight infants, it was still increased, right up until the age of 18 years,” said Dr. Strunk, who is also a faculty member at the University of Western Australia Centre for Research Excellence for Improving Outcomes for Preterm Infants. “In fact, for every week decrease in gestational age less than 40 weeks, there was a 10.5% increased risk in hospitalization with infection. And for every 500 g reduction in birth weight below 3 kg, there was a 19.7% increased risk.”
Rates of rehospitalization with infection were highest for the most preterm infants (defined as those less than 28 weeks’ gestational age) and those with the lowest birth weights (defined as less those weighing less than 1,000 g). Specifically, these infants faced a 13-fold increased risk of lower respiratory tract infection, an 8.5-fold increased risk of viral infection, and a 4.5-fold increased risk of invasive bacterial infection, compared with infants born at term and those born at normal birth weight.
The researchers were surprised to find that the increased risk of hospitalization with infection persisted in all groups up until age 18 years. “So overall, even in infants born near term or near normal birth weight, previously considered at low or no risk, there is an unrecognized increased risk of severe infection right through childhood and the teenage years,” Dr. Strunk said. “The risk is increased for all common types of infection, including lower respiratory tract infections, bacterial infections, and viral infections.”
The researchers acknowledged certain limitations of the study, including the fact that the researchers were unable to assess individual admissions, “so it relies on statutory data from all hospitalizations. These data are less biased by differences in health-seeking behavior and individual physician practice than emergency department visits and office visits, but the study cannot capture the total burden of infection that is managed outside hospital.” Dr. Strunk also noted that the study did not include data from Aboriginal children, “who have higher rates of infectious diseases and in whom social disadvantage contributes to poor health. The study should be repeated in other populations, where we would expect similar results. We are currently collaborating to identify datasets in resource-poor settings globally to assess the possible impact of low birth weight and prematurity on childhood infection in these populations.”
The researchers reported having no relevant financial conflicts of interest.
On Twitter @dougbrunk
AT THE PAS ANNUAL MEETING
Key clinical point: The rate of infection is significantly increased in those born preterm and/or with a low birth weight, and persists into late adolescence.
Major finding: For every week decrease in gestational age less than 40 weeks, there was a 10.5% increased risk in hospitalization with infection. For every 500 g reduction in birth weight below 3 kg, there was a 19.7% increased risk.
Data source: A retrospective evaluation of 719,311 non-Aboriginal singleton births in Western Australia from 1980-2010.
Disclosures: The researchers reported having no relevant financial conflicts.
PAS: Antibiotic risk factors for recurrent C. difficile unique in children
SAN DIEGO – Unlike in adults, inpatient administration of “high-risk” antibiotics such as cephalosporins, clindamycin, and aminopenicillins are not risk factors for recurrent Clostridium difficile infection in children, results from a multicenter study demonstrated.
“There are previous studies in which recurrent Clostridium difficile infection risk prediction models have been compiled for adult hospitalized patients,” lead study author Anthony Goudie, Ph.D., said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “Our work is the first known risk prediction model specifically looking at the pediatric population.”
Dr. Goudie of the Center for Applied Research and Evaluation in the department of pediatrics at the University of Arkansas for Medical Sciences, Little Rock, said that an adult risk prediction model (J. Hosp. Med. 2014;9:418-23) for recurrent Clostridium difficile infection (rCDI) found no association between clinical diagnoses during hospitalization for the incident CDI (iCDI). However, the model did find a strong positive association between rCDI and increasing patient age, number of recent prior hospitalizations, and the administration of gastric acid suppressors, high-risk antibiotics, or fluoroquinolones during the iCDI hospitalization.
To test the validity of adult rCDI risk factors in a pediatric population, Dr. Goudie and his associates conducted a secondary analysis of data from 43 children’s hospitals in the United States reporting to the Pediatric Health Information System between 2009 and 2013. They defined iCDI by the discharge ICD-9 code 00.845 and the administration of metronidazole or oral vancomycin during the hospital stay, and they defined rCDI as readmission with CDI within 42 days of the end of iCDI treatment. Next, the researchers statistically modeled all cases of iCDI for rCDI and tested for predictors among several factors including age, race/ethnicity, insurance status, use of antibiotics including cephalosporins, clindamycin, and aminopenicillins, and all of the predictors associated with rCDI in the adult risk prediction model.
Of 7,798 iCDI cases reported during the study period, 567 (7.3%), experienced a subsequent rCDI. No association between rCDI was seen among children who were administered histamine-2 receptor blocker (H2RB) gastric acid suppressors (P = .81), or any of all generations of cephalosporins, clindamycin, aminopenicillins, aminoglycosides, carbapenems, and fluoroquinolone antibiotics (all P greater than .10). A positive association (P less than .05) was seen among children 1-3 years, those covered by Medicaid insurance, those who had community-acquired iCDI, those who had a prior hospitalization, those administered tetracycline antibiotics, and those who were administered proton pump inhibitors.
In addition, rCDI was significantly associated with a number of iCDI comorbid diagnoses, including Hodgkin’s disease; non-Hodgkin’s lymphoma; spondylosis, intervertebral disk disorders, or other back problems; cancer of bone or connective tissue; cancer of the brain or nervous system; maintenance chemotherapy and radiotherapy; and leukemia (P less than .05 for all).
“Of all of our results, we were especially surprised to find that compared to children covered by private health insurance, those covered by Medicaid had higher odds of having rCDI,” Dr. Goudie said. “While more study is needed, we think that Medicaid in this context may be a proxy for socioeconomic status and at least a part of the solution to reducing rCDI may lie outside the control of hospital-based quality improvement interventions.”
He acknowledged certain limitations of study, including the fact the researchers “have no clinical data on children who may have been seen at other hospitals before or after the incident or rCDI [was] identified at the children’s hospital.”
The researchers reported having no relevant financial conflicts.
On Twitter @dougbrunk
SAN DIEGO – Unlike in adults, inpatient administration of “high-risk” antibiotics such as cephalosporins, clindamycin, and aminopenicillins are not risk factors for recurrent Clostridium difficile infection in children, results from a multicenter study demonstrated.
“There are previous studies in which recurrent Clostridium difficile infection risk prediction models have been compiled for adult hospitalized patients,” lead study author Anthony Goudie, Ph.D., said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “Our work is the first known risk prediction model specifically looking at the pediatric population.”
Dr. Goudie of the Center for Applied Research and Evaluation in the department of pediatrics at the University of Arkansas for Medical Sciences, Little Rock, said that an adult risk prediction model (J. Hosp. Med. 2014;9:418-23) for recurrent Clostridium difficile infection (rCDI) found no association between clinical diagnoses during hospitalization for the incident CDI (iCDI). However, the model did find a strong positive association between rCDI and increasing patient age, number of recent prior hospitalizations, and the administration of gastric acid suppressors, high-risk antibiotics, or fluoroquinolones during the iCDI hospitalization.
To test the validity of adult rCDI risk factors in a pediatric population, Dr. Goudie and his associates conducted a secondary analysis of data from 43 children’s hospitals in the United States reporting to the Pediatric Health Information System between 2009 and 2013. They defined iCDI by the discharge ICD-9 code 00.845 and the administration of metronidazole or oral vancomycin during the hospital stay, and they defined rCDI as readmission with CDI within 42 days of the end of iCDI treatment. Next, the researchers statistically modeled all cases of iCDI for rCDI and tested for predictors among several factors including age, race/ethnicity, insurance status, use of antibiotics including cephalosporins, clindamycin, and aminopenicillins, and all of the predictors associated with rCDI in the adult risk prediction model.
Of 7,798 iCDI cases reported during the study period, 567 (7.3%), experienced a subsequent rCDI. No association between rCDI was seen among children who were administered histamine-2 receptor blocker (H2RB) gastric acid suppressors (P = .81), or any of all generations of cephalosporins, clindamycin, aminopenicillins, aminoglycosides, carbapenems, and fluoroquinolone antibiotics (all P greater than .10). A positive association (P less than .05) was seen among children 1-3 years, those covered by Medicaid insurance, those who had community-acquired iCDI, those who had a prior hospitalization, those administered tetracycline antibiotics, and those who were administered proton pump inhibitors.
In addition, rCDI was significantly associated with a number of iCDI comorbid diagnoses, including Hodgkin’s disease; non-Hodgkin’s lymphoma; spondylosis, intervertebral disk disorders, or other back problems; cancer of bone or connective tissue; cancer of the brain or nervous system; maintenance chemotherapy and radiotherapy; and leukemia (P less than .05 for all).
“Of all of our results, we were especially surprised to find that compared to children covered by private health insurance, those covered by Medicaid had higher odds of having rCDI,” Dr. Goudie said. “While more study is needed, we think that Medicaid in this context may be a proxy for socioeconomic status and at least a part of the solution to reducing rCDI may lie outside the control of hospital-based quality improvement interventions.”
He acknowledged certain limitations of study, including the fact the researchers “have no clinical data on children who may have been seen at other hospitals before or after the incident or rCDI [was] identified at the children’s hospital.”
The researchers reported having no relevant financial conflicts.
On Twitter @dougbrunk
SAN DIEGO – Unlike in adults, inpatient administration of “high-risk” antibiotics such as cephalosporins, clindamycin, and aminopenicillins are not risk factors for recurrent Clostridium difficile infection in children, results from a multicenter study demonstrated.
“There are previous studies in which recurrent Clostridium difficile infection risk prediction models have been compiled for adult hospitalized patients,” lead study author Anthony Goudie, Ph.D., said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “Our work is the first known risk prediction model specifically looking at the pediatric population.”
Dr. Goudie of the Center for Applied Research and Evaluation in the department of pediatrics at the University of Arkansas for Medical Sciences, Little Rock, said that an adult risk prediction model (J. Hosp. Med. 2014;9:418-23) for recurrent Clostridium difficile infection (rCDI) found no association between clinical diagnoses during hospitalization for the incident CDI (iCDI). However, the model did find a strong positive association between rCDI and increasing patient age, number of recent prior hospitalizations, and the administration of gastric acid suppressors, high-risk antibiotics, or fluoroquinolones during the iCDI hospitalization.
To test the validity of adult rCDI risk factors in a pediatric population, Dr. Goudie and his associates conducted a secondary analysis of data from 43 children’s hospitals in the United States reporting to the Pediatric Health Information System between 2009 and 2013. They defined iCDI by the discharge ICD-9 code 00.845 and the administration of metronidazole or oral vancomycin during the hospital stay, and they defined rCDI as readmission with CDI within 42 days of the end of iCDI treatment. Next, the researchers statistically modeled all cases of iCDI for rCDI and tested for predictors among several factors including age, race/ethnicity, insurance status, use of antibiotics including cephalosporins, clindamycin, and aminopenicillins, and all of the predictors associated with rCDI in the adult risk prediction model.
Of 7,798 iCDI cases reported during the study period, 567 (7.3%), experienced a subsequent rCDI. No association between rCDI was seen among children who were administered histamine-2 receptor blocker (H2RB) gastric acid suppressors (P = .81), or any of all generations of cephalosporins, clindamycin, aminopenicillins, aminoglycosides, carbapenems, and fluoroquinolone antibiotics (all P greater than .10). A positive association (P less than .05) was seen among children 1-3 years, those covered by Medicaid insurance, those who had community-acquired iCDI, those who had a prior hospitalization, those administered tetracycline antibiotics, and those who were administered proton pump inhibitors.
In addition, rCDI was significantly associated with a number of iCDI comorbid diagnoses, including Hodgkin’s disease; non-Hodgkin’s lymphoma; spondylosis, intervertebral disk disorders, or other back problems; cancer of bone or connective tissue; cancer of the brain or nervous system; maintenance chemotherapy and radiotherapy; and leukemia (P less than .05 for all).
“Of all of our results, we were especially surprised to find that compared to children covered by private health insurance, those covered by Medicaid had higher odds of having rCDI,” Dr. Goudie said. “While more study is needed, we think that Medicaid in this context may be a proxy for socioeconomic status and at least a part of the solution to reducing rCDI may lie outside the control of hospital-based quality improvement interventions.”
He acknowledged certain limitations of study, including the fact the researchers “have no clinical data on children who may have been seen at other hospitals before or after the incident or rCDI [was] identified at the children’s hospital.”
The researchers reported having no relevant financial conflicts.
On Twitter @dougbrunk
AT THE PAS ANNUAL MEETING
Key clinical point: Some predictors of recurrent C. difficile infection (rCDI) are different in children, compared with adults.
Major finding: No association between rCDI was observed among children who were treated with histamine-2 receptor blocker (H2RB) gastric acid suppressors (P = .81), or any of all generations of cephalosporins, clindamycin, aminopenicillins, aminoglycosides, carbapenems, and fluoroquinolone antibiotics (all P greater than .10).
Data source: A secondary analysis of data from 43 children’s hospitals in the United States reporting to the Pediatric Health Information System between 2009 and 2013.
Disclosures: The researchers reported having no relevant financial conflicts.
PAS: Iron supplementation found to benefit low-birth-weight infants
SAN DIEGO – The administration of iron supplements to marginally low-birth-weight children during infancy may have a positive effect on cognitive development and behavioral problems by the time they reach the age of 7 years, results from a novel randomized study demonstrated.
“It is known that iron is essential for brain development and it is known that low-birth-weight infants are at risk of iron deficiency and they are also at risk of cognitive-behavioral problems,” Dr. Magnus Domellöf, one of the study authors, said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “However, this is the first randomized, controlled intervention trial to show that early iron supplementation of low-birth-weight infants improves brain function at school age. Our focus has been infants with marginally low-birth-weight (2,000-2,500 g), which constitutes about 5% of newborns in the United States, so it is a large group which is seldom studied.”
For the randomized controlled trial, Dr. Domellöf, professor and head of pediatrics in the department of clinical sciences at Umea University, Sweden, and his associates enrolled 285 healthy, marginally low-birth-weight (MLBW) infants to receive 0, 1, or 2 mg/kg per day of iron supplements from 6 weeks to 6 months of age. An additional 95 normal-birth-weight controls were included at 3 years of age. When the cohort reached the age 7, a pediatric psychologist assessed 70 controls and 186 MLBW children with the Wechsler Intelligence Scale of Children (WISC-IV), while parents of 74 controls and 189 MLBW children completed the validated Child Behavior Checklist (CBCL), a widely used tool to identify problem behavior in children.
The researchers found that MLBW children showed signficantly lower WISC-IV scores, compared with controls, in verbal comprehension (104.9 vs. 107.7; P = .039), but not in perceptual reasoning (P = .402), working memory (P = .560), processing speed (P = .896), or full-scale IQ (100.4 vs. 102.9; P = .236). The differences in verbal comprehension remained after controlling for maternal age and education and were not related to iron supplementation in infancy.
Dr. Domellöf went on to report that the CBCL externalizing score was significantly higher in the placebo group, compared with the two iron groups (median scores of 49 and 44, respectively; P = .015). The proportion of children with a total CBCL score above the cut-off for clinical problems was higher in MLBW children than for controls (5.6% vs 2.7%) and the proportion of children in the placebo group was higher than in the 1-mg and 2-mg supplementation groups (10% vs. 1.8% and 4.2%), but these differences did not reach statistical significance.
“Our study suggests that it is actually possible to prevent some behavioral problems at school age in low-birth-weight infants by giving them iron supplements during infancy,” Dr. Domellöf said. “All low-birth-weight infants [less than 2,500 g] should receive iron supplements during the first 6 months of life.”
Dr. Domellöf reported having no relevant financial disclosures.
On Twitter @dougbrunk
SAN DIEGO – The administration of iron supplements to marginally low-birth-weight children during infancy may have a positive effect on cognitive development and behavioral problems by the time they reach the age of 7 years, results from a novel randomized study demonstrated.
“It is known that iron is essential for brain development and it is known that low-birth-weight infants are at risk of iron deficiency and they are also at risk of cognitive-behavioral problems,” Dr. Magnus Domellöf, one of the study authors, said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “However, this is the first randomized, controlled intervention trial to show that early iron supplementation of low-birth-weight infants improves brain function at school age. Our focus has been infants with marginally low-birth-weight (2,000-2,500 g), which constitutes about 5% of newborns in the United States, so it is a large group which is seldom studied.”
For the randomized controlled trial, Dr. Domellöf, professor and head of pediatrics in the department of clinical sciences at Umea University, Sweden, and his associates enrolled 285 healthy, marginally low-birth-weight (MLBW) infants to receive 0, 1, or 2 mg/kg per day of iron supplements from 6 weeks to 6 months of age. An additional 95 normal-birth-weight controls were included at 3 years of age. When the cohort reached the age 7, a pediatric psychologist assessed 70 controls and 186 MLBW children with the Wechsler Intelligence Scale of Children (WISC-IV), while parents of 74 controls and 189 MLBW children completed the validated Child Behavior Checklist (CBCL), a widely used tool to identify problem behavior in children.
The researchers found that MLBW children showed signficantly lower WISC-IV scores, compared with controls, in verbal comprehension (104.9 vs. 107.7; P = .039), but not in perceptual reasoning (P = .402), working memory (P = .560), processing speed (P = .896), or full-scale IQ (100.4 vs. 102.9; P = .236). The differences in verbal comprehension remained after controlling for maternal age and education and were not related to iron supplementation in infancy.
Dr. Domellöf went on to report that the CBCL externalizing score was significantly higher in the placebo group, compared with the two iron groups (median scores of 49 and 44, respectively; P = .015). The proportion of children with a total CBCL score above the cut-off for clinical problems was higher in MLBW children than for controls (5.6% vs 2.7%) and the proportion of children in the placebo group was higher than in the 1-mg and 2-mg supplementation groups (10% vs. 1.8% and 4.2%), but these differences did not reach statistical significance.
“Our study suggests that it is actually possible to prevent some behavioral problems at school age in low-birth-weight infants by giving them iron supplements during infancy,” Dr. Domellöf said. “All low-birth-weight infants [less than 2,500 g] should receive iron supplements during the first 6 months of life.”
Dr. Domellöf reported having no relevant financial disclosures.
On Twitter @dougbrunk
SAN DIEGO – The administration of iron supplements to marginally low-birth-weight children during infancy may have a positive effect on cognitive development and behavioral problems by the time they reach the age of 7 years, results from a novel randomized study demonstrated.
“It is known that iron is essential for brain development and it is known that low-birth-weight infants are at risk of iron deficiency and they are also at risk of cognitive-behavioral problems,” Dr. Magnus Domellöf, one of the study authors, said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “However, this is the first randomized, controlled intervention trial to show that early iron supplementation of low-birth-weight infants improves brain function at school age. Our focus has been infants with marginally low-birth-weight (2,000-2,500 g), which constitutes about 5% of newborns in the United States, so it is a large group which is seldom studied.”
For the randomized controlled trial, Dr. Domellöf, professor and head of pediatrics in the department of clinical sciences at Umea University, Sweden, and his associates enrolled 285 healthy, marginally low-birth-weight (MLBW) infants to receive 0, 1, or 2 mg/kg per day of iron supplements from 6 weeks to 6 months of age. An additional 95 normal-birth-weight controls were included at 3 years of age. When the cohort reached the age 7, a pediatric psychologist assessed 70 controls and 186 MLBW children with the Wechsler Intelligence Scale of Children (WISC-IV), while parents of 74 controls and 189 MLBW children completed the validated Child Behavior Checklist (CBCL), a widely used tool to identify problem behavior in children.
The researchers found that MLBW children showed signficantly lower WISC-IV scores, compared with controls, in verbal comprehension (104.9 vs. 107.7; P = .039), but not in perceptual reasoning (P = .402), working memory (P = .560), processing speed (P = .896), or full-scale IQ (100.4 vs. 102.9; P = .236). The differences in verbal comprehension remained after controlling for maternal age and education and were not related to iron supplementation in infancy.
Dr. Domellöf went on to report that the CBCL externalizing score was significantly higher in the placebo group, compared with the two iron groups (median scores of 49 and 44, respectively; P = .015). The proportion of children with a total CBCL score above the cut-off for clinical problems was higher in MLBW children than for controls (5.6% vs 2.7%) and the proportion of children in the placebo group was higher than in the 1-mg and 2-mg supplementation groups (10% vs. 1.8% and 4.2%), but these differences did not reach statistical significance.
“Our study suggests that it is actually possible to prevent some behavioral problems at school age in low-birth-weight infants by giving them iron supplements during infancy,” Dr. Domellöf said. “All low-birth-weight infants [less than 2,500 g] should receive iron supplements during the first 6 months of life.”
Dr. Domellöf reported having no relevant financial disclosures.
On Twitter @dougbrunk
AT THE PAS ANNUAL MEETING
Key clinical point: MLBW children have lower verbal scores on the Wechsler Intelligence Scale of Children (WISC-IV) at age 7 years, compared with children born at term with normal birth weight.
Major finding: At age 7 years, MLBW children showed significantly lower WISC-IV scores, compared with controls in verbal comprehension (104.9 vs. 107.7; P = .039).
Data source: A randomized controlled trial of 285 healthy MLBW infants who received 0, 1, or 2 mg/kg per day of iron supplements from 6 weeks to 6 months of age.
Disclosures: Dr. Domellof reported having no relevant financial disclosures.
PAS: Fewer readmissions with narrow-spectrum antibiotics in children with appendicitis
SAN DIEGO – Narrow-spectrum antibiotics yield outcomes that are at least comparable, if not better, than broad-spectrum antibiotics when prescribed to children with appendicitis, according to a retrospective cohort study presented at the annual meeting of the Pediatric Academic Societies.
The study found that in cases of both complicated and uncomplicated appendicitis, rates of hospital readmission were consistently lower for children prescribed narrow-spectrum antibiotics versus broad-spectrum ones.
“Current guidelines recommend what we ended up defining as narrow-spectrum antibiotics, such as cefoxitin, for uncomplicated cases of appendicitis,” said Dr. Matthew P. Kronman of Seattle Children’s Hospital and the study’s lead author. “For the complicated appendicitis cases, the guidelines state that you can either use one of the narrow-spectrum antibiotics, or you can use other agents – such as piperacillin, ticarcillin, ceftazidime, cefepime, or a carbapenem, for example – that we defined as “broad spectrum” because they provide a wider range of protection and have so much activity that patients often don’t need.”
Dr. Kronman and his coinvestigators analyzed the records of 27,306 children aged 3-18 years, admitted to 1 of 23 hospitals for at least 1 day between 2011 and 2013 with appendicitis. The primary outcome was readmission within 30 days for any subsequent abdominal infection or procedure.
Uncomplicated cases accounted for 18.939 of subjects (69.9%), and complicated appendicitis was recorded in the remaining 8.367 (30.1%) of children. Subjects were given broad-spectrum antibiotics in 28.1% of uncomplicated cases and 64.7% of complicated cases.
Overall, 1.0% of patients with uncomplicated appendicitis (189) and 6.0% (498) of complicated cases were readmitted within 30 days. Of the uncomplicated cases, the readmission rate for patients who got broad-spectrum antibiotics was 1.4%, compared with 0.9% for patients who received narrow-spectrum ones; in cases of complicated appendicitis, 6.8% of subjects given broad-spectrum antibiotics were readmitted within 30 days, compared with 4.4% for patients on narrow-spectrum antibiotics.
Multivariate logistic regression was performed on the study population, with adjustments for demographic factors, as well as illness severity and length of stay. Investigators found that age, sex, and insurance type were not factors in the association between broad-spectrum antibiotic intake and readmission within 30 days. However, increased length of stay, illness severity, and placement of a peripherally inserted central catheter line, which was used exclusively for patients with complicated appendicitis, were associated with the primary outcome.
The association between broad-spectrum agents and higher readmission rates could be a result of confounding by indication, wherein patients are given broad-spectrum drugs by physicians because the patients present as very sick, Dr. Kronman said. However, that alone means that they’re at a higher risk for developing complications and being readmitted shortly after receiving treatment, he added.
“But I think it’s reasonable to conclude that those treated with narrow-spectrum antibiotics didn’t do worse, and if they didn’t do worse, there’s no reason that every kid with appendicitis shouldn’t be treated with narrow-spectrum antibiotics because we know that those treated with broader-spectrum antibiotics are more likely to develop colonization with resistant organisms, and can have other complications due to the spectrum of activity brought on by the antibiotics they were given,” Dr. Kronman said. “So if narrow-spectrum antibiotics are just as good, we should try to get every hospital to use them.”
Dr. Kronman did not report any financial disclosures. The study was supported by the Clinical Research Scholars Program at Seattle Children’s Hospital and by the National Center of Advancing Translational Sciences at the National Institutes of Health.
SAN DIEGO – Narrow-spectrum antibiotics yield outcomes that are at least comparable, if not better, than broad-spectrum antibiotics when prescribed to children with appendicitis, according to a retrospective cohort study presented at the annual meeting of the Pediatric Academic Societies.
The study found that in cases of both complicated and uncomplicated appendicitis, rates of hospital readmission were consistently lower for children prescribed narrow-spectrum antibiotics versus broad-spectrum ones.
“Current guidelines recommend what we ended up defining as narrow-spectrum antibiotics, such as cefoxitin, for uncomplicated cases of appendicitis,” said Dr. Matthew P. Kronman of Seattle Children’s Hospital and the study’s lead author. “For the complicated appendicitis cases, the guidelines state that you can either use one of the narrow-spectrum antibiotics, or you can use other agents – such as piperacillin, ticarcillin, ceftazidime, cefepime, or a carbapenem, for example – that we defined as “broad spectrum” because they provide a wider range of protection and have so much activity that patients often don’t need.”
Dr. Kronman and his coinvestigators analyzed the records of 27,306 children aged 3-18 years, admitted to 1 of 23 hospitals for at least 1 day between 2011 and 2013 with appendicitis. The primary outcome was readmission within 30 days for any subsequent abdominal infection or procedure.
Uncomplicated cases accounted for 18.939 of subjects (69.9%), and complicated appendicitis was recorded in the remaining 8.367 (30.1%) of children. Subjects were given broad-spectrum antibiotics in 28.1% of uncomplicated cases and 64.7% of complicated cases.
Overall, 1.0% of patients with uncomplicated appendicitis (189) and 6.0% (498) of complicated cases were readmitted within 30 days. Of the uncomplicated cases, the readmission rate for patients who got broad-spectrum antibiotics was 1.4%, compared with 0.9% for patients who received narrow-spectrum ones; in cases of complicated appendicitis, 6.8% of subjects given broad-spectrum antibiotics were readmitted within 30 days, compared with 4.4% for patients on narrow-spectrum antibiotics.
Multivariate logistic regression was performed on the study population, with adjustments for demographic factors, as well as illness severity and length of stay. Investigators found that age, sex, and insurance type were not factors in the association between broad-spectrum antibiotic intake and readmission within 30 days. However, increased length of stay, illness severity, and placement of a peripherally inserted central catheter line, which was used exclusively for patients with complicated appendicitis, were associated with the primary outcome.
The association between broad-spectrum agents and higher readmission rates could be a result of confounding by indication, wherein patients are given broad-spectrum drugs by physicians because the patients present as very sick, Dr. Kronman said. However, that alone means that they’re at a higher risk for developing complications and being readmitted shortly after receiving treatment, he added.
“But I think it’s reasonable to conclude that those treated with narrow-spectrum antibiotics didn’t do worse, and if they didn’t do worse, there’s no reason that every kid with appendicitis shouldn’t be treated with narrow-spectrum antibiotics because we know that those treated with broader-spectrum antibiotics are more likely to develop colonization with resistant organisms, and can have other complications due to the spectrum of activity brought on by the antibiotics they were given,” Dr. Kronman said. “So if narrow-spectrum antibiotics are just as good, we should try to get every hospital to use them.”
Dr. Kronman did not report any financial disclosures. The study was supported by the Clinical Research Scholars Program at Seattle Children’s Hospital and by the National Center of Advancing Translational Sciences at the National Institutes of Health.
SAN DIEGO – Narrow-spectrum antibiotics yield outcomes that are at least comparable, if not better, than broad-spectrum antibiotics when prescribed to children with appendicitis, according to a retrospective cohort study presented at the annual meeting of the Pediatric Academic Societies.
The study found that in cases of both complicated and uncomplicated appendicitis, rates of hospital readmission were consistently lower for children prescribed narrow-spectrum antibiotics versus broad-spectrum ones.
“Current guidelines recommend what we ended up defining as narrow-spectrum antibiotics, such as cefoxitin, for uncomplicated cases of appendicitis,” said Dr. Matthew P. Kronman of Seattle Children’s Hospital and the study’s lead author. “For the complicated appendicitis cases, the guidelines state that you can either use one of the narrow-spectrum antibiotics, or you can use other agents – such as piperacillin, ticarcillin, ceftazidime, cefepime, or a carbapenem, for example – that we defined as “broad spectrum” because they provide a wider range of protection and have so much activity that patients often don’t need.”
Dr. Kronman and his coinvestigators analyzed the records of 27,306 children aged 3-18 years, admitted to 1 of 23 hospitals for at least 1 day between 2011 and 2013 with appendicitis. The primary outcome was readmission within 30 days for any subsequent abdominal infection or procedure.
Uncomplicated cases accounted for 18.939 of subjects (69.9%), and complicated appendicitis was recorded in the remaining 8.367 (30.1%) of children. Subjects were given broad-spectrum antibiotics in 28.1% of uncomplicated cases and 64.7% of complicated cases.
Overall, 1.0% of patients with uncomplicated appendicitis (189) and 6.0% (498) of complicated cases were readmitted within 30 days. Of the uncomplicated cases, the readmission rate for patients who got broad-spectrum antibiotics was 1.4%, compared with 0.9% for patients who received narrow-spectrum ones; in cases of complicated appendicitis, 6.8% of subjects given broad-spectrum antibiotics were readmitted within 30 days, compared with 4.4% for patients on narrow-spectrum antibiotics.
Multivariate logistic regression was performed on the study population, with adjustments for demographic factors, as well as illness severity and length of stay. Investigators found that age, sex, and insurance type were not factors in the association between broad-spectrum antibiotic intake and readmission within 30 days. However, increased length of stay, illness severity, and placement of a peripherally inserted central catheter line, which was used exclusively for patients with complicated appendicitis, were associated with the primary outcome.
The association between broad-spectrum agents and higher readmission rates could be a result of confounding by indication, wherein patients are given broad-spectrum drugs by physicians because the patients present as very sick, Dr. Kronman said. However, that alone means that they’re at a higher risk for developing complications and being readmitted shortly after receiving treatment, he added.
“But I think it’s reasonable to conclude that those treated with narrow-spectrum antibiotics didn’t do worse, and if they didn’t do worse, there’s no reason that every kid with appendicitis shouldn’t be treated with narrow-spectrum antibiotics because we know that those treated with broader-spectrum antibiotics are more likely to develop colonization with resistant organisms, and can have other complications due to the spectrum of activity brought on by the antibiotics they were given,” Dr. Kronman said. “So if narrow-spectrum antibiotics are just as good, we should try to get every hospital to use them.”
Dr. Kronman did not report any financial disclosures. The study was supported by the Clinical Research Scholars Program at Seattle Children’s Hospital and by the National Center of Advancing Translational Sciences at the National Institutes of Health.
AT THE PAS ANNUAL MEETING
Key clinical point: Children with appendicitis who are treated with narrow-spectrum antibiotics experience comparable clinical outcomes to children who receive broad-spectrum antibiotics and, in some instances, achieve even better outcomes.
Major finding: The 30-day readmission rates were consistently higher in children who received broad-spectrum antibiotics, compared with narrow-spectrum antibiotics (1.4% vs. 0.9% in uncomplicated cases, 6.8% vs. 4.4% in complicated cases).
Data source: A retrospective cohort study of 27,306 patients aged 3-18 years, admitted to one of 23 hospitals from 2011 to 2013.
Disclosures: Dr. Kronman did not report any financial conflicts of interest. The study was supported in part by the Clinical Research Scholars Program at Seattle Children’s Hospital, and by the National Center of Advancing Translational Sciences of the National Institutes of Health.