User login
DOAC’s edge over warfarin fades with low adherence
BOSTON – The direct acting oral anticoagulants boost patient adherence compared with warfarin anticoagulation, but when patients did not adhere to their regimens, those prescribed a new, direct-acting oral anticoagulant had worse outcomes than did patients on warfarin – even patients poorly adherent with warfarin – based on data from more than 80,000 U.S. patients.
Among low-adherence patients, defined as those with adherence rates of 40%-80% based on prescriptions filled, patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of at least 2 and treated with warfarin had a 3.37/100 patient-years rate of thromboembolic events–driven hospitalizations or emergency department visits, compared with a 4.05/100 patient-years rate among low-adherence patients receiving a direct-acting oral anticoagulant (DOAC), Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. The incidence of strokes of any kind was also lower in the low-adherence warfarin patients compared with the low-adherence DOAC patients, although the relationship flipped for hemorrhagic strokes and bleeds, which were more common in the warfarin patients.
In contrast, when patients were adherent, taking more than 80% of their prescribed drug, the performance of the DOACs generally surpassed that of warfarin. In an analysis adjusted for several demographic and clinical confounders, and when compared with patients adherent to a warfarin regimen, those adherent to a DOAC had a 7% lower rate of thromboembolic events, a 36% lower rate of hemorrhagic strokes, an 8% lower rate of any stroke, and a 10% lower rate of bleeds (excluding hemorrhagic strokes), all statistically significant differences, reported Dr. Lakkireddy, medical director of the Kansas City Heart Rhythm Institute in Overland Park, Kan.
The message from this analysis is the importance of maximizing patient adherence, Dr. Lakkireddy said.
“We should not make the false assumption that putting a patient on a DOAC will take care of everything. We need to make it a habit to make sure patients are taking their pills,” he said in an interview. “We were surprised. Our assumption was that the DOACs were more forgiving than warfarin” in poorly compliant patients. “We need to make talking about adherence with patients routine.”
The results also documented that adherence to therapy is better with a DOAC, with a 74% rate of good adherence among the nearly 41,000 patients in the database prescribed a DOAC compared with a 63% rate of good compliance among the more than 42,000 patients prescribed warfarin.
“It’s ironic that we might think low-adherence patients should go on warfarin. That’s sort of backwards,” said Andrew D. Krahn, MD, a cardiac electrophysiologist and professor of medicine at the University of British Columbia in Vancouver. “It’s not biologically plausible” to predict that less adherent patients would do better on warfarin, he noted.
The study run by Dr. Lakkireddy and his associates used data collected by IBM Watson Health Market Scan from about 4 million Medicare patients and 47 million American residents with private insurance during 2012-2016. They focused on the more than 600,000 patients prescribed an anticoagulant during 2014 and 2015, and then narrowed the study group down to just over 83,000 adults with nonvalvular atrial fibrillation, a CHA2DS2-VASc score of 2 or more. The patients’ average age was about 74 years.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehringer Ingelheim, Bristol Myers Squibb, EstechPharma, Janssen, Pfizer, SentreHeart, and St. Jude. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-03.
What is clear from this analysis is that adherence to oral anticoagulant regimens is something we need to address. It would help if we could determine why patients are not well adherent, but regardless of the cause, changing patient behavior and improving adherence will require better patient education and better integration of medical care toward better adherence.
This study has several obvious limitations, including its reliance on an administrative database that does not allow for adjudication of outcomes. The analysis presented so far is also limited by not breaking down the direct-acting oral anticoagulant into individual drugs, and without propensity-score matching of the two treatment subgroups.
We must be very alert to the possibility for confounding by indication in a real-world dataset like this. For example, the stroke rate we see in the patients treated with a DOAC may be affected by clinicians who preferentially prescribed one of the direct-acting drugs to patients who had what they thought was a high stroke risk because they felt these drugs might work better for stroke prevention than warfarin.
What is also notable in the results is that, even among the patients with good adherence, the event and adverse effects rates remained high. Among the highly adherent patients the thromboembolic event rate was greater than 3% per year in both the warfarin and direct-acting drug groups, the stroke rate was also greater than 3% per year in both subgroups, and bleeding events occurred at rates of greater than 4% per year among the patients treated with direct-acting drugs and greater than 5% per year in the warfarin group.
Hein Heidbuchel, MD, professor of medicine and chair of cardiology at the University of Antwerp, Belgium, made these comments as designated discussant for the study. He had no current disclosures.
What is clear from this analysis is that adherence to oral anticoagulant regimens is something we need to address. It would help if we could determine why patients are not well adherent, but regardless of the cause, changing patient behavior and improving adherence will require better patient education and better integration of medical care toward better adherence.
This study has several obvious limitations, including its reliance on an administrative database that does not allow for adjudication of outcomes. The analysis presented so far is also limited by not breaking down the direct-acting oral anticoagulant into individual drugs, and without propensity-score matching of the two treatment subgroups.
We must be very alert to the possibility for confounding by indication in a real-world dataset like this. For example, the stroke rate we see in the patients treated with a DOAC may be affected by clinicians who preferentially prescribed one of the direct-acting drugs to patients who had what they thought was a high stroke risk because they felt these drugs might work better for stroke prevention than warfarin.
What is also notable in the results is that, even among the patients with good adherence, the event and adverse effects rates remained high. Among the highly adherent patients the thromboembolic event rate was greater than 3% per year in both the warfarin and direct-acting drug groups, the stroke rate was also greater than 3% per year in both subgroups, and bleeding events occurred at rates of greater than 4% per year among the patients treated with direct-acting drugs and greater than 5% per year in the warfarin group.
Hein Heidbuchel, MD, professor of medicine and chair of cardiology at the University of Antwerp, Belgium, made these comments as designated discussant for the study. He had no current disclosures.
What is clear from this analysis is that adherence to oral anticoagulant regimens is something we need to address. It would help if we could determine why patients are not well adherent, but regardless of the cause, changing patient behavior and improving adherence will require better patient education and better integration of medical care toward better adherence.
This study has several obvious limitations, including its reliance on an administrative database that does not allow for adjudication of outcomes. The analysis presented so far is also limited by not breaking down the direct-acting oral anticoagulant into individual drugs, and without propensity-score matching of the two treatment subgroups.
We must be very alert to the possibility for confounding by indication in a real-world dataset like this. For example, the stroke rate we see in the patients treated with a DOAC may be affected by clinicians who preferentially prescribed one of the direct-acting drugs to patients who had what they thought was a high stroke risk because they felt these drugs might work better for stroke prevention than warfarin.
What is also notable in the results is that, even among the patients with good adherence, the event and adverse effects rates remained high. Among the highly adherent patients the thromboembolic event rate was greater than 3% per year in both the warfarin and direct-acting drug groups, the stroke rate was also greater than 3% per year in both subgroups, and bleeding events occurred at rates of greater than 4% per year among the patients treated with direct-acting drugs and greater than 5% per year in the warfarin group.
Hein Heidbuchel, MD, professor of medicine and chair of cardiology at the University of Antwerp, Belgium, made these comments as designated discussant for the study. He had no current disclosures.
BOSTON – The direct acting oral anticoagulants boost patient adherence compared with warfarin anticoagulation, but when patients did not adhere to their regimens, those prescribed a new, direct-acting oral anticoagulant had worse outcomes than did patients on warfarin – even patients poorly adherent with warfarin – based on data from more than 80,000 U.S. patients.
Among low-adherence patients, defined as those with adherence rates of 40%-80% based on prescriptions filled, patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of at least 2 and treated with warfarin had a 3.37/100 patient-years rate of thromboembolic events–driven hospitalizations or emergency department visits, compared with a 4.05/100 patient-years rate among low-adherence patients receiving a direct-acting oral anticoagulant (DOAC), Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. The incidence of strokes of any kind was also lower in the low-adherence warfarin patients compared with the low-adherence DOAC patients, although the relationship flipped for hemorrhagic strokes and bleeds, which were more common in the warfarin patients.
In contrast, when patients were adherent, taking more than 80% of their prescribed drug, the performance of the DOACs generally surpassed that of warfarin. In an analysis adjusted for several demographic and clinical confounders, and when compared with patients adherent to a warfarin regimen, those adherent to a DOAC had a 7% lower rate of thromboembolic events, a 36% lower rate of hemorrhagic strokes, an 8% lower rate of any stroke, and a 10% lower rate of bleeds (excluding hemorrhagic strokes), all statistically significant differences, reported Dr. Lakkireddy, medical director of the Kansas City Heart Rhythm Institute in Overland Park, Kan.
The message from this analysis is the importance of maximizing patient adherence, Dr. Lakkireddy said.
“We should not make the false assumption that putting a patient on a DOAC will take care of everything. We need to make it a habit to make sure patients are taking their pills,” he said in an interview. “We were surprised. Our assumption was that the DOACs were more forgiving than warfarin” in poorly compliant patients. “We need to make talking about adherence with patients routine.”
The results also documented that adherence to therapy is better with a DOAC, with a 74% rate of good adherence among the nearly 41,000 patients in the database prescribed a DOAC compared with a 63% rate of good compliance among the more than 42,000 patients prescribed warfarin.
“It’s ironic that we might think low-adherence patients should go on warfarin. That’s sort of backwards,” said Andrew D. Krahn, MD, a cardiac electrophysiologist and professor of medicine at the University of British Columbia in Vancouver. “It’s not biologically plausible” to predict that less adherent patients would do better on warfarin, he noted.
The study run by Dr. Lakkireddy and his associates used data collected by IBM Watson Health Market Scan from about 4 million Medicare patients and 47 million American residents with private insurance during 2012-2016. They focused on the more than 600,000 patients prescribed an anticoagulant during 2014 and 2015, and then narrowed the study group down to just over 83,000 adults with nonvalvular atrial fibrillation, a CHA2DS2-VASc score of 2 or more. The patients’ average age was about 74 years.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehringer Ingelheim, Bristol Myers Squibb, EstechPharma, Janssen, Pfizer, SentreHeart, and St. Jude. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-03.
BOSTON – The direct acting oral anticoagulants boost patient adherence compared with warfarin anticoagulation, but when patients did not adhere to their regimens, those prescribed a new, direct-acting oral anticoagulant had worse outcomes than did patients on warfarin – even patients poorly adherent with warfarin – based on data from more than 80,000 U.S. patients.
Among low-adherence patients, defined as those with adherence rates of 40%-80% based on prescriptions filled, patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of at least 2 and treated with warfarin had a 3.37/100 patient-years rate of thromboembolic events–driven hospitalizations or emergency department visits, compared with a 4.05/100 patient-years rate among low-adherence patients receiving a direct-acting oral anticoagulant (DOAC), Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. The incidence of strokes of any kind was also lower in the low-adherence warfarin patients compared with the low-adherence DOAC patients, although the relationship flipped for hemorrhagic strokes and bleeds, which were more common in the warfarin patients.
In contrast, when patients were adherent, taking more than 80% of their prescribed drug, the performance of the DOACs generally surpassed that of warfarin. In an analysis adjusted for several demographic and clinical confounders, and when compared with patients adherent to a warfarin regimen, those adherent to a DOAC had a 7% lower rate of thromboembolic events, a 36% lower rate of hemorrhagic strokes, an 8% lower rate of any stroke, and a 10% lower rate of bleeds (excluding hemorrhagic strokes), all statistically significant differences, reported Dr. Lakkireddy, medical director of the Kansas City Heart Rhythm Institute in Overland Park, Kan.
The message from this analysis is the importance of maximizing patient adherence, Dr. Lakkireddy said.
“We should not make the false assumption that putting a patient on a DOAC will take care of everything. We need to make it a habit to make sure patients are taking their pills,” he said in an interview. “We were surprised. Our assumption was that the DOACs were more forgiving than warfarin” in poorly compliant patients. “We need to make talking about adherence with patients routine.”
The results also documented that adherence to therapy is better with a DOAC, with a 74% rate of good adherence among the nearly 41,000 patients in the database prescribed a DOAC compared with a 63% rate of good compliance among the more than 42,000 patients prescribed warfarin.
“It’s ironic that we might think low-adherence patients should go on warfarin. That’s sort of backwards,” said Andrew D. Krahn, MD, a cardiac electrophysiologist and professor of medicine at the University of British Columbia in Vancouver. “It’s not biologically plausible” to predict that less adherent patients would do better on warfarin, he noted.
The study run by Dr. Lakkireddy and his associates used data collected by IBM Watson Health Market Scan from about 4 million Medicare patients and 47 million American residents with private insurance during 2012-2016. They focused on the more than 600,000 patients prescribed an anticoagulant during 2014 and 2015, and then narrowed the study group down to just over 83,000 adults with nonvalvular atrial fibrillation, a CHA2DS2-VASc score of 2 or more. The patients’ average age was about 74 years.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehringer Ingelheim, Bristol Myers Squibb, EstechPharma, Janssen, Pfizer, SentreHeart, and St. Jude. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-03.
REPORTING FROM HEART RHYTHM 2018
Key clinical point: Good adherence is needed for direct-acting oral anticoagulants to outperform warfarin.
Major finding: Thromboembolic events in low-adherence patients were 4.05/100 patient years with a DOAC and 3.37/100 patient years with warfarin.
Study details: Analysis of 83,168 insured U.S. atrial fibrillation patients treated with an oral anticoagulant in 2014-2015.
Disclosures: Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehringer Ingelheim, Bristol Myers Squibb, EstechPharma, Janssen, Pfizer, SentreHeart, and St. Jude. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific.
Source: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-03.
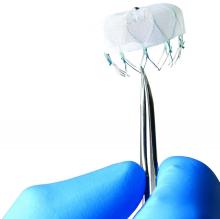
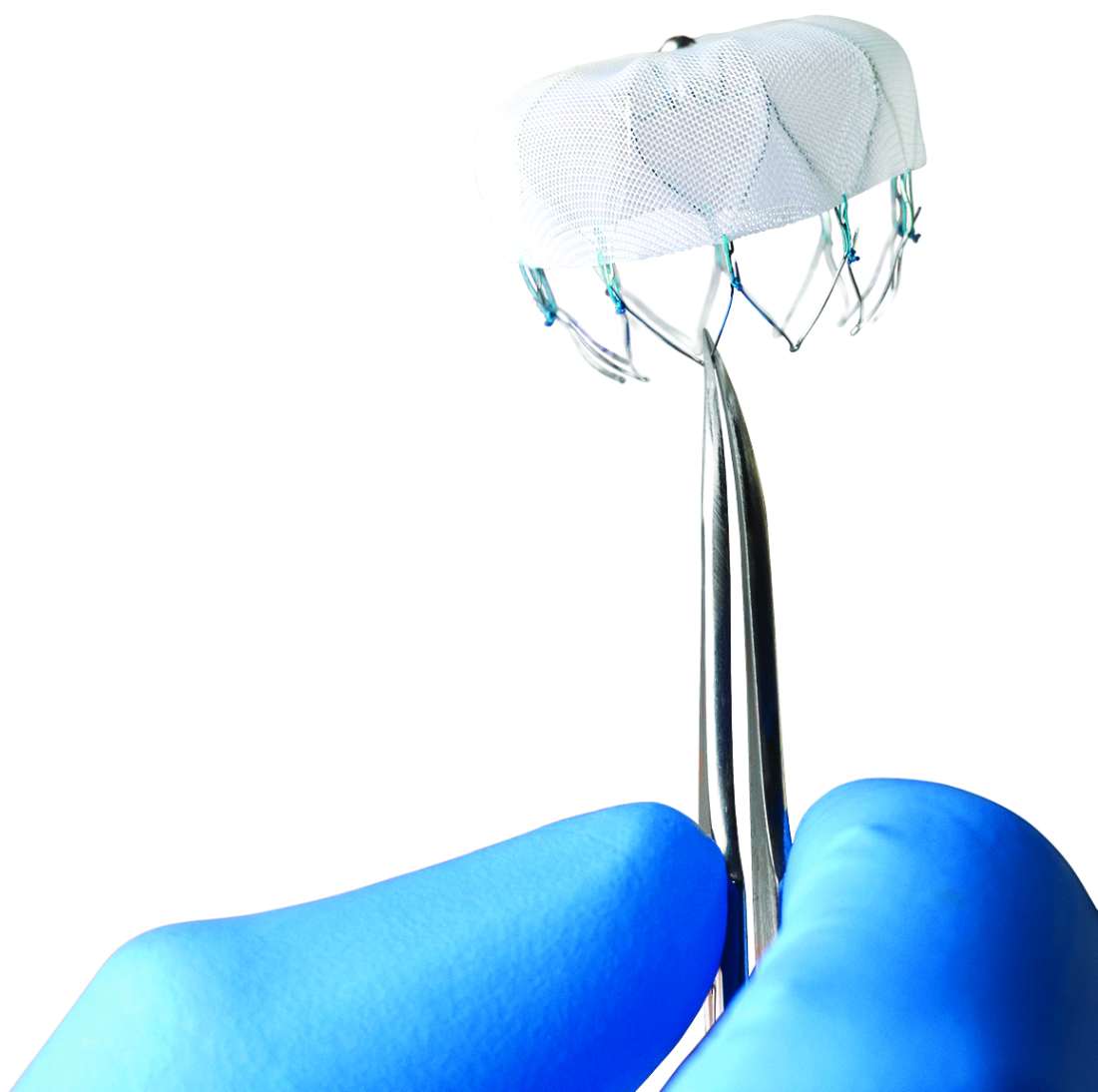
New strategies eliminate cardiac device transvenous leads
reflecting a keen interest in getting leads out of the vascular space and potentially cutting risks for infections, pneumothorax, hematoma, and the possible need for future lead extraction.
Leads are “the Achilles heel” of pacemakers and defibrillators; “there are too many places where the wire can break,” John D. Day, MD, said in an interview during the annual scientific sessions of the Heart Rhythm Society. Eliminating transvenous leads, or any type of lead for that matter, “solves a lot of problems” that currently occur with implanted cardiac devices, said Dr. Day, a cardiac electrophysiologist with Intermountain Health in Murray, Utah.
The Acute Extravascular Defibrillation, Pacing, and Electrogram (ASD2) study tested a substernal lead in 79 patients enrolled at multiple centers in the United States and elsewhere. The lead’s design uses a minimally invasive subxiphoid approach with substernal lead advancement using a blunt tunneling rod, explained Lucas V.A. Boersma, MD, a professor of cardiology at the Academic Medical Center University in Amsterdam. Placed between the sternum and heart, the lead sits just beside the ventricles to provide both ventricular pacing and defibrillation, unlike existing subcutaneously placed leads that do not allow pacing and require high energy for defibrillation. It took a median of 12 minutes to place the lead, Dr. Boersma said.
Testing demonstrated successful ventricular pacing capture in 76 of 78 patients (97%) who underwent this testing, he reported. A single 30-joule shock delivered via the substernal lead resulted in successful defibrillation of induced fibrillation in 102 of 123 fibrillation events (83%). Six patients had seven adverse events that resolved without sequelae for all but two events. The two events with greater clinical impact included one patient with asystolic cardiac arrest 36 hours after lead placement who developed decompensated heart failure and required medical management, and one patient with pericardial effusion and tamponade who required supportive care.
“We are very used to working in the transvenous space” for lead placement, “and leaving that space is outside the comfort zone” for many clinicians, Dr. Boersma noted. “It will take time to adopt these new technologies, and obviously we need more proof” of efficacy and safety.
The second study examined a novel approach that modified the control algorithm of the Micra leadless single-chamber pacing device, approved for U.S. marketing in 2016, so that it used information collected by a built-in accelerometer to detect atrial contractions and produce atrial-ventricular (AV) synchrony in patients with AV block.
The MARVEL (Micra Atrial Tracking Using a Ventricular Accelerometer) study enrolled 70 patients at 12 centers worldwide and collected evaluable data from 64 patients. The average time spent in AV synchrony using this AV pacing was 87% in all patients, with an 80% average rate of AV synchrony in the 33 enrolled patients who had high-degree block, said Larry A. Chinitz, MD, professor of medicine and director of the Heart Rhythm Center at New York University Langone Health. Concurrently with his report at the meeting, the results also appeared in an article online (Heart Rhythm. 2018 May 11. doi: 10.1016/j.hrthm.2018.05.004).
The current leadless pacemaker is very limited as a single-chamber pacing device because it can only help patients who have permanent atrial fibrillation and a slow ventricular response and hence only need single-chamber pacing, about 14% of all patients who need cardiac pacing, said Dr. Chinitz. Using the accelerometer information appears to make the Micra device suitable for the much larger number of patients who have AV block. “You effectively have dual-chamber pacing but with a single pellet. This is a simple and attractive way to do it,” Dr. Day commented. “You get a two-for-one” that potentially could triple the number of patients who could benefit from the Micra leadless device, he estimated.
[email protected]
On Twitter @mitchelzoler
SOURCE: Boersma L et al. Heart Rhythm 2018, Abstract B-LBCT03-03; Chinitz L et al. Heart Rhythm 2018, Abstract B-LBCT03-04.
reflecting a keen interest in getting leads out of the vascular space and potentially cutting risks for infections, pneumothorax, hematoma, and the possible need for future lead extraction.
Leads are “the Achilles heel” of pacemakers and defibrillators; “there are too many places where the wire can break,” John D. Day, MD, said in an interview during the annual scientific sessions of the Heart Rhythm Society. Eliminating transvenous leads, or any type of lead for that matter, “solves a lot of problems” that currently occur with implanted cardiac devices, said Dr. Day, a cardiac electrophysiologist with Intermountain Health in Murray, Utah.
The Acute Extravascular Defibrillation, Pacing, and Electrogram (ASD2) study tested a substernal lead in 79 patients enrolled at multiple centers in the United States and elsewhere. The lead’s design uses a minimally invasive subxiphoid approach with substernal lead advancement using a blunt tunneling rod, explained Lucas V.A. Boersma, MD, a professor of cardiology at the Academic Medical Center University in Amsterdam. Placed between the sternum and heart, the lead sits just beside the ventricles to provide both ventricular pacing and defibrillation, unlike existing subcutaneously placed leads that do not allow pacing and require high energy for defibrillation. It took a median of 12 minutes to place the lead, Dr. Boersma said.
Testing demonstrated successful ventricular pacing capture in 76 of 78 patients (97%) who underwent this testing, he reported. A single 30-joule shock delivered via the substernal lead resulted in successful defibrillation of induced fibrillation in 102 of 123 fibrillation events (83%). Six patients had seven adverse events that resolved without sequelae for all but two events. The two events with greater clinical impact included one patient with asystolic cardiac arrest 36 hours after lead placement who developed decompensated heart failure and required medical management, and one patient with pericardial effusion and tamponade who required supportive care.
“We are very used to working in the transvenous space” for lead placement, “and leaving that space is outside the comfort zone” for many clinicians, Dr. Boersma noted. “It will take time to adopt these new technologies, and obviously we need more proof” of efficacy and safety.
The second study examined a novel approach that modified the control algorithm of the Micra leadless single-chamber pacing device, approved for U.S. marketing in 2016, so that it used information collected by a built-in accelerometer to detect atrial contractions and produce atrial-ventricular (AV) synchrony in patients with AV block.
The MARVEL (Micra Atrial Tracking Using a Ventricular Accelerometer) study enrolled 70 patients at 12 centers worldwide and collected evaluable data from 64 patients. The average time spent in AV synchrony using this AV pacing was 87% in all patients, with an 80% average rate of AV synchrony in the 33 enrolled patients who had high-degree block, said Larry A. Chinitz, MD, professor of medicine and director of the Heart Rhythm Center at New York University Langone Health. Concurrently with his report at the meeting, the results also appeared in an article online (Heart Rhythm. 2018 May 11. doi: 10.1016/j.hrthm.2018.05.004).
The current leadless pacemaker is very limited as a single-chamber pacing device because it can only help patients who have permanent atrial fibrillation and a slow ventricular response and hence only need single-chamber pacing, about 14% of all patients who need cardiac pacing, said Dr. Chinitz. Using the accelerometer information appears to make the Micra device suitable for the much larger number of patients who have AV block. “You effectively have dual-chamber pacing but with a single pellet. This is a simple and attractive way to do it,” Dr. Day commented. “You get a two-for-one” that potentially could triple the number of patients who could benefit from the Micra leadless device, he estimated.
[email protected]
On Twitter @mitchelzoler
SOURCE: Boersma L et al. Heart Rhythm 2018, Abstract B-LBCT03-03; Chinitz L et al. Heart Rhythm 2018, Abstract B-LBCT03-04.
reflecting a keen interest in getting leads out of the vascular space and potentially cutting risks for infections, pneumothorax, hematoma, and the possible need for future lead extraction.
Leads are “the Achilles heel” of pacemakers and defibrillators; “there are too many places where the wire can break,” John D. Day, MD, said in an interview during the annual scientific sessions of the Heart Rhythm Society. Eliminating transvenous leads, or any type of lead for that matter, “solves a lot of problems” that currently occur with implanted cardiac devices, said Dr. Day, a cardiac electrophysiologist with Intermountain Health in Murray, Utah.
The Acute Extravascular Defibrillation, Pacing, and Electrogram (ASD2) study tested a substernal lead in 79 patients enrolled at multiple centers in the United States and elsewhere. The lead’s design uses a minimally invasive subxiphoid approach with substernal lead advancement using a blunt tunneling rod, explained Lucas V.A. Boersma, MD, a professor of cardiology at the Academic Medical Center University in Amsterdam. Placed between the sternum and heart, the lead sits just beside the ventricles to provide both ventricular pacing and defibrillation, unlike existing subcutaneously placed leads that do not allow pacing and require high energy for defibrillation. It took a median of 12 minutes to place the lead, Dr. Boersma said.
Testing demonstrated successful ventricular pacing capture in 76 of 78 patients (97%) who underwent this testing, he reported. A single 30-joule shock delivered via the substernal lead resulted in successful defibrillation of induced fibrillation in 102 of 123 fibrillation events (83%). Six patients had seven adverse events that resolved without sequelae for all but two events. The two events with greater clinical impact included one patient with asystolic cardiac arrest 36 hours after lead placement who developed decompensated heart failure and required medical management, and one patient with pericardial effusion and tamponade who required supportive care.
“We are very used to working in the transvenous space” for lead placement, “and leaving that space is outside the comfort zone” for many clinicians, Dr. Boersma noted. “It will take time to adopt these new technologies, and obviously we need more proof” of efficacy and safety.
The second study examined a novel approach that modified the control algorithm of the Micra leadless single-chamber pacing device, approved for U.S. marketing in 2016, so that it used information collected by a built-in accelerometer to detect atrial contractions and produce atrial-ventricular (AV) synchrony in patients with AV block.
The MARVEL (Micra Atrial Tracking Using a Ventricular Accelerometer) study enrolled 70 patients at 12 centers worldwide and collected evaluable data from 64 patients. The average time spent in AV synchrony using this AV pacing was 87% in all patients, with an 80% average rate of AV synchrony in the 33 enrolled patients who had high-degree block, said Larry A. Chinitz, MD, professor of medicine and director of the Heart Rhythm Center at New York University Langone Health. Concurrently with his report at the meeting, the results also appeared in an article online (Heart Rhythm. 2018 May 11. doi: 10.1016/j.hrthm.2018.05.004).
The current leadless pacemaker is very limited as a single-chamber pacing device because it can only help patients who have permanent atrial fibrillation and a slow ventricular response and hence only need single-chamber pacing, about 14% of all patients who need cardiac pacing, said Dr. Chinitz. Using the accelerometer information appears to make the Micra device suitable for the much larger number of patients who have AV block. “You effectively have dual-chamber pacing but with a single pellet. This is a simple and attractive way to do it,” Dr. Day commented. “You get a two-for-one” that potentially could triple the number of patients who could benefit from the Micra leadless device, he estimated.
[email protected]
On Twitter @mitchelzoler
SOURCE: Boersma L et al. Heart Rhythm 2018, Abstract B-LBCT03-03; Chinitz L et al. Heart Rhythm 2018, Abstract B-LBCT03-04.
REPORTING FROM HEART RHYTHM 2018
Key clinical point: Results from pilot studies showed promise for two different approaches to eliminating transvenous leads from cardiac devices.
Major finding: A substernal lead produced ventricular capture pacing in 97% of patients. Atrial syncing with an accelerometer produced AV synchrony in 87% of patients.
Study details: The ASD2 multicenter study enrolled 79 patients. The MARVEL multicenter study enrolled 64 evaluable patients.
Disclosures: The ASD2 and MARVEL studies were both funded by Medtronic, the company developing the tested devices. Dr. Boersma has been a consultant to Medtronic and Boston Scientific. Dr. Chinitz has been a consultant to Medtronic and several other device companies. Dr. Day has been a consultant to Boston Scientific, Biotronik, and St. Jude.
Source: Boersma L et al. Heart Rhythm 2018, Abstract B-LBCT03-03; Chinitz L et al. Heart Rhythm 2018, Abstract B-LBCT03-04.
Myocarditis shows causal role in frequent PVCs
BOSTON – About half of patients who present with a new onset of frequent premature ventricular contractions without obvious underlying heart disease had an underlying myocardial inflammation that was often responsive to immunosuppressive treatment, according to a single-center series of 107 patients.
“Early diagnosis and appropriate treatment with immunosuppressive therapy can significantly affect the clinical course,” although large-scale, multicenter, randomized trials must confirm this as an effective management approach, Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. He stressed that the anecdotal efficacy seen in this series with immunosuppressive therapy and selected use of ablation treatment for the premature ventricular contractions (PVCs) applies only to patients with new-onset PVCs that occur at a rate of at least 5,000 during 24 hours who also have myocardial inflammation identified by a PET scan showing increased fluorodeoxyglucose (FDG) uptake.
The apparent impact of immunosuppressive treatment was “profound,” Dr. Lakkireddy said. The treatment usually involved prednisone and, in many patients, a second immunosuppressant agent such as azathioprine, cyclophosphamide, or methotrexate. The results suggest “a unique opportunity to intervene early with immunosuppression to change the natural course of the disease. PVCs may be the earliest sign of a disease process” featuring myocardial inflammation.
The data came from the Myocarditis and Ventricular Arrhythmia (MAVERIC) registry that Dr. Lakkireddy and his associates started because “we began seeing patients referred for ablations without underlying heart disease who had suddenly presented with a lot of PVCs,” he recalled, an observation that led them to systematically study these patients in an “arduous” process that involved several tests. One hundred seven patients met the registry’s inclusion criteria for new onset of frequent PVCs without apparent underlying heart disease, and roughly half of these patients showed clear evidence of myocardial inflammation by FDG and PET imaging. “If the PET is negative, I don’t worry about myocarditis, “ Dr. Lakkireddy said.
The 55 patients with apparent myocarditis on PET imaging, out of the 107 patients examined generally, had lower left ventricular (LV) ejection fractions averaging 46%, compared with 51% among the patients without myocarditis The patients with myocarditis further subdivided into 27 with preserved LV function, with an average ejection fraction of 60%, and 28 with a reduced LV function, with an average ejection fraction of 40%. The researchers saw an optimal response to immunosuppressive therapy in 18 of the 23 patients (78%) with preserved ejection fractions who received this treatment and in 13 of the 24 patients (54%) with diminished LV ejection fractions who got immunosuppressive therapy.
Twenty-eight of the 55 patients with myocarditis on PET imaging underwent a right-sided biopsy during their work-up, and 13 of these 28 biopsies (46%) showed a lymphocytic infiltrate of a type often seen in patients with postviral myocarditis. Seven of the 28 biopsied patients (25%) had completely normal-appearing cardiac tissue.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
I was quite taken by this study, which produced results that raise shock and alarm. I believe that the clinical condition that this study highlights is a real biological phenomenon that affects patients who were not on my radar screen.
From now on, I will certainly be more alert for and concerned about patients whom I see with an abrupt onset of frequent premature ventricular contractions, especially those who also have a reduced left ventricular ejection fraction. However the potential need to use serial PET examinations to identify and then follow these patients also raises concern about the cumulative radiation exposure patients could receive from serial PET studies.
David J. Callans, MD , is professor of medicine and associate director of electrophysiology at the University of Pennsylvania in Philadelphia. He has been a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the report.
I was quite taken by this study, which produced results that raise shock and alarm. I believe that the clinical condition that this study highlights is a real biological phenomenon that affects patients who were not on my radar screen.
From now on, I will certainly be more alert for and concerned about patients whom I see with an abrupt onset of frequent premature ventricular contractions, especially those who also have a reduced left ventricular ejection fraction. However the potential need to use serial PET examinations to identify and then follow these patients also raises concern about the cumulative radiation exposure patients could receive from serial PET studies.
David J. Callans, MD , is professor of medicine and associate director of electrophysiology at the University of Pennsylvania in Philadelphia. He has been a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the report.
I was quite taken by this study, which produced results that raise shock and alarm. I believe that the clinical condition that this study highlights is a real biological phenomenon that affects patients who were not on my radar screen.
From now on, I will certainly be more alert for and concerned about patients whom I see with an abrupt onset of frequent premature ventricular contractions, especially those who also have a reduced left ventricular ejection fraction. However the potential need to use serial PET examinations to identify and then follow these patients also raises concern about the cumulative radiation exposure patients could receive from serial PET studies.
David J. Callans, MD , is professor of medicine and associate director of electrophysiology at the University of Pennsylvania in Philadelphia. He has been a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the report.
BOSTON – About half of patients who present with a new onset of frequent premature ventricular contractions without obvious underlying heart disease had an underlying myocardial inflammation that was often responsive to immunosuppressive treatment, according to a single-center series of 107 patients.
“Early diagnosis and appropriate treatment with immunosuppressive therapy can significantly affect the clinical course,” although large-scale, multicenter, randomized trials must confirm this as an effective management approach, Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. He stressed that the anecdotal efficacy seen in this series with immunosuppressive therapy and selected use of ablation treatment for the premature ventricular contractions (PVCs) applies only to patients with new-onset PVCs that occur at a rate of at least 5,000 during 24 hours who also have myocardial inflammation identified by a PET scan showing increased fluorodeoxyglucose (FDG) uptake.
The apparent impact of immunosuppressive treatment was “profound,” Dr. Lakkireddy said. The treatment usually involved prednisone and, in many patients, a second immunosuppressant agent such as azathioprine, cyclophosphamide, or methotrexate. The results suggest “a unique opportunity to intervene early with immunosuppression to change the natural course of the disease. PVCs may be the earliest sign of a disease process” featuring myocardial inflammation.
The data came from the Myocarditis and Ventricular Arrhythmia (MAVERIC) registry that Dr. Lakkireddy and his associates started because “we began seeing patients referred for ablations without underlying heart disease who had suddenly presented with a lot of PVCs,” he recalled, an observation that led them to systematically study these patients in an “arduous” process that involved several tests. One hundred seven patients met the registry’s inclusion criteria for new onset of frequent PVCs without apparent underlying heart disease, and roughly half of these patients showed clear evidence of myocardial inflammation by FDG and PET imaging. “If the PET is negative, I don’t worry about myocarditis, “ Dr. Lakkireddy said.
The 55 patients with apparent myocarditis on PET imaging, out of the 107 patients examined generally, had lower left ventricular (LV) ejection fractions averaging 46%, compared with 51% among the patients without myocarditis The patients with myocarditis further subdivided into 27 with preserved LV function, with an average ejection fraction of 60%, and 28 with a reduced LV function, with an average ejection fraction of 40%. The researchers saw an optimal response to immunosuppressive therapy in 18 of the 23 patients (78%) with preserved ejection fractions who received this treatment and in 13 of the 24 patients (54%) with diminished LV ejection fractions who got immunosuppressive therapy.
Twenty-eight of the 55 patients with myocarditis on PET imaging underwent a right-sided biopsy during their work-up, and 13 of these 28 biopsies (46%) showed a lymphocytic infiltrate of a type often seen in patients with postviral myocarditis. Seven of the 28 biopsied patients (25%) had completely normal-appearing cardiac tissue.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
BOSTON – About half of patients who present with a new onset of frequent premature ventricular contractions without obvious underlying heart disease had an underlying myocardial inflammation that was often responsive to immunosuppressive treatment, according to a single-center series of 107 patients.
“Early diagnosis and appropriate treatment with immunosuppressive therapy can significantly affect the clinical course,” although large-scale, multicenter, randomized trials must confirm this as an effective management approach, Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. He stressed that the anecdotal efficacy seen in this series with immunosuppressive therapy and selected use of ablation treatment for the premature ventricular contractions (PVCs) applies only to patients with new-onset PVCs that occur at a rate of at least 5,000 during 24 hours who also have myocardial inflammation identified by a PET scan showing increased fluorodeoxyglucose (FDG) uptake.
The apparent impact of immunosuppressive treatment was “profound,” Dr. Lakkireddy said. The treatment usually involved prednisone and, in many patients, a second immunosuppressant agent such as azathioprine, cyclophosphamide, or methotrexate. The results suggest “a unique opportunity to intervene early with immunosuppression to change the natural course of the disease. PVCs may be the earliest sign of a disease process” featuring myocardial inflammation.
The data came from the Myocarditis and Ventricular Arrhythmia (MAVERIC) registry that Dr. Lakkireddy and his associates started because “we began seeing patients referred for ablations without underlying heart disease who had suddenly presented with a lot of PVCs,” he recalled, an observation that led them to systematically study these patients in an “arduous” process that involved several tests. One hundred seven patients met the registry’s inclusion criteria for new onset of frequent PVCs without apparent underlying heart disease, and roughly half of these patients showed clear evidence of myocardial inflammation by FDG and PET imaging. “If the PET is negative, I don’t worry about myocarditis, “ Dr. Lakkireddy said.
The 55 patients with apparent myocarditis on PET imaging, out of the 107 patients examined generally, had lower left ventricular (LV) ejection fractions averaging 46%, compared with 51% among the patients without myocarditis The patients with myocarditis further subdivided into 27 with preserved LV function, with an average ejection fraction of 60%, and 28 with a reduced LV function, with an average ejection fraction of 40%. The researchers saw an optimal response to immunosuppressive therapy in 18 of the 23 patients (78%) with preserved ejection fractions who received this treatment and in 13 of the 24 patients (54%) with diminished LV ejection fractions who got immunosuppressive therapy.
Twenty-eight of the 55 patients with myocarditis on PET imaging underwent a right-sided biopsy during their work-up, and 13 of these 28 biopsies (46%) showed a lymphocytic infiltrate of a type often seen in patients with postviral myocarditis. Seven of the 28 biopsied patients (25%) had completely normal-appearing cardiac tissue.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
REPORTING FROM HEART RHYTHM 2018
Key clinical point:
Major finding: Immunosuppressive therapy resolved myocarditis in two-thirds of 51% of patients with new-onset, frequents PVCs.
Study details: Single-center series with 107 patients.
Disclosures: Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
Source: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
Single Botox treatment cuts AF for 3 years
BOSTON – A single set of four injections with botulinum toxin into neuron-containing cardiac fat pads of patients during open-chest cardiac artery bypass surgery led to a long-term cut in the cumulative incidence of atrial tachyarrhythmias during 3-year follow-up in a pilot, sham-controlled study with 60 patients at two Russian centers.
“Because the favorable reduction of atrial fibrillation [AF] outlasted the anticipated botulinum toxin effects on autonomic nervous system activity, this may represent a form of autonomic reverse remodeling” triggered by just one injection of the paralyzing toxin at each of four intracardiac fat pads, Alexander B. Romanov, MD, said at the annual scientific sessions of the Heart Rhythm Society. Botulinum toxin (BT) blocks neuronal release of acetylcholine, thereby interfering with cholinergic neurotransmission and producing hypothesized neurologic remodeling, explained Dr. Romanov, a researcher at the Meshalkin National Medical Research Center in Novosibirsk, Russia.
The 3-year results also showed statistically significant differences or trends favoring BT injections for several other clinical outcomes. Two deaths and two strokes occurred, all among the control patients. Two patients required a total of three hospitalizations during follow-up in the BT-treated group, compared with 10 patients hospitalized a total of 21 times in the control arm. Clinicians prescribed antiarrhythmic drugs to six of the BT-treated patients and to 15 of the controls.
All patients received an implanted heart rhythm monitor during their bypass surgery, and the researchers measured AF burden – the percentage of time during which AF occurred. After 12 months, 24 months, and 36 months, the AF burden averaged 0.2%, 1.6%, and 1.2%, respectively, in the BT-treated patients and 1.9%, 9.5%, and 6.9% in the sham-control patients.

“We don’t know why this works, but it’s a fascinating new approach that is worthy of further study,” commented Kalyanam Shivkumar, MD, professor and director of the Cardiac Arrhythmia Center at the University of California, Los Angeles, and designated discussant for the report.
“This is an extremely exciting study, but it remains inconclusive because how it works is not fully understood,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver.
BOSTON – A single set of four injections with botulinum toxin into neuron-containing cardiac fat pads of patients during open-chest cardiac artery bypass surgery led to a long-term cut in the cumulative incidence of atrial tachyarrhythmias during 3-year follow-up in a pilot, sham-controlled study with 60 patients at two Russian centers.
“Because the favorable reduction of atrial fibrillation [AF] outlasted the anticipated botulinum toxin effects on autonomic nervous system activity, this may represent a form of autonomic reverse remodeling” triggered by just one injection of the paralyzing toxin at each of four intracardiac fat pads, Alexander B. Romanov, MD, said at the annual scientific sessions of the Heart Rhythm Society. Botulinum toxin (BT) blocks neuronal release of acetylcholine, thereby interfering with cholinergic neurotransmission and producing hypothesized neurologic remodeling, explained Dr. Romanov, a researcher at the Meshalkin National Medical Research Center in Novosibirsk, Russia.
The 3-year results also showed statistically significant differences or trends favoring BT injections for several other clinical outcomes. Two deaths and two strokes occurred, all among the control patients. Two patients required a total of three hospitalizations during follow-up in the BT-treated group, compared with 10 patients hospitalized a total of 21 times in the control arm. Clinicians prescribed antiarrhythmic drugs to six of the BT-treated patients and to 15 of the controls.
All patients received an implanted heart rhythm monitor during their bypass surgery, and the researchers measured AF burden – the percentage of time during which AF occurred. After 12 months, 24 months, and 36 months, the AF burden averaged 0.2%, 1.6%, and 1.2%, respectively, in the BT-treated patients and 1.9%, 9.5%, and 6.9% in the sham-control patients.

“We don’t know why this works, but it’s a fascinating new approach that is worthy of further study,” commented Kalyanam Shivkumar, MD, professor and director of the Cardiac Arrhythmia Center at the University of California, Los Angeles, and designated discussant for the report.
“This is an extremely exciting study, but it remains inconclusive because how it works is not fully understood,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver.
BOSTON – A single set of four injections with botulinum toxin into neuron-containing cardiac fat pads of patients during open-chest cardiac artery bypass surgery led to a long-term cut in the cumulative incidence of atrial tachyarrhythmias during 3-year follow-up in a pilot, sham-controlled study with 60 patients at two Russian centers.
“Because the favorable reduction of atrial fibrillation [AF] outlasted the anticipated botulinum toxin effects on autonomic nervous system activity, this may represent a form of autonomic reverse remodeling” triggered by just one injection of the paralyzing toxin at each of four intracardiac fat pads, Alexander B. Romanov, MD, said at the annual scientific sessions of the Heart Rhythm Society. Botulinum toxin (BT) blocks neuronal release of acetylcholine, thereby interfering with cholinergic neurotransmission and producing hypothesized neurologic remodeling, explained Dr. Romanov, a researcher at the Meshalkin National Medical Research Center in Novosibirsk, Russia.
The 3-year results also showed statistically significant differences or trends favoring BT injections for several other clinical outcomes. Two deaths and two strokes occurred, all among the control patients. Two patients required a total of three hospitalizations during follow-up in the BT-treated group, compared with 10 patients hospitalized a total of 21 times in the control arm. Clinicians prescribed antiarrhythmic drugs to six of the BT-treated patients and to 15 of the controls.
All patients received an implanted heart rhythm monitor during their bypass surgery, and the researchers measured AF burden – the percentage of time during which AF occurred. After 12 months, 24 months, and 36 months, the AF burden averaged 0.2%, 1.6%, and 1.2%, respectively, in the BT-treated patients and 1.9%, 9.5%, and 6.9% in the sham-control patients.

“We don’t know why this works, but it’s a fascinating new approach that is worthy of further study,” commented Kalyanam Shivkumar, MD, professor and director of the Cardiac Arrhythmia Center at the University of California, Los Angeles, and designated discussant for the report.
“This is an extremely exciting study, but it remains inconclusive because how it works is not fully understood,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver.
REPORTING FROM HEART RHYTHM 2018
Key clinical point:
Major finding: During 3-year follow-up, atrial tachyarrhythmias occurred in 23% of botulinum toxin-treated patients and in 50% of sham controls.
Study details: Randomized, sham-controlled study with 60 patients at two Russian centers.
Disclosures: The study received no commercial funding. Dr. Romanov, Dr. Shivkumar, and Dr. Krahn had no relevant disclosures.
Source: Romanov A et al. Heart Rhythm 2018, Abstract B-LBCT02-01.
A fib ablation in HFrEF patients gains momentum
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Results from two recent trials suggest that cardiologists may have a new way to improve outcomes in patients with heart failure with reduced ejection fraction if they also have atrial fibrillation: Cut the patient’s atrial fibrillation burden with catheter ablation.
This seemingly off-target approach to improving survival, avoiding heart failure hospitalizations, and possibly reducing other adverse events first gained attention with results from the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) randomized trial, first reported in 2017. The study showed in 363 patients that atrial fibrillation (AF) ablation in patients with heart failure with reduced ejection fraction (HFrEF) led to a statistically significant 38% relative reduction in the primary endpoint of mortality or heart failure hospitalization during a median 38 months of follow-up (N Engl J Med. 2018 Feb 1;378[5]:417-27).
This groundbreaking finding then received some degree of confirmation when Douglas L. Packer, MD, reported primary results from CABANA (Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial) at the annual scientific sessions of the Heart Rhythm Society. CABANA compared upfront ablation against first-line medical management of AF in 2,203 patients. While the primary endpoint of the cumulative rate of all-cause death, disabling stroke, serious bleeding, or cardiac arrest over a median follow-up of just over 4 years was neutral, with no statistically significant difference between the two treatment arms, a subgroup analysis showed a tantalizing suggestion of benefit in the 337 enrolled patients with a history of congestive heart failure (15% of the total study group).
In this subgroup, treatment with ablation cut the primary endpoint by 39% relative to those treated upfront with medical management, an effect that came close to statistical significance. In addition, Dr. Packer took special note of the per-protocol analysis, which censored out the crossover patients who constituted roughly a fifth of all enrolled patients. In the subgroup analysis using the per-protocol data, ablation was linked with a statistically significant 49% relative reduction in the primary endpoint among patients with a history of heart failure.
The patients for whom there may be the quickest shift to upfront ablation to treat AF based on the CABANA results will be those with heart failure and others with high underlying risk, Dr. Packer predicted at the meeting.
“The CASTLE-AF results were interesting, but in fewer than 400 patients. Now we’ve basically seen the same thing” in CABANA, said Dr. Packer, professor and a cardiac electrophysiologist at the Mayo Clinic in Rochester, Minn.
Notably however, the results Dr. Packer reported on the heart failure subgroup did not include any information on how many of these were patients who had HFrEF or heart failure with preserved ejection fraction and how the apparent benefit from AF ablation affected each of these two heart failure types. In addition, the reported CABANA results did not have an endpoint result that completely matched the mortality and heart failure hospitalization composite endpoint used in CASTLE-AF. The closest endpoint that Dr. Packer reported from CABANA was a composite of mortality and cardiovascular hospitalization that showed, for the entire CABANA cohort, a statistically significant 17% relative reduction with ablation in the intention-to-treat analysis. Dr. Packer gave no data on how this outcome shook out in the subgroup of heart failure patients.
Despite these limitations, in trying to synthesize the CABANA and CASTLE-AF results, several electrophysiologists who heard the results agreed with Dr. Packer that the CABANA results confirmed the CASTLE-AF findings and helped strengthen the case for strongly considering AF ablation as first-line treatment in patients with heart failure.
“It’s clear that sinus rhythm is important in patients with heart failure. CASTLE-AF and now these results; that’s very strong to me,” said Eric N. Prystowsky, MD, a cardiac electrophysiologist with the St. Vincent Medical Group in Indianapolis and designated discussant for CABANA at the meeting.
“It’s confirmatory,” said Nassir F. Marrouche, MD, lead investigator for CASTLE-AF, and professor and director of the electrophysiology laboratory at the University of Utah in Salt Lake City.
The “signal” of benefit from AF ablation in heart failure patients in CABANA “replicates what was seen in CASTLE-AF. The results are highly consistent and very important regarding how to treat patients with AF and heart failure,” said Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “The data strongly suggest that catheter ablation is helpful for restoring and preserving [heart] muscle function,” Dr. Ruskin said in a video interview. He noted that AF occurs in at least about a quarter of heart failure patients.
Other cardiologists at the meeting noted that, on the basis of the CASTLE-AF results alone, they have already become more aggressive about treating AF with ablation in patients with heart failure in routine practice.
“It adds to the armamentarium for treatment of patients with heart failure,” said Johannes Brachmann, MD, professor and chief of cardiology at the Coburg (Germany) Clinic and a senior coinvestigator for CASTLE-AF.
William T. Abraham, MD, a heart failure specialist at The Ohio State University in Columbus, offered a broader perspective on where AF diagnosis, treatment, and ablation currently stand in U.S. heart failure practice.
“There is a very tight link between AF burden and worse outcomes in heart failure, so there is something intuitively appealing about restoring sinus rhythm in heart failure patients. I think most heart failure clinicians believe, like me, that heart failure patients with AF benefit from restoration of normal sinus rhythm. But I don’t believe that the CASTLE-AF results have so far had much impact on practice, in part because it was a relatively small study. The heart failure community is looking for some confirmation,” said Dr. Abraham, professor and director of cardiovascular medicine at Ohio State.
“I think the CABANA results are encouraging, but they came from only 15% of the enrolled patients who also had heart failure. CABANA adds to our knowledge, but I’m not sure it’s definitive for the heart failure population. I’m not sure it tells us if you treat patients with heart failure with anti-arrhythmia drugs and successfully maintain sinus rhythm do those patients do just as well as those who get ablated,” he said in an interview. “I’d love to see a study of heart failure patients maintained in sinus rhythm with drugs compared with those treated with ablation.”
For most patients with heart failure, the coexistence of AF is identified because of AF symptoms, or when asymptomatic AF is found in recordings made by an implanted cardiac device. “I’m more aggressive about addressing asymptomatic AF in my heart failure patients, and I believe the heart failure community is moving rapidly in that direction because of the association between higher AF burden and worse heart failure outcomes,” Dr. Abraham said.
A more cautious view came from another heart failure specialist, Clyde Yancy, MD, professor and chief of cardiology at Northwestern University in Chicago. “It’s pretty evident that in certain patients with heart failure AF ablation might be the right treatment, but is it every HFrEF patient with AF?” he wondered. “It’s nice to have more evidence so we can be more comfortable sending heart failure patients for ablation, but I want to see more information about the risk” from ablation in heart failure patients, “the sustainability of the effect, and the consequences of ablation.”
But the reservations expressed by cardiologists like Dr. Yancy contrasted with the views of colleagues who consider the current evidence much more convincing.
“It seems logical to look harder for AF” in heart failure patients, based on the accumulated evidence from CASTLE-AF and CABANA, said Dr. Ruskin. “I don’t think we can offer advice to heart failure physicians to screen their heart failure patients for AF, but if it’s seen I think we have some useful information on how to address it.”
CASTLE-AF was funded by Biotronik. CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and has received research funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude, and also from several other companies. Dr. Prystowsky as been a consultant to CardioNet and Medtronic, he has an equity interest in Stereotaxis, and he receives fellowship support from Medtronic and St. Jude. Dr. Marrouche has been a consultant to Biosense Webster, Biotronik, Boston Scientific, and St. Jude. He has received research support from Medtronic, and he has had financial relationships with several other companies. Dr. Ruskin has been a consultant to Biosense Webster and Medtronic and several other companies, has an ownership interest in Amgen, Cameron Health, InfoBionic, Newpace, Portola, and Regeneron, and has a fiduciary role in Pharmaco-Kinesis. Dr. Russo and Dr. Yancy had no disclosures. Dr. Brachmann has been a consultant to and has received research funding from Biotronik, Boston Scientific, St. Jude, and several other companies. Dr. Abraham has been a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Results from two recent trials suggest that cardiologists may have a new way to improve outcomes in patients with heart failure with reduced ejection fraction if they also have atrial fibrillation: Cut the patient’s atrial fibrillation burden with catheter ablation.
This seemingly off-target approach to improving survival, avoiding heart failure hospitalizations, and possibly reducing other adverse events first gained attention with results from the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) randomized trial, first reported in 2017. The study showed in 363 patients that atrial fibrillation (AF) ablation in patients with heart failure with reduced ejection fraction (HFrEF) led to a statistically significant 38% relative reduction in the primary endpoint of mortality or heart failure hospitalization during a median 38 months of follow-up (N Engl J Med. 2018 Feb 1;378[5]:417-27).
This groundbreaking finding then received some degree of confirmation when Douglas L. Packer, MD, reported primary results from CABANA (Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial) at the annual scientific sessions of the Heart Rhythm Society. CABANA compared upfront ablation against first-line medical management of AF in 2,203 patients. While the primary endpoint of the cumulative rate of all-cause death, disabling stroke, serious bleeding, or cardiac arrest over a median follow-up of just over 4 years was neutral, with no statistically significant difference between the two treatment arms, a subgroup analysis showed a tantalizing suggestion of benefit in the 337 enrolled patients with a history of congestive heart failure (15% of the total study group).
In this subgroup, treatment with ablation cut the primary endpoint by 39% relative to those treated upfront with medical management, an effect that came close to statistical significance. In addition, Dr. Packer took special note of the per-protocol analysis, which censored out the crossover patients who constituted roughly a fifth of all enrolled patients. In the subgroup analysis using the per-protocol data, ablation was linked with a statistically significant 49% relative reduction in the primary endpoint among patients with a history of heart failure.
The patients for whom there may be the quickest shift to upfront ablation to treat AF based on the CABANA results will be those with heart failure and others with high underlying risk, Dr. Packer predicted at the meeting.
“The CASTLE-AF results were interesting, but in fewer than 400 patients. Now we’ve basically seen the same thing” in CABANA, said Dr. Packer, professor and a cardiac electrophysiologist at the Mayo Clinic in Rochester, Minn.
Notably however, the results Dr. Packer reported on the heart failure subgroup did not include any information on how many of these were patients who had HFrEF or heart failure with preserved ejection fraction and how the apparent benefit from AF ablation affected each of these two heart failure types. In addition, the reported CABANA results did not have an endpoint result that completely matched the mortality and heart failure hospitalization composite endpoint used in CASTLE-AF. The closest endpoint that Dr. Packer reported from CABANA was a composite of mortality and cardiovascular hospitalization that showed, for the entire CABANA cohort, a statistically significant 17% relative reduction with ablation in the intention-to-treat analysis. Dr. Packer gave no data on how this outcome shook out in the subgroup of heart failure patients.
Despite these limitations, in trying to synthesize the CABANA and CASTLE-AF results, several electrophysiologists who heard the results agreed with Dr. Packer that the CABANA results confirmed the CASTLE-AF findings and helped strengthen the case for strongly considering AF ablation as first-line treatment in patients with heart failure.
“It’s clear that sinus rhythm is important in patients with heart failure. CASTLE-AF and now these results; that’s very strong to me,” said Eric N. Prystowsky, MD, a cardiac electrophysiologist with the St. Vincent Medical Group in Indianapolis and designated discussant for CABANA at the meeting.
“It’s confirmatory,” said Nassir F. Marrouche, MD, lead investigator for CASTLE-AF, and professor and director of the electrophysiology laboratory at the University of Utah in Salt Lake City.
The “signal” of benefit from AF ablation in heart failure patients in CABANA “replicates what was seen in CASTLE-AF. The results are highly consistent and very important regarding how to treat patients with AF and heart failure,” said Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “The data strongly suggest that catheter ablation is helpful for restoring and preserving [heart] muscle function,” Dr. Ruskin said in a video interview. He noted that AF occurs in at least about a quarter of heart failure patients.
Other cardiologists at the meeting noted that, on the basis of the CASTLE-AF results alone, they have already become more aggressive about treating AF with ablation in patients with heart failure in routine practice.
“It adds to the armamentarium for treatment of patients with heart failure,” said Johannes Brachmann, MD, professor and chief of cardiology at the Coburg (Germany) Clinic and a senior coinvestigator for CASTLE-AF.
William T. Abraham, MD, a heart failure specialist at The Ohio State University in Columbus, offered a broader perspective on where AF diagnosis, treatment, and ablation currently stand in U.S. heart failure practice.
“There is a very tight link between AF burden and worse outcomes in heart failure, so there is something intuitively appealing about restoring sinus rhythm in heart failure patients. I think most heart failure clinicians believe, like me, that heart failure patients with AF benefit from restoration of normal sinus rhythm. But I don’t believe that the CASTLE-AF results have so far had much impact on practice, in part because it was a relatively small study. The heart failure community is looking for some confirmation,” said Dr. Abraham, professor and director of cardiovascular medicine at Ohio State.
“I think the CABANA results are encouraging, but they came from only 15% of the enrolled patients who also had heart failure. CABANA adds to our knowledge, but I’m not sure it’s definitive for the heart failure population. I’m not sure it tells us if you treat patients with heart failure with anti-arrhythmia drugs and successfully maintain sinus rhythm do those patients do just as well as those who get ablated,” he said in an interview. “I’d love to see a study of heart failure patients maintained in sinus rhythm with drugs compared with those treated with ablation.”
For most patients with heart failure, the coexistence of AF is identified because of AF symptoms, or when asymptomatic AF is found in recordings made by an implanted cardiac device. “I’m more aggressive about addressing asymptomatic AF in my heart failure patients, and I believe the heart failure community is moving rapidly in that direction because of the association between higher AF burden and worse heart failure outcomes,” Dr. Abraham said.
A more cautious view came from another heart failure specialist, Clyde Yancy, MD, professor and chief of cardiology at Northwestern University in Chicago. “It’s pretty evident that in certain patients with heart failure AF ablation might be the right treatment, but is it every HFrEF patient with AF?” he wondered. “It’s nice to have more evidence so we can be more comfortable sending heart failure patients for ablation, but I want to see more information about the risk” from ablation in heart failure patients, “the sustainability of the effect, and the consequences of ablation.”
But the reservations expressed by cardiologists like Dr. Yancy contrasted with the views of colleagues who consider the current evidence much more convincing.
“It seems logical to look harder for AF” in heart failure patients, based on the accumulated evidence from CASTLE-AF and CABANA, said Dr. Ruskin. “I don’t think we can offer advice to heart failure physicians to screen their heart failure patients for AF, but if it’s seen I think we have some useful information on how to address it.”
CASTLE-AF was funded by Biotronik. CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and has received research funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude, and also from several other companies. Dr. Prystowsky as been a consultant to CardioNet and Medtronic, he has an equity interest in Stereotaxis, and he receives fellowship support from Medtronic and St. Jude. Dr. Marrouche has been a consultant to Biosense Webster, Biotronik, Boston Scientific, and St. Jude. He has received research support from Medtronic, and he has had financial relationships with several other companies. Dr. Ruskin has been a consultant to Biosense Webster and Medtronic and several other companies, has an ownership interest in Amgen, Cameron Health, InfoBionic, Newpace, Portola, and Regeneron, and has a fiduciary role in Pharmaco-Kinesis. Dr. Russo and Dr. Yancy had no disclosures. Dr. Brachmann has been a consultant to and has received research funding from Biotronik, Boston Scientific, St. Jude, and several other companies. Dr. Abraham has been a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Results from two recent trials suggest that cardiologists may have a new way to improve outcomes in patients with heart failure with reduced ejection fraction if they also have atrial fibrillation: Cut the patient’s atrial fibrillation burden with catheter ablation.
This seemingly off-target approach to improving survival, avoiding heart failure hospitalizations, and possibly reducing other adverse events first gained attention with results from the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) randomized trial, first reported in 2017. The study showed in 363 patients that atrial fibrillation (AF) ablation in patients with heart failure with reduced ejection fraction (HFrEF) led to a statistically significant 38% relative reduction in the primary endpoint of mortality or heart failure hospitalization during a median 38 months of follow-up (N Engl J Med. 2018 Feb 1;378[5]:417-27).
This groundbreaking finding then received some degree of confirmation when Douglas L. Packer, MD, reported primary results from CABANA (Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial) at the annual scientific sessions of the Heart Rhythm Society. CABANA compared upfront ablation against first-line medical management of AF in 2,203 patients. While the primary endpoint of the cumulative rate of all-cause death, disabling stroke, serious bleeding, or cardiac arrest over a median follow-up of just over 4 years was neutral, with no statistically significant difference between the two treatment arms, a subgroup analysis showed a tantalizing suggestion of benefit in the 337 enrolled patients with a history of congestive heart failure (15% of the total study group).
In this subgroup, treatment with ablation cut the primary endpoint by 39% relative to those treated upfront with medical management, an effect that came close to statistical significance. In addition, Dr. Packer took special note of the per-protocol analysis, which censored out the crossover patients who constituted roughly a fifth of all enrolled patients. In the subgroup analysis using the per-protocol data, ablation was linked with a statistically significant 49% relative reduction in the primary endpoint among patients with a history of heart failure.
The patients for whom there may be the quickest shift to upfront ablation to treat AF based on the CABANA results will be those with heart failure and others with high underlying risk, Dr. Packer predicted at the meeting.
“The CASTLE-AF results were interesting, but in fewer than 400 patients. Now we’ve basically seen the same thing” in CABANA, said Dr. Packer, professor and a cardiac electrophysiologist at the Mayo Clinic in Rochester, Minn.
Notably however, the results Dr. Packer reported on the heart failure subgroup did not include any information on how many of these were patients who had HFrEF or heart failure with preserved ejection fraction and how the apparent benefit from AF ablation affected each of these two heart failure types. In addition, the reported CABANA results did not have an endpoint result that completely matched the mortality and heart failure hospitalization composite endpoint used in CASTLE-AF. The closest endpoint that Dr. Packer reported from CABANA was a composite of mortality and cardiovascular hospitalization that showed, for the entire CABANA cohort, a statistically significant 17% relative reduction with ablation in the intention-to-treat analysis. Dr. Packer gave no data on how this outcome shook out in the subgroup of heart failure patients.
Despite these limitations, in trying to synthesize the CABANA and CASTLE-AF results, several electrophysiologists who heard the results agreed with Dr. Packer that the CABANA results confirmed the CASTLE-AF findings and helped strengthen the case for strongly considering AF ablation as first-line treatment in patients with heart failure.
“It’s clear that sinus rhythm is important in patients with heart failure. CASTLE-AF and now these results; that’s very strong to me,” said Eric N. Prystowsky, MD, a cardiac electrophysiologist with the St. Vincent Medical Group in Indianapolis and designated discussant for CABANA at the meeting.
“It’s confirmatory,” said Nassir F. Marrouche, MD, lead investigator for CASTLE-AF, and professor and director of the electrophysiology laboratory at the University of Utah in Salt Lake City.
The “signal” of benefit from AF ablation in heart failure patients in CABANA “replicates what was seen in CASTLE-AF. The results are highly consistent and very important regarding how to treat patients with AF and heart failure,” said Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “The data strongly suggest that catheter ablation is helpful for restoring and preserving [heart] muscle function,” Dr. Ruskin said in a video interview. He noted that AF occurs in at least about a quarter of heart failure patients.
Other cardiologists at the meeting noted that, on the basis of the CASTLE-AF results alone, they have already become more aggressive about treating AF with ablation in patients with heart failure in routine practice.
“It adds to the armamentarium for treatment of patients with heart failure,” said Johannes Brachmann, MD, professor and chief of cardiology at the Coburg (Germany) Clinic and a senior coinvestigator for CASTLE-AF.
William T. Abraham, MD, a heart failure specialist at The Ohio State University in Columbus, offered a broader perspective on where AF diagnosis, treatment, and ablation currently stand in U.S. heart failure practice.
“There is a very tight link between AF burden and worse outcomes in heart failure, so there is something intuitively appealing about restoring sinus rhythm in heart failure patients. I think most heart failure clinicians believe, like me, that heart failure patients with AF benefit from restoration of normal sinus rhythm. But I don’t believe that the CASTLE-AF results have so far had much impact on practice, in part because it was a relatively small study. The heart failure community is looking for some confirmation,” said Dr. Abraham, professor and director of cardiovascular medicine at Ohio State.
“I think the CABANA results are encouraging, but they came from only 15% of the enrolled patients who also had heart failure. CABANA adds to our knowledge, but I’m not sure it’s definitive for the heart failure population. I’m not sure it tells us if you treat patients with heart failure with anti-arrhythmia drugs and successfully maintain sinus rhythm do those patients do just as well as those who get ablated,” he said in an interview. “I’d love to see a study of heart failure patients maintained in sinus rhythm with drugs compared with those treated with ablation.”
For most patients with heart failure, the coexistence of AF is identified because of AF symptoms, or when asymptomatic AF is found in recordings made by an implanted cardiac device. “I’m more aggressive about addressing asymptomatic AF in my heart failure patients, and I believe the heart failure community is moving rapidly in that direction because of the association between higher AF burden and worse heart failure outcomes,” Dr. Abraham said.
A more cautious view came from another heart failure specialist, Clyde Yancy, MD, professor and chief of cardiology at Northwestern University in Chicago. “It’s pretty evident that in certain patients with heart failure AF ablation might be the right treatment, but is it every HFrEF patient with AF?” he wondered. “It’s nice to have more evidence so we can be more comfortable sending heart failure patients for ablation, but I want to see more information about the risk” from ablation in heart failure patients, “the sustainability of the effect, and the consequences of ablation.”
But the reservations expressed by cardiologists like Dr. Yancy contrasted with the views of colleagues who consider the current evidence much more convincing.
“It seems logical to look harder for AF” in heart failure patients, based on the accumulated evidence from CASTLE-AF and CABANA, said Dr. Ruskin. “I don’t think we can offer advice to heart failure physicians to screen their heart failure patients for AF, but if it’s seen I think we have some useful information on how to address it.”
CASTLE-AF was funded by Biotronik. CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and has received research funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude, and also from several other companies. Dr. Prystowsky as been a consultant to CardioNet and Medtronic, he has an equity interest in Stereotaxis, and he receives fellowship support from Medtronic and St. Jude. Dr. Marrouche has been a consultant to Biosense Webster, Biotronik, Boston Scientific, and St. Jude. He has received research support from Medtronic, and he has had financial relationships with several other companies. Dr. Ruskin has been a consultant to Biosense Webster and Medtronic and several other companies, has an ownership interest in Amgen, Cameron Health, InfoBionic, Newpace, Portola, and Regeneron, and has a fiduciary role in Pharmaco-Kinesis. Dr. Russo and Dr. Yancy had no disclosures. Dr. Brachmann has been a consultant to and has received research funding from Biotronik, Boston Scientific, St. Jude, and several other companies. Dr. Abraham has been a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude.
EXPERT ANALYSIS FROM HEART RHYTHM 2018
FDA approval expected for CCM in heart failure patients
BOSTON – Positive results from a confirmatory trial appear to put the Optimizer by Impulse Dynamics, a cardiac contractility modulation (CCM) device for patients with function-limiting heart failure, on track for imminent U.S. marketing approval by the Food and Drug Administration. If that happens, several hundreds of thousands of U.S. heart failure patients would immediately become candidates for this treatment based on the enrolled study populations, the benefits shown, and current treatment options for advanced heart failure, experts predicted.

CCM “promises to meet a very large unmet need in heart failure,” William T. Abraham, MD, said as he presented the confirmatory study’s results at the annual scientific sessions of the Heart Rhythm Society. ”These patients aren’t doing well, but don’t qualify” for a heart transplant, left ventricular assist device, implantable cardioverter defibrillator, or cardiac resynchronization therapy (CRT), noted Dr. Abraham, professor and director of cardiovascular medicine at the Ohio State University in Columbus. In the months following the anticipated FDA approval, Dr. Abraham said he expects the device will be implanted in tens of thousands of U.S. heart failure patients who match the criteria of those who got the biggest benefit from CCM.
“There are few if any evidence-based treatments for patients with an ejection fraction of 35%-45%. This is an underserved population, so the potential of CCM is appropriately high,” Dr. Abraham said.

Researchers designed the study in consultation with the FDA to resolve lingering regulatory concerns following completion of three prior randomized trials with a total of nearly 650 patients. Dr. Abraham simultaneously reported the results at the meeting and published them in a report (JACC Heart Failure. 2018 May 10. doi: 10.1016/j.jchf.2018.04.010); these results from 160 patients – 74 of whom received the device and 86 of whom continued medical therapy – showed the superiority of the device for the primary endpoint of change in exercise capacity (as measured by peak oxygen uptake) and for the secondary endpoints of quality of life (as measured with the Minnesota Living With Heart Failure Questionnaire) and functional status (as measured by New York Heart Association class). The boost in exercise capacity, an average increase of 0.84 ml/kg per min in peak oxygen uptake after 24 weeks, “was similar to the improvement seen with CRT in patients with a wide QRS interval” who thereby qualified for CRT placement, Dr. Abraham said.
The CCM device also met the study’s prespecified safety endpoint of a complication rate of less than 30% – with an actual rate of 10%. “The complications were those we expect from implanted leads and pulse generators and were comparable to what happens with other implanted rhythm devices. In the context of the benefits patients received and their having no other treatment options, I see the complication rate as acceptable,” Dr. Abraham said during his report.

In summing up the trial’s results, Dr. Stevenson noted that “the safety endpoint was met, the primary endpoint and other functional endpoints were met, and functional endpoints are of vital importance to patients. The CCM story is not yet the CRT story,” with CRT having produced even larger effects in its pivotal trial, also led by Dr. Abraham (New Engl J Med. 2002 June 13;346[24]:1845-53), cautioned Dr. Stevenson. But in general she put a positive spin on the CCM device, saying that it “has ingenuity and innovation, and we look forward to a better understanding of which patients benefit from CCM and what we can tell them about the magnitude and duration of the benefit.”

The FIX-HF-5C trial was sponsored by Impulse Dynamics, the company developing the CCM Optimizer device. Dr. Abraham has been a consultant to Impulse Dynamics, as well as to Abbott Vascular, Medtronic, Novartis, and St. Jude Medical. Dr. Singh has been a consultant to Biotronik, Boston Scientific, Liva Nova, Medtronic, and St. Jude. Dr. Stevenson has received research funding from Abbott and Novartis. Dr. Yancy had no disclosures.
SOURCE: Abraham W et al. Heart Rhythm 2018, Abstract B-LBCT01-02.
BOSTON – Positive results from a confirmatory trial appear to put the Optimizer by Impulse Dynamics, a cardiac contractility modulation (CCM) device for patients with function-limiting heart failure, on track for imminent U.S. marketing approval by the Food and Drug Administration. If that happens, several hundreds of thousands of U.S. heart failure patients would immediately become candidates for this treatment based on the enrolled study populations, the benefits shown, and current treatment options for advanced heart failure, experts predicted.

CCM “promises to meet a very large unmet need in heart failure,” William T. Abraham, MD, said as he presented the confirmatory study’s results at the annual scientific sessions of the Heart Rhythm Society. ”These patients aren’t doing well, but don’t qualify” for a heart transplant, left ventricular assist device, implantable cardioverter defibrillator, or cardiac resynchronization therapy (CRT), noted Dr. Abraham, professor and director of cardiovascular medicine at the Ohio State University in Columbus. In the months following the anticipated FDA approval, Dr. Abraham said he expects the device will be implanted in tens of thousands of U.S. heart failure patients who match the criteria of those who got the biggest benefit from CCM.
“There are few if any evidence-based treatments for patients with an ejection fraction of 35%-45%. This is an underserved population, so the potential of CCM is appropriately high,” Dr. Abraham said.

Researchers designed the study in consultation with the FDA to resolve lingering regulatory concerns following completion of three prior randomized trials with a total of nearly 650 patients. Dr. Abraham simultaneously reported the results at the meeting and published them in a report (JACC Heart Failure. 2018 May 10. doi: 10.1016/j.jchf.2018.04.010); these results from 160 patients – 74 of whom received the device and 86 of whom continued medical therapy – showed the superiority of the device for the primary endpoint of change in exercise capacity (as measured by peak oxygen uptake) and for the secondary endpoints of quality of life (as measured with the Minnesota Living With Heart Failure Questionnaire) and functional status (as measured by New York Heart Association class). The boost in exercise capacity, an average increase of 0.84 ml/kg per min in peak oxygen uptake after 24 weeks, “was similar to the improvement seen with CRT in patients with a wide QRS interval” who thereby qualified for CRT placement, Dr. Abraham said.
The CCM device also met the study’s prespecified safety endpoint of a complication rate of less than 30% – with an actual rate of 10%. “The complications were those we expect from implanted leads and pulse generators and were comparable to what happens with other implanted rhythm devices. In the context of the benefits patients received and their having no other treatment options, I see the complication rate as acceptable,” Dr. Abraham said during his report.

In summing up the trial’s results, Dr. Stevenson noted that “the safety endpoint was met, the primary endpoint and other functional endpoints were met, and functional endpoints are of vital importance to patients. The CCM story is not yet the CRT story,” with CRT having produced even larger effects in its pivotal trial, also led by Dr. Abraham (New Engl J Med. 2002 June 13;346[24]:1845-53), cautioned Dr. Stevenson. But in general she put a positive spin on the CCM device, saying that it “has ingenuity and innovation, and we look forward to a better understanding of which patients benefit from CCM and what we can tell them about the magnitude and duration of the benefit.”

The FIX-HF-5C trial was sponsored by Impulse Dynamics, the company developing the CCM Optimizer device. Dr. Abraham has been a consultant to Impulse Dynamics, as well as to Abbott Vascular, Medtronic, Novartis, and St. Jude Medical. Dr. Singh has been a consultant to Biotronik, Boston Scientific, Liva Nova, Medtronic, and St. Jude. Dr. Stevenson has received research funding from Abbott and Novartis. Dr. Yancy had no disclosures.
SOURCE: Abraham W et al. Heart Rhythm 2018, Abstract B-LBCT01-02.
BOSTON – Positive results from a confirmatory trial appear to put the Optimizer by Impulse Dynamics, a cardiac contractility modulation (CCM) device for patients with function-limiting heart failure, on track for imminent U.S. marketing approval by the Food and Drug Administration. If that happens, several hundreds of thousands of U.S. heart failure patients would immediately become candidates for this treatment based on the enrolled study populations, the benefits shown, and current treatment options for advanced heart failure, experts predicted.

CCM “promises to meet a very large unmet need in heart failure,” William T. Abraham, MD, said as he presented the confirmatory study’s results at the annual scientific sessions of the Heart Rhythm Society. ”These patients aren’t doing well, but don’t qualify” for a heart transplant, left ventricular assist device, implantable cardioverter defibrillator, or cardiac resynchronization therapy (CRT), noted Dr. Abraham, professor and director of cardiovascular medicine at the Ohio State University in Columbus. In the months following the anticipated FDA approval, Dr. Abraham said he expects the device will be implanted in tens of thousands of U.S. heart failure patients who match the criteria of those who got the biggest benefit from CCM.
“There are few if any evidence-based treatments for patients with an ejection fraction of 35%-45%. This is an underserved population, so the potential of CCM is appropriately high,” Dr. Abraham said.

Researchers designed the study in consultation with the FDA to resolve lingering regulatory concerns following completion of three prior randomized trials with a total of nearly 650 patients. Dr. Abraham simultaneously reported the results at the meeting and published them in a report (JACC Heart Failure. 2018 May 10. doi: 10.1016/j.jchf.2018.04.010); these results from 160 patients – 74 of whom received the device and 86 of whom continued medical therapy – showed the superiority of the device for the primary endpoint of change in exercise capacity (as measured by peak oxygen uptake) and for the secondary endpoints of quality of life (as measured with the Minnesota Living With Heart Failure Questionnaire) and functional status (as measured by New York Heart Association class). The boost in exercise capacity, an average increase of 0.84 ml/kg per min in peak oxygen uptake after 24 weeks, “was similar to the improvement seen with CRT in patients with a wide QRS interval” who thereby qualified for CRT placement, Dr. Abraham said.
The CCM device also met the study’s prespecified safety endpoint of a complication rate of less than 30% – with an actual rate of 10%. “The complications were those we expect from implanted leads and pulse generators and were comparable to what happens with other implanted rhythm devices. In the context of the benefits patients received and their having no other treatment options, I see the complication rate as acceptable,” Dr. Abraham said during his report.

In summing up the trial’s results, Dr. Stevenson noted that “the safety endpoint was met, the primary endpoint and other functional endpoints were met, and functional endpoints are of vital importance to patients. The CCM story is not yet the CRT story,” with CRT having produced even larger effects in its pivotal trial, also led by Dr. Abraham (New Engl J Med. 2002 June 13;346[24]:1845-53), cautioned Dr. Stevenson. But in general she put a positive spin on the CCM device, saying that it “has ingenuity and innovation, and we look forward to a better understanding of which patients benefit from CCM and what we can tell them about the magnitude and duration of the benefit.”

The FIX-HF-5C trial was sponsored by Impulse Dynamics, the company developing the CCM Optimizer device. Dr. Abraham has been a consultant to Impulse Dynamics, as well as to Abbott Vascular, Medtronic, Novartis, and St. Jude Medical. Dr. Singh has been a consultant to Biotronik, Boston Scientific, Liva Nova, Medtronic, and St. Jude. Dr. Stevenson has received research funding from Abbott and Novartis. Dr. Yancy had no disclosures.
SOURCE: Abraham W et al. Heart Rhythm 2018, Abstract B-LBCT01-02.
REPORTING FROM HEART RHYTHM 2018
VIDEO: CASTLE-AF suggests atrial fibrillation burden better predicts outcomes
BOSTON – From the earliest days of using catheter ablation to treat atrial fibrillation (AF), in the 1990s, clinicians have defined ablation success based on whether patients had recurrence of their arrhythmia following treatment. New findings suggest that this standard was off, and that
The new study used data collected in the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) multicenter trial, which compared the efficacy of AF ablation with antiarrhythmic drug treatment in patients with heart failure for improving survival and freedom from hospitalization for heart failure. The trial’s primary finding showed that, in 363 randomized patients, AF ablation cut the primary adverse event rate by 38% relative to antiarrhythmic drug therapy (New Engl J Med. 2018 Feb 1;378[5]:417-27)
“There have been concerns about the high recurrence rate of AF following ablation,” with reported cumulative recurrence rates running as high as 80% by 5 years after ablation, noted Dr. Brachmann. “The news now is that recurrence alone doesn’t make a difference; we can still help patients” by reducing their AF burden, although he cautioned that this relationship has so far only been seen in patients with heart failure with reduced ejection fraction, the type of patients enrolled in CASTLE-AF.
“This information is very informative for clinicians counseling patients who undergo ablation. Ablation may not eliminate all of a patient’s AF, but it will substantially reduce it, and that’s associated with better outcomes,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver. “Early on using ablation, we had a curative approach and used ablation to ‘clip the wire.’ Now we have growing, objective evidence for ‘debulking’ the problem” working without the need to completely eliminate all AF episodes.
To run the post hoc analysis Dr. Brachmann and his associates categorized the 363 patients randomized in CASTLE-AF by the treatment they received during the study’s first 12 weeks: 150 patients underwent catheter ablation, and 210 received drug treatment, with three patients dropping out. Although this division of the patients diverged from the randomized subgroups, the ablated and drug-treated patients showed no significant differences when compared for several clinical parameters.
Ablation was significantly more effective than drug therapy for cutting atrial fibrillation burden, which started at an average of about 50% in all patients at baseline. AF burden fell to an average of about 10%-15% among the ablated patients when measured at several time points during follow-up, whereas AF burden remained at an average of about 50% or higher among the drug-treated patients.
A receiver operating characteristic analysis showed that change in AF burden after ablation produced a statistically significant 0.66 area-under-the-curve for the primary endpoint, which suggested that reduction in AF burden post ablation could account for about two-thirds of the drop in deaths and hospitalizations for heart failure. Among the nonablated patients the area-under-the-curve was an insignificant 0.49 showing that with drug treatment AF burden had no discernible relationship with outcomes.
One further observation in the new analysis was that a drop in AF burden was linked with improved outcomes regardless of whether or not a “blanking period” was imposed on the data. Researchers applied a 90-day blanking period after ablation when assessing the treatment’s efficacy to censor from the analysis recurrences that occurred soon after ablation. The need for a blanking period during the first 90 days “was put to rest” by this new analysis, Dr. Brachmann said.
CASTLE-AF was sponsored by Biotronik. Dr. Brachmann has been a consultant to and has received research funding from Biotronik and several other companies. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific. Dr. Link had no disclosures.
SOURCE: Brachmann J et al. Heart Rhythm 2018, Abstract B-LBCT02-04.
This new analysis of data from the CASTLE-AF trial is exciting. It shows that, if we reduce the atrial fibrillation burden when we perform catheter ablation of atrial fibrillation in patients with heart failure, patients do better.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Until now, cardiac electrophysiologists who perform atrial fibrillation (AF) ablation have been too hard on themselves by counting as a failure every patient who develops an AF recurrence that lasts for 30 seconds or more. We know that patients who have a substantial drop in their AF burden after catheter ablation report feeling better even if they continue to have some AF events. When their AF burden drops substantially, patients are better able to work and perform activities of daily life. Many options, including noninvasive devices, are now available to monitor patients’ postablation change in AF burden.
We currently tell patients the success rates of catheter ablation on AF based on recurrence rates. Maybe we need to change our definition of success to a cut in AF burden. Based on these new findings, patients don’t need to be perfect after ablation, with absolutely no recurrences. I have patients who are very happy with their outcome after ablation who still have episodes. The success rate of catheter ablation for treating AF may be much better than we have thought.
Andrea M. Russo, MD , is professor and director of the electrophysiology and arrhythmia service at Cooper University Health Care in Camden, N.J. She made these comments during a press conference and in a video interview. She had no relevant disclosures.
This new analysis of data from the CASTLE-AF trial is exciting. It shows that, if we reduce the atrial fibrillation burden when we perform catheter ablation of atrial fibrillation in patients with heart failure, patients do better.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Until now, cardiac electrophysiologists who perform atrial fibrillation (AF) ablation have been too hard on themselves by counting as a failure every patient who develops an AF recurrence that lasts for 30 seconds or more. We know that patients who have a substantial drop in their AF burden after catheter ablation report feeling better even if they continue to have some AF events. When their AF burden drops substantially, patients are better able to work and perform activities of daily life. Many options, including noninvasive devices, are now available to monitor patients’ postablation change in AF burden.
We currently tell patients the success rates of catheter ablation on AF based on recurrence rates. Maybe we need to change our definition of success to a cut in AF burden. Based on these new findings, patients don’t need to be perfect after ablation, with absolutely no recurrences. I have patients who are very happy with their outcome after ablation who still have episodes. The success rate of catheter ablation for treating AF may be much better than we have thought.
Andrea M. Russo, MD , is professor and director of the electrophysiology and arrhythmia service at Cooper University Health Care in Camden, N.J. She made these comments during a press conference and in a video interview. She had no relevant disclosures.
This new analysis of data from the CASTLE-AF trial is exciting. It shows that, if we reduce the atrial fibrillation burden when we perform catheter ablation of atrial fibrillation in patients with heart failure, patients do better.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Until now, cardiac electrophysiologists who perform atrial fibrillation (AF) ablation have been too hard on themselves by counting as a failure every patient who develops an AF recurrence that lasts for 30 seconds or more. We know that patients who have a substantial drop in their AF burden after catheter ablation report feeling better even if they continue to have some AF events. When their AF burden drops substantially, patients are better able to work and perform activities of daily life. Many options, including noninvasive devices, are now available to monitor patients’ postablation change in AF burden.
We currently tell patients the success rates of catheter ablation on AF based on recurrence rates. Maybe we need to change our definition of success to a cut in AF burden. Based on these new findings, patients don’t need to be perfect after ablation, with absolutely no recurrences. I have patients who are very happy with their outcome after ablation who still have episodes. The success rate of catheter ablation for treating AF may be much better than we have thought.
Andrea M. Russo, MD , is professor and director of the electrophysiology and arrhythmia service at Cooper University Health Care in Camden, N.J. She made these comments during a press conference and in a video interview. She had no relevant disclosures.
BOSTON – From the earliest days of using catheter ablation to treat atrial fibrillation (AF), in the 1990s, clinicians have defined ablation success based on whether patients had recurrence of their arrhythmia following treatment. New findings suggest that this standard was off, and that
The new study used data collected in the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) multicenter trial, which compared the efficacy of AF ablation with antiarrhythmic drug treatment in patients with heart failure for improving survival and freedom from hospitalization for heart failure. The trial’s primary finding showed that, in 363 randomized patients, AF ablation cut the primary adverse event rate by 38% relative to antiarrhythmic drug therapy (New Engl J Med. 2018 Feb 1;378[5]:417-27)
“There have been concerns about the high recurrence rate of AF following ablation,” with reported cumulative recurrence rates running as high as 80% by 5 years after ablation, noted Dr. Brachmann. “The news now is that recurrence alone doesn’t make a difference; we can still help patients” by reducing their AF burden, although he cautioned that this relationship has so far only been seen in patients with heart failure with reduced ejection fraction, the type of patients enrolled in CASTLE-AF.
“This information is very informative for clinicians counseling patients who undergo ablation. Ablation may not eliminate all of a patient’s AF, but it will substantially reduce it, and that’s associated with better outcomes,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver. “Early on using ablation, we had a curative approach and used ablation to ‘clip the wire.’ Now we have growing, objective evidence for ‘debulking’ the problem” working without the need to completely eliminate all AF episodes.
To run the post hoc analysis Dr. Brachmann and his associates categorized the 363 patients randomized in CASTLE-AF by the treatment they received during the study’s first 12 weeks: 150 patients underwent catheter ablation, and 210 received drug treatment, with three patients dropping out. Although this division of the patients diverged from the randomized subgroups, the ablated and drug-treated patients showed no significant differences when compared for several clinical parameters.
Ablation was significantly more effective than drug therapy for cutting atrial fibrillation burden, which started at an average of about 50% in all patients at baseline. AF burden fell to an average of about 10%-15% among the ablated patients when measured at several time points during follow-up, whereas AF burden remained at an average of about 50% or higher among the drug-treated patients.
A receiver operating characteristic analysis showed that change in AF burden after ablation produced a statistically significant 0.66 area-under-the-curve for the primary endpoint, which suggested that reduction in AF burden post ablation could account for about two-thirds of the drop in deaths and hospitalizations for heart failure. Among the nonablated patients the area-under-the-curve was an insignificant 0.49 showing that with drug treatment AF burden had no discernible relationship with outcomes.
One further observation in the new analysis was that a drop in AF burden was linked with improved outcomes regardless of whether or not a “blanking period” was imposed on the data. Researchers applied a 90-day blanking period after ablation when assessing the treatment’s efficacy to censor from the analysis recurrences that occurred soon after ablation. The need for a blanking period during the first 90 days “was put to rest” by this new analysis, Dr. Brachmann said.
CASTLE-AF was sponsored by Biotronik. Dr. Brachmann has been a consultant to and has received research funding from Biotronik and several other companies. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific. Dr. Link had no disclosures.
SOURCE: Brachmann J et al. Heart Rhythm 2018, Abstract B-LBCT02-04.
BOSTON – From the earliest days of using catheter ablation to treat atrial fibrillation (AF), in the 1990s, clinicians have defined ablation success based on whether patients had recurrence of their arrhythmia following treatment. New findings suggest that this standard was off, and that
The new study used data collected in the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) multicenter trial, which compared the efficacy of AF ablation with antiarrhythmic drug treatment in patients with heart failure for improving survival and freedom from hospitalization for heart failure. The trial’s primary finding showed that, in 363 randomized patients, AF ablation cut the primary adverse event rate by 38% relative to antiarrhythmic drug therapy (New Engl J Med. 2018 Feb 1;378[5]:417-27)
“There have been concerns about the high recurrence rate of AF following ablation,” with reported cumulative recurrence rates running as high as 80% by 5 years after ablation, noted Dr. Brachmann. “The news now is that recurrence alone doesn’t make a difference; we can still help patients” by reducing their AF burden, although he cautioned that this relationship has so far only been seen in patients with heart failure with reduced ejection fraction, the type of patients enrolled in CASTLE-AF.
“This information is very informative for clinicians counseling patients who undergo ablation. Ablation may not eliminate all of a patient’s AF, but it will substantially reduce it, and that’s associated with better outcomes,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver. “Early on using ablation, we had a curative approach and used ablation to ‘clip the wire.’ Now we have growing, objective evidence for ‘debulking’ the problem” working without the need to completely eliminate all AF episodes.
To run the post hoc analysis Dr. Brachmann and his associates categorized the 363 patients randomized in CASTLE-AF by the treatment they received during the study’s first 12 weeks: 150 patients underwent catheter ablation, and 210 received drug treatment, with three patients dropping out. Although this division of the patients diverged from the randomized subgroups, the ablated and drug-treated patients showed no significant differences when compared for several clinical parameters.
Ablation was significantly more effective than drug therapy for cutting atrial fibrillation burden, which started at an average of about 50% in all patients at baseline. AF burden fell to an average of about 10%-15% among the ablated patients when measured at several time points during follow-up, whereas AF burden remained at an average of about 50% or higher among the drug-treated patients.
A receiver operating characteristic analysis showed that change in AF burden after ablation produced a statistically significant 0.66 area-under-the-curve for the primary endpoint, which suggested that reduction in AF burden post ablation could account for about two-thirds of the drop in deaths and hospitalizations for heart failure. Among the nonablated patients the area-under-the-curve was an insignificant 0.49 showing that with drug treatment AF burden had no discernible relationship with outcomes.
One further observation in the new analysis was that a drop in AF burden was linked with improved outcomes regardless of whether or not a “blanking period” was imposed on the data. Researchers applied a 90-day blanking period after ablation when assessing the treatment’s efficacy to censor from the analysis recurrences that occurred soon after ablation. The need for a blanking period during the first 90 days “was put to rest” by this new analysis, Dr. Brachmann said.
CASTLE-AF was sponsored by Biotronik. Dr. Brachmann has been a consultant to and has received research funding from Biotronik and several other companies. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific. Dr. Link had no disclosures.
SOURCE: Brachmann J et al. Heart Rhythm 2018, Abstract B-LBCT02-04.
REPORTING FROM HEART RHYTHM 2018
Key clinical point: Higher atrial fibrillation (AF) burden was more important than AF recurrence for predicting bad outcomes post ablation.
Major finding: Patients whose AF burden fell to 5% or less had about a threefold higher rate of good outcomes, compared with other patients.
Study details: Post hoc analysis of 360 patients with heart failure and AF enrolled in CASTLE-AF, a multicenter, randomized trial.
Disclosures: CASTLE-AF was sponsored by Biotronik. Dr. Brachmann has been a consultant to and has received research funding from Biotronik and several other companies. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific. Dr. Link had no disclosures.
Source: Brachmann J et al. Heart Rhythm 2018, Abstract B-LBCT02-04.
Device-related thrombus associated with ischemic events
BOSTON – Device-related thrombus (DRT) does not occur often after left atrial appendage closure with the Watchman device. When it does, however, it is associated with a significantly higher rate of stroke and systemic embolism compared with that of patients with no DRT, according to a recent analysis presented at the annual scientific sessions of the Heart Rhythm Society.
Given the negative implications of DRT, a judicious surveillance strategy should be considered, especially when DRT risk factors are present, investigators said in a report on the study, which was published simultaneously in Circulation.
“Certainly, DRT is associated with an increased risk of stroke, and therapeutic anticoagulation should be resumed when discovered with rigorous transesophageal echocardiography follow-up to ensure resolution,” noted senior investigator Vivek Y. Reddy, MD.
Despite the higher rates of all strokes, ischemic strokes, and hemorrhagic strokes linked with DRT, the complication did not link with a higher rate of all-cause mortality compared with patients who never had a DRT. The results also suggested a causal link between DRT and subsequent stroke in about half the patients with DRT because their strokes occurred within a month following DRT diagnosis.
Despite these findings, a majority – 74% – of patients with an identified DRT did not have a stroke, and 87% of the strokes that occurred in the patients who received the Watchman device occurred in the absence of a DRT, reported Dr. Reddy, who presented the findings at the meeting.
The most immediate implication of the findings is the strong case they make for rethinking the timing of planned follow-up transesophageal echocardiography (TEE) examinations of patients after they receive a Watchman device. The current, standard protocol schedules a TEE at 45 days after Watchman placement, when routine anticoagulation usually stops, and then a second TEE 12 months after placement. A better schedule might be to perform the first TEE 3-4 months after Watchman placement to give a potential DRT time to form once oral anticoagulant therapy stops, suggested Dr. Reddy, professor and director of the cardiac arrhythmia service at Mount Sinai Hospital and Health System in New York.
“Surveillance is very important. I don’t think DRT usually occurs unless anticoagulation is suboptimal or stops.” Dr. Reddy noted that he and his associates are analyzing the best time for TEE surveillance in other large databases of patients treated with left atrial appendage (LAA) closure. Newer models of LAA closure devices structurally modified to reduce thrombus formation are nearing clinical use, he added.
The analysis, believed to be the largest to date of DRT following left atrial appendage closure, was based on prospective data from four clinical trials. That included two randomized controlled trials, PROTECT AF and PREVAIL, as well as the CAP and CAP2 prospective registries.
Among 1,739 patients in those studies receiving an implant, 65 (3.74%) had DRT, the investigators found.
Over 1 year of follow-up, 25% of patients with DRT had an ischemic stroke or systemic embolism, versus 6.8% of patients without DRTs (P less than .001), they reported. That worked out to an event rate of 6.28 and 1.65 events per 100 patient years, respectively.
The strongest predictors of DRT in multivariable analysis included vascular disease, history of stroke or transient ischemic attack, permanent atrial fibrillation, and left atrial appendage diameter, according to the report. Conversely, increasing left ventricular ejection fraction was protective against DRT.
Taken together, these data support reevaluating the transesophageal echocardiography strategy, according to Dr. Reddy and his coauthors. Those approaches might include targeting patients with DRT risk factors, routine additional surveillance at 6 months, or delaying the first transesophageal echocardiography to 4 months.
“Importantly, none of these strategies have been rigorously compared, so these suggestions are subject to future studies,” the researchers wrote.
“DRT remains a problem despite increased operator experience with LAA occlusion and improved occluding devices,” commented David B. De Lurgio, MD, a cardiac electrophysiologist at Emory Healthcare in Atlanta. “What is a little alarming is that the risk for DRT extends beyond the period of prescribed anticoagulation. Although the risk from DRT mitigates the benefit of LAA closure compared with warfarin, it does not mitigate the benefit from occlusion compared with no treatment,” said Dr. De Lurgio, designated discussant for the report. “Prevention and management of DRT may require that each patient receive a tailored regimen of anticoagulation and surveillance.”
The Watchman studies were funded by Boston Scientific, the company that markets the device. Dr. Reddy has been a consultant to and has received research funding from Boston Scientific and from Abbott and Biosense-Webster, and he reported having an equity interest in Javelin and Surecor. Coauthors reported disclosures related to Boston Scientific, Johnson & Johnson, Abbott, and other medical device companies. Dr. De Lurgio has been a consultant to Boston Scientific.
Updated, 5/17/18: This article has been updated with reporting from Mitchel L. Zoler at the meeting, and has been revised for clarity and to reflect that the results were presented by Dr. Reddy.
SOURCE: Dukkipati SR et al. Circulation. 2018 May 11. doi: 10.1161/CIRCULATIONAHA.118.035090.
BOSTON – Device-related thrombus (DRT) does not occur often after left atrial appendage closure with the Watchman device. When it does, however, it is associated with a significantly higher rate of stroke and systemic embolism compared with that of patients with no DRT, according to a recent analysis presented at the annual scientific sessions of the Heart Rhythm Society.
Given the negative implications of DRT, a judicious surveillance strategy should be considered, especially when DRT risk factors are present, investigators said in a report on the study, which was published simultaneously in Circulation.
“Certainly, DRT is associated with an increased risk of stroke, and therapeutic anticoagulation should be resumed when discovered with rigorous transesophageal echocardiography follow-up to ensure resolution,” noted senior investigator Vivek Y. Reddy, MD.
Despite the higher rates of all strokes, ischemic strokes, and hemorrhagic strokes linked with DRT, the complication did not link with a higher rate of all-cause mortality compared with patients who never had a DRT. The results also suggested a causal link between DRT and subsequent stroke in about half the patients with DRT because their strokes occurred within a month following DRT diagnosis.
Despite these findings, a majority – 74% – of patients with an identified DRT did not have a stroke, and 87% of the strokes that occurred in the patients who received the Watchman device occurred in the absence of a DRT, reported Dr. Reddy, who presented the findings at the meeting.
The most immediate implication of the findings is the strong case they make for rethinking the timing of planned follow-up transesophageal echocardiography (TEE) examinations of patients after they receive a Watchman device. The current, standard protocol schedules a TEE at 45 days after Watchman placement, when routine anticoagulation usually stops, and then a second TEE 12 months after placement. A better schedule might be to perform the first TEE 3-4 months after Watchman placement to give a potential DRT time to form once oral anticoagulant therapy stops, suggested Dr. Reddy, professor and director of the cardiac arrhythmia service at Mount Sinai Hospital and Health System in New York.
“Surveillance is very important. I don’t think DRT usually occurs unless anticoagulation is suboptimal or stops.” Dr. Reddy noted that he and his associates are analyzing the best time for TEE surveillance in other large databases of patients treated with left atrial appendage (LAA) closure. Newer models of LAA closure devices structurally modified to reduce thrombus formation are nearing clinical use, he added.
The analysis, believed to be the largest to date of DRT following left atrial appendage closure, was based on prospective data from four clinical trials. That included two randomized controlled trials, PROTECT AF and PREVAIL, as well as the CAP and CAP2 prospective registries.
Among 1,739 patients in those studies receiving an implant, 65 (3.74%) had DRT, the investigators found.
Over 1 year of follow-up, 25% of patients with DRT had an ischemic stroke or systemic embolism, versus 6.8% of patients without DRTs (P less than .001), they reported. That worked out to an event rate of 6.28 and 1.65 events per 100 patient years, respectively.
The strongest predictors of DRT in multivariable analysis included vascular disease, history of stroke or transient ischemic attack, permanent atrial fibrillation, and left atrial appendage diameter, according to the report. Conversely, increasing left ventricular ejection fraction was protective against DRT.
Taken together, these data support reevaluating the transesophageal echocardiography strategy, according to Dr. Reddy and his coauthors. Those approaches might include targeting patients with DRT risk factors, routine additional surveillance at 6 months, or delaying the first transesophageal echocardiography to 4 months.
“Importantly, none of these strategies have been rigorously compared, so these suggestions are subject to future studies,” the researchers wrote.
“DRT remains a problem despite increased operator experience with LAA occlusion and improved occluding devices,” commented David B. De Lurgio, MD, a cardiac electrophysiologist at Emory Healthcare in Atlanta. “What is a little alarming is that the risk for DRT extends beyond the period of prescribed anticoagulation. Although the risk from DRT mitigates the benefit of LAA closure compared with warfarin, it does not mitigate the benefit from occlusion compared with no treatment,” said Dr. De Lurgio, designated discussant for the report. “Prevention and management of DRT may require that each patient receive a tailored regimen of anticoagulation and surveillance.”
The Watchman studies were funded by Boston Scientific, the company that markets the device. Dr. Reddy has been a consultant to and has received research funding from Boston Scientific and from Abbott and Biosense-Webster, and he reported having an equity interest in Javelin and Surecor. Coauthors reported disclosures related to Boston Scientific, Johnson & Johnson, Abbott, and other medical device companies. Dr. De Lurgio has been a consultant to Boston Scientific.
Updated, 5/17/18: This article has been updated with reporting from Mitchel L. Zoler at the meeting, and has been revised for clarity and to reflect that the results were presented by Dr. Reddy.
SOURCE: Dukkipati SR et al. Circulation. 2018 May 11. doi: 10.1161/CIRCULATIONAHA.118.035090.
BOSTON – Device-related thrombus (DRT) does not occur often after left atrial appendage closure with the Watchman device. When it does, however, it is associated with a significantly higher rate of stroke and systemic embolism compared with that of patients with no DRT, according to a recent analysis presented at the annual scientific sessions of the Heart Rhythm Society.
Given the negative implications of DRT, a judicious surveillance strategy should be considered, especially when DRT risk factors are present, investigators said in a report on the study, which was published simultaneously in Circulation.
“Certainly, DRT is associated with an increased risk of stroke, and therapeutic anticoagulation should be resumed when discovered with rigorous transesophageal echocardiography follow-up to ensure resolution,” noted senior investigator Vivek Y. Reddy, MD.
Despite the higher rates of all strokes, ischemic strokes, and hemorrhagic strokes linked with DRT, the complication did not link with a higher rate of all-cause mortality compared with patients who never had a DRT. The results also suggested a causal link between DRT and subsequent stroke in about half the patients with DRT because their strokes occurred within a month following DRT diagnosis.
Despite these findings, a majority – 74% – of patients with an identified DRT did not have a stroke, and 87% of the strokes that occurred in the patients who received the Watchman device occurred in the absence of a DRT, reported Dr. Reddy, who presented the findings at the meeting.
The most immediate implication of the findings is the strong case they make for rethinking the timing of planned follow-up transesophageal echocardiography (TEE) examinations of patients after they receive a Watchman device. The current, standard protocol schedules a TEE at 45 days after Watchman placement, when routine anticoagulation usually stops, and then a second TEE 12 months after placement. A better schedule might be to perform the first TEE 3-4 months after Watchman placement to give a potential DRT time to form once oral anticoagulant therapy stops, suggested Dr. Reddy, professor and director of the cardiac arrhythmia service at Mount Sinai Hospital and Health System in New York.
“Surveillance is very important. I don’t think DRT usually occurs unless anticoagulation is suboptimal or stops.” Dr. Reddy noted that he and his associates are analyzing the best time for TEE surveillance in other large databases of patients treated with left atrial appendage (LAA) closure. Newer models of LAA closure devices structurally modified to reduce thrombus formation are nearing clinical use, he added.
The analysis, believed to be the largest to date of DRT following left atrial appendage closure, was based on prospective data from four clinical trials. That included two randomized controlled trials, PROTECT AF and PREVAIL, as well as the CAP and CAP2 prospective registries.
Among 1,739 patients in those studies receiving an implant, 65 (3.74%) had DRT, the investigators found.
Over 1 year of follow-up, 25% of patients with DRT had an ischemic stroke or systemic embolism, versus 6.8% of patients without DRTs (P less than .001), they reported. That worked out to an event rate of 6.28 and 1.65 events per 100 patient years, respectively.
The strongest predictors of DRT in multivariable analysis included vascular disease, history of stroke or transient ischemic attack, permanent atrial fibrillation, and left atrial appendage diameter, according to the report. Conversely, increasing left ventricular ejection fraction was protective against DRT.
Taken together, these data support reevaluating the transesophageal echocardiography strategy, according to Dr. Reddy and his coauthors. Those approaches might include targeting patients with DRT risk factors, routine additional surveillance at 6 months, or delaying the first transesophageal echocardiography to 4 months.
“Importantly, none of these strategies have been rigorously compared, so these suggestions are subject to future studies,” the researchers wrote.
“DRT remains a problem despite increased operator experience with LAA occlusion and improved occluding devices,” commented David B. De Lurgio, MD, a cardiac electrophysiologist at Emory Healthcare in Atlanta. “What is a little alarming is that the risk for DRT extends beyond the period of prescribed anticoagulation. Although the risk from DRT mitigates the benefit of LAA closure compared with warfarin, it does not mitigate the benefit from occlusion compared with no treatment,” said Dr. De Lurgio, designated discussant for the report. “Prevention and management of DRT may require that each patient receive a tailored regimen of anticoagulation and surveillance.”
The Watchman studies were funded by Boston Scientific, the company that markets the device. Dr. Reddy has been a consultant to and has received research funding from Boston Scientific and from Abbott and Biosense-Webster, and he reported having an equity interest in Javelin and Surecor. Coauthors reported disclosures related to Boston Scientific, Johnson & Johnson, Abbott, and other medical device companies. Dr. De Lurgio has been a consultant to Boston Scientific.
Updated, 5/17/18: This article has been updated with reporting from Mitchel L. Zoler at the meeting, and has been revised for clarity and to reflect that the results were presented by Dr. Reddy.
SOURCE: Dukkipati SR et al. Circulation. 2018 May 11. doi: 10.1161/CIRCULATIONAHA.118.035090.
REPORTING FROM HEART RHYTHM 2018
Key clinical point: Device-related thrombus (DRT) following left atrial appendage closure occurs in less than 4% of patients but is associated with a higher rate of stroke and systemic embolism vs. no DRT.
Major finding: The rate of ischemic stroke and systemic embolism was 6.28 and 1.65 per 100 patient years for patients with DRT and with no DRT, respectively (P less than .001).
Study details: Analysis of data from the device arms of four prospective clinical trials.
Disclosures: The Watchman studies were funded by Boston Scientific, the company that markets the device. Dr. Reddy has been a consultant to and has received research funding from Boston Scientific and from Abbott and Biosense-Webster, and he reported having an equity interest in Javelin and Surecor. Coauthors reported disclosures related to Boston Scientific, Johnson & Johnson, Abbott, and other medical device companies. Dr. De Lurgio has been a consultant to Boston Scientific.
Source: Dukkipati SR et al. Circulation. 2018 May 11. doi: 10.1161/CIRCULATIONAHA.118.035090.
CABANA: AF ablation ties drug management, with an asterisk for crossovers
BOSTON – Results from the CABANA trial, the long-awaited, head-to-head comparison of percutaneous catheter ablation with drug therapy for the treatment of atrial fibrillation by restoring sinus rhythm, failed to accomplish what it was designed to prove.
That is, that catheter ablation was superior to medical management for a combined endpoint of all-cause death, stroke, serious bleeding, or cardiac arrest.
The trial results also gave proponents of catheter ablation some tantalizing hints that this approach actually may have been superior to antiarrhythmic drugs, if only the randomization assignments had been more closely followed as the trial proceeded. But that didn’t happen, with about 30% of patients assigned to medical management crossing over to undergo catheter ablation, presumably because they had received inadequate symptom relief from their drug regimens. In addition, 10% of patients assigned to catheter ablation didn’t undergo it, primarily because they reconsidered after randomization and decided to not choose the invasive option. These crossovers produced a disparity in the outcomes between the standard, intention-to-treat analysis, which showed a neutral difference between the two study arms, and the per-protocol analysis that censored out crossover patients. The per-protocol analysis showed a statistically significant, 27% relative risk reduction in the primary endpoint among the patients randomized to and actually treated with catheter ablation, compared with those randomized to and exclusively treated medically.
“A patient can’t receive benefit from ablation if you don’t ablate,” noted the study’s lead investigation, Douglas L. Packer, MD, as he reported the results at the annual scientific sessions of the Heart Rhythm Society. “When you have this many crossovers and so many patients not getting their assigned treatments, then an on-treatment analysis is required, said Dr. Packer, a cardiac electrophysiologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
The prespecified on-treatment analysis, which, instead of censoring crossover patients, analyzed outcomes based on the treatments that patients actually received, showed a statistically significant one-third reduction in the primary endpoint among the ablated patients and a statistically significant 40% relative reduction in all-cause mortality in the ablated arm, compared with those on medical management.
“For symptomatic treatment, and to restore and maintain sinus rhythm, there is no question that ablation is better. We knew that before this trial, and we know it even more convincingly now,” commented Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “To a large extent, we do ablations for symptomatic benefit; to get patients feeling better. And I think this trial will confirm that because this will likely follow the better reduction in atrial fibrillation burden, which was quite impressive in the study.” Dr. Packer said that the quality of life data collected in CABANA will come out in a report later in 2018.
The dilemma that Dr. Ruskin and other physicians who heard the results voiced was how best to interpret the study’s primary results.
“This trial was designed to address whether ablation has an impact [compared with medical management] on hard endpoints, like mortality, and the intention-to-treat analysis showed no difference. I feel bound to adhere to the intention-to-treat analysis, the primary result” the traditional default arbiter of a randomized trial’s outcome, Dr. Ruskin said in an interview, “But intention-to-treat analyses are built on a foundation where most patients are maintained on their assigned treatment.”
The results “tell us that there wasn’t harm from ablation,” Dr. Albert said during a press conference. “That is really important because, before this, we didn’t know for sure. These data make me a little more confident about offering patients ablation. I now have data to discuss with patients that’s useful for decision making.”
“There was certainly no signal whatsoever of harm by taking patients to ablation early” in their management, agreed Dr. Ruskin. “I find that very reassuring and encouraging.”
CABANA (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial) started in 2009 and enrolled 2,204 patients with documented, new-onset paroxysmal or persistent atrial fibrillation (AF) at 110 centers in 10 countries. Patients averaged about 68 years of age, with about 15% at least 75 years old, and in general were what Dr. Packer characterized as a high-risk group, with a high prevalence of comorbidities: 23% with sleep apnea, 10% with cardiomyopathy, 15% with heart failure, 10% with a prior stroke or transient ischemic attack, and just over a third in a New York Heart Association functional class II or III. About 43% had paroxysmal AF, about 47% had persistent AF, and the remaining patients had long-standing persistent AF. The median duration of AF at the time of entry was just over 1 year.
The clinicians treating the patients assigned to medical management could decide on a case-by-case basis whether to use rate or rhythm control, and about 12% of patients received rate control. The trial design specified pulmonary-vein isolation as the method for left atrial ablation.
In the intention-to-treat analyses, ablation was linked to a 14% relative reduction in the composite primary endpoint, a nonsignificant difference. All-cause mortality was a relative 15% lower in the ablation arm, also not statistically significant. A third prespecified, secondary endpoint, all-cause mortality plus cardiovascular hospitalization, was 17% lower in the ablated patients than in those on drug treatment in the intention-to-treat analysis, a statistically significant difference (P = .002).
The adverse event rate in the ablation arm was “surprisingly” low, said Dr. Packer, with a 3.9% rate of complications from catheter insertion (more than half were hematomas), a 3.4% rate of complications from catheter manipulation within the heart (2.2% involved pericardial effusions that required no intervention), and a 1.8% rate of ablation-related events, most commonly severe pericardial chest pain. “The risks of ablation seem to be lower than we thought,” he said, but quickly added the caveat that all ablation operators in CABANA had to have performed at least 100 ablation cases prior to the trial. The observed safety applies to operators “who know what they’re doing,” he said. Adverse events in the medically treated patients were typical for patients treated with amiodarone, Dr. Packer said, with the most common events hyper- or hypothyroidism, in 1.6%, and an allergic reaction, in 0.6%. In the intention-to-treat analysis the incidence of recurrent AF following a 90-day blanking period after ablation was 47% lower in the ablated patients relative to the drug-treated patients (P less than .0001).
Dr. Packer also presented an intriguing subgroup analysis for the primary endpoint that showed ablation had the best performance relative to medical management in patients younger than 65 years, patients with a history of heart failure, minority patients, and those who entered the trial in NYHA functional class II or III. The subgroup analysis showed a signal for worse performance from ablation in patients who were at least 75 years old. “I’m concerned about these older patients; we need to look into this,” Dr. Packer said. He also expressed optimism that the good performance of ablation in heart failure patients, while an exploratory finding, suggested confirmation of the results reported recently from the CASTLE-AF trial, which also showed good outcomes from catheter ablation for treating patients with heart failure and AF (N Engl J Med. 2018 Feb 1;378[5]:417-27).
The main qualification Dr. Packer voiced about the CABANA results is that not every AF patient should get ablation. “All treatments are not right for all patients. Not everyone with AF needs ablation. You need to talk with patients about it.” But despite this caution, he declared that the results had already changed his practice.
“I much less often now say to patients ‘let’s go with a drug and see what happens.’ I’d still do that if I wasn’t sure that a patient’s symptoms were caused by their AF” as opposed to their underlying heart disease, but if I’m pretty certain that their symptoms are caused by their AF over the past few months, I’ve become more likely to say that front-line ablation is reasonable,” Dr. Packer said.
CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and has received research funding from all four of these companies and also from several other companies. Dr. Ruskin has been a consultant to Biosense Webster and Medtronic and several other companies, has an ownership interest in Amgen, Cameron Health, InfoBionic, Newpace, Portola, and Regeneron, and has a fiduciary role in Pharmaco-Kinesis. Dr. Albert has been a consultant to Myokardia and Sanofi Aventis and has received research funding from Roche Diagnostics and St. Jude.
SOURCE: Packer DL et al. HRS 2018, Abstract B-LBCT01-05.
The data from CABANA suggest that at the least, catheter ablation is the equivalent of drug therapy, and I think in many cases, it is probably superior. Patients with atrial fibrillation should be allowed to undergo ablation as their first treatment, performed by operators who know what they’re doing. These are excellent results, but they do not apply to every patient with atrial fibrillation; they apply to patients like those enrolled in the trial.
The results also speak very loudly about the importance of sinus rhythm in patients with heart failure. The results in the subgroup of patients with heart failure appear to support the findings from CASTLE-AF (N Engl J Med. 2018 Feb 1;378[5]:417-27).
Eric N. Prystowsky, MD , is a cardiac electrophysiologist with the St. Vincent Medical Group in Indianapolis. He has been a consultant to CardioNet and Medtronic, has an equity interest in Stereotaxis, and receives fellowship support from Medtronic and St. Jude. He made these comments as designated discussant for CABANA.
The data from CABANA suggest that at the least, catheter ablation is the equivalent of drug therapy, and I think in many cases, it is probably superior. Patients with atrial fibrillation should be allowed to undergo ablation as their first treatment, performed by operators who know what they’re doing. These are excellent results, but they do not apply to every patient with atrial fibrillation; they apply to patients like those enrolled in the trial.
The results also speak very loudly about the importance of sinus rhythm in patients with heart failure. The results in the subgroup of patients with heart failure appear to support the findings from CASTLE-AF (N Engl J Med. 2018 Feb 1;378[5]:417-27).
Eric N. Prystowsky, MD , is a cardiac electrophysiologist with the St. Vincent Medical Group in Indianapolis. He has been a consultant to CardioNet and Medtronic, has an equity interest in Stereotaxis, and receives fellowship support from Medtronic and St. Jude. He made these comments as designated discussant for CABANA.
The data from CABANA suggest that at the least, catheter ablation is the equivalent of drug therapy, and I think in many cases, it is probably superior. Patients with atrial fibrillation should be allowed to undergo ablation as their first treatment, performed by operators who know what they’re doing. These are excellent results, but they do not apply to every patient with atrial fibrillation; they apply to patients like those enrolled in the trial.
The results also speak very loudly about the importance of sinus rhythm in patients with heart failure. The results in the subgroup of patients with heart failure appear to support the findings from CASTLE-AF (N Engl J Med. 2018 Feb 1;378[5]:417-27).
Eric N. Prystowsky, MD , is a cardiac electrophysiologist with the St. Vincent Medical Group in Indianapolis. He has been a consultant to CardioNet and Medtronic, has an equity interest in Stereotaxis, and receives fellowship support from Medtronic and St. Jude. He made these comments as designated discussant for CABANA.
BOSTON – Results from the CABANA trial, the long-awaited, head-to-head comparison of percutaneous catheter ablation with drug therapy for the treatment of atrial fibrillation by restoring sinus rhythm, failed to accomplish what it was designed to prove.
That is, that catheter ablation was superior to medical management for a combined endpoint of all-cause death, stroke, serious bleeding, or cardiac arrest.
The trial results also gave proponents of catheter ablation some tantalizing hints that this approach actually may have been superior to antiarrhythmic drugs, if only the randomization assignments had been more closely followed as the trial proceeded. But that didn’t happen, with about 30% of patients assigned to medical management crossing over to undergo catheter ablation, presumably because they had received inadequate symptom relief from their drug regimens. In addition, 10% of patients assigned to catheter ablation didn’t undergo it, primarily because they reconsidered after randomization and decided to not choose the invasive option. These crossovers produced a disparity in the outcomes between the standard, intention-to-treat analysis, which showed a neutral difference between the two study arms, and the per-protocol analysis that censored out crossover patients. The per-protocol analysis showed a statistically significant, 27% relative risk reduction in the primary endpoint among the patients randomized to and actually treated with catheter ablation, compared with those randomized to and exclusively treated medically.
“A patient can’t receive benefit from ablation if you don’t ablate,” noted the study’s lead investigation, Douglas L. Packer, MD, as he reported the results at the annual scientific sessions of the Heart Rhythm Society. “When you have this many crossovers and so many patients not getting their assigned treatments, then an on-treatment analysis is required, said Dr. Packer, a cardiac electrophysiologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
The prespecified on-treatment analysis, which, instead of censoring crossover patients, analyzed outcomes based on the treatments that patients actually received, showed a statistically significant one-third reduction in the primary endpoint among the ablated patients and a statistically significant 40% relative reduction in all-cause mortality in the ablated arm, compared with those on medical management.
“For symptomatic treatment, and to restore and maintain sinus rhythm, there is no question that ablation is better. We knew that before this trial, and we know it even more convincingly now,” commented Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “To a large extent, we do ablations for symptomatic benefit; to get patients feeling better. And I think this trial will confirm that because this will likely follow the better reduction in atrial fibrillation burden, which was quite impressive in the study.” Dr. Packer said that the quality of life data collected in CABANA will come out in a report later in 2018.
The dilemma that Dr. Ruskin and other physicians who heard the results voiced was how best to interpret the study’s primary results.
“This trial was designed to address whether ablation has an impact [compared with medical management] on hard endpoints, like mortality, and the intention-to-treat analysis showed no difference. I feel bound to adhere to the intention-to-treat analysis, the primary result” the traditional default arbiter of a randomized trial’s outcome, Dr. Ruskin said in an interview, “But intention-to-treat analyses are built on a foundation where most patients are maintained on their assigned treatment.”
The results “tell us that there wasn’t harm from ablation,” Dr. Albert said during a press conference. “That is really important because, before this, we didn’t know for sure. These data make me a little more confident about offering patients ablation. I now have data to discuss with patients that’s useful for decision making.”
“There was certainly no signal whatsoever of harm by taking patients to ablation early” in their management, agreed Dr. Ruskin. “I find that very reassuring and encouraging.”
CABANA (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial) started in 2009 and enrolled 2,204 patients with documented, new-onset paroxysmal or persistent atrial fibrillation (AF) at 110 centers in 10 countries. Patients averaged about 68 years of age, with about 15% at least 75 years old, and in general were what Dr. Packer characterized as a high-risk group, with a high prevalence of comorbidities: 23% with sleep apnea, 10% with cardiomyopathy, 15% with heart failure, 10% with a prior stroke or transient ischemic attack, and just over a third in a New York Heart Association functional class II or III. About 43% had paroxysmal AF, about 47% had persistent AF, and the remaining patients had long-standing persistent AF. The median duration of AF at the time of entry was just over 1 year.
The clinicians treating the patients assigned to medical management could decide on a case-by-case basis whether to use rate or rhythm control, and about 12% of patients received rate control. The trial design specified pulmonary-vein isolation as the method for left atrial ablation.
In the intention-to-treat analyses, ablation was linked to a 14% relative reduction in the composite primary endpoint, a nonsignificant difference. All-cause mortality was a relative 15% lower in the ablation arm, also not statistically significant. A third prespecified, secondary endpoint, all-cause mortality plus cardiovascular hospitalization, was 17% lower in the ablated patients than in those on drug treatment in the intention-to-treat analysis, a statistically significant difference (P = .002).
The adverse event rate in the ablation arm was “surprisingly” low, said Dr. Packer, with a 3.9% rate of complications from catheter insertion (more than half were hematomas), a 3.4% rate of complications from catheter manipulation within the heart (2.2% involved pericardial effusions that required no intervention), and a 1.8% rate of ablation-related events, most commonly severe pericardial chest pain. “The risks of ablation seem to be lower than we thought,” he said, but quickly added the caveat that all ablation operators in CABANA had to have performed at least 100 ablation cases prior to the trial. The observed safety applies to operators “who know what they’re doing,” he said. Adverse events in the medically treated patients were typical for patients treated with amiodarone, Dr. Packer said, with the most common events hyper- or hypothyroidism, in 1.6%, and an allergic reaction, in 0.6%. In the intention-to-treat analysis the incidence of recurrent AF following a 90-day blanking period after ablation was 47% lower in the ablated patients relative to the drug-treated patients (P less than .0001).
Dr. Packer also presented an intriguing subgroup analysis for the primary endpoint that showed ablation had the best performance relative to medical management in patients younger than 65 years, patients with a history of heart failure, minority patients, and those who entered the trial in NYHA functional class II or III. The subgroup analysis showed a signal for worse performance from ablation in patients who were at least 75 years old. “I’m concerned about these older patients; we need to look into this,” Dr. Packer said. He also expressed optimism that the good performance of ablation in heart failure patients, while an exploratory finding, suggested confirmation of the results reported recently from the CASTLE-AF trial, which also showed good outcomes from catheter ablation for treating patients with heart failure and AF (N Engl J Med. 2018 Feb 1;378[5]:417-27).
The main qualification Dr. Packer voiced about the CABANA results is that not every AF patient should get ablation. “All treatments are not right for all patients. Not everyone with AF needs ablation. You need to talk with patients about it.” But despite this caution, he declared that the results had already changed his practice.
“I much less often now say to patients ‘let’s go with a drug and see what happens.’ I’d still do that if I wasn’t sure that a patient’s symptoms were caused by their AF” as opposed to their underlying heart disease, but if I’m pretty certain that their symptoms are caused by their AF over the past few months, I’ve become more likely to say that front-line ablation is reasonable,” Dr. Packer said.
CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and has received research funding from all four of these companies and also from several other companies. Dr. Ruskin has been a consultant to Biosense Webster and Medtronic and several other companies, has an ownership interest in Amgen, Cameron Health, InfoBionic, Newpace, Portola, and Regeneron, and has a fiduciary role in Pharmaco-Kinesis. Dr. Albert has been a consultant to Myokardia and Sanofi Aventis and has received research funding from Roche Diagnostics and St. Jude.
SOURCE: Packer DL et al. HRS 2018, Abstract B-LBCT01-05.
BOSTON – Results from the CABANA trial, the long-awaited, head-to-head comparison of percutaneous catheter ablation with drug therapy for the treatment of atrial fibrillation by restoring sinus rhythm, failed to accomplish what it was designed to prove.
That is, that catheter ablation was superior to medical management for a combined endpoint of all-cause death, stroke, serious bleeding, or cardiac arrest.
The trial results also gave proponents of catheter ablation some tantalizing hints that this approach actually may have been superior to antiarrhythmic drugs, if only the randomization assignments had been more closely followed as the trial proceeded. But that didn’t happen, with about 30% of patients assigned to medical management crossing over to undergo catheter ablation, presumably because they had received inadequate symptom relief from their drug regimens. In addition, 10% of patients assigned to catheter ablation didn’t undergo it, primarily because they reconsidered after randomization and decided to not choose the invasive option. These crossovers produced a disparity in the outcomes between the standard, intention-to-treat analysis, which showed a neutral difference between the two study arms, and the per-protocol analysis that censored out crossover patients. The per-protocol analysis showed a statistically significant, 27% relative risk reduction in the primary endpoint among the patients randomized to and actually treated with catheter ablation, compared with those randomized to and exclusively treated medically.
“A patient can’t receive benefit from ablation if you don’t ablate,” noted the study’s lead investigation, Douglas L. Packer, MD, as he reported the results at the annual scientific sessions of the Heart Rhythm Society. “When you have this many crossovers and so many patients not getting their assigned treatments, then an on-treatment analysis is required, said Dr. Packer, a cardiac electrophysiologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
The prespecified on-treatment analysis, which, instead of censoring crossover patients, analyzed outcomes based on the treatments that patients actually received, showed a statistically significant one-third reduction in the primary endpoint among the ablated patients and a statistically significant 40% relative reduction in all-cause mortality in the ablated arm, compared with those on medical management.
“For symptomatic treatment, and to restore and maintain sinus rhythm, there is no question that ablation is better. We knew that before this trial, and we know it even more convincingly now,” commented Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “To a large extent, we do ablations for symptomatic benefit; to get patients feeling better. And I think this trial will confirm that because this will likely follow the better reduction in atrial fibrillation burden, which was quite impressive in the study.” Dr. Packer said that the quality of life data collected in CABANA will come out in a report later in 2018.
The dilemma that Dr. Ruskin and other physicians who heard the results voiced was how best to interpret the study’s primary results.
“This trial was designed to address whether ablation has an impact [compared with medical management] on hard endpoints, like mortality, and the intention-to-treat analysis showed no difference. I feel bound to adhere to the intention-to-treat analysis, the primary result” the traditional default arbiter of a randomized trial’s outcome, Dr. Ruskin said in an interview, “But intention-to-treat analyses are built on a foundation where most patients are maintained on their assigned treatment.”
The results “tell us that there wasn’t harm from ablation,” Dr. Albert said during a press conference. “That is really important because, before this, we didn’t know for sure. These data make me a little more confident about offering patients ablation. I now have data to discuss with patients that’s useful for decision making.”
“There was certainly no signal whatsoever of harm by taking patients to ablation early” in their management, agreed Dr. Ruskin. “I find that very reassuring and encouraging.”
CABANA (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial) started in 2009 and enrolled 2,204 patients with documented, new-onset paroxysmal or persistent atrial fibrillation (AF) at 110 centers in 10 countries. Patients averaged about 68 years of age, with about 15% at least 75 years old, and in general were what Dr. Packer characterized as a high-risk group, with a high prevalence of comorbidities: 23% with sleep apnea, 10% with cardiomyopathy, 15% with heart failure, 10% with a prior stroke or transient ischemic attack, and just over a third in a New York Heart Association functional class II or III. About 43% had paroxysmal AF, about 47% had persistent AF, and the remaining patients had long-standing persistent AF. The median duration of AF at the time of entry was just over 1 year.
The clinicians treating the patients assigned to medical management could decide on a case-by-case basis whether to use rate or rhythm control, and about 12% of patients received rate control. The trial design specified pulmonary-vein isolation as the method for left atrial ablation.
In the intention-to-treat analyses, ablation was linked to a 14% relative reduction in the composite primary endpoint, a nonsignificant difference. All-cause mortality was a relative 15% lower in the ablation arm, also not statistically significant. A third prespecified, secondary endpoint, all-cause mortality plus cardiovascular hospitalization, was 17% lower in the ablated patients than in those on drug treatment in the intention-to-treat analysis, a statistically significant difference (P = .002).
The adverse event rate in the ablation arm was “surprisingly” low, said Dr. Packer, with a 3.9% rate of complications from catheter insertion (more than half were hematomas), a 3.4% rate of complications from catheter manipulation within the heart (2.2% involved pericardial effusions that required no intervention), and a 1.8% rate of ablation-related events, most commonly severe pericardial chest pain. “The risks of ablation seem to be lower than we thought,” he said, but quickly added the caveat that all ablation operators in CABANA had to have performed at least 100 ablation cases prior to the trial. The observed safety applies to operators “who know what they’re doing,” he said. Adverse events in the medically treated patients were typical for patients treated with amiodarone, Dr. Packer said, with the most common events hyper- or hypothyroidism, in 1.6%, and an allergic reaction, in 0.6%. In the intention-to-treat analysis the incidence of recurrent AF following a 90-day blanking period after ablation was 47% lower in the ablated patients relative to the drug-treated patients (P less than .0001).
Dr. Packer also presented an intriguing subgroup analysis for the primary endpoint that showed ablation had the best performance relative to medical management in patients younger than 65 years, patients with a history of heart failure, minority patients, and those who entered the trial in NYHA functional class II or III. The subgroup analysis showed a signal for worse performance from ablation in patients who were at least 75 years old. “I’m concerned about these older patients; we need to look into this,” Dr. Packer said. He also expressed optimism that the good performance of ablation in heart failure patients, while an exploratory finding, suggested confirmation of the results reported recently from the CASTLE-AF trial, which also showed good outcomes from catheter ablation for treating patients with heart failure and AF (N Engl J Med. 2018 Feb 1;378[5]:417-27).
The main qualification Dr. Packer voiced about the CABANA results is that not every AF patient should get ablation. “All treatments are not right for all patients. Not everyone with AF needs ablation. You need to talk with patients about it.” But despite this caution, he declared that the results had already changed his practice.
“I much less often now say to patients ‘let’s go with a drug and see what happens.’ I’d still do that if I wasn’t sure that a patient’s symptoms were caused by their AF” as opposed to their underlying heart disease, but if I’m pretty certain that their symptoms are caused by their AF over the past few months, I’ve become more likely to say that front-line ablation is reasonable,” Dr. Packer said.
CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and has received research funding from all four of these companies and also from several other companies. Dr. Ruskin has been a consultant to Biosense Webster and Medtronic and several other companies, has an ownership interest in Amgen, Cameron Health, InfoBionic, Newpace, Portola, and Regeneron, and has a fiduciary role in Pharmaco-Kinesis. Dr. Albert has been a consultant to Myokardia and Sanofi Aventis and has received research funding from Roche Diagnostics and St. Jude.
SOURCE: Packer DL et al. HRS 2018, Abstract B-LBCT01-05.
REPORTING FROM HEART RHYTHM 2018
Key clinical point: Catheter atrial fib ablation showed no significant benefit over medical management for the CABANA’s primary endpoint.
Major finding: The composite endpoint that included all-cause death was a nonsignificant 14% lower with ablation than with medical management in the intention-to-treat analysis.
Study details: CABANA, a multicenter, randomized trial with 2,204 patients.
Disclosures: CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and has received research funding from all four of these companies and from several other companies. Dr. Ruskin has been a consultant to Biosense Webster and Medtronic and several other companies, has an ownership interest in Amgen, Cameron Health, InfoBionic, Newpace, Portola, and Regeneron, and has a fiduciary role in Pharmaco-Kinesis. Dr. Albert has been a consultant to Myokardia and Sanofi-Aventis and has received research funding from Roche Diagnostics and St. Jude.
Source: Packer D et al. HRS 2018, Abstract B-LBCT01-05.
Implanted pulse generator ups exercise tolerance in HF
BOSTON – Cardiac contractility modulation (CCM), an electrical device–based modality, may improve exercise tolerance and quality of life in heart failure patients with ejection fraction between 25% and 45%, results of a randomized, controlled trial suggested.
Notably, clinical effectiveness appeared to be even greater in the subset of patient with ejection fractions between 35% and 45%, according to results presented at the annual scientific sessions of the Heart Rhythm Society.
The CCM approach was designed to treat chronic heart failure patients with reduced and midrange ejection fractions. It involves the delivery of electrical signals during the cardiac absolute refractory period to enhance contraction strength. Key components include an atrial lead used for sensing and two ventricular leads used for sensing local electrical activity and delivery of CCM signals.
The device was previously evaluated in FIX-HF-5, a 428-patient randomized trial of CCM in patients with New York Heart Association functional class III/IV and reduced ejection fraction. That study failed to meet its primary efficacy end point, but a subgroup analysis showed significant treatment effects in patients with ejection fractions between 25% and 45%.
Consequently, Dr. Abraham initiated the FIX-HF-5 confirmatory study (FIX-HF-5C) to prospectively evaluate CCM in patients with ejection fractions in that range.
The study included 160 with NYHA class III/IV heart failure, QRS duration less than 130 ms, and ejection fractions of 25%-45% who were randomized either to CCM or to continued optimal medical therapy. The control arm included 86 patients, and the CCM arm included 74 patients, of whom 68 received device implantation.
For evaluating peak VO2, the primary end point of this confirmatory study, investigators used a Bayesian model that allowed them incorporate some data from the corresponding subgroup of patients with ejection fractions between 25% and 45% in the previous FIX-HF-5 randomized trial.
Using this model, they found the mean difference between CCM and medical therapy groups at week 24 was 0.836 mL O2/kg per minute (95% Bayesian credible interval, 0.123-1.552). The probability of CCM treatment’s superiority to control was 0.989, according to investigators, which exceeded the 0.975 threshold for statistical significance.
Dr. Abraham and his coinvestigators found that CCM implantation was safe, with a 90% complication-free rate, and that it improved both quality of life and functional status.
All efficacy endpoints were better in patients with ejection fractions of 35% or greater, confirming the subgroup analysis findings of the previous randomized trial, according to Dr. Abraham and his coinvestigators.
Survival free of cardiac death and heart failure hospitalization at 24 weeks was 97.1% in the CCM arm versus 89.2% in controls, which was a secondary finding that reached statistical significance in favor of CCM (P = .048) in one analysis of Kaplan-Meier estimates, investigators said in their report.
The CCM device could fulfill an unmet need for patients who have persistent heart failure despite optimal, guideline-based medical therapy, according to the investigators.
“Although ICDs are applicable to the broad population of patients with ejection fraction [at least] 35%, they do not deliver a therapy for improving exercise tolerance or quality of life,” they said in their report.
Research grant support for the study came from Impulse Dynamics. Dr. Abraham and several coauthors reported serving as consultants to the company.
SOURCE: Abraham WT et al. JACC Heart Fail. 2018 May 10. doi: 10.1016/j.jchf.2018.04.010.
BOSTON – Cardiac contractility modulation (CCM), an electrical device–based modality, may improve exercise tolerance and quality of life in heart failure patients with ejection fraction between 25% and 45%, results of a randomized, controlled trial suggested.
Notably, clinical effectiveness appeared to be even greater in the subset of patient with ejection fractions between 35% and 45%, according to results presented at the annual scientific sessions of the Heart Rhythm Society.
The CCM approach was designed to treat chronic heart failure patients with reduced and midrange ejection fractions. It involves the delivery of electrical signals during the cardiac absolute refractory period to enhance contraction strength. Key components include an atrial lead used for sensing and two ventricular leads used for sensing local electrical activity and delivery of CCM signals.
The device was previously evaluated in FIX-HF-5, a 428-patient randomized trial of CCM in patients with New York Heart Association functional class III/IV and reduced ejection fraction. That study failed to meet its primary efficacy end point, but a subgroup analysis showed significant treatment effects in patients with ejection fractions between 25% and 45%.
Consequently, Dr. Abraham initiated the FIX-HF-5 confirmatory study (FIX-HF-5C) to prospectively evaluate CCM in patients with ejection fractions in that range.
The study included 160 with NYHA class III/IV heart failure, QRS duration less than 130 ms, and ejection fractions of 25%-45% who were randomized either to CCM or to continued optimal medical therapy. The control arm included 86 patients, and the CCM arm included 74 patients, of whom 68 received device implantation.
For evaluating peak VO2, the primary end point of this confirmatory study, investigators used a Bayesian model that allowed them incorporate some data from the corresponding subgroup of patients with ejection fractions between 25% and 45% in the previous FIX-HF-5 randomized trial.
Using this model, they found the mean difference between CCM and medical therapy groups at week 24 was 0.836 mL O2/kg per minute (95% Bayesian credible interval, 0.123-1.552). The probability of CCM treatment’s superiority to control was 0.989, according to investigators, which exceeded the 0.975 threshold for statistical significance.
Dr. Abraham and his coinvestigators found that CCM implantation was safe, with a 90% complication-free rate, and that it improved both quality of life and functional status.
All efficacy endpoints were better in patients with ejection fractions of 35% or greater, confirming the subgroup analysis findings of the previous randomized trial, according to Dr. Abraham and his coinvestigators.
Survival free of cardiac death and heart failure hospitalization at 24 weeks was 97.1% in the CCM arm versus 89.2% in controls, which was a secondary finding that reached statistical significance in favor of CCM (P = .048) in one analysis of Kaplan-Meier estimates, investigators said in their report.
The CCM device could fulfill an unmet need for patients who have persistent heart failure despite optimal, guideline-based medical therapy, according to the investigators.
“Although ICDs are applicable to the broad population of patients with ejection fraction [at least] 35%, they do not deliver a therapy for improving exercise tolerance or quality of life,” they said in their report.
Research grant support for the study came from Impulse Dynamics. Dr. Abraham and several coauthors reported serving as consultants to the company.
SOURCE: Abraham WT et al. JACC Heart Fail. 2018 May 10. doi: 10.1016/j.jchf.2018.04.010.
BOSTON – Cardiac contractility modulation (CCM), an electrical device–based modality, may improve exercise tolerance and quality of life in heart failure patients with ejection fraction between 25% and 45%, results of a randomized, controlled trial suggested.
Notably, clinical effectiveness appeared to be even greater in the subset of patient with ejection fractions between 35% and 45%, according to results presented at the annual scientific sessions of the Heart Rhythm Society.
The CCM approach was designed to treat chronic heart failure patients with reduced and midrange ejection fractions. It involves the delivery of electrical signals during the cardiac absolute refractory period to enhance contraction strength. Key components include an atrial lead used for sensing and two ventricular leads used for sensing local electrical activity and delivery of CCM signals.
The device was previously evaluated in FIX-HF-5, a 428-patient randomized trial of CCM in patients with New York Heart Association functional class III/IV and reduced ejection fraction. That study failed to meet its primary efficacy end point, but a subgroup analysis showed significant treatment effects in patients with ejection fractions between 25% and 45%.
Consequently, Dr. Abraham initiated the FIX-HF-5 confirmatory study (FIX-HF-5C) to prospectively evaluate CCM in patients with ejection fractions in that range.
The study included 160 with NYHA class III/IV heart failure, QRS duration less than 130 ms, and ejection fractions of 25%-45% who were randomized either to CCM or to continued optimal medical therapy. The control arm included 86 patients, and the CCM arm included 74 patients, of whom 68 received device implantation.
For evaluating peak VO2, the primary end point of this confirmatory study, investigators used a Bayesian model that allowed them incorporate some data from the corresponding subgroup of patients with ejection fractions between 25% and 45% in the previous FIX-HF-5 randomized trial.
Using this model, they found the mean difference between CCM and medical therapy groups at week 24 was 0.836 mL O2/kg per minute (95% Bayesian credible interval, 0.123-1.552). The probability of CCM treatment’s superiority to control was 0.989, according to investigators, which exceeded the 0.975 threshold for statistical significance.
Dr. Abraham and his coinvestigators found that CCM implantation was safe, with a 90% complication-free rate, and that it improved both quality of life and functional status.
All efficacy endpoints were better in patients with ejection fractions of 35% or greater, confirming the subgroup analysis findings of the previous randomized trial, according to Dr. Abraham and his coinvestigators.
Survival free of cardiac death and heart failure hospitalization at 24 weeks was 97.1% in the CCM arm versus 89.2% in controls, which was a secondary finding that reached statistical significance in favor of CCM (P = .048) in one analysis of Kaplan-Meier estimates, investigators said in their report.
The CCM device could fulfill an unmet need for patients who have persistent heart failure despite optimal, guideline-based medical therapy, according to the investigators.
“Although ICDs are applicable to the broad population of patients with ejection fraction [at least] 35%, they do not deliver a therapy for improving exercise tolerance or quality of life,” they said in their report.
Research grant support for the study came from Impulse Dynamics. Dr. Abraham and several coauthors reported serving as consultants to the company.
SOURCE: Abraham WT et al. JACC Heart Fail. 2018 May 10. doi: 10.1016/j.jchf.2018.04.010.
REPORTING FROM HEART RHYTHM 2018
Key clinical point: Compared with continued medical therapy, cardiac contractility modulation (CCM) improved exercise tolerance and quality of life in heart failure patients with ejection fraction between 25% and 45%.
Major finding: The difference in peak VO2 between study arms was 0.836 mL O2/kg per minute (95% Bayesian credible interval, 0.123-1.552).
Study details: A randomized controlled trial including 160 patients with NYHA class III/IV heart failure and ejection fraction between 25% and 45%.
Disclosures: Research grant support for the study came from Impulse Dynamics. Several study authors reported serving as consultants to Impulse Dynamics.
Source: Abraham WT et al. JACC Heart Fail. 2018 May 10. doi: 10.1016/j.jchf.2018.04.010.