User login
Society of Surgical Oncology (SSO): Annual Cancer Symposium
Point/Counterpoint: Should breast MRI be used routinely in the preoperative evaluation of breast cancer?
Yes: MRI should be considered in the preoperative setting for specific clinical indications.
MRI, like any technology, has its strengths and weaknesses, with high sensitivity but low specificity. Importantly, MRI provides excellent soft tissue contrast with anatomic 3-D detail, and is not impeded by high breast density.
Admittedly, MRI only incrementally increases cancer detection rates in either the ipsilateral or contralateral breasts of all patients, and when used in the preoperative setting does not affect short-term surgical outcomes for all patients. Therefore, MRI should not be used for routine screening or routine preoperative screening.
That said, there are specific clinical situations where preoperative MRI may provide surgeons with valuable information. These include patients who have:
• Invasive lobular carcinoma.
• Neoadjuvant chemotherapy.
• Occult primaries in extremely dense breasts.
Invasive lobular carcinomas are more likely to be multi-centric, multi-focal, and/or bilateral than other breast cancer types, and they are more difficult to diagnose because they infiltrate into tissue, making it extremely difficult to determine the extent of disease. In this setting, MRI can more accurately determine tumor size than mammography. Mammography underestimates the tumor size significantly more frequently than does MRI. In addition, among women with this cancer subtype, MRI can significantly reduce the rate of excision (Breast Cancer Res Treat. 2010;119;415-22).
We know that women at risk for systemic recurrence will not be cured with surgery alone. Neoadjuvant therapies give us the opportunity to refine local therapy options, better understand the patient’s response to therapy and prognosis, and accelerate targeted drug development to improve outcomes. To accomplish all of these goals, we need a noninvasive way to assess tumors before, during, and after neoadjuvant treatment. MRI is unsurpassed for evaluating the extent of tumors, showing in larger tumors, for example, the complexity of tumor and stroma.
MRI is also a biomarker for response to therapy and has been shown to be an independent predictor of event-free survival. In addition, MRI is more accurate than either clinical exam, mammography, or ultrasound for determining residual tumor size following neoadjuvant chemotherapy (Radiology. 2012;263:663-72).
Lastly, for patients with an occult primary (by imaging) breast cancer or primary presentation of axillary node involvement, MRI has been found to have an approximately 90% sensitivity for identifying a primary tumor, and a 95% accuracy at locating the tumor in patients who undergo surgical excision. Mammography cannot distinguish a tumor mass that is dense relative to surrounding tissue. However, MRI can distinguish a tumor which is obscured by dense breast tissue because tumors are visualized on MRI by rapid contrast uptake and washout.
MRI is a catalyst for change, but you have to use it and all technology wisely: At the time of diagnosis for select patients, for screening only those patients with very breast dense tissue and very high risk for developing breast cancer, and, perhaps most importantly, for postcancer surveillance only in women at very high risk of recurrence where standard tools such as mammography are expected to have lower performance (for example, very dense breast tissue). Overuse of MRI will increase false positives, anxiety, and cost. However, used appropriately, MRI can be used to help usher in a change in practice through the evaluation of response to neoadjuvant therapy and novel therapeutic approaches to both invasive and in situ lesions.
With improvements in technology and techniques such as diffusion-weighted imaging, the value of MRI in the preoperative setting can only continue to grow. We can also expect greater performance for presurgical staging with more refined technologies for breast imaging, localization, and biopsy, but the costs have to come down. Breast-dedicated MRI technologies may address this need.
Laura Esserman, MD, is a professor of surgery and radiology at the University of California, San Francisco, and Director of the Carol Franc Buck Breast Care Center at the UCSF Mount Zion campus.
No: MRI leads to unnecessary surgeries and does not improve short-term surgical outcomes.
The key word in this debate is “routinely.” I agree that preoperative MRI may have a role in about 5% of all cases – namely in women with occult primaries and those who undergo neoadjuvant chemotherapy. But for the vast majority of patients, the 95%, I would argue that preoperative MRI has the potential to do more harm than good.
Thirty years of experience providing breast conserving therapies without MRI has taught us several important lessons:
• Selection of patients for breast-conserving therapy is not a big problem.
• The incidences of local recurrence and contralateral breast cancers have decreased over time, antedating the use of MRI.
• Surgical excision of all microscopic subclinical disease is not necessary to achieve good long-term outcomes.
In National Surgical Adjuvant Breast and Bowel Project studies from the 1990s, in the era before the use of aromatase inhibitors or HER-2 blockade, the 10-year incidence of ipsilateral breast tumor recurrence ranged from 3.5% to 6.5% in the breast cancer population at large.
In addition, the incidence of contralateral breast cancers has been declining at a rate of approximately 3% per year, thanks to the use of adjuvant systemic therapies.
We have known since the 1960s, thanks to our colleagues in pathology, that somewhere between 30% and 60% of breast cancers that appear to be localized have microscopic subclinical disease which is treated with breast irradiation. More recently, we have recognized that we can leave behind cancer in the axilla in anywhere from 13% to 27% of patients who are not receiving direct axillary radiation, and see failure rates of 1% or less.
In the context, then, of our current understanding of breast cancer biology, what outcomes could MRI be expected to improve, since the purpose of a test is to improve patient outcomes?
We know that MRI will not improve survival, because 30 years ago randomized trials showed us that survival was equal between breast conservation and mastectomy.
MRI has no apparent effect on reducing local recurrences either, as shown in an analysis of data on 3,180 patients published in 2014 (J Clin Oncol. 2014;32:292).
Regarding contralateral breast cancer, a 2007 study (N Engl J Med. 2007;356:1295-303) showed that among 969 women, MRI found unsuspected cancer in the contralateral breast within 1 year of diagnosis in 3.1%, a finding used to support the argument that all women with breast cancer should have an MRI. But a second study using Surveillance, Epidemiology and End Results (SEER) data on 339,790 women with 2.5 million person-years of follow-up found that the 10-year rate of contralateral cancers was 2%-3% of women with estrogen-receptor–positive tumors, and in 5%-6% of those with estrogen-receptor–negative tumors, strongly suggesting that MRI leads to detection and treatment of disease that would never become clinically evident.
Finally, I would point to evidence that MRI does not improve short-term surgical outcomes, as shown in a meta-analysis my colleagues and I conducted of two randomized controlled trials and seven cohort studies, involving a total of 3,112 patients (Ann Surg. 2013;257:249-55).
We found that after adjustment for age, having an MRI was associated with a three-fold greater chance of having a mastectomy, and MRI did not significantly reduce either the need for re-excision or unexpected conversion to mastectomy. We also performed a subanalysis of infiltrating lobular cancers, and found that there was no statistically significant benefit for MRI in these patients.
The overall rate of mastectomy was 25.5% among patients who had an MRI, vs. 18.2% for those who did not.
So to summarize: MRI finds two to three times more cancer than observed rates of local recurrence, leading to unnecessary mastectomies and does not improve short-term surgical outcomes, and there is no evidence indicating that MRI decreases local recurrence.
Monica Morrow, MD, FACS, is chief of the breast service in the department of surgery and holds the Anne Burnett Windfohr Chair of Clinical Oncology at Memorial Sloan Kettering Cancer Center in New York.
Yes: MRI should be considered in the preoperative setting for specific clinical indications.
MRI, like any technology, has its strengths and weaknesses, with high sensitivity but low specificity. Importantly, MRI provides excellent soft tissue contrast with anatomic 3-D detail, and is not impeded by high breast density.
Admittedly, MRI only incrementally increases cancer detection rates in either the ipsilateral or contralateral breasts of all patients, and when used in the preoperative setting does not affect short-term surgical outcomes for all patients. Therefore, MRI should not be used for routine screening or routine preoperative screening.
That said, there are specific clinical situations where preoperative MRI may provide surgeons with valuable information. These include patients who have:
• Invasive lobular carcinoma.
• Neoadjuvant chemotherapy.
• Occult primaries in extremely dense breasts.
Invasive lobular carcinomas are more likely to be multi-centric, multi-focal, and/or bilateral than other breast cancer types, and they are more difficult to diagnose because they infiltrate into tissue, making it extremely difficult to determine the extent of disease. In this setting, MRI can more accurately determine tumor size than mammography. Mammography underestimates the tumor size significantly more frequently than does MRI. In addition, among women with this cancer subtype, MRI can significantly reduce the rate of excision (Breast Cancer Res Treat. 2010;119;415-22).
We know that women at risk for systemic recurrence will not be cured with surgery alone. Neoadjuvant therapies give us the opportunity to refine local therapy options, better understand the patient’s response to therapy and prognosis, and accelerate targeted drug development to improve outcomes. To accomplish all of these goals, we need a noninvasive way to assess tumors before, during, and after neoadjuvant treatment. MRI is unsurpassed for evaluating the extent of tumors, showing in larger tumors, for example, the complexity of tumor and stroma.
MRI is also a biomarker for response to therapy and has been shown to be an independent predictor of event-free survival. In addition, MRI is more accurate than either clinical exam, mammography, or ultrasound for determining residual tumor size following neoadjuvant chemotherapy (Radiology. 2012;263:663-72).
Lastly, for patients with an occult primary (by imaging) breast cancer or primary presentation of axillary node involvement, MRI has been found to have an approximately 90% sensitivity for identifying a primary tumor, and a 95% accuracy at locating the tumor in patients who undergo surgical excision. Mammography cannot distinguish a tumor mass that is dense relative to surrounding tissue. However, MRI can distinguish a tumor which is obscured by dense breast tissue because tumors are visualized on MRI by rapid contrast uptake and washout.
MRI is a catalyst for change, but you have to use it and all technology wisely: At the time of diagnosis for select patients, for screening only those patients with very breast dense tissue and very high risk for developing breast cancer, and, perhaps most importantly, for postcancer surveillance only in women at very high risk of recurrence where standard tools such as mammography are expected to have lower performance (for example, very dense breast tissue). Overuse of MRI will increase false positives, anxiety, and cost. However, used appropriately, MRI can be used to help usher in a change in practice through the evaluation of response to neoadjuvant therapy and novel therapeutic approaches to both invasive and in situ lesions.
With improvements in technology and techniques such as diffusion-weighted imaging, the value of MRI in the preoperative setting can only continue to grow. We can also expect greater performance for presurgical staging with more refined technologies for breast imaging, localization, and biopsy, but the costs have to come down. Breast-dedicated MRI technologies may address this need.
Laura Esserman, MD, is a professor of surgery and radiology at the University of California, San Francisco, and Director of the Carol Franc Buck Breast Care Center at the UCSF Mount Zion campus.
No: MRI leads to unnecessary surgeries and does not improve short-term surgical outcomes.
The key word in this debate is “routinely.” I agree that preoperative MRI may have a role in about 5% of all cases – namely in women with occult primaries and those who undergo neoadjuvant chemotherapy. But for the vast majority of patients, the 95%, I would argue that preoperative MRI has the potential to do more harm than good.
Thirty years of experience providing breast conserving therapies without MRI has taught us several important lessons:
• Selection of patients for breast-conserving therapy is not a big problem.
• The incidences of local recurrence and contralateral breast cancers have decreased over time, antedating the use of MRI.
• Surgical excision of all microscopic subclinical disease is not necessary to achieve good long-term outcomes.
In National Surgical Adjuvant Breast and Bowel Project studies from the 1990s, in the era before the use of aromatase inhibitors or HER-2 blockade, the 10-year incidence of ipsilateral breast tumor recurrence ranged from 3.5% to 6.5% in the breast cancer population at large.
In addition, the incidence of contralateral breast cancers has been declining at a rate of approximately 3% per year, thanks to the use of adjuvant systemic therapies.
We have known since the 1960s, thanks to our colleagues in pathology, that somewhere between 30% and 60% of breast cancers that appear to be localized have microscopic subclinical disease which is treated with breast irradiation. More recently, we have recognized that we can leave behind cancer in the axilla in anywhere from 13% to 27% of patients who are not receiving direct axillary radiation, and see failure rates of 1% or less.
In the context, then, of our current understanding of breast cancer biology, what outcomes could MRI be expected to improve, since the purpose of a test is to improve patient outcomes?
We know that MRI will not improve survival, because 30 years ago randomized trials showed us that survival was equal between breast conservation and mastectomy.
MRI has no apparent effect on reducing local recurrences either, as shown in an analysis of data on 3,180 patients published in 2014 (J Clin Oncol. 2014;32:292).
Regarding contralateral breast cancer, a 2007 study (N Engl J Med. 2007;356:1295-303) showed that among 969 women, MRI found unsuspected cancer in the contralateral breast within 1 year of diagnosis in 3.1%, a finding used to support the argument that all women with breast cancer should have an MRI. But a second study using Surveillance, Epidemiology and End Results (SEER) data on 339,790 women with 2.5 million person-years of follow-up found that the 10-year rate of contralateral cancers was 2%-3% of women with estrogen-receptor–positive tumors, and in 5%-6% of those with estrogen-receptor–negative tumors, strongly suggesting that MRI leads to detection and treatment of disease that would never become clinically evident.
Finally, I would point to evidence that MRI does not improve short-term surgical outcomes, as shown in a meta-analysis my colleagues and I conducted of two randomized controlled trials and seven cohort studies, involving a total of 3,112 patients (Ann Surg. 2013;257:249-55).
We found that after adjustment for age, having an MRI was associated with a three-fold greater chance of having a mastectomy, and MRI did not significantly reduce either the need for re-excision or unexpected conversion to mastectomy. We also performed a subanalysis of infiltrating lobular cancers, and found that there was no statistically significant benefit for MRI in these patients.
The overall rate of mastectomy was 25.5% among patients who had an MRI, vs. 18.2% for those who did not.
So to summarize: MRI finds two to three times more cancer than observed rates of local recurrence, leading to unnecessary mastectomies and does not improve short-term surgical outcomes, and there is no evidence indicating that MRI decreases local recurrence.
Monica Morrow, MD, FACS, is chief of the breast service in the department of surgery and holds the Anne Burnett Windfohr Chair of Clinical Oncology at Memorial Sloan Kettering Cancer Center in New York.
Yes: MRI should be considered in the preoperative setting for specific clinical indications.
MRI, like any technology, has its strengths and weaknesses, with high sensitivity but low specificity. Importantly, MRI provides excellent soft tissue contrast with anatomic 3-D detail, and is not impeded by high breast density.
Admittedly, MRI only incrementally increases cancer detection rates in either the ipsilateral or contralateral breasts of all patients, and when used in the preoperative setting does not affect short-term surgical outcomes for all patients. Therefore, MRI should not be used for routine screening or routine preoperative screening.
That said, there are specific clinical situations where preoperative MRI may provide surgeons with valuable information. These include patients who have:
• Invasive lobular carcinoma.
• Neoadjuvant chemotherapy.
• Occult primaries in extremely dense breasts.
Invasive lobular carcinomas are more likely to be multi-centric, multi-focal, and/or bilateral than other breast cancer types, and they are more difficult to diagnose because they infiltrate into tissue, making it extremely difficult to determine the extent of disease. In this setting, MRI can more accurately determine tumor size than mammography. Mammography underestimates the tumor size significantly more frequently than does MRI. In addition, among women with this cancer subtype, MRI can significantly reduce the rate of excision (Breast Cancer Res Treat. 2010;119;415-22).
We know that women at risk for systemic recurrence will not be cured with surgery alone. Neoadjuvant therapies give us the opportunity to refine local therapy options, better understand the patient’s response to therapy and prognosis, and accelerate targeted drug development to improve outcomes. To accomplish all of these goals, we need a noninvasive way to assess tumors before, during, and after neoadjuvant treatment. MRI is unsurpassed for evaluating the extent of tumors, showing in larger tumors, for example, the complexity of tumor and stroma.
MRI is also a biomarker for response to therapy and has been shown to be an independent predictor of event-free survival. In addition, MRI is more accurate than either clinical exam, mammography, or ultrasound for determining residual tumor size following neoadjuvant chemotherapy (Radiology. 2012;263:663-72).
Lastly, for patients with an occult primary (by imaging) breast cancer or primary presentation of axillary node involvement, MRI has been found to have an approximately 90% sensitivity for identifying a primary tumor, and a 95% accuracy at locating the tumor in patients who undergo surgical excision. Mammography cannot distinguish a tumor mass that is dense relative to surrounding tissue. However, MRI can distinguish a tumor which is obscured by dense breast tissue because tumors are visualized on MRI by rapid contrast uptake and washout.
MRI is a catalyst for change, but you have to use it and all technology wisely: At the time of diagnosis for select patients, for screening only those patients with very breast dense tissue and very high risk for developing breast cancer, and, perhaps most importantly, for postcancer surveillance only in women at very high risk of recurrence where standard tools such as mammography are expected to have lower performance (for example, very dense breast tissue). Overuse of MRI will increase false positives, anxiety, and cost. However, used appropriately, MRI can be used to help usher in a change in practice through the evaluation of response to neoadjuvant therapy and novel therapeutic approaches to both invasive and in situ lesions.
With improvements in technology and techniques such as diffusion-weighted imaging, the value of MRI in the preoperative setting can only continue to grow. We can also expect greater performance for presurgical staging with more refined technologies for breast imaging, localization, and biopsy, but the costs have to come down. Breast-dedicated MRI technologies may address this need.
Laura Esserman, MD, is a professor of surgery and radiology at the University of California, San Francisco, and Director of the Carol Franc Buck Breast Care Center at the UCSF Mount Zion campus.
No: MRI leads to unnecessary surgeries and does not improve short-term surgical outcomes.
The key word in this debate is “routinely.” I agree that preoperative MRI may have a role in about 5% of all cases – namely in women with occult primaries and those who undergo neoadjuvant chemotherapy. But for the vast majority of patients, the 95%, I would argue that preoperative MRI has the potential to do more harm than good.
Thirty years of experience providing breast conserving therapies without MRI has taught us several important lessons:
• Selection of patients for breast-conserving therapy is not a big problem.
• The incidences of local recurrence and contralateral breast cancers have decreased over time, antedating the use of MRI.
• Surgical excision of all microscopic subclinical disease is not necessary to achieve good long-term outcomes.
In National Surgical Adjuvant Breast and Bowel Project studies from the 1990s, in the era before the use of aromatase inhibitors or HER-2 blockade, the 10-year incidence of ipsilateral breast tumor recurrence ranged from 3.5% to 6.5% in the breast cancer population at large.
In addition, the incidence of contralateral breast cancers has been declining at a rate of approximately 3% per year, thanks to the use of adjuvant systemic therapies.
We have known since the 1960s, thanks to our colleagues in pathology, that somewhere between 30% and 60% of breast cancers that appear to be localized have microscopic subclinical disease which is treated with breast irradiation. More recently, we have recognized that we can leave behind cancer in the axilla in anywhere from 13% to 27% of patients who are not receiving direct axillary radiation, and see failure rates of 1% or less.
In the context, then, of our current understanding of breast cancer biology, what outcomes could MRI be expected to improve, since the purpose of a test is to improve patient outcomes?
We know that MRI will not improve survival, because 30 years ago randomized trials showed us that survival was equal between breast conservation and mastectomy.
MRI has no apparent effect on reducing local recurrences either, as shown in an analysis of data on 3,180 patients published in 2014 (J Clin Oncol. 2014;32:292).
Regarding contralateral breast cancer, a 2007 study (N Engl J Med. 2007;356:1295-303) showed that among 969 women, MRI found unsuspected cancer in the contralateral breast within 1 year of diagnosis in 3.1%, a finding used to support the argument that all women with breast cancer should have an MRI. But a second study using Surveillance, Epidemiology and End Results (SEER) data on 339,790 women with 2.5 million person-years of follow-up found that the 10-year rate of contralateral cancers was 2%-3% of women with estrogen-receptor–positive tumors, and in 5%-6% of those with estrogen-receptor–negative tumors, strongly suggesting that MRI leads to detection and treatment of disease that would never become clinically evident.
Finally, I would point to evidence that MRI does not improve short-term surgical outcomes, as shown in a meta-analysis my colleagues and I conducted of two randomized controlled trials and seven cohort studies, involving a total of 3,112 patients (Ann Surg. 2013;257:249-55).
We found that after adjustment for age, having an MRI was associated with a three-fold greater chance of having a mastectomy, and MRI did not significantly reduce either the need for re-excision or unexpected conversion to mastectomy. We also performed a subanalysis of infiltrating lobular cancers, and found that there was no statistically significant benefit for MRI in these patients.
The overall rate of mastectomy was 25.5% among patients who had an MRI, vs. 18.2% for those who did not.
So to summarize: MRI finds two to three times more cancer than observed rates of local recurrence, leading to unnecessary mastectomies and does not improve short-term surgical outcomes, and there is no evidence indicating that MRI decreases local recurrence.
Monica Morrow, MD, FACS, is chief of the breast service in the department of surgery and holds the Anne Burnett Windfohr Chair of Clinical Oncology at Memorial Sloan Kettering Cancer Center in New York.
Evidence-based practices can cut breast cancer costs
HOUSTON – There are at least three evidence-based practices for reducing the costs of locoregional therapy for early breast cancer without compromising the quality of care, according to Dr. Rachel Adams Greenup of the department of surgery at Duke University Medical Center, in Durham, North Carolina.
Management of axilla per the ACOSOG Z0011 study, adherence to joint Society of Surgical Oncology/American Society of Radiation Oncology (SSO/ASTRO) margin guidelines, and alternative radiation regimens following lumpectomy can all cut costs without compromsing quality of care, she said at the annual Society of Surgical Oncology Symposium.
The results of ACOSOG Z0011, published in 2010, were universally acknowledged to be practice changing. They showed that for women undergoing lumpectomy and radiation therapy for T1-2 invasive breast cancer and positive sentinel lymph node biopsy, completion axiallary dissection did not improve either disease-free or overall survival (DFS/OS). There were low rates of locoregional recurrence regardless of whether patients received axillary node dissection.
The potential savings from eliminating the routine practice of axillary dissection were estimated to be a 64% reduction in inpatient days, and an 18% decrease in perioperative costs.
The SSO/ASTRO margin guidelines, published in 2014, were developed by a multidisciplinary panel based on a meta-analysis of 33 studies involving more than 28,000 patients. The guidelines note that positive surgical margins are associated with a 2-fold increase in ipsilateral breast tumor recurrence, with “no ink on tumor” sufficient for a negative margin. The guidelines say that further margin width resections do not decrease same-breast recurrences.
In a related analysis of the cost implications, Dr. Greenup and colleagues noted that there are wide variations in clinical practice, and that 20% of women with close but negative margins were re-excised needlessly. Eliminating 25,000 unnecessary re-excisions annually would save $31 million dollars. These savings do not include cost reductions from an estimated 8% to 12% reduction in conversions to mastectomy that would be avoided, the authors calculated.
The costs of radiation following lumpectomy correlate directly with the number of delivered radiation fractions or treatment sessions, and also with the technique. Alternatives to standard radiation schedules include the following:
Per-patient costs for each of these options in 2011 ranged from $0 for no radiation, as in CALGB 9343, to $5342 for APBI, $9122 for HF-WBI, and $13m358 for conventionally fractionated WBI.
Dr Greenup and colleagues looked at data on 43,247 women in the National Cancer Data Base with T1-T2, NO invasive breast cancers treated with lumpectomy, and compared the actual costs of treatment with the evidence-based alternative. They found that 26% of patients were treated with the least cost-effective radiation, while nearly all of the remaining patients received more expensive radiation than necessary. If every patient were treated with the most cost-effective approach, there would be an estimated 39% reduction in costs, translating into a saving of $164 million over a single year, they reported in an abstract presented at the 2014 San Antonio Breast Cancer Symposium.
“We can’t make decisions based on cost alone, and value is certainly more important, but clinical trials, moving forward, should incorporate cost information. There is an opportunity to have small changes in clinical practice have the potential to make dramatic reductions in health care spending, and there are lots of opportunities in early stage breast cancer to practice evidence-based care while reducing health care spending,” Dr. Greenup concluded.
Dr. Greenup reported having no relevant financial disclosures.
HOUSTON – There are at least three evidence-based practices for reducing the costs of locoregional therapy for early breast cancer without compromising the quality of care, according to Dr. Rachel Adams Greenup of the department of surgery at Duke University Medical Center, in Durham, North Carolina.
Management of axilla per the ACOSOG Z0011 study, adherence to joint Society of Surgical Oncology/American Society of Radiation Oncology (SSO/ASTRO) margin guidelines, and alternative radiation regimens following lumpectomy can all cut costs without compromsing quality of care, she said at the annual Society of Surgical Oncology Symposium.
The results of ACOSOG Z0011, published in 2010, were universally acknowledged to be practice changing. They showed that for women undergoing lumpectomy and radiation therapy for T1-2 invasive breast cancer and positive sentinel lymph node biopsy, completion axiallary dissection did not improve either disease-free or overall survival (DFS/OS). There were low rates of locoregional recurrence regardless of whether patients received axillary node dissection.
The potential savings from eliminating the routine practice of axillary dissection were estimated to be a 64% reduction in inpatient days, and an 18% decrease in perioperative costs.
The SSO/ASTRO margin guidelines, published in 2014, were developed by a multidisciplinary panel based on a meta-analysis of 33 studies involving more than 28,000 patients. The guidelines note that positive surgical margins are associated with a 2-fold increase in ipsilateral breast tumor recurrence, with “no ink on tumor” sufficient for a negative margin. The guidelines say that further margin width resections do not decrease same-breast recurrences.
In a related analysis of the cost implications, Dr. Greenup and colleagues noted that there are wide variations in clinical practice, and that 20% of women with close but negative margins were re-excised needlessly. Eliminating 25,000 unnecessary re-excisions annually would save $31 million dollars. These savings do not include cost reductions from an estimated 8% to 12% reduction in conversions to mastectomy that would be avoided, the authors calculated.
The costs of radiation following lumpectomy correlate directly with the number of delivered radiation fractions or treatment sessions, and also with the technique. Alternatives to standard radiation schedules include the following:
Per-patient costs for each of these options in 2011 ranged from $0 for no radiation, as in CALGB 9343, to $5342 for APBI, $9122 for HF-WBI, and $13m358 for conventionally fractionated WBI.
Dr Greenup and colleagues looked at data on 43,247 women in the National Cancer Data Base with T1-T2, NO invasive breast cancers treated with lumpectomy, and compared the actual costs of treatment with the evidence-based alternative. They found that 26% of patients were treated with the least cost-effective radiation, while nearly all of the remaining patients received more expensive radiation than necessary. If every patient were treated with the most cost-effective approach, there would be an estimated 39% reduction in costs, translating into a saving of $164 million over a single year, they reported in an abstract presented at the 2014 San Antonio Breast Cancer Symposium.
“We can’t make decisions based on cost alone, and value is certainly more important, but clinical trials, moving forward, should incorporate cost information. There is an opportunity to have small changes in clinical practice have the potential to make dramatic reductions in health care spending, and there are lots of opportunities in early stage breast cancer to practice evidence-based care while reducing health care spending,” Dr. Greenup concluded.
Dr. Greenup reported having no relevant financial disclosures.
HOUSTON – There are at least three evidence-based practices for reducing the costs of locoregional therapy for early breast cancer without compromising the quality of care, according to Dr. Rachel Adams Greenup of the department of surgery at Duke University Medical Center, in Durham, North Carolina.
Management of axilla per the ACOSOG Z0011 study, adherence to joint Society of Surgical Oncology/American Society of Radiation Oncology (SSO/ASTRO) margin guidelines, and alternative radiation regimens following lumpectomy can all cut costs without compromsing quality of care, she said at the annual Society of Surgical Oncology Symposium.
The results of ACOSOG Z0011, published in 2010, were universally acknowledged to be practice changing. They showed that for women undergoing lumpectomy and radiation therapy for T1-2 invasive breast cancer and positive sentinel lymph node biopsy, completion axiallary dissection did not improve either disease-free or overall survival (DFS/OS). There were low rates of locoregional recurrence regardless of whether patients received axillary node dissection.
The potential savings from eliminating the routine practice of axillary dissection were estimated to be a 64% reduction in inpatient days, and an 18% decrease in perioperative costs.
The SSO/ASTRO margin guidelines, published in 2014, were developed by a multidisciplinary panel based on a meta-analysis of 33 studies involving more than 28,000 patients. The guidelines note that positive surgical margins are associated with a 2-fold increase in ipsilateral breast tumor recurrence, with “no ink on tumor” sufficient for a negative margin. The guidelines say that further margin width resections do not decrease same-breast recurrences.
In a related analysis of the cost implications, Dr. Greenup and colleagues noted that there are wide variations in clinical practice, and that 20% of women with close but negative margins were re-excised needlessly. Eliminating 25,000 unnecessary re-excisions annually would save $31 million dollars. These savings do not include cost reductions from an estimated 8% to 12% reduction in conversions to mastectomy that would be avoided, the authors calculated.
The costs of radiation following lumpectomy correlate directly with the number of delivered radiation fractions or treatment sessions, and also with the technique. Alternatives to standard radiation schedules include the following:
Per-patient costs for each of these options in 2011 ranged from $0 for no radiation, as in CALGB 9343, to $5342 for APBI, $9122 for HF-WBI, and $13m358 for conventionally fractionated WBI.
Dr Greenup and colleagues looked at data on 43,247 women in the National Cancer Data Base with T1-T2, NO invasive breast cancers treated with lumpectomy, and compared the actual costs of treatment with the evidence-based alternative. They found that 26% of patients were treated with the least cost-effective radiation, while nearly all of the remaining patients received more expensive radiation than necessary. If every patient were treated with the most cost-effective approach, there would be an estimated 39% reduction in costs, translating into a saving of $164 million over a single year, they reported in an abstract presented at the 2014 San Antonio Breast Cancer Symposium.
“We can’t make decisions based on cost alone, and value is certainly more important, but clinical trials, moving forward, should incorporate cost information. There is an opportunity to have small changes in clinical practice have the potential to make dramatic reductions in health care spending, and there are lots of opportunities in early stage breast cancer to practice evidence-based care while reducing health care spending,” Dr. Greenup concluded.
Dr. Greenup reported having no relevant financial disclosures.
EXPERT ANALYSIS AT SSO 2015
Biomarker correlates with pancreatic cancer severity
HOUSTON – A circulating biomarker has the potential to identify metastatic pancreatic cancer and may be able to predict prognosis, investigators said.
Levels of a gene encoding for the gap-junction beta 3 (GJB3) protein were highly elevated in both pancreatic cancer cell lines and in blood samples from patients with pancreatic ductal adenocarcinoma (PDAC), but the gene was undetectable in blood samples from patients without cancer, reported Dr. Raoud Marayati from the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill.
An analysis of gene expression in tumors from patients with PDAC also showed that patients with tumors expressing higher levels of GJB3 had significantly worse overall survival rates.
“GJB3 is highly expressed in blood samples from patients with metastatic versus local pancreatic cancer. GJB3 is a potential circulating biomarker for metastatic pancreatic cancer,” Dr. Marayati said at the annual Society of Surgical Oncology Cancer Symposium.
Markers lacking
PDAC is a highly aggressive cancer with a propensity for early invasion and metastasis. More than half of all patients (53%) have metastatic disease at the time of diagnosis, and 5-year survival for these patients is only 2.3%, according to the Surveillance, Epidemiology, and End Results (SEER) cancer statistics review for 2014, she noted.
The only currently available clinical biomarker for PDAC, carbohydrate antigen 19-9 (CA 19-9), generally correlates with treatment response and disease recurrence but has low sensitivity and specificity, Dr. Marayati said.
Circulating tumor cells (CTCs) – cells shed from the primary tumor into circulation – hold promise for better detection of cancer, but CTCs from pancreatic tumors have proven to be extremely difficult to isolate and count, she added.
To see whether they could identify circulating biomarkers of metastatic PDAC, Dr. Marayati and colleagues working in the laboratory of Dr. Jen Jen Yeh at the university first identified 76 genes that are differentially overexpressed in metastatic PDAC tumors, compared with localized primary tumors and with normal tissues.
The investigators looked for expression of the genes in 11 pancreatic cancer cell lines and in blood samples from 20 patients with pancreatic cancer and four without cancer. As noted, one gene, GJB3, was highly expressed in all of the cell-line samples but could not be detected in white blood cells from patients without PDAC.
To validate GJB3 as a potential cancer marker, the authors looked at expression levels in circulating tumor cells and found that, among the patients with cancer, expression levels of GBJ3 were significantly higher in those with metastatic disease, compared with localized disease (P = .016).
“That would suggest that GJB3 is a potential circulating biomarker specifically for metastatic pancreatic cancer patients,” Dr. Marayati said.
The investigators hypothesized that GJB3 may play a role in the biology of pancreatic cancer metastasis. To test this idea, they looked at resected tumors from 131 patients with primary pancreatic cancer and found that high tumor expression of GJB3 was associated with worse survival. Median overall survival among 32 patients with low levels of gene expression in their tumors was 24 months, compared with 15 months for 99 patients with high levels of GJB3 expression (P = .031).
The investigators plan to further validate the marker by testing it with larger samples from both patients with pancreatic cancer and controls.
The study funding source was not disclosed. Dr. Marayati reported having no disclosures.
HOUSTON – A circulating biomarker has the potential to identify metastatic pancreatic cancer and may be able to predict prognosis, investigators said.
Levels of a gene encoding for the gap-junction beta 3 (GJB3) protein were highly elevated in both pancreatic cancer cell lines and in blood samples from patients with pancreatic ductal adenocarcinoma (PDAC), but the gene was undetectable in blood samples from patients without cancer, reported Dr. Raoud Marayati from the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill.
An analysis of gene expression in tumors from patients with PDAC also showed that patients with tumors expressing higher levels of GJB3 had significantly worse overall survival rates.
“GJB3 is highly expressed in blood samples from patients with metastatic versus local pancreatic cancer. GJB3 is a potential circulating biomarker for metastatic pancreatic cancer,” Dr. Marayati said at the annual Society of Surgical Oncology Cancer Symposium.
Markers lacking
PDAC is a highly aggressive cancer with a propensity for early invasion and metastasis. More than half of all patients (53%) have metastatic disease at the time of diagnosis, and 5-year survival for these patients is only 2.3%, according to the Surveillance, Epidemiology, and End Results (SEER) cancer statistics review for 2014, she noted.
The only currently available clinical biomarker for PDAC, carbohydrate antigen 19-9 (CA 19-9), generally correlates with treatment response and disease recurrence but has low sensitivity and specificity, Dr. Marayati said.
Circulating tumor cells (CTCs) – cells shed from the primary tumor into circulation – hold promise for better detection of cancer, but CTCs from pancreatic tumors have proven to be extremely difficult to isolate and count, she added.
To see whether they could identify circulating biomarkers of metastatic PDAC, Dr. Marayati and colleagues working in the laboratory of Dr. Jen Jen Yeh at the university first identified 76 genes that are differentially overexpressed in metastatic PDAC tumors, compared with localized primary tumors and with normal tissues.
The investigators looked for expression of the genes in 11 pancreatic cancer cell lines and in blood samples from 20 patients with pancreatic cancer and four without cancer. As noted, one gene, GJB3, was highly expressed in all of the cell-line samples but could not be detected in white blood cells from patients without PDAC.
To validate GJB3 as a potential cancer marker, the authors looked at expression levels in circulating tumor cells and found that, among the patients with cancer, expression levels of GBJ3 were significantly higher in those with metastatic disease, compared with localized disease (P = .016).
“That would suggest that GJB3 is a potential circulating biomarker specifically for metastatic pancreatic cancer patients,” Dr. Marayati said.
The investigators hypothesized that GJB3 may play a role in the biology of pancreatic cancer metastasis. To test this idea, they looked at resected tumors from 131 patients with primary pancreatic cancer and found that high tumor expression of GJB3 was associated with worse survival. Median overall survival among 32 patients with low levels of gene expression in their tumors was 24 months, compared with 15 months for 99 patients with high levels of GJB3 expression (P = .031).
The investigators plan to further validate the marker by testing it with larger samples from both patients with pancreatic cancer and controls.
The study funding source was not disclosed. Dr. Marayati reported having no disclosures.
HOUSTON – A circulating biomarker has the potential to identify metastatic pancreatic cancer and may be able to predict prognosis, investigators said.
Levels of a gene encoding for the gap-junction beta 3 (GJB3) protein were highly elevated in both pancreatic cancer cell lines and in blood samples from patients with pancreatic ductal adenocarcinoma (PDAC), but the gene was undetectable in blood samples from patients without cancer, reported Dr. Raoud Marayati from the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill.
An analysis of gene expression in tumors from patients with PDAC also showed that patients with tumors expressing higher levels of GJB3 had significantly worse overall survival rates.
“GJB3 is highly expressed in blood samples from patients with metastatic versus local pancreatic cancer. GJB3 is a potential circulating biomarker for metastatic pancreatic cancer,” Dr. Marayati said at the annual Society of Surgical Oncology Cancer Symposium.
Markers lacking
PDAC is a highly aggressive cancer with a propensity for early invasion and metastasis. More than half of all patients (53%) have metastatic disease at the time of diagnosis, and 5-year survival for these patients is only 2.3%, according to the Surveillance, Epidemiology, and End Results (SEER) cancer statistics review for 2014, she noted.
The only currently available clinical biomarker for PDAC, carbohydrate antigen 19-9 (CA 19-9), generally correlates with treatment response and disease recurrence but has low sensitivity and specificity, Dr. Marayati said.
Circulating tumor cells (CTCs) – cells shed from the primary tumor into circulation – hold promise for better detection of cancer, but CTCs from pancreatic tumors have proven to be extremely difficult to isolate and count, she added.
To see whether they could identify circulating biomarkers of metastatic PDAC, Dr. Marayati and colleagues working in the laboratory of Dr. Jen Jen Yeh at the university first identified 76 genes that are differentially overexpressed in metastatic PDAC tumors, compared with localized primary tumors and with normal tissues.
The investigators looked for expression of the genes in 11 pancreatic cancer cell lines and in blood samples from 20 patients with pancreatic cancer and four without cancer. As noted, one gene, GJB3, was highly expressed in all of the cell-line samples but could not be detected in white blood cells from patients without PDAC.
To validate GJB3 as a potential cancer marker, the authors looked at expression levels in circulating tumor cells and found that, among the patients with cancer, expression levels of GBJ3 were significantly higher in those with metastatic disease, compared with localized disease (P = .016).
“That would suggest that GJB3 is a potential circulating biomarker specifically for metastatic pancreatic cancer patients,” Dr. Marayati said.
The investigators hypothesized that GJB3 may play a role in the biology of pancreatic cancer metastasis. To test this idea, they looked at resected tumors from 131 patients with primary pancreatic cancer and found that high tumor expression of GJB3 was associated with worse survival. Median overall survival among 32 patients with low levels of gene expression in their tumors was 24 months, compared with 15 months for 99 patients with high levels of GJB3 expression (P = .031).
The investigators plan to further validate the marker by testing it with larger samples from both patients with pancreatic cancer and controls.
The study funding source was not disclosed. Dr. Marayati reported having no disclosures.
AT SSO 2015
Key clinical point: GJB3 may be a serum marker for metastatic pancreatic ductal adenocarcinoma.
Major finding: GJB3 was expressed at significantly higher levels in patients with metastatic vs. localized PDAC.
Data source: Gene expression analyses involving patient tumor samples and 11 pancreatic cancer cell lines.
Disclosures: The study funding source was not disclosed. Dr. Marayati reported having no disclosures.
SSIs a factor in postop colon cancer survival
HOUSTON – Surgical-site infections occurring in patients who underwent curative resection for localized colon cancer were associated with worse overall survival in a large retrospective study.
Among nearly 10,000 patients with nonmetastatic colon cancer who underwent surgery with curative intent, surgical-site infections (SSIs) were associated with both worse overall survival and a reduced likelihood of receiving adjuvant chemotherapy, reported Dr. Gala Markia Barden, a surgical resident at Baylor College of Medicine, Houston.
Both SSIs and failure to receive adjuvant chemotherapy are independently associated with worse overall survival, she said at the annual Society of Surgical Oncology Cancer Symposium.
“Future studies and practice guidelines should focus on target areas for improving these potentially preventable problems, including active surveillance for and early recognition of surgical-site infections, as well as vigilant follow-up to ensure treatment completion and to improve the transition between the surgical and medical oncology teams to mitigate losses to follow-up,” she said.
Tapping into the merged Veterans Affairs Surgical Quality Improvement Program and VA Central Cancer Registry (VASQIP-VA) databases, the authors identified 9,946 patients aged 18 years and older who underwent radical resection for colon cancer from 1999 through 2009. Patients with rectal cancers or early postoperative deaths (within 90 days of surgery) were excluded.
The investigators examined the relationships between SSIs and both 5-year overall survival and receipt of adjuvant chemotherapy, which has been documented to improve survival in patients with stage III colon cancer. Delivery of adjuvant chemotherapy in these patients is considered to be a measure of the quality of cancer care, Dr. Barden noted.
Of the 9,946 patients included in the study, 1,340 (13.5%) developed SSIs. These patients were slightly but significantly younger (P < .001), had worse functional status (P = .002), and had higher American Society of Anesthesiologists (ASA) physical status scores (P < .001).
In univariate analysis, the investigators found that, in the entire cohort, SSIs were associated with worse overall survival (OS); in multivariate analysis controlling for sex, nutrition, functional status, ASA score, and number of lymph nodes resected, they saw that SSI was associated with a hazard ratio (HR) for worse overall survival of 1.35 (P < .0001).
When they looked at the association of SSI and OS stratified by cancer stage, however, they found that it was significant only for stage III disease. Patients with stage III who developed an SSI had a median OS of 29 months, compared with 33 months for those with no site infections (P< .001).
Dr. Barden and her associates also found that 42% of patients with infections did not receive adjuvant chemotherapy, compared with 34% of patients without SSIs (P = .002).
To see whether the worse survival among patients with SSI was primarily driven by the failure to deliver chemotherapy, they created a model adjusted for cancer risk factors, which showed that patients with stage III disease who developed an SSI and did not undergo adjuvant chemotherapy had an HR of worse overall survival of 1.59 (P < .0001).
They then added into the model those patients with SSIs who did receive adjuvant therapy but, contrary to their expectations, saw that the HR was only slightly reduced (1.56) and remained significant (P < .0001). The model also confirmed that failure to deliver chemotherapy was associated with worse survival (HR 1.52, P <.0001)
Dr. Barden acknowledged that the study was limited by the retrospective design, predominantly male VA cohort, and the lack of information in the databases about why patients did not receive adjuvant therapy.
The study was internally supported. Dr. Barden reported having no conflicts of interest.
HOUSTON – Surgical-site infections occurring in patients who underwent curative resection for localized colon cancer were associated with worse overall survival in a large retrospective study.
Among nearly 10,000 patients with nonmetastatic colon cancer who underwent surgery with curative intent, surgical-site infections (SSIs) were associated with both worse overall survival and a reduced likelihood of receiving adjuvant chemotherapy, reported Dr. Gala Markia Barden, a surgical resident at Baylor College of Medicine, Houston.
Both SSIs and failure to receive adjuvant chemotherapy are independently associated with worse overall survival, she said at the annual Society of Surgical Oncology Cancer Symposium.
“Future studies and practice guidelines should focus on target areas for improving these potentially preventable problems, including active surveillance for and early recognition of surgical-site infections, as well as vigilant follow-up to ensure treatment completion and to improve the transition between the surgical and medical oncology teams to mitigate losses to follow-up,” she said.
Tapping into the merged Veterans Affairs Surgical Quality Improvement Program and VA Central Cancer Registry (VASQIP-VA) databases, the authors identified 9,946 patients aged 18 years and older who underwent radical resection for colon cancer from 1999 through 2009. Patients with rectal cancers or early postoperative deaths (within 90 days of surgery) were excluded.
The investigators examined the relationships between SSIs and both 5-year overall survival and receipt of adjuvant chemotherapy, which has been documented to improve survival in patients with stage III colon cancer. Delivery of adjuvant chemotherapy in these patients is considered to be a measure of the quality of cancer care, Dr. Barden noted.
Of the 9,946 patients included in the study, 1,340 (13.5%) developed SSIs. These patients were slightly but significantly younger (P < .001), had worse functional status (P = .002), and had higher American Society of Anesthesiologists (ASA) physical status scores (P < .001).
In univariate analysis, the investigators found that, in the entire cohort, SSIs were associated with worse overall survival (OS); in multivariate analysis controlling for sex, nutrition, functional status, ASA score, and number of lymph nodes resected, they saw that SSI was associated with a hazard ratio (HR) for worse overall survival of 1.35 (P < .0001).
When they looked at the association of SSI and OS stratified by cancer stage, however, they found that it was significant only for stage III disease. Patients with stage III who developed an SSI had a median OS of 29 months, compared with 33 months for those with no site infections (P< .001).
Dr. Barden and her associates also found that 42% of patients with infections did not receive adjuvant chemotherapy, compared with 34% of patients without SSIs (P = .002).
To see whether the worse survival among patients with SSI was primarily driven by the failure to deliver chemotherapy, they created a model adjusted for cancer risk factors, which showed that patients with stage III disease who developed an SSI and did not undergo adjuvant chemotherapy had an HR of worse overall survival of 1.59 (P < .0001).
They then added into the model those patients with SSIs who did receive adjuvant therapy but, contrary to their expectations, saw that the HR was only slightly reduced (1.56) and remained significant (P < .0001). The model also confirmed that failure to deliver chemotherapy was associated with worse survival (HR 1.52, P <.0001)
Dr. Barden acknowledged that the study was limited by the retrospective design, predominantly male VA cohort, and the lack of information in the databases about why patients did not receive adjuvant therapy.
The study was internally supported. Dr. Barden reported having no conflicts of interest.
HOUSTON – Surgical-site infections occurring in patients who underwent curative resection for localized colon cancer were associated with worse overall survival in a large retrospective study.
Among nearly 10,000 patients with nonmetastatic colon cancer who underwent surgery with curative intent, surgical-site infections (SSIs) were associated with both worse overall survival and a reduced likelihood of receiving adjuvant chemotherapy, reported Dr. Gala Markia Barden, a surgical resident at Baylor College of Medicine, Houston.
Both SSIs and failure to receive adjuvant chemotherapy are independently associated with worse overall survival, she said at the annual Society of Surgical Oncology Cancer Symposium.
“Future studies and practice guidelines should focus on target areas for improving these potentially preventable problems, including active surveillance for and early recognition of surgical-site infections, as well as vigilant follow-up to ensure treatment completion and to improve the transition between the surgical and medical oncology teams to mitigate losses to follow-up,” she said.
Tapping into the merged Veterans Affairs Surgical Quality Improvement Program and VA Central Cancer Registry (VASQIP-VA) databases, the authors identified 9,946 patients aged 18 years and older who underwent radical resection for colon cancer from 1999 through 2009. Patients with rectal cancers or early postoperative deaths (within 90 days of surgery) were excluded.
The investigators examined the relationships between SSIs and both 5-year overall survival and receipt of adjuvant chemotherapy, which has been documented to improve survival in patients with stage III colon cancer. Delivery of adjuvant chemotherapy in these patients is considered to be a measure of the quality of cancer care, Dr. Barden noted.
Of the 9,946 patients included in the study, 1,340 (13.5%) developed SSIs. These patients were slightly but significantly younger (P < .001), had worse functional status (P = .002), and had higher American Society of Anesthesiologists (ASA) physical status scores (P < .001).
In univariate analysis, the investigators found that, in the entire cohort, SSIs were associated with worse overall survival (OS); in multivariate analysis controlling for sex, nutrition, functional status, ASA score, and number of lymph nodes resected, they saw that SSI was associated with a hazard ratio (HR) for worse overall survival of 1.35 (P < .0001).
When they looked at the association of SSI and OS stratified by cancer stage, however, they found that it was significant only for stage III disease. Patients with stage III who developed an SSI had a median OS of 29 months, compared with 33 months for those with no site infections (P< .001).
Dr. Barden and her associates also found that 42% of patients with infections did not receive adjuvant chemotherapy, compared with 34% of patients without SSIs (P = .002).
To see whether the worse survival among patients with SSI was primarily driven by the failure to deliver chemotherapy, they created a model adjusted for cancer risk factors, which showed that patients with stage III disease who developed an SSI and did not undergo adjuvant chemotherapy had an HR of worse overall survival of 1.59 (P < .0001).
They then added into the model those patients with SSIs who did receive adjuvant therapy but, contrary to their expectations, saw that the HR was only slightly reduced (1.56) and remained significant (P < .0001). The model also confirmed that failure to deliver chemotherapy was associated with worse survival (HR 1.52, P <.0001)
Dr. Barden acknowledged that the study was limited by the retrospective design, predominantly male VA cohort, and the lack of information in the databases about why patients did not receive adjuvant therapy.
The study was internally supported. Dr. Barden reported having no conflicts of interest.
AT SSO 2015
Key clinical point: Surgical site infections in patients with colon cancer are associated with both worse overall survival and lower chance of receiving adjuvant chemotherapy.
Major finding: Median overall survival for stage III patients with SSIs was 29 months, vs. 33 for no SSIs.
Data source: Retrospective cohort study of 9,946 patients who underwent radical colon cancer resection with curative intent.
Disclosures: The study was internally supported. Dr. Barden reported having no conflicts of interest.
Rectal preservation feasible after cancer clinical remission
HOUSTON – Patients who achieve complete clinical responses after neoadjuvant therapy for locally advanced rectal cancer may, in many cases, be safely spared the trauma and morbidity of total mesorectal excision.
That’s the opinion of investigators at Memorial Sloan Kettering Cancer Center, New York, who found that nearly two-thirds of patients followed with nonoperative management (NOM) had durable complete clinical remissions (cCR) for at least 4 years.
Disease-specific survival and overall survival rates among patients who had nonoperative management were similar to those seen in patients with pathologic complete responses (pCR), defined as no viable tumor cells in the resected specimen.
“We know that there is a very good overall and disease-free survival associated with a [pCR]. Clinical complete response is also associated with pathologic complete response. In that regard, this brings up the question: Is an operation always necessary in these patients?” Dr. Jesse Joshua Smith of the cancer center said at the annual Society of Surgical Oncology Cancer Symposium.
Dr. Smith and colleagues conducted a review of their center’s experience to date with NOM for patients with locally advanced rectal cancer, asking whether the data support the approach as oncologically safe and effective for organ preservation.
They identified 442 patients with rectal cancer treated with neoadjuvant chemotherapy from 2006 through 2014 and compared results for 73 who achieved a cCR and were followed with nonoperative management with those of 72 patients who had a pCR following total mesorectal excision.
Demographic and clinical characteristics between the groups were generally similar, although patients in the pCR group were significantly younger (58 years vs. 65 years, P = .01), had a greater tumor distance from the anal verge (median of 6 cm vs. 5.25 cm, P = .02), and higher proportions of clinical stage II (32% vs. 24%) and III (66% vs. 62%, P = .02).
Among the 73 patients managed with NOM, 54 had durable cCR at 4 years. Of the remaining 19 with local tumor regrowth, 2 had local excisions with no further recurrence and 17 went on to have rectal resections. The total number of patients with rectal preservation in this group was 56 (77%).
Of the 19 patients in the conservatively managed group who had local regrowths, all but three of the recurrences were detected within 13 months.
As noted before, neither disease-specific survival nor overall survival were significantly different between patients managed with NOM or with total mesorectal excision.
There were numerically more distant recurrences at both 1 and 4 years among patients treated with NOM compared with total mesorectal excision (7% vs. 2%, and 17% vs. 9%, respectively), but the differences were not statistically significant, the authors found.
Dr. Smith noted that patients who are offered the option of NOM have a discussion with the surgeon emphasizing that the practice is nonstandard management, carries about a 25% risk of local regrowth, and requires increased endoscopic and radiographic surveillance. Patients also are informed about the risks of salvage abdominoperineal resection or extended resections, and about the potential risk of compromising cure.
The study was supported in part by the Berezuk Colorectal Cancer Fund. Dr. Smith reported having no disclosures.
HOUSTON – Patients who achieve complete clinical responses after neoadjuvant therapy for locally advanced rectal cancer may, in many cases, be safely spared the trauma and morbidity of total mesorectal excision.
That’s the opinion of investigators at Memorial Sloan Kettering Cancer Center, New York, who found that nearly two-thirds of patients followed with nonoperative management (NOM) had durable complete clinical remissions (cCR) for at least 4 years.
Disease-specific survival and overall survival rates among patients who had nonoperative management were similar to those seen in patients with pathologic complete responses (pCR), defined as no viable tumor cells in the resected specimen.
“We know that there is a very good overall and disease-free survival associated with a [pCR]. Clinical complete response is also associated with pathologic complete response. In that regard, this brings up the question: Is an operation always necessary in these patients?” Dr. Jesse Joshua Smith of the cancer center said at the annual Society of Surgical Oncology Cancer Symposium.
Dr. Smith and colleagues conducted a review of their center’s experience to date with NOM for patients with locally advanced rectal cancer, asking whether the data support the approach as oncologically safe and effective for organ preservation.
They identified 442 patients with rectal cancer treated with neoadjuvant chemotherapy from 2006 through 2014 and compared results for 73 who achieved a cCR and were followed with nonoperative management with those of 72 patients who had a pCR following total mesorectal excision.
Demographic and clinical characteristics between the groups were generally similar, although patients in the pCR group were significantly younger (58 years vs. 65 years, P = .01), had a greater tumor distance from the anal verge (median of 6 cm vs. 5.25 cm, P = .02), and higher proportions of clinical stage II (32% vs. 24%) and III (66% vs. 62%, P = .02).
Among the 73 patients managed with NOM, 54 had durable cCR at 4 years. Of the remaining 19 with local tumor regrowth, 2 had local excisions with no further recurrence and 17 went on to have rectal resections. The total number of patients with rectal preservation in this group was 56 (77%).
Of the 19 patients in the conservatively managed group who had local regrowths, all but three of the recurrences were detected within 13 months.
As noted before, neither disease-specific survival nor overall survival were significantly different between patients managed with NOM or with total mesorectal excision.
There were numerically more distant recurrences at both 1 and 4 years among patients treated with NOM compared with total mesorectal excision (7% vs. 2%, and 17% vs. 9%, respectively), but the differences were not statistically significant, the authors found.
Dr. Smith noted that patients who are offered the option of NOM have a discussion with the surgeon emphasizing that the practice is nonstandard management, carries about a 25% risk of local regrowth, and requires increased endoscopic and radiographic surveillance. Patients also are informed about the risks of salvage abdominoperineal resection or extended resections, and about the potential risk of compromising cure.
The study was supported in part by the Berezuk Colorectal Cancer Fund. Dr. Smith reported having no disclosures.
HOUSTON – Patients who achieve complete clinical responses after neoadjuvant therapy for locally advanced rectal cancer may, in many cases, be safely spared the trauma and morbidity of total mesorectal excision.
That’s the opinion of investigators at Memorial Sloan Kettering Cancer Center, New York, who found that nearly two-thirds of patients followed with nonoperative management (NOM) had durable complete clinical remissions (cCR) for at least 4 years.
Disease-specific survival and overall survival rates among patients who had nonoperative management were similar to those seen in patients with pathologic complete responses (pCR), defined as no viable tumor cells in the resected specimen.
“We know that there is a very good overall and disease-free survival associated with a [pCR]. Clinical complete response is also associated with pathologic complete response. In that regard, this brings up the question: Is an operation always necessary in these patients?” Dr. Jesse Joshua Smith of the cancer center said at the annual Society of Surgical Oncology Cancer Symposium.
Dr. Smith and colleagues conducted a review of their center’s experience to date with NOM for patients with locally advanced rectal cancer, asking whether the data support the approach as oncologically safe and effective for organ preservation.
They identified 442 patients with rectal cancer treated with neoadjuvant chemotherapy from 2006 through 2014 and compared results for 73 who achieved a cCR and were followed with nonoperative management with those of 72 patients who had a pCR following total mesorectal excision.
Demographic and clinical characteristics between the groups were generally similar, although patients in the pCR group were significantly younger (58 years vs. 65 years, P = .01), had a greater tumor distance from the anal verge (median of 6 cm vs. 5.25 cm, P = .02), and higher proportions of clinical stage II (32% vs. 24%) and III (66% vs. 62%, P = .02).
Among the 73 patients managed with NOM, 54 had durable cCR at 4 years. Of the remaining 19 with local tumor regrowth, 2 had local excisions with no further recurrence and 17 went on to have rectal resections. The total number of patients with rectal preservation in this group was 56 (77%).
Of the 19 patients in the conservatively managed group who had local regrowths, all but three of the recurrences were detected within 13 months.
As noted before, neither disease-specific survival nor overall survival were significantly different between patients managed with NOM or with total mesorectal excision.
There were numerically more distant recurrences at both 1 and 4 years among patients treated with NOM compared with total mesorectal excision (7% vs. 2%, and 17% vs. 9%, respectively), but the differences were not statistically significant, the authors found.
Dr. Smith noted that patients who are offered the option of NOM have a discussion with the surgeon emphasizing that the practice is nonstandard management, carries about a 25% risk of local regrowth, and requires increased endoscopic and radiographic surveillance. Patients also are informed about the risks of salvage abdominoperineal resection or extended resections, and about the potential risk of compromising cure.
The study was supported in part by the Berezuk Colorectal Cancer Fund. Dr. Smith reported having no disclosures.
Key clinical point: Patients with complete clinical response after neoadjuvant chemotherapy for rectal cancer may be able to be safely followed with nonoperative management.
Major finding: There were no significant differences at 4 years in either disease-specific or overall survival among patients with rectal cancer managed conservatively or with total mesorectal excision.
Data source: Review of prospectively collected data on 145 patients with locally advanced rectal cancer.
Disclosures: The study was supported in part by the Berezuk Colorectal Cancer Fund. Dr. Smith reported having no disclosures.
Neoadjuvant therapy facilitates breast conservation
HOUSTON – For some women with breast cancer, neoadjuvant therapy can increase the likelihood of breast-conserving treatment and may limit the extent of axillary dissection, a breast cancer researcher says.
“Neoadjuvant chemotherapy has long been used in the management of inflammatory breast cancer, in patients with locally advanced, or inoperable disease, and it’s increasingly being used in patients who have operable breast cancer,” said Dr. Elizabeth A. Mittendorf of the University of Texas MD Anderson Cancer Center, Houston.
A meta-analysis published in 2007 suggested that neoadjuvant therapy in patients with operable breast cancer reduced the mastectomy rate by 17%, a figure that Dr. Mittendorf said likely underestimates the benefit, because many of the trials included in the analysis did not require patients to be considered for breast conservation at presentation.
The meta-analysis also showed that local recurrence rates did not differ from those seen with mastectomy when patients treated with neoadjuvant therapy were downstaged to breast-conserving therapy, and that there were no differences in local recurrence rates for neoadjuvant vs. adjuvant chemotherapy stratified by type of surgery, Dr. Mittendorf said at the annual Society of Surgical Oncology Cancer Symposium.
Key clinical trials, including the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials, showed that neoadjuvant chemotherapy did not have an effect on either disease-free or overall survival compared with adjuvant chemotherapy, Dr. Mittendorf noted.
Response to neoadjuvant chemotherapy is also a good predictor of prognosis, she said, pointing to a pooled analysis of 12 studies published in 2014 in The Lancet. The authors of the analysis reported that patients with a pathologic complete response (pCR; no invasive disease in either the breast or axilla) after neoadjuvant chemotherapy had significantly improved survival, with the greatest prognostic values seen in patients with aggressive tumor subtypes.
Factors to consider when selecting neoadjuvant chemotherapy include:
• Tumor size.
• Lymph node status.
• Estrogen, progesterone, and/or HER2 status.
• Treatment sensitivity (as measured by Ki-67 or other markers).
• Pathologic complete response rates.
Chemo for HR-positive?
“With respect to hormone receptor–positive breast cancer, I think the most important question for these patients is do they even need chemotherapy?” Dr. Mittendorf said.
Hormone receptor–positive (HR-positive) breast cancers have been shown to be less responsive to neoadjuvant chemotherapy, and pCR is less prognostic of outcome in this tumor subtype. Older patients with HR-positive cancers who are borderline candidates for breast-conserving therapy might benefit from neoadjuvant therapy with an aromatase inhibitor, she noted.
HER2-positive disease
For patients with HER2-positive breast cancers, it may be possible to tailor neoadjuvant therapy, so that patients who achieve a pCR with neoadjuvant trastuzumab (Herceptin) might be spared an additional 6 months of adjuvant therapy. Dr. Mittendorf’s group published a recent study
Combination anti-HER2 therapies (trastuzumab and pertuzumab [Perjeta] as used in the NeoSphere Trial may help to improve pCR rates and outcomes in patients with HER2-positive tumors, Dr. Mittendorf said.
Triple negative disease
Among patients with triple-negative breast cancer (tumors lacking hormonal receptors and HER2), those who have residual cancer after neoadjuvant chemotherapy have a poor prognosis. At MD Anderson, patients with localized triple-negative breast cancer who are scheduled to receive neoadjuvant chemotherapy first have a biopsy with molecular profiling, and are immediately started on an anthracycline-based regimen.
Patients who have a response to the chemotherapy proceed to receive a taxane, while nonresponders will be triaged onto phase II studies based on the subtype of triple-negative breast cancer. Patients who are positive for BRCA mutations will be started on a carboplatin/paclitaxel regimen, while those with mesenchymal tumor subtypes will be started on a phosphoinositide 3-kinase (PI3K) inhibitor, and those with basal-like tumors will be started on an immunotherapy protocol.Better understanding of the biology of different tumor subtypes may also help to reduce the extent of axillary surgery, by helping clinicians to identify those patients who are likely to have a nodal pCR, Dr. Mittendorf said.
HOUSTON – For some women with breast cancer, neoadjuvant therapy can increase the likelihood of breast-conserving treatment and may limit the extent of axillary dissection, a breast cancer researcher says.
“Neoadjuvant chemotherapy has long been used in the management of inflammatory breast cancer, in patients with locally advanced, or inoperable disease, and it’s increasingly being used in patients who have operable breast cancer,” said Dr. Elizabeth A. Mittendorf of the University of Texas MD Anderson Cancer Center, Houston.
A meta-analysis published in 2007 suggested that neoadjuvant therapy in patients with operable breast cancer reduced the mastectomy rate by 17%, a figure that Dr. Mittendorf said likely underestimates the benefit, because many of the trials included in the analysis did not require patients to be considered for breast conservation at presentation.
The meta-analysis also showed that local recurrence rates did not differ from those seen with mastectomy when patients treated with neoadjuvant therapy were downstaged to breast-conserving therapy, and that there were no differences in local recurrence rates for neoadjuvant vs. adjuvant chemotherapy stratified by type of surgery, Dr. Mittendorf said at the annual Society of Surgical Oncology Cancer Symposium.
Key clinical trials, including the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials, showed that neoadjuvant chemotherapy did not have an effect on either disease-free or overall survival compared with adjuvant chemotherapy, Dr. Mittendorf noted.
Response to neoadjuvant chemotherapy is also a good predictor of prognosis, she said, pointing to a pooled analysis of 12 studies published in 2014 in The Lancet. The authors of the analysis reported that patients with a pathologic complete response (pCR; no invasive disease in either the breast or axilla) after neoadjuvant chemotherapy had significantly improved survival, with the greatest prognostic values seen in patients with aggressive tumor subtypes.
Factors to consider when selecting neoadjuvant chemotherapy include:
• Tumor size.
• Lymph node status.
• Estrogen, progesterone, and/or HER2 status.
• Treatment sensitivity (as measured by Ki-67 or other markers).
• Pathologic complete response rates.
Chemo for HR-positive?
“With respect to hormone receptor–positive breast cancer, I think the most important question for these patients is do they even need chemotherapy?” Dr. Mittendorf said.
Hormone receptor–positive (HR-positive) breast cancers have been shown to be less responsive to neoadjuvant chemotherapy, and pCR is less prognostic of outcome in this tumor subtype. Older patients with HR-positive cancers who are borderline candidates for breast-conserving therapy might benefit from neoadjuvant therapy with an aromatase inhibitor, she noted.
HER2-positive disease
For patients with HER2-positive breast cancers, it may be possible to tailor neoadjuvant therapy, so that patients who achieve a pCR with neoadjuvant trastuzumab (Herceptin) might be spared an additional 6 months of adjuvant therapy. Dr. Mittendorf’s group published a recent study
Combination anti-HER2 therapies (trastuzumab and pertuzumab [Perjeta] as used in the NeoSphere Trial may help to improve pCR rates and outcomes in patients with HER2-positive tumors, Dr. Mittendorf said.
Triple negative disease
Among patients with triple-negative breast cancer (tumors lacking hormonal receptors and HER2), those who have residual cancer after neoadjuvant chemotherapy have a poor prognosis. At MD Anderson, patients with localized triple-negative breast cancer who are scheduled to receive neoadjuvant chemotherapy first have a biopsy with molecular profiling, and are immediately started on an anthracycline-based regimen.
Patients who have a response to the chemotherapy proceed to receive a taxane, while nonresponders will be triaged onto phase II studies based on the subtype of triple-negative breast cancer. Patients who are positive for BRCA mutations will be started on a carboplatin/paclitaxel regimen, while those with mesenchymal tumor subtypes will be started on a phosphoinositide 3-kinase (PI3K) inhibitor, and those with basal-like tumors will be started on an immunotherapy protocol.Better understanding of the biology of different tumor subtypes may also help to reduce the extent of axillary surgery, by helping clinicians to identify those patients who are likely to have a nodal pCR, Dr. Mittendorf said.
HOUSTON – For some women with breast cancer, neoadjuvant therapy can increase the likelihood of breast-conserving treatment and may limit the extent of axillary dissection, a breast cancer researcher says.
“Neoadjuvant chemotherapy has long been used in the management of inflammatory breast cancer, in patients with locally advanced, or inoperable disease, and it’s increasingly being used in patients who have operable breast cancer,” said Dr. Elizabeth A. Mittendorf of the University of Texas MD Anderson Cancer Center, Houston.
A meta-analysis published in 2007 suggested that neoadjuvant therapy in patients with operable breast cancer reduced the mastectomy rate by 17%, a figure that Dr. Mittendorf said likely underestimates the benefit, because many of the trials included in the analysis did not require patients to be considered for breast conservation at presentation.
The meta-analysis also showed that local recurrence rates did not differ from those seen with mastectomy when patients treated with neoadjuvant therapy were downstaged to breast-conserving therapy, and that there were no differences in local recurrence rates for neoadjuvant vs. adjuvant chemotherapy stratified by type of surgery, Dr. Mittendorf said at the annual Society of Surgical Oncology Cancer Symposium.
Key clinical trials, including the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials, showed that neoadjuvant chemotherapy did not have an effect on either disease-free or overall survival compared with adjuvant chemotherapy, Dr. Mittendorf noted.
Response to neoadjuvant chemotherapy is also a good predictor of prognosis, she said, pointing to a pooled analysis of 12 studies published in 2014 in The Lancet. The authors of the analysis reported that patients with a pathologic complete response (pCR; no invasive disease in either the breast or axilla) after neoadjuvant chemotherapy had significantly improved survival, with the greatest prognostic values seen in patients with aggressive tumor subtypes.
Factors to consider when selecting neoadjuvant chemotherapy include:
• Tumor size.
• Lymph node status.
• Estrogen, progesterone, and/or HER2 status.
• Treatment sensitivity (as measured by Ki-67 or other markers).
• Pathologic complete response rates.
Chemo for HR-positive?
“With respect to hormone receptor–positive breast cancer, I think the most important question for these patients is do they even need chemotherapy?” Dr. Mittendorf said.
Hormone receptor–positive (HR-positive) breast cancers have been shown to be less responsive to neoadjuvant chemotherapy, and pCR is less prognostic of outcome in this tumor subtype. Older patients with HR-positive cancers who are borderline candidates for breast-conserving therapy might benefit from neoadjuvant therapy with an aromatase inhibitor, she noted.
HER2-positive disease
For patients with HER2-positive breast cancers, it may be possible to tailor neoadjuvant therapy, so that patients who achieve a pCR with neoadjuvant trastuzumab (Herceptin) might be spared an additional 6 months of adjuvant therapy. Dr. Mittendorf’s group published a recent study
Combination anti-HER2 therapies (trastuzumab and pertuzumab [Perjeta] as used in the NeoSphere Trial may help to improve pCR rates and outcomes in patients with HER2-positive tumors, Dr. Mittendorf said.
Triple negative disease
Among patients with triple-negative breast cancer (tumors lacking hormonal receptors and HER2), those who have residual cancer after neoadjuvant chemotherapy have a poor prognosis. At MD Anderson, patients with localized triple-negative breast cancer who are scheduled to receive neoadjuvant chemotherapy first have a biopsy with molecular profiling, and are immediately started on an anthracycline-based regimen.
Patients who have a response to the chemotherapy proceed to receive a taxane, while nonresponders will be triaged onto phase II studies based on the subtype of triple-negative breast cancer. Patients who are positive for BRCA mutations will be started on a carboplatin/paclitaxel regimen, while those with mesenchymal tumor subtypes will be started on a phosphoinositide 3-kinase (PI3K) inhibitor, and those with basal-like tumors will be started on an immunotherapy protocol.Better understanding of the biology of different tumor subtypes may also help to reduce the extent of axillary surgery, by helping clinicians to identify those patients who are likely to have a nodal pCR, Dr. Mittendorf said.
New melanoma therapies may break the bank
HOUSTON – Newer systemic therapies for metastatic malignant melanoma have resulted in significant gains in survival, but at a cost that may be unsustainable in the near future, according to Dr. Jeffrey E. Gershenwald.
Up to one-half of all expenses related to the treatment of malignant melanoma are accounted for by the care of patients with advanced disease, yet patients with distant metastases (stage IV disease) account for only about 2% of all patients, said Dr. Gershenwald, professor of surgical oncology at the University of Texas M.D. Anderson Cancer Center, Houston.
“How best can we achieve the right therapy for the right patient at the right time, and as we learn more and more about some of the therapies, particularly in melanoma, for the right length of time? We can’t really afford to give treatments in perpetuity, so we need to know how long they actually need to be delivered in order to have optimal value for the patient,” he said at the annual Society of Surgical Oncology Cancer Symposium.
Over the last 3 decades, and particularly over the last 5 years, there have been tremendous forward strides in therapy. In 1975, when dacarbazine became the standard of care for metastatic melanoma, it was associated with response rates of only about 6%-15%, durable responses in only 5%-15% of patients, and a median overall survival of about 6-9 months, Dr. Gershenwald reported.
Treatment toxicities, but not response rates, increased with the introduction of interleukin-2 in 1998, which for want of a better drug became the new preferred treatment.
But with the introduction of new systemic therapies, such as immune checkpoint inhibitors (ipilimumab [Yervoy], nivolumab [Opdivo], and pembrolizumab [Keytruda]) and targeted agents (vemurafenib [Zelboraf], dabrafenib [Tafinlar], and trametinib [Mekinist]), response rates have soared, resulting in an improvement in 1-year survival rates from about 30% to 35% in 1970 to as high as 80% in clinical trials in 2014.
Increased survival, higher costs
Dr. Gershenwald pointed to a recently published cost-effectiveness analysis of treatment strategies for BRAF-mutated metastatic melanoma. In it, the authors noted that vemurafenib costs $13,000 per month, translating into $207,000 for a patient with median survival. Patients for whom vemurafenib fails are often put on ipilimumab, at $150,000 per course.
The authors calculated that the incremental cost-effectiveness ratio (ICER) for vemurafenib compared with dacarbazine was nearly $354,993 per quality-adjusted life-year (QALY) gained, a figure that is more than threefold higher than widely accepted thresholds for cost-effective treatment ($50,000-$100,000 per QALY gained).
The ICER for firstline vemurafenib followed by ipilimumab was $158,139, still well above the accepted limits.
The authors of the cost analysis noted that the treatments could become cost effective if drug prices were to drop significantly, or if clinical trials could establish whether it was possible to achieve a durable response without continued therapy.
Going forward, clinicians will need to consider disease burden, including both the extent and growth rate of the disease, as well as the risk of recurrence, in deciding whether to use adjuvant therapies, Dr. Gershenwald said.
In addition, clinical choices will be based on disease biology, predictors of response (although few such predictors currently exist), the likelihood of resistance, and drug toxicities, quality of life, and ease of administration, he said.
Dr. Gershenwald disclosed serving on a Merck advisory board.
HOUSTON – Newer systemic therapies for metastatic malignant melanoma have resulted in significant gains in survival, but at a cost that may be unsustainable in the near future, according to Dr. Jeffrey E. Gershenwald.
Up to one-half of all expenses related to the treatment of malignant melanoma are accounted for by the care of patients with advanced disease, yet patients with distant metastases (stage IV disease) account for only about 2% of all patients, said Dr. Gershenwald, professor of surgical oncology at the University of Texas M.D. Anderson Cancer Center, Houston.
“How best can we achieve the right therapy for the right patient at the right time, and as we learn more and more about some of the therapies, particularly in melanoma, for the right length of time? We can’t really afford to give treatments in perpetuity, so we need to know how long they actually need to be delivered in order to have optimal value for the patient,” he said at the annual Society of Surgical Oncology Cancer Symposium.
Over the last 3 decades, and particularly over the last 5 years, there have been tremendous forward strides in therapy. In 1975, when dacarbazine became the standard of care for metastatic melanoma, it was associated with response rates of only about 6%-15%, durable responses in only 5%-15% of patients, and a median overall survival of about 6-9 months, Dr. Gershenwald reported.
Treatment toxicities, but not response rates, increased with the introduction of interleukin-2 in 1998, which for want of a better drug became the new preferred treatment.
But with the introduction of new systemic therapies, such as immune checkpoint inhibitors (ipilimumab [Yervoy], nivolumab [Opdivo], and pembrolizumab [Keytruda]) and targeted agents (vemurafenib [Zelboraf], dabrafenib [Tafinlar], and trametinib [Mekinist]), response rates have soared, resulting in an improvement in 1-year survival rates from about 30% to 35% in 1970 to as high as 80% in clinical trials in 2014.
Increased survival, higher costs
Dr. Gershenwald pointed to a recently published cost-effectiveness analysis of treatment strategies for BRAF-mutated metastatic melanoma. In it, the authors noted that vemurafenib costs $13,000 per month, translating into $207,000 for a patient with median survival. Patients for whom vemurafenib fails are often put on ipilimumab, at $150,000 per course.
The authors calculated that the incremental cost-effectiveness ratio (ICER) for vemurafenib compared with dacarbazine was nearly $354,993 per quality-adjusted life-year (QALY) gained, a figure that is more than threefold higher than widely accepted thresholds for cost-effective treatment ($50,000-$100,000 per QALY gained).
The ICER for firstline vemurafenib followed by ipilimumab was $158,139, still well above the accepted limits.
The authors of the cost analysis noted that the treatments could become cost effective if drug prices were to drop significantly, or if clinical trials could establish whether it was possible to achieve a durable response without continued therapy.
Going forward, clinicians will need to consider disease burden, including both the extent and growth rate of the disease, as well as the risk of recurrence, in deciding whether to use adjuvant therapies, Dr. Gershenwald said.
In addition, clinical choices will be based on disease biology, predictors of response (although few such predictors currently exist), the likelihood of resistance, and drug toxicities, quality of life, and ease of administration, he said.
Dr. Gershenwald disclosed serving on a Merck advisory board.
HOUSTON – Newer systemic therapies for metastatic malignant melanoma have resulted in significant gains in survival, but at a cost that may be unsustainable in the near future, according to Dr. Jeffrey E. Gershenwald.
Up to one-half of all expenses related to the treatment of malignant melanoma are accounted for by the care of patients with advanced disease, yet patients with distant metastases (stage IV disease) account for only about 2% of all patients, said Dr. Gershenwald, professor of surgical oncology at the University of Texas M.D. Anderson Cancer Center, Houston.
“How best can we achieve the right therapy for the right patient at the right time, and as we learn more and more about some of the therapies, particularly in melanoma, for the right length of time? We can’t really afford to give treatments in perpetuity, so we need to know how long they actually need to be delivered in order to have optimal value for the patient,” he said at the annual Society of Surgical Oncology Cancer Symposium.
Over the last 3 decades, and particularly over the last 5 years, there have been tremendous forward strides in therapy. In 1975, when dacarbazine became the standard of care for metastatic melanoma, it was associated with response rates of only about 6%-15%, durable responses in only 5%-15% of patients, and a median overall survival of about 6-9 months, Dr. Gershenwald reported.
Treatment toxicities, but not response rates, increased with the introduction of interleukin-2 in 1998, which for want of a better drug became the new preferred treatment.
But with the introduction of new systemic therapies, such as immune checkpoint inhibitors (ipilimumab [Yervoy], nivolumab [Opdivo], and pembrolizumab [Keytruda]) and targeted agents (vemurafenib [Zelboraf], dabrafenib [Tafinlar], and trametinib [Mekinist]), response rates have soared, resulting in an improvement in 1-year survival rates from about 30% to 35% in 1970 to as high as 80% in clinical trials in 2014.
Increased survival, higher costs
Dr. Gershenwald pointed to a recently published cost-effectiveness analysis of treatment strategies for BRAF-mutated metastatic melanoma. In it, the authors noted that vemurafenib costs $13,000 per month, translating into $207,000 for a patient with median survival. Patients for whom vemurafenib fails are often put on ipilimumab, at $150,000 per course.
The authors calculated that the incremental cost-effectiveness ratio (ICER) for vemurafenib compared with dacarbazine was nearly $354,993 per quality-adjusted life-year (QALY) gained, a figure that is more than threefold higher than widely accepted thresholds for cost-effective treatment ($50,000-$100,000 per QALY gained).
The ICER for firstline vemurafenib followed by ipilimumab was $158,139, still well above the accepted limits.
The authors of the cost analysis noted that the treatments could become cost effective if drug prices were to drop significantly, or if clinical trials could establish whether it was possible to achieve a durable response without continued therapy.
Going forward, clinicians will need to consider disease burden, including both the extent and growth rate of the disease, as well as the risk of recurrence, in deciding whether to use adjuvant therapies, Dr. Gershenwald said.
In addition, clinical choices will be based on disease biology, predictors of response (although few such predictors currently exist), the likelihood of resistance, and drug toxicities, quality of life, and ease of administration, he said.
Dr. Gershenwald disclosed serving on a Merck advisory board.
AT SSO 2015
Key clinical point: Immunotherapies and targeted agents for metastatic melanoma are effective but very costly.
Major finding: The incremental cost-effectiveness ratio for vemurafenib, compared with dacarbazine, was nearly $354,993 per quality-adjusted life-year gained.
Data source: A review of data on the efficacy and costs of therapy for metastatic malignant melanoma.
Disclosures: Dr. Gershenwald disclosed serving on a Merck advisory board.
Clinical doc improvement ups income, quality
HOUSTON – Results of a pilot program suggest that a surgeon-led Clinical Documentation Improvement (CDI) program can improve the accuracy of diagnostic coding, validate the quality of care delivered, and help ensure that hospitals are fairly compensated for the complexity of care they provide.
A comparison of outcomes of cases from four surgical oncologists from the periods before and after implementation of a CDI suggested that the actual case mix index (CMI) was 9% higher than the original charts indicated, a change that would translate into a more than $700,000 increase in reimbursement, said Dr. Keith Gray from the University of Tennessee Medical Center in Knoxville.
“CDI is low-hanging fruit in the era of pay for quality and dwindling hospital margins. Physicians and hospitals can benefit, and surgical oncologists are the natural physicians in the hospital to lead this process, “ Dr. Gray said at the Society of Surgical Oncology 2015 Cancer Symposium.
CDI programs are collaborative efforts between clinicians and health information management professionals, designed to document the quality of care the institution delivers through improved diagnostic coding, he explained.
The benefits of CDI include more accurate reflection of the severity of illness of patients, better sharing of data among caregivers, optimizing of claims processing, and a stronger bottom line.
“We all think we have the sickest patients in the country, and that’s why our results don’t match up. Clinical documentation is an opportunity for you to prove that,” he said.
In the study, a physician extender trained in CDI audited and update all inpatient diagnoses made by four surgical oncologists in a hospital-based practice from November 2012 through May 2013. The diagnoses were listed as being present on admission or recorded during the inpatient stay.
The investigators looked at data on the CMI, average mortality risk, and average severity of illness for 489 patients treated during the study period. These data were compared with a control set from 482 patients treated from March 2011 through October 2012, the period immediately prior to the implementation of the CDI.
The authors found that with the clinical documentation program in place, the CMI, risk of mortality estimates, and severity of illness index all increased.
The practice’s mean CMI, for example, increased from 2.38 to 2.59 (P < .001), a 9% relative increase. Dr. Gray noted that every 0.1 change in CMI represents a $700/patient difference in reimbursement. Therefore, the change would translate into a $718,830 relative increase in reimbursement.
Similarly, the severity of illness index, based on patient comorbidities, age, procedures, and principal diagnosis also increased, from a mean of 2.32 for controls to 2.54 during the study period, translating into a 9.5% increase (P < .001).
Risk of mortality estimates – the likelihood of in-hospital death based on comorbidities, age, procedures, and principal diagnosis – also increased, from a mean 1.88 to 2.07, a 10% increase (P < .001).
Although the CMI, risk of mortality, and severity of illness all increased during the study period, compared with the control period, the percentage of cases above the average length of stay, a measure of quality care, declined significantly, from 45.6% pre-CDI to 31.1% after CDI was implemented (P = .0001). Other measures of quality such as the observed to expected mortality ratio, length of stay ratio, and percentage of cases above the average cost also improved, but the changes were not statistically significant.
“CDI is relatively easy to implement with the resources that we have in place, and there’s minimal additional training to become efficient in this process,” Dr. Gray said.
The study was internally funded. Dr. Gray did not report potential conflicts of interest.
HOUSTON – Results of a pilot program suggest that a surgeon-led Clinical Documentation Improvement (CDI) program can improve the accuracy of diagnostic coding, validate the quality of care delivered, and help ensure that hospitals are fairly compensated for the complexity of care they provide.
A comparison of outcomes of cases from four surgical oncologists from the periods before and after implementation of a CDI suggested that the actual case mix index (CMI) was 9% higher than the original charts indicated, a change that would translate into a more than $700,000 increase in reimbursement, said Dr. Keith Gray from the University of Tennessee Medical Center in Knoxville.
“CDI is low-hanging fruit in the era of pay for quality and dwindling hospital margins. Physicians and hospitals can benefit, and surgical oncologists are the natural physicians in the hospital to lead this process, “ Dr. Gray said at the Society of Surgical Oncology 2015 Cancer Symposium.
CDI programs are collaborative efforts between clinicians and health information management professionals, designed to document the quality of care the institution delivers through improved diagnostic coding, he explained.
The benefits of CDI include more accurate reflection of the severity of illness of patients, better sharing of data among caregivers, optimizing of claims processing, and a stronger bottom line.
“We all think we have the sickest patients in the country, and that’s why our results don’t match up. Clinical documentation is an opportunity for you to prove that,” he said.
In the study, a physician extender trained in CDI audited and update all inpatient diagnoses made by four surgical oncologists in a hospital-based practice from November 2012 through May 2013. The diagnoses were listed as being present on admission or recorded during the inpatient stay.
The investigators looked at data on the CMI, average mortality risk, and average severity of illness for 489 patients treated during the study period. These data were compared with a control set from 482 patients treated from March 2011 through October 2012, the period immediately prior to the implementation of the CDI.
The authors found that with the clinical documentation program in place, the CMI, risk of mortality estimates, and severity of illness index all increased.
The practice’s mean CMI, for example, increased from 2.38 to 2.59 (P < .001), a 9% relative increase. Dr. Gray noted that every 0.1 change in CMI represents a $700/patient difference in reimbursement. Therefore, the change would translate into a $718,830 relative increase in reimbursement.
Similarly, the severity of illness index, based on patient comorbidities, age, procedures, and principal diagnosis also increased, from a mean of 2.32 for controls to 2.54 during the study period, translating into a 9.5% increase (P < .001).
Risk of mortality estimates – the likelihood of in-hospital death based on comorbidities, age, procedures, and principal diagnosis – also increased, from a mean 1.88 to 2.07, a 10% increase (P < .001).
Although the CMI, risk of mortality, and severity of illness all increased during the study period, compared with the control period, the percentage of cases above the average length of stay, a measure of quality care, declined significantly, from 45.6% pre-CDI to 31.1% after CDI was implemented (P = .0001). Other measures of quality such as the observed to expected mortality ratio, length of stay ratio, and percentage of cases above the average cost also improved, but the changes were not statistically significant.
“CDI is relatively easy to implement with the resources that we have in place, and there’s minimal additional training to become efficient in this process,” Dr. Gray said.
The study was internally funded. Dr. Gray did not report potential conflicts of interest.
HOUSTON – Results of a pilot program suggest that a surgeon-led Clinical Documentation Improvement (CDI) program can improve the accuracy of diagnostic coding, validate the quality of care delivered, and help ensure that hospitals are fairly compensated for the complexity of care they provide.
A comparison of outcomes of cases from four surgical oncologists from the periods before and after implementation of a CDI suggested that the actual case mix index (CMI) was 9% higher than the original charts indicated, a change that would translate into a more than $700,000 increase in reimbursement, said Dr. Keith Gray from the University of Tennessee Medical Center in Knoxville.
“CDI is low-hanging fruit in the era of pay for quality and dwindling hospital margins. Physicians and hospitals can benefit, and surgical oncologists are the natural physicians in the hospital to lead this process, “ Dr. Gray said at the Society of Surgical Oncology 2015 Cancer Symposium.
CDI programs are collaborative efforts between clinicians and health information management professionals, designed to document the quality of care the institution delivers through improved diagnostic coding, he explained.
The benefits of CDI include more accurate reflection of the severity of illness of patients, better sharing of data among caregivers, optimizing of claims processing, and a stronger bottom line.
“We all think we have the sickest patients in the country, and that’s why our results don’t match up. Clinical documentation is an opportunity for you to prove that,” he said.
In the study, a physician extender trained in CDI audited and update all inpatient diagnoses made by four surgical oncologists in a hospital-based practice from November 2012 through May 2013. The diagnoses were listed as being present on admission or recorded during the inpatient stay.
The investigators looked at data on the CMI, average mortality risk, and average severity of illness for 489 patients treated during the study period. These data were compared with a control set from 482 patients treated from March 2011 through October 2012, the period immediately prior to the implementation of the CDI.
The authors found that with the clinical documentation program in place, the CMI, risk of mortality estimates, and severity of illness index all increased.
The practice’s mean CMI, for example, increased from 2.38 to 2.59 (P < .001), a 9% relative increase. Dr. Gray noted that every 0.1 change in CMI represents a $700/patient difference in reimbursement. Therefore, the change would translate into a $718,830 relative increase in reimbursement.
Similarly, the severity of illness index, based on patient comorbidities, age, procedures, and principal diagnosis also increased, from a mean of 2.32 for controls to 2.54 during the study period, translating into a 9.5% increase (P < .001).
Risk of mortality estimates – the likelihood of in-hospital death based on comorbidities, age, procedures, and principal diagnosis – also increased, from a mean 1.88 to 2.07, a 10% increase (P < .001).
Although the CMI, risk of mortality, and severity of illness all increased during the study period, compared with the control period, the percentage of cases above the average length of stay, a measure of quality care, declined significantly, from 45.6% pre-CDI to 31.1% after CDI was implemented (P = .0001). Other measures of quality such as the observed to expected mortality ratio, length of stay ratio, and percentage of cases above the average cost also improved, but the changes were not statistically significant.
“CDI is relatively easy to implement with the resources that we have in place, and there’s minimal additional training to become efficient in this process,” Dr. Gray said.
The study was internally funded. Dr. Gray did not report potential conflicts of interest.
AT SSO 2015
Key clinical point: A surgeon-led clinical documentation program can increase revenue by demonstrating case-mix severity.
Major finding: An audit of previous cases showed that proper documentation would yield $718,830 in additional reimbursements.
Data source: Pilot program and retrospective case audit involving cases of 489 patients and 482 controls.
Disclosures: The study was internally funded. Dr. Gray did not report potential conflicts of interest.
Risk scale predicts mortality after gastric cancer surgery
HOUSTON – A simple preoperative scale can accurately predict a patient’s risk for near-term death following surgery for gastric cancer, investigators say.
The scale accounts for both patient and hospital factors, and is useful as a clinical tool for preoperative counseling of patients, reported Dr. Cristina Harnsberger of the University of California San Diego.
“Male gender, increasing age, and comorbid disease increase risk of in-hospital mortality for patients who undergo gastric resection for malignancy. Additionally, low hospital volume was an independent risk factor,” she said at the annual Society of Surgical Oncology Cancer Symposium.
The scale was able to accurately classify patients as being at low or high risk, and the observed and expected mortality rates for each risk score were well correlated, she said.
Weighing risks
Perioperative mortality rates following resection for gastric malignancies range from 0.6% to 15%. Risk scales and nomograms are intended to help clinicians predict risks for individual patients, but most incorporate postoperative data rather than preoperative or hospital data, Dr. Harnsberger said.
She and her colleagues conducted a study to determine whether a simple preoperative scale based on patient and hospital factors could accurately predict risk for death following gastric resection for malignancy.
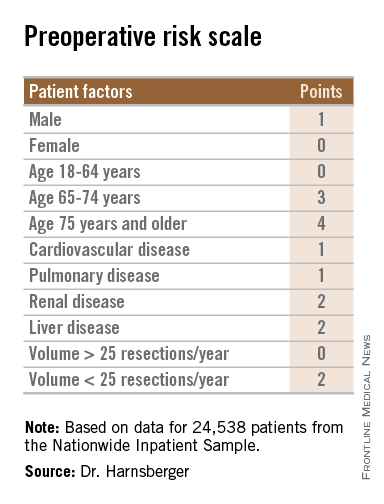
They drew on data from the Nationwide Inpatient Sample database to identify adult patients with a diagnosis of gastric cancer who underwent potentially curative partial or total gastrectomy from 1998 through 2011.
They identified a total 24,538 patients, based on International Classification of Diseases, Revision 9 (ICD-9) diagnosis and procedure codes.
They then created multivariate logistic regression models to identify independent predictors of mortality, create a predictive model, and construct a risk scale. The models controlled for sex, age, race, comorbidities, insurance status, hospital volume (less than 25 vs. 25 or more gastric resections for malignancy per year), laparoscopic vs. open approach, poverty level, alcohol abuse, tobacco use, diabetes mellitus, and year of procedure.
The mean length of stay for the patient sample was 11 days. The overall in-hospital mortality was 5.5%.
The models identified three patient-related factors and one hospital-related factor that were predictive of mortality and when combined in a risk scale proved to be accurate.
The patient factors were male sex, age 65 and older, and comorbid disease, specifically cardiovascular, pulmonary, renal, and/or hepatic.
The hospital factor, expressed as protective, was 25 or more gastric resections for cancer per year.
The maximum possible score is 13. Patients with scores lower than 6 are at low risk for perioperative mortality, while those with scores 6 and higher are at high risk. Among patients with a score of 0-5, the perioperative death rate ranged from 1.3% to 4.5%. In contrast, patients with higher scores had death rates ranging from 6.0% to 23.1%.
Clinical applications for the bedside risk scale include perioperative patient counseling, aiding in informed consent discussions, and as an adjunct to postoperative risk calculators, Dr. Harnsberger said.The study funding source was not disclosed. Dr. Harnsberger reported having no disclosures.
HOUSTON – A simple preoperative scale can accurately predict a patient’s risk for near-term death following surgery for gastric cancer, investigators say.
The scale accounts for both patient and hospital factors, and is useful as a clinical tool for preoperative counseling of patients, reported Dr. Cristina Harnsberger of the University of California San Diego.
“Male gender, increasing age, and comorbid disease increase risk of in-hospital mortality for patients who undergo gastric resection for malignancy. Additionally, low hospital volume was an independent risk factor,” she said at the annual Society of Surgical Oncology Cancer Symposium.
The scale was able to accurately classify patients as being at low or high risk, and the observed and expected mortality rates for each risk score were well correlated, she said.
Weighing risks
Perioperative mortality rates following resection for gastric malignancies range from 0.6% to 15%. Risk scales and nomograms are intended to help clinicians predict risks for individual patients, but most incorporate postoperative data rather than preoperative or hospital data, Dr. Harnsberger said.
She and her colleagues conducted a study to determine whether a simple preoperative scale based on patient and hospital factors could accurately predict risk for death following gastric resection for malignancy.

They drew on data from the Nationwide Inpatient Sample database to identify adult patients with a diagnosis of gastric cancer who underwent potentially curative partial or total gastrectomy from 1998 through 2011.
They identified a total 24,538 patients, based on International Classification of Diseases, Revision 9 (ICD-9) diagnosis and procedure codes.
They then created multivariate logistic regression models to identify independent predictors of mortality, create a predictive model, and construct a risk scale. The models controlled for sex, age, race, comorbidities, insurance status, hospital volume (less than 25 vs. 25 or more gastric resections for malignancy per year), laparoscopic vs. open approach, poverty level, alcohol abuse, tobacco use, diabetes mellitus, and year of procedure.
The mean length of stay for the patient sample was 11 days. The overall in-hospital mortality was 5.5%.
The models identified three patient-related factors and one hospital-related factor that were predictive of mortality and when combined in a risk scale proved to be accurate.
The patient factors were male sex, age 65 and older, and comorbid disease, specifically cardiovascular, pulmonary, renal, and/or hepatic.
The hospital factor, expressed as protective, was 25 or more gastric resections for cancer per year.
The maximum possible score is 13. Patients with scores lower than 6 are at low risk for perioperative mortality, while those with scores 6 and higher are at high risk. Among patients with a score of 0-5, the perioperative death rate ranged from 1.3% to 4.5%. In contrast, patients with higher scores had death rates ranging from 6.0% to 23.1%.
Clinical applications for the bedside risk scale include perioperative patient counseling, aiding in informed consent discussions, and as an adjunct to postoperative risk calculators, Dr. Harnsberger said.The study funding source was not disclosed. Dr. Harnsberger reported having no disclosures.
HOUSTON – A simple preoperative scale can accurately predict a patient’s risk for near-term death following surgery for gastric cancer, investigators say.
The scale accounts for both patient and hospital factors, and is useful as a clinical tool for preoperative counseling of patients, reported Dr. Cristina Harnsberger of the University of California San Diego.
“Male gender, increasing age, and comorbid disease increase risk of in-hospital mortality for patients who undergo gastric resection for malignancy. Additionally, low hospital volume was an independent risk factor,” she said at the annual Society of Surgical Oncology Cancer Symposium.
The scale was able to accurately classify patients as being at low or high risk, and the observed and expected mortality rates for each risk score were well correlated, she said.
Weighing risks
Perioperative mortality rates following resection for gastric malignancies range from 0.6% to 15%. Risk scales and nomograms are intended to help clinicians predict risks for individual patients, but most incorporate postoperative data rather than preoperative or hospital data, Dr. Harnsberger said.
She and her colleagues conducted a study to determine whether a simple preoperative scale based on patient and hospital factors could accurately predict risk for death following gastric resection for malignancy.

They drew on data from the Nationwide Inpatient Sample database to identify adult patients with a diagnosis of gastric cancer who underwent potentially curative partial or total gastrectomy from 1998 through 2011.
They identified a total 24,538 patients, based on International Classification of Diseases, Revision 9 (ICD-9) diagnosis and procedure codes.
They then created multivariate logistic regression models to identify independent predictors of mortality, create a predictive model, and construct a risk scale. The models controlled for sex, age, race, comorbidities, insurance status, hospital volume (less than 25 vs. 25 or more gastric resections for malignancy per year), laparoscopic vs. open approach, poverty level, alcohol abuse, tobacco use, diabetes mellitus, and year of procedure.
The mean length of stay for the patient sample was 11 days. The overall in-hospital mortality was 5.5%.
The models identified three patient-related factors and one hospital-related factor that were predictive of mortality and when combined in a risk scale proved to be accurate.
The patient factors were male sex, age 65 and older, and comorbid disease, specifically cardiovascular, pulmonary, renal, and/or hepatic.
The hospital factor, expressed as protective, was 25 or more gastric resections for cancer per year.
The maximum possible score is 13. Patients with scores lower than 6 are at low risk for perioperative mortality, while those with scores 6 and higher are at high risk. Among patients with a score of 0-5, the perioperative death rate ranged from 1.3% to 4.5%. In contrast, patients with higher scores had death rates ranging from 6.0% to 23.1%.
Clinical applications for the bedside risk scale include perioperative patient counseling, aiding in informed consent discussions, and as an adjunct to postoperative risk calculators, Dr. Harnsberger said.The study funding source was not disclosed. Dr. Harnsberger reported having no disclosures.
AT SSO 2015
Key clinical point: The bedside risk scale can be used in patient counseling prior to surgery for gastric malignancies.
Major finding: Patients with scores of 0-5 had perioperative death rates of 1.3%-4.5%. Patients with higher scores had death rates ranging from 6.0% to 23.1%.
Data source: Review of retrospective data on 24,538 adults who underwent partial or total gastric resection for malignancies.
Disclosures: The study funding source was not disclosed. Dr. Harnsberger reported having no disclosures.
Palliative surgery eases pain at end of life
HOUSTON – Palliative surgery can alleviate pain and improve the quality of life for patients dying from advanced cancers, without compromising performance status, a study showed.
Among 202 patients with stage III or IV cancers who underwent surgery with palliation as the goal, pain scores were significantly improved after surgery, while Karnofsky Performance Status (KPS) scores remained unchanged, said Dr. Anne Falor, a surgical oncology fellow at City of Hope in Duarte, Calif.
“Surgical oncology has not been historically involved in palliative care. If a patient is deemed unresectable, his or her treatment is often the purview of medical or radiation oncology,” she said at the annual Society of Surgical Oncology Cancer Symposium.
But for patients who are likely to have prolonged disease-free intervals, palliative surgery can be performed with low morbidity, she said.
Dr. Falor and her colleagues reviewed their center’s experience with palliative surgery in 2011, during which time 202 patients with a predicted 5-year survival of less than 5% underwent a total of 247 palliative procedures.
The patients had malignancies at various sites, including the large intestine, lung, stomach, breast, prostate, lymph nodes, esophagus, pancreas, and ovaries.
The primary indications for the procedure included dysphagia, pain/wound problems, dyspnea, nausea and vomiting, and dysuria.
Most of the patients (83%) had a single procedure, but 13% had two operations, 4% had three operations, 1% had four procedures, and 0.4% had five or more interventions.
The majority of procedures performed were endoscopic interventions characterized as minor in nature, followed by minor genitourinary and thoracic interventions, although a nearly equal proportion of thoracic interventions (about 28%) were major procedures such as diverting ostomy.
When the investigators looked at 30-day outcomes following palliative surgery, they found that only 13% of patients needed an urgent care visit, 2% required a triage call, 22% were readmitted, and 60% had an institutional supportive care referral.
Total 30-day morbidity of any kind was seen in 37% of patients; 15% of patients died within 30 days of surgery.
Looking at quality of life outcomes, the investigators found no differences in the percentage of patients with KPS scores from 80 to 100 between the presurgery and postsurgery periods (78% and 70%, respectively, P = ns).
There were significant improvements, however, in pain scores, which dropped by a mean of 1.2 points from the preoperative period to discharge (P < .0001), and decreased by 0.6 points before surgery to the first follow-up visit (P = .0037).
Dr. Falor said that it’s important for patients and their care team to have a discussion regarding expectations for surgery and the goals of care.
The study was internally funded. Dr. Falor reported having no conflicts of interest.
Over the past decade palliative surgery has been increasingly discussed and scrutinized, as the concept of palliative care has gained greater traction with medical professionals and the public. Not too long ago the designation “palliative” when applied to a surgical procedure had an apologetic connotation because the operation would fail to heal. In some cases the term was even used to describe positive tumor margins at the conclusion of a resection – something totally irrelevant when assessing the direct impact of the operation upon a patient’s self-designated symptoms.
Only recently has a more positive perspective emerged, helped by data such as these researchers have presented. Palliative surgery now is not failing to cure, but succeeding to comfort.
New perspectives, however, will raise new and necessary questions to better define the role of surgery in the greater context of relief of suffering in all its manifestations. In our wish to respond surgically to pain and other symptoms we must be vigilant against the temptation to “do something” when surgery for cure or palliation is unlikely to help in order to assuage our feelings of hopelessness. Hopelessness is not an indication for surgery – pain, obstruction, and saving life are. Indications for surgery must be more specific, as this article points out, and it is more likely to help with localized and pressing symptoms. A rule of thumb passed down to me from my surgeon grandfather who practiced in an era when the vast majority of operations were palliative, the more pressing and clear the indication for surgery, the better the result.
Dr. Geoffrey Dunn, an ACS Fellow based in Erie, Pa.
Over the past decade palliative surgery has been increasingly discussed and scrutinized, as the concept of palliative care has gained greater traction with medical professionals and the public. Not too long ago the designation “palliative” when applied to a surgical procedure had an apologetic connotation because the operation would fail to heal. In some cases the term was even used to describe positive tumor margins at the conclusion of a resection – something totally irrelevant when assessing the direct impact of the operation upon a patient’s self-designated symptoms.
Only recently has a more positive perspective emerged, helped by data such as these researchers have presented. Palliative surgery now is not failing to cure, but succeeding to comfort.
New perspectives, however, will raise new and necessary questions to better define the role of surgery in the greater context of relief of suffering in all its manifestations. In our wish to respond surgically to pain and other symptoms we must be vigilant against the temptation to “do something” when surgery for cure or palliation is unlikely to help in order to assuage our feelings of hopelessness. Hopelessness is not an indication for surgery – pain, obstruction, and saving life are. Indications for surgery must be more specific, as this article points out, and it is more likely to help with localized and pressing symptoms. A rule of thumb passed down to me from my surgeon grandfather who practiced in an era when the vast majority of operations were palliative, the more pressing and clear the indication for surgery, the better the result.
Dr. Geoffrey Dunn, an ACS Fellow based in Erie, Pa.
Over the past decade palliative surgery has been increasingly discussed and scrutinized, as the concept of palliative care has gained greater traction with medical professionals and the public. Not too long ago the designation “palliative” when applied to a surgical procedure had an apologetic connotation because the operation would fail to heal. In some cases the term was even used to describe positive tumor margins at the conclusion of a resection – something totally irrelevant when assessing the direct impact of the operation upon a patient’s self-designated symptoms.
Only recently has a more positive perspective emerged, helped by data such as these researchers have presented. Palliative surgery now is not failing to cure, but succeeding to comfort.
New perspectives, however, will raise new and necessary questions to better define the role of surgery in the greater context of relief of suffering in all its manifestations. In our wish to respond surgically to pain and other symptoms we must be vigilant against the temptation to “do something” when surgery for cure or palliation is unlikely to help in order to assuage our feelings of hopelessness. Hopelessness is not an indication for surgery – pain, obstruction, and saving life are. Indications for surgery must be more specific, as this article points out, and it is more likely to help with localized and pressing symptoms. A rule of thumb passed down to me from my surgeon grandfather who practiced in an era when the vast majority of operations were palliative, the more pressing and clear the indication for surgery, the better the result.
Dr. Geoffrey Dunn, an ACS Fellow based in Erie, Pa.
HOUSTON – Palliative surgery can alleviate pain and improve the quality of life for patients dying from advanced cancers, without compromising performance status, a study showed.
Among 202 patients with stage III or IV cancers who underwent surgery with palliation as the goal, pain scores were significantly improved after surgery, while Karnofsky Performance Status (KPS) scores remained unchanged, said Dr. Anne Falor, a surgical oncology fellow at City of Hope in Duarte, Calif.
“Surgical oncology has not been historically involved in palliative care. If a patient is deemed unresectable, his or her treatment is often the purview of medical or radiation oncology,” she said at the annual Society of Surgical Oncology Cancer Symposium.
But for patients who are likely to have prolonged disease-free intervals, palliative surgery can be performed with low morbidity, she said.
Dr. Falor and her colleagues reviewed their center’s experience with palliative surgery in 2011, during which time 202 patients with a predicted 5-year survival of less than 5% underwent a total of 247 palliative procedures.
The patients had malignancies at various sites, including the large intestine, lung, stomach, breast, prostate, lymph nodes, esophagus, pancreas, and ovaries.
The primary indications for the procedure included dysphagia, pain/wound problems, dyspnea, nausea and vomiting, and dysuria.
Most of the patients (83%) had a single procedure, but 13% had two operations, 4% had three operations, 1% had four procedures, and 0.4% had five or more interventions.
The majority of procedures performed were endoscopic interventions characterized as minor in nature, followed by minor genitourinary and thoracic interventions, although a nearly equal proportion of thoracic interventions (about 28%) were major procedures such as diverting ostomy.
When the investigators looked at 30-day outcomes following palliative surgery, they found that only 13% of patients needed an urgent care visit, 2% required a triage call, 22% were readmitted, and 60% had an institutional supportive care referral.
Total 30-day morbidity of any kind was seen in 37% of patients; 15% of patients died within 30 days of surgery.
Looking at quality of life outcomes, the investigators found no differences in the percentage of patients with KPS scores from 80 to 100 between the presurgery and postsurgery periods (78% and 70%, respectively, P = ns).
There were significant improvements, however, in pain scores, which dropped by a mean of 1.2 points from the preoperative period to discharge (P < .0001), and decreased by 0.6 points before surgery to the first follow-up visit (P = .0037).
Dr. Falor said that it’s important for patients and their care team to have a discussion regarding expectations for surgery and the goals of care.
The study was internally funded. Dr. Falor reported having no conflicts of interest.
HOUSTON – Palliative surgery can alleviate pain and improve the quality of life for patients dying from advanced cancers, without compromising performance status, a study showed.
Among 202 patients with stage III or IV cancers who underwent surgery with palliation as the goal, pain scores were significantly improved after surgery, while Karnofsky Performance Status (KPS) scores remained unchanged, said Dr. Anne Falor, a surgical oncology fellow at City of Hope in Duarte, Calif.
“Surgical oncology has not been historically involved in palliative care. If a patient is deemed unresectable, his or her treatment is often the purview of medical or radiation oncology,” she said at the annual Society of Surgical Oncology Cancer Symposium.
But for patients who are likely to have prolonged disease-free intervals, palliative surgery can be performed with low morbidity, she said.
Dr. Falor and her colleagues reviewed their center’s experience with palliative surgery in 2011, during which time 202 patients with a predicted 5-year survival of less than 5% underwent a total of 247 palliative procedures.
The patients had malignancies at various sites, including the large intestine, lung, stomach, breast, prostate, lymph nodes, esophagus, pancreas, and ovaries.
The primary indications for the procedure included dysphagia, pain/wound problems, dyspnea, nausea and vomiting, and dysuria.
Most of the patients (83%) had a single procedure, but 13% had two operations, 4% had three operations, 1% had four procedures, and 0.4% had five or more interventions.
The majority of procedures performed were endoscopic interventions characterized as minor in nature, followed by minor genitourinary and thoracic interventions, although a nearly equal proportion of thoracic interventions (about 28%) were major procedures such as diverting ostomy.
When the investigators looked at 30-day outcomes following palliative surgery, they found that only 13% of patients needed an urgent care visit, 2% required a triage call, 22% were readmitted, and 60% had an institutional supportive care referral.
Total 30-day morbidity of any kind was seen in 37% of patients; 15% of patients died within 30 days of surgery.
Looking at quality of life outcomes, the investigators found no differences in the percentage of patients with KPS scores from 80 to 100 between the presurgery and postsurgery periods (78% and 70%, respectively, P = ns).
There were significant improvements, however, in pain scores, which dropped by a mean of 1.2 points from the preoperative period to discharge (P < .0001), and decreased by 0.6 points before surgery to the first follow-up visit (P = .0037).
Dr. Falor said that it’s important for patients and their care team to have a discussion regarding expectations for surgery and the goals of care.
The study was internally funded. Dr. Falor reported having no conflicts of interest.
Key clinical point: Palliative surgery in patients with advanced cancers can relieve pain with minimal morbidity.
Major finding: Pain scores improved significantly from the pre- to postoperative periods, without a significant decline in performance status scores.
Data source: Case series of 202 patients with stage III or IV malignancies who underwent 247 palliative procedures.
Disclosures: The study was internally funded. Dr. Falor reported having no conflicts of interest.




















