User login
Three or more nonadvanced adenomas no longer spell increased CRC risk
SAN ANTONIO – , Carol Rouphael, MD, reported at the annual meeting of the American College of Gastroenterology.
She presented a retrospective study of 3,377 patients who had their first colonoscopies at age 50 or older in 2006 or later, when high-definition colonscopes took over.
The clinical implications of the study are clear: “Our findings suggest the colonoscopy interval for individuals with three or more nonadvanced adenomas should be similar to low-risk adenoma patients; that is, 5-10 years,” said Dr. Rouphael, a gastroenterology fellow at the Cleveland Clinic.
Studies conducted in the early 2000s using standard-definition colonoscopes showed that the risk of metachronous advanced neoplasia (MAN) – that is, colorectal cancer or a pathologically advanced adenoma – was twice as great in patients with three or more small tubular adenomas compared with patients with just one or two of them. Thus, guidelines called for such patients to undergo repeat colonoscopy at a shorter time interval post polypectomy, typically 3 years. But with contemporary colonoscopy using high-definition optics, gastroenterologists are detecting a lot more small adenomas. Dr. Rouphael and coworkers wondered if the definition of high risk established more than a decade ago, prior to the use of high-definition colonoscopes, still held true. They concluded that the answer is no.
Eleven percent of patients in their study had features indicative of high-risk adenoma on the initial colonoscopy. Twenty-four percent of these patients had an adenoma with advanced pathology, meaning villous features or high-grade dysplasia; 51% had an adenoma 10 mm or more in size without advanced pathology; and the remaining 25% of patients were classified as having high-risk adenoma on the basis of having three or more small tubular adenomas.
Follow-up colonoscopy was performed a median of 42 months later in the high-risk adenoma group, 54 months later in the low-risk adenoma patients with one or two small tubular adenomas, and at 61 months in those with no adenomas. At follow-up, MAN was discovered in 3.8% of patients with no adenomas at baseline, 4.6% of the low-risk adenoma group, and 9.3% of the overall high-risk adenoma group. However, within the high-risk adenoma group the risk of MAN varied widely: 6.3% in patients with three or more nonadvanced adenomas, 6.1% in those with three or four nonadvanced adenomas, 7.7% in patients with five or more nonadvanced adenomas, 8.3% in those with a 10-mm or larger adenoma without advanced pathology, and 14.6% in patients with an adenoma with advanced pathology at baseline.
In a multivariate analysis adjusted for age, sex, ethnicity, and time between first and follow-up colonoscopy, the risk of MAN did not differ significantly between patients with three or four nonadvanced adenomas and those with one or two, nor between patients with five or more versus one or two. In addition, there was no significant difference in MAN risk between patients with no adenomas at baseline and those with one or two low-risk, nonadvanced adenomas. In contrast, patients with a 10-mm or larger adenoma without advanced pathology at baseline were 1.9-fold more likely to have MAN at follow-up colonoscopy than were patients with one or two small tubular adenomas. And patients having an adenoma with advanced pathology at baseline were at 3.7-fold greater risk of developing MAN than were those with baseline low-risk adenoma, according to Dr. Rouphael.
She reported having no financial conflicts regarding her study, which won the Fellows-in-Training Award at the annual meeting.
SOURCE: Rouphael C. ACG 2019 Abstract 9.
SAN ANTONIO – , Carol Rouphael, MD, reported at the annual meeting of the American College of Gastroenterology.
She presented a retrospective study of 3,377 patients who had their first colonoscopies at age 50 or older in 2006 or later, when high-definition colonscopes took over.
The clinical implications of the study are clear: “Our findings suggest the colonoscopy interval for individuals with three or more nonadvanced adenomas should be similar to low-risk adenoma patients; that is, 5-10 years,” said Dr. Rouphael, a gastroenterology fellow at the Cleveland Clinic.
Studies conducted in the early 2000s using standard-definition colonoscopes showed that the risk of metachronous advanced neoplasia (MAN) – that is, colorectal cancer or a pathologically advanced adenoma – was twice as great in patients with three or more small tubular adenomas compared with patients with just one or two of them. Thus, guidelines called for such patients to undergo repeat colonoscopy at a shorter time interval post polypectomy, typically 3 years. But with contemporary colonoscopy using high-definition optics, gastroenterologists are detecting a lot more small adenomas. Dr. Rouphael and coworkers wondered if the definition of high risk established more than a decade ago, prior to the use of high-definition colonoscopes, still held true. They concluded that the answer is no.
Eleven percent of patients in their study had features indicative of high-risk adenoma on the initial colonoscopy. Twenty-four percent of these patients had an adenoma with advanced pathology, meaning villous features or high-grade dysplasia; 51% had an adenoma 10 mm or more in size without advanced pathology; and the remaining 25% of patients were classified as having high-risk adenoma on the basis of having three or more small tubular adenomas.
Follow-up colonoscopy was performed a median of 42 months later in the high-risk adenoma group, 54 months later in the low-risk adenoma patients with one or two small tubular adenomas, and at 61 months in those with no adenomas. At follow-up, MAN was discovered in 3.8% of patients with no adenomas at baseline, 4.6% of the low-risk adenoma group, and 9.3% of the overall high-risk adenoma group. However, within the high-risk adenoma group the risk of MAN varied widely: 6.3% in patients with three or more nonadvanced adenomas, 6.1% in those with three or four nonadvanced adenomas, 7.7% in patients with five or more nonadvanced adenomas, 8.3% in those with a 10-mm or larger adenoma without advanced pathology, and 14.6% in patients with an adenoma with advanced pathology at baseline.
In a multivariate analysis adjusted for age, sex, ethnicity, and time between first and follow-up colonoscopy, the risk of MAN did not differ significantly between patients with three or four nonadvanced adenomas and those with one or two, nor between patients with five or more versus one or two. In addition, there was no significant difference in MAN risk between patients with no adenomas at baseline and those with one or two low-risk, nonadvanced adenomas. In contrast, patients with a 10-mm or larger adenoma without advanced pathology at baseline were 1.9-fold more likely to have MAN at follow-up colonoscopy than were patients with one or two small tubular adenomas. And patients having an adenoma with advanced pathology at baseline were at 3.7-fold greater risk of developing MAN than were those with baseline low-risk adenoma, according to Dr. Rouphael.
She reported having no financial conflicts regarding her study, which won the Fellows-in-Training Award at the annual meeting.
SOURCE: Rouphael C. ACG 2019 Abstract 9.
SAN ANTONIO – , Carol Rouphael, MD, reported at the annual meeting of the American College of Gastroenterology.
She presented a retrospective study of 3,377 patients who had their first colonoscopies at age 50 or older in 2006 or later, when high-definition colonscopes took over.
The clinical implications of the study are clear: “Our findings suggest the colonoscopy interval for individuals with three or more nonadvanced adenomas should be similar to low-risk adenoma patients; that is, 5-10 years,” said Dr. Rouphael, a gastroenterology fellow at the Cleveland Clinic.
Studies conducted in the early 2000s using standard-definition colonoscopes showed that the risk of metachronous advanced neoplasia (MAN) – that is, colorectal cancer or a pathologically advanced adenoma – was twice as great in patients with three or more small tubular adenomas compared with patients with just one or two of them. Thus, guidelines called for such patients to undergo repeat colonoscopy at a shorter time interval post polypectomy, typically 3 years. But with contemporary colonoscopy using high-definition optics, gastroenterologists are detecting a lot more small adenomas. Dr. Rouphael and coworkers wondered if the definition of high risk established more than a decade ago, prior to the use of high-definition colonoscopes, still held true. They concluded that the answer is no.
Eleven percent of patients in their study had features indicative of high-risk adenoma on the initial colonoscopy. Twenty-four percent of these patients had an adenoma with advanced pathology, meaning villous features or high-grade dysplasia; 51% had an adenoma 10 mm or more in size without advanced pathology; and the remaining 25% of patients were classified as having high-risk adenoma on the basis of having three or more small tubular adenomas.
Follow-up colonoscopy was performed a median of 42 months later in the high-risk adenoma group, 54 months later in the low-risk adenoma patients with one or two small tubular adenomas, and at 61 months in those with no adenomas. At follow-up, MAN was discovered in 3.8% of patients with no adenomas at baseline, 4.6% of the low-risk adenoma group, and 9.3% of the overall high-risk adenoma group. However, within the high-risk adenoma group the risk of MAN varied widely: 6.3% in patients with three or more nonadvanced adenomas, 6.1% in those with three or four nonadvanced adenomas, 7.7% in patients with five or more nonadvanced adenomas, 8.3% in those with a 10-mm or larger adenoma without advanced pathology, and 14.6% in patients with an adenoma with advanced pathology at baseline.
In a multivariate analysis adjusted for age, sex, ethnicity, and time between first and follow-up colonoscopy, the risk of MAN did not differ significantly between patients with three or four nonadvanced adenomas and those with one or two, nor between patients with five or more versus one or two. In addition, there was no significant difference in MAN risk between patients with no adenomas at baseline and those with one or two low-risk, nonadvanced adenomas. In contrast, patients with a 10-mm or larger adenoma without advanced pathology at baseline were 1.9-fold more likely to have MAN at follow-up colonoscopy than were patients with one or two small tubular adenomas. And patients having an adenoma with advanced pathology at baseline were at 3.7-fold greater risk of developing MAN than were those with baseline low-risk adenoma, according to Dr. Rouphael.
She reported having no financial conflicts regarding her study, which won the Fellows-in-Training Award at the annual meeting.
SOURCE: Rouphael C. ACG 2019 Abstract 9.
REPORTING FROM ACG 2019
Consider hyperbaric oxygen for inflammatory ileal pouchitis
SAN ANTONIO – , Hamna Fahad, MD, reported at the annual meeting of the American College of Gastroenterology.
Dr. Fahad, of the Cleveland Clinic, presented a retrospective case series of 21 consecutive clinic patients who presented with inflammatory bowel disease, a surgically created ileal pouch–anal anastomosis, and medically refractory pouchitis. All patients received 30 hyperbaric oxygen treatment sessions, each an hour long, over the course of 2 months. This intensive regimen worked out to 3-5 sessions per week involving 100% oxygen pressurized to 2.4-3.0 ATA.
Overall, 19 of 21 patients experienced improvement in their modified Pouchitis Disease Activity Index (mPDAI) score. The mean total mPDAI at baseline was 8.71, improving significantly to 5 post treatment. The mPDAI symptoms subscore also showed significant improvement in response to a course of hyperbaric oxygen therapy, decreasing from 4 points to 2. The cuff subscore fell from 3 to 0, and the pouch body subscore improved from 3 to 2.
Thirteen of 21 patients reported subjective symptomatic improvement in stool frequency, bleeding, urgency, and fevers, including 6 with complete symptomatic remission. Seventeen patients demonstrated significant endoscopic improvement upon blinded assessment. Seven of 9 patients with fistulae experienced healing of the fistula tract.
The treatment entailed no side effects. However, the benefits weren’t uniformly durable. Several patients underwent a second 30-session round of hyperbaric oxygen therapy within a year because of recurrent pouchitis symptoms refractory to corticosteroids, biologics, and other medications.
Dr. Fahad said the mechanism of benefit for hyperbaric oxygen in the treatment of chronic inflammatory pouchitis is probably severalfold: reversal of a disordered microbiome through inhibition of the growth of anaerobes, reduced production of tumor necrosis factor–alpha and other inflammatory cytokines, and increased plasma oxygen, which reduces ischemia at the tissue level, thereby promoting tissue healing.
Audience members had a practical question: How can they get this treatment paid for? One gastroenterologist said she has encountered considerable payer resistance when she has sought coverage of hyperbaric oxygen for patients with ulcerative colitis and fistulae, even though there is already published evidence of benefit. But Dr. Fahad’s groundbreaking study provides the first such evidence in pouchitis. So how did she and her coworkers do it? Eighty percent of the pouchitis patients obtained payer approval only upon appeal, which was readily granted, she explained.
Dr. Fahad reported having no financial conflicts regarding her study, conducted without commercial support.
SOURCE: Fahad H. ACG 2019 Abstract 38.
SAN ANTONIO – , Hamna Fahad, MD, reported at the annual meeting of the American College of Gastroenterology.
Dr. Fahad, of the Cleveland Clinic, presented a retrospective case series of 21 consecutive clinic patients who presented with inflammatory bowel disease, a surgically created ileal pouch–anal anastomosis, and medically refractory pouchitis. All patients received 30 hyperbaric oxygen treatment sessions, each an hour long, over the course of 2 months. This intensive regimen worked out to 3-5 sessions per week involving 100% oxygen pressurized to 2.4-3.0 ATA.
Overall, 19 of 21 patients experienced improvement in their modified Pouchitis Disease Activity Index (mPDAI) score. The mean total mPDAI at baseline was 8.71, improving significantly to 5 post treatment. The mPDAI symptoms subscore also showed significant improvement in response to a course of hyperbaric oxygen therapy, decreasing from 4 points to 2. The cuff subscore fell from 3 to 0, and the pouch body subscore improved from 3 to 2.
Thirteen of 21 patients reported subjective symptomatic improvement in stool frequency, bleeding, urgency, and fevers, including 6 with complete symptomatic remission. Seventeen patients demonstrated significant endoscopic improvement upon blinded assessment. Seven of 9 patients with fistulae experienced healing of the fistula tract.
The treatment entailed no side effects. However, the benefits weren’t uniformly durable. Several patients underwent a second 30-session round of hyperbaric oxygen therapy within a year because of recurrent pouchitis symptoms refractory to corticosteroids, biologics, and other medications.
Dr. Fahad said the mechanism of benefit for hyperbaric oxygen in the treatment of chronic inflammatory pouchitis is probably severalfold: reversal of a disordered microbiome through inhibition of the growth of anaerobes, reduced production of tumor necrosis factor–alpha and other inflammatory cytokines, and increased plasma oxygen, which reduces ischemia at the tissue level, thereby promoting tissue healing.
Audience members had a practical question: How can they get this treatment paid for? One gastroenterologist said she has encountered considerable payer resistance when she has sought coverage of hyperbaric oxygen for patients with ulcerative colitis and fistulae, even though there is already published evidence of benefit. But Dr. Fahad’s groundbreaking study provides the first such evidence in pouchitis. So how did she and her coworkers do it? Eighty percent of the pouchitis patients obtained payer approval only upon appeal, which was readily granted, she explained.
Dr. Fahad reported having no financial conflicts regarding her study, conducted without commercial support.
SOURCE: Fahad H. ACG 2019 Abstract 38.
SAN ANTONIO – , Hamna Fahad, MD, reported at the annual meeting of the American College of Gastroenterology.
Dr. Fahad, of the Cleveland Clinic, presented a retrospective case series of 21 consecutive clinic patients who presented with inflammatory bowel disease, a surgically created ileal pouch–anal anastomosis, and medically refractory pouchitis. All patients received 30 hyperbaric oxygen treatment sessions, each an hour long, over the course of 2 months. This intensive regimen worked out to 3-5 sessions per week involving 100% oxygen pressurized to 2.4-3.0 ATA.
Overall, 19 of 21 patients experienced improvement in their modified Pouchitis Disease Activity Index (mPDAI) score. The mean total mPDAI at baseline was 8.71, improving significantly to 5 post treatment. The mPDAI symptoms subscore also showed significant improvement in response to a course of hyperbaric oxygen therapy, decreasing from 4 points to 2. The cuff subscore fell from 3 to 0, and the pouch body subscore improved from 3 to 2.
Thirteen of 21 patients reported subjective symptomatic improvement in stool frequency, bleeding, urgency, and fevers, including 6 with complete symptomatic remission. Seventeen patients demonstrated significant endoscopic improvement upon blinded assessment. Seven of 9 patients with fistulae experienced healing of the fistula tract.
The treatment entailed no side effects. However, the benefits weren’t uniformly durable. Several patients underwent a second 30-session round of hyperbaric oxygen therapy within a year because of recurrent pouchitis symptoms refractory to corticosteroids, biologics, and other medications.
Dr. Fahad said the mechanism of benefit for hyperbaric oxygen in the treatment of chronic inflammatory pouchitis is probably severalfold: reversal of a disordered microbiome through inhibition of the growth of anaerobes, reduced production of tumor necrosis factor–alpha and other inflammatory cytokines, and increased plasma oxygen, which reduces ischemia at the tissue level, thereby promoting tissue healing.
Audience members had a practical question: How can they get this treatment paid for? One gastroenterologist said she has encountered considerable payer resistance when she has sought coverage of hyperbaric oxygen for patients with ulcerative colitis and fistulae, even though there is already published evidence of benefit. But Dr. Fahad’s groundbreaking study provides the first such evidence in pouchitis. So how did she and her coworkers do it? Eighty percent of the pouchitis patients obtained payer approval only upon appeal, which was readily granted, she explained.
Dr. Fahad reported having no financial conflicts regarding her study, conducted without commercial support.
SOURCE: Fahad H. ACG 2019 Abstract 38.
REPORTING FROM ACG 2019
Eluxadoline effective for IBS in loperamide nonresponders
SAN ANTONIO – Darren M. Brenner, MD, reported at the annual meeting of the American College of Gastroenterology.
“From the totality of the clinical trials data we have now, we believe that eluxadoline can be effective both in patients who are naive to other treatments and in patients who have failed loperamide therapy,” concluded Dr. Brenner, a gastroenterologist at Northwestern University, Chicago.
Eluxadoline (Viberzi) is a novel mixed mu- and kappa-opioid receptor agonist and delta-opioid receptor antagonist approved by the Food and Drug Administration for treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults. In contrast, loperamide, a mu-opioid receptor agonist, is not approved for that indication. Yet loperamide is widely prescribed for this purpose, despite the fact that both the Canadian Association of Gastroenterology and the ACG now recommend against this practice.
“There is a lack of conclusive evidence to support the use of loperamide for the relief of global IBS-D symptoms. It works on the stool symptoms – stool frequency and texture – but has never been shown to be beneficial for the abdominal pain symptoms or discomfort or bloating. That being said, as practitioners we continue to see loperamide used as a first-line agent,” Dr. Brenner noted.
RELIEF was a multicenter, prospective, double-blind study which randomized 346 patients with moderate to severe IBS-D to eluxadoline at 100 mg twice daily or placebo for 12 weeks. All participants were required to have an intact gallbladder as per the drug’s labeling guidance, and all had a self-reported recent inadequate response to loperamide.
The primary composite endpoint in the RELIEF trial was a 40% or greater improvement from baseline in the 11-point Daily Worst Abdominal Pain score plus a Bristol Stool Form score below 5 on the same day for at least 50% of study days. At baseline, participants had an average Worst Abdominal Pain score of 6.2 on the 0-10 scale and a Bristol score of 6.2. The primary endpoint was achieved at week 12 in 23% of the eluxadoline group, significantly better than the 10% rate in controls. The eluxadoline group also showed significantly greater improvement on the many secondary endpoints having to do with urgency-free days, stool consistency, bowel movement frequency, abdominal discomfort, bloating, and the experience of adequate relief of symptoms.
The safety profile of eluxadoline mirrored that of placebo, with no serious adverse events recorded in either study arm and a 2.9% study discontinuation rate because of treatment-emergent adverse events in the eluxadoline group. Asked why he thinks eluxadoline was effective in improving the full range of IBS-D symptoms when loperamide wasn’t, even though both drugs are mu-opioid receptor agonists, Dr. Brenner replied, “The problem is mu receptors line the entire GI tract, so you can actually push somebody from diarrhea to opioid-induced constipation – and that’s not the goal. What delta does is alleviate some of the adverse events by binding to the receptor, which results in increased transit time, reduced secretion, and increased absorption. Delta brings things back towards the center. We also believe antagonism of delta potentiates analgesic effects at the mu receptor, improves the pain component, gut symptoms, and stool symptoms.”
Dr. Brenner reported serving as a consultant to and member of a speaker’s bureau for Allergan, which markets eluxadoline and sponsored the RELIEF trial.
SAN ANTONIO – Darren M. Brenner, MD, reported at the annual meeting of the American College of Gastroenterology.
“From the totality of the clinical trials data we have now, we believe that eluxadoline can be effective both in patients who are naive to other treatments and in patients who have failed loperamide therapy,” concluded Dr. Brenner, a gastroenterologist at Northwestern University, Chicago.
Eluxadoline (Viberzi) is a novel mixed mu- and kappa-opioid receptor agonist and delta-opioid receptor antagonist approved by the Food and Drug Administration for treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults. In contrast, loperamide, a mu-opioid receptor agonist, is not approved for that indication. Yet loperamide is widely prescribed for this purpose, despite the fact that both the Canadian Association of Gastroenterology and the ACG now recommend against this practice.
“There is a lack of conclusive evidence to support the use of loperamide for the relief of global IBS-D symptoms. It works on the stool symptoms – stool frequency and texture – but has never been shown to be beneficial for the abdominal pain symptoms or discomfort or bloating. That being said, as practitioners we continue to see loperamide used as a first-line agent,” Dr. Brenner noted.
RELIEF was a multicenter, prospective, double-blind study which randomized 346 patients with moderate to severe IBS-D to eluxadoline at 100 mg twice daily or placebo for 12 weeks. All participants were required to have an intact gallbladder as per the drug’s labeling guidance, and all had a self-reported recent inadequate response to loperamide.
The primary composite endpoint in the RELIEF trial was a 40% or greater improvement from baseline in the 11-point Daily Worst Abdominal Pain score plus a Bristol Stool Form score below 5 on the same day for at least 50% of study days. At baseline, participants had an average Worst Abdominal Pain score of 6.2 on the 0-10 scale and a Bristol score of 6.2. The primary endpoint was achieved at week 12 in 23% of the eluxadoline group, significantly better than the 10% rate in controls. The eluxadoline group also showed significantly greater improvement on the many secondary endpoints having to do with urgency-free days, stool consistency, bowel movement frequency, abdominal discomfort, bloating, and the experience of adequate relief of symptoms.
The safety profile of eluxadoline mirrored that of placebo, with no serious adverse events recorded in either study arm and a 2.9% study discontinuation rate because of treatment-emergent adverse events in the eluxadoline group. Asked why he thinks eluxadoline was effective in improving the full range of IBS-D symptoms when loperamide wasn’t, even though both drugs are mu-opioid receptor agonists, Dr. Brenner replied, “The problem is mu receptors line the entire GI tract, so you can actually push somebody from diarrhea to opioid-induced constipation – and that’s not the goal. What delta does is alleviate some of the adverse events by binding to the receptor, which results in increased transit time, reduced secretion, and increased absorption. Delta brings things back towards the center. We also believe antagonism of delta potentiates analgesic effects at the mu receptor, improves the pain component, gut symptoms, and stool symptoms.”
Dr. Brenner reported serving as a consultant to and member of a speaker’s bureau for Allergan, which markets eluxadoline and sponsored the RELIEF trial.
SAN ANTONIO – Darren M. Brenner, MD, reported at the annual meeting of the American College of Gastroenterology.
“From the totality of the clinical trials data we have now, we believe that eluxadoline can be effective both in patients who are naive to other treatments and in patients who have failed loperamide therapy,” concluded Dr. Brenner, a gastroenterologist at Northwestern University, Chicago.
Eluxadoline (Viberzi) is a novel mixed mu- and kappa-opioid receptor agonist and delta-opioid receptor antagonist approved by the Food and Drug Administration for treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults. In contrast, loperamide, a mu-opioid receptor agonist, is not approved for that indication. Yet loperamide is widely prescribed for this purpose, despite the fact that both the Canadian Association of Gastroenterology and the ACG now recommend against this practice.
“There is a lack of conclusive evidence to support the use of loperamide for the relief of global IBS-D symptoms. It works on the stool symptoms – stool frequency and texture – but has never been shown to be beneficial for the abdominal pain symptoms or discomfort or bloating. That being said, as practitioners we continue to see loperamide used as a first-line agent,” Dr. Brenner noted.
RELIEF was a multicenter, prospective, double-blind study which randomized 346 patients with moderate to severe IBS-D to eluxadoline at 100 mg twice daily or placebo for 12 weeks. All participants were required to have an intact gallbladder as per the drug’s labeling guidance, and all had a self-reported recent inadequate response to loperamide.
The primary composite endpoint in the RELIEF trial was a 40% or greater improvement from baseline in the 11-point Daily Worst Abdominal Pain score plus a Bristol Stool Form score below 5 on the same day for at least 50% of study days. At baseline, participants had an average Worst Abdominal Pain score of 6.2 on the 0-10 scale and a Bristol score of 6.2. The primary endpoint was achieved at week 12 in 23% of the eluxadoline group, significantly better than the 10% rate in controls. The eluxadoline group also showed significantly greater improvement on the many secondary endpoints having to do with urgency-free days, stool consistency, bowel movement frequency, abdominal discomfort, bloating, and the experience of adequate relief of symptoms.
The safety profile of eluxadoline mirrored that of placebo, with no serious adverse events recorded in either study arm and a 2.9% study discontinuation rate because of treatment-emergent adverse events in the eluxadoline group. Asked why he thinks eluxadoline was effective in improving the full range of IBS-D symptoms when loperamide wasn’t, even though both drugs are mu-opioid receptor agonists, Dr. Brenner replied, “The problem is mu receptors line the entire GI tract, so you can actually push somebody from diarrhea to opioid-induced constipation – and that’s not the goal. What delta does is alleviate some of the adverse events by binding to the receptor, which results in increased transit time, reduced secretion, and increased absorption. Delta brings things back towards the center. We also believe antagonism of delta potentiates analgesic effects at the mu receptor, improves the pain component, gut symptoms, and stool symptoms.”
Dr. Brenner reported serving as a consultant to and member of a speaker’s bureau for Allergan, which markets eluxadoline and sponsored the RELIEF trial.
REPORTING FROM ACG 2019
New ustekinumab response predictor in Crohn’s called ‘brilliant’
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.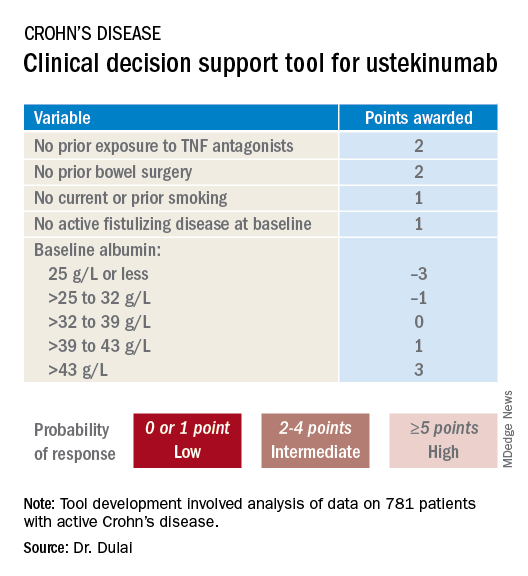
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
REPORTING FROM ACG 2019
New ustekinumab response predictor in Crohn’s called ‘brilliant’
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
REPORTING FROM ACG 2019
Human milk oligosaccharides quell IBS symptoms
SAN ANTONIO – Oral supplementation with a proprietary blend of human milk oligosaccharides improved all of the core symptoms of irritable bowel syndrome in a large open-label study, Magnus Simren, MD, PhD, reported at the annual meeting of the American College of Gastroenterology.
The human milk oligosaccharides (HMOs) were well tolerated, too. Only 2.5% of 317 study participants at 17 U.S. sites discontinued the 12-week study because of side effects, which consisted of flatulence and other mild gastrointestinal symptoms, noted Dr. Simren, a gastroenterologist and professor of medicine at the University of Gothenburg (Sweden).
These positive study results are consistent with the notion that an altered gut microbiota plays a pathophysiological role in irritable bowel syndrome (IBS).
“The challenge is to identify suitable interventions that restore intestinal microbiota composition and functioning,” Dr. Simren observed.
Oral HMOs show promise as one such intervention. In prior small proof-of-concept studies, Dr. Simren and his coworkers demonstrated that HMOs increased gut levels of Bifidobacteria, which are microorganisms important to a healthy gut and are depleted in IBS. The investigators also established that HMOs increased levels of metabolites essential for the gut’s barrier and immune functions.
HMOs are the third-largest constituent in human breast milk. Interest in their potential therapeutic application in IBS grew out of earlier pediatric work demonstrating that HMOs are of great importance in infant health: They bind pathogens and promote gut barrier maturation and immune function.
Dr. Simren reported on 317 patients who met Rome IV criteria for IBS. Nearly two-thirds of them had severe IBS based upon an IBS–Symptom Severity Score above 300. Another third had moderate IBS. Subjects were instructed to take 5 g/day of a 4:1 mix of the HMOs 2’-fucosyllactose and lacto-N-neotetraose, a proprietary nutritional support product available over the counter as Holigos. Participants remained on stable background medications throughout the 12-week study, during which they were evaluated every 4 weeks.
The primary outcome was the effect of daily oral consumption of HMOs on stool consistency as assessed using the Bristol Stool Form Scale. At baseline, 50.3% of subjects had IBS constipation as defined by Bristol type 1-2 stools. By week 4, the proportion of patients with constipation dropped to 32.9%, and at weeks 8 and 12, just under 31%. Similarly, the proportion of patients with diarrhea as reflected in Bristol type 6-7 stools quickly improved from 40.4% at baseline to 27.5% at week 4 and 26% thereafter. Meanwhile, the proportion of patients with normal stools on the Bristol scale jumped from 9.3% at baseline to 39.6% at week 4 and nearly 43% thereafter.
About 77% of patients reported a significant reduction in symptom severity within 4 weeks, and 87% did so by 12 weeks. Bloating decreased by 59%, as did abdominal pain severity. In addition, scores on the IBS Quality of Life Scale improved by 48%.
The observed improvements in symptoms and quality of life were consistent across all IBS subtypes.
“Of course, the next step now is to perform a randomized, placebo-controlled, double-blind study to see if these encouraging results can be confirmed in that setting,” Dr. Simren commented.
Session comoderator Brooks D. Cash, MD, of the University of Texas, Houston, called the HMO study “very provocative” and declared he is looking forward to the randomized, controlled trial, which he hopes will assess the long-term durability of the treatment benefits. That trial is still in the planning stages.
Dr. Simren reported serving on an advisory board for Glycom, the Danish company which markets Holigos and sponsored the open-label U.S. study.
SAN ANTONIO – Oral supplementation with a proprietary blend of human milk oligosaccharides improved all of the core symptoms of irritable bowel syndrome in a large open-label study, Magnus Simren, MD, PhD, reported at the annual meeting of the American College of Gastroenterology.
The human milk oligosaccharides (HMOs) were well tolerated, too. Only 2.5% of 317 study participants at 17 U.S. sites discontinued the 12-week study because of side effects, which consisted of flatulence and other mild gastrointestinal symptoms, noted Dr. Simren, a gastroenterologist and professor of medicine at the University of Gothenburg (Sweden).
These positive study results are consistent with the notion that an altered gut microbiota plays a pathophysiological role in irritable bowel syndrome (IBS).
“The challenge is to identify suitable interventions that restore intestinal microbiota composition and functioning,” Dr. Simren observed.
Oral HMOs show promise as one such intervention. In prior small proof-of-concept studies, Dr. Simren and his coworkers demonstrated that HMOs increased gut levels of Bifidobacteria, which are microorganisms important to a healthy gut and are depleted in IBS. The investigators also established that HMOs increased levels of metabolites essential for the gut’s barrier and immune functions.
HMOs are the third-largest constituent in human breast milk. Interest in their potential therapeutic application in IBS grew out of earlier pediatric work demonstrating that HMOs are of great importance in infant health: They bind pathogens and promote gut barrier maturation and immune function.
Dr. Simren reported on 317 patients who met Rome IV criteria for IBS. Nearly two-thirds of them had severe IBS based upon an IBS–Symptom Severity Score above 300. Another third had moderate IBS. Subjects were instructed to take 5 g/day of a 4:1 mix of the HMOs 2’-fucosyllactose and lacto-N-neotetraose, a proprietary nutritional support product available over the counter as Holigos. Participants remained on stable background medications throughout the 12-week study, during which they were evaluated every 4 weeks.
The primary outcome was the effect of daily oral consumption of HMOs on stool consistency as assessed using the Bristol Stool Form Scale. At baseline, 50.3% of subjects had IBS constipation as defined by Bristol type 1-2 stools. By week 4, the proportion of patients with constipation dropped to 32.9%, and at weeks 8 and 12, just under 31%. Similarly, the proportion of patients with diarrhea as reflected in Bristol type 6-7 stools quickly improved from 40.4% at baseline to 27.5% at week 4 and 26% thereafter. Meanwhile, the proportion of patients with normal stools on the Bristol scale jumped from 9.3% at baseline to 39.6% at week 4 and nearly 43% thereafter.
About 77% of patients reported a significant reduction in symptom severity within 4 weeks, and 87% did so by 12 weeks. Bloating decreased by 59%, as did abdominal pain severity. In addition, scores on the IBS Quality of Life Scale improved by 48%.
The observed improvements in symptoms and quality of life were consistent across all IBS subtypes.
“Of course, the next step now is to perform a randomized, placebo-controlled, double-blind study to see if these encouraging results can be confirmed in that setting,” Dr. Simren commented.
Session comoderator Brooks D. Cash, MD, of the University of Texas, Houston, called the HMO study “very provocative” and declared he is looking forward to the randomized, controlled trial, which he hopes will assess the long-term durability of the treatment benefits. That trial is still in the planning stages.
Dr. Simren reported serving on an advisory board for Glycom, the Danish company which markets Holigos and sponsored the open-label U.S. study.
SAN ANTONIO – Oral supplementation with a proprietary blend of human milk oligosaccharides improved all of the core symptoms of irritable bowel syndrome in a large open-label study, Magnus Simren, MD, PhD, reported at the annual meeting of the American College of Gastroenterology.
The human milk oligosaccharides (HMOs) were well tolerated, too. Only 2.5% of 317 study participants at 17 U.S. sites discontinued the 12-week study because of side effects, which consisted of flatulence and other mild gastrointestinal symptoms, noted Dr. Simren, a gastroenterologist and professor of medicine at the University of Gothenburg (Sweden).
These positive study results are consistent with the notion that an altered gut microbiota plays a pathophysiological role in irritable bowel syndrome (IBS).
“The challenge is to identify suitable interventions that restore intestinal microbiota composition and functioning,” Dr. Simren observed.
Oral HMOs show promise as one such intervention. In prior small proof-of-concept studies, Dr. Simren and his coworkers demonstrated that HMOs increased gut levels of Bifidobacteria, which are microorganisms important to a healthy gut and are depleted in IBS. The investigators also established that HMOs increased levels of metabolites essential for the gut’s barrier and immune functions.
HMOs are the third-largest constituent in human breast milk. Interest in their potential therapeutic application in IBS grew out of earlier pediatric work demonstrating that HMOs are of great importance in infant health: They bind pathogens and promote gut barrier maturation and immune function.
Dr. Simren reported on 317 patients who met Rome IV criteria for IBS. Nearly two-thirds of them had severe IBS based upon an IBS–Symptom Severity Score above 300. Another third had moderate IBS. Subjects were instructed to take 5 g/day of a 4:1 mix of the HMOs 2’-fucosyllactose and lacto-N-neotetraose, a proprietary nutritional support product available over the counter as Holigos. Participants remained on stable background medications throughout the 12-week study, during which they were evaluated every 4 weeks.
The primary outcome was the effect of daily oral consumption of HMOs on stool consistency as assessed using the Bristol Stool Form Scale. At baseline, 50.3% of subjects had IBS constipation as defined by Bristol type 1-2 stools. By week 4, the proportion of patients with constipation dropped to 32.9%, and at weeks 8 and 12, just under 31%. Similarly, the proportion of patients with diarrhea as reflected in Bristol type 6-7 stools quickly improved from 40.4% at baseline to 27.5% at week 4 and 26% thereafter. Meanwhile, the proportion of patients with normal stools on the Bristol scale jumped from 9.3% at baseline to 39.6% at week 4 and nearly 43% thereafter.
About 77% of patients reported a significant reduction in symptom severity within 4 weeks, and 87% did so by 12 weeks. Bloating decreased by 59%, as did abdominal pain severity. In addition, scores on the IBS Quality of Life Scale improved by 48%.
The observed improvements in symptoms and quality of life were consistent across all IBS subtypes.
“Of course, the next step now is to perform a randomized, placebo-controlled, double-blind study to see if these encouraging results can be confirmed in that setting,” Dr. Simren commented.
Session comoderator Brooks D. Cash, MD, of the University of Texas, Houston, called the HMO study “very provocative” and declared he is looking forward to the randomized, controlled trial, which he hopes will assess the long-term durability of the treatment benefits. That trial is still in the planning stages.
Dr. Simren reported serving on an advisory board for Glycom, the Danish company which markets Holigos and sponsored the open-label U.S. study.
REPORTING FROM ACG 2019
Biofeedback corrects dyssynergic constipation in elderly
SAN ANTONIO – Biofeedback for treatment of dyssynergic constipation is highly effective in the elderly, just as it is in younger patients, Samantha Spilman, MD, reported at the annual meeting of the American College of Gastroenterology.
“I think the main point of this study is that older adults have a profound burden of constipation with dyssynergic defecation, and we propose that biofeedback be given strong consideration as first-line therapy for this population, in whom overall we’re trying to reduce medication use,” said Dr. Spilman, a gastroenterology fellow at the University of California, San Diego.
The prevalence of constipation in older patients is estimated to be up to 40%. Yet few prior studies have scrutinized how well older patients with constipation actually respond to biofeedback. It’s a legitimate question, since biofeedback training involves operant conditioning and requires learning new techniques. For this reason, she and her coinvestigators conducted a retrospective analysis of 58 patients over age 65 referred from the university’s gastrointestinal motility and physiology program to the biofeedback program for treatment of dyssynergic defection. The patients’ mean age was 74 years, with a 9.5-year history of constipation. The oldest patient was 88. Most of the subjects were high school graduates. Thirteen of the 58 carried a diagnosis of irritable bowel syndrome.
Numerous studies have demonstrated that 70%-80% of younger adults with dyssynergic constipation experience marked improvement in response to biofeedback training, which typically utilizes an inflated rectal balloon to simulate retained stool. The key finding in Dr. Spilman’s study was that the elderly patients did comparably well in terms of both self-reported outcomes and objective high-resolution anorectal manometric parameters upon completing an average of three biofeedback sessions.
Mean global bowel satisfaction on a 1-10 scale nearly doubled from 2.77 at baseline to 5.01 with biofeedback. Moreover, 79% of seniors demonstrated resolution of their dyssynergia on high-resolution anorectal manometry performed with sensors in the rectum and anal canal. The proportion of patients who reported a feeling of incomplete evacuation after stooling – a sensation individuals with constipation find highly bothersome – improved from 95% to 24% with biofeedback.
The strongest response in terms of the defecation index was observed in older patients with type 2 dyssynergia, characterized by defective propulsion coupled with a paradoxical contraction of the sphincter muscles during defecation. Their defecation index score, derived by dividing intrarectal pressure by residual intra-anal pressure during simulated defection, showed a robust improvement from 0.307 at baseline to 0.793. Patients with types 1 and 3 dyssynergia showed lesser improvements on this objective measure.
Dr. Spilman noted as a study limitation that baseline cognitive status wasn’t formally assessed, so the investigators don’t know how many of the older patients had minimal cognitive impairment. However, baseline quality of life assessment via the Short Form-36 indicated that patients scored average or above for physical and social functioning as well as emotional well-being.
Dr. Spilman reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Spilman S. ACG 2019. Abstract 45.
SAN ANTONIO – Biofeedback for treatment of dyssynergic constipation is highly effective in the elderly, just as it is in younger patients, Samantha Spilman, MD, reported at the annual meeting of the American College of Gastroenterology.
“I think the main point of this study is that older adults have a profound burden of constipation with dyssynergic defecation, and we propose that biofeedback be given strong consideration as first-line therapy for this population, in whom overall we’re trying to reduce medication use,” said Dr. Spilman, a gastroenterology fellow at the University of California, San Diego.
The prevalence of constipation in older patients is estimated to be up to 40%. Yet few prior studies have scrutinized how well older patients with constipation actually respond to biofeedback. It’s a legitimate question, since biofeedback training involves operant conditioning and requires learning new techniques. For this reason, she and her coinvestigators conducted a retrospective analysis of 58 patients over age 65 referred from the university’s gastrointestinal motility and physiology program to the biofeedback program for treatment of dyssynergic defection. The patients’ mean age was 74 years, with a 9.5-year history of constipation. The oldest patient was 88. Most of the subjects were high school graduates. Thirteen of the 58 carried a diagnosis of irritable bowel syndrome.
Numerous studies have demonstrated that 70%-80% of younger adults with dyssynergic constipation experience marked improvement in response to biofeedback training, which typically utilizes an inflated rectal balloon to simulate retained stool. The key finding in Dr. Spilman’s study was that the elderly patients did comparably well in terms of both self-reported outcomes and objective high-resolution anorectal manometric parameters upon completing an average of three biofeedback sessions.
Mean global bowel satisfaction on a 1-10 scale nearly doubled from 2.77 at baseline to 5.01 with biofeedback. Moreover, 79% of seniors demonstrated resolution of their dyssynergia on high-resolution anorectal manometry performed with sensors in the rectum and anal canal. The proportion of patients who reported a feeling of incomplete evacuation after stooling – a sensation individuals with constipation find highly bothersome – improved from 95% to 24% with biofeedback.
The strongest response in terms of the defecation index was observed in older patients with type 2 dyssynergia, characterized by defective propulsion coupled with a paradoxical contraction of the sphincter muscles during defecation. Their defecation index score, derived by dividing intrarectal pressure by residual intra-anal pressure during simulated defection, showed a robust improvement from 0.307 at baseline to 0.793. Patients with types 1 and 3 dyssynergia showed lesser improvements on this objective measure.
Dr. Spilman noted as a study limitation that baseline cognitive status wasn’t formally assessed, so the investigators don’t know how many of the older patients had minimal cognitive impairment. However, baseline quality of life assessment via the Short Form-36 indicated that patients scored average or above for physical and social functioning as well as emotional well-being.
Dr. Spilman reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Spilman S. ACG 2019. Abstract 45.
SAN ANTONIO – Biofeedback for treatment of dyssynergic constipation is highly effective in the elderly, just as it is in younger patients, Samantha Spilman, MD, reported at the annual meeting of the American College of Gastroenterology.
“I think the main point of this study is that older adults have a profound burden of constipation with dyssynergic defecation, and we propose that biofeedback be given strong consideration as first-line therapy for this population, in whom overall we’re trying to reduce medication use,” said Dr. Spilman, a gastroenterology fellow at the University of California, San Diego.
The prevalence of constipation in older patients is estimated to be up to 40%. Yet few prior studies have scrutinized how well older patients with constipation actually respond to biofeedback. It’s a legitimate question, since biofeedback training involves operant conditioning and requires learning new techniques. For this reason, she and her coinvestigators conducted a retrospective analysis of 58 patients over age 65 referred from the university’s gastrointestinal motility and physiology program to the biofeedback program for treatment of dyssynergic defection. The patients’ mean age was 74 years, with a 9.5-year history of constipation. The oldest patient was 88. Most of the subjects were high school graduates. Thirteen of the 58 carried a diagnosis of irritable bowel syndrome.
Numerous studies have demonstrated that 70%-80% of younger adults with dyssynergic constipation experience marked improvement in response to biofeedback training, which typically utilizes an inflated rectal balloon to simulate retained stool. The key finding in Dr. Spilman’s study was that the elderly patients did comparably well in terms of both self-reported outcomes and objective high-resolution anorectal manometric parameters upon completing an average of three biofeedback sessions.
Mean global bowel satisfaction on a 1-10 scale nearly doubled from 2.77 at baseline to 5.01 with biofeedback. Moreover, 79% of seniors demonstrated resolution of their dyssynergia on high-resolution anorectal manometry performed with sensors in the rectum and anal canal. The proportion of patients who reported a feeling of incomplete evacuation after stooling – a sensation individuals with constipation find highly bothersome – improved from 95% to 24% with biofeedback.
The strongest response in terms of the defecation index was observed in older patients with type 2 dyssynergia, characterized by defective propulsion coupled with a paradoxical contraction of the sphincter muscles during defecation. Their defecation index score, derived by dividing intrarectal pressure by residual intra-anal pressure during simulated defection, showed a robust improvement from 0.307 at baseline to 0.793. Patients with types 1 and 3 dyssynergia showed lesser improvements on this objective measure.
Dr. Spilman noted as a study limitation that baseline cognitive status wasn’t formally assessed, so the investigators don’t know how many of the older patients had minimal cognitive impairment. However, baseline quality of life assessment via the Short Form-36 indicated that patients scored average or above for physical and social functioning as well as emotional well-being.
Dr. Spilman reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Spilman S. ACG 2019. Abstract 45.
REPORTING FROM ACG 2019
Some with mild Crohn’s disease need no treatment
Kim L. Isaacs, MD, PhD, said at the annual meeting of the American College of Gastroenterology.
First, it’s essential to establish that a patient truly falls into the mild disease category. And second, during that initial evaluation it’s important to look for the features that signal a high risk of subsequent disease progression.
“Patients with risk factors for disease progression should not be treated as mild Crohn’s disease. These are people that we need to treat perhaps more aggressively up front,” said Dr. Isaacs, professor of medicine and codirector of the Multidisciplinary Center for IBD Research and Treatment at the University of North Carolina, Chapel Hill.
Management of patients with severe Crohn’s disease can be challenging, but Dr. Isaacs finds patients with mild disease flat out “terrifying.”
“The reason I find this situation terrifying is we want to prevent the surgery, the progression from inflammatory disease to more stricturing and penetrating disease. And how can I know that my patient a year from now is not going to be very, very ill and I’ve lost my window to use some of our more potent therapies?” she explained.
Mild Crohn’s disease is characterized clinically by less than 10% weight loss; no fever, tachycardia, or other symptoms of systemic disease; lack of abdominal tenderness; and no signs or symptoms of obstruction. When reading the literature, mild disease is defined by a Crohn’s Disease Activity Index score of 150-220. However, nobody uses that metric outside of research studies; it’s just too cumbersome. More useful in routine clinical practice is the Harvey-Bradshaw Index, where a score of 5-7 indicates mild disease.
Many patients with mild Crohn’s disease will not progress over time. In a recent prospective 29-center European study, just 14% of patients progressed from mild to stricturing and/or penetrating disease within 5 years after diagnosis (Gut. 2018 Jan 23. doi: 10.1136/gutjnl-2017-315568).
Who is likely to progress
Risk factors for disease progression include patients with ileocolonic or perianal disease, smokers, those with inflammatory arthritis or other associated immune-mediated disease, and patients who require corticosteroids in order to maintain remission.
On the other hand, patients with mild Crohn’s disease at low risk for progression have no or only mild symptoms, a limited distribution of bowel inflammation, normal or merely slightly elevated C-reactive protein and/or fecal calprotectin levels, no or only superficial bowel ulceration on colonoscopy, and were diagnosed with inflammatory bowel disease after age 30.
Treatment options
“Our goal in a patient we think is low risk is symptomatic management while avoiding high therapeutic risks in someone we don’t think is going to be progressing,” according to the gastroenterologist.
In patients with mild disease who have symptoms in the ileum or right colon, a good strategy is to induce remission using controlled ileal-release budesonide at 9 mg/day, then follow up with colonoscopy 6-12 months later. The high-dose budesonide regimen has been shown in multiple studies to be more effective than placebo at inducing remission. It’s less effective than conventional oral corticosteroids, but it also causes fewer steroid-related side effects. In fact, the evidence shows that the side effect profile of controlled-release budesonide is no different from that of placebo.
In low-risk, mildly symptomatic patients with diffuse Crohn’s colitis, Dr. Isaacs recommends initial treatment with prednisone and/or sulfasalazine or 5-aminosalicylates. The 5-aminosalicylates aren’t part of most practice guidelines because of conflicting study results. However, a meta-analysis of 22 randomized controlled trials has shown that high-dose mesalamine was 2.29-fold more effective than placebo at inducing remission and is a good option for patients who would rather avoid corticosteroids (Inflamm Bowel Dis. 2017 Mar;23(3):461-72). Prednisone should be tapered after achieving clinical remission. Inability to stop steroids within 6 months after entering remission warrants a switch to maintenance therapy with a biologic or immunomodulatory agent.
Smoking cessation should be a top priority
“I spend a good portion of my time in clinic telling patients, ‘You can do more with stopping smoking than I can do with any of my medications,’ ” Dr. Isaacs said.
Another worthwhile intervention is to check for and correct low serum vitamin D levels. Vitamin D is a potent immunomodulator, and severely low levels below 15 ng/mL are associated with increased risk of Crohn’s disease relapse, more hospitalizations, more active disease activity, and heavier use of corticosteroids than in patients with moderate deficiency in the 15- to 30-ng/mL range (Nutrients. 2019 May 11;11[5]. doi: 10.3390/nu11051059).
Monitoring patients with mild Crohn’s disease
“Monitor closely for mucosal inflammation because if they start to progress, we want to get to them early so they get a good response to their first therapies. If the CRP starts moving up, consider doing something,” she advised.
A CRP above 5 mg/L is associated with a markedly increased risk of relapse. And Spanish investigators have shown in a prospective study of 95 patients in clinical remission for at least 6 months while on a tumor necrosis factor inhibitor that thereafter a fecal calprotein level greater than 300 mcg/g at any point was strongly predictive of relapse within the next 4 months (J Clin Gastroenterol. 2018 Mar;52[3]:229-34). Following a patient with mild Crohn’s disease over time endoscopically, it’s of value to utilize the Simple Endoscopic Score for Crohn’s Disease (SES-CD) to document that in fact the disease is mild. Dr. Isaacs recommended IG-IBD Scores – Calculators in Gastroenterology as “a great site” for assistance in calculating the SES-CD, the Harvey-Bradshaw Index, and a plethora of other inflammatory bowel disease scores.
She reported having no financial conflicts of interest.
Kim L. Isaacs, MD, PhD, said at the annual meeting of the American College of Gastroenterology.
First, it’s essential to establish that a patient truly falls into the mild disease category. And second, during that initial evaluation it’s important to look for the features that signal a high risk of subsequent disease progression.
“Patients with risk factors for disease progression should not be treated as mild Crohn’s disease. These are people that we need to treat perhaps more aggressively up front,” said Dr. Isaacs, professor of medicine and codirector of the Multidisciplinary Center for IBD Research and Treatment at the University of North Carolina, Chapel Hill.
Management of patients with severe Crohn’s disease can be challenging, but Dr. Isaacs finds patients with mild disease flat out “terrifying.”
“The reason I find this situation terrifying is we want to prevent the surgery, the progression from inflammatory disease to more stricturing and penetrating disease. And how can I know that my patient a year from now is not going to be very, very ill and I’ve lost my window to use some of our more potent therapies?” she explained.
Mild Crohn’s disease is characterized clinically by less than 10% weight loss; no fever, tachycardia, or other symptoms of systemic disease; lack of abdominal tenderness; and no signs or symptoms of obstruction. When reading the literature, mild disease is defined by a Crohn’s Disease Activity Index score of 150-220. However, nobody uses that metric outside of research studies; it’s just too cumbersome. More useful in routine clinical practice is the Harvey-Bradshaw Index, where a score of 5-7 indicates mild disease.
Many patients with mild Crohn’s disease will not progress over time. In a recent prospective 29-center European study, just 14% of patients progressed from mild to stricturing and/or penetrating disease within 5 years after diagnosis (Gut. 2018 Jan 23. doi: 10.1136/gutjnl-2017-315568).
Who is likely to progress
Risk factors for disease progression include patients with ileocolonic or perianal disease, smokers, those with inflammatory arthritis or other associated immune-mediated disease, and patients who require corticosteroids in order to maintain remission.
On the other hand, patients with mild Crohn’s disease at low risk for progression have no or only mild symptoms, a limited distribution of bowel inflammation, normal or merely slightly elevated C-reactive protein and/or fecal calprotectin levels, no or only superficial bowel ulceration on colonoscopy, and were diagnosed with inflammatory bowel disease after age 30.
Treatment options
“Our goal in a patient we think is low risk is symptomatic management while avoiding high therapeutic risks in someone we don’t think is going to be progressing,” according to the gastroenterologist.
In patients with mild disease who have symptoms in the ileum or right colon, a good strategy is to induce remission using controlled ileal-release budesonide at 9 mg/day, then follow up with colonoscopy 6-12 months later. The high-dose budesonide regimen has been shown in multiple studies to be more effective than placebo at inducing remission. It’s less effective than conventional oral corticosteroids, but it also causes fewer steroid-related side effects. In fact, the evidence shows that the side effect profile of controlled-release budesonide is no different from that of placebo.
In low-risk, mildly symptomatic patients with diffuse Crohn’s colitis, Dr. Isaacs recommends initial treatment with prednisone and/or sulfasalazine or 5-aminosalicylates. The 5-aminosalicylates aren’t part of most practice guidelines because of conflicting study results. However, a meta-analysis of 22 randomized controlled trials has shown that high-dose mesalamine was 2.29-fold more effective than placebo at inducing remission and is a good option for patients who would rather avoid corticosteroids (Inflamm Bowel Dis. 2017 Mar;23(3):461-72). Prednisone should be tapered after achieving clinical remission. Inability to stop steroids within 6 months after entering remission warrants a switch to maintenance therapy with a biologic or immunomodulatory agent.
Smoking cessation should be a top priority
“I spend a good portion of my time in clinic telling patients, ‘You can do more with stopping smoking than I can do with any of my medications,’ ” Dr. Isaacs said.
Another worthwhile intervention is to check for and correct low serum vitamin D levels. Vitamin D is a potent immunomodulator, and severely low levels below 15 ng/mL are associated with increased risk of Crohn’s disease relapse, more hospitalizations, more active disease activity, and heavier use of corticosteroids than in patients with moderate deficiency in the 15- to 30-ng/mL range (Nutrients. 2019 May 11;11[5]. doi: 10.3390/nu11051059).
Monitoring patients with mild Crohn’s disease
“Monitor closely for mucosal inflammation because if they start to progress, we want to get to them early so they get a good response to their first therapies. If the CRP starts moving up, consider doing something,” she advised.
A CRP above 5 mg/L is associated with a markedly increased risk of relapse. And Spanish investigators have shown in a prospective study of 95 patients in clinical remission for at least 6 months while on a tumor necrosis factor inhibitor that thereafter a fecal calprotein level greater than 300 mcg/g at any point was strongly predictive of relapse within the next 4 months (J Clin Gastroenterol. 2018 Mar;52[3]:229-34). Following a patient with mild Crohn’s disease over time endoscopically, it’s of value to utilize the Simple Endoscopic Score for Crohn’s Disease (SES-CD) to document that in fact the disease is mild. Dr. Isaacs recommended IG-IBD Scores – Calculators in Gastroenterology as “a great site” for assistance in calculating the SES-CD, the Harvey-Bradshaw Index, and a plethora of other inflammatory bowel disease scores.
She reported having no financial conflicts of interest.
Kim L. Isaacs, MD, PhD, said at the annual meeting of the American College of Gastroenterology.
First, it’s essential to establish that a patient truly falls into the mild disease category. And second, during that initial evaluation it’s important to look for the features that signal a high risk of subsequent disease progression.
“Patients with risk factors for disease progression should not be treated as mild Crohn’s disease. These are people that we need to treat perhaps more aggressively up front,” said Dr. Isaacs, professor of medicine and codirector of the Multidisciplinary Center for IBD Research and Treatment at the University of North Carolina, Chapel Hill.
Management of patients with severe Crohn’s disease can be challenging, but Dr. Isaacs finds patients with mild disease flat out “terrifying.”
“The reason I find this situation terrifying is we want to prevent the surgery, the progression from inflammatory disease to more stricturing and penetrating disease. And how can I know that my patient a year from now is not going to be very, very ill and I’ve lost my window to use some of our more potent therapies?” she explained.
Mild Crohn’s disease is characterized clinically by less than 10% weight loss; no fever, tachycardia, or other symptoms of systemic disease; lack of abdominal tenderness; and no signs or symptoms of obstruction. When reading the literature, mild disease is defined by a Crohn’s Disease Activity Index score of 150-220. However, nobody uses that metric outside of research studies; it’s just too cumbersome. More useful in routine clinical practice is the Harvey-Bradshaw Index, where a score of 5-7 indicates mild disease.
Many patients with mild Crohn’s disease will not progress over time. In a recent prospective 29-center European study, just 14% of patients progressed from mild to stricturing and/or penetrating disease within 5 years after diagnosis (Gut. 2018 Jan 23. doi: 10.1136/gutjnl-2017-315568).
Who is likely to progress
Risk factors for disease progression include patients with ileocolonic or perianal disease, smokers, those with inflammatory arthritis or other associated immune-mediated disease, and patients who require corticosteroids in order to maintain remission.
On the other hand, patients with mild Crohn’s disease at low risk for progression have no or only mild symptoms, a limited distribution of bowel inflammation, normal or merely slightly elevated C-reactive protein and/or fecal calprotectin levels, no or only superficial bowel ulceration on colonoscopy, and were diagnosed with inflammatory bowel disease after age 30.
Treatment options
“Our goal in a patient we think is low risk is symptomatic management while avoiding high therapeutic risks in someone we don’t think is going to be progressing,” according to the gastroenterologist.
In patients with mild disease who have symptoms in the ileum or right colon, a good strategy is to induce remission using controlled ileal-release budesonide at 9 mg/day, then follow up with colonoscopy 6-12 months later. The high-dose budesonide regimen has been shown in multiple studies to be more effective than placebo at inducing remission. It’s less effective than conventional oral corticosteroids, but it also causes fewer steroid-related side effects. In fact, the evidence shows that the side effect profile of controlled-release budesonide is no different from that of placebo.
In low-risk, mildly symptomatic patients with diffuse Crohn’s colitis, Dr. Isaacs recommends initial treatment with prednisone and/or sulfasalazine or 5-aminosalicylates. The 5-aminosalicylates aren’t part of most practice guidelines because of conflicting study results. However, a meta-analysis of 22 randomized controlled trials has shown that high-dose mesalamine was 2.29-fold more effective than placebo at inducing remission and is a good option for patients who would rather avoid corticosteroids (Inflamm Bowel Dis. 2017 Mar;23(3):461-72). Prednisone should be tapered after achieving clinical remission. Inability to stop steroids within 6 months after entering remission warrants a switch to maintenance therapy with a biologic or immunomodulatory agent.
Smoking cessation should be a top priority
“I spend a good portion of my time in clinic telling patients, ‘You can do more with stopping smoking than I can do with any of my medications,’ ” Dr. Isaacs said.
Another worthwhile intervention is to check for and correct low serum vitamin D levels. Vitamin D is a potent immunomodulator, and severely low levels below 15 ng/mL are associated with increased risk of Crohn’s disease relapse, more hospitalizations, more active disease activity, and heavier use of corticosteroids than in patients with moderate deficiency in the 15- to 30-ng/mL range (Nutrients. 2019 May 11;11[5]. doi: 10.3390/nu11051059).
Monitoring patients with mild Crohn’s disease
“Monitor closely for mucosal inflammation because if they start to progress, we want to get to them early so they get a good response to their first therapies. If the CRP starts moving up, consider doing something,” she advised.
A CRP above 5 mg/L is associated with a markedly increased risk of relapse. And Spanish investigators have shown in a prospective study of 95 patients in clinical remission for at least 6 months while on a tumor necrosis factor inhibitor that thereafter a fecal calprotein level greater than 300 mcg/g at any point was strongly predictive of relapse within the next 4 months (J Clin Gastroenterol. 2018 Mar;52[3]:229-34). Following a patient with mild Crohn’s disease over time endoscopically, it’s of value to utilize the Simple Endoscopic Score for Crohn’s Disease (SES-CD) to document that in fact the disease is mild. Dr. Isaacs recommended IG-IBD Scores – Calculators in Gastroenterology as “a great site” for assistance in calculating the SES-CD, the Harvey-Bradshaw Index, and a plethora of other inflammatory bowel disease scores.
She reported having no financial conflicts of interest.
REPORTING FROM ACG 2019
New evidence further supports starting CRC screening at age 45
SAN ANTONIO – The American Cancer Society’s 2018 qualified recommendation to lower the starting age for colorectal cancer screening from 50 to 45 years in average-risk individuals has picked up new support from a New Hampshire Colonoscopy Registry analysis.
Data from the population-based statewide colonoscopy registry demonstrated that the prevalence of both advanced adenomas and clinically significant serrated polyps was closely similar for average-risk New Hampshirites age 45-49 years and for those age 50-54, Lynn F. Butterly, MD, reported at the annual meeting of the American College of Gastroenterology.
“The clinical implication is that our data support the recommendation to begin average-risk colorectal cancer screening at age 45,” declared Dr. Butterly, a gastroenterologist at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
The American Cancer Society recommendation to lower the initial screening age was designed to address a disturbing national trend: the climbing incidence of colorectal cancer in young adults. Indeed, the incidence increased by 55% among 20- to 49-year-olds during 1995-2016, even while falling by 38% in individuals age 50 years and older. The 2018 recommendation was billed as “qualified” because it was based upon predictive modeling and National Cancer Institute Surveillance, Epidemiology, and End Results data which have been criticized as subject to potential bias. Several studies conducted in Korea and other Asian countries have reported a lower colorectal cancer risk in the younger adult population than in those age 50 or older, but questions have been raised about the applicability of such data to the U.S. population.
For Dr. Butterly and coinvestigators, the research imperative was clear: “We need to generate U.S. outcomes data for average-risk individuals age 45-49, versus those over age 50, for whom colorectal cancer screening is already strongly recommended.”
Toward that end, the investigators turned to the New Hampshire Colonoscopy Registry, which contains detailed data on 200,000 colonoscopies, with some 400 variables recorded per patient. To zero in on an average-risk population below age 50, they restricted the analysis to patients undergoing their first colonoscopy for evaluation of low-risk conditions including abdominal pain or constipation while excluding those with GI bleeding, iron-deficiency anemia, abnormal imaging, or a family history of colorectal cancer.
The final study population included 42,600 New Hampshire residents who underwent their first colonoscopy. The key outcomes were the prevalence of advanced adenomas, defined as adenomas more than 1 cm in size, or with high-grade dysplasia or villous elements, and the prevalence of clinically significant serrated polyps larger than 1 cm, or larger than 5 mm if proximally located, as well as traditional serrated adenomas and those with sessile features.
The prevalence of advanced adenomas in 1,870 average-risk patients aged 45-49 years was 3.7% and nearly identical at 3.6% in 22,160 individuals undergoing screening colonoscopy at age 50-54. The rate of clinically significant serrated polyps was 5.9% in the 45- to 49-year-olds, closely similar to the 6.1% rate in patients age 50-54.
Of note, the prevalence of advanced adenomas was just 1.1% in individuals younger than age 40 years, jumping to 3.0% among 40- to 44-year-olds, 5.1% in those age 55-59, and 6.9% at age 60 or more. Clinically significant serrated polyps followed a similar pattern, with rates of 3.0% before age 40, 5.1% in 40- to 44-year-olds, 6.6% in 55- to 59-year-olds, and 6.0% in those who were older.
In a multivariate logistic regression analysis adjusted for sex, body mass index, smoking, and other potential confounders, 45- to 49-year-olds were at a 243% increased risk of finding advanced adenomas on colonoscopy, compared with those less than 40 years old, while the 50- to 54-year-olds had a virtually identical 244% increased risk.
Dr. Butterly noted that there are now 15,000 cases of colorectal cancer occurring annually in individuals under age 50 in the United States, with 3,600 deaths.
“Prevention of colorectal cancer in young, productive individuals is an essential clinical imperative that must be addressed,” she concluded.
She reported having no financial conflicts regarding her study.
SAN ANTONIO – The American Cancer Society’s 2018 qualified recommendation to lower the starting age for colorectal cancer screening from 50 to 45 years in average-risk individuals has picked up new support from a New Hampshire Colonoscopy Registry analysis.
Data from the population-based statewide colonoscopy registry demonstrated that the prevalence of both advanced adenomas and clinically significant serrated polyps was closely similar for average-risk New Hampshirites age 45-49 years and for those age 50-54, Lynn F. Butterly, MD, reported at the annual meeting of the American College of Gastroenterology.
“The clinical implication is that our data support the recommendation to begin average-risk colorectal cancer screening at age 45,” declared Dr. Butterly, a gastroenterologist at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
The American Cancer Society recommendation to lower the initial screening age was designed to address a disturbing national trend: the climbing incidence of colorectal cancer in young adults. Indeed, the incidence increased by 55% among 20- to 49-year-olds during 1995-2016, even while falling by 38% in individuals age 50 years and older. The 2018 recommendation was billed as “qualified” because it was based upon predictive modeling and National Cancer Institute Surveillance, Epidemiology, and End Results data which have been criticized as subject to potential bias. Several studies conducted in Korea and other Asian countries have reported a lower colorectal cancer risk in the younger adult population than in those age 50 or older, but questions have been raised about the applicability of such data to the U.S. population.
For Dr. Butterly and coinvestigators, the research imperative was clear: “We need to generate U.S. outcomes data for average-risk individuals age 45-49, versus those over age 50, for whom colorectal cancer screening is already strongly recommended.”
Toward that end, the investigators turned to the New Hampshire Colonoscopy Registry, which contains detailed data on 200,000 colonoscopies, with some 400 variables recorded per patient. To zero in on an average-risk population below age 50, they restricted the analysis to patients undergoing their first colonoscopy for evaluation of low-risk conditions including abdominal pain or constipation while excluding those with GI bleeding, iron-deficiency anemia, abnormal imaging, or a family history of colorectal cancer.
The final study population included 42,600 New Hampshire residents who underwent their first colonoscopy. The key outcomes were the prevalence of advanced adenomas, defined as adenomas more than 1 cm in size, or with high-grade dysplasia or villous elements, and the prevalence of clinically significant serrated polyps larger than 1 cm, or larger than 5 mm if proximally located, as well as traditional serrated adenomas and those with sessile features.
The prevalence of advanced adenomas in 1,870 average-risk patients aged 45-49 years was 3.7% and nearly identical at 3.6% in 22,160 individuals undergoing screening colonoscopy at age 50-54. The rate of clinically significant serrated polyps was 5.9% in the 45- to 49-year-olds, closely similar to the 6.1% rate in patients age 50-54.
Of note, the prevalence of advanced adenomas was just 1.1% in individuals younger than age 40 years, jumping to 3.0% among 40- to 44-year-olds, 5.1% in those age 55-59, and 6.9% at age 60 or more. Clinically significant serrated polyps followed a similar pattern, with rates of 3.0% before age 40, 5.1% in 40- to 44-year-olds, 6.6% in 55- to 59-year-olds, and 6.0% in those who were older.
In a multivariate logistic regression analysis adjusted for sex, body mass index, smoking, and other potential confounders, 45- to 49-year-olds were at a 243% increased risk of finding advanced adenomas on colonoscopy, compared with those less than 40 years old, while the 50- to 54-year-olds had a virtually identical 244% increased risk.
Dr. Butterly noted that there are now 15,000 cases of colorectal cancer occurring annually in individuals under age 50 in the United States, with 3,600 deaths.
“Prevention of colorectal cancer in young, productive individuals is an essential clinical imperative that must be addressed,” she concluded.
She reported having no financial conflicts regarding her study.
SAN ANTONIO – The American Cancer Society’s 2018 qualified recommendation to lower the starting age for colorectal cancer screening from 50 to 45 years in average-risk individuals has picked up new support from a New Hampshire Colonoscopy Registry analysis.
Data from the population-based statewide colonoscopy registry demonstrated that the prevalence of both advanced adenomas and clinically significant serrated polyps was closely similar for average-risk New Hampshirites age 45-49 years and for those age 50-54, Lynn F. Butterly, MD, reported at the annual meeting of the American College of Gastroenterology.
“The clinical implication is that our data support the recommendation to begin average-risk colorectal cancer screening at age 45,” declared Dr. Butterly, a gastroenterologist at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
The American Cancer Society recommendation to lower the initial screening age was designed to address a disturbing national trend: the climbing incidence of colorectal cancer in young adults. Indeed, the incidence increased by 55% among 20- to 49-year-olds during 1995-2016, even while falling by 38% in individuals age 50 years and older. The 2018 recommendation was billed as “qualified” because it was based upon predictive modeling and National Cancer Institute Surveillance, Epidemiology, and End Results data which have been criticized as subject to potential bias. Several studies conducted in Korea and other Asian countries have reported a lower colorectal cancer risk in the younger adult population than in those age 50 or older, but questions have been raised about the applicability of such data to the U.S. population.
For Dr. Butterly and coinvestigators, the research imperative was clear: “We need to generate U.S. outcomes data for average-risk individuals age 45-49, versus those over age 50, for whom colorectal cancer screening is already strongly recommended.”
Toward that end, the investigators turned to the New Hampshire Colonoscopy Registry, which contains detailed data on 200,000 colonoscopies, with some 400 variables recorded per patient. To zero in on an average-risk population below age 50, they restricted the analysis to patients undergoing their first colonoscopy for evaluation of low-risk conditions including abdominal pain or constipation while excluding those with GI bleeding, iron-deficiency anemia, abnormal imaging, or a family history of colorectal cancer.
The final study population included 42,600 New Hampshire residents who underwent their first colonoscopy. The key outcomes were the prevalence of advanced adenomas, defined as adenomas more than 1 cm in size, or with high-grade dysplasia or villous elements, and the prevalence of clinically significant serrated polyps larger than 1 cm, or larger than 5 mm if proximally located, as well as traditional serrated adenomas and those with sessile features.
The prevalence of advanced adenomas in 1,870 average-risk patients aged 45-49 years was 3.7% and nearly identical at 3.6% in 22,160 individuals undergoing screening colonoscopy at age 50-54. The rate of clinically significant serrated polyps was 5.9% in the 45- to 49-year-olds, closely similar to the 6.1% rate in patients age 50-54.
Of note, the prevalence of advanced adenomas was just 1.1% in individuals younger than age 40 years, jumping to 3.0% among 40- to 44-year-olds, 5.1% in those age 55-59, and 6.9% at age 60 or more. Clinically significant serrated polyps followed a similar pattern, with rates of 3.0% before age 40, 5.1% in 40- to 44-year-olds, 6.6% in 55- to 59-year-olds, and 6.0% in those who were older.
In a multivariate logistic regression analysis adjusted for sex, body mass index, smoking, and other potential confounders, 45- to 49-year-olds were at a 243% increased risk of finding advanced adenomas on colonoscopy, compared with those less than 40 years old, while the 50- to 54-year-olds had a virtually identical 244% increased risk.
Dr. Butterly noted that there are now 15,000 cases of colorectal cancer occurring annually in individuals under age 50 in the United States, with 3,600 deaths.
“Prevention of colorectal cancer in young, productive individuals is an essential clinical imperative that must be addressed,” she concluded.
She reported having no financial conflicts regarding her study.
REPORTING FROM ACG 2019
A triple-antibiotic cure for Crohn’s disease?
SAN ANTONIO – A proprietary oral fixed-dose, triple-antibiotic combination pill offers a promising new approach to the treatment of Crohn’s disease, David Y. Graham, MD, declared at the annual meeting of the American College of Gastroenterology.
In the phase 3 MAP US trial, patients with Crohn’s disease who were randomized to the fixed-dose combination of 45 mg rifabutin, 95 mg clarithromycin, and 10 mg clofazimine, known for now as RHB-104, experienced significantly higher rates of clinical remission and improvement in inflammation as assessed endoscopically and via biomarkers, compared with placebo-treated controls, reported Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
RHB-104 is effective against Mycobacterium avium paratuberculosis (MAP) – and therein hangs a tale.
“MAP has been considered as a possible cause of Crohn’s disease since the disease was described by Crohn in 1932,” the gastroenterologist noted. “These randomized trial data provide further evidence suggesting an important role for MAP or similar microorganisms in the pathogenesis of Crohn’s disease.”
For Dr. Graham, this is a case of deja vu all over again. More than a quarter century ago he was lead author of a highly influential randomized, controlled trial which established that treatment with antibiotics directed against Helicobacter pylori cured peptic ulcer disease. As such, he became internationally recognized as a key figure in the resultant revolution in peptic ulcer treatment. He hears an echo of that earlier transformative change in the MAP US results.
“This is either an additional therapy or it’s the beginning of a paradigm shift. I mean, I see this as we’re standing at the same place now as we were standing with Helicobacter pylori 30 years ago, when the question was: Have we found something that we can eradicate and change the natural history of the disease and cure it? You can say this [MAP-directed therapy] is going in that direction, but it certainly hasn’t gotten to the point of proof yet. The results have to be reproduced,” he said.
The MAP US trial included 331 patients with moderate to severely active Crohn’s disease at 92 sites who had failed to achieve an adequate response with conventional therapies. Participants were randomized double blind to twice-daily RHB-104 or placebo for 52 weeks. Those not in remission at 26 weeks could opt for open-label RHB-104. Background concomitant treatment with corticosteroids, tumor necrosis factor inhibitors, and immunosuppressives was permitted.
The primary outcome was clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 at week 26. This was achieved in 36.7% of the active treatment group and 23% of controls, a highly significant difference. The clinical remission rates at week 16 were 42.2% and 29.1%, respectively. At week 26, 44% of RHB-104-treated patients had achieved at least a 100-point reduction in CDAI score, compared with baseline, as did 30.9% of controls. The key symptom score provided by the sum of the abdominal pain and bowel movement components of the CDAI was significantly lower in the RHB-104 group than in controls from week 16 on.
The remission rate at week 26 in the group on RHB-104 was similarly favorable regardless of whether or not they were on anti–tumor necrosis factor therapy.
“This suggests that RHB-104 can be used effectively and safely as an adjunct treatment to other medications to enhance the response to medical therapy,” according to Dr. Graham, who was principal investigator for MAP US.
The composite endpoint of clinical remission plus at least a 50% reduction from baseline in fecal calprotectin or C-reactive protein was achieved in 21.1% of the RHB-104 group and 9.1% of controls at week 26, and by 16.9% on RHB-104 and 7.9% on placebo at week 52.
In the 35 patients who underwent endoscopy at week 26, a 50% or greater reduction in the Simple Endoscopic Score in Crohn’s Disease was documented in 28.6% of patients on RHB-104 versus 4.8% of controls.
Durable remission, defined as a CDAI score below 150 at all study visits from week 16 to week 52, was achieved in 18.7% of the RHB-104 group, compared with 8.5% of controls.
The side effect profiles of RHB-104 and placebo were similar, with no serious adverse events recorded in the 52-week study. An increase in the QT interval on ECG was noted in the RHB-104 group from week 4 on, but it wasn’t associated with any clinical findings. Further study of this ECG finding is underway.
Several audience members rose to urge caution in interpreting the MAP US data.
“We must adhere to Koch’s postulates before we make conclusions about causative agents of an infectious disease, and I didn’t see those data here. So I look forward to a future presentation that shares that,” one gastroenterologist commented.
“I haven’t seen any data here that shows Mycobacterium was present in these patients,” noted another.
Dr. Graham replied that MAP US was a hypothesis-driven clinical trial: Crohn’s disease has much in common with an inflammatory bowel disease occurring in ruminant animals, where RHB-104 has shown treatment efficacy.
“This is a Mycobacterium avium organism, so it’s not something you’re going to cure in 2 weeks or 2 months. But the question is, do you have an effect on the disease, and the answer in MAP US was unquestionably yes. It’s very positive data to further pursue the hypothesis, but the study doesn’t provide a definitive answer,” he said.
Dr. Graham reported serving as a consultant to RedHill Biopharma, the study sponsor.
SAN ANTONIO – A proprietary oral fixed-dose, triple-antibiotic combination pill offers a promising new approach to the treatment of Crohn’s disease, David Y. Graham, MD, declared at the annual meeting of the American College of Gastroenterology.
In the phase 3 MAP US trial, patients with Crohn’s disease who were randomized to the fixed-dose combination of 45 mg rifabutin, 95 mg clarithromycin, and 10 mg clofazimine, known for now as RHB-104, experienced significantly higher rates of clinical remission and improvement in inflammation as assessed endoscopically and via biomarkers, compared with placebo-treated controls, reported Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
RHB-104 is effective against Mycobacterium avium paratuberculosis (MAP) – and therein hangs a tale.
“MAP has been considered as a possible cause of Crohn’s disease since the disease was described by Crohn in 1932,” the gastroenterologist noted. “These randomized trial data provide further evidence suggesting an important role for MAP or similar microorganisms in the pathogenesis of Crohn’s disease.”
For Dr. Graham, this is a case of deja vu all over again. More than a quarter century ago he was lead author of a highly influential randomized, controlled trial which established that treatment with antibiotics directed against Helicobacter pylori cured peptic ulcer disease. As such, he became internationally recognized as a key figure in the resultant revolution in peptic ulcer treatment. He hears an echo of that earlier transformative change in the MAP US results.
“This is either an additional therapy or it’s the beginning of a paradigm shift. I mean, I see this as we’re standing at the same place now as we were standing with Helicobacter pylori 30 years ago, when the question was: Have we found something that we can eradicate and change the natural history of the disease and cure it? You can say this [MAP-directed therapy] is going in that direction, but it certainly hasn’t gotten to the point of proof yet. The results have to be reproduced,” he said.
The MAP US trial included 331 patients with moderate to severely active Crohn’s disease at 92 sites who had failed to achieve an adequate response with conventional therapies. Participants were randomized double blind to twice-daily RHB-104 or placebo for 52 weeks. Those not in remission at 26 weeks could opt for open-label RHB-104. Background concomitant treatment with corticosteroids, tumor necrosis factor inhibitors, and immunosuppressives was permitted.
The primary outcome was clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 at week 26. This was achieved in 36.7% of the active treatment group and 23% of controls, a highly significant difference. The clinical remission rates at week 16 were 42.2% and 29.1%, respectively. At week 26, 44% of RHB-104-treated patients had achieved at least a 100-point reduction in CDAI score, compared with baseline, as did 30.9% of controls. The key symptom score provided by the sum of the abdominal pain and bowel movement components of the CDAI was significantly lower in the RHB-104 group than in controls from week 16 on.
The remission rate at week 26 in the group on RHB-104 was similarly favorable regardless of whether or not they were on anti–tumor necrosis factor therapy.
“This suggests that RHB-104 can be used effectively and safely as an adjunct treatment to other medications to enhance the response to medical therapy,” according to Dr. Graham, who was principal investigator for MAP US.
The composite endpoint of clinical remission plus at least a 50% reduction from baseline in fecal calprotectin or C-reactive protein was achieved in 21.1% of the RHB-104 group and 9.1% of controls at week 26, and by 16.9% on RHB-104 and 7.9% on placebo at week 52.
In the 35 patients who underwent endoscopy at week 26, a 50% or greater reduction in the Simple Endoscopic Score in Crohn’s Disease was documented in 28.6% of patients on RHB-104 versus 4.8% of controls.
Durable remission, defined as a CDAI score below 150 at all study visits from week 16 to week 52, was achieved in 18.7% of the RHB-104 group, compared with 8.5% of controls.
The side effect profiles of RHB-104 and placebo were similar, with no serious adverse events recorded in the 52-week study. An increase in the QT interval on ECG was noted in the RHB-104 group from week 4 on, but it wasn’t associated with any clinical findings. Further study of this ECG finding is underway.
Several audience members rose to urge caution in interpreting the MAP US data.
“We must adhere to Koch’s postulates before we make conclusions about causative agents of an infectious disease, and I didn’t see those data here. So I look forward to a future presentation that shares that,” one gastroenterologist commented.
“I haven’t seen any data here that shows Mycobacterium was present in these patients,” noted another.
Dr. Graham replied that MAP US was a hypothesis-driven clinical trial: Crohn’s disease has much in common with an inflammatory bowel disease occurring in ruminant animals, where RHB-104 has shown treatment efficacy.
“This is a Mycobacterium avium organism, so it’s not something you’re going to cure in 2 weeks or 2 months. But the question is, do you have an effect on the disease, and the answer in MAP US was unquestionably yes. It’s very positive data to further pursue the hypothesis, but the study doesn’t provide a definitive answer,” he said.
Dr. Graham reported serving as a consultant to RedHill Biopharma, the study sponsor.
SAN ANTONIO – A proprietary oral fixed-dose, triple-antibiotic combination pill offers a promising new approach to the treatment of Crohn’s disease, David Y. Graham, MD, declared at the annual meeting of the American College of Gastroenterology.
In the phase 3 MAP US trial, patients with Crohn’s disease who were randomized to the fixed-dose combination of 45 mg rifabutin, 95 mg clarithromycin, and 10 mg clofazimine, known for now as RHB-104, experienced significantly higher rates of clinical remission and improvement in inflammation as assessed endoscopically and via biomarkers, compared with placebo-treated controls, reported Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
RHB-104 is effective against Mycobacterium avium paratuberculosis (MAP) – and therein hangs a tale.
“MAP has been considered as a possible cause of Crohn’s disease since the disease was described by Crohn in 1932,” the gastroenterologist noted. “These randomized trial data provide further evidence suggesting an important role for MAP or similar microorganisms in the pathogenesis of Crohn’s disease.”
For Dr. Graham, this is a case of deja vu all over again. More than a quarter century ago he was lead author of a highly influential randomized, controlled trial which established that treatment with antibiotics directed against Helicobacter pylori cured peptic ulcer disease. As such, he became internationally recognized as a key figure in the resultant revolution in peptic ulcer treatment. He hears an echo of that earlier transformative change in the MAP US results.
“This is either an additional therapy or it’s the beginning of a paradigm shift. I mean, I see this as we’re standing at the same place now as we were standing with Helicobacter pylori 30 years ago, when the question was: Have we found something that we can eradicate and change the natural history of the disease and cure it? You can say this [MAP-directed therapy] is going in that direction, but it certainly hasn’t gotten to the point of proof yet. The results have to be reproduced,” he said.
The MAP US trial included 331 patients with moderate to severely active Crohn’s disease at 92 sites who had failed to achieve an adequate response with conventional therapies. Participants were randomized double blind to twice-daily RHB-104 or placebo for 52 weeks. Those not in remission at 26 weeks could opt for open-label RHB-104. Background concomitant treatment with corticosteroids, tumor necrosis factor inhibitors, and immunosuppressives was permitted.
The primary outcome was clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 at week 26. This was achieved in 36.7% of the active treatment group and 23% of controls, a highly significant difference. The clinical remission rates at week 16 were 42.2% and 29.1%, respectively. At week 26, 44% of RHB-104-treated patients had achieved at least a 100-point reduction in CDAI score, compared with baseline, as did 30.9% of controls. The key symptom score provided by the sum of the abdominal pain and bowel movement components of the CDAI was significantly lower in the RHB-104 group than in controls from week 16 on.
The remission rate at week 26 in the group on RHB-104 was similarly favorable regardless of whether or not they were on anti–tumor necrosis factor therapy.
“This suggests that RHB-104 can be used effectively and safely as an adjunct treatment to other medications to enhance the response to medical therapy,” according to Dr. Graham, who was principal investigator for MAP US.
The composite endpoint of clinical remission plus at least a 50% reduction from baseline in fecal calprotectin or C-reactive protein was achieved in 21.1% of the RHB-104 group and 9.1% of controls at week 26, and by 16.9% on RHB-104 and 7.9% on placebo at week 52.
In the 35 patients who underwent endoscopy at week 26, a 50% or greater reduction in the Simple Endoscopic Score in Crohn’s Disease was documented in 28.6% of patients on RHB-104 versus 4.8% of controls.
Durable remission, defined as a CDAI score below 150 at all study visits from week 16 to week 52, was achieved in 18.7% of the RHB-104 group, compared with 8.5% of controls.
The side effect profiles of RHB-104 and placebo were similar, with no serious adverse events recorded in the 52-week study. An increase in the QT interval on ECG was noted in the RHB-104 group from week 4 on, but it wasn’t associated with any clinical findings. Further study of this ECG finding is underway.
Several audience members rose to urge caution in interpreting the MAP US data.
“We must adhere to Koch’s postulates before we make conclusions about causative agents of an infectious disease, and I didn’t see those data here. So I look forward to a future presentation that shares that,” one gastroenterologist commented.
“I haven’t seen any data here that shows Mycobacterium was present in these patients,” noted another.
Dr. Graham replied that MAP US was a hypothesis-driven clinical trial: Crohn’s disease has much in common with an inflammatory bowel disease occurring in ruminant animals, where RHB-104 has shown treatment efficacy.
“This is a Mycobacterium avium organism, so it’s not something you’re going to cure in 2 weeks or 2 months. But the question is, do you have an effect on the disease, and the answer in MAP US was unquestionably yes. It’s very positive data to further pursue the hypothesis, but the study doesn’t provide a definitive answer,” he said.
Dr. Graham reported serving as a consultant to RedHill Biopharma, the study sponsor.
REPORTING FROM ACG 2019